Table of Contents
As filed with the Securities and Exchange Commission on December 23, 2010
Registration No. 333-170376
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
Amendment No. 2
to
FORM S-4
REGISTRATION STATEMENT
UNDER
THE SECURITIES ACT OF 1933
AMERICAN RENAL HOLDINGS INC.
(Exact name of Registrant as specified in its charter)
SEE TABLE OF ADDITIONAL REGISTRANTS
| Delaware | 8090 | 04-3477845 | ||
| (State or other jurisdiction of incorporation or organization) | (Primary Standard Industrial Classification Code Number) | (I.R.S. Employer Identification Number) |
66 Cherry Hill Drive,
Beverly, Massachusetts 01915
(978) 922-3080
(Address, including zip code, and telephone number, including area code, of registrant’s principal executive offices)
Michael R. Costa
Vice President and General Counsel
American Renal Holdings Inc.
66 Cherry Hill Drive,
Beverly, Massachusetts 01915
(978) 922-3080
(Name, address, including zip code, and telephone number, including area code, of agent for service)
With a copy to:
Stephan J. Feder, Esq.
Simpson Thacher & Bartlett LLP
425 Lexington Avenue
New York, New York 10017
(212) 455-2000
Approximate date of commencement of proposed exchange offers: As soon as practicable after this Registration Statement is declared effective.
If the securities being registered on this Form are being offered in connection with the formation of a holding company and there is compliance with General Instruction G, check the following box. ¨
If this Form is filed to register additional securities for an offering pursuant to Rule 462(b) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ¨
If this Form is a post-effective amendment filed pursuant to Rule 462(d) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ¨
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See the definitions of “large accelerated filer,” “accelerated filer,” and “smaller reporting company” in Rule 12b-2 of the Exchange Act.
| Large accelerated filer | ¨ | Accelerated filer | ¨ | |||||
| Non-accelerated filer | x | (Do not check if a smaller reporting company) | Smaller reporting company | ¨ |
If applicable, place an X in the box to designate the appropriate rule provision relied upon in conducting this transaction:
Exchange Act Rule 13e-4(i) (Cross-Border Issuer Tender Offer) ¨
Exchange Act Rule 14d-1(d) (Cross-Border Third-Party Tender Offer) ¨
CALCULATION OF REGISTRATION FEE
| Title of each class of securities to be registered | Amount to be registered | Proposed maximum offering price per unit (1) | Proposed maximum aggregate offering price (1) | Amount of registration fee | ||||
8.375% Senior Secured Notes due 2018 | $250,000,000 | 100% | $250,000,000 | $17,825(2) | ||||
Guarantees of 8.375% Senior Secured Notes due 2018 (3) | N/A | N/A | N/A | N/A(4) | ||||
| (1) | Estimated solely for purposes of calculating the registration fee pursuant to Rule 457(f) under the Securities Act of 1933, as amended (the “Securities Act”). |
| (2) | Previously paid. |
| (3) | See inside facing page for table of registrant guarantors. |
| (4) | Pursuant to Rule 457(n) under the Securities Act, no separate filing fee is required for the guarantees. |
The registrants hereby amend this Registration Statement on such date or dates as may be necessary to delay its effective date until the Registrants shall file a further amendment which specifically states that this Registration Statement shall thereafter become effective in accordance with Section 8(a) of the Securities Act of 1933, or until this Registration Statement shall become effective on such date as the Securities and Exchange Commission, acting pursuant to said Section 8(a), may determine.
Table of Contents
TABLE OF ADDITIONAL REGISTRANT GUARANTORS
Exact Name of Registrant Guarantor as | State or Other Jurisdiction of Incorporation or Organization | I.R.S. Employer Identification Number | Address and Telephone Number | |||||
C.P. Atlas Intermediate Holdings, LLC | Delaware | 27-2170865 | 375 Park Avenue 12th Floor, New York, NY 10152, (212) 672-5000 | |||||
American Renal Associates LLC | Delaware | 84-1694930 | 66 Cherry Hill Drive, Beverly, Massachusetts 01915, (978) 922-3080. | |||||
American Renal Management LLC | Delaware | 04-3527505 | 66 Cherry Hill Drive, Beverly, Massachusetts 01915, (978) 922-3080. | |||||
AKC Holding LLC | Delaware | 01-0833124 | 66 Cherry Hill Drive, Beverly, Massachusetts 01915, (978) 922-3080. | |||||
JKC Holding LLC | Delaware | 01-0833133 | 66 Cherry Hill Drive, Beverly, Massachusetts 01915, (978) 922-3080. | |||||
ARA-Boca Raton Holding LLC | Delaware | 55-0885438 | 1905 Clint Moore Road, Boca Raton, FL 33496, (561) 893-6878 | |||||
ARA-Ohio Holdings LLC | Delaware | 88-0519793 | 66 Cherry Hill Drive, Beverly, Massachusetts 01915, (978) 922-3080. | |||||
ARA-Rhode Island Dialysis II LLC | Delaware | 05-0598781 | 66 Cherry Hill Drive, Beverly, Massachusetts 01915, (978) 922-3080. | |||||
Texas-ARA LLC | Delaware | 04-3537394 | 66 Cherry Hill Drive, Beverly, Massachusetts 01915, (978) 922-3080. | |||||
American Renal Texas L.P. | Texas | 04-3535002 | 66 Cherry Hill Drive, Beverly, Massachusetts 01915, (978) 922-3080. | |||||
American Renal Texas II, L.P. | Texas | 83-0438331 | 66 Cherry Hill Drive, Beverly, Massachusetts 01915, (978) 922-3080. | |||||
Acute Dialysis Services-ARA LLC | Delaware | 26-1148649 | 66 Cherry Hill Drive, Beverly, Massachusetts 01915, (978) 922-3080. | |||||
Table of Contents
The information in this prospectus is not complete and may be changed. We may not sell these securities until the registration statement filed with the Securities and Exchange Commission is effective. This prospectus is not an offer to sell these securities and it is not soliciting an offer to buy these securities in any state where the offer or sale is not permitted.
SUBJECT TO COMPLETION, DATED DECEMBER 23, 2010
PRELIMINARY PROSPECTUS
$250,000,000

American Renal Holdings Inc.
Offer to Exchange all outstanding $250,000,000 8.375% Senior Secured Notes due 2018 (the “outstanding notes”) for an equal amount of 8.375% Senior Secured Notes due 2018, which have been registered under the Securities Act of 1933, as amended (the “exchange notes”).
We are conducting the exchange offer in order to provide you with an opportunity to exchange your unregistered notes for freely tradable notes that have been registered under the Securities Act of 1933, as amended.
The Exchange Offer
| • | We will exchange all outstanding notes that are validly tendered and not validly withdrawn for an equal principal amount of exchange notes that are freely tradable. |
| • | You may withdraw tenders of outstanding notes at any time prior to the expiration date of the exchange offer. |
| • | The exchange offer expires at 11:59 p.m., New York City time, on , 2011, unless extended. We do not currently intend to extend the expiration date. |
| • | The exchange of outstanding notes for exchange notes in the exchange offer will not be a taxable event for U.S. federal income tax purposes. |
| • | We will not receive any proceeds from the exchange offer. |
The Exchange Notes
| • | The exchange notes are being offered in order to satisfy certain of our obligations under the registration rights agreement entered into in connection with the placement of the outstanding notes. |
| • | The terms of the exchange notes to be issued in the exchange offer are substantially identical to the outstanding notes, except that the exchange notes will be freely tradable. |
| • | The parent and certain subsidiaries of American Renal Holdings Inc. initially jointly and severally, irrevocably and unconditionally guaranteed, on a secured senior basis, the performance and full and punctual payment when due, whether at maturity, by acceleration or otherwise, of all obligations of American Renal Holdings Inc. under the outstanding notes, exchange notes and the indenture governing the notes. |
Resales of Exchange Notes
| • | The exchange notes may be sold in the over-the-counter market, in negotiated transactions or through a combination of such methods. We do not plan to list the exchange notes on a national market. |
All untendered outstanding notes will continue to be subject to the restrictions on transfer set forth in the outstanding notes and in the indenture. In general, the outstanding notes may not be offered or sold, unless registered under the Securities Act of 1933, as amended, except pursuant to an exemption from, or in a transaction not subject to, the Securities Act of 1933, as amended, and applicable state securities laws. Other than in connection with the exchange offer, we do not currently anticipate that we will register the outstanding notes under the Securities Act of 1933, as amended.
You should consider carefully therisk factors beginning on page 21 of this prospectus before participating in the exchange offer.
Neither the Securities and Exchange Commission nor any state securities commission has approved or disapproved of the exchange notes to be distributed in the exchange offer or passed upon the adequacy or accuracy of this prospectus. Any representation to the contrary is a criminal offense.
The date of this prospectus is , 2010.
Table of Contents
| Page | ||||
| ii | ||||
| ii | ||||
| 1 | ||||
| 21 | ||||
| 44 | ||||
| 45 | ||||
| 46 | ||||
| 47 | ||||
| 49 | ||||
| 51 | ||||
MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS | 56 | |||
| 84 | ||||
| 103 | ||||
| 107 | ||||
| 118 | ||||
SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT | 119 | |||
| 120 | ||||
| 122 | ||||
| 124 | ||||
| 134 | ||||
| 135 | ||||
MATERIAL U.S. FEDERAL INCOME TAX CONSEQUENCES OF THE EXCHANGE OFFER | 200 | |||
| 201 | ||||
| 203 | ||||
| 204 | ||||
| 204 | ||||
| 204 | ||||
| F-1 | ||||
This prospectus does not constitute an offer to sell, or a solicitation of an offer to buy, any exchange notes offered hereby in any jurisdiction where, or to any person to whom, it is unlawful to make such offer or solicitation. The information contained in this prospectus speaks only as of the date of this prospectus unless the information specifically indicates that another date applies. No dealer, salesperson or other person has been authorized to give any information or to make any representations other than those contained in this prospectus in connection with the offer contained herein and, if given or made, such information or representations must not be relied upon as having been authorized by American Renal Holdings Inc. Neither the delivery of this prospectus nor any sales made hereunder shall under any circumstances create an implication that there has been no change in our affairs or that of our subsidiaries since the date hereof.
i
Table of Contents
AmericanRenal, American Renal Associates, ARA®, the American Renal Associates logo and other trademarks or service marks of American Renal appearing in this prospectus are our property. All trade names, trademarks and service marks of other companies appearing in this prospectus are the property of the respective holders.
Certain market data and other statistical information used throughout this prospectus are based on the 2009 Annual Data Report prepared by the United States Renal Data System (“USRDS”) and information from the Centers for Medicare and Medicaid Services (“CMS”). Some data are also based on our good faith estimates, which are derived from management’s review of internal data and information, as well as the independent sources such as independent industry publications, government publications, reports by market research firms or other published independent sources. Although we believe these sources are reliable, we have not independently verified the information contained therein and cannot guarantee its accuracy and completeness.
ii
Table of Contents
This summary highlights information contained elsewhere in this prospectus. This summary is not complete and may not contain all of the information that you should consider to make your decisions regarding the exchange offer. You should carefully read the entire prospectus, including the section entitled “Risk Factors.”
Unless the context otherwise requires it or as otherwise indicated, “we,” “us,” “our” and similar terms, as well as references to the “Company,” “American Renal” and “ARA” refer to C.P. Atlas Intermediate Holdings, LLC and its consolidated subsidiaries.
The financial and performance information throughout this prospectus includes amounts derived from our non-guarantor joint venture subsidiaries. See “Management’s Discussion and Analysis of Financial Condition and Results of Operations—Critical Accounting Policies and Estimates,” Note Q of “Audited Financial Statements for the years December 31, 2009, 2008 and 2007—Notes to Consolidated Financial Statements” and Note K of “Unaudited Financial Statements for the periods ended September 30, 2010 and 2009—Notes to Unaudited Consolidated Financial Statements.”
Our Company
We are a national provider of kidney dialysis services for patients suffering from chronic kidney failure, also known as end stage renal disease, or ESRD. As of September 30, 2010, we owned and operated 88 dialysis clinics treating more than 6,100 patients in 17 states and the District of Columbia. Our operating model is based on shared ownership of our facilities with physicians, known as nephrologists, who specialize in kidney-related diseases in the local market served by the clinic. Each clinic is maintained as a separate joint venture, or JV, in which we own a controlling interest and our local nephrologist partners own noncontrolling interests.
We believe we are the largest dialysis services provider in the United States founded and operating exclusively on a joint venture model. We have invested significantly in the development of our senior and field level core management function and operating procedures, and we believe we are well-positioned to continue to realize the benefits of platform scalability, which will allow us to add clinics without significant incremental operating costs. As a result, our revenue and earnings growth will be driven primarily by the growth in the number of our JV clinics and treatment growth at our existing clinics. Since our founding, we have opened 66 new clinics (“de novo clinics”) and have acquired 22 clinics from third parties (“acquired clinics”) that we subsequently converted to our JV model. In 2009 we opened 10 clinics, seven of which are de novo clinics and three are acquired clinics. We plan to continue the expansion of our operations with the use of the JV model primarily through the redeployment of free cash flow to create de novo clinics, to expand existing clinics and to selectively acquire clinics.
We believe our JV model is attractive to the nephrologist community because it provides a platform for favorable clinical outcomes and superior financial results while enabling our nephrologist partners to focus on providing the highest quality of patient care rather than the administration of corporate-directed treatments and protocols. Through our wholly-owned subsidiary, American Renal Management, LLC (“ARM”), we provide our nephrologist partners with the managerial, accounting, financial, technological and administrative support necessary to operate our clinics. In particular, our management services focus on critical revenue cycle management for each patient, which encompasses patient registration, insurance and benefit verification, medical treatment documentation and coding, bill preparation and collections. In addition, our corporate clinical advisory team, which includes our National Medical Director and clinical and regulatory advisors, assists our nephrologist partners in establishing clinical objectives, while our operating model allocates to our nephrologist partners autonomy over the clinical protocols used to achieve those objectives.
According to the United States Renal Data System, or USRDS, the number of dialysis patients in the U.S. is expected to continue growing faster than increases in the general population, primarily due to an aging
1
Table of Contents
population with increased life expectancies. As a result, we believe there will be an increasing need for dialysis clinics. In addition, we believe that the JV model will continue to be one of the fastest growing operating models for dialysis clinics and will represent an increasing percentage of the dialysis clinic industry. We believe that our JV model, with its clinic level autonomy for the physician, together with the comprehensive management services that we have developed, enhance the clinical and operating performance of our clinics and make us a preferred dialysis partner for nephrologists. We believe this will lead to additional JV opportunities with nephrologists seeking the corporate support we provide.
Dialysis Services Industry Overview
End Stage Renal Disease, or ESRD, is characterized by the loss of kidney functionality and is normally irreversible and fatal unless treated. ESRD most commonly results from complications associated with diabetes, hypertension, renal and hereditary diseases, old age and a combination of other risk factors. ESRD requires continued dialysis treatments or a kidney transplant to sustain life. Absent transplantation, the average life expectancy for an ESRD patient on dialysis is approximately five years following diagnosis, according to the USRDS. Scarcity of compatible kidneys has limited the option for transplants, causing most patients suffering from ESRD to rely on dialysis. Dialysis is the removal of toxins and fluids from the blood of ESRD patients by artificial means. Patients suffering from ESRD generally require dialysis at least three times per week, amounting to approximately 156 treatments per year, for the remainder of their lives.
There are two primary methods of dialysis commonly used today, hemodialysis and peritoneal dialysis. Hemodialysis, or the removal of toxins and fluid from the blood through a specially designed filter, is the most common form of ESRD treatment and represented approximately 92% of all dialysis treatments in the United States in 2007. Hemodialysis is typically performed in outpatient dialysis clinics and lasts approximately 3.5 hours per treatment. Treatments are usually performed by teams of licensed nurses and trained technicians pursuant to a physician’s instructions. Almost all patients receive hemodialysis in our outpatient dialysis clinics as their primary ESRD treatment, although we also provide such services in the home. Peritoneal dialysis uses the patient’s peritoneal, or abdominal, cavity to eliminate fluid and toxins. A patient generally performs peritoneal dialysis at home. We also provide peritoneal dialysis services to patients who prefer and are able to receive that form of treatment.
Large, Growing Market for Outpatient Dialysis Services
The total annual cost of providing healthcare services to ESRD patients in the U.S. in 2007 has been estimated by the USRDS to be approximately $35 billion, of which we believe approximately $18 billion represents our currently addressable market. According to the most recent report by the USRDS, there were approximately 527,000 ESRD patients in the United States as of December 31, 2007, with the number of ESRD patients expected to grow at an annual rate consistent with the historical rate of 3-4%, outpacing general population growth. Aside from a relatively small number of individuals who qualify for and successfully undergo kidney transplants, the vast majority of ESRD patients require three dialysis treatments per week, on average, for the remainder of their lives. USRDS data indicates that the prevalence rate in patients ages 65 to 74 increased 24% from 2000 to 2007; while, in patients ages 75 and older the prevalence rate increased 28% over the same period.
According to the USRDS, the increasing percentage of the U.S. population afflicted with ESRD has been primarily caused by:
| • | aging of the general population; |
| • | improved treatment and increased survival rate of patients with diabetes, hypertension and other illnesses that lead to ESRD; |
2
Table of Contents
| • | growth rates of minority populations with higher than average incidence rates of ESRD; and |
| • | improved dialysis technology, that has enabled older patients and those who previously could not tolerate dialysis due to other illnesses, to benefit from this treatment. |
Reimbursement Environment
A majority of reimbursement for dialysis services is provided by the federal government’s Medicare ESRD program based on rates established by the Centers for Medicare and Medicaid Services, or CMS. The coverage criteria for dialysis centers under Medicare are well defined and, as a result, Medicare reimbursement for dialysis services has historically been more predictable than reimbursements for most other services and procedures. ESRD has been classified as a chronic disability since 1972 and patients are entitled to treatment and Medicare benefits regardless of age or financial circumstances. Generally, if a patient does not have employer-sponsored health coverage, Medicare becomes the primary payor either immediately or after a three-month waiting period. For a patient with employer-sponsored health coverage, Medicare generally becomes the primary payor after 33 months, which includes a three-month coordination of benefits period, or earlier if the patient’s employer-sponsored health coverage terminates. Additional sources of reimbursement for dialysis services include other government agencies such as state Medicaid programs as well as private pay options.
Currently, Medicare reimburses dialysis providers separately for treatments and ancillaries. Recently approved Medicare legislation, however, will establish a “bundled” Medicare rate for dialysis reimbursement, starting in 2011. This new bundled rate will combine the current payments for composite rate and the separately billable dialysis services into a single base rate. This rate will include all services such as certain drugs and laboratory tests which are currently billed separately. The initial 2011 bundled rate will be set based on a 2% reduction in the payment rate that providers would have received under the historical fee for service payment methodology and based on the lowest average industry pharmaceutical utilization from 2007 to 2009. Beginning in 2012, the new single bundled payment base rate will be adjusted annually for inflation based upon a market basket index, less a productivity adjustment based on a 10-year moving average of productivity as projected by the Secretary of Health and Human Services. The bundled payment rate will be determined by the Secretary of Health and Human Services, who will have discretion to determine the base payment rate based on the goods and services included in the bundled rate. Dialysis providers, including us, will have the option to move fully to the bundled payment system in 2011 or to phase in the payment system over four years on a facility by facility basis. We believe that the move to bundled payment, together with the introduction of automatic annual rate increases, will result in greater predictability of rates and stability of revenue for the Company. The Congressional Budget Office estimates that these changes will result in an additional $1.5 billion in government payments to the dialysis industry over the next 10 years.
Before Medicare becomes the primary payor, a patient’s commercial insurance plan, if any, is responsible for payment of the dialysis services provided. Although commercial payment rates vary significantly, average commercial payment rates are generally significantly higher than Medicare rates and often include price increases. Payment methods from commercial payors include a single lump-sum per treatment, referred to as bundled rates, and separate payments for ancillary treatments and pharmaceuticals, if used as part of the dialysis treatment, referred to as fee for service rates. We believe commercial payors are generally sensitive to the special circumstances and challenges faced by ESRD patients, particularly because they are responsible for primary coverage for a relatively limited number of ESRD patients for a finite 30-month period of time. We also believe commercial payors are incentivized to facilitate quality dialysis care because patients who do not regularly receive dialysis treatment typically require hospitalization and other more costly acute care treatment. For the year ended December 31, 2009, we derived approximately 43% of our net operating revenues from commercial payors, which represented approximately 14% of the treatments performed. The source of the balance of our treatments and net operating revenues come from Medicare and other governmental payors.
3
Table of Contents
Our Competitive Strengths
Focus On Core Values Leading to Patient Satisfaction and Strong Patient Base
We believe our core values, including our focus on patient satisfaction, have contributed to our loyal patient base, which is the key to stable and dependable revenues. Our values state our commitment to excellent patient care, providing the nephrologists autonomy to make decisions and practice as he/she deems appropriate and treating our clinic staff members as a critical and valuable asset. We believe the satisfaction of our employees encourages them to provide a comfortable environment and better treatment for patients. We also maintain a strong focus on revenue cycle management so that patients may maximize insurance coverage. We believe we provide value to patients by assisting them in navigating the complex healthcare reimbursement climate, and through our knowledge and application of health insurance rules, to consistently obtain high quality insurance coverage for patients.
Differentiated Business Model Attracts Nephrologists, Aligns Interests and Enhances Clinic Level Performance
We seek to partner with leading nephrologists within their local community. To date, none of our nephrologist partners has sought our consent to divest his or her joint venture ownership interest. We believe that our JV business model aligns the interests of the clinic owners with that of the patients and physicians better than the more traditional model of wholly-owned corporate ownership of a clinic. By having an investment in the clinics where their patients are treated, our nephrologist partners have a vested stake in the quality, reputation and performance of the clinics. Through ARM, we provide operational and managerial support to our clinics with rigorous individual patient focus. We strive to maintain the high quality of these support services through the development and implementation of patient care policies and procedures, education, mentoring and audits at each of our clinics. We also invest in state-of-the-art facilities, equipment, and amenities at our clinics. We believe that these management services substantially ease the burden of back-office responsibilities and enable our nephrologist partners to focus on providing the highest quality of patient care, which in turn drives high levels of patient satisfaction and financial performance.
Disciplined Approach to Maintaining Efficient Operations
We maintain a disciplined approach to enhancing performance in key areas such as revenue cycle management. We work to improve our processes with regard to patient registration, facilitation and verification of insurance, payor interaction and arrangements, and billing and collections in an effort to maximize our revenue per treatment. We believe our efforts have resulted in strong revenue per treatment rates, as well as low days’ sales outstanding and bad debt expense. In addition, we believe the rigorous management policies and procedures that we have consistently put in place at each clinic that we own and operate have resulted in operating expenses per treatment that are among the lowest in the industry as well as low general and administrative expenses per treatment.
Experience in Establishing De Novo Clinics and Selectively Completing Acquisitions
Our de novo clinic strategy encompasses all facets of development, from site review to personnel hiring. We have never had a survey deficiency delay Medicare certification for one of our de novo clinics, and we typically achieve EBITDA positive operations within 12 months of opening a de novo clinic. We believe our established de novo clinic model offers turnkey solutions to nephrologist partners who are interested in establishing de novo clinics with us.
In addition, we selectively pursue acquisitions of existing dialysis clinics which we restructure as JVs. Although acquisition costs for an existing dialysis clinic are typically much higher than the cost to develop a de novo clinic, we believe our management policies and procedures for acquiring clinics have allowed us to achieve
4
Table of Contents
significant improvements in key operating metrics in a relatively short period of time. We believe that as a result of our experience with acquiring and restructuring existing dialysis clinics, those clinics have enjoyed improved clinical outcomes and financial performance.
Consistent Financial Performance and Strong Free Cash Flow Generation
We believe our favorable revenue per treatment rates and our focus on efficiency of operations in a stable and growing industry has resulted in consistent, strong financial performance. From 2005 to 2009, we have achieved compound annual net revenue growth of 26%. In addition, from 2007 to 2009, our non-guarantor joint venture subsidiaries achieved compound annual net revenue growth of 24%. As a result, our free cash flow generation has been strong. During 2005 to 2009, we had on average non-acquired treatment growth of approximately 19% annually. We have also been able to increase our Adjusted EBITDA margin to 26% of net sales. We believe this increase in margin illustrates the benefits that are achieved by growing our portfolio of dialysis clinics on our management services platform. In addition, our existing clinics typically require minimal maintenance capital expenditure consisting of less than 2% of net revenues in 2009. In the year ended December 31, 2009, the Company generated $51.3 million in cash flows from operating activities, primarily from distributions from JV shares and management services fees, $13.3 million of which was derived from the issuer and its subsidiary guarantors, and the remaining $38.0 million of which was derived from our non-guarantor joint venture subsidiaries.
Experienced Management Team Supported by Strong Equity Sponsorship
Most of our executive and senior management team has held multiple positions with one or more of our competitors and have strong contacts throughout the dialysis services industry with providers, clinical staff, payors, vendors and other parties. One of our three founding executives and the Chairman of our Board of Directors, Christopher T. Ford, is also the current chairman of the Kidney Care Council. We believe this breadth and depth of experience gives our leadership team the knowledge and resources to manage more effectively relations with our nephrologist partners and other personnel, enhance operating results and promote growth.
We benefit from the sponsorship of Centerbridge Capital Partners, L.P., our majority investor following the close of the Transactions, as described below. Centerbridge Partners, L.P., a private investment firm with over $11 billion of capital under management in Centerbridge Capital Partners, L.P. and certain affiliated entities (“Centerbridge”), is focused on making value-oriented investments in select industries in partnership with strong management teams.
Our Business Strategy
Key components of our business strategy include:
Continued Focus on Achieving Superior Clinical Outcomes
We believe our reputation for providing quality care is a key factor in attracting patients and physicians and in securing contracts with healthcare plans. Our clinical team works routinely with individual physicians, clinic managers and dieticians, and our corporate management team promotes a patient- and physician-focused corporate culture in order to optimize clinical outcomes, improve operating performance, and ensure compliance with applicable laws and regulations. In addition, we engage in organized and systematic efforts through our quality of care management programs to monitor and improve the quality of services we deliver. These efforts include the development and implementation of patient care policies and procedures, clinical education and training programs, education and mentoring related to our clinical guidelines and protocols and audits of the quality of services rendered at each of our centers.
Recruitment and Retention of Leading Physicians
Our effective recruitment of leading nephrologist partners is critical to achieving our growth objectives. We believe we enjoy a strong and growing reputation among the nephrologist community, ensuring that nephrologists who contemplate a migration to the shared-ownership model strongly consider us. We will continue to leverage our market position and reputation to identify, screen and recruit experienced nephrologists.
5
Table of Contents
We enjoy strong retention rates. We believe this is due in large part to nephrologist partner satisfaction with our JV model, which gives nephrologists autonomy over clinical protocols and allows them to focus on providing high quality patient care. Since inception, none of our nephrologist partners have asked for our consent to a transfer of their ownership interests in our clinics. Further, although certain of our nephrologist partners had the option of requiring us to purchase their equity interests as a result of the Transactions, none chose to do so. We believe our existing network of nephrologist partners will be a key resource for us in creating future JVs with nephrologists who are not currently associated with us.
Further Implementation of Rigorous Management Services
A critical component of our business strategy is to enable our nephrologist partners to focus on providing superior individual patient care while we manage the daily operations of the clinic in support of the nephrologist partners. As dialysis clinics begin to implement the new bundled payment rate and other procedures to be adopted pursuant to recently passed healthcare legislation, we believe the administrative services that we provide to our nephrologist partners will be of even greater value. By offering the management services of a large corporation combined with the responsiveness of an entrepreneurial enterprise, we believe we help our clinics achieve desirable clinical outcomes, growth and profitability. These management services are provided under long-term renewable management services agreements and include, among others, operations management, negotiating pricing of products from vendors, coordinating outsourced clinical laboratory testing services, reporting and benchmarking of clinical outcomes, implementing revenue cycle management practices, training and education for our staff, performing back-office finance, accounting and payroll functions, managing compliance programs, and administering human resources support.
Utilization of Internally Generated Cash Flow to Support Expansion
We intend to leverage our JV model and our reputation in the nephrology community to continue the development of de novo clinics in the U.S., both in new and existing markets. Our streamlined approach to opening de novo clinics delivers attractive financial returns to us and our nephrologist partners. Our substantial free cash flow generation provides for a significant portion of the funding for continued expansion. Additionally, nephrologist partners have reinvested a portion of their return on capital from existing sites to expand those sites or invest in de novo clinics together with us. De novo clinics typically reach positive EBITDA results within 12 months of opening, which we believe results in attractive returns on our investment. As a result of our substantial expertise in opening de novo clinics, we have never had a survey deficiency delay Medicare certification for one of our de novo clinics, and further, we have not experienced delays to our development timeline as a result of delays in certification.
Because the acquisition cost for an existing dialysis clinic is typically much higher than the cost to develop a de novo clinic, we typically acquire existing dialysis clinics in very selective situations. We pursue acquisitions that we believe to be an effective use of invested capital, or in situations where other factors we believe to be important, such as our desire to enter a new market and use the acquired clinic as the base from which to open additional de novo clinics in that market, favor such acquisitions. We intend to continue to pursue acquisitions of existing clinics with reputations for strong quality and service as well as leading local market shares. In making these acquisitions, we intend to continue to restructure the ownership of the acquired clinic as a joint venture in accordance with our JV model. In applying our management policies and procedures to acquired clinics, we believe we have been able to achieve significant improvements in key operating metrics in a relatively short period of time, which have resulted in improved clinical outcomes and financial performance at those clinics.
6
Table of Contents
Summary of the Transactions
On March 22, 2010, American Renal Holdings Inc. (“ARH”) entered into a Contribution and Merger Agreement (the “Merger Agreement”) with C.P. Atlas Holdings, Inc. (the “Parent”), C.P. Atlas Intermediate Holdings, LLC, C.P. Atlas Acquisition Corp., certain of ARH’s stockholders party thereto and Pamlico Capital I, L.P. (formerly known as Wachovia Capital Partners GP I, LLC) (“Sellers’ Representative”), pursuant to which ARH agreed that C.P. Atlas Acquisition Corp. shall merge with and into ARH (the “Merger”) and, after which, ARH became the surviving entity and a wholly owned subsidiary of C.P. Atlas Intermediate Holdings, LLC, which is in turn a wholly owned subsidiary of the Parent. The parties agreed to consummate the Merger, subject to the terms and conditions set forth in the Merger Agreement, for an aggregate purchase price of $415 million, subject to adjustments including, without limitation, for working capital, indebtedness, certain specified liabilities and certain tax savings. ARH agreed that $2.5 million of the purchase price be placed into a third-party escrow account as security for working capital adjustments and that $27.5 million of the purchase price be placed into a third-party escrow account as security for indemnification claims that may be made by the Parent or its affiliates.
As of the effective time of the Merger, holders of shares of ARH’s common stock have no further ownership interest in ARH and instead received cash and the right to receive certain future payments from the Sponsor, ARH and/or the escrow agent pursuant to the Merger Agreement (in the case of the escrow agent, in respect of the unused portion of the third-party escrow accounts, if any) (with the exception of certain members of senior management and certain other employees who have agreed not to have certain of their equity interests cashed out in the Merger and holders who perfect their appraisal rights under Delaware law).
In connection with the Merger:
| • | Centerbridge made an aggregate cash equity investment of $161.5 million in the Parent, subject to certain adjustments, and in exchange, received 87% of the common stock of the Parent immediately following the Merger. |
| • | Certain members of ARH’s management exchanged $19.9 million of proceeds that they received from the Transaction for 878,571 newly issued shares of common stock of the Parent and options to purchase 93,186 shares of common stock of the Parent. |
| • | ARH entered into a $25 million revolving credit facility (the “New Revolving Credit Facility”). |
| • | ARH offered the outstanding notes being exchanged hereby. |
The Merger and the financing transactions described above are collectively referred to herein as the “Transactions.”
The aggregate purchase price of approximately $415 million for the Merger, plus related fees and expenses, was funded by the equity investment by Centerbridge and from certain members of management and the net proceeds from the offering of the outstanding notes. See “Use of Proceeds,” “Description of Other Indebtedness,” and “The Transactions.” In connection with the Merger, all of ARH’s debt and preferred stock that existed prior to the closing date of the Merger was repaid or redeemed, except for $11.9 million aggregate amount of third-party debt as of December 31, 2009. Other third-party debt of ARH’s JVs was refinanced through secured intercompany loans. The secured intercompany loans, which total $36.3 million as of December 31, 2009, on a pro forma basis as if the Transactions had occurred on that date, were pledged as collateral for our New Revolving Credit Facility and for the outstanding notes being exchanged hereby. Although certain of ARH’s nephrologist partners had the right to require us to repurchase some or all of the equity interests in certain clinics as a result of the Transactions, these rights were waived.
7
Table of Contents
Ownership Structure and Organizational Chart
The following chart summarizes our organizational structure, equity ownership and our principal indebtedness as of the date of this prospectus. This chart is provided for illustrative purposes only and does not represent all legal entities of the Company and its consolidated subsidiaries or all obligations of such entities.
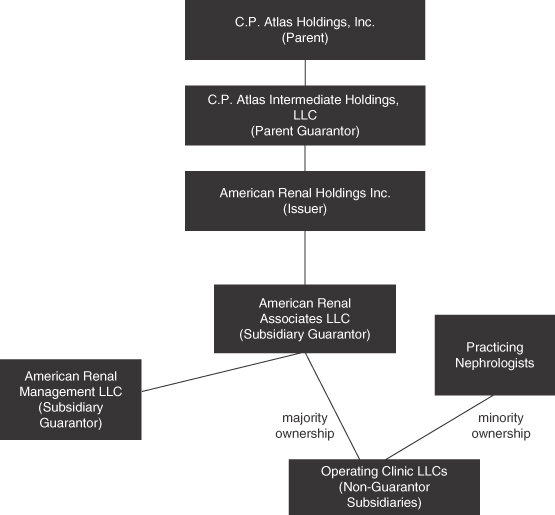
8
Table of Contents
Our Sponsor
Centerbridge Partners, L.P., established in 2005, is a private investment firm that sponsors and manages funds that make traditional private equity investments, distressed investments and other credit investments.
The firm is dedicated to partnering with world-class management teams to invest throughout a company’s capital structure and to employ various strategies to help companies achieve their operating and financial objectives. Through Centerbridge Capital Partners, L.P., the firm has made investments in companies that vary in size and geography and span a broad range of industries. The firm’s professionals have significant experience investing in healthcare companies.
Centerbridge’s limited partners include many prominent financial institutions, university endowments, public and corporate pensions and sovereign wealth funds, as well as foundations and other charitable trusts.
Our Corporate Information
American Renal Holdings Inc. was incorporated in Delaware on July 19, 1999. Our principal executive offices are located at 66 Cherry Hill Drive, Beverly, Massachusetts 01915 and our telephone number is (978) 922-3080. Our corporate website address is www.americanrenal.com. Information contained on our website is not a part of this prospectus.
9
Table of Contents
The Exchange Offer
On May 7, 2010, we completed the private offering of $250,000,000 aggregate principal amount of our 8.375% Senior Secured Notes due 2018, which we refer to in this prospectus as the “outstanding notes.” The term “exchange notes” refers to the 8.375% Senior Secured Notes due 2018 as registered under the Securities Act of 1933, as amended (the “Securities Act”). References to the “notes” in this prospectus are references to both the outstanding notes and the exchange notes. This prospectus is part of a registration statement covering the exchange of the outstanding notes for the exchange notes.
General | American Renal Holdings Inc. and the guarantors of the notes entered into a registration rights agreement with the initial purchasers in the private offering in which American Renal Holdings Inc. and the guarantors of the notes agreed to deliver to you this prospectus as part of the exchange offer and agreed to use all commercially reasonable efforts to have the registration statement covering the exchange to be declared effective on or prior to the date 210 days after the closing of the private offering. You are entitled to exchange in the exchange offer your outstanding notes for exchange notes which are identical in all material respects to the outstanding notes except: |
| • | the exchange notes have been registered under the Securities Act; |
| • | the exchange notes are not entitled to certain registration rights which are applicable to the outstanding notes under the registration rights agreement; and |
| • | certain special interest rate provisions in the registration rights agreement are no longer applicable. |
The Exchange Offer | We are offering to exchange up to $250,000,000 aggregate principal amount of our 8.375% Senior Secured Notes due 2018, which have been registered under the Securities Act, for up to $250,000,000 aggregate principal amount of our existing 8.375% Senior Secured Notes due 2018. Outstanding notes may be exchanged only in denominations of $2,000 and integral multiples of $1,000 in excess of $2,000. |
Resale | Based on an interpretation by the staff of the Securities and Exchange Commission (the “SEC”) set forth in no-action letters issued to third parties, we believe that the exchange notes issued pursuant to the exchange offer in exchange for the outstanding notes may be offered for resale, resold and otherwise transferred by you (unless you are our “affiliate” within the meaning of Rule 405 under the Securities Act) without compliance with the registration and prospectus delivery provisions of the Securities Act, provided that: |
| • | you are acquiring the exchange notes in the ordinary course of your business; and |
| • | you have not engaged in, do not intend to engage in, and have no arrangement or understanding with any person to participate in, a distribution of the exchange notes. |
10
Table of Contents
If you are a broker-dealer and receive exchange notes for your own account in exchange for outstanding notes that you acquired as a result of market-making activities or other trading activities, you must acknowledge that you will deliver this prospectus in connection with any resale of the exchange notes. See “Plan of Distribution.”
Any holder of outstanding notes who:
| • | is our affiliate; |
| • | does not acquire exchange notes in the ordinary course of its business; or |
| • | tenders its outstanding notes in the exchange offer with the intention to participate, or for the purpose of participating, in a distribution of exchange notes; |
cannot rely on the position of the staff of the SEC enunciated inMorgan Stanley & Co. Incorporated(available June 5, 1991) andExxon Capital Holdings Corporation(available May 13, 1988), as interpreted inShearman & Sterling(available July 2, 1993), or similar no-action letters and, in the absence of an exemption therefrom, must comply with the registration and prospectus delivery requirements of the Securities Act in connection with any resale of the exchange notes.
Expiration Date; Withdrawal of Tender | The exchange offer will expire at 11:59 p.m., New York City time, on , 2011, unless extended by us. We do not currently intend to extend the expiration date. You may withdraw the tender of your outstanding notes at any time prior to the expiration of the exchange offer. We will return to you any of your outstanding notes that are not accepted for any reason for exchange, without expense to you, promptly after the expiration or termination of the exchange offer. |
Conditions to the Exchange Offer | The exchange offer is subject to customary conditions, which we may waive. See “The Exchange Offer—Conditions to the Exchange Offer” of this prospectus for more information. |
Procedures for Tendering Outstanding Notes | If you wish to participate in the exchange offer, you must complete, sign and date the accompanying letter of transmittal, or a facsimile of such letter of transmittal, according to the instructions contained in this prospectus and the letter of transmittal. You must then mail or otherwise deliver the letter of transmittal, or a facsimile of such letter of transmittal, together with your outstanding notes and any other required documents, to the exchange agent at the address set forth on the cover page of the letter of transmittal. |
If you hold outstanding notes through The Depository Trust Company (“DTC”) and wish to participate in the exchange offer, you must comply with the Automated Tender Offer Program procedures of
11
Table of Contents
DTC by which you will agree to be bound by the letter of transmittal. By signing, or agreeing to be bound by, the letter of transmittal, you will represent to us that, among other things: |
| • | you are not our “affiliate” within the meaning of Rule 405 under the Securities Act; |
| • | you do not have an arrangement or understanding with any person or entity to participate in the distribution of the exchange notes; |
| • | you are acquiring the exchange notes in the ordinary course of your business; and |
| • | if you are a broker-dealer that will receive exchange notes for your own account in exchange for outstanding notes that were acquired as a result of market-making activities, you will deliver a prospectus, as required by law, in connection with any resale of such exchange notes. |
Special Procedures for Beneficial Owners | If you are a beneficial owner of outstanding notes which are registered in the name of a broker, dealer, commercial bank, trust company or other nominee, and you wish to tender such outstanding notes in the exchange offer, you should contact such registered holder promptly and instruct such registered holder to tender on your behalf. If you wish to tender on your own behalf, you must, prior to completing and executing the letter of transmittal and delivering your outstanding notes, either make appropriate arrangements to register ownership of the outstanding notes in your name or obtain a properly completed bond power from the registered holder. The transfer of registered ownership may take considerable time and may not be able to be completed prior to the expiration date. |
Guaranteed Delivery Procedures | If you wish to tender your outstanding notes and your outstanding notes are not immediately available or you cannot deliver your outstanding notes, the letter of transmittal or any other required documents, or you cannot comply with the procedures under DTC’s Automated Tender Offer Program for transfer of book-entry interests prior to the expiration date, you must tender your outstanding notes according to the guaranteed delivery procedures set forth in this prospectus under “The Exchange Offer—Guaranteed Delivery Procedures.” |
Effect on Holders of Outstanding Notes | As a result of the making of, and upon acceptance for exchange of all validly tendered outstanding notes pursuant to the terms of the exchange offer, we and the guarantors will have fulfilled a covenant contained in the registration rights agreement and, accordingly, there will be no increase in the interest rate on the outstanding notes under the circumstances described in the registration rights agreement. If you are a holder of outstanding notes and you do not tender your outstanding notes in the exchange offer, you will continue to hold |
12
Table of Contents
such outstanding notes and you will be entitled to all the rights and limitations applicable to the outstanding notes as set forth in the indenture, except we and the guarantors will not have any further obligations to you to provide for the exchange and registration of untendered outstanding notes under the registration rights agreement. To the extent that outstanding notes are tendered and accepted in the exchange offer, the trading market for outstanding notes that are not so tendered and accepted could be adversely affected. |
Consequences of Failure to Exchange | All untendered outstanding notes will continue to be subject to the restrictions on transfer provided for in the outstanding notes and in the indenture. In general, the outstanding notes may not be offered or sold, unless registered under the Securities Act, except pursuant to an exemption from, or in a transaction not subject to, the Securities Act and applicable state securities laws. Other than in connection with the exchange offer, we and the guarantors do not currently anticipate that we will register the outstanding notes under the Securities Act. |
Material U.S. Federal Income Tax Consequences | The exchange of outstanding notes for exchange notes in the exchange offer will not constitute a taxable event for U.S. federal income tax purposes. See “Material U.S. Federal Income Tax Consequences of the Exchange Offer.” |
Accounting Treatment | We will record the exchange notes in our accounting records at the same carrying value as the outstanding notes, which is the aggregate principal amount as reflected in our accounting records on the date of exchange. |
Regulatory Approvals | Other than compliance with the Securities Act and qualification of the indentures governing the notes under the Trust Indenture Act, there are no federal or state regulatory requirements that must be complied with or approvals that must be obtained in connection with the exchange offers. |
Use of Proceeds | We will not receive any cash proceeds from the issuance of exchange notes pursuant to the exchange offer. See “Use of Proceeds.” |
Exchange Agent | Wilmington Trust FSB is the exchange agent for the exchange offer. The address and telephone number of the exchange agent are set forth in the section captioned “The Exchange Offer—Exchange Agent” of this prospectus. |
13
Table of Contents
The Exchange Notes
The following summary is provided solely for your convenience. This summary is not intended to be complete. You should read the full text and more specific details contained elsewhere in this prospectus, including a more detailed summary of the terms of the notes under “Description of the Exchange Notes” beginning on page 135. In this section, “we” or the “Company” refers to American Renal Holdings Inc. and not to any of its subsidiaries.
Issuer | American Renal Holdings Inc. |
Notes Offered | $250,000,000 aggregate principal amount of 8.375% Senior Secured Notes due 2018. |
Maturity Date | May 15, 2018. |
Interest Payment Dates | May 15 and November 15, commencing November 15, 2010. |
Guarantees | The notes are guaranteed, jointly and severally, by our direct parent and all of our existing and future wholly owned domestic restricted subsidiaries, other than certain excluded subsidiaries. Our majority owned subsidiaries, which operate the clinics, will not guarantee the notes. |
Collateral | The notes and the guarantees are secured by a first-priority lien (pari passu, subject to the intercreditor agreement described below, with the liens granted under the New Revolving Credit Facility and subject to certain exceptions and permitted liens) on substantially all of our and our guarantors’ tangible and intangible assets, which are valued at approximately $542 million as of September 30, 2010 (such assets, the “Collateral”). With respect to the Collateral, the indebtedness and obligations under the notes, our New Revolving Credit Facility and certain other first lien obligations permitted under the indenture governing the notes have first-priority liens. Under the terms of the intercreditor agreement, however, in the event of a foreclosure on the Collateral or insolvency proceedings, the holders of the notes will receive proceeds from the Collateral only after debt under the New Revolving Credit Facility as well as obligations under certain hedging and cash management arrangements owed to lenders or their affiliates under the New Revolving Credit Facility have been repaid. See “Description of the Exchange Notes—Collateral.” As of the time of the filing of this prospectus, the New Revolving Credit Facility is undrawn and the Company does not have any other obligations that would have priority over the notes pursuant to the intercreditor agreement. |
Ranking | The notes and the guarantees rank: |
| • | pari passu with our and the guarantors’ obligations that are secured by first priority liens, except obligations under our New Revolving Credit Facility and obligations under certain hedging and cash management arrangements will be entitled to proceeds of the Collateral prior to the notes in the event of a foreclosure or in any insolvency proceeding; |
| • | equal in right of payment to all of our and our guarantors’ unsubordinated obligations; |
14
Table of Contents
| • | effectively senior in right of payment to all of our and our guarantors’ existing and future unsecured obligations to the extent of the value of the Collateral; |
| • | senior in right of payment to all of our and our guarantors’ future obligations that are expressly subordinated in right of payment to the notes; |
| • | structurally junior to all obligations of our subsidiaries that are not guarantors except, with respect to any subsidiary, to the extent of any intercompany loans owing by such subsidiary that constitute Collateral. These obligations of non-guarantor subsidiaries totaled approximately $8.2 million as of September 30, 2010. |
The indenture governing the notes permits additional indebtedness or other obligations to be secured by the Collateral either on a pari passu or on a junior priority basis relative to the notes, subject to limitations.
Intercreditor Agreement | The trustee and the collateral agent under the indenture governing the notes and the administrative agent and collateral agent under the New Revolving Credit Facility have entered into an intercreditor agreement as to the relative priorities of their respective entitlement to proceeds of the assets securing the notes and borrowings under the New Revolving Credit Facility and other first-priority lien secured obligations upon a foreclosure or bankruptcy and certain other matters relating to the administration of security interests. See “Description of the Exchange Notes—Intercreditor Agreement.” As of the time of the filing of this prospectus, the New Revolving Credit Facility is undrawn and the Company does not have any other obligations that would have priority over the notes pursuant to the intercreditor agreement. |
Use of Proceeds | There will be no cash proceeds to us from the exchange offer. |
Optional Redemption | On or after May 15, 2013, we may redeem some or all of the notes at any time at the redemption prices specified under “Description of the Exchange Notes—Optional Redemption.” Prior to May 15, 2013, we may redeem some or all of the notes at a redemption price of 100% of the principal amount of each note to be redeemed plus a “make-whole” premium described in “Description of the Exchange Notes—Optional Redemption.” |
In addition, prior to May 15, 2013, we will have the option to redeem, during each 12 month period commencing on the issue date, up to 10% of the aggregate principal amount of the notes at 103% of the aggregate principal amount of the notes redeemed plus accrued and unpaid interest to the date of redemption.
Furthermore, at any time prior to May 15, 2013, we may redeem up to 35% of the notes with the net cash proceeds from specified equity
15
Table of Contents
offerings at a redemption price equal to 108.375% of the principal amount of each Note to be redeemed, plus accrued and unpaid interest, if any, to the date of redemption. |
Change of Control | Upon a change of control, we must offer to repurchase the notes at 101% of the principal amount, plus accrued interest to the purchase date. See “Description of the Exchange Notes—Change of Control.” |
Certain Covenants | The indenture governing the notes contains covenants that, among other things, limit our ability and ability of our restricted subsidiaries to: |
| • | incur additional indebtedness or liens; |
| • | pay dividends or make distributions on our capital stock or repurchase our capital stock; |
| • | make certain investments or other restricted payments; |
| • | place restrictions on the ability of subsidiaries to pay dividends or make other distributions to us; |
| • | sell certain assets or merge with or into other companies; and |
| • | enter into certain types of transactions with shareholders and affiliates. |
These covenants are subject to important exceptions and qualifications, which are described in “Description of the Exchange Notes—Certain Covenants.”
Voting | The exchange notes will be treated along with the outstanding notes as a single class for voting purposes. |
No Prior Market | The exchange notes will be freely transferable but will be new securities for which there will not initially be a market. Accordingly, we cannot assure you whether a market for the exchange notes will develop or as to the liquidity of any such market that may develop. The initial purchasers of the outstanding notes have informed us that they currently intend to make a market in the exchange notes; however, they are not obligated to do so, and they may discontinue any such market-making activities at any time without notice. |
Risk Factors | Potential investors in the notes should carefully consider the matters set forth under the caption “Risk Factors” prior to making an investment decision with respect to the notes. |
16
Table of Contents
Summary Historical and Pro Forma Consolidated Financial and Other Data
Set forth below is our summary historical and pro forma consolidated financial data at the dates and for the periods indicated. The statement of income and other financial data for each of the years in the three-year period ended December 31, 2009 and the balance sheet data as of December 31, 2008 and 2009 have been derived from our historical consolidated financial statements included elsewhere in this prospectus, which have been audited by Grant Thornton LLP.
The summary unaudited pro forma consolidated financial data as of and for the year ended December 31, 2009 and as of and for the nine months ended September 30, 2010 have been prepared to illustrate the estimated effects on our historical results of operations and financial condition of the following (the “Pro Forma Adjustments”):
| • | the Merger, including the cash equity investment by our Sponsor (net of transaction expenses); |
| • | the issuance of the notes being exchanged hereby; |
| • | the results of our four acquisitions in 2009; |
| • | the effect on noncontrolling interests resulting from the purchase and sale of such noncontrolling interests in various clinics; and |
| • | the purchase price allocation and related adjustments based on asset, liability and noncontrolling interest valuations. |
The summary unaudited pro forma consolidated statement of income data and other financial data give effect to the Pro Forma Adjustments as if they had occurred on January 1, 2009. The Pro Forma Adjustments are based upon available information and certain assumptions that we believe are reasonable. The summary unaudited pro forma consolidated financial data are for informational purposes only and do not purport to represent what our results of operations or financial position actually would have been had the Pro Forma Adjustments actually occurred on the dates indicated, and the data do not purport to project our results of operations or financial condition for any future period or as of any future date.
The ratios of earnings to fixed charges for the relevant periods appear in “Selected Consolidated Financial Data.”
17
Table of Contents
| Historical | Pro Forma | |||||||||||||||||||||||||||||||||||
| Predecessor Entity | Successor Entity | Year Ended December 31, 2009 | Nine Months Ended September 30, 2010 | |||||||||||||||||||||||||||||||||
| As of and for the Year Ended December 31, | As of and for the Nine Months Ended September 30, 2009 | As of May 7, 2010 and for the period from January 1, 2010 through May 7, 2010 | As of September 30, 2010 and for the period from May 8, 2010 through September 30, 2010 | |||||||||||||||||||||||||||||||||
(in thousands, except ratios | 2007 | 2008 | 2009 | |||||||||||||||||||||||||||||||||
| (unaudited) | (unaudited) | (unaudited) | (unaudited) | (unaudited) | ||||||||||||||||||||||||||||||||
Statement of Income Data: | ||||||||||||||||||||||||||||||||||||
Net patient service revenue | $ | 178,391 | $ | 217,777 | $ | 262,989 | $ | 193,109 | $ | 102,094 | $ | 120,833 | $ | 265,206 | $ | 222,927 | ||||||||||||||||||||
Operating expenses: | ||||||||||||||||||||||||||||||||||||
Patient care costs | 115,058 | 141,810 | 170,826 | 125,098 | 66,042 | 78,335 | 172,353 | 142,737 | ||||||||||||||||||||||||||||
General and administrative expense | 18,595 | 19,944 | 24,819 | 17,423 | 10,016 | 14,991 | 25,557 | 22,750 | ||||||||||||||||||||||||||||
Merger and related expenses | — | — | — | — | 7,378 | 14,839 | — | — | ||||||||||||||||||||||||||||
Depreciation and amortization | 7,919 | 9,777 | 12,127 | 8,816 | 4,429 | 6,434 | 15,493 | 12,322 | ||||||||||||||||||||||||||||
Provision for doubtful accounts | 3,258 | 4,834 | 3,216 | 3,184 | (334 | ) | 1,021 | 3,280 | 687 | |||||||||||||||||||||||||||
Total operating expenses | 144,830 | 176,365 | �� | 210,988 | 154,521 | 87,531 | 115,620 | 216,683 | 178,496 | |||||||||||||||||||||||||||
Operating income | 33,561 | 41,412 | 52,001 | 38,588 | 14,563 | 5,213 | 48,523 | 44,431 | ||||||||||||||||||||||||||||
Interest expense, net | (13,695 | ) | (13,729 | ) | (14,948 | ) | (11,212 | ) | (5,717 | ) | (9,205 | ) | (22,588 | ) | (16,935 | ) | ||||||||||||||||||||
Income (loss) before income taxes | 19,866 | 27,683 | 37,053 | 27,376 | 8,846 | (3,992 | ) | 25,935 | 27,496 | |||||||||||||||||||||||||||
Income tax expense (benefit) | 4,409 | 6,860 | 9,524 | 7,036 | 2,264 | (649 | ) | 1,880 | 2,992 | |||||||||||||||||||||||||||
Net income (loss) | 15,457 | 20,823 | 27,529 | 20,340 | 6,582 | (3,343 | ) | 24,055 | 24,504 | |||||||||||||||||||||||||||
Less: Net income attributable to noncontrolling interests | (14,706 | ) | (17,179 | ) | (22,391 | ) | (15,823 | ) | (9,266 | ) | (10,949 | ) | (20,964 | ) | (19,583 | ) | ||||||||||||||||||||
Net income (loss) attributable to ARH | $ | 751 | $ | 3,644 | $ | 5,138 | $ | 4,517 | $ | (2,684 | ) | $ | (14,292 | ) | $ | 3,092 | $ | 4,921 | ||||||||||||||||||
Other Financial Data: | ||||||||||||||||||||||||||||||||||||
Adjusted EBITDA (including noncontrolling interests) (1) | $ | 43,660 | $ | 52,671 | $ | 67,724 | $ | 49,161 | $ | 26,589 | $ | 31,193 | $ | 68,612 | $ | 57,929 | ||||||||||||||||||||
Adjusted EBITDA (1) | 28,954 | 35,492 | 45,333 | 33,338 | 17,323 | 20,244 | 47,198 | 38,346 | ||||||||||||||||||||||||||||
Capital expenditures (2) | 15,218 | 21,254 | 15,067 | 9,757 | 15,067 | 11,045 | ||||||||||||||||||||||||||||||
Operating Data: | ||||||||||||||||||||||||||||||||||||
Number of clinics (as of end of period) | 64 | 75 | 83 | 80 | 88 | 83 | 88 | |||||||||||||||||||||||||||||
Patients (as of end of period) | 3,740 | 4,545 | 5,405 | 5,235 | 6,119 | 5,405 | 6,119 | |||||||||||||||||||||||||||||
Number of treatments | 503,576 | 604,888 | 745,812 | 546,825 | 753,596 | 632,556 | ||||||||||||||||||||||||||||||
Non-acquired treatment growth (3) | 16.9 | % | 20.1 | % | 20.6 | % | 22.5 | % | 20.6 | % | 15.5 | % | ||||||||||||||||||||||||
Revenues per treatment (4) | $ | 354 | $ | 360 | $ | 353 | $ | 353 | $ | 352 | $ | 352 | ||||||||||||||||||||||||
Patient care costs per treatment (4) | $ | 228 | $ | 234 | $ | 229 | $ | 229 | $ | 229 | $ | 226 | ||||||||||||||||||||||||
General and administrative expenses per treatment (4) | $ | 37 | $ | 33 | $ | 33 | $ | 32 | $ | 34 | $ | 36 | ||||||||||||||||||||||||
18
Table of Contents
| As of September 30, 2010 (unaudited) | ||||
| (dollars in thousands) | ||||
Consolidated Balance Sheet Data: | ||||
Cash and cash equivalents | $ | 27,579 | ||
Working capital (5) | 29,244 | |||
Total assets | 703,483 | |||
Total debt and capital lease obligations | 250,569 | |||
ARH Equity | 174,541 | |||
| (1) | Adjusted EBITDA is defined as net income attributable to ARH before income taxes, interest expense, depreciation and amortization, and we further adjust for non-cash charges, non-recurring charges and pro forma amounts for acquisitions as if they had been consummated on the first day of each period. We believe Adjusted EBITDA provides information useful for evaluating our businesses and understanding our operation performance in a manner similar to management. We believe Adjusted EBITDA is helpful in highlighting trends because Adjusted EBITDA excludes the results of decisions that are outside the control of operating management and can differ significantly from company to company depending on long-term strategic decisions regarding capital structure, the tax jurisdictions in which companies operate and capital investments. In addition, we present Adjusted EBITDA because it is one of the components used in the calculations under the covenants contained in our revolving credit facility. Adjusted EBITDA is not a measure of operating performance computed in accordance with GAAP and should not be considered as a substitute for operating income, net income, cash flows from operations, or other statement of operations or cash flow data prepared in conformity with GAAP, or as measures of profitability or liquidity. In addition, Adjusted EBITDA may not be comparable to similarly titled measures of other companies. Adjusted EBITDA may not be indicative of historical operating results, and we do not mean for it to be predictive of future results of operations or cash flows. Adjusted EBITDA has limitations as an analytical tool, and you should not consider this item in isolation, or as a substitute for an analysis of our results as reported under GAAP. Some of these limitations are that Adjusted EBITDA: |
| • | does not include interest expense—as we have borrowed money for general corporate purposes, interest expense is a necessary element of our costs and ability to generate profits and cash flows; |
| • | does not include depreciation and amortization—because construction and operation of our dialysis clinics requires significant capital expenditures, depreciation and amortization are a necessary element of our costs and ability to generate profits; |
| • | does not include stock-based compensation expense; |
| • | does not reflect changes in, or cash requirements for, our working capital needs; and |
| • | does not include certain income tax payments that represent a reduction in cash available to us. |
You should not consider Adjusted EBITDA as an alternative to income from operations or net income, determined in accordance with GAAP, as an indicator of our operating performance, or as an alternative to cash flows from operating activities, determined in accordance with GAAP, as an indicator of cash flows or as a measure of liquidity.
19
Table of Contents
The following table presents the reconciliation from net income (loss) to Adjusted EBITDA for the periods indicated:
| Historical | Pro Forma | |||||||||||||||||||||||||||||||||||
| Predecessor Entity | Successor Entity | |||||||||||||||||||||||||||||||||||
| As of and for the Year Ended December 31, | For the Nine Months Ended September 30, 2009 | For the period from January 1, 2010 through May 7, 2010 | For the period from May 8, 2010 through September 30, 2010 | Year Ended December 31, 2009 | Nine Months Ended September 30, 2010 | |||||||||||||||||||||||||||||||
| 2007 | 2008 | 2009 | ||||||||||||||||||||||||||||||||||
| (unaudited) | (unaudited) | (unaudited) | (unaudited) | (unaudited) | ||||||||||||||||||||||||||||||||
Net income (loss) | $ | 15,457 | $ | 20,823 | $ | 27,529 | $ | 20,340 | $ | 6,582 | $ | (3,343 | ) | $ | 24,055 | $ | 24,504 | |||||||||||||||||||
Add: | ||||||||||||||||||||||||||||||||||||
Stock-based compensation | 2,180 | 1,482 | 1,135 | 876 | 219 | 4,487 | 1,135 | 763 | ||||||||||||||||||||||||||||
Depreciation and amortization | 7,919 | 9,777 | 12,127 | 8,816 | 4,429 | 6,434 | 15,493 | 12,322 | ||||||||||||||||||||||||||||
Interest expense, net | 13,695 | 13,729 | 14,948 | 11,212 | 5,717 | 9,205 | 22,588 | 16,935 | ||||||||||||||||||||||||||||
Income tax expense (benefit) | 4,409 | 6,860 | 9,524 | 7,036 | 2,264 | (649 | ) | 2,192 | 2,992 | |||||||||||||||||||||||||||
Merger and related expenses | — | — | — | — | 7,378 | 14,839 | — | — | ||||||||||||||||||||||||||||
Initial public offering transaction expenses | — | — | 1,797 | 508 | — | — | 1,797 | — | ||||||||||||||||||||||||||||
Management fee | — | — | — | — | — | 220 | 550 | 413 | ||||||||||||||||||||||||||||
Specified legal costs | — | — | 664 | 373 | — | — | 664 | — | ||||||||||||||||||||||||||||
Adjusted EBITDA (including noncontrolling interests) | 43,660 | 52,671 | 67,724 | 49,161 | 26,589 | 31,193 | 68,612 | 57,929 | ||||||||||||||||||||||||||||
Less: Net income attributable to noncontrolling interests | (14,706 | ) | (17,179 | ) | (22,391 | ) | (15,823 | ) | (9,266 | ) | (10,949 | ) | (20,964 | ) | (19,583 | ) | ||||||||||||||||||||
Adjusted EBITDA | $ | 28,954 | $ | 35,492 | $ | 45,333 | $ | 33,338 | $ | 17,323 | $ | 20,244 | $ | 47,198 | $ | 38,346 | ||||||||||||||||||||
This presentation of Adjusted EBITDA may not be directly comparable to similarly titled measures of other companies, since not all companies use identical calculations.
| (2) | Includes the following capital expenditure by type: |
| Year Ended December 31, | Nine Months Ended September 30, 2009 | Pro Forma Nine Months Ended September 30, 2010 | ||||||||||||||||||
| 2007 | 2008 | 2009 | ||||||||||||||||||
Development capital expenditures | $ | 12,887 | $ | 18,764 | $ | 11,056 | $ | 9,020 | $ | 8,961 | ||||||||||
Maintenance capital expenditures | 2,331 | 2,490 | 4,011 | 737 | 2,084 | |||||||||||||||
Total capital expenditures | $ | 15,218 | $ | 21,254 | $ | 15,067 | $ | 9,757 | $ | 11,045 | ||||||||||
| (3) | We calculate non-acquired treatment growth by dividing the number of treatments performed during the applicable period by the number of treatments performed during the corresponding prior period, excluding the number of treatments performed at clinics acquired during the applicable period, and expressing the resulting number as a percentage. |
| (4) | We calculate revenues per treatment, patient care costs per treatment and general and administrative expenses per treatment by dividing net operating revenues, patient care costs and general and administrative expenses for the applicable period by the number of treatments performed in the applicable period. |
| (5) | Current assets minus current liabilities. |
20
Table of Contents
Investing in the notes involves a high degree of risk. You should consider carefully the risks and uncertainties described below, together with all of the other information in this prospectus, including the financial statements and the related notes appearing at the end of this prospectus, before making an investment decision. If any of the following risks actually occur, they may materially harm our business, prospects, financial condition and results of operations, which in turn could adversely affect our ability to repay the notes. You may lose all or part of your original investment.
Risks Related to the Exchange Offer
If you choose not to exchange your outstanding notes, the present transfer restrictions will remain in force and the market price of your outstanding notes could decline.
If you do not exchange your outstanding notes for exchange notes in the exchange offer, then you will continue to be subject to the transfer restrictions on the outstanding notes as set forth in the offering circular distributed in connection with the private offering of the outstanding notes. In general, the outstanding notes may not be offered or sold unless they are registered or exempt from registration under the Securities Act and applicable state securities laws. Except as required by the registration rights agreement, we do not intend to register resales of the outstanding notes under the Securities Act. You should refer to “Summary—The Exchange Offer” and “The Exchange Offer” for information about how to tender your outstanding notes.
The tender of outstanding notes under the exchange offer will reduce the principal amount of the outstanding notes outstanding, which may have an adverse effect upon, and increase the volatility of, the market price of the outstanding notes due to reduction in liquidity.
Certain persons who participate in the exchange offer must deliver a prospectus in connection with resales of the exchange notes.
Based on interpretations of the staff of the SEC contained inExxon Capital Holdings Corp., SEC no-action letter (April 13, 1988),Morgan Stanley & Co. Inc., SEC no-action letter (June 5, 1991) andShearman & Sterling, SEC no-action letter (July 2, 1983), we believe that you may offer for resale, resell or otherwise transfer the exchange notes without compliance with the registration and prospectus delivery requirements of the Securities Act. However, in some instances described in this prospectus under “Plan of Distribution,” certain holders of exchange notes will remain obligated to comply with the registration and prospectus delivery requirements of the Securities Act to transfer the exchange notes. If such a holder transfers any exchange notes without delivering a prospectus meeting the requirements of the Securities Act or without an applicable exemption from registration under the Securities Act, such a holder may incur liability under the Securities Act. We do not and will not assume, or indemnify such a holder against, this liability.
Your ability to transfer the notes may be limited by the absence of an active trading market, and an active trading market may not develop for the notes.
We do not intend to apply for a listing of the exchange notes on a securities exchange or on any automated dealer quotation system. There is currently no established market for the exchange notes, and we cannot assure you as to the liquidity of markets that may develop for the exchange notes, your ability to sell the exchange notes or the price at which you would be able to sell the exchange notes. If such markets were to exist, the exchange notes could trade at prices that may be lower than their principal amount or purchase price depending on many factors, including prevailing interest rates, the market for similar notes, our financial and operating performance and other factors. We cannot assure you that an active market for the exchange notes will develop or, if developed, that it will continue. Historically, the market for non-investment grade debt has been subject to disruptions that have caused substantial volatility in the prices of securities similar to the exchange notes. The market, if any, for the exchange notes may experience similar disruptions and any such disruptions may adversely affect the prices at which you may sell your exchange notes.
21
Table of Contents
Risks Relating to Our Business
If the number of patients with commercial insurance declines, or if the average rates paid by commercial payors decline, our operating results and financial condition would be adversely affected.
Commercial payors pay us on terms and at rates that are generally significantly higher than Medicare rates. For the year ended December 31, 2009, we derived approximately 43% of our net operating revenues from commercial payors, even though these payors were the source of reimbursement for approximately 14% of the treatments performed. Medicare payment rates are generally insufficient to cover our total operating expenses allocable to providing dialysis treatments for Medicare patients, although in some circumstances they are sufficient to cover their patient care costs. The dialysis services industry is experiencing a growing number of commercial payors seeking to reduce payment rates and impose limitations on out-of-network access and rates. The downward pressure on commercial payment rates is a result of general conditions in the market, recent and future consolidations among commercial payors, increased focus on dialysis services and other factors. Decreases in the number of patients covered by commercial plans, reductions in commercial payor rates and decreases in out-of-network rates or increases in restrictions on out-of-network access could result in a significant decrease in our overall revenues derived from commercial payors.
We are continuously in the process of negotiating agreements with our commercial payors, who are increasingly aggressive in their negotiations with us. In the event that our continued negotiations result in overall commercial rate reductions in excess of overall commercial rate increases, the cumulative effect could have a material adverse effect on our financial results. Consolidations have significantly increased the negotiating leverage of commercial payors. Our negotiations with payors are also influenced by competitive pressures. It is possible that some of our contracted rates with commercial payors may decrease or that we may experience decreases in patient volume as our negotiations with commercial payors continue. In addition to increasing downward pressure on contracted commercial payor rates, payors have been attempting to impose restrictions and limitations on non-contracted or out-of-network providers. Rates for out-of-network providers are on average higher than rates for in-network providers. Commercial payors may restructure their benefits to create disincentives for patients to select or remain with out-of-network providers or may decrease payment rates for out-of-network providers. Through our affiliation with dialysis provider trade organizations, we are resisting attempts to limit access to out-of-network providers through regulatory, legislative and legal means. Because some of our clinics are currently designated as out of network providers for our current commercial payors, decreases in out-of-network rates and restrictions on out-of-network access combined with decreases in contracted rates could result in a significant decrease in our overall revenue derived from commercial payors. If the average rates that commercial payors pay us decline significantly, it would have a material adverse effect on our revenues, earnings and cash flows.
Additionally, our revenues are sensitive to the percentage of patients with commercial insurance coverage. A patient’s insurance coverage may change for a number of reasons, including as a result of changes in the patient’s or a family member’s employment status. Currently, for a patient covered by an employer group health plan, Medicare generally becomes the primary payor after 33 months, including a 3-month coordination of benefits period. When Medicare becomes the primary payor, the payment rate we receive for that patient shifts from the employer group health plan rate to the lower Medicare payment rate. If there is a significant reduction in the number of patients under higher-paying commercial plans relative to government-based programs, our revenues, earnings and cash flows would be adversely affected.
We have seen an increase in the number of patients who have government-based programs as their primary payors which we believe is largely as a result of the current economic recession which has reduced the percentage of patients covered under commercial insurance plans. To the extent there are sustained or increased job losses in the United States as a result of current economic conditions, we could experience a decrease in the number of patients under commercial plans. We could also experience a further decrease if changes to the healthcare regulatory system result in fewer patients covered under commercial plans. In addition, our continued negotiations with commercial payors could result in a decrease in the number of patients under commercial plans
22
Table of Contents
to the extent that we cannot reach agreement with commercial payors on rates and other terms. If there is a significant reduction in the number of patients under higher-paying commercial plans relative to government-based programs that pay at lower rates, it would have a material adverse effect on our revenues, earnings and cash flows.
Changes in the structure of, and payment rates under, state Medicaid programs and the Medicare ESRD program could adversely affect our operating results and financial condition.
We derived approximately 3% of our revenues for the year ended December 31, 2009 from patients who have Medicaid as their primary coverage. As state governments face increasing budgetary pressure, they may propose reductions in payment rates, delays in the timing of payments, limitations on eligibility or other changes to Medicaid programs. Some states have already taken steps to reduce or delay payments. In addition, Medicaid eligibility requirements mandate that citizen enrollees in Medicaid programs provide documented proof of citizenship. Our revenues, earnings and cash flows could be negatively impacted to the extent that we are not paid by Medicaid or other state programs for services provided to patients who are unable to satisfy the eligibility requirements, including undocumented patients living in the United States. If state governments reduce the rates paid by Medicaid programs for dialysis and related services, delay the timing of payment for services provided, further limit eligibility for Medicaid coverage or adopt changes to the Medicaid payment structure which reduces our overall payments from Medicaid, then our revenues, earnings and cash flows could be adversely affected.
During the year ended December 31, 2009, we derived approximately 57% of our revenues from reimbursement from federal programs, such as Medicare and Medicaid. The Medicare ESRD program pays us for dialysis treatment services at a single composite rate for each dialysis treatment, adjusted based upon geography and individual patient characteristics. The composite rate includes the supplies used in those treatments, specified laboratory tests and certain pharmaceuticals. The existing Medicare ESRD program does not provide for regular percentage increases in the composite rate. Accordingly, the amounts by which we are reimbursed for our services are not adjusted to keep pace with inflation, even if our operating costs, which include labor and supply costs, increase.
Other services and pharmaceuticals, vitamin D analogs, and iron supplements, are separately billed. These separately-billable pharmaceuticals include erythropoietin (“EPO”), a pharmaceutical used to treat anemia, a common complication associated with ESRD. Reductions in the payment rate or changes in the reimbursement methodology for these separately billed items, without a corresponding increase in the composite rate, could have a material adverse effect on our revenues, earnings and cash flows.
In July 2008, the Medicare Improvements for Patients and Providers Act of 2008, or MIPPA, was passed by Congress. This legislation introduced a new payment system for dialysis services beginning in January 2011 whereby ESRD payments will be made under a bundled payment rate which will provide for a fixed rate for all goods and services provided during the dialysis treatment, including laboratory services, the administration of pharmaceuticals and payments for pharmaceuticals such as EPO that are separately billed under the current methodology. On July 23, 2010, CMS released the final rule regarding the new bundled payment rate system. We are still evaluating the various components of the new bundled payment rate system, however, we noted that the initial 2011 bundled rate includes reductions of 2% and 3.1%, respectively, to conform to the provisions of MIPPA and to establish budget neutrality. Further there is a 5.94% reduction tied to an expanded list of case mix adjustors which can be earned back based upon the presence of these co-morbidities at the time of treatment. There are other provisions which may impact payment including an outlier pool and a low volume facility adjustment. Further the new rule requires dialysis facilities to provide new services within the payment bundle such as oral vitamin D medications and an expanded list of laboratory tests. Historically these services were separately billable; now the dialysis facility will be at risk for utilization with reimbursement set at a fixed rate. With regard to the expanded list of case-mix adjustors, these may be difficult or impossible for our dialysis clinics or billing and other systems to document and track resulting in a reduction in the amounts of the payments that we would otherwise be entitled to receive. The rule also requires the new single bundled payment base rate
23
Table of Contents
to be adjusted annually for inflation based upon a market basket index, less a productivity adjustment, beginning in 2012. Also, beginning in 2012, the rule provides for up to a 2% withhold that can be earned back by facilities that meet certain defined clinical performance standards.
Federal or state healthcare reform laws could adversely affect our operating results and financial condition.
On March 30, 2010, President Obama signed into law the Health Care and Education Reconciliation Act that modified the newly enacted Patient Protection and Affordable Care Act, commonly referred to as the Health Care Reform Act. This culmination of a yearlong legislative process will have a significant impact on the health care delivery system. Much of that impact, specifically as related to dialysis services, is unknown.
The Health Care Reform Act, among other things, sets out a plan for a type of universal healthcare coverage. A number of states, including California, Colorado, Connecticut, Massachusetts, New York and Pennsylvania, are also contemplating significant reform of their health insurance markets. Other states have mounted legal challenges to the implementation of certain aspects of the health care reform bill in their respective states. The Health Care Reform Act, along with possible changes at the state level, will affect both public programs and privately-financed health insurance arrangements. Both the new federal law and the state proposals increase the number of insured persons by expanding the eligibility levels for public programs and compelling individuals and employers to purchase health coverage. At the same time, these laws seek to reform the underwriting and marketing practices of health plans. These laws could further increase pricing pressure on existing commercial payors. As a result, commercial payors may likely seek to lower their rates of reimbursement for the services we provide. The state proposals are still being debated in various legislatures and the legal challenges to the Health Care Reform Act are in their earliest stages.
Given the recent enactment of the Health Care Reform Act, and taking into account proposed state reforms and possible legal challenges, we cannot predict how our business will be affected by the full implementation of these and future actions. The Health Care Reform Act, in connection with state initiatives, may increase our costs, decrease our revenues, expose us to expanded liability or require us to revise the ways in which we conduct our business, any of which could adversely affect our operating results and financial condition.
If we fail to adhere to all of the complex federal, state and local government regulations that apply to our business, we could suffer severe consequences and it could adversely affect our operating results and financial condition.
Our dialysis operations are subject to extensive federal, state and local government regulations, all of which are subject to change. These government regulations currently relate, among other things, to:
| • | government healthcare program participation requirements and reimbursement for patient services, including Medicare and Medicaid reimbursement rules and regulations; |
| • | federal and state anti-kickback laws, the federal Stark Law’s prohibition on physician self-referrals and analogous state physician self-referral statutes; |
| • | false claims prohibitions for healthcare reimbursement programs and other fraud and abuse laws and regulations, including amendments to the federal False Claims Act in 2009; |
| • | federal and state laws regarding record keeping requirements, privacy and security protections applicable to the collection, use and disclosure of protected health information, security breach notification requirements relating to protected health information, and standards for the exchange of electronic health information, electronic transactions and code sets and unique identifiers for providers; |
| • | corporate practice of medicine; |
| • | licensing and certification requirements applicable to our dialysis clinics; and |
| • | regulation related to health, safety and environmental compliance, including medical waste disposal. |
24
Table of Contents
We endeavor to comply with all of the requirements for receiving Medicare and Medicaid payments and to structure all of our relationships with physicians to comply with state and federal anti-kickback laws, the federal Stark Law and applicable state self-referral laws. However, the laws and regulations in this area are complex and subject to varying interpretations. For example, if a governmental agency were to challenge the level of compensation that we pay our medical directors, we could be required to change our practices, face criminal or civil penalties, pay substantial fines or otherwise experience a material adverse effect as a result of a challenge to these arrangements.
Both federal and state government agencies, as well as private payors, have heightened and coordinated audits and administrative, civil, and criminal enforcement efforts as part of numerous ongoing investigations of healthcare organizations. These investigations relate to a wide variety of topics, including the following:
| • | cost reporting and billing practices; |
| • | quality of care; |
| • | financial reporting; |
| • | financial relationships with referral sources; and |
| • | medical necessity of services provided. |
In addition, CMS has increased the frequency and intensity of its certification inspections of dialysis clinics. State and federal governments have increased enforcement efforts and discussions related to proposed federal and state healthcare reform raise the possibility of significantly increased funding for enforcement of anti-fraud, physician self-referral, and false claims laws against healthcare providers. We may be especially susceptible to state law regulation risks in states where we have large concentrations of business and in states in which we establish new JVs but in which we may be unfamiliar with the regulatory requirements. For example, we are required to provide substantial documentation related to the administration of pharmaceuticals, including EPO. To the extent that any of this documentation is found insufficient, we may be required to refund any amounts received from this administration by government or commercial payors, and be subject to substantial penalties under applicable laws or regulations. In addition, fiscal intermediaries have increased their prepayment and post-payment reviews. Medicare Administrative Contractors are now active in many, but not all, states and are responsible for performing the functions previously performed by fiscal intermediaries. Medicare is also in the process of implementing a network of privately contracted auditors, called Recovery Audit Contractors, or RACs, which will conduct post-payment reviews to identify improper payments made by Medicare to providers. The RACs have been assigned to each of the four regional jurisdictions covering the United States under the Recovery Audit Contractor Program. They are scheduled to be fully functional by 2010, but are already operating in several states and are performing limited post-payment reviews. RACs are to be paid on a contingency basis for all overpayments identified and recovered. If any of these enforcement actions or payment reviews identifies non-compliance or overpayments by us, they could adversely affect our operating results and financial condition.
The Medicare and Medicaid reimbursement rules related to claims submission, licensing requirements, cost reporting, and payment processes impose complex and extensive requirements upon dialysis providers. A violation or departure from these requirements may result in government audits, lower reimbursements, recoupments or voluntary repayments, and the potential loss of certification. Further, if any of our operations are found to violate these or other government regulations, we could suffer severe consequences that would have a material adverse effect on our revenues, earnings and cash flows including:
| • | suspension or termination of our participation in government payment programs; |
| • | refunds to the government and third-party payors of amounts received in violation of law or applicable payment program requirements; |
| • | loss of required government certifications or exclusion from government payment programs; |
25
Table of Contents
| • | loss of licenses required to operate healthcare clinics in some of the states in which we operate; |
| • | reductions in payment rates or coverage for dialysis and ancillary services and related pharmaceuticals; |
| • | fines, damages or monetary penalties for anti-kickback law violations, federal Stark Law violations, violations of state self-referral and anti-kickback prohibitions, submission of false claims, civil or criminal liability based on violations of law, or other failures to meet regulatory requirements; |
| • | claims for monetary damages from or on behalf of patients who believe their protected health information has been used or disclosed in violation of federal or state patient privacy laws, or the imposition of fines and penalties for violations of those laws; |
| • | mandated practice changes that significantly increase operating expenses; and |
| • | termination of relationships with medical directors or healthcare providers. |
In addition, the Office of the Inspector General of the Department of Health and Human Services and the Department of Justice have, from time to time, undertaken national enforcement initiatives that focus on specific billing practices or other suspected areas of abuse. If such enforcement initiatives are undertaken or if we become subject to any federal or state audits or investigations we are unable to predict the impact such actions would have on our business, financial position or results of operations. See “Business—Government Regulation.”
The laws and regulations relating to our operations vary from state to state, and many states prohibit general business corporations, as we are, from practicing medicine, controlling physicians’ medical decisions or engaging in some practices such as splitting professional fees with physicians. Possible sanctions for violation of these restrictions include loss of license and civil and criminal penalties. In addition, agreements between the corporation and the physician may be considered void and unenforceable. We have structured our activities and operations to avoid conflict with state law restrictions on the corporate practice of medicine, and we have structured all of our corporate and operational agreements to conform to any licensure requirements, fee-splitting and related corporate practice of medicine prohibitions. See “Business—Government Regulation.” However, other parties may assert that we are engaged in the corporate practice of medicine or unlawful fee-splitting despite the way we are structured. Were such allegations to be asserted successfully before the appropriate judicial or administrative forums, we could be subject to adverse judicial or administrative penalties, certain contracts could be determined to be unenforceable and we may be required to restructure our contractual arrangements.
Our attempt to expand through development of de novo clinics or acquisition of existing dialysis clinics entails risks to our growth, as well as our operating results and financial condition.
We have experienced rapid growth since our inception. We have grown primarily through the development of de novo dialysis clinics as JVs with nephrologists or nephrologist groups, but we have also grown through the acquisition of existing clinics that we have restructured as JVs. Growth through development and acquisition places significant demands on our financial and management resources. Inability on our part to address these demands could adversely affect our growth, as well as our operating results and financial condition.
We generally expand by seeking appropriate locations for a dialysis clinic, taking into consideration the potential patient base, payor types, the availability of a nephrologist to be our medical director and nephrologist partner, and a skilled work force including qualified and cost-effective nursing and technical personnel. The inability to identify suitable locations, suitable nephrologist partners and workforce personnel for our dialysis clinics could adversely affect our growth as well as our operating results and financial condition.
The cost of developing a de novo dialysis clinic can be expensive and may include costs related to construction, equipment and initial working capital. In general, acquiring an existing dialysis clinic is more costly than developing a de novo dialysis clinic, but has historically been a faster means for achieving profitability. De novo dialysis clinics are subject to various risks, including risks associated with the availability and terms of
26
Table of Contents
financing for development and securing appropriate licenses. In addition, our de novo clinics may not become cash flow positive or profitable on a timely basis or at all. The inability to develop de novo clinics or acquire existing clinics on reasonable terms or in a cost-effective manner could adversely affect our growth as well as our operating results and financial condition. There is no assurance that we will be able to continue to successfully expand our business through establishing de novo clinics, or that de novo clinics will be able to achieve profitability that is consistent with our past results.
Our business strategy includes the selective acquisition of existing dialysis clinics. Businesses we acquire may have unknown or contingent liabilities or liabilities that are in excess of the amounts that we originally estimated. Although we generally seek indemnification from the sellers of businesses we acquire for matters that are not properly disclosed to us, we may not be successful. In addition, even in cases where we are able to obtain indemnification, we may be subject to liabilities greater than the contractual limits of our indemnification or the financial resources of the indemnifying party. In the event that we are responsible for liabilities substantially in excess of any amounts recovered through rights to indemnification, we could suffer severe consequences that could adversely impact our operating results.
If physicians cease referring patients to our dialysis clinics, our revenues would decrease.
Our dialysis services business is dependent upon patients choosing our clinics as the location for their treatments. Patients may select a clinic based, in whole or in part, on the recommendation of their physician. We believe that physicians and other clinicians typically consider a number of factors when recommending a particular dialysis facility to an ESRD patient, including, but not limited to, the quality of care at a clinic, the competency of a clinic’s staff, convenient scheduling, and a clinic’s location and physical condition. Physicians, including our nephrologist partners who are our medical directors, may change their facility recommendations at any time, which may result in the transfer of our existing patients to competing clinics, including clinics established by the physicians themselves. Our dialysis care business also depends on recommendations by hospitals, managed care plans and other health care institutions. If a significant number of providers cease referring their patients to our clinics, this would reduce our dialysis care revenue and could materially adversely affect our overall operations.
Our arrangements with our nephrologist partners and medical directors do not satisfy all the elements of a safe harbor to the federal anti-kickback statute and certain state anti-kickback laws and as a result may subject us to government scrutiny.
We take great efforts to ensure that our JV arrangements and medical director agreements comply with applicable laws and government regulations or fall within applicable safe harbors. Our business model is focused exclusively on JVs with nephrologist partners, which we have sought to structure in compliance with the federal anti-kickback statute, the Stark Law, and analogous state anti-kickback and self-referral laws, including the exceptions applicable to Medicare ESRD services. Many of our JVs with physicians or physician groups also involve the nephrologist partners providing medical director services to those clinics. Under Medicare regulations, each of our dialysis clinics is required to have an active medical director who is responsible for decision-making in analyzing core processes and patient outcomes and in stimulating a team approach to Continuous Quality Improvement and patient safety. For these services, we retain a physician on an independent contractor basis at an annual fixed fee to serve as the medical director. In the majority of cases, the medical director is also a nephrologist partner of ours in the particular dialysis clinic in question.
We believe that our relationships with our nephrologist partners, which includes our medical directors, meet most but not all of the elements of one of the safe harbors to the federal anti-kickback statute and may not meet all of the elements of analogous state safe harbors. Arrangements that do not meet all of the elements of a safe harbor are not prohibited under the federal anti-kickback statute but are susceptible to government scrutiny. The Office of Inspector General, or OIG, of the U.S. Department of Health and Human Services has indicated a concern about joint venture arrangements between physicians and other healthcare providers that may benefit
27
Table of Contents
from referrals of patients by those physicians or other business generated between the parties. Accordingly, there is some risk that the OIG or another government agency might investigate our JV arrangements and medical director contracts. In addition, if physician self-referral laws were to be interpreted differently than they currently are, we may be found to be in violation of such laws. If our arrangements with our medical directors were investigated and determined to violate the federal anti-kickback statute or analogous state laws, we could be required to restructure these relationships. We could also be subjected to severe monetary consequences that could adversely affect our operating results and financial condition, including the repayment of amounts received from Medicare by the offending clinics and the payment of penalties.
If we cannot renew our medical director agreements or enforce the noncompetition agreements with our medical directors, whether due to regulatory or other reasons, our operating results and financial condition could be materially and adversely affected.
Our medical director contracts are for fixed initial ten-year periods with automatic renewal options. Medical directors have no obligation to extend their agreements with us. We may take actions to restructure existing relationships or take positions in negotiating extensions of relationships to assure compliance with the safe harbor provisions of the anti-kickback statute, Stark Law and other similar laws. These actions could negatively impact the decision of physicians to extend their medical director agreements with us or to recommend us to their patients. If the terms of any existing agreement are found to violate applicable laws, we may not be successful in restructuring the relationship which could lead to the early termination of the agreement, or cause the physician to stop recommending our dialysis clinics to their patients. If a significant number of physicians were to cease recommending our dialysis clinics to their patients, whether due to regulatory or other reasons, then our revenues, earnings and cash flows would be substantially reduced.
If a medical director agreement terminates, whether before or at the end of its term, it may negatively impact that former medical director’s decision to treat his or her patients at one of our clinics. All of our medical director agreements provide for noncompetition restrictions prohibiting the medical directors from owning an interest in or serving as a medical director of a competing facility within specified geographical areas for specified periods of time. If we are unable to enforce the noncompetition provisions contained in our medical director agreements, it is possible that these medical directors may choose to provide medical director services for competing providers or establish their own dialysis clinics in competition with ours.
Delays in state Medicare and Medicaid certification of our dialysis clinics could adversely affect our operating results and financial condition.
We are required to obtain state and federal certification for participation in the Medicare and Medicaid programs before we can begin billing for patients treated in our clinics who are enrolled in government-based programs. Due to budgetary pressures, significant delays in certification have occurred in some states and additional delays may occur in the future. For example, the state of Texas stopped certifying dialysis clinics during a portion of 2009, and the state of California experienced significant delays in certifying dialysis clinics during 2009. Failures or delays in obtaining certification could cause significant delays in our ability to bill for services provided to patients covered under government programs, cause us to incur write-offs of investments or accelerate the recognition of lease obligations in the event we have to close clinics or our clinics’ operating performance deteriorates. This could have an adverse effect on our operating results and financial condition.
Our business is subject to substantial competition, which could adversely impact our operating results and financial condition as well as growth.
The development, acquisition and operation of dialysis clinics is highly competitive. Our competition comes from other dialysis clinics, many of which are owned by much larger public companies, small to mid-sized private companies, acute care hospitals, nursing homes and physician groups. The dialysis industry is rapidly consolidating, resulting in several large dialysis companies competing with us for the acquisition of existing
28
Table of Contents
dialysis clinics and the development of relationships with nephrologists to serve as medical directors for new clinics. Over the past few years, several dialysis companies, including some of our largest competitors, have adopted a JV model of dialysis clinic ownership resulting in increased competition in the development, acquisition and operation of our JV dialysis clinics. The addition of competitors using a JV model could adversely affect our growth as well as our operating results and financial condition. Some of our competitors have significantly greater financial resources, more dialysis clinics and a significantly larger patient base. Some of our competitors may also be able to achieve better economies of scale by asserting leverage against their suppliers and other commercial parties.
Changes in clinical practices relating to EPO, the use and marketing of alternatives to EPO and payment rates or rules for EPO and other pharmaceuticals could adversely affect our operating results and financial condition as well as our ability to care for patients.
Payments for the administration of EPO and other pharmaceuticals accounted for approximately 34% of our revenues for the year ended December 31, 2009, with payments for EPO accounting for approximately 22% of our revenue. Changes in clinical practices that result in decreased utilization of prescribed pharmaceuticals or changes in payment rates for those pharmaceuticals could adversely affect our operating results and financial condition. Since late 2006, there has been significant media discussion and government scrutiny regarding anemia management practices in the United States which has created confusion and concern in the nephrology community. In late 2006, the House Ways and Means Committee held a hearing on the issue of the utilization of erythropoiesis stimulating agents, or ESAs, which include EPO, and in 2007, the FDA required changes to the labeling of EPO and Aranesp® to include a black box warning, the FDA’s strongest form of warning label. The FDA held additional hearings to revisit these label changes as they apply to ESRD and has indicated that they will convene in 2010 to further review ESA labeling. CMS also reviewed its EPO reimbursement policies and in January 2008, changes to the EPO monitoring policy went into effect which further limited reimbursement and which impacted the prescribing habits of our physicians and which has in the past and may in the future result in lower pharmaceutical intensities. Most recently, on March 24, 2010, the CMS Medical Evidence Development & Coverage Committee (“MEDCAC”) met to examine currently available evidence on the use of erythropoiesis stimulating agents (“ESAs”), including EPO. MEDCAC is an independent body established to provide guidance and expert advice to CMS on clinical issues. At the meeting, evidence was presented from current and recently completed clinical studies regarding the effectiveness of use of ESAs to manage anemia in patients who have chronic kidney disease. No policy determinations or recommendations concerning the manner in which ESAs, such as EPO, are covered or reimbursed under Medicare were made at the meeting, although MEDCAC is empowered to submit future recommendations to CMS following its review of the available evidence. Commercial payors have also increasingly examined their administration policies for EPO and, in some cases have modified those policies. Further changes in labeling of EPO and other pharmaceuticals in a manner that alters physician practice patterns or accepted clinical practices, changes in private and government payment criteria, including the introduction of EPO administration policies or the conversion to alternate types of administration of EPO or other pharmaceuticals that result in further decreases in utilization or reimbursement for EPO and other pharmaceuticals could have a material adverse effect on our revenues, earnings and cash flows.
Amgen Inc. (“Amgen”) is the sole supplier of EPO. We purchase EPO from Amgen through a group purchasing organization that negotiates the terms on which we purchase EPO. The available supply of EPO could be delayed or reduced, whether by Amgen itself, through unforeseen circumstances or as a result of excessive demand. In addition, our group purchasing organization may be unable to negotiate acceptable terms for us to purchase EPO from Amgen, or Amgen may unilaterally decide to increase its price for EPO at any time. If that occurs, we may have no ability on our part to pass any price increases to our payors. Future changes in the cost of EPO could have a material adverse effect on our earnings and cash flows and ultimately reduce our income. Although our agreements with our group purchasing organization and Amgen for EPO include potential rebates that depend upon our achievement of certain criteria, we cannot predict whether we will continue to receive rebates for EPO at the levels that we currently receive, or whether we will continue to achieve the same levels of
29
Table of Contents
rebates within that structure as we have historically achieved. Our agreements with our group purchasing organization and Amgen provide for specific rebates off list price based on a combination of factors, including data submission. Factors that could impact our ability to qualify for rebates provided for in our agreements with our group purchasing organization and Amgen in the future include our ability to track data elements. Failure to meet targets and earn the specified rebates could adversely affect our operating results and financial condition.
Amgen has developed and obtained FDA approval for Aranesp®, and Roche has developed Mircera®, each of which are pharmaceuticals used to treat anemia that may replace EPO or reduce its use with dialysis patients. Unlike EPO, which is generally administered in conjunction with each dialysis treatment, Aranesp® and Mircera® are administered less frequently. If Aranesp®, Mircera® or any future alternatives to EPO are marketed for the treatment of dialysis patients, we may realize lower margins on the administration of these pharmaceuticals than we currently realize with EPO. A significant increase in the development and use of similar alternatives to EPO, or a change in drug administration practices, could adversely affect our operating results and financial condition.
Most of our EPO reimbursement is from government programs. In recent years, the Centers for Medicare & Medicaid Services, or CMS, revised its rules for reimbursement of pharmaceuticals, including EPO, which resulted in a net reduction of average Medicare payment rates. In 2006, reimbursement for EPO was set at the average sales price plus 6%, which resulted in a decreased reimbursement for pharmaceuticals, but was offset by a 1.6% composite rate increase. If government or commercial payors further reduce reimbursement for EPO, then our revenues, earnings and cash flows may decline.
The Medicare Improvements for Patients and Providers Act of 2008 provides for marginal increases in the dialysis composite rate for 2009 and 2010, but also establishes “bundled” payments for goods and services provided during a dialysis treatment beginning in 2011. By statute, the items and services in the new dialysis bundled rate will include in its calculation, among other things, EPO and other erythropoiesis stimulating agents.
Current economic conditions, including the current recession in the United States and the worldwide economic slowdown, as well as further disruptions in the financial markets, could adversely impact our operating results and financial condition.
The current economic recession in the United States and worldwide economic slowdown could adversely affect our operating results and financial condition. Among other things, the potential decline in federal and state revenues that may result from these conditions may create additional pressures to contain or reduce reimbursements for our services from Medicare, Medicaid and other government sponsored programs. Our business may be particularly sensitive to economic conditions in certain states in which we operate a large number of clinics, such as Florida, Rhode Island or others. The increased job losses and elevated unemployment rates in the United States resulting from the recession could result in a smaller percentage of patients being covered by commercial payors and a larger percentage being covered by lower-paying Medicare and Medicaid programs. Employers may also begin to select more restrictive commercial plans with lower reimbursement rates. To the extent that payors are adversely affected by a decline in the economy, we may experience further pressure on commercial rates, delays in fee collections and a reduction in the amounts we are able to collect. In addition, if the current turmoil in the financial markets continues, interest rates may increase and it could be more difficult to obtain credit in the future. Any or all of these factors, as well as other consequences of the current economic conditions which currently cannot be anticipated, could adversely impact our operating results and financial condition.
We may be subject to liability claims for damages and other expenses not covered by insurance that could adversely impact our operating results.
The administration of dialysis services to patients may subject us to litigation and liability for damages based on an allegation of malpractice, professional negligence in the performance of our treatment and related services, the acts or omissions of our employees, or other matters. Our exposure to this litigation and liability for
30
Table of Contents
damages increases with growth in the number of our clinics and treatments performed. Potential judgments, settlements or costs relating to potential future claims, complaints or lawsuits could result in substantial damages and could subject us to the incurrence of significant fees and costs. Our insurance may not be sufficient or available to cover these damages, costs or expenses. Our business, profitability and growth prospects could suffer if we face negative publicity or we pay damages or defense costs in connection with a claim that is outside the scope of any applicable insurance coverage, including claims related to contractual disputes and professional and general liability claims.
Our insurance costs have been increasing substantially over the last several years, and our coverage may not be sufficient to cover claims and losses.
We maintain a program of insurance coverage against a broad range of risks in our business. In particular, we maintain professional liability insurance, subject to deductibles. The premiums and deductibles under our insurance program have been increasing over the last several years as a result of general business rate increases. We are unable to predict further increases in premiums and deductibles, but based on recent experience, we anticipate further increases in this area, which could adversely impact earnings. The liability exposure of operations in the healthcare services industry has increased, resulting not only in increased premiums, but in limited liability on behalf of the insurance carriers. Our ability to obtain the necessary and sufficient insurance coverage for our operations upon expiration of our insurance policies may be limited, and sufficient insurance may not be available on favorable terms, if at all. We could be materially and adversely affected by any of the following:
| • | our inability to obtain sufficient insurance for our operations; |
| • | the collapse or insolvency of our insurance carriers; |
| • | further increases in premiums and deductibles; and |
| • | an inability to obtain one or more types of insurance on acceptable terms. |
There are significant risks associated with estimating the amount of revenues that we recognize that could impact the timing of our recognition of revenues or have a significant impact on our operating results and financial condition.
There are significant risks associated with estimating the amount of revenues that we recognize in a reporting period. Ongoing insurance coverage changes, geographic coverage differences, differing interpretations of contract coverage, and other payor issues complicate the billing and collection process. Determining applicable primary and secondary coverage for an extensive number of patients at any point in time, together with the changes in patient coverage that occur each month, requires complex, resource-intensive processes. Errors in determining the correct coordination of benefits may result in refunds to payors. Revenues associated with Medicare and Medicaid programs also are subject to estimating risk related to the amounts not paid by the primary government payor that will ultimately be collectible from other government programs paying secondary coverage, the patient’s commercial health plan secondary coverage or the patient. Collections, refunds and payor retractions typically continue to occur for up to three years and longer after services are provided. If our estimates of revenues are materially inaccurate, it could impact the timing of our recognition of revenues and have a significant impact on our operating results and financial condition.
We may be the subject of inquiries by governmental authorities, any of which could result in substantial penalties against us.
From time to time, we may be the subject of inquiries or investigations by governmental authorities. Any negative findings could result in substantial financial penalties against us, exclusion from future participation in the Medicare and Medicaid programs, and, in some cases, criminal penalties. Further, in many cases the mere existence or announcement of any such inquiry could have a material adverse effect on our business. While some
31
Table of Contents
proceedings against companies in our industry may be filed under seal, we have no notice or knowledge that proceedings have been initiated against us at this time. Although we cannot predict whether or when proceedings might be initiated or when these matters may be resolved, it is not unusual for these investigations to continue for a considerable period of time. Responding to these investigations can require substantial management attention and significant legal expense, which could materially adversely affect our operations.
If our key supplier is unable to meet our needs or if we are unable effectively to access new technology it could adversely affect our operating results and financial condition.
Amgen Inc. is the sole supplier of EPO, the administration of which makes up a significant portion of our revenues. If this supplier is unable to meet our needs for EPO or EPO alternatives, including in the event of a product recall, and we are not able to find adequate alternative sources, it could adversely affect our operating results and financial condition.
In addition, the technology related to EPO is subject to new developments that may result in superior products. If we are not able to access these superior products on a cost-effective basis or if suppliers are not able to fulfill our requirements for products, we could face patient attrition which could adversely affect our operating results and financial condition.
Material decisions regarding our dialysis clinics may require consent or trigger rights of our nephrologist partners, and we may be required to purchase the ownership interests of our nephrologist partners or be unable to take actions that we believe are in our best interest.
Our nephrologist partners participate in material strategic and operating decisions we make for our clinics. For example, we generally must obtain the consent of our nephrologist partners before making any material amendments to the operating agreement for the dialysis clinic or admitting additional members. See “Business—JV Operating Agreements”. In addition, some of our JV operating agreements grant our nephrologist partners rights to require us to purchase—at fair market value or book value depending on the circumstances— their ownership interests at certain set times or upon the occurrence of certain triggering events. Event-based triggers of these rights in various JV operating agreements may include sale of assets, closure of the clinic, acquisitions over a certain dollar amount, failure to make distributions and other events. The consummation of the Transactions triggered some of these rights of our nephrologist partners, but such rights were waived. Time-based triggers give nephrologist partners at certain of our clinics the option to require us to purchase previously agreed upon percentages of their ownership interests at certain set dates.
The rights of our nephrologist partners to approve material decisions could limit our ability to take actions that we believe are in our best interest and the best interest of the dialysis clinic. We may not be able to resolve favorably, or at all, any dispute regarding material decisions with our nephrologist partners.
The funds required to honor our put obligations may make it difficult for us to meet our other debt obligations, including borrowings under the New Revolving Credit Facility and the payments of the notes being exchanged hereby. As a result of the exercise of these rights by nephrologist partners, we may also lose the JV ownership structure in some of our clinics. As of September 30, 2010, we have recorded liabilities of approximately $34 million for all existing time-based obligations and approximately $9 million for all existing event-based obligations to our nephrologist partners, which amounts reflect our estimation of fair market value as of September 30, 2010.
Shortages of qualified skilled clinical personnel, or higher than normal turnover rates, could affect our ability to grow and deliver quality, timely and cost-effective care services.
We depend on qualified nurses and other skilled clinical personnel to provide quality service to patients in our clinics. Competition is intense for qualified nursing, technical staff and nephrologists. We depend on our
32
Table of Contents
ability to attract and retain skilled clinical personnel to support our growth and generate revenues. There is currently a shortage of skilled clinical personnel in many of the markets in which we operate our clinics as well as markets in which we are considering opening new clinics. This nursing shortage may adversely affect our ability to grow or, in some cases, to replace existing staff, thereby leading to disruptions in our services. In addition, this shortage of skilled clinical personnel and the more stressful working conditions it creates for those remaining in the profession are increasingly viewed as a threat to patient safety and may trigger the adoption of state and federal laws and regulations intended to reduce that risk. For example, some states have adopted or are considering legislation that would prohibit forced overtime for nurses or establish mandatory staffing level requirements.
In response to the shortage of skilled clinical personnel, we have increased and are likely to have to continue to increase our wages and benefits to recruit and retain nurses or to engage contract nurses at a higher expense until we hire permanent staff nurses. We may not be able to increase the rates we charge to offset increased costs. The shortage of skilled clinical personnel may in the future delay our ability to achieve our operational goals at a dialysis clinic by limiting the number of patients we are able to service. The shortage of skilled clinical personnel also makes it difficult for us in some markets to reduce personnel expense at our clinics by implementing a reduction in the size of the skilled clinical personnel staff during periods of reduced patient admissions and procedure volumes. In addition, we believe that retention of skilled clinical personnel is an important factor in a patient’s decision to continue receiving treatment at one of our clinics. If we are unable to hire skilled clinical personnel when needed, or if we experience a higher than normal turnover rate for our skilled clinical personnel, our operations and treatment growth will be negatively impacted, which would result in reduced revenues, earnings and cash flows.
Growing numbers of skilled clinical personnel are also joining unions that threaten and sometimes call work stoppages. Currently, none of our employees belong to a union. Union organizing activities at our clinics could adversely affect our operating costs, our employee relations, productivity, earnings and cash flows.
Because our senior management has been key to our growth and success, we may be adversely affected if we lose any member of our senior management.
We are highly dependent on our senior management. Although we have employment agreements with our Chairman, Chief Executive Officer, President and Chief Financial Officer, we do not maintain “key man” life insurance policies on any of our officers. Because our senior management has contributed greatly to our growth since inception, the loss of key management personnel or our inability to attract, retain and motivate sufficient members of qualified management or other personnel could have a material adverse effect on us.
We depend on our relationships with our medical directors. Our ability to provide medical services at our facilities would be impaired and our revenues reduced if we were not able to maintain these relationships.
Our business depends on the efforts and success of the physicians who are medical directors at our clinics and the strength of our relationships with these physicians. The efforts of our medical directors directly correlate to the patient satisfaction and operating metrics of our clinics. Our revenues would be reduced if we lost our relationship with one of these key medical directors or group of medical directors. In addition, any failure of these medical directors to maintain the quality of medical care provided or to otherwise adhere to professional guidelines at our clinics or any damage to the reputation of a key medical director or group of medical directors could damage our reputation, subject us to liability and significantly reduce our revenues.
In addition our ability to attract physicians to enter into JV partnerships with us is essential to the growth of our business. If we became unable to attract physicians to enter into JV partnerships it would have a material adverse effect on us.
33
Table of Contents
We may make significant loans to, and are generally liable for a proportionate share of the debts and other obligations of, our JV clinics.
We own and operate our JV clinics through arrangements in which we own, generally, between 51% and 75% of the ownership interests and our nephrologist partners own the remainder interests. In each of our JVs we are liable for our proportionate share (based upon interests) of the third-party debts and other obligations of the JV. Our nephrologist partners guarantee their proportionate share of these non-consolidated third-party debts, which are commonly secured by a lien on substantially all of the clinics’ assets. As of December 31, 2009, we guaranteed approximately $1.9 million of the third-party debt owed by these clinics. A failure of a clinic to repay these debts, or the occurrence of an event of default under the applicable debt arrangements for that clinic, could result in a loss of the applicable clinic. Further, a failure of our nephrologist partners to satisfy their obligations under their guarantees of these debts could also result in the forfeiture of the applicable clinic(s) or may require us to provide further funds in excess of our guarantee to prevent such loss. Our financial condition and results of operations would be materially adversely affected if our clinics are unable to pay these third-party debts, or if our nephrologist partners are unable to satisfy their associated guarantees.
Following the Transactions, we expect that indebtedness at the clinic level will, in most cases, be funded through intercompany loans that we provide. Pursuant to these intercompany lending arrangements we will obtain a secured interest in substantially all of the clinic’s assets to secure the repayment of the intercompany loan. However, our financial condition and results of operations would be materially adversely affected if our clinics are unable to repay these intercompany loans.
We will be required to evaluate the effectiveness of our internal controls when we become subject to the Sarbanes-Oxley Act of 2002.
As a privately held company (“non-issuer”) we have not been and are not currently subject to the requirements of the Securities Act, the Securities Exchange Act of 1934, as amended (the “Exchange Act”), the rules and regulations of the SEC or the Sarbanes-Oxley Act of 2002, which require, among other things, public companies to have and maintain effective disclosure controls and procedures to ensure timely disclosure of material information, and have management review the effectiveness of those controls on a quarterly basis. The Sarbanes-Oxley Act of 2002 also requires public companies to have and maintain effective internal controls over financial reporting to provide reasonable assurance regarding the reliability of financial reporting and preparation of financial statements, and have management review the effectiveness of those controls on an annual basis (and, in certain cases, have the independent auditor attest to the effectiveness of such internal controls). We are not currently required to comply with these requirements, and therefore may not have comparable procedures in place as compared to public companies.
Following the effectiveness of our registration statement (see “The Exchange Offer”) we will, to the extent required by Section 404 of the Sarbanes-Oxley Act of 2002, be required to annually complete an evaluation of our internal controls over financial reporting. Because we have not completed such evaluations in the past, we cannot predict the outcome of this testing and our internal controls over financial reporting may not be effective. If our internal controls are ineffective, our financial results could be adversely affected. We will incur additional expenses and commitment of management’s time in connection with these evaluations.
Risks Related to the Notes
The following risks apply to the outstanding notes and will apply equally to the exchange notes.
Our substantial level of indebtedness could adversely affect our financial condition and prevent us from fulfilling our obligations under the notes and our other indebtedness.
We have substantial indebtedness. As of September 30, 2010, we have a total aggregate principal amount of indebtedness of $258.2 million, and an additional $25.0 million available to be borrowed under our New
34
Table of Contents
Revolving Credit Facility. Our high level of indebtedness could have important consequences. For example, it could:
| • | make it more difficult for us to satisfy our obligations under the notes offered hereby, as well as our New Revolving Credit Facility, exposing us to the risk of default, which could result in a foreclosure on our assets, which, in turn, would negatively affect our ability to operate as a going concern; |
| • | require us to dedicate a substantial portion of our cash flow from operations to interest and principal payments on our indebtedness, reducing the availability of our cash flow for other purposes, such as capital expenditures, acquisitions and working capital; |
| • | limit our flexibility in planning for, or reacting to, changes in our business and the industries in which we operate; |
| • | increase our vulnerability to general adverse economic and industry conditions; |
| • | place us at a disadvantage compared to our competitors that have less debt; |
| • | expose us to fluctuations in the interest rate environment because the interest rates on borrowings under our New Revolving Credit Facility will be variable; |
| • | increase our cost of borrowing; |
| • | limit our ability to borrow additional funds; and |
| • | require us to sell assets to raise funds, if needed, for working capital, capital expenditures, acquisitions or other purposes. |
Despite our current indebtedness levels, we may still incur significant additional indebtedness, which could increase the risks associated with our substantial indebtedness.
We may be able to incur substantial additional indebtedness, including additional secured indebtedness, in the future. The terms of the indenture governing the notes being exchanged hereby, and our New Revolving Credit Facility limit, but do not prohibit, us from incurring additional indebtedness. In addition, the indenture allows us to issue additional notes under certain circumstances which is also guaranteed by the guarantors and shares in the Collateral that secures the notes and guarantees. The indenture also allows our non-guarantor subsidiaries to incur a certain amount of additional debt, which would be structurally senior to the notes. In addition, the indenture does not prevent us from incurring other liabilities that do not constitute indebtedness. See “Description of the Exchange Notes.” This may have the effect of reducing the amounts available to pay interest on the notes. If we incur new debt or other liabilities, the related risks that we now face could intensify.
We may not be able to generate sufficient cash to service our indebtedness, including the notes, and we may be forced to take other actions to satisfy our payment obligations under our indebtedness, which may not be successful.
Our ability to make scheduled payments on or to refinance our debt obligations depends on our future performance, which will be affected by financial, business, economic, competitive, legislative, regulatory and other factors. We will not be able to control many of these factors, such as economic conditions in the industry in which we operate, regulatory changes and competitive pressures. Our cash flow may not be sufficient to allow us to pay principal and interest on our debt and to meet our other obligations. If our cash flows and capital resources are insufficient to fund our debt service obligations, we may be forced to reduce or delay investments and capital expenditures, or to sell assets, seek additional capital or restructure or refinance our indebtedness, including the notes. These alternative measures may not be successful and may not permit us to meet our scheduled debt service obligations. In addition, the terms of existing or future debt agreements, including our New Revolving Credit Facility and the indenture relating to the notes, may restrict us from pursuing any of these alternatives. Any limitation on our ability to pay principal of and interest on the notes would likely reduce the value of the notes.
35
Table of Contents
Our New Revolving Credit Facility and the indenture governing the notes being exchanged hereby impose significant operating and financial restrictions on us and our future subsidiaries, which may prevent us from capitalizing on business opportunities and taking some actions.
The agreements that govern the terms of our debt, including the indenture that governs the notes and our New Revolving Credit Facility, impose significant operating and financial restrictions on us. These restrictions limit our ability to, among other things:
| • | incur additional indebtedness; |
| • | incur liens; |
| • | make investments and sell assets; |
| • | pay dividends and make other distributions; |
| • | purchase our stock; |
| • | engage in business activities unrelated to our current business; |
| • | enter into transactions with affiliates; or |
| • | consolidate, merge or sell all or substantially all of our assets. |
In addition, under our New Revolving Credit Facility we are required to satisfy and maintain specified financial ratios and other financial condition tests. Our ability to meet those financial ratios and tests can be affected by events beyond our control, and we cannot assure you that we will meet those ratios and tests. A breach of any of these covenants could result in a default under our New Revolving Credit Facility. Upon the occurrence of an event of default under our New Revolving Credit Facility, our lenders could elect to declare all amounts outstanding under our New Revolving Credit Facility to be immediately due and payable and terminate all commitments to extend further credit.
As a result of these covenants and restrictions, we will be limited in how we conduct our business, and we may be unable to raise additional debt or equity financing to compete effectively or to take advantage of new business opportunities. The terms of any future indebtedness we may incur could include more restrictive covenants. A breach of any of these covenants could result in a default in respect of the related indebtedness. If a default occurs, the relevant lenders could elect to declare the indebtedness, together with accrued interest and other fees, to be due and payable immediately and proceed against any Collateral securing that indebtedness.
This, in turn, could cause our other debt to become due and payable as a result of cross-acceleration provisions contained in the agreements governing such other debt. In the event that some or all of our debt is accelerated and becomes immediately due and payable, we may not have the funds to repay, or the ability to refinance, such debt.
The issuer and guarantors of the notes (other than American Renal Management LLC) are holding companies with no operations of their own.
The issuer and the guarantors of the notes (other than American Renal Management LLC) are holding companies, and their ability to service our debt is dependent upon the earnings from the business conducted by our non-guarantor subsidiaries that operate the clinics. The effect of this structure is that we depend on the earnings of our clinics, and the distribution or payment to us of a portion of these earnings to meet our obligations, including those under our New Revolving Credit Facility, the notes being exchanged hereby and any of our other debt obligations. The distributions of those earnings or advances or other distributions of funds by these entities to us, all of which are contingent upon the clinics’ earnings, are subject to various business considerations. In addition, distributions by our clinics could be subject to statutory restrictions, including state laws requiring that such subsidiaries be solvent, or contractual restrictions. Some of our clinics may become
36
Table of Contents
subject to agreements that restrict the sale of assets and significantly restrict or prohibit the payment of dividends or the making of distributions, loans or other payments to stockholders, partners or members. The indenture governing the notes permits our clinics to enter into agreements with similar prohibitions and restrictions in the future, subject to certain limitations.
The right to receive payments on the notes is structurally subordinated to the liabilities of our non-guarantor subsidiaries and substantially all of our operations are conducted through non-guarantor subsidiaries.
The notes are structurally subordinated to all indebtedness of our subsidiaries that are not guarantors of the notes. While the indenture governing the notes limits the indebtedness and activities of these non-guarantor subsidiaries, they are able to incur a certain amount of debt and their ability to incur non-debt obligations is not limited. The indenture governing the notes does not require our joint venture subsidiaries or our subsidiaries that are controlled foreign corporations to guarantee the notes.
In the event that any of the non-guarantor subsidiaries or joint venture entities becomes insolvent, liquidates or otherwise reorganizes:
| • | the creditors of the guarantors (including the holders of the notes) have no right to proceed against such subsidiary’s assets; and |
| • | the creditors of such non-guarantor subsidiary, including trade creditors, generally are entitled to payment in full from the sale or other disposal of assets of such subsidiary, before any guarantor as direct or indirect shareholder, is entitled to receive any distributions from such subsidiary. |
The notes are secured only to the extent of the value of the assets that have been granted as security for the notes and in the event that the security is enforced against the Collateral, the holders of the notes will receive proceeds from the Collateral only after the lenders under our New Revolving Credit Facility.
If we default on the notes, the holders of the notes are secured only to the extent of the value of the assets underlying their security interest. Not all of our assets secure the notes and we have not and will not take action to perfect all liens on assets which do secure the notes. Furthermore, upon enforcement against any Collateral or in insolvency, under the terms of the intercreditor agreement proceeds of such enforcement will first be used to pay obligations outstanding under our New Revolving Credit Facility (including post-petition interest, whether or not allowable in any bankruptcy case) and certain hedging and cash management arrangements prior to paying the notes.
The rights of holders of notes in the Collateral may be adversely affected by the intercreditor agreement.
Under the terms of the intercreditor agreement by and among the collateral agent for the holders of notes, the collateral agent under the New Revolving Credit Facility and the other parties from time to time thereto, the liens securing the obligations under the New Revolving Credit Facility and obligations under certain hedging and cash management arrangements on any assets of ours or the guarantors will generally rank equally with the liens on such assets securing our and the guarantors’ obligations under the notes and the guarantees. However, the intercreditor agreement provides that the obligations under the New Revolving Credit Facility (including post-petition interest, whether or not allowable in any bankruptcy case) as well as under such hedging and cash management arrangements will be paid prior to the obligations under the notes and guarantees in the event of any foreclosure or bankruptcy event.
Additionally, the intercreditor agreement generally permits each of the collateral agent for the holders of the notes, the collateral agent for the lenders under the New Revolving Credit Facility and the collateral agent for any other holders of pari passu lien indebtedness to independently enforce their liens on the Collateral (provided that distributions received on enforcement are applied as provided in the intercreditor agreement). It is possible
37
Table of Contents
that disputes may occur between the holders of the notes and lenders under our New Revolving Credit Facility or other secured parties as to the appropriate manner of pursuing enforcement remedies with respect to the Collateral which may delay enforcement of the Collateral, result in litigation and/or result in enforcement actions against the Collateral that are not approved by the holders of the notes. See “Description of the Exchange Notes—Intercreditor Agreement.”
The value of the Collateral securing the notes may not be sufficient to satisfy our obligations under the notes and the other obligations secured by an equal and ratable lien on the Collateral.
Obligations under the notes are secured by a lien on the Collateral. No appraisal of the value of the Collateral has been made in connection with this offering, and the fair market value is subject to fluctuations based on factors that include, among others, changing economic conditions, competition and other future trends. By its nature, some or all of the Collateral may be illiquid and may have no readily ascertainable market value. In the event of a foreclosure, liquidation, bankruptcy or similar proceeding, no assurance can be given that the proceeds from any sale or liquidation of the Collateral will be sufficient to pay our obligations under the notes, in full or at all, after first satisfying our obligations in full under the New Revolving Credit Facility. There also can be no assurance that the Collateral will be saleable and, even if saleable, the timing of its liquidation would be uncertain. To the extent that liens, rights or easements granted to third parties encumber assets located on property owned by us, such third parties have or may exercise rights and remedies with respect to the property subject to such liens that could adversely affect the value of the Collateral and the ability of the collateral agent to foreclose on the Collateral. In addition, we may not have liens perfected on all of the Collateral securing the notes prior to the closing of this offering. Accordingly, there may not be sufficient Collateral to pay all or any of the amounts due on the notes. Any claim for the difference between the amount, if any, realized by holders of the notes from the sale of the Collateral securing the notes and the obligations under the notes will rank equally in right of payment with any second lien debt, all of our other unsecured unsubordinated indebtedness and other obligations, including trade payables. With respect to some of the Collateral, the collateral agent’s security interest and ability to foreclose will also be limited by the need to meet certain requirements, such as obtaining third-party consents and making additional filings. If we are unable to obtain these consents or make these filings, the security interests may be invalid and the holders will not be entitled to the Collateral or any recovery with respect thereto. We cannot assure you that any such required consents can be obtained on a timely basis or at all. These requirements may limit the number of potential bidders for certain Collateral in any foreclosure and may delay any sale, either of which events may have an adverse effect on the sale price of the Collateral. Therefore, the practical value of realizing on the Collateral may, without the appropriate consents and filings, be limited.
In addition, as noted above, the Issuer and the Guarantors (other than American Renal Management LLC) are holding companies for our joint venture subsidiaries that operate the clinics. Our joint venture subsidiaries are not guarantors, and their assets do not secure the notes obligations.
The pledge of the capital stock, other securities and similar items of our subsidiaries that secure the notes may automatically be released from the lien on them and no longer constitute collateral for so long as the pledge of such capital stock or such other securities would require the filing of separate financial statements with the SEC for that subsidiary.
The notes are secured by a pledge of the stock of some of our subsidiaries. Under the SEC regulations in effect as of the issue date of the notes, if the par value, book value as carried by us or market value (whichever is greatest) of the capital stock, other securities or similar items of a subsidiary pledged as part of the collateral is greater than or equal to 20% of the aggregate principal amount of the notes then outstanding, such subsidiary would be required to provide separate financial statements to the SEC. Therefore, the indenture and the collateral documents provide that any capital stock and other securities of any of our subsidiaries will be excluded from the collateral for so long as the pledge of such capital stock or other securities to secure the notes would cause such subsidiary to be required to file separate financial statements with the SEC pursuant to Rule 3-16 of Regulation
38
Table of Contents
S-X (as in effect from time to time). As a result, holders of the notes could lose a portion or all of their security interest in the capital stock or other securities of those subsidiaries during such period. It may be more difficult, costly and time-consuming for holders of the notes to foreclose on the assets of a subsidiary than to foreclose on its capital stock or other securities, so the proceeds realized upon any such foreclosure could be significantly less than those that would have been received upon any sale of the capital stock or other securities of such subsidiary. In addition, in the event that the capital stock or other securities of a subsidiary is included in the Collateral as described above, the collateral agent under the New Revolving Credit Facility would be entitled to maintain possession of such capital stock or other securities until the payment in full of the obligations under the New Revolving Credit Facility. See “Description of the Exchange Notes—Collateral.”
There are circumstances other than repayment or discharge of the notes under which Collateral could be released automatically without the consent of the holders of the notes, which could be adverse to holders of notes.
Under various circumstances, all or a portion of the Collateral may be released, including:
| • | to enable the sale, transfer or other disposal of such Collateral in a transaction not prohibited under the indenture, including the sale of any entity in its entirety that owns or holds such Collateral; and |
| • | with respect to Collateral held by a guarantor, upon the release of such guarantor from its guarantee. |
Our wholly owned domestic subsidiaries are required to be subsidiary guarantors and guarantee the notes. The guarantee of any subsidiary guarantor will be released in connection with a sale of such subsidiary guarantor in a transaction not prohibited by the indenture. The indenture also permits us to designate one or more of our restricted subsidiaries that is a guarantor of the notes as an unrestricted subsidiary. If we designate a subsidiary guarantor as an unrestricted subsidiary, all of the liens on any Collateral owned by such subsidiary or any of its subsidiaries and any guarantees of the notes by such subsidiary or any of its subsidiaries will be released under the indenture but not under the New Revolving Credit Facility. Designation of an unrestricted subsidiary will reduce the aggregate value of the Collateral with respect to the notes to the extent that liens on the assets of the unrestricted subsidiary and its subsidiaries are released. In addition, the creditors of the unrestricted subsidiary and its subsidiaries will have a senior claim on the assets of such unrestricted subsidiary and its subsidiaries. See “Description of the Exchange Notes.”
Rights of holders of notes in the Collateral may be adversely affected by bankruptcy proceedings and holders of notes may not be entitled to post-petition interest in any bankruptcy proceeding.
The right of the collateral agent for the notes to repossess and dispose of the Collateral securing the notes upon acceleration is likely to be significantly impaired by federal bankruptcy law if bankruptcy proceedings are commenced by or against us prior to or possibly even after the collateral agent has repossessed and disposed of the Collateral. Under the U.S. Bankruptcy Code, a secured creditor, such as the collateral agent for the notes, is prohibited from repossessing its security from a debtor in a bankruptcy case, or from disposing of security repossessed from a debtor, without bankruptcy court approval. Moreover, bankruptcy law permits the debtor to continue to retain and to use Collateral, and the proceeds, products, rents, or profits of the Collateral, even though the debtor is in default under the applicable debt instruments, provided that the secured creditor is given “adequate protection.” The meaning of the term “adequate protection” may vary according to circumstances, but it is intended in general to protect the value of the secured creditor’s interest in the Collateral and may include cash payments or the granting of additional security, if and at such time as the court in its discretion determines, for any diminution in the value of the Collateral as a result of the stay of repossession or disposition or any use of the Collateral by the debtor during the pendency of the bankruptcy case. In view of the broad discretionary powers of a bankruptcy court, it is impossible to predict how long payments under the notes could be delayed following commencement of a bankruptcy case, whether or when the collateral agent would repossess or dispose of the Collateral, or whether or to what extent holders of the notes would be compensated for any delay in payment of loss of value of the Collateral through the requirements of “adequate protection.” Furthermore, in the
39
Table of Contents
event the bankruptcy court determines that the value of the Collateral is not sufficient to repay all amounts due on the notes, the holders of the notes would have “undersecured claims” as to the difference. Federal bankruptcy laws do not permit the payment or accrual of interest, costs, and attorneys’ fees for “undersecured claims” during the debtor’s bankruptcy case.
We may not be able to repurchase the notes upon a change of control or pursuant to an asset sale offer.
Upon a change of control, as defined under the indenture governing the notes, the holders of notes have the right to require us to offer to purchase all of the notes then outstanding at a price equal to 101% of their principal amount plus accrued and unpaid interest. We cannot assure you that we would have sufficient funds available to repay all of our indebtedness or other put rights that would become payable upon a change of control and to repurchase all of the notes. Our failure to offer to purchase all outstanding notes or to purchase all validly tendered notes would be an event of default under the indenture. Such an event of default may cause the acceleration of our other debt. Our other debt also may contain restrictions on repayment requirements with respect to specified events or transactions that constitute a change of control under the indenture.
In addition, in certain circumstances specified in the indenture governing the notes, we are required to commence an asset sale offer, or an intercompany loan refinancing offer, each as defined in the indenture, pursuant to which we are obligated to offer to purchase the applicable notes at a price equal to 100% of their principal amount plus accrued and unpaid interest. Our other debt may contain restrictions that would limit or prohibit us from completing any such asset sale offer. Our failure to purchase any such notes when required under the indenture would be an event.
Certain laws and regulations may impose restrictions or limitations on foreclosure.
Our obligations under the notes are secured only by the Collateral described in this prospectus. The collateral agent’s ability to foreclose on the Collateral on your behalf may be subject to perfection, priority issues, state law requirements and practical problems associated with the realization of the collateral agent’s security interest or lien in the Collateral, including cure rights, foreclosing on the Collateral within the time periods permitted by third parties or prescribed by laws, obtaining third-party consents, making additional filings, statutory rights of redemption, and the effect of the order of foreclosure. We cannot assure you that the consents of any third parties and approvals by governmental entities will be given when required to facilitate a foreclosure on such assets. Therefore, we cannot assure you that foreclosure on the Collateral will be sufficient to make all payments on the notes.
In addition, our business requires numerous registrations, licenses and permits. Continued operation of our dialysis clinics that are significant to the operations of our business depends on the maintenance of such registrations, licenses and permits. Our business is subject to substantial regulation and registration, license and permit requirements and may be adversely affected if we are unable to comply with existing regulations or requirements or changes in applicable regulations or requirements. In the event of an acquisition, sale or foreclosure of a dialysis clinic, the transfer of such registrations, licenses and permits may be prohibited and may require us to incur significant cost and expense. Further, we cannot assure you that the applicable governmental authorities will consent to the transfer of such registrations, licenses and permits. If the regulatory approvals required for such transfers are not obtained or are delayed, the acquisition, sale or foreclosure may be delayed, a temporary shutdown of operations may result.
Rights of holders of notes in the Collateral may be adversely affected by the failure to perfect liens on certain Collateral acquired in the future.
Applicable law requires that certain property and rights acquired after the grant of a general security interest or lien can only be perfected at the time such property and rights are acquired and identified. Although the indenture contains customary further assurances covenants, there can be no assurance that the trustee or the
40
Table of Contents
collateral agent will monitor, or that we will inform the trustee or the collateral agent of the future acquisition of property and rights that constitute Collateral, and that the necessary action will be taken to properly perfect the lien on such after-acquired Collateral. Neither the trustee nor the collateral agent for the notes has any obligation to monitor the acquisition of additional property or rights that constitute Collateral or the perfection of any security interests therein. Such failure may result in the loss of the practical benefits of the liens thereon or of the priority of the liens securing the notes.
The secured intercompany loans may be avoided or subordinated in the event of our bankruptcy.
The collateral includes a pledge by ARA of its right under secured intercompany loans made from time to time to our clinic subsidiaries. The enforceability of intercompany loans may be avoided or equitably subordinated if we are a debtor in a bankruptcy case.
Any future pledge by a clinic of collateral in favor of ARA to secure existing obligations under that intercompany debt might be avoidable by the pledgor (as debtor in possession) or by its trustee in bankruptcy if certain events or circumstances exist or occur, including if the pledgor is insolvent at the time of the pledge, the pledge permits ARA to receive a greater recovery than they would have received in a hypothetical liquidation under Chapter 7 of the U.S. Bankruptcy Code if the pledge had not been given and a bankruptcy proceeding in respect of the pledge is commenced within one year following the pledge. To the extent that the grant of any such mortgage or other security interest is avoided as a preference, you would lose the benefit of the mortgage or security interest as security for the intercompany loan. Similarly, payments made by a clinic under its intercompany loans and within one year of the commencement of a bankruptcy case of that clinic may be recaptured to the extent such payments exceed the value of the collateral for that intercompany loan.
Last, under the doctrine of equitable subordination, in the event of a bankruptcy case involving a clinic, the obligation of a clinic to repay its intercompany loan may be subordinated to the payment of a clinic’s other debts if it is determined that ARA engaged in inequitable conduct and subordination of the intercompany claim is proper.
Any future pledge of Collateral might be avoidable in bankruptcy.
Any future pledge of Collateral in favor of the collateral agent (for its benefit and for the benefit of the trustee and the holders of the notes), including pursuant to security documents delivered after the date of the indenture governing the notes, might be avoidable by the pledgor (as debtor in possession) or by its trustee in bankruptcy if certain events or circumstances exist or occur, including if the pledgor is insolvent at the time of the pledge, the pledge permits the holders of the notes to receive a greater recovery than they would have received in a hypothetical liquidation under Chapter 7 of the U.S. Bankruptcy Code if the pledge had not been given and a bankruptcy proceeding in respect of the pledge is commenced within 90 days following the pledge, or, in certain circumstances, a longer period. To the extent that the grant of any such mortgage or other security interest is avoided as a preference, you would lose the benefit of the mortgage or security interest. As more fully described herein, certain of the assets securing the notes were not subject to a valid and perfected security interest on the closing date. We have agreed to use commercially reasonable efforts to obtain a valid and perfected security interest on such assets.
A court could void our subsidiaries’ guarantees of the notes under fraudulent transfer laws.
Although the guarantees provide you with a direct claim against the assets of the subsidiary guarantors, under the federal bankruptcy laws and comparable provisions of state fraudulent transfer laws, a guarantee could be voided, or claims with respect to a guarantee could be subordinated to all other debts of that guarantor. In addition, a bankruptcy court could void (i.e., cancel) any payments by that guarantor pursuant to its guarantee and require those payments to be returned to the guarantor or to a fund for the benefit of the other creditors of the guarantor.
41
Table of Contents
The bankruptcy court might take these actions if it found, among other things, that when a subsidiary guarantor executed its guarantee (or, in some jurisdictions, when it became obligated to make payments under its guarantee):
| • | such subsidiary guarantor received less than reasonably equivalent value or fair consideration for the incurrence of its guarantee; and |
| • | such subsidiary guarantor: |
| • | was (or was rendered) insolvent by the incurrence of the guarantee; |
| • | was engaged or about to engage in a business or transaction for which its assets constituted unreasonably small capital to carry on its business; |
| • | intended to incur, or believed that it would incur, obligations beyond its ability to pay as those obligations matured; or |
| • | was a defendant in an action for money damages, or had a judgment for money damages docketed against it and, in either case, after final judgment, the judgment was unsatisfied. |
A bankruptcy court would likely find that a subsidiary guarantor received less than fair consideration or reasonably equivalent value for its guarantee to the extent that it did not receive direct or indirect benefit from the issuance of the notes. A bankruptcy court could also void a guarantee if it found that the subsidiary issued its guarantee with actual intent to hinder, delay, or defraud creditors. Although courts in different jurisdictions measure solvency differently, in general, an entity would be deemed insolvent if the sum of its debts, including contingent and unliquidated debts, exceeds the fair value of its assets, or if the present fair saleable value of its assets is less than the amount that would be required to pay the expected liability on its debts, including contingent and unliquidated debts, as they become due.
We cannot predict what standard a court would apply in order to determine whether a subsidiary guarantor was insolvent as of the date it issued the guarantee or whether, regardless of the method of valuation, a court would determine that the subsidiary guarantor was insolvent on that date, or whether a court would determine that the payments under the guarantee constituted fraudulent transfers or conveyances on other grounds.
The indenture governing the notes contains a “savings clause” intended to limit each subsidiary guarantor’s liability under its guarantee to the maximum amount that it could incur without causing the guarantee to be a fraudulent transfer under applicable law. There can be no assurance that this provision will be upheld as intended. In a recent case, the U.S. Bankruptcy Court in the Southern District of Florida found this kind of provision in that case to be ineffective, and held the subsidiary guarantees to be fraudulent transfers and voided them in their entirety.
If a guarantee is deemed to be a fraudulent transfer, it could be voided altogether, or it could be subordinated to all other debts of the subsidiary guarantor. In such case, any payment by the subsidiary guarantor pursuant to its guarantee could be required to be returned to the subsidiary guarantor or to a fund for the benefit of the creditors of the subsidiary guarantor. If a guarantee is voided or held unenforceable for any other reason, holders of the notes would cease to have a claim against the subsidiary guarantor based on the guarantee and would be creditors only of the Company and any subsidiary guarantor whose guarantee was not similarly voided or otherwise held unenforceable.
Your ability to transfer the notes may be limited by the absence of an active trading market, and there is no assurance that any active trading market will develop for the notes.
There is currently no established public market for the notes, we do not intend to apply for the notes to be listed on any securities exchange or to arrange for their quotation on any automated dealer quotation system and we cannot assure you as to the liquidity of markets that may develop for the notes, your ability to sell the notes or
42
Table of Contents
the price at which you would be able to sell the exchange notes. If such markets were to exist, the notes could trade at prices that may be lower than their principal amount or purchase price depending on many factors, including prevailing interest rates, the market for similar notes, our financial and operating performance and other factors The initial purchasers have advised us that they intend to make a market in the exchange notes as permitted by applicable laws and regulations; however, the initial purchasers are not obligated to make a market in the exchange notes, and they may discontinue their market-making activities at any time without notice. In addition, such market making activity may be limited during the pendency of the exchange offer or the effectiveness of a shelf registration statement in lieu thereof. Therefore, an active market for the notes may not develop or, if developed, it may not continue. Historically, the market for non-investment grade debt has been subject to disruptions that have caused substantial volatility in the prices of securities similar to the notes. The market, if any, for the notes may experience similar disruptions and any such disruptions may adversely affect the prices at which you may sell your notes. Any disruptions may have a negative effect on noteholders, regardless of our prospects and financial performance. In addition, subsequent to their initial issuances, the exchange notes may trade at discounts from their initial offering prices, depending upon prevailing interest rates, the market for similar notes, our financial and operating performance and other factors.
We are indirectly owned and controlled by the Sponsor, and the Sponsor’s interests as equity holders may conflict with yours as a creditor.
The Sponsor owns a significant majority of our parent company’s equity and, accordingly, will have the ability to control our policies and operations. The Sponsor will not have any liability for any obligations under the notes, and the interests of the Sponsor may not in all cases be aligned with your interests. For example, if we encounter financial difficulties or are unable to pay our debts as they mature, the interests of our equity holders might conflict with your interests as a noteholder. In addition, our equity holders may have an interest in pursuing acquisitions, divestitures, financings or other transactions that, in their judgment, could enhance their equity investments, even though such transactions might involve risks to you as a holder of the notes. Furthermore, the Sponsor may in the future own equity or debt interests in entities that directly or indirectly compete with us. The Sponsor also may pursue acquisition opportunities that may be complementary to our business, and as a result, those acquisition opportunities may not be available to us. For information concerning our arrangements with the Sponsor following the Transactions, see “Certain Relationships and Related Party Transactions.”
43
Table of Contents
This prospectus contains “forward-looking statements” within the meaning of the federal securities laws which are intended to be covered by the safe harbors created thereby. Forward-looking statements are those statements that are based upon management’s current plans and expectations as opposed to historical and current facts and are often identified in this report by use of words including but not limited to “may,” “believe,” “will,” “project,” “expect,” “estimate,” “anticipate,” and “plan.” These statements are based upon estimates and assumptions made by our management that, although believed to be reasonable, are subject to numerous factors, risks and uncertainties that could cause actual outcomes and results to be materially different from those projected. These and other important factors, including those discussed in this prospectus under the headings “Summary,” “Risk Factors,” “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and “Business,” may cause our actual results, performance or achievements to differ materially from any future results, performance or achievements expressed or implied by these forward-looking statements. Some of the factors that could cause actual results to differ materially from those expressed or implied by the forward-looking statements include, among others, the following:
| • | Our high degree of leverage and interest rate risk; |
| • | Our ability to incur substantially more debt; |
| • | Operating and financial restrictions in our debt agreements; |
| • | Our ability to successfully implement our business strategies; |
| • | Our ability to successfully develop future de novo clinics or to integrate future acquisitions; |
| • | The highly competitive nature of the healthcare industry; |
| • | Governmental regulation of the industry, including Medicare and Medicaid reimbursement levels, and potential federal or state reform of healthcare; |
| • | Our ability to attract and retain qualified management and healthcare professionals, including physicians and nurses; |
| • | The availability of capital to fund our corporate growth strategy; |
| • | Potential lawsuits or other claims asserted against us; |
| • | Our ability to maintain or increase patient membership and control costs of our managed healthcare plans; |
| • | Pressures to contain costs by managed care organizations and other insurers and our ability to negotiate acceptable terms with these third-party payors; |
| • | Changes in general economic conditions; |
| • | Our exposure to the increased amounts of and collection risks associated with uninsured accounts and the co-pay and deductible portions of insured accounts; and |
| • | Dependence on our senior management team and local management personnel. |
Our forward-looking statements speak only as of the date made. Except as required by law, we undertake no obligation to publicly update or revise any forward-looking statements contained herein, whether as a result of new information, future events or otherwise. You are cautioned to not rely on such forward-looking statements when evaluating the information contained in this report. In light of the significant uncertainties inherent in the forward-looking statements included in this prospectus, you should not regard the inclusion of such information as a representation by us that our objectives and plans anticipated by the forward-looking statements will occur or be achieved, or if any of them do, what impact they will have on our results of operations and financial condition.
44
Table of Contents
We will not receive any cash proceeds from the issuance of the exchange notes pursuant to the exchange offer. In consideration for issuing the exchange notes as contemplated in this prospectus, we will receive in exchange a like principal amount of outstanding notes, the terms of which are identical in all material respects to the exchange notes, except that the exchange notes are registered under the Securities Act, are not entitled to the registration rights which are applicable to the outstanding notes, and are not subject to certain special interest rate provisions applicable to the outstanding notes. The outstanding notes surrendered in exchange for the exchange notes will be retired and canceled and cannot be reissued. Accordingly, issuance of the exchange notes will not result in any change in our capitalization.
45
Table of Contents
The following table sets forth our cash and cash equivalents and capitalization as of September 30, 2010. The table below should be read in conjunction with “The Transactions,” “Summary Historical and Pro Forma Consolidated Financial and Other Data,” “Selected Consolidated Financial Data,” “Management’s Discussion and Analysis of Financial Condition and Results of Operations,” “Use of Proceeds,” “Description of Other Indebtedness” and our historical consolidated financial statements, including the related notes, appearing elsewhere in this offering memorandum.
| September 30, 2010 | ||||
| (in thousands) | ||||
Cash and cash equivalents | $ | 27,579 | ||
Debt: | ||||
New Revolving Credit Facility | $ | — | ||
Notes being exchanged hereby (1) | 250,000 | |||
Debt and capital lease obligations, including current portion (1) | 8,212 | |||
Total equity | 329,174 | |||
Total capitalization | $ | 587,386 | ||
| (1) | Represents principal amount. |
46
Table of Contents
General
On March 22, 2010, ARH entered into the Merger Agreement with the Parent, C.P. Atlas Intermediate Holdings, LLC, C.P. Atlas Acquisition Corp., certain of ARH’s stockholders party thereto and the Sellers’ Representative pursuant to which ARH agreed that C.P. Atlas Acquisition Corp. merged with and into ARH and, after which, ARH became the surviving entity and a wholly owned subsidiary of C.P. Atlas Intermediate Holdings, LLC, which is in turn a wholly owned subsidiary of the Parent. The parties agreed to consummate the Merger, subject to the terms and conditions set forth in the Merger Agreement, for an aggregate purchase price of $415 million, subject to adjustments, including, without limitation, for working capital, indebtedness, certain specified liabilities and certain tax savings. ARH agreed that $2.5 million of the purchase price was to be placed into a third-party escrow account as security for working capital adjustments and that $27.5 million of the purchase price was to be placed into a third-party escrow account as security for indemnification claims, as described below under “—Merger Agreement.”
As of the effective time of the Merger, holders of shares of ARH’s common stock have no further ownership interest in ARH and instead received cash and the right to receive certain future payments from Centerbridge, ARH and/or the escrow agent pursuant to the Merger Agreement (in the case of the escrow agent, in respect of the unused portion of the third-party escrow accounts, if any) (with the exception of certain members of senior management and certain other employees who have agreed not to have certain of their equity interests cashed out in the Merger and holders who perfect their appraisal rights under Delaware law). These future payments consist of the right to receive tax benefits, estimated to be worth $17.0 million, which the former shareholders are entitled to receive when they are received by Centerbridge, ARH and/or the escrow agent, as applicable.
In connection with the Merger:
| • | Centerbridge made an aggregate cash equity investment of $161.5 million in the Parent, subject to certain adjustments, and in exchange received 87% of the common stock of the Parent immediately following the Merger. |
| • | Certain members of ARH’s management contributed a portion of their existing equity ownership in ARH in exchange for newly issued shares of common stock and options to purchase common stock of the Parent. |
| • | ARH entered into the New Revolving Credit Facility. |
| • | ARH offered the notes being exchanged hereby. |
In connection with the Merger, all of ARH’s debt and preferred stock that existed prior to the closing date of the Merger was repaid or redeemed with the proceeds of the notes being exchanged hereby and our borrowings under the New Revolving Credit Facility. All of ARH’s JVs’ debt was refinanced through secured intercompany loans, except for $11.9 million aggregate amount, as of December 31, 2009, of third-party debt. The secured intercompany loans, which total $36.3 million as of December 31, 2009, were pledged as collateral for our New Revolving Credit Facility and for the notes being exchanged hereby. The aggregate purchase price of approximately $415 million for the Merger (including the related repayment of ARA’s existing debt) and related fees and expenses were funded by the cash equity investment by Centerbridge and the net proceeds from the outstanding notes. Although certain of ARH’s nephrologist partners had the right to require ARH to repurchase some or all of the equity interests in certain clinics as a result of the Transactions, these rights were waived.
Merger Agreement
The Merger Agreement provides for indemnification for losses relating to specified events, circumstances or matters. ARH’s selling stockholders, optionholders and warrantholders (collectively, the “Sellers”) are obligated to indemnify C.P. Atlas Holdings, Inc., and after the consummation of the Merger, ARH, for any losses arising out of, relating to or resulting from the following: (1) any inaccuracy or breach of any representation or warranty
47
Table of Contents
made by ARH in the Merger Agreement or any certificate delivered in connection with the conditions to closing of the Merger Agreement; (2) any breach by ARH (or any of ARH’s subsidiaries) of a covenant or an agreement set forth in the Merger Agreement, other than post-closing covenants or agreements; (3) any breach by the selling stockholders or the Sellers’ Representative of any of their respective post-closing covenants or agreements, specified tax losses or liabilities; (4) any and all income taxes imposed on ARH or any of ARH’s subsidiaries attributable to a pre-closing tax period; (5) any and all taxes imposed on ARH or any of ARH’s subsidiaries attributable to certain specified tax positions; and (6) certain specified matters. The right to indemnification generally for a breach by ARH of a representation or warranty is not effective until the aggregate dollar amount of all losses exceeds $1,500,000, with each individual claim exceeding $50,000, subject to certain limited exceptions. Subject to limited exceptions, the aggregate indemnification obligations of the Sellers for losses will not exceed $27.5 million.
48
Table of Contents
SELECTED CONSOLIDATED FINANCIAL DATA
The following table presents our selected consolidated financial and operating information, which you should read in conjunction with, and is qualified in its entirety by reference to, our historical financial statements, the notes to those statements, and “Management’s Discussion and Analysis of Financial Condition and Results of Operations.” The selected consolidated financial information set forth below as of December 31, 2008 and 2009 and for each of the years ended December 31, 2007, 2008 and 2009 has been derived from our audited consolidated financial statements included in this prospectus. The selected consolidated financial information set forth below as of December 31, 2005 and 2006 and for each of the years ended December 31, 2005 and 2006 has been derived from our unaudited consolidated financial statements not including this prospectus. Historical results are not indicative of future performance.
| Historical | ||||||||||||||||||||||||||||||||||||
| Predecessor Entity | Successor Entity | |||||||||||||||||||||||||||||||||||
| As of and for the Year Ended December 31, | As of and for the Nine Months Ended September 30, 2009 | As of May 7, 2010 and for the period from January 1, 2010 through May 7, 2010 | As of September 30, 2010 and for the period from May 8, 2010 through September 30, 2010 | |||||||||||||||||||||||||||||||||
(in thousands, except ratios | 2005 | 2006 | 2007 | 2008 | 2009 | |||||||||||||||||||||||||||||||
| (unaudited) | (unaudited) | (unaudited) | (unaudited) | (unaudited) | ||||||||||||||||||||||||||||||||
Statement of Income Data: | ||||||||||||||||||||||||||||||||||||
Net patient service revenue | $ | 104,314 | $ | 145,118 | $ | 178,391 | $ | 217,777 | $ | 262,989 | $ | 193,109 | $ | 102,094 | $ | 120,833 | ||||||||||||||||||||
Operating expenses: | ||||||||||||||||||||||||||||||||||||
Patient care costs | 69,989 | 91,859 | 115,058 | 141,810 | 170,826 | 125,098 | 66,042 | 78,335 | ||||||||||||||||||||||||||||
General and administrative expense | 11,966 | 14,576 | 18,595 | 19,944 | 24,819 | 17,423 | 10,016 | 14,991 | ||||||||||||||||||||||||||||
Merger and related expenses | — | — | — | — | — | — | 7,378 | 14,839 | ||||||||||||||||||||||||||||
Depreciation and amortization | 4,598 | 6,682 | 7,919 | 9,777 | 12,127 | 8,816 | 4,429 | 6,434 | ||||||||||||||||||||||||||||
Provision for doubtful accounts | 2,085 | 2,781 | 3,258 | 4,834 | 3,216 | 3,184 | (334 | ) | 1,021 | |||||||||||||||||||||||||||
Total operating expenses | 88,638 | 115,898 | 144,830 | 176,365 | 210,988 | 154,521 | 87,531 | 115,620 | ||||||||||||||||||||||||||||
Operating income | 15,676 | 29,220 | 33,561 | 41,412 | 52,001 | 38,588 | 14,563 | 5,213 | ||||||||||||||||||||||||||||
Interest expense, net | (4,545 | ) | (12,845 | ) | (13,695 | ) | (13,729 | ) | (14,948 | ) | (11,212 | ) | (5,717 | ) | (9,205 | ) | ||||||||||||||||||||
Income (loss) before income taxes | 11,131 | 16,375 | 19,866 | 27,683 | 37,053 | 27,376 | 8,846 | (3,992 | ) | |||||||||||||||||||||||||||
Income tax expense (benefit) | 709 | 309 | 4,409 | 6,860 | 9,524 | 7,036 | 2,264 | (649 | ) | |||||||||||||||||||||||||||
Net income (loss) | 10,422 | 16,066 | 15,457 | 20,823 | 27,529 | 20,340 | 6,582 | (3,343 | ) | |||||||||||||||||||||||||||
Less: Net income attributable to noncontrolling interests | (7,198 | ) | (11,833 | ) | (14,706 | ) | (17,179 | ) | (22,391 | ) | (15,823 | ) | (9,266 | ) | (10,949 | ) | ||||||||||||||||||||
Net income (loss) attributable to ARH | $ | 3,224 | $ | 4,233 | $ | 751 | $ | 3,644 | $ | 5,138 | $ | 4,517 | $ | (2,684 | ) | $ | (14,292 | ) | ||||||||||||||||||
Other Financial Data: | ||||||||||||||||||||||||||||||||||||
Capital expenditures (1) | 6,707 | 9,296 | 15,218 | 21,254 | 15,067 | 9,757 | ||||||||||||||||||||||||||||||
Ratio of earnings to fixed charges (2) | 1.68 | 1.31 | 1.33 | 1.64 | 1.81 | 2.69 | 0.94 | (3) | 0.55 | (4) | ||||||||||||||||||||||||||
Operating Data: | ||||||||||||||||||||||||||||||||||||
Number of clinics (as of end of period) | 43 | 53 | 64 | 75 | 83 | 80 | 88 | |||||||||||||||||||||||||||||
Patients (as of end of period) | 2,548 | 3,041 | 3,740 | 4,545 | 5,405 | 5,235 | 6,119 | |||||||||||||||||||||||||||||
Number of treatments | 322,739 | 418,584 | 503,576 | 604,888 | 745,812 | 546,825 | ||||||||||||||||||||||||||||||
Revenues per treatment (5) | $ | 323 | $ | 347 | $ | 354 | $ | 360 | $ | 353 | $ | 353 | ||||||||||||||||||||||||
Patient care costs per treatment (5) | $ | 217 | $ | 219 | $ | 228 | $ | 234 | $ | 229 | $ | 229 | ||||||||||||||||||||||||
General and administrative expenses per treatment (5) | $ | 37 | $ | 35 | $ | 37 | $ | 33 | $ | 33 | $ | 32 | ||||||||||||||||||||||||
49
Table of Contents
| (1) | Includes the following capital expenditure type: |
| Year Ended December 31, | Nine Months Ended September 30, 2009 | |||||||||||||||||||||||
| 2005 | 2006 | 2007 | 2008 | 2009 | ||||||||||||||||||||
Development capital expenditures | $ | 6,027 | $ | 7,846 | $ | 12,887 | $ | 18,764 | $ | 11,056 | $ | 9,020 | ||||||||||||
Maintenance capital expenditures | 680 | 1,450 | 2,331 | 2,490 | 4,011 | 737 | ||||||||||||||||||
Total capital expenditures | $ | 6,707 | $ | 9,296 | $ | 15,218 | $ | 21,254 | $ | 15,067 | $ | 9,757 | ||||||||||||
| (2) | For the purposes of calculating the ratio of earnings to fixed charges, earnings consists of income from operations before income taxes plus fixed charges less amounts attributable to noncontrolling interests. Fixed charges consist of interest expense and interest portion of rent expense. |
| (3) | Represents a deficiency of approximately $420,000. |
| (4) | Represents a deficiency of approximately $4,736,000. |
| (5) | We calculate revenues per treatment, patient care costs per treatment and general and administrative expenses per treatment by dividing net operating revenues, patient care costs and general and administrative expenses for the applicable period by the number of treatments performed in the applicable period. |
50
Table of Contents
UNAUDITED PRO FORMA CONDENSED CONSOLIDATED FINANCIAL DATA
Set forth below is our summary historical and pro forma consolidated financial data at the dates and for the periods indicated. The statement of operations and other financial data for each of the years in the three-year period ended December 31, 2009 and the balance sheet data as of December 31, 2008 and 2009 have been derived from our historical consolidated financial statements included elsewhere in this prospectus, which have been audited by Grant Thornton LLP. The unaudited interim summary historical and pro forma consolidated financial data for the period from January 1, 2010 through May 7, 2010, and the period from May 8, 2010 through September 30, 2010 were derived from our historical consolidated financial statements included elsewhere in this prospectus.
The unaudited condensed consolidated statements of income give effect to the Pro Forma Adjustments as if they had occurred on January 1, 2009. The Pro Forma Adjustments are described below and in the accompanying notes, which should be read in conjunction with these unaudited condensed consolidated financial statements.
The unaudited pro forma condensed consolidated statements of income give effect to the Transactions and the following transactions (together, the “Pro Forma Adjustments”):
| (i) | the Merger, including the cash equity investment by our Sponsor as well as related transaction expenses, |
| (ii) | the issuance of the notes being exchanged hereby with related interest expense and amortization of deferred financing costs and fees capitalized, |
| (iii) | the results of our four acquisitions in 2009, |
| (iv) | the effect on noncontrolling interests resulting from the purchase and sale of such noncontrolling interests in various clinics, |
| (v) | the annual fee paid to Centerbridge Advisors, L.L.C. in accordance with the new management agreement entered into in connection with the Transactions, and |
| (vi) | the asset, liability and non-controlling interest valuations and the related purchase price allocations. |
The unaudited pro forma adjustments are based upon available information and certain assumptions that we believe are reasonable under the circumstances. The unaudited pro forma condensed consolidated financial information is presented for informational purposes only. The unaudited pro forma condensed consolidated financial information does not purport to represent what our results of operations would have been had the pro forma adjustments actually occurred on the dates indicated, and they do not purport to project our results of operations for any future period or as of any future date. Further, the unaudited pro forma consolidated financial statements do not reflect the impact of restructuring activities, cost savings, employee termination costs and other exit costs that may result from or in connection with the Transactions. The unaudited pro forma condensed consolidated financial information should be read in conjunction with the information contained in other sections of this offering memorandum, particularly the sections entitled “The Transactions,” “Selected Consolidated Financial Data” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and our consolidated financial statements and related notes thereto included elsewhere in this prospectus. All pro forma adjustments and their underlying assumptions are described more fully in the notes to our unaudited pro forma condensed consolidated financial statements.
The Merger was accounted for as a business combination using the acquisition method of accounting. The pro forma information presented includes all adjustments required to reflect the fair value of assets, both tangible and intangible, acquired, liabilities assumed and noncontrolling interests based upon valuations of assets acquired, liabilities assumed and noncontrolling interests.
51
Table of Contents
American Renal Holdings Inc.
Unaudited Pro Forma Consolidated Statement of Income
For the Year Ended December 31, 2009
(in thousands) | Historical (a) | Pro Forma Adjustments | Pro Forma Consolidated | |||||||||||||
Net patient service revenue | $ | 262,989 | $ | 2,217 | (b) | $ | 265,206 | |||||||||
Operating expenses: | ||||||||||||||||
Facility operating expenses | 170,826 | 1,527 | (c) | 172,353 | ||||||||||||
General and administrative expense | 24,819 | 738 | (d) | 25,557 | ||||||||||||
Depreciation and amortization | 12,127 | 3,366 | (e) | 15,493 | ||||||||||||
Provision for doubtful accounts | 3,216 | 64 | (f) | 3,280 | ||||||||||||
Total operating expenses | 210,988 | 5,695 | 216,683 | |||||||||||||
Operating income | 52,001 | (3,478 | ) | 48,523 | ||||||||||||
Interest expense, net | (14,948 | ) | (7,640 | ) | (g) | (22,588 | ) | |||||||||
Income before income taxes | 37,053 | (11,118 | ) | 25,935 | ||||||||||||
Income tax expense | 9,524 | (7,644 | ) | (h) | 1,880 | |||||||||||
Net income | 27,529 | (3,474 | ) | 24,055 | ||||||||||||
Less: Net income attributable to noncontrolling interests | (22,391 | ) | 1,427 | (i | ) | (20,964 | ) | |||||||||
Net income attributable to ARH | $ | 5,138 | $ | (2,046 | ) | $ | 3,092 | |||||||||
See accompanying notes to the unaudited pro forma financial information.
52
Table of Contents
American Renal Holdings Inc.
Unaudited Pro Forma Consolidated Statement of Income
For the Nine Months Ended September 30, 2010
| Historical (a) | Pro Forma Adjustments | Pro Forma Results | ||||||||||||||||||||
| Successor | Predecessor | |||||||||||||||||||||
(in thousands) | Period from May 8 through September 30, 2010 | Period from January 1 through May 7, 2010 | ||||||||||||||||||||
Net patient service revenue | $ | 120,833 | $ | 102,094 | $ | — | $ | 222,927 | ||||||||||||||
Operating expenses: | ||||||||||||||||||||||
Patient care costs | 78,335 | 66,042 | (1,640 | ) | (c) | 142,737 | ||||||||||||||||
General and administrative expense | 14,991 | 10,016 | (2,257 | ) | (d) | 22,750 | ||||||||||||||||
Merger and related expenses | 14,839 | 7,378 | (22,217 | ) | (j) | — | ||||||||||||||||
Depreciation and amortization | 6,434 | 4,429 | 1,459 | (e) | 12,322 | |||||||||||||||||
Provision for (recoveries of) doubtful accounts | 1,021 | (334 | ) | — | 687 | |||||||||||||||||
Total operating expenses | 115,620 | 87,531 | (24,655 | ) | 178,496 | |||||||||||||||||
Operating income (loss) | 5,213 | 14,563 | 24,655 | 44,431 | ||||||||||||||||||
Interest expense, net | (9,205 | ) | (5,717 | ) | (2,013 | ) | (g) | (16,935 | ) | |||||||||||||
Income (loss) before income taxes | (3,992 | ) | 8,846 | 22,642 | 27,496 | |||||||||||||||||
Income tax expense (benefit) | (649 | ) | 2,264 | 1,377 | (h) | 2,992 | ||||||||||||||||
Net income (loss) | (3,343 | ) | 6,582 | 21,265 | 24,504 | |||||||||||||||||
Less: Net income attributable to noncontrolling interests | (10,949 | ) | (9,266 | ) | 632 | (i) | (19,583 | ) | ||||||||||||||
Net income (loss) attributable to ARH | $ | (14,292 | ) | $ | (2,684 | ) | $ | 21,897 | $ | 4,921 | ||||||||||||
See accompanying notes to the unaudited pro forma financial information.
53
Table of Contents
American Renal Holdings Inc.
Notes to Unaudited Pro Forma Financial Information
(dollars in thousands)
| (a) | The amounts in these columns represent our historical balances and results for the periods reflected. |
| (b) | Reflects the effect of our 2009 acquisitions as if they had occurred on January 1, 2009. |
| (c) | Reflects the following adjustments to facility operating expenses: |
| Year Ended December 31, 2009 | Nine Months Ended September 30, 2010 | |||||||
Acquisitions if occurred on January 1, 2009 | $ | 1,697 | $ | — | ||||
Decreased rent expense from the valuation of leases | (170 | ) | (54 | ) | ||||
Exclude costs associated with the accelerated vesting of equity awards | — | (1,586 | ) | |||||
Total Facility operating expense adjustments | $ | 1,527 | $ | (1,640 | ) | |||
| (d) | Reflects the following adjustments to general and administrative expenses: |
| Year Ended December 31, 2009 | Nine Months Ended September 30, 2010 | |||||||
Acquisitions if occurred on January 1, 2009 | $ | 188 | $ | — | ||||
Sponsor management fee | 550 | 193 | ||||||
Exclude costs associated with the accelerated vesting of equity awards | — | (2,450 | ) | |||||
Total General and administrative expense adjustments | $ | 738 | $ | (2,257 | ) | |||
| (e) | Reflects the following adjustments to depreciation and amortization: |
| Year Ended December 31, 2009 | Nine Months Ended September 30, 2010 | |||||||
Acquisitions if occurred on January 1, 2009 | $ | 51 | $ | — | ||||
Allocation of purchase price to tangible and intangible assets | 3,315 | 1,459 | ||||||
Total Depreciation and amortization expense adjustments | $ | 3,366 | $ | 1,459 | ||||
| (f) | Reflects the effect of our acquisitions as if they occurred on January 1, 2009. |
54
Table of Contents
American Renal Holdings Inc.
Notes to Unaudited Pro Forma Financial Information—(Continued)
(dollars in thousands)
| (g) | Reflects the following adjustments to interest expense, net: |
| Year Ended December 31, 2009 | Nine Months Ended September 30, 2010 | |||||||
Senior Secured Notes (1) | $ | 20,938 | $ | 15,703 | ||||
New Revolving Credit Facility (2) | 188 | 141 | ||||||
Rollover clinic debt (3) | 826 | 614 | ||||||
Amortization of capitalized debt issuance costs and discount (4) | 644 | 477 | ||||||
Total pro forma interest expense | 22,596 | 16,935 | ||||||
Less: actual interest expense for the period | 14,948 | 14,922 | ||||||
Net adjustment to interest expense | $ | 7,648 | $ | 2,013 | ||||
| (1) | Reflects pro forma interest expense on the senior secured notes. |
| (2) | Reflects no outstanding balance on the $25,000 New Revolving Credit Facility and includes annual commitment fee of 75 basis points on undrawn commitments under the New Revolving Credit Facility. |
| (3) | Reflects actual interest expense on third-party clinic debt that was not refinanced as part of the Transactions, including the amortization of debt issuance costs. |
| (4) | Represents amortization of debt issuance costs of $5,085 and discount of $1,800 associated with the senior secured notes amortized over eight years. |
| h) | Reflects the effects on our income tax provision of the pro forma adjustments to result in an effective tax rate of 10.9%, which approximates our actual effective tax rate in the successor period excluding non-deductible Merger-related expenses. Our income tax provision principally relates to our share of pre-tax income from our majority owned subsidiaries as these subsidiaries are all pass-through entities for tax purposes. Therefore, we do not incur tax on the share of pre-tax income attributable to noncontrolling interests. This results in a substantially lower effective rate when our income tax provision is calculated as a percentage of total pre-tax income. When our income tax provision is calculated as a percentage of our share of pre-tax income, the pro forma effective tax rate estimated was 37.8% which was based on our statutory federal rate of 35% and an estimated state effective rate, net of federal benefit, of approximately 2.8%. |
| (i) | Reflects the following adjustments to net income attributable to noncontrolling interests: |
| Year Ended December 31, 2009 | Nine Months Ended September 30, 2010 | |||||||
Acquisitions if occurred on January 1, 2009 | $ | (102 | ) | $ | — | |||
Changes in ownership since January 1, 2009 | 114 | — | ||||||
Allocation of purchase price to tangible and intangible assets | 1,415 | 632 | ||||||
Total Net income attributable to noncontrolling interest adjustments | $ | 1,427 | $ | 632 | ||||
| (j) | Reflects a pro forma adjustment to exclude Transaction costs incurred and expensed by us resulting from the Transactions. |
55
Table of Contents
MANAGEMENT’S DISCUSSION AND ANALYSIS OF
FINANCIAL CONDITION AND RESULTS OF OPERATIONS
You should read the following discussion and analysis of our financial condition and results of operations together with our financial statements and related notes appearing at the beginning of this document. Some of the information contained in this discussion and analysis or set forth elsewhere in this document, including information with respect to our plans and strategy for our business and related financing, includes forward-looking statements that involve risks and uncertainties.
Merger and Presentation
On March 22, 2010, ARH entered into the Merger Agreement with C.P. Atlas Holdings, Inc (the “Parent”), C.P. Atlas Intermediate Holdings, LLC, C.P. Atlas Acquisition Corp., certain of ARH’s stockholders party thereto and the Sellers’ Representative pursuant to which ARH agreed that C.P. Atlas Acquisition Corp. merged with and into ARH (the “Merger”) and, after which, ARH became the surviving entity and a wholly owned subsidiary of C.P. Atlas Intermediate Holdings, LLC, which is in turn a wholly owned subsidiary of the Parent. The parties agreed to consummate the Merger, subject to the terms and conditions set forth in the Merger Agreement, for an aggregate purchase price of $415 million, subject to adjustments, including, without limitation, for working capital, indebtedness, certain specified liabilities and certain tax savings. ARH agreed that $2.5 million of the purchase price was to be placed into a third-party escrow account as security for working capital adjustments and that $27.5 million of the purchase price was to be placed into a third-party escrow account as security for indemnification claims, as described below under “—Merger Agreement.”
The aggregate purchase price of approximately $415 million for the Merger, plus related fees and expenses, was funded by the equity investment by Centerbridge as well as from certain members of management and the net proceeds from the offering of $250.0 million of Senior Secured Notes (the “Senior Secured Notes”) due 2018 which bear interest at 8.375%. The Merger and the financing transaction described above are collectively referred to herein as the “Transactions.”
The Transactions were consummated on May 7, 2010. Although ARH continued as the surviving corporation and same legal entity after the merger, the accompanying consolidated balance sheet, statements of operations and cash flows are presented for two periods: predecessor and successor, which relate to the periods preceding the Merger and the period succeeding the Merger, respectively. The Merger resulted in a new basis of accounting beginning on May 8, 2010. The Merger was accounted for under the acquisition method of accounting in accordance with ASC Topic 805,Business Combinations. Accordingly, the assets acquired, liabilities assumed and noncontrolling interests in the Merger were recorded at fair value. As a result of the Merger and the associated acquisition accounting, the Consolidated Financial Statements of the Successor are not comparable to periods preceding the Merger.
Executive Overview
We are a national provider of kidney dialysis services for patients suffering from chronic kidney failure, also known as end stage renal disease, or ESRD. As of September 30, 2010 we owned and operated 88 dialysis clinics treating more than 6,100 patients in 17 states and the District of Columbia. Our operating model is based on shared ownership of our facilities with physicians, known as nephrologists, who specialize in kidney-related diseases in the local market served by the clinic. Each clinic is maintained as a separate joint venture in which we own a controlling interest and our local nephrologist partners own noncontrolling interests, although during the development stages or under certain circumstances we may temporarily own up to 100% of the interests of a clinic. The results of operations for all of clinics are currently included in our consolidated financial statements.
We endeavor to provide our nephrologist partners with comprehensive management and operational tools in order to enable them (and other referring physicians) to focus on providing quality care to patients.
56
Table of Contents
We derive our revenues from providing both outpatient and inpatient dialysis treatments as well as from ancillary services such as administering dialysis-related pharmaceuticals, but not including services that we outsource to third-party vendors such as lab services. The sources of these revenues are principally government-based programs, including Medicare, Medicaid and Medicare-certified HMO plans as well as commercial insurance plans.
The Competitive Strength of Our JV Model
We operate our clinics exclusively through our JV model, in which we share the ownership and operational responsibility of our dialysis clinics with physicians known as nephrologists who specialize in kidney-related diseases. In each of our JVs, we own a controlling interest in the clinic and our nephrologist partners own noncontrolling interests. We believe that our exclusive focus on a JV model makes us well-positioned to increase our market share by attracting nephrologists who are not only interested in our service platform but also want greater clinical autonomy and a potential return on capital investment associated with ownership of a noncontrolling interest in a dialysis clinic. We believe our JV model best aligns our interests with those of our nephrologist partners and their patients. By owning a portion of the clinics where their patients are treated, our nephrologist partners have a vested stake in the quality, reputation and performance of the clinics. We believe that this enhances patient and staff satisfaction and retention, clinical outcomes, patient growth, and operational and financial performance.
Clinic Development
We have experienced significant growth since opening our first clinic in December 2000. Since that date, we have developed 64 new clinics, commonly referred to as de novo clinics. The following chart shows the number of de novo and acquired clinics over the three years ended December 31, 2009 and nine months ended September 30, 2010:
| Nine Months Ended September 30, | Years Ended December 31, | |||||||||||||||
| 2010 | 2009 | 2008 | 2007 | |||||||||||||
De novo clinics (1) | 5 | 7 | 12 | 12 | ||||||||||||
Acquired clinics (2) | 1 | 3 | — | 2 | ||||||||||||
Total new clinics | 6 | 10 | 12 | 14 | ||||||||||||
| (1) | Clinics formed by us which began to operate and dialyze patients in the applicable period. |
| (2) | Clinics acquired by us which began to operate and dialyze patients in the applicable period. |
Selective recruitment of new nephrologist partners who have an established practice is critical in achieving our growth objectives. We also believe we enjoy a strong and growing reputation among the nephrologist community, ensuring that nephrologists who contemplate a migration to the shared-ownership model strongly consider us.
We also believe that the return earned by our nephrologist partners on the clinic investment can drive organic growth by enabling our nephrologist partners to reinvest in their practices, including by adding new nephrologists, which provides us with the opportunity to expand existing clinics, develop new clinics or acquire competing clinics in the same market.
We will continue to incur start-up losses associated with the opening of de novo clinics. It has been our experience that newly established dialysis centers, although contributing to increased revenues, have adversely affected our results of operations in the short term due to start-up fixed operating expenses and a smaller patient base. We consider new dialysis centers to be “start-up centers” through their initial 12 months of operations, or when they achieve consistent profitability, whichever is sooner.
57
Table of Contents
Effect of the Transactions
In connection with the Transactions we incurred significant additional indebtedness, including $250.0 million aggregate principal amount of the Senior Secured Notes being exchanged hereby and $25.0 million of borrowing availability under our Credit Facility (the “New Revolving Credit Facility”). As of September 30, 2010, we had $258.2 million aggregate principal amount of indebtedness outstanding including approximately $8.2 million of third-party debt owed by our clinic subsidiaries. We estimate this additional indebtedness will result in annualized interest expense, including amortization of deferred financing costs and discounts, of approximately $23.0 million which is significantly higher than our historical annual interest expense and which may result in lower net income in future periods.
The following discussion and analysis of our historical financial condition and results of operations covers periods prior to and after the consummation of the Transactions. After the Transactions, we are highly leveraged. Significant additional liquidity requirements, resulting primarily from increased interest expense, and other factors related to the Transactions, such as increased amortization of identified intangible assets as a result of the application of acquisition accounting will continue to significantly affect our financial condition, results of operations and liquidity going forward.
Components of Revenues and Expenses
Net Operating Revenues
The major components of our net operating revenues include both base charges for dialysis services and the administration of separately-billable pharmaceuticals as part of the dialysis treatment, such as erythropoietin (EPO). As a result, changes in physician practice patterns, including the prescription of pharmaceuticals, and changes in private and governmental payment rates for EPO and other separately-billable pharmaceuticals significantly influence our level of revenues. During the nine months ended September 30, 2010, we derived approximately 88% of our net operating revenues from outpatient hemodialysis treatments, approximately 10% from home-based hemodialysis and peritoneal dialysis and approximately 2% from hospital inpatient hemodialysis. The administration of EPO and other pharmaceuticals as part of the dialysis treatment represented approximately 33% of net operating revenues during the nine months ended September 30, 2010.
Operating Expenses
Patient Care Costs. Patient care costs are those costs directly associated with operating and supporting our dialysis clinics and ancillary operations. Patient care costs consist principally of labor, pharmaceuticals, medical supplies and facility costs. The principal drivers of patient care costs are clinical staff hours per treatment, labor rates, vendor pricing of pharmaceuticals and business infrastructure and compliance costs. However, other cost categories, such as employee benefit costs and insurance costs, can result in significant cost changes from period to period. Our average clinical staff hours per treatment have remained stable over the past several years primarily because of limited staff turnover. For the past several years we have been able to negotiate relatively stable pharmaceutical pricing with our vendors. In addition, our agreements with our group purchasing organization and Amgen for the purchase of EPO provide for specific rebates off list price and discounted pricing based data submission, which could negatively impact our earnings if we are unable to qualify for these rebates and discounts. Our ability to influence the pricing of our services is limited. Profitability depends on our ability to grow but also on our ability to manage labor, drug and supply costs. For mature clinics, we believe there is limited opportunity for productivity improvements beyond the levels currently achieved.
General and Administrative Costs. General and administrative costs consist of expenses for clinic and corporate administration, including accounting, billing and cash collection functions, and regulatory compliance oversight. Our general and administrative expenses, both on an absolute dollar basis and as a percentage of our net operating revenues, have increased following the issuance of the notes as a result of our agreement to register the notes with the Securities and Exchange Commission.
58
Table of Contents
Operating Environment
Current regulation and proposed changes
Proposed and pending regulatory changes could significantly change the reimbursement rates we receive for our dialysis services from government-based programs and, indirectly, from commercial payors. In July 2008, the Medicare Improvements for Patients and Providers Act of 2008, or MIPPA, was passed by Congress. This legislation provides for an increase in the composite rate of 1% which went into effect on January 1, 2009 and an additional 1% increase that went into effect on January 1, 2010. In addition, MIPPA introduced a new payment system for dialysis services beginning in January 2011 whereby ESRD payments will be made under a bundled payment rate which will provide for a fixed rate for all goods and services provided during the dialysis treatment, including laboratory services, the administration of pharmaceuticals and payments for pharmaceuticals such as EPO that are separately billed under the current methodology. The initial 2011 bundled rate will be set based on a 2% reduction in the payment rate that providers would have received under the historical fee for service payment methodology and based on the lowest average industry pharmaceutical utilization from 2007 to 2009. The combined effect of these adjustments could result in a bundled rate that represents a greater than 2% reduction in the payment rate that we would have received for our services prior to bundling. Beginning in 2012, a new single bundled payment base rate will be adjusted annually for inflation based upon a market basket index, less a productivity adjustment based on a 10-year moving average of productivity as projected by the Secretary of Health and Human Services. The bundled payment rate will be determined by the Secretary of Health and Human Services, who will have discretion to determine the base payment rate based on the goods and services included in the bundled rate. Dialysis providers will have the option to move fully to the bundled payment system in 2011 or to phase in the payment system over four years.
Results of Operations
The following table presents our operating results for the periods indicated, expressed both in dollars and as a percentage of net operating revenues. Historical results for the successor period during the three months ended September 30, 2010 are compared and discussed in relation to the historical predecessor results during the three months ended September 30, 2009. Historical results for the period from January 1, 2010 to May 7, 2010 (Predecessor period) and from May 8, 2010 to September 30, 2010 (Successor period) are compared and discussed in relation to the historical predecessor results during the nine month period ended September 30, 2009. Supplementally, pro forma results for the nine months ended September 30, 2010 are compared and discussed in relation to the historical predecessor results during the nine month period ended September 30, 2009.
59
Table of Contents
Successor Three Months Ended September 30, 2010 as compared with the Predecessor Three Months Ended September 30, 2009
| Successor | Predecessor | |||||||||||||||||||
| Three Months Ended September 30, 2010 | Three Months Ended September 30, 2009 | |||||||||||||||||||
(in thousands) | Dollar Change | Percent Change | ||||||||||||||||||
Statement of Operations Data: | ||||||||||||||||||||
Net patient service revenue | $ | 77,231 | $ | 68,084 | $ | 9,147 | 13.4 | % | ||||||||||||
Operating expenses: | ||||||||||||||||||||
Patient care costs | 49,321 | 44,061 | 5,260 | 11.9 | ||||||||||||||||
General and administrative expense | 8,001 | 6,393 | 1,608 | 25.2 | ||||||||||||||||
Merger and related expenses | 152 | — | 152 | 100.0 | ||||||||||||||||
Depreciation and amortization | 4,085 | 2,814 | 1,271 | 45.2 | ||||||||||||||||
Provision for uncollectible accounts | 371 | 1,016 | (645 | ) | -63.5 | |||||||||||||||
Total operating expenses | 61,930 | 54,284 | 7,646 | 14.1 | ||||||||||||||||
Operating income | 15,301 | 13,800 | 1,501 | 10.9 | ||||||||||||||||
Interest expense, net | (5,885 | ) | (3,755 | ) | (2,130 | ) | 56.7 | |||||||||||||
Income before income taxes | 9,416 | 10,045 | (629 | ) | -6.3 | |||||||||||||||
Income tax expense | 977 | 2,582 | (1,605 | ) | -62.2 | |||||||||||||||
Net income | 8,439 | 7,463 | 976 | 13.1 | ||||||||||||||||
Less: Net income attributable to noncontrolling interests | (6,907 | ) | (5,756 | ) | (1,151 | ) | 20.0 | |||||||||||||
Net income attributable to ARH | $ | 1,532 | $ | 1,707 | $ | (175 | ) | -10.3 | % | |||||||||||
Net Operating Revenues
Net operating revenues for the three months ended September 30, 2010 were $77.2 million, an increase of 13.4% from $68.1 million for the three months ended September 30, 2009. The increase in net operating revenues was primarily due to an increase of approximately 12.8% in the number of dialysis treatments for the three months ended September 30, 2010. The increase in treatments resulted principally from non-acquired treatment growth from existing clinics and de novo clinics. Net operating revenue per treatment for the three months ended September 30, 2010 was $352, compared with $350 for the three months ended September 30, 2009. The increase in revenue per treatment was primarily due to a slight improvement in commercial payor reimbursement rates. The administration of EPO represented approximately 22% of net operating revenues for both the three months ended September 30, 2010 and 2009.
Our net operating revenues are driven by the number of treatments performed and our average revenue per treatment. The following table summarizes our net operating revenues and net operating revenue per treatment for each of the periods indicated:
| Three Months Ended September 30, | ||||||||
| 2010 | 2009 | |||||||
Net operating revenues (in thousands) | $ | 77,231 | $ | 68,084 | ||||
Net operating revenue per treatment | $ | 352 | $ | 350 | ||||
The number of treatments that we performed during the three months ended September 30, 2010 increased by approximately 12.8% over the number of treatments that we performed during the three months ended September 30, 2009. This treatment growth has been driven by increasing the patient base at our existing clinics,
60
Table of Contents
adding new patients through the opening of de novo clinics and the acquisition of existing dialysis clinics in which we acquired a controlling interest. The following table summarizes the sources of our treatment growth for the periods indicated.
| Three Months Ended September 30, | ||||||||
| 2010 | 2009 | |||||||
Source of Treatment Growth: | ||||||||
Existing clinics (1) | 8.9 | % | 17.1 | % | ||||
De novo clinics opened (2) | 3.2 | % | 5.7 | % | ||||
Non-acquired treatment growth | 12.1 | % | 22.8 | % | ||||
Clinics acquired (3) | 0.7 | % | 4.0 | % | ||||
Total treatment growth | 12.8 | % | 26.8 | % | ||||
| (1) | Represents net growth in treatments at clinics operating at end of the period that were also opened at the end of the prior period. |
| (2) | Represents additional treatments provided at clinics opened since the end of the prior period, compared to the total number. |
| (3) | Represents treatments performed at clinics acquired since the end of the prior period. |
Our net operating revenue per treatment is principally driven by our mix of commercial and government (principally Medicare and Medicaid) patients, the mix and amount of physician-prescribed pharmaceuticals, and commercial and government payment rates. We are generally paid more for services provided to patients covered by commercial healthcare plans than we are for patients covered by Medicare or Medicaid. Patients covered by employer group health plans transition to Medicare coverage after a maximum of 33 months. As a result, our ability to generate operating earnings is substantially dependent on revenues derived from commercial payors, some of which pay negotiated payment rates and others of which pay based on our usual and customary fee schedule. Additionally, as a patient transitions from commercial coverage to Medicare coverage, the payment rates typically decline substantially.
The following table summarizes our net operating revenues and treatments by source for each of the periods indicated.
| Three Months Ended September��30, | ||||||||
| 2010 | 2009 | |||||||
Source of revenues: | ||||||||
Government-based programs and other | 57.5 | % | 57.5 | % | ||||
Commercial payors | 42.5 | % | 42.5 | % | ||||
| 100.0 | % | 100.0 | % | |||||
Source of treatments: | ||||||||
Government-based programs and other | 86.3 | % | 86.1 | % | ||||
Commercial payors | 13.7 | % | 13.9 | % | ||||
| 100.0 | % | 100.0 | % | |||||
Operating Expenses
Patient care costs. Patient care costs for the three months ended September 30, 2010 were $49.3 million, an increase of 11.9% from $44.1 million in the three months ended September 30, 2009. This increase was primarily due to an increase in the number of treatments. As a percentage of net operating revenues, patient care costs were approximately 63.8% for the three months ended September 30, 2010 compared to 64.7% for the three months ended September 30, 2009. Patient care costs per treatment were $225 for the three months ended September 30, 2010 and $227 for the three months ended September 30, 2009.
61
Table of Contents
General and administrative expenses. General and administrative expenses for the three months ended September 30, 2010 were $8.0 million, an increase of 25.2% from $6.4 million in the three months ended September 30, 2009. The increase is primarily attributable to approximately $0.8 million of increased charitable contributions and approximately $0.2 million of increased stock-based compensation. As a percentage of net operating revenues, general and administrative costs were approximately 10.3% for the three months ended September 30, 2010 compared to 9.4% for the three months ended September 30, 2009. General and administrative costs per treatment for the three months ended September 30, 2010 were $36, compared to $33 for the three months ended September 30, 2009, reflecting an increase of $3 per treatment.
Depreciation and amortization. Depreciation and amortization expense represents expense attributable to our clinics’ equipment and leasehold improvements and amortization of intangible assets. We calculate depreciation and amortization expense using a straight-line method over the assets’ useful lives. Depreciation and amortization expense for the three months ended September 30, 2010 was $4.1 million, an increase of 45.2% from $2.8 million in the three months ended September 30, 2009 due to the amortization in intangible assets acquired as part of the Transactions. As a percentage of operating revenues, depreciation and amortization expense was approximately 5.3% during the three months ended September 30, 2010 and 4.1% during the three months ended September 30, 2009.
Provision for uncollectible accounts. Provision for uncollectible accounts represents reserves established for amounts for which patients are primarily responsible which we believe will not be collectible. Provision for uncollectible accounts for the three months ended September 30, 2010 was $0.4 million as compared to $1.0 million in the three months ended September 30, 2009. The decrease in our provision is primarily due determining in 2010 that we have developed sufficient and relevant historical experience with Medicare bad debt cost reporting to use as a basis for reliably estimating Medicare bad debt cost recoveries. Our accounts receivable, net of the bad debt allowance, represented approximately 54 days of revenues as of September 30, 2010 and approximately 60 days of revenues as of September 30, 2010.
Interest Expense, net. Interest expense represents charges for interest associated with our corporate level debt and credit facilities entered into by our dialysis clinics, as well as accumulated dividends attributable during the predecessor period to our Series X preferred stock. Interest expense, net for the three months ended September 30, 2010 was $5.9 million, an increase of 56.7% from $3.8 million in the three months ended September 30, 2009 primarily related to interest on the Senior Secured Notes issued as part of the transactions.
Income Tax Expense.The income tax expense included in the accompanying consolidated statements of operations principally relates to our proportionate share of the pre-tax income of our majority-owned subsidiaries. The provision for income taxes for the three months ended September 30, 2010 represented an effective annualized tax rate of 10.4% compared with 25.7% for the three months ended September 30, 2009. The decrease in our effective income tax rate is primarily due to nondeductible Series X dividends and discount amortization accumulated during the predecessor period. The Series X redeemable preferred stock was settled in the Transactions.
Net income attributable to noncontrolling interests. Noncontrolling interests represent the equity interests in our consolidated entities that we do not own, which is primarily the equity interests of our nephrologist partners in our JV clinics. Net income attributable to noncontrolling interests for the three months ended September 30, 2010 was $6.9 million, an increase of 20.0% from $5.8 million in the three months ended September 30, 2009. The increase was primarily due to the addition of de novo clinics and growth in the earnings of our existing JVs.
62
Table of Contents
Nine Months Ended September 30, 2010 as compared with the Nine Months Ended September 30, 2009
(in thousands) | Pro Forma | Historical | Pro Forma Nine Months Ended September 30, 2010 vs. Historical Nine Months Ended September 30, 2009 | |||||||||||||||||||||||||
| Successor | Predecessor | |||||||||||||||||||||||||||
| Nine Months Ended September 30, 2010 | Period from May 8 through September 30, 2010 | Period from January 1 through May 7, 2010 | Nine Months Ended September 30, 2009 | |||||||||||||||||||||||||
| Dollar Change | Percent Change | |||||||||||||||||||||||||||
Statement of Operations Data: | ||||||||||||||||||||||||||||
Net patient service revenue | $ | 222,927 | $ | 120,833 | $ | 102,094 | $ | 193,109 | $ | 29,818 | 15.4 | % | ||||||||||||||||
Operating expenses: | ||||||||||||||||||||||||||||
Patient care costs | 142,737 | 78,335 | 66,042 | 125,098 | 17,639 | 14.1 | ||||||||||||||||||||||
General and administrative expense | 22,750 | 14,991 | 10,016 | 17,423 | 5,327 | 30.6 | ||||||||||||||||||||||
Merger and related expenses | — | 14,839 | 7,378 | — | — | N/A | ||||||||||||||||||||||
Depreciation and amortization | 12,322 | 6,434 | 4,429 | 8,816 | 3,506 | 39.8 | ||||||||||||||||||||||
Provision for (recoveries of) uncollectible accounts | 687 | 1,021 | (334 | ) | 3,184 | (2,497 | ) | -78.4 | ||||||||||||||||||||
Total operating expenses | 178,496 | 115,620 | 87,531 | 154,521 | 23,975 | 15.5 | ||||||||||||||||||||||
Operating income | 44,431 | 5,213 | 14,563 | 38,588 | 5,843 | 15.1 | ||||||||||||||||||||||
Interest expense, net | (16,935 | ) | (9,205 | ) | (5,717 | ) | (11,212 | ) | (5,723 | ) | 51.0 | |||||||||||||||||
Income (loss) before income taxes | 27,496 | (3,992 | ) | 8,846 | 27,376 | 120 | 0.4 | |||||||||||||||||||||
Income tax expense (benefit) | 2,992 | (649 | ) | 2,264 | 7,036 | (4,044 | ) | -57.4 | ||||||||||||||||||||
Net income (loss) | 24,504 | (3,343 | ) | 6,582 | 20,340 | 4,164 | 20.5 | |||||||||||||||||||||
Less: Net income attributable to noncontrolling interests | (19,583 | ) | (10,949 | ) | (9,266 | ) | (15,823 | ) | (3,760 | ) | 23.8 | |||||||||||||||||
Net income (loss) attributable to ARH | $ | 4,921 | $ | (14,292 | ) | $ | (2,684 | ) | $ | 4,517 | $ | 404 | 8.9 | % | ||||||||||||||
Net Operating Revenues
Net operating revenues for the nine months ended September 30, 2010 increased $29.8 million, an increase of 15.4%, including $102.1 million in the Predecessor period and $20.8 million in the Successor period, from $193.1 million for the nine months ended September 30, 2009. The increase in net operating revenues was primarily due to an increase of approximately 15.7% in the number of dialysis treatments for the nine months ended September 30, 2010. The increase in treatments resulted principally from non-acquired treatment growth from existing clinics and de novo clinics. Net operating revenue per treatment for the nine months ended September 30, 2010 was $352, compared with $353 for the nine months ended September 30, 2009. The decrease in revenue per treatment was primarily due to a change in our commercial payor mix resulting from the relatively high rates of unemployment in the current economic climate. The administration of EPO represented approximately 22% of net operating revenues for both the nine months ended September 30, 2010 and September 30, 2009.
Our net operating revenues are driven by the number treatments performed and our average revenue per treatment. The following table summarizes our net operating revenues and net operating revenue per treatment for each of the periods indicated.
| Nine Months Ended September 30, | ||||||||
| 2010 | 2009 | |||||||
Net operating revenues (in thousands) | $ | 222,927 | $ | 193,109 | ||||
Net operating revenue per treatment | $ | 352 | $ | 353 | ||||
63
Table of Contents
The number of treatments that we performed during the nine months ended September 30, 2010 increased by approximately 15.7% over the number of treatments that we performed during the nine months ended September 30, 2009. This treatment growth has been driven by increasing the patient base at our existing clinics, adding new patients through the opening of de novo clinics and the acquisition of existing dialysis clinics in which we acquired a controlling interest. The following table summarizes the sources of our treatment growth for the periods indicated.
| Nine Months Ended September 30, | ||||||||
| 2010 | 2009 | |||||||
Source of Treatment Growth: | ||||||||
Existing clinics (1) | 13.0 | % | 18.4 | % | ||||
De novo clinics opened (2) | 2.5 | % | 4.1 | % | ||||
Non-acquired treatment growth | 15.5 | % | 22.5 | % | ||||
Clinics acquired (3) | 0.2 | % | 2.3 | % | ||||
Total treatment growth | 15.7 | % | 24.8 | % | ||||
| (1) | Represents net growth in treatments at clinics operating at end of the period that were also opened at the end of the prior period. |
| (2) | Represents additional treatments provided at clinics opened since the end of the prior period, compared to the total number. |
| (3) | Represents treatments performed at clinics acquired since the end of the prior period. |
Our net operating revenue per treatment is principally driven by our mix of commercial and government (principally Medicare and Medicaid) patients, the mix and amount of physician-prescribed pharmaceuticals, and commercial and government payment rates. We are generally paid more for services provided to patients covered by commercial healthcare plans than we are for patients covered by Medicare or Medicaid. Patients covered by employer group health plans transition to Medicare coverage after a maximum of 33 months. As a result, our ability to generate operating earnings is substantially dependent on revenues derived from commercial payors, some of which pay negotiated payment rates and others of which pay based on our usual and customary fee schedule. Additionally, as a patient transitions from commercial coverage to Medicare coverage, the payment rates typically decline substantially.
The following table summarizes our net operating revenues and treatments by source for each of the periods indicated.
| Nine Months Ended September 30, | ||||||||
| 2010 | 2009 | |||||||
Source of revenues: | ||||||||
Government-based programs and other | 57.6 | % | 56.6 | % | ||||
Commercial payors | 42.4 | % | 43.4 | % | ||||
| 100.0 | % | 100.0 | % | |||||
Source of treatments: | ||||||||
Government-based programs and other | 86.6 | % | 85.6 | % | ||||
Commercial payors | 13.4 | % | 14.4 | % | ||||
| 100.0 | % | 100.0 | % | |||||
Operating Expenses
Patient care costs. Pro forma patient care costs for the nine months ended September 30, 2010 were $142.7 million, an increase of 14.1% from $125.1 million in the nine months ended September 30, 2009. This increase was primarily due to an increase in the number of treatments. As a percentage of net operating revenues, pro forma
64
Table of Contents
patient care costs were approximately 64.0% for the nine months ended September 30, 2010 compared to 64.8% for the nine months ended September 30, 2009. Pro forma patient care costs per treatment for the nine months ended September 30, 2010 were $226, compared to $229 for the nine months ended September 30, 2009.
Patient care costs were $66.0 million in the Predecessor period and $78.3 million in the Successor period or $144.4 for the nine months ended September 30, 2010, an increase of $19.3 million or 15.4% as compared to $125.1 million during the nine month period ended September 30, 2009. This increase was primarily due to the number of treatments as well as $1.6 million of stock-based compensation related to the Merger.
General and administrative expenses. Pro forma general and administrative expenses for the nine months ended September 30, 2010 were $22.8 million, an increase of 30.6% from $17.4 million in the nine months ended September 30, 2009. The increase is primarily attributable to approximately $2.8 million of increased charitable contributions and approximately $1.2 million of increased stock compensation. As a percentage of net operating revenues, pro forma general and administrative costs were approximately 10.2% for the nine months ended September 30, 2010 compared to 9.0% for the nine months ended September 30, 2009. Pro forma general and administrative costs per treatment for the nine months ended September 30, 2010 were $36, compared to $32 for the nine months ended September 30, 2009, reflecting an increase of $4 per treatment.
General and administrative expenses were $10.0 million in the Predecessor period and $15.0 million in the Successor period or $25.0 for the nine months ended September 30, 2010, an increase of $7.6 million or 43.7% as compared to $17.4 million during the nine month period ended September 30, 2009. This increase was primarily due the increases discussed previously as well as $2.5 million of stock-based compensation related to the Merger.
Depreciation and amortization.Depreciation and amortization expense represents expense attributable to our clinics’ equipment and leasehold improvements and amortization of intangible assets. We calculate depreciation and amortization expense using a straight-line method over the assets’ useful lives. Pro forma depreciation and amortization expense for the nine months ended September 30, 2010 was $12.3 million, an increase of 39.8% from $8.8 million in the nine months ended September 30, 2009 primarily related to intangible assets acquired as part of the Merger. As a percentage of operating revenues, pro forma depreciation and amortization expense was approximately 5.5% for the nine months ended September 30, 2010 compared to 4.6% for the nine months ended September 30, 2009.
Depreciation and amortization was $4.4 million in the Predecessor period and $6.4 million in the Successor period or $10.8 million for the nine months ended September 30, 2010, an increase of $2.0 million or 22.7% as compared to $8.8 million during the nine month period ended September 30, 2009. This increase is primarily related to intangible assets acquired as part of the Merger.
Provision for uncollectible accounts.Provision for uncollectible accounts represents reserves established for amounts for which patients are primarily responsible which we believe will not be collectible. Provision for uncollectible accounts for the nine months ended September 30, 2010 was $0.7 million, a decrease of 78.4% from $3.2 million in the nine months ended September 30, 2009. In the period from April 1, 2010 to May 7, 2010, we determined that we have developed sufficient and relevant historical experience with Medicare bad debt cost reporting to use as a basis for reliably estimating successful Medicare bad debt cost recoveries. As such, we revised our estimate on the collectability of Medicare bad debt claims related to 2008 and 2009 which resulted in a favorable adjustment of $1.8 million to our provision for doubtful accounts. Our accounts receivable, net of the bad debt allowance, represented approximately 54 days of revenues as of September 30, 2010 and approximately 60 days of revenues as of September 30, 2009.
Interest Expense, net.Interest expense represents charges for interest associated with our corporate level debt and credit facilities entered into by our dialysis clinics, as well as accumulated dividends and amortization of discount attributable to our Series X preferred stock during the predecessor period. Pro forma interest expense, net for the nine months ended September 30, 2010 was $16.9 million, an increase of 51.0% from $11.2 million in the nine months ended September 30, 2009 primarily related to interest related to the Senior Secured Notes.
65
Table of Contents
Interest expense was $5.7 million in the Predecessor period and $9.2 million in the Successor period or $14.9 million for the nine months ended September 30, 2010, an increase of $3.7 million or 33.0% as compared to $11.2 million during the nine month period ended September 30, 2009. This increase is primarily related to interest related to the Senior Notes.
Income Tax Expense.The pro forma provision for income taxes for the nine months ended September 30, 2010 represented an effective annualized tax rate of 10.9% compared with 25.6% in 2009. The variation from the statutory federal rate of 35% on our share of pre-tax income during the nine months ended September 30, 2009 is primarily due to nondeductible Series X dividends and discount amortization accumulated during the predecessor period, state taxes and the tax impact of the noncontrolling interest.
The provision for income taxes in Predecessor period was 25.6% and during the Successor period was 16.2%. The variation from the statutory federal rate during the Predecessor period was primarily due to nondeductible Series X dividends and discount amortization accumulated during the Predecessor period. The variation from the statutory rate in the Successor period was primarily due to the nondeductible transaction expenses related to the Merger.
Net income attributable to noncontrolling interests. Noncontrolling interests represent the equity interests in our consolidated entities that we do not own, which is primarily the equity interests of our nephrologist partners in our JV clinics. Pro forma net income attributable to noncontrolling interests for the nine months ended September 30, 2010 was $19.6 million, an increase of 23.8% from $15.8 million in the nine months ended September 30, 2009. The increase was primarily due to the addition of de novo clinics and growth in the earnings of our existing JVs.
Net income attributable to noncontrolling interests was $9.3 million in the Predecessor period and $10.9 million in the Successor period for $20.2 million for the nine months ended September 30, 2010, an increase of $4.4 million or 27.8%, as compared to $15.8 million during the nine month period ended September 30, 2009. This increase is primarily due to the addition of de novo clinics and growth in earnings of our existing JVs.
Year ended December 31, 2009, as compared with year ended December 31, 2008
| Year Ended December 31, | Dollar Change | Percent Change | ||||||||||||||
(in thousands) | 2009 | 2008 | ||||||||||||||
Net patient service revenue | $ | 262,989 | $ | 217,777 | $ | 45,212 | 20.8 | % | ||||||||
Operating expenses: | ||||||||||||||||
Facility operating expenses | 170,826 | 141,810 | 29,016 | 20.5 | ||||||||||||
General and administrative expense | 24,819 | 19,944 | 4,875 | 24.4 | ||||||||||||
Depreciation and amortization | 12,127 | 9,777 | 2,350 | 24.0 | ||||||||||||
Provision for doubtful accounts | 3,216 | 4,834 | (1,618 | ) | -33.5 | |||||||||||
Total operating expenses | 210,988 | 176,365 | 34,623 | 19.6 | ||||||||||||
Operating income | 52,001 | 41,412 | 10,589 | 25.6 | ||||||||||||
Interest expense, net | (14,948 | ) | (13,729 | ) | (1,219 | ) | 8.9 | |||||||||
Income before income taxes | 37,053 | 27,683 | 9,370 | 33.8 | ||||||||||||
Income tax expense | 9,524 | 6,860 | 2,664 | 38.8 | ||||||||||||
Net income | 27,529 | 20,823 | 6,706 | 32.2 | ||||||||||||
Less: Net income attributable to noncontrolling interests | (22,391 | ) | (17,179 | ) | (5,212 | ) | 30.3 | |||||||||
Net income attributable to ARH | $ | 5,138 | $ | 3,644 | $ | 1,494 | 41.0 | % | ||||||||
66
Table of Contents
Net Operating Revenues
Net operating revenues for the year ended December 31, 2009 were $263.0 million, an increase of 20.8% from $217.8 million for the year ended December 31, 2008. The increase in net operating revenues was primarily due to an increase of approximately 23.3% in the number of dialysis treatments, but was partially offset by a 1.9% decrease in the average revenues per treatment. The increase in treatments resulted principally from non-acquired treatment growth from existing clinics and de novo clinics. Net operating revenues per treatment for the year ended December 31, 2009 was $353, compared with $360 for the year ended December 31, 2008. The decrease in revenues per treatment was primarily due to a decrease in the administration of EPO, which represented approximately 22% of net operating revenues for the year ended December 31, 2009, compared to 24% for the year ended December 31, 2008, and a change in our commercial payor mix resulting from the relatively high rates of unemployment in the current economic climate. The decrease in the administration of EPO was due to a change in our goal in hemoglobin levels as a result of new target hemoglobin levels in patients set by the Centers for Medicare and Medicaid Services. Our net operating revenues are driven by the number treatments performed and our average revenues per treatment. The following table summarizes our net operating revenues and net operating revenues per treatment for each of the periods indicated.
| Years Ended December 31, | ||||||||
| 2009 | 2008 | |||||||
Net operating revenues (in thousands) | $ | 262,989 | $ | 217,777 | ||||
Net operating revenues per treatment | $ | 353 | $ | 360 | ||||
The number of treatments that we performed during the year ended December 31, 2009 increased by approximately 23.3% over the number of treatments that we performed during the year ended December 31, 2008. This treatment growth has been driven by increasing the patient base at our existing clinics, adding new patients through the opening of de novo clinics and the acquisition of existing dialysis clinics in which we acquired a controlling interest. The following table summarizes the sources of our treatment growth for the period indicated.
| Years Ended December 31, | ||||||||
| 2009 | 2008 | |||||||
Source of Treatment Growth: | ||||||||
Existing clinics (1) | 18.9 | % | 16.5 | % | ||||
De novo clinics opened (2) | 1.7 | % | 3.6 | % | ||||
Non-acquired treatment growth | 20.6 | % | 20.1 | % | ||||
Clinics acquired (3) | 2.7 | % | — | % | ||||
Total treatment growth | 23.3 | % | 20.1 | % | ||||
| (1) | Represents net growth in treatments at clinics operating at end of the period that were also opened at the end of the prior period. |
| (2) | Represents additional treatments provided at clinics opened since the end of the prior period, compared to the total number. |
| (3) | Represents treatments performed at clinics acquired since the end of the prior period. |
Our net operating revenues per treatment is principally driven by our mix of commercial and government (principally Medicare and Medicaid) payor patients, the mix and amount of physician-prescribed pharmaceuticals, and commercial and government payment rates. We are generally paid more for services provided to patients covered by commercial healthcare plans than we are for patients covered by Medicare or Medicaid. Patients covered by employer group health plans transition to Medicare coverage after a maximum of 33 months. During the year ended December 31, 2009, the Medicare ESRD dialysis reimbursement rates for patients at our clinics were between $126 and $154 per treatment, excluding the administration of separately-billable pharmaceuticals, such as EPO. Medicare payment rates are generally insufficient to cover our total operating expenses allocable to providing dialysis treatments for Medicare patients, although in some circumstances they are sufficient to cover their patient care costs. As a result, our ability to generate operating
67
Table of Contents
earnings is substantially dependent on revenues derived from commercial payors, some of which pay negotiated payment rates and others of which pay based on our usual and customary fee schedule. Additionally, as a patient transitions from commercial coverage to Medicare coverage, the payment rates typically decline substantially.
The following table summarizes our net operating revenues and treatments by source for each of the periods indicated. These numbers are largely driven by the nephrologist partners we choose to partner with and the overall economic environment, particularly unemployment.
| Years Ended December 31, | ||||||||
| 2009 | 2008 | |||||||
Source of revenues: | ||||||||
Government-based programs and other | 57.0 | % | 57.9 | % | ||||
Commercial payors | 43.0 | % | 42.1 | % | ||||
| 100.0 | % | 100 | % | |||||
Source of treatments: | ||||||||
Government-based programs and other | 85.9 | % | 86.1 | % | ||||
Commercial payors | 14.1 | % | 13.9 | % | ||||
| 100.0 | % | 100.0 | % | |||||
Operating Expenses
Patient care costs. Patient care costs for the year ended December 31, 2009 were $170.8 million, an increase of 20.5% from $141.8 million in the year ended December 31, 2008. This increase was primarily due to an increase in the number of treatments. As a percentage of net operating revenues, patient care costs were approximately 64.9% for the year ended December 31, 2009 compared to 65.1% for the year ended December 31, 2008. Patient care costs per treatment for the year ended December 31, 2009 were $229, compared to $234 for the year ended December 31, 2008, reflecting a decrease of $5 per treatment. This decrease was primarily due to decreased pharmacy costs associated with the decrease in the administration of EPO.
General and administrative expenses. General and administrative expenses for the year ended December 31, 2009 were $24.8 million, an increase of 24.4% from $19.9 million in the year ended December 31, 2008. The increase is primarily attributable to an increase in headcount associated with our total treatment growth of 23.3%. As a percentage of net operating revenues, general and administrative costs were approximately 9.4% during the year ended December 31, 2009 and 9.1% during the year ended December 31, 2008. General and administrative costs per treatment were $33 for the year ended December 31, 2009 and for the year ended December 31, 2008.
Depreciation and amortization. Depreciation and amortization expense represents expense attributable to our clinics’ equipment and leasehold improvements. We calculate depreciation and amortization expense using a straight-line method over the assets’ useful lives. Depreciation and amortization expense for the year ended December 31, 2009 was $12.1 million, an increase of 23.5% from $9.8 million in the year ended December 31, 2008. The increase is primarily due to the increase in the number of clinics from 64 as of January 1, 2008 to 83 as of December 31, 2009. As a percentage of net operating revenues, depreciation and amortization expense was approximately 4.6% for the year ended December 31, 2009 compared to 4.5% for the year ended December 31, 2008.
Provision for uncollectible accounts. Provision for uncollectible accounts represents reserves established for amounts for which patients are primarily responsible which we believe will not be collectible. Provision for uncollectible accounts for the year ended December 31, 2009 was $3.2 million, a decrease of 33.3% from $4.8 million in the year ended December 31, 2008. As a percentage of net operating revenues, provision for uncollectible accounts was approximately 1.2% for the year ended December 31, 2009, compared to 2.2% for the year ended December 31, 2008. The decrease was primarily due to uncollectible amounts in the corresponding period of 2008 from CMS for a new facility which did not receive its Medicare certification until July 2008. Our accounts receivable, net of the bad debt allowance, represented approximately 59 days of revenues as of December 31, 2009 and as of December 31, 2008.
68
Table of Contents
Interest Expense, net. Interest expense represents charges for interest associated with our corporate level debt and credit facilities entered into by our dialysis clinics, as well as accumulated dividends attributable to our Series X preferred stock. Interest expense, net for the year ended December 31, 2009 was $14.9 million, an increase of 8.8% from $13.7 million in the year ended December 31, 2008. Interest expense for the year ended December 31, 2009 and 2008 consisted of $5.7 million and $5.9 million, respectively, attributable to interest on long-term debt, $8.1 million and $7.2 million, respectively, of a non-cash charge attributable to Series X preferred stock dividends and discount accretion, and $1.1 million and $0.6 million, respectively, attributable to amortization of deferred financing costs. Interest expense on long-term debt decreased due to a decrease in our weighted average interest rate to 6.8% during 2009 from 7.1% during 2008 as a result of lower LIBOR rates. Series X preferred stock dividends increased as dividends compound quarterly. The increase in amortization of deferred financing costs is attributable to expensing the remaining unamortized deferred financing costs of $0.6 million in connection with the amendment of certain loans during 2009.
Income Tax Expense. The provision for income taxes for the year ended December 31, 2009 represented an effective annualized tax rate of 25.7% compared with 24.8% in 2008. The income tax expense included in the accompanying consolidated statements of income principally relates to our proportionate share of the pre-tax income of our majority-owned subsidiaries. The principal difference between our effective tax rate and the statutory tax rate is the non-deductible interest expense, including original issue discount, related to the Series X redeemable preferred stock, which amounted to $8.1 million and $7.2 million in 2009 and 2008, respectively.
Net income attributable to noncontrolling interests. Noncontrolling interests represent the equity interests in our consolidated entities that we do not own, primarily the equity interests of our nephrologist partners in our JV clinics. Net income attributable to noncontrolling interests for the year ended December 31, 2009 was $22.4 million, an increase of 30.2% from $17.2 million in the year ended December 31, 2008. The increase was primarily due to de novo clinics and growth in the earnings of our existing JVs. Net income attributable to noncontrolling interests as a percentage of operating income was 43.1% in the year ended December 31, 2009 and 41.5% in the year ended December 31, 2008.
Year ended December 31, 2008, as compared with year ended December 31, 2007
| Year Ended December 31, | Dollar Change | Percent Change | ||||||||||||||
(in thousands) | 2008 | 2007 | ||||||||||||||
Net patient service revenue | $ | 217,777 | $ | 178,391 | $ | 39,386 | 22.1 | % | ||||||||
Operating expenses: | ||||||||||||||||
Facility operating expenses | 141,810 | 115,058 | 26,752 | 23.3 | % | |||||||||||
General and administrative expense | 19,944 | 18,595 | 1,349 | 7.3 | % | |||||||||||
Depreciation and amortization | 9,777 | 7,919 | 1,858 | 23.5 | % | |||||||||||
Provision for doubtful accounts | 4,834 | 3,258 | 1,576 | 48.4 | % | |||||||||||
Total operating expenses | 176,365 | 144,830 | 31,535 | 21.8 | % | |||||||||||
Operating Income | 41,412 | 33,561 | 7,851 | 23.4 | % | |||||||||||
Interest expense, net | (13,729 | ) | (13,695 | ) | (34 | ) | 0.2 | % | ||||||||
Income before income taxes | 27,683 | 19,866 | 7,817 | 39.3 | % | |||||||||||
Income tax expense | 6,860 | 4,409 | 2,451 | 55.6 | % | |||||||||||
Net income | 20,823 | 15,457 | 5,366 | 34.7 | % | |||||||||||
Less: Net income attributable to noncontrolling interests | (17,179 | ) | (14,706 | ) | (2,473 | ) | 16.8 | % | ||||||||
Net income attributable to ARH | $ | 3,644 | $ | 751 | $ | 2,893 | 385.2 | % | ||||||||
69
Table of Contents
Net Operating Revenues
Net operating revenues for the year ended December 31, 2008 were $217.8 million, an increase of 22.1% from $178.4 million for the year ended December 31, 2007. The increase in net operating revenues was primarily due to an increase of approximately 20.1% in the number of dialysis treatments and a 1.7% increase in the average dialysis revenues per treatment. The increase in treatments resulted principally from non-acquired treatment growth from existing clinics and de novo clinics. Net operating revenues per treatment for the year ended December 31, 2008 was $360, compared with $354 for the year ended December 31, 2007. The increase in revenues per treatment was primarily due to a favorable increase in our commercial treatment mix in 2008.
| Years Ended December 31, | ||||||||
| 2008 | 2007 | |||||||
Net operating revenues (in thousands) | $ | 217,777 | $ | 178,391 | ||||
Net operating revenues per treatment | $ | 360 | $ | 354 | ||||
The number of treatments that we performed during the year ended December 31, 2008 increased by approximately 20.1% over the number of treatments that we performed during the year ended December 31, 2007. This treatment growth has been driven by increasing the patient base at our existing clinics, adding new patients through the opening of de novo clinics and the acquisition of existing dialysis clinics in which we acquired a controlling interest. The following table summarizes the sources of our treatment growth for the period indicated.
| Years Ended December 31, | ||||||||
| 2008 | 2007 | |||||||
Source of Treatment Growth: | ||||||||
Existing clinics (1) | 16.5 | % | 11.3 | % | ||||
De novo clinics opened (2) | 3.6 | % | 5.6 | % | ||||
Non-acquired treatment growth | 20.1 | % | 16.9 | % | ||||
Clinics acquired (3) | — | % | 3.4 | % | ||||
Total treatment growth | 20.1 | % | 20.3 | % | ||||
| (1) | Represents net growth in treatments at clinics operating at end of the period that were also opened at the end of the prior period. |
| (2) | Represents additional treatments provided at clinics opened since the end of the prior period, compared to the total number. |
| (3) | Represents treatments performed at clinics acquired since the end of the prior period. |
Our net operating revenues per treatment is principally driven by our mix of commercial and government (principally Medicare and Medicaid) payor patients, the mix and amount of physician-prescribed pharmaceuticals, and commercial and government payment rates. We are generally paid more for services provided to patients covered by commercial healthcare plans than we are for patients covered by Medicare or Medicaid. Patients covered by employer group health plans transition to Medicare coverage after a maximum of 33 months. Medicare payment rates are generally insufficient to cover our total operating expenses allocable to providing dialysis treatments for Medicare patients, although in some circumstances they are sufficient to cover their patient care costs. As a result, our ability to generate operating earnings is substantially dependent on revenues derived from commercial payors, some of which pay negotiated payment rates and others of which pay based on our usual and customary fee schedule. Additionally, as a patient transitions from commercial coverage to Medicare coverage, the payment rates typically decline substantially.
70
Table of Contents
The following table summarizes our net operating revenues and treatments by source for each of the periods indicated. These numbers are largely driven by the nephrologist partners we choose to partner with and the overall economic environment, particularly unemployment.
| Years Ended December 31, | ||||||||
| 2008 | 2007 | |||||||
Source of revenues: | ||||||||
Government-based programs and other | 57.9 | % | 57.7 | % | ||||
Commercial payors | 42.1 | % | 42.3 | % | ||||
| 100 | % | 100.0 | % | |||||
Source of treatments: | ||||||||
Government-based programs and other | 86.1 | % | 87.6 | % | ||||
Commercial payors | 13.9 | % | 12.4 | % | ||||
| 100.0 | % | 100.0 | % | |||||
Operating Expenses
Patient care costs. Patient care costs for the year ended December 31, 2008 were $141.8 million, an increase of 23.2% from $115.1 million in the year ended December 31, 2007. This increase was primarily attributable to an increase in the number of treatments. As a percentage of net operating revenues, patient care costs were approximately 65.1% for the year ended December 31, 2008 compared to 64.4% for the year ended December 31, 2007. Patient care costs per treatment for the year ended December 31, 2008 were $234, compared to $228 for the year ended December 31, 2007, reflecting an increase of $6 per treatment. This increase was primarily due to increased salaries and benefits costs for personnel and increased medical supply costs.
General and administrative expenses. General and administrative expenses for the year ended December 31, 2008 were $19.8 million, an increase of 7.3% from $18.6 million in the year ended December 31, 2007. The increase is primarily attributable to an increase in headcount associated with our treatment growth of 20.1%. As a percentage of net operating revenues, general and administrative costs were approximately 9.1% for the year ended December 31, 2008 and 10.4% for the year ended December 31, 2007. General and administrative costs per treatment for the year ended December 31, 2008 were $32, compared to $37 for the year ended December 31, 2007 primarily related to lower stock-based compensation in 2008 as compared to 2007.
Depreciation and amortization. Depreciation and amortization expense for the year ended December 31, 2008 was $9.8 million, an increase of 24.1% from $7.9 million in the year ended December 31, 2007. The increase is primarily attributable to the increase in the number of clinics from 53 as of January 1, 2007 to 75 as of December 31, 2008. As a percentage of net operating revenues, depreciation and amortization expense was approximately 4.5% for the year ended December 31, 2008 and 4.4% for the year ended December 31, 2007.
Provision for uncollectible accounts. Provision for uncollectible accounts for the year ended December 31, 2008 was $4.8 million, an increase of 45.4% from $3.3 million in the year ended December 31, 2007. As a percentage of net operating revenues, provision for uncollectible accounts was 2.2% during the year ended December 31, 2008 and 1.8% during the year ended December 31, 2007. The increase was primarily due to uncollectible amounts in the corresponding period of 2008 from CMS for a new facility which did not receive its Medicare certification until July 2008. Provision for uncollectible accounts per treatment for the year ended December 31, 2008 was $8, compared to $6 for the year ended December 31, 2007. Our accounts receivable, net of the bad debt allowance, represented approximately 59 days of revenues as of December 31, 2008, and 58 days as of December 31, 2007.
Interest Expense, net. Interest expense was $13.7 million for the year ended December 31, 2008 and in the year ended December 31, 2007. Interest expense for the year ended December 31, 2008 and 2007 consisted of $5.9 million and $6.4 million, respectively, attributable to interest on long-term debt, $7.2 million and
71
Table of Contents
$6.5 million, respectively, of a non-cash charge attributable to Series X preferred stock dividends and discount accretion, and $0.6 million and $0.8 million, respectively, attributable to amortization of deferred financing costs. Interest expense on long-term debt decreased 7.8% due to a decrease in our weighted average interest rate to 7.1% during 2008 from 8.6% during 2007 due to lower LIBOR rates but offset by an increase in our long-term debt in 2008. Series X preferred stock dividends increased as dividends compound quarterly.
Income Tax Expense. The provision for income taxes for the year ended December 31, 2008 represented an effective tax rate of 24.8%, compared with 22.2% for the year ended December 31, 2007. The income tax expense included in the accompanying consolidated statements of income principally relates to our proportionate share of the pre-tax income of our majority-owned subsidiaries. The principal difference between our effective tax rate and the statutory federal tax rate of 35% is the non-deductible interest expense, including original issue discount, related to the Series X redeemable preferred stock, which amounted to $7.2 million and $6.5 million in 2008 and 2007, respectively, state taxes and the tax impact of the noncontrolling interest.
Net income attributable to noncontrolling interests. Net income attributable to noncontrolling interests for the year ended December 31, 2008 was $17.2 million, an increase of 17.0% from $14.7 million in the year ended December 31, 2007. The increase was primarily due to de novo clinics and growth in the earnings of our existing JVs. Net income attributable to noncontrolling interests as a percentage of operating income was 41.5% for the year ended December 31, 2008, compared with 43.8% for the year ended December 31, 2007.
Liquidity and Capital Resources
We own controlling interests in our JV clinics and our nephrologist partners own noncontrolling interests, typically between 25% and 49%. Except as otherwise indicated, the following discussion of our liquidity and capital resources presents information on a consolidated basis, without giving effect to any noncontrolling interests in our JV clinics.
Cash Flows
The following discussion highlights our cash flow activities during the successor period from May 8, 2010 toSeptember 30, 2010, the predecessor periods from January 1, 2010 through May 7, 2010 and theninemonth period endedSeptember 30, 2009.
Cash Flows from Operations:
| Successor | Predecessor | |||||||||||
| May 8 through September 30, 2010 | January 1 through May 7, 2010 | Nine months Ended September 30, 2009 | ||||||||||
Source / (Use) | ||||||||||||
Net (loss) income | $ | (3,343 | ) | $ | 6,582 | $ | 20,340 | |||||
Depreciation and amortization | 6,434 | 4,429 | 8,816 | |||||||||
Non-cash and non-operating items | 5,662 | 4,877 | 8,580 | |||||||||
Increase (decrease) in cash resulting in changes from: | ||||||||||||
Accounts receivable | 2,132 | (2,465 | ) | (4,827 | ) | |||||||
Inventories | 424 | (6 | ) | 192 | ||||||||
Other assets | 3,668 | (1,506 | ) | 2,985 | ||||||||
Accounts payable and accrued expenses | 245 | (6,546 | ) | 3,306 | ||||||||
Other liabilities | 1,615 | (70 | ) | (1,254 | ) | |||||||
Net cash provided by operating activities | $ | 16,837 | $ | 5,295 | $ | 37,222 | ||||||
72
Table of Contents
The decrease in our net income is primarily attributable to expenses related to the Merger. In the successor period, other non-cash and non-operating items such as stock-based compensation expense and expenses related to the Merger. In the predecessor period, other non-cash and non-operating items is primarily non-cash Series X preferred stock interest expense. The increase in accounts receivable in the predecessor periods resulted from the timing of collections compared to billings.
Cash Flows from Investing Activities:
| Successor | Predecessor | |||||||||||
| May 8 through September 30, 2010 | January 1 through May 7, 2010 | Nine months Ended September 30, 2009 | ||||||||||
Source / (Use) | ||||||||||||
Purchases of property and equipment | $ | (6,141 | ) | $ | (4,904 | ) | $ | (9,757 | ) | |||
Cash paid for acquisitions | (275 | ) | (144 | ) | (3,964 | ) | ||||||
Merger with C.P. Atlas Holdings, Inc. | (249,716 | ) | — | — | ||||||||
Net cash used in investing activities | $ | (256,132 | ) | $ | (5,048 | ) | $ | (13,721 | ) | |||
The increase in our cash used in investing activities is due to the Merger. Cash paid for acquisitions during the nine months ended September 30, 2009 represents the acquisition of three clinics.
Cash Flows provided by (used in) Financing Activities:
| Successor | Predecessor | |||||||||||
| May 8 through September 30, 2010 | January 1 through May 7, 2010 | Nine months Ended September 30, 2009 | ||||||||||
Source / (Use) | ||||||||||||
Payments on long-term debt | $ | (65,104 | ) | $ | (5,391 | ) | $ | (8,687 | ) | |||
Proceeds from issuance of common stock | 161,732 | 8 | 81 | |||||||||
Issuance of debt, net of issuance cost of 11,426 | 236,774 | — | — | |||||||||
Payoff of Series X Preferred Stock | (68,088 | ) | — | — | ||||||||
Distributions to noncontrolling interests | (9,934 | ) | (11,394 | ) | (15,016 | ) | ||||||
Other | (747 | ) | (408 | ) | 485 | |||||||
Net cash provided by (used in) financing activities | $ | 254,633 | $ | (17,185 | ) | $ | (23,137 | ) | ||||
The increase in our cash provided by financing activities is primarily due to the Merger. In connection with the Merger:
| • | Centerbridge made an aggregate cash equity investment of $161.5 million in the Parent, subject to certain adjustments, and in exchange received 87% of the common stock of the Parent immediately following the Merger; |
| • | Certain members of ARH’s management contributed a portion of their existing equity ownership in ARH in exchange for newly issued shares of common stock and options to purchase common stock of the Parent; |
| • | ARH entered into the New Revolving Credit Facility; |
| • | ARH offered the Senior Secured Notes; |
| • | ARH repaid approximately $63.0 million of debt related to the Predecessor; and |
| • | ARH purchased all of the outstanding shares as of May 7, 2010 of the Series X Preferred Stock for $65.2 million. |
73
Table of Contents
Capital Resources
Our future needs for liquidity will arise primarily from funding the development of new clinics, operating expenses, capital expenditures, payment of the advisory fee and other expenses payable under the management agreement with Centerbridge and to service our debt, including our New Revolving Credit Facility and the Senior Secured Notes. Our primary sources of liquidity will be funds generated from our operations, short-term borrowing under our New Revolving Credit Facility and borrowings of long-term debt.
We believe our cash flows from operations, combined with availability under our New Revolving Credit Facility, provides sufficient liquidity to fund our current obligations, projected working capital requirements and capital spending for a period that includes the next 12 months. If existing cash and cash generated from operations and borrowings under the New Revolving Credit Facility are insufficient to satisfy our liquidity requirements, we may seek to obtain additional debt or equity financing. If additional funds are raised through the issuance of debt securities, these securities could contain covenants that would restrict our operations. Any financing may not be available in amounts or on terms acceptable to us. If we are unable to obtain required financing, we may be required to reduce the scope of our planned growth efforts, which could harm our financial condition and operating results.
As of September 30, 2010 we have outstanding $258.2 million in aggregate principal amount of indebtedness, with an additional $25.0 million of borrowing capacity available under our New Revolving Credit Facility (not giving effect to any outstanding letters of credit, which would reduce the amount available under our New Revolving Credit Facility).
Our liquidity requirements will be significant, primarily due to debt service requirements in connection with the Transactions. In addition, we pay Centerbridge a yearly advisory services fee beginning for the fiscal year 2010 of the greater of (i) an amount equal to the greater of (x) $550,000 and (y) the amount of the previous fiscal year’s advisory fee and (ii) an amount equal to 1.25% of our EBITDA for that fiscal year.
Historically, our principal needs for liquidity have been to fund the development and acquisition of new clinics, to pay our operating expenses, to fund capital expenditures and to service our debt. Our primary sources of liquidity are funds generated from our operations and from borrowings of long-term debt. Our cash and cash equivalent balances were the following for each period:
| December 31, | September 30, 2010 | |||||||||||
| 2008 | 2009 | |||||||||||
| (in thousands) | ||||||||||||
Cash and cash equivalents at issuer and guarantor subsidiaries | $ | 11,869 | $ | 11,423 | $ | 5,379 | ||||||
Cash and cash equivalents at non-guarantor subsidiaries | 17,313 | 17,756 | 22,200 | |||||||||
Consolidated cash and cash equivalents | $ | 29,182 | $ | 29,179 | $ | 27,579 | ||||||
The decrease in cash and cash equivalents of the issuer and guarantor subsidiaries is primarily attributable to the issuer financing the Merger by approximately $8.4 million on May 7, 2010.
At September 30, 2010, our subsidiary level long-term debt, on an aggregated basis, consisted of term loans, lines of credit and mortgages totaling $8.2 million with maturities ranging from December 2011 to February 2015 and interest rates ranging from 3.06% to 8.67%.
Senior Secured Notes
In connection with the Transactions, we issued $250.0 million of Senior Secured Notes (the notes) at an offering price of 99.28%. The notes are secured, subject to certain exceptions, by (i) all of the our capital stock and (ii) substantially all of our assets of our wholly owned subsidiary guarantors. The notes are guaranteed by the our direct parent, C. P. Atlas Intermediate Holdings, LLC and all of our existing and future wholly owned domestic subsidiaries. The notes mature on May 15, 2018. Interest is payable semi-annually at 8.375% per annum, commencing November 15, 2010.
On or after May 15, 2015, we may redeem the notes at our option, subject to certain notice periods, at a price equal to 100% of the principal amount of the notes. Prior to May 15, 2013, we have the option to redeem during each 12-month period commencing on the issue date of May 7, 2010 up to 10% of the aggregate principal
74
Table of Contents
amount of the notes at 103% of the aggregate principal amount of the notes redeemed plus accrued and unpaid interest to the date of redemption. In addition, we may redeem up to 35% of the notes before May 15, 2013, with the net cash proceeds from certain equity offerings. We may also redeem some or all of the notes before May 15, 2013 at a redemption price of 100% of the principal amount thereof plus accrued and unpaid interest, if any, to the redemption date, plus a “make-whole” premium. Upon a change in control, we must offer to purchase the notes at 101% of the principal amount, plus accrued interest to the purchase date.
New Revolving Credit Facility
In connection with the Transactions, we entered into a $25.0 million senior secured revolving credit facility (the New Revolving Credit Facility). As of September 30, 2010, there were no borrowings outstanding under the New Revolving Credit Facility. The New Revolving Credit Facility expires on May 7, 2015. Borrowings under the New Revolving Credit Facility bear interest at a rate equal to, at our option, either (a) an alternate base rate determined by reference to the higher of (1) the prime rate in effect on such day, (2) the federal funds effective rate plus 0.50% and (3) the Eurodollar rate applicable for an interest period of one month plus 1.00% or (b) the LIBOR rate, plus an applicable margin. The initial applicable margin for borrowings under the New Revolving Credit Facility is 3.50% with respect to alternate base rate borrowings and 4.50% with respect to LIBOR borrowings. In addition to paying interest on outstanding principal under the New Revolving Credit Facility, we are required to pay a commitment fee, initially 0.75% per annum, in respect of the unutilized revolving credit commitments thereunder.
Earnings before Interest, Taxes, Depreciation and Amortization and other Adjustments
We believe earnings before interest, taxes, depreciation and amortization and other adjustments, which we refer to as Adjusted EBITDA, provides information useful for evaluating our businesses and understanding our operation performance in a manner similar to management. We define Adjusted EBITDA as net income attributable to ARH before income taxes, interest expense, depreciation and amortization, and we further adjust for non-cash charges, non-recurring charges and pro forma amounts for acquisitions as if they had been consummated on the first day of each period. We believe Adjusted EBITDA is helpful in highlighting trends because Adjusted EBITDA excludes the results of decisions that are outside the control of operating management and can differ significantly from company to company depending on long-term strategic decisions regarding capital structure, the tax jurisdictions in which companies operate and capital investments. In addition, we present Adjusted EBITDA because it is one of the components used in the calculations under the covenants contained in our revolving credit facility. Adjusted EBITDA is not a measure of operating performance computed in accordance with GAAP and should not be considered as a substitute for operating income, net income, cash flows from operations, or other statement of operations or cash flow data prepared in conformity with GAAP, or as measures of profitability or liquidity. In addition, Adjusted EBITDA may not be comparable to similarly titled measures of other companies. Adjusted EBITDA may not be indicative of historical operating results, and we do not mean for it to be predictive of future results of operations or cash flows. Adjusted EBITDA has limitations as an analytical tool, and you should not consider this item in isolation, or as a substitute for an analysis of our results as reported under GAAP. Some of these limitations are that Adjusted EBITDA:
| • | does not include interest expense—as we have borrowed money for general corporate purposes, interest expense is a necessary element of our costs and ability to generate profits and cash flows; |
| • | does not include depreciation and amortization—because construction and operation of our dialysis clinics requires significant capital expenditures, depreciation and amortization are a necessary element of our costs and ability to generate profits; |
| • | does not include stock-based compensation expense; |
| • | does not reflect changes in, or cash requirements for, our working capital needs; and |
| • | does not include certain income tax payments that represent a reduction in cash available to us. |
You should not consider Adjusted EBITDA as an alternative to income from operations or net income, determined in accordance with GAAP, as an indicator of our operating performance, or as an alternative to cash flows from operating activities, determined in accordance with GAAP, as an indicator of cash flows or as a measure of liquidity.
75
Table of Contents
The following table presents the reconciliation from net income to Adjusted EBITDA for the periods indicated:
| Historical | Pro Forma | Historical | ||||||||||||||
| Three-Months Ended September 30, | Nine-Months Ended September 30, | |||||||||||||||
(in thousands) | 2010 | 2009 | 2010 | 2009 | ||||||||||||
| Successor | Predecessor | Successor | Predecessor | |||||||||||||
Net income | $ | 8,439 | $ | 7,463 | $ | 24,504 | $ | 20,340 | ||||||||
Interest expense | 5,885 | 3,755 | 16,935 | 11,212 | ||||||||||||
Income tax expense | 977 | 2,582 | 2,992 | 7,036 | ||||||||||||
Depreciation and amortization | 4,085 | 2,814 | 12,322 | 8,816 | ||||||||||||
Merger and related expenses | 152 | — | — | — | ||||||||||||
Stock-based compensation | 544 | 293 | 763 | 876 | ||||||||||||
Initial public offering transaction expenses | — | 462 | — | 508 | ||||||||||||
Management fee | 138 | — | 413 | — | ||||||||||||
Specified legal costs | — | 130 | — | 373 | ||||||||||||
Adjusted EBITDA (including noncontrolling interests) | $ | 20,220 | $ | 17,499 | $ | 57,929 | $ | 49,161 | ||||||||
Less: Net income attributable to noncontrolling interests | (6,907 | ) | (5,756 | ) | (19,583 | ) | (15,823 | ) | ||||||||
Adjusted EBITDA | $ | 13,313 | $ | 11,743 | $ | 38,346 | $ | 33,338 | ||||||||
Covenant Compliance
Under the New Revolving Credit Facility, certain limitations, restrictions and defaults could occur if we are not able to satisfy and remain in compliance with specified financial ratios. We have agreed that beginning on September 30, 2010, we will not permit the Consolidated Net Debt to Consolidated EBITDA (both as defined in the agreement) Ratio for any 12 month period (last four fiscal quarters) ending during a period set forth below to be greater than the ratio set forth below opposite such period:
Period | Ratio | |||
September 30, 2010 through September 30, 2011 | 5.75:1.00 | |||
December 31, 2011 through September 30, 2012 | 5.50:1.00 | |||
December 31, 2012 through September 30, 2013 | 5.00:1.00 | |||
December 31, 2013 through September 30, 2014 | 4.75:1.00 | |||
December 31, 2014 and thereafter | 4.25:1.00 | |||
We have also agreed that beginning on September 30, 2010, we will not permit the Consolidated EBITDA to Fixed Charges (both as defined in the agreement) Ratio for any 12 month period (last four fiscal quarters) ending during a period set forth below to be lower than the ratio set forth below opposite such period:
Period | Ratio | |||
September 30, 2010 through September 30, 2011 | 1.80:1.00 | |||
December 31, 2011 through September 30, 2012 | 1.80:1.00 | |||
December 31, 2012 through September 30, 2013 | 2.00:1.00 | |||
December 31, 2013 through September 30, 2014 | 2.25:1.00 | |||
December 31, 2014 and thereafter | 2.50:1.00 | |||
The breach of these covenants could result in a default under the New Revolving Credit Facility and the lenders could elect to declare all amounts borrowed due and payable. As of September 30, 2010, we are in compliance with our covenants.
76
Table of Contents
In determining Consolidated EBITDA, EBITDA is calculated by reference to net income plus interest and other financing costs, net, provision for income taxes, depreciation and amortization and stock-based compensation. Consolidated EBITDA as defined in the agreement is calculated by adjusting EBITDA to exclude unusual items and other adjustments permitted in calculating covenant compliance under the indentures and the credit facilities. The adjustments made in determining Consolidated EBITDA include those used in determining Adjusted EBITDA, and further include adjustments for net income attributable to noncontrolling interests with clinic-level debt and other adjustments. We believe that the inclusion of supplementary adjustments to EBITDA applied in presenting Consolidated EBITDA are appropriate to provide additional information to investors to demonstrate our ability to comply with the financial covenants of the credit facility. The calculation of Consolidated EBITDA under the New Revolving Credit Facility is as follows (in thousands):
| Last Twelve months ended September 30, 2010 | ||||
Net loss | $ | (16,359 | ) | |
Interest expense, net (1) | 18,658 | |||
Provision for income taxes | 4,103 | |||
Depreciation and amortization | 14,174 | |||
Stock-based compensation | 5,058 | |||
Transaction expenses (2) | 22,217 | |||
Initial public offering transaction expenses | 1,289 | |||
Specified legal costs (3) | 299 | |||
Adjusted EBITDA (5) | $ | 49,439 | ||
Net income attributable to noncontrolling interests with clinic-level | 4,025 | |||
Other | 235 | |||
Consolidated EBITDA (5) | $ | 53,699 | ||
| (1) | Includes interest expense and interest income. |
| (2) | Reflects expenses related to the Transactions. |
| (3) | Represents adjustments for certain legal costs as a specified exclusion. |
| (4) | Reflects net income attributable to noncontrolling interests at those clinics with $8.2 million of third-party debt as of September 30, 2010. The agreement permits this adjustment up to the amount of third-party debt that is included in certain coverage and leverage ratios. |
| (5) | Adjusted EBITDA is defined as net income plus net interest expense, income taxes, depreciation and amortization, stock-based compensation transaction expenses, initial public offering transaction expenses and specified legal costs. Adjusted EBITDA is not a recognized term under GAAP and does not purport to be an alternative to net income as a measure of operating performance or to cash flows from operating activities as a measure of liquidity. Additionally, Adjusted EBITDA is not intended to be a measure of free cash flow available for management’s discretionary use as it does not consider certain cash requirements such as interest payments, tax payments and debt service requirements. The presentation of Adjusted EBITDA has limitations as an analytical tool and should not be considered in isolation or as a substitute for analysis of the Company’s results as reported under GAAP. Management believes Adjusted EBITDA is helpful in highlighting trends because Adjusted EBITDA excludes the results of decisions that are outside the control of operating management and can differ significantly from company to company depending on long-term strategic decisions regarding capital structure, the tax jurisdictions in which companies operate and capital investments. In addition, Adjusted EBITDA provides more comparability between our predecessor results and our successor results that reflect acquisition accounting and our new capital structure. Management compensates for the limitations of using non-GAAP financial measures by using them to supplement GAAP results to provide a more complete understanding of the factors and trends affecting the business than GAAP results alone. |
77
Table of Contents
Consolidated EBITDA (or debt covenant EBITDA) is defined as Adjusted EBITDA plus net income attributable to noncontrolling interests with clinic-level debt and other adjustments. Consolidated EBITDA, not Adjusted EBITDA, is used in calculating covenant compliance under the agreements governing the New Revolving Credit Facility. The Company believes that the inclusion of supplementary adjustments to Adjusted EBITDA are appropriate to provide additional information to investors about items that will impact the calculation of EBITDA that is used to determine covenant compliance under the agreements governing the New Revolving Credit Facility. Since not all companies use identical calculations, this presentation of Consolidated EBITDA may not be comparable to other similarly titled measures of other companies.
Off Balance Sheet Arrangements, Contractual Obligations and Commitments
The following is a summary of historical contractual obligations and commitments as of September 30, 2010 (in thousands)
Scheduled payments under contractual obligations | Total | July 1 to December 31, 2010 | 2011 & 2012 | 2013 & 2014 | 2015 | After 2015 | ||||||||||||||||||
Clinic level third-party long-term debt and capital lease obligations (including current portion) | $ | 8,212 | $ | 849 | $ | 5,757 | $ | 1,602 | $ | 4 | $ | — | ||||||||||||
Senior Secured Notes (1) | 250,000 | — | — | — | — | 250,000 | ||||||||||||||||||
Operating leases (2) | 66,376 | 2,490 | 17,893 | 15,190 | 6,650 | 24,153 | ||||||||||||||||||
Total | $ | 324,588 | $ | 3,339 | $ | 23,650 | $ | 16,792 | $ | 6,654 | $ | 274,153 | ||||||||||||
| (1) | Bear interest at 8.375% with interest payment dates of May 15 and November 15, commencing November 15, 2010. As of September 30, 2010, accrued interest totals approximately $8.4 million. |
| (2) | Net of estimated sublease proceeds of $0.3 million remaining in 2010, $1.0 million per year from 2011 to 2015 and $6.9 million thereafter. |
We also have potential acquisition obligations for some of our non-wholly-owned subsidiaries in the form of put provisions, which are exercisable at our nephrologist partners’ future discretion within specified periods (“time-based puts”) or upon the occurrence of certain events (“event-based puts”) as set forth in each specific put provision. See Note H—Noncontrolling Interests Subject to Put Provisions in the Notes to our Consolidated Financial Statements, included elsewhere in this prospectus, for discussion of these put provisions. These put obligations are not customary in the operating agreements with our JV partners. Although no assurance can be given, we do not expect a significant increase in the number of future JV arrangements to contain put provisions.
Since our inception, $15.5 million of time-based obligations have become exercisable by our nephrologist partners, but only $2.4 million of these puts have been exercised. The following is a summary of the estimated cash obligations in each of the specified years under all time-based puts existing as of September 30, 2010 (in thousands) and reflects the payments that would be made, assuming (a) all vested puts as of September 30, 2010 are exercised on October 1, 2010 and paid accordingly and (b) all puts exercisable thereafter are exercised as soon as they vest and are paid accordingly.
Year | Amount Exercisable | |||
2010 (remainder) | $ | 6,552 | ||
2011 | $ | 6,234 | ||
2012 | $ | 10,148 | ||
2013 | $ | 7,966 | ||
2014 | $ | 2,263 | ||
Thereafter | $ | 721 | ||
We do not have any off balance sheet arrangements.
78
Table of Contents
Critical Accounting Policies and Estimates
We believe that the accounting policies described below are critical to understanding our business, results of operations and financial condition because they involve significant judgments and estimates used in the preparation of our consolidated financial statements. An accounting policy is deemed to be critical if it requires a judgment or accounting estimate to be made based on assumptions about matters that are highly uncertain, and if different estimates that could have been used, or if changes in the accounting estimates that are reasonably likely to occur periodically, could materially impact our consolidated financial statements. Other significant accounting policies, primarily those with lower levels of uncertainty than those discussed below, are also critical to understanding our consolidated financial statements. The notes to our consolidated financial statements contain additional information related to our accounting policies and should be read in conjunction with this discussion.
Net Operating Revenues and Allowance for Doubtful Accounts
We recognize net operating revenues as services are provided to patients. Net operating revenues consist primarily of reimbursement for dialysis and ancillary services provided to patients. We maintain a usual and customary fee schedule for dialysis treatment and other patient services; however, actual collectible revenues are normally at a discount to the fee schedule. We bill Medicare and Medicaid programs at predetermined net realizable rates per treatment that are established by statute or regulation. We record contracted payors at contracted rates and bill other payors at usual and customary rates, and record a contractual allowance to reflect the expected net realizable revenues for services provided. We estimate contractual allowances, along with provisions for uncollectible amounts, based upon contractual terms, regulatory compliance, and historical collection experience. Recognition of net revenues and allowances for uncollectible billings require the use of estimates of the amounts that will actually be realized considering among other items, retroactive adjustments that may be associated with regulatory reviews, audits, billing reviews, and other matters. As a result, there is at least a reasonable possibility that our recorded estimates will change by a material amount in the near term. We separately disclose changes in estimates of our revenues for prior periods if these changes are material.
Approximately 56% of our total net patient service revenues for the nine months ended September 30, 2010 were accounted for by net patient service revenues associated with patients whose primary coverage is under governmental programs, including Medicare and Medicaid and Medicare or Medicaid Managed Care programs. Laws and regulations governing the Medicare and Medicaid programs are extremely complex and subject to interpretation. As a result, there is at least a reasonable possibility that our recorded estimates will change by a material amount in the near term. Patient accounts receivable from these programs was $39.8 million at September 30, 2010. No other single payor accounted for more than 5% of total patient accounts receivable.
Noncontrolling Interests
We own a controlling interest in each of our 88 clinics, and our local nephrologist partners own the remaining noncontrolling interests. Effective January 1, 2009, we were required to treat noncontrolling interests as a separate component of equity, but apart from our equity, and not as a liability or other item outside of equity. We were also required to identify and retrospectively present consolidated net income attributable to us and to noncontrolling interests on the face of the consolidated statement of income. In addition beginning on January 1, 2009, changes in our ownership interest while we retain a controlling financial interest are prospectively accounted for as equity transactions. We were also required to expand disclosures in the financial statements to include a reconciliation of the beginning and ending balances of the equity attributable to us and the noncontrolling owners and a schedule showing the effects of changes in our ownership interest in a subsidiary on the equity attributable to us. This change did not have a material impact on our consolidated financial statements; however, it did change the presentation of noncontrolling interests in our consolidated financial statements.
In conjunction with adopting these requirements, we were required to classify securities with redemption features that are not solely within our control, such as the noncontrolling interests subject to put provisions,
79
Table of Contents
outside of permanent equity and to measure these noncontrolling interests at fair value. See Note E—Noncontrolling Interests Subject to Put Provisions in the Notes to our Consolidated Financial Statements for further details. These put provisions, if exercised, would require us to purchase our nephrologist partners’ interests at the appraised fair value. We estimate the fair value of the noncontrolling interests subject to these put provisions using an average multiple of earnings, based on historical earnings, development stage of the underlying business and other factors. The estimate of the fair values of the interests subject to these put provisions is a critical accounting estimate that involves significant judgments and assumptions and may not be indicative of the actual values at which these obligations may ultimately be settled in the future. The estimated fair values of the interests subject to these put provisions can also fluctuate and the implicit multiple of earnings at which these obligations may be settled will vary depending upon market conditions access to the credit and capital markets, which can impact the level of competition for dialysis and non-dialysis related businesses, the economic performance of these businesses and the restricted marketability of the nephrologist partners’ interests.
Stock-based Compensation
We measure and recognize compensation expense for all share-based payment awards based on estimated fair values at the date of grant. Determining the fair value of share-based awards requires judgment in developing assumptions, which involve a number of variables. We calculate fair value by using the Black-Scholes option-pricing model, which requires estimates for expected volatility, expected dividends, the risk-free interest rate and the expected term of the option. In addition, estimates of the number of share-based awards that are expected to be forfeited must be made. We also estimate the expected service period over which our stock option awards will vest, as well as make estimates regarding whether or not performance-based restricted stock will vest. Vesting of share-based awards issued with performance-based vesting criteria must be “probable” before we begin recording compensation expense. At each reporting period, we review the likelihood that these awards will vest and if the vesting is deemed probable, we begin to recognize compensation expense at that time. If ultimately performance goals are not met, for any awards where vesting was deemed probable, previously recognized compensation cost will be reversed. Each of these assumptions, while reasonable, requires a certain degree of judgment and the fair value estimates could vary if actual results are materially different than those initially applied.
Fair Value of Common Stock Determination by Predecessor
We granted stock options with the following exercise prices during the predecessor period from January 1, 2009 through May 7, 2010:
Option Grant Dates | Number of Shares Underlying Options | Exercise Price Per Share | Fair Market Value Per Underlying Share as of Grant Date | |||||||||
February 2009 | 257,500 | $ | 4.72 | $ | 4.72 | |||||||
May 2009 | 147,500 | 5.20 | 5.20 | |||||||||
September 2009 | 207,500 | 5.24 | 5.24 | |||||||||
November 2009 | 95,500 | 5.24 | 5.24 | |||||||||
The fair value of the common stock underlying stock options granted during this predecessor period was estimated by our board of directors, which intended all options granted to be exercisable at a price per share not less than the per share fair value of our common stock underlying those options on the date of grant. In the absence of a public trading market, our board of directors considered numerous objective and subjective factors to determine its best estimate of the fair market value of our common stock as of the date of each option grant, including but not limited to, the following factors: (i) the rights, preferences and privileges of our preferred stock relative to the common stock; (ii) our performance and stage of development; (iii) valuations of our common stock; and (iv) the likelihood of achieving a liquidity event for the shares of common stock underlying these stock options, such as an initial public offering or sale of our company, given prevailing market conditions. The
80
Table of Contents
assumptions we use in the valuation model are based on subjective future expectations combined with management judgment. If any of the assumptions used in the Black-Scholes model changes significantly, stock-based compensation for future awards may differ materially compared with the awards granted previously.
Every six months (June 30th and December 31st), we performed a concurrent valuation analysis to determine the fair value to be used for options to be granted in the successive six month period. The valuation analysis consists of two major steps: the estimation of the aggregate value of the entire company (referred to as Business Enterprise Value, or BEV) and the allocation of this aggregate value to each element of the contemporary capital structure (preferred stock, common stock, options and warrants). As described below, the BEV was estimated using a combination of income and market-based methods. The allocation of the BEV to equity classes was performed using an option-based method as of each valuation date.
Significant Factors, Assumptions and Methodologies Used in Determining Fair Value
In determining the fair value of our BEV and common stock, we used a combination of the income approach and the market approach to estimate our aggregate BEV at each valuation date.
The income approach is an estimate of the present value of the future monetary benefits expected to flow to the owners of a business. It requires a projection of the cash flows that the business is expected to generate over a forecast period and an estimate of the present value of cash flows beyond that period, which is referred to as residual value. These cash flows are converted to present value by means of discounting, using a rate of return that accounts for the time value of money and the appropriate degree of risks inherent in the business. The market approach considers multiples of financial metrics based on both acquisitions and trading multiples of a peer group of companies. These multiples are then applied to our financial metrics to derive an indication of value.
Our indicated BEV at each valuation date was first reduced by the amount of long-term debt outstanding and then, the residual value was allocated to the preferred stock and common stock, using a contingent claim methodology. This methodology treats the various components of our capital structure as a series of call options on the proceeds expected from the sale of the company or the liquidation of our assets at some future date. These call options are then valued using the Black-Scholes option pricing model. This model defines the securities’ fair values as functions of the current fair value of the company and assumptions based on the securities’ rights and preferences. As a result, the option-pricing method requires assumptions regarding the anticipated timing of a potential liquidity event, such as an initial public offering, and the estimated volatility of our equity securities. The anticipated timing of a liquidity event utilized in these valuations was based on then current plans and estimates of our board of directors and management regarding an initial public offering. Estimates of the volatility of our stock were based on available information on the volatility of capital stock of comparable publicly traded companies.
Fair Value of Common Stock Determination by Successor
During the successor period from May 8, 2010, we granted stock options to purchase 1,184,050 shares in July 2010 at an exercise price of $21.00 per share, the fair value on the date of the grant. The fair value was determined by our board of directors as $21.00 per share as this represented the price per share of common stock paid by C.P. Atlas Holdings, Inc. in the Merger, on an as converted basis.
Accounting for income taxes
We estimate our income tax provision to recognize our tax expense for the current year, and our deferred tax liabilities and assets for future tax consequences of events that have been recognized in our financial statements, measured using enacted tax rates and laws expected to apply in the periods when the deferred tax liabilities or assets are expected to be realized. Deferred tax assets are assessed based upon the likelihood of recoverability from future taxable income and, to the extent that recovery is not likely, a valuation allowance is established. We
81
Table of Contents
regularly review and update the allowance for changes in circumstances that would cause a change in judgment about the realizability of the related deferred tax assets. These calculations and assessments involve complex estimates and judgments because the ultimate tax outcome can be uncertain and future events unpredictable.
Acquisition accounting valuation estimates
We make various assumptions and estimates regarding the valuation of tangible and intangible assets, liabilities and contractual as well as non-contractual contingencies associated with our acquisitions. These assumptions can have a material effect on our balance sheet valuations and the related amount of depreciation and amortization expense that will be recognized in the future. Long-lived tangible and intangible assets are subject to our regular ongoing impairment assessments.
Goodwill impairment
The excess of aggregate purchase price over the fair value of the net tangible and specifically identifiable intangible assets of businesses acquired in purchase transactions is recorded as goodwill.
We evaluate goodwill for impairment at least on an annual basis and more frequently if certain indicators are encountered. We assess goodwill for impairment as of October 1. Goodwill is to be tested at the reporting unit level, defined as an operating segment or one level below an operating segment (referred to as a component), with the fair value of the reporting unit being compared to its carrying amount, including goodwill. If the fair value of a reporting unit exceeds its carrying amount, goodwill of the reporting unit is not considered to be impaired. We have determined that we have one operating, as well as one reportable, segment. For impairment testing purposes, our individual clinics qualify as components of that operating segment. Because they have similar economic characteristics, the components are aggregated and deemed a single reporting unit. No impairment was identified during the years ended December 31, 2009 and 2008.
Recent Accounting Pronouncements
In January 2010, the FASB issued ASC Topic 820,Fair Value Measurements and Disclosures—Improving Disclosures About Fair Value Measurements. The ASU requires new disclosures about transfers into and out of Levels 1 and 2 and separate disclosures about purchases, sales, issuances, and settlements related to Level 3 measurements. It also clarifies existing fair value disclosures about the level of disaggregations and about inputs and valuation techniques used to measure fair value. The new disclosures and clarifications of existing disclosures are effective January 1, 2010, except for the disclosures about purchases, sales, issuances, and settlements relating to Level 3 measurements, which are effective January 1, 2011. Other than requiring additional disclosures, the adoption of this new guidance is not expected to have a material impact on our consolidated results of operations and financial position.
In August 2010, the FASB issued guidance that requires health care entities to use cost as the measurement basis for charity care disclosures and defines cost as the direct and indirect costs of providing charity care. The amended disclosure requirements are effective for fiscal years beginning after December 15, 2010 and must be applied retrospectively. The Company will adopt the amended disclosure requirements for their fiscal year beginning January 1, 2011. Since the new guidance amends disclosure requirements only, its adoption will not impact the Company’s statement of financial position, statement of operations, or cash flow statement.
In August 2010, the FASB issued ASU 2010-24 to address how health care entities should account for medical malpractice claims and related anticipated insurance recoveries. The new guidance, which amends ASC 954-450,Contingencies, and ASC 954-720,Other Expenses, also applies to similar contingent liabilities. Under the new guidance, health care entities are prohibited from presenting claim liabilities net of insurance recoveries. Further, the guidance requires health care entities to disregard insurance recoveries when measuring the amount of a claim liability. However, a related insurance receivable should be recorded
82
Table of Contents
concurrently with the liability, subject to a valuation allowance, if necessary. The new guidance is effective beginning January 1, 2011. In the period of adoption, entities must recognize in opening retained earnings any cumulative-effect adjustment resulting from the application of the new guidance. Entities may apply the new guidance retrospectively, and early application is permitted. We are currently assessing the impact this may have on our consolidated financial statements.
Quantitative and Qualitative Disclosure About Market Risk
Our investments include cash and cash equivalents. Cash and cash equivalents consist of cash, money market accounts, certificates of deposit and commercial paper. The primary objective of our investment activities is to preserve principal while maximizing income without significantly increasing risk. We do not enter into investments for trading or speculative purposes. Our investments are exposed to market risk due to a fluctuation in interest rates, which may affect our interest income and the fair market value of our investments. Due to the short-term nature of our investment portfolio, we do not believe an immediate 10% increase in interest rates would have a material effect on the fair market value of our portfolio, and therefore we do not expect our operating results or cash flows to be materially affected to any degree by a sudden change in market interest rates.
Interest Rate Risk
Our New Revolving Credit Facility contains multiple interest rate options which allow us to choose between a U.S. prime rate based interest rate or a London Interbank Offered Rate based interest rate. As of September 30, 2010, there were no borrowings under our New Revolving Credit Facility. If we were to draw on it, then we would be subject to changes in interest rates. As of September 30, 2010, we did not maintain any interest rate swap agreements.
Inflation Risk
We do not believe that inflation has had a material effect on our business, financial condition or results of operations. If our costs were to become subject to significant inflationary pressures, we may not be able to fully offset such higher costs through price increases. Our inability or failure to do so could harm our business, financial condition and results of operations.
83
Table of Contents
Our Company
We are a national provider of kidney dialysis services for patients suffering from chronic kidney failure, also known as end stage renal disease, or ESRD. As of September 30, 2010 we owned and operated 88 dialysis clinics treating more than 6,100 patients in 17 states and the District of Columbia. Our operating model is based on shared ownership of our facilities with physicians, known as nephrologists, who specialize in kidney-related diseases in the local market served by the clinic. Each clinic is maintained as a separate joint venture, or JV, in which we own a controlling interest and our local nephrologist partners own noncontrolling interests.
We believe we are the largest dialysis services provider in the United States founded and operating exclusively on a joint venture model. We have invested significantly in the development of our senior and field level core management function and operating procedures, and we believe we are well-positioned to continue to realize the benefits of platform scalability, which will allow us to add clinics without significant incremental operating costs. As a result, our revenue and earnings growth will be driven primarily by the growth in the number of our JV clinics and treatment growth at our existing clinics. Since our founding, we have opened 64 new clinics (“de novo clinics”) and have acquired 21 clinics from third parties (“acquired clinics”) that we subsequently converted to our JV model. In 2009 we opened 10 clinics, seven of which are de novo clinics and three are acquired clinics. We plan to continue the expansion of our operations with the use of the JV model primarily through the redeployment of free cash flow to create de novo clinics, to expand existing clinics and to selectively acquire clinics.
We believe our JV model is attractive to the nephrologist community because it provides a platform for favorable clinical outcomes and superior financial results while enabling our nephrologist partners to focus on providing the highest quality of patient care rather than the administration of corporate-directed treatments and protocols. Through our wholly-owned subsidiary, American Renal Management, LLC (“ARM”), we provide our nephrologist partners with the managerial, accounting, financial, technological and administrative support necessary to operate our clinics. In particular, our management services focus on critical revenue cycle management for each patient, which encompasses patient registration, insurance and benefit verification, medical treatment documentation and coding, bill preparation and collections. In addition, our corporate clinical advisory team, which includes our National Medical Director and clinical and regulatory advisors, assists our nephrologist partners in establishing clinical objectives, while our operating model allocates to our nephrologist partners autonomy over the clinical protocols used to achieve those objectives.
According to the United States Renal Data System, or USRDS, the number of dialysis patients in the U.S. is expected to continue growing faster than increases in the general population, primarily due to an aging population with increased life expectancies. As a result, we believe there will be an increasing need for dialysis clinics. In addition, we believe that the JV model will continue to be one of the fastest growing operating models for dialysis clinics and will represent an increasing percentage of the dialysis clinic industry. We believe that our JV model, with its clinic level autonomy for the physician, together with the comprehensive management services that we have developed, enhance the clinical and operating performance of our clinics and make us a preferred dialysis partner for nephrologists. We believe this will lead to additional JV opportunities with nephrologists seeking the corporate support we provide.
Dialysis Services Industry Overview
End Stage Renal Disease, or ESRD, is characterized by the loss of kidney functionality and is normally irreversible and fatal unless treated. ESRD most commonly results from complications associated with diabetes, hypertension, renal and hereditary diseases, old age and a combination of other risk factors. ESRD requires continued dialysis treatments or a kidney transplant to sustain life. Absent transplantation, the average life expectancy for an ESRD patient on dialysis is approximately five years following diagnosis, according to the USRDS. Scarcity of compatible kidneys has limited the option for transplants, causing most patients suffering
84
Table of Contents
from ESRD to rely on dialysis. Dialysis is the removal of toxins and fluids from the blood of ESRD patients by artificial means. Patients suffering from ESRD generally require dialysis at least three times per week, amounting to approximately 156 treatments per year, for the remainder of their lives.
There are two primary methods of dialysis commonly used today, hemodialysis and peritoneal dialysis. Hemodialysis, or the removal of toxins and fluid from the blood through a specially designed filter, is the most common form of ESRD treatment and represented approximately 92% of all dialysis treatments in the United States in 2007. Hemodialysis is typically performed in outpatient dialysis clinics and lasts approximately 3.5 hours per treatment. Treatments are usually performed by teams of licensed nurses and trained technicians pursuant to a physician’s instructions. Almost all patients receive hemodialysis in our outpatient dialysis clinics as their primary ESRD treatment, although we also provide such services in the home. Peritoneal dialysis uses the patient’s peritoneal, or abdominal, cavity to eliminate fluid and toxins. A patient generally performs peritoneal dialysis at home. We also provide peritoneal dialysis services to patients who prefer and are able to receive that form of treatment.
Large, Growing Market for Outpatient Dialysis Services
The total annual cost of providing healthcare services to ESRD patients in the U.S. in 2007 has been estimated by the USRDS to be approximately $35 billion, of which we believe approximately $18 billion represents our currently addressable market. According to the most recent report by the USRDS, there were approximately 527,000 ESRD patients in the United States as of December 31, 2007, with the number of ESRD patients expected to grow at an annual rate consistent with the historical rate of 3-4%, outpacing general population growth. Aside from a relatively small number of individuals who qualify for and successfully undergo kidney transplants, the vast majority of ESRD patients require three dialysis treatments per week, on average, for the remainder of their lives. USRDS data indicates that the prevalence rate in patients ages 65 to 74 increased 24% from 2000 to 2007; while, in patients ages 75 and older the prevalence rate increased 28% over the same period.
According to the USRDS, the increasing percentage of the U.S. population afflicted with ESRD has been primarily caused by:
| • | aging of the general population; |
| • | improved treatment and increased survival rate of patients with diabetes, hypertension and other illnesses that lead to ESRD; |
| • | growth rates of minority populations with higher than average incidence rates of ESRD; and |
| • | improved dialysis technology, that has enabled older patients and those who previously could not tolerate dialysis due to other illnesses, to benefit from this treatment. |
Reimbursement Environment
A majority of reimbursement for dialysis services is provided by the federal government’s Medicare ESRD program based on rates established by the Centers for Medicare and Medicaid Services, or CMS. The coverage criteria for dialysis centers under Medicare are well defined and, as a result, Medicare reimbursement for dialysis services has historically been more predictable than reimbursements for most other services and procedures. ESRD has been classified as a chronic disability since 1972 and patients are entitled to treatment and Medicare benefits regardless of age or financial circumstances. Generally, if a patient does not have employer-sponsored health coverage, Medicare becomes the primary payor either immediately or after a three-month waiting period. For a patient with employer-sponsored health coverage, Medicare generally becomes the primary payor after 33 months, which includes a three-month coordination of benefits period, or earlier if the patient’s employer-sponsored health coverage terminates. Additional sources of reimbursement for dialysis services include other government agencies such as state Medicaid programs as well as private pay options.
85
Table of Contents
Currently, Medicare reimburses dialysis providers separately for treatments and ancillaries. Recently approved Medicare legislation, however, will establish a “bundled” Medicare rate for dialysis reimbursement, starting in 2011. This new bundled rate will combine the current payments for composite rate and the separately billable dialysis services into a single base rate. This rate will include all services such as certain drugs and laboratory tests which are currently billed separately. The initial 2011 bundled rate will be set based on a 2% reduction in the payment rate that providers would have received under the historical fee for service payment methodology and based on the lowest average industry pharmaceutical utilization from 2007 to 2009. Beginning in 2012, the new single bundled payment base rate will be adjusted annually for inflation based upon a market basket index, less a productivity adjustment based on a 10-year moving average of productivity as projected by the Secretary of Health and Human Services. The bundled payment rate will be determined by the Secretary of Health and Human Services, who will have discretion to determine the base payment rate based on the goods and services included in the bundled rate. Dialysis providers, including us, will have the option to move fully to the bundled payment system in 2011 or to phase in the payment system over four years on a facility by facility basis. We believe that the move to bundled payment, together with the introduction of automatic annual rate increases of 1.5% per year, will result in greater predictability of rates and stability of revenue for the Company. The Congressional Budget Office estimates that these changes will result in an additional $1.5 billion in government payments to the dialysis industry over the next 10 years, which is directly related to the automatic annual rate increases.
Before Medicare becomes the primary payor, a patient’s commercial insurance plan, if any, is responsible for payment of the dialysis services provided. Although commercial payment rates vary significantly, average commercial payment rates are generally significantly higher than Medicare rates and often include price increases. Payment methods from commercial payors include a single lump-sum per treatment, referred to as bundled rates, and separate payments for ancillary treatments and pharmaceuticals, if used as part of the dialysis treatment, referred to as fee for service rates. We believe commercial payors are generally sensitive to the special circumstances and challenges faced by ESRD patients, particularly because they are responsible for primary coverage for a relatively limited number of ESRD patients for a finite 30-month period of time. We also believe commercial payors are incentivized to facilitate quality dialysis care because patients who do not regularly receive dialysis treatment typically require hospitalization and other more costly acute care treatment. For the year ended December 31, 2009, we derived approximately 43% of our net operating revenues from commercial payors, which represented approximately 14% of the treatments performed. The source of the balance of our treatments and net operating revenues come from Medicare and other governmental payors.
Our Operations
Our JV Operating Model
We believe we are the only dialysis service provider founded and operating exclusively on a shared-ownership model. Each of our clinics is maintained as a separate joint venture in which we own a controlling interest typically between 51% and 75%, and our nephrologist partners, who may be single practitioners or an affiliated group of nephrologists, own the noncontrolling interest. As of December 31, 2009, on average we owned a 55% interest in our clinics and our nephrologist partners owned a 45% interest.
Our operating model stresses the following ideals for our company:
| • | Take excellent care of the patients and the financial success will follow; |
| • | Enable the nephrologist to practice as he/she deems appropriate; |
| • | Provide the nephrologist the autonomy to make operational decisions; |
| • | Acknowledge that clinic staff members are a critical and valuable asset; do everything possible to hire and retain the best possible staff; |
| • | Listen to the practitioners and provide the tools needed to take excellent care of their patients; and |
| • | The corporate office works for our staff, our doctors and patients. |
86
Table of Contents
Our relationships with physicians and other referral sources relating to our joint ventures are required to comply with the anti-kickback statute. Although there is a safe harbor for certain investment interests in “small entities,” it is not clear if any of our joint ventures satisfies all of the requirements for protection by this safe harbor. Under current law, physician joint ventures are not prohibited but instead require a case-by-case evaluation under the anti-kickback statute. We have structured our joint ventures to satisfy as many safe harbor requirements as we believe are reasonably possible and we believe that these investments are offered on a fair market value basis and provide returns to the physician investors only in proportion to their actual investment in the venture. We believe that our agreements do not violate the federal anti-kickback statute; however, since the arrangements do not satisfy all of the requirements for safe harbor protection, these arrangements could be challenged. See “Risk Factors—Our arrangements with our nephrologist partners and medical directors do not satisfy all the elements of a safe harbor to the federal anti-kickback statute and certain state anti-kickback laws and as a result may subject us to government scrutiny.”
As required by law, our clinics contract with a nephrologist partner or a group of affiliated nephrologists who are our nephrologist partners to provide medical director services at each of our clinics. Our medical director arrangements are typically for a ten-year term with provision for optional renewals at the end of the term.
Our JV clinics are most commonly de novo clinics. In a typical de novo clinic, the total capital required to develop the clinic is approximately $1.5 million, of which a portion is equity capital funded by us and our nephrologist partners in proportion to our respective ownership interests. Historically, the balance of such development cost was funded primarily through third-party debt financing that we and our nephrologist partners guaranty on a basis proportionate to our respective ownership interests, and in some cases through intercompany loans. We began to finance such balances primarily through intercompany loans in 2008 in part as a response to the unpredictability of the financial market at that time, and we expect to continue financing a significant portion of our clinical development costs through intercompany loans. Historically, our de novo clinics become EBITDA positive within an average of 12 months of opening. Once a clinic becomes EBITDA positive, the JV clinic begins to distribute excess cash to us and our nephrologist partners.
We equip our clinics with technologically advanced dialysis equipment and amenities. Our clinics generally contain between 15 and 20 dialysis stations, one or more nurses’ stations, a patient waiting area, examination rooms, a supply room, a water treatment space to purify water used in hemodialysis treatments, a dialyzer reprocessing room, staff work areas, offices and a staff lounge. Our clinics are also typically outfitted with amenities including personal cable televisions and fully reclining chairs.
Each of our JVs is organized as a limited liability company, typically organized in the state in which the clinics are located. Although the terms on which each JV is owned and operated vary to some extent, our JV arrangements have many common features. See “—JV Operating Agreements” and “—Management Services.”
Our Services
As of September 30, 2010, we owned and operated 88 dialysis clinics treating more than 6,100 patients in 17 states and the District of Columbia. As required by law, our clinics contract with a nephrologist partner or a group of affiliated nephrologists who are our nephrologist partners to provide medical director services at each of our clinics. The duties of the medical director generally include responsibility for the oversight of the clinic, its staff, patient care and treatment. In addition, other nephrologists may apply for practice privileges to treat their patients at our centers.
In addition to a medical director, each clinic has a clinic manager, typically a registered nurse, who supervises the day-to-day operations of the center and its staff. The staff of each center typically consists of registered nurses, patient care technicians, a social worker, a registered dietician, biomedical technician support and other administrative and support personnel.
87
Table of Contents
Many of our outpatient dialysis centers offer services for dialysis patients who prefer and are able to receive either hemodialysis treatments in their homes or peritoneal dialysis. Home-based dialysis services consist of providing equipment and supplies, training, patient monitoring, on-call support services and follow-up assistance. Registered nurses train patients and their families or other caregivers to perform either hemodialysis at home or peritoneal dialysis.
Under Medicare regulations, we cannot and do not promote, develop or maintain any kind of contractual relationship with patients that would directly or indirectly obligate a patient to use or continue to use our dialysis services, or which would give us any preferential rights other than those related to collecting payments for our services.
Location and Capacity of Our Clinics
As of September 30, 2010 we owned and operated 88 dialysis clinics treating more than 6,100 patients in 17 states and the District of Columbia, each of which is consolidated in our financial statements. The locations of these clinics at September 30, 2010 were as follows:
State | Centers | State | Centers | State | Centers | |||||||||||
Florida | 23 | Colorado | 5 | Louisiana | 1 | |||||||||||
Ohio | 11 | Virginia | 4 | South Carolina | 1 | |||||||||||
Rhode Island | 9 | Maryland | 2 | California | 1 | |||||||||||
Pennsylvania | 9 | Texas | 3 | District of Columbia | 1 | |||||||||||
Massachusetts | 7 | Illinois | 2 | Missouri | 1 | |||||||||||
Georgia | 5 | Wisconsin | 2 | Michigan | 1 | |||||||||||
| TOTAL | 88 | |||||||||||||||
We have developed our clinics in a manner that we believe promotes high-quality patient care. Our typical clinic is approximately 6,500 to 7,000 square feet. We select clinic locations to maximize appeal and convenience based on demographic and other factors. One of the primary considerations in selecting a clinic location is finding an area with an existing patient base under the current treatment of local nephrologists interested in working with a dialysis clinic. Other considerations include:
| • | the types of commercial insurance in the area and the dialysis patient census they serve; |
| • | the availability and cost of qualified and skilled personnel, particularly nursing and technical staff; |
| • | the size and condition of the proposed clinic and its equipment; |
| • | the atmosphere for the patients; |
| • | the area’s demographics and population growth estimates; |
| • | state regulation of dialysis and healthcare services; and |
| • | the existence of competitive factors such as existing outpatient dialysis clinics within reasonable proximity to the proposed clinic. |
We are able to increase our capacity by extending hours at our existing centers, expanding our existing centers, relocating our centers, developing new centers and by acquiring centers. The development of a typical de novo clinic by us generally requires approximately $1.5 million for leasehold improvements, equipment and first-year working capital. Based on our experience, a de novo clinic typically opens within a year after the property lease is signed and normally achieves operating profitability in the first 12 months after certification. Acquiring an existing clinic or group of clinics requires a substantially greater initial investment, but profitability and cash flow are generally initially more predictable.
Some of our dialysis centers are operating at or near capacity. However, we believe that we have adequate capacity within most of our existing dialysis centers to accommodate additional patient volume through increased hours and/or days of operation, or, if additional space is available within an existing facility, by adding dialysis stations.
88
Table of Contents
All of our clinics lease their space on terms that we believe are customary in the industry. We can usually relocate existing centers to larger facilities or open new centers if existing centers reach capacity. With respect to relocating centers or building new centers, we believe that we can generally lease space at economically reasonable rates in the areas planned for each of these centers, although there can be no assurances in this regard. Expansion of existing centers or relocation of our dialysis centers is subject to review for compliance with conditions relating to participation in the Medicare ESRD program. In states that require a certificate of need or center license, additional approvals would generally be necessary for expansion or relocation.
Medical Directors And Other Nephrologists
In order for our clinics to be eligible to participate in the Medicare ESRD program, a qualified physician or group of physicians must act as medical director for each our clinics. We engage practicing, board-certified or board-eligible nephrologists to serve as medical directors. Medical directors also own a noncontrolling interest in the clinic as a result of our JV model. Medical directors are responsible for:
| • | supervising medical aspects of a clinic’s operations; |
| • | administering and monitoring patient care policies; |
| • | administration of dialysis treatments; |
| • | administration of staff development and training programs; and |
| • | assessment of all patients. |
Our medical directors are given autonomy and play an important role in quality assurance activities at our clinics and in coordinating the delivery of care. In addition, at some clinics one or more other nephrologists serve as assistant or associate medical directors or direct specific programs, such as home dialysis training programs. Our medical directors, as well as assistant or associate medical directors, receive fair market value compensation for their services. We base this compensation on independent third-party valuations. Our medical director arrangements are typically for a ten-year term with provision for optional renewals at the end of the term. Our medical director arrangements also include restrictions similar to those of other dialysis service providers that restrict our medical directors from competing with us. These agreements not to compete restrict the physicians from owning or providing medical director services to other dialysis clinics, but do not prohibit our medical directors from providing direct patient care services at other locations. Consistent with federal and state law, such agreements do not require our medical directors to recommend our dialysis clinics to their patients.
Local nephrologists are a key factor in the success of our clinics. Caring for ESRD patients is typically the primary clinical activity of a nephrologist, although a nephrologist may have other clinical activities including the post-surgical care of kidney transplant patients and the diagnosis, treatment and management of kidney disorders other than ESRD. Most nephrologists practice in small single-specialty groups covering a relatively large geographic service area. Most nephrologists also have a significant office practice, consult on numerous hospitalized patients who are not on dialysis and follow the progress of kidney transplant patients. An ESRD patient generally seeks treatment at a clinic where his or her nephrologist has privileges to admit patients. Nephrologists at our clinics typically include our nephrologist partners, as well as other nephrologists that apply for and receive practice privileges to treat their patients at our clinics. As of December 31, 2009, there were 218 nephrologists with privileges to practice at one or more of our clinics.
JV Operating Agreements
Many of the features of our JVs are set forth in agreements we enter with our clinics and our local nephrologist partners. Agreements that we typically enter into in connection with our clinics include a joint venture operating agreement and a management services agreement pursuant to which we provide various support services to our clinics. See “—Management Services” below. These agreements allocate ownership, rights and responsibilities in our clinics. These agreements also provide, among other things, for distributions on
89
Table of Contents
the ownership interests in our JVs. Under these JV agreements, the JV’s net profits, if any, or net losses, if any, are typically distributed no less often than annually in proportion to holdings of membership interests. These distributions are made out of the JV’s net cash flows in an amount determined by a majority in interest of the JV members (the Company, as majority member in the JV clinics, can therefore typically determine the distribution amount). Because distributions are limited to net cash flow available, the JV clinics are generally unable to distribute amounts that would result in the JV having insufficient capital to pay debt, interest obligations or general operating expenses or have insufficient working capital reserves. In addition, statutory restrictions including state laws, may prevent the JV clinics from distributing amounts that would result in the JV becoming insolvent. Our JV arrangements do not require the consent of our nephrologist partners prior to the making of any distributions from our JVs, so long as a pro rata distribution is made to our partners with one exception that we do not believe is material.
The operating agreements for some clinics give the nephrologist partners put rights that, when exercised, require us to purchase some or all of that nephrologist partner’s interest in that clinic. Some of these put rights are exercisable upon certain dates, and some are exercisable or accelerable upon the occurrence of certain events, including the transfer of a majority of ARA’s equity interest to a third-party. Nephrologist partners in certain of our clinics have the right to accelerate the exercise date of certain time-based puts. The Transactions triggered the acceleration of the exercise date of the put rights of certain of our nephrologist partners, but these put rights were waived by our partners having such rights.
From time to time our operating agreement may provide that we cannot take certain specified actions affecting a clinic without the consent of the nephrologist partner(s) for that clinic. Such actions may include (i) a sale, transfer, liquidation or reorganization of all or substantially of the clinic, a merger or dissolution of the clinic, (ii) a lease of all or substantially all of the clinic, (iii) the admission of a new or substituted member, (iv) an amendment or modification of the applicable operating agreement or the constituent documents for the clinic, (vi) certain transactions with affiliates, (vii) any capital calls except to the extent specifically provided, (viii) any hiring or firing of employees of the clinic, (ix) entering into borrowing on behalf of the clinic for a principal amount exceeding a specified principal amount, and (x) entering into any lease agreements on behalf of the clinic where annual payments exceed a specified amount.
Management Services
Our executive and senior management team operates out of our Beverly, Massachusetts headquarters. Executive management located at our corporate headquarters includes our Chairman, Chief Executive Officer, President, Chief Financial Officer and General Counsel. Other corporate staff includes personnel responsible for the management of operations, clinical and regulatory services, corporate compliance, technical services, project management and business development. Our national medical director and five regional directors are dispersed geographically throughout the United States.
Our corporate management is focused on supporting the operation of our dialysis clinics and the practices of our nephrologists. Through ARA, we enter agreements to provide management services to our clinics. For these services, we are typically entitled to the greater of $50,000 or 4-7% of the clinic’s net revenues. In addition, depending on state law, these agreements typically provide for us to employ clinical staff and provide them to the clinic. Our management agreements are typically for a ten-year term with provision for optional renewals at the end of the term. Pursuant to these agreements we provide our nephrologist partners with all of the managerial, accounting, financial, technological and administrative support necessary to operate our clinics, which enables our nephrologist partners to focus on delivering high quality healthcare. We strive to improve the clinical outcomes and operating and financial performance of our dialysis clinics, ensure compliance with applicable laws and regulations, and identify opportunities that are consistent with our growth strategy. The management services we provide to our clinics generally include:
| • | supervising site searches and negotiating leases; |
90
Table of Contents
| • | obtaining and maintaining licenses, permits and certifications; |
| • | providing manuals, policies and procedures; |
| • | performing payroll processing, personnel and benefit administration, billing and collection and payment of accounts receivable; |
| • | providing staff training programs; |
| • | recommending and purchasing or leasing equipment; |
| • | preparing and filing cost reports; |
| • | preparing annual operating budgets; |
| • | administering financial and clinical information systems; |
| • | procuring and maintaining insurance policies; |
| • | performing legal and compliance services; |
| • | providing strategic marketing; and |
| • | employing clinical staff. |
Quality Care
Our corporate management team promotes a patient and physician-focused corporate culture and other founding philosophies. We believe our culture and founding principles improve the clinical outcomes and operating performance of our dialysis clinics, and ensure our clinics’ compliance with applicable laws and regulations. For example, we believe that our culture of compliance, implemented by facilitating internal compliance audits, compliance hotlines, HIPAA compliance safeguards, as well as through management services such as manuals, policies and procedures and training, has contributed to our clinics’ strong track record in regulatory matters.
We maintain a monthly schedule with senior management and our national medical director to review clinical outcomes on a clinic by clinic basis and individual planning for continuous improvement. Our clinical team works routinely with individual physicians, clinic managers, and dieticians in an effort to optimize clinical outcomes such as anemia management, adequacy of the dialysis treatment (KT/V), nutrition (albumin levels), arterial venous fistula (AV fistula), and other important indicators. Based on the review of outcomes data, action plans, including clinical programs and educational offerings, are developed and implemented. The company has created a clinical ladder system that is used to track key performance data and effect improvement. We believe this system encourages clinic managers to strive for excellence, thereby enhancing quality of care and improving patient outcomes.
EPO and Other Pharmaceuticals
Patients receiving dialysis are also typically administered one or more pharmaceuticals and supplements. Patients are commonly treated with EPO. EPO is a genetically engineered form of a naturally occurring protein that stimulates the production of red blood cells. EPO is used in connection with all forms of dialysis to treat anemia, a medical complication most ESRD patients experience. Anemia involves a shortage of oxygen-carrying red blood cells. Because red blood cells bring oxygen to all the cells in the body, untreated anemia can cause severe fatigue, heart disorders, difficulty concentrating, reduced immune function, and other problems. Anemia is common among renal patients, caused by insufficient erythropoietin, iron deficiency, repeated blood losses, and other factors. Patients are also commonly treated with vitamin D analogs and iron supplements.
EPO is produced by a single manufacturer, Amgen, and any interruption of supply or product cost increases could adversely affect our operations. We purchase EPO from Amgen through a group purchasing organization that negotiates the terms on which we purchase EPO. We have entered into agreements with our group purchasing organization and Amgen that provide for specific rebates based on a combination of factors, including process improvement and data submission.
91
Table of Contents
Amgen has also developed and obtained U.S. Food and Drug Administration, or FDA, approval for Aranesp®, a pharmaceutical used to treat anemia that may replace EPO or reduce its use with dialysis patients. Roche has also developed a drug, Mircera®, used to treat anemia, although it is currently unavailable to patients in the United States due to Amgen’s patent on EPO.
Unlike EPO, which is generally administered in conjunction with each dialysis treatment, Aranesp® can be administered less frequently. In the event that alternatives to EPO are marketed for treatment of dialysis patients, we may realize lower margins on the administration of these pharmaceuticals than are currently realized on EPO. A significant increase in the development and use of other or similar alternatives to EPO, or a change in administration practices, could have a material impact on our operating results.
Over the past few years there has been significant media discussion and government scrutiny regarding anemia management practices for ESRD patients in the United States, mainly as a result of clinical studies that identified risks in patient populations related to the utilization of EPO and similar pharmaceuticals. As a result, the FDA required warning labels for EPO and Aranesp® and changes were made to EPO reimbursement and payment coverage policies. As new information is obtained from research and clinical trials, practice guidelines may change over the next several years. Even though we believe that our anemia management practices have been compliant with existing laws and regulations, we may be subject to further inquiries from a variety of government bodies as these payment policies and practicing guidelines evolve. In addition, reimbursement rates for the administration of EPO and other pharmaceuticals may change in connection with the implementation of bundled reimbursement rates for dialysis services beginning in 2011.
Competition
The dialysis industry is highly competitive. Because of the ease of entry into the dialysis business and the ability of physicians to be medical directors for their own clinics, competition for growth in existing and expanding markets is not limited to large competitors with substantial financial resources. According to the USRDS, there were in excess of 5,200 dialysis clinics in the United States at the end of 2009. We face competition from large and medium-sized providers for the identification and retention of patients and for the acquisition of existing dialysis clinics. We face particularly intense competition for the identification and retention of nephrologists, whether as physicians, medical directors or physician partners. In many instances, our competitors have taken steps to include comprehensive non-competition provisions within their medical director agreements, thereby limiting the ability of physicians to serve as medical directors or potential joint venture partners for competing dialysis clinics. These non-competition provisions often contain both time and geographic limitations during the term of the agreement and for a period of years thereafter. Our competitors have threatened and filed litigation seeking to intervene in our relationships with nephrologists based on alleged violations of these non-competition provisions. The ability to successfully attract and retain nephrologist partners and referring physicians is critical to increasing the number of treatments we perform at our clinics which, in turn, is key to driving revenue growth.
The dialysis services industry has undergone rapid consolidation. As of 2007, Fresenius Medical Care, or Fresenius, and DaVita Inc., or DaVita, accounted for approximately 63% of outpatient dialysis patients in the United States. The largest not-for-profit provider of dialysis services, Dialysis Clinic, Inc., accounted for approximately 0.5% of outpatient dialysis patients in the United States. A number of small and medium sized dialysis companies, including our company, collectively account for the remaining outpatient dialysis patients in the United States, with no company accounting for more than 5% of these outpatient dialysis patients.
Because services to the majority of patients in the United States are primarily reimbursed under government programs, competition for patients is based primarily on quality and accessibility of service and the ability to obtain referrals from physicians and hospitals. However, extension of periods during which commercial insurers are primarily responsible for reimbursement and the growth of managed care has placed greater emphasis on service costs for patients with private insurance coverage.
92
Table of Contents
Reimbursement
We derive our revenues from providing both outpatient and inpatient dialysis treatments as well as from ancillary services such as administering dialysis-related pharmaceuticals. The sources of these revenues are principally government-based programs, including Medicare, Medicaid and Medicare-certified HMO plans and commercial insurance plans.
Medicare Reimbursement
Under the Medicare ESRD program, the payment methodology for dialysis services is established by Congress. The Medicare composite rate, currently set by CMS, pays freestanding dialysis clinics for services provided to Medicare beneficiaries under two methods:
| • | the composite payment which includes a base payment, adjusted for case-mix that links payments more closely with illness severity and regional geography differences, and a drug add-on payment, which is updated annually to account for changes in drug prices and utilization; and |
| • | separately billable ancillary services such as certain physician-ordered tests and pharmaceuticals. |
Accordingly, clinics receive a composite payment rate per treatment to cover routine dialysis services, certain pharmaceuticals, routine lab work, and other supplies, as well as a separate payment for some pharmaceuticals, which include EPO, vitamin D analogs and iron supplements that are not included in the composite payment rate. Included in the composite rate payment is a drug add-on adjustment for certain pharmaceuticals, which is based upon average acquisition costs for certain pharmaceuticals used to treat patients with ESRD. Other pharmaceuticals are reimbursed at their average sale price, or ASP, plus 6% based upon prices set by Medicare.
A majority of dialysis patients are covered under Medicare. Dialysis patients become eligible for primary Medicare coverage at various times, depending on their age or disability status, as well as whether they are covered by an employer group health plan. Generally, for a patient not covered by an employer group health plan, Medicare becomes the primary payor either immediately or after a three-month waiting period. For a patient covered by an employer group health plan, Medicare generally becomes the primary payor after 33 months, which includes the coordination of benefits period, or earlier if the patient’s employer group health plan coverage terminates. When Medicare becomes a patient’s primary payor, the payment rate for that patient shifts from the employer group health plan rate to the Medicare payment rate.
For each covered treatment, Medicare pays 80% of the amount set by the Medicare system. The patient is responsible for the remaining 20%. In most cases, a secondary payor, such as Medicare supplemental insurance, a state Medicaid program or a commercial health plan, covers all or part of these balances. Some patients, who do not qualify for Medicaid but otherwise cannot afford secondary insurance, can apply for premium payment assistance from charitable organizations through a program offered by the American Kidney Fund. If a patient does not have secondary insurance coverage, we endeavor to collect payment from the patient using reasonable collection efforts consistent with federal and state law. However, in these cases we are generally unsuccessful in collecting from the patient the 20% portion of the composite rate that Medicare does not pay.
During the year ended December 31, 2009, the Medicare ESRD dialysis reimbursement rates for patients at our clinics were between $126 and $154 per treatment, excluding the administration of separately-billable pharmaceuticals, such as EPO. However, Congress and CMS have addressed the impact of inflation more consistently since 2000, with several increases in the composite rate having occurred through April 2007. In July 2008, the Medicare Improvements for Patients and Providers Act of 2008, or MIPPA, was passed by Congress. This legislation introduced a new payment system for dialysis services beginning in January 2011 whereby ESRD payments will be made under a bundled payment rate which will provide for a fixed rate for all goods and services provided during the dialysis treatment, including laboratory services, the administration of pharmaceuticals and payments for pharmaceuticals such as EPO that are separately billed under the current methodology. On July 23, 2010, CMS released the final rule regarding the new bundled payment rate system. We
93
Table of Contents
are still evaluating the various components of the new bundled payment rate system, however, we noted that the initial 2011 bundled rate includes reductions of 2% and 3.1%, respectively, to conform to the provisions of MIPPA and to establish budget neutrality. Further there is a 5.94% reduction tied to an expanded list of case mix adjustors which can be earned back based upon the presence of these co–morbidities at the time of treatment. There are other provisions which may impact payment including an outlier pool and a low volume facility adjustment. Further the new rule requires dialysis facilities to provide new services within the payment bundle such as oral vitamin D medications and an expanded list of laboratory tests. Historically these services were separately billable; now the dialysis facility will be at risk for utilization with reimbursement set at a fixed rate. With regard to the expanded list of case–mix adjustors, these may be difficult or impossible for our dialysis clinics or billing and other systems to document and track resulting in a reduction in the amounts of the payments that we would otherwise be entitled to receive. The rule also requires the new single bundled payment base rate to be adjusted annually for inflation based upon a market basket index, less a productivity adjustment, beginning in 2012. Also, beginning in 2012, the rule provides for up to a 2% withhold that can be earned back by facilities that meet certain defined clinical performance standards.
Medicaid Reimbursement
Medicaid programs are state-administered programs partially funded by the federal government. These programs are intended to provide health coverage for patients whose income and assets fall below state-defined levels and who are otherwise uninsured. These programs also serve as supplemental reimbursement sources for the co-insurance payments due from Medicaid-eligible patients with primary coverage under Medicare. Some Medicaid programs also pay for additional services, including some oral medications that are not covered by Medicare. We are an authorized Medicaid provider in all of the states in which our clinics are located.
Commercial Insurance
Before Medicare becomes the primary payor, a patient’s employer group health plan or private insurance plan, if any, is responsible for payment. Although commercial payment rates vary, average commercial payment rates are generally higher than Medicare reimbursement rates. Commercial payment rates are the result of negotiations between us and insurers or third-party administrators. We are continuously in the process of negotiating agreements with our commercial payors and if our negotiations result in overall commercial rate reductions in excess of our commercial rate increases, our revenues and operating results could be negatively impacted. Payment methods include a single lump-sum per treatment, referred to as bundled rates, and separate payments for treatments and pharmaceuticals, if used as part of the treatment, referred to as fee for service rates. In certain circumstances, we may bill commercial payors as out-of-network providers.
Reimbursement for EPO and Other Pharmaceuticals
Over the past several years, CMS has changed its reimbursement and payment coverage policies for EPO. Effective April 2006, CMS implemented a policy for monitoring the dosage of EPO based on the patient’s hematocrit level and for limiting the quantity of EPO that can be administered in any one month. This policy restricts payments based on EPO doses and hemoglobin levels for certain patients and has resulted in overall decreases in the amount of reimbursement payments that we have received from CMS. In addition, effective January 1, 2008, CMS implemented changes to the existing EPO monitoring policy that further limited reimbursement payments.
Congress passed the Medicare Improvements for Patients and Providers Act of 2008 in July 2008. This statute provides for marginal increases in the dialysis composite rate for 2009 and 2010, but also establishes “bundled” payments for goods and services provided during a dialysis treatment beginning in 2011. By statute, the calculation of the new dialysis bundled rate will include, among other items and services, EPO and other erythropoiesis stimulating agents.
94
Table of Contents
Government Regulation
Our operations are subject to extensive federal, state and local governmental regulations. These regulations require us to meet various standards relating to, among other things, government payment programs, dialysis clinics and equipment, management of clinics, personnel qualifications, maintenance of proper records and quality assurance programs and patient care.
We have structured our activities and operations to avoid conflict with state law restrictions on the corporate practice of medicine, which generally prohibit non-licensed persons or corporations from employing physicians to practice medicine, restrict the delivery of medical services to those entities owned and controlled only by licensed professionals and prohibit the division or splitting of professional fees between licensed medical doctors and non-licensed individuals or entities. Neither we nor the JVs directly employ physicians, but rather establish relationships on an independent contractor basis through our Medical Director Agreements. Further, we have structured all of our corporate and operational agreements to conform to any licensure requirements, fee-splitting and related corporate practice of medicine prohibitions.
Because we are subject to a number of governmental regulations, our business could be adversely impacted by:
| • | loss or suspension of federal certifications; |
| • | loss or suspension of licenses under the laws of any state or governmental authority from which we generate substantial revenues; |
| • | exclusion from government healthcare programs including Medicare and Medicaid; |
| • | significant reductions or lack of inflation-adjusted increases in payment rates by government programs or reduction of coverage for dialysis and ancillary services and related pharmaceuticals; |
| • | fines, damages and monetary penalties for federal anti-kickback law violations, Stark Law violations, submission of false claims, civil or criminal liability based on violations of law or other failures to meet regulatory requirements, and similar sanctions under applicable state laws; |
| • | claims by or on behalf of patients who believe their protected health information has been used or disclosed in violation of federal and state patient privacy laws, or enforcement fines or penalties for violations of these laws; |
| • | government mandated practice changes that significantly increase our operating expenses or materially alter relationships with our medical directors or nephrologist partners in our dialysis clinics; or |
| • | refunds of payments received from government payors and government healthcare program beneficiaries because of overpayments or any failures to meet applicable requirements. |
We expect that our industry will continue to be subject to substantial regulation, the scope and effect of which are difficult to predict. Our activities could be reviewed or challenged by regulatory authorities at any time in the future. This regulation and scrutiny could materially adversely impact us.
Licensure and Certification
Our clinics are certified by CMS, as is required for the receipt of Medicare payments. In some states, we are also required to secure additional state licenses and permits for our clinics. Governmental authorities, primarily state departments of health, periodically inspect our clinics to determine if we satisfy applicable federal and state standards and requirements, including the conditions of participation in the Medicare ESRD program.
To date, we have not experienced significant difficulty in obtaining certifications from CMS or in maintaining our licenses or our Medicare and Medicaid authorizations.
95
Table of Contents
CMS continues to study the regulations applicable to Medicare certification to provide dialysis services. In April 2008, CMS issued new regulations, referred to as Conditions for Coverage, for Medicare-certified ESRD clinics to provide dialysis services. The Conditions for Coverage were effective October 14, 2008, with some provisions having a phased-in implementation date of February 1, 2009 or later. The new regulations are patient, quality and outcomes focused. Among other things, the Conditions for Coverage establish performance expectations for clinics and staff, eliminate procedural requirements and promote continuous quality improvement and patient safety measures. We have established an interdisciplinary work group to facilitate implementation of the Conditions of Coverage and have developed comprehensive auditing processes, policies and procedures to monitor ongoing compliance. We continue to assess the impact these changes will have on our revenues, earnings and cash flows.
Federal Anti-kickback Statute
The federal anti-kickback statute contained in the Social Security Act imposes criminal and civil sanctions on persons who receive, make, offer or solicit payments in return for any of the following with respect to items or services that are paid for in whole or in part by Medicare, Medicaid or similar federal and state programs:
| • | the referral of a Medicare or Medicaid patient for treatment; |
| • | the ordering or purchasing of items or services; or |
| • | arranging for or recommending the ordering or purchasing of these items. |
Federal criminal penalties for the violation of these laws include imprisonment, fines and exclusion of the provider from future participation in the Medicare and Medicaid programs. Violations of the anti-kickback statute are punishable by imprisonment for up to five years, fines of up to $250,000 or both. Larger fines can be imposed upon corporations under the provisions of the U.S. Sentencing Guidelines and the Alternate Fines Statute. Individuals and entities convicted of violating the anti-kickback statute are subject to mandatory exclusion from participation in Medicare, Medicaid and other federal healthcare programs for a minimum of five years. Civil penalties for violations of these laws include up to $50,000 in monetary penalties per violation, repayments of up to three times the total payments between the parties and suspension from future participation in Medicare and Medicaid. Some state anti-kickback statutes also include criminal penalties. The federal statute expressly prohibits traditionally criminal transactions, such as kickbacks, rebates or bribes for patient referrals. Court decisions have also held that the statute is violated whenever one of the purposes of remuneration is to induce referrals.
The Department of Health and Human Services regulations create exceptions, known as safe harbors, for some business transactions and arrangements. Transactions and arrangements that satisfy every element of a safe harbor are deemed not to violate the anti-kickback statute. Transactions and arrangements that do not satisfy all elements of a relevant safe harbor do not necessarily violate the statute, but can be subject to greater scrutiny by enforcement agencies.
The medical directors for our clinics refer patients to our clinics. Accordingly, the agreements under which we engage our medical directors must be in compliance with the federal anti-kickback statute. Among the available safe harbors is one for personal services furnished for fair market value. However, most of our agreements with our medical directors do not satisfy all seven of the requirements of the personal services safe harbor. In particular, because of the nature of our medical directors’ duties, we believe it is impossible to satisfy the safe-harbor requirement that if the services are provided on a part-time basis, as they are with our medical directors, the agreement must specify the schedule of intervals of service, their precise length and the exact charge for these intervals. Accordingly, while we believe that our agreements with our medical directors satisfy as many of the elements of the personal services safe harbor as we believe is reasonably possible, our arrangements do not qualify for safe harbor protection. We also note that there is little guidance available as to what constitutes fair market value for medical director services. We believe that our medical director agreements do not violate the federal anti-kickback statute. However, since the arrangements do not satisfy all of the requirements for safe harbor protection, these arrangements could be challenged.
96
Table of Contents
We operate all our clinics in accordance with our JV model, in which we own a controlling interest in our clinics. Our relationships with our nephrologist partners and other referral sources relating to these JVs are required to comply with the anti-kickback statute. Although there is a safe harbor for investment interests in small entities, our JVs do not satisfy every element of this safe harbor. Under current law, physician JVs are not prohibited but instead require a case by case evaluation under the anti-kickback statute. We have structured our JVs to satisfy as many safe harbor requirements as we believe are reasonably possible and we believe that these investments are offered on a fair market value basis. Our JVs provide returns to our nephrologist partners only in proportion to their actual investment in the venture. We believe that our JVs do not violate the federal anti-kickback statute. However, since the arrangements do not satisfy every element of the applicable safe harbors, these arrangements could be challenged.
We finance the balance of the capital required to construct and operate the clinics through intercompany loans. Even though there is not a safe harbor for these loans, the intercompany loans do not necessarily violate the anti-kickback statute. They can, however, be subject to greater scrutiny by enforcement agencies. See “Risk Factor—Our arrangements with our nephrologist partners and medical directors do not satisfy all the elements of a safe harbor to the federal anti-kickback statute and certain state anti-kickback laws and as a result may subject us to government scrutiny.”
We lease space for approximately 16 of our clinics from entities in which physicians hold ownership interests and we sublease space to referring physicians at approximately 28 of our dialysis clinics. These arrangements must be in compliance with the anti-kickback statute. We believe that we meet the elements of the safe harbor for space rentals.
Because we purchase and sell items and services in the operation of our clinics that may be paid for, in whole or in part, by Medicare or a state healthcare program and because we acquire items and services at a discount, we must structure these arrangements in compliance with the federal anti-kickback statute. Subject to requirements and limitations, discounts representing reductions in the amounts we are charged or that we charge for items or services based on arm’s-length transactions can qualify for safe harbor protection if we fully and accurately report the discounts in the applicable invoices or Medicare cost reports. While some of the safe harbor elements are subject to interpretation, we believe that our vendor contracts that contain discount provisions are in compliance with the anti-kickback statute and the discount safe harbor.
Stark Law
Another federal law, known as the Stark Law, prohibits a physician who has a financial relationship, or who has an immediate family member who has a financial relationship, with entities, including ESRD providers, providing “designated health services,” from referring Medicare patients to these entities for the furnishing of these services, with limited exceptions. Designated health services under the Stark Law include durable medical equipment and supplies, home health services, outpatient prescription drugs, inpatient and outpatient hospital services and clinical laboratory services.
Dialysis services are not included within the definition of designated health services. However, clinical laboratory services, outpatient prescription drugs and inpatient hospital services sometimes are rendered in connection with dialysis. Accordingly, depending on the relationships between physicians and the providers of these designated health services associated with dialysis, the Stark Law could apply. There are several Stark Law exceptions that apply to these associated services. First, the Stark Law includes an exception for all services that are covered as part of a composite rate payment. Because reimbursement for dialysis services is primarily paid under a composite rate, all services included in those composite payments are excepted from the Stark Law prohibitions. The “bundled” Medicare rate for dialysis reimbursement, described under MIPPA, and which will be implemented in 2011, is a composite rate payment. Second, the Stark Law includes a specific exception for EPO and other dialysis-related drugs, which are identified on a list of Current Procedural Terminology, which is published by CMS and revised on an annual basis.
97
Table of Contents
The Stark Law also prohibits the entity receiving a prohibited referral from filing a claim or billing for the services arising out of the prohibited referral. The prohibition applies regardless of the reasons for the financial relationship and the referral. Therefore, unlike the federal anti-kickback statute, an intention to induce referrals is not required. Sanctions for violations of the Stark Law include denial of payment for the services provided in violation of the law, refunds of amounts collected in violation of the law, a civil penalty of up to $15,000 for each service arising out of the prohibited referral, exclusion from the federal healthcare programs, including Medicare and Medicaid, and a civil penalty of up to $100,000 against parties that enter into a scheme to circumvent the Stark Law. Violations of the Stark law also can form the basis for False Claims Act liability if a person acts with the requisite intent under the False Claims Act. The types of financial arrangements between a physician and an entity that trigger the self-referral prohibitions of the Stark Law are broad and include direct and indirect ownership and investment interests and compensation arrangements.
CMS has adopted regulations under the Stark Law implementing the Stark Law’s application to all designated health services. These are commonly referred to as the Stark II regulations.
In August 2008, CMS issued final changes to its Hospital Inpatient Prospective Payment Systems and Fiscal Year 2009 Rates final rules, or IPPS Rules. The IPPS rules contained significant changes to the Stark II regulations. One of these changes will prohibit compensation payable under space or equipment leases based on either:
| • | a percentage of revenues attributable to services performed in connection with the lease; or |
| • | a per unit of services charge to the extent that charge reflects services provided to patients referred by the lessor to the lessee. |
These changes became effective on October 1, 2009. While the final rule only affects equipment and space leases, CMS has indicated that they are concerned about other percentage based compensation arrangements, but at this point they have not proposed regulations to prohibit such arrangements.
In addition, the IPPS rules restrict the provision of certain services furnished “under arrangement” by a provider pursuant to an agreement with a hospital, effective October 1, 2009. Several of our JVs have agreements with acute care hospitals to provide dialysis services to the hospitals’ inpatients. As of October 1, 2009, if covered by the Stark Law, these arrangements likely would have had to be unwound pursuant to the IPPS rules. However, the IPPS rules and prior Stark II regulations contain an exception which allows the JVs to continue providing such services under the agreements with the hospitals. Specifically, dialysis services furnished by a hospital that is not certified to provide ESRD services under applicable law, are not considered designated health services for purposes of the Stark Law. Accordingly, the Stark Law prohibitions do not apply to these services. Thus, the physicians affiliated with the applicable JVs may continue to provide these services to hospital inpatients unless and until the Stark II regulations are modified to prohibit the provision of these services.
We believe that various exceptions under the Stark II regulations apply to our provision of dialysis services in our clinics. However, it is possible that CMS could interpret such exceptions not to apply to aspects of our operations. If that were the case, CMS could determine that the Stark II regulations require us to restructure existing compensation agreements with our medical directors and to repurchase or to request the sale of ownership interests in our JVs held by referring physicians or, alternatively, to refuse to accept referrals for designated health services from these physicians. If CMS were to interpret the Stark II regulations to apply to aspects of our operations and we were not able to achieve compliance with the Stark II regulations, it would have a material adverse effect on our operations.
If any of our business transactions or arrangements including those described above were found to violate the federal anti-kickback statute or the Stark Law, we could face criminal, civil and administrative sanctions, including possible exclusion from participation in Medicare, Medicaid and other state and federal healthcare programs. Any findings that we have violated these laws could have a material adverse impact on our earnings.
98
Table of Contents
Fraud and Abuse Under State Law
Many states in which we operate dialysis clinics have statutes prohibiting physicians from holding financial interests in various types of medical clinics to which they refer patients. Some states also have laws similar to the federal anti-kickback statute that may affect our ability to receive referrals from physicians with whom we have financial relationships, such as our medical directors or nephrologist partners. Some of these statutes include exemptions applicable to our medical directors and other physician relationships or for financial interests in our common stock or interests in our JVs. Some, however, include no explicit exemption for medical director services or other services for which we contract with and compensate referring physicians or for joint ownership interests of the type held by some of our referring physicians or for financial interests in our common stock. If these statutes change or are interpreted to apply to referring physicians with whom we contract for medical director and similar services, to our nephrologist partners or to physicians who hold interests in our common stock, we may be required to terminate or restructure some or all of our relationships with, purchase some or all of the ownership interests of, or refuse referrals from these referring physicians and could be subject to civil and administrative sanctions, refund requirements and exclusions from government healthcare programs, including Medicare and Medicaid. Such events could have a material adverse impact on our business.
Federal Laws Related to Fraud and False Statements Relating to Healthcare.
Federal laws, including HIPAA and the False Claims Act, make it unlawful to make false statements or commit fraud in connection with a health benefit program, including Medicare and Medicaid. These federal laws include a prohibition on false statements in connection with compliance with Medicare conditions for coverage, a prohibition on making false statements or submitting false documents in connection with a healthcare benefit program, and a prohibition on making or attempting to make a scheme or artifice to defraud any healthcare benefit program. Any violation of these laws may lead to significant penalties and may have a material adverse effect upon our business.
The False Claims Act
The federal False Claims Act is a means of policing false bills or false requests for payment in the healthcare delivery system. In part, the False Claims Act authorizes the imposition of civil penalties on any person who:
| • | knowingly presents or causes to be presented to the federal government, a false or fraudulent claim for payment or approval; |
| • | knowingly makes, uses or causes to be made or used, a false record or statement to get a false or fraudulent claim paid or approved by the federal government; |
| • | conspires to defraud the federal government by getting a false or fraudulent claim allowed or paid; or |
| • | knowingly makes, uses or causes to be made or used, a false record or statement to conceal, avoid or decrease an obligation to pay or transmit money or property to the federal government. |
Congress revised the False Claims Act in 2009 to make it unlawful knowingly to file a false claim with the federal government or with a government contractor that is spending money on the government’s behalf or to advance a government interest. In addition, Congress revised the False Claims Act to impose a duty on providers to repay to the federal government any overpayments that it receives from the federal government. A provider may incur substantial penalties for knowingly failing to repay an overpayment to the federal government. As amended, the False Claims Act requires that providers allocate resources to identify overpayments and to train employees on the potential repercussions of filing false claims to the federal government or government contractors and monitor employee actions to detect potential false claims.
The penalties for a violation of the False Claims Act range from $5,500 to $11,000 for each false claim plus three times the amount of damages caused by each false claim. The federal government has used the False
99
Table of Contents
Claims Act to prosecute a wide variety of alleged false claims and fraud allegedly perpetrated against Medicare and state healthcare programs, including coding errors, billing for services not rendered, the submission of false cost reports, billing for services at a higher payment rate than appropriate, billing under a comprehensive code as well as under one or more component codes included in the comprehensive code and billing for care that is not considered medically necessary. Although still subject to dispute, several courts have also determined that a violation of the federal anti-kickback statute or the Stark Law can form the basis for liability under the False Claims Act. In addition to the provisions of the False Claims Act, which provide for civil enforcement, the federal government can use several criminal statutes to prosecute persons who are alleged to have submitted false or fraudulent claims for payment to the federal government.
The Health Insurance Portability and Accountability Act of 1996
The Department of Health and Human Services, or HHS, has enacted extensive regulations under the Health Insurance Portability and Accountability Act of 1996, or HIPAA, relating to the privacy and security of medical information. These regulations require us to maintain extensive policies and procedures and to implement administrative, physical and technical safeguards with respect to protected health information, referred to as PHI, for which we are responsible. HIPAA regulations also include provisions relating to standards for the security of the storage and transmission of electronic-PHI. The regulations were strengthened significantly under the Health Information Technology for Economic and Clinical Health Act, known as the HITECH Act, enacted as part of the American Recovery and Reinvestment Act of 2009. Included in the HITECH Act are significant new requirements for the notification of patients, and other compliance actions, in the event of a breach with respect to unsecured PHI. HHS issued a new breach notification regulation on August 24, 2009, effective on September 23, 2009, that imposes significant notification requirements on covered entity healthcare providers, such as us, that experience breaches of unsecured PHI. On April 27, 2009, HHS provided guidance that set forth elective standards that provide for a “safe harbor” for rendering PHI secure such that an inappropriate use or disclosure involving such PHI would not be subject to the breach notification requirements. We have not yet taken action to meet the safe harbor described under this recently issued guidance. Thus, a breach involving unsecured PHI for which we are responsible, might be subject to the breach notification requirements, which would be expensive. Many of the other requirements set forth in the HITECH Act have not yet been implemented by regulations. If additional implementing regulations under the HITECH Act are especially burdensome, they could have a materially adverse impact on our operations.
State False Claims Laws
Many states have passed their own false claims laws, which generally mirror the False Claims Act and are designed to prevent false claims from being submitted to state healthcare programs and commercial insurers. Violations of these laws may result in monetary penalties or other sanctions to the violator. We believe that we are in material compliance with these laws and regulations. However, violation of these laws and the imposition of related consequences could have a materially adverse impact on our operations.
State Privacy and Medical Record Retention Laws
State patient privacy and confidentiality laws generally require providers to keep confidential certain patient information, including information contained in medical records. Violations of these laws could lead to monetary penalties against providers and sanctions against licensed individuals.
Similarly, medical record retention laws place a duty on providers to retain medical records for certain periods of time and dispose of records in a certain manner. Violations of these duties may result in sanctions from state agencies or from the Medicare program.
We believe that we are in material compliance with these laws and regulations. However, violation of these laws and the imposition of related consequences could have a materially adverse impact on our operations.
100
Table of Contents
Other Regulations
Our operations are subject to various state hazardous waste and non-hazardous medical waste disposal laws and regulations. These laws and regulations do not classify as hazardous most of the waste produced from dialysis services, although we can be subject to liability under both federal and state laws, as well as under contracts with those who haul our wastes, with respect to our waste disposal. Occupational Safety and Health Administration laws and regulations also apply to us, including, for example, those that require employers to provide workers who are occupationally subject to blood or other potentially infectious materials with prescribed protections. These requirements apply to all healthcare clinics, including dialysis clinics, and also require employers to determine which employees may be exposed to blood or other potentially infectious materials and to have in effect a written exposure control plan. In addition, employers are required to provide or employ hepatitis B vaccinations, personal protective equipment and other safety devices, infection control training, post-exposure evaluation and follow-up, waste disposal techniques and procedures and work practice controls, as well as comply with various record-keeping requirements. We are not aware of material liabilities affecting us under any of these requirements.
A few states have certificate of need programs regulating the establishment or expansion of healthcare clinics, including dialysis clinics.
As discussed below, we lease many and own several properties, all in the United States. If contamination is discovered in our buildings or in the surface or subsurface or in the groundwater beneath any of our facilities, whether leased or owned, we may be liable for the investigation or cleanup of the contamination and for damages arising out it, pursuant to applicable state and/or federal law and/or under the terms of our leases. Such liability may arise even when we do not cause or contribute to the contamination (for example, where it is caused by a prior occupant or a neighbor). We believe we take reasonable precautions to avoid contamination in or affecting our facilities, and we are not aware of any such issues that have resulted in material liability to us. We cannot assure you, though, that such conditions will not affect us in the future.
Corporate Compliance Programs
We have adopted and maintain an active corporate compliance program, including a plan document, corporate compliance officer, compliance hotline, the policies and procedures designed to ensure compliance with applicable healthcare laws and proper billing of claims, and employee training regarding such policies and procedures.
In addition, we have adopted and maintained a HIPAA compliance plan document, privacy and security officers, policies and procedures designed to ensure compliance with HIPAA and the privacy and security rules and employee training regarding such policies and procedures.
Properties and Clinics
Our corporate headquarters are located at 66 Cherry Hill Drive, Beverly, MA 01915-1072 in a 30,649 square foot leased portion of an office building. The lease for our headquarters expires on December 31, 2012 and includes a five year renewal option.
As of September 30, 2010 we had 88 dialysis clinics located in California, Colorado, Florida, Georgia, Illinois, Louisiana, Maryland, Massachusetts, Michigan, Missouri, Ohio, Pennsylvania, Rhode Island, South Carolina, Texas, Virginia, Washington, D.C. and Wisconsin. Our dialysis clinics range in average size from approximately 6,500 to 7,000 square feet. We own the land and buildings for one of our dialysis clinics, and our JVs own the land and buildings for two of our dialysis clinics. Our remaining dialysis clinics are located on premises that we lease. The majority of our dialysis clinics are leased under non-cancelable operating leases expiring in various years through 2023. Most lease agreements cover periods from five to ten years, and contain renewal options of five to ten years at the fair rental value at the time of renewal.
101
Table of Contents
Employees
As of September 30, 2010, we had 1,769 employees, consisting of 607 nurses, 771 patient care and equipment technicians and 391 other employees. Our 125 nephrologist partners are not our employees, nor are our medical directors, who are paid pursuant to their contractual arrangements. None of our employees are subject to collective bargaining agreements. We consider our relationships with our employees to be good.
Legal Proceedings
We are subject to a Decision and Order entered In the Matter of American Renal Associates Inc. and Fresenius Medical Care Holdings, Inc. by the Federal Trade Commission. The Decision and Order was entered in 2007 following a nonpublic, informal investigation by the Federal Trade Commission into proposed dialysis clinic acquisition activities in Rhode Island and the execution of an Agreement Containing Consent Order by the parties. The Decision and Order prohibits us for a period of ten years from September 7, 2007, without prior notice to the Federal Trade Commission from: (1) acquiring dialysis clinics located in ZIP codes in and around the cities of Cranston and Warwick, Rhode Island, and/or (2) entering into any contract to manage or operate dialysis clinics in ZIP codes in and around the cities of Cranston and Warwick. These prohibitions are subject to a number of exceptions that permit us to develop, own, manage or operate de novo dialysis clinics or dialysis clinics owned or operated as of the date the Decision and Order was entered, or to perform specified services, including offsite laboratory services, bookkeeping services, accounting services, billing services, supply services and purchasing and logistics services with the adherence to confidentiality requirements. We have complied and intend to continue to comply with the terms of the Decision and Order. We do not believe that compliance with the Decision and Order will have a material impact on our revenues, earnings or cash flows.
We are also subject to various claims and legal actions in the ordinary course of our business. Some of these matters include professional liability, employee-related matters and inquiries and investigations by governmental agencies regarding our employment practices. We are not aware of any pending or threatened litigation that we believe is reasonably likely to have a material adverse effect on us.
102
Table of Contents
MANAGEMENT AND BOARD OF DIRECTORS
The following table sets forth the name, age and position of each of our executive officers and directors as of September 30, 2010.
Name | Age | Position(s) | ||||
Joseph A. Carlucci | 57 | Chief Executive Officer and Director | ||||
Christopher T. Ford | 60 | Chairman of the Board of Directors | ||||
Syed T. Kamal | 58 | President and Director | ||||
John J. McDonough | 46 | Executive Vice President and Chief Financial Officer | ||||
Michael R. Costa | 40 | Vice President and General Counsel | ||||
Steven M. Silver | 42 | Director | ||||
Jared S. Hendricks | 30 | Director | ||||
Michael E. Boxer | 49 | Director | ||||
Christopher T. Ford, 60, Chairman of the Board of Directors. Mr. Ford is a founder, served as our Chief Executive Officer from our inception in 1999 to 2005 and has also served as Chairman of our Board of Directors since our inception. Mr. Ford has more than 37 years of experience in the dialysis services industry. Prior to founding our company, from 1989 to 1997, Mr. Ford served as President of Fresenius Medical Care’s International Services Division. From 1986 to 1989, Mr. Ford served as Senior Vice President of Operations for the Dialysis Services Division of Fresenius Medical Care. Mr. Ford holds a B.S. degree in Business Administration from Northeastern University.
Joseph A. Carlucci, 57, Chief Executive Officer and Director. Mr. Carlucci is a founder, served as our Chief Operating Officer and Treasurer from our inception in 1999 to 2005 and our Chief Executive Officer since 2006. Mr. Carlucci has more than 30 years of experience in the dialysis services industry. Prior to founding our company, Mr. Carlucci served as President and CEO of Optimal Renal Care, a JV in disease management between Fresenius Medical Care North America (FMCNA) and Kaiser Permanente of Southern California. Prior to that, Mr. Carlucci served as Vice President of Administration at Fresenius and was responsible nationally for managed care, medical director relations and facility development. He has operations experience from Facility Administrator to assumption of U.S. Operations at Fresenius. Mr. Carlucci holds a B.S. degree in Accounting from Bentley College.
Syed T. Kamal, 58, President and Director. Mr. Kamal is a founder, served as our Executive Vice President from our inception in 1999 to 2005 and has also served as our President and as a Director since our inception. Mr. Kamal has more than 30 years of experience in the dialysis services industry. Prior to founding our company, from 1997 to 1999, Mr. Kamal served as President of Fresenius Medical Care North America’s southern business unit. From 1995 to 1997, Mr. Kamal served as Vice President of Fresenius’ Operations, North America. From 1993 to 1995, Mr. Kamal served as Director and Vice President of Operations for Fresenius’ International division. From 1985 to 1993, Mr. Kamal served as Regional Manager of Fresenius’ Mid-Atlantic and Southeast regions (U.S.). From 1980 to 1985, Mr. Kamal served as Facility Administrator in Louisiana with Fresenius. Mr. Kamal holds B.A. and M.B.A. degrees from the University of Punjab in Pakistan.
John J. McDonough, 46, Executive Vice President and Chief Financial Officer. Mr. McDonough has served as our Executive Vice President and Chief Financial Officer since 2003. Mr. McDonough has more than 20 years of experience in accounting and finance. From 1998 to 2001, Mr. McDonough served as Vice President and Chief Accounting Officer at DaVita Inc. Prior to joining our company, from 1995 to 1997, Mr. McDonough served as Chief Financial Officer at Palatin Technologies, Inc. From 1990 to 1995, Mr. McDonough served as Chief Financial Officer at MedChem Products Inc. From 1986 to 1990, Mr. McDonough served as Audit Manager and held other positions at KPMG Peat Marwick. Mr. McDonough holds a B.S. degree in Accounting from Bentley College and a M.B.A. from the Harvard University Graduate School of Business Administration.
Michael R. Costa, Esq., M.P.H., 40, Vice President and General Counsel. Mr. Costa has served as our Vice President and General Counsel since 2007. Mr. Costa has more than 12 years’ experience as a corporate
103
Table of Contents
healthcare attorney. Prior to joining our company, from 2001 to 2005, Mr. Costa served as an Associate and, from 2006 to 2007, as Senior Counsel in the Health Business Group of Greenberg Traurig LLP. From 1999 to 2001, Mr. Costa served as an Associate Healthcare Attorney at Behar & Kalman in Boston, Massachusetts. Mr. Costa holds a B.S. degree in Legal Studies and Business Management from Roger Williams University, an M.P.H. from Boston University School of Public Health and a J.D. from Suffolk University Law School.
Steven M. Silver, 42, Director. Mr. Silver has been a Senior Managing Director at Centerbridge Partners, L.P. since 2006 where he focuses on originating and managing private equity and credit investments in the healthcare and industrial sectors. Prior to joining Centerbridge Partners L.P., Mr. Silver was a Managing Director and Partner of Vestar Capital Partners, a private equity investment firm. At Vestar, Mr. Silver was responsible for originating, negotiating and financing private equity investments in multiple industries including healthcare services and industrial and manufacturing as well as providing ongoing strategic direction to various portfolio companies. Mr. Silver began his career as a member of the Mergers and Acquisitions department of Wasserstein Perella & Co. in New York and London. While at Wasserstein Perella, Mr. Silver focused on managing the firm’s merchant banking portfolio as well as advising corporate clients on all aspects of mergers and acquisitions and corporate finance. Mr. Silver received a B.A. from Yale College and an M.B.A. with high distinction from Harvard Business School in 1995, where he was a George F. Baker Scholar. He currently serves on the Board of Directors of GSI Holdings Corp.
Jared S. Hendricks, 30, Director. Mr. Hendricks joined Centerbridge Partners, L.P. in 2006 and is a Managing Director responsible for originating, negotiating and financing private equity and credit investments across multiple industries. Prior to joining Centerbridge Partners L.P., Mr. Hendricks was an Associate at Silver Lake Partners, a private equity firm focused on investments in technology and related growth companies. Prior to Silver Lake, he was an investment banking analyst within the Global Industrial and Services group at Credit Suisse First Boston. Mr. Hendricks graduated summa cum laude from The Wharton School of the University of Pennsylvania, where he received a B.S. in Economics.
Michael E. Boxer, 49, Director. Mr. Boxer has been a Special Advisor to Centerbridge Partners L.P. since 2009. Mr. Boxer has served as President of The Enterprise Group, Ltd., a health care financial advisory firm, since 2008. Previously, Mr. Boxer served as the Chief Financial Officer of HealthMarkets, Inc., a provider of health and life insurance products to individuals and small groups. Mr. Boxer served as Executive Vice President and Chief Financial Officer of Mariner Health Care, Inc., a provider of skilled nursing and long-term health care services. Prior to Mariner, Mr. Boxer served as Senior Vice President and Chief Financial Officer of Watson Pharmaceuticals, Inc., a NYSE-listed specialty pharmaceuticals company. Mr. Boxer was an investment banker at Furman Selz, LLC, a New York-based investment bank. Mr. Boxer holds an M.B.A. from the University of Chicago Booth School of Business and a B.S. in Business Administration from Colorado State University. He is a Director of Skilled Healthcare Group Inc.
Except as indicated above, none of the directors holds any other directorship.
Pursuant to our bylaws, our directors are elected by ballot at the annual meeting of the stockholders, which election may take place by written consent. Our sole stockholder is C.P. Atlas Intermediate Holdings, LLC, the sole member of which is Parent. Each member of our current Board of Directors also serves as a director of Parent, although there is no requirement that persons who serve as directors of Parent also serve on our Board of Directors. Parent entered into an amended and restated stockholders agreement (the “New Stockholders Agreement of Parent”) on June 28, 2010 with the Sponsor, certain affiliates of the Sponsor, and certain other stockholders. The New Stockholders Agreement of Parent provides that the Sponsor has the right to designate five directors of Parent and that each of Messrs. Carlucci, Ford and Kamal (collectively, the “Founder Directors”) would also serve as directors of Parent. Each of Messrs. Silver, Hendricks and Boxer (collectively, the “Sponsor Directors”) was designated as a director of Parent by the Sponsor pursuant to the New Stockholders Agreement of Parent. The remaining two director positions are currently vacant.
As a group, the Sponsor Directors possess experience in owning and managing enterprises like the Company and are familiar with corporate finance, strategic business planning activities and issues involving stakeholders more generally.
104
Table of Contents
The Founder Directors bring leadership, extensive business, operating, legal and policy experience, and tremendous knowledge of our Company and the Company’s industry, to the Board. In addition, the Founder Directors bring their broad strategic vision for our Company to the Board. Mr. Ford’s service as the Chairman and as previous Chief Executive Officer of the Company, Mr. Carlucci’s service as previous Chief Operating Officer, Treasurer, and current Chief Executive Officer and Mr. Kamal’s service as previous Executive Vice President and current President and Director creates a critical link between management and the Board, enabling the Board to perform its oversight function with the benefits of management’s perspectives on the business.
Equity-Based Arrangements
In connection with the closing of the Merger, the Parent has adopted new equity incentive arrangements for directors, executives and other senior management employees including a new stock incentive plan, the 2010 C.P. Atlas Holdings, Inc. Stock Incentive Plan, and an arrangement for equity contributions from senior management. The 2010 C.P. Atlas Holdings, Inc. Stock Incentive Plan is designed to promote our interests by providing eligible persons with the opportunity to receive an equity interest and/or equity-based awards in one of our direct or indirect parent entities as an incentive for them to remain in our service. New equity awards may also be granted under the new stock incentive plan to members of our senior management.
In addition, consistent with these arrangements, Messrs. Carlucci, Kamal and Ford each executed an equity contribution, exchange and subscription agreement pursuant to which each individual made an equity contribution of approximately $6 million to the Parent, through the rollover of common stock and/or preferred stock of ARH in exchange for common stock of the Parent. Mr. McDonough also entered into an equity contribution, exchange and subscription agreement pursuant to which he contributed approximately $450,000 and Mr. McDonough also contributed and rolled over 25% of the intrinsic value of his outstanding stock options in ARH into options to acquire common stock of the Parent.
New Employment Agreements
In connection with the execution of the Merger Agreement, we have entered into new employment agreements with Messrs. Carlucci, Kamal, Ford, and McDonough, which become effective upon the closing of the Merger. These new employment agreements contain restrictive covenants in our favor and ensure management’s commitment to our interests. We have summarized the terms of these new agreements below.
Joseph A. Carlucci. We entered into a new employment agreement, dated as of March 22, 2010 and effective as of the closing of the Merger, with Mr. Carlucci (the “Carlucci Agreement”) pursuant to which Mr. Carlucci serves as our Chief Executive Officer. The Carlucci Agreement has a three-year term with automatic one-year successive renewals. Mr. Carlucci is entitled to receive a salary of $525,360, an annual bonus opportunity based on achievement of performance goals set by our board of directors, customary employee benefits and payment severance in the event of a termination without cause, for good reason, or due to death or disability (each as defined in the Carlucci Agreement).
The Carlucci Agreement also provides for certain restrictive covenants which apply during the term of the Carlucci Agreement and through the third anniversary of the date of termination of Mr. Carlucci’s employment, provided that solely in the case of a change in control (as defined in the Carlucci Agreement) the restrictive period will end on the later of (i) the third anniversary of the change in control and (ii) the first anniversary of the date of termination of employment, provided further that solely in the case of a change in control, we have the right to extend such period until the later of (a) the fifth anniversary of the change in control and (b) the first anniversary of the date of termination if we make a timely election and pay Mr. Carlucci an amount equal to 300% of his then current base salary.
During the restrictive period, Mr. Carlucci is prohibited from (1) soliciting any employee or contracting physician to terminate his or her relationship with us, (2) hiring any person who was employed by us at any time during Mr. Carlucci’s employment with us, (3) competing, directly or indirectly with us as an owner of any
105
Table of Contents
business (x) engaged in the kidney dialysis business and/or the operation of kidney dialysis facilities within 10 miles of any of our facilities, (y) engaged in the kidney dialysis business and/or the operation of kidney dialysis facilities where Mr. Carlucci is involved in a program to establish joint ventures with nephrologists in the United States of America, and (z) in the case of a termination of employment that occurs on or before the third anniversary of the date of the Carlucci Agreement or which occurs after a change in control, engaged in the kidney dialysis business and/or the operation of kidney dialysis facilities in the United States of America, and (4) representing any other entity or business in conducting substantial negotiations with any nephrologists with whom Mr. Carlucci had conducted substantial negotiations on behalf of us during the one year period immediately prior to the termination of Mr. Carlucci’s employment with us.
Mr. Carlucci is also subject to a confidentiality covenant prohibiting him from disclosing our confidential information for an indefinite period and Mr. Carlucci will agree that all inventions, patents, discoveries, and work product created by him while employed by us will belong to us.
Syed T. Kamal. We entered into a new employment agreement, dated March 22, 2010 and effective as of the closing of the Merger, with Mr. Kamal pursuant to which Mr. Kamal serves as our President. Mr. Kamal’s employment agreement has a three-year term with automatic one-year successive renewals. Mr. Kamal is entitled to receive a salary of $525,360, an annual bonus opportunity based on achievement of performance goals set by our board of directors, customary employee benefits and payment severance in the event of a termination without cause, for good reason, or due to death or disability (each as defined in the Carlucci Agreement). Mr. Kamal is also subject to the same restrictive covenants that are contained in the Carlucci Agreement.
Christopher T. Ford. We entered into a new employment agreement, dated March 22, 2010 and effective as of the closing of the Merger, with Mr. Ford pursuant to which Mr. Ford serves as our Chairman of the Board on a part-time basis. Mr. Ford’s employment agreement has a two-year term with automatic one-year successive renewals. Mr. Ford is entitled to receive a salary of $175,000, employee benefits accruing on a part-time basis and payment severance in the event of a termination without cause, for good reason, or due to death or disability (each as defined in Mr. Ford’s employment agreement). Mr. Ford will also be subject to the same restrictive covenants that are contained in the Carlucci Agreement.
John J. McDonough. We entered into a new employment agreement, dated March 22, 2010 and effective as of the closing of the Merger, with Mr. McDonough pursuant to which Mr. McDonough serves as our Chief Financial Officer. Mr. McDonough’s employment agreement has a three-year term with automatic one-year successive renewals. Mr. McDonough is entitled to receive a salary of $440,720, an annual bonus opportunity based on achievement of performance goals set by our board of directors, customary employee benefits and payment severance in the event of a termination without cause, for good reason, or due to death or disability (each as defined in the Carlucci Agreement). Mr. McDonough is also subject to the same restrictive covenants that are contained in the Carlucci Agreement.
106
Table of Contents
Compensation Discussion and Analysis
The following discussion and analysis of the compensation arrangements of our named executive officers should be read together with the compensation tables and related disclosures regarding our current plans, considerations, expectations and determinations regarding future compensation programs.
Executive Summary
The primary objectives of our executive compensation programs are to attract and retain talented executives to effectively manage and lead our company and create value for our equityholders. The compensation packages for our named executive officers generally include a base salary, annual cash bonuses, equity awards and other benefits and perquisites.
The discussion below includes a review of our compensation decisions with respect to 2009. Our named executive officers for 2009 were Joseph A. Carlucci, our Chief Executive Officer; Christopher T. Ford, our Chairman; Syed T. Kamal, our President; John J. McDonough, our Executive Vice President, Chief Financial Officer and Treasurer and Michael R. Costa, our Vice President, General Counsel and Secretary.
On March 22, 2010, we entered into a Merger Agreement pursuant to which we became a wholly-owned subsidiary of C.P. Atlas Intermediate Holdings, LLC, which is in turn a wholly-owned subsidiary of C.P. Atlas Holdings, Inc. In connection with this transaction: (1) Centerbridge, as sponsor in the Merger Agreement, made an aggregate cash equity investment of $161.5 million in C.P. Atlas Holdings, Inc., subject to certain adjustments, and acquired 87% of the common stock of C.P. Atlas Holdings, Inc; and (2) certain members of our management contributed a portion of their existing equity ownership in us in exchange for cash and newly issued shares of common stock of C.P. Atlas Holdings, Inc.
As further described below, in connection with the Centerbridge merger, C.P. Atlas Holdings, Inc. adopted new equity incentive arrangements applicable to our directors, executives and other senior management employees, including a new stock incentive plan and an arrangement for equity contributions from senior management, and we entered into new employment agreements with Messrs. Carlucci, Kamal, Ford and McDonough. Accordingly, the compensation arrangements for our named executive officers following the Centerbridge merger differ from those in place during 2009.
Compensation Determination Process
Our compensation programs for our named executive officers are designed to reflect our view that all components of executive compensation should be set at levels that are necessary, within reasonable parameters, to successfully attract, retain and motivate optimally talented and experienced executives and that are fair and equitable in light of market practices. We believe that the ownership by management of equity interests in our business is the most effective mechanism for providing incentives to maximize gains for equityholders and that annual cash incentive compensation should be linked to metrics that create value for our equityholders. In setting an individual executive officer’s initial compensation package and the relative allocation among different types of compensation, we consider the nature of the position being filled, the scope of associated responsibilities, the individual’s prior experience and skills and the individual’s compensation expectations, as well as the compensation of existing executive officers at our company and our general impressions of prevailing conditions in the market for executive talent.
The base salary levels for our named executive officers were established by our Compensation Committee at the commencement of each such executive officer’s employment with us and are subject to adjustment by our
107
Table of Contents
Compensation Committee based upon the recommendation of the supervising executive officer and/or our Chief Executive Officer. The amounts of the annual cash bonuses for Messrs. Carlucci, Kamal, Ford and McDonough for 2009 were determined on the basis of our Compensation Committee’s allocation of an overall bonus pool, the size of which was calculated in accordance with our achievement of certain EBITDA performance targets and company growth objectives as contemplated in the named executive officers’ respective employment agreements. The amount of Mr. Costa’s annual cash bonus for 2009 was based upon the recommendation of the supervising executive officer and/or our Chief Executive Officer and was approved by our Compensation Committee. The amounts of equity grants to our named executive officers are determined by our Compensation Committee.
Compensation Risk Assessment
We do not believe that risks arising from our compensation policies and practices for our employees are reasonably likely to have a material adverse effect on us.
Elements of Compensation
Base Salaries
Base salaries are intended to provide a fixed level of compensation sufficient to attract and retain an effective management team when considered in combination with other components of our executive compensation programs. We believe that the base salary element of compensation is required to provide our named executive officers with a stable income stream that is commensurate with their experience and responsibilities. Annual base salaries are established on the basis of market conditions at the time we hire an executive. Any subsequent modifications to annual base salaries are influenced by the performance of the executive and by significant changes in market conditions.
During 2009, the base salaries of our named executive officers were as follows: Mr. Carlucci, $420,000; Mr. Costa, $266,200; Mr. Ford, $420,000; Mr. Kamal, $420,000; and Mr. McDonough, $340,000. Following the effectiveness of the new employment agreements entered into with Messrs. Carlucci, Ford, Kamal and McDonough on March 22, 2010 in connection with the Centerbridge merger, the base salaries of our named executive officers are as follows: Mr. Carlucci, $525,360; Mr. Costa, $292,820 (effective August 3, 2010); Mr. Ford, $175,000; Mr. Kamal, $525,360; and Mr. McDonough, $440,720. These increases in base salaries in connection with the Centerbridge merger were attributable to improved operational results, sustained growth and our objective of aligning named executive officer compensation with our impressions of prevailing base salaries for similarly situated executives at companies of our size in our industry, although we did not evaluate base salaries at any specific peer group of companies.
Annual Cash Bonuses
The employment agreements effective during 2009 provided for annual cash bonuses (the “Bonus Plan”) for each of Messrs. Carlucci, Ford, Kamal and McDonough. Each of Messrs. Carlucci, Ford, Kamal and McDonough was entitled to participate in a bonus pool to be allocated among themselves (the “Bonus Pool”). The aggregate target amount of the Bonus Pool was the sum of 50% of the base salary of each of Messrs. Carlucci, Ford, Kamal and McDonough. For 2009, this aggregate target amount was $800,000. The actual amount of the Bonus Pool for 2009 was determined in part (75%) based on our achievement of an annual, fiscal year consolidated EBITDA performance goal set by our Board of Directors and in part (25%) based on our achievement of an annual target with respect to the number of dialysis patients treated during the year. The 2009 target for EBITDA performance was $66,114,738, and our actual EBITDA achievement was $67,050,000. The target for the number of dialysis patients treated during 2009 was 5,351, and our actual achievement was 5,405 patients.
In determining the actual amount of the Bonus Pool, the Compensation Committee makes adjustments to the target amount based on our performance in relation to our EBITDA and number of patients treated targets and has substantial discretion as to the amounts of these adjustments based on the facts and circumstances surrounding our performance. If our performance targets had not been met, the Bonus Pool would have been funded at less than the $800,000 target amount. For example, if 90% of the performance targets had been met, the Bonus Pool might have been funded at 80% of the target amount, subject to the exercise of discretion by the
108
Table of Contents
Compensation Committee and with primary emphasis placed upon EBITDA performance. If 100% of the performance targets had been met, the Bonus Pool might have been funded at 100%, again subject to the exercise of discretion by the Compensation Committee. In 2009, actual performance exceeded 100% of each of the performance targets. In recognition of these superior results, which were achieved in a very challenging business environment, the Compensation Committee elected to fund the Bonus Pool in the aggregate amount of $1,000,000. This decision to fund the Bonus Pool at $200,000 greater than the $800,000 target amount reflects the Compensation Committee’s intention to reward the Bonus Pool participants for their extraordinary efforts to exceed our performance targets and recognizes the participants’ downside risk that the Bonus Pool would likely have been funded significantly below the target amount if the performance targets had been missed.
The actual portion of the Bonus Pool payable to each of Messrs. Carlucci, Ford, Kamal and McDonough was determined by our Board of Directors based upon the recommendation of our Compensation Committee, subject to the requirement that the entire amount of the Bonus Pool be divided among the four executives. In 2009, the annual cash payments made from the Bonus Pool were as follows: Mr. Carlucci, $325,000; Mr. Ford, $75,000; Mr. Kamal, $325,000; and Mr. McDonough, $275,000. Our Board of Directors determined these allocation amounts on a discretionary basis through an informal process in which the Board of Directors assessed the contributions of each executive towards our achievement in surpassing our performance goals for EBITDA and number of dialysis patients treated during 2009, with our founders, Messrs. Carlucci and Kamal, receiving higher payments in recognition of their contributions in building our business from a long-term perspective. Messrs. Carlucci, Kamal and McDonough were primarily responsible for our number of dialysis patients treated during 2009. Mr. Ford’s allocation reflects substantial changes in his role with us during 2009, particularly his transition from a focus on revenue-generating activities to instead assuming primary responsibility for serving as our liaison to the Kidney Care Council and related trade associations. Mr. Costa did not participate in the Bonus Pool for 2009 but was awarded a discretionary cash bonus of $100,000 for 2009. All of our Vice Presidents, including Mr. Costa, received cash bonuses pursuant to their offer letters. Mr. Costa’s bonus, which was based upon the recommendation of the Chief Executive Officer and was approved by the Compensation Committee, reflects his successful performance in handling various transactions, including the Merger, and the strong performance of the law department as a whole.
In connection with the Centerbridge merger, we entered into new employment agreements with Messrs. Carlucci, Kamal, Ford and McDonough which provide for a new bonus plan (the “2010 Bonus Plan”). Under the 2010 Bonus Plan, each of Messrs. Carlucci, Kamal, Ford and McDonough is eligible to earn an annual cash bonus award with a minimum threshold of 37.5% of his base salary, a target bonus of 75% of his base salary and a maximum bonus of 150% of his base salary based upon our achievement of annual, fiscal year consolidated EBITDA performance goals established by our Compensation Committee. To date, no payments have been made under the 2010 Bonus Plan.
Long-Term Equity Incentives
We believe that our long-term financial success is achieved in part through an ownership culture that encourages our named executive officers to focus on our long-term performance through the use of equity-based compensation tools. Our Equity Incentive Plan that was in place during 2009 was initially established in 2005. We adopted this plan to align the interests of our equityholders with those of our employees and to provide for grants of options to key employees, directors, advisors and consultants as either incentive stock options or nonqualified stock options as determined by our Board of Directors. The only equity award granted to a named executive officer during 2009 was a grant of 20,000 stock options made to Mr. Costa on May 20, 2009 in accordance with our practice of granting equity awards to certain employees each year on the anniversary of the date on which we hired each such employee.
In connection with the Centerbridge merger, our parent entered into the 2010 C.P. Atlas Holdings, Inc. Stock Incentive Plan. This 2010 Stock Incentive Plan is designed to promote our interests by providing eligible persons with the opportunity to receive an equity interest and/or equity-based awards in one of our direct or indirect parent entities as an incentive for them to remain in our service. Together with the substantial investments made in our parent by our senior management, these arrangements demonstrate the commitment of our employees and management team to our long-term growth.
109
Table of Contents
Consistent with these arrangements, each of Messrs. Carlucci, Kamal and Ford executed an equity contribution, exchange and subscription agreement pursuant to which each such executive made an equity contribution with a value of $6,000,000 to our parent, C.P. Atlas Holdings, Inc., through the rollover of common stock and/or preferred stock of our company in exchange for common stock of C.P. Atlas Holdings, Inc. Mr. McDonough entered into an equity contribution, exchange and subscription agreement pursuant to which he made an equity contribution with a value of $450,000 to C.P. Atlas Holdings, Inc. through the rollover of common and /or preferred stock of our company in exchange for common stock of C.P. Atlas Holdings, Inc. Mr. McDonough also contributed and rolled over 25% of the spread value of his then outstanding stock options in our company in exchange for options to acquire common stock in C.P. Atlas Holdings, Inc. Mr. Costa entered into an equity contribution, exchange and subscription agreement pursuant to which he made an equity contribution with a value of $125,000 to C.P. Atlas Holdings, Inc. through the rollover of stock options in our company in exchange for stock options in C.P. Atlas Holdings, Inc.
In addition to the forgoing, in connection with the Centerbridge merger, each of Messrs. Carlucci, Ford, Kamal and McDonough received approximately $185,899 on May 10, 2010 in exchange for 46,250 shares of our common stock that each of the executives purchased from us pursuant to Notes issued on March 4, 2008. A portion of such sales proceeds were used by the executives to repay their indebtedness to us under the Notes.
Other Compensation
We also provide various other benefits to certain of our named executive officers that are intended to be part of a competitive compensation program. In 2009, these benefits for each of Messrs. Costa, Ford, Kamal and McDonough included director and officer liability insurance as well as a company-provided automobile allowance and reimbursements for related expenses (including insurance, taxes and fuel), provided that the cost of providing such automobile allowance could not exceed $12,000 per fiscal year, plus all costs for insurance and fuel. We believe that these benefits are comparable to those offered by other companies that compete with us for executive talent.
Payments Upon Termination
Each of our named executive officers, other than Mr. Costa, is entitled to certain benefits upon the termination of his employment with us, the terms of which are described below under “Summary of Employment Agreements” and “Potential Payments Upon Termination or Change in Control.” We believe that these benefits are valuable as they address the valid concern that it may be difficult for these executives to find comparable employment in a short period of time in the event of termination and provide an incentive for these executives to comply with non-competition and non-solicitation covenants.
Anticipated Actions
We expect to continue to evaluate and revisit the structure of our executive compensation programs and may make adjustments to them from time to time.
110
Table of Contents
Summary Compensation Table
The following table sets forth the cash and other compensation that we paid to our named executive officers, or that was otherwise earned by our named executive officers, for their services in all capacities during the last fiscal year.
Name and Principal Position | Year | Salary ($) | Bonus ($) | Option Awards ($) (1) | Non-Equity Incentive Plan Compensation ($) (2) | All Other Compensation ($) (3) | Total ($) | |||||||||||||||||||||
Joseph A. Carlucci | 2009 | 420,000 | 0 | 0 | 325,000 | 18,148 | 763,148 | |||||||||||||||||||||
Chief Executive Officer | ||||||||||||||||||||||||||||
Christopher T. Ford | 2009 | 420,000 | 0 | 0 | 75,000 | 16,571 | 511,571 | |||||||||||||||||||||
Chairman | ||||||||||||||||||||||||||||
Syed T. Kamal | 2009 | 420,000 | 0 | 0 | 325,000 | 17,300 | 762,300 | |||||||||||||||||||||
President | ||||||||||||||||||||||||||||
John J. McDonough | 2009 | 340,000 | 0 | 0 | 275,000 | 0 | 615,000 | |||||||||||||||||||||
Executive Vice President, Chief Financial Officer and Treasurer | ||||||||||||||||||||||||||||
Michael R. Costa | 2009 | 266,200 | 100,000 | 104,000 | 0 | 0 | 470,200 | |||||||||||||||||||||
Vice President, General Counsel and Secretary | ||||||||||||||||||||||||||||
| (1) | The amount set forth in this column represent the aggregate grant date fair value of option awards granted in fiscal year 2009 as calculated pursuant to Financial Accounting Standards Board (FASB) Accounting Standards Codification Topic 718,Compensation—Stock Compensation (FASB ASC Topic 718). This amount has been determined based on the assumptions set forth in Note N to our consolidated financial statements for the fiscal year ended December 31, 2009. |
| (2) | The amounts set forth in this column reflect awards under our Bonus Plan. For more information regarding the Bonus Plan, see the Compensation Discussion and Analysis section above. |
| (3) | See the All Other Compensation Table below. |
All Other Compensation
| Car Allowance ($) | Gasoline Allowance ($) | Car Insurance Allowance ($) | Life Insurance Premiums ($) | Total ($) | ||||||||||||||||
Mr. Carlucci | 12,000 | 4,629 | 0 | 1,519 | 18,148 | |||||||||||||||
Mr. Ford | 12,000 | 2,746 | 1,825 | 0 | 16,571 | |||||||||||||||
Mr. Kamal | 12,000 | 3,473 | 959 | 868 | 17,300 | |||||||||||||||
Mr. McDonough | 0 | 0 | 0 | 0 | 0 | |||||||||||||||
Mr. Costa | 0 | 0 | 0 | 0 | 0 | |||||||||||||||
Grants of Plan-Based Awards in 2009
The following table sets forth the individual grants of plan-based awards made to each of our named executive officers during 2009.
Name | Grant Date | Estimated Future Payouts Under Non-Equity Incentive Plan Awards | All Other Option Awards: Number of Securities Underlying Options (#) | Exercise or Base Price of Option Awards ($/Sh) | Grant Date Fair Value of Stock and Option Awards ($) | |||||||||||||||
| Target ($) | ||||||||||||||||||||
Mr. Carlucci | 325,000 | (1) | ||||||||||||||||||
Mr. Ford | 75,000 | (1) | ||||||||||||||||||
Mr. Kamal | 325,000 | (1) | ||||||||||||||||||
Mr. McDonough | 275,000 | (1) | ||||||||||||||||||
Mr. Costa | 5/20/09 | 20,000 | (2) | 5.20 | (3) | 104,000 | (4) | |||||||||||||
111
Table of Contents
| (1) | Reflects the anticipated opportunity of each of Messrs. Carlucci, Ford, Kamal and McDonough to participate in the Bonus Plan. There were no threshold, target or maximum awards payable under the Bonus Plan for 2009. Instead, each executive was entitled to participate in a Bonus Pool funded based on our achievement of pre-established performance goals as discussed in the Compensation Discussion & Analysis section above. In 2009, the aggregate amount available in the Bonus Pool to be allocated among Messrs. Carlucci, Ford, Kamal and McDonough was $1,000,000. Actual amounts earned by and paid to Messrs. Carlucci, Ford, Kamal and McDonough are shown in the “Non-Equity Incentive Plan Compensation” column of the Summary Compensation Table. For additional information regarding the Bonus Plan, see the Compensation Discussion and Analysis section above. |
| (2) | Represents an award of time-vesting stock options granted under our 2005 Equity Incentive Plan. |
| (3) | Reflects the per-share exercise price of the options, which is equal to the fair market value of a share of our common stock on the grant date as determined by our Board of Directors. A description of the process used in determining the fair market value of our common stock is set forth above under the subheading “Management’s Discussion and Analysis of Financial Condition and Results of Operations—Stock-based Compensation.” |
| (4) | Reflects the aggregate grant date fair value the award of stock options determined pursuant to FASB ASC Topic 718. |
Summary of Employment Agreements
Employment Agreements Effective During 2009
We maintain employment agreements with each of our named executive officers, with the exception of Mr. Costa. The employment agreements in effect during 2009 were originally executed on August 25, 2004 and were amended on December 16, 2005 and March 1, 2007 respectively. The employment agreements provided for an initial term of five years and automatic renewal for successive one-year periods unless notice of non-renewal was provided in writing by either party at least 60 days before the end of the then-current term.
As of 2009, the employment agreements provided for base salaries in the following amounts subject to increases in the sole discretion of our Compensation Committee: Mr. Carlucci, $420,000; Mr. Ford, $420,000; Mr. Kamal, $420,000; and Mr. McDonough, $340,000. The executives were also entitled to participate in any employee benefit plans that we offered to our executive officers. In addition, each of Messrs. Ford, Kamal and McDonough received a company-provided automobile allowance and reimbursements for related expenses (including insurance, taxes and fuel), provided that the cost of providing such automobile allowance could not exceed $12,000 per fiscal year, plus all costs for insurance and fuel.
The employment agreements also included certain restrictive covenants which applied during their term and through the third anniversary of the date of termination of the employment agreements, provided that, solely in the case of a Sale (as defined therein), the restrictive period would end on the later of (1) the third anniversary of the Sale and (2) the first anniversary of the date of termination of employment, provided further that, solely in the case of a Sale, we would have the right to extend the restrictive covenant periods with each executive (1) until the later of the fifth anniversary of the sale or the first anniversary of the executive’s termination of employment, in the case of Messrs. Carlucci, Ford and Kamal; or (2) through the third anniversary of the termination of the executive’s employment, in the case of Mr. McDonough. In the event that we chose to extend the restrictive covenant periods, Messrs. Carlucci, Ford and Kamal would receive a lump sum payment equal to $751,666, and Mr. McDonough would receive a lump sum payment equal to his then-current base salary.
During the restrictive period, the executive would be prohibited from (1) soliciting any employee or contracting physician to terminate his or her relationship with us; (2) hiring any person who was employed by us at any time during the executive’s employment with us; (3) competing, directly or indirectly with us as an owner of any business (x) engaged in the kidney dialysis business and/or the operation of kidney dialysis facilities within 10 miles of any of our facilities, (y) engaged in the kidney dialysis business and/or the operation of kidney dialysis facilities where the executive is involved in a program to establish joint ventures with nephrologists in
112
Table of Contents
the United States of America and (z) in the case of a termination of employment that occurred on or before the third anniversary of the date of the employment agreement or that occurred after a change in control, engaged in the kidney dialysis business and/or the operation of kidney dialysis facilities in the United States of America; and (4) representing any other entity or business in conducting substantial negotiations with any nephrologists with whom the executive had conducted substantial negotiations on behalf of us during the one-year period immediately prior to the termination of the executive’s employment with us.
The executives were also subject to a confidentiality covenant prohibiting them from disclosing our confidential information for an indefinite period, and the executives agreed that all inventions, patents, discoveries and work product created by them while employed by us would belong to us.
Current Employment Agreements
In connection with the execution of the Merger Agreement, we have entered into new employment agreements with Messrs. Carlucci, Kamal, Ford, and McDonough, which become effective upon the closing of the Merger. These new employment agreements contain restrictive covenants in our favor and ensure management’s commitment to our interests. We have summarized the terms of these new agreements below.
Joseph A. Carlucci. We entered into a new employment agreement, dated as of March 22, 2010 and effective as of the closing of the Merger, with Mr. Carlucci (the “Carlucci Agreement”) pursuant to which Mr. Carlucci serves as our Chief Executive Officer. The Carlucci Agreement has a three-year term with automatic one-year successive renewals. Mr. Carlucci is entitled to receive a salary of $525,360, an annual bonus opportunity based on achievement of performance goals set by our board of directors, customary employee benefits and payment severance in the event of a termination without cause, for good reason, or due to death or disability (each as defined in the Carlucci Agreement).
The Carlucci Agreement also provides for certain restrictive covenants which apply during the term of the Carlucci Agreement and through the third anniversary of the date of termination of Mr. Carlucci’s employment, provided that solely in the case of a change in control (as defined in the Carlucci Agreement) the restrictive period will end on the later of (i) the third anniversary of the change in control and (ii) the first anniversary of the date of termination of employment, provided further that solely in the case of a change in control, we have the right to extend such period until the later of (a) the fifth anniversary of the change in control and (b) the first anniversary of the date of termination if we make a timely election and pay Mr. Carlucci an amount equal to 300% of his then current base salary.
During the restrictive period, Mr. Carlucci is prohibited from (1) soliciting any employee or contracting physician to terminate his or her relationship with us, (2) hiring any person who was employed by us at any time during Mr. Carlucci’s employment with us, (3) competing, directly or indirectly with us as an owner of any business (x) engaged in the kidney dialysis business and/or the operation of kidney dialysis facilities within 10 miles of any of our facilities, (y) engaged in the kidney dialysis business and/or the operation of kidney dialysis facilities where Mr. Carlucci is involved in a program to establish joint ventures with nephrologists in the United States of America, and (z) in the case of a termination of employment that occurs on or before the third anniversary of the date of the Carlucci Agreement or which occurs after a change in control, engaged in the kidney dialysis business and/or the operation of kidney dialysis facilities in the United States of America, and (4) representing any other entity or business in conducting substantial negotiations with any nephrologists with whom Mr. Carlucci had conducted substantial negotiations on behalf of us during the one year period immediately prior to the termination of Mr. Carlucci’s employment with us.
Mr. Carlucci is also subject to a confidentiality covenant prohibiting him from disclosing our confidential information for an indefinite period and Mr. Carlucci will agree that all inventions, patents, discoveries, and work product created by him while employed by us will belong to us.
Syed T. Kamal. We entered into a new employment agreement, dated March 22, 2010 and effective as of the closing of the Merger, with Mr. Kamal pursuant to which Mr. Kamal serves as our President. Mr. Kamal’s
113
Table of Contents
employment agreement has a three-year term with automatic one-year successive renewals. Mr. Kamal is entitled to receive a salary of $525,360, an annual bonus opportunity based on achievement of performance goals set by our board of directors, customary employee benefits and payment severance in the event of a termination without cause, for good reason, or due to death or disability (each as defined in the Carlucci Agreement). Mr. Kamal is also subject to the same restrictive covenants that are contained in the Carlucci Agreement.
Christopher T. Ford. We entered into a new employment agreement, dated March 22, 2010 and effective as of the closing of the Merger, with Mr. Ford pursuant to which Mr. Ford serves as our Chairman of the Board on a part-time basis. Mr. Ford’s employment agreement has a two-year term with automatic one-year successive renewals. Mr. Ford is entitled to receive a salary of $175,000, employee benefits accruing on a part-time basis and payment severance in the event of a termination without cause, for good reason, or due to death or disability (each as defined in Mr. Ford’s employment agreement). Mr. Ford will also be subject to the same restrictive covenants that are contained in the Carlucci Agreement.
John J. McDonough. We entered into a new employment agreement, dated March 22, 2010 and effective as of the closing of the Merger, with Mr. McDonough pursuant to which Mr. McDonough serves as our Chief Financial Officer. Mr. McDonough’s employment agreement has a three-year term with automatic one-year successive renewals. Mr. McDonough is entitled to receive a salary of $440,720, an annual bonus opportunity based on achievement of performance goals set by our board of directors, customary employee benefits and payment severance in the event of a termination without cause, for good reason, or due to death or disability (each as defined in the Carlucci Agreement). Mr. McDonough is also subject to the same restrictive covenants that are contained in the Carlucci Agreement.
Outstanding Equity Awards at 2009 Fiscal Year-End
The following table provides information concerning unexercised options and stock awards outstanding as of December 31, 2009 for each of our named executive officers.
| Option Awards | Stock Awards | |||||||||||||||||||||||
Name | Number of Securities Underlying Unexercised Options (#) Exercisable | Number of Securities Underlying Unexercised Options (#) Unexercisable | Option Exercise Price ($) | Option Expiration Date | Number of Shares or Units of Stock That Have Not Vested (#) (9) | Market Value of Shares or Units of Stock That Have Not Vested ($) (10) | ||||||||||||||||||
Mr. Carlucci | 419,067 | (1) | 209,533 | (1) | 3.50 | 3/1/17 | 30,833.4 | 161,567 | ||||||||||||||||
Mr. Ford | 419,067 | (1) | 209,533 | (1) | 3.50 | 3/1/17 | 30,833.4 | 161,567 | ||||||||||||||||
Mr. Kamal | 419,067 | (1) | 209,533 | (1) | 3.50 | 3/1/17 | 30,833.4 | 161,567 | ||||||||||||||||
Mr. McDonough | 150,000 | (2) | 0.14 | 8/4/13 | 30,833.4 | 161,567 | ||||||||||||||||||
| 100,000 | (3) | 0.14 | 04/01/14 | |||||||||||||||||||||
| 150,000 | (4) | 50,000 | (4) | 1.50 | 12/16/15 | |||||||||||||||||||
| 100,000 | (5) | 300,000 | (5) | 3.50 | 05/11/17 | |||||||||||||||||||
Mr. Costa | 18,750 | (6) | 56,250 | (6) | 3.77 | 08/06/11 | ||||||||||||||||||
| 5,000 | (7) | 15,000 | (7) | 4.26 | 06/10/12 | |||||||||||||||||||
| 20,000 | (8) | 5.20 | 05/20/13 | |||||||||||||||||||||
| (1) | Reflects stock options granted under our 2005 Equity Incentive Plan to each of Messrs. Carlucci, Ford and Kamal on March 1, 2007, which were scheduled to fully vest on March 5, 2011. |
| (2) | Reflects stock options granted under our 2000 Equity Incentive Plan (post stock split) to Mr. McDonough on August 4, 2003, which were fully vested on August 4, 2007. |
| (3) | Reflects stock options granted under our 2000 Equity Incentive Plan to Mr. McDonough on April 1, 2004, which were fully vested on August 1, 2007. |
114
Table of Contents
| (4) | Reflects stock options granted under our 2000 Equity Incentive Plan to Mr. McDonough on December 16, 2005, which were scheduled to vest in four equal annual installments beginning on the first anniversary of the grant date. |
| (5) | Reflects stock options granted under our 2005 Equity Incentive Plan to Mr. McDonough on May 11, 2007, which were scheduled to vest in four equal annual installments beginning on the first anniversary of the grant date. |
| (6) | Reflects stock options granted under our 2005 Equity Incentive Plan to Mr. Costa on September 6, 2007, which were scheduled to vest in four equal annual installments beginning on August 6, 2007. |
| (7) | Reflects stock options granted under our 2005 Equity Incentive Plan to Mr. Costa on June 11, 2008, which were scheduled to vest in four equal annual installments beginning on the first anniversary of the grant date. |
| (8) | Reflects stock options granted under our 2005 Equity Incentive Plan to Mr. Costa on May 20, 2009, which were scheduled to vest in four equal annual installments beginning on the first anniversary of the grant date. |
| (9) | Reflects the portion of shares of restricted stock granted to each named executive officer, other than Mr. Costa, on March 4, 2008 that were unvested as of December 31, 2009. The awards were scheduled to vest as to one-third of the shares on each of March 5, 2009, March 5, 2010 and March 5, 2011. |
| (10) | Reflects the value as calculated using the fair market value of our common stock as of December 31, 2009 ($5.24 per share). |
Option Exercises and Stock Vested in 2009
None of our named executive officers had options that were exercised during 2009. The following table summarizes amounts realized upon the vesting of restricted stock for each of our named executive officers during 2009.
| Stock Awards (1) | ||||||||
Name | Number of Shares Acquired on Vesting (#) | Value Realized on Vesting ($) | ||||||
Mr. Carlucci | 15,416.6 | 72,766 | ||||||
Mr. Ford | 15,416.6 | 72,766 | ||||||
Mr. Kamal | 15,416.6 | 72,766 | ||||||
Mr. McDonough | 15,416.6 | 72,766 | ||||||
Mr. Costa | 0 | 0 | ||||||
| (1) | Represents the number of restricted shares that vested in 2009 and the aggregate value of such shares of common stock based upon the fair market value of our common stock on the March 5, 2009 vesting date ($4.72 per share). |
Pension Benefits for 2009
We do not offer pension benefits to our named executive officers.
Non-Qualified Deferred Compensation for 2009
We do not offer non-qualified deferred compensation to our named executive officers.
Potential Benefits Upon Termination or Change in Control
Termination of Employment
As discussed above, we maintain employment agreements with each of our named executive officers, other than Mr. Costa, that provide for certain payments and benefits upon their termination of employment for various reasons. The following disclosure reflects terms of the employment arrangements in place on December 31, 2009 rather than those provided for in the employment agreements entered into in connection with the Centerbridge merger.
115
Table of Contents
Payments Made Upon Any Termination of Employment. Regardless of the manner in which his employment terminates, each executive officer is entitled to receive amounts earned and accrued during his term of employment, including accrued but unpaid base salary through the date of termination and unreimbursed employment-related expenses owed to the executive officer under our policies. These payments do not differ from payments made upon termination to all employees.
Payments Made Upon Termination Without Cause or Good Reason.Messrs. Carlucci, Ford, Kamal and McDonough’s employment agreements in effect on December 31, 2009 provided that, if we terminated the executive without Cause, or the executive terminated his employment with us for Good Reason, he would be entitled to receive:
| • | continuation of his base salary, at the then-current level, for a period of 12 months, payable in installments in accordance with our normal payroll practices; |
| • | continuation of employee benefits for 12 months following the date of termination; and |
| • | a pro rata portion of his bonus for the then-current fiscal year based upon actual performance, payable at the time at which bonuses are normally paid. |
Cause generally means the executive’s (1) conviction of, or plea of guilty to, a crime if, as a result of his continued association with us, such crime is injurious to our business or reputation; (2) breach of duty of loyalty that is detrimental to us and involves his personal profit; (3) willful failure to perform his duties or to follow lawful directives of the Board of Directors; or (4) gross negligence or willful misconduct in the performance of his duties.
Good Reason generally means any substantial diminution of or substantial detrimental change in the executive’s responsibilities, salary or benefits, or re-location of the executive’s principal office from the metropolitan Boston area.
Payments Made Upon Death or Termination of Employment by Reason of Disability.If Messrs. Carlucci, Ford, Kamal or McDonough’s employment was terminated on December 31, 2009 by reason of his death or disability, he would have been entitled to receive the same payments and benefits that he would have received had he been terminated without Cause or for Good Reason, as described above.
Restrictive Covenants. Each of the employment agreements in effect on December 31, 2009 contained confidentiality, non-competition and employee non-solicitation covenants that applied during the executive’s employment with us and for a period of time after his termination of employment (36 months, in the case of Messrs. Carlucci, Ford and Kamal, and 24 months, in the case of Mr. McDonough). In the case of a sale of our company, Messrs. Carlucci, Ford and Kamal’s restrictive covenants would terminate on the later of the third anniversary of the sale or the first anniversary of the executive’s termination of employment.
Upon the occurrence of a sale of our company, we would have the right to extend the restrictive covenant periods with each executive (1) until the later of the fifth anniversary of the sale or the first anniversary of the executive’s termination of employment, in the case of Messrs. Carlucci, Ford and Kamal; or (2) through the third anniversary of the termination of the executive’s employment, in the case of Mr. McDonough. In the event that we chose to extend the restrictive covenant periods, Messrs. Carlucci, Ford and Kamal would receive a lump sum payment equal to $751,666, and Mr. McDonough would receive a lump sum payment equal to his then-current base salary.
Summary of Termination Payments and Benefits.The following table summarizes the approximate value of the termination payments and benefits that each of our named executive officers would have received if his employment had been terminated on December 31, 2009 by the executive for Good Reason, by us
116
Table of Contents
without Cause or upon Death or Disability. The table does not include certain amounts that the named executive officer would be entitled to receive under certain plans or arrangements that do not discriminate in scope, terms or operation, in favor of our executive officers and that are generally available to all salaried employees.
| Mr. Carlucci ($) | Mr. Ford ($) | Mr. Kamal ($) | Mr. McDonough ($) | Mr. Costa ($) | ||||||||||||||||
Continuation of base salary for 12 months | 420,000 | 420,000 | 420,000 | 340,000 | 0 | |||||||||||||||
Continuation of employee benefits for 12 months (1) | 14,056 | 14,056 | 12,706 | 13,784 | 0 | |||||||||||||||
Bonus for year despite executive no longer being employed on payment date (2) | 325,000 | 75,000 | 325,000 | 275,000 | 0 | |||||||||||||||
Total | 759,056 | 509,056 | 757,706 | 628,784 | 0 | |||||||||||||||
| (1) | These benefits include long-term and short-term disability insurance, dental insurance, medical insurance and life insurance. The amounts in the table above reflect the cost to our company of providing such benefits during 2009 and, accordingly, are only an estimate of the costs our company would have incurred to provide such benefits for 12 months following a termination of employment on December 31, 2009. |
| (2) | In the event of a termination by the executive for Good Reason, by us without Cause or upon Death or Disability, the executive would have been entitled to a bonus for the year of termination through the date of termination on a pro-rated basis. In the event of a termination by the executive without good reason or by us for Cause, the executive would not have been entitled to a pro-rated bonus for the fiscal year of termination but would have been entitled to any bonus earned for any fiscal year prior to the year of termination. |
Change in Control
As of December 31, 2009, our named executive officers were not entitled to any additional benefits following a change in control of our company or upon a termination of employment following a change in control of our company.
117
Table of Contents
The following table sets forth the cash and other compensation paid by us to the members of our Board of Directors for all services in all capacities during 2009. Messrs. Carlucci, Ford and Kamal were named executive officers for 2009 and were not separately compensated for their service as directors. D. Neal Morrison and Scott B. Perper also served on our Board of Directors during 2009 and were not compensated for service during 2009. Dr. Donald Leeber was the only member of our Board of Directors who was compensated for service during 2009. The form of such compensation was cash payments of $7,500 per quarter and a December 17, 2009 grant of options to purchase 5,000 shares of our common stock at an exercise price of $5.24 per share, for an aggregate grant date fair value of $26,200. These options are scheduled to vest in four equal annual installments beginning on December 17, 2010. We have not yet determined what, if any, changes we will make to our director compensation arrangements following the Centerbridge merger.
Name | Fees Earned or Paid in Cash ($) | Option Awards ($) (1) | Total ($) | |||||||||
Messrs. Morrison | 0 | 0 | 0 | |||||||||
Perper | 0 | 0 | 0 | |||||||||
Donald Leeber, M.D | 30,000 | 26,200 | 56,200 | |||||||||
| (1) | The amount set forth in this column represents the aggregate grant date fair value of option awards granted in fiscal year 2009 as calculated pursuant to FASB ASC Topic 718. This amount has been determined based on the assumptions set forth in Note N to our consolidated financial statements for the fiscal year ended December 31, 2009. As of December 31, 2009, Dr. Leeber held options exercisable into 57,500 shares of our common stock. Of these options, 12,500 had an exercise price of $0.48 per share; 25,000 had an exercise price of $1.50 per share; 10,000 had an exercise price of $3.50 per share; 5,000 had an exercise price of $3.77 per share; and 5,000 had an exercise price of $4.72 per share. |
118
Table of Contents
SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT
C.P. Atlas Holdings, Inc. owns 100% of the capital stock of C.P. Intermediate Holdings, LLC, which owns 100% of the capital stock of American Renal Holdings Inc. The following table sets forth information regarding the beneficial ownership of the common stock of C.P. Atlas Holdings, Inc. as of September 7, 2010.
| • | each person who is known by us to own beneficially more than 5% of the outstanding shares of our common stock; |
| • | each of our directors; |
| • | each of our executive officers named in the Summary Compensation Table; and |
| • | all of our directors and executive officers as a group. |
The percentages of shares outstanding provided in the tables are based on 8,855,065 shares of C.P. Atlas Holdings, Inc. common stock, par value $0.01 per share, outstanding as of September 7, 2010. Beneficial ownership is determined in accordance with the rules of the SEC and generally includes voting or investment power with respect to securities. Shares issuable upon the exercise of options that are exercisable within 60 days of September 7, 2010 are considered outstanding for the purpose of calculating the percentage of outstanding shares of our common stock held by the individual, but not for the purpose of calculating the percentage of outstanding shares held by any other individual. The address of each of our directors and executive officers listed below is c/o American Renal Holdings Inc., 66 Cherry Hill Drive, Beverly, Massachusetts 01915.
Name of Beneficial Owner | Beneficial Ownership of C.P. Atlas Holdings, Inc. Common Stock | Percentage of C.P. Atlas Holdings, Inc. Common Stock | ||||||
Centerbridge Capital Partners L.P. and certain affiliated entities1 | 7,692,505 | 86.9 | % | |||||
Christopher T. Ford . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . | 285,714 | 2 | 3.2 | % | ||||
Syed Kamal. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . | 285,714 | 3.2 | % | |||||
Joseph A. Carlucci . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . | 285,714 | 3.2 | % | |||||
John J. McDonough . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . | 21,429 | * | ||||||
Michael R. Costa. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . | 5,952 | * | ||||||
Steven M. Silver1 . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . | 7,692,505 | 86.9 | % | |||||
Jared S. Hendricks1 . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . | 7,692,505 | 86.9 | % | |||||
Michael E. Boxer. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . | 14,286 | 3 | * | |||||
All directors and executive officers as a group (8 persons). . . . . . . . | 898,809 | 10.2 | % | |||||
| * | Less than one percent. |
| 1 | C.P. Atlas Holdings, Inc., a Delaware limited liability company, is 83.3% owned by Centerbridge Capital Partners, L.P., 1.0% owned by Centerbridge Capital Partners SBS, L.P., 2.6% owned by Centerbridge Capital Partners Strategic, L.P., and 13.1% owned by other persons. Steven Silver, a Senior Managing Director of Centerbridge Partners, L.P., and Jared Hendricks, a Managing Director of Centerbridge Partners, L.P., both owners of interests in Centerbridge Capital Partners, L.P., Centerbridge Capital Partners SBS, L.P., and Centerbridge Capital Partners Strategic, L.P., disclaim beneficial ownership of such shares, except to the extent of their pecuniary interest therein. The address of C.P. Atlas Holdings, Inc. and each of the entities and individuals listed in this footnote is c/o Centerbridge Partners, L.P., 375 Park Avenue, New York, New York 10152. |
| 2 | Comprised of 128,790 shares under the Christopher T. Ford 2005 Grantor Retained Annuity Trust and 156,924 shares under the Christopher T. Ford 2008 Grantor Retained Annuity Trust. |
| 3 | Beneficially owned through Black Diamond Partners LLC, JJ Bark LLC and Tribeca Investments LLC. |
119
Table of Contents
CERTAIN RELATIONSHIPS AND RELATED PARTY TRANSACTIONS
Lease Agreements with Entities Owned by Related Parties
We own the land and buildings of our dialysis clinic located in Chillicothe, Ohio and our JVs own the land and buildings of our dialysis clinics in Beaumont and Jasper, Texas. ARA-Chillicothe Dialysis, the dialysis clinic located in Chillicothe, Ohio in which we have a controlling interest, leases 8,100 square feet of space from ARA-Ohio Holdings LLC. We own all of the outstanding equity of ARA-Ohio Holdings LLC. The lease in Chillicothe, Ohio was entered in 2005 for a term of ten years with five year renewal options. The current minimum annual rent for our dialysis clinic in Chillicothe, Ohio is $72,900. In 2010, the minimum annual rent increases to $81,000.
We have other lease agreements for dialysis clinics with our nephrologist partners or entities under the control of our nephrologist partners in such clinics. The amount of rent expense under these lease arrangements was approximately $2.5 million, $1.5 million and $1.1 million in 2009, 2008 and 2007, respectively.
Indemnification of Officers and Directors
In connection with this offering, we have entered into indemnification agreements with each of our directors and officers, and we have purchased a policy of directors’ and officers’ liability insurance that insures our directors and officers against the cost of defense, settlement or payment of a judgment under certain circumstances.
Transaction Fee and Advisory Services Fee Arrangements with Centerbridge
In connection with the Transactions, we entered into a transaction fee and advisory services agreement with Centerbridge Advisors, L.L.C., the investment manager of Centerbridge, our principal stockholder after the consummation of the Merger. Pursuant to the transaction fee and advisory services agreement, we paid to Centerbridge a fee of $4 million plus out-of-pocket expenses upon the closing of the Merger as consideration for the financial and structural analysis, due diligence investigations, corporate strategy, and other advice and assistance rendered by Centerbridge to us.
In addition, under this agreement, Centerbridge (including through its affiliates) agreed to provide advisory and consulting services related to our affairs and the affairs of our subsidiaries, including, without limitation,
| • | advice regarding the structure, terms, conditions and other provisions, distribution and timing of debt and equity offerings and advice regarding relationships with us and our subsidiaries’ lenders and bankers, |
| • | advice regarding our strategy, |
| • | advice regarding dispositions and/or acquisitions, and |
| • | such other advice directly related or ancillary to the above advisory services as may be reasonably requested by us. |
In consideration for these services, we pay Centerbridge Advisors, L.L.C., commencing upon the closing of the Merger, a per annum advisory services fee (payable quarterly) for each fiscal year from and including fiscal year 2010 of the greater of (i) an amount equal to the greater of (x) $550,000 and (y) the advisory services fee of the previous fiscal year and (ii) an amount equal to 1.25% of our EBITDA,minus a personnel expense deduction, if applicable. In addition, Centerbridge Advisors, L.L.C. is entitled to receive an additional fee equal to 1.0% of the enterprise value and/or aggregate value, as applicable, for any future fundamental or significant transactions (e.g., a sale of, or acquisition by, the Parent or its subsidiaries) in which Centerbridge is involved.
The transaction fee and advisory services agreement will continue until the earliest of (i) the tenth anniversary of the date of the agreement, (ii) the date on which Centerbridge owns less than 20% of the
120
Table of Contents
outstanding common stock of the Parent, or (iii) such date as Centerbridge may specify in writing. We have agreed to indemnify Centerbridge and its affiliates partners, members, stockholders, directors, officers, employees, agents and representatives from and against all liabilities relating to the services contemplated by the transaction fee and advisory services agreement.
Upon a change of control in our ownership, an initial public offering or a sale of all or substantially all of the outstanding common stock of the parent or of the businesses and assets of the Parent (or at such other time as Centerbridge and the Company may agree), Centerbridge or Centerbridge Advisors, L.L.C., as the case may be, may elect to receive, in lieu of annual payments of the advisory services and related fees, a single lump sum cash payment equal to the then present value of all then current and future advisory services and related fees payable under this agreement. The indenture governing the notes permits the lump sum payment in the case of a change of control, an initial public offering or a sale of all or substantially all of our assets, but to the extent Centerbridge and the Company agree to pay a lump sum, such payment would only be payable to the extent that it is permitted under the indenture. The lump sum payment is also subject to other agreements governing our indebtedness.
Stockholders Agreement of C.P. Atlas Holdings, Inc.
In connection with the Merger, the Parent entered into a stockholders agreement with Centerbridge and the other holders of common stock of the Parent (the “Stockholders Agreement”). Holders of common stock of the Parent will become party to the Stockholders Agreement from time to time, by executing a joinder to the Stockholders Agreement.
Under the Stockholders Agreement, each of the stockholders of the Parent party thereto agreed to vote in favor of, and to take all other necessary and appropriate actions to cause to occur, the election to the board of directors of Parent of each director designated in accordance with the provisions of the Stockholders Agreement. The parties to the Stockholders Agreement also agreed to vote the securities of Parent as Centerbridge directs in connection with any merger or consolidation of the Parent, the sale of all or substantially all of its assets, the amendment of its organizational documents and certain other matters.
The Stockholders Agreement restricts employee stockholders party thereto from transferring their securities of the Parent prior to the earlier of (i) 180 days after a qualified public offering or (ii) a change of control, subject to certain exceptions. The Stockholders Agreement also provides employee stockholders party thereto customary tag-along rights with respect to sales of securities of the Parent held by Centerbridge and gives Centerbridge customary drag-along rights, each subject to a minimum threshold of securities of the Parent sold by Centerbridge.
121
Table of Contents
DESCRIPTION OF OTHER INDEBTEDNESS
New Revolving Credit Facility
Overview
In connection with the Transactions, ARH entered into a senior secured credit agreement with Bank of America, N.A., as administrative agent, the lenders and the other parties thereto.
The senior secured credit facility provided senior secured financing consisting of a $25.0 million revolving credit facility (the New Revolving Credit Facility).
The New Revolving Credit Facility includes borrowing capacity available for letters of credit and for borrowings on same-day notice, referred to as the swingline loans and will be available in U.S. dollars. Currently, the revolving credit facility is undrawn.
Interest Rate and Fees
Borrowings under the New Revolving Credit Facility bear interest at a rate equal to an applicable margin plus, at our option, either (a) a base rate determined by reference to the highest of (1) the corporate base rate of interest of Bank of America, N.A., (2) the federal funds rate plus 0.50% and (3) a Eurodollar rate determined by reference to the costs of funds for deposits in U.S. dollars for the interest period of one month plus 1.00%, or (b) a LIBOR rate determined by reference to the costs of funds for deposits in U.S. dollars for the interest period relevant to such borrowing adjusted for certain additional costs. In no event will the LIBOR rate be less than 2.00% per annum nor will the base rate be less than 3.00% per annum.
ARH is required to pay a commitment fee to the lenders under the revolving credit facility in respect of the unutilized commitments thereunder. The commitment fee rate is 0.75% per annum. ARH must also pay customary letter of credit fees.
Prepayments
The New Revolving Credit Facility requires ARH to prepay outstanding loans under the revolving credit facility at any time the aggregate outstanding amount of loans and letter of credit obligations under the New Revolving Credit Facility exceeds the aggregate commitment of all lenders under the New Revolving Credit Facility, by an amount equal to such excess. ARH may voluntarily repay outstanding loans under the New Revolving Credit Facility at any time without premium or penalty, other than customary “breakage” costs with respect to LIBOR loans.
Prepayments and commitment reductions may be required in connection with certain asset sales and refinancings of intercompany debt.
Repayment
Principal amounts outstanding under the revolving credit facility are due and payable in full at maturity, five years from the date of the closing of the senior secured credit facility.
Guarantees and Security
All obligations under the New Revolving Credit Facility will be unconditionally guaranteed by C.P. Atlas Intermediate Holdings, LLC (“Holdings”) and, subject to certain exceptions, each of Holdings’ existing and future domestic wholly owned subsidiaries (other than ARH) (the “Subsidiary Guarantors” and, together with Holdings, the “Guarantors”).
122
Table of Contents
Subject to certain exceptions, all obligations under the New Revolving Credit Facility, and the guarantees of those obligations, are secured by:
| • | a pledge of 100% of the capital stock of ARH, 100% of the capital stock of each of the Guarantors (other than Holdings) and 65% of the capital stock of each wholly owned foreign subsidiary that is directly owned by any of the Guarantors; and |
| • | a security interest in, and mortgages on, substantially all material tangible and intangible assets of ARH and the Guarantors. |
Certain Covenants and Events of Default
The New Revolving Credit Facility contains a number of covenants that, among other things, restrict, subject to certain exceptions, ARH’s ability and the ability of ARH’s subsidiaries to:
| • | incur additional indebtedness or issue certain preferred stock; |
| • | create liens on assets; |
| • | enter into sale and leaseback transactions; |
| • | engage in mergers or consolidations with or into other companies; |
| • | sell assets; |
| • | pay dividends and distributions or repurchase ARH’s capital stock; |
| • | make investments, loans or advances; |
| • | make certain acquisitions; |
| • | engage in certain transactions with affiliates; |
| • | amend material agreements (including the organizational documents of ARH and its subsidiaries); |
| • | repay certain indebtedness (including the notes offered hereby); |
| • | change its lines of business; |
| • | change its fiscal year; and |
| • | change the status of Holdings as a passive holding company. |
| • | In addition, the revolving credit facility will require ARH to maintain the following financial covenants: |
| • | a maximum consolidated leverage ratio; and |
| • | a minimum fixed charge coverage ratio. |
The New Revolving Credit Facility also contains certain customary affirmative covenants and events of default.
Our Assumed Third-Party Clinic Level Debt
From time to time we have and in the future (subject to certain limitations under the indenture governing the notes offered hereby) may incur third-party working capital indebtedness in connection with the development of a clinic. In connection with the Merger we assumed approximately $11.9 million, as of December 31, 2009, of such indebtedness; the remainder of such third-party debt was repaid at the closing. As of September 30, 2010, $8.2 million of this debt is outstanding. Our assumed third-party clinic debt is represented by 40 facilities at 25 clinics and with 8 lending institutions. No assumed third-party debt at a single clinic exceeds $2 million. This assumed debt has variable interest rates, generally based upon a floating rate of interest, and is generally secured by all of the assets of the respective clinic. Further information about this assumed indebtedness is set forth under “Management’s Discussion and Analysis of Financial Condition and Results of Operations—Off-Balance Sheet Arrangements, Contractual Obligations and Commitments.”
123
Table of Contents
Purpose and Effect of the Exchange Offer
We and the guarantors have entered into a registration rights agreement with the initial purchasers of the outstanding notes in which we and the guarantors agreed, under some circumstances, to use all commercially reasonable efforts to (i) no later 180 days after the issuance of the outstanding notes, file a registration statement with respect to a registered exchange offer to exchange the outstanding notes for new exchange notes, (ii) no later than 210 days after the issuance of the outstanding notes, cause the exchange offer registration statement for the exchange notes to become effective and (iii) no later than 240 days after the issuance of the outstanding notes, to consummate the exchange and to hold the exchange offer open for at least 20 days, or longer, if required by the federal securities laws. The exchange notes will have terms substantially identical to the outstanding notes, except that the exchange notes will not contain terms with respect to transfer restrictions, registration rights and special interest for failure to observe certain obligations in the exchange and registration rights agreement. The outstanding notes were issued on May 7, 2010.
Under the circumstances set below, we will use all commercially reasonable efforts to, no later than 240 days after the issuance of the outstanding notes, file with the SEC a shelf registration statement with respect to the resale of the outstanding notes and cause such shelf registration statement to be declared effective and keep such shelf registration statement continuously effective until the second anniversary of the effective date of the shelf registration statement or such earlier time as there are no longer any outstanding notes to be registered. These circumstances include:
| • | if we and the guarantors are not permitted to consummate the exchange offer because the exchange offer is not permitted by applicable law or SEC policy; and |
| • | if any holder of outstanding notes to be registered notifies us prior to the 20th business day following consummation of the exchange offer that (A) such holder was prohibited by law or SEC policy from participating in the exchange offer, (B) such holder may not resell the exchange notes acquired by it in the exchange offer to the public without delivering a prospectus and this prospectus is not appropriate or available for such resales by such holder or (C) such holder is a broker-dealer and holds outstanding notes to be registered acquired directly from us or one of our affiliates. |
If (i) we have not filed an exchange offer registration statement with the SEC on or prior to the 180th day after the issuance of the outstanding notes, (ii) any such exchange offer registration statement has not been declared effective on or before the 210th day after the issuance of the outstanding notes, (iii) we have not exchanged the exchange notes for all notes validly tendered in accordance with the terms of an exchange offer on or before the 240th day after the issuance of the outstanding notes or, if applicable, (iv) a shelf registration statement covering resales of the notes has not been declared effective or such shelf registration statement ceases to be effective at any time during the shelf registration period (subject to certain exceptions), then additional interest shall accrue on the principal amount of the notes at a rate of 0.25% per annum for the first 180-day period immediately following such date and by an additional 0.25% per annum with respect to each subsequent 90-day period, up to a maximum additional rate of 1.50% per annum thereafter, until the exchange offer is completed, the shelf registration statement is declared effective or, if such shelf registration statement ceased to be effective, again becomes effective or until the second anniversary of the original issue date of the notes, unless such period is extended, as described in the registration rights agreement.
If we fail to comply with certain obligations under the registration rights agreement, we will be required to pay special interest to holders of the outstanding notes.
If you wish to exchange your outstanding notes for exchange notes in the exchange offer, you will be required to make the following written representations:
| • | you are not our affiliate or an affiliate of any guarantor within the meaning of Rule 405 of the Securities Act; |
124
Table of Contents
| • | you have no arrangement or understanding with any person to participate in a distribution (within the meaning of the Securities Act) of the exchange notes in violation of the provisions of the Securities Act; |
| • | you are not engaged in, and do not intend to engage in, a distribution of the exchange notes; and |
| • | you are acquiring the exchange notes in the ordinary course of your business. |
Each broker-dealer that receives exchange notes for its own account in exchange for outstanding notes, where the broker-dealer acquired the outstanding notes as a result of market-making activities or other trading activities, must acknowledge that it will deliver a prospectus in connection with any resale of such exchange notes. See “Plan of Distribution.”
Resale of Exchange Notes
Based on interpretations by the SEC set forth in no-action letters issued to third parties, we believe that you may resell or otherwise transfer exchange notes issued in the exchange offer without complying with the registration and prospectus delivery provisions of the Securities Act if:
| • | you are not our affiliate or an affiliate of any guarantor within the meaning of Rule 405 under the Securities Act; |
| • | you do not have an arrangement or understanding with any person to participate in a distribution of the exchange notes; |
| • | you are not engaged in, and do not intend to engage in, a distribution of the exchange notes; and |
| • | you are acquiring the exchange notes in the ordinary course of your business. |
If you are our affiliate or an affiliate of any guarantor, or are engaging in, or intend to engage in, or have any arrangement or understanding with any person to participate in, a distribution of the exchange notes, or are not acquiring the exchange notes in the ordinary course of your business:
| • | you cannot rely on the position of the SEC set forth inMorgan Stanley & Co. Incorporated (available June 5, 1991) andExxon Capital Holdings Corporation (available May 13, 1988), as interpreted in the SEC’s letter toShearman & Sterling, dated July 2, 1993, or similar no-action letters; and |
| • | in the absence of an exception from the position stated immediately above, you must comply with the registration and prospectus delivery requirements of the Securities Act in connection with any resale of the exchange notes. |
This prospectus may be used for an offer to resell, resale or other transfer of exchange notes only as specifically set forth in this prospectus. With regard to broker-dealers, only broker-dealers that acquired the outstanding notes as a result of market-making activities or other trading activities may participate in the exchange offer. Each broker-dealer that receives exchange notes for its own account in exchange for outstanding notes, where such outstanding notes were acquired by such broker-dealer as a result of market-making activities or other trading activities, must acknowledge that it will deliver a prospectus in connection with any resale of the exchange notes. Please read “Plan of Distribution” for more details regarding the transfer of exchange notes.
Terms of the Exchange Offer
On the terms and subject to the conditions set forth in this prospectus and in the letter of transmittal, we will accept for exchange in the exchange offer any outstanding notes that are properly tendered and not withdrawn prior to the expiration date. Outstanding notes may only be tendered in minimum denominations of $2,000 and integral multiples of $1,000 in excess of $2,000. We will issue exchange notes in principal amount identical to outstanding notes surrendered in the exchange offer.
125
Table of Contents
The form and terms of the exchange notes will be substantially identical to the form and terms of the outstanding notes except the exchange notes will be registered under the Securities Act, will not bear legends restricting their transfer and will not provide for any special interest upon our failure to fulfill our obligations under the exchange and registration rights agreement to complete the exchange offer, or file, and cause to be effective, a registration statement, if required thereby, within the specified time period described above. The exchange notes will evidence the same debt as the outstanding notes. The exchange notes will be issued under and entitled to the benefits of the same indenture that authorized the issuance of the outstanding notes. Consequently, the outstanding notes and the exchange notes will be treated as a single class of debt securities under the indenture. For a description of the indenture, see “Description of the Exchange Notes.”
The exchange offer is not conditioned upon any minimum aggregate principal amount of outstanding notes being tendered for exchange.
As of the date of this prospectus, $250 million aggregate principal amount of the 8.375% Senior Secured Notes due 2018 are outstanding. This prospectus and a letter of transmittal are being sent to all registered holders of outstanding notes. There will be no fixed record date for determining registered holders of outstanding notes entitled to participate in the exchange offer. We intend to conduct the exchange offer in accordance with the provisions of the registration rights agreement, the applicable requirements of the Securities Act and the Securities Exchange Act of 1934, as amended (the “Exchange Act”), and the rules and regulations of the SEC. Outstanding notes that are not tendered for exchange in the exchange offer will remain outstanding and continue to accrue interest and will be entitled to the rights and benefits the holders have under the indenture relating to the outstanding and the exchange and registration rights agreement, except for any rights under the registration rights agreement that by their terms terminate upon the consummation of the exchange offer.
We will be deemed to have accepted for exchange properly tendered outstanding notes when we have given oral or written notice of the acceptance to the exchange agent. The exchange agent will act as agent for the tendering holders for the purposes of receiving the exchange notes from us and delivering exchange notes to holders. Subject to the terms of the exchange and registration rights agreement, we expressly reserve the right to amend or terminate the exchange offer and to refuse to accept for exchange any outstanding notes not previously accepted for exchange, upon the occurrence of any of the conditions specified below under “—Conditions to the Exchange Offer.”
If you tender your outstanding notes in the exchange offer, you will not be required to pay brokerage commissions or fees or, subject to the instructions in the letter of transmittal, transfer taxes with respect to the exchange of outstanding notes. We will pay all charges and expenses, other than certain applicable taxes described below in connection with the exchange offer. It is important that you read “—Fees and Expenses” below for more details regarding fees and expenses incurred in the exchange offer.
Expiration Date, Extensions and Amendments
As used in this prospectus, the term “expiration date” means 11:59 p.m., New York City time, on, , 2011. However, if we, in our sole discretion, extend the period of time for which the exchange offer is open, the term “expiration date” will mean the latest time and date to which we shall have extended the expiration of the exchange offer.
To extend the period of time during which the exchange offer is open, we will notify the exchange agent of any extension by oral or written notice, followed by notification by press release or other public announcement to the registered holders of the outstanding notes no later than 9:00 a.m., New York City time, on the next business day after the previously scheduled expiration date.
We reserve the right, in our sole discretion:
| • | to delay accepting for exchange any outstanding notes (only in the case that we amend or extend the exchange offer); |
126
Table of Contents
| • | to extend the exchange offer or to terminate the exchange offer and refuse to accept outstanding notes not previously accepted if any of the conditions set forth below under “—Conditions to the Exchange Offer” have not been satisfied, by giving oral or written notice of such delay, extension or termination to the exchange agent; and |
| • | subject to the terms of the exchange and registration rights agreement, to amend the terms of the exchange offer in any manner. In the event of a material change in the exchange offer, including the waiver of a material condition, we will extend the offer period, if necessary, so that at least five business days remain in such offer period following notice of the material change. |
Any delay in acceptance, extension, termination or amendment will be followed as promptly as practicable by oral or written notice to the registered holders of the outstanding notes. If we amend the exchange offer in a manner that we determine to constitute a material change, we will promptly disclose the amendment in a manner reasonably calculated to inform the holders of the outstanding notes of that amendment.
Without limiting the manner in which we may choose to make public announcements of any delay in acceptance, extension, termination or amendment of the exchange offer, we will have no obligation to publish, advertise, or otherwise communicate any public announcement, other than by making a timely release to a financial news service.
Conditions to the Exchange Offer
Despite any other term of the exchange offer, we will not be required to accept for exchange, or to issue exchange notes in exchange for, any outstanding notes and we may terminate or amend the exchange offer as provided in this prospectus prior to the expiration date if in our reasonable judgment:
| • | the exchange offer or the making of any exchange by a holder violates any applicable law or interpretation of the SEC; or |
| • | any action or proceeding has been instituted or threatened in writing in any court or by or before any governmental agency with respect to the exchange offer that, in our judgment, would reasonably be expected to impair our ability to proceed with the exchange offer. |
In addition, we will not be obligated to accept for exchange the outstanding notes of any holder that has not made to us:
| • | the representations described under “—Purpose and Effect of the Exchange Offer,” “—Procedures for Tendering Outstanding Notes” and “Plan of Distribution”; or |
| • | any other representations as may be reasonably necessary under applicable SEC rules, regulations or interpretations to make available to us an appropriate form for registration of the exchange notes under the Securities Act. |
We expressly reserve the right at any time or at various times to extend the period of time during which the exchange offer is open. Consequently, we may delay acceptance of any outstanding notes by giving oral or written notice of such extension to their holders. We will return any outstanding notes that we do not accept for exchange for any reason without expense to their tendering holder promptly after the expiration or termination of the exchange offer.
We expressly reserve the right to amend or terminate the exchange offer and to reject for exchange any outstanding notes not previously accepted for exchange, upon the occurrence of any of the conditions of the exchange offer specified above. We will give oral or written notice of any extension, amendment, non-acceptance or termination to the holders of the outstanding notes as promptly as practicable. In the case of any extension, such notice will be issued no later than 9:00 a.m., New York City time, on the next business day after the previously scheduled expiration date.
127
Table of Contents
These conditions are for our sole benefit, and we may assert them regardless of the circumstances that may give rise to them or waive them in whole or in part at any or at various times prior to the expiration date in our sole discretion. If we fail at any time to exercise any of the foregoing rights, this failure will not constitute a waiver of such rights. Each such right will be deemed an ongoing right that we may assert at any time or at various times prior to the expiration date.
In addition, we will not accept for exchange any outstanding notes tendered, and will not issue exchange notes in exchange for any such outstanding notes, if at such time any stop order is threatened or in effect with respect to the registration statement of which this prospectus constitutes a part or the qualification of the indenture under the Trust Indenture Act of 1939 (the “TIA”).
Procedures for Tendering Outstanding Notes
To tender your outstanding notes in the exchange offer, you must comply with either of the following:
| • | complete, sign and date the letter of transmittal, or a facsimile of the letter of transmittal, have the signature(s) on the letter of transmittal guaranteed if required by the letter of transmittal and mail or deliver such letter of transmittal or facsimile thereof to the exchange agent at the address set forth below under “—Exchange Agent” prior to the expiration date; or |
| • | comply with DTC’s Automated Tender Offer Program procedures described below. |
In addition, either:
| • | the exchange agent must receive certificates for the outstanding notes along with the letter of transmittal prior to the expiration date; |
| • | the exchange agent must receive a timely confirmation of book-entry transfer of the outstanding notes into the exchange agent’s account at DTC according to the procedures for book-entry transfer described below or a properly transmitted agent’s message prior to the expiration date; or |
| • | you must comply with the guaranteed delivery procedures described below. |
Your tender, if not withdrawn prior to the expiration date, constitutes an agreement between us and you upon the terms and subject to the conditions described in this prospectus and in the letter of transmittal.
The method of delivery of outstanding notes, letter of transmittal and all other required documents to the exchange agent is at your election and risk. We recommend that instead of delivery by mail, you use an overnight or hand delivery service, properly insured. In all cases, you should allow sufficient time to assure timely delivery to the exchange agent before the expiration date. You should not send letters of transmittal or certificates representing outstanding notes to us. You may request that your broker, dealer, commercial bank, trust company or nominee effect the above transactions for you.
If you are a beneficial owner whose outstanding notes are registered in the name of a broker, dealer, commercial bank, trust company or other nominee and you wish to tender your outstanding notes, you should promptly contact the registered holder and instruct the registered holder to tender on your behalf. If you wish to tender the outstanding notes yourself, you must, prior to completing and executing the letter of transmittal and delivering your outstanding notes, either:
| • | make appropriate arrangements to register ownership of the outstanding notes in your name; or |
| • | obtain a properly completed bond power from the registered holder of outstanding notes. |
The transfer of registered ownership may take considerable time and may not be able to be completed prior to the expiration date.
128
Table of Contents
Signatures on the letter of transmittal or a notice of withdrawal, as the case may be, must be guaranteed by a member firm of a registered national securities exchange or of the Financial Industry Regulatory Authority, Inc., a commercial bank or trust company having an office or correspondent in the United States or another “eligible guarantor institution” within the meaning of Rule 17A(d)-15 under the Exchange Act unless the outstanding notes surrendered for exchange are tendered:
| • | by a registered holder of the outstanding notes who has not completed the box entitled “Special Registration Instructions” or “Special Delivery Instructions” on the letter of transmittal; or |
| • | for the account of an eligible guarantor institution. |
If the letter of transmittal is signed by a person other than the registered holder of any outstanding notes listed on the outstanding notes, such outstanding notes must be endorsed or accompanied by a properly completed bond power. The bond power must be signed by the registered holder as the registered holder’s name appears on the outstanding notes, and an eligible guarantor institution must guarantee the signature on the bond power.
If the letter of transmittal, any certificates representing outstanding notes or bond powers are signed by trustees, executors, administrators, guardians, attorneys-in-fact, officers of corporations or others acting in a fiduciary or representative capacity, those persons should also indicate when signing and, unless waived by us, they should also submit evidence satisfactory to us of their authority to so act.
The exchange agent and DTC have confirmed that any financial institution that is a participant in DTC’s system may use DTC’s Automated Tender Offer Program to tender outstanding notes. Participants in the program may, instead of physically completing and signing the letter of transmittal and delivering it to the exchange agent, electronically transmit their acceptance of the exchange by causing DTC to transfer the outstanding notes to the exchange agent in accordance with DTC’s Automated Tender Offer Program procedures for transfer. DTC will then send an agent’s message to the exchange agent. The term “agent’s message” means a message transmitted by DTC, received by the exchange agent and forming part of the book-entry confirmation, which states that:
| • | DTC has received an express acknowledgment from a participant in its Automated Tender Offer Program that is tendering outstanding notes that are the subject of the book-entry confirmation; |
| • | the participant has received and agrees to be bound by the terms of the letter of transmittal, or in the case of an agent’s message relating to guaranteed delivery, that such participant has received and agrees to be bound by the notice of guaranteed delivery; and |
| • | we may enforce that agreement against such participant. DTC is referred to herein as a “book-entry transfer facility.” |
Acceptance of Exchange Notes
In all cases, we will promptly issue exchange notes for outstanding notes that we have accepted for exchange under the exchange offer only after the exchange agent timely receives:
| • | outstanding notes or a timely book-entry confirmation of such outstanding notes into the exchange agent’s account at the book-entry transfer facility; and |
| • | a properly completed and duly executed letter of transmittal and all other required documents or a properly transmitted agent’s message. |
By tendering outstanding notes pursuant to the exchange offer, you will represent to us that, among other things:
| • | you are not our affiliate or an affiliate of any guarantor within the meaning of Rule 405 under the Securities Act; |
129
Table of Contents
| • | you do not have an arrangement or understanding with any person or entity to participate in a distribution of the exchange notes; and |
| • | you are acquiring the exchange notes in the ordinary course of your business. |
In addition, each broker-dealer that is to receive exchange notes for its own account in exchange for outstanding notes must represent that such outstanding notes were acquired by that broker-dealer as a result of market-making activities or other trading activities and must acknowledge that it will deliver a prospectus that meets the requirements of the Securities Act in connection with any resale of the exchange notes. The letter of transmittal states that by so acknowledging and by delivering a prospectus, a broker-dealer will not be deemed to admit that it is an “underwriter” within the meaning of the Securities Act. See “Plan of Distribution.”
We will interpret the terms and conditions of the exchange offer, including the letter of transmittal and the instructions to the letter of transmittal, and will resolve all questions as to the validity, form, eligibility, including time of receipt and acceptance of outstanding notes tendered for exchange. Our determinations in this regard will be final and binding on all parties. We reserve the absolute right to reject any and all tenders of any particular outstanding notes not properly tendered or to not accept any particular outstanding notes if the acceptance might, in its or its counsel’s judgment, be unlawful. We also reserve the absolute right to waive any defects or irregularities as to any particular outstanding notes prior to the expiration date.
Unless waived, any defects or irregularities in connection with tenders of outstanding notes for exchange must be cured within such reasonable period of time as we determine. Neither we, the exchange agent nor any other person will be under any duty to give notification of any defect or irregularity with respect to any tender of outstanding notes for exchange, nor will any of them incur any liability for any failure to give notification. Any outstanding notes received by the exchange agent that are not properly tendered and as to which the irregularities have not been cured or waived will be returned by the exchange agent to the tendering holder, unless otherwise provided in the letter of transmittal, promptly after the expiration date.
Book-Entry Delivery Procedures
Promptly after the date of this prospectus, the exchange agent will establish an account with respect to the outstanding notes at DTC and, as the book-entry transfer facility, for purposes of the exchange offer. Any financial institution that is a participant in the book-entry transfer facility’s system may make book-entry delivery of the outstanding notes by causing the book-entry transfer facility to transfer those outstanding notes into the exchange agent’s account at the facility in accordance with the facility’s procedures for such transfer. To be timely, book-entry delivery of outstanding notes requires receipt of a confirmation of a book-entry transfer, a “book-entry confirmation,” prior to the expiration date. In addition, although delivery of outstanding notes may be effected through book-entry transfer into the exchange agent’s account at the book-entry transfer facility, the letter of transmittal or a manually signed facsimile thereof, together with any required signature guarantees and any other required documents, or an “agent’s message,” as defined below, in connection with a book-entry transfer, must, in any case, be delivered or transmitted to and received by the exchange agent at its address set forth on the cover page of the letter of transmittal prior to the expiration date to receive exchange notes for tendered outstanding notes, or the guaranteed delivery procedure described below must be complied with. Tender will not be deemed made until such documents are received by the exchange agent. Delivery of documents to the book-entry transfer facility does not constitute delivery to the exchange agent.
Holders of outstanding notes who are unable to deliver confirmation of the book-entry tender of their outstanding notes into the exchange agent’s account at the book-entry transfer facility or all other documents required by the letter of transmittal to the exchange agent on or prior to the expiration date must tender their outstanding notes according to the guaranteed delivery procedures described below.
130
Table of Contents
Guaranteed Delivery Procedures
If you wish to tender your outstanding notes but your outstanding notes are not immediately available or you cannot deliver your outstanding notes, the letter of transmittal or any other required documents to the exchange agent or comply with the procedures under DTC’s Automatic Tender Offer Program in the case of outstanding notes, prior to the expiration date, you may still tender if:
| • | the tender is made through an eligible guarantor institution; |
| • | prior to the expiration date, the exchange agent receives from such eligible guarantor institution either a properly completed and duly executed notice of guaranteed delivery, by facsimile transmission, mail, or hand delivery or a properly transmitted agent’s message and notice of guaranteed delivery, that (1) sets forth your name and address, the certificate number(s) of such outstanding notes and the principal amount of outstanding notes tendered; (2) states that the tender is being made thereby; and (3) guarantees that, within three New York Stock Exchange trading days after the expiration date, the letter of transmittal, or facsimile thereof, together with the outstanding notes or a book-entry confirmation, and any other documents required by the letter of transmittal, will be deposited by the eligible guarantor institution with the exchange agent; and |
| • | the exchange agent receives the properly completed and executed letter of transmittal or facsimile thereof, as well as certificate(s) representing all tendered outstanding notes in proper form for transfer or a book-entry confirmation of transfer of the outstanding notes into the exchange agent’s account at DTC and all other documents required by the letter of transmittal within three New York Stock Exchange trading days after the expiration date. |
Upon request, the exchange agent will send to you a notice of guaranteed delivery if you wish to tender your outstanding notes according to the guaranteed delivery procedures.
Withdrawal Rights
Except as otherwise provided in this prospectus, you may withdraw your tender of outstanding notes at any time prior to 11:59 p.m., New York City time, on the expiration date.
For a withdrawal to be effective:
| • | the exchange agent must receive a written notice, which may be by telegram, telex, facsimile or letter, of withdrawal at its address set forth below under “—Exchange Agent”; or |
| • | you must comply with the appropriate procedures of DTC’s Automated Tender Offer Program system. |
Any notice of withdrawal must:
| • | specify the name of the person who tendered the outstanding notes to be withdrawn; |
| • | identify the outstanding notes to be withdrawn, including the certificate numbers and principal amount of the outstanding notes; and |
| • | where certificates for outstanding notes have been transmitted, specify the name in which such outstanding notes were registered, if different from that of the withdrawing holder. |
If certificates for outstanding notes have been delivered or otherwise identified to the exchange agent, then, prior to the release of such certificates, you must also submit:
| • | the serial numbers of the particular certificates to be withdrawn; and |
| • | a signed notice of withdrawal with signatures guaranteed by an eligible institution unless you are an eligible guarantor institution. |
131
Table of Contents
If outstanding notes have been tendered pursuant to the procedures for book-entry transfer described above, any notice of withdrawal must specify the name and number of the account at the book-entry transfer facility to be credited with the withdrawn outstanding notes and otherwise comply with the procedures of the facility. We will determine all questions as to the validity, form and eligibility, including time of receipt of notices of withdrawal, and our determination will be final and binding on all parties. Any outstanding notes so withdrawn will be deemed not to have been validly tendered for exchange for purposes of the exchange offer. Any outstanding notes that have been tendered for exchange but that are not exchanged for any reason will be returned to their holder, without cost to the holder, or, in the case of book-entry transfer, the outstanding notes will be credited to an account at the book-entry transfer facility, promptly after withdrawal, rejection of tender or termination of the exchange offer. Properly withdrawn outstanding notes may be retendered by following the procedures described under “—Procedures for Tendering Outstanding Notes” above at any time on or prior to the expiration date.
Exchange Agent
Wilmington Trust FSB has been appointed as the exchange agent for the exchange offers. You should direct all executed letters of transmittal and all questions and requests for assistance, requests for additional copies of this prospectus or of the letter of transmittal and requests for notices of guaranteed delivery to the exchange agent addressed as follows:
By Registered or Certified Mail: | By Regular Mail: |
| By Overnight Courier or Hand Delivery: |
| ||
Wilmington Trust FSB c/o Wilmington Trust Company Rodney Square North 1100 North Market Street Wilmington, DE 19890-1626 Attn: Sam Hamed Telephone: (302) 636-6181 | Wilmington Trust FSB c/o Wilmington Trust Company Rodney Square North 1100 North Market Street Wilmington, DE 19890-1626 Attn: Sam Hamed Telephone: (302) 636-6181 |
| Wilmington Trust FSB c/o Wilmington Trust Company Rodney Square North 1100 North Market Street Wilmington, DE 19890-1626 Attn: Sam Hamed Telephone: (302) 636-6181 |
| ||
| By Facsimile Transmission (eligible institutions only): | ||||||
(302) 636-4139, Attention: Sam Hamed | ||||||
Telephone Inquiries: (302) 636-6181 | ||||||
If you deliver the letter of transmittal to an address other than the one set forth above or transmit instructions via facsimile to a number other than the one set forth above, that delivery or those instructions will not be effective.
Fees and Expenses
The registration rights agreement provides that we will bear all expenses in connection with the performance of our obligations relating to the registration of the exchange notes and the conduct of the exchange offer. These expenses include registration and filing fees, accounting and legal fees and printing costs, among others. We will pay the exchange agent reasonable and customary fees for its services and reasonable out-of-pocket expenses. We will also reimburse brokerage houses and other custodians, nominees and fiduciaries for customary mailing and handling expenses incurred by them in forwarding this prospectus and related documents to their clients that are holders of outstanding notes and for handling or tendering for such clients.
We have not retained any dealer-manager in connection with the exchange offer and will not pay any fee or commission to any broker, dealer, nominee or other person, other than the exchange agent, for soliciting tenders of outstanding notes pursuant to the exchange offer.
132
Table of Contents
Accounting Treatment
We will record the exchange notes in our accounting records at the same carrying value as the outstanding notes, which is the aggregate principal amount as reflected in our accounting records on the date of exchange. Accordingly, we will not recognize any gain or loss for accounting purposes upon the consummation of the exchange offer. We will record the expenses of the exchange offer as incurred.
Transfer Taxes
We will pay all transfer taxes, if any, applicable to the exchange of outstanding notes under the exchange offer. The tendering holder, however, will be required to pay any transfer taxes, whether imposed on the registered holder or any other person, if:
| • | certificates representing outstanding notes for principal amounts not tendered or accepted for exchange are to be delivered to, or are to be issued in the name of, any person other than the registered holder of outstanding notes tendered; |
| • | tendered outstanding notes are registered in the name of any person other than the person signing the letter of transmittal; or |
| • | a transfer tax is imposed for any reason other than the exchange of outstanding notes under the exchange offer. |
If satisfactory evidence of payment of such taxes is not submitted with the letter of transmittal, the amount of such transfer taxes will be billed to that tendering holder.
Holders who tender their outstanding notes for exchange will not be required to pay any transfer taxes. However, holders who instruct us to register exchange notes in the name of, or request that outstanding notes not tendered or not accepted in the exchange offer be returned to, a person other than the registered tendering holder will be required to pay any applicable transfer tax.
Consequences of Failure to Exchange
If you do not exchange your outstanding notes for exchange notes under the exchange offer, your outstanding notes will remain subject to the restrictions on transfer of such outstanding notes:
| • | as set forth in the legend printed on the outstanding notes as a consequence of the issuance of the outstanding notes pursuant to the exemption from, or in transactions not subject to, the registration requirements of the Securities Act and applicable state securities laws; and |
| • | as otherwise set forth in the prospectus distributed in connection with the private offering of the outstanding notes. |
In general, you may not offer or sell your outstanding notes unless they are registered under the Securities Act or if the offer or sale is exempt from registration under the Securities Act and applicable state securities laws. Except as required by the exchange and registration rights agreement, we do not intend to register resales of the outstanding notes under the Securities Act.
Other
Participating in the exchange offer is voluntary, and you should carefully consider whether to accept. You are urged to consult your financial and tax advisors in making your own decision on what action to take.
We may in the future seek to acquire untendered outstanding notes in open market or privately negotiated transactions, through subsequent exchange offers or otherwise. We have no present plans to acquire any outstanding notes that are not tendered in the exchange offer or to file a registration statement to permit resales of any untendered outstanding notes.
133
Table of Contents
The Company is filing this registration statement in connection with its obligations under the registration rights agreement, which the Company and its guarantor subsidiaries entered into with the initial purchasers with respect to the outstanding notes on the original issue date of the outstanding notes, pursuant to which the Company and its guarantor subsidiaries have agreed, for the benefit of the holders of the outstanding notes, that they will, at their own expense, (i) file an exchange offer registration statement with the SEC with respect to an offer to exchange the outstanding notes for exchange notes, having certain identical terms in all material respects to the outstanding notes offered hereby and which will evidence the same continuing indebtedness (except that the exchange notes will not contain terms with respect to transfer restrictions or interest rate increases as described under “The Exchange Offer—Purpose and Effect of the Exchange Offer”), (ii) use their reasonable efforts to cause the exchange offer registration statement to be declared effective by the SEC under the Securities Act and (iii) use their reasonable efforts to consummate the exchange offer not later than 240 days following the closing date of the original issuance of the outstanding notes. Once the exchange offer registration statement has been declared effective, we will offer the exchange notes in exchange for surrender of the outstanding notes. We will keep the exchange offer open for at least 20 business days (or longer if required by applicable law) after the date that notice of the exchange offer is mailed to holders of the outstanding notes.
In the event that (i) any changes in law or the applicable interpretations of the staff of the SEC do not permit us to effect the exchange offer, (ii) for any other reason the exchange offer is not consummated within 240 days of the original issue date of the outstanding notes, (iii) under certain circumstances, the initial purchasers shall so request or (iv) any holder of outstanding notes (other than the initial purchasers) is not eligible to participate in the exchange offer, we will, at our expense, (a) promptly after the filing obligation takes effect, file with the SEC a shelf registration statement covering resales of the outstanding notes, (b) use our commercially reasonable efforts to cause the shelf registration statement to be declared effective and (c) use our commercially reasonable efforts to keep the shelf registration statement effective until the earlier of the second anniversary of the closing of this offering and the date all outstanding notes covered by the shelf registration statement have either been sold in the manner set forth and as contemplated in the shelf registration statement or become eligible for resale pursuant to Rule 144 under the Securities Act without volume restrictions. We will, in the event of the filing of the shelf registration statement, provide to each holder of the outstanding notes copies of the prospectus which is a part of the shelf registration statement, notify each such holder when the shelf registration statement has become effective and take certain other actions as are required to permit unrestricted resales of the outstanding notes. A holder of the outstanding notes that sells its outstanding notes pursuant to the shelf registration statement generally (i) will be required to be named as a selling security holder in the related prospectus and to deliver a prospectus to purchasers, (ii) will be subject to certain of the civil liability provisions under the Securities Act in connection with such sales and (iii) will be bound by the provisions of the registration rights agreement that are applicable to such a holder (including certain indemnification rights and obligations thereunder). In addition, each holder of the outstanding notes will be required to deliver information to be used in connection with the shelf registration statement and to provide comments on the shelf registration statement within the time periods set forth in the registration rights agreement to have their outstanding notes included in the shelf registration statement and to benefit from certain provisions regarding liquidated damages.
This summary of certain provisions of the registration rights agreement does not purport to be complete and is subject to, and is qualified in its entirety by, the complete provisions of the registration rights agreement, a copy of which we will make available to holders of outstanding notes upon request.
134
Table of Contents
DESCRIPTION OF THE EXCHANGE NOTES
You can find the definitions of certain terms used in this description under the subheading “—Certain Definitions.” In this description, (1) the term “Issuer” refers only to American Renal Holdings Inc. and not to any of its subsidiaries and (2) the term “Notes” refers to the outstanding notes and the exchange notes.
The outstanding notes were, and the exchange notes will be, issued under an Indenture (the “Indenture”), dated as May 7, 2010, among the Issuer, the Guarantors and Wilmington Trust FSB, as trustee (the “Trustee”). The terms of the Notes will include those stated in the Indenture and those made part of the Indenture by reference to the Trust Indenture Act of 1939, as amended (the “Trust Indenture Act”).
The following description is a summary of the material provisions of the Indenture, the Security Documents and the Intercreditor Agreement. It does not restate the Indenture, the Security Documents and the Intercreditor Agreement in their entirety. We urge you to read the Indenture, the Security Documents and the Intercreditor Agreement because the Indenture, the Security Documents and the Intercreditor Agreement, and not this description, define your rights as holders of the Notes. Copies of the Indenture, the Security Documents and the Intercreditor Agreement are available as set forth below under “—Additional Information.” Certain defined terms used in this description but not defined below under “—Certain Definitions” have the meanings assigned to them in the Indenture.
The registered holder of a note will be treated as the owner of it for all purposes. Only registered holders will have rights under the Indenture. For additional information, see “The Exchange Offer.”
Brief Description of the Notes and the Guarantees of the Notes
The Notes:
| • | are secured, subject to Permitted Liens, by the Collateral, which will also secure, on an equal and ratable basis, any Pari Passu Lien Indebtedness and the Priority Payment Lien Obligations (although the Holders of Notes will receive proceeds of Collateral after the payment in full of the Priority Payment Lien Obligations in the event of a foreclosure or in any bankruptcy, insolvency or similar event); |
| • | are structurally subordinated to all Indebtedness (other than Indebtedness owing to the Issuer or a Guarantor) of Non-Guarantor Subsidiaries, including all of the Subsidiaries that operate the clinics; |
| • | rank equally in right of payment with any existing and future senior Indebtedness of the Issuer; |
| • | will be effectively senior to any future Indebtedness of the Issuer that is unsecured or secured by Liens that are junior to the Liens securing the Notes, in each case, to the extent of the value of the Collateral (after giving effect to Liens securing the Priority Payment Lien Obligations and any other Lien on the Collateral); |
| • | are senior in right of payment to any future obligations of the Issuer that are expressly subordinated in right of payment to the Notes; |
| • | are subject in right of payment to the prior payment of Priority Payment Lien Obligations in the event of a foreclosure or in any insolvency proceeding with proceeds of the Collateral; and |
| • | are entitled to registration rights pursuant to the Registration Rights Agreement. |
The Notes are unconditionally guaranteed, jointly and severally, by Holdings and each of Issuer’s existing and future Subsidiaries other than Excluded Subsidiaries.
135
Table of Contents
The Guarantee of each Guarantor:
| • | are secured, subject to Permitted Liens, by the Collateral, which will also secure, on an equal and ratable basis, any Pari Passu Lien Indebtedness and the Priority Payment Lien Obligations (although the Holders will receive proceeds of Collateral after the payment in full of the Priority Payment Lien Obligations in the event of a foreclosure or in any bankruptcy, insolvency or similar event); |
| • | are subject in right of payment to the prior payment of Priority Payment Lien Obligations in the event of a foreclosure or in any insolvency proceeding with proceeds of the Collateral; |
| • | rank equally in right of payment with all existing and future senior Indebtedness of that Guarantor; |
| • | are effectively senior to any future Indebtedness of that Guarantor that is unsecured or secured by Liens that are junior to the Liens securing the Guarantees, in each case, to the extent of the value of the Collateral (after giving effect to Liens securing the Priority Payment Lien Obligations and any other senior Lien on the Collateral); and |
| • | are senior in right of payment to any future Indebtedness of that Guarantor that is expressly subordinated in right of payment to the Guarantee of that Guarantor. |
As of September 30, 2010, the Issuer and the Guarantors had aggregate principal amount of Indebtedness (other than intercompany Indebtedness) of $250.0 million. The Issuer was also been able to borrow up to an additional $25.0 million of secured Indebtedness under the Credit Agreement, which constitutes Priority Payment Lien Obligations.
Holders of the Notes are only creditors of the Issuer and the Guarantors, and not of our Non-Guarantor Subsidiaries. In the event of a bankruptcy, liquidation or reorganization of any of the Non-Guarantor Subsidiaries, the Non-Guarantor Subsidiaries will pay the holders of their debt, including their trade creditors, secured creditors and creditors holding indebtedness or guarantees issued by those subsidiaries, before they will be able to distribute any of their assets to us. As a result, all the existing and future liabilities of our Non-Guarantor Subsidiaries, including any claims of trade creditors, are effectively senior to the Notes.
The Issuer and the Guarantors (other than American Renal Management LLC) are currently holding companies for our joint venture subsidiaries that operate the clinics. Non-Guarantor Subsidiaries account for substantially all of our consolidated revenues, cash flows and assets. As of September 30, 2010, the Non-Guarantor Subsidiaries had approximately $98.4 million of total balance sheet liabilities. Although the Indenture contains limitations on the amount of additional Indebtedness that we and the Restricted Subsidiaries may incur, the amounts of this Indebtedness could be substantial. In addition, because the Indenture does not materially limit our ability to purchase equity interests of Strategic Investors in our Qualified Restricted Subsidiaries, the holders of those interests may have the ability to obtain a return on their investment senior to the holders of the Notes. Our ability to service our debt, including the Notes, is dependent upon the earnings of our Non-Guarantor Subsidiaries and their ability to distribute those earnings to us. See “Risk Factors—Risks Related to the Notes—The right to receive payments on the notes is structurally subordinated to the liabilities of our non-guarantor subsidiaries and substantially all of our operations are conducted through non-guarantor subsidiaries.”
Under the circumstances described below under the caption “—Certain Covenants—Designation of Restricted and Unrestricted Subsidiaries,” we were permitted to designate certain of our Subsidiaries as “Unrestricted Subsidiaries.” Our Unrestricted Subsidiaries will not be subject to many of the restrictive covenants in the Indenture and will not guarantee the Notes. There are no Unrestricted Subsidiaries as of the Issue Date.
136
Table of Contents
Principal, Maturity and Interest
The Issuer issued $250.0 million aggregate principal amount of Notes in a private transaction that was not subject to the registration requirements of the Securities Act. The Notes were issued in initial denominations of $2,000 principal amount and integral multiples of $1,000 principal amount in excess thereof. The Notes will mature on May 15, 2018. Subject to our compliance with the covenant described under the subheading “—Certain Covenants—Incurrence of Indebtedness and Issuance of Disqualified Stock and Preferred Stock” and “—Certain Covenants—Liens,” we are permitted to issue an unlimited amount of additional Notes under the Indenture (the “Additional Notes”) on the same terms and conditions as the Notes being offered hereby. Any Additional Notes will be secured, equally and ratably with the Notes offered hereby, by liens on the Collateral. The Notes and the Additional Notes, if any, will be treated as a single class for all purposes of the Indenture, including waivers, amendments, redemptions and offers to purchase;provided that, if any Additional Notes subsequently issued are not fungible for U.S. federal income tax purposes or securities law purposes with any Notes previously issued, such Additional Notes shall trade separately from such previously issued Notes under a separate CUSIP number but shall otherwise be treated as a single class with all other Notes issued under the Indenture. Unless the context otherwise requires, for all purposes of the Indenture and this “Description of the Exchange Notes,” references to the Notes include any Additional Notes actually issued.
Interest on the Notes accrues at the rate of 8.375% per annum from May 7, 2010, or from the most recent date to which interest has been paid or provided for, and will be payable semiannually in arrears on May 15 and November 15 of each year, commencing on November 15, 2010. We will make each interest payment to the holders of record of the Notes on the immediately preceding May 1 and November 1. We will pay interest on overdue principal at 2% per annum in excess of the above rate and will pay interest on overdue installments of interest at such higher rate to the extent lawful. Interest will be computed on the basis of a 360-day year comprised of twelve 30-day months.
Methods of Receiving Payments on the Notes
Principal of, premium, if any, and interest and Additional Interest on the Notes are payable, and the Notes may be exchanged or transferred, at the office or agency of the Issuer (which initially will be an office of an affiliate of the Trustee);provided, however, payment of interest and Additional Interest may, at the option of the Issuer, be made by check mailed to the address of the holders as such address appears in the register of holders, and in addition, if a holder of at least $1.0 million in aggregate principal amount of Notes has given wire transfer instructions to us prior to the record date for a payment, the Issuer will make such payment of principal of, premium, if any, and interest and Additional Interest on such holder’s Notes in accordance with those instructions. Payment of principal of, premium, if any, and interest and Additional Interest on, Notes in global form registered in the name of or held by DTC or any successor depositary or its nominee will be made by wire transfer of immediately available funds to such depositary or its nominee, as the case may be, as the registered holder of such global note.
Paying Agent and Registrar for the Notes
The Trustee will initially act as paying agent and registrar. The Issuer may change the paying agent or registrar without prior notice to the holders of the Notes, and the Issuer or any of its Subsidiaries may act as paying agent or registrar.
Transfer and Exchange
A holder may transfer or exchange Notes in accordance with the provisions of the Indenture. The registrar and the Trustee may require a holder, among other things, to furnish appropriate endorsements and transfer documents in connection with a transfer of Notes. No service charge will be made for any registration of transfer or exchange of Notes, but the Issuer may require payment of a sum sufficient to cover any transfer tax or other
137
Table of Contents
similar governmental charge payable in connection therewith. The Issuer is not be required to transfer or exchange any note selected for redemption. Also, the Issuer is not be required to transfer or exchange any note for a period of 15 days before a selection of Notes to be redeemed.
Optional Redemption
Prior to May 15, 2013, not more than once in each twelve-month period, the Issuer may, at its option, redeem up to 10% of the aggregate principal amount of the Notes issued under the Indenture at a redemption price equal to 103% of the principal amount of the Notes to be redeemed, plus accrued and unpaid interest and Additional Interest thereon, if any, to the date of redemption.
At any time prior to May 15, 2013, the Issuer may, on one or more occasions, redeem up to 35% of the aggregate principal amount of Notes issued under the Indenture at a redemption price of 108.375% of the principal amount, plus accrued and unpaid interest and Additional Interest, if any, to the redemption date, with the net cash proceeds of one or more Equity Offerings by the Issuer or a cash contribution to the equity capital of the Issuer (other than Disqualified Stock) from the net cash proceeds of one or more Equity Offerings by the Issuer, Holdings or any other direct or indirect parent of the Issuer;provided that:
(1) at least 65% of the aggregate principal amount of Notes issued under the Indenture (excluding Notes held by the Issuer and its Affiliates) remains outstanding immediately after the occurrence of such redemption; and
(2) the redemption occurs within 90 days of the date of the closing of such Equity Offering.
On or after May 15, 2013, the Issuer may, on one or more occasions, redeem all or any portion of the Notes, at the redemption prices (expressed as percentages of principal amount) set forth below plus accrued and unpaid interest and Additional Interest, if any, on the Notes to be redeemed, to the applicable redemption date, if redeemed during the twelve-month period beginning on May 15 of the years indicated below, subject to the rights of holders of Notes on the relevant record date to receive interest on the relevant interest payment date:
Year | Percentage | |||
2013 | 104.188 | % | ||
2014 | 102.094 | % | ||
2015 and thereafter | 100.000 | % | ||
Before May 15, 2013, the Issuer may, on one or more occasions, redeem all or any portion of the Notes, at a redemption price equal to 100% of the principal amount thereof plus the Applicable Premium as of, and accrued and unpaid interest and Additional Interest, if any, to, the date of redemption (a “Make-Whole Redemption Date”).
“Applicable Premium” means, with respect to any Note on any Make-Whole Redemption Date, the greater of (i) 1.0% of the principal amount of such Note and (ii) the excess of (A) the present value at such Make-Whole Redemption Date of (1) the redemption price of such Note at May 15, 2013 as set forth in the table above (exclusive of accrued interest), plus (2) all scheduled interest payments due on such Note from the Make-Whole Redemption Date through May 15, 2013, computed using a discount rate equal to the Treasury Rate at such Make-Whole Redemption Date, plus 50 basis points over (B) the principal amount of such Note.
“Treasury Rate” means, with respect to any Make-Whole Redemption Date, the yield to maturity at the time of computation of United States Treasury securities with a constant maturity (as compiled and published in the most recent Federal Reserve Statistical Release H.15(519) that has become publicly available at least two business days prior to such Make-Whole Redemption Date (or, if such Statistical Release is no longer published, any publicly available source of similar market data)) most nearly equal to the period from such Make-Whole Redemption Date to May 15, 2013;provided, however, that if the period from such Make-Whole Redemption
138
Table of Contents
Date to May 15, 2013 is not equal to the constant maturity of a United States Treasury security for which a weekly average yield is given, the Treasury Rate shall be obtained by linear interpolation (calculated to the nearest one-twelfth of a year) from the weekly average yields of United States Treasury securities for which such yields are given, except that if the period from such Make-Whole Redemption Date to May 15, 2013 is less than one year, the weekly average yield on actually traded United States Treasury securities adjusted to a constant maturity of one year shall be used.
Unless the Issuer defaults in the payment of the redemption price, interest and Additional Interest will cease to accrue on the Notes or portions thereof called for redemption on the applicable redemption date.
The Issuer or any of its Restricted Subsidiaries may acquire Notes by means other than a redemption, whether pursuant to a tender offer, open market purchase, privately negotiated purchase or otherwise, so long as the acquisition does not otherwise violate the terms of the Indenture.
Selection and Notice
If less than all of the Notes are to be redeemed at any time, the Trustee will select Notes for redemption on a pro rata basis unless otherwise required by law or applicable stock exchange requirements;provided, however, that no Notes in an unauthorized denomination will be redeemed in part.
No Notes of $2,000 or less can be redeemed in part. Notices of redemption will be mailed by first class mail at least 30 but not more than 60 days before the redemption date to each holder of Notes to be redeemed at its registered address, except that redemption notices may be mailed more than 60 days prior to a redemption date if the notice is issued in connection with a defeasance of the Notes or a satisfaction and discharge of the Indenture. Notices of redemption may be conditional.
If any Note is to be redeemed in part only, the notice of redemption that relates to that Note will state the portion of the principal amount of that Note that is to be redeemed. A new Note in principal amount equal to the unredeemed portion of the original Note will be issued in the name of the holder of Notes upon cancellation of the original Note. Notes called for redemption become due on the date fixed for redemption. On and after the redemption date, interest and Additional Interest will cease to accrue on Notes or portions of Notes called for redemption.
Mandatory Redemption
The Issuer is not required to make mandatory redemption or sinking fund payments with respect to the Notes.
Guarantees
The Notes are guaranteed, on a senior secured basis, by Holdings and each of the Issuer’s Subsidiaries, other than Excluded Subsidiaries. Future Subsidiaries (other than Excluded Subsidiaries) will also become Guarantors under the Indenture governing the Notes. The Guarantees are joint and several, irrevocable and unconditional obligations of the Guarantors. The obligations of each Subsidiary Guarantor under its Guarantee are limited to the extent practicable to prevent that Guarantee from constituting a fraudulent conveyance under applicable law. The validity of this “savings clause,” however, is not free from doubt. See “Risk Factors—Risks Related to the Notes—A court could void our subsidiaries’ guarantees of the notes under fraudulent transfer laws.”
139
Table of Contents
A Guarantor may not sell or otherwise dispose of all or substantially all of its assets to, or consolidate with or merge with or into (whether or not such Guarantor is the surviving Person) another Person, other than the Issuer or a Subsidiary Guarantor, unless:
(1) the Person (if other than the Issuer or a Subsidiary Guarantor) acquiring the property in any such sale or disposition or the Person (if other than the Issuer of a Subsidiary Guarantor) formed by or surviving any such consolidation or merger assumes all the obligations of that Guarantor under its Guarantee, the Indenture, the Security Documents and the Registration Rights Agreement pursuant to a supplemental indenture satisfactory to the Trustee; or
(2) such transaction does not violate the provisions of the Indenture and the Net Proceeds of such sale or other disposition are applied in accordance with the applicable provisions of the Indenture; and
(3) immediately after such transaction, no Default or Event of Default exists.
The Guarantee of a Subsidiary Guarantor will be automatically released:
(1) in connection with any sale or other disposition of all or substantially all of the assets of that Guarantor (including by way of merger or consolidation) to a Person that is not (either before or after giving effect to such transaction) the Issuer or a Subsidiary Guarantor, if the sale or other disposition does not violate the provisions of the Indenture;
(2) in connection with any sale or other disposition of the Capital Stock of that Guarantor to a Person that is not (either before or after giving effect to such transaction) the Issuer or a Guarantor after which the Guarantor whose Capital Stock is sold or otherwise disposed of is no longer a Restricted Subsidiary, if the sale or other disposition does not violate the provisions of the Indenture;
(3) if the Issuer designates any Restricted Subsidiary that is a Guarantor to be an Unrestricted Subsidiary in accordance with the applicable provisions of the Indenture;
(4) upon payment in full, legal defeasance, covenant defeasance or satisfaction and discharge of the Indenture as provided below under the captions “—Legal Defeasance and Covenant Defeasance” or “—Satisfaction and Discharge,” respectively; or
(5) upon such Subsidiary Guarantor becoming an Excluded Subsidiary in a transaction not prohibited by the Indenture.
If any Subsidiary Guarantor is released from its Guarantee, any of its Subsidiaries that are Guarantors will be released from their Guarantees, if any, and it and any of its Subsidiaries will be released from their obligations under the Security Documents.
Collateral
Subject to the limitations described under “—Intercreditor Agreement” below, the obligations of the Issuer with respect to the Notes, the obligations of the Guarantors under the Guarantees, and the performance of all other obligations of the Issuer and the Guarantors under the Indenture are secured equally and ratably with the obligations of the Issuer and the Guarantors under any Pari Passu Lien Indebtedness and Priority Payment Lien Obligations by a security interest (subject only to Permitted Liens and a prior right of payment afforded to Priority Payment Lien Obligations in the event of a foreclosure or in any bankruptcy, insolvency or similar event) in the following assets of the Issuer and the Guarantors, in each case whether now owned or hereafter acquired (other than Excluded Property) (the “Collateral”):
(a) 100% of the Equity Interests in Subsidiaries (other than CFCs) owned by the Issuer or any Guarantor and 66% of the voting stock and 100% of the non-voting stock of CFCs owned by the Issuer or any Guarantor;
(b) all accounts, chattel paper, certain deposit accounts, documents, general intangibles (including contract rights, including an assignment of benefits of physician guarantees of intercompany loans),
140
Table of Contents
instruments, inventory, equipment, cash, investment property, letter-of-credit rights and any supporting obligations related to any of the foregoing (each as defined in the Uniform Commercial Code);
(c) present and future intercompany debt owed to the Issuer or any Guarantor;
(d) certain commercial tort claims;
(e) all books and records pertaining to Collateral;
(f) owned real properties owned by the Issuer or any Guarantor with a cost or book value (whichever is greater) in excess of $2.0 million (“Material Real Property”);
(g) all intellectual property and intellectual property licenses of the Issuer or any Guarantor (including without limitation patents, trademarks and copyrights);
(h) all other tangible and intangible assets of the Issuer or any Guarantor other than Excluded Property;
(i) all property of the Issuer or any Guarantor held by the Collateral Agent or any collateral agent for any class of Pari Passu Lien Indebtedness or Priority Payment Lien Obligations, including all property of every description, in the custody of or in transit to the Collateral Agent or any such collateral agent for any purpose, including safekeeping, collection or pledge, for the account of the Issuer or such Guarantor as to which the Issuer or such Guarantor may have any right or power, including, but not limited to, cash; and
(j) to the extent not otherwise included, all proceeds of the foregoing.
“Excluded Property” means, collectively,
(i) any rights or interests in any permit, license, lease or contract entered into by the Issuer or any Guarantor (A) that prohibits, or requires the consent of any Person other than the Issuer and its Affiliates which has not been obtained as a condition to, the creation by the Issuer or the applicable Guarantor of a Lien on any right, title or interest in such permit, license, lease or contract or (B) to the extent that any requirement of law applicable thereto prohibits the creation of a Lien thereon, but only, with respect to the prohibition in clauses (A) and (B), to the extent, and for as long as, such prohibition is not terminated or rendered unenforceable or otherwise deemed ineffective by the Uniform Commercial Code, any other requirement of law or principles of equity;
(ii) the Equity Interests of and other assets of Persons that are not Wholly Owned Subsidiaries to the extent and for so long as the granting of security interests in such Equity Interests and assets would be prohibited by a shareholders agreement or similar contract governing such Persons to the extent, and for as long as, such prohibition is not terminated or rendered unenforceable or otherwise deemed ineffective by the Uniform Commercial Code, any other requirement of law or principles of equity;
(iii) property owned by the Issuer or any Guarantor that is subject to a Lien permitted by clause (5) of the definition of “Permitted Liens” if the contractual obligation pursuant to which such Lien is granted (or in the document providing for such Lien) prohibits or requires the consent of any Person other than the Issuer and its Affiliates which has not been obtained as a condition to the creation of any other Lien on such item of property;
(iv) motor vehicles and other assets subject to certificates of title;
(v) any real property owned by the Issuer or any Guarantor that is not a Material Real Property;
(vi) leasehold real property interests;
(vii) any Equity Interests consisting of voting stock in any CFC directly owned by the Issuer or any Guarantor in excess of 66% of the outstanding voting stock of such CFC;
(viii) any “intent-to-use” application for registration of a trademark filed pursuant to Section 1(b) of the Lanham Act, 15 U.S.C. Section 1051, prior to the filing of a “Statement of Use” pursuant to Section 1(d) of the Lanham Act or an “Amendment to Allege Use” pursuant to Section 1(c) of the Lanham Act with respect
141
Table of Contents
thereto, solely to the extent, if any, that, and solely during the period, if any, in which, the grant of a security interest therein would impair the validity or enforceability of any registration that issues from such intent-to-use application under applicable federal law;
(ix) Excluded Deposit Accounts; and
(x) those assets as to which (x) the collateral agent under the Credit Agreement determines that the cost of obtaining such a security interest or perfection thereof is excessive in relation to the benefit to the lenders under the Credit Agreement of the security to be afforded thereby and (y) the Issuer reasonably and in good faith determines that the cost of obtaining such a security interest or perfection thereof is excessive in relation to the benefit to the lenders under the Credit Agreement and the holders of the security to be afforded thereby, such determinations to be certified in a Officers’ Certificate delivered by the Issuer to the Trustee.
For the avoidance of doubt, “Excluded Property” shall not include any proceeds, products, substitutions or replacements of Excluded Property (unless such proceeds, products, substitutions or replacements would otherwise constitute Excluded Property).
The Collateral was pledged pursuant to Security Documents substantially identical to the security documents to secure the Priority Payment Lien Obligations, with such conforming changes necessary to reflect that the obligations secured by the Security Documents are the Notes Obligations and other changes, if any, requested by the Collateral Agent or the Trustee. For the avoidance of doubt, no assets of any Subsidiary that is not a Guarantor (including any Equity Interests owned by any such Subsidiary) shall constitute Collateral.
In addition, in the event that Rule 3-16 of Regulation S-X under the Securities Act (as amended or modified from time to time) is interpreted by the SEC to require (or is replaced with another rule or regulation, or any other law, rule or regulation is adopted, which would require) the filing with the SEC (or any other governmental agency) of separate financial statements of any Subsidiary of the Issuer due to the fact that such Subsidiary’s Capital Stock secures the Notes, then the Capital Stock of such Subsidiary shall automatically be deemed not to be part of the Collateral but only to the extent necessary to not be subject to such requirement (the “Rule 3-16 Exception”). In such event, the Security Documents may be amended or modified, without the consent of any Holder, to the extent necessary to release the security interests in favor of the Collateral Agent on the shares of Capital Stock that are so deemed to no longer constitute part of the Collateral. In the event that Rule 3-16 of Regulation S-X under the Securities Act (as amended or modified from time to time) is interpreted by the SEC to permit (or are replaced with another rule or regulation, or any other law, rule or regulation is adopted, which would permit) such Subsidiary’s Capital Stock to secure the Notes in excess of the amount then pledged without the filing with the SEC (or any other governmental agency) of separate financial statements of such Subsidiary, then the Capital Stock of such Subsidiary shall automatically be deemed to be a part of the Collateral but only to the extent necessary to not be subject to any such financial statement requirement.
We may not have taken the actions required to perfect the security interests in all of the Collateral by the Issue Date. To the extent not completed by the Issue Date, such actions are required to be taken after the Issue Date within periods specified in the Indenture or the Security Documents.
Certain Limitations on the Collateral. The right of the Collateral Agent to take possession and dispose of the Collateral following an Event of Default is likely to be significantly impaired by applicable bankruptcy law if a bankruptcy proceeding were to be commenced by or against the Issuer or any Guarantor prior to the Collateral Agent having taken possession and disposed of the Collateral. Under the U.S. Bankruptcy Code, a secured creditor is prohibited from taking its security from a debtor in a bankruptcy case, or from disposing of security taken from such debtor, without bankruptcy court approval. Moreover, the U.S. Bankruptcy Code permits the debtor in certain circumstances to continue to retain and to use collateral owned as of the date of the bankruptcy filing (and the proceeds, products, offspring, rents or profits of such collateral) even though the debtor is in default under the applicable debt instruments, provided that the secured creditor is given “adequate protection.”
142
Table of Contents
The meaning of the term “adequate protection” may vary according to circumstances. In view of the lack of a precise definition of the term “adequate protection” and the broad discretionary powers of a bankruptcy court, it is impossible to predict how long payments under the Notes could be delayed following commencement of a bankruptcy case, whether or when the Collateral Agent could repossess or dispose of the Collateral, or whether or to what extent Holders would be compensated for any delay in payment or loss of value of the Collateral pursuant to the requirement of “adequate protection.”
Furthermore, in the event a U.S. bankruptcy court determines the value of the Collateral (after giving effect to any prior Liens) is not sufficient to repay all amounts due on the Priority Payment Lien Obligations, the Notes and any Pari Passu Lien Indebtedness, the Priority Payment Lien Obligations would be repaid in full prior to any payments being made on the Notes and any Pari Passu Lien Indebtedness and then the holders of the Notes and any Pari Passu Lien Indebtedness would hold secured claims to the extent of the remaining value of the Collateral, and would hold unsecured claims with respect to any shortfall. Applicable U.S. bankruptcy laws permit the payment and/or accrual of post-petition interest, costs and attorneys’ fees during a debtor’s bankruptcy case only to the extent the claims are oversecured or the debtor is solvent at the time of reorganization. In addition, if the Issuer or any Guarantor were to become the subject of a bankruptcy case, the bankruptcy court, among other things, may avoid certain pre-petition transfers made by the entity that is the subject of the bankruptcy filing, including, without limitation, transfers held to be preferences or fraudulent conveyances.
The Issuer and the Guarantors generally are not required to take any actions to perfect the security interest of the Collateral Agent in the Collateral beyond the filing of UCC financing statements, mortgages on Material Real Properties, delivery of certificates and instruments evidencing pledges of Equity Interests and Indebtedness and delivering control agreements as described in the second paragraph under “—Use and Release of Collateral” and filings with respect to U.S. registered intellectual property. To the extent the Collateral Agent does not have a perfected security interest in any Collateral, the Collateral Agent’s security interest will not be enforceable against third parties.
Use and Release of Collateral
Unless an Event of Default shall have occurred and be continuing and the Collateral Agent shall have commenced enforcement of remedies under the Security Documents, the Issuer and the Guarantors have the right to remain in possession and retain exclusive control of the Collateral, to freely operate the Collateral and to collect, invest and dispose of any income thereon.
The Indenture and the Security Documents does, however, generally require the Issuer and the Guarantors to deliver to the collateral agent under the Credit Agreement or, if the Credit Agreement is not in effect, other Priority Payment Lien Obligations and for such collateral agent to maintain in its possession certificates and instruments evidencing pledges of Equity Interests and Indebtedness and, subject to exceptions specified in the Indenture and the Security Documents, to maintain all cash and cash equivalents of the Issuer and the Guarantors at an account or accounts with a financial institution that has entered into a control agreement in favor of the Collateral Agent with respect to such account(s).
Release of Collateral. The Indenture provides that the Liens on the Collateral securing the Notes will automatically and without the need for any further action by any Person be released:
(1) in whole or in part, as applicable, as to all or any portion of property subject to such Liens which has been taken by eminent domain, condemnation or other similar circumstances;
(2) in whole upon:
(a) satisfaction and discharge of the Indenture as described below under “—Satisfaction and Discharge”; or
143
Table of Contents
(b) legal defeasance or covenant defeasance of the Indenture as described below under “—Legal Defeasance and Covenant Defeasance”;
(3) in part, as to any property that (a) is sold, transferred or otherwise disposed of by the Issuer or any Guarantor (other than to the Issuer or another Guarantor) in a transaction not prohibited by the Indenture at the time of such sale, transfer or disposition or (b) is owned or at any time acquired by a Guarantor that has been released from its Guarantee in accordance with the Indenture, concurrently with the release of such Guarantee (including in connection with the designation of a Guarantor as an Unrestricted Subsidiary);
(4) as set forth under “—Amendment, Supplement and Waiver,” as to property that constitutes less than all or substantially all of the Collateral, with the consent of holders of at least a majority in aggregate principal amount of the Notes outstanding (or, in the case of a release of all or substantially all of the Collateral, with the consent of the holders of at least seventy-five percent (75%) in aggregate principal amount of the Notes outstanding), including consents obtained in connection with a tender offer or exchange offer for, or purchase of, Notes; and
(5) in part, in accordance with the applicable provisions of the Security Documents and as described below with respect to the Intercreditor Agreement.
To the extent applicable, the Issuer will cause Trust Indenture Act § 313(b), relating to reports, and Trust Indenture Act § 314(d), relating to the release of property or securities or relating to the substitution therefor of any property or securities to be subjected to the Lien of the security documents, to be complied with. Any certificate or opinion required by Trust Indenture Act § 314(d) may be made by an officer of the Issuer except in cases where Trust Indenture Act § 314(d) requires that such certificate or opinion be made by an independent Person, which Person will be an independent engineer, appraiser or other expert selected or reasonably satisfactory to the Trustee.
Notwithstanding anything to the contrary in the preceding paragraph, the Issuer is not required to comply with all or any portion of Trust Indenture Act § 314(d) if it determines, in good faith based on advice of counsel, that under the terms of Trust Indenture Act § 314(d) and/or any interpretation or guidance as to the meaning thereof of the SEC and its staff, including “no action” letters or exemptive orders, all or any portion of Trust Indenture Act § 314(d) is inapplicable to one or a series of released Collateral.
Notwithstanding anything to the contrary contained in the preceding two paragraphs, the Trustee and Collateral Agent shall be entitled to receive an officer’s certificate and opinion of counsel stating that the release is authorized or permitted by the terms of the Indenture and that all conditions precedent to such release required by the Indenture have been complied with.
Intercreditor Agreement
On the Issue Date, the Collateral Agent and the collateral agent under the Credit Agreement entered into an intercreditor agreement (the “Intercreditor Agreement”) that was acknowledged by the Issuer and the Guarantors. Following the Issue Date, additional collateral agents for the holders of Pari Passu Lien Indebtedness may become party to the Intercreditor Agreement subject to compliance with certain procedural requirements in the Intercreditor Agreement. The Notes and other obligations secured by the Liens in favor of the Collateral Agent and the Priority Payment Lien Obligations secured by Liens in favor of the collateral agent under the Credit Agreement and the obligations in respect of any Pari Passu Lien Indebtedness secured by Liens in favor of any other collateral agent that becomes party to the Intercreditor Agreement are each referred to as a “class” of First Lien Obligations in this section.
The Intercreditor Agreement provides that, notwithstanding the date, time, method, manner or order of grant, attachment or perfection of any liens on any Collateral in which the Collateral Agent and one or more collateral agents for any class of Priority Payment Lien Obligations or Pari Passu Lien Indebtedness have
144
Table of Contents
perfected security interests (any such Collateral as to which the Collateral Agent and any other collateral agent have such a perfected security interest being referred to as “Shared Collateral”), the Collateral Agent and each other collateral agent with respect to such Shared Collateral has equal rights to enforce the respective security interests in the Shared Collateral subject to certain other provisions of the Intercreditor Agreement;provided that the Priority Payment Lien Obligations have priority in right of payment upon a foreclosure or a bankruptcy, insolvency or similar event and will be repaid prior to payment of the Notes Obligations and the Pari Passu Lien Indebtedness.
The Intercreditor Agreement provides that none of the Collateral Agent, the collateral agent under the Credit Agreement, any additional collateral agent for the holders of Pari Passu Lien Indebtedness or any holders of First Lien Obligations shall contest or support any person in contesting in any proceeding (including a bankruptcy proceeding) the perfection, priority, validity, attachment or enforceability of a lien held by or on behalf of any other collateral agent or any holders of First Lien Obligations in the Shared Collateral;provided that the foregoing shall not impair the right of any collateral agent or holder of First Lien Obligations to enforce the Intercreditor Agreement. In addition, the Intercreditor Agreement provides that the Issuer and the Guarantors shall not, and shall not permit any Subsidiary to, grant or permit or suffer to exist any additional liens on any asset or property to secure the Priority Payment Lien Obligations, the Notes Obligations or any class of Pari Passu Lien Indebtedness unless it has granted a lien on such asset or property to secure the Priority Payment Lien Obligations, the Notes Obligations and each other class of Pari Passu Lien Indebtedness, as the case may be;provided that the foregoing shall not prohibit the Priority Payment Lien Obligations and any Pari Passu Lien Obligations from being secured by any Capital Stock that does not secure the Notes Obligations or any Pari Passu Lien Obligations due to the Rule 3-16 Exception.
If (i) an event of default under any document governing any First Lien Obligations shall have occurred and be continuing and any of the Collateral Agent, the collateral agent for the Credit Agreement or the collateral agent for any class of Pari Passu Lien Indebtedness or any secured party in respect of any class of First Lien Obligations is taking action to enforce rights or exercise remedies in respect of any Shared Collateral, (ii) any distribution is made in respect of any Shared Collateral in any insolvency or liquidation proceeding of the Issuer or any Guarantor or (iii) the Collateral Agent, any other such collateral agent or any such secured party receives any payment with respect to any Shared Collateral pursuant to any intercreditor agreement (other than the Intercreditor Agreement), then the proceeds of any sale, collection or other liquidation of any Shared Collateral obtained by such Collateral Agent, any other such collateral agent or any such secured party in respect of any First Lien Obligations on account of such enforcement of rights or exercise of remedies, and any such distributions or payments received by such Collateral Agent, any other such collateral agent or any such secured party in respect of any First Lien Obligations shall be applied as follows (1) first, to (a) the payment of all amounts owing to such collateral agent (in its capacity as such) pursuant to the terms of any document related to the First Lien Obligations, (b) in the case of any such enforcement of rights or exercise of remedies, to the payment of all costs and expenses incurred by such collateral agent or any of its related secured parties in respect of First Lien Obligations in connection therewith and (c) in the case of any such payment pursuant to any such intercreditor agreement, to the payment of all costs and expenses incurred by such collateral agent or any of its related secured parties in enforcing its rights thereunder to obtain such payment, (2) second, to the payment in full of any Priority Payment Lien Obligations (including any post-petition interest with respect thereto, whether or not allowable in any bankruptcy case) and the termination of any commitments thereunder, (3) third, to the payment in full of the Notes Obligations and all Pari Passu Lien Indebtedness secured by a valid and perfected lien on such Shared Collateral at the time due and payable (the amounts so applied to be distributed, as among such classes of First Lien Obligations, ratably in accordance with the amounts of the First Lien Obligations of each such class on the date of such application), (4) fourth, after payment in full of all the First Lien Obligations secured by such Shared Collateral, to the holders of junior liens on the Shared Collateral and (5) fifth, to the Issuer and the other Guarantors or their successors or assigns or as a court of competent jurisdiction may direct.
Nothing in the Intercreditor Agreement shall affect the ability of any of the Collateral Agent, the collateral agent for the Credit Agreement, other collateral agent in respect of any class of Pari Passu Lien Indebtedness or
145
Table of Contents
any of the secured parties in respect of any First Lien Obligations (i) to enforce any rights and exercise any remedies with respect to any Shared Collateral available under the documents related to such Priority Payment Lien Obligations, Notes Obligations or the Pari Passu Lien Indebtedness or applicable law or (ii) to commence any action or proceeding with respect to such rights or remedies;provided that, notwithstanding the foregoing, (a) each collateral agent and its related secured parties shall remain subject to, and bound by, all covenants or agreements made in the Intercreditor Agreement, (b) each collateral agent has agreed, on behalf of itself and its related secured parties, that, prior to the commencement of any enforcement of rights or any exercise of remedies with respect to any Shared Collateral by such collateral agent or any of its related secured parties, such collateral agent or its related secured party, as the case may be, shall provide written notice thereof to each other collateral agent as far in advance of such commencement as reasonably practicable, and shall consult with each collateral agent on a regular basis in connection with such enforcement or exercise, and (c) each collateral agent agrees, on behalf of itself and its related secured parties, that such collateral agent and its related secured parties shall cooperate in a commercially reasonable manner with each other collateral agent and its related secured parties in any enforcement of rights or any exercise of remedies with respect to any Shared Collateral.
With respect to any Shared Collateral on which a Lien can be perfected by the possession or control of such Shared Collateral or of any deposit, securities or other account in which such Shared Collateral is held, then the applicable collateral agent in respect of Priority Payment Lien Obligations, Notes Obligations or the Pari Passu Lien Indebtedness that holds or controls such Shared Collateral shall also hold such Shared Collateral as gratuitous bailee and sub-agent for each other collateral agent in respect of the Notes Obligations, Pari Passu Lien Indebtedness and Priority Payment Lien Obligations. Any such collateral agent that holds Shared Collateral as gratuitous bailee and sub-agent shall be entitled to deal with the applicable pledged or controlled Shared Collateral as if the liens thereon of the collateral agent or secured parties of any other class of First Priority Obligations did not exist;provided that any proceeds arising from such pledged or controlled Shared Collateral shall be subject to the waterfall provisions set forth in the fourth paragraph of this section. Until the payment in full of the obligations under the Credit Agreement, the collateral agent under the Credit Agreement shall hold all such Collateral (for itself and as bailee for the Collateral Agent) which can be perfected by control or possession and, after the payment in full of such obligations, the collateral agent with respect to the class of First Priority Obligations of the largest principal amount at such time shall hold such Collateral.
The Intercreditor Agreement provides that the Collateral Agent and each collateral agent under the First Priority Obligations will have a right to exercise any remedies under any control agreement over a deposit account or securities account to which it is party;provided that the proceeds of any such exercise of remedies will be subject to the terms of the Intercreditor Agreement.
The Intercreditor Agreement shall not limit the Issuer’s ability to amend or refinance the Notes, Priority Payment Lien Obligations or Pari Passu Lien Indebtedness (although the Issuer will remain subject to the restrictions contained in the Credit Agreement, the Indenture and the documents governing any Priority Payment Lien Obligations or Pari Passu Lien Indebtedness).
Sufficiency of Collateral
There can be no assurance that the proceeds from the sale of the Collateral in whole or in part pursuant to the Security Documents following an Event of Default would be sufficient to satisfy the Notes Obligations. The fair market value of the Collateral is subject to fluctuations based on factors that include, among others, the condition of the dialysis industry, the ability to sell the Collateral in an orderly sale, general economic conditions, the availability of buyers and similar factors. The amount to be received upon a sale of the Collateral will also be dependent on numerous factors, including, but not limited to, the actual fair market value of the Collateral at such time and the timing and the manner of the sale. By their nature, portions of the Collateral may be illiquid and may have no readily ascertainable market value. In addition, the fact that the lenders under the Priority Payment Lien Obligations will receive proceeds from enforcement of the Collateral before holders of the Notes and that other Persons may have first-priority Liens in respect of Collateral pursuant to Permitted Liens could have a
146
Table of Contents
material adverse effect on the amount that holders of the Notes would receive upon a sale or other disposition of the Collateral. Accordingly, there can be no assurance that the Collateral can be sold in a short period of time or in an orderly manner. If the proceeds from a sale or other disposition of the Collateral were not sufficient to repay all amounts due on the Notes, the holders of the Notes (to the extent not repaid from the proceeds of the sale of the Collateral) would have only an unsecured claim against the remaining assets of the Issuer and the Guarantors. See “Risk Factors—Risk Related to the Notes—The value of the Collateral securing the Notes may not be sufficient to satisfy our obligations under the Notes and the other obligations secured by an equal and ratable lien on the Collateral.”
To the extent that third parties hold Liens permitted by the Security Documents and the Indenture, such third parties will have rights and remedies with respect to the assets subject to such Liens that, if exercised, could adversely affect the value of the Collateral or the ability of the Collateral Agent acting for the benefit of the Trustee or the holders of the Notes to realize or foreclose on the Collateral. In addition, the ability of the Collateral Agent acting for the benefit of the Trustee and the holders of Notes to realize on the Collateral may be subject to certain bankruptcy law limitations in the event of a bankruptcy. See “—Collateral—Certain Limitations on the Collateral.”
In addition, as noted above, the Issuer and the Guarantors (other than American Renal Management LLC) are holding companies for our joint venture subsidiaries that operate the clinics. Our joint venture subsidiaries are not Guarantors, and their assets do not secure the Notes Obligations.
Further Assurances; After-Acquired Collateral
Subject to the limitations described above, the Security Documents and the Indenture provides that the Issuer and the Guarantors shall, at their expense, duly execute and deliver, or cause to be duly executed and delivered, such further agreements, documents and instruments, and do or cause to be done such further acts as may be necessary or proper, or which the Collateral Agent may reasonably request, to evidence, perfect, maintain and enforce the lien in the Collateral granted to the Collateral Agent and the priority thereof and benefits intended to be conferred as contemplated by, and to otherwise effectuate the provisions or purposes of, the Indenture and the Security Documents.
Upon the acquisition by the Issuer or any Guarantor after the Issue Date of (1) any after-acquired assets, including, but not limited to, any after-acquired Material Real Property or any equipment or fixtures which constitute accretions, additions or technological upgrades to the equipment or fixtures or any working capital assets that, in any such case, form part of the Collateral, or (2) any replacement assets in compliance with the covenant described below under “—Repurchase at the Option of Holders—Asset Sales,” the Issuer or such Guarantor shall execute and deliver, (i) with regard to any Material Real Property, a mortgage and supporting documentation specified in the Indenture within 90 days of the date of acquisition, and (ii) to the extent required by the Security Documents, any information, documentation, financing statements or other certificates and opinions of counsel as may be necessary to vest in the Collateral Agent a perfected security interest, subject only to Permitted Liens, in such after-acquired property (other than Excluded Property) and to have such after-acquired property added to the Collateral, and thereupon all provisions of the Indenture, the Security Documents and the Intercreditor Agreement relating to the Collateral shall be deemed to relate to such after-acquired property to the same extent and with the same force and effect.
Repurchase at the Option of Holders
Change of Control
If a Change of Control occurs, each holder of Notes will have the right to require the Issuer to repurchase all or any part (equal to $2,000 or an integral multiple of $1,000 in excess thereof) of that holder’s Notes pursuant to a Change of Control Offer on the terms set forth in the Indenture. In the Change of Control Offer, the Issuer will
147
Table of Contents
offer a change of control payment (the “Change of Control Payment”) in cash equal to 101% of the aggregate principal amount of Notes repurchased plus accrued and unpaid interest and Additional Interest, if any, on the Notes repurchased to the date of purchase, subject to the rights of holders of Notes on the relevant record date to receive interest due on the relevant interest payment date. Within 30 days following any Change of Control, the Issuer will mail a notice to each holder describing the transaction or transactions that constitute the Change of Control and offering to repurchase Notes on the date specified in the notice (the “Change of Control Payment Date”), which date will be no earlier than 30 days and no later than 60 days from the date such notice is mailed, pursuant to the procedures required by the Indenture and described in such notice. The Issuer will comply with the requirements of Rule 14e-1 under the Exchange Act and any other securities laws and regulations thereunder to the extent those laws and regulations are applicable in connection with the repurchase of the Notes as a result of a Change of Control. To the extent that the provisions of any securities laws or regulations conflict with the Change of Control provisions of the Indenture, the Issuer will comply with the applicable securities laws and regulations and will not be deemed to have breached its obligations under the Change of Control provisions of the Indenture by virtue of such compliance.
On the Change of Control Payment Date, the Issuer will, to the extent lawful:
(1) accept for payment all Notes or portions of Notes properly tendered pursuant to the Change of Control Offer;
(2) deposit with the paying agent an amount equal to the Change of Control Payment in respect of all Notes or portions of Notes properly tendered; and
(3) deliver or cause to be delivered to the Trustee the Notes properly accepted together with and Officers’ Certificate stating the aggregate principal amount of Notes or portions of Notes being purchased by the Issuer.
The paying agent will promptly mail to each holder of Notes properly tendered the Change of Control Payment for such Notes, and the Trustee will promptly authenticate and mail (or cause to be transferred by book entry) to each holder a new Note equal in principal amount to any unpurchased portion of the Notes surrendered, if any. The Issuer will publicly announce the results of the Change of Control Offer on or as soon as practicable after the Change of Control Payment Date.
The provisions described above that require the Issuer to make a Change of Control Offer following a Change of Control are applicable whether or not any other provisions of the Indenture are applicable. Except as described above with respect to a Change of Control, the Indenture does not contain provisions that permit the holders of the Notes to require that the Issuer repurchase or redeem the Notes in the event of a takeover, recapitalization or similar transaction.
The Issuer will not be required to make a Change of Control Offer upon a Change of Control if (1) a third party makes the Change of Control Offer in the manner, at the times and otherwise in compliance with the requirements set forth in the Indenture applicable to a Change of Control Offer made by the Issuer and purchases all Notes properly tendered and not withdrawn under the Change of Control Offer or (2) notice of redemption has been given pursuant to the Indenture as described above under the caption “—Optional Redemption,” unless and until there is a default in payment of the applicable redemption price. Notwithstanding anything to the contrary herein, a Change of Control Offer may be made in advance of a Change of Control, conditional upon such Change of Control, if a definitive agreement is in place for the Change of Control at the time of making of the Change of Control Offer.
The definition of Change of Control includes a phrase relating to the direct or indirect sale, lease, transfer, conveyance or other disposition of “all or substantially all” of the properties or assets of the Issuer and its Subsidiaries taken as a whole. Although there is a limited body of case law interpreting the phrase “substantially all,” there is no precise established definition of the phrase under applicable law. Accordingly, the ability of a
148
Table of Contents
holder of Notes to require the Issuer to repurchase its Notes as a result of a sale, lease, transfer, conveyance or other disposition of less than all of the assets of the Issuer and its Subsidiaries taken as a whole to another Person or group may be uncertain.
Asset Sales
The Issuer will not, and will not permit any of its Restricted Subsidiaries to, consummate an Asset Sale unless:
(1) the Issuer (or the Restricted Subsidiary, as the case may be) receives consideration at the time of the Asset Sale at least equal to the Fair Market Value of the assets or Equity Interests issued or sold or otherwise disposed of;
(2) if such Asset Sale involves the disposition of Collateral, the Issuer or such Restricted Subsidiary has complied with the provisions of the Indenture and the Security Documents; and
(3) at least 75% of the consideration received in the Asset Sale by the Issuer or such Restricted Subsidiary is in the form of cash. For purposes of this paragraph (3), each of the following will be deemed to be cash:
(a) Cash Equivalents;
(b) any liabilities (as shown on the Issuer’s most recent consolidated balance sheet) of the Issuer or any Restricted Subsidiary (other than contingent liabilities and liabilities that are by their terms subordinated to the Notes or any Guarantee) that are assumed by the transferee of any such assets pursuant to an agreement that releases the Issuer or such Restricted Subsidiary from further liability;
(c) any securities, notes or other obligations received by the Issuer or any such Restricted Subsidiary from such transferee that are converted by the Issuer or such Restricted Subsidiary into cash within 180 days of receipt, to the extent of the cash received in that conversion;
(d)(i) any Designated Noncash Consideration received by the Issuer or a Restricted Subsidiary;provided, however, that (x) any such Designated Noncash Consideration that is converted into Cash Equivalents shall be treated as Net Proceeds in the manner set forth below and (y) in the event such Designated Noncash Consideration is an Investment, such Designated Noncash Consideration shall reduce amounts available under clause (16) of the definition of “Permitted Investments,” as determined by the Issuer, and (ii) any Designated Noncash Consideration the Fair Market Value of which, when taken together with all other Designated Noncash Consideration received pursuant to this clause (ii) (and not subsequently converted into Cash Equivalents that are treated as Net Proceeds of an Asset Sale), does not exceed the greater of $15.0 million and 3% of Total Assets at the time of receipt since the Issue Date, with the Fair Market Value of each item of Designated Noncash Consideration being measured at the time received and without giving effect to subsequent changes in value; and
(e) any Capital Stock, Investments or assets permitted by clause (2) or (4) of the next paragraph.
Within 365 days after the receipt of any Net Proceeds from an Asset Sale the Issuer (or the applicable Restricted Subsidiary, as the case may be) may apply such Net Proceeds at its option:
(1) to repay or prepay any (x) Priority Payment Lien Obligations;provided that if the Priority Payment Lien Obligation is revolving credit Indebtedness, the Issuer shall effect a corresponding reduction of commitments with respect thereto or (y) Indebtedness secured by a Permitted Lien on the assets sold whose prepayment is required by its terms upon such Asset Sale;
(2) to (x) acquire Capital Stock of a Person primarily engaged in a Permitted Business, if, after giving effect thereto, such Person is or becomes a Subsidiary Guarantor or a Qualified Restricted Subsidiary of the Issuer or (y) make Investments pursuant to clause (16) of the definition of “Permitted Investments”;
149
Table of Contents
(3) to make capital expenditures; or
(4) to acquire assets (other than Indebtedness and Capital Stock) to be used by the Issuer or the applicable Restricted Subsidiary in a Permitted Business;
provided that (i) the requirements of clauses (2) through (4) above shall be deemed to be satisfied if an agreement (including a lease, whether a capital lease or an operating lease) committing to make the acquisitions or expenditures referred to in any of clauses (2) through (4) above is entered into by the Issuer or the applicable Restricted Subsidiary within 365 days after the receipt of such Net Proceeds with the good faith expectation that such Net Proceeds will be applied to satisfy such commitment in accordance with such agreement within 180 days of such commitment and if such Net Proceeds are not so applied within such 180-day period, then such Net Proceeds shall constitute Excess Proceeds (as defined below), and (ii) the requirement of clause (2)(x) shall be deemed to be satisfied with respect to any Net Proceeds from the sale or issuance of Equity Interests of a Qualified Restricted Subsidiary to the extent an amount equal to such Net Proceeds was used as described in clause (2)(x) within 365 days prior to the receipt of such Net Proceeds. In addition, to the extent that the assets that were the subject of the Asset Sale constituted Collateral, the assets acquired with the proceeds pursuant to any of clauses (2) through (4) above or acquired as consideration for such Assets Sale shall constitute Collateral.
Pending the final application of any Net Proceeds, the Issuer may temporarily reduce revolving credit borrowings or otherwise invest the Net Proceeds in any manner that is not prohibited by the Indenture.
Any Net Proceeds from Asset Sales that are not applied or invested as provided in the third paragraph of this covenant will constitute “Excess Proceeds.” When the aggregate amount of Excess Proceeds exceeds $10.0 million, within ten business days thereof, the Issuer will make an Asset Sale Offer to:
(i) in the case of Net Proceeds from Collateral, all holders of First Lien Obligations, and
(ii) in the case of any other Net Proceeds, all holders of First Lien Obligations and all holders of other Indebtedness that ranks pari passu with the Notes containing provisions similar to those set forth in this Indenture with respect to offers to purchase or redeem with the proceeds of sales of assets (“Pari Passu Debt”).
The offer price in any Asset Sale Offer will be equal to 100% of the principal amount plus accrued and unpaid interest and Additional Interest, if any, to the date of purchase, and will be payable in cash. If any Excess Proceeds remain after consummation of an Asset Sale Offer, the Issuer may use those Excess Proceeds for any purpose not otherwise prohibited by the Indenture. If the aggregate principal amount of First Lien Obligations (in the case of Net Proceeds from Collateral) or First Lien Obligations and Pari Passu Debt (in the case of any other Net Proceeds) tendered into such Asset Sale Offer exceeds the amount of Excess Proceeds, the Trustee will select the Notes to be purchased, and the representative(s) for the holders of other First Lien Obligations shall select such other First Lien Obligations to be purchased and prepaid, on apro rata basis. Upon completion of each Asset Sale Offer, the amount of Excess Proceeds will be reset at zero.
The Issuer will comply with the requirements of Rule 14e-1 under the Exchange Act and any other securities laws and regulations thereunder to the extent those laws and regulations are applicable in connection with each repurchase of Notes pursuant to an Asset Sale Offer. To the extent that the provisions of any securities laws or regulations conflict with the Asset Sale provisions of the Indenture, the Issuer will comply with the applicable securities laws and regulations and will not be deemed to have breached its obligations under the Asset Sale provisions of the Indenture by virtue of such compliance.
The Credit Agreement provides that certain change of control or asset sale events with respect to the Issuer or repurchases of or other prepayments in respect of the Notes would constitute a default under the Credit Agreement. Any future credit agreements or other agreements relating to Indebtedness to which the Issuer becomes a party may contain similar restrictions and provisions. In the event a Change of Control or Asset Sale
150
Table of Contents
occurs at a time when the Issuer is prohibited from purchasing Notes, the Issuer could seek the consent of its senior lenders to the purchase of Notes or could attempt to refinance the borrowings that contain such prohibition. If the Issuer does not obtain such a consent or repay such borrowings, the Issuer will remain prohibited from purchasing Notes. In such case, the Issuer’s failure to purchase tendered Notes would constitute an Event of Default under the Indenture which would, in turn, constitute a default under the Credit Agreement and other agreements governing the Issuer’s Indebtedness.
Intercompany Loan Refinancing
Subject to the following proviso, promptly following receipt of Net Proceeds from any Intercompany Loan Refinancing, the Issuer shall, to the extent thereof:
(1) prepay, or make an offer to prepay term loans that are Priority Payment Lien Obligations, and prepay such term loans to the extent the lenders thereof shall accept such offer in accordance with the terms of the applicable credit agreement, or reduce, or make an offer to reduce, the commitments under any revolving credit facility that is a Priority Payment Lien Obligation (and prepay any borrowings thereunder to the extent that the aggregate amount of credit exposures thereunder exceeds the aggregate amount of commitments) and, in the case of an offer to reduce, reduce the commitments of the lenders that accept such offer (and prepay such borrowings); and/or
(2) make an offer to purchase the Notes at a price in cash equal to 100% of the principal amount thereof, plus accrued and unpaid interest and Additional Interest, if any, to the date of purchase and if applicable, to make an offer to purchase or prepay to the holders of other First Lien Obligations that by their terms require the Issuer to make an offer to purchase or prepay such First Lien Obligations upon an Intercompany Loan Refinancing (the “Intercompany Loan Refinancing Offer”), and to purchase or prepay such First Lien Obligations on a pro rata basis with the Notes;
provided that the Issuer is not required to prepay, reduce commitments, make offers to prepay, reduce commitments or purchase Notes pursuant to clauses (1) and/or (2) above until the aggregate amount of proceeds received from one or more Intercompany Loan Refinancings (“Proceeds Amount”) exceeds $4.0 million.
Pending the final application of any such proceeds, the Issuer may temporarily reduce revolving credit borrowings or otherwise invest such proceeds in any manner that is not prohibited by the Indenture. If any such proceeds remain after consummation of the offer(s), as applicable, referred to above, the Issuer may use those proceeds for any purposes not prohibited by the Indenture and the Proceeds Amount shall be reset at zero.
Agreements relating to Indebtedness to which the Issuer becomes a party in the future may contain restrictions on the Issuer’s ability to purchase Notes. In the event an Intercompany Loan Refinancing occurs at a time when the Issuer is prohibited from purchasing Notes, the Issuer could seek the consent of its senior lenders to the purchase of Notes or could attempt to refinance the borrowings that contain such prohibition. If the Issuer does not obtain such a consent or repay such borrowings, the Issuer will remain prohibited from purchasing Notes. In such case, the Issuer’s failure to purchase tendered Notes would constitute an Event of Default under the Indenture which would, in turn, constitute a default under the Credit Agreement and other agreements governing the Issuer’s Indebtedness.
The Issuer will comply with the requirements of Rule 14e-1 under the Exchange Act and any other securities laws and regulations thereunder to the extent such laws or regulations are applicable in connection with the purchase of Notes pursuant to an Intercompany Loan Refinancing Offer. To the extent that the provisions of any applicable securities laws or regulations conflict with the Intercompany Loan Refinancing Offer provisions of the Indenture, the Issuer will comply with the applicable securities laws and regulations and shall not be deemed to have breached its obligations under the Intercompany Loan Refinancing Offer provisions of the Indenture by virtue of such compliance.
151
Table of Contents
Certain Covenants
Restricted Payments
The Issuer will not, and will not permit any of its Restricted Subsidiaries to, directly or indirectly:
(A) declare or pay any dividend or make any other payment or distribution on account of the Issuer’s or any of its Restricted Subsidiaries’ Equity Interests (including, without limitation, any payment in connection with any merger or consolidation involving the Issuer or any of its Restricted Subsidiaries) or to the direct or indirect holders of the Issuer’s or any of its Restricted Subsidiaries’ Equity Interests in their capacity as such (other than dividends or distributions payable in Equity Interests (other than Disqualified Stock) of the Issuer);
(B) purchase, redeem or otherwise acquire or retire for value (including, without limitation, in connection with any merger or consolidation involving the Issuer) any Equity Interests of the Issuer, Holdings or any other direct or indirect parent of the Issuer;
(C) make any payment on or with respect to, or purchase, repurchase, redeem, defease or otherwise acquire or retire for value any Indebtedness of the Issuer or any Guarantor that is contractually subordinated to the Notes or to any Guarantee (excluding any intercompany Indebtedness between or among the Issuer and any of its Restricted Subsidiaries), except (i) a payment of interest or principal at the Stated Maturity thereof or (ii) the purchase, repurchase, redemption, defeasance or other acquisition or retirement of any such subordinated Indebtedness purchased in anticipation of satisfying a sinking fund obligation, principal installment or payment at final maturity, in each case within one year of the date of such purchase, repurchase, redemption, defeasance or other acquisition or retirement; or
(D) make any Restricted Investment
(all such payments and other actions set forth in these clauses (A) through (D) above being collectively referred to as “Restricted Payments”), unless, at the time of and after giving effect to such Restricted Payment:
(1) no Default or Event of Default has occurred and is continuing or would occur as a consequence of such Restricted Payment;
(2) the Issuer would, at the time of such Restricted Payment and after giving pro forma effect thereto as if such Restricted Payment had been made at the beginning of the Measurement Period, have been permitted to incur at least $1.00 of additional Indebtedness pursuant to the Fixed Charge Coverage Ratio test set forth in the first paragraph of the covenant described below under the caption “—Incurrence of Indebtedness and Issuance of Disqualified Stock and Preferred Stock”; and
(3) such Restricted Payment, together with the aggregate amount of all other Restricted Payments made by the Issuer and its Restricted Subsidiaries since the Issue Date (including Restricted Payments permitted by clauses (1) and (14) of the next succeeding paragraph, but excluding all other clauses of next succeeding paragraph), is less than the sum, without duplication, of:
(a) 50% of the Consolidated Net Income of the Issuer for the period (taken as one accounting period) from the beginning of the fiscal quarter commencing after the Issue Date to the end of the Issuer’s most recently ended fiscal quarter for which internal financial statements are available at the time of such Restricted Payment (or, if such Consolidated Net Income for such period is a deficit, less 100% of such deficit);plus
(b) 100% of the aggregate Qualified Proceeds received by the Issuer since the Issue Date as a contribution to its equity capital (other than Disqualified Stock) or from the issue or sale (other than to a Subsidiary of the Issuer) of Equity Interests of the Issuer (other than Disqualified Stock and Excluded Contributions) or from the issue or sale (other than to a Subsidiary of the Issuer) of convertible or exchangeable Disqualified Stock or convertible or exchangeable debt securities of the Issuer that have been converted into or exchanged for such Equity Interests of the Issuer (other than Disqualified Stock);plus
152
Table of Contents
(c) an amount equal to the net reduction in Investments by the Issuer and its Restricted Subsidiaries resulting from (A) the sale or other disposition (other than to the Issuer or a Restricted Subsidiary) of any Restricted Investment that was made after the Issue Date and (B) repurchases, redemptions and repayments of such Restricted Investments and the receipt of any dividends or distributions from such Restricted Investments (other than, in each case, to the extent such Investment was made by the Issuer or any Restricted Subsidiary of the Issuer pursuant to clause (15) or (18) below and such amounts received have been applied to increase availability under either such clause);plus
(d) to the extent that any Unrestricted Subsidiary of the Issuer designated as such after the Issue Date is redesignated as a Restricted Subsidiary after the Issue Date, an amount equal to the lesser of (A) the Fair Market Value of the Issuer’s interest in such Subsidiary immediately prior to such redesignation and (B) the aggregate amount of the Issuer’s Investments in such Subsidiary that was previously treated as a Restricted Payment (other than, in each case, to the extent such Investment was made by the Issuer or any Restricted Subsidiary of the Issuer pursuant to clause (15) or (18) below and such amounts received have been applied to increase availability under either such clause);plus
(e) in the event the Issuer and/or any Restricted Subsidiary of the Issuer makes any Investment in a Person that, as a result of or in connection with such Investment, becomes a Restricted Subsidiary of the Issuer, an amount equal to the existing Investment of the Issuer and/or any of its Restricted Subsidiaries in such Person that was previously treated as a Restricted Payment (other than to the extent such Investment was made by the Issuer or any Restricted Subsidiary of the Issuer pursuant to clause (15) or (18) below and such amounts received have been applied to increase availability under either such clause).
The preceding provisions will not prohibit:
(1) the payment of any dividend or other distribution or the consummation of any irrevocable redemption within 60 days after the date of declaration of the dividend or giving of the redemption notice, as the case may be, if at the date of declaration or notice, the dividend or redemption payment would have complied with the provisions of the Indenture;
(2) the making of any Restricted Payment in exchange for, or out of the net cash proceeds of the substantially concurrent sale (other than to a Subsidiary of the Issuer) of, Equity Interests of the Issuer (other than Disqualified Stock) or from the substantially concurrent contribution of equity capital to the Issuer (other than Disqualified Stock);provided that the amount of any such net cash proceeds that are utilized for any such Restricted Payment will be excluded from clause (3)(b) of the preceding paragraph;
(3) the repurchase, redemption, defeasance or other acquisition or retirement for value of Indebtedness of the Issuer or any Subsidiary Guarantor that is contractually subordinated to the Notes or to any Guarantee with the net cash proceeds from a substantially concurrent incurrence of Permitted Refinancing Indebtedness, or from the substantially concurrent sale (other than to a Subsidiary of the Issuer) of, Equity Interests of the Issuer (other than Disqualified Stock) or from the substantially concurrent contribution of equity capital to the Issuer (other than Disqualified Stock);provided that the amount of any such net cash proceeds that are utilized for any such Restricted Payment will be excluded from clause (3)(b) of the preceding paragraph;
(4) the declaration and payment of regularly scheduled or accrued dividends to holders of any class or series of Disqualified Stock of the Issuer or any Restricted Subsidiary of the Issuer which Disqualified Stock was issued after the Issue Date in accordance with the provisions of the covenant described below under the caption “—Incurrence of Indebtedness and Issuance of Disqualified Stock and Preferred Stock”;
(5) the repurchase, redemption or other acquisition or retirement for value of Disqualified Stock of the Issuer or any Restricted Subsidiary of the Issuer made by exchange for, or out of the proceeds of the substantially concurrent sale of Replacement Preferred Stock that is permitted to be incurred pursuant to the covenant described below under “—Incurrence of Indebtedness and Issuance of Disqualified Stock and Preferred Stock”;
153
Table of Contents
(6) the payment of any dividend (or any similar distribution) by a Restricted Subsidiary of the Issuer to the holders of its Equity Interests, including payments to non-Affiliates on apro rata basis;
(7) the repurchase, redemption or other acquisition or retirement for value of any Equity Interests of a Qualified Restricted Subsidiary owned by a Strategic Investor if such repurchase, redemption or other acquisition or retirement for value is made for consideration not in excess of the Fair Market Value of such Equity Interests (a) pursuant to any repurchase obligation to such Strategic Investor or (b) if no Default exists or would result therefrom;
(8) the repurchase, redemption or other acquisition or retirement for value of any Equity Interests of the Issuer or any Restricted Subsidiary of the Issuer held by any current or former officer, director, employee or consultant of the Issuer or any of its Restricted Subsidiaries, and any dividend payment or other distribution by the Issuer or a Restricted Subsidiary to Holdings or any other direct or indirect parent holding company of the Issuer utilized for the repurchase, redemption or other acquisition or retirement for value of any Equity Interests of Holdings or such other direct or indirect parent holding company held by any current or former officer, director, employee or consultant of the Issuer or any of its Restricted Subsidiaries or Holdings or such other parent holding company, in each case, pursuant to any equity subscription agreement, stock option agreement, shareholders’ agreement or similar agreement or benefit plan or other agreement of any kind;provided that the aggregate price paid for all such repurchased, redeemed, acquired or retired Equity Interests may not exceed $5.0 million in any fiscal year (it being understood, however, that unused amounts permitted to be paid pursuant to this proviso are available to be carried over to subsequent fiscal years but in no event shall the aggregate price paid for all such repurchased, redeemed, acquired or retired Equity Interests exceed $10.0 million in any year);provided further that such amount in any fiscal year may be further increased by an amount not to exceed:
(a) the net cash proceeds from the sale of Equity Interests of the Issuer (other than Disqualified Stock) and, to the extent contributed to the Issuer as equity capital (other than Disqualified Stock), Equity Interests of Holdings or any other direct or indirect parent company of the Issuer, in each case to members of management, directors or consultants of the Issuer, any of its Subsidiaries, Holdings or any other direct or indirect parent company of the Issuer that occurs after the Issue Date, to the extent the cash proceeds from the sale of such Equity Interests have not otherwise been applied to the payment of Restricted Payments by virtue of clause (3)(b) of the preceding paragraph (and, to the extent utilized pursuant to this clause (8), such amount will be excluded from clause (3)(b) of the preceding paragraph), and excluding Excluded Contributions,plus
(b) the cash proceeds of key man life insurance policies received by the Issuer and its Restricted Subsidiaries after the Issue Date, less
(c) the amount of any Restricted Payments previously made pursuant to clauses (a) and (b) of this clause (8);
andprovided, further, that cancellation of Indebtedness owing to the Issuer or any Restricted Subsidiary from members of management of the Issuer, any of the Issuer’s direct or indirect parent companies or any of the Issuer’s Restricted Subsidiaries in connection with a repurchase of Equity Interests of the Issuer or any of its direct or indirect parent companies will not be deemed to constitute a Restricted Payment for purposes of this covenant or any other provision of the Indenture;
(9) the repurchase of Equity Interests deemed to occur upon the exercise of options, rights or warrants to the extent such Equity Interests represent a portion of the exercise price of those options, rights or warrants;
(10) the repurchase, redemption, defeasance or other acquisition or retirement for value of Indebtedness of the Issuer or any Subsidiary Guarantor that is contractually subordinated to the Notes or to any Guarantee with any Excess Proceeds that remain after consummation of an Asset Sale Offer;
(11) after the occurrence of a Change of Control and within 60 days after the completion of the offer to repurchase the Notes pursuant to the covenant described above under “—Repurchase at the Option of
154
Table of Contents
Holders—Change of Control” (including the purchase of the Notes tendered), any purchase or redemption of Indebtedness of the Issuer or any Subsidiary Guarantor that is contractually subordinated to the Notes or to any Guarantee required pursuant to the terms thereof as a result of such Change of Control at a purchase or redemption price not to exceed 101% of the outstanding principal amount thereof, plus any accrued and unpaid interest;
(12) cash payments in lieu of fractional shares issuable as dividends on preferred stock or upon the conversion of any preferred stock or convertible debt securities of the Issuer or any of its Restricted Subsidiaries;
(13) Permitted Payments to Parent;
(14) the payment:
(a) by the Issuer or any Restricted Subsidiary to Holdings or any other direct or indirect parent of the Issuer, which payment is used by the Person receiving such payment, following the first initial public offering of common Equity Interests by such Person, to pay dividends of up to 6% per annum of the net proceeds received by such Person in such public offering (or any subsequent public offering of common Equity Interests of such Person) that are contributed to the Issuer as equity capital (other than Disqualified Stock), or
(b) by the Issuer, following the first initial public offering of common Equity Interests by the Issuer, to pay dividends of up to 6% per annum of the net proceeds received by or contributed to the Issuer in such public offering (or any subsequent public offering of common Equity Interests by the Issuer)
(excluding, in the case of both clause (a) and clause (b), public offerings of common Equity Interests registered on Form S-8 and any other public sale to the extent the proceeds thereof are Excluded Contributions);
(15) Investments that are made with Excluded Contributions;
(16) payment of fees and reimbursement of other expenses to the Permitted Holders in connection with the Transactions as described in this prospectus under the caption “Certain Relationships and Related Transactions” or dividends to any direct or indirect parent of the Issuer to fund such payments;
(17) all payments to be made under the Merger Agreement and all other payments made or to be made in connection with the Transactions (including payments made to C.P. Atlas Holdings, Inc. to permit it to make such payments) as set forth in this prospectus, including payments to stockholders, and holders of options and warrants for common stock, of the merger consideration (or, in the case of options and warrants, the merger consideration less the exercise price thereof), and all payments made to former stockholders of the Issuer who have validly exercised appraisal rights, in connection with the Transactions;
(18) other Restricted Payments in an aggregate amount taken together with all other Restricted Payments made pursuant to this clause (18) not to exceed the greater of (a) $15.0 million and (b) 3.0% of Total Assets at the time made; and
(19) purchases of receivables pursuant to a Receivables Repurchase Obligation and distributions or payments of Receivables Fees and any other payments, in each case, in connection with a Qualified Receivables Transaction;
provided that in the case of clause (4), (10), (11), (14) or (18), no Default shall exist or result therefrom.
The amount of all Restricted Payments (other than cash) will be the Fair Market Value on the date of the Restricted Payment of the asset(s) or securities proposed to be transferred or issued by the Issuer or such Restricted Subsidiary, as the case may be, pursuant to the Restricted Payment. The Fair Market Value of any assets or securities that are required to be valued by this covenant will, if the Fair Market Value thereof exceeds $15.0 million, be determined by the Board of Directors of the Issuer, whose resolution with respect thereto will be delivered to the Trustee.
155
Table of Contents
For purposes of determining compliance with the provisions set forth above, in the event that a Restricted Payment meets the criteria of more than one of the types of Restricted Payments described in the above clauses, the Issuer, in its sole discretion, may order and classify, and from time to time may reorder and reclassify, such Restricted Payment if it would have been permitted at the time such Restricted Payment was made and at the time of any such reclassification.
Incurrence of Indebtedness and Issuance of Disqualified Stock and Preferred Stock
The Issuer will not, and will not permit any of its Restricted Subsidiaries to, directly or indirectly, create, incur, issue, assume, guarantee or otherwise become directly or indirectly liable, contingently or otherwise, with respect to (collectively, “incur”) any Indebtedness (including Acquired Debt), and the Issuer will not issue any Disqualified Stock and will not permit any of its Restricted Subsidiaries to issue any shares of preferred stock;provided, however, that the Issuer or any Guarantor may incur Indebtedness (including Acquired Debt) or issue Disqualified Stock or preferred stock, if the Fixed Charge Coverage Ratio would be at least 2.00 to 1.00.
The first paragraph of this covenant will not prohibit the incurrence of any of the following items of Indebtedness or the issuance of any of the following items of Disqualified Stock or preferred stock (collectively, “Permitted Debt”):
(1) the incurrence by the Issuer and/or any Guarantor of Indebtedness under Credit Facilities in an aggregate principal amount at any one time outstanding under this clause (1) not to exceed the greater of (a) $25.0 million and (b) an amount equal to 50% of Consolidated EBITDA, calculated on a Pro Forma basis, for the most recently ended four full fiscal quarters for which internal financial statements are available immediately preceding the date of any incurrence under this clause (1);less the aggregate amount of all Net Proceeds of Asset Sales of the Issuer or any Restricted Subsidiary applied since the Issue Date to repay any Indebtedness under a Credit Facility pursuant to the covenant described above under the caption “—Repurchase at the Option of Holders—Asset Sales,”less the aggregate amount of term loans prepaid or revolving commitments reduced pursuant to the covenant described above under the caption “—Repurchase at the Option of Holders—Intercompany Loan Refinancings”;
(2) the incurrence by the Issuer and the Subsidiary Guarantors of the Existing Indebtedness outstanding on the Issue Date;
(3) the incurrence by the Issuer and the Guarantors of Indebtedness represented by the Notes to be issued on the Issue Date, the Guarantees of all of the Notes and the Exchange Notes, and the Exchange Notes to be issued pursuant to the Registration Rights Agreement;
(4)(x) Acquired Debt or Disqualified Stock or preferred stock of any Person that is acquired by the Issuer or any of its Restricted Subsidiaries or that consolidates or merges with or into a Restricted Subsidiary in accordance with the terms of the Indenture;provided, however, that (i) such Acquired Debt, Disqualified Stock or preferred stock existed prior to such acquisition, consolidation or merger and was not incurred or issued in connection therewith, or in contemplation thereof; and (ii) after giving effect thereto, the Issuer would be permitted to incur at least $1.00 of additional Indebtedness under the first paragraph of this covenant; or
(y) Acquired Debt or Disqualified Stock or preferred stock of any Person that consolidates or merges into the Issuer or any Guarantor in accordance with the terms of the Indenture;provided, however, that (i) such Acquired Debt, Disqualified Stock or preferred stock existed prior to such consolidation or merger and was not incurred or issued in connection therewith, or in contemplation thereof; and (ii) after giving effect thereto, the Consolidated Fixed Charge Coverage Ratio of the Issuer and the Restricted Subsidiaries is equal to or greater than immediately prior to the incurrence of such Acquired Debt, Disqualified Stock or preferred stock;
(5) the incurrence by the Issuer or any of its Restricted Subsidiaries of Permitted Refinancing Indebtedness or Replacement Preferred Stock in exchange for, or the net proceeds of which are used to
156
Table of Contents
renew, refund, refinance, replace, defease or discharge any Indebtedness (other than intercompany Indebtedness) or any Disqualified Stock or preferred stock that was permitted by the Indenture to be incurred under the first paragraph of this covenant or clause (2) or (3) of this paragraph or this clause (5);
(6) the incurrence by the Issuer, any Subsidiary Guarantor, any Qualified Restricted Subsidiary or any Restricted Subsidiary that meets the requirements of clauses (1), (2) and (3) of the definition of “Qualified Restricted Subsidiary” of Indebtedness owing to the Issuer, any Subsidiary Guarantor and any Qualified Restricted Subsidiary;provided, however, that:
(a) if the Issuer or any Subsidiary Guarantor is the obligor on such Indebtedness and the payee is not the Issuer or a Subsidiary Guarantor, such Indebtedness must be expressly subordinated to the prior payment in full in cash of all Obligations with respect to the Notes, in the case of the Issuer or the Guarantee, in the case of a Guarantor, except to the extent such subordination would violate any applicable law, rule or regulation; and
(b)(i) any subsequent issuance or transfer of Equity Interests that results in any such Indebtedness being owed to a Person other than the Issuer, a Subsidiary Guarantor or a Qualified Restricted Subsidiary of the Issuer and (ii) any sale or other transfer of any such Indebtedness to a Person that is not either the Issuer, a Subsidiary Guarantor or a Qualified Restricted Subsidiary of the Issuer, will be deemed, in each case, to constitute a new incurrence of such Indebtedness by the Issuer or such Restricted Subsidiary, as the case may be, which new incurrence is not permitted by this clause (6);
(7) the issuance by any Subsidiary Guarantor or any Qualified Restricted Subsidiary to the Issuer, any Subsidiary Guarantor or to any Qualified Restricted Subsidiary of shares of preferred stock;provided, however, that:
(a) any subsequent issuance or transfer of Equity Interests that results in any such preferred stock being held by a Person other than the Issuer, a Subsidiary Guarantor or a Qualified Restricted Subsidiary of the Issuer, and
(b) any sale or other transfer of any such preferred stock to a Person that is not either the Issuer, a Subsidiary Guarantor or a Qualified Restricted Subsidiary of the Issuer,
will be deemed, in each case, to constitute a new issuance of such preferred stock by such Restricted Subsidiary which new issuance is not permitted by this clause (7);
(8) the incurrence by the Issuer or any of its Restricted Subsidiaries of Hedging Obligations for the purpose of limiting interest rate, currency or commodity risk;
(9) the guarantee:
(a) by the Issuer or any Subsidiary Guarantor of Indebtedness of the Issuer or a Guarantor that was permitted to be incurred by another provision of this covenant;provided that if the Indebtedness being guaranteed is subordinated to the Notes, then the guarantee shall be subordinated to the same extent as the Indebtedness guaranteed;
(b)(i) by any Non-Guarantor Subsidiary that is a Qualified Restricted Subsidiary of Indebtedness of a Non-Guarantor Subsidiary that is a Qualified Restricted Subsidiary and (ii) by any Non-Guarantor Subsidiary that is not a Qualified Restricted Subsidiary of Indebtedness of a Non-Guarantor Subsidiary that is not a Qualified Restricted Subsidiary; and
(c) by the Issuer or any Subsidiary Guarantor of Indebtedness of any Qualified Restricted Subsidiary incurred pursuant to clause (15) or (16) of this covenant (up to the indirect or indirect proportionate ownership interest in such Qualified Restricted Subsidiary by the Issuer);
(10) the incurrence by the Issuer or any of its Restricted Subsidiaries of Indebtedness in respect of workers’ compensation claims, self-insurance obligations, bankers’ acceptances, letters of credit, performance bonds, surety bonds, appeal bonds or other similar bonds in the ordinary course of business;
157
Table of Contents
(11) the incurrence by the Issuer or any of its Restricted Subsidiaries of Indebtedness arising from the honoring by a bank or other financial institution of a check, draft or similar instrument inadvertently (except in the case of daylight overdrafts) drawn against insufficient funds in the ordinary course of business, so long as such Indebtedness is extinguished within five business days;
(12) the incurrence of Indebtedness arising from agreements of the Issuer or a Restricted Subsidiary providing for indemnification, adjustment of purchase price, holdback, contingency payment obligations or similar obligations, in each case, incurred or assumed in connection with the disposition or acquisition of any business, assets or Capital Stock of the Issuer or any Restricted Subsidiary;
(13) Indebtedness of the Issuer or any of its Restricted Subsidiaries supported by a letter of credit issued pursuant to any Credit Facilities, in a principal amount not in excess of the stated amount of such letter of credit;
(14) the incurrence of Indebtedness resulting from endorsements of negotiable instruments for collection in the ordinary course of business;
(15) the incurrence of Indebtedness or Disqualified Stock by Qualified Restricted Subsidiaries, in an aggregate amount not to exceed (net of unrestricted cash and Cash Equivalents held by any Qualified Restricted Subsidiary not included in the calculation under clause (16) below, up to the amount of Indebtedness of such Qualified Restricted Subsidiary under this clause (15)) at any time outstanding, the greater of (i) $25.0 million and (ii) an amount equal to 50% of Consolidated EBITDA for the most recently ended four full fiscal quarters for which internal financial statements are available immediately preceding the date of any incurrence under this clause (15);
(16) if the Consolidated Leverage Ratio of the Issuer and its Restricted Subsidiaries would not be greater than 4.50 to 1.00, (a) the incurrence of Permitted Refinancing Indebtedness or Replacement Preferred Stock by Qualified Restricted Subsidiaries incurred to refinance Indebtedness owed, or Disqualified Stock issued, to the Issuer or a Subsidiary Guarantor in accordance with clause (6) of this paragraph, or (b) the sale to any Person that is not Holdings or any of its Subsidiaries of any Indebtedness owed, or Disqualified Stock issued, by a Qualified Restricted Subsidiary to the Issuer or a Subsidiary Guarantor in accordance with clause (6) of this paragraph (either clause (a) or (b), an “Intercompany Loan Refinancing”), in an aggregate principal amount under this clause (16) not to exceed (net of unrestricted cash and Cash Equivalents held by any Qualified Restricted Subsidiary not included in the calculation under clause (15) above, up to the amount of Indebtedness of such Qualified Restricted Subsidiary under this clause (16)) at any time outstanding, the greater of (i) $25.0 million and (ii) an amount equal to 50% of Consolidated EBITDA, calculated on a Pro Forma Basis, for the most recently ended of four full fiscal quarters for which internal financial statements are available immediately preceding the date of any incurrence under this clause (16);
(17) Indebtedness of the Issuer or a Restricted Subsidiary in respect of netting services, overdraft protection and otherwise in connection with deposit accounts;provided that such Indebtedness remains outstanding for ten business days or less;
(18) Purchase Money Indebtedness incurred by the Issuer or any Restricted Subsidiary, and refinancings thereof, in an aggregate amount not to exceed at any time outstanding $15.0 million;
(19) the incurrence or issuance by the Issuer or any of its Restricted Subsidiaries of additional Indebtedness, Disqualified Stock or preferred stock in an aggregate principal amount (or accreted value or liquidation preference, as applicable) at any time outstanding, including all Permitted Refinancing Indebtedness and all Replacement Preferred Stock incurred to renew, refund, refinance, replace, defease or discharge any Indebtedness, Disqualified Stock and preferred stock incurred or issued pursuant to this clause (19), not to exceed $15.0 million;
(20) Indebtedness in respect of promissory notes issued to Strategic Investors in connection with repurchases of Equity Interests permitted by clause (7) of the second paragraph under the caption “—Restricted Payments”;
158
Table of Contents
(21) Indebtedness owed by the Issuer or any Restricted Subsidiary to future, current or former officers, directors, employees or consultants thereof, their respective estates, spouses or former spouses, in each case to finance the purchase or redemption of Equity Interests of the Issuer or any direct or indirect parent company of the Issuer to the extent described in clause (8) of the second paragraph under the caption “—Restricted Payments”;
(22) Indebtedness representing deferred compensation to employees of the Issuer and the Restricted Subsidiaries incurred in the ordinary course of business;
(23) Indebtedness of the Issuer or any Restricted Subsidiary consisting of (i) the financing of insurance premiums or (ii) take-or-pay obligations contained in supply arrangements, in each case, in the ordinary course of business;
(24) Indebtedness incurred by the Issuer or any Restricted Subsidiary constituting reimbursement obligations with respect to letters of credit issued in the ordinary course of business, including letters of credit in respect of workers’ compensation claims, health, disability or other employee benefits, or property, casualty or liability insurance, or other Indebtedness with respect to reimbursement-type obligations regarding workers’ compensation claims;provided that upon the drawing of such letters of credit or the incurrence of such Indebtedness, such obligations are reimbursed within 45 days following such drawing or incurrence;
(25) Indebtedness of the Issuer or any Restricted Subsidiary to the extent the proceeds of such Indebtedness are substantially concurrent with the incurrence thereof deposited and used to defease the Notes as provided for below under “—Legal Defeasance and Covenant Defeasance” or “—Satisfaction and Discharge”;
(26) incurrence by the Issuer or any of its Restricted Subsidiaries of Contribution Indebtedness;
(27) Indebtedness in respect of bid, performance or surety bonds or obligations of a similar nature issued for the account of the Issuer or any Restricted Subsidiary in the ordinary course of business, including guarantees or obligations of the Issuer or any Restricted Subsidiary with respect to letters of credit supporting such bid, performance or surety obligations (in each case other than for an obligation for money borrowed);
(28) Indebtedness in the form of earn-outs, contingent payments, seller notes, indemnification, incentive, non-compete, consulting or similar arrangements in connection with Permitted Investments or in connection with the acquisition or disposition of any business or assets of the Issuer or any Restricted Subsidiary or Equity Interests of a Restricted Subsidiary, other than guarantees of Indebtedness incurred by any Person acquiring all or any portion of such business, assets or Equity Interests for the purpose of financing or in contemplation of any such acquisition;provided that (a) any amount of such obligations included on the face of the balance sheet of the Issuer or any Restricted Subsidiary shall not be permitted under this clause (28) and (b) in the case of a disposition, the maximum aggregate liability in respect of all such obligations outstanding under this clause (28) shall at no time exceed the gross proceeds actually received by the Issuer and the Restricted Subsidiaries in connection with such disposition; or
(29) Standard Securitization Undertakings incurred in a Qualified Receivables Transaction permitted under the Indenture.
For purposes of determining compliance with this “Incurrence of Indebtedness and Issuance of Disqualified Stock and Preferred Stock” covenant, in the event that an item of proposed Indebtedness meets the criteria of more than one of the categories of Permitted Debt described in clauses (1) through (29) above, or is entitled to be incurred pursuant to the first paragraph of this covenant, the Issuer will be permitted to classify such item of Indebtedness on the date of its incurrence, or later reclassify all or a portion of such item of Indebtedness, in any manner that complies with this covenant except that Indebtedness under the Credit Agreement outstanding on the Issue Date will be deemed to have been incurred in reliance on the exception provided by clause (1) of the definition of “Permitted Debt” above. The accrual of interest, the accretion or amortization of original issue
159
Table of Contents
discount, the payment of interest on any Indebtedness in the form of additional Indebtedness with the same terms, the reclassification of preferred stock as Indebtedness due to a change in accounting principles, and the payment of dividends on Disqualified Stock or preferred stock in the form of additional shares of the same class of Disqualified Stock or preferred stock will not be deemed to be an incurrence of Indebtedness or an issuance of Disqualified Stock or preferred stock for purposes of this covenant; provided, in each such case, that the amount thereof is included in Fixed Charges of the Issuer as accrued (other than the reclassification of preferred stock as Indebtedness due to a change in accounting principles).
The amount of any Indebtedness outstanding as of any date will be:
(1) the accreted value of the Indebtedness, in the case of any Indebtedness issued with original issue discount;
(2) the principal amount of the Indebtedness, in the case of any other Indebtedness; and
(3) in respect of Indebtedness of another Person secured by a Lien on the assets of the specified Person, the lesser of:
(a) the Fair Market Value of such assets at the date of determination; and
(b) the amount of the Indebtedness of the other Person.
No Layering of Debt
The Issuer will not, and will not permit any Guarantor to, directly or indirectly, incur any Indebtedness that is or purports to be by its terms (or by the terms of any agreement governing such Indebtedness) subordinated to any other Indebtedness of the Issuer or of such Guarantor, as the case may be, unless such Indebtedness is also by its terms (or by the terms of any agreement governing such Indebtedness) made expressly subordinate to the Notes or the Guarantee of such Guarantor, to the same extent and in the same manner as such Indebtedness is subordinated to such other Indebtedness of the Issuer or such Guarantor, as the case may be.
For purposes of the foregoing, no Indebtedness will be deemed to be subordinated in right of payment to any other Indebtedness of the Issuer or any Guarantors solely by virtue of being unsecured or secured by a junior priority Lien or by virtue of the fact that the holders of such Indebtedness have entered into intercreditor agreements or other arrangements giving one or more of such holders priority over the other holders in the collateral held by them.
Liens
The Issuer will not, and will not permit any of its Restricted Subsidiaries to, directly or indirectly, create, incur, assume or otherwise cause or suffer to exist or become effective any Lien of any kind, on or with respect to the Collateral except Permitted Collateral Liens. Subject to the immediately preceding sentence, the Issuer will not, and will not permit any of the Restricted Subsidiaries to, create, incur, assume or otherwise cause or suffer to exist or become effective any Lien of any kind securing Indebtedness upon any of their property or assets, now owned or hereafter acquired, other than Permitted Liens.
Dividend and Other Payment Restrictions Affecting Restricted Subsidiaries
The Issuer will not, and will not permit any of its Restricted Subsidiaries to, directly or indirectly, create or permit to exist or become effective any consensual encumbrance or restriction on the ability of any Restricted Subsidiary to:
(1) pay dividends or make any other distributions on its Capital Stock to the Issuer or any of its Restricted Subsidiaries, or with respect to any other interest or participation in, or measured by, its profits, or pay any indebtedness owed to the Issuer or any of its Restricted Subsidiaries;
160
Table of Contents
(2) make loans or advances to the Issuer or any of its Restricted Subsidiaries; or
(3) sell, lease or transfer any of its properties or assets to the Issuer or any of its Restricted Subsidiaries.
However, the preceding restrictions will not apply to encumbrances or restrictions existing under or by reason of:
(1) agreements governing Existing Indebtedness and the Credit Agreement as in effect on the Issue Date;
(2) the Indenture, the Notes, the Guarantees, the Security Documents and the Intercreditor Agreement;
(3) applicable law, rule, regulation or order, including any requirement of any governmental healthcare programs;
(4) any instrument or agreement governing Indebtedness or Capital Stock of a Restricted Subsidiary acquired by the Issuer or any of its Restricted Subsidiaries as in effect at the time of such acquisition (except to the extent such Indebtedness or Capital Stock was incurred in connection with or in contemplation of such acquisition), which encumbrance or restriction is not applicable to any Person, or the properties or assets of any Person, other than the Person or any of its Subsidiaries, or the property or assets of the Person or any of its Subsidiaries, so acquired;provided that, in the case of Indebtedness, such Indebtedness was permitted by the terms of the Indenture to be incurred;
(5) customary non-assignment provisions in contracts, leases, subleases, licenses and sublicenses entered into in the ordinary course of business;
(6) customary restrictions in leases (including capital leases), security agreements or mortgages or other purchase money obligations for property acquired in the ordinary course of business that impose restrictions on the property purchased or leased of the nature described in clause (3) of the preceding paragraph;
(7) any agreement for the sale or other disposition of all or substantially all the Capital Stock or the assets of a Restricted Subsidiary that restricts distributions by that Restricted Subsidiary pending the sale or other disposition;
(8) any instrument or agreement governing Permitted Refinancing Indebtedness;provided that the restrictions contained therein are not materially more restrictive (as determined in good faith by the Issuer), taken as a whole, than those contained in the agreements governing the Indebtedness being refinanced;
(9) Liens permitted to be incurred under the provisions of the covenant described above under the caption “—Liens” that limit the right of the debtor to dispose of the assets subject to such Liens;
(10) restrictions on cash or other deposits or net worth imposed by customers under contracts entered into in the ordinary course of business;
(11) customary provisions imposed on the transfer of copyrighted or patented materials;
(12) customary provisions restricting dispositions of real property interests set forth in any reciprocal easement agreements of the Issuer or any Restricted Subsidiary;
(13) contracts entered into in the ordinary course of business, not relating to any Indebtedness, and that do not, individually or in the aggregate, detract from the value of property or assets of the Issuer or any Restricted Subsidiary of the Issuer in any manner material to the Issuer or any Restricted Subsidiary of the Issuer;
(14) restrictions on the transfer of property or assets required by any regulatory authority having jurisdiction over the Issuer or any Restricted Subsidiary of the Issuer or any of their businesses;
(15) any instrument or agreement governing Indebtedness or preferred stock of any Restricted Subsidiary that is incurred or issued subsequent to the Issue Date and not in violation of the covenant
161
Table of Contents
described under “—Incurrence of Indebtedness and Issuance of Disqualified Stock and Preferred Stock”;provided that the Issuer’s Board of Directors determines in good faith that restrictions are not reasonably likely to have a materially adverse effect on the Issuer’s and/or Guarantors’ ability to make principal and interest payments on the Notes;
(16) customary provisions in joint venture and other similar agreements, including agreements related to the ownership and operation of dialysis clinics, relating solely to such joint venture or facilities or the Persons who own Equity Interests therein;
(17) any amendments, modifications, restatements, renewals, increases, supplements, refundings, replacements or refinancings of the Indebtedness, preferred stock, Liens, agreements, contracts, licenses, leases, subleases, instruments or obligations referred to in clauses (1), (2), (4) and (15) above;provided, however, that such amendments, modifications, restatements, renewals, increases, supplements, refundings, replacements or refinancings are not materially more restrictive, taken as a whole, (as determined by the Issuer in good faith) than those restrictions contained in the Indebtedness, preferred stock, Liens, agreements, contracts, licenses, leases, subleases, instruments or obligations referred to in clauses (1), (2), (4) and (15) above, as applicable prior to such amendment, modification, restatement, renewal, increase, supplement, refunding, replacement or refinancing;
(18) customary provisions in connection with a Qualified Receivables Transaction; and
(19) restrictions in Management Agreements that require the payment of management fees to the Issuer or one of its Restricted Subsidiaries prior to payment of dividends or distributions.
For purposes of determining compliance with this covenant, (i) the priority of any preferred stock in receiving dividends or liquidating distributions prior to dividends or liquidating distributions being paid on common stock shall not be deemed a restriction on the ability to make distributions on Capital Stock and (ii) the subordination of loans or advances made to the Issuer or a Restricted Subsidiary of the Issuer to other Indebtedness incurred by the Issuer or any such Restricted Subsidiary shall not be deemed a restriction on the ability to make loans or advances.
Distributions by Qualified Restricted Subsidiaries
Except to the extent restricted pursuant to any Permitted Payment Restrictions, the Issuer shall, and shall cause each Restricted Subsidiary to, cause each Qualified Restricted Subsidiary to declare and pay regular monthly, quarterly, semiannual or annual dividends or distributions to the holders of its Capital Stock in an amount equal to substantially all of the available cash flow of such Restricted Subsidiary for such period as determined in good faith by the board of directors, board of governors or such other individuals performing similar functions, subject to fiduciary duties applicable to such board or individual and such ordinary and customary reserves and other amounts as, in the good faith judgment of such individuals, may be necessary so that the business of such Restricted Subsidiary may be properly and advantageously conducted at all times, including amounts necessary for operations, capital expenditures, debt service and other needs.
If, at any time, any Restricted Subsidiary would fail to meet the requirements set forth in the definition of “Qualified Restricted Subsidiary,” it will thereafter cease to be a Qualified Restricted Subsidiary for purposes of the Indenture governing the Notes and any Indebtedness of such Subsidiary will be deemed to be incurred by a Restricted Subsidiary that is not a Qualified Restricted Subsidiary as of such date and, if such Indebtedness is not permitted to be incurred as of such date under the covenant described under the caption “—Incurrence of Indebtedness and Issuance of Disqualified Stock and Preferred Stock,” the Issuer will be in default of such covenant. The Board of Directors of the Issuer may at any time designate any Restricted Subsidiary not to be a Qualified Restricted Subsidiary;provided that such designation will be deemed to be an incurrence of Indebtedness by such Restricted Subsidiary of any outstanding Indebtedness of such Restricted Subsidiary, and such designation will only be permitted if (1) such Indebtedness is permitted under the covenant described under
162
Table of Contents
the caption “—Incurrence of Indebtedness and Issuance of Disqualified Stock and Preferred Stock,” and (2) no Default would be in existence following such designation. In the event (x) a Restricted Subsidiary fails to meet the requirements to be a Qualified Restricted Subsidiary or (y) the Board of Directors designates a Qualified Restricted Subsidiary not to be a Qualified Restricted Subsidiary, then all Investments in such Subsidiary since the Issue Date shall be deemed to have been acquired and consequently reduce the amount available for Restricted Payments under the covenant described above under the caption “—Restricted Payments” or the amount available for Restricted Investments under clause (16) of the definition of “Permitted Investments,” as determined by the Issuer. As of the Issue Date, all of the Issuer’s Restricted Subsidiaries are Qualified Restricted Subsidiaries.
Merger, Consolidation or Sale of Assets
The Issuer will not, directly or indirectly: (1) consolidate or merge with or into another Person (whether or not the Issuer is the surviving Person); or (2) sell, assign, transfer, convey or otherwise dispose of all or substantially all of the properties or assets of the Issuer and its Restricted Subsidiaries taken as a whole, in one or more related transactions, to another Person, unless:
(1) either: (a) the Issuer is the surviving Person; or (b) the Person formed by or surviving any such consolidation or merger (if other than the Issuer) or to which such sale, assignment, transfer, conveyance or other disposition has been made (collectively, the “Successor”) is a corporation or a limited liability company organized or existing under the laws of the United States, any state of the United States or the District of Columbia;
(2) the Successor assumes all the obligations of the Issuer under the Notes, the Indenture, the Security Documents and the Registration Rights Agreement pursuant to a supplement indenture and other agreements reasonably satisfactory to the Trustee;provided, however, that at all times, a corporation organized and existing under the laws of the United States of America, any State thereof or the District of Columbia must be a co-issuer or the issuer of the Notes if such surviving Person is not a corporation;
(3) immediately after such transaction, no Default exists;
(4) the Issuer or the Successor would, on the date of such transaction after giving pro forma effect thereto and any related financing transactions as if the same had occurred at the beginning of the applicable four-quarter period:
(a) be permitted to incur at least $1.00 of additional Indebtedness pursuant to the Fixed Charge Coverage Ratio test set forth in the first paragraph of the covenant described above under the caption “—Incurrence of Indebtedness and Issuance of Disqualified Stock and Preferred Stock”; or
(b) have a Fixed Charge Coverage Ratio that is greater than the Fixed Charge Coverage Ratio of the Issuer immediately prior to such transaction;
(5) the Successor promptly causes such amendments, supplements or other instruments to be executed, delivered, filed and recorded in such jurisdictions as may be required by applicable law to preserve and protect the Liens of the Security Documents on the Collateral owned by or transferred to the Successor, together with such financing statements as may be required to perfect any security interests in such Collateral which may be perfected by filing of a financing statement under the Uniform Commercial Code of the relevant states;
(6) the Issuer shall have delivered to the Trustee an Officers’ Certificate stating that such consolidation, merger, amalgamation or transfer and such supplemental indenture, if any, comply with the Indenture and that such supplemental indenture, amendments, supplements or other instruments relating to the applicable Security Documents, if any, comply with the Indenture and an Officers’ Certificate and an Opinion of Counsel in a customary form including customary qualifications to the effect that such amendments, supplements or other instruments are enforceable;
163
Table of Contents
(7) the Collateral owned by or transferred to the Successor shall:
(a) continue to constitute Collateral under the Indenture and the Security Documents,
(b) be subject to the Liens in favor of the Collateral Agent for its benefit and the benefit of the holders of the First Lien Obligations, and
(c) not be subject to any Lien other than Permitted Liens; and
(8) the property and assets of the Person which is merged or consolidated with or into the Successor, to the extent that they are property or assets of the types that would constitute Collateral under the Security Documents, shall be treated as after acquired property and the Successor shall take such action as may be reasonably necessary to cause such property and assets to be made subject to the Lien of the Security Documents in the manner and to the extent required in the Indenture.
In addition, the Issuer will not, directly or indirectly, lease all or substantially all of the properties and assets of it and its Restricted Subsidiaries taken as a whole, in one or more related transactions, to any other Person.
Clauses (3) and (4) above will not apply to:
(1) a merger of the Issuer with an Affiliate for the sole purpose and effect of reincorporating the Issuer in another jurisdiction;
(2) any consolidation or merger, or any sale, assignment, transfer, conveyance, lease or other disposition of assets between or among the Issuer and the Subsidiary Guarantors; and
(3) the consolidation or merger, or sale, assignment, transfer, conveyance, lease or other disposition of all or part of its assets, by any Restricted Subsidiary to the Issuer or a Subsidiary Guarantor.
Holdings will not, directly or indirectly: (1) consolidate or merge with or into another Person (whether or not the Holdings is the surviving Person); or (2) sell, assign, transfer, convey or otherwise dispose of all or substantially all of the properties or assets of Holdings and its Subsidiaries taken as a whole, in one or more related transactions, to another Person, unless:
(1) either: (a) Holdings is the surviving Person; or (b) the Person formed by or surviving any such consolidation or merger (if other than the Issuer) or to which such sale, assignment, transfer, conveyance or other disposition has been made (collectively, the “Successor Holdings”) is a corporation or a limited liability company organized or existing under the laws of the United States, any state of the United States or the District of Columbia;
(2) the Successor Holdings assumes all the obligations of Holdings under the Notes, the Indenture, the Security Documents and the Registration Rights Agreement pursuant to a supplemental indenture and other agreements reasonably satisfactory to the Trustee;
(3) immediately after such transaction, no Default exists;
(4) the Successor Holdings promptly causes such amendments, supplements or other instruments to be executed, delivered, filed and recorded in such jurisdictions as may be required by applicable law to preserve and protect the Liens of the Security Documents on the Collateral owned by or transferred to the Successor Holdings, together with such financing statements as may be required to perfect any security interests in such Collateral which may be perfected by filing of a financing statement under the Uniform Commercial Code of the relevant states;
(5) Holdings shall have delivered to the Trustee an Officers’ Certificate stating that such consolidation, merger, amalgamation or transfer and such amendments, supplements or other instruments, if any, comply with the Indenture and that such amendments, supplements or other instruments, relating to the applicable Security Documents, if any, comply with the Indenture and an Officers’ Certificate and an Opinion of Counsel in a customary form including customary qualifications to the effect that such amendments, supplements or other instruments are enforceable;
164
Table of Contents
(6) the Collateral owned by or transferred to the Successor Holdings shall:
(a) continue to constitute Collateral under the Indenture and the Security Documents,
(b) be subject to the Liens in favor of the Collateral Agent for its benefit and the benefit of the holders of the First Lien Obligations, and
(c) not be subject to any Lien other than Permitted Liens; and
(7) the property and assets of the Person which is merged or consolidated with or into the Successor Holdings, to the extent that they are property or assets of the types that would constitute Collateral under the Security Documents, shall be treated as after acquired property and the Successor Holdings shall take such action as may be reasonably necessary to cause such property and assets to be made subject to the Lien of the Security Documents in the manner and to the extent required in the Indenture.
Transactions with Affiliates
The Issuer will not, and will not permit any of its Restricted Subsidiaries to, make any payment to, or sell, lease, transfer or otherwise dispose of any of its properties or assets to, or purchase any property or assets from, or enter into or make or amend any transaction, contract, agreement, understanding, loan, advance or guarantee with, or for the benefit of, any Affiliate of the Issuer involving aggregate consideration in excess of $2.5 million (each, an “Affiliate Transaction”), unless:
(a) the Affiliate Transaction is on terms that, taken as a whole, are not materially less favorable to the Issuer or the relevant Restricted Subsidiary than those that would have been obtained in a comparable transaction by the Issuer or such Restricted Subsidiary with an unrelated Person; and
(b) the Issuer delivers to the Trustee (i) with respect to any Affiliate Transaction or series of related Affiliate Transactions involving aggregate consideration in excess of $15.0 million, an Officers’ Certificate certifying that such Affiliate Transaction complies with this covenant and that such Affiliate Transaction has been approved by a majority of the members of the Board of Directors of the Issuer, together with a certified copy of the resolutions of the Board of Directors of the Issuer approving such Affiliate Transaction or Affiliate Transactions; and (ii) with respect to any Affiliate Transaction or series of related Affiliate Transactions involving aggregate consideration in excess of $20.0 million, an opinion as to the fairness to the Issuer or such Restricted Subsidiary of such Affiliate Transaction from a financial point of view issued by an accounting, appraisal or investment banking firm of national standing.
The following items will not be deemed to be Affiliate Transactions and, therefore, will not be subject to the provisions of the prior paragraph:
(1) any employment agreement, employee benefit plan, officer or director indemnification agreement or any similar arrangement entered into by the Issuer or any of its Restricted Subsidiaries in the ordinary course of business and payments pursuant thereto;
(2) transactions between or among the Issuer and/or its Restricted Subsidiaries;
(3) transactions with a Person (other than an Unrestricted Subsidiary of the Issuer) that is an Affiliate of the Issuer solely because the Issuer owns, directly or through a Restricted Subsidiary, an Equity Interest in, or controls, such Person;
(4) payment of reasonable directors’ fees;
(5) any issuance of Equity Interests (other than Disqualified Stock) of the Issuer to Affiliates of the Issuer;
(6) Permitted Investments or Restricted Payments that do not violate the provisions of the Indenture described above under the caption “—Restricted Payments”;
165
Table of Contents
(7) payment of fees and the reimbursement of other expenses to the Permitted Holders in connection with the Transactions as described in this prospectus under the caption “Certain Relationships and Related Transactions”;
(8) loans (or cancellation of loans) or advances to employees in the ordinary course of business;
(9) transactions with joint ventures, Unrestricted Subsidiaries, customers, suppliers, contractors, joint venture partners (including, without limitation, physicians) or purchasers or sellers of goods or services, in each case which are in the ordinary course of business (including, without limitation, pursuant to joint venture agreements) and otherwise in compliance with the terms of the Indenture, and which are fair to the Issuer or its Restricted Subsidiaries, as applicable, in the reasonable determination of the Board of Directors, chief executive officer or chief financial officer of the Issuer or its Restricted Subsidiaries, as applicable, or are on terms at least as favorable as might reasonably have been obtained at such time from an unaffiliated party;
(10) the existence of, or the performance by the Issuer or any Restricted Subsidiary of their obligations, if any, or obligations of Holdings under the terms of, any subscription, registration rights or stockholders agreement, partnership agreement or limited liability company agreement or similar agreement to which Holdings, the Issuer or any Restricted Subsidiary is a party as of the Issue Date and any similar agreements which the Issuer, any Restricted Subsidiary, Holdings or any other direct or indirect parent company of the Issuer may enter into thereafter;provided, however, that the entering into by the Issuer or any Restricted Subsidiary or the performance by the Issuer or any Restricted Subsidiary of obligations under any future amendment to any such existing agreement or under any similar agreement entered into after the Issue Date will only be permitted by this clause to the extent that the terms of any such amendment or new agreement, taken as a whole, are not materially disadvantageous to the holders of the Notes, as determined in good faith by the Board of Directors, chief executive officer or chief financial officer of the Issuer;
(11) the Transactions, including all payments made or to be made in connection with the Transactions as described in this prospectus;
(12) the entering into of any tax sharing agreement or arrangement and any Permitted Payments to Parent;
(13)(i) payments by the Issuer or any of its Restricted Subsidiaries to the Sponsor and/or any of its Affiliates for any financial advisory, financing, underwriting or placement services or in respect of other investment banking activities, including, without limitation, in connection with acquisitions or divestitures, which payments are approved by the majority of the disinterested members of the Board of Directors of the Issuer in good faith in an aggregate amount for all such fees for any transaction not to exceed 2.00% of the aggregate value of such transaction and (ii) fees payable pursuant to the Sponsor Management Agreement as in effect on the Issue Date or as amended in a manner not adverse in any material respect to the holders of Notes;
(14) the issuance of Equity Interests (other than Disqualified Stock) in the Issuer or any Restricted Subsidiary for compensation purposes in the ordinary course of business;
(15) any lease or sublease entered into between the Issuer or any Restricted Subsidiary, as lessee, and any Affiliate of the Issuer, as lessor or sublessor, which is approved by a majority of the disinterested members of the Board of Directors of the Issuer in good faith;
(16) intellectual property licenses in the ordinary course of business;
(17) Existing Indebtedness and any other obligations pursuant to an agreement existing on the Issue Date as described in this prospectus, including any amendment thereto (so long as such amendment is not adverse to the holders of the Notes in any material respect);
(18) transactions in which the Issuer or any Restricted Subsidiary delivers to the Trustee a letter from an accounting, appraisal or investment banking firm of national standing stating that such transaction is fair
166
Table of Contents
to the Issuer or such Restricted Subsidiary from a financial point of view and which are approved by a majority of the disinterested members of the Board of Directors of the Issuer in good faith; and
(19) customary transactions pursuant to a Qualified Receivables Transactions.
Business Activities
The Issuer will not, and will not permit any of its Restricted Subsidiaries to, engage in any business other than Permitted Businesses, except to such extent as would not be material to the Issuer and its Restricted Subsidiaries taken as a whole.
Activities of Holdings
Holdings may not hold any material assets, become liable for any material obligations, engage in any trade or business, or conduct any business activity, other than (1) the issuance of its Equity Interests to its shareholders, (2) the incurrence of Indebtedness as a guarantor of the Notes, the Credit Agreement and any other Indebtedness that is permitted to be incurred by the Issuer under the covenant described under “—Incurrence of Indebtedness and Issuance of Disqualified Stock and Preferred Stock”;provided that the net proceeds of such Indebtedness are received by the Issuer as primary obligor and not retained by Holdings, and (3) activities incidental thereto. Neither the Issuer nor any Restricted Subsidiary shall engage in any transactions with Holdings in violation of the immediately preceding sentence.
Additional Guarantors
If, after the Issue Date, (i) the Issuer shall, directly or indirectly, create a Subsidiary (other than an Excluded Subsidiary), (ii) any Person shall become a Subsidiary of the Issuer that is not an Excluded Subsidiary (including by way of acquisition of Equity Interests of such Person by the Issuer or a Restricted Subsidiary of the Issuer or redesignation of any Unrestricted Subsidiary as a Restricted Subsidiary) or (iii) the Issuer otherwise elects to have any Restricted Subsidiary become a Guarantor, then, in each such case, and, in the case of clauses (i) and (ii), within 30 days of the creation of such Subsidiary or such Person becoming a Subsidiary that is not an Excluded Subsidiary, the Issuer shall cause such Restricted Subsidiary to: (a) execute and deliver to the Trustee a Guarantee Agreement and any joinders to the Security Documents and/or additional Security Documents and effect all filings and other actions, in each case, necessary to grant valid and perfected security interests in the Collateral of such Restricted Subsidiary to secure the First Lien Obligations, and (b) deliver an Opinion of Counsel to the Trustee that such Guarantee Agreement and Security Documents (x) have been duly authorized, executed and delivered by such Restricted Subsidiary and (y) constitute valid and legally binding obligations of such Restricted Subsidiary in accordance with its terms.
Designation of Restricted and Unrestricted Subsidiaries
The Board of Directors of the Issuer may designate any Restricted Subsidiary to be an Unrestricted Subsidiary if that designation would not cause a Default. If a Restricted Subsidiary is designated as an Unrestricted Subsidiary, the aggregate Fair Market Value of all outstanding Investments owned by the Issuer and its Restricted Subsidiaries in the Subsidiary designated as an Unrestricted Subsidiary will be deemed to be an Investment made as of the time of the designation and will reduce the amount available for Restricted Payments under the covenant described above under the caption “—Restricted Payments” or under one or more clauses of the definition of Permitted Investments, as determined by the Issuer. That designation will only be permitted if the Investment would be permitted at that time and if the Restricted Subsidiary otherwise meets the definition of an Unrestricted Subsidiary.
Any designation of a Subsidiary of the Issuer as an Unrestricted Subsidiary will be evidenced to the Trustee by filing with the Trustee a certified copy of a resolution of the Board of Directors of the Issuer giving effect to
167
Table of Contents
such designation and an Officers’ Certificate certifying that such designation complied with the preceding conditions and was permitted by the covenant described above under the caption “—Restricted Payments.” If, at any time, any Unrestricted Subsidiary would fail to meet the preceding requirements as an Unrestricted Subsidiary, it will thereafter cease to be an Unrestricted Subsidiary for purposes of the Indenture and any Indebtedness of such Subsidiary will be deemed to be incurred by a Restricted Subsidiary of the Issuer as of such date and, if such Indebtedness is not permitted to be incurred as of such date under the covenant described under the caption “—Incurrence of Indebtedness and Issuance of Disqualified Stock and Preferred Stock,” the Issuer will be in default of such covenant. The Board of Directors of the Issuer may at any time designate any Unrestricted Subsidiary to be a Restricted Subsidiary of the Issuer;provided that such designation will be deemed to be an incurrence of Indebtedness and Liens by a Restricted Subsidiary of the Issuer of any outstanding Indebtedness and Liens of such Unrestricted Subsidiary, and such designation will only be permitted if (1) such Indebtedness and Liens are permitted under the covenants described under the captions “—Incurrence of Indebtedness and Issuance of Disqualified Stock and Preferred Stock” and “ —Liens” and (2) such designation would not cause a Default.
Payments for Consent
Holdings will not, and will not permit any of its Subsidiaries to, directly or indirectly, pay or cause to be paid any consideration to or for the benefit of any holder of Notes for or as an inducement to any consent, waiver or amendment of any of the terms or provisions of the Indenture, the Notes, the Guarantees, the Security Documents, the Intercreditor Agreement or the Registration Rights Agreement unless such consideration is offered to be paid and is paid to all holders of the Notes that consent, waive or agree to amend in the time frame set forth in the solicitation documents relating to such consent, waiver or agreement.
Reports
From and After the Exchange Offer Filing Date
From and after the Exchange Offer Filing Date, and whether or not required by the rules and regulations of the SEC, so long as any Notes are outstanding, the Issuer will furnish to the Trustee and to Cede & Co., the nominee of DTC, and the holders of Notes, within the time periods that are applicable to the Issuer (or, if not applicable, would be if the Issuer were required to file such reports under Section 13(a) or 15(d) of the Exchange Act as a non-accelerated filer):
(1) all quarterly and annual financial information that would be required to be contained in a filing with the SEC on Forms 10-Q and 10-K, if the Issuer were required to file such Forms, including a “Management’s Discussion and Analysis of Financial Condition and Results of Operations” that describes the Issuer’s consolidated financial condition and results of operation and, with respect to the annual information only, a report thereon by the Issuer’s independent registered public accountants but not any assessment, attestation or audit of internal controls pursuant to Section 404 of the Sarbanes-Oxley Act or rules and regulations promulgated thereunder; and
(2) all current reports that would be required to be filed with the SEC on Form 8-K if the Issuer were required to file such reports.
In addition, the Issuer agrees that, for so long as any Notes remain outstanding, the Issuer will furnish to any beneficial owner of Notes or to any prospective purchaser of Notes in connection with any sale thereof, upon their request, the information required to be delivered pursuant to Rule 144A(d)(4) under the Securities Act. The Issuer shall also comply with the provisions of Section 314(a) of the Trust Indenture Act.
In addition, whether or not required by the SEC, the Issuer will file a copy of all of the information and reports referred to in clauses (1) and (2) above with the SEC for public availability within the time periods specified in the SEC’s rules and regulations for a company required to file reports under Section 13(a) or 15(d) of
168
Table of Contents
the Exchange Act (unless the SEC will not accept such a filing) and make such information available to securities analysts and prospective investors upon request. The Issuer agrees that it will not take any action for the purpose of causing the SEC not to accept such filings. If, notwithstanding the foregoing, the SEC will not accept such filings for any reason, the Issuer will post the reports specified in the preceding sentence on its website within the time periods that would apply if the Issuer were required to file those reports with the SEC.
Notwithstanding the foregoing, such requirements shall be deemed satisfied prior to the commencement of the Exchange Offer or the effectiveness of the shelf registration statement described in the Registration Rights Agreement (1) by the filing with the SEC of the exchange offer registration statement or shelf registration statement (or any other similar registration statement), and any amendments thereto, with such financial information that satisfies Regulation S-X, subject to exceptions consistent with the presentation of financial information in this prospectus, to the extent filed within the times specified above, or (2) by posting reports that would be required to be filed substantially in the form required by the SEC (subject to the limitations set forth above) on the Issuer’s website (or that of any of its parent companies) or providing such reports to the Trustee within 15 days after the time the Issuer would be required to file such information with the SEC if it were subject to Section 13 or 15(d) of the Exchange Act, the financial information (including a “Management’s Discussion and Analysis of Financial Condition and Results of Operation”) that would be required to be included in such reports, subject to exceptions consistent with the presentation of financial information in this prospectus, to the extent filed within the times specified above.
Before and After the Exchange Offer Filing Date
For so long as any notes are outstanding, the Issuer shall:
(1) beginning with the fiscal quarter ended September 30, 2010, within 15 business days after filing with the Trustee or filing with the SEC the annual and quarterly information required pursuant to the above, hold a conference call for Permitted Parties to discuss such reports and the results of operations for the relevant reporting period; and
(2) issue a press release to any U.S. nationally recognized wire service or employ other means commercially reasonably expected to reach Permitted Parties no fewer than three business days prior to the date of the conference call required to be held in accordance with clause (1) above, announcing the time and date of such conference call and either including all information necessary to access the call or directing Permitted Parties to contact the appropriate person at the Issuer to obtain such information;
provided that, after an initial public offering of common stock of the Issuer or any direct or indirect parent company of the Issuer, no separate press release or conference call shall be required to comply with the foregoing if the Issuer or any direct or indirect parent company of the Issuer conducts customary earnings conference calls and issues press releases in connection therewith.
Events of Default and Remedies
Each of the following is an Event of Default:
(1) default for 30 days in the payment when due of interest on, or Additional Interest, if any, with respect to, the Notes;
(2) default in the payment when due (at maturity, upon redemption or otherwise) of the principal of, or premium, if any, on, the Notes;
(3) failure by the Issuer or any of its Restricted Subsidiaries to comply with the provisions described above under the caption “—Certain Covenants—Merger, Consolidation or Sale of Assets”;
(4) failure by the Issuer or any of its Restricted Subsidiaries for 60 days after notice to the Issuer by the Trustee or the holders of at least 25% in aggregate principal amount of the Notes then outstanding to comply with any of the other agreements or covenants in the Indenture or the Security Documents;
169
Table of Contents
(5) default under any mortgage, indenture or instrument under which there may be issued or by which there may be secured or evidenced any Indebtedness for money borrowed by the Issuer or any of its Significant Subsidiaries (or the payment of which is guaranteed by the Issuer or any of its Significant Subsidiaries), whether such Indebtedness or guarantee now exists, or is created after the Issue Date, if that default;
(a) is caused by a failure to pay principal at the final Stated Maturity of such Indebtedness (a “Payment Default”); or
(b) results in the acceleration of such Indebtedness prior to its express maturity;
and, in each case, the principal amount of such Indebtedness, together with the principal amount of any other such Indebtedness under which there has been a Payment Default or the maturity of which has been so accelerated, aggregates $10.0 million or more;
(6) with respect to any judgment or decree for the payment of money (net of any amount covered by insurance issued by a reputable and creditworthy insurer that has not contested coverage or reserved rights with respect to an underlying claim) in excess of $10.0 million or its foreign currency equivalent against the Issuer or any Significant Subsidiary, the failure by the Issuer or such Significant Subsidiary, as applicable, to pay such judgment or decree, which judgment or decree has remained outstanding for a period of 60 days after such judgment or decree became final and nonappealable without being paid, discharged, waived or stayed;
(7) except as permitted by the Indenture, any Guarantee of any Significant Subsidiary is declared to be unenforceable or invalid by any final and nonappealable judgment or decree or ceases for any reason to be in full force and effect, or any Guarantor or any Person acting on behalf of any Guarantor denies or disaffirms its obligations in writing under its Guarantee and such Default continues for 10 days after receipt of the notice specified in the Indenture;
(8) certain events of bankruptcy or insolvency described in the Indenture with respect to the Issuer, Holdings or any Subsidiary that is a Significant Subsidiary;
(9) any written repudiation or disaffirmation by the Issuer or any Guarantor of any of its obligations under the Security Documents or the Issuer or any Guarantor asserts, in any pleading in any court of competent jurisdiction, that any security interest in the Collateral is invalid and unenforceable; and
(10) with respect to any Collateral having a fair market value in excess of $3.0 million, individually or in the aggregate:
(a) the security interest under the Security Documents, at any time, ceases to be in full force and effect for any reason other than in accordance with the terms of the Indenture, the Security Documents and the Intercreditor Agreement, or
(b) any security interest created thereunder or under the Indenture is declared invalid or unenforceable by a court of competent jurisdiction.
In the case of an Event of Default arising from certain events of bankruptcy or insolvency, with respect to Holdings, the Issuer or any Guarantor that is a Significant Subsidiary, all outstanding Notes will become due and payable immediately without further action or notice. If any other Event of Default occurs and is continuing, the Trustee or the holders of at least 25% in aggregate principal amount of the then outstanding Notes may direct the Trustee to declare all the Notes to be due and payable immediately.
Subject to certain limitations, holders of a majority in aggregate principal amount of the then outstanding Notes may direct the Trustee in its exercise of any trust or power. The Trustee may withhold from holders of the Notes notice of any continuing Default or Event of Default if it determines that withholding notice is in their interest, except a Default or Event of Default relating to the payment of principal, interest or premium or Additional Interest, if any.
170
Table of Contents
Subject to the provisions of the Indenture relating to the duties of the Trustee, in case an Event of Default occurs and is continuing, the Trustee will be under no obligation to exercise any of the rights or powers under the Indenture at the request or direction of any holders of Notes unless such holders have offered to the Trustee indemnity or security satisfactory to it against any loss, liability or expense. Except to enforce the right to receive payment of principal, premium, if any, or interest or Additional Interest, if any, when due, no holder of a note may pursue any remedy with respect to the Indenture or the Notes unless:
(1) such holder has previously given the Trustee notice that an Event of Default is continuing;
(2) holders of at least 25% in aggregate principal amount of the then outstanding Notes have requested the Trustee to pursue the remedy;
(3) such holders have offered the Trustee security or indemnity satisfactory to it against any loss, liability or expense;
(4) the Trustee has not complied with such request within 60 days after the receipt of the request and the offer of security or indemnity; and
(5) holders of a majority in aggregate principal amount of the then outstanding Notes have not given the Trustee a written direction inconsistent with such request within such 60-day period.
The holders of a majority in aggregate principal amount of the then outstanding Notes by written notice to the Trustee may, on behalf of the holders of all of the Notes, rescind an acceleration or waive any existing Default or Event of Default and its consequences under the Indenture except a continuing Default or Event of Default in the payment of interest or premium or Additional Interest, if any, on, or the principal of, the Notes.
The Issuer is required to deliver to the Trustee annually a statement regarding compliance with the Indenture. Upon becoming aware of any Default or Event of Default, the Issuer is required to deliver to the Trustee within 30 days a statement specifying such Default or Event of Default.
No Personal Liability of Directors, Officers, Employees and Stockholders
No director, officer, employee, incorporator, stockholder, member, partner or other holder of Equity Interests of the Issuer or any Guarantor, as such, will have any liability for any obligations of the Issuer or the Guarantors under the Notes, the Indenture, the Guarantees, the Security Documents, the Intercreditor Agreement or the Registration Rights Agreement or for any claim based on, in respect of, or by reason of, such obligations or their creation. Each holder of Notes by accepting a note waives and releases all such liability. The waiver and release are part of the consideration for issuance of the Notes. The waiver may not be effective to waive liabilities under the federal securities laws.
Legal Defeasance and Covenant Defeasance
The Issuer may at any time, elect to have all of its obligations discharged with respect to the outstanding Notes and all obligations of the Guarantors discharged with respect to their Guarantees (“Legal Defeasance”) except for:
(1) the rights of holders of outstanding Notes to receive payments in respect of the principal of, or interest or premium and Additional Interest, if any, on, such Notes when such payments are due from the trust referred to below;
(2) the Issuer’s obligations with respect to the Notes concerning issuing temporary Notes, registration of Notes, mutilated, destroyed, lost or stolen Notes and the maintenance of an office or agency for payment and money for security payments held in trust;
(3) the rights, powers, trusts, duties and immunities of the Trustee, and the Issuer’s and the Guarantors’ obligations in connection therewith; and
(4) the Legal Defeasance provisions of the Indenture.
171
Table of Contents
In addition, the Issuer may, at its option and at any time, elect to have the obligations of the Issuer and the Guarantors released (“Covenant Defeasance”) with respect to the Security Documents and the covenants described under “—Repurchase at the Option of Holders—Change of Control,” “—Repurchase at the Option of Holders—Asset Sales,” “—Repurchase at the Option of Holders—Intercompany Loan Refinancing,” and “—Certain Covenants” and with respect to certain Events of Default (including bankruptcy default with respect to Significant Subsidiaries, cross-default and judgment default) and thereafter any omission to comply with those covenants will not constitute a Default or Event of Default with respect to the Notes. In the event Covenant Defeasance occurs, certain events (not including nonpayment and bankruptcy, receivership, rehabilitation and insolvency events with respect to the Issuer) described under “—Events of Default and Remedies” will no longer constitute an Event of Default with respect to the Notes.
In order to exercise either Legal Defeasance or Covenant Defeasance:
(1) the Issuer must irrevocably deposit with the Trustee, in trust, for the benefit of the holders of the Notes, cash in U.S. dollars, non-callable Government Securities, or a combination of cash in U.S. dollars and non-callable Government Securities, in amounts as will be sufficient (without consideration of any reinvestment of interest), in the opinion of a nationally recognized investment bank, appraisal firm or firm of independent public accountants, to pay the principal of, or interest and premium and Additional Interest, if any, on, the outstanding Notes on the stated date for payment thereof or on the applicable redemption date, as the case may be, and the Issuer must specify whether the Notes are being defeased to such stated date for payment or to a particular redemption date;
(2) in the case of Legal Defeasance, the Issuer must deliver to the Trustee an Opinion of Counsel confirming that (a) the Issuer has received from, or there has been published by, the Internal Revenue Service a ruling or (b) since the Issue Date, there has been a change in the applicable federal income tax law, in either case to the effect that, and based thereon such Opinion of Counsel will confirm that, the holders of the outstanding Notes will not recognize income, gain or loss for federal income tax purposes as a result of such Legal Defeasance and will be subject to federal income tax on the same amounts, in the same manner and at the same times as would have been the case if such Legal Defeasance had not occurred;
(3) in the case of Covenant Defeasance, the Issuer must deliver to the Trustee an Opinion of Counsel confirming that the holders of the outstanding Notes will not recognize income, gain or loss for federal income tax purposes as a result of such Covenant Defeasance and will be subject to federal income tax on the same amounts, in the same manner and at the same times as would have been the case if such Covenant Defeasance had not occurred;
(4) such Legal Defeasance or Covenant Defeasance will not result in a breach or violation of, or constitute a default under, any material agreement (including, without limitation, the Credit Agreement) or instrument (other than the Indenture) to which the Issuer or any of its Subsidiaries is a party or by which the Issuer or any of its Subsidiaries is bound;
(5) the Issuer must deliver to the Trustee an Officers’ Certificate stating that the deposit was not made by the Issuer with the intent of preferring the holders of Notes over the other creditors of the Issuer with the intent of defeating, hindering, delaying or defrauding any creditors of the Issuer or others; and
(6) the Issuer must deliver to the Trustee an Officers’ Certificate and an Opinion of Counsel, each stating that all conditions precedent relating to the Legal Defeasance or the Covenant Defeasance have been complied with.
Amendment, Supplement and Waiver
Subject to certain exceptions below, the Indenture, the Notes, the Guarantees, the Intercreditor Agreement or the Security Documents may be amended or supplemented with the consent of the holders of at least a majority in aggregate principal amount of the Notes then outstanding (including, without limitation, consents
172
Table of Contents
obtained in connection with a purchase of, or tender offer or exchange offer for, Notes), and any existing Default or Event of Default or compliance with any provision of the Indenture or the Notes or the Guarantees may be waived with the consent of the holders of a majority in aggregate principal amount of the then outstanding Notes (including, without limitation, consents obtained in connection with a purchase of, or tender offer or exchange offer for, Notes);provided that such amendments may not, without the consent of the holders of 75% in principal amount of the Notes outstanding, release all or substantially all of the Collateral other than in accordance with the Indenture, the Intercreditor Agreement and the Security Documents.
Without the consent of each holder of Notes affected, an amendment, supplement or waiver may not (with respect to any Notes held by a non-consenting holder):
(1) reduce the principal amount of Notes whose holders must consent to an amendment, supplement or waiver;
(2) reduce the principal of or change the fixed maturity of any Note or alter the provisions with respect to the optional redemption of the Notes as described under the caption “—Optional Redemption�� (other than provisions relating to the notice period for consummating an optional redemption of the Notes);
(3) reduce the rate of or change the time for payment of interest, including default interest, on any Note;
(4) waive a Default or Event of Default in the payment of principal of, or interest or premium, or Additional Interest, if any, on, the Notes (except a rescission of acceleration of the Notes by the holders of at least a majority in aggregate principal amount of the then outstanding Notes and a waiver of the payment default that resulted from such acceleration);
(5) make any note payable in money other than that stated in the Notes;
(6) make any change in the provisions of the Indenture relating to waivers of past Defaults or the rights of holders of Notes to receive payments of principal of, or interest or premium or Additional Interest, if any, on, the Notes;
(7) make the Notes or the Guarantees subordinated in right of payment to any other obligations or subordinate the Lien securing the Notes Obligations to any other obligations;
(8) release Holdings or any Subsidiary Guarantor that is a Significant Subsidiary from any of its obligations under its Guarantee or the Indenture, except as permitted by the Indenture;
(9) make any change in the preceding amendment and waiver provisions; or
(10) after the obligation to make an Asset Sale Offer, Intercompany Loan Refinancing Offer or Change of Control Offer, as applicable, arises, amend, change or modify the obligations of the Issuer to make and consummate an Asset Sale Offer with respect to any Asset Sale in accordance with the “Repurchase at the Option of Holders—Asset Sales” covenant, obligations of the Issuer to make and consummate an Intercompany Loan Refinancing Offer with respect to any Intercompany Loan Refinancing in accordance with the “Repurchase at the Option of Holders—Intercompany Loan Refinancing” covenant or obligation of the Issuer to make and consummate a Change of Control Offer in the event of a Change of Control in accordance with the “Repurchase at the Option of Holders— Change of Control” covenant including, in each case, amending, changing or modifying any definition relating thereto.
Notwithstanding the preceding, without the consent of any holder of Notes, the Issuer, the Guarantors and the Trustee may amend or supplement the Indenture, the Notes, the Guarantees, the Intercreditor Agreement or the Security Documents:
(1) to cure any ambiguity, defect or inconsistency;
(2) to provide for uncertificated Notes in addition to or in place of certificated Notes;
173
Table of Contents
(3) to provide for the assumption of the Issuer’s or a Guarantor’s obligations to holders of Notes and Guarantees in the case of a merger or consolidation or sale of all or substantially all of the Issuer’s or such Guarantor’s assets, as applicable;
(4) to make any change that would provide any additional rights or benefits to the holders of Notes or that does not adversely affect the legal rights under the Indenture of any such holder;
(5) to comply with requirements of the SEC in order to effect or maintain the qualification of the Indenture under the Trust Indenture Act;
(6) to conform the text of the Indenture, the Guarantees or the Notes to any provision of this Description of the Exchange Notes;
(7) to allow any Guarantor to execute a supplemental indenture and/or a Guarantee with respect to the Notes;
(8) to add to the Collateral securing the Notes, to add a Guarantor or to release a Guarantor in accordance with the Indenture;
(9) to release Collateral from the Lien of the Indenture and the Security Documents where permitted by the Indenture and by the Security Documents; or
(10) to appoint a successor collateral agent with respect to the Collateral in favor of the holders of Notes, in accordance with the Indenture and the Security Documents.
In connection with any amendment, the Trustee shall be entitled to receive an Officer’s Certificate and Opinion of Counsel each stating that such amendment is authorized or permitted by the terms of the Indenture and that all conditions precedent to such amendment required by the Indenture have been complied with.
Satisfaction and Discharge
The Indenture and the Security Documents and related Liens will be discharged and will cease to be of further effect as to all Notes issued thereunder, when:
(1) either:
(a) all Notes that have been authenticated, except lost, stolen or destroyed Notes that have been replaced or paid and Notes for whose payment money has been deposited in trust and thereafter repaid to the Issuer, have been delivered to the Trustee for cancellation; or
(b) all Notes that have not been delivered to the Trustee for cancellation have become due and payable by reason of the mailing of a notice of redemption or otherwise or will become due and payable within one year and the Issuer or any Guarantor has irrevocably deposited or caused to be deposited with the Trustee as trust funds in trust solely for the benefit of the holders, cash in U.S. dollars, non-callable Government Securities, or a combination of cash in U.S. dollars and non-callable Government Securities, in amounts as will be sufficient, without consideration of any reinvestment of interest, to pay and discharge the entire Indebtedness on the Notes not delivered to the Trustee for cancellation for principal, premium and Additional Interest, if any, and accrued interest to the date of maturity or redemption;
(2) no Default or Event of Default has occurred and is continuing on the date of the deposit (other than a Default or Event of Default resulting from the borrowing of funds to be applied to such deposit) and the deposit will not result in a breach or violation of, or constitute a default under, any other instrument to which the Issuer or any Guarantor is a party or by which the Issuer or any Guarantor is bound;
(3) the Issuer or any Guarantor has paid or caused to be paid all sums payable by it under the Indenture; and
(4) the Issuer has delivered irrevocable instructions to the Trustee under the Indenture to apply the deposited money toward the payment of the Notes at maturity or on the redemption date, as the case maybe.
174
Table of Contents
In addition, the Issuer must deliver an Officers’ Certificate and an Opinion of Counsel to the Trustee stating that all conditions precedent to satisfaction and discharge have been satisfied.
Concerning the Trustee
If the Trustee becomes a creditor of the Issuer or any Guarantor, the Indenture limits the right of the Trustee to obtain payment of claims in certain cases, or to realize on certain property received in respect of any such claim as security or otherwise. The Trustee is permitted to engage in other transactions; however, if it acquires any conflicting interest it must eliminate such conflict within 90 days, apply to the SEC for permission to continue as Trustee (if the Indenture has been qualified under the Trust Indenture Act) or resign.
The holders of a majority in aggregate principal amount of the then outstanding Notes have the right to direct the time, method and place of conducting any proceeding for exercising any remedy available to the Trustee, subject to certain exceptions. The Indenture provides that in case an Event of Default occurs and is continuing, the Trustee will be required, in the exercise of its power, to use the degree of care of a prudent man in the conduct of his own affairs. Subject to such provisions, the Trustee will be under no obligation to exercise any of its rights or powers under the Indenture at the request of any holder of Notes, unless such holder has offered to the Trustee security and indemnity satisfactory to it against any loss, liability or expense.
Additional Information
Anyone who receives this prospectus may obtain a copy of the Indenture without charge by writing to American Renal Holdings Inc., 66 Cherry Hill Drive, Beverly, MA 01915, Attention: Secretary.
Certain Definitions
Set forth below are certain defined terms used in the Indenture. Reference is made to the Indenture for a full disclosure of all defined terms used therein, as well as any other capitalized terms used herein for which no definition is provided.
“Acquired Debt” means, with respect to any specified Person:
(1) Indebtedness of any other Person existing at the time such other Person is merged with or into or became a Restricted Subsidiary of such specified Person, whether or not such Indebtedness is incurred in connection with, or in contemplation of, such other Person merging with or into, or becoming a Restricted Subsidiary of, such specified Person; and
(2) Indebtedness secured by a Lien encumbering any asset acquired by such specified Person.
“Additional Interest” means all additional interest then owing pursuant to the Registration Rights Agreement.
“Affiliate” of any specified Person means any other Person directly or indirectly controlling or controlled by or under direct or indirect common control with such specified Person. For purposes of this definition, “control,” as used with respect to any Person, means the possession, directly or indirectly, of the power to direct or cause the direction of the management or policies of such Person, whether through the ownership of voting securities, by agreement or otherwise. For purposes of this definition, the terms “controlling,” “controlled by” and “under common control with” have correlative meanings. No Person in whom a Receivables Subsidiary makes an Investment in connection with a Qualified Receivables Transaction will be deemed to be an Affiliate of the Issuer or any of its Subsidiaries solely by reason of such Investment.
“Applicable Premium” has the meaning set forth under “—Optional Redemption.”
175
Table of Contents
“Asset Sale” means:
(1) the sale, lease (other than operating leases), conveyance or other disposition of any assets or rights outside of the ordinary course of business (including any Event of Loss);provided that the sale, lease, conveyance or other disposition of all or substantially all of the assets of the Issuer and its Restricted Subsidiaries taken as a whole will be governed by the provisions of the Indenture described above under the caption “—Repurchase at the Option of Holders—Change of Control” and/or the provisions described above under the caption “—Certain Covenants—Merger, Consolidation or Sale of Assets” and not by the provisions of the Asset Sale covenant; and
(2) the issuance of Equity Interests in any of the Issuer’s Restricted Subsidiaries or the sale of Equity Interests in any of its Restricted Subsidiaries (other than directors’ qualifying Equity Interests or Equity Interests required by applicable law to be held by a Person other than the Issuer or a Restricted Subsidiary).
Notwithstanding the preceding, none of the following items will be deemed to be an Asset Sale:
(1) any single transaction or series of related transactions that involves assets having a Fair Market Value of less than $5.0 million;
(2) a transfer of assets between or among the Issuer and its Restricted Subsidiaries;
(3) an issuance or sale of Equity Interests of a Restricted Subsidiary of the Issuer to the Issuer or to a Restricted Subsidiary of the Issuer or of a Qualified Restricted Subsidiary to Strategic Investors in connection with the start-up of such Qualified Restricted Subsidiary;
(4) the sale or lease of products, services or accounts receivable (including at a discount) in the ordinary course of business and any sale or other disposition of damaged, worn-out, negligible, surplus or obsolete assets in the ordinary course of business;
(5) the sale or other disposition of Cash Equivalents;
(6) a Restricted Payment that does not violate the covenant described above under the caption “—Certain Covenants—Restricted Payments” or a Permitted Investment;
(7) the sale or disposition of any assets or property received as a result of a foreclosure by the Issuer or any of its Restricted Subsidiaries on any secured Investment or any other transfer of title with respect to any secured Investment in default;
(8) the licensing of intellectual property in the ordinary course of business or in accordance with industry practice;
(9) surrender or waiver of contract rights or the settlement, release or surrender of contract, tort or other claims of any kind;
(10) leases or subleases to third persons in the ordinary course of business that do not interfere in any material respect with the business of the Issuer or any of its Restricted Subsidiaries;
(11) transfers of accounts receivable and related assets of the type specified in the definition of Qualified Receivables Transaction (or a fractional undivided interest therein) by a Receivables Subsidiary in a Qualified Receivables Transaction;
(12) any sale or disposition deemed to occur in connection with creating or granting any Permitted Liens (but not the sale or other disposition of the property subject to such Permitted Lien);
(13) any disposition of an account receivable in connection with the collection or compromise thereof; and
(14) any Intercompany Loan Refinancing if and to the extent the proceeds thereof are utilized as described in “—Repurchase at the Option of Holders—Intercompany Loan Refinancing.”
“Asset Sale Offer” has the meaning assigned to that term in the Indenture governing the Notes.
176
Table of Contents
“Beneficial Owner” has the meaning assigned to such term in Rule 13d-3 and Rule 13d-5 under the Exchange Act, except that in calculating the beneficial ownership of any particular “person” (as that term is used in Section 13(d)(3) of the Exchange Act), such “person” will be deemed to have beneficial ownership of all securities that such “person” has the right to acquire by conversion or exercise of other securities, whether such right is currently exercisable or is exercisable only after the passage of time.
“Board of Directors” means:
(1) with respect to a corporation, the board of directors of the corporation or, other than for purposes of the definition of Change of Control, any committee thereof duly authorized to act on behalf of such board;
(2) with respect to a partnership, the board of directors or board of managers of the general partner of the partnership;
(3) with respect to a limited liability company, the managing member or members or any controlling committee of managing members thereof; and
(4) with respect to any other Person, the board or committee of such Person serving a similar function.
“Capital Lease Obligation” means, at the time any determination is to be made, the amount of the liability in respect of a capital lease that would at that time be required to be capitalized on a balance sheet prepared in accordance with GAAP, and the Stated Maturity thereof shall be the date of the last payment of rent or any other amount due under such lease prior to the first date upon which such lease may be prepaid by the lessee without payment of a penalty.
“Capital Stock” means:
(1) in the case of a corporation, corporate stock;
(2) in the case of an association or business entity, any and all shares, interests, participations, rights or other equivalents (however designated) of corporate stock;
(3) in the case of a partnership or limited liability company, partnership interests (whether general or limited) or membership interests; and
(4) any other interest or participation that confers on a Person the right to receive a share of the profits and losses of, or distributions of assets of, the issuing Person, but excluding from all of the foregoing any debt securities convertible into Capital Stock, whether or not such debt securities include any right of participation with Capital Stock.
“Cash Equivalents” means:
(1) United States dollars or, in the case of any Restricted Subsidiary which is not a Domestic Subsidiary, any other currencies held from time to time in the ordinary course of business;
(2) securities issued or directly and fully guaranteed or insured by the United States government or any agency or instrumentality of the United States government (provided that the full faith and credit of the United States is pledged in support of those securities) having maturities of not more than 12 months from the date of acquisition;
(3) direct obligations issued by any state of the United States of America or any political subdivision of any such state, or any public instrumentality thereof, in each case having maturities of not more than 12 months from the date of acquisition;
(4) certificates of deposit and eurodollar time deposits with maturities of 12 months or less from the date of acquisition, bankers’ acceptances with maturities not exceeding 12 months and overnight bank deposits, in each case, with any lender party to the Credit Agreement or with any domestic commercial bank that has capital and surplus of not less than $500.0 million;
177
Table of Contents
(5) repurchase obligations with a term of not more than one year for underlying securities of the types described in clauses (2) and (4) above entered into with any financial institution meeting the qualifications specified in clause (4) above;
(6) commercial paper having one of the two highest ratings obtainable from Moody’s Investors Service, Inc. or Standard & Poor’s Rating Services and, in each case, maturing within 12 months after the date of acquisition; and
(7) money market funds at least 95% of the assets of which constitute Cash Equivalents of the kinds described in clauses (1) through (6) of this definition or money market funds that comply with the criteria set forth in SEC Rule 2a-7 under the Investment Company Act of 1940, as amended.
“CFC” means a Restricted Subsidiary that is a controlled foreign corporation (as such term is defined in Section 957(a) of the United States Internal Revenue Code of 1986, as amended).
“Change of Control” means the occurrence of any of the following:
(1) the direct or indirect sale, lease, transfer, conveyance or other disposition (other than by way of merger or consolidation), in one or a series of related transactions, of all or substantially all of the properties or assets of the Issuer and its Subsidiaries taken as a whole to any “person” (as that term is used in Section 13(d) of the Exchange Act) other than Permitted Holders; or
(2) the consummation of any transaction (including, without limitation, any merger or consolidation), the result of which is that any “person” (as defined above), other than Permitted Holders, becomes the Beneficial Owner, directly or indirectly, of 50% or more of the Voting Stock of the Issuer, measured by voting power rather than number of shares;provided, however, for purposes of this clause (2), each Person will be deemed to beneficially own any Voting Stock of another Person held by one or more of its Subsidiaries.
“Change of Control Offer” has the meaning assigned to that term in the Indenture governing the Notes.
“Collateral Agent” means Wilmington Trust FSB, acting in its capacity as collateral agent under the Security Documents, or any successor thereto.
“Consolidated Adjusted Secured Debt Ratio” means, as of any date of determination, the ratio of
(1) Consolidated Adjusted Secured Net Debt as of such date after giving effect to all incurrences and repayments or discharges of Indebtedness and Liens to occur on such date to (2) the Issuer’s Consolidated EBITDA for the Measurement Period calculated on a Pro Forma Basis.
“Consolidated Adjusted Secured Net Debt” means, as of any date, (a) the aggregate principal amount of Indebtedness of the type specified in clauses (a), (b) and (g) of the definition thereof of the Issuer and its Restricted Subsidiaries outstanding as of such date determined on a consolidated basis, if such Indebtedness is (x) secured by a Lien or (y) owing by a Non-Guarantor Subsidiary,minus (b) the amount of unrestricted cash and Cash Equivalents held, on such date, by the Issuer and the Subsidiary Guarantors,minus (c) the amount of unrestricted cash and Cash Equivalents held, on such date, by any Non-Guarantor Subsidiary, up to the greater of (x) the aggregate principal amount of Indebtedness of such Non-Guarantor Subsidiary included in clause (a) of this definition and (y) the amount of such unrestricted cash and Cash Equivalents of such Non-Guarantor Subsidiary times the percentage of such Non-Guarantor Subsidiary owned directly or indirectly by the Issuer or a Subsidiary Guarantor.
178
Table of Contents
“Consolidated EBITDA” means, with respect to any specified Person for any period, Consolidated Net Income for such Person for such periodplus
(a) without duplication and to the extent deducted in determining such Consolidated Net Income for such period, the sum of:
(i) consolidated interest expense (and solely for purposes of calculating the Fixed Charge Coverage Ratio, other Fixed Charges) of the Issuer and its Restricted Subsidiaries for such period and, to the extent not reflected in such total interest expense, increased by payments made in respect of Hedging Obligations or other derivative instruments entered into for the purpose of hedging interest rate risk, minus any payments received in respect of such Hedging Obligations or other derivative instruments,
(ii) consolidated tax expense of the Issuer and its Restricted Subsidiaries based on income, profits or capital, including state, franchise, capital and similar taxes and withholding taxes paid or accrued during such period,
(iii) all amounts attributable to depreciation and amortization expense of the Issuer and its Restricted Subsidiaries for such period,
(iv) any Non-Cash Charges for such period,
(v) costs associated with the Transactions made or incurred by the Issuer and its Restricted Subsidiaries in connection with the Transactions for such period that are paid, accrued or reserved for within 365 days of the consummation of the Transactions,
(vi) any restructuring charges (including restructuring costs related to acquisitions after the Issue Date and to closure or consolidation of facilities) for such period and any “Specified Payments” as defined in Schedule 11.2(a)(vi) to the Merger Agreement made during such period,
(vii) any unusual or nonrecurring fees, cash charges and other cash expenses for such period (A) made or incurred by the Issuer and its Restricted Subsidiaries in connection with any acquisition or investment not prohibited by the Indenture, including severance, relocation and facilities closing costs, including any earnout payments, whether or not accounted for as such, that are paid, accrued or reserved for within 365 days of such transaction, or (B) incurred in connection with the issuance of Equity Interests or Indebtedness,
(viii) cash expenses incurred during such period in connection with an acquisition not prohibited by the Indenture to the extent that such expenses are reimbursed in cash during such period pursuant to indemnification provisions of any agreement relating to such transaction,
(ix) periodic management fees that are permitted by clause (13)(ii) under the covenant described under “—Certain Covenants—Transactions with Affiliates,”
(x) cash expenses incurred during such period in connection with extraordinary casualty events to the extent such expenses are reimbursed in cash by insurance during such period, and
(xi) the amount of any minority interest expense consisting of Restricted Subsidiary income attributable to minority equity interests of third parties in any non-Wholly Owned Restricted Subsidiary to the extent the Indebtedness owed by such Restricted Subsidiary is included in the Indebtedness of the Issuer;minus
(b) without duplication and, in the case of clause (ii) below, to the extent included in determining such Consolidated Net Income,
(i) any cash payments made during such period in respect of Non-Cash Charges described in clause (a)(iv) taken in a prior period or taken in such period,
(ii) any non-cash items of income for such period (other than the accrual of revenue or recording of receivables in the ordinary course of business);
179
Table of Contents
provided that (I) in no event shall any charge, expense or loss relating to write-downs, write-offs or reserves with respect to current assets be added back and (II) the aggregate amount added back pursuant to clauses (vi) and (vii) shall not exceed 10% of Consolidated EBITDA (calculated before giving effect to such clauses) for any period.
“Consolidated Leverage Ratio” means, as of any date of determination, the ratio of (1) Consolidated Net Debt as of such date after giving effect to all incurrences and repayments of Indebtedness to occur on such date to (2) the Issuer’s Consolidated EBITDA for the Measurement Period calculated on a Pro Forma Basis.
“Consolidated Net Debt” means, as of any date, (a) the aggregate principal amount of Indebtedness of the type specified in clauses (a), (b) and (g) of the definition thereof of the Issuer and its Restricted Subsidiaries outstanding as of such date determined on a consolidated basis or is owing by Non-Guarantor Subsidiaries, minus (b) the amount of unrestricted cash and Cash Equivalents held, on such date, by the Issuer and the Subsidiary Guarantors, minus (c) the amount of unrestricted cash and Cash Equivalents held, on such date, by any Non-Guarantor Subsidiary, up to, the greater of (x) the aggregate principal amount of Indebtedness of such Non-Guarantor Subsidiary included in clause (a) of this definition and (y) the amount of such unrestricted cash and Cash Equivalents of such Non-Guarantor Subsidiary times the percentage of such Non-Guarantor Subsidiary owned directly or indirectly by the Issuer or a Subsidiary Guarantor.
“Consolidated Net Income” means, with respect to any specified Person for any period, the aggregate of the Net Income attributable to such specified Person and its Restricted Subsidiaries for such period, on a consolidated basis, determined in accordance with GAAP;provided that:
(1) the Net Income (and net loss) of any other Person (other than a Restricted Subsidiary of the Issuer) in which the Issuer or any of its Restricted Subsidiaries has an ownership interest will be excluded, except to the extent that any such Net Income is actually received in cash by the Issuer or a Qualified Restricted Subsidiary in the form of dividends or similar distributions in respect of such period;
(2) the cumulative effect of a change in accounting principles will be excluded;
(3) the amortization of any premiums, fees or expenses incurred in connection with the Transactions or any amounts required or permitted by Accounting Principles Board Opinions No. 16 (including non-cash write-ups and non-cash charges relating to inventory and fixed assets, in each case arising in connection with the Transactions) and No. 17 (including non-cash charges relating to intangibles and goodwill), in each case in connection with the Transactions, will be excluded;
(4) any gain or loss, together with any related provision for taxes on such gain or loss, realized in connection with: (a) any sales of assets out of the ordinary course of business (it being understood that a sale of assets comprising discontinued operations shall be deemed a sale of assets out of the ordinary course of business); or (b) the disposition of any securities by such Person or any of its Restricted Subsidiaries or the extinguishment of any Indebtedness of such Person or any of its Restricted Subsidiaries will be excluded;
(5) any extraordinary gain or loss, together with any related provision for taxes on such extraordinary gain or loss, will be excluded;
(6) income or losses attributable to discontinued operations (including without limitation, operations disposed during such period whether or not such operations were classified as discontinued) will be excluded;
(7) any non-cash charges (i) attributable to applying the purchase method of accounting in accordance with GAAP, (ii) resulting from the application of FAS 142 or FAS 144, and (iii) relating to the amortization of intangibles resulting from the application of FAS 141, will be excluded;
(8) all non-cash charges relating to employee benefit or other management or stock compensation plans of the Issuer or a Restricted Subsidiary (excluding any such non-cash charge to the extent that it represents an accrual of or reserve for cash expenses in any future period or amortization of a prepaid cash expense
180
Table of Contents
incurred in a prior period) will be excluded to the extent that such non-cash charges are deducted in computing such Consolidated Net Income;provided, further, that if the Issuer or any Restricted Subsidiary of the Issuer makes a cash payment in respect of such non-cash charge in any period, such cash payment will (without duplication) be deducted from the Consolidated Net Income of the Issuer for such period;
(9) all unrealized gains and losses relating to hedging transactions and mark-to-market of Indebtedness denominated in foreign currencies resulting from the application of FAS 52 shall be excluded;
(10) the Net Income for such period of any Restricted Subsidiary (other than a Guarantor) shall be excluded to the extent that the declaration or payment of dividends or similar distributions by that Restricted Subsidiary of its Net Income is not at the date of determination permitted without any prior governmental approval (which has not been obtained) or, directly or indirectly, by the operation of the terms of its charter or any agreement, instrument, judgment, decree, order, statute, rule, or governmental regulation applicable to that Restricted Subsidiary or its stockholders, except to the extent such restriction with respect to the payment of dividends or similar distributions has been legally waived; and
(11) Consolidated Net Income shall be reduced by the amount of any payments described in clause (2) of the definition of “Permitted Payments to Parent.”
“Contribution Indebtedness” means Indebtedness of the Issuer in an aggregate principal amount not to exceed the aggregate net cash proceeds received by the Issuer after the date of the Indenture from the sale of its Equity Interests (other than Disqualified Stock) or as a contribution to its common equity capital (in each case, other than to or from a Subsidiary of the Issuer);provided that such Indebtedness (a) is incurred within 180 days after the sale of such Equity Interests or the making of such capital contribution and (b) is designated as “Contribution Indebtedness” pursuant to an Officers’ Certificate delivered to the Trustee within one business day of the date of its incurrence. Any sale of Equity Interests or capital contribution that forms the basis for an incurrence of Contribution Indebtedness will not be considered to be a sale of Capital Stock and will be disregarded for purposes of the “Restricted Payments” covenant and will not be considered to be an Equity Offering for purposes of the “Optional Redemption” provisions of the Indenture.
“Credit Agreement” means that certain Credit Agreement, dated on or about the Issue Date, by and among the Issuer, as borrower, Holdings, the Guarantors, Bank of America, N.A., as administrative agent and collateral agent, and various lenders from time to time party thereto, including any related notes, guarantees, collateral documents, instruments and agreements executed in connection therewith, and, in each case, as amended, restated, modified, renewed, refunded, replaced (whether upon or after termination or otherwise) or refinanced from time to time.
“Credit Facilities” means one or more debt facilities (including, without limitation, the Credit Agreement) or commercial paper facilities or indentures, in each case, with banks or other institutional lenders providing for revolving credit loans, notes, term loans, receivables financing (including through the sale of receivables to such lenders or to special purpose entities formed to borrow from such lenders against such receivables) or letters of credit or any other Indebtedness, in each case, as amended, restated, modified, renewed, refunded, replaced (whether upon or after termination or otherwise) or refinanced (including by means of sales of debt securities and including any amendment, restatement, modification, renewal, refunding, replacement or refinancing that increases the amount borrowed thereunder or extends the maturity thereof) in whole or in part from time to time.
“Default” means any event that is, or with the passage of time or the giving of notice or both would be, an Event of Default.
“Designated Noncash Consideration” means any non-cash consideration received by the Issuer or a Restricted Subsidiary in connection with an Asset Sale that is designated as Designated Noncash Consideration pursuant to an Officers’ Certificate.
181
Table of Contents
“Disqualified Stock” means any Capital Stock that, by its terms (or by the terms of any security into which it is convertible, or for which it is exchangeable, in each case, at the option of the holder of the Capital Stock), or upon the happening of any event, matures or is mandatorily redeemable, pursuant to a sinking fund obligation or otherwise, or is redeemable at the option of the holder of the Capital Stock, in whole or in part, on or prior to the date that is 91 days after the date on which the Notes mature. Notwithstanding the preceding sentence, (x) any Capital Stock that would constitute Disqualified Stock solely because the holders of the Capital Stock have the right to require the Issuer or the Subsidiary that issued such Capital Stock to repurchase such Capital Stock upon the occurrence of a change of control or an asset sale will not constitute Disqualified Stock if the terms of such Capital Stock provide that the Issuer may not repurchase such Capital Stock unless the Issuer would be permitted to do so in compliance with the covenant described under “—Certain Covenants—Restricted Payments,” (y) any Capital Stock that would constitute Disqualified Stock solely as a result of any redemption feature that is conditioned upon, and subject to, compliance with the covenant described above under “—Certain Covenants—Restricted Payments” will not constitute Disqualified Stock and (z) any Capital Stock issued to any plan for the benefit of employees will not constitute Disqualified Stock solely because it may be required to be repurchased by the Issuer or the Subsidiary that issued such Capital Stock in order to satisfy applicable statutory or regulatory obligations. The amount of Disqualified Stock deemed to be outstanding at any time for purposes of the Indenture will be the maximum amount that the Issuer and its Restricted Subsidiaries may become obligated to pay upon the maturity of, or pursuant to any mandatory redemption provisions of, such Disqualified Stock, exclusive of accrued dividends.
“Domestic Subsidiary” means any Restricted Subsidiary of the Issuer that was formed under the laws of the United States or any state of the United States.
“Equity Interests” means Capital Stock and all warrants, options or other rights to acquire Capital Stock (but excluding any debt security that is convertible into, or exchangeable for, Capital Stock).
“Equity Offering” means a public or private offering of Qualified Capital Stock of the Issuer, Holdings or any other direct or indirect parent of the Issuer, other than:
(1) a public offering with respect to the Issuer’s or any direct or indirect parent company’s Qualified Capital Stock registered on Form S-4 or Form S-8;
(2) issuances to any Subsidiary of the Issuer; and
(3) any such public or private offering that constitutes an Excluded Contribution.
“Event of Loss” means, with respect to any property or asset (tangible or intangible, real or personal) constituting Collateral, any of the following:
(i) any loss, destruction or damage of such property or asset;
(ii) any institution of any proceeding for the condemnation or seizure of such property or asset or for the exercise of any right of eminent domain;
(iii) any actual condemnation, seizure or taking by exercise of the power of eminent domain or otherwise of such property or asset, or confiscation of such property or asset or the requisition of the use of such property or asset; or
(iv) any settlement in lieu of clauses (ii) or (iii) above.
“Exchange Act” means the Securities Exchange Act of 1934, as amended and the rules and regulations of the SEC promulgated thereunder.
“Exchange Notes” means the notes issued in the Exchange Offer pursuant to the Registration Rights Agreement.
182
Table of Contents
“Exchange Offer” has the meaning set forth for such term in the Registration Rights Agreement.
“Excluded Contributions” means net cash proceeds, marketable securities or Qualified Proceeds received by the Issuer from (i) contributions to its equity capital (other than Disqualified Stock) or (ii) the sale (other than to a Subsidiary of the Issuer or to any management equity plan or stock option plan or any other management or employee benefit plan or agreement of the Issuer) of Equity Interests (other than Disqualified Stock) of the Issuer, in each case designated as Excluded Contributions pursuant to an Officers’ Certificate on the date such capital contributions are made or the date such Equity Interests are sold, as the case may be, that are excluded from the calculation set forth in clause (3) of the first paragraph under “—Certain Covenants—Restricted Payments.”
“Excluded Deposit Accounts” means (1) any deposit accounts specially and exclusively used for payroll, payroll taxes and other employee wage and benefit payments to or for the benefit of the Issuer’s or any Guarantor’s employees, (2) any deposit account of the Issuer or any Guarantor into which proceeds from any receivables in respect of participation in federal and state healthcare programs, including the Medicare or Medicaid programs, are paid into, so long as the balance of such deposit account is swept at the end of each business day into a deposit account subject to a control agreement and (3) any deposit account specially and exclusively used to hold cash deposits required to be held in escrow, (which escrow is not prohibited by the Indenture) and which by terms of the agreement creating the escrow obligations shall not be subject to any other Liens.
“Excluded Subsidiary” means any Subsidiary of the Issuer that is (a) an Immaterial Subsidiary so long as such Subsidiary remains an Immaterial Subsidiary, (b) not a Wholly Owned Subsidiary so long as such Subsidiary is not a Wholly Owned Subsidiary, (c) an Unrestricted Subsidiary so long as such Subsidiary remains an Unrestricted Subsidiary, (d) a Receivables Subsidiary so long as such Subsidiary remains a Receivables Subsidiary or (e) a CFC so long as such Subsidiary remains a CFC.
“Existing Indebtedness” means Indebtedness (other than the Indebtedness under the Credit Agreement and Indebtedness owed to Holdings or any of its Subsidiaries), existing on the Issue Date.
“Fair Market Value” means the value that would be paid by a willing buyer to an unaffiliated willing seller in a transaction not involving distress or necessity of either party, determined in good faith by the Board of Directors, chief executive officer or chief financial officer of the Issuer (unless otherwise provided in the Indenture).
“First Lien Obligations” means Priority Payment Lien Obligations, the Notes Obligations and Pari Passu Lien Indebtedness.
“Fixed Charge Coverage Ratio” means, with respect to any specified Person at any date of determination, the ratio of the Consolidated EBITDA of such Person for the Measurement Period to the Fixed Charges of such Person for the Measurement Period, in each case, calculated on a Pro Forma Basis. In the event that the specified Person or any of its Restricted Subsidiaries incurs, assumes, guarantees, repays, repurchases, redeems, defeases or otherwise discharges any Indebtedness (other than ordinary working capital borrowings) or issues, repurchases or redeems preferred stock or Disqualified Stock subsequent to the commencement of the Measurement Period, then the Fixed Charge Coverage Ratio will be calculated giving pro forma effect to such incurrence, assumption, Guarantee, repayment, repurchase, redemption, defeasance or other discharge of Indebtedness, or such issuance, repurchase or redemption of preferred stock or Disqualified Stock, and the use of the proceeds therefrom, as if the same had occurred at the beginning of the Measurement Period.
“Fixed Charges” means, with respect to any specified Person for any period, the sum, without duplication, of:
(1) the consolidated interest expense of such Person and its Restricted Subsidiaries for such period, net of interest income, whether paid or accrued, including, without limitation, original issue discount, non-cash
183
Table of Contents
interest payments, the interest component of all payments associated with Capital Lease Obligations, commissions, discounts and other fees and charges incurred in respect of letter of credit or bankers’ acceptance financings, and net of the effect of all cash payments made or received pursuant to Hedging Obligations in respect of interest rates, and excluding (v) amortization of deferred financing costs, (w) accretion or accrual of discounted liabilities not constituting Indebtedness, (x) any expense resulting from the discounting of any Indebtedness in connection with the application of purchase accounting in connection with any acquisition, (y) any expensing of bridge, commitment and other financing fees and (z) to the extent included in Fixed Charges, the portion of consolidated interest expense of such Person and its Restricted Subsidiaries attributable to Fixed Charges incurred in connection with the acquisition of discontinued operations;plus
(2) any interest on Indebtedness of another Person that is Guaranteed by such Person or one of its Restricted Subsidiaries or secured by a Lien on assets of such Person or one of its Restricted Subsidiaries, but only to the extent such Guarantee or Lien is called upon;plus
(3) the product of (A) all cash dividends paid on any series of preferred stock of such Person or any of its Restricted Subsidiaries (other than to the Issuer or any Qualified Restricted Subsidiary), in each case, determined on a consolidated basis in accordance with GAAP,multiplied by (B) a fraction, the numerator of which is one and the denominator of which is one minus the then current combined federal, state and local statutory tax rate of the Issuer and its Restricted Subsidiaries expressed as a decimal.
“GAAP” means generally accepted accounting principles set forth in the opinions and pronouncements of the Accounting Principles Board of the American Institute of Certified Public Accountants and statements and pronouncements of the Financial Accounting Standards Board or in such other statements by such other entity as have been approved by a significant segment of the accounting profession, which are in effect on the Issue Date. For the purposes of the Indenture, the term “consolidated” with respect to any Person shall mean such Person consolidated with its Restricted Subsidiaries, and shall not include any Unrestricted Subsidiary, but the interest of such Person in an Unrestricted Subsidiary will be accounted for as an Investment.
“Government Securities” means direct obligations of, or obligations guaranteed by, the United States of America (including any agency or instrumentality thereof) and the payment for which the United States pledges its full faith and credit.
“guarantee” of or by any Person (the “guarantor”) means any obligation, contingent or otherwise, of the guarantor guaranteeing or having the economic effect of guaranteeing any Indebtedness or other obligation of any other Person (the “primary obligor”) in any manner, whether directly or indirectly, and including any obligation of the guarantor, direct or indirect, (a) to purchase or pay (or advance or supply funds for the purchase or payment of) such Indebtedness or other obligation or to purchase (or to advance or supply funds for the purchase of) any security for the payment thereof, (b) to purchase or lease property, securities or services for the purpose of assuring the owner of such Indebtedness or other obligation of the payment thereof, (c) to maintain working capital, equity capital or any other financial statement condition or liquidity of the primary obligor so as to enable the primary obligor to pay such Indebtedness or other obligation or (d) as an account party or applicant in respect of any letter of credit or letter of guaranty issued to support such Indebtedness or obligation,provided that the term “guarantee” shall not include endorsements for collection or deposit in the ordinary course of business. The amount of any guarantee of any guarantor shall be deemed to be the lower of (a) an amount equal to the stated or determinable amount of the primary obligation in respect of which the guarantee is made and (b) the maximum amount for which such guarantor may be liable pursuant to the terms of the instrument embodying such guarantee.
“Guarantee” means the guarantee by each Guarantor of the Issuer’s obligations under the Indenture and the Notes, executed pursuant to the provisions of the Indenture.
184
Table of Contents
“Guarantee Agreement” means a supplemental indenture or a notation of guarantee, in a form satisfactory to the Trustee, pursuant to which a Guarantor guarantees the Issuer’s obligations with respect to the Notes on the terms provided for in the Indenture.
“Guarantors” means Holdings and the Subsidiary Guarantors.
“Hedging Obligations” means, with respect to any specified Person, the obligations of such Person
under:
(1) interest rate swap agreements (whether from fixed to floating or from floating to fixed), interest rate cap agreements and interest rate collar agreements;
(2) other agreements or arrangements designed to manage interest rates or interest rate risk; and
(3) other agreements or arrangements designed to protect such Person against fluctuations in currency exchange rates or commodity prices.
“Holder” means any registered holder, from time to time, of the Notes.
“Holdings” means C.P. Atlas Intermediate Holdings, LLC, a Delaware limited liability company.
“Immaterial Subsidiary” means, at any date of determination, any Subsidiary of the Issuer that accounts for less than 1.0% of the Issuer’s consolidated revenues and less than 1.0% of the Issuer’s Net Income for the Measurement Period and has less than $10,000 of assets;provided that at no time shall Immaterial Subsidiaries in the aggregate account for more than 3.0% of the Issuer’s consolidated revenues or more than 3.0% of the Issuer’s Net Income for any Measurement Period.
“Indebtedness” of any Person means, without duplication, (a) all obligations of such Person for borrowed money, (b) all obligations of such Person evidenced by bonds, debentures, notes or similar instruments, (c) all obligations of such Person under conditional sale or other title retention agreements relating to property acquired by such Person, (d) all obligations of such Person in respect of the deferred purchase price of property or services (excluding trade accounts payable and accrued obligations incurred in the ordinary course of business), (e) all obligations of others secured by any Lien on property owned or acquired by such Person, whether or not the obligations secured thereby have been assumed, but limited, in the event such secured obligations are nonrecourse to such Person, to the fair value of such property, (f) all Guarantees by such Person of the Indebtedness of any other Person, (g) all Capital Lease Obligations of such Person, (h) all reimbursement obligations of such Person as an account party or applicant in respect of letters of credit and letters of guaranty, (i) all obligations, contingent or otherwise, of such Person in respect of bankers’ acceptances and (j) all obligations of such Person in respect of Disqualified Stock. The Indebtedness of any Person shall include the Indebtedness of any other entity (including any partnership in which such Person is a general partner) to the extent such Person is liable therefor as a result of such Person’s ownership interest in or other relationship with such entity, except to the extent the terms of such Indebtedness provide that such Person is not liable therefor. For the avoidance of doubt, any right of Strategic Investors in an Qualified Restricted Subsidiary to require the Issuer or any Qualified Restricted Subsidiary to repurchase the Equity Interests in such Qualified Restricted Subsidiary held by such Strategic Investors does not constitute Indebtedness.
“Intercompany Loan Refinancing” has the meaning given to such term in clause (16) of the second paragraph of the covenant under “—Certain Covenants—Incurrence of Indebtedness and Issuance of Disqualified Stock and Preferred Stock.”
“Investments” means, with respect to any Person, all direct or indirect investments by such Person in other Persons (including Affiliates) in the forms of loans (including Guarantees or other obligations), advances or
185
Table of Contents
capital contributions (excluding accounts receivable, trade credit, advances to customers and commission, travel, relocation and similar advances to officers and employees made in the ordinary course of business), purchases or other acquisitions for consideration of Indebtedness, Equity Interests or other securities, together with all items that are or would be classified as investments on a balance sheet (excluding the footnotes) prepared in accordance with GAAP. If the Issuer or any Restricted Subsidiary of the Issuer sells or otherwise disposes of any Equity Interests of any direct or indirect Restricted Subsidiary of the Issuer such that, after giving effect to any such sale or disposition, such Person is no longer a Subsidiary of the Issuer, the Issuer will be deemed to have made an Investment on the date of any such sale or disposition equal to the Fair Market Value of the Issuer’s Investments in such Subsidiary that were not sold or disposed of in an amount determined as provided in the penultimate paragraph of the covenant described above under the caption “—Certain Covenants—Restricted Payments.” The acquisition by the Issuer or any Restricted Subsidiary of the Issuer of a Person that holds an Investment in a third Person will not be deemed to be an Investment by the Issuer or such Restricted Subsidiary in such third Person, unless such third Person’s Investment was made in contemplation of the acquisition by the Issuer or a Restricted Subsidiary, in which case it shall be an Investment in an amount equal to the Fair Market Value of the Investments held by the acquired Person in such third Person in an amount determined as provided in the penultimate paragraph of the covenant described above under the caption “—Certain Covenants—Restricted Payments.” The outstanding amount of any Investment shall be the original cost thereof, reduced by all returns on such Investment (including dividends, interest, distributions, returns of principal and profits on sale).
“Issue Date” means the date the Notes are first issued under the Indenture. “Issuer” means American Renal Holdings Inc., a Delaware corporation.
“Lien” means, with respect to any asset, (a) any mortgage, deed of trust, lien, pledge, hypothecation, encumbrance, charge or security interest in, on or of such asset and (b) the interest of a vendor or a lessor under any conditional sale agreement, capital lease or title retention agreement (or any financing lease having substantially the same economic effect as any of the foregoing) relating to such asset.
“Make-Whole Redemption Date” has the meaning set forth under “—Optional Redemption.”
“Management Agreements” means the management, service or similar agreements pursuant to which the Issuer or any of its Qualified Restricted Subsidiaries manages the assets and businesses of any of its Restricted Subsidiaries.
“Measurement Period” means, at any date of determination, the period of four full fiscal quarters for which internal financial statements are available immediately preceding such date.
“Merger Agreement” means the Agreement and Plan of Merger by and among, inter alia, Holdings, the Issuer, Pamlico Capital I, L.P. (formerly known as Wachovia Capital Partners GP I, LLC) and selling shareholders named therein, dated as of March 22, 2010, as amended or modified from time to time prior to the Issue Date.
“Net Income” means, with respect to any specified Person, the net income (loss) attributable to such Person (which shall exclude, for the avoidance of doubt, the income (loss) attributable to minority interests), determined in accordance with GAAP and before any reduction in respect of preferred stock dividends.
“Net Proceeds” means the aggregate cash proceeds received by the Issuer or any of its Restricted Subsidiaries in respect of any Asset Sale (including, without limitation, any cash received upon the sale or other disposition of any non-cash consideration received in any Asset Sale and, in the case of an Event of Loss, insurance proceeds, condemnation awards or damages awarded by any judgment), net of the direct costs relating to such Asset Sale, including, without limitation, legal, accounting and investment banking fees, payments made in order to obtain a necessary consent or required by applicable law, and sales commissions, and any relocation
186
Table of Contents
expenses incurred as a result of the Asset Sale, taxes paid or payable as a result of the Asset Sale, including taxes resulting from the transfer of the proceeds of such Asset Sale to the Issuer, in each case, after taking into account:
(1) any available tax credits or deductions and any tax sharing arrangements;
(2) any reserve for adjustment in respect of the sale price of such asset or assets established in accordance with GAAP;
(3) any reserve for adjustment in respect of any liabilities associated with the asset disposed of in such transaction and retained by the Issuer or any Restricted Subsidiary after such sale or other disposition thereof;
(4) any distributions and other payments required to be made to minority interest holders in Subsidiaries or joint ventures as a result of such Asset Sale; and
(5) in the event that a Restricted Subsidiary consummates an Asset Sale and makes a pro rata payment of dividends to all of its stockholders from any cash proceeds of such Asset Sale, the amount of dividends paid to any stockholder other than the Issuer or any other Restricted Subsidiary,provided that any net proceeds of an Asset Sale by a Non-Guarantor Subsidiary that are subject to legal or contractual restrictions on repatriation to the Issuer will not be considered Net Proceeds for so long as such proceeds are subject to such restrictions,provided, however, that any such contractual restrictions on repatriation were not entered into in contemplation of such Asset Sale.
“Non-Cash Charges” means (a) losses on asset sales, disposals or abandonments, (b) any impairment charge or asset write-off or write-down related to intangible assets, goodwill, long-lived assets, and investments in debt and equity securities pursuant to GAAP, (c) all losses from investments recorded using the equity method, (d) stock-based awards compensation expense, and (e) other non-cash charges (provided that if any non-cash charges, expenses and write-downs referred to in this clause (e) represent an accrual or reserve for potential cash items in any future period, the cash payment in respect thereof in such future period shall be subtracted from Consolidated EBITDA to such extent, and excluding amortization of a prepaid cash item that was paid in a prior period).
“Non-Guarantor Subsidiary” means any Subsidiary of the Issuer that is not a Subsidiary Guarantor.
“Non-Recourse Debt” means Indebtedness:
(1) as to which neither the Issuer nor any of its Restricted Subsidiaries (a) provides credit support of any kind (including any undertaking, agreement or instrument that would constitute Indebtedness), (b) is directly or indirectly liable as a guarantor or (c) otherwise constitutes the lender;
(2) no default with respect to which (including any rights that the holders of the Indebtedness may have to take enforcement action against an Unrestricted Subsidiary) would permit upon notice, lapse of time or both any holder of any other Indebtedness of the Issuer or any of its Restricted Subsidiaries to declare a default on such other Indebtedness or cause the payment of such other Indebtedness to be accelerated or payable prior to its Stated Maturity; and
(3) as to which the lenders have been notified in writing or have agreed in writing (in the agreement relating thereto or otherwise) that they will not have any recourse to the stock or assets of the Issuer or any of its Restricted Subsidiaries.
“Notes Obligations” means Obligations in respect of the Notes, the Guarantees and the Indenture.
“Obligations” means any principal, interest, penalties, fees, indemnifications, reimbursements, damages and other liabilities payable under the documentation governing any Indebtedness.
“Officer” means any of the following of the Issuer: the Chairman of the Board of Directors, the Chief Executive Officer, the Chief Financial Officer, the President, any Vice President, the Treasurer or the Secretary.
187
Table of Contents
“Officers’ Certificate” means a certificate signed by two Officers.
“Opinion of Counsel” means a written opinion from legal counsel who is acceptable to the Trustee. The counsel may be an employee of or counsel to the Issuer or the Trustee.
“Pari Passu Lien Indebtedness” means Obligations with respect to Indebtedness permitted to be incurred under the covenant described under “—Incurrence of Indebtedness and Issuance of Disqualified Stock and Preferred Stock” and “—Liens” and under the Credit Agreement which is by its terms intended to be secured on a pari passu basis with the Liens securing the Notes; provided such Lien is permitted to be incurred under the Indenture and the Credit Agreement and such Indebtedness has a stated maturity that is no earlier than the stated maturity of the Notes.
“Permitted Debt” has the meaning given to such term in the second paragraph of the covenant under “—Certain Covenants—Incurrence of Indebtedness and Issuance of Disqualified Stock and Preferred Stock.”
“Permitted Business” means (i) any business engaged in by the Issuer or any of its Restricted Subsidiaries on the Issue Date, and (ii) any business or other activities that are reasonably similar, ancillary, complementary or related to, or a reasonable extension, development or expansion of, the businesses in which the Issuer and its Restricted Subsidiaries are engaged on the Issue Date.
“Permitted Collateral Liens” means (a) in the case of Collateral other than mortgaged real property and any pledged securities, Permitted Liens, (b) in the case of mortgaged real property, “Permitted Collateral Liens” means the Liens described in clauses (1), (7), (8), (9), (10), (16), (17), (25) and (28) of the definition of “Permitted Liens” and (c) in the case of Collateral consisting of pledged securities, means the Liens described in clauses (10), (25) and (28) of the definition of “Permitted Liens.”
“Permitted Holder” means the Sponsor and its Affiliates (other than portfolio companies or holding companies of other portfolio companies).
“Permitted Investments” means:
(1)(a) any Investment in the Issuer, in a Subsidiary Guarantor or in a Qualified Restricted Subsidiary of the Issuer and (b) any loans or advances to any Restricted Subsidiary that meets the requirements of clauses (1), (2) and (3) of the definition of Qualified Restricted Subsidiary;
(2) any Investment in Cash Equivalents;
(3) any Investment by the Issuer or any Restricted Subsidiary of the Issuer in a Person (other than the Issuer, a Subsidiary Guarantor or a Qualified Restricted Subsidiary of the Issuer) that is engaged as its primary business in a Permitted Business, if as a result of such Investment:
(a) such Person becomes a Qualified Restricted Subsidiary of the Issuer; or
(b) such Person, in one transaction or a series of transactions, is merged, consolidated or amalgamated with or into, or transfers or conveys substantially all of its assets to, or is liquidated into, the Issuer, or a Qualified Restricted Subsidiary of the Issuer;
(4) any Investment received in connection with a disposition of assets not constituting an Asset
Sale;
(5) any Investment solely in exchange for the issuance of Equity Interests (other than Disqualified Stock) of the Issuer or any parent of the Issuer;
(6) any Investments received in compromise, settlement or resolution of (A) obligations of trade debtors or customers that were incurred in the ordinary course of business of the Issuer or any of its
188
Table of Contents
Restricted Subsidiaries, including pursuant to any plan of reorganization or similar arrangement upon the bankruptcy or insolvency of any trade debtor or customer, (B) litigation, arbitration or other disputes with Persons who are not Affiliates or (C) as a result of a foreclosure by the Issuer or any Restricted Subsidiary with respect to any secured Investment or other transfer of title with respect to any secured Investment in default;
(7) Investments represented by Hedging Obligations entered into to protect against fluctuations in interest rates, exchange rates and commodity prices;
(8) any Investment in payroll, travel and similar advances to cover business-related travel expenses, moving expenses or other similar expenses, in each case incurred in the ordinary course of business;
(9) Investments in receivables owing to the Issuer or any Restricted Subsidiary if created or acquired in the ordinary course of business and payable or dischargeable in accordance with customary trade terms;provided, however, that such trade terms may include such concessionary trade terms as the Issuer or any such Restricted Subsidiary deems reasonable under the circumstances;
(10) Investments in prepaid expenses, negotiable instruments held for collection and lease, utility and workers compensation, performance and similar deposits entered into as a result of the operations of the business in the ordinary course of business;
(11) obligations of one or more officers or other employees of the Issuer or any of its Restricted Subsidiaries in connection with such officer’s or employee’s acquisition of shares of Capital Stock of the Issuer or Capital Stock of Holdings (or any other direct or indirect parent company of the Issuer) so long as no cash or other assets are paid by the Issuer or any of its Restricted Subsidiaries to such officers or employees in connection with the acquisition of any such obligations;
(12) loans or advances to and guarantees provided for the benefit of employees made in the ordinary course of business of the Issuer or the Restricted Subsidiary of the Issuer in an aggregate principal amount not to exceed $2.0 million at any one time outstanding;
(13) Investments existing as of the Issue Date or an Investment consisting of any extension, modification or renewal of any Investment existing as of the Issue Date (excluding any such extension, modification or renewal involving additional advances, contributions or other investments of cash or property or other increases thereof unless it is a result of the accrual or accretion of interest or original issue discount or payment-in-kind pursuant to the terms, as of the Issue Date, of the original Investment so extended, modified or renewed) and pursuant to any binding commitment outstanding as of the Issue Date;
(14) repurchases of the Notes;
(15) the acquisition by a Receivables Subsidiary in connection with a Qualified Receivables Transaction of Equity Interests of a trust or other Person established by such Receivables Subsidiary to effect such Qualified Receivables Transaction; and any other Investment by the Issuer or a Subsidiary of the Issuer in a Receivables Subsidiary or any Investment by a Receivables Subsidiary in any other Person in connection with a Qualified Receivables Transaction customary for such transactions;
(16) Investments not otherwise permitted by the foregoing clauses in an amount, taken together with all other Investments made pursuant to this clause, not to exceed, in the aggregate at any time outstanding, the greater of (i) $20.0 million and (ii) 3.75% of Total Assets at the time of any Investment pursuant to this clause;
(17) Investments consisting of amounts potentially due from a seller of property in an acquisition that (i) relate to customary post-closing adjustments with respect to accounts receivable, accounts payable and similar items typically subject to post-closing adjustments in similar transactions and (ii) are outstanding for a period of one hundred twenty (120) days or less following the closing of such acquisition;
(18) good faith deposits in connection with any acquisition, joint venture or acquisition of assets and escrowed money in connection with Asset Sales, acquisitions or joint ventures;
189
Table of Contents
(19) Investments of a Restricted Subsidiary of the Issuer acquired after the Issue Date or of a Person merged into, amalgamated with or consolidated with a Restricted Subsidiary of the Issuer in a transaction that is not prohibited by the covenant described under “—Certain Covenants—Merger, Consolidation or Sale of Assets” after the Issue Date to the extent that such Investments were not made in contemplation of such acquisition, merger, amalgamation or consolidation and were in existence on the date of such merger, acquisition, amalgamation or consolidation;
(20) any investment in a Receivables Subsidiary or any Investment by a Receivables Subsidiary in any other Person in connection with a Qualified Receivables Transaction, including, without limitation, Investments of funds held in accounts permitted or required by the arrangements governing such Qualified Receivables Transaction or any related indebtedness; and
(21) guarantees of obligations (other than Indebtedness) incurred by Qualified Restricted Subsidiaries in the ordinary course of business and not otherwise prohibited by the Indenture;
provided that any Investment in a Restricted Subsidiary that is not a Subsidiary Guarantor which is made in the form of loans or advances shall be secured by substantially all assets of such Restricted Subsidiary and evidenced by an intercompany note that is pledged as part of the Collateral.
“Permitted Liens” means:
(1) Liens in favor of the Issuer or any Subsidiary Guarantor;
(2) Liens on property or assets of a Person, existing at the time such Person is merged with or into, consolidated with or acquired by the Issuer or any Restricted Subsidiary of the Issuer;provided that
such Liens were in existence prior to, and were not incurred in contemplation of, such merger, consolidation or acquisition and do not extend to any assets other than those of the Person merged into, consolidated with or acquired by the Issuer or such Subsidiary, plus renewals and extensions of such Liens on the same assets;
(3) Liens on property (including Capital Stock) existing at the time of acquisition of the property by the Issuer or any Restricted Subsidiary of the Issuer;provided that such Liens were in existence prior to such acquisition, and were not incurred in contemplation of, such acquisition, plus renewals and extensions of such Liens on the same assets;
(4) Liens (including deposits and pledges) to secure the performance of public or statutory obligations, progress payments, surety or appeal bonds, performance bonds or other obligations of a like nature incurred in the ordinary course of business;
(5) Liens to secure Purchase Money Indebtedness permitted by clause (18) of the definition of “Permitted Debt” covering only the assets acquired, constructed or improved with or financed by such Indebtedness;
(6) Liens existing on the Issue Date (other than Liens in favor of the lenders under the Credit Agreement), plus renewals and extensions of such Liens on the same assets;
(7) Liens for taxes, assessments or governmental charges or claims that are not yet delinquent or that are being contested in good faith by appropriate proceedings promptly instituted and diligently concluded;provided that any reserve or other appropriate provision as is required in conformity with GAAP has been made therefor;
(8) Liens imposed by law, such as carriers’, warehousemen’s, landlord’s, materialmen’s, laborers’, employees’, suppliers’ and mechanics’ Liens, in each case, incurred in the ordinary course of business;
(9) survey exceptions, title defects, encumbrances, easements or reservations of, or rights of others for, licenses, rights-of-way, sewers, electric lines, telegraph and telephone lines and other similar purposes, or zoning or other restrictions as to the use of real property that do not materially interfere with the ordinary conduct of the business of the Issuer and its Subsidiaries, taken as a whole;
190
Table of Contents
(10) Liens securing the Notes Obligations relating to Notes (and the Guarantees) issued on the Issue Date and the Exchange Notes (and the Guarantees) issued pursuant to an Exchange Offer;
(11) Liens to secure any Permitted Refinancing Indebtedness permitted to be incurred under the Indenture;provided, however, that the new Lien shall be limited to all or part of the same property and assets that secured or, under the written agreements pursuant to which the original Lien arose, could secure the original Indebtedness (plus improvements and accessions to, such property or proceeds or distributions thereof);
(12) Liens securing Indebtedness that does not exceed $2.5 million at any one time outstanding, and Obligations in respect thereof;
(13) Liens on assets of any Qualified Restricted Subsidiary securing Indebtedness of such Qualified Restricted Subsidiary incurred under clause (15) or (16) of the definition of “Permitted Debt,” and Obligations in respect thereof;
(14) security for the payment of workers’ compensation, unemployment insurance, other social security benefits or other insurance-related obligations (including, but not limited to, in respect of deductibles, self-insured retention amounts and premiums and adjustments thereto) entered into in the ordinary course of business;
(15) deposits or pledges in connection with bids, tenders, leases and contracts (other than contracts for the payment of money) entered into in the ordinary course of business;
(16) zoning restrictions, easements, licenses, reservations, provisions, encroachments, encumbrances, protrusion permits, servitudes, covenants, conditions, waivers, restrictions on the use of property or minor irregularities of title (and with respect to leasehold interests, mortgages, obligations, liens and other encumbrances incurred, created, assumed or permitted to exist and arising by, through or under a landlord or owner of the leased property, with or without consent of the lessee), in each case, not materially interfering with the ordinary conduct of the business of the Issuer and its Subsidiaries, taken as a whole;
(17) leases, subleases, licenses or sublicenses to third parties entered into in the ordinary course of business;
(18) Liens securing Hedging Obligations entered into to protect against fluctuations in interest rates, exchange rates and commodity prices permitted under the Indenture;provided that the holders of such Obligations (or their representative) are party to, such Liens are subject to, the Intercreditor Agreement;
(19) Liens arising out of judgments, decrees, orders or awards (to the extent not constituting an Event of Default) in respect of which the Issuer shall in good faith be prosecuting an appeal or proceedings for review which appeal or proceedings shall not have been finally terminated, or if the period within which such appeal or proceedings may be initiated shall not have expired;
(20) Liens on Capital Stock of an Unrestricted Subsidiary that secure Indebtedness or other obligation of such Unrestricted Subsidiary;
(21) Liens securing Treasury Management Obligations;provided that the holders of such Obligations (or their representative) are party to, and such Liens are subject to, the Intercreditor Agreement;
(22) any Liens arising from the precautionary filing of Uniform Commercial Code financing statements regarding leases;
(23) Liens (i) of a collection bank arising under Section 4-210 of the Uniform Commercial Code on items in the course of collection and (ii) in favor of banking institution encumbering deposits (including the right of set-off) and which are within the general parameters customary in the banking industry;
(24) Liens encumbering reasonable customary initial deposits and margin deposits and similar Liens attaching to brokerage accounts incurred in the ordinary course of business and not for speculative purposes;
191
Table of Contents
(25) Liens on the Collateral securing Indebtedness (including Liens securing any Obligations in respect thereof) permitted to be incurred pursuant to clause (1) of the definition of “Permitted Debt”;provided that the holders of such Indebtedness (or their representative) are party to, and such Liens are subject to, the Intercreditor Agreement;
(26) Liens created or deemed to exist by the establishment of trusts for the purpose of satisfying government reimbursement program costs and other actions or claims pertaining to the same or related matters or other medical reimbursement programs;
(27) Liens solely on any cash earned money deposits made by the Issuer or any Restricted Subsidiary with any letter of intent or purchase agreement permitted hereunder;
(28) Liens securing Indebtedness (including Liens securing any Obligations in respect thereof) permitted to be incurred pursuant to the “Incurrence of Indebtedness and Issuance of Disqualified Stock and Preferred Stock” covenant if the Consolidated Adjusted Secured Debt Ratio of the Issuer and its Restricted Subsidiaries would not be greater than 4.50 to 1.00;provided that (a) an authorized representative of the holders of such Indebtedness shall have executed (i) a joinder to the Intercreditor Agreement (in the form attached thereto) as a holder of Pari Passu Lien Indebtedness or (ii) another intercreditor agreement pursuant to which such representative shall agree with the Trustee and other representatives of First Lien Obligations that the Liens securing such Indebtedness are subordinated to the Liens securing the First Lien Obligations and (b) the Issuer may elect, pursuant to an Officers’ Certificate delivered to the Trustee, to treat all or any portion of the commitment under any Indebtedness which is to be secured by a Lien permitted by this clause (28) as being Incurred at the time the Lien is Incurred and any subsequent Incurrence of Indebtedness under such commitment shall not be deemed to be an Incurrence at such subsequent time; and
(29) Liens incurred in connection with a Qualified Receivables Transaction (which, in the case of the Issuer and its Restricted Subsidiaries (other than Receivables Subsidiaries), shall be limited to receivables and related assets referred to in the definition of “Qualified Receivables Transaction”).
“Permitted Payment Restriction” means, with respect to any Subsidiary, any restriction that (i) becomes effective only upon the occurrence of (x) specified events under its charter or (y) a default by such Subsidiary in the payment of principal of or interest, a bankruptcy default, a default on any financial covenant or any other material event of default, in each case on Indebtedness that was incurred by such Subsidiary in compliance with the covenant under “—Incurrence of Indebtedness and Issuance of Disqualified Stock and Preferred Stock” and (ii) does not materially impair the Issuer’s ability to make scheduled payments of cash interest and fees and to make required principal payments on the Notes, as determined in good faith by the Board of Directors.
“Permitted Payments to Parent” means
(1) payments, directly or indirectly, to Holdings or any other direct or indirect parent company of the Issuer (including C.P. Atlas Holdings, Inc.) to be used by Holdings (or any other direct or indirect parent company of the Issuer) to pay (x) consolidated, combined or similar Federal, state and local taxes payable by Holdings (or such parent company) and directly attributable to (or arising as a result of) the operations of the Issuer and its Subsidiaries and (y) franchise or similar taxes and fees of Holdings (or such parent company) required to maintain Holdings’ (or such parent company’s) corporate or other existence;provided that:
(a) the amount of such dividends, distributions or advances paid shall not exceed the amount (x) that would be due with respect to a consolidated, combined or similar federal, state or local tax return for the Issuer and its Subsidiaries if the Issuer were the parent of such group for federal, state and local tax purposes plus (y) the actual amount of such franchise or similar taxes and fees of Holdings (or such parent company) required to maintain Holdings’ (or such parent company’s) corporate or other existence, each as applicable; and
(b) such payments are used by Holdings (or such parent company) for such purposes within 90 days of the receipt of such payments;
192
Table of Contents
(2) payments, directly or indirectly, to Holdings or any other direct or indirect parent company of the Issuer if the proceeds thereof are used to pay general corporate and overhead expenses (including salaries and other compensation of employees) incurred in the ordinary course of its business or of the business of Holdings or such other parent company of the Issuer as a direct or indirect holding company for the Issuer or used to pay fees and expenses (other than to Affiliates) relating to any unsuccessful debt or equity financing, in each case, only to the extent directly attributable to the operations of Holdings, the Issuer and its Restricted Subsidiaries; and
(3) so long as no Default exists at the time of such payment or would result therefrom, payments, directly or indirectly, to Holdings or any other direct or indirect parent company of the Issuer if the proceeds thereof are used to pay amounts payable to the Permitted Holders to the extent permitted by clause (13) of “—Transactions with Affiliates,” solely to the extent such amounts are not paid directly by the Issuer or any of its Subsidiaries;provided that any accelerated payment of periodic management fees under the Sponsor Management Agreement (other than upon termination thereof upon an initial public offering of common stock, or change of control, of the Issuer or any direct or indirect parent company of the Issuer) shall constitute a Restricted Payment (whether or not such payment is made by the Issuer directly or through a dividend or distribution to Holdings) not permitted by this clause (3) and shall be permitted only if the Issuer would be permitted to make a Restricted Payment under another exception under the “Restricted Payments” covenant.
“Permitted Refinancing Indebtedness” means any Indebtedness of the Issuer or any of its Restricted Subsidiaries issued in exchange for, or the net proceeds of which are used to extend, renew, refund, refinance, replace, defease or discharge other Indebtedness of the Issuer or any of its Restricted Subsidiaries;provided that:
(1) the principal amount (or accreted value, if applicable) of such Permitted Refinancing Indebtedness does not exceed the principal amount (or accreted value, if applicable) of the Indebtedness extended, renewed, refunded, refinanced, replaced, defeased or discharged (plus all accrued interest on the Indebtedness and the amount of all fees, commissions, discounts and expenses, including premiums, incurred in connection therewith);
(2) either (a) such Permitted Refinancing Indebtedness has a final maturity date equal to or later than the final maturity date of, and has a Weighted Average Life to Maturity equal to or greater than the Weighted Average Life to Maturity of, the Indebtedness being extended, renewed, refunded, refinanced, replaced, defeased or discharged or (b) all scheduled payments on or in respect of such Permitted Refinancing Indebtedness (other than interest payments) shall be at least 91 days following the final scheduled maturity of the Notes;
(3) if the Indebtedness being extended, renewed, refunded, refinanced, replaced, defeased or discharged is subordinated in right of payment to the Notes, such Permitted Refinancing Indebtedness is subordinated in right of payment to the Notes on terms at least as favorable to the holders of Notes as those contained in the documentation governing the Indebtedness being extended, renewed, refunded, refinanced, replaced, defeased or discharged;
(4) such Indebtedness is incurred
(a) by the Issuer or by the Restricted Subsidiary who is the obligor on the Indebtedness being renewed, refunded, refinanced, replaced, defeased or discharged;
(b) by the Issuer or any Subsidiary Guarantor if the obligor on the Indebtedness being renewed, refunded, refinanced, replaced, defeased or discharged is the Issuer or a Subsidiary Guarantor; or
(c) by any Non-Guarantor Subsidiary if the obligor on the Indebtedness being renewed, refunded, refinanced, replaced, defeased or discharged is a Non-Guarantor Subsidiary; and
(5) such Indebtedness is only secured if and to the extent and with the priority the Indebtedness being extended, renewed, refunded, refinanced, replaced, defeased or discharged is secured.
193
Table of Contents
“Person” means any natural person, corporation, limited liability company, trust, joint venture, association, company, partnership, governmental authority or other entity.
“Priority Payment Lien Obligations” means (i) Obligations secured by Liens permitted by clause (25) of the definition of “Permitted Liens” and (ii) to the extent secured equally and ratably with the Obligations referred to in the foregoing clause (i), Obligations secured by Liens permitted by clause (18) or clause (21) of the definition of “Permitted Liens.”
“Pro Forma Basis” means, with respect to any calculation for any Measurement Period:
(1) Investments, acquisitions, mergers, consolidations and dispositions that have been made by the specified Person or any of its Restricted Subsidiaries, or any Person or any of its Restricted Subsidiaries acquired by, merged or consolidated with the specified Person or any of its Restricted Subsidiaries, and including any related financing transactions and including increases in ownership of Restricted Subsidiaries, during such Measurement Period or subsequent to the Measurement Period and on or prior to the date for which the calculation is being made (the “Calculation Date”) will be given pro forma effect, including giving effect to Pro Forma Cost Savings, as if they had occurred on the first day of the Measurement Period;
(2) for purposes of the Fixed Charge Coverage Ratio, the Fixed Charges attributable to discontinued operations, as determined in accordance with GAAP, and operations or businesses (and ownership interests therein) disposed of prior to the Calculation Date, will be excluded, but only to the extent that the obligations giving rise to such Fixed Charges will not be obligations of the specified Person or any of its Restricted Subsidiaries following the Calculation Date;
(3) any Person that is a Restricted Subsidiary on the Calculation Date will be deemed to have been a Restricted Subsidiary at all times during such Measurement Period;
(4) any Person that is not a Restricted Subsidiary on the Calculation Date will be deemed not to have been a Restricted Subsidiary at any time during such Measurement Period; and
(5) if any Indebtedness bears a floating rate of interest, the interest expense on such Indebtedness will be calculated as if the rate in effect on the Calculation Date had been the applicable rate for the entire Measurement Period (taking into account any Hedging Obligation applicable to such Indebtedness).
The calculations above shall be made in good faith by a responsible financial or accounting officer of the Issuer and shall take into account any Hedging Obligations of the Issuer and its Restricted Subsidiaries. Interest on a Capital Lease Obligation shall be deemed to accrue at an interest rate reasonably determined by a responsible financial or accounting officer of the Issuer to be the rate of interest implicit in such Capital Lease Obligation in accordance with GAAP. For purposes of making the computation referred to above, interest on any Indebtedness under a revolving credit facility computed on a pro forma basis shall be computed based upon the average daily balance of such Indebtedness during the applicable period. Interest on Indebtedness that may optionally be determined at an interest rate based upon a factor of a prime or similar rate, a eurocurrency interbank offered rate, or other rate, shall be deemed to have been based upon the rate actually chosen, or, if none, then based upon such optional rate chosen as the Issuer may designate.
“Pro Forma Cost Savings” means, with respect to any period, the reduction in net costs and related adjustments that were directly attributable to an acquisition, merger, consolidation or disposition that (i) occurred during the four-quarter reference period or subsequent to the four-quarter reference period and on or prior to the date of determination and calculated on a basis that is consistent with Regulation S-X under the Securities Act as in effect and applied as of the date of the Indenture, (ii) were actually implemented within 12 months after the date of the acquisition, merger, consolidation or disposition and prior to the date of determination that are supportable and quantifiable by the underlying accounting records of such business or (iii) the Issuer reasonably determines are probable based upon specifically identifiable actions to be taken within 12 months of the date of the acquisition, merger, consolidation or disposition and, in the case of each of (i), (ii) and (iii), are described, as
194
Table of Contents
provided below, in an Officers’ Certificate, as if all such reductions in costs had been effected as of the beginning of such period. Pro Forma Cost Savings described above shall be accompanied by an Officers’ Certificate delivered to the Trustee from the chief financial officer of the Issuer that outlines the actions taken or to be taken, the net cost savings achieved or to be achieved from such actions and that, in the case of clause (iii) above, such savings have been determined to be probable.
“Purchase Money Indebtedness” means Indebtedness, including Capital Lease Obligations, Disqualified Stock or preferred stock of the Issuer or any Restricted Subsidiary incurred for the purpose of financing all or any part of the purchase price of assets used in the business of the Issuer or any Restricted Subsidiary or the cost of installation, construction or improvement thereof;provided, however, that (1) the amount of such Indebtedness shall not exceed such purchase price or cost and (2) such Indebtedness shall be incurred within 90 days after such acquisition of such asset by the Issuer or such Restricted Subsidiary or such installation, construction or improvement.
“Qualified Capital Stock” means any Capital Stock that is not Disqualified Stock. “Qualified Proceeds” means any of the following or any combination of the following:
(1) cash or Cash Equivalents;
(2) the Fair Market Value of assets that are used or useful in the Permitted Business; and
(3) the Fair Market Value of the Capital Stock of any Person engaged primarily in a Permitted Business if, in connection with the receipt by the Issuer or any of its Restricted Subsidiaries of such Capital Stock, such Person becomes a Subsidiary Guarantor or a Qualified Restricted Subsidiary or such Person is merged or consolidated into the Issuer or a Subsidiary Guarantor or a Qualified Restricted Subsidiary;
provided that (i) for purposes of clause (3) of the first paragraph under “—Certain Covenants—Restricted Payments,” Qualified Proceeds shall not include Excluded Contributions and (ii) the amount of Qualified Proceeds shall be reduced by the amount of payments made in respect of the applicable transaction which are permitted under clause (13)(i) of the covenant described under “—Certain Covenants—Transactions with Affiliates.”
“Qualified Receivables Transaction” means any transaction or series of transactions entered into by the Issuer or any of its Subsidiaries pursuant to which the Issuer or any of its Subsidiaries sells, conveys or otherwise transfers, or grants a security interest, to:
(1) a Receivables Subsidiary (in the case of a transfer by the Issuer or any of its Subsidiaries, which transfer may be effected through the Issuer or one or more of its Subsidiaries); and
(2) if applicable, any other Person (in the case of a transfer by a Receivables Subsidiary),
in each case, in any accounts receivable (including health care insurance receivables), instruments, chattel paper, general intangibles and similar assets (whether now existing or arising in the future, the “Receivables”) of the Issuer or any of its Subsidiaries, and any assets related thereto, including, without limitation, all collateral securing such Receivables, all contracts, contract rights and all guarantees or other obligations in respect of such Receivables, proceeds of such Receivables and any other assets, which are customarily transferred or in respect of which security interests are customarily granted in connection with receivables financings and asset securitization transactions of such type, together with any related transactions customarily entered into in receivables financings and asset securitizations, including servicing arrangements. All determinations under the Indenture as to whether a particular provision in respect of a receivables transaction is customary shall be made by the Issuer in good faith (which determination shall be conclusive).
“Qualified Restricted Subsidiary” means a majority-owned Restricted Subsidiary or a Wholly Owned Restricted Subsidiary that satisfies each of the following requirements: (1) except for Permitted Payment
195
Table of Contents
Restrictions, there are no consensual encumbrances or restrictions, directly or indirectly, on the ability of such Restricted Subsidiary to (a) pay dividends or make any other distributions on its equity interest to the Issuer or a Restricted Subsidiary or pay any Indebtedness owed to the Issuer or a Restricted Subsidiary or (b) make any loans or advances to the Issuer or a Restricted Subsidiary; (2) the Equity Interests of such Restricted Subsidiary are owned by the Issuer and/or one or more of its Qualified Restricted Subsidiaries (without giving effect to the proviso in this definition) and, if it is not a Wholly Owned Restricted Subsidiary, one or more of (A) Strategic Investors, (B) directors of such Restricted Subsidiary (only to the extent holding directors’ qualifying shares) and (C) any other Person to the extent ownership by such other Person is required as a result of changes in law occurring after the Issue Date; and (3) the primary business of such Restricted Subsidiary is a Permitted Business; provided that, so long as the laws or regulations of the State of New York require that membership interests in limited liability companies that own dialysis clinics in the State of New York be owned by individuals, a Restricted Subsidiary that operates one or more clinics located only in the State of New York shall be deemed a Qualified Restricted Subsidiary if (i) the requirements of clause (1) and (3) of this definition are satisfied, (ii) a majority of its Equity Interests are owned by an officer of the Issuer who is party to a written contract with the Issuer or a Subsidiary Guarantor pursuant to which the Issuer or such Subsidiary Guarantor shall have the right to repurchase all of such Equity Interests owned by such officer for a nominal amount, (iii) the Issuer or a Subsidiary Guarantor receives dividends and distributions from such Restricted Subsidiary as if it owned all of the Equity Interests owned by such officer and (iv) such officer pledges such Equity Interests as part of the Collateral to the extent such Equity Interests would have been pledged if they were owned by the Issuer or a Guarantor.
“Receivables Fees” means distributions or payments made directly or by means of discounts with respect to any participation interest issued or sold in connection with, and other fees paid to a Person that is not a Restricted Subsidiary in connection with, any Qualified Receivables Transaction.
“Receivables Repurchase Obligation” means any obligation of a seller of receivables in a Qualified Receivables Transaction to repurchase receivables arising as a result of a breach of a representation, warranty or covenant or otherwise, including as a result of a receivable or portion thereof becoming subject to any asserted defense, dispute, off-set or counterclaim of any kind as a result of any action taken by, any failure to take action by or any other event relating to the seller.
“Receivables Subsidiary” means a Subsidiary of the Issuer which engages in no activities other than in connection with the financing of accounts receivable and in businesses related or ancillary thereto and that is designated by the Board of Directors of the Issuer (as provided below) as a Receivables Subsidiary (A) no portion of the Indebtedness or any other Obligations (contingent or otherwise) of which:
(1) is guaranteed by the Issuer or any Subsidiary of the Issuer (excluding guarantees of Obligations (other than the principal of, and interest on, Indebtedness) pursuant to Standard Securitization Undertakings);
(2) is recourse to or obligates the Issuer or any Subsidiary of the Issuer in any way other than pursuant to Standard Securitization Undertakings; or
(3) subjects any property or asset of the Issuer or any Subsidiary of the Issuer (other than accounts receivable and related assets as provided in the definition of Qualified Receivables Transaction), directly or indirectly, contingently or otherwise, to the satisfaction thereof, other than pursuant to Standard Securitization Undertakings; and
(B) with which neither the Issuer nor any Subsidiary of the Issuer has any material contract, agreement, arrangement or understanding other than on terms no less favorable to the Issuer or such Subsidiary than those that might be obtained at the time from Persons who are not Affiliates of the Issuer, other than as may be customary in a Qualified Receivables Transaction including for fees payable in the ordinary course of business in connection with servicing accounts receivable; and (C) with which neither the Issuer nor any Subsidiary of the Issuer has any obligation to maintain or preserve such Subsidiary’s financial condition or cause such Subsidiary
196
Table of Contents
to achieve certain levels of operating results other than pursuant to representations, warranties, covenants and indemnities entered into in connection with a Qualified Receivables Transaction. Any such designation by the Board of Directors of the Issuer will be evidenced to the Trustee by filing with the Trustee a certified copy of the resolution of the Board of Directors of the Issuer giving effect to such designation and an Officers’ Certificate certifying that such designation complied with the foregoing conditions.
“Registration Rights Agreement” means (i) the Registration Rights Agreement dated the Issue Date by and among the Issuer, the Guarantors, Banc of America Securities LLC, Barclays Capital and Wells Fargo Securities, LLC, as amended, supplemented or otherwise modified from time to time and (ii) any other registration rights agreement entered into in connection with an issuance of Additional Notes in a private offering after the Issue Date.
“Replacement Preferred Stock” means any Disqualified Stock of the Issuer or any of its Restricted Subsidiaries issued in exchange for, or the net proceeds of which are used to redeem, refund, refinance, replace or discharge any Disqualified Stock of the Issuer or any of its Restricted Subsidiaries (other than intercompany Disqualified Stock); provided that such Replacement Preferred Stock (i) is issued by the Issuer or by the Restricted Subsidiary who is the Issuer of the Disqualified Stock being redeemed, refunded, refinanced, replaced or discharged, (ii) does not have an initial liquidation preference in excess of the liquidation preference plus accrued and unpaid dividends on the Disqualified Stock being redeemed, refunded, refinanced, replaced or discharged and (iii) does not require redemption, repurchase or discharge at any time prior to the date on which the Disqualified Stock being redeemed, refunded, refinanced, replaced or discharged is required to be redeemed, repurchased or discharged.
“Restricted Investment” means an Investment other than a Permitted Investment.
“Restricted Subsidiary” of a Person means any Subsidiary of the referent Person that is not an Unrestricted Subsidiary. Unless otherwise specified, references to Restricted Subsidiaries shall refer to Restricted Subsidiaries of the Issuer.
“Secured Party” means (i) the Holders, (ii) the Trustee, (iii) the Collateral Agent and (iv) any successors, indorsees, transferees and assigns of each of the foregoing.
“Security Agreement” means the security agreement by and between the Issuer and the Collateral Agent, dated as of the Issue Date, as amended, supplemented or otherwise modified from time to time.
“Security Documents” means the Security Agreement, each mortgage and any other instruments and documents executed and delivered pursuant to the Indenture or any of the foregoing, as the same may be amended, supplemented or otherwise modified from time to time and pursuant to which Collateral is pledged, assigned or granted to or on behalf of the Collateral Agent for the benefit of the Secured Parties.
“Significant Subsidiary” means any Subsidiary that would be a “significant subsidiary” as defined in Article 1, Rule 1-02(w)(1) or (2) of Regulation S-X, promulgated pursuant to the Securities Act, as such Regulation is in effect on the Issue Date. For purposes of determining whether an Event of Default has occurred, if any group of Restricted Subsidiaries as to which a particular event has occurred and is continuing at any time would be, taken as a whole, a “Significant Subsidiary” then such event shall be deemed to have occurred with respect to a Significant Subsidiary.
“Sponsor” means Centerbridge Capital Partners, L.P. and certain affiliated entities.
“Sponsor Management Agreement” means the Management Agreement between the Issuer and the Sponsor dated as of the Issue Date.
“Standard Securitization Undertakings” means representations, warranties, covenants and indemnities entered into by the Issuer or any Subsidiary of the Issuer which the Issuer has determined in good faith to be
197
Table of Contents
customary in a Qualified Receivables Transaction including, without limitation, those relating to the servicing of the assets of a Receivables Subsidiary, it being understood that any Receivables Repurchase Obligation shall be deemed to be a Standard Securitization Undertaking.
“Stated Maturity” means, with respect to any installment of interest or principal on any series of Indebtedness, the date on which the payment of interest or principal was scheduled to be paid in the documentation governing such Indebtedness, and will not include any contingent obligations to repay, redeem or repurchase any such interest or principal prior to the date originally scheduled for the payment thereof.
“Strategic Investors” means physicians, hospitals, health systems, other healthcare providers, other healthcare companies and other similar strategic joint venture partners which joint venture partners are, directly or indirectly, actively involved in the day-to-day operations of providing dialysis-related services, or, in the case of physicians, that have retired therefrom, individuals who are former owners or employees of dialysis clinics purchased by the Issuer, any of its Restricted Subsidiaries, and consulting firms that receive common stock solely as consideration for consulting services performed.
“subsidiary” means, with respect to any Person (the “parent”) at any date, any corporation, limited liability company, partnership, association or other entity the accounts of which would be consolidated with those of the parent in the parent’s consolidated financial statements if such financial statements were prepared in accordance with GAAP as of such date.
“Subsidiary” means any subsidiary of the Issuer, which, for the avoidance of doubt, includes Non-Guarantor Subsidiaries.
“Subsidiary Guarantors” means the Restricted Subsidiaries of the Issuer that have executed and delivered a Guarantee of the Notes and their respective successors and assigns, in each case, until the Guarantee of such Person has been released in accordance with the provisions of the Indenture.
“Total Assets” means the total consolidated assets of the Issuer and its Restricted Subsidiaries as set forth on the most recent consolidated balance sheet of the Issuer and its Restricted Subsidiaries prepared in accordance with GAAP.
“Transactions” means the transactions contemplated by the Merger Agreement and the other related transactions described in this prospectus.
“Treasury Management Obligations” means obligations under any agreement governing the provision of treasury or cash management services, including deposit accounts, funds transfer, automated clearinghouse, zero balance accounts, returned check concentration, controlled disbursement, lockbox, account reconciliation and reporting and trade finance services.
“Treasury Rate” has the meaning set forth under “—Optional Redemption.”
“Unrestricted Subsidiary” means any Subsidiary of the Issuer that is designated by the Board of Directors of the Issuer as an Unrestricted Subsidiary pursuant to a resolution of the Board of Directors and any Subsidiary of an Unrestricted Subsidiary.
The Issuer may designate any Subsidiary of the Issuer (including any existing Subsidiary and any newly acquired or newly formed Subsidiary) to be an Unrestricted Subsidiary unless such Subsidiary or any of its Subsidiaries owns any Equity Interests or Indebtedness of, or owns or holds any Lien on, any property of, the Issuer or any Subsidiary of the Issuer (other than solely any Subsidiary of the Subsidiary to be so designated);provided that
(1) such designation complies with the covenants described under “Certain Covenants— Restricted Payments”;
198
Table of Contents
(2) each of (a) the Subsidiary to be so designated and (b) its Subsidiaries has not at the time of designation, and does not thereafter, incur any Indebtedness other than Non-Recourse Debt;
(3) is not party to any agreement, contract, arrangement or understanding with the Issuer or any Restricted Subsidiary of the Issuer unless the terms of any such agreement, contract, arrangement or understanding are no less favorable to the Issuer or such Restricted Subsidiary than those permitted under the covenant described above under the caption “—Certain Covenants—Transactions with Affiliates”;
(4) is a Person with respect to which neither the Issuer nor any of its Restricted Subsidiaries has any direct or indirect obligation to maintain or preserve such Person’s financial condition or to cause such Person to achieve any specified levels of operating results; and
(5) has not guaranteed or otherwise directly or indirectly provided credit support for any Indebtedness of the Issuer or any of its Restricted Subsidiaries.
“Voting Stock” of any specified Person as of any date means the Capital Stock of such Person that is at the time entitled to vote in the election of the Board of Directors of such Person.
“Weighted Average Life to Maturity” means, when applied to any Indebtedness at any date, the number of years obtained by dividing:
(1) the sum of the products obtained by multiplying (a) the amount of each then remaining installment, sinking fund, serial maturity or other required payments of principal, including payment at final maturity, in respect of the Indebtedness, by (b) the number of years (calculated to the nearest one-twelfth) that will elapse between such date and the making of such payment; by
(2) the then outstanding principal amount of such Indebtedness.
“Wholly Owned Restricted Subsidiary” of any specified Person means a Restricted Subsidiary of such Person all of the outstanding Capital Stock or other ownership interest of which (other than directors’ qualifying shares) will at that time be owned by such Person or by one or more Wholly Owned Restricted Subsidiaries of such Person.
“Wholly Owned Subsidiary” of any specified Person means a Subsidiary of such Person all of the outstanding Capital Stock or other ownership interest of which (other than directors’ qualifying shares) will at that time be owned by such Person or by one or more Wholly Owned Subsidiaries of such Person.
199
Table of Contents
MATERIAL U.S. FEDERAL INCOME TAX CONSEQUENCES OF THE EXCHANGE OFFER
The following is a summary of the material U.S. federal income tax consequences of the exchange of the outstanding notes for the exchange notes in the exchange offer. The exchange of outstanding notes for exchange notes in the exchange offer will not constitute a taxable event to holders for U.S. federal income tax purposes. Consequently, you will not recognize gain or loss upon receipt of an exchange note, the holding period of the exchange note will include the holding period of the outstanding note for which it was exchanged and the basis of the exchange note will be the same as the basis of the outstanding note immediately before the exchange.
In any event, persons considering the exchange of outstanding notes for exchange notes should consult their own tax advisors concerning the U.S. federal income tax consequences in light of their particular situations as well as any consequences arising under the laws of any other taxing jurisdiction.
200
Table of Contents
The following is a summary of certain considerations associated with the purchase of the notes and exchange notes by employee benefit plans that are subject to Title I of the Employee Retirement Income Security Act of 1974, as amended (“ERISA”), plans, individual retirement accounts and other arrangements that are subject to Section 4975 of the Internal Revenue Code of 1986, as amended (the “Code”) or provisions under any other federal, state, local, non-U.S. or other laws or regulations that are similar to such provisions of ERISA or the Code (collectively, “Similar Laws”), and entities whose underlying assets are considered to include “plan assets” of any such plan, account or arrangement (each, a “Plan”).
General fiduciary matters
ERISA and the Code impose certain duties on persons who are fiduciaries of a Plan subject to Title I of ERISA or Section 4975 of the Code (an “ERISA Plan”) and prohibit certain transactions involving the assets of an ERISA Plan and its fiduciaries or other interested parties. Under ERISA and the Code, any person who exercises any discretionary authority or control over the administration of an ERISA Plan or the management or disposition of the assets of an ERISA Plan, or who renders investment advice for a fee or other compensation to an ERISA Plan, is generally considered to be a fiduciary of the ERISA Plan.
In considering an investment in the notes and exchange notes using a portion of the assets of any Plan, a fiduciary should determine whether the investment is in accordance with the documents and instruments governing the Plan and the applicable provisions of ERISA, the Code or any Similar Law relating to a fiduciary’s duties to the Plan including, without limitation, the prudence, diversification, delegation of control and prohibited transaction provisions of ERISA, the Code and any other applicable Similar Laws.
Prohibited transaction issues
Section 406 of ERISA and Section 4975 of the Code prohibit ERISA Plans from engaging in specified transactions involving plan assets with persons or entities who are “parties in interest,” within the meaning of ERISA, or “disqualified persons,” within the meaning of Section 4975 of the Code, unless an exemption is available. A party in interest or disqualified person who engages in a non-exempt prohibited transaction may be subject to excise taxes and other penalties and liabilities under ERISA and the Code. In addition, the fiduciary of an ERISA Plan that engages in such a non-exempt prohibited transaction may be subject to penalties and liabilities under ERISA and the Code. The acquisition and/or holding of notes or exchange notes by an ERISA Plan with respect to which the issuer, an initial purchaser, or a guarantor is considered a party in interest or a disqualified person may constitute or result in a direct or indirect prohibited transaction under Section 406 of ERISA and/or Section 4975 of the Code, unless the investment is acquired and is held in accordance with an applicable statutory, class or individual prohibited transaction exemption. In this regard, the U.S. Department of Labor has issued prohibited transaction class exemptions, or “PTCEs,” that may apply to the acquisition and holding of the notes or exchange notes. These class exemptions include, without limitation, PTCE 84-14 respecting transactions determined by independent qualified professional asset managers, PTCE 90-1 respecting insurance company pooled separate accounts, PTCE 91-38 respecting bank collective investment funds, PTCE 95-60 respecting life insurance company general accounts and PTCE 96-23 respecting transactions determined by in-house asset managers. In addition, Section 408(b)(17) of ERISA and Section 4975(d)(20) of the Code provide relief from the prohibited transaction provisions of ERISA and Section 4975 of the Code for certain transactions between an ERISA Plan and a person that is a party in interest and/or a disqualified person (other than a fiduciary or an affiliate that, directly or indirectly, has or exercises) any discretionary authority or control or renders any investment advice with respect to the assets of any ERISA Plan involved in the transaction solely by reason of providing services to the Plan or by relationship to a service provider, provided that the ERISA Plan pays no more than adequate consideration in connection with the transaction. There can be no assurance that all of the conditions of any such exemptions will be satisfied.
201
Table of Contents
Because of the foregoing, the notes and the exchange notes should not be purchased or held by any person investing “plan assets” of any Plan, unless such purchase and holding (and the exchange of notes for exchange notes) will not constitute a non-exempt prohibited transaction under ERISA and the Code or a similar violation of any applicable Similar Laws.
Representation
Accordingly, by acceptance of a note or an exchange note, each purchaser and subsequent transferee will be deemed to have represented and warranted that either (i) no portion of the assets used by such purchaser or transferee to acquire or hold the notes or the exchange notes constitutes assets of any Plan or (ii) the acquisition and holding of the notes and the exchange notes (and the exchange of notes for exchange notes) by such purchaser or transferee will not constitute a non-exempt prohibited transaction under Section 406 of ERISA or Section 4975 of the Code or a similar violation under any applicable Similar Laws.
The foregoing discussion is general in nature and is not intended to be all-inclusive. Due to the complexity of these rules and the penalties that may be imposed upon persons involved in non-exempt prohibited transactions, it is particularly important that fiduciaries, or other persons considering acquiring the notes or exchange notes (and holding the notes or exchange notes) on behalf of, or with the assets of, any Plan, consult with their counsel regarding the potential applicability of ERISA, Section 4975 of the Code and any Similar Laws to such investment and whether an exemption would be applicable to the purchase and holding of the notes or exchange notes.
202
Table of Contents
Each broker–dealer that receives exchange notes for its own account pursuant to the exchange offer must acknowledge that it will deliver a prospectus in connection with any resale of the exchange notes. This prospectus, as it may be amended or supplemented from time to time, may be used by a broker–dealer in connection with resales of exchange notes received in exchange for outstanding notes where the outstanding notes were acquired as a result of market–making activities or other trading activities. In addition, all dealers effecting transactions in the exchange notes may be required to deliver a prospectus.
We will not receive any proceeds from any exchange of outstanding notes for exchange notes or from any sale of exchange notes by broker–dealers. Exchange notes received by broker–dealers for their own accounts pursuant to the exchange offer may be sold from time to time in one or more transactions in the over–the–counter market, in negotiated transactions, through the writing of options on the exchange notes or a combination of these methods of resale, at market prices prevailing at the time of resale, at prices related to the prevailing market prices or at negotiated prices. Any such resale may be made directly to purchasers or through brokers or dealers who may receive compensation in the form of commissions or concessions from any broker–dealer and/or the purchasers of any exchange notes. Any broker–dealer that resells exchange notes that were received by it for its own account pursuant to the exchange offer and any broker or dealer that participates in a distribution of the exchange notes may be deemed to be an “underwriter” within the meaning of the Securities Act and any profit of any resale of exchange notes and any commissions or concessions received by these persons may be deemed to be underwriting compensation under the Securities Act. The letter of transmittal states that by acknowledging that it will deliver and by delivering a prospectus, a broker–dealer will not be deemed to admit that it is an “underwriter” within the meaning of the Securities Act.
We have agreed to pay all expenses incident to the exchange offer other than commissions or concessions of any brokers or dealers and, except in certain circumstances, the expenses of counsel and other advisors of the holders and will indemnify the holders of outstanding notes, including any broker–dealers, against certain liabilities, including liabilities under the Securities Act.
203
Table of Contents
Simpson Thacher & Bartlett LLP will pass upon the legality of the exchange notes and the related guarantees to be offered hereby.
INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
The financial statements and schedule as of December 31, 2009 and 2008 and for each of the three years in the period ended December 31, 2009, included in this prospectus and elsewhere in the registration statement have been audited by Grant Thornton LLP, independent registered public accountants, as stated in its report appearing herein.
After the consummation of the exchange offer, we will file certain reports with the Securities Exchange Commission (“the SEC”), including annual reports on Form 10-K, quarterly reports on Form 10-Q and current reports on Form 8-K. You may read and copy any materials we file with the SEC at the SEC’s Public Reference Room at 100 F Street, N.E., Washington, D.C., 20549. Please call 1-800-SEC-0330 for further information on the operation of the Public Reference Room. Our SEC filings are also available to the public from the SEC’s web site at www.sec.gov.
204
Table of Contents
Audited Financial Statements for the years ended December 31, 2009, 2008 and 2007
| F-2 | ||||
Consolidated Balance Sheets as of December 31, 2009 and 2008 | F-3 | |||
Consolidated Statements of Income for the years ended December 31, 2009, 2008 and 2007 | F-4 | |||
Consolidated Statements of Changes in Deficit for the years ended December 31, 2009, 2008 and 2007 | F-5 | |||
Consolidated Statements of Cash Flows for the years ended December 31, 2009, 2008 and 2007 | F-8 | |||
| F-9 | ||||
| F-39 | ||||
Unaudited Financial Statements for the periods ended September 30, 2010 and 2009 | ||||
Consolidated Balance Sheets as of September 30, 2010 (unaudited) and December 31, 2009 | F-40 | |||
| F-41 | ||||
| F-42 | ||||
| F-44 | ||||
| F-45 | ||||
F-1
Table of Contents
Report of Independent Registered Public Accountants
Board of Directors
American Renal Holdings Inc.
We have audited the accompanying consolidated balance sheets of American Renal Holdings Inc. (a Delaware corporation) and subsidiaries (collectively the “Company”) as of December 31, 2009 and 2008, and the related consolidated statements of income, changes in deficit, and cash flows for each of the three years in the period ended December 31, 2009. Our audits of the basic financial statements included the financial statement schedule listed in the index on page F-1. These consolidated financial statements and financial statement schedule are the responsibility of the Company’s management. Our responsibility is to express an opinion on these financial statements and financial statement schedule based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. An audit includes consideration of internal control over financial reporting as a basis for designing audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion. An audit also includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the consolidated financial statements referred to above present fairly, in all material respects, the financial position of American Renal Holdings Inc. and subsidiaries as of December 31, 2009 and 2008, and the results of their operations and their cash flows for each of the three years in the period ended December 31, 2009 in conformity with accounting principles generally accepted in the United States of America. Also in our opinion, the related financial statement schedule, when considered in relation to the basic financial statements taken as a whole, presents fairly, in all material respects, the information set forth therein.
As discussed in Note B, the Company adopted new accounting guidance on January 1, 2009 related to the accounting for non-controlling interests on a prospective basis except for the presentation and disclosure requirements which were applied retrospectively for all periods presented.
As discussed in Note C, the Company adopted new accounting guidance on January 1, 2009 related to the accounting for business combinations.
/S/ GRANT THORNTON LLP
Boston, Massachusetts
November 4, 2010
F-2
Table of Contents
AMERICAN RENAL HOLDINGS INC. AND SUBSIDIARIES
Consolidated Balance Sheets
December 31, 2009 and 2008
(in thousands, except share and per share data)
| 2009 | 2008 | |||||||
Assets | ||||||||
Current assets: | ||||||||
Cash and cash equivalents | $ | 29,179 | $ | 29,182 | ||||
Accounts receivable, less allowance for doubtful accounts of $8,165 and $7,833 at December 31, 2009 and 2008, respectively | 45,654 | 38,979 | ||||||
Inventories | 2,294 | 1,978 | ||||||
Prepaid expenses and other current assets | 2,366 | 6,030 | ||||||
Deferred tax assets | 4,845 | 3,803 | ||||||
Total current assets | 84,338 | 79,972 | ||||||
Property and equipment, net | 59,405 | 54,819 | ||||||
Deferred financing costs, net | 1,044 | 1,878 | ||||||
Amortizable intangible assets, net | 1,418 | 1,056 | ||||||
Other long-term assets | 3,319 | 2,883 | ||||||
Goodwill | 24,198 | 15,762 | ||||||
Total assets | $ | 173,722 | $ | 156,370 | ||||
Liabilities and Deficit | ||||||||
Current liabilities: | ||||||||
Accounts payable | $ | 18,158 | $ | 17,644 | ||||
Accrued compensation and benefits | 7,170 | 7,262 | ||||||
Accrued expenses and other current liabilities | 15,646 | 11,233 | ||||||
Current portion of long-term debt | 14,309 | 11,556 | ||||||
Current portion of capital lease obligations | 334 | 630 | ||||||
Total current liabilities | 55,617 | 48,325 | ||||||
Long-term debt, less current portion | 64,261 | 76,137 | ||||||
Capital lease obligations, less current portion | 214 | 293 | ||||||
Other long-term liabilities | 7,664 | 7,570 | ||||||
Deferred tax liabilities | 4,548 | 3,613 | ||||||
Series X mandatorily redeemable preferred stock, 43,000 shares authorized; 40,500 shares issued and outstanding at December 31, 2009 and 2008 | 62,799 | 54,730 | ||||||
Commitments and contingencies (notes K and P) | ||||||||
Noncontrolling interests subject to put provisions | 38,431 | 34,881 | ||||||
Deficit: | ||||||||
Series A convertible preferred stock, which accrue dividends at 10%, $.001 par value, 7,300,000 shares authorized, issued and outstanding (liquidation value of $74,322 at December 31, 2009) | 7 | 7 | ||||||
Series B convertible preferred stock, $.001 par value, 10,700,000 shares authorized; 2,675,000 shares issued and outstanding (liquidation value of $18,524 at December 31, 2009) | 3 | 3 | ||||||
Common stock, $.0005 par value, 39,982,000 shares authorized; 1,330,250 and 1,062,500 shares issued and 1,083,350 and 873,600 shares outstanding at December 31, 2009 and 2008, respectively | 1 | 1 | ||||||
Additional paid-in capital | 23,704 | 27,395 | ||||||
Notes receivable from stockholders | (735 | ) | (713 | ) | ||||
Accumulated deficit | (97,784 | ) | (102,922 | ) | ||||
Treasury stock, at cost, 246,900 and 188,900 common shares held at December 31, 2009 and 2008, respectively | (1,065 | ) | (791 | ) | ||||
Total American Renal Holdings Inc. deficit | (75,869 | ) | (77,020 | ) | ||||
Noncontrolling interests not subject to put provisions | 16,057 | 7,841 | ||||||
Total deficit | (59,812 | ) | (69,179 | ) | ||||
Total liabilities and deficit | $ | 173,722 | $ | 156,370 | ||||
See the accompanying notes to the consolidated financial statements.
F-3
Table of Contents
AMERICAN RENAL HOLDINGS INC. AND SUBSIDIARIES
Consolidated Statements of Income
For the years ended December 31, 2009, 2008 and 2007
(in thousands)
| 2009 | 2008 | 2007 | ||||||||||
Net operating revenues | $ | 262,989 | $ | 217,777 | $ | 178,391 | ||||||
Operating expenses: | ||||||||||||
Patient care costs | 170,826 | 141,810 | 115,058 | |||||||||
General and administrative | 24,819 | 19,944 | 18,595 | |||||||||
Depreciation and amortization | 12,127 | 9,777 | 7,919 | |||||||||
Provision for uncollectible accounts | 3,216 | 4,834 | 3,258 | |||||||||
Total operating expenses | 210,988 | 176,365 | 144,830 | |||||||||
Operating income | 52,001 | 41,412 | 33,561 | |||||||||
Interest expense, net | (14,948 | ) | (13,729 | ) | (13,695 | ) | ||||||
Income before income taxes | 37,053 | 27,683 | 19,866 | |||||||||
Income tax expense | 9,524 | 6,860 | 4,409 | |||||||||
Net income | 27,529 | 20,823 | 15,457 | |||||||||
Less: Net income attributable to noncontrolling interests | (22,391 | ) | (17,179 | ) | (14,706 | ) | ||||||
Net income attributable to American Renal Holdings Inc. | $ | 5,138 | $ | 3,644 | $ | 751 | ||||||
See the accompanying notes to the consolidated financial statements.
F-4
Table of Contents
AMERICAN RENAL HOLDINGS INC. AND SUBSIDIARIES
Consolidated Statements of Changes in Deficit
For the years ended December 31, 2009, 2008 and 2007
(in thousands, except share and per share data)
| Non- controlling interests subject to put provisions | Total American Renal Holdings Inc. Deficit | Non- controlling interests not subject to put provisions | Compre- hensive Income | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Series A Convertible Preferred Stock | Series B Convertible Preferred Stock | Common Stock | Treasury Stock | Additional Paid-in Capital | Notes Receivable from Stock- holders | Accumu- lated Deficit | Accumu- lated Other Compre- hensive Loss | Total | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Shares | Amount | Shares | Amount | Shares | Amount | Shares | Cost | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Balance at December 31, 2006 | $ | 29,819 | 7,300,000 | $ | 7 | 2,675,000 | $ | 3 | 285,000 | $ | 1 | 5,000 | $ | (8 | ) | $ | 28,643 | $ | — | $ | (106,317 | ) | $ | (57 | ) | $ | (77,728 | ) | $ | 6,567 | ||||||||||||||||||||||||||||||||||
Comprehensive income: | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Interest rate swap, net of tax | — | — | — | — | — | — | — | — | — | — | — | (143 | ) | (143 | ) | $ | (143 | ) | ||||||||||||||||||||||||||||||||||||||||||||||
Net income | 4,424 | — | — | — | — | — | — | — | — | — | — | 751 | — | 751 | 10,282 | 15,457 | ||||||||||||||||||||||||||||||||||||||||||||||||
Total comprehensive income | $ | 15,314 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Redemption of Series B preferred stock | — | — | — | — | — | — | — | — | — | — | (1,000 | ) | — | (1,000 | ) | |||||||||||||||||||||||||||||||||||||||||||||||||
Stock-based compensation expense | — | — | — | — | — | — | — | — | 2,180 | — | — | — | 2,180 | |||||||||||||||||||||||||||||||||||||||||||||||||||
Distributions to noncontrolling interests | (5,253 | ) | — | — | — | — | — | — | — | — | — | — | — | — | — | (10,775 | ) | |||||||||||||||||||||||||||||||||||||||||||||||
Contributions from noncontrolling interests | 348 | — | — | — | — | — | — | — | — | — | — | — | — | — | �� | 1,228 | ||||||||||||||||||||||||||||||||||||||||||||||||
Acquisitions of noncontrolling interests | — | — | — | — | — | — | — | — | — | — | — | — | — | — | 287 | |||||||||||||||||||||||||||||||||||||||||||||||||
Changes in fair value of noncontrolling interests | 2,216 | — | — | — | — | — | — | — | — | (2,216 | ) | — | — | — | (2,216 | ) | ||||||||||||||||||||||||||||||||||||||||||||||||
Balance at December 31, 2007 | $ | 31,554 | 7,300,000 | $ | 7 | 2,675,000 | $ | 3 | 285,000 | $ | 1 | 5,000 | $ | (8 | ) | $ | 28,607 | $ | — | $ | (106,566 | ) | $ | (200 | ) | $ | (78,156 | ) | $ | 7,589 | ||||||||||||||||||||||||||||||||||
See the accompanying notes to the consolidated financial statements.
F-5
Table of Contents
AMERICAN RENAL HOLDINGS INC. AND SUBSIDIARIES
Consolidated Statements of Changes in Deficit—(Continued)
For the years ended December 31, 2009, 2008 and 2007
(in thousands, except share and per share data)
| Non- controlling interests subject to put provisions | Total American Renal Holdings Inc. Deficit | Non- controlling interests not subject to put provisions | Compre- hensive Income | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Series A Convertible Preferred Stock | Series B Convertible Preferred Stock | Common Stock | Treasury Stock | Additional Paid-in Capital | Notes Receivable from Stock- holders | Accumu- lated Deficit | Accumu- lated Other Compre- hensive Loss | Total | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Shares | Amount | Shares | Amount | Shares | Amount | Shares | Cost | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Balance at December 31, 2007 | $ | 31,554 | 7,300,000 | $ | 7 | 2,675,000 | $ | 3 | 285,000 | $ | 1 | 5,000 | $ | (8 | ) | $ | 28,607 | $ | — | $ | (106,566 | ) | $ | (200 | ) | $ | (78,156 | ) | $ | 7,589 | ||||||||||||||||||||||||||||||||||
Comprehensive income: | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Interest rate swap, net of tax | — | — | — | — | — | — | — | — | — | — | — | 200 | 200 | $ | 200 | |||||||||||||||||||||||||||||||||||||||||||||||||
Net income | 4,908 | — | — | — | — | — | — | — | — | — | — | 3,644 | — | 3,644 | 12,271 | 20,823 | ||||||||||||||||||||||||||||||||||||||||||||||||
Total comprehensive income | $ | 21,023 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Issuance of common stock | — | — | — | — | 185,000 | — | — | — | 697 | (697 | ) | — | — | — | ||||||||||||||||||||||||||||||||||||||||||||||||||
Purchase of common stock for treasury | — | — | — | — | — | — | 183,900 | (783 | ) | — | — | — | — | (783 | ) | |||||||||||||||||||||||||||||||||||||||||||||||||
Stock options exercised | — | — | — | — | 592,500 | — | — | — | 170 | — | — | — | 170 | |||||||||||||||||||||||||||||||||||||||||||||||||||
Excess tax benefits from stock awards exercised | — | — | — | — | — | — | — | — | 351 | — | — | — | 351 | |||||||||||||||||||||||||||||||||||||||||||||||||||
Interest due from note receivable | — | — | — | — | — | — | — | — | — | (16 | ) | — | — | (16 | ) | |||||||||||||||||||||||||||||||||||||||||||||||||
Stock-based compensation expense | — | — | — | — | — | — | — | — | 1,482 | — | — | — | 1,482 | |||||||||||||||||||||||||||||||||||||||||||||||||||
Distributions to noncontrolling interests | (5,602 | ) | — | — | — | — | — | — | — | — | — | — | — | — | — | (12,806 | ) | |||||||||||||||||||||||||||||||||||||||||||||||
Contributions from noncontrolling interests | 109 | — | — | — | — | — | — | — | — | — | — | — | — | — | 861 | |||||||||||||||||||||||||||||||||||||||||||||||||
Sales and assumptions of additional noncontrolling interests | — | — | — | — | — | — | — | — | — | — | — | — | — | — | (74 | ) | ||||||||||||||||||||||||||||||||||||||||||||||||
Changes in fair value of noncontrolling interests | 3,912 | — | — | — | — | — | — | — | — | (3,912 | ) | — | — | — | (3,912 | ) | ||||||||||||||||||||||||||||||||||||||||||||||||
Balance at December 31, 2008 | $ | 34,881 | 7,300,000 | $ | 7 | 2,675,000 | $ | 3 | 1,062,500 | $ | 1 | 188,900 | $ | (791 | ) | $ | 27,395 | $ | (713 | ) | $ | (102,922 | ) | $ | — | $ | (77,020 | ) | $ | 7,841 | ||||||||||||||||||||||||||||||||||
See the accompanying notes to the consolidated financial statements.
F-6
Table of Contents
AMERICAN RENAL HOLDINGS INC. AND SUBSIDIARIES
Consolidated Statements of Changes in Deficit—(Continued)
For the years ended December 31, 2009, 2008 and 2007
(in thousands, except share and per share data)
| Non- controlling interests subject to put provisions | Total American Renal Holdings Inc. Deficit | Non- controlling interests not subject to put provisions | Compre- hensive Income | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Series A Convertible Preferred Stock | Series B Convertible Preferred Stock | Common Stock | Treasury Stock | Additional Paid-in Capital | Notes Receivable from Stock- holders | Accumu- lated Deficit | Accumu- lated Other Compre- hensive Loss | Total | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Shares | Amount | Shares | Amount | Shares | Amount | Shares | Cost | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Balance at December 31, 2008 | $ | 34,881 | 7,300,000 | $ | 7 | 2,675,000 | $ | 3 | 1,062,500 | $ | 1 | 188,900 | $ | (791 | ) | $ | 27,395 | $ | (713 | ) | $ | (102,922 | ) | $ | — | $ | (77,020 | ) | $ | 7,841 | ||||||||||||||||||||||||||||||||||
Comprehensive income: | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Net income | 5,100 | — | — | — | — | — | — | — | — | — | — | 5,138 | — | 5,138 | 17,291 | $ | 27,529 | |||||||||||||||||||||||||||||||||||||||||||||||
Total comprehensive income | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Issuance of common stock | — | — | — | — | 267,750 | — | — | — | 96 | — | — | — | 96 | |||||||||||||||||||||||||||||||||||||||||||||||||||
Purchase of common stock for treasury | — | — | — | — | — | — | 58,000 | (274 | ) | — | — | — | — | (274 | ) | |||||||||||||||||||||||||||||||||||||||||||||||||
Adjustment for tax benefits from stock awards exercised | — | — | — | — | — | — | — | — | (204 | ) | — | — | — | (204 | ) | |||||||||||||||||||||||||||||||||||||||||||||||||
Interest due from note receivable | — | — | — | — | — | — | — | — | — | (22 | ) | — | — | (22 | ) | |||||||||||||||||||||||||||||||||||||||||||||||||
Stock-based compensation expense | — | — | — | — | — | — | — | — | 1,135 | — | — | — | 1,135 | |||||||||||||||||||||||||||||||||||||||||||||||||||
Distributions to noncontrolling interests | (5,618 | ) | — | — | — | — | — | — | — | — | — | — | — | — | — | (15,476 | ) | |||||||||||||||||||||||||||||||||||||||||||||||
Contributions from noncontrolling interests | 292 | — | — | — | — | — | — | — | — | — | — | — | — | — | 1,303 | |||||||||||||||||||||||||||||||||||||||||||||||||
Acquisitions of noncontrolling interests | — | — | — | — | — | — | — | — | — | — | — | — | — | — | 5,098 | |||||||||||||||||||||||||||||||||||||||||||||||||
Purchases of noncontrolling interests | (942 | ) | — | — | — | — | — | — | — | — | — | — | — | — | — | — | ||||||||||||||||||||||||||||||||||||||||||||||||
Changes in fair value of noncontrolling interests | 4,718 | — | — | — | — | — | — | — | — | (4,718 | ) | — | — | — | (4,718 | ) | — | |||||||||||||||||||||||||||||||||||||||||||||||
Balance at December 31, 2009 | $ | 38,431 | 7,300,000 | $ | 7 | 2,675,000 | $ | 3 | 1,330,250 | $ | 1 | 246,900 | $ | (1,065 | ) | $ | 23,704 | $ | (735 | ) | $ | (97,784 | ) | $ | — | $ | (75,869 | ) | $ | 16,057 | ||||||||||||||||||||||||||||||||||
See the accompanying notes to the consolidated financial statements.
F-7
Table of Contents
AMERICAN RENAL HOLDINGS INC. AND SUBSIDIARIES
Consolidated Statements of Cash Flows
For the years ended December 31, 2009, 2008 and 2007
(in thousands)
| 2009 | 2008 | 2007 | ||||||||||
Operating activities | ||||||||||||
Net income | $ | 27,529 | $ | 20,823 | $ | 15,457 | ||||||
Adjustments to reconcile net income to net cash provided by operating activities: | ||||||||||||
Depreciation and amortization | 12,127 | 9,777 | 7,919 | |||||||||
Amortization of deferred financing costs | 1,212 | 611 | 795 | |||||||||
Stock-based compensation expense | 1,135 | 1,482 | 2,180 | |||||||||
Excess tax benefit for stock options exercised, net | 204 | (351 | ) | — | ||||||||
Change in deferred taxes | (108 | ) | 1,117 | 876 | ||||||||
Accrued interest on obligations under financing agreements | 377 | 300 | 438 | |||||||||
Non cash change related to interest rate swap | — | 200 | — | |||||||||
Accrued interest on obligations under Series X preferred stock agreement | 8,069 | 7,268 | 6,536 | |||||||||
Gain on nonmonetary exchange | (611 | ) | (542 | ) | (483 | ) | ||||||
Non-cash rent charges | 822 | 1,161 | 400 | |||||||||
Loss (gain) on disposal of assets | 18 | (66 | ) | 217 | ||||||||
Change in operating assets and liabilities, net of acquisitions: | ||||||||||||
Increase in accounts receivable | (5,439 | ) | (7,661 | ) | (7,446 | ) | ||||||
(Increase) decrease in inventories | (316 | ) | 4,274 | (4,057 | ) | |||||||
Decrease (increase) in prepaid expenses and other current assets | 4,040 | (2,573 | ) | (1,458 | ) | |||||||
Increase in other assets | (424 | ) | (2,023 | ) | (219 | ) | ||||||
Increase (decrease) in accounts payable | 67 | (216 | ) | 5,329 | ||||||||
(Decrease) increase in accrued compensation and benefits | (236 | ) | 1,049 | 1,457 | ||||||||
Increase (decrease) in accrued expenses and other current liabilities | 4,389 | 1,435 | (2,253 | ) | ||||||||
(Decrease) increase in other liabilities | (1,524 | ) | 947 | 2,956 | ||||||||
Net cash provided by operating activities | 51,331 | 37,012 | 28,644 | |||||||||
Investing activities | ||||||||||||
Purchases of property and equipment | (15,067 | ) | (21,254 | ) | (15,218 | ) | ||||||
Cash paid for acquisitions | (3,964 | ) | — | (1,995 | ) | |||||||
Proceeds from divestiture of dialysis center | — | 507 | — | |||||||||
Net cash used in investing activities | (19,031 | ) | (20,747 | ) | (17,213 | ) | ||||||
Financing activities | ||||||||||||
Borrowings, net | $ | 1,184 | $ | 17,363 | $ | 14,239 | ||||||
Payments on long-term debt | (12,001 | ) | (7,952 | ) | (7,026 | ) | ||||||
Payments on capital lease obligations | (663 | ) | (675 | ) | (1,537 | ) | ||||||
Proceeds from issuance of common stock | 96 | 170 | — | |||||||||
Purchase of treasury stock | (274 | ) | (783 | ) | — | |||||||
Excess tax benefits for stock options exercised, net | (204 | ) | 351 | — | ||||||||
Payments made for Series B stock redemption | — | — | (1,000 | ) | ||||||||
Decrease in cash held in escrow | — | — | 1,000 | |||||||||
Distributions to noncontrolling interests | (21,094 | ) | (18,408 | ) | (16,028 | ) | ||||||
Purchases of noncontrolling interests | (942 | ) | (678 | ) | — | |||||||
Contributions from noncontrolling interests | 1,595 | 970 | 1,576 | |||||||||
Net cash used in financing activities | (32,303 | ) | (9,642 | ) | (8,776 | ) | ||||||
Net (decrease) increase in cash and cash equivalents | (3 | ) | 6,623 | 2,655 | ||||||||
Cash and cash equivalents at beginning of year | 29,182 | 22,559 | 19,904 | |||||||||
Cash and cash equivalents at end of year | $ | 29,179 | $ | 29,182 | $ | 22,559 | ||||||
Supplemental disclosure of cash flow information | ||||||||||||
Cash paid during the year for income taxes | $ | 7,125 | $ | 7,361 | $ | 4,369 | ||||||
Cash paid during the year for interest | 5,572 | 5,651 | 6,166 | |||||||||
Supplemental disclosure of non-cash financing activities | ||||||||||||
Issuance of common stock in exchange for long-term notes | — | 697 | — | |||||||||
Issuance of notes to sellers in connection with acquisitions | 1,342 | — | — | |||||||||
Noncontrolling interest in net assets of acquisitions | 5,098 | — | 1,413 | |||||||||
See the accompanying notes to the consolidated financial statements.
F-8
Table of Contents
AMERICAN RENAL HOLDINGS INC. AND SUBSIDIARIES
Notes to Consolidated Financial Statements
December 31, 2009 and 2008
(dollars in thousands, except per share amounts)
NOTE A—DESCRIPTION OF BUSINESS
On December 16, 2005, American Renal Holding LLC (ARH) was merged with American Renal Associates, Inc., which was renamed American Renal Holdings Inc. (the Company). Immediately thereafter, the Company contributed all of its assets, subject to all of its liabilities, to ARA Subsidiary Corp., which was renamed American Renal Associates Inc. (ARA). On December 31, 2007, ARA converted to a limited liability company (American Renal Associates LLC). All financial statements presented reflect the merger of ARH into the Company and the contribution of all of the assets, subject to all of the liabilities, from the Company to ARA. Prior to the merger, ARH owned 99.8% of the common stock of the Company and the balance of the common stock was owned by employees of the Company who exercised stock options. The Company was incorporated as a Delaware corporation in July 1999, and was formed to open, staff, and operate outpatient kidney dialysis centers and to provide related medical services in selected markets. The Company’s first dialysis center opened on December 8, 2000. On December 31, 2009, the Company operated 83 dialysis centers, which are located in 16 states and Washington, D.C.
The Company owns and operates dialysis centers in partnership with nephrologists. The ownership and management of each center is established through operating and management services agreements. Medical director services are provided separately through a medical director agreement. The Company maintains a majority ownership interest in each of its centers. The Company and the partners contribute capital to the centers in proportion to their ownership interest. Financing for the centers is obtained through third-party lenders, collateralized by the assets of the centers. If additional financing is required and is not reasonably available, additional capital contributions may be required from the Company and its partners to meet operating needs.
NOTE B—SIGNIFICANT ACCOUNTING POLICIES
Basis of Presentation and Consolidation
These consolidated financial statements and accompanying notes are prepared in accordance with accounting principles generally accepted in the United States. The financial statements include the Company’s subsidiaries and partnerships that are wholly owned or majority owned. All significant intercompany transactions have been eliminated in consolidation. The Company has evaluated subsequent events through November 4, 2010, which is the date these consolidated financial statements were issued.
As of January 1, 2009, the Company adopted the new accounting standard for noncontrolling interests and changed the reporting for minority interests, which are now characterized as noncontrolling interests. Noncontrolling interests are classified in the accompanying balance sheet either as a component of equity whenever such interests are not subject to a put provision or as temporary equity whenever such interests are subject to a put provision. The amounts of consolidated net income attributable to both parent and the noncontrolling interest are now separately presented in the accompanying statement of income. The presentation and disclosure requirements were retroactively applied to minority interest amounts existing as of December 31, 2006, 2007 and 2008, and for the years ended December 31, 2007 and 2008 in the consolidated financial statements. See “Noncontrolling Interests” disclosure in Note B and Note H for additional information.
Use of Estimates
The preparation of financial statements in conformity with accounting principles generally accepted in the United States requires the use of estimates and assumptions that affect the reported amounts of revenues,
F-9
Table of Contents
AMERICAN RENAL HOLDINGS INC. AND SUBSIDIARIES
Notes to Consolidated Financial Statements—(Continued)
December 31, 2009 and 2008
(dollars in thousands, except per share amounts)
NOTE B—SIGNIFICANT ACCOUNTING POLICIES—(Continued)
expenses, assets, liabilities, and contingencies. Although actual results in subsequent periods will differ from these estimates, such estimates are developed based on the best information available to management and management’s best judgments at the time made. All significant assumptions and estimates underlying the reported amounts in the consolidated financial statements and accompanying notes are regularly reviewed and updated. Changes in estimates are reflected in the financial statements based upon ongoing actual experience, trends, or subsequent settlements and realizations, depending on the nature and predictability of the estimates and contingencies.
The most significant assumptions and estimates underlying these financial statements and accompanying notes involve revenue recognition and provisions for uncollectible accounts, impairments and valuation adjustments, the useful lives of property and equipment, fair value measurements, accounting for income taxes, acquisition accounting valuation estimates and stock-based compensation. Specific risks and contingencies related to these estimates are further addressed within the notes to the consolidated financial statements.
Net Operating Revenues and Allowance for Doubtful Accounts
Net operating revenues are recognized as services are provided to patients and consist primarily of reimbursement for dialysis and ancillary services provided to patients. A usual and customary fee schedule is maintained for dialysis treatment and other patient services; however, actual collectible revenue is normally at a discount to the fee schedule. Medicare and Medicaid programs are billed at predetermined net realizable rates per treatment that are established by statute or regulation. Revenue for contracted payors is recorded at contracted rates and other payors are billed at usual and customary rates, and a contractual allowance is recorded to reflect the expected net realizable revenue for services provided. Contractual allowances, along with provisions for uncollectible amounts, are estimated based upon contractual terms, regulatory compliance, and historical collection experience. Net revenue recognition and allowances for uncollectible billings require the use of estimates of the amounts that will actually be realized considering among other items, retroactive adjustments that may be associated with regulatory reviews, audits, billing reviews, and other matters.
Net operating service revenues associated with patients whose primary coverage is under governmental programs, including Medicare and Medicaid, Veterans Administration, and Medicare or Medicaid Managed Care programs, accounted for approximately 57%, 56% and 59% of total net operating service revenues for the years ended December 31, 2009, 2008 and 2007, respectively.
Laws and regulations governing the Medicare and Medicaid programs are extremely complex and subject to interpretation. As a result, there is at least a reasonable possibility that recorded estimates will change by a material amount in the near term. Patient accounts receivable from these programs were $30,389 and $25,053 at December 31, 2009 and 2008, respectively. No other single payor accounted for more than 5% of total patient accounts receivable.
Management services are provided to dialysis centers not owned by the Company. The management fees are typically determined as a percentage of the centers’ patient revenues and are included in net revenues as earned. Any costs incurred in performing these management services are recognized in general and administrative expenses. Management fees that are charged to noncontrolling shareholders are eliminated in consolidation. The noncontrolling share of expenses is included in net income attributable to noncontrolling interests.
F-10
Table of Contents
AMERICAN RENAL HOLDINGS INC. AND SUBSIDIARIES
Notes to Consolidated Financial Statements—(Continued)
December 31, 2009 and 2008
(dollars in thousands, except per share amounts)
NOTE B—SIGNIFICANT ACCOUNTING POLICIES—(Continued)
A significant physician-prescribed pharmaceutical administered during dialysis, EPO (Erythropoietin), is provided by a sole supplier and accounted for approximately 22%, 24% and 25% of gross operating revenues for the years ended December 31, 2009, 2008 and 2007, respectively. Although the Company currently receives discounted prices for EPO, the supplier has unilateral pricing discretion, and in the future, the Company may not be able to achieve the same cost levels historically obtained.
Segment Information
Operating segments are identified as components of an enterprise about which separate discrete financial information is available for evaluation by the chief operating decision maker, or decision-making group, in making decisions how to allocate resources and assess performance. The Company’s chief decision-maker is a combination of the Chief Executive Officer and the Chief Financial Officer. The Company views its operations and manages its business as one reportable business segment, the ownership and operation of dialysis clinics, all of which are located in the United States.
Cash and Cash Equivalents
The Company considers all highly liquid investments with an original maturity of three months or less at time of purchase to be cash equivalents.
Fair Value Measurements
The Company measures the fair value of certain assets, liabilities and noncontrolling interests subject to put provisions based upon certain valuation techniques that include observable or unobservable inputs and assumptions that market participants would use in pricing these assets, liabilities and commitments. The Company also has classified certain assets, liabilities and noncontrolling interests subject to put provisions that are measured at fair value into the appropriate fair value hierarchy levels. The Company’s financial instruments consist of cash equivalents, accounts receivable, accounts payable, accrued liabilities and long-term debt. The Company’s estimate of fair value for these financial instruments approximates their carrying values at December 31, 2009 and 2008.
Inventories
Inventories are stated at the lower of cost (first in, first out) or market, and consist principally of pharmaceuticals and dialysis-related supplies.
Property and Equipment
Property and equipment are recorded at cost. Depreciation expense is calculated using the straight-line method over the following useful lives:
Buildings | 39 years | |
Leasehold improvements | Shorter of lease term or useful lives | |
Equipment and information systems | 3 to 7 years |
Upon retirement or sale, the cost and related accumulated depreciation are removed from the accounts, and any resulting gain or loss is credited or charged to income. Maintenance and repairs are charged to expense as incurred. The Company capitalizes interest on funds borrowed to finance facility construction.
F-11
Table of Contents
AMERICAN RENAL HOLDINGS INC. AND SUBSIDIARIES
Notes to Consolidated Financial Statements—(Continued)
December 31, 2009 and 2008
(dollars in thousands, except per share amounts)
NOTE B—SIGNIFICANT ACCOUNTING POLICIES—(Continued)
Interest capitalized was $49, $328 and $231 during 2009, 2008 and 2007, respectively.
Deferred Financing Costs
Costs incurred in connection with a stock exchange and recapitalization, debt issuances, and capital lease arrangements have been deferred, and are being amortized over the term of the related instrument as interest expense.
Amortizable Intangible Assets
Amortizable intangible assets include a right of first refusal waiver, noncompete agreements and certificates of need. Each of these assets is amortized on a straight-line basis over the term of the agreement, which is generally five to ten years.
Goodwill
The excess of aggregate purchase price over the fair value of the net tangible and specifically identifiable intangible assets acquired in business combinations is recorded as goodwill. Goodwill is not amortized, but required to be assessed for possible impairment as circumstances warrant, but at least annually. The Company assesses goodwill for impairment as of October 1st.
The Company evaluates goodwill for impairment at least on an annual basis and more frequently if certain indicators are encountered. Goodwill is to be tested at the reporting unit level, defined as an operating segment or one level below an operating segment (referred to as a component), with the fair value of the reporting unit being compared to its carrying amount, including goodwill. If the fair value of a reporting unit exceeds its carrying amount, goodwill of the reporting unit is not considered to be impaired. The Company has determined that it has one operating, as well as one reportable, segment. For impairment testing purposes, the Company’s individual clinics qualify as components of that operating segment. Because they have similar economic characteristics, the components are aggregated and deemed a single reporting unit. No impairment was identified during the years ended December 31, 2009 and 2008.
Impairment of Long-Lived Assets
Long-lived assets include property and equipment and intangibles, except for goodwill. In the event that facts and circumstances indicate that these assets may be impaired, an evaluation of recoverability would be performed. If an evaluation is required, the estimated future undiscounted cash flows associated with the asset would be compared to the asset’s carrying amount to determine if a write-down to market value or discounted cash flow value is required. No long-lived assets were identified as impaired in 2009 and 2008.
Income Taxes
The Company accounts for income taxes under a liability approach. Under this approach, deferred tax assets and liabilities are recognized based upon temporary differences between the financial statement and tax bases of assets and liabilities, as measured by the enacted tax rates, which will be in effect when these differences reverse. Deferred tax expense or benefit is the result of changes between deferred tax assets and liabilities. A valuation allowance is established when, based on an evaluation of objective verifiable evidence, there is a likelihood that some portion or all of the deferred tax assets will not be realized.
F-12
Table of Contents
AMERICAN RENAL HOLDINGS INC. AND SUBSIDIARIES
Notes to Consolidated Financial Statements—(Continued)
December 31, 2009 and 2008
(dollars in thousands, except per share amounts)
NOTE B—SIGNIFICANT ACCOUNTING POLICIES—(Continued)
On January 1, 2007, the Company adopted the provisions of Financial Accounting Standards Board (FASB) Accounting Standards Codification (ASC) Topic 740-10, Accounting for Income Tax Uncertainties which Interpretation prescribes a recognition threshold of more-likely-than-not and a measurement attribute on all tax positions taken or expected to be taken in a tax return in order to be recognized in the financial statements. In making this assessment, a company must determine whether it is more-likely-than-not that a tax position will be sustained upon examination, including resolution of any related appeals or litigation processes, based solely on the technical merits of the position and must assume that the tax position will be examined by the appropriate taxing authority that would have full knowledge of all relevant information. Once the recognition threshold is met, the tax position is then measured to determine the actual amount of benefit to recognize in the financial statements. In addition, the recognition threshold of more-likely-than-not must continue to be met in each reporting period to support continued recognition of the tax benefit. Tax positions that previously failed to meet the more-likely-than-not recognition threshold should be recognized in the first financial reporting period in which that threshold is met. Previously recognized tax positions that no longer meet the more-likely-than-not recognition threshold should be derecognized in the financial reporting period in which that threshold is no longer met. The Company recognizes interest and penalties related to unrecorded tax positions in its income tax expense. See Note L for a discussion of the impact of this new accounting standard on the accompanying financial statements.
Noncontrolling Interests
Noncontrolling interests represent the proportionate equity interests of other partners in the Company’s consolidated entities, which are not wholly owned. Effective January 1, 2009, the Company is required to treat noncontrolling interests not subject to put provisions as a separate component of equity, but apart from the Company’s equity, and not as a liability or other item outside of equity. The Company is also required to identify and present consolidated net income attributable to the Company and to noncontrolling interests on the face of the consolidated statement of income. In addition, changes in the Company’s ownership interest while the Company retains a controlling financial interest should be accounted for as equity transactions. The Company was also required to expand disclosures in the financial statements to include a reconciliation of the beginning and ending balances of the equity attributable to the Company and the noncontrolling owners and a schedule showing the effects of changes in the Company’s ownership interest in a subsidiary on the equity attributable to the Company. This change did not have a material impact on the Company’s consolidated financial statements; however, it did change the presentation of minority interests (noncontrolling interests) in the Company’s consolidated financial statements. In conjunction with adopting these requirements, the Company was required to classify member interests with redemption features that are not solely within the Company’s control, such as the Company’s noncontrolling interests that are subject to put provisions, outside of permanent equity and to measure these noncontrolling interests at fair value. Changes in the fair value of noncontrolling interests subject to put provisions are accounted for as equity transactions. See Note H for further details. These consolidated financial statements have been recast for all prior periods presented for the retrospective application of these presentation and disclosure requirements.
F-13
Table of Contents
AMERICAN RENAL HOLDINGS INC. AND SUBSIDIARIES
Notes to Consolidated Financial Statements—(Continued)
December 31, 2009 and 2008
(dollars in thousands, except per share amounts)
NOTE B—SIGNIFICANT ACCOUNTING POLICIES—(Continued)
The effects of the change upon the retrospective application of these presentation and disclosure requirements were as follows:
Consolidated Balance Sheet:
| December 31, 2008 | ||||||||||||||||
| Minority Interest | Noncontrolling Interests subject to put provisions | Noncontrolling Interests not subject to put provisions | Additional paid-in capital | |||||||||||||
Balance | $ | 8,911 | $ | — | $ | — | $ | 61,208 | ||||||||
Net change | (8,911 | ) | 34,881 | 7,843 | (33,813 | ) | ||||||||||
Balance as adjusted | $ | — | $ | 34,881 | $ | 7,843 | $ | 27,395 | ||||||||
Consolidated Statements of Cash Flows:
For the year ended December 31, 2008
| Operating Activities | Financing Activities | |||||||
Net cash provided by operating/financing activities | $ | 18,604 | $ | 9,444 | ||||
Reclassification of distributions to noncontrolling interests | 18,407 | (18,407 | ) | |||||
Reclassification of purchase of noncontrolling interest from investing activities | — | (678 | ) | |||||
Net cash provided by (used in) operating/financing activities as adjusted | $ | 37,011 | $ | (9,641 | ) | |||
In addition to the reclassifications noted above, the retrospective application in the accounting for noncontrolling interests resulted in an increase of $678 in cash flows from investing activities in 2008 as compared to the amount previously presented.
For the year ended December 31, 2007
| Operating Activities | Financing Activities | |||||||
Net cash provided by operating/financing activities | $ | 12,616 | $ | 7,252 | ||||
Reclassification of distributions to noncontrolling interests | 16,028 | (16,028 | ) | |||||
Net cash provided by (used in) operating/financing activities as adjusted | $ | 28,644 | $ | (8,776 | ) | |||
Stock-Based Compensation
The Company measures and recognizes the cost for all share-based awards made to employees and directors, including stock options, stock appreciation rights, stock units, and discounted employee stock purchases. The Company’s stock-based compensation awards are measured at their estimated grant-date fair
F-14
Table of Contents
AMERICAN RENAL HOLDINGS INC. AND SUBSIDIARIES
Notes to Consolidated Financial Statements—(Continued)
December 31, 2009 and 2008
(dollars in thousands, except per share amounts)
NOTE B—SIGNIFICANT ACCOUNTING POLICIES—(Continued)
value and recognized as compensation expense on the straight-line method over their requisite service periods. The amount of compensation cost recognized in the consolidated statements of income is based on the awards that vest, and therefore, is reduced for forfeitures.
Recent Accounting Pronouncements
In January 2010, the FASB issued ASC Topic 820,Fair Value Measurements and Disclosures—Improving Disclosures About Fair Value Measurements. The ASU requires new disclosures about transfers into and out of Levels 1 and 2 and separate disclosures about purchases, sales, issuances, and settlements relating to Level 3 measurements. It also clarifies existing fair value disclosures about the level of disaggregation and about inputs and valuation techniques used to measure fair value. The new disclosures and clarifications of existing disclosures are effective January 1, 2010, except for the disclosures about purchases, sales, issuances, and settlements relating to Level 3 measurements, which are effective January 1, 2011. Other than requiring additional disclosures, the adoption of this new guidance is not expected to have a material impact on the Company’s consolidated results of operations and financial position.
In August 2010, the FASB issued guidance that requires health care entities to use cost as the measurement basis for charity care disclosures and defines cost as the direct and indirect costs of providing charity care. The amended disclosure requirements are effective for fiscal years beginning after December 15, 2010 and must be applied retrospectively. The Company will adopt the amended disclosure requirements for their fiscal year beginning January 1, 2011. Since the new guidance amends disclosure requirements only, its adoption will not impact the Company’s statement of financial position, statement of operations, or cash flow statement.
NOTE C—ACQUISITIONS
The Company periodically acquires assets and liabilities of dialysis centers. The results of operations for these acquisitions are included in the Company’s consolidated statements of income from the respective acquisition dates.
All acquisitions occurring after January 1, 2009 are required to be accounted for under the acquisition method (previously referred to as the purchase method). Under this method, the acquirer recognizes the assets acquired, the liabilities assumed, contractual contingencies, as well as any noncontrolling interest in the acquiree at their fair values at the acquisition date. Transaction costs are excluded from the acquisition cost and are expensed as incurred. Under accounting standards in effect prior to January 1, 2009, noncontrolling interests in the acquiree were recorded at historical cost and transaction costs were included in the acquisition cost.
Fiscal Year 2009
On May 1, 2009, the Company entered into three joint ventures with nephrologists to acquire the assets and assume certain liabilities of three dialysis centers in Georgia for cash and notes payable. The Company has a 51% interest in each of the three joint ventures.
On July 29, 2009, the Company entered into a joint venture with a nephrologist to acquire the assets and assume certain liabilities of a dialysis center in Michigan for cash. The Company has a 51% interest in the joint venture.
F-15
Table of Contents
AMERICAN RENAL HOLDINGS INC. AND SUBSIDIARIES
Notes to Consolidated Financial Statements—(Continued)
December 31, 2009 and 2008
(dollars in thousands, except per share amounts)
NOTE C—ACQUISITIONS—(Continued)
The purchase price, on a combined basis for both acquisitions consummated in 2009, was allocated preliminarily as follows:
Accounts receivable | $ | 1,182 | ||
Other receivables and supplies | 462 | |||
Property and equipment | 1,433 | |||
Noncompete agreements | 593 | |||
Goodwill | 8,436 | |||
Less liabilities assumed | (1,703 | ) | ||
Less noncontrolling interest in net assets acquired | (5,098 | ) | ||
Purchase price (including notes to sellers of $1,341) | $ | 5,305 | ||
The goodwill above is deductible for income tax purposes. The weighted average amortization period for the acquired noncompete agreements is ten years.
Fiscal Year 2008
On January 1, 2008, the Company purchased an additional 8% membership interest from a noncontrolling interest partner. After the purchase of this interest, the Company has a 59% interest in this subsidiary. The consideration paid in excess of the seller’s book value in the amount of $604 was recorded as goodwill.
Fiscal Year 2007
On January 1, 2007, the Company entered into a joint venture with a nephrologist to acquire the assets and assume certain liabilities of a dialysis center in Missouri for cash. The Company has a 51% interest in the partnership.
On May 1, 2007, the Company entered into a joint venture with three nephrologists to acquire the assets of a dialysis center in Maryland. The Company has a 51% interest in the partnership.
On September 1, 2007, the Company acquired the assets of a chronic kidney disease business in Rhode Island for cash. The Company has a 100% interest in the entity.
The purchase price, on a combined basis for all acquisitions consummated in 2007, was allocated as follows:
Property and equipment | $ | 944 | ||
Other receivables and supplies | 91 | |||
Noncompete agreements | 670 | |||
Goodwill | 1,171 | |||
Less liabilities assumed | (594 | ) | ||
Less noncontrolling interest in assets acquired | (287 | ) | ||
Cash purchase price | $ | 1,995 | ||
The goodwill above is deductible for income tax purposes. The weighted average amortization period for the acquired noncompete agreements is 8 years.
F-16
Table of Contents
AMERICAN RENAL HOLDINGS INC. AND SUBSIDIARIES
Notes to Consolidated Financial Statements—(Continued)
December 31, 2009 and 2008
(dollars in thousands, except per share amounts)
NOTE D—FAIR VALUE OF FINANCIAL INSTRUMENTS
The Company’s financial instruments are classified and disclosed in one of the following three categories:
Level 1: Financial instruments with unadjusted, quoted prices listed on active market exchanges. The Company’s Level 1 assets include cash equivalent investments held in money market funds.
Level 2: Financial instruments determined using prices for recently traded financial instruments with similar underlying terms, as well as directly or indirectly observable inputs, such as interest rates and yield curves that are observable at commonly quoted intervals.
Level 3: Financial instruments not actively traded on a market exchange. This category includes situations where there is little, if any, market activity for the financial instrument. The prices are determined using significant unobservable inputs or valuation techniques.
The asset’s or liability’s fair value measurement level within the fair value hierarchy is based on the lowest level of any input that is significant to the fair value measurement. The following is a description for the valuation methodologies used for assets and liability’s measured at fair value. There were no changes in the methodologies used at December 31, 2009 and 2008.
Cash equivalent—The cash equivalent represents investments in a money market fund and U.S. government obligations which are recorded at fair value based on their quoted market prices.
Noncontrolling interest subject to put provision—See Note H to the consolidated financial statements for a discussion of the Company’s methodology for estimating fair value of noncontrolling interest subject to put obligations.
Interest rate swap—The fair value of the interest rate swap was determined using a pricing model with inputs that are observable in the market.
| December 31, 2009 | ||||||||||||||||
| Total | Level 1 | Level 2 | Level 3 | |||||||||||||
Assets | ||||||||||||||||
Cash equivalent | $ | 9,506 | $ | 9,506 | $ | — | $ | — | ||||||||
Temporary Equity | ||||||||||||||||
Noncontrolling interests subject to put provisions | $ | 38,431 | $ | — | $ | — | $ | 38,431 | ||||||||
| December 31, 2008 | ||||||||||||||||
| Total | Level 1 | Level 2 | Level 3 | |||||||||||||
Assets | ||||||||||||||||
Cash equivalent | $ | 10,004 | $ | 10,004 | $ | — | $ | — | ||||||||
Liabilities | ||||||||||||||||
Interest rate swap | $ | 190 | $ | — | $ | 190 | $ | — | ||||||||
Temporary Equity | ||||||||||||||||
Noncontrolling interests subject to put provisions | $ | 34,881 | $ | — | $ | — | $ | 34,881 | ||||||||
F-17
Table of Contents
AMERICAN RENAL HOLDINGS INC. AND SUBSIDIARIES
Notes to Consolidated Financial Statements—(Continued)
December 31, 2009 and 2008
(dollars in thousands, except per share amounts)
NOTE E—PROPERTY AND EQUIPMENT
Property and equipment consist of the following at December 31:
| 2009 | 2008 | |||||||
Land | $ | 380 | $ | 380 | ||||
Buildings | 1,312 | 1,312 | ||||||
Leasehold improvements | 54,045 | 45,712 | ||||||
Equipment and information systems | 43,669 | 37,101 | ||||||
Construction in progress, new centers | 2,250 | 1,519 | ||||||
| 101,656 | 86,024 | |||||||
Less accumulated depreciation | (42,251 | ) | (31,205 | ) | ||||
| $ | 59,405 | $ | 54,819 | |||||
Depreciation of property and equipment was $11,897, $9,548 and $7,757 for the years ended December 31, 2009, 2008 and 2007, respectively. Included in construction in progress for the Company’s new centers are items related to property and equipment during the development stage of new dialysis clinics. At December 31, 2009, the Company had commitments of $1,102 to complete projects relating to capital construction.
During 2006, the Company exchanged old dialysis machines for new dialysis machines, which qualified as a nonmonetary asset exchange. The assets received in the exchange were accounted for using fair value and resulted in a deferred gain of $2,391. A purchase agreement with this vendor for dialysis supplies was revised in contemplation of this nonmonetary exchange of assets. As of December 31, 2009 and 2008, $324 and $934, respectively, of the unrecognized gain was deferred related to additional dialysis supplies purchases on this contract. During 2009, 2008 and 2007, $611, $542 and $483, respectively, of the gain was recognized as a reduction to patient care costs.
NOTE F—DEFERRED FINANCING COSTS AND AMORTIZABLE INTANGIBLE ASSETS
Deferred financing costs and amortizable intangible assets consist of the following at December 31:
| 2009 | 2008 | |||||||
Deferred financing costs | $ | 2,347 | $ | 4,166 | ||||
Less accumulated amortization | (1,303 | ) | (2,288 | ) | ||||
| $ | 1,044 | $ | 1,878 | |||||
| 2009 | 2008 | |||||||
Noncompete agreements | $ | 1,794 | $ | 1,201 | ||||
Other intangible assets | 426 | 426 | ||||||
| 2,220 | 1,627 | |||||||
Less accumulated amortization | (802 | ) | (571 | ) | ||||
| $ | 1,418 | $ | 1,056 | |||||
F-18
Table of Contents
AMERICAN RENAL HOLDINGS INC. AND SUBSIDIARIES
Notes to Consolidated Financial Statements—(Continued)
December 31, 2009 and 2008
(dollars in thousands, except per share amounts)
NOTE F—DEFERRED FINANCING COSTS AND AMORTIZABLE INTANGIBLE ASSETS—(Continued)
Amortization of amortizable intangible assets was $231, $230 and $162 for the years ended December 31, 2009, 2008 and 2007, respectively. Amortization expense for deferred financing costs was $585, $611 and $795 for the years ended December 31, 2009, 2008 and 2007, respectively, and is included as part of interest expense. In connection with the amendment of certain loans during 2009, the Company expensed the remaining unamortized deferred financing costs of $627 associated with these loans.
The estimated annual amortization expense related to amortizable intangible assets is as follows for the years ending December 31:
2010 | $ | 261 | ||
2011 | 250 | |||
2012 | 196 | |||
2013 | 170 | |||
2014 | 149 | |||
Thereafter | 392 | |||
| $ | 1,418 | |||
NOTE G—ACCRUED EXPENSES
Accrued compensation and benefits consist of the following at December 31:
| 2009 | 2008 | |||||||
Accrued payroll | $ | 4,038 | $ | 4,266 | ||||
Other | 3,132 | 2,996 | ||||||
| $ | 7,170 | $ | 7,262 | |||||
Accrued expenses and other current liabilities consist of the following at December 31:
| 2009 | 2008 | |||||||
Payor refunds and retractions | $ | 9,928 | $ | 6,802 | ||||
Other | 5,718 | 4,432 | ||||||
| $ | 15,646 | $ | 11,234 | |||||
NOTE H—NONCONTROLLING INTERESTS SUBJECT TO PUT PROVISIONS
The Company has potential obligations to purchase the noncontrolling interests held by third parties in several of its consolidated subsidiaries. These obligations are in the form of put provisions and are exercisable at the third-party owners’ discretion within specified periods as outlined in each specific put provision. If these put provisions were exercised, the Company would be required to purchase the third-party owners’ noncontrolling interests at the appraised fair value. The methodology the Company used to estimate the fair value of the noncontrolling interests subject to these put provisions assumes an average multiple of earnings, based on historical earnings, development stage of the underlying business and other factors. The estimated fair values of the noncontrolling interests subject to these put provisions can also fluctuate and the implicit multiple of earnings
F-19
Table of Contents
AMERICAN RENAL HOLDINGS INC. AND SUBSIDIARIES
Notes to Consolidated Financial Statements—(Continued)
December 31, 2009 and 2008
(dollars in thousands, except per share amounts)
NOTE H—NONCONTROLLING INTERESTS SUBJECT TO PUT PROVISIONS—(Continued)
at which these noncontrolling interest obligations may ultimately be settled could vary significantly from our current estimates depending upon market conditions including potential purchasers’ access to the credit and capital markets, which can impact the level of competition for dialysis and non-dialysis related businesses, the economic performance of these businesses and the restricted marketability of the third-party owners’ noncontrolling interests.
As of December 31, 2009 and 2008, the Company’s potential obligations under these put provisions totaled approximately $29,295 and $25,770, respectively. Additionally, the Company has certain put agreements which are exercisable upon the occurrence of specific events, including third-party members’ death, disability, bankruptcy, retirement, or if third-party members are dissolved. As of December 31, 2009 and 2008, the Company’s potential additional obligations under these put provisions were approximately $9,136 and $9,111, respectively. The Company’s potential obligations for all of these put provisions are included in noncontrolling interests subject to put provisions in the accompanying consolidated balance sheets.
During 2009, the Company purchased an additional 7.5% membership interest from a noncontrolling interest partner for $942. This purchase has been accounted for as an equity transaction.
NOTE I—LONG-TERM DEBT
Long-term debt consists of the following at December 31:
| 2009 | 2008 | |||||||
Term loan A, principal payments of $550 and interest at variable rates (8.50% as of December 31, 2009) payable quarterly through December 2010. | $ | 2,750 | $ | 4,950 | ||||
Term loan B (converted into Term loan D in 2009) | — | 17,505 | ||||||
Term loan C (converted into Term loan D in 2009) | — | 22,000 | ||||||
Term loan D, principal payments of $45 and interest at variable rates (8.50% as of December 31, 2009) payable quarterly through September 2011, with a $39,010 balloon payment due December 2011. | 39,325 | — | ||||||
Term loans, principal payments of $33 and interest at variable monthly rates (3.60% as of December 31, 2009) payable monthly through November 2011, with a balloon payment of $1,983 due December 2011. | 2,065 | 3,120 | ||||||
Term loans, principal payments of $9 and interest at variable rates (3.01% as of December 31, 2009) through May 2014, with a $536 balloon payment due May 2014 | 1,001 | — | ||||||
Term loans, principal and interest payable monthly at rates between 5.9% and 8.67% over varying periods through December 2013. | 29,802 | 25,914 | ||||||
Term loans, interest payable monthly at variable rates (between 4.51% and 7.90% as of December 31, 2008) during the progress periods, which converted in 2009 to term loans | — | 8,401 | ||||||
Mortgage payable, principal and interest due monthly through February 2012 at a rate of 7% | 509 | 528 | ||||||
Mortgage payable, principal and interest due monthly through March 2014 at a rate of 4.79% | 268 | 321 | ||||||
Lines of credit, variable interest (4.25% as of December 31, 2009), due monthly through September 2010, convertible to term loans payable over an additional term of 36 months through September 2013. | 2,850 | 4,954 | ||||||
| 78,570 | 87,693 | |||||||
Less current maturities | (14,309 | ) | (11,556 | ) | ||||
| $ | 64,261 | $ | 76,137 | |||||
F-20
Table of Contents
AMERICAN RENAL HOLDINGS INC. AND SUBSIDIARIES
Notes to Consolidated Financial Statements—(Continued)
December 31, 2009 and 2008
(dollars in thousands, except per share amounts)
NOTE I—LONG-TERM DEBT—(Continued)
Scheduled maturities of long-term debt are as follows for the years ending December 31:
2010 | $ | 14,309 | ||
2011 | 52,024 | |||
2012 | 7,826 | |||
2013 | 3,617 | |||
2014 | 794 | |||
| $ | 78,570 | |||
In December 2005, the Company’s wholly owned subsidiary, ARA, entered into a credit agreement with a financial institution. The financial institution extended three term loans (term loans A, B, and C), totaling $51,000, and a revolving line of credit of $10,000, to fund the repayment of certain indebtedness, provide for working capital and acquisition funding, and for the stock recapitalization (see Note M). During 2007, the Company and the financial institution amended the credit agreement reducing the applicable variable interest rate margins by three quarters of a percent on term loan A, one and one quarter of a percent on term loan B, three and three quarters percent on term loan C and three quarters of a percent on the revolving line of credit. In 2009, the Company amended its revolving line of credit with the financial institution which decreased borrowing availability under the line of credit from $10,000 to $6,000 and combined term loan B and term loan C into one new term loan (term loan D) with a variable interest rate based on the 30—day LIBOR (with a LIBOR floor of 2.5%) plus 6.00%. This amendment also permitted the Company to increase its intercompany loans from $12,000 to $15,000. The unused portion of the line of credit was $6,000 at December 31, 2009 and $10,000 at December 31, 2008.
The loans are guaranteed by the Company, and by each wholly owned subsidiary of ARA. Additionally, the loans are secured by (i) all of the assets of ARA and each wholly owned subsidiary of ARA (other than their membership interests in the dialysis clinic subsidiaries that are owned in partnership with nephrologists,) and; (ii) all of the capital stock of ARA and the membership interests of each wholly owned subsidiary of ARA. The loans from the financial institution were subject to certain debt covenants beginning March 31, 2006, which were primarily based on ARA’s financial results. The Company was in compliance with the covenants of these loans as of and for the year ended December 31, 2009.
During 2006, the Company entered into an interest rate swap agreement with an amortizing notional amount of $20,000. The agreement has the economic effect of converting the LIBOR-based variable interest rate to a fixed rate of 12.27% for $20,000 of term loan C. The swap agreement expired on March 31, 2009, and required quarterly settlement payments which were recorded as an increase or decrease to interest expense. During 2008, management determined the interest rate swap could no longer be considered an effective cash flow hedge. As a result, the change in the fair value of the interest rate swap of $144 was reflected in the accompanying consolidated statement of income in 2008 as a component of interest expense. In addition, the accumulated other comprehensive loss balance on the interest rate swap has also been recognized as a component of interest expense in 2008. The interest rate swap is recorded at its fair value of $190 as of December 31, 2008 (see Note J).
F-21
Table of Contents
AMERICAN RENAL HOLDINGS INC. AND SUBSIDIARIES
Notes to Consolidated Financial Statements—(Continued)
December 31, 2009 and 2008
(dollars in thousands, except per share amounts)
NOTE J—OTHER LIABILITIES
Other liabilities consist of the following at December 31:
| 2009 | 2008 | |||||||
Deferred gain from nonmonetary asset exchange | $ | 324 | $ | 934 | ||||
Deferred straight-line rent | 3,234 | 2,411 | ||||||
Liability from tenant allowances | 4,483 | 4,914 | ||||||
Accrued professional liability | 527 | 487 | ||||||
Interest rate swap liability | — | 190 | ||||||
Other | 110 | — | ||||||
| 8,678 | 8,936 | |||||||
Less current portion of other liabilities | (1,013 | ) | (1,366 | ) | ||||
| $ | 7,665 | $ | 7,570 | |||||
NOTE K—LEASES
Operating Leases
The majority of the Company’s facilities are leased under noncancelable operating leases expiring in various years through 2023. Most lease agreements cover periods from five to fifteen years, and contain renewal options of five to ten years at the fair rental value at the time of renewal. Certain leases are subject to rent holidays and/or escalation clauses. The Company expenses rent using the straight-line method over the lease term. Tenant allowances received from lessors are amortized over the term of the leases. Rental expense under all operating leases for the years ended December 31, 2009, 2008 and 2007 was $9,152, $8,242 and $5,760, respectively. The Company also subleases space to related party tenants at fair values under noncancelable operating leases expiring in various years through 2023. Rental income under all subleases for the years ended December 31, 2009, 2008 and 2007 was $1,091, $643 and $244, respectively.
Future minimum lease payments under non-cancelable operating leases, net of sublease receipts, are as follows as of December 31, 2009:
| Operating Leases | Less: Sublease Receipts | Net Lease Obligation | ||||||||||
2010 | $ | 9,603 | $ | 1,038 | $ | 8,565 | ||||||
2011 | 9,240 | 1,034 | 8,206 | |||||||||
2012 | 8,430 | 1,027 | 7,403 | |||||||||
2013 | 7,710 | 1,040 | 6,670 | |||||||||
2014 | 7,103 | 1,017 | 6,086 | |||||||||
Thereafter | 31,580 | 7,626 | 23,954 | |||||||||
| $ | 73,666 | $ | 12,782 | $ | 60,884 | |||||||
Capital Leases
Capital leases are recorded as an asset and an obligation at an amount equal to the lesser of the present value of the minimum lease payments during the lease term or the fair market value of the leased asset. Assets recorded
F-22
Table of Contents
AMERICAN RENAL HOLDINGS INC. AND SUBSIDIARIES
Notes to Consolidated Financial Statements—(Continued)
December 31, 2009 and 2008
(dollars in thousands, except per share amounts)
NOTE K—LEASES—(Continued)
under capital leases totaled $2,804 and $2,822, with accumulated amortization of $1,811 and $1,679 at December 31, 2009 and 2008, respectively. The cost and accumulated amortization of the equipment are included in property and equipment. Amortization expense for these assets is included in depreciation and amortization expense in the consolidated statements of operations.
The future minimum commitments due under capital leases, as of December 31, 2009 are as follows for the years ending December 31:
2010 | $ | 358 | ||
2011 | 59 | |||
2012 | 59 | |||
2013 | 59 | |||
2014 | 59 | |||
Thereafter | 5 | |||
Total minimum lease payments | 599 | |||
Less portion representing interest | (51 | ) | ||
Present value of minimum lease payments | 548 | |||
Less amount due in one year | (334 | ) | ||
Long-term capital lease obligations | $ | 214 | ||
NOTE L—INCOME TAXES
The provision for income taxes consisted of the following:
| 2009 | 2008 | 2007 | ||||||||||
Current: | ||||||||||||
Federal | $ | 7,705 | $ | 4,372 | $ | 2,299 | ||||||
State | 1,926 | 1,371 | 1,234 | |||||||||
| 9,631 | 5,743 | 3,533 | ||||||||||
Deferred: | ||||||||||||
Federal | (22 | ) | 1,019 | 1,108 | ||||||||
State | (85 | ) | 98 | (232 | ) | |||||||
| (107 | ) | 1,117 | 876 | |||||||||
Total provision for income taxes | $ | 9,524 | $ | 6,860 | $ | 4,409 | ||||||
F-23
Table of Contents
AMERICAN RENAL HOLDINGS INC. AND SUBSIDIARIES
Notes to Consolidated Financial Statements—(Continued)
December 31, 2009 and 2008
(dollars in thousands, except per share amounts)
NOTE L—INCOME TAXES—(Continued)
The significant components of the Company’s deferred tax assets and liabilities are as follows at December 31:
| 2009 | 2008 | |||||||
Deferred tax assets: | ||||||||
Bad debt reserve | $ | 1,582 | $ | 1,075 | ||||
Stock-based compensation | 1,883 | 1,437 | ||||||
Accrued expenses | 168 | 251 | ||||||
Lease | 657 | 429 | ||||||
Net operating loss carryforwards | — | 19 | ||||||
Other | 555 | 592 | ||||||
Total deferred tax assets | 4,845 | 3,803 | ||||||
Deferred tax liabilities: | ||||||||
Goodwill amortization | (2,716 | ) | (2,090 | ) | ||||
Depreciation | (1,748 | ) | (1,523 | ) | ||||
Other | (84 | ) | — | |||||
Total deferred tax liabilities | (4,548 | ) | (3,613 | ) | ||||
Net deferred tax asset | $ | 297 | $ | 190 | ||||
The income tax expense included in the accompanying consolidated statements of income principally relates to the Company’s proportionate share of the pre-tax income of its majority-owned subsidiaries. A reconciliation of the federal statutory rate to the Company’s effective tax rate is as follows for the years ended December 31:
| 2009 | 2008 | 2007 | ||||||||||
Income tax provision at federal statutory rate | 35.0 | % | 35.0 | % | 35.0 | % | ||||||
Increase (decrease) in tax resulting from: | ||||||||||||
State taxes, net of federal benefit | 4.2 | 3.5 | 4.1 | |||||||||
Noncontrolling interests | (21.3 | ) | (21.9 | ) | (25.1 | ) | ||||||
Nondeductible Series X interest expense | 7.6 | 9.2 | 11.1 | |||||||||
Other | 0.2 | (1.0 | ) | (2.9 | ) | |||||||
Effective income tax rate | 25.7 | % | 24.8 | % | 22.2 | % | ||||||
The Company and its subsidiaries file U.S. federal income tax returns and various state returns. The Company is no longer subject to U.S. federal, state and local examinations by tax authorities for years before 2006.
As of January 1, 2007 (date of adoption) the Company did not have any uncertain tax positions. In 2009, the Company recognized a liability for uncertain tax positions totaling $470 attributable to a bad debt deduction taken. This liability is expected to be paid in the next year. The resolution of this uncertain tax position will have no impact on the provision for income taxes.
F-24
Table of Contents
AMERICAN RENAL HOLDINGS INC. AND SUBSIDIARIES
Notes to Consolidated Financial Statements—(Continued)
December 31, 2009 and 2008
(dollars in thousands, except per share amounts)
NOTE M—SERIES X REDEEMABLE PREFERRED STOCK
As part of the December 16, 2005 recapitalization transaction, certain investors, including holders of Series A and Series B convertible preferred stock, purchased 40,500 shares of Series X redeemable preferred stock at $1,000 per share. Series X redeemable preferred stock accrues dividends at a rate of 12% per annum, compounding quarterly. Accrued dividends on Series X redeemable preferred stock were $24,726 and $17,478 at December 31, 2009 and 2008, respectively. Dividends recorded during 2009, 2008 and 2007 were $7,248, $6,447 and $5,715, respectively, and are included in interest expense. Series X redeemable preferred stock is redeemable for cash at the earlier occurrence of the Company’s initial public offering, liquidation event, as defined, or December 16, 2012, equal to the face value of the stock plus accrued and unpaid dividends. The Series X redeemable preferred stock is classified as long-term debt in the accompanying consolidated balance sheets. In connection with the recapitalization, the purchasers of the Series X redeemable preferred stock received 3,844,000 detachable common stock warrants which have an exercise price of $.005 per share. These warrants are valued at $1.50 per share ($5,747 in the aggregate), and included in additional paid-in capital in the accompanying consolidated balance sheets. Through December 31, 2009, none of these warrants have been exercised.
The allocation of proceeds to the warrants resulted in an original issue discount on the Series X redeemable preferred stock, which is being amortized through December 2012. Amortization of $821 was recorded in interest expense for each of the years ended December 31, 2009, 2008 and 2007.
The carrying value of the Series X redeemable preferred stock consisted of the following at December 31, 2009 and 2008:
| 2009 | 2008 | |||||||
Series X preferred stock, $.001 par value, 43,000 shares authorized; 40,500 shares issued and outstanding at December 31, 2009 and 2008 | $ | 40,500 | $ | 40,500 | ||||
Accrued dividends | 24,726 | 17,478 | ||||||
| 65,226 | 57,978 | |||||||
Original issue discount, net of amortization | (2,427 | ) | (3,248 | ) | ||||
| $ | 62,799 | $ | 54,730 | |||||
NOTE N—PREFERRED AND COMMON STOCK
Series A Convertible Preferred Stock
The Series A convertible preferred stock is initially convertible on a 2:1 basis into shares of the Company’s common stock. The Series A convertible preferred stock has a liquidation preference equal to $3.4625 per share plus any accrued and unpaid dividends (the Series A Liquidation Value), and ranks junior in priority to the Series X redeemable preferred stock and senior in priority to all other equity securities of the Company. Each share of Series A convertible preferred stock is convertible into the Company’s common stock at any time at the option of the holder thereof, and is automatically converted into the Company’s common stock upon a qualified initial public offering. The Series A convertible preferred stock accrues cumulative dividends at a rate of 10% per year compounded annually on the then-effective Series A Liquidation Value. Dividends will be payable quarterly in arrears, when, as, and if declared by the Board. The amount of undeclared dividends for the years ended December 31, 2009 and 2008 was $6,757 and $6,142, respectively, and as of December 31, 2009 cumulative undeclared dividends were $23,770.
F-25
Table of Contents
AMERICAN RENAL HOLDINGS INC. AND SUBSIDIARIES
Notes to Consolidated Financial Statements—(Continued)
December 31, 2009 and 2008
(dollars in thousands, except per share amounts)
NOTE N—PREFERRED AND COMMON STOCK—(Continued)
Series B Convertible Preferred Stock
The Series B convertible preferred stock is initially convertible on a 2:1 basis into shares of the Company’s common stock. The Series B convertible preferred stock has a liquidation preference equal to $3.4625 per share (the Series B Liquidation Value) and ranks junior in priority to the Series X redeemable preferred stock and the Series A convertible preferred stock and senior in priority to all other equity securities of the Company. Each share of Series B convertible preferred stock is convertible into the Company’s common stock at any time at the option of the holder thereof, and shall be automatically converted into the Company’s common stock upon a qualified initial public offering. The Series B convertible preferred stock does not accumulate dividends.
Common Stock
Determining the fair value of the Company’s common stock requires making complex and subjective judgments. The Company hired a third-party valuation firm to assist with the valuation of the common stock. The Company’s approach to valuation is based on discounted future cash flow that uses estimates of revenue, driven by assumed market quote rates and estimated costs, as well as appropriate discount rates. The Company also considers market multiples of Earnings Before Income Tax, Depreciation and Amortization (EBITDA) and third-party transactions in determining fair value.
The estimated enterprise fair value is allocated to preferred and common shares using the option-pricing method. The option-pricing method involves making estimates of the anticipated timing of a potential liquidity event, such as a sale of the Company or an initial public offering, and estimates of the volatility of the equity securities. The Company estimates volatility based on available information on volatility of stocks of publicly traded companies in its industry.
Stock Option Plans
The Company adopted the American Renal Associates, Inc. 2000 Stock Option Plan (the 2000 Plan), under which 4,000,000 shares of the Company’s common stock were reserved for issuance to employees, directors, and consultants. Options granted under the 2000 Plan may be incentive stock options or nonstatutory stock options. Stock purchase rights may also be granted under the 2000 Plan. The Board of Directors determines the period over which options become exercisable; however, except in the case of options granted to officers, directors, and consultants, options generally become exercisable at a rate of not less than 25% per year over four years from the date the options are granted. The exercise price of incentive stock options and nonstatutory stock options shall be no less than 100% and 85%, respectively, of the fair market value per share of the Company’s common stock on the grant date. The term of the options is ten years.
On December 16, 2005, the Company established the American Renal Holdings Inc. 2005 Equity Incentive Plan (the 2005 Plan), under which 3,659,800 shares of the Company’s common stock were reserved for issuance to employees, directors, and consultants. Options granted under the 2005 Plan may be incentive stock options or nonstatutory stock options. The Board of Directors determines the period over which options become exercisable; however, except in the case of options granted to officers, directors, and consultants, options generally become exercisable at a rate of not less than 25% per year over four years from the date the options are granted. The exercise price of incentive stock options and nonstatutory stock options shall be no less than 100% and 85%, respectively, of the fair market value per share of the Company’s common stock on the grant date. The term of the options is 10 years.
F-26
Table of Contents
AMERICAN RENAL HOLDINGS INC. AND SUBSIDIARIES
Notes to Consolidated Financial Statements—(Continued)
December 31, 2009 and 2008
(dollars in thousands, except per share amounts)
��
NOTE N—PREFERRED AND COMMON STOCK—(Continued)
The following tables summarize the activity of the Company’s stock option plans:
| Number of Options | Weighted- Average Exercise Price per Share | |||||||
2000 Stock Option Plan | ||||||||
Outstanding at December 31, 2007 | 3,530,000 | $ | 0.49 | |||||
Exercised | (592,500 | ) | 0.29 | |||||
Canceled | (65,000 | ) | 0.77 | |||||
Outstanding at December 31, 2008 | 2,872,500 | 0.52 | ||||||
Exercised | (265,000 | ) | 0.32 | |||||
Canceled | (40,000 | ) | 0.48 | |||||
Outstanding at December 31, 2009 | 2,567,500 | $ | 0.54 | |||||
Vested and exercisable at December 31, 2009 | 2,532,500 | $ | 0.54 | |||||
Vested and exercisable at December 31, 2008 | 2,621,875 | $ | 0.45 | |||||
In 2009, the aggregate intrinsic value of 2000 Plan stock awards exercised was $1,304. In 2008, the aggregate intrinsic value of 2000 Plan stock awards exercised was $2,904. At December 31, 2009, the aggregate intrinsic value of 2000 Plan stock awards outstanding was $12,067, and the aggregate intrinsic value of exercisable 2000 Plan stock awards was $11,903.
| Number of Options | Weighted- Average Exercise Price per Share | |||||||
2005 Stock Option Plan | ||||||||
Outstanding at December 31, 2007 | 2,734,550 | $ | 3.52 | |||||
Granted | 192,500 | 3.99 | ||||||
Canceled | (48,750 | ) | 3.87 | |||||
Outstanding at December 31, 2008 | 2,878,300 | 3.55 | ||||||
Granted | 707,500 | 5.04 | ||||||
Exercised | (2,500 | ) | 4.19 | |||||
Canceled | (140,000 | ) | 4.07 | |||||
Outstanding at December 31, 2009 | 3,443,300 | $ | 3.83 | |||||
Vested and exercisable at December 31, 2009 | 2,359,925 | $ | 3.54 | |||||
Vested and exercisable at December 31, 2008 | 1,515,325 | $ | 3.51 | |||||
In 2009, the aggregate intrinsic value of 2005 Plan stock awards exercised was $2,888. At December 31, 2009, the aggregate intrinsic value of 2005 Plan stock awards outstanding was $4,855 and the aggregate intrinsic value of exercisable 2005 Plan stock awards was $4,083.
F-27
Table of Contents
AMERICAN RENAL HOLDINGS INC. AND SUBSIDIARIES
Notes to Consolidated Financial Statements—(Continued)
December 31, 2009 and 2008
(dollars in thousands, except per share amounts)
NOTE N—PREFERRED AND COMMON STOCK—(Continued)
The aggregate intrinsic values disclosed herein represent the total pretax intrinsic value (the difference between the estimated per share fair value of the Company’s common stock on December 31, 2009, and the stock option exercise price, multiplied by the number of in-the-money stock options) that would have been received by the stock option holders had all stock option holders exercised their stock options on December 31, 2009. The amount of aggregate intrinsic value will change based on the fair market value of the Company’s common stock.
The following table summarizes information relating to current outstanding and exercisable options under all plans as of December 31, 2009:
Exercise Price | Weighted- Average Remaining Contractual Life (in Years) | Number of Options Outstanding | Number of Options Vested and Exercisable | |||||||||
$0.14 | 3 | 1,530,000 | 1,530,000 | |||||||||
$0.48 | 5 | 372,500 | 372,500 | |||||||||
$1.50 | 6 | 665,000 | 630,000 | |||||||||
$3.50 | 7 | 2,505,550 | 2,216,800 | |||||||||
$3.77 | 8 | 197,500 | 81,250 | |||||||||
$4.26 | 8 | 75,000 | 16,875 | |||||||||
$4.72 | 9 | 215,000 | 35,625 | |||||||||
$5.20 | 9 | 147,500 | 1,875 | |||||||||
$5.24 | 9 | 302,500 | 7,500 | |||||||||
| 6,010,550 | 4,892,425 | |||||||||||
Stock-Based Compensation
In 2009, 2008 and 2007, the Company recorded an aggregate of $1,135, $1,482 and $2,180 in stock-based compensation expense ($738, $955 and $1,326 net of taxes).
Weighted-average assumptions used to estimate fair values of stock options on the dates of grant are as follows:
| 2009 | December 31 2008 | 2007 | ||||||||||
Risk-free interest rates | 2.7 | % | 4.9 | % | 4.9 | % | ||||||
Expected dividend yield | 0 | % | 0 | % | 0 | % | ||||||
Expected term of option | 7 years | 7 years | 7 years | |||||||||
Expected volatility | 39 | % | 38 | % | 38 | % | ||||||
Weighted-average grant date fair value per share of options granted | $ | 1.42 | $ | 1.57 | $ | 1.28 | ||||||
Expected volatility is based on comparable companies’ volatility over the expected term. Expected life of an option is based on the Company’s historical experience of stock options. The risk-free interest rate represents the
F-28
Table of Contents
AMERICAN RENAL HOLDINGS INC. AND SUBSIDIARIES
Notes to Consolidated Financial Statements—(Continued)
December 31, 2009 and 2008
(dollars in thousands, except per share amounts)
NOTE N—PREFERRED AND COMMON STOCK—(Continued)
implied yields available from the U.S. Treasury zero-coupon yield curve. Expected dividend yield is 0% because the Company has not paid dividends in the past, and currently has no known intention to do so in the future. Compensation expense is recognized net of forfeitures.
Based on equity awards outstanding as of December 31, 2009, there was $743 of unrecognized compensation costs, net of forfeitures, related to unvested share-based compensation arrangements that are expected to vest. Such costs are expected to be recognized over a weighted-average period of three years. The following table summarizes the estimated future annual stock-based compensation expense related to share-based arrangements, existing as of December 31, 2009, that are expected to vest:
2010 | $ | 480 | ||
2011 | 190 | |||
2012 | 61 | |||
2014 | 12 | |||
Total | $ | 743 | ||
NOTE O—RELATED PARTY TRANSACTIONS
The Company has lease agreements for dialysis clinics with noncontrolling interest members or entities under the control of noncontrolling interest members. The amount of rent expense under these lease arrangements was approximately $2,468, $1,500 and $1,114 in 2009, 2008 and 2007, respectively. In addition, in 2008, the Company sub-leased space at one of its dialysis clinics to the noncontrolling interest member. Rent income under this sub-lease arrangement, which extends to 2023, amounted to $510 and $510 in 2009 and 2008, respectively. Future rental receipts of $7,258 due from this related party are included in sub-lease receipts as presented in Note K.
During 2008, 185,000 shares of restricted common stock were sold to the Company’s executives at a per share price equal to the fair value of the Company’s common stock on the date of issuance. All shares were unvested as of December 31, 2008. The purchases were financed through secured promissory notes with the Company in the amount of $697, which accrue interest at a rate of 3% per annum. The notes are full recourse and become due and payable March 4, 2011.
NOTE P—COMMITMENTS AND CONTINGENCIES
Healthcare provider revenues may be subject to adjustment as a result of (i) examination by government agencies or contractors, for which the resolution of any matters raised may take extended periods of time to finalize; (ii) differing interpretations of government regulations by different fiscal intermediaries or regulatory authorities; (iii) differing opinions regarding a patient’s medical diagnosis or the medical necessity of service provided; (iv) retroactive applications or interpretations of governmental requirements; and (v) claims for refund from private payors, including as the result of government actions. Management believes it has recorded adequate provisions to consider potential revenue adjustments.
The Company had obligations under contracts related to the construction of its clinics totaling approximately $1,102 as of December 31, 2009 which are expected to be paid in 2010.
F-29
Table of Contents
AMERICAN RENAL HOLDINGS INC. AND SUBSIDIARIES
Notes to Consolidated Financial Statements—(Continued)
December 31, 2009 and 2008
(dollars in thousands, except per share amounts)
NOTE P—COMMITMENTS AND CONTINGENCIES—(Continued)
Professional Liability Coverage
The Company maintains professional liability insurance coverage on a claims-made basis. Under this type of policy, claims based on occurrences during its term, but reported subsequently, will be uninsured should the policy not be renewed or replaced with other coverage. Management expects to be able to obtain renewal or other coverage in future periods, and has accrued the estimated cost associated with coverage of past occurrences reported in subsequent periods.
Litigation
The Company is a defendant in various legal actions in the normal course of business. In the opinion of the Company’s management, based in part on the advice of outside counsel, the resolution of these matters will not have a material effect on the Company’s financial position, results of operations or cash flow.
Regulatory
The healthcare industry is subject to numerous laws and regulations of federal, state, and local governments. Recently, government activity has increased with respect to investigations and allegations concerning possible violations by healthcare providers of fraud and abuse statutes and regulations, which could result in the imposition of significant fines and penalties, as well as significant repayments for patient services previously billed. Compliance with such laws and regulations are subject to government review and interpretations, as well as regulatory actions unknown or unasserted at this time.
NOTE Q—SUPPLEMENTAL INFORMATION
The following information is presented in accordance with Rule 3-10 of Regulation S-X. The operating and investing activities of the separate legal entities included in the Company’s consolidated financial statements are fully interdependent and integrated. Revenues and operating expenses of the separate legal entities include the Company and were guaranteed by substantially all of its wholly-owned subsidiaries. Non-wholly-owned subsidiaries were not guarantors of these obligations.
F-30
Table of Contents
AMERICAN RENAL HOLDINGS INC. AND SUBSIDIARIES
Notes to Consolidated Financial Statements—(Continued)
December 31, 2009 and 2008
(amounts in thousands, except per share amounts)
NOTE Q—SUPPLEMENTAL INFORMATION—(Continued)
Condensed Consolidating Balance Sheet—December 31, 2009
| ARH | Subsidiary Guarantors | Non- Guarantors | Eliminations | Predecessor Consolidated | ||||||||||||||||
Assets | ||||||||||||||||||||
Cash and cash equivalents | $ | — | $ | 11,423 | $ | 17,756 | $ | — | $ | 29,179 | ||||||||||
Accounts receivable | — | 215 | 45,439 | — | 45,654 | |||||||||||||||
Inventories | — | 22 | 2,272 | — | 2,294 | |||||||||||||||
Prepaid expenses and other current assets | — | 2,313 | 3,107 | (3,054 | ) | 2,366 | ||||||||||||||
Deferred tax assets | 4,845 | — | — | — | 4,845 | |||||||||||||||
Total current assets | 4,845 | 13,973 | 68,574 | (3,054 | ) | 84,338 | ||||||||||||||
Property and equipment, net | — | 2,054 | 57,351 | — | 59,405 | |||||||||||||||
Deferred financing costs, net | — | 1,000 | 44 | — | 1,044 | |||||||||||||||
Intangible assets, net | — | 363 | 1,055 | — | 1,418 | |||||||||||||||
Other long-term assets | — | 5,161 | 3,251 | (5,093 | ) | 3,319 | ||||||||||||||
Receivables from subsidiaries | — | 12,267 | — | (12,267 | ) | — | ||||||||||||||
Investment in subsidiaries | 38,021 | — | — | (38,021 | ) | — | ||||||||||||||
Goodwill, net | (13,349 | ) | — | 32,973 | 4,574 | 24,198 | ||||||||||||||
Total assets | $ | 29,517 | $ | 34,818 | $ | 163,248 | $ | (53,861 | ) | $ | 173,722 | |||||||||
Liabilities and Deficit | ||||||||||||||||||||
Current liabilities | $ | — | $ | 7,095 | $ | 63,814 | $ | (15,292 | ) | $ | 55,617 | |||||||||
Long-term debt, less current portion | — | 40,185 | 29,169 | (5,093 | ) | 64,261 | ||||||||||||||
Capital lease obligations, less current portion | — | — | 214 | — | 214 | |||||||||||||||
Other long-term liabilities | — | 137 | 7,527 | — | 7,664 | |||||||||||||||
Deferred tax liability | 4,548 | — | — | — | 4,548 | |||||||||||||||
Series X preferred stock | 62,799 | — | — | — | 62,799 | |||||||||||||||
Noncontrolling interests subject to put provisions | — | 750 | (99,184 | ) | 136,865 | 38,431 | ||||||||||||||
Total American Renal Holdings Inc. equity (deficit) | (37,830 | ) | (13,349 | ) | 145,651 | (170,341 | ) | (75,869 | ) | |||||||||||
Noncontrolling interests not subject to put provisions | — | — | 16,057 | — | 16,057 | |||||||||||||||
Total equity (deficit) | (37,830 | ) | (13,349 | ) | 161,708 | (170,341 | ) | (59,812 | ) | |||||||||||
Total liabilities and equity (deficit) | $ | 29,517 | $ | 34,818 | $ | 163,248 | $ | (53,861 | ) | $ | 173,722 | |||||||||
F-31
Table of Contents
AMERICAN RENAL HOLDINGS INC. AND SUBSIDIARIES
Notes to Consolidated Financial Statements—(Continued)
December 31, 2009 and 2008
(amounts in thousands, except per share amounts)
NOTE Q—SUPPLEMENTAL INFORMATION—(Continued)
Condensed Consolidating Balance Sheet—December 31, 2008
| ARH | Subsidiary Guarantors | Non- Guarantors | Eliminations | Consolidated | ||||||||||||||||
Assets | ||||||||||||||||||||
Cash and cash equivalents | $ | — | $ | 11,869 | $ | 17,313 | $ | — | $ | 29,182 | ||||||||||
Accounts receivable, net | — | 226 | 38,753 | — | 38,979 | |||||||||||||||
Inventories | — | 19 | 1,959 | — | 1,978 | |||||||||||||||
Prepaid expenses and other current assets | — | 3,413 | 3,735 | (1,118 | ) | 6,030 | ||||||||||||||
Deferred tax asset | 3,803 | — | — | — | 3,803 | |||||||||||||||
Total current assets | 3,803 | 15,527 | 61,760 | (1,118 | ) | 79,972 | ||||||||||||||
Property and equipment, net | — | 2,338 | 52,481 | — | 54,819 | |||||||||||||||
Deferred financing costs, net | — | 1,825 | 53 | — | 1,878 | |||||||||||||||
Amortizable intangible assets, net | — | 435 | 621 | — | 1,056 | |||||||||||||||
Other long-term assets | — | 89 | 2,794 | — | 2,883 | |||||||||||||||
Receivables from subsidiaries | — | 11,060 | — | (11,060 | ) | — | ||||||||||||||
Investment in subsidiaries | 29,152 | — | (29,152 | ) | — | |||||||||||||||
Goodwill, net | (17,822 | ) | — | 24,537 | 9,047 | 15,762 | ||||||||||||||
Total assets | $ | 15,133 | $ | 31,274 | $ | 142,246 | $ | (32,283 | ) | $ | 156,370 | |||||||||
Liabilities and Deficit | ||||||||||||||||||||
Current liabilities: | $ | — | $ | 5,757 | $ | 54,715 | $ | (12,147 | ) | $ | 48,325 | |||||||||
Long-term debt, less current portion | — | 42,584 | 33,553 | — | 76,137 | |||||||||||||||
Capital lease obligations, less current portion | — | — | 293 | — | 293 | |||||||||||||||
Other long-term liabilities | — | 4 | 7,566 | — | 7,570 | |||||||||||||||
Deferred tax liability | 3,613 | — | — | — | 3,613 | |||||||||||||||
Series X preferred stock | 54,730 | — | — | — | 54,730 | |||||||||||||||
Noncontrolling interests subject to put provisions | — | 750 | (60,030 | ) | 94,161 | 34,881 | ||||||||||||||
Total ARH deficit | (43,210 | ) | (17,822 | ) | 98,308 | (114,296 | ) | (77,020 | ) | |||||||||||
Noncontrolling interests not subject to put provisions | — | — | 7,841 | — | 7,841 | |||||||||||||||
Total deficit | (43,210 | ) | (17,822 | ) | 106,149 | (114,296 | ) | (69,179 | ) | |||||||||||
Total liabilities & deficit | $ | 15,133 | $ | 31,273 | $ | 142,246 | $ | (32,282 | ) | $ | 156,370 | |||||||||
F-32
Table of Contents
AMERICAN RENAL HOLDINGS INC. AND SUBSIDIARIES
Notes to Consolidated Financial Statements—(Continued)
December 31, 2009 and 2008
(amounts in thousands, except per share amounts)
NOTE Q—SUPPLEMENTAL INFORMATION—(Continued)
Condensed Consolidating Statement of Operations—Year Ended December 31, 2009
| ARH | Subsidiary Guarantors | Non- Guarantors | Eliminations | Consolidated | ||||||||||||||||
Net operating revenues | $ | — | $ | 1,489 | $ | 261,500 | $ | — | $ | 262,989 | ||||||||||
Total operating expenses | — | 5,760 | 205,228 | 210,988 | ||||||||||||||||
Operating income | (4,271 | ) | 56,272 | — | 52,001 | |||||||||||||||
Interest expense, net | (11,218 | ) | (36 | ) | (3,694 | ) | — | (14,948 | ) | |||||||||||
Income before taxes | (11,218 | ) | (4,307 | ) | 52,578 | — | 37,053 | |||||||||||||
Income tax expense | 8,521 | — | 1,003 | — | 9,524 | |||||||||||||||
Net income (loss) before earnings in subsidiaries | (19,739 | ) | (4,307 | ) | 51,575 | — | 27,529 | |||||||||||||
Equity earnings in subsidiaries | 24,875 | 29,184 | — | (54,059 | ) | — | ||||||||||||||
Net income | 5,136 | 24,877 | 51,575 | (54,059 | ) | 27,529 | ||||||||||||||
Less: Net income attributable to noncontrolling interests | — | — | — | (22,391 | ) | (22,391 | ) | |||||||||||||
Net income attributable to American Renal Holdings Inc. | $ | 5,136 | $ | 24,877 | $ | 51,575 | $ | (76,450 | ) | $ | 5,138 | |||||||||
Condensed Consolidating Statement of Operations—Year Ended December 31, 2008
| ARH | Subsidiary Guarantors | Non- Guarantors | Eliminations | Consolidated | ||||||||||||||||
Net operating revenues | $ | — | $ | 1,486 | $ | 216,291 | $ | — | $ | 217,777 | ||||||||||
Total operating expenses | — | 16,120 | 160,245 | — | 176,365 | |||||||||||||||
Operating income | — | (14,634 | ) | 56,046 | — | 41,412 | ||||||||||||||
Interest expense, net | (10,607 | ) | (38 | ) | (3,084 | ) | — | (13,729 | ) | |||||||||||
Income before taxes | (10,607 | ) | (14,672 | ) | 52,962 | — | 27,683 | |||||||||||||
Income tax expense | 6,284 | — | 576 | — | 6,860 | |||||||||||||||
Net income (loss) before earnings in subsidiaries | (16,891 | ) | (14,672 | ) | 52,386 | — | 20,823 | |||||||||||||
Equity earnings in subsidiaries | 20,535 | 35,207 | — | (55,742 | ) | — | ||||||||||||||
Net income . | 3,644 | 20,535 | 52,386 | (55,742 | ) | 20,823 | ||||||||||||||
Less: Net income attributable to noncontrolling interests | — | — | — | (17,179 | ) | (17,179 | ) | |||||||||||||
Net income attributable to American Renal Holdings Inc. | $ | 3,644 | $ | 20,535 | $ | 52,386 | $ | (72,921 | ) | $ | 3,644 | |||||||||
F-33
Table of Contents
AMERICAN RENAL HOLDINGS INC. AND SUBSIDIARIES
Notes to Consolidated Financial Statements—(Continued)
December 31, 2009 and 2008
(amounts in thousands, except per share amounts)
NOTE Q—SUPPLEMENTAL INFORMATION—(Continued)
Condensed Consolidating Statement of Operations—Year Ended December 31, 2007
| ARH | Subsidiary Guarantors | Non- Guarantors | Eliminations | Consolidated | ||||||||||||||||
Net operating revenues | $ | — | $ | 249 | $ | 178,142 | $ | — | $ | 178,391 | ||||||||||
Total operating expenses | — | 13,528 | 131,302 | — | 144,830 | |||||||||||||||
Operating income | — | (13,279 | ) | 46,840 | — | 33,561 | ||||||||||||||
Interest expense, net | (11,012 | ) | (42 | ) | (2,641 | ) | — | (13,695 | ) | |||||||||||
Income before taxes | (11,012 | ) | (13,321 | ) | 44,199 | — | 19,866 | |||||||||||||
Income tax expense | 3,922 | — | 487 | — | 4,409 | |||||||||||||||
Net income (loss) before earnings in subsidiaries | (14,934 | ) | (13,321 | ) | 43,712 | — | 15,457 | |||||||||||||
Equity earnings in subsidiaries | 15,684 | 29,006 | — | (44,690 | ) | — | ||||||||||||||
Net income | 750 | 15,685 | 43,712 | (44,690 | ) | 15,457 | ||||||||||||||
Less: Net income attributable to noncontrolling interests | — | — | — | (14,706 | ) | (14,706 | ) | |||||||||||||
Net income attributable to American Renal Holdings Inc. | $ | 750 | $ | 15,685 | $ | 43,712 | $ | (59,396 | ) | $ | 751 | |||||||||
F-34
Table of Contents
AMERICAN RENAL HOLDINGS INC. AND SUBSIDIARIES
Notes to Consolidated Financial Statements—(Continued)
December 31, 2009 and 2008
(amounts in thousands, except per share amounts)
NOTE Q—SUPPLEMENTAL INFORMATION—(Continued)
Condensed Consolidated Statement of Cash Flows—Year Ended December 31, 2009
| ARH | Subsidiary Guarantors | Non- Guarantors | Eliminations | Consolidated | ||||||||||||||||
Operating activities | ||||||||||||||||||||
Net income | $ | 5,137 | $ | 24,875 | $ | 51,576 | $ | (54,059 | ) | $ | 27,529 | |||||||||
Change in operating assets and liabilities and noncash items included in net income | (15,800 | ) | (924 | ) | 9,383 | 31,143 | 23,802 | |||||||||||||
Net cash provided by (used in) operating activities | (10,663 | ) | 23,951 | 60,959 | (22,916 | ) | 51,331 | |||||||||||||
Investing Activities | ||||||||||||||||||||
Other | — | — | — | — | — | |||||||||||||||
Purchases of property and equipment | — | (68 | ) | (14,999 | ) | — | (15,067 | ) | ||||||||||||
Cash paid for acquisitions | — | — | (3,964 | ) | — | (3,964 | ) | |||||||||||||
Net cash provided by (used in) investing activities | — | (68 | ) | (18,963 | ) | — | (19,031 | ) | ||||||||||||
Financing Activities | ||||||||||||||||||||
Proceeds from borrowings | — | (323 | ) | 1,507 | — | 1,184 | ||||||||||||||
Payments on long-term debt obligations | — | (2,398 | ) | (9,603 | ) | — | (12,001 | ) | ||||||||||||
Other | 10,663 | (21,610 | ) | (33,455 | ) | 22,916 | (21,486 | ) | ||||||||||||
Net cash provided by (used in) financing activities | 10,663 | (24,331 | ) | (41,551 | ) | 22,916 | (32,303 | ) | ||||||||||||
Net increase (decrease) in cash | — | (448 | ) | 445 | — | (3 | ) | |||||||||||||
Cash, at beginning of year | — | 11,869 | 17,313 | — | 29,182 | |||||||||||||||
Cash, at ending of year | $ | — | $ | 11,421 | $ | 17,758 | $ | — | $ | 29,179 | ||||||||||
F-35
Table of Contents
AMERICAN RENAL HOLDINGS INC. AND SUBSIDIARIES
Notes to Consolidated Financial Statements—(Continued)
December 31, 2009 and 2008
(amounts in thousands, except per share amounts)
NOTE Q—SUPPLEMENTAL INFORMATION—(Continued)
Condensed Consolidated Statement of Cash Flows—Year Ended December 31, 2008
| ARH | Subsidiary Guarantors | Non- Guarantors | Eliminations | Consolidated | ||||||||||||||||
Operating activities | ||||||||||||||||||||
Net income | $ | 3,643 | $ | 20,535 | $ | 52,387 | $ | (55,742 | ) | $ | 20,823 | |||||||||
Change in operating assets and liabilities and noncash items included in net income | (25,593 | ) | 2,109 | (3,694 | ) | 43,367 | 16,189 | |||||||||||||
Net cash provided by (used in) operating activities | (21,950 | ) | 22,644 | 48,693 | (12,375 | ) | 37,012 | |||||||||||||
Investing Activities | ||||||||||||||||||||
Other | — | (12,375 | ) | 507 | 12,375 | 507 | ||||||||||||||
Purchases of property and equipment | — | (318 | ) | (20,936 | ) | — | (21,254 | ) | ||||||||||||
Cash paid for acquisitions | — | — | — | — | — | |||||||||||||||
Net cash provided by (used in) investing activities | — | (12,693 | ) | (20,429 | ) | 12,375 | (20,747 | ) | ||||||||||||
Financing Activities | ||||||||||||||||||||
Proceeds from borrowings | — | 14 | 17,349 | — | 17,363 | |||||||||||||||
Payments on long-term debt obligations | — | (1,802 | ) | (6,150 | ) | — | (7,952 | ) | ||||||||||||
Other | 21,950 | (3,927 | ) | (37,076 | ) | — | (19,053 | ) | ||||||||||||
Net cash provided by (used in) financing activities | 21,950 | (5,715 | ) | (25,877 | ) | — | (9,642 | ) | ||||||||||||
Net increase (decrease) in cash | — | 4,236 | 2,387 | — | 6,623 | |||||||||||||||
Cash, at beginning of year | — | 7,634 | 14,925 | — | 22,559 | |||||||||||||||
Cash, at ending of year | $ | — | $ | 11,870 | $ | 17,312 | $ | — | $ | 29,182 | ||||||||||
F-36
Table of Contents
AMERICAN RENAL HOLDINGS INC. AND SUBSIDIARIES
Notes to Consolidated Financial Statements—(Continued)
December 31, 2009 and 2008
(amounts in thousands, except per share amounts)
NOTE Q—SUPPLEMENTAL INFORMATION—(Continued)
Condensed Consolidated Statement of Cash Flows—Year Ended December 31, 2007
| ARH | Subsidiary Guarantors | Non- Guarantors | Eliminations | Consolidated | ||||||||||||||||
Operating activities | ||||||||||||||||||||
Net income | $ | 750 | $ | 15,684 | $ | 43,712 | $ | (44,689 | ) | $ | 15,457 | |||||||||
Change in operating assets and liabilities, and noncash items included in net income | (19,663 | ) | (8,636 | ) | (3,953 | ) | 45,439 | 13,187 | ||||||||||||
Net cash provided (used) by operating activities | (18,913 | ) | 7,048 | 39,759 | 750 | 28,644 | ||||||||||||||
Investing activities | ||||||||||||||||||||
Other | — | — | — | — | — | |||||||||||||||
Purchases of property and equipment | — | (1,740 | ) | (13,478 | ) | — | (15,218 | ) | ||||||||||||
Cash paid for acquisitions | — | — | (1,995 | ) | — | (1,995 | ) | |||||||||||||
Net cash provided (used) for investing activities | — | (1,740 | ) | (15,473 | ) | — | (17,213 | ) | ||||||||||||
Financing activities | ||||||||||||||||||||
Proceeds from borrowings | — | — | 14,239 | — | 14,239 | |||||||||||||||
Payments on long-term debt obligations | — | (2,396 | ) | (4,630 | ) | — | (7,026 | ) | ||||||||||||
Other | 18,913 | 147 | (34,299 | ) | (750 | ) | (15,989 | ) | ||||||||||||
Net Cash provided (used) in financing activities | 18,913 | (2,249 | ) | (24,690 | ) | (750 | ) | (8,776 | ) | |||||||||||
Net increase (decrease) in cash | — | 3,059 | (404 | ) | — | 2,655 | ||||||||||||||
Cash, at beginning of period | — | 4,576 | 15,328 | — | 19,904 | |||||||||||||||
Cash, at ending of period | $ | — | $ | 7,635 | $ | 14,924 | $ | — | $ | 22,559 | ||||||||||
NOTE R—SUBSEQUENT EVENTS
On March 22, 2010, the Company entered into a Contribution and Merger Agreement (the “Merger Agreement”) with C.P. Atlas Holdings, Inc. (the “Parent”), C.P. Atlas Intermediate Holdings LLC, C.P. Atlas Acquisition Corp., certain of the Company’s stockholders and Pamlico Capital I, LLC (formerly known as Wachovia Capital Partners GP I, LLC), pursuant to which C.P. Atlas Acquisition Corp. merged with and into the Company (the “Merger”) and, after which, American Renal Holdings Inc. (“ARH”) is the surviving entity and a wholly owned subsidiary of C.P. Atlas Intermediate Holdings, LLC, which is in turn a wholly owned subsidiary of the Parent.
C.P. Atlas Intermediate Holdings, LLC is an entity formed in the Merger and prior to the Merger had no assets or liabilities other than the shares of CP Atlas Acquisition Corp. and its rights and obligations under and in connection with the Merger Agreement. As a result of the Merger, all of the Company’s issued and outstanding capital stock is owned by CP Atlas Intermediate Holdings, LLC which is CP Atlas Intermediate Holdings, LLC’s only asset. As such, the consolidated financial statements of CP Atlas Intermediate Holdings, LLC are identical to the Company’s consolidated financial statements.
F-37
Table of Contents
AMERICAN RENAL HOLDINGS INC. AND SUBSIDIARIES
Notes to Consolidated Financial Statements—(Continued)
December 31, 2009 and 2008
(dollars in thousands, except per share amounts)
NOTE R—SUBSEQUENT EVENTS—(Continued)
The parties agreed to consummate the Merger, subject to the terms and conditions set forth in the Merger Agreement, for an aggregate purchase price of $415 million, subject to adjustments for working capital, indebtedness, certain specified liabilities and certain tax savings. The aggregate purchase price of approximately $415 million for the Merger, plus related fees and expenses, was funded by the equity investment by Centerbridge Capital Partners, L.P. and certain affiliated entities (“Centerbridge”) as well as from certain members of management and the net proceeds from the offering of $250 million of Senior Secured Notes (the “Senior Secured Notes) due 2018 which bear interest at 8.375%. The Merger and the financing transaction described above were consummated on May 7, 2010.
F-38
Table of Contents
Schedule II—Valuation and Qualifying Accounts
| (in thousands) | Balance at Beginning of Year | Charged to Expense | Deductions | Balance at End of Year | ||||||||||||
Allowance for Doubtful Accounts | ||||||||||||||||
Year Ended December 31, 2009 | $ | 7,833 | $ | 3,216 | $ | (2,884 | ) | $ | 8,165 | |||||||
Year Ended December 31, 2008 | $ | 7,356 | $ | 4,834 | $ | (4,357 | ) | $ | 7,833 | |||||||
Year Ended December 31, 2007 | $ | 5,037 | $ | 3,258 | $ | (939 | ) | $ | 7,356 | |||||||
F-39
Table of Contents
AMERICAN RENAL HOLDINGS INC. AND SUBSIDIARIES
Consolidated Balance Sheets
(in thousands, except share and per share data)
| Successor | Predecessor | |||||||||||
| September 30, 2010 | December 31, 2009 | |||||||||||
| (Unaudited) | ||||||||||||
Assets | ||||||||||||
Current assets: | ||||||||||||
Cash and cash equivalents | $ | 27,579 | $ | 29,179 | ||||||||
Accounts receivable, less allowance for doubtful accounts of $10,398 and $8,165 at September 30, 2010 and December 31, 2009, respectively | 45,987 | 45,654 | ||||||||||
Inventories | 1,977 | 2,294 | ||||||||||
Prepaid expenses and other current assets | 3,830 | 2,366 | ||||||||||
Income tax receivable | 10,303 | — | ||||||||||
Deferred tax assets | 4,043 | 4,845 | ||||||||||
Total current assets | 93,719 | 84,338 | ||||||||||
Property and equipment, net | 56,631 | 59,405 | ||||||||||
Deferred financing costs, net | 4,993 | 1,044 | ||||||||||
Intangible assets, net | 39,112 | 1,418 | ||||||||||
Deferred tax asset | 3,625 | — | ||||||||||
Other long-term assets | 2,043 | 3,319 | ||||||||||
Goodwill | 503,360 | 24,198 | ||||||||||
Total assets | $ | 703,483 | $ | 173,722 | ||||||||
Liabilities and Equity (Deficit) | ||||||||||||
Current liabilities: | ||||||||||||
Accounts payable | $ | 18,150 | $ | 18,158 | ||||||||
Accrued compensation and benefits | 10,116 | 7,170 | ||||||||||
Accrued expenses and other current liabilities | 19,371 | 15,646 | ||||||||||
Amount due to sellers | 13,928 | — | ||||||||||
Current portion of long-term debt | 2,863 | 14,309 | ||||||||||
Current portion of capital lease obligations | 47 | 334 | ||||||||||
Total current liabilities | 64,475 | 55,617 | ||||||||||
Long-term debt, less current portion | 247,484 | 64,261 | ||||||||||
Capital lease obligations, less current portion | 175 | 214 | ||||||||||
Other long-term liabilities | 2,468 | 7,664 | ||||||||||
Deferred tax liabilities | 16,968 | 4,548 | ||||||||||
Series X mandatorily redeemable preferred stock, 43,000 shares authorized at December 31, 2009; 40,500 shares issued and outstanding at December 31, 2009 | — | 62,799 | ||||||||||
Commitments and contingencies (note J) | ||||||||||||
Noncontrolling interests subject to put provisions | 42,739 | 38,431 | ||||||||||
Equity (Deficit) | ||||||||||||
Series A convertible preferred stock, which accrue dividends at 10%, $.001 par value, 7,300,000 shares authorized, issued and outstanding at December 31, 2009 and no shares issued or outstanding at September 30, 2010 (liquidation value of $74,322 at December 31, 2009) | — | 7 | ||||||||||
Series B convertible preferred stock, $.001 par value, 10,700,000 shares authorized; 2,675,000 shares issued and outstanding at December 31, 2009 and no shares issued or outstanding at September 30, 2010 (liquidation value of $18,524 at December 31, 2009) | — | 3 | ||||||||||
Common stock, $.0005 par value, 39,982,000 shares authorized at December 31, 2009; 1,330,250 shares issued and 1,083,350 shares outstanding at December 31, 2009 and 1,000 shares authorized, issued and outstanding at September 30, 2010 | — | 1 | ||||||||||
Notes receivable from stockholders | — | (735 | ) | |||||||||
Additional paid-in capital | 188,833 | 23,704 | ||||||||||
Accumulated deficit | (14,292 | ) | (97,784 | ) | ||||||||
Treasury stock, at cost, 246,900 common shares held at December 31, 2009 and no shares held at September 30, 2010 | — | (1,065 | ) | |||||||||
Total American Renal Holdings Inc. equity (deficit) | 174,541 | (75,869 | ) | |||||||||
Noncontrolling interests not subject to put provisions | 154,633 | 16,057 | ||||||||||
Total equity (deficit) | 329,174 | (59,812 | ) | |||||||||
Total liabilities and equity (deficit) | $ | 703,483 | $ | 173,722 | ||||||||
See accompanying notes to the unaudited consolidated financial statements.
F-40
Table of Contents
AMERICAN RENAL HOLDINGS INC. AND SUBSIDIARIES
Unaudited Consolidated Statements of Operations
(in thousands)
| Successor | Predecessor | |||||||||||||||||||||||
| Three months Ended September 30, 2010 | May 8 through September 30, 2010 | January 1 through May 7, 2010 | Nine months Ended September 30, 2009 | Three months Ended September 30, 2009 | ||||||||||||||||||||
Net operating revenues | $ | 77,231 | $ | 120,833 | $ | 102,094 | $ | 193,109 | $ | 68,084 | ||||||||||||||
Operating expenses: | ||||||||||||||||||||||||
Patient care costs | 49,321 | 78,335 | 66,042 | 125,098 | 44,061 | |||||||||||||||||||
General and administrative | 8,001 | 14,991 | 10,016 | 17,423 | 6,393 | |||||||||||||||||||
Merger and transaction-related costs | 152 | 14,839 | 7,378 | — | — | |||||||||||||||||||
Depreciation and amortization | 4,085 | 6,434 | 4,429 | 8,816 | 2,814 | |||||||||||||||||||
Provision for (recoveries of) uncollectible accounts | 371 | 1,021 | (334 | ) | 3,184 | 1,016 | ||||||||||||||||||
Total operating expenses | 61,930 | 115,620 | 87,531 | 154,521 | 54,284 | |||||||||||||||||||
Operating income | 15,301 | 5,213 | 14,563 | 38,588 | 13,800 | |||||||||||||||||||
Interest expense, net | (5,885 | ) | (9,205 | ) | (5,717 | ) | (11,212 | ) | (3,755 | ) | ||||||||||||||
Income (loss) before income taxes | 9,416 | (3,992 | ) | 8,846 | 27,376 | 10,045 | ||||||||||||||||||
Income tax expense (benefit) | 977 | (649 | ) | 2,264 | 7,036 | 2,582 | ||||||||||||||||||
Net income (loss) | 8,439 | (3,343 | ) | 6,582 | 20,340 | 7,463 | ||||||||||||||||||
Less: Net income attributable to noncontrolling interests | (6,907 | ) | (10,949 | ) | (9,266 | ) | (15,823 | ) | (5,756 | ) | ||||||||||||||
| �� | ||||||||||||||||||||||||
Net income (loss) attributable to American Renal Holdings Inc. | $ | 1,532 | $ | (14,292 | ) | $ | (2,684 | ) | $ | 4,517 | $ | 1,707 | ||||||||||||
See accompanying notes to the unaudited consolidated financial statements.
F-41
Table of Contents
AMERICAN RENAL HOLDINGS INC. AND SUBSIDIARIES
Unaudited Consolidated Statements of Changes in Equity (Deficit)
For the predecessor period from December 31, 2009 to May 7, 2010 and
for the successor period from May 8, 2010 to September 30, 2010
(in thousands, except share data)
| Non- controlling interests subject to put provisions | Total American Renal Holdings Inc. Equity (Deficit) | Non- controlling interests not subject to put provisions | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Series A Convertible Preferred Stock | Series B Convertible Preferred Stock | Common Stock | Note Receivable from Stock- holders | Treasury Stock | Additional Paid-in Capital | Accumu- lated Deficit | Total | |||||||||||||||||||||||||||||||||||||||||||||||||
| Shares | Amount | Shares | Amount | Shares | Par Value | Shares | Cost | |||||||||||||||||||||||||||||||||||||||||||||||||
Balance at December 31, 2009 | $ | 38,431 | 7,300,000 | $ | 7 | 2,675,000 | $ | 3 | 1,330,250 | $ | 1 | $ | (735 | ) | 246,900 | $ | (1,065 | ) | $ | 23,704 | $ | (97,784 | ) | $ | (75,869 | ) | $ | 16,057 | ||||||||||||||||||||||||||||
Net income (loss) | 1,942 | — | — | — | — | — | — | — | — | — | — | (2,684 | ) | (2,684 | ) | 7,324 | ||||||||||||||||||||||||||||||||||||||||
Issuance of common stock | — | — | — | — | 328,490 | — | — | — | — | 7 | — | 7 | ||||||||||||||||||||||||||||||||||||||||||||
Purchase of common stock for treasury | — | — | — | — | — | — | — | — | — | — | — | — | ||||||||||||||||||||||||||||||||||||||||||||
Tax benefits from stock awards exercised | — | — | — | — | — | — | — | — | — | 9,140 | — | 9,140 | ||||||||||||||||||||||||||||||||||||||||||||
Stock-based compensation expense | — | — | — | — | — | — | — | — | — | 219 | — | 219 | ||||||||||||||||||||||||||||||||||||||||||||
Distributions to noncontrolling interests | (2,487 | ) | — | — | — | — | — | — | — | — | — | — | — | — | (8,907 | ) | ||||||||||||||||||||||||||||||||||||||||
Contributions from noncontrolling interests | 128 | — | — | — | — | — | — | — | — | — | — | — | — | 520 | ||||||||||||||||||||||||||||||||||||||||||
Sales of noncontrolling interests | — | — | — | — | — | — | — | — | — | — | (250 | ) | — | (250 | ) | 626 | ||||||||||||||||||||||||||||||||||||||||
Purchases of noncontrolling interests | (1,183 | ) | — | — | — | — | — | — | — | — | — | — | — | — | — | |||||||||||||||||||||||||||||||||||||||||
Changes in fair value of noncontrolling interests | 5,386 | — | — | — | — | — | — | — | — | — | (5,386 | ) | — | (5,386 | ) | — | ||||||||||||||||||||||||||||||||||||||||
Balance at May 7, 2010 | $ | 42,217 | 7,300,000 | $ | 7 | 2,675,000 | $ | 3 | 1,658,740 | $ | 1 | (735 | ) | 246,900 | $ | (1,065 | ) | $ | 27,434 | $ | (100,468 | ) | $ | (74,823 | ) | $ | 15,620 | |||||||||||||||||||||||||||||
See accompanying notes to the unaudited consolidated financial statements.
F-42
Table of Contents
AMERICAN RENAL HOLDINGS INC. AND SUBSIDIARIES
Unaudited Consolidated Statements of Changes in Equity (Deficit)—(Continued)
For the predecessor period from December 31, 2009 to May 7, 2010 and
for the successor period from May 8, 2010 to September 30, 2010
(in thousands, except share data)
| Non- controlling interests subject to put provisions | Total American Renal Holdings Inc. Equity (Deficit) | Non- controlling interests not subject to put provisions | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Series A Convertible Preferred Stock | Series B Convertible Preferred Stock | Common Stock | Note Receivable from Stock- holders | Treasury Stock | Additional Paid-in Capital | Accumu- lated Deficit | Total | |||||||||||||||||||||||||||||||||||||||||||||||||
| Shares | Amount | Shares | Amount | Shares | Par Value | Shares | Cost | |||||||||||||||||||||||||||||||||||||||||||||||||
Successor Entity Acquisition transaction | — | (7,300,000 | ) | (7 | ) | (2,675,000 | ) | (3 | ) | (1,658,740 | ) | (1 | ) | 735 | (246,900 | ) | 1,065 | (27,434 | ) | 100,468 | 74,823 | 141,182 | ||||||||||||||||||||||||||||||||||
Successor Entity Opening Equity | $ | 42,217 | — | $ | — | — | $ | — | — | $ | — | — | $ | — | $ | — | $ | — | $ | — | $ | — | 156,802 | |||||||||||||||||||||||||||||||||
Capital contribution | — | — | — | — | — | 1,000 | — | — | — | — | 186,433 | — | 186,433 | — | ||||||||||||||||||||||||||||||||||||||||||
Net income (loss) | 2,768 | — | — | — | — | — | — | — | — | — | — | (14,292 | ) | (14,292 | ) | 8,181 | ||||||||||||||||||||||||||||||||||||||||
Stock-based compensation | — | — | — | — | — | — | — | — | — | — | 544 | — | 544 | |||||||||||||||||||||||||||||||||||||||||||
Parent capital costs | — | — | — | — | — | — | — | — | — | — | (137 | ) | — | (137 | ) | |||||||||||||||||||||||||||||||||||||||||
Distributions to noncontrolling interests | (2,407 | ) | — | — | — | — | — | — | — | — | — | — | — | — | (7,527 | ) | ||||||||||||||||||||||||||||||||||||||||
Contributions from noncontrolling interests | 362 | — | — | — | — | — | — | — | — | — | — | — | — | 1,564 | ||||||||||||||||||||||||||||||||||||||||||
Sales of noncontrolling interests | 28 | — | — | — | — | — | — | — | — | — | 93 | — | 93 | — | ||||||||||||||||||||||||||||||||||||||||||
Purchases of noncontrolling interests | (229 | ) | — | — | — | — | — | — | — | — | — | 1,900 | — | 1,900 | (4,387 | ) | ||||||||||||||||||||||||||||||||||||||||
Balance at September 30, 2010 | $ | 42,739 | — | $ | — | — | $ | — | 1,000 | $ | — | — | $ | — | $ | — | $ | 188,833 | $ | (14,292 | ) | $ | 174,541 | $ | 154,633 | |||||||||||||||||||||||||||||||
See accompanying notes to the unaudited consolidated financial statements.
F-43
Table of Contents
AMERICAN RENAL HOLDINGS INC. AND SUBSIDIARIES
Unaudited Consolidated Statements of Cash Flows
(in thousands)
| Successor | Predecessor | |||||||||||||||
| May 8 through September 30, 2010 | January 1 through May 7, 2010 | Nine months ended September 30, 2009 | ||||||||||||||
Operating activities | ||||||||||||||||
Net (loss) income | $ | (3,343 | ) | $ | 6,582 | $ | 20,340 | |||||||||
Adjustments to reconcile net (loss) income to net cash provided by operating activities: | ||||||||||||||||
Depreciation and amortization | 6,434 | 4,429 | 8,816 | |||||||||||||
Amortization of deferred financing costs | 752 | 205 | 1,066 | |||||||||||||
Stock-based compensation expense | 4,487 | 219 | 876 | |||||||||||||
Change in deferred taxes | — | 1,130 | — | |||||||||||||
Accrued interest on obligations under financing agreements | — | — | 377 | |||||||||||||
Accrued interest on obligations under Series X preferred stock agreement | — | 3,137 | 5,955 | |||||||||||||
Non-cash rent charges | 423 | 186 | 306 | |||||||||||||
Change in operating assets and liabilities, net of acquisitions: | ||||||||||||||||
Decrease (increase) in accounts receivable | 2,132 | (2,465 | ) | (4,827 | ) | |||||||||||
Decrease (increase) in inventories | 424 | (6 | ) | 192 | ||||||||||||
Decrease (increase) in prepaid expenses and other current assets | 730 | (1,529 | ) | 2,946 | ||||||||||||
Decrease in other assets | 2,938 | 23 | 39 | |||||||||||||
Increase (decrease) in accounts payable | 846 | (855 | ) | (1,650 | ) | |||||||||||
Increase in accrued compensation and benefits | 2,161 | 754 | 853 | |||||||||||||
(Decrease) increase in accrued expenses and other current liabilities | (2,762 | ) | (6,445 | ) | 4,103 | |||||||||||
Increase (decrease) in other liabilities | 1,615 | (70 | ) | (1,254 | ) | |||||||||||
Net cash provided by operating activities | 16,837 | 5,295 | 37,222 | |||||||||||||
Investing activities | ||||||||||||||||
Purchases of property and equipment | (6,141 | ) | (4,904 | ) | (9,757 | ) | ||||||||||
Cash paid for acquisitions | (275 | ) | (200 | ) | (3,964 | ) | ||||||||||
Merger with C.P. Atlas Holdings, Inc. | (249,716 | ) | 56 | — | ||||||||||||
Net cash used in investing activities | (256,132 | ) | (5,048 | ) | (13,721 | ) | ||||||||||
Financing activities | ||||||||||||||||
Proceeds from borrowings | — | — | 1,008 | |||||||||||||
Payments on long-term debt | (65,104 | ) | (5,391 | ) | (8,687 | ) | ||||||||||
Payments on capital lease obligations | (78 | ) | (249 | ) | (504 | ) | ||||||||||
Proceeds from issuance of common stock | 161,732 | 8 | 81 | |||||||||||||
Purchase of treasury stock | — | — | (274 | ) | ||||||||||||
Issuance of debt | 248,200 | — | — | |||||||||||||
Debt issuance costs | (11,426 | ) | ||||||||||||||
Payoff of Series X mandatorily redeemable preferred stock | (68,088 | ) | — | — | ||||||||||||
Distributions to noncontrolling interests | (9,934 | ) | (11,394 | ) | (15,016 | ) | ||||||||||
Contributions from noncontrolling interests | 1,926 | 648 | 1,197 | |||||||||||||
Purchases of noncontrolling interests | (2,716 | ) | (1,183 | ) | (942 | ) | ||||||||||
Proceeds from sales of additional noncontrolling interests | 121 | 376 | — | |||||||||||||
Net cash provided by (used in) financing activities | 254,633 | (17,185 | ) | (23,137 | ) | |||||||||||
Net increase (decrease) in cash and cash equivalents | 15,338 | (16,938 | ) | 364 | ||||||||||||
Cash and cash equivalents at beginning of period | 12,241 | 29,179 | 29,182 | |||||||||||||
Cash and cash equivalents at end of period | $ | 27,579 | $ | 12,241 | $ | 29,546 | ||||||||||
See accompanying notes to the unaudited consolidated financial statements.
F-44
Table of Contents
AMERICAN RENAL HOLDINGS INC. AND SUBSIDIARIES
Notes to Unaudited Consolidated Financial Statements
September 30, 2010
(dollars in thousands, except per share amounts)
NOTE A—MERGER AND PRESENTATION
On March 22, 2010, American Renal Holdings Inc. (“ARH” or “the Company”) entered into a Contribution and Merger Agreement (the “Merger Agreement”) with C.P. Atlas Holdings, Inc. (the “Parent”), C.P. Atlas Intermediate Holdings, LLC, C.P. Atlas Acquisition Corp., certain of ARH’s stockholders party thereto and Pamlico Capital I, LLC (formerly known as Wachovia Capital Partners GP I, LLC), pursuant to which C.P. Atlas Acquisition Corp. merged with and into ARH (the “Merger”) and, after which, ARH became the surviving entity and a wholly owned subsidiary of C.P. Atlas Intermediate Holdings, LLC, which is in turn a wholly owned subsidiary of the Parent.
C.P. Atlas Intermediate Holdings, LLC is an entity formed in the Merger and prior to the Merger had no assets or liabilities other than the shares of CP Atlas Acquisition Corp. and its rights and obligations under and in connection with the Merger Agreement. As a result of the Merger, all of ARH’s issued and outstanding capital stock became owned by CP Atlas Intermediate Holdings, LLC which is CP Atlas Intermediate Holdings, LLC’s only asset. As such, the consolidated financial statements of CP Atlas Intermediate Holdings, LLC are identical to the consolidated financial statements of ARH.
The parties agreed to consummate the Merger, subject to the terms and conditions set forth in the Merger Agreement, for an aggregate purchase price of $415 million, subject to adjustments for working capital, indebtedness, certain specified liabilities and certain tax savings. The aggregate purchase price of approximately $415 million for the Merger, plus related fees and expenses, was funded by the equity investment by Centerbridge Capital Partners, L.P. and its affiliates (“Centerbridge”) as well as from certain members of management and the net proceeds from the offering of $250.0 million of Senior Secured Notes (the “Senior Secured Notes”) due 2018 which bear interest at 8.375%. The Merger and the financing transaction described above are collectively referred to herein as the “Transactions.” See Note F for a summary of the terms of the Senior Secured Notes.
A reconciliation of the base price to the acquisition-date fair value of consideration is as follows:
Base Price | $ | 415,000 | ||
Adjustment for cash and working capital | 11,233 | |||
Acquisition-related deferred consideration | 17,015 | |||
Post-combination service compensation | (4,035 | ) | ||
Acquisition-date fair value of consideration | $ | 439,213 | ||
The Transactions were consummated on May 7, 2010. Although ARH continued as the surviving corporation and same legal entity after the Merger, the accompanying unaudited consolidated financial statements are presented for two periods: predecessor and successor, which relate to the periods preceding the Merger and the period succeeding the Merger, respectively. The Merger resulted in a new basis of accounting beginning on May 8, 2010 and the financial reporting periods are presented as follows:
| • | The nine month period ended September 30, 2010 includes the predecessor period of the Company from January 1, 2010 to May 7, 2010 and the successor period, reflecting the Merger of ARH and Parent, from May 8, 2010 to September 30, 2010. |
| • | The 2009 periods presented are predecessor. The consolidated financial statements for all predecessor periods have been prepared using the historical basis of accounting for the Company. As a result of the Merger and the associated acquisition accounting, the consolidated financial statements of the successor are not comparable to periods preceding the Merger. |
F-45
Table of Contents
AMERICAN RENAL HOLDINGS INC. AND SUBSIDIARIES
Notes to Unaudited Consolidated Financial Statements—(Continued)
September 30, 2010
(dollars in thousands, except per share amounts)
NOTE A—MERGER AND PRESENTATION—(Continued)
Total fees and expenses related to the Transactions aggregated approximately $27.7 million consisting of $22.4 million of acquisition costs and $5.3 million of deferred financing costs. Of the $22.4 million, $7.4 million was recognized in the predecessor period and $15.0 million was recognized in the successor period.
The Merger has been accounted for under the acquisition method of accounting, whereby the purchase price was allocated to the assets, liabilities and noncontrolling interests based on the estimated fair values on the date of acquisition. The Company has evaluated and allocated the purchase price as the appraisal associated with the valuation of certain assets, liabilities and noncontrolling interests is complete.
The Company calculated the fair value of its noncontrolling interests not subject to put provisions at the acquisition date using a discounted cash flow approach for interest based on income forecasts for all clinics. The Company utilized a weighted average cost of capital of 13.25%. The discounted cash flow for each interest was then reduced by discounts for a lack of control and marketability. The lack of control discount applied was estimated at 10% as determined through an analysis of the premiums for control paid in transactions of similar companies. A discount for lack of marketability of 9% was estimated utilizing the Black-Scholes model. The application of this model was based on the following assumptions: a maturity period of 1 year, a volatility of 22.5% based on this maturity period for comparable companies, and a risk free rate of 0.38%, which also corresponds with the maturity period.
F-46
Table of Contents
AMERICAN RENAL HOLDINGS INC. AND SUBSIDIARIES
Notes to Unaudited Consolidated Financial Statements—(Continued)
September 30, 2010
(dollars in thousands, except per share amounts)
NOTE A—MERGER AND PRESENTATION—(Continued)
A reconciliation of the final closing balance sheet of the Predecessor entity (the “Predecessor”) prior to the effect of the Transactions, including other simultaneous transactions, such as the repayment of certain indebtedness of the Predecessor and the capital contribution made by the Parent, with the beginning consolidated balance sheet of the Successor entity (the “Successor”) (after the application of the acquisition method of accounting) is presented below:
| Predecessor | Successor | |||||||||||
| May 7, 2010 | Adjustments | May 8, 2010 | ||||||||||
Assets | ||||||||||||
Current assets: | ||||||||||||
Cash and cash equivalents | $ | 12,241 | $ | — | $ | 12,241 | ||||||
Accounts receivable, net | 48,119 | — | 48,119 | |||||||||
Inventories | 2,300 | — | 2,300 | |||||||||
Prepaid expenses and other current assets | 3,606 | — | 3,606 | |||||||||
Deferred transaction expense | — | 18,982 | 18,982 | |||||||||
Income tax receivable | 11,706 | 1,595 | 13,301 | |||||||||
Deferred tax assets | 3,429 | 593 | 4,022 | |||||||||
Total current assets | 81,401 | 21,170 | 102,571 | |||||||||
Property and equipment, net | ||||||||||||
Land and buildings | 1,692 | (250 | ) | 1,442 | ||||||||
Leasehold improvements | 55,859 | (27,948 | ) | 27,911 | ||||||||
Equipment and information systems | 43,640 | (20,647 | ) | 22,993 | ||||||||
Construction in progress | 3,047 | — | 3,047 | |||||||||
| 104,238 | (48,845 | ) | 55,393 | |||||||||
Less: accumulated depreciation and amortization | (44,338 | ) | 44,338 | — | ||||||||
| 59,900 | (4,507 | ) | 55,393 | |||||||||
Deferred financing costs, net | 882 | 4,323 | 5,205 | |||||||||
Intangible assets, net | 1,408 | 39,081 | 40,489 | |||||||||
Deferred tax asset | — | 3,625 | 3,625 | |||||||||
Other long-term assets | 3,287 | (2,012 | ) | 1,275 | ||||||||
Goodwill | 33,157 | 470,181 | 503,338 | |||||||||
Total assets | $ | 180,035 | $ | 531,861 | $ | 711,896 | ||||||
Liabilities and Deficit | ||||||||||||
Current liabilities: | ||||||||||||
Accounts payable | $ | 17,304 | $ | — | $ | 17,304 | ||||||
Accrued compensation and benefits | 7,924 | — | 7,924 | |||||||||
Accrued expenses and other current liabilities | 20,523 | (6,911 | ) | 13,612 | ||||||||
Amount due to sellers | — | 17,016 | 17,016 | |||||||||
Current portion of long-term debt | 13,148 | (9,678 | ) | 3,470 | ||||||||
Current portion of capital lease obligations | 105 | 105 | ||||||||||
Total current liabilities | 59,004 | 427 | 59,431 | |||||||||
Long-term debt, less current portion | 60,030 | 188,556 | 248,586 | |||||||||
Capital lease obligations, less current portion | 195 | — | 195 | |||||||||
Other long-term liabilities | 7,595 | (6,331 | ) | 1,264 | ||||||||
Deferred tax liabilities | 4,261 | 12,707 | 16,968 | |||||||||
Series X mandatorily redeemable preferred stock | 65,936 | (65,936 | ) | — | ||||||||
Noncontrolling interests subject to put provisions | 42,217 | — | 42,217 | |||||||||
Equity (deficit): | ||||||||||||
Additional paid-in capital | 27,434 | 158,999 | 186,433 | |||||||||
Accumulated deficit | (100,468 | ) | 100,468 | — | ||||||||
Other stockholders’ deficit | (1,789 | ) | 1,789 | — | ||||||||
Total American Renal Holdings Inc. equity (deficit) | (74,823 | ) | 261,256 | 186,433 | ||||||||
Noncontrolling interests not subject to put provisions | 15,620 | 141,182 | 156,802 | |||||||||
Total equity (deficit) | (59,203 | ) | 402,438 | 343,235 | ||||||||
Total liabilities and equity (deficit) | $ | 180,035 | $ | 531,861 | $ | 711,896 | ||||||
F-47
Table of Contents
AMERICAN RENAL HOLDINGS INC. AND SUBSIDIARIES
Notes to Unaudited Consolidated Financial Statements—(Continued)
September 30, 2010
(dollars in thousands, except per share amounts)
NOTE B—SIGNIFICANT ACCOUNTING POLICIES
As a result of the Merger, we have recorded approximately $503.3 million of goodwill. The components of this goodwill are primarily patient relationships and our assembled work force. The goodwill is not deductible for income tax purposes.
Fair Value of Consideration Transferred
At the closing, C.P. Atlas made a cash payment to the former shareholders of the Company equal to the sum of the initial purchase price of $217.9 million plus $1.8 million based on an estimated working capital adjustment and other adjustments in accordance with the Merger Agreement. The final working capital adjustment was settled during the third quarter of fiscal 2010 for an additional payment of $379,000. In addition, at the close, C.P. Atlas made a cash payment to an escrow agent for $30.0 million. Based on the conditions of the escrow release, the Company has determined the release of the escrow amount is probable.
Of the $217.9 million, approximately $45.3 million was paid to the Company’s holders of stock-options. In connection with the Merger, vesting was accelerated for all outstanding stock options. As a result of this acceleration, the Company determined that approximately $4.1 million of the $45.3 million payment was attributable to post-combination services and, as such, recorded as an expense in the successor income statement.
The total acquisition date fair value of the consideration transferred was estimated at $439.2 million as follows:
Cash consideration to debtholders | $ | 63,600 | ||
Cash consideration to Series X redeemable preferred stockholders | 68,088 | |||
Cash consideration to stockholders at close | 249,716 | |||
Acquisition-related deferred consideration | 17,015 | |||
Equity consideration to shareholders | 24,089 | |||
Assumed liabilities | 16,705 | |||
Acquisition-date fair value of consideration | $ | 439,213 | ||
The Company’s stockholders are additionally entitled to certain future tax benefits realized as a result of the Merger. The Company has estimated this amount as $17.0 million for which a liability was recognized for the deferred consideration. A liability was recognized for the acquisition date fair value of the acquisition-related consideration for this deferred fixed purchase price. Any change in the fair value of the acquisition-related consideration subsequent to the acquisition date, including changes from events after the acquisition date will be recognized in earnings in the period the estimated fair value changes.
In connection with the Merger, C.P. Atlas assumed $16.7 million of liabilities which consisted of $10.1 million of term loans at the Company’s clinics and $6.6 million of Merger-related transaction expenses which C.P. Atlas settled on behalf of the Company.
In connection with the Merger, the Company’s shareholders were allowed to receive shares of C.P. Atlas in exchange for foregoing cash payments. The fair value of the shares issued in connection with such payments were approximately $24.1 million.
F-48
Table of Contents
AMERICAN RENAL HOLDINGS INC. AND SUBSIDIARIES
Notes to Unaudited Consolidated Financial Statements—(Continued)
September 30, 2010
(dollars in thousands, except per share amounts)
NOTE B—SIGNIFICANT ACCOUNTING POLICIES—(Continued)
Basis of Presentation and Consolidation
The accompanying unaudited consolidated financial statements have been prepared in accordance with U.S. generally accepted accounting principles (U.S. GAAP) for interim financial reports and the instructions for Form 10-Q and Rule 10-01 of Regulation S-X. Accordingly, certain information and footnote disclosures normally included in financial statements prepared under generally accepted accounting principles have been condensed or omitted pursuant to such regulations. However, the Company believes that the disclosures are adequate to make the information presented not misleading. In the opinion of management, the accompanying financial statements include all adjustments necessary to present a fair presentation of the consolidated financial statements for the periods shown. These statements should be read in conjunction with the Company’s annual audited consolidated statements, including Note B which discusses principles of consolidation and summary of significant accounting policies. These statements have been prepared in accordance with generally accepted accounting principles, which require management to make certain estimates and assumptions that affect reported amounts of assets, liabilities and noncontrolling interests and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. The most significant estimates include valuation of net operating revenues (contractual allowances) and the allowance for doubtful accounts, valuation of goodwill and intangibles, fair value of noncontrolling interests, provision for income taxes, stock-based compensation and contingencies. Actual results could differ from these estimates and actual results are not necessarily indicative of results for the remainder of 2010 or future periods thereafter. The Company has evaluated subsequent events through December 6, 2010, which is the date these unaudited consolidated financial statements were issued.
During the period from January 1, 2010 to May 7, 2010, the Company determined that it had developed sufficient and relevant historical experience with Medicare bad debt cost reporting to use as a basis for reliably estimating Medicare bad debt cost recoveries. As such, the Company revised its estimate on the collectability of Medicare bad debt claims related to 2008 and 2009 which resulted in a favorable adjustment of $1.8 million to our provision for uncollectible accounts.
Recent Accounting Pronouncements
In January 2010, the FASB issued ASC Topic 820,Fair Value Measurements and Disclosures—Improving Disclosures About Fair Value Measurements. The Accounting Standards Update (“ASU”) requires new disclosures about transfers into and out of Levels 1 and 2 and separate disclosures about purchases, sales, issuances, and settlements relating to Level 3 measurements. It also clarifies existing fair value disclosures about the level of disaggregation and about inputs and valuation techniques used to measure fair value. The new disclosures and clarifications of existing disclosures are effective January 1, 2010, except for the disclosures about purchases, sales, issuances, and settlements relating to Level 3 measurements, which are effective January 1, 2011. Other than requiring additional disclosures, the adoption of this new guidance did not have a material impact on the Company’s consolidated results of operations and financial position.
In August 2010, the FASB issued guidance that requires health care entities to use cost as the measurement basis for charity care disclosures and defines cost as the direct and indirect costs of providing charity care. The amended disclosure requirements are effective for fiscal years beginning after December 15, 2010 and must be applied retrospectively. The Company will adopt the amended disclosure requirements for their fiscal year beginning January 1, 2011. Since the new guidance amends disclosure requirements only, its adoption will not impact the Company’s statement of financial position, statement of operations, or cash flow statement.
F-49
Table of Contents
AMERICAN RENAL HOLDINGS INC. AND SUBSIDIARIES
Notes to Unaudited Consolidated Financial Statements—(Continued)
September 30, 2010
(dollars in thousands, except per share amounts)
NOTE B—SIGNIFICANT ACCOUNTING POLICIES—(Continued)
In August 2010, the FASB issued ASU 2010-24 to address how health care entities should account for medical malpractice claims and related anticipated insurance recoveries. The new guidance, which amends ASC 954-450,Contingencies, and ASC 954-720,Other Expenses, also applies to similar contingent liabilities. Under the new guidance, health care entities are prohibited from presenting claim liabilities net of insurance recoveries. Further, the guidance requires health care entities to disregard insurance recoveries when measuring the amount of a claim liability. However, a related insurance receivable should be recorded concurrently with the liability, subject to a valuation allowance, if necessary. The new guidance is effective beginning January 1, 2011. In the period of adoption, entities must recognize in opening retained earnings any cumulative-effect adjustment resulting from the application of the new guidance. Entities may apply the new guidance retrospectively, and early application is permitted. The Company is currently assessing the impact this may have on its consolidated financial statements.
NOTE C—FAIR VALUE MEASUREMENTS
Certain of the Company’s assets and noncontrolling interests are accounted for at fair value on a recurring basis and are classified and disclosed in one of the following three categories:
Level 1: Financial instruments with unadjusted, quoted prices listed on active market exchanges. The Company’s Level 1 assets include cash equivalent investments held in money market funds.
Level 2: Financial instruments determined using prices for recently traded financial instruments with similar underlying terms, as well as directly or indirectly observable inputs, such as interest rates and yield curves that are observable at commonly quoted intervals.
Level 3: Financial instruments not actively traded on a market exchange. This category includes situations where there is little, if any, market activity for the financial instrument. The prices are determined using significant unobservable inputs or valuation techniques.
The asset or liabilities fair value measurement level within the fair value hierarchy is based on the lowest level of any input that is significant to the fair value measurement. The following is a description for the valuation methodologies used for assets and noncontrolling interests measured at fair value. There were no changes in the methodologies used at September 30, 2010.
Cash equivalent—The cash equivalent represents investments in a money market fund and U.S. government obligations with an original maturity of less than 90 days which are recorded at fair value based on their quoted market prices.
Noncontrolling interest subject to put provision—See Note E for a discussion of the Company’s methodology for estimating fair value of noncontrolling interest subject to put obligations.
F-50
Table of Contents
AMERICAN RENAL HOLDINGS INC. AND SUBSIDIARIES
Notes to Unaudited Consolidated Financial Statements—(Continued)
September 30, 2010
(dollars in thousands, except per share amounts)
NOTE C—FAIR VALUE MEASUREMENTS—(Continued)
| September 30, 2010 | ||||||||||||||||
| Total | Level 1 | Level 2 | Level 3 | |||||||||||||
Temporary Equity | ||||||||||||||||
Noncontrolling interests subject to put provisions | $ | 42,739 | $ | — | $ | — | $ | 42,739 | ||||||||
| December 31, 2009 | ||||||||||||||||
| Total | Level 1 | Level 2 | Level 3 | |||||||||||||
Assets | ||||||||||||||||
Cash equivalent | $ | 9,506 | $ | 9,506 | $ | — | $ | — | ||||||||
Temporary Equity | ||||||||||||||||
Noncontrolling interests subject to put provisions | $ | 38,431 | $ | — | $ | — | $ | 38,431 | ||||||||
NOTE D—INTANGIBLE ASSETS
In connection with the Transactions, the Company’s intangible assets were revalued based on an appraisal of estimated fair values at the date of acquisition. The carrying amount and accumulated amortization of identifiable intangible assets at September 30, 2010 and December 31, 2009 was:
| Estimated Life (Years) | Successor | Predecessor | ||||||||||||||
| September 30, 2010 | December 31, 2009 | |||||||||||||||
Noncompete agreements | 5 years | $ | 19,156 | $ | 1,794 | |||||||||||
Other intangible assets | 138 | 426 | ||||||||||||||
| 19,294 | 2,220 | |||||||||||||||
Less accumulated amortization | (1,516 | ) | (802 | ) | ||||||||||||
Net intangible assets subject to amortization | 17,778 | 1,418 | ||||||||||||||
Indefinite-lived trademarks | 21,334 | — | ||||||||||||||
| $ | 39,112 | $ | 1,418 | |||||||||||||
For the period from May 8, 2010 through September 30, 2010, the three months ended September 30, 2010, the period from January 1, 2010 through May 7, 2010 and the three and nine months ended September 30, 2009, amortization expense of $1.5 million, $0.9 million, $0.1 million, $0.1 million and $0.1 million, respectively, was recognized by the Company.
F-51
Table of Contents
AMERICAN RENAL HOLDINGS INC. AND SUBSIDIARIES
Notes to Unaudited Consolidated Financial Statements—(Continued)
September 30, 2010
(dollars in thousands, except per share amounts)
NOTE D—INTANGIBLE ASSETS—(Continued)
The remaining amortization of identifiable intangible assets, net, by year as of September 30, 2010 is as follows:
Fiscal Year | Amortization | |||
2010 (remainder) | $ | 958 | ||
2011 | 3,859 | |||
2012 | 3,859 | |||
2013 | 3,859 | |||
2014 | 3,859 | |||
2015 and thereafter | 1,384 | |||
Total | $ | 17,778 | ||
NOTE E—NONCONTROLLING INTERESTS SUBJECT TO PUT PROVISIONS
The Company has potential obligations to purchase the noncontrolling interests held by third parties in several of its consolidated subsidiaries. These obligations are in the form of put provisions and are exercisable at the third-party owners’ discretion within specified periods as outlined in each specific put provision. If these put provisions were exercised, the Company would be required to purchase the third-party owners’ noncontrolling interests at the appraised fair value. In connection with the Transactions, the obligations were recorded based on an appraisal of estimated fair values as of the date of the acquisition. The appraisal estimated the fair value of the noncontrolling interests subject to these put provisions using a discounted cash flow approach and other factors. The estimated fair values of the noncontrolling interests subject to these put provisions can also fluctuate. The amount for which noncontrolling interests may ultimately be settled could vary significantly from our current estimates depending upon market conditions including potential purchasers’ access to the credit and capital markets, which can impact the level of competition for dialysis and non-dialysis related businesses, the economic performance of these businesses and the restricted marketability of the third-party owners’ noncontrolling interests.
As of September 30, 2010 and December 31, 2009, the Company’s potential obligations under these put provisions totaled approximately $33,883 and $29,295, respectively. Additionally, the Company has certain put agreements which are exercisable upon the occurrence of specific events, including third-party members’ death, disability, bankruptcy, retirement, or if third-party members are dissolved. As of September 30, 2010 and December 31, 2009, the Company’s potential additional obligations under these put provisions were approximately $8,856 and $9,136, respectively. The Company’s potential obligations for all of these put provisions are included in noncontrolling interests subject to put provisions in the accompanying consolidated balance sheets.
F-52
Table of Contents
AMERICAN RENAL HOLDINGS INC. AND SUBSIDIARIES
Notes to Unaudited Consolidated Financial Statements—(Continued)
September 30, 2010
(dollars in thousands, except per share amounts)
NOTE E—NONCONTROLLING INTERESTS SUBJECT TO PUT PROVISIONS—(Continued)
During 2010, the Company purchased the following additional membership interests from a noncontrolling interest partner. These purchases have been accounted for as equity transactions.
Membership interest | Amount | |||
7.5% during predecessor period | $ | 870 | ||
3.8% during predecessor period | 168 | |||
3.5% during predecessor period | 145 | |||
5.4% during successor period | 234 | |||
10.0% during successor period | 1,880 | |||
24.5% during successor period | 464 | |||
3.8% during successor period | 138 | |||
During the nine months ended September 30, 2009, the Company purchased an additional 7.5% membership interest from a noncontrolling interest partner for $942. This purchase has been accounted for as an equity transaction.
F-53
Table of Contents
AMERICAN RENAL HOLDINGS INC. AND SUBSIDIARIES
Notes to Unaudited Consolidated Financial Statements—(Continued)
September 30, 2010
(dollars in thousands, except per share amounts)
NOTE F—LONG-TERM DEBT
Long-term debt consists of the following at December 31, 2009 and September 30, 2010:
| Successor | Predecessor | |||||||||||
| September 30, 2010 | December 31, 2009 | |||||||||||
Senior secured notes issued May 7, 2010, interest payable semi-annually at a fixed rate of 8.375% with a balloon payment due on May 15, 2018 | $ | 250,000 | $ | — | ||||||||
Term loan A, principal payments of $550 and interest at variable rates (8.50% as of December 31, 2009) payable quarterly through December 2010, repaid in May 2010 in connection with the Transactions | — | 2,750 | ||||||||||
Term loan D, principal payments of $45 and interest at variable rates (8.5% as of December 31, 2009) payable quarterly through September 2011, with a $39,010 balloon payment due December 2011, repaid in May 2010 in connection with the Transactions | — | 39,325 | ||||||||||
Term loans, principal payments due of $25 and interest at variable monthly rates (2.04% as of September 30, 2010) payable monthly through November 2011, with a balloon payment of $1,983 due December 2011 | 1,844 | 2,065 | ||||||||||
Term loan, principal payments of $9 and interest at variable rates (2.38% as of September 30, 2010) through May 2014, with a $536 balloon payment due May 2014 | 920 | 1,001 | ||||||||||
Term loans, principal and interest payable monthly at rates between 3.85% and 8.67% over varying periods through December 2013 | 4,804 | 29,802 | ||||||||||
Mortgage payable, principal and interest due monthly through February 2012 at a rate of 7%, repaid in May 2010 in connection with the Transactions | — | 509 | ||||||||||
Mortgage payable, principal and interest due monthly through March 2014 at a rate of 4.79% | 222 | 268 | ||||||||||
Lines of credit, variable interest (4.25% as of September 30, 2010), due monthly through January 2011 convertible to term loans payable over an additional term of 36 months through January 2014 | 200 | 2,850 | ||||||||||
| 257,990 | 78,570 | |||||||||||
Less: discounts and fees, net of accumulated amortization | (7,643 | ) | — | |||||||||
Less: current maturities | (2,863 | ) | (14,309 | ) | ||||||||
| $ | 247,484 | $ | 64,261 | |||||||||
Scheduled maturities of long-term debt as of September 30, 2010 are as follows for the periods ending December 31:
2010 (remainder) | $ | 841 | ||
2011 | 4,095 | |||
2012 | 1,563 | |||
2013 | 895 | |||
2014 | 596 | |||
Thereafter | 250,000 | |||
| $ | 257,990 | |||
F-54
Table of Contents
AMERICAN RENAL HOLDINGS INC. AND SUBSIDIARIES
Notes to Unaudited Consolidated Financial Statements—(Continued)
September 30, 2010
(dollars in thousands, except per share amounts)
NOTE F—LONG-TERM DEBT—(Continued)
Senior Secured Notes
In connection with the Transactions, the Company issued $250.0 million of Senior Secured Notes (the “Notes”) at an offering price of 99.28%. The Notes are secured, subject to certain exceptions, by (i) all of the Company’s capital stock and (ii) substantially all of the assets of our wholly owned subsidiary guarantors. The Notes are guaranteed by the Company’s direct parent, C. P. Atlas Intermediate Holdings, LLC and all of our existing and future wholly owned domestic subsidiaries. The Notes mature on May 15, 2018. Interest is payable semi-annually at 8.375% per annum, commencing November 15, 2010.
On or after May 15, 2015, the Company may redeem the Notes at its option, subject to certain notice periods, at a price equal to 100% of the aggregate principal amount of the Notes plus accrued and unpaid interest. From May 15, 2013 to May 14, 2015, the Company may redeem the Notes at prices ranging from 102.094% to 104.188% of the aggregate principal balance of the Notes plus accrued and unpaid interest prior to May 15, 2013, the Company has the option to redeem during each 12-month period commencing on the issue date of May 7, 2010 up to 10% of the aggregate principal amount of the notes at 103% of the aggregate principal amount of the Notes redeemed plus accrued and unpaid interest to the date of redemption. In addition, the Company may redeem up to 35% of the Notes before May 15, 2013, with the net cash proceeds from certain equity offerings at 108.375% of the aggregate principal amount of the Notes redeemed plus accrued and unpaid interest. The Company may also redeem some or all of the Notes before May 15, 2013 at a redemption price of 100% of the principal amount thereof plus accrued and unpaid interest, if any, to the redemption date, plus a “make-whole” premium. Upon a change in control, the Company must offer to purchase the Notes at 101% of the principal amount, plus accrued interest to the purchase date.
Credit Facility
In connection with the Transactions, the Company entered into a $25.0 million Senior Secured Revolving Credit Facility (the Credit Facility). The Credit Facility expires on May 7, 2015. Borrowings under the Credit Facility bear interest at a rate equal to, at the Company’s option, either (a) an alternate base rate determined by reference to the higher of (1) the prime rate in effect on such day, (2) the federal funds effective rate plus 0.50% and (3) the Eurodollar rate applicable for an interest period of one month plus 1.00% or (b) the LIBOR rate, plus an applicable margin. The initial applicable margin for borrowings under the Credit Facility is 3.50% with respect to alternate base rate borrowings and 4.50% with respect to LIBOR borrowings. In addition to paying interest on outstanding principal under the Credit Facility, the Company is required to pay a commitment fee, initially 0.75% per annum, in respect of the unutilized revolving credit commitments thereunder. Beginning September 30, 2010, the Company is subject to leverage and fixed charge financial covenants under the Credit Facility as set forth below.
We have agreed that beginning on September 30, 2010, we will not permit the Consolidated Net Debt to Consolidated EBITDA (both as defined in the agreement) Ratio for any 12 month period (last four fiscal quarters) ending during a period set forth below to be greater than the ratio set forth below opposite such period:
Period | Ratio | |||
September 30, 2010 through September 30, 2011 | 5.75:1.00 | |||
December 31, 2011 through September 30, 2012 | 5.50:1.00 | |||
December 31, 2012 through September 30, 2013 | 5.00:1.00 | |||
December 31, 2013 through September 30, 2014 | 4.75:1.00 | |||
December 31, 2014 and thereafter | 4.25:1.00 | |||
F-55
Table of Contents
AMERICAN RENAL HOLDINGS INC. AND SUBSIDIARIES
Notes to Unaudited Consolidated Financial Statements—(Continued)
September 30, 2010
(dollars in thousands, except per share amounts)
NOTE F—LONG-TERM DEBT—(Continued)
We have also agreed that beginning on September 30, 2010, we will not permit the Consolidated EBITDA to Fixed Charges (both as defined in the agreement) Ratio for any 12 month period (last four fiscal quarters) ending during a period set forth below to be lower than the ratio set forth below opposite such period:
Period | Ratio | |||
September 30, 2010 through September 30, 2011 | 1.80:1.00 | |||
December 31, 2011 through September 30, 2012 | 1.80:1.00 | |||
December 31, 2012 through September 30, 2013 | 2.00:1.00 | |||
December 31, 2013 through September 30, 2014 | 2.25:1.00 | |||
December 31, 2014 and thereafter | 2.50:1.00 | |||
As of September 30, 2010, there were no borrowings outstanding under the Credit Facility and the Company is in compliance with its covenants.
NOTE G—INCOME TAXES
The income tax expense included in the accompanying consolidated statements of operations principally relates to the Company’s proportionate share of the pre-tax income of its majority-owned subsidiaries. The Predecessor’s effective income tax rate during the period from January 1, 2010 through May 7, 2010 was 25.6% which differed from the statutory federal rate of 35% primarily due to non-deductible transaction costs and interest expense, including original issue discount related to the Series X Preferred Stock. The Successor’s effective income tax rate for the three months ended September 30, 2010 was 10.4% which approximates the statutory federal tax rate on the Company’s proportionate share of pre-tax income from our majority-owned subsidiaries. The Successor’s effective income tax rate for the period from May 8, 2010 through September 30, 2010 was (16.3)%. This differs from the statutory federal tax rate primarily due to non-deductible transaction costs.
NOTE H—STOCK-BASED COMPENSATION
The Predecessor issued stock options and other stock awards to executive management and employees under its stock-based compensation plans. During the three and nine months ended September 30, 2009, the Company recognized $0.3 million and $0.9 million, respectively, of stock-based compensation. Related tax benefits of $0.1 million and $0.2 million, respectively, were recognized for those periods. During the period from January 1, 2010 to May 7, 2010, the Predecessor recognized $0.2 million of stock-based compensation. A summary of the activity in the Company’s stock option plans for the period from January 1, 2010 through May 7, 2010 is presented below:
| Number of Options | Weighted- Average Exercise Price per Share | |||||||
2000 Stock Option Plan | ||||||||
Outstanding at December 31, 2009 | 2,567,500 | $ | 0.54 | |||||
Exercised | (43,750 | ) | 0.14 | |||||
Converted/Rolled-over | (2,523,750 | ) | 0.55 | |||||
Outstanding at May 7, 2010 | — | $ | — | |||||
F-56
Table of Contents
AMERICAN RENAL HOLDINGS INC. AND SUBSIDIARIES
Notes to Unaudited Consolidated Financial Statements—(Continued)
September 30, 2010
(dollars in thousands, except per share amounts)
NOTE H—STOCK-BASED COMPENSATION—(Continued)
| Number of Options | Weighted- Average Exercise Price per Share | |||||||
2005 Stock Option Plan | ||||||||
Outstanding at December 31, 2009 | 3,443,300 | $ | 3.83 | |||||
Canceled | (12,750 | ) | 4.93 | |||||
Converted/Rolled-over | (3,430,550 | ) | 3.83 | |||||
Outstanding at May 7, 2010 | — | $ | — | |||||
In connection with the Merger, all unvested options were vested and all outstanding stock options were either converted into the right to receive the difference between $9.50 and the exercise price of the stock option or were rolled-over into fully vested options in the Successor. As a result, the Company paid approximately $45.3 million related to the conversion of stock options of which $3.7 million was recorded as stock-based compensation expense during the period from May 8, 2010 to June 30, 2010 and the remaining $41.6 million was accounted for as part of purchase price of the Transaction. In connection with the conversion of these options, the Company recognized a tax benefit of $9.1 million in the period from January 1, 2010 to May 7, 2010 for compensation deducted in tax returns filed for this period which has been credited to additional paid-in capital in the predecessor period. Under the terms of the Merger Agreement, this tax savings is to be paid to the selling shareholders as part of the purchase price. A summary of the activity in the Company’s option plan activity for the 2000 Stock Option Plan and the 2005 Stock Option Plan on a combined basis for the period from May 8, 2010 through September 30, 2010 is presented below:
| Number of Options | Weighted- Average Exercise Price per Share | |||||||
2000 Stock Option Plan and 2005 Stock Option Plan | ||||||||
Converted in connection with the Merger | 214,308 | $ | 4.51 | |||||
Exercised | (5,149 | ) | 3.01 | |||||
Outstanding at September 30, 2010 | 209,159 | $ | 4.59 | |||||
In May 2010, the Parent adopted the C.P. Atlas Holdings, Inc. 2010 Stock Incentive Plan (the 2010 Plan) under which 1,574,782 shares of the Parent’s common stock were reserved for issuance to the Company’s employees, directors and consultants. Options granted under the 2010 Plan must be nonstatutory stock options. Stock appreciation rights may also be granted under the 2010 Plan. The Board of Directors determines the period over which options become exercisable; however, such awards generally require that certain performance conditions and service conditions be met before the awards would vest. During the period from May 8, 2010 to June 30, 2010, the Company’s Parent did not grant any awards under the 2010 Plan. During the three months ended September 30, 2010, the Company’s Parent granted 1,184,050 options under the 2010 Plan to the Company’s employees at an exercise price of $21.00 per share, all of which were outstanding as of September 30, 2010 and none of which were vested as of September 30, 2010. During the three months ended September 30, 2010, the Company recorded $0.5 million in stock-based compensation expense ($0.3 million net of taxes) related to these grants. Vesting of share-based awards issued with performance-based vesting criteria must be deemed “probable” before we begin recording compensation expense. At each reporting period, we review the likelihood that these awards will vest and if the vesting is deemed probable, we begin to recognize
F-57
Table of Contents
AMERICAN RENAL HOLDINGS INC. AND SUBSIDIARIES
Notes to Unaudited Consolidated Financial Statements—(Continued)
September 30, 2010
(dollars in thousands, except per share amounts)
NOTE H—STOCK-BASED COMPENSATION—(Continued)
��
compensation expense at that time. If ultimately performance goals are not met, for any awards where vesting was deemed probable, previously recognized compensation cost will be reversed.
The weighted-average assumptions used to estimate the fair values of 2010 Plan stock options on the dates of grant are as follows:
Risk-free interest rates | 2.4 | % | ||
Expected dividend yield | 0 | % | ||
Expected term of option | 6.5 years | |||
Expected volatility | 44 | % | ||
Weighted-average grant date fair value per share of options granted | $ | 9.86 |
Expected volatility is based on a selected group of comparable companies’ volatility over the expected term. Expected life of an option is based on the “short-cut method” as prescribed by SEC SAB No. 110 as the Company has never granted performance-based awards and, as such, the Company does not have any historical experience from which to estimate an expected life. The risk-free interest rate represents the implied yields available from the U.S. Treasury zero-coupon yield curve. Expected dividend yield is 0% because the Company has not paid dividends in the past, and currently has no known intention to do so in the future. Compensation expense is recognized net of forfeitures.
Based on equity awards outstanding as of September 30, 2010, there was $10.3 million of unrecognized compensation costs, net of estimated forfeitures, related to unvested share-based compensation arrangements. Such costs are expected to be recognized over a period of five years if certain performance criteria continue to be deemed probable and are met. The following table summarizes the estimated future annual stock-based compensation expense related to share-based arrangements, existing as of September 30, 2010, that are expected to vest:
2010 (remainder) | $ | 544 | ||
2011 | 2,178 | |||
2012 | 2,178 | |||
2013 | 2,178 | |||
2014 | 2,178 | |||
2015 | 1,089 | |||
| $ | 10,345 | |||
NOTE I—RELATED PARTY TRANSACTIONS
The Company has lease agreements for dialysis clinics with noncontrolling interest members or entities under the control of noncontrolling interest members. The amount of rent expense under these lease arrangements was approximately $0.9 million and $1.0 million for the period from January 1, 2010 to May 7, 2010 and May 8, 2010 to September 30, 2010, respectively. In addition, in 2008, the Company sub-leased space at one of its dialysis clinics to the noncontrolling interest member. Rent income under this sub-lease arrangement, which extends to 2023, was approximately $0.2 million and $0.2 million for the period from January 1, 2010 to May 7, 2010, and May 8, 2010 to September 30, 2010, respectively.
F-58
Table of Contents
AMERICAN RENAL HOLDINGS INC. AND SUBSIDIARIES
Notes to Unaudited Consolidated Financial Statements—(Continued)
September 30, 2010
(dollars in thousands, except per share amounts)
NOTE I—RELATED PARTY TRANSACTIONS—(Continued)
Upon consummation of the Merger, the Company entered into a management services agreement with Centerbridge. Under this management services agreement, Centerbridge agreed to provide to the Company certain investment banking, management, consulting, and financing planning services on an ongoing basis. In consideration for these services, the Company will pay Centerbridge, commencing May 7, 2010 an annual advisory services fee (payable quarterly) for each fiscal year from and including fiscal year 2010 of the greater of (i) an amount equal to the greater of (x) $550,000 and (y) the advisory services fee of the previous fiscal year or (ii) an amount equal to 1.25% of EBITDA, minus a personnel expense deduction, if applicable. During the period from May 8, 2010 to September 30, 2010, the Company recorded $0.2 million of expense related to this agreement. In addition, Centerbridge is entitled to receive an additional fee equal to 1.0% of the enterprise value and/or aggregate value, as applicable, for any future fundamental or significant transactions in which Centerbridge is involved. The Company paid a transaction fee of $4.8 million (including reimbursement of expenses) to Centerbridge for financial advisory services rendered in connection with the Merger all of which was expensed as a merger and transaction related cost in the period May 8, 2010 to June 30, 2010. These services included assisting the Company in structuring the Merger, taking into account tax considerations and optimal access to financing, and assisting in the negotiation of the Company’s material agreements and financing arrangements in connection with the Merger.
NOTE J—COMMITMENTS AND CONTINGENCIES
Healthcare provider revenues may be subject to adjustment as a result of (i) examination by government agencies or contractors, for which the resolution of any matters raised may take extended periods of time to finalize; (ii) differing interpretations of government regulations by different fiscal intermediaries or regulatory authorities; (iii) differing opinions regarding a patient’s medical diagnosis or the medical necessity of service provided; (iv) retroactive applications or interpretations of governmental requirements; and (v) claims for refund from private payors, including as the result of government actions. Management believes it has recorded adequate provisions to consider potential revenue adjustments.
Professional Liability Coverage
The Company maintains professional liability insurance coverage on a claims-made basis. Under this type of policy, claims based on occurrences during its term, but reported subsequently, will be uninsured should the policy not be renewed or replaced with other coverage. Management expects to be able to obtain renewal or other coverage in future periods, and has accrued the estimated cost associated with coverage of past occurrences reported in subsequent periods.
Litigation
The Company is a defendant in various legal actions in the normal course of business. In the opinion of the Company’s management, based in part on the advice of outside counsel, the resolution of these matters will not have a material effect on the Company’s financial position, results of operations or cash flows.
Regulatory
The healthcare industry is subject to numerous laws and regulations of federal, state, and local governments. Recently, government activity has increased with respect to investigations and allegations concerning possible violations by healthcare providers of fraud and abuse statutes and regulations, which could result in the
F-59
Table of Contents
AMERICAN RENAL HOLDINGS INC. AND SUBSIDIARIES
Notes to Unaudited Consolidated Financial Statements—(Continued)
September 30, 2010
(dollars in thousands, except per share amounts)
NOTE J—COMMITMENTS AND CONTINGENCIES—(Continued)
imposition of significant fines and penalties, as well as significant repayments for patient services previously billed. Compliance with such laws and regulations are subject to government review and interpretations, as well as regulatory actions unknown or unasserted at this time.
NOTE K—SUPPLEMENTAL INFORMATION
As set forth in Note F, the Notes are guaranteed, jointly and severally, by all wholly-owned domestic current or future subsidiaries of the Company and C.P. Atlas Intermediate Holdings, LLC. (the Guarantors).
The following tables present the condensed consolidating financial information for ARH, the subsidiary Guarantors and the Non-Guarantors, together with eliminations, as of and for the periods indicated. As a result of the Merger, all of ARH’s issued and outstanding capital stock is owned by CP Atlas Intermediate Holdings, LLC which is CP Atlas Intermediate Holdings, LLC’s only asset. As such, the consolidated financial statements of CP Atlas Intermediate Holdings, LLC are identical to the consolidated financial statements of ARH.
The information as of September 30, 2010 and for the period from May 8, 2010 to September 30, 2010 presents the financial position, results of operations and cash flows of the Successor entity. The information as of December 31, 2009, and for the period from January 1, 2010 through May 7, 2010 presents the financial position, results of operations and cash flows of the Predecessor entity. The consolidating information may not necessarily be indicative of the financial position, results of operations or cash flows had ARH, Guarantors and Non-Guarantors operated as independent entities.
Condensed Consolidating Balance Sheet—September 30, 2010
| ARH | Subsidiary Guarantors | Non- Guarantors | Eliminations | Successor Consolidated | ||||||||||||||||
Assets | ||||||||||||||||||||
Cash and cash equivalents | $ | — | $ | 5,379 | $ | 22,200 | $ | — | $ | 27,579 | ||||||||||
Accounts receivable | — | 181 | 45,806 | — | 45,987 | |||||||||||||||
Inventories | — | 16 | 1,961 | — | 1,977 | |||||||||||||||
Prepaid expenses and other current assets | 10,303 | 14,156 | 3,874 | (14,200 | ) | 14,133 | ||||||||||||||
Deferred tax assets | 4,043 | — | — | — | 4,043 | |||||||||||||||
Total current assets | 14,346 | 19,732 | 73,841 | (14,200 | ) | 93,719 | ||||||||||||||
Property and equipment, net | — | 1,963 | 54,668 | — | 56,631 | |||||||||||||||
Deferred financing costs, net | 4,993 | — | — | — | 4,993 | |||||||||||||||
Intangible assets, net | — | 26,735 | 12,377 | — | 39,112 | |||||||||||||||
Other long-term assets | 3,625 | 25,468 | 1,983 | (25,408 | ) | 5,668 | ||||||||||||||
Receivables from subsidiaries | — | 12,339 | — | (12,339 | ) | — | ||||||||||||||
Investment in subsidiaries | 43,844 | — | — | (43,844 | ) | — | ||||||||||||||
Goodwill, net | 389,087 | — | 892 | 113,381 | 503,360 | |||||||||||||||
Total assets | $ | 455,895 | $ | 86,237 | $ | 143,761 | $ | 17,590 | $ | 703,483 | ||||||||||
F-60
Table of Contents
AMERICAN RENAL HOLDINGS INC. AND SUBSIDIARIES
Notes to Unaudited Consolidated Financial Statements—(Continued)
September 30, 2010
(dollars in thousands, except per share amounts)
NOTE K—SUPPLEMENTAL INFORMATION—(Continued)
| ARH | Subsidiary Guarantors | Non- Guarantors | Eliminations | Successor Consolidated | ||||||||||||||||
Liabilities and Shareholders Equity (Deficit) | ||||||||||||||||||||
Current liabilities | $ | 22,291 | $ | 2,765 | $ | 65,928 | $ | (26,509 | ) | $ | 64,475 | |||||||||
Long-term debt, less current portion | 242,327 | 469 | 30,096 | (25,408 | ) | 247,484 | ||||||||||||||
Capital lease obligations, less current portion | — | — | 175 | — | 175 | |||||||||||||||
Other long-term liabilities | — | 312 | 2,156 | — | 2,468 | |||||||||||||||
Deferred tax liability | 16,968 | — | — | — | 16,968 | |||||||||||||||
Noncontrolling interests subject to put provisions | — | 650 | (133,658 | ) | 175,747 | 42,739 | ||||||||||||||
Total American Renal Holdings Inc. equity | 174,309 | 82,041 | 24,431 | (106,240 | ) | 174,541 | ||||||||||||||
Noncontrolling interests not subject to put provisions | — | — | 154,633 | — | 154,633 | |||||||||||||||
Total equity | 174,309 | 82,041 | 179,064 | (106,240 | ) | 329,174 | ||||||||||||||
Total liabilities and equity | $ | 455,895 | $ | 86,237 | $ | 143,761 | $ | 17,590 | $ | 703,483 | ||||||||||
Condensed Consolidating Balance Sheet—December 31, 2009
| ARH | Subsidiary Guarantors | Non- Guarantors | Eliminations | Predecessor Consolidated | ||||||||||||||||
Assets | ||||||||||||||||||||
Cash and cash equivalents | $ | — | $ | 11,423 | $ | 17,756 | $ | — | $ | 29,179 | ||||||||||
Accounts receivable | — | 215 | 45,439 | — | 45,654 | |||||||||||||||
Inventories | — | 22 | 2,272 | — | 2,294 | |||||||||||||||
Prepaid expenses and other current assets | — | 2,313 | 3,107 | (3,054 | ) | 2,366 | ||||||||||||||
Deferred tax assets | 4,845 | — | — | — | 4,845 | |||||||||||||||
Total current assets | 4,845 | 13,973 | 68,574 | (3,054 | ) | 84,338 | ||||||||||||||
Property and equipment, net | — | 2,054 | 57,351 | — | 59,405 | |||||||||||||||
Deferred financing costs, net | — | 1,000 | 44 | — | 1,044 | |||||||||||||||
Intangible assets, net | — | 363 | 1,055 | — | 1,418 | |||||||||||||||
Other long-term assets | — | 5,161 | 3,251 | (5,093 | ) | 3,319 | ||||||||||||||
Receivables from subsidiaries | — | 12,267 | — | (12,267 | ) | — | ||||||||||||||
Investment in subsidiaries | 38,021 | — | — | (38,021 | ) | — | ||||||||||||||
Goodwill, net | (13,349 | ) | — | 32,973 | 4,574 | 24,198 | ||||||||||||||
Total assets | $ | 29,517 | $ | 34,818 | $ | 163,248 | $ | (53,861 | ) | $ | 173,722 | |||||||||
F-61
Table of Contents
AMERICAN RENAL HOLDINGS INC. AND SUBSIDIARIES
Notes to Unaudited Consolidated Financial Statements—(Continued)
September 30, 2010
(dollars in thousands, except per share amounts)
NOTE K—SUPPLEMENTAL INFORMATION—(Continued)
| ARH | Subsidiary Guarantors | Non- Guarantors | Eliminations | Predecessor Consolidated | ||||||||||||||||
Liabilities and Deficit | ||||||||||||||||||||
Current liabilities | $ | — | $ | 7,095 | $ | 63,814 | $ | (15,292 | ) | $ | 55,617 | |||||||||
Long-term debt, less current portion | — | 40,185 | 29,169 | (5,093 | ) | 64,261 | ||||||||||||||
Capital lease obligations, less current portion | — | — | 214 | — | 214 | |||||||||||||||
Other long-term liabilities | — | 137 | 7,527 | — | 7,664 | |||||||||||||||
Deferred tax liability | 4,548 | — | — | — | 4,548 | |||||||||||||||
Series X preferred stock | 62,799 | — | — | — | 62,799 | |||||||||||||||
Noncontrolling interests subject to put provisions | — | 750 | (99,184 | ) | 136,865 | 38,431 | ||||||||||||||
Total American Renal Holdings Inc. equity (deficit) | (37,830 | ) | (13,349 | ) | 145,651 | (170,341 | ) | (75,869 | ) | |||||||||||
Noncontrolling interests not subject to put provisions | — | — | 16,057 | — | 16,057 | |||||||||||||||
Total equity (deficit) | (37,830 | ) | (13,349 | ) | 161,708 | (170,341 | ) | (59,812 | ) | |||||||||||
Total liabilities and deficit | $ | 29,517 | $ | 34,818 | $ | 163,248 | $ | (53,861 | ) | $ | 173,722 | |||||||||
Condensed Consolidating Statement of Operations—May 8, 2010 to September 30, 2010
| ARH | Subsidiary Guarantors | Non- Guarantors | Eliminations | Successor Consolidated | ||||||||||||||||
Net operating revenues | $ | — | $ | 582 | $ | 120,251 | $ | — | $ | 120,833 | ||||||||||
Total operating expenses | 14,840 | 6,617 | 94,163 | — | 115,620 | |||||||||||||||
Operating income (loss) | (14,840 | ) | (6,035 | ) | 26,088 | — | 5,213 | |||||||||||||
Interest expense, net | (7,777 | ) | (36 | ) | (1,392 | ) | (9,205 | ) | ||||||||||||
Income before (loss) taxes | (22,617 | ) | (6,071 | ) | 24,696 | (3,992 | ) | |||||||||||||
Income tax expense (benefit) | (913 | ) | — | 264 | (649 | ) | ||||||||||||||
Net income (loss) before earnings in subsidiaries | (21,704 | ) | (6,071 | ) | 24,432 | — | (3,343 | ) | ||||||||||||
Equity earnings in subsidiaries | 7,411 | 13,483 | — | (20,894 | ) | — | ||||||||||||||
Net income (loss) | (14,293 | ) | 7,412 | 24,432 | (20,894 | ) | (3,343 | ) | ||||||||||||
Less: Net income attributable to noncontrolling interests | — | — | — | (10,949 | ) | (10,949 | ) | |||||||||||||
Net income (loss) attributable to American Renal Holdings Inc. | $ | (14,293 | ) | $ | 7,412 | $ | 24,432 | $ | (31,843 | ) | $ | (14,292 | ) | |||||||
F-62
Table of Contents
AMERICAN RENAL HOLDINGS INC. AND SUBSIDIARIES
Notes to Unaudited Consolidated Financial Statements—(Continued)
September 30, 2010
(dollars in thousands, except per share amounts)
NOTE K—SUPPLEMENTAL INFORMATION—(Continued)
Condensed Consolidating Statement of Operations—three months ended September 30, 2010
| ARH | Subsidiary Guarantors | Non- Guarantors | Eliminations | Successor Consolidated | ||||||||||||||||
Net operating revenues | $ | — | $ | 312 | $ | 76,919 | $ | — | $ | 77,231 | ||||||||||
Total operating expenses | 22,218 | (20,537 | ) | 60,249 | — | 61,930 | ||||||||||||||
Operating income (loss) | (22,218 | ) | 20,849 | 16,670 | — | 15,301 | ||||||||||||||
Interest expense, net | (4,978 | ) | (31 | ) | (876 | ) | — | (5,885 | ) | |||||||||||
Income before (loss) taxes | (27,196 | ) | 20,818 | 15,794 | — | 9,416 | ||||||||||||||
Income tax expense (benefit) | 837 | — | 140 | 977 | ||||||||||||||||
Net income (loss) before earnings in subsidiaries | (28,033 | ) | 20,818 | 15,654 | — | 8,439 | ||||||||||||||
Equity earnings in subsidiaries | 29,564 | 8,747 | — | (38,311 | ) | — | ||||||||||||||
Net income | 1,531 | 29,565 | 15,654 | (38,311 | ) | 8,439 | ||||||||||||||
Less: Net income attributable to noncontrolling interests | — | — | — | (6,907 | ) | (6,907 | ) | |||||||||||||
Net income attributable to American Renal Holdings Inc. | $ | 1,531 | $ | 29,565 | $ | 15,654 | $ | (45,218 | ) | $ | 1,532 | |||||||||
Condensed Consolidating Statement of Operations—January 1, 2010 to May 7, 2010
| ARH | Subsidiary Guarantors | Non- Guarantors | Eliminations | Predecessor Consolidated | ||||||||||||||||
Net operating revenues | $ | — | $ | 587 | $ | 101,507 | $ | — | $ | 102,094 | ||||||||||
Total operating expenses | 7,378 | 1,040 | 79,113 | — | 87,531 | |||||||||||||||
Operating income (loss) | (7,378 | ) | (453 | ) | 22,394 | — | 14,563 | |||||||||||||
Interest expense, net | (4,119 | ) | (10 | ) | (1,588 | ) | — | (5,717 | ) | |||||||||||
Income (loss) before taxes | (11,497 | ) | (463 | ) | 20,806 | — | 8,846 | |||||||||||||
Income tax expense | 1,963 | — | 301 | — | 2,264 | |||||||||||||||
Net income (loss) before earnings in subsidiaries | (13,460 | ) | (463 | ) | 20,505 | — | 6,582 | |||||||||||||
Equity earnings in subsidiaries | 10,776 | 11,240 | — | (22,016 | ) | — | ||||||||||||||
Net income (loss) | (2,684 | ) | 10,777 | 20,505 | (22,016 | ) | 6,582 | |||||||||||||
Less: Net income attributable to noncontrolling interests | — | — | — | (9,266 | ) | (9,266 | ) | |||||||||||||
Net income (loss) attributable to American Renal Holdings Inc. | $ | (2,684 | ) | $ | 10,777 | $ | 20,505 | $ | (31,282 | ) | $ | (2,684 | ) | |||||||
F-63
Table of Contents
AMERICAN RENAL HOLDINGS INC. AND SUBSIDIARIES
Notes to Unaudited Consolidated Financial Statements—(Continued)
September 30, 2010
(dollars in thousands, except per share amounts)
NOTE K—SUPPLEMENTAL INFORMATION—(Continued)
Condensed Consolidating Statement of Operations—Three Months Ended September 30, 2009
| ARH | Subsidiary Guarantors | Non- Guarantors | Eliminations | Predecessor Consolidated | ||||||||||||||||
Net operating revenues | $ | — | $ | 378 | $ | 67,706 | $ | — | $ | 68,084 | ||||||||||
Total operating expenses | — | 1,216 | 53,068 | — | 54,284 | |||||||||||||||
Operating income (loss) | — | (838 | ) | 14,638 | — | 13,800 | ||||||||||||||
Interest expense, net | (2,058 | ) | (738 | ) | (959 | ) | — | (3,755 | ) | |||||||||||
Income (loss) before taxes | (2,058 | ) | (1,576 | ) | 13,679 | — | 10,045 | |||||||||||||
Income tax expense | 2,275 | — | 307 | — | 2,582 | |||||||||||||||
Net income (loss) before earnings in subsidiaries | (4,333 | ) | (1,576 | ) | 13,372 | — | 7,463 | |||||||||||||
Equity earnings in subsidiaries | 6,042 | 7,618 | — | (13,660 | ) | — | ||||||||||||||
Net income | 1,709 | 6,042 | 13,372 | (13,660 | ) | 7,463 | ||||||||||||||
Less: Net income attributable to noncontrolling interests | — | — | — | (5,756 | ) | (5,756 | ) | |||||||||||||
Net income attributable to American Renal Holdings Inc. | $ | 1,709 | $ | 6,042 | $ | 13,372 | $ | (19,416 | ) | $ | 1,707 | |||||||||
Condensed Consolidating Statement of Operations—Nine Months Ended September 30, 2009
| ARH | Subsidiary Guarantors | Non- Guarantors | Eliminations | Predecessor Consolidated | ||||||||||||||||
Net operating revenues | $ | — | $ | 1,092 | $ | 192,017 | $ | — | $ | 193,109 | ||||||||||
Total operating expenses | — | 3,050 | 151,471 | — | 154,521 | |||||||||||||||
Operating income (loss) | — | (1,958 | ) | 40,546 | — | 38,588 | ||||||||||||||
Interest expense, net | (5,955 | ) | (2,445 | ) | (2,812 | ) | — | (11,212 | ) | |||||||||||
Income (loss) before taxes | (5,955 | ) | (4,403 | ) | 37,734 | — | 27,376 | |||||||||||||
Income tax expense | 6,255 | — | 781 | — | 7,036 | |||||||||||||||
Net income (loss) before earnings in subsidiaries | (12,210 | ) | (4,403 | ) | 36,953 | — | 20,340 | |||||||||||||
Equity earnings in subsidiaries | 16,729 | 21,133 | — | (37,862 | ) | — | ||||||||||||||
Net income | 4,519 | 16,730 | 36,953 | (37,862 | ) | 20,340 | ||||||||||||||
Less: Net income attributable to noncontrolling interests | — | — | — | (15,823 | ) | (15,823 | ) | |||||||||||||
Net income attributable to American Renal Holdings Inc. | $ | 4,519 | $ | 16,730 | $ | 36,953 | $ | (53,685 | ) | $ | 4,517 | |||||||||
F-64
Table of Contents
AMERICAN RENAL HOLDINGS INC. AND SUBSIDIARIES
Notes to Unaudited Consolidated Financial Statements—(Continued)
September 30, 2010
(dollars in thousands, except per share amounts)
NOTE K—SUPPLEMENTAL INFORMATION—(Continued)
Condensed Consolidating Statement of Cash Flows—May 8, 2010 to September 30, 2010
| ARH | Subsidiary Guarantors | Non- Guarantors | Eliminations | Successor Consolidated | ||||||||||||||||
Operating activities | ||||||||||||||||||||
Net income (loss) | $ | (14,293 | ) | $ | 7,411 | $ | 24,432 | $ | (20,893 | ) | $ | (3,343 | ) | |||||||
Change in operating assets and liabilities and noncash items included in net income (loss) | 12,005 | (4,742 | ) | 8,263 | 4,654 | 20,180 | ||||||||||||||
Net cash provided by (used in) operating activities | (2,288 | ) | 2,669 | 32,695 | (16,239 | ) | 16,837 | |||||||||||||
Investing Activities | ||||||||||||||||||||
Other | — | 4,654 | — | (4,654 | ) | — | ||||||||||||||
Purchases of property and equipment | — | (70 | ) | (6,071 | ) | — | (6,141 | ) | ||||||||||||
Merger | (315,957 | ) | 61,834 | (9,239 | ) | 13,646 | (252,608 | ) | ||||||||||||
Cash paid for acquisitions | — | — | (275 | ) | — | (275 | ) | |||||||||||||
Net cash provided by (used in) investing activities | (315,957 | ) | 66,418 | (15,585 | ) | 8,992 | (259,024 | ) | ||||||||||||
Financing Activities | ||||||||||||||||||||
Proceeds from borrowings | — | — | — | — | — | |||||||||||||||
Payments on long-term debt obligations | — | (40,893 | ) | (24,211 | ) | — | (65,104 | ) | ||||||||||||
Other | 318,245 | (24,240 | ) | 18,485 | 7,247 | 322,629 | ||||||||||||||
Net cash provided by (used in) financing activities | 318,245 | (65,133 | ) | (5,726 | ) | 7,247 | 257,525 | |||||||||||||
Net increase (decrease) in cash | — | 3,954 | 11,384 | — | 15,338 | |||||||||||||||
Cash, at beginning of year | — | 1,426 | 10,815 | — | 12,241 | |||||||||||||||
Cash, at ending of year | $ | — | $ | 5,380 | $ | 22,199 | — | $ | 27,579 | |||||||||||
F-65
Table of Contents
AMERICAN RENAL HOLDINGS INC. AND SUBSIDIARIES
Notes to Unaudited Consolidated Financial Statements—(Continued)
September 30, 2010
(dollars in thousands, except per share amounts)
NOTE K—SUPPLEMENTAL INFORMATION—(Continued)
Condensed Consolidating Statement of Cash Flows—January 1, 2010 to May 7, 2010
| ARH | Subsidiary Guarantors | Non- Guarantors | Eliminations | Predecessor Consolidated | ||||||||||||||||
Operating activities | ||||||||||||||||||||
Net income (loss) | $ | (2,685 | ) | $ | 10,776 | $ | 20,507 | ($ | 22,016 | ) | $ | 6,582 | ||||||||
Change in operating assets and liabilities and noncash items included in net income (loss) | (6,300 | ) | (6,067 | ) | (371 | ) | 11,451 | (1,287 | ) | |||||||||||
Net cash provided by (used in) operating activities | (8,985 | ) | (4,709 | ) | 20,136 | (10,565 | ) | 5,295 | ||||||||||||
Investing Activities | ||||||||||||||||||||
Other | (2,691 | ) | (8,493 | ) | — | 11,240 | 56 | |||||||||||||
Purchases of property and equipment | — | (187 | ) | (4,717 | ) | — | (4,904 | ) | ||||||||||||
Cash paid for acquisitions | — | — | (200 | ) | — | (200 | ) | |||||||||||||
Net cash provided by (used in) investing activities | (2,691 | ) | (8,680 | ) | (4,917 | ) | 11,240 | (5,048 | ) | |||||||||||
Financing Activities | ||||||||||||||||||||
Proceeds from borrowings | — | — | 1,250 | (1,250 | ) | — | ||||||||||||||
Payments on long-term debt obligations | — | (1,201 | ) | (4,765 | ) | 575 | (5,391 | ) | ||||||||||||
Other | 11,676 | (4,825 | ) | (18,645 | ) | — | (11,794 | ) | ||||||||||||
Net cash provided by (used in) financing activities | 11,676 | (6,026 | ) | (22,160 | ) | (675 | ) | (17,185 | ) | |||||||||||
Net increase (decrease) in cash | — | (9,997 | ) | (6,941 | ) | — | (16,938 | ) | ||||||||||||
Cash, at beginning of period | — | 11,423 | 17,756 | — | 29,179 | |||||||||||||||
Cash, at ending of period | $ | — | $ | 1,426 | $ | 10,815 | $ | — | $ | 12,241 | ||||||||||
F-66
Table of Contents
AMERICAN RENAL HOLDINGS INC. AND SUBSIDIARIES
Notes to Unaudited Consolidated Financial Statements—(Continued)
September 30, 2010
(dollars in thousands, except per share amounts)
NOTE K—SUPPLEMENTAL INFORMATION—(Continued)
Consolidating Statement of Cash Flows—Nine Months Ended September 30, 2009
| ARH | Subsidiary Guarantors | Non- Guarantors | Eliminations | Predecessor Consolidated | ||||||||||||||||
Operating activities | ||||||||||||||||||||
Net income | $ | 4,519 | $ | 16,726 | $ | 36,956 | $ | (37,861 | ) | $ | 20,340 | |||||||||
Change in operating assets and liabilities, and non-cash items included in net income | 6,820 | 2,845 | 3,986 | 3,231 | 16,882 | |||||||||||||||
Net cash provided by (used in) operating activities | 11,339 | 19,571 | 40,942 | (34,630 | ) | 37,222 | ||||||||||||||
Investing Activities | ||||||||||||||||||||
Purchase of other intangible assets | — | — | — | — | — | |||||||||||||||
Purchases of property and equipment | — | (119 | ) | (9,638 | ) | — | (9,757 | ) | ||||||||||||
Acquisition | — | — | — | — | — | |||||||||||||||
Cash paid for Acquisitions | — | — | (3,964 | ) | — | (3,964 | ) | |||||||||||||
Net cash provided by (used in) investing activities | — | (119 | ) | (13,602 | ) | — | (13,721 | ) | ||||||||||||
Financing Activities | ||||||||||||||||||||
Proceeds from borrowings | — | (246 | ) | 1,254 | — | 1,008 | ||||||||||||||
Payments on long-term debt obligations | — | (1,799 | ) | (6,888 | ) | — | (8,687 | ) | ||||||||||||
Other | (11,339 | ) | (17,067 | ) | (21,682 | ) | 34,630 | (15,458 | ) | |||||||||||
Net cash provided by (used in) financing activities | (11,339 | ) | (19,112 | ) | (27,316 | ) | 34,630 | (23,137 | ) | |||||||||||
Net increase (decrease) in cash | — | 340 | 24 | — | 364 | |||||||||||||||
Cash, at beginning of period | — | 11,869 | 17,313 | — | 29,182 | |||||||||||||||
Cash, at ending of period | $ | — | $ | 12,209 | $ | 17,337 | $ | — | $ | 29,546 | ||||||||||
F-67
Table of Contents

PROSPECTUS
Offer to Exchange
$250,000,000 principal amount of our 8.375% Senior Secured Notes due 2018, which have been registered under the Securities Act of 1933, for any and all of our outstanding 8.375% Senior Secured Notes due 2018.
Until the date that is 90 days from the date of this prospectus, all dealers that effect transactions in these securities, whether or not participating in this offering, may be required to deliver a prospectus. This is in addition to the dealers’ obligation to deliver a prospectus when acting as underwriters with respect to their unsold allotments or subscriptions or otherwise.
Table of Contents
II. INFORMATION NOT REQUIRED IN PROSPECTUS
| Item 20. | Indemnification of Directors and Officers |
(a) American Renal Holdings Inc. is incorporated under the laws of Delaware.
Section 145 of the Delaware General Corporation Law (the “DGCL”) grants each corporation organized thereunder the power to indemnify any person who is or was a director, officer, employee or agent of a corporation or enterprise, against expenses, including attorneys’ fees, judgments, fines and amounts paid in settlement actually and reasonably incurred by him in connection with any threatened, pending or completed action, suit or proceeding, whether civil, criminal, administrative or investigative, other than an action by or in the right of the corporation, by reason of being or having been in any such capacity, if he acted in good faith in a manner reasonably believed to be in, or not opposed to, the best interests of the corporation, and, with respect to any criminal action or proceeding, had no reasonable cause to believe his conduct was unlawful. Section 102(b)(7) of the DGCL enables a corporation in its certificate of incorporation or an amendment thereto to eliminate or limit the personal liability of a director to the corporation or its stockholders of monetary damages for violations of the directors’ fiduciary duty of care, except (i) for any breach of the directors’ duty of loyalty to the corporation or its stockholders, (ii) for acts or omissions not in good faith or that involve intentional misconduct or a knowing violation of law, (iii) pursuant to Section 174 of the DGCL (providing for liability of directors for unlawful payment of dividends or unlawful stock purchases or redemptions) or (iv) for any transaction from which a director derived an improper personal benefit.
The Certificate of Incorporation and the By-Laws of American Renal Holdings Inc. indemnifies and holds harmless, to the fullest extent permitted by the DGCL, as the same exists or may hereafter be amended (but only to the extent such amendment permits broader indemnification rights), each person who was or is made a party or is threatened to be made a party to or is or was involved in any action, suit or proceeding, whether, civil, criminal, administrative or investigative, by reason of the fact that he or she or a person of whom he or she is the legal representative is or was a director of American Renal Holdings Inc. or is or was serving at the request of American Renal Holdings Inc. as a director of another entity. The Articles of Incorporation and By-Laws of American Renal Holdings Inc. also permit American Renal Holdings Inc. to maintain insurance, at its expense, to protect itself and any director, officer, employee or agent of American Renal Holdings Inc. or another entity against any expense, liability or loss, whether or not American Renal Holdings Inc. would have the power to indemnify such person under the DGCL.
(b) C.P. Atlas Intermediate Holdings, LLC, American Renal Associates LLC, American Renal Management LLC, AKC Holdings LLC, JKC Holdings LLC, ARA-Boca Raton Holding LLC, ARA-Ohio Holdings LLC, ARA-Rhode Island Dialysis II LLC, Texas-ARA LLC and Acute Dialysis Services-ARA LLC are limited liability companies organized under the laws of Delaware.
Section 18-108 of the Delaware Limited Liability Company Act empowers a Delaware limited liability company to indemnify and hold harmless any member or manager of the limited liability company from and against any and all claims and demands whatsoever.
The Limited Liability Company Agreement of C.P. Atlas Intermediate Holdings, LLC provides that the sole LLC member, the officers and any respective affiliates or agents of C.P. Atlas Intermediate Holdings, LLC are entitled to indemnification from C.P. Atlas Intermediate Holdings, LLC, to the fullest extent permitted by applicable law, for any loss, damage or claim incurred by reason of any act or omission performed or omitted by such person in good faith on behalf of C.P. Atlas Intermediate Holdings, LLC and in a manner reasonably believed to be within the scope of authority conferred upon such person.
(c) American Renal Texas L.P. and American Renal Texas II, L.P. are limited partnerships formed under the laws of Texas.
II-1
Table of Contents
American Renal Texas L.P. and American Renal Texas II, L.P. are limited partnerships formed under the laws of Texas.
Chapter 8 of the Texas Business Organizations Code (“TBOC”) requires a limited partnership to indemnify a general partner or former general partner who incurs expenses in connection with a legal proceeding relating to such current or former general partner’s position with the partnership. Indemnification is mandatory only if (i) the current or former general partner is wholly successful in the underlying legal proceeding, and (ii) such indemnification is not otherwise prohibited by a written partnership agreement.
Additionally, Chapter 8 permits a limited partnership to indemnify a general partner or former general partner who acted in good faith and reasonably believed that (i) the conduct was in the partnership’s best interests (if performed in the general partner’s official capacity), or (ii) the conduct was not opposed to the partnership’s best interests (if performed outside of the general partner’s official capacity). In the case of a criminal proceeding, indemnification is permitted only if the general partner had no reasonable belief that the conduct was unlawful. Chapter 8 permits indemnification of a general partner without the necessity of indemnification provisions in the partnership agreement. In the absence of such provisions, however, the partnership must make the determination to indemnify a general partner according to the guidelines provided in Section 8.103 of the TBOC.
In all instances, Chapter 8 prohibits a limited partnership from indemnifying a general partner or former general partner in relation to a proceeding in which the general partner is found to be liable for (i) willful or intentional misconduct, (ii) breach of the duty of loyalty or (iii) an act or omission not in good faith constituting a breach of the general partner’s duty to the partnership.
Chapter 8 provides that limited partners, employees and others who are not also general partners may be indemnified by provisions in the partnership agreement, by contract, by common law or through other action by the partnership’s governing authority.
The Agreement of Limited Partnership of American Renal Texas L.P. and the Agreement of Limited Partnership of American Renal Texas II, L.P. provide for the indemnification of officers, employees and current and former general partners so long the person seeking indemnification meets certain standards of conduct consistent with those set forth in Chapter 8.
EXHIBIT | EXHIBIT DESCRIPTION | |
| 2.1* | Contribution and Merger Agreement, dated as of March 22, 2010, by and among American Renal Holdings Inc., the rollover stockholders named therein, Wachovia Capital Partners GP I, LLC, C.P. Atlas Holdings, Inc., C.P. Atlas Intermediate Holdings, LLC and C.P. Atlas Acquisition Corp. | |
| 3.1* | Restated Certificate of Incorporation of American Renal Holdings Inc., as amended. | |
| 3.2* | American Renal Associates Inc. By-Laws. | |
| 3.3* | Certificate of Formation of C.P. Atlas Intermediate Holdings, LLC. | |
| 3.4* | Limited Liability Company Agreement of C.P. Atlas Intermediate Holdings, LLC. | |
| 3.5* | Certificate of Formation of American Renal Associates LLC. | |
| 3.6* | Limited Liability Company Agreement of American Renal Associates LLC. | |
| 3.7* | Certificate of Formation of Texas-ARA LLC. | |
| 3.8* | Texas-ARA LLC Operating Agreement. | |
| 3.9* | Certificate of Formation of JKC Holding LLC. | |
II-2
Table of Contents
EXHIBIT | EXHIBIT DESCRIPTION | |
| 3.10* | JKC Holding LLC Operating Agreement. | |
| 3.11* | Certificate of Formation of ARA-Rhode Island Dialysis II LLC. | |
| 3.12* | ARA-Rhode Island Dialysis II LLC Operating Agreement. | |
| 3.13* | Certificate of Formation of ARA-Ohio Holdings LLC. | |
| 3.14* | ARA-Ohio Holding LLC Operating Agreement. | |
| 3.15* | Certificate of Formation of ARA-Boca Raton Holding LLC. | |
| 3.16* | ARA-Boca Raton Holding LLC Operating Agreement. | |
| 3.17* | Certificate of Formation of American Renal Management LLC. | |
| 3.18* | American Renal Management LLC Operating Agreement. | |
| 3.19* | Certificate of Formation of AKC Holding LLC | |
| 3.20* | AKC Holding LLC Operating Agreement. | |
| 3.21* | Certificate of Formation of Acute Dialysis Services-ARA LLC. | |
| 3.22* | Acute Dialysis Services-ARA LLC Operation Agreement. | |
| 3.23* | Certificate of Limited Partnership of American Renal Texas L.P. | |
| 3.24* | American Renal Texas L.P. Agreement of Limited Partnership. | |
| 3.25* | Certificate of Limited Partnership of American Renal Texas II, L.P. | |
| 3.26* | American Renal Texas II, L.P. Agreement of Limited Partnership. | |
| 3.27* | Form of Joint Venture Operating Agreement. | |
| 4.1* | Indenture, dated as May 7, 2010, among American Renal Holdings Inc., the guarantors named therein and Wilmington Trust FSB, as trustee. | |
| 4.2* | Registration Rights Agreement, dated as May 7, 2010, among American Renal Holdings Inc., the guarantors named therein, and Banc of America Securities LLC, Barclays Capital Inc. and Wells Fargo Securities, LLC, as representatives to the several initial purchasers. | |
| 4.3* | Form of 8.375% Senior Secured Note due 2018 (included in Exhibit 4.1). | |
| 4.4* | Security Agreement dated as May 7, 2010, among American Renal Holdings Inc., the guarantors named therein and Wilmington Trust FSB, as collateral agent. | |
| 4.5* | Trademark Security Agreement, dated as of May 7, 2010, between American Renal Associates LLC and Wilmington Trust FSB, as collateral agent. | |
| 4.6* | Intercreditor Agreement among American Renal Holdings Inc., C.P. Atlas Acquisition Corp., the guarantors named therein, Bank of America, N.A., as credit agreement administrative agent, and Wilmington Trust FSB, as collateral agent. | |
| 5.1* | Opinion of Simpson Thacher & Bartlett LLP. | |
| 5.2* | Opinion of Greenberg Traurig, LLP. | |
| 10.1* | Credit Agreement, dated as of May 7, 2010, among C.P. Atlas Acquisition Corp. (to be merged with and into American Renal Holdings Inc.), C.P. Intermediate Holdings, LLC, Bank of America, N.A. and the other lenders party thereto. | |
| 10.2* | Guaranty, dated as of May 7, 2010, among C.P. Atlas Acquisition Corp. (to be merged with and into American Renal Holdings Inc.), the guarantors named therein, Bank of America, N.A. and the other secured parties named therein. | |
II-3
Table of Contents
EXHIBIT | EXHIBIT DESCRIPTION | |
| 10.3* | Security Agreement, dated as of May 7, 2010, among C.P. Atlas Acquisition Corp. (to be merged with and into American Renal Holdings Inc.), the guarantors party thereto and Bank of America, N.A., as administrative agent. | |
| 10.4* | Employment Agreement, dated as of March 22, 2010, among American Renal Management LLC, American Renal Holdings Inc. and Christopher T. Ford. | |
| 10.5* | Employment Agreement, dated as of March 22, 2010, among American Renal Management LLC, American Renal Holdings Inc. and Syed T. Kamal. | |
| 10.6* | Employment Agreement, dated as of March 22, 2010, among American Renal Management LLC, American Renal Holdings Inc. and Joseph A. Carlucci. | |
| 10.7* | Employment Agreement, dated as of March 22, 2010, among American Renal Management LLC, American Renal Holdings Inc. and John J. McDonough. | |
| 10.8* | 2010 C.P. Atlas Holdings, Inc. Stock Incentive Plan. | |
| 10.9* | Subscription Agreement, dated as of May 7, 2010, by and between C.P. Atlas Holdings, Inc., Centerbridge Capital Partners, L.P., Centerbridge Capital Partners SBS, L.P., Centerbridge Capital Partners Strategic, L.P., AFOS Equity LLC, Black Diamond Partners LLC, JJ Bark LLC and Tribeca Investments LLC. | |
| 10.10* | Equity Contribution, Exchange and Subscription Agreement, dated as of March 22, 2010, by and between C.P. Atlas Holdings, Inc. and Joseph A. Carlucci. | |
| 10.11* | Equity Contribution, Exchange and Subscription Agreement, dated as of March 22, 2010, by and between C.P. Atlas Holdings, Inc. and Christopher T. Ford 2005 Grantor Retained Annuity Trust. | |
| 10.12* | Equity Contribution, Exchange and Subscription Agreement, dated as of March 22, 2010, by and between C.P. Atlas Holdings, Inc. and Christopher T. Ford 2008 Grantor Retained Annuity Trust. | |
| 10.13* | Equity Contribution, Exchange and Subscription Agreement, dated as of April 30, 2010, by and between C.P. Atlas Holdings, Inc. and Wesley V. Forgue. | |
| 10.14* | Equity Contribution, Exchange and Subscription Agreement, dated as of March 22, 2010, by and between C.P. Atlas Holdings, Inc. and Syed T. Kamal. | |
| 10.15* | Equity Contribution, Exchange and Subscription Agreement, dated as of March 22, 2010, by and between C.P. Atlas Holdings, Inc. and John McDonough. | |
| 10.16* | Equity Contribution, Exchange and Subscription Agreement, dated as of April 29, 2010, by and between C.P. Atlas Holdings, Inc. and Lakhan Saha. | |
| 10.17* | Transaction Fee and Advisory Services Agreement, dated as of May 7, 2010, by and among C.P. Atlas Holdings, Inc., C.P. Atlas Intermediate Holdings, LLC, American Renal Holdings Inc. and Centerbridge Advisors, LLC. | |
| 12.1* | Statement of Computation of Ratio of Earnings to Fixed Charges. | |
| 21.1* | Subsidiaries of American Renal Holdings Inc. | |
| 23.1* | Consent of Simpson Thacher & Bartlett LLP (included as part of its opinion in Exhibit 5.1). | |
| 23.2* | Consent of Greenberg Traurig, LLP (included as part of its opinion in Exhibit 5.2). | |
| 23.3† | Consent of Grant Thornton LLP. | |
| 24.1* | Power of Attorney (included in the signature pages of this registration statement). | |
II-4
Table of Contents
EXHIBIT | EXHIBIT DESCRIPTION | |
| 25.1* | Form T-1 Statement of Eligibility under the Trust Indenture Act of 1939 of Wilmington Trust FSB as trustee under the Indenture, dated May 7, 2010, among American Renal Holdings Inc., the guarantors named therein and Wilmington Trust FSB, as trustee. | |
| 99.1* | Form of Letter of Transmittal. | |
| 99.2* | Form of Letter to Brokers, Dealers, Commercial Banks, Trust Companies and Other Nominees. | |
| 99.3* | Form of Letter to Clients. | |
| 99.4* | Form of Notice of Guaranteed Delivery. | |
| * | Previously filed. |
| † | Filed herewith. |
| Item 22. | Undertakings. |
| (a) | Each of the undersigned registrant hereby undertakes: |
| (1) | To file, during any period in which offers or sales are being made, a post-effective amendment to this registration statement: |
| (i) | to include any prospectus required by section 10(a)(3) of the Securities Act of 1933; |
| (ii) | to reflect in the prospectus any facts or events arising after the effective date of the registration statement (or the most recent post-effective amendment thereof) which, individually or in the aggregate, represent a fundamental change in the information set forth in the registration statement. Notwithstanding the foregoing, any increase or decrease in volume of securities offered (if the total dollar value of securities offered would not exceed that which was registered) and any deviation from the low or high end of the estimated maximum offering range may be reflected in the form of prospectus filed with the Commission pursuant to Rule 424(b) if, in the aggregate, the changes in volume and price represent no more than 20% change in the maximum aggregate offering price set forth in the “Calculation of Registration Fee” table in the effective registration statement; and |
| (iii) | to include any material information with respect to the plan of distribution not previously disclosed in the registration statement or any material change to such information in the registration statement. |
| (2) | that, for the purpose of determining any liability under the Securities Act of 1933, each such post-effective amendment shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof; |
| (3) | to remove from registration by means of a post-effective amendment any of the securities being registered which remain unsold at the termination of the offering; |
| (4) | that, for the purpose of determining liability under the Securities Act of 1933 to any purchaser: each prospectus filed pursuant to Rule 424(b) as part of a registration statement relating to an offering, other than registration statements relying on Rule 430B or other than prospectuses filed in reliance on Rule 430A, shall be deemed to be part of and included in the registration statement as of the date it is first used after effectiveness. Provided, however, that no statement made in a registration statement or prospectus that is part of the registration statement or made in a document incorporated or deemed incorporated by reference into the registration statement or prospectus that is part of the registration statement will, as to a purchaser with a time of contract of sale prior to such first use, supersede or modify any statement that was made in the registration statement or prospectus that was part of the registration statement or made in any such document immediately prior to such date of first use; and |
| (5) | that, for the purpose of determining liability of the registrant under the Securities Act of 1933 to any purchaser in the initial distribution of the securities: The undersigned registrant undertakes that in a |
II-5
Table of Contents
primary offering of securities of the undersigned registrant pursuant to this registration statement, regardless of the underwriting method used to sell the securities to the purchaser, if the securities are offered or sold to such purchaser by means of any of the following communications, the undersigned registrant will be a seller to the purchaser and will be considered to offer or sell such securities to such purchaser: |
| (i) | any preliminary prospectus or prospectus of the undersigned registrant relating to the offering required to be filed pursuant to Rule 424; |
| (ii) | any free writing prospectus relating to the offering prepared by or on behalf of the undersigned registrant or used or referred to by the undersigned registrant; |
| (iii) | the portion of any other free writing prospectus relating to the offering containing material information about the undersigned registrant or its securities provided by or on behalf of the undersigned registrant; and |
| (iv) | any other communication that is an offer in the offering made by the undersigned registrant to the purchaser. |
| (b) | Insofar as indemnification for liabilities arising under the Securities Act of 1933 may be permitted to directors, officers and controlling persons of the registrant pursuant to the foregoing provisions, or otherwise, the registrant has been advised that in the opinion of the Securities and Exchange Commission such indemnification is against public policy as expressed in the Act and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities (other than the payment by the registrant of expenses incurred or paid by a director, officer or controlling person of the registrant in the successful defense of any action, suit or proceeding) is asserted by such director, officer or controlling person in connection with the securities being registered, the registrant will, unless in the opinion of its counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question whether such indemnification by it is against public policy as expressed in the Act and will be governed by the final adjudication of such issue. |
| (c) | The undersigned registrant hereby undertakes to respond to requests for information that is incorporated by reference into the prospectus pursuant to Items 4, 10(b), 11, or 13 of this Form, within one business day of receipt of such request, and to send the incorporated documents by first class mail or other equally prompt means. This includes information contained in documents filed subsequent to the effective date of the registration statement through the date of responding to the request. |
| (d) | Each of the undersigned registrant hereby undertakes to supply by means of a post-effective amendment all information concerning a transaction, and the company being acquired involved therein, that was not the subject of and included in the registration statement when it became effective. |
II-6
Table of Contents
SIGNATURES
Pursuant to the requirements of the Securities Act, the registrant has duly caused this registration statement to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of Beverly, State of Massachusetts, on this 23rd day of December, 2010.
| AMERICAN RENAL HOLDINGS INC. | ||
/S/ MICHAEL R. COSTA | ||
| Name: | Michael R. Costa | |
| Title: | Vice President, General Counsel | |
Pursuant to the requirements of the Securities Act of 1933, this registration statement has been signed by the following persons in the capacities and on the dates indicated.
Signature | Title | Date | ||
* Joseph A. Carlucci | Chief Executive Officer, Director (Principal Executive Officer) | December 23, 2010 | ||
* John J. McDonough | Chief Financial Officer, Executive Vice President (Principal Financial Officer) | December 23, 2010 | ||
* Syed T. Kamal | President, Director | December 23, 2010 | ||
* Christopher T. Ford | Chairman of the Board | December 23, 2010 | ||
/S/ MICHAEL R. COSTA Michael R. Costa | Vice President, General Counsel | December 23, 2010 | ||
* Steven M. Silver | Director | December 23, 2010 | ||
* Jared S. Hendricks | Director | December 23, 2010 | ||
* Michael E. Boxer | Director | December 23, 2010 | ||
*By: | /S/ MICHAEL R. COSTA Michael R. Costa Attorney-in-Fact | |||||
Table of Contents
SIGNATURES
Pursuant to the requirements of the Securities Act, the registrant has duly caused this registration statement to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of Beverly, State of Massachusetts, on this 23rd day of December, 2010.
| C.P. ATLAS INTERMEDIATE, LLC | ||
| By: | C.P. Atlas, Inc., its Sole Member | |
/S/ MICHAEL R. COSTA | ||
| Name: | Michael R. Costa | |
| Title: | Secretary | |
Pursuant to the requirements of the Securities Act of 1933, this registration statement has been signed by the following persons in the capacities and on the dates indicated.
Signature | Title | Date | ||
* Joseph A. Carlucci | President (Principal Executive Officer) | December 23, 2010 | ||
* John J. McDonough | Treasurer (Principal Financial Officer) | December 23, 2010 | ||
*By: | /S/ MICHAEL R. COSTA Michael R. Costa Attorney-in-Fact |
Table of Contents
SIGNATURES
Pursuant to the requirements of the Securities Act, the registrant has duly caused this registration statement to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of Beverly, State of Massachusetts, on this 23rd day of December, 2010.
| AMERICAN RENAL ASSOCIATES LLC | ||
| By: American Renal Holdings Inc. | ||
| AMERICAN RENAL MANAGEMENT LLC | ||
| AKC HOLDING LLC | ||
| JKC HOLDING LLC | ||
| ARA-BOCA RATON HOLDING LLC | ||
| ARA-OHIO HOLDINGS LLC | ||
| ARA-RHODE ISLAND DIALYSIS II LLC | ||
| TEXAS-ARA LLC | ||
| ACUTE DIALYSIS SERVICES-ARA LLC | ||
| By: American Renal Associates LLC | ||
| AMERICAN RENAL TEXAS L.P. | ||
| AMERICAN RENAL TEXAS II, L.P. | ||
| By: Texas-ARA LLC | ||
* | ||
Name: | Joseph A. Carlucci | |
Title: | Chief Executive Officer | |
Pursuant to the requirements of the Securities Act of 1933, this registration statement has been signed by the following persons in the capacities and on the dates indicated.
Signature | Title | Date | ||
* Joseph A. Carlucci | Chief Executive Officer (Principal Executive Officer) | December 23, 2010 | ||
* John J. McDonough | Chief Financial Officer (Principal Financial Officer) | December 23, 2010 | ||
*By: | /S/ MICHAEL R. COSTA Michael R. Costa Attorney-in-Fact |
Table of Contents
EXHIBIT INDEX
EXHIBIT | EXHIBIT DESCRIPTION | |
| 2.1* | Contribution and Merger Agreement, dated as of March 22, 2010, by and among American Renal Holdings Inc., the rollover stockholders named therein, Wachovia Capital Partners GP I, LLC, C.P. Atlas Holdings, Inc., C.P. Atlas Intermediate Holdings, LLC and C.P. Atlas Acquisition Corp. | |
| 3.1* | Restated Certificate of Incorporation of American Renal Holdings Inc., as amended. | |
| 3.2* | American Renal Associates Inc. By-Laws. | |
| 3.3* | Certificate of Formation of C.P. Atlas Intermediate Holdings, LLC. | |
| 3.4* | Limited Liability Company Agreement of C.P. Atlas Intermediate Holdings, LLC. | |
| 3.5* | Certificate of Formation of American Renal Associates LLC. | |
| 3.6* | Limited Liability Company Agreement of American Renal Associates LLC. | |
| 3.7* | Certificate of Formation of Texas-ARA LLC. | |
| 3.8* | Texas-ARA LLC Operating Agreement. | |
| 3.9* | Certificate of Formation of JKC Holding LLC. | |
| 3.10* | JKC Holding LLC Operating Agreement. | |
| 3.11* | Certificate of Formation of ARA-Rhode Island Dialysis II LLC. | |
| 3.12* | ARA-Rhode Island Dialysis II LLC Operating Agreement. | |
| 3.13* | Certificate of Formation of ARA-Ohio Holdings LLC. | |
| 3.14* | ARA-Ohio Holding LLC Operating Agreement. | |
| 3.15* | Certificate of Formation of ARA-Boca Raton Holding LLC. | |
| 3.16* | ARA-Boca Raton Holding LLC Operating Agreement. | |
| 3.17* | Certificate of Formation of American Renal Management LLC. | |
| 3.18* | American Renal Management LLC Operating Agreement. | |
| 3.19* | Certificate of Formation of AKC Holding LLC | |
| 3.20* | AKC Holding LLC Operating Agreement. | |
| 3.21* | Certificate of Formation of Acute Dialysis Services-ARA LLC. | |
| 3.22* | Acute Dialysis Services-ARA LLC Operation Agreement. | |
| 3.23* | Certificate of Limited Partnership of American Renal Texas L.P. | |
| 3.24* | American Renal Texas L.P. Agreement of Limited Partnership. | |
| 3.25* | Certificate of Limited Partnership of American Renal Texas II, L.P. | |
| 3.26* | American Renal Texas II, L.P. Agreement of Limited Partnership. | |
| 3.27* | Form of Joint Venture Operating Agreement. | |
| 4.1* | Indenture, dated as May 7, 2010, among American Renal Holdings Inc., the guarantors named therein and Wilmington Trust FSB, as trustee. | |
| 4.2* | Registration Rights Agreement, dated as May 7, 2010, among American Renal Holdings Inc., the guarantors named therein, and Banc of America Securities LLC, Barclays Capital Inc. and Wells Fargo Securities, LLC, as representatives to the several initial purchasers. | |
Table of Contents
EXHIBIT | EXHIBIT DESCRIPTION | |
| 4.3* | Form of 8.375% Senior Secured Note due 2018 (included in Exhibit 4.1). | |
| 4.4* | Security Agreement dated as May 7, 2010, among American Renal Holdings Inc., the guarantors named therein and Wilmington Trust FSB, as collateral agent. | |
| 4.5* | Trademark Security Agreement, dated as of May 7, 2010, between American Renal Associates LLC and Wilmington Trust FSB, as collateral agent. | |
| 4.6* | Intercreditor Agreement among American Renal Holdings Inc., C.P. Atlas Acquisition Corp., the guarantors named therein, Bank of America, N.A., as credit agreement administrative agent, and Wilmington Trust FSB, as collateral agent. | |
| 5.1* | Opinion of Simpson Thacher & Bartlett LLP. | |
| 5.2* | Opinion of Greenberg Traurig, LLP. | |
| 10.1* | Credit Agreement, dated as of May 7, 2010, among C.P. Atlas Acquisition Corp. (to be merged with and into American Renal Holdings Inc.), C.P. Intermediate Holdings, LLC, Bank of America, N.A. and the other lenders party thereto. | |
| 10.2* | Guaranty, dated as of May 7, 2010, among C.P. Atlas Acquisition Corp. (to be merged with and into American Renal Holdings Inc.), the guarantors named therein, Bank of America, N.A. and the other secured parties named therein. | |
| 10.3* | Security Agreement, dated as of May 7, 2010, among C.P. Atlas Acquisition Corp. (to be merged with and into American Renal Holdings Inc.), the guarantors party thereto and Bank of America, N.A., as administrative agent. | |
| 10.4* | Employment Agreement, dated as of March 22, 2010, among American Renal Management LLC, American Renal Holdings Inc. and Christopher T. Ford. | |
| 10.5* | Employment Agreement, dated as of March 22, 2010, among American Renal Management LLC, American Renal Holdings Inc. and Syed T. Kamal. | |
| 10.6* | Employment Agreement, dated as of March 22, 2010, among American Renal Management LLC, American Renal Holdings Inc. and Joseph A. Carlucci. | |
| 10.7* | Employment Agreement, dated as of March 22, 2010, among American Renal Management LLC, American Renal Holdings Inc. and John J. McDonough. | |
| 10.8* | 2010 C.P. Atlas Holdings, Inc. Stock Incentive Plan. | |
| 10.9* | Subscription Agreement, dated as of May 7, 2010, by and between C.P. Atlas Holdings, Inc., Centerbridge Capital Partners, L.P., Centerbridge Capital Partners SBS, L.P., Centerbridge Capital Partners Strategic, L.P., AFOS Equity LLC, Black Diamond Partners LLC, JJ Bark LLC and Tribeca Investments LLC. | |
| 10.10* | Equity Contribution, Exchange and Subscription Agreement, dated as of March 22, 2010, by and between C.P. Atlas Holdings, Inc. and Joseph A. Carlucci. | |
| 10.11* | Equity Contribution, Exchange and Subscription Agreement, dated as of March 22, 2010, by and between C.P. Atlas Holdings, Inc. and Christopher T. Ford 2005 Grantor Retained Annuity Trust. | |
| 10.12* | Equity Contribution, Exchange and Subscription Agreement, dated as of March 22, 2010, by and between C.P. Atlas Holdings, Inc. and Christopher T. Ford 2008 Grantor Retained Annuity Trust. | |
| 10.13* | Equity Contribution, Exchange and Subscription Agreement, dated as of April 30, 2010, by and between C.P. Atlas Holdings, Inc. and Wesley V. Forgue. | |
| 10.14* | Equity Contribution, Exchange and Subscription Agreement, dated as of March 22, 2010, by and between C.P. Atlas Holdings, Inc. and Syed T. Kamal. | |
Table of Contents
EXHIBIT | EXHIBIT DESCRIPTION | |
| 10.15* | Equity Contribution, Exchange and Subscription Agreement, dated as of March 22, 2010, by and between C.P. Atlas Holdings, Inc. and John McDonough. | |
| 10.16* | Equity Contribution, Exchange and Subscription Agreement, dated as of April 29, 2010, by and between C.P. Atlas Holdings, Inc. and Lakhan Saha. | |
| 10.17* | Transaction Fee and Advisory Services Agreement, dated as of May 7, 2010, by and among C.P. Atlas Holdings, Inc., C.P. Atlas Intermediate Holdings, LLC, American Renal Holdings Inc. and Centerbridge Advisors, LLC. | |
| 12.1* | Statement of Computation of Ratio of Earnings to Fixed Charges. | |
| 21.1* | Subsidiaries of American Renal Holdings Inc. | |
| 23.1* | Consent of Simpson Thacher & Bartlett LLP (included as part of its opinion in Exhibit 5.1). | |
| 23.2* | Consent of Greenberg Traurig, LLP (included as part of its opinion in Exhibit 5.2). | |
| 23.3† | Consent of Grant Thornton LLP. | |
| 24.1* | Power of Attorney (included in the signature pages of this registration statement). | |
| 25.1* | Form T-1 Statement of Eligibility under the Trust Indenture Act of 1939 of Wilmington Trust FSB as trustee under the Indenture, dated May 7, 2010, among American Renal Holdings Inc., the guarantors named therein and Wilmington Trust FSB, as trustee. | |
| 99.1* | Form of Letter of Transmittal. | |
| 99.2* | Form of Letter to Brokers, Dealers, Commercial Banks, Trust Companies and Other Nominees. | |
| 99.3* | Form of Letter to Clients. | |
| 99.4* | Form of Notice of Guaranteed Delivery. | |
| * | Previously filed. |
| † | Filed herewith. |