Exhibit 99.2

PRIMA BIOMED
ASX/Media Release (Code: ASX: PRR; NASDAQ: PBMD)
13 October 2015
Appendix 3B & Cleansing Notice
SYDNEY, AUSTRALIA - Prima BioMed Ltd (ASX: PRR; NASDAQ: PBMD) (“Prima” or the “Company”) Prima advises that in relation to the issuance of ordinary shares as further detailed in the attached Appendix 3B, the Company gives notice under section 708A(5)(e) of the Corporations Act 2001(Cth) (the “Corporations Act”) that:
1. the abovementioned ordinary shares were issued without disclosure to the investor under Part 6D.2 of the Corporations Act;
2. as at the date of this notice the Company has complied with:
(a) the provisions of Chapter 2M Corporations Act as they apply to the Company; and
(b) section 674 Corporations Act; and
3. As at the date of this notice there is no “excluded information” (as defined in subsection 708A(7) of the Corporations Act) which is required to be disclosed by the Company.
Yours faithfully
Prima BioMed Ltd
Deanne Miller
General Counsel & Company Secretary
Prima BioMed Ltd, Level 7, 151 Macquarie Street, Sydney NSW 2000
Phone: +61 2 9276 1224 Fax: +61 2 8569 1880
www.primabiomed.com.au ABN: 90 009 237 889

Rule 2.7, 3.10.3, 3.10.4, 3.10.5
Appendix 3B
New issue announcement, application for quotation of additional securities and agreement
Information or documents not available now must be given to ASX as soon as available. Information and documents given to ASX become ASX’s property and may be made public.
Introduced 1/7/96. Origin: Appendix 5. Amended 1/7/98, 1/9/99, 1/7/2000, 30/9/2001, 11/3/2002, 1/1/2003, 24/10/2005.
Name of entity
Prima BioMed Ltd (Company)
ABN
90 009 237 889
We (the entity) give ASX the following information.
Part 1 - All issues
You must complete the relevant sections (attach sheets if there is not enough space).
1 +Class of +securities issued or to be issued Ordinary Shares
2 Number of +securities issued or to be issued (if known) or maximum number which may be issued 31,022,181 Ordinary Shares

3 Principal terms of the +securities (e.g., if options, exercise price and expiry date; if partly paid +securities, the amount outstanding and due dates for payment; if +convertible securities, the conversion price and dates for conversion) Pari passu with existing Ordinary Shares (PRR)
4 Do the +securities rank equally in all respects from the date of allotment with an existing +class of quoted +securities? Yes (PRR)
If the additional securities do not rank equally, please state:
the date from which they do
the extent to which they participate for the next dividend, (in the case of a trust, distribution) or interest payment
the extent to which they do not rank equally, other than in relation to the next dividend, distribution or interest payment
5 Issue price or consideration 5 cents per share
Prima BioMed Ltd, Level 7, 151 Macquarie Street, Sydney NSW 2000
Phone: +61 2 9276 1224 Fax: +61 2 8569 1880
www.primabiomed.com.au ABN: 90 009 237 889

6 Purpose of the issue (If issued as consideration for the acquisition of assets, clearly identify those assets) The purpose of the issue is to raise capital for general corporate and working capital purposes.
6a Is the entity an +eligible entity that has obtained security holder approval under rule 7.1A? Yes
If Yes, complete sections 6b – 6h in relation to the +securities the subject of this Appendix 3B, and comply with section 6i
y
6b The date the security holder resolution under rule 7.1A was passed 14 November 2014
6c Number of +securities issued without security holder approval under rule 7.1 Not applicable
6d Number of +securities issued with security holder approval under rule 7.1A 31,022,181 Ordinary Shares
6e Number of +securities issued with security holder approval under rule 7.3, or another specific security holder approval (specify date of meeting) Not applicable
6f Number of +securities issued under an exception in rule 7.2 Not applicable
6g If +securities issued under rule 7.1A, was issue price at least 75% of 15 day VWAP as calculated under rule 7.1A.3? Include the +issue date and both values. Include the source of the VWAP calculation. Yes Issue price = 5 cents 75% of 15 day VWAP = 4.37 cents Source - IRESS
Prima BioMed Ltd, Level 7, 151 Macquarie Street, Sydney NSW 2000
Phone: +61 2 9276 1224 Fax: +61 2 8569 1880
www.primabiomed.com.au ABN: 90 009 237 889

6h If +securities were issued under rule 7.1A for non-cash consideration, state date on which valuation of consideration was released to ASX Market Announcements Not applicable
6i Calculate the entity’s remaining issue capacity under rule 7.1 and rule 7.1A – complete Annexure 1 and release to ASX Market Announcements Refer Annexure 1
7 Dates of entering +securities into uncertificated holdings or despatch of certificates 13 October 2015
Number +Class
8 Number and +class of all +securities quoted on ASX (including the securities in clause 2 if applicable) 2,018,297,608 Ordinary fully paid shares (ASX: PRR)
77,378,696 Options exercisable at $0.20 on or before 19 June 2017 (PRRO)
Number +Class - Options
9 Number and +class of all Amount Exercise Price Expiration Date
740,741 $0.3390 1 February 2016
200,000 $0.1730 20 February 2016
1,515,752 $0.0774 30 June 2018
165,116 $0.0774 30 June 2018
147,628,500 $0.05019 12 December 2018
371,445,231 $0.02374 August 2020
8,475,995 $0.0254 August 2025
Number +Class – Performance Rights
Amount Type Expiration Date
30,918,333 LTI 30 October 2018
Prima BioMed Ltd, Level 7, 151 Macquarie Street, Sydney NSW 2000
Phone: +61 2 9276 1224 Fax: +61 2 8569 1880
www.primabiomed.com.au ABN: 90 009 237 889
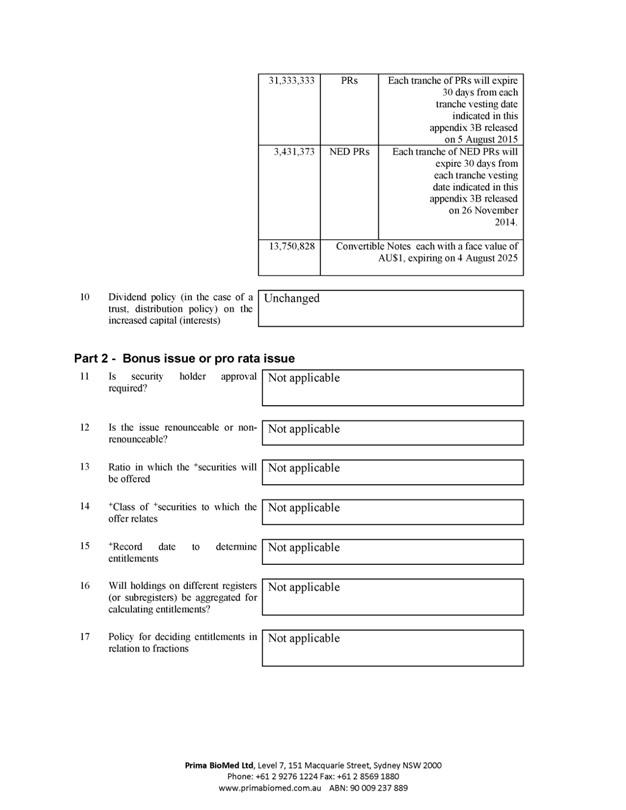
31,333,333 PRs Each tranche of PRs will expire 30 days from each tranche vesting date indicated in this appendix 3B released on 5 August 2015
3,431,373 NED PRs Each tranche of NED PRs will expire 30 days from each tranche vesting date indicated in this appendix 3B released on 26 November 2014.
13,750,828 Convertible Notes each with a face value of AU$1, expiring on 4 August 2025
10 Dividend policy (in the case of a trust, distribution policy) on the increased capital (interests) Unchanged
Part 2 - Bonus issue or pro rata issue
11 Is security holder approval required? Not applicable
12 Is the issue renounceable or non- renounceable? Not applicable
13 Ratio in which the +securities will be offered Not applicable
14 +Class of +securities to which the offer relates Not applicable
15 +Record date to determine entitlements Not applicable
16 Will holdings on different registers (or subregisters) be aggregated for calculating entitlements? Not applicable
17 Policy for deciding entitlements in relation to fractions Not applicable
Prima BioMed Ltd, Level 7, 151 Macquarie Street, Sydney NSW 2000
Phone: +61 2 9276 1224 Fax: +61 2 8569 1880
www.primabiomed.com.au ABN: 90 009 237 889

18 Names of countries in which the entity has +security holders who will not be sent new issue documents Not applicable
Note: Security holders must be told how their entitlements are to be dealt with.
Cross reference: rule 7.7.
19 Closing date for receipt of acceptances or renunciations Not applicable
20 Names of any underwriters Not applicable
21 Amount of any underwriting fee or commission Not applicable
22 Names of any brokers to the issue Not applicable
23 Fee or commission payable to the broker to the issue Not applicable
24 Amount of any handling fee payable to brokers who lodge acceptances or renunciations on behalf of +security holders Not applicable
25 If the issue is contingent on +security holders’ approval, the date of the meeting Not applicable
26 Date entitlement and acceptance form and prospectus or Product Disclosure Statement will be sent to persons entitled Not applicable
27 If the entity has issued options, and the terms entitle option holders to participate on exercise, the date on which notices will be sent to option holders Not applicable
28 Date rights trading will begin (if applicable) Not applicable
29 Date rights trading will end (if applicable) Not applicable
Prima BioMed Ltd, Level 7, 151 Macquarie Street, Sydney NSW 2000
Phone: +61 2 9276 1224 Fax: +61 2 8569 1880
www.primabiomed.com.au ABN: 90 009 237 889

30 How do +security holders sell their entitlements in full through a broker? Not applicable
31 How do +security holders sell part of their entitlements through a broker and accept for the balance? Not applicable
32 How do +security holders dispose of their entitlements (except by sale through a broker)? Not applicable
33 +Despatch date Not applicable
Part 3 - Quotation of securities
You need only complete this section if you are applying for quotation of securities
34 Type of securities
(tick one)
(a)x Securities described in Part 1
(b)¨ All other securities
Example: restricted securities at the end of the escrowed period, partly paid securities that become fully paid, employee incentive share securities when restriction ends, securities issued on expiry or conversion of convertible securities
Entities that have ticked box 34(a)
Additional securities forming a new class of securities
Tick to indicate you are providing the information or documents
35¨ If the +securities are +equity securities, the names of the 20 largest holders of the additional +securities, and the number and percentage of additional +securities held by those holders
36¨ If the +securities are +equity securities, a distribution schedule of the additional +securities setting out the number of holders in the categories
1 - 1,000
1,001 - 5,000
5,001 - 10,000
10,001 - 100,000
Prima BioMed Ltd, Level 7, 151 Macquarie Street, Sydney NSW 2000
Phone: +61 2 9276 1224 Fax: +61 2 8569 1880
www.primabiomed.com.au ABN: 90 009 237 889

100,001 and over
37¨ A copy of any trust deed for the additional +securities
Entities that have ticked box 34(b)
38 Number of securities for which +quotation is sought Not applicable
39 Class of +securities for which quotation is sought Not applicable
40 Do the +securities rank equally in all respects from the date of allotment with an existing +class of quoted +securities? Not applicable
If the additional securities do not rank equally, please state:
the date from which they do
the extent to which they participate for the next dividend, (in the case of a trust, distribution) or interest payment
the extent to which they do not rank equally, other than in relation to the next dividend, distribution or interest payment
41 Reason for request for quotation now Not applicable
Example: In the case of restricted securities, end of restriction period
(if issued upon conversion of another security, clearly identify that other security)
Number +Class
42 Number and +class of all +securities quoted on ASX (including the securities in clause 38) Not applicable
Prima BioMed Ltd, Level 7, 151 Macquarie Street, Sydney NSW 2000
Phone: +61 2 9276 1224 Fax: +61 2 8569 1880
www.primabiomed.com.au ABN: 90 009 237 889

Quotation agreement
1 +Quotation of our additional +securities is in ASX’s absolute discretion. ASX may quote the +securities on any conditions it decides.
2 We warrant the following to ASX.
The issue of the +securities to be quoted complies with the law and is not for an illegal purpose.
There is no reason why those +securities should not be granted +quotation.
An offer of the +securities for sale within 12 months after their issue will not require disclosure under section 707(3) or section 1012C(6) of the Corporations Act.
Note: An entity may need to obtain appropriate warranties from subscribers for the securities in order to be able to give this warranty
Section 724 or section 1016E of the Corporations Act does not apply to any applications received by us in relation to any +securities to be quoted and that no-one has any right to return any +securities to be quoted under sections 737, 738 or 1016F of the Corporations Act at the time that we request that the +securities be quoted.
If we are a trust, we warrant that no person has the right to return the +securities to be quoted under section 1019B of the Corporations Act at the time that we request that the +securities be quoted.
3 We will indemnify ASX to the fullest extent permitted by law in respect of any claim, action or expense arising from or connected with any breach of the warranties in this agreement.
4 We give ASX the information and documents required by this form. If any information or document not available now, will give it to ASX before +quotation of the +securities begins. We acknowledge that ASX is relying on the information and documents. We warrant that they are (will be) true and complete.
Sign here: Date: 13 October 2015
Company secretary
Print name: Deanne Miller
Prima BioMed Ltd, Level 7, 151 Macquarie Street, Sydney NSW 2000
Phone: +61 2 9276 1224 Fax: +61 2 8569 1880
www.primabiomed.com.au ABN: 90 009 237 889

Appendix 3B – Annexure 1
Calculation of placement capacity under
rule 7.1 and rule 7.1A for eligible entities
Introduced 01/08/12 Amended 04/03/13
Part 1
Rule 7.1 – Issues exceeding 15% of capital
Step 1: Calculate “A”, the base figure from which the placement capacity is calculated
Insert number of fully paid +ordinary securities on issue 12 months before the +issue date or date of agreement to issue
1,258,301,929
Add the following:
Number of fully paid +ordinary securities issued in that 12 month period under an exception in rule 7.2
Number of fully paid +ordinary securities issued in that 12 month period with shareholder approval
728,973,498
Number of partly paid +ordinary securities that became fully paid in that 12 month period
Note:
Include only ordinary securities here – other classes of equity securities cannot be added
Include here (if applicable) the securities the subject of the Appendix 3B to which this form is annexed
It may be useful to set out issues of securities on different dates as separate line items
Subtract the number of fully paid +ordinary securities cancelled during that 12 month period
Nil
“A”
1,987,275,427
Prima BioMed Ltd, Level 7, 151 Macquarie Street, Sydney NSW 2000
Phone: +61 2 9276 1224 Fax: +61 2 8569 1880
www.primabiomed.com.au ABN: 90 009 237 889

Step 2: Calculate 15% of “A”
“B” 0.15 [Note: this value cannot be changed]
Multiply “A” by 0.15 298,091,314
Step 3: Calculate “C”, the amount of placement capacity under rule 7.1 that has already been used
Insert number of +equity securities issued or agreed to be issued in that 12 month period not counting those issued: Nil
Under an exception in rule 7.2
Under rule 7.1A
With security holder approval under rule 7.1 or rule 7.4
Note:
This applies to equity securities, unless specifically excluded – not just ordinary securities
Include here (if applicable) the securities the subject of the Appendix 3B to which this form is annexed
It may be useful to set out issues of securities on different dates as separate line items
“C” Nil
Step 4: Subtract “C” from [“A” x “B”] to calculate remaining placement capacity under rule 7.1
“A” x 0.15 298,091,314
Note: number must be same as shown in Step 2
Subtract “C” Nil
Note: number must be same as shown in Step 3
Total [“A” x 0.15] – “C” 298,091,314
[Note: this is the remaining placement capacity under rule 7.1]
Prima BioMed Ltd, Level 7, 151 Macquarie Street, Sydney NSW 2000
Phone: +61 2 9276 1224 Fax: +61 2 8569 1880
www.primabiomed.com.au ABN: 90 009 237 889
Part 2
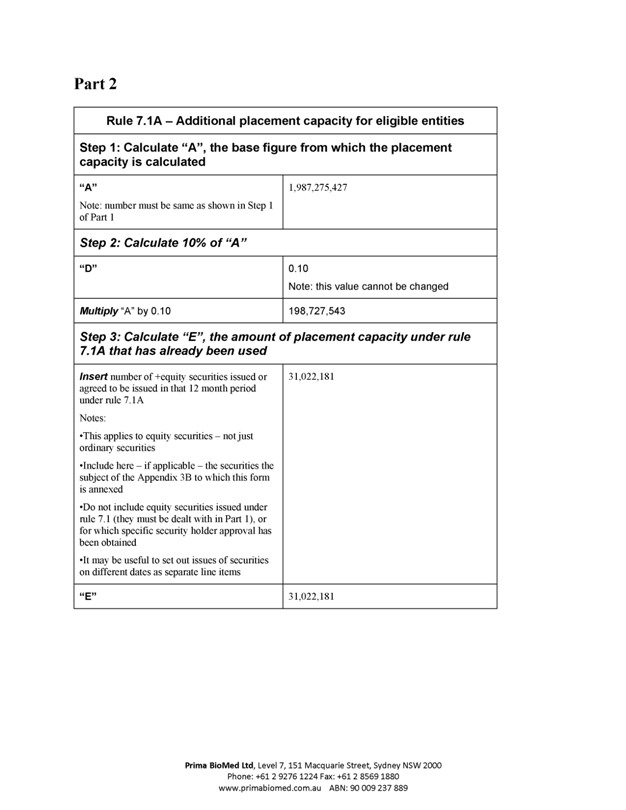
Rule 7.1A – Additional placement capacity for eligible entities
Step 1: Calculate “A”, the base figure from which the placement capacity is calculated
“A” 1,987,275,427
Note: number must be same as shown in Step 1 of Part 1
Step 2: Calculate 10% of “A”
“D” 0.10
Note: this value cannot be changed
Multiply “A” by 0.10 198,727,543
Step 3: Calculate “E”, the amount of placement capacity under rule 7.1A that has already been used
Insert number of +equity securities issued or agreed to be issued in that 12 month period under rule 7.1A 31,022,181
Notes:
This applies to equity securities – not just ordinary securities
Include here – if applicable – the securities the subject of the Appendix 3B to which this form is annexed
Do not include equity securities issued under rule 7.1 (they must be dealt with in Part 1), or for which specific security holder approval has been obtained
It may be useful to set out issues of securities on different dates as separate line items
“E” 31,022,181
Prima BioMed Ltd, Level 7, 151 Macquarie Street, Sydney NSW 2000
Phone: +61 2 9276 1224 Fax: +61 2 8569 1880
www.primabiomed.com.au ABN: 90 009 237 889

Step 4: Subtract “E” from [“A” x “D”] to calculate remaining placement capacity under rule 7.1A
“A” x 0.10
198,727,543
Note: number must be same as shown in Step 2
Subtract “E”
31,022,181
Note: number must be same as shown in Step 3
Total [“A” x 0.10] – “E”
167,705,362
Note: this is the remaining placement capacity under rule 7.1A
Prima BioMed Ltd, Level 7, 151 Macquarie Street, Sydney NSW 2000
Phone: +61 2 9276 1224 Fax: +61 2 8569 1880
www.primabiomed.com.au ABN: 90 009 237 889













