Immutep CEO Marc Voigt said: “We are delighted that patient outcomes are improved with the combination of efti plus pembrolizumab across different patient groups. The data is encouraging for patients, as there is an unmet medical need particularly for those with NSCLC with no or low PD-L1 expression. We enlarged this part of the study in order to see if the strong earlier results in a smaller group of patients are holding true in more than a hundred patients. By biotech standards, we consider this to be a large patient population for a Phase II trial.”
“For Immutep, these highly favourable results are of strategic importance, as they support late stage development for an attractive and very large adressible market,” he said.
Trial endpoints
The primary endpoint was ORR according to iRECIST and local read. The data announced today represents the primary analysis of mature data of this endpoint. Secondary endpoints include ORR by RECIST 1.1., DCR, Duration of Response (DoR), PFS, Overall Survival (OS), and Safety assessments.
Patient population and condition
A total of 114 patients with 1st line NSCLC were enrolled and treated with efti plus pembrolizumab in 6 countries across 19 trial sites throughout Europe, the United States, and Australia.
Importantly, the patients were enrolled without any selection for PD-L1 status (PD-L1 all-comers), a biomarker indicating the likelihood of response to pembrolizumab. The trial was confirmed as a “PD-L1 all-comer trial” with ~70% of patients having a Tumour Progression Score (TPS) of < 50%. 93% of patients had metastatic disease at study entry and the patients had an ECOG performance status of 0 (37.7%) or 1 (62.3%). Treatment prior to study start included radiotherapy (33%), surgery (20%) and systemic therapy (22%) for non-metastatic disease. The trial reflects a typical patient population for this indication, including a mix of squamous/non-squamous disease and male/female representation.
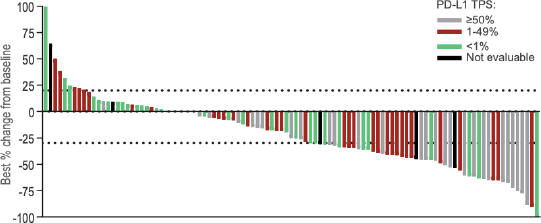
Key Findings from 1st line NSCLC patients in TACTI-002 – data cut-off date 15 April 2022
Primary analysis of primary endpoint by iRECIST – ORR
| • | | ORR of 38.6% in the intent to treat (ITT) group (44/114 patients) and 42.7% for evaluable patients (44/103) by local read, see Table 1 |
| • | | Responses across all PD-L1 status groups in this all-comer trial (by central lab assessment): |
| | • | | ORR of 28.1% (9/32) in PD-L1 negative patients |
| | • | | ORR of 41.7% (15/36) in patients with PD-L1 status of 1-49% |
| | • | | ORR of 45.5% (25/55) in patients with PD-L1 status of ≥ 1% |
| | • | | ORR of 52.6% (10/19) in patients with PD-L1 status of ≥ 50% |
| • | | Comparable ORR in squamous (35%) or non-squamous (38.9%) tumour type |
| • | | RECIST 1.1 results are comparable to the iRECIST results |
| • | | ORR is favourable compared to historical trials of anti-PD-1 monotherapy for all-comer population and PD-L1 status groups1 |
| 1 | See, for example, KN-001 and KN-042 trials with pembrolizumab monotherapy reporting a ORR of 19.4% in the all-comer population and 27.3% in the PD-L1 ≥ 1% population: |
Leighl et al, The Lancet 2019, http://dx.doi.org/10.1016/S2213-2600(18)30500-9
Mok et al, The Lancet 2019, http://dx.doi.org/10.1016/S0140-6736(18)32409-7