Exhibit 99.1

ASX/Media Release
Immutep Announces Compelling Clinical Results from Phase II Trial Utilizing its
First-in-Class Soluble LAG-3 Protein, Eftilagimod Alpha, in 1st Line NSCLC
at SITC 2022 Annual Meeting
Overall response rate (ORR) increases to 40.4%, according to iRECIST, in TACTI-002 all-comer PD-L1 Phase II trial in 1st line non-small cell lung cancer (1L NSCLC)
ORR improved across all PD-L1 status groups by central assessment compared with data reported at ASCO 2022, including 48.3%, 44.7%, 55.0%, and 31.3% ORR in patients with PD-L1 TPS of >1%, 1-49%, >50%, and <1%, respectively
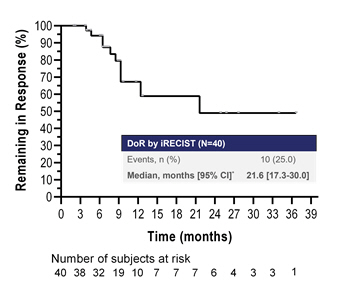
Interim median Duration of Response (DoR) of 21.6 months, compares favourably to historical controls including anti-PD-1 therapy combined with chemotherapy
Despite ~75% patients in trial having PD-L1 TPS <50% who are less likely to respond to anti-PD-1 monotherapy, promising results achieved in secondary endpoint of interim median Progression Free Survival (PFS) with overall PFS of 6.6 months and 9.3 months PFS in TPS >1%
Significant increase in IFN-y and CXCL10 serum biomarkers for systemic TH1 response; substantiates efti’s unique stimulation of the immune system also seen in the randomized AIPAC Phase IIb trial in Breast Cancer
Webcast to Discuss Results at 5pm ET (9am AEDT) Today
SYDNEY, AUSTRALIA – 11 November 2022 – Immutep Limited (ASX: IMM; NASDAQ: IMMP) (“Immutep” or “the Company”), a clinical-stage biotechnology company developing novel LAG-3 immunotherapies for cancer and autoimmune disease, today announces compelling new clinical data from the TACTI-002 all-comer PD-L1 Phase II trial evaluating Immutep’s lead product candidate, eftilagimod alpha (“efti” or “IMP321”) in combination with MSD’s (Merck & Co., Inc., Rahway, NJ., USA) anti-PD-1 therapy KEYTRUDA® (pembrolizumab) in 114 patients with 1L NSCLC.
The new data was presented today in a late-breaking abstract oral presentation (Abstract #1470) at the 37th Annual Society of Immunotherapy of Cancer (SITC) Meeting by Wade T. Iams, MD, Assistant Professor of Medicine, Vanderbilt-Ingram Cancer Center Division of Hematology/Oncology. Today’s presentation followed the abstract data that had been discussed at the SITC 2022 Press Conference on 8 November 2022.
Dr. Iams stated, “The encouraging ORR, PFS and DCR presented today at SITC build on the promise of efti, a first-in-class soluble LAG-3 protein, to uniquely engage the innate and adaptive immune system to enhance the clinical effect of pembrolizumab. Responses in TACTI-002 were seen across all PD-L1 expression levels and histologic types, including in patients with PD-L1 TPS less than 50%. The deep and durable responses driven by efti plus pembrolizumab continue to be favorable with median DoR of 21.6 months.”