
Global Webcast Presentation (ASX: IMM, NASDAQ: IMMP) Update on new TACTI-002 Phase II and initial INSIGHT-003 Phase 1 data presented at SITC 2022 A Global Leader in LAG-3 Therapeutics in Oncology and Autoimmune Disease Date & Time Thursday, 10 November 2022, at 5 pm U.S. ET Friday, November 11, at 9 am Australian Eastern Daylight Time (AEDT) Registration Webcast Link Exhibit 99.1

The purpose of the presentation is to provide an update of the business of Immutep Limited ACN 009 237 889 (ASX:IMM; NASDAQ:IMMP). These slides have been prepared as a presentation aid only and the information they contain may require further explanation and/or clarification. Accordingly, these slides and the information they contain should be read in conjunction with past and future announcements made by Immutep and should not be relied upon as an independent source of information. Please refer to the Company's website and/or the Company’s filings to the ASX and SEC for further information. The views expressed in this presentation contain information derived from publicly available sources that have not been independently verified. No representation or warranty is made as to the accuracy, completeness or reliability of the information. Any forward looking statements in this presentation have been prepared on the basis of a number of assumptions which may prove incorrect and the current intentions, plans, expectations and beliefs about future events are subject to risks, uncertainties and other factors, many of which are outside Immutep’s control. Important factors that could cause actual results to differ materially from assumptions or expectations expressed or implied in this presentation include known and unknown risks. Because actual results could differ materially to assumptions made and Immutep’s current intentions, plans, expectations and beliefs about the future, you are urged to view all forward looking statements contained in this presentation with caution. This presentation should not be relied on as a recommendation or forecast by Immutep. Nothing in this presentation should be construed as either an offer to sell or a solicitation of an offer to buy or sell shares in any jurisdiction. This presentation is authorised for release by the CEO of Immutep Limited. Forward-Looking Statements

Eftilagimod Alpha (efti): A First-in-Class Soluble LAG-3 Protein
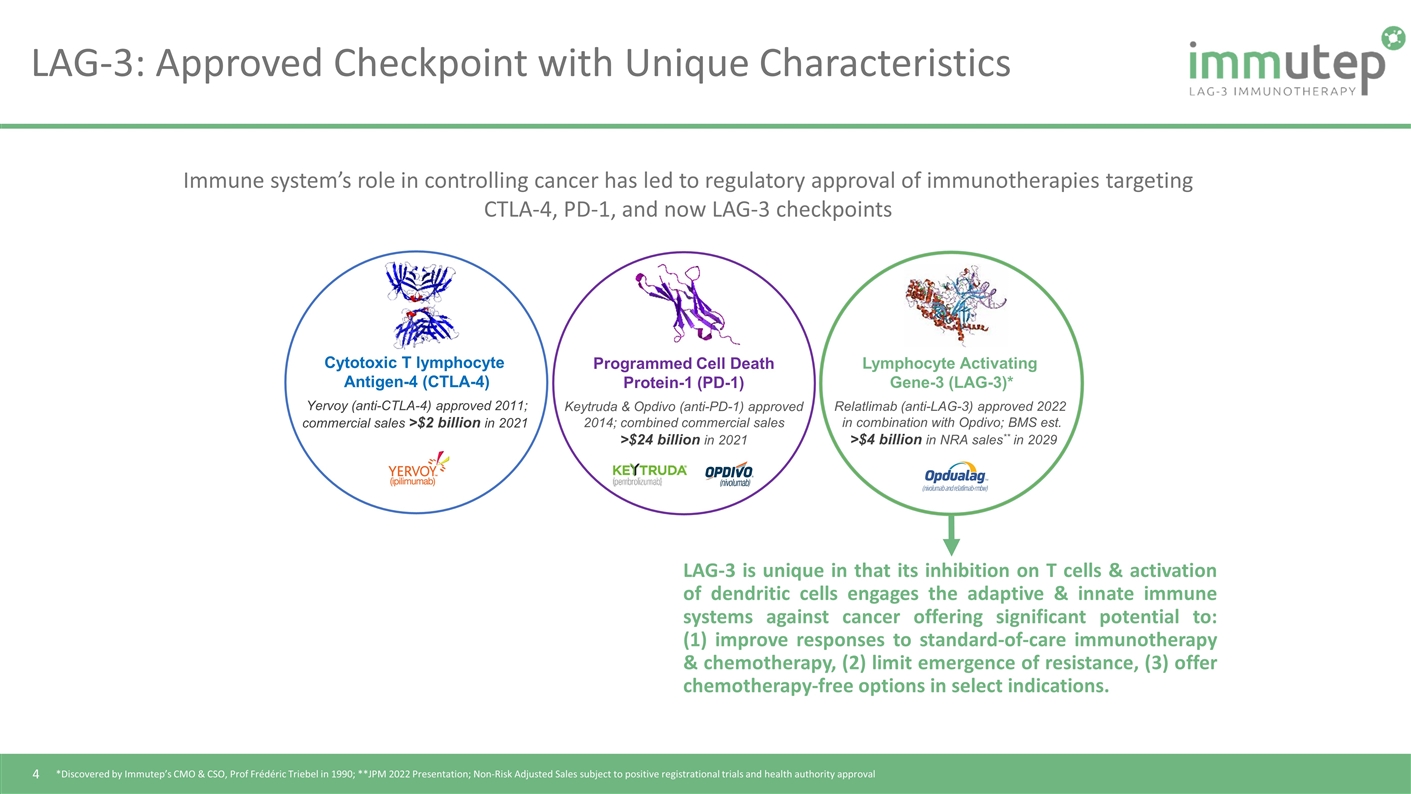
Cytotoxic T lymphocyte Antigen-4 (CTLA-4) Yervoy (anti-CTLA-4) approved 2011; commercial sales >$2 billion in 2021 Programmed Cell Death Protein-1 (PD-1) Keytruda & Opdivo (anti-PD-1) approved 2014; combined commercial sales >$24 billion in 2021 Lymphocyte Activating Gene-3 (LAG-3)* Relatlimab (anti-LAG-3) approved 2022 in combination with Opdivo; BMS est. >$4 billion in NRA sales** in 2029 Immune system’s role in controlling cancer has led to regulatory approval of immunotherapies targeting CTLA-4, PD-1, and now LAG-3 checkpoints *Discovered by Immutep’s CMO & CSO, Prof Frédéric Triebel in 1990; **JPM 2022 Presentation; Non-Risk Adjusted Sales subject to positive registrational trials and health authority approval LAG-3: Approved Checkpoint with Unique Characteristics LAG-3 is unique in that its inhibition on T cells & activation of dendritic cells engages the adaptive & innate immune systems against cancer offering significant potential to: (1) improve responses to standard-of-care immunotherapy & chemotherapy, (2) limit emergence of resistance, (3) offer chemotherapy-free options in select indications.
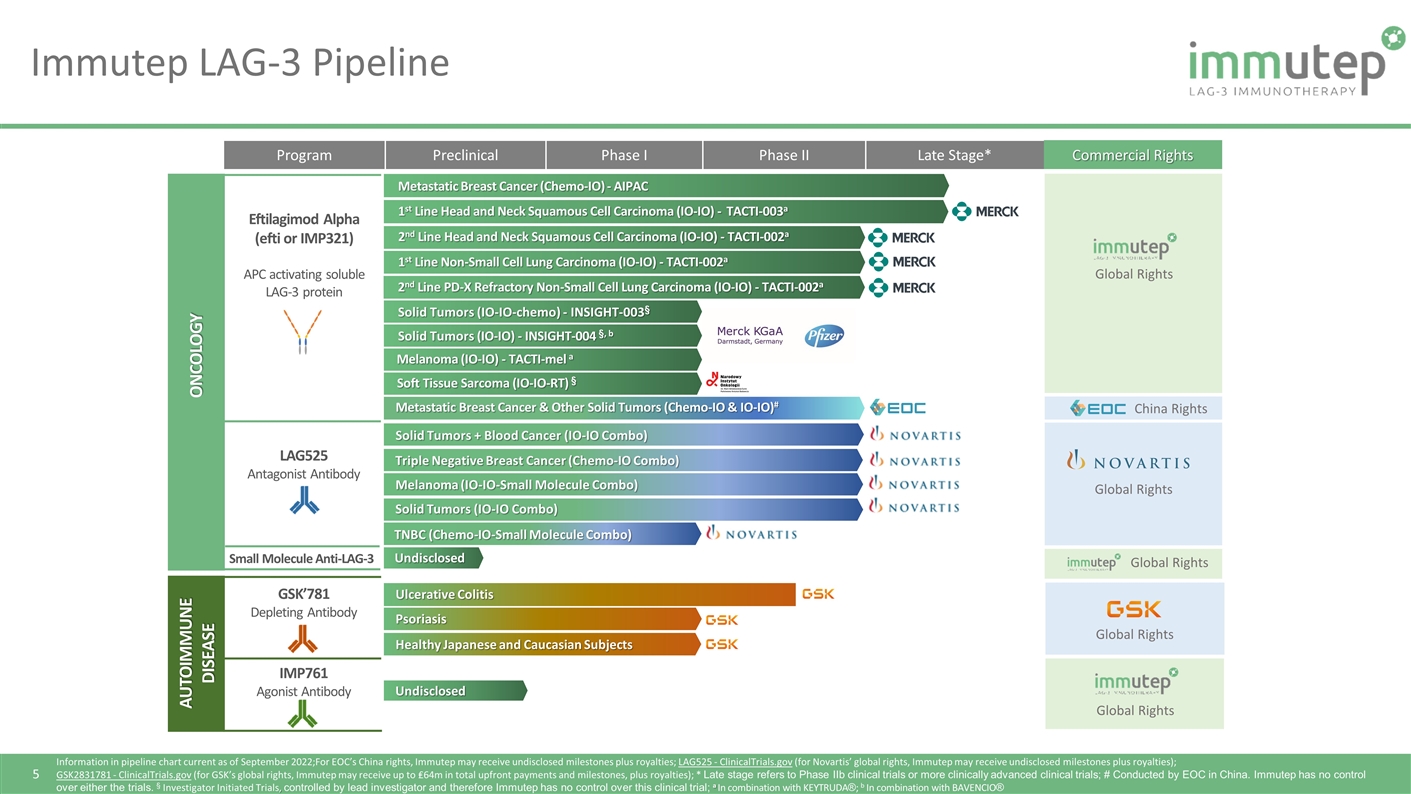
Program Preclinical Phase I Phase II Late Stage* Commercial Rights Solid Tumors (IO-IO-chemo) - INSIGHT-003§ Metastatic Breast Cancer (Chemo-IO) - AIPAC 1st Line Head and Neck Squamous Cell Carcinoma (IO-IO) - TACTI-003a 2nd Line Head and Neck Squamous Cell Carcinoma (IO-IO) - TACTI-002a 1st Line Non-Small Cell Lung Carcinoma (IO-IO) - TACTI-002a Solid Tumors (IO-IO) - INSIGHT-004 §, b Undisclosed Global Rights Global Rights China Rights Undisclosed Global Rights Global Rights Global Rights Solid Tumors + Blood Cancer (IO-IO Combo) Triple Negative Breast Cancer (Chemo-IO Combo) Melanoma (IO-IO-Small Molecule Combo) Solid Tumors (IO-IO Combo) TNBC (Chemo-IO-Small Molecule Combo) Ulcerative Colitis Psoriasis Healthy Japanese and Caucasian Subjects Eftilagimod Alpha (efti or IMP321) APC activating soluble LAG-3 protein LAG525 Antagonist Antibody Small Molecule Anti-LAG-3 ONCOLOGY AUTOIMMUNE DISEASE GSK’781 Depleting Antibody IMP761 Agonist Antibody Immutep LAG-3 Pipeline Information in pipeline chart current as of September 2022;For EOC’s China rights, Immutep may receive undisclosed milestones plus royalties; LAG525 - ClinicalTrials.gov (for Novartis’ global rights, Immutep may receive undisclosed milestones plus royalties); GSK2831781 - ClinicalTrials.gov (for GSK’s global rights, Immutep may receive up to ₤64m in total upfront payments and milestones, plus royalties); * Late stage refers to Phase IIb clinical trials or more clinically advanced clinical trials; # Conducted by EOC in China. Immutep has no control over either the trials. § Investigator Initiated Trials, controlled by lead investigator and therefore Immutep has no control over this clinical trial; a In combination with KEYTRUDA®; b In combination with BAVENCIO® 2nd Line PD-X Refractory Non-Small Cell Lung Carcinoma (IO-IO) - TACTI-002a Soft Tissue Sarcoma (IO-IO-RT) § Metastatic Breast Cancer & Other Solid Tumors (Chemo-IO & IO-IO)# Melanoma (IO-IO) - TACTI-mel a
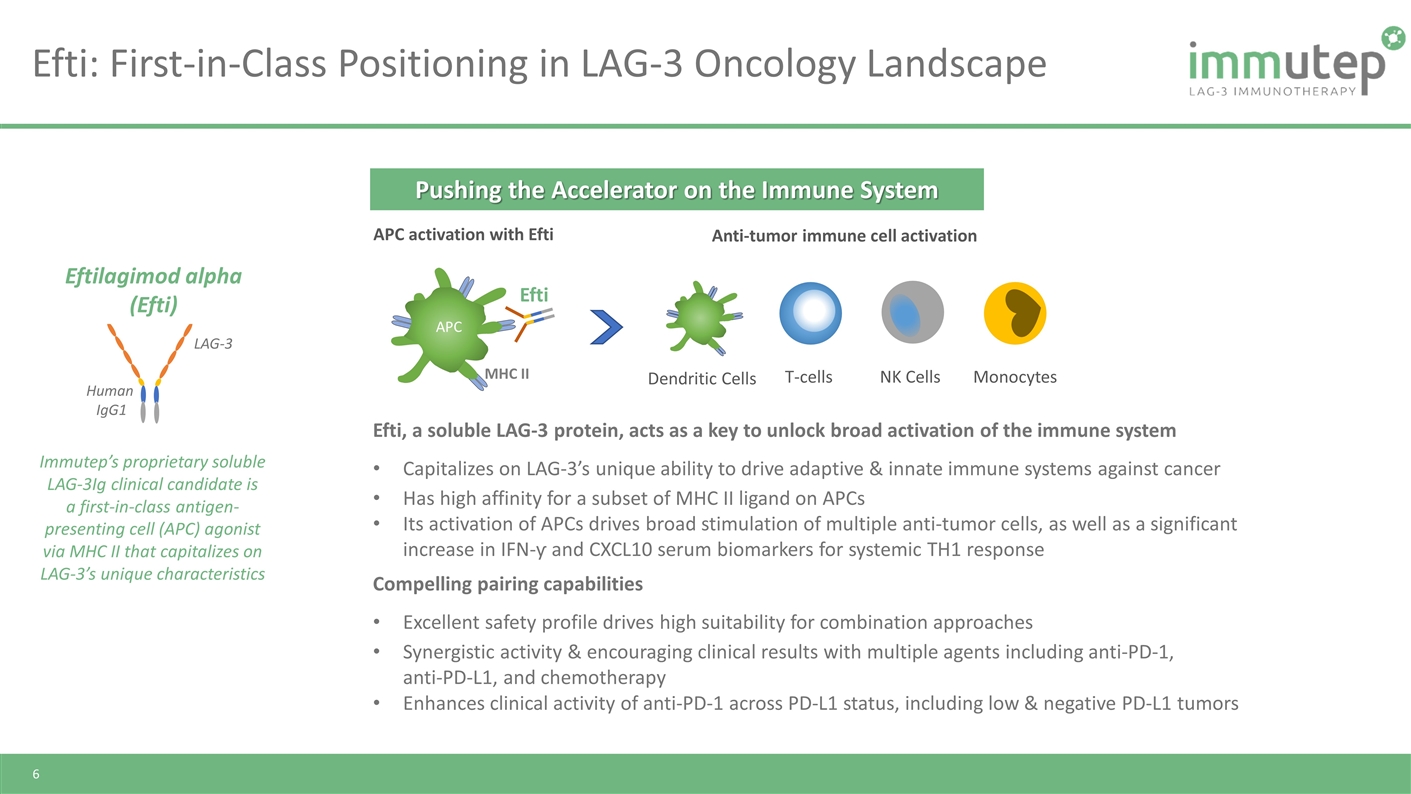
Efti: First-in-Class Positioning in LAG-3 Oncology Landscape Immutep’s proprietary soluble LAG-3Ig clinical candidate is a first-in-class antigen-presenting cell (APC) agonist via MHC II that capitalizes on LAG-3’s unique characteristics LAG-3 Human IgG1 Eftilagimod alpha (Efti) Efti, a soluble LAG-3 protein, acts as a key to unlock broad activation of the immune system Capitalizes on LAG-3’s unique ability to drive adaptive & innate immune systems against cancer Has high affinity for a subset of MHC II ligand on APCs Its activation of APCs drives broad stimulation of multiple anti-tumor cells, as well as a significant increase in IFN-ƴ and CXCL10 serum biomarkers for systemic TH1 response Compelling pairing capabilities Excellent safety profile drives high suitability for combination approaches Synergistic activity & encouraging clinical results with multiple agents including anti-PD-1, anti-PD-L1, and chemotherapy Enhances clinical activity of anti-PD-1 across PD-L1 status, including low & negative PD-L1 tumors MHC II APC Dendritic Cells T-cells Monocytes NK Cells APC activation with Efti Anti-tumor immune cell activation Efti Pushing the Accelerator on the Immune System
TACTI-002 Phase II Trial – Part A: Efti + Pembrolizumab Combination in 1st Line Non-Small Cell Lung Cancer (1L NSCLC)
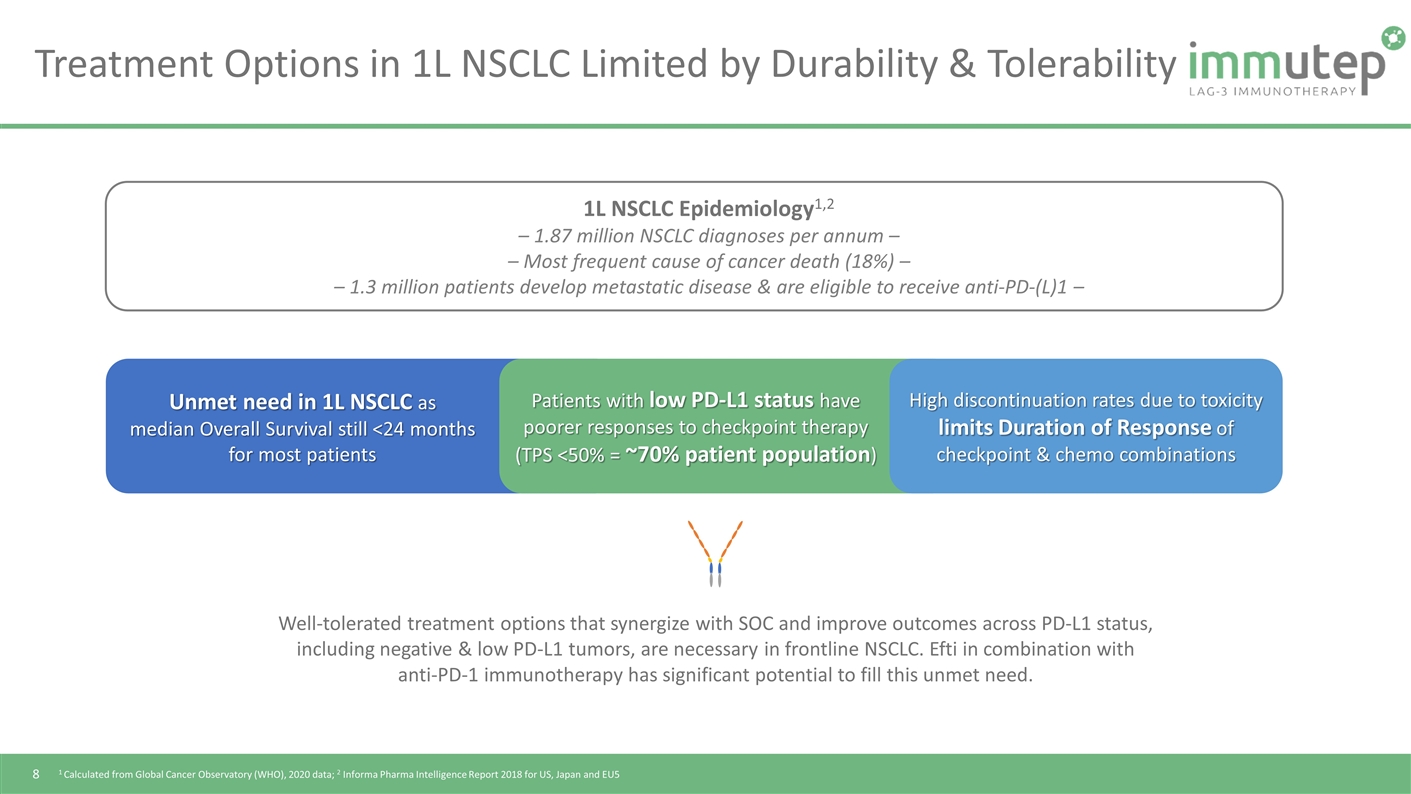
Treatment Options in 1L NSCLC Limited by Durability & Tolerability Well-tolerated treatment options that synergize with SOC and improve outcomes across PD-L1 status, including negative & low PD-L1 tumors, are necessary in frontline NSCLC. Efti in combination with anti-PD-1 immunotherapy has significant potential to fill this unmet need. 1L NSCLC Epidemiology1,2 – 1.87 million NSCLC diagnoses per annum – – Most frequent cause of cancer death (18%) – – 1.3 million patients develop metastatic disease & are eligible to receive anti-PD-(L)1 – 1 Calculated from Global Cancer Observatory (WHO), 2020 data; 2 Informa Pharma Intelligence Report 2018 for US, Japan and EU5 Unmet need in 1L NSCLC as median Overall Survival still <24 months for most patients High discontinuation rates due to toxicity limits Duration of Response of checkpoint & chemo combinations Patients with low PD-L1 status have poorer responses to checkpoint therapy (TPS <50% = ~70% patient population)
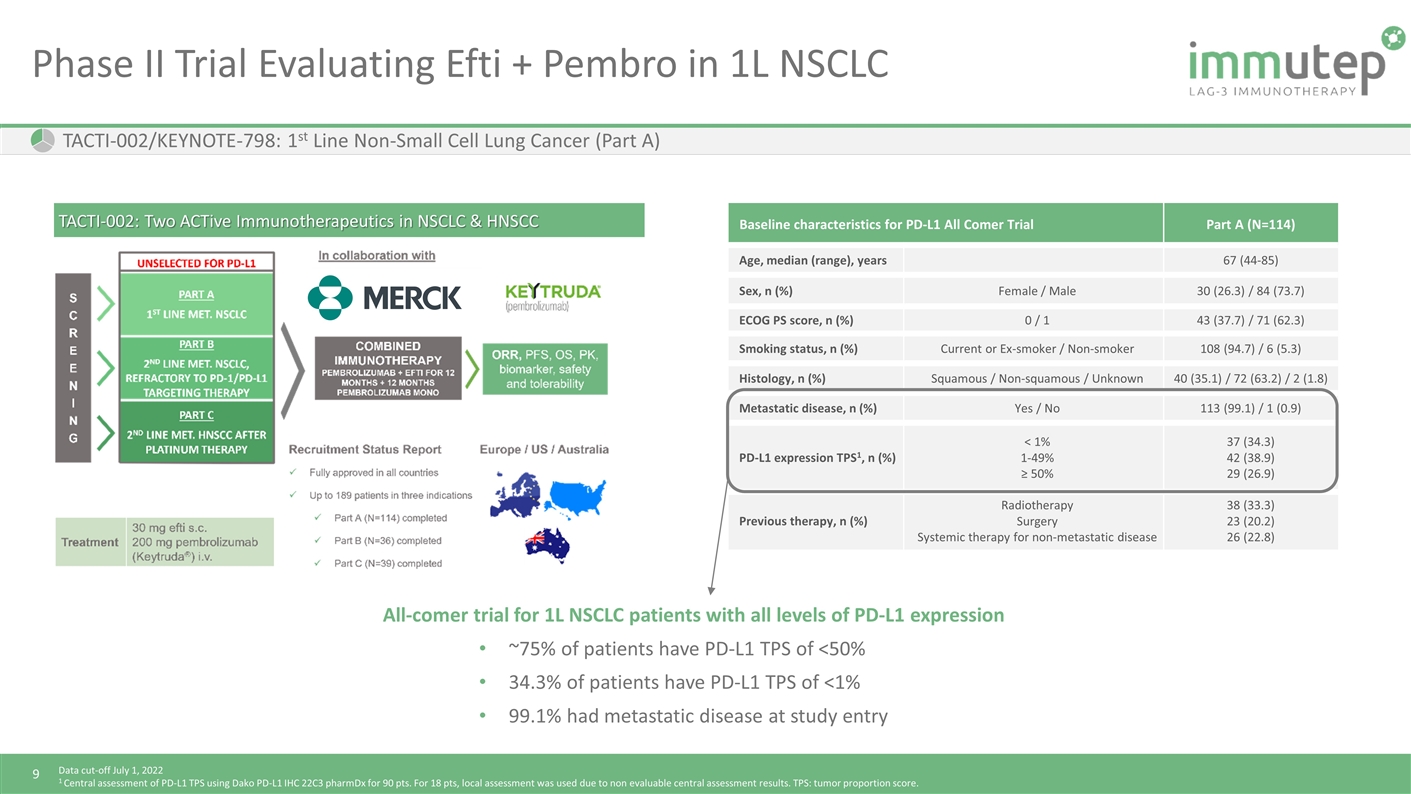
Phase II Trial Evaluating Efti + Pembro in 1L NSCLC TACTI-002: Two ACTive Immunotherapeutics in NSCLC & HNSCC Baseline characteristics for PD-L1 All Comer Trial Part A (N=114) Age, median (range), years 67 (44-85) Sex, n (%) Female / Male 30 (26.3) / 84 (73.7) ECOG PS score, n (%) 0 / 1 43 (37.7) / 71 (62.3) Smoking status, n (%) Current or Ex-smoker / Non-smoker 108 (94.7) / 6 (5.3) Histology, n (%) Squamous / Non-squamous / Unknown 40 (35.1) / 72 (63.2) / 2 (1.8) Metastatic disease, n (%) Yes / No 113 (99.1) / 1 (0.9) PD-L1 expression TPS1, n (%) < 1% 1-49% ≥ 50% 37 (34.3) 42 (38.9) 29 (26.9) Previous therapy, n (%) Radiotherapy Surgery Systemic therapy for non-metastatic disease 38 (33.3) 23 (20.2) 26 (22.8) Data cut-off July 1, 2022 1 Central assessment of PD-L1 TPS using Dako PD-L1 IHC 22C3 pharmDx for 90 pts. For 18 pts, local assessment was used due to non evaluable central assessment results. TPS: tumor proportion score. TACTI-002/KEYNOTE-798: 1st Line Non-Small Cell Lung Cancer (Part A) All-comer trial for 1L NSCLC patients with all levels of PD-L1 expression ~75% of patients have PD-L1 TPS of <50% 34.3% of patients have PD-L1 TPS of <1% 99.1% had metastatic disease at study entry
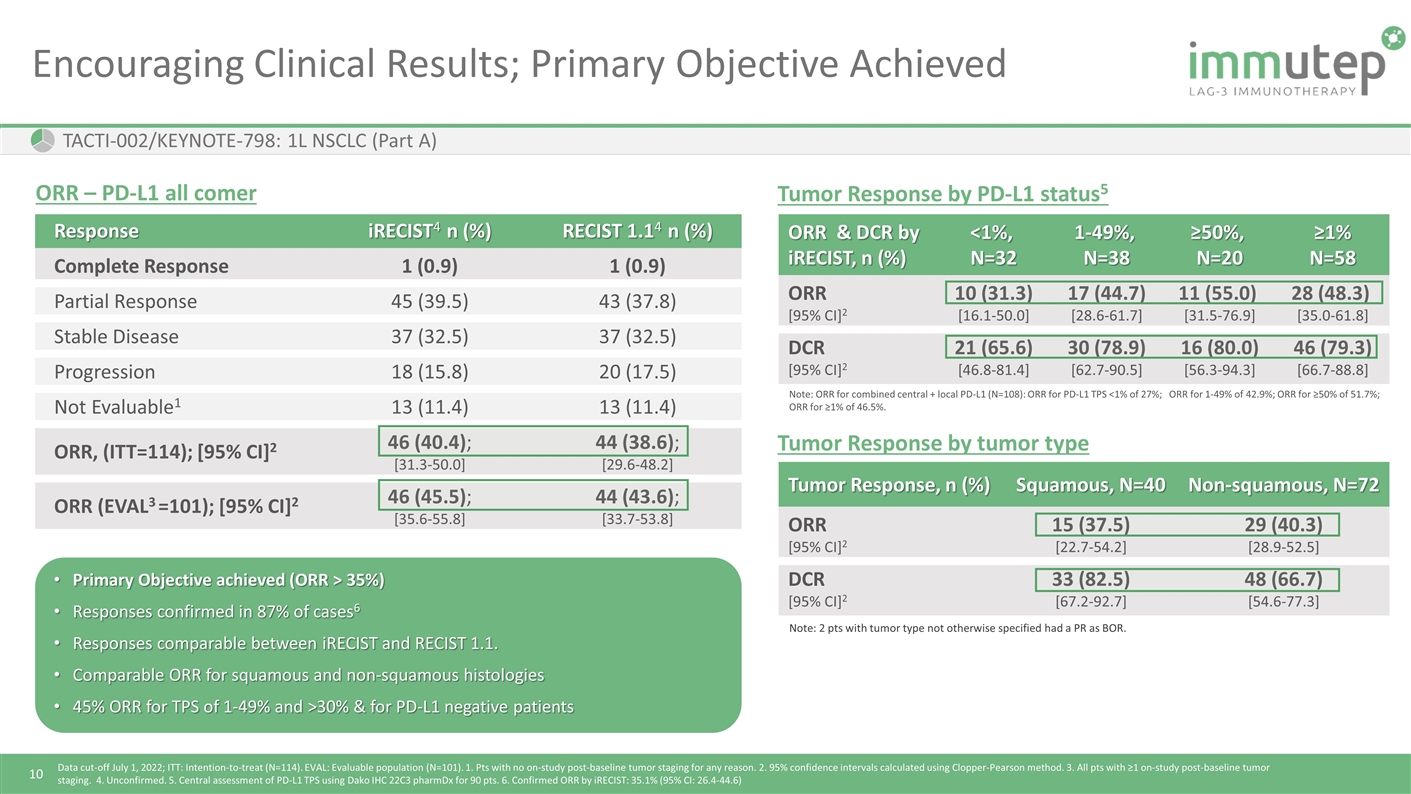
Encouraging Clinical Results; Primary Objective Achieved TACTI-002/KEYNOTE-798: 1L NSCLC (Part A) Response iRECIST4 n (%) RECIST 1.14 n (%) Complete Response 1 (0.9) 1 (0.9) Partial Response 45 (39.5) 43 (37.8) Stable Disease 37 (32.5) 37 (32.5) Progression 18 (15.8) 20 (17.5) Not Evaluable1 13 (11.4) 13 (11.4) ORR, (ITT=114); [95% CI]2 46 (40.4); [31.3-50.0] 44 (38.6); [29.6-48.2] ORR (EVAL3 =101); [95% CI]2 46 (45.5); [35.6-55.8] 44 (43.6); [33.7-53.8] ORR & DCR by iRECIST, n (%) <1%, N=32 1-49%, N=38 ≥50%, N=20 ≥1% N=58 ORR [95% CI]2 10 (31.3) [16.1-50.0] 17 (44.7) [28.6-61.7] 11 (55.0) [31.5-76.9] 28 (48.3) [35.0-61.8] DCR [95% CI]2 21 (65.6) [46.8-81.4] 30 (78.9) [62.7-90.5] 16 (80.0) [56.3-94.3] 46 (79.3) [66.7-88.8] Note: ORR for combined central + local PD-L1 (N=108): ORR for PD-L1 TPS <1% of 27%; ORR for 1-49% of 42.9%; ORR for ≥50% of 51.7%; ORR for ≥1% of 46.5%. Tumor Response by PD-L1 status5 ORR – PD-L1 all comer Data cut-off July 1, 2022; ITT: Intention-to-treat (N=114). EVAL: Evaluable population (N=101). 1. Pts with no on-study post-baseline tumor staging for any reason. 2. 95% confidence intervals calculated using Clopper-Pearson method. 3. All pts with ≥1 on-study post-baseline tumor staging. 4. Unconfirmed. 5. Central assessment of PD-L1 TPS using Dako IHC 22C3 pharmDx for 90 pts. 6. Confirmed ORR by iRECIST: 35.1% (95% CI: 26.4-44.6) Primary Objective achieved (ORR > 35%) Responses confirmed in 87% of cases6 Responses comparable between iRECIST and RECIST 1.1. Comparable ORR for squamous and non-squamous histologies 45% ORR for TPS of 1-49% and >30% & for PD-L1 negative patients Tumor Response, n (%) Squamous, N=40 Non-squamous, N=72 ORR [95% CI]2 15 (37.5) [22.7-54.2] 29 (40.3) [28.9-52.5] DCR [95% CI]2 33 (82.5) [67.2-92.7] 48 (66.7) [54.6-77.3] Note: 2 pts with tumor type not otherwise specified had a PR as BOR. Tumor Response by tumor type
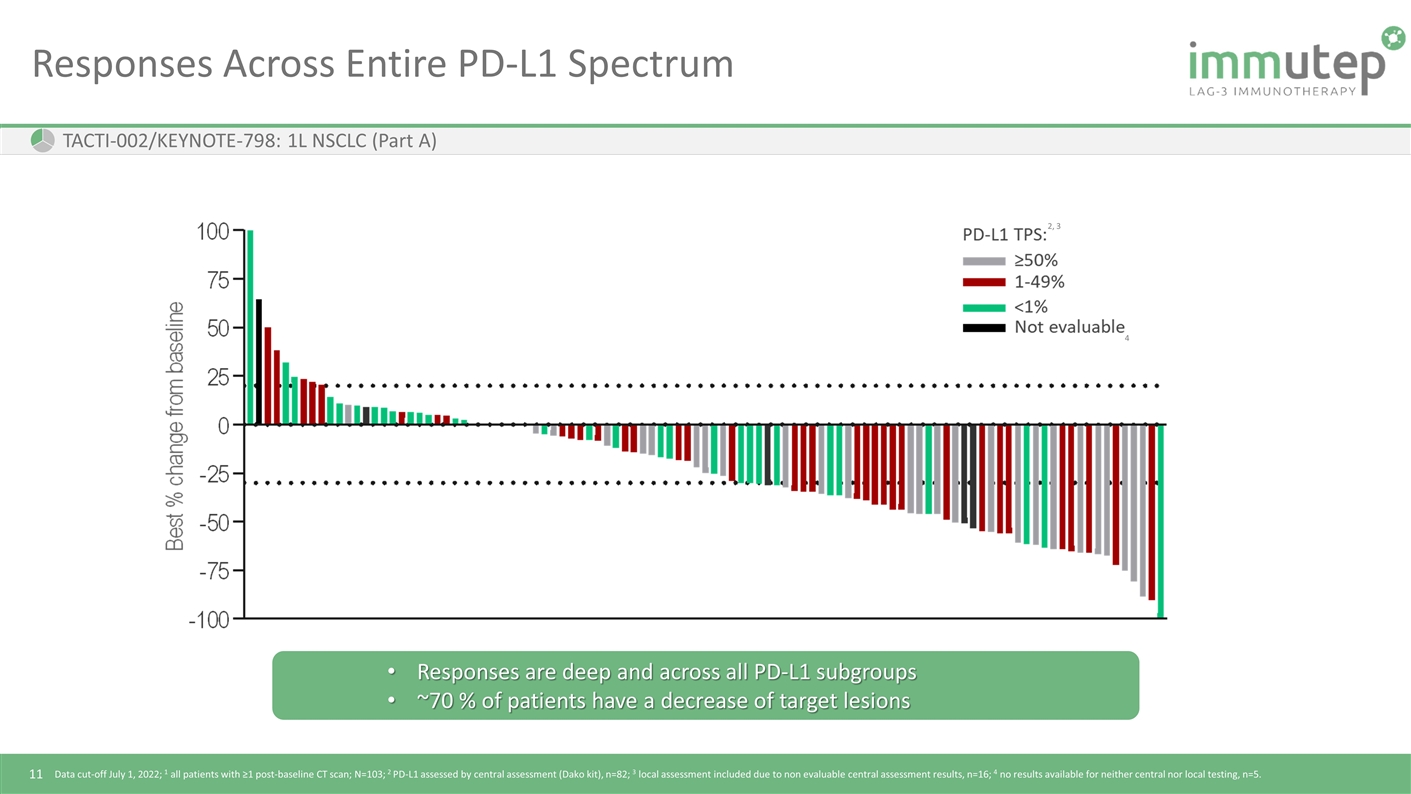
Responses Across Entire PD-L1 Spectrum Data cut-off July 1, 2022; 1 all patients with ≥1 post-baseline CT scan; N=103; 2 PD-L1 assessed by central assessment (Dako kit), n=82; 3 local assessment included due to non evaluable central assessment results, n=16; 4 no results available for neither central nor local testing, n=5. TACTI-002/KEYNOTE-798: 1L NSCLC (Part A) 4 2, 3 Responses are deep and across all PD-L1 subgroups ~70 % of patients have a decrease of target lesions
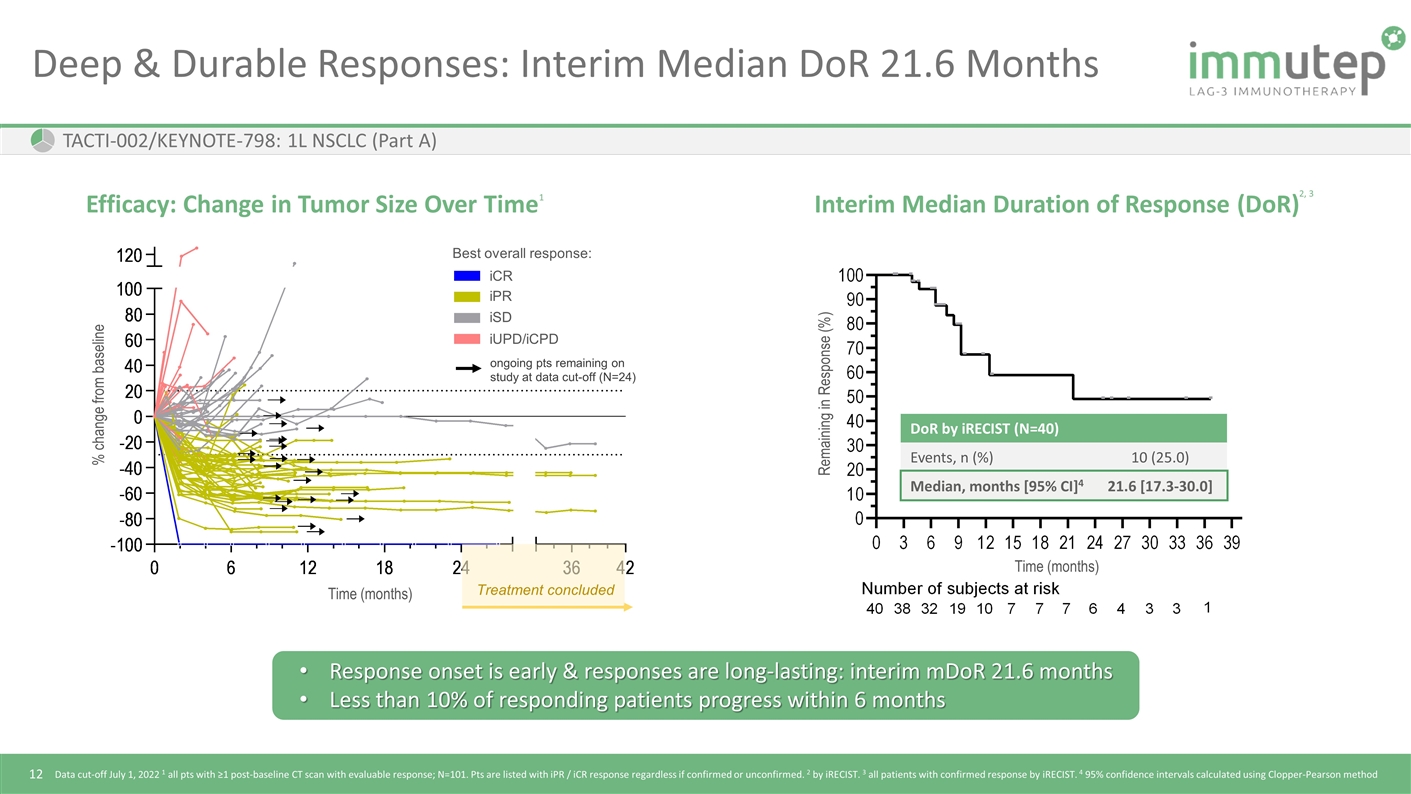
Time (months) Remaining in Response (%) Deep & Durable Responses: Interim Median DoR 21.6 Months TACTI-002/KEYNOTE-798: 1L NSCLC (Part A) Efficacy: Change in Tumor Size Over Time Interim Median Duration of Response (DoR) DoR by iRECIST (N=40) Events, n (%) 10 (25.0) Median, months [95% CI]4 21.6 [17.3-30.0] Treatment concluded Time (months) % change from baseline Response onset is early & responses are long-lasting: interim mDoR 21.6 months Less than 10% of responding patients progress within 6 months Data cut-off July 1, 2022 1 all pts with ≥1 post-baseline CT scan with evaluable response; N=101. Pts are listed with iPR / iCR response regardless if confirmed or unconfirmed. 2 by iRECIST. 3 all patients with confirmed response by iRECIST. 4 95% confidence intervals calculated using Clopper-Pearson method Best overall response: iUPD/iCPD iCR iPR iSD ongoing pts remaining on study at data cut-off (N=24) 2, 3 1
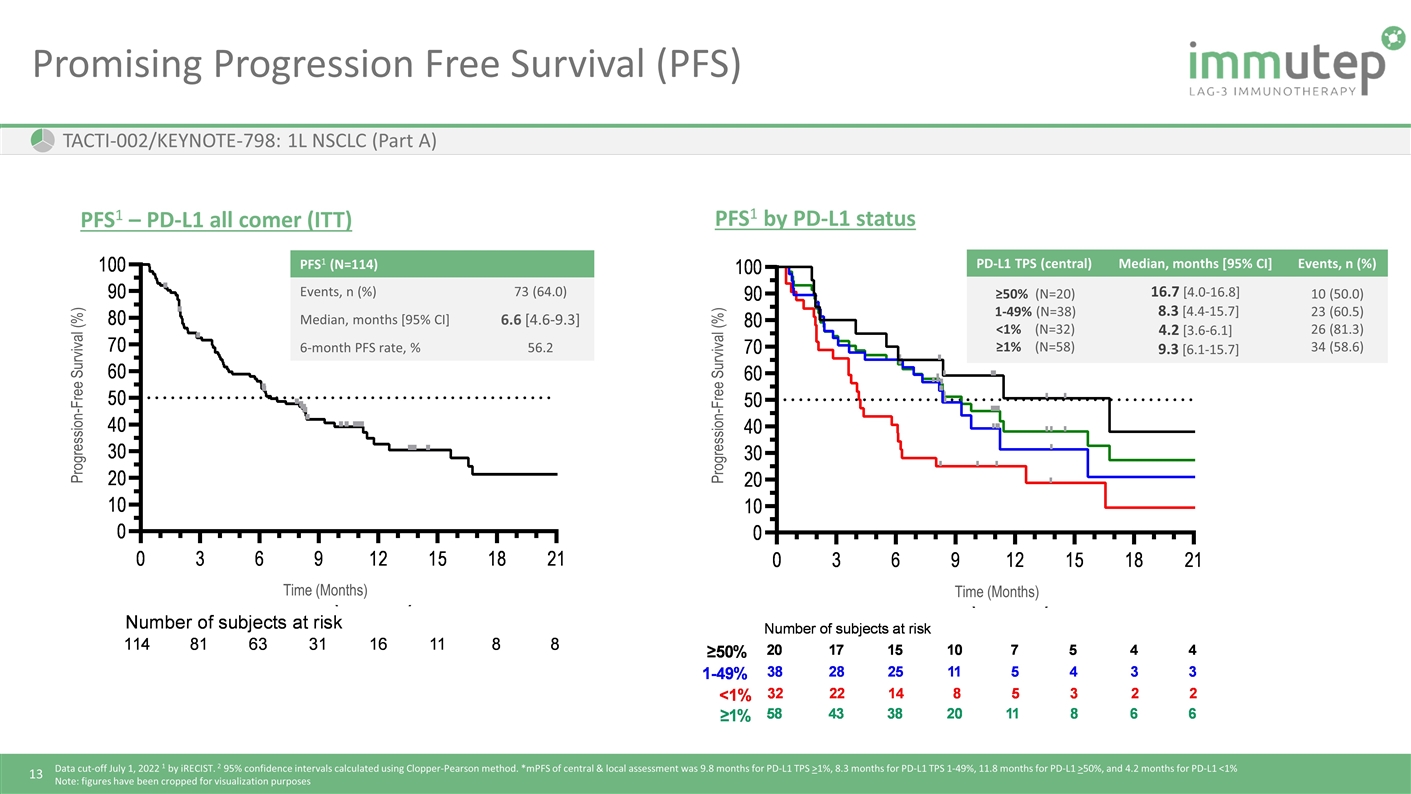
Promising Progression Free Survival (PFS) TACTI-002/KEYNOTE-798: 1L NSCLC (Part A) PD-L1 TPS (central) Median, months [95% CI] Events, n (%) ≥50% (N=20) 1-49% (N=38) <1% (N=32) ≥1% (N=58) 16.7 [4.0-16.8] 8.3 [4.4-15.7] 4.2 [3.6-6.1] 9.3 [6.1-15.7] 10 (50.0) 23 (60.5) 26 (81.3) 34 (58.6) PFS1 by PD-L1 status PFS1 – PD-L1 all comer (ITT) PFS1 (N=114) Events, n (%) 73 (64.0) Median, months [95% CI] 6.6 [4.6-9.3] 6-month PFS rate, % 56.2 Progression-Free Survival (%) Progression-Free Survival (%) Time (Months) Time (Months) Data cut-off July 1, 2022 1 by iRECIST. 2 95% confidence intervals calculated using Clopper-Pearson method. *mPFS of central & local assessment was 9.8 months for PD-L1 TPS >1%, 8.3 months for PD-L1 TPS 1-49%, 11.8 months for PD-L1 >50%, and 4.2 months for PD-L1 <1% Note: figures have been cropped for visualization purposes
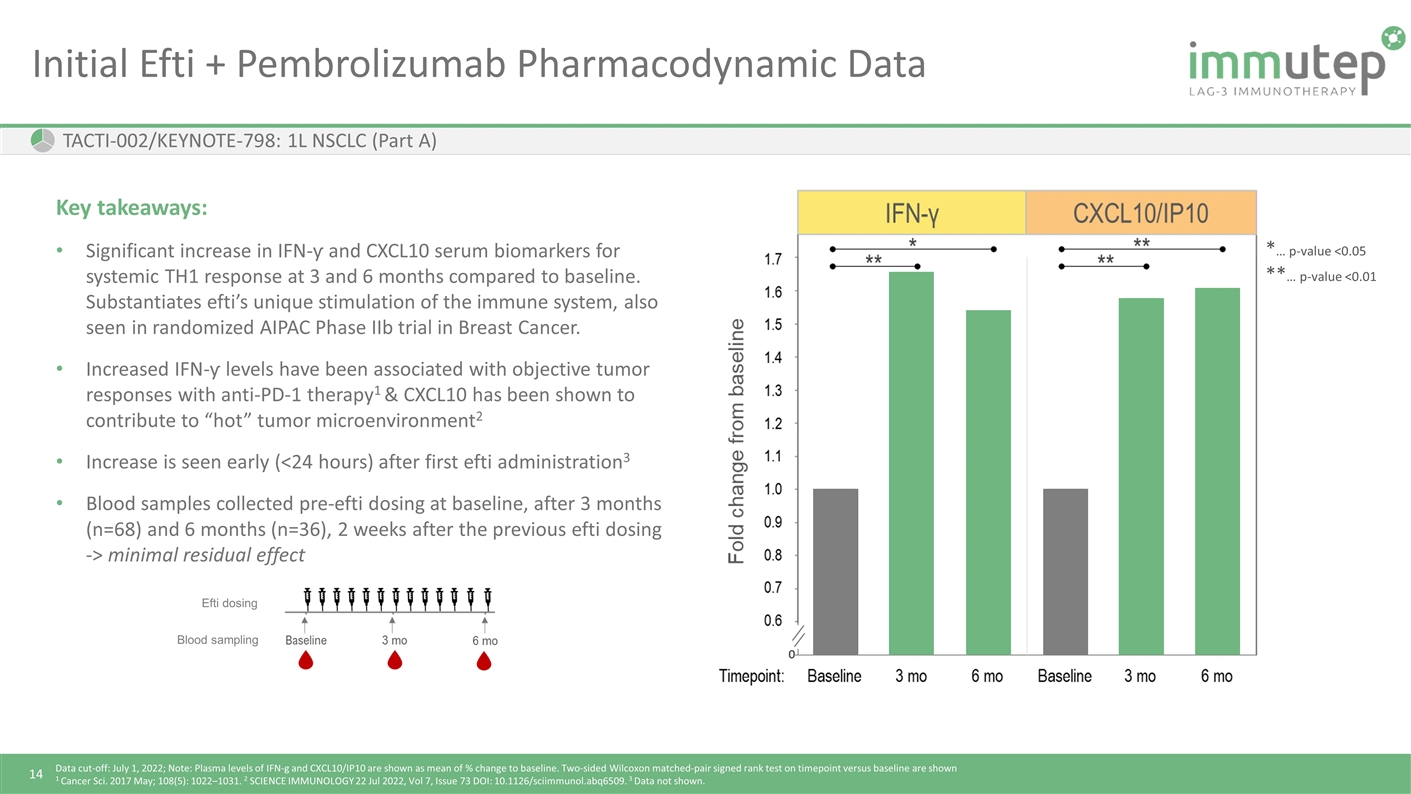
Initial Efti + Pembrolizumab Pharmacodynamic Data TACTI-002/KEYNOTE-798: 1L NSCLC (Part A) Key takeaways: Significant increase in IFN-ƴ and CXCL10 serum biomarkers for systemic TH1 response at 3 and 6 months compared to baseline. Substantiates efti’s unique stimulation of the immune system, also seen in randomized AIPAC Phase IIb trial in Breast Cancer. Increased IFN-ƴ levels have been associated with objective tumor responses with anti-PD-1 therapy1 & CXCL10 has been shown to contribute to “hot” tumor microenvironment2 Increase is seen early (<24 hours) after first efti administration3 Blood samples collected pre-efti dosing at baseline, after 3 months (n=68) and 6 months (n=36), 2 weeks after the previous efti dosing -> minimal residual effect Data cut-off: July 1, 2022; Note: Plasma levels of IFN-g and CXCL10/IP10 are shown as mean of % change to baseline. Two-sided Wilcoxon matched-pair signed rank test on timepoint versus baseline are shown 1 Cancer Sci. 2017 May; 108(5): 1022–1031. 2 SCIENCE IMMUNOLOGY 22 Jul 2022, Vol 7, Issue 73 DOI: 10.1126/sciimmunol.abq6509. 3 Data not shown. *… p-value <0.05 **… p-value <0.01 Efti dosing Blood sampling
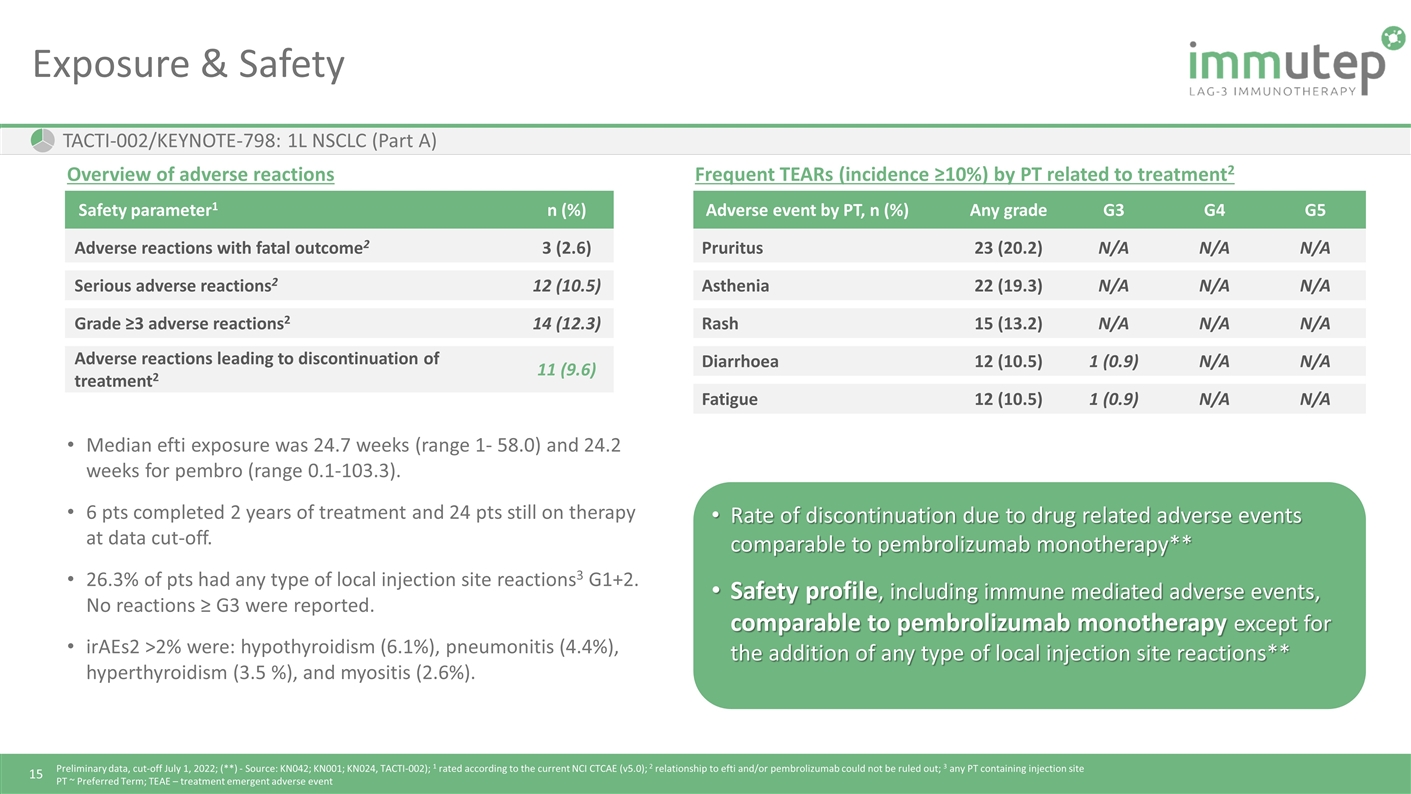
Exposure & Safety TACTI-002/KEYNOTE-798: 1L NSCLC (Part A) Safety parameter1 n (%) Adverse reactions with fatal outcome2 3 (2.6) Serious adverse reactions2 12 (10.5) Grade ≥3 adverse reactions2 14 (12.3) Adverse reactions leading to discontinuation of treatment2 11 (9.6) Frequent TEARs (incidence ≥10%) by PT related to treatment2 Adverse event by PT, n (%) Any grade G3 G4 G5 Pruritus 23 (20.2) N/A N/A N/A Asthenia 22 (19.3) N/A N/A N/A Rash 15 (13.2) N/A N/A N/A Diarrhoea 12 (10.5) 1 (0.9) N/A N/A Fatigue 12 (10.5) 1 (0.9) N/A N/A Overview of adverse reactions Median efti exposure was 24.7 weeks (range 1- 58.0) and 24.2 weeks for pembro (range 0.1-103.3). 6 pts completed 2 years of treatment and 24 pts still on therapy at data cut-off. 26.3% of pts had any type of local injection site reactions3 G1+2. No reactions ≥ G3 were reported. irAEs2 >2% were: hypothyroidism (6.1%), pneumonitis (4.4%), hyperthyroidism (3.5 %), and myositis (2.6%). Rate of discontinuation due to drug related adverse events comparable to pembrolizumab monotherapy** Safety profile, including immune mediated adverse events, comparable to pembrolizumab monotherapy except for the addition of any type of local injection site reactions** Preliminary data, cut-off July 1, 2022; (**) - Source: KN042; KN001; KN024, TACTI-002); 1 rated according to the current NCI CTCAE (v5.0); 2 relationship to efti and/or pembrolizumab could not be ruled out; 3 any PT containing injection site PT ~ Preferred Term; TEAE – treatment emergent adverse event
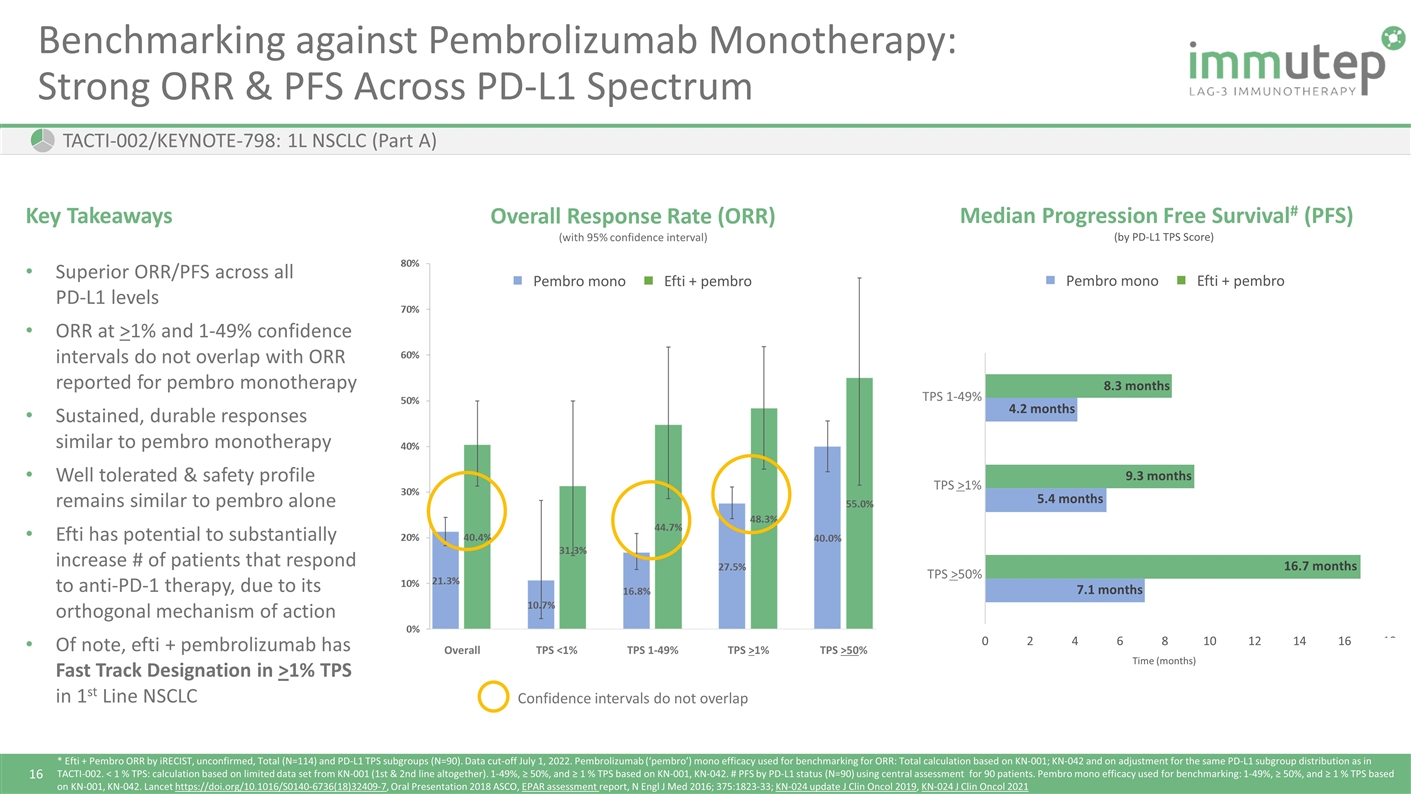
TACTI-002/KEYNOTE-798: 1L NSCLC (Part A) * Efti + Pembro ORR by iRECIST, unconfirmed, Total (N=114) and PD-L1 TPS subgroups (N=90). Data cut-off July 1, 2022. Pembrolizumab (‘pembro’) mono efficacy used for benchmarking for ORR: Total calculation based on KN-001; KN-042 and on adjustment for the same PD-L1 subgroup distribution as in TACTI-002. < 1 % TPS: calculation based on limited data set from KN-001 (1st & 2nd line altogether). 1-49%, ≥ 50%, and ≥ 1 % TPS based on KN-001, KN-042. # PFS by PD-L1 status (N=90) using central assessment for 90 patients. Pembro mono efficacy used for benchmarking: 1-49%, ≥ 50%, and ≥ 1 % TPS based on KN-001, KN-042. Lancet https://doi.org/10.1016/S0140-6736(18)32409-7, Oral Presentation 2018 ASCO, EPAR assessment report, N Engl J Med 2016; 375:1823-33; KN-024 update J Clin Oncol 2019, KN-024 J Clin Oncol 2021 Benchmarking against Pembrolizumab Monotherapy: Strong ORR & PFS Across PD-L1 Spectrum 8.3 months 4.2 months 9.3 months 5.4 months 16.7 months 7.1 months (by PD-L1 TPS Score) Efti + pembro Pembro mono TPS 1-49% TPS >1% TPS >50% Median Progression Free Survival# (PFS) Time (months) Overall Response Rate (ORR) (with 95% confidence interval) TPS 1-49% TPS >1% TPS >50% TPS <1% Overall Efti + pembro Pembro mono Key Takeaways Superior ORR/PFS across all PD-L1 levels ORR at >1% and 1-49% confidence intervals do not overlap with ORR reported for pembro monotherapy Sustained, durable responses similar to pembro monotherapy Well tolerated & safety profile remains similar to pembro alone Efti has potential to substantially increase # of patients that respond to anti-PD-1 therapy, due to its orthogonal mechanism of action Of note, efti + pembrolizumab has Fast Track Designation in >1% TPS in 1st Line NSCLC Confidence intervals do not overlap
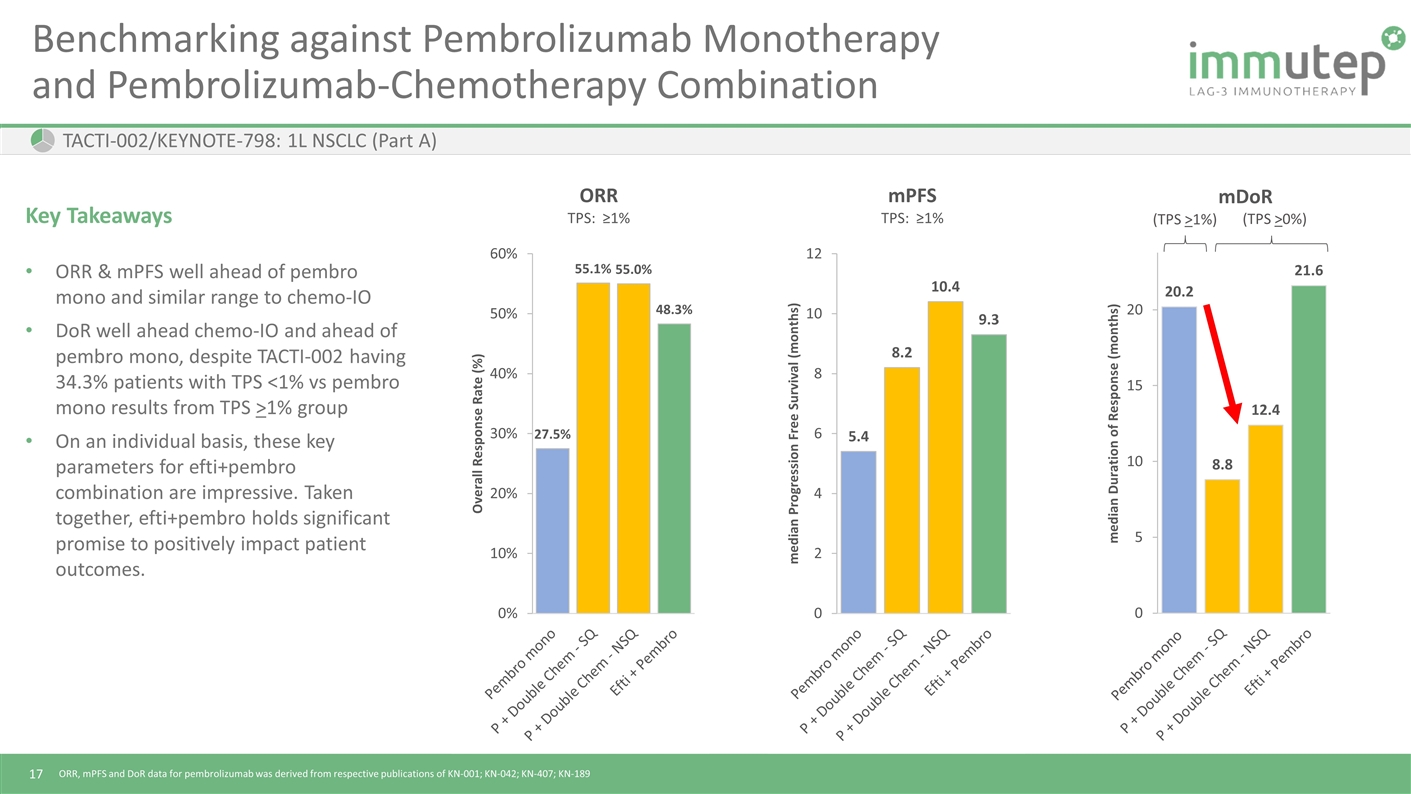
ORR, mPFS and DoR data for pembrolizumab was derived from respective publications of KN-001; KN-042; KN-407; KN-189 Benchmarking against Pembrolizumab Monotherapy and Pembrolizumab-Chemotherapy Combination Key Takeaways ORR & mPFS well ahead of pembro mono and similar range to chemo-IO DoR well ahead chemo-IO and ahead of pembro mono, despite TACTI-002 having 34.3% patients with TPS <1% vs pembro mono results from TPS >1% group On an individual basis, these key parameters for efti+pembro combination are impressive. Taken together, efti+pembro holds significant promise to positively impact patient outcomes. TACTI-002/KEYNOTE-798: 1L NSCLC (Part A) (TPS >0%) Pembro mono (TPS >1%)

INSIGHT-003 Phase 1 Trial: Efti + Pembrolizumab + Chemotherapy Combination in 1L NSCLC
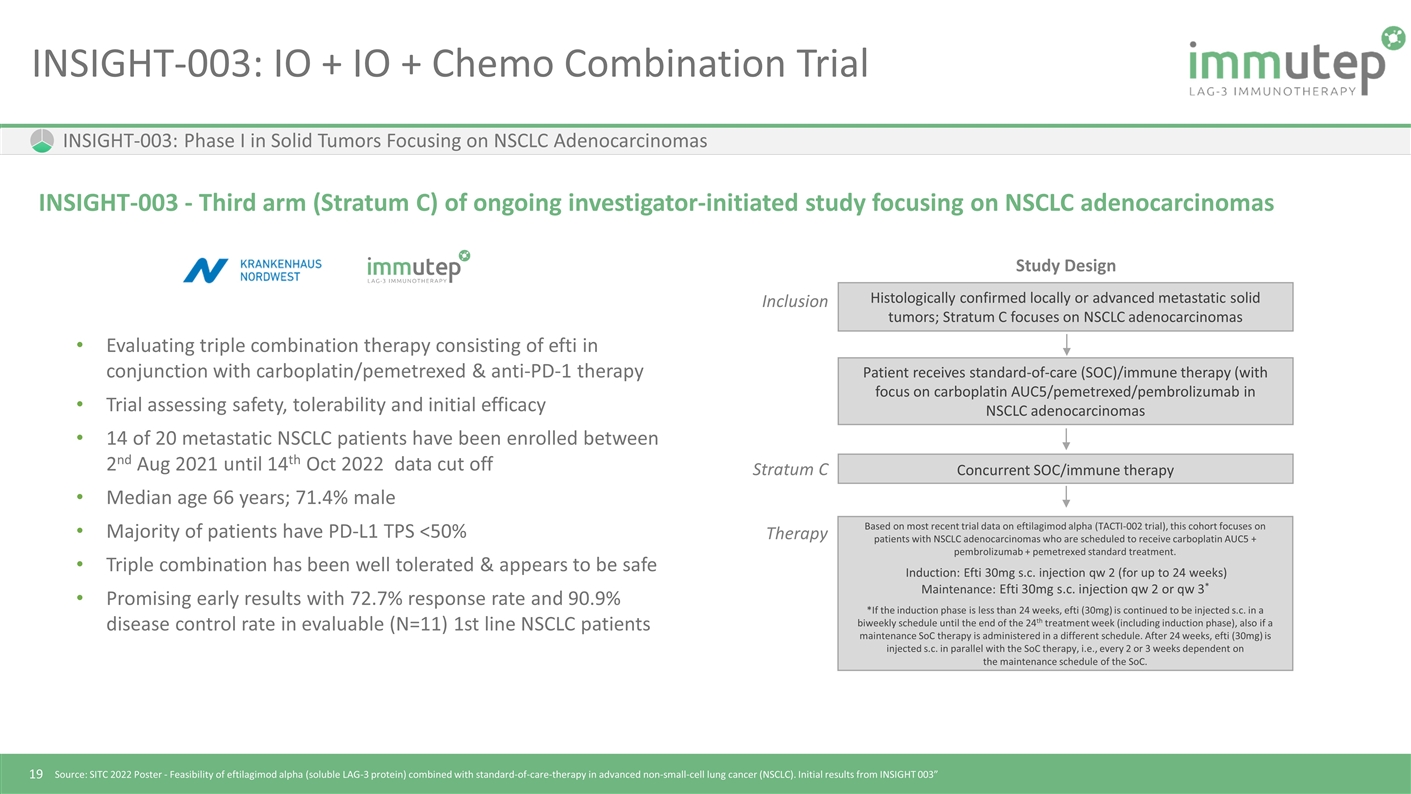
INSIGHT-003: IO + IO + Chemo Combination Trial INSIGHT-003: Phase I in Solid Tumors Focusing on NSCLC Adenocarcinomas INSIGHT-003 - Third arm (Stratum C) of ongoing investigator-initiated study focusing on NSCLC adenocarcinomas Evaluating triple combination therapy consisting of efti in conjunction with carboplatin/pemetrexed & anti-PD-1 therapy Trial assessing safety, tolerability and initial efficacy 14 of 20 metastatic NSCLC patients have been enrolled between 2nd Aug 2021 until 14th Oct 2022 data cut off Median age 66 years; 71.4% male Majority of patients have PD-L1 TPS <50% Triple combination has been well tolerated & appears to be safe Promising early results with 72.7% response rate and 90.9% disease control rate in evaluable (N=11) 1st line NSCLC patients Source: SITC 2022 Poster - Feasibility of eftilagimod alpha (soluble LAG-3 protein) combined with standard-of-care-therapy in advanced non-small-cell lung cancer (NSCLC). Initial results from INSIGHT 003” Histologically confirmed locally or advanced metastatic solid tumors; Stratum C focuses on NSCLC adenocarcinomas Inclusion Patient receives standard-of-care (SOC)/immune therapy (with focus on carboplatin AUC5/pemetrexed/pembrolizumab in NSCLC adenocarcinomas Stratum C Concurrent SOC/immune therapy Therapy Based on most recent trial data on eftilagimod alpha (TACTI-002 trial), this cohort focuses on patients with NSCLC adenocarcinomas who are scheduled to receive carboplatin AUC5 + pembrolizumab + pemetrexed standard treatment. Induction: Efti 30mg s.c. injection qw 2 (for up to 24 weeks) Maintenance: Efti 30mg s.c. injection qw 2 or qw 3* *If the induction phase is less than 24 weeks, efti (30mg) is continued to be injected s.c. in a biweekly schedule until the end of the 24th treatment week (including induction phase), also if a maintenance SoC therapy is administered in a different schedule. After 24 weeks, efti (30mg) is injected s.c. in parallel with the SoC therapy, i.e., every 2 or 3 weeks dependent on the maintenance schedule of the SoC. Study Design
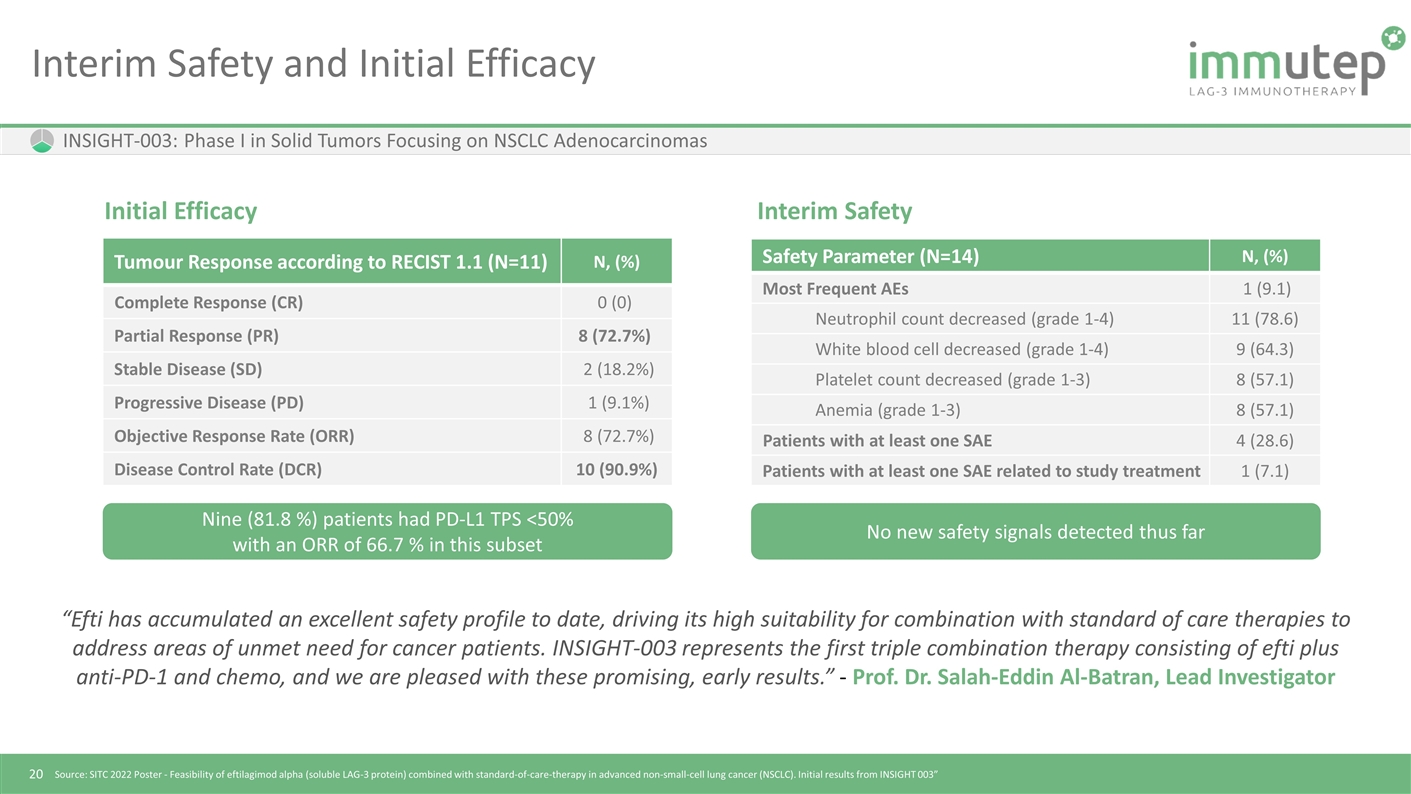
Interim Safety and Initial Efficacy INSIGHT-003: Phase I in Solid Tumors Focusing on NSCLC Adenocarcinomas Tumour Response according to RECIST 1.1 (N=11) N, (%) Complete Response (CR) 0 (0) Partial Response (PR) 8 (72.7%) Stable Disease (SD) 2 (18.2%) Progressive Disease (PD) 1 (9.1%) Objective Response Rate (ORR) 8 (72.7%) Disease Control Rate (DCR) 10 (90.9%) “Efti has accumulated an excellent safety profile to date, driving its high suitability for combination with standard of care therapies to address areas of unmet need for cancer patients. INSIGHT-003 represents the first triple combination therapy consisting of efti plus anti-PD-1 and chemo, and we are pleased with these promising, early results.” - Prof. Dr. Salah-Eddin Al-Batran, Lead Investigator Safety Parameter (N=14) N, (%) Most Frequent AEs 1 (9.1) Neutrophil count decreased (grade 1-4) 11 (78.6) White blood cell decreased (grade 1-4) 9 (64.3) Platelet count decreased (grade 1-3) 8 (57.1) Anemia (grade 1-3) 8 (57.1) Patients with at least one SAE 4 (28.6) Patients with at least one SAE related to study treatment 1 (7.1) Initial Efficacy Interim Safety Source: SITC 2022 Poster - Feasibility of eftilagimod alpha (soluble LAG-3 protein) combined with standard-of-care-therapy in advanced non-small-cell lung cancer (NSCLC). Initial results from INSIGHT 003” Nine (81.8 %) patients had PD-L1 TPS <50% with an ORR of 66.7 % in this subset No new safety signals detected thus far

Conclusion: Large data set for efti+pembro (+chemo) combination with 120+ pts in 1st line NSCLC provides robust basis for phase III Efti+pembro nearly doubles ORR of pembro mono (40.4% vs 21.3%), extends PFS, & maintains long DoR/safety profile of pembro mono Due to orthogonal MoA, efti has potential to greatly increase # of patients who respond to anti-PD-1, including low/negative TPS Initial pharmacodynamic data of efti+pembro reveals significant increase in IFN-ƴ & CXCL10 (Th1 biomarker) à proof of principle Initial data shows efti + SoC doublet chemo + anti-PD-1 is feasible & safe with promising efficacy à increases flexibility for Phase 3 planning Strength of efti+pembro clinical data has led to Fast Track Designation in >1% TPS in 1st Line NSCLC 1 Mok et al, The Lancet 2019, http://dx.doi.org/10.1016/S0140-6736(18)32409-7 Outlook: Planning around intelligent registrational relevant trials (e.g. adaptive Phase 2/3) is in progress and under discussion with regulators. Potential design options are proceeding and include: Superiority in terms of OS against pembro monotherapy restricted to patients where this combination could potentially demonstrate superiority Superiority in terms of OS vs. doublet chemo + anti-PD-1/PD-L1 in appropriate 1st line NSCLC patients Trials are being planned in most cost-effective and time-efficient manner Final trial design and patient population will depend on feedback from agencies Expected timelines are design feedback in H1 2023 concluded and trial start in H2 2023 1L NSCLC: Conclusion & Outlook

Progress in HNSCC & MBC
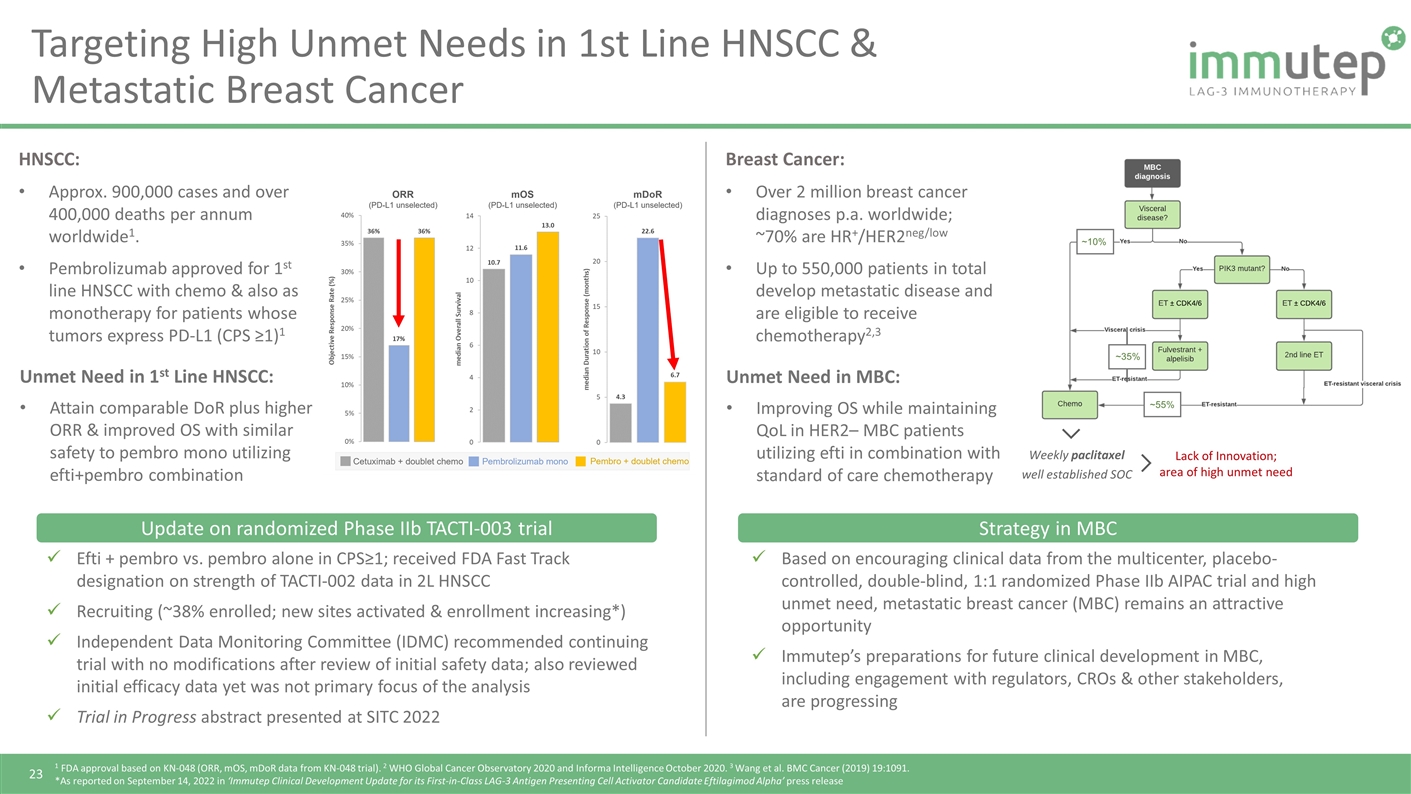
Targeting High Unmet Needs in 1st Line HNSCC & Metastatic Breast Cancer 1 FDA approval based on KN-048 (ORR, mOS, mDoR data from KN-048 trial). 2 WHO Global Cancer Observatory 2020 and Informa Intelligence October 2020. 3 Wang et al. BMC Cancer (2019) 19:1091. *As reported on September 14, 2022 in ‘Immutep Clinical Development Update for its First-in-Class LAG-3 Antigen Presenting Cell Activator Candidate Eftilagimod Alpha’ press release Breast Cancer: Over 2 million breast cancer diagnoses p.a. worldwide; ~70% are HR+/HER2neg/low Up to 550,000 patients in total develop metastatic disease and are eligible to receive chemotherapy2,3 HNSCC: Approx. 900,000 cases and over 400,000 deaths per annum worldwide1. Pembrolizumab approved for 1st line HNSCC with chemo & also as monotherapy for patients whose tumors express PD‑L1 (CPS ≥1)1 Efti + pembro vs. pembro alone in CPS≥1; received FDA Fast Track designation on strength of TACTI-002 data in 2L HNSCC Recruiting (~38% enrolled; new sites activated & enrollment increasing*) Independent Data Monitoring Committee (IDMC) recommended continuing trial with no modifications after review of initial safety data; also reviewed initial efficacy data yet was not primary focus of the analysis Trial in Progress abstract presented at SITC 2022 Based on encouraging clinical data from the multicenter, placebo-controlled, double-blind, 1:1 randomized Phase IIb AIPAC trial and high unmet need, metastatic breast cancer (MBC) remains an attractive opportunity Immutep’s preparations for future clinical development in MBC, including engagement with regulators, CROs & other stakeholders, are progressing Unmet Need in MBC: Improving OS while maintaining QoL in HER2– MBC patients utilizing efti in combination with standard of care chemotherapy ~10% ~35% ~55% Weekly paclitaxel well established SOC Lack of Innovation; area of high unmet need Unmet Need in 1st Line HNSCC: Attain comparable DoR plus higher ORR & improved OS with similar safety to pembro mono utilizing efti+pembro combination Update on randomized Phase IIb TACTI-003 trial Strategy in MBC
Year to date: 1L NSCLC Oral Presentation at ASCO (TACTI-002; Part A) 2L NSCLC PD-X refractory data at ELCC & WCLC 2022 (TACTI-002; Part B) Fast Track Designation granted in 1L NSCLC New, significant data from AIPAC study IP expansion for eftilagimod alpha New data from Phase II TACTI-002 in 1L NSCLC at SITC 2022 Initial results from INSIGHT-003 (triple-combo) at SITC 2022 Trial in progress poster on randomized trial in 1L HNSCC at SITC 2022 Expansion of existing programs (i.e., new sarcoma trial) Regulatory updates Manufacturing scale up to 2,000L Updates on IMP761 & partnered programs 2022 Milestones Pioneering LAG-3 portfolio in oncology and autoimmune diseases with four product candidates in multiple clinical trials First-in-class positioning with eftilagimod alpha (efti) Multiple big pharma partnerships & collaborations, while retaining full control of efti (ex-China) & IMP761 Well funded with ~A$73.9 million* in cash Cash runway to early CY2024* Market cap ~A$280M / $185M US** Ticker symbols: IMM (ASX) & IMMP (NASDAQ) Total institutional ownership of ~57% includes Fidelity (FIL Ltd.) ~7.41% and Australian Ethical ~4.98%*** Corporate Snapshot Summary *As reported in Quarterly Activities Report for quarter ended 30 September, 2022 (Q1 FY23); ** As of 8 November, 2022. 26.76% of ordinary shares outstanding are represented by ADSs listed on NASDAQ. ***Based on latest substantial holder notices and Orient Capital Report reflecting the register as at 14 October 2022.

Thank You
























