Filed pursuant to Rule 424(b)(3)
Registration No. 333-216030
PROSPECTUS

11,444,204 Shares
Common Stock
This prospectus relates to the sale or other disposition from time to time of up to 11,444,204 shares of our common stock, $0.001 par value per share, held by the selling stockholders named in this prospectus. We are not selling any shares of common stock under this prospectus and will not receive any of the proceeds from the sale of shares of common stock by the selling stockholders.
The selling stockholders may sell or otherwise dispose of the shares of common stock covered by this prospectus in a number of different ways and at varying prices. We provide more information about how the selling stockholders may sell or otherwise dispose of their shares of common stock in the section entitled “Plan of Distribution” on page 64. The selling stockholders will pay all brokerage fees and commissions and similar expenses. We will pay all expenses (except brokerage fees and commissions and similar expenses) relating to the registration of the shares with the Securities and Exchange Commission.
Our common stock is listed on the OTCQB Venture Market operated by OTC Markets Group, Inc. (or OTCQB) under the ticker symbol “CTXR.QB.” On March 30, 2017, the last reported sale price of our common stock on the OTCQB was $0.45.
Investing in our common stock involves a high degree of risk. You should review carefully the risks and uncertainties described under the heading “Risk Factors” beginning on page 9 of this prospectus, and under similar headings in any amendments or supplements to this prospectus.
Neither the Securities and Exchange Commission nor any state securities commission has approved or disapproved of these securities or passed upon the adequacy or accuracy of this prospectus. Any representation to the contrary is a criminal offense.
The date of this prospectus is April 11, 2017.
|
| Page |
| |
|
| 1 |
| |
|
|
|
|
|
|
| 8 |
| |
|
|
|
|
|
|
| 9 |
| |
|
|
|
|
|
|
| 28 |
| |
|
|
|
|
|
|
| 29 |
| |
|
|
|
|
|
|
| 29 |
| |
|
|
|
|
|
|
| 31 |
| |
|
|
|
|
|
Management’s Discussion and Analysis of Financial Condition and Results of Operations. |
|
| 32 |
|
|
|
|
|
|
|
| 41 |
| |
|
|
|
|
|
|
| 51 |
| |
|
|
|
|
|
|
| 62 |
| |
|
|
|
|
|
|
| 64 |
| |
|
|
|
|
|
Security Ownership of Certain Beneficial Owners and Management |
|
| 66 |
|
|
|
|
|
|
|
| 68 |
| |
|
|
|
|
|
|
| 71 |
| |
|
|
|
|
|
|
| 71 |
| |
|
|
|
|
|
|
| 71 |
| |
|
|
|
|
|
|
| F-1 |
| |
i |
You should rely only on the information contained in this prospectus, as supplemented and amended. We have not, and the selling stockholders have not, authorized anyone to provide you with information that is different. This prospectus may only be used where it is legal to sell these securities. The information in this prospectus may only be accurate on the date of this prospectus.
We urge you to read carefully this prospectus, as supplemented and amended, before deciding whether to invest in any of the common stock being offered.
Unless the context indicates otherwise, as used in this prospectus, the terms “Citius,” “we,” “us,” “our,” “our company” and “our business” refer to Citius Pharmaceuticals, Inc.
We own or have rights to various U.S. federal trademark registrations and applications, and unregistered trademarks and servicemarks, including Mino-Lok™. All other trade names, trademarks and service marks appearing in this prospectus are the property of their respective owners. We have assumed that the reader understands that all such terms are source-indicating. Accordingly, such terms, when first mentioned in this prospectus, appear with the trade name, trademark or service mark notice and then throughout the remainder of this prospectus without trade name, trademark or service mark notices for convenience only and should not be construed as being used in a descriptive or generic sense.
ii |
| Table of Contents |
This summary highlights certain information about us and this offering contained elsewhere in this prospectus. Because it is only a summary, it does not contain all of the information that you should consider before investing in shares of our securities and it is qualified in its entirety by, and should be read in conjunction with, the more detailed information appearing elsewhere in this prospectus. Before you decide to invest in our securities, you should read the entire prospectus carefully, including “Risk Factors” beginning on page 9, and the financial statements and related notes included in this prospectus.
Company Overview
Citius Pharmaceuticals, Inc. (“Citius” or the “Company”) headquartered in Cranford, New Jersey, is a specialty pharmaceutical company dedicated to the development and commercialization of critical care products targeting important medical needs with a focus on anti-infective products, adjunctive cancer care, and unique prescription products. Our goal is to achieve leading market positions in our targeted markets by providing therapeutic products that address unmet medical needs. New formulations of previously approved drugs with substantial safety and efficacy data is a core focus as we seek to reduce development and clinical risks associated with drug development. Our strategy keys on products that have intellectual property and regulatory exclusivity protection, while providing competitive advantages over other existing therapeutic approaches.
The Company was founded as Citius Pharmaceuticals, LLC, a Massachusetts limited liability company, on January 23, 2007. On September 12, 2014, Citius Pharmaceuticals, LLC entered into a Share Exchange and Reorganization Agreement, with Citius Pharmaceuticals, Inc. (formerly Trail One, Inc.), a publicly traded company incorporated under the laws of the State of Nevada. Citius Pharmaceuticals, LLC became a wholly-owned subsidiary of Citius. On March 30, 2016, Citius acquired Leonard-Meron Biosciences, Inc. (“LMB”) as a wholly-owned subsidiary. LMB was a pharmaceutical company focused on the development and commercialization of critical care products with a concentration on anti-infectives.
Since its inception, the Company has devoted substantially all of its efforts to business planning, research and development, recruiting management and technical staff, and raising capital. We are developing two proprietary products: Mino-Lok™, an antibiotic lock solution used to treat patients with catheter-related bloodstream infections by salvaging the infected catheter, and a Hydrocortisone-Lidocaine topical formulation that is intended to provide anti-inflammatory and anesthetic relief to persons suffering from hemorrhoids. We believe the markets for our products are large, growing, and underserved by current prescription products or procedures.
References to “we,” “us,” “our” and similar words refer to the Company and its wholly-owned subsidiaries Citius Pharmaceuticals LLC and LMB, taken as a whole. References to “Trail One” refer to the Company and its business prior to the Reverse Acquisition.
Strategy
Our goal is to build a successful specialty pharmaceutical company through the commercialization of innovative, efficacious and cost-effective mid- to late-term development products that address compelling market opportunities in the critical care field. We will seek to achieve this goal by:
| · | identifying new drug product candidates that are typically prescribed and used by a small, identified number of specialists, which we believe can therefore be successfully commercialized by a small, specialty sales force, and a focused educational program for innovation adoption; | |
|
| |
| · | obtaining licenses for the most relevant and advanced technologies in critical care medicine that address unmet or urgent medical needs for underserved markets with limited competition and have at least 10 years of intellectual property protection; | |
|
| |
| · | leveraging our in-house clinical and regulatory expertise to advance the development of product candidates in our pipeline more rapidly and take advantage of expedited regulatory pathways; | |
|
| |
| · | leveraging our commercialization and marketing expertise in prescription drugs and critical care medicine to develop and monetize markets that are in need of new product solutions; and | |
|
| |
| · | managing our business in a financially disciplined and cost-conscious manner. |
| 1 |
| Table of Contents |
Our Business
We seek to achieve our business objectives by utilizing the U.S. Food and Drug Administration’s, or FDA’s, 505(b)(2) pathway for our new drug approvals. We believe this pathway is faster, has lower risk and is less expensive than the FDA’s traditional new drug approval pathway. In addition to focusing on new drug approvals, we focus on obtaining intellectual property protection with the objective of listing relevant patents in the FDA Orange Book in order to limit generic competition.
By using previously approved drugs with substantial safety and efficacy data already available, we seek to reduce the risks associated with pharmaceutical product development. We have two development candidates. Our Mino-Lok product for the treatment of catheter related bloodstream infections, or CRBSIs, has completed Phase 2b and is entering Phase 3 trials. We are also developing a topical product containing both hydrocortisone and lidocaine (Hydro-Lido) for the treatment of mild to moderate hemorrhoids. We are reformulating this product and will be entering Phase 2b trials in 2017.
In July 2016, the Company decided to discontinue Suprenza, its FDA-approved phentermine-based product for weight loss, due to a strategic change in direction following the acquisition of LMB and the Mino-Lok product. In September 2016, Citius notified the FDA of its decision to voluntarily withdraw both the Investigative New Drug Application and New Drug Application for commercial reasons and not due to safety concerns, effective immediately. The Company had received no royalties from Suprenza and believed costs associated with the ongoing regulatory expenses were depleting resources from our more promising Mino-Lok and Hydro-Lido product candidates.
Our Product Candidates
Product |
| Indication |
| Current Status |
| Patent Expiry; Patent Number |
|
|
|
|
|
|
|
Mino-Lok |
| Antibiotic Lock Therapy |
| Phase 3 study upcoming |
| June 7, 2024; 7,601,731 |
|
|
|
|
|
|
|
Mino-Lok |
| Antibiotic Lock Therapy |
| Enhanced Stability |
| Pending |
|
|
|
|
|
|
|
Hydrocortisone-Lidocaine Cream |
| Hemorrhoids |
| Phase 2b study upcoming |
| Patent to be filed upon finalization of formulation. |
Mino-LokTM
Overview
Mino-Lok is a patented solution containing minocycline, disodium ethylenediaminetetraacetic acid (edetate), and ethyl alcohol, all of which act synergistically to treat and salvage infected central venous catheters (“CVCs”) in patients with catheter related bloodstream infections (“CRBIs”). Mino-Lok breaks down biofilm barriers formed by bacterial colonies, eradicate the bacteria, and provide anti-clotting properties to maintain patency in CVCs.
The administration of Mino-Lok consists of filling the lumen of the catheter with 0.8 ml to 2.0 ml of Mino-Lok solution, with a lock (dwell-time) of two hours while the catheter is not in use. If the catheter has multiple lumens, all lumens may be locked with the Mino-Lok solution either simultaneously or sequentially. If patients are receiving continuous infusion therapy, the catheters alternate between being locked with the Mino-Lok solution and delivering therapy. The Mino-Lok therapy is two hours per day for at least five days, usually with additional locks in the subsequent two weeks. After locking the catheter for two hours, the Mino-Lok solution is aspirated, and the catheter is flushed with normal saline. At that time, either the infusion will be continued, or will be locked with the standard-of-care lock solution until further use of the catheter is required. In a clinical study conducted by MD Anderson Cancer Center (“MDACC”), there were no serum levels of either minocycline or edetate detected in the sera of several patients who underwent daily catheter lock solution with minocycline and edetate (“M-EDTA”) at the concentration level proposed in Mino-Lok treatment. Thus, it has been demonstrated that the amount of either minocycline or edetate that leaks into the serum is very low or none at all.
| 2 |
| Table of Contents |
Phase 2b Results
From April 2013 to July 2014, 30 patients with CVC-related bloodstream infection were enrolled at MDACC in a prospective Phase 2b study. Patients received Mino-Lok therapy for two hours once daily for a minimum of five days within the first week followed by two additional locks within the next two weeks. Patients were followed for one month post lock therapy. Demographic information, clinical characteristics, laboratory data, therapy, as well as adverse events and outcome were collected for each patient. Median age at diagnosis was 56 years (range: 21-73 years). In all patients, prior to the use of lock therapy, systemic treatment with a cultured-directed, first-line intravenous was started. Microbiological eradication was achieved in all cases. None of the patients experienced any serious adverse event related to the lock therapy.
The active arm was then compared to 60 patients in a matched cohort that experienced removal and replacement of their CVCs within the same contemporaneous timeframe. The patients were matched for cancer type, infecting organism, and level of neutropenia. All patients were cancer patients and treated at the MDACC. The efficacy of Mino-Lok therapy was 100% in salvaging CVCs, demonstrating equal effectiveness to removing the infected CVC and replacing with a new catheter.
However, the main purpose of the study was to show that Mino-Lok therapy was at least as safe as the removal and replacement of CVCs when CRBSIs are present, and that the complications of removing an infected catheter and replacing with a new one could be avoided. In addition to having a 100% efficacy rate with all CVCs being salvaged, Mino-Lok therapy had no significant adverse events (“SAEs”), compared to an 18% serious adverse event rate in the matched cohort where patients had the infected CVCs removed and replaced (“R&R”). There were no overall complication rates in the Mino-Lok arm group compared to 11 events (18%) in the control group. These events included bacterial relapse (5%) and a number of complications associated with mechanical manipulation in the removal or replacement procedure for the catheter (10%) or development of deep seated infections such as septic thrombophlebitis and osteomyelitis (8%). It was noted that six (6) patients had more than one (1) complication in the control arm group.
Phase 3 Initiation
In November 2016, the Company initiated site recruitment for Phase 3 clinical trials. It is expected that patient enrollment will commence in the Company’s third quarter 2017.
Market Opportunity
In spite of best clinical practice, catheters contribute to approximately 70% of blood stream infections that occur in the ICU, or are associated with hemodialysis or cancer patients (approximately 470,000 per year). Bacteria enter the catheter either from the skin or intraluminally through the catheter hub. Once in the catheter, bacteria tend to form hard biofilm on the surface of the catheter that is resistant to most antimicrobial solutions. The most frequently used maintenance flush, heparin, actually stimulates biofilm formation. Heparin is widely used as a prophylactic lock solution, in spite of the evidence that it contributes to the promotion of biofilm formation. The formation of bacterial biofilm usually precedes CRBSIs.
The standard of care (SOC) in the management of CRBSI patients consists of removing the infected CVC and replacing it with a new catheter at a different vascular access site. However, in cancer and hemodialysis patients with long-term surgically implantable silicone catheters, removal of the CVC and reinsertion of a new one at a different site might be difficult, or even impossible, because of the unavailability of other accessible vascular sites and the need to maintain infusion therapy. Furthermore, critically ill patients with short-term catheters often have underlying coagulopathy, which makes reinsertion of a new CVC at a different site, in the setting of CRBSIs, risky in terms of mechanical complications, such as pneumothorax, misplacement, or arterial puncture. Studies have also revealed that CRBSI patients may be associated with serious complications, including septic thrombosis, endocarditis and disseminated infection, particularly if caused by Staphylococcus aureus or Candida species. Furthermore, catheter retention in patients with CRBSIs is associated with a higher risk of relapse and poor response to antimicrobial therapy.
According to Maki et al., published in the Mayo Clinic Proceedings in 2006, there are approximately 250,000 CRBSIs annually in the U.S. Subsequent to this study, our estimates have ranged upwards to over 450,000 central line-associated blood stream infections (“CLABSIs”) annually (see analysis in the table below). CRBSIs are associated with a 12% to 35% mortality rate and an attributable cost of $35,000 to $56,000 per episode.
| 3 |
| Table of Contents |
We estimate that the potential market for Mino-Lok in the U.S. to be approximately $500 million to $1 billion as shown in the table below.
| Short-Term CVC | Long-Term CVC | Total |
No. of Catheters | 3 million | 4 million | 7 million |
Avg. Duration (Days) | 12 | 100 | N/A |
Catheter Days | 36 million | 400 million | 436 million |
Infection Rate | 2/1,000 days | 1/1,000 days | N/A |
Catheters Infected | 72,000 | 400,000 | 472,000 |
Flushes/Catheter | 5 | 7 | 6.7 |
Total Salvage Flushes | 360,000 | 2,800,000 | 3,160,000 |
Sources: Ann Intern Med 2000; 132:391–402, Clev Clin J Med 2011; 78(1):10-17, JAVA 2007; 12(1):17-27, J Inf Nurs 2004;27(4):245-250, Joint Commission website Monograph, CLABSI and Internal Estimates.
Under various plausible pricing scenarios, we believe that Mino-Lok would be cost saving to the healthcare system given that the removal of an infected CVC and replacement of a new catheter in a different venous access site is estimated by the Company to cost between $8,000 and $10,000. Furthermore, there are potential additional medical benefits and reduction in patient discomfort with the Mino-Lok approach. We believe there will be an economic argument to enhance the adoption of Mino-Lok by infection control committees at acute care institutions.
In January of 2017, the Company commissioned a third party survey of 31 physicians to qualify the need for catheter salvage in patients with infected, indwelling central venous lines, especially when the catheter is a tunneledor an implanted port. There were 19 Infection Disease experts and 12 Intensivists surveyed who all agreed that salvage would be preferable to catheter exchange to avoid catheter misplacements, blood clots, or vessel punctures that can potentially occur during reinsertion. Most were also concerned that viable venous access may not be available in patients who were vitally dependenton a central line.
Hydro-Lido
Overview
Hydro-Lido is a topical formulation of hydrocortisone and lidocaine that is intended for the treatment of hemorrhoids. To our knowledge, there are currently no FDA-approved prescription drug products for the treatment of hemorrhoids. Some physicians are known to prescribe topical steroids for the treatment of hemorrhoids. In addition, there are various strengths of topical combination prescription products containing hydrocortisone along with lidocaine or pramoxine, each a topical anesthetic, that are prescribed by physicians for the treatment of hemorrhoids. These products contain drugs that were in use prior to the start of the Drug Efficacy Study Implementation (DESI) program and are commonly referred to as DESI drugs. However, none of these single-agent or combination prescription products have been clinically evaluated for safety and efficacy and approved by the FDA for the treatment of hemorrhoids. Further, many hemorrhoid patients use over the counter (OTC) products as their first line therapy. OTC products contain any one of several active ingredients including glycerin, phenylephrine, pramoxine, white petrolatum, shark liver oil and/or witch hazel, for symptomatic relief.
Development of Hemorrhoids Drugs
Hemorrhoids are a common gastrointestinal disorder, characterized by anal itching, pain, swelling, tenderness, bleeding and difficulty defecating. In the U.S., hemorrhoids affect nearly 5% of the population, with approximately 10 million persons annually admitting to having symptoms of hemorrhoidal disease. Of these persons, approximately one third visit a physician for evaluation and treatment of their hemorrhoids. The data also indicate that for both sexes a peak of prevalence occurs from age 45 to 65 years with a subsequent decrease after age 65 years. Caucasian populations are affected significantly more frequently than African Americans, and increased prevalence rates are associated with higher socioeconomic status in men but not women. Development of hemorrhoids before age 20 is unusual. In addition, between 50% and 90% of the general U.S., Canadian and European population will experience hemorrhoidal disease at least once in life. Although hemorrhoids and other anorectal diseases are not life-threatening, individual patients can suffer from agonizing symptoms which can limit social activities and have a negative impact on the quality of life.
Hemorrhoids are defined as internal or external according to their position relative to the dentate line. Classification is important for selecting the optimal treatment for an individual patient. Accordingly, physicians use the following grading system:
Grade I | Hemorrhoids not prolapsed but bleeding. |
Grade II | Hemorrhoids prolapse and reduce spontaneously with or without bleeding. |
Grade III | Prolapsed hemorrhoids that require reduction manually. |
Grade IV | Prolapsed and cannot be reduced including both internal and external hemorrhoids that are confluent from skin tag to inner anal canal. |
Development Activities to Date
In the fall of 2015, we completed dosing patients in a double-blind dose ranging placebo controlled Phase 2 study where six different formulations containing hydrocortisone and lidocaine in various strengths were tested against the vehicle control. The objectives of this study were to: 1) demonstrate the safety and efficacy of the formulations when applied twice daily for two weeks in subjects with Grade I or II hemorrhoids and 2) assess the potential contribution of lidocaine hydrochloride and hydrocortisone acetate, alone or in combination for the treatment of symptoms of Goligher’s Classification Grade I or II hemorrhoids.
| 4 |
| Table of Contents |
Symptom improvement was observed based on a global score of disease severity (“GSDS”), and based on some of the individual signs and symptoms of hemorrhoids, specifically itching and overall pain and discomfort. Within the first few days of treatment, the combination products (containing both hydrocortisone and lidocaine) were directionally favorable versus the placebo and their respective individual active treatment groups (e.g., hydrocortisone or lidocaine alone) in achieving ‘almost symptom free’ or ‘symptom free’ status according to the GSDS scale. These differences suggest the possibility of a benefit for the combination product formulation.
Overall, results from adverse event reporting support the safety profile of all test articles evaluated in this study and demonstrate similar safety profiles as compared to the vehicle. The safety findings were unremarkable. There was a low occurrence of adverse events and a similar rate of treatment related adverse events across all treatment groups. The majority of adverse events were mild and only one was severe. None of the adverse events were serious and the majority of adverse events were recovered/resolved at the end of the study. There were only two subjects who were discontinued from the study due to adverse events.
In addition to the safety and dose-ranging information, information was obtained relating to the use of the GSDS as an assessment tool for measuring the effectiveness of the test articles. Individual signs and symptoms were also assessed but can vary from patient to patient. Therefore, the goal of the GSDS was to provide an assessment tool that could be used for all patients regardless of which signs and symptoms they are experiencing. The GSDS proved to be a more effective tool for assessing the severity of the disease and the effectiveness of the drug when compared to the assessment of the individual signs and symptoms. Citius believes that we can continue to develop this assessment tool as well as other patient reported outcome endpoints for use in the next trials.
Information was also obtained about the formulation of the drug and the vehicle. As a result of this study, we believe that the performance of the active arms of the study relative to the vehicle can be improved by re-formulating our topical preparation. Therefore, we have initiated work on vehicle formulation and evaluation of higher potency steroids.
We recently conducted primary market research to better understand the symptoms that are most bothersome to patients. We also learned about the factors that drive patients to seek medical attention for hemorrhoids in an effort to understand the disease impact on quality of life. The results of this survey are able to help us develop patient reported outcome evaluation tools. These tools can be used in clinical trials to evaluate the patients’ conditions and to assess the performance of the test articles.
A Phase 2b study will begin once the new formulation is completed and the updated evaluation tools are developed. This study will be a 300 patient four arm study. The cost is estimated at approximately $3.0 – 5.0 million and is expected to require approximately one year to complete.
Market Opportunity
The current market for OTC and topical DESI formulations of hydrocortisone and lidocaine is highly fragmented, and includes approximately 20 million units of OTC hemorrhoid products and over 4 million prescriptions for non-approved prescription treatments. Several topical combination prescription products for the treatment of hemorrhoids are available containing hydrocortisone in strengths ranging from 0.5% to 3.0%, combined with lidocaine in strengths ranging from 1.0% to 3.0%. The various topical formulations include creams, ointments, gels, lotions, enemas, pads, and suppositories. The most commonly prescribed topical combination gel, is sold as a branded generic product and contains 2.5% hydrocortisone and 3.0% lidocaine.
We believe there are currently no FDA-approved prescription drug products for the treatment of hemorrhoids. Although there are numerous prescription and OTC products commonly used to treat hemorrhoids, none possess proven safety and efficacy data generated from rigorously conducted clinical trials. We believe that a novel topical formulation of hydrocortisone and lidocaine designed to provide anti-inflammatory and anesthetic relief and which has an FDA-approved label specifically claiming the treatment of hemorrhoids will become an important treatment option for physicians who want to provide their patients with a therapy that has demonstrated safety and efficacy in treating this uncomfortable and often recurring disease. We believe that our Hydro-Lido product represents an attractive, low-risk product opportunity with meaningful upside potential.
Market Exclusivity
We believe that we will be the first company to conduct rigorous clinical trials and receive FDA approval of a topical hydrocortisone-lidocaine combination product for the treatment of hemorrhoids. If we receive FDA approval, we will qualify for 3 years of market exclusivity for our dosage strength and formulation. In addition, we will also be the only product on the market specifically proven to be safe and effective for the treatment of hemorrhoids. Generally, if a company conducts clinical trials and receives FDA approval of a product for which there are similar, but non FDA-approved, prescription products on the market, the manufacturers of the unapproved but marketed products are required to withdraw them from the market. However, the FDA has significant latitude in determining how to enforce its regulatory powers in these circumstances. We have not had any communication with the FDA regarding this matter and cannot predict what action, if any, the FDA will take with respect to the unapproved products.
| 5 |
| Table of Contents |
We believe that should our product receive an FDA approval and demonstrate, proven safety and efficacy data, and if our products obtain 3 years of market exclusivity based on our dosage strength and formulation, Citius is likely to have a meaningful advantage in its pursuit of achieving a significant position in the market for topical combination prescription products for the treatment of hemorrhoids.
Recent Developments
The Company issued demand promissory notes in favor of Leonard Mazur, Chairman of the Board, from 2016 through March 30, 2017 in the aggregate principal amount of $1,850,000 (collectively, the “Notes”). The Notes mature on the earlier of December 31, 2017 or demand by the lender and accrue interest at the prime rate plus 1%.
In February 2017, the Company completed an offering (the “2016 Offering”) and sold 1,920,250 units (the “2016 Offering Units”), each 2016 Offering Unit consists of (i) one share of common stock and (ii) a warrant to purchase one share of common stock (the “2016 Offering Warrants”) for gross proceeds of $768,100. Each 2016 Offering Unit was sold at a negotiated price of $0.40. Each 2016 Offering Warrant has an exercise price of $0.55 and is exercisable for a period of five years from the date of issuance. The Placement Agent received a 10% cash commission on the gross proceeds of each sale of the 2016 Offering Units. In addition, the Placement Agent also received (i) an expense allowance equal to 3% of the proceeds of the sale, and (ii) warrants to purchase a number of shares of common stock equal to 10% of the 2016 Offering Units sold at an exercise price of $0.55 per share.
On January 17, 2017, the Company issued 445,932 shares of common stock to IRTH Communications for services related to strategic consulting and investor relations.
On January 17, 2017, we issued an option to purchase 20,000 shares of common stock to each of Dr. Mark Rupp and Dr. Robert Sheretz, both members of our scientific advisory boards, that vests over twenty-four (24) months. Also on this same date, the Company issued an option to purchase 40,000 shares of common stock to the chairman of the scientific advisory board, Dr. Issam Raad, that vests over thirty-six (36) months. The exercise price for each of these options is $0.67 per share.
On January 17, 2017, we issued an option to purchase 50,000 shares of common stock to consultant Dr. Howard Maibach that vests over twelve (12) months and has an exercise price of $0.67 per share.
Risk Factors
Our business is subject to a number of risks you should be aware of before making an investment decision. These risks and others related to the purchase of the Securities are discussed more fully in “Risk Factors” beginning on page 9 and include:
| · | we have no source of revenue, had an accumulated deficit of $19,512,145 as of December 31, 2016, may never become profitable and may incur substantial and increasing net losses for the foreseeable future as we seek development, regulatory approval and commercialization of our products and any future product candidates; | |
|
| |
| · | we may need to obtain additional capital to complete the development of existing products, acquire new products, and to continue operations; | |
|
| |
| · | our success is primarily dependent on the successful completion of the upcoming Mino-Lok Phase 3 trial, our second registration trial and regulatory approval by the FDA, and the upcoming Hydro-Lido Phase 2b trial; | |
|
| |
| · | we are subject to regulatory approval processes that are lengthy, time consuming and inherently unpredictable; we may not obtain approval for our current products or any of our future product candidates from the FDA or any foreign regulatory authorities; | |
|
| |
| · | it is difficult and costly to protect our intellectual property rights; | |
|
| |
| · | we may be unable to recruit or retain key employees, including our senior management team; and | |
|
| |
| · | we depend on the performance of third parties, including contract research organizations and manufacturers, for the clinical testing and production of Mino-Lok, our Hydro-Lido formulation and any other product candidates. |
| 6 |
| Table of Contents |
Corporate Information
The Company was founded as Citius Pharmaceuticals, LLC, a Massachusetts limited liability company, on January 23, 2007. On September 12, 2014, Citius Pharmaceuticals, LLC entered into a Share Exchange and Reorganization Agreement, with Citius Pharmaceuticals, Inc. (formerly Trail One, Inc.), a publicly traded company incorporated under the laws of the State of Nevada. Citius Pharmaceuticals, LLC became a wholly-owned subsidiary of Citius. On March 30, 2016, Citius acquired Leonard-Meron Biosciences, Inc. (“LMB”) as a wholly-owned subsidiary. LMB was a pharmaceutical company focused on the development and commercialization of critical care products with a concentration on anti-infectives.
The Company’s principal executive offices are located at 11 Commerce Drive, Cranford, New Jersey 07016 and its telephone number is (908) 976-6677.
SUMMARY CONSOLIDATED FINANCIAL DATA
The following selected financial information is derived from the Company's Financial Statements appearing elsewhere in this Prospectus and should be read in conjunction with the Company's Financial Statements, including the notes thereto, appearing elsewhere in this Prospectus. The results indicated below are not necessarily indicative of our future performance.
You should read this information together with the sections entitled "Capitalization", "Management's Discussion and Analysis of Financial Condition and Results of Operations" and our consolidated financial statements and related notes included elsewhere in this prospectus.
Summary of Statements of Operations
|
| Three Months Ended |
|
| Three Months Ended |
|
| Year Ended September 30, 2016 |
|
| Year Ended September 30, 2015 |
| ||||
|
| (unaudited) |
|
| (unaudited) |
|
|
|
|
|
|
| ||||
Revenues |
| $ | - |
|
| $ | - |
|
| $ | - |
|
| $ | - |
|
Loss from operations |
| $ | (2,784,856 | ) |
| $ | (1,244,676 | ) |
| $ | (7,449,291 | ) |
| $ | (3,229,929 | ) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Other income (expense), net |
| $ | 608,958 |
|
| $ | 23,955 |
|
| $ | (846,407 | ) |
| $ | 327,661 |
|
Net loss |
| $ | (2,175,898 | ) |
| $ | (1,220,721 | ) |
| $ | (8,295,698 | ) |
| $ | (2,902,268 | ) |
Net loss per common share-basic and diluted |
| $ | (0.03 | ) |
| $ | (0.04 | ) |
| $ | (0.15 | ) |
| $ | (0.09 | ) |
Weighted average number of common shares outstanding – basic and diluted |
|
| 73,551,357 |
|
|
| 34,414,988 |
|
|
| 54,348,120 |
|
|
| 31,835,440 |
|
Summary Balance Sheet Information
|
| December 31, |
|
| September 30, |
|
| September 30, |
| |||
|
| 2016 |
|
| 2016 |
|
| 2015 |
| |||
|
| (unaudited) |
|
|
|
|
|
|
| |||
Cash and cash equivalents |
| $ | 46,764 |
|
| $ | 294,351 |
|
| $ | 676,137 |
|
Total Assets |
| $ | 21,482,323 |
|
| $ | 21,950,341 |
|
| $ | 741,538 |
|
Current Liabilities |
| $ | 6,318,711 |
|
| $ | 5,183,958 |
|
| $ | 1,376,751 |
|
Long-Term Debt |
| $ | - |
|
| $ | - |
|
| $ | - |
|
Stockholders' Equity (Deficit) |
| $ | 15,163,612 |
|
| $ | 16,766,383 |
|
| $ | (635,213 | ) |
| 7 |
| Table of Contents |
This prospectus relates to the resale by the selling stockholders identified in this prospectus of up to 11,444,204 shares of our common stock, as follows:
Common stock offered by the selling stockholders | 11,444,204 shares | ||
Common stock outstanding before the offering (1) (2) | 75,504,242 shares | ||
Common stock to be outstanding after the offering (2) | 75,504,242 shares | ||
Common stock OTCQB Symbol | CTXR.QB | ||
______________ |
|
| |
(1) Based on the number of shares outstanding as of February 28, 2017. |
|
| |
(2) Does not include up to 9,523,954 shares of common stock issuable on exercise of the warrants. |
|
| |
Use of Proceeds
The 11,444,204 shares that are being offered for resale by the selling stockholders represent 1,920,250 currently outstanding shares and 9,523,954 shares underlying currently outstanding warrants, and will be sold for the accounts of the selling stockholders named in this prospectus. As a result, all proceeds from the sales of the 11,444,204 shares of common stock and offered for resale hereby will go to the selling stockholders and we will not receive any proceeds from the resale of those shares of common stock by the selling stockholders.
We may receive up to a total of $5,618,360 in gross proceeds if all of the warrants are exercised hereunder for cash. However, as we are unable to predict the timing or amount of potential exercises of the warrants, we have not allocated any proceeds of such exercises to any particular purpose. Accordingly, all such proceeds are allocated to working capital. It is possible that the warrants may expire and may never be exercised.
After the exercise of any of the warrants, we would not receive any proceeds from the resale of those shares by the selling stockholders because those shares will be sold for the accounts of the selling stockholders named in this prospectus.
We will incur all costs associated with this registration statement and prospectus.
Dividend Policy
We have never paid dividends on our capital stock and do not anticipate paying any dividends for the foreseeable future. See “Dividend Policy.”
| 8 |
| Table of Contents |
Investing in our securities includes a high degree of risk. Prior to making a decision about investing in our securities, you should consider carefully the specific factors discussed below, together with all of the other information contained in this prospectus. If any of the following risks actually occurs, our business, financial condition, results of operations and future prospects would likely be materially and adversely affected. This could cause the market price of our common stock to decline and could cause you to lose all or part of your investment.
Risks related to our Business and our Industry
Citius has a history of net losses and expects to incur losses for the foreseeable future. We may never generate revenues or, if we are able to generate revenues, achieve profitability.
Citius was formed as a limited liability company in 2007 and since its inception has incurred net loss in each of its previous operating years. Our ability to become profitable depends upon our ability to generate revenues from sales of our product candidates. Citius has been focused on product development and has not generated any revenues to date. Citius has incurred losses in each period of our operations, and we expect to continue to incur losses for the foreseeable future. These losses are likely to continue to adversely affect our working capital, total assets and shareholders’ equity (deficit). The process of developing our products requires significant clinical, development and laboratory testing and clinical trials. In addition, commercialization of our product candidates will require that we obtain necessary regulatory approvals and establish sales, marketing and manufacturing capabilities, either through internal hiring or through contractual relationships with others. We expect to incur substantial losses for the foreseeable future as a result of anticipated increases in our research and development costs, including costs associated with conducting preclinical testing and clinical trials, and regulatory compliance activities. Citius incurred net losses of $2,175,898 for the three months ended December 31, 2016, $8,295,698 and $2,902,268 for the years ended September 30, 2016 and 2015, respectively, and a net loss of $737,727 for the nine months ended September 30, 2014. At December 31, 2016, Citius had stockholders’ equity of $15,163,612 and an accumulated deficit of $19,512,145. Citius’ net cash used for operating activities was $1,314,792 for the three months ended December 31, 2016, $5,900,421 and $2,385,416 for the years ended September 30, 2016 and 2015, respectively, and $183,164 for the nine months ended September 30, 2014.
Our ability to generate revenues and achieve profitability will depend on numerous factors, including success in:
| · | developing and testing product candidates; | |
|
| |
| · | receiving regulatory approvals; | |
|
| |
| · | commercializing our products; | |
|
| |
| · | manufacturing commercial quantities of our product candidates at acceptable cost levels; and | |
|
| |
| · | establishing a favorable competitive position. |
Many of these factors will depend on circumstances beyond our control. We cannot assure you that any of our products will be approved by the FDA, that we will successfully bring any product to market or, if so, that we will ever become profitable.
Our auditors have issued a “going concern” audit opinion.
Our independent registered accountants have indicated, in their report on our September 30, 2016 financial statements, that there is substantial doubt about our ability to continue as a going concern. A “going concern” opinion indicates that the financial statements have been prepared assuming we will continue as a going concern and do not include any adjustments to reflect the possible future effects on the recoverability and classification of assets, or the amounts and classification of liabilities that may result if we do not continue as a going concern. Currently, we do not have sufficient capital to continue our operations for the next twelve months. You should not rely on our consolidated balance sheet as an indication of the amount of proceeds that would be available to satisfy claims of creditors, and potentially be available for distribution to shareholders, in the event of liquidation.
| 9 |
| Table of Contents |
We need to secure additional financing.
We anticipate that we will incur operating losses for the foreseeable future. We have received gross proceeds of approximately $7.8 million from our private placement offerings through February 2017. Additionally, in connection with the acquisition of LMB our Executive Chairman Leonard Mazur made an equity investment of $3.0 million in March 2016. Additionally, Leonard Mazur has loaned the Company $1,850,000 in the form of demand promissory notes. We may need to seek additional financing, including from affiliates, to continue our clinical programs and manufacturing for clinical programs.
The amount and timing of our future funding requirements will depend on many factors, including, but not limited to:
| · | the rate of progress and cost of our trials and other product development programs for our product candidates; | |
|
| |
| · | the costs and timing of obtaining licenses for additional product candidates or acquiring other complementary technologies; | |
|
| |
| · | the timing of any regulatory approvals of our product candidates; | |
|
| |
| · | the costs of establishing sales, marketing and distribution capabilities; and | |
|
| |
| · | the status, terms and timing of any collaborative, licensing, co-promotion or other arrangements. |
We will need to access the capital markets in the future for additional capital for research and development and for operations. Traditionally, pharmaceutical companies have funded their research and development expenditures through raising capital in the equity markets. Declines and uncertainties in these markets over the past several years have severely restricted raising new capital and have affected companies’ ability to continue to expand or fund existing research and development efforts. If these economic conditions continue or become worse, our future cost of equity or debt capital and access to the capital markets could be adversely affected. If we are not successful in securing additional financing, we may be required to delay significantly, reduce the scope of or eliminate one or more of our research or development programs, downsize our general and administrative infrastructure, or seek alternative measures to avoid insolvency, including arrangements with collaborative partners or others that may require us to relinquish rights to certain of our technologies or product candidates.
We are a late-stage development company with an unproven business strategy and may never achieve commercialization of our therapeutic products or profitability.
Our strategy of using collaborative partners to assist us in the development of our therapeutic products is unproven. Our success will depend upon our ability to enter into additional collaboration agreements on favorable terms and to select an appropriate commercialization strategy for each potential therapeutic product we and our collaborators choose to pursue. If we are not successful in implementing our strategy to commercialize our potential therapeutic products, we may never achieve, maintain or increase profitability. Our ability to successfully commercialize any of our products or product candidates will depend, among other things, on our ability to:
| · | successfully complete our clinical trials; | |
|
| |
| · | produce, through a validated process, sufficiently large quantities of our drug compound(s) to permit successful commercialization; | |
|
| |
| · | receive marketing approvals from the FDA and similar foreign regulatory authorities; | |
|
| |
| · | establish commercial manufacturing arrangements with third-party manufacturers; | |
|
| |
| · | build and maintain strong sales, distribution and marketing capabilities sufficient to launch commercial sales of the drug(s) or establish collaborations with third parties for such commercialization; | |
|
| |
| · | secure acceptance of the drug(s) from physicians, health care payers, patients and the medical community; and | |
|
| |
| · | manage our spending as costs and expenses increase due to clinical trials, regulatory approvals and commercialization. |
| 10 |
| Table of Contents |
There are no guarantees that we will be successful in completing these tasks. If we are unable to successfully complete these tasks, we may not be able to commercialize any of our product candidates in a timely manner, or at all, in which case we may be unable to generate sufficient revenues to sustain and grow our business. If we experience unanticipated delays or problems, our development costs could substantially increase and our business, financial condition and results of operations will be adversely affected.
We may fail to realize any of the anticipated benefits of the recent merger.
The success of our recent merger with Leonard-Meron Biosciences, Inc. (“LMB”) will depend on, among other things, our ability to realize anticipated benefits and to combine the businesses of the Company and LMB in a manner that achieves synergy and a shared strategy but that does not materially disrupt the existing activities of the companies. If we are not able to successfully achieve these objectives, the anticipated benefits of the merger may not be realized fully, if at all, or may take longer to realize than expected.
We face significant risks in our product candidate development efforts.
Our business depends on the successful development and commercialization of our product candidates. We are not permitted to market any of our product candidates in the United States until we receive approval of an NDA from the FDA, or in any foreign jurisdiction until we receive the requisite approvals from such jurisdiction. The process of developing new drugs and/or therapeutic products is inherently complex, unpredictable, time-consuming, expensive and uncertain. We must make long-term investments and commit significant resources before knowing whether our development programs will result in drugs that will receive regulatory approval and achieve market acceptance. Product candidates that appear to be promising at all stages of development may not reach the market for a number of reasons that may not be predictable based on results and data of the clinical program. Product candidates may be found ineffective or may cause harmful side effects during clinical trials, may take longer to progress through clinical trials than had been anticipated, may not be able to achieve the pre-defined clinical endpoints due to statistical anomalies even though clinical benefit may have been achieved, may fail to receive necessary regulatory approvals, may prove impracticable to manufacture in commercial quantities at reasonable cost and with acceptable quality, or may fail to achieve market acceptance.
We cannot predict whether or when we will obtain regulatory approval to commercialize our product candidates that are under development and will be further developed using the proceeds of our private placements and we cannot, therefore, predict the timing of any future revenues from these product candidates, if any. The FDA has substantial discretion in the drug approval process, including the ability to delay, limit or deny approval of a product candidate for many reasons. For example, the FDA:
| · | could determine that we cannot rely on Section 505(b)(2) for any of our product candidates; | |
|
| |
| · | could determine that the information provided by us was inadequate, contained clinical deficiencies or otherwise failed to demonstrate the safety and effectiveness of any of our product candidates for any indication; | |
|
| |
| · | may not find the data from clinical trials sufficient to support the submission of an NDA or to obtain marketing approval in the United States, including any findings that the clinical and other benefits of our product candidates outweigh their safety risks; | |
|
| |
| · | may disagree with our trial design or our interpretation of data from preclinical studies or clinical trials, or may change the requirements for approval even after it has reviewed and commented on the design for our trials; | |
|
| |
| · | may determine that we have identified the wrong reference listed drug or drugs or that approval of our Section 505(b)(2) application for any of our product candidates is blocked by patent or non-patent exclusivity of the reference listed drug or drugs; | |
|
| |
| · | may identify deficiencies in the manufacturing processes or facilities of third-party manufacturers with which we enter into agreements for the manufacturing of our product candidates; | |
|
| |
| · | may approve our product candidates for fewer or more limited indications than we request, or may grant approval contingent on the performance of costly post-approval clinical trials; | |
|
| |
| · | may change its approval policies or adopt new regulations; or | |
|
| |
| · | may not approve the labeling claims that we believe are necessary or desirable for the successful commercialization of our product candidates. |
Any failure to obtain regulatory approval of our product candidates would significantly limit our ability to generate revenues, and any failure to obtain such approval for all of the indications and labeling claims we deem desirable could reduce our potential revenues.
| 11 |
| Table of Contents |
The results of pre-clinical studies and completed clinical trials are not necessarily predictive of future results, and our current product candidates may not have favorable results in later studies or trials.
Pre-clinical studies and Phase 1 and Phase 2 clinical trials are not primarily designed to test the efficacy of a product candidate in the general population, but rather to test initial safety, to study pharmacokinetics and pharmacodynamics, to study limited efficacy in a small number of study patients in a selected disease population, and to identify and attempt to understand the product candidate’s side effects at various doses and dosing schedules. Success in pre-clinical studies or completed clinical trials does not ensure that later studies or trials, including continuing pre-clinical studies and large-scale clinical trials, will be successful nor does it necessarily predict future results. Favorable results in early studies or trials may not be repeated in later studies or trials, and product candidates in later stage trials may fail to show acceptable safety and efficacy despite having progressed through earlier trials. In addition, the placebo rate in larger studies may be higher than expected.
We may be required to demonstrate through large, long-term outcome trials that our product candidates are safe and effective for use in a broad population prior to obtaining regulatory approval.
There is typically a high rate of attrition from the failure of product candidates proceeding through clinical trials. In addition, certain subjects in our clinical trials may respond positively to placebo treatment - these subjects are commonly known as “placebo responders” - making it more difficult to demonstrate efficacy of the test drug compared to placebo. This effect is likely to be observed in the treatment of hemorrhoids. If any of our product candidates fail to demonstrate sufficient safety and efficacy in any clinical trial, we will experience potentially significant delays in, or may decide to abandon development of that product candidate. If we abandon or are delayed in our development efforts related to any of our product candidates, we may not be able to generate any revenues, continue our operations and clinical studies, or become profitable. Our reputation in the industry and in the investment community would likely be significantly damaged. It may not be possible for us to raise funds in the public or private markets, and our stock price would likely decrease significantly.
If we are unable to file for approval under Section 505(b)(2) of the Federal Food, Drug and Cosmetic Act or if we are required to generate additional data related to safety and efficacy in order to obtain approval under Section 505(b)(2), we may be unable to meet our anticipated development and commercialization timelines.
Our current plans for filing additional NDAs for our product candidates include efforts to minimize the data we will be required to generate in order to obtain marketing approval for our additional product candidates and therefore possibly obtain a shortened review period for the applications. The timeline for filing and review of our NDAs is based upon our plan to submit those NDAs under Section 505(b)(2) of the Federal Food, Drug and Cosmetic Act, wherein we will rely in part on data in the public domain or elsewhere. Depending on the data that may be required by the FDA for approval, some of the data may be related to products already approved by the FDA. If the data relied upon is related to products already approved by the FDA and covered by third-party patents we would be required to certify that we do not infringe the listed patents or that such patents are invalid or unenforceable. As a result of the certification, the third party would have 45 days from notification of our certification to initiate an action against us. In the event that an action is brought in response to such a certification, the approval of our NDA could be subject to a stay of up to 30 months or more while we defend against such a suit. Approval of our product candidates under Section 505(b)(2) may therefore be delayed until patent exclusivity expires or until we successfully challenge the applicability of those patents to our product candidates. Alternatively, we may elect to generate sufficient additional clinical data so that we no longer rely on data which triggers a potential stay of the approval of our product candidates. Even if no exclusivity periods apply to our applications under Section 505(b)(2), the FDA has broad discretion to require us to generate additional data on the safety and efficacy of our product candidates to supplement third-party data on which we may be permitted to rely. In either event, we could be required, before obtaining marketing approval for any of our product candidates, to conduct substantial new research and development activities beyond those we currently plan to engage in order to obtain approval of our product candidates. Such additional new research and development activities would be costly and time consuming.
| 12 |
| Table of Contents |
We may not be able to obtain shortened review of our applications, and the FDA may not agree that our products qualify for marketing approval. If we are required to generate additional data to support approval, we may be unable to meet our anticipated development and commercialization timelines, may be unable to generate the additional data at a reasonable cost, or at all, and may be unable to obtain marketing approval of our product candidates. In addition, notwithstanding the approval of many products by the FDA pursuant to Section 505(b)(2), over the last few years, some pharmaceutical companies and others have objected to the FDA’s interpretation of Section 505(b)(2). If the FDA changes its interpretation of Section 505(b)(2), or if the FDA’s interpretation is successfully challenged in court, this could delay or even prevent the FDA from approving any Section 505(b)(2) application that we submit.
Even if we receive regulatory approval to commercialize our product candidates, post-approval marketing and promotion of products is highly regulated by the FDA, and marketing campaigns which violate FDA standards may result in adverse consequences including regulatory enforcement action by the FDA as well as follow-on actions filed by consumers and other end-payers, which could result in substantial fines, sanctions and damage awards against us, any of which could harm our business.
Post-approval marketing and promotion of drugs, standards and regulations for direct-to-consumer advertising, dissemination of off-label product information, industry-sponsored scientific and educational activities and promotional activities via the Internet are heavily scrutinized and regulated by the FDA. Drugs may only be marketed for approved indications and in accordance with provisions of the FDA approved labels. Failure to comply with such requirements may result in adverse publicity, warning letters issued by the FDA, and civil or criminal penalties.
In the event the FDA discovers new violations, we could face penalties in the future including the FDA’s issuance of a cease and desist order, impounding of our products, and civil or criminal penalties. As a follow-on to such governmental enforcement activities, consumers and other end-payers of the product may initiate action against us claiming, among other things, fraudulent misrepresentation, civil RICO, unfair competition, violation of various state consumer protection statues and unjust enrichment. If the plaintiffs in such follow-on actions are successful, we could be subject to various damages, including compensatory damages, treble damages, punitive damages, restitution, disgorgement, prejudgment and post-judgment interest on any monetary award, and the reimbursement of the plaintiff’s legal fees and costs, any of which could have an adverse effect on our revenue, business and financial prospects.
Even if we receive regulatory approval to commercialize our product candidates, our ability to generate revenues from any resulting drugs will be subject to a variety of risks, many of which are out of our control.
Even if our product candidates obtain regulatory approval, those drugs may not gain market acceptance among physicians, patients, healthcare payers or the medical community. The indication may be limited to a subset of the population or we may implement a distribution system and patient access program that is limited. Coverage and reimbursement of our product candidates by third-party payers, including government payers, generally is also necessary for optimal commercial success. We believe that the degree of market acceptance and our ability to generate revenues from such drugs will depend on a number of factors, including:
| · | timing of market introduction of competitive drugs; | |
|
| |
| · | prevalence and severity of any side effects; | |
|
| |
| · | results of any post-approval studies of the drug; | |
|
| |
| · | potential or perceived advantages or disadvantages over alternative treatments including generics; | |
|
| |
| · | the relative convenience and ease of administration and dosing schedule; | |
|
| |
| · | strength of sales, marketing and distribution support; | |
|
| |
| · | price of any future drugs, if approved, both in absolute terms and relative to alternative treatments; |
| 13 |
| Table of Contents |
| · | the effectiveness of our or any future collaborators’ sales and marketing strategies; | |
|
| |
| · | the effect of current and future healthcare laws on our product candidates; | |
|
| |
| · | availability of coverage and reimbursement from government and other third-party payers; | |
|
| |
| · | patient access programs that require patients to provide certain information prior to receiving new and refill prescriptions; | |
|
| |
| · | requirements for prescribing physicians to complete certain educational programs for prescribing drugs; | |
|
| |
| · | the willingness of patients to pay out of pocket in the absence of government or third-party coverage; and | |
|
| |
| · | product labeling or product insert requirements of the FDA or other regulatory authorities. |
If approved, our product candidates may fail to achieve market acceptance or generate significant revenue to achieve or sustain profitability. In addition, our efforts to educate the medical community and third-party payers on the benefits of our product candidates may require significant resources and may never be successful.
Even if approved for marketing by applicable regulatory bodies, we will not be able to create a market for any of our products if we fail to establish marketing, sales and distribution capabilities, or fail to enter into arrangements with third parties.
Our strategy with our product candidates is to outsource to third parties, all or most aspects of the product development process, as well as marketing, sales and distribution activities. Currently, we do not have any sales, marketing or distribution capabilities. In order to generate sales of any product candidates that receive regulatory approval, we must either acquire or develop an internal marketing and sales force with technical expertise and with supporting distribution capabilities or make arrangements with third parties to perform these services for us. The acquisition or development of a sales and distribution infrastructure would require substantial resources, which may divert the attention of our management and key personnel and defer our product development efforts. To the extent that we enter into marketing and sales arrangements with other companies, our revenues will depend on the efforts of others. These efforts may not be successful. If we fail to develop sales, marketing and distribution channels, or enter into arrangements with third parties, we will experience delays in product sales and incur increased costs.
The markets in which we operate are highly competitive and we may be unable to compete successfully against new entrants or established companies.
Competition in the pharmaceutical and medical products industries is intense and is characterized by costly and extensive research efforts and rapid technological progress. We are aware of several pharmaceutical companies also actively engaged in the development of therapies for the same conditions we are targeting. Many of these companies have substantially greater research and development capabilities as well as substantially greater marketing, financial and human resources than we do. In addition, many of these companies have significantly greater experience than us in undertaking pre-clinical testing, human clinical trials and other regulatory approval procedures. Our competitors may develop technologies and products that are more effective than those we are currently marketing or researching and developing. Such developments could render our products, if approved, less competitive or possibly obsolete. We are also competing with respect to marketing capabilities and manufacturing efficiency, areas in which we have limited experience. Mergers, acquisitions, joint ventures and similar events may also significantly increase the competition. New developments, including the development of other drug technologies and methods of preventing the incidence of disease, occur in the pharmaceutical and medical technology industries at a rapid pace. These developments may render our products and product candidates obsolete or noncompetitive. Compared to us, many of our potential competitors have substantially greater:
| 14 |
| Table of Contents |
| · | research and development resources, including personnel and technology; | |
|
| |
| · | regulatory experience; | |
|
| |
| · | product candidate development and clinical trial experience; | |
|
| |
| · | experience and expertise in exploitation of intellectual property rights; and | |
|
| |
| · | access to strategic partners and capital resources. |
As a result of these factors, our competitors may obtain regulatory approval of their products more rapidly than we can or may obtain patent protection or other intellectual property rights that limit our ability to develop or commercialize our product candidates. Our competitors may also develop drugs or surgical approaches that are more effective, more useful and less costly than ours and may also be more successful in manufacturing and marketing their products. In addition, our competitors may be more effective than us in commercializing their products and as a result, our business and prospects might be materially harmed.
Physicians and patients might not accept and use any of our products for which regulatory approval is obtained.
Even if the FDA approves one of our product candidates, physicians and patients might not accept and use it. Acceptance and use of our products will depend upon a number of factors, including:
| · | perceptions by members of the health care community, including physicians, about the safety and effectiveness of our products; | |
|
| |
| · | cost-effectiveness of our product relative to competing product or therapies; | |
|
| |
| · | availability of reimbursement for our product from government or other healthcare payers; and | |
|
| |
| · | effective marketing and distribution efforts by us and our licensees and distributors, if any. |
If our current product candidates are approved, we expect their sales to generate substantially all of our revenues for the foreseeable future, and as a result, the failure of these products to find market acceptance would harm our business and would require us to seek additional financing.
Our two product candidates, Mino-Lok and Hydro-Lido, are combination products consisting of components that have each been separately approved by the FDA for other indications and which are commercially available and marketed by other companies. Our approval under 505(b)(2) does not preclude physicians, pharmacists and patients from obtaining individual drug products and titrating the dosage of these drug products as close to our approved dose as possible.
Our Hydro-Lido product candidate for the treatment of hemorrhoids is a combination product consisting of two drugs, hydrocortisone and lidocaine, that have each been separately approved by the FDA for other indications and which are commercially available and marketed by other companies. Hydrocortisone creams are available from strengths ranging from 0.5% to 2.5% and lidocaine creams are also available in strengths up to 5%. From our market analysis and discussions with a limited number of physicians, we know that patients sometimes obtain two separate cream products and co-administer them as prescribed, giving them a combination treatment which could be very similar to what we intend to study and seek approval for. As a branded, FDA-approved product with safety and efficacy data, we intend to price our product substantially higher than the generically available individual creams. We will then have to convince third-party payers and pharmacy benefit managers of the advantages of our product and justify our premium pricing. We may encounter resistance from these entities and will then be dependent on patients’ willingness to pay the premium and not seek alternatives. In addition, pharmacists often suggest lower cost prescription treatment alternatives to both physicians and patients. Our 505(b)(2) approval and the market exclusivity we may receive will not guarantee that such alternatives will not exist, that substitution will not occur, or that there will be immediate acceptance to our pricing by payer formularies.
Our Mino-Lok solution contains minocycline, disodium ethylenediaminetetraacetic acid (edetate), and ethyl alcohol, all of which have been separately approved by the FDA for other indications, or are used as excipients in other parenteral products.
| 15 |
| Table of Contents |
Our ability to generate product revenues will be diminished if our products sell for inadequate prices or patients are unable to obtain adequate levels of reimbursement.
Our ability to commercialize our products, alone or with collaborators, will depend in part on the extent to which reimbursement will be available from:
| · | government and health administration authorities; | |
|
| |
| · | private health maintenance organizations and health insurers; and | |
|
| |
| · | other healthcare payers. |
Significant uncertainty exists as to the reimbursement status of newly approved healthcare products. Healthcare payers, including Medicare, are challenging the prices charged for medical products and services. Government and other healthcare payers increasingly attempt to contain healthcare costs by limiting both coverage and the level of reimbursement for drugs. Even if our product candidates are approved by the FDA, insurance coverage might not be available, and reimbursement levels might be inadequate, to cover our products. If government and other healthcare payers do not provide adequate coverage and reimbursement levels for our products, once approved, market acceptance of such products could be reduced. Proposals to modify the current health care system in the U.S. to improve access to health care and control its costs are continually being considered by the federal and state governments. In March 2010, the U.S. Congress passed landmark healthcare legislation. We cannot predict what impact on federal reimbursement policies this legislation will have in general or on our business specifically. Members of the U.S. Congress and some state legislatures are seeking to overturn at least portions of the legislation and we expect they will continue to review and assess this legislation and possibly alternative health care reform proposals. We cannot predict whether new proposals will be made or adopted, when they may be adopted or what impact they may have on us if they are adopted.
Health administration authorities in countries other than the U.S. may not provide reimbursement for our products at rates sufficient for us to achieve profitability, or at all. Like the U.S., these countries have considered health care reform proposals and could materially alter their government-sponsored health care programs by reducing reimbursement rates. Any reduction in reimbursement rates under Medicare or foreign health care programs could negatively affect the pricing of our products. If we are not able to charge a sufficient amount for our products, then our margins and our profitability will be adversely affected.
We rely exclusively on third parties to formulate and manufacture our product candidates.
We do not have and do not intend to establish our own manufacturing facilities. Consequently, we lack the physical plant to formulate and manufacture our own product candidates, which are currently being manufactured entirely by a commercial third party. If any additional product candidate we might develop or acquire in the future receives FDA approval, we will rely on one or more third-party contractors to manufacture our products. If, for any reason, we become unable to rely on our current source or any future source to manufacture our product candidates, either for clinical trials or, for commercial quantities, then we would need to identify and contract with additional or replacement third-party manufacturers to manufacture compounds for preclinical, clinical and commercial purposes. We might not be successful in identifying additional or replacement third-party manufacturers, or in negotiating acceptable terms with any that we do identify. If we are unable to secure and maintain third-party manufacturing capacity, the development and sales of our products and our financial performance might be materially affected.
| 16 |
| Table of Contents |
In addition, before any of our collaborators can begin to commercially manufacture our product candidates, each must obtain regulatory approval of the manufacturing facility and process. Manufacturing of drugs for clinical and commercial purposes must comply with the FDA’s Current Good Manufacturing Practices, or cGMP, and applicable non-U.S. regulatory requirements. The cGMP requirements govern quality control and documentation policies and procedures. Complying with cGMP and non-U.S. regulatory requirements will require that we expend time, money, and effort in production, recordkeeping, and quality control to assure that the product meets applicable specifications and other requirements. Our contracted manufacturing facilities must also pass a pre-approval inspection prior to FDA approval. Failure to pass a pre- approval inspection might significantly delay FDA approval of our products. If any of our collaborators fails to comply with these requirements, we would be subject to possible regulatory action which could limit the jurisdictions in which we are permitted to sell our products. As a result, our business, financial condition, and results of operations might be materially harmed.
Our reliance on a limited number of third-party manufacturers exposes us to the following risks:
| · | We might be unable to identify manufacturers for commercial supply on acceptable terms or at all because the number of potential manufacturers is limited and the FDA must approve any replacement contractor. This approval would generally require compliance inspections. In addition, a new manufacturer would have to be educated in, or develop substantially equivalent processes for, production of our products after receipt of FDA approval, if any; | |
|
| |
| · | Our third-party manufacturers might be unable to formulate and manufacture our drugs in the volume and of the quality required to meet our clinical and commercial needs, if any; | |
|
| |
| · | Our contract manufacturers might not perform as agreed or might not remain in the contract manufacturing business for the time required to supply our clinical trials or to successfully produce, store and distribute our products; | |
|
| |
| · | Currently, our contract manufacturer is foreign, which increases the risk of shipping delays and adds the risk of import restrictions; | |
|
| |
| · | Drug manufacturers are subject to ongoing periodic unannounced inspection by the FDA and corresponding state agencies to ensure strict compliance with cGMP and other government regulations and corresponding foreign standards. We do not have complete control over third-party manufacturers’ compliance with these regulations and standards; | |
|
| |
| · | If any third-party manufacturer makes improvements in the manufacturing process for our products, we might not own, or might have to share, the intellectual property rights to the innovation with our licensors; | |
|
| |
| · | Operations of our third-party manufacturers or suppliers could be disrupted by conditions unrelated to our business or operations, including a bankruptcy of the manufacturer or supplier, and | |
|
| |
| · | We might compete with other companies for access to these manufacturers’ facilities and might be subject to manufacturing delays if the manufacturers give other clients higher priority than us. |
Each of these risks could delay our clinical trials or the approval, if any, of our product candidates by the FDA or the commercialization of our product candidates and could result in higher costs or deprive us of potential product revenues. As a result, our business, financial condition, and results of operations might be materially harmed.
| 17 |
| Table of Contents |
We will be dependent on third-party contract research organizations to conduct all of our future human studies.
We will be dependent on third-party research organizations to conduct all of our human studies with respect to pharmaceutical products that we may develop in the future. If we are unable to obtain any necessary testing services on acceptable terms, we may not complete our product development efforts in a timely manner. If we rely on third parties for human studies, we may lose some control over these activities and become too dependent upon these parties. These third parties may not complete testing activities on schedule or when we so request. We may not be able to secure and maintain suitable research organizations to conduct our human studies. We are responsible for confirming that each of our clinical trials is conducted in accordance with our general plan and protocol. Moreover, the FDA and foreign regulatory agencies require us to comply with regulations and standards, commonly referred to as good clinical practices, for conducting, recording and reporting the results of clinical trials to assure that data and reported results are credible and accurate and that the trial participants are adequately protected. Our reliance on third parties does not relieve us of these responsibilities and requirements. If these third parties do not successfully carry out their contractual duties or regulatory obligations or meet expected deadlines, if the third parties need to be replaced or if the quality or accuracy of the data they obtain is compromised due to the failure to adhere to our clinical protocols or regulatory requirements or for other reasons, our preclinical development activities or clinical trials may be extended, delayed, suspended or terminated, and we may not be able to obtain regulatory approval for our future product candidates.
Any termination or breach by or conflict with our strategic partners or licensees could harm our business.
If we or any of our collaborators or licensees fail to renew or terminate any of our collaboration or license agreements or if either party fails to satisfy its obligations under any of our collaboration or license agreements or complete them in a timely manner, we could lose significant sources of revenue, which could result in volatility in our future revenue. In addition, our agreements with our collaborators and licensees may have provisions that give rise to disputes regarding the rights and obligations of the parties. These and other possible disagreements could lead to termination of the agreement or delays in collaborative research, development, supply or commercialization of certain products, or could require or result in litigation or arbitration. Any such conflicts with our collaborators could reduce our ability to obtain future collaboration agreements and could have a negative impact on our relationship with existing collaborators, adversely affecting our business and revenues. Finally, any of our collaborations or license agreements may prove to be unsuccessful.
If we are unable to retain or hire additional qualified personnel, our ability to grow our business might be harmed.
We utilize the services of a clinical management team on part-time basis to assist us in managing our Phase 2 and Phase 3 trials. While we believe this will provide us with sufficient staffing for our current development efforts, we will need to hire or contract with additional qualified personnel with expertise in preclinical testing, clinical research and testing, government regulation, formulation and manufacturing and sales and marketing in connection with the continued development, regulatory approval and commercialization of our product candidates. We compete for qualified individuals with numerous pharmaceutical and biopharmaceutical companies, universities and other research institutions. Competition for these individuals is intense, and we cannot be certain that our search for such personnel will be successful. Attracting and retaining qualified personnel will be critical to our success.
In addition, we may be unable to attract and retain those qualified officers, directors and members of board committees required to provide for effective management because of the rules and regulations that govern publicly held companies, including, but not limited to, certifications by principal executive officers. The enactment of the Sarbanes-Oxley Act has resulted in the issuance of a series of related rules and regulations and the strengthening of existing rules and regulations by the SEC, as well as the adoption of new and more stringent rules by the stock exchanges. The perceived increased personal risk associated with these changes may deter qualified individuals from accepting roles as directors and executive officers. Further, some of these changes heighten the requirements for board or committee membership, particularly with respect to an individual’s independence from the corporation and level of experience in finance and accounting matters. The Company may have difficulty attracting and retaining directors with the requisite qualifications. If we are unable to attract and retain qualified officers and directors, the management of our business and our ability to obtain or retain listing of the shares of our Common Stock on any stock exchange or quotation platform other than OTC Markets or the OTCQB where the Company’s shares are currently quoted (assuming we elect to seek and are successful in obtaining such listing) could be adversely affected.
| 18 |
| Table of Contents |
We will need to increase the size of our organization, and we may experience difficulties in managing growth.
We will need to manage our anticipated growth and increased operational activity. Our personnel, systems and facilities currently in place may not be adequate to support this future growth. Our need to effectively execute our growth strategy will require that we:
| · | manage our regulatory approval trials effectively; | |
|
| |
| · | manage our internal development efforts effectively while complying with our contractual obligations to licensors, licensees, contractors, collaborators and other third parties; | |
|
| |
| · | develop internal sales and marketing capabilities or establish collaborations with third parties with such capabilities; | |
|
| |
| · | commercialize our product candidates; | |
|
| |
| · | improve our operational, financial and management controls, reporting systems and procedures; and | |
|
| |
| · | attract and motivate sufficient numbers of talented employees. |
This future growth could place a strain on our administrative and operational infrastructure and may require our management to divert a disproportionate amount of its attention away from our day-to-day activities. We may not be able to effectively manage the expansion of our operations or recruit and train additional qualified personnel, which may result in weaknesses in our infrastructure, and give rise to operational mistakes, loss of business opportunities, loss of employees and reduced productivity among remaining employees. We may not be able to make improvements to our management information and control systems in an efficient or timely manner and may discover deficiencies in existing systems and controls. If our management is unable to effectively manage our expected growth, our expenses may increase more than expected, our ability to generate or increase our revenues could be reduced and we may not be able to implement our business strategy. Our future financial performance and our ability to compete effectively will depend, in part, on our ability to effectively manage any future growth.
Risks Related to Our Regulatory and Legal Environment
We are subject to extensive and costly government regulation.
Product candidates and approved products such as ours are subject to extensive and rigorous domestic government regulation including regulation by the FDA, the Centers for Medicare and Medicaid Services, other divisions of the U.S. Department of Health and Human Services, the U.S. Department of Justice, state and local governments, and their respective foreign equivalents. The FDA regulates the research, development, preclinical and clinical testing, manufacture, safety, effectiveness, record keeping, reporting, labeling, storage, approval, advertising, promotion, sale, distribution, import, and export of pharmaceutical products. The FDA regulates small molecule chemical entities, whether administered orally, topically or by injection, as drugs, subject to an NDA, under the Federal Food, Drug, and Cosmetic Act. If product candidates and approved products such as ours are marketed abroad, they will also be subject to extensive regulation by foreign governments, whether or not they have obtained FDA approval. Such foreign regulation might be equally or more demanding than corresponding U.S. regulation. Government regulation substantially increases the cost and risk of researching, developing, manufacturing, and selling our products. The regulatory review and approval process, which includes preclinical testing and clinical trials of each product candidate, is lengthy, expensive, and uncertain. Our collaborators or we must obtain and maintain regulatory authorization to conduct clinical trials and approval for each product we intend to market, and the manufacturing facilities used for the products must be inspected and meet legal requirements. Securing regulatory approval requires submitting extensive preclinical and clinical data and other supporting information for each proposed therapeutic indication in order to establish the product’s safety and efficacy for each intended use. The development and approval process might take many years, requires substantial resources, and might never lead to the approval of a product. Even if we are able to obtain regulatory approval for a particular product, the approval might limit the indicated medical uses for the product, limit our ability to promote, sell, and distribute the product, require that we conduct costly post-marketing surveillance, and/or require that we conduct ongoing post-marketing studies. Material changes to an approved product, such as, for example, manufacturing changes or revised labeling, might require further regulatory review and approval. Once obtained, any approvals might be withdrawn, including, for example, if there is a later discovery of previously unknown problems with the product, such as a previously unknown safety issue.
| 19 |
| Table of Contents |
If we, our collaborators, or our contract manufacturers fail to comply with applicable regulatory requirements at any stage during the regulatory process, such noncompliance could result in, among other things, delays in the approval of applications or supplements to approved applications; refusal of a regulatory authority, including the FDA, to review pending market approval applications or supplements to approved applications; warning letters; fines; import and export restrictions; product recalls or seizures; injunctions; total or partial suspension of production; civil penalties; withdrawals of previously approved marketing applications or licenses; recommendations by the FDA or other regulatory authorities against governmental contracts; and/or criminal prosecutions.
We might not obtain the necessary U.S. regulatory approvals to commercialize any product candidates.
We cannot assure you that we will receive the approvals necessary to commercialize for sale any product candidates we acquire or develop in the future. We will need FDA approval to commercialize our product candidates in the U.S. In order to obtain FDA approval of any product candidate, we must submit to the FDA an NDA demonstrating that the product candidate is safe for humans and effective for its intended use. This demonstration requires significant research, pre-clinical studies, and clinical trials. Satisfaction of the FDA’s regulatory requirements typically takes many years, depends upon the type, complexity and novelty of the product candidate and requires substantial resources for research, development and testing. We cannot predict whether our research and clinical approaches will result in additional drugs that the FDA considers safe for humans and effective for their indicated uses. The FDA has substantial discretion in the product approval process and might require us to conduct additional pre-clinical and clinical testing, perform post-marketing studies or otherwise limit or impose conditions on any additional approvals we obtain. The approval process might also be delayed by changes in government regulation, future legislation or administrative action or changes in FDA policy that occur prior to or during our regulatory review. Delays in obtaining regulatory approvals might:
| · | delay commercialization of, and our ability to derive product revenues from, our product candidates; | |
|
| |
| · | impose costly procedures on us; and | |
|
| |
| · | diminish any competitive advantages that we might otherwise enjoy. |
Even if we comply with all FDA requests, the FDA might ultimately reject one or more of our NDAs. We cannot be sure that we will ever obtain regulatory clearance for our product candidates. Failure to obtain FDA approval of our product candidates will severely undermine our business by leaving us without saleable products, and therefore without any potential sources of revenues, until another product candidate could be developed or obtained. There is no guarantee that we will ever be able to develop or acquire another product candidate
Following regulatory approval of any product candidates, we will be subject to ongoing regulatory obligations and restrictions, which may result in significant expense and limit our ability to commercialize our potential drugs.
If one of our product candidates is approved by the FDA or by another regulatory authority for a territory outside of the U.S., we will be required to comply with extensive regulations for product manufacturing, labeling, packaging, adverse event reporting, storage, distribution, advertising, promotion and record keeping. Regulatory approvals may also be subject to significant limitations on the indicated uses or marketing of the product candidates or to whom and how we may distribute our products. Even if U.S. regulatory approval is obtained, the FDA may still impose significant restrictions on a drug’s indicated uses or marketing or impose ongoing requirements for potentially costly post-approval studies. For example, the label ultimately approved for our products, if any, may include restrictions on use, including restrictions based on level of obesity and duration of treatment. If so, we may be subject to ongoing regulatory obligations and restrictions, which may result in significant expense and limit our ability to commercialize our products. The FDA could also require a registry to track the patients utilizing the drug or implement a Risk Evaluation and Mitigation Strategy, or REMS, that could restrict access to the drug, reduce our revenues and/or increase our costs. Potentially costly post-marketing clinical studies may be required as a condition of approval to further substantiate safety or efficacy, or to investigate specific issues of interest to the regulatory authority.
| 20 |
| Table of Contents |
Manufacturers of drug products and their facilities are subject to continual review and periodic inspections by the FDA and other regulatory authorities for compliance with current good manufacturing practices, or cGMP, regulations, which include requirements relating to quality control and quality assurance as well as the corresponding maintenance of records and documentation. Further, regulatory agencies must approve these manufacturing facilities before they can be used to manufacture our future approved drugs, if any, and these facilities are subject to ongoing regulatory inspections. In addition, regulatory agencies subject a drug, its manufacturer and the manufacturer’s facilities to continual review and inspections. The subsequent discovery of previously unknown problems with a drug, including adverse events of unanticipated severity or frequency, or problems with the facility where the drug is manufactured, may result in restrictions on the marketing of that drug, up to and including, withdrawal of the drug from the market. If the manufacturing facilities of our suppliers fail to comply with applicable regulatory requirements, it could result in regulatory action and additional costs to us. Failure to comply with applicable FDA and other regulatory requirements may, either before or after product approval, if any, subject our company to administrative or judicially imposed sanctions, including:
| · | issuance of Form 483 notices, warning letters and adverse publicity by the FDA or other regulatory agencies; | |
|
| |
| · | imposition of fines and other civil penalties due to product liability or other issues; | |
|
| |
| · | criminal prosecutions; | |
|
| |
| · | injunctions, suspensions or revocations of regulatory approvals; | |
|
| |
| · | suspension of any ongoing clinical trials; | |
|
| |
| · | total or partial suspension of manufacturing; | |
|
| |
| · | delays in commercialization; | |
|
| |
| · | refusal by the FDA to approve pending applications or supplements to approved applications filed by us or our collaborators; | |
|
| |
| · | refusals to permit drugs to be imported into or exported from the U.S.; | |
|
| |
| · | restrictions on operations, including costly new manufacturing requirements; and | |
|
| |
| · | product recalls or seizures. |
In addition, the law or regulatory policies governing pharmaceuticals may change. New statutory requirements may be enacted or additional regulations may be enacted that could prevent or delay regulatory approval of our product candidates. Contract Manufacturing Organizations, or CMOs, and their vendors or suppliers may also face changes in regulatory requirements from governmental agencies in the U.S. and other countries. We cannot predict the likelihood, nature, extent or effects of government regulation that may arise from future legislation or administrative action, either in the U.S. or elsewhere. If we are not able to maintain regulatory compliance, we might not be permitted to market any future approved products and our business could suffer.
| 21 |
| Table of Contents |
We could be forced to pay substantial damage awards if product liability claims that may be brought against us are successful.
The use of any of our product candidates in clinical trials, and the sale of any approved products, may expose us to liability claims and financial losses resulting from the use or sale of our products. We have obtained limited product liability insurance coverage for our clinical trials of $2 million per occurrence and in the aggregate, subject to a deductible of $50,000 per occurrence. There can be no assurance that our existing insurance coverage will extend to our other products in the future. Any product liability insurance coverage may not be sufficient to satisfy all liabilities resulting from product liability claims. A successful claim may prevent us from obtaining adequate product liability insurance in the future on commercially desirable terms, if at all. Even if a claim is not successful, defending such a claim would be time consuming and expensive, may damage our reputation in the marketplace, and would likely divert management’s attention.
Risks Related to our Intellectual Property
Our business depends on protecting our intellectual property.
If we do not obtain protection for our intellectual property rights, our competitors might be able to take advantage of our research and development efforts to develop competing drugs. Our success, competitive position and future revenues, if any, depend in part on our ability and the abilities of our licensors to obtain and maintain patent protection for our products, methods, processes and other technologies, to preserve our trade secrets, to prevent third parties from infringing on our proprietary rights and to operate without infringing the proprietary rights of third parties. We anticipate filing additional patent applications both in the U.S. and in other countries, as appropriate. However, the patent process is subject to numerous risks and uncertainties, and there can be no assurance that we will be successful in protecting our products by obtaining and defending patents. These risks and uncertainties include the following:
| · | Our patent rights might be challenged, invalidated, or circumvented, or otherwise might not provide any competitive advantage; | |
|
| |
| · | Our competitors, many of which have substantially greater resources than we do and many of which might make significant investments in competing technologies, might seek, or might already have obtained, patents that will limit, interfere with, or eliminate our ability to make, use, and sell our potential products either in the U.S. or in international markets; | |
|
| |
| · | As a matter of public policy regarding worldwide health concerns, there might be significant pressure on the U.S. government and other international governmental bodies to limit the scope of patent protection both inside and outside the U.S. for disease treatments that prove successful; and | |
|
| |
| · | Countries other than the U.S. might have less restrictive patent laws than those upheld by U.S. courts, allowing foreign competitors the ability to exploit these laws to create, develop, and market competing products. |
In addition, the U.S. Patent and Trademark Office and patent offices in other jurisdictions have often required that patent applications concerning pharmaceutical and/or biotechnology-related inventions be limited or narrowed substantially to cover only the specific innovations exemplified in the patent application, thereby limiting the scope of protection against competitive challenges. Thus, even if we or our licensors are able to obtain patents, the patents might be substantially narrower than anticipated.
Because the time period from filing a patent application to the issuance, if ever, of the patent is often more than three years and because any regulatory approval and marketing for a drug often occurs several years after the related patent application is filed, the resulting market exclusivity afforded by any patent on our drug candidates and technologies will likely be substantially less than 20 years. In the United States, the European Union and some other jurisdictions, patent term extensions are available for certain delays in either patent office proceedings or marketing and regulatory approval processes. However, due to the specific requirements for obtaining these extensions, there is no assurance that our patents will be granted extensions even if we encounter significant delays in patent office proceedings or marketing and regulatory approval.
| 22 |
| Table of Contents |
In addition to patents, we also rely on trade secrets and proprietary know-how. Although we take measures to protect this information by entering into confidentiality and inventions agreements with our employees, scientific advisors, consultants, and collaborators, we cannot provide any assurances that these agreements will not be breached, that we will be able to protect ourselves from the harmful effects of disclosure if they are breached, or that our trade secrets will not otherwise become known or be independently discovered by competitors. If any of these events occurs, or we otherwise lose protection for our trade secrets or proprietary know-how, the value of this information may be greatly reduced.
Patent and other intellectual property protection is crucial to the success of our business and prospects, and there is a substantial risk that such protections will prove inadequate. Our business and prospects will be harmed if these protections prove insufficient.
We rely on trade secret protections through confidentiality agreements with our employees, customers and other parties, and the breach of these agreements could adversely affect our business and prospects.
We rely on trade secrets, which we seek to protect, in part, through confidentiality and non-disclosure agreements with our employees, collaborators, suppliers, and other parties. There can be no assurance that these agreements will not be breached, that we would have adequate remedies for any such breach or that our trade secrets will not otherwise become known to or independently developed by our competitors. We might be involved from time to time in litigation to determine the enforceability, scope and validity of our proprietary rights. Any such litigation could result in substantial cost and divert management’s attention from our operations.
If we infringe the rights of third parties we might have to forgo selling our future products, pay damages, or defend against litigation.
If our product candidates, methods, processes and other technologies infringe the proprietary rights of other parties, we could incur substantial costs and we might have to:
| · | obtain licenses, which might not be available on commercially reasonable terms, if at all; | |
|
| |
| · | abandon an infringing product candidate; | |
|
| |
| · | redesign our products or processes to avoid infringement; | |
|
| |
| · | stop using the subject matter claimed in the patents held by others; | |
|
| |
| · | pay damages, and/or | |
|
| |
| · | defend litigation or administrative proceedings which might be costly whether we win or lose, and which could result in a substantial diversion of our financial and management resources. |
Any of these events could substantially harm our earnings, financial condition and operations.
| 23 |
| Table of Contents |
Risks Related to Our Common Stock, Liquidity Risks and Reverse Acquisition
Our securities will be deemed to be “Penny Stock” and subject to specific rules governing their sale.
The SEC has adopted Rule 15g-9 which establishes the definition of a “penny stock,” for the purposes relevant to Company, as any equity security that has a market price of less than $5.00 per share, subject to certain exceptions. For any transaction involving a penny stock, unless exempt, the rules require that a broker or dealer approve a person’s account for transactions in penny stocks, and the broker or dealer receive from the investor a written agreement to the transaction, setting forth the identity and quantity of the penny stock to be purchased.
In order to approve a person’s account for transactions in penny stocks, the broker or dealer must obtain financial information and investment experience objectives of the person, and make a reasonable determination that the transactions in penny stocks are suitable for that person and the person has sufficient knowledge and experience in financial matters to be capable of evaluating the risks of transactions in penny stocks.
The broker or dealer must also deliver, prior to any transaction in a penny stock, a disclosure schedule prescribed by the SEC relating to the penny stock market, which, in highlight form sets forth the basis on which the broker or dealer made the suitability determination, and that the broker or dealer received a signed, written agreement from the investor prior to the transaction.
Generally, brokers may be less willing to execute transactions in securities subject to the “penny stock” rules. This may make it more difficult for shareholders to dispose of the Company’s Common Stock if and when such shares are eligible for sale and may cause a decline in the market value of its stock.
Disclosure also has to be made about the risks of investing in penny stocks in both public offerings and in secondary trading and about the commissions payable to both the broker-dealer and the registered representative, current quotations for the securities and the rights and remedies available to an investor in cases of fraud in penny stock transactions. Finally, monthly statements have to be sent disclosing recent price information for the penny stock held in the account and information on the limited market in penny stocks.
Compliance with the reporting requirements of federal securities laws can be expensive.
While the Company was not previously subject to the filing requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, it filed certain reports with the Securities and Exchange Commission on a voluntary basis. On October 22, 2015, the Company registered its Common Stock under the Exchange Act and the filing of the reports with the SEC became mandatory. The quotation of the Company’s Common Stock on the OTCQB is contingent upon the Company staying current on such Exchange Act filings. The costs of preparing and filing annual and quarterly reports and other information with the SEC and furnishing audited reports to stockholders will cause our expenses to be higher than they would be if we remained privately-held.
If the Company fails to maintain an effective system of internal controls, it may not be able to accurately report its financial results or detect fraud. Consequently, shareholders could lose confidence in the Company’s financial reporting and this may decrease the trading price of its stock.
The Company must maintain effective internal controls to provide reliable financial reports and to be able to detect fraud. The Company has been assessing its internal controls to identify areas that need improvement. Our management concluded that our disclosure controls and procedures were, and continue to be, ineffective and as of September 30, 2016 identified a material weakness in our internal controls. While the Company is in the process of implementing changes to internal controls, it has not yet completed implementing these changes and there is no assurance that the changes will remediate the material weakness or that the controls will prevent or defect future material weakness. Failure to implement these changes to the Company’s internal controls or any others that it identifies as necessary to maintain an effective system of internal controls could harm its operating results and cause shareholders to lose confidence in the Company’s reported financial information. Any such loss of confidence would have a negative effect on the trading price of the Company’s stock.
| 24 |
| Table of Contents |
The price of the Common Stock may become volatile, which could lead to losses by shareholders and costly securities litigation.
The trading price of the Common Stock is likely to be highly volatile and could fluctuate in response to factors such as:
| · | actual or anticipated variations in the Company’s operating results; | |
|
| |
| · | announcements of developments by the Company or its competitors; | |
|
| |
| · | the completion and/or results of the Company’s clinical trials; | |
|
| |
| · | regulatory actions regarding the Company’s products | |
|
| |
| · | announcements by the Company or its competitors of significant acquisitions, strategic partnerships, joint ventures or capital commitments; | |
|
| |
| · | adoption of new accounting standards affecting the Company’s industry; | |
|
| |
| · | additions or departures of key personnel; | |
|
| |
| · | introduction of new products by the Company or its competitors; | |
|
| |
| · | sales of the Company’s Common Stock or other securities in the open market; and | |
|
| |
| · | other events or factors, many of which are beyond the Company’s control. |
The stock market is subject to significant price and volume fluctuations. In the past, following periods of volatility in the market price of a company’s securities, securities class action litigation has often been initiated against such a company. Litigation initiated against the Company, whether or not successful, could result in substantial costs and diversion of its management’s attention and resources, which could harm the Company’s business and financial condition.
If we complete a reverse stock split of our shares of common stock, it may reduce and may limit the market trading liquidity of the shares due to the reduced number of shares outstanding, and may potentially have an anti-takeover effect.
In September 2016 our shareholders granted our Board of Directors the authority, in its sole discretion, to effect a reverse stock split of our Common Stock by a ratio of not less than 1-for-8 and not more than 1-for-20 at any time prior to September 15, 2017, with the exact ratio to be set at a whole number within this range as determined by the Board of Directors. The liquidity of our Common Stock may be adversely affected by the reverse stock split as a result of the reduced number of shares outstanding following the reverse stock split. In addition, the reverse stock split may increase the number of stockholders who own odd lots of our Common Stock, creating the potential for such stockholders to experience an increase in the cost of selling their shares and greater difficulty effecting such sales. Reducing the number of outstanding shares of our Common Stock through the reverse stock split is intended, absent other factors, to increase the per share market price of our Common Stock. However, other factors, such as our financial results, market conditions and the market perception of our business may adversely affect the market price of our Common Stock. As a result, there can be no assurance that the reverse stock split will result in the intended benefits, that the market price of our Common Stock will increase following the reverse stock split or that the market price of our Common Stock will not decrease in the future. Further, since the reverse stock split will be accompanied by a corresponding increase in the number of shares authorized for issuance under our Amended and Restated Articles of Incorporation, the relative increase in the number of shares authorized for issuance could, under certain circumstances, have an anti-takeover effect by enabling the Board of Directors to issue additional shares of Common Stock in a transaction making it more difficult for a party to obtain control of us by tender offer or other means.
| 25 |
| Table of Contents |
You may experience dilution of your ownership interests because of the future issuance of additional shares of the Common Stock.
In the future, the Company may issue additional authorized but previously unissued equity securities, resulting in the dilution of the ownership interests of its present stockholders. The Company is currently authorized to issue an aggregate of 200,000,000 shares of Common Stock and 10,000,000 shares of preferred stock. As of February 28, 2017, there are 75,504,242 shares of Common Stock outstanding, 20,171,370 shares underlying warrants with a weighted average exercise price of $0.58 per share, and 8,732,770 shares underlying options with a weighted average exercise price of $0.54 per share. The Company may also issue additional shares of its Common Stock or other securities that are convertible into or exercisable for Common Stock in connection with hiring or retaining employees, future acquisitions, future sales of its securities for capital raising purposes, or for other business purposes. The future issuance of any such additional shares of Common Stock may create downward pressure on the trading price of the Common Stock. There can be no assurance that the Company will not be required to issue additional shares, warrants or other convertible securities in the future in conjunction with any capital raising efforts, including at a price (or exercise prices) below the price at which shares of the Common Stock are currently quoted on the OTCQB, which is one of OTC Markets’ three marketplaces for trading over-the-counter stocks.
The Common Stock is controlled by insiders.
As of February 28, 2017, the former managing members of Citius Pharmaceuticals, LLC beneficially own approximately 20.5% of our outstanding shares of Common Stock and the Company’s current officers and directors beneficially own approximately 42.3% of our outstanding shares of Common Stock. Such concentrated control of the Company may adversely affect the price of the Common Stock. If you acquire Common Stock, you may have no effective voice in the management of the Company. Sales by insiders or affiliates of the Company, along with any other market transactions, could affect the market price of the Common Stock.
We do not intend to pay dividends for the foreseeable future.
We have paid no dividends on our Common Stock to date and it is not anticipated that any dividends will be paid to holders of our Common Stock in the foreseeable future. While our future dividend policy will be based on the operating results and capital needs of the business, it is currently anticipated that any earnings will be retained to finance our future expansion and for the implementation of our business plan. The lack of a dividend can further affect the market value of our stock, and could significantly affect the value of any investment in our Company.
Our Certificate of Incorporation allows for the board of directors to create new series of preferred stock without further approval by stockholders, which could adversely affect the rights of the holders of the Common Stock.
The Company’s Board of Directors has the authority to fix and determine the relative rights and preferences of preferred stock. The Company’s Board of Directors has the authority to issue up to 10,000,000 shares of preferred stock without further stockholder approval. As a result, the Company’s Board of Directors could authorize the issuance of a series of preferred stock that would grant to holders the preferred right to our assets upon liquidation, the right to receive dividend payments before dividends are distributed to the holders of Common Stock and the right to the redemption of the shares, together with a premium, prior to the redemption of the Common Stock. In addition, the Company’s Board of Directors could authorize the issuance of a series of preferred stock that has greater voting power than the Common Stock or that is convertible into our Common Stock, which could decrease the relative voting power of the Common Stock or result in dilution to our existing stockholders.
| 26 |
| Table of Contents |
There are a significant number of shares of Common Stock eligible for sale, which could depress the market price of such shares.
A large number of shares of Common Stock will be available for sale in the public market, which could harm the market price of the stock. Further, shares may be offered from time to time in the open market pursuant to Rule 144, and these sales may have a depressive effect as well.
Risks Related to Our Common Stock
There is not an active liquid trading market for the Company’s Common Stock.
The Company files reports under the Exchange Act and its Common Stock is eligible for quotation on the OTCQB. However, there is no regular active trading market in the Company’s Common Stock, and we cannot give any assurance that an active trading market will develop. If an active market for the Company’s Common Stock develops, there is a significant risk that the Company’s stock price may fluctuate dramatically in the future in response to any of the following factors, some of which are beyond our control:
| · | variations in our quarterly operating results; | |
|
| |
| · | announcements that our revenue or income are below analysts’ expectations; | |
|
| |
| · | general economic slowdowns; | |
|
| |
| · | sales of large blocks of the Company’s Common Stock; and | |
|
| |
| · | announcements by us or our competitors of significant contracts, acquisitions, strategic partnerships, joint ventures or capital commitments. |
Because we became a public company by means of a reverse acquisition, we may not be able to attract the attention of brokerage firms.
Because we became public through a “reverse acquisition”, securities analysts of brokerage firms may not provide coverage of us since there is little incentive to brokerage firms to recommend the purchase of our Common Stock. No assurance can be given that brokerage firms will want to conduct any secondary offerings on behalf of the Company in the future.
Applicable regulatory requirements, including those contained in and issued under the Sarbanes-Oxley Act of 2002, may make it difficult for the Company to retain or attract qualified officers and directors, which could adversely affect the management of its business and its ability to obtain or retain listing of its Common Stock.
The Company may be unable to attract and retain those qualified officers, directors and members of board committees required to provide for effective management because of the rules and regulations that govern publicly held companies, including, but not limited to, certifications by principal executive officers. The enactment of the Sarbanes-Oxley Act has resulted in the issuance of a series of related rules and regulations and the strengthening of existing rules and regulations by the SEC, as well as the adoption of new and more stringent rules by the stock exchanges. The perceived increased personal risk associated with these changes may deter qualified individuals from accepting roles as directors and executive officers.
| 27 |
| Table of Contents |
Further, some of these changes heighten the requirements for board or committee membership, particularly with respect to an individual’s independence from the corporation and level of experience in finance and accounting matters. The Company may have difficulty attracting and retaining directors with the requisite qualifications. If the Company is unable to attract and retain qualified officers and directors, the management of its business and its ability to obtain or retain listing of our shares of Common Stock on any stock exchange (assuming the Company elects to seek and are successful in obtaining such listing) could be adversely affected.
As an issuer of “penny stock”, the protection provided by the federal securities laws relating to forward looking statements does not apply to us.
Although federal securities laws provide a safe harbor for forward-looking statements made by a public company that files reports under the federal securities laws, this safe harbor is not available to issuers of penny stocks. As a result, we will not have the benefit of this safe harbor protection in the event of any legal action based upon a claim that the material provided by us contained a material misstatement of fact or was misleading in any material respect because of our failure to include any statements necessary to make the statements not misleading. Such an action could hurt our financial condition.
SPECIAL NOTE REGARDING FORWARD-LOOKING STATEMENTS
This prospectus, including the sections entitled “Risk Factors,” “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and “Business,” contains forward-looking statements that are based on our management’s belief and assumptions and on information currently available to our management. Although we believe that the expectations reflected in these forward-looking statements are reasonable, these statements relate to future events or our future financial performance, and involve known and unknown risks, uncertainties and other factors that may cause our actual results, levels of activity, performance or achievements to be materially different from any future results, levels of activity, performance or achievements expressed or implied by these forward-looking statements. Forward-looking statements in this prospectus include, but are not limited to, statements about:
| · | the commercial success and market acceptance of any of our products and product candidates that are approved for marketing in the United States or other countries; |
| · | the accuracy of our estimates of the size and characteristics of the markets that may be addressed by our products and product candidates; |
| · | our ability to manufacture sufficient amounts of our product candidates for clinical trials and our products for commercialization activities; |
| · | our need for, and ability to raise, additional capital; |
| · | the number, designs, results and timing of our clinical trials; |
| · | the regulatory review process and any regulatory approvals that may be issued or denied by the FDA or other regulatory agencies; |
| · | our need to secure collaborators to license, manufacture, market and sell any products for which we receive regulatory approval in the future; |
| · | our ability to protect our intellectual property and operate our business without infringing upon the intellectual property rights of others; |
| · | the medical benefits, effectiveness and safety of our products and product candidates; |
| · | the safety and efficacy of medicines or treatments introduced by competitors that are targeted to indications which our products and product candidates have been developed to treat; |
| · | our current or prospective collaborators’ compliance or non-compliance with their obligations under our agreements with them; and |
| · | other factors discussed elsewhere in this prospectus. |
| 28 |
| Table of Contents |
In some cases, you can identify forward-looking statements by terminology such as “may,” “will,” “should,” “expects,” “intends,” “plans,” “anticipates,” “believes,” “estimates,” “predicts,” “potential,” “continue” or the negative of these terms or other comparable terminology. These statements are only predictions. You should not place undue reliance on forward-looking statements because they involve known and unknown risks, uncertainties and other factors, which are, in some cases, beyond our control and which could materially affect results. Factors that may cause actual results to differ materially from current expectations include, among other things, those listed under “Risk Factors” and elsewhere in this prospectus. Actual events or results may vary significantly from those implied or projected by the forward-looking statements. No forward-looking statement is a guarantee of future performance. You should read this prospectus and the documents that we reference in this prospectus and have filed with the Securities and Exchange Commission as exhibits to this prospectus completely and with the understanding that our actual future results may be materially different from any future results expressed or implied by these forward-looking statements.
The forward-looking statements in this prospectus represent our views as of the date of this prospectus. We anticipate that subsequent events and developments will cause our views to change. However, while we may elect to update these forward-looking statements at some point in the future, we have no current intention of doing so except to the extent required by applicable law. You should therefore not rely on these forward-looking statements as representing our views as of any date subsequent to the date of this prospectus.
The 11,444,204 shares that are being offered for resale by the selling stockholders represent 1,920,250 currently outstanding shares and 9,523,954 shares underlying currently outstanding warrants, and will be sold for the accounts of the selling stockholders named in this prospectus. As a result, all proceeds from the sales of the 11,444,204 shares of common stock currently outstanding and offered for resale hereby will go to the selling stockholders and we will not receive any proceeds from the resale of those shares of common stock by the selling stockholders.
We may receive up to a total of $5,618,360 in gross proceeds if all of the warrants are exercised hereunder for cash. However, as we are unable to predict the timing or amount of potential exercises of the warrants, we have not allocated any proceeds of such exercises to any particular purpose. Accordingly, all such proceeds are allocated to working capital. It is possible that the warrants may expire and may never be exercised.
After the exercise of any of the warrants, we would not receive any proceeds from the resale of those shares by the selling stockholders because those shares will be sold for the accounts of the selling stockholders named in this prospectus.
We will incur all costs associated with this registration statement and prospectus.
Our common stock is quoted on the OTCQB under the symbol “CTXR.QB.” Our common stock was not traded during the nine months ended September 30, 2014 and traded on a limited basis during the year ended September 30, 2015 and through the six months ended March 31, 2016. Since the acquisition of Leonard-Meron Biosciences, Inc. on March 30, 2016, the trading volume of our Common Stock has started to increase. We were quoted under the ticker symbol TRLO.QB through October 9, 2014 and on October 10, 2014, our ticker symbol changed to CTXR.QB.
The following table sets forth the range of the high and low bid quotations of our Common Stock for the last five fiscal quarters, as reported by the OTCQB. The over-the-counter market quotations reflect inter-dealer prices, without retail mark-up, mark-down or commission and may not necessarily represent actual transactions.
|
| Year Ended September 30, 2016 |
| |||||
|
| High |
|
| Low |
| ||
First Quarter |
| $ | 1.85 |
|
| $ | 1.00 |
|
Second Quarter |
| $ | 2.50 |
|
| $ | 1.55 |
|
Third Quarter |
| $ | 2.50 |
|
| $ | 0.78 |
|
Fourth Quarter |
| $ | 1.20 |
|
| $ | 0.58 |
|
| 29 |
| Table of Contents |
|
| Year Ended September 30, 2017 |
| |||||
|
| High |
|
| Low |
| ||
First Quarter |
| $ | 0.99 |
|
| $ | 0.17 |
|
Second Quarter |
| $ | 0.98 |
|
| $ | 0.36 |
|
On March 30, 2017, the closing price as reported on the OTCQB of our common stock was $0.45.
Holders of Common Stock
We are authorized to issue 200,000,000 shares of Common Stock, $0.001 par value per share. As of February 28, 2017, we have 75,504,242 shares of Common Stock issued and outstanding and there are approximately 121 shareholders of record of the Company’s Common Stock.
Each share of Common Stock shall have one (1) vote per share for all purposes. The holders of a majority of the shares entitled to vote, present in person or represented by proxy shall constitute a quorum at all meetings of our shareholders. Our Common Stock does not provide preemptive, subscription or conversion rights and there are no redemption or sinking fund provisions or rights. Our Common Stock holders are not entitled to cumulative voting for election of the board of directors.
Holders of Common Stock are entitled to receive ratably such dividends as may be declared by the board of directors out of funds legally available therefore as well as any distributions to the security holder. We have never paid cash dividends on our Common Stock, and do not expect to pay such dividends in the foreseeable future.
In the event of a liquidation, dissolution or winding up of our company, holders of Common Stock are entitled to share ratably in all of our assets remaining after payment of liabilities. Holders of Common Stock have no preemptive or other subscription or conversion rights. There are no redemption or sinking fund provisions applicable to the Common Stock.
Equity Compensation Plan Information
The following table provides information about the securities authorized for issuance under the Company’s equity compensation plan as of September 30, 2016:
Plan category |
| Number of securities to be issued upon exercise of outstanding options, warrants and rights |
|
| Weighted average exercise price of outstanding options, warrants and rights |
|
| Number of securities remaining available for future issuance |
| |||
|
| (a) |
|
| (b) |
|
| (c) |
| |||
Equity compensation plans approved by security holders (1) Stock options |
|
| 8,732,770 |
|
| $ | 0.54 |
|
|
| 4,267,230 |
|
Equity compensation plans not approved by security holders |
|
| - |
|
|
|
|
|
|
| - |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total |
|
| 8,732,770 |
|
| $ | 0.54 |
|
|
| 4,267,230 |
|
__________
(1)
| On September 12, 2014, the Board approved the Citius Pharmaceuticals, Inc. 2014 Stock Incentive Plan pursuant to which the Company may grant stock options, stock appreciation rights, restricted stock, restricted stock units, other stock-based awards and cash-based awards covering an aggregate of 13,000,000 shares of its Common Stock. On September 12, 2014, the Company received a written consent in lieu of a meeting from the holders of a majority of the Common Stock of the Company ratifying the Citius Pharmaceuticals, Inc. 2014 Stock Incentive Plan. |
| 30 |
| Table of Contents |
Adoption of Citius Pharmaceuticals, Inc. 2014 Stock Incentive Plan
On September 12, 2014, the Board approved the Citius Pharmaceuticals, Inc. 2014 Stock Incentive Plan (the “2014 Plan”). The purpose of the 2014 Plan is to promote the interests of the Company and its stockholders by providing (i) officers and employees, (ii) advisors, and (iii) non-employee directors with appropriate incentives and rewards.
The 2014 Plan provides for the granting of stock options, stock appreciation rights, restricted stock, restricted stock units, other stock-based awards and cash-based awards. The 2014 Plan also provides for the granting of performance stock awards so that the Board may use performance criteria in establishing specific targets to be attained as a condition to the grant or vesting of awards under the 2014 Plan.
The 2014 Plan provides for the grant of stock awards to employees, directors and consultants of the Company and its affiliates covering an aggregate of 13,000,000 shares of Common Stock, subject to adjustments in the event of certain changes to the Company’s capitalization.
The Common Stock subject to the 2014 Plan may be unissued shares or reacquired shares, including shares purchased on the open market. If a stock award granted under the 2014 Plan is forfeited, expires or is canceled or settled without issuance of Common Stock it shall not count against the maximum number of shares that may be issued under the 2014 Plan.
The Board has broad discretion in making grants under the 2014 Plan and may make grants subject to such terms and conditions as determined by the Board or a duly appointed committee thereof. Grants under the 2014 Plan will be subject to the terms and conditions set forth in the document making the award, including, without limitation any applicable purchase price and provisions pursuant to which the grant may be forfeited.
The Board may terminate or amend the 2014 Plan at any time, except for certain actions that may not be taken without stockholder approval. The 2014 Plan is scheduled to terminate on September 12, 2024.
We have not paid any cash dividends on our common stock and our Board of Directors presently intends to continue a policy of retaining earnings, if any, for use in our operations. The declaration and payment of dividends in the future, of which there can be no assurance, will be determined by the Board of Directors in light of conditions then existing, including earnings, financial condition, capital requirements and other factors. Nevada law prohibits us from declaring dividends where, if after giving effect to the distribution of the dividend:
| · | we would not be able to pay our debts as they become due in the usual course of business; or |
| · | except as otherwise specifically allowed by our articles of incorporation, our total assets would be less than the sum of our total liabilities plus the amount that would be needed to satisfy the rights of stockholders who have preferential rights superior to those receiving the distribution. |
Except as set forth above, there are no restrictions that currently materially limit our ability to pay dividends or which we reasonably believe are likely to limit materially the future payment of dividends on common stock.
Our Board of Directors has the right to authorize the issuance of preferred stock, without further stockholder approval, the holders of which may have preferences over the holders of our common stock as to payment of dividends.
| 31 |
| Table of Contents |
MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION
The following discussion and analysis of our financial condition and results of operations should be read together with our financial statements and related notes included elsewhere in this Prospectus. Management’s discussion and analysis contains forward-looking statements, such as statements of our plans, objectives, expectations and intentions. Any statements that are not statements of historical fact are forward-looking statements. When used, the words “believe,” “plan,” “intend,” “anticipate,” “target,” “estimate,” “expect” and the like, and/or future tense or conditional constructions (“will,” “may,” “could,” “should,” etc.), or similar expressions, identify certain of these forward-looking statements. These forward-looking statements are subject to risks and uncertainties including those under “Risk Factors” in this Prospectus that could cause actual results or events to differ materially from those expressed or implied by the forward-looking statements. Our actual results and the timing of events could differ materially from those anticipated in these forward-looking statements as a result of several factors. The Company does not undertake any obligation to update forward-looking statements to reflect events or circumstances occurring after the date of this Prospectus.
Historical Background
Citius Pharmaceuticals, Inc. (“Citius” or the “Company”) is a specialty pharmaceutical company dedicated to the development and commercialization of critical care products targeting unmet needs with a focus on anti-infectives, cancer care and unique prescription products. On September 12, 2014, we acquired Citius Pharmaceuticals, LLC as a wholly-owned subsidiary.
Citius Pharmaceuticals, LLC was founded in Massachusetts in January 2007. Activities since Citius Pharmaceuticals, LLC’s inception through December 31, 2016 were devoted primarily to the development and commercialization of therapeutic products for large and growing markets using innovative patented or proprietary formulations and novel drug delivery technology.
On March 30, 2016, the Company acquired all of the outstanding stock of Leonard-Meron Biosciences, Inc. (“LMB”) by issuing 29,136,839 shares of its common stock. As of March 30, 2016, the stockholders of LMB received approximately 41% of the issued and outstanding common stock of the Company. In addition, the Company converted the outstanding common stock warrants of LMB into 3,645,297 common stock warrants of the Company and converted the outstanding common stock options of LMB into 1,158,770 common stock options of the Company. Management estimated the fair value of the purchase consideration to be $19,015,073.
In connection with the acquisition, the Company acquired net assets of $17,428,277, including identifiable intangible assets of $19,400,000 related to in-process research and development and other assets and liabilities. The Company recorded goodwill of $1,586,796 for the excess of the purchase price over the net assets acquired.
In-process research and development represents the value of LMB’s leading drug candidate, which is an antibiotic solution used to treat catheter-related bloodstream infections. Goodwill represents the value of LMB’s industry relationships and its assembled workforce. In-process research and development is expected to be amortized on a straight-line basis over a period of eight years commencing upon revenue generation. Goodwill will not be amortized, but will be tested at least annually for impairment.
Through December 31, 2016, the Company has devoted substantially all of its efforts to product development, raising capital, building infrastructure through strategic alliances and coordinating activities relating to its first commercial product Suprenza. On July 1, 2016, the Company announced that it was discontinuing Suprenza and was focusing on the Phase 3 development of Mino-Lok™, an antibiotic lock solution used to treat patients with catheter-related bloodstream infections, and the Phase 2b development of Hydro-Lido for hemorrhoids. The Company has not yet realized any revenues from its planned principal operations.
| 32 |
| Table of Contents |
Business Agreements
Patent and Technology License Agreement
In May 2014, LMB entered into a patent and technology license agreement with Novel Anti-Infective Therapeutics, Inc., (“NAT”) to develop and commercialize Mino-Lok™ on an exclusive, worldwide (except for South America), sub licensable basis. On March 20, 2017, LMB entered into an amendment to the license agreement that expanded the licensed territory to include South America, providing LMB with worldwide rights. Since May 2014, LMB has paid an annual maintenance fee of $30,000 that increases over five years to $90,000, until commercial sales of a product subject to the license. LMB will also pay annual royalties on net sales of licensed products, with royalties ranging from the mid-single digits to the low double digits. In limited circumstances in which the licensed product is not subject to a valid patent claim and a competitor is selling a competing product, the royalty rate is in the low-single digits. After a commercial sale is obtained, LMB must pay minimum aggregate annual royalties that increase in subsequent years. LMB must also pay NAT up to $1,050,000 upon achieving specified regulatory and sales milestones. Finally, LMB must pay NAT a specified percentage of payments received from any sub licensees.
Suprenza Business Agreements
On June 12, 2008, the Company entered into a collaboration and license agreement (the “Alpex Agreement”) with Alpex Pharma S.A. (“Alpex”), in which Alpex granted the Company an exclusive right and license to use certain Alpex intellectual property in order to develop and commercialize orally disintegrating tablet formulations of pharmaceutical products in United States, Canada and Mexico. In addition, Alpex manufactured Suprenza, the Company’s commercialized pharmaceutical product, on a contract basis. The agreement was amended on November 15, 2011 as part of an Amendment and Coordination Agreement, pursuant to which Prenzamax and the Company agreed to each pay a portion of certain regulatory filing fees for as long as Prenzamax is purchasing Suprenza from Alpex. Both agreements were terminated in September 2016. During the three months ended March 31, 2016, the Company received $292,575 from Alpex as reimbursement for regulatory filing fees that were previously expensed during the three months ended December 31, 2015. The reimbursement was recorded as a reduction of research and development expenses. No other milestone, royalty or other payments have been earned or received by the Company through September 30, 2016 pursuant to these agreements.
On November 15, 2011, the Company entered into an exclusive license agreement (the “Sublicense Agreement”) with Prenzamax, LLC (“Prenzamax”), in which the Company granted Prenzamax and its affiliates the exclusive right to commercialize Suprenza in the United States. Prenzamax is an affiliate of Akrimax, a related party and was formed for the specific purpose of managing the Sublicense Agreement. The Sublicense Agreement was terminated in September 2016. The Company never received any payments under the Sublicense Agreement.
On July 1, 2016, the Company announced that it notified the Food and Drug Administration (“FDA”), Alpex and Prenzamax that it was discontinuing Suprenza.
| 33 |
| Table of Contents |
Three months ended December 31, 2016 compared with the three months ended December 31, 2015
|
| Three Months Ended December 31, 2016 |
|
| Three Months Ended December 31, 2015 |
| ||
Revenues |
| $ | — |
|
| $ | — |
|
|
|
|
|
|
|
|
|
|
Operating expenses: |
|
|
|
|
|
|
|
|
Research and development |
|
| 1,411,159 |
|
|
| 829,156 |
|
General and administrative |
|
| 1,132,183 |
|
|
| 294,221 |
|
Stock-based compensation |
|
| 241,514 |
|
|
| 121,299 |
|
Total operating expenses |
|
| 2,784,856 |
|
|
| 1,244,676 |
|
Operating loss |
|
| (2,784,856 | ) |
|
| (1,244,676 | ) |
Interest income |
|
| — |
|
|
| 15 |
|
Gain on revaluation of derivative warrant liability |
|
| 622,186 |
|
|
| 23,940 |
|
Interest expense |
|
| (13,228 | ) |
|
| — |
|
Net loss |
| $ | (2,175,898 | ) |
| $ | (1,220,721 | ) |
Revenues
We did not generate any revenues for the three months ended December 31, 2016 and 2015.
Research and Development Expenses
For the three months ended December 31, 2016, research and development expenses were $1,411,159 as compared to $829,156 during the three months ended December 31, 2015. The $582,003 increase in 2016 was primarily due to the $1,343,635 in costs incurred by LMB on the development of Mino-Lok™ offset by a decrease of $761,632 in costs incurred in the development of our product for the treatment of hemorrhoids and costs related to Suprenza. We are actively seeking to raise additional capital in order to fund our research and development efforts.
General and Administrative Expenses
For the three months ended December 31, 2016, general and administrative expenses were $1,132,183 as compared to $294,221 during the three months ended December 31, 2015. The $837,962 increase in 2016 was primarily due to the acquisition of LMB which resulted in increased compensation costs, increased consulting fees incurred for financing activities and corporate development services, and increased investor relations fees.
Stock-based Compensation Expense
For the three months ended December 31, 2016, stock-based compensation expense was $241,514 as compared to $121,299 for the three months ended December 31, 2015. The $120,215 increase in expense includes the expense for options assumed in the acquisition of LMB, as well as recent grants to new directors and new employees.
Other Income (Expense)
There was no interest income earned on our cash balances for the three months ended December 31, 2016 and only $15 in interest income earned for the three months ended December 31, 2015.
Gain on revaluation of derivative warrant liability for the three months ended December 31, 2016 was $622,186 compared to $23,940 for the three months ended December 31, 2015. The fair value of the derivative warrant liability fluctuates with changes in our stock price, volatility, remaining lives of the warrants, and interest rates. The gain for the three months ended December 31, 2016 was primarily due to a decrease in the fair value of our stock from $0.63 per share at September 30, 2016 to $0.44 per share at December 31, 2016.
Interest expense on the notes payables acquired in the acquisition of LMB and recent borrowings from our Chairman was $13,228 for the three months ended December 31, 2016. There was no interest expense for the three months ended December 31, 2015.
Net Loss
For the three months ended December 31, 2016, we incurred a net loss of $2,175,898 compared to a net loss for the three months ended December 31, 2015 of $1,220,721. The $955,177 increase in the net loss was primarily due to the $582,003 increase in research and development expenses and the increase of $837,962 in general and administrative expenses offset by the $598,246 increase in the gain on the revaluation of derivative warrant liability.
| 34 |
| Table of Contents |
Results of Operations for Year Ended September 30, 2016 compared to Year Ended September 30, 2015
|
| Year Ended September 30, |
|
| Year Ended September 30, |
| ||
Revenues |
| $ | - |
|
| $ | - |
|
|
|
|
|
|
|
|
|
|
Operating expenses: |
|
|
|
|
|
|
|
|
Research and development |
|
| 2,933,199 |
|
|
| 1,797,045 |
|
General and administrative |
|
| 3,783,941 |
|
|
| 946,613 |
|
Stock-based compensation – general and administrative |
|
| 732,151 |
|
|
| 486,271 |
|
Total operating expenses |
|
| 7,449,291 |
|
|
| 3,229,929 |
|
Operating loss |
|
| (7,449,291 | ) |
|
| (3,229,929 | ) |
Interest income |
|
| 806 |
|
|
| 3,066 |
|
Gain (loss) on revaluation of derivative warrant liability |
|
| (838,219 | ) |
|
| 332,095 |
|
Interest expense |
|
| (8,994 | ) |
|
| (7,500 | ) |
Net loss |
| $ | (8,295,698 | ) |
| $ | (2,902,268 | ) |
Revenues
We did not generate any revenues for the years ended September 30, 2016 and 2015. Beginning in May 2012, our strategic sales and marketing partner, Prenzamax, generated revenues from the sale of Suprenza, our first commercial product. Under the partnering agreement, we were not entitled to any revenues during the years ended September 30, 2016 and 2015. On July 1, 2016, the Company announced that it was discontinuing Suprenza and was focusing on the Phase 3 development of Mino-Lok™, an antibiotic lock solution used to treat patients with catheter-related bloodstream infections, and the Phase 2b development of Hydro-Lido for hemorrhoids.
Research and Development Expenses
For the year ended September 30, 2016, research and development expenses were $2,933,199 as compared to $1,797,045 for the year ended September 30, 2015. The $1,136,154 increase in 2016 was primarily due to the $1,912,745 in costs incurred in the development of Mino-Lok™ offset by a decrease in the costs on our product for the treatment of hemorrhoids and the reimbursement of $292,575 from Alpex for regulatory filing fees. We are actively seeking additional capital in order to fund our research and development efforts.
General and Administrative Expenses
For the year ended September 30, 2016, general and administrative expenses were $3,783,941 as compared to $946,613 for the year ended September 30, 2015. The increase of $2,837,328 in 2016 was primarily due to the acquisition of LMB which resulted in increased compensation costs, increased consulting fees incurred for financing activities and corporate development services, and increased investor relations fees.
| 35 |
| Table of Contents |
Stock-based Compensation Expense
For the year ended September 30, 2016, stock-based compensation expense was $732,151 as compared to $486,271 for the year ended September 30, 2015, an increase of $245,880. The $732,151 expense for the year ended September 30, 2016 includes the expenses for our Chairman’s options, an option granted to a consultant, options granted to six directors (including our current Chief Executive Officer), options granted to three employees, and options granted in connection with the acquisition of LMB. The $486,271 expense for the year ended September 30, 2015 was due to the stock options granted to our Chairman in connection with his employment agreement and options granted to two consultants.
Other Income (Expense)
Interest income earned was $806 for the year ended September 30, 2016 compared to $3,066 for the year September 30, 2015. The interest income was earned on the proceeds of our private offerings that were invested in money market accounts.
Loss on revaluation of derivative warrant liability for the year ended September 30, 2016 was $838,219 compared to a gain of $332,095 for the year ended September 30, 2015. The $838,219 loss for the year ended September 30, 2016 was primarily due to the increase in the fair value of our Common Stock from $0.54 per share at September 30, 2015 to $0.63 per share at September 30, 2016 and an increase in volatility from 57% at September 30, 2015 to 73% at September 30, 2016. The $332,095 gain for the year ended September 30, 2015 was primarily due to the decrease in our stock price used to calculate the fair value of the derivative liability from $0.60 at September 30, 2014 to $0.54 at September 30, 2015.
For the year ended September 30, 2016, interest expense increased by $1,494 in comparison to the year ended September 30, 2015. Interest expense for the year ended September 30, 2016 related to the demand notes payable assumed in the acquisition of LMB and the new $500,000 demand note payable issued in September 2016. For the year ended September 30, 2015, interest expense related to promissory notes issued to two existing investors. On December 31, 2014, the outstanding $600,000 promissory notes and accrued interest of $33,333 were converted into 1,055,554 shares of Common Stock at a conversion price of $0.60 per share. From December 31, 2014 to March 30, 2016, the Company had no outstanding interest bearing debt.
Net Loss
For the year ended September 30, 2016, we incurred a net loss of $8,295,698 compared to a net loss of $2,902,268 for the year ended September 30, 2015. The $5,393,430 increase in the net loss was primarily due to the $2,837,328 increase in general and administrative expenses, the $1,136,154 increase in research and development expenses and the $1,170,314 change in the gain (loss) on revaluation of derivative warrant liability.
Results of Operations for Year Ended September 30, 2015 compared to Nine Months Ended September 30, 2014
|
| Year Ended September 30, 2015 |
|
| Nine Months Ended September 30, 2014 |
| ||
Revenues |
| $ | - |
|
| $ | - |
|
|
|
|
|
|
|
|
|
|
Operating expenses: |
|
|
|
|
|
|
|
|
Research and development |
|
| 1,797,045 |
|
|
| 574 |
|
General and administrative |
|
| 946,613 |
|
|
| 183,044 |
|
Stock-based compensation – general and administrative |
|
| 486,271 |
|
|
| 470,185 |
|
Total operating expenses |
|
| 3,229,929 |
|
|
| 653,803 |
|
Operating loss |
|
| (3,229,929 | ) |
|
| (653,803 | ) |
Interest income |
|
| 3,066 |
|
|
| 555 |
|
Gain on revaluation of derivative warrant liability |
|
| 332,095 |
|
|
| 8,588 |
|
Interest expense |
|
| (7,500 | ) |
|
| (93,067 | ) |
Net loss |
| $ | (2,902,268 | ) |
| $ | (737,727 | ) |
| 36 |
| Table of Contents |
Revenues
We did not generate any revenues for the year ended September 30, 2015 and the nine months ended September 30, 2014. Beginning in May 2012, our strategic sales and marketing partner, Prenzamax, generated revenues from the sale of Suprenza, our first commercial product. Under the partnering agreement, we were not entitled to any revenues during the year ended September 30, 2015 and the nine months ended September 30, 2014.
Research and Development Expenses
For the year ended September 30, 2015, research and development expenses were $1,797,045 as compared to $574 during the nine months ended September 30, 2014. The $1,796,471 increase in 2015 was primarily due to costs incurred in the development of our product for the treatment of hemorrhoids in the current year and our limited working capital in the prior period.
General and Administrative Expenses
For the year ended September 30, 2015, general and administrative expenses were $946,613, as compared to $183,044 for the nine months ended September 30, 2014. The increase of $763,569 was attributable to additional compensation costs for our new Chief Executive Officer, plus additional financial and consulting expenses, higher insurance costs and increases in professional fees due to being a public company. Expense increases in the year ended September 30, 2015 were also attributable to our ability to fund our efforts as a result of the working capital raised in our private placements. Expenses were limited in 2014 as we focused our efforts solely on raising new capital to fund operations.
Stock-based Compensation Expense
For the year ended September 30, 2015, stock-based compensation expense was $486,271 compared to $470,185 for the nine months ended September 30, 2014. The $16,086 increase in 2015 was primarily due to options granted to two consultants during the year ended September 30, 2015. A majority of the stock-based compensation expense for the year ended September 30, 2015 and all of the stock-based compensation expense for the nine month period ended September 30, 2014 relates to options granted to our Chief Executive Officer in September 2014 in connection with his employment agreement to purchase 3,300,000 shares of the Company’s Common Stock.
Other Income (Expense)
Interest income earned was $3,066 for the year ended September 30, 2015 compared to $555 for the nine months ended September 30, 2014. The interest income was earned on the proceeds of our private offerings that were invested in money market accounts.
Gain on revaluation of derivative warrant liability for the year ended September 30, 2015 was $332,095 compared to a gain of $8,588 for the nine months ended September 30, 2014. The $332,095 gain for the year ended September 30, 2015 was primarily due to the decrease in our stock price used to calculate the fair value of the derivative liability from $0.60 at September 30, 2014 to $0.54 at September 30, 2015. The $8,588 gain for the nine months ended September 30, 2014 was due to the change in the fair value of the derivative warrant liability that we recognized in connection with the first closing of a private offering on September 12, 2014.
For the year ended September 30, 2015, interest expense decreased by $85,567 in comparison to the nine months ended September 30, 2014. On July 31, 2014, $2,035,000 of convertible promissory notes and accrued interest of $196,058 were converted to equity, and on December 31, 2014, $600,000 of promissory notes and accrued interest of $33,333 were converted to equity. From December 31, 2014 through September 30, 2015, the Company had no outstanding interest bearing debt.
Net Loss
For the year ended September 30, 2015, we incurred a net loss of $2,902,268 compared to a net loss of $737,727 for the nine months ended September 30, 2014. The $2,164,541 increase in the net loss was primarily due to our $1,796,471 increase in research and development expenses.
| 37 |
| Table of Contents |
LIQUIDITY AND CAPITAL RESOURCES
Going Concern Uncertainty and Working Capital
Citius has incurred operating losses since inception and incurred a net loss of $2,175,898 for the three months ended December 31, 2016. At December 31, 2016, Citius had an accumulated deficit of $19,512,145. Citius’ net cash used in operations during the three months ended December 31, 2016 was $1,314,792.
Citius has incurred operating losses and incurred a net loss of $8,295,698, $2,902,268 and $737,727 for the years ended September 30, 2016 and 2015, and the nine months ended September 30, 2014, respectively. Citius’ net cash used in operations during the years ended September 30, 2016 and 2015, and the nine months ended September 30, 2014 was $5,900,421, $2,385,416 and $183,164, respectively.
As of December 31, 2016, Citius had a working capital deficit of $5,828,421. The working capital deficit was attributable to the operating losses incurred by the Company since inception offset by our capital raising activities. At December 31, 2016, Citius had cash and cash equivalents of $46,764 available to fund its operations. The Company’s primary sources of cash flow since inception have been from financing activities. During the three months ended December 31, 2016, the Company received net proceeds of $247,205 from the issuance of equity and $820,000 from the issuance of notes payable to our Chairman, Leonard Mazur.
During the years ended September 30, 2016 and 2015, and the nine months ended September 30, 2014, the Company received net proceeds of $5,427,688, $1,509,493 and $1,680,834, respectively from the issuance of equity. Our primary uses of operating cash were for product development and commercialization activities, regulatory expenses, employee compensation, consulting fees, legal and accounting fees, and insurance and travel expenses.
On July 31, 2014, the note holders demanded conversion of the outstanding $1,685,000 in Convertible Notes, the $350,000 Subordinated Note and the accrued interest of $196,058 into 3,667,886 membership interests of Citius. Citius and the two note holders agreed to convert the Convertible Notes and accrued interest at the 2014 Private Offering price of $0.60 per share of Common Stock while the Subordinated Note issued in the 2013 private placement converted at $0.65 per share. All the Citius membership interests were exchanged on a one for one basis for shares of Common Stock in the Reverse Acquisition.
On September 12, 2014, the Company sold 3,400,067 units (“Units”) for a purchase price of $0.60 per Unit for gross proceeds of $2,040,040 and net proceeds of $1,630,834. Each Unit consists of one share of Common Stock and one five-year warrant (the “Investor Warrants”) to purchase one share of Common Stock at an exercise price of $0.60, (the “Private Offering”). The exercise price of the Investor Warrants is subject to adjustment, for up to one year, if the Company issues Common Stock at a price lower than the exercise price, subject to certain exceptions. The Investor Warrants will be redeemable by the Company at a price of $0.001 per Investor Warrant at any time subject to the conditions that (i) the Common Stock has traded for twenty (20) consecutive trading days with a closing price of at least $1.50 per share with an average trading volume of 50,000 shares per day and (ii) the Company provides 20 trading days prior notice of the redemption and the closing price of the Common Stock is not less than $1.17 for more than any 3 days during such notice period and (iii) the underlying shares of Common Stock are registered.
On December 31, 2014, the note holders requested conversion of $600,000 in Promissory Notes and accrued interest of $33,333 into 1,055,554 shares of Common Stock at a conversion price of $0.60 per share.
Between March 19, 2015 and September 14, 2015, the Company sold an additional 2,837,037 Units for a purchase price of $0.54 per Unit and 200,000 Units for a purchase price of $0.60 per Unit for gross proceeds of $1,652,000.
During the year ended September 30, 2016, the Company sold an additional 4,350,001 Units for a purchase price of $0.54 per Unit and 266,667 Units for a purchase price of $0.60 per Unit for gross proceeds of $2,509,000.
| 38 |
| Table of Contents |
On March 22, 2016, the Company sold 5,000,000 shares of Common Stock at $0.60 per share to its Chairman of the Board, Leonard Mazur, for gross proceeds of $3,000,000.
On September 7, 2016, the Company issued a $500,000 demand promissory note to our Chairman, Leonard Mazur which matures on demand by the lender. The Company issued $820,000 of additional demand promissory notes to Leonard Mazur during the three months ended December 31, 2016 which mature on the earlier of December 31, 2017 or demand by the lender. These notes accrue interest at the prime rate plus 1%. The Board of Directors has authorized additional revolving demand promissory notes with Leonard Mazur on substantially similar terms in an aggregate principal amount of up to $2,500,000, of which $1,320,000 is outstanding at December 31, 2016.
In October 2016, the Company commenced an offering (the “2016 Offering”) of up to 15,000,000 units at a price of $0.40 (the “2016 Offering Units”), each 2016 Offering Unit consists of (i) one share of common stock and (ii) a warrant to purchase one share of common stock (the “2016 Offering Warrants”) for gross proceeds of up to $6,000,000 with an over-subscription allotment of up to $2,000,000. Each 2016 Offering Warrant has an exercise price of $0.55 and is exercisable for five years from the date of issuance. The Placement Agent will receive a 10% cash commission on the gross proceeds of each sale of the 2016 Offering Units. In addition, on each closing the Placement Agent will also receive (i) an expense allowance equal to 3% of the proceeds of the sale, and (ii) warrants to purchase a number of shares of common stock equal to 10% of the 2016 Offering Units sold at an exercise price of $0.55 per share.
On November 23, 2016, the Company sold 975,000 2016 Offering Units for gross proceeds of $390,000. Additionally, a warrant to purchase 97,500 shares of common stock was granted to the Placement Agent pursuant to the above pricing terms. The Placement agent was paid commissions and an expense allowance of $50,700. Other costs of the placement were $156,896.
We expect that we will have sufficient funds to continue our operations for the next three months. We plan to raise additional capital in the future to support our operations. There is no assurance, however, that we will be successful in raising the needed capital or that the proceeds will be received in a timely manner to fully support our operations.
Inflation
Our management believes that inflation has not had a material effect on our results of operations.
Off Balance Sheet Arrangements
We do not have any off balance sheet arrangements.
CRITICAL ACCOUNTING POLICIES
Our discussion and analysis of our financial condition and results of operations is based on our financial statements, which have been prepared in accordance with accounting principles generally accepted in the United States. The preparation of these financial statements requires us to make estimates and judgments that affect the reported amounts of assets, liabilities and expenses and related disclosure of contingent assets and liabilities. We review our estimates on an ongoing basis. We base our estimates on historical experience and on various other factors that we believe to be reasonable under the circumstances. Actual results may differ from these estimates. We believe the judgments and estimates required by the following accounting policies to be critical in the preparation of our financial statements.
| 39 |
| Table of Contents |
Principles of Consolidation
As a result of the Reverse Acquisition, the accompanying consolidated financial statements include the operations of Citius Pharmaceuticals, LLC (the accounting acquirer). The accompanying consolidated financial statements also include the operations of Citius Pharmaceuticals, Inc. (formerly Trail One, Inc.) since the September 12, 2014 Reverse Acquisition and the operations of Leonard-Meron Biosciences, Inc. (“LMB”) since the March 30, 2016 acquisition. All significant inter-company balances and transactions have been eliminated in consolidation.
Research and Development
Research and development costs, including upfront fees and milestones paid to collaborators who are performing research and development activities under contractual agreement with us, are expensed as incurred. We defer and capitalize our nonrefundable advance payments that are for research and development activities until the related goods are delivered or the related services are performed. When we are reimbursed by a collaboration partner for work we perform, we record the costs incurred as research and development expenses and the related reimbursement as a reduction to research and development expenses in our statement of operations. Research and development expenses primarily consist of clinical and non-clinical studies, materials and supplies, third-party costs for contracted services, and payments related to external collaborations and other research and development related costs.
In-process Research and Development and Goodwill
In-process research and development represents the value of LMB’s leading drug candidate which is an antibiotic solution used to treat catheter-related bloodstream infections (Mino-Lok™) and is expected to be amortized on a straight-line basis over a period of eight years commencing upon revenue generation. Goodwill represents the value of LMB’s industry relationships and its assembled workforce. Goodwill will not be amortized but will be tested for impairment annually or more frequently if events or changes in circumstances indicate that the carrying value of the asset might be impaired.
Derivative Warrant Liability
The FASB ASC 815-40: Derivatives and Hedging-Contracts in Entity’s Own Equity requires freestanding contracts that are settled in a company’s own stock, including Common Stock warrants, to be designated as an equity instrument, asset or a liability. Under the provisions of ASC 815-40, a contract designated as an asset or a liability must be carried at fair value on a company’s balance sheet, with any changes in fair value recorded in the company’s results of operations. A contract designated as an equity instrument must be included within equity, and no fair value adjustments are required from period to period. The 3,400,067 Investor Warrants, the 680,013 warrants underlying the placement agent’s Unit warrants and the 1,000,000 warrants issued for investment banking services in the Private Offering on September 12, 2014 were separately accounted for as liabilities at issuance. In addition, the 3,037,037 Investor Warrants issued during the year ended September 30, 2015 and the 4,616,668 Investor Warrants issued during the year ended September 30, 2016 were accounted for as liabilities at issuance. The warrants were classified as liabilities at issuance because the exercise price of the warrants is subject to adjustment in the event that the Company issues Common Stock for less than $0.60 per share within one-year of the issuance of the warrants. The 2015 and 2016 private placements did not result in an adjustment of the exercise price.
The Company performs valuations of the warrants issued in the Private Offering using a probability weighted Black-Scholes Pricing Model which value was compared to a Binomial Option Pricing Model for reasonableness. The model uses market-sourced inputs such as underlying stock prices, risk-free interest rates, volatility, expected life and dividend rates and has also considered the likelihood of “down-round” financings. Selection of these inputs involves management’s judgment and may impact net income. Due to our limited operating history and limited number of sales of our Common Stock, we estimate our volatility based on a number of factors including the volatility of comparable publicly traded pharmaceutical companies. The volatility factor used in the Black-Scholes Pricing Model has a significant effect on the resulting valuation of the derivative liabilities on our balance sheet. The volatility calculated at September 30, 2016 was 73%. We used a risk-free interest rate of 1.14% and estimated lives of 4.10 to 4.57 years, which are the remaining contractual lives of the warrants. The volatility calculated at September 30, 2015 was 57%. We used a risk-free interest rate of 1.37% and estimated lives of 4.47 to 4.96 years, which are the remaining contractual lives of the warrants.
| 40 |
| Table of Contents |
On September 12, 2015, anti-dilution rights related to warrants to purchase 5,080,080 shares of Common Stock expired which resulted in a reclassification from derivative warrant liability to additional paid-in capital of $1,148,328. During the year ended September 30, 2016, anti-dilution rights related to warrants to purchase 3,037,037 shares of Common Stock expired which resulted in a reclassification from derivative warrant liability to additional paid-in capital of $1,093,765.
Income Taxes
Citius Pharmaceuticals, LLC was treated as a partnership for federal and state income taxes prior to the September 12, 2014 Reverse Acquisition. A partnership’s income or loss is allocated directly to the Members for income tax purposes.
We follow accounting guidance regarding the recognition, measurement, presentation and disclosure of uncertain tax positions in the financial statements. Tax positions taken or expected to be taken in the course of preparing our tax returns, including the position that Citius Pharmaceuticals, LLC qualified as a pass-through entity, are required to be evaluated to determine whether the tax positions are “more-likely-than-not” of being sustained by the applicable tax authorities. Tax positions not deemed to meet a more-likely-than-not threshold would be recorded in the financial statements. There are no uncertain tax positions that require accrual or disclosure as of September 30, 2016.
Any interest or penalties are charged to expense. None have been recognized in these financial statements. Generally, we are subject to federal and state tax examinations by tax authorities for all years subsequent to December 31, 2012.
After the Reverse Acquisition, we recognize deferred tax assets and liabilities based on differences between the financial reporting and tax basis of assets and liabilities using the enacted tax rates and laws that are expected to be in effect when the differences are expected to reverse. We provide a valuation allowance for deferred tax assets for which we do not consider realization of such assets to be more likely than not.
Business Overview
Citius Pharmaceuticals, Inc. (“Citius” or the “Company”) headquartered in Cranford, New Jersey, is a specialty pharmaceutical company dedicated to the development and commercialization of critical care products targeting important medical needs with a focus on anti-infective products in adjunct cancer care and unique prescription products. Our goal is to achieve leading market positions by providing therapeutic products that address unmet medical needs. New formulations of previously approved drugs with substantial safety and efficacy data is a core focus as we seek to reduce development and clinical risks associated with drug development. Our strategy keys on products that have intellectual property and regulatory exclusivity protection, while providing competitive advantages over other existing therapeutic approaches.
The Company was founded as Citius Pharmaceuticals, LLC, a Massachusetts limited liability company, on January 23, 2007. On September 12, 2014, Citius Pharmaceuticals, LLC entered into a Share Exchange and Reorganization Agreement, with Citius Pharmaceuticals, Inc. (formerly Trail One, Inc.), a publicly traded company incorporated under the laws of the State of Nevada. Citius Pharmaceuticals, LLC became a wholly-owned subsidiary of Citius. On March 30, 2016, Citius acquired Leonard-Meron Biosciences, Inc. (“LMB”) as a wholly-owned subsidiary. LMB was a pharmaceutical company focused on the development and commercialization of critical care products with a concentration on anti-infectives.
Since its inception, the Company has devoted substantially all of its efforts to business planning, research and development, recruiting management and technical staff, and raising capital. We are developing two proprietary products: Mino-Lok™, an antibiotic lock solution used to treat patients with catheter-related bloodstream infections by salvaging the infected catheter, and a Hydrocortisone-Lidocaine topical formulation that is intended to provide anti-inflammatory and anesthetic relief to persons suffering from hemorrhoids. We believe the markets for our products are large, growing, and underserved by current prescription products or procedures.
| 41 |
| Table of Contents |
In July 2016, the Company decided to discontinue Suprenza, its FDA-approved phentermine-based product for weight loss, due to a strategic change in direction following the acquisition of LMB and the Mino-Lok product. In September 2016, Citius notified the FDA of its decision to voluntarily withdraw both the Investigative New Drug Application and New Drug Application for commercial reasons and not due to safety concerns, effective immediately. The Company had received no royalties from Suprenza and believed costs associated with the ongoing regulatory expenses were depleting resources from our more promising Mino-Lok and Hydro-Lido product candidates.
Citius is subject to a number of risks common to companies in the pharmaceutical industry including, but not limited to, risks related to the development by Citius or its competitors of research and development stage products, market acceptance of its products, competition from larger companies, dependence on key personnel, dependence on key suppliers and strategic partners, the Company’s ability to obtain additional financing and the Company’s compliance with governmental and other regulations.
Mino-LokTM
Overview
Mino-Lok is a patented solution containing minocycline, disodium ethylenediaminetetraacetic acid (edetate), and ethyl alcohol, all of which act synergistically to treat and salvage infected central venous catheters (“CVCs”) in patients with catheter related bloodstream infections (“CRBIs”). Mino-Lok breaks down biofilm barriers formed by bacterial colonies, eradicate the bacteria, and provide anti-clotting properties to maintain patency in CVCs.
The administration of Mino-Lok consists of filling the lumen of the catheter with 0.8 ml to 2.0 ml of Mino-Lok solution, with a lock (dwell-time) of two hours while the catheter is not in use. If the catheter has multiple lumens, all lumens may be locked with the Mino-Lok solution either simultaneously or sequentially. If patients are receiving continuous infusion therapy, the catheters alternate between being locked with the Mino-Lok solution and delivering therapy. The Mino-Lok therapy is two hours per day for at least five days, usually with additional locks in the subsequent two weeks. After locking the catheter for two hours, the Mino-Lok solution is aspirated, and the catheter is flushed with normal saline. At that time, either the infusion will be continued, or will be locked with the standard-of-care lock solution until further use of the catheter is required. In a clinical study conducted by MD Anderson Cancer Center (“MDACC”), there were no serum levels of either minocycline or edetate detected in the sera of several patients who underwent daily catheter lock solution with minocycline and edetate (“M-EDTA”) at the concentration level proposed in Mino-Lok treatment. Thus, it has been demonstrated that the amount of either minocycline or edetate that leaks into the serum is very low or none at all.
Phase 2b Results
From April 2013 to July 2014, 30 patients with CVC-related bloodstream infection were enrolled at MDACC in a prospective Phase 2b study. Patients received Mino-Lok therapy for two hours once daily for a minimum of five days within the first week followed by two additional locks within the next two weeks. Patients were followed for one month post lock therapy. Demographic information, clinical characteristics, laboratory data, therapy, as well as adverse events and outcome were collected for each patient. Median age at diagnosis was 56 years (range: 21-73 years). In all patients, prior to the use of lock therapy, systemic treatment with a cultured-directed, first-line intravenous was started. Microbiological eradication was achieved in all cases. None of the patients experienced any serious adverse event related to the lock therapy.
The active arm was then compared to 60 patients in a matched cohort that experienced removal and replacement of their CVCs within the same contemporaneous timeframe. The patients were matched for cancer type, infecting organism, and level of neutropenia. All patients were cancer patients and treated at the MDACC. The efficacy of Mino-Lok therapy was 100% in salvaging CVCs, demonstrating equal effectiveness to removing the infected CVC and replacing with a new catheter.
| 42 |
| Table of Contents |
However, the main purpose of the study was to show that Mino-Lok therapy was at least as safe as the removal and replacement of CVCs when CRBSIs are present, and that the complications of removing an infected catheter and replacing with a new one could be avoided. In addition to having a 100% efficacy rate with all CVCs being salvaged, Mino-Lok therapy had no significant adverse events (“SAEs”), compared to an 18% serious adverse event rate in the matched cohort where patients had the infected CVCs removed and replaced (“R&R”). There were no overall complication rates in the Mino-Lok arm group compared to 11 events (18%) in the control group. These events included bacterial relapse (5%) and a number of complications associated with mechanical manipulation in the removal or replacement procedure for the catheter (10%) or development of deep seated infections such as septic thrombophlebitis and osteomyelitis (8%). As footnoted, six (6) patients had more than one (1) complication in the control arm group.
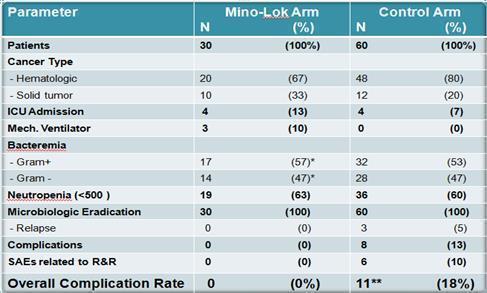
Phase 3 Initiation
In November 2016, the Company initiated site recruitment for Phase 3 clinical trials. It is expected that patient enrollment will commence in the Company’s third quarter 2017.
| 43 |
| Table of Contents |
Market Opportunity
In spite of best clinical practice, catheters contribute to approximately 70% of blood stream infections that occur in the ICU, or are associated with hemodialysis or cancer patients (approximately 470,000 per year). Bacteria enter the catheter either from the skin or intraluminally through the catheter hub. Once in the catheter, bacteria tend to form hard biofilm on the surface of the catheter that is resistant to most antimicrobial solutions. The most frequently used maintenance flush, heparin, actually stimulates biofilm formation. Heparin is widely used as a prophylactic lock solution, in spite of the evidence that it contributes to the promotion of biofilm formation. The formation of bacterial biofilm usually precedes CRBSIs.
The standard of care (“SOC”) in the management of CRBSI patients consists of removing the infected CVC and replacing it with a new catheter at a different vascular access site. However, in cancer and hemodialysis patients with long-term surgically implantable silicone catheters, removal of the CVC and reinsertion of a new one at a different site might be difficult, or even impossible, because of the unavailability of other accessible vascular sites and the need to maintain infusion therapy. Furthermore, critically ill patients with short-term catheters often have underlying coagulopathy, which makes reinsertion of a new CVC at a different site, in the setting of CRBSIs, risky in terms of mechanical complications, such as pneumothorax, misplacement, or arterial puncture. Studies have also revealed that CRBSI patients may be associated with serious complications, including septic thrombosis, endocarditis and disseminated infection, particularly if caused by Staphylococcus aureus or Candida species. Furthermore, catheter retention in patients with CRBSIs is associated with a higher risk of relapse and poor response to antimicrobial therapy.
According to Maki et al., published in the Mayo Clinic Proceedings in 2006, there are approximately 250,000 CRBSIs annually in the U.S. Subsequent to this study, our estimates have ranged upwards to over 450,000 central line-associated blood stream infections (“CLABSIs”) annually (see analysis in the table below). CRBSIs are associated with a 12% to 35% mortality rate and an attributable cost of $35,000 to $56,000 per episode.
We estimate that the potential market for Mino-Lok in the U.S. to be approximately $500 million to $1 billion as shown in the table below.
| Short-Term CVC |
| Long-Term CVC |
| Total |
No. of Catheters | 3 million |
| 4 million |
| 7 million |
Avg. Duration (Days) | 12 |
| 100 |
| N/A |
Catheter Days | 36 million |
| 400 million |
| 436 million |
Infection Rate | 2/1,000 days |
| 1/1,000 days |
| N/A |
Catheters Infected | 72,000 |
| 400,000 |
| 472,000 |
Flushes/Catheter | 5 |
| 7 |
| 6.7 |
Total Salvage Flushes | 360,000 |
| 2,800,000 |
| 3,160,000 |
Sources: Ann Intern Med 2000; 132:391–402, Clev Clin J Med 2011; 78(1):10-17, JAVA 2007; 12(1):17-27, J Inf Nurs 2004;27(4):245-250, Joint Commission website Monograph, CLABSI and Internal Estimates.
Under various plausible pricing scenarios, we believe that Mino-Lok would be cost saving to the healthcare system given that the removal of an infected CVC and replacement of a new catheter in a different venous access site is estimated by the Company to cost between $8,000 and $10,000. Furthermore, there are potential additional medical benefits and reduction in patient discomfort with the Mino-Lok approach. We believe there will be an economic argument to enhance the adoption of Mino-Lok by infection control committees at acute care institutions.
In January of 2017, the Company commissioned a third party survey of 31 physicians to qualify the need for catheter salvage in patients with infected, indwelling central venous lines, especially when the catheter is a tunneled or an implanted port. There were 19 Infection Disease experts and 12 Intensivists surveyed who all agreed that salvage would be preferable to catheter exchange to avoid catheter misplacements, blood clots, or vessel punctures that can potentially occur during reinsertion. Most were also concerned that viable venous access may not be available in patients who were vitally dependent on a central line.
| 44 |
| Table of Contents |
Hydro-Lido
Overview
Hydro-Lido is a topical formulation of hydrocortisone and lidocaine that is intended for the treatment of hemorrhoids. To our knowledge, there are currently no FDA-approved prescription drug products for the treatment of hemorrhoids. Some physicians are known to prescribe topical steroids for the treatment of hemorrhoids. In addition, there are various strengths of topical combination prescription products containing hydrocortisone along with lidocaine or pramoxine, each a topical anesthetic, that are prescribed by physicians for the treatment of hemorrhoids. These products contain drugs that were in use prior to the start of the Drug Efficacy Study Implementation (“DESI”) program and are commonly referred to as DESI drugs. However, none of these single-agent or combination prescription products have been clinically evaluated for safety and efficacy and approved by the FDA for the treatment of hemorrhoids. Further, many hemorrhoid patients use over the counter (“OTC”) products as their first line therapy. OTC products contain any one of several active ingredients including glycerin, phenylephrine, pramoxine, white petrolatum, shark liver oil and/or witch hazel, for symptomatic relief.
Development of Hemorrhoids Drugs
Hemorrhoids are a common gastrointestinal disorder, characterized by anal itching, pain, swelling, tenderness, bleeding and difficulty defecating. In the U.S., hemorrhoids affect nearly 5% of the population, with approximately 10 million persons annually admitting to having symptoms of hemorrhoidal disease. Of these persons, approximately one third visit a physician for evaluation and treatment of their hemorrhoids. The data also indicate that for both sexes a peak of prevalence occurs from age 45 to 65 years with a subsequent decrease after age 65 years. Caucasian populations are affected significantly more frequently than African Americans, and increased prevalence rates are associated with higher socioeconomic status in men but not women. Development of hemorrhoids before age 20 is unusual. In addition, between 50% and 90% of the general U.S., Canadian and European population will experience hemorrhoidal disease at least once in life. Although hemorrhoids and other anorectal diseases are not life-threatening, individual patients can suffer from agonizing symptoms which can limit social activities and have a negative impact on the quality of life.
Hemorrhoids are defined as internal or external according to their position relative to the dentate line. Classification is important for selecting the optimal treatment for an individual patient. Accordingly, physicians use the following grading system:
Grade I | Hemorrhoids not prolapsed but bleeding. |
Grade II | Hemorrhoids prolapse and reduce spontaneously with or without bleeding. |
Grade III | Prolapsed hemorrhoids that require reduction manually. |
Grade IV | Prolapsed and cannot be reduced including both internal and external hemorrhoids that are confluent from skin tag to inner anal canal. |
| 45 |
| Table of Contents |
Development Activities to Date
In the fall of 2015, we completed dosing patients in a double-blind dose ranging placebo controlled Phase 2 study where six different formulations containing hydrocortisone and lidocaine in various strengths were tested against the vehicle control. The objectives of this study were to: 1) demonstrate the safety and efficacy of the formulations when applied twice daily for two weeks in subjects with Grade I or II hemorrhoids and 2) assess the potential contribution of lidocaine hydrochloride and hydrocortisone acetate, alone or in combination for the treatment of symptoms of Goligher’s Classification Grade I or II hemorrhoids.
Symptom improvement was observed based on a global score of disease severity (“GSDS”), and based on some of the individual signs and symptoms of hemorrhoids, specifically itching and overall pain and discomfort. Within the first few days of treatment, the combination products (containing both hydrocortisone and lidocaine) were directionally favorable versus the placebo and their respective individual active treatment groups (e.g., hydrocortisone or lidocaine alone) in achieving ‘almost symptom free’ or ‘symptom free’ status according to the GSDS scale. These differences suggest the possibility of a benefit for the combination product formulation.
Overall, results from adverse event reporting support the safety profile of all test articles evaluated in this study and demonstrate similar safety profiles as compared to the vehicle. The safety findings were unremarkable. There was a low occurrence of adverse events and a similar rate of treatment related adverse events across all treatment groups. The majority of adverse events were mild and only one was severe. None of the adverse events were serious and the majority of adverse events were recovered/resolved at the end of the study. There were only two subjects who were discontinued from the study due to adverse events.
In addition to the safety and dose-ranging information, information was obtained relating to the use of the GSDS as an assessment tool for measuring the effectiveness of the test articles. Individual signs and symptoms were also assessed but can vary from patient to patient. Therefore, the goal of the GSDS was to provide an assessment tool that could be used for all patients regardless of which signs and symptoms they are experiencing. The GSDS proved to be a more effective tool for assessing the severity of the disease and the effectiveness of the drug when compared to the assessment of the individual signs and symptoms. Citius believes that we can continue to develop this assessment tool as well as other patient reported outcome endpoints for use in the next trials.
Information was also obtained about the formulation of the drug and the vehicle. As a result of this study, we believe that the performance of the active arms of the study relative to the vehicle can be improved by re-formulating our topical preparation. Therefore, we have initiated work on vehicle formulation and evaluation of higher potency steroids.
We recently conducted primary market research to better understand the symptoms that are most bothersome to patients. We also learned about the factors that drive patients to seek medical attention for hemorrhoids in an effort to understand the disease impact on quality of life. The results of this survey are able to help us develop patient reported outcome evaluation tools. These tools can be used in clinical trials to evaluate the patients’ conditions and to assess the performance of the test articles.
A Phase 2b study will begin once the new formulation is completed and the updated evaluation tools are developed. This study will be a 300 patient four arm study. The cost is estimated at approximately $3.0 – 5.0 million and is expected to require approximately one year to complete.
Market Opportunity
The current market for OTC and topical DESI formulations of hydrocortisone and lidocaine is highly fragmented, and includes approximately 20 million units of OTC hemorrhoid products and over 4 million prescriptions for non-approved prescription treatments. Several topical combination prescription products for the treatment of hemorrhoids are available containing hydrocortisone in strengths ranging from 0.5% to 3.0%, combined with lidocaine in strengths ranging from 1.0% to 3.0%. The various topical formulations include creams, ointments, gels, lotions, enemas, pads, and suppositories. The most commonly prescribed topical combination gel, is sold as a branded generic product and contains 2.5% hydrocortisone and 3.0% lidocaine.
| 46 |
| Table of Contents |
We believe there are currently no FDA-approved prescription drug products for the treatment of hemorrhoids. Although there are numerous prescription and OTC products commonly used to treat hemorrhoids, none possess proven safety and efficacy data generated from rigorously conducted clinical trials. We believe that a novel topical formulation of hydrocortisone and lidocaine designed to provide anti-inflammatory and anesthetic relief and which has an FDA-approved label specifically claiming the treatment of hemorrhoids will become an important treatment option for physicians who want to provide their patients with a therapy that has demonstrated safety and efficacy in treating this uncomfortable and often recurring disease. We believe that our Hydro-Lido product represents an attractive, low-risk product opportunity with meaningful upside potential.
Market Exclusivity
We believe that we will be the first company to conduct rigorous clinical trials and receive FDA approval of a topical hydrocortisone-lidocaine combination product for the treatment of hemorrhoids. If we receive FDA approval, we will qualify for 3 years of market exclusivity for our dosage strength and formulation. In addition, we will also be the only product on the market specifically proven to be safe and effective for the treatment of hemorrhoids. Generally, if a company conducts clinical trials and receives FDA approval of a product for which there are similar, but non FDA-approved, prescription products on the market, the manufacturers of the unapproved but marketed products are required to withdraw them from the market. However, the FDA has significant latitude in determining how to enforce its regulatory powers in these circumstances. We have not had any communication with the FDA regarding this matter and cannot predict what action, if any, the FDA will take with respect to the unapproved products.
We believe that should our product receive an FDA approval and demonstrate, proven safety and efficacy data, and if our products obtain 3 years of market exclusivity based on our dosage strength and formulation, Citius is likely to have a meaningful advantage in its pursuit of achieving a significant position in the market for topical combination prescription products for the treatment of hemorrhoids.
Sales and Marketing
We are primarily focused on identifying opportunities within the critical care and cancer care market segments. In our product acquisition criteria, we concentrate on markets that are highly influenced by key opinion leaders (KOLs) and have products that are prescribed by a relatively small number of physicians, yet provide large opportunities for growth and market share. This strategy allows for a manageable commercialization effort for our Company in terms of resources and capital. We also seek to provide cost-effective therapies that would be endorsed by payers, patients, and providers. We believe that we will be able to commercialize products within the scope of these criteria ourselves, and that we can create marketing synergies by having a common narrow audience for our marketing efforts (“several products in the bag for the same customer”).
For products that we own that fall out of the narrow scope criteria, we have identified pharmaceutical companies with large sales forces, experienced sales and marketing management teams, direct-to-consumer (“DTC”) capabilities, significantly larger resources than ours, and non-competing product portfolios that we believe would make excellent sales and marketing partners for us. We intend to license our mass audience, non-specialty products to such companies for sales and marketing.
Intellectual Property
We rely on a combination of patent, trade secret, copyright, and trademark laws, as well as confidentiality, licensing and other agreements, to establish and protect our proprietary rights. Our policy is to actively seek to obtain, where appropriate, the broadest intellectual property protection possible for our current product candidates and any future product candidates both in the U.S. and abroad. However, patent protection may not provide us with complete protection against competitors who seek to circumvent our patents. To help protect our proprietary know-how, which is not patentable, and for inventions for which patents may be difficult to enforce, we currently rely and will in the future rely on trade secret protection and confidentiality agreements to protect our interests.
| 47 |
| Table of Contents |
Mino-Lok Intellectual Property
Mino-Lok is covered by an issued U.S. patent (no. 7,601,731), “Antimicrobials in Combination with Chelators and Ethanol for the Rapid Eradication of Microorganisms Embedded in Biofilm,” which was issued on October 13, 2009. This patent is a composition of matter patent and provides intellectual property protection until June 7, 2024. There are corresponding applications pending in Europe and Canada (European Application No. EP 1644024; Canadian Patent Application No. 0252852). On April 15, 2014, a patent application was filed for an enhanced formulation that provides greater stability of the reconstituted Mino-Lok solution.
On May 14, 2014, LMB entered into a patent and technology license agreement with Novel Anti-Infective Therapeutics, Inc., (“NAT”) to develop and commercialize Mino-Lok on an exclusive, worldwide (except for South America), sub licensable basis. LMB incurred a one-time license fee in May 2014. Under the license agreement, the Company will pay (i) an annual maintenance fee until commercial sales of a product subject to the license, (ii) upon commercialization, we will pay annual royalties on net sales of licensed products, (iii) and certain regulatory and milestone payments. Unless earlier terminated, the license agreement remains in effect until the date that all patents licensed under the agreement have expired and all patent applications within the licensed patent rights have been cancelled, withdrawn or expressly abandoned.
Mino-Lok has received a Qualified Infectious Disease Product (“QIDP”) designation. QIDP provides New Drug Applications an additional 5 years of market exclusivity with Hatch-Waxman for a combined total of 10 years regardless of patent protection.
Hydro-Lido Intellectual Property
We are developing a new formulation of Hydro-Lido which will have a unique combination of excipients as well as unique concentrations of the active ingredients. The goal is to have a product that is optimized for stability and activity. Once the formulation development is completed and data is obtained, we will apply for a patent on this new topical formulation.
We seek to achieve approval for Hydro-Lido by utilizing the FDA’s 505(b)(2) pathway. This pathway will provide 3 years of market exclusivity.
Competition
We operate in a highly competitive and regulated industry which is subject to rapid and frequent changes. We face significant competition from organizations that are pursuing drugs that would compete with the drug candidates that we are developing and the same or similar products that target the same conditions we intend to treat. Due to our limited resources, we may not be able to compete successfully against these organizations, which include many large, well-financed and experienced pharmaceutical and biotechnology companies, as well as academic and research institutions and government agencies.
Mino-Lok Competition
Currently, the only alternative to Mino-Lok in the treatment of infected CVCs in CRBSI/CLABSI patients of which we are aware, is the SOC of removing the culprit CVC and replacing a new CVC at a different vascular site. Citius is not aware of any Investigational New Drug Applications (“INDs”) for a salvage antibiotic lock solution and does not expect any to be forthcoming due to the difficulty of meeting the necessary criteria to be effective and practical.
| 48 |
| Table of Contents |
At this time, there are no pharmacologic agents approved in the U.S. for the prevention or treatment of CLABSIs in central venous catheters. Citius is aware that there are several agents in development for prevention but none for salvage. The most prominent of these appear to be Neutrolin from CorMedix and B-Lock from Great Lakes Pharmaceuticals, Inc. (“GLP”).
NeutrolinÒ (CorMedix Inc.)
Neutrolin is a formulation of Taurolidine 1.35%, Citrate 3.5%, and Heparin 1000 units/mL. Neutrolin is an anti-microbial catheter lock solution being developed by CorMedix to prevent CRBSIs and to prevent clotting. In January 2015, the U.S. Food and Drug Administration (the “FDA”) granted Fast Track and Qualified Infectious Disease Product (“QIDP”) designations for Neutrolin. In December 2015, CorMedix initiated its Phase 3 clinical trial in hemodialysis patients in the United States. The clinical trial named Catheter Lock Solution Investigational Trial, or LOCK-IT-100 is a prospective, multicenter, randomized, double-blind, placebo-controlled, active control trial designed to show efficacy and safety of Neutrolin in preventing CRBSIs in subjects receiving hemodialysis therapy. CorMedix has also announced that it plans to conduct a second Phase 3 trial in parenteral nutrition patients and is working closely with the FDA. This clinical trial is expected to commence in the first quarter of 2017.
B-Lock™ (Great Lakes Pharmaceuticals, Inc.)
B-Lock is a triple combination of trimethoprim, EDTA and ethanol from Great Lakes Pharmaceuticals, Inc. (“GLP”). On July 24, 2012, GLP announced the initiation of a clinical study of B-Lock. GLP has stated that it has developed B-Lock as a device/drug combination product capable of effective prevention of CRBSIs. The study that was announced is a prospective, randomized, active control clinical investigation to be conducted in 22 clinical sites in Hungary and Poland and involves up to 400 patients on renal dialysis who required a central venous catheter for vascular access. GLP has stated that the clinical data would be used for CE Mark approval in the European Union. We are unaware as to the progress or results of these studies. In addition, we are not aware of any IND being filed in the US for B-Lock, nor are we aware of any clinical studies to support salvage of infected catheters in bacteremic patients.
Neither of these lock solutions have been shown to be effective in salvaging catheters in bacteremic patients as Mino-Lok is intended to do, and Citius does not expect that either would be pursued for this indication.
Hydro-Lido Competition
The primary competition in the hemorrhoid market is non-prescription over the counter products. When approved, Hydro-Lido will be the only prescription product for the treatment of hemorrhoids.
Supply and Manufacturing
We do not currently have and we do not intend to set up our own manufacturing facilities. We expect to use approved contract manufacturers for manufacturing our products in all stages of development after we file for FDA approval. Each of our domestic and foreign contract manufacturing establishments, including any contract manufacturers we may decide to use, must be listed in the New Drug Application (“NDA”) and must be registered with the FDA. Also, the FDA imposes substantial annual fees on manufacturers of branded products.
In general, our suppliers purchase raw materials and supplies on the open market. Substantially all such materials are obtainable from a number of sources so that the loss of any one source of supply would not have a material adverse effect on us.
| 49 |
| Table of Contents |
If we elect to conduct product development and manufacturing, we will be subject to regulation under various federal and state laws, including the Occupational Safety and Health Act, the Environmental Protection Act, the Toxic Substances Control Act, the Resource Conservation and Recovery Act, the Controlled Substances Act and other present and potential future federal, state or local regulations.
We have contracted with proven suppliers and manufacturers for active pharmaceutical ingredient, development and packaging. We are confident that all materials meet or will meet specifications discussed at the chemistry, manufacturing and controls meeting with the FDA.
Regulatory Strategy
United States Government Regulation
The research, development, testing, manufacture, labeling, promotion, advertising, distribution and marketing, among other things, of our products are extensively regulated by governmental authorities in the United States and other countries. Citius’ products may be classified by the FDA as a drug or a medical device depending upon the indications for use or claims. Because certain of our product candidates are considered as medical devices and others are considered as drugs for regulatory purposes, we intend to submit applications to regulatory agencies for approval or clearance of both medical devices and pharmaceutical product candidates.
In the United States, the FDA regulates drugs and medical devices under the Federal Food, Drug, and Cosmetic Act and the agency’s implementing regulations. If Citius fails to comply with the applicable United States requirements at any time during the product development process, clinical testing, and the approval process or after approval, we may become subject to administrative or judicial sanctions. These sanctions could include the FDA’s refusal to approve pending applications, license suspension or revocation, withdrawal of an approval, warning letters, adverse publicity, product recalls, product seizures, total or partial suspension of production or distribution, injunctions, fines, civil penalties or criminal prosecution. Any agency enforcement action could have a material adverse effect on Citius.
Foreign Regulatory Requirements
Citius and any collaborative partners may be subject to widely varying foreign regulations, which may be different from those of the FDA, governing clinical trials, manufacture, product registration and approval and pharmaceutical sales. Whether or not FDA approval has been obtained, Citius or its collaboration partners must obtain a separate approval for a product by the comparable regulatory authorities of foreign countries prior to the commencement of product marketing in such countries. In certain countries, regulatory authorities also establish pricing and reimbursement criteria. The approval process varies from country to country, and the time may be longer or shorter than that required for FDA approval. In addition, under current United States law, there are restrictions on the export of products not approved by the FDA, depending on the country involved and the status of the product in that country.
International sales of medical devices manufactured in the U.S. that are not approved by the FDA for use in the U.S., or are banned or deviate from lawful performance standards, are subject to FDA export requirements. Exported devices are subject to the regulatory requirements of each country to which the device is exported. Some countries do not have medical device regulations, but in most foreign countries, medical devices are regulated. Frequently, regulatory approval may first be obtained in a foreign country prior to application in the U.S. to take advantage of differing regulatory requirements. Most countries outside of the U.S. require that product approvals be recertified on a regular basis, generally every 5 years. The recertification process requires that Citius evaluate any device changes and any new regulations or standards relevant to the device and conduct appropriate testing to document continued compliance. Where recertification applications are required, they must be approved in order to continue selling Citius’ products in those countries.
| 50 |
| Table of Contents |
In the European Union, in order for a product to be marketed and sold, it is required to comply with the Medical Devices Directive and obtain CE Mark certification. The CE Mark certification encompasses an extensive review of the applicant’s quality management system which is inspected by a notified body’s auditor as part of a stage 1 and 2 International Organization for Standardization (“ISO”) 13485:2016 audit, in accordance with worldwide recognized ISO standards and applicable European Medical Devices Directives for quality management systems for medical device manufacturers. Once the quality management system and design dossier has been successfully audited by a notified body and reviewed and approved by a competent authority, a CE certificate for the medical device will be issued. Applicants are also required to comply with other foreign regulations such as the requirement to obtain Ministry of Health, Labor and Welfare approval before a new product can be launched in Japan. The time required to obtain these foreign approvals to market Citius’ products may vary from U.S. approvals, and requirements for these approvals may differ from those required by the FDA.
Medical device laws and regulations are in effect in many of the countries in which Citius may do business outside the United States. These laws and regulations range from comprehensive device approval requirements for Citius’ medical device product to requests for product data or certifications. The number and scope of these requirements are increasing. Citius may not be able to obtain regulatory approvals in such countries and may be required to incur significant costs in obtaining or maintaining its foreign regulatory approvals. In addition, the export of certain of Citius’ products which have not yet been cleared for domestic commercial distribution may be subject to FDA export restrictions. Any failure to obtain product approvals in a timely fashion or to comply with state or foreign medical device laws and regulations may have a serious adverse effect on Citius’ business, financial condition or results of operations.
Employees
As of September 30, 2016, the Company had 6 employees and various consultants providing support. Through our consulting and collaboration arrangements, and including our Scientific Advisory Board, we have access to more than 30 additional professionals, who possess significant expertise in business development, legal, accounting, regulatory affairs, clinical operations and manufacturing. We also rely upon a network of consultants to support our clinical studies and manufacturing efforts.
Properties
We maintain our offices at 11 Commerce Drive, Cranford, NJ 07016. We do not intend to expand our operations for the foreseeable future and do not intend to lease additional space.
Legal Proceedings
We are not involved in any litigation that we believe could have a material adverse effect on our financial position or results of operations. There is no action, suit, proceeding, inquiry or investigation before or by any court, public board, government agency, self-regulatory organization or body pending or, to the knowledge of our executive officers, threatened against or affecting our company or our officers or directors in their capacities as such.
Below are the names and certain information regarding the Company’s executive officers and directors.
Name | Age | Position(s) | ||
Myron Holubiak | 70 | President and Chief Executive Officer and Director | ||
Leonard Mazur | 72 | Executive Chairman of the Board of Directors | ||
Suren Dutia | 72 | Director | ||
Carol Webb | 70 | Director | ||
Dr. William Kane | 73 | Director | ||
Howard Safir | 75 | Director | ||
Dr. Eugene Holuka | 57 | Director |
| 51 |
| Table of Contents |
Myron Holubiak is the President, Chief Executive Officer and has been a member of the Board since October 2015. Mr. Holubiak has extensive experience in managing and advising large and emerging pharmaceutical and life sciences companies. Mr. Holubiak was the President of Roche Laboratories, Inc. (“Roche”), a major research-based pharmaceutical company, from December 1998 to August 2001. Prior to that, he held sales and marketing positions at Roche during his 19-year tenure. From September, 2002 to July, 2016, Mr. Holubiak served on the board of directors and for the last 2 years was the Chairman of the board of directors of BioScrip, Inc. (“BioScrip”) (Nasdaq: BIOS). BioScrip is a leading national provider of infusion and home care management solutions. Since July 2010, Mr. Holubiak has served as a member of the board of directors of Assembly Biosciences, Inc. (“Assembly”) (Nasdaq: ASMB) and its predecessor Ventrus Biosciences, Inc. (“Ventrus”). Assembly is a biopharmaceutical company developing innovative treatments for hepatitis B virus infection (HBV) and C. difficile-associated diarrhea (CDAD). In March, 2013, Mr. Holubiak founded Leonard-Meron Biosciences, Inc. (“LMB”), the Company’s wholly-owned subsidiary, and he served as the Chief Executive Officer and President of LMB until March, 2016. In addition, Mr. Holubiak was also a trustee of the Academy of Managed Care Pharmacy Foundation until the current year. Mr. Holubiak received a B.S. in Molecular Biology and Biophysics from the University of Pittsburgh.
Leonard Mazur is the Executive Chairman and Secretary of the Company and has been a member of the Board since September 2014. Mr. Mazur is the cofounder and Vice Chairman of Akrimax Pharmaceuticals, LLC (“Akrimax”), a privately held pharmaceutical company specializing in producing cardiovascular and general pharmaceutical products. Akrimax was founded in September 2008 and has successfully launched prescription drugs while acquiring drugs from major pharmaceutical companies. From January 2005 to May 2012, Mr. Mazur also co-founded and served as the Chief Operating Officer of Triax Pharmaceuticals LLC (“Triax”), a specialty pharmaceutical company producing prescription dermatological drugs. Prior to joining Triax, he was the founder and, from 1995 to 2005, Chief Executive Officer of Genesis Pharmaceutical, Inc. (“Genesis”), a dermatological products company that marketed its products through dermatologists’ offices as well as co-promoting products for major pharmaceutical companies. In 2003, Mr. Mazur successfully sold Genesis to Pierre Fabre, a leading pharmaceutical company. Mr. Mazur has extensive sales, marketing and business development experience from his tenures at Medicis Pharmaceutical Corporation as executive vice president, ICN Pharmaceuticals, Inc. as vice president, sales & marketing, Knoll Pharma (a division of BASF), and Cooper Laboratories, Inc. Mr. Mazur is a member of the Board of Trustees of Manor College, is a recipient of the Ellis Island Medal of Honor and was previously the chairman of the board of directors of LMB, the Company’s wholly-owned subsidiary. Mr. Mazur received both his BA and MBA from Temple University and has served in the U.S. Marine Corps Reserves.
Suren Dutia has been a member of the Board since October 2015. Mr. Dutia has served as Senior Fellow of the Ewing Mario Kauffman Foundation since March 2011 and as Senior Fellow of Skandalaris Center for Entrepreneurial Studies at Washington University, St. Louis since 2013. He has served as a member of the advisory board of Center for Digital Transformation, University of California, Irvine since May 2012 and as chairman of the board of directors of AccelPath, LLC since October 2009. From February 2006 to May 2010, Mr. Dutia served as the Chief Executive Officer of TiE Global, a non-profit organization involved in globally fostering entrepreneurship. From February 2011 to May 2013, Mr. Dutia served as a director of LifeProof Cases and from July 2000 to December 2011, he served as a director of Anvita Health. From 1989 to 1998, Mr. Dutia served as the Chief Executive Officer and chairman of the board of directors of Xscribe Corporation. Prior to his positions with Xscibe Corporation, Mr. Dutia held several positions with Dynatech Corporation, and in addition, he was the president of a medical instruments company. Previously, Mr. Dutia worked for the U.S. Department of Education. Mr. Dutia received his B.S. and M.S. degrees in chemical engineering and B.A. in political science from Washington University, St. Louis. In addition, he obtained an M.B.A. from University of Dallas.
Carol Webb has served as a director of LMB and, upon LMB’s acquisition by the Company in March 2016, a director of the Company. From 2000 to 2005, she served as Company Group Chairman of Johnson & Johnson, and from 1987 to 2000 she served in capacities including President, Vice President, Executive Director, Product Management and Senior Product Director of Ortho Biotech. Ms. Webb has worked in various positions including Sales Representative, Sales Trainer, Product Manager and Manager of Public Policy at Roche Laboratories from 1972 to 1983. Ms. Webb received her B.S. in Biology from Bowling Green State University.
| 52 |
| Table of Contents |
Dr. William (Terry) Kane has served as a director of LMB and, upon LMB’s acquisition by the Company in March 2016, a director of the Company. He has served as a Clinical Professor at Duke University Medical Center since 2003. From 2008 to 2012, Dr. Kane was on the Board of the First Fight Venture Center ("FFVC") in Research Triangle Park, NC and chaired the FFVC Board from 2012 to 2016. From 2006 to 2009, he served as the Chief Executive Officer of RadarFind Corporation, and from 2002 to 2003, he served as the Interim Chief Medical Officer of Mercy Fitzgerald Hospital. From 1996 until 2002, Dr. Kane served as the President and Chief Executive Officer of InteCardia, Inc., and from 1995 until 1996, he was a Health Care Consultant. From 1993 to 1995, Dr. Kane served in various capacities at Sharp Healthcare including Executive Vice President, Operations and Executive Vice President, Community Care. From 1992 to 1993, he was the Senior Vice President, Medical Affairs at Independence Blue Cross, and from 1990 to 1992, he served in various capacities at CentraState Medical Center including President, Chief Executive Officer, Executive Vice President and Chief Operating Officer. From 1989 to 1990, Dr. Kane was with Cain Brothers, Shattuck & Co., and from 1988 to 1989, he was the Senior Vice President, Health Services Division of American International Healthcare (formerly JBI). From 1986 to 1987, Dr. Kane was the Executive Vice President and Corporate Medical Director of CIGNA Healthplan, Inc., and from 1984 to 1986, he was at U.S. Healthcare, Inc. and served in various capacities including Senior Vice President Medical Delivery, President and Senior Medical Director. Dr. Kane is currently the chair of the board of directors of Research Triangle Park and was a past member of the board of directors of Pisacano Leadership Foundation and Make-A-Wish Foundation. In addition, he previously served on the Management Advisory Committee of Cornucopia House Cancer Support Center. Dr. Kane received his B.S. in Biology from the University of Scranton and his M.D. with Honors from the Temple University School of Medicine.
Howard Safir has served as a director of LMB since April 2014 and, upon LMB’s acquisition by the Company in March 2016, a director of the Company. He has served as Chairman and Chief Executive Officer of VRI Technologies LLC, a security consulting and law enforcement integrator since July 2010. From 2001 until 2010, Mr. Safir served as the Chairman and Chief Executive Officer of Safir Rosetti, a provider of security and investigation services and a wholly-owned subsidiary of Global Options Group Inc. Mr. Safir served as the Vice Chairman of Global Options Group Inc. from its 2005 acquisition of Safir Rosetti until 2010. He served as Chief Executive Officer of Bode Technology, also a wholly-owned subsidiary of Global Options Group Inc., from 2007 to 2010. Mr. Safir currently serves as a director of Implant Sciences Corporation, an explosives device detection company, and LexisNexis Special Services, Inc., a leading provider of information and technology solutions to governments, as well as Verint Systems Inc. During his career, Mr. Safir served as the 39th Police Commissioner of the City of New York, as Associate Director for Operations, U.S. Marshals Service and as Assistant Director of the Drug Enforcement Administration.
Dr. Eugene Holuka has served as a director of the Company since June 2016. Dr. Holuka is an internist and has practiced in critical care medicine for almost thirty years. He is presently an attending physician at the Staten Island University Hospital where he has practiced since 1991. Dr. Holuka has also served as an Adjunct Clinical Assistant Professor at the Touro College of Osteopathic Medicine since 2011. Prior to the acquisition of LMB by the Company in March 2016, he was a member of the LMB Scientific Advisory Board from April 2014 until the present day. Dr. Holuka received the Ellis Island Medal of Honor in 2000 and has served on the NECO Committee Board since 2005. He was an Executive Committee Member on the Forum’s Children Foundation from 2000 until 2008.
Family Relationships
There are no family relationships among or between any of our current or former executive officers and directors.
Code of Ethics
We have adopted a code of ethics relating to the conduct of our business by all of our employees, officers and directors. We have also adopted a corporate communications policy for our employees and directors establishing guidelines for the disclosure of information related to the Company to the investing public, market analysts, brokers, dealers, investment advisors, the media, and any persons who are not our employees or directors. Additionally, we have adopted an insider trading policy to establish guidelines for our employees, officers, directors, and consultants regarding transactions in our securities and the disclosure of material nonpublic information related to our Company. Each of these policies is posted under the Investors section of our website at www.citiuspharma.com.
| 53 |
| Table of Contents |
Section 16(a) Beneficial Ownership Reporting Compliance
Section 16(a) of the Exchange Act, requires the Company’s directors and named executive officers, and persons who beneficially own more than ten percent of our Common Stock, to file initial reports of ownership and reports of changes in ownership of our Common Stock and our other equity securities with the SEC. As a practical matter, the Company assists its directors and officers by monitoring transactions and completing and filing Section 16 reports on their behalf. Based solely on a review of the copies of such forms in our possession and on written representations from reporting persons, we believe that during the year ended September 30, 2016 all of our named executive officers and directors filed the required reports on a timely basis under Section 16(a) of the Exchange Act except that, due to administrative errors, option grants to Eugene Holuka in June 2016 were reported 2 days late.
Involvement in Certain Legal Proceedings
To our knowledge, our directors and executive officers have not been involved in any of the following events during the past ten years:
1. | any bankruptcy petition filed by or against such person or any business of which such person was a general partner or executive officer either at the time of the bankruptcy or within two years prior to that time; | |
| 2. | any conviction in a criminal proceeding or being subject to a pending criminal proceeding (excluding traffic violations and other minor offenses); |
| 3. | being subject to any order, judgment, or decree, not subsequently reversed, suspended or vacated, of any court of competent jurisdiction, permanently or temporarily enjoining him from or otherwise limiting his involvement in any type of business, securities or banking activities or to be associated with any person practicing in banking or securities activities; |
| 4. | being found by a court of competent jurisdiction in a civil action, the SEC or the Commodity Futures Trading Commission to have violated a Federal or state securities or commodities law, and the judgment has not been reversed, suspended, or vacated; |
| 5. | being subject of, or a party to, any Federal or state judicial or administrative order, judgment decree, or finding, not subsequently reversed, suspended or vacated, relating to an alleged violation of any Federal or state securities or commodities law or regulation, any law or regulation respecting financial institutions or insurance companies, or any law or regulation prohibiting mail or wire fraud or fraud in connection with any business entity; or |
| 6. | being subject of or party to any sanction or order, not subsequently reversed, suspended, or vacated, of any self-regulatory organization, any registered entity or any equivalent exchange, association, entity or organization that has disciplinary authority over its members or persons associated with a member. |
Board Committees
In June 2016, the Company formed an Audit Committee, Compensation Committee, and Nominating and Governance Committee. The Board of Directors has determined that each of the members of the Audit and Risk, Compensation, and Nominating and Governance Committees is independent. In June 2016, our Board of Directors adopted written charters for each of its permanent committees, all of which are available under the Investors section of our website at www.citiuspharma.com. Each committee is required to perform an annual evaluation of its charter, and each committee may engage outside independent advisors as the committee deems appropriate. A brief description of the responsibilities of the Audit and Risk, Compensation, and Nominating and Governance Committees follows.
| 54 |
| Table of Contents |
Audit and Risk Committee
Our Audit and Risk Committee consists of Messrs. Dutia (Chair) and Safir and Dr. Kane. Each of Messrs. Dutia and Safir and Dr. Kane satisfy the independence requirements of Rule 5605(a)(2) of the NASDAQ Stock Market listing rules and SEC Rule 10A-3. Our Audit and Risk Committee is responsible for, among other things:
| · | appointing, terminating, compensating, and overseeing the work of any accounting firm engaged to prepare or issue an audit report or other audit, review or attest services; |
| · | reviewing and approving, in advance, all audit and non-audit services to be performed by the independent auditor, taking into consideration whether the independent auditor's provision of non-audit services to us is compatible with maintaining the independent auditor's independence; |
| · | reviewing and discussing the adequacy and effectiveness of our accounting and financial reporting processes and controls and the audits of our financial statements; |
| · | establishing and overseeing procedures for the receipt, retention, and treatment of complaints received by us regarding accounting, internal accounting controls or auditing matters, including procedures for the confidential, anonymous submission by our employees regarding questionable accounting or auditing matters; |
| · | investigating any matter brought to its attention within the scope of its duties and engaging independent counsel and other advisors as the audit committee deems necessary; |
| · | determining compensation of the independent auditors and of advisors hired by the audit committee and ordinary administrative expenses; |
| · | reviewing and discussing with management and the independent auditor the annual and quarterly financial statements prior to their release; |
| · | monitoring and evaluating the independent auditor's qualifications, performance, and independence on an ongoing basis; |
| · | reviewing reports to management prepared by the internal audit function, as well as management's response; |
| · | reviewing and assessing the adequacy of the formal written charter on an annual basis; |
| · | reviewing and approving related-party transactions for potential conflict of interest situations on an ongoing basis; and |
| · | handling such other matters that are specifically delegated to the audit committee by our Board of Directors from time to time. |
Our Board of Directors has affirmatively determined that Mr. Dutia is designated as the “audit committee financial expert”. The designation does not impose on Mr. Dutia any duties, obligations or liabilities that are greater than those generally imposed on members of our audit committee and our Board of Directors.
Compensation Committee
Our Compensation Committee consists of Mr. Safir (Chair), Ms. Webb and Dr. Holuka. Our Compensation Committee is responsible for, among other things:
| · | reviewing and approving the compensation, employment agreements and severance arrangements, and other benefits of all of our executive officers and key employees; |
| · | reviewing and approving, on an annual basis, the corporate goals and objectives relevant to the compensation of the executive officers, and evaluating their performance in light thereof; |
| · | reviewing and making recommendations, on an annual basis, to the Board of Directors with respect to director compensation; |
| · | reviewing any analysis or report on executive compensation required to be included in the annual proxy statement and periodic reports pursuant to applicable federal securities rules and regulations, and recommending the inclusion of such analysis or report in our proxy statement and period reports; |
| · | reviewing and assessing, periodically, the adequacy of the formal written charter; and |
| · | such other matters that are specifically delegated to the compensation committee by our Board of Directors from time to time. |
| 55 |
| Table of Contents |
Pursuant to its written charter, our Compensation Committee has the authority to engage the services of outside advisors as it deems appropriate to assist it in the evaluation of the compensation of our directors, principal executive officer or other executive and non-executive officers, and in the fulfillment of its other duties. Additionally, our Compensation Committee has the authority to review and approve the compensation of our other officers and employees and may delegate its authority to review and approve the compensation of other non-executive officer employees to specified executive officers.
Nominating and Governance Committee
Our Nominating and Governance Committee consists of Dr. Kane (Chair), Dr. Holuka and Ms. Webb. It is responsible for, among other things:
| · | identifying and screening candidates for our Board of Directors, and recommending nominees for election as directors; |
| · | establishing procedures to exercise oversight of the evaluation of the Board of Directors and management; |
| · | reviewing the structure of the Board of Directors’ committees and recommending to the Board of Directors for its approval directors to serve as members of each committee, and where appropriate, making recommendations regarding the removal of any member of any committee; |
| · | developing and reviewing our code of conduct, evaluating management's communication of the importance of our code of conduct, and monitoring compliance with our code of conduct; |
| · | reviewing and assessing the adequacy of the formal written charter on an annual basis; and |
| · | generally advising our Board of Directors on corporate governance and related matters. |
Nomination of Directors
The Nominating and Governance Committee is responsible for establishing the criteria for recommending which directors should stand for re-election to the Board and the selection of new directors to serve on the Board of Directors. In addition, the Committee is responsible for establishing the procedures for our stockholders to nominate candidates to the Board. Board candidates are typically identified by existing directors or members of management. The Nominating and Governance Committee considers the needs for the Board of Directors as a whole when identifying and evaluating nominees and, among other things, considers diversity in background, age, experience, qualifications, attributes and skills in identifying nominees, although it does not have a formal policy regarding the consideration of diversity. Each director nominee is recommended by the Nominating and Governance Committee and the Chairman of the Board.
Pursuant to the Company's Bylaws, our stockholders may select individuals to be nominated for election to the Board of Directors by providing written notice to the Company no more than 90 and not less than 60 days before the meeting. Such notice must set forth the following:
| · | Information with respect to the proposed director nominee; |
| · | the name and address of the stockholder, as it appears on the Company's books; |
| · | the class and number of shares of the Company that are beneficially owned by such stockholder; |
| · | a representation that the stockholder is a holder of record of stock of the Company entitled to vote at the meeting and intends to appear in person or by proxy at the meeting to propose the foregoing nomination; and |
| · | any material interest of the stockholder in the proposed nomination; |
Our Nominating and Governance Committee will evaluate a nominee recommended by a stockholder in the same manner in which the Committee evaluates nominees recommended by other persons as well as its own nominee recommendations.
| 56 |
| Table of Contents |
Compensation of Directors
No director of the Company received any compensation for services as a director during the year ended September 30, 2015 and the nine month period ended September 30, 2014.
On October 1 and October 8, 2015, the Company appointed Myron Holubiak and Suren Dutia, respectively to the board of directors. Mr. Holubiak and Mr. Dutia each received an option to purchase 400,000 shares of Common Stock at an exercise price of $0.54 per share in consideration for their services as members of the board of directors. The options were issued pursuant to the Company’s 2014 Stock Incentive Plan.
On June 23, 2016, the board of directors issued options to purchase 200,000 shares of Common Stock with an exercise price of $0.80 per share, to each of Mr. Safir, Ms. Webb, Dr. Kane and Dr. Holuka in consideration for their services as members of the board of directors. The options vest in full on June 23, 2017. The options were issued pursuant to the Company’s 2014 Stock Incentive Plan.
On June 23, 2016, the board approved a director compensation plan for non-employee directors. Non-employee directors will each receive (1) an annual retainer of $10,000, (2) $2,000 for each meeting attended, and (3) $500 for each telephone meeting. In addition; (i) the Lead Independent Director and the Audit and Risk Committee chairman will each receive an additional annual retainer of $10,000, (ii) the Compensation Committee, and Nominating and Corporate Governance Committee chairmen will each receive an additional annual retainer of $5,000 and (iii) each committee member will receive an annual retainer of $2,500.
Director compensation for the year ended September 30, 2016 was as follows:
Name |
| Fees Earned or Paid in Cash ($) (1) |
|
| Stock Awards |
|
| Option Awards |
|
| All Other Compensation |
|
| Total |
| |||||
Suren Dutia |
|
| 12,500 |
|
|
| -- |
|
|
| -- |
|
|
| -- |
|
|
| 12,500 |
|
Carol Webb |
|
| 9,500 |
|
|
| 87,798 |
|
|
| -- |
|
|
| -- |
|
|
| 97,298 |
|
Dr. William Kane |
|
| 11,250 |
|
|
| 87,798 |
|
|
| -- |
|
|
| -- |
|
|
| 99,048 |
|
Howard Safir |
|
| 15,750 |
|
|
| 87,798 |
|
|
| -- |
|
|
| -- |
|
|
| 103,548 |
|
Dr. Eugene Holuka |
|
| 9,500 |
|
|
| 87,798 |
|
|
| -- |
|
|
| -- |
|
|
| 97,298 |
|
____________
(1) Includes half of the annual retainer fee ($5,000) since the director compensation plan was approved mid-year.
(2) The director was awarded options to purchase 200,000 shares of Common Stock on June 23, 2016. The value in this column represents the grant date fair value ($0.44 per option). The exercise price of each option is $0.80 per share.
Executive Compensation
In accordance with Item 402 of Regulation S-K promulgated by the SEC, we are required to disclose certain information regarding the makeup of and compensation for our company’s directors, former directors and named executive officers, in certain cases for each of the last three completed fiscal years.
In establishing executive compensation, our Board is guided by the following goals:
| · | compensation should consist of a combination of cash and equity awards that are designed to fairly pay the executive officers and directors for work required for a company of our size and scope; |
| · | compensation should align the executive officers’ and directors’ interests with the long-term interests of stockholders; |
| · | compensation should assist with attracting and retaining qualified executive officers and directors. |
| 57 |
| Table of Contents |
Summary Compensation Table
The following table sets forth information regarding compensation paid to our executive officers for the years ended September 30, 2016 and 2015, and the nine months ended September 30, 2014. Trail One, Inc. did not pay any compensation to its Chief Executive Officer for its fiscal year ended September 30, 2014.
Name & Position |
| Fiscal Year |
| Salary |
|
| Bonus |
|
| Option Awards |
|
| All Other Compensation |
|
| Total |
| |||||
Myron Holubiak (1) |
| 2016 |
|
| 225,000 |
|
|
| 112,500 |
|
|
| 95,346 | (4) |
|
| 0 |
|
|
| 432,846 |
|
President and CEO |
| 2015 |
|
| 0 |
|
|
| 0 |
|
|
| 0 |
|
|
| 0 |
|
|
| 0 |
|
|
| 2014 |
|
| 0 |
|
|
| 0 |
|
|
| 0 |
|
|
| 0 |
|
|
| 0 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Leonard Mazur (2) |
| 2016 |
|
| 250,000 |
|
|
| 120,000 |
|
|
| 187,653 | (5) |
|
| 0 |
|
|
| 557,653 |
|
Executive Chairman |
| 2015 |
|
| 250,000 |
|
|
| 0 |
|
|
| 420,710 | (5) |
|
| 0 |
|
|
| 670,710 |
|
|
| 2014 |
|
| 20,833 |
|
|
| 0 |
|
|
| 470,185 | (5) |
|
| 0 |
|
|
| 491,018 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Reinier Beeuwkes (3) |
| 2016 |
|
| 0 |
|
|
| 0 |
|
|
| 0 |
|
|
| 0 |
|
|
| 0 |
|
Chief Executive Officer |
| 2015 |
|
| 0 |
|
|
| 0 |
|
|
| 0 |
|
|
| 0 |
|
|
| 0 |
|
|
| 2014 |
|
| 0 |
|
|
| 0 |
|
|
| 0 |
|
|
| 0 |
|
|
| 0 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Geoffrey Clark (3) |
| 2016 |
|
| 0 |
|
|
| 0 |
|
|
| 0 |
|
|
| 0 |
|
|
| 0 |
|
Chief Medical Officer |
| 2015 |
|
| 0 |
|
|
| 0 |
|
|
| 0 |
|
|
| 0 |
|
|
| 0 |
|
|
| 2014 |
|
| 0 |
|
|
| 0 |
|
|
| 0 |
|
|
| 0 |
|
|
| 0 |
|
(1) Appointed as President and Chief Executive Officer on March 30, 2016.
(2) Appointed as Chief Executive Officer on September 12, 2014 and on March 30, 2016 became Executive Chairman of the Board of Directors.
(3) Resigned as executive officer and director on September 12, 2014.
(4) On October 1, 2015, Myron Holubiak was granted options to purchase 400,000 shares of Common Stock at an exercise price of $0.54 per share that vest 40,000 shares on the grant date and then 30,000 shares per month commencing on December 31, 2015. The dollar amount set forth in the table represents the dollar amount recognized for financial statement reporting purposes with respect to the fiscal year in accordance with FASB ASC Topic 718.
(5) On September 12, 2014, Leonard Mazur was granted options to purchase 3,300,000 shares of Common Stock at an exercise price of $0.45 per share that vest 1,300,000 shares on the grant date; 500,000 shares on September 12, 2015; 500,000 shares on March 12, 2016; 500,000 shares on September 12, 2016; and 500,000 shares on September 12, 2017. The dollar amount set forth in the table represents the dollar amount recognized for financial statement reporting purposes with respect to the fiscal year in accordance with FASB ASC Topic 718.
| 58 |
| Table of Contents |
Outstanding Equity Awards at Fiscal Year-End
OUTSTANDING EQUITY AWARDS AT FISCAL YEAR-END | ||||||||||||||||||||||||||||||||||
OPTION AWARDS |
| STOCK AWARDS |
| |||||||||||||||||||||||||||||||
Name (a) |
| Number of Securities Underlying Unexercised Options (#) Exercisable (b) |
|
| Number of Securities Underlying Unexercised Options (#) Unexercisable (c) |
|
| Equity Incentive Plan Awards: Number of Securities Underlying Unexercised Unearned Options (#) (d) |
|
| Option Exercise Price ($) (e) |
|
| Option Expiration Date (f) |
| Number of Shares or Units of Stock That Have Not Vested (#) (g) |
|
| Market Value of Shares or Units of Stock That Have Not Vested ($) (h) |
|
| Equity Incentive Plan Awards: Number of Unearned Shares, Units or Other Rights That Have Not Vested (#) (i) |
|
| Equity Incentive Plan Awards: Market or Payout Value of Unearned Shares, Units or Other Rights That Have Not Vested (#) (j) |
| ||||||||
Myron Holubiak |
|
| 340,000 |
|
|
| – |
|
|
| 60,000 | (2) |
| $ | 0.54 |
|
| 10/1/25 |
|
| – |
|
|
| – |
|
|
| – |
|
|
| – |
|
Leonard Mazur |
|
| 2,800,000 |
|
|
| – |
|
|
| 500,000 | (1) |
| $ | 0.45 |
|
| 9/12/24 |
|
| – |
|
|
| – |
|
|
| – |
|
|
| – |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
(1) On September 12, 2014, Leonard Mazur was granted options to purchase 3,300,000 shares of Common Stock at an exercise price of $0.45 per share that vest 1,300,000 shares on the grant date; 500,000 shares on September 12, 2015; 500,000 shares on March 12, 2016; 500,000 shares on September 12, 2016; and 500,000 shares on September 12, 2017.
(2) On October 1, 2015, Myron Holubiak was granted options to purchase 400,000 shares of Common Stock at an exercise price of $0.54 per share that vest 40,000 shares on the grant date and then 30,000 shares per month commencing on December 31, 2015.
| 59 |
| Table of Contents |
Employment Agreements
Leonard Mazur
Mr. Leonard Mazur, our Executive Chairman, and the Company entered into an employment agreement on September 12, 2014. Below are the material terms of his employment agreement:
| · | a term of three years beginning on September 12, 2014 and upon expiration, the agreement shall automatically renew for successive periods of one-year; |
| · | an initial base salary of $250,000 per year; |
| · | a $120,000 cash bonus if the Company is successful in raising $2,000,000 in equity financing during the term (such bonus was earned and accrued in the year ended September 30, 2016); |
| · | a stock option grant dated September 12, 2014 to purchase 3,300,000 shares of Common Stock under the Company’s 2014 Stock Incentive Plan at $0.45 per share vesting over a three-year term; and |
| · | participation in any regular Company benefits, such as medical insurance plans, life insurance plans, disability income plans, retirement plans, vacation and other paid time off plans, in addition to reimbursement for ordinary and necessary business expenses. |
The employment agreement provides that if Mr. Mazur is terminated by the Company without cause, or that if Mr. Mazur resigns for “Good Reason” (as defined in the agreement), the Company would continue to pay Mr. Mazur’s salary and health insurance for a period of six months from the date of termination, and fully vest any options that would have vested at the next immediate vesting event following termination. In the event that Mr. Mazur was terminated as a result of a “Change of Control” (as defined in the agreement), he would be entitled to receive his salary and health insurance for a period of twelve months and any options would become fully vested. In the event that Mr. Mazur’s employment was terminated for any other reason, there would be no continuation of salary or health insurance.
Myron Holubiak
On March 30, 2016, the Company entered into an employment agreement with Myron Holubiak to serve as Chief Executive Officer for a term of 3 years, which term will automatically be extended for additional one year periods unless earlier terminated. Mr. Holubiak will receive (i) an annual base salary equal to $450,000, (ii) a discretionary bonus on each anniversary of the effective date in an amount up to 50% of the current base salary based on the attainment of certain financial, clinical development and business milestones as established annually by the Board of Directors and (iii) an incentive bonus based upon market capitalization of the Company as defined in the employment agreement. Upon termination of employment, Mr. Holubiak may be entitled to receive certain severance as further described in the employment agreement.
TRANSACTIONS WITH RELATED PERSONS
Related Party Transactions
Our headquarters were located in the office space of Ischemix, LLC (“Ischemix”), a company majority-owned by Dr. Geoffrey Clark and Dr. Reinier Beeuwkes until March 30, 2016. Although Dr. Clark and Dr. Beeuwkes resigned as officers and directors of the Company effective as of September 12, 2014, the Company had an oral agreement with Ischemix to continue to maintain its headquarters in the office spare of Ischemix. The Company was not required to pay for use of the space.
| 60 |
| Table of Contents |
As of September 30, 2016, the Company owes $27,637 to Ischemix LLC for expenses paid on the Company’s behalf and services performed by Ischemix. Ischemix is owned by Reinier Beeuwkes and Geoffrey Clark who were both officers and directors, as well as principal stockholders of the Company. Reinier Beeuwkes and Geoffrey Clark have resigned as both officers and directors effective September 12, 2014.
In November 2011, we entered into an exclusive license agreement with Prenzamax LLC (“Prenzamax”), pursuant to which we granted to Prenzamax a license for sales of Suprenza in the U.S. Prenzamax’s performance of this agreement is guaranteed by Akrimax LLC (“Akrimax”), a specialty pharmaceuticals sales and marketing company. The exclusive license agreement provides that all of the sales and marketing expenses will be incurred and borne by Prenzamax. Both we and Prenzamax will equally share the expenses related to FDA establishment fees, product fees and post-marketing studies and the resulting earnings will be shared equally by us and Prenzamax. The co-founder and Vice Chairman of Akrimax is Leonard Mazur, our Executive Chairman of the Board of Directors. On July 1, 2016, the Company announced that it notified Prenzamax that it was discontinuing Suprenza.
In May 2014, Citius sold Membership Interests that converted to 200,000 shares of Common Stock to Leonard Mazur for an aggregate purchase price of $50,000.
Between July 12, 2010 and March 25, 2013, Citius issued convertible promissory notes in the aggregate principal amount of $1,685,000, including $850,000 to Geoffrey Clark and $835,000 to Reinier Beeuwkes. On July 31, 2014, the note holders demanded conversion of the outstanding $1,685,000 notes and accrued interest of $151,813 into 3,061,355 shares of Common Stock at a conversion price of $0.60 per share.
On November 19, 2013, Citius issued two promissory notes, each in the principal amount of $300,000, to Geoffrey Clark and Reinier Beeuwkes, respectively. On December 31, 2014, the note holders requested conversion of $600,000 in notes and accrued interest of $33,333 into 1,055,554 shares of Common Stock at a conversion price of $0.60 per share, which is the same price that the Company sold Units for in the September 2014 Private Placement.
Effective as of September 1, 2014, the Company entered into a consulting agreement (the “Consulting Agreement”) with Neeta Wadekar, a stockholder of the Company. Pursuant to the terms of the Consulting Agreement, Mrs. Wadekar shall receive $4,000 per month and shall: (i) maintain and manage the Company’s accounts including, but not limited to, accounts payable and accounts receivable, (ii) prepare bank reconciliations, (iii) assist with the preparation of quarterly and annual financial statements to be filed with the Securities and Exchange Commission (the “SEC”) and (iv) assist with the preparation of filings required by the SEC including, but not limited to, registration statements, current reports and proxy statement. Consulting expenses pursuant to the Consulting Agreement for the years ended September 30, 2016 and 2015 and the nine months ended September 30, 2014 were $48,000, $48,000 and $4,000, respectively.
On March 30, 2016, the Company entered into that certain Agreement and Plan of Merger by and among the Company, Citius LMB Acquisition Corp., a Delaware corporation and wholly-owned subsidiary of the Company (“SubCo”), and Leonard-Meron Biosciences, Inc., a Delaware corporation (“LMB”), pursuant to which SubCo was merged with and into LMB, with LMB continuing as the surviving corporation. Our Chairman of the Board, Leonard Mazur, and our Chief Executive Officer, Myron Holubiak, were co-founders and significant shareholders in LMB. In connection with the acquisition of LMB, our Chairman purchased an additional 5,000,000 shares of the Company for a purchase price of $3,000,000.
The Company entered into three-year employment agreements with Leonard Mazur and Myron Holubiak and granted options to certain of our Directors as more fully described, in all cases, in our Proxy Statement.
The Company executed demand promissory notes in favor of Leonard Mazur, Chairman of the Board, from 2016 through March 30, 2017 in the principal amount of $1,850,000 (the “Notes”). The Notes bear interest at the “Prime Rate” as published in the Wall Street Journal on the last day of the month plus 1%.
| 61 |
| Table of Contents |
Review, Approval or Ratification of Transactions with Related Persons
We intend to adopt a written related person transactions policy that our executive officers, directors, nominees for election as a director, beneficial owners of more than 5% of our Common Stock, and any members of the immediate family of and any entity affiliated with any of the foregoing persons, are not permitted to enter into a material related person transaction with us without the review and approval of our audit committee, or a committee composed solely of independent directors in the event it is inappropriate for our audit committee to review such transaction due to a conflict of interest. We expect the policy to provide that any request for us to enter into a transaction with an executive officer, director, nominee for election as a director, beneficial owner of more than 5% of our Common Stock or with any of their immediate family members or affiliates, in which the amount involved exceeds $120,000 will be presented to our audit committee for review, consideration and approval. In approving or rejecting any such proposal, we expect that our audit committee will consider the relevant facts and circumstances available and deemed relevant to the audit committee, including, but not limited to, whether the transaction is on terms no less favorable than terms generally available to an unaffiliated third party under the same or similar circumstances and the extent of the related person’s interest in the transaction.
Although we have not had a written policy for the review and approval of transactions with related persons, our board of directors has historically reviewed and approved any transaction where a director or officer had a financial interest, including all of the transactions described above. Prior to approving such a transaction, the material facts as to a director’s or officer’s relationship or interest as to the agreement or transaction were disclosed to our board of directors. Our board of directors would take this information into account when evaluating the transaction and in determining whether such transaction was fair to us and in the best interest of all of our stockholders.
Director Independence
After review of all relevant transactions or relationships between each director and nominee for director, or any of his or her family members, and the Company, its senior management and its Independent Registered Public Accounting Firm, the Board of Directors has determined that all of the Company's directors are independent within the meaning of the applicable NASDAQ listing standards, except Mr. Holubiak, the Chief Executive Officer, Chief Operating Officer, President and director of the Company and Leonard Mazur, the Executive Chairman and Secretary of the Company. Although the Company is not currently listed on an exchange, we believe it is in the Company's interests to comply with these standards both as a matter of good governance and to facilitate any future listing.
Audit Committee members must satisfy the independence criteria set forth in Rule 10A-3 under the Securities Exchange Act of 1934, as amended, for listed companies. In order to be considered to be independent for purposes of Rule 10A-3, a member of an audit committee of a listed company may not, other than in his or her capacity as a member of the audit committee, the Board of Directors or any other board committee: (1) accept, directly or indirectly, any consulting, advisory or other compensatory fee from the listed company or any of its subsidiaries; or (2) be an affiliated person of the listed company or any of its subsidiaries.
The following table set forth certain information regarding the selling stockholders and the shares of common stock beneficially owned by them, which information is available to us as of February 28, 2017. The selling stockholders may offer the shares under this prospectus from time to time and may elect to sell some, all or none of the shares set forth under this prospectus. However, for the purposes of the table below, we have assumed that, after completion of the offering, none of the shares covered by this prospectus will be held by the selling stockholders. In addition, a selling stockholder may have sold, transferred or otherwise disposed of all or a portion of that holder’s shares of common stock since the date on which the selling stockholder provided information for this table. We have not made independent inquiries about such transfers or dispositions. See the section entitled “Plan of Distribution” beginning on page 64.
| 62 |
| Table of Contents |
Beneficial ownership is determined in accordance with Rule 13d-3(d) promulgated by the SEC under the Securities Exchange Act of 1934, or the Exchange Act. The percentage of shares beneficially owned prior to the offering is based on 75,504,242 shares of our common stock outstanding as of February 28, 2017.
Selling Stockholder |
| Number of Shares of Common Stock Beneficially Owned Before Any Sale |
|
| % of Class |
|
| Number of Share of Common Stock Offering |
|
| Shares of Common Stock Beneficially Owned After |
| ||||||||
|
|
|
|
|
|
| Number of Shares |
|
| % of Class |
| |||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||
Antoni, David 1 |
|
| 1,000,000 |
|
|
| 1.32 | % |
|
| 1,000,000 |
|
|
| - |
|
|
| 0.00 | % |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Scott, Duncan 2 |
|
| 250,000 |
|
| * |
|
|
| 250,000 |
|
|
| - |
|
|
| 0.00 | % | |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
McLoughlin, Mick 3 |
|
| 325,000 |
|
| * |
|
|
| 325,000 |
|
|
| - |
|
|
| 0.00 | % | |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Deas, Richard R. 4 |
|
| 625,000 |
|
| * |
|
|
| 625,000 |
|
|
| - |
|
|
| 0.00 | % | |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Lipson MD, Adam C. 5 |
|
| 250,000 |
|
| * |
|
|
| 250,000 |
|
|
| - |
|
|
| 0.00 | % | |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Healy, Kevin 6 |
|
| 100,000 |
|
| * |
|
|
| 100,000 |
|
|
| - |
|
|
| 0.00 | % | |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Trafford, John 7 |
|
| 500,000 |
|
| * |
|
|
| 500,000 |
|
|
| - |
|
|
| 0.00 | % | |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Altshuler, Howard 8 |
|
| 100,000 |
|
| * |
|
|
| 100,000 |
|
|
| - |
|
|
| 0.00 | % | |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Dunlavy, Stanley 9 |
|
| 190,500 |
|
| * |
|
|
| 190,500 |
|
|
| - |
|
|
| 0.00 | % | |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Intracoastal Capital, LLC 10 |
|
| 500,000 |
|
| * |
|
|
| 500,000 |
|
|
| - |
|
|
| 0.00 | % | |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
CITIUS INVESTMENT FUND, LP 11 |
|
| 2,633,331 |
|
|
| 3.38 | % |
|
| 2,466,665 |
|
|
| 166,666 |
|
|
| 0.22 | % |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
CITIUS SPECIAL PURPOSE FUND, LLC 12 |
|
| 8,945,745 |
|
|
| 11.15 | % |
|
| 4,720,372 |
|
|
| 4,225,373 |
|
|
| 5.30 | % |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
THOMAS W. GENRICH 13 |
|
| 200,000 |
|
| * |
|
|
| 100,000 |
|
|
| 100,000 |
|
|
| 0.13 | % | |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
THE ENTRUST GROUP, INC FBO ROBERT G. CURTIN 14 |
|
| 100,000 |
|
| * |
|
|
| 50,000 |
|
|
| 50,000 |
|
|
| 0.07 | % | |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
THE ENTRUST GROUP, INC FBO SUSAN M. CURTIN 15 |
|
| 100,000 |
|
| * |
|
|
| 50,000 |
|
|
| 50,000 |
|
|
| 0.07 | % | |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
THE ENTRUST GROUP, INC. F/B/O ORVILLE A. WHITE 16 |
|
| 383,334 |
|
| * |
|
|
| 166,667 |
|
|
| 216,667 |
|
|
| 0.29 | % | |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
EQUITY DYNAMICS INC. F/B/O MATT KINLEY 401K 17 |
|
| 100,000 |
|
| * |
|
|
| 50,000 |
|
|
| 50,000 |
|
|
| 0.07 | % | |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
TOTAL |
|
| 16,302,910 |
|
|
| 19.17 | % |
|
| 11,444,204 |
|
|
| 4,858,706 |
|
|
| 6.05 | % |
* Represents beneficial ownership of less than one percent of the outstanding shares of our common stock.
____________
| 1) | Includes warrants to purchase 500,000 shares of common stock. |
| 2) | Includes warrants to purchase 125,000 shares of common stock. |
| 3) | Includes warrants to purchase 162,500 shares of common stock. |
| 63 |
| Table of Contents |
| 4) | Includes warrants to purchase 312,500 shares of common stock. |
| 5) | Includes warrants to purchase 125,000 shares of common stock. |
| 6) | Includes warrants to purchase 50,000 shares of common stock. |
| 7) | Includes warrants to purchase 250,000 shares of common stock. |
| 8) | Includes warrants to purchase 50,000 shares of common stock. |
| 9) | Includes (i) 49,750 shares of common stock and warrants to purchase 49,750 shares of common stock held by Stanley Dunlavy and (ii) 45,500 shares of common stock and warrants to purchase 45,500 shares of common stock hold by Stanley Dunlavy IRA. Includes warrants to purchase 95,250 shares of common stock. |
| 10) | Mitchell P. Kopin (“Mr. Kopin”) and Daniel B. Asher (“Mr. Asher”), each of whom are managers of Intracoastal Capital LLC (“Intracoastal”), have shared voting control and investment discretion over the securities reported herein that are held by Intracoastal. As a result, each of Mr. Kopin and Mr. Asher may be deemed to have beneficial ownership (as determined under Section 13(d) of the Securities Exchange Act of 1934, as amended (the “Exchange Act”)) of the securities reported herein that are held by Intracoastal. Includes warrants to purchase 250,000 shares of common stock. |
| 11) | Frank Cardia is the control person. Includes warrants to purchase 2,466,665 shares of common stock. |
| 12) | Joe McGowan is the control person. Includes warrants to purchase 4,720,372 shares of common stock. |
| 13) | Includes warrants to purchase 100,000 shares of common stock. |
| 14) | Robert G. Curtin may be deemed to beneficially own 200,000 shares of common stock, which consists of (i) 50,000 shares of common stock and warrants to purchase 50,000 shares of common stock held by The Entrust Group Inc. FBO Susan M. Curtin, and (ii) 50,000 shares of common stock and warrants to purchase 50,000 shares of common stock held by The Entrust Group Inc. FBO Robert G. Curtin.15) Susan M. Curtin may be deemed to beneficially own 200,000 shares of common stock, which consists of (i) 50,000 shares of common stock and warrants to purchase 50,000 shares of common stock held by The Entrust Group Inc. FBO Susan M. Curtin, and (ii) 50,000 shares of common stock and warrants to purchase 50,000 shares of common stock held by The Entrust Group Inc. FBO Robert G. Curtin. |
| 16) | Includes warrants to purchase 166,667 shares of common stock. |
| 17) | Includes warrants to purchase 50,000 shares of common stock. |
Information about any other selling stockholders will be included in prospectus supplements or post-effective amendments, if required. Information about the selling stockholders may change from time to time. Any changed information with respect to which we are given notice will be included in prospectus supplements.
PLAN OF DISTRIBUTION
We are registering the shares of common stock offered in this prospectus on behalf of the selling stockholders. The term selling stockholders, which as used herein includes pledgees, donees, transferees or other successors-in-interest selling shares received from the selling stockholders as a gift, pledge, partnership distribution or other transfer after the date of this prospectus, may, from time to time, sell, transfer or otherwise dispose of any or all of their shares of common stock or interests in common stock on any stock exchange, market or trading facility on which the shares are traded or in private transactions. The selling stockholders will pay any brokerage commissions and similar selling expenses attributable to the sale of the shares. We will not receive any of the proceeds from the sale of the shares by the selling stockholders.
These dispositions may be at fixed prices, at prevailing market prices at the time of sale, at prices related to the prevailing market price, at varying prices determined at the time of sale, or at negotiated prices. To the extent a selling stockholder gifts, pledges or otherwise transfers the shares offered hereby, such transferees may offer and sell the shares from time to time under this prospectus, provided that this prospectus has been amended under Rule 424(b)(3) or other applicable provision of the Securities Act to include the name of such transferee in the list of selling stockholders under this prospectus.
The selling stockholders may use any one or more of the following methods when disposing of shares or interests therein:
| · | on any national securities exchange or quotation service on which the common stock may be listed or quoted at the time of sale; |
| · | in the over-the-counter market; |
| · | in transactions otherwise than on these exchanges or systems or in the over-the-counter market; |
| · | ordinary brokerage transactions and transactions in which the broker-dealer solicits purchasers; |
| · | block trades in which the broker-dealer will attempt to sell the shares as agent, but may position and resell a portion of the block as principal to facilitate the transaction; |
| 64 |
| Table of Contents |
| · | purchases by a broker-dealer as principal and resale by the broker-dealer for its account; |
| · | an exchange distribution in accordance with the rules of the applicable exchange; |
| · | privately negotiated transactions; |
| · | short sales; |
| · | sales pursuant to Rule 144; |
| · | through the writing or settlement of options or other hedging transactions, whether through an options exchange or otherwise; |
| · | broker-dealers may agree with a selling stockholder to sell a specified number of such shares at a stipulated price per share; |
| · | a combination of any such methods of sale; and |
| · | any other method permitted pursuant to applicable law. |
The selling stockholders may, from time to time, pledge or grant a security interest in some or all of the shares of common stock owned by them and, if they default in the performance of their secured obligations, the pledgees or secured parties may offer and sell the shares of common stock, from time to time, under this prospectus, or under an amendment to this prospectus under Rule 424(b)(3) or other applicable provision of the Securities Act amending the list of selling stockholders to include the pledgee, transferee or other successors in interest as selling stockholders under this prospectus.
In connection with the sale of our common stock or interests therein, the selling stockholders may enter into hedging transactions with broker-dealers or other financial institutions, which may in turn engage in short sales of the common stock in the course of hedging the positions they assume. The selling stockholders may also sell shares of common stock short and deliver these securities to close out its short positions, or loan or pledge the common stock to broker-dealers that in turn may sell these securities. The selling stockholders may also enter into option or other transactions with broker-dealers or other financial institutions or the creation of one or more derivative securities which require the delivery to such broker-dealer or other financial institution of shares offered by this prospectus, which shares such broker-dealer or other financial institution may resell pursuant to this prospectus (as supplemented or amended to reflect such transaction).
The aggregate proceeds to a selling stockholder from the sale of the shares of common stock offered by it will be the purchase price of the common stock less discounts or commissions, if any. Each selling stockholder reserves the right to accept and, together with its agents from time to time, to reject, in whole or in part, any proposed purchase of common stock to be made directly or through agents.
To the extent required, the shares of common stock to be sold, the names of the selling stockholders, the respective purchase prices and public offering prices, the names of any agents, dealer or underwriter, any applicable commissions or discounts with respect to a particular offer will be set forth in an accompanying prospectus supplement or, if appropriate, a post-effective amendment to the registration statement that includes this prospectus.
| 65 |
| Table of Contents |
In order to comply with the securities laws of some states, if applicable, the common stock may be sold in these jurisdictions only through registered or licensed brokers or dealers. In addition, in some states the common stock may not be sold unless it has been registered or qualified for sale or an exemption from registration or qualification requirements is available and is complied with.
We have advised the selling stockholders that the anti-manipulation rules of Regulation M under the Exchange Act may apply to sales of shares in the market and to the activities of the selling stockholders and its affiliates. In addition, we will make copies of this prospectus (as it may be supplemented or amended from time to time) available to the selling stockholders for the purpose of satisfying the prospectus delivery requirements of the Securities Act.
The selling stockholders and any broker dealers that act in connection with the sale of the shares might be deemed to be “underwriters” as the term is defined in Section 2(11) of the Securities Act. Consequently, any commissions received by these broker dealers and any profit on the resale of the shares sold by them while acting as principals might be deemed to be underwriting discounts or commissions under the Securities Act. Because a selling stockholder may be deemed to be an “underwriter” as defined in Section 2(11) of the Securities Act, the selling stockholders may be subject to the prospectus delivery requirements of the Securities Act.
The selling stockholders also may resell all or a portion of the shares in open market transactions in reliance upon Rule 144 under the Securities Act of 1933, provided that such sale meets the criteria and conform to the requirements of that Rule.
Broker-dealers engaged by the selling stockholders may arrange for other broker-dealers to participate in sales. Broker-dealers may receive commissions or discounts from the selling stockholders (or, if any broker-dealer acts as agent for the purchaser of shares, from the purchaser) in amounts to be negotiated. The selling stockholders do not expect these commissions and discounts to exceed what is customary in the types of transactions involved. No such broker-dealer will receive compensation in excess of that permitted by NASD Rule 2440 and IM-2440. Any profits on the resale of shares of common stock by a broker-dealer acting as principal might be deemed to be underwriting discounts or commissions under the Securities Act. Discounts, concessions, commissions and similar selling expenses, if any, attributable to the sale of shares will be borne by the selling stockholders. The selling stockholders may agree to indemnify any agent, dealer or broker-dealer that participates in transactions involving sales of the shares if liabilities are imposed on that agent, dealer or broker-dealer under the Securities Act.
SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT
The following table sets forth information known to us with respect to the beneficial ownership of Citius Pharmaceuticals, Inc. Common Stock as of January 31, 2017, unless otherwise noted, by:
· | each stockholder known to own beneficially more than 5% of our Common Stock; | |
· | each of our directors and executive officers; and | |
· | all of our current directors and executive officers as a group. |
Beneficial ownership is determined in accordance with the rules of the SEC and generally includes voting or dispositive power with respect to securities. Shares relating to options or warrants currently exercisable, or exercisable within 60 days of February 28, 2017, are deemed outstanding for computing the percentage of the person holding such securities but are not deemed outstanding for computing the percentage of any other person. Percentage of ownership is based on 75,504,242 shares of Common Stock outstanding on February 28, 2017. Except as indicated by footnote and subject to the community property laws where applicable, the persons or entities named in the tables have sole voting and investment power with respect to all shares shown as beneficially owned by them. Except as otherwise noted below, the address for each director or executive officer listed in the table is c/o Citius Pharmaceuticals, Inc., 11 Commerce Drive, First Floor, Cranford, NJ 07016.
| 66 |
| Table of Contents |
Name of Beneficial Owner |
| Number of Shares of Common Stock Beneficially Owned |
| Percentage of Shares of Common Stock Beneficially Owned | ||||
| ||||||||
Named Executive Officers and Directors: |
| |||||||
Myron Holubiak |
| 8,448,715 |
| 11.09 | %(1) | |||
Leonard Mazur |
| 25,280,223 |
| 31.44 | %(2) | |||
Suren Dutia |
| 400,000 |
| * | (3) | |||
Carol Webb |
| 181,057 |
| * | (4) | |||
Dr. William Kane |
| 181,057 |
| * | (4) | |||
Dr. Eugene Holuka |
| 36,212 |
| * | (5) | |||
Howard Safir |
| 181,057 |
| * | (6) | |||
All executive officers and directors as a group |
| 34,708,321 |
| 42.28 | % | |||
| ||||||||
Other Stockholders: |
| |||||||
Geoffrey Clark |
| 7,432,506 |
| 9.84 | %(7) | |||
Reinier Beeuwkes |
| 8,013,959 |
| 10.61 | %(8) | |||
Citius Special Purpose Fund |
| 8,995,745 |
| 11.15 | %(9) | |||
|
|
|
|
|
|
|
|
|
Lifestyle Healthcare LLC |
| 5,262,392 |
| 6.82 | %(10) | |||
___________________
(1) | Includes (i) 7,754,498 shares of Common Stock, (ii) an option to purchase 400,000 shares of Common Stock at an exercise price of $.54 per share and (iii) a warrant to purchase 294,217 shares of Common Stock at an exercise price of $.41 per share. |
(2) | Includes (i) 20,373,889 shares of Common Stock held by Leonard Mazur, (ii) an option to purchase 2,800,000 shares of Common Stock at an exercise price of $.45 per share and (iii) warrants to purchase an aggregate of 2,106,334 shares of Common Stock at a weighted average exercise price of $.47. |
(3) | Includes an option to purchase 400,000 shares of Common Stock at an exercise price of $.54 per share. |
(4) | Includes an option to purchase 181,057 shares of Common Stock at an exercise price of $.001 per share. |
(5) | Includes an option to purchase 36,212 shares of Common Stock at an exercise price of $.001 per share. |
(6) | Includes an option to purchase 181,057 shares of Common Stock at an exercise price of $.001 per share. |
(7) | Geoffrey Clark is the trustee of Geoffrey C. Clark Revocable Trust, and in such capacities he is deemed to hold voting and dispositive power over the securities held by such entity. Includes 7,432,506 shares of Common Stock held by Geoffrey C. Clark Revocable Trust. Geoffrey Clark resigned as executive officer and director upon completion of the Reverse Acquisition on September 12, 2014. |
(8) | Reinier Beeuwkes resigned as executive officer and director upon completion of the Reverse Acquisition on September 12, 2014. |
(9) | Joe McGowan is the control person, and in such capacity he is deemed to hold voting and dispositive power over the securities held by such entity. Includes (i) 4,225,373 shares of Common Stock and (ii) a warrant to purchase 4,720,372 shares of Common Stock at an exercise price of $.60 per share. |
(10) | Nickolay Kukekov is the manager, and in such capacity he is deemed to hold voting and dispositive power over the securities held by such entity. Includes (i) 3,562,325 shares of Common Stock and (ii) a warrant to purchase 1,700,067 shares of Common Stock at an exercise price of $.60 per share. |
* | Less than 1%. |
| 67 |
| Table of Contents |
The following description summarizes the material terms of Citius capital stock as of the date of this Memorandum. Because it is only a summary, it does not contain all the information that may be important to you. For a complete description of our capital stock, you should refer to our certificate of incorporation and our bylaws, and to the provisions of applicable Nevada law.
General
Our authorized capital stock consists of 200,000,000 shares of Common Stock, par value $0.001, 75,504,242 shares of which are issued and outstanding as of February 28, 2017, and 10,000,000 shares of preferred stock, none of which are issued and outstanding. Our preferred stock and/or Common Stock may be issued from time to time without prior approval by our stockholders. Our preferred stock and/or Common Stock may be issued for such consideration as may be fixed from time to time by our Board of Directors. Our Board of Directors may issue such shares of our preferred stock and/or Common Stock in one or more series, with such voting powers, designations, preferences and rights or qualifications, limitations or restrictions thereof as shall be stated in the resolution or resolutions.
Common Stock
The Company, a Nevada corporation, is authorized to issue 200,000,000 shares of Common Stock, $0.001 par value. Each share of Common Stock shall have one (1) vote per share for all purposes. The holders of a majority of the shares entitled to vote, present in person or represented by proxy shall constitute a quorum at all meetings of our stockholders. Our Common Stock does not provide preemptive, subscription or conversion rights and there are no redemption or sinking fund provisions or rights. Our Common Stock holders are not entitled to cumulative voting for election of the Board of Directors.
Holders of Common Stock are entitled to receive ratably such dividends as may be declared by the Board of Directors out of funds legally available therefore as well as any distributions to the security holder. We have never paid cash dividends on our Common Stock, and do not expect to pay such dividends in the foreseeable future.
In the event of a liquidation, dissolution or winding up of our company, holders of Common Stock are entitled to share ratably in all of our assets remaining after payment of liabilities. Holders of Common Stock have no preemptive or other subscription or conversion rights. There are no redemption or sinking fund provisions applicable to the Common Stock.
Preferred Stock
Our Board of Directors is authorized to cause us to issue, from our authorized but unissued shares of preferred stock, one or more series of preferred stock, to establish from time to time the number of shares to be included in each such series, as well as to fix the designation and any preferences, conversion and other rights and limitations of such series. These rights and limitations may include voting powers, limitations as to dividends, and qualifications and terms and conditions of redemption of the shares of each such series.
Units
In a private offering commenced in October 2016 (the “2016 Offering”), we sold 1,920,250 Units, each Unit consisting of (i) one share of Common Stock and (ii) Warrants to purchase one share of Common Stock (a “Warrant Share”). Each Warrant has an exercise price of $0.55 and is exercisable for a period of five years from the date of issuance.
| 68 |
| Table of Contents |
Warrants Issued as Part of the Units
Each Warrant issued to investors in the 2016 Offering entitles the registered holder to purchase one share of our Common Stock at a price of $0.55 per share, with such exercise price to be subject to adjustment as set forth in the warrant agreement. The Warrants have a five-year term and a cashless exercise if there is no effective registration statement covering the resale of the shares underlying the warrants. The Warrants are redeemable at the Company’s option provided the shares underlying the warrants can be sold pursuant to an effective registration statement filed with the SEC, upon the date the Company’s Common Stock has traded for $2.00 per share for any 17 out of 20 consecutive days and the aggregate trading volume per day during that period is a minimum of 100,000 shares. The Warrants shall not provide for price or share based adjustments due to dilutive issuances of equity securities, other than stock splits, cash dividends, or the like.
Options
On September 12, 2014, our stockholders approved the Company’s 2014 Stock Incentive Plan, which provides for the award of stock options, stock appreciation rights, restricted stock and other equity awards for up to an aggregate of 13,000,000 shares of common stock. The shares of common stock underlying any awards that are forfeited, canceled, reacquired by us prior to vesting, satisfied without any issuance of stock, expire or are otherwise terminated (other than by exercise) under the 2014 Plan will be added back to the shares of common stock available for issuance under the 2014 Plan.
As of December 31, 2016, we had outstanding options to purchase an aggregate of 8,732,770 shares of our common stock at a weighted average exercise price of $0.54 per share. Of these, an aggregate of 5,286,654 are exercisable. The remainder has vesting requirements.
The 2014 Plan is administered by our Board or a committee designated by the Board (as applicable, the Administrator). The Administrator has full power to select, from among the individuals eligible for awards, the individuals to whom awards will be granted, to make any combination of awards to participants, and to determine the specific terms and conditions of each award, subject to the provisions of the 2014 Plan. The Administrator may delegate to our Chief Executive Officer the authority to grant stock options and other awards to employees who are not subject to the reporting and other provisions of Section 16 of the Exchange Act and not subject to Section 162(m) of the Code, subject to certain limitations and guidelines.
Persons eligible to participate in the 2014 Plan are full or part-time officers, employees, non-employee directors, directors and other key persons (including consultants and prospective officers) of our company and its subsidiaries as selected from time to time by the Administrator in its discretion.
The 2014 Plan provides that upon the effectiveness of a “sale event” as defined in the 2014 Plan, except as otherwise provided by the Administrator in the award agreement, all stock options, stock appreciation rights and other awards will be assumed or continued by the successor entity and adjusted accordingly to take into account the impact of the transaction. To the extent, however, that the parties to such sale event do not agree that all stock options, stock appreciation rights or any other awards shall be assumed or continued, then such stock options and stock appreciation rights shall become fully exercisable and the restrictions and conditions on all such other awards with time-based conditions will automatically be deemed waived. Awards with conditions and restrictions relating to the attainment of performance goals may become vested and non-forfeitable in connection with a sale event in the Administrator’s discretion. In addition, in the case of a sale event in which our stockholders will receive cash consideration, we may make or provide for a cash payment to participants holding options and stock appreciation rights equal to the difference between the per share cash consideration and the exercise price of the options or stock appreciation rights in exchange for the cancellation thereto.
Trading Market
The shares of our Common Stock are currently quoted on the OTCQB under the symbol “CTXR.QB”.
| 69 |
| Table of Contents |
Transfer Agent
The transfer agent of our common stock is VStock Transfer. Their address is 18 Lafayette Place, Woodmere, NY 11598.
Nevada’s Anti-Takeover Law and Provisions of Our Articles of Incorporation and Bylaws
Acquisition of Controlling Interest Statutes. Nevada’s “acquisition of controlling interest” statutes contain provisions governing the acquisition of a controlling interest in certain Nevada corporations. These “control share” laws provide generally that any person that acquires a “controlling interest” in certain Nevada corporations may be denied certain voting rights, unless a majority of the disinterested stockholders of the corporation elects to restore such voting rights. These statutes provide that a person acquires a “controlling interest” whenever a person acquires shares of a subject corporation that, but for the application of these provisions of the Nevada Revised Statutes, would enable that person to exercise (1) one-fifth or more, but less than one-third, (2) one-third or more, but less than a majority or (3) a majority or more, of all of the voting power of the corporation in the election of directors. Once an acquirer crosses one of these thresholds, shares which it acquired in the transaction taking it over the threshold and within the 90 days immediately preceding the date when the acquiring person acquired or offered to acquire a controlling interest become “control shares” to which the voting restrictions described above apply. Our articles of incorporation and bylaws currently contain no provisions relating to these statutes, and unless our articles of incorporation or bylaws in effect on the tenth day after the acquisition of a controlling interest were to provide otherwise, these laws would apply to us if we were to (i) have 200 or more stockholders of record (at least 100 of which have addresses in the State of Nevada appearing on our stock ledger) and (ii) do business in the State of Nevada directly or through an affiliated corporation. As of August 1, 2016, we have 111 record stockholders and do not have 100 stockholders of record with Nevada addresses appearing on our stock ledger. If these laws were to apply to us, they might discourage companies or persons interested in acquiring a significant interest in or control of the Company, regardless of whether such acquisition may be in the interest of our stockholders.
Combination with Interested Stockholders Statutes. Nevada’s “combinations with interested stockholders” statutes prohibit certain business “combinations” between certain Nevada corporations and any person deemed to be an “interested stockholder” for two years after such person first becomes an “interested stockholder” unless (i) the corporation’s Board of Directors approves the combination (or the transaction by which such person becomes an “interested stockholder”) in advance, or (ii) the combination is approved by the Board of Directors and sixty percent of the corporation’s voting power not beneficially owned by the interested stockholder, its affiliates and associates. Furthermore, in the absence of prior approval certain restrictions may apply even after such two-year period. For purposes of these statutes, an “interested stockholder” is any person who is (x) the beneficial owner, directly or indirectly, of ten percent or more of the voting power of the outstanding voting shares of the corporation, or (y) an affiliate or associate of the corporation and at any time within the two previous years was the beneficial owner, directly or indirectly, of ten percent or more of the voting power of the then outstanding shares of the corporation. The definition of the term “combination” is sufficiently broad to cover most significant transactions between the corporation and an “interested stockholder”. Subject to certain timing requirements set forth in the statutes, a corporation may elect not to be governed by these statutes. We have not included any such provision in our articles of incorporation.
The effect of these statutes may be to potentially discourage parties interested in taking control of the Company from doing so if it cannot obtain the approval of our Board of Directors
Articles of Incorporation and Bylaws. Provisions of our certificate of incorporation and bylaws may delay or discourage transactions involving an actual or potential change of control or change in our management, including transactions in which stockholders might otherwise receive a premium for their shares, or transactions that our stockholders might otherwise deem to be in their best interests. Therefore, these provisions could adversely affect the price of our common stock. Among other things, these provisions include:
| · | the authorization of 10,000,000 shares of “blank check” preferred stock, the rights, preferences and privileges of which may be established and shares of which may be issued by our Board of Directors at its discretion from time to time and without stockholder approval; |
| · | limiting the removal of directors by the stockholders; |
| · | allowing for the creation of a staggered Board of Directors; |
| · | eliminating the ability of stockholders to call a special meeting of stockholders; and |
| · | establishing advance notice requirements for nominations for election to the Board of Directors or for proposing matters that can be acted upon at stockholder meetings. |
| 70 |
| Table of Contents |
The validity of the shares of common stock being offered by this prospectus will be passed upon for us by Wyrick Robbins Yates & Ponton, LLP, Raleigh, North Carolina.
The financial statements of Citius Pharmaceuticals, Inc. included in this prospectus at September 30, 2016 and 2015, and for each of the two years in the period ended September 30, 2016 and the nine months ended September 30, 2014, have been audited by Wolf & Company, P.C., independent registered public accounting firm, as set forth in their report thereon appearing elsewhere herein, and are included in reliance upon such report given on the authority of such firm as experts in accounting and auditing.
WHERE YOU CAN FIND MORE INFORMATION
This prospectus, which constitutes a part of the registration statement on Form S-1 that we have filed with the SEC under the Securities Act, does not contain all of the information in the registration statement and its exhibits. For further information with respect to us and the common stock offered by this prospectus, you should refer to the registration statement and the exhibits filed as part of that document. Statements contained in this prospectus as to the contents of any contract or any other document referred to are not necessarily complete, and in each instance, we refer you to the copy of the contract or other document filed as an exhibit to the registration statement. Each of these statements is qualified in all respects by this reference.
We are subject to the reporting requirements of the Securities Exchange Act of 1934, as amended, and file annual, quarterly and current reports, proxy statements and other information with the SEC. You can read our SEC filings, including the registration statement, over the Internet at the SEC’s website at http://www.sec.gov. We also maintain a website at http://www.citiuspharma.com, at which you may access these materials free of charge as soon as reasonably practicable after they are electronically filed with, or furnished to, the SEC. The information contained in, or that can be accessed through, our website is not part of this prospectus.
You may also read and copy any document we file with the SEC at its public reference facilities at 100 F Street, N.E., Room 1580, Washington, D.C. 20549. You may also obtain copies of these documents at prescribed rates by writing to the Public Reference Section of the SEC at 100 F Street, N.E., Washington, D.C. 20549. Please call the SEC at 1-800-SEC-0330 for further information on the operation of the public reference facilities. You may also request a copy of these filings, at no cost, by writing or telephoning us at: 11 Commerce Drive, First Floor, Cranford, New Jersey 07016, (908) 967-6677.
| 71 |
| Table of Contents |
INDEX TO THE FINANCIAL STATEMENTS
CITIUS PHARMACEUTICALS, INC.
|
| Page |
| |
|
|
|
| |
Audited Financial Statements |
|
|
| |
|
| F-2 |
| |
|
| F-3 |
| |
|
| F-4 |
| |
Condensed Consolidated Statements of Changes in Stockholders’ Equity (Deficit) |
|
| F-5 |
|
|
| F-6 |
| |
|
| F-7 |
| |
|
| Page |
| |
|
|
|
| |
| Unaudited Interim Financial Statements |
|
|
| |
|
| F-21 |
| |
|
| F-22 |
| |
Condensed Consolidated Statements of Changes in Stockholders’ Equity (Deficit) |
|
| F-23 |
|
|
| F-24 |
| |
|
| F-25 |
| |
| F-1 |
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors and Stockholders of Citius Pharmaceuticals, Inc.
We have audited the accompanying consolidated balance sheets of Citius Pharmaceuticals, Inc. as of September 30, 2016 and 2015, and the related consolidated statements of operations, changes in stockholders' equity (deficit) and cash flows for the years ended September 30, 2016 and 2015, and the nine month period ended September 30, 2014. These financial statements are the responsibility of the Company's management. Our responsibility is to express an opinion on these consolidated financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audits to obtain reasonable assurance about whether the financial statements are free of material misstatement. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. Our audits included consideration of internal control over financial reporting as a basis for designing audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion. An audit also includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the consolidated financial statements referred to above present fairly, in all material respects, the financial position of Citius Pharmaceuticals, Inc. as of September 30, 2016 and 2015, and the results of its operations and its cash flows for the years ended September 30, 2016 and 2015, and the nine month period ended September 30, 2014, in conformity with U.S. generally accepted accounting principles.
The accompanying consolidated financial statements have been prepared assuming that the Company will continue as a going concern. As discussed in Note 2 to the consolidated financial statements, the Company has suffered recurring losses from operations, has negative cash flows from operations, and has a significant accumulated deficit. These conditions raise substantial doubt about the Company’s ability to continue as a going concern. Management’s plans in regard to these matters are also described in Note 2. The consolidated financial statements do not include any adjustments that might result from the outcome of this uncertainty.
/s/ Wolf & Company, P.C.
Boston, Massachusetts
December 23, 2016
| F-2 |
| Table of Contents |
CITIUS PHARMACEUTICALS, INC.
CONSOLIDATED BALANCE SHEETS
SEPTEMBER 30, 2016 AND 2015
|
| 2016 |
|
| 2015 |
| ||
|
|
|
|
|
|
| ||
ASSETS | ||||||||
|
|
|
|
|
|
| ||
Current Assets: |
|
|
|
|
|
| ||
Cash and cash equivalents |
| $ | 294,351 |
|
| $ | 676,137 |
|
Prepaid expenses |
|
| 598,484 |
|
|
| 60,000 |
|
Total Current Assets |
|
| 892,835 |
|
|
| 736,137 |
|
|
|
|
|
|
|
|
|
|
Property and Equipment, Net of Accumulated Depreciation of $4,780 |
|
| 3,742 |
|
|
| — |
|
|
|
|
|
|
|
|
|
|
Other Assets: |
|
|
|
|
|
|
|
|
Trademarks |
|
| — |
|
|
| 5,401 |
|
Deposits |
|
| 2,167 |
|
|
| — |
|
Deferred offering costs |
|
| 64,801 |
|
|
| — |
|
In-process research and development |
|
| 19,400,000 |
|
|
| — |
|
Goodwill |
|
| 1,586,796 |
|
|
| — |
|
Total Other Assets |
|
| 21,053,764 |
|
|
| 5,401 |
|
|
|
|
|
|
|
|
|
|
Total Assets |
| $ | 21,950,341 |
|
| $ | 741,538 |
|
|
|
|
|
|
|
|
|
|
LIABILITIES AND STOCKHOLDERS’ EQUITY (DEFICIT) | ||||||||
|
|
|
|
|
|
|
|
|
Current Liabilities: |
|
|
|
|
|
|
|
|
Accounts payable |
| $ | 909,156 |
|
| $ | 559,150 |
|
Accrued expenses |
|
| 958,101 |
|
|
| 8,260 |
|
Accrued compensation |
|
| 903,250 |
|
|
| — |
|
Accrued interest |
|
| 30,871 |
|
|
| — |
|
Notes payable – related parties |
|
| 672,970 |
|
|
| — |
|
Derivative warrant liability |
|
| 1,681,973 |
|
|
| 738,955 |
|
Due to related party |
|
| 27,637 |
|
|
| 70,386 |
|
Total Current Liabilities |
|
| 5,183,958 |
|
|
| 1,376,751 |
|
|
|
|
|
|
|
|
|
|
Commitments and Contingencies |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Stockholders’ Equity (Deficit): |
|
|
|
|
|
|
|
|
Preferred stock - $0.001 par value; 10,000,000 shares authorized; no shares issued and outstanding |
|
| — |
|
|
| — |
|
Common stock - $0.001 par value; 200,000,000 shares authorized; 73,138,060 and 34,117,886 shares issued and outstanding at September 30, 2016 and 2015, respectively |
|
| 73,138 |
|
|
| 34,118 |
|
Additional paid-in capital |
|
| 34,029,492 |
|
|
| 8,371,218 |
|
Accumulated deficit |
|
| (17,336,247 | ) |
|
| (9,040,549 | ) |
Total Stockholders’ Equity (Deficit) |
|
| 16,766,383 |
|
|
| (635,213 | ) |
|
|
|
|
|
|
|
|
|
Total Liabilities and Stockholders’ Equity (Deficit) |
| $ | 21,950,341 |
|
| $ | 741,538 |
|
See accompanying report of independent registered public accounting firm and notes to the consolidated financial statements.
| F-3 |
| Table of Contents |
CONSOLIDATED STATEMENTS OF OPERATIONS
|
| Year September 30, 2016 |
|
| Year September 30, 2015 |
|
| Nine Months September 30, 2014 |
| |||
|
|
|
|
|
|
|
|
|
| |||
Revenues |
| $ | — |
|
| $ | — |
|
| $ | — |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Operating Expenses: |
|
|
|
|
|
|
|
|
|
|
|
|
Research and development |
|
| 2,933,199 |
|
|
| 1,797,045 |
|
|
| 574 |
|
General and administrative |
|
| 3,783,941 |
|
|
| 946,613 |
|
|
| 183,044 |
|
Stock-based compensation – general and administrative |
|
| 732,151 |
|
|
| 486,271 |
|
|
| 470,185 |
|
Total Operating Expenses |
|
| 7,449,291 |
|
|
| 3,229,929 |
|
|
| 653,803 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Operating Loss |
|
| (7,449,291 | ) |
|
| (3,229,929 | ) |
|
| (653,803 | ) |
|
|
|
|
|
|
|
|
|
|
|
|
|
Other Income (Expense), Net: |
|
|
|
|
|
|
|
|
|
|
|
|
Interest income |
|
| 806 |
|
|
| 3,066 |
|
|
| 555 |
|
Gain (loss) on revaluation of derivative warrant liability |
|
| (838,219 | ) |
|
| 332,095 |
|
|
| 8,588 |
|
Interest expense |
|
| (8,994 | ) |
|
| (7,500 | ) |
|
| (93,067 | ) |
Total Other Income (Expense), Net |
|
| (846,407 | ) |
|
| 327,661 |
|
|
| (83,924 | ) |
|
|
|
|
|
|
|
|
|
|
|
|
|
Loss before Income Taxes |
|
| (8,295,698 | ) |
|
| (2,902,268 | ) |
|
| (737,727 | ) |
Income tax benefit |
|
| — |
|
|
| — |
|
|
| — |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net Loss |
| $ | (8,295,698 | ) |
| $ | (2,902,268 | ) |
| $ | (737,727 | ) |
|
|
|
|
|
|
|
|
|
|
|
|
|
Net Loss Per Share - Basic and Diluted |
| $ | (0.15 | ) |
| $ | (0.09 | ) |
| $ | (0.04 | ) |
|
|
|
|
|
|
|
|
|
|
|
|
|
Weighted Average Common Shares Outstanding |
|
|
|
|
|
|
|
|
|
|
|
|
Basic and diluted |
|
| 54,348,120 |
|
|
| 31,835,440 |
|
|
| 19,322,206 |
|
See accompanying report of independent registered public accounting firm and notes to the consolidated financial statements.
| F-4 |
| Table of Contents |
CONSOLIDATED STATEMENTS OF CHANGES IN STOCKHOLDERS' EQUITY (DEFICIT)
FOR THE YEARS ENDED SEPTEMBER 30, 2016 AND 2015, AND
THE NINE MONTHS ENDED SEPTEMBER 30, 2014
|
|
|
|
|
|
|
|
|
|
|
|
|
| Total |
| |||||||||
|
|
|
|
|
|
|
| Additional |
|
|
|
|
| Stockholders' |
| |||||||||
|
| Preferred |
|
| Common Stock |
|
| Paid-In |
|
| Accumulated |
|
| Equity |
| |||||||||
|
| Stock |
|
| Shares |
|
| Amount |
|
| Capital |
|
| Deficit |
|
| (Deficit) |
| ||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| ||||||
Balance, January 1, 2014 |
| $ | — |
|
|
| 17,757,342 |
|
| $ | 17,757 |
|
| $ | 2,481,043 |
|
| $ | (5,400,554 | ) |
| $ | (2,901,754 | ) |
Issuance of common stock |
|
| — |
|
|
| 200,000 |
|
|
| 200 |
|
|
| 49,800 |
|
|
| — |
|
|
| 50,000 |
|
Conversion of subordinated convertible promissory note and accrued interest |
|
| — |
|
|
| 606,531 |
|
|
| 607 |
|
|
| 393,638 |
|
|
| — |
|
|
| 394,245 |
|
Conversion of convertible promissory notes and accrued interest |
|
| — |
|
|
| 3,061,355 |
|
|
| 3,061 |
|
|
| 1,833,752 |
|
|
| — |
|
|
| 1,836,813 |
|
Issuance of common stock in private placement, net of costs |
|
| — |
|
|
| 3,400,067 |
|
|
| 3,400 |
|
|
| 142,903 |
|
|
| — |
|
|
| 146,303 |
|
Issuance of common stock in reverse acquisition |
|
| — |
|
|
| 5,000,000 |
|
|
| 5,000 |
|
|
| (5,000 | ) |
|
| — |
|
|
| — |
|
Stock-based compensation |
|
| — |
|
|
| — |
|
|
| — |
|
|
| 470,185 |
|
|
| — |
|
|
| 470,185 |
|
Net loss |
|
| — |
|
|
| — |
|
|
| — |
|
|
| — |
|
|
| (737,727 | ) |
|
| (737,727 | ) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance, September 30, 2014 |
|
| — |
|
|
| 30,025,295 |
|
|
| 30,025 |
|
|
| 5,366,321 |
|
|
| (6,138,281 | ) |
|
| (741,935 | ) |
Conversion of promissory notes and accrued interest |
|
| — |
|
|
| 1,055,554 |
|
|
| 1,056 |
|
|
| 632,277 |
|
|
| — |
|
|
| 633,333 |
|
Issuance of common stock in private placement, net of costs |
|
| — |
|
|
| 3,037,037 |
|
|
| 3,037 |
|
|
| 738,021 |
|
|
| — |
|
|
| 741,058 |
|
Reclassification of derivative warrant liability to additional paid-in capital |
|
| — |
|
|
| — |
|
|
| — |
|
|
| 1,148,328 |
|
|
| — |
|
|
| 1,148,328 |
|
Stock-based compensation |
|
| — |
|
|
| — |
|
|
| — |
|
|
| 486,271 |
|
|
| — |
|
|
| 486,271 |
|
Net loss |
|
| — |
|
|
| — |
|
|
| — |
|
|
| — |
|
|
| (2,902,268 | ) |
|
| (2,902,268 | ) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance, September 30, 2015 |
|
| — |
|
|
| 34,117,886 |
|
| $ | 34,118 |
|
| $ | 8,371,218 |
|
| $ | (9,040,549 | ) |
| $ | (635,213 | ) |
Issuance of common stock in private placement, net of costs |
|
| — |
|
|
| 9,616,668 |
|
|
| 9,616 |
|
|
| 4,219,508 |
|
|
| — |
|
|
| 4,229,124 |
|
Issuance of common stock for services |
|
| — |
|
|
| 266,667 |
|
|
| 267 |
|
|
| 149,733 |
|
|
| — |
|
|
| 150,000 |
|
Issuance of common stock, warrants and stock options for acquisition |
|
| — |
|
|
| 29,136,839 |
|
|
| 29,137 |
|
|
| 18,985,936 |
|
|
| — |
|
|
| 19,015,073 |
|
Issuance of warrants for services |
|
| — |
|
|
| — |
|
|
| — |
|
|
| 477,181 |
|
|
| — |
|
|
| 477,181 |
|
Reclassification of derivative warrant liability to additional paid-in capital |
|
| — |
|
|
| — |
|
|
| — |
|
|
| 1,093,765 |
|
|
| — |
|
|
| 1,093,765 |
|
Stock-based compensation |
|
| — |
|
|
| — |
|
|
| — |
|
|
| 732,151 |
|
|
| — |
|
|
| 732,151 |
|
Net loss |
|
| — |
|
|
| — |
|
|
| — |
|
|
| — |
|
|
| (8,295,698 | ) |
|
| (8,295,698 | ) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance, September 30, 2016 |
|
| — |
|
|
| 73,138,060 |
|
| $ | 73,138 |
|
| $ | 34,029,492 |
|
| $ | (17,336,247 | ) |
| $ | 16,766,383 |
|
See accompanying report of independent registered public accounting firm and notes to the consolidated financial statements.
| F-5 |
| Table of Contents |
CONSOLIDATED STATEMENTS OF CASH FLOWS
|
| Year |
|
| Year |
|
| Nine Months |
| |||
|
|
|
|
|
|
|
|
|
| |||
Cash Flows From Operating Activities: |
|
|
|
|
|
|
|
|
| |||
Net loss |
| $ | (8,295,698 | ) |
| $ | (2,902,268 | ) |
| $ | (737,727 | ) |
Adjustments to reconcile net loss to net cash used in operating activities: |
|
|
|
|
|
|
|
|
|
|
|
|
Amortization of debt issuance costs |
|
| — |
|
|
| — |
|
|
| 14,000 |
|
Stock-based compensation |
|
| 732,151 |
|
|
| 486,271 |
|
|
| 470,185 |
|
(Gain) loss on revaluation of derivative warrant liability |
|
| 838,219 |
|
|
| (332,095 | ) |
|
| (8,588 | ) |
Stock issued for services |
|
| 150,000 |
|
|
| — |
|
|
| — |
|
Depreciation |
|
| 1,343 |
|
|
| — |
|
|
| — |
|
Loss on abandoned trademarks |
|
| 5,401 |
|
|
| — |
|
|
| — |
|
Changes in operating assets and liabilities: |
|
|
|
|
|
|
|
|
|
|
|
|
Prepaid expenses |
|
| (40,759 | ) |
|
| (60,000 | ) |
|
| 9,174 |
|
Accounts payable |
|
| 105,230 |
|
|
| 452,981 |
|
|
| (66,320 | ) |
Accrued expenses |
|
| 351,182 |
|
|
| (52,057 | ) |
|
| 56,764 |
|
Accrued compensation |
|
| 288,250 |
|
|
| — |
|
|
| — |
|
Accrued interest |
|
| 7,009 |
|
|
| 7,500 |
|
|
| 79,067 |
|
Due to related party |
|
| (42,749 | ) |
|
| 14,252 |
|
|
| 281 |
|
Net Cash Used In Operating Activities |
|
| (5,900,421 | ) |
|
| (2,385,416 | ) |
|
| (183,164 | ) |
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash Flows From Investing Activities: |
|
|
|
|
|
|
|
|
|
|
|
|
Cash acquired in acquisition |
|
| 255,748 |
|
|
| — |
|
|
| — |
|
Net Cash Used In Investing Activities |
|
| 255,748 |
|
|
| — |
|
|
| — |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash Flows From Financing Activities: |
|
|
|
|
|
|
|
|
|
|
|
|
Proceeds from notes payable |
|
| 500,000 |
|
|
| — |
|
|
| — |
|
Repayment of notes payable |
|
| (600,000 | ) |
|
| — |
|
|
| — |
|
Proceeds from issuance of common stock |
|
| — |
|
|
| — |
|
|
| 50,000 |
|
Net proceeds from private placement |
|
| 5,427,688 |
|
|
| 1,509,493 |
|
|
| 1,630,834 |
|
Deferred offering costs |
|
| (64,801 | ) |
|
| — |
|
|
| — |
|
Net Cash Provided by Financing Activities |
|
| 5,262,887 |
|
|
| 1,509,493 |
|
|
| 1,680,834 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Increase (Decrease) in Cash and Cash Equivalents |
|
| (381,786 | ) |
|
| (875,923 | ) |
|
| 1,497,670 |
|
Cash and Cash Equivalents – Beginning of Period |
|
| 676,137 |
|
|
| 1,552,060 |
|
|
| 54,390 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cash and Cash Equivalents – End of Period |
| $ | 294,351 |
|
| $ | 676,137 |
|
| $ | 1,552,060 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Supplemental Disclosures of Cash Flow Information and Non-cash Transactions: |
|
|
|
|
|
|
|
|
|
|
|
|
Interest paid |
| $ | 1,985 |
|
| $ | — |
|
| $ | — |
|
Income taxes paid |
| $ | — |
|
| $ | — |
|
| $ | — |
|
Fair value of warrants recorded as derivative warrant liability |
| $ | 1,198,564 |
|
| $ | 768,435 |
|
| $ | 1,459,531 |
|
Fair value of warrants issued for services |
| $ | 477,181 |
|
|
| — |
|
|
| — |
|
Reclassification of derivative warrant liability to additional paid-in capital |
| $ | 1,093,765 |
|
| $ | 1,148,328 |
|
| $ | — |
|
Conversion of promissory notes and accrued interest into common stock |
| $ | — |
|
| $ | 633,333 |
|
| $ | — |
|
Conversion of convertible promissory notes and accrued interest into common stock |
| $ | — |
|
| $ | — |
|
| $ | 1,836,813 |
|
Conversion of subordinated convertible promissory note and accrued interest into common stock |
| $ | — |
|
| $ | — |
|
| $ | 394,245 |
|
See Note 1 for supplemental cash flow information related to the acquisition of Leonard-Meron Biosciences, Inc.
See accompanying report of independent registered public accounting firm and notes to the consolidated financial statements.
| F-6 |
| Table of Contents |
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEARS ENDED SEPTEMBER 30, 2016 AND 2015, AND
THE NINE MONTHS ENDED SEPTEMBER 30, 2014
1. NATURE OF OPERATIONS AND BASIS OF PRESENTATION
Business
Citius Pharmaceuticals, Inc. (“Citius” or the “Company”) is a specialty pharmaceutical company dedicated to the development and commercialization of critical care products targeting unmet needs with a focus on anti-infectives, cancer care and unique prescription products. The Company was founded as Citius Pharmaceuticals, LLC, a Massachusetts limited liability company, on January 23, 2007. On September 12, 2014, Citius Pharmaceuticals, LLC entered into a Share Exchange and Reorganization Agreement (the “Exchange Agreement”), with Citius Pharmaceuticals, Inc. (formerly Trail One, Inc.), a publicly traded company incorporated under the laws of the State of Nevada. Citius Pharmaceuticals, LLC became a wholly-owned subsidiary of Citius (see “Reverse Acquisition” below).
On March 30, 2016, Citius acquired Leonard-Meron Biosciences, Inc. (“LMB”) as a wholly-owned subsidiary (see “Acquisition of Leonard-Meron Biosciences, Inc.” below).
The Company had one approved and marketed product, Suprenza (phentermine hydrochloride), which it had out licensed for promotion in the United States, Canada and Mexico. On July 1, 2016, the Company announced that it was discontinuing Suprenza. Since its inception, the Company has devoted substantially all of its efforts to business planning, research and development, recruiting management and technical staff, and raising capital.
Citius is subject to a number of risks common to companies in the pharmaceutical industry including, but not limited to, risks related to the development by Citius or its competitors of research and development stage products, market acceptance of its products, competition from larger companies, dependence on key personnel, dependence on key suppliers and strategic partners, the Company’s ability to obtain additional financing and the Company’s compliance with governmental and other regulations.
Reverse Acquisition
On September 12, 2014, Citius completed a reverse acquisition transaction with Citius Pharmaceuticals, LLC, which became a wholly-owned subsidiary of Citius. As part of the reverse acquisition, the former members of Citius Pharmaceuticals, LLC received 21,625,219 shares of the Company’s common stock in exchange for their interest in Citius Pharmaceuticals, LLC and, immediately after the transaction, owned 72% of the outstanding common stock. Immediately prior to the transaction, Citius had 5,000,000 shares of common stock outstanding. In connection with the Exchange Agreement, the Company completed the first closing of a Private Offering (see Note 7). Following the acquisition, Citius Pharmaceuticals, LLC began operating as a wholly-owned subsidiary of Citius Pharmaceuticals, Inc.
Accounting principles generally accepted in the United States generally require that a company whose security holders retain the majority voting interest in the combined business be treated as the acquirer for financial reporting purposes. The acquisition was accounted for as a reverse acquisition whereby Citius Pharmaceuticals, LLC was deemed to be the accounting acquirer. Accordingly, the historical consolidated financial statements are those of Citius Pharmaceuticals, LLC as the accounting acquirer. The post-merger combination of Citius Pharmaceuticals, Inc. and Citius Pharmaceuticals, LLC is referred to throughout these notes to consolidated financial statements as the “Company.” As the accounting acquirer, Citius Pharmaceuticals, LLC did not acquire any tangible assets from Citius and did not assume any liabilities of Citius. This transaction is not considered a business combination because Citius, the non-operating public corporation, did not meet the definition of a business. Instead, this transaction is considered to be a capital transaction of Citius Pharmaceuticals, LLC and is equivalent to the issuance of shares by Citius Pharmaceuticals, LLC for the net assets of Citius accompanied by a recapitalization.
In connection with the reverse acquisition, Citius Pharmaceuticals, LLC adopted the fiscal year end of Citius, thereby changing our fiscal year end from December 31 to September 30.
Acquisition of Leonard-Meron Biosciences, Inc.
On March 30, 2016, the Company acquired all of the outstanding stock of Leonard-Meron Biosciences, Inc. (“LMB”) by issuing 29,136,839 shares of its common stock. As of March 30, 2016, the stockholders of LMB received approximately 41% of the issued and outstanding common stock of the Company. In addition, the Company converted the outstanding common stock warrants of LMB into 3,645,297 common stock warrants of the Company and converted the outstanding common stock options of LMB into 1,158,770 common stock options of the Company.
| F-7 |
| Table of Contents |
The Company acquired tangible assets consisting of cash of $255,748, prepaid expenses of $20,544, property and equipment of $5,085, deposits of $2,167, and identifiable intangible assets of $19,400,000 related to in-process research and development. The Company assumed accounts payable of $244,776, accrued expenses of $598,659, accrued compensation of $615,000, accrued interest of $23,862, and notes payable of $772,970. Accordingly, the net assets acquired amounted to $17,428,277.
The fair value of LMB’s net assets acquired on the date of the acquisition, based on management’s analysis of the fair value of the 29,136,839 shares of the Company’s common stock issued for LMB’s outstanding stock, the 3,645,297 Company common stock warrants issued for LMB’s outstanding common stock warrants, and the vested portion of the 1,158,770 Company common stock options issued for LMB’s outstanding common stock options was $19,015,073. The fair value of the common stock issued was estimated at $17,482,093, the fair value of the warrants issued was estimated at $1,071,172 and the fair value of the vested options was estimated at $461,808.
The Company recorded goodwill of $1,586,796 for the excess of the purchase price of $19,015,073 over the net assets acquired of $17,428,277.
In-process research and development represents the value of LMB’s leading drug candidate which is an antibiotic solution used to treat catheter-related bloodstream infections (Mino-Lok™). Goodwill represents the value of LMB’s industry relationships and its assembled workforce. In-process research and development and goodwill will not be amortized but will be tested at least annually for impairment.
Unaudited pro forma operating results, assuming the acquisition of LMB had been made as of October 1, 2014, are as follows:
|
| Year Ended September 30, |
| |||||
|
| 2016 |
|
| 2015 |
| ||
|
|
|
|
|
|
| ||
Revenues |
| $ | — |
|
| $ | — |
|
Net loss |
| $ | (11,548,647 | ) |
| $ | (6,640,600 | ) |
Net loss per share – basic and diluted |
| $ | (0.17 | ) |
| $ | (0.11 | ) |
Basis of Presentation
As a result of the reverse acquisition, the accompanying consolidated financial statements include the operations of Citius Pharmaceuticals, LLC (the accounting acquirer). The accompanying consolidated financial statements also include the operations of Citius Pharmaceuticals, Inc. (formerly Trail One, Inc.) since the September 12, 2014 reverse acquisition and the operations of Leonard-Meron Biosciences, Inc. (“LMB”) since the March 30, 2016 acquisition. All significant inter-company balances and transactions have been eliminated in consolidation.
All share and per share amounts presented in these consolidated financial statements reflect the one-for-one exchange ratio of Citius Pharmaceuticals, LLC member interests to common shares in the reverse acquisition.
2. GOING CONCERN UNCERTAINTY AND MANAGEMENT’S PLAN
The accompanying consolidated financial statements have been prepared on a going concern basis, which contemplates the realization of assets and the satisfaction of liabilities in the normal course of business. The Company experienced negative cash flows from operations of $5,900,421, $2,385,416, and $183,164, for the years ended September 30, 2016 and 2015, and the nine months ended September 30, 2014, respectively. At September 30, 2016, the Company had a working capital deficit of $4,291,123. The Company has no revenue and has relied on proceeds from equity transactions and debt to finance its operations. At September 30, 2016, the Company had limited capital to fund its operations. This raises substantial doubt about the Company’s ability to continue as a going concern.
The Company plans to raise capital through equity financings from outside investors as well as raise additional funds from existing investors. There is no assurance, however, that that the Company will be successful in raising the needed capital and, if funding is available, that it will be available on terms acceptable to the Company.
The accompanying consolidated financial statements do not include any adjustments that might result from the outcome of the above uncertainty.
| F-8 |
| Table of Contents |
3. BUSINESS AGREEMENTS
Alpex Pharma S.A.
On June 12, 2008, the Company entered into a collaboration and license agreement (the “Alpex Agreement”) with Alpex Pharma S.A. (“Alpex”), in which Alpex granted the Company an exclusive right and license to use certain Alpex intellectual property in order to develop and commercialize orally disintegrating tablet formulations of pharmaceutical products in United States, Canada and Mexico. In addition, Alpex manufactures Suprenza, the Company’s commercialized pharmaceutical product, on a contract basis. The agreement was amended on November 15, 2011 as part of an Amendment and Coordination Agreement (see the “Three-Party Agreement” below).
Under the terms of the Alpex Agreement, as amended by the Three-Party Agreement dated November 15, 2011 (see below), Alpex is entitled to a payment per tablet manufactured and a percentage of all milestone, royalty and other payments received by the Company from Prenzamax, LLC, pursuant to a sublicense agreement (see below). A milestone is generally understood as a completion of a specific defined task towards the completion of a project or performance of a contract. For example, pursuant to the Company’s agreement with Alpex, the Company is required to pay Alpex for the completion of certain tasks including, but not limited to, the development of the analytical methods, formulations and filings of the NDA. In addition, under the terms of the Alpex Agreement, Alpex retained the right to use the clinical data generated by the Company to file for regulatory approval and market Suprenza in the rest of the world. In the event that Alpex has such sales, Alpex will pay the Company a percentage royalty on net sales, as defined (“Alpex Revenue”). No milestone, royalty or other payments have been earned or received by the Company through September 30, 2016 except for the reimbursement of regulatory fees under the Three-Party Agreement.
On July 1, 2016, the Company announced that it notified the Food and Drug Administration (“FDA”) and Alpex that it was discontinuing Suprenza.
Prenzamax, LLC
On November 15, 2011, the Company entered into an exclusive license agreement (the “Sublicense Agreement”) with Prenzamax, LLC (“Prenzamax”), in which the Company granted Prenzamax and its affiliates the exclusive right to commercialize Suprenza in the United States. Prenzamax is an affiliate of Akrimax, a related party (see Note 8) and was formed for the specific purpose of managing the Sublicense Agreement. Under the terms of the Sublicense Agreement, Prenzamax is to pay the Company a percentage of the product’s EBITDA, as defined (“Profit Share Payments”). In addition, Prenzamax is to reimburse the Company directly for certain development costs. These payments are to commence once Prenzamax has achieved profitability, as defined in the Sublicense Agreement. Further, under the terms of the Sublicense Agreement, Prenzamax is required to share in the royalty payment due to Alpex under the Alpex Agreement. In addition, Prenzamax is entitled to a percentage of the Alpex Revenue received by the Company. The Company has not been reimbursed for any development costs nor has it earned any Profit Share Payments through September 30, 2016.
On July 1, 2016, the Company announced that it notified Prenzamax that it was discontinuing Suprenza.
Three-Party Agreement
On November 15, 2011, the Company, Alpex and Prenzamax entered into the Three-Party Agreement wherein the terms of the Alpex Agreement were modified and Prenzamax and the Company agreed to each pay a portion of certain regulatory filing fees for as long as Prenzamax is purchasing Suprenza from Alpex pursuant to the Three-Party Agreement. During the three months ended March 31, 2016, the Company received $292,575 from Alpex as reimbursement for regulatory filing fees that were previously expensed during the three months ended December 31, 2015. The reimbursement was recorded as a reduction of research and development expenses.
On July 1, 2016, the Company announced that it notified Alpex and Prenzamax that it was discontinuing Suprenza.
Patent and Technology License Agreement
LMB has a patent and technology license agreement with Novel Anti-Infective Therapeutics, Inc., (“NAT”) to develop and commercialize Mino-Lok™ on an exclusive, worldwide (except for South America), sub licensable basis. LMB expensed a one-time license fee of $350,000 during the year ended May 31, 2014. LMB will pay an annual maintenance fee of $30,000 that increases over five years to $90,000, until commercial sales of a product subject to the license. LMB will also pay annual royalties on net sales of licensed products, with royalties ranging from the mid-single digits to the low double digits. In limited circumstances in which the licensed product is not subject to a valid patent claim and a competitor is selling a competing product, the royalty rate is in the low-single digits. After a commercial sale is obtained, LMB must pay minimum aggregate annual royalties that increase in subsequent years. LMB must also pay NAT up to $1,050,000 upon achieving specified regulatory and sales milestones. Finally, LMB must pay NAT a specified percentage of payments received from any sub licensees.
| F-9 |
| Table of Contents |
4. SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
A summary of the significant accounting policies followed by the Company in the preparation of the consolidated financial statements is as follows:
Use of Estimates
The process of preparing financial statements in conformity with accounting principles generally accepted in the United States of America (“GAAP”) requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of assets and liabilities at the date of financial statements and the reported amounts of revenues and expenses during the reporting period. Estimates having relatively higher significance include the accounting for acquisitions, stock-based compensation, valuation of warrants, and income taxes. Actual results could differ from those estimates and changes in estimates may occur.
Cash and Cash Equivalents
The Company considers all highly liquid instruments with maturities of less than three months at the time of purchase to be cash equivalents. From time to time, the Company may have cash balances in financial institutions in excess of insurance limits. The Company has never experienced any losses related to these balances.
Property and Equipment
Property and equipment are valued at cost and are being depreciated over their useful lives using the straight-line method for financial reporting purposes. Routine maintenance and repairs are charged to expense as incurred. Expenditures which materially increase the value or extend useful lives are capitalized. Property and equipment are depreciated over estimated useful lives of three to five years.
Property and equipment consisted of the following at September 30, 2016:
|
| 2016 |
| |
|
|
|
| |
Computer equipment |
| $ | 8,522 |
|
Less accumulated depreciation |
|
| (4,780 | ) |
|
| $ | 3,742 |
|
Depreciation and amortization expense for the year ended September 30, 2016 was $1,343. There was no depreciation and amortization expense for the year ended September 30, 2015 and the nine months ended September 30, 2014.
Research and Development
Research and development costs, including upfront fees and milestones paid to collaborators who are performing research and development activities under contractual agreement with the Company, are expensed as incurred. The Company defers and capitalizes its nonrefundable advance payments that are for research and development activities until the related goods are delivered or the related services are performed. When the Company is reimbursed by a collaboration partner for work the Company performs, it records the costs incurred as research and development expenses and the related reimbursement as a reduction to research and development expenses in its consolidated statement of operations. Research and development expenses primarily consist of clinical and non-clinical studies, materials and supplies, third-party costs for contracted services, and payments related to external collaborations and other research and development related costs.
In-process Research and Development and Goodwill
In-process research and development represents the value of LMB’s leading drug candidate which is an antibiotic solution used to treat catheter-related bloodstream infections (Mino-Lok™). Goodwill represents the value of LMB’s industry relationships and its assembled workforce. In-process research and development and goodwill will not be amortized but will be tested at least annually for impairment.
The Company reviews intangible assets annually to determine if any adverse conditions exist or a change in circumstances has occurred that would indicate impairment or a change in the remaining useful life of any intangible asset. If the carrying value of an asset exceeds its undiscounted cash flows, the Company writes down the carrying value of the intangible asset to its fair value in the period identified. No triggering events occurred since the acquisition of LMB that would suggest that a potential impairment may have occurred through September 30, 2016.
The Company evaluates the recoverability of goodwill annually or more frequently if events or changes in circumstances indicate that the carrying value of an asset might be impaired. Goodwill is first qualitatively assessed to determine whether further impairment testing is necessary. Factors that management considers in this assessment include macroeconomic conditions, industry and market considerations, overall financial performance (both current and projected), changes in management and strategy and changes in the composition or carrying amount of net assets. If this qualitative assessment indicates that it is more likely than not that the fair value of a reporting unit is less than its carrying amount, a two-step process is then performed.
| F-10 |
| Table of Contents |
The Company performed a qualitative assessment for our 2016 analysis of goodwill. Based on this assessment, management does not believe that it is more likely than not that the carrying value of the reporting unit exceeds its fair value. Accordingly, no further testing was performed as management believes that there are no impairment issues in regards to goodwill as of September 30, 2016.
Patents and Trademarks
Certain costs of outside legal counsel related to obtaining trademarks for the Company are capitalized. Patent costs are amortized over the legal life of the patents, generally twenty years, starting at the patent issuance date. The costs of unsuccessful and abandoned applications are expensed when abandoned. The cost of maintaining existing patents are expensed as incurred.
Revenue Recognition
The Company recognizes revenue using the four basic criteria that must be met before revenue can be recognized: (1) persuasive evidence of an arrangement exists, (2) delivery has occurred, (3) the selling price is fixed and determinable, and (4) collectability is reasonably assured. Provisions for discounts, rebates, estimated returns and allowances, and other adjustments are provided in the period that the revenue is recorded.
The Company’s license and collaboration agreements with certain partners also provide for contingent payments to us based solely upon the performance of the respective partner. For such contingent amounts we expect to recognize the payments as revenue when earned under the applicable contract, which is generally upon completion of performance by the respective partner, provided that collection is reasonably assured.
The Company’s license and collaboration agreements with its partners also provide for payments to us upon the achievement of specified sales volumes of approved drugs. We consider these payments to be similar to royalty payments and we will recognize such sales-based payments upon achievement of such sales volumes, provided that collection is reasonably assured.
Stock-Based Compensation
The Company recognizes compensation costs resulting from the issuance of stock-based awards to employees and directors, net of expected forfeitures, as an expense in the consolidated statement of operations over the requisite service period based on the fair value for each stock award on the grant date. The fair value of each option grant is estimated as of the date of grant using the Black-Scholes option pricing model. Due to its limited operating history, limited number of sales of its common stock and limited history of its shares being publicly traded, the Company estimates its volatility in consideration of a number of factors including the volatility of comparable public companies. The estimated forfeiture rate is based on historical forfeiture information as well as subsequent events occurring prior to the issuance of the financial statements. Because our stock options have characteristics significantly different from those of traded options, and because changes in the input assumptions can materially affect the fair value estimate, the existing model may not necessarily provide a reliable single measure of fair value of our stock options
The Company recognizes compensation costs resulting from the issuance of stock-based awards to non-employees as an expense in the consolidated statement of operations over the service period based on the measurement of fair value for each stock award.
Derivative Instruments
The Company generally does not use derivative instruments to hedge exposures to cash-flow or market risks; however, certain warrants to purchase common stock that do not meet the requirements for classification as equity are classified as liabilities. In such instances, net-cash settlement is assumed for financial reporting purposes, even when the terms of the underlying contracts do not provide for a net-cash settlement. Such financial instruments are initially recorded at fair value with subsequent changes in fair value charged (credited) to operations in each reporting period. If these instruments subsequently meet the requirements for classification as equity, the Company reclassifies the fair value to equity.
Income Taxes
Citius Pharmaceuticals, LLC was treated as a partnership for federal and state income taxes prior to the September 12, 2014 Reverse Acquisition. A partnership’s income or loss is allocated directly to the Members for income tax purposes.
The Company follows accounting guidance regarding the recognition, measurement, presentation and disclosure of uncertain tax positions in the consolidated financial statements. Tax positions taken or expected to be taken in the course of preparing our tax returns, including the position that Citius Pharmaceuticals, LLC qualified as a pass-through entity, are required to be evaluated to determine whether the tax positions are “more-likely-than-not” of being sustained by the applicable tax authorities. Tax positions not deemed to meet a more-likely-than-not threshold would be recorded in the consolidated financial statements. There are no uncertain tax positions that require accrual or disclosure as of September 30, 2016.
| F-11 |
| Table of Contents |
Any interest or penalties are charged to expense. During the years ended September 30, 2016 and 2015, and the nine months ended September 30, 2014, the Company did not recognize any interest and penalties. Tax years subsequent to December 31, 2012 are subject to examination by federal and state authorities.
After the Reverse Acquisition, we recognize deferred tax assets and liabilities based on differences between the financial reporting and tax basis of assets and liabilities, and operating loss and tax credit carry forwards. Deferred tax assets and liabilities are measured using the enacted tax rates and laws that are expected to be in effect when the differences are expected to reverse. We provide a valuation allowance, if necessary, for deferred tax assets for which we do not consider realization of such assets to be “more-likely-than-not”. The deferred tax benefit or expense for the period represents the change in the deferred tax asset or liability from the beginning to the end of the period.
Basic and Diluted Net Loss per Common Share
Basic and diluted net loss per common share is computed by dividing net loss in each period by the weighted average number of shares of common stock outstanding during such period. For the periods presented, common stock equivalents, consisting of options, warrants and convertible securities were not included in the calculation of the diluted loss per share because they were anti-dilutive.
Fair Value of Financial Instruments
The financial statements include various estimated fair value information. Financial instruments are initially recorded at historical cost. If subsequent circumstances indicate that a decline in the fair value of a financial asset is other than temporary, the financial asset is written down to its fair value.
Unless otherwise indicated, the fair values of financial instruments approximate their carrying amounts. By their nature, all financial instruments involve risk, including credit risk for non-performance by counterparties. The fair values of cash and cash equivalents, accounts payable, accrued interest, accrued expenses, notes payable and due to related party approximate their recorded amounts because of their relatively short settlement terms.
The Company groups its financial assets and financial liabilities generally measured at fair value in three levels, based on the markets in which the assets and liabilities are traded and the reliability of the assumptions used to determine fair value.
Level 1: | Valuation is based on quoted prices in active markets for identical assets or liabilities. Level 1 assets and liabilities generally include debt and equity securities that are traded in an active exchange market. Valuations are obtained from readily available pricing sources for market transactions involving identical assets or liabilities. |
|
|
Level 2: | Valuation is based on observable inputs other than Level 1 prices, such as quoted prices for similar assets or liabilities; quoted prices in markets that are not active; or other inputs that are observable or can be corroborated by observable market data for substantially the full term of the assets or liabilities. For example, Level 2 assets and liabilities may include debt securities with quoted prices that are traded less frequently than exchange-traded instruments. |
|
|
Level 3: | Valuation is based on unobservable inputs that are supported by little or no market activity and that are significant to the fair value of the assets or liabilities. Level 3 assets and liabilities include financial instruments whose value is determined using pricing models, discounted cash flow methodologies, or similar techniques, as well as instruments for which the determination of fair value requires significant management judgment or estimation. This category generally includes certain private equity investments and long-term derivative contracts. |
The Company's financial liabilities measured at fair value on September 30, 2016 and 2015 consists solely of the derivative warrant liability which is classified as Level 3 in fair value hierarchy (see Note 6). The Company uses a valuation method, the Black-Scholes option pricing model, and the requisite assumptions in estimating the fair value for the warrants considered to be derivative instruments. The Company has no financial assets measured at fair value.
The Company may also be required, from time to time, to measure certain other financial assets at fair value on a nonrecurring basis. These adjustments to fair value usually result from application of lower-of-cost-or-market accounting or write-downs of individual assets. There were no such adjustments in the years ended September 30, 2016 and 2015, and the nine month period ended September 30, 2014.
| F-12 |
| Table of Contents |
Segment Reporting
The Company currently operates as a single segment.
Concentrations of Credit Risk
The Company has no significant off-balance-sheet concentration of credit risk such as foreign exchange contracts, option contracts or other hedging arrangements.
Recently Issued Accounting Standards
In August 2014, the FASB issued ASU No. 2014-15, “Presentation of Financial Statements—Going Concern (Subtopic 205-40); Disclosure of Uncertainties about an Entity’s Ability to Continue as a Going Concern” which applies should a company be facing probable liquidation within one year of the issuance of the financial statements, but is not actually in liquidation at the time of issuance. The applicable accounting basis for presentation remains as a going concern, but if liquidation within one year is probable, then certain disclosures must be included in the financial statement presentation. ASU 2014-15 is effective for annual and interim periods beginning after December 15, 2016, with early adoption permitted. We are currently in the process of evaluating the impact of adoption of this ASU on the consolidated financial statements.
In August 2015, the FASB also issued ASU No. 2015-14, “Revenue from Contracts with Customers (Topic 606) Deferral of the Effective Date which deferred the effective date of ASU 2014-09 by one year. Originally scheduled to be effective for fiscal years beginning after December 15, 2016, ASU 2015-14 is effective for the year ended September 30, 2019.
In February 2016, the FASB issued ASU No. 2016-02, “Leases” (Topic 842). The guidance in this ASU supersedes the leasing guidance in Topic 840, Leases. Under the new guidance, lessees are required to recognize lease assets and lease liabilities on the balance sheet for all leases with terms longer than 12 months. Leases will be classified as either finance or operating, with classification affecting the pattern of expense recognition in the statement of operations. The new standard is effective for annual reporting periods beginning after December 15, 2019. The Company is currently evaluating the impact of the adoption of this ASU on the financial statements.
In March 2016, the FASB issued ASU No. 2016-09, “Compensation – Stock Compensation” to require changes to several areas of employee share-based payment accounting in an effort to simplify share-based reporting. The update revises requirements in the following areas: minimum statutory withholding, accounting for income taxes, forfeitures, and intrinsic value accounting for private entities. ASU 2016-09 is effective for annual reporting periods beginning after December 15, 2017. The Company is currently evaluating the impact of the adoption of this ASU on the financial statements.
In August 2016, the FASB issued ASU No. 2016-15, “Classification of Certain Cash Receipts and Cash Payments” to address how certain cash receipts and cash payments are presented and classified in the statement of cash flows in an effort to reduce existing diversity in practice. The update includes eight specific cash flow issues and provides guidance on the appropriate cash flow presentation for each. ASU 2016-15 is effective for annual reporting periods beginning after December 15, 2017. The Company does not expect the adoption of this guidance to have a material impact on the financial statements.
5. NOTES PAYABLE
Convertible Promissory Notes
Between July 12, 2010 and November 30, 2012, the Company issued several convertible promissory notes (collectively the “Convertible Notes”) to two existing investors in aggregate total principal amount of $1,460,000. The Convertible Notes accrued interest at 3.00% per annum and were payable on demand only after their respective 10-year maturities. Between January 1, 2013 and March 25, 2013, the Company issued additional Convertible Notes to existing investors in aggregate total principal amount of $225,000. The additional Convertible Notes accrued interest at 5.00% per annum and were payable on demand only after their respective 10-year maturities. The unpaid principal and accrued interest were only convertible into common stock following a reorganization or conversion into a corporation at the option of the holder. The unpaid principal and accrued interest will convert into common stock at the greater of the fair value of the common stock on the date of the conversion or $0.25 ($0.69 if the Company’s common stock is admitted to trade on a national exchange prior to the date of conversion).
On July 31, 2014, in anticipation of the completion of the reverse acquisition and the Private Offering, the note holders demanded conversion of the outstanding $1,685,000 Convertible Notes and accrued interest of $151,813 into 3,061,355 shares of common stock at a conversion price of $0.60 per share.
| F-13 |
| Table of Contents |
Promissory Notes
In November 2013, the Company issued two promissory notes (the “Promissory Notes”) to two existing investors in aggregate total principal amount of $600,000. The Promissory Notes accrued interest at 5.00% per annum and were due at the earliest of (1) December 19, 2014, (2) the occurrence of an event of default as defined in the Promissory Notes, (3) an initial installment of $100,000 principal amount, to each investor, upon the receipt by the Company of a minimum $6,500,000 in aggregate proceeds under any financing transaction, (4) a second installment of $100,000 principal amount, to each investor, upon the receipt by the Company of a minimum $8,500,000 in aggregate proceeds under any financing transaction, and (5) a third installment of $100,000 principal amount, to each investor, upon the receipt by the Company of a minimum $10,000,000 in aggregate proceeds under any financing transaction. At September 30, 2014, the Promissory Notes had an outstanding aggregate principal balance of $600,000.
On December 31, 2014, the note holders requested conversion of the outstanding $600,000 Promissory Notes and accrued interest of $33,333 into 1,055,554 shares of common stock at a conversion price of $0.60 per share.
Subordinated Convertible Promissory Note
In 2013, the Company entered into an investment banking agreement to raise up to $6 million of 10% subordinated convertible promissory notes. The agreement contemplated a reverse acquisition with a public company and an automatic conversion of the notes into units of common stock and warrants, as defined therein. In April 2013, the Company issued a $350,000 subordinated convertible promissory note (the “Subordinated Note”). The Subordinated Note accrued interest at 10% per annum and was payable on demand any time after April 2014. If the Company has not repaid the Subordinated Note at the closing of a reverse acquisition, the unpaid principal and accrued interest will automatically convert into common stock by dividing the amount due by a price per unit of $0.65. Also, upon automatic conversion, the purchaser of the Subordinated Note will receive a warrant to purchase the same number of shares in to which the Subordinated Note converts.
On July 31, 2014, in anticipation of the completion of the reverse acquisition and the Private Offering, the note holder demanded conversion of the outstanding $350,000 Subordinated Note and accrued interest of $44,245 into 606,531 shares of common stock at a conversion price of $0.65 per share.
Notes Payable – Related Parties
On March 30, 2016, the Company assumed $772,970 of demand notes payable in the acquisition of LMB. The principal balance of the notes payable to our Chairman, Leonard Mazur, was $760,470 and the principal balance of the notes payable to our Chief Executive Officer, Myron Holubiak, was $12,500. Notes with a principal balance of $704,000 accrue interest at the “Prime Rate”, as published in the Wall Street Journal on the last day of each month plus 1% and notes with a principal balance of $68,970 accrue interest at 12% per annum. In April 2016, $600,000 of the “Prime Rate” plus 1% demand notes payable and accrued interest of $1,985 was repaid to Leonard Mazur.
On September 7, 2016, the Company issued a $500,000 demand promissory note to our Chairman, Leonard Mazur which matures on the earlier of December 31, 2016 or demand by the lender. The note accrues interest at the “Prime Rate”, as published in the Wall Street Journal on the last day of each month, plus 1%.
Interest Expense
During 2013, the Company incurred $42,000 of debt issuance costs related to the Subordinated Note which was amortized over the term of the underlying debt. Amortization of debt issuance costs recorded as interest expense for the nine months ended September 30, 2014 amounted to $14,000.
Interest expense on the notes for the years ended September 30, 2016 and 2015, and the nine months ended September 30, 2014, including non-cash interest related to debt issuance costs, was $8,994, $7,500, and $93,067, respectively.
6. DERIVATIVE WARRANT LIABILITY
Derivative financial instruments are recognized as a liability on the consolidated balance sheet and measured at fair value. At September 30, 2016 and 2015, the Company had outstanding warrants to purchase 4,616,668 and 3,037,037 shares, respectively, of its common stock that are considered to be derivative instruments since the agreements contain “down round” provisions whereby the exercise price of the warrants is subject to adjustment in the event that the Company issues common stock for less than $0.60 per share within one-year of the issuance of the warrants (see Note 7).
| F-14 |
| Table of Contents |
The Company performs valuations of the warrants using a probability weighted Black-Scholes option pricing model which value was also compared to a Binomial Option Pricing Model for reasonableness. This model requires input of assumptions including the risk-free interest rates, volatility, expected life and dividend rates, and has also considered the likelihood of “down-round” financings. Selection of these inputs involves management’s judgment and may impact net income. Due to our limited operating history and limited number of sales of our common stock, we estimate our volatility based on a number of factors including the volatility of comparable publicly traded pharmaceutical companies. The volatility factor used in the Black-Scholes option pricing model has a significant effect on the resulting valuation of the derivative liabilities on our balance sheet. The volatility calculated at September 30, 2016 was 73% and we used a risk-free interest rate of 1.14%, estimated lives of 4.10 to 4.57 years, which are the remaining contractual lives of the warrants subject to “down-round” provisions, and no dividends to our common stock. The volatility calculated at September 30, 2015 was 57% and we used a risk-free interest rate of 1.37%, estimated lives of 4.47 to 4.96 years, which are the remaining contractual lives of the warrants subject to “down-round” provisions, and no dividends to our common stock.
On September 12, 2015, anti-dilution rights related to warrants to purchase 5,080,080 shares of common stock expired which resulted in a reclassification from derivative warrant liability to additional paid-in capital of $1,148,328. During the year ended September 30, 2016, anti-dilution rights related to warrants to purchase 3,037,037 shares of common stock expired which resulted in a reclassification from derivative warrant liability to additional paid-in capital of $1,093,765.
The table below presents the changes in the derivative warrant liability, which is measured at fair value on a recurring basis and classified as Level 3 in fair value hierarchy (see Note 4):
|
| Year |
|
| Year |
|
| Nine Months |
| |||
|
|
|
|
|
|
|
|
|
| |||
Derivative warrant liability, beginning of period |
| $ | 738,955 |
|
| $ | 1,450,943 |
|
| $ | — |
|
Fair value of warrants issued |
|
| 1,198,564 |
|
|
| 768,435 |
|
|
| 1,459,531 |
|
Total realized/unrealized losses (gains) included in net loss |
|
| 838,219 |
|
|
| (332,095 | ) |
|
| (8,588 | ) |
Reclassification of liability to additional paid-in capital |
|
| (1,093,765 | ) |
|
| (1,148,328 | ) |
|
| — |
|
Derivative warrant liability, end of period |
| $ | 1,681,973 |
|
| $ | 738,955 |
|
| $ | 1,450,943 |
|
7. COMMON STOCK, STOCK OPTIONS AND WARRANTS
Common Stock
In May 2014, the Company issued 200,000 shares of common stock for $50,000, or $0.25 per share.
On September 12, 2014, in connection with the Reverse Acquisition, 5,000,000 shares of common stock were recorded in the financial statements of Citius Pharmaceuticals, LLC, the accounting acquirer (See Note 1 – Reverse Acquisition).
On September 15, 2016, the stockholders approved an increase in the number of shares of authorized common stock from 90,000,000 shares to 200,000,000 shares. In addition, the stockholders granted the Board of Directors the authority to effect a reverse stock split of our common stock by a ratio of not less than 1-for-8 and not more than 1-for-20 at any time prior to September 15, 2017.
Private Offerings
In 2014, the Company entered into an investment banking agreement to raise up to $5.1 million and issue up to 8,500,000 Units described below. The agreement contemplated a Reverse Acquisition with a public company. As of December 31, 2013, the Company capitalized as deferred offering costs a $25,000 retainer for legal costs associated with this offering. The $25,000 retainer was charged to additional paid-in capital on completion of the first closing of the offering.
On September 12, 2014, the Company sold 3,400,067 Units for a purchase price of $0.60 per Unit for gross proceeds of $2,040,040. Each Unit consists of one share of common stock and one five-year warrant (the “Investor Warrants”) to purchase one share of common stock at an exercise price of $0.60, (the “Private Offering”). The exercise price of the Investor Warrants is subject to adjustment, for up to one year, if the Company issues common stock at a price lower than the exercise price, subject to certain exceptions. The 2015 private placement described below did not result in an adjustment of the exercise price of the Investor Warrants. The Investor Warrants will be redeemable by the Company at a price of $0.001 per Investor Warrant at any time subject to the conditions that (i) the common stock has traded for twenty (20) consecutive trading days with a closing price of at least $1.50 per share with an average trading volume of 50,000 shares per day and (ii) the Company provides 20 trading days prior notice of the redemption and the closing price of the common stock is not less than $1.17 for more than any 3 days during such notice period and (iii) the underlying shares of common stock are registered.
| F-15 |
| Table of Contents |
The Placement Agent was paid a commission of ten percent (10%) and a non-accountable expense allowance of three percent (3%) of the funds raised in the Private Offering. As a result of the foregoing arrangement, the Placement Agent was paid commissions and expenses of $265,206. In addition, the Company issued to the Placement Agent and their designees five-year warrants (the “Placement Agent Unit Warrants”) to purchase 680,013 Units at an exercise price of $0.60 per Unit. The Placement Agent Unit Warrants are exercisable on a cash or cashless basis with respect to purchase of the Units, and will be exercisable only for cash with respect to warrants received as part of the Units. The exercise price of the warrants underlying the Placement Agent Unit Warrants is subject to adjustment, for up to one year, if the Company issues common stock at a price lower than the exercise price, subject to certain exceptions.
In addition, the Placement Agent was issued warrants to purchase 1,000,000 shares of common stock exercisable for cash at $0.60 per share for investment banking services provided in connection with the transaction (the “Placement Agent Share Warrants”). Other cash expenses related to the private placement totaled $169,000. The Placement Agent may, while the Placement Agent Unit Warrants are outstanding, appoint one person to the Board of Directors, and designate one person who may attend meetings of the Board of Directors as an observer. On November 2, 2015, the Placement Agent waived its right to appoint a person to the Board of Directors.
In connection with the Private Offering, the Company entered into a Registration Rights Agreement pursuant to which the Company is required to file a registration statement (the “Registration Statement”), registering for resale all shares of common stock (i) included in the Units; and (ii) issuable upon exercise of the Investor Warrants. The Company has agreed to use its reasonable efforts to cause the Registration Statement to be filed no later than 60 days after the completion of the Private Offering (the “Filing Deadline”), and to have the Registration Statement declared effective within 180 days of the Filing Deadline. Any holders of the shares of common stock removed from the Registration Statement as a result of a Section 415 comment from the SEC shall be included in a subsequent registration statement the Company will file no later than six months after the prior registration statement (or such other period as permitted by SEC rules). The Company filed the Registration Statement on September 11, 2015 and it was declared effective on January 21, 2016.
During the year ended September 30, 2015, the Company sold an additional 2,837,037 Units for a purchase price of $0.54 per Unit and 200,000 Units for a purchase price of $0.60 per Unit for gross proceeds of $1,652,000. Each Unit consists of one share of common stock and one Investor Warrant (see description above). There was no placement agent for the 2015 private placements and other cash expenses related to the placements were $142,507. In connection with these placements, the Company credited $741,058 to stockholders’ equity (deficit) and $768,435 to derivative warrant liability.
During the year ended September 30, 2016, the Company sold an additional 4,350,001 Units for a purchase price of $0.54 per Unit and 266,667 Units for a purchase price of $0.60 per Unit for gross proceeds of $2,509,000. Each Unit consists of one share of common stock and one Investor Warrant (see description above). There was no placement agent for these private placements and other cash expenses related to the placements were $81,312. In connection with these placements, the Company credited $1,229,124 to stockholders’ equity (deficit) and $1,198,564 to derivative warrant liability.
On March 22, 2016, the Company sold 5,000,000 shares of common stock at $0.60 per share to its Chairman of the Board, Leonard Mazur, for gross proceeds of $3,000,000. There were no expenses related to this placement.
Stock Options
On September 12, 2014, the Board of Directors adopted the 2014 Stock Incentive Plan (the “2014 Plan”) and reserved 13,000,000 shares of common stock for issuance to employees, directors and consultants. On September 12, 2014, the stockholders approved the plan. Pursuant to the 2014 Plan, the Board of Directors (or committees and/or executive officers delegated by the Board of Directors) may grant stock options, stock appreciation rights, restricted stock, restricted stock units, other stock-based awards and cash-based awards. As of September 30, 2016, there were options to purchase an aggregate of 8,732,770 shares of common stock outstanding under the 2014 Plan and 4,267,230 shares available for future grants.
The fair value of each stock option award is estimated on the date of grant using the Black-Scholes option pricing model. Due to its limited operating history and limited number of sales of its common stock, the Company estimated its volatility in consideration of a number of factors including the volatility of comparable public companies. The Company uses historical data, as well as subsequent events occurring prior to the issuance of the consolidated financial statements, to estimate option exercises and employee terminations within the valuation model. The risk-free interest rate is based on the U.S. Treasury yield curve in effect at the time of grant commensurate with the expected term assumption. The expected term of stock options granted to employees and directors, all of which qualify as “plain vanilla,” is based on the average of the contractual term (generally 10 years) and the vesting period. For non-employee options, the expected term is the contractual term.
| F-16 |
| Table of Contents |
The following assumptions were used in determining the fair value of stock option grants:
|
| Year |
|
| Year |
|
| Nine Months |
| |||
|
|
|
|
|
|
|
|
|
| |||
Risk-free interest rate |
| 0.95 – 1.40 | % |
| 1.37 – 1.52 | % |
|
| 1.83 | % | ||
Expected dividend yield |
|
| 0 | % |
|
| 0 | % |
|
| 0 | % |
Expected term |
| 4.75 – 9 years |
|
| 2.5 – 6 years |
|
| 5 – 6 years |
| |||
Forfeiture rate |
|
| 0 | % |
|
| 0 | % |
|
| 0 | % |
Expected volatility |
| 57 – 74 | % |
| 53 – 58 | % |
|
| 54 | % | ||
A summary of option activity under the 2014 Plan is presented below:
Options |
| Shares |
|
| Weighted- Average Exercise Price |
|
| Weighted- Average Remaining Contractual Term |
| Aggregate Intrinsic Value |
| |||
|
|
|
|
|
|
|
|
|
|
|
| |||
Outstanding at January 1, 2014 |
|
| — |
|
| $ | — |
|
|
|
|
|
| |
Granted |
|
| 3,300,000 |
|
|
| 0.45 |
|
|
|
|
|
| |
Exercised |
|
| — |
|
|
| — |
|
|
|
|
|
| |
Forfeited or expired |
|
| — |
|
|
| — |
|
|
|
|
|
| |
Outstanding at September 30, 2014 |
|
| 3,300,000 |
|
|
| 0.45 |
|
| 9.96 years |
| $ | 495,000 |
|
Granted |
|
| 600,000 |
|
|
| 0.60 |
|
|
|
|
|
|
|
Exercised |
|
| — |
|
|
| — |
|
|
|
|
|
|
|
Forfeited or expired |
|
| — |
|
|
| — |
|
|
|
|
|
|
|
Outstanding at September 30, 2015 |
|
| 3,900,000 |
|
|
| 0.47 |
|
| 8.94 years |
| $ | 297,000 |
|
Granted |
|
| 3,674,000 |
|
|
| 0.76 |
|
|
|
|
|
|
|
Assumed in acquisition |
|
| 1,158,770 |
|
|
| 0.07 |
|
|
|
|
|
|
|
Exercised |
|
| — |
|
|
| — |
|
|
|
|
|
|
|
Forfeited or expired |
|
| — |
|
|
| — |
|
|
|
|
|
|
|
Outstanding at September 30, 2016 |
|
| 8,732,770 |
|
| $ | 0.54 |
|
| 8.59 years |
| $ | 1,355,924 |
|
Exercisable at September 30, 2016 |
|
| 5,136,654 |
|
| $ | 0.45 |
|
| 8.14 years |
| $ | 1,059,615 |
|
On September 12, 2014, the Board of Directors granted stock options to purchase 3,300,000 shares of common stock at an exercise price of $0.45 per share. The weighted average grant-date fair value of the options granted was estimated at $0.34 per share. These options vest over three years and have a term of 10 years.
On April 1, 2015, the Board of Directors granted stock options to purchase 100,000 shares of common stock at an exercise price of $0.60 per share. The weighted average grant-date fair value of the options granted was estimated at $0.16 per share. These options vested immediately and have a term of 5 years. On June 1, 2015, the Board of Directors granted stock options to purchase 500,000 shares of common stock at an exercise price of $0.60 per share. The weighted average grant-date fair value of the options granted was estimated at $0.27 per share. These options vest over three years and have a term of 10 years.
In October 2015, the Company appointed two new directors. Each director received an option to purchase 400,000 shares of common stock at an exercise price of $0.54 per share in consideration for their services as members of the Board of Directors. The weighted average grant-date fair value of the options was estimated at $0.28 per share. These options vest over 14 months and have a term of 10 years.
On March 30, 2016, the Company assumed stock options to purchase 1,158,770 shares of common stock in connection with the acquisition of LMB. The LMB option holders received stock options to purchase 1,068,241 shares at an exercise price of $0.001 per share and 90,529 shares at an exercise price of $0.91 per share. Pursuant to the original grants, options to purchase 72,423 shares were immediately vested and options to purchase 1,086,347 shares vest over three years. The March 30, 2016 estimated fair value of the stock options was $670,242. The fair value of the vested options was estimated at $461,808 and has been included in the purchase price of LMB. The March 30, 2016 fair value of the unvested options was estimated at $208,434 per share and will be expensed over the remaining vesting period of the options. These options all had original terms of 10 years.
| F-17 |
| Table of Contents |
On June 23, 2016, the Board of Directors granted stock options to four directors. Each director received an option to purchase 200,000 shares of common stock at an exercise price of $0.80 per share in consideration for their services as members of the Board of Directors. The weighted average grant-date fair value of the options was estimated at $0.44 per share. These options vest in full on June 23, 2017 and have a term of 10 years.
In July 2016, the Board of Directors granted stock options to purchase a total of 2,074,000 shares to three employees at prices ranging from $0.70 to $0.90 per share. The weighted average grant date fair value of the options was estimated at $0.52 per share. These options vest over terms of 19 to 48 months and have a term of 10 years.
Stock-based compensation expense for the years ended September 30, 2016 and 2015, and the nine months ended September 30, 2014 was $732,151, $486,271 and $470,185, respectively.
At September 30, 2016, unrecognized total compensation cost related to unvested awards of $1,510,923 is expected to be recognized over a weighted average period of 1.67 years.
Warrants
The Company has reserved 18,059,095 shares of common stock for the exercise of outstanding warrants. The following table summarizes the warrants outstanding at September 30, 2016:
|
| Exercise price |
|
| Number |
|
| Expiration Dates | |||
|
|
|
|
|
|
|
|
|
| ||
Investor Warrants |
| $ | 0.60 |
|
|
| 3,400,067 |
|
| September 12, 2019 | |
Placement Agent Unit Warrants |
|
| 0.60 |
|
|
| 680,013 |
|
| September 12, 2019 | |
Warrants underlying Placement Agent Unit Warrants |
|
| 0.60 |
|
|
| 680,013 |
|
| September 12, 2019 | |
Placement Agent Share Warrants |
|
| 0.60 |
|
|
| 1,000,000 |
|
| September 12, 2019 | |
Investor Warrants |
|
| 0.60 |
|
|
| 2,145,371 |
|
| March 19, 2020 – June 26, 2020 | |
Investor Warrants |
|
| 0.60 |
|
|
| 891,666 |
|
| July 2, 2020 – September 14,2020 | |
Investor Warrants |
|
| 0.60 |
|
|
| 583,334 | (1) |
| November 5, 2020 – November 20, 2020 | |
Investor Warrants |
|
| 0.60 |
|
|
| 2,133,334 | (1) |
| January 7, 2021 – March 21, 2021 | |
Investor Warrants |
|
| 0.60 |
|
|
| 1,900,000 | (1) |
| April 15, 2021 – April 25, 2021 | |
LMB Warrants |
|
| 0.41 |
|
|
| 1,352,266 |
|
| June 12, 2019 – March 2, 2021 | |
LMB Warrants |
|
| 0.66 |
|
|
| 122,319 |
|
| September 30, 2019 – January 8, 2020 | |
LMB Warrants |
|
| 1.38 |
|
|
| 265,814 |
|
| November 3, 2019 – March 6, 2020 | |
LMB Warrants |
|
| 0.50 |
|
|
| 1,108,249 |
|
| August 18, 2020 – March 14, 2021 | |
LMB Warrants |
|
| 0.91 |
|
|
| 796,649 |
|
| March 24, 2022 – April 29, 2022 | |
Financial Advisor Warrants |
|
| 0.20 |
|
|
| 1,000,000 |
|
| August 15, 2021 | |
|
|
|
|
|
|
| 18,059,095 |
|
|
| |
_____________________
| (1) | Fair value of these warrants are included in the derivative warrant liability |
On March 30, 2016, the Company granted warrants to purchase 3,645,297 shares of common stock in connection with the acquisition of LMB. The warrants have exercise prices between $0.41 and $1.38 per share. All warrants were vested at March 30, 2016. The fair value of the warrants was estimated at $1,071,172 and has been included in the purchase price of LMB.
On August 16, 2016, the Company granted warrants to purchase 1,000,000 shares of common stock in connection with a one year financial advisory agreement. The warrants were vested on issuance, have an exercise price of $0.20 per share and are exercisable on a cash or cashless basis. The fair value of the warrants was estimated at $477,181 and recorded as a prepaid expense on the issuance date. During the year ended September 30, 2016, the Company expensed $60,000 of the initial prepaid expense amount and the balance will be expensed over the remaining term of the agreement.
At September 30, 2016, the weighted average remaining life of all of the outstanding warrants is 3.77 years, all warrants are exercisable, and the aggregate intrinsic value for the warrants outstanding was $1,273,985.
8. RELATED PARTY TRANSACTIONS
The Company’s headquarters were previously located in Maynard, MA in the office space of a company affiliated through common ownership. In connection with the March 30, 2016 acquisition of LMB, the Company moved its principal executive offices to Cranford, NJ. The Company did not record any revenue or expense related to the use of the Maynard, MA office space as management has determined the usage to be immaterial and the affiliate has not charged for the usage.
| F-18 |
| Table of Contents |
As of September 30, 2016 and 2015, the Company owed $27,637 and $70,386, respectively, to a company affiliated through common ownership for the expenses the related party paid on the Company’s behalf and services performed by the related party.
Our Chairman of the Board, Leonard Mazur, is the cofounder and Vice Chairman of Akrimax Pharmaceuticals, LLC (“Akrimax”), a privately held pharmaceutical company specializing in producing cardiovascular and general pharmaceutical products (see Note 3).
Our Chairman of the Board, Leonard Mazur, and our Chief Executive Officer, Myron Holubiak, are co-founders and were significant shareholders in LMB. In connection with the acquisition of LMB, our Chairman purchased an additional 5,000,000 shares of the Company.
9. EMPLOYMENT AND CONSULTING AGREEMENTS
Employment Agreements
The Company entered into a three year employment agreement with its Chief Executive Officer, Leonard Mazur, effective September 12, 2014. Upon expiration, the agreement automatically renews for successive periods of one-year. The agreement requires the Company to pay base compensation plus incentives over the employment term plus severance benefits upon the occurrence of certain events as described in the agreement. Under the agreement, Leonard Mazur was granted options to purchase 3,300,000 shares of common stock (see Note 7 – Stock Options). On March 30, 2016, in connection with the acquisition of LMB, Leonard Mazur resigned as Chief Executive Officer but will continue to serve as Chairman of the Board under the current employment agreement.
On March 30, 2016, in connection with the acquisition of LMB, the Company entered into a three year employment agreement with Myron Holubiak to serve as Chief Executive Officer. Upon expiration, the agreement automatically renews for successive periods of one-year. The agreement requires the Company to pay base compensation plus incentives over the employment term plus severance benefits upon the occurrence of certain events as described in the agreement.
The Company has employment agreements with certain other employees that require the Company to pay base compensation plus incentives over the employment term plus severance benefits upon the occurrence of certain events as described in the agreement.
Consulting Agreements
Effective September 1, 2014, the Company entered into three consulting agreements. Two of the agreements are for financial consulting services including accounting, preparation of financial statements and filings with the SEC. The third agreement is for financing activities, product development strategies and corporate development. The agreements may be terminated by the Company or the consultant with 90 days written notice.
Consulting expense under the agreements for the years ended September 30, 2016 and 2015, and the nine months ended September 30, 2014 was $460,000, $348,000 and $29,000, respectively. Consulting expense for the years ended September 30, 2016 and 2015, and the nine months ended September 30, 2014 includes $48,000, $48,000 and $4,000, respectively, paid to a financial consultant who is a stockholder of the Company. In addition, one financial consulting services agreement provides for the grant of options to purchase 500,000 shares of common stock contingent upon approval by the Board of Directors. The options were granted on June 1, 2015.
10. COMMITMENTS AND CONTINGENCIES
Operating Lease
The Company leases office space from Akrimax (see Note 8) in Cranford, New Jersey at a monthly rental rate of $2,167 pursuant to an agreement which currently expires on October 31, 2017. Rent expense for the year ended September 30, 2016 was $13,002. There was no rent expense for the year ended September 30, 2015 and the nine months ended September 30, 2014. Future minimum rentals for the years ending September 30, 2017 and 2018 are $26,004 and $2,167, respectively
Legal Proceedings
The Company is not involved in any litigation that we believe could have a material adverse effect on our financial position or results of operations. There is no action, suit, proceeding, inquiry or investigation before or by any court, public board, government agency, self-regulatory organization or body pending or, to the knowledge of our executive officers, threatened against or affecting our company or our officers or directors in their capacities as such.
| F-19 |
| Table of Contents |
11. INCOME TAXES
There was no provision for federal or state income taxes for the years ended September 30, 2016 and 2015, and the nine months ended September 30, 2014 due to the Company’s operating losses and a full valuation reserve on deferred tax assets. In addition, Citius Pharmaceuticals, LLC (the accounting acquirer) was treated as a partnership for federal and state income taxes from inception until the Reverse Acquisition was completed on September 12, 2014. A partnership’s income or loss is allocated directly to the partners for income tax purposes.
The income tax benefit differs from the amount of income tax determined by applying the U.S. federal income tax rate to pretax income due to the following:
|
| Year |
|
| Year |
|
| Nine Months Ended September 30, 2014 |
| |||
|
|
|
|
|
|
|
|
|
| |||
Computed “expected” tax benefit |
|
| (35.0 | )% |
|
| (35.0 | )% |
|
| (35.0 | )% |
Increase (decrease) in income taxes resulting from: |
|
|
|
|
|
|
|
|
|
|
|
|
State taxes, net of federal benefit |
|
| (5.2 | )% |
|
| (5.2 | )% |
|
| (5.2 | )% |
Permanent differences |
|
| 4.2 | % |
|
| (4.6 | )% |
|
| — | % |
Tax reporting differences due to the reverse acquisition |
|
| — | % |
|
| — | % |
|
| 11.3 | % |
Increase in the valuation reserve |
|
| 36.0 | % |
|
| 44.8 | % |
|
| 28.9 | % |
|
|
| 0.0 | % |
|
| 0.0 | % |
|
| 0.0 | % |
Deferred income taxes reflect the net tax effects of temporary differences between the carrying amounts of assets and liabilities for financial reporting purposes and the amounts used for income tax purposes. Significant components of the Company's deferred tax assets and liabilities are as follows:
|
| September 30, 2016 |
|
| September 30, 2015 |
| ||
Deferred tax assets: |
|
|
|
|
|
| ||
Net operating loss carryforward |
| $ | 3,801,000 |
|
| $ | 1,131,000 |
|
Stock-based compensation |
|
| 703,000 |
|
|
| 384,000 |
|
Valuation allowance |
|
| (4,504,000 | ) |
|
| (1,515,000 | ) |
Deferred tax assets |
| $ | — |
|
| $ | — |
|
The Company has recorded a valuation allowance against deferred tax assets as the utilization of the net operating loss carryforward and other deferred tax assets is uncertain. There were no deferred tax assets or liabilities carried forward from Trail One, Inc. (the legal acquirer in the Reverse Acquisition) as the Company did not acquire any assets or liabilities in the Reverse Acquisition. Accordingly, during the nine months ended September 30, 2014, the valuation allowance increased by $216,000. During the years ended September 30, 2016 and 2015, the valuation allowance increased by $2,989,000 and $1,299,000, respectively. The increase in the valuation allowance during the years ended September 30, 2016 and 2015, and the nine months ended September 30, 2014 was due to the Company’s net operating loss. At September 30, 2016, the Company has a net operating loss carryforward of approximately $9,456,000 which begins expiring in 2034.
12. SUBSEQUENT EVENTS
The Company issued demand promissory notes in favor of Leonard Mazur, Chairman of the Board, on October 20, 2016 in the principal amount of $500,000, on December 9, 2016 in the principal amount of $50,000 and on December 14, 2016 in the principal amount of $100,000 (collectively, the “Notes”). The Notes mature on the earlier of December 31, 2017 or demand by the lender. And accrue interest at the prime rate plus 1%. The Board of Directors has authorized additional revolving demand promissory notes with Leonard Mazur on substantially similar terms in an aggregate principal amount of up to $2,500,000, of which $1,150,000 is outstanding at December 15, 2016.
In October 2016, the Company commenced an offering (the “2016 Offering”) of up to 15,000,000 units (the “2016 Offering Units”), each 2016 Offering Unit consists of (i) one share of common stock and (ii) a warrant to purchase one share of common stock (the “2016 Offering Warrants”) for gross proceeds of up to $6,000,000 with an over-subscription allotment of up to $2,000,000. Each 2016 Offering Unit will be sold at a negotiated price of $0.40. Each 2016 Offering Warrant shall have an exercise price of $0.55 (the “Exercise Price”). Each 2016 Offering Warrant is exercisable for a period of five years from the date of issuance. The Placement Agent will receive a 10% cash commission on the gross proceeds of each sale of the 2016 Offering Units. In addition, on each closing the Placement Agent will also receive (i) an expense allowance equal to 3% of the proceeds of the sale, and (ii) warrants to purchase a number of shares of common stock equal to 10% of the 2016 Offering Units sold at an exercise price of $0.55 per share.
On November 23, 2016, the Company sold 975,000 2016 Offering Units for gross proceeds of $390,000. Additionally, a warrant to purchase 97,500 shares of common stock was granted to the Placement Agent pursuant to the above pricing terms.
| F-20 |
| Table of Contents |
CONDENSED CONSOLIDATED BALANCE SHEETS
(Unaudited)
|
| December 31, |
|
| September 30, |
| ||
|
| 2016 |
|
| 2016 |
| ||
ASSETS | ||||||||
Current Assets: |
|
|
|
|
|
| ||
Cash and cash equivalents |
| $ | 46,764 |
|
| $ | 294,351 |
|
Prepaid expenses |
|
| 443,526 |
|
|
| 598,484 |
|
Total Current Assets |
|
| 490,290 |
|
|
| 892,835 |
|
|
|
|
|
|
|
|
|
|
Property and Equipment, Net of Accumulated Depreciation of $5,452 and $4,780 |
|
| 3,070 |
|
|
| 3,742 |
|
|
|
|
|
|
|
|
|
|
Other Assets: |
|
|
|
|
|
|
|
|
Deposits |
|
| 2,167 |
|
|
| 2,167 |
|
Deferred offering costs |
|
| — |
|
|
| 64,801 |
|
In-process research and development |
|
| 19,400,000 |
|
|
| 19,400,000 |
|
Goodwill |
|
| 1,586,796 |
|
|
| 1,586,796 |
|
Total Other Assets |
|
| 20,988,963 |
|
|
| 21,053,764 |
|
|
|
|
|
|
|
|
|
|
Total Assets |
| $ | 21,482,323 |
|
| $ | 21,950,341 |
|
|
|
|
|
|
|
|
|
|
LIABILITIES AND STOCKHOLDERS’ EQUITY | ||||||||
Current Liabilities: |
|
|
|
|
|
|
|
|
Accounts payable |
| $ | 985,865 |
|
| $ | 909,156 |
|
Accrued expenses |
|
| 1,847,062 |
|
|
| 958,101 |
|
Accrued compensation |
|
| 1,010,500 |
|
|
| 903,250 |
|
Accrued interest |
|
| 44,099 |
|
|
| 30,871 |
|
Notes payable – related parties |
|
| 1,492,970 |
|
|
| 672,970 |
|
Derivative warrant liability |
|
| 910,578 |
|
|
| 1,681,973 |
|
Due to related party |
|
| 27,637 |
|
|
| 27,637 |
|
Total Current Liabilities |
|
| 6,318,711 |
|
|
| 5,183,958 |
|
|
|
|
|
|
|
|
|
|
Commitments and Contingencies |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Stockholders’ Equity: |
|
|
|
|
|
|
|
|
Preferred stock – $0.001 par value; 10,000,000 shares authorized; no shares issued and outstanding |
|
| — |
|
|
| — |
|
Common stock – $0.001 par value; 200,000,000 shares authorized; 74,113,060 and 73,138,060 shares issued and outstanding at December 31, 2016 and September 30, 2016, respectively |
|
| 74,113 |
|
|
| 73,138 |
|
Additional paid-in capital |
|
| 34,601,644 |
|
|
| 34,029,492 |
|
Accumulated deficit |
|
| (19,512,145 | ) |
|
| (17,336,247 | ) |
Total Stockholders’ Equity |
|
| 15,163,612 |
|
|
| 16,766,383 |
|
|
|
|
|
|
|
|
|
|
Total Liabilities and Stockholders’ Equity |
| $ | 21,482,323 |
|
| $ | 21,950,341 |
|
See notes to unaudited condensed consolidated financial statements.
| F-21 |
| Table of Contents |
CONDENSED CONSOLIDATED STATEMENTS OF OPERATIONS
FOR THE THREE MONTHS ENDED DECEMBER 31, 2016 AND 2015
(Unaudited)
|
| Three Months Ended |
| |||||
|
| December 31, |
|
| December 31, |
| ||
|
| 2016 |
|
| 2015 |
| ||
|
|
|
|
|
|
| ||
Revenues |
| $ | — |
|
| $ | — |
|
|
|
|
|
|
|
|
|
|
Operating Expenses |
|
|
|
|
|
|
|
|
Research and development |
|
| 1,411,159 |
|
|
| 829,156 |
|
General and administrative |
|
| 1,132,183 |
|
|
| 294,221 |
|
Stock-based compensation – general and administrative |
|
| 241,514 |
|
|
| 121,299 |
|
Total Operating Expenses |
|
| 2,784,856 |
|
|
| 1,244,676 |
|
|
|
|
|
|
|
|
|
|
Operating Loss |
|
| (2,784,856 | ) |
|
| (1,244,676 | ) |
|
|
|
|
|
|
|
|
|
Other Income (Expense), Net |
|
|
|
|
|
|
|
|
Interest income |
|
| — |
|
|
| 15 |
|
Gain on revaluation of derivative warrant liability |
|
| 622,186 |
|
|
| 23,940 |
|
Interest expense |
|
| (13,228 | ) |
|
| — |
|
Total Other Income (Expense), Net |
|
| 608,958 |
|
|
| 23,955 |
|
|
|
|
|
|
|
|
|
|
Loss before Income Taxes |
|
| (2,175,898 | ) |
|
| (1,220,721 | ) |
Income tax benefit |
|
| — |
|
|
| — |
|
|
|
|
|
|
|
|
|
|
Net Loss |
| $ | (2,175,898 | ) |
| $ | (1,220,721 | ) |
|
|
|
|
|
|
|
|
|
Net Loss Per Share - Basic and Diluted |
| $ | (0.03 | ) |
| $ | (0.04 | ) |
|
|
|
|
|
|
|
|
|
Weighted Average Common Shares Outstanding |
|
|
|
|
|
|
|
|
Basic and diluted |
|
| 73,551,375 |
|
|
| 34,414,988 |
|
See notes to unaudited condensed consolidated financial statements.
| F-22 |
| Table of Contents |
CONDENSED CONSOLIDATED STATEMENT OF CHANGES IN STOCKHOLDERS' EQUITY
FOR THE THREE MONTHS ENDED DECEMBER 31, 2016
(Unaudited)
|
|
|
|
|
|
|
| Additional |
|
|
|
|
| Total |
| |||||||||
|
| Preferred |
|
| Common Stock |
|
| Paid-In |
|
| Accumulated |
|
| Stockholders' |
| |||||||||
|
| Stock |
|
| Shares |
|
| Amount |
|
| Capital |
|
| Deficit |
|
| Equity |
| ||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| ||||||
Balance, October 1, 2016 |
| $ | — |
|
|
| 73,138,060 |
|
| $ | 73,138 |
|
| $ | 34,029,492 |
|
| $ | (17,336,247 | ) |
| $ | 16,766,383 |
|
Issuance of common stock in private placements, net of costs |
|
| — |
|
|
| 975,000 |
|
|
| 975 |
|
|
| 181,429 |
|
|
| — |
|
|
| 182,404 |
|
Reclassification of derivative warrant liability to additional paid-in capital |
|
| — |
|
|
| — |
|
|
| — |
|
|
| 149,209 |
|
|
| — |
|
|
| 149,209 |
|
Stock-based compensation |
|
| — |
|
|
| — |
|
|
| — |
|
|
| 241,514 |
|
|
| — |
|
|
| 241,514 |
|
Net loss |
|
| — |
|
|
| — |
|
|
| — |
|
|
| — |
|
|
| (2,175,898 | ) |
|
| (2,175,898 | ) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance, December 31, 2016 |
| $ | — |
|
|
| 74,113,060 |
|
| $ | 74,113 |
|
| $ | 34,601,644 |
|
| $ | (19,512,145 | ) |
| $ | 15,163,612 |
|
See notes to unaudited condensed consolidated financial statements.
| F-23 |
| Table of Contents |
CONDENSED CONSOLIDATED STATEMENTS OF CASH FLOWS
FOR THE THREE MONTHS ENDED DECEMBER 31, 2016 AND 2015
(Unaudited)
|
| 2016 |
|
| 2015 |
| ||
Cash Flows From Operating Activities: |
|
|
|
|
|
| ||
Net loss |
| $ | (2,175,898 | ) |
| $ | (1,220,721 | ) |
Adjustments to reconcile net loss to net cash used in operating activities: |
|
|
|
|
|
|
|
|
Gain on revaluation of derivative warrant liability |
|
| (622,186 | ) |
|
| (23,940 | ) |
Stock-based compensation expense |
|
| 241,514 |
|
|
| 121,299 |
|
Depreciation |
|
| 672 |
|
|
| — |
|
Changes in operating assets and liabilities: |
|
|
|
|
|
|
|
|
Prepaid expenses |
|
| 154,958 |
|
|
| — |
|
Accounts payable |
|
| 76,709 |
|
|
| (100,464 | ) |
Accrued expenses |
|
| 888,961 |
|
|
| 526,740 |
|
Accrued compensation |
|
| 107,250 |
|
|
| — |
|
Accrued interest |
|
| 13,228 |
|
|
| — |
|
Net Cash Used In Operating Activities |
|
| (1,314,792 | ) |
|
| (697,086 | ) |
|
|
|
|
|
|
|
|
|
Cash Flows From Financing Activities: |
|
|
|
|
|
|
|
|
Proceeds from notes payable - related parties |
|
| 820,000 |
|
|
| — |
|
Net proceeds from private placements |
|
| 247,205 |
|
|
| 302,438 |
|
Net Cash Provided By Financing Activities |
|
| 1,067,205 |
|
|
| 302,438 |
|
|
|
|
|
|
|
|
|
|
Net Change in Cash and Cash Equivalents |
|
| (247,587 | ) |
|
| (394,648 | ) |
Cash and Cash Equivalents - Beginning of Period |
|
| 294,351 |
|
|
| 676,137 |
|
|
|
|
|
|
|
|
|
|
Cash and Cash Equivalents - End of Period |
| $ | 46,764 |
|
| $ | 281,489 |
|
|
|
|
|
|
|
|
|
|
Supplemental Disclosures Of Cash Flow Information and Non-cash Transactions: |
|
|
|
|
|
|
|
|
Interest paid |
| $ | — |
|
| $ | — |
|
Income taxes paid |
| $ | — |
|
| $ | — |
|
Fair value of warrants recorded as derivative warrant liability |
| $ | — |
|
| $ | 157,984 |
|
Reclassification of derivative warrant liability to additional paid-in capital |
| $ | 149,209 |
|
| $ | — |
|
See notes to unaudited condensed consolidated financial statements.
| F-24 |
| Table of Contents |
NOTES TO CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
FOR THE THREE MONTHS ENDED DECEMBER 31, 2016 AND 2015
(Unaudited)
1. NATURE OF OPERATIONS, BASIS OF PRESENTATION AND SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
Business
Citius Pharmaceuticals, Inc. (“Citius” or the “Company”) is a specialty pharmaceutical company dedicated to the development and commercialization of critical care products targeting unmet needs with a focus on anti-infectives, cancer care and unique prescription products. The Company was founded as Citius Pharmaceuticals, LLC, a Massachusetts limited liability company, on January 23, 2007. On September 12, 2014, Citius Pharmaceuticals, LLC entered into a Share Exchange and Reorganization Agreement with Citius Pharmaceuticals, Inc. (formerly Trail One, Inc.), a publicly traded company incorporated under the laws of the State of Nevada. Citius Pharmaceuticals, LLC became a wholly-owned subsidiary of Citius.
On March 30, 2016, Citius acquired Leonard-Meron Biosciences, Inc. (“LMB”) as a wholly-owned subsidiary (see “Acquisition of Leonard-Meron Biosciences, Inc.” below).
The Company had one approved product, Suprenza (phentermine hydrochloride), which it licensed out for promotion in the United States, Canada and Mexico. On July 1, 2016, the Company announced that it was discontinuing Suprenza. Since its inception, the Company has devoted substantially all of its efforts to business planning, research and development, recruiting management and technical staff, and raising capital.
Citius is subject to a number of risks common to companies in the pharmaceutical industry including, but not limited to, risks related to the development by Citius or its competitors of research and development stage products, market acceptance of its products, competition from larger companies, dependence on key personnel, dependence on key suppliers and strategic partners, the Company’s ability to obtain additional financing and the Company’s compliance with governmental and other regulations.
Acquisition of Leonard-Meron Biosciences, Inc.
On March 30, 2016, the Company acquired all of the outstanding stock of Leonard-Meron Biosciences, Inc. (“LMB”) by issuing 29,136,839 shares of its common stock. As of March 30, 2016, the stockholders of LMB received approximately 41% of the issued and outstanding common stock of the Company. In addition, the Company converted the outstanding common stock warrants of LMB into 3,645,297 common stock warrants of the Company and converted the outstanding common stock options of LMB into 1,158,770 common stock options of the Company.
The Company acquired tangible assets consisting of cash of $255,748, prepaid expenses of $20,544, property and equipment of $5,085, deposits of $2,167, and identifiable intangible assets of $19,400,000 related to in-process research and development. The Company assumed accounts payable of $244,776, accrued expenses of $598,659, accrued compensation of $615,000, accrued interest of $23,862, and notes payable of $772,970. Accordingly, the net assets acquired amounted to $17,428,277.
The fair value of LMB’s net assets acquired on the date of the acquisition, based on management’s analysis of the fair value of the 29,136,839 shares of the Company’s common stock issued for LMB’s outstanding stock, the 3,645,297 Company common stock warrants issued for LMB’s outstanding common stock warrants, and the vested portion of the 1,158,770 Company common stock options issued for LMB’s outstanding common stock options was $19,015,073. The fair value of the common stock issued was estimated at $17,482,093, the fair value of the warrants issued was estimated at $1,071,172 and the fair value of the vested options was estimated at $461,808.
The Company recorded goodwill of $1,586,796 for the excess of the purchase price of $19,015,073 over the net assets acquired of $17,428,277.
In-process research and development represents the value of LMB’s leading drug candidate which is an antibiotic solution used to treat catheter-related bloodstream infections (Mino-Lok™) and is expected to be amortized on a straight-line basis over a period of eight years commencing upon revenue generation. Goodwill represents the value of LMB’s industry relationships and its assembled workforce. Goodwill will not be amortized but will be tested at least annually for impairment.
| F-25 |
| Table of Contents |
Unaudited pro forma operating results for the three months ended December 31, 2015, assuming the acquisition of LMB had been made as of October 1, 2015, are as follows:
|
| 2015 |
| |
Revenues |
| $ | — |
|
Net loss |
| $ | (1,640,688 | ) |
Net loss per share – basic and diluted |
| $ | (0.03 | ) |
Basis of Presentation and Summary of Significant Accounting Policies
Basis of Preparation — The accompanying consolidated financial statements include the operations of Citius Pharmaceuticals, Inc., and its wholly-owned subsidiaries, Citius Pharmaceuticals, LLC, and LMB since the March 30, 2016 acquisition. All significant inter-company balances and transactions have been eliminated in consolidation.
The accompanying unaudited consolidated financial statements of the Company have been prepared in accordance with accounting principles generally accepted in the United States of America for interim financial information, without being audited, pursuant to the rules and regulations of the Securities and Exchange Commission. Accordingly, they do not include all of the information and footnotes required by accounting principles generally accepted in the United States of America for complete financial statements. In the opinion of management, all adjustments considered necessary to make the financial statements not misleading have been included. Operating results for the three months ended December 31, 2016 are not necessarily indicative of the results that may be expected for the year ending September 30, 2017. The unaudited consolidated financial statements should be read in conjunction with the audited consolidated financial statements and notes thereto included in the Company's Annual Report on Form 10-K for the year ended September 30, 2016 filed with the Securities and Exchange Commission.
Use of Estimates — Our accounting principles require our management to make estimates and assumptions that affect the reported amounts of assets and liabilities and the disclosure of assets and liabilities at the date of the financial statements, and reported amounts of revenues and expenses during the reporting period. Estimates having relatively higher significance include the accounting for acquisitions, stock-based compensation, valuation of warrants, and income taxes. Actual results could differ from those estimates and changes in estimates may occur.
Basic and Diluted Net Loss per Common Share — Basic and diluted net loss per common share is computed by dividing net loss in each period by the weighted average number of shares of common stock outstanding during such period. For the periods presented, common stock equivalents, consisting of options, warrants and convertible securities were not included in the calculation of the diluted loss per share because they were anti-dilutive.
Recently Issued Accounting Standards
In January 2017, the Financial Accounting Standards Board (“FASB”) issued Accounting Standards Update (“ASU”) No. 2017-01, Business Combinations (Topic 805): Clarifying the Definition of a Business, in an effort to clarify the definition of a business with the objective of adding guidance to assist entities with evaluating whether transactions should be accounted for as acquisitions (or disposals) of assets or businesses. The amendments of this ASU are effective for fiscal years beginning after December 15, 2017, and interim periods within those fiscal years. The adoption of this guidance is not expected to have a material impact on our consolidated financial statements.
In January 2017, the FASB issued ASU No. 2017-04, Intangibles – Goodwill and Other (Topic 350). This ASU eliminates step 2 from the goodwill impairment test by comparing the fair value of a reporting unit with the carrying amount of the reporting unit. If the carrying amount exceeds the fair value, an impairment charge for the excess is recorded. The amendments of this ASU are effective for annual or any interim goodwill impairment tests in fiscal years beginning after December 15, 2019. Early adoption is permitted for interim or annual goodwill impairment tests performed on testing dates after January 1, 2017. The Company is currently evaluating the impact of the adoption of this ASU on the consolidated financial statements.
2. GOING CONCERN UNCERTAINTY AND MANAGEMENT’S PLAN
The accompanying condensed consolidated financial statements have been prepared on a going concern basis, which contemplates the realization of assets and the satisfaction of liabilities in the normal course of business. The Company experienced negative cash flows from operations of $1,314,792 and $697,086 for the three months ended December 31, 2016 and 2015, respectively. At December 31, 2016, the Company had a working capital deficit of $5,828,421. The Company has no revenue and has relied on proceeds from equity transactions and debt to finance its operations. At December 31, 2016, the Company had limited capital to fund its operations. This raises substantial doubt about the Company’s ability to continue as a going concern.
| F-26 |
| Table of Contents |
The Company plans to raise capital through equity financings from outside investors as well as raise additional funds from existing investors and continued borrowings under related party debt agreements. There is no assurance, however, that the Company will be successful in raising the needed capital and, if funding is available, that it will be available on terms acceptable to the Company.
The accompanying condensed consolidated financial statements do not include any adjustments that might result from the outcome of the above uncertainty.
3. BUSINESS AGREEMENTS
Alpex Pharma S.A.
On June 12, 2008, the Company entered into a collaboration and license agreement (the “Alpex Agreement”) with Alpex Pharma S.A. (“Alpex”), in which Alpex granted the Company an exclusive right and license to use certain Alpex intellectual property in order to develop and commercialize orally disintegrating tablet formulations of pharmaceutical products in United States, Canada and Mexico. In addition, Alpex manufactures Suprenza, the Company’s commercialized pharmaceutical product, on a contract basis. The agreement was amended on November 15, 2011 as part of an Amendment and Coordination Agreement (see the “Three-Party Agreement” below).
Under the terms of the Alpex Agreement, as amended by the Three-Party Agreement dated November 15, 2011 (see below), Alpex is entitled to a payment per tablet manufactured and a percentage of all milestone, royalty and other payments received by the Company from Prenzamax, LLC, pursuant to a sublicense agreement (see below). In addition, under the terms of the Alpex Agreement, Alpex retained the right to use the clinical data generated by the Company to file for regulatory approval and market Suprenza in the rest of the world. In the event that Alpex has such sales, Alpex will pay the Company a percentage royalty on net sales, as defined (“Alpex Revenue”). No milestone, royalty or other payments were earned or received by the Company except for the reimbursement of certain regulatory fees under the Three-Party Agreement.
On July 1, 2016, the Company announced that it notified the Food and Drug Administration (“FDA”) and Alpex that it was discontinuing Suprenza.
Prenzamax, LLC
On November 15, 2011, the Company entered into an exclusive license agreement (the “Sublicense Agreement”) with Prenzamax, LLC (“Prenzamax”), in which the Company granted Prenzamax and its affiliates the exclusive right to commercialize Suprenza in the United States. Prenzamax is an affiliate of Akrimax, a related party (see Note 7) and was formed for the specific purpose of managing the Sublicense Agreement. Under the terms of the Sublicense Agreement, Prenzamax is to pay the Company a percentage of the product’s EBITDA, as defined (“Profit Share Payments”). In addition, Prenzamax is to reimburse the Company directly for certain development costs. These payments are to commence once Prenzamax has achieved profitability, as defined in the Sublicense Agreement. Further, under the terms of the Sublicense Agreement, Prenzamax is required to share in the royalty payment due to Alpex under the Alpex Agreement. In addition, Prenzamax is entitled to a percentage of the Alpex Revenue received by the Company. The Company has not been reimbursed for any development costs nor has it earned any Profit Share Payments.
On July 1, 2016, the Company announced that it notified Prenzamax that it was discontinuing Suprenza.
Three-Party Agreement
On November 15, 2011, the Company, Alpex and Prenzamax entered into the Three-Party Agreement wherein the terms of the Alpex Agreement were modified and Prenzamax and the Company agreed to each pay a portion of certain regulatory filing fees for as long as Prenzamax is purchasing Suprenza from Alpex pursuant to the Three-Party Agreement. During the three months ended March 31, 2016, the Company received $292,575 from Alpex as reimbursement for regulatory filing fees that were previously expensed during the three months ended December 31, 2015. The reimbursement was recorded as a reduction of research and development expenses.
On July 1, 2016, the Company announced that it notified Alpex and Prenzamax that it was discontinuing Suprenza.
| F-27 |
| Table of Contents |
Patent and Technology License Agreement
LMB has a patent and technology license agreement with Novel Anti-Infective Therapeutics, Inc., (“NAT”) to develop and commercialize Mino-Lok™ on an exclusive, worldwide (except for South America), sub licensable basis. Since May 2014, LMB has paid an annual maintenance fee of $30,000 that increases over five years to $90,000, until commercial sales of a product subject to the license. LMB will also pay annual royalties on net sales of licensed products, with royalties ranging from the mid-single digits to the low double digits. In limited circumstances in which the licensed product is not subject to a valid patent claim and a competitor is selling a competing product, the royalty rate is in the low-single digits. After a commercial sale is obtained, LMB must pay minimum aggregate annual royalties that increase in subsequent years. LMB must also pay NAT up to $1,050,000 upon achieving specified regulatory and sales milestones. Finally, LMB must pay NAT a specified percentage of payments received from any sub licensees.
4. NOTES PAYABLE – RELATED PARTIES
On March 30, 2016, the Company assumed $772,970 of demand notes payable in the acquisition of LMB. The principal balance of the notes payable to our Chairman, Leonard Mazur, was $760,470 and the principal balance of the notes payable to our Chief Executive Officer, Myron Holubiak, was $12,500. Notes with a principal balance of $704,000 accrue interest at the prime rate plus 1.0% per annum and notes with a principal balance of $68,970 accrue interest at 12% per annum. In April 2016, $600,000 of the prime rate plus 1.0% demand notes payable and accrued interest of $1,985 was repaid to Leonard Mazur.
The Board of Directors has authorized revolving demand promissory notes with Leonard Mazur in an aggregate principal amount of up to $2,500,000, of which $1,320,000 is outstanding at December 31, 2016.
On September 7, 2016, the Company issued a $500,000 demand promissory note to our Chairman, Leonard Mazur which matures on demand by the lender. The Company then issued $820,000 of additional demand promissory notes to Leonard Mazur during the three months ended December 31, 2016 which mature on the earlier of December 31, 2017 or demand by the lender. These notes accrue interest at the prime rate plus 1%.
Interest expense on notes payable – related parties was $13,228 for the three months ended December 31, 2016.
5. DERIVATIVE WARRANT LIABILITY
Derivative financial instruments are recognized as a liability on the consolidated balance sheet and measured at fair value. At December 31, 2016 and September 30, 2016, the Company had outstanding warrants to purchase 4,033,334 shares and 4,616,668 shares, respectively, of its common stock that are considered to be derivative instruments since the agreements contain “down round” provisions whereby the exercise price of the warrants is subject to adjustment in the event that the Company issues common stock for less than $0.60 per share within one-year of the original issuance of the warrants (see Note 6).
The Company performs valuations of the warrants using the Black-Scholes option pricing model which value was also compared to a Binomial Option Pricing Model for reasonableness. The Black-Scholes option pricing model requires input of assumptions including the risk-free interest rates, volatility, expected life and dividends. Selection of these inputs involves management’s judgment and may impact net loss. Due to our limited operating history and limited number of sales of our common stock, we estimate our volatility based on a number of factors including the volatility of comparable publicly traded pharmaceutical companies. The volatility factor used in the Black-Scholes option pricing model has a significant effect on the resulting valuation of the derivative liabilities on our balance sheet. The volatility calculated at December 31, 2016 was 76%. We used a risk-free interest rate of 1.93%, estimated lives of 4.02 to 4.32 years, which are the remaining contractual lives of the warrants subject to “down round” provisions, and no dividends to our common stock. The volatility calculated at September 30, 2016 was 73%. We used a risk-free interest rate of 1.14%, estimated lives of 4.10 to 4.57 years, which are the remaining contractual lives of the warrants subject to “down round” provisions, and no dividends to our common stock.
During the three months ended December 31, 2016, anti-dilution rights related to warrants to purchase 583,334 shares of common stock expired which resulted in a reclassification from derivative warrant liability to additional paid-in capital of $149,209.
The table below presents the changes in the derivative warrant liability, which is measured at fair value on a recurring basis and classified as Level 3 in the fair value hierarchy:
|
| Three Months Ended December 31, 2016 |
|
| Three Months Ended December 31, 2015 |
| ||
Derivative warrant liability, beginning of period |
| $ | 1,681,973 |
|
| $ | 738,955 |
|
Fair value of warrants issued |
|
| — |
|
|
| 157,984 |
|
Total realized/unrealized gains included in net loss |
|
| (622,186 | ) |
|
| (23,940 | ) |
Reclassification of liability to additional paid-in capital |
|
| (149,209 | ) |
|
| — |
|
Derivative warrant liability, end of period |
| $ | 910,578 |
|
| $ | 872,999 |
|
| F-28 |
| Table of Contents |
6. COMMON STOCK, STOCK OPTIONS AND WARRANTS
Common Stock
On September 15, 2016, the stockholders approved an increase in the number of shares of authorized common stock from 90,000,000 shares to 200,000,000 shares. In addition, the stockholders granted the Board of Directors the authority to affect a reverse stock split of our common stock by a ratio of not less than 1-for-8 and not more than 1-for-20 at any time prior to September 15, 2017.
Private Offerings
On September 12, 2014, the Company sold 3,400,067 Units for a purchase price of $0.60 per Unit for gross proceeds of $2,040,040. Each Unit consists of one share of common stock and one five-year warrant (the “Investor Warrants”) to purchase one share of common stock at an exercise price of $0.60 (the “Private Offering”). The Investor Warrants will be redeemable by the Company at a price of $0.001 per Investor Warrant at any time subject to the conditions that (i) the common stock has traded for twenty (20) consecutive trading days with a closing price of at least $1.50 per share with an average trading volume of 50,000 shares per day and (ii) the Company provides 20 trading days prior notice of the redemption and the closing price of the common stock is not less than $1.17 for more than any 3 days during such notice period and (iii) the underlying shares of common stock are registered.
The Company issued the Placement Agent and their designees five-year warrants (the “Placement Agent Unit Warrants”) to purchase 680,013 Units at an exercise price of $0.60 per Unit. The Placement Agent Unit Warrants are exercisable on a cash or cashless basis with respect to purchase of the Units, and will be exercisable only for cash with respect to warrants received as part of the Units.
In addition, the Placement Agent was issued warrants to purchase 1,000,000 shares of common stock exercisable for cash at $0.60 per share for investment banking services provided in connection with the transaction (the “Placement Agent Share Warrants”).
In connection with the Private Offering, the Company entered into a Registration Rights Agreement pursuant to which the Company filed a registration statement, registering for resale all shares of common stock (i) included in the Units; and (ii) issuable upon exercise of the Investor Warrants. The Company filed the Registration Statement on September 11, 2015 and it was declared effective on January 21, 2016.
During the year ended September 30, 2015, the Company sold an additional 2,837,037 Units for a purchase price of $0.54 per Unit and 200,000 Units for a purchase price of $0.60 per Unit for gross proceeds of $1,652,000. Each Unit consists of one share of common stock and one Investor Warrant (see description above).
During the year ended September 30, 2016, the Company sold an additional 4,350,001 Units for a purchase price of $0.54 per Unit and 266,667 Units for a purchase price of $0.60 per Unit for gross proceeds of $2,509,000. Each Unit consists of one share of common stock and one Investor Warrant (see description above). On May 12, 2016, the Company announced that it had completed the final phase of the Private Offering.
On March 22, 2016, the Company sold 5,000,000 shares of common stock at $0.60 per share to its Chairman of the Board, Leonard Mazur, for gross proceeds of $3,000,000. There were no expenses related to this placement.
In October 2016, the Company commenced an offering (the “2016 Offering”) of up to 15,000,000 Units at a price of $0.40 per Unit (the “2016 Offering Units”), each 2016 Offering Unit consists of (i) one share of common stock and (ii) a warrant to purchase one share of common stock (the “2016 Offering Warrants”) for gross proceeds of up to $6,000,000 with an over-subscription allotment of up to $2,000,000. Each 2016 Offering Warrant has an exercise price of $0.55 and is exercisable for five years from the date of issuance. The Placement Agent will receive a 10% cash commission on the gross proceeds of each sale of the 2016 Offering Units. In addition, on each closing the Placement Agent will also receive (i) an expense allowance equal to 3% of the proceeds of the sale, and (ii) warrants to purchase a number of shares of common stock equal to 10% of the 2016 Offering Units sold at an exercise price of $0.55 per share.
On November 23, 2016, the Company sold 975,000 2016 Offering Units for gross proceeds of $390,000. The estimated fair value of the warrants included in the 2016 Offering Units sold to the investors was $234,505. Additionally, a warrant to purchase 97,500 shares of common stock was granted to the Placement Agent pursuant to the above pricing terms. The estimated fair value of the warrant granted to the Placement Agent was $23,451. The Placement agent was paid commissions and an expense allowance of $50,700. Other costs of the placement were $156,896.
| F-29 |
| Table of Contents |
Stock Options
On September 12, 2014, the Board of Directors adopted the 2014 Stock Incentive Plan (the “2014 Plan”) and reserved 13,000,000 shares of common stock for issuance to employees, directors and consultants. On September 12, 2014, the stockholders approved the plan. Pursuant to the 2014 Plan, the Board of Directors (or committees and/or executive officers delegated by the Board of Directors) may grant stock options, stock appreciation rights, restricted stock, restricted stock units, other stock-based awards and cash-based awards. As of December 31, 2016, there were options to purchase an aggregate of 8,732,770 shares of common stock outstanding under the 2014 Plan and 4,267,230 shares available for future grants.
The fair value of each stock option award is estimated on the date of grant using the Black-Scholes option pricing model. Due to its limited operating history and limited number of sales of its Common Stock, the Company estimated its volatility in consideration of a number of factors including the volatility of comparable public companies. The Company uses historical data, as well as subsequent events occurring prior to the issuance of the consolidated financial statements, to estimate option exercises and employee terminations within the valuation model. The risk-free interest rate is based on the U.S. Treasury yield curve in effect at the time of grant commensurate with the expected term assumption. The expected term of stock options granted, all of which qualify as “plain vanilla,” is based on the average of the contractual term (generally 10 years) and the vesting period. For non-employee options, the expected term is the contractual term.
A summary of option activity under the 2014 Plan as of December 31, 2016 and the changes during the three months then ended is presented below:
Options |
| Shares |
|
| Weighted- Average Exercise Price |
|
| Weighted- Average Remaining Contractual Term |
| Aggregate Intrinsic Value |
| |||
Outstanding at October 1, 2016 |
|
| 8,732,770 |
|
| $ | 0.54 |
|
| 8.59 years |
| $ | 1,355,924 |
|
Granted |
|
| — |
|
|
| — |
|
|
|
|
|
|
|
Exercised |
|
| — |
|
|
| — |
|
|
|
|
|
|
|
Forfeited or expired |
|
| — |
|
|
| — |
|
|
|
|
|
|
|
Outstanding at December 31, 2016 |
|
| 8,732,770 |
|
| $ | 0.54 |
|
| 8.34 years |
| $ | 468,958 |
|
Exercisable at December 31, 2016 |
|
| 5,286,654 |
|
| $ | 0.45 |
|
| 7.91 years |
| $ | 336,484 |
|
Stock-based compensation expense for the three months ended December 31, 2016 and 2015 was $241,514 and $121,299, respectively.
At December 31, 2016, unrecognized total compensation cost related to unvested awards of $1,145,334 is expected to be recognized over a weighted average period of 1.58 years.
Warrants
The Company has reserved 19,131,595 shares of common stock for the exercise of outstanding warrants. The following table summarizes the warrants outstanding at December 31, 2016:
Exercise price | Number | Expiration Dates | ||||||
Investor Warrants | $ | 0.60 | 3,400,067 | September 12, 2019 | ||||
Placement Agent Unit Warrants | 0.60 | 680,013 | September 12, 2019 | |||||
Warrants underlying Placement Agent Unit Warrants | 0.60 | 680,013 | September 12, 2019 | |||||
Placement Agent Share Warrants | 0.60 | 1,000,000 | September 12, 2019 | |||||
Investor Warrants | 0.60 | 2,145,371 | March 19, 2020 – June 26, 2020 | |||||
Investor Warrants | 0.60 | 891,666 | July 2, 2020 – September 14, 2020 | |||||
Investor Warrants | 0.60 | 583,334 | November 5, 2020 – November 20, 2020 | |||||
Investor Warrants | 0.60 | 2,133,334 | (1) | January 7, 2021 – March 21, 2021 | ||||
Investor Warrants | 0.60 | 1,900,000 | (1) | April 15, 2021 – April 25, 2021 | ||||
LMB Warrants | 0.41 | 1,352,266 | June 12, 2019 - March 2, 2021 | |||||
LMB Warrants | 0.66 | 122,319 | September 30, 2019 - January 8, 2020 | |||||
LMB Warrants | 1.38 | 265,814 | November 3, 2019 - March 6, 2020 | |||||
LMB Warrants | 0.50 | 1,108,249 | August 18, 2020 – March 14, 2021 | |||||
LMB Warrants | 0.91 | 796,649 | March 24, 2022 – April 29, 2022 | |||||
Financial Advisor Warrants | 0.20 | 1,000,000 | August 15, 2021 | |||||
2016 Offering Warrants | 0.55 | 975,000 | November 23, 2021 | |||||
2016 Offering Placement Agent Warrants | 0.55 | 97,500 | November 23, 2021 | |||||
19,131,595 | ||||||||
__________
| (1) | Fair value of these warrants are included in the derivative warrant liability |
| F-30 |
| Table of Contents |
On November 23, 2016, the Company sold 975,000 2016 Offering Units, at a price of $0.40 per Unit, consisting of (i) one share of common stock and (ii) a warrant to purchase one share of common stock. Each 2016 Offering Warrant has an exercise price of $0.55 and is exercisable for five years from the date of issuance. Additionally, a warrant to purchase 97,500 shares of common stock was granted to the Placement Agent pursuant to the above pricing terms.
At December 31, 2016, the weighted average remaining life of all of the outstanding warrants is 3.59 years, all warrants are exercisable, and the aggregate intrinsic value for the warrants outstanding was $280,568.
7. RELATED PARTY TRANSACTIONS
As of December 31, 2016 and September 30, 2016, the Company owed $27,637 to a company affiliated through common ownership for the expenses the related party paid on the Company’s behalf and services performed by the related party.
Our Chairman of the Board, Leonard Mazur, is the cofounder and Vice Chairman of Akrimax Pharmaceuticals, LLC (“Akrimax”), a privately held pharmaceutical company specializing in producing cardiovascular and general pharmaceutical products (see Note 3).
Our Chairman of the Board, Leonard Mazur, and our Chief Executive Officer, Myron Holubiak, were co-founders and significant shareholders in LMB. In connection with the acquisition of LMB, our Chairman purchased an additional 5,000,000 shares of the Company.
The Company has outstanding debt due to Leonard Mazur and Myron Holubiak (see Note 4).
General and administrative expense for each of the three months ended December 31, 2016 and 2015 includes $12,000 paid to a financial consultant who is a stockholder of the Company.
8. OPERATING LEASE
LMB leases office space from Akrimax (see Note 7) in Cranford, New Jersey at a monthly rental rate of $2,167 pursuant to an agreement which currently expires on October 31, 2017. Rent expense for the three months ended December 31, 2016 was $6,501. There was no rent expense for the three months ended December 31, 2015.
9. SUBSEQUENT EVENTS
During January 2017, the Company issued additional demand notes to its Chairman in the aggregate amount of $260,000 under the same terms as the notes issued during the three months ending December 31, 2016.
During January 2017, the Company issued 445,932 shares of its common stock for investor relations services.
During February 2017, the Company sold an additional 399,750 Offering Units for gross proceeds of $159,900. Additionally, a warrant to purchase 39,975 shares of common stock was granted to the Placement Agent pursuant to the 2016 Offering pricing terms.
F-31