Exhibit 99.1

INVESTOR PRESENTATION OTCPink: BNKL August 2022

OTCPink: BNKL Forward Looking Statements This document is private and confidential, and contains sensitive business information of BIONIK Laboratories Corp. (“BIONIK”) and is not for public distribution. This document has been prepared for informational purposes only and is provided personally to you. By accepting this document you agree to keep its contents strictly confidential, not to copy any portion of this document and to return it to BIONIK promptly upon its request. This document does not constitute an offer to sell or a solicitation of an offer to purchase any securities of BIONIK Any such offer will be made only pursuant to an effective registration statement or an exemption from registration. This document contains forward - looking statements relating to future events or the future financial performance and operations of BIONIK. Forward - looking statements, which involve assumptions and describe BIONIK’s intent, belief or current expectations about its business opportunities, prospects, performance and results, are generally identifiable by use of the words “may,” “could,” “should,” “will,” “would,” “expect,” “anticipate,” “plan,” “potential,” “estimate,” “believe,” “intend,” “project,” “forecast,” the negative of such words and other variations on such words or similar terminology. These forward - looking statements are not guarantees of future performance and by their nature involve known and unknown risks and uncertainties that may cause actual opportunities, prospects, performance and results to vary from those presented in this document, and those variances may be material. In evaluating such statements, prospective investors should carefully consider the various risks and uncertainties identified in BIONIK’s public filings with the Securities and Exchange Commission, such as market risk, liquidity risk, competitive risk, regulatory risk and other commonly recognized forms of risk relating to BIONIK and its securities. In light of these risks, uncertainties and assumptions, the forward - looking events discussed in this document might not occur, BIONIK is not obligated to publicly update or revise any forward - looking statements, whether as a result of new information, future events or otherwise.

Investor presentation Company Overview BIONIK Laboratories (OTCPink: BNKL) is a global pioneering healthcare company working to transform neurorehabilitation by helping millions reclaim their mobility through cloud - connected, robotic evaluation and therapy. BIONIK’s suite of robotic rehabilitation products are the result of groundbreaking medical engineering research and development at the Massachusetts Institute of Technology (MIT). 3

Investor presentation Investment Highlights Gold standard for robotic upper extremity rehabilitation Expanding distribution to new international markets including the EU, China, Canada & Brazil Expanding portfolio of products and AI capabilities to increase connectivity, productivity and patient care outcomes In process of launching “Centers of Excellence Neuro - Recovery Care Centers”, a showcase for BIONIK technology and solutions Increasing market opportunity from growing global market for recovery and rehabilitation, projected at $17.5 billion in 2025 Growing installed base of BIONIK devices increasing awareness and usage Strong balance sheet with $2.2 million working capital as of June 30, 2022 Management team and Board with significant experience 4

Investor presentation Market Opportunity BIONIK is targeting a growing global market for recovery and rehabilitation: 5 The global market for rehabilitation devices is projected to reach $16.6 billion in 2025 The rehabilitation robotics market is forecast to be valued at $2.6 billion by 2026 The global neurorehabilitation devices market is projected to reach $4.9 billion by 2028 The physical therapy market is expected to reach $45 billion by 2023

Investor presentation Stroke by Numbers 6 Someone in the United States has a stroke every 40 seconds Worldwide, 15 million people suffer a stroke each year Up to 30% of stroke survivors are left permanently disabled, requiring specialized treatment for rehabilitation Heart disease and stroke cost the US healthcare system $214 billion per year An aging population, worldwide, means the prevalence of stroke is expected to increase 7.8 million Americans have experienced a stroke in their lifetime.

Investor presentation Overview 7 BIONIK InMotion systems are the gold standard for robotic upper extremity rehabilitation as supported by 200 peer reviewed clinical studies. 450 BIONIK devices now installed in 20 countries worldwide, with more than 300 of these in the United States. A collaboration with the Kindred Hospital Network has resulted in approximately 30 Kindred locations that are currently equipped with InMotion devices Robust IP portfolio includes 5 issued US patents granted, and 3 US patent applications pending BIONIK devices were originally developed by medical engineering experts at MIT and are backed by data gathered from over 1,000 patients in clinical trials. MIT - MANUS – Cambridge, MA

Investor presentation The BIONIK Solution 8 BIONIK's devices deliver compelling economic benefits with a ~25% reduction in healthcare costs following InMotion therapy. The company's robotic devices provide hope and motivation for patients and their families as they work through recovery. BIONIK Robotic solutions provide high repetition (dosage) by a factor of 10 as compared to traditional treatments. BIONIK InMotion Therapy is a safe and effective treatment for both acute and chronic patients with a wide range of impairments ranging from mild to severe BIONIK is in process of positioning itself as a stroke care expert with owned and operated Centers of Excellence to be located throughout the U.S.

Investor presentation InMotion Therapy is the Established Standard of Care for Upper Extremity Neuro Rehab 9 • Supported by more than 200 independent, peer - reviewed articles beginning with ground - breaking MIT research. 1 • Significant improvements in chronic stroke 2 • Improvements seen 3 years after ceasing Rx 3 • 34% reduction healthcare expenses at 36 weeks 4 • Robotic therapy recommended as a Best Practice in the American Heart Assoc. and Veterans Administration/DoD Guidelines for Stroke Care 5 • Adopted by leading Health Care Systems as part of the Standard of Care • Used in over 250 Rehabilitation Centers Worldwide 1. See a broad sample of relevant articles at: bioniklabs.com/published - research 2. Krebs et al, 2008 3. Volpe et al., 1999 4. Lo et al, 2010 AKA the VA Study 5. VA/DoD Clinical Practice Guideline for the Management of Stroke Rehabilitation, Guideline Summary.

Investor presentation Focus on Clinical Results 10

Investor presentation Product Portfolio BIONIK’s product portfolio includes two robotic devices and one cloud - based data software solution 11 Provides safe and effective sensorimotor therapy to patients with real - time "assistance - as - needed” Clinicians can customize therapy to the specific needs of each patient Incorporates a hand grasping training component to InMotion ARM, combining isolated movement patterns into reach and grasp retraining activities Monitors the patient's movements during therapy, helping them complete the hundreds of repetitions required for neural plasticity. A cloud - based software solution that enables real - time monitoring of InMotion robot usage and generates valuable data that can be used in decision - making by hospital administrators In the future, InMotion connect will use the data gathered from each patient to optimize individual treatments BIONIK has plans to develop an app and patient portal to facilitate patient and family connectivity with treatment

Investor presentation Competitive Advantages BIONIK's robotic devices and InMotion cloud - based software solution offer evidence - based therapy intervention, making rehabilitation methods and processes smarter and more intuitive, and potentially facilitating faster patient recovery. 12 Suite of products backed by 20 years of clinical research Smart, intuitive rehabilitation with outcomes of 1000 patients demonstrated in clinical trials Patient data tracked over 200 times per second, with benefits for patients and clinicians as therapy can be individualized Compelling economic benefits for insurers and providers Cost - effective solutions for facility administrators More than 300 devices currently installed in the United States, with a 72% increase reported in patient sessions on devices used in Kindred facilities nationwide in the past year
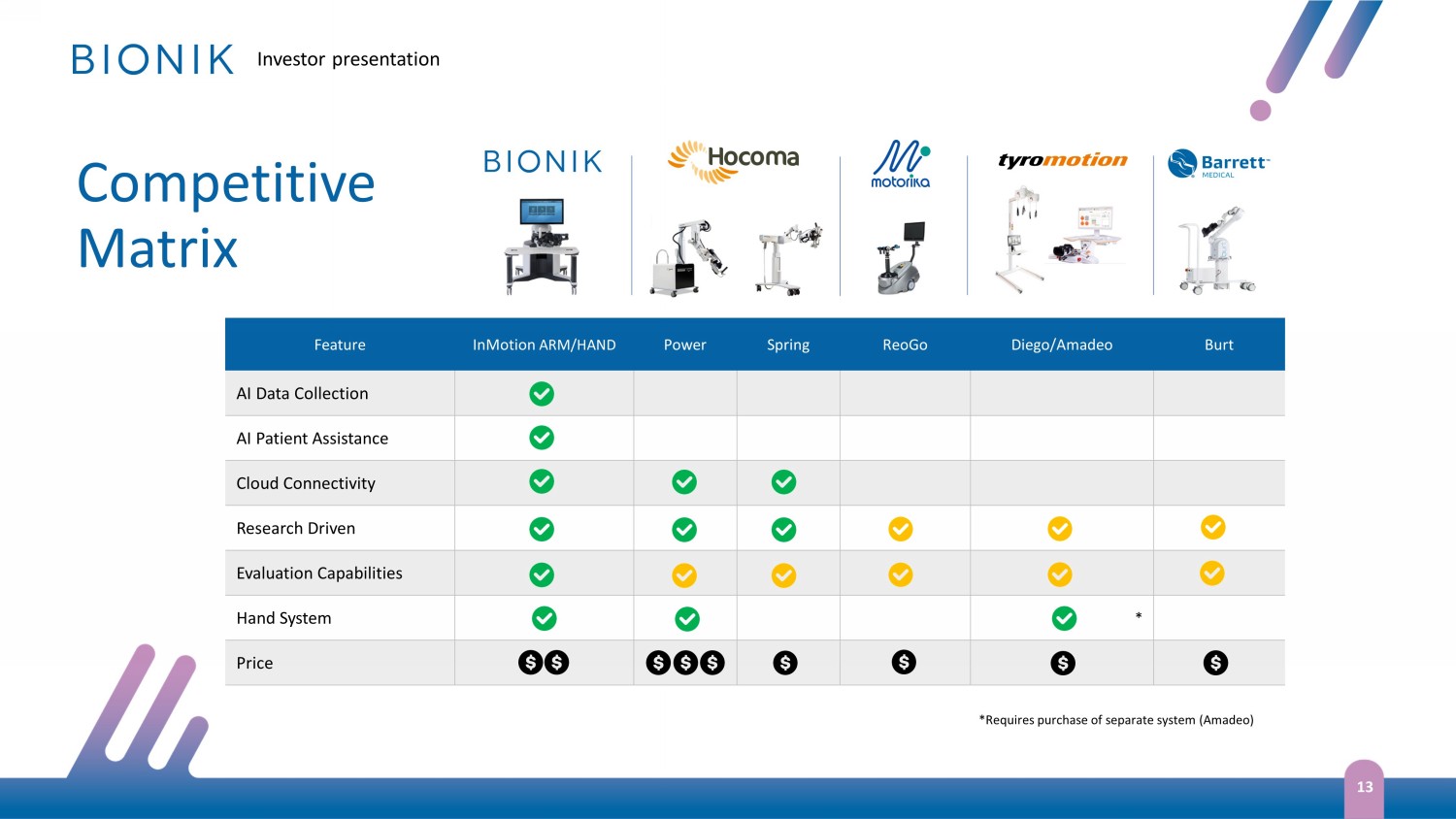
Investor presentation Feature InMotion ARM/HAND Power Spring ReoGo Diego/Amadeo Burt AI Data Collection AI Patient Assistance Cloud Connectivity Research Driven Evaluation Capabilities Hand System * Price Competitive Matrix 13 *Requires purchase of separate system (Amadeo)

Investor presentation Growth Strategy 14 DATA ENHANCEMENT • IMC - Data to Patient • New SaaS Products CENTERS OF EXCELLENCE • Neuro - recovery Care Continuum • BIONIK Technology COMMERCIAL • Expansion of InMotion Tech to New Markets • EU, China, Canada & Brazil NEW TECHNOLOGY AND PRODUCTS • M & A • IoT / Data Integration with existing products

Investor presentation Centers of Excellence 15 Neuro - recovery Care Centers Showcase for BIONIK technology and solutions which will act as a vehicle for data strategy and bringing data directly to patients Goal is to expand the continuum of care beyond the boundaries of insurance through robotics Top - line revenues expected instantly with a net profit margin between 20 - 40% Allows for recurring revenues with Patient Care Model Growing opportunity from expanding Physical Therapy market estimated at $45B in 2023

Investor presentation InMotion Connect TM Data is Just the Tip of the Iceberg BIONIK - Products & Technology 2.6 16 InMotion Connect TM : Today • Tracking of patient usage • Tracking number of repetitions • Static evaluations • Anonymized data InMotion Connect TM : Tomorrow • Detailed real - time tracking of patient usage • Tracking all activities • Seamless evaluations • Anonymized and non - anonymized data • Real - time patient movement • Real - time performance of all evaluation metrics • Tracking of key activities • Key performance metrics on two evaluations • Clinician usage • 200 times per second tracking of coordination and force of patient • Integration of other healthcare data from outside of InMotion robotic devices • Prediction of treatment protocol • Prediction of key metric outcomes • Recommendations on dosage • Large enough data sets to build meaningful artificial intelligence - based metrics, outcomes, and predictions BIG DATA Bringing Big Data to the Surface

Investor presentation 4.7 ๏ Each market segment / geography is evaluated based on key market variables (size, regulatory and reimbursement, appetite for advanced technologies, effectiveness of local 3 rd party distribution and existing base . ๏ Current Market Priorities » Strategic accounts development for US Market is most significant and most attainable market over next two years » Continued support and realization of market opportunity for Korea » Assessment of Europe based on country by country match to our key market variables with reference to our existing client base : target is to have 2 to 4 countries being directly operated by Bionik and the rest with distributors 3 4 2 1 Decision Methodology for Market Expansion 4.6 5 BIONIK - Business Model & Strategy Planned Commercial Expansion Boston, USA (Watertown) – Headquarters, Engineering, R&D, Quality and regulatory, Service and Maintenance & Contract Manufacturing North America 1 2 Korea 3 Customers in 5 countries, multiple locations Developing Europe based sales organization Evaluating 3 rd Party Distribution Relationships CE Mark Certification achieved Europe & Middle East 4 Distribution relationship with Curexo Regulatory approval for InMotion Arm TM Existing customer base in place 5 Customers in 7 countries outside of China and Korea Evaluating 3 rd Party Distribution Relationships Rest of Asia China Preparing for a new Joint Venture partner Continued positive market signs in terms of size and market pricing 17 17

Investor presentation Pathway to Success BIONIK is positioning for growth in the near, medium and long term: 18 Expansion of sales team throughout the world via distributor partnerships and entry into new markets Developing a joint - venture in China to address the Chinese market Planning to launch of Centers of Excellence strategy in Fall 2022 with purchase of first center Development of scalable business processes Over the next 24 months, BIONIK plans to position itself as a stroke care expert with a chain of Centers of Excellence Centers of Excellence expected to be a showcase for BIONIK robotics and technology Expect to foster relationships with insurance providers and local medical professionals for patient referrals Per clinic revenue estimated at $0.5 - 2 million annually Data Offering: BIONIK is developing its data and AI capabilities via a partnership with machine learning expert, Bitstrapped M&A: BIONIK to explore opportunities to acquire complementary technology or facilities Near Term Medium Term Long Term

Investor presentation Impacting Insurance Reimbursement Now and in the Future InMotion Eval – objective data used to extend coverage period Private Insurances and CMS moving towards Valued Based Care Model • Medicare payments based on the quality of care provided, rather than on the quantity of services performed. • Data supports robotic therapy as a more effective, value - based quality treatment. 1 French Rehabilitation Center Funding Reform – Pending 2023 • Goal – Better Insurance reimbursement for specialized care • Annex 4: Specialized technical platform - Specifications for an assisted rehabilitation platform for the upper limb Promote and drive higher reimbursement amounts with evidence - based, cost - effective robotic therapy. • Cost effective standard robotic treatments 19 1. Using IoT Data to Quantify InMotion Therapy Gains on Upper Extremity Motor Impairments (Bionik Labs - March 2022)

Investor presentation 20 André Jacques Auberton - Hervé Chairman of the Board Rémi Gaston Dreyfus Independent Director Peter Gerald Malone Independent Director Audrey D. Thévenon Independent Director Joseph Martin Independent Director Charles Martine Independent Director Michal Prywata Co - Founder Professor Neville Hogan Scientific Advisor Richard Russo, Jr. CFO & Interim CEO Richard brings over 20 years of finance and accounting leadership experience. VP of finance and US CFO of ICarbonX, where he was responsible for the merger of 3 companies, fundraising and the ultimate dissolution of the US companies. He started his career as an auditor at PwC Mark Eiseman Director of Sales Mark started with BIONIK in 2022 and serves as our Sales Director leading the Sales and Clinical Teams. Mark graduated from the University of Nebraska at Omaha and has worked with advanced rehabilitation technology companies for over 10 years, such as AlterG, Ekso Bionics and most recently as a Total Solutions Specialists for DIH (Hocoma). Dan Gonsalves Corporate Controller John Smathers Director of Operations John brings over 27 years of experience in development of market leading medical devices for radiation oncology, neurosurgery, and neurocritical care. He joined BIONIK from the Codman Specialty Surgical division of Integra LifeScience. * Visit www.bioniklabs.com for full biographies Dan has over 16 years of Finance and Accounting leadership experience and is a licensed CPA. Dan spent 4 years with DXL Group where he held Director roles in Accounting and Financial Planning and Analysis. Prior to DXL Group, Dan spent 6 years with CFGI advising companies across a variety of industries on technical accounting issues, financial statement preparation and IPO readiness, and internal controls. Dan started his career with Deloitte in the Audit practice. Management Team & Board of Directors*
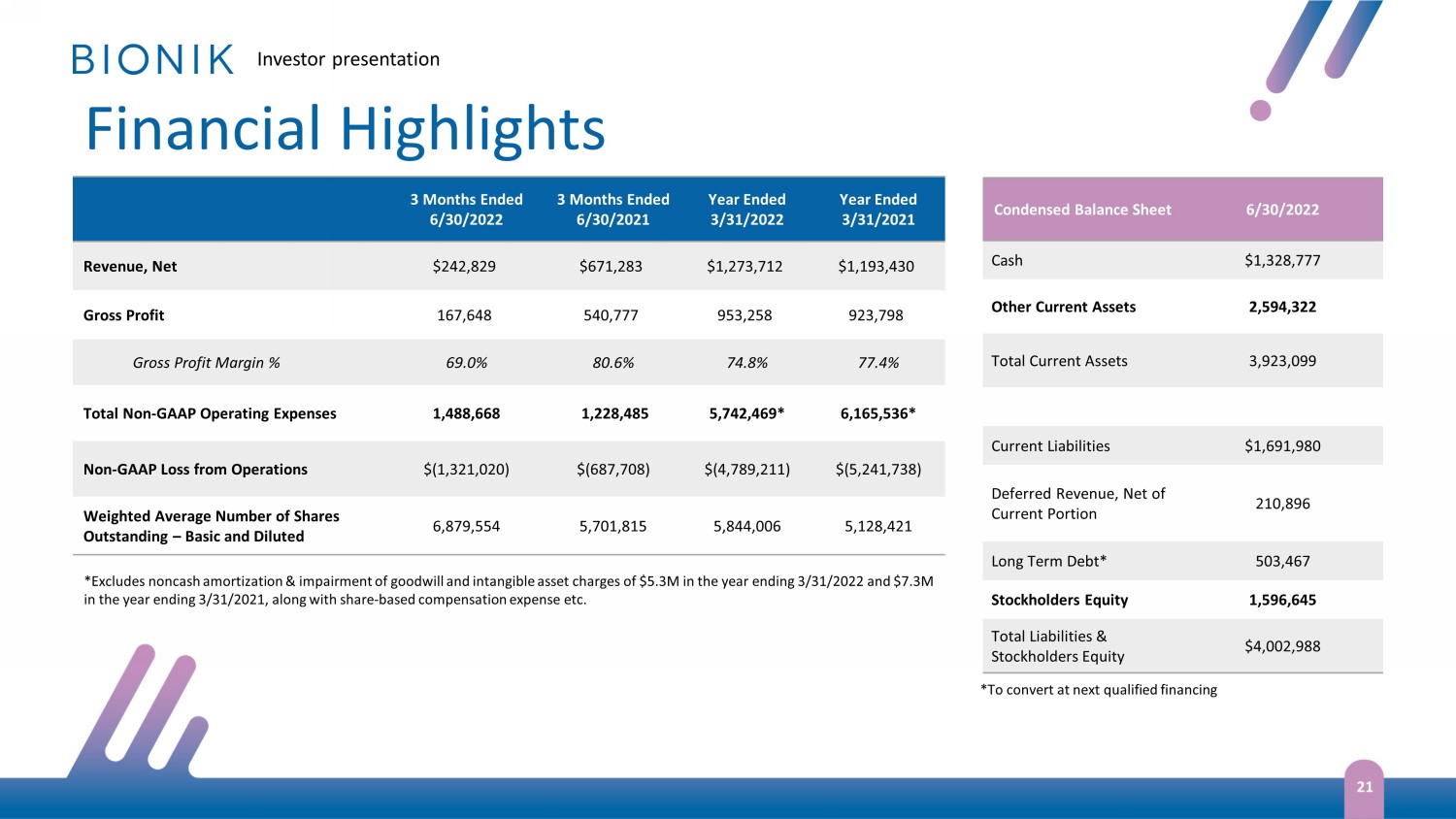
Investor presentation Financial Highlights 21 3 Months Ended 6/30/2022 3 Months Ended 6/30/2021 Year Ended 3/31/2022 Year Ended 3/31/2021 Revenue, Net $242,829 $671,283 $1,273,712 $1,193,430 Gross Profit 167,648 540,777 953,258 923,798 Gross Profit Margin % 69.0% 80.6% 74.8% 77.4% Total Non - GAAP Operating Expenses 1,488,668 1,228,485 5,742,469* 6,165,536* Non - GAAP Loss from Operations $(1,321,020) $(687,708) $(4,789,211) $(5,241,738) Weighted Average Number of Shares Outstanding – Basic and Diluted 6,879,554 5,701,815 5,844,006 5,128,421 Condensed Balance Sheet 6/30/2022 Cash $1,328,777 Other Current Assets 2,594,322 Total Current Assets 3,923,099 Current Liabilities $1,691,980 Deferred Revenue, Net of Current Portion 210,896 Long Term Debt* 503,467 Stockholders Equity 1,596,645 Total Liabilities & Stockholders Equity $4,002,988 * Exc ludes noncash amortization & impairment of goodwill and intangible asset charges of $5.3M in the year ending 3 /31/2022 and $7.3M in the year ending 3 /31/2021, along with share - based compensation expense etc. *To convert at next qualified financing

Investor presentation Key Takeaways 22 Gold standard for robotic upper extremity rehabilitation Expanding distribution to new international markets including the EU, China, Canada & Brazil Expanding portfolio of products and AI capabilities to increase connectivity, productivity and patient care outcomes In process of launching “Centers of Excellence Neuro - Recovery Care Centers”, a showcase for BIONIK technology and solutions Increasing market opportunity from growing global market for recovery and rehabilitation, projected at $17.5 billion in 2025 Growing installed base of BIONIK devices increasing awareness and usage Strong balance Sheet with $2.2 million working capital as of June 30, 2022

Global Headquarters 80 Coolidge Hill Road Watertown, MA 02472 United States Tel: +1 617.926.4800 support@bioniklabs.com OTCPink: BNKL






















