Exhibit 99.2

Exhibit 99.2
ARATANA
THERAPEUTICS
A new beginning ... in pet biologics October 2013
Vet
THERAPEUTICS

Safe Harbor Statement
Special Note Regarding Forward-Looking Statements
This presentation contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. All statements contained in this presentation that do not relate to matters of historical fact should be considered forward-looking statements, including statements regarding our proposed acquisition of Vet Therapeutics; proposed private placement of shares of common stock; expectations regarding the approval of products; the prospects for Vet Therapeutics; expected future cash balance and liquidity; expectations regarding development programs, trials, studies, and approval; expectations regarding in-license initiatives; and expectations regarding the Company’s plans and opportunities.
These forward-looking statements are based on management’s current expectations. These statements are neither promises nor guarantees, but involve known and unknown risks, uncertainties and other important factors that may cause our actual results, performance or achievements to be materially different from any future results, performance or achievements expressed or implied by the forward-looking statements, including, but not limited to, the following: our limited operating history and expectations of losses for the foreseeable future; our lack of commercial sales; our failure to obtain any necessary additional financing; our substantial dependence on the success of our current compounds, AT-001, AT-002 and AT-003, which are still in development; our inability to identify, license, develop and commercialize additional product candidates; our inability to obtain regulatory approval for our existing or future product candidates; the lack of commercial success of our current or future product candidates; uncertainties regarding the outcomes of studies regarding our products; effects of competition; our failure to attract and keep senior management and key scientific personnel; our complete reliance on third-party manufacturers and third parties to conduct all our target animal studies and certain other development efforts; our lack of a sales organization; our significant costs of operating as a public company; our lack of effective internal control over financial reporting; changes in distribution channels for pet therapeutics; consolidation of our customers; impacts of generic products; unanticipated safety or efficacy concerns; our limited patents and patent rights; our failure to comply with our intellectual property license obligations; our infringement of third party patents and challenges to our patents or rights; our failure to comply with regulatory requirements; our failure to report adverse medical events related to our products; legislative or regulatory changes; the volatility of our stock price; our status as an “emerging growth company,” as defined in the JOBS Act; the potential for dilution if we sell shares of our common stock in future financings; the significant control over our business by our principal stockholders and management; the potential that a significant portion of our total outstanding shares could be sold into the market in the near future; effects of anti-takeover provisions in our charter documents and under Delaware law; and our intention not to pay dividends. These and other important factors discussed under the caption “Risk Factors” in the Company’s final prospectus filed with the Securities and Exchange Commission, or SEC, on June 27, 2013 relating to its Registration Statement on Form S-1, and our other reports filed with the SEC could cause actual results to differ materially from those indicated by the forward-looking statements made in this presentation. Any such forward-looking statements represent management’s estimates as of the date of this presentation. While we may elect to update such forward-looking statements at some point in the future, we disclaim any obligation to do so, even if subsequent events cause our views to change. These forward-looking statements should not be relied upon as representing our views as of any date subsequent to the date of this presentation.
ARATANA
THERAPEUTICS
2

Investment Highlights
Large, growing market
De-risked drug development
Private-pay
Portfolio approach
Scalable and capital-efficient
Pure-play, first mover
The new beginning in pet therapeutics.
ARATANA
THERAPEUTICS
3

Vet Therapeutics
Strategic Rationale
Biologics (specifically, monoclonal antibodies) promising “second front”
- Fast to market, targeted, high-value therapies
- Vet Therapeutics has a leading IP position
Oncology is a key therapeutic area
- Good fit with PETX portfolio (AT-002, AT-003)
- Dog lymphoma is a leading indication
Early commercialization potential
- Licensure anticipated within next twelve months
- If licensure achieved, limited launch in 2014 to gain clinical experience with the product pending full licensure
- Full launch may be possible as early as 2016 for T-cell product
Pipeline beyond lymphoma and cancer
- Mast Cell Tumor, Atopic Dermatitis, Feline lymphoma
- Diagnostic opportunity
ARATANA
THERAPEUTICS
4
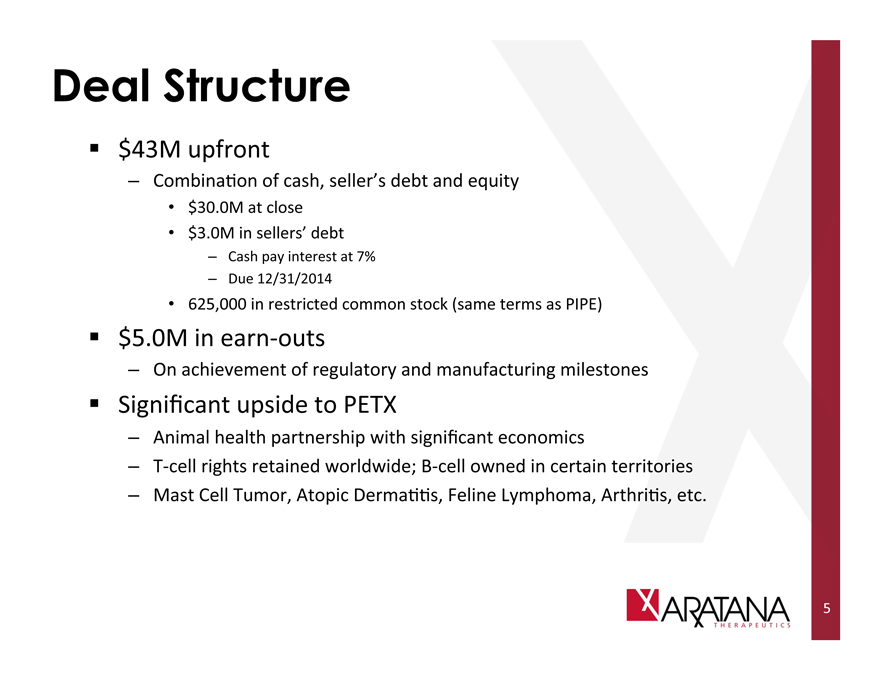
Deal Structure
$43M upfront
- Combination of cash, seller’s debt and equity
$30.0M at close
$3.0M in sellers’ debt
- Cash pay interest at 7%
- Due 12/31/2014
625,000 in restricted common stock (same terms as PIPE)
$5.0M in earn-outs
- On achievement of regulatory and manufacturing milestones
Significant upside to PETX
- Animal health partnership with significant economics
- T-cell rights retained worldwide; B-cell owned in certain territories
- Mast Cell Tumor, Atopic Dermatitis, Feline Lymphoma, Arthritis, etc.
ARATANA
THERAPEUTICS
5
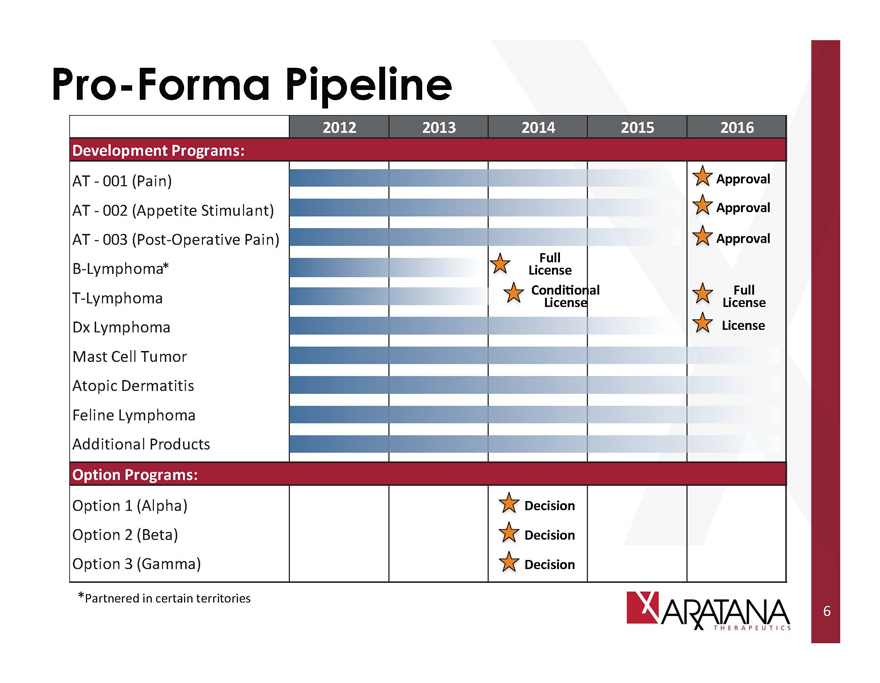
Pro-Forma Pipeline
2012 2013 2014 2015 2016
Development Programs:
AT - 001 (Pain)
AT - 002 (Appetite Stimulant)
AT - 003 (Post-Operative Pain)
B-Lymphoma*
T-Lymphoma
Dx Lymphoma
Mast Cell Tumor
Atopic Dermatitis
Feline Lymphoma
Additional Products
Option Programs:
Option 1 (Alpha)
Option 2 (Beta)
Option 3 (Gamma)
Full License
Conditional License
Decision
Decision
Decision
Approval
Approval
Approval
Full License
License
*Partnered in certain territories
ARATANA THERAPEUTICS
6
The Promise of Biologics
TRANSLATING THE BEST OF HUMAN INNOVATION TO PETS
Biology underlying cancer and immune conditions are similar
Human Biologics Standard of Care
20 years of knowledge, and experience
Pet Biologics
Engineered biologics for pets based on existing human blockbuster products
ARATANA THERAPEUTICS
7
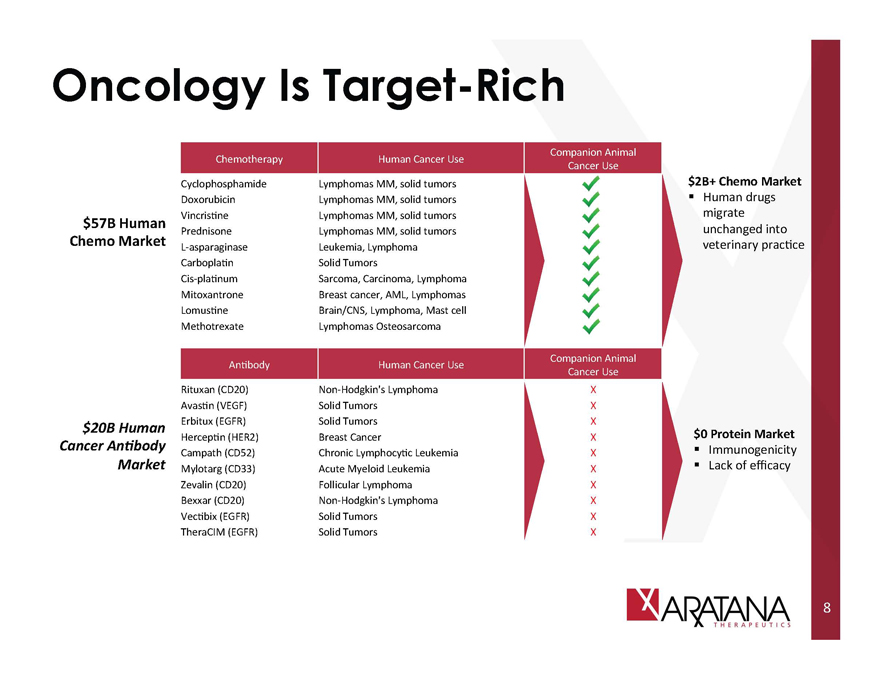
Oncology Is Target-Rich
$57B Human Chemo Market
$20B Human Cancer Antibody Market
Chemotheraphy
Cyclophosphamide
Doxorubicin
Vincristine
Prednisone
L-asparaginase
Carboplatin
Cis-platinum
Mitoxantrone
Lomustine
Methotrexate
Antibody
Rituxan (CD20)
Avastin (VEGF)
Erbitux (EGFR)
Herceptin (HER2)
Campath (CD52)
Mylotarg (CD33)
Zevalin (CD20)
Bexxar (CD20)
Vectibix (EGFR)
TheraCIM (EGFR)
Human Cancer Use
Lymphomas MM, solid tumors
Lymphomas MM, solid tumors
Lymphomas MM, solid tumors
Lymphomas MM, solid tumors
Leukemia, Lymphoma
Solid Tumors
Sarcoma, Carcinoma, Lymphoma
Breast cancer, AML, Lymphomas
Brain/CNS, Lymphoma, Mast cell
Lymphomas Osteosarcoma
Human Cancer Use
Non-Hodgkin’s Lymphoma
Solid Tumors
Solid Tumors
Breast Cancer
Chronic Lymphocytic Leukemia
Acute Myeloid Leukemia
Follicular Lymphoma
Non-Hodgkin’s Lymphoma
Solid Tumors
Solid Tumors
Companion Animal Cancer Use
Companion Animal Cancer Use
$2B+ Chemo Market
Human drugs migrate unchanged into veterinary practice
$0 Protein Market
Immunogenicity
Lack of efficacy
ARATANA THERAPEUTICS
8
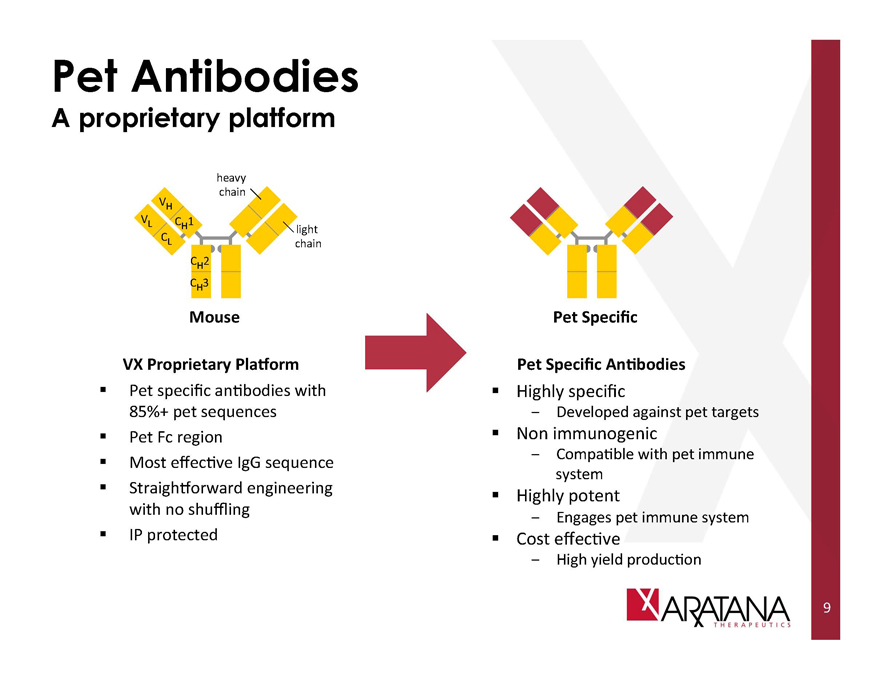
Pet Antibodies
A proprietary platform
VL VH
CL CH1
CH2 CH3
heavy chain
light chain
Mouse
VX Proprietary Platform
Pet specific antibodies with 85%+ pet sequences
Pet Fc region
Most effective IgG sequence
Straightforward engineering with no shuffling
IP protected
Pet Specific
Pet Specific Antibodies
Highly specific
- Developed against pet targets
Non immunogenic
- Compatible with pet immune system
Highly potent
- Engages pet immune system
Cost effective
- High yield production
ARATANA THERAPEUTICS
9
Intellectual Property
US Patent No. 8,337,842 (speciesization of Abs)
- Family of patents (expiration 2029)
Claims directed to heterochimeric Ab
Covers all VX products
- Dependent claims directed to both CD52 & CD20
- Additionally, a divisional directed to methods of treatment
Antibody Constant Domain Regions and Uses Thereof
- Notice of Allowance (at least 2030)
- Covers all VX products
Monoclonal Antibodies Directed to CD20 (at least 2031)
Monoclonal Antibodies Directed to CD52 (at least 2031)
Unpublished applications against IL-6, Her2 Receptor
ARATANA THERAPEUTICS
10
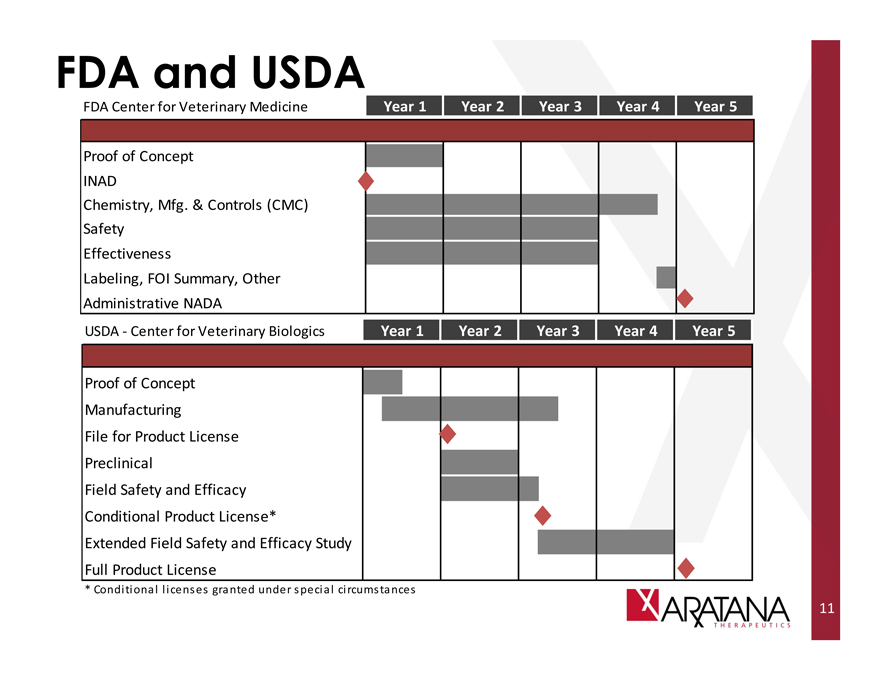
FDA and USDA
FDA Center for Veterinary Medicine
Proof of Concept INAD
Chemistry, Mfg. & Controls (CMC) Safety
Effectiveness
Labeling, FOI Summary, Other Administrative NADA
USDA - Center for Veterinary Biologics
Proof of Concept
Manufacturing
File for Product License
Preclinical
Field Safety and Efficacy
Conditional Product License*
Extended Field Safety and Efficacy Study
Full Product License
Year 1 Year 2 Year 3 Year 4 Year 5
Year 1 Year 2 Year 3 Year 4 Year 5
* Conditional licenses granted under special circumstances
ARATANA
THERAPEUTICS
11
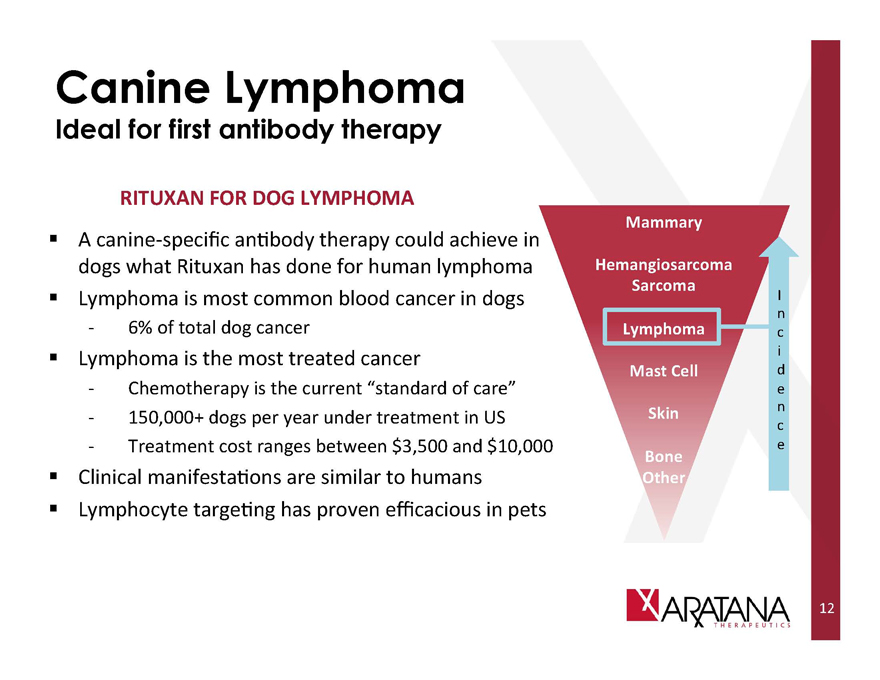
Canine Lymphoma
Ideal for first antibody therapy
RITUXAN FOR DOG LYMPHOMA
A canine-specific antibody therapy could achieve in dogs what Rituxan has done for human lymphoma
Lymphoma is most common blood cancer in dogs
6% of total dog cancer
Lymphoma is the most treated cancer
Chemotherapy is the current “standard of care” 150,000+ dogs per year under treatment in US Treatment cost ranges between $3,500 and $10,000
Clinical manifestations are similar to humans
Lymphocyte targeting has proven efficacious in pets
Mammary
Hemangiosarcoma
Sarcoma
Lymphoma
Mast Cell
Skin
Bone
Other
Incidence
ARATANA
THERAPEUTICS
12
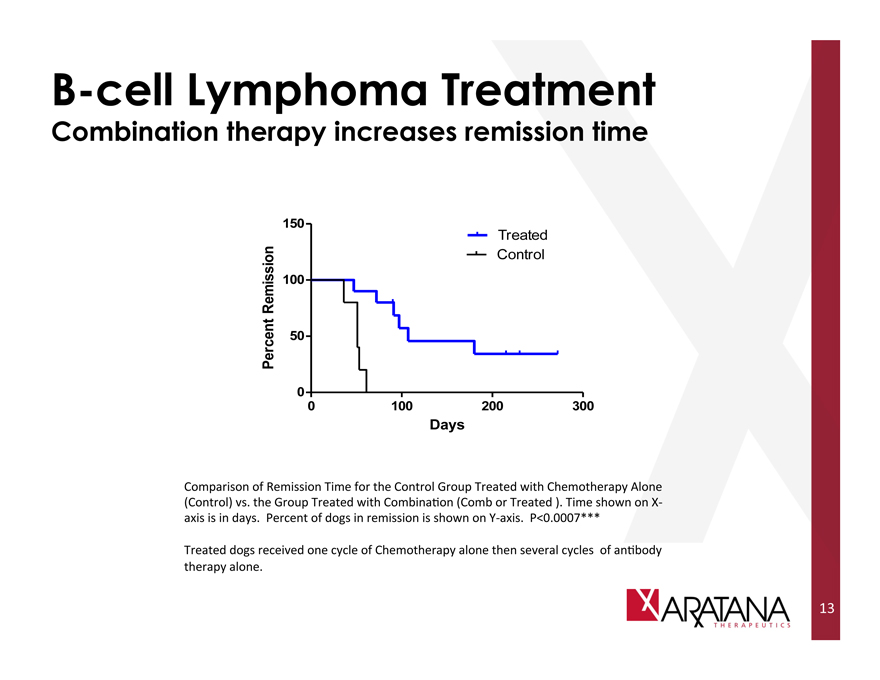
B-cell Lymphoma Treatment
Combination therapy increases remission time
Percent Remission
150
100
50
0
0
100
200
300
Days
Treated
Control
Comparison of Remission Time for the Control Group Treated with Chemotherapy Alone (Control) vs. the Group Treated with Combination (Comb or Treated). Time shown on X-axis is in days. Percent of dogs in remission is shown on Y-axis. P<0.0007***
Treated dogs received one cycle of Chemotherapy alone then several cycles of antibody therapy alone.
ARATANA
THERAPEUTICS
13
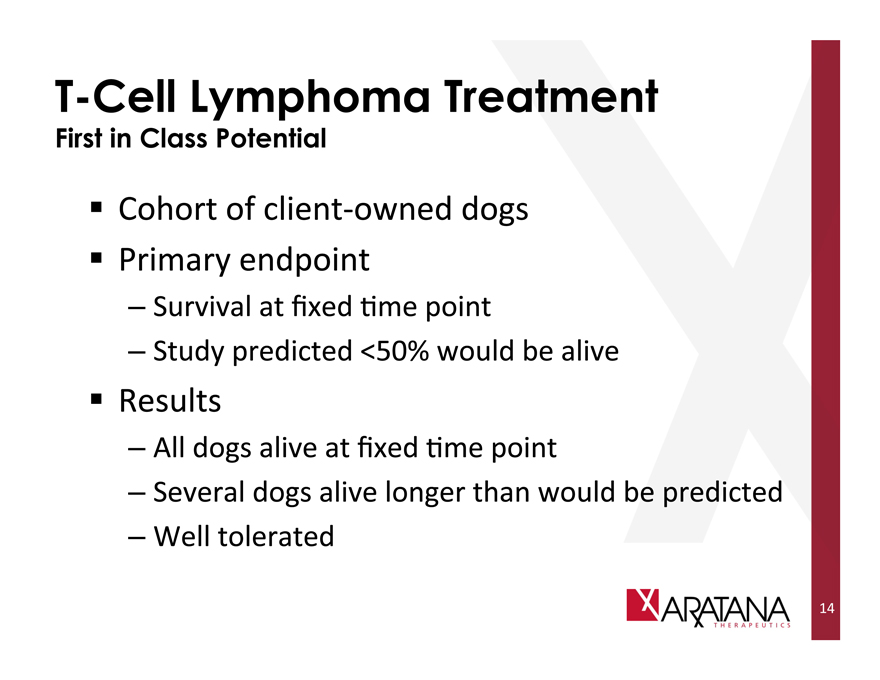
T-Cell Lymphoma Treatment
First in Class Potential
Cohort of client-owned dogs
Primary endpoint
Survival at fixed time point
Study predicted <50% would be alive
Results
All dogs alive at fixed time point
Several dogs alive longer than would be predicted
Well tolerated
ARATANA
THERAPEUTICS
14
Antibody Positioning
Standard of Care in human lymphoma comes to veterinary medicine
Canine Rituxan
Canine Campath
Lymphoma phenotyping allows B-cell and T-cell lymphomas to be treated differently
CD20 and CD52 are more precise immunotherapy targets
Value will be determined by efficacy, toxicity and ease of use
ARATANA
THERAPEUTICS
15

Strategic Fit
Pet therapeutics as the exclusive focus
Cutting edge science and innovation
— AT-001 Pain EP4 Receptor Antagonist
— AT-002 Appetite Stimulant Ghrelin Agonist
— AT-003 Post-Operative Pain Bupivicaine Liposome
PETX portfolio complementary to cancer
ARATANA
THERAPEUTICS
16
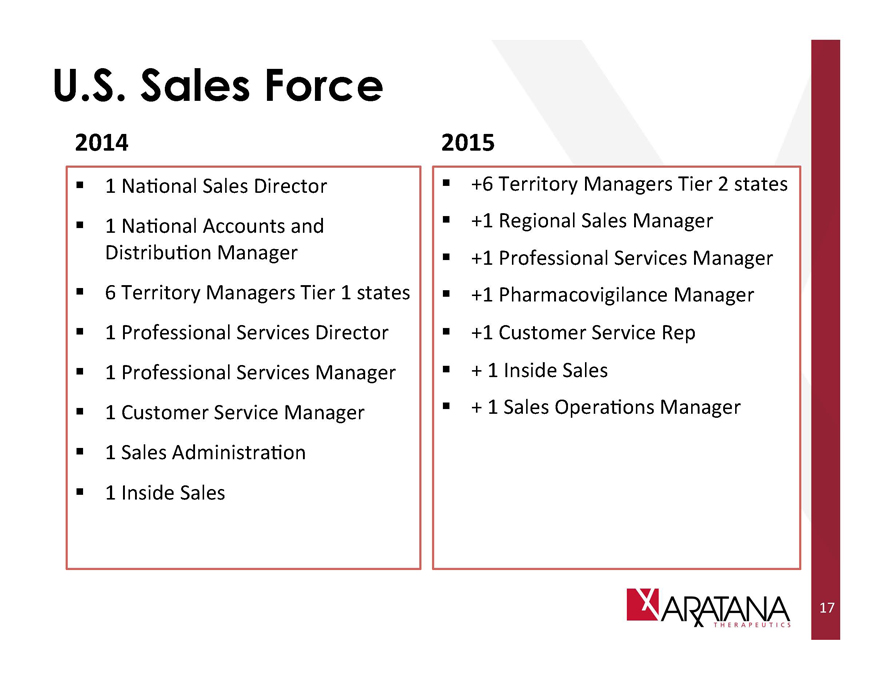
U.S. Sales Force
2014
1 National Sales Director
1 National Accounts and Distribution Manager
6 Territory Managers Tier 1 states
1 Professional Services Director
1 Professional Services Manager
1 Customer Service Manager
1 Sales Administration
1 Inside Sales
2015
+6 Territory Managers Tier 2 states
+1 Regional Sales Manager
+1 Professional Services Manager
+1 Pharmacovigilance Manager
+1 Customer Service Rep
+ 1 Inside Sales
+1 Sales Operations Manager
ARATANA
THERAPEUTICS
17
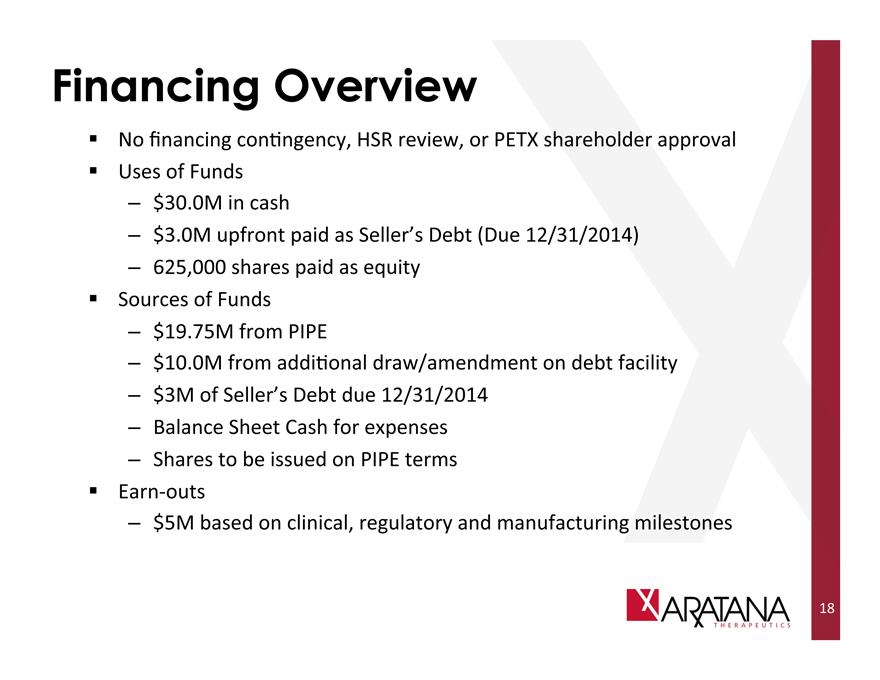
Financing Overview
No financing contingency, HSR review, or PETX shareholder approval
Uses of Funds
— $30.0M in cash
— $3.0M upfront paid as Seller’s Debt (Due 12/31/2014)
— 625,000 shares paid as equity
Sources of Funds
— $19.75M from PIPE
— $10.0M from additional draw/amendment on debt facility
— $3M of Seller’s Debt due 12/31/2014
— Balance Sheet Cash for expenses
— Shares to be issued on PIPE terms
Earn-outs
— $5M based on clinical, regulatory and manufacturing milestones
ARATANA
THERAPEUTICS
18
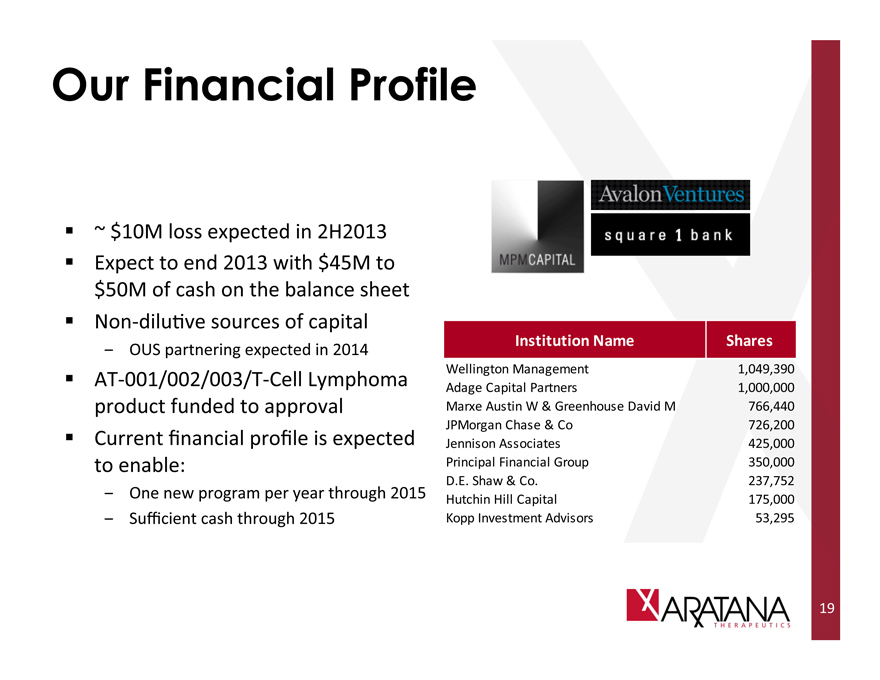
Our Financial Profile
~ $10M loss expected in 2H2013
Expect to end 2013 with $45M to $50M of cash on the balance sheet
Non-dilutive sources of capital
- OUS partnering expected in 2014
AT-001/002/003/T-Cell Lymphoma product funded to approval
Current financial profile is expected to enable:
- One new program per year through 2015
- Sufficient cash through 2015
MPMCAPITAL
Avalon
square 1 bank
Institution Name
Wellington Management
Adage Capital Partners
Marxe Austin W & Greenhouse David M
JPMorgan Chase & Co
Jennison Associates
Principal Financial Group
D.E. Shaw & Co.
Hutchin Hill Capital
Kopp Investment Advisors
Shares
1,049,390
1,000,000
766,440
726,200
425,000
350,000
237,752
175,000
53,295
ARATANA
THERAPEUTICS
19

Investment Highlights
Large, growing market
De-risked drug development
Private-pay
Portfolio approach
Scalable and capital-efficient
Pure-play, first mover
The new beginning in pet therapeutics.
ARATANA
THERAPEUTICS
20



















