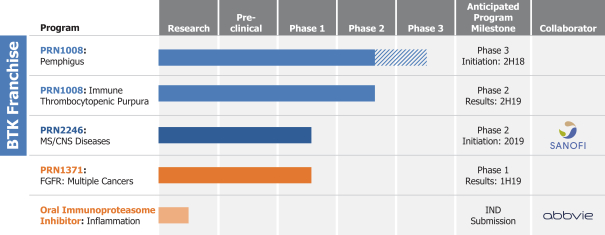
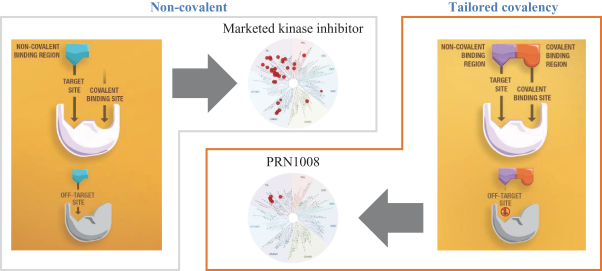
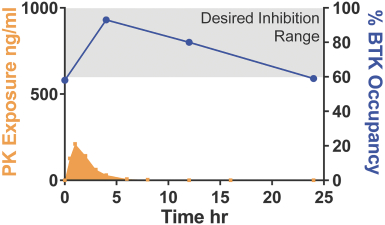
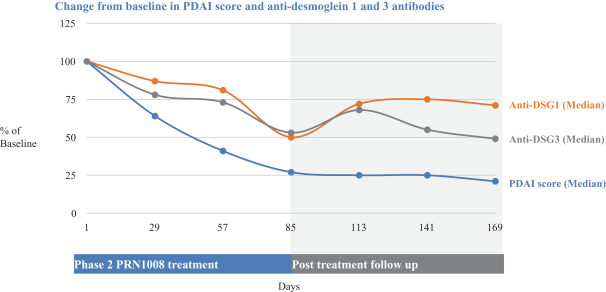
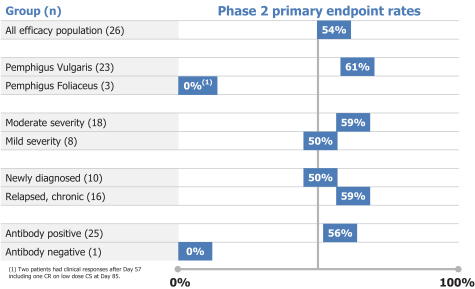
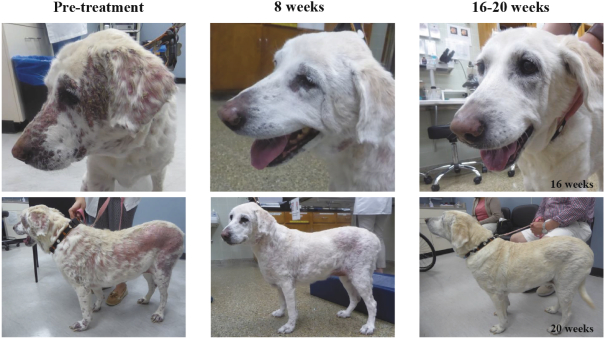
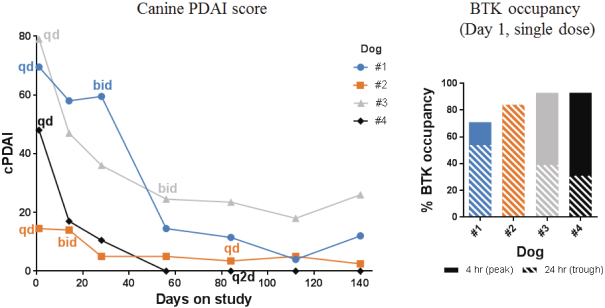
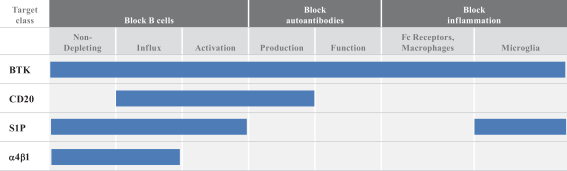
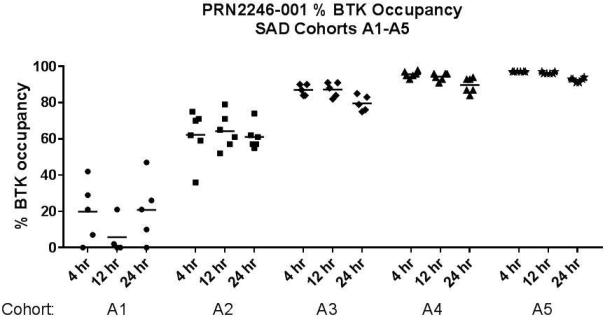
Other U.S. Regulatory Matters
Research and development activities prior to product approval and manufacturing, sales, promotion and other activities following product approval are also subject to regulation by numerous regulatory authorities in the United States in addition to the FDA, including the Centers for Medicare & Medicaid Services, other divisions of the Department of Health and Human Services, or HHS, the Department of Justice, the Drug Enforcement Administration, the Consumer Product Safety Commission, the Federal Trade Commission, the Occupational Safety & Health Administration, the Environmental Protection Agency and state and local governments.
For example, in the United States, prescription drug manufacturers must comply with federal fraud and abuse, data privacy, transparency, and other healthcare laws. These laws include the federal Anti-Kickback Statute, which makes it illegal for any person, including a prescription drug manufacturer (or a party acting on its behalf), to knowingly and willfully solicit, receive, offer or pay any remuneration that is intended to induce or reward referrals, including the purchase, recommendation, order or prescription of a particular drug, for which payment may be made under a federal healthcare program, such as Medicare or Medicaid. Moreover, the government may assert that a claim including items or services resulting from a violation of the federal Anti-Kickback Statute constitutes a false or fraudulent claim for purposes of the civil False Claims Act.
Federal civil and criminal false claims laws, including the civil False Claims Act, which can be enforced by private citizens through civil whistleblower and qui tam actions, prohibit, among other things, any person or entity from knowingly presenting, or causing to be presented, a false or fraudulent claim for payment or approval to the federal government or knowingly making, using or causing to be made or used a false record or statement material to a false or fraudulent claim to the federal government. Pharmaceutical companies have been prosecuted under these laws for, among other things, allegedly providing free product to customers with the expectation that the customers would bill federal programs for the product. Other companies have been prosecuted for causing false claims to be submitted because of the companies’ marketing of the product for unapproved, and thusnon-covered, uses.
Federal civil and criminal statutes prohibit knowingly and willfully executing, or attempting to execute, a scheme to defraud any healthcare benefit program, including private third-party payors and knowingly and willfully falsifying, concealing or covering up a material fact or making any materially false, fictitious or fraudulent statement in connection with the delivery of, or payment for, healthcare benefits, items or services.
Pricing and rebate programs must comply with the Medicaid rebate requirements of the Omnibus Budget Reconciliation Act of 1990 and more recent requirements in the Patient Protection and Affordable Care Act, as amended by the Health Care and Education Reconciliation Act of 2010, or collectively the ACA. If products are made available to authorized users of the Federal Supply Schedule of the General Services Administration, additional laws and requirements apply. Products must meet applicable child-resistant packaging requirements under the U.S. Poison Prevention Packaging Act. Manufacturing, sales, promotion and other activities also are potentially subject to federal and state consumer protection and unfair competition laws.
Payments made to institutions, physicians and other healthcare providers are subject to federal and state regulations, including the Physician Payments Sunshine Act, or the Sunshine Act. The Sunshine Act requires certain manufacturers of drugs, devices, biologicals and medical supplies, with certain exceptions, to report annually to CMS information related to payments and other transfers of value to physicians and teaching hospitals and ownership and investment interests held by physicians and their immediate family members.
Personally identifiable information, under certain conditions, is also subject to data privacy and security regulation by both federal and state governments. The federal Health Insurance Portability and Accountability Act of 1996 (HIPAA) as amended by the Health Information Technology for Economic and Clinical Health Act (HITECH), and their implementing regulations, imposes certain requirements relating to the privacy, security and
122