UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10
GENERAL FORM FOR REGISTRATION OF SECURITIES
Pursuant to Section 12(b) or 12(g) of the Securities Exchange Act of 1934
ASIA LEECHDOM HOLDING CORPORATION
(Exact name of small business issuer as specified in its charter)
| Nevada | 26-3552219 |
| (State or other jurisdiction of | (I.R.S. Employer Identification No.) |
| incorporation or organization) | |
No.55 Miyun Road
Nankai District, Tianjin City, 300111
People’s Republic of China
(Address of Principal Executive Offices; Zip Code)
| Registrant’s Telephone Number, Including Area Code:+86 22 27640191 |
| | |
| Securities to be registered under Section 12(b) of the Act: |
| | |
| Title of each class | Name of each exchange on which |
| to be so registered | each class is to be registered |
| None | None |
Securities registered pursuant to Section 12(g) of the Act:
Common Stock, par value $0.001
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See the definition for “large accelerated filer,” “accelerated filer,” and “smaller reporting company” in Rule 12b-2 of the Exchange Act. (Check one):
| Large Accelerated Filer [ ] | Accelerated Filer [ ] |
| Non-Accelerated Filer [X] | Smaller Reporting Company [ ] |
Table of Contents
| | Page |
| SPECIAL NOTE REGARDING FORWARD LOOKING STATEMENTS | 1 |
| OUR BUSINESS | 2 |
| RISK FACTORS | 17 |
| SELECTED FINANCIAL DATA | 30 |
| MANAGEMENT'S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS | 31 |
| SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT | 44 |
| DIRECTORS AND EXECUTIVE OFFICERS | 45 |
| EXECUTIVE COMPENSATION | 49 |
| CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE | 50 |
| LEGAL PROCEEDINGS | 52 |
| MARKET PRICE OF AND DIVIDENDS ON THE REGISTRANT'S COMMON EQUITY AND RELATED STOCKHOLDER MATTERS | 53 |
| RECENT SALES OF UNREGISTERED SECURITIES | 53 |
| DESCRIPTION OF REGISTRANT'S SECURITIES TO BE REGISTERED | 54 |
| INDEMNIFICATION OF DIRECTORS AND OFFICERS | 56 |
| FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA | 57 |
| CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE | 57 |
i
SPECIAL NOTE REGARDING FORWARD LOOKING STATEMENTS
This report contains forward-looking statements. The forward-looking statements are contained principally in the sections entitled “Our Business,” “Risk Factors,” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations.” These statements involve known and unknown risks, uncertainties and other factors which may cause our actual results, performance or achievements to be materially different from any future results, performances or achievements expressed or implied by the forward-looking statements. These risks and uncertainties include, but are not limited to, the factors described in the section captioned “Risk Factors” above. In some cases, you can identify forward-looking statements by terms such as “anticipates,” “believes,” “could,” “estimates,” “expects,” “intends,” “may,” “plans,” “potential,” “predicts,” “projects,” “should,” “would” and similar expressions intended to identify forward-looking statements. Forward-looking statements reflect our current views with respect to future events and are based on assumptions and subject to risks and uncertainties. Given these uncertainties, you should not place undue reliance on these forward-looking statements. These forward-looking statements include, among other things, statements relating to:
our ability to overcome competition in our market;
the impact that a downturn or negative changes in nutraceutical prices could have on our business and profitability;
our ability to simultaneously fund the implementation of our business plan and invest in new projects;
economic, political, regulatory, legal and foreign exchange risks associated with international expansion; or
the loss of key members of our senior management.
Also, forward-looking statements represent our estimates and assumptions only as of the date of this registration statement. You should read this registration statement and the documents that we reference and filed as exhibits to the registration statement completely and with the understanding that our actual future results may be materially different from what we expect. Except as required by law, we assume no obligation to update any forward-looking statements publicly, or to update the reasons actual results could differ materially from those anticipated in any forward-looking statements, even if new information becomes available in the future.
Use of Certain Defined Terms
Except as otherwise indicated by the context, references in this report to:
"ALH" are to Asia Leechdom Holding Corporation, a New Jersey corporation;
"Company," "we," "us," or "our," are references to the combined business of Asia Leechdom Holding Corporation, a Nevada corporation, together with its wholly-owned subsidiary, ALH, and ALH’s wholly-owned subsidiary, BOAI Pharm, and BOAI Pharm’s wholly-owned subsidiary, BOAI Leechdom, and majority-owned subsidiary, BOAI Bio-Pharm;
“China,��� the “state” and “PRC” are references to the People’s Republic of China;
the “Exchange Act” are to the Securities Exchange Act of 1934, as amended;
“BOAI Bio-Pharm” are references to Tianjin BOAI Bio-Pharmaceutical Co., Ltd., a PRC limited company;
“BOAI Pharm” are references to Tianjin BOAI Pharmaceutical Co., Ltd., a PRC limited company;
“BOAI Leechdom” are references to Tianjin BOAI Leechdom Technique Co., Ltd., a PRC limited company;
“RMB” are to Renminbi, the legal currency of China; and “U.S. dollar,” “USD” or “$,” are to the legal currency of the United States of America (RMB 6.8129 = $1.00 for its December 31, 2010 audited balance sheet, with the exception of the equity accounts, and RMB 6.8449 = $1.00 for its December 31, 2009 audited balance sheets, with the exception of the equity accounts; the equity accounts were stated at their historical rate; the average exchange rates applied to the income statement and statement of cash flows for the years ended December 31, 2010 and 2009 were RMB 6.8280 and RMB 6.8482, respectively); and
the “Securities Act” are to Securities Act of 1933, as amended.
- 1 -
In this registration statement we are relying on and we refer to information and statistics regarding the pharmaceutical industry that we have obtained from cited sources throughout. This information is publicly available for free and has not been specifically prepared for us for use or incorporation in this Registration Statement or otherwise.
OUR BUSINESS
Overview
We are a producer and distributor of pharmaceutical products including a variety of medical supplies, prescription and over-the-counter, or OTC, drugs in China and abroad. Through our wholly-owned PRC subsidiary, BOAI Pharm and BOAI Pharm’s wholly-owned subsidiary, BOAI Leechdom, we currently produce and sell 42 medicines and distribute over 7,000 medicines and medical devices for third-party producers and manufacturers. We market our products through a sales network covering 23 provinces and municipalities in China, including Beijing, Shanghai, Shandong, and Guangdong. We also export our products through agents to customers in North America, Europe and East Asia.
We produce our products at our 3,428.4 square meter production facility located in Tianjin, China. We currently operate 9 production lines, each serving the following product formats: (1) ointment, (2) pill, (3) tablet, (4) syrup, (5) granule, (6) capsule, (7) orally taken liquid, (8) plaster and (9) extract; and further separated into liquid preparations (such as ointment and syrup) and solid preparations (such as tablets, pills and granules). We recently obtained land use rights for another 85,938.92 square meters of property for construction of an additional production facility in Tianjin. We expect to complete the initial phase of construction for this new facility by August 2011.
We generate revenues from the sale and distribution of our products and other third party products in China and abroad. For the fiscal years ended June 30, 2010, 2009 and 2008, total revenue was $68,166,997, $49,489,066 and $32,381,120, respectively, and our net income for the same periods was $21,750,626, $15,460,736 and $11,125,217, respectively.
Our Corporate History and Background
We were organized under the laws of the State of Arizona, in 2004, as VT French Services, Inc., a wholly owned subsidiary of Visitalk Capital Corporation, (“VCC”), which in turn was a wholly owned subsidiary of Visitalk.com (“Visitalk”). As part of Visitalk’s Chapter 11 reorganization plan, we were established as a shell company with no assets or operations and VCC was authorized by the Visitalk plan to be the reorganized debtor. Effective September 11, 2008, our name was changed from VT French Services, Inc. to Bay Peak 6 Acquisition Corp., in connection with our redomestication to Nevada. On July 28, 2010, we changed our name to Asia Leechdom Holding Corp., in connection with our reverse acquisition of Asia Leechdom Holding Corp. (“ALH”), a New Jersey corporation, discussed in more detail below. As a result of the reverse acquisition, we are now engaged in the production and distribution of pharmaceutical products including a variety of medical supplies, prescription and over-the-counter, or OTC, drugs. We currently produce and sell 42 medicines and distribute over 7,000 medicines and medical devices for third-party producers and manufacturers.
Reverse Acquisition and Warrant Financing
Reverse Acquisition
Prior to May 28, 2010, we were a shell company and had no operations. On May 28, 2010, we completed a reverse acquisition through a share exchange with ALH, whereby we issued the sole shareholder of ALH, 32,310,758 shares of our common stock, par value $0.001, for 100% of the issued and outstanding capital stock of ALH. ALH thereby became our wholly-owned subsidiary and its subsidiary, BOAI Pharm, became the Company’s indirect subsidiary. In accordance with Financial Accounting Standards Board (“FASB”) Accounting Standards Codification (“ASC”) 805, “Business Combinations,” the Company recorded this merger using the recapitalization method which consolidated the Company, ALH and BOAI Pharm and treated the Company as a shell at the time of the merger. According to ASC 805-10-55-13, “the acquirer usually is the combining entity whose relative size (measured in, for example, assets, revenues, or earnings), is significantly larger than that of the other.” Since the Company was a shell at the time of the merger while ALH had operations through its acquisition of BOAI Pharm and was significantly larger than the Company was, under ASC 805, ALH was considered the acquirer.
- 2 -
Upon the closing of the reverse acquisition on May 28, 2010, Mr. Cory Roberts, our Director and former President and Chief Financial Officer resigned from his offices as President and Chief Financial Officer, effective immediately, and Mr. Lanny R. Lang, our former Secretary and Director, resigned from all positions held by him, effective immediately, and our Board of Directors appointed Ms. Xuecheng Xia to serve as our Chief Executive Officer and Chief Financial Officer in Mr. Roberts’ stead, effective immediately. Also upon the closing of the reverse acquisition, our Board of Directors increased its size to 3 members and, in addition to Mr. Roberts, who remains a director, the board appointed Ms. Xia and Ms. Haiwei He to fill the vacancies created by such increase, effective immediately.
As a condition to the closing of the reverse acquisition, we filed a certificate of amendment to our articles of incorporation with the Nevada Secretary of State on July 28, 2010, to change our name from Bay Peak 6 Acquisition Corp. to Asia Leechdom Holding Corporation.
Warrant Financing
As part of the implementation of Visitalk’s Chapter 11 reorganization plan, Visitalk issued six series of warrants, designated as Series A through F, together the Plan Warrants, to certain Visitalk creditors. Each Plan Warrant entitled its holder to purchase, subject to the terms and conditions set forth in a Plan Warrant Agreement, effective as of June 22, 2004, one fully paid and non-assessable share of our common stock at a purchase price equal to the designated exercise price of such Plan Warrant. The Plan Warrants were exercisable as follows:
| Warrant | Exercise Price |
| Series A | $2.00 |
| Series B | $2.00 |
| Series C | $3.00 |
| Series D | $3.00 |
| Series E | $4.00 |
| Series F | $4.00 |
The Plan Warrants were exercisable as of September 17, 2004 and were to expire on November 30, 2008, unless such date was extended by us. From November 2008 through June 30, 2010, our Board of Directors approved extensions of the expiration or termination date of the outstanding Plan Warrants through June 30, 2010 when the Plan Warrants expired. In addition, our Board of Directors approved a reduction of the exercise price for the Series C through F Plan Warrants to $2.51 per share of common stock. We extended the exercise date and adjusted the exercise price in accordance with the terms and conditions of the Plan Warrant Agreement and the Plan. In March, May and June 2010, certain holders of the Plan Warrants, each a Warrant Investor, exercised their right to purchase an aggregate of 110,000 shares of common stock at $2.00 per share and an aggregate of 4,177,381 shares of common stock at $2.51 per share, for aggregate proceeds of approximately $10.7 million.
Pursuant to Side Letter Agreements, dated May 28, 2010 and June 30, 2010, among the Company, the controlling shareholder and each of the Warrant Investors, we agreed to issue to each of the Warrant Investors, on a pro rata basis, new five-year warrants, the New Warrants, to purchase an aggregate of 835,476 shares of common stock at $2.51 per share. The controlling shareholder also agreed to (1) surrender an amount of shares equal to 10% of our issued and outstanding shares as at the closing of the reverse acquisition and the warrant financing, if the Company failed to reach certain income thresholds for the years ending June 30, 2009, 2010 and 2011; and (2) surrender an amount of shares equal to 2% of our issued and outstanding shares, if the Company was not a “reporting company” within the meaning of Rule 144 under the Securities Act of 1933, as amended, within 180 days following the closing of such financing, and had not filed a registration statement on Form 10 with respect to its Common Stock with the U.S. Securities and Exchange Commission, or the SEC, before December 31, 2010. We have met the 2009 and 2010 income thresholds but we did not meet our obligation to become a reporting company by the required date. As a result, our controlling shareholders were obligated to either deliver approximately 796,812 shares of Company’s common stock owned by them to the investors. On February, 11, 2011, the Company, the controlling stockholders and the investors entered into an amendment and waiver agreement, pursuant to which: the investors agreed to extend the Company’s requirement to become a reporting company to February 15, 2011; we agreed to list our Common Stock on the Nasdaq Stock Market, the NYSE Amex or the New York Stock Exchange, on or before October 31, 2011; and the controlling stockholders agreed to surrender an amount of shares equal to 2% of our issued and outstanding fully diluted common stock, if the Company’s actual 2011 net income is not equal to or greater than $27,500,000. We also agreed to appoint a bilingual (English and Mandarin) Chief Financial Officer with prior experience with U.S. public companies and United States generally accepted accounting principles, or U.S. GAAP, within 90 days of the closing of the financing. On October 14, 2010, Ms. Xia resigned from her position as Chief Financial Officer and our Board of Directors appointed Mr. Shuyuan Chang to serve as Chief Financial Officer in her stead.
- 3 -
As consideration for its services as financial advisor in connection with the May 2010 and June 2010 financing, at the closing of such financing we also paid Bay Peak, LLC, the company’s financial advisor, $935,000 in cash and issued them 675,317 shares of our Common Stock valued at $2.51 per share. Bay Peak is beneficially owned and controlled by our Director, Cory Roberts.
China BOI Hunter Settlement
ALH was obligated to repay $1,500,000 due to China BOI Hunter, LLC, or BOI Hunter, under a certain Secured Convertible Promissory Note, due December 20, 2008, issued pursuant to a certain Credit and Security Agreement, dated as of June 20, 2008, among BOI Hunter, ALH and the other ALH affiliates signatory thereto. The note was convertible into warrants to purchase up an amount of shares of ALH common stock equal to $750,000. The note remained outstanding and unconverted until the parties agreed to a settlement agreement, dated May 25, 2010, among the Company, ALH, BOAI Pharm and BOI Hunter, pursuant to which the controlling stockholder agreed to allocate to BOI Hunter as settlement of the Obligations, 239,044 shares of our common stock to be issued to him in exchange for his equity interests in ALH. We also agreed to issue BOI Hunter, a three-year warrant to purchase 298,805 shares of common stock at an exercise price of $2.51 per share.
Acquisition of BOAI Pharm
ALH was incorporated on June 20, 2007 in New Jersey. On July 5, 2007, ALH entered into an equity transfer agreements with the original shareholders of BOAI Pharm, pursuant to which ALH acquired 100% of the outstanding equity interest of BOAI Pharm. The transaction was approved on August 17, 2007, by the Tianjin Commission of Economy and Trade, subject to the PRCRegulations on Merger with and Acquisition of Domestic Enterprises by Foreign Investors, or the Merger Regulations, which require the equity interest transfer price to be paid in full by a date certain, or in the absence of such express date, within three months commencing from the issuance of the new business license. On November 22, 2007, a renewed business license was issued by the Tianjin Administration for Industry and Commerce, or AIC, and BOAI Pharm was converted into a wholly foreign owned enterprise. BOAI Pharm’s new business license is valid through August 5, 2021 and is subject to renewal 180 days prior to such expiration date. We expect that such renewal will be granted within 30 days of applying so long as we continue operating within the scope of the business license.
BOAI Pharm was incorporated on August 6, 2001, and engages in the production and sale of 42 medicines. BOAI Pharm products are sold under its registered trademarks, referred to as Branded Products, and under third party trademarks pursuant to Original Design Manufacture, or ODM arrangements. BOAI Pharm acquired BOAI Leechdom on August 10, 2007, when the original shareholders of BOAI Leechdom transferred their respective equity in BOAI Leechdom to BOAI Pharm, pursuant to equity transfer agreements, dated August 10, 2007, among BOAI Pharm and each of them. After the acquisition, BOAI Pharm became the sole shareholder of BOAI Leechdom. BOAI Leechdom was incorporated on March 17, 1999, and engages in the wholesale and distribution of BOAI Pharm’s Branded Products, ODM products and the pharmaceutical products and medical devices manufactured by third party companies.
Acquisition of Qi Shi Leather
On November 20, 2010, we consummated a property purchase agreement, dated on May 8, 2010, between the Company and Tianjin Qi Shi Leather Co., Ltd., or Qi Shi Leather, pursuant to which we acquired 100% of the equity interest in Qi Shi Leather, for an aggregate purchase price of RMB 80,000,000 Chinese Renminbi (approximately $11.7 million) in cash. Ninety-three percent of the equity interest in Qi Shi Leather was transferred to BOAI Pharm and the remaining 7% was transferred to BOAI Leechdom. Qi Shi Leather was incorporated on July 22, 2003 and at the closing date did not have any business operations, assets or liabilities, other than its land use rights for 85,938.92 square meters of property and 22,138.22 square meters of building space in Jinghai District, Tianjin. Our purpose for acquiring Qi Shi Leather was to acquire such land use rights and property. The acquisition of the Qi Shi Leather equity interest was in lieu of an asset acquisition which would have required us to engage in a more time-consuming process for the transfer of Qi Shi Leather's properties. We intend to use the property acquired with Qi Shi Leather to enhance our current production facilities and increase our production capacity. The additional production facilities are under construction and are expected to be completed by August, 2011. In connection with the closing of the acquisition, we have changed Qi Shi Leather's name to Tianjin BOAI Bio-Pharmaceutical Co., Ltd., or BOAI Bio-Pharm.
- 4 -
Call Option Exercises
On February 27, 2010, Chenghai Du, our former controlling stockholder, entered into a series of call option agreements with each of Ma Dan, Xia Xuecheng, Wang Yan, Wang Yansheng, He Haiwei and Tian Mengchun, the original shareholders and managers of BOAI Pharm, pursuant to which Mr. Du granted each of them an option to acquire an amount of shares equal to 90% of the shares of our common stock issued to Mr. Du in the reverse acquisition, at an exercise price of $0.0001 per share, and on January 10, 2011, Mr. Du entered into an additional call option with Ms. Jianping Lu, pursuant to which Mr. Du granted her an option to acquire an amount of shares equal to the remaining 10% of the shares of our common stock issued to Mr. Du in the reverse acquisition, on the same terms and conditions as the other option holders. Each of the option holders had the right to exercise their option during the period commencing on the date of the option agreement and ending on the fifth anniversary of the date thereof.
On January 11, 2011, the option holders transferred and assigned their options to purchase an aggregate of 9,454,183 shares of our Common Stock to Dragon Core Limited, a British Virgin Islands company, or Dragon Core, and an aggregate of 22,059,762 shares of our Common Stock to Neo Profit Limited, a British Virgin Islands company, or Neo Profit. On the same date each of Dragon Core and Neo Profit exercised their options to purchase their respective shares from Mr. Du. As a result of the exercises, Dragon Core and Neo Profit hold an aggregate of 31,513,945, or 79.1%, of our issued and outstanding common stock and Mr. Du is no longer affiliated with, and no longer holds any equity interest in the Company or its subsidiaries. Dragon Core Limited is 67% beneficially owned and controlled by our Chief Executive Officer and Director, Ms. Xuecheng Xia, and 33% beneficially owned and controlled by Ms. Jianping Lu, an executive employee of BOAI Pharm and Neo Profit Limited is wholly-owned and controlled by Ms. Xia.
Our Corporate Structure
The following chart reflects our organizational structure as of the date of this registration statement.
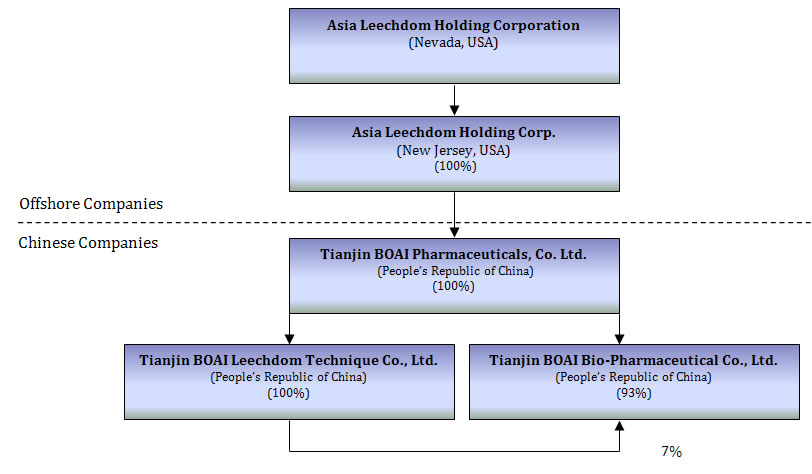
- 5 -
Our corporate headquarters are located at No.55 Miyun Road, Nankai District, Tianjin City, 300111, China. Our telephone number is (+86) 22 27640191. We maintain a website at www.tjboai-pharma.com that contains information about our Company, but that information is not incorporated into, or otherwise considered a part of, this registration statement.
Our Industry and Market Trends
Through our wholly-owned PRC subsidiary, BOAI Pharm and BOAI Pharm’s wholly-owned subsidiary, BOAI Leechdom, we are a producer and distributor of pharmaceutical products including a variety of medical supplies, prescription and over-the-counter, or OTC, drugs in China and abroad. We currently produce and sell 42 medicines and distribute over 7,000 medicines and medical devices. As a result pharmaceutical industry trends in China and the availability of domestic insurance coverage can affect our business and operations.
China’s Pharmaceutical Industry
According to the 2009 Blue Book on China’s Pharmaceutical Market, the value of China’s pharmaceutical market has increased from RMB 7.9 billion in 1978 to RMB 66.79 billion in 2007, with an average growth of 16.8% per year, as compared to an average growth of 8%-10% in the global pharmaceutical industry. In 2008, the value of the Chinese pharmaceutical market reached RMB 86.7 billion, an annual growth rate of 25.74%. The 30-year domestic pharmaceutical industry growth rate from 1978 to 2008 outpaced the record growth rate of China’s gross domestic product, or GDP, which was 9.85%. Despite the global financial crisis, in 2009 the gross industrial output value for China’s pharmaceutical market was over RMB 1,000 billion for the first time, with a growth rate of 21.18%, compared to the 2008 growth rate. Furthermore, over the last 8 years, the domestic pharmaceutical market has experienced a compound annual growth rate of 20.45%, as compared to the 8.45% growth rate of the global pharmaceutical market.
Insurance Coverage in China
In China, eligible participants in the national medical insurance program, mainly consisting of urban residents, are entitled to buy medicines when presenting their medical insurance cards in an authorized pharmacy, provided that the medicines they purchase are included in the national or provincial medical insurance catalogs. The pharmacy in turn obtains reimbursement from the relevant government social security bureaus. The provincial and municipal authorities responsible for administering social medical insurance funds to cover such reimbursements have gradually increased funding in recent years.
According to statistics from China’s Ministry of Human Resources and Social Security, in 2009, 182.1 million people were already covered by medical insurance in rural and urban areas nationwide. We expect that, in light of the expanded availability of medical insurance, coverage should increase to over 90% both in rural and urban areas by 2014.
Our Growth Strategy
Our goal is to become one of the leading companies in the field of research and development, production, sale and distribution of pharmaceutical products in China. We intend to pursue the following strategies to achieve our goal:
Research and Development and Branding: We plan to develop our brand reputation through continual improvement in the quality and technology of our products through continued investment in research and development. We will also continue to implement Good Manufacturing Practices, or GMP, to ensure the standardization of production of our pharmaceutical products.
New Products and Expansion of Production Capacity: We intend to increase our production capacity through the construction of a new manufacturing facility. We also plan to develop new products through effective cooperation with local universities and institutes to increase our competitiveness and profit margins.
- 6 -
- Increase of Market Share: We plan to expand our share of the domestic and international pharmaceutical market through the establishment of broader distribution networks and the expansion of sales channels. We also plan to identify new and unique product portfolios that suit market demands, and provide after-sale services to our customers.
Our Products
Through our subsidiaries, BOAI Pharm and BOAI Leechdom, we are engaged in the production and sale of 42 medicines, some of which are sold under our registered trademarks, referred to as Branded Products, and others which are sold under third party trademarks pursuant to ODM arrangements. We are also engaged in the distribution of more than 7,000 medicines and medical devices produced and manufactured by third-party companies.
Branded Products
Our subsidiary, BOAI Pharm, is primarily engaged in the production and sale of 30 Branded Products, including 27 traditional Chinese medicinal, or TCM, products, and 3 western medicines. According to the PRC Academy of Chinese Medical Sciences, TCMs are characterized by the extraction of active ingredients from Chinese herbs and plants, and from animals and minerals to a lesser extent. TCM are widely used in China and are recognized for their remarkable effectiveness and minimal side effects, as compared to western medicines.
Among BOAI Pharm’s Branded Products, 13 require a physician’s prescription and the remaining 17 are OTCs. All Branded Products have received approval from the PRC State Food and Drug Administration, or SFDA, and are sold in 10 provinces and municipalities across China, 15 of them are included in the PRC Insurance Catalogue of the National Medical Insurance Program, and 3 of them are included in the PRC National Essential Drugs List.
For the fiscal year ended June 30, 2009 and 2010, the revenue from the sale of our Branded Products was $16.3 million and $22.8 million, respectively, accounting for 33.05% and 33.48% of our total revenue. Our top five Branded Products for the fiscal year ended June 30, 2010 are discussed in the following table:
Product Name
| Description
| Prescription
Needed
| Covered by
Medical
Insurance | 2010
Sales
| 2010
Sales Ratio
|
FuFangYiMuCao
Gel | For regulating
menstruation | X
| X
| $5.5 million
| 8.08%
|
YiQiRunChang
Gel
| For relieving stomach
discomfort, dry mouth,
and poor digestion
associated with
constipation and to relax
the bowels for relief
from constipation. | X
| X
| $3.6 million
| 5.33%
|
YangYinQingFei
Gel
| For resolving phlegm,
cough and relieving sore
throat | X
| X
| $3.3 million
| 4.89%
|
PuDiLanXiaoYan
Tablet | For reducing fever and
Tonsillitis | X
| X
| $2.0 million
| 2.95%
|
LianQiaoBaiDu
Tablet | For dispelling toxins
and for detoxification | X | X | $1.6 million | 2.38% |
- 7 -
ODM Products
BOAI Pharm manufactures 12 ODM Products for both domestic and international pharmaceutical companies. Its ODM Products include products sold under the “American Ginseng Tea” brands by the Prince of Peace Company in the United States through the Northern International Group Tianjin Pharmaceutical Import and Export Co., Ltd, and products sold under the “Chitin Capsule” and “Cordyceps.sinensis” and “Job's Tears Powder” brands, for the Tianjin Tiens Group Co., Ltd., which are sold nationwide across China. Other ODM Products are sold to customers in Canada, Europe and Asia. Revenue from ODM products accounted for about 8.33% and 7.81% of total revenue for the fiscal year ended June 30, 2009 and 2010, respectively.
Marketing and Distribution Efforts
Sales Strategy
We have adopted a sales strategy that utilizes internal and external sales agents. We expect to expand our sales network by:
advertising, organizing or participating in professional lectures and exhibitions to publicize and promote our products as well as broaden our brand recognition;
coordinating with more qualified new sales agents nationwide benefiting from their efficient management and existing network;
establishing online sales network system; and
extending the reach of our distribution network by setting up agents for clinical sales and services in hospital pharmacies.
Through these methods, we expect to build a distribution network nationwide, and achieve an increased market share. Our objective is to become the leading brand in the Chinese pharmaceutical industry.
Distribution
Our subsidiary, BOAI Leechdom, sells BOAI Pharm’s Branded and ODM Products, and distributes more than 7,000 medicines produced by third party companies, for 70 of which BOAI Leechdom has exclusive distribution rights. The range of products includes not only prescription and OTC medicines, but also healthcare products and devices such as massage machines and blood pressure monitors. We sell these products to hospitals, clinics, pharmacies and other wholesalers across China.
For the fiscal years ended June 30, 2009 and 2010, the revenue from BOAI Leechdom’s distribution of pharmaceutical products was 59.77% and 59.01% of total revenue, respectively. For the fiscal year ended June 30, 2010, the top two products distributed by BOAI Leechdom were as follows:
Product Name
| Description
| Prescription
Needed
| Covered by
Medical
Insurance | 2010 Sales
| Sales Ratio 2010
|
| | | | | | |
| Snakegourd Peel | | X | X | $2.3 million | 3.33% |
| | | | | | |
| Ciluostazot Tablets | | X | X | $1.2 million | 1.83% |
| | | | | | |
- 8 -
Production
We currently own a 3,428.4 square meter production facility located in Tianjin. We operate 9 production lines, each serving the following specific product formats: (1) concentrated decoctions, (2) pills, (3) tablets, (4) syrups, (5) granules, (6) capsules, (7) oral solutions, (8) plasters and (9) extracts, and further separated into liquid preparations (such as ointment and syrup) and solid preparations (such as tablets, pills and granules). We have received GMP certification for the above (1) to (7) production lines.
We intend to use the 85,938.92 square meters of property acquired with Qi Shi Leather to enhance our current production facilities and increase our production capacity. The additional production facilities are under construction and are expected to be completed by August, 2011.
Quality Control Measures
Our production facility is a contamination-free zone, controlled by a centrally controlled closed-circuit air conditioning system, which not only controls temperature and humidity but also continually purifies and disinfects the circulating air.
According to the new 2011 GMP standard, a workshop for the production of liquid and solid preparations must reach class 100,000 cleanliness. This means that the maximum permissible dust must be 3.5million micrometer per cubic meter (μm/m3), and the maximum permissible microorganisms must be not more than 500 μm /m3 of planktonic bacteria (bacteria that are suspended or growing in a fluid environment as opposed to those attached to a surface) and not more than 10/utensil for depositing bacteria (bacteria that deposit in a variety of environments). We are in compliance with the above standard and have GMP certificates for all of our production lines.
Raw Materials and Suppliers
We use 49 different herbs and 12 ancillary materials as the primary raw material for our products. Our raw materials usually account for over half of our production costs. We select eligible raw material suppliers near our production facilities in order reduce the cost of transportation and control product quality. None of our suppliers were responsible for more than 20% of our raw material expenses for the year ended June 30, 2010.
Competition
The pharmaceutical industry is characterized by rapid product development and technology change. We face direct competition from other producers of our pharmaceutical products and indirect competition from producers of other products having similar medical efficacy as our products. Our pharmaceuticals could be rendered obsolete or made uneconomical due to the development of new pharmaceuticals to treat the same conditions, technological advances affecting the cost of production, or marketing or pricing actions by one or more of our competitors. Our business, results of operations and financial condition could be materially adversely affected by any one or more of these developments. Our competitors may also be able to obtain regulatory approval for new products more quickly than we are and, therefore, may begin to market their products in advance of our products. We believe that competition among pharmaceuticals in China will continue to be based on, among other things, brand name recognition, product efficacy, safety, reliability, availability, promotional activities and price.
Many of our existing and potential competitors have substantially greater financial, technical, manufacturing or other resources than we do. Our competitors’ greater size in some cases provides them with a competitive advantage with respect to manufacturing costs because of their economies of scale and their ability to purchase raw materials at lower prices. Many of our competitors may also have greater brand name recognition, more established distribution networks, larger customer base, or have more extensive knowledge of our customer groups. As a result, they may be able to devote greater resources on research, development, promotion and sale of their products and respond more quickly to evolving industry standards and changes in market conditions than we can. In addition, certain of our competitors may adopt low-margin sales strategies and compete against us based on lower prices. Furthermore, as a result of China’s admission to the WTO in 2001 and subsequent changes in PRC government laws and regulations, we may also face increasing competition from foreign manufacturers in addition to domestic manufacturers.
- 9 -
Subsequent to the reduction of import tariffs pursuant to China’s WTO obligations, the selling prices in China of imported pharmaceuticals have become more competitive. Also, some foreign pharmaceutical producers have set up domestic production bases in China leading to increasing direct competition.
Our generic pharmaceuticals are not protected by patents and are subject to competition from other generic pharmaceuticals. However, the SFDA may at its discretion, subject to certain limitations, grant first-to-market generic pharmaceuticals the protection of a multiple-year monitoring period, or a protection period under the prior regulation, during which other pharmaceutical companies cannot apply for the registration of pharmaceuticals with the same chemical structure, dosage form and indication. Once the transitional protection period elapses, other manufacturers will be able to produce pharmaceuticals with the same chemical structure, dosage form and indication, and may be able to sell such products at a lower price. As a result, hospitals, clinics, pharmacies and other retail outlets may choose the lower priced products over our pharmaceuticals, resulting in a commensurate loss in sales of our products. Most of our products are branded generics, which can be manufactured and sold by other pharmaceutical producers in China once the relevant protection or monitoring periods elapse.
We believe that the combination of our manufacturing capabilities, ODM client base and regional distribution network gives us leverage to capture a substantial portion of the value chain. This value chain allows us to negotiate procurement pricing with other producers on a periodic basis, thus providing us with favorable discounts.
Research and Development
Research and development, or R&D, efforts are critical to the future of our business so we have focused our efforts on R&D partnerships with Tsinghua University, Tianjin University, the Tianjin University of Technology and the Tianjin Nankai Science and Technology Park. Our joint research and development efforts have resulted in our development of the following products:
Paclitaxel –We produce Paclitaxel, or “Taxol,” an anti-cancer raw material originally derived from the bark of the Pacific Yew tree and currently under production in small quantities. Paclitaxel is used to treat patients with lung, ovarian, breast cancer, head and neck cancer and advanced forms of Kaposi’s sarcoma. Paclitaxel binds to microtubules and inhibits their molecular disassembly into tubulin, which blocks a cell’s ability to break down into mitotic spindle during mitosis. This stops the cell from dividing and combats cancerous cells from spreading.
We process Paclitaxel through a new technique developed during our cooperation with Tsinghua University. We own this new technology pursuant to a Technology Transfer Agreement, dated September 30, 2008, whereby Tsinghua University agreed to transfer the proprietary technology to us. The proprietary technology involves a “solid to liquid” cell cultivation method that increases the success ratio and consistency of the product.
According to the Chinese Ministry of Health, the cancer mortality rate of urban Chinese residents is 140.47 per 100,000, or 21.82%, of all urban deaths and the cancer mortality rate of rural Chinese residents was 111.57 per 100,000, or 16.50%, of all rural deaths. The anti-cancer drug market in China has been growing at a 22.24% annual growth rate as of 2007, and the total market for anti-cancer drugs was RMB 25.45 billion, or $3.63 billion USD, based on research from Nan Fang Institute, a direct subsidiary of the Chinese SFDA. We are currently capable of “pilot” scale production of Paclitaxel, but we intend to significantly ramp up our scale of production to 100 kg once our additional production facility is completed in August 2011, and 200-300 kg within the next 3 years.
- SuBing Oral Suspension –SuBing is an OTC cardiovascular drug to relieve the symptoms of cardio- cerebral-vascular diseases. SuBing was co developed with Tianjin University and is currently in the final stage of SFDA approval. According to a Development Agreement, dated June 20, 2007, between BOAI Pharm and The Pharmaceutical College of Tianjin University, we will be the sole owner of all the proprietary technologies related to SuBing, including a 20-year patent currently owned by Tianjin University covering the “oral suspension” form with an added benefit of extending the shelf life from 18 months to 36 months after the SFDA’s approval is granted. The delivery method of the most popular SuBing drug on the market, the Fu Fang Danshen Pill, is also available in an oral suspension form but takes upwards of 3 to 5 minutes to dissolve, while our patent covers the effective delivery in a pill which dissolves in 30 seconds. Compared to the sales performance of other similar products in the market, we believe that the potential annual sales of SuBing within our current sales channel will reach $21 million in its first year of release and $150 million within 3 years.
- 10 -
- Bing WuSuanNa Syrup –Bing WuSuanNa, or sodium valproate, our solely developed SFDA approved product, is designed to treat epilepsy symptoms such as partial seizures, nmyoclonic epilepsy and tonic- clonic seizures. Our formula is in a syrup form and is designed to be easier to administer to children.
The following table outlines our research and development work in progress:
Products Currently in
Development * | Cure/Use
| Status of Product
Development | SFDA Stage **
|
| SuBing Oral Suspension | Used to relieve the symptoms of cardio- cerebral-vascular diseases | Completed | In the last stage of SFDA approval |
| Paclitaxel | Raw materials for cancer treatment | Completed | No SFDA approval required |
| Bing WuSuanNa Syrup | Used to treat epilepsy symptoms | Completed | SFDA approval granted |
____________
* Under PRC law, each variation in the packaging, dosage and concentration of medical products requires registration and approval by the SFDA. During this process the altered product is not commercially available for sale.
** These stages refer to the stages in the regulatory approval process for our products disclosed under the heading “Regulation” in this report.
For the fiscal years ended June 30, 2010 and 2009, total research and development expenses amounted to approximately $1.6 million and $0.5 million, respectively, representing approximately 2.3% and 1.0%, respectively, of our revenues.
Intellectual Property
We hold 2 registered trademarks for our DongFang and BOAI labels. We also currently hold 2 proprietary technologies and 2 external design patents.
Patents
| Description Appearance Design | Patent No | Issue Date | Expiration Date |
| | | | |
Appearance Design: Package:
Fufang Yimucao Gao | ZL 02 3 30403.8
| December 4, 2002
| June 24, 2012
|
Appearance Design: Package:
Yangyin Qingfei Gao | ZL 02 3 30429.4
| December 11, 2002
| July 24, 2012
|
Trademarks
| Description Trade Mark | Trade Mark No | Term of the Trade Mark |
| | | |
| Trade Mark Bo Ai | 1974880 | December 7, 2002 to December 6, 2012 |
| Trade Mark Dong Fang | 1014753 | May 28, 2007 to May 27, 2017 |
- 11 -
There can be no assurance that third parties will not assert infringement or other claims against us with respect to any of our existing or future products or processes. There can be no assurance that licenses would be available if any of our technology was successfully challenged by a third party, or if it became desirable to use any third-party technology to enhance our products. Litigation to protect our proprietary information or to determine the validity of any third-party claims could result in a significant expense and divert the efforts of our technical and management personnel, whether or not such litigation is determined in our favor.
While we have no knowledge that we are infringing upon the proprietary rights of any third party, there can be no assurance that such claims will not be asserted in the future with respect to existing or future products or processes. Any such assertion by a third party could require us to pay royalties, to participate in costly litigation and defend licensees in any such suit pursuant to indemnification agreements, or to refrain from selling an alleged infringing product.
Employees
As of June 30, 2010, we had 344 employees. The payment of salaries conforms to the local minimum salary standard. We are not subject to any collective bargaining agreements and we believe our relationship with our employees is excellent. We have not experienced any significant problems or disruption to our operations due to labor disputes, nor have we experienced any difficulties in recruitment and retention of experienced staff.
Our ability to achieve our operational and financial objectives depends in part upon our ability to retain key technical, marketing and operational personnel, and to attract new employees as required to support growth. Working capital constraints may impair our ability to retain and attract the staff needed to maintain current operations and meet the needs of anticipated growth.
As required by applicable Chinese laws, we have entered into employment contracts with all of our officers, managers and employees.
Our employees in China participate in a state pension scheme organized by Chinese municipal and provincial governments. In addition, we are required by Chinese laws to cover employees in China with various types of social insurance. We have purchased social insurances for all of our employees.
Regulations
Regulation of Pharmaceuticals
The testing, approval, manufacturing, labeling, advertising and marketing, post-approval safety reporting, of our products or product candidates are extensively regulated by governmental authorities in the PRC. Our main sales market is presently in China. We are subject to the Drug Administration Law of China, which governs the licensing, production, marketing and distribution of pharmaceutical products in China and sets penalties for violations of the law. Additionally, we are subject to various regulations and permit systems by the Chinese government. These regulations and their impact on the business of us are set forth in more detail below.
Drug Administration Law of the PRC
The PRC Drug Administration Law was promulgated by the Standing Committee of National People’s Congress on February 28, 2001 and was effective as of December 1, 2001, and its implemental rules were promulgated by the State Council on August 4, 2004 and were effective as of September 15, 2002. According to the Drug Administration Law and its implemental rules, a pharmaceutical producer is required to obtain a Pharmaceutical Manufacturing Permit and a Drug Approval Number for each manufactured medicine from its SFDA provincial branch. Such permit and approval number are valid for five years and are renewable upon application before expiration. We have obtained such permits and approvals for each of our medicines and we renew them prior to expiration.
- 12 -
Administration Regulations for Drug Registration
The Administration Regulations for Drug Registration was promulgated by the SFDA on July 10, 2007, and was effective as of October 1, 2007. The regulations specify the requirements and procedures of obtaining a Drug Approval Number for new drugs, including the requirements for the clinical trial of new drugs, procedures for registering imported medicines and reporting and approval procedures for generic medicines.A Drug Approval Number is valid for five years and can be re-registered upon expiration. We have obtained a Drug Approval Number for each of our drugs and have applied for re-registration prior to their expiration.
GMP for Pharmaceutical Products and Authentication Regulations for Drug GMP
As revised in 1998,GMP for Pharmaceutical Products was promulgated by the SFDA on June 18, 1999 and became effective as of August 1, 1999, and the Authentication Regulations for Drug GMP was promulgated by the SFDA on September 7, 2005 and became effective on of October 1, 2005.A pharmaceutical producer must meet the GMP standards and obtain a GMP Certificate with a five-year validity period from the SFDA. Before GMP Certification expires, the pharmaceutical producer must apply for recertification and complete the relevant procedures in order to maintain its pharmaceutical production permit. The recertification process can take up to 120 business days to complete. Our GMP Certification will expire on January 13, 2015.
Administration Regulations for Drug Recall
The regulations on Drug Recall were promulgated by the SFDA on December 10, 2007 and were immediately effective. According to the regulations, pharmaceutical producers must establish drug recall procedures and collect information regarding the safety of their pharmaceutical products. If a producer learns that any of its drugs pose unreasonable danger to public health and safety, it must immediately stop the manufacturing and sale of such drug, notify its distributors and report its findings to the applicable SFDA branch. The regulations also establish standards for the required drug recall procedures and the standards by which pharmaceutical producers may evaluate whether its drug poses a danger to the public. None of our products has been recalled to date.
Administration Regulations for Drug Instructions and Labels
The regulations for Drug Instructions and Labels were promulgated by the SFDA on March 15, 2006 and were effective as of June 1, 2006. According to the regulations, the SFDA must approve the content of all drug labels and instructions, and that even the smallest packing unit of drug must include such instructions. We have received approval and we maintain labeling for our products in conformity with such regulations.
Regulations for Drug Advertisement Censoring and Publication
The regulations for Drug Advertisement Censoring and Publication were promulgated by the SFDA and by the State Administration for Industry and Commerce, or SAIC, on March 3, 2007, and were effective as of May 1, 2007. According to the regulations, a pharmaceutical producer must obtain a Drug Advertisement Approval Number from its relevant provincial branch of the SFDA if a drug advertisement will describes the functions or benefits of a drug. This approval number is valid for a one-year period and is not required if (1) such publication involves an OTC drug advertisement in any media, or a prescription drug advertisement in a professional medical magazine or (2) such publication only refers to the name of the drug, including the generic name and commercial name, without any additional promotional information. We are currently in compliance with all regulations relating to the advertisement of our products.
The PRC Drug Approval Process
The SFDA and China Traditional Medicine Administration Bureau regulate the new drug approval and licensing in China. The process can involve many layers of authority, lacks transparency, and presents one of the greatest obstacles to the introduction of new drugs into the market. A pharmaceutical producer must take the steps below to obtain approval for its newly developed drugs. The new drug approval process usually lasts between three and four years.
- 13 -
| Stage | Description | Approximate Time/Duration |
| 1. | A new drug applicant prepares documentation of a pharmacological study, toxicity study and pharmacokinetics and drug metabolism study for the proposed drug and submit such documentation along with samples of the drug to its provincial branch of the SFDA, or the Local SFDA | Timing depends on the category and class of the new drug and the applicant’s internal processes. Our process usually takes 18 – 24 months |
| 2. | The Local SFDA sends its officials to the applicant to inspect the applicant’s research and development facilities and arranges to convene a local new drug examination committee meeting, consisting of government officials, for approval deliberations. If approved by the committee, the local SFDA will submit the approved documentation and samples to the SFDA | 3 months |
| 3. | The SFDA examines the documentation, tests the drug samples and arranges to convene a state new drug examination committee meeting for approval deliberations. If the application is approved by the committee, the SFDA will issue a clinical trial license to the applicant for clinical trials | 1 year |
| 4. | The applicant conducts and completes clinical trial and prepares documentation and files for submission to the SFDA for new drug approval. | 1 – 2 years (depending on the category and class of the new drug) |
| 5. | The SFDA examines the documentation, gives final approval for the new drug and issues a new drug license to the applicant. | 8 months |
A preliminary aspect of the foregoing application process involves a review of the PRC market’s need for a particular drug. If the SFDA determines that the market for a particular drug is saturated, the drug will not receive further consideration and the licensing application will be denied.
Compliance with Environmental Law
Our operation and facilities are subject to environmental laws and regulations stipulated by the national and the local environment protection bureaus in China, including the PRC Environmental Protection Law. Relevant laws and regulations include provisions governing air emissions, water discharges and the management and disposal of hazardous substances and wastes. The PRC regulatory authorities require pharmaceutical companies to carry out environmental impact studies before engaging in new construction projects to ensure that their production processes meet the required environmental standards.
We maintain controls at our production facilities to facilitate compliance with such environmental rules and regulations. In addition to such compliance, we actively ensure the environmental sustainability of our operations. The Company is not aware of any investigations, prosecutions, disputes, claims or other proceedings in respect of environmental protection, nor has it been subject to any action made by any environmental administration authorities of the PRC. To management’s knowledge, the Company’s operation meets or exceeds the existing requirements of the PRC.
Regulation of Foreign Currency Exchange
The principal regulations governing foreign currency exchange in China are the Foreign Exchange Administration Regulations (1996), as amended, and the Administration Rules of the Settlement, Sale and Payment of Foreign Exchange (1996). Under these regulations, the Renminbi is freely convertible for current account items, including the distribution of dividends, interest payments, trade and service-related foreign exchange transactions, but not for most capital account items, such as direct investment, loan, repatriation of investment and investment in securities outside China, unless the prior approval of SAFE or its local counterparts is obtained. In addition, any loans to an operating subsidiary in China that is a foreign invested enterprise, cannot, in the aggregate, exceed the difference between its respective approved total investment amount and its respective approved registered capital amount.
- 14 -
Furthermore, any foreign loan must be registered with SAFE or its local counterparts for the loan to be effective. Any increase in the amount of the total investment and registered capital must be approved by the PRC Ministry of Commerce or its local counterpart. We may not be able to obtain these government approvals or registrations on a timely basis, if at all, which could result in a delay in the process of making these loans.
Dividends paid by the PRC subsidiary to its foreign shareholder are deemed shareholder income and are taxable in China. Pursuant to the Administration Rules of the Settlement, Sale and Payment of Foreign Exchange (1996), foreign-invested enterprises in China may purchase or remit foreign exchange, subject to a cap approved by SAFE, for settlement of current account transactions without the approval of SAFE. Foreign exchange transactions under the capital account are still subject to limitations and require approvals from, or registration with, SAFE and other relevant PRC governmental authorities.
Regulation of Dividend Distribution
The principal regulations governing the distribution of dividends by foreign holding companies include the Foreign Investment Enterprise Law (1986), as amended, and the Administrative Rules under the Foreign Investment Enterprise Law (2001).
Under these regulations, foreign investment enterprises in China may pay dividends only out of their retained profits, if any, determined in accordance with PRC accounting standards and regulations. In addition, foreign investment enterprises in China are required to allocate at least 10% of their respective retained profits each year, if any, to fund certain reserve funds unless these reserves have reached 50% of the registered capital of the enterprises. These reserves are not distributable as cash dividends.
Taxation
PRC enterprise income tax is calculated based on taxable income determined under PRC accounting principles. According to the Foreign-invested Enterprises and Foreign Enterprises Income Tax Law (the “FIE Income Tax Law”) and the related implementing rules, both of which issued in 1991, foreign-invested enterprises established in China are generally subject to an income tax rate of 33% (consisting of 30% enterprise income tax and 3% local income tax). The FIE Income Tax Law and the related implementing rules provide certain favorable tax treatments to qualified foreign invested enterprises.
Under the EIT Law, a unified enterprise income tax rate of 25% and unified tax deduction standards will be applied equally to both domestic-invested enterprises and foreign-invested enterprises. Enterprises established prior to March 16, 2007 eligible for preferential tax treatment in accordance with the currently prevailing tax laws and administrative regulations shall, under the regulations of the State Council, gradually become subject to the EIT Law rate over a five-year transition period starting from the date of effectiveness of the EIT Law. The details of the transitional arrangement for the five-year period from January 1, 2008 to December 31, 2012 applicable to enterprises approved for establishment prior to March 16, 2007, such as our company, were adopted in January 2008.
Furthermore, under the EIT Law, an enterprise established outside of the PRC with “de facto management bodies” within the PRC is considered a resident enterprise and will normally be subject to the enterprise income tax at the rate of 25% on its global income. If the PRC tax authorities subsequently determine that we or any of our non-PRC subsidiaries should be classified as a PRC resident enterprise, then such entity’s global income will be subject to PRC income tax at a tax rate of 25%. In addition, under the EIT Law, payments from BDL to us may be subject to a withholding tax. The EIT Law currently provides for a withholding tax rate of 20%. We are actively monitoring the proposed withholding tax and are evaluating appropriate organizational changes to minimize the corresponding tax impact.
- 15 -
Value Added Tax
Pursuant to the Provisional Regulation of China on Value Added Tax and its implementing rules, issued in December 1993, all entities and individuals that are engaged in the businesses of sales of goods, provision of repair and placement services and importation of goods into China are generally subject to a 17% VAT (with the exception of certain goods which are subject to a rate of 13%) of the gross sales proceeds received, less any VAT already paid or borne by the taxpayer on the goods or services purchased by it and utilized in the production of goods or provisions of services that have generated the gross sales proceeds.
Business Tax
Companies in China are generally subject to business tax and related surcharges by various local tax authorities at rates ranging from 3% to 20% of revenue generated from providing services and revenue generated from the transfer of intangibles.
Labor Contract Law
The new Labor Contract Law took effect January 1, 2008, and governs standard terms and conditions for employment, including termination and lay-off rights, contract requirements, compensation levels and consultation with labor unions, among other topics. In addition, the law limits non-competition agreements with senior management and other employees with knowledge of trade secrets to two years and imposes restrictions or geographical limits.
Our Properties and Facilities
There is no private ownership of land in China and all land ownership is held by the government of the PRC, its agencies and collectives. Land use rights can be obtained from the government for a period of up to 50 years, and are typically renewable. Land use rights can be transferred upon approval by the land administrative authorities of the PRC (State Land Administration Bureau) upon payment of the required land transfer fee.
The table below provides a summary of our land use rights to: Xi Qing Dan Guo Yong (2003) No.132 located in Yonghong Industry, Nanhe Town, Xiqing District, Tianjin, China; Fang Di Zheng Jin Zi No. 104030753702 and Jing Dan Guo Yong (2006) No. 46, located at Ke Ji Street in the Tianyu Tranquility Science and Technology Parks, Jinghai District, Tianjin, China (respectively, “Land 1”, “Land 2”, and “Land 3”):
| Land | Usage | Area (m2) | Type of Land Use Right | Term of Use |
| Land 1 | Industrial | 3,438.4 | Granted | Expiration on October 27, 2053 |
| Land 2 | Industrial | 2,524.3 | Granted | Expiration on September 3, 2057 |
| Land 3 | Industrial | 85,938.9 | Granted | Expiration on June 24, 2054 |
We also own the property titles to six buildings located at Tianjin City, China. “Tianjin Property Ownership Certificate No. 110029331” includes 2 buildings with a total area of 1325.8 square meters, “Fang Di Zheng Jin Zi No. 104030753702” includes 1 building with a total area of, 2,856.8 square meters. The properties are used for industrial and office purposes.We also own “Fang Quan Zheng Jin Fang Zi No.000011279" for property in the Jinghai District that includes 3 buildings with a total area of 22,138.2 square meters.
- 16 -
RISK FACTORS
RISKS RELATED TO OUR BUSINESS
Our products and product candidates may not achieve or maintain widespread market acceptance.
Success of our products is highly dependent on the needs and preferences of healthcare practitioners and patients and market acceptance, and we may not achieve or maintain widespread market acceptance of our products or product candidates among healthcare practitioners and patients. We believe that market acceptance of our products will depend on many factors, including:
the perceived advantages of our products over competing products and the availability and success of competing products;
the effectiveness of our sales and marketing efforts;
the safety and efficacy of our products and the prevalence and severity of adverse side effects, if any;
our product pricing and cost effectiveness;
publicity concerning our products, product candidates or competing products;
whether or not patients routinely use our products, refill prescriptions and purchase additional products;
our ability to respond to changes in healthcare practitioner and patient preferences; and
the continued inclusion of our products in the Medical Insurance Catalogs.
If our products fail to achieve or maintain market acceptance, or if new products are introduced by others that are more favorably received than our products, which are more cost effective or otherwise render our products obsolete, we may experience a decline in the demand for our products. If we were unable to market and sell our products successfully, our business, financial condition, results of operation and future growth would be adversely affected.
Most of our products are branded generics that can be manufactured and sold by other pharmaceutical producers in China once the relevant protection or monitoring periods, if any, elapse.
Most of our products are branded generic pharmaceuticals and are not protected by patents. As a result, if other pharmaceutical companies sell equivalent products at a lower cost, this might adversely affect sales of our branded generic products. Certain of our generic products are subject to a protection or monitoring period. The maximum monitoring period currently granted by the SFDA is five years. During such period, the SFDA, will not accept applications for new medicine certificates for the same product by other pharmaceutical companies or approve the production or import of the same product by other pharmaceutical companies. However, once such protection or monitoring periods expire, other manufacturers may obtain relevant production approvals and will be entitled to sell generic pharmaceutical products with similar formulae or production methods in China.
Delays in production due to regulatory restrictions or other factors could have a material adverse impact on our business.
We manufacture a large number of our products in our own manufacturing facilities. The manufacture of pharmaceutical products requires precise and reliable controls and regulatory authorities in China have imposed significant compliance obligations to regulate the manufacturing of pharmaceutical products. As a result, we may face delays in production due to regulatory restrictions or other factors. Failure by our own manufacturing facility or any third party product supplier to comply with regulatory requirements could adversely affect our ability to provide products. All facilities and manufacturing techniques used for the manufacture of pharmaceutical products must be operated in conformity with GMPs. In complying with GMP requirements, we and our product suppliers must continually spend time, money and effort in production, record-keeping and quality assurance and control to ensure that the product meets applicable specifications and other requirements for product safety, efficacy and quality. Manufacturing facilities are subject to periodic unannounced inspections by the SFDA and other regulatory authorities. In addition, adverse experiences with the use of products must be reported to the SFDA and could result in the imposition of market restrictions through labeling changes or in product removal.
- 17 -
Suppliers of certain active and inactive pharmaceutical ingredients and certain packaging materials used in our products are required to obtain SFDA approval before they may supply us with such materials. The development and regulatory approval of our products are dependent upon our ability to procure these ingredients, packaging materials and finished products from SFDA-approved sources. SFDA approval of a new supplier would be required if, for example, an existing supplier breached its obligations to us, active ingredients, packaging materials or finished products were no longer available from the initially approved supplier or if a supplier had its approval from the SFDA withdrawn. The qualification of a new product supplier could potentially delay the manufacture of the product involved. Furthermore, we may not be able to obtain active ingredients, packaging materials or finished products from a new supplier on terms that are at least as favorable to us as those agreed with the initially approved supplier or at reasonable prices.
A delay in supplying, or failure to supply, products by any product supplier could result in our inability to meet the demand for our products and adversely affect our revenues, financial condition, results of operations and cash flows.
Counterfeit pharmaceuticals in China could negatively impact our revenues, brand reputation, business and results of operations.
Our products are also subject to competition from counterfeit pharmaceuticals, which are pharmaceuticals manufactured without proper licenses or approvals and are fraudulently mislabeled with respect to their content and/or manufacturer. Counterfeiters may illegally manufacture and market pharmaceuticals under our brand name or that of our competitors. Counterfeit pharmaceuticals are generally sold at lower prices than the authentic products due to their low production costs, and in some cases are very similar in appearance to the authentic products. Counterfeit pharmaceuticals may or may not have the same chemical content as their authentic counterparts. If counterfeit pharmaceuticals illegally sold under our brand name results in adverse side effects to consumers, we may be associated with any negative publicity resulting from such incidents. In addition, consumers may buy counterfeit pharmaceuticals that are in direct competition with our pharmaceuticals, which could have an adverse impact on our revenues, business and results of operations. Although the PRC government has recently been increasingly active in policing counterfeit pharmaceuticals, there is not yet an effective counterfeit pharmaceutical regulation control and enforcement system in China. The proliferation of counterfeit pharmaceuticals has grown in recent years and may continue to grow in the future. Any such increase in the sales and production of counterfeit pharmaceuticals in China, or the technological capabilities of the counterfeiters, could negatively impact our revenues, brand reputation, business and results of operations.
We may be subject to damages resulting from claims that we or our employees have wrongfully used or disclosed alleged trade secrets of their former employers.
Certain of our employees and consultants were previously employed at other biotechnology or pharmaceutical companies, including our competitors or potential competitors, or at universities or other research institutions. Although no claims against us are currently pending, we may be subject to claims that these employees, consultants or we have inadvertently or otherwise used or disclosed trade secrets or other proprietary information of their former employers. Litigation may be necessary to defend against these claims. Even if we are successful in defending against these claims, litigation could result in substantial costs and be a distraction to our management. If we fail in defending such claims, in addition to paying monetary damages, we may lose valuable intellectual property rights or personnel. A loss of key research personnel or their work product could delay or prevent us from commercializing one or more of our product candidates.
- 18 -
Our future research and development projects may not be successful.
The successful development of pharmaceutical products can be affected by many factors. Products that appear to be promising at their early phases of research and development may fail to be commercialized for various reasons, including the failure to obtain the necessary regulatory approvals. In addition, the research and development cycle for new products for which we may obtain an approval certificate is long. The process of conducting basic research and various stages of tests and trials of a new product before obtaining an approval certificate and commercializing the product may require ten years or longer. Many of our product candidates are in the early stages of pre-clinical studies or clinical trials and we must conduct significant additional clinical trials before we can seek the necessary regulatory approvals to begin commercial production and sales of these products. There is no assurance that our future research and development projects will be successful or completed within the anticipated time frame or budget or that we will receive the necessary approvals from relevant authorities for the production of these newly developed products, or that these newly developed products will achieve commercial success. Even if such products can be successfully commercialized, we may not achieve the level of market acceptance that we expect.
In addition, the pharmaceutical industry is characterized by rapid changes in technology, constant enhancement of industrial know-how and frequent emergence of new products. Future technological improvements and continual product developments in the pharmaceutical market may render our existing products obsolete or affect their viability and competitiveness. Therefore, our future success will largely depend on our research and development capability, including our ability to improve our existing products, diversify our product range and develop new and competitively priced products that can meet the requirements of the changing market. Should we fail to respond to these frequent technological advances by improving our existing products or developing new products in a timely manner or these products do not achieve a desirable level of market acceptance, our business and profitability will be materially and adversely affected.
We may not be successful in competing with other manufacturers of pharmaceuticals in the tender processes for the purchase of medicines by state-owned and state-controlled hospitals.
A substantial portion of the products we sell to our distributor customers are sold to hospitals owned and controlled by counties or higher-level government authorities in China. These hospitals must implement collective tender processes for the purchase of medicines listed in the Medical Insurance Catalogs and medicines that are consumed in large volumes and commonly prescribed for clinical uses. During a collective tender process, the hospitals will establish a committee consisting of recognized pharmaceutical experts. The committee will assess the bids submitted by the pharmaceutical producers, taking into consideration, among other things, the quality and price of the medicine and the service and reputation of the manufacturers. For the same type of pharmaceutical, the committee usually selects from among two to three different brands. Only pharmaceuticals that have won in the collective tender processes may be purchased by these hospitals. The collective tender process for pharmaceuticals with the same chemical composition must be conducted at least annually, and pharmaceuticals that have won in the collective tender processes previously must participate and win in the collective tender processes in the following period before new purchase orders can be issued. If we are unable to win purchase contracts through the collective tender processes in which we decide to participate, we will lose market share to our competitors, and our revenue and profitability will be adversely affected.
Key employees are essential to growing our business.
Ms. Xuecheng Xia, our Board Chair and Chief Executive Officer, Mr. Yansheng Wang, the General Manager of our PRC subsidiaries, and Mr. Shuyuan Chang, Chief Financial Officer are essential to our ability to continue to grow our business. Mrs. Xia and Wang have established relationships within the industries in which we operate. If either of them were to leave us, our growth strategy might be hindered, which could limit our ability to increase revenue. In addition, we face competition for attracting skilled personnel. If we fail to attract and retain qualified personnel to meet current and future needs, this could slow our ability to grow our business, which could result in a decrease in market share.
- 19 -
Our marketing activities are critical to the success of our products, and if we fail to grow our marketing capabilities or maintain adequate spending on marketing activities, the market share of our products and our brand name and product reputation would be materially adversely affected.
A large percentage of our products are branded generic pharmaceuticals and the success and lifespan of our products are dependent on our efforts in the marketing of our products. Our marketing professionals regularly visit hospitals, clinics and pharmacies to explain the therapeutic value of our pharmaceuticals and to keep healthcare professionals up to date as to any developments relating to our pharmaceuticals. We organize in-person product presentations, conferences and seminars for physicians and other healthcare professionals and participate in trade shows to generate market awareness of our existing and new prescription pharmaceuticals. We are also engaged in advertising and educational campaigns through various media channels to educate the public as to our pharmaceuticals. These various marketing activities are critical to the success of our products. However, we cannot assure you that our current and planned spending on marketing activities will be adequate to support our future growth. Any factors adversely affecting our ability to grow our marketing capabilities or our ability to maintain adequate spending on marketing activities will have an adverse effect on the market share of our products and the brand name and reputation of our products, which may result in decreased demand for our products and negatively affect our business and results of operations.
There is no assurance that our existing products will continue to be included or new products developed by us will be included in the medical insurance catalogs.
Eligible participants in the national basic medical insurance program in China, which consists of mostly urban residents, are entitled to reimbursement from the social medical insurance fund for up to the entire cost of medicines that are included in the Medical Insurance Catalogs. As of June 30, 2010, 15 of our principal products that were manufactured and sold were included in the national Medical Insurance Catalog. The inclusion of a medicine in the Medical Insurance Catalogs can substantially improve the sales of the medicine. The Ministry of Human Sources and Social Security in China, or the Ministry of Human Resources, together with other government authorities from time to time, selects medicines to be included in the Medical Insurance Catalogs based on factors including treatment requirements, frequency of use, effectiveness and price. The Ministry of Human Resources also occasionally removes medicines from such catalogs. There can be no assurance that our existing products will continue to be included in the Medical Insurance Catalogs. The removal or exclusion of our products from the Medical Insurance Catalogs may adversely affect our sales. In addition, there is significant uncertainty related to the coverage and reimbursement of newly approved pharmaceutical products. The commercial success of our potential products is substantially dependent on whether reimbursement is available for the ordering of our potential products by hospitals for use by their patients. Our failure to obtain inclusion of our potential products to the Medical Insurance Catalogs may adversely affect the future sales of those products.
We have a limited operating history as a separate wholly-owned foreign company.
Our limited operating history makes it difficult to evaluate our business and future prospects. Although our revenues have grown rapidly, we cannot assure you that we will maintain our profitability or that we will not incur net losses in the future. We expect that our operating expenses will increase as we expand. Any significant failure to realize anticipated revenue growth could result in significant operating losses. We will continue to encounter risks and difficulties in implementing our business model, including potential failure to:
increase awareness of our products, protect our reputation and develop customer loyalty;
manage our expanding operations and service offerings, including the integration of any future acquisitions;
maintain adequate control of our expenses; and
- 20 -
- anticipate and adapt to changing conditions in the markets in which it operates as well as the impact of any changes in government regulation, mergers and acquisitions involving our competitors, technological developments and other significant competitive and market dynamics.
If we are not successful in addressing any or all of these risks, our business may be materially and adversely affected.
We have limited insurance coverage and may incur losses resulting from product liability claims or business interruptions.
The nature of our business exposes us to the risk of product liability claims that is inherent in the research and development, manufacturing and marketing of pharmaceutical products. Using product candidates in clinical trials also exposes us to product liability claims. These risks are greater for our products that receive regulatory approval for commercial sale. Even if a product were approved for commercial use by an appropriate governmental agency, there can be no assurance that users will not claim effects other than those intended resulted from the use of our products. While to date no material claim for personal injury resulting from allegedly defective products has been brought against us, a substantial claim or a substantial number of claims, if successful, could have a material adverse impact on our business, financial condition and results of operations. Such lawsuits may divert the attention of our management from our business strategies and may be costly to defend. In addition, we do not maintain product liability insurance or insurance covering potential liability relating to the release of hazardous materials. In the event of allegations that any of our products are harmful, we may experience reduced consumer demand for our products or our products may be recalled from the market. We may also be forced to defend lawsuits and, if unsuccessful, to pay a substantial amount in damages. In addition, business interruption insurance available in China offers limited coverage compared to that offered in many other countries. We do not have any business interruption insurance. Any business disruption or natural disaster could result in substantial costs and diversion of resources.
We expect to need additional financing, which may not be available on satisfactory terms or at all.
We believe that our existing cash, including the net proceeds from the Warrant Financing, will be sufficient to support our current operating plan for at least the next 12 months. As of June 30, 2010, we had approximately $3.7 million in cash. We expect to need additional capital to support our future development programs. Our capital requirements may be accelerated as a result of many factors, including timing of development activities, underestimates of budget items, unanticipated expenses or capital expenditures, limitation of development of new potential products, future product opportunities with collaborators, future licensing opportunities and future business combinations. Consequently, we may need to seek additional debt or equity financing, which may not be available on favorable terms, if at all, and which may be dilutive to you.
We may seek to raise additional capital through public or private equity offerings, debt financings or additional corporate collaboration and licensing arrangements. To the extent that we raise additional capital by issuing equity securities, our stockholders may experience dilution. To the extent that we raise additional capital by issuing debt securities, it would incur substantial interest obligations, may be required to pledge assets as security for the debt and may be constrained by restrictive financial and/or operational covenants. Debt financing would also be superior to your interest in bankruptcy or liquidation. To the extent that we raises additional funds through collaboration and licensing arrangements, it may be necessary to relinquish some rights to our technologies or product candidates, or grant licenses on unfavorable terms.
If we fail to protect adequately or enforce our intellectual property rights, or to secure rights to patents of others, the value of our intellectual property rights could diminish.
Our success, competitive position and future revenues will depend in part on our ability, and the ability of our licensors, to obtain and maintain patent protection for our products, methods, processes and other technologies, to preserve our trade secrets, to prevent third parties from infringing on our proprietary rights and to operate without infringing the proprietary rights of third parties.
- 21 -
To date, we have filed 10 patents with the National Intellectual Property Administration of the PRC. We anticipate filing additional patent applications both in the PRC and in other countries, as appropriate. However, we cannot predict the degree and range of protection patents will afford us against competitors, including whether third parties will find ways to invalidate or otherwise circumvent our patents, if and when patents will issue, whether or not others will obtain patents claiming aspects similar to our patent applications, or if we will need to initiate litigation or administrative proceedings, which may be costly whether we win or lose.
Our success also depends on the skills, knowledge and experience of our scientific and technical personnel, consultants, advisors, licensors and contractors. To help protect our proprietary know-how and inventions for which patents may be unobtainable or difficult to obtain, we rely on trade secret protection and confidentiality agreements. To this end, we require all of our employees, consultants, advisors and contractors to enter into confidentiality and, where applicable, grant-back agreements. These agreements may not provide adequate protection in the event of unauthorized use or disclosure or the lawful development by others of such information. If any of our intellectual property is disclosed, our value would be significantly impaired, and our business and competitive position would suffer.
If we infringe the rights of third parties, we could be prevented from selling products, forced to pay damages and compelled to defend against litigation.
If our products, methods, processes and other technologies infringe proprietary rights of other parties, we could incur substantial costs, and may have to obtain licenses (which may not be available on commercially reasonable terms, if at all), redesign our products or processes, stop using the subject matter claimed in the asserted patents, pay damages, or defend litigation or administrative proceedings, which may be costly whether we win or lose. All these could result in a substantial diversion of valuable management resources.
We believe that we have taken reasonable steps, including comprehensive internal and external prior patent searches, to ensure that we have freedom to operate and that our development and commercialization efforts can be carried out as planned without infringing others’ proprietary rights. However, we cannot guarantee that no third party patent has been filed or will be filed that may contain subject matter of relevance to our development, causing a third party patent holder to claim infringement. Resolving such issues has traditionally resulted, and could in our case result, in lengthy and costly legal proceedings, the outcome of which cannot be predicted accurately.
We have never paid cash dividends and are not likely to do so in the foreseeable future.
We have never declared or paid any cash dividends on our common stock. We currently intend to retain any future earnings for use in the operation and expansion of our business. We do not expect to pay any cash dividends in the foreseeable future but will review this policy as circumstances dictate.
Acquisitions and strategic investments could adversely affect our operations and result in unanticipated liabilities.
We may in the future acquire or make strategic investments in a number of companies, websites or content providers. Such transactions may result in dilutive issuances of equity securities, use of cash resources, incurrence of debt and amortization of expenses related to intangible assets. Our acquisitions and strategic investments would be accompanied by a number of risks, including:
the difficulty of assimilating operations and personnel of acquired companies into our operations;
the potential disruption of ongoing business and distraction of management;
additional operating losses and expenses of the businesses acquired or in which we invest;
the potential for patent and trademark infringement claims against the acquired company;
- 22 -
the impairment of relationships with customers, supplier and partners of the companies we acquired or with our customers, suppliers and partners as a result of the integration of acquired operations;
the impairment of relationships with employees of the acquired companies or our employees as a result of integration of new management personnel;
the difficulty of integrating the acquired company’s accounting, management information, human resources and other administrative systems;
in the case of foreign acquisitions, uncertainty regarding foreign laws and regulations and difficulty integrating operations and systems as a result of cultural, systems and operational differences; and
the impact of known potential liabilities or unknown liabilities associated with the companies, website or content we acquire or in which we invest.
Our failure adequately to address such risks in connection with future acquisitions and strategic investments could prevent us from realizing the anticipated benefits of such acquisitions or investments, causing us to incur unanticipated liabilities and harming our business generally.
RISKS ASSOCIATED WITH DOING BUSINESS IN GREATER CHINA
Our operations and assets in greater china are subject to significant political and economic uncertainties.
Changes in PRC laws and regulations, or their interpretation, or the imposition of confiscatory taxation, restrictions on currency conversion, imports and sources of supply, devaluations of currency or the nationalization or other expropriation of private enterprises could have a material adverse effect on our business, results of operations and financial condition. Under its current leadership, the Chinese government has been pursuing economic reform policies that encourage private economic activity and greater economic decentralization. There is no assurance, however, that the Chinese government will continue to pursue these policies, or that it will not significantly alter these policies from time to time without notice.
China’s economic policies could affect our business.
All of our assets are located in China and all of our revenue is derived from our operations in China. Accordingly, our results of operations and prospects are subject, to a significant extent, to the economic, political and legal developments in China. While China’s economy has experienced significant growth in the past twenty years, such growth has been uneven, both geographically and among various sectors of the economy. The Chinese government has implemented various measures to encourage economic growth and guide the allocation of resources. Some of these measures benefit the overall economy of the PRC, but they may also have a negative effect on us. For example, our operating results and financial condition may be adversely affected by the government control over capital investments or changes in tax regulations. The economy of the PRC has been changing from a planned economy to a more market-oriented economy. In recent years the Chinese government has implemented measures emphasizing the utilization of market forces for economic reform and the reduction of state ownership of productive assets, and the establishment of corporate governance in business enterprises.
However, a substantial portion of productive assets in the PRC are still owned by the Chinese government. In addition, the Chinese government continues to play a significant role in regulating industry development by imposing industrial policies. It also exercises significant control over the PRC’s economic growth through the allocation of resources, the control of payment of foreign currency-denominated obligations, the setting of monetary policy and the provision of preferential treatment to particular industries or companies.
If the Chinese government finds that the structure for operating our Chinese businesses do not comply with Chinese governmental restrictions on foreign investment, or if these regulations or the interpretation of existing regulations change in the future, we could be subject to severe penalties or be forced to relinquish our interests in those operations.
- 23 -
All of our operations are conducted through our subsidiary in China. If we, our subsidiary, or our corporate structure is found to be in violation of any existing or future PRC laws or regulations (for example, if we are deemed to be holding equity interests in an entity in which direct foreign ownership is restricted) the relevant PRC regulatory authorities, including the administration of industry and commerce, the administration of foreign exchange and relevant agencies of the Ministry of Commerce, would have broad discretion in dealing with such violations, including:
revoking business and operating licenses
confiscating income and imposing fines and other penalties
requiring us to restructure our corporate structure or operations
restricting or prohibiting our use of proceeds from our financings to finance our business and operations in China
imposing conditions with which we or our subsidiaries may not be able to comply
forcing us to relinquish our interests in our subsidiaries
The imposition of any of these penalties could result in a material and adverse effect on our ability to conduct our business.
We may not be able to obtain regulatory approval for any of the products resulting from our development efforts and failure to obtain these approvals could materially harm our business.
All new medicines must be approved by the SFDA before they can be marketed and sold in China. The SFDA requires successful completion of clinical trials and demonstrated manufacturing capability before it grants approval. Clinical trials are expensive and their results are uncertain. It often takes a number of years before a medicine can be ultimately approved by the SFDA. In addition, the SFDA and other regulatory authorities may apply new standards for safety, manufacturing, packaging, and distribution of future product candidates. Complying with such standards may be time-consuming and expensive and could result in delays in obtaining SFDA approval for our future product candidates, or possibly preclude us from obtaining SFDA approval altogether. Furthermore, our future products may not be effective or may prove to have undesirable or unintended side effects, toxicities or other characteristics that may preclude us from obtaining regulatory approval or prevent or limit commercial use. The SFDA and other regulatory authorities may not approve the products that we develop and even if we do obtain regulatory approvals, such regulatory approvals may be subject to limitations on the indicated uses for which we may market a product, which may limit the size of the market for such product.
We may have difficulty establishing adequate management, legal and financial controls in China.
China historically has not adopted a Western style of management, financial reporting concepts and practices, modern banking, computer or other control systems. We may have difficulty in hiring and retaining a sufficient number of qualified employees to work in China. As a result of these factors, we may experience difficulty in establishing management, legal and financial controls, and instituting business practices that meet Western standards. We may also experience difficulty in collecting financial data and preparing financial statements, books of account and corporate records.
Currency fluctuations and restrictions on currency exchange may adversely affect our business, including limiting our ability to convert Chinese renminbi into foreign currencies and, if Chinese renminbi were to decline in value, reducing our revenue in U.S. dollar terms.
Our reporting currency is the U.S. dollar and our operations in China use their local currency as their functional currencies. Substantially all of our revenue and expenses are in Chinese renminbi. We are subject to the effects of exchange rate fluctuations with respect to any of these currencies. For example, the value of the renminbi depends to a large extent on Chinese government policies and China’s domestic and international economic and political developments, as well as supply and demand in the local market. Since 1994, the official exchange rate for the conversion of renminbi to the U.S. dollar had generally been stable and the renminbi had appreciated slightly against the U.S. dollar. However, on July 21, 2005, the Chinese government changed its policy of pegging the value of Chinese renminbi to the U.S. dollar. Under the new policy, Chinese renminbi may fluctuate within a narrow and managed band against a basket of certain foreign currencies. As a result of this policy change, Chinese renminbi appreciated approximately 2.5% against the U.S. dollar in 2005 and 3.3% in 2006. It is possible that the Chinese government could adopt a more flexible currency policy, which could result in more significant fluctuation of Chinese renminbi against the U.S. dollar. We can offer no assurance that Chinese renminbi will be stable against the U.S. dollar or any other foreign currency.
- 24 -
The income statements of our international operations are translated into U.S. dollars at the average exchange rates in each applicable period. To the extent the U.S. dollar strengthens against foreign currencies, the translation of these foreign currencies denominated transactions results in reduced revenue, operating expenses and net income for our international operations. Similarly, to the extent the U.S. dollar weakens against foreign currencies, the translation of these foreign currency denominated transactions results in increased revenue, operating expenses and net income for our international operations. We are also exposed to foreign exchange rate fluctuations as we convert the financial statements of our foreign subsidiaries into U.S. dollars in consolidation. If there is a change in foreign currency exchange rates, the conversion of the foreign subsidiaries’ financial statements into U.S. dollars will lead to a translation gain or loss which is recorded as a component of other comprehensive income. In addition, we have certain assets and liabilities that are denominated in currencies other than the relevant entity’s functional currency. Changes in the functional currency value of these assets and liabilities create fluctuations that will lead to a transaction gain or loss. We have not entered into agreements or purchased instruments to hedge our exchange rate risks, although we may do so in the future. The availability and effectiveness of any hedging transaction may be limited and we may not be able to successfully hedge our exchange rate risks.
Although Chinese governmental policies were introduced in 1996 to allow the convertibility of Chinese renminbi into foreign currency for current account items, conversion of Chinese renminbi into foreign exchange for capital items, such as foreign direct investment, loans or securities, requires the approval of the State Administration of Foreign Exchange, or SAFE, which is under the authority of the People’s Bank of China. These approvals, however, do not guarantee the availability of foreign currency conversion. We cannot be sure that it will be able to obtain all required conversion approvals for our operations or that Chinese regulatory authorities will not impose greater restrictions on the convertibility of Chinese renminbi in the future. Because a significant amount of our future revenue may be in the form of Chinese renminbi, our inability to obtain the requisite approvals or any future restrictions on currency exchanges could limit our ability to utilize revenue generated in Chinese renminbi to fund our business activities outside of China, or to repay foreign currency obligations, including our debt obligations, which would have a material adverse effect on our financial condition and results of operations.
We may have limited legal recourse under PRC law if disputes arise under our contracts with third parties.
The Chinese government has enacted some laws and regulations dealing with matters such as corporate organization and governance, foreign investment, commerce, taxation and trade. However, their experience in implementing, interpreting and enforcing these laws and regulations is limited, and our ability to enforce commercial claims or to resolve commercial disputes is unpredictable. If our new business ventures are unsuccessful, or other adverse circumstances arise from these transactions, we face the risk that the parties to these ventures may seek ways to terminate the transactions, or, may hinder or prevent us from accessing important information regarding the financial and business operations of these acquired companies. The resolution of these matters may be subject to the exercise of considerable discretion by agencies of the Chinese government, and forces unrelated to the legal merits of a particular matter or dispute may influence their determination. Any rights we may have to specific performance, or to seek an injunction under PRC law, in either of these cases, are severely limited, and without a means of recourse by virtue of the Chinese legal system, we may be unable to prevent these situations from occurring. The occurrence of any such events could have a material adverse effect on our business, financial condition and results of operations.
- 25 -
We may be exposed to liabilities under the Foreign Corrupt Practices Act and Chinese anti-corruption laws, and any determination that we violated these laws could have a material adverse effect on our business.
We are subject to the Foreign Corrupt Practice Act, or FCPA, and other laws that prohibit improper payments or offers of payments to foreign governments and their officials and political parties by U.S. persons and issuers as defined by the statute, for the purpose of obtaining or retaining business. We have operations, agreements with third parties and we make most of our sales in China. PRC law also strictly prohibits bribery of government officials. Our activities in China create the risk of unauthorized payments or offers of payments by the employees, consultants, sales agents or distributors of our Company, even though they may not always be subject to our control. It is our policy to implement safeguards to discourage these practices by our employees. However, our existing safeguards and any future improvements may prove to be less than effective, and the employees, consultants, sales agents or distributors of our Company may engage in conduct for which we might be held responsible. Violations of the FCPA or Chinese anti-corruption laws may result in severe criminal or civil sanctions, and we may be subject to other liabilities, which could negatively affect our business, operating results and financial condition. In addition, the U.S. government may seek to hold our Company liable for successor liability in connection with FCPA violations committed by companies in which we invest or that we acquire.
New labor laws in the PRC may adversely affect our results of operations.
On June 29, 2007, the PRC government promulgated a new labor law, namely, the Labor Contract Law of the PRC, or the New Labor Contract Law, which became effective on January 1, 2008. The Implementation Rules of the New Labor Contract Law was subsequently promulgated and became effective on September 18, 2008. The PRC government also promulgated the Law on Mediation and Arbitration of Labor Disputes on December 29, 2007 that came into effect on May 1, 2008. These newly enacted labor laws and regulations impose greater liabilities on employers and significantly impact the cost of an employer’s decision to reduce our workforce. Further, they require certain terminations to be based upon seniority but not merit. In the event we decide to significantly change or decrease our workforce, the New Labor Contract Law could adversely affect our ability to enact such changes in a manner that is most advantageous to our business or in a timely and cost effective manner, thus materially and adversely affecting our financial condition and results of operations.
Future inflation in china may inhibit our ability to conduct business in China.
In recent years, the Chinese economy has experienced periods of rapid expansion and highly fluctuating rates of inflation. During the past ten years, the rate of inflation in China has been as high as 5.9% and as low as - -0.8%. These factors have led to the adoption by the Chinese government, from time to time, of various corrective measures designed to restrict the availability of credit or regulate growth and contain inflation. High inflation may in the future cause the Chinese government to impose controls on credit and/or prices, or to take other action, which could inhibit economic activity in China, and thereby harm the market for our products.
Failure to comply with PRC regulations relating to the establishment of offshore special purpose companies by PRC residents may subject our PRC resident stockholders to personal liability, limit our ability to acquire PRC companies or to inject capital into PRC subsidiaries, limit our PRC subsidiary's ability to distribute profits to us or otherwise materially adversely affect us.
Recent Chinese regulations relating to the establishment of offshore special purpose companies by Chinese residents and registration requirements for employee stock ownership plans or share option plans may subject our China resident shareholders to personal liability and limit our ability to acquire Chinese companies or to inject capital into our operating subsidiaries in China, limit our subsidiary’s ability to distribute profits to us, or otherwise materially and adversely affect us.
The State Administration of Foreign Exchange (SAFE) issued a public notice in October 2005, requiring PRC residents, including both legal persons and natural persons, to register with the competent local SAFE branch before establishing or controlling any company outside of China, referred to as an “offshore special purpose company,” for the purpose of acquiring any assets of or equity interest in PRC companies and raising funds from overseas. In addition, any PRC resident that is the shareholder of an offshore special purpose company is required to amend his or her SAFE registration with the local SAFE branch, with respect to that offshore special purpose company in connection with any increase or decrease of capital, transfer of shares, merger, division, equity investment or creation of any security interest over any assets located in China. To further clarify the implementation of Circular 75, the SAFE issued Circular 124 on November 24, 2005. If these shareholders fail to comply, the PRC subsidiaries are required to report to the local SAFE authorities. If the PRC subsidiaries of the offshore parent company do not report to the local SAFE authorities, they may be prohibited from distributing their profits and proceeds from any reduction in capital, share transfer or liquidation to their offshore parent company and the offshore parent company may be restricted in our ability to contribute additional capital into our PRC subsidiaries. Moreover, failure to comply with the above SAFE registration requirements could result in liabilities under PRC laws for evasion of foreign exchange restrictions. Some of our PRC resident beneficial owners have not registered with the local SAFE branch as required under SAFE regulations. The failure or inability of these PRC resident beneficial owners to comply with the applicable SAFE registration requirements may subject these beneficial owners or us to fines, legal sanctions and restrictions described above.
- 26 -
On March 28, 2007, SAFE released detailed registration procedures for employee stock ownership plans or share option plans to be established by overseas listed companies and for individual plan participants. Any failure to comply with the relevant registration procedures may affect the effectiveness of our employee stock ownership plans or share option plans and subject the plan participants, the companies offering the plans or the relevant intermediaries, as the case may be, to penalties under PRC foreign exchange regime. These penalties may subject us to fines and legal sanctions, prevent us from being able to make distributions or pay dividends, as a result of which our business operations and our ability to distribute profits to you could be materially and adversely affected.
In addition, the National Development and Reform Commission ("NDRC") promulgated a rule in October 2004, or the NDRC Rule, which requires NDRC approvals for overseas investment projects made by PRC entities. The NDRC Rule also provides that approval procedures for overseas investment projects of PRC individuals must be implemented with reference to this rule. However, there exist extensive uncertainties in terms of interpretation of the NDRC Rule with respect to its application to a PRC individual’s overseas investment, and in practice, we are not aware of any precedents that a PRC individual’s overseas investment has been approved by the NDRC or challenged by the NDRC based on the absence of NDRC approval. Our current beneficial owners who are PRC individuals did not apply for NDRC approval for investment in us. We cannot predict how and to what extent this will affect our business operations or future strategy. For example, the failure of our shareholders who are PRC individuals to comply with the NDRC Rule may subject these persons or our PRC subsidiary to certain liabilities under PRC laws, which could adversely affect our business.
Changes in economic conditions and consumer confidence in China may influence consumer preferences and spending patterns, and accordingly, our results of operations.
Our business and revenue growth primarily depend on the size of the pharmaceutical industry in China. As a result, our revenue and profitability may be negatively affected by changes in national, regional or local economic conditions and consumer confidence in China. In particular, as we focus on our expansion of sales in metropolitan markets, where living standards and consumer purchasing power are higher than rural areas, we are especially susceptible to changes in economic conditions, consumer confidence and customer preferences of the urban Chinese population. External factors beyond our control that affect consumer confidence include unemployment rates, levels of personal disposable income, national, regional or local economic conditions and acts of war or terrorism. Changes in economic conditions and consumer confidence could adversely affect consumer preferences, purchasing power and spending patterns. For example, the recent global economic and financial market crisis may cause, among other things, lower customer spending in metropolitan markets. In addition, the timing and nature of any recovery in the credit and financial markets remains uncertain, and there can be no assurance that market conditions will improve in the near future or that our results will not be materially and adversely affected. In addition, acts of war or terrorism may cause damage to our facilities, disrupt the supply of the products and services we offer or adversely impact consumer demand. Any of these factors could have a material adverse effect on our business, financial condition and results of operations.
We face intense competition that may prevent us from maintaining or increasing market share for our existing products and gaining market acceptance for our future products. Our competitors may develop or commercialize products before us or more successfully.
The pharmaceutical market in China is intensely competitive, rapidly evolving and highly fragmented. Our competitors may develop products that are superior to ours or may be more effective in marketing products that are competitive with our products. We face competition from other pharmaceutical companies, including multinational companies as well as manufacturers of traditional Chinese medicines with similar curative effects that can be used as substitutes for certain of our products.
- 27 -
Many of our existing and potential competitors may have greater financial, technical, manufacturing and other resources than we do. In addition, certain competitors, which were established by multinational pharmaceutical companies, have more extensive research and development and technical capabilities than we do. Furthermore, China’s industry reforms aimed to meet the World Trade Organization, or the WTO, requirements may foster increased competition from multinational pharmaceutical companies. Such competitors may also have greater brand name recognition, more established distribution networks, larger customer bases or more extensive knowledge of our target markets. Our competitors’ greater size in some cases provides them with a competitive advantage with respect to manufacturing costs because of their economies of scale and their ability to purchase raw materials at lower prices. As a result, they may be able to devote greater resources to the research, development, promotion and sale of their products or respond more quickly to evolving industry standards and changes in market conditions than we can. In addition, certain of our competitors may adopt low-margin sales strategies and compete against us based on lower prices. Our failure to adapt to changing market conditions and to compete successfully with existing or new competitors may materially and adversely affect our financial condition and results of operations.
In addition, to increase sales, certain manufacturers or distributors of pharmaceuticals may engage in questionable practices in order to influence procurement decisions of our customers. As a result, as competition intensifies in the pharmaceutical industry in China, we may lose sales, customers or contracts to competitors that engage in these practices.
The retail prices of certain of our products are subject to control, including periodic downward adjustment, by PRC government authorities.
Certain of our pharmaceutical products, primarily those included in the national and provincial Medical Insurance Catalogs, are subject to price controls in the form of fixed retail prices or retail price ceilings. In addition, the maximum retail prices of products that are included in the Medical Insurance Catalogs are also subject to periodic downward adjustments as the PRC government authorities aim to make pharmaceuticals more affordable to the general public. However, PRC government authorities impose no control over the prices at which pharmaceutical producers sell their products to their distributors. Since May 1998, the relevant PRC government authorities have ordered price reductions of various pharmaceuticals 24 times. The latest price reductions occurred in January, March, April and May of 2007 and affected a total of 466 different Chinese medicines and 614 different the western pharmaceuticals. However, in the long term, the prices at which pharmaceutical producers in China sell their products to distributors, including the prices of our products, will be affected by the relevant fixed retail prices or retail price ceilings. Government price controls, especially downward price adjustments, may have a material adverse effect on our revenues and profitability.
Pharmaceutical companies in china require a number of permits and licenses in order to carry on their business.
All pharmaceutical manufacturing and distribution companies in China are required to obtain certain permits and licenses from various PRC governmental authorities, including, in the case of manufacturing companies, a pharmaceutical manufacturing permit and, in the case of distribution companies, a pharmaceutical distribution permit. We have obtained permits and licenses and GMP certifications required for the manufacture of our pharmaceutical products. In addition, we have obtained permits, licenses and Good Supply Practice, or GSP, certifications for the distribution of our products. Each of these permits and licenses held by us is valid for a limited number of years and subject to periodic renewal and/or reassessment by the relevant PRC government authorities and the standards of compliance required in relation thereto may from time to time be subject to changes. We intend to apply for the renewal of such permits and licenses when required by applicable laws and regulations. Any failure by us to obtain such renewals may have a material adverse effect on the operation of our business, and prevent us from continuing to carry on our business. Furthermore, any changes in compliance standards, or any new laws or regulations may prohibit or render it more restrictive for us to conduct our business or may increase our compliance costs, which may adversely affect our operations or profitability.
RISKS RELATING TO OUR COMMON STOCK
There is not now, and there may not ever be, an active market for our common stock and we cannot assure you that the common stock will become liquid or that it will be listed on a securities exchange.
There currently is no market for our common stock. We plan to list our common stock as soon as practicable. However, we cannot assure you that we will be able to meet the initial listing standards of any stock exchange, or that we will be able to maintain any such listing. In this venue, however, an investor may find it difficult to obtain accurate quotations as to the market value of the common stock and trading of our common stock may be extremely sporadic. For example, several days may pass before any shares may be traded. A more active market for the common stock may never develop. In addition, if we failed to meet the criteria set forth in SEC regulations, various requirements would be imposed by law on broker-dealers who sell our securities to persons other than established customers and accredited investors. Consequently, such regulations may deter broker-dealers from recommending or selling the common stock, which may further affect our liquidity. This would also make it more difficult for us to raise additional capital.
- 28 -
We may be subject to penny stock regulations and restrictions and you may have difficultyselling shares of our common stock.
The SEC has adopted regulations which generally define so-called “penny stocks” to be an equity security that has a market price less than $5.00 per share or an exercise price of less than $5.00 per share, subject to certain exemptions. When listed or quoted on an over-the-counter electronic exchange, our common stock may be deemed to be a “penny stock” subject to Rule 15g-9 under the Exchange Act, or the Penny Stock Rule. This rule imposes additional sales practice requirements on broker-dealers that sell such securities to persons other than established customers and “accredited investors” (generally, individuals with a net worth in excess of $1,000,000 or annual incomes exceeding $200,000, or $300,000 together with their spouses). For transactions covered by Rule 15g-9, a broker-dealer must make a special suitability determination for the purchaser and have received the purchaser's written consent to the transaction prior to sale. As a result, this rule may affect the ability of broker-dealers to sell our securities and may affect the ability of purchasers to sell any of our securities in the secondary market, thus possibly making it more difficult for us to raise additional capital.
For any transaction involving a penny stock, unless exempt, the rules require delivery, prior to any transaction in penny stock, of a disclosure schedule prepared by the SEC relating to the penny stock market. Disclosure is also required to be made about sales commissions payable to both the broker-dealer and the registered representative and current quotations for the securities. Finally, monthly statements are required to be sent disclosing recent price information for the penny stock held in the account and information on the limited market in penny stock.
There can be no assurance that our common stock will qualify for exemption from the Penny Stock Rule. In any event, even if our common stock were exempt from the Penny Stock Rule, we would remain subject to Section 15(b)(6) of the Exchange Act, which gives the SEC the authority to restrict any person from participating in a distribution of penny stock, if the SEC finds that such a restriction would be in the public interest.
Our holding company structure may limit the payment of dividends.
We have no direct business operations, other than our ownership of our subsidiaries. While we have no current intention of paying dividends, should we decide in the future to do so, as a holding company, our ability to pay dividends and meet other obligations depends upon the receipt of dividends or other payments from our operating subsidiary and other holdings and investments. In addition, our operating subsidiary, from time to time, may be subject to restrictions on their ability to make distributions to us, including restrictions on the conversion of local currency into U.S. dollars or other hard currency and other regulatory restrictions as discussed below. We currently intend to retain any future earnings for funding growth and, therefore, do not expect to pay any dividends in the foreseeable future. If we determine that we will pay dividends to the holders of our common stock, we cannot assure that such dividends will be paid on a timely basis. As a result, you will not receive any return on your investment prior to selling your shares in our company and, for the other reasons discussed in this “Risk Factors ” section, you may not receive any return on your investment even when you sell your shares in our company and your shares may become worthless. If future dividends are paid in RMB, fluctuations in the exchange rate for the conversion of RMB into U.S. dollars may reduce the amount received by U.S. stockholders upon conversion of the dividend payment into U.S. dollars.
- 29 -
The management team collectively has the power to make all major decisions regarding the company without the need to get consent from any stockholder or other person. This discretion could lead to decisions that are not necessarily in the best interests of minority shareholders.
Our management team collectively owns 79.1% of our outstanding common stock. Management, therefore, has the power to make all major decisions regarding our affairs, including decisions regarding whether or not to issue stock and for what consideration, whether or not to sell all or substantially all of our assets and for what consideration and whether or not to authorize more stock for issuance or otherwise amend our charter or bylaws. The management team is in a position to elect all of our directors and to dictate all of our policies.
We may be exposed to potential risks relating to our internal controls over financial reporting and our ability to have those controls attested to by our independent auditors.
As directed by Section 404 of the Sarbanes-Oxley Act of 2002, or SOX 404, the SEC adopted rules requiring public companies to include a report of management on the company’s internal controls over financial reporting in their annual reports, including Form 10-K. Since we just completed the acquisition of ALH on May 28, 2010, we have not evaluated ALH and its consolidated subsidiaries’ internal control systems in order to allow our management to report on our internal controls on a consolidated basis as required by these requirements of SOX 404. Under current law, we will be subject to these requirements beginning with our annual report for the fiscal year ending June 30, 2011. We can provide no assurance that we will comply with all of the requirements imposed thereby. In the event we identify significant deficiencies or material weaknesses in our internal controls that we cannot remediate in a timely manner, investors and others may lose confidence in the reliability of our financial statements.
SELECTED FINANCIAL DATA
The selected consolidated statement of income and comprehensive income data for the years ended June 30, 2008, 2009 and 2010 and the selected balance sheet data as of June 30, 2009 and 2010 are derived from our audited consolidated financial statements included elsewhere in this report. The selected consolidated financial data for the years ended June 30, 2006 and 2007 are derived from our unaudited consolidated financial statements not included in this report.
The following selected historical financial information should be read in conjunction with our consolidated financial statements and related notes and the information contained under "Management’s Discussion and Analysis of Financial Condition and Results of Operations."
(All amounts, except for share and per share amounts, in U.S. dollars)
| | | 2010 | | | 2009 | | | 2008 | | | 2007 | | | 2006 | |
| | | | | | | | | | | | (Unaudited) | | | (Unaudited) | |
| | | | | | | | | | | | | | | | |
| Revenues | $ | 68,166,997 | | $ | 49,489,066 | | $ | 32,381,120 | | $ | 19,450,426 | | $ | 14,926,611 | |
| | | | | | | | | | | | | | | | |
| Income From Operations | $ | 30,351,709 | | $ | 20,775,222 | | $ | 13,232,446 | | $ | 4,687,846 | | $ | 2,792,665 | |
| | | | | | | | | | | | | | | | |
| Net Income | $ | 21,750,626 | | $ | 15,460,736 | | $ | 11,125,217 | | $ | 3,674,657 | | $ | 2,083,213 | |
| | | | | | | | | | | | | | | | |
| Income from Operations Per Share | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | |
Basic | $ | 0.85 | | $ | 0.64 | | $ | 0.41 | | $ | 0.15 | | $ | 0.09 | |
| | | | | | | | | | | | | | | | |
Diluted | $ | 0.85 | | $ | 0.64 | | $ | 0.41 | | $ | 0.15 | | $ | 0.09 | |
| | | | | | | | | | | | | | | | |
| Total Assets | $ | 77,429,655 | | $ | 48,591,732 | | $ | 29,015,101 | | $ | 9,247,968 | | $ | 8,760,911 | |
| | | | | | | | | | | | | | | | |
| Total Current Liabilities | $ | 14,923,296 | | $ | 18,561,901 | | $ | 14,525,468 | | $ | 5,263,003 | | $ | 9,042,403 | |
| | | | | | | | | | | | | | | | |
| Total Long Term Liabilities | $ | 146,780 | | $ | 0 | | $ | 0 | | $ | 0 | | $ | 0 | |
- 30 -
| Net Assets | $ | 62,359,579 | | $ | 30,029,831 | | $ | 14,489,633 | | $ | 3,984,965 | | | (281,492 | ) |
| | | | | | | | | | | | | | | | |
| Capital Stock (excluding long term debt and treasury stock) | $ | 62,359,578 | | $ | 30,029,832 | | $ | 14,489,633 | | $ | 3,984,965 | | | (281,492 | ) |
| | | | | | | | | | | | | | | | |
| Number of Shares Issued and Outstanding | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | |
Basic | | 35,783,112 | | | 32,310,758 | | | 32,310,758 | | | 32,310,758 | | | 32,310,758 | |
| | | | | | | | | | | | | | | | |
Diluted | | 35,783,112 | | | 32,310,758 | | | 32,310,758 | | | 32,310,758 | | | 32,310,758 | |
| | | | | | | | | | | | | | | | |
| Net Income Per Share | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | |
Basic | $ | 0.61 | | $ | 0.48 | | $ | 0.34 | | $ | 0.11 | | $ | 0.06 | |
| | | | | | | | | | | | | | | | |
Diluted | $ | 0.61 | | $ | 0.48 | | $ | 0.34 | | $ | 0.11 | | $ | 0.06 | |
MANAGEMENT’S DISCUSSION AND ANALYSIS OF
FINANCIAL CONDITION AND RESULTS OF OPERATIONS
The following discussion and analysis of our financial condition and results of operations should be read in conjunction with our audited consolidated financial statements, related notes, and other detailed information included elsewhere in this Registration Statement. Our financial statements have been prepared in accordance with United States generally accepted accounting principles (“GAAP). Certain information contained below and elsewhere in this Registration Statement, including information regarding our plans and strategy for our business, constitute forward-looking statements. See "Cautionary Statement Regarding Forward-Looking Statements.”
Overview
We are a producer and distributor of pharmaceutical products including a variety of medical supplies, prescription and over-the-counter, or OTC, drugs in China and abroad. We were organized under the laws of the State of Arizona, in 2004, as VT French Services, Inc., a wholly owned subsidiary of Visitalk Capital Corporation, which in turn was a wholly owned subsidiary of Visitalk.com. As part of Visitalk’s Chapter 11 reorganization plan, we were a shell company with no assets or operations. VCC was authorized by the Visitalk plan to be the reorganized debtor. Effective September 11, 2008, our name was changed from VT French Services, Inc. to Bay Peak 6 Acquisition Corp., in connection with our redomestication to Nevada. On July 28, 2010, we changed our name to Asia Leechdom Holding Corp., in connection with our reverse acquisition of ALH. As a result of the reverse acquisition, we are now engaged in the production and distribution of pharmaceutical products including a variety of medical supplies, prescription and over-the-counter, or OTC, drugs.
Through our wholly-owned PRC subsidiary, BOAI Pharm and BOAI Pharm’s wholly-owned subsidiary, BOAI Leechdom, we currently produce and sell 42 medicines and distribute over 7,000 medicines and medical devices for third-party producers and manufacturers. We market our products through a sales network covering 23 provinces and municipalities in China, including Beijing, Shanghai, Shandong, and Guangdong. We also export our products through agents to customers in North America, Europe and East Asia.
- 31 -
We produce our products at our 3,428.4 square meter production facility located in Tianjin, China. We currently operate 9 production lines, each serving the following product formats: (1) ointment, (2) pill, (3) tablet, (4) syrup, (5) granule, (6) capsule, (7) orally taken liquid, (8) plaster and (9) extract; and further separated into liquid preparations (such as ointment and syrup) and solid preparations (such as tablets, pills and granules). We recently obtained land use rights for another 85,938.92 square meters of property for construction of an additional production facility in Tianjin. We expect to complete the initial phase of construction for this new facility by August 2011.
We generate revenues from the sale and distribution of our products and other third party products in China and abroad. For the fiscal years ended June 30, 2010, 2009 and 2008, total revenue was $68,166,997, 49,489,066 and $32,381,120, respectively, and our net income for the same periods was $21,750,626, $15,460,736 and $11,125,217, respectively.
Recent Developments
BOI Hunter Settlement
ALH was obligated to repay $1,500,000 due to China BOI Hunter, LLC, or BOI Hunter, under a certain Secured Convertible Promissory Note, due December 20, 2008, issued pursuant to a certain Credit and Security Agreement, dated as of June 20, 2008, among BOI Hunter, ALH and the other ALH affiliates signatory thereto. The note was convertible into warrants to purchase up an amount of shares of ALH common stock equal to $750,000. The note remained outstanding and unconverted until the parties agreed to a settlement agreement, dated May 25, 2010, among the Company, ALH, BOAI Pharm and BOI Hunter, pursuant to which the controlling stockholder agreed to allocate to BOI Hunter as settlement of the Obligations, 239,044 shares of our common stock to be issued to him in exchange for his equity interests in ALH. We also agreed to issue BOI Hunter, a three-year warrant to purchase 298,805 shares of common stock at an exercise price of $2.51 per share.
Reverse Acquisition and Warrant Financing
Prior to May 28, 2010, we were a shell company and had no operations. On May 28, 2010, we completed a reverse acquisition through a share exchange with ALH, whereby we issued the sole shareholder of ALH, 32,310,758 shares of our common stock, par value $0.001, for 100% of the issued and outstanding capital stock of ALH. ALH thereby became our wholly-owned subsidiary and its subsidiary, BOAI Pharm, became the Company’s indirect subsidiary. In accordance with Financial Accounting Standards Board (“FASB”) Accounting Standards Codification (“ASC”) 805, “Business Combinations,” the Company recorded this merger using the recapitalization method which consolidated the Company, ALH and BOAI Pharm and treated the Company as a shell at the time of the merger. According to ASC 805-10-55-13, “the acquirer usually is the combining entity whose relative size (measured in, for example, assets, revenues, or earnings), is significantly larger than that of the other.” Since the Company was a shell at the time of the merger while ALH had operations through its acquisition of BOAI Pharm and was significantly larger than the Company was, under ASC 805, ALH was considered the acquirer.
Immediately following closing of the reverse acquisition of ALH, Mr. Du transferred an aggregate of 796,813 of the shares issued to him under the share exchange to certain persons who provided prior services to ALH and its subsidiaries, pursuant to an allocation agreement, dated May 28, 2010, among ALH, Mr. Du and such service providers, and an additional 239,044 of the shares issued to him to BOI Hunter pursuant to the BOI Hunter settlement agreement. As a result of these share transfers, Mr. Du held 31,513,946 shares of our common stock constituting 79.1% of our issued and outstanding capital stock on a fully-diluted basis until exercise of certain call options discussed in more detail below.
Upon the closing of the reverse acquisition on May 28, 2010, Mr. Cory Roberts, our Director and former President and Chief Financial Officer resigned from his offices as President and Chief Financial Officer, effective immediately, and Mr. Lanny R. Lang, our former Secretary and Director, resigned from all positions held by him, effective immediately, and our Board of Directors appointed Ms. Xuecheng Xia to serve as our Chief Executive Officer and Chief Financial Officer in Mr. Roberts’ stead, effective immediately. Also upon the closing of the reverse acquisition, our Board of Directors increased its size to 3 members and, in addition to Mr. Roberts, who remains a director, the board appointed Ms. Xia and Ms. Haiwei He to fill the vacancies created by such increase, effective immediately.
- 32 -
In March, May and June 2010, certain holders of our Plan Warrants, each a Warrant Investor, exercised their right to purchase an aggregate of 110,000 shares of common stock at $2.00 per share and an aggregate of 4,177,381 shares of common stock at $2.51 per share, for aggregate proceeds of approximately $10.7 million. Pursuant to Side Letter Agreements, dated May 28, 2010 and June 30, 2010, among the Company, the controlling shareholder and each of the Warrant Investors, we agreed to issue to each of the Warrant Investors, on a pro rata basis, new five-year warrants, the New Warrants, to purchase an aggregate of 835,476 shares of common stock at $2.51 per share.
Acquisition of Qi Shi Leather
On November 20, 2010, we consummated a property purchase agreement, dated on May 8, 2010, between the Company and Tianjin Qi Shi Leather Co., Ltd., pursuant to which we acquired 100% of the equity interest in Qi Shi Leather, for an aggregate purchase price of RMB 80,000,000 Chinese Renminbi (approximately $11.7 million) in cash. Ninety-three percent of the equity interest in Qi Shi Leather was transferred to BOAI Pharm and the remaining 7% was transferred to BOAI Leechdom. Qi Shi Leather was incorporated on July 22, 2003 and at the closing date did not have any business operations, assets or liabilities, other than its land use rights for 85,938.92 square meters of property and 22,138.22 square meters of building space in Jinghai District, Tianjin. Our purpose for acquiring Qi Shi Leather was to acquire such land use rights and property. The acquisition of the Qi Shi Leather equity interest was in lieu of an asset acquisition which would have required us to engage in a more time-consuming process for the transfer of Qi Shi Leather's properties. We intend to use the property acquired with Qi Shi Leather to enhance our current production facilities and increase our production capacity. The additional production facilities are under construction and are expected to be completed by August, 2011. In connection with the closing of the acquisition, we have changed Qi Shi Leather's name to Tianjin BOAI Bio-Pharmaceutical Co., Ltd.
Call Option Exercises
On February 27, 2010, Chenghai Du, our former controlling stockholder, entered into a series of call option agreements with each of Ma Dan, Xia Xuecheng, Wang Yan, Wang Yansheng, He Haiwei and Tian Mengchun, the original shareholders and managers of BOAI Pharm, pursuant to which Mr. Du granted each of them an option to acquire an amount of shares equal to 90% of the shares of our common stock issued to Mr. Du in the reverse acquisition, at an exercise price of $0.0001 per share, and on January 10, 2011, Mr. Du entered into an additional call option with Ms. Jianping Lu, pursuant to which Mr. Du granted her an option to acquire an amount of shares equal to the remaining 10% of the shares of our common stock issued to Mr. Du in the reverse acquisition, on the same terms and conditions as the other option holders. Each of the option holders had the right to exercise their option during the period commencing on the date of the option agreement and ending on the fifth anniversary of the date thereof.
On January 11, 2011, the option holders transferred and assigned their options to purchase an aggregate of 9,454,183 shares of our Common Stock to Dragon Core Limited, a British Virgin Islands company, or Dragon Core, and an aggregate of 22,059,762 shares of our Common Stock to Neo Profit Limited, a British Virgin Islands company, or Neo Profit. On the same date each of Dragon Core and Neo Profit exercised their options to purchase their respective shares from Mr. Du. As a result of the exercises, Dragon Core and Neo Profit hold an aggregate of 31,513,945, or 79.1%, of our issued and outstanding common stock and Mr. Du is no longer affiliated with, and no longer holds any equity interest in the Company or its subsidiaries. Dragon Core Limited is 67% beneficially owned and controlled by our Chief Executive Officer and Director, Ms. Xuecheng Xia, and 33% beneficially owned and controlled by Ms. Jianping Lu, an executive employee of BOAI Pharm and Neo Profit Limited is wholly-owned and controlled by Ms. Xia.
Principal Factors Affecting Our Financial Performance
Our operating results are primarily affected by the following factors:
- Growth in the Chinese Economy. We operate our manufacturing facilities in China and derive over 100% of our revenues from sales to customers in China. Economic conditions in China, therefore, affect virtually all aspects of our operations, including the demand for our products, the availability and prices of our raw materials and our other expenses. Despite the global economic turmoil which resulted in a slowing of its growth rate, China experienced significant economic growth in recent years, achieving a 8.7% growth in gross domestic product in 2009, with a fourth quarter growth of 10.7% on an annualized basis. China appears to be emerging from the economic slowdown and is expected to experience continued growth in all areas of investment and consumption.
- 33 -
Product Development and Brand Recognition. We believe that in order to compete effectively in this market, we need to constantly improve the quality of our products and deliver new products. As such, we face the challenge of expanding our research and development capacity. We need to maintain a strong and sufficient research and development team and identify the right directions for our research and development. We also face the long-term challenge of developing our brand recognition. We plan to focus on building a reputation for quality and excellent customer service within our industry instead of advertising. We believe that our sales and service team are key in developing the company’s brand recognition and value.
- Taxation. We are subject to United States tax at a tax rate of 34%. No provision for income taxes in the United States has been made as we have no income taxable in the United States. We are also subject to PRC taxes, pursuant to the New EIT Law discussed in more detail under the heading “Regulation –Taxation” heading elsewhere in this registration statement.
Results of Operations
Since we were a shell at the time of the merger while ALH had operations through its acquisition of BOAI Pharm, ALH was considered the acquirer for accounting purposes.
Three Months Ended September 30, 2010 Compared to Three Months Ended September 30, 2009
The following table sets forth key components of ALH’s results of operations for the three months ended September 30, 2010 and 2009, both in dollars and as a percentage of our net revenues.
| | | | | | Three Months Ended | | | | |
| | | | | | September 30, | | | | |
| | | 2010 | | | 2009 | |
| | | | | | As a | | | | | | As a | |
| | | | | | percentage of | | | | | | percentage | |
| | | In Dollars | | | net revenues | | | In Dollars | | | of net revenues | |
| Revenues | $ | 21,111,429 | | | | | $ | 14,173,138 | | | | |
| Cost of Goods Sold | | 10,288,924 | | | 48.7% | | | 6,967,539 | | | 49.2% | |
| Gross Profit | | 10,822,505 | | | 51.3% | | | 7,205,599 | | | 50.8% | |
| Operating Expenses | | 1,047,681 | | | 5.0% | | | 680,593 | | | 4.8% | |
| Operating Income | | 9,774,824 | | | 46.3% | | | 6,525,006 | | | 46.0% | |
| Income Taxes | | 2,389,140 | | | 11.3% | | | 1,672,055 | | | 11.8% | |
| Net Income | $ | 6,837,251 | | | 32.4% | | $ | 4,866,111 | | | 34.3% | |
Revenues.We had revenues of $21,111,429 for the three months ended September 30, 2010, an increase of $6,938,291, or 49.0%, compared to $14,173,138 for the three months ended September 30 2009. The increase was due to the sales generated from sale of our pharmaceutical products and our wholesale distribution businesses. Sales of our pharmaceutical products, including inter-company sales, for the three months ended September 30, 2010 increased by $4,291,042, or 79.9%, compared to the 2009 period. We also recorded $11,469,999 in revenue for the three months ended September 30, 2010, from our wholesale distribution business, an increase of $2,659,448, or 30.2%, compared to the 2009 period. The sales growth in the 2010 period was primarily due to our continuous marketing efforts and our increase in new products offerings.
- 34 -
Cost of Goods Sold and Gross Profit.Our cost of goods sold consists of raw materials, labor costs and production overhead. In the three months ended September 30, 2010, our revenue increased by 49.0% and our cost of goods sold increased by 47.7%, from $6,967,539 to $10,288,924, compared to the 2009 period. The increase of cost of sales in the 2010 period is attributed to our increase in sales. However, this slight disparity in growth between revenue and cost of goods sold is mainly attributable to the stronger sales of our higher margin pharmaceutical products in the 2010 period. During the three months ended September 30 2010, the gross margin of our pharmaceutical production business was 66.4%, compared to the 65.6% margin in the 2009 period. The overall result was an increase in our gross margin from 50.8% in the three months ended September 30 2009 to 51.3% in the 2010 period.
Operating expenses.Our operating expenses increased by 53.9%, from $680,593 in the three months ended September 30, 2009, to $1,047,681 in the 2010 period. The increase is primarily due to higher general and administrative (“G&A) expenses for the three months ended September 30, 2010. G&A expenses increased 147.3%, from $270,366 for the three months ended September 30, 2009, to $668,483 in the 2010 period. G&A expenses as a percentage of revenues was 3.2% in 2010 and 1.9% in 2009. The increase in G&A expenses in 2010 compared to 2009 was primarily due to higher payroll expenses, professional fees (such as accounting, legal and financial consultant fee), and maintenance and repair fees as a result of more stringent GMP manufacturing standards and to improve production efficiency. Our total “other income (expense)” for the three months ended September 30, 2010 did not change significantly as compared to the 2009 period, except for our recognition of a $580,758 other expense for the three months ended September 30, 2010 for a one time inventory allowance due to losses caused by a natural disaster, compared to $1,395 for the 2009 period.
Income Taxes.Income taxes for the three months ended September 30, 2010 was $2,389,140, compared to $1,672,055 for the 2009 period. The Company’s effective tax rate was 25.6% for the three months ended September 30, 2009 and 25.9% for the 2010 period.
Net Income.Our net income was $6,837,251 for the three months ended September 30, 2010, as compared to $4,866,111 for the three months ended September 30, 2009. The 40.5% increase in net income was during the 2010 period was mainly due to significant sales growth.
Year Ended June 30, 2010 Compared to Year Ended June 30, 2009
The following table sets forth key components of ALH’s results of operations for the fiscal years ended June 30, 2010 and 2009, both in dollars and as a percentage of our net revenues.
| | | Fiscal Year EndedJune 30, | |
| | | 2010 | | | 2009 | |
| | | | | | As a | | | | | | As a | |
| | | | | | percentage of | | | | | | percentage | |
| | | In Dollars | | | net revenues | | | In Dollars | | | of net | |
| | | | | | | | | | | | revenues | |
| Revenues | $ | 68,166,997 | | | | | $ | 49,489,066 | | | | |
| Cost of Goods Sold | | 34,217,906 | | | 50.2% | | | 26,094,624 | | | 52.7% | |
| Gross Profit | | 33,949,091 | | | 49.8% | | | 23,394,442 | | | 47.3% | |
| Operating Expenses | | 3,597,382 | | | 5.3% | | | 2,619,220 | | | 5.3% | |
| Operating Income | | 30,351,709 | | | 44.5% | | | 20,775,222 | | | 42.0% | |
| Research and Development Costs | | 1,561,310 | | | 2.3% | | | 473,559 | | | 1.0% | |
| General and Administrative Expense | | 1,382,099 | | | 2.0 % | | | 1,008,764 | | | 2.0% | |
| Other Expense | | (938,455 | ) | | (1.4)% | | | (110,939 | ) | | (0.2)% | |
| Income Taxes | | 7,662,628 | | | 11.2% | | | 5,203,547 | | | 10.5% | |
| Net Income | $ | 21,750,626 | | | 31.9% | | $ | 15,460,736 | | | 31.2% | |
- 35 -
Revenues.We generate revenues from the sale and distribution of our pharmaceutical products and other third party medicines and medical devices in China and abroad. We had total revenues of $68,166,997 for the year ended June 30, 2010, an increase of $18,677,931, or 37.7%, compared to $49,489,066 for the year ended June 30, 2009. The increase in revenues was due to sales generated both from sales of our pharmaceutical products and from our wholesale business. During the year ended June 30, 2010, sales of our pharmaceutical products (including inter-company sales) increased by $7,587,264, or 37.2%, compared to $20,419,490 in the year ended June 30, 2009, and we earned $40,226,335 in revenue from our wholesale business for the year ended June 30, 2010, an increase of $10,646,919, or 36.0%, compared to the year ended June 30, 2009. Our sales growth was primarily due to our continuous marketing efforts, increase in new products offerings, as well as the expansion of our sales to previously unaddressed geographic locations.
Cost of Goods Sold and Gross Profit.Our cost of goods sold consists of the cost of raw materials, labor costs and production overhead. In the year ended June 30, 2010, our revenue increased by 37.7% and our cost of goods sold increased by 31.1%, from $26,094,624 to $34,217,906, compared to the period ended June 30, 2009. The increase of cost of sales in 2010 compared to 2009 is attributable to our increase in sales. However, the disparity in growth between revenue and cost of goods sold is mainly attributable to the stronger sales of our higher margin products in 2010 than in 2009. As a result, during the year ended June 30 2010, the gross margin of our medicine wholesales business was 42.0%, compared to the 36.2% margin in the 2009 period. The overall result was a 47.3% increase in our gross margin, from the year ended June 30 2009, to 49.8% in 2010.
Operating Expenses.The Company’s operating expenses increased by 37.3%, from $2,619,220 in the year ended June 30, 2009 to $3,597,382 in the 2010 period. The increase is primarily due to higher research and development (“R&D”) expense and general and administrative (G&A”) expense, partially offset by lower selling expense, for the year ended June30, 2010, compared to 2009.
Research and Development Costs.R&D costs consist of salary and benefits, fees paid to research organizations, supplies and facility costs. R&D costs increased by $1,087,751, or 230%, from $473,559 in the year ended June 30, 2009, to $1,561,310 in the 2010 period. Expressed as a percentage of revenue, R&D cost was 2.3% for the year ended June 30, 2010, compared to 1.0% in 2009. The increase in R&D costs reflected increased expense incurred during the 2010 period in connection with trial production of new products.
General and Administrative Expenses.G&A expenses increased from $1,008,764 in the year ended June 30, 2009, to $1,382,099 in the 2010 period, a 37.0% increase. G&A expenses expressed as a percentage of revenues was 2.0% in the year ended June 30, 2010, which is the same as in 2009. The increase in G&A expenses in the year ended June 30, 2010 was primarily due to higher professional fees (such as legal and accounting fees), maintenance and repair fees, and bad debt allowance for accounts receivables.
Other Expense. Other expense for the year ended June 30, 2010 increased to $(938,455), from $(110,939) for the year ended June 30, 2009, a $827,516, or 745.9%, increase. The increase was mainly due to our recognition of $826,947 in interest expense the default settlement of our $1.5 million bridge loan with BOI Hunter during 2010. Pursuant to the settlement agreement, our controlling shareholder transferred 239,044 shares of our common stock issued to him in the reverse merger with ALH, and the Company delivered three-year warrants to purchase 298,805 shares of the Company’s common stock. The fair value of these settlement securities was $826,947.
Income Taxes.Income tax expense for the year ended June 30, 2010 was $7,662,628, compared to $5,203,547 for 2009. The Company’s effective tax rates were 26.0% and 25.2% for the years ended June 30 2010 and 2009, respectively.
- 36 -
Net Income.Our net income was $21,750,626 for the year ended June 30, 2010, representing a 40.7% increase over 2009. The increase was due to our increased sales and the continued growth of our business.
Year Ended June 30, 2009 Compared to Year Ended June 30, 2008
The following tables present certain consolidated statement of operations information. Financial information is presented for the years ended June 30, 2009 and 2008 respectively.
| | | Fiscal Year Ended June 30, | |
| | | 2009 | | | 2008 | |
| | | | | | As a | | | | | | As a | |
| | | | | | percentage of | | | | | | percentageof | |
| | | In Dollars | | | net revenues | | | In Dollars | | | net revenues | |
| Revenues | $ | 49,489,066 | | | | | $ | 32,381,120 | | | | |
| Cost of Goods Sold | | 26,094,624 | | | 52.7% | | | 17,015,697 | | | 52.5% | |
| Gross Profit | | 23,394,442 | | | 47.3% | | | 15,365,423 | | | 47.5% | |
| Operating Expenses | | 2,619,220 | | | 5.3% | | | 2,132,977 | | | 6.6% | |
| Operating Income | | 20,775,222 | | | 42.0% | | | 13,232,446 | | | 40.9% | |
| Income Taxes | | 5,203,547 | | | 10.5% | | | 2,122,178 | | | 6.6% | |
| Net Income | $ | 15,460,736 | | | 31.2% | | $ | 11,125,217 | | | 34.4% | |
Revenues.We had total revenues of $49,489,066 for the year ended June 30, 2009, an increase of $17,107,946, or 52.8%, compared to $32,381,120 for the year ended June 30 2008. The increase in revenues was due to sales generated both from sales of our pharmaceutical products and from our wholesale business. During the year ended June 30, 2009, sales of our pharmaceutical products (including inter-company sales) was $20,419,490, an increase of $6,516,516, or 46.9%, compared to the year ended June 30, 2008, and we earned $29,579,416 in revenue from our wholesale business for the year ended June 30, 2009, an increase of $10,595,163, or 55.8%, compared to the year ended June 30, 2008. The sales growth was primarily due to our continuous marketing efforts and our increase in new products offering.
Cost of Goods Sold and Gross Profit.Our cost of goods sold consists of the cost of raw materials, labor costs and production overhead. In the year ended June 30, 2009, our revenue increased by 52.8% and our cost of goods sold increased by 53.4%, from $17,015,697 to $26,094,624, compared to the 2008 period. The increase of cost of sales in 2009 is attributable to our increase in sales. The overall result was a decrease in our gross margin from 47.5% in the year ended June 30, 2008 to 47.3% for 2009.
Operating Expenses.The Company’s operating expenses increased by 22.8%, from $2,132,977 in the year ended June 30, 2008, to $2,619,220 in the 2009 period. The increase is primarily due to higher general and administrative expense to support higher sales for the year ended June 30, 2009 than the 2008 period. G&A expenses increased from $502,432 in 2008 to $1,008,764 in 2009, or a 100.8% increase. The increase in G&A expenses in 2009 compared to 2008 was primarily due to higher payroll expenses, administrative expense, and maintenance and repair fees as a result of more stringent GMP manufacturing standards and to improve production efficiency. G&A expenses expressed as a percentage of revenues was 2.0% in 2009 and 1.6% in 2008. Other expense for the year ended June 30, 2009 did not change significantly as compared to the prior period, however, for the year ended June 30, 2009, the interest expense increased by 65.1%, from 165,471 to $273,172, mainly due to more loans received by us.
Income Taxes.Income tax expense for the year ended June 30, 2009 was $5,203,547, compared to $2,122,178 for the 2008 period. The Company’s effective tax rate was 25.2% and 16.0% for the years ended June 30, 2009 and 2008, respectively. The 16% tax rate for the year ended June 30, 2008 was due to the Company enjoyment of a 12.5% on its PRC income tax rate through December 31, 2007.
- 37 -
Net Income. Our net income was $15,460,736 for the year ended June 30, 2009, representing a 39.0% increase over 2008.
Liquidity and Capital Resources
The following table sets forth a summary of our cash flows for the periods indicated:
Cash Flow
(all amounts in U.S. dollars)
| | | Three Months Ended | | | Fiscal Year Ended | |
| | | September 30, | | | | | | June 30, | | | | |
| | | 2010 | | | 2009 | | | 2010 | | | 2009 | | | 2008 | |
| Net cash provided by (used in) operating activities | $ | 3,135,036 | | $ | 3,515,415 | | $ | 16,256,711 | | $ | 8,966,640 | | $ | 11,513,021 | |
| Net cash (used in) provided by in investing activities | | (5,933,038 | ) | | 1,132,936 | | | (13,301,286 | ) | | (10,571,744 | ) | | (8,506,054 | ) |
| Net cash provided by financing activities | | 4,353,219 | | | 374,711 | | | 6,002,970 | | | 1,955,778 | | | 1,312,593 | |
| Effects of exchange rate change in cash | | 304,767 | | | (1,445 | ) | | 45,135 | | | 14,224 | | | 164,720 | |
| Net increase (decrease) in cash and cash equivalents | | 1,555,217 | | | 5,023,062 | | | 8,958,395 | | | 350,674 | | | 4,319,560 | |
| Cash and cash equivalent at beginning of the year | | 14,498,707 | | | 5,495,176 | | | 5,495,176 | | | 5,130,278 | | | 645,997 | |
| Cash and cash equivalent at end of the year | $ | 16,358,691 | | $ | 10,516,793 | | $ | 14,498,707 | | $ | 5,495,176 | | $ | 5,130,278 | |
At September 30, 2010, the Company had working capital of $27,778,190, an improvement from $26,142,433 at June 30, 2010. Our cash balance at September 30, 2010 was $16,358,691, an increase of $1,859,984, or 12.8%, compared with our cash balance of $14,498,707 at June 30, 2010. The increase was mainly attributable to the financing and operating activities, partially offset by our investment activities.
Operating activities
Net cash provided by operating activities was $3,135,036 for the three months ended September 30, 2010, as compared to $3,515,415 in net cash provided by operating activities for the same period in 2009. The decrease in net cash provided by operating activities between the 2010 and 2009 periods was mainly due to more cash demand for accounts receivable and due from related parties in order to support higher sales in 2010, partially offset by the cash demand for inventory.
Net cash used in operating activities was $16,256,711 for the years ended June 30, 2010, as compared to $8,966,640 in net cash provided by operating activities for the same period in 2009 and $11,513,021 provided in 2008. The increase in net cash provided by operating activities between 2010 and 2009 was mainly due to higher net income and lower working capital demand for inventory and other payables. The decrease in net cash provided by operating activities between 2009 and 2008 was mainly due to higher working capital demand for advances to material suppliers, inventory and other payables in order to support higher sales in 2009.
Investing activities
Our net cash used in investing activities was $5,933,038 during the three months ended September 30, 2010, compared to net cash provided by investing activities of $1,132,936 in the 2009 period. The changes in net cash used in investing activities were primarily attributable to deposits for asset acquisition of $4,342,140 and additional investment of $1,565,533 in construction in progress.
Net cash used in investing activities for the years ended June 30, 2010 was $13,301,286, as compared to $10,571,744 in net cash used in investing activities for the same period in 2009 and $8,506,054 used in 2008. The increase in net cash used in investing activities between 2010 and 2009 was mainly due to higher advances to equipment suppliers and deposit for asset acquisition. The increase in net cash used in investing activities between 2009 and 2008 was mainly due to our purchase of an intangible asset, particularly the Paclitaxel technology.
- 38 -
Financing activities
Our cash provided by financing activities during the three months ended September 30, 2010 was $4,353,219, compared to $374,711 for the 2009 period. The increase in net cash provided by financing activities was mainly attributable to the collections on subscriptions receivable of $3,007,224 and increase of bank loans of $1,535,995, partially offset by the return of a $190,000 loan during the 2010 period.
Net cash provided by financing activities for the years ended June 30, 2010 was $6,002,970, as compared to $1,955,778 in net cash provided by financing activities for the same period in 2009 and $1,312,593 provided in 2008. The increase in net cash provided by financing activities between 2010 and 2009 was mainly due to our warrant financing during the 2010 period. The increase in net cash provided by financing activities between 2009 and 2008 was mainly due to more bank loans during the 2009 period as compared to 2008.
Capital Expenditures
Our capital expenditures were $13,301,286, $10,571,744 and $8,506,054 for the fiscal years ended June 30, 2010, 2009 and 2008, respectively. Our capital expenditures in 2010 were mainly used to acquire land usage rights in Tianjin and advances to equipment suppliers in connection with our planned expansion of production capacity. The capital expenditures in 2009 were mainly used to purchase property and equipments and complete construction of our facilities. As of September 30, 2010 and June 30, 2010, we had cash and cash equivalents of $16,358,691 and $14,498,707, respectively, primarily consisting of cash on hand and demand deposits. As of September 30, 2010, we expect to make additional capital expenditures of RMB 107 million (unaudited) (approximately, $16.1 million) for the construction of buildings and purchase of equipment relating to construction of our new production facilities in Tianjin. To date, we have financed our operations primarily through cash flows from operations, augmented by short-term bank borrowings and equity contributions by our stockholders.
We believe that our cash on hand and cash flow from operations will meet part of our present cash needs and we will require additional cash resources, including loans, to meet our expected capital expenditure and working capital for the next 12 months. We may, however, in the future, require additional cash resources due to changed business conditions, implementation of our strategy to ramp up our marketing efforts and increase brand awareness, or acquisitions we may decide to pursue. If our own financial resources are insufficient to satisfy our capital requirements, we may seek to sell additional equity or debt securities or obtain additional credit facilities. The sale of additional equity securities could result in dilution to our stockholders. The incurrence of indebtedness would result in increased debt service obligations and could require us to agree to operating and financial covenants that would restrict our operations. Financing may not be available in amounts or on terms acceptable to us, if at all. Any failure by us to raise additional funds on terms favorable to us, or at all, could limit our ability to expand our business operations and could harm our overall business prospects.
Loan Commitments
The growth of our Company has been funded by capital contributions, initially from our founders and in May and June 2010 approximately $10.7 million capital raised by the sale of our securities to investors. As of September 30, 2010 and June 30, 2010 we had $4,971,936 and $3,536,584, respectively, in short-term loans and no long term liabilities. All our short term loans are secured by our properties.
The following table summarizes our short term loans outstanding as of September 30, 2010.
Bank
| Amount
| Maturity
Date | Interest
Rate | Duration
|
| Tianjin Agriculture Credit Union Bank | $284,538 | March 8, 2011 | 5.841% | 1 year |
| Tianjin Agriculture Credit Union Bank | $359,417 | May 10, 2011 | 5.841% | 1 year |
| Tianjin Agriculture Credit Union Bank | $359,417 | July 7, 2011 | 5.841% | 1 year |
| Tianjin Bank | $2,695,628 | August 1, 2011 | 4.425% | 1 year |
| Tianjin Bank | $1,272,935 | November 23, 2011 | 5.841% | 1 year |
| Total Short-term Loans | $4,971,936 | -- | -- | -- |
- 39 -
Obligations under Material Contracts
Except with respect to the loan obligations disclosed under the “Loan Commitments” heading and the obligations discussed below, we have no obligations to pay cash or deliver cash to any other party.
On September 30, 2008, we entered into a technology transfer agreement with Tsinghua University, pursuant to which we obtained the Paclitaxel cell cultivation method for an aggregate purchase price of RMB 43 million (approximately $6.4 million) .We received the technology in December 2008 in connection with our initial payment of approximately $2.9 million, and made a second payment of approximately $2.9 million in March, 2009, when we successfully completed trial production. As of September 30, 2010, we had paid approximately $5.8 million and we are obligated to make a third and final payment of approximately$0.6 million once we are able to successfully produce on a large scale.
Inflation
Inflation and changing prices have not had a material effect on our business and we do not expect that inflation or changing prices will materially affect our business in the foreseeable future. However, our management will closely monitor the price change in travel industry and continually maintain effective cost control in operations.
Off Balance Sheet Arrangements
We do not have any off balance sheet arrangements that have or are reasonably likely to have a current or future effect on our financial condition, changes in financial condition, revenues or expenses, results of operations, liquidity or capital expenditures or capital resources that is material to an investor in our securities.
Seasonality
Our operating results and operating cash flows historically have not been subject to seasonal variations. This pattern may change, however, as a result of new market opportunities or new product introductions.
Critical Accounting Policies
The preparation of financial statements in conformity with U.S. GAAP requires our management to make assumptions, estimates and judgments that affect the amounts reported, including the notes thereto, and related disclosures of commitments and contingencies, if any. We have identified certain accounting policies that are significant to the preparation of our financial statements. These accounting policies are important for an understanding of our financial condition and results of operation. Critical accounting policies are those that are most important to the portrayal of our financial conditions and results of operations and require management’s difficult, subjective, or complex judgment, often as a result of the need to make estimates about the effect of matters that are inherently uncertain and may change in subsequent periods. Certain accounting estimates are particularly sensitive because of their significance to financial statements and because of the possibility that future events affecting the estimate may differ significantly from management’s current judgments.
- 40 -
We believe the following critical accounting policies involve the most significant estimates and judgments used in the preparation of our financial statements:
Estimates Affecting Accounts Receivable and Inventories.At September 30, 2010, the Company provided $149,757 reserve against accounts receivable. Management’s estimate of the appropriate reserve on accounts receivable at September 30, 2010 was based on the aged nature of these accounts receivable. In making its judgment, management assessed our customers’ ability to continue to pay their outstanding invoices on a timely basis, and whether their financial position might deteriorate significantly in the future, which would result in their inability to pay their debts to the Company. At September 30, 2010, the Company provided $0 allowance against its inventories. Management determination of this allowance was based on potential impairments to the current carrying value of the inventories due to potential obsolescence of aged inventories. In making its estimate, management considered the probable demand for our products in the future and historical trends in the turnover of our inventories. While the Company currently believes that there is little likelihood that actual results will differ materially from these current estimates, if customer demand for our products significantly decreases in the near future, or if the financial condition of our customers deteriorates in the near future, the Company could realize significant write downs for slow-moving inventories or uncollectible accounts receivable.
Policy Affecting Recognition of Revenue.Among the most important accounting policies affecting our consolidated financial statements is our policy of recognizing revenue in accordance with the Financial Accounting Standards Board (“FASB”) Accounting Standards Codification (“ASC”) 605 “Revenue Recognition”. Under this policy, all of the following criteria must be met in order for us to recognize revenue: (1) persuasive evidence of an arrangement exists; (2) delivery has occurred or services have been rendered; (3) the seller’s price to the buyer is fixed or determinable; and (4) collectability is reasonably assured. The majority of the Company’s revenue that results from sales contracts with distributors and revenue is recorded upon the shipment of goods. Management conducts credit background checks for new customers as a means to reduce the subjectivity of assuring collectability. Based on these factors, the Company believes that it can apply the provisions of FASB ASC 605 with minimal subjectivity.
Impairment of Long-Lived Assets.We adopted Statement of Financial Accounting Standards No. 144, “Accounting for the Impairment or Disposal of Long-Lived Assets” (SFAS 144), which addresses financial accounting and reporting for the impairment or disposal of long-lived assets and supersedes SFAS No. 121, “Accounting for the Impairment of Long-Lived Assets and for Long-Lived Assets to be Disposed Of,” and the accounting and reporting provision of APB Opinion No. 30, “reporting the Results of Operations for a Disposal of a Segment of a Business.” We periodically evaluate the carrying value of long-lived assets to be held and used in accordance with SFAS 144. SFAS 144 requires impairment losses to be recorded on long-lived assets used in operations when indicators of impairment are present and the undiscounted cash flows estimated to be generated are present and the undiscounted cash flows estimated to be generated by those assets are less than the assets’ carrying amounts. In that event, a loss is recognized based on the amount by which the carrying amount exceeds the fair market value of the long-lived assets. Loss on long- lived assets to be disposed of is determined in a similar manner, except that fair market values are reduced for the cost of disposal.
Foreign Currency Translations.The functional currency of the company is Chinese Yuan Renminbi (CNY). We maintain our financial statements in the functional currency. Our financial statements are translated into U.S. Dollars in accordance with Statement of Financial Accounting Standards (SFAS) No. 52, “Foreign Currency Translation,” with the CNY as the functional currency. Monetary assets and liabilities denominated in currencies other than the functional currency are translated into the functional currency at rates of exchange prevailing at the balance sheet dates. Transactions denominated in currencies other than the functional currency are translated into the functional currency at the exchange rates prevailing at the dates of the transaction. Exchange gains or losses arising from foreign currency transactions are included in the determination of net income for the respective periods. The resulting translation adjustments are reported under other comprehensive income in accordance with SFAS No. 130, “Reporting Comprehensive Income,” as a component of shareholders’ equity.
- 41 -
Recent Accounting Pronouncements
On April 1, 2009, the FASB approved FSP FAS 141(R)-1, “Accounting for Assets Acquired and Liabilities Assumed in a Business Combination That Arise from Contingencies”, which amends FAS 141(R), as incorporated into ASC 805, and eliminated the distinction between contractual and non-contractual contingencies. Under the updated standard an acquirer is required to recognize at fair value an asset acquired or liability assumed in a business combination that arises from a contingency if the acquisition-date fair value of that asset or liability can be determined during the measurement period. If the acquisition-date fair value cannot be determined, the acquirer applies the recognition criteria in ASC 450, “Contingencies” to determine whether the contingency should be recognized as of the acquisition date or after it. This guidance is effective for assets or liabilities arising from contingencies in business combinations for which the acquisition date is on or after the beginning of the first annual reporting period beginning on or after December 15, 2008. The adoption of such standard had no material impact on our consolidated financial statements.
In April 2009, the FASB issued FSP No. FAS 157-4, “Determining Fair Value When the Volume and Level of Activity for the Asset or Liability Have Significantly Decreased and Identifying Transactions That Are Not Orderly” as incorporated into ASC 820, “Fair Value Measurements and Disclosures”. The guidance relates to determining fair values when there is no active market or where the price inputs being used represent distressed sales. It reaffirms what FASB ASC 820 states is the objective of fair value measurement—to reflect how much an asset would be sold for in an orderly transaction (as opposed to a distressed or forced transaction) at the date of the financial statements under current market conditions. Specifically, it reaffirms the need to use judgment to ascertain if a formerly active market has become inactive and in determining fair values when markets have become inactive. This guidance is effective for interim and annual periods ended after June 15, 2009, but entities may early adopt this guidance for the interim and annual periods ended after March 15, 2009. The adoption of such standard had no material impact on our consolidated financial statements.
In May 2009, the FASB issued ASC 855, “Subsequent Events,” which establishes general standards of accounting for and disclosure of events that occur after the balance sheet date but before financial statements. The statement is effective for interim and annual periods ended after June 15, 2009. The standard was subsequently amended by FASB Accounting Standards Update (“ASU”) 2010-09 which exempts an entity that is an SEC filer from the requirement to disclose the date through which subsequent events have been evaluated.
In June 2009, the FASB issued FASB ASU 2009-01, which amends ASC 105, “Generally Accepted Accounting Principles” (“Statement of Financial Accounting Standards (“SFAS”) No. 168, “The FASB Accounting Standards Codification TM and the Hierarchy of Generally Accepted Accounting Principles – a replacement of FASB Statement No. 162,” ) , which establishes the FASB Accounting Standards Codification TM (“Codification”) as the source of authoritative generally accepted accounting principles in the United States (“GAAP”) for nongovernmental entities. The Codification does not change GAAP. Instead, it takes the thousands of individual pronouncements that currently comprise GAAP and reorganizes them into approximately 90 accounting Topics, and displays all Topics using a consistent structure. Contents in each Topic are further organized first by Subtopic, then Section and finally Paragraph. The Paragraph level is the only level that contains substantive content. Citing particular content in the Codification involves specifying the unique numeric path to the content through the Topic, Subtopic, Section and Paragraph structure. FASB suggests that all citations begin with “FASB ASC”. Changes to the ASC subsequent to June 30, 2009 are referred to as Accounting Standards Updates.
In August 2009, the FASB issued ASU 2009-5, “Measuring Liabilities at Fair Value”. ASU 2009-05 amends ASC 820, “Fair Value Measurements”. Specifically, ASU 2009-05 provides clarification that in circumstances in which a quoted price in an active market for the identical liability is not available, a reporting entity is required to measure fair value using one or more of the following methods: 1) a valuation technique that uses a) the quoted price of the identical liability when traded as an asset or b) quoted prices for similar liabilities or similar liabilities when traded as assets and/or 2) a valuation technique that is consistent with the principles of ASC 820 of the Accounting Standards Codification (e.g. an income approach or market approach). ASU 2009-05 also clarifies that when estimating the fair value of a liability, a reporting entity is not required to adjust to include inputs relating to the existence of transfer restrictions on that liability. The adoption of such standard had no material impact on our consolidated financial statements.
- 42 -
In September 2009, the Emerging Issues Task Force reached final consensus on ASU 2009-13, “Revenue Arrangements with Multiple Deliverables”. ASU 2009-13 addresses how to determine whether an arrangement involving multiple deliverables contains more than one unit of accounting, and how the arrangement consideration should be allocated among the separate units of accounting. This ASU will be effective prospectively for revenue arrangements entered into or materially modified in years beginning on or after June 15, 2010. Early adoption is permitted. The adoption of such standard had no material impact on our consolidated financial statements.
In December 2009, the FASB issued ASU 2009-17, “Improvements to Financial Reporting by Enterprises Involved with Variable Interest Entities”. This ASU amends ASC 810, “Consolidation”, eliminates exceptions to consolidating qualifying special-purpose entities, contains new criteria for determining the primary beneficiary, and increases the frequency of required reassessments to determine whether a company is the primary beneficiary of a variable interest entity. This ASU is effective for years beginning after November 15, 2009, which for the Company is January 1, 2010, with earlier adoption prohibited. The Company does not expect the adoption of ASU 2009-17 to have a material effect on its consolidated financial statements.
In January 2010, FASB amended ASC 820, “Fair Value Measurements and Disclosures.” The new disclosures and clarifications of existing disclosures are effective for interim and annual reporting periods beginning after December 15, 2009, except for the disclosures about purchases, sales, issuances, and settlements in the roll forward of activity in Level 3 fair value measurements. Those disclosures are effective for years beginning after December 15, 2010, and for interim periods within those years. The Company determined the adoption of this ASU had no material impact on its financial statements.
Quantitative and Qualitative Disclosures about Market Risk
Interest Rate Risk
The Company deposits surplus funds with Chinese banks earning daily interest. The Company does not invest in any instruments for trading purposes. Most of the Company’s outstanding debt instruments carry fixed rates of interest. The Company’s operations generally are not directly sensitive to fluctuations in interest rates as we have no long term debt. Management monitors the banks’ prime rates in conjunction with our cash requirements to determine the appropriate level of debt balances relative to other sources of funds. We have not entered into any hedging transactions in an effort to reduce our exposure to interest rate risk.
Foreign Exchange Risk
While our reporting currency is the U.S. dollar, substantially all of our consolidated revenues and consolidated costs and expenses are denominated in RMB. Substantially all of our assets are denominated in RMB except for cash. As a result, we are exposed to foreign exchange risk as our revenues and results of operations may be affected by fluctuations in the exchange rate between U.S. dollars and RMB. If the RMB depreciates against the U.S. dollar, the value of our RMB revenues, earnings and assets as expressed in our U.S. dollar financial statements will decline. Assets and liabilities are translated at exchange rates at the balance sheet dates and revenue and expenses are translated at the average exchange rates and equity is translated at historical exchange rates. Any resulting translation adjustments are not included in determining net income but are included in determining other comprehensive income, a component of equity. An average appreciation (depreciation) of the RMB against the U.S. dollar of 5% would increase (decrease) our comprehensive income by $2.0 million based on our outstanding revenues, costs and expenses, assets and liabilities denominated in RMB as of June 30, 2010. As of June 30, 2010, our accumulated other comprehensive income was $1.46 million. We have not entered into any hedging transactions in an effort to reduce our exposure to foreign exchange risk.
The value of the Renminbi against the U.S. dollar and other currencies is affected by, among other things, changes in China’s political and economic conditions. Since July 2005, the Renminbi has not been pegged to the U.S. dollar. Although the People’s Bank of China regularly intervenes in the foreign exchange market to prevent significant short-term fluctuations in the exchange rate, the Renminbi may appreciate or depreciate significantly in value against the U.S. dollar in the medium to long term. Moreover, it is possible that in the future, PRC authorities may lift restrictions on fluctuations in the Renminbi exchange rate and lessen intervention in the foreign exchange market.
- 43 -
Inflation
Inflationary factors such as increases in the cost of our product and overhead costs may adversely affect our operating results. Although we do not believe that inflation has had a material impact on our financial position or results of operations to date, a high rate of inflation in the future may have an adverse effect on our ability to maintain current levels of gross margin and selling, general and administrative expenses as a percentage of net revenues if the selling prices of our products do not increase with these increased costs.
SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT
The following table sets forth information regarding beneficial ownership of our common stock as of February 1, 2011 (i) by each person who is known by us to beneficially own more than 5% of our common stock; (ii) by each of our officers and directors; and (iii) by all of our officers and directors as a group. Unless otherwise specified, the address of each of the persons set forth below is in care of the Company, No.55 Miyun Road, Nankai District, Tianjin City, 300111, People’s Republic of China.
Name and Address of Beneficial
Owner |
Office, If Any
|
Title of Class
| Amount and
Nature of
Beneficial
Ownership(1) |
Percent
of
Class(2) |
| Officers and Directors |
| Xuecheng Xia | Chairwoman, CEO and President | Common stock, $0.001 par value | 31,513,945(3) | 79.1% |
| Shuyuan Chang | CFO, Treasurer and Secretary | Common stock, $0.001 par value | 0 | * |
| Yansheng Wang | Chief Operating Officer | Common stock, $0.001 par value | 0 | * |
| Cory Roberts | Director | Common stock, $0.001 par value | 2,754,350(6) | 6.8% |
| Haiwei He | Director | Common stock, $0.001 par value | 0 | * |
| All officers and directors as a group (5 persons namedabove) | | Commonstock, $0.001par value | 0 | 85.9% |
| 5% Security Holders |
| Dragon Core Limited | -- | Common stock, $0.001 par value | 9,454,183(4) | 23.7% |
| Xuecheng Xia | Director | Common stock, $0.001 par value | 31,513,945(3) | 79.1% |
| Jianping Lu | -- | Common stock, $0.001 par value | 9,454,183(4) | 23.7% |
- 44 -
| Neo Profit Limited | -- | Common stock, $0.001 par value | 22,059,762(5) | 55.3% |
| Bay Peak, LLC | -- | Common stock, $0.001 par value | 2,754,350(6) | 6.8% |
| Cory Roberts | Director | Common stock, $0.001 par value | 2,754,350(6) | 6.8% |
_____________________
* Less than 1%
| (1) | Beneficial Ownership is determined in accordance with the rules of the SEC and generally includes voting or investment power with respect to securities. Each of the beneficial owners listed above has direct ownership of and sole voting power and investment power with respect to the shares of our common stock. |
| | |
| (2) | A total of 39,840,605 shares of our common stock are considered to be outstanding pursuant to SEC Rule 13d-3(d)(1) as of February 1, 2011. For each beneficial owner above, any options exercisable within 60 days have been included in the denominator. |
| | |
| (3) | The shares attributed to Ms. Xuecheng Xia are held indirectly through Dragon Core Limited and Neo Profit Limited, which are both beneficially owned and controlled by her. |
| | |
| (4) | Dragon Core Limited is beneficially owned and controlled by Ms. Xia and Ms. Jianping Lu. |
| | |
| (5) | Neo Profit Limited is beneficially owned and controlled by Ms. Xia. |
| | |
| (6) | The shares attributed to Mr. Roberts include 2,541,535 shares of our common stock and warrants to purchase 212,815 shares of our common stock held indirectly through Bay Peak, LLC. Bay Peak, LLC is beneficially owned and controlled by Mr. Roberts and he is deemed to have voting and dispositive control over the shares held by it. |
Changes in Control
We do not currently have any arrangements which if consummated may result in a change of control of our Company.
DIRECTORS AND EXECUTIVE OFFICERS
| Name | Age
| Position |
| Xuecheng Xia | 54 | Chief Executive Officer and Chairman |
| Shuyuan Chang | 36 | Chief Financial Officer |
| Yansheng Wang | 55 | Chief Operating Officer |
| Haiwei He | 48 | Director |
| Cory Roberts | 46 | Director |
Ms. Xuecheng Xia.Ms. Xia has been our President, Chief Executive Officer and Board Chair since May 28, 2010 and has served as the President and Chief Executive Officer of our indirect PRC subsidiary, BOAI Pharm, since its inception in August 2001. Ms. Xia has an extensive background in the PRC pharmaceutical industry and is a member of the Tianjin Association of Entrepreneurs, the Tianjin Medicine Association and the People’s Political Consultative Conference of Tianjin Nankai. She also serves as commissioner of the Tianjin Union Association of Industrial and Commercial Enterprises.
- 45 -
Mr. Shuyuan Chang. Mr. Chang has served as our Chief Financial Officer since October 15, 2010. He has extensive corporate finance and extensive capital market experience, including experience with Initial Public Offerings, private placements and follow-on offerings. Prior to joining us, Mr. Chang has served since March 2009 as Senior Vice President of Finance for Advanced Battery Technology, Inc. (NASDAQ: ABAT), where he was responsible for overseeing its financial reporting, capital market fundraising, (including the completion of $72 million in financing), SEC compliance and investor relationships.Prior to that, Mr. Chang served, from April, 2007 to March 2009, as Senior Vice President of China Natural Gas, Inc. (NASDAQ: CHNG), where he was responsible for managing all aspects of its New York office, including assisting in the completion of two financing transactions, totaling $55 million, and its successful listing on NASDAQ. Prior to joining CHNG, Mr. Chang served, from 2000 to 2004, as associate manager at investment banking groups of Taiwan Securities Group and Polaris Securities Group in Taipei, Taiwan. Mr. Chang holds an MBA from the National Cheng-Chi University in Taiwan, and a Masters Degree in Accounting from Pace University (New York). Mr. Chang is also a U.S. Certified Financial Analyst (CFA).
Mr. Yansheng Wang.Mr. Wang has served as our Chief Operating Officer since May 28, 2010, and as served in the same capacity for our subsidiary, BOAI Pharm, since its inception in August 2001.
Ms. Haiwei He.Mr. He has served as our Director since July 15, 2010 and has 29 years’ experience in finance and accounting. Mr. He is a co-founder of our subsidiary BOAI Pharm and has served as the Comptroller of our subsidiary BOAI Pharm, since its inception in August 2001. Mr. He holds an Accounting Diploma from Tianjin University.
Mr. Cory Roberts.Mr. Roberts has served as our Director since September 2008 and has served as the founder and principal of Bay Peak LLC, private company providing investment for and financial advisory services to PRC companies going public in the U.S., since 2005. Mr. Roberts has a 20-year career in finance over which time he has invested in private companies that have raised over $300 million in expansion capital and that have subsequently commanded over $3 billion in public market value. Prior to establishing Bay Peak LLC, from 2003 to 2005, Mr. Roberts co-managed Accela International, an investment firm, where he assisted in the development of a Hong Kong listed private equity fund, and from 1999 to 2003, as co-managing partner of Elevation Capital, a San Francisco-based investment management company, where he was responsible for overseeing its investment fund, the Elevation Venture Fund. Mr. Roberts holds a Bachelors Degree in Finance from the University of Oklahoma and began his career in the Private Client Group at Salomon Smith Barney.
Family Relationships
There are no family relationships among any of our officers or directors.
Involvement in Certain Legal Proceedings
To the best of our knowledge, none of our directors or executive officers has been convicted in a criminal proceeding, excluding traffic violations or similar misdemeanors, or has been a party to any judicial or administrative proceeding during the past ten years that resulted in a judgment, decree or final order enjoining the person from future violations of, or prohibiting activities subject to, federal or state securities laws, or a finding of any violation of federal or state securities laws, except for matters that were dismissed without sanction or settlement. Except as set forth in our discussion below in “Certain Relationships and Related Transactions, and Director Independence – Transactions with Related Persons,” none of our directors, director nominees or executive officers has been involved in any transactions with us or any of our directors, executive officers, affiliates or associates which are required to be disclosed pursuant to the rules and regulations of the SEC.
Governance Structure
The Company is governed by a Board of Directors that currently consists of three members: Xuecheng Xia, Cory Roberts, and Haiwei He, with Ms. Xia serving as Board Chair and Mr. Roberts serving as Lead Director. None of the Company’s directors are “independent” as that term is defined by the Nasdaq Stock Market Rules, however the Company intends to appoint one or more independent directors in the near future. The Board may also establish and delegate some of its functions to various committees.
- 46 -
The Board believes the interests of all stockholders are best served at the present time through a leadership model with a combined Chairman/CEO position and a Lead Director. However, the Board retains authority to amend the By-Laws to separate the positions of Chairman and CEO at any time. The current CEO possesses an in-depth knowledge of the Company, its integrated operations in China, and the array of challenges to be faced, gained through over 15 years of successful experience. The Board believes that these experiences and other insights put the CEO in the best position to provide broad leadership for the Board as it considers strategy and as it exercises its fiduciary responsibilities to stockholders.
The Board established the role of Lead Director and has appointed Mr. Cory Roberts, to serve in this capacity to remain in the position at least through the annual meeting of stockholders. The Leading Director’s duties include chairing executive sessions of the independent directors; chairing meetings of the Board in the absence of the Chairman; and, working closely with the Chairman in developing Board agendas, topics, schedules, and in reviewing materials provided to the directors.
The Board’s Role in Risk Oversight
The Board is charged with oversight of and safeguarding the assets of the Company and with maintaining appropriate financial and other controls, and conducting the Company’s business wisely and in compliance with applicable laws and regulations and proper governance. Included in these responsibilities is the Board of Directors’ oversight of the various risks facing the Company. In this regard, the Board seeks to understand and oversee critical business risks. The Board does not view risk in isolation. Risks are considered in virtually every business decision and as part of the Company’s business strategy. The Board recognizes that it is neither possible nor prudent to eliminate all risk. Indeed, purposeful and appropriate risk-taking is essential for the Company to be competitive on a global basis and to achieve its objectives.
While the Board oversees risk management, Company management is charged with managing risk. The Company has robust internal processes and a strong internal control environment to identify and manage risks and to communicate with the Board. The Board monitors and evaluate the effectiveness of the internal controls and the risk management program at least annually. Management communicates routinely with the Board and individual Directors on the significant risks identified and how they are being managed. Directors are free to, and indeed often do, communicate directly with senior management. The Board implements its risk oversight function as a whole but intends to establish and delegate this risk oversight function to various committees in the future.
Director Qualifications
Directors are responsible for overseeing the Company’s business consistent with their fiduciary duty to shareowners. This significant responsibility requires highly-skilled individuals with various qualities, attributes and professional experience. The Board believes that there are general requirements for service on the Company’s Board of Directors that are applicable to all Directors and that there are other skills and experience that should be represented on the Board as a whole but not necessarily by each Director. The Board considers the qualifications of Directors and Director candidates individually and in the broader context of the Board’s overall composition and the Company’s current and future needs.
Qualifications for All Directors
In its assessment of each potential candidate, the Board considers the nominee’s judgment, integrity, experience, independence, understanding of the Company’s business or other related industries and such other factors the Board determines are pertinent in light of the current needs of the Board. The Board also takes into account the ability of a Director to devote the time and effort necessary to fulfill his or her responsibilities to the Company.
- 47 -
The Board requires that each Director be a recognized person of high integrity with a proven record of success in his or her field. Each Director must demonstrate respect for and an ability and willingness to learn corporate governance requirements and practices, an appreciation of multiple cultures and a commitment to ethical business practices. In addition to the qualifications required of all Directors, the Board assesses intangible qualities including the individual’s ability to ask difficult questions and, simultaneously, to work collegially.
The Board does not have a specific diversity policy, but considers diversity of professional and academic experiences in evaluating candidates for Board membership. Diversity is important because a variety of points of view contribute to a more effective decision-making process.
Qualifications, Attributes, Skills and Experience to be Represented on the Board as a Whole
The Board has identified particular qualifications, attributes, skills and experience that are important to be represented on the Board as a whole, in light of the Company’s current needs and business priorities. The Company is a U.S. public company that produces and distributes pharmaceutical products in China and abroad. Therefore, the Board believes that a diversity of professional experiences in domestic and international pharmaceutical industry, specific knowledge of key geographic growth areas, and knowledge of U.S. capital markets and of U.S. accounting and financial reporting standards should be represented on the Board.
Set forth below is a tabular disclosure summarizing some of the specific qualifications, attributes, skills and experiences of our directors.
| Director | Titles | Material Qualifications |
| Xuecheng Xia | Board Chair and Chief Executive Officer | • | Co-founder of BOAI Pharm |
| • | Chief Executive Officer of the Company’s subsidiary, BOAI Pharm, since its incorporation |
| • | Member of the Tianjin Medicine Association |
| • | Ms. Xia contributes invaluable long-term knowledge of the Company’s business and operations and extensive experience in the PRC pharmaceutical industry |
| Haiwei He | Director and Comptroller | • | Co-founder of BOAI Pharm |
| • | Has 29 years’ experience in finance and accounting |
| • | Holds a Diploma in Accounting from Tianjin University. |
| • | Ms. He contributes invaluable long-term knowledge of the Company’s business and operations and extensive experience in the PRC pharmaceutical industry |
| Cory Roberts | Lead Director | • | Principal of Bay Peak LLC, private company providing investment for and financial advisory services to PRC companies going public in the U.S. |
| • | Has invested in private companies that have raised over $300 million in expansion capital and that have subsequently commanded over $3 billion in public market value |
| • | holds a B.A. in Finance from the University of Oklahoma and has over 20 years experience in finance in the U.S. capital markets which provides invaluable guidance and perspective to the Board |
Stockholder Communication with the Board of Directors
Stockholders may communicate with the Board by sending a letter to our board of directors, c/o Corporate Secretary, Asia Leechdom Holding Corporation, No.55 Miyun Road, Nankai District, Tianjin City, 300111, People's Republic of China, for submission to the board or committee or to any specific director to whom the correspondence is directed. Stockholders communicating through this means should include with the correspondence evidence, such as documentation from a brokerage firm, that the sender is a current record or beneficial stockholder of the Company. All communications received as set forth above will be opened by the Corporate Secretary or his designee for the sole purpose of determining whether the contents contain a message to one or more of our directors.
- 48 -
Any contents that are not advertising materials, promotions of a product or service, patently offensive materials or matters deemed, using reasonable judgment, inappropriate for the Board will be forwarded promptly to the chairman of the Board, the appropriate committee or the specific director, as applicable.
Code of Ethics
We have adopted a written code of ethics that applies to all of our officers, directors and employees, including our principal executive officer and principal financial officer, or persons performing similar functions, a copy of which is attached as an exhibit to this Registration Statement.
EXECUTIVE COMPENSATION
Summary Compensation Table — Fiscal Years Ended June 30, 2010 and 2009
The following table sets forth information concerning all cash and non-cash compensation awarded to, earned by or paid to the named persons for services rendered in all capacities during the noted periods. No other executive officer received total annual salary and bonus compensation in excess of $100,000.
Name and
Principal
Position |
Year |
Salary
($) |
Bonus
($) |
Stock
Awards
($) |
Option
Awards
($) |
Non-Equity
Incentive Plan
Compensation
Earnings
($) | Non-
Qualified
Deferred
Compensation
Earnings
($) |
All Other
Compensation
($) |
Total
($) |
Xuecheng Xia,
Chief Executive Officer(1) | 2010 | 240,000 | 0 | 0 | 0 | 0 | 0 | 0 | 240,000 |
| 2009 | 26,800 | 0 | 0 | 0 | 0 | 0 | 0 | 26,800 |
Shuyuan Chang,
Chief Financial Officer(2) | 2010 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 2009 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
Cory Roberts,
Former CEO, CFO and President(3) | 2009 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 2008 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| (1) | On May 28, 2010, we acquired ALH in a reverse acquisition that was structured as a share exchange and in connection with that transaction, Ms. Xia became our Chief Executive Officer, Chief Financial Officer and Chairwoman, effective immediately. Prior to the effective date of the reverse acquisition, Ms. Xia served as President of ALH and its subsidiary BOI Pharm. The annual, long term and other compensation shown in this table include the amount Ms. Xia received from such subsidiary prior to the consummation of the reverse acquisition. |
| |
| (2) | On October 15, 2010, Ms. Xia resigned from her position as our Chief Financial Officer and Mr. Chang was appointed Chief Financial Officer in her stead. |
| |
| (3) | Mr. Cory Roberts served as our President from September 11, 2008 until his resignation from this position and appointment of Ms. Xia on May 28, 2010. Mr. Roberts continues to serve as a Director on our Board of Directors. |
Summary of Employment Agreements and Material Terms
Prior to our reverse acquisition, our operating subsidiary was a private limited company organized under the laws of the PRC, and in accordance with PRC regulations, the salary of our executives was determined by our shareholders. In addition, each employee is required to enter into an employment agreement executed by our human resources department and our financial department. Accordingly, all our employees, including our Chief Executive Officer, Ms. Xuecheng Xia, our Chief Financial Officer, Mr. Shuyuan Chang, and our Chief Operating Officer, Mr. Yansheng Wang, have executed our employment agreement. Our employment agreements with our executives provide the amount of each executive officer’s salary and establish their eligibility to receive a bonus. Ms. Xia’s employment agreement provides for an annual salary of RMB 1,632,000 (approximately $240,000), Mr. Yang’s employment agreement provides for an annual salary of RMB 850,000 (approximately $125,000) and Mr. Wang’s employment agreement provides for an annual salary of RMB 100,000 (approximately $15,000).
- 49 -
Other than the salary and necessary social benefits required by the government, which are defined in the employment agreement, we currently do not provide other benefits to the officers at this time. Our executive officers are not entitled to severance payments upon the termination of their employment agreements or following a change in control.
We have not provided retirement benefits (other than a state pension scheme in which all of our employees in China participate) or severance or change of control benefits to our named executive officers.
Outstanding Equity Awards at Fiscal Year End
For the year ended June 30, 2010, no director or executive officer has received compensation from us pursuant to any compensatory or benefit plan. There is no plan or understanding, express or implied, to pay any compensation to any director or executive officer pursuant to any compensatory or benefit plan, although we anticipate that we will compensate our officers and directors for services to us with stock or options to purchase stock, in lieu of cash.
Director Compensation
No member of our board of directors received any compensation for his or her services as a director during the year ended June 30, 2010.
CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE
Transactions with Related Persons
The following includes a summary of transactions since the beginning of the 2009 year, or any currently proposed transaction, in which we were or are to be a participant and the amount involved exceeded or exceeds the lesser of $120,000 or one percent of the average of our total assets at year end for the last two completed fiscal years, and in which any related person had or will have a direct or indirect material interest (other than compensation described under “Executive Compensation”). We believe the terms obtained or consideration that we paid or received, as applicable, in connection with the transactions described below were comparable to terms available or the amounts that would be paid or received, as applicable, in arm’s-length transactions
ALH was obligated to repay $1,500,000 due to China BOI Hunter, LLC, or BOI Hunter, under a certain Secured Convertible Promissory Note, due December 20, 2008, issued pursuant to a certain Credit and Security Agreement, dated as of June 20, 2008, among BOI Hunter, ALH and the other ALH affiliates signatory thereto. The note was convertible into warrants to purchase up an amount of shares of ALH common stock equal to $750,000. The note remained outstanding and unconverted until the parties agreed to a settlement agreement, dated May 25, 2010, among the Company, ALH, BOAI Pharm and BOI Hunter, pursuant to which the controlling stockholder agreed to allocate to BOI Hunter as settlement of the Obligations, 239,044 shares of our common stock to be issued to him in exchange for his equity interests in ALH. We also agreed to issue BOI Hunter, a three-year warrant to purchase 298,805 shares of common stock at an exercise price of $2.51 per share.
In March, May and June 2010, certain holders of our Plan Warrants, each a Warrant Investor, exercised their right to purchase an aggregate of 110,000 shares of common stock at $2.00 per share and an aggregate of 4,177,381 shares of common stock at $2.51 per share, for aggregate proceeds of approximately $10.7 million. Pursuant to Side Letter Agreements, dated May 28, 2010 and June 30, 2010, among the Company, the controlling shareholder and each of the Warrant Investors, we agreed to issue to each of the Warrant Investors, on a pro rata basis, new five-year warrants, the New Warrants, to purchase an aggregate of 835,476 shares of common stock at $2.51 per share. As consideration for its services as financial advisor in connection with the May 2010 and June 2010 financing, at the closing of such financing we also paid Bay Peak, LLC, the company’s financial advisor, $935,000 in cash and issued them 675,317 shares of our Common Stock valued at $2.51 per share. Bay Peak is beneficially owned and controlled by our Director, Cory Roberts.
- 50 -
On February 27, 2010, Chenghai Du, our former controlling stockholder, entered into a series of call option agreements with each of Ma Dan, Xia Xuecheng, Wang Yan, Wang Yansheng, He Haiwei and Tian Mengchun, the original shareholders and managers of BOAI Pharm, pursuant to which Mr. Du granted each of them an option to acquire an amount of shares equal to 90% of the shares of our common stock issued to Mr. Du in the reverse acquisition, at an exercise price of $0.0001 per share, and on January 10, 2011, Mr. Du entered into an additional call option with Ms. Jianping Lu, pursuant to which Mr. Du granted her an option to acquire an amount of shares equal to the remaining 10% of the shares of our common stock issued to Mr. Du in the reverse acquisition, on the same terms and conditions as the other option holders. Each of the option holders had the right to exercise their option during the period commencing on the date of the option agreement and ending on the fifth anniversary of the date thereof.
On January 11, 2011, the option holders transferred and assigned their options to purchase an aggregate of 9,454,183 shares of our Common Stock to Dragon Core Limited and an aggregate of 22,059,762 shares of our Common Stock to Neo Profit Limited. On the same date each of Dragon Core and Neo Profit exercised their options to purchase their respective shares from Mr. Du. As a result of the exercises, Dragon Core and Neo Profit hold an aggregate of 31,513,945, or 79.1%, of our issued and outstanding common stock and Mr. Du is no longer affiliated with, and no longer holds any equity interest in the Company or its subsidiaries Dragon Core Limited is 67% beneficially owned and controlled by our Chief Executive Officer and Director, Ms. Xuecheng Xia, and 33% beneficially owned and controlled by Ms. Jianping Lu, an executive employee of BOAI Pharm and Neo Profit Limited is wholly-owned and controlled by Ms. Xia.
- For the year ended June 30, 2010, the Company advanced $1,893,114 to drug chain stores, owned and controlled by Ms. Xuecheng Xia, our CEO and controlling shareholder. These loans were unsecured and were due on demand. They were paid in full on December 31, 2010. Now that the Company will be a public company, the management understands that future loans or advances for the benefit of officers and directors of the Company are generally not permitted under the Sarbanes-Oxley Act.
Policies and Procedures Relating to Transactions with Related Persons
On February 1, 2011, our Board of Directors adopted Code of Business Ethics and Conduct which includes our policies and procedures for review, approval or ratification of relationships or transactions with related persons.
Our Code of Business Ethics and Conduct provides a number of specific procedures, requirements, and prohibitions relating to related party transactions. It prohibits directors, officers and employees from accepting simultaneous employment with a Company supplier, customer, developer, or competitor, or from taking part in any activity that enhances or supports a competitor’s position. All directors, officers and employees must disclose to the Company any interest that may conflict with the Company. A director, officer and employee may accept a position as a director of another company only if he or she obtains approval from the Board of Directors; the other company does not compete with the Company; and any compensation received in this capacity is commensurate with its responsibilities.
- 51 -
Our Code of Business Ethics and Conduct generally requires directors, officers, and employees of the Company to avoid conducting Company business with a relative or significant other, or with a business in which a relative or significant other is associated in any significant manner. Where a Company director, officer or employee believes that such a transaction is unavoidable, he or she must fully disclose it to the Company’s Chief Executive Officer. If the Chief Executive Officer deems it material to the Company, the Nominating and Corporate Governance Committee must review and provide advance written approval for such transactions. The Board subsequently reassigned part of this responsibility to the Audit Committee pursuant to the Audit Committee Charter. The most significant related party transactions, especially those involving the Company’s directors or executive officers, must be reviewed and approved in writing in advance by the Company’s Board of Directors. The Board also delegated part of this function to the Audit Committee under the Audit Committee Charter, as explained above. The Company must report all such material related party transactions under applicable accounting rules, federal securities laws, SEC rules and regulations, and securities market rules. Any dealings with a related party must be conducted in such a way that no preferential treatment is given.
In addition, our Code of Business Ethics and Conduct discourages the employment of relatives and significant others in positions or assignments within the same department and prohibits the employment of such persons in positions that have influence (e.g., an auditing or control relationship, or a supervisor/subordinate relationship). Our Human Resources department is primarily responsible for administering this aspect of our related party transactions policy. If any covered relationship develops or exists between two employees, the senior employee must advise his or her supervisor. The Company may separate the employees either by reassignment or termination, if necessary.
Our Code of Business Ethics and Conduct permits waivers of the above policies and procedures as to directors or executive officers only if they are approved in writing by the Board of Directors and promptly disclosed. Any waiver with respect to any employee, agent or contractor must be approved in writing by the Company. Under its Charter, our Nominating and Corporate Governance Committee is also responsible for reviewing requests for any waivers and recommending to the Board whether a particular request should be granted.
Our Board of Directors will monitor and manage potential conflicts of interest of management in accordance with this Code. Any type of related party transaction not expressly covered by the above policies and procedures is subject to separate review and approval of our Board of Directors.
Promoters and Certain Control Persons
We did not have any promoters at any time during the past five fiscal years.
Director Independence
As of the date of this Registration Statement, we have no standing committees and our entire Board of Directors serves as our audit and compensation committees. We have determined that none of our directors are "independent" directors, as that term is defined in Rule 5605(a)(2) of the Listing Rules of The Nasdaq Stock Market, Inc. If we undertake to qualify our common stock for quotation in the over-the-counter market, we may need to ensure we meet any eligibility requirements with respect to independent directors.
LEGAL PROCEEDINGS
In the normal course of business, we are subject to claims and litigation. We are not a party to any material legal proceedings nor are we aware of any circumstance that may reasonably lead a third party to initiate legal proceedings against us.
- 52 -
MARKET PRICE OF AND DIVIDENDS ON THE REGISTRANT’S COMMON EQUITY
AND RELATED STOCKHOLDER MATTERS
Market
There is no established public trading market in our Common Stock. Our securities are not listed for trading on any national securities exchange nor are bid or asked quotations reported in any over-the-counter quotation service.
Holders of Record
As of February 1, 2011, we had 39,840,605 shares of our common stock issued and outstanding, held by 232 holders.
In general, pursuant to Rule 144 adopted under the Securities Act of 1933, as amended, a shareholder who owns restricted shares of a company which files periodic reports with the Securities and Exchange Commission and who has a holding period of at least six months, is entitled to sell such shares in accordance with the provisions of Rule 144. In the event the shareholder is a non-affiliate of the issuer, he or she may make unlimited public resales of shares under Rule 144 provided that the current public information requirement is satisfied. A non-affiliate who has a holding period of more than one year, may make unlimited resales of shares without compliance with any other requirement of Rule 144. Persons who are affiliates of the issuer must comply with all requirements of Rule 144 in conjunction with resales of their shares including the current public information requirement, the volume limitations, the manner of sale requirements and the filing of a Form 144. Therefore, the possible sale of our currently outstanding shares pursuant to Rule 144 may, in the future, have a depressive effect on the price of our common stock in the over-the-counter market.
Dividends
We have never paid any dividends and we plan to retain earnings, if any, for use in the development of the business. Payment of future dividends, if any, will be at the discretion of the Board of Directors after taking into account various factors, including current financial condition, operating results, current and anticipated cash needs and regulations governing dividend distributions by wholly-foreign owned enterprises in China.
RECENT SALES OF UNREGISTERED SECURITIES
On May 28, 2010, we completed a reverse acquisition through a share exchange with ALH, whereby we issued the sole shareholder of ALH, 32,310,758 shares of our common stock, par value $0.001, for 100% of the issued and outstanding capital stock of ALH. ALH thereby became our wholly-owned subsidiary and its subsidiary, BOAI Pharmaceutical, became the Company’s indirect subsidiary. Upon the closing of the reverse acquisition on May 28, 2010, Mr. Cory Roberts, our Director and former President and Chief Financial Officer resigned from his offices as President and Chief Financial Officer, effective immediately, and Mr. Lanny R. Lang, our former Secretary and Director, resigned from all positions held by him, effective immediately, and our Board of Directors appointed Ms. Xuecheng Xia to serve as our Chief Executive Officer and Chief Financial Officer in Mr. Roberts’ stead, effective immediately. Also upon the closing of the reverse acquisition, our Board of Directors increased its size to 3 members and, in addition to Mr. Roberts, who remains a director, the board appointed Ms. Xia and Ms. Hiawei He to fill the vacancies created by such increase, effective immediately.
ALH was obligated to repay a principal amount of $1,500,000 due to BOI Hunter under a certain Secured Convertible Promissory Note, due December 20, 2008, issued pursuant to a certain Credit and Security Agreement, dated as of June 20, 2008, among BOI Hunter, ALH and the other ALH affiliates signatory thereto. The note was convertible into warrants to purchase up an amount of shares of ALH common stock equal to $750,000. The note remained outstanding and unconverted until the parties agreed to a settlement agreement, dated May 25, 2010, among the Company, ALH, BOAI Pharm and BOI Hunter, pursuant to which the controlling stockholder agreed to allocate to BOI Hunter as settlement of the Obligations, 239,044 shares of our common stock to be issued to him in exchange for his equity interests in ALH. We also agreed to issue BOI Hunter, three-year warrants to purchase 298,805 shares of common stock at an exercise price of $2.51 per share.
In March, May and June 2010, certain holders of our Plan Warrants, each a Warrant Investor, exercised their right to purchase an aggregate of 110,000 shares of common stock at $2.00 per share and an aggregate of 4,177,381 shares of common stock at $2.51 per share, for aggregate proceeds of approximately $10.7 million. Pursuant to Side Letter Agreements, dated May 28, 2010 and June 30, 2010, among the Company, the controlling shareholder and each of the Warrant Investors, we agreed to issue to each of the Warrant Investors, on a pro rata basis, new five-year warrants, the New Warrants, to purchase an aggregate of 835,476 shares of common stock at $2.51 per share. As consideration for its services as financial advisor in connection with the May 2010 and June 2010 financing, at the closing of such financing we also paid Bay Peak, LLC, the company’s financial advisor, $935,000 in cash and issued them 675,317 shares of our Common Stock valued at $2.51 per share. Bay Peak is beneficially owned and controlled by our Director, Cory Roberts.
- 53 -
The issuance of common stock to ALH, the Warrant Investors and Bay Peak, LLC was exempt from the registration requirements provided by Section 4(2) of the Securities Act for the offer and sale of securities not involving a public offering and Regulation D promulgated thereunder. Our reliance upon Section 4(2) of the Securities Act in issuing securities was based upon the following factors: (a) the issuance of the securities was an isolated private transaction by us which did not involve a public offering; (b) there were only a limited number of offerees; (c) there were no subsequent or contemporaneous public offerings of the securities by us; (d) the securities were not broken down into smaller denominations; and (e) the negotiations for the sale of the stock took place directly between the offeree and us.
DESCRIPTION OF REGISTRANT’S SECURITIES TO BE REGISTERED
Common Stock
We are authorized to issue 190,000,000 shares of Common Stock, par value $0.001, of which 39,840,605 shares of shares are issued and outstanding. Each outstanding share of common stock entitles the holder thereof to one vote per share on all matters. Our bylaws provide that any vacancy occurring in the Board of Directors may be filled by the affirmative vote of a majority of the remaining directors though less than a quorum of the Board of Directors. Stockholders do not have preemptive rights to purchase shares in any future issuance of our common stock.
The holders of shares of our common stock are entitled to dividends out of funds legally available when and as declared by our Board of Directors. Our Board of Directors has never declared a dividend and does not anticipate declaring a dividend in the foreseeable future. Should we decide in the future to pay dividends, as a holding company, our ability to do so and meet other obligations depends upon the receipt of dividends or other payments from our operating subsidiary and other holdings and investments. In addition, our operating subsidiary in the PRC, from time to time, may be subject to restrictions on their ability to make distributions to us, including as a result of restrictive covenants in loan agreements, restrictions on the conversion of local currency into U.S. dollars or other hard currency and other regulatory restrictions. In the event of our liquidation, dissolution or winding up, holders of our common stock are entitled to receive, ratably, the net assets available to stockholders after payment of all creditors.
All of the issued and outstanding shares of our common stock are duly authorized, validly issued, fully paid and non-assessable. To the extent that additional shares of our common stock are issued, the relative interests of existing stockholders will be diluted.
Preferred Stock
We are authorized to issue up to 10,000,000 shares of preferred stock, par value $0.001 per share, in one or more classes or series within a class as may be determined by our Board of Directors, who may establish, from time to time, the number of shares to be included in each class or series, may fix the designation, powers, preferences and rights of the shares of each such class or series and any qualifications, limitations or restrictions thereof. Any preferred stock so issued by the Board of Directors may rank senior to the common stock with respect to the payment of dividends or amounts upon liquidation, dissolution or winding up of us, or both. Moreover, while providing desirable flexibility in connection with possible acquisitions and other corporate purposes, under certain circumstances, the issuance of preferred stock or the existence of the unissued preferred stock might tend to discourage or render more difficult a merger or other change of control. As of February 1, 2011, no shares of our preferred stock are considered to be outstanding pursuant to SEC Rule 13d-3(d)(1).
- 54 -
Warrants
In the May 2010 and June 2010 financing we sold 4,287,381 shares of Common Stock and warrants to purchase an aggregate of 835,477 shares of Common Stock to the Investors for a purchase price of $10,705,311. The Warrants expire five years after issuance and permit the holder to purchase shares of our Common Stock at an exercise price of $2.51 per share. The Warrants contain customary antidilution provisions.
Anti-takeover Effects of Our Articles of Incorporation and By-laws
Our amended and restated articles of incorporation and bylaws contain certain provisions that may have anti-takeover effects, making it more difficult for or preventing a third party from acquiring control of the Company or changing its Board of Directors and management. According to our bylaws and articles of incorporation, neither the holders of the Company’s common stock nor the holders of the Company’s preferred stock have cumulative voting rights in the election of our directors. The combination of the present ownership by a few stockholders of a significant portion of the Company’s issued and outstanding common stock and lack of cumulative voting makes it more difficult for other stockholders to replace the Company’s Board of Directors or for a third party to obtain control of the Company by replacing its Board of Directors.
Anti-takeover Effects of Nevada Law
Business Combinations
The “business combination” provisions of Sections 78.411 to 78.444 of Nevada’s Combinations with Interested Stockholders statute prohibit a Nevada corporation with at least 200 stockholders from engaging in various “combination” transactions with any interested stockholder: for a period of three years after the date of the transaction in which the person became an interested stockholder, unless the transaction is approved by the Board of Directors prior to the date the interested stockholder obtained such status; or after the expiration of the three-year period, unless:
the transaction is approved by the Board of Directors or a majority of the voting power held by disinterested stockholders, or
if the consideration to be paid by the interested stockholder is at least equal to the highest of: (a) the highest price per share paid by the interested stockholder within the three years immediately preceding the date of the announcement of the combination or in the transaction in which it became an interested stockholder, whichever is higher, (b) the market value per share of common stock on the date of announcement of the combination and the date the interested stockholder acquired the shares, whichever is higher, or (c) for holders of preferred stock, the highest liquidation value of the preferred stock, if it is higher.
A “combination” is defined to include mergers or consolidations or any sale, lease exchange, mortgage, pledge, transfer or other disposition, in one transaction or a series of transactions, with an “interested stockholder” having: (a) an aggregate market value equal to 5% or more of the aggregate market value of the assets of the corporation, (b) an aggregate market value equal to 5% or more of the aggregate market value of all outstanding shares of the corporation, or (c) 10% or more of the earning power or net income of the corporation.
In general, an “interested stockholder” is a person who, together with affiliates and associates, owns (or within three years, did own) 10% or more of a corporation’s voting stock. The statute could prohibit or delay mergers or other takeover or change in control attempts and, accordingly, may discourage attempts to acquire our company even though such a transaction may offer our stockholders the opportunity to sell their stock at a price above the prevailing market price.
- 55 -
Control Share Acquisitions
Nevada’s Acquisition of Controlling Interest statute (NRS Sections 78.378 -78.3793) applies only to Nevada corporations with at least 200 stockholders, including at least 100 stockholders of record who are Nevada residents, and which conduct business directly or indirectly in Nevada. The Acquisition of Controlling Interest statute prohibits an acquirer, under certain circumstances, from voting its shares of a target corporation’s stock after crossing certain ownership threshold percentages, unless the acquirer obtains approval of the target corporation’s disinterested stockholders. The statute specifies three thresholds: one-fifth or more but less than one-third, one-third but less than a majority, and a majority or more, of the outstanding voting power. Once an acquirer crosses one of the above thresholds, those shares in an offer or acquisition and acquired within 90 days thereof become “control shares” and such Control Shares are deprived of the right to vote until disinterested stockholders restore the right. The Acquisition of Controlling Interest statute also provides that if control shares are accorded full voting rights and the acquiring person has acquired a majority or more of all voting power, all other stockholders who do not vote in favor of authorizing voting rights to the control shares are entitled to demand payment for the fair value of their shares in accordance with statutory procedures established for dissenters’ rights.
Transfer Agent and Registrar
The transfer agent and registrar for our common stock is Interwest Transfer Co., Inc., 1981 East 4800 South, Suite 100, Salt Lake City, Utah 84117, telephone number (801) 277-3147.
INDEMNIFICATION OF DIRECTORS AND OFFICERS
Section 78.138 of the Nevada Revised Statutes, or NRS, provides that a director or officer will not be individually liable unless it is proven that (i) the director’s or officer’s acts or omissions constituted a breach of his or her fiduciary duties, and (ii) such breach involved intentional misconduct, fraud or a knowing violation of the law.
Section 78.7502 of NRS permits a company to indemnify its directors and officers against expenses, judgments, fines and amounts paid in settlement actually and reasonably incurred in connection with a threatened, pending or completed action, suit or proceeding if the officer or director (i) is not liable pursuant to NRS 78.138 or (ii) acted in good faith and in a manner the officer or director reasonably believed to be in or not opposed to the best interests of the corporation and, if a criminal action or proceeding, had no reasonable cause to believe the conduct of the officer or director was unlawful.
Section 78.751 of NRS permits a Nevada company to indemnify its officers and directors against expenses incurred by them in defending a civil or criminal action, suit or proceeding as they are incurred and in advance of final disposition thereof, upon receipt of an undertaking by or on behalf of the officer or director to repay the amount if it is ultimately determined by a court of competent jurisdiction that such officer or director is not entitled to be indemnified by the company. Section 78.751 of NRS further permits the company to grant its directors and officers additional rights of indemnification under its articles of incorporation or bylaws or otherwise.
Section 78.752 of NRS provides that a Nevada company may purchase and maintain insurance or make other financial arrangements on behalf of any person who is or was a director, officer, employee or agent of the company, or is or was serving at the request of the company as a director, officer, employee or agent of another company, partnership, joint venture, trust or other enterprise, for any liability asserted against him and liability and expenses incurred by him in his capacity as a director, officer, employee or agent, or arising out of his status as such, whether or not the company has the authority to indemnify him against such liability and expenses.
Our articles of incorporation provide that no director or officer of the Company will be personally liable to the Company or any of its stockholders for damages for breach of fiduciary duty as a director or officer; provided, however, that the foregoing provision shall not eliminate or limit the liability of a director or officer (i) for acts or omissions which involve intentional misconduct, fraud or knowing violation of law, or (ii) the payment of dividends in violation of Section 78.300 of NRS. In addition, our bylaws implement the indemnification and insurance provisions permitted by Chapter 78 of the Nevada Revised Statutes by providing that: the Company shall indemnify its directors to the fullest extent permitted by the Nevada Revised Statutes and may, if and to the extent authorized by the Board of Directors, so indemnify its officers and any other person whom it has the power to indemnify against liability, reasonable expense or other matter whatsoever. The Company may at the discretion of the Board of Directors purchase and maintain insurance on behalf of any person who holds or who has held any position identified in the paragraph above against any and all liability incurred by such person in any such position or arising out of his status as such.
- 56 -
Insofar as indemnification by us for liabilities arising under the Securities Act may be permitted to our directors, officers or persons controlling the company pursuant to provisions of our articles of incorporation and bylaws, or otherwise, we have been advised that in the opinion of the SEC, such indemnification is against public policy as expressed in the Securities Act and is therefore unenforceable. In the event that a claim for indemnification by such director, officer or controlling person of us in the successful defense of any action, suit or proceeding is asserted by such director, officer or controlling person in connection with the securities being offered, we will, unless in the opinion of our counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question whether such indemnification by us is against public policy as expressed in the Securities Act and will be governed by the final adjudication of such issue.
At the present time, there is no pending litigation or proceeding involving a director, officer, employee or other agent of ours in which indemnification would be required or permitted. We are not aware of any threatened litigation or proceeding, which may result in a claim for such indemnification.
FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA
The information required by this item may be found beginning on page F-1 of this Form 10.
CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON
ACCOUNTING AND FINANCIAL DISCLOSURE
We have had no changes in or disagreements with our accountants required to be disclosed pursuant to Item 304 of Regulation S-K.
- 57 -
ASIA LEECHDOM HOLDING CORPORATION
Index to Financial Statements
| | Page |
| ASIA LEECHDOM HOLDING CORPORATION AND SUBSIDIARIES CONSOLIDATED FINANCIALSTATEMENTS FOR THE THREE MONTHS ENDED SEPTEMBER 30, 2010 (UNAUDITED) | F-2 |
| Consolidated Balance Sheets September 30 and June 30, 2010 (Unaudited) | F-3 |
| Consolidated Statements of Income and Comprehensive Income Three Months ended September 30, 2010 and 2009 (Unaudited) | F-4 |
| Consolidated Statements of Cash Flows Three Months Ended September 30, 2010 and 2009 | F-5 |
| Notes to Consolidated Financial Statements (Unaudited) | F-6 |
| ASIA LEECHDOM HOLDING CORP. AND SUBSIDIARIES CONSOLIDATED FINANCIALSTATEMENTS FOR THE YEARS ENDED JUNE 30, 2010 AND 2009 | F-19 |
| Report of Independent Registered Public Accounting Firm | F-20 |
| Consolidated Balance Sheets as at June 30, 2010 and 2009 | F-21 |
| Consolidated Statements of Income and Comprehensive Income for the Years Ended June 30, 2010, 2009 and 2008 | F-22 |
| Consolidated Statements of Cash Flows for the Years Ended June 30, 2010, 2009 and 2008 | F-23 |
| Consolidated Statements of Stockholders' Equity for the Years Ended June 30, 2010, 2009 and 2008 | F-25 |
| Notes to Consolidated Financial Statements | F-27 |
F-1
ASIA LEECHDOM HOLDING CORPORATION AND SUBSIDIARIES
CONSOLIDATED FINANCIALSTATEMENTS
FOR THE THREE MONTHS ENDED SEPTEMBER 30, 2010 (UNAUDITED)
F-2
ASIA LEECHDOM HOLDING CORPORATION AND SUBSIDIARIES
CONSOLIDATED BALANCE SHEETS (UNAUDITED)
| ASSETS | |
| | | | | | | |
| | | September 30,2010 | | | June 30,2010 | |
| | | (Unaudited) | | | | |
| Current Assets: | | | | | | |
| Cash and equivalents | $ | 16,358,691 | | $ | 14,498,707 | |
| Accounts receivable, net | | 15,274,361 | | | 11,442,034 | |
| Other receivables | | 385,647 | | | 113,923 | |
| Advances to material suppliers | | 3,032,805 | | | 2,849,511 | |
| Inventory | | 6,075,260 | | | 7,261,216 | |
| Due from related party | | 3,865,835 | | | 1,893,114 | |
| Subscriptions receivable | | - | | | 3,007,224 | |
| Total Current Assets | | 44,992,599 | | | 41,065,729 | |
| | | | | | | |
| Property, Plant & Equipment, net | | 15,582,255 | | | 15,457,266 | |
| Advances to equipment suppliers | | 6,746,228 | | | 6,612,116 | |
| Deposit for asset acquisition | | 10,093,628 | | | 5,577,640 | |
| Construction in Progress | | 3,874,315 | | | 2,241,428 | |
| Intangible Assets, net | | 6,617,828 | | | 6,475,476 | |
| Total Assets | $ | 87,906,853 | | $ | 77,429,655 | |
| | | | | | | |
| LIABILITIES AND STOCKHOLDERS' EQUITY | |
| | | | | | | |
| Current Liabilities: | | | | | | |
| Short-term loans | $ | 4,971,936 | | $ | 3,536,584 | |
| Accounts payable | | 2,494,232 | | | 2,170,676 | |
| Unearned revenue | | 2,227,788 | | | 1,255,195 | |
| Other payables | | 903,691 | | | 1,985,857 | |
| Taxes payable | | 3,958,044 | | | 3,257,483 | |
| Due to related party | | 2,448,879 | | | 2,506,118 | |
| Accrued expenses | | 209,839 | | | 211,383 | |
| Total Current Liabilities | | 17,214,409 | | | 14,923,296 | |
| | | | | | | |
| Long Term Liabilities | | | | | | |
| Deferred tax liability | | 162,237 | | | 146,780 | |
| Total Liabilities | | 17,376,646 | | | 15,070,076 | |
| | | | | | | |
| Stockholders' Equity: | | | | | | |
Common stock,$0.001 par value, 200,000,000 shares
authorized; 39,840,605 shares issued and outstanding
as of September 30 and June 30, 2010 | | 39,841 | | | 39,841 | |
| Additional paid-in-capital | | 10,139,120 | | | 10,139,120 | |
| Accumulated other comprehensive income | | 2,790,358 | | | 1,456,983 | |
| Statutory reserve | | 2,298,207 | | | 1,904,135 | |
| Retained earnings | | 55,262,681 | | | 48,819,499 | |
| Total Stockholders' Equity | | 70,530,206 | | | 62,359,578 | |
| Total Liabilities and Stockholders' Equity | $ | 87,906,853 | | $ | 77,429,655 | |
The accompanying notes are an integral part of these consolidated financial statements.
F-3
ASIA LEECHDOM HOLDING CORPORATION AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF INCOME AND OTHER COMPREHENSIVE INCOME
(UNAUDITED)
| | | Three months Ended September 30, | |
| | | 2010 | | | 2009 | |
| | | | | | | |
| Net Revenue | $ | 21,111,429 | | $ | 14,173,138 | |
| | | | | | | |
| Cost of Goods Sold | | 10,288,924 | | | 6,967,539 | |
| | | | | | | |
| Gross Profit | | 10,822,505 | | | 7,205,599 | |
| | | | | | | |
| Operating Expenses | | | | | | |
| Selling | | 247,069 | | | 138,012 | |
| Research and development | | 132,129 | | | 272,215 | |
| General and administrative | | 668,483 | | | 270,366 | |
| Total operating expenses | | 1,047,681 | | | 680,593 | |
| | | | | | | |
| Income From Operations | | 9,774,824 | | | 6,525,006 | |
| | | | | | | |
| Other Income (Expense) | | | | | | |
| Interest income | | 1,345 | | | 0 | |
| Interest expense | | (47,438 | ) | | (69,395 | ) |
| Other income | | 78,418 | | | 83,950 | |
| Other expense | | (580,758 | ) | | (1,395 | ) |
| Total other (expenses) income | | (548,433 | ) | | 13,160 | |
| | | | | | | |
| Income Before Income Taxes | | 9,226,391 | | | 6,538,166 | |
| | | | | | | |
| Provision For Income Taxes | | 2,389,140 | | | 1,672,055 | |
| | | | | | | |
| Net Income | | 6,837,251 | | | 4,866,111 | |
| | | | | | | |
| Other Comprehensive Income | | 1,333,375 | | | 256,461 | |
| | | | | | | |
| Comprehensive Income | $ | 8,170,626 | | $ | 5,122,572 | |
| | | | | | | |
| Earnings Per Share | | | | | | |
| Basic | $ | 0.17 | | $ | 0.15 | |
| Diluted | $ | 0.17 | | $ | 0.15 | |
| | | | | | | |
| Weighted average number of common shares outstanding | | | | | | |
| Basic | | 39,840,605 | | | 32,310,758 | |
| Diluted | | 39,840,605 | | | 32,310,758 | |
The accompanying notes are an integral part of these consolidated financial statements.
F-4
ASIA LEECHDOM HOLDING CORPORATION AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF CASH FLOWS
(UNAUDITED)
| | | Three Months Ended September 30, | |
| | | 2010 | | | 2009 | |
| CASH FLOWS FROM OPERATING ACTIVITIES | | | | | | |
| Net Income | $ | 6,837,251 | | $ | 4,866,111 | |
Adjustments to reconcile net income to net cash
provided by operating activities: | | | | | | |
| Depreciation and amortization | | 200,430 | | | 188,313 | |
| Deferred tax liability | | 12,308 | | | 0 | |
| (Increase) / decrease in current assets: | | | | | | |
| Accounts receivable | | (3,550,602 | ) | | (487,368 | ) |
| Other receivables | | (265,698 | ) | | (12,176 | ) |
| Advances to material suppliers | | (123,768 | ) | | 113,702 | |
| Due from related party | | (1,907,649 | ) | | 717,344 | |
| Inventory | | 1,314,847 | | | (1,918,500 | ) |
| Increase / (decrease) in current liabilities: | | | | | | |
| Accounts payable | | 275,674 | | | 479,984 | |
| Other payables | | (1,123,548 | ) | | (1,462,803 | ) |
| Due to related party | | (100,578 | ) | | (63,788 | ) |
| Taxes payable | | 638,048 | | | (499,706 | ) |
| Accrued expenses | | (5,751 | ) | | 9,067 | |
| Unearned revenue | | 934,072 | | | 1,585,235 | |
| Total Adjustments | | (3,702,215 | ) | | (1,350,696 | ) |
| Net cash provided by operating activities | | 3,135,036 | | | 3,515,415 | |
| | | | | | | |
| CASH FLOWS FROM INVESTING ACTIVITIES | | | | | | |
| Purchase of intangible assets | | (20,227 | ) | | (17,448 | ) |
| Purchase of property, plant & equipment | | (5,138 | ) | | (587,111 | ) |
| Investments in construction in progress | | (1,565,533 | ) | | 1,737,495 | |
| Deposit for asset acquisition | | (4,342,140 | ) | | 0 | |
| Net cash used in investing activities | | (5,933,038 | ) | | 1,132,936 | |
| | | | | | | |
| CASH FLOWS FROM FINANCING ACTIVITIES | | | | | | |
| Borrowing of short-term loans | | 1,535,995 | | | 374,711 | |
| Repayment of short-term loan | | (190,000 | ) | | 0 | |
| Collections on subscriptions receivable | | 3,007,224 | | | 0 | |
| Net cash provided by financing activities | | 4,353,219 | | | 374,711 | |
| | | | | | | |
| NET (DECREASE)/ INCREASE IN CASH AND EQUIVALENTS | | 1,555,217 | | | 5,023,062 | |
| | | | | | | |
| EFFECT OF EXCHANGE RATE CHANGES ON CASH AND EQUIVALENTS | | 304,767 | | | (1,445 | ) |
| | | | | | | |
| CASH AND EQUIVALENTS, BEGINNING BALANCE | | 14,498,707 | | | 5,495,176 | |
| | | | | | | |
| CASH AND EQUIVALENTS, ENDING BALANCE | $ | 16,358,691 | | $ | 10,516,793 | |
| | | | | | | |
| SUPPLEMENTAL DISCLOSURES: | | | | | | |
| | | | | | | |
| Cash paid during the period for: | | | | | | |
| | | | | | | |
| Interest | $ | 24,490 | | $ | 35,575 | |
| Income taxes | $ | 2,018,647 | | $ | 1,946,042 | |
The accompanying notes are an integral part of these combined financials statements.
F-5
ASIA LEECHDOM HOLDING CORPORATION AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
September 30, 2010
Note 1 - ORGANIZATION AND DESCRIPTION OF BUSINESS
Asia Leechdom Holding Corp. (hereinafter referred to as “Asia Leechdom” or the “Company”), formerly, Bay Peak 6 Acquisition Corp., was organized under the laws of the State of Arizona, in 2004, as VT French Services, Inc. (“VTFS”), a wholly owned subsidiary of Visitalk Capital Corporation, (“VCC”), which in turn was a wholly owned subsidiary of Visitalk.com (“Visitalk”). As part of Visitalk’s Chapter 11 reorganization plan, Asia Leechdom was a shell company with no assets or operations. VCC was authorized by the Visitalk plan to be the reorganized debtor. Effective September 11, 2008, the name of the Company was changed from VT French Services, Inc. to Bay Peak 6 Acquisition Corp., in connection with the Company’s redomestication to Nevada. On June July 28, 2010, the Company changed its name to Asia Leechdom Holding Corp., in connection with its reverse acquisition of Asia Leechdom Holding Corp. (“ALH”), a New Jersey corporation. As a result of the reverse acquisition, the Company now conducts its operations in the People’s Republic of China (the “PRC”) through ALH’s wholly owned PRC subsidiary, Tianjin BOAI Pharmaceutical Company Ltd. (“BOAI Pharmaceutical”) and its second tier subsidiary, Tianjin BOAI Leechdom Technique Co., Ltd. (“BOAI Leechdom”).
On May 28, 2010, the Company completed a reverse acquisition through a share exchange with ALH, whereby the Company issued the sole shareholder of ALH, 32,310,758 shares of the Company’s common stock, par value $0.001, for 100% of ALH’s issued and outstanding capital stock. ALH thereby became the Company’s wholly owned subsidiary and its subsidiary, BOAI Pharmaceutical, became the Company’s indirect subsidiary. In accordance with Financial Accounting Standards Board (“FASB”) Accounting Standards Codification (“ASC”) 805, “Business Combinations”, the Company recorded this merger as a recapitalization which consolidated the Company, ALH and BOAI Pharmaceutical and treated the Company as a shell at the time of the merger. According to 805-10-55-13, “the acquirer usually is the combining entity whose relative size (measured in, for example, assets, revenues, or earnings), is significantly larger than that of the other.” Since the Company was a shell at the time of the merger while ALH had operations through its acquisition of BOAI Pharmaceutical and was significantly larger than the Company was, under ASC 805, ALH was considered the acquirer. Because the transaction was accounted for as a recapitalization and not a business combination, pro forma information is not presented.
ALH was incorporated on June 20, 2007 in New Jersey. On July 5, 2007, ALH acquired 100% of the shares of BOAI Pharmaceutical for $1,260,000. The acquisition of BOAI Pharmaceutical was treated as an acquisition by an entity under common control as Mrs. Xuecheng Xia, the major shareholder of BOAI Pharmaceutical was also an ALH director and held an option to purchase equity interests in ALH from ALH’s sole shareholder. As a result the acquisition was accounted for at historical cost in a manner similar to that in pooling of interests accounting.
ALH, through its Chinese subsidiaries BOAI Pharmaceutical and Tianjin BOAI Leechdom Technique Co., Ltd., is engaged in the research and development, manufacture, distribution and technical support of pharmaceutical products.
BOAI Pharmaceutical was formed as a PRC company on August 6, 2001. On June 24, 2010, BOAI Pharmaceutical increased its registered capital to $7,175,405. The operations of BOAI Pharmaceutical are based in Tianjin, China.
BOAI Leechdom was formed as a PRC company on March 17, 1999 in Tianjin China. On May 10, 2005, pursuant to a shareholder resolution, BOAI Leechdom reduced its registered capital by $120,788 which was owned by Huan Bo Hai Investment Service Company. After this capital reduction, the registered capital of BOAI Leechdom is now $483,150.
On August 10, 2007, the original shareholders of BOAI Leechdom transferred their respective equity in BOAI Leechdom to BOAI Pharmaceutical, pursuant to equity transfer agreements, dated August 10, 2007, among BOAI Pharmaceutical and each of them. After the acquisition, BOAI Pharmaceutical became the sole shareholder of BOAI Leechdom. BOAI Pharmaceutical and BOAI Leechdom were under common control prior to the acquisition as the shareholders of BOAI Pharmaceutical were also the owners of BOAI Leechdom before the acquisition. Accordingly, the acquisition of the 100% equity interest in BOAI Leechdom by BOAI Pharmaceutical was accounted for at historical cost in a manner similar to that in pooling of interests accounting with ALH being the ultimate parent entity of BOAI Leechdom.
On February 27, 2010, Chenghai Du, the Company’s controlling stockholder, entered into a series of call option agreements with each of Ma Dan, Xia Xuecheng, Wang Yan, Wang Yansheng, He Haiwei and Tian Mengchun, the original shareholders and managements of BOAI Pharmaceutical (the “Option Holders”), pursuant to which Mr. Du granted each of them an option to acquire an amount of shares equal to 90% of the shares the Company’s common stock issued to Mr. Du in the reverse acquisition, at an exercise price of $0.0001 per share. Each of them had the right to exercise their option during the period commencing on the date of the option agreement and ending on the fifth anniversary of the date thereof.
F-6
Note 2 - BASIS OF PRESENTATION AND SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
Basis of presentation
The Consolidated Financial Statements were prepared in accordance with generally accepted accounting principles in the United States of America ("US GAAP"). The Company’s functional currency is the Chinese Renminbi; however the accompanying consolidated financial statements have been translated and presented in United States Dollars ($).
Principle of consolidation
The accompanying consolidated financial statements include the accounts of the Company, and its wholly-owned subsidiaries BOAI Pharmaceutical and BOAI Leechdom. All significant inter-company accounts and transactions were eliminated in the combination.
Use of estimates
The preparation of consolidated financial statements in conformity with US GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the consolidated financial statements and the amount of revenues and expenses during the reporting periods. Management makes these estimates using the best information available when the estimates are made. However, actual results could differ materially from those results.
Foreign currency transactions and comprehensive income (loss)
The accounts of BOAI Pharmaceutical and BOAI Leechdom were maintained, and its financial statements were expressed, in Chinese Yuan Renminbi (CNY). Such financial statements were translated into U.S. Dollars (USD) in accordance with ASC 830, “Foreign Currency Matters,” with the CNY as the functional currency. According to the Statement, all assets and liabilities were translated at the exchange rate on the balance sheet date, stockholder’s equity are translated at the historical rates and income statement items are translated at the average exchange rate for the period. The resulting translation adjustments are reported under other comprehensive income in accordance with ASC 220, “Comprehensive Income” as a component of shareholders’ equity.
Exchange gains and losses are recognized for the different foreign exchange rates applied when the foreign currency assets and liabilities are settled. Transaction gains and losses that arise from exchange rate fluctuations on transactions denominated in a currency other than the functional currency are included in the results of operations as incurred.
Cash and equivalents
For Statement of Cash Flows purposes, BOAI Pharmaceutical considers all cash on hand and in banks, certificates of deposit and other highly-liquid investments with maturities of three months or less, when purchased, to be cash and equivalents.
Accounts receivable
The Company maintains allowances for potential credit losses on accounts receivable. Management reviews the composition of accounts receivable and analyzes historical bad debts, customer concentrations, customer credit worthiness, current economic trends and changes in customer payment patterns to evaluate the adequacy of these reserves. The allowance for accounts receivable was $149,757 and $146,780 as of September 30 (unaudited) and June 30, 2010.
Inventory
Inventory is valued at the lower of cost (determined on a weighted average basis) or market. The management compares the cost of inventory with the net realizable value and an allowance is made for writing down the inventory to its net realizable value, if lower than the cost.
F-7
Property, plant and equipment
Property, plant and equipment are recorded at cost. Gains or losses on disposals are reflected as gain or loss in the year of disposal. The cost of improvements that extend the life of plant, property, and equipment are capitalized. These capitalized costs may include structural improvements, equipment, and fixtures. All ordinary repair and maintenance costs are expensed as incurred.
Depreciation for financial reporting purposes is provided using the straight-line method over the estimated useful lives of the assets:
| | Estimated Useful Life |
| Buildings | 20 years |
| Machinery and equipments | 5-10 years |
| Office equipments | 5 years |
| Vehicle | 8-10 years |
Impairment of long-lived assets
Finite-lived intangible assets are amortized over their respective useful lives and, along with other long-lived assets, are evaluated for impairment at least annually, or periodically whenever events or changes in circumstances indicate that their related carrying amounts may not be recoverable in accordance with ASC 360, “Property, Plant and Equipment.” In evaluating long-lived assets for recoverability, including finite-lived intangibles and property and equipment, the Company uses its best estimate of future cash flows expected to result from the use of the asset and eventual disposition in accordance with ASC 360. To the extent estimated future undiscounted net cash flows attributable to the asset are less than its carrying amount, an impairment loss is recognized equal to the difference between the carrying value of such asset and its fair value. Assets to be disposed whether through sale or abandonment, are reported at the lower of carrying value or fair value less costs to sell. The Company estimates fair value based on the information available in making whatever estimates, judgments and projections are considered necessary. There was no impairment of long-lived assets for the three months ended September 30, 2010 and 2009, respectively.
Revenue recognition
The Company's revenue recognition policies are in compliance with Securities and Exchange Commission (“SEC”) Staff Accounting Bulletin (SAB) 104. Sales revenue is recognized at the date of shipment to customers when a formal arrangement exists, the price is fixed or determinable, the delivery is completed, no other significant obligations of the Company exist and collectability is reasonably assured. Payments received before all of the relevant criteria for revenue recognition are satisfied are recorded as unearned revenue. The Company's revenue consists of invoiced value of goods, net of a value-added tax (VAT) and product return or sales discount allowance. The Company does not offer unconditional right of return to its customers.
Income taxes
The Company accounts for income taxes using an asset and liability approach which allows for the recognition and measurement of deferred tax assets based upon the likelihood of realization of tax benefits in future years. Under the asset and liability approach, deferred taxes are provided for the net tax effects of temporary differences between the carrying amounts of assets and liabilities for financial reporting purposes and the amounts used for income tax purposes. A valuation allowance is provided for deferred tax assets if it is more likely than not these items will either expire before the Company is able to realize their benefits, or that future deductibility is uncertain.
The Company records a valuation allowance for deferred tax assets, if any, based on its estimates of its future taxable income as well as its tax planning strategies when it is more likely than not that a portion or all of its deferred tax assets will not be realized.
If the Company is able to utilize more of its deferred tax assets than the net amount previously recorded when unanticipated events occur, an adjustment to deferred tax assets would increase the Company net income when those events occur. The Company does not have any significant deferred tax asset or liabilities in the PRC tax jurisdiction.
In China, beginning January 1, 2008, the new Enterprise Income Tax (“EIT”) law replaced the existing laws for Domestic Enterprises (“DES”) and Foreign Invested Enterprises (“FIEs”). The new standard EIT rate of 25% replaced the 33% rate formerly applicable to both DES and FIEs. The two years tax exemption, three years 50% tax reduction tax holiday for production-oriented FIEs are eliminated.
F-8
Fair value of financial instruments
The Company adopted the provisions of Accounting Standards Codification (“ASC”) 820, “Fair Value Measurements and Disclosures.” ASC 820 clarifies the definition of fair value, prescribes methods for measuring fair value, and establishes a fair value hierarchy to classify the inputs used in measuring fair value as follows:
Level 1-Inputs are unadjusted quoted prices in active markets for identical assets or liabilities available at the measurement date.
Level 2-Inputs are unadjusted quoted prices for similar assets and liabilities in active markets, quoted prices for identical or similar assets and liabilities in markets that are not active, inputs other then quoted prices that are observable, and inputs derived from or corroborated by observable market data.
Level 3-Inputs are unobservable inputs which reflect the reporting entity’s own assumptions on what assumptions the market participants would use in pricing the asset or liability based on the best available information.
The carrying amounts reported in the balance sheets for cash, accounts receivable, loan receivables, other receivables, advances to material suppliers, short-term loan, accounts payable, advance from customers, other payables and accrued expenses, approximate their fair market value due to the short-term nature of these instruments. The Company uses Level 3 method to measure fair value of its warrant liability.
As of the balance sheet dates, the estimated fair values of the financial instruments were not materially different from their carrying values as presented on the balance sheet. This is attributed to the short maturities of the instruments and that interest rates on the borrowings approximate those that would have been available for loans of similar remaining maturity and risk profile at respective balance sheet dates.
Construction in progress
Construction in progress are stated at cost, unless events or circumstances indicate that cost cannot be recovered, in which case the carrying value is reduced to estimated fair value. Estimated fair value: (i) is based on the Company’s plans for the development of each property; and (ii) considers the cost to complete and the estimated fair value of the completed projects.
Direct construction, development costs, property taxes and other related costs to the construction projects are capitalized during periods those activities which are necessary to get the projects ready for its intended use. The capitalization ends when the construction is substantially completed and the projects are ready for its intended use.
Research and development
Research and development costs are expensed as incurred.
Segment reporting
The Company follows the provisions of ASC 280, “Segment Reporting,” for reporting information about operating segments. Operating segments are defined as components of an enterprise about which separate financial information is available that is evaluated regularly by the chief operating decision maker in deciding how to allocate resources and assess performance. The Company’s chief operating decision maker has been identified as the Chief Executive Officer.
The Company operates in two reportable business segments - (1) the pharmaceutical manufacturing segment and (2) medicine wholesales segment. The Company's reportable segments are strategic business units that offer different products. The Company's reportable segments, although integral to the success of the others, offer distinctly different products and services and require different types of management focus. As such, these segments are managed separately.
The following table presents a summary of operating information for the three months ended September 30, 2010 (unaudited) and 2009 (unaudited) and balance sheet information as of September 30, 2010 (unaudited) and June 30, 2010:
F-9
Three Months Ended September
30, 2010 | | Pharmaceutical
Manufacturing | | | Medicine Wholesales | | | Inter-segment
Elimination | | | Non-operating
Entities | | | Consolidated Total | |
| | | | | | | | | | | | | | | | |
| Net Sales | $ | 9,658,270 | | $ | 11,469,999 | | $ | (16,840 | ) | $ | - | | $ | 21,111,429 | |
| | | | | | | | | | | | | | | | |
| Interest Income (expense) | | (27,631 | ) | | (18,462 | ) | | - | | | - | | | (46,093 | ) |
| | | | | | | | | | | | | | | | |
| Depreciation and Amortization | | 108,817 | | | 91,613 | | | - | | | 0 | | | 200,430 | |
| | | | | | | | | | | | | | | | |
| Segment assets | | 61,834,266 | | | 39,168,719 | | | (22,235,220 | ) | | 9,139,087 | | | 87,906,852 | |
| | | | | | | | | | | | | | | | |
| Segment net income (loss) before tax | | 5,255,514 | | | 4,296,452 | | | (1,050 | ) | | (324,525 | ) | | 9,226,391 | |
| | | | | | | | | | | | | | | | |
| Three Months Ended September30, 2009 | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | |
| Net Sales | | 5,367,228 | | | 8,810,551 | | | (4,641 | ) | | 0 | | | 14,173,138 | |
| | | | | | | | | | | | | | | | |
| Interest Income (expense) | | (34,273 | ) | | (11,697 | ) | | - | | | (23,426 | ) | | (69,395 | ) |
| | | | | | | | | | | | | | | | |
| Depreciation and Amortization | | 106,870 | | | 81,443 | | | - | | | 0 | | | 188,313 | |
| | | | | | | | | | | | | | | | |
| Segment assets | | 38,582,529 | | | 27,695,475 | | | (13,516,025 | ) | | 1,260,187 | | | 54,022,166 | |
| | | | | | | | | | | | | | | | |
| Segment net income (loss) before tax | $ | 2,946,285 | | $ | 3,593,543 | | $ | 22,931 | | $ | (24,593 | ) | $ | 6,538,166 | |
F-10
| Reconciliation of segment to | | Three Months Ended | | | Three Months Ended | |
| consolidated incomes | | September 30, 2010 | | | September 30, 2009 | |
| | | | | | | |
| Total segment income (Operating entities) | $ | 9,551,966 | | $ | 6,539,828 | |
| Total segment income (Non- operating entities) | | (324,525 | ) | | (24,593 | ) |
| | | | | | | |
| Elimination of intersegment profits | | (1,050 | ) | | 22,931 | |
| Consolidated income before income taxes | $ | 9,226,391 | | $ | 6,538,166 | |
| Reconciliation of segment to | | As of September 30, | | | | |
| consolidated assets | | 2010 | | | As of June 30, 2010 | |
| | | | | | | |
| Total segment assets (Operating entities) | $ | 101,002,985 | | $ | 66,278,004 | |
| Total segment assets (Non- operating entities) | | 9,139,087 | | | 1,260,187 | |
| Elimination of intersegment receivables | | (22,235,220 | ) | | (13,516,025 | ) |
| Consolidated assets | $ | 87,906,852 | | $ | 54,022,166 | |
F-11
Recent accounting pronouncements
On April 1, 2009, the FASB approved FSP FAS 141(R)-1, “Accounting for Assets Acquired and Liabilities Assumed in a Business Combination That Arise from Contingencies”, which amends FAS 141(R), as incorporated into ASC 805, and eliminated the distinction between contractual and non-contractual contingencies. Under the updated standard an acquirer is required to recognize at fair value an asset acquired or liability assumed in a business combination that arises from a contingency if the acquisition-date fair value of that asset or liability can be determined during the measurement period. If the acquisition-date fair value cannot be determined, the acquirer applies the recognition criteria in ASC 450, “Contingencies” to determine whether the contingency should be recognized as of the acquisition date or after it. This guidance is effective for assets or liabilities arising from contingencies in business combinations for which the acquisition date is on or after the beginning of the first annual reporting period beginning on or after December 15, 2008. The adoption of such standard had no material impact on our consolidated financial statements.
In April 2009, the FASB issued FSP No. FAS 157-4, “Determining Fair Value When the Volume and Level of Activity for the Asset or Liability Have Significantly Decreased and Identifying Transactions That Are Not Orderly” as incorporated into ASC 820, “Fair Value Measurements and Disclosures”. The guidance relates to determining fair values when there is no active market or where the price inputs being used represent distressed sales. It reaffirms what FASB ASC 820 states is the objective of fair value measurement—to reflect how much an asset would be sold for in an orderly transaction (as opposed to a distressed or forced transaction) at the date of the financial statements under current market conditions. Specifically, it reaffirms the need to use judgment to ascertain if a formerly active market has become inactive and in determining fair values when markets have become inactive. This guidance is effective for interim and annual periods ended after June 15, 2009, but entities may early adopt this guidance for the interim and annual periods ended after March 15, 2009. The adoption of such standard had no material impact on our consolidated financial statements.
In May 2009, the FASB issued ASC 855, “Subsequent Events,” which establishes general standards of accounting for and disclosure of events that occur after the balance sheet date but before financial statements. The statement is effective for interim and annual periods ended after June 15, 2009. The standard was subsequently amended by FASB Accounting Standards Update (“ASU”) 2010-09 which exempts an entity that is an SEC filer from the requirement to disclose the date through which subsequent events have been evaluated.
In June 2009, the FASB issued FASB ASU 2009-01, which amends ASC 105, “Generally Accepted Accounting Principles” (“Statement of Financial Accounting Standards (“SFAS”) No. 168, “The FASB Accounting Standards Codification TM and the Hierarchy of Generally Accepted Accounting Principles – a replacement of FASB Statement No. 162,” ) , which establishes the FASB Accounting Standards Codification TM (“Codification”) as the source of authoritative generally accepted accounting principles in the United States (“GAAP”) for nongovernmental entities. The Codification does not change GAAP. Instead, it takes the thousands of individual pronouncements that currently comprise GAAP and reorganizes them into approximately 90 accounting Topics, and displays all Topics using a consistent structure. Contents in each Topic are further organized first by Subtopic, then Section and finally Paragraph. The Paragraph level is the only level that contains substantive content. Citing particular content in the Codification involves specifying the unique numeric path to the content through the Topic, Subtopic, Section and Paragraph structure. FASB suggests that all citations begin with “FASB ASC”. Changes to the ASC subsequent to June 30, 2009 are referred to as Accounting Standards Updates.
In August 2009, the FASB issued ASU 2009-5, “Measuring Liabilities at Fair Value”. ASU 2009-05 amends ASC 820, “Fair Value Measurements”. Specifically, ASU 2009-05 provides clarification that in circumstances in which a quoted price in an active market for the identical liability is not available, a reporting entity is required to measure fair value using one or more of the following methods: 1) a valuation technique that uses a) the quoted price of the identical liability when traded as an asset or b) quoted prices for similar liabilities or similar liabilities when traded as assets and/or 2) a valuation technique that is consistent with the principles of ASC 820 of the Accounting Standards Codification (e.g. an income approach or market approach). ASU 2009-05 also clarifies that when estimating the fair value of a liability, a reporting entity is not required to adjust to include inputs relating to the existence of transfer restrictions on that liability. The adoption of such standard had no material impact on our consolidated financial statements.
In September 2009, the Emerging Issues Task Force reached final consensus on ASU 2009-13, “Revenue Arrangements with Multiple Deliverables”. ASU 2009-13 addresses how to determine whether an arrangement involving multiple deliverables contains more than one unit of accounting, and how the arrangement consideration should be allocated among the separate units of accounting. This ASU will be effective prospectively for revenue arrangements entered into or materially modified in years beginning on or after June 15, 2010. Early adoption is permitted. The adoption of such standard had no material impact on our consolidated financial statements.
F-12
In December 2009, the FASB issued ASU 2009-17, “Improvements to Financial Reporting by Enterprises Involved with Variable Interest Entities”. This ASU amends ASC 810, “Consolidation”, eliminates exceptions to consolidating qualifying special-purpose entities, contains new criteria for determining the primary beneficiary, and increases the frequency of required reassessments to determine whether a company is the primary beneficiary of a variable interest entity. This ASU is effective for years beginning after November 15, 2009, which for the Company is January 1, 2010, with earlier adoption prohibited. The Company believes the adoption of ASU 2009-17 would not have a material effect on its consolidated financial statements.
In January 2010, FASB amended ASC 820, “Fair Value Measurements and Disclosures.” The new disclosures and clarifications of existing disclosures are effective for interim and annual reporting periods beginning after December 15, 2009, except for the disclosures about purchases, sales, issuances, and settlements in the roll forward of activity in Level 3 fair value measurements. Those disclosures are effective for years beginning after December 15, 2010, and for interim periods within those years. The Company determined the adoption of this ASU had no material impact on its financial statements.
Note 3 - INVENTORY
Inventory consists of the following as of September 30 (unaudited) and June 30, 2010:
| | | September 30 | | | June 30 | |
| Supplies, packing and raw materials | $ | 1,108,376 | | $ | 1,177,056 | |
| Finished goods | | 4,720,429 | | | 5,311,841 | |
| Work in process | | 246,455 | | | 772,319 | |
| Total | $ | 6,075,260 | | $ | 7,261,216 | |
Note 4 - ADVANCE TO MATERIAL SUPPLIERS
The Company advanced payments to several medicine and package materials suppliers in order to support increasing production with competitive procurement cost. As of September 30, 2010, the Company made total deposits in the amount of $3,032,805. The deposit will be reclassified to the respective accounts under inventory upon delivery and transfer of legal titles.
Note 5 - PROPERTY, PLANT AND EQUIPMENT
Property, plant and equipment consist of the following as of September 30 (unaudited) and June 30, 2010:
| | | September 30 | | | June 30 | |
| Building and improvements | $ | 16,603,373 | | $ | 16,273,307 | |
| Machinery and equipment | | 934,967 | | | 915,816 | |
| Office equipment | | 98,153 | | | 91,660 | |
| Vehicles | | 489,925 | | | 480,185 | |
| Total | | 18,126,418 | | | 17,760,968 | |
| Less: Accumulated Depreciation | | (2,544,163 | ) | | (2,303,702 | ) |
| Net | $ | 15,582,255 | | $ | 15,457,266 | |
Depreciation (unaudited) for the three months ended September 30, 2010 and 2009 was $191,064 and $160,834, respectively.
As of September 30 and June 30, 2010, the net book value of buildings pledged as collateral for bank loans were $9,702,762 and $9,509,877, respectively. See Note 10.
Note 6 - ADVANCE TO EQUIPMENT SUPPLIERS
The Company advanced payments to several equipment materials suppliers in order to support increasing production with competitive procurement cost. As of September 30, 2010, the Company made total deposits in the amount of $6,746,228. The deposit will be reclassified to the respective accounts under fixed asset upon delivery and transfer of legal titles.
F-13
Note 7 – DEPOSIT FOR ASSET ACQUISITION
The Company signed a property purchase contract with Tianjin Qi Shi Leather Ltd. ("Qi Shi Leather") to acquire land use rights and buildings for RMB 80 million (approximately, $11.7 million). As of September 30, 2010, the Company had made $10,093,628 in aggregate deposits towards the purchase. See Note 21.
Note 8 - CONSTRUCTION IN PROGRESS
As of September 30 (unaudited) and June 30, 2010, the construction in progress of the Company consisted of the following:
| | | September 30 | | | June 30 | |
| Office building | $ | 3,874,315 | | $ | 2,241,428 | |
| Total construction in progress | $ | 3,874,315 | | $ | 2,241,428 | |
As of September 30, 2010 (unaudited), BOAI Leechdom expects to pay an additional $93,341 in order to complete the construction of office building.
Note 9- INTANGIBLE ASSETS
As of September 30 (unaudited) and June 30, 2010, the Company has intangible assets including Paclitaxel ongoing project, pharmaceutical production permits, land use right and software.
| | | September 30 | | | June 30 | |
| Pharmaceutical production permits | $ | 344,441 | | $ | 337,592 | |
| Paclitaxel– In-process research and development | | 6,439,555 | | | 6,311,540 | |
| Land use right | | 103,846 | | | 101,782 | |
| Software | | 22,694 | | | 2,132 | |
| Total | | 6,910,536 | | | 6,753,046 | |
| Less: Accumulated Amortization | | (292,708 | ) | | (277,570 | ) |
| Net | $ | 6,617,828 | | $ | 6,475,476 | |
The pharmaceutical production permits were obtained in December 2001, updated in January 2006 and valid until January 2011. The Company expects to renew permits in January 2011, which will be effective until January 2016. With these production permits, the Company is authorized to manufacture certain types of Chinese medicines.
The Company obtained the Paclitaxel cell cultivation method from Tsinghua University in 2009 and paid approximately $5.8 million as of September 30, 2010 (unaudited). The first payment of approximately $2.9 million was paid in early 2009 when the Company obtained all Paclitaxel cell cultivation information. The second payment of approximately $2.9 million was paid in 2009 after the Company successfully completed trial production. The third payment will be paid once the scale production is proven successful. At September 30, 2010, the amount due was in other payables. See Note 10.
Our technology could increase the success ratio and consistency of the produced product to treat patients with lung, ovarian, breast, head and neck cancer. We intend to ramp up the scale and enter into commercial production once the new facility for Paclitaxel production is available.
Note 10 – SHORT TERM LOANS
The loans payable at September 30 (unaudited) and June 30, 2010 comprised of the following:
| Description | | September 30 | | | June 30 | |
| Loans payable to Tianjin Agriculture Credit Union Bank: | | | | | | |
| Due by January 28, 2011. Interest at 5.841% p.a. | $ | | | $ | 1,467,800 | |
| Due by March 8, 2011. Interest at 5.841% p.a. | | 284,538 | | | 278,882 | |
| Due by May 10, 2011. Interest at 5.841% p.a. | | 359,417 | | | 352,272 | |
| Due by July 7, 2011. Interest at 5.841% p.a. | | 359,417 | | | - | |
F-14
| Loans payable to Tianjin Bank : | | | | | | |
| Due by January 28, 2011. Interest at 5.841% p.a. | | | | | 1,247,630 | |
| Due by August 1, 2011. Interest at 4.425% p.a. | | 2,695,628 | | | | |
| Due by November 23, 2011. Interest at 5.841% p.a. | | 1,272,935 | | | | |
| Loan payable to Midi Investment Inc | | - | | | 190,000 | |
| Total Short-term Loans | $ | 4,971,936 | | $ | 3,536,584 | |
The short-term bank loans are secured by buildings owned by the Company. See Notes 5.
Note 11- OTHER PAYABLES
Other payables consist of the following as of September 30 (unaudited) and June 30, 2010:
| | | September 30 | | | June 30 | |
| Non-related party company payable(a) | $ | 159,500 | | $ | 544,786 | |
| Acquired Paclitaxel ongoing project | | 449,271 | | | 440,340 | |
| Employees payable(b) | | 150,345 | | | 147,334 | |
| Funds and Insurance payable(c) | | 51,893 | | | 754,723 | |
| Welfare payable | | 92,682 | | | 98,674 | |
| Total | $ | 903,691 | | $ | 1,985,857 | |
(a) The 2010 payable is primarily for the purchase of a building. It is to be paid upon transfer of land and building certification to the Company. This payable bears no interest.
(b) Employees payable represents advances from employees for various payments made on behalf of the Company. This payable is due on demand and bears no interest.
(c) Funds and insurance payable includes $682,109 financing fees as of June 30, 2010 and was paid in July 2010.
Note 12 - TAXES PAYABLE
Taxes payable consist of the following as of September 30 (unaudited) and June 30, 2010:
| | | September 30 | | | June 30 | |
| VAT payable | $ | 1,240,894 | | $ | 977,278 | |
| Income tax payable | | 2,432,286 | | | 2,057,856 | |
| Other levies | | 284,864 | | | 222,349 | |
| Total | $ | 3,958,044 | | $ | 3,257,483 | |
Note 13 - INCOME TAXES
The Company utilizes ASC 740, "Income Taxes," which requires recognition of deferred tax assets and liabilities for the expected future tax consequences of events that were included in the financial statements or tax returns. Under this method, deferred income taxes are recognized for the tax consequences in future years of differences between the tax bases of assets and liabilities and their financial reporting amounts at each period end based on enacted tax laws and statutory tax rates applicable to the periods in which the differences are expected to affect taxable income. Valuation allowances are established, when necessary, to reduce deferred tax assets to the amount expected to be realized.
Local PRC income tax
The Company is governed by the Income Tax Law of the PRC concerning Chinese registered limited liability companies. Under the Income Tax Laws of the PRC, Chinese enterprises are generally subject to an income tax at an effective rate of 33% (30% state income taxes plus 3% local income taxes) on income reported in the statutory financial statements after appropriate tax adjustments, unless the enterprise is located in a specially designated region for which more favorable effective tax rates are applicable.
Beginning January 1, 2008, in the PRC the new Enterprise Income Tax (“EIT”) law replaced the existing laws for Domestic Enterprises (“DES”) and Foreign Invested Enterprises (“FIEs”). The new standard EIT rate of 25% replaced the 33% rate applicable to both DES and FIEs. The two years tax exemption and three years 50% tax reduction tax holiday for production-oriented FIEs were eliminated.
F-15
The provision for income taxes for the three months ended September 30, 2010 (unaudited) and 2009 (unaudited) consisted of the following:
| | | 2010 | | | 2009 | |
| Income tax expense- current | $ | 2,376,832 | | $ | 1,635,463 | |
| Income tax expense- deferred | | 12,308 | | | 36,592 | |
| Total provision for income taxes | $ | 2,389,140 | | $ | 1,672,055 | |
The following table reconciles the U.S. statutory rates to the Company’s effective tax rate for the three months ended September 30, 2010 (unaudited) and 2009 (unaudited):
| | | 2010 | | | 2009 | |
| Tax at statutory rate | | 35% | | | 35% | |
| Foreign tax rate difference | | (10% | ) | | (10% | ) |
| Other items(a) | | 0.9% | | | 0.6% | |
| Total | | 25.9% | | | 25.6% | |
| | (a) | Primarily for expenses incurred by the Company’s overseas holding companies not subject to PRC income tax |
Note 14 - RELATED PARTY TRANSACTIONS
Due from related parties
As of September 30, 2010 (unaudited), the Company advanced $2,342,970 to drug chain stores and $1,522,865 to its Chief Executive Officer, Xuecheng Xia. The major shareholder of the Company is also the main shareholder of these chain stores. These loans were unsecured and due on demand.
Due to related parties
As of September 30, 2010 (unaudited), the Company received loans totaling $1,577,957 from its major shareholder and Chief Executive Officer, Xuecheng Xia. The Company also had $870,922 payable to drug chain stores, whose major shareholder is the main shareholder of the Company. The loans and the payables were unsecured and due on demand.
Note 15 - STATUTORY RESERVES
As stipulated by Law of the PRC, net income after taxation can only be distributed as dividends after appropriation has been made for the following:
| | i) | Making up cumulative prior years' losses, if any; |
| | ii) | Allocations to the "Statutory surplus reserve" of at least 10% of income after tax, as determined under PRC accounting rules and regulations, until the fund amounts to 50% of the Company's registered capital; and |
| | iii) | Allocations to the discretionary surplus reserve, if approved in the shareholders' general meeting. |
In accordance with the Chinese Company Law, the Company’s subsidiaries allocated 10% of their net income to surplus.
Note 16 - EARNINGS PER SHARE
Earnings per share is determined by dividing net income for the periods by the weighted average number of both basic and diluted shares of common stock and common stock equivalents outstanding pursuant to ASC 260, “Earnings Per Share.” The following demonstrates the calculation for earnings per share for the three months ended September 30, 2010 (unaudited) and 2009 (unaudited):
| | | | 2010 | | | 2009 | |
| | Basic earnings per share | | | | | | |
| | Net Income | $ | 6,837,251 | | $ | 4,866,111 | |
| | Weighted average number of common shares outstanding-Basic | | 39,840,605 | | | 32,310,758 | |
| | Earnings per share-Basic | $ | 0.17 | | $ | 0.15 | |
F-16
| | Diluted earnings per share | | | | | | |
| | Net Income | $ | 6,837,252 | | $ | 4,866,111 | |
| | Weighted average number of common shares outstanding-Basic | | 39,840,605 | | | 32,310,758 | |
| | Effect of exercise of options and warrants | | 0 | | | 0 | |
| | Weighted average number of common shares outstanding-Diluted | | 39,840,605 | | | 32,310,758 | |
| | Earnings per share-Diluted | $ | 0.17 | | $ | 0.15 | |
Note 17 - EQUITY PLACEMENTS
From March to June 2010, the Company sold 4,287,381 shares of common stock and 835,477 common stock warrants for $10,705,311. Each Warrant permits the holder to purchase one share of common stock from the Company for $2.51. The Warrants expire in five years after issuance. The Company paid the Placement Agent a fee of $935,000 and 675,317 shares of common stock, at $2.51 a share, a total of $2,630,046.
In connection with this financing, the Company and its controlling shareholders entered into Side Letter Agreements with the investors which required the Company's controlling shareholders to surrender Company shares to the Investors if the Company failed to reach certain income thresholds for the years ending June 30, 2009, 2010 and 2011. The 2009 and 2010 thresholds were met. Another condition was that the Company become a “reporting company” within the meaning of Rule 144 under the Securities Act of 1933, as amended, within 180 days following the closing of such financing, and file a registration statement with respect to its Common Stock on Form 10 with the U.S. Securities and Exchange Commission before December 31, 2010. The Company did not meet this obligation on the required date and so, in accordance with the Side Letter Agreements, the controlling shareholders were obligated to either deliver 796,812 shares of Company’s common stock owned by them to the investors.
Pursuant to a bridge loan settlement agreement dated May 25, 2010 with China Boai Hunter, LLC, the Company issued 239,044 shares of common stock and 298,805 common stock purchase 3-year warrants. Each Warrant permits the holder to purchase one share of common stock from the Company for $2.51. The fair value of shares and warrants issued for this settlement of $600,000 and $ 226,947 was accounted for as additional interest expense on the settlement date. The fair value of the warrant was calculated using the Black-Scholes options pricing model using the following assumptions: Volatility: 43.9%; Risk free interest rate: 1%, Expected term: 2.92 years.
The fair value of outstanding warrants related to June 2010 equity financing was $731,568 as of September 30, 2010 (unaudited). The fair value of the warrants at September 30, 2010 was calculated using the Black-Scholes options pricing model using the following assumptions: Volatility: 39.2%; Risk free interest rate: 1.27%, Expected term: 4.67 -4.75 years. The fair value of the warrants has been accounted for as cost of raising equity.
Note 18 - CURRENT VULNERABILITY DUE TO CERTAIN CONCENTRATIONS
The Company's operations are all carried out in the PRC. Accordingly, the Company's business, financial condition and results of operations may be influenced by the political, economic and legal environments in the PRC, and by the general state of the PRC's economy.
The Company's operations in the PRC are subject to specific considerations and significant risks not typically associated with companies in the North America and Western Europe. These include risks associated with, among others, the political, economic and legal environments and foreign currency exchange. The Company's results may be adversely affected by changes in governmental policies with respect to laws and regulations, anti-inflationary measures, currency conversion and remittance abroad, and rates and methods of taxation, among other things.
Note 19 - MAJOR CUSTOMERS AND VENDORS
Major customers and vendors represent those which accounted for 10% or more of the Company’s net revenue or purchase, respectively.
No customer accounted for 10% or more of net revenue for the three months ended September 30, 2010 (unaudited) and 2009 (unaudited).
F-17
There was one vendor which provided 58.0% of the Company’s purchases of raw materials for the three months ended September 30, 2010 (unaudited). The Company had $18,533 accounts payable as of September 30, 2010 (unaudited) to this vendor.
Note 20 - COMMITMENTS AND CONTINGENCIES
The Company’s sales, purchases and expenses transactions are denominated in RMB and all of the Company’s assets and liabilities are also denominated in RMB. The RMB is not freely convertible into foreign currencies under the current law. In China, foreign exchange transactions are required by law to be transacted only by authorized financial institutions at exchange rates set by the People’s Bank of China, the central bank of China. Remittances in currencies other than RMB may require certain supporting documentation in order to affect the remittance.
BOAI Leechdom leases unused offices to several non-related parties from 2007. These leases were expected to continue year by year. Total rental revenue for the three months ended September 30, 2010 and 2009 for $76,277 and $76,443, respectively.
As of September 30, 2010 (unaudited), the Company has RMB 107 million (unaudited) (approximately, $16.1 million) in capital commitments for the construction of buildings and purchase of equipment relating to construction of its new production facilities in Tianjin.
As of September 30, 2010 (unaudited), the Company has no significant litigation claims. Also, the Company does not have significant operating-lease commitments as of September 30, 2010 (unaudited).
Note 21 – SUBSEQUENT EVENTS
On November 20, 2010, the Company consummated a purchase agreement, dated on May 8, 2010, between the Company and Qi Shi Leather, pursuant to which the Company acquired 100% of the equity interest in Qi Shi Leather, for an aggregate purchase price of RMB 80,000,000 Chinese Renminbi (approximately $11.7 million) in cash. Ninety-three percent of the equity interest in Qi Shi Leather was transferred to BOAI Pharmaceutical and the remaining 7% was transferred to BOAI Leechdom. Qi Shi Leather was incorporated on July 22, 2003 and at the closing date did not have any business operations, assets or liabilities, other than its land use rights for 85,938.92 square meters of property and 22,138.22 square meters of building space in Jinghai District, Tianjin. The Company’s purpose for acquiring Qi Shi Leather was to acquire such land use rights and property. The acquisition of the Qi Shi Leather equity interest was in lieu of an asset acquisition which would have required the Company to engage in a more time-consuming process for the transfer of Qi Shi Leather's properties. The Company intends to use the property acquired with Qi Shi Leather to enhance its current production facilities and increase its production capacity. The additional production facilities are under construction and are expected to be completed by August, 2011. In connection with the closing of the acquisition, the Company has changed Qi Shi Leather's name to Tianjin BOAI Bio-Pharmaceutical Co., Ltd.
On January 10, 2011, Mr. Chenghai Du, the Company’s controlling stockholder entered into an additional call option agreement with Ms. Jianping Lu, an executive employee of BOAI Pharm, pursuant to which Mr. Du granted her an option to acquire an amount of shares equal to the remaining 10% of the shares of our common stock issued to Mr. Du in the reverse acquisition, on the same terms and conditions as the other option holders discussed under Note 1 hereof.
On January 11, 2011, the option holders transferred and assigned their options to purchase an aggregate of 9,454,183 shares of our Common Stock to Dragon Core Limited and an aggregate of 22,059,762 shares of our Common Stock to Neo Profit Limited. On the same date each of Dragon Core and Neo Profit exercised their options to purchase their respective shares from Mr. Du. Dragon Core Limited is 67% beneficially owned and controlled by our Chief Executive Officer and Director, Ms. Xuecheng Xia, and 33% beneficially owned and controlled by Ms. Jianping Lu, and Neo Profit Limited is wholly-owned and controlled by Ms. Xia.
As a result of the exercises, Dragon Core and Neo Profit hold an aggregate of 31,513,945, or 79.1%, of our issued and outstanding common stock and Mr. Du is no longer affiliated with, and no longer holds any equity interest in the Company or its subsidiaries.
On February, 11, 2011, the Company, the controlling stockholders and the investors entered into an amendment and waiver agreement, pursuant to which: the investors agreed to extend the Company’s requirement to become a reporting company to February 15, 2011; we agreed to list our Common Stock on the Nasdaq Stock Market, the NYSE Amex or the New York Stock Exchange, on or before October 31, 2011; and the controlling stockholders agreed to surrender an amount of shares equal to 2% of our issued and outstanding fully diluted common stock, if the Company’s actual 2011 net income is not equal to or greater than $27,500,000.
F-18
ASIA LEECHDOM HOLDING CORPORATION AND SUBSIDIARIES
CONSOLIDATED FINANCIAL STATEMENTS
JUNE 30, 2010
F-19
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
Board of Directors and Shareholders of
Asia Leechdom Holding Corporation and Subsidiaries
We have audited the accompanying consolidated balance sheets of Asia Leechdom Holding Corporation and Subsidiaries as of June 30, 2010 and 2009 and the related consolidated statements of income and other comprehensive income, stockholders’ equity and cash flows for each of the three years ended June 30, 2010, 2009 and 2008. These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these financial statements based on our audits.
We conducted our audits in accordance with standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe our audits provide a reasonable basis for our opinion.
In our opinion, the financial statements referred to above present fairly, in all material respects, the financial position of Asia Leechdom Holding Corp. and Subsidiaries as of June 30, 2010 and 2009, and the results of their operations and their cash flows for the three years ended June 30, 2010, 2009 and 2008, in conformity with U.S. generally accepted accounting principles.
Goldman Kurland and Mohidin LLP
Encino, California
January 12, 2011, except for Note 21, for which the date is February 11, 2011
F-20
ASIA LEECHDOM HOLDING CORPORATION AND SUBSIDIARIES
CONSOLIDATED BALANCE SHEETS
| ASSETS | |
| | | June 30,2010 | | | June 30,2009 | |
| Current Assets: | | | | | | |
| Cash and equivalents | $ | 14,498,707 | | $ | 5,495,176 | |
| Accounts receivable, net | | 11,442,034 | | | 9,222,096 | |
| Other receivables | | 113,923 | | | 87,971 | |
| Advances to material suppliers | | 2,849,511 | | | 1,158,998 | |
| Inventory | | 7,261,216 | | | 7,180,019 | |
| Due from related party | | 1,893,114 | | | 1,764,593 | |
| Subscriptions receivable | | 3,007,224 | | | - | |
| Total Current Assets | | 41,065,729 | | | 24,908,853 | |
| | | | | | | |
| Property, Plant & Equipment, net | | 15,457,266 | | | 13,200,505 | |
| Advances to equipment suppliers | | 6,612,116 | | | - | |
| Deposit for asset acquisition | | 5,577,640 | | | - | |
| Construction in Progress | | 2,241,428 | | | 3,998,753 | |
| Intangible Assets, net | | 6,475,476 | | | 6,483,621 | |
| Total Assets | $ | 77,429,655 | | $ | 48,591,732 | |
| | | | | | | |
| LIABILITIES AND STOCKHOLDERS' EQUITY | |
| | | | | | | |
| Current Liabilities: | | | | | | |
| Short-term loans | $ | 3,536,584 | | $ | 4,737,293 | |
| Accounts payable | | 2,170,676 | | | 4,041,737 | |
| Unearned revenue | | 1,255,195 | | | 1,179,852 | |
| Other payables | | 1,985,857 | | | 3,167,033 | |
| Taxes payable | | 3,257,483 | | | 2,730,764 | |
| Due to related party | | 2,506,118 | | | 2,055,538 | |
| Accrued expenses | | 211,383 | | | 649,684 | |
| Total Current Liabilities | | 14,923,296 | | | 18,561,901 | |
| | | | | | | |
| Long Term Liabilities | | | | | | |
| Deferred tax liability | | 146,780 | | | - | |
| Total Liabilities | | 15,070,076 | | | 18,561,901 | |
| | | | | | | |
| Stockholders' Equity: | | | | | | |
Common stock,$0.001 par value, 200,000,000 shares authorized;
39,840,605 shares issued and outstanding as of June 30, 2010
and 32,310,758 shares issued and outstanding as of June 30, 2009 | | 39,841 | | | 32,311 | |
| Additional paid-in-capital (deficit) | | 10,139,120 | | | (240,729 | ) |
| Accumulated other comprehensive income | | 1,456,983 | | | 1,265,242 | |
| Statutory reserve | | 1,904,135 | | | 838,340 | |
| Retained earnings | | 48,819,499 | | | 28,134,668 | |
| Total Stockholders' Equity | | 62,359,578 | | | 30,029,832 | |
| Total Liabilities and Stockholders' Equity | $ | 77,429,655 | | $ | 48,591,732 | |
The accompanying notes are an integral part of these consolidated financial statements.
F-21
ASIA LEECHDOM HOLDING CORPORATION AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF INCOME AND OTHER COMPREHENSIVE INCOME
| | | YEARS ENDED JUNE 30, | |
| | | 2010 | | | 2009 | | | 2008 | |
| | | | | | | | | | |
| Net Revenue | $ | 68,166,997 | | $ | 49,489,066 | | $ | 32,381,120 | |
| | | | | | | | | | |
| Cost of Goods Sold | | 34,217,906 | | | 26,094,624 | | | 17,015,697 | |
| | | | | | | | | | |
| Gross Profit | | 33,949,091 | | | 23,394,442 | | | 15,365,423 | |
| | | | | | | | | | |
| Operating Expenses | | | | | | | | | |
| Selling | | 653,973 | | | 1,136,897 | | | 1,065,179 | |
| Research and development | | 1,561,310 | | | 473,559 | | | 565,366 | |
| General and administrative | | 1,382,099 | | | 1,008,764 | | | 502,432 | |
| Total operating expenses | | 3,597,382 | | | 2,619,220 | | | 2,132,977 | |
| | | | | | | | | | |
| Income From Operations | | 30,351,709 | | | 20,775,222 | | | 13,232,446 | |
| | | | | | | | | | |
| Other Income ( Expense) | | | | | | | | | |
| Interest income | | 39,514 | | | 21,699 | | | 19,730 | |
| Interest expense | | (1,143,569 | ) | | (273,172 | ) | | (165,471 | ) |
| Other income | | 291,982 | | | 233,868 | | | 208,703 | |
| Other expense | | (126,382 | ) | | (93,334 | ) | | (48,013 | ) |
| Total other income (expense) | | (938,455 | ) | | (110,939 | ) | | 14,949 | |
| | | | | | | | | | |
| Income Before Income Taxes | | 29,413,254 | | | 20,664,283 | | | 13,247,395 | |
| | | | | | | | | | |
| Provision For Income Taxes | | 7,662,628 | | | 5,203,547 | | | 2,122,178 | |
| | | | | | | | | | |
| Net Income | | 21,750,626 | | | 15,460,736 | | | 11,125,217 | |
| | | | | | | | | | |
| Other Comprehensive Income/ (Loss) | | 191,741 | | | 79,466 | | | 1,018,155 | |
| | | | | | | | | | |
| Comprehensive Income | $ | 21,942,367 | | $ | 15,540,202 | | | 12,143,372 | |
| | | | | | | | | | |
| Earnings Per Share | | | | | | | | | |
| Basic | $ | 0.61 | | $ | 0.48 | | $ | 0.34 | |
| Diluted | $ | 0.61 | | $ | 0.48 | | $ | 0.34 | |
| | | | | | | | | | |
| Weighted average number of common shares outstanding | | | | | | | | | |
| Basic | | 35,783,112 | | | 32,310,758 | | | 32,310,758 | |
| Diluted | | 35,783,112 | | | 32,310,758 | | | 32,310,758 | |
The accompanying notes are an integral part of these consolidated financial statements.
F-22
ASIA LEECHDOM HOLDING CORPORATION AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF CASH FLOWS
| | | YEARS ENDED JUNE 30, | |
| | | 2010 | | | 2009 | | | 2008 | |
| CASH FLOWS FROM OPERATING ACTIVITIES | | | | | | | | | |
| Net Income | $ | 21,750,626 | | $ | 15,460,736 | | $ | 11,125,217 | |
Adjustments to reconcile net income to net cash
provided by operating activities: | | | | | | | | | |
| Depreciation and amortization | | 756,182 | | | 587,306 | | | 391,972 | |
| Deferred tax liability | | 146,456 | | | 0 | | | 0 | |
| Fair value of stock issued for settlement of debt | | 826,947 | | | 0 | | | 0 | |
| (Increase) / decrease in current assets: | | | | | | | | | |
| Accounts receivable | | (2,171,981 | ) | | (2,276,373 | ) | | (3,398,350 | ) |
| Other receivables | | (25,484 | ) | | 12,287 | | | 835,093 | |
| Advances to material suppliers | | (1,681,374 | ) | | (973,372 | ) | | (174,936 | ) |
| Due from related party | | (119,997 | ) | | (970,031 | ) | | (344,475 | ) |
| Inventory | | (47,491 | ) | | (2,691,926 | ) | | (1,432,685 | ) |
| Increase / (decrease) in current liabilities: | | | | | | | | | |
| Accounts payable | | (1,885,808 | ) | | 1,990,198 | | | (416,156 | ) |
| Other payables | | (1,874,121 | ) | | (4,345,451 | ) | | 3,441,490 | |
| Due to related party | | 440,648 | | | 540,562 | | | 162,708 | |
| Taxes payable | | 512,807 | | | 666,264 | | | 1,383,340 | |
| Unearned revenue | | 69,669 | | | 339,760 | | | (78,403 | ) |
| Accrued expenses | | (440,368 | ) | | 626,680 | | | 18,206 | |
| Total Adjustments | | (5,493,915 | ) | | (6,494,096 | ) | | 387,804 | |
| Net cash provided by operating activities | | 16,256,711 | | | 8,966,640 | | | 11,513,021 | |
| | | | | | | | | | |
| CASH FLOWS FROM INVESTING ACTIVITIES | | | | | | | | | |
| Advances to equipment suppliers | | (6,597,538 | ) | | 0 | | | 0 | |
| Purchase of intangible assets | | 0 | | | (6,274,260 | ) | | (46,324 | ) |
| Purchase of property, plant & equipment | | (640,453 | ) | | (1,489,661 | ) | | (7,335,773 | ) |
| Investment in construction in progress | | (497,952 | ) | | (2,807,823 | ) | | (1,123,957 | ) |
| Deposit for asset acquisition | | (5,565,343 | ) | | 0 | | | 0 | |
| Net cash used in investing activities | | (13,301,286 | ) | | (10,571,744 | ) | | (8,506,054 | ) |
F-23
| CASH FLOWS FROM FINANCING ACTIVITIES | | | | | | | | | |
| Borrowing of short-term loans | | 267,653 | | | 1,955,778 | | | 1,312,593 | |
| Repayment of short-term loans | | (1,500,000 | ) | | 0 | | | 0 | |
| Proceeds from issuance of stock, net | | 7,235,317 | | | 0 | | | 0 | |
| Net cash provided by (used in) financing | | 6,002,970 | | | 1,955,778 | | | 1,312,593 | |
| activities | | | | | | | | | |
| | | | | | | | | | |
| NET INCREASE IN CASH AND EQUIVALENTS | | 8,958,395 | | | 350,674 | | | 4,319,560 | |
| | | | | | | | | | |
| EFFECT OF EXCHANGE RATE CHANGES ON CASH AND | | 45,135 | | | 14,224 | | | 164,720 | |
| EQUIVALENTS | | | | | | | | | |
| | | | | | | | | | |
| CASH AND EQUIVALENTS, BEGINNING BALANCE | | 5,495,176 | | | 5,130,278 | | | 645,997 | |
| | | | | | | | | | |
| CASH AND EQUIVALENTS, ENDING BALANCE | $ | 14,498,707 | | $ | 5,495,176 | | $ | 5,130,278 | |
| SUPPLEMENTAL DISCLOSURES: | | | | | | | | | |
| Cash paid during the year for: | | | | | | | | | |
| Interest | $ | 492,809 | | $ | 168,529 | | $ | 158,734 | |
| Income taxes | $ | 7,242,177 | | $ | 4,791,151 | | $ | 1,611,559 | |
The accompanying notes are an integral part of these consolidated financial statements.
F-24
ASIA LEECHDOM HOLDING CORPORATION AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF STOCKHOLDERS' EQUITY (DEFICIT)
YEARS ENDED JUNE 30, 2010, 2009 and 2008
| | | Common Stock | | | Additional | | | Retained | | | Statutory | | | Accumulated Other | | | Shareholders' | |
| | | Shares | | | Par Value | | | Paid in Capital (Deficit) | | | Earnings | | | Reserve | | | Comprehensive Income | | | Equity (Deficit) | |
| | | | | | | | | | | | | | | | | | | | | | |
| Balance, July 1, 2007 | | 32,310,758 | | $ | 32,311 | | $ | 1,019,271 | | $ | 2,029,607 | | $ | 357,450 | | $ | 167,621 | | $ | 3,606,259 | |
| | | | | | | | | | | | | | | | | | | | | | |
| Changes due to recapitalization | | | | | | | | (1,260,000 | ) | | | | | | | | | | | (1,260,000 | ) |
| | | | | | | | | | | | | | | | | | | | | | |
| Transfer to statutory reserve | | | | | | | | | | | (480,890 | ) | | 480,890 | | | | | | - | |
| | | | | | | | | | | | | | | | | | | | | | |
| Foreign currency translation adjustment | | | | | | | | | | | | | | | | | 1,018,155 | | | 1,018,155 | |
| | | | | | | | | | | | | | | | | | | | | | |
| Net income for year | | | | | | | | | | | 11,125,217 | | | | | | | | | 11,125,217 | |
| | | | | | | | | | | | | | | | | | | | | | |
| Balance, June 30, 2008 | | 32,310,758 | | | 32,311 | | | (240,729 | ) | | 12,673,935 | | | 838,340 | | | 1,185,776 | | | 14,489,633 | |
| | | | | | | | | | | | | | | | | | | | | | |
| Foreign currency translation adjustment | | | | | | | | | | | | | | | | | 79,466 | | | 79,466 | |
| | | | | | | | | | | | | | | | | | | | | | |
| Net income for year | | | | | | | | | | | 15,460,733 | | | | | | | | | 15,460,733 | |
| | | | | | | | | | | | | | | | | | | | | | |
| Balance, June 30, 2009 | | 32,310,758 | | | 32,311 | | | (240,729 | ) | | 28,134,668 | | | 838,340 | | | 1,265,242 | | | 30,029,832 | |
| | | | | | | | | | | | | | | | | | | | | | |
| Changes due to capitalization | | 2,328,105 | | | 2,328 | | | (2,328 | ) | | | | | | | | | | | - | |
| | | | | | | | | | | | | | | | | | | | | | |
| Issuance of common stock for settlement | | | | | | | | 599,761 | | | | | | | | | | | | 600,000 | |
| | | 239,044 | | | 239 | | | | | | | | | | | | | | | | |
| Issuance of warrants for settlement | | - | | | - | | | 226,947 | | | | | | | | | | | | 226,947 | |
| | | | | | | | | | | | | | | | | | | | | | |
| Issuance of common stock for warrant financing | | 110,000 | | | 110 | | | 219,890 | | | | | | | | | | | | 220,000 | |
F-25
| Issuance of common stock | | 4,177,381 | | | 4,177 | | | 9,336,254 | | | | | | | | | | | | 9,340,432 | |
| | | | | | | | | | | | | | | | | | | | | | |
| Stock issued for services | | 675,317 | | | 675 | | | (675 | ) | | | | | | | | | | | - | |
| | | | | | | | | | | | | | | | | | | | | | |
| Transfer to statutory reserve | | | | | | | | | | | (1,065,795 | ) | | 1,065,795 | | | | | | - | |
| | | | | | | | | | | | | | | | | | | | | | |
| Foreign currency translation adjustment | | | | | | | | | | | | | | | | | 191,741 | | | 191,741 | |
| | | | | | | | | | | | | | | | | | | | | | |
| Net income for year | | | | | | | | | | | 21,750,626 | | | | | | | | | 21,750,626 | |
| | | | | | | | | | | | | | | | | | | | | | |
| Balance, June 30, 2010 | | 39,840,605 | | $ | 39,841 | | $ | 10,139,120 | | $ | 48,819,499 | | $ | 1,904,135 | | $ | 1,456,983 | | $ | 62,359,578 | |
The accompanying notes are an integral part of these consolidated financial statements.
F-26
ASIA LEECHDOM HOLDING CORPORATION AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
June 30, 2010, 2009 and 2008
Note 1 - ORGANIZATION AND DESCRIPTION OF BUSINESS
Asia Leechdom Holding Corp. (hereinafter referred to as “Asia Leechdom” or the “Company”), formerly, Bay Peak 6 Acquisition Corp., was organized under the laws of the State of Arizona, in 2004, as VT French Services, Inc., a wholly owned subsidiary of Visitalk Capital Corporation, (“VCC”), which in turn was a wholly owned subsidiary of Visitalk.com (“Visitalk”). As part of Visitalk’s Chapter 11 reorganization plan, Asia Leechdom was a shell company with no assets or operations. VCC was authorized by the Visitalk plan to be the reorganized debtor. Effective September 11, 2008, the name of the Company was changed from VT French Services, Inc. to Bay Peak 6 Acquisition Corp., in connection with the Company’s redomestication to Nevada. On June July 28, 2010, the Company changed its name to Asia Leechdom Holding Corp., in connection with its reverse acquisition of Asia Leechdom Holding Corp. (“ALH”), a New Jersey corporation. As a result of the reverse acquisition, the Company now conducts its operations in the People’s Republic of China (the “PRC”) through ALH’s wholly owned PRC subsidiary, Tianjin BOAI Pharmaceutical Company Ltd. (“BOAI Pharmaceutical”) and its second tier subsidiary, Tianjin BOAI Leechdom Technique Co., Ltd. (“BOAI Leechdom”).
On May 28, 2010, the Company completed a reverse acquisition through a share exchange with ALH, whereby the Company issued the sole shareholder of ALH, 32,310,758 shares of the Company’s common stock, par value $0.001, for 100% of the issued and outstanding capital stock of ALH. ALH thereby became the Company’s wholly owned subsidiary and its subsidiary, BOAI Pharmaceutical, became the Company’s indirect subsidiary. In accordance with Financial Accounting Standards Board (“FASB”) Accounting Standards Codification (“ASC”) 805, “Business Combinations”, the Company recorded this merger using the recapitalization method which consolidated the Company, ALH and BOAI Pharmaceutical and treated the Company as a shell at the time of the merger. According to 805-10-55-13, “the acquirer usually is the combining entity whose relative size (measured in, for example, assets, revenues, or earnings), is significantly larger than that of the other.” Since the Company was a shell at the time of the merger while ALH had operations through its acquisition of BOAI Pharmaceutical and was significantly larger than the Company was, under ASC 805, ALH was considered the acquirer. Because the transaction was accounted for as a recapitalization and not a business combination, pro forma information is not presented.
ALH was incorporated on June 20, 2007 in New Jersey. On July 5, 2007, ALH agreed to acquire 100% outstanding shares of BOAI Pharmaceutical for $1,260,000. The acquisition of BOAI Pharmaceutical was treated as an acquisition by an entity under common control as Mrs. Xuecheng Xia, the major shareholder of BOAI Pharmaceutical was also an ALH director and held an option to purchase equity interests in ALH from ALH’s sole shareholder. As a result the acquisition was accounted for at historical cost in a manner similar to that in pooling of interests accounting.
ALH, through its Chinese subsidiaries BOAI Pharmaceutical and BOAI Leechdom, is engaged in the research and development, manufacture, distribution of and technical support for pharmaceutical products. BOAI Pharmaceutical was formed as a PRC company on August 6, 2001. On June 24, 2010, BOAI Pharmaceutical increased its registered capital to $7,175,405. The operations of BOAI Pharmaceutical are based in Tianjin, China. BOAI Leechdom was formed as a PRC company on March 17, 1999 in Tianjin China. On May 10, 2005, pursuant to a shareholder resolution, BOAI Leechdom reduced its registered capital by $120,788 which was owned by Huan Bo Hai Investment Service Company. After this capital reduction, the registered capital of BOAI Leechdom is now $483,150.
On August 10, 2007, the original shareholders of BOAI Leechdom transferred their respective equity in BOAI Leechdom to BOAI Pharmaceutical, pursuant to equity transfer agreements, dated August 10, 2007, among BOAI Pharmaceutical and each of them.. After the acquisition, BOAI Pharmaceutical became the sole shareholder in BOAI Leechdom. BOAI Pharmaceutical and BOAI Leechdom were under common control prior to the acquisition as the shareholders of BOAI Pharmaceutical were also the owners of BOAI Leechdom before the acquisition. Accordingly, the acquisition of the 100% equity interest in BOAI Leechdom by BOAI Pharmaceutical was accounted for at historical cost in a manner similar to that in pooling of interests accounting, with ALH being the ultimate parent entity of BOAI Leechdom.
On February 27, 2010, Chenghai Du, the Company’s controlling stockholder, entered into a series of call option agreements with each of Ma Dan, Xia Xuecheng, Wang Yan, Wang Yansheng, He Haiwei and Tian Mengchun, the original shareholders and managements of BOAI Pharmaceutical (the “Option Holders”), pursuant to which Mr. Du granted each of them an option to acquire an amount of shares equal to 90% of the shares of the Company’s common stock issued to Mr. Du in the reverse acquisition, at an exercise price of $0.0001 per share. Each of them had the right to exercise their option during the period commencing on the date of the option agreement and ending on the fifth anniversary of the date thereof.
F-27
Note 2 - BASIS OF PRESENTATION AND SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
Basis of presentation
The consolidated financial statements are prepared in accordance with generally accepted accounting principles in the United States of America ("US GAAP"). The Company’s functional currency is the Chinese Renminbi (“CNY”); however the accompanying consolidated financial statements were translated and presented in United States Dollars (“USD” or “$”).
Principle of consolidation
The accompanying consolidated financial statements for the years ended June 30, 2010, 2009 and 2008 include the accounts of the Company, and its wholly-owned subsidiaries BOAI Pharmaceutical and BOAI Leechdom. All significant inter-company accounts and transactions were eliminated in consolidation.
Use of estimates
The preparation of consolidated financial statements in conformity with US GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the consolidated financial statements and the amount of revenues and expenses during the reporting periods. Management makes these estimates using the best information available at the time the estimates are made. However, actual results could differ materially from those results.
Foreign currency transactions and comprehensive income (loss)
As of June 30, 2010, 2009 and 2008, the accounts of BOAI Pharmaceutical and BOAI Leechdom were maintained, and their financial statements were expressed, in CNY. Such financial statements were translated into USD in accordance with ASC 830, “Foreign Currency Matters,” with the CNY as the functional currency. According to the Statement, all assets and liabilities were translated at the exchange rate on the balance sheet date, stockholders’ equity is translated at the historical rates and income statement items are translated at the average exchange rate for the period. The resulting translation adjustments are reported under other comprehensive income in accordance with ASC 220, “Comprehensive Income” as a component of stockholders’ equity.
During the years ended June 30, 2010, 2009 and 2008, the transactions of BOAI Pharmaceutical and BOAI Leechdom were denominated and recorded in CNY at the rates of exchange in effect when the transactions occurred. Exchange gains and losses are recognized for the different foreign exchange rates applied when the foreign currency assets and liabilities are settled. Transaction gains and losses that arise from exchange rate fluctuations on transactions denominated in a currency other than the functional currency are included in the results of operations as incurred.
Cash and equivalents
For statement of cash flows purposes, the Company considers all cash on hand and in banks, certificates of deposit and other highly-liquid investments with maturities of three months or less, when purchased, to be cash and equivalents.
Accounts receivable
The Company maintains reserves for potential credit losses on accounts receivable. Management reviews the composition of accounts receivable and analyzes historical bad debts, customer concentrations, customer credit worthiness, current economic trends and changes in customer payment patterns to evaluate the adequacy of these reserves. The allowance for accounts receivable bad debts was $146,780 and $0 as of June 30, 2010 and 2009, respectively.
Inventory
Inventory is valued at the lower of cost (determined on a weighted average basis) or market. Management compares the cost of inventories with their net realizable value and an allowance is made for writing down the inventory to its net realizable value, if lower than the cost.
Property, plant and equipment
Property, plant and equipment are recorded at cost. Gains or losses on disposals are reflected as gain or loss in the year of disposal. The cost of improvements that extend the life of plant, property, and equipment are capitalized. These capitalized costs may include structural improvements, equipment, and fixtures. All ordinary repair and maintenance costs are expensed as incurred.
F-28
Depreciation for financial reporting purposes is provided using the straight-line method over the estimated useful lives of the assets, as follows:
| | Estimated Useful Life |
| Buildings | 20 years |
| Machinery and equipment | 5-10 years |
| Office equipment | 5 years |
| Vehicles | 8-10 years |
Impairment of long-lived assets
Finite-lived intangible assets are amortized over their respective useful lives and, along with other long-lived assets, are evaluated for impairment at least annually, or periodically whenever events or changes in circumstances indicate that their related carrying amounts may not be recoverable in accordance with ASC 360, “Property, Plant and Equipment.”
In evaluating long-lived assets for recoverability, including finite-lived intangibles and property and equipment, the Company uses its best estimate of future cash flows expected to result from the use of the asset and eventual disposition in accordance with ASC 360. To the extent that estimated future undiscounted net cash flows attributable to the asset are less than the carrying amount, an impairment loss is recognized in an amount equal to the difference between the carrying value of such asset and its fair value. Assets to be disposed through sale or abandonment, are reported at the lower of carrying value or fair value less costs to sell. The Company estimates fair value based on the information available in making whatever estimates, judgments and projections are considered necessary. There was no impairment of long-lived assets for the years ended June 30, 2010, 2009 and 2008.
Revenue recognition
The Company's revenue recognition policies are in compliance with Securities and Exchange Commission (“SEC”) Staff Accounting Bulletin (“SAB”) 104. Sales revenue is recognized at the date of shipment to customers when a formal arrangement exists, the price is fixed or determinable, the delivery is completed, no other significant obligations of the Company exist and collectability is reasonably assured. Payments received before all of the relevant criteria for revenue recognition are satisfied are recorded as unearned revenue. The Company's revenue consists of invoiced value of goods, net of value-added tax (“VAT”) and product return or sales discount allowance. The Company does not offer unconditional right of return to its customers.
Income taxes
The Company accounts for income taxes using an asset and liability approach which allows for the recognition and measurement of deferred tax assets based upon the likelihood of realization of tax benefits in future years. Under the asset and liability approach, deferred taxes are provided for the net tax effects of temporary differences between the carrying amounts of assets and liabilities for financial reporting purposes and the amounts used for income tax purposes. A valuation allowance is provided for deferred tax assets if it is more likely than not these items will either expire before the Company is able to realize their benefits, or that future deductibility is uncertain.
The Company records a valuation allowance for deferred tax assets, if any, based on its estimates of its future taxable income as well as its tax planning strategies when it is more likely than not that a portion or all of its deferred tax assets will not be realized.
If the Company is able to utilize more of its deferred tax assets than the net amount previously recorded when unanticipated events occur, an adjustment to deferred tax assets would increase the Company net income when those events occur. The Company does not have any significant deferred tax asset or liabilities in the PRC tax jurisdiction.
In China, beginning January 1, 2008, the new Enterprise Income Tax (“EIT”) law replaced the existing laws for Domestic Enterprises (“DES”) and Foreign Invested Enterprises (“FIEs”). The new standard EIT rate of 25% replaced the 33% rate formerly applicable to both DES and FIEs. The two years tax exemption and the three years 50% tax reduction tax holiday for production-oriented FIEs were eliminated.
F-29
Statement of Cash Flows
In accordance with ASC 230, “Statement of Cash Flows”, cash flows from the Company’s operations are calculated based upon local currencies. As a result, amounts related to assets and liabilities reported on the statement of cash flows may not necessarily agree with changes in the corresponding balances on the balance sheet. During the year ended June 30, 2009, cash from investing and financing activities excluded non-cash items such as purchase of a building through assumption of liabilities for $2,268,493.
Fair value of financial instruments
The Company adopted the provisions of ASC 820, “Fair Value Measurements and Disclosures.” ASC 820 clarifies the definition of fair value, prescribes methods for measuring fair value, and establishes a fair value hierarchy to classify the inputs used in measuring fair value as follows:
Level 1-Inputs are unadjusted quoted prices in active markets for identical assets or liabilities available at the measurement date.
Level 2-Inputs are unadjusted quoted prices for similar assets and liabilities in active markets, quoted prices for identical or similar assets and liabilities in markets that are not active, inputs other then quoted prices that are observable, and inputs derived from or corroborated by observable market data.
Level 3-Inputs are unobservable inputs which reflect the reporting entity’s own assumptions on what assumptions the market participants would use in pricing the asset or liability based on the best available information.
The carrying amounts reported in the balance sheets for cash, accounts receivable, loan receivables, other receivables, advances to material suppliers, short-term loans, accounts payable, unearned revenue, other payables and accrued expenses, approximate their fair market value due to their short-term nature. The Company uses Level 3 method to measure fair value of its warrants.
As of the balance sheet dates, the estimated fair values of the financial instruments were not materially different from their carrying values as presented on the balance sheets. This is due to the short maturities of the instruments and that interest rates on the borrowings approximate those that would have been available for loans of similar remaining maturity and risk profile at the respective balance sheet dates.
Constructions in progress
Construction in progress is stated at cost, unless events or circumstances indicate that cost cannot be recovered, in which case the carrying value is reduced to estimated fair value. Estimated fair value: (i) is based on the Company’s plans for the development of each property; and (ii) considers the cost to complete and the estimated fair value of the completed projects.
Direct construction, development costs, property taxes and other related costs of construction projects are capitalized during periods those activities which are necessary to get the projects ready for its intended use. The capitalization ends when the construction is substantially completed and the projects are ready for its intended use.
Research and development
Research and development costs are incurred on a project specific basis and expensed as incurred.
Segment reporting
The Company follows ASC 280, “Segment Reporting,” which establishes standards for reporting about operating segments. Operating segments are defined as components of an enterprise about which separate financial information is available that is evaluated regularly by the chief operating decision maker in deciding how to allocate resources and assess performance. The Company’s chief operating decision maker was identified as the Chief Executive Officer.
During the years ended June 30, 2010, 2009 and 2008, the Company operated in two reportable business segments - (1) the pharmaceutical manufacturing segment and (2) medicine wholesales segment. The Company's reportable segments are strategic business units that offer different products. The Company's reportable segments, although integral to the success of the others, offer distinctly different products and services and require different types of management focus. As such, these segments are managed separately.
F-30
The following table presents a summary of operating information for the years ended June 30, 2010, 2009 and 2008 and year-end balance sheet information for the years ended June 30, 2010 and 2009:
F-31
| | | Pharmaceutical | | | Medicine | | | Inter-segment | | | Non-operating | | | Consolidated | |
| For the Year Ended June 30, 2010 | | Manufacturing | | | Wholesales | | | Elimination | | | Entities | | | Total | |
| | | | | | | | | | | | | | | | |
| Net Sales | $ | 28,006,754 | | $ | 40,226,335 | | $ | (66,092 | ) | $ | - | | $ | 68,166,997 | |
| | | | | | | | | | | | | | | | |
| Interest Income (expense) | | (112,028 | ) | | (71,376 | ) | | - | | | (920,651 | ) | | (1,104,055 | ) |
| | | | | | | | | | | | | | | | |
| Depreciation and Amortization | | 428,707 | | | 327,475 | | | - | | | - | | | 756,182 | |
| | | | | | | | | | | | | | | | |
| Segment assets | | 51,935,131 | | | 34,988,610 | | | (19,829,808 | ) | | 10,335,722 | | | 77,429,655 | |
| | | | | | | | | | | | | | | | |
| Segment net income (loss) before tax | | 14,211,912 | | | 16,432,178 | | | (150,880 | ) | | (1,079,956 | ) | | 29,413,254 | |
| | | | | | | | | | | | | | | | |
| For the Year Ended June 30, 2009 | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | |
| Net Sales | | 20,419,490 | | | 29,579,416 | | | (509,840 | ) | | - | | | 49,489,066 | |
| | | | | | | | | | | | | | | | |
| Interest Income (expense) | | (115,085 | ) | | (42,684 | ) | | - | | | (93,704 | ) | | (251,473 | ) |
| | | | | | | | | | | | | | | | |
| Depreciation and Amortization | | 311,907 | | | 275,399 | | | - | | | - | | | 587,306 | |
| | | | | | | | | | | | | | | | |
| Segment assets | | 32,716,539 | | | 24,064,231 | | | (9,450,392 | ) | | 1,261,354 | | | 48,591,732 | |
| | | | | | | | | | | | | | | | |
| Segment net income (loss) before tax | | 10,508,496 | | | 10,302,257 | | | (1,602 | ) | | (144,868 | ) | | 20,664,283 | |
| | | | | | | | | | | | | | | | |
| For the Year Ended June 30, 2008 | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | |
| Net Sales | | 13,902,974 | | | 18,984,253 | | | (506,107 | ) | | - | | | 32,381,120 | |
| | | | | | | | | | | | | | | | |
| Interest Income (expense) | | (150,445 | ) | | 4,704 | | | - | | | - | | | (145,741 | ) |
| | | | | | | | | | | | | | | | |
| Depreciation and Amortization | | 219,342 | | | 172,630 | | | - | | | - | | | 391,972 | |
| | | | | | | | | | | | | | | | |
| Segment assets | | 15,585,611 | | | 16,735,413 | | | (4,618,441 | ) | | 1,312,518 | | | 29,015,101 | |
| | | | | | | | | | | | | | | | |
| Segment net income (loss) before tax | $ | 6,156,909 | | $ | 7,110,807 | | $ | (20,246 | ) | $ | (75 | ) | $ | 13,247,395 | |
F-32
| | | | | | | | | | |
| | | For the Year | | | For the Year | | | For the Year | |
| Reconciliation of segment incomes to consolidated incomes | | Ended June 30, | | | Ended June 30, | | | Ended June 30, | |
| | | 2010 | | | 2009 | | | 2008 | |
| | | | | | | | | | |
| Total segment income (Operating entities) | $ | 30,644,090 | | $ | 20,810,753 | | $ | 13,267,716 | |
| Total segment income (Non- operating entities) | | (1,079,956 | ) | | (144,868 | ) | | (75 | ) |
| | | | | | | | | | |
| Elimination of intersegment profits | | (150,880 | ) | | (1,602 | ) | | (20,246 | ) |
| Consolidated income before income taxes | $ | 29,413,254 | | $ | 20,664,283 | | $ | 13,247,395 | |
| Reconciliation of segment assets to consolidated assets | | As of June 30, | | | As of June 30, | |
| | | 2010 | | | 2009 | |
| | | | | | | |
| Total segment assets (Operating entities) | $ | 86,923,741 | | $ | 56,780,770 | |
| Total segment assets (Non-operating entities) | | 10,335,722 | | | 1,261,354 | |
| Elimination of intersegment receivables | | (19,829,808 | ) | | (9,450,392 | ) |
| Consolidated assets | $ | 77,429,655 | | $ | 48,591,732 | |
F-33
Recent accounting pronouncements
On April 1, 2009, the FASB approved FSP FAS 141(R)-1, “Accounting for Assets Acquired and Liabilities Assumed in a Business Combination That Arise from Contingencies”, which amends FAS 141(R), as incorporated into ASC 805, and eliminated the distinction between contractual and non-contractual contingencies. Under the updated standard an acquirer is required to recognize at fair value an asset acquired or liability assumed in a business combination that arises from a contingency if the acquisition-date fair value of that asset or liability can be determined during the measurement period. If the acquisition-date fair value cannot be determined, the acquirer applies the recognition criteria in ASC 450, “Contingencies” to determine whether the contingency should be recognized as of the acquisition date or after it. This guidance is effective for assets or liabilities arising from contingencies in business combinations for which the acquisition date is on or after the beginning of the first annual reporting period beginning on or after December 15, 2008. The adoption of such standard had no material impact on our consolidated financial statements.
In April 2009, the FASB issued FSP No. FAS 157-4, “Determining Fair Value When the Volume and Level of Activity for the Asset or Liability Have Significantly Decreased and Identifying Transactions That Are Not Orderly” as incorporated into ASC 820, “Fair Value Measurements and Disclosures”. The guidance relates to determining fair values when there is no active market or where the price inputs being used represent distressed sales. It reaffirms what FASB ASC 820 states is the objective of fair value measurement—to reflect how much an asset would be sold for in an orderly transaction (as opposed to a distressed or forced transaction) at the date of the financial statements under current market conditions. Specifically, it reaffirms the need to use judgment to ascertain if a formerly active market has become inactive and in determining fair values when markets have become inactive. This guidance is effective for interim and annual periods ended after June 15, 2009, but entities may early adopt this guidance for the interim and annual periods ended after March 15, 2009. The adoption of such standard had no material impact on our consolidated financial statements.
In May 2009, the FASB issued ASC 855, “Subsequent Events,” which establishes general standards of accounting for and disclosure of events that occur after the balance sheet date but before financial statements. The statement is effective for interim and annual periods ended after June 15, 2009. The standard was subsequently amended by FASB Accounting Standards Update (“ASU”) 2010-09 which exempts an entity that is an SEC filer from the requirement to disclose the date through which subsequent events have been evaluated.
In June 2009, the FASB issued FASB ASU 2009-01, which amends ASC 105, “Generally Accepted Accounting Principles” (“Statement of Financial Accounting Standards (“SFAS”) No. 168, “The FASB Accounting Standards Codification TM and the Hierarchy of Generally Accepted Accounting Principles – a replacement of FASB Statement No. 162,” ) , which establishes the FASB Accounting Standards Codification TM (“Codification”) as the source of authoritative generally accepted accounting principles in the United States (“GAAP”) for nongovernmental entities. The Codification does not change GAAP. Instead, it takes the thousands of individual pronouncements that currently comprise GAAP and reorganizes them into approximately 90 accounting Topics, and displays all Topics using a consistent structure. Contents in each Topic are further organized first by Subtopic, then Section and finally Paragraph. The Paragraph level is the only level that contains substantive content. Citing particular content in the Codification involves specifying the unique numeric path to the content through the Topic, Subtopic, Section and Paragraph structure. FASB suggests that all citations begin with “FASB ASC”. Changes to the ASC subsequent to June 30, 2009 are referred to as Accounting Standards Updates.
In August 2009, the FASB issued ASU 2009-5, “Measuring Liabilities at Fair Value”. ASU 2009-05 amends ASC 820, “Fair Value Measurements”. Specifically, ASU 2009-05 provides clarification that in circumstances in which a quoted price in an active market for the identical liability is not available, a reporting entity is required to measure fair value using one or more of the following methods: 1) a valuation technique that uses a) the quoted price of the identical liability when traded as an asset or b) quoted prices for similar liabilities or similar liabilities when traded as assets and/or 2) a valuation technique that is consistent with the principles of ASC 820 of the Accounting Standards Codification (e.g. an income approach or market approach). ASU 2009-05 also clarifies that when estimating the fair value of a liability, a reporting entity is not required to adjust to include inputs relating to the existence of transfer restrictions on that liability. The adoption of such standard had no material impact on our consolidated financial statements.
F-34
In September 2009, the Emerging Issues Task Force reached final consensus on ASU 2009-13, “Revenue Arrangements with Multiple Deliverables”. ASU 2009-13 addresses how to determine whether an arrangement involving multiple deliverables contains more than one unit of accounting, and how the arrangement consideration should be allocated among the separate units of accounting. This ASU will be effective prospectively for revenue arrangements entered into or materially modified in years beginning on or after June 15, 2010. Early adoption is permitted. The adoption of such standard had no material impact on our consolidated financial statements.
In December 2009, the FASB issued ASU 2009-17, “Improvements to Financial Reporting by Enterprises Involved with Variable Interest Entities”. This ASU amends ASC 810, “Consolidation”, eliminates exceptions to consolidating qualifying special-purpose entities, contains new criteria for determining the primary beneficiary, and increases the frequency of required reassessments to determine whether a company is the primary beneficiary of a variable interest entity. This ASU is effective for years beginning after November 15, 2009, which for the Company is January 1, 2010, with earlier adoption prohibited. The Company does not expect the adoption of ASU 2009-17 to have a material effect on its consolidated financial statements.
In January 2010, FASB amended ASC 820, “Fair Value Measurements and Disclosures.” The new disclosures and clarifications of existing disclosures are effective for interim and annual reporting periods beginning after December 15, 2009, except for the disclosures about purchases, sales, issuances, and settlements in the roll forward of activity in Level 3 fair value measurements. Those disclosures are effective for years beginning after December 15, 2010, and for interim periods within those years. The Company determined the adoption of this ASU had no material impact on its financial statements.
Note 3 - INVENTORY
Inventory consisted of the following as of June 30, 2010 and 2009:
| | | 2010 | | | 2009 | |
| Supplies, packing and raw materials | $ | 1,177,056 | | $ | 1,133,502 | |
| Finished goods | | 5,311,841 | | | 3,626,321 | |
| Work in process | | 772,319 | | | 2,420,196 | |
| Total | $ | 7,261,216 | | $ | 7,180,019 | |
Note 4 - ADVANCES TO MATERIAL SUPPLIERS
The Company advanced payments to several medicine and package materials suppliers to support increasing production with competitive procurement cost. As of June 30, 2010, the Company made total deposits of $2,849,511 to materials suppliers. The deposit will be reclassified to the respective accounts under inventory upon delivery and transfer of legal titles.
Note 5 – SUBSCRIPTIONS RECEIVABLE
As of June 30, 2010, subscriptions receivable represent amounts received on July 7, 2010 for the sale of shares.
Note 6 - PROPERTY, PLANT AND EQUIPMENT
Property, plant and equipment consist of the following as of June 30, 2010 and 2009:
| | | 2010 | | | 2009 | |
| Building and improvements | $ | 16,273,307 | | $ | 13,346,026 | |
| Machinery and equipment | | 915,816 | | | 881,662 | |
| Office equipment | | 91,660 | | | 78,428 | |
| Vehicles | | 480,185 | | | 468,751 | |
| Total | | 17,760,968 | | | 14,774,867 | |
| Less: Accumulated Depreciation | | (2,303,702 | ) | | (1,574,362 | ) |
| Net Book Value | $ | 15,457,266 | | $ | 13,200,505 | |
F-35
Depreciation for the years ended June 30, 2010, 2009 and 2008 was $720,380, $551,609 and $358,565, respectively.
As of June 30, 2010 and 2009, the net book value of buildings pledged as collateral for bank loans were $9,509,877 and $9,496,918, respectively. See Note 11.
Note 7 - ADVANCES TO EQUIPMENT SUPPLIERS
The Company advanced payments to several equipment suppliers to support increasing production with competitive procurement cost. As of June 30, 2010, the Company made total deposits of $6,612,116 to equipment suppliers. The deposit will be reclassified to the respective accounts under fixed assets upon delivery and transfer of legal titles.
Note 8 – DEPOSIT FOR ASSET ACQUISITION
The Company signed a property purchase agreement with Tianjin Qi Shi Leather Ltd. on May 8, 2010 to acquire land use rights and buildings for RMB 80 million ($11.7 million). As of June 30, 2010, the Company made $5,577,640 deposit to Tianjin Qi Shi Leather Ltd for future acquisition. See Note 22.
Note 9 - CONSTRUCTION IN PROGRESS
As of June 30, 2010 and 2009, construction in progress of the Company consisted of the following:
| | | 2010 | | | 2009 | |
| Office building | $ | 2,241,428 | | $ | 1,734,260 | |
| Workshop of Paclitaxel | | - | | | 2,264,493 | |
| Total construction in progress | $ | 2,241,428 | | $ | 3,998,753 | |
On June 15, 2009, BOAI Pharmaceutical signed a Construction Agreement with Tianjin Zhongjin Environmental Technology Co., Ltd. to build a workshop for manufacturing Paclitaxel. The construction was completed in October 2009.
As of June 30, 2010, BOAI Leechdom expects to pay an additional $400,612 in order to complete the construction of the office building.
Note 10- INTANGIBLE ASSETS
As of June 30, 2010 and 2009, the Company has intangible assets including Paclitaxel ongoing project, pharmaceutical production permits, land use rights and software.
| | | 2010 | | | 2009 | |
| Pharmaceutical production permits | $ | 337,594 | | $ | 336,022 | |
| Paclitaxel - In-process research and development | | 6,311,540 | | | 6,282,141 | |
| Land use rights | | 101,781 | | | 101,307 | |
| Software | | 2,141 | | | 4,724 | |
| Total | | 6,753,056 | | | 6,724,194 | |
| Less: Accumulated Amortization | | (277,580) | | | (240,573) | |
| Net book value | $ | 6,475,476 | | $ | 6,483,621 | |
F-36
The pharmaceutical production permits were obtained in December 2001, updated in January 2006 and valid until January 2011. The Company expects to renew permits in January 2011, which will be effective until January 2016. With these production permits, the Company is authorized to manufacture certain Chinese medicines.
The Company obtained Paclitaxel cell cultivation method from Tsinghua University in 2009 and paid approximately $5.8 million as of June 30, 2010. The first payment of approximately $2.9 million was paid in early 2009 when the Company obtained all Paclitaxel cell cultivation information. The second payment of approximately $2.9 million was paid in 2009 after the Company successfully completed trial production. The third payment will be paid once the scale production is proven successful. At June 30, 2010, the amount due was in other payables. See Note 12.
Our technology could increase the success ratio and consistency of the produced product to treat patients with lung, ovarian, breast, head and neck cancer. We intend to ramp up the scale and enter into commercial production once the new facility for Paclitaxel production is available. The technology was valued on December 18, 2010 by a licensed appraiser in China and the fair value was approximately $6.8 million.
Note 11 – SHORT TERM LOANS
The loans payable at June 30, 2010 and 2009 comprised of the following:
| Description | | 2010 | | | 2009 | |
| Loans payable to Tianjin Agriculture Credit Union Bank: | | | | | | |
| Due by January 28, 2011. Interest at 5.841% | $ | 1,467,800 | | $ | - | |
| Due by March 8, 2011. Interest at 5.841% | | 278,882 | | | - | |
| Due by May 10, 2011. Interest at 5.841% | | 352,272 | | | - | |
| Due by February 27, 2010. Interest at 5.841% | | - | | | 1,460,963 | |
| Due by March 30, 2010. Interest at 5.841% | | - | | | 277,583 | |
| Due by May 20, 2010. Interest at 5.841% | | - | | | 350,631 | |
| Loans payable to Tianjin Bank: | | | | | | |
| Due by January 28, 2011. Interest at 5.841% | | 1,247,630 | | | - | |
| Due by November 23, 2009. Interest at 6.96% | | | | | 1,241,819 | |
| Loan payable to Midi Investment Inc | | 190,000 | | | - | |
| Loan payable to China BOI Hunter, LLC: interest imputed at 14.95% (a) | | - | | | 1,406,297 | |
| Total Short-term Loans | $ | 3,536,584 | | $ | 4,737,293 | |
(a) This loan, net of unamortized discount of $93,703, was originally obtained on June 20, 2008 and was past due in 2009. Pursuant to a settlement agreement, it was paid in June 2010.
The short-term bank loans are secured by buildings owned by the Company. See Notes 6.
Note 12 - OTHER PAYABLES
Other payables consisted of the following as of June 30, 2010 and 2009:
| | | 2010 | | | 2009 | |
| Non-related party company payable (a) | $ | 544,786 | | $ | 2,425,177 | |
| Acquired Paclitaxel ongoing project | | 440,340 | | | 438,289 | |
| Employees payable (b) | | 147,333 | | | 146,026 | |
| Funds and Insurance payable (c) | | 754,723 | | | 48,403 | |
| Welfare payable | | 98,675 | | | 109,138 | |
| Total | $ | 1,985,857 | | $ | 3,167,033 | |
(a) This payable is primarily for the purchase of a building, to be paid upon transfer of the land and building certificate to the Company. It bears no interest.
F-37
(b) Employees payable represents advances from employees for various payments made on behalf of the Company. This payable is due on demand and bears no interest.
(c) Funds and insurance payable includes $682,109 financing fees as of June 30, 2010, paid in July 2010.
Note 13 - TAXES PAYABLE
Taxes payables consist of the following as of June 30, 2010 and 2009:
| | | 2010 | | | 2009 | |
| VAT payable | $ | 977,278 | | $ | 827,518 | |
| Income tax payable | | 2,057,856 | | | 1,767,645 | |
| Other | | 222,349 | | | 135,601 | |
| Total | $ | 3,257,483 | | $ | 2,730,764 | |
Note 14 - INCOME TAXES
The Company utilizes ASC 740, "Income Taxes," which requires recognition of deferred tax assets and liabilities for the expected future tax consequences of events that have been included in the financial statements or tax returns. Under this method, deferred income taxes are recognized for the tax consequences in future years of differences between the tax bases of assets and liabilities and their financial reporting amounts at each period end based on enacted tax laws and statutory tax rates applicable to the periods in which the differences are expected to affect taxable income. Valuation allowances are established, when necessary, to reduce deferred tax assets to the amount expected to be realized.
Local PRC income tax
The Company is governed by the Income Tax Law of the PRC concerning Chinese registered limited liability companies. Under the Income Tax Laws of the PRC, Chinese enterprises are generally subject to an income tax at an effective rate of 33% (30% state income taxes plus 3% local income taxes) on income reported in the statutory financial statements after appropriate tax adjustments, unless the enterprise is located in a specially designated region for which more favorable effective tax rates are applicable.
Beginning January 1, 2008, in the PRC the new Enterprise Income Tax (“EIT”) law replaced the existing laws for Domestic Enterprises (“DES”) and Foreign Invested Enterprises (“FIEs”). The new standard EIT rate of 25% replaced the 33% rate applicable to both DES and FIEs. The two years tax exemption and three years 50% tax reduction tax holiday for production-oriented FIEs were eliminated.
The provision for income taxes for the years ended June 30, 2010, 2009 and 2008 consisted of the following:
| | | 2010 | | | 2009 | | | 2008 | |
| Income tax expense- current | $ | 7,515,848 | | $ | 5,203,547 | | $ | 2,122,178 | |
| Income tax expense- deferred | | 146,780 | | | - | | | - | |
| Total provision for income taxes | $ | 7,662,628 | | $ | 5,203,547 | | $ | 2,122,178 | |
This table reconciles the U.S. statutory rates to the Company’s effective tax rate for the years ended June 30, 2010, 2009 and 2008:
| | | 2010 | | | 2009 | | | 2008 | |
| Tax at statutory rate | | 35% | | | 35% | | | 35% | |
| Foreign tax rate difference | | (10% | ) | | (10% | ) | | (10% | ) |
| China income tax exemption (a) | | - | | | - | | | (9% | ) |
F-38
| Other items (b) | | 1.0% | | | 0.2% | | | - | |
| Total | | 26.0% | | | 25.2% | | | 16.0% | |
| | (a) | The (9%) tax exemption was due to Government tax policy for certain FIEs, reducing taxes to 12.5% from July to December 2007. |
| | | |
| | (b) | The 1.0% and 0.2% represent $1,079,956 and $144,868 expenses incurred by overseas holding companies not subject to PRC income tax for the years ended June 30, 2010 and 2009, respectively. |
Note 15 - RELATED PARTY TRANSACTIONS
Due from related party
For the year ended June 30, 2010, the Company advanced $1,893,114 to drug chain stores. The major shareholder of the Company is also the main shareholder of these chain stores. These loans were unsecured and due on demand.
Due to related party
As of June 30, 2010, the Company received loans totaling $1,552,552 from its major shareholder and Chief Executive Officer, Ms. Xuecheng Xia. The Company also had $953,566 payable to drug chain stores, whose major shareholder is the main shareholder of the Company. The loans and the payables were unsecured and due on demand.
Note 16 - STATUTORY RESERVES
As stipulated by Law of the PRC, net income after taxation can only be distributed as dividends after appropriation has been made for the following:
| | i) | Making up cumulative prior years' losses, if any; |
| | ii) | Allocations to the "Statutory surplus reserve" of at least 10% of income after tax, as determined under PRC accounting rules and regulations, until the fund amounts to 50% of the Company's registered capital; and |
| | iii) | Allocations to the discretionary surplus reserve, if approved in the shareholders' general meeting. |
In accordance with the Chinese Company Law, the Company’s subsidiaries allocated 10% of their net income to surplus.
Note 17 - EARNINGS PER SHARE
Earnings per share is determined by dividing net income for the periods by the weighted average number of both basic and diluted shares of common stock and common stock equivalents outstanding pursuant to ASC 260, “Earnings Per Share.”
The following demonstrates the calculation for earnings per share for the years ended June 30, 2010, 2009 and 2008:
| | | 2010 | | | 2009 | | | 2008 | |
| Basic earnings per share | | | | | | | | | |
| Net Income | $ | 21,750,626 | | $ | 15,460,736 | | $ | 11,125,217 | |
| Weighted average number of common shares outstanding-Basic | | 35,783,112 | | | 32,310,758 | | | 32,310,758 | |
| Earnings per share-Basic | $ | 0.61 | | $ | 0.48 | | $ | 0.34 | |
| | | | | | | | | | |
| Diluted earnings per share | | | | | | | | | |
| Net Income | $ | 21,750,626 | | $ | 15,460,736 | | $ | 11,125,217 | |
| Weighted average number of common shares outstanding- Diluted | | 35,783,112 | | | 32,310,758 | | | 32,310,758 | |
| Earnings per share-Diluted | $ | 0.61 | | $ | 0.48 | | $ | 0.34 | |
F-39
Note 18 - EQUITY PLACEMENTS
From March to June 2010, the Company sold 4,287,381 shares of common stock and 835,477 common stock warrants for $10,705,311. Each Warrant permits the holder to purchase one share of common stock from the Company for $2.51. The Warrants expire five years after issuance. The Company paid the Placement Agent a fee of $935,000 and 675,317 shares of common stock, at $2.51 a share, for a total of $2,630,046.
The Company and its controlling shareholders entered into Side Letter Agreements with the investors which required the Company's controlling shareholders to surrender Company shares to the Investors if the Company failed to reach certain income thresholds for the years ending June 30, 2009, 2010 and 2011. The 2009 and 2010 thresholds were met. Another condition was that the Company become a “reporting company” within the meaning of Rule 144 under the Securities Act of 1933, as amended, within 180 days following the closing of such financing, and file a registration statement with respect to its Common Stock on Form 10 with the U.S. Securities and Exchange Commission before December 31, 2010. The Company did not meet this obligation on the required date and so, in accordance with the Side Letter Agreements, the controlling shareholders were obligated to either deliver 796,812 shares of Company’s common stock owned by them to the investors.
Pursuant to a bridge loan settlement agreement dated May 25, 2010 with China BOI Hunter, LLC, the Company issued 239,044 shares of common stock and 298,805 common stock purchase 3-year warrants. Each Warrant permits the holder to purchase one share of common stock from the Company for $2.51. The fair value of shares and warrants issued for this settlement of $600,000 and $226,947 was accounted for as additional interest expense on the settlement date. The fair value of the warrant was calculated using the Black-Scholes options pricing model using the following assumptions: Volatility: 43.9%; Risk free interest rate: 1%, Expected term: 2.92 years.
The fair value of outstanding warrants from the June 2010 equity financing was $843,141 as of June 30, 2010. The fair value of the warrants at June 30, 2010 was calculated using the Black-Scholes options pricing model using the following assumptions: Volatility: 43.9%; Risk free interest rate: 1.79%, Expected term: 4.92 -5.00 years. The fair value of the warrants was accounted for as cost of raising equity.
Note 19 - CURRENT VULNERABILITY DUE TO CERTAIN CONCENTRATIONS
The Company's operations are all carried out in the PRC. Accordingly, the Company's business, financial condition and results of operations may be influenced by the political, economic and legal environments in the PRC, and by the general state of the PRC's economy.
The Company's operations in the PRC are subject to specific considerations and significant risks not typically associated with companies in the North America and Western Europe. These include risks associated with, among others, the political, economic and legal environments and foreign currency exchange. The Company's results may be adversely affected by changes in governmental policies with respect to laws and regulations, anti-inflationary measures, currency conversion and remittance abroad, and rates and methods of taxation, among other things.
F-40
Note 20 - MAJOR CUSTOMERS AND VENDORS
Major customers and vendors represent those which accounted for 10% or more of the Company’s total net revenue or purchases, respectively.
No customer accounted for 10% or more of our net revenue for the years ended June 30, 2010, 2009 and 2008.
One vendor provided 15.5% of the Company’s purchases of raw materials for the year ended June 30, 2010. The Company had $78,103 payable to this vendor as of June 30, 2010.
Three vendors provided 23%, 21% and 11% of the Company’s purchases of raw materials for the year ended June 30, 2009. The Company had $2,279,381, no and $517,682 payable to these vendors as of June 30, 2009 to the vendors.
No vendor provided 10% or more of the Company’s purchase of raw materials for the year ended June 30, 2008.
Note 21 - COMMITMENTS AND CONTINGENCIES
The Company’s sales, purchases and expenses transactions are denominated in RMB and all of the Company’s assets and liabilities are also denominated in RMB. The RMB is not freely convertible into foreign currencies under the current law. In China, foreign exchange transactions are required by law to be transacted only by authorized financial institutions at exchange rates set by the People’s Bank of China, the central bank of China. Remittances in currencies other than RMB may require certain supporting documentation in order to affect the remittance.
BOAI Leechdom leased offices to several non-related parties from 2007. These leases are expected to continue year by year. Total rental revenue for the years ended June 30, 2010, 2009 and 2008 was $106,634, $191,847 and $78,846, respectively.
As of June 30, 2010, the Company has RMB 107 million (unaudited) (approximately, $16.1 million) in capital commitments for the construction of buildings and purchase of equipment relating to construction of its new production facilities in Tianjin.
As of June 30, 2010, the Company has no significant litigation claims. Also, the Company does not have significant operating-lease commitments as of June 30, 2010.
Note 22 – SUBSEQUENT EVENTS
On November 20, 2010, the Company consummated purchase agreement, dated on May 8, 2010, between the Company and Tianjin Qi Shi Leather Co., Ltd. ("Qi Shi Leather"), pursuant to which the Company acquired 100% of the equity interest in Qi Shi Leather, for an aggregate purchase price of RMB 80,000,000 Chinese Renminbi (approximately $11.7 million) in cash. Ninety-three percent of the equity interest in Qi Shi Leather was transferred to BOAI Pharmaceutical and the remaining 7% was transferred to BOAI Leechdom. Qi Shi Leather was incorporated on July 22, 2003 and at the closing date did not have any business operations, assets or liabilities, other than its land use rights for 85,938.92 square meters of property and 22,138.22 square meters of building space in Jinghai District, Tianjin. The Company’s purpose for acquiring Qi Shi Leather was to acquire such land use rights and property. The acquisition of the Qi Shi Leather equity interest was in lieu of an asset acquisition which would have required the Company to engage in a more time-consuming process for the transfer of Qi Shi Leather's properties. The Company intends to use the property acquired with Qi Shi Leather to enhance its current production facilities and increase its production capacity. The additional production facilities are under construction and are expected to be completed by August, 2011. In connection with the closing of the acquisition, the Company has changed Qi Shi Leather's name to Tianjin BOAI Bio-Pharmaceutical Co., Ltd.
On January 10, 2011, Mr. Chenghai Du, the Company’s controlling stockholder entered into an additional call option agreement with Ms. Jianping Lu, an executive employee of BOAI Pharm, pursuant to which Mr. Du granted her an option to acquire an amount of shares equal to the remaining 10% of the shares of our common stock issued to Mr. Du in the reverse acquisition, on the same terms and conditions as the other option holders discussed under Note 1 hereof.
F-41
On January 11, 2011, the option holders transferred and assigned their options to purchase an aggregate of 9,454,183 shares of our Common Stock to Dragon Core Limited and an aggregate of 22,059,762 shares of our Common Stock to Neo Profit Limited. On the same date each of Dragon Core and Neo Profit exercised their options to purchase their respective shares from Mr. Du. Dragon Core Limited is 67% beneficially owned and controlled by our Chief Executive Officer and Director, Ms. Xuecheng Xia, and 33% beneficially owned and controlled by Ms. Jianping Lu, and Neo Profit Limited is wholly-owned and controlled by Ms. Xia.
As a result of the exercises, Dragon Core and Neo Profit hold an aggregate of 31,513,945, or 79.1%, of our issued and outstanding common stock and Mr. Du is no longer affiliated with, and no longer holds any equity interest in the Company or its subsidiaries.
On February, 11, 2011, the Company, the controlling stockholders and the investors entered into an amendment and waiver agreement, pursuant to which: the investors agreed to extend the Company’s requirement to become a reporting company to February 15, 2011; we agreed to list our Common Stock on the Nasdaq Stock Market, the NYSE Amex or the New York Stock Exchange, on or before October 31, 2011; and the controlling stockholders agreed to surrender an amount of shares equal to 2% of our issued and outstanding fully diluted common stock, if the Company’s actual 2011 net income is not equal to or greater than $27,500,000.
F-42
SIGNATURES
Pursuant to the requirements of Section 12 of the Securities Exchange Act of 1934, the registrant has duly caused this registration statement to be signed on its behalf by the undersigned, thereunto duly authorized.
| ASIA LEECHDOM HOLDING CORPORATION |
| | |
| By: | /s/ Xuecheng Xia |
| | Xuecheng Xia |
| Date:February 11, 2011 | | CEO, President and Board Chair |
POWER OF ATTORNEY
Pursuant to the requirements of the Securities Act of 1933, this registration statement has been signed by the following persons in the capacities and on the dates indicated. Each person whose signature appears below constitutes and appoints Xuecheng Xia his or her true and lawful attorneys-in-fact and agents, with full power of substitution and resubstitution, for him or her and in his or her name, place and stead, in any and all capacities, to sign any and all amendments (including post-effective amendments) to this registration statement, and to file the same, with all exhibits thereto, and other documents in connection therewith, with the Securities and Exchange Commission, granting unto said attorneys-in-fact and agents, and each of them, full power and authority to do and perform each and every act and thing requisite and necessary to be done in connection therewith, as fully to all intents and purposes as he might or could do in person, hereby ratifying and confirming all that said attorneys-in-fact and agents, or any of them, or their or his or her substitute or substitutes, may lawfully do or cause to be done by virtue hereof.
| Signature | | Title | | Date |
| /s/ Xuecheng Xia | | CEO, President and Board Chair (Principal Executive | | February 11, 2011 |
| Xuecheng Xia | | Officer) | | |
| | | | | |
| /s/ Shuyuan Chang | | Chief Financial Officer (Principal Financial Officer and | | February 11, 2011 |
| Shuyuan Chang | | Principal Accounting Officer) | | |
| | | | | |
| /s/ Yansheng Wang | | Chief Operating Officer | | February 11, 2011 |
| Yansheng Wang | | | | |
| | | | | |
| /s/ Haiwei He | | Director | | February 11, 2011 |
| Haiwei He | | | | |
| | | | | |
| /s/ Cory Roberts | | Director | | February 11, 2011 |
| Cory Roberts | | | | |
EXHIBITS
ExhibitNo. | Description |
2.1 | Share Exchange Agreement, dated as of May 28, 2010, as amended, among Bay Peak 6Acquisition Corp., Asia Leechdom Holding Corp.,Tianjin BOAI Pharmaceutical Co.,Ltd., Tianjin BOAI Leechdom Technique Co., Ltd. and the shareholder of AsiaLeechdom Holding Corp. |
3.1 | Articles of Incorporation, as amended, as filed with the Secretary of State of Nevada |
3.2 | Bylaws, adopted September 11, 2008 |
4.1 | Visitalk Capital Corporation Bankruptcy Plan, Order and Decree |
4.2 | Plan Warrant Agreement of Visitalk Capital Corporation, effective as of June 22, 2004 |
4.3 | VT French 2004 Equity Incentive Plan |
4.4 | Form of Warrant (May and June 2010 Warrant Financing) |
10.1 | Side Letter Agreement, dated May 28, 2010, between Asia Leechdom Holding Corp.and the investors signatory thereto |
10.2 | Side Letter Agreement, dated June 30, 2010, between Asia Leechdom Holding Corp.and the investors signatory thereto |
10.3 | Settlement Agreement, dated May 25, 2010, among Bay Peak 6 Acquisition Corp., AsiaLeechdom Holding Corp., Tianjin BOAI Pharmaceutical Co., Ltd. and China BOIHunter, LLC |
10.4 | Property Purchase Agreement, dated May 8, 2010, between Tianjin BOAIPharmaceutical Co., Ltd. and Tianjin Qi Shi Leather Co., Ltd. (English Translation) |
10.5 | Sales Agreement, dated January 7, 2010, between Baoding Huida Medicine Co, Ltd. andTianjin Boai Leechdom Technique Co., Ltd. (English Translation) |
10.6 | Product Purchase and Sale Contract, July 1, 2010, between Jinnan Hengfeng WeiyeMedicine Co., Ltd. and Tianjin Boai Leechdom Technique Co., Ltd. (EnglishTranslation) |
10.7 | Technology Transfer Agreement, dated September 30, 2008, between TsinghuaUniversity and Tianjin BOAI Pharmaceutical Co., Ltd. (English Translation) |
10.8 | Supply Agreement, dated January 4, 2010, between Tianjin BOAI Pharmaceutical Co.,Ltd. and Baoding Zhongjing Medicine Co, Ltd. (English Translation) |
10.9 | Supply Agreement, dated January 6, 2010, between Tianjin BOAI Pharmaceutical Co.,Ltd. and Tianjin Pengda Printing Adhesive Products Factory. (English Translation) |
10.10 | Employment Agreement, dated March 25, 2010, between Tianjin BOAI PharmaceuticalCo., Ltd. and Ms. Xuecheng Xia |
10.11 | Employment Agreement, dated October 15, 2010, between Tianjin BOAIPharmaceutical Co., Ltd. and Mr. Shuyuan Chang |
10.12 | Employment Agreement, dated March 25, 2010, between Tianjin BOAI PharmaceuticalCo., Ltd. and Mr. Yansheng Wang |
10.13 | Loan Contract, dated March 15, 2010, between Tianjin BOAI Pharmaceutical Co., Ltd.and Tianjin Rural Cooperative Bank, Zhexing Branch (English Translation) |
10.14 | Loan Contract, dated February 8, 2010, between Tianjin BOAI Pharmaceutical Co., Ltd.and Tianjin Binhai Rural Commercial Bank Ningfa Sub-branch (English Translation) |
10.15 | Loan Contract, dated August 2, 2010, between Tianjin BOAI Pharmaceutical Co., Ltd.and Bank of Tianjin Co., Ltd., Yinlian Sub-branch (English Translation) |
10.16 | Loan Contract, dated May 20, 2010, between Tianjin BOAI Pharmaceutical Co., Ltd.and Tianjin Rural Cooperative Bank, Zhexing Branch (English Translation) |
10.17 | Loan Contract, dated July 12, 2010, between Tianjin BOAI Pharmaceutical Co., Ltd.and Tianjin Rural Cooperative Bank, Zhexing Branch (English Translation) |
10.18 | Loan Contract, dated November 9, 2010, between BOAI Medicine Technology Co., Ltd. and Bank of Tianjin Co., Ltd.Yinlian Sub-branch |
10.19 | Sales Contract, dated January 5, 2010, between Tianjin BOAI Pharmaceutical Co. andBeifang International Holding Tianjin Medicines and Health Products Import & Export Co., Ltd. (English Translation) |
10.20 | Sales Contract, dated July 13, 2010, between Tianjin BOAI Leechdom Technique Co.,Ltd. and Tianjin Tianshili Medicine Company (English Translation) |
10.21 | Sales Agreement, between Tianjin BOAI Leechdom Technique Co., Ltd. and TianjinOffice of Sinopharm Holdings (English Translation) |
10.22 | Sales Agreement, dated January 4, 2010, between Tianjin BOAI Pharmaceutical Co.and Hebei Dongsheng Yinghua Medicine Co, Ltd. (English Translation) |
10.23 | Equity Transfer Agreement, dated July 5, 2007, between Asia Leechdom Holding Corp. (formerly, Keytech Holding Inc.) and Xuecheng Xia (English Translation) |
10.24 | Equity Transfer Agreement, dated July 5, 2007, between Asia Leechdom Holding Corp. (formerly, Keytech Holding Inc.) and Yansheng Wang (English Translation) |
10.25 | Development Agreement, dated June 30, 2007, between Tianjin BOAI Pharmaceutical Co., Ltd. and The Pharmaceutical College of Tianjin University (English Translation) |
10.26 | Construction Agreement, dated June 15, 2009, between Tianjin BOAI Pharmaceutical Co., Ltd. and Tianjin Zhongjin Environmental Technology Co., Ltd. |
10.27 | Financial Advisory Agreement, dated July 1, 2010, between Bay Peak, LLC and Tianjin BOAI Pharmaceutical Co., Ltd. |
10.28 | Equity Transfer Agreements, dated August 10, 2007, between Tianjin Boai Pharmaceutical Co., Ltd. and each of Chao Ma, Xuecheng Xia, Haiwei He and Yansheng Wang(English Translation) |
10.29 | Side Letter Amendment and Waiver, dated January 11, 2011, among Asia Leechdom Holding Corp., Xuecheng Xia, Jianping Lu, and the investor signatory thereto. |
14 | Code of Ethics, adopted February 1, 2011 |
21 | List of Subsidiaries |
24 | Power of Attorney (included on the signature page of this registration statement) |
