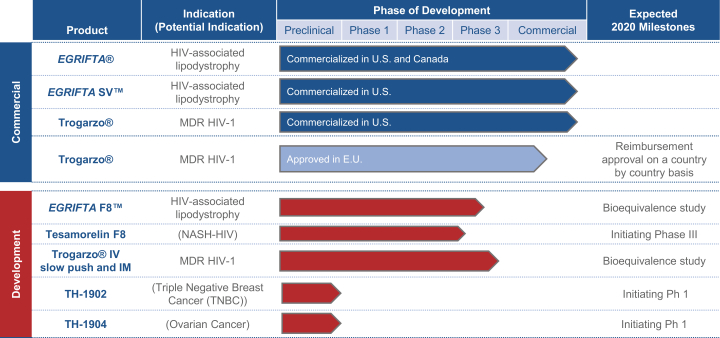
If we are unable to successfully launch EGRIFTA SVTM, sales of this product will not contribute to an increase in our revenues and this would have a material adverse effect on our business, prospect, operating results and financial condition.
Significant safety problems may arise with respect to EGRIFTA SVTM which could result in restrictions in EGRIFTA SVTM’s label, product recall or withdrawal of such product from the market, any of which would materially adversely impact our business and our future business prospects.
New safety issues may arise as EGRIFTA SVTM is used over longer periods of time by a wider group of patients, some of whom may be taking numerous other medicines, or may suffer from additional underlying health problems. Such safety issues could include an increase in the severity or frequency of known problems or the discovery of previously unknown problems, and may result in a variety of adverse regulatory actions. Under U.S. laws, the FDA has broad authority over drug manufacturers to compel any number of actions if safety problems arise, including, but not limited to: (i) requiring manufacturers to conduct post-approval clinical studies to assess known risks or signals of serious risks, or to identify unexpected serious risks; (ii) mandating labeling changes to a product based on new safety information; or (iii) requiring manufacturers to implement a risk evaluation mitigation strategy where necessary to assure safe use of the drug. Previously unknown safety problems could also result in product recalls, restrictions on the product’s permissible use, or withdrawal of the product from the United States. If new safety issues are discovered, sales of EGRIFTA SVTM may decrease and result in a material adverse effect on our business, operating results and financial condition.
The development of a vaccine against HIV or of any cure against HIV would have a material adverse effect on our business, operating results and financial condition.
Although there exists no known vaccine and cure of HIV, we are aware that there are research and development activities carried out in order to eradicate this disease. If a vaccine or a cure was found to prevent or cure HIV, sales of our products would be materially adversely impacted and our revenue growth would be hampered. The discovery of any vaccine or cure against HIV would have a material adverse effect on our business, operating results and financial condition.
The effects of Brexit are still unknown to us and it is difficult to assess how it will affect our commercialization plan for Trogarzo® in the United Kingdom, the cost associated with such commercialization and the potential conduct of clinical trials in this country.
The effects of Brexit will depend in part on the adoption of the proposed agreement the United Kingdom (“UK”) made with the European Union with respect to its access to European Union markets either during a transitional period or permanently. However, based on guidance and publications issued by the Medicines and Healthcare Products Regulatory Agency (“MHRA”), in the event there is no agreement between the UK and the European Union, Trogarzo® would be grandfathered and could be commercialized in the UK. However, we will have to incur various costs to keep this authorization valid in the UK through the filings of various documents with the MHRA. In addition, various requirements regarding the UK residency of individuals and entities carrying out pharmacovigilance activities, batch analysis, release of batches, and other similar functions would force us to contract with additional suppliers. We may not be able to negotiate the terms and conditions of such contracts to our advantage or enter into any contract at all. Under both circumstances, our management team will have to spend time not otherwise spent on other projects. Overall, we will incur additional costs that may adversely impact our business, operating results and financial condition.
In addition, there exists uncertainty regarding the acceptability by the MHRA of results obtained from the conduct of clinical trials in European Union’s countries if no UK patients are included in those clinical trials. We are not certain whether clinical trials will need to include patients residing in the UK in order to seek the approval of a product in the UK. If we need to enroll UK patients in our clinical trials in order to be able to present our results to the MHRA if we decide to seek approval in the UK, this may delay the conduct of our clinical trials and require more financial resources, both of which could have a material adverse effect on our business, operating results and financial condition.
We currently obtain over 95% of our revenues from the sale of our products in the United States from one client which also acts as our warehouser and exclusive distributor of our products in this territory. Any material adverse issue such client may incur in connection with the operation of its business may materially adversely affect our business, operating results and financial condition.
Sales of EGRIFTA® and Trogarzo® in the United States are made exclusively by RxCrossroads which also acts as our third-party logistic warehouser and the exclusive distributor of our products in the United States. RxCrossroads purchases our products based on orders it receives from wholesalers or certain specialty pharmacies we have agreements with. Sales of EGRIFTA SVTM will also be handled by RxCrossroads.
We do not have state licensure in the United States to distribute EGRIFTA®, Trogarzo® and EGRIFTA SVTM or any other product we may acquire or in-license and we do not currently intend to pursue applications to obtain the licenses required in order to distribute a drug product in the United States. Our supply chain model is based upon that fact and the distribution of our products in the United States is done through RxCrossroads which currently holds all state licensure required to distribute a drug product in every American state. Although potential alternative third-party service providers have been identified to replace RxCrossroads in the event that it becomes unable to distribute our products, we have not entered into any agreements with them and no assurance can be given that such providers would enter into any agreement with us on terms satisfactory to us.
Our reliance on RxCrossroads as our sole client and our sole distributor could be detrimental to our business, operating results and financial condition if RxCrossroads becomes unable to perform its services under the terms of our agreement in the United States if, for example, the following events were to occur:
| | • | | Violation of laws by RxCrossroads in connection with its warehousing or distribution activities which could entail that RxCrossroads could suspend those activities until it regains compliance with such laws; |
| | • | | Loss or non-renewal in due time by RxCrossroads of state licensure; |
| | • | | Damages to RxCrossroads facilities and/or its operations due to a natural disaster such as flooding, hurricanes, tornados, power supply failure, fire or earthquake; |
| | • | | Corporate restructuring or insolvency of RxCrossroads; or |
| | • | | Material disagreements between us and RxCrossroads on the terms and conditions of our agreement. |
If securities or industry analysts do not publish research or reports, or publish unfavorable research or reports about our business, the price of our common shares and trading volume may decline.
The trading market for our Common Shares will rely in part on the research and reports that industry or financial analysts publish about us, our business, our markets and our competitors. We do not control these analysts. If securities analysts do not cover our Common Shares, the lack of research coverage may adversely affect the market price of our Common Shares. Furthermore, if one or more of the analysts who do cover us downgrade our Common Shares or if those analysts issue other unfavorable commentary about us or our business, the price of our Common Shares would likely decline. If one or more of these analysts cease coverage of us or fails to regularly publish reports on us, we could lose visibility in the market and interest in our Common Shares could decrease, which in turn could cause our share price or trading volume to decline and may also impair our ability to expand our business with existing customers and attract new customers.
10