
Investor Day | September 15, 2016

Welcome Jay Shepard | Chief Executive Officer

Safe Harbor This presentation and the accompanying oral commentary contain forward-looking statements for purposes of the safe harbor provisions of the Private Securities Litigation Reform Act of 1995. Forward-looking statements are not guarantees of future performance and are subject to risks and uncertainties that could cause actual results and events to differ materially from those anticipated, including, but not limited to, risks and uncertainties related to: our success being heavily dependent on somavaratan; the risk that somavaratan may not have favorable results in clinical trials or receive regulatory approval; potential delays in our clinical trials due to regulatory requirements or difficulty identifying qualified investigators or enrolling patients; the risk that somavaratan may have properties that delay or prevent regulatory approval or limit its commercial potential; the risk that we may encounter difficulties in manufacturing somavaratan; if somavaratan is approved, risks associated with its market acceptance, including pricing and reimbursement; potential difficulties enforcing our intellectual property rights; and our need for additional funds to support our operations. Forward-looking statements involve known and unknown risks, uncertainties, assumptions and other factors that may cause our actual results, performance or achievements to be materially different from any future results, performance or achievements expressed or implied by the forward-looking statements. We discuss many of these risks in greater detail under the heading "Risk Factors" contained in our Annual Report on Form 10-K for the year ended December 31, 2015 and in our Quarterly Report on Form 10‐Q for the three months ended June 30, 2016, which are on file with the Securities and Exchange Commission. Forward-looking statements are not guarantees of future performance, and our actual results of operations, financial condition and liquidity, and the development of the industry in which we operate, may differ materially from the forward-looking statements contained in this contained in this presentation and the accompanying oral commentary. Any forward-looking statements that we make in this presentation and the accompanying oral commentary speak only as of the date of this presentation. We assume no obligation to update our forward-looking statements whether as a result of new information, future events or otherwise.

Endocrine-Focused Biopharmaceutical Company Developing Somavaratan, a Novel, Long-Acting form of Recombinant Human Growth Hormone to Treat the Orphan Disease of Growth Hormone Deficiency

Today’s Agenda 8:00 am Welcome, Vision & Strategy Jay Shepard Chief Executive Officer, Versartis, Inc. Introductions: PGHD Speakers Colin Hislop, MD Chief Medical Officer, Versartis, Inc. Pediatric Growth Hormone Deficiency: A 50 Year Perspective Alan Rogol, MD, PhD Professor Emeritus, Pediatrics and Pharmacology University of Virginia School of Medicine Somavaratan in Pediatric Growth Hormone Deficiency Michael Stalvey, MD Assistant Professor & Fellowship Program Director, Department of Pediatrics, University of Alabama Birmingham School of Medicine PGHD R&D Program Colin Hislop, MD PGHD Panel Q&A Session 1 Moderator: Josh Brumm Chief Financial Officer, Versartis, Inc. 9:30 am AGHD Speaker Introduction Colin Hislop, MD Adult Growth Hormone Deficiency (AGHD) and Long-acting Growth Hormone (LAGH) for Adults Beverly M. K. Biller, MD, FACP Physician in Medicine, Neuroendocrine Unit, Mass General Hospital & Professor of Medicine at Harvard Medical School AGHD R&D Program Colin Hislop, MD AGHD Panel Q&A Session 2 Moderator: Josh Brumm 10:15 am Final Remarks Jay Shepard

Introducing the Versartis Team: Significant Expertise in GHD Manufacturing, Development & Commercialization Jay Shepard President & CEO >33 Years Pharmaceutical, Biotech, Drug Delivery Expertise Commercialization of Specialty Products, M&A Expertise Joshua Brumm CFO Led Successful IPO and Follow on Offering Leadership Role in Finance and 2 Approved Products Colin Hislop, MD CMO Numerous In-License and Out-License Deals for Development Compounds; Purchase of CV Therapeutics by Gilead for $1.4B Multiple Successful Regulatory Interactions from Pre-IND to Advisory Committee Meetings for Approval Paul Westberg SVP, Corporate Dev Numerous In-License/Out-License Deals; >$350M GNE Deal Private Equity Financings Totaling >$200M & NOVC IPO Bert Bakker, MD, PhD SVP, Medical Affairs 15 Years of Industry Experience Covers both Medical Affairs and Drug Development; >25 Years Pediatric Endocrinology Experience Clinical Lead for Previous Sustained Release rhGH product Mike Burdick SVP, Regulatory Affairs >30 Years Development & LCM Experience (>20 approved products) Supported Genotropin sNDA and orphan drug applications for SGA and Prader-Willi Syndrome Keith Lui VP, Marketing Led Multiple Successful Pharmaceutical Product Launches Commercial Build–Out and Organizational Leadership Experience Patrick Murphy VP, Manufacturing >35 Years of Manufacturing Experience (10 Approved Products) Developed Processes & Plants for Numerous Biologics Development and Launch (Long Acting) Clinical Development Manufacturing rhGH Expertise

2016: Achieving Our Objectives PGHD AGHD Activities Supporting BLA Filing & Launch Readiness Complete enrollment PGHD Phase 3 VELOCITY trial-mid 2016 ANNOUNCED August 22 (n=137) Complete Phase 2 portion of J14vr5 Japan study, move to Phase 3 Phase 2 enrolled; Phase 3 initiation on track Q4 2016 Complete VITAL Phase 2; identify dose/schedule for Phase 3 Enrollment complete Top line results: Phase 3 starting dose, schedule & population identified Dialogue with FDA to ensure clarity on clinical/CMC/device expectations for regulatory submission Meetings/correspondence during Q2-Q3 2016 with CMC, device and clinical teams within FDA: Ongoing CMC/manufacturing preparations Boehringer Ingelheim manufacturing to commercial scale Auto injector developed; Owen Mumford manufacturing/supply agreement announced Defining commercial strategy Signed strategic alliance w/Teijin for Japan Evaluating EU and US strategy Expanding team and medical affairs activities Colin Hislop, MD appointed as CMO Full journal publication of Phase 2a PGHD results in JCEM 24 month data at ENDO 2016 Safety, efficacy & adherence data (≤30 mo.) at ICE & ESPE 2016 Full KOL & Medical Education plan in place

2017-2018 Strategic Objectives Complete Phase 3 VELOCITY trial J14vr5 Phase 3 trial in Japan Initiate AGHD Phase 3 Continued KOL development Device & CMC validation Prepare for US/EU regulatory submissions Continue growing organization & executing on plan Commercial strategy & launch preparation Expected BLA & MAA filings Preparations for potential early 2019 PGHD launch Product lifecycle management Execute AGHD Phase 3 [image placeholder] 2018 2017
Somavaratan’s Data to Date Focus For Today’s Program Market Expectations Commercial Readiness Somavaratan continues to demonstrate a strong safety and efficacy profile as seen in 24-month data VISTA study patients starting 4th year of Treatment on Somavaratan Evidence that Somavaratan is working in patients Somavaratan is well positioned to address market Expectations, context for a “successful” VELOCITY data outcome in Q3’17 Market research and KOL perspectives Clinical program designed to support commercial success Robust data package for regulatory filings Auto-injector device ready at launch CMC/manufacturing at commercial scale Teijin alliance for Japan Commercial Preparation Market Needs & Expectations Data to Date
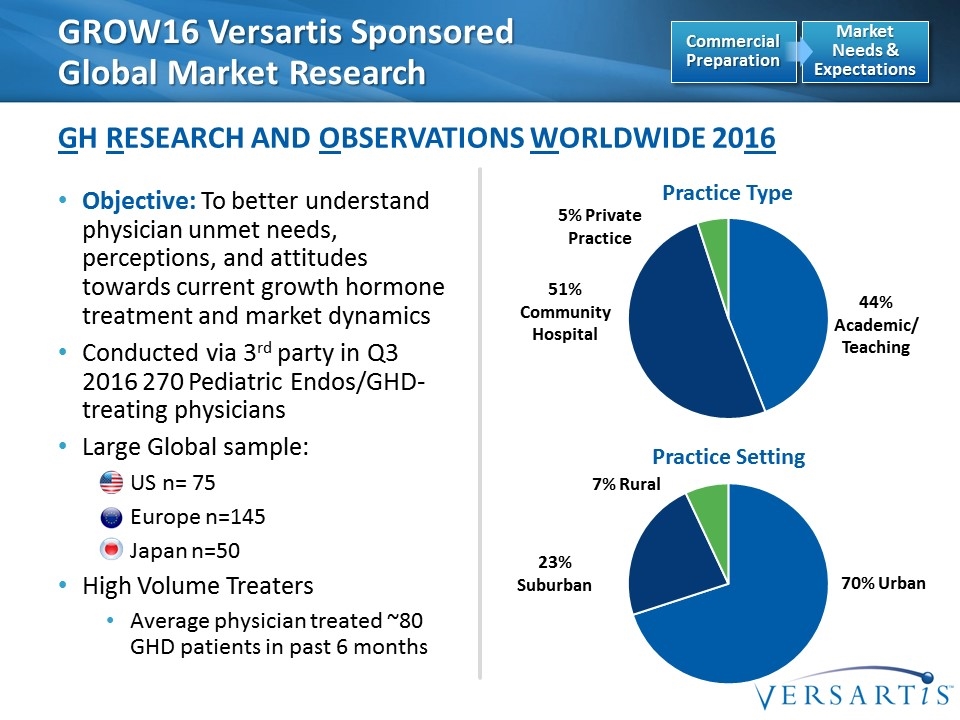
GROW16 Versartis Sponsored Global Market Research GH Research and Observations Worldwide 2016 Market Needs & Expectations Commercial Preparation Objective: To better understand physician unmet needs, perceptions, and attitudes towards current growth hormone treatment and market dynamics Conducted via 3rd party in Q3 2016 270 Pediatric Endos/GHD-treating physicians Large Global sample: US n= 75 Europe n=145 Japan n=50 High Volume Treaters Average physician treated ~80 GHD patients in past 6 months Practice Type Practice Setting 70% Urban 23% Suburban 7% Rural 44% Academic/ Teaching 5% Private Practice 51% Community Hospital
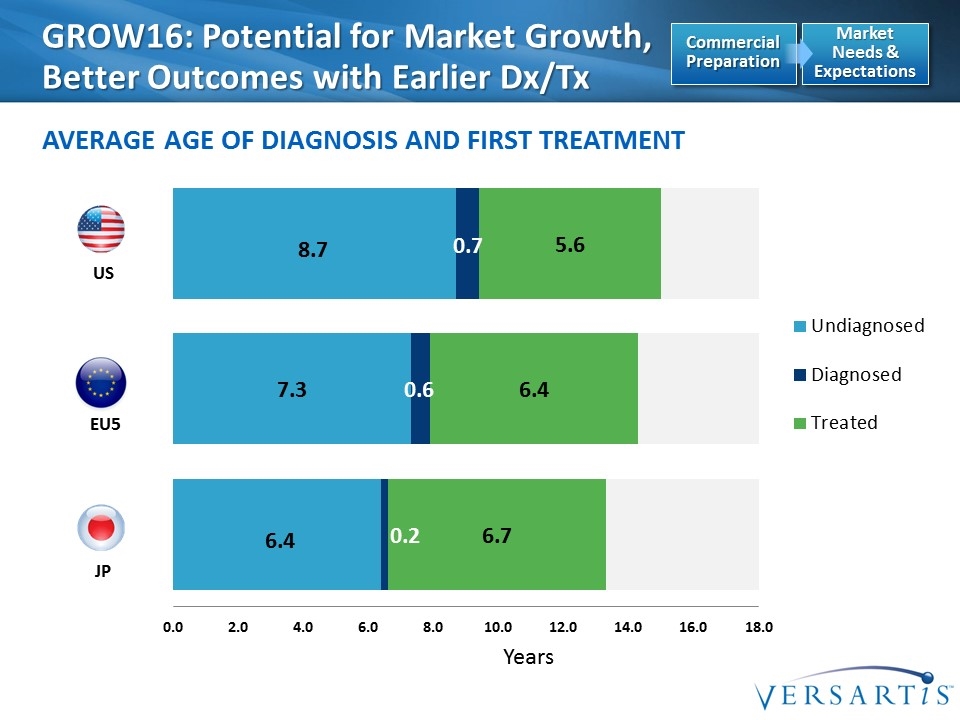
Years EU5 US JP GROW16: Potential for Market Growth, Better Outcomes with Earlier Dx/Tx Average Age of Diagnosis and First Treatment Market Needs & Expectations Commercial Preparation
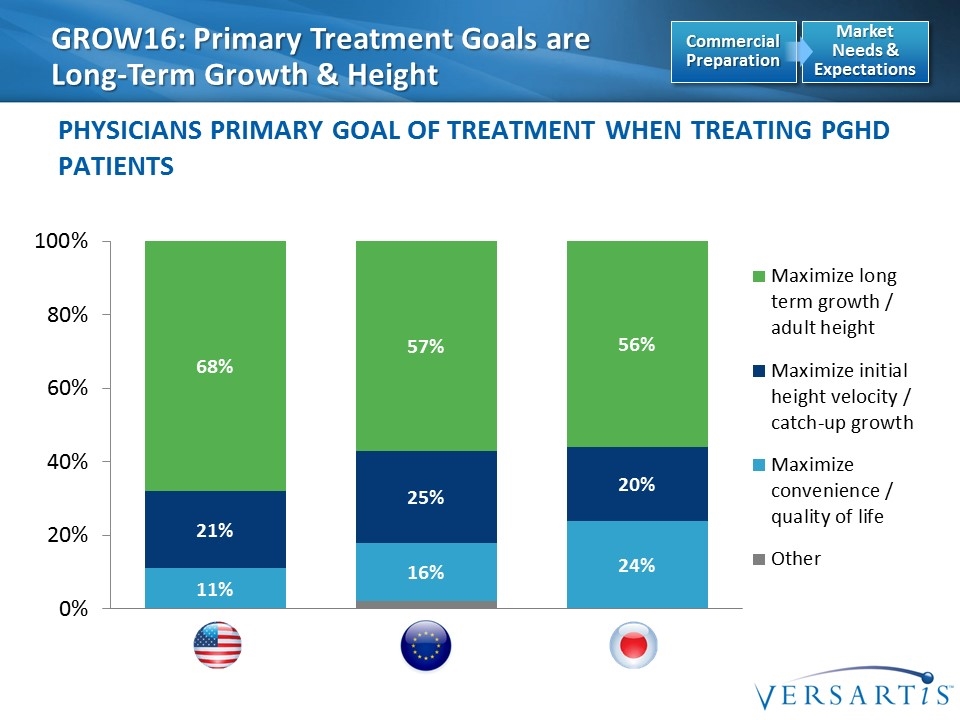
GROW16: Primary Treatment Goals are Long-Term Growth & Height Physicians Primary Goal of Treatment when Treating PGHD Patients EU5 (B) US (A) JP (C) Market Needs & Expectations Commercial Preparation
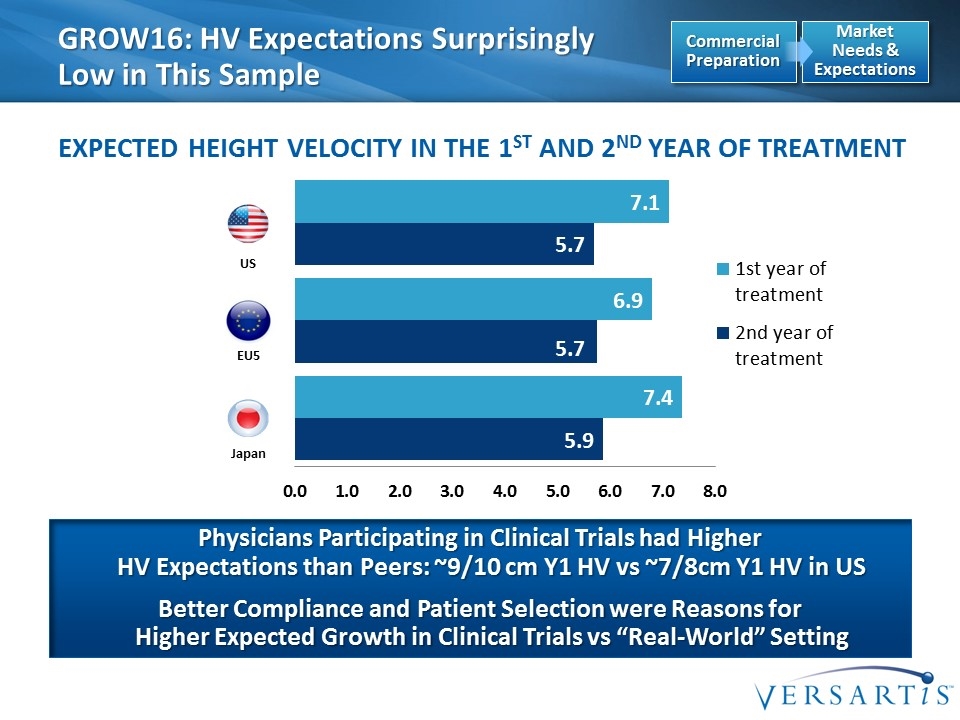
EU5 US Japan GROW16: HV Expectations Surprisingly Low in This Sample Expected Height Velocity in the 1st and 2nd Year of Treatment Physicians Participating in Clinical Trials had Higher HV Expectations than Peers: ~9/10 cm Y1 HV vs ~7/8cm Y1 HV in US Better Compliance and Patient Selection were Reasons for Higher Expected Growth in Clinical Trials vs “Real-World” Setting Market Needs & Expectations Commercial Preparation
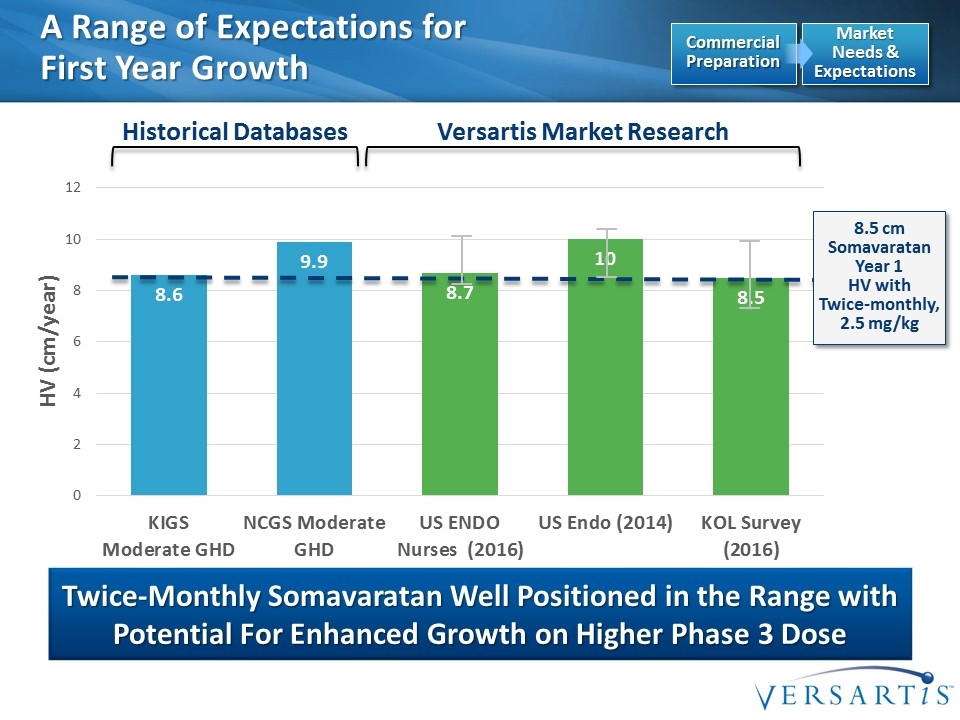
A Range of Expectations for First Year Growth Twice-Monthly Somavaratan Well Positioned in the Range with Potential For Enhanced Growth on Higher Phase 3 Dose Historical Databases Versartis Market Research Market Needs & Expectations Commercial Preparation 8.5 cm Somavaratan Year 1 HV with Twice-monthly, 2.5 mg/kg
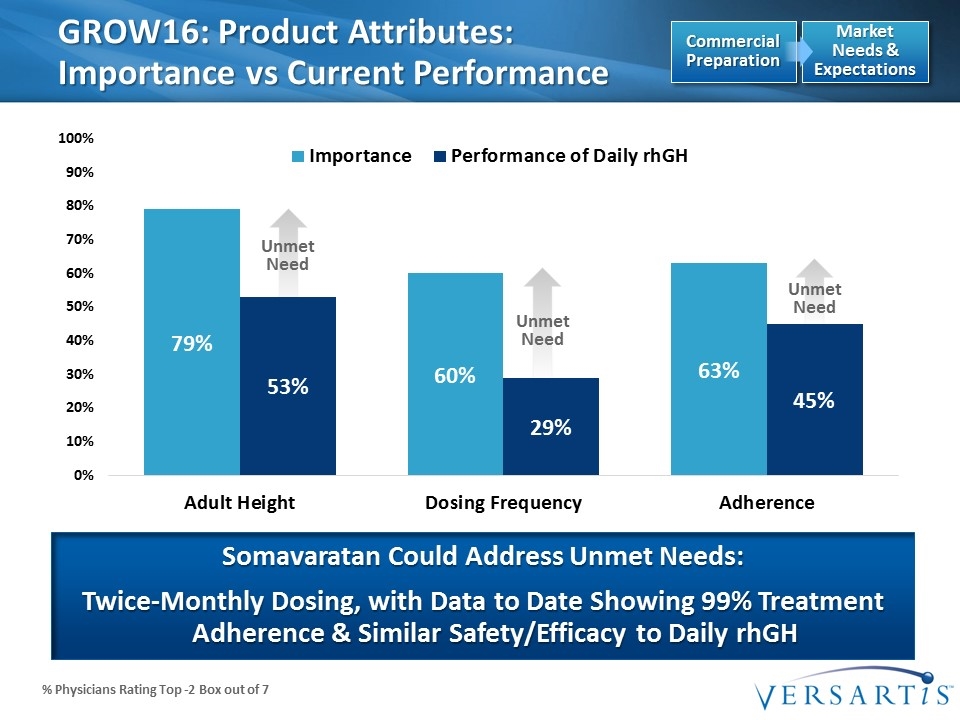
Unmet Need Unmet Need Unmet Need % Physicians Rating Top -2 Box out of 7 GROW16: Product Attributes: Importance vs Current Performance Somavaratan Could Address Unmet Needs: Twice-Monthly Dosing, with Data to Date Showing 99% Treatment Adherence & Similar Safety/Efficacy to Daily rhGH Market Needs & Expectations Commercial Preparation

Manufacturing and Supply Agreement with Owen Mumford Over six decades of experience in developing medical device solutions for own brands & for pharmaceutical/diagnostic companies including Johnson & Johnson, Janssen, Novartis, Bayer, Merck Sorono and others (one of the top 3 in the world) Manufactures proprietary disposable auto-injector device exclusively for Versartis Assembles & supplies final combination product which includes the device and somavaratan Proven Global Leader in Medical Device Design and Manufacturing Auto-Injector Commercial Preparation Versartis provides prefilled syringes for incorporating into device
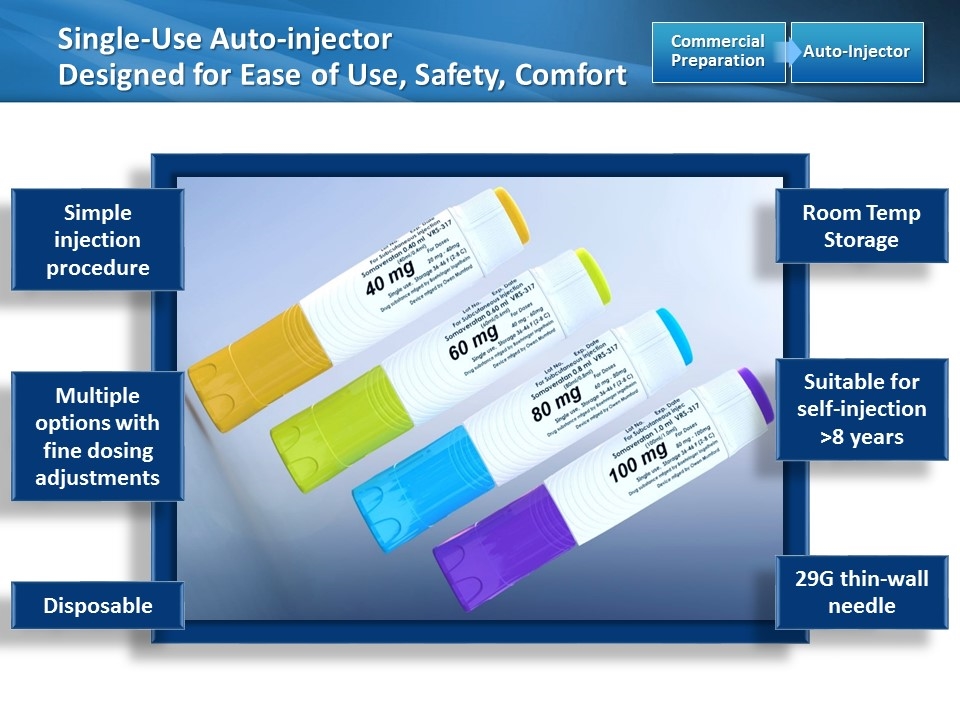
Single-Use Auto-injector Designed for Ease of Use, Safety, Comfort Auto-Injector Commercial Preparation [image placeholder] Simple injection procedure Suitable for self-injection >8 years Room Temp Storage Disposable 29G thin-wall needle Multiple options with fine dosing adjustments

Versartis and Teijin Establish Strategic Alliance in Japan Teijin receives exclusive license to commercialize & further develop somavaratan in Japan Versartis to receive $40 million upfront, and up to $125 million in potential milestones: Development milestone of $35 million Regulatory milestones up to $55 million Sales milestones up to $35 million As exclusive supplier of somavaratan to Teijin, Versartis to receive attractive transfer pricing and a royalty calculated on net sales in Japan Versartis responsible for completing & funding ongoing J14VR5 Ph2/3 Japan trial and the ongoing pediatric VELOCITY & adult VITAL trials outside of Japan Teijin responsible for executing and funding regulatory and commercialization activities in all indications within Japan Teijin’S THERAPEUTIC FOCUS AND Regulatory/Commercial Infrastructure ideally Suited to capitalize on Somavaratan & its lead as the 1st Potential LAGH to enter Japanese market Japan Commercial Preparation

Teijin Transaction is a Strong Strategic Fit 6th largest Japanese company in Pharmaceutical sales Licensing partnerships with Merck, Ipsen, Boehringer Ingelheim, Takeda & Sanofi Highly Successful commercial track record in very competitive product areas of ENDOCRINE & METABOLIC DISEASES Existing franchise supporting Somatuline® (lanreotide) commercialization in acromegaly & potential approval in NET Teijin’s #1 selling product is Feburic for gout and hyperuricemia; #2 is Bonalon (Fosamax) in osteoporosis 4 endocrine/metabolic candidates in P1-3 trials Strong potential to grow current $600M market for rhGH in Japan and capture significant share Validation of science and safety/efficacy data behind somavaratan JAPAN Japan Commercial Preparation

Summary Data to Date suggest somavaratan is working Executing on the plan Somavaratan is well positioned to address market Clinical program designed to support commercial success


Introductions: PGHD Speakers Colin Hislop, MD | Chief Medical Officer

Alan D. Rogol, MD, PhD Dr. Rogol is Professor Emeritus, Pediatrics and Pharmacology, at the University of Virginia School of Medicine. He received his doctorates in medicine and physiology (Endocrinology) from Duke University and completed a residency in pediatrics at the Johns Hopkins Hospital. Following a fellowship in endocrinology at the National Institutes of Health, he moved to the University of Virginia, ultimately serving as Professor of Pediatrics and Pharmacology and Chief of the Division of Endocrinology In recent years, Dr. Rogol has spent time in the pharmaceutical industry and as a safety monitor, but has continued to practice pediatric endocrinology and teach at UVA and Riley Hospital for Children in Indianapolis Dr. Rogol has been a member and Chief of the American Board of Pediatrics’ sub-board in endocrinology, served as Secretary of the Pediatric Endocrine Society and also as Vice-President of The Endocrine Society. He is a Fellow of the American Academy of Pediatrics and the American College of Sports Medicine and a member of the American Pediatric Society. His interests in endocrinology have included growth and puberty as well as the endocrinology of sport and exercise. Dr. Rogol has published more than 500 scientific articles and is a member of several editorial boards

Michael Stalvey, MD Dr. Michael Stalvey is an Assistant Professor and Fellowship Program Director in the Department of Pediatrics at the University of Alabama at Birmingham, School of Medicine. He received his M.D. from the University of Florida College of Medicine, conducted his residency there in the Department of Pediatrics and then completed a fellowship in the Division of Pediatric Endocrinology. His clinical and research interests focus on endocrine disorders, particularly growth, diabetes, and bone disease as they relate to cystic fibrosis. He currently leads a research and clinical care effort at the University of Alabama at Birmingham focusing on CF endocrine disorders, and recently studied the effect of therapeutically correcting the underlying CF defect and its impact on linear growth in children. UAB is one of only a few centers worldwide studying CF bone metabolism, and the vanguard in the study of the mechanisms of poor growth in CF Dr. Stalvey serves as the endocrine representative to the Translational Advisory Group for the Cystic Fibrosis Foundation Therapeutics Development Network. He has published and presented extensively on endocrine disorders and CF, including invited symposium presentations at the North American Cystic Fibrosis Conference and the Pediatric Endocrine Society Meeting

Pediatric Growth Hormone Deficiency A 50 Year Perspective Alan D. Rogol, MD, PhD Professor, Emeritus University of Virginia Charlottesville, VA USA
Growth Hormone (GH) 191 amino acid single chain peptide consisting of several isoforms with the 22 kDa for the most prominent Regulates production of IGF-I Promotes skeletal growth

Growth Hormone (GH) Metabolic actions Rapid and large increase in REE Increased fat oxidation Increased protein and glucose synthesis Anabolic, lipolytic and weakly natriuretic Increases muscle mass, bone formation and total body water Decreases fat mass

Which Children Require Evaluation for GH Deficiency? Growth failure suggested by a height- for-age curve has deviated downwards across two major height percentile curves eg, from above the 25th percentile to below the 10th percentile Or if the child is growing slower than the following rates: Age two to four years: HV less than 5.5 cm/year (<2.2 inches/year) Age four to six years: HV less than 5 cm/year (<2 inches/year) Age six years to puberty: HV less than 4 cm/year for boys (<1.6 inches/year) HV less than 4.5 cm/year for girls (<1.8 inches/year)
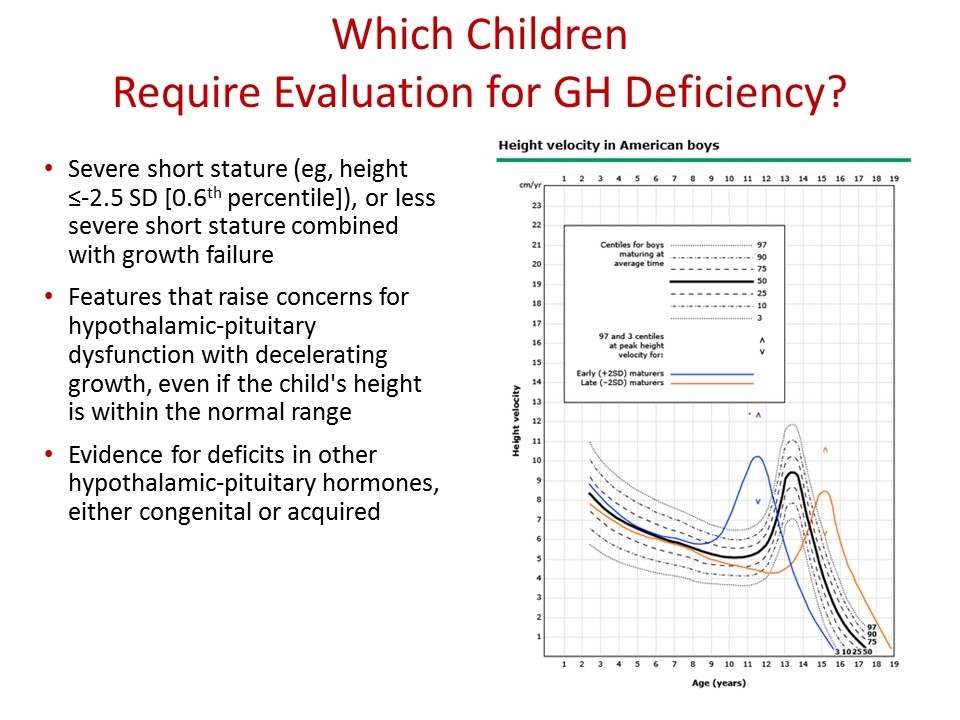
Which Children Require Evaluation for GH Deficiency? Severe short stature (eg, height ≤-2.5 SD [0.6th percentile]), or less severe short stature combined with growth failure Features that raise concerns for hypothalamic-pituitary dysfunction with decelerating growth, even if the child's height is within the normal range Evidence for deficits in other hypothalamic-pituitary hormones, either congenital or acquired

GH Therapy for Childhood GH Deficiency Current Daily rhGH Treatment Approach in the US Routine administration by daily injection Administered subcutaneously Nocturnal administration Storage considerations Relatively benign safety profile Great variety in injection devices Injection practices determine treatment adherence Treatment with rhGH should be started at the youngest possible age, to achieve the best growth response US Starting dose: ~40 mcg/kg/day, given subcutaneously once daily ~20 mcg/kg/day for patients with severe GHD

GH Therapy for Childhood GH Deficiency Daily administration remains inconvenient: Painful Distressing to some patients May lead to: non-compliance Reduced efficacy Increased health-care costs
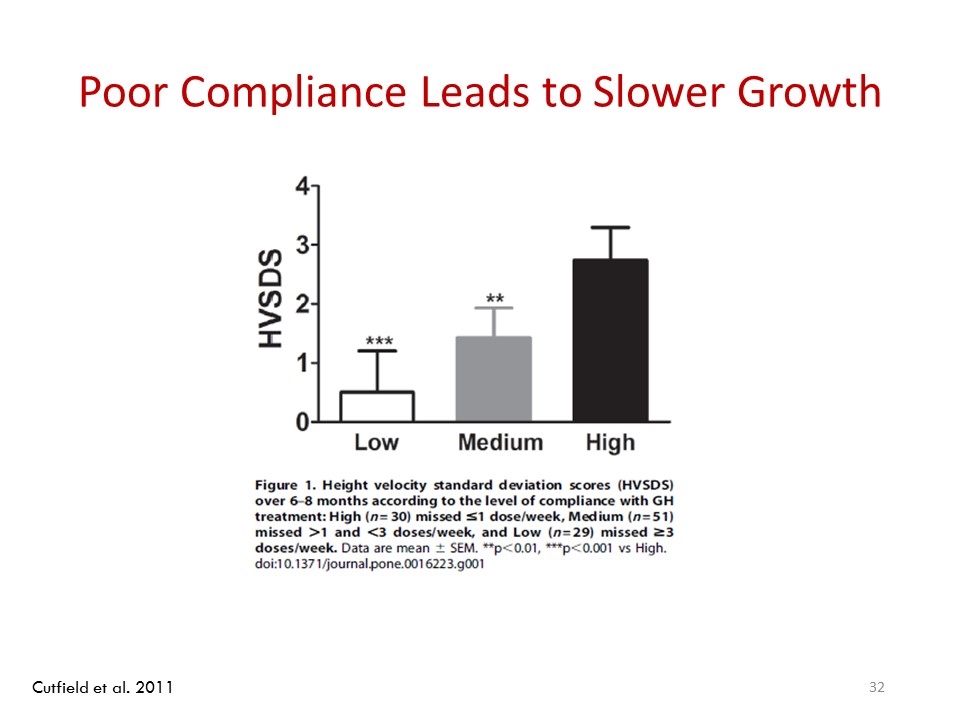
Poor Compliance Leads to Slower Growth Cutfield et al. 2011
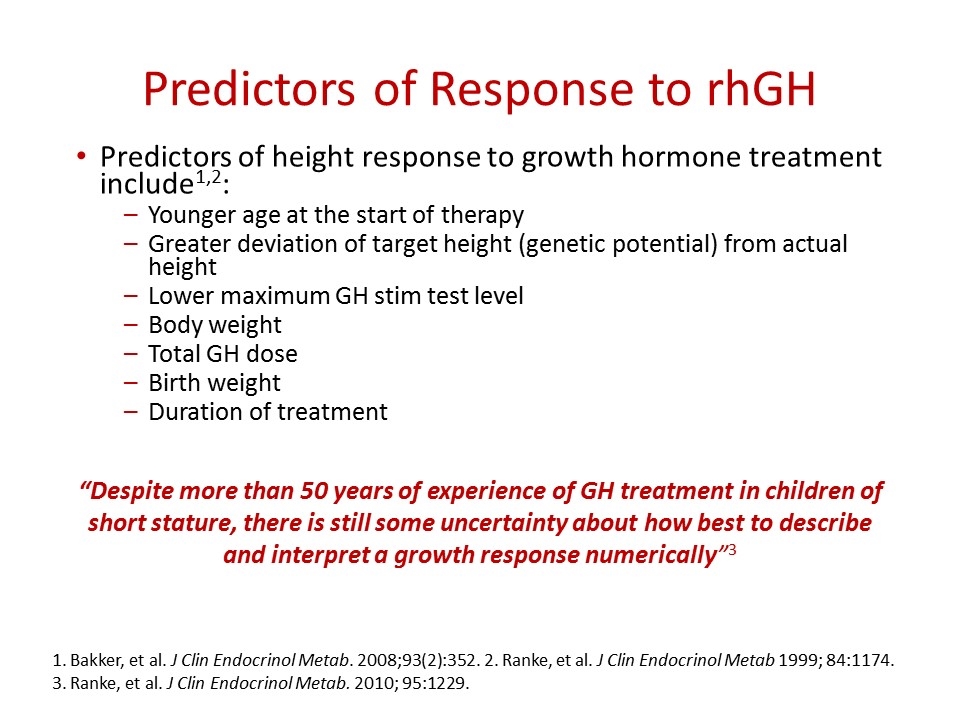
Predictors of Response to rhGH Predictors of height response to growth hormone treatment include1,2: Younger age at the start of therapy Greater deviation of target height (genetic potential) from actual height Lower maximum GH stim test level Body weight Total GH dose Birth weight Duration of treatment 1. Bakker, et al. J Clin Endocrinol Metab. 2008;93(2):352. 2. Ranke, et al. J Clin Endocrinol Metab 1999; 84:1174. 3. Ranke, et al. J Clin Endocrinol Metab. 2010; 95:1229. “Despite more than 50 years of experience of GH treatment in children of short stature, there is still some uncertainty about how best to describe and interpret a growth response numerically”3

Predictors of Response to GH Therapy Factors predicting response to GH are derived from two very large databases: KIGS: Pfizer International Growth Database (previously known as the Kabi International Growth Study)1 NCGS: National Childhood Growth Study in North America2 Together, these factors predicted 61% of the variance in growth response during the first year of treatment For the 2nd, 3rd and 4th years of treatment, positive predictors of growth response were HV during the previous year, body weight, younger age, and GH dose1 1. Ranke, et al. J Clin Endocrinol Metab 1999; 84:1174. 2. Blethen, et al. J Clin Endocrinol Metab 1993; 76:574. 3. Zadik, et al. Pediatrics 2005; 116:68.

Expected Response to GH Therapy When given for the treatment of growth hormone deficiency, growth hormone therapy is clearly effective If GH is administered at an early age, patients can achieve adult height within the midparental target height range1,2 Prediction of adult height as a function of the dose has proven impossible Variations in treatment regimens Response demonstrated in large studies is not linear (ie, the increase in growth rate is not proportional to the increase in dose)3 Adherence is a factor in non-response 1. Root, et al. J Pediatr Endocrinol Metab 1998; 11:403. 2. Reiter, et al. J Clin Endocrinol Metab 2006; 91:2047. 3. Frasier, et al. J Clin Endocrinol Metab 1981; 53:1213.

Defining “Good” Response HV should be monitored to determine whether the growth response is adequate Growth should be compared with curves generated from a population of similarly treated children with growth hormone deficiency Target HV >75th percentile curve or >1 SD above the mean HV HV can also be compared with curves showing normal height velocity for age in non GHD children Much less accurate
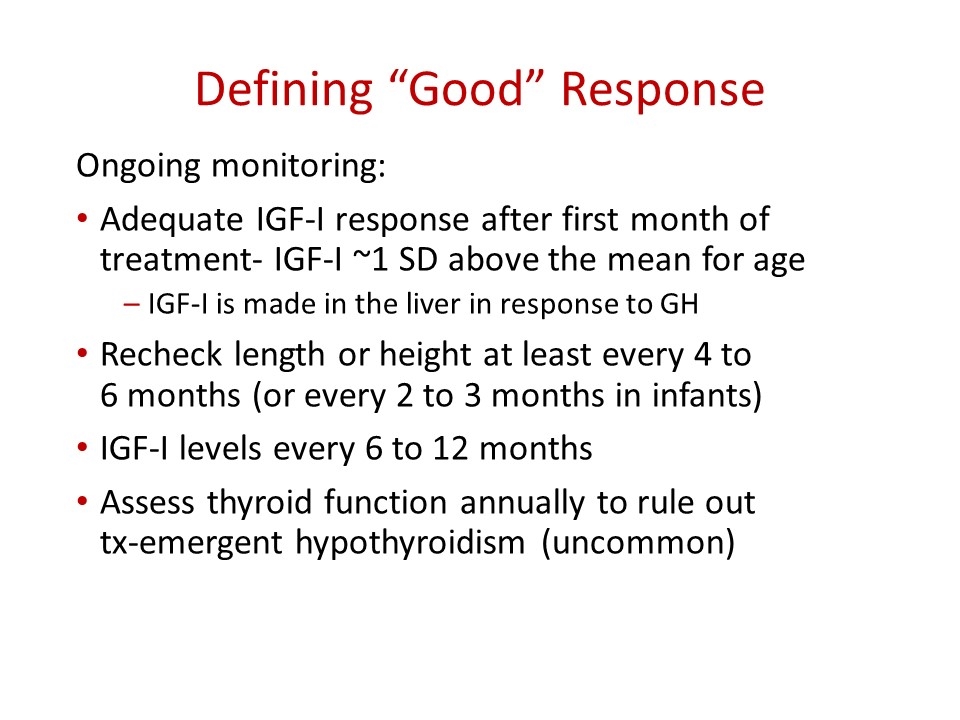
Defining “Good” Response Ongoing monitoring: Adequate IGF-I response after first month of treatment- IGF-I ~1 SD above the mean for age IGF-I is made in the liver in response to GH Recheck length or height at least every 4 to 6 months (or every 2 to 3 months in infants) IGF-I levels every 6 to 12 months Assess thyroid function annually to rule out tx-emergent hypothyroidism (uncommon)
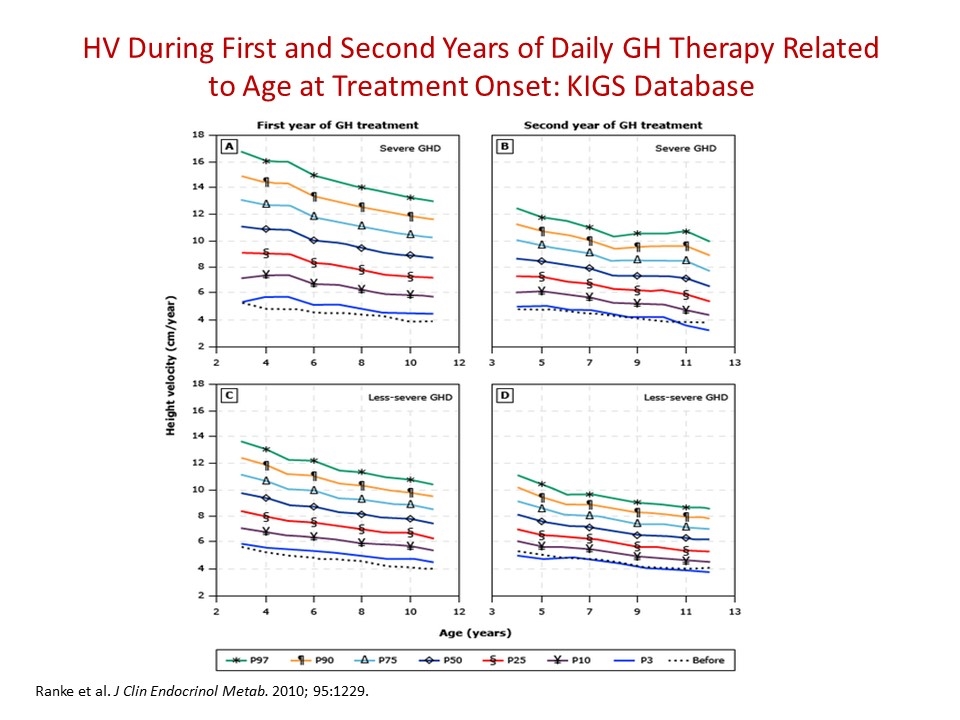
HV During First and Second Years of Daily GH Therapy Related to Age at Treatment Onset: KIGS Database Ranke et al. J Clin Endocrinol Metab. 2010; 95:1229.
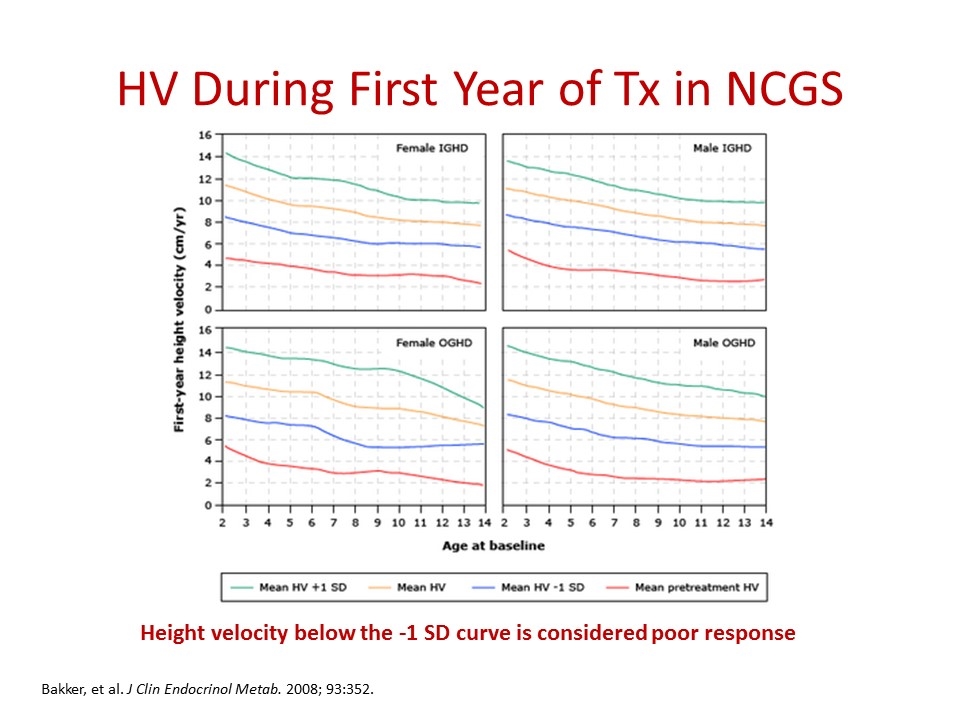
HV During First Year of Tx in NCGS Height velocity below the -1 SD curve is considered poor response Bakker, et al. J Clin Endocrinol Metab. 2008; 93:352.

Treatment Goals: HV & Adult Height First year HV matters: Make as normal as possible “now” Most important for the smallest children The end goal is adult height An estimate of a child's genetic height potential can be obtained by calculation of the midparental height Based upon the heights of both parents and adjusted for the sex of the child This estimate is also known as the "target height"

Long-acting Endocrine Agents Daily GH does not mimic physiologic growth hormone secretion and it works Does this matter? Formulations for endocrine-active agents have long been part of the therapeutic armamentarium of endocrinologists GnRH agonist analogs Testosterone Medroxyprogesterone acetate hCG (as long acting LH) Multiple analogs of insulin

Long-acting Growth Hormone What are we looking for from a Long-Acting form of GH? Safety considerations No substantial differences in clinical effects when compared to daily administration Adherence/Compliance

Growth Hormone Research Society Perspective on the Development of Long-Acting GH There is a clinical need for a safe and effective long-acting growth hormone treatment for which improved treatment adherence is attainable1 Development of a long-acting form of rhGH with long-term effectiveness may potentially reduce treatment burden, improve adherence issues, and improve overall treatment outcomes1 Christiansen, et al. Eur J Endocrinol. 2016 Jun;174(6).

Investor Day | September 15, 2016

Somavaratan (VRS-317) Treatment for Pediatric Growth Hormone Deficiency (GHD): VISTA (13VR3) Study Results Michael Stalvey, MD Assistant Professor, Fellowship Program Director Department of Pediatrics, Division of Pediatric Endocrinology University of Alabama at Birmingham

Growth Hormone Research Society Consensus Statement1 “Long-acting growth hormone compounds may represent an advance over daily GH injections because of increased convenience and differing pharmacodynamic properties, providing the potential for improved adherence and outcomes.” Growth Hormone Research Society – 2016 1. Christiansen et al. Eur J Endocrinol. 174;C1-C8
Recombinant Human Growth Hormone (rhGH) for Treatment of Pediatric Growth Hormone Deficiency (GHD) Therapeutic potential of daily rhGH is well established and has been the primary treatment for pediatric GHD for three decades1,2 Current challenges with daily rhGH preparations include burden of daily subcutaneous (SC) injections3 Noncompliance has been reported in up to 77% of adults and children with GHD4,5 Reduced efficacy (decreasing HV-SDS) is significantly associated with number of missed doses per week3,5,6 HV-SDS, height velocity-standard deviation score 1. Ergun-Longmire and Wajnrajch. Endotext. http://www.ncbi.nlm.nih.gov/books/NBK279142; 2. Ranke et al. J Clin Endocrinol Metabol. 2005;90:1966; 3. Saenger and Mejia-Corletto. Adv Ther Ped Endocrinol Diabetes. 2016;30:79-97; 4. Rosenfeld and Bakker. Endocr Pract. 2008;14(2)143-54; 5. Cutfield et al. PloS One. 2011;6(1):e16223. 6. De Pedro et al. Growth Hormone & IGF Research.2016; 26:32-35

Challenges With Adherence to Daily Growth Hormone Treatment Children with growth hormone deficiency (GHD) are often treated for multiple years with daily injections of recombinant human growth hormone (rhGH) replacement therapy, currently the only treatment available in North America and Europe 7 years old How long will we need to treat? The majority of growth will occur up till about 16 years of age. In GHD this may be longer. 16 years (typical target) -7 years (current age) +2 years (delayed bone age) Treatment Duration: 11 years 11 years x 1 shot per day x 365 days/yr Total number of injections: 4015 injections
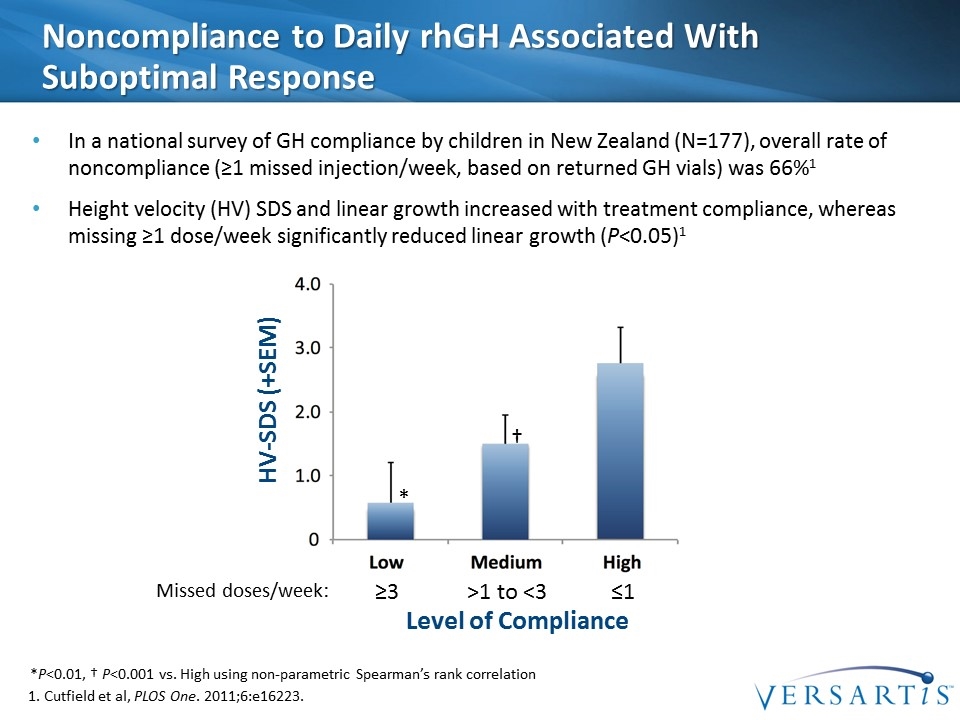
Noncompliance to Daily rhGH Associated With Suboptimal Response In a national survey of GH compliance by children in New Zealand (N=177), overall rate of noncompliance (≥1 missed injection/week, based on returned GH vials) was 66%1 Height velocity (HV) SDS and linear growth increased with treatment compliance, whereas missing ≥1 dose/week significantly reduced linear growth (P<0.05)1 *P<0.01, † P<0.001 vs. High using non-parametric Spearman’s rank correlation HV-SDS (+SEM) Level of Compliance ≥3 ≤1 >1 to <3 Missed doses/week: * † 1. Cutfield et al, PLOS One. 2011;6:e16223.
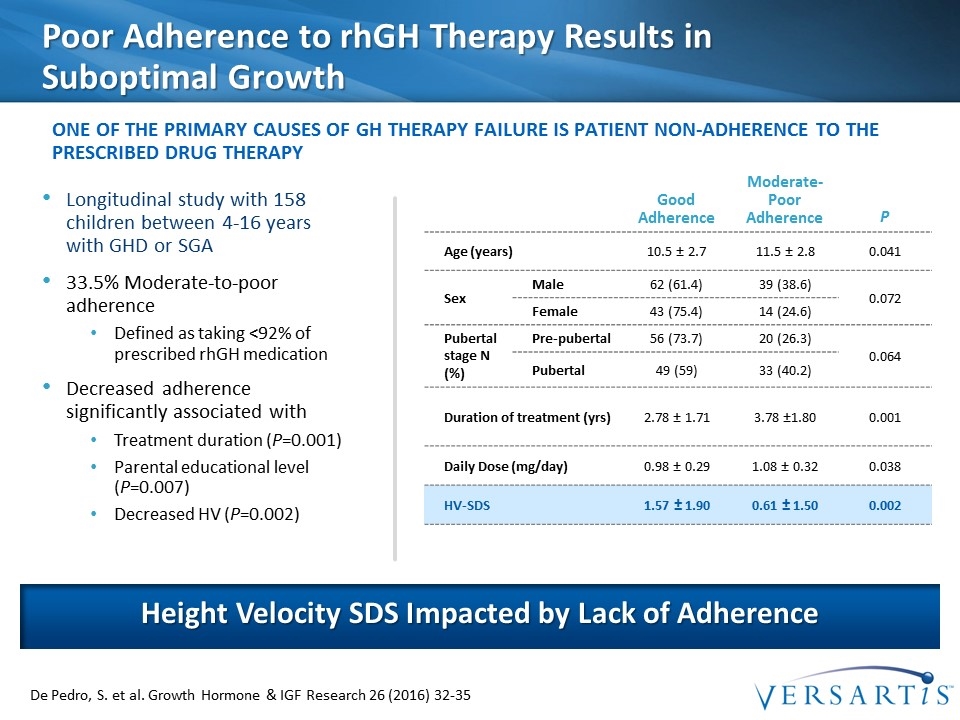
Poor Adherence to rhGH Therapy Results in Suboptimal Growth Longitudinal study with 158 children between 4-16 years with GHD or SGA 33.5% Moderate-to-poor adherence Defined as taking <92% of prescribed rhGH medication Decreased adherence significantly associated with Treatment duration (P=0.001) Parental educational level (P=0.007) Decreased HV (P=0.002) Height Velocity SDS Impacted by Lack of Adherence one of the primary causes of GH therapy failure is patient non-adherence to the prescribed drug therapy De Pedro, S. et al. Growth Hormone & IGF Research 26 (2016) 32-35 Good Adherence Moderate-Poor Adherence P Age (years) 10.5 ± 2.7 11.5 ± 2.8 0.041 Sex Male 62 (61.4) 39 (38.6) 0.072 Female 43 (75.4) 14 (24.6) Pubertal stage N (%) Pre-pubertal 56 (73.7) 20 (26.3) 0.064 Pubertal 49 (59) 33 (40.2) Duration of treatment (yrs) 2.78 ± 1.71 3.78 ±1.80 0.001 Daily Dose (mg/day) 0.98 ± 0.29 1.08 ± 0.32 0.038 HV-SDS 1.57 ± 1.90 0.61 ± 1.50 0.002
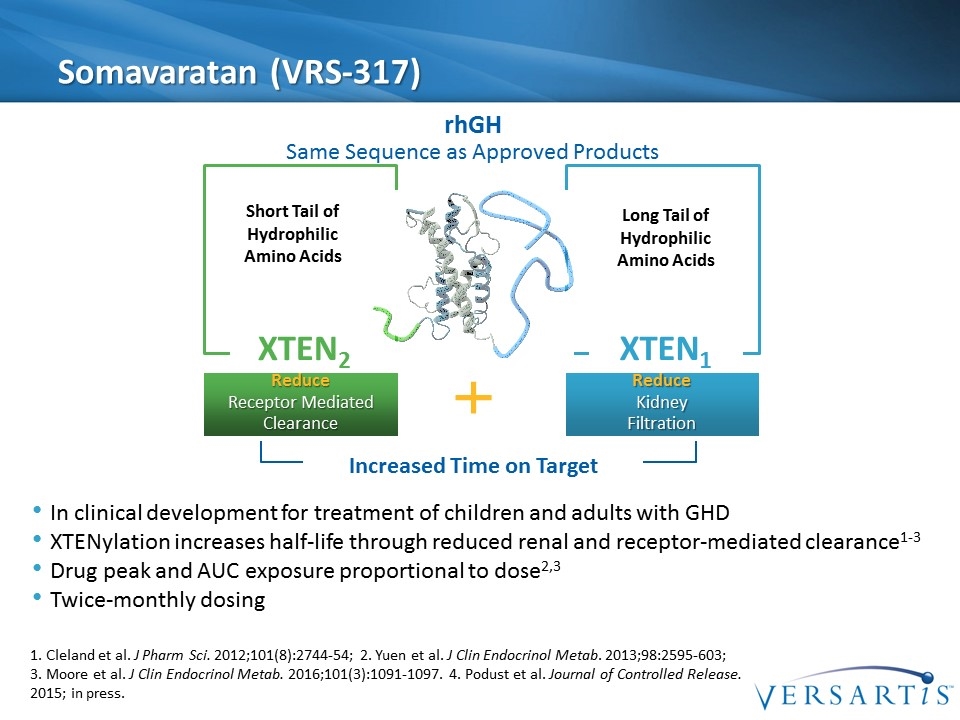
Somavaratan (VRS-317) Increased Time on Target XTEN1 Long Tail of Hydrophilic Amino Acids Reduce Kidney Filtration XTEN2 Short Tail of Hydrophilic Amino Acids Reduce Receptor Mediated Clearance rhGH Same Sequence as Approved Products In clinical development for treatment of children and adults with GHD XTENylation increases half-life through reduced renal and receptor-mediated clearance1-3 Drug peak and AUC exposure proportional to dose2,3 Twice-monthly dosing 1. Cleland et al. J Pharm Sci. 2012;101(8):2744-54; 2. Yuen et al. J Clin Endocrinol Metab. 2013;98:2595-603; 3. Moore et al. J Clin Endocrinol Metab. 2016;101(3):1091-1097. 4. Podust et al. Journal of Controlled Release. 2015; in press.
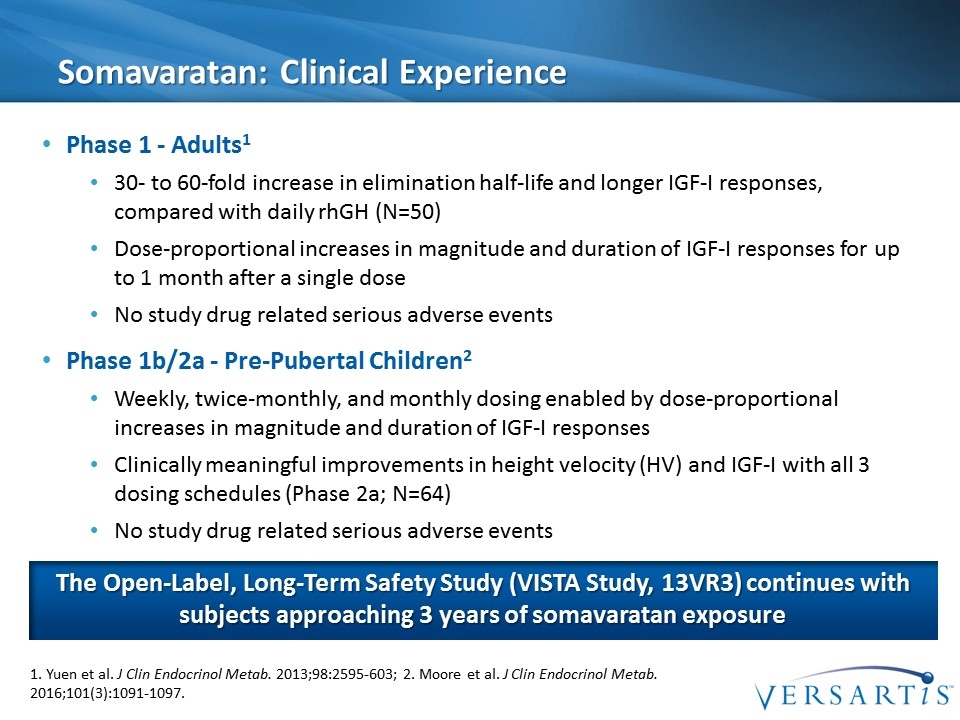
Somavaratan: Clinical Experience Phase 1 - Adults1 30- to 60-fold increase in elimination half-life and longer IGF-I responses, compared with daily rhGH (N=50) Dose-proportional increases in magnitude and duration of IGF-I responses for up to 1 month after a single dose No study drug related serious adverse events Phase 1b/2a - Pre-Pubertal Children2 Weekly, twice-monthly, and monthly dosing enabled by dose-proportional increases in magnitude and duration of IGF-I responses Clinically meaningful improvements in height velocity (HV) and IGF-I with all 3 dosing schedules (Phase 2a; N=64) No study drug related serious adverse events 1. Yuen et al. J Clin Endocrinol Metab. 2013;98:2595-603; 2. Moore et al. J Clin Endocrinol Metab. 2016;101(3):1091-1097. The Open-Label, Long-Term Safety Study (VISTA Study, 13VR3) continues with subjects approaching 3 years of somavaratan exposure
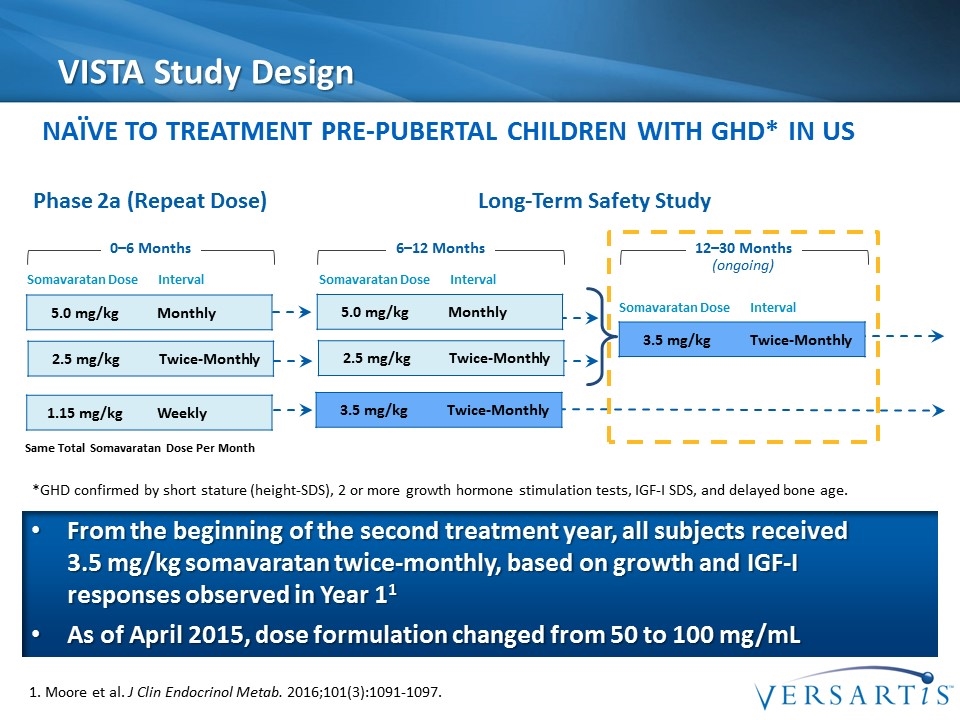
VISTA Study Design 1. Moore et al. J Clin Endocrinol Metab. 2016;101(3):1091-1097. 5.0 mg/kg Monthly 3.5 mg/kg Twice-Monthly 6–12 Months 12–30 Months (ongoing) 0–6 Months Somavaratan Dose Interval Phase 2a (Repeat Dose) Long-Term Safety Study 2.5 mg/kg Twice-Monthly 1.15 mg/kg Weekly 5.0 mg/kg Monthly 2.5 mg/kg Twice-Monthly 3.5 mg/kg Twice-Monthly Somavaratan Dose Interval Somavaratan Dose Interval Naïve to Treatment Pre-Pubertal Children with GHD* IN US Same Total Somavaratan Dose Per Month From the beginning of the second treatment year, all subjects received 3.5 mg/kg somavaratan twice-monthly, based on growth and IGF-I responses observed in Year 11 As of April 2015, dose formulation changed from 50 to 100 mg/mL *GHD confirmed by short stature (height-SDS), 2 or more growth hormone stimulation tests, IGF-I SDS, and delayed bone age.
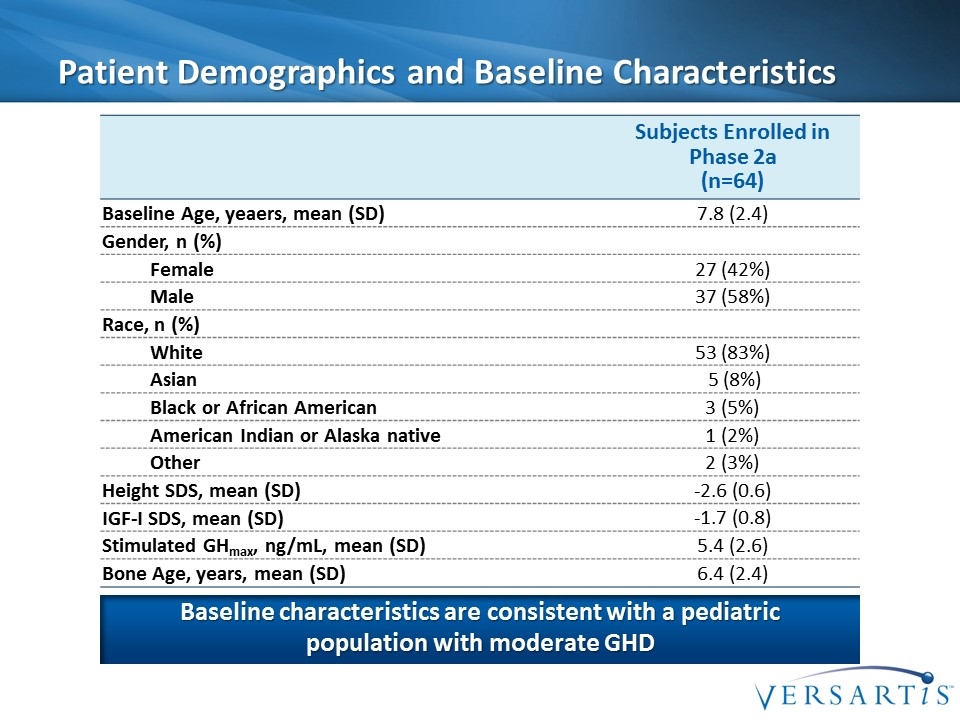
Patient Demographics and Baseline Characteristics Subjects Enrolled in Phase 2a (n=64) Baseline Age, yeaers, mean (SD) 7.8 (2.4) Gender, n (%) Female 27 (42%) Male 37 (58%) Race, n (%) White 53 (83%) Asian 5 (8%) Black or African American 3 (5%) American Indian or Alaska native 1 (2%) Other 2 (3%) Height SDS, mean (SD) -2.6 (0.6) IGF-I SDS, mean (SD) -1.7 (0.8) Stimulated GHmax, ng/mL, mean (SD) 5.4 (2.6) Bone Age, years, mean (SD) 6.4 (2.4) Baseline characteristics are consistent with a pediatric population with moderate GHD
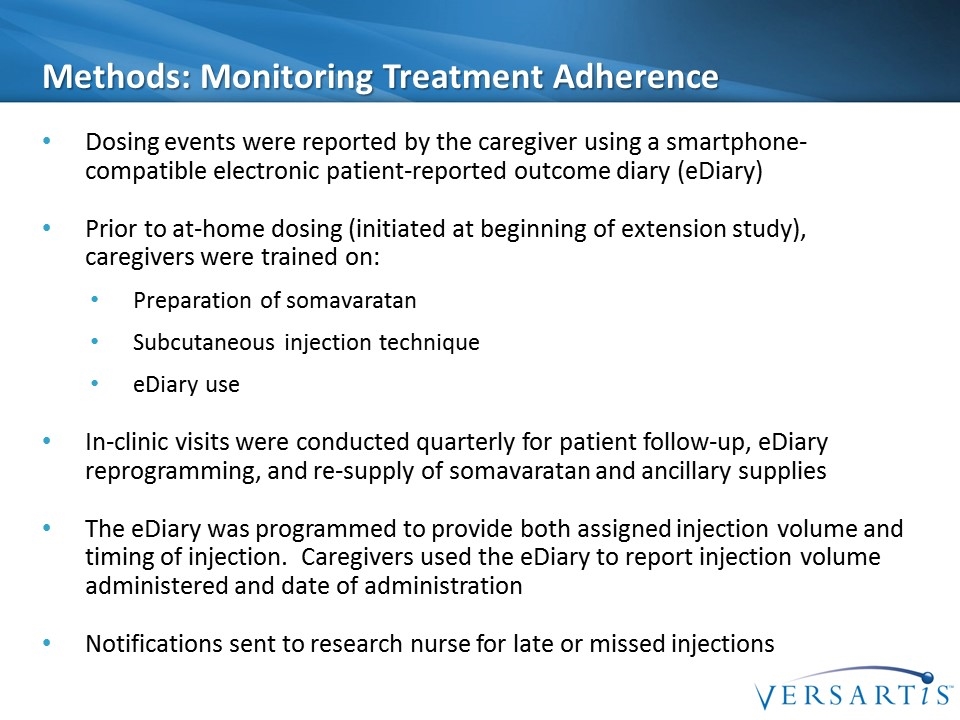
Methods: Monitoring Treatment Adherence Dosing events were reported by the caregiver using a smartphone-compatible electronic patient-reported outcome diary (eDiary) Prior to at-home dosing (initiated at beginning of extension study), caregivers were trained on: Preparation of somavaratan Subcutaneous injection technique eDiary use In-clinic visits were conducted quarterly for patient follow-up, eDiary reprogramming, and re-supply of somavaratan and ancillary supplies The eDiary was programmed to provide both assigned injection volume and timing of injection. Caregivers used the eDiary to report injection volume administered and date of administration Notifications sent to research nurse for late or missed injections
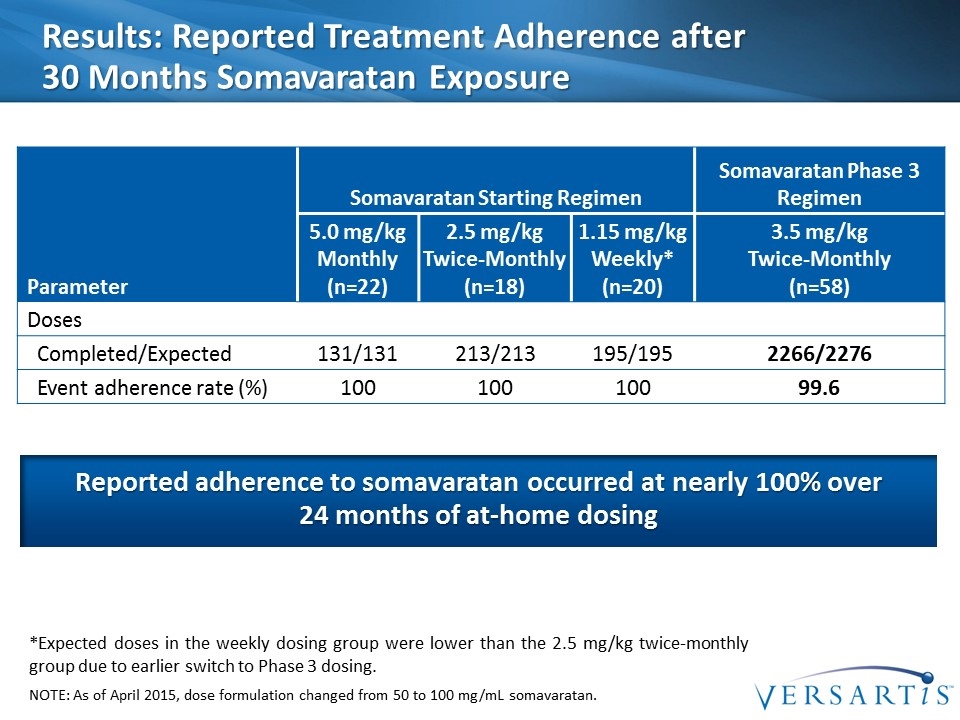
Results: Reported Treatment Adherence after 30 Months Somavaratan Exposure Parameter Somavaratan Starting Regimen Somavaratan Phase 3 Regimen 5.0 mg/kg Monthly (n=22) 2.5 mg/kg Twice-Monthly (n=18) 1.15 mg/kg Weekly* (n=20) 3.5 mg/kg Twice-Monthly (n=58) Doses Completed/Expected 131/131 213/213 195/195 2266/2276 Event adherence rate (%) 100 100 100 99.6 *Expected doses in the weekly dosing group were lower than the 2.5 mg/kg twice-monthly group due to earlier switch to Phase 3 dosing. NOTE: As of April 2015, dose formulation changed from 50 to 100 mg/mL somavaratan. Reported adherence to somavaratan occurred at nearly 100% over 24 months of at-home dosing
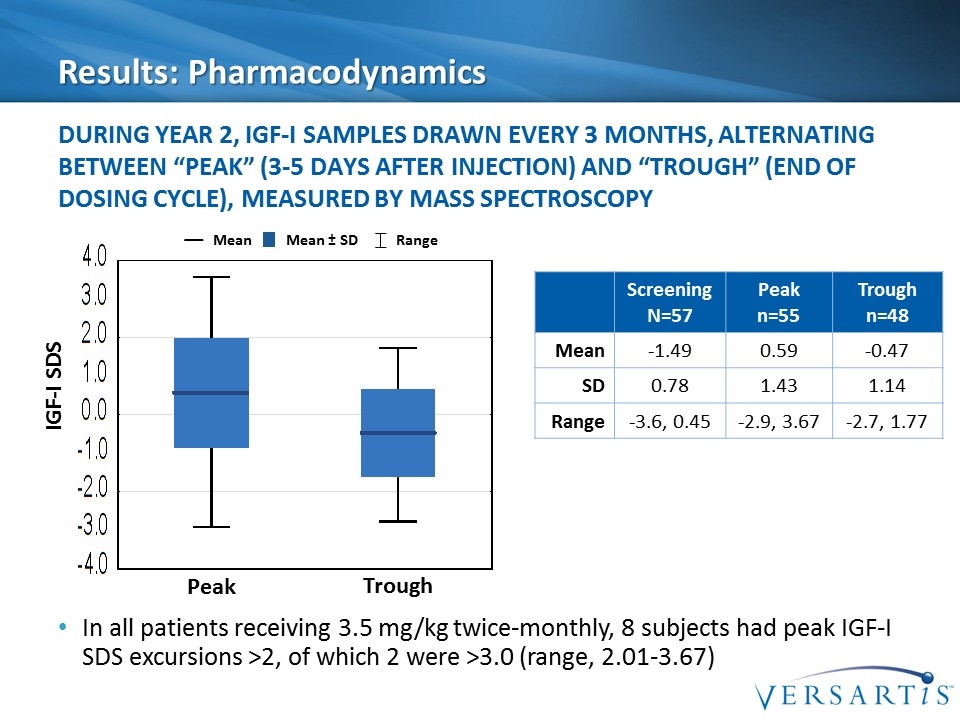
Results: Pharmacodynamics In all patients receiving 3.5 mg/kg twice-monthly, 8 subjects had peak IGF-I SDS excursions >2, of which 2 were >3.0 (range, 2.01-3.67) During Year 2, IGF-I samples drawn every 3 months, alternating between “peak” (3-5 days after injection) and “trough” (end of dosing cycle), measured by mass spectroscopy Mean Mean ± SD Range Screening N=57 Peak n=55 Trough n=48 Mean -1.49 0.59 -0.47 SD 0.78 1.43 1.14 Range -3.6, 0.45 -2.9, 3.67 -2.7, 1.77 Peak Trough IGF-I SDS
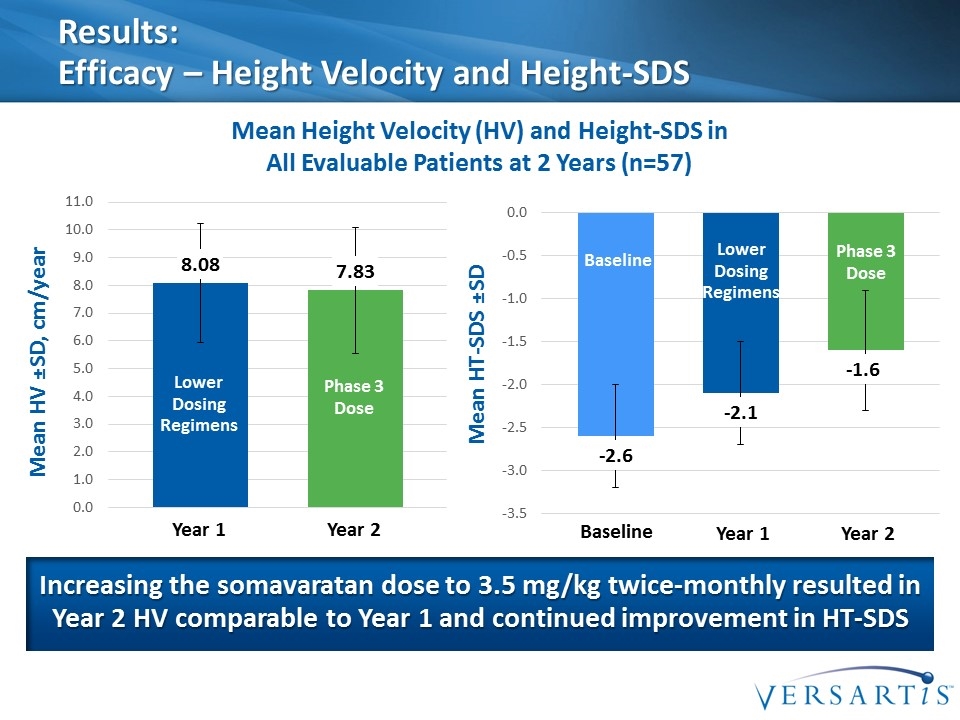
Results: Efficacy – Height Velocity and Height-SDS Mean Height Velocity (HV) and Height-SDS in All Evaluable Patients at 2 Years (n=57) Mean HV ±SD, cm/year Increasing the somavaratan dose to 3.5 mg/kg twice-monthly resulted in Year 2 HV comparable to Year 1 and continued improvement in HT-SDS Lower Dosing Regimens Phase 3 Dose Year 1 Year 2 Mean HT-SDS ±SD Lower Dosing Regimens Phase 3 Dose Year 1 Year 2 Baseline Baseline
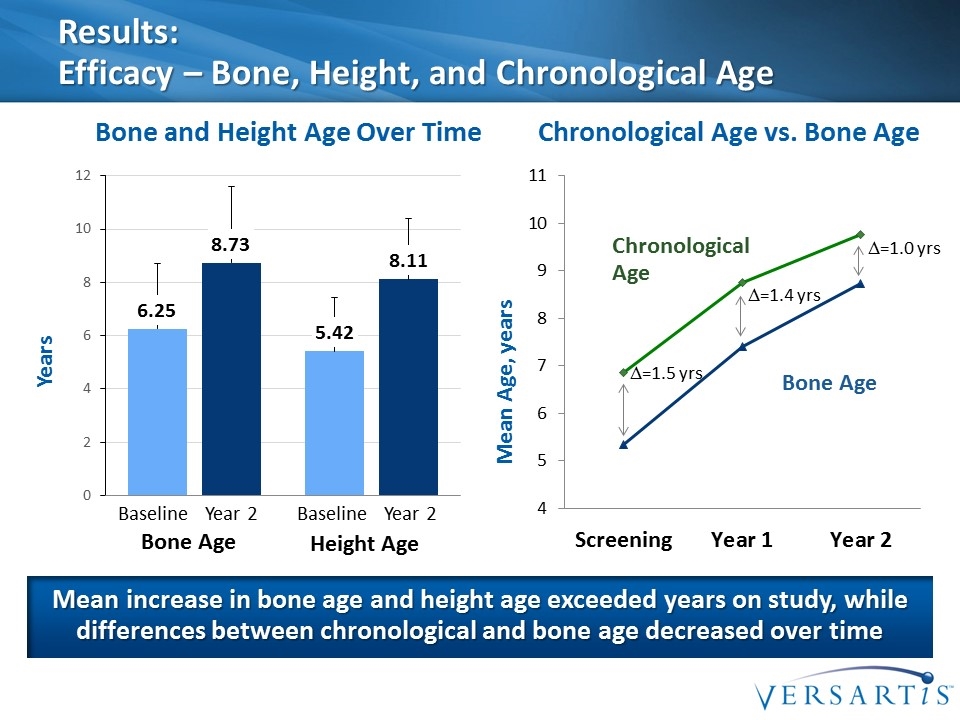
Results: Efficacy – Bone, Height, and Chronological Age Mean increase in bone age and height age exceeded years on study, while differences between chronological and bone age decreased over time Years Bone and Height Age Over Time Chronological Age vs. Bone Age Bone Age Height Age Baseline Year 2 Baseline Year 2 Bone Age Chronological Age Mean Age, years D=1.5 yrs D=1.4 yrs D=1.0 yrs
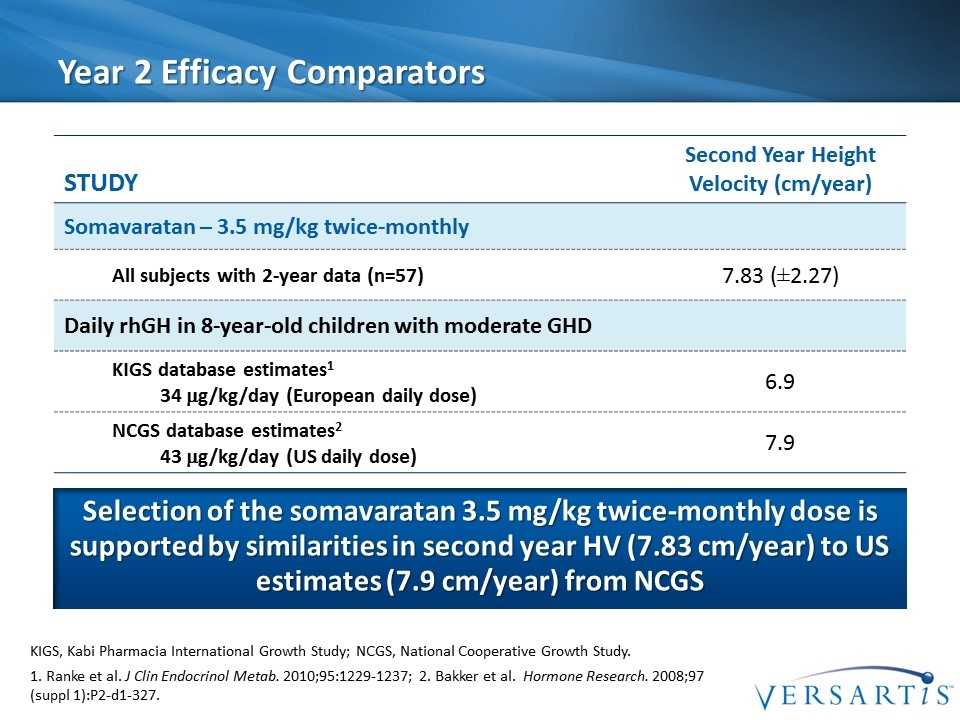
Year 2 Efficacy Comparators Selection of the somavaratan 3.5 mg/kg twice-monthly dose is supported by similarities in second year HV (7.83 cm/year) to US estimates (7.9 cm/year) from NCGS Study Second Year Height Velocity (cm/year) Somavaratan – 3.5 mg/kg twice-monthly All subjects with 2-year data (n=57) 7.83 (±2.27) Daily rhGH in 8-year-old children with moderate GHD KIGS database estimates1 34 mg/kg/day (European daily dose) 6.9 NCGS database estimates2 43 mg/kg/day (US daily dose) 7.9 1. Ranke et al. J Clin Endocrinol Metab. 2010;95:1229-1237; 2. Bakker et al. Hormone Research. 2008;97 (suppl 1):P2-d1-327. KIGS, Kabi Pharmacia International Growth Study; NCGS, National Cooperative Growth Study.
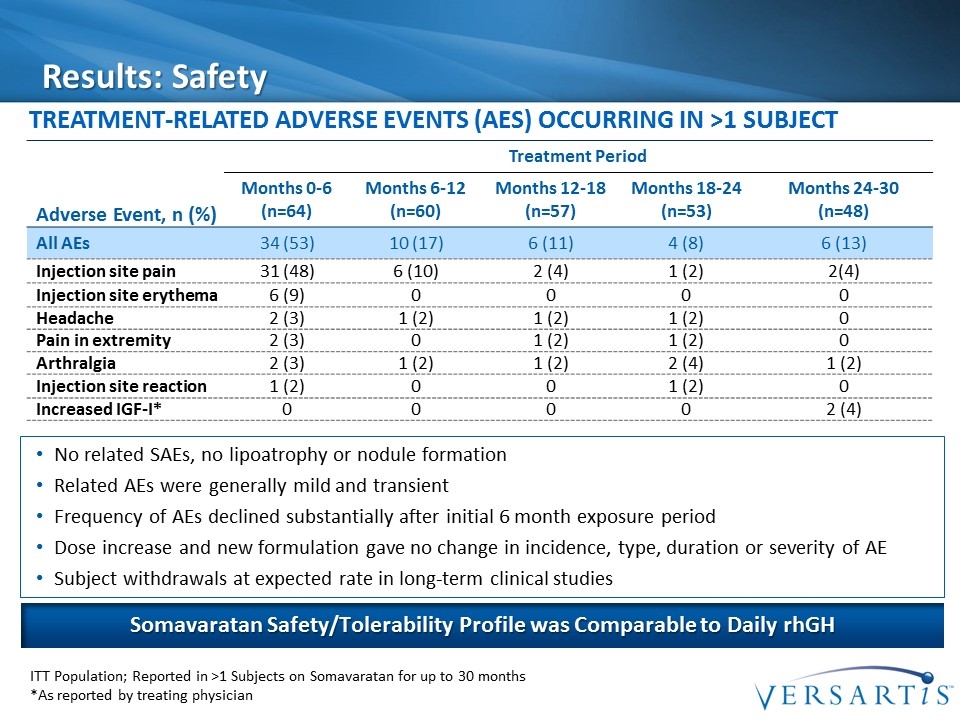
Results: Safety Treatment-Related Adverse Events (AEs) Occurring in >1 Subject Adverse Event, n (%) Treatment Period Months 0-6 (n=64) Months 6-12 (n=60) Months 12-18 (n=57) Months 18-24 (n=53) Months 24-30 (n=48) All AEs 34 (53) 10 (17) 6 (11) 4 (8) 6 (13) Injection site pain 31 (48) 6 (10) 2 (4) 1 (2) 2(4) Injection site erythema 6 (9) 0 0 0 0 Headache 2 (3) 1 (2) 1 (2) 1 (2) 0 Pain in extremity 2 (3) 0 1 (2) 1 (2) 0 Arthralgia 2 (3) 1 (2) 1 (2) 2 (4) 1 (2) Injection site reaction 1 (2) 0 0 1 (2) 0 Increased IGF-I* 0 0 0 0 2 (4) ITT Population; Reported in >1 Subjects on Somavaratan for up to 30 months *As reported by treating physician Somavaratan Safety/Tolerability Profile was Comparable to Daily rhGH No related SAEs, no lipoatrophy or nodule formation Related AEs were generally mild and transient Frequency of AEs declined substantially after initial 6 month exposure period Dose increase and new formulation gave no change in incidence, type, duration or severity of AE Subject withdrawals at expected rate in long-term clinical studies
Conclusions Phase 3 dose selection is supported by VISTA study results for subjects switched to 3.5 mg/kg twice-monthly Mean peak IGF-I SDS at Phase 3 dose was in upper half of normal range Catch-up growth supported by mean increase in bone age and height age exceeding years on study Improvement in HT-SDS continued in Year 2 Year 2 HV comparable to US daily dosing data from NCGS Phase 3 dose (3.5 mg/kg, twice-monthly) is safe and well tolerated in this study Frequency and severity of treatment-related adverse events indicate no safety concerns Somavaratan, 3.5 mg/kg twice-monthly, is now under study in a randomized, Phase 3, non-inferiority trial versus daily rhGH in pre-pubertal children with GHD (NCT02339090) - The VELOCITY Trial

Investor Day | September 15, 2016

PGHD R&D Program Colin Hislop, MD | Chief Medical Officer
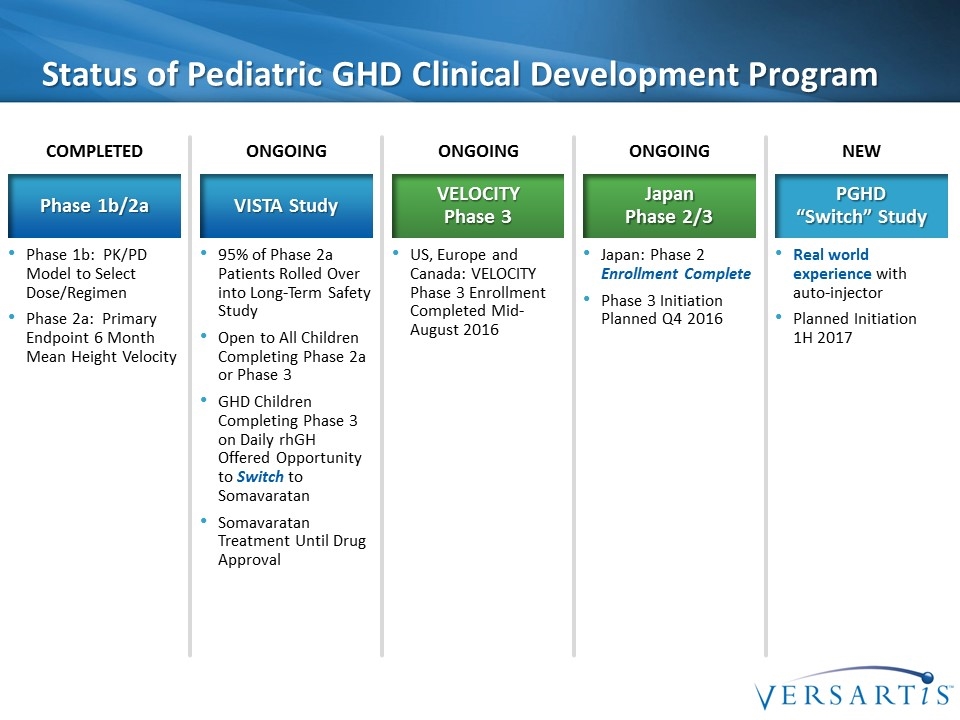
Status of Pediatric GHD Clinical Development Program Phase 1b: PK/PD Model to Select Dose/Regimen Phase 2a: Primary Endpoint 6 Month Mean Height Velocity Phase 1b/2a COMPLETED 95% of Phase 2a Patients Rolled Over into Long-Term Safety Study Open to All Children Completing Phase 2a or Phase 3 GHD Children Completing Phase 3 on Daily rhGH Offered Opportunity to Switch to Somavaratan Somavaratan Treatment Until Drug Approval VISTA Study ONGOING US, Europe and Canada: VELOCITY Phase 3 Enrollment Completed Mid-August 2016 VELOCITY Phase 3 ONGOING Real world experience with auto-injector Planned Initiation 1H 2017 PGHD “Switch” Study NEW Japan: Phase 2 Enrollment Complete Phase 3 Initiation Planned Q4 2016 Japan Phase 2/3 ONGOING
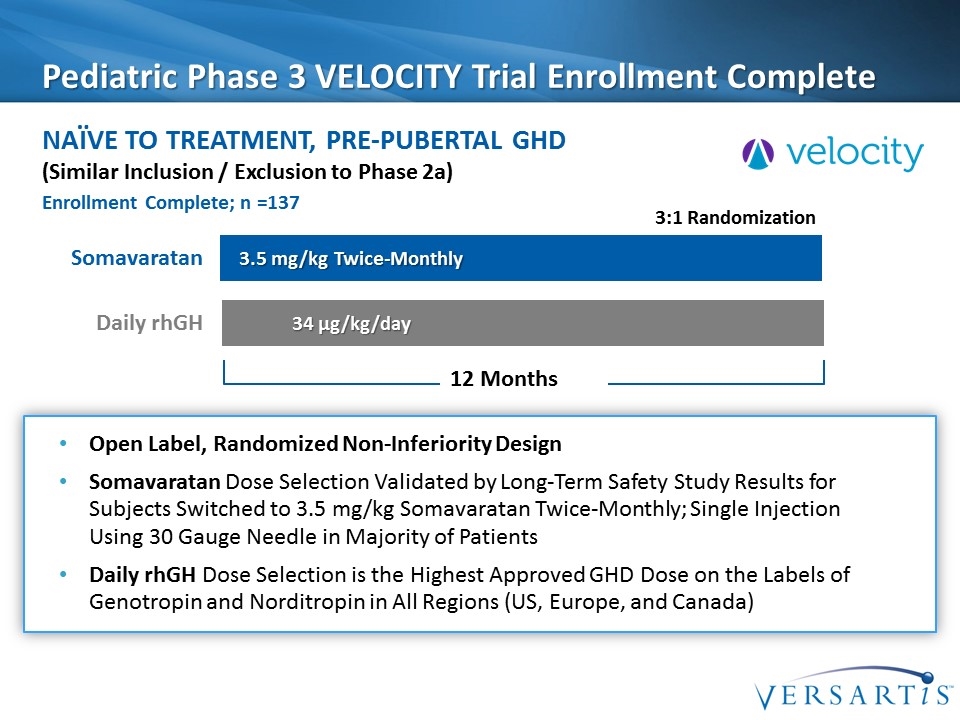
Pediatric Phase 3 VELOCITY Trial Enrollment Complete Naïve to treatment, Pre-pubertal GHD Somavaratan 3.5 mg/kg Twice-Monthly 34 µg/kg/day 3:1 Randomization Daily rhGH (Similar Inclusion / Exclusion to Phase 2a) Open Label, Randomized Non-Inferiority Design Somavaratan Dose Selection Validated by Long-Term Safety Study Results for Subjects Switched to 3.5 mg/kg Somavaratan Twice-Monthly; Single Injection Using 30 Gauge Needle in Majority of Patients Daily rhGH Dose Selection is the Highest Approved GHD Dose on the Labels of Genotropin and Norditropin in All Regions (US, Europe, and Canada) 12 Months Enrollment Complete; n =137
The VISTA Study Previously known as the 13VR3 Extension Study United States, Canada and Europe GHD children completing a somavaratan study 95% of Phase 2a patients rolled over into long-term safety study Currently 48 Subjects continue in study GHD children completing Phase 3 on daily rhGH offered opportunity to switch to somavaratan Somavaratan dosed 3.5 mg/kg twice-monthly Subjects in VISTA will roll-over to auto-injector over time Somavaratan treatment until drug approval (up to four years of safety data at submission; five years at launch) Versartis Long-Term Extension Study of Somavaratan
Japan Pediatric GHD Phase 2/3 Study J14VR5 Phase 2 portion enrollment complete n=24 subjects Single dose administration PK/PD assessment over 30 days Comparison between Japanese and Western subjects Subjects transitioning to extension study at 3.5 mg/kg twice-monthly Phase 3 initiation planned Q4 2016
16VR9: PGHD Switch Study Study to gain real-world experience: Switch from daily Broad PGHD population Auto-injector/Commercial Presentation Provide physicians additional clinical data on how best to use somavaratan for children currently on therapy N=~60 FPI: 1H 2017 A Versartis Study of Somavaratan in Children with GHD Previously Treated with daily RHgh

PGHD Development Summary Versartis clinical development program has been strategic and intentional: Long term safety data - 4 years at time of BLA submission, 5 years at time of launch Clinical studies in geographies representative of current PGHD market Hands on somavaratan experience for PGHD treating physicians "Real world" patient experience with drug/device Strong, Robust Data Package To Support a Regulatory Submission and Commercial Launch

Q & A Session


Introduction: AGHD Speaker Colin Hislop, MD | Chief Medical Officer

Beverly MK Biller, MD Dr. Beverly Biller is an endocrinologist and faculty member in the Neuroendocrine Clinical Center at Massachusetts General Hospital. She is also a Professor of Medicine at Harvard Medical School. Dr. Biller received her M.D from the University of Oklahoma College of Medicine. She completed her internship and residency at Beth Israel Hospital in Boston and fellowships in medicine and endocrinology at MGH and Harvard Medical School. Dr. Biller currently serves on the leadership council of the Endocrine Society. As her Society biography aptly describes, “she has had a longstanding interest in the diagnosis and treatment of pituitary tumors, with a special interest in Cushing’s disease and treatment of growth hormone deficiency in adults, and has published both primary scientific research and clinical guidelines on these topics.” As a clinical investigator, her major research interests include the diagnosis and treatment of adult GHD as well as Cushing's, prolactinomas, acromegaly, and other pituitary tumors and pituitary hormone deficiencies. Over her 25 years as member of the Endocrine Society, Dr. Biller has served as an Associate Editor of the Journal of Clinical Endocrinology and Metabolism, as Chair of the Annual Meeting Steering Committee and as Chair of the Scientific and Educational Programs Core Committee, among other roles. She has also served as President of the Association of Program Directors of Endocrinology and Metabolism and on the Council of the Growth Hormone Research Society. In addition to numerous published articles, Dr. Biller has authored multiple books and texts on endocrine disorders, including adult GHD.

Beverly MK Biller, MD Professor of Medicine Harvard Medical School Neuroendocrine Unit Massachusetts General Hospital Adult growth hormone deficiency (AGHD) and long-acting growth hormone (LAGH) for adults

Adult growth hormone deficiency (AGHD) and long-acting growth hormone (LAGH) for adults What is adult GHD? Will LAGH fill an unmet need in the treatment of AGHD?
Case 43 year old man - surgery, radiation for benign pituitary tumor Panhypopituitary on triple hormone replacement (thyroid, cortisol, testosterone); otherwise healthy Read about GHD in a well-known publication: “sounds just like me, especially the excess abdominal fat” Worried about his risk of cardiovascular disease and osteoporosis with a strong family history of both Asked endocrinologist for treatment with GH What journal had he read?

Newspaper: USA TODAY

Illicit use by athletes Bodybuilding Baseball Olympics
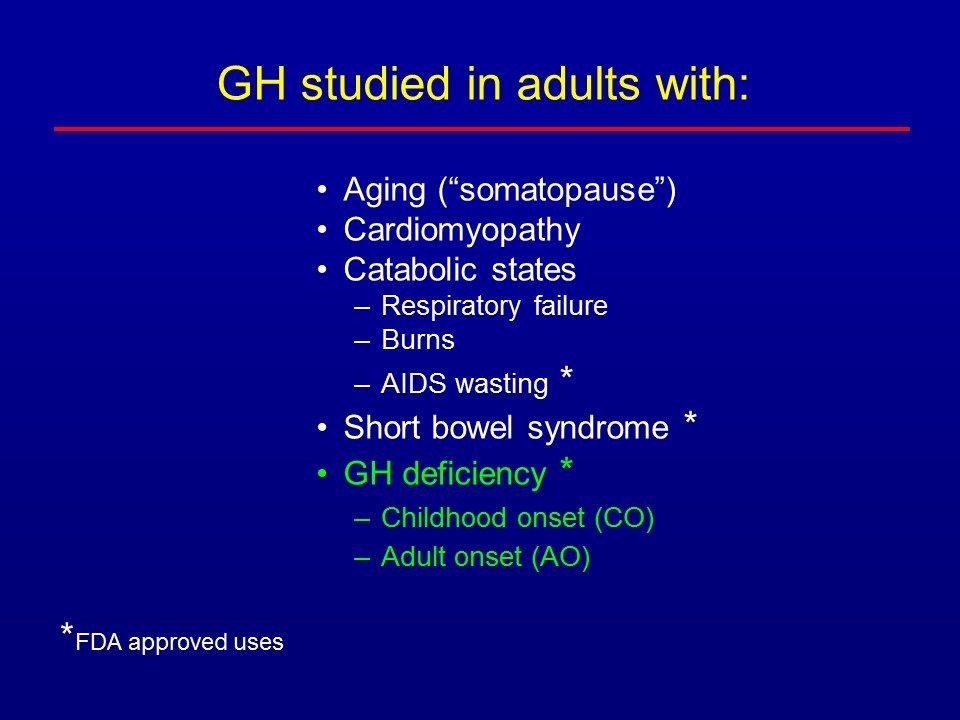
GH studied in adults with: Aging (“somatopause”) Cardiomyopathy Catabolic states Respiratory failure Burns AIDS wasting * Short bowel syndrome * GH deficiency * Childhood onset (CO) Adult onset (AO) *FDA approved uses
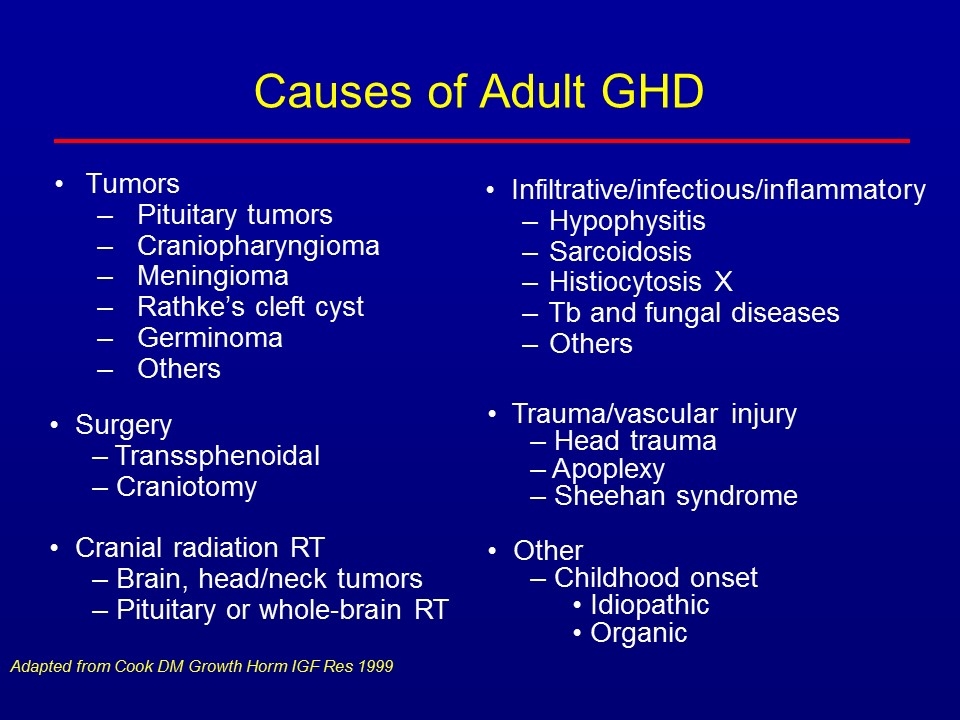
Causes of Adult GHD Tumors Pituitary tumors Craniopharyngioma Meningioma Rathke’s cleft cyst Germinoma Others Infiltrative/infectious/inflammatory Hypophysitis Sarcoidosis Histiocytosis X Tb and fungal diseases Others Adapted from Cook DM Growth Horm IGF Res 1999 Surgery Transsphenoidal Craniotomy Cranial radiation RT Brain, head/neck tumors Pituitary or whole-brain RT Trauma/vascular injury Head trauma Apoplexy Sheehan syndrome Other Childhood onset Idiopathic Organic
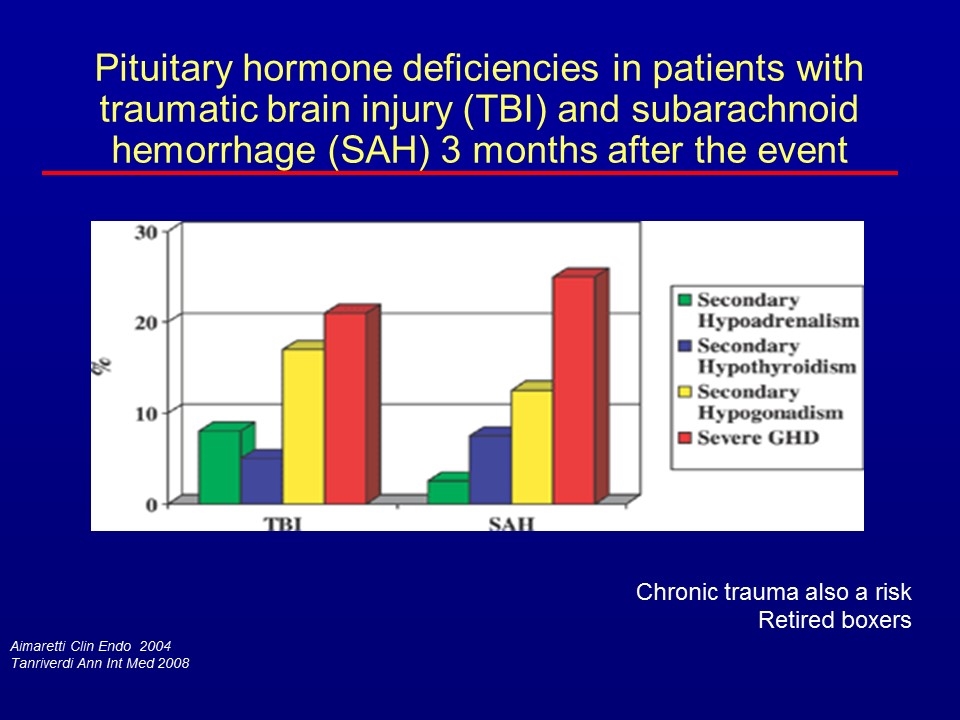
Aimaretti Clin Endo 2004 Tanriverdi Ann Int Med 2008 Chronic trauma also a risk Retired boxers Pituitary hormone deficiencies in patients with traumatic brain injury (TBI) and subarachnoid hemorrhage (SAH) 3 months after the event
From Harris Location of pituitary gland
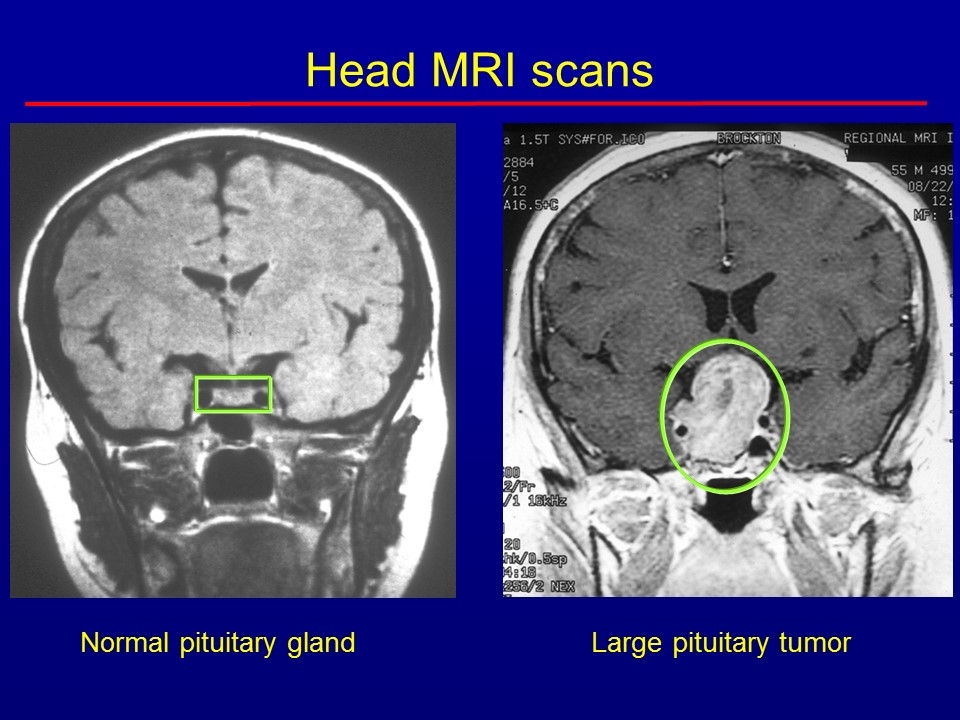
Normal pituitary gland Large pituitary tumor Head MRI scans
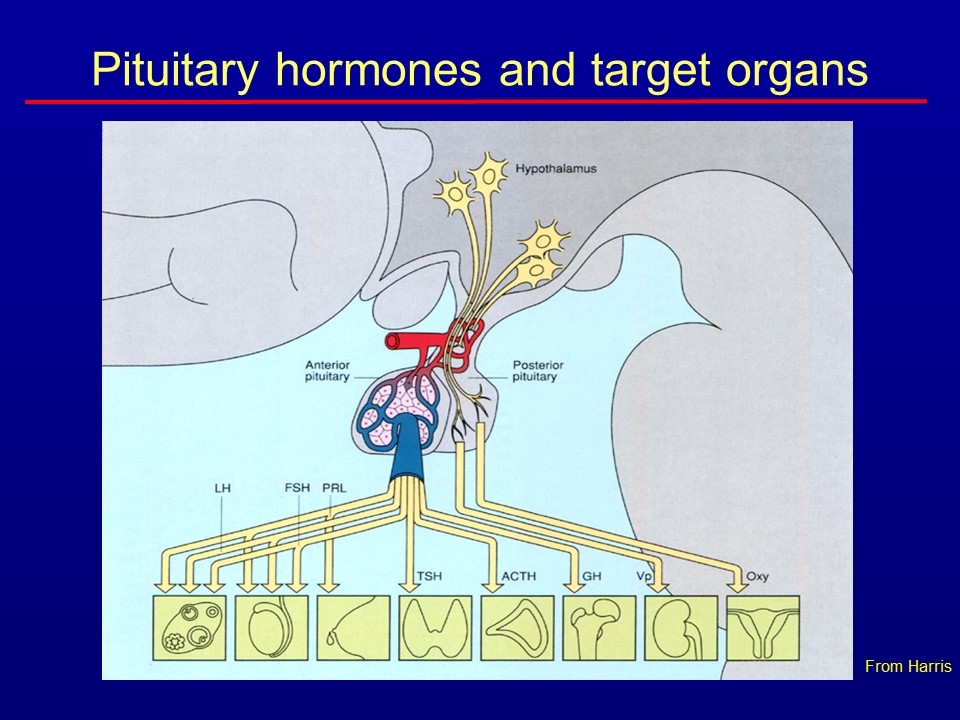
Pituitary hormones and target organs From Harris
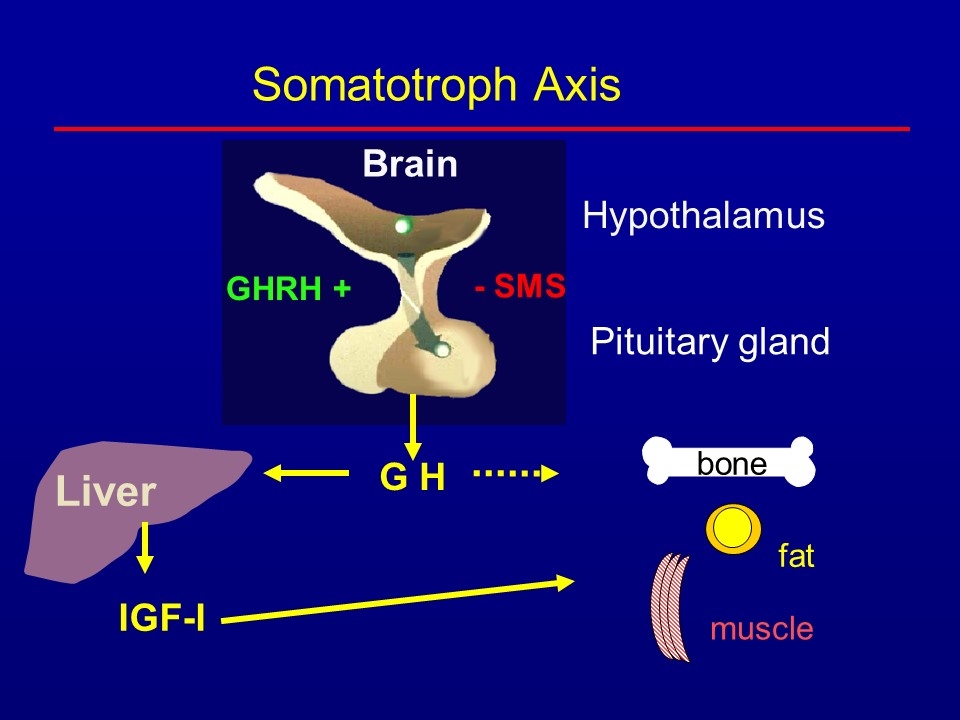
Brain Hypothalamus GHRH + G H Liver Somatotroph Axis Pituitary gland - SMS IGF-I bone fat muscle
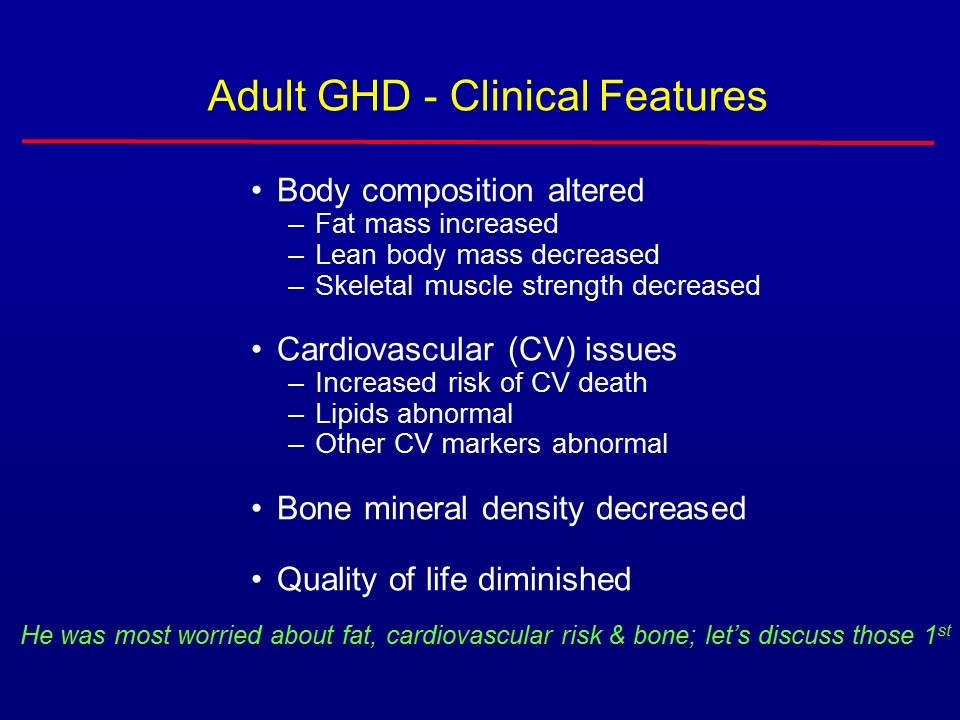
Adult GHD - Clinical Features Body composition altered Fat mass increased Lean body mass decreased Skeletal muscle strength decreased Cardiovascular (CV) issues Increased risk of CV death Lipids abnormal Other CV markers abnormal Bone mineral density decreased Quality of life diminished He was most worried about fat, cardiovascular risk & bone; let’s discuss those 1st
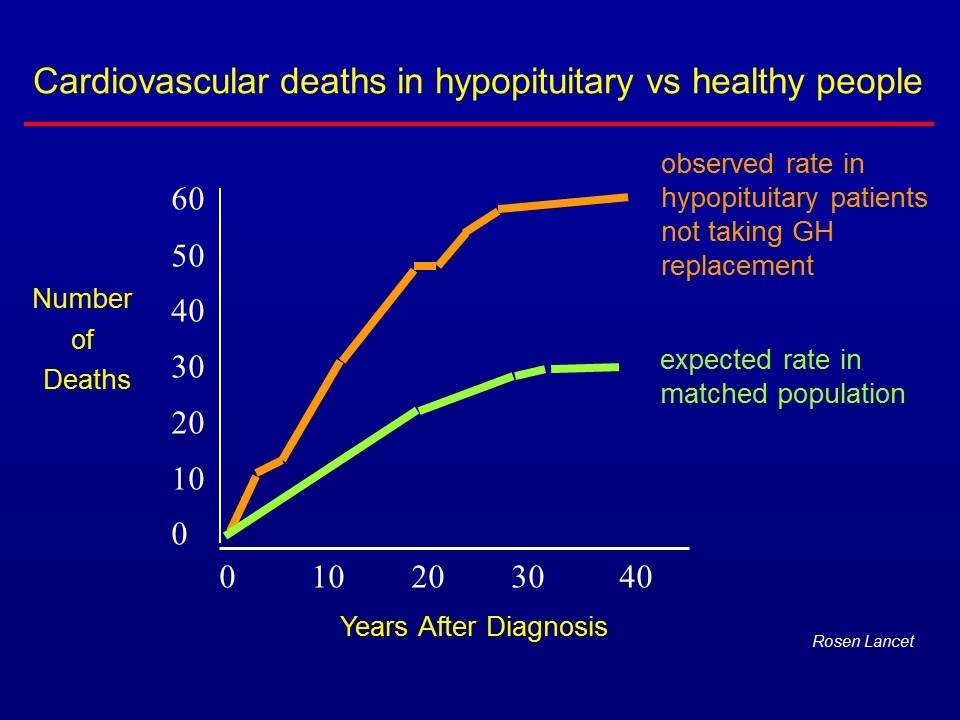
0 10 20 30 40 60 50 40 30 20 10 0 Number of Deaths Years After Diagnosis Rosen Lancet Cardiovascular deaths in hypopituitary vs healthy people observed rate in hypopituitary patients not taking GH replacement expected rate in matched population
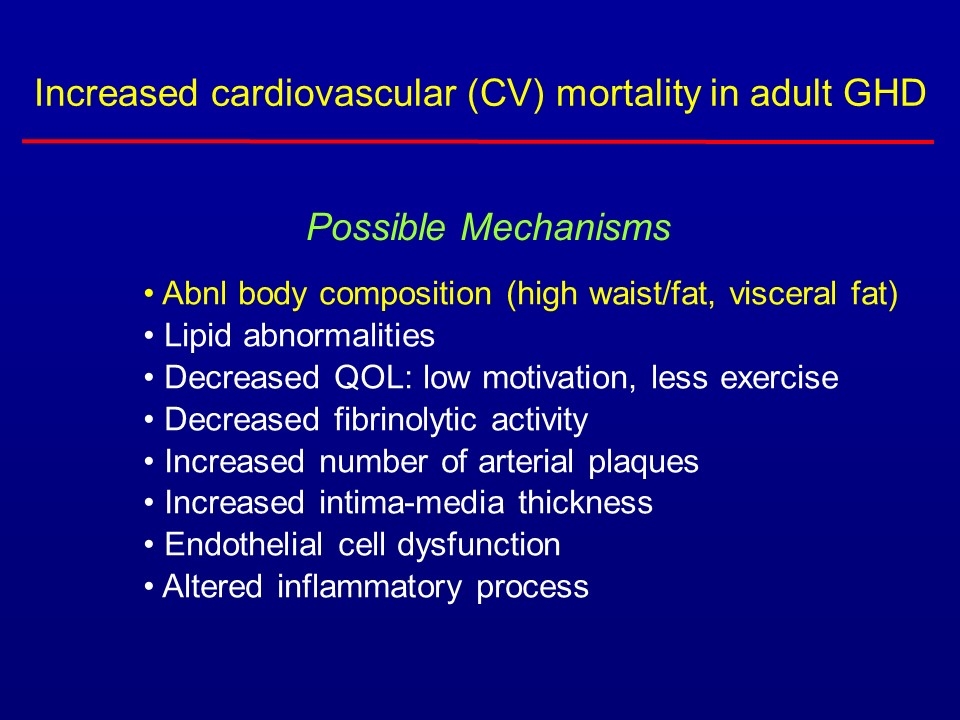
Abnl body composition (high waist/fat, visceral fat) Lipid abnormalities Decreased QOL: low motivation, less exercise Decreased fibrinolytic activity Increased number of arterial plaques Increased intima-media thickness Endothelial cell dysfunction Altered inflammatory process Possible Mechanisms Increased cardiovascular (CV) mortality in adult GHD
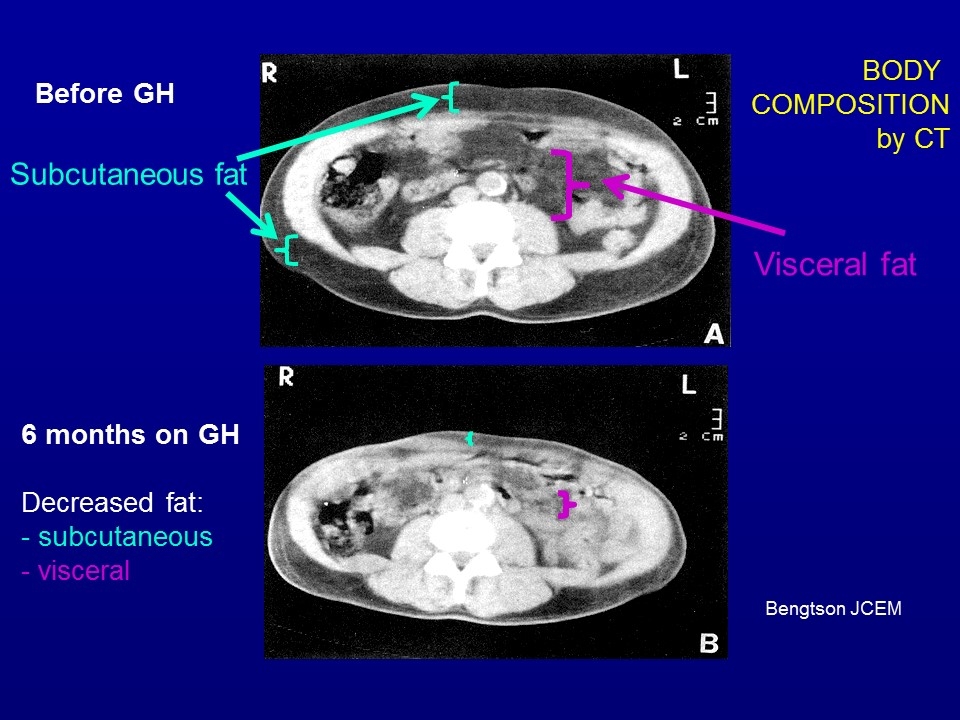
Bengtson JCEM Before GH 6 months on GH Decreased fat: - subcutaneous - visceral Subcutaneous fat Visceral fat BODY COMPOSITION by CT
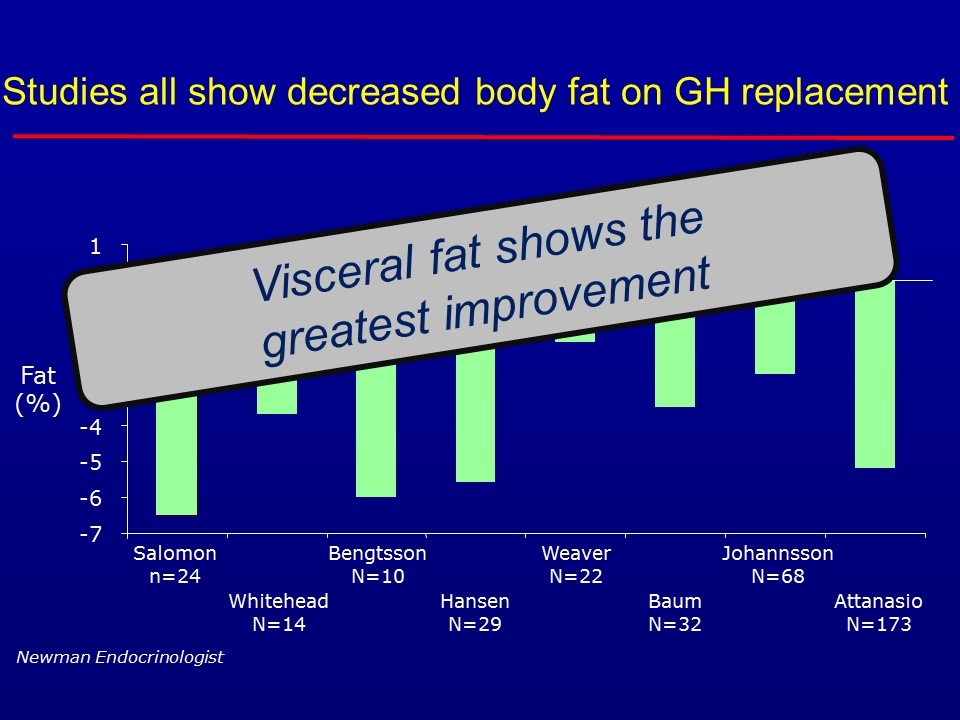
Studies all show decreased body fat on GH replacement -7 -6 -5 -4 -3 -2 -1 0 1 Salomon n=24 Whitehead N=14 Bengtsson N=10 Hansen N=29 Weaver N=22 Baum N=32 Johannsson N=68 Attanasio N=173 Fat (%) Newman Endocrinologist Visceral fat shows the greatest improvement
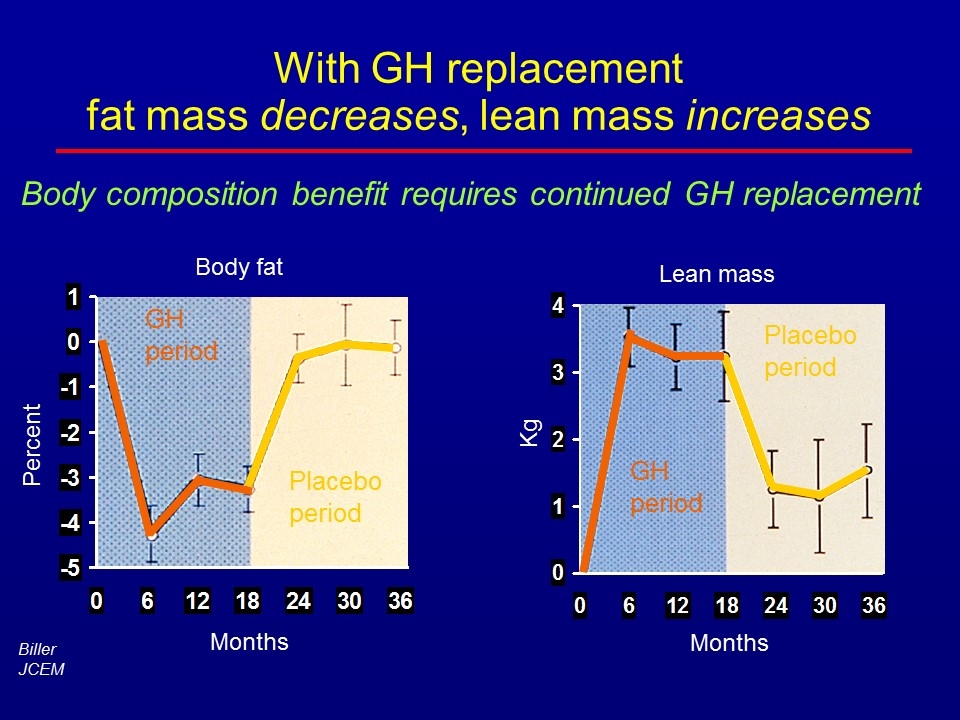
With GH replacement fat mass decreases, lean mass increases Body fat Percent Months GH period Placebo period Lean mass Kg Months GH period Placebo period Body composition benefit requires continued GH replacement Biller JCEM
Abnl body composition (high waist/fat, visceral fat) Lipid abnormalities Decreased QOL: low motivation, less exercise Decreased fibrinolytic activity Increased number of arterial plaques Increased intima-media thickness Endothelial cell dysfunction Altered inflammatory process Possible Mechanisms Increased cardiovascular (CV) mortality in adult GHD
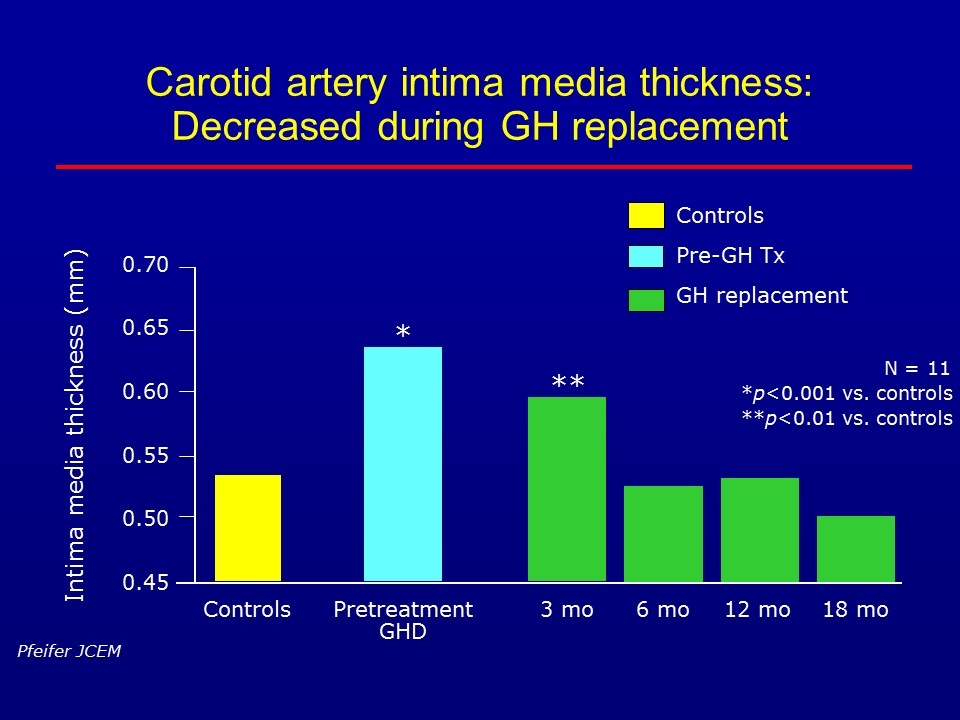
Carotid artery intima media thickness: Decreased during GH replacement Controls Pre-GH Tx GH replacement N = 11 *p<0.001 vs. controls **p<0.01 vs. controls Pfeifer JCEM 6 mo 0.70 3 mo Intima media thickness (mm) 12 mo 18 mo * ** 0.65 0.60 0.55 0.50 0.45 Controls Pretreatment GHD
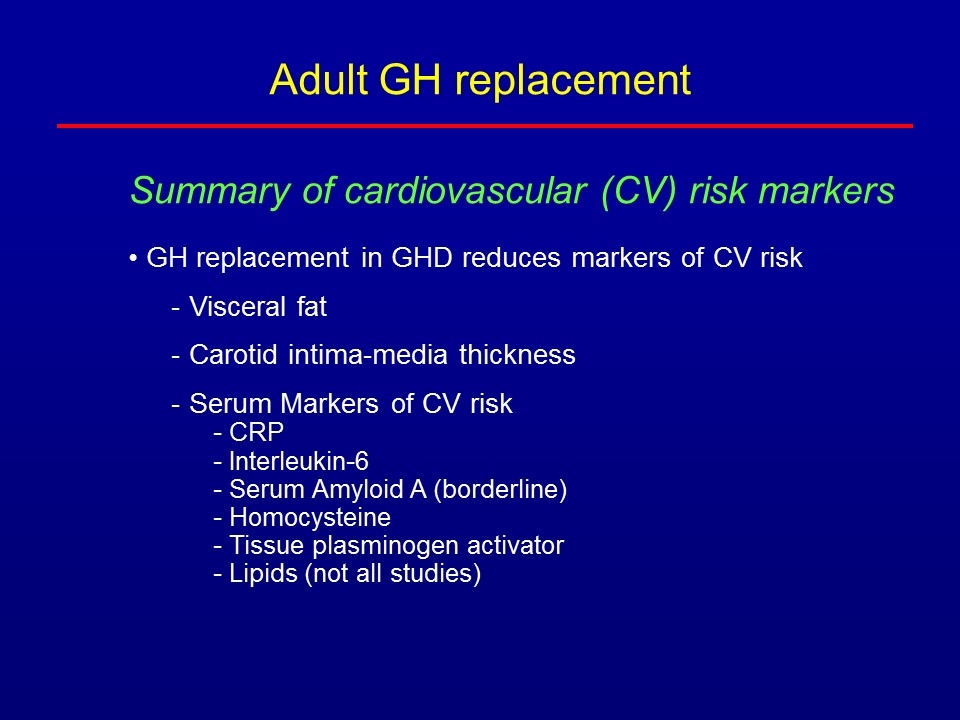
Summary of cardiovascular (CV) risk markers GH replacement in GHD reduces markers of CV risk Visceral fat Carotid intima-media thickness Serum Markers of CV risk CRP Interleukin-6 Serum Amyloid A (borderline) Homocysteine Tissue plasminogen activator Lipids (not all studies) Adult GH replacement
Adult GHD - Clinical Features Body composition altered Fat mass increased Lean body mass decreased Skeletal muscle strength decreased Cardiovascular (CV) issues Increased risk of CV death Lipids abnormal Other CV markers abnormal Bone mineral density decreased
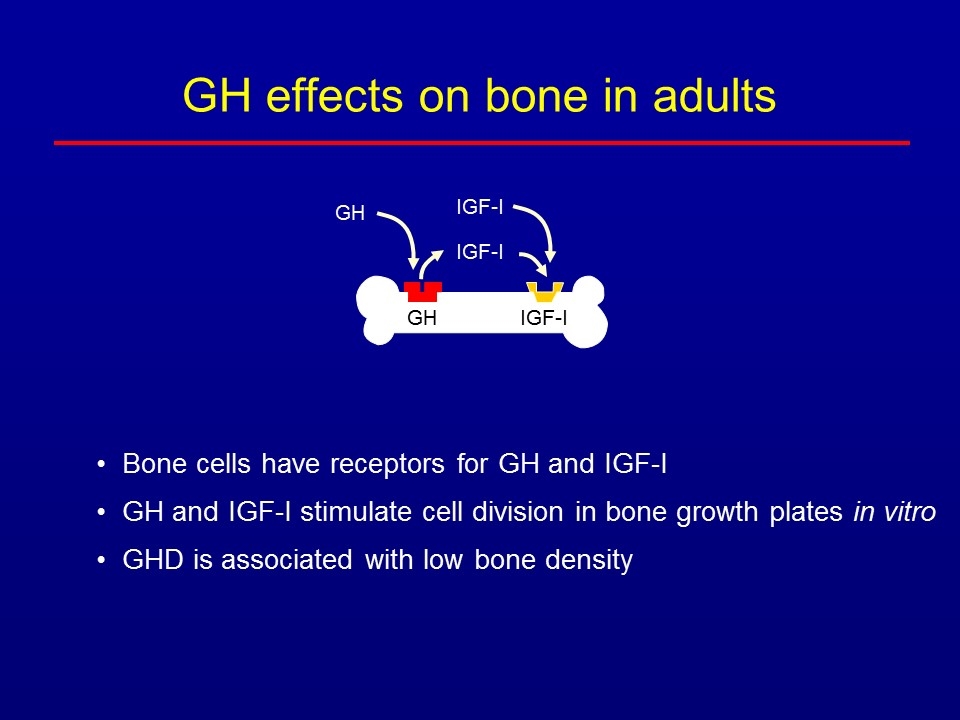
GH effects on bone in adults Bone cells have receptors for GH and IGF-I GH and IGF-I stimulate cell division in bone growth plates in vitro GHD is associated with low bone density GH IGF-I IGF-I GH IGF-I
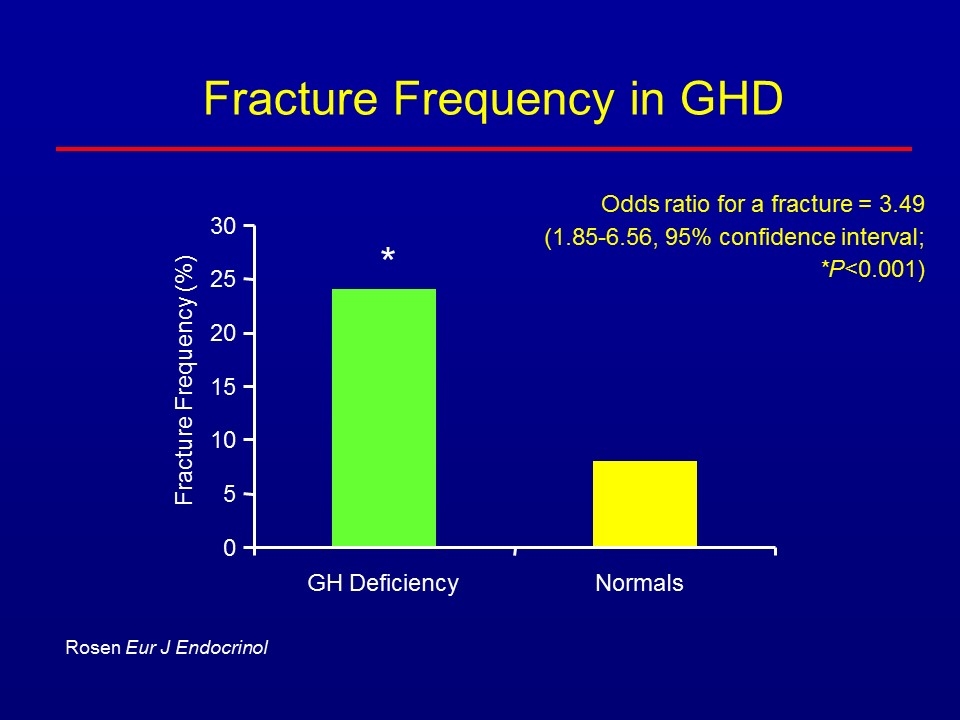
Fracture Frequency in GHD 0 5 10 15 20 25 30 GH Deficiency Normals Fracture Frequency (%) * Rosen Eur J Endocrinol Odds ratio for a fracture = 3.49 (1.85-6.56, 95% confidence interval; *P<0.001)
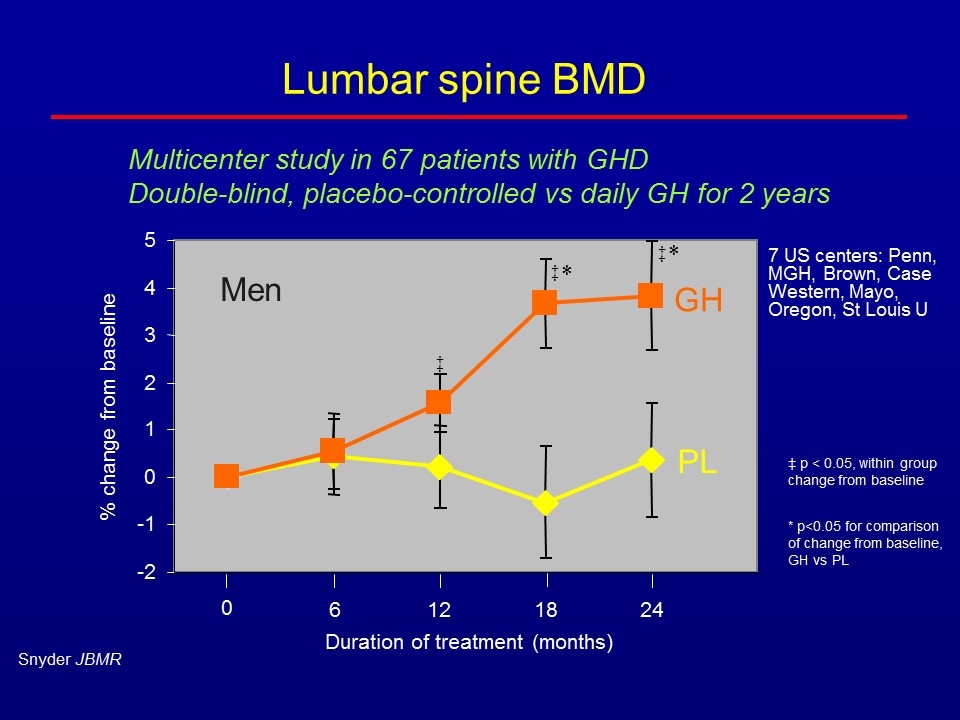
Lumbar spine BMD % change from baseline Duration of treatment (months) GH PL 6 12 18 24 0 ‡ ‡ ‡ * * Men -2 -1 0 1 2 3 4 5 Snyder JBMR ‡ p < 0.05, within group change from baseline * p<0.05 for comparison of change from baseline, GH vs PL 7 US centers: Penn, MGH, Brown, Case Western, Mayo, Oregon, St Louis U Multicenter study in 67 patients with GHD Double-blind, placebo-controlled vs daily GH for 2 years
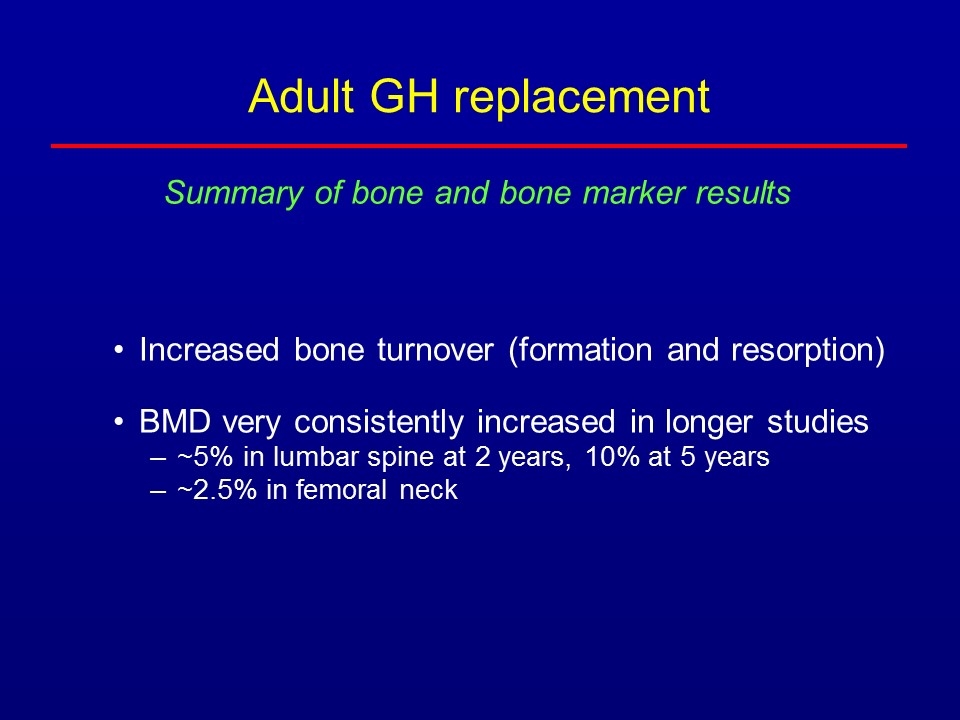
Adult GH replacement Increased bone turnover (formation and resorption) BMD very consistently increased in longer studies ~5% in lumbar spine at 2 years, 10% at 5 years ~2.5% in femoral neck Summary of bone and bone marker results
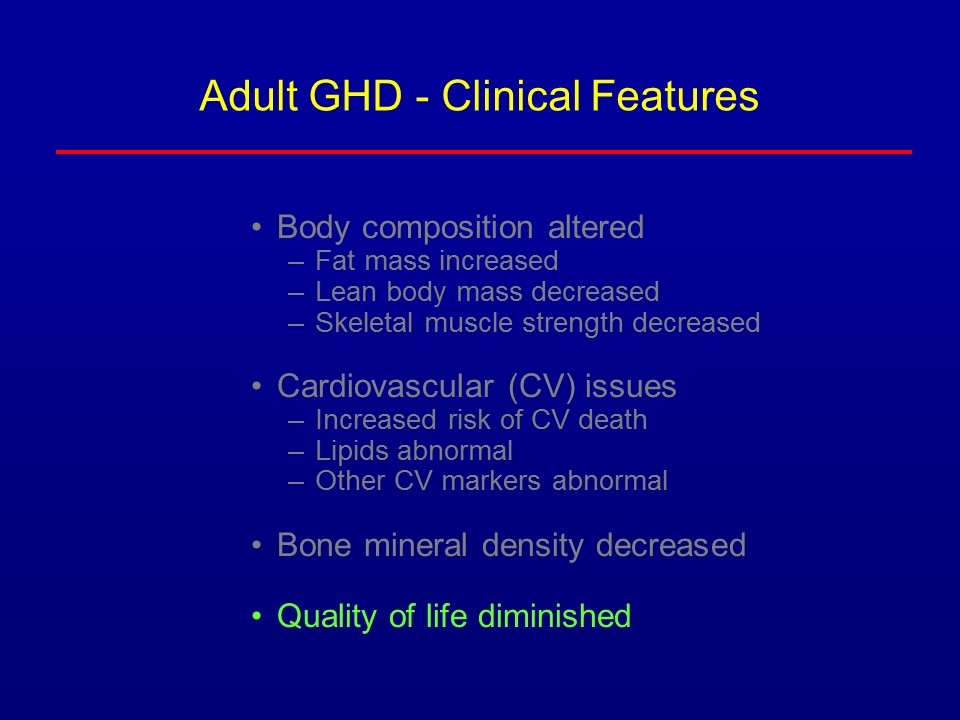
Adult GHD - Clinical Features Body composition altered Fat mass increased Lean body mass decreased Skeletal muscle strength decreased Cardiovascular (CV) issues Increased risk of CV death Lipids abnormal Other CV markers abnormal Bone mineral density decreased Quality of life diminished
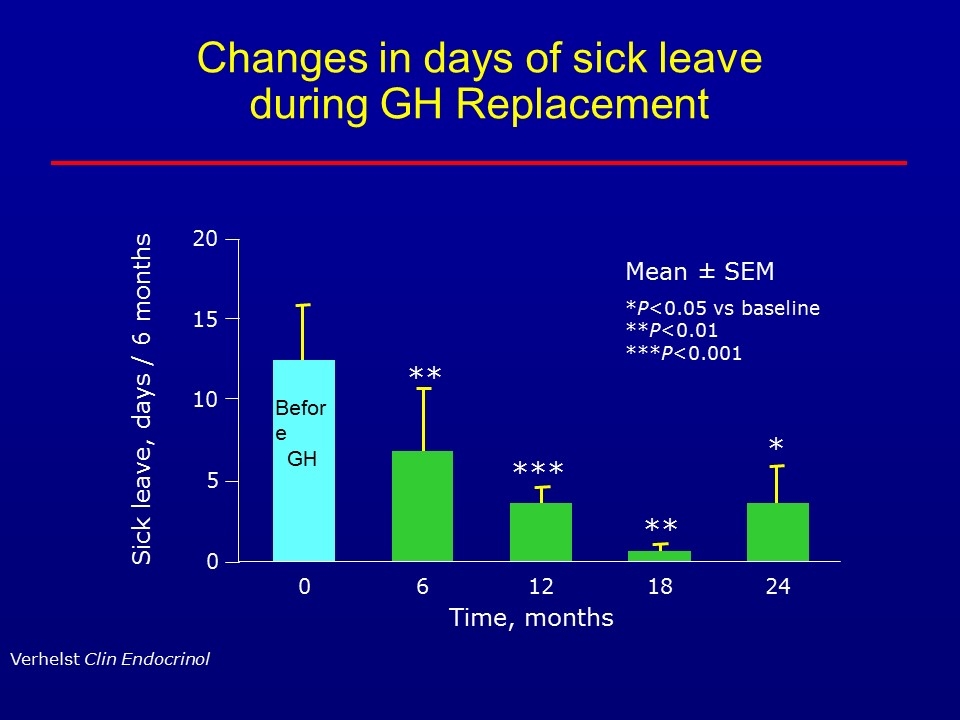
Changes in days of sick leave during GH Replacement Time, months Sick leave, days / 6 months 0 15 20 10 5 0 Mean ± SEM *P<0.05 vs baseline **P<0.01 ***P<0.001 ** *** ** * Verhelst Clin Endocrinol 6 12 18 24 Before GH
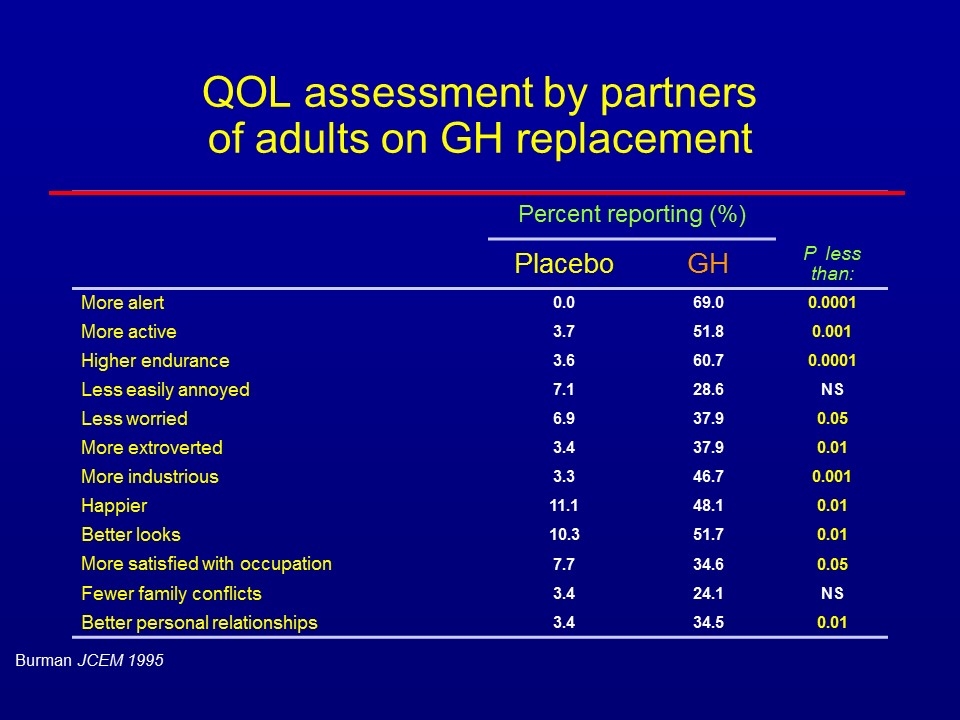
QOL assessment by partners of adults on GH replacement Percent reporting (%) Placebo GH P less than: More alert 0.0 69.0 0.0001 More active 3.7 51.8 0.001 Higher endurance 3.6 60.7 0.0001 Less easily annoyed 7.1 28.6 NS Less worried 6.9 37.9 0.05 More extroverted 3.4 37.9 0.01 More industrious 3.3 46.7 0.001 Happier 11.1 48.1 0.01 Better looks 10.3 51.7 0.01 More satisfied with occupation 7.7 34.6 0.05 Fewer family conflicts 3.4 24.1 NS Better personal relationships 3.4 34.5 0.01 Burman JCEM 1995
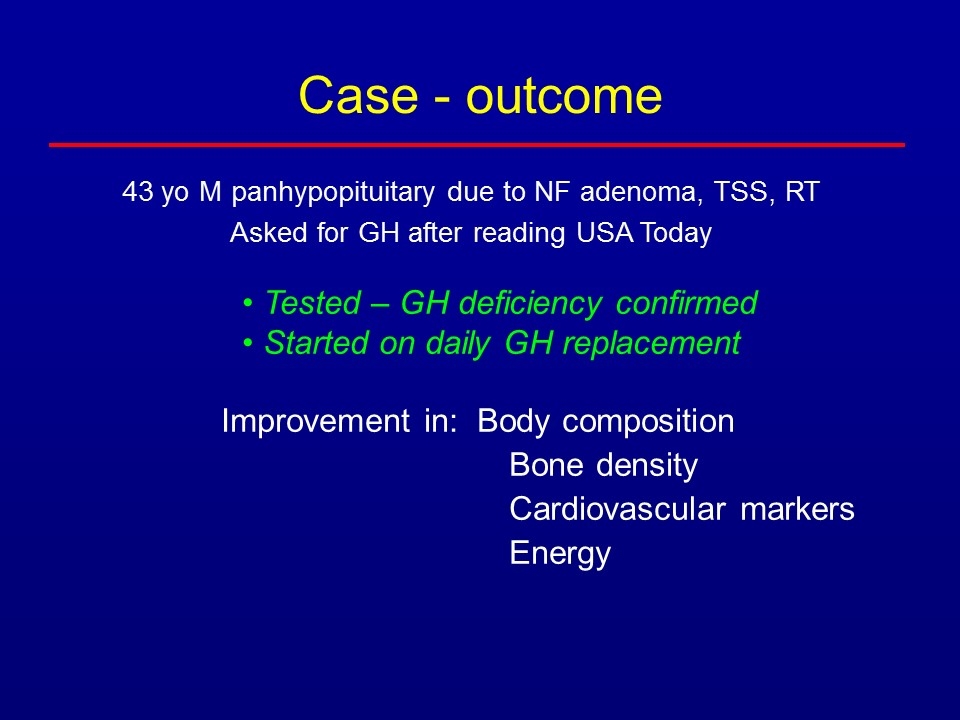
43 yo M panhypopituitary due to NF adenoma, TSS, RT Asked for GH after reading USA Today Tested – GH deficiency confirmed Started on daily GH replacement Improvement in: Body composition Bone density Cardiovascular markers Energy Case - outcome
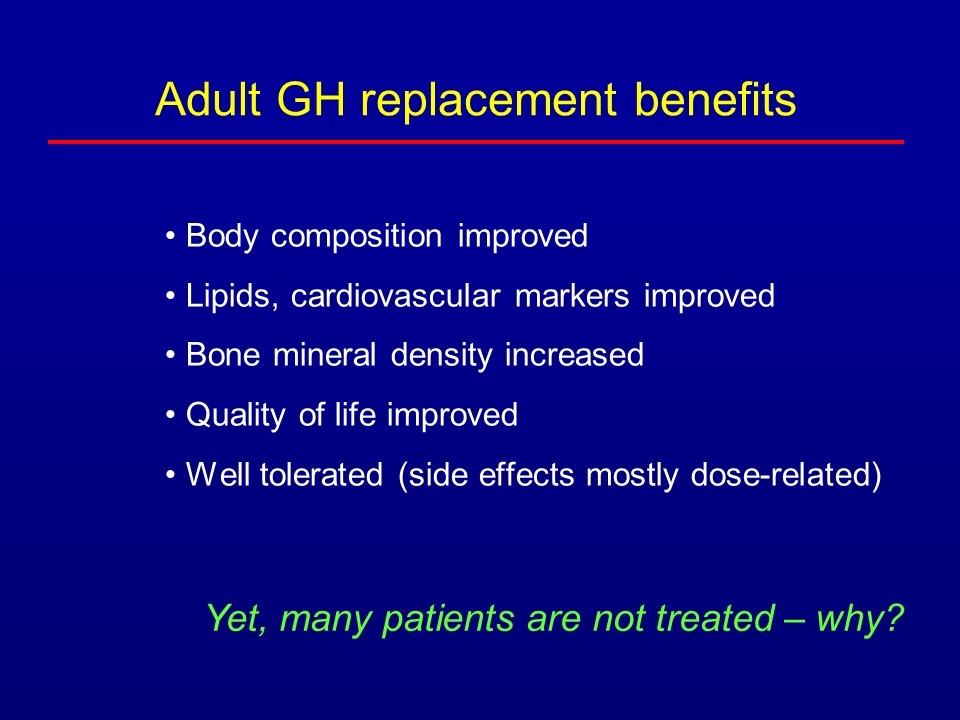
Adult GH replacement benefits Body composition improved Lipids, cardiovascular markers improved Bone mineral density increased Quality of life improved Well tolerated (side effects mostly dose-related) Yet, many patients are not treated – why?
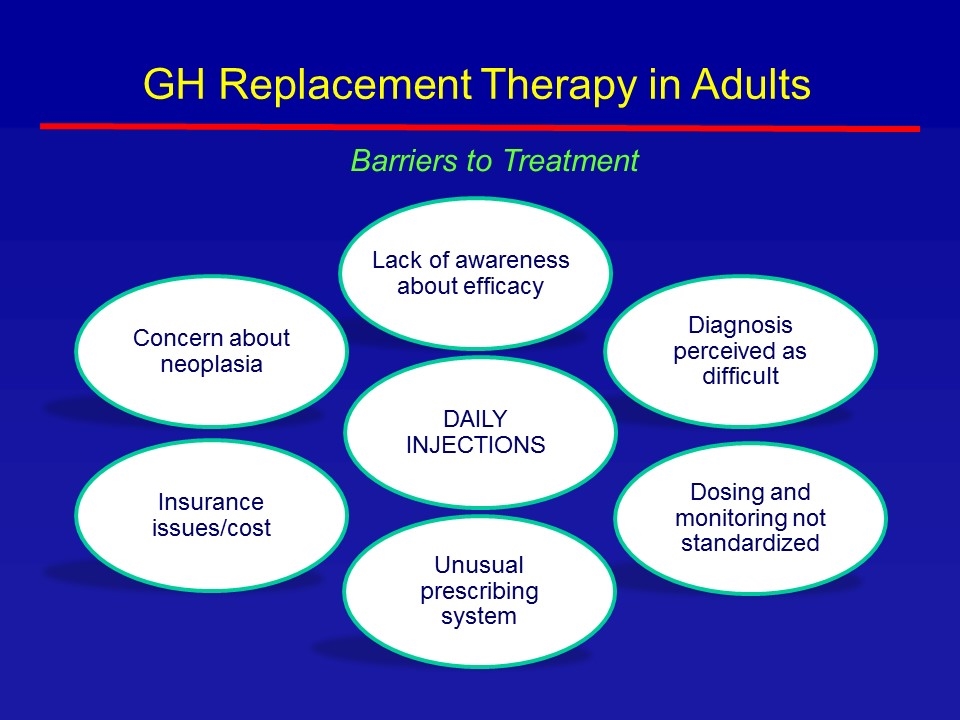
Lack of awareness about efficacy Dosing and monitoring not standardized Diagnosis perceived as difficult Unusual prescribing system Insurance issues/cost Concern about neoplasia Barriers to Treatment GH Replacement Therapy in Adults DAILY INJECTIONS

Case 55 year old man with large pituitary tumor Panhypopituitary on cortisol, thyroid, testosterone Enrolled in a study of weekly long-acting GH Reduction in body fat, increase in lean mass, BMD, QOL When study ended, started commercial daily GH Lost job à no insurance à stopped daily GH New job à insurance à advised to resume daily GH “Didn’t like the shots - I’ll wait until your next long-acting GH study”
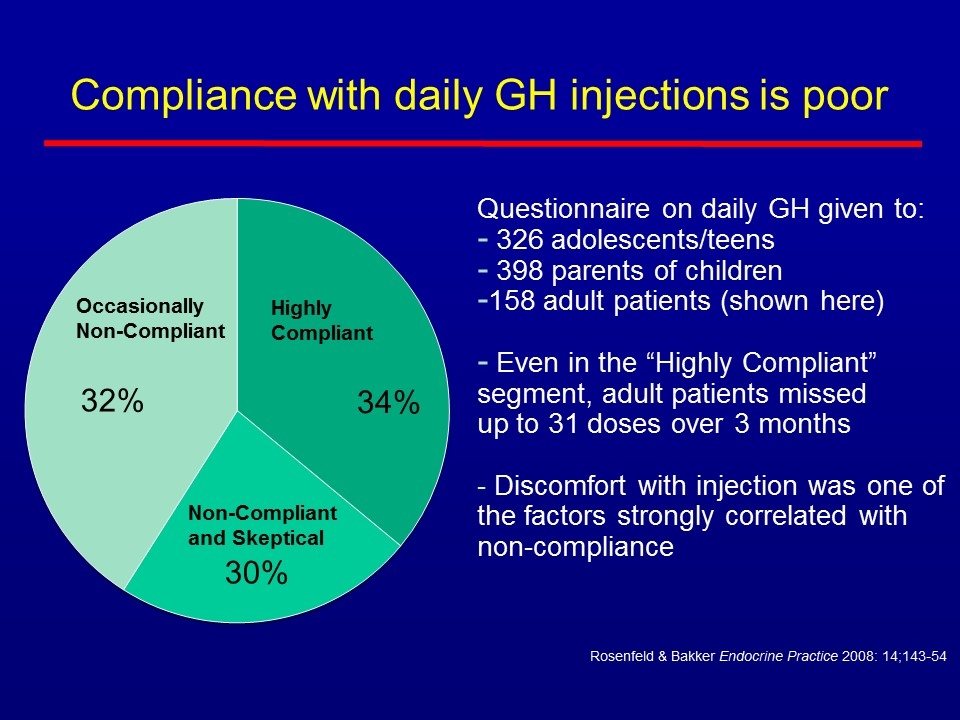
Compliance with daily GH injections is poor Rosenfeld & Bakker Endocrine Practice 2008: 14;143-54 Questionnaire on daily GH given to: 326 adolescents/teens 398 parents of children 158 adult patients (shown here) Even in the “Highly Compliant” segment, adult patients missed up to 31 doses over 3 months - Discomfort with injection was one of the factors strongly correlated with non-compliance Non-Compliant and Skeptical Occasionally Non-Compliant Highly Compliant

Case 70 year old woman from a New England state Surgery and radiation for craniopharyngioma Partial hypopituitarism (central hypothyroidism and GHD) Enrolled in a study of GH vs PL 2004-5, did well on GH After study, declined daily commercial GH due to shots Enrolled in weekly LAGH study 2006-7, did well After study, again declined daily GH Wanted to join new LAGH study but did not qualify Still waiting for commercial LAGH

Hormone deficiency associated with pituitary and neurologic disorders in adults Common in patients with other pituitary deficiencies Affect many tissues throughout the body GH replacement improves: body composition muscle strength, endurance bone mineral density cardiovascular markers quality of life What is AGHD?

My patients would answer YES! Will LAGH fill an unmet need in the treatment of AGHD?


Topline AGHD Phase 2 Data: Results of Ongoing VITAL (15VR7) Study Colin Hislop, MD | Chief Medical Officer
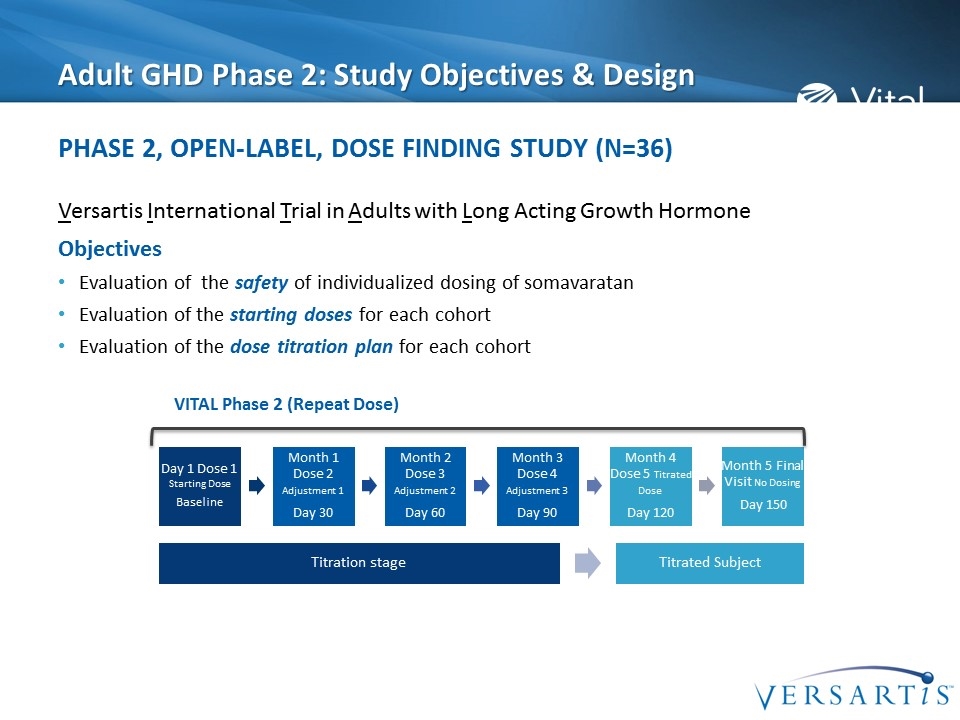
Adult GHD Phase 2: Study Objectives & Design Versartis International Trial in Adults with Long Acting Growth Hormone Objectives Evaluation of the safety of individualized dosing of somavaratan Evaluation of the starting doses for each cohort Evaluation of the dose titration plan for each cohort Phase 2, Open-Label, Dose Finding Study (N=36) VITAL Phase 2 (Repeat Dose) Month 1 Dose 2 Adjustment 1 Day 30 Month 2 Dose 3 Adjustment 2 Day 60 Month 3 Dose 4 Adjustment 3 Day 90 Month 4 Dose 5 Titrated Dose Day 120 Month 5 Final Visit No Dosing Day 150 Day 1 Dose 1 Starting Dose Baseline Titration stage Titrated Subject
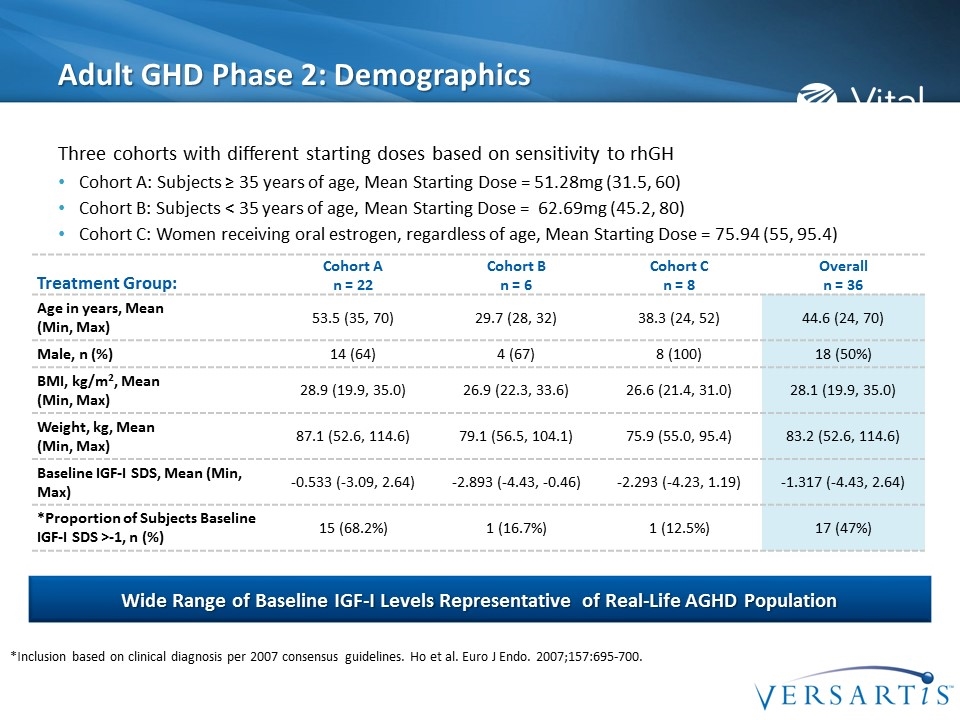
Adult GHD Phase 2: Demographics Three cohorts with different starting doses based on sensitivity to rhGH Cohort A: Subjects ≥ 35 years of age, Mean Starting Dose = 51.28mg (31.5, 60) Cohort B: Subjects < 35 years of age, Mean Starting Dose = 62.69mg (45.2, 80) Cohort C: Women receiving oral estrogen, regardless of age, Mean Starting Dose = 75.94 (55, 95.4) Wide Range of Baseline IGF-I Levels Representative of Real-Life AGHD Population Treatment Group: Cohort A n = 22 Cohort B n = 6 Cohort C n = 8 Overall n = 36 Age in years, Mean (Min, Max) 53.5 (35, 70) 29.7 (28, 32) 38.3 (24, 52) 44.6 (24, 70) Male, n (%) 14 (64) 4 (67) 8 (100) 18 (50%) BMI, kg/m2, Mean (Min, Max) 28.9 (19.9, 35.0) 26.9 (22.3, 33.6) 26.6 (21.4, 31.0) 28.1 (19.9, 35.0) Weight, kg, Mean (Min, Max) 87.1 (52.6, 114.6) 79.1 (56.5, 104.1) 75.9 (55.0, 95.4) 83.2 (52.6, 114.6) Baseline IGF-I SDS, Mean (Min, Max) -0.533 (-3.09, 2.64) -2.893 (-4.43, -0.46) -2.293 (-4.23, 1.19) -1.317 (-4.43, 2.64) *Proportion of Subjects Baseline IGF-I SDS >-1, n (%) 15 (68.2%) 1 (16.7%) 1 (12.5%) 17 (47%) *Inclusion based on clinical diagnosis per 2007 consensus guidelines. Ho et al. Euro J Endo. 2007;157:695-700.
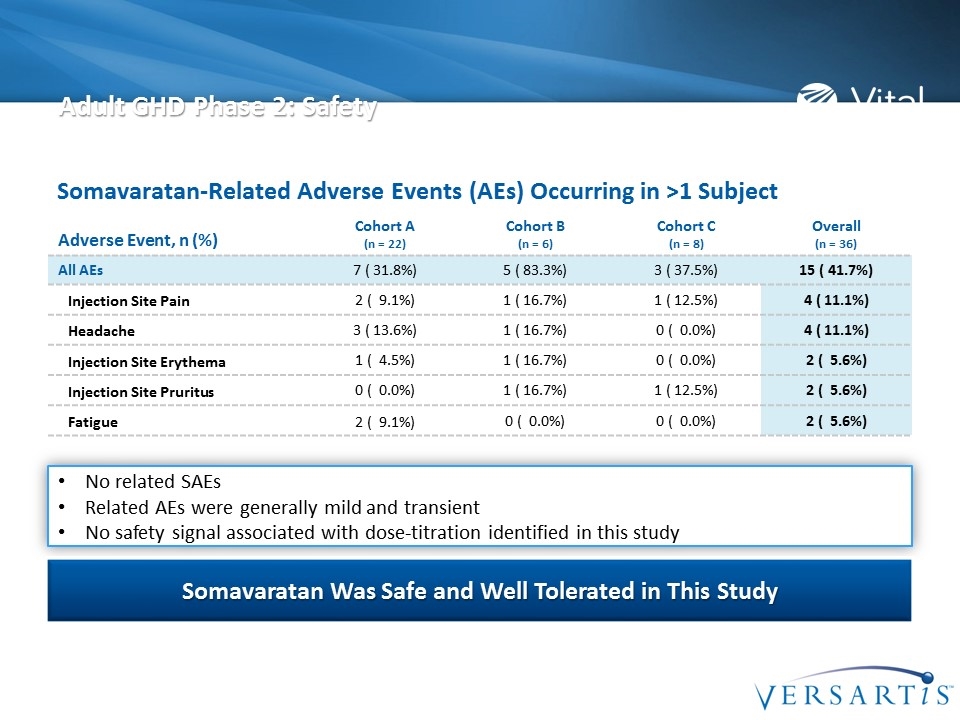
Adult GHD Phase 2: Safety Adverse Event, n (%) Cohort A (n = 22) Cohort B (n = 6) Cohort C (n = 8) Overall (n = 36) All AEs 7 ( 31.8%) 5 ( 83.3%) 3 ( 37.5%) 15 ( 41.7%) Injection Site Pain 2 ( 9.1%) 1 ( 16.7%) 1 ( 12.5%) 4 ( 11.1%) Headache 3 ( 13.6%) 1 ( 16.7%) 0 ( 0.0%) 4 ( 11.1%) Injection Site Erythema 1 ( 4.5%) 1 ( 16.7%) 0 ( 0.0%) 2 ( 5.6%) Injection Site Pruritus 0 ( 0.0%) 1 ( 16.7%) 1 ( 12.5%) 2 ( 5.6%) Fatigue 2 ( 9.1%) 0 ( 0.0%) 0 ( 0.0%) 2 ( 5.6%) No related SAEs Related AEs were generally mild and transient No safety signal associated with dose-titration identified in this study Somavaratan Was Safe and Well Tolerated in This Study Somavaratan-Related Adverse Events (AEs) Occurring in >1 Subject
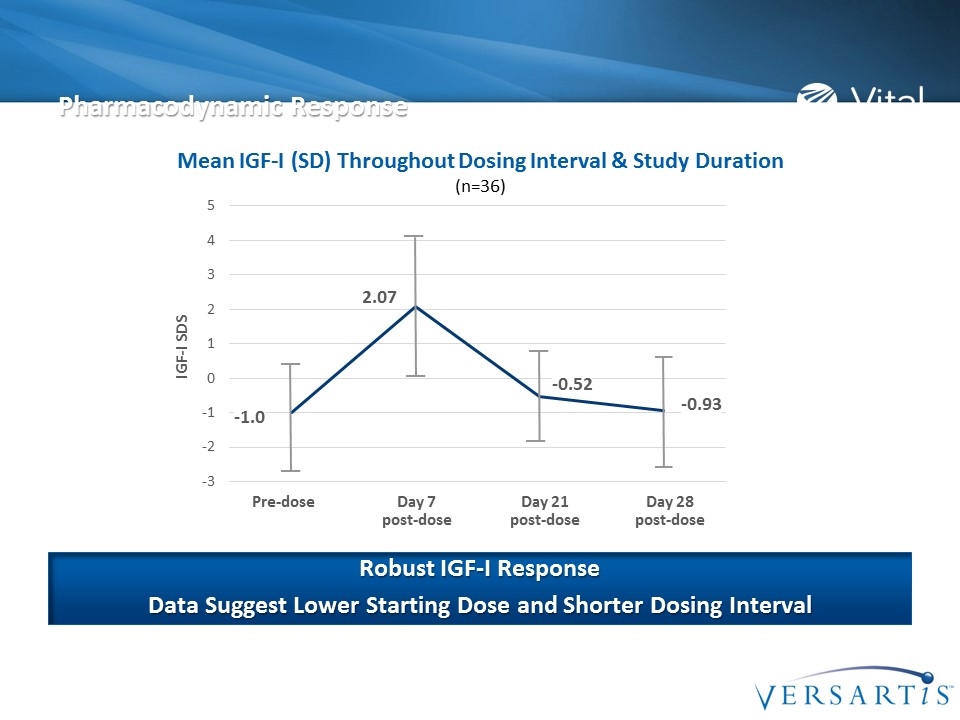
Pre-dose Day 7 post-dose Day 21 post-dose Day 28 post-dose Pharmacodynamic Response Mean IGF-I (SD) Throughout Dosing Interval & Study Duration (n=36) Robust IGF-I Response Data Suggest Lower Starting Dose and Shorter Dosing Interval -1.0 2.07 -0.52 -0.93 IGF-I SDS
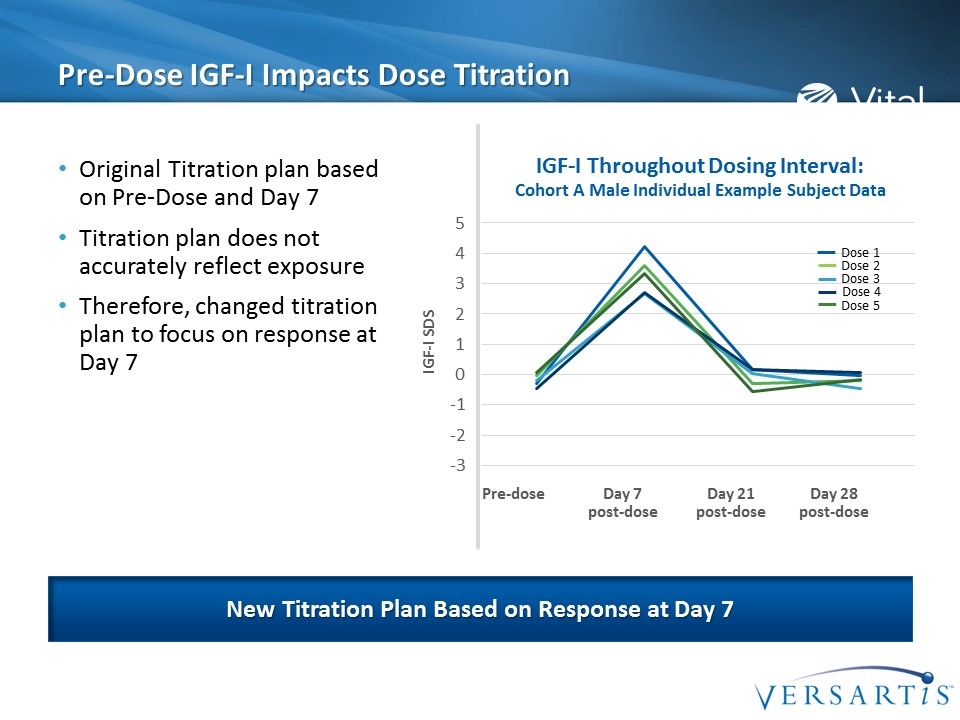
Pre-Dose IGF-I Impacts Dose Titration Original Titration plan based on Pre-Dose and Day 7 Titration plan does not accurately reflect exposure Therefore, changed titration plan to focus on response at Day 7 New Titration Plan Based on Response at Day 7 IGF-I Throughout Dosing Interval: Cohort A Male Individual Example Subject Data IGF-I SDS Dose 5 Dose 1 Dose 2 Dose 3 Dose 4 Pre-dose Day 7 post-dose Day 21 post-dose Day 28 post-dose

Starting Dose & Titration Plan Somavaratan IGF-I response was robust IGF-I values returned to pre-dose levels by 21 days post dose Titration plan was not ideal to address the high IGF-I at Day 7 No IGF-I associated clinically relevant adverse events 20 mg is the starting dose selected to maintain IGF-I in target range 40 mg is selected for women on oral estrogen Based on these data: A lower starting dose was identified Titration plan based on Day 7 Value A more frequent administration (twice-monthly) is required to provide pharmacologic effect throughout the dosing interval
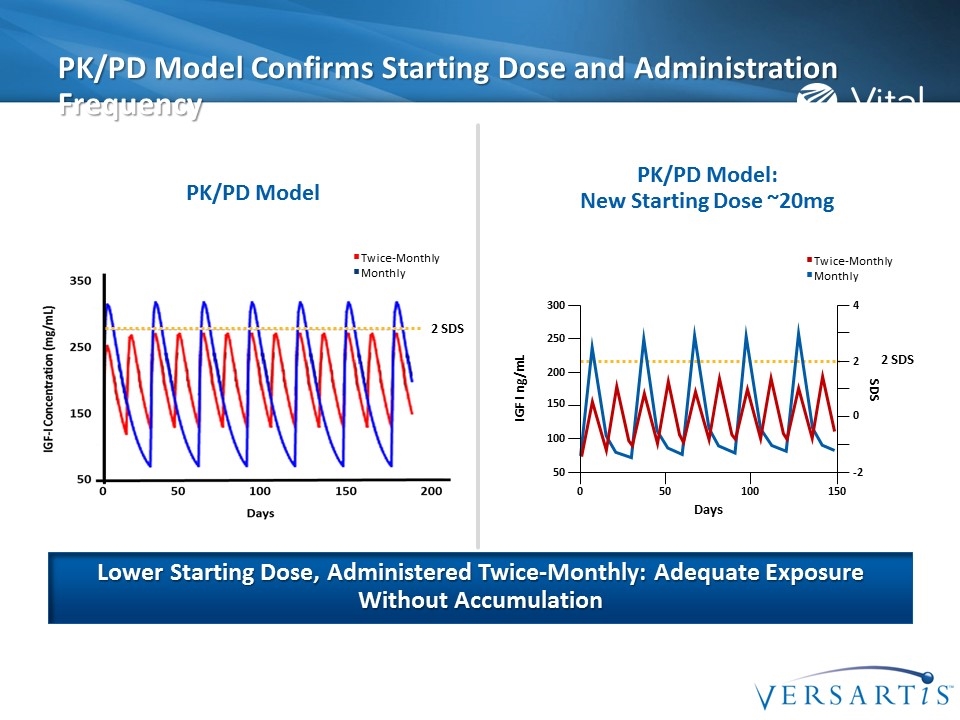
PK/PD Model Confirms Starting Dose and Administration Frequency Lower Starting Dose, Administered Twice-Monthly: Adequate Exposure Without Accumulation Twice-Monthly Monthly PK/PD Model PK/PD Model: New Starting Dose ~20mg 2 SDS 2 SDS Twice-Monthly Monthly SDS 250 IGF I ng/mL 200 150 100 50 300 2 0 -2 4 Days 0 50 100 150
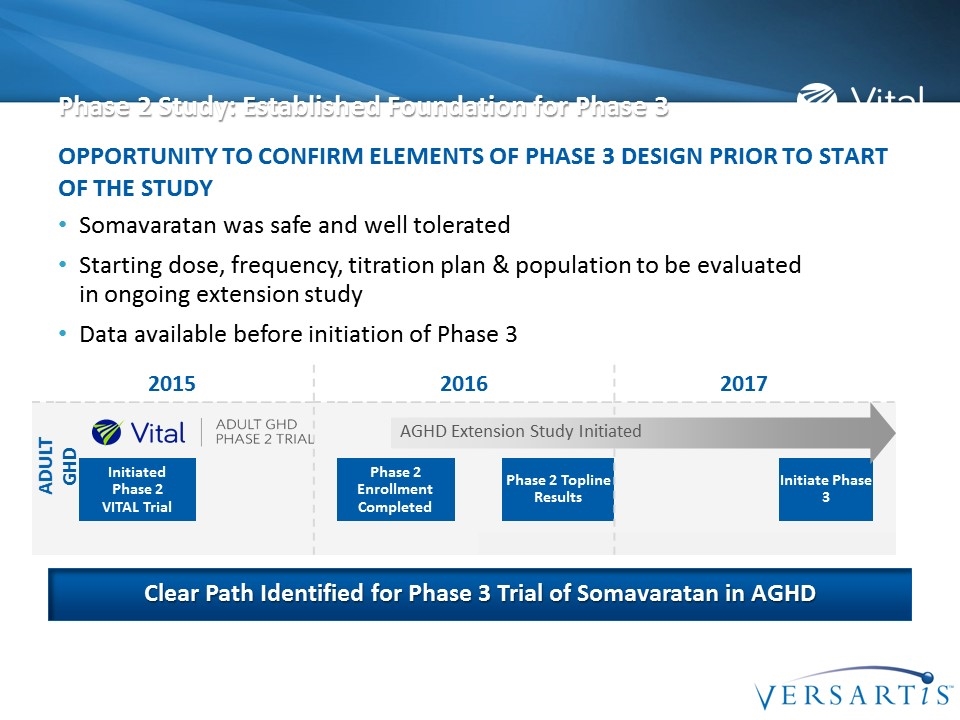
Phase 2 Study: Established Foundation for Phase 3 Somavaratan was safe and well tolerated Starting dose, frequency, titration plan & population to be evaluated in ongoing extension study Data available before initiation of Phase 3 Opportunity to confirm elements of Phase 3 design prior to start of the study Clear Path Identified for Phase 3 Trial of Somavaratan in AGHD 2015 2016 2017 Initiated Phase 2 VITAL Trial Phase 2 Enrollment Completed Phase 2 Topline Results Initiate Phase 3 ADULT GHD AGHD Extension Study Initiated


Q & A Session

Closing Remarks Jay Shepard | Chief Executive Officer
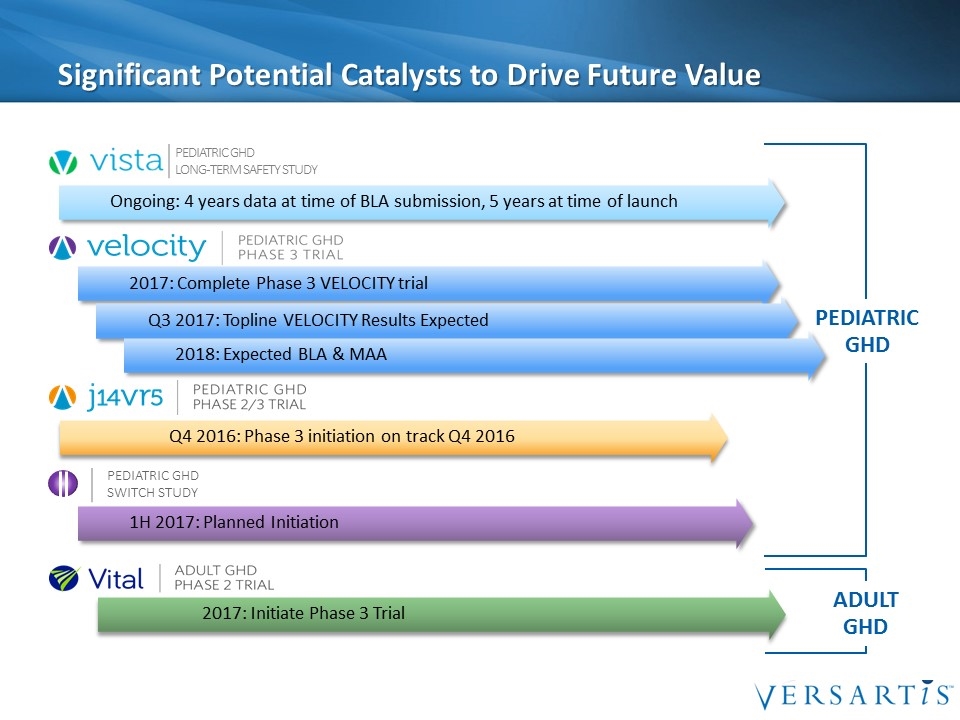
Significant Potential Catalysts to Drive Future Value PEDIATRIC GHD 2017: Initiate Phase 3 Trial 2017: Complete Phase 3 VELOCITY trial Q4 2016: Phase 3 initiation on track Q4 2016 Q3 2017: Topline VELOCITY Results Expected 2018: Expected BLA & MAA Ongoing: 4 years data at time of BLA submission, 5 years at time of launch Pediatric GHD Long-Term Safety Study 1H 2017: Planned Initiation Pediatric GHD Switch Study ADULT GHD

Thank You!































































































































