
Halting Disease Progression in its Tracks © 2020 Aravive, Inc. Aravive Corporate Presentation March 2020 Exhibit 99.2

Important Information © 2020 Aravive, Inc. Forward-Looking Statements This presentation contains forward-looking statements that may discuss Aravive’s plans, goals, intentions and expectations as to future trends, events, results of operations, financial condition or other matters. Forward- looking statements generally include statements that are predictive in nature and depend upon or refer to future events or conditions, and they often include words such as “may,” “will,” “should,” “would,” “expect,” “anticipate,” “plan,” “likely,” “believe,” “estimate,” “project,” “intend,” and other similar expressions. Statements that are not historical facts are forward-looking statements. Forward-looking statements included in this presentation include statements regarding Aravive’s planned clinical activities, including the design, initiation, patient enrollment and availability of data from clinical studies, future indications and cash position and the anticipated safety, activity and manufacturability of Aravive’s product candidates. Forward-looking statements are based on Aravive’s current beliefs and assumptions, are subject to risks and uncertainties and are not guarantees of future performance. Actual results could differ materially from those contained in any forward-looking statement as a result of various factors, including, without limitation: the unpredictability of clinical development activities; risks related to Aravive’s ability to estimate and control its operating expenses; Aravive’s ability to protect its intellectual property rights; changes in the competitive environment; and legislative, regulatory, political and economic developments. The foregoing review of important factors that could cause actual events to differ from expectations should not be construed as exhaustive and should be read in conjunction with statements that are included herein and elsewhere, including the risk factors included in Aravive’s most recent Annual Report on Form 10-K, Quarterly Report on Form 10-Q and Current Reports on Form 8-K filed with the Securities and Exchange Commission. Except as required by law, Aravive undertakes no obligation to revise or update any forward-looking statement or to make any other forward-looking statements, whether as a result of new information, future events or otherwise.

Company Overview The Company Lead Clinical Program (AVB-500) Aravive is a clinical stage company developing treatments designed to halt the progression of life-threatening diseases, including cancer and fibrosis NASDAQ-listed company (ARAV) Offices in Houston, TX (hdqtrs.) and Palo Alto, CA $20 million Product Development Award from the Cancer Prevention & Research Institute of Texas (CPRIT) Led by a team of industry veterans with decades of target biology, drug development, & commercialization expertise Multiple clinical and program milestones during 2020 A first-in-class, ultra-high affinity, Fc-fusion protein designed to selectively block the novel GAS6-AXL signaling pathway Proof-of-mechanism established in clinic using proprietary biomarker for target engagement Early proof-of-concept for anti-tumor activity demonstrated in platinum-resistant ovarian cancer Currently in expanded Phase 1b/2 for platinum-resistant ovarian cancer; Fast-track designation granted by the FDA Ideal profile for combination with chemo, check-point inhibitors and PARP inhibitors in broad range of indications IP coverage through 2033 and beyond Our approach draws on novel insights into targeting signaling pathways that drive the activation, migration and invasion of abnormal cells into healthy tissues © 2020 Aravive, Inc.
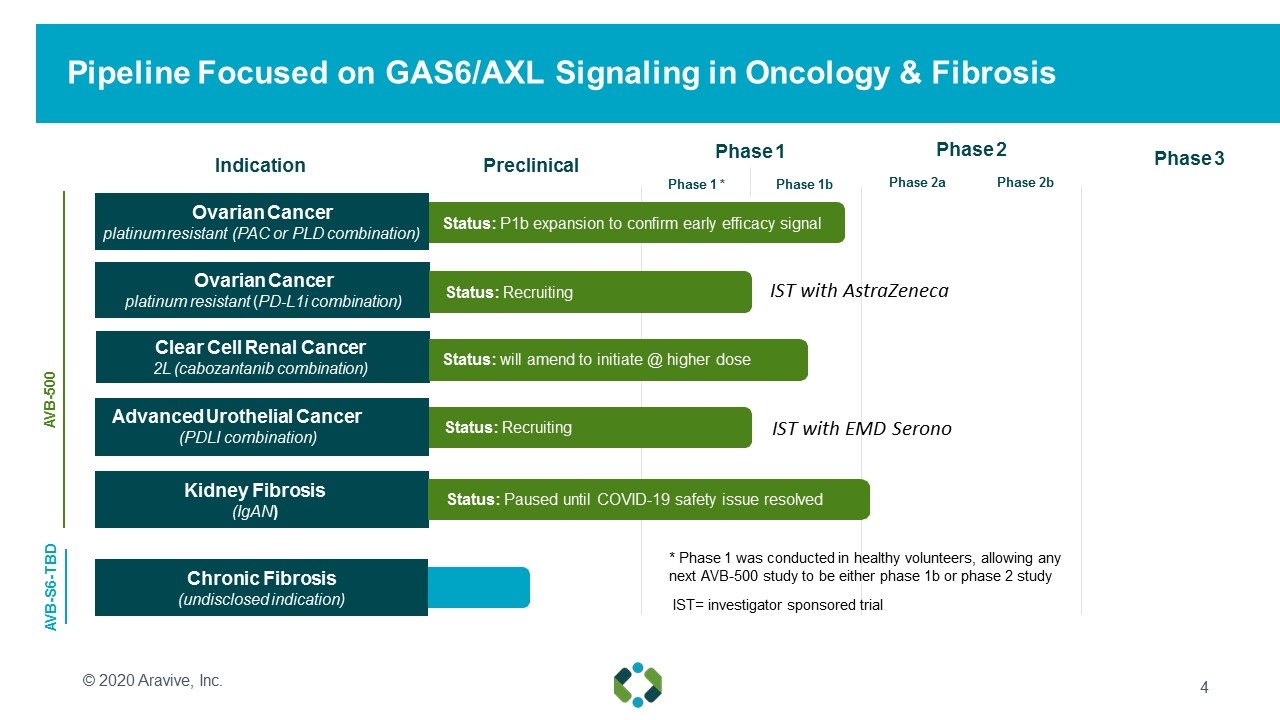
Pipeline Focused on GAS6/AXL Signaling in Oncology & Fibrosis Indication Preclinical Phase 1 Phase 1 *Phase 1b Phase 3 AVB-S6-TBD Ovarian Cancer platinum resistant (PAC or PLD combination) Clear Cell Renal Cancer 2L (cabozantanib combination) Advanced Urothelial Cancer (PDLI combination) Kidney Fibrosis (IgAN) Chronic Fibrosis (undisclosed indication) AVB-500 Status: will amend to initiate @ higher dose Status: P1b expansion to confirm early efficacy signal Status: Paused until COVID-19 safety issue resolved © 2020 Aravive, Inc. Ovarian Cancer platinum resistant (PD-L1i combination) Status: Recruiting IST= investigator sponsored trial Status: Recruiting * Phase 1 was conducted in healthy volunteers, allowing any next AVB-500 study to be either phase 1b or phase 2 study Phase 2 Phase 2aPhase 2b IST with AstraZeneca IST with EMD Serono
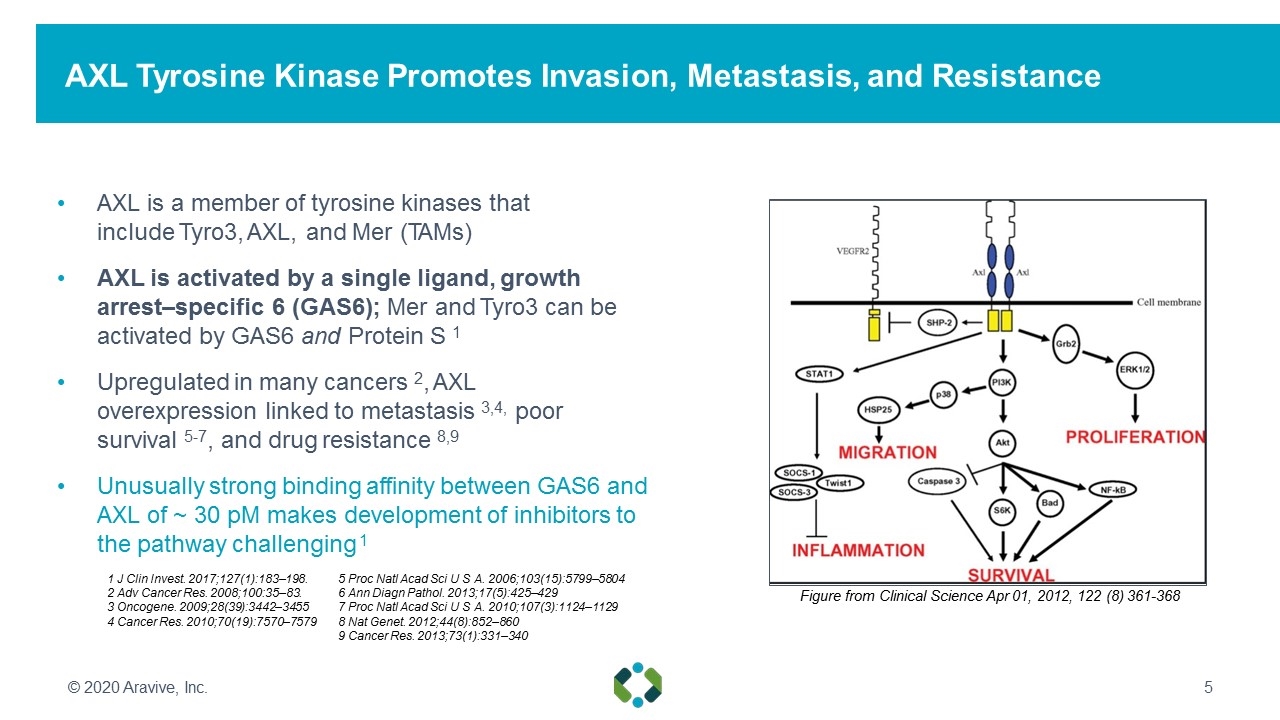
AXL Tyrosine Kinase Promotes Invasion, Metastasis, and Resistance AXL is a member of tyrosine kinases that include Tyro3, AXL, and Mer (TAMs) AXL is activated by a single ligand, growth arrest–specific 6 (GAS6); Mer and Tyro3 can be activated by GAS6 and Protein S 1 Upregulated in many cancers 2, AXL overexpression linked to metastasis 3,4, poor survival 5-7, and drug resistance 8,9 Unusually strong binding affinity between GAS6 and AXL of ~ 30 pM makes development of inhibitors to the pathway challenging 1 Figure from Clinical Science Apr 01, 2012, 122 (8) 361-368 © 2020 Aravive, Inc. 1 J Clin Invest. 2017;127(1):183–198. 2 Adv Cancer Res. 2008;100:35–83. 3 Oncogene. 2009;28(39):3442–3455 4 Cancer Res. 2010;70(19):7570–7579 5 Proc Natl Acad Sci U S A. 2006;103(15):5799–5804 6 Ann Diagn Pathol. 2013;17(5):425–429 7 Proc Natl Acad Sci U S A. 2010;107(3):1124–1129 8 Nat Genet. 2012;44(8):852–860 9 Cancer Res. 2013;73(1):331–340
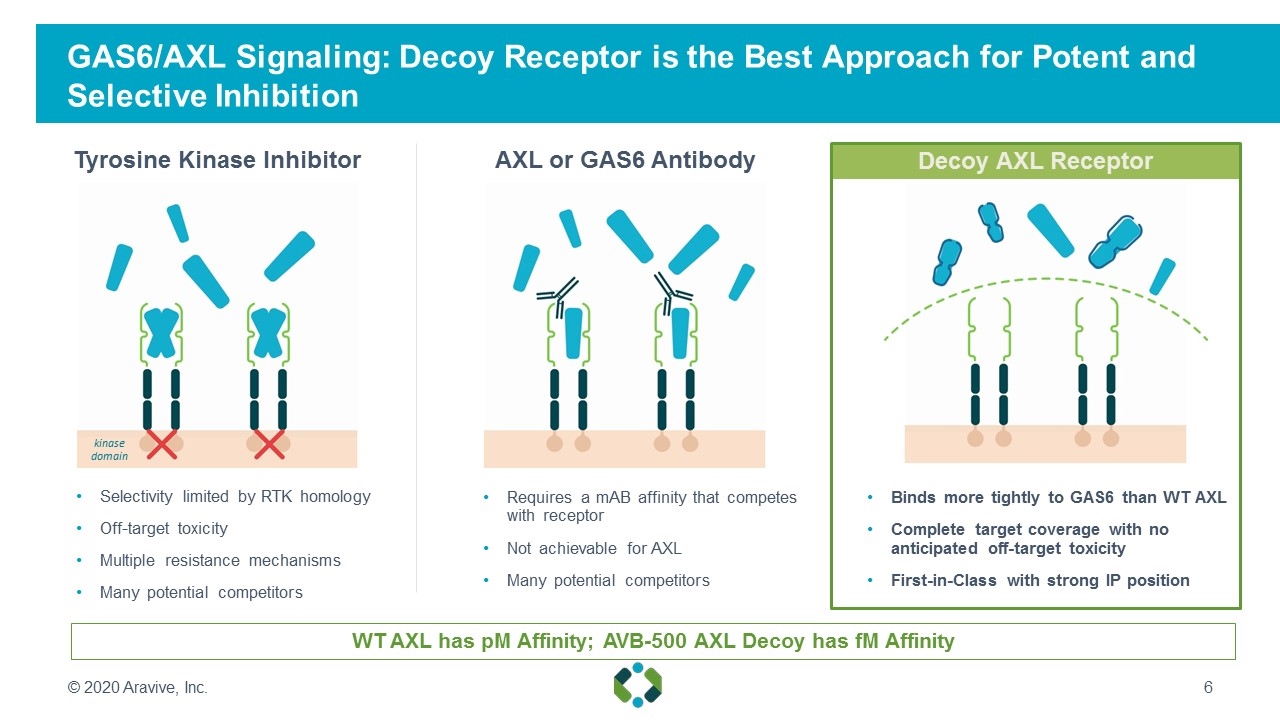
GAS6/AXL Signaling: Decoy Receptor is the Best Approach for Potent and Selective Inhibition WT AXL has pM Affinity; AVB-500 AXL Decoy has fM Affinity Selectivity limited by RTK homology Off-target toxicity Multiple resistance mechanisms Many potential competitors AXL or GAS6 Antibody Tyrosine Kinase Inhibitor Requires a mAB affinity that competes with receptor Not achievable for AXL Many potential competitors Binds more tightly to GAS6 than WT AXL Complete target coverage with no anticipated off-target toxicity First-in-Class with strong IP position kinase domain © 2020 Aravive, Inc. Decoy AXL Receptor
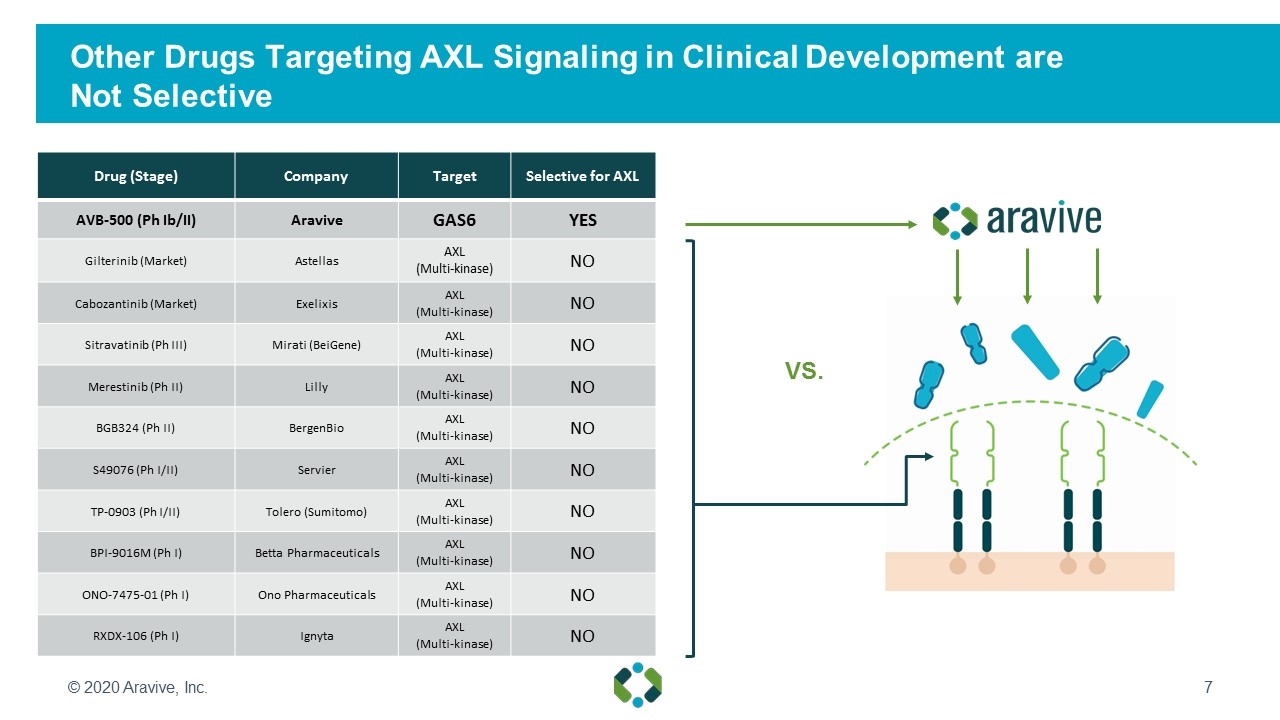
Other Drugs Targeting AXL Signaling in Clinical Development are Not Selective Drug (Stage) Company Target Selective for AXL AVB-500 (Ph Ib/II) Aravive GAS6 YES Gilterinib (Market) Astellas AXL (Multi-kinase) NO Cabozantinib (Market) Exelixis AXL (Multi-kinase) NO Sitravatinib (Ph III) Mirati (BeiGene) AXL (Multi-kinase) NO Merestinib (Ph II) Lilly AXL (Multi-kinase) NO BGB324 (Ph II) BergenBio AXL (Multi-kinase) NO S49076 (Ph I/II) Servier AXL (Multi-kinase) NO TP-0903 (Ph I/II) Tolero (Sumitomo) AXL (Multi-kinase) NO BPI-9016M (Ph I) Betta Pharmaceuticals AXL (Multi-kinase) NO ONO-7475-01 (Ph I) Ono Pharmaceuticals AXL (Multi-kinase) NO RXDX-106 (Ph I) Ignyta AXL (Multi-kinase) NO © 2020 Aravive, Inc. VS.

AVB-500: A Novel Approach to Selectively Inhibit AXL Signaling Decoy receptor engineered for very high affinity for GAS6 – ~200 fold greater than native AXL Favorable safety and PK profile GAS6 not needed by normal tissue GLP preclinical studies demonstrate ≥ 30-fold safety margin (relative to max feasible dose in tox; 3-month tox study completed) As biologic, does not compete for metabolism with chemotherapies; facilitates combination with other therapies Small molecules targeting AXL can have off target activities Strong IP position on GAS6 inhibition Proprietary biomarker tests to monitor GAS6 levels; serum GAS6 levels associated with efficacy in preclinical studies Robust CMC Drug candidate manufactured at high yield and purity AVB-500 Demonstrated Favorable Safety Profile and Proof-of-Mechanism in FIH Phase 1 Trial J Clin Invest. 2017;127(1):183–198 © 2020 Aravive, Inc.
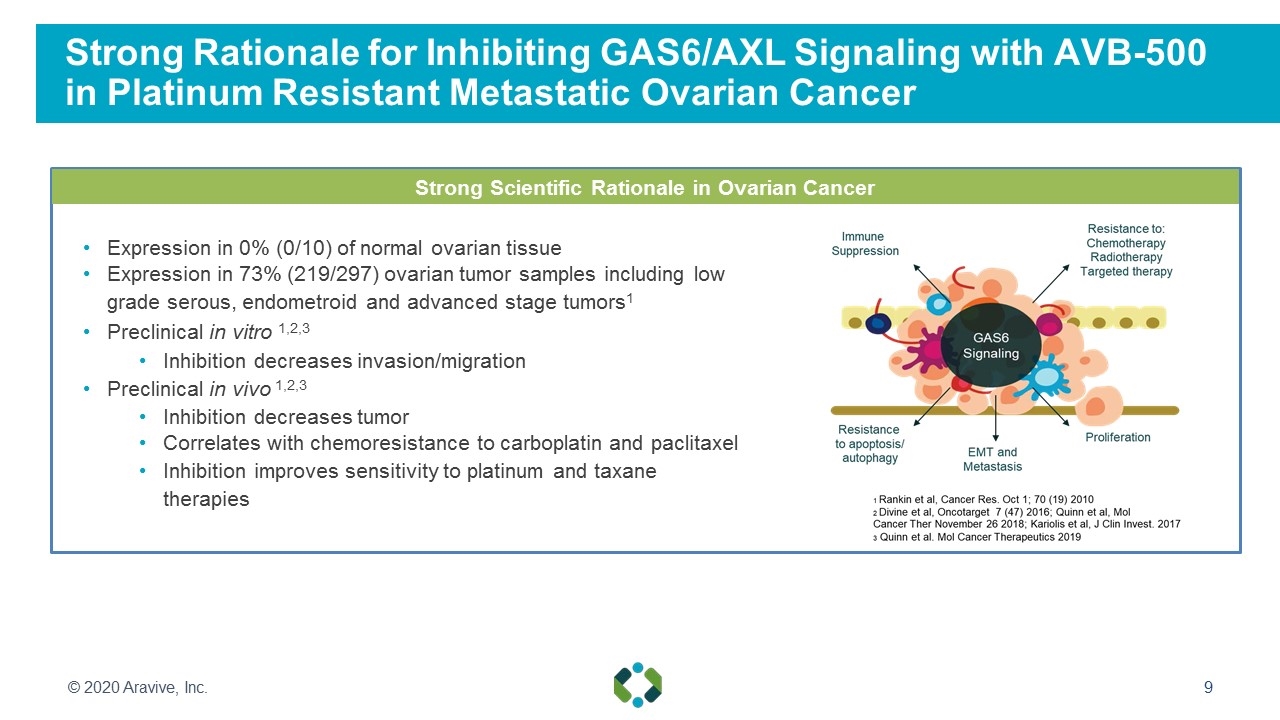
Strong Rationale for Inhibiting GAS6/AXL Signaling with AVB-500 in Platinum Resistant Metastatic Ovarian Cancer Expression in 0% (0/10) of normal ovarian tissue Expression in 73% (219/297) ovarian tumor samples including low grade serous, endometroid and advanced stage tumors1 Preclinical in vitro 1,2,3 Inhibition decreases invasion/migration Preclinical in vivo 1,2,3 Inhibition decreases tumor Correlates with chemoresistance to carboplatin and paclitaxel Inhibition improves sensitivity to platinum and taxane therapies Strong Scientific Rationale in Ovarian Cancer © 2020 Aravive, Inc.
AVB-500: Clinical Program Currently in Expanded Phase 1b/2 Trial With Early Proof-of-Concept Data Fast-track designation granted by the FDA for platinum-resistant ovarian cancer; 10 and 15 mg/kg dose enrollment completed & now enrolling 20 mg/kg dose Well-tolerated to date and no anticipated drug-drug interactions facilitate combination studies (unlike small molecules) The ability to combine with common standards of care allows access to a large total accessible market Robust chemistry manufacturing control with high yield and purity © 2020 Aravive, Inc.
Key Eligibility Criteria for P1b Study in Platinum-Resistant Ovarian Cancer NOS= Not Otherwise Specified | ECOG= Eastem Cooperative Oncology Group © 2020 Aravive, Inc. 1-3 prior lines Measurable disease Platinum free interval ≤ 6mo after most recent platinum-containing regimen Adenocarcinoma NOS, high grade endometroid adenocarcinoma, mixed epithelial (≥ 80% high grade serous), high grade serous, or undifferentiated carcinoma ECOG performance status 0-1
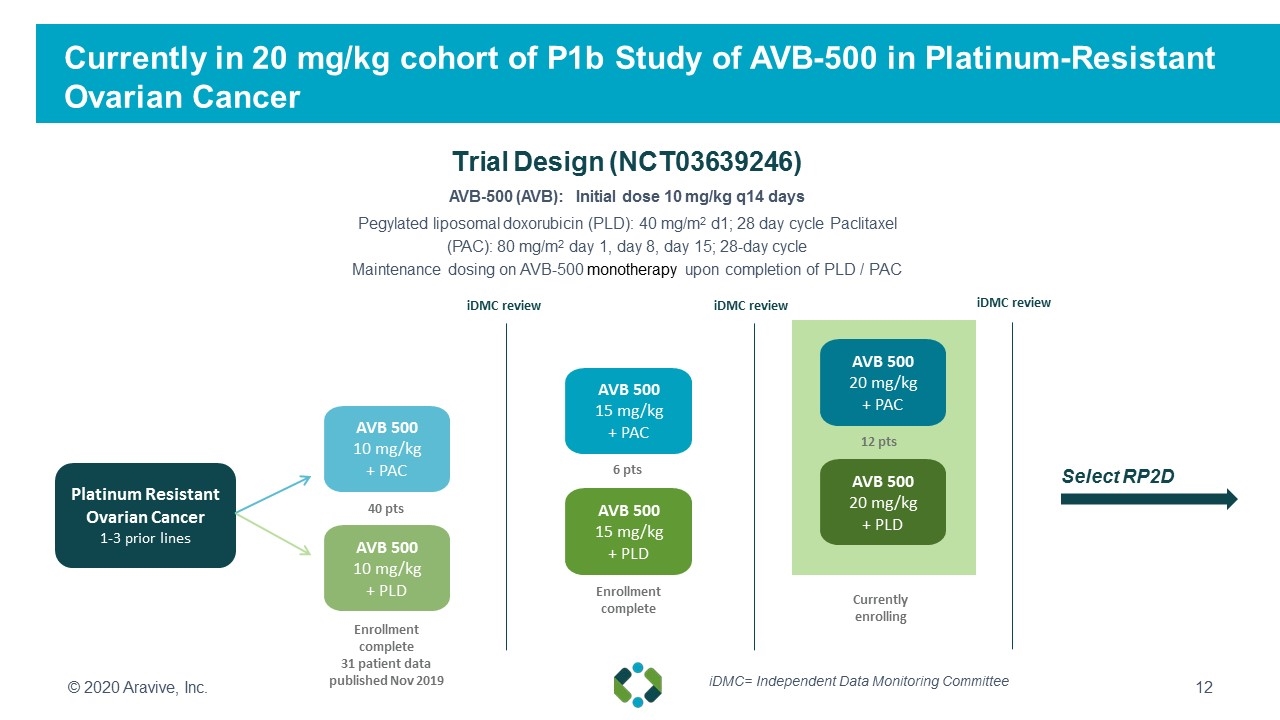
Currently in 20 mg/kg cohort of P1b Study of AVB-500 in Platinum-Resistant Ovarian Cancer AVB-500 (AVB): Initial dose 10 mg/kg q14 days Pegylated liposomal doxorubicin (PLD): 40 mg/m2 d1; 28 day cycle Paclitaxel (PAC): 80 mg/m2 day 1, day 8, day 15; 28-day cycle Maintenance dosing on AVB-500 monotherapy upon completion of PLD / PAC Trial Design (NCT03639246) © 2020 Aravive, Inc. AVB 500 10 mg/kg + PLD AVB 500 15 mg/kg + PLD AVB 500 20 mg/kg + PLD Enrollment complete 31 patient data published Nov 2019 Enrollment complete iDMC review iDMC review Select RP2D iDMC review Platinum Resistant Ovarian Cancer 1-3 prior lines 40 pts 6 pts 12 pts AVB 500 10 mg/kg + PAC AVB 500 15 mg/kg + PAC AVB 500 20 mg/kg + PAC Currently enrolling iDMC= Independent Data Monitoring Committee
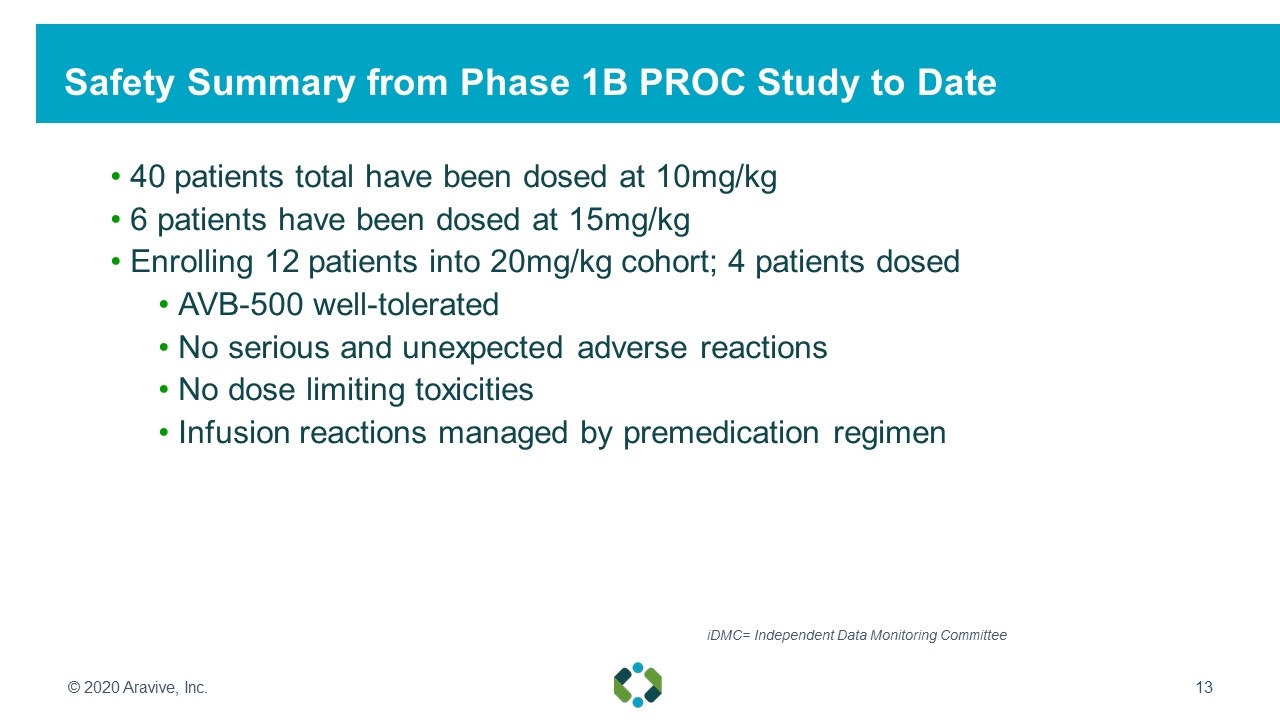
40 patients total have been dosed at 10mg/kg 6 patients have been dosed at 15mg/kg Enrolling 12 patients into 20mg/kg cohort; 4 patients dosed AVB-500 well-tolerated No serious and unexpected adverse reactions No dose limiting toxicities Infusion reactions managed by premedication regimen © 2020 Aravive, Inc. Safety Summary from Phase 1B PROC Study to Date iDMC= Independent Data Monitoring Committee
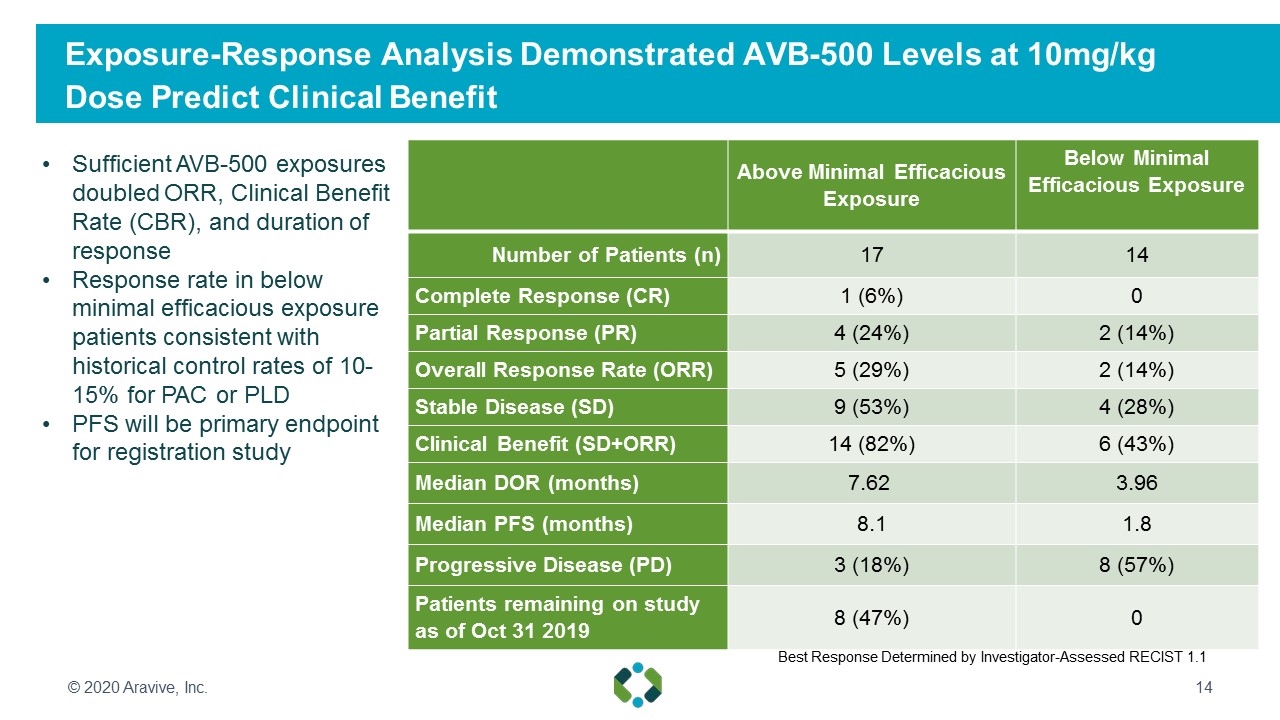
Exposure-Response Analysis Demonstrated AVB-500 Levels at 10mg/kg Dose Predict Clinical Benefit © 2020 Aravive, Inc. Above Minimal Efficacious Exposure Below Minimal Efficacious Exposure Number of Patients (n) 17 14 Complete Response (CR) 1 (6%) 0 Partial Response (PR) 4 (24%) 2 (14%) Overall Response Rate (ORR) 5 (29%) 2 (14%) Stable Disease (SD) 9 (53%) 4 (28%) Clinical Benefit (SD+ORR) 14 (82%) 6 (43%) Median DOR (months) 7.62 3.96 Median PFS (months) 8.1 1.8 Progressive Disease (PD) 3 (18%) 8 (57%) Patients remaining on study as of Oct 31 2019 8 (47%) 0 Sufficient AVB-500 exposures doubled ORR, Clinical Benefit Rate (CBR), and duration of response Response rate in below minimal efficacious exposure patients consistent with historical control rates of 10-15% for PAC or PLD PFS will be primary endpoint for registration study Best Response Determined by Investigator-Assessed RECIST 1.1
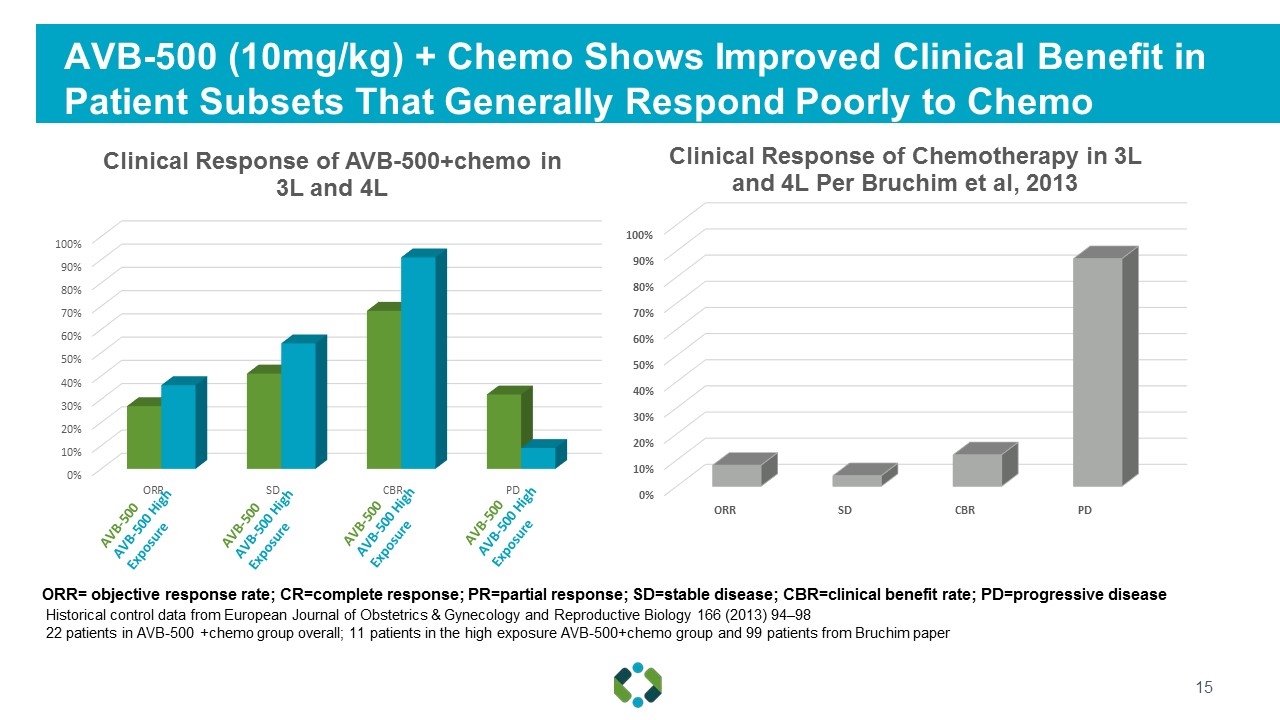
AVB-500 (10mg/kg) + Chemo Shows Improved Clinical Benefit in Patient Subsets That Generally Respond Poorly to Chemo ORR= objective response rate; CR=complete response; PR=partial response; SD=stable disease; CBR=clinical benefit rate; PD=progressive disease Historical control data from European Journal of Obstetrics & Gynecology and Reproductive Biology 166 (2013) 94–98 22 patients in AVB-500 +chemo group overall; 11 patients in the high exposure AVB-500+chemo group and 99 patients from Bruchim paper AVB-500 AVB-500 High Exposure AVB-500 AVB-500 High Exposure AVB-500 AVB-500 High Exposure AVB-500 AVB-500 High Exposure
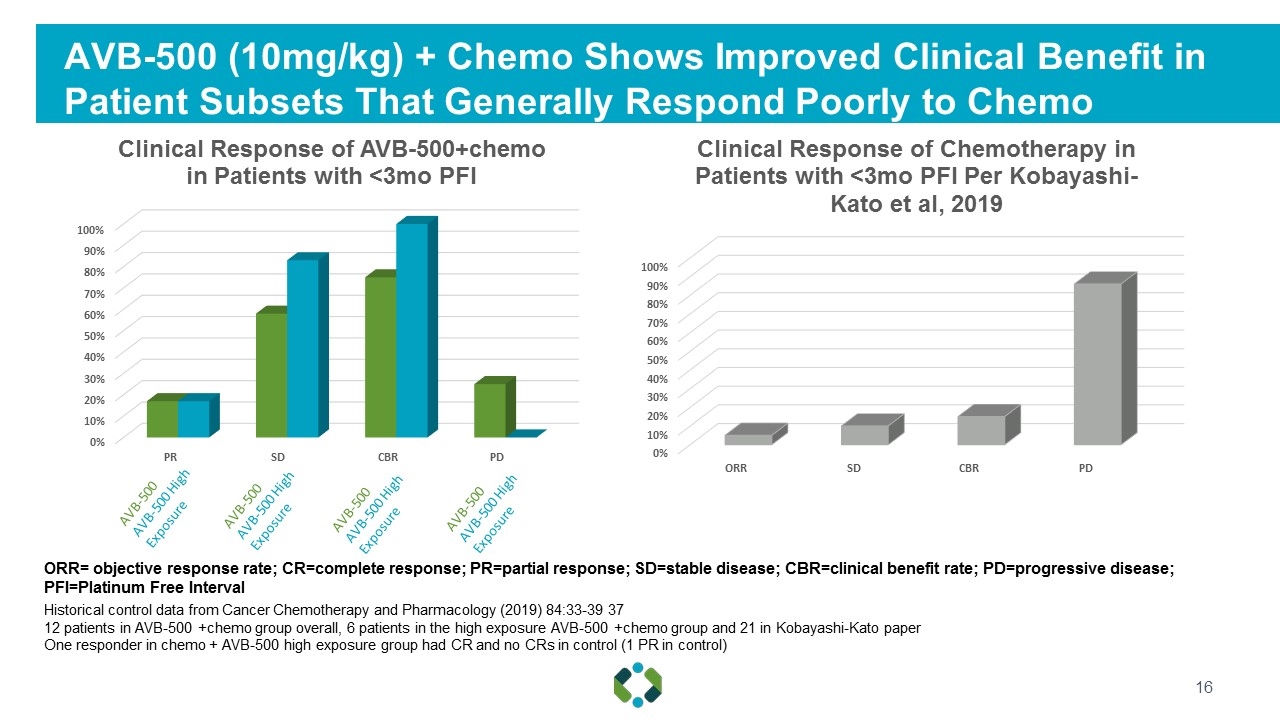
AVB-500 (10mg/kg) + Chemo Shows Improved Clinical Benefit in Patient Subsets That Generally Respond Poorly to Chemo Historical control data from Cancer Chemotherapy and Pharmacology (2019) 84:33-39 37 12 patients in AVB-500 +chemo group overall, 6 patients in the high exposure AVB-500 +chemo group and 21 in Kobayashi-Kato paper One responder in chemo + AVB-500 high exposure group had CR and no CRs in control (1 PR in control) ORR= objective response rate; CR=complete response; PR=partial response; SD=stable disease; CBR=clinical benefit rate; PD=progressive disease; PFI=Platinum Free Interval AVB-500 AVB-500 High Exposure AVB-500 AVB-500 High Exposure AVB-500 AVB-500 High Exposure AVB-500 AVB-500 High Exposure
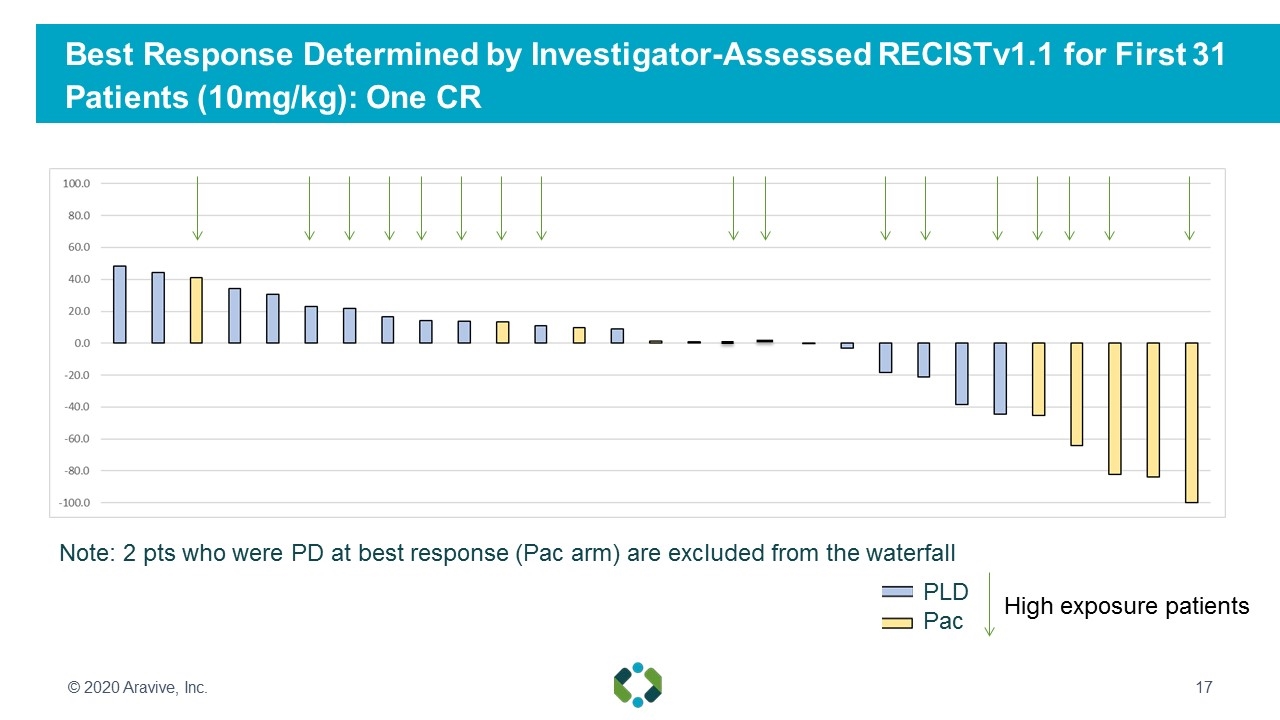
Best Response Determined by Investigator-Assessed RECISTv1.1 for First 31 Patients (10mg/kg): One CR © 2020 Aravive, Inc. Note: 2 pts who were PD at best response (Pac arm) are excluded from the waterfall PLD Pac High exposure patients
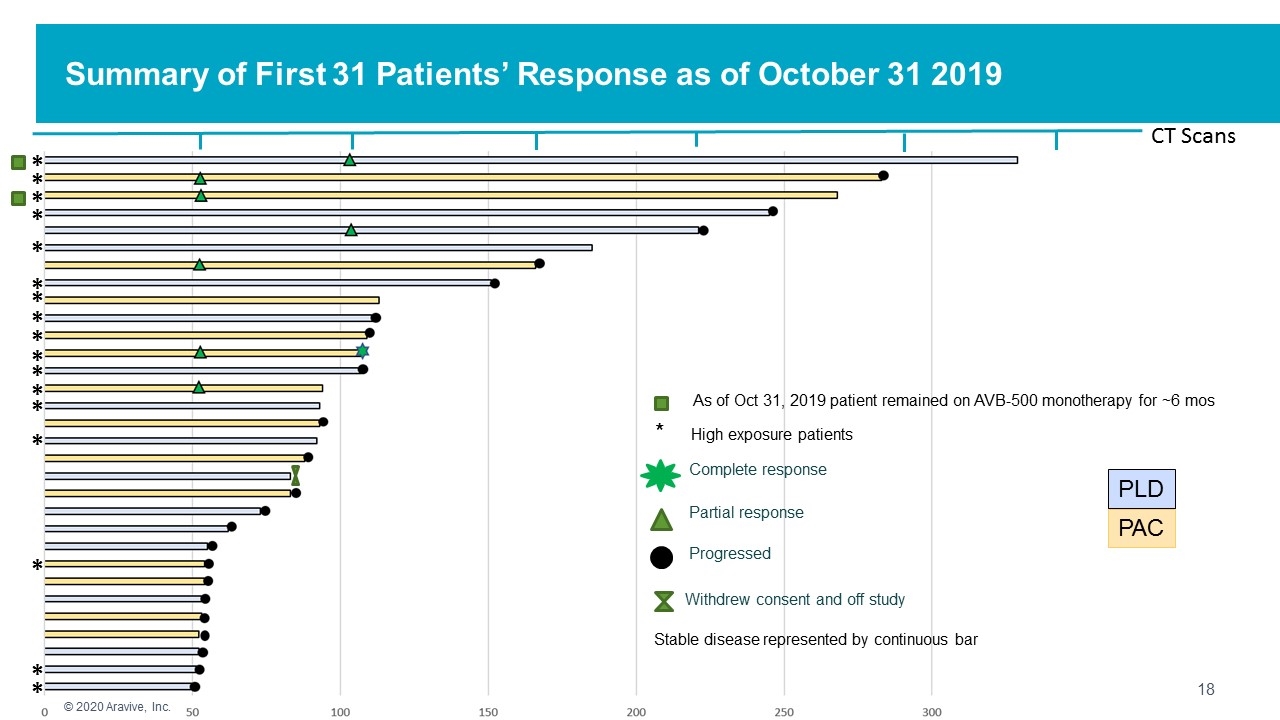
Summary of First 31 Patients’ Response as of October 31 2019 CT Scans PAC PLD Partial response Progressed Complete response Stable disease represented by continuous bar * * * * * * * * * * * * * * * * * * High exposure patients Withdrew consent and off study © 2020 Aravive, Inc. As of Oct 31, 2019 patient remained on AVB-500 monotherapy for ~6 mos
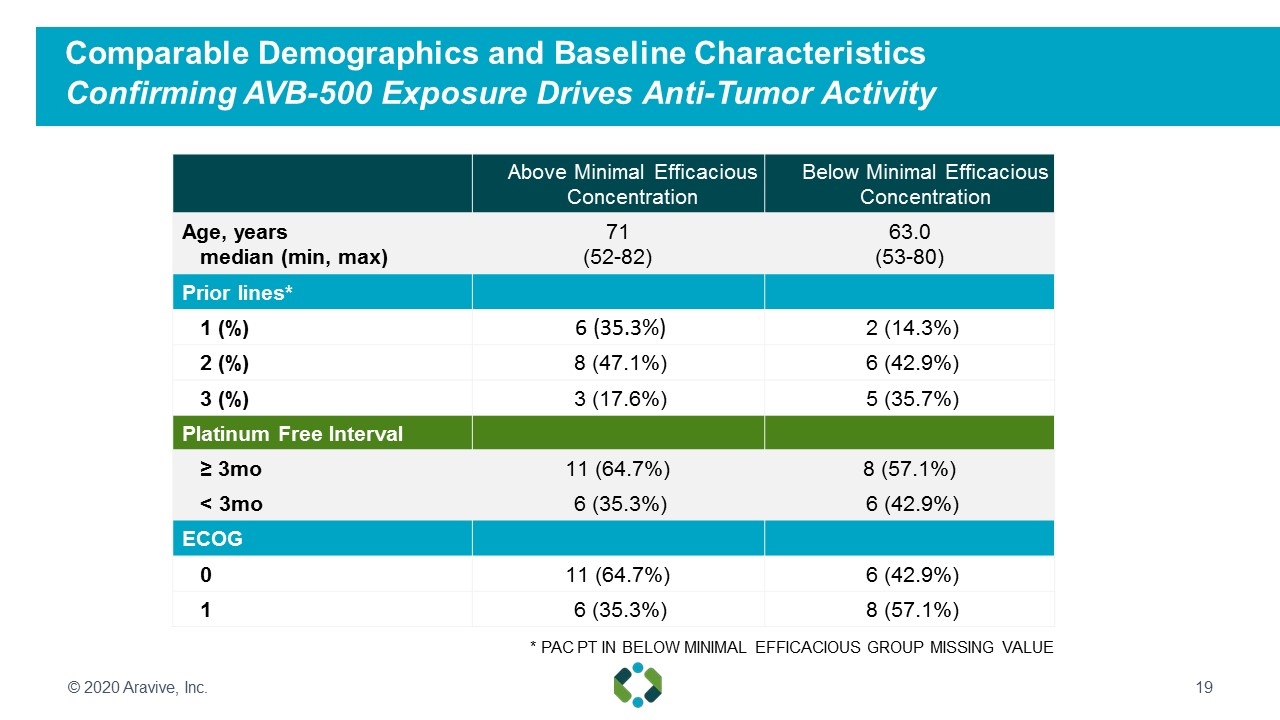
Comparable Demographics and Baseline Characteristics Confirming AVB-500 Exposure Drives Anti-Tumor Activity Above Minimal Efficacious Concentration Below Minimal Efficacious Concentration Age, years median (min, max) 71 (52-82) 63.0 (53-80) Prior lines* 1 (%) 6 (35.3%) 2 (14.3%) 2 (%) 8 (47.1%) 6 (42.9%) 3 (%) 3 (17.6%) 5 (35.7%) Platinum Free Interval ≥ 3mo 11 (64.7%) 8 (57.1%) < 3mo 6 (35.3%) 6 (42.9%) ECOG 0 11 (64.7%) 6 (42.9%) 1 6 (35.3%) 8 (57.1%) * PAC PT IN BELOW MINIMAL EFFICACIOUS GROUP MISSING VALUE © 2020 Aravive, Inc.

Next Steps Data confirm strategy to investigate higher doses in the current Phase 1b study to determine if a greater proportion of patients achieve high drug exposure levels PK modeling predict a dose of 20 mg/kg should allow >90% of patients to achieve desired high exposure levels Anticipate PK and safety read-out mid-2020 for 15 and 20mg/kg, subject to the impact of COVID-19 © 2020 Aravive, Inc.
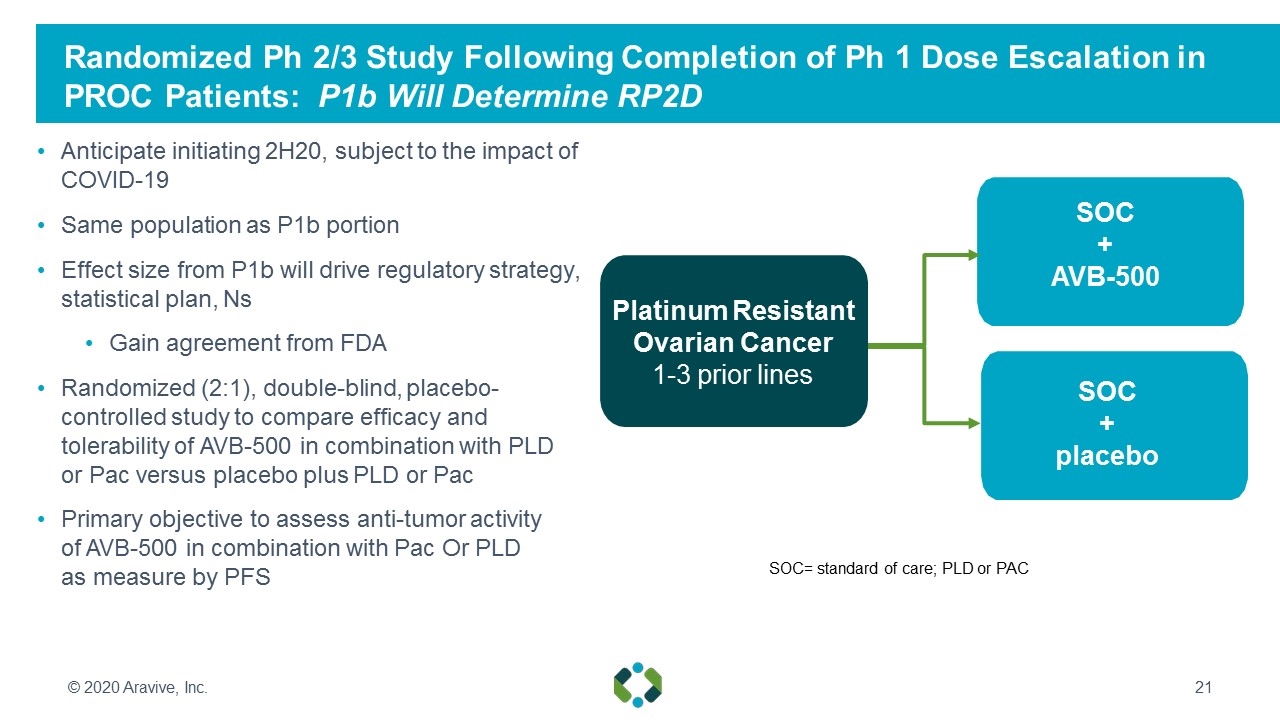
Randomized Ph 2/3 Study Following Completion of Ph 1 Dose Escalation in PROC Patients: P1b Will Determine RP2D Anticipate initiating 2H20, subject to the impact of COVID-19 Same population as P1b portion Effect size from P1b will drive regulatory strategy, statistical plan, Ns Gain agreement from FDA Randomized (2:1), double-blind, placebo- controlled study to compare efficacy and tolerability of AVB-500 in combination with PLD or Pac versus placebo plus PLD or Pac Primary objective to assess anti-tumor activity of AVB-500 in combination with Pac Or PLD as measure by PFS Platinum Resistant Ovarian Cancer 1-3 prior lines PLD SOC + placebo SOC= standard of care; PLD or PAC SOC + AVB-500 © 2020 Aravive, Inc.
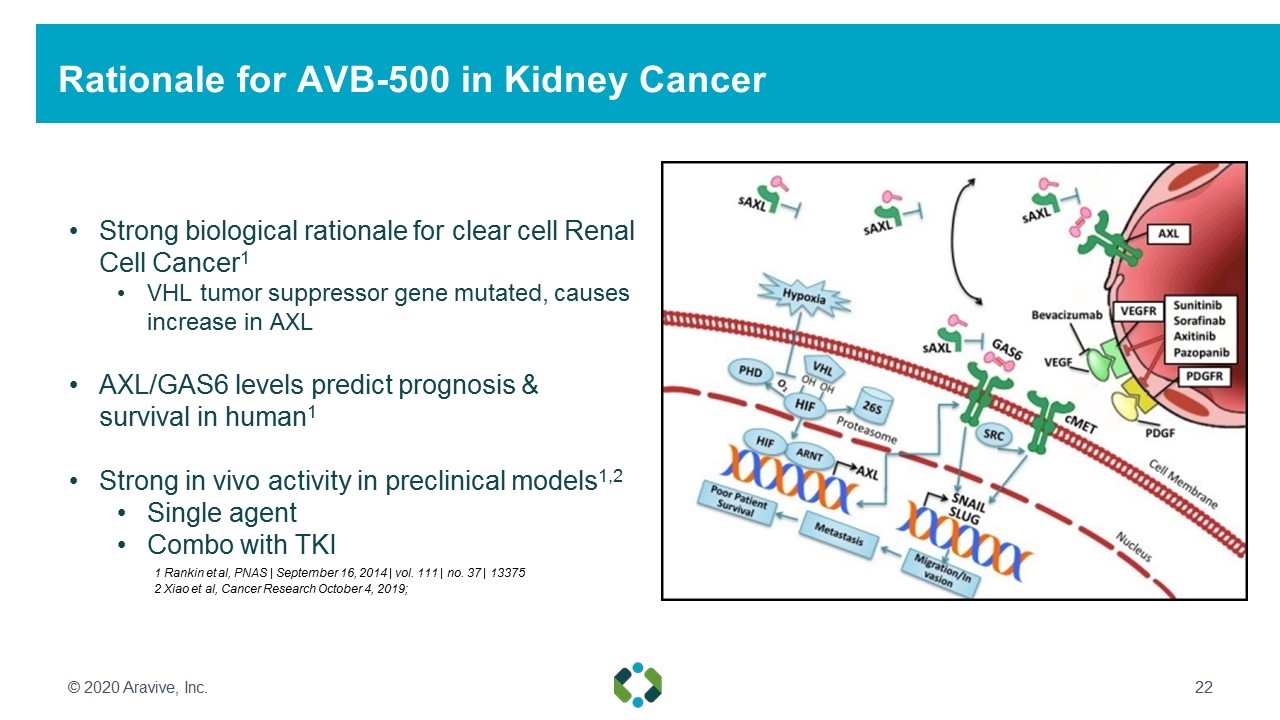
Rationale for AVB-500 in Kidney Cancer Strong biological rationale for clear cell Renal Cell Cancer1 VHL tumor suppressor gene mutated, causes increase in AXL AXL/GAS6 levels predict prognosis & survival in human1 Strong in vivo activity in preclinical models1,2 Single agent Combo with TKI 2 Xiao et al, Cancer Research October 4, 2019; © 2020 Aravive, Inc. 1 Rankin et al, PNAS | September 16, 2014 | vol. 111 | no. 37 | 13375
Overview of P1b/P2 Clear Cell Renal Cell Carcinoma Positioning AVB-500 in the Evolving Treatment Landscape Histologically confirmed stage IV ccRCC (according to the American Joint Commission on Cancer, seventh edition, classification) progressed on/after or intolerant to 1L Cabozantinib is used most frequently after IO Cabozantinib being tested in 1L with nivo in CM-9ER vs sunitinib Positive 2L data of AVB-500 combined with cabozantinib creates opportunities for expansion Phase 1b safety run-in with AVB-500 in combination with cabozantinib 90 patients in P2 controlled, randomized portion Primary endpoint in P2 is PFS Amending study to allow initiation at 15 or 20mg/kg AVB-500 following iDMC review of 20mg/kg PROC cohort; plan to initiate later in 2020, depending on COVID-19 impact
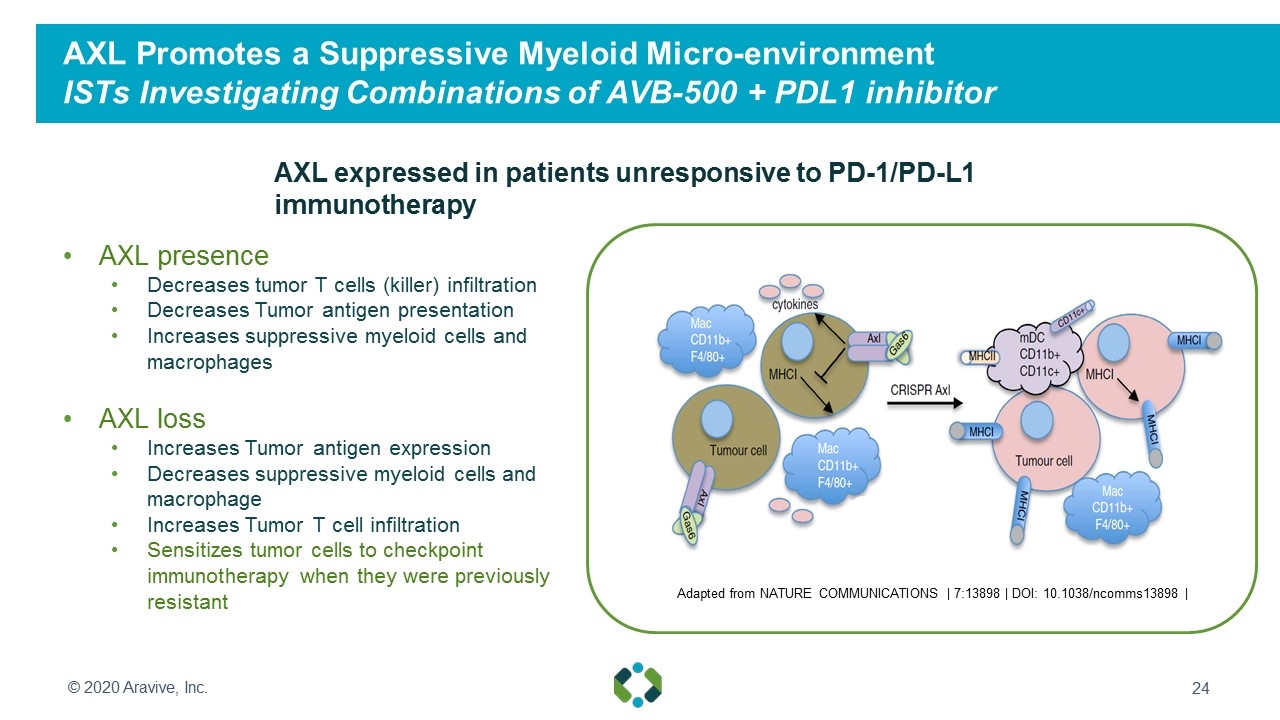
AXL Promotes a Suppressive Myeloid Micro-environment ISTs Investigating Combinations of AVB-500 + PDL1 inhibitor AXL presence Decreases tumor T cells (killer) infiltration Decreases Tumor antigen presentation Increases suppressive myeloid cells and macrophages AXL loss Increases Tumor antigen expression Decreases suppressive myeloid cells and macrophage Increases Tumor T cell infiltration Sensitizes tumor cells to checkpoint immunotherapy when they were previously resistant AXL expressed in patients unresponsive to PD-1/PD-L1 immunotherapy Adapted from NATURE COMMUNICATIONS | 7:13898 | DOI: 10.1038/ncomms13898 | © 2020 Aravive, Inc.
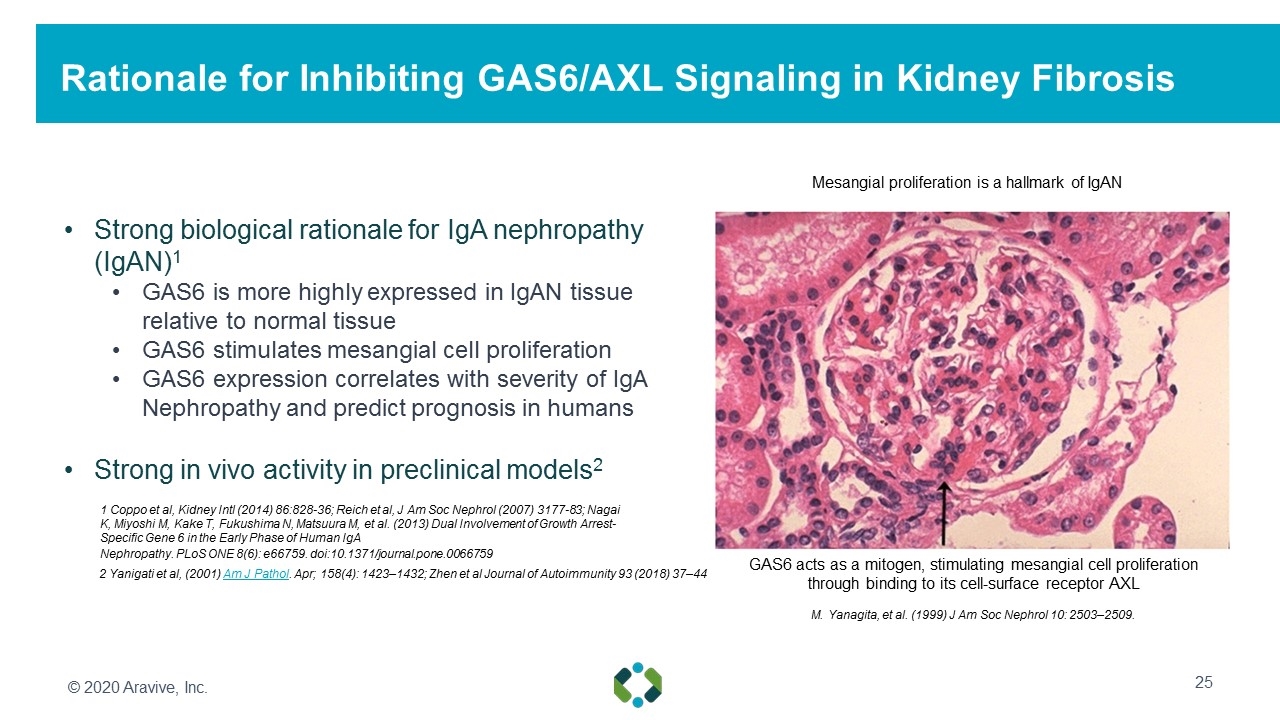
Strong biological rationale for IgA nephropathy (IgAN)1 GAS6 is more highly expressed in IgAN tissue relative to normal tissue GAS6 stimulates mesangial cell proliferation GAS6 expression correlates with severity of IgA Nephropathy and predict prognosis in humans Strong in vivo activity in preclinical models2 Rationale for Inhibiting GAS6/AXL Signaling in Kidney Fibrosis 1 Coppo et al, Kidney Intl (2014) 86:828-36; Reich et al, J Am Soc Nephrol (2007) 3177-83; Nagai K, Miyoshi M, Kake T, Fukushima N, Matsuura M, et al. (2013) Dual Involvement of Growth Arrest-Specific Gene 6 in the Early Phase of Human IgA Nephropathy. PLoS ONE 8(6): e66759. doi:10.1371/journal.pone.0066759 GAS6 acts as a mitogen, stimulating mesangial cell proliferation through binding to its cell-surface receptor AXL Mesangial proliferation is a hallmark of IgAN M. Yanagita, et al. (1999) J Am Soc Nephrol 10: 2503–2509. 2 Yanigati et al, (2001) Am J Pathol. Apr; 158(4): 1423–1432; Zhen et al Journal of Autoimmunity 93 (2018) 37–44 © 2020 Aravive, Inc.
Overview of Phase 2A IgAN Study Small (N of 12-24) Proof of Concept Study in IgA patients Sites in USA and Ukraine Diagnosis of biopsy-proven IgAN Proteinuria ≥ 1g to 3g/24hr Monitor safety, PK/PD Ensure sGAS6 levels suppressed throughout dosing interval Open-label Monitor proteinuria and other renal functions Ability to increase dose based on data Putting on hold to ensure safety of patients and study integrity; will open when safety issue resolves, depending on COVID-19 situation © 2020 Aravive, Inc.
Experienced, Methodical, Efficient Development Execution Strong Clinical Development Team © 2020 Aravive, Inc. 2Q 2019- POM in Ovarian Cancer 2Q 2019- Tolerability in Ovarian Cancer 3Q 2019- Early POC in Ovarian Cancer 3Q 2019- Expanded P1b Ovarian Cancer 4Q2019- Kidney fibrosis trial initiation 1Q 2020- IND open for ccRCC study Mid-2020- Safety and PK from Ph1b Ovarian (from 15 and 20 mg/kg doses) 2H-2020- P2/P3 Platinum-resistant ovarian cancer initiation 2H-2020- P1b/P2 ccRCC initiation at higher dose TBD Re-open enrollment of IgAN Above milestones may be impacted by COVID-19 2019 Achieved MilestonesUpcoming Milestones IND= investigational new drug application; POM=proof of mechanism; POC=proof of concept
Financials and Capitalization NASDAQ: ARAV; Shares Outstanding*: 15.0M basic Cash and cash equivalents*: $65M Cash and cash equivalents expected to enable the company to fund its operating plans into 2022 © 2020 Aravive, Inc. * As of December 31, 2019

AVB-500 Offers First- and Best-In-Class Opportunity in Oncology Uniquely positioned in competitive landscape Robust pipeline addressing significant drivers of mortality in oncology and fibrosis: metastasis and drug resistance © 2020 Aravive, Inc. AVB-500: high affinity decoy protein, a potent and selective drug targets GAS6/AXL signaling Validated pathway drives activation, migration and invasion of abnormal cells Suppresses GAS6 to undetectable levels in early clinical studies; prolonged target engagement Safety and tolerability profile to date support use as combination and/or maintenance therapy Compelling early efficacy signal in platinum-resistant ovarian cancer No adverse events that limit dosing High AVB-500 levels predictive of anti-tumor activity; statistically significant association with PFS In first 31 patients, median PFS for patients with high drug levels was statistically significantly longer than those with low drug levels Expanded P1b portion to validate efficacy signal, evaluate higher doses and explore the most efficient regulatory path to approval Additional programs advancing into the clinic in high need indications Kidney fibrosis (IgA nephropathy) trial on hold due to COVID-19 and expected to re-open later this year Renal cell carcinoma IND open and expect to initiate with higher dose after 20mg/kg PROC cohort is reviewed by iDMC ISTs Recruitment and retention in clinical trials may be impacted by COVID-19

Halting Disease Progression in its Tracks © 2020 Aravive, Inc. Thank You





























