
© 2020 Aravive, Inc. Corporate Presentation July 2020 Halting Disease Progression in its Tracks Exhibit 99.2

© 2020 Aravive, Inc. Forward-Looking Statements This presentation contains forward-looking statements that may discuss Aravive’s plans, goals, intentions and expectations as to future trends, events, results of operations, financial condition or other matters. Forward- looking statements generally include statements that are predictive in nature and depend upon or refer to future events or conditions, and they often include words such as “may,” “will,” “should,” “would,” “expect,” “anticipate,” “plan,” “likely,” “believe,” “estimate,” “project,” “intend,” and other similar expressions. Statements that are not historical facts are forward-looking statements. Forward-looking statements included in this presentation include statements regarding Aravive’s planned clinical activities, including the design, initiation, patient enrollment and availability of data from clinical studies, potential pipeline, future indications and cash position and the anticipated safety, activity and manufacturability of Aravive’s product candidates. Forward-looking statements are based on Aravive’s current beliefs and assumptions, are subject to risks and uncertainties and are not guarantees of future performance. Actual results could differ materially from those contained in any forward-looking statement as a result of various factors, including, without limitation: the unpredictability of clinical development activities; the impact of the COVID-19 pandemic; risks related to Aravive’s ability to estimate and control its operating expenses; Aravive’s ability to protect its intellectual property rights; changes in the competitive environment; and legislative, regulatory, political and economic developments. The foregoing review of important factors that could cause actual events to differ from expectations should not be construed as exhaustive and should be read in conjunction with statements that are included herein and elsewhere, including the risk factors included in Aravive’s most recent Annual Report on Form 10-K, Quarterly Report on Form 10-Q and Current Reports on Form 8-K filed with the Securities and Exchange Commission. Except as required by law, Aravive undertakes no obligation to revise or update any forward-looking statement or to make any other forward-looking statements, whether as a result of new information, future events or otherwise.
© 2020 Aravive, Inc. Aravive is a clinical stage company developing treatments designed to halt the progression of life-threatening diseases, including cancer and fibrosis. Nasdaq-listed company (ARAV) Offices in Houston, TX (HQ) and RTP, NC $20 million Product Development Award from the Cancer Prevention & Research Institute of Texas (CPRIT) Led by a team of industry veterans with decades of target biology, drug development & commercialization expertise Multiple milestones anticipated during 2020 & 2021 Lead Clinical program is GAS6/AXL inhibitor and preclinical program in CTGF, both important targets in cancer and fibrosis Company Overview Our approach draws on novel insights into targeting signaling pathways that drive the activation, migration, and invasion of abnormal cells into healthy tissues.
© 2020 Aravive, Inc. A first-in-class, ultra-high affinity, Fc-fusion protein designed to selectively block the novel GAS6-AXL signaling pathway Proof-of-mechanism established in clinic using proprietary biomarker for target engagement Early proof-of-concept for anti-tumor activity demonstrated in platinum-resistant ovarian cancer (PROC) Fast-track designation granted by the FDA Completed Phase 1b and plan to initiate P2/3 trial for platinum-resistant ovarian cancer 2H20/Q121 following discussion with the FDA Ideal profile for combination with chemotherapy, checkpoint inhibitors and PARP inhibitors in broad range of indications IP coverage through 2033 and beyond AVB-500: Lead Clinical Program
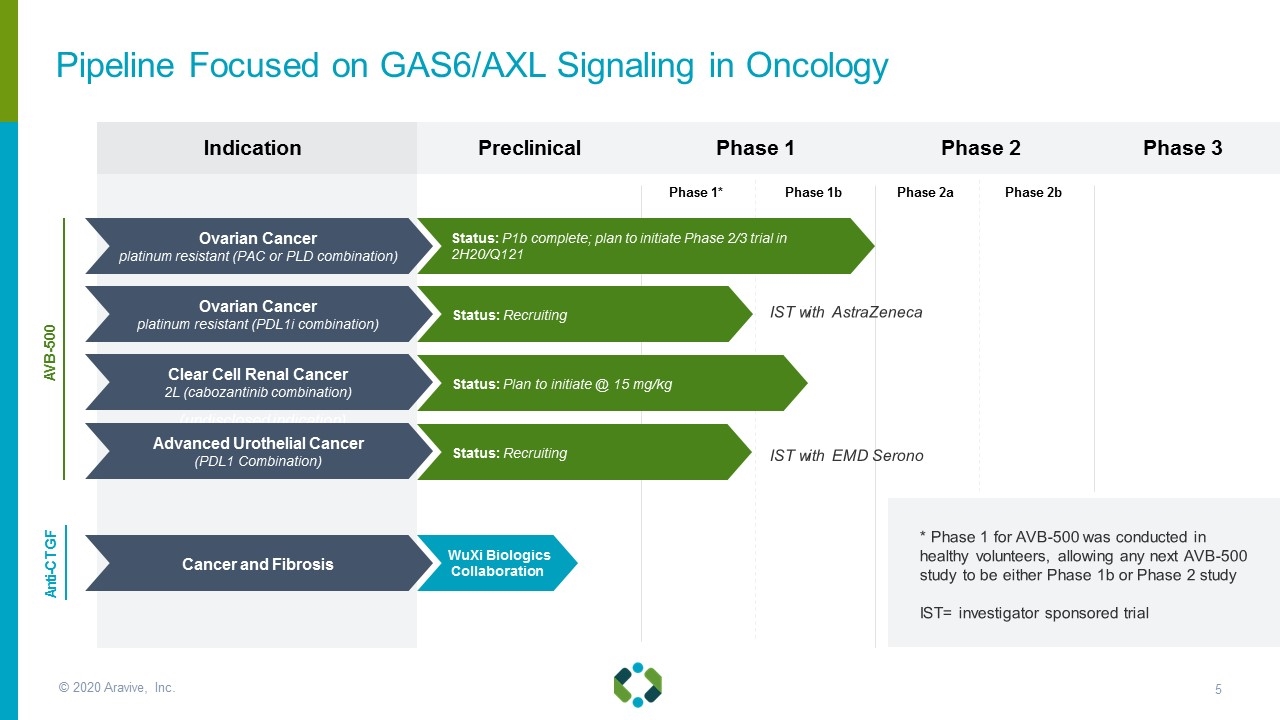
© 2020 Aravive, Inc. Indication Preclinical 3rd Oncology (undisclosed indication) Status: IND Open Status: Recruiting Status: Recruiting Phase 1 Phase 3 Phase 2 Phase 2a Phase 2b Anti-CTGF AVB-500 IST with AstraZeneca Ovarian Cancer platinum resistant (PAC or PLD combination) Ovarian Cancer platinum resistant (PDL1i combination) Clear Cell Renal Cancer 2L (cabozantinib combination) Advanced Urothelial Cancer (PDL1 Combination) Cancer and Fibrosis WuXi Biologics Collaboration Status: P1b complete; plan to initiate Phase 2/3 trial in 2H20/Q121 Status: Recruiting Status: Plan to initiate @ 15 mg/kg Status: Recruiting IST with EMD Serono * Phase 1 for AVB-500 was conducted in healthy volunteers, allowing any next AVB-500 study to be either Phase 1b or Phase 2 study IST= investigator sponsored trial Pipeline Focused on GAS6/AXL Signaling in Oncology Phase 1* Phase 1b
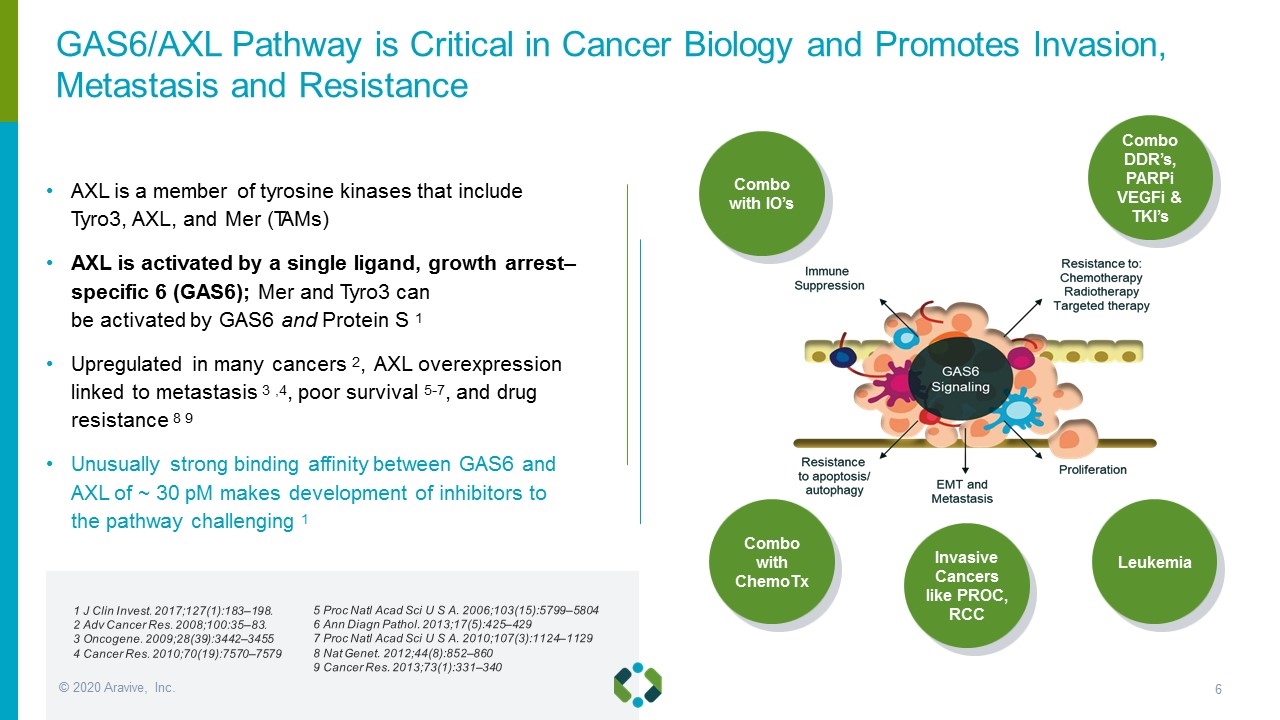
AXL is a member of tyrosine kinases that include Tyro3, AXL, and Mer (TAMs) AXL is activated by a single ligand, growth arrest– specific 6 (GAS6); Mer and Tyro3 can be activated by GAS6 and Protein S 1 Upregulated in many cancers 2, AXL overexpression linked to metastasis 3 ,4, poor survival 5-7, and drug resistance 8 9 Unusually strong binding affinity between GAS6 and AXL of ~ 30 pM makes development of inhibitors to the pathway challenging 1 Combo with ChemoTx 1 J Clin Invest. 2017;127(1):183–198. 2 Adv Cancer Res. 2008;100:35–83. 3 Oncogene. 2009;28(39):3442–3455 4 Cancer Res. 2010;70(19):7570–7579 5 Proc Natl Acad Sci U S A. 2006;103(15):5799–5804 6 Ann Diagn Pathol. 2013;17(5):425–429 7 Proc Natl Acad Sci U S A. 2010;107(3):1124–1129 8 Nat Genet. 2012;44(8):852–860 9 Cancer Res. 2013;73(1):331–340 GAS6/AXL Pathway is Critical in Cancer Biology and Promotes Invasion, Metastasis and Resistance Invasive Cancers like PROC, RCC Leukemia Combo with IO’s Combo DDR’s, PARPi VEGFi & TKI’s © 2020 Aravive, Inc.
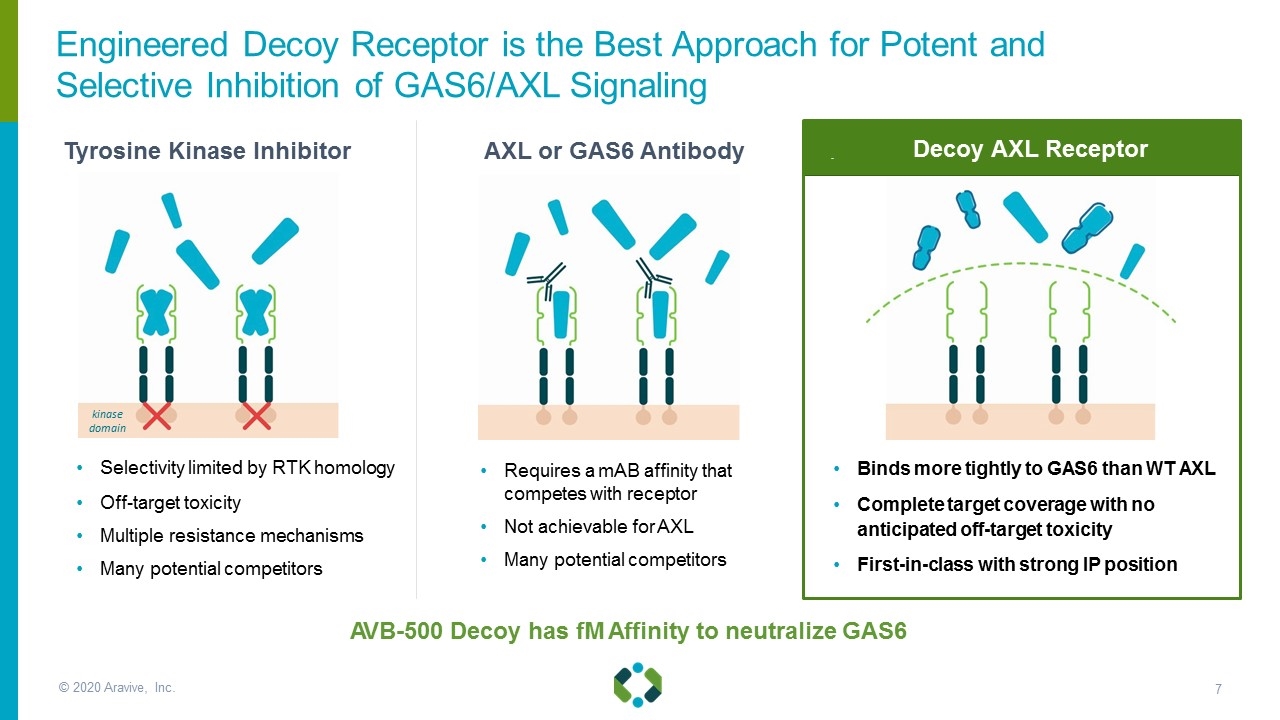
© 2020 Aravive, Inc. AVB-500 Decoy has fM Affinity to neutralize GAS6 Selectivity limited by RTK homology Off-target toxicity Multiple resistance mechanisms Many potential competitors Requires a mAB affinity that competes with receptor Not achievable for AXL Many potential competitors Binds more tightly to GAS6 than WT AXL Complete target coverage with no anticipated off-target toxicity First-in-class with strong IP position kinase domain Tyrosine Kinase Inhibitor Engineered Decoy Receptor is the Best Approach for Potent and Selective Inhibition of GAS6/AXL Signaling Decoy AXL Receptor AXL or GAS6 Antibody
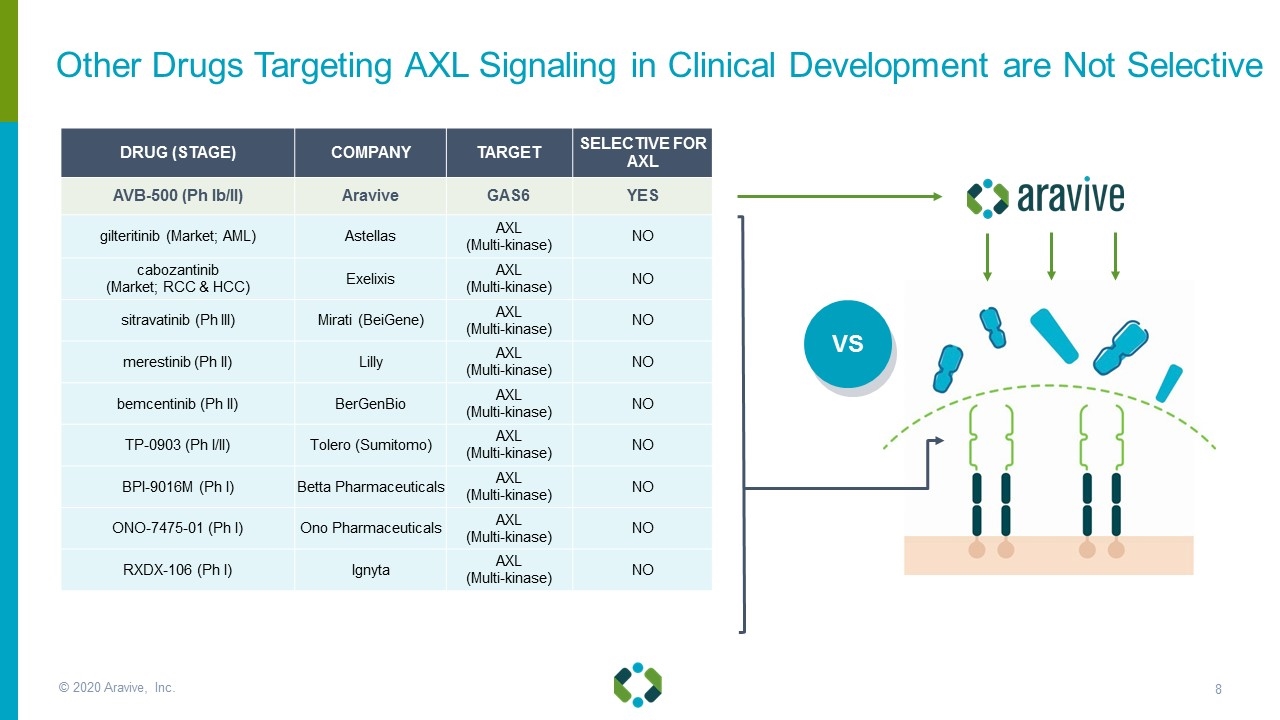
© 2020 Aravive, Inc. DRUG (STAGE) COMPANY TARGET SELECTIVE FOR AXL AVB-500 (Ph Ib/II) Aravive GAS6 YES gilteritinib (Market; AML) Astellas AXL (Multi-kinase) NO cabozantinib (Market; RCC & HCC) Exelixis AXL (Multi-kinase) NO sitravatinib (Ph III) Mirati (BeiGene) AXL (Multi-kinase) NO merestinib (Ph II) Lilly AXL (Multi-kinase) NO bemcentinib (Ph II) BerGenBio AXL (Multi-kinase) NO TP-0903 (Ph I/II) Tolero (Sumitomo) AXL (Multi-kinase) NO BPI-9016M (Ph I) Betta Pharmaceuticals AXL (Multi-kinase) NO ONO-7475-01 (Ph I) Ono Pharmaceuticals AXL (Multi-kinase) NO RXDX-106 (Ph I) Ignyta AXL (Multi-kinase) NO VS Other Drugs Targeting AXL Signaling in Clinical Development are Not Selective
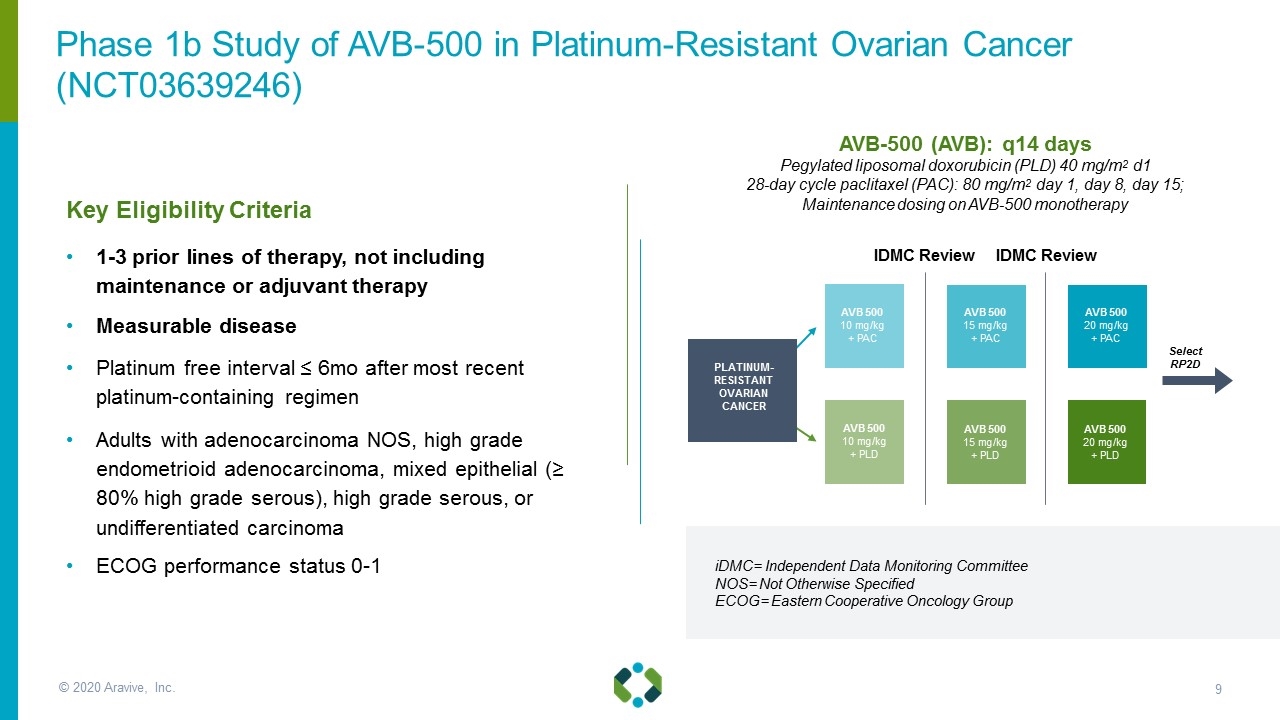
© 2020 Aravive, Inc. AVB-500 (AVB): q14 days Pegylated liposomal doxorubicin (PLD) 40 mg/m2 d1 28-day cycle paclitaxel (PAC): 80 mg/m2 day 1, day 8, day 15; Maintenance dosing on AVB-500 monotherapy AVB 500 10 mg/kg + PLD AVB 500 15 mg/kg + PLD Select RP2D AVB 500 10 mg/kg + PAC AVB 500 15 mg/kg + PAC PLATINUM-RESISTANT OVARIAN CANCER AVB 500 20 mg/kg + PLD AVB 500 20 mg/kg + PAC Key Eligibility Criteria 1-3 prior lines of therapy, not including maintenance or adjuvant therapy Measurable disease Platinum free interval ≤ 6mo after most recent platinum-containing regimen Adults with adenocarcinoma NOS, high grade endometrioid adenocarcinoma, mixed epithelial (≥ 80% high grade serous), high grade serous, or undifferentiated carcinoma ECOG performance status 0-1 iDMC= Independent Data Monitoring Committee NOS= Not Otherwise Specified ECOG= Eastern Cooperative Oncology Group IDMC Review IDMC Review Phase 1b Study of AVB-500 in Platinum-Resistant Ovarian Cancer (NCT03639246)
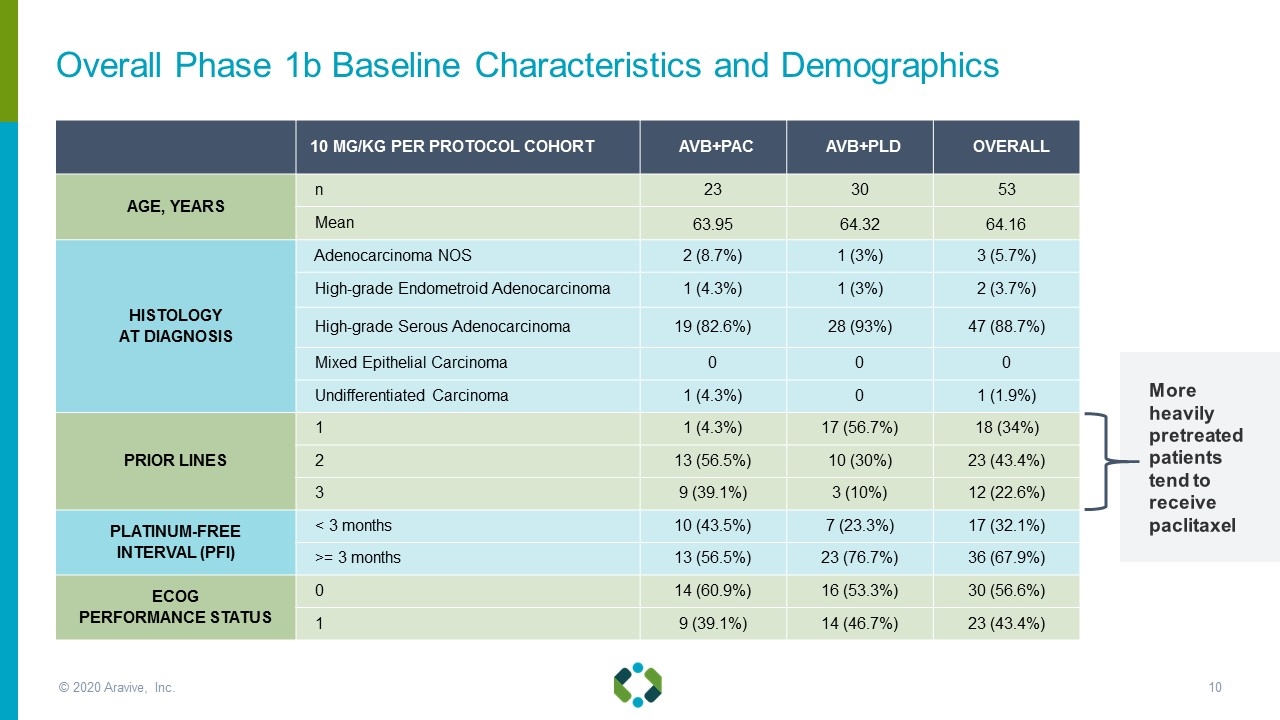
Overall Phase 1b Baseline Characteristics and Demographics 10 MG/KG PER PROTOCOL COHORT AVB+PAC AVB+PLD OVERALL AGE, YEARS n 23 30 53 Mean 63.95 64.32 64.16 HISTOLOGY AT DIAGNOSIS Adenocarcinoma NOS 2 (8.7%) 1 (3%) 3 (5.7%) High-grade Endometroid Adenocarcinoma 1 (4.3%) 1 (3%) 2 (3.7%) High-grade Serous Adenocarcinoma 19 (82.6%) 28 (93%) 47 (88.7%) Mixed Epithelial Carcinoma 0 0 0 Undifferentiated Carcinoma 1 (4.3%) 0 1 (1.9%) PRIOR LINES 1 1 (4.3%) 17 (56.7%) 18 (34%) 2 13 (56.5%) 10 (30%) 23 (43.4%) 3 9 (39.1%) 3 (10%) 12 (22.6%) PLATINUM-FREE INTERVAL (PFI) < 3 months 10 (43.5%) 7 (23.3%) 17 (32.1%) >= 3 months 13 (56.5%) 23 (76.7%) 36 (67.9%) ECOG PERFORMANCE STATUS 0 14 (60.9%) 16 (53.3%) 30 (56.6%) 1 9 (39.1%) 14 (46.7%) 23 (43.4%) More heavily pretreated patients tend to receive paclitaxel © 2020 Aravive, Inc.
Safety report for trial AVB500-OC-002 generated by independent medical monitor concluded: “Based on a review of the safety data, no unexpected safety trends have been detected and the reported AE severity and frequency are consistent with expectations of the concomitant treatment (PAC or PLD) and the disease under study (ovarian cancer).” AVB-500 well-tolerated: fatigue and infusion reactions appeared to be related to treatment No serious events that required expedited reporting per Sponsor No drug related SAEs reported to date No dose-limiting toxicities Premedication regimen designed to manage potential infusion reactions employed during the trial 53 PROC Patients Dosed: 10 mg/kg (40); 15 mg/kg (6); and 20 mg/kg (7) Safety Data Summary from Phase 1b Study © 2020 Aravive, Inc.
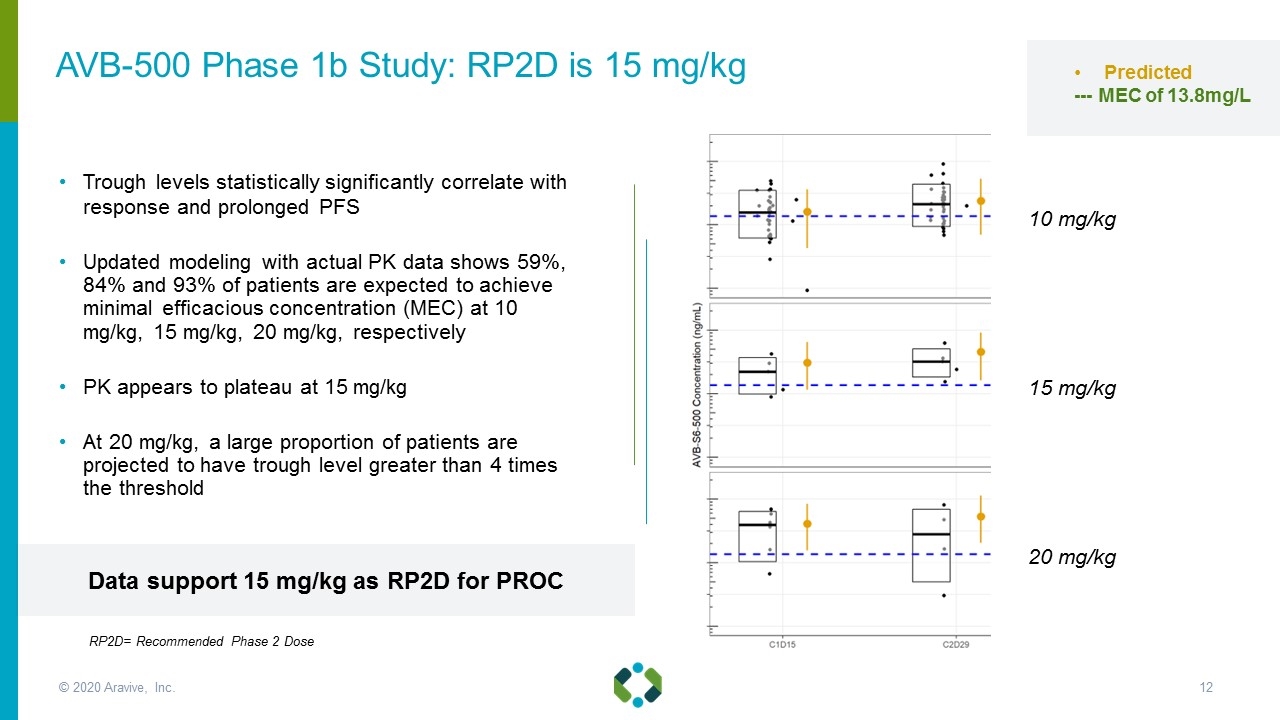
Trough levels statistically significantly correlate with response and prolonged PFS Updated modeling with actual PK data shows 59%, 84% and 93% of patients are expected to achieve minimal efficacious concentration (MEC) at 10 mg/kg, 15 mg/kg, 20 mg/kg, respectively PK appears to plateau at 15 mg/kg At 20 mg/kg, a large proportion of patients are projected to have trough level greater than 4 times the threshold 10 mg/kg 15 mg/kg 20 mg/kg Predicted --- MEC of 13.8mg/L AVB-500 Phase 1b Study: RP2D is 15 mg/kg Data support 15 mg/kg as RP2D for PROC © 2020 Aravive, Inc. RP2D= Recommended Phase 2 Dose

INVESTIGATOR-ASSESSED BEST RESPONSE PER RECIST V1.1 PAC (N=23) PLD (N=26) CR 2 (8.7%) 0 PR 6 (26%) 4 (15%) ORR 8 (35%) 4 (15%) SD 6 (26%) 12 (46%) CBR 14 (61%) 16 (61.5%) PD 9 (39%) 10 (38.5%) 1 A. Davis et al. / Gynecologic Oncology 133 (2014) 624–631 Response Higher in PAC than Historical Data of 10-15%1 Summary of all Cohorts, Evaluable Per Protocol All responses confirmed except the one PAC patient at 20 mg/kg ORR across all dose cohorts of 12/49 patients (24%) © 2020 Aravive, Inc.
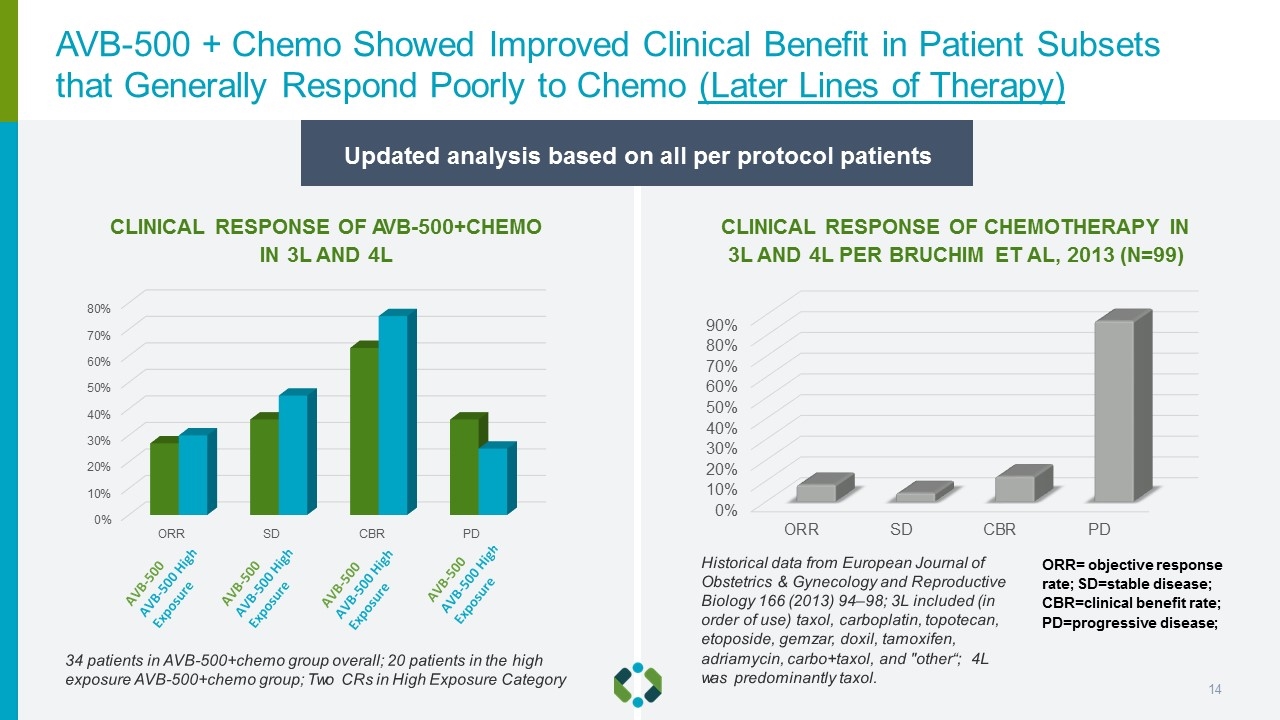
CLINICAL RESPONSE OF AVB-500+CHEMO IN 3L AND 4L CLINICAL RESPONSE OF CHEMOTHERAPY IN 3L AND 4L PER BRUCHIM ET AL, 2013 (N=99) 34 patients in AVB-500+chemo group overall; 20 patients in the high exposure AVB-500+chemo group; Two CRs in High Exposure Category Updated analysis based on all per protocol patients ORR= objective response rate; SD=stable disease; CBR=clinical benefit rate; PD=progressive disease; Historical data from European Journal of Obstetrics & Gynecology and Reproductive Biology 166 (2013) 94–98; 3L included (in order of use) taxol, carboplatin, topotecan, etoposide, gemzar, doxil, tamoxifen, adriamycin, carbo+taxol, and "other“; 4L was predominantly taxol. AVB-500 + Chemo Showed Improved Clinical Benefit in Patient Subsets that Generally Respond Poorly to Chemo (Later Lines of Therapy)
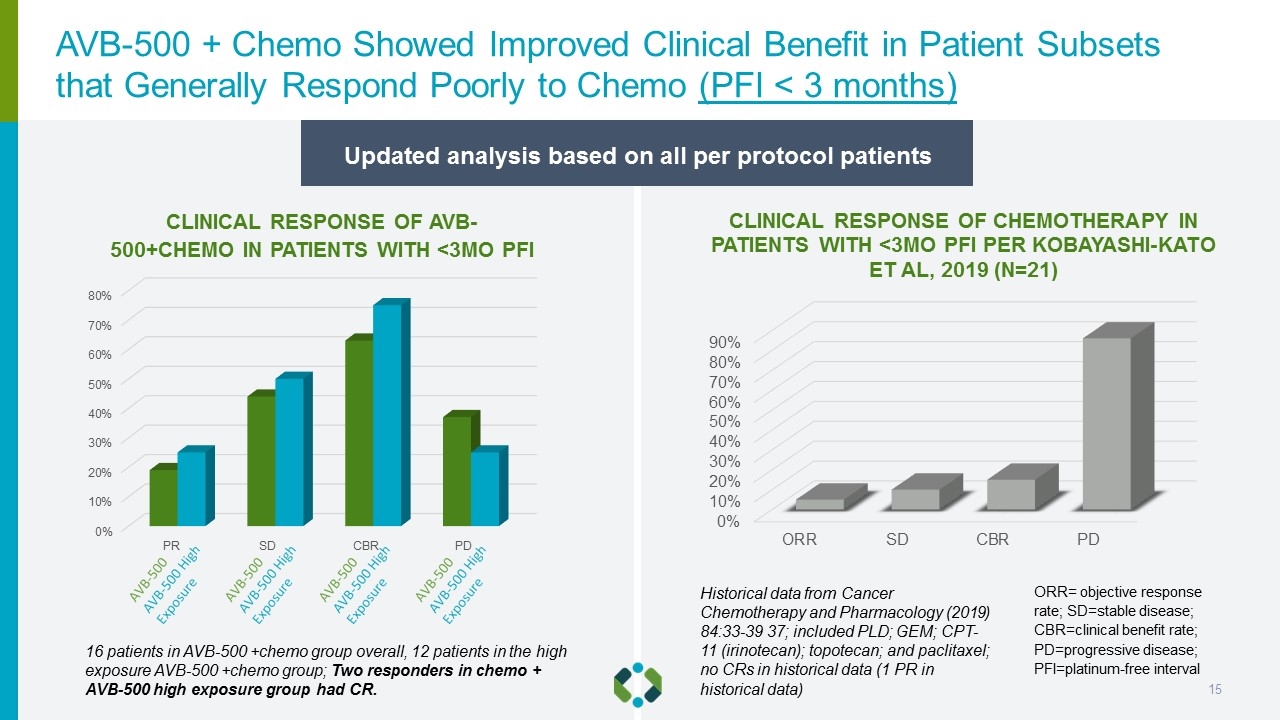
Updated analysis based on all per protocol patients CLINICAL RESPONSE OF CHEMOTHERAPY IN PATIENTS WITH <3MO PFI PER KOBAYASHI-KATO ET AL, 2019 (N=21) CLINICAL RESPONSE OF AVB-500+CHEMO IN PATIENTS WITH <3MO PFI ORR= objective response rate; SD=stable disease; CBR=clinical benefit rate; PD=progressive disease; PFI=platinum-free interval 16 patients in AVB-500 +chemo group overall, 12 patients in the high exposure AVB-500 +chemo group; Two responders in chemo + AVB-500 high exposure group had CR. Historical data from Cancer Chemotherapy and Pharmacology (2019) 84:33-39 37; included PLD; GEM; CPT-11 (irinotecan); topotecan; and paclitaxel; no CRs in historical data (1 PR in historical data) AVB-500 + Chemo Showed Improved Clinical Benefit in Patient Subsets that Generally Respond Poorly to Chemo (PFI < 3 months)
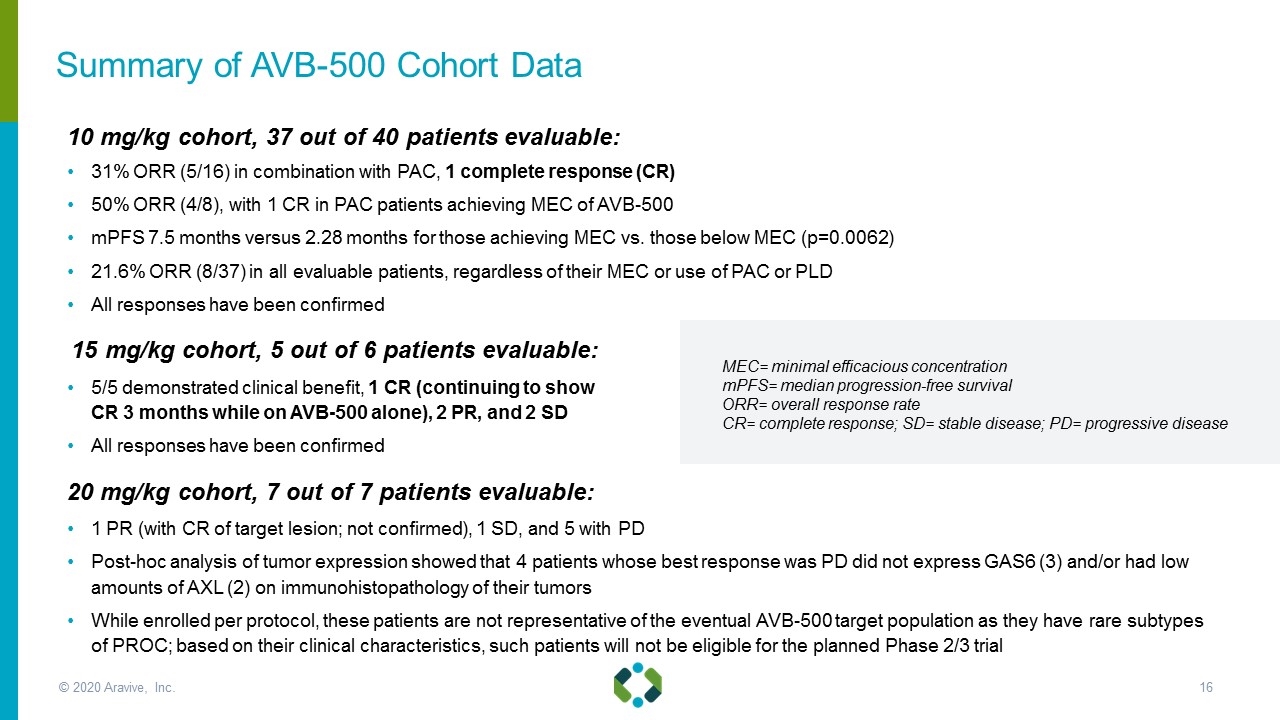
10 mg/kg cohort, 37 out of 40 patients evaluable: 31% ORR (5/16) in combination with PAC, 1 complete response (CR) 50% ORR (4/8), with 1 CR in PAC patients achieving MEC of AVB-500 mPFS 7.5 months versus 2.28 months for those achieving MEC vs. those below MEC (p=0.0062) 21.6% ORR (8/37) in all evaluable patients, regardless of their MEC or use of PAC or PLD All responses have been confirmed 15 mg/kg cohort, 5 out of 6 patients evaluable: 5/5 demonstrated clinical benefit, 1 CR (continuing to show CR 3 months while on AVB-500 alone), 2 PR, and 2 SD All responses have been confirmed 20 mg/kg cohort, 7 out of 7 patients evaluable: 1 PR (with CR of target lesion; not confirmed), 1 SD, and 5 with PD Post-hoc analysis of tumor expression showed that 4 patients whose best response was PD did not express GAS6 (3) and/or had low amounts of AXL (2) on immunohistopathology of their tumors While enrolled per protocol, these patients are not representative of the eventual AVB-500 target population as they have rare subtypes of PROC; based on their clinical characteristics, such patients will not be eligible for the planned Phase 2/3 trial MEC= minimal efficacious concentration mPFS= median progression-free survival ORR= overall response rate CR= complete response; SD= stable disease; PD= progressive disease Summary of AVB-500 Cohort Data © 2020 Aravive, Inc.
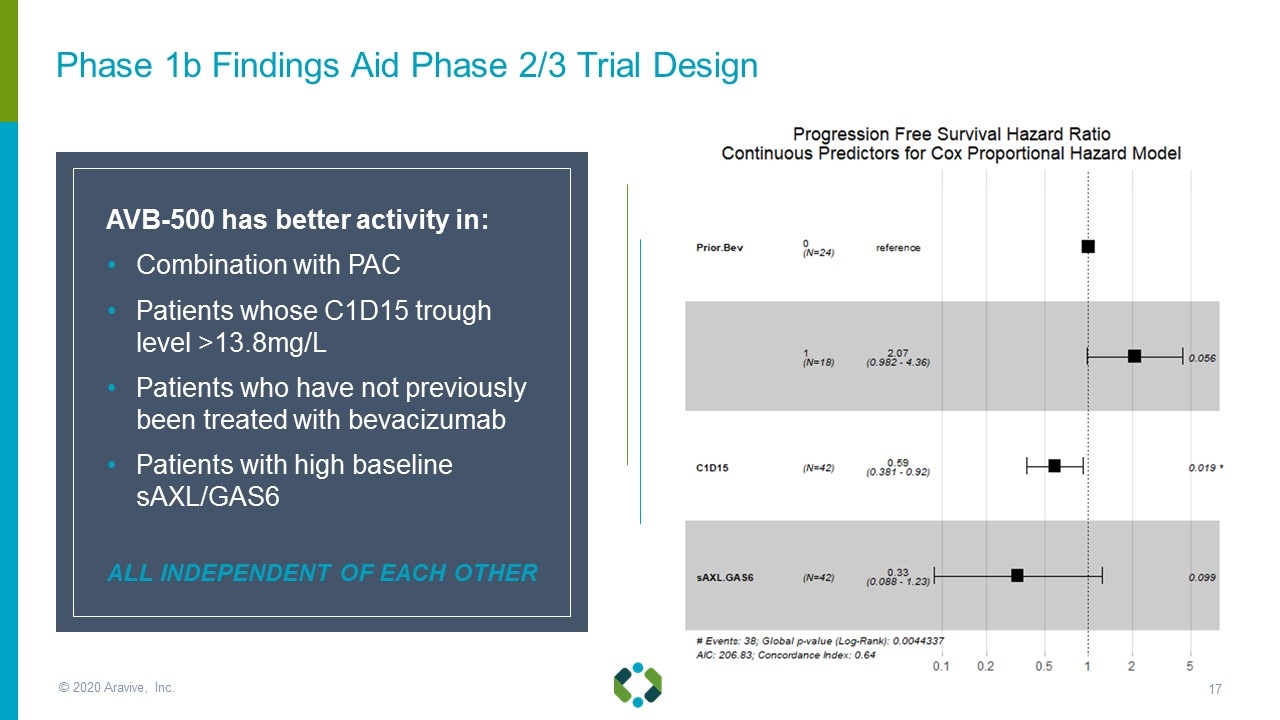
AVB-500 has better activity in: Combination with PAC Patients whose C1D15 trough level >13.8mg/L Patients who have not previously been treated with bevacizumab Patients with high baseline sAXL/GAS6 ALL INDEPENDENT OF EACH OTHER Phase 1b Findings Aid Phase 2/3 Trial Design © 2020 Aravive, Inc.
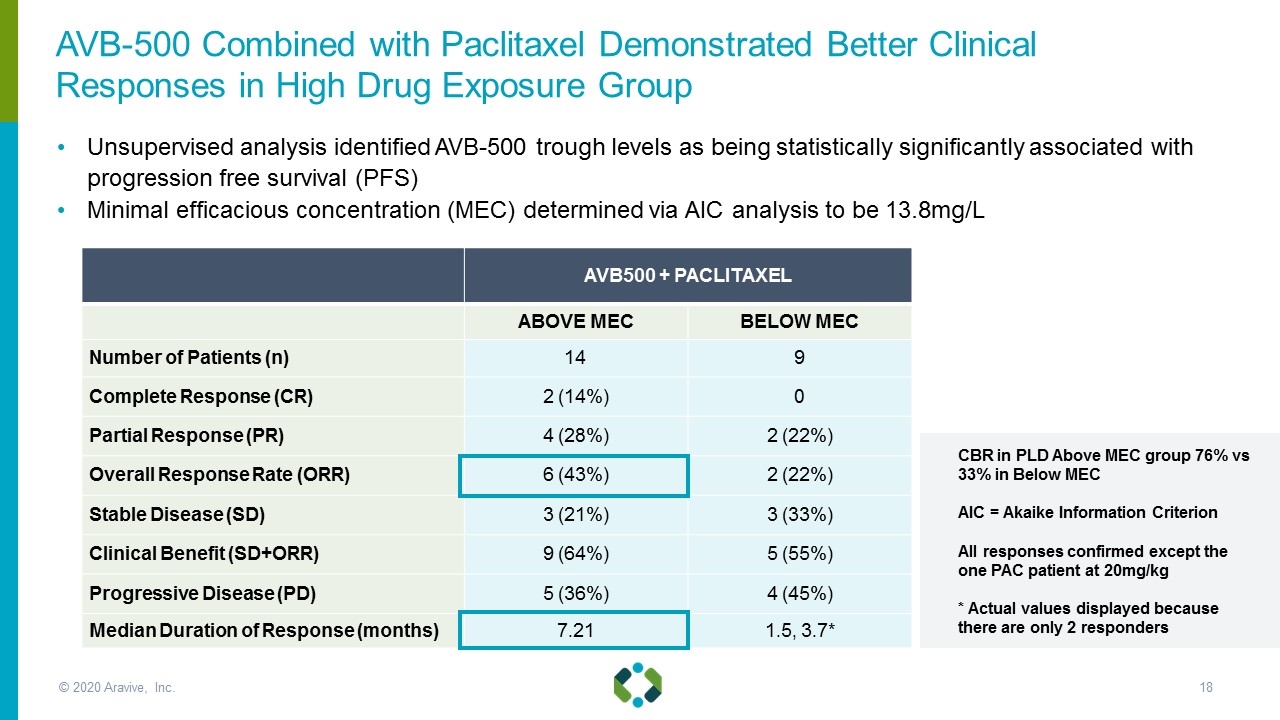
AVB500 + PACLITAXEL ABOVE MEC BELOW MEC Number of Patients (n) 14 9 Complete Response (CR) 2 (14%) 0 Partial Response (PR) 4 (28%) 2 (22%) Overall Response Rate (ORR) 6 (43%) 2 (22%) Stable Disease (SD) 3 (21%) 3 (33%) Clinical Benefit (SD+ORR) 9 (64%) 5 (55%) Progressive Disease (PD) 5 (36%) 4 (45%) Median Duration of Response (months) 7.21 1.5, 3.7* Unsupervised analysis identified AVB-500 trough levels as being statistically significantly associated with progression free survival (PFS) Minimal efficacious concentration (MEC) determined via AIC analysis to be 13.8mg/L CBR in PLD Above MEC group 76% vs 33% in Below MEC AIC = Akaike Information Criterion All responses confirmed except the one PAC patient at 20mg/kg * Actual values displayed because there are only 2 responders AVB-500 Combined with Paclitaxel Demonstrated Better Clinical Responses in High Drug Exposure Group © 2020 Aravive, Inc.
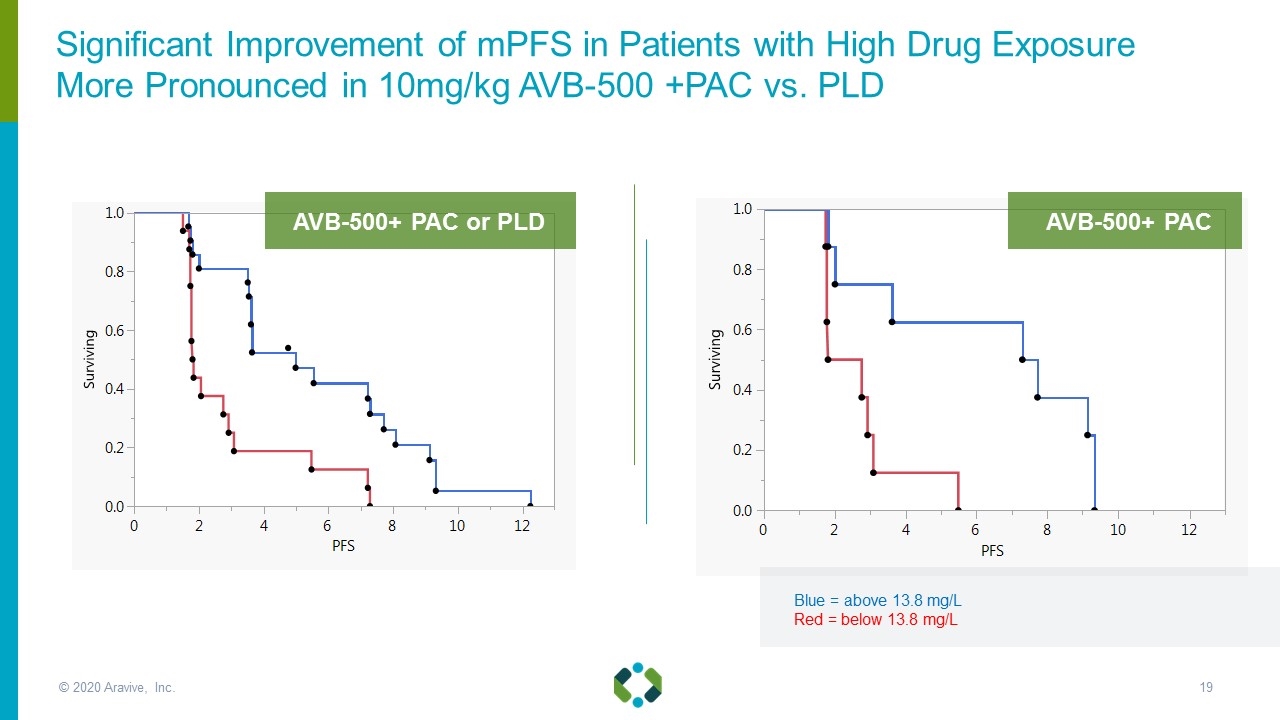
Blue = above 13.8 mg/L Red = below 13.8 mg/L AVB-500+ PAC AVB-500+ PAC or PLD Significant Improvement of mPFS in Patients with High Drug Exposure More Pronounced in 10mg/kg AVB-500 +PAC vs. PLD © 2020 Aravive, Inc.

49 pts in per protocol population, no quantitative CT data for 08-0001 (patient shown on x-axis all the way to right in figure); 4 pts with target lesions that were stable were determined to be PD as best response 10 mg/kg 15 mg/kg 20 mg/kg Target lesions in ~70% patients were stable (i.e, w/in +20 to -30% ) or decreased (≥ -30% ) from baseline % Change in Target Lesions at Best Response by AVB Dose Level © 2020 Aravive, Inc.

Days 50 100 150 200 250 300 350 * * * # # Trough above MEC Trough below MEC Per protocol population * Still on study # on AVB-500 alone after 6 mos chemo # Duration of Treatment by Trough above MEC: Patients with Higher Exposures Stayed on Treatment Longer © 2020 Aravive, Inc.
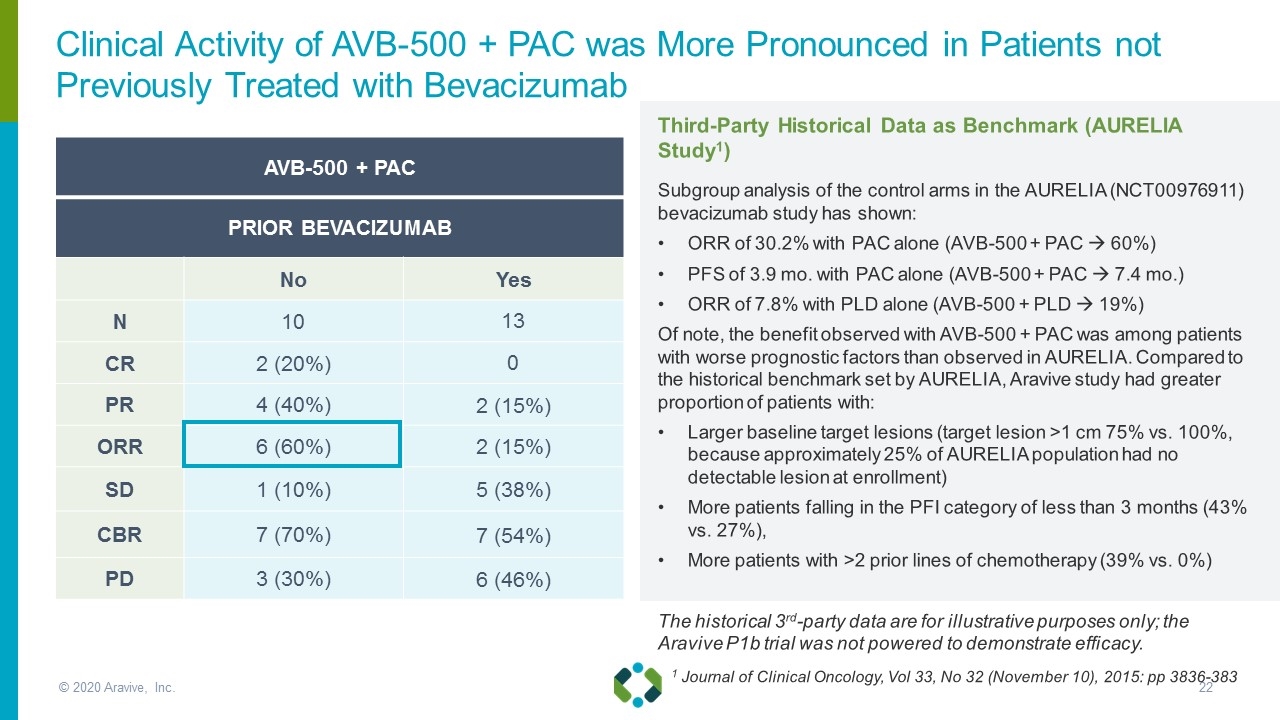
AVB-500 + PAC PRIOR BEVACIZUMAB No Yes N 10 13 CR 2 (20%) 0 PR 4 (40%) 2 (15%) ORR 6 (60%) 2 (15%) SD 1 (10%) 5 (38%) CBR 7 (70%) 7 (54%) PD 3 (30%) 6 (46%) 1 Journal of Clinical Oncology, Vol 33, No 32 (November 10), 2015: pp 3836-383 Third-Party Historical Data as Benchmark (AURELIA Study1) Subgroup analysis of the control arms in the AURELIA (NCT00976911) bevacizumab study has shown: ORR of 30.2% with PAC alone (AVB-500 + PAC à 60%) PFS of 3.9 mo. with PAC alone (AVB-500 + PAC à 7.4 mo.) ORR of 7.8% with PLD alone (AVB-500 + PLD à 19%) Of note, the benefit observed with AVB-500 + PAC was among patients with worse prognostic factors than observed in AURELIA. Compared to the historical benchmark set by AURELIA, Aravive study had greater proportion of patients with: Larger baseline target lesions (target lesion >1 cm 75% vs. 100%, because approximately 25% of AURELIA population had no detectable lesion at enrollment) More patients falling in the PFI category of less than 3 months (43% vs. 27%), More patients with >2 prior lines of chemotherapy (39% vs. 0%) The historical 3rd-party data are for illustrative purposes only; the Aravive P1b trial was not powered to demonstrate efficacy. Clinical Activity of AVB-500 + PAC was More Pronounced in Patients not Previously Treated with Bevacizumab © 2020 Aravive, Inc.
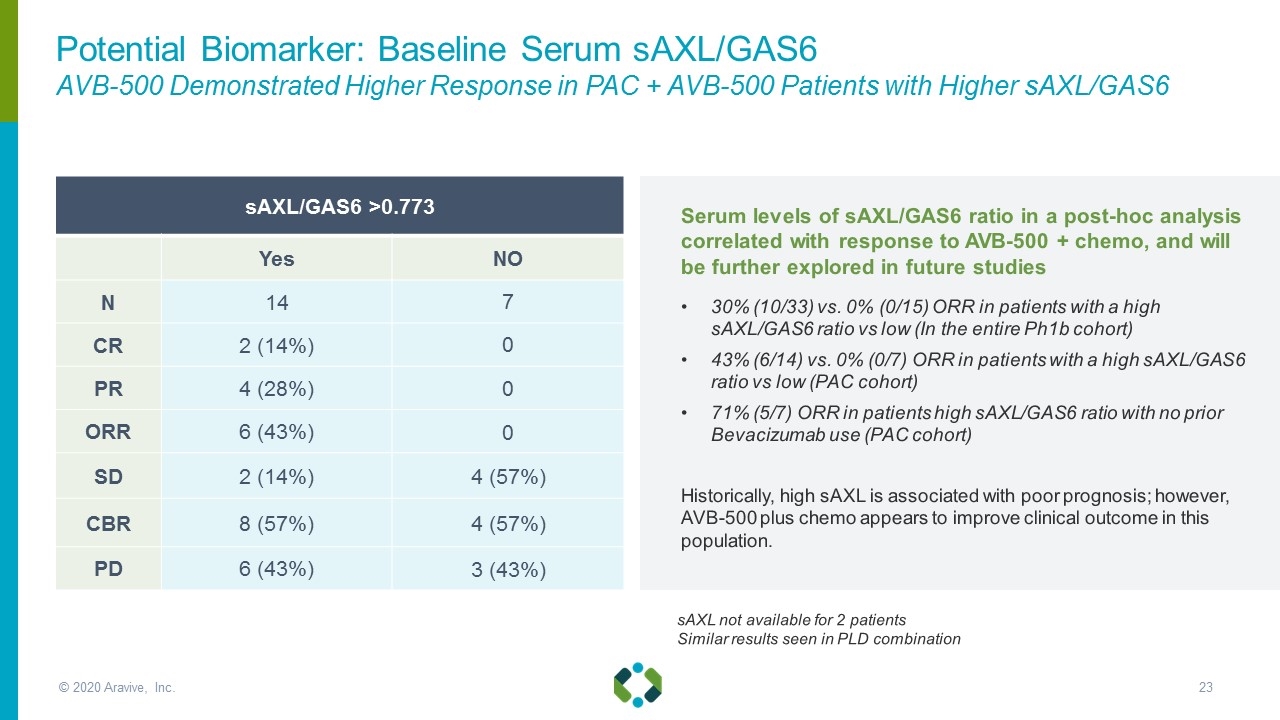
sAXL/GAS6 >0.773 Yes NO N 14 7 CR 2 (14%) 0 PR 4 (28%) 0 ORR 6 (43%) 0 SD 2 (14%) 4 (57%) CBR 8 (57%) 4 (57%) PD 6 (43%) 3 (43%) sAXL not available for 2 patients Similar results seen in PLD combination Serum levels of sAXL/GAS6 ratio in a post-hoc analysis correlated with response to AVB-500 + chemo, and will be further explored in future studies 30% (10/33) vs. 0% (0/15) ORR in patients with a high sAXL/GAS6 ratio vs low (In the entire Ph1b cohort) 43% (6/14) vs. 0% (0/7) ORR in patients with a high sAXL/GAS6 ratio vs low (PAC cohort) 71% (5/7) ORR in patients high sAXL/GAS6 ratio with no prior Bevacizumab use (PAC cohort) Historically, high sAXL is associated with poor prognosis; however, AVB-500 plus chemo appears to improve clinical outcome in this population. Potential Biomarker: Baseline Serum sAXL/GAS6 AVB-500 Demonstrated Higher Response in PAC + AVB-500 Patients with Higher sAXL/GAS6 © 2020 Aravive, Inc.

Potential Registrational Study Primary objective to assess anti-tumor activity of AVB-500 in combination with PAC as measured by PFS FDA’s preliminary feedback Current RCT design is expected to be appropriate Investigating adaptive designs / interim analyses exploring potential biomarkers or alternative end points Anticipate FDA meeting by end of 2020 Anticipate initiating 2H20/Q121, subject to the impact of COVID-19 Exploratory Biomarkers: serum GAS6, AVB-500 drug levels, serum sAXL/GAS6 ratio © 2020 Aravive, Inc. Randomized, Double-Blind, Placebo-Controlled Trial to Compare Efficacy and Tolerability of AVB-500 + PAC versus PAC Planned Phase 2/3 Trial AVB-500 + PAC Placebo + PAC Platinum-Resistant Ovarian Cancer 1-3 prior lines Measurable disease
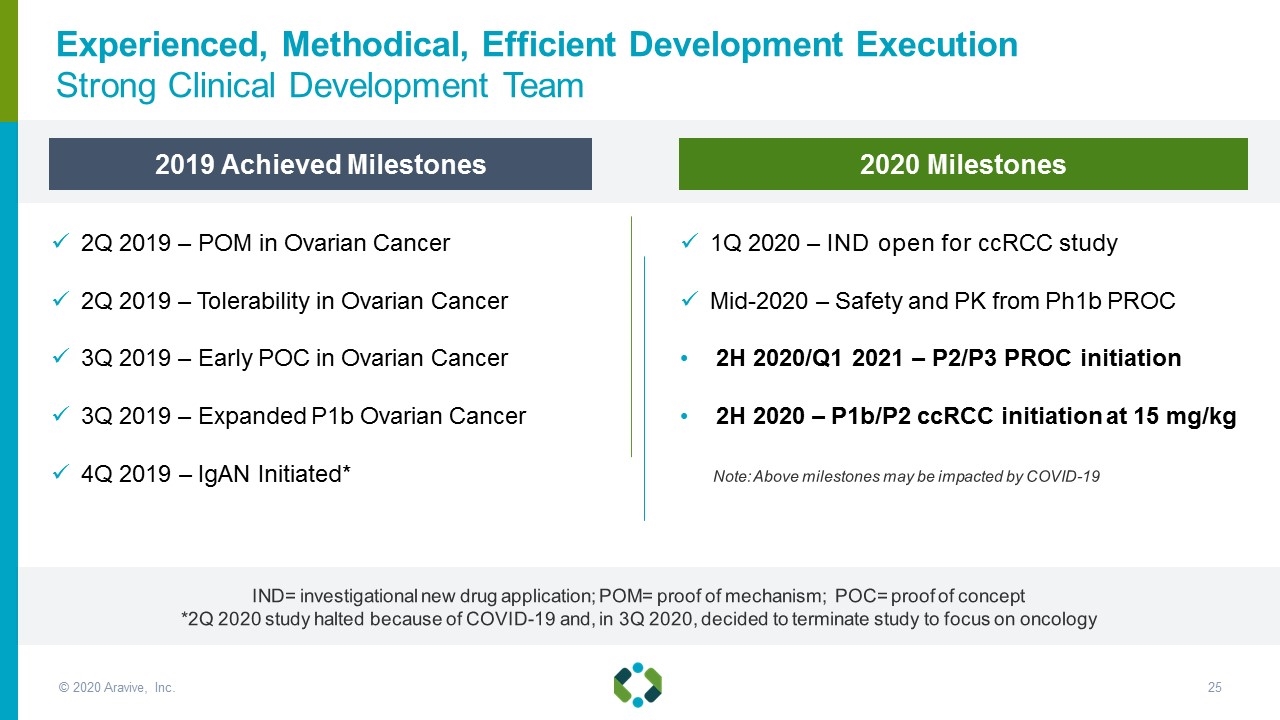
2Q 2019 – POM in Ovarian Cancer 2Q 2019 – Tolerability in Ovarian Cancer 3Q 2019 – Early POC in Ovarian Cancer 3Q 2019 – Expanded P1b Ovarian Cancer 4Q 2019 – IgAN Initiated* 1Q 2020 – IND open for ccRCC study Mid-2020 – Safety and PK from Ph1b PROC 2H 2020/Q1 2021 – P2/P3 PROC initiation 2H 2020 – P1b/P2 ccRCC initiation at 15 mg/kg Note: Above milestones may be impacted by COVID-19 2019 Achieved Milestones IND= investigational new drug application; POM= proof of mechanism; POC= proof of concept *2Q 2020 study halted because of COVID-19 and, in 3Q 2020, decided to terminate study to focus on oncology Experienced, Methodical, Efficient Development Execution Strong Clinical Development Team 2020 Milestones © 2020 Aravive, Inc.

Expand Board of Directors Opportunistic with respect to business development deals around our pipeline ex-U.S. Focus on oncology: Advance PROC Phase 2/3 trial Initiate ccRCC trial Potential pipeline expansion in high unmet oncology indications, all of which GAS6/AXL pathway is active and relates to poor prognosis Advance CTGF discovery program to clinic Other potential discovery programs Plan to expand development team to execute company strategy Growth Strategy: Strengthen Company and Build Pipeline © 2020 Aravive, Inc.
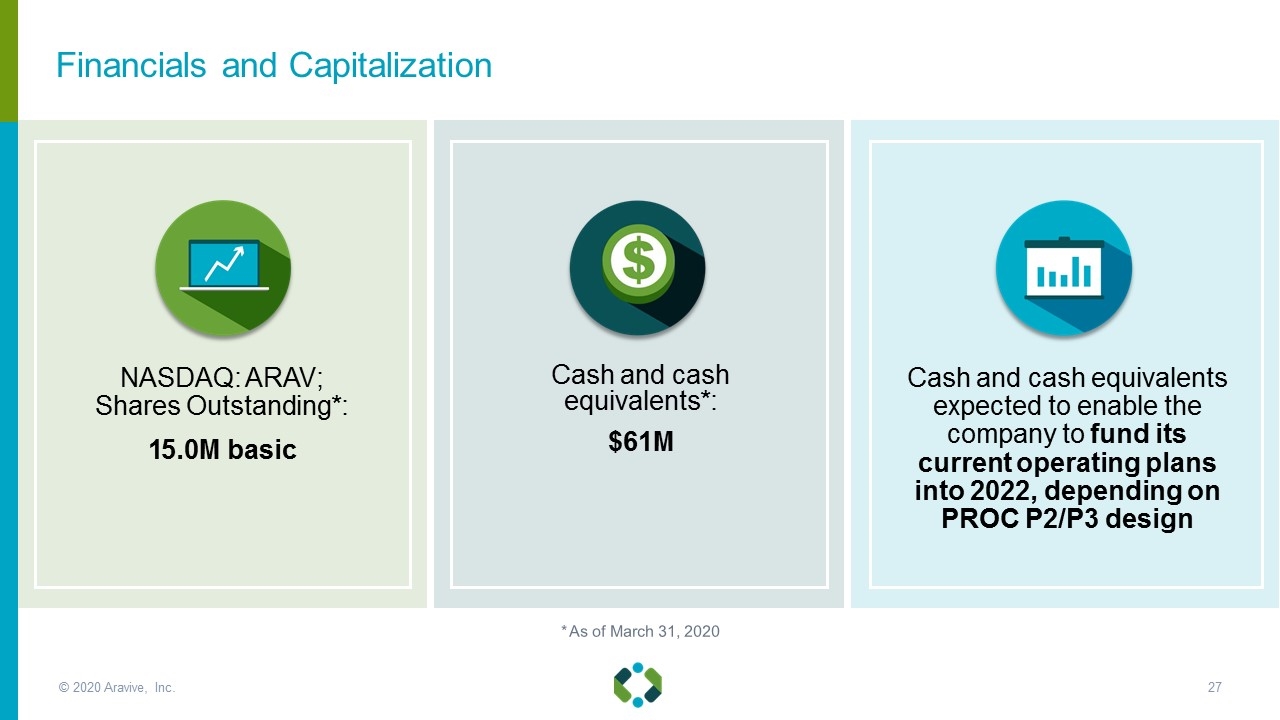
© 2020 Aravive, Inc. NASDAQ: ARAV; Shares Outstanding*: 15.0M basic Cash and cash equivalents*: $61M Cash and cash equivalents expected to enable the company to fund its current operating plans into 2022, depending on PROC P2/P3 design * As of March 31, 2020 Financials and Capitalization

© 2020 Aravive, Inc. Uniquely positioned in competitive landscape Robust pipeline addressing significant drivers of mortality in oncology: metastasis and drug resistance AVB-500: high affinity decoy protein, a potent and selective drug that targets GAS6/AXL signaling Validated pathway drives activation, migration, and invasion of abnormal cells Suppresses GAS6 to undetectable levels in early clinical studies; prolonged target engagement Safety and tolerability profile to date support use as combination and/or maintenance therapy Compelling early efficacy signal in platinum-resistant ovarian cancer No adverse events that limit dosing High AVB-500 levels predictive of anti-tumor activity; exposure-response relationship RP2D identified AVB-500 performs well in late line therapy, where the clinical need is great Plan to discuss Phase 2/3 trial with FDA later this year Additional programs advancing into the clinic in high need indications* Renal cell carcinoma IND open and expect to initiate later this year ISTs (bladder cancer and PROC) CTGF preclinical program *Recruitment and retention in clinical trials may be impacted by COVID-19 AVB-500 Offers First- and Potential Best-In-Class Opportunity in Oncology

© 2020 Aravive, Inc. Thank You Halting Disease Progression in its Tracks




























