
Corporate Presentation April 2021 Halting Disease Progression in its Tracks ARAV (NASDAQ) © 2021 Aravive, Inc. 1 Exhibit 99.2

Forward-Looking Statements Forward Looking Statements © 2021 Aravive, Inc. 2 This presentation contains forward-looking statements that may discuss Aravive’s plans, goals, intentions and expectations as to future trends, events, results of operations, financial condition or other matters. Forward- looking statements generally include statements that are predictive in nature and depend upon or refer to future events or conditions, and they often include words such as “may,” “will,” “should,” “would,” “expect,” “anticipate,” “plan,” “likely,” “believe,” “estimate,” “project,” “intend,” and other similar expressions. Statements that are not historical facts are forward-looking statements. Forward-looking statements included in this presentation include statements regarding Aravive’s planned clinical activities and the timing of such activities, including the design, patient enrollment and availability of data from clinical studies, the potential pipeline, future indications and cash position, the anticipated safety, benefits, activity and manufacturability of Aravive’s product candidates and the ability to obtain regulatory approval, including the Phase 3 study supporting full approval in PROC. Forward-looking statements are based on Aravive’s current beliefs and assumptions, are subject to risks and uncertainties and are not guarantees of future performance. Actual results could differ materially from those contained in any forward-looking statement as a result of various factors, including, without limitation: The trial supporting the submission of a biologics license application to the FDA, Aravive’s ability to conduct an interim analysis early next year, as planned, Aravive’s ability to enroll approximately 300-400 patients with high-grade serous ovarian cancer who have received one to four prior lines of therapy as planned, Aravive’s ability to conduct the trial in the U.S. and Europe as planned, the impact of COVID-19 on Aravive's clinical strategy, clinical trials, supply chain and fundraising, Aravive's ability to expand development into additional oncology indications, Aravive's dependence upon AVB-500, AVB-500's ability to have favorable results in clinical trials and ISTs, the clinical trials of AVB-500 having results that are as favorable as those of preclinical and clinical trials, the ability to receive regulatory approval, potential delays in Aravive's clinical trials due to regulatory requirements or difficulty identifying qualified investigators or enrolling patients especially in light of the COVID-19 pandemic; the risk that AVB-500 may cause serious side effects or have properties that delay or prevent regulatory approval or limit its commercial potential; the risk that Aravive may encounter difficulties in manufacturing AVB-500; if AVB-500 is approved, risks associated with its market acceptance, including pricing and reimbursement; potential difficulties enforcing Aravive's intellectual property rights; Aravive's reliance on its licensor of intellectual property and financing needs. The foregoing review of important factors that could cause actual events to differ from expectations should not be construed as exhaustive and should be read in conjunction with statements that are included herein and elsewhere, including the risk factors included in Aravive’s most recent Annual Report on Form 10-K, Quarterly Report on Form 10-Q and Current Reports on Form 8-K filed with the Securities and Exchange Commission. Except as required by law, Aravive undertakes no obligation to revise or update any forward-looking statement or to make any other forward-looking statements, whether as a result of new information, future events or otherwise.

Experienced Management and Board of Directors Board of Directors Senior Management CEO Gail McIntyre, Ph.D., DABT CMO Reshma Rangwala, M.D., Ph.D. Chairman of the Board Fred Eshelman, Pharm D. Director Amato Giaccia, Ph.D. Director Michael Rogers Director Eric Zhang CFO Vinay Shah SVP of Data Sciences Randy Anderson, Ph.D. VP, Translational Medicine Elisabeth Gardiner, Ph.D. Director Gail McIntyre, Ph.D., DABT VP, Regulatory Jody Gould, Ph.D. VP, Clinical Operations Amy Franke, MBE © 2021 Aravive, Inc. 3
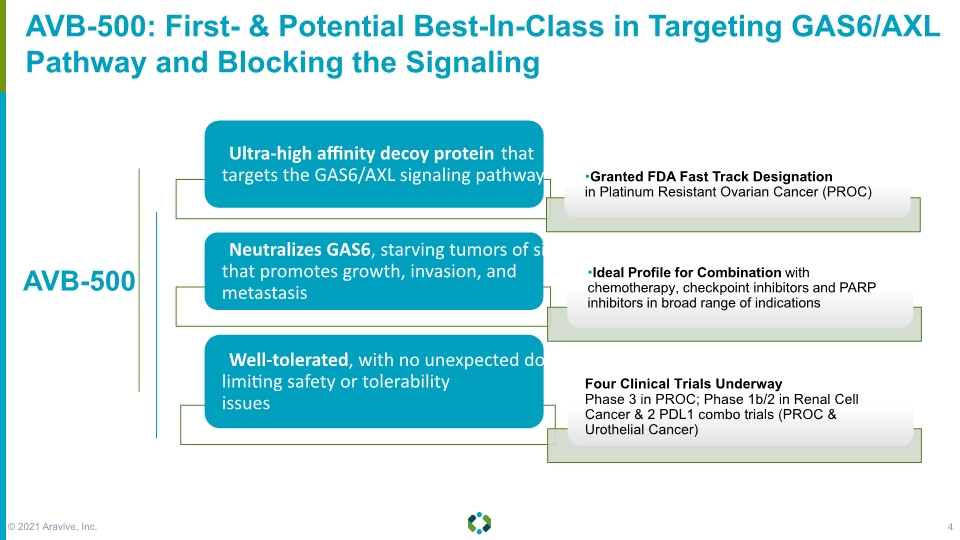
AVB-500: First- & Potential Best-In-Class in Targeting GAS6/AXL Pathway and Blocking the Signaling AVB-500 © 2021 Aravive, Inc. 4
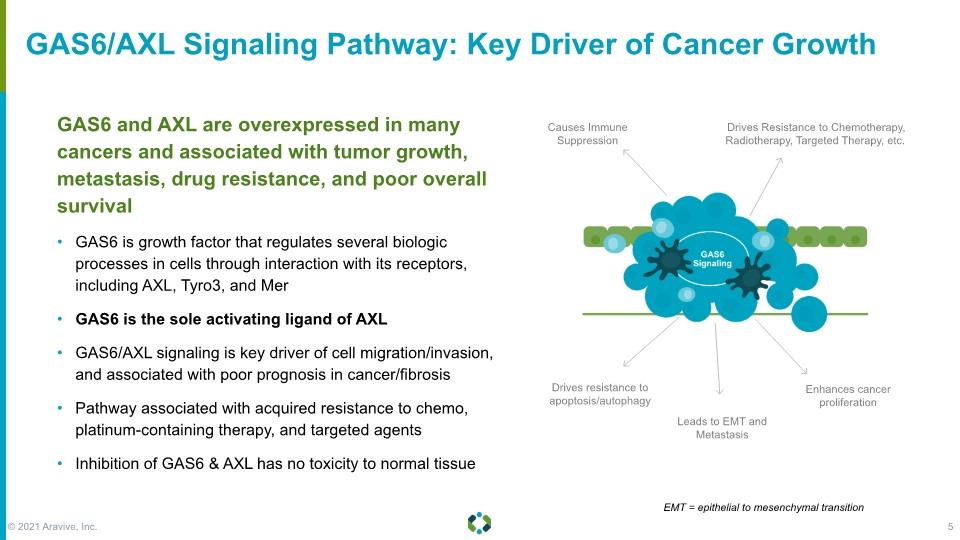
GAS6/AXL Signaling Pathway: Key Driver of Cancer Growth GAS6 and AXL are overexpressed in many cancers and associated with tumor growth, metastasis, drug resistance, and poor overall survival GAS6 is growth factor that regulates several biologic processes in cells through interaction with its receptors, including AXL, Tyro3, and Mer GAS6 is the sole activating ligand of AXL GAS6/AXL signaling is key driver of cell migration/invasion, and associated with poor prognosis in cancer/fibrosis Pathway associated with acquired resistance to chemo, platinum-containing therapy, and targeted agents Inhibition of GAS6 & AXL has no toxicity to normal tissue Drives Resistance to Chemotherapy, Radiotherapy, Targeted Therapy, etc. Causes Immune Suppression Drives resistance to apoptosis/autophagy Leads to EMT and Metastasis Enhances cancer proliferation EMT = epithelial to mesenchymal transition © 2021 Aravive, Inc. 5
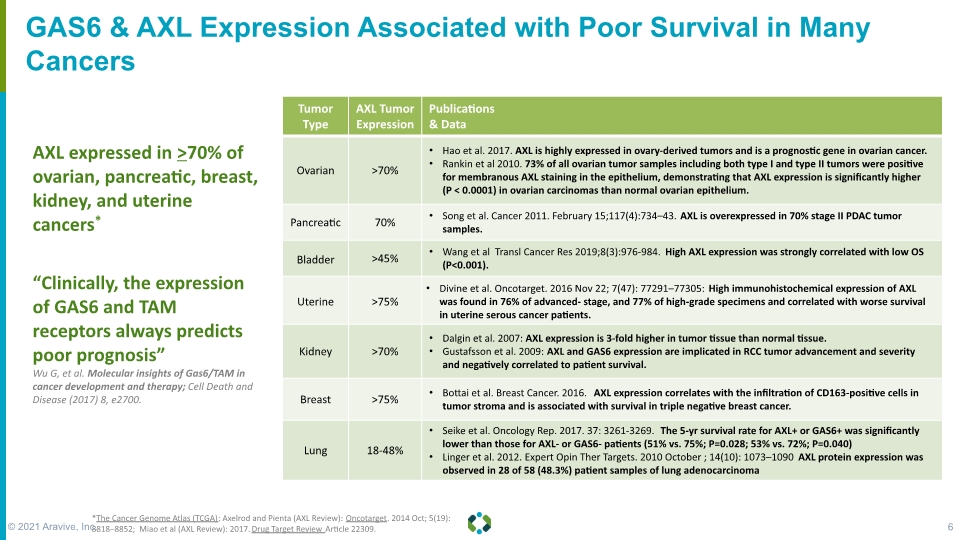
GAS6 & AXL Expression Associated with Poor Survival in Many Cancers “Clinically, the expression of GAS6 and TAM receptors always predicts poor prognosis” Wu G, et al. Molecular insights of Gas6/TAM in cancer development and therapy; Cell Death and Disease (2017) 8, e2700. AXL expressed in >70% of ovarian, pancreatic, breast, kidney, and uterine cancers* *The Cancer Genome Atlas (TCGA); Axelrod and Pienta (AXL Review): Oncotarget. 2014 Oct; 5(19): 8818–8852; Miao et al (AXL Review): 2017. Drug Target Review Article 22309. © 2021 Aravive, Inc. 6
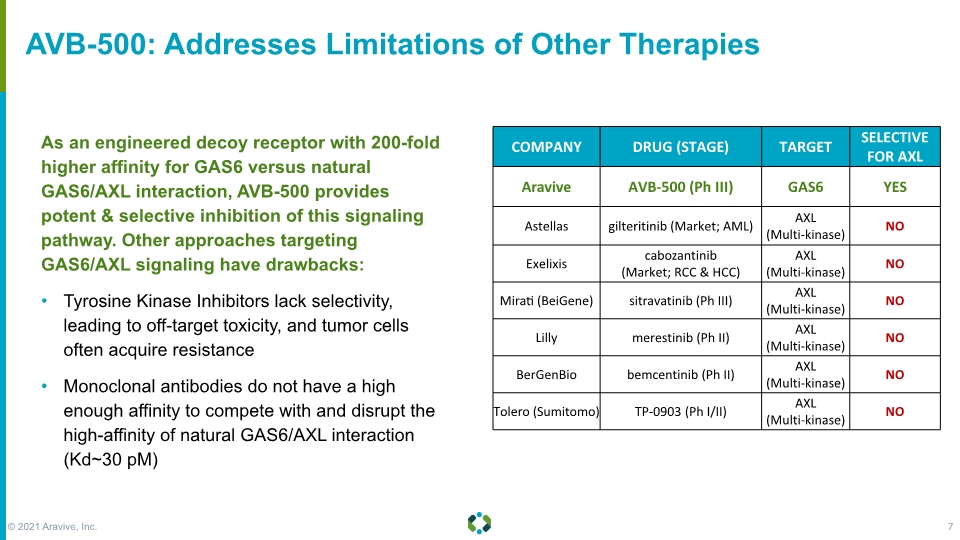
As an engineered decoy receptor with 200-fold higher affinity for GAS6 versus natural GAS6/AXL interaction, AVB-500 provides potent & selective inhibition of this signaling pathway. Other approaches targeting GAS6/AXL signaling have drawbacks: Tyrosine Kinase Inhibitors lack selectivity, leading to off-target toxicity, and tumor cells often acquire resistance Monoclonal antibodies do not have a high enough affinity to compete with and disrupt the high-affinity of natural GAS6/AXL interaction (Kd~30 pM) AVB-500: Addresses Limitations of Other Therapies © 2021 Aravive, Inc. 7
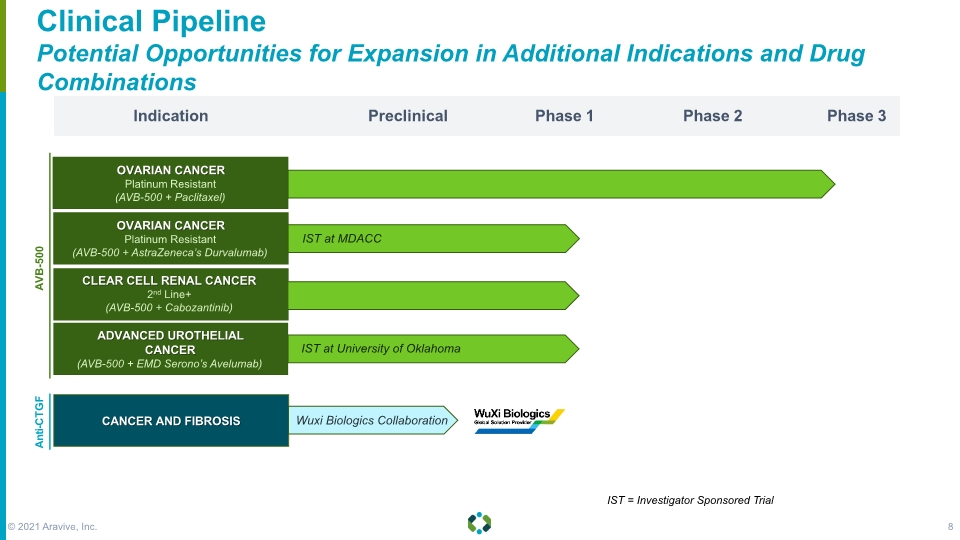
OVARIAN CANCER Platinum Resistant (AVB-500 + AstraZeneca’s Durvalumab) Forward-Looking Statements Clinical Pipeline Potential Opportunities for Expansion in Additional Indications and Drug Combinations Indication Preclinical Phase 1 Phase 3 Phase 2 Anti-CTGF AVB-500 IST at MDACC CANCER AND FIBROSIS OVARIAN CANCER Platinum Resistant (AVB-500 + Paclitaxel) CLEAR CELL RENAL CANCER 2nd Line+ (AVB-500 + Cabozantinib) ADVANCED UROTHELIAL CANCER (AVB-500 + EMD Serono’s Avelumab) IST at University of Oklahoma Wuxi Biologics Collaboration IST = Investigator Sponsored Trial © 2021 Aravive, Inc. 8
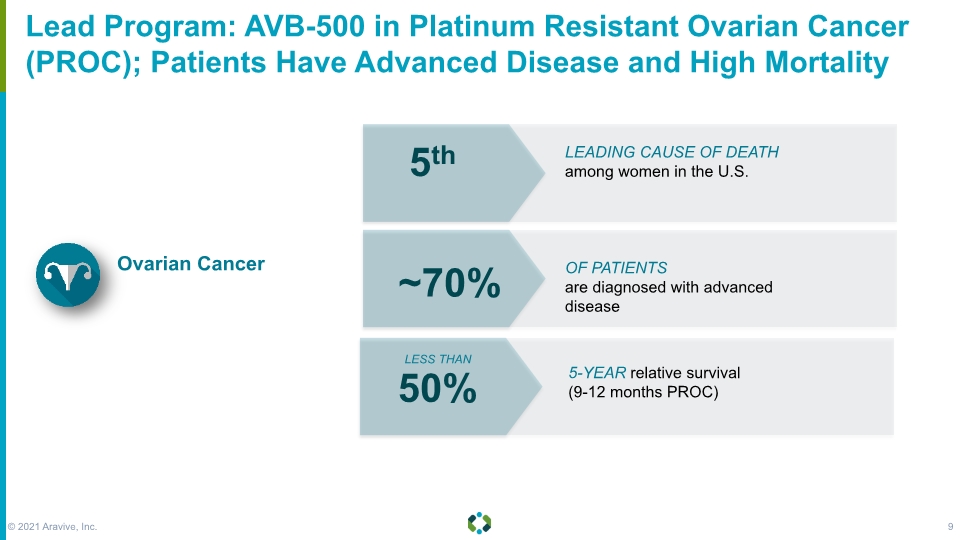
Forward-Looking Statements Lead Program: AVB-500 in Platinum Resistant Ovarian Cancer (PROC); Patients Have Advanced Disease and High Mortality 5th LEADING CAUSE OF DEATH among women in the U.S. ~70% OF PATIENTS are diagnosed with advanced disease 50% 5-YEAR relative survival (9-12 months PROC) LESS THAN Ovarian Cancer © 2021 Aravive, Inc. 9
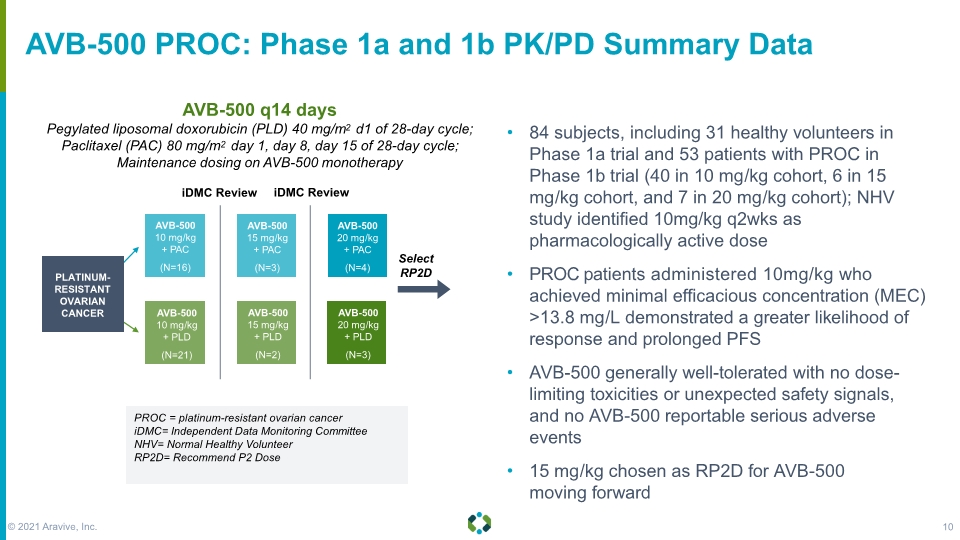
AVB-500 PROC: Phase 1a and 1b PK/PD Summary Data AVB-500 q14 days Pegylated liposomal doxorubicin (PLD) 40 mg/m2 d1 of 28-day cycle; Paclitaxel (PAC) 80 mg/m2 day 1, day 8, day 15 of 28-day cycle; Maintenance dosing on AVB-500 monotherapy PROC = platinum-resistant ovarian cancer iDMC= Independent Data Monitoring Committee NHV= Normal Healthy Volunteer RP2D= Recommend P2 Dose iDMC Review iDMC Review 84 subjects, including 31 healthy volunteers in Phase 1a trial and 53 patients with PROC in Phase 1b trial (40 in 10 mg/kg cohort, 6 in 15 mg/kg cohort, and 7 in 20 mg/kg cohort); NHV study identified 10mg/kg q2wks as pharmacologically active dose PROC patients administered 10mg/kg who achieved minimal efficacious concentration (MEC) >13.8 mg/L demonstrated a greater likelihood of response and prolonged PFS AVB-500 generally well-tolerated with no dose- limiting toxicities or unexpected safety signals, and no AVB-500 reportable serious adverse events 15 mg/kg chosen as RP2D for AVB-500 moving forward © 2021 Aravive, Inc. 10
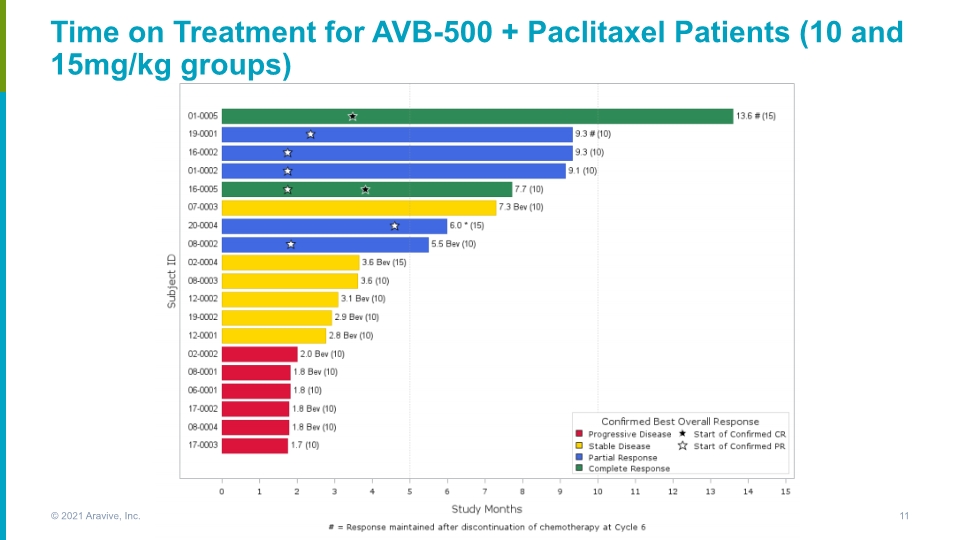
Time on Treatment for AVB-500 + Paclitaxel Patients (10 and 15mg/kg groups) © 2021 Aravive, Inc. 11
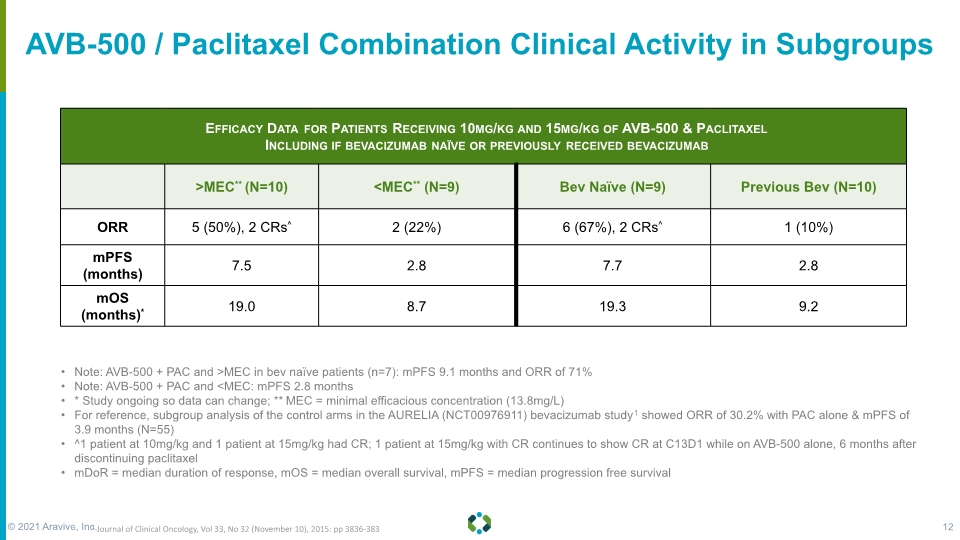
AVB-500 / Paclitaxel Combination Clinical Activity in Subgroups Note: AVB-500 + PAC and >MEC in bev naïve patients (n=7): mPFS 9.1 months and ORR of 71% Note: AVB-500 + PAC and <MEC: mPFS 2.8 months * Study ongoing so data can change; ** MEC = minimal efficacious concentration (13.8mg/L) For reference, subgroup analysis of the control arms in the AURELIA (NCT00976911) bevacizumab study1 showed ORR of 30.2% with PAC alone & mPFS of 3.9 months (N=55) ^1 patient at 10mg/kg and 1 patient at 15mg/kg had CR; 1 patient at 15mg/kg with CR continues to show CR at C13D1 while on AVB-500 alone, 6 months after discontinuing paclitaxel mDoR = median duration of response, mOS = median overall survival, mPFS = median progression free survival 1 Journal of Clinical Oncology, Vol 33, No 32 (November 10), 2015: pp 3836-383 © 2021 Aravive, Inc. 12
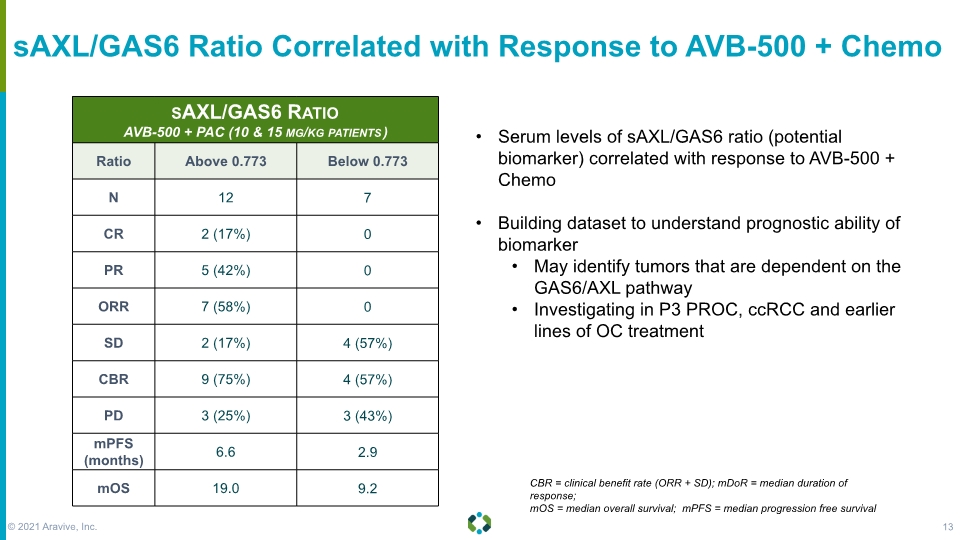
sAXL/GAS6 Ratio Correlated with Response to AVB-500 + Chemo Serum levels of sAXL/GAS6 ratio (potential biomarker) correlated with response to AVB-500 + Chemo Building dataset to understand prognostic ability of biomarker May identify tumors that are dependent on the GAS6/AXL pathway Investigating in P3 PROC, ccRCC and earlier lines of OC treatment CBR = clinical benefit rate (ORR + SD); mDoR = median duration of response; mOS = median overall survival; mPFS = median progression free survival © 2021 Aravive, Inc. 13
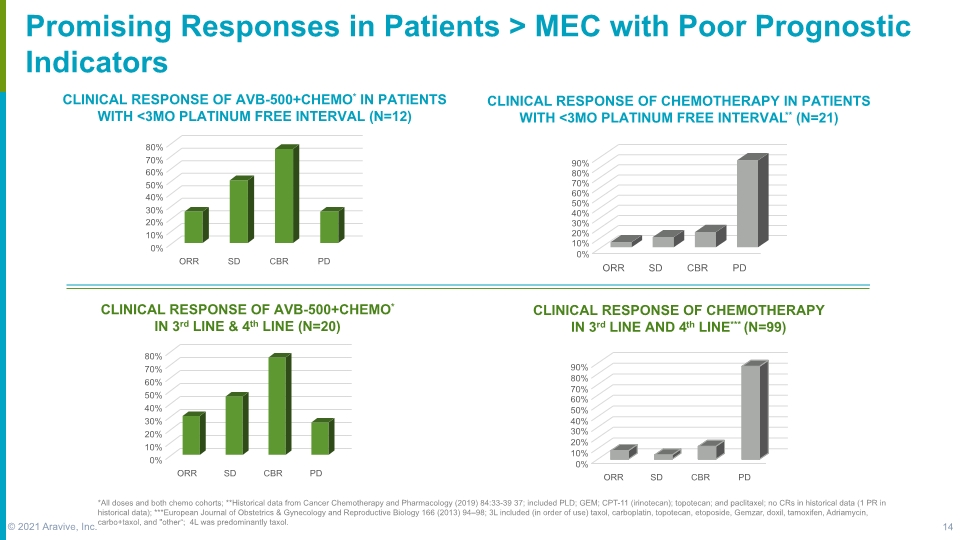
Promising Responses in Patients > MEC with Poor Prognostic Indicators CLINICAL RESPONSE OF CHEMOTHERAPY IN PATIENTS WITH <3MO PLATINUM FREE INTERVAL** (N=21) CLINICAL RESPONSE OF CHEMOTHERAPY IN 3rd LINE AND 4th LINE*** (N=99) *All doses and both chemo cohorts; **Historical data from Cancer Chemotherapy and Pharmacology (2019) 84:33-39 37; included PLD; GEM; CPT-11 (irinotecan); topotecan; and paclitaxel; no CRs in historical data (1 PR in historical data); ***European Journal of Obstetrics & Gynecology and Reproductive Biology 166 (2013) 94–98; 3L included (in order of use) taxol, carboplatin, topotecan, etoposide, Gemzar, doxil, tamoxifen, Adriamycin, carbo+taxol, and "other“; 4L was predominantly taxol. © 2021 Aravive, Inc. 14
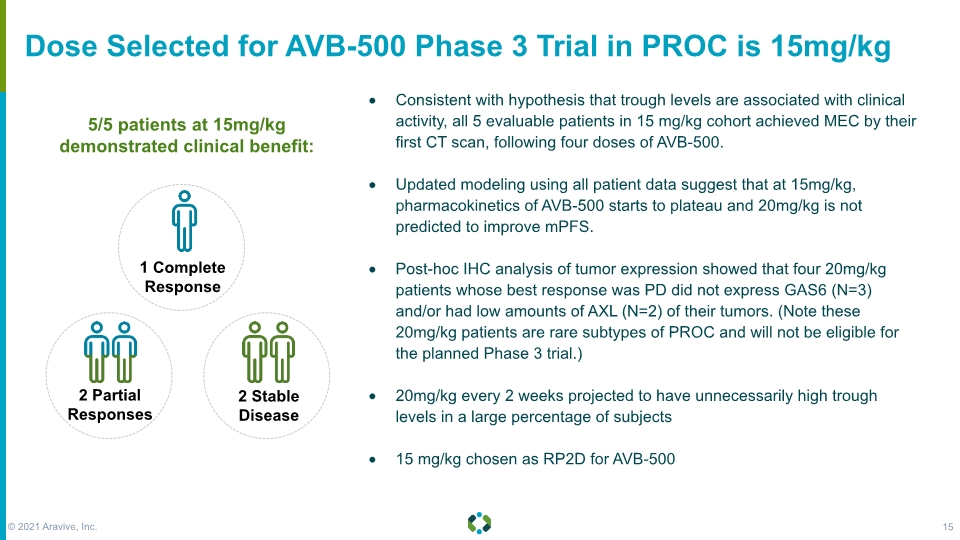
Dose Selected for AVB-500 Phase 3 Trial in PROC is 15mg/kg 5/5 patients at 15mg/kg demonstrated clinical benefit: Consistent with hypothesis that trough levels are associated with clinical activity, all 5 evaluable patients in 15 mg/kg cohort achieved MEC by their first CT scan, following four doses of AVB-500. Updated modeling using all patient data suggest that at 15mg/kg, pharmacokinetics of AVB-500 starts to plateau and 20mg/kg is not predicted to improve mPFS. Post-hoc IHC analysis of tumor expression showed that four 20mg/kg patients whose best response was PD did not express GAS6 (N=3) and/or had low amounts of AXL (N=2) of their tumors. (Note these 20mg/kg patients are rare subtypes of PROC and will not be eligible for the planned Phase 3 trial.) 20mg/kg every 2 weeks projected to have unnecessarily high trough levels in a large percentage of subjects 15 mg/kg chosen as RP2D for AVB-500 © 2021 Aravive, Inc. 15
Strong Safety Profile of AVB-500 Safety report for trial AVB500-OC-002 generated by independent medical monitor concluded: “Based on a review of the safety data, no unexpected safety trends have been detected and the reported AE severity and frequency are consistent with expectations of the concomitant treatment (PAC or PLD) and the disease under study (ovarian cancer).” AVB-500 well-tolerated: fatigue and infusion reactions appeared to be related to treatment No serious events that required expedited reporting per Sponsor No dose-limiting toxicities 53 PROC Patients Dosed: 10 mg/kg (40); 15 mg/kg (6); and 20 mg/kg (7) © 2021 Aravive, Inc. 16
Competitive Advantages of AVB-500 in PROC: Favorable Safety Profile and Compelling Anti-Tumor Activity Favorable Safety Profile to Date NO DLTs like chemotherapies and ADCs NO dose reductions or discontinuations to manage toxicities NO toxicities that have been seen with ADCs: neutropenia, ocular toxicities, peripheral neuropathy, and/or liver enzyme elevation Administered with therapies that physicians can manage Infusion reactions and fatigue appeared to be related to treatment Compelling anti-tumor activity Saw ORR of 50% with 2 CRs, mPFS of 7.5 months, and mOS of 19 months in 10 & 15mg/kg AVB-500/PAC patients when trough levels exceeded MEC Promising clinical activity improved in bevacizumab naïve patients ORR of 37% in 10 and 15mg/kg AVB-500/PAC patients, regardless of trough levels © 2021 Aravive, Inc. 17
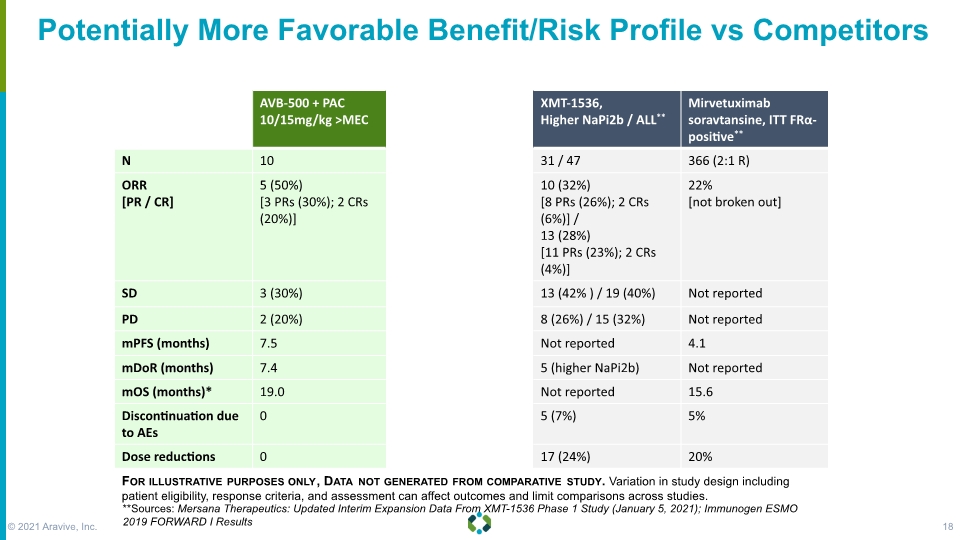
Potentially More Favorable Benefit/Risk Profile vs Competitors For illustrative purposes only, Data not generated from comparative study. Variation in study design including patient eligibility, response criteria, and assessment can affect outcomes and limit comparisons across studies. **Sources: Mersana Therapeutics: Updated Interim Expansion Data From XMT-1536 Phase 1 Study (January 5, 2021); Immunogen ESMO 2019 FORWARD I Results © 2021 Aravive, Inc. 18
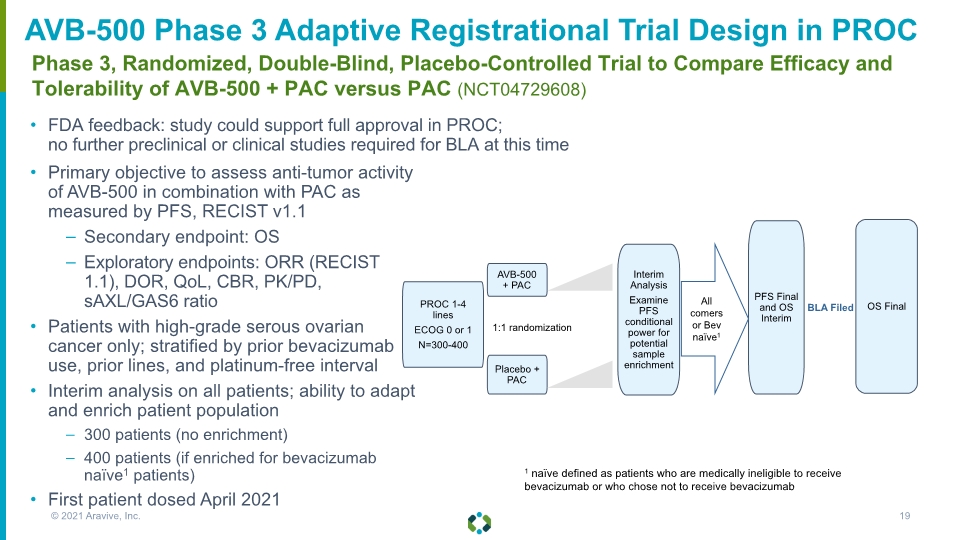
FDA feedback: study could support full approval in PROC; no further preclinical or clinical studies required for BLA at this time Phase 3, Randomized, Double-Blind, Placebo-Controlled Trial to Compare Efficacy and Tolerability of AVB-500 + PAC versus PAC (NCT04729608) AVB-500 Phase 3 Adaptive Registrational Trial Design in PROC Primary objective to assess anti-tumor activity of AVB-500 in combination with PAC as measured by PFS, RECIST v1.1 Secondary endpoint: OS Exploratory endpoints: ORR (RECIST 1.1), DOR, QoL, CBR, PK/PD, sAXL/GAS6 ratio Patients with high-grade serous ovarian cancer only; stratified by prior bevacizumab use, prior lines, and platinum-free interval Interim analysis on all patients; ability to adapt and enrich patient population 300 patients (no enrichment) 400 patients (if enriched for bevacizumab naïve1 patients) First patient dosed April 2021 All comers or Bev naïve1 © 2021 Aravive, Inc. 19 1 naïve defined as patients who are medically ineligible to receive bevacizumab or who chose not to receive bevacizumab
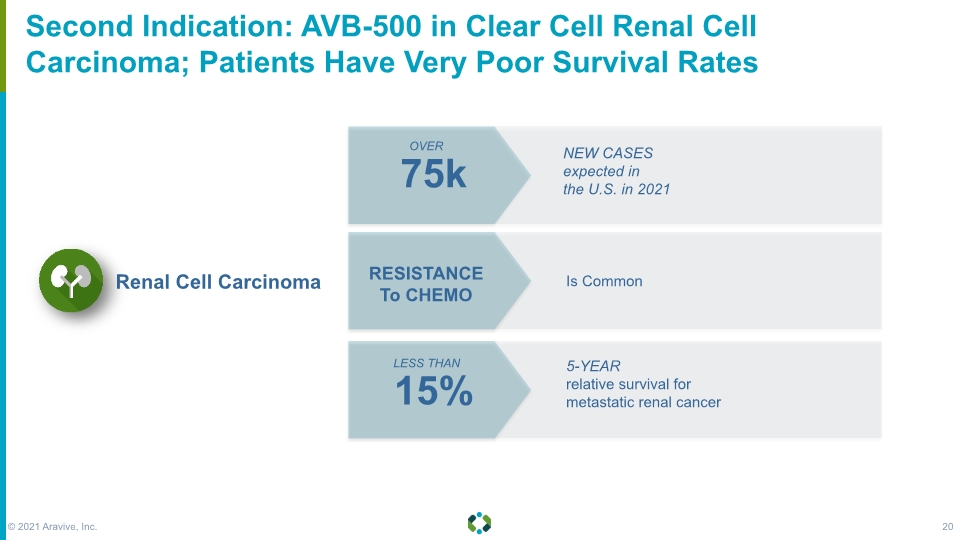
Forward-Looking Statements Second Indication: AVB-500 in Clear Cell Renal Cell Carcinoma; Patients Have Very Poor Survival Rates Renal Cell Carcinoma 15% 75k RESISTANCE To CHEMO OVER LESS THAN 5-YEAR relative survival for metastatic renal cancer NEW CASES expected in the U.S. in 2021 Is Common © 2021 Aravive, Inc. 20
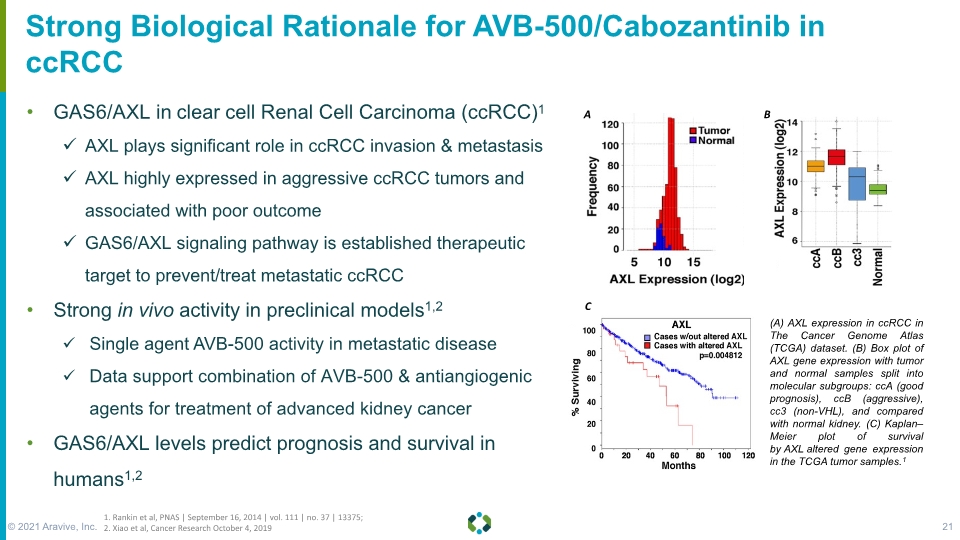
Forward-Looking Statements Strong Biological Rationale for AVB-500/Cabozantinib in ccRCC GAS6/AXL in clear cell Renal Cell Carcinoma (ccRCC)1 AXL plays significant role in ccRCC invasion & metastasis AXL highly expressed in aggressive ccRCC tumors and associated with poor outcome GAS6/AXL signaling pathway is established therapeutic target to prevent/treat metastatic ccRCC Strong in vivo activity in preclinical models1,2 Single agent AVB-500 activity in metastatic disease Data support combination of AVB-500 & antiangiogenic agents for treatment of advanced kidney cancer GAS6/AXL levels predict prognosis and survival in humans1,2 1. Rankin et al, PNAS | September 16, 2014 | vol. 111 | no. 37 | 13375; 2. Xiao et al, Cancer Research October 4, 2019 © 2021 Aravive, Inc. 21
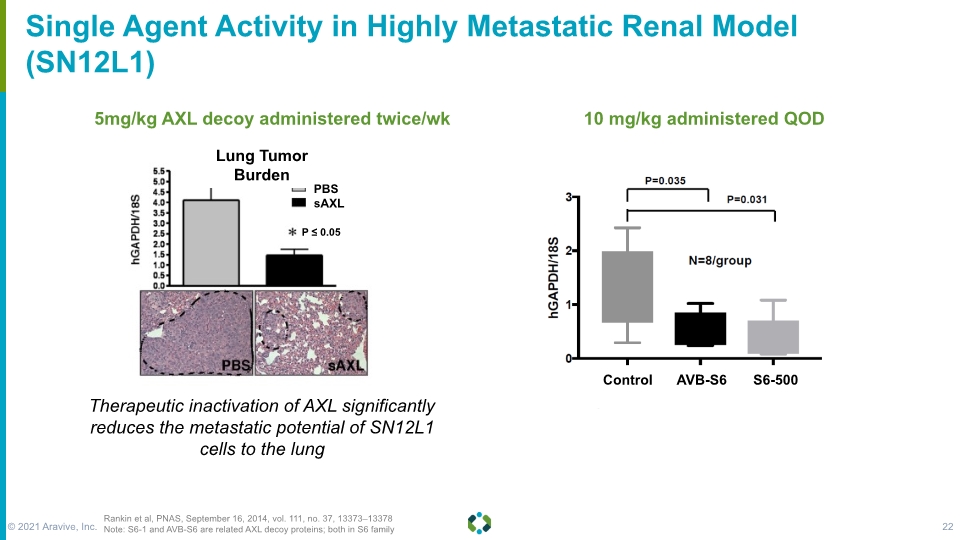
Forward-Looking Statements Single Agent Activity in Highly Metastatic Renal Model (SN12L1) 5mg/kg AXL decoy administered twice/wk 10 mg/kg administered QOD Rankin et al, PNAS, September 16, 2014, vol. 111, no. 37, 13373–13378 Note: S6-1 and AVB-S6 are related AXL decoy proteins; both in S6 family Therapeutic inactivation of AXL significantly reduces the metastatic potential of SN12L1 cells to the lung P ≤ 0.05 Lung Tumor Burden PBS sAXL © 2021 Aravive, Inc. 22
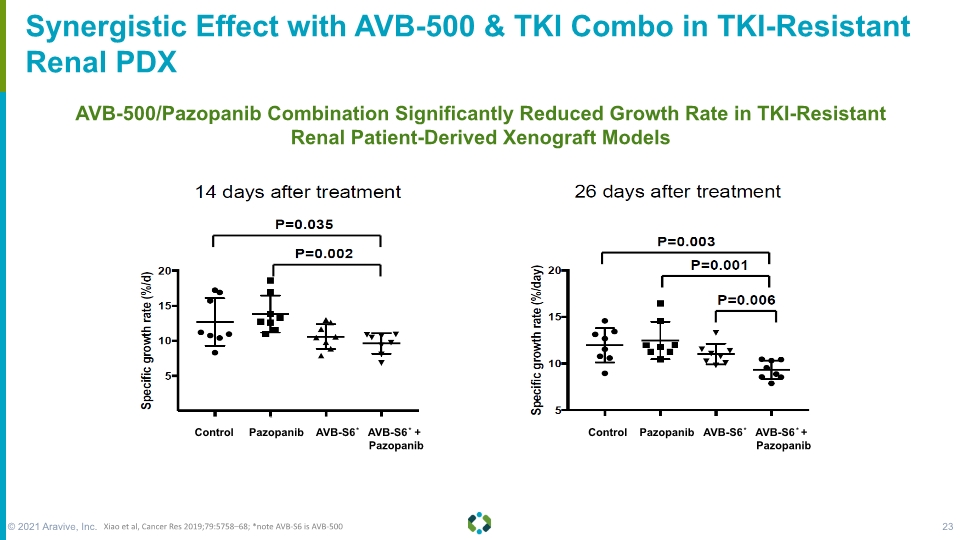
Forward-Looking Statements Synergistic Effect with AVB-500 & TKI Combo in TKI-Resistant Renal PDX AVB-500/Pazopanib Combination Significantly Reduced Growth Rate in TKI-Resistant Renal Patient-Derived Xenograft Models Xiao et al, Cancer Res 2019;79:5758–68; *note AVB-S6 is AVB-500 © 2021 Aravive, Inc. 23
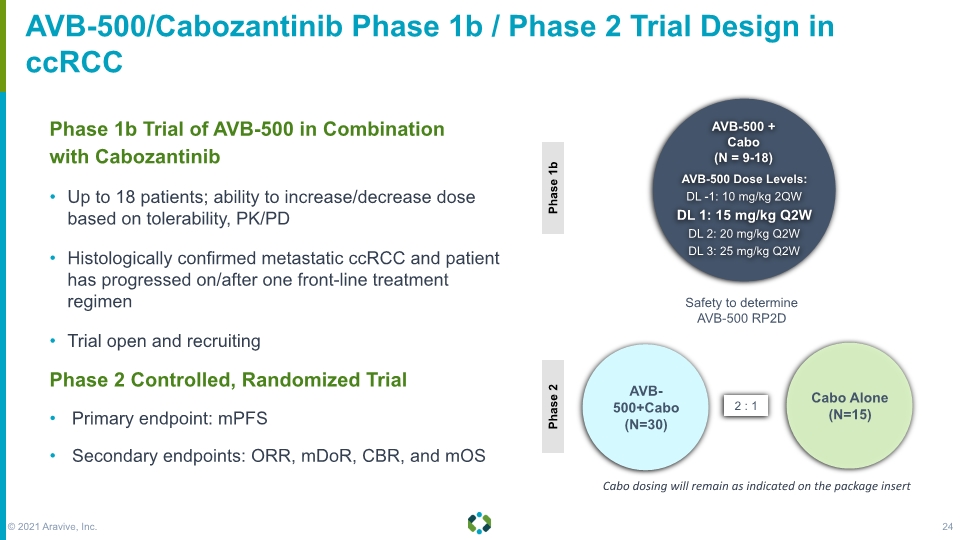
Forward-Looking Statements AVB-500/Cabozantinib Phase 1b / Phase 2 Trial Design in ccRCC Phase 1b Trial of AVB-500 in Combination with Cabozantinib Up to 18 patients; ability to increase/decrease dose based on tolerability, PK/PD Histologically confirmed metastatic ccRCC and patient has progressed on/after one front-line treatment regimen Trial open and recruiting Phase 2 Controlled, Randomized Trial Primary endpoint: mPFS Secondary endpoints: ORR, mDoR, CBR, and mOS AVB-500 + Cabo (N = 9-18) AVB-500 Dose Levels: DL -1: 10 mg/kg 2QW DL 1: 15 mg/kg Q2W DL 2: 20 mg/kg Q2W DL 3: 25 mg/kg Q2W AVB-500+Cabo (N=30) Cabo Alone (N=15) Safety to determine AVB-500 RP2D Cabo dosing will remain as indicated on the package insert © 2021 Aravive, Inc. 24
Preclinical data suggests that AVB-500 has potential in multiple combinations and indications: Combination trials with agents currently embedded as standard of care Earlier lines of therapy Multiple tumor types © 2021 Aravive, Inc. 25 Forward-Looking Statements Development Strategy: Conduct Additional Trials to Expand Pipeline Preclinical data on AVB-500 includes: Reduction of metastases to lung (breast and renal models) Reduction of metastases to peritoneum (ovarian and pancreatic models) Decreased fibrosis in pancreatic model Decreased metastasis in pancreatic model Survival tripled in pancreatic model relative to control Reduction in number and weight of metastases in combination with doxorubicin, paclitaxel, bevacizumab and PARP inhibitors Reversal of CPI- and radiation-resistance in TNBC model
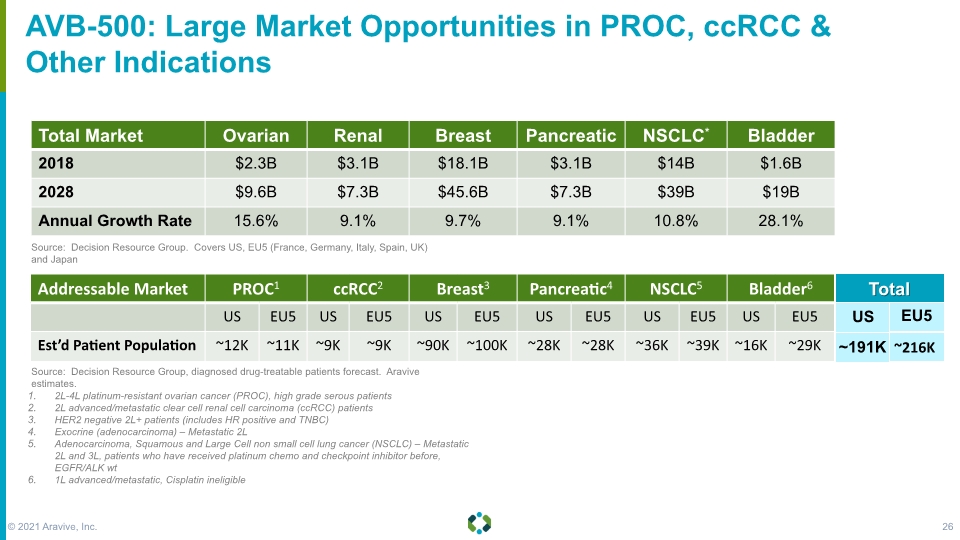
AVB-500: Large Market Opportunities in PROC, ccRCC & Other Indications 2L-4L platinum-resistant ovarian cancer (PROC), high grade serous patients 2L advanced/metastatic clear cell renal cell carcinoma (ccRCC) patients HER2 negative 2L+ patients (includes HR positive and TNBC) Exocrine (adenocarcinoma) – Metastatic 2L Adenocarcinoma, Squamous and Large Cell non small cell lung cancer (NSCLC) – Metastatic 2L and 3L, patients who have received platinum chemo and checkpoint inhibitor before, EGFR/ALK wt 1L advanced/metastatic, Cisplatin ineligible Source: Decision Resource Group. Covers US, EU5 (France, Germany, Italy, Spain, UK) and Japan Source: Decision Resource Group, diagnosed drug-treatable patients forecast. Aravive estimates. Total US EU5 ~191K ~216K © 2021 Aravive, Inc. 26

Forward-Looking Statements Strategic Collaboration with WuXi Biologics for High-Affinity Bispecific Antibodies Objective is to identify/develop novel, high-affinity bispecific antibodies against CCN2, also known as connective tissue growth factor (CTGF), targeting cancer/fibrosis using WuXiBody™ platform CTGF over-expression is hallmark of fibrosis in multiple tissues and is widely thought to be required to mediate profibrotic effects of TGFβ Overexpression associated with poor prognosis in many cancers, including aggressive/invasive breast cancer, osteolytic breast cancer, pancreatic cancer, and glioblastoma Biomarker potential for CTGF as levels are increased in certain cancers Domain-focused strategy expected to generate a best-in-class clinical therapeutic targeting desmoplasia and tumor growth in 2023 Initial target anticipated to be pancreatic ductal adenocarcinoma (PDAC), the fourth most frequent cause of cancer-related deaths worldwide with a 5-year overall survival of less than 8% CTGF may play key role in PDAC progression & blocking it could prevent/reverse PDAC desmoplasia Therapeutic could work as part of life cycle management with AVB-500 in certain cancers © 2021 Aravive, Inc. 27

Forward-Looking Statements 3D Medicines Collaboration to Develop/Commercialize AVB-500 in China for Oncology Indications Exclusive collaboration and license agreement for development & commercialization of AVB-500 across all oncology indications in mainland China, Hong Kong, Macau, and Taiwan (Greater China) Aravive received upfront payment of $12M and is eligible to receive additional $207M in development and commercial milestone payments Potential for near-term milestone payments of $6M Aravive will receive tiered-royalties ranging from low double-digit to mid-teens as a % of annual net sales 3D Medicines will be responsible for all costs associated with development and commercialization activities for AVB-500 in Greater China “We believe that AVB-500, used in combination with existing standard of care therapeutics or Envafolimab, an innovative subcutaneous PD-L1 antibody to be launched in China soon, could alter the treatment paradigm across various tumor types. We are committed to working closely with Aravive to further advance the development of AVB-500 and bring this important potential therapy to patients living with cancer in China.” - John Gong, M.D., Ph.D., Chairman/CEO of 3DMedicines* *Source: Aravive & 3D Medicines press release November 10, 2020 © 2021 Aravive, Inc. 28

Forward-Looking Statements Financial Summary: Solid Financial Position Shares outstanding – 19.7 million* Cash and cash equivalents as of 12/31/20 – $60.5 million (Does not include approximately $21 million investment from Eshelman Ventures in Q1’2021) No debt Cash runway expected to fund current operating plans into H2’2022** * As of 3/10/2021 ** Includes cash proceeds from Eshelman Ventures investment. © 2021 Aravive, Inc. 29
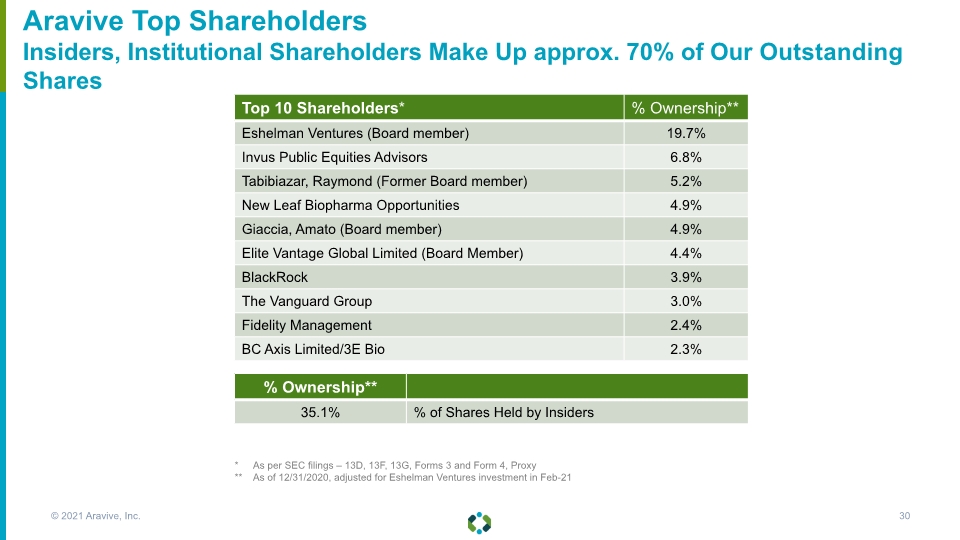
Aravive Top Shareholders Insiders, Institutional Shareholders Make Up approx. 70% of Our Outstanding Shares * As per SEC filings – 13D, 13F, 13G, Forms 3 and Form 4, Proxy ** As of 12/31/2020, adjusted for Eshelman Ventures investment in Feb-21 © 2021 Aravive, Inc. 30

Forward-Looking Statements Upcoming Key Milestones Advancing Our Clinical Development Program Q3’2021 – Anticipate completion of enrollment in Phase 1b ccRCC trial Q4’2021 – Anticipate initiation of Phase 2 ccRCC trial Q1’2022 – Anticipate interim analysis for Phase 3 PROC trial 2021 and beyond: Data from IST-sponsored trials & anti-CTGF discovery program Pipeline expansion in oncology Execution of global business development opportunities © 2021 Aravive, Inc. 31

Focused on difficult-to-treat cancers: AVB-500 lead indication; advanced stage ovarian cancer AVB-500 targets and inhibits GAS6/AXL signaling which is associated with tumor growth, metastasis, drug resistance, and poor patient survival AVB-500 is a differentiated innovative approach that addresses the inadequacies and limitations of other therapies Potential for expansion in multiple indications and multiple combinations with AVB-500 Strong Management Team and Board of Directors Solid financial position Upcoming key milestones to advance our clinical development program Dedicated to Developing Innovative Treatments for Life-Threatening Diseases to Increase Patient Survival © 2021 Aravive, Inc. 32

Thank you! Halting Disease Progression in its Tracks © 2021 Aravive, Inc. 33
































