Exhibit 99.1

Corporate Presentation Nasdaq: SILO 1 Fall 2022

Nasdaq: SILO | | | | | | | | | | | Statement This presentation contains "forward - looking statements" within the meaning of the “safe - harbor” provisions of the Private Securities Litigation Reform Act of 1995 . These statements are identified by the use of words “could”, “believe”, “anticipate”, “intend”, “estimate”, “expect”, “may”, “continue”, “predict”, “potential” and similar expressions that are intended to identify forward - looking statements . Such statements involve known and unknown risks, uncertainties and other factors that could cause the actual results of Silo Pharma, Inc . (“Silo” or “the Company”) to differ materially from the results expressed or implied by such statements, including changes to anticipated sources of revenues, future economic and competitive conditions, difficulties in developing the Company’s technology platforms, retaining and expanding the Company’s customer base, fluctuations in consumer spending on the Company’s products and other factors . Accordingly, although the Company believes that the expectations reflected in such forward - looking statements are reasonable, there can be no assurance that such expectations will prove to be correct . The Company disclaims any obligations to publicly update or release any revisions to the forward - looking information contained in this presentation, whether as a result of new information, future events or otherwise, after the date of this presentation or to reflect the occurrence of unanticipated events except as required by law . 2
| | | | | | | | | | | | | | | | | | | | | | | Psychedelic Research Silo Pharma is a developmental stage biopharmaceutical company focused on merging traditional therapeutics with psychedelic research. Five novel drug candidates are under development for large, underserved medical markets: ◉ Alzheimer’s Disease ◉ Multiple sclerosis (MS) ◉ Rheumatoid arthritis (RA) ◉ Stress - induced psychiatric disorders ◉ Fibromyalgia Exclusive collaborations with world - class medical research partners provide valuable IP, assets, and time - to - market advantages . 3 Nasdaq: SILO

| | | | | | | | | | Considerations 4 Nasdaq: SILO ◉ Established collaborations with leading academic institutions facilitate streamlined 505(b)(2) regulatory pathway for drug approval. ◉ Disruptive market potential for novel IP - protected technologies and assets. ◉ Burgeoning psychedelic drugs market valued at $2.8 billion in 2021. 1 ◉ Valuable intellectual property and technology rights for the treatment of rare diseases. ◉ Strong balance sheet with cash of $8.7 million and zero debt as of June 30, 2022. ◉ Leadership with deep expertise in drug development and licensing, asset acquisition, biotech management, capital markets, and finance. 1 Data Bridge Market Research, September 2022
SPU - 16 | Multiple Sclerosis ◉ Central nervous system homing peptide ◉ Only targets tissue affected by certain conditions | | | | | | | | | | Collaborations and Assets 5 Nasdaq: SILO SP - 26 | Multiple indications ◉ Time - released psilocybin, ketamine ◉ Topical drug delivery system developed at Albert Einstein College of Medicine ◉ Initial indication is fibromyalgia SPU - 21 | Rheumatoid Arthritis ◉ Arthritogenic joint homing peptides ◉ Identifies markers of arthritic inflammation in joints SPC - 14 | Alzheimer’s Disease ◉ 505(b)(2) pathway drug combining two FDA - approved therapeutics ◉ Studying ketamine in combination with other drugs SPC - 15 | Stress - Induced Affective Disorders ◉ Targeted prophylactic treatment ◉ Utilizes ketamine compositions as a method for treatment and prevention Generic psilocybin ◉ Investigator - sponsored study of the effect of psilocybin on inflammation in the blood ◉ Findings may support UMB homing peptide studies

| | | | | | | | | | Development Timeline 6 Nasdaq: SILO Drug Indication Optimization/ Proof of Concept Preclinical Phase I Phase II Phase III Launch SP - 26 Fibromyalgia, Multiple SPU - 16 Peptide Multiple Sclerosis (MS) SPU - 21 Peptide Rheumatoid Arthritis (RA) SPC - 14 Alzheimer’s Disease (AD) SPC - 15 Stress - Induced Affective Disorders Clinical Study Inflammation

| | | | | | | | | | | | | | | | Property Position > Five patents issued and provisional US Patent issued 11 / 16 / 21 # 11174287 Central Nervous System Homing Peptides US # 8 , 623 , 377 Joint - Homing peptide U . S . Provisional Patent Application No . 63 / 060 , 573 , titled “Central Nervous System Delivery of Psilocybin,” filed August 3 , 2020 U . S . Provisional Patent Application No . 63 / 060 , 569 , titled “Central Nervous System Delivery of Nonsteroidal Anti - Inflammatory Drugs and Psilocybin,” filed August 3 , 2020 U . S . Provisional Patent Application No . 63 / 060 , 577 , titled “Central Nervous System Delivery of Nonsteroidal Anti - Inflammatory Drugs and Psilocybin,” filed August 3 , 2020 U . S . Provisional Patent Application No . 63 / 24 , 827 , titled “Use of Psilocybin in Cancer Treatment,” filed December 13 , 2020 . + Four patents pending 7 Nasdaq: SILO

Asset Portfolio Nasdaq: | SILO 8
SP - 26 Time - Released Psilocybin, Ketamine ▪ Fibromyalgia and multiple other i ndications Market Opportunity: ▪ Deliver ketamine or psilocybin in a time - released manner ▪ Determining if time - release will diminish hallucinogenic effects of these psychedelics ▪ Preclinical study underway shows Z - pod can hold and distribute ketamine ▪ Efficacy study in animals underway Applications ▪ Joint Venture with Zylö Therapeutics, Inc. ▪ Clinical development of psilocybin using ZTI’s Z - pod technology ▪ Clinical development of Zylo’s sustained release topical delivery system Technology 9 Nasdaq: SILO | | | | | | | | | | | | | | | | | Joint Venture

SPU - 16 CNS Homing Peptide ▪ A pproximately 400,000 Americans and 2.5 million people worldwide with MS ▪ M ost widespread disabling neurological condition of young adults ▪ Global market for MS drugs expected to reach $25.3 billion by 2027 Market Opportunity: Multiple Sclerosis (MS) ▪ May be used as a delivery tool to target current therapies to detect inflammation in the spinal cord ▪ May be used for diagnosing and monitoring MS ▪ Decreases toxicity of existing therapeutics ▪ Animal study results show much improved delivery of therapeutics and decreased toxicity Regulatory Pathway and Results 505(b)(2) Pathway ▪ Licensed from University of Maryland, Baltimore ▪ Patent issued ▪ Central nervous system homing peptides ▪ Use for investigation and treatment of MS and other neuroinflammatory pathology Technology 10 Nasdaq: SILO | | | | | License Agreement

SPU - 21 Joint Homing Peptides Targeting Rheumatoid Arthritis ▪ 1.3M U.S. adults suffer from RA ▪ The most common autoimmune disease in U.S. ▪ U.S. market for RA drugs expected to reach $63 billion by 2027 Market Opportunity: Rheumatoid Arthritis (RA) ▪ Identify markers of arthritic inflammation in joints ▪ Isolate phage clones that preferentially target inflamed joints of arthritic Lewis rats ▪ Peptide significantly inhibited arthritic progression in this animal model ▪ Further studies underway at UMB Applications ▪ Development plan to utilize liposomal homing peptide to deliver targeted psilocybin ▪ Ability to target inflamed epithelium suggests possibility to target drug delivery ▪ Approach could enhance therapeutic effect of current and future therapies and decrease potential systemic toxicity despite systemic administration of the drug ▪ Potential to develop fusion imaging molecules and/or nanoparticles to study arthritic pathogenesis ▪ Could be customizable and used to deliver nanoparticles for precise imaging ▪ Could be used to treat autoimmune diseases other than RA Technology 11 Nasdaq: SILO UMB CELA (Commercial Evaluation License Agreement)

SPC - 14 Targeting Alzheimer's ▪ 6.5 million Americans suffer from Alzheimer’s and related diseases ▪ ~1 in 9 Americans 65+ have Alzheimer’s ▪ U.S. market for relevant drugs expected to reach $5 billion by 2027 Market Opportunity: Alzheimer’s Disease ▪ 505(b)(2) Pathway ▪ Preclinical testing and proof - of - concept led by inventor Dr. Christine Denny of Columbia University ▪ Drug showed reduced anxiety in animal studies ▪ May reduce behavioral despair ▪ Scientific research agreement with Dr. Denny’s lab Regulatory Pathway and Results ▪ Developed by Columbia University ▪ Novel drug combines two approved therapeutics ▪ Targets NDMARS and 5HT4Rs to treat cognitive and neuropsychiatric symptoms in Alzheimer’s Technology 12 Nasdaq: SILO | | | | | | | | | | | | | | | | | | CELA

SPC - 15 Targeted Prophylactic Treatment — Stress - Induced Affective Disorders ▪ 26% of Americans 18+ suffer from anxiety, PTSD and other disorders ▪ This number has escalated post - COVID - 19 ▪ U.S. market for relevant drugs expected to reach $13 billion by 2027 Market Opportunity: Stress - induced Affective Disorders ▪ Sponsored research agreement with Columbia University ▪ Prevention of stress - induced affective disorders ▪ Increasing stress resilience in military, first responders, and other populations at high risk of PTSD ▪ Predicting level of severity or progression of such disorders ▪ Molecular targets for use in drug discovery of innovative treatments Applications ▪ Metabolomic biomarkers predict response to pharmacological treatments ▪ Utilizes ketamine compositions as a method for treatment and prevention Technology 13 Nasdaq: SILO | | | | | | | | | | | | | | | | | | CELA

Clinical Study: Effect of Psilocybin on Inflammation in the Blood ▪ Parkinson’s Disease, chronic pain, and bipolar disorder Market Opportunity: Multiple Indications ▪ Examine effects of psilocybin on inflammatory activity in humans ▪ Accelerate its implementation as a potential treatment Applications ▪ Repeated low doses of psilocybin Technology 14 Nasdaq: SILO | | | | | | Sponsored Research Agreement

Leadership Nasdaq: | SILO 15

| | | | | | | | | Leaders Eric Weisblum CEO ◉ 20+ years investing, building and managing businesses ◉ Prior president of Sableridge Capital ◉ Former board member of Aikido Pharma., a Nasdaq - listed biotech company focused on the commercialization of oncology therapeutics James Kuo , M.D. Vice President of R&D ◉ Current managing director of Athena Bioventures ◉ Formerly held executive positions in private and listed bioscience companies in the U.S., Canada, and Europe ◉ Chairman of the Board of ImmunoPrecise Antibodies and board director of Tryp Therapeutics. ◉ Former CEO of Tryp Therapeutics, Synthetic Biologics, BioMicro Systems, and Discovery Laboratories Daniel Ryweck CFO ◉ Certified Public Accountant ◉ Formerly chief compliance officer of Mill City Ventures III Ltd, interim chief financial officer of Sun BioPharma, Inc., and director of Dala Petroleum Corp. 16 Nasdaq: SILO

| | | | | | | | | Advisory Board Charles B. Nemeroff M.D., Ph.D. ◉ Chair, Department of Psychiatry and Behavioral Sciences, Dell Medical School, University of Texas at Austin; director, Institute for Early Life Adversity Research, Department of Psychiatry and Behavioral Sciences, Mulva Clinic for the Neurosciences ◉ University of North Carolina (UNC) School of Medicine, Duke University ◉ Brain and Behavior Research Foundation; Anxiety and Depression Association of America (ADAA); National Academy of Medicine 17 Nasdaq: SILO Josh Woolley M.D., Ph.D. ◉ Associate professor, Department of Psychiatry and Behavioral Sciences, UCSF; licensed psychiatrist, San Francisco Veterans Affairs Medical Center; director and founder, Bonding and Attunement in Neuropsychiatric Disorders (BAND) Laboratory; director, Translational Psychedelic Research ( TrPR ) Program, UCSF ◉ UCSF, Brown University ◉ American College of Neuropsychopharmacology

| | | | | | | | Directors Wayne Linsley Director ◉ 40 years in business management ◉ Wide and varied skillset including sales and sales management, finance (for both public and private companies), accounting, audit support and financial reporting ◉ Independent director for Hoth Therapeutics Inc. (NASDAQ: HOTH) and DatChat Inc. (NASDAQ: DATS) Kevin Muñoz M.D. Director ◉ Director of Operations at Physical Medicine and Rehabilitation ◉ Former researcher with Harlem Health Promotion Center in New York. ◉ Doctor of Medicine from Xavier University School of Medicine, B.S. from University of Michigan with distinction Jeff Pavell D.O. Director ◉ Site proctor for Rusk Institute residents training for Rehabilitation Medicine and Chief of Rehabilitation Medicine at Englewood Hospital and Medical Center in New Jersey. ◉ Residency and chief residency at New York University Medical Center’s Rusk Institute of Rehabilitation and Bellevue Hospital, respectively, New York College of Osteopathic Medicine with honors 18 Nasdaq: SILO

Key Takeaways Nasdaq: | SILO 19

| | | | | | | | | Highlights 20 Nasdaq: SILO ◉ 505(b)(2) regulatory pathway provides competitive time - to - market advantage over other early psychedelic drug market entrants. ◉ Diversified pipeline drugs showing promise in underserved rare disease, neurological, and mental health indications. ◉ Massive addressable market projected to grow at a 17% CAGR to $9.8 billion in 2029. 1 ◉ Assets well protected with more than five issued and provisional patents and four patent applications pending. ◉ Ample capital resources to support upcoming catalysts. ◉ Deep expertise and strong governance through highly credentialed management, board, and advisory. 1 Data Bridge Market Research, September 2022

| | | | | | | | | | Priorities 21 Nasdaq: SILO Clinical study update. Advance SP - 26 topical time - released ketamine to th e clinic . Pursue initial indication of fibromyalgia for SP - 26 . Advance studies of SPU - 21 in progress at UMB. Ongoing intellectual property and patent expansion. Data from p re - clinical studies.
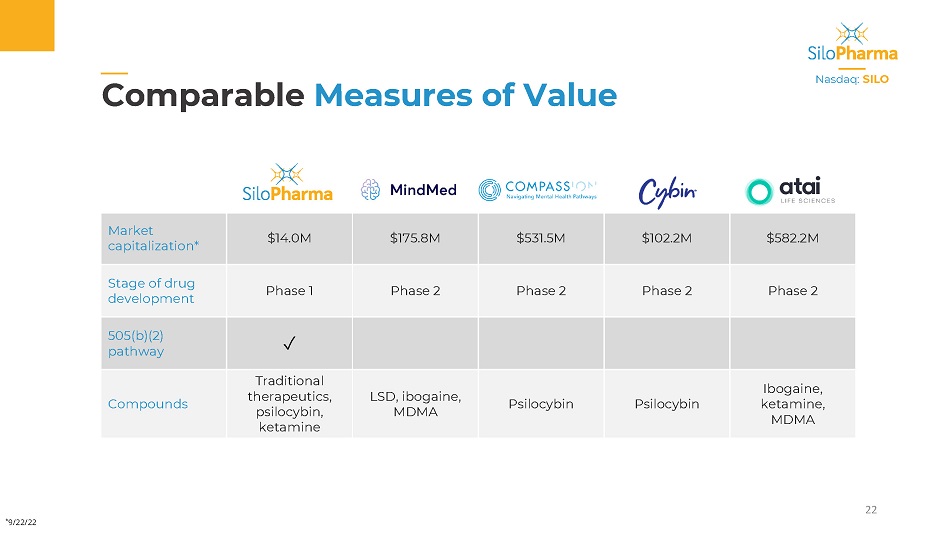
| | | | | | | | | | | Measures of Value 22 Nasdaq: SILO Market capitalization* $14.0M $175.8M $531.5M $102.2M $582.2M Stage of drug development Phase 1 Phase 2 Phase 2 Phase 2 Phase 2 505(b)(2) pathway ✓ Compounds Traditional therapeutics, psilocybin, ketamine LSD, i bogaine, MDMA Psilocybin Psilocybin Ibogaine, ketamine, MDMA * 9/22/22
| | | | | | | | | | Summary Established collaborations with leading academic institutions Experienced drug development team Multiple large disease markets being pursued Well - capitalized balance sheet Recent uplisting to Nasdaq Strong intellectual property position 23 Nasdaq: SILO

Thank you. 560 Sylvan Ave, Suite 3160 Englewood Cliffs, NJ 07632 Nasdaq: | SILO 800 - 705 - 0120 investors@silopharma.com























