As filed with the U.S. Securities and Exchange Commission on July 17, 2024
Registration No. 333-
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM S-1
REGISTRATION STATEMENT
UNDER THE
SECURITIES ACT OF 1933
SILO PHARMA, INC.
(Exact name of registrant as specified in its charter)
| Nevada | | 2834 | | 27-3046338 |
(State or other jurisdiction of
incorporation or organization) | | (Primary Standard Industrial
Classification Code Number) | | (I.R.S. Employer Identification Number) |
677 N. Washington Boulevard
Sarasota, Florida 34236
(718) 400-9031
(Address, including zip code, and telephone number, including area code, of registrant’s principal executive offices)
Eric Weisblum
Chief Executive Officer
Silo Pharma, Inc.
677 N. Washington Boulevard
Sarasota, Florida 34236
(718) 400-9031
(Name, address including zip code, and telephone number, including area code, of agent for service)
With copies to:
Richard Friedman, Esq.
Sheppard Mullin Richter & Hampton LLP
30 Rockefeller Plaza
New York, NY 10112
(212) 653-8700
Approximate date of commencement of proposed sale to the public: From time to time after the effective date of this registration statement.
If any of the securities being registered on this Form are to be offered on a delayed or continuous basis pursuant to Rule 415 under the Securities Act of 1933, check the following box: ☒
If this Form is filed to register additional securities for an offering pursuant to Rule 462(b) under the Securities Act, please check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
If this Form is a post-effective amendment filed pursuant to Rule 462(c) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
If this Form is a post-effective amendment filed pursuant to Rule 462(d) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company, or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company,” and “emerging growth company” in Rule l2b-2 of the Exchange Act.
Large accelerated filer ☐ Accelerated filer ☐ Non-accelerated filer ☒ Smaller reporting company ☒ Emerging Growth Company ☐
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 7(a)(2)(B) of the Securities Act. ☐
The registrant hereby amends this registration statement on such date or dates as may be necessary to delay its effective date until the registrant shall file a further amendment which specifically states that this registration statement shall thereafter become effective in accordance with Section 8(a) of the Securities Act of 1933, as amended, or until the registration statement shall become effective on such date as the Securities and Exchange Commission, acting pursuant to said Section 8(a), may determine.
The information in this prospectus is not complete and may be changed. These securities may not be sold until the registration statement filed with the Securities and Exchange Commission is effective. This prospectus is not an offer to sell these securities and it is not soliciting an offer to buy these securities in any state where the offeror sale is not permitted.
| PRELIMINARY PROSPECTUS | SUBJECT TO COMPLETION | DATED JULY 17, 2024 |

Silo Pharma, Inc.
Up to 1,043,739 Shares of Upon Exercise of Certain Common Stock Purchase Warrants
This prospectus relates to the offer and resale of up to an aggregate of 1,043,739 shares (the “Shares”) of common stock, par value $0.0001 per share (“Common Stock”), of Silo Pharma, Inc. (the “Company”, “we”, “us” or “our”), consisting of shares of Common Stock issuable upon the exercise of: (i) common stock purchase warrants (the “June 2024 Investor Warrants”), to purchase up to 917,432 shares of Common Stock (the “June 2024 Investor Warrant Shares”), at an exercise price of $2.06 per share; issued by us to certain accredited investors on June 6, 2024 in a concurrent private placement and registered direct transaction pursuant to a securities purchase agreement, dated as of June 4, 2024 (the “June 2024 Purchase Agreement”); (ii) common stock purchase warrants (the “June 2024 Placement Agent Warrants”) to purchase 68,807 shares of Common Stock (the “June 2024 Placement Agent Warrant Shares”) issued to H.C. Wainwright & Co., LLC, as exclusive placement agent (the “Placement Agent”), at an exercise price of $2.725 per share and (iii) certain common stock purchase warrants (the “September 2022 Underwriter Warrants,” together with the June 2024 Investor Warrants and the June 2024 Placement Agent Warrants, the “Warrants”), to purchase up to 57,500 shares of Common Stock (the “September 2022 Underwriter Warrant Shares,” together with the June 2024 Investor Warrant Shares and the June 2024 Placement Agent Warrant Shares, the “Warrant Shares”), at an exercise price of $6.25 per share, issued by us to Laidlaw & Company (UK) Ltd. on September 29, 2022 in a public offering pursuant to an underwriting agreement, dated as of September 26, 2022 (the “Underwriting Agreement”). The June 2024 Warrants and the 2024 Placement Agent Warrants are exercisable immediately upon issuance for a five-year period and the September 2022 Underwriter Warrants are initially exercisable on March 25, 2023, and expire on September 26, 2027. The holders of the Warrants and the underlying Warrant Shares are each referred to herein as a “Selling Shareholder” and collectively as the “Selling Shareholders.”
This prospectus describes the general manner in which the Shares may be offered and sold. If necessary, the specific manner in which the Warrant Shares may be offered and sold will be described in a supplement to this prospectus. The June 2024 Investor Warrants and the June 2024 Private Placement Warrants were each issued to the applicable Selling Shareholders in connection with private placement offerings pursuant to Section 4(a)(2) of the Securities Act of 1933, as amended (the “Securities Act”), and/or Regulation D promulgated thereunder. The September 2022 Underwriter Warrants were issued to the applicable Selling Shareholders in connection with our September 2022 Public Offering For additional information regarding the issuance of the Warrants and Warrant Shares, see “June 2024 Registered Direct Offering and Concurrent Private Placement,” and “September 2022 Public Offering,” beginning on page 10.
The Shares will be resold from time to time by the Selling Shareholders listed in the section titled “Selling Shareholders” beginning on page 11.
The Selling Shareholders, or their respective transferees, pledgees, donees or other successors-in-interest, will sell the Shares through public or private transactions at prevailing market prices, at prices related to prevailing market prices or at privately negotiated prices. The Selling Shareholders may sell any, all or none of the securities offered by this prospectus, and we do not know when or in what amount the Selling Shareholders may sell their Shares hereunder following the effective date of this registration statement. We provide more information about how a Selling Shareholder may sell its Shares in the section titled “Plan of Distribution” on page 16.
We are registering the Shares on behalf of the Selling Shareholders, to be offered and sold by them from time to time. While we will not receive any proceeds from the sale of our Common Stock by the Selling Shareholders in the offering described in this prospectus, we may receive up to (ii) $2.06 per share upon the cash exercise of the June 2024 Investor Warrants; (iii) $2.725 per share upon the cash exercise of the June 2024 Placement Agent Warrants and (iii) $6.25 per share upon the cash exercise of the September 2022 Underwriter Warrants. Upon the exercise of the Warrants for all 1,043,739 Shares by payment of cash, we would receive aggregate gross proceeds of approximately $2.44 million. However, we cannot predict when and in what amounts or if the Warrants will be exercised, and it is possible that the Warrants may expire and never be exercised, in which case we would not receive any cash proceeds. We have agreed to bear all of the expenses incurred in connection with the registration of the Shares. The Selling Shareholders will pay or assume discounts, commissions, fees of underwriters, selling brokers or dealer managers and similar expenses, if any, incurred for the sale of the Shares.
The Common Stock is currently listed on the Nasdaq Capital Market under the symbol “SILO” On July 15, 2024, the last reported sale price of our Common Stock was $1.07.
This offering will terminate on the earlier of (i) the date when all of the Securities registered hereunder have been sold pursuant to this prospectus or Rule 144 under the Securities Act, and (ii) the date on which all of such securities may be sold pursuant to Rule 144 without volume or manner-of-sale restrictions, unless we terminate it earlier.
Investing in our Common Stock involves risks. You should carefully review the risks described under the heading “Risk Factors” beginning on page 9 and in the documents which are incorporated by reference herein before you invest in our Common Stock.
Neither the Securities and Exchange Commission nor any state securities commission has approved or disapproved of these securities or determined if this prospectus is truthful or complete. Any representation to the contrary is a criminal offense.
The date of this prospectus is, 2024.
TABLE OF CONTENTS
ABOUT THIS PROSPECTUS
This prospectus relates to the offer and resale of up to an aggregate of 1,043,739 shares (the “Shares”) of common stock, par value $0.0001 per share (“Common Stock”), of Silo Pharma, Inc. (the “Company”, “we”, “us” or “our”), consisting of shares of Common Stock issuable upon exercise of: (i) common stock purchase warrants (the “June 2024 Investor Warrants”), to purchase up to 917,432 shares of Common Stock (the “June 2024 Investor Warrant Shares”), at an exercise price of $2.06 per share; issued by us to certain accredited investors on June 6, 2024 in a concurrent private placement and registered direct transaction pursuant to a securities purchase agreement, dated as of June 4, 2024 (the “June 2024 Purchase Agreement”); (ii) common stock purchase warrants (the “June 2024 Placement Agent Warrants”) to purchase 68,807 shares of Common Stock (the “June 2024 Placement Agent Warrant Shares”) issued to H.C. Wainwright & Co., LLC, as exclusive placement agent (the “Placement Agent”), at an exercise price of $2.725 per share and (iii) certain common stock purchase warrants (the “September 2022 Underwriter Warrants,” together with the June 2024 Investor Warrants and the June 2024 Placement Agent Warrants, the “Warrants”), to purchase up to 57,500 shares of Common Stock (the “September 2022 Underwriter Warrant Shares,” together with the June 2024 Investor Warrant Shares and the June 2024 Placement Agent Warrant Shares, the “Warrant Shares”)), at an exercise price of $6.25 per share, issued by us to Laidlaw & Company (UK) Ltd. on September 29, 2022 in a public offering pursuant to an underwriting agreement, dated as of September 26, 2022 (the “Underwriting Agreement”). The June 2024 Warrants and the 2024 Placement Agent Warrants were exercisable immediately upon issuance for a five-year period and the September 2022 Underwriter Warrants were initially exercisable on March 25, 2023, and expire on September 26, 2027. The holders of the Warrants and the underlying Warrant Shares are each referred to herein as a “Selling Shareholder” and collectively as the “Selling Shareholders.” For additional information regarding the issuance of the Warrants and Warrant Shares, see “June 2024 Registered Direct Offering and Concurrent Private Placement,” and “September 2022 Public Offering,” beginning on page 10. You should rely only on the information contained in this prospectus and the related exhibits, any prospectus supplement or amendment thereto and the documents incorporated by reference, or to which we have referred you, before making your investment decision. Neither we nor the Selling Shareholders have authorized anyone to provide you with different information. If anyone provides you with different or inconsistent information, you should not rely on it. This prospectus, any prospectus supplement or amendments thereto do not constitute an offer to sell, or a solicitation of an offer to purchase, the shares of Common Stock offered by this prospectus, any prospectus supplement or amendments thereto in any jurisdiction to or from any person to whom or from whom it is unlawful to make such offer or solicitation of an offer in such jurisdiction. You should not assume that the information contained in this prospectus, any prospectus supplement or amendments thereto, as well as information we have previously filed with the U.S. Securities and Exchange Commission (the “SEC”), is accurate as of any date other than the date on the front cover of the applicable document.
If necessary, the specific manner in which the shares of our common stock may be offered and sold will be described in a supplement to this prospectus, which supplement may also add, update or change any of the information contained in this prospectus. To the extent there is a conflict between the information contained in this prospectus and any prospectus supplement, you should rely on the information in such prospectus supplement, provided that if any statement in one of these documents is inconsistent with a statement in another document having a later date - for example, a document incorporated by reference in this prospectus or any prospectus supplement - the statement in the document having the later date modifies or supersedes the earlier statement.
Neither the delivery of this prospectus nor any distribution of shares of our common stock pursuant to this prospectus shall, under any circumstances, create any implication that there has been no change in the information set forth or incorporated by reference into this prospectus or in our affairs since the date of this prospectus. Our business, financial condition, results of operations and prospects may have changed since such date.
When used herein, unless the context requires otherwise, references to “SILO”, the “Company”, “we”, “our” or “us” refer to Silo Pharma, Inc., a Nevada corporation, and its subsidiaries on a consolidated basis.
SPECIAL NOTE REGARDING FORWARD-LOOKING STATEMENTS
This prospectus, any amendment and the information incorporated by reference into this prospectus contain various forward-looking statements within the meaning of Section 27A of the Securities Act and Section 21E of the Securities and Exchange Act of 1934, as amended (the “Exchange Act”), which represent our expectations or beliefs concerning future events. Forward-looking statements include statements that are predictive in nature, which depend upon or refer to future events or conditions, and/or which include words such as “believes,” “plans,” “intends,” “anticipates,” “estimates,” “expects,” “may,” “will” or similar expressions. In addition, any statements concerning future financial performance, ongoing strategies or prospects, and possible future actions including any potential strategic transaction involving us, which may be provided by our management, are also forward-looking statements. Forward-looking statements are based on current expectations and projections about future events and are subject to risks, uncertainties, and assumptions about our company, economic and market factors, and the industry in which we do business, among other things. These statements are not guarantees of future performance, and we undertake no obligation to publicly update any forward-looking statements, whether as a result of new information, future events, or otherwise, except as required by law. Actual events and results may differ materially from those expressed or forecasted in forward-looking statements due to a number of factors. Factors that could cause our actual performance, future results and actions to differ materially from any forward-looking statements include, but are not limited to, those discussed under the heading “Risk Factors” in this prospectus and in any of our filings with the SEC pursuant to Sections 13(a), 13(c), 14 or 15(d) of the Exchange Act incorporated by reference into this prospectus. The forward-looking statements in this prospectus, and the information incorporated by reference herein represent our views as of the date such statements are made. These forward-looking statements should not be relied upon as representing our views as of any date subsequent to the date such statements are made.
INDUSTRY AND MARKET DATA
Unless otherwise indicated, information contained in this prospectus concerning our industry and the market in which we operate, including our market position, market opportunity and market size, is based on information from various sources, on assumptions that we have made based on such data and other similar sources and on our knowledge of the markets for our products. These data sources involve a number of assumptions and limitations, and you are cautioned not to give undue weight to such estimates.
We have not independently verified any third-party information. While we believe the market position, market opportunity and market size information included in this prospectus is generally reliable, such information is inherently imprecise. In addition, projections, assumptions and estimates of our future performance and the future performance of the industry in which we operate is necessarily subject to a high degree of uncertainty and risk due to a variety of factors, including those described in the section titled “Risk Factors” and elsewhere in this prospectus and in any documents that we incorporate by reference into this prospectus and the registration statement of which this prospectus forms a part. These and other factors could cause results to differ materially from those expressed in the estimates made by the independent parties and by us.
PROSPECTUS SUMMARY
This summary highlights selected information contained elsewhere in this prospectus or incorporated by reference into this prospectus. This summary does not contain all of the information that you should consider before investing in our Common Stock. You should carefully read this entire prospectus, and our other filings with the SEC, including the following sections, which are either included herein and/or incorporated by reference herein, “Risk Factors”, “Special Note Regarding Forward-Looking Statements”, “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and the consolidated financial statements incorporated by reference herein, before making a decision about whether to invest in our securities.
Overview
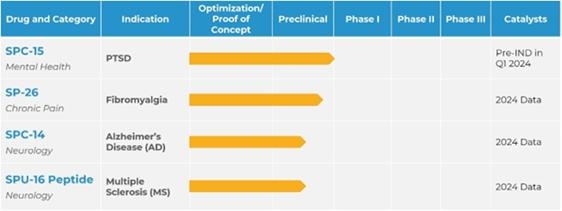
We are a developmental stage biopharmaceutical company developing novel therapeutics that address underserved conditions including PTSD, stress-induced anxiety disorders, fibromyalgia, and central nervous system (CNS) diseases. We are focused on developing novel therapies that include conventional drugs and psychedelic formulations. The Company’s lead program, SPC-15, is an intranasal drug targeting PTSD and stress-induced anxiety disorders. SP-26 is a time-release ketamine-based loaded implant for fibromyalgia and chronic pain relief. Silo’s two preclinical programs are SPC-14, an intranasal compound for the treatment of Alzheimer’s disease, and SPU-16, a CNS-homing peptide targeting the central nervous system with initial research indication in multiple sclerosis (MS).
Rare Disease Therapeutics
We seek to acquire and/or develop intellectual property or technology rights from leading universities and researchers to treat rare diseases, including the use of psychedelic drugs, such as psilocybin, ketamine, and the potential benefits they may have in certain cases involving depression, mental health issues and neurological disorders. We are focused on developing traditional therapeutics and psychedelic medicine. We concentrate on the development and commercialization of therapies for unmet needs from indications such as depression, post-traumatic stress disorder (“PTSD”), and other rare neurological disorders. Our mission is to identify assets to license and fund the research which we believe will be transformative to the well-being of patients and the health care industry.
Psilocybin is considered a serotonergic hallucinogen and is an active ingredient in some species of mushrooms. Recent industry studies using psychedelics, such as psilocybin, have been promising, and we believe there is a large unmet need with many people suffering from depression, mental health issues and neurological disorders. While classified as a Schedule I substance under the Controlled Substances Act (“CSA”), there is an accumulating body of evidence that psilocybin may have beneficial effects on depression and other mental health conditions. Therefore, the U.S. Food and Drug Administration (“FDA”) and U.S. Drug Enforcement Agency (“DEA”) have permitted the use of psilocybin in clinical studies for the treatment of a range of psychiatric conditions.
The potential of psilocybin therapy in mental health conditions has been demonstrated in a number of academic-sponsored studies over the last decade. In these early studies, it was observed that psilocybin therapy provided rapid reductions in depression symptoms after a single high dose, with antidepressant effects lasting for up to at least six months for a number of patients. These studies assessed symptoms related to depression and anxiety through a number of widely used and validated scales. The data generated by these studies suggest that psilocybin is generally well-tolerated and may have the potential to treat depression when administered with psychological support.
We have engaged in discussions with a number of world-renowned educational institutions and advisors regarding potential opportunities and have formed a scientific advisory board that is intended to help advise management regarding potential acquisition and development of products.
In addition, as more fully described below, we have entered into a license agreement with the University of Maryland, Baltimore, and are developing a Ketamine polymer implant. In addition, we have recently entered into a sponsored research agreement with Columbia University pursuant to which we have been granted an option to license certain patents and inventions relating to the treatment of Alzheimer’s disease and stress-induced affective disorders using Ketamine in combination with certain other compounds.
We plan to actively pursue the acquisition and/or development of intellectual property or technology rights to treat rare diseases, and to ultimately expand our business to focus on this new line of business.
Product Candidates
We are currently focusing on four product candidates: (i) SPC-15 for treatment of depression disorders; (ii) SP-26 for treatments of chronic pain; (iii)SPC-14 for the treatment of Alzheimer’s disease and (iv) SPU-16 for the treatment of CNS disorders with an initial indication for multiple sclerosis.
SPC-15
On October 1, 2021, we entered into a sponsored research agreement with Columbia University pursuant to which we have been granted an option to license certain assets currently under development, including assets related to SPC-15 for the treatment of depression disorders. On September 22, 2022, we entered into a First Amendment to Sponsored Research Agreement with Columbia to extend the term of the Columbia Agreement to conduct further research studies, which extension runs through March 31, 2024. On April 11, 2023 the assets under development for which we have the option to license as described above were issued a patent from the U.S. Patent & Trademark Office (USPTO) for “Biomarkers for Efficacy of Prophylactic Treatments Against Stress-Induced Affective Disorders” (US 11,622,948, B2). We exercised our option for an exclusive license agreement for SPC-15, a prophylactic treatment for stress-induced affective disorders including anxiety and PTSD pursuant to which we were granted an exclusive license to further develop, manufacture, and commercialize SPC-15 worldwide pursuant to an exclusive license agreement entered into on July 1, 2024. See “----License Agreements between the Company and Vendor—Exclusive License Agreement with Columbia University.”
SPC-15 is a targeted prophylactic therapeutic composition for the treatment and prevention for stress-induced affective disorders including PTSD. The treatment predicts levels of severity or progression of such disorders and their metabolomic biomarkers’ response to pharmacological treatments. We intend to develop SPC-15 under the Section 505(b)(2) regulatory pathway of the FDA rules. Section 505(b)(2) of the Federal Food, Drug, and Cosmetic Act (“FDCA”) was enacted to enable sponsors to seek New Drug Application (“NDA”) approval for novel repurposed drugs without the need for such sponsors to undertake time consuming and expensive pre-clinical safety studies and Phase 1 safety studies. Proceeding under this regulatory pathway, we will be able to rely upon publicly available data with respect to our active ingredient in our NDA submission to the FDA for marketing approval.
On November 15, 2023, we entered an exclusive license agreement with Medspray Pharma BV for its proprietary patented soft mist nasal spray technology, as the delivery mechanism for SPC-15, which agreement has an effective date of October 31, 2023. Preclinical and formulation studies are expected to be completed in the first quarter of 2024 and we intend to submit a pre-IND meeting request to FDA in the first half of 2024.
SP-26
In March 2023, we filed a provisional patent application with the USPTO to use SP-26 for treatment of chronic pain, including fibromyalgia. Fibromyalgia is a chronic condition causing pain to the connective tissues through the body including muscles, ligaments, and tendons. Musculoskeletal pain is often accompanied by sleep difficulties, fatigue, mood disorders, and problems with memory and concentration. Fibromyalgia affects about 4 million American adults, or about 2% of the adult population.
We intend to develop SP-26 following the Section 505(b)(2) regulatory pathway of the FDA rules. Section 505(b)(2) of the FDCA was enacted to enable sponsors to seek NDA approval for novel repurposed drugs without the need for such sponsors to undertake time consuming and expensive pre-clinical safety studies and Phase 1 safety studies. Proceeding under this regulatory pathway, we will be able to rely upon publicly available data with respect to our active ingredient in our NDA submission to the FDA for marketing approval.
SPC-14
On October 1, 2021, we entered into a sponsored research agreement with Columbia University (“Columbia”) pursuant to which Columbia shall conduct two different studies related to the use of SPC-14 for the treatment of Alzheimer’s. See “Investigator-Sponsored Study Agreements between the Company and Vendors—Sponsored Research Agreement with Columbia University for the Study of Ketamine in Combination with Other Drugs for Treatment of Alzheimer’s and Depression Disorders” for additional details. In addition, Company has been granted an option to license certain assets currently under development, including SPC-14 for the treatment of Alzheimer’s disease. We exercised our option for an exclusive license agreement for SPC-15, a prophylactic treatment for stress-induced affective disorders including anxiety and PTSD pursuant to which were granted an exclusive license to further develop, manufacture, and commercialize SPC-15 worldwide pursuant to an exclusive license agreement entered into on July 1, 2024. See “----License Agreements between the Company and Vendor—Exclusive License Agreement with Columbia University.” We expect to have the complete license agreement with Columbia completed in the first half of 2024.
SPC-14 is a novel drug combining two approved therapeutics, so we intend to develop SPC-14 following the Section 505(b)(2) regulatory pathway of the FDA rules. Section 505(b)(2) of the FDCA was enacted to enable sponsors to seek NDA approval for novel repurposed drugs without the need for such sponsors to undertake time consuming and expensive pre-clinical safety studies and Phase 1 safety studies. Proceeding under this regulatory pathway, we will be able to rely upon publicly available data with respect to our active ingredient in our NDA submission to the FDA for marketing approval.
On October 13, 2022, we extended the term of the sponsored research agreement with Columbia to conduct further research studies into the mechanism of action of SPC-14 in the treatment of Alzheimer’s disease. We expect the results from further preclinical studies in 2024.
SPU-16
On February 12, 2021, we entered into a Master License Agreement (the “UMB License Agreement”) with the University of Maryland, Baltimore (“UMB”) pursuant to which UMB granted us an exclusive, worldwide, sublicensable, royalty-bearing license to certain intellectual property (i) to make, have made, use, sell, offer to sell, and import certain licensed products and (ii) to use the invention titled “Central nervous system-homing peptides in vivo and their use for the investigation and treatment of multiple sclerosis and other neuroinflammatory pathology,” or SPU-16. See “License Agreements between the Company and Vendors—Vendor License Agreement with the University of Maryland, Baltimore for CNS Homing Peptide” for additional details. On April 11, 2023 certain intellectual property under the UMB License Agreement described above were issued a patent from the U.S. Patent & Trademark Office (USPTO) for “Peptide-Targeted Liposomal Delivery For Treatment, Diagnosis, and Imaging of Diseases and Disorders” (US 11,766,403, B2).
SPU-16 is a novel peptide homing specifically to inflamed CNS areas. It may be used to diagnose neuroinflammation in patients, and targeted delivery of drugs into the spinal cord. The initial indication is for multiple sclerosis (MS). The peptides have been tested in the EAE mouse model of human MS, where they show homing specifically to inflamed CNS areas.
Product Development Pipeline
The following table summarizes our product development pipeline.
License Agreements between the Company and Vendor
Vendor License Agreement with the University of Maryland, Baltimore for CNS Homing Peptide
On February 12, 2021, we entered into a Master License Agreement (the “UMB License Agreement”) with the University of Maryland, Baltimore (“UMB”) pursuant to which UMB granted us an exclusive, worldwide, sublicensable, royalty-bearing license to certain intellectual property (i) to make, have made, use, sell, offer to sell, and import certain licensed products and (ii) to use the invention titled, “Central nervous system-homing peptides in vivo and their use for the investigation and treatment of multiple sclerosis and other neuroinflammatory pathology” (the “Invention”) and UMB’s confidential information to develop and perform certain licensed processes for the therapeutic treatment of neuroinflammatory disease. The term of the License Agreement shall commence on the UMB Effective Date and shall continue until the latest of (i) ten years from the date of First Commercial Sale (as defined in the Sublicense Agreement) of the Licensed Product in such country and (ii) the date of expiration of the last to expire claim of the Patent Rights (as defined in the UMB License Agreement) covering such Licensed Product in such country, or (iii) the expiration of data protection, new chemical entity, orphan drug exclusivity, regulatory exclusivity, or other legally enforceable market exclusivity, if applicable, unless terminated earlier pursuant to the terms of the agreement. Pursuant to the UMB License Agreement, we agreed to pay UMB (i) a license fee of $75,000, (ii) certain event-based milestone payments, (iii) royalty payments, depending on net revenues, (iv) minimum royalty payments, and (v) a tiered percentage of sublicense income. The UMB License Agreement will remain in effect until the later of: (a) the last patent covered under the UMB License Agreement expires, (b) the expiration of data protection, new chemical entity, orphan drug exclusivity, regulatory exclusivity, or other legally enforceable market exclusivity, if applicable, or (c) ten years after the first commercial sale of a licensed product in that country, unless earlier terminated in accordance with the provisions of the UMB License Agreement. The term of the UMB License Agreement shall expire 15 years after the effective date in which (a) there were never any patent rights, (b) there was never any data protection, new chemical entity, orphan drug exclusivity, regulatory exclusivity, or other legally enforceable market exclusivity or (c) there was never a first commercial sale of a licensed product.
As described below, we have entered into an investigator sponsored research agreement with UMB related to a clinical study to examine a novel peptide-guided drug delivery approach for the treatment of Multiple Sclerosis.
Commercial Evaluation License and Option Agreement with UMB for Joint Homing Peptide
Effective as of February 26, 2021, we, through our wholly-subsidiary, Silo Pharma, Inc., a Florida corporation, and University of Maryland, Baltimore (“UMB”), entered into a commercial evaluation license and option agreement (“License Agreement”), which we were granted an exclusive, non-sublicensable, non-transferable license to with respect to the exploration of the potential use of joint-homing peptides for use in the investigation and treatment of arthritogenic processes. The License Agreement also granted us an exclusive option to negotiate and obtain an exclusive, sublicensable, royalty-bearing license (“Exclusive Option”) to with respect to the subject technology. The License Agreement had a term of six months from the effective date. Both parties could have terminated the License Agreement within thirty days by giving a written notice.
On July 6, 2021, we entered into a First Amendment Agreement (“Amended License Agreement”) with UMB to extend the term of the original License Agreement by an additional six months such that the Amended License Agreement was effective until February 25, 2022 however, if we exercise the Exclusive Option, the License Agreement shall expire at the end of the negotiation period (as defined in the License Agreement) or upon execution of a master license agreement, whichever occurs first. We paid a license fee of $10,000 to UMB in March 2021 pursuant to the License Agreement, which was expensed, since we could not conclude that such costs would be recoverable for this early-stage venture.
On January 28, 2022, we entered into a second amendment to the License Agreement with UMB dated February 26, 2021 (“Second Amendment”). The Second Amendment extended the term of the License Agreement until December 31, 2022. However, if we exercise the Exclusive Option, the License Agreement shall expire at the end of the negotiation period (as defined in the License Agreement) or upon execution of a master license agreement, whichever occurs first.
On June 22, 2022, we entered into a third amendment to the License Agreement with UMB dated February 26, 2021 under which UMB agreed, to expand the scope of the license granted in the CELA to add additional Patent Rights with respect to an invention generally known as “Peptide-Targeted Liposomal Delivery for Treatment Diagnosis, and Imaging of Diseases and Disorders.” On December 16, 2022, we entered into a fourth amendment to License Agreement with UMB (the “Fourth Amendment”) dated February 26, 2021 to extend the term of the License Agreement until March 31, 2023. In addition, the parties agreed in the Fourth Amendment to allow us to extend the term of the License Agreement to June 30, 2023 by paying UMB a fee of $1,000 on or before February 28, 2023. This fee was paid and thus the term of the License Agreement was extended to June 30, 2023. We let this license expire by its terms on December 31, 2023.
Joint Venture Agreement with Zylö Therapeutics, Inc. for Z-pod™ Technology
On April 22, 2021, we entered into a Joint Venture Agreement with Zylö Therapeutics, Inc. (“ZTI”) pursuant to which the parties agreed to form a joint venture entity, to be named Ketamine Joint Venture, LLC, to, among other things, focus on the clinical development of ketamine using ZTI’s Z-pod™ technology. Pursuant to the Joint Venture Agreement, we shall act as the manager of the Joint Venture. The Venture shall terminate if the development program does not meet certain specifications and milestones as set forth in the Joint Venture Agreement within 30 days of the date set forth in the Joint Venture Agreement. Notwithstanding the foregoing, the Manager may, in its sole discretion, terminate the Venture at any time.
Pursuant to the terms of the Joint Venture Agreement, (A) we shall contribute (1) $225,000 and (2) its expertise and the expertise of its science advisory board and (B) ZTI shall contribute (1) certain rights to certain of its patented technology as set forth in the JV Agreement, (2) a license to the know-how and trade secrets with respect to its Z-pod™ technology for the loading and release of ketamine, (3) ketamine to be used for clinical purposes, (4) reasonable use of its facilities and permits and (5) its expertise and know-how. Pursuant to the Joint Venture Agreement, 51% of the interest in the Joint Venture shall initially be owned by the Company and 49% of the interest in the Joint Venture shall initially be owned by ZTI, subject to adjustment in the event of additional contributions by either party. Notwithstanding the foregoing, in no event shall either party own more than 60% of the interest in the Joint Venture. As of March 31, 2024 and as of the date hereof, the joint venture entity has not been formed yet.
Furthermore, pursuant to the terms of the JV Agreement, ZTI shall grant the Joint Venture a sublicense pursuant to its license agreement (the “License Agreement”) with Albert Einstein College of Medicine dated November 27, 2017, in the event that we or a third party makes a request indicating that the patented technology (the “Patented Technology”) licensed to ZTI pursuant to the License Agreement is needed to advance the development of the Joint Venture or it is contemplated or determined that the Patented Technology will be sold. Furthermore, pursuant to the JV Agreement, ZTI granted the Company an exclusive option to enter into a separate joint venture for the clinical development of psilocybin using ZTI’s Z-pod™ technology on the same terms and conditions set forth in the JV Agreement, which option shall expire 24 months after the JV Effective Date. We do not intend to continue with Zylo and have entered into an agreement to develop a polymer implant for dosage and time release of Ketamine for chronic pain and Fibromyalgia.
Exclusive License Agreement between Medspray Pharma BV and the Company
On November 15, 2023, we entered into an Exclusive License Agreement (the “Medspray License Agreement”) with Medspray Pharma BV (“Medspray”) pursuant to which Medspray granted us an exclusive, non-revocable, worldwide royalty bearing license for Medspray’s proprietary patented soft mist nasal spray technology for marketing, promotion, sale and distribution of the products licensed by Medspray to us under the Medspray License Agreement. The Medspray License Agreement has an effective date of October 31, 2023 and expires on the earlier of (i) termination of the Medspray License Agreement or expiry of all Medspray license rights in the United States, Germany, United Kingdom, Spain, Italy and France. In consideration of the exclusive rights granted by Medspray to us, we agreed to pay Medspray a royalty on a quarterly basis equal to 5% of net sales. The term of the agreement commences on the effective date and continues until the earlier of (i) expiration of the last to expire of Medspray’s patent rights or (ii) December 31, 2023 (the “Initial Term”) at which time, the Medspray License Agreement will automatically renew for a successive period of three (3) years, unless terminated by either party upon one year prior written notice prior to the end of any term; provided, however, the Medspray may terminate the Medspray License Agreement immediately if fail to have any licensed product under the Medspray License Agreement registered with the FDA or EMA by July 1, 2028 or has filed to reach the point of first sale of any licensed product under the Medspray License Agreement by July 1, 2028.
Exclusive License Agreement with Columbia University
On July 1, 2024, we entered into an exclusive license agreement (the “Columbia License Agreement”) with Columbia University (“Columbia”) effective as of June 28, 2024 (the “Effective Date”) and pursuant to which we have been granted exclusive rights to certain patents and technical information to develop, manufacture and commercialize Products related to our SPC-15 (as defined in the Columbia License Agreement), including therapies for stress-induced affective disorders and other conditions.
The term of the Columbia License Agreement shall commence on the Effective Date and shall continue on a country-by-country and product-by-product basis until the latest of: (a) the date of expiration of the last to expire of the issued Patents (as defined in the Columbia License Agreement), (b) twenty (20) years after the first bona fide commercial sale of the Product in the country in question, or (c) expiration of any market exclusivity period granted by a regulatory agency for a Product in the country in question. Pursuant to the Columbia License Agreement, we agreed to pay Columbia (i) initial and annual license fees ranging from the low five figures to mid five figures that are creditable to earned royalties and milestone payments due to Columbia in the same calendar year, (ii) certain development-based and other milestone payments, (iii) royalty payments, depending on net revenues, (iv) minimum royalty payments, and (v) certain non-royalty sublicense income. Royalties on each particular Product are payable on a country-by-country and product-by-product basis until the later of (i) twenty (20) years after the first bona fide commercial sale of such particular Technology Product in each country and (ii) expiration of any market exclusivity period granted by a regulatory agency of such particular Product in such country.
Investigator-Sponsored Study Agreements between the Company and Vendors
Sponsored Research Agreement with Columbia University for the Study of Ketamine in Combination with Other Drugs for Treatment of Alzheimer’s and Depression Disorders
On October 1, 2021, we entered into a sponsored research agreement with Columbia University (“Columbia”) pursuant to which Columbia shall conduct two different studies related to all uses of Ketamine or its metabolites in combination with Prucalopride, one of which is related to Alzheimer’s and the other of which is related to Depression, PTSD and Stress Projects. In addition, Company has been granted an option to license certain assets currently under development, including Alzheimer’s disease. The term of the option will commence on the effective date of this agreement and will expire upon the earlier of (i) 90 days after the date of the Company’s receipt of a final research report for each specific research proposal as defined in the agreement or (ii) termination of the research. If we elect to exercise the option, both parties will commence negotiation of a license agreement and will execute a license agreement no later than 3 months after the dated of the exercise of the option. We exercised our option for an exclusive license agreement for SPC-15, a prophylactic treatment for stress-induced affective disorders including anxiety and PTSD pursuant to which we were granted an exclusive license to further develop, manufacture, and commercialize SPC-15 worldwide. See “Prospectus Summary-License Agreements between the Company and Vendor—Exclusive License Agreement with Columbia University.” Columbia University and the Company will work towards developing a therapeutic treatment for patients suffering from Alzheimer’s disease to posttraumatic stress disorder. During a one-year period from the date of this agreement, we shall pay a total of $1,436,082 to Columbia University for the support of the research according to the payment schedule as follows: (i) 30% at signing, (ii) 30% at four and half months after the start of the project, (iii) 30% at nine months after the start of the project and, (iv)10% at completion of the project. On October 13, 2022, we entered into an amendment of the sponsored research agreement pursuant to which the parties agreed to extend the payment schedule until March 31, 2024. We paid the first payment of $430,825 in November 2021 and the second payment of $430,825 in July 2022.
Sponsored Research Agreement with University of Maryland, Baltimore for the Study of Targeted liposomal drug delivery for rheumatoid arthritis
On July 6, 2021, we entered into a sponsored research agreement (the “July 2021 Sponsored Research Agreement”) with UMB pursuant to which UMB shall evaluate the pharmacokinetics of dexamethasone delivered to arthritic rats via liposome. The research pursuant to the July 2021 Sponsored Research Agreement commenced on September 1, 2021 and will continue until the substantial completion thereof, subject to renewal upon written consent of the parties with a project timeline of twelve months. The July 2021 Sponsored Research Agreement may be terminated by either party upon 30 days’ prior written notice to the other party. In addition, if either party commits any material breach of or defaults with respect to any terms or conditions of the July 2021 Sponsored Research Agreement and fails to remedy such default or breach within 10 business days after written notice from the other party, the party giving notice may terminate the July 2021 Sponsored Research Agreement as of the date of receipt of such notice by the other party. If we terminate the July 2021 Sponsored Research Agreement for any reason other than an uncured material breach by UMB, we shall relinquish any and all rights it may have in the Results (as defined in the July 2021 Sponsored Research Agreement) to UMB. In addition, if the July 2021 Sponsored Research Agreement is terminated early, we, among other things, will pay all costs incurred and accrued by UMB as of the date of termination. Pursuant to the terms of the July 2021 Sponsored Research Agreement, UMB granted us an option (the “Option”) to negotiate and obtain an exclusive license to any UMB Arising IP (as defined in the July 2021 Sponsored Research Agreement) and UMB’s rights in any Joint Arising IP (as defined in the July 2021 Sponsored Research Agreement) (collectively, the “UMB IP”). We may exercise the Option by giving UMB written notice within 60 days after it receives notice from UMB of the UMB IP. We shall pay total fees of $276,285 as set forth in the July 2021 Sponsored Research Agreement. We paid the first payment of $92,095 on September 1, 2021 and on August 31, 2022, we paid the second payment of $92,095.
Sponsored Research Agreement with The Regents of the University of California for the Effect of Psilocybin on Inflammation in the Blood
On June 1, 2021, we entered into a sponsored research agreement (“Sponsored Research Agreement”) with The Regents of the University of California, on behalf of its San Francisco Campus (“UCSF”) pursuant to which UCSF shall conduct a study to examine psilocybin’s effect on inflammatory activity in humans to accelerate its implementation as a potential treatment for Parkinson’s Disease, chronic pain, and bipolar disorder. The purpose of this is to show what effect psilocybin has on inflammation in the blood. We believe that this study will help support the UMB homing peptide study. Pursuant to the Agreement, we shall pay UCSF a total fee of $342,850 to conduct the research over the two-year period. The Agreement shall be effective for a period of two years from the effective date, subject to renewal or earlier termination as set forth in the Sponsored Research Agreement. During the years ended December 31, 2022 and 2021, pursuant to the Sponsored Research Agreement, we paid to UCSF $181,710 and $100,570, respectively. We have notified UCSF we do not plan to continue this study.
Investigator-Sponsored Study Agreement with UMB for CNS Homing Peptide
On January 5, 2021, we entered into an investigator-sponsored study agreement with UMB. The research project is a clinical study to examine a novel peptide-guided drug delivery approach for the treatment of Multiple Sclerosis (“MS”). More specifically, the study is designed to evaluate (1) whether MS-1-displaying liposomes can effectively deliver dexamethasone to the central nervous system and (2) whether MS-1-displaying liposomes are superior to plain liposomes, also known as free drug, in inhibiting the relapses and progression of Experimental Autoimmune Encephalomyelitis. Pursuant to the agreement, the research commenced on March 1, 2021 and will continue until substantial completion, subject to renewal upon mutual written consent of the parties. The total cost under the investigator-sponsored study agreement shall not exceed $81,474. which is payable in two equal installments of $40,737 upon execution of the Sponsored Study Agreement and $40,737 upon completion of the project with an estimated project timeline of nine months. We paid $40,737 on January 13, 2021. This project is postponed until further notice and the second payment is not due.
Recent Developments
Exclusive License Agreement with Columbia University
On July 1, 2024, we entered into an Exclusive License Agreement with Columbia University under which we were granted an exclusive license to further develop, manufacture, and commercialize SPC-15 worldwide. See “----License Agreements between the Company and Vendor—Exclusive License Agreement with Columbia University.”
Pre-IND Submission
On June 4, 2024, we submitted a pre-Investigational New Drug (pre-IND) briefing package and meeting request to the U.S. Food and Drug Administration (FDA) for SPC-15, Silo’s intranasal prophylactic treatment for post-traumatic stress disorder (PTSD) and stress-induced anxiety disorder.
June 2024 Registered Direct Offering and Concurrent Private Placement
For a discussion of this transaction please see “June 2024 Registered Direct Offering And Concurrent Private Placement” beginning on page 10
Our Corporate Information
We were incorporated as in the State of New York on July 13, 2010. On January 24, 2013, we changed our state of incorporation from New York to Delaware. On December 19, 2023, we changed our state of incorporation from Delaware to Nevada. Our principal executive offices are located at 677 N Washington Blvd Sarasota FL 34236 and our telephone number is (718) 400-9031.
ABOUT THIS OFFERING
This prospectus relates to the offer and resale by the Selling Shareholders of up to 1,043,739 shares of restricted Common Stock and Common Stock issuable upon the exercise of the Warrants. All of the Shares, if and when sold, will be sold by the Selling Shareholders. The Selling Shareholders may sell the Shares from time to time at prevailing market prices or at privately negotiated prices.
| Shares offered by the Selling Shareholders: | | Up to 1,043,739 shares of Common Stock consisting of: (i) 917,432 shares of Common Stock upon exercise of the June 2024 Investor Warrants; (ii) 68,807 shares of Common Stock issuable upon exercise of the June 2024 Placement Agent Warrants; and (iii) 57,500 shares of Common Stock issuable upon exercise of the September 2022 Underwriter Warrants. |
Shares of Common Stock outstanding after completion of this offering (assuming full exercise of the Warrants that are exercisable for the Warrant Shares offered hereby): | | 4,764,557 shares(1) |
| Use of proceeds: | | We will not receive any proceeds from any sale of the Shares by the Selling Shareholders. We will receive proceeds in the event that any of the Warrants are exercised at the exercise prices per share for cash which would result in gross proceeds of approximately $2.44 million. Any proceeds that we receive from the exercise of the Warrants will be used for working capital, capital expenditures, product development, and other general corporate purposes, including investments in sales and marketing in the United States and internationally. See “Use of Proceeds.” |
| | | |
| Risk factors: | | An investment in the shares of Common Stock offered under this prospectus is highly speculative and involves substantial risk. Please carefully consider the “Risk Factors” section on page 9, other information in this prospectus and in the documents incorporated by reference herein for a discussion of risks. Additional risks and uncertainties not presently known to us or that we currently deem to be immaterial may also impair our business and operations |
| NASDAQ symbol: | | SILO |
| (1) | The number of shares of Common Stock to be outstanding immediately after this offering is based on 3,720,818 shares of Common Stock outstanding as of July 17, 2024, and excludes, as of such date: |
| ● | 22,850 shares of Common Stock issuable upon exercise of options outstanding under the Silo Pharma, Inc. Amended and Restated 2020 Omnibus (the “2020 Plan”) with a weighted-average exercise price of $0.005 per share; |
| ● | 6,000 shares of Common Stock issuable upon exercise of non-plan options outstanding with a weighted-average exercise price of $9.19 per share; |
| ● | 447,150 shares of Common Stock reserved for future issuance under the 2020 Plan; |
| ● | 347,080 shares of Common Stock issuable upon the exercise of warrants outstanding at an exercise price of $15.50 per share; |
RISK FACTORS
An investment in our securities involves a high degree of risk. Before deciding whether to invest in our securities, you should consider carefully the risks described below, together with other information in this prospectus supplement, the accompanying prospectus and the information and documents incorporated by reference. You should also consider the risks, uncertainties and assumptions discussed under the heading “Risk Factors” included in our most recent Annual Report on Form 10-K and the subsequent reports that we file with the SEC which are on file with the SEC and are incorporated herein by reference, and which may be amended, supplemented or superseded from time to time by other reports we file with the SEC in the future. If any of these risks actually occur, our business, financial condition, results of operations or cash flow could be adversely effected. This could cause the trading price of our common stock to decline, resulting in a loss of all or part of your investment. The risks and uncertainties described below are not the only ones facing us. Additional risks and uncertainties not presently known to us, or that we currently see as immaterial, may also harm our business. Please also read carefully the section above entitled “Special Note Regarding Forward-Looking Statements.”
Risks Related to the Resale of the Shares
The Selling Shareholders may choose to sell the Shares at prices below the current market price.
The Selling Shareholders are not restricted as to the prices at which they may sell or otherwise dispose of the Shares covered by this prospectus. Sales or other dispositions of the Shares below the then-current market prices could adversely affect the market price of our Common Stock.
A large number of shares of Common Stock may be sold in the market following this offering, which may significantly depress the market price of our Common Stock.
The Shares sold in the offering will be freely tradable without restriction or further registration under the Securities Act. As a result, a substantial number of shares of Common Stock may be sold in the public market following this offering. If there are significantly more shares of Common Stock offered for sale than buyers are willing to purchase, then the market price of our Common Stock may decline to a market price at which buyers are willing to purchase the offered Common Stock and sellers remain willing to sell Common Stock.
Neither we nor the Selling Shareholders have authorized any other party to provide you with information concerning us or this offering.
You should carefully evaluate all of the information in this prospectus, including the documents incorporated by reference herein and therein. We may receive media coverage regarding our Company, including coverage that is not directly attributable to statements made by our officers, that incorrectly reports on statements made by our officers or employees, or that is misleading as a result of omitting information provided by us, our officers or employees. Neither we nor the Selling Shareholders have authorized any other party to provide you with information concerning us or this offering, and recipients should not rely on this information.
You may experience future dilution as a result of issuance of the Shares, future equity offerings by us and other issuances of our Common Stock or other securities. In addition, the issuance of the Shares and future equity offerings and other issuances of our Common Stock or other securities may adversely affect our Common Stock price.
In order to raise additional capital, we may in the future offer additional shares of our Common Stock or other securities convertible into or exchangeable for our Common Stock at prices that may not be the same as the price per share as prior issuances of Common Stock. We may not be able to sell shares or other securities in any other offering at a price per share that is equal to or greater than the price per share previously paid by investors, and investors purchasing shares or other securities in the future could have rights superior to existing stockholders. The price per share at which we sell additional shares of our Common Stock or securities convertible into Common Stock in future transactions may be higher or lower than the prices per share per share. ln addition, the exercise price of the Warrants for the Warrant Shares may be or greater than the price per share previously paid by certain investors. You will incur dilution upon exercise of any outstanding stock options, warrants or upon the issuance of shares of Common Stock under our stock incentive programs. In addition, the issuance of the Shares and any future sales of a substantial number of shares of our Common Stock in the public market, or the perception that such sales may occur, could adversely affect the price of our Common Stock. We cannot predict the effect, if any, that market sales of those shares of Common Stock or the availability of those shares for sale will have on the market price of our Common Stock.
JUNE 2024 REGISTERED DIRECT OFFERING AND CONCURRENT PRIVATE PLACEMENT
On June 4, 2024, we entered into a securities purchase agreement (the “Purchase Agreement”) with certain institutional investors, pursuant to which we agreed to sell to such investors 883,395 shares (the “Shares”) of Common Stock, pre-funded warrants (the “Pre-Funded Warrants”) to purchase up to 34,037 shares of Common Stock (the “Pre-Funded Warrant Shares”), having an exercise price of $0.0001 per share, at a purchase price of $2.18 per share of Common Stock and a purchase price of $2.1799 per Pre-Funded Warrant (the “Offering”). The shares of Common Stock and Pre-Funded Warrants (and shares of common stock underlying the Pre-Funded Warrants) were offered by us pursuant to its shelf registration statement on Form S-3 (File No. 333-276658), which was declared effective by the Securities and Exchange Commission on January 30, 2024.
Concurrently with the sale of Common Stock and/or the Pre-Funded Warrants, pursuant to the Purchase Agreement in a private placement, for each share of Common Stock and/or Pre-Funded Warrant purchased by the investors, such investors received an unregistered warrant (the “Common Warrant”) to purchase one share of Common Stock (the “Common Warrant Shares”). The warrants have an exercise price of $2.06 per share, and are exercisable immediately upon issuance for a five-year period.
The closing of the sales of these securities under the Purchase Agreement took place on June 6, 2024. The gross proceeds from the offering were approximately $2 million, prior to deducting placement agent’s fees and other offering expenses payable by the Company. We intend to use the net proceeds from the offering for working capital and other general corporate purposes.
On April 23, 2024, we entered into an engagement agreement with H.C. Wainwright & Co., LLC, as exclusive placement agent (the “Placement Agent”), pursuant to which the Placement Agent agreed to act as placement agent on a reasonable “best efforts” basis in connection with the Offering. We agreed to pay the Placement Agent an aggregate cash fee equal to 7.5% of the gross proceeds from the sale of securities in the Offering and a management fee equal to 1.0% of the gross proceeds raised in the Offering. We also agreed to issue the Placement Agent (or its designees) a warrant (the “Placement Agent Warrant”) to purchase up to 7.5% of the aggregate number of shares of Common Stock and/or Pre-Funded Warrants sold in the offering, or warrants to purchase up to 68,807 shares of Common Stock, at an exercise price equal to 125.0% of the offering price per share of Common Stock, or $2.725 per share. The Placement Agent Warrant are exercisable immediately upon issuance for a period of five years following the commencement of the sales pursuant to the Offering. In addition, we agreed to pay the Placement Agent $25,000 for non-accountable expenses, $50,000 for legal expenses and other out-of-pocket expenses and $15,950 for clearing fees.
SEPTEMBER 2022 PUBLIC OFFERING
On September 26, 2022, we entered into an underwriting agreement (the “Underwriting Agreement”) with Laidlaw & Company (UK) Ltd., as representative of the several underwriters identified therein (the “Underwriters”), relating to the public offering (the “Offering”) of 1,000,000 shares of Common Stock, at a public offering price of $5.00 per share. Under the terms of the Underwriting Agreement, we granted the Underwriters an option, exercisable for 45 days following the closing of the Offering, to purchase up to an additional 150,000 shares of common stock at the public offering price to cover over-allotments, if any. On September 28, 2022, the Underwriters fully exercised their over-allotment option and purchased an additional 150,000 shares (the “Option Shares”).
On September 29, 2022, concurrently with the closing of the Offering, we also issued warrants (the “September 2022 Underwriter Warrants”) to purchase an aggregate of up to 57,500 shares of its common stock to the representative of the Underwriters or its designees, at an exercise price of $6.25 per share (the “September 2022 Underwriter Warrant Shares”). The September 2022 Underwriter Warrants are exercisable beginning on March 25, 2023, and expire on September 26, 2027, pursuant to the terms and conditions of the Representative’s Warrants. The September 2022 Underwriter Warrant Shares were offered, issued and sold to the public pursuant to a registration statement on Form S-1 (File No. 333-261532) filed with the Securities and Exchange Commission (“SEC”), which was declared effective by the SEC on September 26, 2022, and the prospectus forming a part thereof.
SELLING SHAREHOLDERS
The Shares being offered by the Selling Shareholders are those issuable upon the exercise of the Warrants. For additional information regarding the issuance of these securities, see “June 2024 Registered Direct Offering and Concurrent Private Placement,” and “September 2022 Public Offering” beginning on page 10 of this prospectus. We are registering the Warrant Shares issuable upon exercise of the Warrants in order to permit the Selling Shareholders to offer such shares for resale from time to time. Except for the ownership of the Warrants, none of the Selling Shareholders have had any material relationship with us within the past three (3) years except as set forth below.
The following table sets forth certain information with respect to each Selling Shareholder, including (i) the shares of Common Stock beneficially owned by the Selling Shareholder prior to this offering, (ii) the number of Shares, being offered by the Selling Shareholder pursuant to this prospectus and (iii) the Selling Shareholder’s beneficial ownership after completion of this offering. The second column lists the number of shares of Common Stock beneficially owned by each selling stockholder, based on its ownership of the shares of our securities, as of July 17, 2024, assuming full exercise of all Warrants held by the selling stockholders on that date, without regard to any limitations on exercise. The registration of the Shares issuable to the Selling Shareholders upon the exercise of the Warrants, does not necessarily mean that the Selling Shareholders will sell all or any of such shares, but the number of shares of Common Stock and percentages set forth in the final two columns below assume that all shares of Common Stock being offered by the Selling Shareholders are sold. The final two columns also assume the exercise of all of the Warrants held by the Selling Shareholders as of July 17, 2024, without regard to any limitations on exercise described in this prospectus or in the Warrants. See “Plan of Distribution.”
The table is based on information supplied to us by the Selling Shareholders, with beneficial ownership and percentage ownership determined in accordance with the rules and regulations of the SEC and includes voting or investment power with respect to shares of Common Stock. This information does not necessarily indicate beneficial ownership for any other purpose. In computing the number of shares of Common Stock beneficially owned by a Selling Shareholder and the percentage ownership of that Selling Shareholder, shares of Common Stock subject to warrants held by that Selling Shareholder that are exercisable for shares of Common Stock within 60 days after July 17, 2024, are deemed outstanding. Such shares, however, are not deemed outstanding for the purposes of computing the percentage ownership of any other stockholder.
This prospectus covers the resale of up to an aggregate of 1,043,739 shares of Common Stock, consisting of:: (i) 917,432 shares of Common Stock issuable upon exercise of the June 2024 Investor Warrants; (ii) 68,807 shares of Common Stock issuable upon exercise of the June 2024 Placement Agent Warrants and (iii) 57,500 shares of Common Stock issuable upon exercise of the September 2022 Underwriter Warrants to purchase up to 57,500 shares of Common Stock. See “June 2024 Registered Direct Offering and Concurrent Private Placement” and “September 2022 Public Offering” beginning on page 10 of this prospectus for further details relating to the Warrant Shares and the Warrants.
| Name of Selling Shareholder | | Number of
Shares of
Common
Stock
Beneficially
Owned
Prior to
Offering(1) | | | Maximum
Number of
Shares of Common Stock to be Sold Pursuant to this Prospectus(2) | | | Number of Shares of Common Stock Beneficially Owned
After Offering(3) | | | Percentage Beneficially Owned
After Offering(3) | |
| Armistice Capital, Master Fund Ltd.(4) | | | 344,037 | )(5) | | | 344,037 | | | | - | | | | * | |
| Intracoastal Capital LLC(6) | | | 383,537 | (7) | | | 344,037 | | | | 39,500 | | | | * | |
| Hudson Bay Master Fund Ltd.(8) | | | 229,358 | (9) | | | 229,358 | | | | - | | | | * | |
| Michael Vasinkevich(10) | | | 44,122 | (11) | | | 44,122 | | | | - | | | | * | |
| Noam Rubinstein(10) | | | 21,674 | (12) | | | 21,674 | | | | - | | | | * | |
| Craig Schwabe(10) | | | 2,323 | (13) | | | 2,323 | | | | - | | | | * | |
| Charles Worthman(10) | | | 688 | (14) | | | 688 | | | | - | | | | * | |
| Laidlaw & Company (UK) Ltd.(15) | | | 57,500 | (16) | | | 57,500 | | | | - | | | | * | |
| (1) | All of the Warrants that are exercisable for the Warrant Shares offered hereby (other than any of the September 2022 Warrants) contain certain beneficial ownership limitations, which provide that a holder of the Warrants will not have the right to exercise any portion of its Warrants if such holder, together with its affiliates, would beneficially own in excess of 4.99% or 9.99%, as applicable, of the number of shares of Common Stock outstanding immediately after giving effect to such exercise, provided that upon at least 61 days’ prior notice to us, a holder may increase or decrease such limitation up to a maximum of 9.99% of the number of shares of Common Stock outstanding (each such limitation, a “Beneficial Ownership Limitation”). As a result, the number of shares of Common Stock reflected in this column as beneficially owned by each Selling Shareholder includes (i) any outstanding shares of Common Stock held by such Selling Shareholder, and (ii) if any, the number of shares of Common Stock subject to the Warrants exercisable for the Warrant Shares offered hereby and any other warrants that may be held by such Selling Shareholder, in each case which such Selling Shareholder has the right to acquire as of July 17, 2024 or within 60 days thereafter and without it or any of its affiliates beneficially owning more than 4.99% or 9.99%, as applicable, of the number of outstanding shares of Common Stock as of July 17, 2024. |
| (2) | Represents shares of Common Stock owned by the Selling Shareholders upon full exercise of the Warrants offered hereby. |
| (3) | The number of shares owned and the percentage of beneficial ownership after this offering set forth in these columns are based on 4,764,557 shares of Common Stock, which includes 3,720,818 shares of Common Stock outstanding as of July 17, 2024 and assumes full exercise of the Warrants that are exercisable for the 1,043,739 Warrant Shares offered hereby. The calculation of beneficial ownership reported in such columns takes into account the effect of the Beneficial Ownership Limitations in any warrants held by the Selling Shareholders after this offering. We do not know when or in what amounts a Selling Shareholder may offer shares for sale. The Selling Shareholders may choose not to sell any or all of the shares offered by this prospectus. Because the Selling Shareholders may offer all or some of the Shares pursuant to this offering, we cannot estimate the number of the Shares that will be held by the Selling Shareholders after completion of the offering. However, for purposes of this table, we have assumed that, after completion of the offering, all of the Shares covered by this prospectus will be sold by the Selling Shareholders and that the Selling Stockholders do not acquire beneficial ownership of any additional shares. |
| (4) | The securities are directly held by Armistice Capital Master Fund Ltd., a Cayman Islands exempted company (the “Master Fund”), and may be deemed to be indirectly beneficially owned by: (i) Armistice Capital, LLC (“Armistice”), as the investment manager of the Master Fund; and (ii) Steven Boyd, as the Managing Member of Armistice Capital. The warrants are subject to a beneficial ownership limitation of 4.99%, which such limitation restricts the Selling Stockholder from exercising that portion of the warrants that would result in the Selling Stockholder and its affiliates owning, after exercise, a number of shares of common stock in excess of the beneficial ownership limitation. The address of Armistice Capital Master Fund Ltd. is c/o Armistice Capital, LLC, 510 Madison Avenue, 7th Floor, New York, NY 10022. |
| (5) | Common stock beneficially owned prior to this offering consists of common stock underlying the June 2024 Investor Warrants to purchase 344,037 shares of common stock being offered by the selling shareholder pursuant to this prospectus. |
| (6) | Mitchell P. Kopin (“Mr. Kopin”) and Daniel B. Asher (“Mr. Asher”), each of whom are managers of Intracoastal Capital LLC (“Intracoastal”), have shared voting control and investment discretion over the securities reported herein that are held by Intracoastal. As a result, each of Mr. Kopin and Mr. Asher may be deemed to have beneficial ownership (as determined under Section 13(d) of the Securities Exchange Act of 1934, as amended (the “Exchange Act”)) of the securities reported herein that are held by Intracoastal. The address of Intracoastal is 245 Palm Trail, Delray Beach, FL 33483. |
| (7) | Common stock beneficially owned prior to this offering consists of (i) common stock underlying the June 2024 Investor Warrants to purchase 344,037 shares of common stock being offered by the selling stockholder pursuant to this prospectus and (ii) 39,500 shares of common stock. |
| (8) | Hudson Bay Capital Management LP, the investment manager of Hudson Bay Master Fund Ltd., has voting and investment power over these securities. Sander Gerber is the managing member of Hudson Bay Capital GP LLC, which is the general partner of Hudson Bay Capital Management LP. Each of Hudson Bay Master Fund Ltd. and Sander Gerber disclaims beneficial ownership over these securities. The address of Hudson Bay Master Fund Ltd. is 28 Havemeyer Pl, 2nd Floor, Greenwich, CT 06830. |
| (9) | Common stock beneficially owned prior to this offering consists of common stock underlying the June 2024 Investor Warrants to purchase 229,358 shares of common stock being offered by the selling stockholder pursuant to this prospectus. |
| (10) | Each of the selling shareholders is affiliated with H.C. Wainwright & Co., LLC, a registered broker dealer with a registered address of c/o H.C. Wainwright & Co., 430 Park Ave, 3rd Floor, New York, NY 10022, and has sole voting and dispositive power over the securities held. The number of shares to be sold in this offering consists of shares of common stock issuable upon exercise of June 2024 Placement Agent Warrants, which were received as compensation for our private placement. The selling shareholder acquired the June 2024 Placement Agent Warrants in the ordinary course of business and, at the time the June 2024 Placement Agent Warrants were acquired, the selling shareholder had no agreement or understanding, directly or indirectly, with any person to distribute such securities. |
| | |
| (11) | Common stock beneficially owned prior to this offering consists of common stock underlying the June 2024 Placement Agent Warrants to purchase 44,122 shares of common stock being offered by the selling stockholder pursuant to this prospectus. |
| | |
| (12) | Common stock beneficially owned prior to this offering consists of common stock underlying the June 2024 Placement Agent Warrants to purchase 21,674 shares of common stock being offered by the selling stockholder pursuant to this prospectus. |
| | |
| (13) | Common stock beneficially owned prior to this offering consists of common stock underlying the June 2024 Placement Agent Warrants to purchase 2,323 shares of common stock being offered by the selling stockholder pursuant to this prospectus |
| (14) | Common stock beneficially owned prior to this offering consists of common stock underlying the June 2024 Placement Agent Warrants to purchase 688 shares of common stock being offered by the selling stockholder pursuant to this prospectus |
| | |
| (15) | Laidlaw & Company (UK) Ltd. (“Laidlaw”) holds outstanding warrants to purchase an aggregate of up to 57,500 shares of common stock. Pursuant to the terms of the warrants, Laidlaw cannot exercise the warrants to the extent it would beneficially own, after any such exercise, more than 9.99% of the outstanding shares of common stock (the “Blocker”) and the percentage set forth above gives effect to the Blocker. The warrants were issued to Laidlaw as partial compensation for its services as sole book-running manager for the Company’s initial public offering. The selling shareholder is a registered broker-dealer and acquired the Placement Agent Warrants in the ordinary course of business and, at the time the September 2022 Underwriter Warrants were acquired, the selling shareholder had no agreement or understanding, directly or indirectly, with any person to distribute such securities. The business address of Laidlaw & Company (UK) Ltd. is 521 5th Avenue, 12th Floor, New York, NY 10175. |
| | |
| (16) | Common stock beneficially owned prior to this offering consists of common stock underlying the September 2022 Underwriter Warrants to purchase 57,500 shares of common stock being offered by the selling stockholder pursuant to this prospectus. |
USE OF PROCEEDS
We will not receive any of the proceeds from the sale of the Shares by the Selling Shareholders pursuant to this prospectus. We may receive up to approximately $2.44 million in aggregate gross proceeds from cash exercises of the Warrants, based on the per share exercise price of the Warrants. We intend to use any net proceeds we receive for working capital, capital expenditures, product development, and other general corporate purposes, including investments in sales and marketing in the United States and internationally. We have not allocated specific amounts of net proceeds for any of these purposes.
The Selling Shareholders will pay any agent’s commissions and expenses they incur for brokerage, accounting, tax or legal services or any other expenses that they incur in disposing of the shares of Common Stock. We will bear all other costs, fees and expenses incurred in effecting the registration of the shares of Common Stock covered by this prospectus and any prospectus supplement. These may include, without limitation, all registration and filing fees, SEC filing fees and expenses of compliance with state securities or “blue sky” laws.
We cannot predict when or if the Warrants will be exercised, and it is possible that the Warrants may expire and never be exercised. In addition, the Warrants are exercisable on a cashless basis after six (6) months from the date of issuance if at the time of exercise there is no effective registration statement registering, or the prospectus contained therein is not available for, the issuance of the Shares. As a result, we may never receive meaningful, or any, cash proceeds from the exercise of the Warrants, and we cannot plan on any specific uses of any proceeds we may receive beyond the purposes described herein.
See “Plan of Distribution” elsewhere in this prospectus for more information.
DIVIDEND POLICY
We have never declared or paid any dividends on our Common Stock. We currently intend to retain all available funds and any future earnings for the operation and expansion of our business and, therefore, we do not anticipate declaring or paying dividends in the foreseeable future. The payment of dividends will be at the discretion of our Board and will depend on our results of operations, capital requirements, financial condition, prospects, contractual arrangements, any limitations on payment of dividends present in our future debt agreements, and other factors that our Board may deem relevant.
SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT
The following table sets forth certain information regarding beneficial ownership of shares of our common stock as of July 17, 2024 by (i) each person known to beneficially own more than 5% of our outstanding common stock, (ii) each of our directors, (iii) each of our named executive officers and (iv) all of our directors and named executive officers as a group.
The percentage ownership information is based upon 3,720,818 common shares outstanding as of July 17, 2024. The percentage ownership information shown in the table after this offering is based upon 4,764,557 shares of Common Stock (assuming full exercise of the Warrants to purchase 1,043,779 Warrant Shares). Information with respect to beneficial ownership has been furnished by each director, officer or beneficial owner of more than 5% of our common stock. We have determined beneficial ownership in accordance with the rules of the SEC. These rules generally attribute beneficial ownership of securities to persons who possess sole or shared voting power or investment power with respect to those securities. In addition, the rules attribute beneficial ownership of securities as of a particular date to persons who hold convertible preferred stock, options or warrants to purchase shares of common stock and that are exercisable within 60 days of such date. These shares are deemed to be outstanding and beneficially owned by the person holding those convertible preferred stock, options or warrants for the purpose of computing the percentage ownership of that person, but they are not treated as outstanding for the purpose of computing the percentage ownership of any other person. Except as otherwise indicated, the persons named in the table below have sole voting and investment power with respect to all shares beneficially owned, subject to community property laws, where applicable.
Except as otherwise noted below, the address for each person or entity listed in the table is c/o Silo Pharma, Inc., 677 N. Washington Boulevard, Sarasota, FL 34236.
| Name and Address of Beneficial Owner | | Number
of Shares | | | Percentage of
Common
Stock | | | Percentage
of
Common
Stock
Beneficially
Owned
After this
Offering | |
| 5% Stockholders: | | | | | | | | | |
| None | | | | | | | | | |
| Officers and Directors | | | | | | | | | |
| Eric Weisblum | | | 186,432 | (1) | | | 5.00 | % | | | 3.91 | % |
| Daniel Ryweck | | | 5,000 | (2) | | | | | | | | |
| Wayne D. Linsley | | | 3,425 | (2) | | | * | | | | * | |
| Kevin Munoz | | | 3,425 | (2) | | | * | | | | * | |
| Jeff Pavell | | | - | | | | * | | | | * | |
| All directors and executive officers as a group (5 persons) | | | 198,282 | | | | 5.31 | % | | | 4.15 | % |
| * | Represents beneficial ownership of less than 1% of the outstanding shares of our common stock. |
| (1) | Includes 6,000 shares of common stock issuable upon exercise of presently exercisable options. |
| | |
| (2) | Includes options to purchase 3,425 shares of common stock, all of which are presently exercisable. |
DESCRIPTION OF SECURITIES THAT THE SELLING SHAREHOLDERS ARE OFFERING
The Selling Shareholders are offering for resale up to an aggregate of 1,043,739 shares of Common Stock consisting of: (i) 917,432 shares of Common Stock upon exercise of the June 2024 Investor Warrants; (ii) 68,807 shares of Common Stock issuable upon exercise of the June 2024 Placement Agent Warrants; and (iii) 57,500 shares of Common Stock issuable upon exercise of the September 2022 Underwriter Warrants. The following summary of the terms of our shares of Common Stock is based upon our Articles of Incorporation and our Bylaws. The summary is not complete and is qualified by reference to our Articles of Incorporation and our Bylaws, which were filed as exhibits to our Annual Report on Form 10-K for the fiscal year ended December 31, 2023. For a description of our Common Stock, Exhibit 4.1-Description of Securities, to our Annual Report on Form 10-K for the fiscal year ended December 31, 2023, filed with the SEC on March 25, 2024.
Our Articles of Incorporation authorizes the issuance of up to 100,000,000 shares of Common Stock, par value $0.0001 per share, and up to 5,000,000 shares of blank check preferred stock, par value $0.0001 per share. Our Board may establish the rights and preferences of the preferred stock from time to time.
PLAN OF DISTRIBUTION
The Selling Shareholders and any of their respective pledgees, assignees and successors-in-interest may, from time to time, sell any or all of their securities covered hereby on any trading market, stock exchange or other trading facility on which the securities are traded or in private transactions. These sales may be at fixed or negotiated prices. The Selling Shareholders may use any one or more of the following methods when selling securities:
| | ● | ordinary brokerage transactions and transactions in which the broker-dealer solicits purchasers; |
| | ● | block trades in which the broker-dealer will attempt to sell the securities as agent but may position and resell a portion of the block as principal to facilitate the transaction; |
| | ● | purchases by a broker-dealer as principal and resale by the broker-dealer for its account; |
| | ● | an exchange distribution in accordance with the rules of the applicable exchange; |
| | ● | privately negotiated transactions; |
| | ● | settlement of short sales; |
| | ● | in transactions through broker-dealers that agree with the Selling Shareholders to sell a specified number of such securities at a stipulated price per security; |
| | ● | through the writing or settlement of options or other hedging transactions, whether through an options exchange or otherwise; |
| | ● | a combination of any such methods of sale; or |
| | ● | any other method permitted pursuant to applicable law. |
The Selling Shareholders may also sell securities under Rule 144 under the Securities Act, if available, rather than under this prospectus.
Broker-dealers engaged by the Selling Shareholders may arrange for other brokers-dealers to participate in sales. Broker-dealers may receive commissions or discounts from the Selling Shareholders (or, if any broker-dealer acts as agent for the purchaser of securities, from the purchaser) in amounts to be negotiated, but, except as set forth in a supplement to this prospectus, in the case of an agency transaction not in excess of a customary brokerage commission in compliance with FINRA Rule 2440; and in the case of a principal transaction a markup or markdown in compliance with FINRA IM-2440.
In connection with the sale of the securities covered hereby, the Selling Shareholders may enter into hedging transactions with broker-dealers or other financial institutions, which may in tum engage in short sales of the securities in the course of hedging the positions they assume. The Selling Shareholders may also sell securities short and deliver these securities to close out their short positions, or loan or pledge the securities to broker-dealers that in tum may sell these securities. The Selling Shareholders may also enter into option or other transactions with broker-dealers or other financial institutions or create one or more derivative securities which require the delivery to such broker-dealer or other financial institution of securities offered by this prospectus, which securities such broker-dealer or other financial institution may resell pursuant to this prospectus (as supplemented or amended to reflect such transaction).
The Selling Shareholders and any broker-dealers or agents that are involved in selling the securities may be deemed to be “underwriters” within the meaning of the Securities Act in connection with such sales. In such event, any commissions received by such broker-dealers or agents and any profit on the resale of the securities purchased by them may be deemed to be underwriting commissions or discounts under the Securities Act. We are requesting that each Selling Shareholder inform us that it does not have any written or oral agreement or understanding, directly or indirectly, with any person to distribute the securities. We will pay certain fees and expenses incurred by us incident to the registration of the securities.
Because the Selling Shareholders may be deemed to be “underwriters” within the meaning of the Securities Act, they will be subject to the prospectus delivery requirements of the Securities Act, including Rule 172 thereunder. In addition, any securities covered by this prospectus which qualify for sale pursuant to Rule 144 under the Securities Act may be sold under Rule 144 rather than under this prospectus. We are requesting that each Selling Shareholder confirm that there is no underwriter or coordinating broker acting in connection with the proposed sale of the resale securities by the Selling Shareholder.
We intend to keep this prospectus effective until the earlier of (i) the date on which the securities may be resold by the Selling Shareholders without registration and without regard to any volume or manner-of-sale limitations by reason of Rule 144, without the requirement for us to be in compliance with the current public information requirement under Rule 144 under the Securities Act or any other rule of similar effect or (ii) all of the securities have been sold pursuant to this prospectus or Rule 144 under the Securities Act or any other rule of similar effect. The resale securities will be sold only through registered or licensed brokers or dealers if required under applicable state securities laws. In addition, in certain states, the resale securities covered hereby may not be sold unless they have been registered or qualified for sale in the applicable state or an exemption from the registration or qualification requirement is available and is complied with.
Under applicable rules and regulations under the Exchange Act, any person engaged in the distribution of the resale securities may not simultaneously engage in market making activities with respect to the Common Stock for the applicable restricted period, as defined in Regulation M, prior to the commencement of the distribution. In addition, the Selling Shareholders will be subject to applicable provisions of the Exchange Act and the rules and regulations thereunder, including Regulation M, which may limit the timing of purchases and sales of the Common Stock by the Selling Shareholders or any other person. We will make copies of this prospectus available to the Selling Shareholders and are informing the Selling Shareholders of the need to deliver a copy of this prospectus to each purchaser at or prior to the time of the sale (including by compliance with Rule 172 under the Securities Act).
DISCLOSURE OF COMMISSION POSITION ON INDEMNIFICATION FOR SECURITIES ACT LIABILITY
Insofar as indemnification for liabilities arising under the Securities Act may be permitted to directors, officers or persons controlling the registrant pursuant to the foregoing provisions, the registrant has been informed that in the opinion of the SEC such indemnification is against public policy as expressed in the Securities Act and is, therefore, unenforceable.
LEGAL MATTERS
The validity of the issuance of the securities offered hereby will be passed upon for us by Sheppard Mullin Richter & Hampton LLP of New York, New York.
EXPERTS
The consolidated financial statements of Silo Pharma, Inc. as of December 31, 2023 and 2022 and for each of the two years in the period ended December 31, 2023, incorporated into this prospectus and the Registration Statement on Form S-1 of which it forms a part by reference to the Annual Report on Form 10-K for the year ended December 31, 2023, have been so incorporated in reliance on the report of Salberg & Company, P.A., an independent registered public accounting firm, given on the authority of said firm as experts in auditing and accounting.
WHERE YOU CAN FIND MORE INFORMATION
This prospectus constitutes a part of a registration statement on Form S-1 filed under the Securities Act. As permitted by the SEC’s rules, this prospectus and any prospectus supplement, which form a part of the registration statement, do not contain all the information that is included in the registration statement. You will find additional information about us in the registration statement and its exhibits. Any statements made in this prospectus or any prospectus supplement concerning legal documents are not necessarily complete and you should read the documents that are filed as exhibits to the registration statement or otherwise filed with the SEC for a more complete understanding of the document or matter.
You can read our electronic SEC filings, including such registration statement, on the internet at the SEC’s website at www.sec.gov. We are subject to the information reporting requirements of the Exchange Act, and we file reports, proxy statements and other information with the SEC. These reports, proxy statements and other information will be available at the website of the SEC referred to above. We also maintain a website at www.wisatechnologies.com, at which you may access these materials free of charge as soon as reasonably practicable after they are electronically filed with, or furnished to, the SEC. However, the information contained in or accessible through our website is not part of this prospectus or the registration statement of which this prospectus forms a part, and investors should not rely on such information in making a decision to purchase our securities in this offering.
INCORPORATION OF DOCUMENTS BY REFERENCE
This prospectus is part of the registration statement but the registration statement includes and incorporates by reference additional information and exhibits. The SEC permits us to “incorporate by reference” the information contained in documents we file with the SEC, which means that we can disclose important information to you by referring you to those documents rather than by including them in this prospectus. Information that is incorporated by reference is considered to be part of this prospectus and you should read it with the same care that you read this prospectus. Information that we file later with the SEC will automatically update and supersede the information that is either contained, or incorporated by reference, in this prospectus, and will be considered to be a part of this prospectus from the date those documents are filed. We have filed with the SEC, and incorporate by reference in this prospectus:
| ● | our Annual Report on Form 10-K for the year ended December 31, 2023 filed with the SEC on March 25, 2024; |
| ● | our Quarterly Reports on Form 10-Q for the quarter ended March 31, 2024 filed with the SEC on May 13, 2024; |
| ● | the description of our common stock, par value $0.0001 per share, contained in Exhibit 4.1 to our Annual Report on Form 10-K filed with the SEC on March 25, 2024, including any amendment or report filed for the purpose of updating such description. |
In addition, all documents subsequently filed by us pursuant to Sections 13(a), 13(c), 14 or 15(d) of the Exchange Act, as amended, prior to the termination of the offering (excluding any information furnished rather than filed) shall be deemed to be incorporated by reference into this prospectus.
Notwithstanding the statements in the preceding paragraphs, no document, report or exhibit (or portion of any of the foregoing) or any other information that we have “furnished” to the SEC pursuant to the Exchange Act shall be incorporated by reference into this prospectus.
We will furnish without charge to you, on written or oral request, a copy of any or all of the documents incorporated by reference in this prospectus, including exhibits to these documents. You should direct any requests for documents to:
Silo Pharma, Inc.
677 N. Washington Boulevard
Sarasota, Florida 34236
Attn.: Secretary Copies of these filings are also available through our website at https://silopharma.com/sec-filings. For other ways to obtain a copy of these filings, please refer to “Where You Can Find More Information” above. We do not incorporate the information on our website into this prospectus or any supplement to this prospectus and you should not consider any information on, or that can be accessed through, our website as part of this prospectus or any supplement to this prospectus (other than those filings with the SEC that we specifically incorporate by reference into this prospectus or any supplement to this prospectus).
Any statement contained in a document incorporated or deemed to be incorporated by reference in this prospectus will be deemed modified, superseded or replaced for purposes of this prospectus to the extent that a statement contained in this prospectus modifies, supersedes or replaces such statement. Any statement contained herein or in any document incorporated or deemed to be incorporated by reference shall be deemed to be modified or superseded for purposes of the registration statement of which this prospectus forms a part to the extent that a statement contained in any other subsequently filed document which also is or is deemed to be incorporated by reference modifies or supersedes such statement. Any such statement so modified or superseded shall not be deemed to constitute a part of the registration statement of which this prospectus forms a part, except as so modified or superseded.

Silo Pharma, Inc.
Up to 1,043,739 Shares of Upon Exercise of Certain Common Stock Purchase Warrants
PROSPECTUS
The date of this prospectus is , 2024.
PART II - INFORMATION NOT REQUIRED IN PROSPECTUS
Item 13. Other Expenses of Issuance and Distribution.
The following table sets forth an estimate of the fees and expenses relating to the issuance and distribution of the securities being registered hereby, other than underwriting discounts and commissions, all of which shall be borne by the registrant. All of such fees and expenses, except for the SEC registration fee, are estimated:
| SEC registration fee | | $ | 146.35 | |
| Transfer agent and registrar fees and expenses | | $ | 5,000.00 | |
| Legal fees and expenses | | $ | 20,000.00 | |
| Printing fees and expenses | | $ | 5,000.00 | |
| Accounting fees and expenses | | $ | 10,000.00 | |
| Miscellaneous fees and expenses | | $ | 1,500.00 | |
| Total | | $ | 41,646.35 | |
Item 14. Indemnification of Officers and Directors.
We are a Nevada corporation and generally governed by the Nevada Private Corporations Code, Title 78 of the Nevada Revised Statutes (the “NRS”).
Section 78.138 of the NRS provides that, unless the corporation’s articles of incorporation provide otherwise, a director or officer will not be individually liable unless it is proven that (i) the director’s or officer’s acts or omissions constituted a breach of his or her fiduciary duties, and (ii) such breach involved intentional misconduct, fraud, or a knowing violation of the law. Our articles of incorporation provide the personal liability of our directors is eliminated to the fullest extent permitted under the NRS.
Section 78.7502 of the NRS permits a company to indemnify its directors and officers against expenses, judgments, fines, and amounts paid in settlement actually and reasonably incurred in connection with a threatened, pending, or completed action, suit, or proceeding, if the officer or director (i) is not liable pursuant to NRS 78.138, or (ii) acted in good faith and in a manner the officer or director reasonably believed to be in or not opposed to the best interests of the corporation and, if a criminal action or proceeding, had no reasonable cause to believe the conduct of the officer or director was unlawful. Section 78.7502 of the NRS requires a corporation to indemnify a director or officer that has been successful on the merits or otherwise in defense of any action or suit. Section 78.7502 of the NRS precludes indemnification by the corporation if the officer or director has been adjudged by a court of competent jurisdiction, after exhaustion of all appeals, to be liable to the corporation or for amounts paid in settlement to the corporation, unless and only to the extent that the court determines that in view of all the circumstances, the person is fairly and reasonably entitled to indemnity for such expenses and requires a corporation to indemnify its officers and directors if they have been successful on the merits or otherwise in defense of any claim, issue, or matter resulting from their service as a director or officer.
Section 78.751 of the NRS permits a Nevada company to indemnify its officers and directors against expenses incurred by them in defending a civil or criminal action, suit, or proceeding as they are incurred and in advance of final disposition thereof, upon determination by the stockholders, the disinterested board members, or by independent legal counsel. If so provided in the corporation’s articles of incorporation, bylaws, or other agreement, Section 78.751 of the NRS requires a corporation to advance expenses as incurred upon receipt of an undertaking by or on behalf of the officer or director to repay the amount if it is ultimately determined by a court of competent jurisdiction that such officer or director is not entitled to be indemnified by the company. Section 78.751 of the NRS further permits the company to grant its directors and officers’ additional rights of indemnification under its articles of incorporation, bylaws, or other agreement.
Section 78.752 of the NRS provides that a Nevada company may purchase and maintain insurance or make other financial arrangements on behalf of any person who is or was a director, officer, employee, or agent of the company, or is or was serving at the request of the company as a director, officer, employee, or agent of another company, partnership, joint venture, trust, or other enterprise, for any liability asserted against him and liability and expenses incurred by him in his capacity as a director, officer, employee, or agent, or arising out of his status as such, whether or not the company has the authority to indemnify him against such liability and expenses.
Our articles of incorporation provide for indemnification of our officers and directors to the fullest extent permissible under Nevada General Corporation Law, in accordance with the Company’s Bylaws. Our Bylaws provide for indemnification of our officers and directors to the fullest extent not prohibited by the Nevada; provided however, that we may modify the extent of such indemnification by individual contracts with its directors and officers; and provided, further, that we shall not be required to indemnify any director or officer in connection with any proceeding (or part thereof) initiated by such person unless (i) such indemnification is expressly required to be made by law; (ii) the proceeding was authorized by the board of directors; (iii) such indemnification is provided by the Company, in its sole discretion, pursuant to the powers vested in the corporation under the Nevada General Corporation Law or; (iv) such indemnification is a result of the enforcement of a contractual right.
See “Item 17. Undertakings” for a description of the SEC’s position regarding such indemnification provisions.
Item 15. Recent Sales of Unregistered Securities.
Set forth below is information regarding shares of capital stock issued by us within the past three years. Also included is the consideration received by us for such shares and information relating to the section of the Securities Act, or rule of the SEC, under which exemption from registration was claimed.
The following is a list of unregistered sales of our equity securities during the prior three years.
On March 31, 2022, we converted 227 shares of Series C Convertible preferred stock into 15,167 shares of common stock. The issuance was exempt under Section 3(a)(9) and/or Section 4(a)(2) of the Securities Act of 1933, as amended.
On August 29, 2022, we entered into a one-year consulting agreement with an entity for investor relations services. In connection with this consulting agreement, we issued 20,000 restricted common shares to the consultant. These shares vest immediately. These shares were valued at $135,100, or $6.755 per common share, based on contemporaneous common share sales by the Company.
On December 29, 2021 and effective January 1, 2022, the Board granted an aggregate of 6,849 incentive stock options under the 2020 Plan, to two non-employee board members, exercisable at $7.30 per share which expire on December 26, 2026 and vest on the first anniversary date of the grant date.
On January 27, 2022, pursuant to an Employment Agreement, an aggregate of 16,000 incentive stock options were issued under the 2020 Plan, to Dr. Kou, exercisable at $10.00 per share and expires on January 31, 2032.
On June 6, 2024, we issued common stock purchase warrants to purchase 917,432 shares of common stock with an exercise price of $2.06 per share in a private placement to certain institutional investors.
On June 6, 2024, we issued common stock purchase warrants to purchase 68,807 shares of common stock at an exercise price of $2.725 to designees of H.C. Wainwright & Co, LLC, as placement agent.
Unless otherwise indicated, the foregoing securities were offered and issued in reliance on the exemption from registration requirements under the Securities Act afforded by Section 4(a)(2) thereof and/or Rule 506 of Regulation D promulgated thereunder.
Item 16. Exhibits.
The list of exhibits in the Exhibit Index to this registration statement is incorporated herein by reference.
Item 17. Undertakings.
The undersigned registrant hereby undertakes:
| (1) | To file, during any period in which offers or sales are being made, a post-effective amendment to this registration statement: |
| (i) | To include any prospectus required by Section 10(a)(3) of the Securities Act of 1933, as amended; |
| (ii) | To reflect in the prospectus any facts or events arising after the effective date of this registration statement (or the most recent post-effective amendment thereof) which, individually or in the aggregate, represent a fundamental change in the information set forth in this registration statement. Notwithstanding the foregoing, any increase or decrease in the volume of securities offered (if the total dollar value of the securities offered would not exceed that which was registered) and any deviation from the low or high end of the estimated maximum offering range may be reflected in the form of prospectus filed with the Securities and Exchange Commission pursuant to Rule 424(b) if, in the aggregate, the changes in volume and price represent no more than a 20% change in the maximum aggregate offering price set forth in the “Calculation of Registration Fee” table in the effective registration statement; and |
| (iii) | To include any material information with respect to the plan of distribution not previously disclosed in this registration statement or any material change to such information in this registration statement; |
provided, however, that the undertakings set forth in paragraphs (1)(i), (1)(ii) and (1)(iii) above do not apply if the information required to be included in a post-effective amendment by those paragraphs is contained in reports filed with or furnished to the Securities and Exchange Commission by the registrant pursuant to Section 13 or Section 15(d) of the Securities Exchange Act of 1934, as amended, that are incorporated by reference in this registration statement or is contained in a form of prospectus filed pursuant to Rule 424(b) that is part of this registration statement;
| (2) | That, for the purpose of determining any liability under the Securities Act of 1933, as amended, each such post-effective amendment shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof; |
| (3) | To remove from registration by means of a post-effective amendment any of the securities being registered which remain unsold at the termination of the offering; |
| (4) | That, for the purpose of determining liability under the Securities Act of 1933, as amended, to any purchaser: |
| (i) | Each prospectus filed by the registrant pursuant to Rule 424 (b)(3) shall be deemed to be part of this registration statement as of the date the filed prospectus was deemed part of and included in this registration statement; and |
| (ii) | Each prospectus required to be filed pursuant to Rule 424 (b)(2), (b)(5), or (b)(7) as part of a registration statement in reliance on Rule 430B relating to an offering made pursuant to Rule 415(a)(l)(i), (vii) or (x) for the purpose of providing the information required by Section 10(a) of the Securities Act of 1933, as amended, shall be deemed to be part of and included in the registration statement as of the earlier of the date such prospectus is first used after effectiveness or the date of the first contract of sale of securities in the offering described in the prospectus. As provided in Rule 430B, for liability purposes of the issuer and any person that is at that date an underwriter, such date shall be deemed to be a new effective date of the registration statement relating to the securities in the registration statement to which that prospectus relates, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof; provided, however, that no statement made in a registration statement or prospectus that is part of the registration statement or made in a document incorporated or deemed incorporated by reference into the registration statement or prospectus that is part of the registration statement will, as to a purchaser with a time of contract of sale prior to such effective date, supersede or modify any statement that was made in the registration statement or prospectus that was part of the registration statement or made in any such document immediately prior to such effective date; |
| (5) | That, for the purpose of determining liability of the registrant under the Securities Act of 1933, as amended, to any purchaser in the initial distribution of the securities, the undersigned registrant undertakes that in a primary offering of securities of the undersigned registrant pursuant to this registration statement, regardless of the underwriting method used to sell the securities to the purchaser, if the securities are offered or sold to such purchaser by means of any of the following communications, the undersigned registrant will be a seller to the purchaser and will be considered to offer or sell such securities to such purchaser: |
| | (i) | Any preliminary prospectus or prospectus of the undersigned registrant relating to the offering required to be filed pursuant to Rule 424; |
| | (ii) | Any free writing prospectus relating to the offering prepared by or on behalf of the undersigned registrant or used or referred to by the undersigned registrant; |
| | (iii) | The portion of any other free writing prospectus relating to the offering containing material information about the undersigned registrant or its securities provided by or on behalf of the undersigned registrant; and |
| | (iv) | Any other communication that is an offer in the offering made by the undersigned registrant to the purchaser; |
| (6) | That, for purposes of determining any liability under the Securities Act of 1933, as amended, each filing of the registrant’s annual report pursuant to Section 13(a) or Section 15(d) of the Securities Exchange Act of 1934, as amended (and, where applicable, each filing of an employee benefit plan’s annual report pursuant to Section 15(d) of the Securities Exchange Act of 1934, as amended) that is incorporated by reference in the registration statement shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof; |
| (7) | Insofar as indemnification for liabilities arising under the Securities Act of 1933, as amended, may be permitted to directors, officers and controlling persons of the registrant pursuant to the foregoing provisions, or otherwise, the registrant has been advised that in the opinion of the Securities and Exchange Commission such indemnification is against public policy as expressed in the Securities Act of 1933, as amended, and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities (other than the payment by the registrant of expenses incurred or paid by a director, officer or controlling person of the registrant in the successful defense of any action, suit or proceeding) is asserted by such director, officer or controlling person in connection with the securities being registered, the registrant will, unless in the opinion of its counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question whether such indemnification by it is against public policy as expressed in the Securities Act of 1933, as amended, and will be governed by the final adjudication of such issue. |
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, as amended, the registrant has duly caused this registration statement to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of Sarasota, State of Florida, on July 17, 2024.
| | SILO PHARMA, INC. |
| | | |
| | By: | /s/ Eric Weisblum |
| | | Eric Weisblum |
| | | Chief Executive Officer and Chairman |
I, the undersigned officers and directors of Silo Pharma, Inc., hereby severally and appoint Eric Weisblum and Daniel Ryweck, and each of them singly (with full power to each of them to act alone), our true and lawful attorneys-in-fact and agents, with full power of substitution and re-substitution in each of them for him or her and in his or her name, place and stead, and in any and all capacities, to sign any and all amendments (including post-effective amendments) to this registration statement (or any other registration statement for the same offering that is to be effective upon filing pursuant to Rule 462(b) under the Securities Act of 1933), and to file the same, with all exhibits thereto and other documents in connection therewith, with the Securities and Exchange Commission, granting unto said attorneys-in-fact and agents, and each of them, full power and authority to do and perform each and every act and thing requisite or necessary to be done in and about the premises, as full to all intents and purposes as he might or could do in person, hereby ratifying and confirming all that said attorneys-in-fact and agents or any of them, or their or his substitute or substitutes, may lawfully do or cause to be done by virtue hereof.
Pursuant to the requirements of the Securities Act of 1933, this Registration Statement has been signed by the following persons in the capacities held on the dates indicated.
| Signature | | Title | | Date |
| | | | | |
| /s/ Eric Weisblum | | Chairman and Chief Executive Officer | | July 17, 2024 |
| Eric Weisblum | | (principal executive officer) | | |
| | | | | |
| /s/ Daniel Ryweck | | Chief Financial Officer | | July 17, 2024 |
| Daniel Ryweck | | (principal financial officer) | | |
| | | | | |
| /s/ Wayne Linsley | | Director | | July 17, 2024 |
| Wayne Linsley | | | | |
| | | | | |
| /s/ Dr. Kevin Muñoz | | Director | | July 17, 2024 |
| Dr. Kevin Muñoz | | | | |
| | | | | |
| /s/ Jeff Pavell | | Director | | July 17, 2024 |
| Jeff Pavell | | | | |
EXHIBIT INDEX
| Exhibits | | Description |
| 2.1 | | Plan of Conversion dated December 19, 2023, filed as Exhibit 2.1 to the Company’s Current Report on Form 8-K, filed with the Commission on December 20, 2023 and incorporated herein by reference |
| 3.1 | | Articles of Incorporation of Silo Pharma, Inc., a Nevada corporation, filed as an Exhibit 3.3 to the Company’s Current Report on Form 8-K, filed with the Commission on December 20, 2023 and incorporated herein by reference. |
| 3.2 | | Bylaws of Silo Pharma, Inc., a Nevada corporation, filed as an Exhibit 3.4 to the Company’s Current Report on Form 8-K, filed with the Commission on December 20, 2023 and incorporated herein by reference. |
| 3.3 | | Articles of Conversion filed with the Nevada Secretary of State on December 19, 2023, filed as an Exhibit 3.1 to the Company’s Current Report on Form 8-K, filed with the Commission on December 20, 2023 and incorporated herein by reference. |
| 3.4 | | Certificate of Conversion filed with the Delaware Secretary of State on December 19, 2023, filed as an Exhibit 3.2 to the Company’s Current Report on Form 8-K, filed with the Commission on December 20, 2023 and incorporated herein by reference. |
| 4.1 | | Description of the Registrant’s Securities, filed as Exhibit 4.1 to the Company’s Annual Report on Form 10-K for the year ended December 31, 2023 filed with the Commission on March 25, 2024 and incorporated herein by reference. |
| 4.2 | | Form of Representative’s Warrant, filed as Exhibit 4.1 to the Company’s Current Report on Form 8-K filed with the SEC on September 30, 2022 and incorporated herein by reference. |
| 4.3 | | Form of Warrant, filed as Exhibit 4.2 to the Company’s Current Report on Form 8-K filed with the SEC on June 6, 2024 and incorporated herein by reference. |
| 4.4 | | Form of Placement Agent Warrant, filed as Exhibit 4.3 to the Company’s Current Report on Form 8-K filed with the SEC on June 6, 2024 and incorporated herein by reference. |
| 5.1* | | Opinion of Sheppard Mullin Richter & Hampton LLP |
| 10.1 | | Stock Purchase Agreement dated April 24, 2013 between Point Capital, Inc. and Alpha Capital Anstalt, filed as an exhibit to the Current Report on Form 8-K, filed with the Commission on April 30, 2013 and incorporated herein by reference. |
| 10.2 | | Corrected Asset Purchase Agreement with Blind Faith Concepts Holdings, Inc. dated September 28, 2018, filed as an exhibit to the Current Report on Form 8-K, filed with the Commission on December 20, 2018 and incorporated herein by reference. |
| 10.3 | | Form of Return to Treasury Agreement, filed as an exhibit to the Current Report on Form 8-K, filed with the Commission on December 20, 2018 and incorporated herein by reference. |
| 10.4 | | Form of Securities Purchase Agreement, dated October 2019, between Uppercut Brands, Inc., and Investors, filed as an exhibit to the Annual Report on Form 10-K filed with the Commission on March 20, 2020 and incorporated herein by reference. |
| 10.5 | | Form of convertible note agreement with Investors dated October 2019, filed as an exhibit to the Annual Report on Form 10-K filed with the Commission on March 20, 2020 and incorporated herein by reference. |
| 10.6 | | Form of Warrant, dated October 2019, filed as an exhibit to the Quarterly Report on Form 10-Q filed with the Commission on November 13, 2019 and incorporated herein by reference. |
| 10.7 | | Form of Securities Purchase Agreement for the purchase of Series B preferred shares, dated November 2019, between Uppercut Brands, Inc., and Investors, filed as an exhibit to the Annual Report on Form 10-K filed with the Commission on March 20, 2020 and incorporated herein by reference. |
| 10.8 | | Form of Warrant related to Series B preferred shares, dated November 2019, between Uppercut Brands, Inc., and Investors, filed as an exhibit to the Annual Report on Form 10-K, filed with the Commission on March 20, 2020 and incorporated herein by reference. |
| 10.9 | | Form of registration rights agreement related to Series B preferred shares, dated November 2019, between Uppercut Brands, Inc., and Investors, filed as an exhibit to the Annual Report on Form 10-K filed with the Commission on March 20, 2020 and incorporated herein by reference. |
| 10.10 | | Form of Exchange Agreement for Convertible Notes, dated as of April 15, 2020, filed as an exhibit to the Current Report on Form 8-K/A, filed with the Commission on April 22, 2020 and incorporated herein by reference. |
| 10.11 | | Form of Exchange Agreement for Series B Preferred Stock, dated as of April 15, 2020, filed as an exhibit to the Current Report on Form 8-K/A, filed with the Commission on April 22, 2020 and incorporated herein by reference. |
| 10.12 | | Form of Subscription Agreement, dated as of April 17, 2020, filed as an exhibit to the Current Report on Form 8-K/A, filed with the Commission on April 22, 2020 and incorporated herein by reference. |
| 10.13 | | Form of Consulting Agreement, dated as of April 17, 2020, filed as an exhibit to the Current Report on Form 8-K/A, filed with the Commission on April 22, 2020 and incorporated herein by reference. |
| 10.14 | | Form of Advisory Agreement, dated as of April 17, 2020, filed as an exhibit to the Current Report on Form 8-K/A, filed with the Commission on April 22, 2020 and incorporated herein by reference. |
| 10.15+ | | Employment Agreement by and between the Company and Eric Weisblum, dated as of April 17, 2020, filed as an exhibit to the Current Report on Form 8-K/A, filed with the Commission on April 22, 2020 and incorporated herein by reference. |
| 10.16 | | Form of Securities Purchase Agreement, dated as of April 28, 2020, filed as an exhibit to the Current Report on Form 8-K, filed with the Commission on April 28, 2020 and incorporated herein by reference. |
| 10.17 | | Form of Registration Rights Agreement, dated as of April 28, 2020, filed as an exhibit to the Current Report on Form 8-K, filed with the Commission on April 28, 2020 and incorporated herein by reference. |
| 10.18 | | Form of Lock-Up Agreement, dated as of April 28, 2020, filed as an exhibit to the Current Report on Form 8-K, filed with the Commission on April 28, 2020 and incorporated herein by reference. |
| 10.19 | | Patent License Agreement by and among the Company and Silo Pharma, Inc., a Florida corporation and their affiliates and subsidiaries and AIkido Pharma Inc., filed as an exhibit to the Current Report on Form 8-K filed with the Commission on January 11, 2021 and incorporated herein by reference. |
| 10.20 | | Sponsored Research Agreement by and between the Company and the University of Maryland, Baltimore, filed as an exhibit to the Current Report on Form 8-K filed with the Commission on January 11, 2021 and incorporated herein by reference. |
| 10.21+ | | Silo Pharma, Inc. 2020 Omnibus Equity Incentive Plan, filed as an exhibit to the Current Report on Form 8-K filed with the Commission on January 28, 2021 and incorporated herein by reference. |
| 10.22+ | | First Amendment to Employment Agreement, dated January 18, 2021, by and between the Company and Eric Weisblum, filed as an exhibit to the Current Report on Form 8-K filed with the Commission on January 28, 2021 and incorporated herein by reference. |
| 10.23 | | Form of Securities Purchase Agreement, dated as of February 9, 2021, between Silo Pharma, Inc. and the signatories thereto (Incorporated by reference to Exhibit 10.1 to the Company’s Current Report on Form 8-K filed on February 10, 2021) |
| 10.24 | | Form of Warrant (Incorporated by reference to Exhibit 10.2 to the Company’s Current Report on Form 8-K filed on February 10, 2021) |
| 10.25 | | Form of Registration Rights Agreement, dated as of February 9, 2021, between Silo Pharma, Inc. and the signatories thereto (Incorporated by reference to Exhibit 10.3 to the Company’s Current Report on Form 8-K filed on February 10, 2021) |
| 10.26 | | Form of Lock-Up Agreement, dated as of February 9, 2021 (Incorporated by reference to Exhibit 10.4 to the Company’s Current Report on Form 8-K filed on February 10, 2021) |
| 10.27 | | Form of Placement Agent Warrant (Incorporated by reference to Exhibit 10.5 to the Company’s Current Report on Form 8-K/A filed on February 12, 2021) |
| 10.28# | | Master License Agreement, dated February 12, 2021, by and between the Company and the University of Maryland, Baltimore (Incorporated by reference to Exhibit 10.1 to the Company’s Current Report on Form 8-K filed on February 16, 2021) |
| 10.29# | | Letter of Intent, dated February 12, 2021, by and between the Company and Aikido Pharma, Inc. (Incorporated by reference to Exhibit 10.2 to the Company’s Current Report on Form 8-K filed on February 16, 2021) |
| 10.30 | | Patent License Agreement by and among the Company and Silo Pharma, Inc., a Florida corporation and their affiliates and subsidiaries and Aikido Pharma Inc. (Incorporated by reference to Exhibit 10.1 to the Company’s Current Report on Form 8-K filed with the SEC on January 11, 2021). |
| 10.31 | | Sponsored Research Agreement by and between the Company and the University of Maryland, Baltimore (Incorporated by reference to Exhibit 10.1 to the Company’s Current Report on Form 8-K filed with the SEC on January 11, 2021). |
| 10.32 | | Underwriting Agreement by and between the Company and Laidlaw & Company (UK) Ltd., as representative of the several underwriters named therein, dated September 26, 2022. (Incorporated by reference to Exhibit 1.1 to the Company’s Current Report on Form 8-K filed on September 30, 2022.) |
| 10.33 | | Representative’s Warrant, dated as of September 29, 2022. (Incorporated by reference to Exhibit 4.1 to the Company’s Current Report on Form 8-K filed on September 30, 2022.) |
| 10.34 | | Ryweck Employment Agreement, dated September 28, 2022. (Incorporated by reference to Exhibit 10.1 to the Company’s Current Report on Form 8-K filed on September 30, 2022.) |
| 10.35 | | Form of First Amendment to Sponsored Research Agreement by and between the Company and Columbia University. (Incorporated by reference to Exhibit 10.1 to the Company’s Current Report om Form 8-K filed with the SEC on October 18, 2022). |
| 10.36 | | Employment Agreement by and between the Company and Eric Weisblum, dated October 12, 2022. (Incorporated by reference to Exhibit 10.2 to the Company’s Current Report on Form 8-K filed with the SEC on October 18, 2022). |
| 10.37 | | First Amendment to Employment Agreement by and between the Company and Daniel Ryweck, dated October 12, 2022. (Incorporated by reference to Exhibit 10.3 to the Company’s Current Report on Form 8-K filed with the SEC on October 18, 2022). |
| 10.38 | | Silo Pharma, Inc. Amended and Restated 2020 Omnibus Equity Incentive Plan, filed as Appendix A to the Company Definitive Proxy Statement on Schedule 14A filed with the SEC on October 23, 2023 and incorporated herein by reference. |
| 10.39 | | Form of Securities Purchase Agreement, filed as Exhibit 10.1 to the Company’s Current Report on Form 8-K filed with the SEC on June 6, 2024 and incorporated herein by reference. |
| 10.40 | | Form of Lock-Up Agreement, filed as Exhibit 10.2 to the Company’s Current Report on Form 8-K filed with the SEC on June 6, 2024 and incorporated herein by reference. |
| 10.41** | | Exclusive License Agreement dated June 28, 2024 with Columbia University, filed as Exhibit 10.1 to the Company’s Current Report on Form 8-K filed with the SEC on July 8, 2024 and incorporated herein by reference. |
| 21.1 | | Subsidiaries, filed as Exhibit 21.1 to the Company’s Annual Report on Form 10-K for the year ended December 31, 2023 filed with the Commission on March 25, 2024 and incorporated herein by reference. |
| 23.1* | | Consent of Salberg & Company, P.A. |
| 23.2* | | Consent of Sheppard Mullin Richter & Hampton LLC (included in Exhibit 5.1) |
| 24.1* | | Power of Attorney (included on signature page) |
| 107* | | Filing fee table |
| * | Filed herewith |
| ** | Certain confidential portions of this exhibit have been redacted from the publicly filed document because such portions are (i) not material and (ii) would be competitively harmful if publicly disclosed. |
| + | Indicates a management contract or any compensatory plan, contract or arrangement. |
| # | Portions of this exhibit (indicated by asterisks) have been redacted in compliance with Regulation S-K Item 601(b)(10)(iv). |


