April 20, 2012
VIA EDGAR
United States Securities
and Exchange Commission
100 F Street, NE
Mail Stop 4720
Washington, D.C. 20549
Attention: Jeffrey P. Riedler
Assistant Director
Re: Caldera Pharmaceuticals, Inc.
Registration Statement on Form S-1
Filed February 14, 2012
File No. 333-179508
Dear Mr. Riedler:
Thank you for your March 8, 2012 letter regarding Caldera Pharmaceuticals, Inc. (“Caldera”). In order to assist you in your review of Caldera’s Form S-1, we hereby submit a letter responding to the comments and Amendment No. 1 to Form S-1 marked to show changes. For your convenience, we have set forth below the staff’s numbered comments in their entirety followed by our responses thereto.
Registration Statement on Form S-1
General
1. Please note that where we provide examples to illustrate what we mean by our comments, they are examples and not complete lists. If our comments are applicable to portions of the filing that we have not cited as examples, please make the appropriate changes in accordance with our comments.
United States Securities
and Exchange Commission
April 20, 2012
Page 2
Response: Complied with. We have made appropriate changes in accordance with your comments throughout the filing.
2. Please provide the disclosure required by Item 505(a) of Regulation S-K regarding the determination of offering price.
Response: Complied with. We have added an additional section to the filing under “Determination of Offering Price.”
3. Please provide the disclosure required by Item 506 of Regulation S-K regarding dilution.
Response: Complied with. We have added the disclosure required by Item 506.
Prospectus Summary, page 1
4. Please briefly describe the phrase “micro X-ray fluorescence spectroscopy” on page 1.
Response: Complied with. We have added the requested disclosure.
5. You state on page 1 that you expect your future revenue will be derived, in part, from sale or lease of new drug candidates and sale of your drug discovery instruments. Please expand your Prospectus Summary to state that you have not yet sold any drug discovery instruments, nor have you identified new drug candidates.
Response: Complied with. We have added the requested disclosure regarding the sale of drug discovery instruments and have clarified the fact that none of the new drug candidates that have been discovered have received FDA approval.
6. Please expand your prospectus summary to briefly describe your manufacturing agreement with Bruker Nano GmbH. Your description should include the name of your principal manufacturer and each parties’ material obligations.
Response: Complied with. We have added the requested disclosure.
Risk Factors, page 3
General
7. Please update the Risk Factors section to provide financial information for the fiscal year ended December 31, 2011 where appropriate.
United States Securities
and Exchange Commission
April 20, 2012
Page 3
Response: Complied with. We have updated the Risk Factors where appropriate with financial information for the fiscal year ended December 31, 2011.
8. Please add risk factor disclosure regarding the costs and obligations of becoming a public company.
Response: Complied with. We have added the requested disclosure.
9. Please add a risk factor discussing your pending legal proceedings.
Response: Complied with. We have added the requested risk factor.
“We have a history of losses and there can be no assurance . . .,” page 3
10. In this risk factor, you refer to the “problems, expenses, difficulties, complications and delays frequently encountered in connection with any emerging business operations.” Please briefly identify the different problems, expenses, difficulties, complications and delays encountered by emerging businesses, adding separate risk factors if these problems are material and not already addressed in your Risk Factors section.
Response: We have deleted the referenced language.
11. In this risk factor, you refer to various factors including the success, if any, of “expansion plans.” If you have acquisition or expansion plans, please describe those plans in the Business section.
Response: Complied with. We have clarified that our expansion plans involve the sale of our current products and services to biotech and pharmaceutical companies.
“If we cannot establish profitable operations, we will need to raise additional capital . . .,” page 3
12. Please disclose how long your existing resources will fund your operations.
Response: Complied with. We have added the requested disclosure.
“Our financial statements have been prepared assuming that the Company . . .,” page 3
13. Please add in this risk factor that the going concern opinion issued to you by your independent registered public accounting firm may affect your ability to obtain financing.
Response: Complied with. We have added the requested disclosure.
United States Securities
and Exchange Commission
April 20, 2012
Page 4
“We may not receive the benefit of the use of our net operating loss carryforward, page 4”
14. Please briefly describe net operating loss carryforward.
Response: Complied with. We have amended our disclosure in the risk factor.
15. Please quantify the amount of net operating loss carryforwards that may be subject to limitation.
Response: Complied with. We have added the requested disclosure in the risk factor.
16. Please briefly describe why you may be subject to Section 382 limitations and therefore may not receive the benefit of net operating loss carryforwards in your financial statements.
Response: Complied with. We have added the requested disclosure in the risk factor.
17. Please clearly state in this risk factor that any limitation on your ability to use your net operating loss carryforwards to offset United States federal taxable income could potentially result in increased future tax liability to you.
Response: Complied with. We have added the requested disclosure in the risk factor.
“There is uncertainty as to market acceptance of our technology and products,” page 4
18. On page 4, you discuss possible delay in releasing products or providing services to third parties. Please expand your discussion to indicate when you plan to begin providing services to third parties.
Response: Complied with. We have added language to clarify that we are currently providing such services.
United States Securities
and Exchange Commission
April 20, 2012
Page 5
“We rely heavily on a single source for a major part of our product, . . .,” page 4
19. On page 4, you list several risks relevant to your reliance upon a sole supplier. If Bruker Nano GmnH has previously failed to supply you with an adequate supply of required parts or failed to timely deliver materials, please so disclose.
Response: Complied with. We have added language to clarify that to date we have not experienced any such problem with Bruker; however we have not yet placed any orders with Bruker.
“We must expend a significant amount of time and resources to develop new products, . . .,” page 5
20. Please expand this risk factor to quantify the amount of time you have devoted to the development of your XRpro instruments.
Response: Complied with. We have added the requested disclosure.
21. Please expand this risk factor to disclose the amount spent on research and development in 2011.
Response: Complied with. We have added the requested disclosure.
22. Please describe the development progress of your new products.
Response: Complied with. We have added language clarifying that we do not anticipate developing new products unless modifications to existing products are requested by customers.
“Our Chief Executive Officer beneficially owns and controls . . .,” page 5
23. Please disclose the percent of outstanding shares held by the Chief Executive Officer as of the latest practicable date.
Response: Complied with. We have added the requested disclosure.
24. You state on page 5 that certain matters require shareholder approval. Please expand this risk factor to identify whether the matters described require a majority vote by shareholders to be approved, etc.
Response: Complied with. We have added the requested disclosure.
United States Securities
and Exchange Commission
April 20, 2012
Page 6
“If we deliver products with defects, our credibility will be harmed . . .,” page 5
25. If applicable, please describe instances in which material resources, etc. were expended to correct the problems referred to in this risk factor.
Response: Complied with. We have added language to clarify that we have not yet had any such instances.
“Most of our potential customers are from the pharmaceutical . . .,” page 5
26. Please revise the title of this risk factor. In your Prospectus Summary, you indicate that substantially all of your revenue is derived from sales to two government agencies, although you plan to sell products and services to third parties in the future.
Response: We have revised the title, please note that the pharmaceutical sector includes the government.
“We will need to depend on credit terms and lines of credit . . .,” page 6
27. If you have a line of credit with Bruker Nano GmnH, please describe the material terms of this credit line in an appropriate place in your filing.
Response: Complied with. We have added language to clarify that we do not have any such line of credit with Bruker.
“We depend on our key personnel, the loss of whom would impair . . .,” page 6
28. If you have experienced difficulty hiring qualified employees in the past, please so disclose.
Response: Complied with. We have deleted the language regarding difficulty hiring employees as the Company has not experienced any such difficulty to date.
“We have initiated and may in the future need to initiate lawsuits . . .,” page 7
29. Please expand this risk factor to quantify how much you have spent on litigation, if material.
Response: Complied with. We have added the requested disclosure.
30. Please expand this risk factor to describe how the loss of intellectual property that may be the subject of such pending litigation could affect you.
United States Securities
and Exchange Commission
April 20, 2012
Page 7
Response: We have clarified in previous risk factors that the risk associated with the litigation is a loss of compensation for a lost opportunity and not the loss of intellectual property.
“We may be subject to the risks of doing business internationally,” page 8
31. Please expand this risk factor to describe when you plan to offer your products outside of the United States and when you intend to manufacture products at Bruker’s facility in Germany.
Response: Complied with. We have clarified that we are currently offering the products outside the United States and intend to manufacture products in the Bruker facility upon receipt of orders.
32. Given your current relationship with the German manufacturer Bruker and your future intentions, please consider whether you would like to include separate risk factors for the bullet points listed in your next amendment. To the extent that you have been materially impacted by any of the bulleted points, you should specifically describe these instances instead of providing a generic reference.
Response: We have deleted certain bulleted points that we believe are not significant risks; however, although the remaining bulleted points are risks that we face, we do not believe that the bulleted points require their own risk factors.
“We have no independent audit committee nor do we have an audit . . .,” page 10
33. In this risk factor, and on page 26 under the heading “Director Independence,” you refer to the independence requirements of Rule 10A-3 of the Exchange Act. However, this rule directs the national securities exchanges and national securities associations to prohibit the listing of any security of an issuer that is not in compliance with the audit committee requirements mandated by the Sarbanes-Oxley Act of 2002. Given that your common stock is not yet listed on a national exchange, Rule 10A-3 is not appropriate. Please remove all references to Rule 10A-3. Guidance regarding the appropriate standard for director independence is provided by Item 407(a) of Regulation S-K. When referring to the dependence of your directors in this risk factor and on page 26, please provide the disclosure required by Item 407(a)(1)(ii) of Regulation S-K.
Response: Complied with. We have revised the disclosure as requested.
United States Securities
and Exchange Commission
April 20, 2012
Page 8
34. In addition, please consider whether the risk factor heading and discussion should be revised to indicate the effect of your lack of an independent audit committee and audit committee financial expert on your ability to obtain a listing.
Response: Complied with. We have revised the heading.
“Our board of directors acts as our compensation committee . . .,” page 10
35. Please describe the fiduciary obligations owed by board members with respect to compensation of executive officers.
Response: Please see the response to comment number 36 below.
36. In addition, please advise us as to your basis for the statement that all directors owe fiduciary duties with respect to compensation matters. Otherwise, please revise your disclosure in the risk factor to delete any reference to an existing fiduciary duty with respect to compensation matters.
Response: Complied with. We have deleted the reference to fiduciary duty.
“The rights of our preferred stock could negatively affect holders of common stock . . .,” page 11
37. Please expand this risk factor to disclose the number of preferred stock authorized for issuance.
Response: Complied with. We have added the requested information.
“We may not be able to attract the attention of major brokerage firms . . .,” page 12
38. This risk factor appears to address a risk substantially similar to the risk discussed in the risk factor entitled “If equity research analysts do not publish research or reports about our business . . .” on page 9. Please revise your prospectus to combine these two risk factors.
Response: Complied with. We have combined the two risk factors.
“Future sales of our equity securities could put downward selling pressure . . .,” page 12
39. This risk factor appears to address a risk substantially similar to the risk discussed in the risk factor entitled “Future sales of our common stock by existing shareholders could cause our stock price to decline” on page 9. Please revise your prospectus to combine these two risk factors.
United States Securities
and Exchange Commission
April 20, 2012
Page 9
Response: Complied with. We have combined the two risk factors.
Use of Proceeds, page 12
40. Please expand your discussion to address how the proceeds from the exercise of warrants will be allocated.
Response: Complies with. We have added the requested information.
Business, page 12
General
41. Please confirm that you have no disclosure to make under Item 101(h)(4)(viii)-(iv) of Regulation S-K regarding government approval and government regulation.
Response: We confirm that the company does not require any government approval to conduct its operations and it is not subject to any governmental regulation for the conduct of its day-to-day operations.
History, page 12
42. Please provide more information regarding Los Alamos National Laboratory. This information should include a description of its business, identification of its owner(s), and a discussion of its relationship, if any, with the United States government.
Response: Complied with. We have added the requested information.
Our Current and Future Business, page 13
43. You state on page 13 that substantially all of your revenue to date has been derived from analytical services performed for United States governmental agencies. Please expand your disclosure to discuss the material terms of your agreements with these agencies. Your disclosure in the Business section should include the following information:
The name of each party to each agreement;
The material obligations of each party, including any financial obligations;
The nature of the drug discovery or analytical services you currently provide to the U.S. government;
United States Securities
and Exchange Commission
April 20, 2012
Page 10
A description of the royalty rates payable under the agreement, expressed within a ten percent range (for example, “single digits,” “teens”, “twenties,” etc), if any;
The potential aggregate milestones payable, if any; The amounts paid to date;
The rights as to each party regarding the drug candidates identified;
The term of the agreement; and
The termination provisions of the agreement.
In addition, please file all agreements with the government agencies as exhibits to your Form S-1, or provide us with a legal analysis as to why these agreements need not be filed pursuant to Item 601(b)(10)(2)(ii) of Regulation S-K.
Response: Complied with. We have provided the requested disclosure for all current agreements and have added such agreements as exhibits.
44. Please refer to the statement, “We also anticipate deriving revenue from the development of drugs that are presented to us when conducting our chemical analysis.” Please clarify how you anticipate deriving revenue from this.
Response: Complied with. We have further clarified the statement.
XRpro Drug Discovery Instruments, page 13
45. You state on page 13 that you “have not sold any XRpro instruments” and that you have “not shipped XRpro instruments to the private sector.” Please clarify whether your instruments have been leased to third parties, as opposed to sold, and whether any XRpro instruments have been shipped to the government sector.
Response: Complied with. We have clarified that the instruments have not been leased to third parties.
46. You indicate that you have begun initial marketing of the XRpro instrument. Please expand your Business section to describe your marketing capabilities. If your marketing capabilities are limited, please add a risk factor discussing this limitation.
Response: Complied with. We have added language regarding our limited marketing capabilities in this section and in the second risk factor.
United States Securities
and Exchange Commission
April 20, 2012
Page 11
New Drug Candidates, page 13
47. Please clarify the introductory sentence, “We are developing new drug candidates, primarily paid for by Federal contracts.” It appears from disclosure elsewhere in your prospectus that you evaluate drug candidates for safety and efficacy. The molecules you develop, as described on page 13, do not appear to be drug candidates, but rather assist in the analytical services you provide to your clients.
Response: Complied with. We have further clarified the statement.
48. You state on page 13 that you obtained a $3,000,000 grant in August 2011. Please identify the party from whom you received the grant, and describe the nature of the drug candidate for which you received the grant.
Response: Complied with. We have added the requested disclosure.
Our Analysis Technology, page 14
49. You state on page 14 that the use of a reagent in the analysis process, for a typical high-throughput campaign, can typically cost up to $0.50 per compound. Please provide a source for this information.
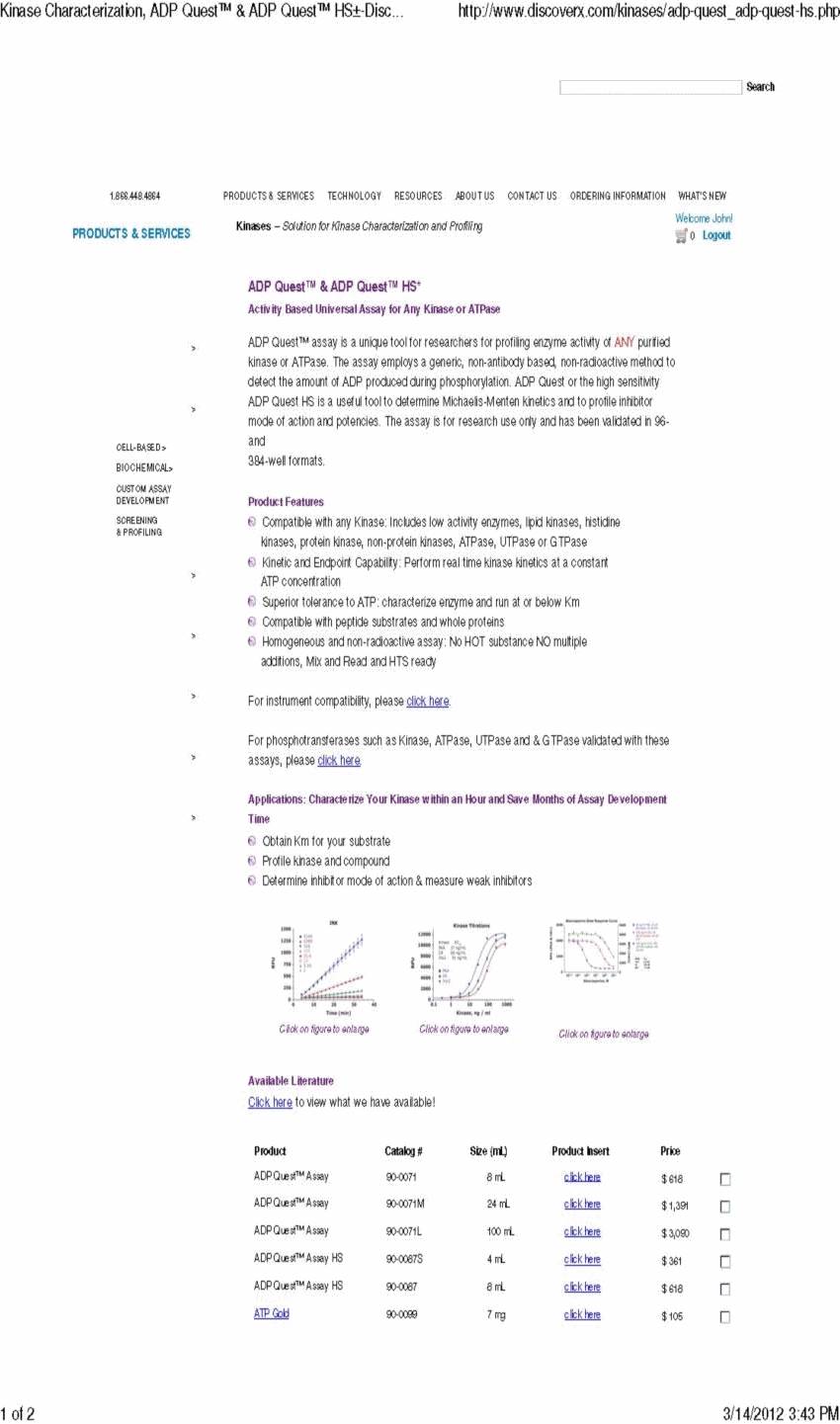
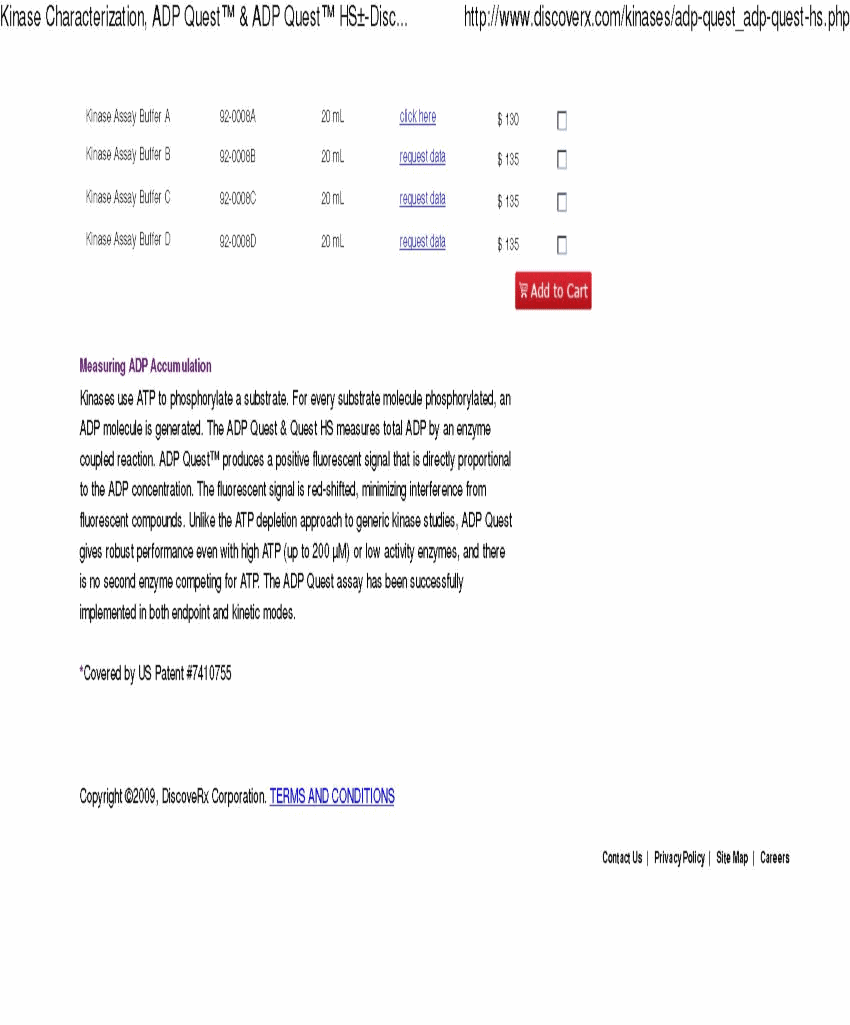
Response: Complied with. We have revised the disclosure as requested. In addition, we have included with this letter a page from the DiscoveRx Corporation sales catalog that can be found on its website as well as a page from the Fisher Scientific sales catalog that can be found on its website, each of which contain the pricing information of reagents.
Intellectual Property Rights, page 15
50. Please provide a range of expiration dates for all material patents in the patent portfolio that is the subject of the license agreement with Los Alamos National Security, LLC.
Response: Complied with. We have added the requested disclosure.
51. It is not clear whether the bulleted lists on page 16 encompass issued patents, patent applications, or both. Please revise to clearly identify all issued patents as such. In addition, please provide specific expiration dates for all issued, material patents. Please refer to Item 101(h)(4)(vii) of Regulation S-K.
United States Securities
and Exchange Commission
April 20, 2012
Page 12
Response: Complied with. We have added the requested disclosure.
52. If applicable, please expand your disclosure in this subsection to describe the U.S. government’s interest in the inventions subject to your patent applications. Please further describe the nature of such interest.
Response: Complied with. We have expanded the disclosure as requested.
53. Please clarify which, if any, of the indicated patent applications on page 16 have been granted.
Response: Complied with. We have added the requested disclosure.
54. You indicate that you entered into an agreement with The Regents of the University of California that was subsequently assigned to the Los Alamos National Security.
Please file the assignment agreement between the Regents of the University of California and Los Alamos National Security, LLC as an exhibit to your Form S-1
Please disclose, if known, the reasons for the assignment, and the consideration paid.
Response: Complied with. We have filed the assignment letter that we received and disclosed the reason stated in the letter.
Backlog, page 16
55. You disclose here that you do not have any material order backlog. However, you disclose in the last paragraph on page 19 that you expect 2012 revenues will increase due to your current back log of government contracts. You also disclose the existence of a $3 million grant in August 2011 in the last paragraph on page 13. Please revise your disclosure to provide all the information required by Item 101(c)(1)(viii) of Regulation SK.
Response: Complied with. We have changed the disclosure to clarify that we do not have backlog of orders that are not fulfilled but rather future commitments for orders.
United States Securities
and Exchange Commission
April 20, 2012
Page 13
Property, page 16
56. Please file the lease agreement related to your laboratory as an exhibit to your Form S-1 pursuant to Item 601(b)(10)(2)(iv) of Regulation S-K.
Response: Complied with. We have filed copies of our two leases and an amendment thereto as exhibits.
Legal Proceedings, page 16
57. Please update the progress of your legal proceedings as of the latest practicable date, and discuss the relief you seek for each proceeding. We note that you have not provided updated disclosure regarding the September 2011 suit against the Regents of the University of California and LANS, and the March 2010 suit against Joel Bellows and Bellows and Bellows P.C.
Response: Complied with. We have added the requested disclosure.
58. Please briefly describe the terms of the settlement entered into with Seddie Bastanipour. Please clarify whether the settlement pertains to the October 2008 litigation against the company, the December 2010 litigation by the company against the individual, or both.
Response: Complied with. Since we signed an agreement not to disclose the terms of the settlement, we have provided limited information about the settlement. We are more than willing to provide the terms of the settlement to the Commission on a confidential basis. Please note, the terms of the settlement did not have a material impact on the company or its financial position.
Equity Compensation Plans, page 17
59. Please file the 2005 Stock Option Plan as an exhibit to your Form S-1.
Response: Complied with. We filed a copy of the Stock Option Plan as Exhibit 4.3 to the company’s Registration Statement on Form S-1 filed on February 14, 2012, but inadvertently omitted to include it on the list of exhibits.
United States Securities
and Exchange Commission
April 20, 2012
Page 14
Management’s Discussion and Analysis of Financial Condition and Results of Operations, page
Liquidity and Capital Resources, page 21
60. Please expand your discussion of liquidity and capital resources to include the full fiscal years presented in the financial statements pursuant to Item 303 of Regulation S-K. For example, in your next amendment, please include a discussion of cash provided by/used in operating activities, investing activities and financing activities for the year ended December 31, 2011 compared to December 31, 2010.
Response: Complied with. We have expanded our discussion with updated information.
Directors, Executive Officers, Promoters and Control Persons, page 23
61. Please expand the biographical information of Mr. Roffman to describe his business experience from April 2006 until the present. Please refer to Item 401(e)(1) of Regulation S-K.
Response: Complied with. We have expanded Mr. Roffman’s biography.
Executive Compensation and Other Information, page 24
62. We note that certain amounts have been awarded under “All Other Compensation.” Please expand your disclosure to identify, to the extent material, the amounts included under All Other Compensation. Please refer to Item 402(o)(7) of Regulation S-K.
Response: Complied with. We have provided detail as to the compensation included in All Other Compensation.
63. You state on page 25 that the employment agreement with Dr. Warner provides that Dr. Warner is to be paid a base salary of $200,000. However, according to the Summary Compensation Table, Dr. Warner was paid less in base salary than $200,000 in both 2011 and 2010. Similarly, your discussion of “Employment Agreements” indicates that Lori Peterson was to be paid $100,000 per year pursuant to her employment agreement. However, according to the Summary Compensation Table, Ms. Peterson was paid more than $100,000 in base salary in 2011 and 2010. Please clarify why these officers’ actual compensation differed from the levels agreed upon in their employment contracts.
United States Securities
and Exchange Commission
April 20, 2012
Page 15
Response: Complied with. We have clarified that the discrepancy in Ms. Peterson’s compensation was due to increases in her salary.
64. You indicate that Dr. Warner’s employment agreement provides that he is to be paid a base salary of $200,000. However, this amount is not reflected in the employment agreement filed as Exhibit 10.1. Please clarify.
Response: We have provided a copy of Dr. Warner’s employment agreement which discloses the amount of his salary. The copy of the agreement that had been filed had been submitted to various lenders and for such purposes the salary amount had been redacted; however the agreement that has been filed as an exhibit to the amendment is the complete agreement.
Security Ownership of Certain Beneficial Owners and Management, page 26
65. Please expand your disclosure to include Mr. Warner’s address. Please refer to Item 403(a) of Regulation S-K.
Response: Complied with. We have added Mr. Warner’s address.
Selling Stockholders, page 27
66. Please identify the persons with voting and dispositive power for each selling shareholder that is not an individual.
Response: Complied with. We have identified the persons with voting and dispositive power for each selling shareholder that is not an individual.
Consolidated Interim Financial Statements, page F-1
67. Please update your financial statements as required by Rule 8-08 of Regulation S-X.
Response: Complied with. We have provided audited financial statements for the year ended December 31, 2011.
United States Securities
and Exchange Commission
April 20, 2012
Page 16
Unaudited Consolidated Balance Sheets, page F-1
68. Please explain why the number of authorized Series A Convertible Preferred Stock is disclosed on the Balance Sheet as 10,000,000 shares but is disclosed on page 31 as 500,000 shares.
Response: The number of authorized Series A Convertible Preferred stock was subsequently amended to 400,000 shares. The amount was incorrectly disclosed in the balance sheet as 10,000,000 shares, and this error has been corrected.
69. Please disclose on the face of this statement the aggregate liquidation preference of the preferred stock. Refer to ASC 505-10-50-4.
Response: Complied with. We have provided the liquidation preference of the preferred stock on the face of the balance sheet.
70. When you update your financial statements please ensure that the periods disclosed in the Common Stock line item are correct. For example, the Common Stock line item on the unaudited balance sheet currently discloses that the shares outstanding are as of December 31, 2010 and 2009 whereas it should have stated that the shares issued and outstanding are as of September 30, 2011 and December 31, 2010. Please reconcile the difference between the 4,895,620 shares issued and outstanding as of September 30, 2011 and the 4,291,620 shares issued and outstanding as of the date of this filing disclosed on page 32 and elsewhere in the filing.
Response: Complied with. The error on the balance sheet has been corrected and the number of shares reconciles to that disclosed elsewhere in the prospectus other than 10,650 shares which were issued as a dividend to preferred stockholders subsequent to the date of the financial statements.
Notes to Consolidated Financial Statements
10. Series A 8% Convertible Preferred Stock and Common Stock Purchase Warrants, page F-18
United States Securities
and Exchange Commission
April 20, 2012
Page 17
71. In the second to last paragraph of this note, you indicate that the preferred stockholders have the option to force redemption of their shares if you receive at least $3 million in proceeds from your litigation against the managers of Los Alamos National Laboratory. Please tell us why this contingency does not apparently necessitate classification of your preferred stock in mezzanine equity under ASR 268 and ASC 480-10-S99. In your response, please clarify whether you are obligated to continue to pursue litigation against Los Alamos National Laboratory. Please reference the authoritative literature you rely upon to support your classification.
Response: Complied with. The preferred stock has been reclassified as mezzanine equity under ASR268 and the disclosure in the notes to the consolidated financial statements has been amended accordingly.
Consolidated Annual Financial Statements
Report of Independent Registered Public Accounting Firm, page F-1
72. Please explain the statement on page 3 that “The report of our independent registered public accounting firm for the years ended December 31, 2010 and 2009 when originally issued included an explanatory paragraph expressing substantial doubt about our ability to continue as a going concern in their audit report included herein.” Also, on page 21 you state that the independent public accountants have included a discussion related to going concern in their opinion on the financial statements through December 31, 2010. However, the final accountant’s report issued on page F-1 does not include a going concern uncertainty. We note that you include language that is of a going concern nature in note 3 of your audited and interim financial statements. Please explain to us the consideration that your accountants made in not including such an explanatory paragraph in the final audit opinion included in this registration statement.
Response: Complied with. The going concern explanatory paragraph was unnecessary for the 2010 and 2009 periods as the financial statements were more than one year old, and the disclosure was inadvertently left in the footnotes. The Going concern explanatory paragraph has been reinstated in the audit opinion for the year ended December 31, 2011.
Consolidated Balance Sheets, page F-2
73. Please present accounts payable as a separate line item on the face of the balance sheet.
United States Securities
and Exchange Commission
April 20, 2012
Page 18
Response: Complied with. Accounts payable has been separately disclosed on the face of the balance sheet.
Notes to the Consolidated Financial Statements
2. Accounting Policies and Estimates
Concentration of Major Customers, page F-10
74. Please revise your disclosure to include the amount of revenue from each major customer as required by ASC 280-10-50-42 for each year for which financial statements are presented. The disclosure should include the amount from each of the two government agencies referenced on page 3 of the filing.
Response: Complied with. The note has been amended to disclose major revenue per customer.
Revenue Recognition, page F-11
75. Please expand your policy for the recognition of government contract revenue to clarify how your policy is in compliance with ASC 912-605-25.
Response: Complied with. The accounting policy wording has been amended to comply with ASC 912-605-25.
76. Please clarify the items that have been recorded as a reduction to revenue since the line item on the statement of operations is ‘Sales, net.’
Response: Complied with. The word "net" has been removed from Sales in the statement of operations.
4. Intangible Assets, page F-13
77. Please expand your disclosure to include the termination provisions for your Patent License Agreement with Los Alamos National Security as well as the total amount of the potential milestone payments in connection with the agreement.
United States Securities
and Exchange Commission
April 20, 2012
Page 19
Response: Complied with. The note has been amended to include the requested disclosures.
78. In the first paragraph on page F-14 you disclose that the Licensor is entitled to anti-dilution clauses which ensure that it maintains at least a 3% equity interest in your stock until you raise at least $20 million in equity financing. It is unclear from Section 3b of appendix B of your patent license agreement filed as Exhibit 10.3 whether you are obligated to issue additional equity for no consideration if the Licensor’s ownership interest falls below 3%. Please clarify for us how this clause operates. In addition, please analyze for us whether this clause should be treated as a liability under either ASC 480 or ASC 815. If not, please tell us how you would account for any equity issuance under this clause. In your response, please reference the authoritative literature you rely upon to support your position.
Response: Complied with. The licensee is obligated to issue additional shares to the licensor for no consideration if the licensor’s shareholding in the issued common stock of the company falls below 3% of the issued common stock until the threshold of $20 million of financing is reached. The company has treated this as a derivative financial liability in terms of ASC815. Please refer to the additional disclosure in note 7 to the company’s financial statements.
15. Litigation
Seddie Bastanipour and Joel Bellows Suit, page F-23
79. Please revise your disclosure to include an estimate of the possible loss or range of loss or a statement that such an estimate cannot be made for loss contingencies that are at least reasonably possible but not accrued, either because it is not probable that a loss has been incurred or the amount of loss cannot be reasonably estimated. Please refer to ASC 45020-50-3 and 50-4.
Response: Complied with. The disclosure in the footnote has been amended to state that we cannot estimate the amount of the loss, if any, at this point in time.
Item 16. Exhibits
80. Please note that Exhibit 10.3, the Exclusive Patent License Agreement between the Company and The Regents of the University of California, and Exhibit 10.6, the OEM Agreement between the Company and Bruker Nano GmbH, has been filed with redactions. However, the exhibit index does not indicate that you intended to omit certain confidential information from these exhibits pursuant to a request for confidential treatment; in addition, it does not appear that the Company has submitted an application for confidential treatment of portions of these exhibits.
United States Securities
and Exchange Commission
April 20, 2012
Page 20
Since no request for confidential treatment has been made, it appears that you have impermissibly filed partial exhibits. Please amend your Form S-1 to file Exhibits 10.3 and 10.6 in unredacted form.
If you choose to submit an application for confidential treatment for portions of these exhibits, please be advised that all comments relating to your application will need to be fully resolved before we take final action on the registration statement.
Response: Please note an application for confidential treatment has been submitted.
Signatures
81. We note that your chief executive officer has signed this Form S-1, but that the registration statement has not been signed by your chief financial officer and controller or principal accounting officer in those capacities pursuant to Instruction 1 to “Signatures” in Form S-1. Please amend your Form S-1 to have your chief financial officer and controller or principal accounting officer sign this Form S-1 in those respective capacities. In addition, if Dr. Warner is also your chief financial officer and controller or principal accounting officer please indicate beneath his signature that he is signing the Form S-1 in the capacity of chief executive officer, chief financial officer, and controller or principal accounting officer.
Response: Complied with Dr. Warner has signed the Form S-1 in his capacity as the Chief Financial Officer and the Chief Executive Officer.
* * *
We acknowledge that the adequacy and accuracy of the disclosure in our filings is our responsibility. We acknowledge that the staff comments or changes to disclosure do not foreclose the Commission from taking any action with respect to the filings. We acknowledge that the company may not assert staff comments as a defense in any proceedings initiated by the Commission or any person under the federal securities laws of the United States.
United States Securities
and Exchange Commission
April 20, 2012
Page 21
If you have any questions or need additional information, please contact the undersigned at (516) 496-2223 or (212) 907-6457.
Sincerely,
/s/ Hank Gracin
Hank Gracin
HG:ckg
Enclosures
cc: Caldera Pharmaceutcals, Inc.