Table of Contents
As filed with the Securities and Exchange Commission on June 3, 2014
Registration No. 333-195825
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
Amendment No. 1
to
FORM S-1
REGISTRATION STATEMENT
Under
The Securities Act of 1933
DERMTECH INTERNATIONAL
(Exact name of registrant as specified in its charter)
| California | 3841 | 27-2053069 | ||
(State or other jurisdiction of incorporation or organization) | (Primary Standard Industrial Classification Code Number) | (I.R.S. Employer Identification Number) |
11099 N. Torrey Pines Road, Suite 100
La Jolla, California 92037
(858) 450-4222
(Address, including zip code, and telephone number, including area code, of registrant’s principal executive offices)
John Dobak, M.D.
President and Chief Executive Officer
11099 N. Torrey Pines Road, Suite 100
La Jolla, California 92037
(858) 450-4222
(Name, address, including zip code, and telephone number, including area code, of agent for service)
Copies to:
Martin J. Waters Wilson Sonsini Goodrich & Rosati, P.C. 12235 El Camino Real, Suite 100 San Diego, California 92130 (858) 350-2300 | Steven Kemper Chief Financial Officer 11099 N. Torrey Pines Road, Suite 100 La Jolla, California 92037 (858) 450-4222 | Steven M. Skolnick Lowenstein Sandler LLP 1251 Avenue of the Americas New York, New York 10020 (212) 262-6700 |
Approximate date of commencement of proposed sale to the public: As soon as practicable after this registration statement becomes effective.
If any of the securities being registered on this Form are to be offered on a delayed or continuous basis pursuant to Rule 415 under the Securities Act of 1933 check the following box: ¨
If this Form is filed to register additional securities for an offering pursuant to Rule 462(b) under the Securities Act, please check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ¨
If this Form is a post effective amendment filed pursuant to Rule 462(c) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ¨
If this Form is a post effective amendment filed pursuant to Rule 462(d) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ¨
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See the definitions of “large accelerated filer”, “accelerated filer” and “smaller reporting company” in Rule 12b-2 of the Exchange Act. (Check one):
| Large accelerated filer | ¨ | Accelerated filer | ¨ | |||
| Non-accelerated filer | ¨ (do not check if a smaller reporting company) | Smaller reporting company | x | |||
CALCULATION OF REGISTRATION FEE
| ||||
Title of Each Class of Securities to be Registered | Proposed Maximum Aggregate Offering Price(1)(2) | Amount of Registration Fee(3) | ||
Common Stock, par value $0.0001 per share | $15,000,000 | $1,932.00 | ||
| ||||
| ||||
| (1) | Includes offering price of any additional shares that the underwriters have the option to purchase. |
| (2) | Estimated solely for the purpose of calculating the registration fee in accordance with Rule 457(o) under the Securities Act of 1933, as amended. |
| (3) | Calculated pursuant to Rule 457(o) based on an estimate of the proposed maximum aggregate offering price of the securities registered hereunder. $3,220 was previously paid by the Registrant upon the initial filing of this Registration Statement. |
The registrant hereby amends this registration statement on such date or dates as may be necessary to delay its effective date until the registrant shall file a further amendment which specifically states that this registration statement shall thereafter become effective in accordance with Section 8(a) of the Securities Act of 1933 or until the registration statement shall become effective on such date as the Securities and Exchange Commission, acting pursuant to said Section 8(a), may determine.
Table of Contents
The information in this preliminary prospectus is not complete and may be changed. These securities may not be sold until the registration statement filed with the Securities and Exchange Commission is effective. This preliminary prospectus is not an offer to sell nor does it seek an offer to buy these securities in any jurisdiction where the offer or sale is not permitted.
Subject to Completion: Dated June 3, 2014.
Shares of Common Stock

This is an initial public offering of shares of common stock of DermTech International.
We are offering shares of our common stock in the offering.
Prior to this offering, there has been no public market for our common stock. It is currently estimated that the initial public offering price per share will be between $ and $ . We intend to apply to list our common stock on The NASDAQ Capital Market under the symbol “DMTK.”
We are an “emerging growth company” as that term is used in the Jumpstart Our Business Startups Act of 2012 and, as such, have elected to comply with certain reduced public company reporting requirements for this prospectus and future filings. See “Prospectus Summary—Implications of Being an Emerging Growth Company.”
Investing in our common stock involves risks. See “Risk Factors” beginning on page 12 to read about factors you should consider before buying shares of our common stock.
Neither the Securities and Exchange Commission nor any other regulatory body has approved or disapproved of these securities or passed upon the accuracy or adequacy of this prospectus. Any representation to the contrary is a criminal offense.
| Per Share | Total | |||||||
Initial public offering price | $ | $ | ||||||
Underwriting discount (1) | $ | $ | ||||||
Proceeds, before expenses, to us | $ | $ | ||||||
| (1) | See “Underwriting” for a description of the compensation payable to the underwriters. |
The underwriters have the option to purchase up to an additional shares from us at the initial public offering price less the underwriting discount within 45 days of the date on which the shares are delivered against payment to cover overallotments, if any.
The underwriters expect to deliver the shares against payment in New York, New York on , 2014.
Sole Booking–Running Manager
Maxim Group LLC
Co-Manager
Feltl and Company
Prospectus dated , 2014.
Table of Contents
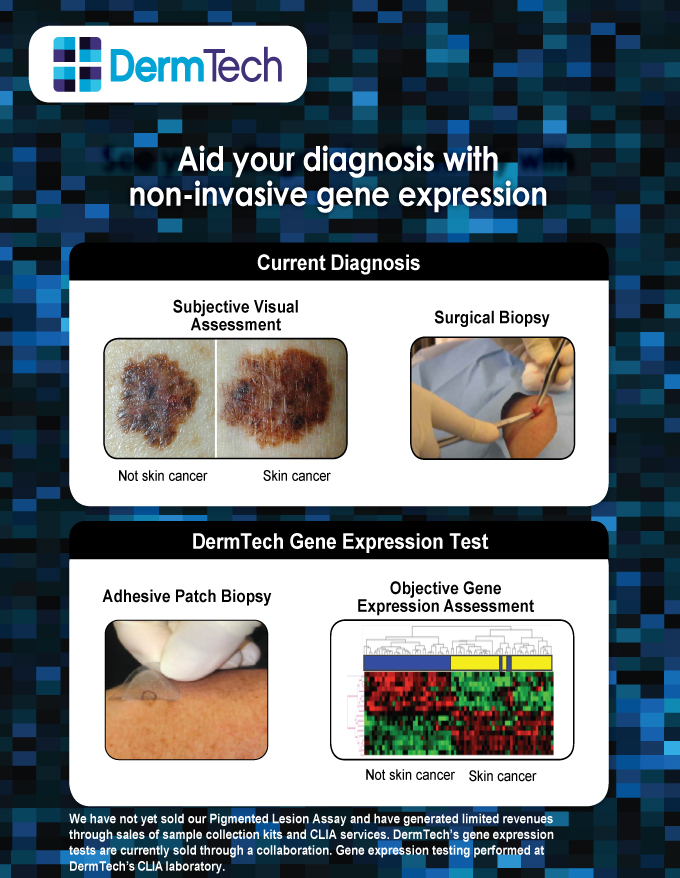
Table of Contents
| Page | ||||
| 1 | ||||
| 12 | ||||
| 47 | ||||
| 48 | ||||
| 49 | ||||
| 50 | ||||
| 52 | ||||
| 54 | ||||
Management’s Discussion and Analysis of Financial Condition and Results of Operations | 56 | |||
| 66 | ||||
| 87 | ||||
| 94 | ||||
| 104 | ||||
| 107 | ||||
| 109 | ||||
| 114 | ||||
Material U.S. Federal Income Tax and Estate Tax Consequences to Non-U.S. Holders | 117 | |||
| 121 | ||||
| 127 | ||||
| 127 | ||||
| 127 | ||||
| F-1 | ||||
Through and including , 2014 (the 25th day after the date of this prospectus), all dealers effecting transactions in these securities, whether or not participating in this offering, may be required to deliver a prospectus. This is in addition to a dealer’s obligation to deliver a prospectus when acting as an underwriter and with respect to an unsold allotment or subscription.
Neither we nor the underwriters have authorized anyone to provide any information or to make any representations other than those contained in this prospectus or in any free writing prospectuses we have prepared. We take no responsibility for, and can provide no assurance as to the reliability of, any other information that others may give you. This prospectus is an offer to sell only the shares offered hereby, but only under circumstances and in jurisdictions where it is lawful to do so. The information contained in this prospectus is current only as of its date.
For investors outside the United States: neither we nor any of the underwriters have done anything that would permit this offering or possession or distribution of this prospectus in any jurisdiction where action for that purpose is required, other than in the United States. You are required to inform yourselves about, and to observe any restrictions relating to, this offering and the distribution of this prospectus.
-i-
Table of Contents
This summary highlights selected information appearing elsewhere in this prospectus and is qualified in its entirety by the more detailed information and financial statements included elsewhere in this prospectus. This summary may not contain all the information you should consider before investing in our common stock. You should carefully read this prospectus in its entirety before investing in our common stock, including the sections titled “Risk Factors” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and our financial statements and related notes included elsewhere in this prospectus. Unless the context otherwise requires, we use the terms “DermTech,” “we,” “us,” and “our” in this prospectus to refer to DermTech International.
Business Overview
We are an emerging growth diagnostics company developing and marketing novel non-invasive gene expression tests to aid in the diagnosis of various skin conditions, including skin cancer, inflammatory diseases, and aging-related conditions. Our technology provides a highly accurate alternative to surgical biopsy, eliminating patient pain, scarring, and risk of infection, while maximizing convenience. Our scalable gene expression assays have been designed to work with a proprietary “adhesive patch biopsy” that provides a tissue sample non-invasively. Current dermatologic diagnosis is primarily based on subjective visual pattern recognition that is prone to error and results in a substantial number of unnecessary surgical biopsies.
We analyze the skin cells taken from the patient using our adhesive patch with a proprietary process that allows us to extract ribonucleic acid, or RNA, from the patch with sufficient quality and quantity to run our gene expression analysis. Our quantitative polymerase chain reaction, or qPCR, tests provide objective, biology-based information that is highly accurate and can significantly reduce unnecessary surgical biopsies. We offer our gene expression tests through our 6,000 square foot state-of-the-art government-licensed laboratory located in our company headquarters. We believe we will be the first company to offer a non-invasive gene expression test to the clinical dermatology market, which is in contrast to the vast majority of molecular diagnostic tests that are sold into the highly saturated pathology and oncology markets.
We are initially commercializing tests that will address unmet needs in the diagnostic pathway of pigmented skin lesions, such as moles or dark colored skin spots. Our products are designed to facilitate the clinical assessment of pigmented skin lesions for melanoma, aid the histopathologic staging of melanoma, and determine the metastatic potential of melanoma. Our first product in this area is the Pigmented Lesion Assay, which can be used to reduce unnecessary surgical biopsy procedures by ruling out false positives based on visual assessment prior to performing a surgical biopsy. The development of our Pigmented Lesion Assay has involved over 500 lesion samples, including over 250 melanomas. We have completed the development of our Pigmented Lesion Assay and plan to introduce our product in the second half of 2014. We plan to initially market this test directly to a concentrated group of 250 to 500 dermatologists that treat a majority of the 750,000 patients with the highest risk of melanoma in the United States. While we have not yet generated any sales from our Pigmented Lesion Assay, and have a history of net losses of $39.4 million since our inception. We have begun generating revenue of $31,000 from our sample collection kits and CLIA testing services during the first quarter of 2014 through research collaborations with Biogen Idec and others.
Over the next three years, we also plan to commercialize additional solutions in skin cancer and pursue additional partnering and licensing opportunities for our platform technology in areas such, inflammatory skin diseases, aesthetic/cosmetic indications, and acne. We have developed proof-of-concept tests and gene expression profiles and classifiers in some of these areas that demonstrate the potential utility of our technology. These opportunities involve clinical trial support and can produce revenue from sample processing. In addition, they may lead to companion diagnostic products for new or existing drug therapies. We have begun recognizing revenue with customers who purchase our sample collection kits and CLIA testing services.
-1-
Table of Contents
Our proposed products have been validated as Laboratory Developed Tests, or LDTs, for use in our laboratory, which has been certified under the Clinical Laboratory Improvement Amendments of 1988, or CLIA. Our sample collection kit is a Class I medical device that is manufactured by a supplier approved by the Food and Drug Administration, or FDA. Our validation studies have been performed under Quality System Regulations, or QSR, standards. Our current plans are to launch our assay as an LDT in our CLIA laboratory. There is uncertainty surrounding the regulatory path for new healthcare technologies, and if the FDA required us to submit our product for pre-market approval or 510K clearance, there would be significant delays in our product launch and our ability to generate revenue, while we would incur additional costs for clinical trials and related expenses. The FDA indicated that it is reviewing the regulatory requirements that apply to LDTs. In July 2010, the FDA held a two-day public meeting to obtain input from stakeholders on how it should apply its authority to implement a reasonable, risk-based, and effective regulatory framework for LDTs, including genetic tests. However, the FDA has not yet issued the additional guidance, and they do not have such guidance in their current fiscal year goals, but may generate such guidance in the future.
Our Competitive Advantages
We believe we are strategically positioned to address the unmet medical needs in dermatologic diagnosis. Our first product for the assessment of pigmented skin lesions highlights the potential impact of our technology platform. Our competitive advantages include:
| • | Highly accurate tests that can significantly reduce unnecessary surgical biopsies. Our Pigmented Lesion Assay test demonstrates an accuracy of 91.0% in differentiating melanoma from non-melanoma using histopathology as the reference standard. Our prevalence-weighted negative predictive value, or NPV, exceeds 99.4% such that the probability that a negative test may actually be positive is less than 0.6%. This compares with the current standard of care’s estimated NPV of 76%. |
| • | Superior ease of use. Our non-invasive adhesive patch biopsy procedure can be performed in less than five minutes by the physician. All the necessary items including adhesive patches, instructions, a marking pen for outlining, and a pre-addressed and prepaid return shipping label are contained in our kit. |
| • | Physician services can be reimbursed using existing Current Procedure Terminology, or CPT, codes. Unlike competing solutions for which there are no CPT reimbursement codes, physicians using our tests can seek reimbursement under an existing biopsy CPT code. |
| • | Lower cost of dermatologic diagnosis. We believe our Pigmented Lesion Assay highlights how our technology can provide better care at a reduced cost. We have performed an economic analysis that demonstrates an average direct cost savings of more than $50 per lesion tested, plus additional benefits, such as reductions in lost productivity and improved quality of life. |
| • | Simple integration into clinical practice. Our test and adhesive patch aim to replace the scalpel in the initial assessment. Unlike alternative device technologies, we do not require the installation and maintenance of capital equipment. The nursing support, documentation and specimen processing, are substantially similar to current practice. These issues are critical in a busy clinical practice where, on average, clinicians see a new patient every five-to-seven minutes. |
| • | Highly differentiated diagnostic technology. Our tests measure objective biological changes in gene expression rather than subjective visual pattern changes. Consequently, we believe the performance of our tests is superior to that of other technologies. Because we are assessing the changes in cellular biology, we can identify other gene expression profiles that are associated with the cancer stage and its probability of metastasis. |
| • | Technology platform that can address other skin conditions. We are pursuing partnering and licensing opportunities for our platform technology in other areas such as inflammatory skin diseases, |
-2-
Table of Contents
aesthetic/cosmetic indications, and acne. We are currently partnering with Biogen Idec, Inc., a large multi-national biotechnology company to assist in developing a drug product for an inflammatory disease of the skin, and have generated revenues from this collaboration. In connection with this collaboration, we are providing services, such as assay development, adhesive patch testing using gene expression, and sample storage to Biogen Idec. |
| • | Strong intellectual property protection. We have five issued U.S. patents, of which two are broadly directed to the use of an adhesive to collect samples containing RNA from the skin for analysis. We also have patent applications pending on unique gene expression profiles and classifiers. We have developed processes and know-how that allow us to extract RNA material from adhesive patch samples suitable for qPCR analysis. |
Strategy
Our objective is to be the leader in non-invasive molecular tests for dermatology. To achieve this objective, we intend to:
| • | Build a specialized sales force to introduce our products, starting with the Pigmented Lesion Assay. We intend to build a specialty sales force. This sales force will initially call on the 250 to 500 pigmented lesion specialists that treat the more than 750,000 patients with the highest risk for melanoma. We will then expand our sales force to penetrate 80% of the approximately 7,500 U.S. dermatologists with active clinical practices. |
| • | Integrate our products into the standard of care. We intend to conduct rigorous clinical and basic science research, and publish the results of this research in peer-reviewed journals. We will work with, and establish, boards of key opinion leaders in fields relevant to our products. We have assembled a world-renowned board of melanoma experts and will work with these advisors to incorporate our melanoma products into the diagnostic pathway guidelines for melanoma. |
| • | Secure broad reimbursement coverage for our assays. We plan to target regional and national payors to secure favorable coverage decisions for the reimbursement of our tests. We will focus on completing our clinical utility studies, building an in-house reimbursement team, publishing additional papers, and completing our Medicare dossier. National Medicare coverage is currently determined through the MolDx coverage decision program and we plan to submit our application in the next 12 months. |
| • | Expand our product offerings. We have identified other gene expression profiles and classifiers in other areas of dermatology. We have also developed gene expression profiles that can assess the stage of melanoma and determine if the tumor has become invasive or remains confined to the outermost skin layer. In addition, we have identified differential gene expression that should form the basis for a test that can determine the metastatic potential of the lesion. Development of this test could reduce the need for sentinel lymph node surgery, which can be extensive, can cause significant morbidity, and is costly. |
| • | Develop new products and explore licensing and partnership opportunities. In 2013, we initiated a collaboration with Biogen Idec, Inc., or Biogen Idec, to support the development of a new drug for an inflammatory skin disease. We will continue to explore through our own research and with partners, the use of our technology in other dermatologic conditions, including other skin cancers, anti-aging treatments, acne, and inflammatory skin diseases. |
Market Opportunity—Melanoma
Melanoma is currently one of the fastest growing cancers and the subject of significant attention in the medical community. The incidence of melanoma has doubled since 1973. While there has been a 20% decline in
-3-
Table of Contents
cancer deaths overall since 1991, melanoma is one of three cancers facing increasing rates. According to a study from the Mayo Clinic, the incidence of melanoma increased eightfold among women under 40 and fourfold among men under 40 from 1970 to 2009.
The American Cancer Society projects that 9,710 people will die in the United States from melanoma in 2014. If diagnosed and removed early in its evolution, when confined to the outermost skin layer and deemed to be “in situ (Stage 0),” patients are expected to have a survival rate of almost 100%. Invasive melanomas that are thin and extend into the uppermost regions of the second skin layer still have cure rates that are greater than 90%. However, once the cancer advances into the deeper layers of skin, the risk of it spreading to other parts of the body, or metastasizing, increases significantly, as does the risk of death.
We estimate that approximately 3.0 million surgical biopsies are performed in the United States each year to diagnose about 138,000 cases of melanoma—more than 61,000 non-invasive (in situ) and 77,000 invasive. Based on those volumes, we estimate the total U.S. market opportunity for our tests and products for pigmented skin lesions and melanoma exceeds $1.0 billion annually, and the select worldwide market opportunity outside of the United States exceeds $500 million annually. The Surveillance, Epidemiology, and End Results (SEER) program of the National Cancer Institute (NCI) is a source of epidemiologic information on the incidence and survival rates of cancer in the United States. According to the SEER Registries database, biopsies per patient can range from one per year to over fifty per year for certain conditions.
Limitations of Current Melanoma Diagnostic Pathway
Pigmented skin lesions suspicious for melanoma are clinically atypical and meet one or more subjective visual grading criteria. If identified as suspicious for melanoma, a lesion may be surgically biopsied or followed for further change. Patients can have many atypical lesions, which can complicate the decision of which lesions to surgically biopsy, and it may not be practical to surgically biopsy all lesions. Due to the low sensitivity of clinical assessment, on average 80%, we believe lesions with a lower suspicion for melanoma may not be selected for biopsy, such that approximately 20% may have a delayed diagnosis. Studies show that the current standard clinical assessment of pigmented lesions for biopsy demonstrate an accuracy of less than 20%, with sensitivities on average of 80% and specificities ranging from 5% to 20%. Technologies that involve image, spectral, or electrical impedance analysis, can be used at this point to provide further assessment for biopsy need, but typically lack the accuracy needed to meaningfully improve clinical assessment.
Lesions selected for surgical biopsy are ideally subjected to an excisional procedure. However, physicians looking to minimize patient discomfort and recovery time will employ simpler, but less accurate biopsy procedures known as “shave” and “punch” biopsies approximately 70% of the time. Shave and punch biopsies can confound the histopathologic diagnosis, can lead to a delayed diagnosis, and can result in a substantial number of rebiopsies (approximately 20% or greater).
After a lesion is surgically biopsied it is sent for histopathologic assessment, which is also subjective and challenging. Consequently, there is a high rate of discordant diagnosis (two pathologists do not make the same diagnosis when evaluating the same tissue), which is estimated to be as high as 20% to 30%. Studies have demonstrated that pathology, the current standard of care, has a false negative rate and false positive rate of approximately 10% and 10%, respectively, and samples can have ambiguous findings which can preclude a definitive pathologic diagnosis.
Our Platform Technology and Diagnostic Solutions
Cancer and many skin conditions result from changes in gene expression that alter cell growth, function, and ability of cells to invade and metastasize. The information provided by gene expression analysis is both objective and biologically-based. This shift from subjective pattern recognition-based information to objective biological information provides highly relevant information to clinicians about skin conditions.
-4-
Table of Contents
Adhesive Skin Sample Collection Kit
The adhesive patch allows for the collection of skin samples with minimal patient discomfort. A single kit contains all of the necessary components to complete the sample collection for our analysis, including the adhesive patches, instructions for use, a marking pen for lesion outlining, and a pre-addressed and prepaid return shipping pack. The unique properties of the tape maximize the collection of informative cellular material for our Pigmented Lesion Assay and the entire procedure takes less than five minutes.
Pigmented Lesion Assay
The development of our Pigmented Lesion Assay has involved over 500 lesion samples, including over 250 melanomas. The initial effort involved a genome wide screen (approximately 47,000 genes) to identify genes that were differentially expressed between melanoma and non-melanoma. We utilize a qPCR technology platform to test and identify gene expression profiles for our commercial test result.
The Pigmented Lesion Assay produces a PLA score derived from the company’s proprietary algorithm that utilizes the expression data from our assay. The PLA Score steadily increases from non-melanoma lesions, to melanomain situ, to invasive melanoma, demonstrating the progression of the gene expression as the cancer advances. In the validation studies involving 158 lesions (80 melanoma and 78 non-melanoma), statistically significant differences (p£ 0.00001) in gene expression between melanoma and non-melanoma samples were measured. A p-value as shown above, of less than or equal to 0.00001 means that there is at least a 99.999% probability that the increased PLA score in the sample group is a result of the melanoma tested and not due to chance.
Additional Opportunities
We are in various stages of developing and exploring additional applications for our technology platform including:
| • | In situ vs. invasive staging (Micro Staging); |
| • | metastatic potential; |
| • | other skin cancers; |
| • | inflammatory indications; |
| • | aesthetic and cosmetic indications; and |
| • | acne. |
Risks That We Face
An investment in our common stock involves a high degree of risk. You should carefully consider the risks summarized below. The risks are discussed more fully in the “Risk Factors” section of this prospectus immediately following this prospectus summary. These risks include, but are not limited to, the following:
| • | we are an early-stage company with a history of substantial net losses, we have never been profitable and we have an accumulated deficit of approximately $39.4 million as of March 31, 2014. We expect to incur net losses in the future, and we may never achieve sustained profitability; |
| • | our business depends on us being able to recruit and hire a sales and marketing team, scientists, licensed laboratory personnel, and other personnel with extensive experience in our field, who are in short supply; |
| • | our business depends on the launch and commercial success of our Pigmented Lesion Assay; |
-5-
Table of Contents
| • | our business may depend on our ability to continue to develop and commercialize novel, innovative, and non-invasive diagnostic tests and services; |
| • | our current cash resources are insufficient to fund our operations beyond August 2014 without raising additional capital; |
| • | our business depends on executing our sales and marketing strategy for our cancer diagnostic tests and on gaining acceptance of our current test and potentially future tests in the market; |
| • | our business depends on being able to obtain adequate reimbursement from governmental and other third-party payors for tests and services; |
| • | our business could be subject to FDA regulation or enforcement actions; |
| • | if our sole laboratory facility becomes damaged or inoperable, or we are required to vacate the facility, our ability to sell and provide molecular tests and pursue our research and development efforts may be jeopardized; |
| • | our business depends on satisfying numerous applicable United States and international regulatory requirements; |
| • | our business depends on our ability to effectively compete with other diagnostic tests, methods and services that now exist or may hereafter be developed; and |
| • | we need to obtain or maintain patents or other appropriate intellectual property protection for the intellectual property utilized in our current and planned tests and services, and we must avoid infringement of third-party intellectual property rights. |
Corporate and Other Information
We were incorporated in California in 1995. In connection with this offering, we plan to reincorporate in Delaware. Our principal offices are located at 11099 North Torrey Pines Road, Suite 100, La Jolla, California 92037. Our telephone number is (858) 450-4222 and our website address iswww.dermtech.com. Information contained on or accessible through our website is not a part of this prospectus, and the inclusion of our website address in this prospectus is an inactive textual reference only.
Implications of Being an Emerging Growth Company
As a company with less than $1.0 billion in revenue during our last fiscal year, we qualify as an “emerging growth company” as defined in the Jumpstart Our Business Startups Act, or JOBS Act, enacted in April 2012. An “emerging growth company” may take advantage of reduced reporting requirements that are otherwise applicable to public companies. These provisions include, but are not limited to:
| • | being permitted to present only two years of audited financial statements and only two years of related Management’s Discussion and Analysis of Financial Condition and Results of Operations in this prospectus; |
| • | not being required to comply with the auditor attestation requirements of Section 404 of the Sarbanes-Oxley Act; |
| • | reduced disclosure obligations regarding executive compensation in our periodic reports, proxy statements, and registration statements; and |
| • | exemptions from the requirements of holding a nonbinding advisory vote on executive compensation and stockholder approval of any golden parachute payments not previously approved. |
-6-
Table of Contents
We may take advantage of these provisions until the last day of our fiscal year following the fifth anniversary of the date of the first sale of our common equity securities pursuant to an effective registration statement under the Securities Act of 1933, as amended, or the Securities Act, which such fifth anniversary will occur in 2019. However, if certain events occur before the end of such five-year period, including if we become a “large accelerated filer,” our annual gross revenues exceed $1.0 billion, or we issue more than $1.0 billion of non-convertible debt in any three-year period, we will cease to be an emerging growth company before the end of such five-year period.
We have elected to take advantage of certain of the reduced disclosure obligations and may elect to take advantage of other reduced reporting requirements in future filings. As a result, the information that we provide to our stockholders may be different than the information you might receive from other public reporting companies in which you hold equity interests.
-7-
Table of Contents
The Offering
Common stock we are offering | shares of common stock |
Common stock outstanding after the offering | shares of common stock |
Offering price
Underwriters’ option to purchase additional shares | shares of common stock |
Use of proceeds | We currently intend to use the net proceeds of the offering: to hire sales and marketing personnel and support increased sales and marketing activities; to fund further research and development, clinical utility studies, and future enhancements of our current test; to fund research and development and clinical studies for our planned products; to acquire equipment, implement automation, and scale our capabilities to prepare for significant test volume; and for general corporate purposes and to fund ongoing operations and the expansion of our business. See “Use of Proceeds.” |
Risk Factors | See the section entitled “Risk Factors” and any other information included in this prospectus for a discussion of factors you should consider carefully before deciding to invest in shares of our common stock. |
Proposed NASDAQ Capital Market Symbol | DMTK |
The number of shares of our common stock to be outstanding immediately after this offering is based on 1,885,647 shares of our common stock outstanding as of March 31, 2014 (including preferred stock on an as-converted basis), and excludes:
| • | 394,563 shares of common stock issuable upon exercise of options outstanding as of March 31, 2014, at a weighted average exercise price of $0.75 per common share; and |
| • | 295,623 shares of common stock issuable upon exercise of warrants outstanding as of March 31, 2014, at a weighted average exercise price of $5.25 per common share. |
Unless the context otherwise requires, all share and per share amounts in this prospectus have been adjusted on a pro forma basis to give effect to our one-for-seventy-five reverse split which we plan to effect prior to the completion of this offering and unless otherwise indicated, all information in this prospectus assumes:
| • | the conversion of all outstanding shares of our convertible preferred stock into an aggregate of shares of common stock upon the closing of this offering; |
| • | the filing of our amended and restated certificate of incorporation immediately prior to the closing of this offering; |
-8-
Table of Contents
| • | no exercise by the underwriters of their option to purchase additional shares; and |
| • | no options, warrants or shares of common stock were issued after March 31, 2014, and no outstanding options were exercised after March 31, 2014. |
In April and May, 2014, the Registrant sold 240,425 shares of its Series B preferred stock in connection with the exercise of outstanding warrants at a per share exercise price of $6.00.
-9-
Table of Contents
Summary Financial Data
The following table summarizes our selected financial data for the periods and as of the dates indicated. Our selected statements of operations data for the years ended December 31, 2012 and 2013 and our selected balance sheet data as of December 31, 2012 and 2013 have been derived from our audited financial statements and their related notes, which are included elsewhere in this prospectus. All “Weighted average shares outstanding” data and all “Net loss per common share” data, whether derived from our audited financial statements or from our unaudited financial statements, have been adjusted to reflect a 1-for-75 reverse stock split which we plan to effect prior to the completion of this offering. Our selected statements of operations data for the three months ended March 31, 2013 and 2014 and our selected balance sheet data as of March 31, 2014 have been derived from our unaudited interim financial statements which are included elsewhere in this prospectus. Our historical results are not necessarily indicative of the results to be expected for any future periods and our interim results are not necessarily indicative of the results to be expected for the full fiscal year. Our unaudited financial statements have been prepared on the same basis as the audited financial statements and, in the opinion of management, include all adjustments, consisting of normal recurring adjustments necessary for a fair presentation of our financial condition as of such dates and our results of operations for such periods. Our summary financial data should be read together with the section entitled “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and with our financial statements and their related notes, which are included elsewhere in this prospectus.
| Year Ended December 31, | Three Months Ended March 31, | |||||||||||||||
| 2012 | 2013 | 2013 | 2014 | |||||||||||||
| (in thousands, except per share data) | ||||||||||||||||
| (unaudited) | ||||||||||||||||
Statement of Operations Data: | ||||||||||||||||
Revenues | $ | — | $ | — | $ | — | $ | 31 | ||||||||
Cost of revenues | — | — | — | 10 | ||||||||||||
|
|
|
|
|
|
|
| |||||||||
Gross profit | — | — | — | 21 | ||||||||||||
Operating expenses: | ||||||||||||||||
Sales and marketing | 126 | 31 | 11 | 81 | ||||||||||||
Research and development | 976 | 1,958 | 368 | 728 | ||||||||||||
General and administrative | 1,064 | 1,273 | 295 | 585 | ||||||||||||
|
|
|
|
|
|
|
| |||||||||
Loss from operations | (2,166 | ) | (3,262 | ) | (674 | ) | (1,373 | ) | ||||||||
Total other income/(expense) | (62 | ) | (1,916 | ) | (50 | ) | (1 | ) | ||||||||
|
|
|
|
|
|
|
| |||||||||
Net loss | $ | (2,228 | ) | $ | (5,178 | ) | $ | (724 | ) | $ | (1,374 | ) | ||||
|
|
|
|
|
|
|
| |||||||||
Weighted average shares outstanding used in computing net loss per common share, basic and diluted(1) | 45,884 | 62,906 | 45,884 | 106,052 | ||||||||||||
Net loss per common share outstanding, basic and diluted | $ | (48.55 | ) | $ | (82.32 | ) | $ | (15.79 | ) | $ | (12.96 | ) | ||||
Weighted average shares outstanding used in computing pro forma net loss per share basic and diluted (unaudited) (2) | 902,449 | 1,993,289 | ||||||||||||||
Pro forma net loss per common share, basic and diluted (3) | $ | (5.74 | ) | $ | (0.69 | ) | ||||||||||
| (1) | Basic and diluted net loss per common share is determined by dividing net loss applicable to common shareholders by the weighted average common shares outstanding during the period. Because there is a net loss attributable to common shareholders for the years ended December 31, 2012 and 2013 and the three |
-10-
Table of Contents
| months ended March 31, 2013 and 2014, the outstanding shares of Preferred stock, warrants, and common stock options have been excluded from the calculation of diluted loss per common share because their effect would be anti-dilutive. Therefore, the weighted average shares used to calculate both basic and diluted loss per share are the same. Net loss per common share, diluted, excludes the effect of anti-dilutive equity instruments including 191,136, 735,414, 193,942 and 1,623,895 shares of common stock issuable upon conversion of our Preferred stock, and 8,241, 433,763, 7,087 and 690,185 shares of common stock issuable upon the exercise of outstanding warrants and stock options, for 2012, 2013 and the three months ended March 31, 2013 and 2014, respectively. |
| (2) | The weighted average shares outstanding used in computing pro forma net loss per share assumes the conversion of all outstanding preferred stock and preferred stock warrants into an aggregate of 856,565 and 1,887,236 shares of common stock, respectively, for 2013 and the three months ended March 31, 2014. |
| (3) | The pro forma net loss per share, basic and diluted, is calculated using the net loss, adjusted to eliminate the interest on convertible debt during the period, divided by the pro forma weighted average shares outstanding, respectively for 2013 and the three months ended March 31, 2014. |
| As of December 31, | As of March 31, | |||||||||||||
| 2012 | 2013 | 2014 | Pro Forma As Adjusted(1) | |||||||||||
| (in thousands) | (in thousands, unaudited) | |||||||||||||
Balance Sheet Data: | ||||||||||||||
Cash and cash equivalents | $ | 1,353 | $ | 1,105 | $ | 1,487 | ||||||||
Total assets | 1,429 | 1,200 | 1,769 | |||||||||||
Current liabilities | 3,240 | 637 | 1,136 | |||||||||||
Total liabilities | 3,240 | 637 | 1,138 | |||||||||||
Convertible preferred stock | 0.0 | 0.2 | 0.2 | |||||||||||
Accumulated deficit | (32,819 | ) | (37,997 | ) | (39,371 | ) | ||||||||
Total stockholders’ equity (deficit) | (1,810 | ) | 564 | 631 | ||||||||||
| (1) | The pro forma as adjusted balance sheet data above reflects the conversion of all outstanding shares of preferred stock into an aggregate of 1,779,595 shares of our common stock, plus the sale of shares of our common stock in this offering and application of the net proceeds at an assumed initial public offering price of $ per share, the midpoint of the pricing range set forth on the cover of this prospectus, after deducting the estimated underwriting discount and estimated offering expenses payable by us. |
-11-
Table of Contents
An investment in our common stock involves a high degree of risk. You should consider carefully the specific risk factors described below in addition to the other information contained in this prospectus, including our financial statements and related notes and Management’s Discussion and Analysis of Financial Condition and Results of Operations included elsewhere in the prospectus, before making your investment decision. The occurrence of any of the events or developments described below could harm our business, financial condition, results of operations, and growth prospects. In such an event, the market price of our common stock could decline, and you may lose all or part of your investment. Additional risks and uncertainties not presently known to us or that we currently deem immaterial also may impair our business operations.
Risks Relating to Our Financial Condition and Capital Requirements
We are an emerging growth company with a history of net losses; we expect to incur net losses in the future, and we may never achieve sustained profitability.
We have historically incurred substantial net losses, including net losses of $2.2 million in 2012 and $5.2 million in 2013; and we have never been profitable. As of March 31, 2014, our accumulated deficit is $39.4 million.
We expect our losses to continue as a result of costs relating to ongoing research and development expenses, for increased sales and marketing costs and related to the planned launch of our first commercial product. These losses have had, and will continue to have, an adverse effect on our working capital, total assets, and stockholders’ equity. Because of the numerous risks and uncertainties associated with our commercialization efforts, we are unable to predict when we will become profitable, and we may never become profitable. Even if we do achieve profitability, we may not be able to sustain or increase profitability on a quarterly or annual basis. Our inability to achieve and then maintain profitability would negatively affect our business, financial condition, results of operations, and cash flows.
Our independent registered public accounting firm’s report for the year ended December 31, 2013 includes an explanatory paragraph regarding substantial doubt about our ability to continue as a going concern.
Due to the deficit that we have accumulated since our inception, in their report on our audited annual financial statements as of and for the year ended December 31, 2013, our auditors included an explanatory paragraph regarding concerns about our ability to continue as a going concern. Recurring losses from operations raise substantial doubt about our ability to continue as a going concern. If we are unable to obtain sufficient funding at acceptable terms, we may be forced to significantly curtail our operations, and the lack of sufficient funding may have a material adverse impact on our ability to continue as a going concern. If we are unable to continue as a going concern, we might have to liquidate our assets and the values we receive for our assets in liquidation or dissolution could be significantly lower than the values reflected in our financial statements. In addition, the inclusion of an explanatory paragraph regarding substantial doubt about our ability to continue as a going concern and our lack of cash resources may materially adversely affect our share price and our ability to raise new capital or to enter into critical contractual relations with third parties.
We have a limited operating history and we expect a number of factors to cause our operating results to fluctuate on a quarterly and annual basis, which may make it difficult to predict our future performance.
We are an emerging molecular diagnostics company with a limited operating history. Our operations to date have been primarily focused on developing our technology. We have not obtained regulatory approvals for any of our tests. Consequently, if regulatory approval is determined to be necessary, any predictions made about our future success or viability may not be as accurate as they could be if we had a longer operating history or commercialized products. Our financial condition and operating results have varied significantly in the past and
-12-
Table of Contents
will continue to fluctuate from quarter-to-quarter or year-to-year due to a variety of factors, many of which are beyond our control. Factors relating to our business that may contribute to these fluctuations include other factors described elsewhere in this prospectus and also include:
| • | our ability to obtain additional funding to develop our products and tests; |
| • | our ability to obtain and maintain any necessary regulatory approval for any of our tests in the United States and foreign jurisdictions; |
| • | potential side effects of tests that could delay or prevent commercialization, limit the indications for any of our tests, require the establishment of risk evaluation and mitigation strategies, or cause any of our commercialize tests to be taken off the market; |
| • | our dependence on third-party suppliers and manufacturers, to supply or manufacture our products; |
| • | our ability to establish or maintain collaborations, licensing, or other arrangements; |
| • | market acceptance of our products and tests; |
| • | our ability to establish and maintain an effective sales and marketing infrastructure, either through the creation of a commercial infrastructure or through strategic collaborations; |
| • | competition from existing products or new products that may emerge; |
| • | the ability of patients or healthcare providers to obtain coverage of or sufficient reimbursement for our products; |
| • | our ability to leverage our proprietary technology platform to discover and develop additional product candidates; |
| • | our ability to successfully obtain, maintain, defend, and enforce intellectual property rights important to our business; |
| • | our ability to attract and retain key personnel to manage our business effectively; |
| • | our ability to build our finance infrastructure and improve our accounting systems and controls; |
| • | potential product liability claims; |
| • | potential liabilities associated with hazardous materials; and |
| • | our ability to obtain and maintain adequate insurance policies. |
Accordingly, the results of any quarterly or annual periods should not be relied upon as indications of future operating performance.
We will need to raise additional capital to fund our existing operations, commercialize our products, and expand our operations.
As of March 31, 2014, our cash and cash equivalents totaled approximately $1.5 million. Based on our current business operations, we believe the net proceeds from this offering, together with our current cash and cash equivalents, will be sufficient to meet our anticipated cash requirements for the 24-month period following this offering. If our available cash balances, net proceeds from this offering, and anticipated cash flow from operations are insufficient to satisfy our liquidity requirements including due to changes in our business operations, a lengthier sales cycle, lower demand for our products, or other risks described in this prospectus, we may seek to raise additional capital through equity offerings, debt financings, collaborations, or licensing arrangements. We may also consider raising additional capital in the future to expand our business, to pursue strategic investments, to take advantage of financing opportunities, or for other reasons, including to:
| • | increase our efforts to drive market adoption of our tests and address competitive developments; |
| • | fund development activities and efforts of any future products; |
-13-
Table of Contents
| • | acquire, license, or invest in technologies; |
| • | acquire or invest in complementary businesses or assets; and |
| • | finance capital expenditures and general and administrative expenses. |
Our present and future funding requirements will depend on many factors, including:
| • | our revenue growth rate and ability to generate cash flows from operating activities; |
| • | our sales and marketing and research and development activities; |
| • | effects of competing technological and market developments; |
| • | costs of and potential delays in product development; |
| • | changes in regulatory oversight applicable to our tests; and |
| • | costs related to international expansion. |
The various ways we could raise additional capital carry potential risks. If we raise funds by issuing equity securities, dilution to our stockholders could result. Any equity securities issued also could provide for rights, preferences, or privileges senior to those of holders of our common stock. If we raise funds by issuing debt securities, those debt securities would have rights, preferences, and privileges senior to those of holders of our common stock. The terms of debt securities issued or borrowings pursuant to a credit agreement could impose significant restrictions on our operations. If we raise funds through collaborations and licensing arrangements, we might be required to relinquish significant rights to our platform technologies or products, or grant licenses on terms that are not favorable to us. The credit markets and the financial services industry have experienced a period of unprecedented turmoil and upheaval characterized by the bankruptcy, failure, collapse, or sale of various financial institutions and an unprecedented level of intervention from the U.S. federal government. These events have generally made equity and debt financing more difficult to obtain. Accordingly, additional equity or debt financing might not be available on reasonable terms, if at all. If we cannot secure additional funding when needed, we may have to delay, reduce the scope of, or eliminate one or more research and development programs or sales and marketing initiatives. In addition, we may have to work with a partner on one or more of our development programs, which could lower the economic value of those programs to us.
We will also need to raise additional capital to expand our business to meet our long-term business objectives. Additional financing may be from the sale of equity or convertible or other debt securities in a public or private offering, from a credit facility or strategic partnership coupled with an investment in us, or a combination of both. For further discussion of our liquidity requirements as they relate to our long-term plans, see the section entitled “Management’s Discussion and Analysis of Financial Condition and Results of Operations—Liquidity and Capital Resources.”
We expect to continue to incur significant expenses to develop and market our tests, which could make it difficult for us to achieve and sustain profitability.
In recent years, we have incurred significant costs in connection with the development of our tests. For the year ended December 31, 2013, our research and development expenses were $2.0 million and our general and administrative expenses were $1.3 million. For the year ended December 31, 2012, our research and development expenses were $1.0 million and our general and administrative expenses were $1.1 million. We expect our expenses to continue to increase for the foreseeable future as we conduct studies of our current test and our planned other tests, establish a sales and marketing organization, drive adoption of and reimbursement for our tests, and develop new tests. As a result, we need to generate significant revenues in order to achieve sustained profitability.
-14-
Table of Contents
Risks Relating to Our Business and Strategy
We may not be able to generate sufficient revenue from the commercialization of our Pigmented Lesion Assay or successfully develop and commercialize other tests to achieve or sustain profitability.
We recorded our first revenues from our planned principal operations in the three months ended March 31, 2014. We are in varying stages of research and development for other tests that we may offer in the future. We believe that our commercialization success is dependent upon our ability to significantly increase the number of customers that are using our tests. In addition, demand for our tests may not increase as quickly as planned and we may be unable to increase our revenue levels as expected. We are currently not profitable. Even if we succeed in increasing adoption of our Pigmented Lesion Assay by dermatologists, in maintaining and creating relationships with our existing and new customers, and developing and commercializing additional molecular diagnostic testing products, we may not be able to generate sufficient revenue to achieve or sustain profitability.
If we are unable to execute our sales and marketing strategy for our Pigmented Lesion Assay and are unable to gain acceptance in the market, we may be unable to generate sufficient revenue to sustain our business.
Although we believe that our current test and our planned future tests represent a promising commercial opportunity, our tests may never gain significant acceptance in the marketplace and therefore may never generate substantial revenue or profits for us. We will need to establish a market for our tests and build that market through physician education, awareness programs, and the publication of clinical trial results. Gaining acceptance in medical communities requires publication in leading peer-reviewed journals of results from studies using our current test and/or our planned future tests. The process of publication in leading medical journals is subject to a peer-review process and peer-reviewers may not consider the results of our studies sufficiently novel or worthy of publication. Failure to have our studies published in peer-reviewed journals would limit the adoption of our current test and our planned tests.
Our ability to successfully market the tests that we develop will depend on numerous factors, including:
| • | conducting clinical utility studies of such tests in collaboration with key thought leaders to demonstrate their use and value in important medical decisions such as treatment selection; |
| • | the success of the sales force that we intend to hire with some of the proceeds of this offering; |
| • | whether healthcare providers believe such tests provide clinical utility; |
| • | whether the medical community accepts that such tests are sufficiently sensitive and specific to be meaningful in patient care and treatment decisions; and |
| • | whether health insurers, government health programs, and other third-party payors will cover and pay for such tests and, if so, whether they will adequately reimburse us. |
Failure to achieve widespread market acceptance of our current test and our planned future tests would materially harm our business, financial condition, and results of operations.
If we cannot develop tests to keep pace with rapid advances in technology, medicine and science, our operating results and competitive position could be harmed.
In recent years, there have been numerous advances in technologies relating to the molecular diagnosis for cancer and other medical conditions. Several new cancer drugs have been approved, including two for melanoma, and a number of new drugs in clinical development may increase patient survival time. There have also been advances in methods used to identify patients likely to benefit from these drugs based on analysis of biomarkers. We must continuously develop new tests and enhance any existing tests to keep pace with evolving standards of care. Our current test and our planned tests could become obsolete unless we continually innovate and expand them to demonstrate benefit in the diagnosis, monitoring, or prognosis of patients with cancer and
-15-
Table of Contents
other dermatologic conditions. If we cannot adequately demonstrate the applicability of our current test and our planned future tests to new diagnostic and treatment developments, sales of our tests could decline, which would have a material adverse effect on our business, financial condition, and results of operations.
Our future success will depend in part upon our ability to enhance our Pigmented Lesion Assay and to develop, introduce, and commercialize other novel innovative and non-invasive diagnostics tests and services. New test development involves a lengthy and complex process and we may be unable to commercialize new or improved tests or any other products we may develop on a timely basis, or at all.
Our future success will depend in part upon our ability to enhance our Pigmented Lesion Assay and to develop new innovative products. Our failure to successfully develop new products on a timely basis could have a material adverse effect on our revenue, results of operations, and business.
The development of new or enhanced tests is a complex and uncertain process requiring precise technological execution. In addition, the successful development of new products may depend on the development of new technologies. We may be required to undertake time-consuming and costly development activities. We may experience difficulties that could delay or prevent the successful development, commercialization, and marketing of these new products. Before we can commercialize any new products, we will need to expend significant funds in order to conduct substantial research and development, including validation studies.
Our product development process involves a high degree of risk, and product development efforts may fail for many reasons, including a failure to demonstrate the performance of the product or an inability to obtain any required certification or regulatory approval.
As we develop products, we will have to make significant investments in product development, as well as sales and marketing resources. In addition, competitors may develop and commercialize competing products faster than we are able to do so, which could have a material adverse effect on our revenue, results of operations and business.
We have limited experience manufacturing our tests on a commercial scale and there are no established standards for their manufacture. As a result, manufacturing issues may arise that could cause delays or increase costs, and if we are unable to adequately address our customers’ needs, it could negatively impact sales and market acceptance of our product and we may never generate sufficient revenue to achieve or sustain profitability.
We have limited experience manufacturing our tests on a commercial scale. We have developed manufacturing processes that have never been tested in commercial production; however, there is no established generally accepted manufacturing or quality standard for the production of our tests. Also, as we scale-up manufacturing of any approved product, our suppliers and manufacturers may encounter unexpected issues relating to the manufacturing process or the quality, purity, and stability of our tests and we may be required to refine or alter our manufacturing processes to address these issues. Resolving these issues could result in significant delays and may result in significantly increased costs. If we experience delays or other obstacles in producing any of our products for commercial scale, our ability to market and sell any products may be adversely affected and our business could suffer. Further, if we are unable to adequately address our customers’ needs, it could negatively impact sales and market acceptance of our products and we may never generate sufficient revenue to achieve or sustain profitability.
Our tests employ a novel diagnostic platform and may never be accepted by their intended markets.
Our activities have focused on the discovery and development of novel gene expression tests and preparation for commercial activities. Our future success depends on our ability to successfully commercialize our tests, as well as our ability to develop and market other tests that use our proprietary technology platform.
-16-
Table of Contents
The scientific discoveries that form the basis of our proprietary technology platform and our tests are relatively new. We are not aware of any other gene expression tests such as ours and there can be no assurance that physicians will be willing to use them. If we do not successfully develop and commercialize our tests based upon our technological approach, we may not become profitable and the value of our common stock may decline.
The novel nature of our tests also means that fewer people are trained in or experienced with products of this type, which may make it difficult to find, hire, and retain capable personnel for research, development, and manufacturing positions.
Further, our focus solely on gene expression tests, as opposed to multiple, more proven technologies for patient diagnosis, increases the risks associated with the ownership of our common stock. If we do not achieve market acceptance for our tests, we may be required to change the scope and direction of our product development activities. In that case, we may not be able to identify and implement successfully an alternative product development strategy.
If our current test and our planned tests do not to perform as expected, as a result of human error or otherwise, it could have a material adverse effect on our operating results, reputation, and business.
Our success depends on the market’s confidence that we can provide reliable, high-quality diagnostic results. There is no guarantee that any accuracy we have demonstrated to date will continue, particularly as the number of tests using our assays increases and as the number of different tests that we develop and commercialize expands. We believe that our customers are likely to be particularly sensitive to test defects and errors. As a result, the failure of our current or planned tests to perform as expected could significantly impair our reputation and the public image of our tests. As a result, the failure or perceived failure of our products to perform as expected could have a material adverse effect on our business, financial condition and results of operation.
We may be unable to manage our future growth effectively, which could make it difficult to execute our business strategy.
As part of our strategy, we expect to increase our number of employees as our business grows. This future growth could create strain on our organizational, administrative, and operational infrastructure, including laboratory operations, quality control, customer service, and sales and marketing. Our ability to manage our growth properly will require us to continue to improve our operational, financial, and management controls, as well as our reporting systems and procedures. If our current infrastructure is unable to handle our growth, we may need to further expand our infrastructure and staff and implement new reporting systems. The time and resources required to implement such expansion and systems could adversely affect our operations. Our expected future growth will impose significant added responsibilities on members of management, including the need to identify, recruit, maintain, and integrate additional employees. Our future financial performance and our ability to commercialize our products and to compete effectively will depend, in part, on our ability to manage this potential future growth effectively, without compromising quality.
If our sole laboratory facility becomes damaged or inoperable, or we are required to vacate the facility, our ability to sell and provide molecular tests and pursue our research and development efforts may be jeopardized.
We do not have any clinical reference laboratory facilities outside of our facility in La Jolla, California. Our facilities and equipment could be harmed or rendered inoperable by natural or man-made disasters, including fire, earthquake, flooding, and power outages, which may render it difficult or impossible for us to perform our diagnostic tests for some period of time. The inability to perform our current test, our planned tests, or the backlog of tests that could develop if our facility is inoperable for even a short period of time may result in the loss of customers or harm to our reputation or relationships with scientific or clinical collaborators, and we may
-17-
Table of Contents
be unable to regain those customers or repair our reputation in the future. Furthermore, our facilities and the equipment we use to perform our research and development work could be costly and time-consuming to repair or replace.
The San Diego area has recently experienced serious fires and power outages, and is considered to lie in an area with earthquake risk.
Additionally, a key component of our research and development process involves using biological samples as the basis for our diagnostic test development. In some cases, these samples are difficult to obtain. If the parts of our laboratory facility where we store these biological samples were damaged or compromised, our ability to pursue our research and development projects, as well as our reputation, could be jeopardized. We carry insurance for damage to our property and the disruption of our business, but this insurance may not be sufficient to cover all of our potential losses and may not continue to be available to us on acceptable terms, if at all.
Further, if our Clinical Laboratory Improvement Amendments of 1988, or CLIA, laboratory became inoperable we may not be able to license or transfer our technology to another facility with the necessary state licensure and CLIA certification under the scope of which our current test and our planned future tests could be performed. Even if we find a facility with such qualifications to perform our tests, it may not be available to us on commercially reasonable terms.
If we cannot compete successfully with our competitors, we may be unable to increase or sustain our revenues or achieve and sustain profitability.
Our principal competition comes from mainstream clinical diagnostic methods, used by dermatologists for many years, which focus on visual tumor tissue analysis. It may be difficult to change the methods or behavior of dermatologists to incorporate our Pigmented Lesion Assay and Adhesive Skin Sample Collection Kit into their practices in conjunction with, or instead of, tissue biopsies and analysis. In addition, companies offering capital equipment and kits or reagents to local dermatologists represent another source of potential competition. These tests are used directly by the dermatologists, which can facilitate adoption. We plan to focus our marketing and sales efforts on medical dermatologists rather than pathologists.
We also face competition from companies that offer device products or are conducting research to develop device products for analysis of pigmented lesions. In particular, MELA Sciences, Inc., markets its MelaFind® device to dermatologists. Scibase AB and Verisante Technology, Inc., have devices under development and may market their medical products directly to dermatologists if and when they obtain Food and Drug Administration, or FDA, approval. In addition to these companies, our competitors also include other device companies selling photographic technologies, whole body photography services, dermatoscopes, or confocal microscopy, such as Fotofinder, Molemate, Canfield Scientific, MedX, and Caliber I.D. Many of these groups, in addition to operating research and development laboratories, are selling equipment and devices.
In addition to these device companies, Myriad Genetics, Inc., offers an expression test for melanoma that is used on surgical biopsy specimens. Myriad Genetics, Inc., could also try and market their test as a biopsy aid at the point-of-care. Gene expression testing is a relatively new area of science, especially in dermatology and we cannot predict what tests others will develop that may compete with or provide results similar or superior to the results we are able to achieve with the tests we develop. There are a number of companies that are focused on the oncology diagnostic market and expression tests including Veracyte, Inc., Genomic Health, Inc., Trovagene, Inc., and others.
Additionally, projects related to cancer diagnostics and particularly genomics have received increased government funding, both in the United States and internationally. As more information regarding cancer genomics becomes available to the public, we anticipate that more products aimed at analyzing pigmented lesions and identifying melanoma may be developed and that these products may compete with ours. In addition, competitors may develop their own versions of our current or planned tests in countries where we did not apply
-18-
Table of Contents
for patents or where our patents have not issued or have expired and may compete with us in those countries, including encouraging the use of their test by physicians or patients in other countries. In addition, one or more competitors may seek to invalidate or render unenforceable any of our patents in a court of competent jurisdiction or at the United States Patent and Trademark Office. If any such proceeding were to be successful and result in the invalidation or unenforceability of one or more patents in our intellectual property portfolio, we may be unable to prevent unlicensed third-party competition in the marketplace with respect to our current and planned future tests.
Some of our present and potential competitors have widespread brand recognition and substantially greater financial and technical resources and development, production, and marketing capabilities than we do. Others may develop lower-priced, less complex tests that payors and dermatologists could view as functionally equivalent to our current or planned tests, which could force us to lower the list price of our tests and impact our operating margins and our ability to achieve and maintain profitability. In addition, technological innovations that result in the creation of enhanced diagnostic tools that are more sensitive or specific than ours may enable other clinical laboratories, hospitals, physicians, or medical providers to provide specialized diagnostic tests similar to ours in a more patient-friendly, efficient, or cost-effective manner than is currently possible. If we cannot compete successfully against current or future competitors, we may be unable to increase or create market acceptance and sales of our current or planned tests, which could prevent us from increasing or sustaining our revenues or achieving or sustaining profitability.
Our competitors may be able to respond more quickly and effectively than we can to new or changing opportunities, technologies, standards, or customer requirements. We anticipate that we will face increased competition in the future as existing companies and competitors develop new or improved products and distribution strategies and as new companies enter the market with new technologies and distribution strategies. We may not be able to compete effectively against these organizations. Our ability to compete successfully and to increase our market share is dependent upon our reputation for providing responsive, professional, and high-quality products and services and achieving strong customer satisfaction. Increased competition in the future could adversely affect our revenue, revenue growth rate, if any, margins and market share.
If dermatologists decide not to order the Pigmented Lesion Assay, or our future tests, we may be unable to generate sufficient revenue to sustain our business.
To generate demand for our current test and our planned tests, we will need to educate dermatologists and other health care professionals on the clinical utility, benefits, and value of the tests we provide through published papers, presentations at scientific conferences, educational programs, and one-on-one education sessions by members of our sales force. In addition, we need to assure dermatologists of their ability to obtain and maintain adequate reimbursement coverage from third-party payors for the adhesive patch sample collection method. If we cannot convince medical practitioners to order our current test and our planned tests, we will likely be unable to create demand in sufficient volume for us to achieve sustained profitability.
If we are unable to identify collaborators willing to work with us to conduct clinical utility studies, or the results of those studies do not demonstrate that a test provides clinically meaningful information and value, commercial adoption of our tests may be slow, which would negatively impact our business.
We believe clinical utility studies will show how the Pigmented Lesion Assay changes the decision-making of the dermatologist toward making a surgical biopsy decision, in particularly to avoid making a surgical biopsy when the test is negative. Clinical utility studies also show the impact of the test results on patient care and management. Clinical utility studies are typically performed with collaborating dermatologists at medical centers and hospitals, analogous to a clinical trial, and generally result in peer-reviewed publications. Sales and marketing representatives use these publications to demonstrate to customers how to use a clinical test, as well as why they should use it. These publications are also used with payors to obtain coverage for a test, helping to assure there is appropriate reimbursement.
-19-
Table of Contents
We are currently conducting a clinical utility study for our Pigmented Lesion Assay with investigators from the University of Pittsburgh Medical Center and Northwestern University Medical School. We will need to conduct additional studies for this test, as well as other tests we plan to introduce, to drive test adoption in the marketplace and reimbursement. Should we not be able to perform these studies, or should their results not provide clinically meaningful data and value for oncologists, adoption of our tests could be impaired and we may not be able to obtain reimbursement for them.
We are undergoing a management transition.
We have recently added several new senior management positions, including Chief Financial Officer and Chief Operating Officer. As of April 1, 2014, Steven Kemper became our Chief Financial Officer and Michael Willis, Ph.D. became our Chief Operating Officer. We have also recruited and hired other senior staff. Such a management transition subjects us to a number of risks, including risks pertaining to coordination of responsibilities and tasks, creation of new management systems and processes, differences in management style, effects on corporate culture, and the need for transfer of historical knowledge. In addition, our operations will be adversely affected if our management does not work together harmoniously, do not efficiently allocate responsibilities between themselves, or do not implement and abide by effective controls.
The loss of key members of our executive management team could adversely affect our business.
Our success in implementing our business strategy depends largely on the skills, experience, and performance of key members of our executive management team and others in key management positions, including John Dobak, M.D., our Chief Executive Officer and President, Steve Kemper, our Chief Financial Officer, John Alsobrook, Ph.D., our Chief Scientific Officer, and Mike Willis, Ph.D., our Chief Operating Officer. The collective efforts of each of these persons and others working with them as a team are critical to us as we continue to develop our technologies, tests, and research and development and sales programs. As a result of the difficulty in locating qualified new management, the loss or incapacity of existing members of our executive management team could adversely affect our operations. If we were to lose one or more of these key employees, we could experience difficulties in finding qualified successors, competing effectively, developing our technologies, and implementing our business strategy. Our Chief Executive Officer and President, Chief Financial Officer, Chief Operating Officer, and Chief Scientific Officer have employment agreements; however, the existence of an employment agreement does not guarantee retention of members of our executive management team and we may not be able to retain those individuals for the duration of or beyond the end of their respective terms. We do not maintain “key person” life insurance on any of our employees.
In addition, we rely on collaborators, consultants, and advisors, including scientific and clinical advisors, to assist us in formulating our research and development and commercialization strategy. Our collaborators, consultants, and advisors are generally employed by employers other than us and may have commitments under agreements with other entities that may limit their availability to us.
The loss of a key employee, the failure of a key employee to perform in his or her current position, or our inability to attract and retain skilled employees could result in our inability to continue to grow our business or to implement our business strategy.
There is a scarcity of experienced professionals in our industry. If we are not able to retain and recruit personnel with the requisite technical skills, we may be unable to successfully execute our business strategy.
The specialized nature of our industry results in an inherent scarcity of experienced personnel in the field. Our future success depends upon our ability to attract and retain highly skilled personnel, including scientific, technical, laboratory, sales, marketing, business, regulatory, and administrative personnel necessary to support our anticipated growth, develop our business, and perform certain contractual obligations. Given the scarcity of professionals with the scientific knowledge that we require and the competition for qualified personnel among life science businesses, we may not succeed in attracting or retaining the personnel we require to continue and grow our operations.
-20-
Table of Contents
Our inability to attract, hire, and retain a sufficient number of qualified sales professionals would hamper our ability to launch and increase demand for our Pigmented Lesion Assay, to expand geographically, and to successfully commercialize any other tests or products we may develop.
To succeed in selling our Pigmented Lesion Assay, and any other tests or products that we are able to develop, we must expand our sales force in the United States and/or internationally by recruiting sales representatives with extensive experience in dermatology and close relationships with medical dermatologists, dermatopathologists, and other hospital personnel. To achieve our marketing and sales goals, we will need to substantially build our sales and commercial infrastructure, with which to date we have had little experience. Sales professionals with the necessary technical and business qualifications are in high demand, and there is a risk that we may be unable to attract, hire, and retain the number of sales professionals with the right qualifications, scientific backgrounds, and relationships with decision-makers and potential customers needed to achieve our sales goals. We expect to face competition from other companies in our industry, some of whom are much larger than us and who can pay greater compensation and benefits than we can, in seeking to attract and retain qualified sales and marketing employees. If we are unable to hire and retain qualified sales and marketing personnel, our business will suffer.
We currently rely on third-party suppliers for critical materials needed to perform our current test and our planned future tests and any problems experienced by them could result in a delay or interruption of their supply to us.
We currently purchase raw materials for our tests and products under purchase orders and do not have long-term contracts with most of the suppliers of these materials. If suppliers were to delay or stop producing our materials or reagents, or if the prices they charge us were to increase significantly, or if they elected not to sell to us, we would need to identify other suppliers. We could experience delays in manufacturing or performing our tests while finding another acceptable supplier, which could impact our results of operations. The changes could also result in increased costs associated with qualifying the new materials or reagents and in increased operating costs. Further, any prolonged disruption in a supplier’s operations could have a significant negative impact on our ability to perform tests in a timely manner.
Some of the components used in our current or planned products are currently sole-source, and substitutes for these components might not be able to be obtained easily or may require substantial design or manufacturing modifications. Any significant problem experienced by one of our sole source suppliers may result in a delay or interruption in the supply of components to us until that supplier cures the problem or an alternative source of the component is located and qualified. Any delay or interruption would likely lead to a delay or interruption in our manufacturing operations. The inclusion of substitute components must meet our product specifications and could require us to qualify the new supplier with the appropriate government regulatory authorities. In addition, one or more components used in our current, or future planned, products may be patented by a third-party and may not have any substantial non-infringing uses, in which case, any inability to secure a license to such components on terms that are commercially reasonable to us may have a material impact on our business and render it difficult or impracticable for us to continue to offer our current and future planned tests and products.
We may encounter manufacturing problems or delays that could result in lost revenue.
The adhesive patch sample collection kit we distribute is manufactured by a third-party in Connecticut. This manufacturer assembles several components, including the key adhesive patch trifold, into a finished product, then labels, stores, and ships this finished product. The adhesive tape subcomponent of the adhesive patches is provided by a single source third-party. This tape is assembled into the individual adhesive patches by another third-party supplier. We manage the production of this adhesive patch trifold component with our third-party suppliers.
We believe we currently have adequate manufacturing capacity for our adhesive patch sample collection kits through our third-party manufacturer. If demand for our current test and our planned future tests increases significantly, we will need to either expand our manufacturing capabilities through our third-party manufacturer
-21-
Table of Contents
or outsource to other manufacturers. If our third-party or other manufacturers engaged by us fail to manufacture and deliver our adhesive patch kits or certain reagents in a timely manner, our relationships with our customers could be seriously harmed. We cannot assure you that manufacturing or quality control problems will not arise as we attempt to increase the production of our sample collection kit or that we can increase our manufacturing capabilities and maintain quality control in a timely manner or at commercially reasonable costs. If we cannot manufacture our adhesive patch sample collection kits consistently on a timely basis because of these or other factors, it could have a significant negative impact on our ability to perform tests and generate revenues.
If we cannot support demand for our current test and our planned future tests, including successfully managing the evolution of our technology and manufacturing platforms, our business could suffer.
As our test volume grows, we will need to increase our testing capacity, implement automation, increase our scale and related processing, customer service, billing, collection, and systems process improvements, and expand our internal quality assurance program and technology to support testing on a larger scale. We will also need additional technicians, certified laboratory scientists, and other scientific and technical personnel to process these additional tests. Any increases in scale, related improvements and quality assurance may not be successfully implemented and appropriate personnel may not be available. As additional tests are commercialized, we may need to bring new equipment on line, implement new systems, technology, controls and procedures, and hire personnel with different qualifications. Failure to implement necessary procedures or to hire the necessary personnel could result in a higher cost of processing or an inability to meet market demand. We cannot assure you that we will be able to perform tests on a timely basis at a level consistent with demand, that our efforts to scale our commercial operations will not negatively affect the quality of our test results or that we will respond successfully to the growing complexity of our testing operations. If we encounter difficulty meeting market demand or quality standards for our current test and our planned future tests, our reputation could be harmed and our future prospects and business could suffer, which may have a material adverse effect on our financial condition, results of operations and cash flows.
Failure to manufacture our tests in accordance with product specifications could result in increased costs, lost revenue, customer dissatisfaction, or voluntary product recalls, any of which could have a material adverse effect on our revenue, results of operations, and business.
Properly manufacturing our Pigmented Lesion Assay requires precise technological execution and strict compliance with our guidelines. We may experience problems in the manufacturing process for a number of reasons, such as equipment malfunction, failure to follow specific protocols, or human error. If problems arise during the production of a particular product lot, that product lot may need to be discarded or destroyed. This could, among other things, result in increased costs, lost revenue, customer dissatisfaction, and injury to our reputation. If problems are not discovered before the product lot is released to the market, we may incur recall and product liability costs. Any failure to manufacture our products in accordance with product specifications could have a material adverse effect on our revenue, results of operations, and business.
If we are unable to manufacture our tests in sufficient quantities, on a timely basis, and at acceptable costs, our ability to sell our tests will be harmed.
Our Pigmented Lesion Assay must be manufactured in sufficient quantities and on a timely basis, while maintaining product quality and acceptable manufacturing costs and complying with our requirements. In determining the required quantities of tests and the manufacturing schedule, we must make significant judgments and estimates based on historical experience, inventory levels, and other related factors. There could be significant differences between our estimates and the actual amounts of products we and our customers require, which could have a material adverse effect on our revenue, results of operations, and business.
Additional work will be required for scaling-up manufacturing of each new test prior to commercialization, and we may not successfully complete this work. Manufacturing and quality control problems may arise in the future as we attempt to scale-up our manufacturing of a new test, and we may not achieve scale-up in a timely
-22-
Table of Contents
manner, at a commercially reasonable cost, or at all. New tests that detect or quantify more than one target condition may contain significantly more complex reagents, which will increase the cost of our manufacturing processes and quality control testing. We may not be able to manufacture these products at a cost or in quantities that would make these products commercially viable. If we are unable to develop or contract for manufacturing capabilities on acceptable terms for our products under development, we may not be able to expand our product offerings.
If we were sued for product liability or professional liability, we could face substantial liabilities that exceed our resources.
The marketing, sale, and use of our current test and our planned future diagnostic tests could lead to the filing of product liability claims against us if someone alleges that our tests failed to perform as designed. We may also be subject to liability for errors in the test results we provide to physicians or for a misunderstanding of, or inappropriate reliance upon, the information we provide. A product liability or professional liability claim could result in substantial damages and be costly and time-consuming for us to defend.
Although we believe that our existing product and professional liability insurance is adequate, our insurance may not fully protect us from the financial impact of defending against product liability or professional liability claims. Any product liability or professional liability claim brought against us, with or without merit, could increase our insurance rates or prevent us from securing insurance coverage in the future. Additionally, any product liability lawsuit could damage our reputation, result in the recall of tests, or cause current partners to terminate existing agreements and potential partners to seek other partners, any of which could impact our results of operations.
If we use biological and hazardous materials in a manner that causes injury, we could be liable for damages.
Our activities currently require the controlled use of potentially harmful biological materials and chemicals. We cannot eliminate the risk of accidental contamination or injury to employees or third parties from the use, storage, handling or disposal of these materials. In the event of contamination or injury, we could be held liable for any resulting damages, and any liability could exceed our resources or any applicable insurance coverage we may have. Additionally, we are subject to, on an ongoing basis, federal, state, and local laws and regulations governing the use, storage, handling, and disposal of these materials and specified waste products. The cost of compliance with these laws and regulations may become significant and could have a material adverse effect on our financial condition, results of operations and cash flows. In the event of an accident or if we otherwise fail to comply with applicable regulations, we could lose our permits or approvals or be held liable for damages or penalized with fines.
We may acquire other businesses, form joint ventures, or make investments in other companies or technologies that could harm our operating results, dilute our stockholders’ ownership, increase our debt, or cause us to incur significant expense.
As part of our business strategy, we may pursue acquisitions of businesses and assets. We also may pursue strategic alliances and joint ventures that leverage our core technology and industry experience to expand our offerings or distribution. We have no experience with acquiring other companies and limited experience with forming strategic alliances and joint ventures. We may not be able to find suitable partners or acquisition candidates, and we may not be able to complete such transactions on favorable terms, if at all. If we make any acquisitions, we may not be able to integrate these acquisitions successfully into our existing business, and we could assume unknown or contingent liabilities. Any future acquisitions also could result in significant write-offs or the incurrence of debt and contingent liabilities, any of which could have a material adverse effect on our financial condition, results of operations and cash flows. Integration of an acquired company also may disrupt ongoing operations and require management resources that would otherwise focus on developing our existing
-23-
Table of Contents
business. We may experience losses related to investments in other companies, which could have a material negative effect on our results of operations. We may not identify or complete these transactions in a timely manner, on a cost-effective basis, or at all, and we may not realize the anticipated benefits of any acquisition, technology license, strategic alliance, or joint venture.
To finance any acquisitions or joint ventures, we may choose to issue shares of our common stock as consideration, which would dilute the ownership of our stockholders. If the price of our common stock is low or volatile, we may not be able to acquire other companies or fund a joint venture project using our stock as consideration. Alternatively, it may be necessary for us to raise additional funds for acquisitions through public or private financings. Additional funds may not be available on terms that are favorable to us, or at all.
International expansion of our business would expose us to business, regulatory, political, operational, financial, and economic risks associated with doing business outside of the United States.
Our business strategy contemplates possible international expansion, including partnering with academic and commercial testing laboratories, and introducing the Pigmented Lesion Assay or other future products outside the United States and exporting our adhesive patch sample collection kit. Doing business internationally involves a number of risks, including:
| • | multiple, conflicting, and changing laws and regulations such as tax laws, export and import restrictions, employment laws, intellectual property laws, regulatory requirements, and other governmental approvals, permits and licenses; |
| • | failure by us or our distributors to obtain regulatory approvals for the sale or use of our current test and our planned future tests in various countries; |
| • | difficulties in managing foreign operations; |
| • | complexities associated with managing government payor systems, multiple payor-reimbursement regimes, or self-pay systems; |
| • | logistics and regulations associated with shipping blood samples, including infrastructure conditions and transportation delays; |
| • | limits on our ability to penetrate international markets if our current test and our planned future diagnostic tests cannot be processed by an appropriately qualified local laboratory; |
| • | financial risks, such as longer payment cycles, difficulty enforcing contracts and collecting accounts receivable and exposure to foreign currency exchange rate fluctuations; |
| • | reduced protection for intellectual property rights, or lack of them in certain jurisdictions, forcing more reliance on any trade secrets we may have, if such protection is available; |
| • | natural disasters, political and economic instability, including wars, terrorism and political unrest, outbreak of disease, boycotts, curtailment of trade, and other business restrictions; and |
| • | failure to comply with the Foreign Corrupt Practices Act, including its books and records provisions and its anti-bribery provisions, by maintaining accurate information and control over sales activities and distributors’ activities. |
Any of these risks, if encountered, could significantly harm our future international expansion and operations and, consequently, have a material adverse effect on our financial condition, results of operations, and cash flows.
Declining general economic or business conditions may have a negative impact on our business.
Continuing concerns over United States health care reform legislation and energy costs, geopolitical issues, the availability and cost of credit, and government stimulus programs in the United States and other countries have contributed to increased volatility and diminished expectations for the global economy. These factors,
-24-
Table of Contents
combined with low business and consumer confidence and high unemployment, precipitated an economic slowdown and recession. If the economic climate does not improve, or it deteriorates, our business, including our access to patient samples and the addressable market for diagnostic tests that we may successfully develop, as well as the financial condition of our suppliers and our third-party payors, could be adversely affected, resulting in a negative impact on our business, financial condition, and results of operations.
Intrusions into our computer systems could result in compromise of confidential information.
Despite the implementation of security measures, our technology or systems that we interface with, including the Internet and related systems, may be vulnerable to physical break-ins, hackers, improper employee or contractor access, computer viruses, programming errors, or similar problems. Any of these might result in confidential medical, business or other information of other persons or of ourselves being revealed to unauthorized persons.
There are a number of state, federal and international laws protecting the privacy and security of health information and personal data. As part of the American Recovery and Reinvestment Act 2009, or ARRA, Congress amended the privacy and security provisions of the Health Insurance Portability and Accountability Act of 1996, or HIPAA. HIPAA imposes limitations on the use and disclosure of an individual’s healthcare information by healthcare providers, healthcare clearinghouses, and health insurance plans, collectively referred to as covered entities. The HIPAA amendments also impose compliance obligations and corresponding penalties for non-compliance on individuals and entities that provide services to healthcare providers and other covered entities, collectively referred to as business associates. ARRA also made significant increases in the penalties for improper use or disclosure of an individual’s health information under HIPAA and extended enforcement authority to state attorneys general. The amendments also create notification requirements for individuals whose health information has been inappropriately accessed or disclosed: notification requirements to federal regulators and in some cases, notification to local and national media. Notification is not required under HIPAA if the health information that is improperly used or disclosed is deemed secured in accordance with encryption or other standards developed by the U.S. Department of Health and Human Services, or HHS. Most states have laws requiring notification of affected individuals and state regulators in the event of a breach of personal information, which is a broader class of information than the health information protected by HIPAA. Many state laws impose significant data security requirements, such as encryption or mandatory contractual terms to ensure ongoing protection of personal information. Activities outside of the United States implicate local and national data protection standards, impose additional compliance requirements, and generate additional risks of enforcement for non-compliance. We may be required to expend significant capital and other resources to ensure ongoing compliance with applicable privacy and data security laws, to protect against security breaches and hackers or to alleviate problems caused by such breaches.
We depend on our information technology and telecommunications systems, and any failure of these systems could harm our business.
We depend on information technology and telecommunications systems for significant aspects of our operations. In addition, our third-party billing and collections provider depends upon telecommunications and data systems provided by outside vendors and information we provide on a regular basis. These information technology and telecommunications systems support a variety of functions, including test processing, sample tracking, quality control, customer service and support, billing and reimbursement, research and development activities, and our general and administrative activities. Information technology and telecommunications systems are vulnerable to damage from a variety of sources, including telecommunications or network failures, malicious human acts, and natural disasters. Moreover, despite network security and back-up measures, some of our servers are potentially vulnerable to physical or electronic break-ins, computer viruses, and similar disruptive problems. Despite the precautionary measures we have taken to prevent unanticipated problems that could affect our information technology and telecommunications systems, failures or significant downtime of our information technology or telecommunications systems, or those used by our third-party service providers could prevent us
-25-
Table of Contents
from processing tests, providing test results to oncologists, pathologists, billing payors, processing reimbursement appeals, handling patient or physician inquiries, conducting research and development activities, and managing the administrative aspects of our business. Any disruption or loss of information technology or telecommunications systems on which critical aspects of our operations depend could have an adverse effect on our business.
We rely on Federal Express Corporation, or FedEx, for the distribution of our products and, if FedEx incurs any damage to the facilities where our products are located or is unable to distribute our products as needed, it could have a material adverse effect on our results of operations and business.
We rely on FedEx for the distribution of our products. The FedEx facilities where our products are located may be harmed or rendered inoperable by natural or man-made disasters, including earthquakes, power outages, communications failure, or terrorism. Any material destruction to the facilities where our products are located could adversely affect the ability of FedEx to meet the needs of our customers. In addition, a disruption or slowdown in the operations of FedEx, including as a result of damage to the facilities of FedEx or a strike by FedEx employees, could cause delays in our ability to fulfill customer orders and may cause orders to be canceled, lost, or delivered late, our products to be returned, or receipt of products to be refused, any of which could adversely affect our business and our results of operations. If our shipping costs were to increase as a result of an increase by FedEx or as a result of obtaining a new third-party logistics company and if we are unable to pass on these higher costs to our customers, it could have a material adverse effect on our results of operations and business.
Regulatory Risks Relating to Our Business
Healthcare policy changes, including recently enacted legislation reforming the U.S. health care system, may have a material adverse effect on our financial condition, results of operations, and cash flows.
The 2010 Patient Protection and Affordable Care Act, as amended by the Health Care and Education Reconciliation Act, or collectively the ACA, makes a number of substantial changes in the way health care is financed by both governmental and private insurers. Among other things, the ACA:
| • | Mandates a reduction in payments for clinical laboratory services paid under the Medicare Clinical Laboratory Fee Schedule Annual Consumer Price Index update of 1.75% for the years 2011 through 2015. In addition, a permanent productivity adjustment is made to the fee schedule payment amount, which could range from 1.1% to 1.4% each year over the next 10 years. These changes in payments may apply to some or all of the tests we furnish to Medicare beneficiaries; |
| • | Establishes an Independent Payment Advisory Board to reduce the per capita rate of growth in Medicare spending if spending exceeds a target growth rate. The Independent Payment Advisory Board has broad discretion to propose policies, which may have a negative impact on payment rates for services, including clinical laboratory services, beginning in 2016, and for hospital services beginning in 2020; |
| • | Requires each medical device manufacturer to pay an excise tax equal to 2.3% of the price for which such manufacturer sells its medical devices, beginning in 2013; nevertheless, this could change in the future if either the FDA or the Internal Revenue Service, which regulates the payment of this excise tax, changes its position; and |
| • | Established the Physician Payments Sunshine Act which imposes new reporting and disclosure requirements for applicable device manufacturers of covered products and those entities under common ownership that provide assistance and support to applicable manufacturers, with regard to payments or other transfers of value made to certain practitioners (including physicians and teaching hospitals) and certain investment ownership interests held by physicians in the reporting entity. |
Although some of these provisions may negatively impact payment rates for clinical laboratory tests, the ACA also extends coverage to over 30 million previously uninsured people, which may result in an increase in
-26-
Table of Contents
the demand for our current test and our planned future tests. The mandatory purchase of insurance has been strenuously opposed by a number of state governors, resulting in lawsuits challenging the constitutionality of certain provisions of the ACA. In 2012, the Supreme Court upheld the constitutionality of the ACA, with the exception of certain provisions dealing with the expansion of Medicaid coverage under the law. Therefore, most of the law’s provisions will go into effect in 2013 and 2014. Congress has also proposed a number of legislative initiatives, including possible repeal of the ACA. At this time, it remains unclear whether there will be any changes made to the ACA, whether in part or in its entirety.
In addition, other legislative changes have been proposed and adopted since the ACA was enacted. The Budget Control Act of 2011, among other things, created the Joint Select Committee on Deficit Reduction to recommend proposals in spending reductions to Congress. The Joint Select Committee did not achieve a projected targeted deficit reduction of at least $1.2 trillion for the years 2013 through 2021, triggering the legislation’s automatic reduction to several government programs. This includes aggregate reductions to Medicare payments to providers and suppliers of up to 2% per fiscal year, starting in 2013. The full impact on our business of the ACA and the sequester law is uncertain. In addition, the Middle Class Tax Relief and Job Creation Act of 2012, or MCTRJCA, mandated an additional change in Medicare reimbursement for clinical laboratory tests. This legislation requires a rebasing of the Medicare Clinical Laboratory Fee Schedule to effect a 2% reduction in payment rates otherwise determined for 2013. This will serve as a base for 2014 and subsequent years. In January 2013, as a result of the changes mandated by the ACA and MCTRJCA, the Centers for Medicare & Medicaid Services, or CMS, reduced its reimbursement for laboratory tests for 2013 by approximately 3%.
Some of our laboratory test business is subject to the Medicare Physician Fee Schedule and, under the current statutory formula, the rates for these services are updated annually. For the past several years, the application of the statutory formula would have resulted in substantial payment reductions if Congress failed to intervene. In the past, Congress passed interim legislation to prevent the decreases. On November 1, 2013, CMS issued its 2014 Physician Fee Schedule Final Rule, or the 2014 Final Rule. In the 2014 Final Rule, CMS called for a reduction of approximately 23.7% in the 2014 conversion factor that is used to calculate physician reimbursement. If in future years Congress does not adopt interim legislation to block or offset, and/or CMS does not moderate, any substantial CMS-proposed reimbursement reductions, the resulting decrease in payments from Medicare could adversely impact our revenues and results of operations.
In addition, many of the Current Procedure Terminology, or CPT, codes were revised by the American Medical Association, effective January 1, 2013. In the 2014 Final Rule, CMS announced that it has decided to keep the new molecular codes on the Clinical Laboratory Fee Schedule rather than move them to the Physician Fee Schedule as some stakeholders had urged. Our reimbursement could be adversely affected by CMS’ actions. If it reduces reimbursement for the new test codes or does not pay for our codes, then our revenues would be adversely affected. There can be no guarantees that Medicare and other payors will establish positive or adequate coverage policies or reimbursement rates.
Currently, we are not subject to the Physician Payments Sunshine Act provisions of the ACA. However, if in the future the FDA determines that our Adhesive Skin Sample Collection Kit or any of our current or future products are subject to premarket clearance or approval process, we would be subject to the Physician Payments Sunshine Act.
The final rule implementing the Physician Payments Sunshine Act is complex, ambiguous, and broad in scope. If we are considered an “applicable manufacturer” under the Physician Payments Sunshine Act we will be subject to reporting requirements. In addition, certain of our subsidiaries may be found to be subject to the reporting requirements to the extent they provide assistance and support to our Company with respect to the manufacturing, marketing promotion, sale or distribution of the Company’s covered products. It is difficult to predict how the new requirements may impact existing relationships among manufacturers, distributors, physicians, and teaching hospitals. The Physician Payments Sunshine Act preempts similar state reporting laws,
-27-
Table of Contents
although we or our subsidiaries may be required to continue to report under certain provisions of such state laws. Failure to comply with the Physician Payments Sunshine Act may subject the Company to civil monetary penalties.
We cannot predict whether future health care initiatives will be implemented at the federal or state level, or how any future legislation or regulation may affect us. The expansion of government’s role in the U.S. health care industry as a result of the ACA’s implementation, and changes to the reimbursement amounts paid by Medicare and other payors for our current test and our planned future tests, may reduce our profits, if any, and have a materially adverse effect on our business, financial condition, results of operations, and cash flows. Moreover, Congress has proposed on several occasions to impose a 20% coinsurance payment requirement on patients for clinical laboratory tests reimbursed under the Medicare Clinical Laboratory Fee Schedule, which would require us to bill patients for these amounts. In the event that Congress were to ever enact such legislation, the cost of billing and collecting for our tests could often exceed the amount actually received from the patient.
Our commercial success could be compromised if customers do not pay our invoices or if third-party payors, including managed care organizations and Medicare, do not provide coverage and reimbursement, breach, rescind, or modify their contracts or reimbursement policies, or delay payments for our current test and our planned future tests.
Dermatologists may not order our current Pigmented Lesion Assay and our planned tests unless third-party payors, such as managed care organizations and government payors (e.g., Medicare and Medicaid), pay a substantial portion of the test price. Coverage and reimbursement by a third-party payor may depend on a number of factors, including a payor’s determination that tests using our technologies are:
| • | not experimental or investigational; |
| • | medically necessary; |
| • | appropriate for the specific patient; |
| • | cost-effective; |
| • | supported by peer-reviewed publications; and |
| • | included in clinical practice guidelines. |
Uncertainty surrounds third-party payor reimbursement of any test incorporating new technology, including tests developed using our technologies. Technology assessments of new medical tests conducted by research centers and other entities may be disseminated to interested parties for informational purposes. Third-party payors and health care providers may use such technology assessments as grounds to deny coverage for a test or procedure. Technology assessments can include evaluation of clinical utility studies, which define how a test is used in a particular clinical setting or situation.
Because each payor generally determines for its own enrollees or insured patients whether to cover or otherwise establish a policy to reimburse our tests, seeking payor approvals is a time-consuming and costly process. We cannot be certain that coverage for our current test and our planned future tests will be provided in the future by additional third-party payors or that existing agreements, policy decisions, or reimbursement levels will remain in place or be fulfilled under existing terms and provisions. If we cannot obtain coverage and reimbursement from private and governmental payors such as Medicare and Medicaid for our current test, or new tests or test enhancements that we may develop in the future, our ability to generate revenues could be limited, which may have a material adverse effect on our financial condition, results of operations, and cash flow. Further, we have experienced in the past, and will likely experience in the future, delays and interruptions in the receipt of payments from third-party payors due to missing documentation and/or other issues, which could cause delay in collecting our revenue.
-28-
Table of Contents
In addition, to the extent that our testing is ordered for Medicare inpatients and outpatients, only the hospital may receive payment from the Medicare program for the technical component of pathology services and any clinical laboratory services that we perform, unless the testing is ordered at least 14 days after discharge and certain other requirements are met. We therefore must look to the hospital for payment for these services under these circumstances. If hospitals refuse to pay for the services or fail to pay in a timely manner, our ability to generate revenues could be limited, which may have a material adverse effect on our financial condition, results of operations and cash flow.
We expect to depend on Medicare and a limited number of private payors for a significant portion of our revenues and if these or other payors stop providing reimbursement or decrease the amount of reimbursement for our current test and our planned future tests, our revenues could decline.
We believe that a portion of the patients for whom we would expect to perform our tests will have Medicare as their primary medical insurance. Medicare and other third-party payors may change their coverage policies or cancel future contracts with us at any time, review and adjust the rate of reimbursement, or stop paying for our tests altogether, which would reduce our total revenues. Payors have increased their efforts to control the cost, utilization, and delivery of health care services. In the past, measures have been undertaken to reduce payment rates for and decrease utilization of the clinical laboratory testing generally. Because of the cost-trimming trends, third-party payors that cover and provide reimbursement for our test and our planned tests may suspend, revoke, or discontinue coverage at any time, or may reduce the reimbursement rates payable to us. Any such action could have a negative impact on our revenues, which may have a material adverse effect on our financial condition, results of operations, and cash flows.
In addition, we are currently considered a “non-contracted provider” by private third-party payors because we have not entered into a specific contract to provide tests to their insured patients at specified rates of reimbursement. If we were to become a contracted provider with one more payors in the future, the amount of overall reimbursement we receive would likely decrease because we could be reimbursed less money per test performed at a contracted rate than at a non-contracted rate, which could have a negative impact on our revenues. Further, we typically are unable to collect payments from patients beyond that which is paid by their insurance and will continue to experience lost revenue as a result.
Because of certain Medicare billing policies, we may not receive complete reimbursement for tests provided to Medicare patients. Medicare reimbursement revenues are an important component of our business model, and private payors sometimes look to Medicare determinations when making their own payment determinations; therefore, incomplete or inadequate reimbursement from Medicare would negatively affect our business.
Medicare has coverage policies that can be national or regional in scope. Coverage means that the test or assay is approved as a benefit for Medicare beneficiaries. If there is no coverage, neither the supplier nor any other party, such as a reference laboratory, may bill Medicare or the beneficiary for the service. There is currently no national coverage policy regarding the Pigmented Lesion Assay. Because our laboratory is in California, the regional Medicare Administrative Contractor, or MAC, for California is the relevant MAC for all our testing. The current MAC for California, Noridian Healthcare Solutions, LLC, is adopting the coverage policies from Palmetto GBA. In the latter half of 2014, we plan to submit a comprehensive dossier explaining to Palmetto GBA and Noridian the benefits of the Pigmented Lesion Assay in order to persuade the MACs to allow coverage our test.
We cannot assure that, even if the Pigmented Lesion Assay and our future planned tests are otherwise successful, reimbursement from Medicare will be obtained. Even if Medicare reimbursement is obtained, we cannot assure that payment will be sufficient to produce revenues to enable us to reach profitability and achieve our other commercial objectives.
The processing of Medicare claims is subject to change at CMS’ discretion at any time. Cost containment initiatives may be a threat to Medicare reimbursement levels for the foreseeable future.
-29-
Table of Contents
Billing for our tests is complex and we must dedicate substantial time and resources to the billing process to be paid for our tests; long payment cycles of Medicare, Medicaid, and/or other third-party payors, or other payment delays, could hurt our cash flows and increase our need for working capital.
Billing for clinical laboratory testing services is complex, time-consuming, and expensive. Depending on the billing arrangement and applicable law, we bill various payors, including Medicare, Medicaid, private insurance companies, and patients, all of which have different billing requirements. To the extent laws or contracts require us to bill patient co-payments or co-insurance, we must also comply with these requirements. We may also face increased risks in our collection efforts, including potential write-offs of doubtful accounts, long collection cycles, and failure by third parties to properly process payment of claims in a timely manner which could adversely affect our business, results of operations, and financial condition. Several factors make the billing practice complex, including:
| • | compliance with complex federal and state regulations related to Medicare billing; |
| • | disputes among payors as to which party is responsible for payment; |
| • | differences in coverage among payors and effect of patient co-payments or co-insurance; |
| • | differences in information and billing requirements among payors; |
| • | incorrect or missing billing information; and |
| • | the resources required to manage the billing and claims appeals process. |
Additionally our billing activities require us to implement compliance procedures and oversight, train and monitor our employees, challenge coverage and payment denials, assist patients in appealing claims, and undertake internal audits to evaluate compliance with applicable laws and regulations as well as internal compliance policies and procedures. Payors also conduct external audits to evaluate payments, which add further complexity to the billing process.
Failure to comply with these billing requirements may result in non-payment, refunds, exclusion from government healthcare programs, and civil or criminal liabilities, any of which may have a material adverse effect on our revenues and earnings. These billing complexities and the related uncertainties in obtaining reimbursement could negatively affect our cash flow and our ability to achieve profitability.
Complying with numerous regulations pertaining to our business is an expensive and time-consuming process, and any failure to comply could result in substantial penalties.
Our laboratory is subject to CLIA, a federal law regulating clinical laboratories that perform testing on specimens derived from humans for the purpose of providing information for the diagnosis, prevention, or treatment of disease. Our clinical laboratory must be certified under CLIA in order for us to perform testing on human specimens. CLIA is intended to ensure the quality and reliability of clinical laboratories in the United States by mandating specific standards in the areas of personnel qualifications, administration, and participation in proficiency testing, patient test management, quality control, quality assurance, and inspections. We have a current certificate of registration from CMS to perform high-complexity testing, which is managed by California Laboratory Field Services, or CA LFS. To renew this certificate, we are subject to survey and inspection every two years. Moreover, CA LFS/CLIA inspectors may make periodic inspections of our clinical laboratory outside of the renewal process.
As we expand our geographic focus, we may need to obtain laboratory licenses from additional states to perform testing in our California lab on samples from those respective states. California laws establish standards for operation of our clinical laboratory, including the training and skills required of personnel, and quality control. In addition, California laws mandate proficiency testing, which involves testing of specimens that have been specifically prepared for the laboratory. Florida, Maryland, New York, and Rhode Island are states that require that we hold licenses to test specimens from patients in those states or received from ordering physicians
-30-
Table of Contents
in those states. As part of the various and variable State licensure processes, New York requires validation of our tests. We currently do not have the New York, Florida, Maryland and Rhode Island licenses, but we intend to apply for them and see no reason why they would not be granted. Other states may have similar requirements or may adopt similar requirements in the future. Finally, we may be subject to regulation in foreign jurisdictions if we seek to expand international distribution of our tests outside the United States.
We also intend to seek accreditation from the College of American Pathologists, or CAP, in 2014, which is a higher standard than that of the CLIA regulations. CAP is an independent, non-governmental organization of board-certified pathologists that accredits laboratories nationwide on a voluntary basis and that has been recognized by CMS as an accreditation organization to inspect laboratories to determine adherence to the CLIA standards. Since the CAP has deemed status with CA LFS, our post-CAP re-accreditation inspections will be performed by teams formed by the CAP.
If we were to lose our CLIA certification or California laboratory license, whether as a result of a revocation, suspension, or limitation, we would no longer be able to offer our tests, which would limit our revenues and harm our business. If we were to lose our license in any other state where we are required to hold a license, we would not be able to test specimens from those states.
If the FDA were to begin requiring approval or clearance of our current test and our planned future tests, we could incur substantial costs and time delays associated with meeting requirements for pre-market clearance or approval or we could experience decreased demand for, or reimbursement of, our tests.
Although the FDA maintains that it has authority to regulate the development and use of laboratory developed tests, or LDTs, such as ours and many other laboratories’ test as medical devices, it has not exercised its authority with respect to most LDTs as a matter of enforcement discretion. The FDA could, at any time, change its policy with regard to this matter.
We believe that our tests, as utilized in our clinical laboratory, are and would be LDTs. As a result, we believe that pursuant to the FDA’s current policies and guidance, the FDA does not require that we obtain regulatory clearances or approvals for our LDTs. The Adhesive Skin Sample Collection kit we provide for collection and transport of skin samples from a health care provider to our clinical laboratory is considered a Class I medical device subject to the FDA regulation, but is currently exempt from pre-market review by the FDA. However, the FDA could assert this device is Class II, which would subject it and our assay to pre-market clearance or approval processes, which could be time-consuming and expensive. While we believe that we are currently in material compliance with applicable laws and regulations, we cannot assure you that the FDA, or other regulatory agencies, would agree with our determination, and a determination that we have violated these laws, or a public announcement that we are being investigated for possible violations of these laws, could adversely affect our business, prospects, results of operations, or financial condition.
Moreover, FDA policy pertaining to diagnostic testing is continuing to evolve and is subject to ongoing review and revision. A significant change in any of the laws, regulations, or policies may require us to achieve regulatory compliance. At various times since 2006, the FDA has issued guidance documents or announced draft guidance regarding initiatives that may require varying levels of FDA oversight of our current test and our planned future tests. For example, in June 2010, the FDA announced a public meeting to discuss the agency’s oversight of LDTs prompted by the increased complexity of LDTs and their increasingly important role in clinical decision-making and disease management, particularly in the context of personalized medicine. The FDA indicated that it was considering a risk-based application of oversight to LDTs and that, following public input and discussion, it might issue separate draft guidance on the regulation of LDTs, which ultimately could require that we seek and obtain either pre-market clearance or approval of LDTs, depending upon the risk-based approach the FDA adopts. The public meeting was held in July 2010 and further public comments were submitted to the FDA through September 2010. The FDA has stated it is continuing to develop draft guidance in this area, however, such guidance did not appear in the FDA list of objectives for the current year. Section 1143
-31-
Table of Contents
of the Food and Drug Administration Safety and Innovation Act of 2012 requires the FDA to notify U.S. Congress at least 60 days before issuing a draft or final guidance regulating LDTs and to provide details of the anticipated action.
We cannot provide any assurance that FDA regulation, including pre-market review, will not be required in the future for our current test and our planned future tests, whether through additional guidance issued by the FDA, new enforcement policies adopted by the FDA or new legislation enacted by Congress. We believe it is possible that legislation will be enacted into law or guidance could be issued by the FDA, which may result in increased regulatory burdens for us to continue to offer our Pigmented Lesion Test or to develop and introduce new tests. Given the attention Congress continues to give to these issues, legislation affecting this area may be enacted and may result in increased regulatory burdens on us as we continue to offer our test and to develop and introduce new tests.
The requirement of pre-market review could negatively affect our business until such review is completed and clearance to market or approval is obtained. The FDA could require that we stop selling our tests pending pre-market clearance or approval. If the FDA allows our tests to remain on the market but there is uncertainty about our tests, if they are labeled investigational by the FDA or if labeling claims the FDA allows us to make are very limited, orders from dermatologists or reimbursement may decline. The regulatory approval process may involve, among other things, successfully completing additional clinical trials and making a 510(k) submission, or filing a pre-market approval, or PMA, application with the FDA. If the FDA requires pre-market review, our tests may not be cleared or approved on a timely basis, if at all. We may also decide voluntarily to pursue FDA pre-market review of our tests if we determine that doing so would be appropriate.
Additionally, should future regulatory actions affect any of the reagents we obtain from suppliers and use in conducting our tests, our business could be adversely affected in the form of increased costs of testing or delays, limits, or prohibitions on the purchase of reagents necessary to perform our testing.
Our business is subject to many laws and government regulations governing the manufacture and sale of medical devices.
The Adhesive Skin Sample Collection Kit we provide for collection and transportation of skin samples from a healthcare provider to our clinical laboratory is considered a Class I medical device subject to FDA regulations, but is currently exempt from pre-market review by FDA.
If the FDA decides to regulate LDTs, such as our Pigmented Lesion Assay, as medical devices we will be subject to increased regulatory burdens. Before a new medical device can be marketed in the United States, it must first receive either pre-market approval pursuant to a PMA or 510(k) clearance from the FDA, unless an exemption exists. The same rule applies when a manufacturer plans to market a medical device for a new intended use or significant modifications to the device are made. The process can be costly and time-consuming. The FDA’s goal is to respond to a 510(k) notification in 90 days, but often takes much longer. In addition, the FDA may request additional information in order to complete its review. Responding to such additional information requests can be time-consuming and burdensome, and can delay clearance of the 510(k). The PMA process usually takes six months to three years, but may take longer. We cannot assure that our Pigmented Lesion Assay or any new medical devices that we may develop or new uses for our products that we develop will be cleared or approved in a timely or cost-effective manner, if cleared or approved at all. Even if such devices are cleared or approved, the products may not be cleared or approved for all indications. Because medical devices may only be marketed for cleared or approved indications, this could significantly limit the market for that product and may adversely affect our results of operations.
From the time that our test entered commercial distribution, numerous regulatory requirements applied. These include the Quality System Regulation, or QSR, which imposes extensive design, testing, control, documentation, and other quality assurance requirements on the manufacturers of medical devices; labeling
-32-
Table of Contents
regulations; the FDA’s general prohibition against promoting products for unapproved or “off-label” uses; and the Medical Device Reporting regulation, which requires that manufacturers report to the FDA if their device may have caused or contributed to a death or serious injury. The FDA has broad post-market and regulatory and enforcement powers. Failure to comply with applicable United States medical device regulatory requirements could result in, among other things, warning letters, fines, injunctions, consent decrees, civil penalties, repairs, replacements, refunds, recalls or seizures of products, total or partial suspension of production, the FDA’s refusal to grant future premarket clearances or approvals, withdrawals or suspensions of current product applications, and criminal prosecution.
If we were required to conduct additional clinical studies or trials before continuing to offer tests that we have developed or may develop as LDTs, those studies or trials could lead to delays or failure to obtain necessary regulatory approval, which could cause significant delays in commercializing any future products and harm our ability to achieve sustained profitability.
If the FDA decides to require that we obtain 510(k) clearance or premarket approvals pursuant to a PMA to commercialize our current Pigmented Lesion Assay, or our planned future tests, we may be required to conduct additional pre-market clinical testing before submitting a regulatory notification or application for commercial sales. In addition, as part of our long-term strategy we may plan to seek FDA clearance or approval; however, we would need to conduct additional clinical validation activities on our tests before we can submit an application for FDA approval or clearance. Clinical trials must be conducted in compliance with FDA regulations or the FDA may take enforcement action or reject the data. The data collected from these clinical trials may ultimately be used to support market clearance or approval for our tests. We believe it would likely take two years or more to conduct the clinical studies and trials necessary to obtain approval from the FDA to commercially launch our current test and our planned future tests outside of our clinical laboratory. Even if our clinical trials are completed as planned, we cannot be certain that their results will support our test claims or that the FDA or foreign authorities will agree with our conclusions regarding our test results. Success in early clinical trials does not ensure that later clinical trials will be successful, and we cannot be sure that the later trials will replicate the results of prior clinical trials and studies. If we are required to conduct pre-market clinical trials, whether using prospectively acquired samples or archival samples, delays in the commencement or completion of clinical testing could significantly increase our test development costs and delay commercialization. Many of the factors that may cause or lead to a delay in the commencement or completion of clinical trials may also ultimately lead to delay or denial of regulatory clearance or approval. The commencement of clinical trials may be delayed due to insufficient patient enrollment, which is a function of many factors, including the size of the patient population, the nature of the protocol, the proximity of patients to clinical sites and the eligibility criteria for the clinical trial. Moreover, the clinical trial process may fail to demonstrate that our current test and our planned future tests are effective for the proposed indicated uses, which could cause us to abandon a test candidate and may delay development of other tests.
We may find it necessary to engage contract research organizations to perform data collection and analysis and other aspects of our clinical trials, which would increase the cost and complexity of our trials. We may also depend on clinical investigators, medical institutions, and contract research organizations to perform the trials properly. If these parties do not successfully carry out their contractual duties or obligations or meet expected deadlines, or if the quality, completeness, or accuracy of the clinical data they obtain is compromised due to the failure to adhere to our clinical protocols or for other reasons, our clinical trials may have to be extended, delayed or terminated. Many of these factors would be beyond our control. We may not be able to enter into replacement arrangements without undue delays or considerable expenditures. If there are delays in testing or approvals as a result of the failure to perform by third parties, our research and development costs would increase, and we may not be able to obtain regulatory clearance or approval for our current test and our planned future tests. In addition, we may not be able to establish or maintain relationships with these parties on favorable terms, if at all. Each of these outcomes would harm our ability to market our tests or to achieve sustained profitability.
-33-
Table of Contents
Our products may in the future be subject to product recalls. A recall of our products, either voluntarily or at the direction of government authorities, or the discovery of serious safety or effectiveness issues with our products, could have a significant adverse impact on our business.
Government authorities, including the FDA, have the authority to require the recall of commercialized products, including medical devices, in the event of material deficiencies in safety or effectiveness, defects in design or manufacture, failure to comply with QSR requirements, or failure to comply with other regulatory requirements. In the case of the FDA, the authority to require a recall must be based on an FDA finding that there is reasonable probability that the device would cause serious injury or death. In addition, if we conduct sales outside of the United States, certain non-U.S. governmental authorities would have the authority to require the recall of our products under certain circumstances, including in the event of material deficiencies or defects in design or manufacture. Manufacturers may, under their own initiative, recall a product if any material deficiency in a device is found. A government-mandated or voluntary recall by us could occur as a result of an unacceptable risk to health, product failures, malfunctions, manufacturing errors, design or labeling defects, or other deficiencies and issues. Recalls of any of our products would divert managerial and financial resources and would have an adverse effect on our reputation, results of operations, and financial condition, which could impair our ability to produce our products in a cost-effective and timely manner in order to meet our customers’ demands. As a result of a product recall, we may also be subject to liability claims, be required to bear other costs, or take other actions that may be a negative impact on our future sales and our ability to reach profitability.
We are subject to federal and state healthcare fraud and abuse laws and regulations and could face substantial penalties if we are unable to fully comply with such laws.
We are subject to health care fraud and abuse regulation and enforcement by both the federal government and the states in which we conduct our business. These health care laws and regulations include, for example:
| • | the federal Anti-Kickback Statute, which prohibits, among other things, persons or entities from soliciting, receiving, offering or providing remuneration, directly or indirectly, in return for or to induce either the referral of an individual for, or the purchase order or recommendation of, any item or services for which payment may be made under a federal health care program such as the Medicare and Medicaid programs; |
| • | the federal physician self-referral prohibition, commonly known as the Stark Law, which prohibits physicians from referring Medicare or Medicaid patients to providers of “designated health services” with whom the physician or a member of the physician’s immediate family has an ownership interest or compensation arrangement, unless a statutory or regulatory exception applies; |
| • | HIPAA, which establishes federal crimes for knowingly and willfully executing a scheme to defraud any health care benefit program or making false statements in connection with the delivery of or payment for health care benefits, items or services; |
| • | federal false claims laws, which, prohibit, among other things, individuals or entities from knowingly presenting, or causing to be presented, false or fraudulent claims for payment to the federal government; and |
| • | state law equivalents of each of the above federal laws, such as anti-kickback and false claims laws, which may apply to items or services reimbursed by any third-party payor, including commercial insurers. |
The risk of our being found in violation of these laws and regulations is increased by the fact that many of them have not been fully interpreted by the regulatory authorities or the courts, and their provisions are open to a variety of interpretations. Further, the ACA, among other things, amends the intent requirement of the federal Anti-Kickback Statute and certain criminal health care fraud statutes. Where the intent requirement has been lowered, a person or entity no longer needs to have actual knowledge of this statute or specific intent to violate it. In addition, because of amendments enacted in 2009 as part of the Fraud Enforcement and Recovery Act, the
-34-
Table of Contents
government may now assert that a claim including items or services resulting from a violation of the federal Anti-Kickback Statute constitutes a false or fraudulent claim for purposes of the false claims statutes. Any action brought against us for violation of these laws or regulations, even if we successfully defend against it, could cause us to incur significant legal expenses and divert our management’s attention from the operation of our business. If our operations are found to be in violation of any of these laws and regulations, we may be subject to any applicable penalty associated with the violation, including civil and criminal penalties, damages and fines, and/or exclusion from participation in Medicare, the California Medical Assistance Program (Medi-Cal—the California Medicaid program) or other state or federal health care programs. Additionally, we could be required to refund payments received by us, and we could be required to curtail or cease our operations. Any of the foregoing consequences could seriously harm our business and our financial results.
We may be required to comply with laws governing the transmission, security, and privacy of health information that require significant compliance costs, and any failure to comply with these laws could result in material criminal and civil penalties.
Under the administrative simplification provisions of HIPAA, HHS has issued regulations which establish uniform standards governing the conduct of certain electronic health care transactions and protecting the privacy and security of Protected Health Information used or disclosed by health care providers and other covered entities.
The privacy regulations regulate the use and disclosure of Protected Health Information by health care providers engaging in certain electronic transactions or “standard transactions.” They also set forth certain rights that an individual has with respect to his or her Protected Health Information maintained by a covered health care provider, including the right to access or amend certain records containing Protected Health Information or to request restrictions on the use or disclosure of Protected Health Information. The HIPAA security regulations establish administrative, physical, and technical standards for maintaining the integrity and availability of Protected Health Information in electronic form. These standards apply to covered health care providers and also to “business associates” or third parties providing services involving the use or disclosure of Protected Health Information. The HIPAA privacy and security regulations establish a uniform federal “floor” and do not supersede state laws that are more stringent or provide individuals with greater rights with respect to the privacy or security of, and access to, their records containing Protected Health Information. As a result, we may be required to comply with both HIPAA privacy regulations and varying state privacy and security laws.
Moreover, the Health Information Technology for Economic and Clinical Health Act, or HITECH, among other things, established certain health information security breach notification requirements. In the event of a breach of unsecured Protected Health Information, a covered entity must notify each individual whose Protected Health Information is breached, federal regulators and in some cases, must publicize the breach in local or national media. Breaches affecting 500 individuals or more are publicized by federal regulators who publicly identify the breaching entity, the circumstances of the breach and the number of individuals affected.
These laws contain significant fines and other penalties for wrongful use or disclosure of Protected Health Information. Given the complexity of HIPAA and HITECH and their overlap with state privacy and security laws, and the fact that these laws are rapidly evolving and are subject to changing and potentially conflicting interpretation, our ability to comply with the HIPAA, HITECH and state privacy requirements is uncertain and the costs of compliance are significant. Adding to the complexity is that our operations are evolving and the requirements of these laws will apply differently depending on such things as whether or not we bill electronically for our services, or provide services involving the use or disclosure of Protected Health Information and incur compliance obligations as a business associate. The costs of complying with any changes to the HIPAA, HITECH and state privacy restrictions may have a negative impact on our operations. Noncompliance could subject us to criminal penalties, civil sanctions and significant monetary penalties as well as reputational damage.
-35-
Table of Contents
Clinical research is heavily regulated and failure to comply with human subject protection regulations may disrupt our research program leading to significant expense, regulatory enforcement, private lawsuits, and reputational damage.
Clinical research is subject to federal, state, and, for studies conducted outside of the United States, international regulation. At the federal level, the FDA imposes regulations for the protection of human subjects and requirements such as initial and ongoing institutional review board review; informed consent requirements, adverse event reporting and other protections to minimize the risk and maximize the benefit to research participants. Many states impose human subject protection laws that mirror or in some cases exceed federal requirements. HIPAA also regulates the use and disclosure of Protected Health Information in connection with research activities. Research conducted overseas is subject to a variety of national protections such as mandatory ethics committee review, as well as laws regulating the use, disclosure and cross-border transfer of personal data. The costs of compliance with these laws may be significant and compliance with regulatory requirements may result in delay. Noncompliance may disrupt our research and result in data that is unacceptable to regulatory authorities, data lock, or other sanctions that may significantly disrupt our operations.
Violation of a state’s prohibition on the corporate practice of medicine could result in a material adverse effect on our business.
A number of states, including California, do not allow business corporations to employ physicians to provide professional services. This prohibition against the “corporate practice of medicine” is aimed at preventing corporations such as us from exercising control over the medical judgments or decisions of physicians. The state licensure statutes and regulations and agency and court decisions that enumerate the specific corporate practice rules vary considerably from state to state and are enforced by both the courts and regulatory authorities, each with broad discretion. If regulatory authorities or other parties in any jurisdiction successfully assert that we are engaged in the unauthorized corporate practice of medicine, we could be required to restructure our contractual and other arrangements. In addition, violation of these laws may result in sanctions imposed against us and/or the professional through licensure proceedings, and we could be subject to civil and criminal penalties that could result in exclusion from state and federal health care programs.
If we are sued for product liability or errors and omissions liability, we could face substantial liabilities that exceed our resources.
The marketing, sale and use of our Pigmented Lesion Assay could lead to product liability claims if someone were to allege that it failed to perform as it was designed. We may also be subject to liability for errors in results we provide to physicians or for misunderstanding of, or inappropriate reliance upon, the information we provide. We may also be subject to similar types of claims related to products we may develop in the future. A product liability or errors and omissions liability claim could result in substantial damages and be costly and time-consuming for us to defend. Although we maintain product liability and errors and omissions insurance, we cannot assure you that our insurance would fully protect us from the financial impact of defending against these types of claims, or any judgments, fines or settlement costs arising out of any such claims. Any product liability or errors and omissions liability claim brought against us, with or without merit, could increase our insurance rates or prevent us from securing insurance coverage in the future. Additionally, any product liability lawsuit could cause injury to our reputation or cause us to suspend sales of our product. The occurrence of any of these events could have an adverse effect on our business results of operations.
Intellectual Property Risks Related to Our Business
Our collaborators may assert ownership or commercial rights to inventions we develop from our use of the biological materials which they provide to us, or otherwise arising from the collaboration.
We collaborate with several institutions, physicians, and researchers in scientific matters. Also, we rely on numerous third parties to provide us with adhesive patch samples and biological materials that we use to develop tests. If we cannot successfully negotiate sufficient ownership, licensing, and/or commercial rights to any inventions that result from our use of a third-party collaborator’s materials, or if disputes arise with respect to the
-36-
Table of Contents
intellectual property developed with the use of a collaborator’s samples, or data developed in a collaborator’s study, our ability to capitalize on the market potential of these inventions or developments may be limited or precluded altogether.
If we are unable to maintain intellectual property protection, our competitive position could be harmed.
Our ability to protect our discoveries and technologies affects our ability to compete and to achieve sustained profitability. Currently, we rely on a combination of U.S. and foreign patents and patent applications, copyrights, trademarks and trademark applications, confidentiality or non-disclosure agreements, material transfer agreements, licenses, consulting agreements, work-for-hire agreements, and invention assignment agreements to protect our intellectual property rights. We also maintain certain company know-how, trade secrets, and technological innovations designed to provide us with a competitive advantage in the marketplace as trade secrets. Currently, we own five issued U.S. patents, eight pending U.S. patent applications, and their corresponding foreign counterpart patents and patent applications, relevant to our testing methodology and expression profiles. While we intend to pursue additional patent applications, it is possible that our pending patent applications and any future applications may not result in issued patents. Even if patents are issued, third parties may independently develop similar or competing technology that avoids our patents. Further, we cannot be certain that the steps we have taken will prevent the misappropriation of our trade secrets and other confidential information as well as the misuse of our patents and other intellectual property, particularly in foreign countries where we have not filed for patent protection.
From time-to-time the U.S. Supreme Court, other federal courts, the U.S. Congress or the U.S. Patent and Trademark Office, or the USPTO, may change the standards of patentability and any such changes could have a negative impact on our business. For instance, in 2008, the Court of Appeals for the Federal Circuit issued a decision that methods or processes cannot be patented unless they are tied to a machine or involve a physical transformation. The U.S. Supreme Court later reversed that decision inBilski v. Kappos, finding that the “machine-or-transformation” test is not the only test for determining patent eligibility. The Court, however, declined to specify how and when processes are patentable. In 2012, in the caseMayo Collaborative Services v. Prometheus Laboratories, Inc., the U.S. Supreme Court reversed the Federal Circuit’s application ofBilski and invalidated a patent focused on a diagnostic process because the patent claim embodied a law of nature. It is unclear at this time how the USPTO will apply its patent prosecution guidelines for determining patentability of diagnostic or other processes, and how lower courts will implement the decision. Some aspects of our technology involve processes that may be subject to this evolving standard and we cannot guarantee that any of our pending process claims will be patentable as a result of such evolving standards.
In 2013, inAssociation for Molecular Pathology v. Myriad Genetics, the Supreme Court unanimously ruled that, “[a] naturally occurring DNA segment is a product of nature and not patent eligible merely because it has been isolated,” thereby invalidating Myriad Genetics’ patents on the BRCA1 and BRCA2 breast cancer genes. However, the Supreme Court also held that manipulation of a gene to create something not found in nature, such as a strand of synthetically-produced complementary DNA, or cDNA, could still be eligible for patent protection. The Supreme Court noted that method patents, which concern technical procedures for carrying out a certain process, are not affected by the ruling.
It should also be noted that in 2010, the Secretary’s Advisory Committee on Genetics, Health and Society voted to approve a report entitled “Gene Patents and Licensing Practices and Their Impact on Patient Access to Genetic Tests.” That report defines “patent claims on genes” broadly to include claims to isolated nucleic acid molecules as well as methods of detecting particular sequences or mutations. The report also contains six recommendations, including the creation of an exemption from liability for infringement of patent claims on genes for anyone making, using, ordering, offering for sale, or selling a test developed under the patent for patient care purposes, or for anyone using the patent-protected genes in the pursuit of research. The report also recommended that HHS should explore, identify, and implement mechanisms that will encourage more voluntary adherence to current guidelines that promote nonexclusive in-licensing of diagnostic genetic and genomic technologies. It is unclear whether HHS will act upon these recommendations, or if the recommendations would
-37-
Table of Contents
result in a change in law or process that could negatively impact our patent portfolio or future research and development efforts. If acted upon, implementation of such provisions could have a material negative impact on our business.
We may face intellectual property infringement claims that could be time-consuming and costly to defend, and could result in the loss of significant rights, the implementation of an injunction, and the assessment of treble damages.
From time-to-time we may face intellectual property infringement or misappropriation claims from third parties. Some of these claims may lead to litigation. The outcome of any such litigation can never be guaranteed, and an adverse outcome could affect us negatively. For example, were a third party to succeed on an infringement claim against us, we may be required to pay substantial damages, including treble damages if such infringement were found to be willful. In addition, we could face an injunction barring us from conducting the allegedly infringing activity, including an order preventing us from offering our current test and future planned test in the marketplace. The outcome of the litigation could require us to enter into a license agreement which may not be pursuant to acceptable or commercially reasonable or practical terms or which may not be available at all.
It is also possible that an adverse finding of infringement against us may require us to dedicate substantial resources and time in developing non-infringing alternatives, which may or may not be possible. In the case of diagnostic tests, we would also need to include non-infringing technologies, which would require us to re-validate the test. Any such re-validation, in addition to being costly and time-consuming, may be unsuccessful.
Finally, we may initiate claims to assert or defend our own intellectual property against third parties. Any intellectual property litigation, irrespective of whether we are the plaintiff or the defendant, and regardless of the outcome, is expensive and time-consuming, and could divert and distract our management’s attention from our business and negatively affect our operating results or financial condition.
Risks Relating to Our Common Stock and This Offering
The price of our common stock may be volatile, and the market price of our common stock after this offering may drop below the initial public offering price.
The initial public offering price per share may vary from the market price of our common stock after the offering. If an active market for our stock develops and continues, our stock price nevertheless may be volatile. Market prices for securities of early-stage life sciences companies have historically been particularly volatile. As a result of this volatility, you may not be able to sell your common stock at or above the initial public offering price per share. The factors that may cause the market price of our common stock to fluctuate include, but are not limited to:
| • | actual or anticipated fluctuation in our financial condition and operating results; |
| • | progress, or lack of progress, in developing and commercializing our current Pigmented Lesion Assay and our planned future tests; |
| • | favorable or unfavorable decisions about our tests from government regulators, insurance companies, or other third-party payors; |
| • | our ability to recruit and retain qualified research and development personnel; |
| • | failure to meet or exceed financial estimates and projections of the investment community or that we provide to the public; |
| • | changes in investors’ and securities analysts’ perception of the business risks and conditions of our business; |
| • | changes in our relationship with key collaborators; |
-38-
Table of Contents
| • | changes in the market valuation or earnings of our competitors or companies viewed as similar to us; |
| • | competition from existing products or new products that may emerge; |
| • | changes in key personnel; |
| • | depth of the trading market in our common stock; |
| • | termination of the lock-up agreements or other restrictions on the ability of our existing stockholders to sell shares after this offering; |
| • | changes in our capital structure, such as future issuances of securities or the incurrence of additional debt; |
| • | the granting or exercise of employee stock options or other equity awards; |
| • | realization of any of the risks described under this section entitled “Risk Factors”; and |
| • | general market and economic conditions. |
In addition, the equity markets have experienced significant price and volume fluctuations that have affected the market prices for the securities of newly public companies for a number of reasons, including reasons that may be unrelated to our business or operating performance. These broad market fluctuations may result in a material decline in the market price of our common stock and you may not be able to sell your shares at prices you deem acceptable. In the past, following periods of volatility in the equity markets, securities class action lawsuits have been instituted against public companies. Such litigation, if instituted against us, could result in substantial cost and the diversion of management attention.
The NASDAQ Capital Market may not list our securities, which could limit investors’ ability to transact in our securities and subject us to additional trading restrictions.
We anticipate that our securities will be listed on The NASDAQ Capital Market, a national securities exchange, upon consummation of this offering. Although, after giving effect to this offering, we expect to meet, on a pro forma basis, The NASDAQ Capital Market’s minimum initial listing standards, which generally mandate that we meet certain requirements relating to stockholders’ equity, market capitalization, aggregate market value of publicly held shares, and distribution requirements, we cannot assure you that we will be able to meet those initial listing requirements. If The NASDAQ Capital Market does not list our securities for trading on its exchange, we could face significant material adverse consequences, including:
| • | a limited availability of market quotations for our securities; |
| • | reduced liquidity with respect to our securities; |
| • | a determination that our shares of common stock are “penny stock” which will require brokers trading in our shares of common stock to adhere to more stringent rules, possibly resulting in a reduced level of trading activity in the secondary trading market for our shares of common stock; |
| • | a limited amount of news and analyst coverage for our company; and |
| • | a decreased ability to issue additional securities or obtain additional financing in the future. |
The National Securities Markets Improvement Act of 1996, which is a federal statute, prevents or preempts the states from regulating the sale of certain securities, which are referred to as “covered securities.” Assuming our common stock will be listed on The NASDAQ Capital Market, our common stock will be covered securities. Although the states are preempted from regulating the sale of our securities, the federal statute does allow the states to investigate companies if there is a suspicion of fraud, and, if there is a finding of fraudulent activity, then the states can regulate or bar the sale of covered securities in a particular case. Further, if we were no longer listed on The NASDAQ Capital Market, our common stock would not be covered securities and we would be subject to regulation in each state in which we offer our securities.
-39-
Table of Contents
Our failure to meet the continued listing requirements of The NASDAQ Capital Market could result in a de-listing of our common stock.
If after listing we fail to satisfy the continued listing requirements of The NASDAQ Capital Market, such as the corporate governance requirements or the minimum closing bid price requirement, NASDAQ may take steps to de-list our common stock. Such a de-listing would likely have a negative effect on the price of our common stock and would impair your ability to sell or purchase our common stock when you wish to do so. In the event of a de-listing, we would take actions to restore our compliance with NASDAQ’s listing requirements, but we can provide no assurance that any such action taken by us would allow our common stock to become listed again, stabilize the market price or improve the liquidity of our common stock, prevent our common stock from dropping below the NASDAQ minimum bid price requirement or prevent future non-compliance with NASDAQ’s listing requirements.
If our shares become subject to the penny stock rules, it would become more difficult to trade our shares.
The SEC has adopted rules that regulate broker-dealer practices in connection with transactions in penny stocks. Penny stocks are generally equity securities with a price of less than $5.00, other than securities registered on certain national securities exchanges or authorized for quotation on certain automated quotation systems, provided that current price and volume information with respect to transactions in such securities is provided by the exchange or system. If we do not obtain or retain a listing on The NASDAQ Capital Market and if the price of our common stock is less than $5.00, our common stock will be deemed a penny stock. The penny stock rules require a broker-dealer, before a transaction in a penny stock not otherwise exempt from those rules, to deliver a standardized risk disclosure document containing specified information. In addition, the penny stock rules require that before effecting any transaction in a penny stock not otherwise exempt from those rules, a broker-dealer must make a special written determination that the penny stock is a suitable investment for the purchaser and receive (i) the purchaser’s written acknowledgment of the receipt of a risk disclosure statement; (ii) a written agreement to transactions involving penny stocks; and (iii) a signed and dated copy of a written suitability statement. These disclosure requirements may have the effect of reducing the trading activity in the secondary market for our common stock, and therefore stockholders may have difficulty selling their shares.
Our quarterly operating results may fluctuate significantly.
We are in the early stage of commercializing our Pigmented Lesion Assay, and the rate of market acceptance of our Pigmented Lesion Assay could vary considerably. As a result, we expect our operating results to be subject to quarterly fluctuations. Our net loss and other operating results will be affected by numerous factors, including:
| • | the rate of adoption and/or continued use of our current Pigmented Lesion Assay and our planned future tests; |
| • | variations in the level of expenses related to our development programs; |
| • | addition or reduction of resources for sales and marketing; |
| • | addition or termination of clinical utility studies; |
| • | any intellectual property infringement lawsuit in which we may become involved; |
| • | third-party payor determinations affecting our tests; and |
| • | regulatory developments affecting our tests. |
If our quarterly operating results fall below the expectations of investors or securities analysts, the price of our common stock could decline substantially. Furthermore, any quarterly fluctuations in our operating results may, in turn, cause the price of our stock to fluctuate substantially.
-40-
Table of Contents
The shares you purchase in this offering will experience immediate and substantial dilution and may also be diluted by exercises of outstanding options and warrants.
The initial public offering price per share of our common stock will be substantially higher than the net tangible book value per share of our common stock immediately after the offering. At an assumed initial public offering price of $ per share, purchasers of our common stock will effectively incur dilution of $ per share in the net tangible book value of their purchased shares. Conversely, the shares of our common stock that our existing stockholders currently own will receive a material increase in net tangible book value per share. As of March 31, 2014, we had outstanding options to purchase an aggregate of 394,563 shares of our common stock at a weighted average exercise price of $0.75 per share and warrants to purchase an aggregate of 295,623 shares of our common stock at an exercise price of $5.25 per share, including preferred stock warrants on an as-converted basis. The exercise of such outstanding options and warrants will result in further dilution of your investment. In addition, you may experience additional dilution if we issue common stock in the future. As a result of this dilution, you may receive significantly less than the full purchase price you paid for the shares in the event of liquidation.
Future sales of our common stock, or the perception that future sales may occur, may cause the market price of our common stock to decline, even if our business is doing well.
Sales of substantial amounts of our common stock after this offering, or the perception that these sales may occur, could materially and adversely affect the price of our common stock and could impair our ability to raise capital through the sale of additional equity securities. The shares of common stock sold in this offering will be freely tradable, without restriction, in the public market, except for any shares sold to our affiliates.
In connection with this offering, we have agreed, subject to limited exceptions, not to issue, sell, or transfer any shares of common stock for 180 days after the date of this prospectus without the consent of the underwriters. Our officers, directors, and certain stockholders have agreed before the commencement of this offering, subject to limited exceptions, not to sell or transfer any shares of common stock for 180 days after the date of this prospectus without the consent of the underwriters. However, the underwriters may release these shares from any restrictions at any time. We cannot predict what effect, if any, market sales of shares held by any stockholder or the availability of shares for future sale will have on the market price of our common stock.
Approximately million shares of common stock may be sold in the public market by existing stockholders on or about 181 days after the date of this prospectus, subject to volume and other limitations imposed under the federal securities laws. Sales of substantial amounts of our common stock in the public market after the completion of this offering, or the perception that such sales could occur, could adversely affect the market price of our common stock and could materially impair our ability to raise capital through offerings of our common stock. See the section entitled “Shares Eligible for Future Sale” for a more detailed description of the restrictions on selling shares of our common stock after this offering.
In addition, as of March 31, 2014, we had outstanding options to purchase 394,563 shares of our common stock and outstanding warrants to purchase shares of our common stock and of our Series B preferred stock overlying an estimated aggregate of 295,623 common stock equivalents. We plan to register for offer and sale the shares of common stock that are reserved for issuance pursuant to outstanding options. Shares covered by such registration statements upon the exercise of stock options generally will be eligible for sale in the public market, except that affiliates will continue to be subject to volume limitations and other requirements of Rule 144 under the Securities Act. The issuance or sale of such shares could depress the market price of our common stock.
In addition, we are registering the shares of our common stock underlying the warrants to be issued to the representative of the underwriters in connection with this offering as described in the “Underwriting—Representative’s Warrants” section of this prospectus.
-41-
Table of Contents
We also intend to register all shares of common stock that we may issue under our equity compensation plans. Once we register these shares, they can be freely sold in the public market upon issuance, subject to volume limitations applicable to affiliates and the lock-up agreements described in the “Underwriting” section of this prospectus.
No public market for our common stock currently exists and an active trading market may not develop for our common stock, and you may not be able to sell your stock at or above the initial public offering price per share.
There is currently no established trading market for our common stock, the market for our common stock will likely be highly volatile, and the market price of our common stock may decline regardless of our operating performance. Before this offering, you could not buy or sell our securities publicly. Although we anticipate that our common stock will be approved for listing on The NASDAQ Capital Market, an active public market for our common stock may not develop or be sustained after this offering. We cannot predict the extent to which investor interest in our company will lead to the development of an active trading market in our common stock or how liquid that market might become. If a market does not develop or is not sustained, it may be difficult for you to sell your shares of common stock at the time you wish to sell them, at a price that is attractive to you, or at all.
The initial public offering price per share has been determined through negotiation between us and representatives of the underwriters, and may not be indicative of the market price for our common stock after this offering. You may not be able to sell your shares at or above the initial public offering price per share.
If securities or industry analysts do not publish research or reports or publish unfavorable research or reports about our business, our stock price and trading volume could decline.
The trading market for our common stock will depend in part on the research and reports that securities or industry analysts publish about us, our business, our market or our competitors. We do not currently have and may never obtain research coverage by securities and industry analysts. If no securities or industry analysts commence coverage of our company, the trading price for our stock would be negatively impacted. In the event we obtain securities or industry analyst coverage, if one or more of the analysts who covers us downgrades our stock, our stock price would likely decline. If one or more of these analysts ceases to cover us or fails to regularly publish reports on us, interest in our stock could decrease, which could cause our stock price or trading volume to decline.
Our largest stockholder will continue to have substantial influence over us after this offering and could delay or prevent a change in corporate control.
Gary Jacobs, a member of board of directors, beneficially owns approximately 64% of our common stock and, upon the closing of this offering, assuming million shares of common stock are sold in this offering and there is no exercise of the underwriters’ option to purchase additional shares, will beneficially own approximately % of our common stock. Mr. Jacobs has significant influence over the outcome of matters submitted to our stockholders for approval, including the election of directors and any merger, consolidation, or sale of all or substantially all of our assets. Accordingly, this concentration of ownership might harm the market price of our common stock by:
| • | delaying, deferring, or preventing a change in control; |
| • | impeding a merger, consolidation, takeover, or other business combination involving us; or |
| • | discouraging a potential acquirer from making a tender offer or otherwise attempting to obtain control of us. |
-42-
Table of Contents
If we are unable to favorably assess the effectiveness of our internal control over financial reporting, investors may lose confidence in our financial reporting and our stock price could be materially adversely affected.
As a private company, we were not subject to the requirements of Section 404 of the Sarbanes-Oxley Act of 2002. After completion of this offering, we will be required to document and test our internal control over financial reporting. If we fail to remediate any significant deficiencies or material weaknesses in internal controls that may be identified in the future, we may be unable to conclude that our internal control over financial reporting is effective. Under the Jumpstart Our Business Startups Act of 2012, or the JOBS Act, issuers that qualify as “emerging growth companies” under the JOBS Act will not be required to provide an auditor’s attestation report on internal controls for so long as the issuer qualifies as an emerging growth company. We currently qualify as an emerging growth company under the JOBS Act and we may choose not to provide an auditor’s attestation report on internal controls. However, if we cannot favorably assess the effectiveness of our internal control over financial reporting, or if we require an attestation report from our independent registered public accounting firm in the future and that firm is unable to provide an unqualified attestation report on the effectiveness of our internal controls over financial reporting, investor confidence and, in turn, our stock price could be materially adversely affected.
We are an “emerging growth company,” and we cannot be certain if the reduced reporting requirements applicable to emerging growth companies will make our common stock less attractive to investors.
We are an emerging growth company as defined in the JOBS Act. For as long as we continue to be an emerging growth company, we may take advantage of exemptions from various reporting requirements that are applicable to other public companies that are not emerging growth companies, including not being required to comply with the auditor attestation requirements of Section 404 of the Sarbanes-Oxley Act of 2002, reduced disclosure obligations regarding executive compensation in this prospectus, and our periodic reports and proxy statements and exemptions from the requirements of holding non-binding advisory votes on executive compensation and stockholder approval of any golden parachute payments not previously approved. We could be an emerging growth company for up to five years, although circumstances could cause us to lose that status earlier, including if the market value of our common stock held by non-affiliates exceeds $700 million as of any June 30 before that time or if we have total annual gross revenue of $1.0 billion or more during any fiscal year before that time, in which cases we would no longer be an emerging growth company as of the following December 31 or, if we issue more than $1.0 billion in non-convertible debt during any three year period before that time, we would cease to be an emerging growth company immediately. Even after we no longer qualify as an emerging growth company, we may still qualify as a “smaller reporting company” which would allow us to take advantage of many of the same exemptions from disclosure requirements including not being required to comply with the auditor attestation requirements of Section 404 of the Sarbanes-Oxley Act and reduced disclosure obligations regarding executive compensation in this prospectus and our periodic reports and proxy statements. We cannot predict if investors will find our common stock less attractive because we may rely on these exemptions. If some investors find our common stock less attractive as a result, there may be a less active trading market for our common stock and our stock price may be more volatile.
Under the JOBS Act, emerging growth companies can also delay adopting new or revised accounting standards until such time as those standards apply to private companies. We have irrevocably elected not to avail ourselves of this exemption from new or revised accounting standards and, therefore, will be subject to the same new or revised accounting standards as other public companies that are not emerging growth companies. As a result, changes in rules of U.S. generally accepted accounting principles or their interpretation, the adoption of new guidance or the application of existing guidance to changes in our business could significantly affect our financial position and results of operations.
-43-
Table of Contents
We will incur significant increased costs as a result of operating as a public company, and our management will be required to devote substantial time to new compliance initiatives.
As a public company, we will incur significant legal, accounting and other expenses that we did not incur as a private company. We will be subject to the reporting requirements of the Securities Exchange Act of 1934, as amended, or the Exchange Act, which will require, among other things, that we file with the Securities and Exchange Commission, or the SEC, annual, quarterly and current reports with respect to our business and financial condition. In addition, the Sarbanes-Oxley Act, as well as rules subsequently adopted by the SEC and The NASDAQ Capital Market to implement provisions of the Sarbanes-Oxley Act, impose significant requirements on public companies, including requiring establishment and maintenance of effective disclosure and financial controls and changes in corporate governance practices. Further, there are significant corporate governance and executive compensation related provisions in the Dodd-Frank Wall Street Reform and Consumer Protection Act, enacted in 2010, that require the SEC to adopt additional rules and regulations in these areas such as “say on pay” and proxy access. Recent legislation permits smaller “emerging growth companies” to implement many of these requirements over a longer period and up to five years from the pricing of this offering. We intend to take advantage of this new legislation, but cannot guarantee that we will not be required to implement these requirements sooner than budgeted or planned and thereby incur unexpected expenses. Stockholder activism, the current political environment, and the current high level of government intervention and regulatory reform may lead to substantial new regulations and disclosure obligations, which may lead to additional compliance costs and impact the manner in which we operate our business in ways we cannot currently anticipate.
We expect the rules and regulations applicable to public companies to substantially increase our legal and financial compliance costs and to make some activities more time-consuming and costly. If these requirements divert the attention of our management and personnel from other business concerns, they could have a material adverse effect on our business, financial condition, and results of operations. The increased costs will decrease our net income or increase our consolidated net loss, and may require us to reduce costs in other areas of our business or increase the prices of our products or services. For example, we expect these rules and regulations to make it more difficult and more expensive for us to obtain director and officer liability insurance and we may be required to incur substantial costs to maintain the same or similar coverage. We cannot predict or estimate the amount or timing of additional costs we may incur to respond to these requirements. The impact of these requirements could also make it more difficult for us to attract and retain qualified persons to serve on our board of directors, our board committees, or as executive officers.
Our management will have broad discretion over the use of the proceeds we receive in this offering, and may not apply the proceeds in ways that increase the value of your investment.
We estimate that net proceeds of the sale of the common stock that we are offering will be approximately $ million and $ million, if the underwriters exercise their option to purchase additional shares in this offering in full. We currently intend to use the net proceeds of the offering to hire sales and marketing personnel and support increased sales and marketing activities, to fund further research and development, clinical utility studies and future enhancements of our tests, to acquire equipment, implement automation and scale our capabilities to prepare for significant test volume, and for general corporate purposes and to fund ongoing operations and expansion of our business. We will have broad discretion in the application of the net proceeds in the category of “for general corporate purposes and to fund ongoing operations and expansion of our business,” and investors will be relying on our judgment regarding the application of the proceeds of this offering. The actual amounts and timing of our actual expenditures depend on numerous factors, including the success of our efforts to market the Pigmented Lesion Assay, the timing and progress of our discovery, research, and development activities for the tests in our pipeline, our ability to reduce operating costs through the implementation of automation and economies of scale, changes in regulatory requirements for LDTs, and other unforeseen regulatory or compliance costs. The costs and timing of development activities, particularly conducting clinical utility studies and validation studies are highly uncertain, subject to substantial risks and can often change. Depending on the outcome of these activities and other unforeseen events, our plans and priorities
-44-
Table of Contents
may change and we may apply the net proceeds of this offering in different proportions than we currently anticipate. Moreover, you will not have the opportunity to influence our decision on how to use the proceeds from this offering. We may use the proceeds for corporate purposes that do not immediately enhance our prospects for the future or increase the value of your investment. See the section entitled “Use of Proceeds.”
Existing stockholders may view our initial public offering process unfavorably.
The process of effecting an initial public offering has taken considerable time and is associated with several personnel and other changes, including a reverse common stock split. Some of our current stockholders have invested in our securities at prices which are at or above the initial public offering price per share. No assurances can be given as to whether any stockholders will seek to take actions against our company or the board with respect to our initial public offering process.
Anti-takeover provisions of our certificate of incorporation, our bylaws, and Delaware law could make an acquisition of us, which may be beneficial to our stockholders, more difficult and may prevent attempts by our stockholders to replace or remove the current members of our board and management.
Certain provisions of our amended certificate of incorporation and amended and restated bylaws that will be in effect upon the completion of this offering could discourage, delay, or prevent a merger, acquisition or other change of control that stockholders may consider favorable, including transactions in which you might otherwise receive a premium for your shares. Furthermore, these provisions could prevent or frustrate attempts by our stockholders to replace or remove members of our board of directors. For example, Delaware law provides that if a corporation has a classified board of directors, stockholders cannot remove any director during his or her term without cause. These provisions also could limit the price that investors might be willing to pay in the future for our common stock, thereby depressing the market price of our common stock. Stockholders who wish to participate in these transactions may not have the opportunity to do so. These provisions, among other things:
| • | classify our board of directors into three classes of equal (or roughly equal) size, with all directors serving for a three-year term and the directors of only one class being elected at each annual meeting of stockholders, so that the terms of the classes of directors are “staggered”; |
| • | allow the authorized number of directors to be changed only by resolution of our board of directors; |
| • | authorize our board of directors to issue, without stockholder approval, preferred stock, the rights of which will be determined at the discretion of the board of directors and that, if issued, could operate as a “poison pill” to dilute the stock ownership of a potential hostile acquirer to prevent an acquisition that our board of directors does not approve; |
| • | establish advance notice requirements for stockholder nominations to our board of directors or for stockholder proposals that can be acted on at stockholder meetings; and |
| • | limit who may call a stockholders meeting. |
In addition, after we reincorporate in the state of Delaware in connection with this offering, we will be governed by the provisions of Section 203 of the Delaware General Corporation Law, or DGCL, which may, unless certain criteria are met, prohibit large stockholders, in particular those owning 15% or more of the voting rights on our common stock, from merging or combining with us for a prescribed period of time.
Because we do not expect to pay cash dividends for the foreseeable future, you must rely on appreciation of our common stock price for any return on your investment. Even if we change that policy, we may be restricted from paying dividends on our common stock.
We do not intend to pay cash dividends on shares of our common stock for the foreseeable future. Any determination to pay dividends in the future will be at the discretion of our board of directors and will depend upon results of operations, financial performance, contractual restrictions, restrictions imposed by applicable law,
-45-
Table of Contents
and other factors our board of directors deems relevant. Accordingly, you will have to rely on capital appreciation, if any, to earn a return on your investment in our common stock. Investors seeking cash dividends in the foreseeable future should not purchase our common stock.
Investors in this offering will pay a higher price than the book value of our common stock.
If you purchase common stock in this offering, you will pay more for your shares than the amounts paid by existing stockholders for their shares. You will incur immediate and substantial dilution of $ per share, representing the difference between our pro forma as adjusted net tangible book value per share after giving effect to this offering and an assumed initial public offering price of $ per share, the midpoint of the estimated price range set forth on the cover of this prospectus. In the past, we issued options and warrants to acquire capital stock at prices significantly below the assumed initial public offering price. To the extent any outstanding options or warrants are ultimately exercised, you will sustain further dilution. For further information on this calculation, see the section entitled “Dilution.”
Our ability to use our net operating loss carryforwards and certain other tax attributes may be limited.
Subject to certain limitations, a corporation may offset a net operating loss carryforward against profit earned in a future year to determine its U.S. federal income tax expenses for such year. Our ability to utilize our federal net operating loss carryforwards and federal tax credit may be limited under Sections 382 and 383 of the Internal Revenue Code of 1986, as amended. The limitations apply if an “ownership change,” as defined by Section 382, occurs. Generally, an ownership change occurs if the percentage of the value of the stock that is owned by one or more direct or indirect “five percent shareholders” increases by more than 50 percentage points over their lowest ownership percentage at any time during the applicable testing period, typically three years. If we have experienced an “ownership change” at any time since our formation, we may already be subject to limitations on our ability to utilize our existing net operating losses and other tax attributes to offset taxable income. In addition, future changes in our stock ownership, which may be outside of our control, may trigger an “ownership change” and, consequently, Section 382 and 383 limitations. As a result, if we earn net taxable income, our ability to use our pre-change net operating loss carryforwards and other tax attributes to offset United States federal taxable income may be subject to limitations, which could potentially result in increased future tax liability to us. State net operating loss carryforwards (and certain other tax attributes) may be similarly limited. As of December 31, 2013, we had federal and state net operating loss carryforwards of approximately $29.5 million and $25.4 million, respectively, and federal and California research and development and other tax credits of $456,000 and $490,000, respectively, which could be limited if we experience an “ownership change.”
We could be subject to securities class action litigation.
In the past, securities class action litigation has often been brought against a company following a decline in the market price of its securities. This risk is especially relevant for us because early-stage life sciences companies have experienced significant stock price volatility in recent years. If we face such litigation, it could result in substantial costs and a diversion of management’s attention and resources, which could harm our business.
-46-
Table of Contents
SPECIAL NOTE REGARDING FORWARD-LOOKING STATEMENTS
This prospectus contains forward-looking statements. All statements other than statements of historical facts contained in this prospectus, including statements regarding our future results of operations and financial position, business strategy, prospective tests, test approvals, timing and likelihood of success, plans and objectives of management for future operations, and future results of current and anticipated tests are forward-looking statements. These statements involve known and unknown risks, uncertainties and other important factors that may cause our actual results, performance, or achievements to be materially different from any future results, performance, or achievements expressed or implied by the forward-looking statements.
In some cases, you can identify forward-looking statements by terms such as “may,” “should,” “expect,” “plan,” “anticipate,” “could,” “intend,” “target,” “project,” “contemplates,” “believes,” “estimates,” “predicts,” “potential,” or “continue” or the negative of these terms or other similar expressions. The forward-looking statements in this prospectus are only predictions. We have based these forward-looking statements largely on our current expectations and projections about future events and financial trends that we believe may affect our business, financial condition, and results of operations. These forward-looking statements speak only as of the date of this prospectus and are subject to a number of risks, uncertainties, and assumptions described under the sections in this prospectus entitled “Risk Factors” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and elsewhere in this prospectus. Because forward-looking statements are inherently subject to risks and uncertainties, some of which cannot be predicted or quantified and some of which are beyond our control, you should not rely on these forward-looking statements as predictions of future events. The events and circumstances reflected in our forward-looking statements may not be achieved or occur and actual results could differ materially from those projected in the forward-looking statements. Moreover, we operate in an evolving environment. New risk factors and uncertainties may emerge from time-to-time, and it is not possible for management to predict all risk factors and uncertainties. Except as required by applicable law, we do not plan to publicly update or revise any forward-looking statements contained herein, whether as a result of any new information, future events, changed circumstances, or otherwise. The forward-looking statements contained in this prospectus are excluded from the safe harbor protection provided by the Private Securities Litigation Reform Act of 1995 and Section 27A of the Securities Act.
This prospectus also contains estimates and other statistical data made by independent parties and by us relating to market size and growth and other data about our industry. This data involves a number of assumptions and limitations, and you are cautioned not to give undue weight to such estimates. In addition, projections, assumptions, and estimates of our future performance and the future performance of the markets in which we operate are necessarily subject to a high degree of uncertainty and risk.
-47-
Table of Contents
We estimate that we will receive net proceeds from this offering of approximately $ million, or approximately $ million if the underwriters exercise their over-allotment option in full, assuming an initial public offering price of $ per share, the midpoint of the price range listed on the cover page of this prospectus, and after deducting the estimated underwriting discounts and commissions and estimated offering expenses payable by us. Each $1.00 increase (decrease) in the assumed initial public offering price of $ per share would increase (decrease) the net proceeds to us from this offering by approximately $ million, or approximately $ million if the underwriters exercise their over-allotment option in full, assuming the number of shares offered by us, as set forth on the cover page of this prospectus, remains the same and after deducting estimated underwriting discounts and commissions and estimated offering expenses payable by us.
We currently intend to use the net proceeds of the offering as follows:
| • | approximately $ million to hire sales and marketing personnel and support increased sales and marketing activities; |
| • | approximately $ million to fund further research and development, clinical utility studies, and future enhancements of our current test; |
| • | approximately $ million to fund research and development and clinical studies for our planned products; |
| • | approximately $ million to acquire equipment, implement automation, and scale our capabilities to prepare for significant test volume; and |
| • | the balance for general corporate purposes and to fund ongoing operations and the expansion of our business. |
The expected use of net proceeds of this offering represents our current intentions based upon our present plan and business conditions. As of the date of this prospectus, we cannot specify with certainty all of the particular uses for the net proceeds to be received upon the completion of this offering. We will have broad discretion in the application of the net proceeds in the category of “for general corporate purposes and to fund ongoing operations and the expansion of our business,” and investors will be relying on our judgment regarding the application of the proceeds of this offering. For example, if we identify opportunities that we believe are in the best interests of our stockholders, we may use a portion of the net proceeds from this offering to acquire, invest in, or license complementary products, technologies, or businesses although we have no current commitments, understandings, or agreements to do so.
-48-
Table of Contents
We have never declared dividends on our equity securities, and currently do not plan to declare dividends on shares of our common stock in the foreseeable future. We expect to retain our future earnings, if any, for use in the operation and expansion of our business. Subject to the foregoing, the payment of cash dividends in the future, if any, will be at the discretion of our board of directors and will depend upon such factors as earnings levels, capital requirements, our overall financial condition, and any other factors deemed relevant by our board of directors.
-49-
Table of Contents
The following table sets forth our capitalization as of March 31, 2014:
| • | on an actual basis; |
| • | on a pro forma basis as of March 31, 2014, to reflect (i) the conversion of all outstanding shares of our convertible preferred stock into shares of common stock, and (ii) the filing of our amended and restated certificate of incorporation upon the closing of this offering; and |
| • | on a pro forma as adjusted basis, to further reflect the sale and issuance by us of shares of common stock in this offering at an assumed initial public offering price of $ per share, the midpoint of the range reflected on the cover page of this prospectus, after deducting estimated underwriting discounts and commissions and estimated offering expenses. |
You should read this table together with the sections entitled “Selected Financial Data” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations” as well as our financial statements and the related notes, which appear elsewhere in this prospectus. All shares shown below have been adjusted for a one-for-seventy-five reverse stock split which we plan to effect prior to the completion of this Offering.
| As of March 31, 2014 | ||||||||||
| Actual | Pro Forma | Pro Forma, as Adjusted | ||||||||
(dollars in thousands, except share amounts) | ||||||||||
Series B convertible preferred stock, par value $0.0001 per share, 2,053,333 shares authorized; 1,645,821 shares issued and outstanding | $ | 0.2 | ||||||||
|
|
|
|
| ||||||
Series A-3 convertible preferred stock, par value $0.0001 per share, 59,030 shares authorized; 27,809 shares issued and outstanding | 0.0 | |||||||||
Series A-2 convertible preferred stock, par value $0.0001 per share, 58,486 shares authorized; 49,999 shares issued and outstanding | 0.0 | |||||||||
Series A-1 convertible preferred stock, par value $0.0001 per share, 76,427 shares authorized; 55,966 shares issued and outstanding | 0.0 | |||||||||
Common stock, $0.0001 par value, 2,804,619 shares authorized; 106,052 shares issued and outstanding | 0.0 | |||||||||
Additional paid-in capital | 40,002.6 | |||||||||
Accumulated deficit | (39,371.4 | ) | ||||||||
|
|
|
|
| ||||||
Total stockholders’ equity (deficit) | 631.4 | |||||||||
|
|
|
|
| ||||||
Total capitalization | $ | 631.4 | $ | |||||||
|
|
|
|
| ||||||
A $1.00 increase (decrease) in the assumed initial public offering price of $ per share, the midpoint of the estimated price range shown on the cover page of this prospectus, would increase (decrease) the amount of cash and cash equivalents, additional paid-in capital, total stockholders’ equity (deficit), and total capitalization on a pro forma as adjusted basis by approximately $ million, assuming the number of shares offered by us, as set forth on the cover page of this prospectus, remains the same and after deducting estimated underwriting discounts and commissions and estimated offering expenses payable by us. Similarly, each increase (decrease) of one million shares offered by us would increase (decrease) cash and cash equivalents, total stockholders’ equity (deficit), and total capitalization on a pro forma as adjusted basis by approximately $ million, assuming the assumed initial public offering price remains the same, and after deducting estimated underwriting discounts and commissions and estimated offering expenses payable by us. An increase in the number of shares offered by us together with a concomitant $1.00 increase in the assumed initial public offering price of $ per share, the
-50-
Table of Contents
midpoint of the price range set forth on the cover of this prospectus, would increase each of cash and cash equivalents and total stockholders’ (deficit) equity by approximately $ million after deducting underwriting discounts and commissions and any estimated offering expenses payable by us. Conversely, a decrease in the number of shares offered by us together with a concomitant $1.00 decrease in the assumed initial public offering price of $ per share, the midpoint of the price range set forth on the cover of this prospectus, would decrease each of cash and cash equivalents and total stockholders’ (deficit) equity by approximately $ million after deducting underwriting discounts and commissions and any estimated offering expenses payable by us. The pro forma as adjusted information discussed above is illustrative only and will be adjusted based on the actual public offering price and other terms of this offering determined at pricing.
The number of shares of common stock to be outstanding after the offering is based on the pro forma number of shares outstanding as of March 31, 2014 after giving effect to (i) the sale of shares of common stock in this offering, (ii) the conversion of all outstanding shares of our Series A preferred stock into shares of common stock, (iii) the conversion of all outstanding shares of our Series B preferred stock into shares of common stock, and (iv) the conversion of all outstanding warrants for our Series B preferred stock into shares of common stock, assuming for all such items an initial public offering price of $ per share. This number excludes:
| • | 394,563 shares of common stock issuable upon the exercise of stock options outstanding as of March 31, 2014, with a weighted average exercise price of $0.75 per share; |
| • | 32,281 shares of common stock issuable upon the exercise of warrants outstanding as of March 31, 2014, with a weighted average exercise price of $0.75 per share; |
| • | any shares of our common stock issuable upon exercise of the underwriters’ over-allotment option; |
| • | other shares of our common stock reserved for future issuance under our 2010 Stock Plan. |
-51-
Table of Contents
If you invest in our common stock in this offering, your ownership interest will be diluted to the extent of the difference between the public offering price per share of our common stock in this offering and our pro forma net tangible book value per share immediately after this offering. We calculate net tangible book value per share by dividing our net tangible book value, which is tangible assets less total liabilities less debt discounts, by the number of outstanding shares of our common stock. Before considering the effects of the proceeds of this offering, but giving effect to the completion of our initial public offering of shares of our common stock at $ per share, the conversion of our outstanding shares of Series A and Series B preferred stock into shares of our common stock in connection with our initial public offering our pro forma net tangible book value (deficit) as of March 31, 2014 was $ , or approximately $ per share. Upon completion of this offering, our pro forma net tangible book value as of March 31, 2014 will be approximately $ million or approximately $ per share. This represents an immediate increase in pro forma net tangible book value of $ per share to our existing stockholders and an immediate dilution of $ per share to new investors purchasing our common stock in this offering. The following table illustrates the per share dilution (unaudited):
Assumed initial public offering price per share | $ | |||||||
Historical net tangible book deficit per share as of March 31, 2014 | $ | |||||||
Pro forma net tangible book value per share as of March 31, 2014 | $ | |||||||
Increase in pro forma net tangible book value per share attributable to new investors in this offering | ||||||||
|
| |||||||
Pro forma as adjusted net tangible book value per share immediately after this offering | $ | |||||||
|
| |||||||
Dilution in pro forma net tangible book value per share to new investors in this offering | $ | |||||||
|
|
The information above assumes that the underwriters do not exercise their over-allotment option. If the underwriters exercise their over-allotment option in full, the pro forma net tangible book value will increase to $ per share, representing an immediate increase to existing stockholders of $ per share and an immediate dilution of $ per share to new investors. If any shares are issued upon exercise of outstanding options or warrants, new investors will experience further dilution.
A $1.00 increase (decrease) in the assumed initial public offering price of $ per share would increase (decrease) the pro forma as adjusted net tangible book value, by $ per share and the dilution to new investors by $ per share, assuming the number of shares offered by us, as set forth on the cover page of this prospectus, remains the same, and after deducting underwriting discounts and commissions and estimated expenses payable by us. Similarly, each increase (decrease) of one million shares offered by us would increase (decrease) the pro forma as adjusted net tangible book value by $ per share and the dilution to new investors by $ per share, assuming the assumed initial public offering price remains the same and after deducting underwriting discounts and commissions and estimated expenses payable by us. An increase in the number of shares offered by us together with a concomitant $1.00 increase in the assumed initial public offering price of $ per share, the midpoint of the price range set forth on the cover of this prospectus, would increase the pro forma as adjusted net tangible book value by approximately $ million after deducting underwriting discounts and commissions and any estimated offering expenses payable by us. Conversely, a decrease in the number of shares offered by us together with a concomitant $1.00 decrease in the assumed initial public offering price of $ per share, the midpoint of the price range set forth on the cover of this prospectus, would decrease the pro forma as adjusted net tangible book value by approximately $ million after deducting underwriting discounts and commissions and any estimated offering expenses payable by us. If the underwriters exercise their over-allotment option in full, the pro forma as adjusted net tangible book value would be $ per share, and the dilution in pro forma net tangible book value per share to investors in this offering would be $ per share.
-52-
Table of Contents
The following table summarizes, on a pro forma as adjusted basis as of March 31, 2014, the differences between the number of shares of common stock purchased from us, the total consideration and the average price per share paid by existing stockholders and by investors participating in this offering, after deducting estimated underwriting discounts and commissions and estimated offering expenses, at an initial public offering price of $ per share (unaudited).
| Shares Purchased | Total Consideration | Average Price Per Share | ||||||||||||||||
| Number | Percent | Amount | Percent | |||||||||||||||
Existing stockholders | % | $ | % | $ | ||||||||||||||
New investors | ||||||||||||||||||
|
|
|
|
|
|
| ||||||||||||
Totals | 100 | % | $ | 100 | % | |||||||||||||
|
|
|
|
|
|
| ||||||||||||
A $1.00 increase (decrease) in the assumed initial public offering price of $ per share, the midpoint of the price range set forth on the cover of this prospectus, would increase (decrease) the total consideration paid by new investors by approximately $ million, assuming the number of shares offered by us, as set forth on the cover page of this prospectus, remains the same and after deducting underwriting discounts and commissions and estimated offering expenses payable by us.
The number of shares of common stock to be outstanding after the offering is based on the pro forma as adjusted number of shares outstanding as of March 31, 2014 after giving effect to (i) the sale of shares of common stock in this offering, (ii) the conversion of all outstanding shares of our Series A preferred stock into 133,774 shares of common stock, (iii) the conversion of 1,645,821 shares of our Series B preferred stock into shares of common stock, and (iv) the conversion of all outstanding warrants for our Series B preferred stock into 263,341 shares of common stock, assuming for all such items an initial public offering price of $ per share, and excludes:
| • | 394,563 shares of common stock issuable upon the exercise of options outstanding as of March 31, 2014, with a weighted average exercise price of $0.75 per share; |
| • | 32,281 shares of common stock issuable upon the exercise of common stock warrants outstanding as of March 31, 2014, with a weighted average exercise price of $0.75 per share; |
| • | any shares of our common stock issuable upon exercise of the underwriters’ over-allotment option; and |
| • | shares of our common stock reserved for future issuance under our 2010 Stock Plan. |
-53-
Table of Contents
The following table summarizes our selected financial data for the periods and as of the dates indicated. Our selected statements of operations data for the years ended December 31, 2012 and 2013, and our selected balance sheet data as of December 31, 2012 and 2013, have been derived from our audited financial statements and their related notes, which are included elsewhere in this prospectus. All “Weighted average shares outstanding” data and all “Net loss per common share” data, whether derived from our audited financial statements or from our unaudited financial statements, have been adjusted to reflect a 1-for-75 reverse stock split which we plan to effect prior to the completion of this offering. Our selected statements of operations data for the three months ended March 31, 2013 and 2014 and our selected balance sheet data as of March 31, 2014 have been derived from our unaudited interim financial statements which are included elsewhere in this prospectus. Our unaudited financial statements have been prepared on the same basis as the audited financial statements and, in the opinion of management, include all adjustments, consisting of normal recurring adjustments necessary for a fair presentation of our financial condition as of such dates and our results of operations for such periods. Our historical results are not necessarily indicative of the results to be expected for any future periods and our interim results are not necessarily indicative of the results to be expected for the full fiscal year. Our selected financial data should be read together with the section entitled “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and with our financial statements and their related notes, which are included elsewhere in this prospectus.
Year Ended December 31, | Three Months Ended March 31, | |||||||||||||||
| 2012 | 2013 | 2013 | 2014 | |||||||||||||
| (in thousands, except per share data) | ||||||||||||||||
| (unaudited) | ||||||||||||||||
Statement of Operations Data: | ||||||||||||||||
Revenues | $ | — | $ | — | $ | — | $ | 31 | ||||||||
Cost of revenues | — | — | — | 10 | ||||||||||||
|
|
|
|
|
|
|
| |||||||||
Gross profit | — | — | — | 21 | ||||||||||||
Operating expenses: | ||||||||||||||||
Sales and marketing | 126 | 31 | 11 | 81 | ||||||||||||
Research and development | 976 | 1,958 | 368 | 728 | ||||||||||||
General and administrative | 1,064 | 1,273 | 295 | 585 | ||||||||||||
|
|
|
|
|
|
|
| |||||||||
Loss from operations | (2,166 | ) | (3,262 | ) | (674 | ) | (1,373 | ) | ||||||||
Total other income/(expense) | (62 | ) | (1,916 | ) | (50 | ) | (1 | ) | ||||||||
|
|
|
|
|
|
|
| |||||||||
Net loss | $ | (2,228 | ) | $ | (5,178 | ) | $ | (724 | ) | $ | (1,374 | ) | ||||
|
|
|
|
|
|
|
| |||||||||
Weighted average shares outstanding used in computing net loss per common share, basic and diluted (1) | 45,884 | 62,906 | 45,884 | 106,052 | ||||||||||||
Net loss per common share outstanding, basic and diluted | $ | (48.55 | ) | $ | (82.32 | ) | $ | (15.79 | ) | $ | (12.96 | ) | ||||
Weighted average shares outstanding used in computing pro forma net loss per share basic and diluted (unaudited) (2) | 902,449 | 1,993,289 | ||||||||||||||
Pro forma net loss per common share, basic and diluted (3) | $ | (5.74 | ) | $ | (0.69 | ) | ||||||||||
| (1) | Basic and diluted net loss per common share is determined by dividing net loss applicable to common shareholders by the weighted average common shares outstanding during the period. Because there is a net loss attributable to common shareholders for the years ended December 31, 2012 and 2013 and the three months ended March 31, 2013 and 2014, the outstanding shares of Preferred stock, warrants, and common stock options have been excluded from the calculation of diluted loss per common share because their effect would be anti-dilutive. Therefore, the weighted average shares used to calculate both basic and diluted loss per share are the same. Net loss per common share, diluted, excludes the effect of anti-dilutive equity instruments including 191,136, 735,414, 193,942 and 1,623,895 shares of common stock issuable upon conversion of our Preferred stock, and 8,241, 433,763, 7,087 and 690,185 shares of common stock issuable upon the exercise of outstanding warrants and stock options, for 2012, 2013 and the three months ended March 31, 2013 and 2014, respectively. |
-54-
Table of Contents
| (2) | The weighted average shares outstanding used in computing pro forma net loss per share assumes the conversion of all outstanding preferred stock and preferred stock warrants into an aggregate of 856,565 and 1,887,236 shares of common stock, respectively, for 2013 and the three months ended March 31, 2014. |
| (3) | The pro forma net loss per share, basic and diluted, is calculated using the net loss, adjusted to eliminate the interest on convertible debt during the period, divided by the pro forma weighted average shares outstanding, respectively for 2013 and the three months ended March 31, 2014. |
| As of December 31, | As of March 31, | |||||||||||||
| 2012 | 2013 | 2014 | Pro Forma As Adjusted(1) | |||||||||||
| (in thousands) | (in thousands, unaudited) | |||||||||||||
Balance Sheet Data: | ||||||||||||||
Cash and cash equivalents | $ | 1,353 | $ | 1,105 | $ | 1,487 | ||||||||
Total assets | 1,429 | 1,200 | 1,769 | |||||||||||
Current liabilities | 3,240 | 637 | 1,136 | |||||||||||
Total liabilities | 3,240 | 637 | 1,138 | |||||||||||
Convertible preferred stock | 0.0 | 0.2 | 0.2 | |||||||||||
Accumulated deficit | (32,819 | ) | (37,997 | ) | (39,371 | ) | ||||||||
Total stockholders’ equity (deficit) | (1,810 | ) | 564 | 631 | ||||||||||
| (1) | The pro forma as adjusted balance sheet data above reflects the conversion of all outstanding shares of preferred stock into an aggregate of 1,779,595 shares of our common stock, plus the sale of shares of our common stock in this offering and application of the net proceeds at an assumed initial public offering prices of $ per share, the midpoint of the pricing range set forth on the cover of this prospectus, after deducting the estimated underwriting discount and estimated offering expenses payable by us. |
-55-
Table of Contents
MANAGEMENT’S DISCUSSION AND ANALYSIS OF
FINANCIAL CONDITION AND RESULTS OF OPERATIONS
The following discussion of our financial condition and results of operations should be read together with our financial statements and related notes included elsewhere in the prospectus. This discussion contains forward-looking statements based upon our current plans, estimates, beliefs, and expectations that involve risks, uncertainties, and assumptions. Our actual results may differ materially from those anticipated in these forward-looking statements as a result of various factors, including those set forth under the sections entitled “Risk Factors,” “Special Note Regarding Forward-Looking Statements,” and elsewhere in this prospectus.
Overview
We are an emerging growth diagnostics company developing and marketing novel non-invasive gene expression tests to aid in the diagnosis of various skin conditions, including skin cancer, inflammatory diseases, and aging-related conditions. Our technology provides a highly accurate alternative to surgical biopsy, eliminating patient pain, scarring, and risk of infection, while maximizing convenience. Our scalable gene expression assays have been designed to work with a proprietary “adhesive patch biopsy” that provides a tissue sample non-invasively. Current dermatologic diagnosis is primarily based on subjective visual pattern recognition that is prone to error and results in a substantial number of unnecessary surgical biopsies.
We analyze the skin cells taken from the patient using our adhesive patch with a proprietary process that allows us to extract ribonucleic acid, or RNA, from the patch with sufficient quality and quantity to run our gene expression analysis. Our quantitative polymerase chain reaction, or qPCR, tests provide objective, biology-based information that is highly accurate and can significantly reduce unnecessary surgical biopsies. We offer our gene expression tests through our 6,000 square foot state-of-the-art government-licensed laboratory located in our company headquarters. We believe we will be the first company to offer a non-invasive gene expression test to the clinical dermatology market, which is in contrast to the vast majority of molecular diagnostic tests that are sold into the highly saturated pathology and oncology markets.
We are initially commercializing tests that will address unmet needs in the diagnostic pathway of pigmented skin lesions, such as moles or dark colored skin spots. Our products will facilitate the clinical assessment of pigmented skin lesions for melanoma, aid the histopathologic staging of melanoma, and determine the metastatic potential of melanoma. Our first product in this area is the Pigmented Lesion Assay, which can be used to reduce unnecessary surgical biopsy procedures by ruling out false positives based on visual assessment prior to performing a surgical biopsy. The development of our Pigmented Lesion Assay has involved over 500 lesion samples, including over 250 melanomas. We have completed the development of our Pigmented Lesion Assay and plan to introduce our product in the second half of 2014. We plan to initially market this test directly to a concentrated group of 250 to 500 dermatologists that treat a majority of the 750,000 patients with the highest risk of melanoma in the United States. While we have not yet generated any sales of our pigmented lesion assay, and have a history of net losses since our inception, we have begun generating revenue from our sample collection kits and CLIA testing services.
Financial Overview
Revenue
We are an emerging growth company and have begun to recognize revenue in 2014. We sell CLIA laboratory services which may include sample collection kits, assay development, gene expression analysis, data analysis and reporting.
-56-
Table of Contents
Operating Expenses
Sales and Marketing expenses
To date, our sales and marketing expenses have been related to market research, reimbursement efforts, trade show attendance, public relations, and general marketing. We do not have sales and marketing employees, but plan on establishing our initial sales force, executives and marketing support team during the second half of 2014. We expect our sales and marketing expenses to increase significantly as we implement a staged sales force expansion.
Research and Development Expenses
Since inception, we have focused our research and development on our gene classifier and related CLIA laboratory development. Our expenses consist primarily of salaries and fringe benefits, consulting costs, facilities, laboratory costs, equipment expense, and depreciation. More recently we have been incurring expenses relating to the clinical tests that we perform to validate the performance characteristics of our tests and to show medical cost benefit in support of our reimbursement efforts. We expect these expenses to increase significantly as we continue to develop new products and expand the use of our existing products.
General and Administrative Expenses
Our general and administrative expenses consist of senior management compensation, consulting, legal, human resources, information technology, accounting, insurance, and general business expenses. We expect our general and administrative expenses, including insurance, accounting, and legal fees, to increase after we become a public company.
Critical Accounting Policies and Significant Judgments and Estimates
Our management’s discussion and analysis of financial condition and results of operations is based on our financial statements, which have been prepared in accordance with accounting principles generally accepted in the United States or GAAP. The preparation of our financial statements requires us to make estimates and judgments that affect the reported amounts of assets, liabilities, revenues and expenses and related disclosure of contingent assets and liabilities. On an ongoing basis, we evaluate our estimates based on historical experience and make various assumptions, which management believes to be reasonable under the circumstances, which form the basis for judgments about the carrying values of assets and liabilities that are not readily apparent from other sources. Actual results may differ from these estimates under different assumptions or conditions.
The notes to our audited and unaudited financial statements, which are included elsewhere in this prospectus, contain a summary of our significant accounting policies. We consider the following accounting policies critical to the understanding of the results of our operations.
Stock based compensation
We have granted stock options to employees and consultants for the purchase of common stock. These options generally have a ten-year life during which the option holder can exercise at any time; they generally vest over a four-year service period at the rate of 25% per year, and their exercise price equals the fair market value of our common stock on the date they are granted. Certain options, used in lieu of cash compensation, vest more quickly or vest upon completion of specific milestones.
Compensation costs associated with stock option awards and other forms of equity compensation are measured at the grant-date fair value of the awards and recognized over the requisite service period of the awards on a straight-line basis. No related tax benefits of the share-based compensation costs have been utilized since the date our company entered the development stage.
-57-
Table of Contents
The stock options grants have exercise prices equal to the market value of the underlying stock, as determined by the board of directors and outside valuation experts on the date the equity award was granted. The board of directors determined the value of the underlying stock by considering a number of factors, including historical and projected financial results, the risks our company faced at the time, the preferences of our company’s preferred stockholders, and the lack of liquidity of our company’s common stock.
The fair value of each option award is estimated on the date of grant using the Black-Scholes-Merton valuation model. The expected term of options is based on the simplified method set out in Staff Accounting Bulletin (SAB) No. 110, Share-Based Payment (SAB No. 110). The simplified method defines the life as the average of the contractual term of the options and the weighted-average vesting period for all option tranches. The expected volatility of stock options is based upon the historical volatility of a number of publicly traded companies in similar stages of development. The risk-free interest rate is based on the average yield of U.S. Treasury securities with remaining terms similar to the expected term of the share-based awards. The assumed dividend yield was based on our company’s expectation of not paying dividends in the foreseeable future.
Equity instruments awarded to non-employees are periodically re-measured as the underlying awards vest, unless the instruments are fully vested, immediately exercisable, and nonforfeitable on the date of grant.
Our company granted 164,000 options on November 7, 2013 with an exercise price of $0.75 and an estimated fair value of $0.75.
The fair value of each option granted during 2013 was estimated on the date of grant using the following assumptions:
Year Ended | ||
Assumed risk-free interest rate | 1.3% to 2.7% | |
Assumed volatility | 60.0% to 63.0% | |
Expected option term (years) | 4.8 years to 10 years | |
Expected dividend yield | — % |
Our company recorded stock based compensation expense of $22,037 for the year ended December 31, 2013. The total compensation cost related to nonvested awards not yet recognized at December 31, 2013 was $48,099 which is expected to be recognized on a straight-line basis over a weighted-average term of 1.1 years.
The fair value of the common stock underlying our equity awards was assessed on each grant date by our board of directors. Given the absence of an active market for our common stock prior to this offering, our board of directors determined the estimated fair value of our common stock based on an analysis of a number of objective and subjective factors that we believe market participants would consider, including the following:
| • | the contemporaneous valuations of our common stock by an unrelated third-party valuation firm; |
| • | the going-concern opinion received by our auditors pending then and confirmed on March 6, 2014 for the 2012 fiscal year and subsequently for the 2013 fiscal year; |
| • | the lack of existing investors’ willingness to continue to support our company; |
| • | the prices of our convertible preferred stock sold to outside investors in arms-length transactions; |
| • | the rights, preferences and privileges of our convertible preferred stock relative to those of our common stock; |
| • | the rights of freestanding warrants and other similar instruments related to our securities; |
| • | the hiring of key personnel; |
-58-
Table of Contents
| • | the stage of development and status of commercialization with respect to our products; |
| • | the risks inherent in the development and expansion of our products and services; |
| • | the fact that the option grants involve illiquid securities in a private company; |
| • | our results of operations, history of losses and other financial metrics; |
| • | our capital resources and financial condition; |
| • | and the deteriorating general market conditions for biotechnology and medical technology companies; and |
| • | the likelihood of achieving a liquidity event, such as an initial public offering or sale of our company given prevailing market conditions. |
We believe that our board of directors’ estimate of the fair value of our common stock was reasonable and consistent with the risks and preferences of our company’s stockholders and option holders at each period measured.
Valuation of Common Stock
Our equity structure was re-organized in July 2010. At that time, the majority of our preferred stock was converted into common stock and then we effected a 20-for-1 reverse stock split. Our company had been funded primarily by convertible promissory notes, which were segregated into two tranches. The first tranche of convertible promissory notes, or Junior notes, were converted into common stock and subject to a reverse split. The second tranche of convertible promissory notes, or Senior notes, were converted into Series A Preferred stock at a conversion price of one share for $30.75 for the 2010 convertible promissory notes, and one share for $56.25 for the 2008/2009 convertible promissory notes. At that time, and through 2012, the board of directors determined the value of the common stock and no external valuation experts were utilized. Stock options were granted with an exercise price of $10.50 per underlying common share.
The economic conditions related to the 2008/2009 financial crisis, and thereafter, exerted substantial negative effects on our company’s ability to raise capital and continue its development activities and impacted the value of its equity securities.
In 2013, our company engaged an experienced valuation firm to value the common stock for the purpose of complying with the Internal Revenue Code Section 409A, or IRC 409A. These experts performed an analysis of the fair value of our common stock for financial reporting and tax reporting purposes as outlined under Financial Accounting Standards Board (FASB) Accounting Standards Codification (ASC) Topic 718:Compensation–Stock Compensationand the IRC 409A, in relation to the valuation of privately-held equity securities issued as compensation. The valuation date of this analysis was October 15, 2013.
The principal procedures used in formulating the estimates of fair value included, without limitation, the following:
| • | the nature of and business operations of our company, including historical performance; |
| • | recent arm’s-length transactions involving the sale or transfer of our company’s stock; |
| • | financial statements of our company; |
| • | analyses of the economy and the industry within which our company operates; |
| • | estimation of fair value using various valuation approaches and methodologies; and |
| • | other relevant factors such as discounts for lack of marketability. |
Given the early stage of our company, the “backsolve method” was used to estimate the fair value of our securities. This method is a market approach that derives an implied market value of invested capital from a
-59-
Table of Contents
transaction involving a company’s own securities. The price of a company’s security that was involved in a recent arms-length transaction is used as a reference point in an allocation of value. Our Series B offering included six new investors and twenty existing investors. New money investors paid $6.00 for one share of Series B preferred stock and one-half of one share of a Series B preferred stock warrant (50% warrant coverage). Existing investors with outstanding convertible promissory notes were converted into shares of Series B preferred stock at a conversion price of $3.00 per share. The backsolve method requires considering the rights and preferences of each class of equity and solving for the total market value of invested capital that is consistent with a recent transaction in the company’s own securities, considering the rights and preferences of each class of equity. Per the American Institute of CPAs, or AICPA, Accounting and Valuation Guide for the Valuation of Privately-Held-Company Equity Securities Issued as Compensation, or the AICPA Accounting Guide, the backsolve method is generally the most reliable indicator of value of early-stage enterprises with no product revenue or cash flow, if relevant and reliable transactions have occurred in the company’s equity securities. This methodology is also prescribed by the AICPA when a valuation is conducted in close proximity to the date of a financing transaction, and when other methodologies are deemed less reliable.
The stage of development of the company’s technology was reflected in our selection of the term and volatility estimates used in the backsolve option pricing method analysis. The estimate of the term considers the company’s existing cash runway and the time to the next potential financing or liquidity event, while the volatility estimate reflects the relative riskiness of the company’s equity securities (or asset base) relative to the general stock market.
On August 15, 2013, we raised additional capital through the sale of Series B preferred stock. Investors paid $6.00 for one share of Series B preferred stock and, in connection with their purchase, investors also received a warrant to purchase an amount of shares of Series B preferred stock equal to 50% of the total shares they purchased, (50% warrant coverage). Our board of directors approved an amendment to the Series B preferred warrants such that if the warrants were exercised prior to March 1, 2014, then the warrant holders would receive one additional warrant. Effectively, the investors paid $6.00 for one share of Series B preferred stock and one warrant to purchase one share of a Series B preferred stock. Investors who had funded our company with convertible debt during 2012 were given a discount upon conversion of their debt into Series B preferred stock of 50%. These investors in our convertible debt paid $3.00 per share of Series B preferred stock.
Our experts estimated the implied market value of invested capital of our company by backsolving for the purchase price of $6.00 for one share of Series B preferred stock and one warrant to purchase one share of Series B preferred stock through the option-pricing method. The premise of this method is that the transaction implied a market price for a share which in turn implied values for the other classes of equity based on relative claims on equity value, such as liquidation preferences and conversion rights. The application of the backsolve method considering our company’s capital structure yielded a total market value of invested capital of $9,175,850 of which $137,999 was allocated to the total value of common stock.
After estimating the market value of invested capital, it must be allocated to the various equity classes comprising the subject company’s capitalization table. This process ultimately results in creating a final estimate of value for the subject company’s underlying equity interests. While there are many different value allocation methods, these various methods can be grouped into three general categories as defined by the AICPA Accounting Guide:
| • | Probability-Weighted Expected Return Method (PWERM); |
| • | The Option-Pricing Method (OPM); and |
| • | The Current-Value Method. |
We used the OPM to allocate market value of invested capital to the various equity classes and debt comprising our company’s capitalization structure. We chose the OPM over the PWERM and Current Value
-60-
Table of Contents
methods due to the complex capital structure of our company, the uncertainty related to market conditions, and the lack of visibility on an imminent exit event. Under the OPM, each equity class is modeled as a call option with a distinct claim on the equity of the company. The option’s exercise price is based on the company’s total equity value available for each participating equity holder. The characteristics of each equity class determine the equity class’ claim on the total equity value. By constructing a series of options in which the exercise price is set at incremental levels of value, which correspond to the equity value necessary for each level of equity to participate, we determined the incremental option value of each series. When multiplied by the percentage of ownership of each equity class participating under that series, the result is the incremental value allocated to each class under that series.
The OPM relies on the Black-Scholes option-pricing model to value the call options on the company’s invested capital. The following inputs were applied in the Black-Scholes calculations of the OPM:
| • | Underlying Security Price of $9,175,850; |
| • | Expected Term of 1.75 years; |
| • | Expected Volatility as 90.0%. The volatility relies on an analysis of historical volatilities derived from a peer set of reasonably similar guideline public companies; and |
| • | Risk-Free Rate 0.16% based on the yield on U.S. Treasuries with a 1.75-year term. |
Discounts were applied for lack of control and lack of marketability for the common stock. The calculation resulted in a fair value for the common stock of less than $0.01 per share. This amount was rounded up to $0.01 per share.
The table below details the historical valuations of our Preferred and Common stock:
Equity Series | 2010 | 2011 | 2012 | 2013 | ||||||||||||
Series A | $ | 30.75-$60.00 | $ | 30.75-$60.00 | $ | 30.75-$60.00 | $ | 6.00 | ||||||||
Series B | $ | 3.00-$6.00 | ||||||||||||||
Common | $ | 10.50 | $ | 10.50 | $ | 10.50 | $ | 0.75 | ||||||||
Based on the significant decline in the value of our company’s preferred and common stock and the 409A common stock valuation, our company re-priced the outstanding stock options in January 2014.
Results of Operations
Comparison of the years ended December 31, 2012 and 2013
The following table sets forth the results of operations for the years ended December 31, 2012 and 2013:
| Year ended December 31, | ||||||||||||
| 2012 | 2013 | Change | ||||||||||
| (dollars in thousands) | ||||||||||||
Revenues | $ | — | $ | — | $ | — | ||||||
Sales and marketing | 126 | 31 | (95 | ) | ||||||||
Research and development | 976 | 1,958 | 982 | |||||||||
General and administrative | 1,064 | 1,273 | 209 | |||||||||
|
|
|
|
|
| |||||||
Loss from operations | (2,166 | ) | (3,262 | ) | 1,096 | |||||||
Interest expense, net and other | (62 | ) | (1,916 | ) | 1,854 | |||||||
|
|
|
|
|
| |||||||
Net loss | $ | (2,228 | ) | $ | (5,178 | ) | $ | 2,950 | ||||
|
|
|
|
|
| |||||||
-61-
Table of Contents
Operating expenses
Sales and marketing expense of $31,000 for the year ended December 31, 2013 declined $95,000 compared to the expense of $126,000 during the year ended December 31, 2012. The reduction in expenses was due to reduced sales, marketing, and business development contractors.
Research and development expense of $2.0 million for the year ended December 31, 2013 increased $1.0 million compared to an expense of $1.0 million for the year ended December 31, 2012. The increase was primarily related to an increase of $378,000 for product development, $230,000 for clinical efforts, $99,000 for clinical software development, and accelerated analysis of specimens including in-vitro analysis and pathology services. We also increased assay development activities and hired a consulting Chief Scientific Officer and laboratory technicians, and completed two additional studies for assay validation.
General and administrative expense of $1.3 million for the year ended December 31, 2013 increased $209,000 compared to $1.1 million in expense for the year ended December 31, 2012. Payroll and consulting costs increased $230,000 and facility costs increased $113,000, and these were partially offset by $118,000 in lower legal expenses. We hired our Chief Executive Officer in June 2012 resulting in one-half year of this cost in 2012 compared to a full-year in 2013. In April 2013, we moved into a new facility generating additional expense for relocation and rent.
Interest expenseof $1.9 million for the year ended December 31, 2013 was an increase of $1.9 million compared to a $62,000 expense for the year ended December 31, 2012. We recorded a beneficial conversion charge to interest expense in 2013 of $1.8 million that reflected the 50% discount that convertible debt holders received upon their conversion into Series B preferred stock. We also had an increase of $63,000 in convertible debt interest in 2013 compared to 2012 due to higher levels of convertible debt.
Comparison of the three months ended March 31, 2013 and 2014
The following table sets forth the results of operations for the three months ended March 31, 2013 and 2014:
Three Months ended March 31, | ||||||||||||
| 2013 | 2014 | Change | ||||||||||
| (dollars in thousands) | ||||||||||||
Revenues | $ | — | $ | 31 | $ | 31 | ||||||
Cost of revenues | — | 10 | 10 | |||||||||
|
|
|
|
|
| |||||||
Gross margin | — | 21 | 21 | |||||||||
Sales and marketing | 11 | 81 | 70 | |||||||||
Research and development | 368 | 728 | 360 | |||||||||
General and administrative | 295 | 585 | 290 | |||||||||
|
|
|
|
|
| |||||||
Loss from operations | (674 | ) | (1,373 | ) | (699 | ) | ||||||
Interest expense, net and other | (50 | ) | (1 | ) | 49 | |||||||
|
|
|
|
|
| |||||||
Net loss | $ | (724 | ) | $ | (1,374 | ) | $ | (650 | ) | |||
|
|
|
|
|
| |||||||
Revenues, cost of revenue and gross margin
Revenue of $31,000 was recorded for the three months ended March 31, 2014, compared to zero for the three months ended March 31, 2013. The revenue in 2014 was related to our collaboration agreement with Biogen Idec and sale of sample collection kits. Cost of revenues of $10,000 was recorded for the three months ended March 31, 2014 compared to zero for the same period in 2013. The cost of revenues was primarily for payroll and consulting costs incurred for laboratory validation and assay development. Gross margin of $21,000 for the three months ended March 31, 2014 reflected an increase of $21,000 compared to zero for the same period in 2013.
-62-
Table of Contents
Operating expenses
Sales and marketing expense of $81,000 for the three months ended March 31, 2014 increased $70,000 compared to the expense of $11,000 during the three months ended March 31, 2013. The increase in expense was due to $61,000 for reimbursement related consulting for our cost utility study and our Medicare strategy and a $9,000 increase in trade show expenses.
Research and development expense of $728,000 for the three months ended March 31, 2014 increased $360,000 compared to an expense of $368,000 for the three months ended March 31, 2013. The increase was due to $199,000 of legal expense related to our patent efforts, a $39,000 increase in facility costs related to our new laboratory, $38,000 in higher payroll related costs and $31,000 in higher consulting for product development and clinical activities. During the three months ended March 31, 2014, we accelerated efforts on our natural prevalence and reader studies.
General and administrative expense of $585,000 for the three months ended March 31, 2014 increased $290,000 compared to $295,000 in expense for the three months ended March 31, 2013. Payroll and consulting costs increased $205,000 due to increases in accounting, finance and administrative efforts to prepare for public company reporting. Audit costs increased $64,000 and facility expenses increased $45,000 related to our new facility that was occupied in April of 2013.
Interest expenseof $1,000 for the three months ended March 31, 2014 declined $49,000 compared to an expense of $50,000 for the three months ended March 31, 2013. We had outstanding convertible debt during the three months ended March 31, 2013 that was converted into equity in August 2013.
Liquidity and Capital Resources
We have incurred net losses since our company’s formation and have funded our business through the sale of our convertible debt and equity. As of March 31, 2014, we had an accumulated deficit of $39.4 million and we expect to continue to record net losses for the foreseeable future. We currently do not have any debt, but may enter into debt arrangements as an ordinary part of financing our business.
In February 2014, the majority of our warrant holders exercised their warrants, resulting in proceeds of $1.4 million. The warrants had an expiration date of August 2014 and were issued to the purchasers of our Series B preferred stock. In order to compel our warrant holders to exercise their warrants six months early, we offered an additional warrant, with the same exercise price and underlying share amount, of the existing warrant, to each holder who exercised their warrant prior to March 1, 2014. The additional warrants had an expiration date of May 31, 2014. In May 2014, a majority of the holders of these additional warrants exercised their warrants, resulting in proceeds of $1.4 million.
In August 2013, we sold $3.2 million of our Series B preferred stock at a price of $6.00 per share. At the same time, the holders of our convertible debt converted $2.6 million of their principal and interest into shares of Series B preferred stock at a price of $3.00 per share. As a result, all of our convertible debt was extinguished. Each investor who purchased shares of Series B preferred stock directly at a price of $6.00 per share also received a one-year warrant equal to 50% of their investment that is exercisable into shares of Series B preferred stock at a price of $6.00 per share. In October 2013, we sold an additional $30,000 of Series B preferred stock at $6.00 per share.
In August 2012, we entered into a Convertible Note Purchase Agreement and sold convertible promissory notes which were converted into shares of preferred stock issued in the next equity financing. The notes were issued in August 2012 and October 2012 in the amount of $1,500,000 and $969,351, respectively. The notes were due and payable upon written demand by the holder at any time after the first anniversary of the issuance date and accrued interest at 8%. In the event of a transaction in which our company sells equity or debt securities for
-63-
Table of Contents
aggregate gross proceeds of at least $5 million, or a Qualified Financing, the notes convert into the same securities issued in the Qualified Financing at the lowest price paid per share reduced by 50%. All of the notes were converted into shares of Series B preferred stock in August 2013.
Our net cash flow from operating, investing and financing activities for the periods below were as follows:
| Year Ended December 31, | Three months ended March 31, | |||||||||||||||
| 2012 | 2013 | 2013 | 2014 | |||||||||||||
| (dollars in thousands) | ||||||||||||||||
| (unaudited) | ||||||||||||||||
Cash provided by (used in): | ||||||||||||||||
Operating activities | $ | (2,159 | ) | $ | (3,099 | ) | $ | (639 | ) | $ | (1,039 | ) | ||||
Investing activities | (7 | ) | (17 | ) | — | (4 | ) | |||||||||
Financing activities | 3,165 | 2,869 | — | 1,425 | ||||||||||||
|
|
|
|
|
|
|
| |||||||||
Net increase (decrease) in cash | $ | 999 | $ | (247 | ) | $ | (639 | ) | $ | 382 | ||||||
|
|
|
|
|
|
|
| |||||||||
Cash Used in Operating Activities.Net cash used in operating activities of $1.0 million for the three months ended March 31, 2014 increased $400,000 compared to a use of $639,000 for the three months ended March 31, 2013. The increase was primarily related to a $650,000 increase in our net loss and $191,000 of deferred IPO costs, partially offset by $448,000 in higher payroll accruals and other accrued expenses. Net cash used in operating activities increased $0.9 million for fiscal year 2013, to $3.1 million, compared to $2.2 million for fiscal year 2012. The primary reason was due to our net loss increasing by $3.0 million for 2013. The $3.0 million increase was due to $1.1 million in increased expenses and a $1.8 million non-cash beneficial conversion expense related to the 50% discount on shares of Series B preferred stock offered to holders of our convertible notes.
Cash Used in Investing Activities.Net cash used in investing activities was $4,000 for the three months ended March 31, 2014, compared to zero for the three months ended March 31, 2013, due to the purchase of equipment. Net cash used in investing activities increased $10,000 for fiscal year 2013, to $17,000, compared to $7,000 in fiscal year 2012, due to increased purchases of laboratory equipment.
Cash Provided by Financing Activities.Net cash provided by financing activities was $1.4 million for the three months ended March 31, 2014 compared to zero for the three months ended March 31, 2013. The $1.4 million was the result of the exercise of warrants to purchase our Series B preferred stock. Net cash provided by financing activities declined $297,000 for fiscal 2013, to $2.9 million, compared to $3.2 million for fiscal 2012. The decline was due to differences in timing on the issuance of our convertible notes and Series B preferred stock financings.
Contractual Obligations and Commitments
We currently have lease commitments on our laboratory equipment. A summary of our obligations as of March 31, 2014 are as follows:
| Payments Due by Period | ||||||||||||||||||||
| Total | Less than One year | 1-3 years | 3-5 years | More than 5 years | ||||||||||||||||
Office lease | 959,018 | 329,588 | 629,430 | — | — | |||||||||||||||
Equipment lease | 113,335 | 71,580 | 41,755 | — | — | |||||||||||||||
|
|
|
|
|
|
|
|
|
| |||||||||||
Total | $ | 1,072,353 | $ | 401,168 | $ | 671,185 | $ | — | $ | — | ||||||||||
|
|
|
|
|
|
|
|
|
| |||||||||||
In January 2014, our company signed an amendment to the lease on its operating facilities to extend the term through January 2017.
-64-
Table of Contents
Off Balance Sheet Arrangements
None.
Other Items
We have limited capital resources and have experienced negative cash flows from operations and have incurred net losses since our formation. We expect to continue to experience negative cash flows from operations and incur net losses in the foreseeable future as we devote substantially all of our efforts to commercializing our products and continuing product development. We expect future operating, investment, and financing activities to be funded by our product revenue, our existing cash and cash equivalents, bank debt, and the net proceeds of this offering. Based on our current business plan, we believe that the net proceeds from this offering, existing cash and cash equivalents, and bank debt, will be sufficient to fund our projected operating requirements for at least 24 months following the closing of this offering. Our liquidity requirements have and will continue to consist of research and development expenses, sales and marketing expenses, capital expenditures, working capital, and general corporate expenses. As demand for our products increases, we expect that our capital requirements will also increase in order to fund working capital requirements such as inventory and accounts receivable. We expect that we will use a portion of the net proceeds of this offering, in combination with our existing cash and cash equivalents, for these purposes and for increased general and administrative expenses, such as higher insurance costs and professional fees associated with being a public company.
Net Operating Loss Carryforwards
We have deferred tax assets of approximately $413,000 as of December 31, 2013, which have been fully offset by a valuation allowance due to uncertainties surrounding our ability to realize these tax benefits. The deferred tax assets are primarily composed of timing differences for depreciation between U.S. GAAP and U.S. tax treatments. As of December 31, 2013, we had gross federal net operating loss, or NOL, carryforwards of approximately $29.5 million and gross state NOL carryforwards of approximately $25.4 million available to reduce future taxable income, if any. These federal and state NOL carryforwards expire at various times beginning in 2029. In general, if we experience a greater than 50% aggregate change in ownership of certain significant stockholders over a three-year period, or a Section 382 ownership change, utilization of our pre-change NOL carryforwards are subject to an annual limitation under Section 382 of the Internal Revenue Code of 1986, as amended, and similar state laws. Such limitations may result in expiration of a portion of the NOL carryforwards before utilization and may be substantial. If we experience a Section 382 ownership change in connection with this offering or as a result of future changes in our stock ownership, some of which changes are outside our control, the tax benefits related to the NOL carryforwards may be limited or lost.
-65-
Table of Contents
Our Company
We are an emerging growth diagnostics company developing and marketing novel non-invasive gene expression tests to aid in the diagnosis of various skin conditions, including skin cancer, inflammatory diseases, and aging-related conditions. Our technology provides a highly accurate alternative to surgical biopsy, eliminating pain, scarring, and risk of infection, while providing convenience. Our scalable gene expression assays have been designed to work with a proprietary adhesive patch biopsy that provides a tissue sample non-invasively. Current dermatologic diagnosis is primarily based on subjective visual pattern recognition that is prone to error and results in a substantial number of unnecessary invasive surgical biopsies. Our quantitative polymerase chain reaction, or qPCR, tests provide objective, biology-based information that is highly accurate and can significantly reduce surgical biopsies. We believe we will be the first company to offer a non-invasive gene expression test to the clinical dermatology market, which is in contrast to the vast majority of molecular diagnostic tests that are sold into the highly saturated pathology and oncology markets.
We are initially commercializing tests that will address unmet needs in the diagnostic pathway of pigmented skin lesions such as moles or dark colored skin spots. Our products are designed to facilitate the clinical assessment of pigmented skin lesions for melanoma, aid the histopathologic staging of melanoma, and determine the metastatic potential of melanoma. Our first product in this area is the Pigmented Lesion Assay, which can be used to reduce unnecessary surgical biopsy procedures and improve diagnostic accuracy by ruling out false positives based on visual assessment prior to the surgical biopsy. We believe that our adhesive patch biopsy should be the initial biopsy used in the diagnostic pathway of pigmented skin lesions suspicious for melanoma. Of the approximate 3 million surgical biopsies performed each year on pigmented skin lesions, over 95% are negative for melanoma and represent an unnecessary surgical procedure.
As shown below, our technology platform is easy to use and integrates seamlessly into the current clinical diagnostic pathway by providing (i) simple and rapid tissue collection and shipping via standard express mail, (ii) sample processing via qPCR, and (iii) physician reporting within 48 to 72 hours. In addition, physicians can bill for their services using an existing biopsy CPT code. We believe our products and tests will provide better care at a lower cost. Moreover, we have performed an economic analysis that supports our view that the Pigmented Lesion Assay will provide value-added and necessary diagnostic information while reducing overall costs.

In addition, we are expanding the use of our technology to other areas of dermatology through a collaboration with Biogen Idec, Inc., or Biogen Idec, a large multi-national biotechnology company developing a drug product for an inflammatory disease of skin. We have also developed a mobile photoinformatic software platform for capturing clinical images and data, which may also be useful in disease management by documenting and following pigmented lesions treated with our tests.
We offer our gene expression tests through our 6,000 square foot state-of-the-art CLIA-licensed laboratory located in our company headquarters. We have been developing our technology over the last seven years and
-66-
Table of Contents
have created a tissue archive of approximately 5,000 skin samples, including more than 900 melanomas to facilitate this development. Our technology analyzes ribonucleic acid, or RNA, from skin samples collected with our adhesive patches, which maximize collection of tissue with minimum patient discomfort. We have developed significant intellectual property and know-how around the use of adhesives for non-invasive biopsy and the transportation and handling of this type of sample. We have developed a proprietary process that allows us to extract informative RNA from the patch with sufficient quality and quantity to run gene expression analysis. The results of these efforts will allow us to introduce our platform technology to facilitate the diagnosis of a broad array of dermatologic conditions.
We plan to initiate commercialization of our Pigmented Lesion Assay in the second half of 2014. We plan to market this test directly to a concentrated group of approximately 250 to 500 dermatologists that treat a majority of the 750,000 high-risk melanoma survivors in the United States. We then plan to expand this effort by building a direct sales force to penetrate a majority of the 7,500 active clinical dermatologists that see more than 2,000,000 patients with other melanoma risk factors. We believe the total annual U.S. market opportunity for this product exceeds $750 million and that the select annual worldwide market consisting of Australia, Europe, and Canada exceeds $500 million. While we have not yet generated any sales from our Pigmented Lesion Assay, and have a history of net losses since our inception, we have begun generating revenue from our sample collection kits and CLIA testing services.
Over the next eighteen to twenty four months we plan to introduce two additional products into the melanoma diagnostic pathway. Our micro staging product will facilitate the differentiation between a tumor that is confined to the epidermis, or in situmelanoma, and a tumor that has penetrated into the dermis, or invasive melanoma, which can be challenging. Invasive melanoma is associated with more significant surgical treatment, higher costs, and lower survival. We believe the U.S. market opportunity for this product exceeds $100 million. In addition, we plan to introduce a test to determine the metastatic potential of melanoma. Current assessment of metastatic potential involves an extensive and costly surgical procedure on the lymph nodes that can cause substantial recovery for the patient. We believe the U.S. market opportunity for this product exceeds $150 million.
We also plan to explore partnering and licensing opportunities for our platform technology in areas such as other skin cancers (basal cell or squamous cell), inflammatory skin diseases, aesthetic/cosmetic indications, and acne. We have developed proof-of-concept tests and gene expression profiles/classifiers in some of these areas that demonstrate the potential utility of our technology. These opportunities may involve clinical trial support and produce revenue from sample processing. In addition, they may lead to companion diagnostic products for new or existing drug therapies.
-67-
Table of Contents
Our Competitive Advantages
Highly accurate tests that can significantly reduce unnecessary biopsies. Our Pigmented Lesion Assay test demonstrates an accuracy of 91.0% in differentiating melanoma from non-melanoma using histopathology as the reference standard. Our prevalence-weighted negative predictive value, or NPV, exceeds 99.4% such that the probability of a negative test actually being positive is less than 0.6%. The table below compares our Pigmented Lesion Assay with other techniques that represent the current standard of care.
| Visual Assessment | Diagnostic Devices | Surgical Specimen Biomarkers | DermTech (Gene Expression) | Pathology (Current Standard) | ||||||
Mechanism | Pattern Recognition | Pattern Recognition | Tumor Biology | Tumor Biology | Pattern Recognition | |||||
Platform Tech | No | No | N/A | Yes | N/A | |||||
Complete Solution | No | No | No | Yes | Yes | |||||
Physician Payment | Yes | No | No | Yes | Yes | |||||
Better Performance | ||||||||||
NPV (1) | 76% | 95-97% | >99% | >99% | >99% | |||||
Accuracy (2) | 8% | 15% | 90% | 91% | 90% | |||||
Sensitivity (3) | 70% | 80-98% | 89% | 93% | >90% | |||||
Specificity (4) | 5-10% | 10-20% | 93% | 90% | >90% |
| (1) | Negative Predictive Value, or NPV, measures the probability that a negative result is truly negative. |
| (2) | Accuracy measures the proportion of lesions correctly identified. |
| (3) | Sensitivity measures the proportion of actual positives that are correctly identified as such. |
| (4) | Specificity measures the proportion of negatives that are correctly identified as such. |
Superior ease of use. Our non-invasive biopsy procedure can be performed in less than five minutes. All the necessary items including adhesive patches, instructions, a marking pen for outlining, and a preaddressed and prepaid return shipping label are contained in our kit.
Physician services can be reimbursed using existing payment (CPT) codes. Unlike competing solutions for which there are no CPT reimbursement codes, physicians using our tests can seek reimbursement under an existing biopsy CPT code.
Lower cost of dermatologic diagnosis. We believe our Pigmented Lesion Assay will provide better care at a reduced cost. Our analysis demonstrates an average direct cost savings of more than $50 per lesion tested, assuming a $400 payer reimbursement, plus additional cost effectiveness through reductions in lost productivity and improved quality of life. According to the Styperek, MD and Kimball, MD publication “Malignant Melanoma: The Implications of Cost for Stakeholder Innovation,” published in the American Journal of Pharmacy Benefits, March/April 2012, the accurate micro staging melanoma could achieve as much as $350 to $500 in direct savings per lesion tested due to reduced treatment and surveillance costs. Determining metastatic potential could save $2,000 to $5,000 in additional cost and can reduce the number of lymph node surgeries, which can average approximately $7,000 per procedure.
Simple integration into clinical practice. Our test and adhesive patch replaces the scalpel in the initial assessment. Unlike device technologies, we do not require the installation and maintenance of capital equipment. The nursing support, documentation, specimen processing, and requisition post procedure are substantially similar to current practice. These issues are critical in a busy clinical practice where clinicians see patients every five to seven minutes.
Highly differentiated diagnostic technology. Our tests measure objective biological changes in gene expression rather than subjective visual pattern changes. Consequently, the performance of our tests, particularly the specificity, is superior to that of other technologies.
-68-
Table of Contents
Technology platform that can address other skin conditions. We are pursuing partnering and licensing opportunities for our platform technology in other areas such as inflammatory skin diseases, aesthetic/cosmetic indications, and acne. We are currently partnering with Biogen Idec, Inc., a large multi-national biotechnology company to assist in developing a drug product for an inflammatory disease of the skin, and have generated revenues from this collaboration. In connection with this collaboration, we are providing services, such as assay development, adhesive patch testing using gene expression, and sample storage to Biogen Idec.
Strong intellectual property protection. We have five issued U.S. patents of which two are broadly directed to the use of an adhesive to collect samples containing RNA from the skin for analysis. Foreign counterparts to the first issued of these patents have been granted in many countries around the world, including what we believe to be the world’s major melanoma markets. In addition, we have patent applications pending on unique gene expression profiles and classifiers that differentiate melanoma from non-melanoma, which if issued, should not expire until 2034. We have developed processes and expertise that allow us to extract RNA material from adhesive patch samples suitable for qPCR analysis.
Our Strategy
Our goal is to become the leader in non-invasive molecular testing for dermatologic conditions. We believe our dominant intellectual property portfolio, platform technology, first-to-market advantage, and groundbreaking research will facilitate the achievement of this goal. Specifically we will focus on the following objectives:
Build a specialized sales force to introduce our products starting with our Pigmented Lesion Assay. We intend to build a specialty sales force, starting with five to ten representatives and growing to forty once broad reimbursement is achieved. This sales force will initially call on the 250 to 500 pigmented lesion specialists that treat the more than 750,000 patients with the highest risk for melanoma. We will then expand our sales force to penetrate 80% of the approximately 7,500 dermatologists with active clinical practices in the United States.
Integrate our products into the standard of care. We conduct rigorous clinical and basic science research, and publish the results of this research in peer-reviewed journals. We will work with, and establish, boards of key opinion leaders in fields relevant to our products. We have assembled and world-renowned board of melanoma experts and will work with these advisors to incorporate our melanoma products into the diagnostic pathway guidelines for melanoma.
Secure broad reimbursement coverage for our assays. We plan to target regional and national payors to secure favorable coverage decisions for the reimbursement of our tests. We will focus on completing our clinical utility studies, building an in-house reimbursement team, publishing additional papers and completing our Medicare dossier. National Medicare coverage is currently determined through the MolDx coverage decision program and we plan to submit our application in the next 12 to 18 months.
Expand our product offerings. We have identified other gene expression profiles and classifiers in other areas of dermatology. We have also developed gene expression profiles that can assess the stage of melanoma and determine if the tumor has become invasive or remainsin situ. This micro-staging test may reduce the need for more extensive surgical treatment when invasive melanoma is found. In addition, we have identified differential gene expression that may form the basis for a test that can determine the metastatic potential of the lesion. Development of this test could reduce the need for a sentinel lymph node surgery, which can be extensive, cause significant morbidity and is costly.
Develop new products and explore licensing and partnerships for other indications. In 2013, we initiated a collaboration with Biogen Idec to support the development of a new drug for an inflammatory skin disease. In 2014 we have begun to recognize revenue from this customer and from the sale of our sample collection kits to other customers. We will continue to explore with our own research and with partners the use of our technology in other dermatologic conditions, including other skin cancers, anti-aging treatments, acne, and inflammatory skin diseases.
-69-
Table of Contents
Market Opportunity—Melanoma
Melanoma is currently one of the fastest growing cancers and the subject of significant attention in the medical community. The incidence of melanoma has doubled since 1973. While there has been a 20% decline in cancer deaths overall since 1991, melanoma is one of three cancers facing increasing rates. According to a study from the Mayo Clinic, the incidence of melanoma increased eightfold among women under 40 and fourfold among men under 40 from 1970 to 2009.
Melanoma is one of the deadliest form of skin cancer. On average, melanoma causes more than one death every hour of every day of the year in the United States. The American Cancer Society projects that 9,710 people will die from melanoma in 2014. If diagnosed and removed early in its evolution, when confined to the outermost skin layer and deemed to be “in situ (Stage 0),” patients are expected to have a survival rate of almost 100%. Invasive melanomas that are thin and extend into the uppermost regions of the second skin layer still have cure rates that are greater than 90%. However, once the cancer advances into the deeper layers of skin, the risk of it spreading to other parts of the body, or metastasis, and death increases. The table below depicts the survival rate of melanoma based on the stage of the cancer at initial diagnosis.
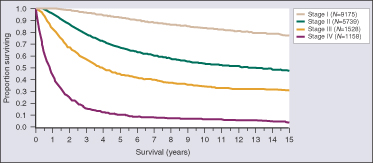
From Balch CM, Buzaid AC, Soong S-j et al: Final Version of the American Joint Committee on Cancer Staging System for Cutaneous Melanoma. Journal of Clinical Oncology, August 2001.
We estimate that approximately 3.0 million surgical biopsies are performed in the United States each year to diagnose about 138,000 cases of melanoma—more than 61,000 non-invasive (in situ) and more than 77,000 invasive. As a result, we estimate that the total United States annual market opportunity for our tests and products for pigmented skin lesions and melanoma exceeds $1.0 billion. Outside the United States, the incidence of melanoma is highest in Western Europe, Australia, and Canada. We estimate that these select worldwide markets perform over 1.5 million surgical biopsies annually to diagnose approximately 75,000 melanomas creating additional market opportunity that we believe exceeds $500 million.
Limitations of Current Melanoma Diagnostic Pathway
Pigmented skin lesions suspicious for melanoma are clinically atypical and meet one or more subjective visual grading criteria, including: Asymmetry, Border Irregularity, Color Variation, Diameter > 6 mm, and Evolving changes (ABCDE criteria). Lesions identified as suspicious for melanoma may be surgically biopsied or followed for further change. Because patients can have many atypical lesions the decision of which lesions to surgically biopsy can be challenging and it is not practical to surgically biopsy all lesions. Lesions with a lower suspicion for melanoma, and likely earlier stages, may not be selected for biopsy such that 20% to 30% may have a delayed diagnosis. Studies show that the current standard clinical assessment of pigmented lesions for biopsy demonstrate an accuracy of less than 20%, with sensitivities on average of 80% and specificities ranging from 5 to 20%. Technologies that involve image, spectral or electrical impedance analysis, can be used at this point to provide further assessment for biopsy need, but typically lack the accuracy needed to meaningfully improve clinical assessment.
-70-
Table of Contents
Lesions selected for surgical biopsy are ideally subjected to an excisional procedure, which is more extensive and time-consuming. More than 95% of surgical biopsies for suspicion of melanoma are negative and may be considered unnecessary. Consequently, approximately 70% of the time, a less optimal biopsy procedure is often performed, such as a shave or punch biopsy, in an effort by physicians to reduce pain and recovery time. These biopsies can confound the histopathologic diagnosis, lead to a delayed diagnosis, and result in a substantial number of rebiopsies (approximately 20% or greater). It has been shown that the vast majority of biopsies leave residual tumor and therefore cannot be considered treatment. Even after local treatment for melanoma, which is a wide local excision, residual tumor can be found in 5 to 6% of patients.
After a lesion is surgically biopsied, it is sent for histopathology where the definitive diagnosis of melanoma is made. Similar to the clinical evaluation of pigmented lesions, histopathologic assessment is also subjective and challenging. Consequently there is a high rate of discordant diagnosis (two pathologists do not make the same diagnosis when evaluating the same tissue), which is estimated to be as high as 20 to 30%. Studies have demonstrated that pathology, the gold standard, has a false negative rate and false positive rate of 10% or more. In addition, up to 15% of samples can have ambiguous findings, which can preclude a definitive pathologic diagnosis. Current expression technology can be used to assist the histopathologic diagnostics, but requires a surgical biopsy sample.
Lesions positive for melanoma are subsequently staged to determine if the tumor remainsin situ or if it has undergone invasion. For early melanoma, this staging can be difficult and is also dependent on the area selected for examination. It is important to identify the stage accurately, because invasive melanoma has a lower survival rate, requires more extensive medical treatment, surveillance, and has higher costs. The medical work up for invasive melanoma may include a sentinel lymph node biopsy surgical procedure to determine if the melanoma has metastatic potential. This is a significant surgical procedure with associated high cost and morbidity with results that can be ambiguous. Existing gene expression technologies that are used to determine metastatic risk require a surgical biopsy and further clinical validation.
Our Platform Technology and Diagnostic Solutions
Because we measure gene expression, our technology platform can address the unmet needs in dermatologic diagnosis. Cancer and other skin conditions can result from changes in gene expression that alter cell growth, function and the ability of cells to invade and metastasize. The information provided by gene expression analysis is both objective and biologically-based. This shift from subjective pattern recognition-based information to objective biology-based information provides highly relevant information to clinicians about skin conditions.
The limitations associated with the diagnosis of melanoma can be summarized into two significant unmet needs. There is a need to improve the clinical assessment of pigmented skin lesions to reduce the number of unnecessary surgical biopsies while improving the early detection of melanoma. There is also a need to improve the accuracy of melanoma diagnosis and staging. Melanoma is one of the top three misdiagnosed cancers. The potential errors can be traced throughout the entire diagnostic pathway, including clinical assessment, histopathologic diagnosis, and disease staging.
-71-
Table of Contents
Adhesive Skin Sample Collection Kit
We are the inventors and own the intellectual property for the Adhesive Skin Sample Collection Kit (pictured below). We have contracted with a Food and Drug Administration, or FDA, registered supplier to produce our kit and we control the exclusive distribution rights. The adhesive patch allows for the collection of skin samples with minimal patient discomfort. A single kit contains all of the necessary components to complete the sample collection for our analysis, including the adhesive patches, instructions for use, a marking pen for lesion outlining, and a pre-addressed and prepaid return shipping pack. The unique properties of the tape maximize the collection of informative cellular material for our Pigmented Lesion Assay. The entire procedure for sample collection takes less than five minutes.
Pigmented Lesion Assay
The development of our Pigmented Lesion Assay has involved over 500 lesion samples, including over 250 melanomas. Our development studies and the performance metrics of the gene profiles are outlined in the table below. The initial effort involved a genome wide screen (47,000 genes) to identify genes that were differentially expressed between melanoma and non-melanoma. Of the 312 genes identified in this study, 17 genes were shown to accurately differentiate melanoma from non-melanoma based on their expression levels. This initial testing was carried out using a microarray gene chip technology and was subsequently transferred and validated using qPCR technology that is better suited for commercial use. On this qPCR platform, the original 17 genes were further narrowed down to two genes that serve as the expression profile for our commercial test. This two-gene expression profile was independently validated in two subsequent studies.
Summary of Pigmented Lesion Assay Development & Validation Data
Study | Platform | Classifier | # Samples | ROC + | NPV* | Sensitivity | Specificity | |||||||||||||||||
Genome Screen | MicroArray | N/A | 82 | | Melanoma genes found in stratum corneum 312 differentially expressed genes identified | | ||||||||||||||||||
Proof of Concept† | MicroArray | 17 genes | 202 | 0.92 | >99 | % | 100 | % | 88 | % | ||||||||||||||
Platform Change | qPCR | 15 genes | 118 | 0.91 | >99 | % | 100 | % | 85 | % | ||||||||||||||
Refine Gene Classifier | qPCR | 2 genes | 130 | 0.96 | >99 | % | 99 | % | 80 | % | ||||||||||||||
13-01** | qPCR | 2 genes | 64 | 0.92 | >99 | % | 97.5 | % | 73 | % | ||||||||||||||
13-02‡ | qPCR | 2 genes | 94 | 0.93 | >99 | % | 87 | % | 94 | % | ||||||||||||||
Overall Performance(2-genes) | qPCR | 2 genes | 288 | 0.93 | >99 | % | 96 | % | 81 | % | ||||||||||||||
| * | Negative Predictive Value-assumes prevalence of 7% in average dermatology practice |
| † | Published British Journal of Dermatology |
| ** | Accepted for publication, Journal American Academy of Dermatology |
| ‡ | 13-01 and 13-02 serve as validation studies |
| + | ROC is receiver operator curve |
The Pigmented Lesion Assay produces a PLA score derived from the company’s proprietary algorithm that utilizes the expression data from our assay. The PLA Score steadily increases from non-melanoma lesions, to
-72-
Table of Contents
melanomain situ, to invasive melanoma, demonstrating the progression of the gene expression as the cancer advances (see excerpt from physician test report below). In the validation studies involving 158 lesions (80 melanoma and 78 non-melanoma), statistically significant differences (p<0.00001) in gene expression between melanoma and non-melanoma samples were measured. A p-value, as shown above, of less than or equal to 0.00001 means that there is greater than a 99.999% probability that the increased PLA score in the sample group is a result of the melanoma tested and not chance.
Proprietary Pigmented Lesion Assay Score as Report (sample)
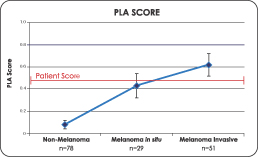
Using histopathology as a reference standard, the gene expression profile of the Pigmented Lesion Assay differentiated non-melanoma lesions from melanoma lesions with an accuracy of 91%, a sensitivity of 93%, and a specificity of 90%. At a projected melanoma prevalence of 7%, the NPV is greater than 99%. To minimize potential diagnostic error by histopathology, only lesions that had a consensus diagnosis by at least three dermatopathologists were included in the analysis. These results indicate that our test may substantially improve the diagnostic pathway for pigmented skin lesions.
Melanoma In Situ vs. Invasive Staging (Micro Staging)
Because of the extremely high cure rate and reduced medical costs ofin situ melanoma it is important to accurately differentiate this stage from invasive melanoma. Additionally, the surgical treatment and follow on tests are more extensive and expensive for invasive melanoma versusin situ. Subtle changes in the way tissue is processed and the anatomic architecture of pigmented lesions can obscure accurate differentiation of these stages. Just as the cancer transformation of benign cells is controlled by gene expression, the development of invasive tumor properties is also controlled by gene expression. We have developed a gene expression profile for melanoma lesions that have become invasive. In a study involving 77 lesions the test was shown to accurately differentiate invasive fromin situmelanoma with 100% sensitivity and 77% specificity.
Metastatic Potential
Metastatic potential is also controlled through gene expression and we have identified a group of differentially expressed genes in a sample of nine patients that correctly identified tumors that had metastasized to the lymph node versus those that did not. Sentinel lymph node surgery, which is currently performed to determine metastatic risk, has a high morbidity and high cost. More accurate and less invasive methods to determine this risk would address this significant unmet need.
Clinical Research Products
We plan to offer a suite of products to facilitate clinical research using our company’s technology. Research on the gene expression of diseases has increased significantly over the last decade. Expression analysis can facilitate drug development by identifying drug targets and stratifying patients into groups that will maximize drug response. Characterizing gene expression is part of the effort to personalize medical therapy to patient’s
-73-
Table of Contents
individual needs. Consequently, tools to facilitate this type of research are in high demand. We offer our Adhesive Skin Sample Collection Kit for sale to clinicians that need a non-invasive means to collect clinical trial samples. We also offer RNA extraction services from the adhesive patch, which can be a challenging process for research labs. We also have developed a smart phone based photoinformatic platform that allows the capture of clinical images and the attendant clinical information and organizes this data in the cloud. These products are currently offered for sale through our website atwww.dermtech.com.
Leveraging our Platform for Other Indications
Our adhesive patch gene expression platform is applicable to numerous other indications in dermatology. While we are focused initially on pigmented lesion and melanoma products, we believe there are significant business development opportunities. The effort will focus on providing support for clinical trials and licensing and partnering for the development of companion diagnostics. Our business development activities will benefit from our sales and marketing initiatives in pigmented lesion and melanoma. In addition, because the processing of samples is the same regardless of the indication, it will leverage our laboratory operations.
Other Skin Cancers
Visual assessment of basal cell and squamous cell cancer can be confused with other benign skin conditions, including dermatitis, seborrheic keratosis, actinic keratosis, and others. This can lead to unnecessary biopsies, particularly in the primary care setting. Because these cancers often occur on face and other cosmetically sensitive areas, avoiding unnecessary biopsies can be important. We plan to expand our product development to include these additional skin cancers and believe there is a significant market opportunity with over 2.5 million basal cell and 700,000 squamous cell cancers diagnosed each year in the U.S.
Inflammatory Indications
Biogen Idec has developed a strategic initiative to develop drugs for inflammatory skin diseases. In 2013 we commenced a collaboration with Biogen Idec to use our technology for the development of a treatment for an inflammatory skin condition. In connection with this collaboration, we are providing services, such as assay development, adhesive patch testing using gene expression, and sample storage to Biogen Idec. Because the targeted condition affects the face, non-invasive sample collection to assess the condition is preferred. Under the agreement, our gene expression assays will be used to track the targeted drug effects of this therapy. This information may help identify responders to the therapy and identify new drug targets. The effort may result in the development of a companion diagnostic product for use with the drug therapy.
We have also demonstrated that our technology may be used to assess inflammatory gene expression in psoriasis. We have shown that RNA targets found with the adhesive patch were not found in surgical biopsy specimens, suggesting that our technology may uniquely identify certain gene targets. In addition, we have shown that the technology may be useful for identifying responders to biologic treatment of treatment of psoriasis.
Aesthetic and Cosmetic Indications
We have developed an expression profile that correlates with the age of the skin. Our profile could be used to stratify patients to a particular anti aging treatment, to identify potential drug targets, and to assess the performance of different anti-aging treatments. Over $10 billion annually is spent in the United States on anti-aging topical treatments creating a significant market opportunity.
We have found over 300 genes that are differentially expressed between the skin of patients greater than 60 years old and those less than 30 years old. In a study of over 100 patients, a 16-gene expression profile could be used to stratify patients into 10-year increments. In addition, the profile demonstrated that some individuals show gene expression that is not consistent with their chronological age, and have older or younger gene expression.
-74-
Table of Contents
Acne
We have successfully isolated RNA from acne lesions on the face. In addition, we have successfully identified differences in gene expression between inflammatory and non-inflammatory acne. We believe our technology could be used to stratify patients for appropriate treatment and to assess therapeutic response.
Mobile Phone Clinical Application
As part of our clinical trial activities we developed a mobile phone and cloud based application for use in documenting and archiving photos of lesions as well as storing the clinical and diagnostic history of a patient. We believe that the application will be attractive to dermatologists for use in their practice. We have designed it to interface with certain clinical information systems and believe that it will help us drive adoption of our product by offering a more seamless solution than exists with the current systems.
Sales and Marketing
The vast majority of molecular diagnostic tests are sold to pathology and oncology practitioners. These markets are fast becoming saturated with products, services, and sales calls. We believe that we have a unique opportunity to be the first company to market a novel molecular test to the dermatologist. We believe there are fewer barriers to adoption in this customer base because our product fits within the current diagnostic and reimbursement pathway for various skin conditions.
We are in the process of recruiting a senior sales and marketing executive with significant experience in launching and growing dermatology products, including dermatologic devices. We will start building our initial specialty sales force in the second half of 2014 and we believe that ultimately a specialty sales force of approximately forty representatives, strategically placed in regions across the United States, can address a significant amount of dermatologists. Concurrently, we are developing in-house reimbursement capabilities, including claims submittal, appeals, collection, and contracting.
There are approximately 7,500 dermatologists in the United States in active clinical practices. We segment these practices into three categories: cosmetic only practices (10-15%), mixed medical and cosmetic practices (50 to 75%), and medical only practices (15-25%). Our initial call point will be the approximately 250-500 clinicians that we consider pigmented lesion specialists. We stratify our patient population into high-risk patients and low-risk patients. Patients seen by pigmented lesion specialists tend to be high-risk and have a personal history of melanoma, a family history of melanoma, and/or multiple atypical pigmented lesions or atypical nevi syndrome. This high-risk population pool is growing due to an increasing number of melanomain situ and stage 1 invasive melanoma diagnoses that have a favorable 10-year survival. This group of high-risk patients may be subject to multiple biopsies each year. We have analyzed the SEER Registries database and cross referenced this with the Medicare Claims database and estimate that there are approximately 3.0 million biopsies performed on pigmented lesions each year.
Our marketing will initially be focused on dermatology trade shows and scientific symposiums as well as in-person physician education and peer-to-peer education. We plan to utilize key opinion leaders in our industry to help integrate our tests into the current standard of care. We also plan to continue to expand the validation of our tests with additional clinical trials and we will publish the results of our scientific and clinical work in peer-reviewed dermatology medical journals. Through these efforts we believe we can incorporate our tests into various diagnostic guidelines, including the pigmented lesion and melanoma diagnostic guidelines. As the market progresses we expect to begin advertising in medical journals and utilizing social media campaigns to rally the extensive patient advocacy support that exists today for a variety of skin conditions and melanoma sufferers.
We plan to engage in the marketing of our product outside the United States only after we have established the United States market. We expect to focus our initial effort outside the United States in countries that have a
-75-
Table of Contents
high incidence of melanoma such as Australia, Canada, and Western Europe. We will likely seek distribution partners in these select countries for the sales and marketing of our tests. We believe the stability of the skins samples on our adhesive patch is suitable for shipping from countries outside the United States
Our products to support clinical research are primarily made available through our website. We plan to add additional headcount to support a more expanded effort in this area. We plan to focus this effort initially by offering sample processing services to support clinical trials with pharmaceutical, biotechnology, and medical device companies with clinical development programs in dermatology. We believe that this may lead to partnering opportunities for companion diagnostic and/or prognostic products that incorporate our products and technology. We expect there to be significant marketing synergy with our Pigmented Lesion Assay as many of these potential customers and partners will be present at the same major dermatology and scientific symposia meetings.
Reimbursement Strategy.
We plan to use the existing process for submitting for reimbursement under the pathology CPT code 84999. We are completing our documentation package that includes letters of medical necessity (that will be approved by the physician), cost justification, peer-reviewed scientific literature, and patient advocacy materials. In addition, we work with a leading health care economics firm who has developed a detailed analysis of the utility, pricing, and cost savings that could be achieved upon adoption of our test. In situations where payment is denied, we plan to work through the claims appeals process to secure payment for services performed. The appeals process can require several cycles and culminate in an independent committee review for blocks of claims. If we are successful at achieving payment under the appeals process, we plan to pursue coverage decisions from these payors. We also plan to look to obtain a discounted co-payment from the patient in situations where payment will not occur.
We plan to seek contractual reimbursement coverage decisions from third-party payors. Reimbursement coverage decisions for clinical tests are supported with clinical utility studies. Clinical utility of a molecular test is established by demonstrating that information from the test changes the behavior of the physician. One aspect of the utility of our first product is to reduce the use of surgical biopsies performed by physicians in the diagnosis of melanoma. Our high level of specificity is the first step of evidence demonstrating this utility. Because the standard of care using visual grading has such a low specificity (<10%), nearly all lesions (>90%) are surgically biopsied. With a specificity of 90%, we would expect that only approximately 10% of lesions would require a biopsy, giving us a significant margin for utility.
We anticipate completing additional clinical utility studies to support our first product over the next 18 months. The first of these studies is our 13-04 study. This study is designed as a reader study and will use clinical, photographic, and dermoscopic data from our 13-03 study, combined with the assay data, to assess the reduction in surgical biopsy decision expected with the use of our Pigmented Lesion Assay. We also anticipate starting a second reimbursement study in the second half of 2014 to further support our reimbursement effort with payors. This study will compare the biopsy rates between two groups of physicians: one treating patients according to the current surgical biopsy standard of care and the other incorporating the assay into the diagnostic pathway. We believe that favorable outcomes of these utility studies will advance our products toward becoming the standard of care for pigmented lesions assessment.
A Medicare coverage decision is obtained through the MolDx program administered by Medicare contractor Palmetto GBA. The MolDx program requires the submission and review of three publications, including a clinical utility manuscript and manuscripts that demonstrate analytical and clinical validity. Additional supporting manuscripts, such as economic models and analysis, can facilitate this process and we are working on these publications. Medicare reimbursement coverage decisions require one to two six-month review cycles from the time of submission of the initial dossier. In addition to our previously published manuscripts on clinical validity, we have analytical and clinical validation manuscripts in preparation that will be derived from clinical studies that are ongoing or initiated in 2014.
-76-
Table of Contents
Patient Self-Pay
We believe that some patients will pay out-of-pocket for our test. The model for patient pay in cosmetic dermatology is well-established with products such as injectable muscle relaxants and dermal fillers achieving substantial market adoption and sales. We believe the non-scarring attributes of our technology may appeal to this customer segment. We conducted a survey at two dermatology offices to determine the amount of money patients would pay to avoid a surgical biopsy on the face. Approximately 20% of respondents indicated that they would pay more than $250 for the test. We are exploring distribution strategies to target these customers including potential sales distribution relationships with firms that specialize in cosmetic dermatology products.
Key Opinion Leaders
Our sales and marketing strategy will leverage our extensive network of key opinion leaders in the fields of dermatology, pathology, biostatistics, healthcare economics and reimbursement. We will use our experts to perform peer-to-peer education, to publish papers utilizing our tests and become early adopters of our technology. We will also utilize our opinion leaders to help drive broad reimbursement for our tests by providing statistical justification for our projected cost savings, by interfacing with healthcare coverage payers to by educating them on the cost and quality of life benefits of our technology. Our key opinion leader group includes Allan C. Halpern, M.D., Scott Menzies, Ph.D., David Polsky, M.D., Ph.D., Pedram Gerami, M.D., Laura Ferris, M.D., Ph.D., Michael Walker, Ph.D., Bruce Quinn, M.D., Ph.D., and John Hornberger, M.D., M.S.
Competition
The molecular diagnostics market is highly competitive. We compete with a number of manufacturers and distributors of molecular diagnostic tests as well as new and traditional medical devices and other technologies that are used to assist physicians with the diagnosis of melanoma and other diseases by testing and analyzing skin tissue samples. In the area of melanoma, Myriad Genetics, Inc., recently launched a gene expression assay as a validated CLIA laboratory test for surgical biopsy specimens. MELA Sciences, Inc. markets a device that utilizes varying wavelengths to capture lesion images at different depths and algorithmic image analysis to determine the degree of lesion disorganization and the need for biopsy. SciBase AB is marketing an epidermal electrical impedance spectrometer to diagnose melanoma in Europe and Australia. Verisante Technology, Inc. has received regulatory approval in Europe and Australia to market a device that uses real-time Raman spectroscopy to assess changes in the chemical composition of skin tissue. Welch-Allen, Inc. and various others manufacture dermatoscopes, which provide magnified views of a pigmented lesion during diagnosis. Of the devices mentioned above, we believe that only the MELA Sciences, Inc. device has received FDA approval. While FDA approval is an important factor considered by physicians when evaluating a technology, we believe that factors such as accuracy, cost and ease of use are also given significant weight.
Research and Development
We have expertise in the development of gene expression profiles for the diagnosis of dermatologic disease. In addition, we have developed know-how related to the collection of skin samples using adhesives. We have also developed expertise in statistical programs and algorithms that are used to process gene expression data.
Our process of product development involves several stages. The first stage involves a genome-wide screen for differential gene expression. These differentially expressed genes are then narrowed down to specific gene sets that categorize disease states. These genes sets are then validated by comparison to clinical reference standards to produce a clinical product. We have developed substantial expertise in the designing and conducting clinical validation studies.
In 2014, we expect to have two additional validation studies completed for the Pigmented Lesion Assay. The first study (13-02, part 2, n≈100) will utilize the remaining samples in our tissue archive and be used to test the clinical limits of our test across different areas of the body and with lesions less than 6 mm and greater than
-77-
Table of Contents
15 mm. The second study (13-03, n≈150) will be used to validate the performance of newly collected samples in our commercially-licensed laboratory and with our most recent lot of manufactured adhesive patches. We have identified additional gene targets that may further improve the performance of our Pigmented Lesion Assay. The qPCR assays for these genes are under development and may be added in the future if their performance is validated in additional clinical samples.
We will also continue the development of our staging products for melanoma. Our product for identifying invasive tumor was initially developed on a microarray gene expression technology platform and tested on 100 samples. The gene expression profile in this proof-of-concept study demonstrated 100% sensitivity and over 77% specificity in detecting invasive melanoma when compared against pathology. We plan to initially transfer this work to a qPCR technology platform and undertake gene expression profile refinement and independent validation testing of this in clinical samples.
Our metastatic staging product is in early stages of development. We have demonstrated differential expression of genes between a small number of melanoma patients that had tumor metastasis to the lymph node and those that did not. Further development will require transfer to a qPCR technology platform and gene expression profile refinement and validation in clinical samples.
We also plan to expand our platform to the diagnosis of other skin lesions and anticipate beginning a development program for other epidermal skin cancers. We have also identified gene expression profiles for other conditions, such as psoriasis, acne, and aging of the skin. Should we determine that there are viable market opportunities for these products we plan to consider development of gene expression tests for these conditions. Alternatively, we may seek development partners or licensing opportunities for these potential products.
Intellectual Property
We have developed a comprehensive portfolio of intellectual property. This intellectual property estate includes five issued and eight pending U.S. patents and applications and corresponding granted and pending foreign patents and patent applications. The intellectual property portfolio also includes trademark rights, trade secrets, and industry know-how. We believe this intellectual property adequately protects our technology and products, and that it may prevent others from developing similar products to ours.
Two of our issued U.S. patents (U.S. Pat. Nos. 6,949,338 and 7,183,057) and their corresponding foreign counterpart patents and applications are directed to methods of using an adhesive to collect skin samples to quantitate RNA and determine disease or pathological state, and are not limited by specific species of RNA or by the use of specific types of adhesives. Corresponding foreign patents have been granted in Europe, Australia, and Canada. Subject to payment of all maintenance fees, U.S. Pat. No. 6,949,338 is expected to expire in 2019 and U.S. Pat. No. 7,183,057 is expected to expire in 2024, unless either patent is disclaimed or rendered invalid by a court of competent jurisdiction or by the USPTO. These patents are not encumbered by licensing agreements nor subject to any royalty payments.
In addition, we have four U.S. patents pending on specific RNA species and expression profiles and classifiers associated with various melanoma disease states. These patent applications provide potential patent protection of our proprietary two-gene classifier for melanoma and also describe additional gene targets that we may add to the expression profile in the future. Counterpart foreign patent applications have been or will be filed in what our company believes to be the major foreign countries for melanoma. In addition to our core patent filings around our melanoma assay and our Pigmented Lesion Test, we have also filed patent applications in other areas, including acne, inflammatory conditions of the skin, and age ranges of the skin. These applications are directed to methods for differentiating these conditions and specific gene profiles and classifiers used in such assays.
The remaining three issued patents that we own are directed to methods that differ from, but are related to, our current and planned products. U.S. Pat. No. 6,720,145 is directed to methods of distinguishing an irritant contact dermatitis from an allergic contact dermatitis in human subject, and is expected to expire in 2019. U.S.
-78-
Table of Contents
Pat. No. 7,279,480 is directed to non-invasive methods for detecting early stage melanoma in a skin sample of a human subject by detecting the level of lnterleukin-1 RI RNA in the skin sample, and is expected to expire in 2023. U.S. Pat. No. 7,989,165 is directed to non-invasive methods for isolating or detecting a protein from an epidermal sample of a human subject and is expected to expire in 2024.
Laboratory Operations
Our CLIA laboratory occupies approximately 6,000 square feet and is divided into an accession area, prePCR-lab, and postPCR-lab area as per CLIA standards. Access to all areas is controlled and requires gowning. The laboratories employ commercial state-of-the-art equipment including a high-throughput qPCR machine from Life Technologies. We use a laboratory information system to track all of our samples. We employ clinical laboratory scientist with appropriate state licensing to perform the assay.
Our assay utilizes qPCR techniques and involves the four main steps set forth below. In addition, we have developed a proprietary and unique extraction method, consisting of:
| • | RNA extraction using our proprietary process to maximize the yields and quantity of RNA from the cells on the patch; |
| • | reverse transcription, which converts the RNA into cDNA; |
| • | pre-amplification, which utilizes PCR to enrich the sample for the target genes in our expression profile; and |
| • | expression level quantification, which utilizes (quantitative) qPCR to determine the expression levels of the target genes in our expression profile. |
After testing is complete, a written laboratory report is prepared, and reviewed by our CA-licensed and ABMG board certified Laboratory Director. This report is made available to the physician by HIPAA compliant methods such as fax or via an internet portal. The reports are generated in industry-standard pdf formats that allows for high definition figures to be reproduced clearly.
We expect to work to automate various steps in our test process. Much of this automation will come from purchasing and qualifying off-shelf and customized laboratory equipment such as liquid handlers and pipetting robots. We have commenced the development of a laser-cutting robot to automate the cutting of the lesion area circumscribed on the adhesive patch by the clinician. We expect these automation efforts to improve sample throughput at the same or reduced processing time and to reduce the need for direct labor, as well and improve quality by reducing human error.
Third-Party Suppliers and Manufacturers
We are the owners of intellectual property for the Adhesive Skin Sample Collection Kit with our company’s logo and have contracted with an FDA registered supplier to produce our kits. This kit is considered a class 1 FDA device and is exempt from FDA premarket notification requirements. This product is manufactured according to FDA’s applicable Quality System manufacturing requirements. Our FDA registered supplier conducts the assembly and labeling of this kit. All of our suppliers are high quality medical component and finished product suppliers accustomed to working on high volume disposable FDA regulated products. Our product has a shelf life tested to three years that allows us to build inventory to mitigate against disruptions.
Governmental Regulation
The services that we provide are regulated by federal, state and foreign governmental authorities. Failure to comply with the applicable laws and regulations can subject us to repayment of amounts previously paid to us, significant civil and criminal penalties, loss of licensure, certification, or accreditation, or exclusion from government health care programs.
-79-
Table of Contents
Our Adhesive Skin Sample Collection kit is a Class 1 FDA device and is manufactured by an FDA registered supplier according to applicable regulations and is exempt from obtaining premarket approval or clearance by the FDA. The FDA could exert enforcement over our product by declaring our sample collection kit a Class 2 device, which would require us to submit an application for pre-market clearance or approval, which may require us to develop additional clinical data to support premarket clearance or approval, which could come at substantial expense and could delay our commercialization effort. We, however, do not believe that our kit as it is designed and used today warrants a Class 2 designation by the FDA under the applicable regulations.
Our qPCR gene expression assay is considered a laboratory developed test (LDT) and currently regulated under the Clinical Laboratory Improvement Amendments of 1988. While the FDA has claimed authority to regulate LDTs it has generally exercised enforcement discretion and is not otherwise regulating most tests developed and performed by high complexity CLIA-certified laboratories. We plan to commercialize our test as an LDT and will process all tests in our CLIA-certified central laboratory. We may at sometime in the future seek FDA clearance or approval for our qPCR gene expression assay. We believe the data we have collected in the development of our LDT will support any FDA medical device clearance or approval process, but cannot guarantee that the FDA will find this data sufficient to support clearance or approval as a medical device under the applicable FDA regulations. This may require us to collect additional clinical data, which could come at substantial expense and could delay our commercialization effort.
Clinical Laboratory Improvement Amendments of 1988 and State Regulation
Clinical laboratories must hold certain federal, state, and local licenses, certifications, and permits to conduct business. Laboratories that perform testing on human specimens for the purpose of providing information for the diagnosis, prevention, or treatment of disease are subject to CLIA. CLIA requires such laboratories to be certified by the federal government and mandates compliance with various operational, personnel, facilities administration, quality, and proficiency testing requirements intended to ensure that testing services are accurate, reliable and timely. CLIA certification also is a prerequisite to be eligible to bill state and federal health care programs, as well as many private insurers, for laboratory testing services.
Standards for testing under CLIA vary based on the test and level of test complexity. Laboratories performing high complexity testing must comply with more stringent requirements than laboratories performing waived or moderate complexity testing. In addition, CLIA requires each certified laboratory to enroll in an approved proficiency-testing program if it performs testing in any category for which proficiency testing is required. Such laboratories must periodically test specimens received from an outside proficiency testing organization and then must submit the results back to that organization for evaluation. A laboratory that fails to achieve a passing score on a proficiency test may lose its right to perform testing in the category at issue. Further, failure to comply with other proficiency testing regulations, such as the prohibition on referral of a proficiency-testing specimen to another laboratory for analysis, can result in revocation of the referring laboratory’s CLIA certification.
As a condition of CLIA certification, our laboratory is subject to survey and inspection every other year, in addition to being subject to additional unannounced inspections. The biennial survey is conducted by the CMS, a CMS agent (typically a state agency), or, if the laboratory holds a CLIA Certificate of Accreditation, a CMS-approved accreditation organization. We will seek accreditation by the College of American Pathologists, or CAP, which is a CMS-approved accreditation organization. Consequently our laboratory must comply with all CLIA requirements as well as with any additional requirements imposed by CAP.
CLIA provides that a state may adopt laboratory regulations that are more stringent than those under federal law. In some cases, state licensure programs actually substitute for the federal CLIA program. In other instances, the state’s regulations may be in addition to the CLIA program. Our laboratory is licensed by the appropriate state agencies in the states in which we operate, if such licensure is required. If a laboratory is out of compliance with state laws or regulations governing licensed laboratories, penalties for violation vary from state to state but
-80-
Table of Contents
may include suspension, limitation, revocation or annulment of the license, assessment of financial penalties or fines, or imprisonment. We believe that we are in material compliance with all applicable licensing laws and regulations.
We may become aware from time to time of other states that require out-of-state laboratories to obtain licensure to accept specimens from the state, and other states may impose such requirements in the future. If we identify any other state with such requirements, or if we are contacted by any other state advising us of such requirements, we intend to follow all instructions from the state regulators regarding compliance with such requirements.
Food and Drug Administration
Although the FDA has historically claimed that it has the authority to regulate laboratory developed tests, or LDTs, that are validated by the developing laboratory and performed only by that laboratory, it has generally exercised enforcement discretion in not otherwise regulating most tests developed and performed by high complexity CLIA-certified laboratories. Nevertheless, the FDA indicated that it is reviewing the regulatory requirements that apply to LDTs. In July 2010, the FDA held a two-day public meeting to obtain input from stakeholders on how it should apply its authority to implement a reasonable, risk-based, and effective regulatory framework for LDTs, including genetic tests. However, the FDA has not yet issued the promised additional guidance, and they do not have such guidance in their current fiscal year goals, but may generate such guidance in the future. Before any draft or final guidance is issued, however, the FDA is required to provide Congress at least sixty days prior notice in accordance with the requirements of the Food and Drug Administration Safety and Innovation Act, or FDASIA. The notice must include anticipated details of the action. FDASIA was signed into law on July 9, 2012, and the notice requirement will sunset five years thereafter. If the FDA decides to regulate LDTs, such as our Pigmented Lesion Assay, as medical devices we will be subject to increased regulatory burdens. Before a new medical device can be marketed in the United States, it must first receive either premarket approval pursuant to a PMA or 510(k) clearance from the FDA, unless an exemption exists. The same rule applies when a manufacturer plans to market a medical device for a new intended use or significant modifications to the device are made. The process can be costly and time-consuming. The FDA’s goal is to respond to a 510(k) notification in ninety days, but often takes much longer. In addition, the FDA may request additional information in order to complete its review. Responding to such additional information requests can be time-consuming and burdensome, and can delay clearance of the 510(k). The PMA process usually takes six months to three years, but may take longer. We cannot assure that our Pigmented Lesion Assay or any new medical devices that we may develop or new uses for our products that we develop will be cleared or approved in a timely or cost-effective manner, if cleared or approved at all. Even if such devices are cleared or approved, the products may not be cleared or approved for all indications. Because medical devices may only be marketed for cleared or approved indications, this could significantly limit the market for that product and may adversely affect our results of operations.
The adhesive skin sample collection kit we provide for collection and transport of skin samples from a healthcare provider to our clinical laboratory is considered a Class I medical device subject to FDA regulations but is currently exempt from pre-market review by the FDA. From the time that our tests entered commercial distribution, numerous regulatory requirements applied. These include the Quality System Regulation, or QSR, which imposes extensive design, testing, control, documentation and other quality assurance requirements on the manufacturers of medical devices; labeling regulations; the FDA’s general prohibition against promoting products for unapproved or “off-label” uses; and the Medical Device Reporting regulation, which requires that manufacturers report to the FDA if their device may have caused or contributed to a death or serious injury or malfunctioned in a way that would likely cause or contribute to a death or serious injury. The FDA has broad post-market and regulatory and enforcement powers. Failure to comply with applicable United States medical device regulatory requirements could result in, among other things, warning letters, fines, injunctions, consent decrees, civil penalties, repairs, replacements, refunds, recalls or seizures of products, total or partial suspension of production, the FDA’s refusal to grant future premarket clearances or approvals, withdrawals or suspensions of current product applications, and criminal prosecution.
-81-
Table of Contents
HIPAA and other privacy laws
HIPAA established for the first time comprehensive federal protection for the privacy and security of health information. The HIPAA standards apply to three types of organizations, or Covered Entities: health plans, healthcare clearing houses, and healthcare providers that conduct certain healthcare transactions electronically. Title II of HIPAA, the Administrative Simplification Act, contains provisions that address the privacy of health data, the security of health data, the standardization of identifying numbers used in the healthcare system and the standardization of certain healthcare transactions. The privacy regulations protect medical records and other protected health information by limiting their use and release, giving patients the right to access their medical records and limiting most disclosures of health information to the minimum amount necessary to accomplish an intended purpose. The HIPAA security standards require the adoption of administrative, physical, and technical safeguards and the adoption of written security policies and procedures. HIPAA requires Covered Entities to obtain a written assurance of compliance from individuals or organizations who provide services to Covered Entities involving the use or disclosure of protected health information, or Business Associates.
On February 17, 2009, Congress enacted Subtitle D of the Health Information Technology for Economic and Clinical Health Act, or HITECH, provisions of the American Recovery and Reinvestment Act of 2009. HITECH amends HIPAA and, among other things, expands and strengthens HIPAA, creates new targets for enforcement, imposes new penalties for noncompliance and establishes new breach notification requirements for Covered Entities and Business Associates. Regulations implementing major provisions of HITECH were finalized on January 25, 2013 through publication of the HIPAA Omnibus Rule, or the Omnibus Rule. The Omnibus Rule contained significant changes for Covered Entities and Business Associates with respect to permitted uses and disclosures of Protected Health Information.
Under HITECH’s new breach notification requirements, Covered Entities must report breaches of protected health information that has not been encrypted or otherwise secured in accordance with guidance from the Secretary of the U.S. Department of Health and Human Services, or the Secretary. Required breach notices must be made as soon as is reasonably practicable, but no later than sixty days following discovery of the breach. Reports must be made to affected individuals and to the Secretary and in some cases, they must be reported through local and national media, depending on the size of the breach. We are currently subject to the HIPAA regulations and maintain an active compliance program. We are subject to audit under HHS’s HITECH-mandated audit program. We may also be audited in connection with a privacy complaint. We are subject to prosecution and/or administrative enforcement and increased civil and criminal penalties for non-compliance, including a new, four-tiered system of monetary penalties adopted under HITECH. We are also subject to enforcement by state attorneys general who were given authority to enforce HIPAA under HITECH. To avoid penalties under the HITECH breach notification provisions, we must ensure that breaches of protected health information are promptly detected and reported within the company, so that we can make all required notifications on a timely basis. However, even if we make required reports on a timely basis, we may still be subject to penalties for the underlying breach.
In addition to the federal privacy regulations, there are a number of state laws regarding the privacy and security of health information and personal data that are applicable to clinical laboratories. The compliance requirements of these laws, including additional breach reporting requirements, and the penalties for violation vary widely and new privacy and security laws in this area are evolving. Many states have also implemented genetic testing and privacy laws imposing specific patient consent requirements and protecting test results. In some cases, we are prohibited from conducting certain tests without a certification of patient consent by the physician ordering the test. Requirements of these laws and penalties for violations vary widely. We believe that we have taken the steps required of us to comply with health information privacy and security statutes and regulations in all jurisdictions, both state and federal. However, we may not be able to maintain compliance in all jurisdictions where we do business. Failure to maintain compliance, or changes in state or federal laws regarding privacy or security, could result in civil and/or criminal penalties and could have a material adverse effect on our business.
-82-
Table of Contents
We are subject to laws and regulations related to the protection of the environment, the health and safety of employees and the handling, transportation and disposal of medical specimens, infectious and hazardous waste, and radioactive materials. For example, the U.S. Occupational Safety and Health Administration, or OSHA, has established extensive requirements relating specifically to workplace safety for healthcare employers in the United States. This includes requirements to develop and implement multi-faceted programs to protect workers from exposure to blood-borne pathogens, such as HIV and hepatitis B and C, including preventing or minimizing any exposure through needle stick injuries. For purposes of transportation, some biological materials and laboratory supplies are classified as hazardous materials and are subject to regulation by one or more of the following agencies: the U.S. Department of Transportation, the U.S. Public Health Service, the U.S. Postal Service, and the International Air Transport Association. We generally use third-party vendors to dispose of regulated medical waste, hazardous waste, and radioactive materials and contractually require them to comply with applicable laws and regulations.
Federal and State Self-Referral Prohibitions
We are subject to the federal self-referral prohibitions, commonly known as the Stark Law, and to similar state restrictions such as California’s Physician Ownership and Referral Act, commonly known as PORA. Together these restrictions generally prohibit us from billing a recipient or any governmental or private payor for any test when the physician ordering the test, or any member of such physician’s immediate family, has an investment interest in, or compensation arrangement with, us, unless the arrangement meets an exception to the prohibition.
Both the Stark Law and PORA contain an exception for compensation paid to a physician for personal services rendered by the physician, provided that certain conditions are satisfied. We have compensation arrangements with a number of physicians for personal services, such as speaking engagements and specimen tissue preparation. We have structured these arrangements with terms intended to comply with the requirements of the personal services exception to the Stark Law and PORA. However, we cannot be certain that regulators would find these arrangements to be in compliance with the Stark Law, PORA or similar state laws.
Sanctions for a violation of the Stark Law include the following:
| • | denial of payment for the services provided in violation of the prohibition; |
| • | refunds of amounts collected by an entity in violation of the Stark Law; |
| • | a civil penalty of up to $15,000 for each service arising out of the prohibited referral; |
| • | exclusion from federal healthcare programs, including the Medicare and Medicaid programs; and |
| • | a civil penalty of up to $100,000 against parties that enter into a scheme to circumvent the Stark Law’s prohibition. |
These prohibitions apply regardless of the reasons for the financial relationship and the referral. No finding of intent to violate the Stark Law is required to commit a violation. In addition, knowing violations of the Stark Law may also serve as the basis for liability under the Federal False Claims Act.
Further, a violation of PORA is a misdemeanor and could result in civil penalties and criminal fines. Finally, other states have self-referral restrictions with which we have to comply that differ from those imposed by federal and California law. While we have attempted to comply with the Stark Law, PORA and similar laws of other states, it is possible that some of our financial arrangements with physicians could be subject to regulatory scrutiny at some point in the future, and we cannot provide an assurance that we will be found to be in compliance with these laws following any such regulatory review.
-83-
Table of Contents
Federal and State Fraud and Abuse Laws
Because of the significant federal funding involved in Medicare and Medicaid, Congress and the states have enacted, and actively enforce, a number of laws to eliminate fraud and abuse in federal healthcare programs. Our business is subject to compliance with these laws. In March 2010, the Recipient Protection and Affordable Care Act, as amended by the Healthcare and Education Affordability Reconciliation Act, which we refer to collectively as the Affordable Care Act, was enacted in the United States. The provisions of the Affordable Care Act are effective on various dates. The Affordable Care Act expands the government’s investigative and enforcement authority and increases the penalties for fraud and abuse, including amendments to both the Anti-Kickback Statute and the False Claims Act, to make it easier to bring suit under these statutes. The Affordable Care Act also allocates additional resources and tools for the government to police healthcare fraud, with expanded subpoena power for HHS, additional funding to investigate fraud and abuse across the healthcare system, and expanded use of recovery audit contractors for enforcement.
Anti-Kickback Statutes
The federal healthcare programs’ Anti-Kickback Statute prohibits persons from knowingly and willfully soliciting, offering, receiving, or providing remuneration, directly or indirectly, in exchange for or to induce either the referral of an individual, or the furnishing or arranging for a good or service, for which payment may be made under a federal healthcare program such as Medicare or Medicaid.
The definition of “remuneration” has been broadly interpreted to include anything of value, including, for example, gifts, certain discounts, the furnishing of free supplies, equipment or services, credit arrangements, payment of cash, and waivers of payments. Several courts have interpreted the statute’s intent requirement to mean that if any one purpose of an arrangement involving remuneration is to induce referrals of federal healthcare covered businesses, the statute has been violated. Penalties for violations include criminal penalties and civil sanctions such as fines, imprisonment, and possible exclusion from Medicare, Medicaid and other federal healthcare programs. In addition some kickback allegations have been claimed to violate the Federal False Claims Act, discussed in more detail below.
The Anti-Kickback Statute is broad and prohibits many arrangements and practices that are otherwise lawful in businesses outside of the healthcare industry. Recognizing that the Anti- Kickback Statute is broad and may technically prohibit many innocuous or beneficial arrangements, Congress authorized the Office of Inspector General, or OIG, of the HHS to issue a series of regulations known as “safe harbors.” These safe harbors set forth provisions that, if all their applicable requirements are met, will assure healthcare providers and other parties that they will not be prosecuted under the Anti-Kickback Statute. The failure of a transaction or arrangement to fit precisely within one or more safe harbors does not necessarily mean that it is illegal or that prosecution will be pursued. However, conduct and business arrangements that do not fully satisfy an applicable safe harbor may result in increased scrutiny by government enforcement authorities such as OIG.
Many states have adopted laws similar to the Anti-Kickback Statute. Some of these state prohibitions apply to referral of recipients for healthcare items or services reimbursed by any source, not only the Medicare and Medicaid programs.
Government officials have focused their enforcement efforts on the marketing of healthcare services and products, among other activities, and recently have brought cases against companies, and certain individual sales, marketing, and executive personnel, for allegedly offering unlawful inducements to potential or existing customers in an attempt to procure their business.
Federal False Claims Act
Another development affecting the healthcare industry is the increased use of the federal False Claims Act, and in particular, action brought pursuant to the False Claims Act’s “whistleblower” or “qui tam” provisions. The False Claims Act imposes liability on any person or entity that, among other things, knowingly presents, or
-84-
Table of Contents
causes to be presented, a false or fraudulent claim for payment by a federal healthcare program. The qui tam provisions of the False Claims Act allow a private individual to bring actions on behalf of the federal government alleging that the defendant has violated the False Claims Act and to share in any monetary recovery. In recent years, the number of suits brought against healthcare providers by private individuals has increased dramatically. In addition, various states have enacted false claims law analogous to the False Claims Act, many of these state laws apply where a claim is submitted to any third-party payor and not merely a federal healthcare program.
When an entity is determined to have violated the False Claims Act, it may be required to pay up to three times the actual damages sustained by the government, plus civil penalties of between $5,500 and $11,000 for each separate instance of false claim. There are many potential bases for liability under the False Claims Act. Liability arises, primarily, when an entity knowingly submits, or causes another to submit, a false claim for reimbursement to the federal government. The federal government has used the False Claims Act to assert liability on the basis of inadequate care, kick-backs and other improper referrals, and improper use of Medicare numbers when detailing the provider of services, in addition to the more predictable allegations as to misrepresentations with respect to the services rendered. In addition, the federal government has prosecuted companies under the False Claims Act in connection with off-label promotion of products. Our future activities relating to the reporting of wholesale or estimated retail prices of our products, the reporting of discount and rebate information and other information affecting federal, state and third-party reimbursement of our products and the sale and marketing of our products may be subject to scrutiny under these laws.
While we are unaware of any current matters, we are unable to predict whether we will be subject to actions under the False Claims Act or a similar state law, or the impact of such actions. However, the costs of defending such claims, as well as any sanctions imposed, could significantly affect our financial performance.
The Sunshine Act
The Physician Payment Sunshine Act, or the Sunshine Act, which was enacted as part of the Affordable Care Act, requires all pharmaceutical manufacturers that participate in Medicare, Medicaid or the Children’s Health Insurance Program to report annually to the Secretary payments or other transfers of value made by that entity, or by a third party as directed by that entity, to physicians and teaching hospitals or to third parties on behalf of physicians or teaching hospitals. The payments required to be reported include the cost of meals provided to a physician, travel reimbursements, and other transfers of value provided as part of contracted services such as speaker programs, advisory boards, consultation services, and clinical trial services. The final rule implementing the Sunshine Act requires data collection on payments to begin on August 1, 2013. The first annual report, comprised of data collected from August 1, 2013 to December 31, 2013, is due March 31, 2014. The statute requires the federal government to make reported information available to the public starting September 2014.
Failure to comply with the reporting requirements can result in significant civil monetary penalties ranging from $1,000 to $10,000 for each payment or other transfer of value that is not reported (up to a maximum per annual report of $150,000) and from $10,000 to $100,000 for each knowing failure to report (up to a maximum per annual report of $1.0 million). Additionally, there are criminal penalties if an entity intentionally makes false statements in such reports. We are subject to the Sunshine Act and the information we disclose may lead to greater scrutiny, which may result in modifications to established practices and additional costs. Additionally, similar reporting requirements have also been enacted on the state level domestically, and an increasing number of countries worldwide either have adopted or are considering similar laws requiring transparency of interactions with healthcare professionals.
Foreign Corrupt Practices Act
The Foreign Corrupt Practices Act, or FCPA, prohibits any U.S. individual or business from paying, offering, or authorizing payment or offering of anything of value, directly or indirectly, to any foreign official, political party, or candidate for the purpose of influencing any act or decision of the foreign entity in order to
-85-
Table of Contents
assist the individual or business in obtaining or retaining business. The FCPA also obligates companies whose securities are listed in the United States to comply with accounting provisions requiring us to maintain books and records that accurately and fairly reflect all transactions of the corporation, including international subsidiaries, and to devise and maintain an adequate system of internal accounting controls for international operations.
Foreign regulations
When we market our tests outside of the United States we will be subject to foreign regulatory requirements governing laboratory licensure, human clinical testing, use of tissue, privacy and data security, and marketing approval for our tests. These requirements vary by jurisdiction, differ from those in the United States and may require us to implement additional compliance measures or perform additional pre-clinical or clinical testing. On September 26, 2012, the European Commission, or EC, released the first drafts of the new EU regulations for medical devices and in vitro diagnostic, or IVDs, that if finalized will impose additional regulatory requirements on IVDs used in the EU. In addition, we will also be required to participate in the Department of Commerce “Safe Harbor” program, which documents our compliance to the HIPAA-like clinical data privacy requirements overseas. In many countries outside of the United States, coverage, pricing and reimbursement approvals are also required. We are also required to maintain accurate information and control over sales and distributors’ activities that may fall within the purview of the FCPA, its books and records provisions and its anti-bribery provisions.
Employees
As of May 31, 2014, we had 20 full-time and contract employees, including eight engaged in research and development, five in general and administrative, five in laboratory operations, and two in marketing. We also engage consultants in regulatory, information technology, and software development. None of our employees are represented by a labor union and we believe that our relationship with our employees and contractors is good.
Facilities
We lease approximately 9,600 square feet of space at 11099 North Torrey Pines Road, Suite 100, San Diego, California 92037. Our lease expires in 2017, but includes a single three-year extension at our option to 2020. We believe these facilities are adequate to meet our current and reasonably foreseeable requirements. We believe that we would be able to obtain additional space, if required, on commercially reasonable terms.
Legal Proceedings
We are not currently a party to any legal proceedings.
Corporate and Other Information
We were incorporated in California in 1996. In connection with this offering, we plan to reincorporate in Delaware. Our principal offices are located at 11099 North Torrey Pines Road, Suite 100, La Jolla, California 92037. Our telephone number is (858) 450-4222 and our website address iswww.dermtech.com. Information contained on or accessible through our website is not a part of this prospectus, and the inclusion of our website address in this prospectus is an inactive textual reference only.
-86-
Table of Contents
Executive Officers and Directors
The following table sets forth the names, ages, and positions of our executive officers and directors as of March 31, 2014:
Name | Age | Position | ||||
Executive Officers | ||||||
John Dobak, M.D. | 48 | Chief Executive Officer and Director | ||||
Steven Kemper, CPA, MBA | 59 | Chief Financial Officer, Treasurer, and Secretary | ||||
Michael Willis, Ph.D. | 50 | Chief Operating Officer | ||||
John Alsobrook, Ph.D. | 55 | Chief Scientific Officer | ||||
Non-Employee Directors | ||||||
Gary Jacobs 1, 2 | 57 | Chairman of the Board | ||||
Scott Pancoast 1, 2 | 55 | Director | ||||
Carl Peck, M.D. | 72 | Director | ||||
Gene Salkind, M.D. | 60 | Director | ||||
| (1) | Member of the audit committee |
| (2) | Member of the compensation committee |
Executive Officers
John Dobak, M.D. has served as a director on our board of directors since June 2012 and currently serves as such. Dr. Dobak has served as our Chief Executive Officer since June 2012. From 2006 until 2011 Dr. Dobak served as the founder and CEO of Lithera, Inc., a pharmaceutical company developing an injectable product for dermatology. Dr. Dobak is the founder and President of the JAKK Group, a life sciences technology accelerator, which has created several companies including Lithera, Inc., INNERCOOL Therapies, Inc., CryoGen, Inc., and CryoCor, Inc. Dr. Dobak’s companies have developed and marketed therapeutics devices for endovascular hypothermia, cryosurgical cardiac catheters, and endometrial ablation. Dr. Dobak and these companies have received several awards for entrepreneurship and innovation, including UCSD Connect’s Most Innovative New Product and MIT’s TR 100. Dr. Dobak received a Bachelor’s Degree from the University of California, Los Angeles and a Medical Doctorate from the University of California, San Diego.
We believe Dr. Dobak is qualified to sit on our board of directors because of his extensive experience in the medical, life sciences, and biotechnology industries.
Steven Kemper, CPA, MBA has served as our Chief Financial Officer and Treasurer since April 1, 2014 and started as our Consulting CFO in December 2013. From 1996 to present, Mr. Kemper has served as President of Pacific Financial Consulting, an advisory firm that assists emerging growth companies raise capital and grow. Since 2011, Mr. Kemper has also served as adjunct professor of Finance at the University of San Diego. Mr. Kemper was Chief Financial Officer of GenMark Diagnostics, Inc., a medical devices company focusing on molecular diagnostic testing, and Sr. Vice President, Finance for its predecessor company, Osmetech plc, from November 2009 until November 2010. From December 2007 to June 2009 Mr. Kemper was Chief Financial Officer of The Active Network, Inc., a activity and participation management software company. He was the founding Chief Financial Officer and Treasurer of DexCom, Inc., a medical device company focusing on glucose monitoring systems, where he served from March 2003 until July 2007. Mr. Kemper has also served as a director of Open Energy Corporation, a renewable energy company focusing on solar energy, and several private companies. He holds a Bachelor’s Degree from the University of California, San Diego, an MBA in Finance from Loyola Marymount University, an MS in Accountancy from San Diego State University, and is a licensed CPA.
Michael Willis, Ph.D. has served as our Chief Operating Officer since April 2014 and started as our Consulting COO in January 2013. From 2005 to December 2013, Dr. Willis served in a variety of senior
-87-
Table of Contents
management roles including President and COO, Vice President and General Manager at Rigaku Automation, Inc., a leader in protein crystallography automation. Dr. Willis has previously served as Director of Chemistry and Senior Director, Chemistry and Manufacturing for Sequenom, Inc., a biotechnology company focusing on genotyping and quantitative gene expression, and Associate Director, Chemistry, at NeXstar Pharmaceuticals Inc., a pharmaceutical company. Dr. Willis holds nine patents and has had numerous publications. He received his B.A. in Chemistry from Point Loma University and his Ph.D. in Chemistry from the University of Colorado.
John P. Alsobrook II, Ph.D., DABCC, FACBhas served as our Chief Scientific Officer since April 2014 and started as our Consulting CSO in January 2013. Prior to that he held several leadership roles at Exagen Diagnostics, Inc., a biotechnology company focused on Rheumatology and Gastroenterology, from 2005 to 2012, including Director of Discovery, VP Clinical Laboratory Operations, and CEO, where he led the company from a development-stage company to commercial-stage company with revenue. Prior to joining Exagen, Dr. Alsobrook was an independent consultant for G.E. Healthcare, a healthcare company focusing on transformational medical technologies, Amersham, a pharmaceutical company specializing in medical diagnostics and life science products, and Genaissance Pharmaceuticals, Inc., biotechnology company specializing in genomics. Dr. Alsobrook is board-certified in molecular diagnostics by the American Board of Clinical Chemists, and he is a Fellow of the Academy of Clinical Biochemistry. He is an inventor on 11 biomarker patents and has published extensively in biomedical journals. Dr. Alsobrook received B.S. degrees in Biochemistry and Physics from California State University, Los Angeles, an MS in Human Genetics from Yale University, and a Ph.D. in Quantitative Genetics from Yale University, and served on the Yale School of Medicine faculty as an Associate Scientist.
Board of Directors
Gary Jacobshas served as Chairman of our board of directors since 2006. Since 2004, Mr. Jacobs has served on the boards of Nutrinia Ltd., a biotechnology company focusing on oral insulin based therapies for infants. Since 2004, Mr. Jacobs has served on the board of Next Generation Technologies, Inc., a technology company specializing in speech recognition software. Since 2007, Mr. Jacobs has served on the board of GEO2 Technologies Inc., a material sciences company providing a microstructure for complex chemical reactions. Since 2009, Mr. Jacobs has served on the board of Bio2 Technologies, Inc., a medical device company specializing in fiber bonding for biocompatible materials. Since 2012, Mr. Jacobs has served on the board of Motus GI Medical Technologies Ltd., a medical device company that develops endoscopy devices. Since 2008, Mr. Jacobs has served on the board of Medical Surgical Technologies, Ltd., another medical device company that develops laparoscopy manipulator systems. Since 2004, Mr. Jacobs has served on the board of Fallbrook Technologies Inc., an automotive company that manufactures transmissions. Mr. Jacobs is an active investor and philanthropist and is Chairman of the Board of Trustees of High Tech High (HTH) as well as a board member of the HTH Graduate School of Education. He serves on the boards of the Lawrence Family Jewish Community Center and Jewish Community Centers Association. Mr. Jacobs also chairs the Dean’s Advisory Council for the Social Sciences at University of California, San Diego, the UCSD Athletic Board, the UCSD Board of Overseers, and UCSD Foundation Board. Mr. Jacobs received his Bachelor of Arts degree in Management Science from the University of California, San Diego.
We believe Mr. Jacobs is qualified to sit on our board of directors because of his experience as a venture capitalist and business leader, his current and past service as a board member for public and private companies, and his experience in the biotechnology and lifesciences industries.
Scott Pancoast has served on our board of directors since 2013. He has served as the President and Chief Executive Officer of Lpath Inc. (Lpath), a biotechnology company that lipodomic-based therapeutic antibodies, since March 2005 and as a Director of Lpath since 1998. Prior to joining Lpath, from 1994 to 2005, Mr. Pancoast was the Executive Vice President of Western States Investment Corporation, a private San Diego based venture capital fund. He has served as the CEO or interim CEO for six start-up companies, and has been a member of the boards of directors for over 15 companies, including two public companies. From 1986 to 1994, Mr. Pancoast
-88-
Table of Contents
was with National Sanitary Supply Company, a supplier of janitorial supplies, where he was a member of the board of directors and served in various management positions, including Senior Vice President of Operations and Chief Financial Officer. He is a graduate of the Harvard Business School and the University of Virginia.
We believe Mr. Pancoast is qualified to sit on our board of directors because of his experience as a venture capitalist and business leader, his current and past service as a member of our board of directors, and his current and past service as a CEO and board member for public and private companies.
Carl C. Peck, M.D., Dr. h.c.(Uppsala) has served on our board of directors since 2005 and is currently Adjunct Professor, Department of Bioengineering and Therapeutic Sciences at the University of California San Francisco. He is Chairman and Co-founder of NDA Partners LLC, a consulting firm specializing in product development and regulatory advice for medical product industries. Dr. Peck joined the FDA as Director, Center for Drug Evaluation and Research in October 1987. He was promoted to Assistant Surgeon General in the Public Health Service in October 1990. Retiring from FDA in late 1993, Dr. Peck was appointed “Boerhaave” Professor of Clinical Drug Research at Leiden University in The Netherlands. Throughout his career, he has mentored more than 40 postdoctoral fellows and graduate students and co-founded the American and Chinese courses in Drug Development and Regulatory Science (ACDRS, CCDRS). In March 2012, Dr. Peck received the ASCPT Gary Neal Prize for Innovation in Drug Development. He is an author of more than 150 original research papers, chapters, and books.
We believe Dr. Peck is qualified to sit on our board of directors because of his extensive experience in the medical, life sciences, and biotechnology industries.
Gene Salkind, M.D.has served on our board of directors since 2007. Dr. Gene Salkind’s background includes more than 25 years as a practicing neurosurgeon in a private practice, with academic affiliation in Pennsylvania. He is the Chairman of Neurosurgery at the Holy Redeemer Hospital and Medical Center and is the former Chairman of Neurosurgery at the Albert Einstein Medical Center. Dr. Salkind has held professorships at the University of Pennsylvania and at the Temple University School of Medicine. Dr. Salkind has published multiple papers on general neurosurgical topics, has been a guest lecturer worldwide, and sits on numerous boards. Dr. Salkind received his B.A. (cum laude) from the University of Pennsylvania and his M.D. from the Temple University School of Medicine, with distinction. He completed his training in neurological surgery at the Hospital of the University of Pennsylvania where he became the Chief Resident in 1985.
We believe Dr. Salkind is qualified to sit on our board of directors because of his extensive experience in the medical, life sciences, and biotechnology industries.
There are no family relationships among any of our executive officers or directors.
Board Composition
Our business and affairs are managed under the direction of our board of directors. The number of directors will be fixed by our board of directors, subject to the terms of our amended and restated certificate of incorporation and amended and restated bylaws that will become effective immediately prior to the completion of this offering. Our board of directors currently consists of five directors, four of whom will qualify as “independent” under The NASDAQ Capital Market listing standards.
In accordance with our amended and restated certificate of incorporation and our amended and restated bylaws, immediately after the completion of this offering, our board of directors will be divided into three classes with staggered three-year terms. Only one class of directors will be elected at each annual meeting of our stockholders, with the other classes continuing for the remainder of their respective three-year terms. Our directors will be divided among the three classes as follows:
| • | the Class I directors will be , and their terms will expire at the annual meeting of stockholders to be held in 2017; |
-89-
Table of Contents
| • | the Class II directors will be , and their terms will expire at the annual meeting of stockholders to be held in 2018; and |
| • | the Class III directors will be , and their terms will expire at the annual meeting of stockholders to be held in 2019. |
The division of our board of directors into three classes with staggered three-year terms may delay or prevent a change of our management or a change of control. Under Delaware law, following our reincorporation in connection with this offering, our directors may be removed for cause by the affirmative vote of the holders of a majority of our outstanding voting stock. Directors may not be removed by our stockholders without cause.
Any increase or decrease in the number of directors will be distributed among the three classes so that, as nearly as possible, each class will consist of one-third of the directors.
Director Independence
Our board of directors has undertaken a review of the independence of each director. Based on information provided by each director concerning his or her background, employment and affiliations, our board of directors determined that Messrs. Jacobs, Pancoast, Peck, and Salkind do not have a relationship that would interfere with the exercise of independent judgment in carrying out the responsibilities of a director and that each of these directors is “independent” as that term is defined under the applicable rules and regulations of the SEC and the listing requirements and rules of The NASDAQ Capital Market. In making these determinations, our board of directors considered the current and prior relationships that each non-employee director has with our company and all other facts and circumstances our board of directors deemed relevant in determining their independence, including the beneficial ownership of our capital stock by each non-employee director and the transactions involving them, as described in the section titled “Certain Relationships, Related Party and Other Transactions.”
Board Committees
Our board of directors currently has an audit committee and a compensation committee. We plan to create a nominating and corporate governance committee prior to the completion of this offering. The composition and responsibilities of each of the committees of our board of directors is described below. Members will serve on these committees until their resignation or until otherwise determined by our board of directors.
Audit Committee
Our audit committee is comprised of Mr. Jacobs and Mr. Pancoast. Mr. Pancoast serves as our audit committee chairperson and meets the requirements for independence of audit committee members under current NASDAQ listing standards and SEC rules and regulations, and before the expiration of the phase-in period applicable to initial public offerings under the applicable NASDAQ rules, all members of our audit committee will be independent for audit committee purposes. Each member of our audit committee meets the financial literacy requirements of the current listing standards. In addition, our board of directors has determined that Mr. Pancoast is an audit committee financial expert within the meaning of Item 407(d) of Regulation S-K under the Securities Act. The responsibilities of our audit committee include, among other things:
| • | selecting and hiring the independent registered public accounting firm to audit our financial statements; |
| • | helping to ensure the independence and performance of the independent registered public accounting firm; |
| • | approving audit and non-audit services and fees; |
| • | reviewing financial statements and discussing with management and the independent registered public accounting firm our annual audited and quarterly financial statements, the results of the independent audit and the quarterly reviews, and the reports and certifications regarding internal controls over financial reporting and disclosure controls; |
-90-
Table of Contents
| • | preparing the audit committee report that the SEC requires to be included in our annual proxy statement; |
| • | reviewing reports and communications from the independent registered public accounting firm; |
| • | reviewing the adequacy and effectiveness of our internal controls and disclosure controls and procedures; |
| • | reviewing our policies on risk assessment and risk management; |
| • | reviewing related party transactions; |
| • | establishing and overseeing procedures for the receipt, retention, and treatment of accounting related complaints and the confidential submission by our employees of concerns regarding questionable accounting or auditing matters; and |
| • | reviewing annually the audit committee charter and the committee’s performance. |
Our audit committee will operate under a written charter, to be effective prior to the completion of this offering, that satisfies the applicable rules of the SEC and the listing standards of The NASDAQ Capital Market. We intend to comply with future requirements to the extent they become applicable to us.
Compensation Committee
Our compensation committee is comprised of Mr. Jacobs and Mr. Pancoast. Mr. Pancoast serves as our compensation committee chairperson. The composition of our compensation committee meets the requirements for independence under current NASDAQ listing standards and SEC rules and regulations. Each member of the compensation committee is also a non-employee director, as defined pursuant to Rule 16b-3 promulgated under the Exchange Act, and an outside director, as defined pursuant to Section 162(m) of the Internal Revenue Code, as amended, or the Code. The purpose of our compensation committee is to oversee our compensation policies, plans, and benefit programs and to discharge the responsibilities of our board of directors relating to compensation of our executive officers. The responsibilities of our compensation committee include, among other things:
| • | overseeing our overall compensation philosophy and compensation policies, plans, and benefit programs; |
| • | reviewing and approving for our executive officers (other than the CEO, for which the committee makes a recommendation to the board of directors): the annual base salary, annual incentive bonus (including the specific goals and amounts), equity compensation, employment agreements, severance agreements, change in control arrangements, and any other benefits, compensation, or arrangements; |
| • | preparing the compensation committee report that the SEC requires to be included in our annual proxy statement; and |
| • | administering our equity compensation plans. |
Our compensation committee will operate under a written charter, to be effective prior to the completion of this offering, that satisfies the applicable rules of the SEC and the listing standards of The NASDAQ Capital Market. We intend to comply with future requirements to the extent they become applicable to us.
Nominating and Corporate Governance Committee
We plan to create a nominating and corporate governance committee prior to the completion of this offering. The composition of our nominating and corporate governance committee will meet the requirements for independence under current NASDAQ listing standards and SEC rules and regulations. The responsibilities of our nominating and corporate governance committee will include, among other things:
| • | identifying, evaluating, and making recommendations to our board of directors regarding nominees for election to our board of directors and its committees; |
-91-
Table of Contents
| • | considering and making recommendations to our board of directors regarding the composition of our board of directors and its committees; |
| • | reviewing developments in corporate governance practices; |
| • | evaluating the adequacy of our corporate governance practices and reporting; |
| • | developing and making recommendations to our board of directors regarding our corporate governance guidelines; |
| • | reviewing the succession planning for each of our executive officers; and |
| • | evaluating the performance of our board of directors and of individual directors. |
Our nominating and corporate governance committee will operate under a written charter, to be effective prior to the completion of this offering, that satisfies the applicable rules of the SEC and the listing standards of The NASDAQ Capital Market. We intend to comply with future requirements to the extent they become applicable to us.
Compensation Committee Interlocks and Insider Participation
No member of our compensation committee has ever been an executive officer or employee of ours. None of our executive officers currently serve, or have served during the last completed year, on the compensation committee or board of directors of any other entity that has one or more executive officers serving as a member of our board of directors or compensation committee.
Code of Business Conduct and Ethics
We plan to adopt a Code of Business Conduct and Ethics, to be effective upon the completion of this offering, that is applicable to all of our employees, officers, and directors, including our Chief Executive Officer, Chief Financial Officer, and other executive and senior financial officers.
Non-Employee Director Compensation
Prior to March 31, 2014, we had not implemented a formal policy with respect to compensation payable to our non-employee directors for service as directors. From time-to-time, we have granted stock options to those non-employee directors for their service on our board of directors. We have not paid cash compensation to any of our non-employee directors. We do, however, reimburse our directors for expenses associated with attending meetings of our board and meetings of committees of our board.
Directors who are also our employees receive no additional compensation for their service as a director. During the year ended December 31, 2013, one director, John Dobak, M.D., our Chief Executive Officer, was an employee. Dr. Dobak’s compensation is discussed in the section titled “Executive Compensation.”
-92-
Table of Contents
The following table lists all outstanding equity awards held by our non-employee directors as of March 31, 2014.
Name | Option Grant Date | Number of Securities Underlying Unexercised Options Exercisable | Number of Securities Underlying Unexercised Options Unexercisable | Option Exercise Price Per Share | Option Expiration Date | |||||||||||||||
Gary Jacobs | 11/5/2010 | 1,000 | — | $ | 0.75 | 11/5/2020 | ||||||||||||||
| 3/10/2012 | 1,000 | — | $ | 0.75 | 3/10/2022 | |||||||||||||||
| 11/7/2013 | — | 5,771 | $ | 0.75 | 11/7/2023 | |||||||||||||||
| 2/25/2014 | 160 | 5,610 | $ | 0.75 | 2/25/2024 | |||||||||||||||
Scott Pancoast | 11/7/2013 | — | 5,771 | $ | 0.75 | 11/7/2023 | ||||||||||||||
| 2/25/2014 | 160 | 5,610 | $ | 0.75 | 2/25/2024 | |||||||||||||||
Carl Peck | 11/5/2010 | 1,333 | — | $ | 0.75 | 11/5/2020 | ||||||||||||||
| 11/7/2013 | — | 3,462 | $ | 0.75 | 11/7/2023 | |||||||||||||||
| 2/25/2014 | 96 | 3,366 | $ | 0.75 | 2/25/2024 | |||||||||||||||
Gene Salkind | 11/5/2010 | 1,000 | — | $ | 0.75 | 11/5/2020 | ||||||||||||||
| 11/7/2013 | — | 3,462 | $ | 0.75 | 11/7/2023 | |||||||||||||||
| 2/25/2014 | 96 | 3,366 | $ | 0.75 | 2/25/2024 | |||||||||||||||
-93-
Table of Contents
Our named executive officers for 2013, which consist of our principal executive officer and the next two most highly compensated executive officers, are:
John Dobak, our Chief Executive Officer;
Steven Kemper, our Chief Financial Officer, Treasurer, and Secretary; and
John Alsobrook, our Chief Scientific Officer.
Summary Compensation Table
The following table provides information regarding the compensation of our principal executive officer and the next two most highly compensated officers during 2013.
Summary Compensation Table
Name and Principal Position | Year | Salary | Bonus | All Other Compensation | Total | |||||||||||||||
John Dobak Chief Executive Officer | 2013 | $ | 251,611 | $ | 36,873 | $ | 21,600 | $ | 310,084 | |||||||||||
Steven Kemper Chief Financial Officer | 2013 | — | — | 1,500 | 1,500 | |||||||||||||||
John Alsobrook Chief Scientific Officer | 2013 | — | — | 127,656 | 127,656 | |||||||||||||||
Executive Employment Arrangements
We have entered into employment agreements with all of our named executive officers. These agreements provide for at-will employment and generally include the named executive officer’s initial base salary, an indication of eligibility for an annual cash incentive award opportunity, and equity awards. In addition, four of our employees, including each of our named executive officers has executed a management retention agreement with us which provides for potential payments and benefits due upon a termination of employment or a change in control. These employment arrangements are described below.
John Dobak
Our President and Chief Executive Officer, John Dobak, received an annual base salary of $251,611 and a bonus of $36,873 in 2013. We entered into an employment agreement with Dr. Dobak in June 2012, which was amended in February 2014. The agreement, as amended, provides for, among other things: (1) a base salary of $250,000 per year, (2) an increase in base salary to $325,000 per year upon the closing of at least $5,000,000 of equity financing from new investors, or a Qualified Financing, (3) a bonus payment of $8,333 per month for the time elapsed in months from August 8, 2013 and the Qualified Financing, (4) a bonus of up to 30% of his base salary based on objectives set by the board of director, and (5) reimbursement of $1,800 per month for health care coverage and up to $15,000 of reimbursement for membership in his professional organization. The agreement provides for a stock option grant equal to 5% of the fully diluted capitalization of our company, adjusted for the next Qualified Financing to maintain the 5% award level. One half of the option award vests over a four year period, monthly, and the remaining one half of the grant shall vest in three equal parts according to completion of the following milestones: (i) CLIA laboratory certification, (ii) $5 million in new capital raised,
-94-
Table of Contents
and (iii) the completion of a clinical utility study. Dr. Dobak will be entitled to receive the following severance payments if his employment terminates under certain conditions, subject to his execution of a release of claims against our company:
| • | in the event that our company terminates Dr. Dobak’s employment without “cause” or Dr. Dobak terminates his employment for “good reason,” in each case outside of a “change in control period,” Dr. Dobak will receive a lump-sum payment equal to six months of his then current salary, our company will pay the COBRA premiums necessary to retain group health plan coverage for Dr. Dobak and his eligible for six months, and Dr. Doback will be granted a twelve month extension to exercise his vested stock options; and |
| • | in the event that our company terminates Dr. Dobak’s employment without “cause” or Dr. Dobak terminates his employment for “good reason” during the period that is between 3 months prior to and 18 months following a change in control (the change in control period), Dr. Dobak will receive the severance payments set forth in the preceding bulletpoint and immediate vesting of all shares underlying Dr. Dobak’s option grant. |
Steven Kemper
Mr. Kemper served as a consultant to our company for the year 2013 and earned $1,500. In February 2014 he was granted a warrant to purchase 18,948 shares of our common stock at a price of $0.75 per share subject to time based and performance based vesting requirements. We entered into an employment agreement with Mr. Kemper on April 1, 2014, pursuant to which Mr. Kemper agreed to serve as our Chief Financial Officer and Treasurer. Mr. Kemper’s agreement provides for, among other things: (1) a base salary of $125,000 per year, (2) eligibility to receive a target bonus of up to 20% of Mr. Kemper’s base salary based on objectives set by the board of directors, (3) an equity award of options to purchase up to 18,948 shares of our common stock at a price of $0.75 per share with vesting based on performance milestones, and (4) reimbursement of $1,500 per month for medical insurance. Upon the occurrence of a Qualified Financing, Mr. Kemper’s salary will be increased to $250,000 per year and he will be paid a one-time bonus in an amount equal to $10,417 per calendar month from April 1, 2014 to the date the Qualified Financing occurs, subject to Mr. Kemper’s continued employment with our company through such date. After a Qualified Financing has occurred, Mr. Kemper will be entitled to receive the following severance payments if his employment terminates under certain conditions, subject to his execution of a release of claims against our company:
| • | in the event that our company terminates Mr. Kemper’s employment other than for “cause”, death or disability or Mr. Kemper terminates his employment for “good reason,” in each case outside of a “change in control period”, Mr. Kemper will receive a lump-sum payment equal to six months of his then current salary, reimbursement of COBRA premium payments for six months to provide group health insurance to Mr. Kemper and his eligible dependents, stock option vesting as to the number of shares that would have vested had Mr. Kemper remained an employee for six months beyond his date of termination, and Mr. Kemper will be granted an eighteen month extension to exercise his vested stock options; and |
| • | in the event that our company terminates Mr. Kemper’s employment other than for “cause”, death or disability, or Mr. Kemper terminates his employment for “good reason” during the period that is between 3 months prior to and 18 months following a change in control (the change in control period), Mr. Kemp will receive a lump-sum payment equal to 12 months of his current salary, reimbursement of COBRA premium payments for twelve months to provide group health insurance to Mr. Kemper and his eligible dependents, and immediate vesting of all shares underlying his option grant. |
| • | in the event that our company terminates Mr. Kemper’s employment other than for “cause”, death or disability outside of a change in control period, subsequent to a change in the identity of the Company’s chief executive officer from John Dobak to any other person, then Mr. Kemper will receive the payments set forth in the preceding bulletpoint and 100% of Mr. Kemper’s unvested options shall immediately vest. |
-95-
Table of Contents
Michael Willis
We entered into an employment agreement with Dr. Willis on April 1, 2014, pursuant to which Dr. Willis agreed to serve as our Chief Operating Officer. Dr. Willis’s agreement provides for, among other things: (1) an annual base salary of no less than $125,000 per year, (2) eligibility to receive a target bonus of up to 20% of Dr. Willis’s base salary based on objectives set by the board of directors, and (3) an equity award of options to purchase up to 31,580 shares of our common stock at a price of $0.75 per share with vesting based on performance milestones. Upon the occurrence of a Qualified Financing, Dr. Willis’s salary will be increased to $250,000 per year and he will be paid a one-time bonus in an amount equal to $10,416.67 per calendar month from April 1, 2014 to the date the Qualified Financing occurs, subject to Dr. Willis’ continued employment with our company through such date. After a Qualified Financing has occurred, Dr. Willis will be entitled to receive the following severance payments if his employment terminates under certain conditions, subject to his execution of a release of claims against our company:
| • | in the event that our company terminates Dr. Willis’s employment other than for “cause”, death or disability, or Dr. Willis terminates his employment for “good reason,” in each case outside of a “change in control period”, Dr. Willis will receive a lump-sum payment equal to six months of his then current salary, reimbursement of COBRA premium payments for six months to provide group health insurance to Dr. Willis and his eligible dependents, and stock option vesting as to the number of shares that would have vested had Dr. Willis remained an employee for six months beyond his date of termination; and |
| • | in the event that our company terminates Dr. Willis’s employment other than for “cause”, death or disability, or Dr. Willis terminates his employment for “good reason” during the period that is between 3 months prior to and 18 months following a change in control (the change in control period), Dr. Willis will receive the severance payments set forth in the preceding bulletpoint and immediate vesting of all shares underlying Dr. Willis’ option grant provided that the acquiring company does not assume his stock option. |
John Alsobrook
Dr. Alsobrook served as a consultant to our company for the year 2013 and earned $127,656 in consulting fees. We entered into an employment agreement with Dr. Alsobrook on April 1, 2014, pursuant to which Dr. Alsobrook agreed to serve as our Chief Scientific Officer. Our employment agreement with Dr. Alsobrook provides for, among other things: (1) an annual base salary of no less than $125,000 per year, (2) eligibility to receive a target bonus of up to 20% of Dr. Alsobrook’s base salary based on objectives set by the board of directors, and (3) an equity award of options to purchase up to 22,738 shares of our common stock at a price of $0.75 per share with vesting based on performance milestones. Upon the occurrence of a Qualified Financing, Dr. Alsobrook’s annual base salary will be increased to $250,000 per year and he will be paid a one-time bonus in an amount equal to $10,417 per calendar month from April 1, 2014 to the date the Qualified Financing occurs, subject to Dr. Alsobrook’s continued service to our company through such date. After a Qualified Financing has occurred, Dr. Alsobrook will be entitled to receive the following severance payments if his employment terminates under certain conditions, subject to his execution of a release of claims against our company:
| • | in the event that our company terminates Dr. Alsobrook’s employment other than for “cause”, death or disability, or Dr. Alsobrook terminates his employment for “good reason”, in each case outside of a “change in control period”, Dr. Alsobrook will receive a lump-sum payment equal to six months of his then current salary, reimbursement of COBRA premium payments for six months to provide group health insurance to Dr. Alsobrook and his eligible dependents, and stock option vesting as to the number of shares that would have vested had Dr. Alsobrook remained an employee for six months beyond his date of termination; and |
| • | in the event that our company terminates Dr. Alsobrook’s employment other than for “cause”, death or disability, or Dr. Alsobrook terminates his employment for “good reason” during the |
-96-
Table of Contents
period that is between 3 months prior to and 18 months following a change in control (the change in control period), Dr. Alsobrook will receive the severance payments set forth in the preceding bulletpoint and immediate vesting of all shares underlying Dr. Alsobrook’s option grant provided that the acquiring company does not assume his stock option. |
Outstanding Equity Awards at Fiscal Year-End
The following table provides information regarding equity awards held by our named executive officers at March 31, 2014.
| Option Awards | ||||||||||||||||||||
| Vesting Commencement Date | Number of Securities Underlying Unexercised Options | Option Exercise Price | Option Expiration Date | |||||||||||||||||
Name | Exercisable | Unexercisable | ||||||||||||||||||
John Dobak | Various | 44,481 | 208,159 | $ | 0.75 | 2024 | ||||||||||||||
Steven Kemper | Various | 1,579 | 36,317 | $ | 0.75 | 2024 | ||||||||||||||
John Alsobrook | Various | 1,923 | 24,276 | $ | 0.75 | 2024 | ||||||||||||||
Employee Benefit and Stock Plans
2014 Equity Incentive Plan
We expect our board of directors to adopt, and our stockholders to approve, a 2014 Equity Incentive Plan, or the 2014 Plan, prior to the registration date. Subject to stockholder approval, the 2014 Plan will be effective one business day prior to the effective date of the registration statement of which this prospectus forms a part, but is not expected to be utilized until after the completion of this offering. Our 2014 Plan permits the grant of incentive stock options, within the meaning of Section 422 of the Code to our employees and any parent and subsidiary corporations’ employees, and for the grant of nonstatutory stock options, restricted stock, restricted stock units, stock appreciation rights, performance units and performance shares to our employees, directors and consultants and our parent and subsidiary corporations’ employees and consultants.
Authorized shares
A total of shares of our common stock have been reserved for issuance pursuant to the 2014 Plan, of which no awards are issued and outstanding. In addition, the shares to be reserved for issuance under our 2014 Plan will also include shares subject to stock options or similar awards granted under the 2010 Stock Plan, or the 2010 Plan, that, after the registration date, expire or terminate without having been exercised in full and shares issued pursuant to awards granted under the 2010 Plan that after the completion of the offering are forfeited to or repurchased by us (provided that the maximum number of shares that may be added to the 2014 Plan pursuant such previsouly granted awards under the 2010 Plan is ). In addition, shares may become available under the 2014 Plan under the following two paragraphs.
The number of shares available for issuance under the 2014 Plan will also include an annual increase on the first day of each fiscal year beginning in 2015, equal to the least of:
| • | shares; |
| • | 5% of the outstanding shares of common stock as of the last day of our immediately preceding fiscal year; or |
| • | such other amount as our board of directors may determine. |
If an award expires or becomes unexercisable without having been exercised in full, is surrendered pursuant to an exchange program, or, with respect to restricted stock, restricted stock units, performance units or
-97-
Table of Contents
performance shares, is forfeited or repurchased due to failure to vest, the unpurchased shares (or for awards other than stock options or stock appreciation rights, the forfeited or repurchased shares) will become available for future grant or sale under our 2014 Plan. With respect to stock appreciation rights, the net shares issued will cease to be available under the 2014 Plan and all remaining shares will remain available for future grant or sale under the 2014 Plan. Shares used to pay the exercise price of an award or satisfy the tax withholding obligations related to an award will become available for future grant or sale under our 2014 Plan. To the extent an award is paid out in cash rather than shares, such cash payment will not result in reducing the number of shares available for issuance under our 2014 Plan.
Plan administration
Our board of directors or one or more committees appointed by our board of directors will administer our 2014 Plan. Our board of directors has appointed the compensation committee of our board of directors to administer our 2014 Plan. In the case of awards intended to qualify as “performance-based compensation” within the meaning of Section 162(m) of the Code, the committee will consist of two or more “outside directors” within the meaning of Section 162(m). In addition, if we determine it is desirable to qualify transactions under the 2014 Plan as exempt under Rule 16b-3 of the Securities Exchange Act of 1934, as amended, or Rule 16b-3, such transactions will be structured to satisfy the requirements for exemption under Rule 16b-3. Subject to the provisions of our 2014 Plan, the administrator has the power to administer the plan, including but not limited to, the power to interpret the terms of our 2014 Plan and awards granted under it, to create, amend and revoke rules relating to our 2014 Plan, including creating sub-plans, and to determine the terms of the awards, including the exercise price, the number of shares subject to each such award, the exercisability of the awards and the form of consideration, if any, payable upon exercise. The administrator also has the authority to amend existing awards to reduce or increase their exercise price, to allow participants the opportunity to transfer outstanding awards to a financial institution or other person or entity selected by the administrator and to institute an exchange program by which outstanding awards may be surrendered in exchange for awards of the same type which may have a higher or lower exercise price or different terms, awards of a different type and/or cash.
Stock options
Stock options may be granted under our 2014 Plan. The exercise price of options granted under our 2014 Plan must at least be equal to the fair market value of our common stock on the date of grant. The term of an incentive stock option may not exceed 10 years, except that with respect to any participant who owns more than 10% of the voting power of all classes of our outstanding stock, the term must not exceed five years and the exercise price must equal at least 110% of the fair market value on the grant date. The administrator will determine the methods of payment of the exercise price of an option, which may include cash, shares or other property acceptable to the administrator, as well as other types of consideration permitted by applicable law. After the termination of service of an employee, director or consultant, he or she may exercise his or her option for the period of time stated in his or her option agreement. Generally, if termination is due to death or disability, the option will remain exercisable for 12 months. In all other cases, the option will generally remain exercisable for three months following the termination of service. However, in no event may an option be exercised later than the expiration of its term. Subject to the provisions of our 2014 Plan, the administrator determines the other terms of options.
Stock appreciation rights
Stock appreciation rights may be granted under our 2014 Plan. Stock appreciation rights allow the recipient to receive the appreciation in the fair market value of our common stock between the exercise date and the date of grant. Stock appreciation rights may not have a term exceeding 10 years. After the termination of service of an employee, director or consultant, he or she may exercise his or her stock appreciation right for the period of time stated in his or her option agreement. However, in no event may a stock appreciation right be exercised later than the expiration of its term. Subject to the provisions of our 2014 Plan, the administrator determines the other terms of stock appreciation rights, including when such rights become exercisable and whether to pay any increased
-98-
Table of Contents
appreciation in cash or with shares of our common stock, or a combination thereof, except that the per share exercise price for the shares to be issued pursuant to the exercise of a stock appreciation right will be no less than 100% of the fair market value per share on the date of grant.
Restricted stock
Restricted stock may be granted under our 2014 Plan. Restricted stock awards are grants of shares of our common stock that vest in accordance with terms and conditions established by the administrator. The administrator will determine the number of shares of restricted stock granted to any employee, director or consultant and, subject to the provisions of our 2014 Plan, will determine the terms and conditions of such awards. The administrator may impose whatever conditions to vesting it determines to be appropriate (for example, the administrator may set restrictions based on the achievement of specific performance goals or continued service to us); provided, however, that the administrator, in its sole discretion, may accelerate the time at which any restrictions will lapse or be removed. Recipients of restricted stock awards generally will have voting and dividend rights with respect to such shares upon grant without regard to vesting, unless the administrator provides otherwise. Shares of restricted stock that do not vest are subject to our right of repurchase or forfeiture.
Restricted stock units
Restricted stock units may be granted under our 2014 Plan. Restricted stock units are bookkeeping entries representing an amount equal to the fair market value of one share of our common stock. Subject to the provisions of our 2014 Plan, the administrator will determine the terms and conditions of restricted stock units, including the vesting criteria (which may include accomplishing specified performance criteria or continued service to us) and the form and timing of payment. Notwithstanding the foregoing, the administrator, in its sole discretion, may accelerate the time at which any restrictions will lapse or be removed.
Performance units and performance shares
Performance units and performance shares may be granted under our 2014 Plan. Performance units and performance shares are awards that will result in a payment to a participant only if performance goals established by the administrator are achieved or the awards otherwise vest. The administrator will establish organizational or individual performance goals or other vesting criteria in its discretion, which, depending on the extent to which they are met, will determine the number and/or the value of performance units and performance shares to be paid out to participants. After the grant of a performance unit or performance share, the administrator, in its sole discretion, may reduce or waive any performance criteria or other vesting provisions for such performance units or performance shares. Performance units shall have an initial dollar value established by the administrator prior to the grant date. Performance shares shall have an initial value equal to the fair market value of our common stock on the grant date. The administrator, in its sole discretion, may pay earned performance units or performance shares in the form of cash, in shares or in some combination thereof.
Outside directors
Our 2014 Plan provides that all non-employee directors are eligible to receive all types of awards (except for incentive stock options) under the 2014 Plan. Our 2014 Plan provides that in any given fiscal year, a non-employee director may not receive under the 2014 Plan (i) cash-settled awards having a grant date fair value greater than $ , increased to $ in connection with her or her initial service; and (ii) stock-settled awards having a grant date fair value greater than $ , increased to $ in connection with his or her initial service, in each case, as grant fair value is determined under generally accepted accounting principles.
Non-transferability of awards
Unless the administrator provides otherwise, our 2014 Plan generally does not allow for the transfer of awards and only the recipient of an award may exercise an award during his or her lifetime.
-99-
Table of Contents
Certain adjustments
In the event of certain changes in our capitalization, to prevent diminution or enlargement of the benefits or potential benefits available under our 2014 Plan, the administrator will adjust the number and class of shares that may be delivered under our 2014 Plan and/or the number, class and price of shares covered by each outstanding award and the numerical share limits set forth in our 2014 Plan. In the event of our proposed liquidation or dissolution, the administrator will notify participants as soon as practicable and all awards will terminate immediately prior to the consummation of such proposed transaction.
Merger or change in control
Our 2014 Plan provides that in the event of a merger or change in control, as defined under the 2014 Plan, each outstanding award will be treated as the administrator determines, except that if a successor corporation or its parent or subsidiary does not assume or substitute an equivalent award for any outstanding award, then such award will fully vest, all restrictions on the shares subject to such award will lapse, all performance goals or other vesting criteria applicable to the shares subject to such award will be deemed achieved at 100% of target levels and all of the shares subject to such award will become fully exercisable, if applicable, for a specified period prior to the transaction. The award will then terminate upon the expiration of the specified period of time.
Amendment, termination
The administrator will have the authority to amend, suspend or terminate the 2014 Plan provided such action will not impair the existing rights of any participant. Our 2014 Plan will automatically terminate in 2024, unless we terminate it sooner.
2010 Stock Plan, as amended
Our board of directors adopted our 2010 Plan in July 2010, and our stockholders approved the 2010 Plan in November 2010. The 2010 Plan provides for the grant of incentive stock options, within the meaning of Section 422 of the Internal Revenue Code, to our employees and any parent and subsidiary corporations’ employees, and for the grant of nonstatutory stock options, restricted stock, and restricted stock purchase rights to our employees, directors, and consultants. Our 2010 Plan will terminate in connection with this offering and, accordingly, no shares are available for issuance under this plan. The 2010 Plan will continue to govern outstanding awards granted thereunder.
Authorized shares
We have reserved an aggregate of 424,225 shares of our common stock for issuance under the 2010 Plan. If shares subject to an award under the 2010 Plan expire or otherwise terminate without having been exercised in full, or are forfeited to or repurchased by our company for an amount not greater than the participant’s exercise or purchase price, the shares will again be available for issuance under the 2010 Plan. Additionally, shares will not be deemed to have been issued pursuant to the 2010 Plan with respect to any portion of an award that is settled in cash or to the extent such shares are withheld or reacquired by us in satisfaction of tax withholding obligations.
Plan administration
Our board of directors or one or more committees appointed by our board of directors administers the 2010 Plan. Following this offering, we anticipate that our compensation committee will administer the 2010 Plan with respect to outstanding awards. Subject to the provisions of our 2010 Plan, the administrator has the power to administer the plan, including the power to: (1) determine the recipients of awards and the number of shares subject to awards; (2) determine the type of award granted; (3) determine the fair market value of our common stock, subject to the provisions of the 2010 Plan; (4) determine the terms of awards, including the exercise or
-100-
Table of Contents
purchase price, the vesting schedule and exercisability schedule, the method of satisfaction of any tax withholding obligations, the time of expiration, the effect of a participant’s termination of service, and all other applicable terms, conditions, and restrictions; (5) approve forms of award agreements; (6) amend or modify any award or waive any restrictions applicable to any award; (7) reprice or otherwise adjust the exercise price of any option, or grant a new award in substitution for such option; (8) accelerate, continue, extend, or defer the exercisability or vesting of awards; and (9) prescribe, amend, or rescind rules relating to the 2010 Plan, or adopt subplans or alternative versions. The administrator may also make all other determinations deemed necessary or advisable for administering the 2010 Plan, to the extent not inconsistent with the provisions of the 2010 Plan or applicable law.
Options
The administrator has the power to grant options under our 2010 Plan. The exercise price per share of options generally must equal at least 100% of the fair market value per share of our common stock on the date of grant. The term of an option may not exceed ten years. An incentive stock option held by a participant who owns more than 10% of the total combined voting power of all classes of our stock, or any parent or subsidiary corporations, may not have a term in excess of five years and must have an exercise price of at least 110% of the fair market value per share of our common stock on the date of grant.
After the termination of service, a participant may generally exercise his or her option, to the extent vested as of such date of termination, for the period of time stated in his or her option agreement. Generally, if termination is due to disability or death, the option will remain exercisable, to the extent vested as of such date of termination, for 12 months or such longer period of time as is specified in the option agreement. In all other cases, other than termination for cause, the option generally will remain exercisable for 3 months following termination of service. However, in no event may an option be exercised later than the expiration of its term.
Restricted stock
The administrator may grant restricted stock awards and restricted stock purchase rights under our 2010 Plan. Restricted stock awards are grants of shares of our common stock that vest in accordance with terms and conditions established by the administrator. Restricted stock purchase rights are the right to purchase restricted stock. The administrator may determine the number of shares of restricted stock and purchase rights granted to any employee, director, or consultant and, subject to the provisions of our 2010 Plan, will determine the terms and conditions of such awards. The administrator may impose whatever conditions to vesting it determines to be appropriate (for example, the administrator may set restrictions based on the achievement of specific performance goals or continued service to us); provided, however, that the administrator, in its sole discretion, may accelerate the time at which any restrictions will lapse or be removed. Recipients of restricted stock awards and restricted stock acquired through the exercise of restricted stock purchase rights generally will have voting and dividend rights with respect to such shares upon grant without regard to vesting, unless the administrator provides otherwise. Shares of restricted stock that do not vest are subject to our right of repurchase or forfeiture. Notwithstanding the foregoing, the administrator, in its sole discretion, may accelerate the time at which any restrictions will lapse or be removed.
Non-transferability of awards
Our 2010 Plan generally does not allow for the transfer of awards except by will or by the laws of descent and distribution, and awards are exercisable during the lifetime of a recipient only by the recipient, provided that, if permitted by the administrator and set forth in the applicable award agreement, an option may be transferrable as permitted by Rule 701 of the Securities Act.
-101-
Table of Contents
Certain adjustments
In the event of certain changes in our capitalization without our receipt of consideration, to prevent dilution or enlargement of participants’ rights under the 2010 Plan, the administrator will adjust the number and class of shares that may be delivered under the 2010 Plan and/or the number, class, and price of shares covered by each outstanding award, and the numerical share limits set forth in the 2010 Plan.
Change in control
Our 2010 Plan provides that in the event of a change in control, as defined in the 2010 Plan, each outstanding award will be treated as the administrator determines, including, without limitation, that awards may be assumed or substituted for by the acquiring or succeeding corporation, awards may be terminated immediately prior to the consummation of the change in control, awards may vest in whole or in part prior to or upon consummation of the change in control and, to the extent the administrator determines, terminate on or immediately prior to the effectiveness of the change in control, or awards may be terminated in exchange for cash or property or replaced with other rights or property. If a successor corporation does not assume or substitute an award, such award will terminate upon the consummation of the change of control.
Amendment or termination
The administrator may amend, suspend, or terminate the 2010 Plan at any time, provided such action does not impair the rights of any participant with respect to an outstanding award. As noted above, in connection with this offering, the 2010 Plan will terminate and no further awards will be granted thereunder. All outstanding awards will continue to be governed by their existing terms.
Limitation on Liability and Indemnification Matters
Our amended and restated certificate of incorporation and amended and restated bylaws, each to be effective upon the completion of this offering, will provide that we will indemnify our directors and officers, and may indemnify our employees and other agents, to the fullest extent permitted by Delaware law. Delaware law prohibits our amended and restated certificate of incorporation from limiting the liability of our directors for the following:
| • | any breach of the director’s duty of loyalty to us or to our stockholders; |
| • | acts or omissions not in good faith or that involve intentional misconduct or a knowing violation of law; |
| • | unlawful payment of dividends or unlawful stock repurchases or redemptions; and |
| • | any transaction from which the director derived an improper personal benefit. |
If Delaware law is amended to authorize corporate action further eliminating or limiting the personal liability of a director, then the liability of our directors will be eliminated or limited to the fullest extent permitted by Delaware law, as so amended. Our amended and restated certificate of incorporation does not eliminate a director’s duty of care and, in appropriate circumstances, equitable remedies, such as injunctive or other forms of non-monetary relief, remain available under Delaware law. This provision also does not affect a director’s responsibilities under any other laws, such as the federal securities laws or other state or federal laws. Under our amended and restated bylaws, we will also be empowered to purchase insurance on behalf of any person whom we are required or permitted to indemnify.
In addition to the indemnification required in our amended and restated certificate of incorporation and amended and restated bylaws, we have entered into an indemnification agreement with each member of our board of directors and each of our officers. These agreements provide for the indemnification of our directors, officers, and some employees for certain expenses and liabilities incurred in connection with any action, suit,
-102-
Table of Contents
proceeding or alternative dispute resolution mechanism, or hearing, inquiry or investigation that may lead to the foregoing, to which they are a party, or are threatened to be made a party, by reason of the fact that they are or were a director, officer, employee, agent, or fiduciary of our company, or any of our subsidiaries, by reason of any action or inaction by them while serving as an officer, director, agent, or fiduciary, or by reason of the fact that they were serving at our request as a director, officer, employee, agent, or fiduciary of another entity. In the case of an action or proceeding by or in the right of our company or any of our subsidiaries, no indemnification will be provided for any claim where a court determines that the indemnified party is prohibited from receiving indemnification. We believe that these charter and bylaw provisions and indemnification agreements are necessary to attract and retain qualified persons as directors and officers.
The limitation of liability and indemnification provisions in our amended and restated certificate of incorporation and amended and restated bylaws may discourage stockholders from bringing a lawsuit against directors for breach of their fiduciary duties. They may also reduce the likelihood of derivative litigation against directors and officers, even though an action, if successful, might benefit us and our stockholders. Moreover, a stockholder’s investment may be harmed to the extent we pay the costs of settlement and damage awards against directors and officers pursuant to these indemnification provisions. Insofar as indemnification for liabilities arising under the Securities Act may be permitted for our directors, officers, and controlling persons pursuant to the foregoing provisions, or otherwise, we have been advised that, in the opinion of the SEC, such indemnification is against public policy as expressed in the Securities Act, and is therefore unenforceable. There is no pending litigation or proceeding naming any of our directors or officers as to which indemnification is being sought, nor are we aware of any pending or threatened litigation that may result in claims for indemnification by any director or officer.
-103-
Table of Contents
CERTAIN RELATIONSHIPS, RELATED PARTY AND OTHER TRANSACTIONS
In addition to the director and executive officer compensation arrangements and indemnification arrangements discussed above in the sections titled “Management” and “Executive Compensation” and the registration rights described in the section titled “Description of Capital Stock—Registration Rights,” the following is a description of each transaction since January 1, 2011 and each currently proposed transaction in which:
| • | we have been or are to be a participant; |
| • | the amount involved exceeded or exceeds $120,000; and |
| • | any of our directors, executive officers, or holders of more than 5% of our capital stock, or any immediate family member of or person sharing the household with any of these individuals, had or will have a direct or indirect material interest. |
Private Placements
Series B Preferred Stock Financing
Between August 8, 2013 and May 30, 2014, we sold an aggregate of 1,886,245 shares of our Series B preferred stock and warrants to purchase 500,849 shares of our Series B preferred, at a per share purchase price of $6.00, subject to certain adjustments, pursuant to a stock purchase agreement. Purchasers of our Series B preferred stock and warrants to purchase Series B preferred stock included certain of our directors, stockholders who hold greater than 5% of our outstanding capital stock, and the Jacobs Investment Company LLC, which holds 5% or more of our outstanding capital stock and is also affiliated with Gary E. Jacobs, a member of our board of directors, referred to collectively herein after as the Investors. The following table presents the number of shares issued to our directors, officers, and holders of more than 5% of our capital stock or entities affiliated with them:
Purchaser | Number of Series B Preferred Shares | Warrants to Purchase Series B Preferred Shares(3) | Total Purchase Price(1) | |||||||||
Jacobs Company Trust LLC (2) | 1,239,676 | 0 | $ | 5,719,030 | ||||||||
Gene Salkind | 155,427 | 0 | $ | 666,282 | ||||||||
Peter Brundage | 147,093 | 0 | $ | 616,280 | ||||||||
| (1) | In some cases, all or a portion of the purchase price was accounted for by conversion of promissory notes issued as part of the 2012-2013 bridge financing. |
| (2) | Includes 537,479 Series B Preferred shares held by Gary E. Jacobs. |
| (3) | All outstanding warrants held were exercised by May 30, 2014 |
2012-2013 Bridge Financing
Between August 31, 2012 and July 31, 2013, we sold an aggregate of $3.0 million in convertible promissory notes to certain of the Investors. On August 8, 2013, these convertible promissory notes including interest converted into 960,813 shares of our series B preferred stock pursuant to the terms of the notes. The following table presents the principal amount of the convertible promissory notes sold to our directors, officers, and holders of more than 5% of our capital stock or entities affiliated with them:
Purchaser | Principal Amount of Convertible Notes | |||
Jacobs Investment Company LLC (1) | $ | 2,000,000 | ||
Gene Salkind | $ | 300,000 | ||
Peter Brundage | $ | 275,000 | ||
| (1) | Includes $1,500,000 of convertible promissory notes purchased by Gary E. Jacobs. |
-104-
Table of Contents
Series A Preferred Stock Financing
Between January 1, 2011 and September 31, 2012, we sold an aggregate of 31,518 shares of series A-3 preferred stock, at per share purchase price of $60.00, subject to certain adjustments, pursuant to a stock purchase agreement. Purchasers of the series A-3 preferred stock include certain of the Investors. The following table presents the number of shares issued to our directors, officers, and holders of more than 5% of our capital stock or entities affiliated with them:
Purchaser | Number of Series A-3 Preferred Shares | Total Purchase Price | ||||||
Jacobs Investment Company LLC | 9,167 | $ | 550,000 | |||||
Gene Salkind | 3,750 | $ | 225,000 | |||||
Peter Brundage | 5,417 | $ | 325,000 | |||||
Investors’ Rights Agreement
We entered into an investors’ rights agreement, dated August 8, 2013, with certain of our directors, officers, and stockholders, including the Jacobs Investment Company LLC, which holds 5% or more of our capital stock and is also affiliated with Gary E. Jacobs, a member of our board of directors. Such agreement provides, among other things, for certain rights relating to the registration of their shares of common stock, including those issued upon conversion of their preferred stock, including the right to demand that we file a registration statement or request that their shares be covered by a registration statement that we are otherwise filing. For a more detailed description of these registration rights, see “Description of Capital Stock—Registration Rights.”
Voting Agreement
We entered into a voting agreement, dated August 8, 2013, with certain of our officers, directors, and stockholders, including the Jacobs Investment Company LLC, which holds 5% or more of our capital stock and is also affiliated with Gary E. Jacobs, a member of our board of directors. The parties to the voting agreement have agreed, subject to certain conditions, to vote their shares so as to elect as directors our company’s chief executive officer and one director designated by a majority of the other then-elected members of our board of directors. Upon the consummation of this offering, the obligations of the parties to the voting agreement to vote their shares so as to elect these nominees, as well as the other rights and obligations under this agreement, will terminate and none of our stockholders will have any special rights regarding the nomination, election, or designation of members of our board of directors. Our existing certificate of incorporation contains provisions that correspond to the voting agreement; however, such provisions will be removed in the amended and restated certificate of incorporation that will be effective at the closing of the offering.
Lease Transaction
We leased our office space from an entity controlled by Gary Jacobs, a member of our board of directors and a holder of more than 5% of our outstanding stock. Rent expense on this facility was $14,000, $51,000, and $156,485, in 2013, 2012, and 2011, respectively. This lease terminated in April 2013.
Indemnification of Officers and Directors
We have also entered into indemnification agreements with each of our directors, executive officers, and certain controlling persons. The indemnification agreements and our amended restated certificate of incorporation and amended and restated bylaws require us to indemnify our directors, executive officers, and certain controlling persons to the fullest extent permitted by Delaware law. See “Executive and Director Compensation—Limitations on Liability and Indemnification Matters” above.
-105-
Table of Contents
Related-Person Transactions Policy
Prior to the completion of this offering, we plan to adopt a written Related Person Transactions Policy that sets forth our policies and procedures regarding the identification, review, consideration, approval, and oversight of “related person transactions.” For purposes of our policy only, a “related person transaction” is a past, present, or future transaction, arrangement, or relationship (or any series of similar transactions, arrangements, or relationships) in which we and any “related person” are participants, the amount involved exceeds $120,000, and a related person has a direct or indirect material interest. Transactions involving compensation for services provided to us as an employee, director, consultant, or similar capacity by a related person are not covered by this policy. A “related person,” as determined since the beginning of our last fiscal year, is any executive officer, director or nominee to become director, or a holder of more than 5% of our common stock, including any immediate family members of such persons. Any related person transaction may only be consummated if approved or ratified by our audit committee in accordance with the policy guidelines set forth below.
Under the policy, where a transaction has been identified as a related person transaction, management must present information regarding the proposed related person transaction to our audit committee for review and approval. In considering related person transactions, our audit committee will take into account the relevant available facts and circumstances including, but not limited to, whether the terms of such transaction are no less favorable than terms generally available to an unaffiliated third-party under the same or similar circumstances and the extent of the related person’s interest in the transaction. In the event a director has an interest in the proposed transaction, the director must recuse himself from the deliberations and approval process.
-106-
Table of Contents
The following table sets forth information regarding beneficial ownership of our common stock as of May 31, 2014, as adjusted to reflect the shares of common stock to be issued and sold by us in this offering, by:
| • | each person or group of affiliated persons known by us to be the beneficial owner of more than 5% of our common stock; |
| • | each of our named executive officers; |
| • | each of our directors; and |
| • | all executive officers and directors as a group. |
The percentage ownership information prior to the offering shown in the table is based on an aggregate of 2,126,063 shares of our common stock and is comprised of 106,052 shares of our common stock outstanding as of May 31, 2014 and 2,020,011 shares of our common stock issuable upon conversion of our outstanding shares of preferred stock. The percentage ownership information after the offering assumes the issuance of shares of common stock in this offering. The table does not reflect any potential purchases of the shares reserved for sale by the underwriters by our directors, officers, or other existing investors as described in “Underwriting.”
We have determined beneficial ownership in accordance with SEC rules. These rules generally attribute beneficial ownership of securities to persons who possess sole or shared voting power or investment power with respect to those securities. In addition, the rules include shares of common stock issuable pursuant to the exercise of stock options and warrants that are either immediately exercisable or exercisable on or before July 31, 2014, which is 60 days after May 31, 2014. These shares are deemed to be outstanding and beneficially owned by the person holding those options and warrants for the purpose of computing the percentage ownership of that person, but they are not treated as outstanding for the purpose of computing the percentage ownership of any other person. Unless otherwise indicated, the persons or entities identified in this table have sole voting and investment power with respect to all shares shown as beneficially owned by them, subject to applicable community property laws.
Unless otherwise indicated, the address of each beneficial owner listed on the table below is c/o DermTech International, 11099 N. Torrey Pines Road, Suite 100, La Jolla, California 92037.
| Shares Beneficially Owned Prior to This Offering | Shares Beneficially Owned After This Offering | |||||||||||
Name of Beneficial Owner | Shares | Percentage | Shares | Percentage | ||||||||
5% Stockholders: | ||||||||||||
Gary Jacobs(1) | 1,320,673 | 62.0 | % | |||||||||
Gene Salkind, M.D.(2) | 195,789 | 9.2 | % | |||||||||
Peter Brundage(3) | 182,924 | 8.6 | % | |||||||||
Named Executive Officers and Directors: | ||||||||||||
John Dobak(4) | 74,290 | 3.4 | % | |||||||||
Steven Kemper(5) | 3,158 | * | ||||||||||
John Alsobrook(6) | 2,693 | * | ||||||||||
Gary Jacobs(1) | 1,320,673 | 62.0 | % | |||||||||
Scott Pancoast(7) | 801 | * | ||||||||||
Carl Peck, M.D.(8) | 1,834 | * | ||||||||||
Gene Salkin, M.D.(2) | 195,789 | 9.2 | % | |||||||||
All executive officers and directors as a group (8 persons) (9) | 1,599,239 | 73.0 | % | |||||||||
| * | Represents beneficial ownership of less than 1% |
-107-
Table of Contents
| (1) | Consists of 537,479 shares and 2,481 options to acquire shares held by Gary Jacobs; 779,490 shares and 0 warrants to acquire shares held by the Jacobs Investment Company LLC and 902 shares held by the Gary E. and Jerri-Ann Jacobs, trustees of the Jacobs Family Trust dated 11-9-1999. |
| (2) | Consists of 194,308 shares and 1,289 options to acquire shares. |
| (3) | Consists of 182,924 shares. |
| (4) | Consists of 25,000 shares and 46,884 options to acquire shares. |
| (5) | Consists of 2,368 warrants to acquire shares. |
| (6) | Consists of 2,308 options to acquire shares. |
| (7) | Consists of 481 options to acquire shares. |
| (8) | Consists of 20 shares and 1,622 options to acquire shares. |
| (9) | Consists of all the shares and options and warrants to acquire shares reflected in footnotes 1, 2, and 4 through 8. |
-108-
Table of Contents
General
The following description summarizes the most important terms of our capital stock, as they are expected to be in effect upon the completion of this offering. We expect to adopt an amended and restated certificate of incorporation and amended and restated bylaws in connection with the completion of this offering, and this description summarizes the provisions that are expected to be included in such documents. This summary does not purport to be complete and is qualified in its entirety by the provisions of our amended and restated certificate of incorporation and amended and restated bylaws, copies of which have been filed as exhibits to the registration statement of which this prospectus is a part. For a complete description of our capital stock, you should refer to our amended and restated certificate of incorporation, amended and restated bylaws, and investor rights agreement, that are or will be included as exhibits to the registration statement of which this prospectus forms a part, and to the applicable provisions of Delaware law. Immediately following the completion of this offering, our authorized capital stock will consist of shares of common stock, $0.001 par value per share, and shares of undesignated preferred stock, $0.001 par value per share.
Assuming the conversion of all outstanding shares of our convertible preferred stock into an aggregate of 1,779,595 shares of our common stock, which will occur upon the completion of this offering, as of March 31, 2014, there were 1,885,647 shares of our common stock outstanding, held by approximately 170 stockholders of record, and no shares of our preferred stock outstanding. Our board of directors is authorized, without stockholder approval, to issue additional shares of our capital stock.
Common Stock
Dividend Rights
Subject to preferences that may be applicable to any then outstanding preferred stock, holders of our common stock are entitled to receive dividends, if any, as may be declared from time-to-time by our board of directors out of legally available funds. We have never declared or paid cash dividends on any of our capital stock and currently do not anticipate paying any cash dividends after this offering or in the foreseeable future.
Voting Rights
Each holder of our common stock is entitled to one vote for each share on all matters submitted to a vote of the stockholders, including the election of directors. Our stockholders do not have cumulative voting rights in the election of directors. Accordingly, holders of a majority of the voting shares are able to elect all of the directors.
Right to Receive Liquidation Distributions
In the event of our liquidation, dissolution, or winding up, holders of our common stock will be entitled to share ratably in the net assets legally available for distribution to stockholders after the payment of all of our debts and other liabilities and the satisfaction of any liquidation preference granted to the holders of any then outstanding shares of preferred stock.
Rights and Preferences
Holders of our common stock have no preemptive, conversion, subscription, or other rights, and there are no redemption or sinking fund provisions applicable to our common stock. The rights, preferences, and privileges of the holders of our common stock are subject to and may be adversely affected by, the rights of the holders of shares of any series of our preferred stock that we may designate in the future.
-109-
Table of Contents
Preferred Stock
Each currently outstanding share of our convertible preferred stock will be converted into common stock on a one-to-one basis immediately prior to the completion of this offering pursuant to the conversion provisions in our certificate of incorporation based on the proposed offering size or based on the consent of the holders of at least a majority of the outstanding shares of certain series of preferred stock. Holders of a majority of each series of our convertible preferred stock have provided their written consent to approve this conversion contingent upon this offering.
Immediately after the completion of this offering, our board of directors will have the authority, without further action by our stockholders, to issue up to shares of preferred stock in one or more series and to fix the rights, preferences, privileges and restrictions thereof. These rights, preferences, and privileges could include dividend rights, conversion rights, voting rights, terms of redemption, liquidation preferences, sinking fund terms, and the number of shares constituting any series or the designation of such series, any or all of which may be greater than the rights of common stock. The issuance of preferred stock by us could adversely affect the voting power of holders of common stock and the likelihood that such holders will receive dividend payments and payments upon liquidation. In addition, the issuance of preferred stock could have the effect of delaying, deferring or preventing a change of control of our company or other corporate action. Upon the completion of this offering, no shares of preferred stock will be outstanding, and we have no present plan to issue any shares of preferred stock.
Stock Options
As of March 31, 2014, there were 394,563 shares of our common stock issuable upon exercise of outstanding stock options pursuant to our 2010 Stock Plan with a weighted average exercise price of $0.75 per share.
Warrants
As of March 31, 2014, we had outstanding warrants to purchase an aggregate of 295,623 shares of our common stock at a weighted-average exercise price of $5.25, including preferred stock warrants on an as-converted basis.
Exclusive Jurisdiction
Unless we consent in writing to the selection of an alternative forum, the Court of Chancery of the State of Delaware will be the sole and exclusive forum for (i) any derivative action or proceeding brought on behalf of us; (ii) any action asserting a claim of breach of a fiduciary duty owed by any of our directors, officers, or other employees or agents to the us or the our stockholders; (iii) any action asserting a claim against us arising pursuant to any provision of the DGCL or our amended and restated certificate of incorporation or amended and restated bylaws; (iv) any action to interpret, apply, enforce, or determine the validity of our amended and restated certificate of incorporation or our amended and restated bylaws; or (v) any action asserting a claim against us governed by the internal affairs doctrine, in each such case, subject to said Court of Chancery having personal jurisdiction over the indispensable parties named as defendants therein. The enforceability of similar choice of forum provisions in other companies’ certificates of incorporation has been challenged in legal proceedings, and it is possible that, in connection with any action, a court could find the choice of forum provisions contained in our amended and restated certificate of incorporation to be inapplicable or unenforceable in such action.
Registration Rights
After the completion of this offering, the holders of an aggregate of shares of our common stock, or their permitted transferees, are entitled to rights with respect to the registration of such shares under the
-110-
Table of Contents
Securities Act. We refer to these shares as “registrable securities.” These rights are provided under the terms of our amended and restated investors’ rights agreement between us and the holders of registrable securities, and include demand registration rights, short form registration rights, and piggyback registration rights.
These registration rights will terminate (i) 4 years following the completion of this offering, or (ii), with respect to any holder of registrable securities, when such holder (together with its affiliates with whom it must aggregate its sales under Rule 144 of the Securities Act) is able to sell all of its registrable securities during a three-month period without registration in compliance with Rule 144 of the Securities Act.
We will pay the registration expenses (other than underwriters’ and brokers’ discounts and commissions) in connection with the registrations described below, including the reasonable fees and disbursements of one counsel for the selling holder or holders of registrable securities. In an underwritten offering, the underwriters have the right to limit the number of shares registered by these holders for marketing reasons, subject to certain limitations. In connection with the completion of this offering, each holder of registrable securities has agreed or will agree not to sell or otherwise dispose of any securities without the prior written consent of the underwriters for a period of 180 days after the date of this prospectus, subject to certain terms and conditions. See “Shares Eligible for Future Sale—Lock-Up Agreements” for additional information.
Demand Registration Rights
Six months after the completion of this offering, the holders of 50% or more of the then outstanding registrable securities can request that we register the offer and sale of their shares if the anticipated aggregate offering price of such shares is at least $10 million. We are not required to effect more than two demand registrations. If we determine that it would be seriously detrimental to us and our stockholders to effect a demand registration, we have the right to defer such registration, not more than once in any 12-month period, for a period of up to 90 days, provided that we do not register any of our securities or those of any other stockholder during such 90-day period, other than with respect to a registration related to a company stock plan, a registration related to a corporate reorganization or transaction under Rule 145 of the Securities Act, a registration on any form that does not include substantially the same information as would be required to be included in a registration statement covering the sale of registrable securities or a registration in which the only common stock being registered is common stock issuable upon conversion of debt securities that are also being registered. Additionally, we are not required to effect a demand registration during the period beginning with the date 90 days prior to our good faith estimate of the date of filing, and ending 180 days following the effectiveness of a registration statement relating to the initial public offering of our securities, nor are we required to effect a demand registration if the initiating holders propose to dispose of shares of registrable securities that may be immediately registered on Form S-3 pursuant to their S-3 Registration Rights.
Piggyback Registration Rights
If we propose to register the offer and sale of any of our securities under the Securities Act in connection with the public offering of such securities, the holders of registrable securities will be entitled to certain “piggyback” registration rights allowing such holders to include their shares in such registration, subject to certain limitations. As a result, whenever we propose to file a registration statement under the Securities Act, other than with respect to a registration related to a company stock plan, a registration related to a corporate reorganization or transaction under Rule 145 of the Securities Act, a registration on any form that does not include substantially the same information as would be required to be included in a registration statement covering the sale of registrable securities or a registration in which the only common stock being registered is common stock issuable upon conversion of debt securities that are also being registered, the holders of these shares are entitled to notice of the registration and have the right to include their shares in the registration.
-111-
Table of Contents
Form S-3 Registration Rights
Any holder or holders of our registrable securities may make a written request that we register the offer and sale of their shares on Form S-3 if we are eligible to file a registration statement on Form S-3 so long as the sale of securities registered pursuant to such request would result in an aggregate price to the public (net of any underwriters’ discounts and commission) of at least $1 million. We are not required to effect more than one Form S-3 registration in any 12-month period. Additionally, if we determine that it would be materially detrimental to our stockholders to effect such a registration, we have the right to defer such registration, not more than once in any 12-month period, for a period of up to 90 days, provided that we do not register any of our securities or those of any other stockholder during such 90-day period, other than with respect to a registration related to a company stock plan, a registration related to a corporate reorganization or transaction under Rule 145 of the Securities Act, a registration on any form that does not include substantially the same information as would be required to be included in a registration statement covering the sale of registrable securities, or a registration in which the only common stock being registered is common stock issuable upon conversion of debt securities that are also being registered.
Anti-Takeover Effects of Delaware Law and Our Certificate of Incorporation and Bylaws
The provisions of Delaware law, our amended and restated certificate of incorporation, and our amended and restated bylaws may have the effect of delaying, deferring, or discouraging another person from acquiring control of our company. These provisions, which are summarized below, may have the effect of discouraging takeover bids. They are also designed, in part, to encourage persons seeking to acquire control of us to negotiate first with our board of directors. We believe that the benefits of increased protection of our potential ability to negotiate with an unfriendly or unsolicited acquirer outweigh the disadvantages of discouraging a proposal to acquire us because negotiation of these proposals could result in an improvement of their terms.
Delaware Law
In connection with this offering, we plan to reincorporate in Delaware. After we reincorporate, we will be governed by the provisions of Section 203 of the DGCL. In general, Section 203 prohibits a public Delaware corporation from engaging in a “business combination” with an “interested stockholder” for a period of three years after the date of the transaction in which the person became an interested stockholder, unless the business combination is approved in a prescribed manner. A “business combination” includes mergers, asset sales, or other transactions resulting in a financial benefit to the stockholder. An “interested stockholder” is a person who, together with affiliates and associates, owns, or within three years of the date on which it is sought to be determined whether such person is an “interested stockholder,” did own, 15% or more of the corporation’s outstanding voting stock. These provisions may have the effect of delaying, deferring or preventing a change in our control.
Amended and Restated Certificate of Incorporation and Amended and Restated Bylaw Provisions
Our amended and restated certificate of incorporation and our amended and restated bylaws include a number of provisions that could deter hostile takeovers or delay or prevent changes in control of our management team, including the following:
| • | Board of Directors Vacancies. Our amended and restated certificate of incorporation and amended and restated bylaws will authorize only our board of directors to fill vacant directorships, including newly created seats. In addition, the number of directors constituting our board of directors will be permitted to be set only by a resolution adopted by our board of directors. These provisions would prevent a stockholder from increasing the size of our board of directors and then gaining control of our board of directors by filling the resulting vacancies with its own nominees. This makes it more difficult to change the composition of our board of directors but promotes continuity of management. |
| • | Classified Board. Our amended and restated certificate of incorporation and amended and restated bylaws will provide that our board is classified into three classes of directors. A third party may be |
-112-
Table of Contents
discouraged from making a tender offer or otherwise attempting to obtain control of us as it is more difficult and time-consuming for stockholders to replace a majority of the directors on a classified board of directors. See “Management—Board Composition” for additional information. |
| • | Stockholder Action; Special Meeting of Stockholders. Our amended and restated certificate of incorporation will provide that our stockholders may not take action by written consent, but may only take action at annual or special meetings of our stockholders. As a result, a holder controlling a majority of our capital stock would not be able to amend our amended and restated bylaws or remove directors without holding a meeting of our stockholders called in accordance with our amended and restated bylaws. Our amended and restated bylaws will further provide that special meetings of our stockholders may be called only by a majority of our board of directors, the Chairman of our board of directors, our Chief Executive Officer or our president, thus prohibiting a stockholder from calling a special meeting. These provisions might delay the ability of our stockholders to force consideration of a proposal or for stockholders controlling a majority of our capital stock to take any action, including the removal of directors. |
| • | Advance Notice Requirements for Stockholder Proposals and Director Nominations. Our amended and restated bylaws will provide advance notice procedures for stockholders seeking to bring business before our annual meeting of stockholders or to nominate candidates for election as directors at our annual meeting of stockholders. Our amended and restated bylaws will also specify certain requirements regarding the form and content of a stockholder’s notice. These provisions might preclude our stockholders from bringing matters before our annual meeting of stockholders or from making nominations for directors at our annual meeting of stockholders if the proper procedures are not followed. We expect that these provisions may also discourage or deter a potential acquirer from conducting a solicitation of proxies to elect the acquirer’s own slate of directors or otherwise attempting to obtain control of our company. |
| • | No Cumulative Voting. The DGCL provides that stockholders are not entitled to the right to cumulate votes in the election of directors unless a corporation’s certificate of incorporation provides otherwise. Our amended and restated certificate of incorporation will not provide for cumulative voting. |
| • | Directors Removed Only for Cause. Our amended and restated certificate of incorporation will provide that stockholders may remove directors only for cause. |
| • | Amendment of Charter Provisions. Any amendment of the above provisions in our amended and restated certificate of incorporation would require approval by holders of at least two-thirds of our then outstanding common stock. |
| • | Issuance of Undesignated Preferred Stock. Our board of directors will have the authority, without further action by the stockholders, to issue up to shares of undesignated preferred stock with rights and preferences, including voting rights, designated from time to time by our board of directors. The existence of authorized but unissued shares of preferred stock would enable our board of directors to render more difficult or to discourage an attempt to obtain control of us by means of a merger, tender offer, proxy contest or other means. |
Transfer Agent and Registrar
Upon the completion of this offering, the transfer agent and registrar for our common stock will be American Stock Transfer & Trust Company, LLC, or AST. The transfer agent and registrar’s address is 6201 15th Avenue, Brooklyn, New York 11219. Our shares of common stock will be issued in uncertificated form only, subject to limited circumstances.
Market Listing
We intend to apply to list our common stock on The NASDAQ Capital Market under the symbol “DMTK.”
-113-
Table of Contents
SHARES ELIGIBLE FOR FUTURE SALE
Prior to this offering, there has been no public market for our common stock, and we cannot predict the effect, if any, that market sales of shares of our common stock or the availability of shares of our common stock for sale will have on the market price of our common stock prevailing from time to time. Future sales of our common stock in the public market, or the availability of such shares for sale in the public market, could adversely affect market prices prevailing from time to time. As described below, only a limited number of shares will be available for sale shortly after this offering due to contractual and legal restrictions on resale. Nevertheless, sales of our common stock in the public market after such restrictions lapse, or the perception that those sales may occur, could adversely affect the prevailing market price at such time and our ability to raise equity capital in the future.
Following the completion of this offering, and after giving effect to the conversion of all outstanding shares of our convertible preferred stock which will occur upon the completion of this offering. Of these outstanding shares, all of the shares of common stock sold in this offering, plus any shares sold upon exercise of the underwriters’ option to purchase up to an additional shares of common stock from us in this offering, will be freely tradable, except that any shares purchased in this offering by our affiliates, as that term is defined in Rule 144 under the Securities Act, would only be able to be sold in compliance with the Rule 144 limitations described below.
The remaining outstanding shares of our common stock will be deemed “restricted securities” as defined in Rule 144. Restricted securities may be sold in the public market only if they are registered or if they qualify for an exemption from registration under Rule 144 or Rule 701 under the Securities Act, which rules are summarized below. In addition, holders of all or substantially all of our equity securities have entered into or will enter into lock-up agreements with the underwriters under which they have agreed, subject to specific exceptions, not to sell any of our stock for at least 180 days following the date of this prospectus, as described below. As a result of these agreements, subject to the provisions of Rule 144 or Rule 701, based on an assumed offering date of shares will be available for sale in the public market as follows:
| • | beginning on the date of this prospectus, the shares of common stock sold in this offering will be immediately available for sale in the public market; |
| • | beginning 181 days after the date of this prospectus, additional shares of common stock will become eligible for sale in the public market, of which shares will be held by affiliates and subject to the volume and other restrictions of Rule 144, as described below; and |
| • | the remainder of the shares of common stock will be eligible for sale in the public market from time to time thereafter, subject in some cases to the volume and other restrictions of Rule 144, as described below. |
Lock-Up Agreements
We, our officers and directors, and holders of substantially all of our common stock and securities convertible into or exchangeable for our common stock, have agreed or will agree that, subject to certain exceptions and under certain conditions, for a period of 180 days after the date of this prospectus, we and they will not, without the prior written consent of Maxim Group LLC, dispose of or hedge any shares or any securities convertible into or exchangeable for shares of our capital stock. Maxim Group LLC may, in their discretion, release any of the securities subject to these lock-up agreements at any time.
In the event that any of our officers or directors or holders of 1% or more of our common stock (including any securities convertible into or exercisable or exchangeable for common stock) are granted an early release, then certain persons or groups who have executed a lock-up agreement automatically will be granted an early release from their obligations under the lock-up agreement on a pro rata basis.
-114-
Table of Contents
Rule 144
In general, under Rule 144 as currently in effect, once we have been subject to the public company reporting requirements of Section 13 or Section 15(d) of the Exchange Act for at least 90 days, a person who is not deemed to have been one of our affiliates for purposes of the Securities Act at any time during the 90 days preceding a sale and who has beneficially owned the shares proposed to be sold for at least six months, including the holding period of any prior owner other than our affiliates, is entitled to sell those shares without complying with the manner of sale, volume limitation, or notice provisions of Rule 144, subject to compliance with the public information requirements of Rule 144. If such a person has beneficially owned the shares proposed to be sold for at least one year, including the holding period of any prior owner other than our affiliates, then that person would be entitled to sell those shares without complying with any of the requirements of Rule 144.
In general, under Rule 144, as currently in effect, and upon expiration of the lock-up agreements described above, our affiliates or persons selling shares on behalf of our affiliates are entitled to sell within any three-month period, a number of shares that does not exceed the greater of:
| • | 1% of the number of shares of our common stock then outstanding, which will equal approximately shares immediately after this offering assuming no exercise by the underwriters of their option to purchase up to an additional shares of common stock from us in this offering; or |
| • | the average weekly trading volume of our common stock during the four calendar weeks preceding the filing of a notice on Form 144 with respect to that sale. |
Provided, in each case, that we have been subject to the Exchange Act periodic reporting requirements for at least 90 days before the sale. Sales under Rule 144 by our affiliates or persons selling shares on behalf of our affiliates are also subject to certain manner of sale provisions and notice requirements and to the availability of current public information about us.
Rule 701
Rule 701 generally allows a stockholder who purchased shares of our common stock pursuant to a written compensatory plan or contract and who is not deemed to have been an affiliate of our company during the immediately preceding 90 days to sell these shares in reliance upon Rule 144, but without being required to comply with the public information, holding period, volume limitation or notice provisions of Rule 144. Rule 701 also permits affiliates of our company to sell their Rule 701 shares under Rule 144 without complying with the holding period requirements of Rule 144. All holders of Rule 701 shares, however, are required by that rule to wait until 90 days after the date of this prospectus before selling those shares pursuant to Rule 701.
Registration Rights
On the date beginning 180 days after the date of this prospectus, the holders of shares of our common stock, or their transferees, will be entitled to various rights with respect to the registration of these shares under the Securities Act. Registration of these shares under the Securities Act would result in these shares becoming fully tradable without restriction under the Securities Act immediately upon the effectiveness of the registration, except for shares purchased by affiliates. See “Description of Capital Stock—Registration Rights” for additional information.
Stock Plan
Following the completion of this offering, we intend to file a registration statement on Form S-8 under the Securities Act to register shares of our common stock issued or reserved for issuance under our 2010 Plan. The registration statement on Form S-8 will become effective immediately upon filing, and shares covered by such registration statement will thereupon be eligible for sale in the public markets, subject to vesting restrictions, the lock-up agreements described above and Rule 144 limitations applicable to affiliates. See “Executive Compensation—Employee Benefit and Stock Plans” for additional information.
-115-
Table of Contents
Warrants
Upon closing of this offering, warrants entitling holders to purchase an aggregate of shares of our common stock at a weighted-average exercise price of $ per share will be outstanding. See “Description of Capital Stock” for additional information. Such shares issued upon exercise of the warrants may be able to be sold after the expiration.
-116-
Table of Contents
MATERIAL U.S. FEDERAL INCOME TAX AND
ESTATE TAX CONSEQUENCES TO NON-U.S. HOLDERS
The following is a summary of the material U.S. federal income tax consequences of the ownership and disposition of our common stock to non-U.S. holders (as defined below), but does not purport to be a complete analysis of all the potential tax considerations relating thereto. This summary is based upon the provisions of the Internal Revenue Code of 1986, as amended, or the Code, Treasury regulations promulgated thereunder, administrative rulings and judicial decisions, all as of the date hereof. These authorities may be changed, possibly retroactively, so as to result in U.S. federal income tax consequences different from those set forth below. We have not sought any ruling from the Internal Revenue Service (IRS) with respect to the statements made and the conclusions reached in the following summary, and there can be no assurance that the IRS will agree with such statements and conclusions.
This summary also does not address the tax considerations arising under the laws of any non-U.S. state or local jurisdiction or under U.S. federal gift and estate tax laws, except to the limited extent set forth below. In addition, this discussion does not address the potential application of the Medicare contribution or any tax considerations applicable to an investor’s particular circumstances or to investors that may be subject to special tax rules, including, without limitation:
| • | banks, insurance companies, or other financial institutions; |
| • | persons subject to the alternative minimum tax; |
| • | tax-exempt organizations; |
| • | foreign controlled corporations, passive foreign investment companies, and corporations that accumulate earnings to avoid U.S. federal income tax; |
| • | brokers or dealers in securities or currencies; |
| • | traders in securities that elect to use a mark-to-market method of accounting for their securities holdings; |
| • | persons that own, or are deemed to own, more than 5% of our capital stock (except to the extent specifically set forth below); |
| • | regulated investment companies; |
| • | real estate investment trusts; |
| • | pension plans; |
| • | individual retirement or tax-deferred accounts; |
| • | controlled foreign corporations; |
| • | passive foreign investment companies; |
| • | corporations that accumulate earnings to avoid U.S. federal income tax; |
| • | hybrid entities; |
| • | integral parts or controlled entities of a foreign sovereign; |
| • | certain former citizens or long-term residents of the United States; |
| • | persons who hold our common stock as a position in a hedging transaction, “straddle,” “conversion transaction,” or other risk reduction transaction; |
| • | persons who do not hold our common stock as a capital asset within the meaning of Section 1221 of the Code; or |
| • | persons deemed to sell our common stock under the constructive sale provisions of the Code. |
-117-
Table of Contents
In addition, if a partnership or entity classified as a partnership for U.S. federal income tax purposes holds our common stock, the tax treatment of a partner generally will depend on the status of the partner and upon the activities of the partnership. Accordingly, partnerships that hold our common stock, and partners in such partnerships, should consult their tax advisors.
You are urged to consult your tax advisor with respect to the application of the U.S. federal income tax laws to your particular situation, as well as any tax consequences of the purchase, ownership, and disposition of our common stock arising under the U.S. federal estate or gift tax rules or under the laws of any state, local, non-U.S., or other taxing jurisdiction or under any applicable tax treaty.
Non-U.S. Holder Defined
For purposes of this discussion, you are a non-U.S. holder if you are any holder other than a partnership (or other entity classified as a partnership for U.S. federal income tax purposes) or:
| • | an individual citizen or resident of the United States (for tax purposes); |
| • | a corporation or other entity taxable as a corporation created or organized in the United States or under the laws of the United States or any political subdivision thereof; |
| • | an estate whose income is subject to U.S. federal income tax regardless of its source; or |
| • | a trust (x) whose administration is subject to the primary supervision of a U.S. court and that has one or more U.S. persons who have the authority to control all substantial decisions of the trust or (y) that has made a valid election under applicable Treasury Regulations to be treated as a U.S. person. |
An individual may be a resident alien for U.S. federal income tax purposes in any calendar year if the individual was present in the United States for at least 31 days in that calendar year and for an aggregate of at least 183 days during the three-year period ending with the current calendar year. For purposes of this calculation, all of the days present in the current year, one-third of the days present in the immediately preceding year and one-sixth of the days present in the second preceding year are counted. Residents are taxed for U.S. federal income tax purposes as if they were U.S. citizens.
Distributions
We do not anticipate making any distributions on our common stock following the completion of this offering. However, if we do make distributions on our common stock, those payments will constitute dividends for U.S. tax purposes to the extent paid from our current or accumulated earnings and profits, as determined under U.S. federal income tax principles. To the extent those distributions exceed both our current and our accumulated earnings and profits, they will constitute a return of capital and will first reduce your basis in our common stock, but not below zero, and then will be treated as gain from the sale of stock.
Any dividend paid to you generally will be subject to U.S. withholding tax either at a rate of 30% of the gross amount of the dividend or such lower rate as may be specified by an applicable income tax treaty. In order to receive a reduced treaty rate, you must provide us with an IRS Form W-8BEN or other appropriate version of IRS Form W-8 certifying qualification for the reduced rate. A non-U.S. holder of shares of our common stock eligible for a reduced rate of U.S. withholding tax pursuant to an income tax treaty may obtain a refund of any excess amounts withheld by filing an appropriate claim for refund with the IRS. If the non-U.S. holder holds the stock through a financial institution or other agent acting on the non-U.S. holder’s behalf, the non-U.S. holder will be required to provide appropriate documentation to the agent, which then will be required to provide certification to us or our paying agent, either directly or through other intermediaries.
Dividends received by you that are effectively connected with your conduct of a U.S. trade or business (and, if an income tax treaty applies, such dividends are attributable to a permanent establishment maintained by you in the United States), are includible in your gross income in the taxable year received, and are exempt from such
-118-
Table of Contents
withholding tax. In order to obtain this exemption, you must provide us with an IRS Form W-8ECI or other applicable IRS Form W-8 properly certifying such exemption. Such effectively connected dividends, although not subject to withholding tax, are taxed at the same graduated rates applicable to U.S. persons, net of certain deductions and credits, subject to an applicable income tax treaty providing otherwise. In addition, if you are a corporate non-U.S. holder, dividends you receive that are effectively connected with your conduct of a U.S. trade or business may also be subject to a branch profits tax at a rate of 30% or such lower rate as may be specified by an applicable income tax treaty.
Gain on Disposition of Common Stock
You generally will not be required to pay U.S. federal income tax on any gain realized upon the sale or other disposition of our common stock unless:
| • | the gain is effectively connected with your conduct of a U.S. trade or business (and, if an income tax treaty applies, the gain is attributable to a permanent establishment maintained by you in the United States); |
| • | you are an individual who is present in the United States for a period or periods aggregating 183 days or more during the calendar year in which the sale or disposition occurs and certain other conditions are met; or |
| • | our common stock constitutes a U.S. real property interest by reason of our status as a “United States real property holding corporation,” or USRPHC, for U.S. federal income tax purposes at any time within the shorter of the five-year period preceding your disposition of, or your holding period for, our common stock. |
We believe that we are not currently and will not become a USRPHC. However, because the determination of whether we are a USRPHC depends on the fair market value of our U.S. real property relative to the fair market value of our other business assets, there can be no assurance that we will not become a USRPHC in the future. Even if we become a USRPHC, however, as long as our common stock is regularly traded on an established securities market, your common stock will be treated as U.S. real property interests only if you actually or constructively hold more than 5% of such regularly traded common stock at any time during the shorter of the five-year period preceding your disposition of, or your holding period for, our common stock.
If you are a non-U.S. holder described in the first bullet above, you will be required to pay tax on the net gain derived from the sale under regular graduated U.S. federal income tax rates, and a corporate non-U.S. holder described in the first bullet above also may be subject to the branch profits tax at a 30% rate, or such lower rate as may be specified by an applicable income tax treaty. If you are an individual non-U.S. holder described in the second bullet above, you will be required to pay a flat 30% tax on the gain derived from the sale, which tax may be offset by U.S. source capital losses for the year. You should consult any applicable income tax or other treaties that may provide for different rules.
Federal Estate Tax
Our common stock beneficially owned by an individual who is not a citizen or resident of the United States (as defined for U.S. federal estate tax purposes) at the time of their death will generally be includable in the decedent’s gross estate for U.S. federal estate tax purposes, unless an applicable estate tax treaty provides otherwise.
Backup Withholding and Information Reporting
Generally, we must report annually to the IRS the amount of dividends paid to you, your name and address, and the amount of tax withheld, if any. A similar report will be sent to you. Pursuant to applicable income tax treaties or other agreements, the IRS may make these reports available to tax authorities in your country of residence.
-119-
Table of Contents
Payments of dividends or of proceeds on the disposition of stock made to you may be subject to information reporting and backup withholding at a current rate of 28% unless you establish an exemption, for example, by properly certifying your non U.S. status on a Form W-8BEN or another appropriate version of IRS Form W-8. Notwithstanding the foregoing, backup withholding and information reporting may apply if either we or our paying agent has actual knowledge, or reason to know, that you are a U.S. person.
Information reporting and backup withholding generally will apply to the proceeds of a disposition of our common stock by a non-U.S. holder effected by or through the U.S. office of any broker, U.S. or foreign, unless the holder certifies its status as a non-U.S. holder and satisfies certain other requirements, or otherwise establishes an exemption. Generally, information reporting and backup withholding will not apply to a payment of disposition proceeds to a non-U.S. holder where the transaction is effected outside the United States through a non-U.S. office of a broker. However, for information reporting purposes, dispositions effected through a non-U.S. office of a broker with substantial U.S. ownership or operations generally will be treated in a manner similar to dispositions effected through a U.S. office of a broker. Non-U.S. holders should consult their own tax advisors regarding the application of the information reporting and backup withholding rules to them.
Backup withholding is not an additional tax; rather, the U.S. income tax liability of persons subject to backup withholding will be reduced by the amount of tax withheld. If withholding results in an overpayment of taxes, a refund or credit may generally be obtained from the IRS, provided that the required information is furnished to the IRS in a timely manner
Legislation Affecting Taxation of our Common Stock Held by or through Foreign Entities
The Foreign Account Tax Compliance Act, or FATCA, codified in Code Sections 1471-1474 and the regulations issued thereunder, generally will impose a U.S. federal withholding tax of 30% on dividends, and the gross proceeds of a disposition of our common stock, paid to a “foreign financial institution” (as specially defined under these rules), unless such institution enters into an agreement with the U.S. government to withhold on certain payments and to collect and provide to the U.S. tax authorities substantial information regarding the U.S. account holders of such institution (which includes certain equity and debt holders of such institution, as well as certain account holders that are foreign entities with U.S. owners). The legislation also generally will impose a U.S. federal withholding tax of 30% on dividends and the gross proceeds of a disposition of our common stock paid to a non-financial foreign entity unless such entity provides the withholding agent with a certification identifying the direct and indirect U.S. owners of the entity. This withholding obligation under this legislation with respect to dividends on our common stock will not begin until July 1, 2014 and with respect to the gross proceeds of a sale or other disposition of our common stock will not begin until January 1, 2017. Under certain circumstances, a non-U.S. holder might be eligible for refunds or credits of such taxes. An intergovernmental agreement between the United States and an applicable foreign country may modify the requirements described in this paragraph. Prospective investors are encouraged to consult with their own tax advisors regarding the possible implications of this legislation on their investment in our common stock.
The preceding discussion of U.S. federal tax considerations is for general information only. It is not tax advice. Each prospective investor should consult its own tax advisor regarding the particular U.S. federal, state and local, and non-U.S. tax consequences of purchasing, holding, and disposing of our common stock, including the consequences of any proposed change in applicable laws.
-120-
Table of Contents
We have entered into an underwriting agreement, or the Underwriting Agreement, with Maxim Group LLC, or Maxim, acting as the sole book-runner and lead managing underwriter, with respect to the shares subject to this offering. Subject to the terms and conditions of the Underwriting Agreement, the underwriters named below have agreed to purchase, and we have agreed to sell to them, the number of shares provided below opposite its name.
Underwriters | Number of Shares | |
Maxim Group LLC | ||
Feltl and Company, Inc. | ||
Total |
The underwriters are offering the shares, subject to acceptance of the shares from us and subject to prior sale. The Underwriting Agreement provides that the obligations of the underwriters to pay for and accept delivery of the shares offered by this prospectus are subject to the approval of certain legal matters by its counsel and to certain other conditions. The underwriters are obligated to take and pay for all of the shares if any such securities are taken, other than those shares covered by the over-allotment option described below.
Over-Allotment
The Underwriting Agreement provides that our company will grant to Maxim an option, exercisable within 45 days after the closing of this offering, to acquire up to an additional shares, or 15% of the total number of shares to be offered by our company pursuant to this offering, solely for the purpose of covering over-allotments.
Commission and Expenses
Maxim has advised us that the underwriters propose to offer the shares to the public at the initial public offering price set forth on the cover page of this prospectus and to certain dealers at that price less a concession not in excess of $ per Share. The underwriters may allow, and certain dealers may re-allow, a discount from the concession not in excess of $ per Share to certain brokers and dealers. After this offering, the initial public offering price, concession and reallowance to dealers may be reduced by Maxim. No such reduction shall change the amount of proceeds to be received by us as set forth on the cover page of this prospectus. The shares are offered by the underwriters as stated herein, subject to receipt and acceptance by them and subject to their right to reject any order in whole or in part. Maxim has informed us that the underwriters do not intend to confirm sales to any accounts over which they exercise discretionary authority.
The following table shows the underwriting discounts and commissions payable to the underwriters by us in connection with this offering.
| Per Share | Total Without Exercise of Over-Allotment | Total With Exercise of Over-Allotment | ||||||||||
Public offering price | $ | $ | $ | |||||||||
Discount(1) | $ | $ | $ | |||||||||
| (1) | The discount does not include the underwriter warrants or expense reimbursement as described below. |
We have agreed to reimburse Maxim for certain out-of-pocket expenses it incurs in connection with this offering, including, but not limited to, filing offering materials with FINRA, any lucite memorabilia to be acquired by the underwriters in connection with this offering, background checks, “road show” expenses, costs of
-121-
Table of Contents
book-building, prospectus tracking and compliance software, and the fees and disbursements of its counsel, accountants and other agents and representatives, up to a maximum aggregate of $125,000. Of such amount, we paid a $50,000 advance to Maxim’s counsel, which will be applied against the anticipated legal expenses incurred by Maxim in connection with this offering.
We estimate that expenses payable by us in connection with the offering of our Shares, other than the underwriting discounts and commissions and the counsel fees and disbursement reimbursement provisions referred to above, will be approximately $ .
Upon the closing of this offering, Maxim will have the right of first refusal to act as a co-manager or placement agent on customary terms for such transactions and with at least 15% of the economics for each and every public equity, equity-linked and debt (excluding commercial bank debt) financing of our company, or any successor to or any subsidiary of our company, that takes place within a period of 12 months from the effective date of the registration statement.
We have also agreed to issue to Maxim or its designees, at the closing of this offering, warrants, or the Representative’s Warrants, to purchase that number of shares of our common stock equal to up to 4% of the aggregate number of shares sold in the offering. The Representative’s Warrants will be exercisable at any time and from time-to-time, in whole or in part, during a period commencing six months from the effective date of this prospectus and will expire five years after the effective date of the registration statement filed in connection with this offering. The Representative’s Warrants will be exercisable at a price equal to 120% of the public offering price of the shares in connection with the offering. The Representative’s Warrants and the common stock underlying the Representative’s Warrants have been deemed compensation by FINRA and are, therefore, subject to a 180-day lock-up pursuant to Rule 5110(g)(1) of FINRA. Maxim or permitted assignees, pursuant to such Rule, will not sell, transfer, assign, pledge, or hypothecate the Representative’s Warrants or the common stock underlying the Representative’s Warrants, nor will it engage in any hedging, short sale, derivative, put or call transaction that would result in the effective economic disposition of the Representative’s Warrants or the common stock underlying the Representative’s Warrants for a period of 180 days from the effective date of the registration statement, except that they may be assigned, in whole or in part, as specifically set forth in the Underwriting Agreement. The Representative’s Warrants will provide for cashless exercise, “piggyback” registration rights for three years from the effective date of the registration statement, and customary anti-dilution provisions (for share dividends, splits and recapitalizations, and the like) consistent with FINRA Rule 5110.
Indemnification
We have agreed to indemnify the underwriters against certain liabilities, including liabilities under the Securities Act and liabilities arising from breaches of representations and warranties contained in the Underwriting Agreement, or to contribute to payments that the underwriters may be required to make in respect of those liabilities.
Lock-Up Agreements
Officers, directors and shareholders of our company holding an aggregate of shares of our common stock prior to the offering, have agreed to a 180-day “lock-up” period from the closing of this offering of the shares with respect to the common stock that they beneficially own, including the issuance of shares upon the exercise of convertible securities and options that are currently outstanding or which may be issued. This means that, for a period of 180 days following the closing of the offering of the shares, such persons may not offer, sell, pledge, or otherwise dispose of these securities without the prior written consent of Maxim.
Maxim has no present intention to waive or shorten the lock-up period; however, the terms of the lock-up agreements may be waived at its discretion. In determining whether to waive the terms of the lock-up agreements, Maxim may base its decision on its assessment of the relative strengths of the securities markets and companies similar to ours in general, and the trading pattern of, and demand for, our securities in general.
-122-
Table of Contents
In addition, the Underwriting Agreement provides that we will not, for a period of 180 days following the closing of the offering of the shares, offer, sell or distribute any of our securities, without the prior written consent of Maxim.
Listing
We will apply to list our common stock on The NASDAQ Capital Market under the symbol “DMTK.”
Electronic Distribution
A prospectus in electronic format may be made available on websites or through other online services maintained by Maxim or by its affiliates. Other than the prospectus in electronic format, the information on Maxim’s website and any information contained in any other website maintained by it is not part of this prospectus or the registration statement of which this prospectus forms a part, has not been approved and/or endorsed by us or Maxim in its capacity as an underwriter, and should not be relied upon by investors.
Price Stabilization, Short Positions, and Penalty Bids
In connection with the offering the underwriters may engage in stabilizing transactions, over-allotment transactions, syndicate covering transactions, and penalty bids in accordance with Regulation M under the Exchange Act:
| • | stabilizing transactions permit bids to purchase the underlying security so long as the stabilizing bids do not exceed a specified maximum; |
| • | over-allotment involves sales by the underwriters of shares in excess of the number of shares the underwriters are obligated to purchase, which creates a syndicate short position. The short position may be either a covered short position or a naked short position. In a covered short position, the number of shares over-allotted by the underwriters is not greater than the number of shares that may be purchased in the over-allotment option. In a naked short position, the number of shares involved is greater than the number of shares in the over-allotment option. Maxim may close out any covered short position by either exercising the over-allotment option and/or purchasing shares in the open market; |
| • | syndicate covering transactions involve purchases of shares in the open market after the distribution has been completed in order to cover syndicate short positions. In determining the source of shares to close out the short position, Maxim will consider, among other things, the price of shares available for purchase in the open market as compared to the price at which it may purchase shares through the over-allotment option. If the underwriters sell more shares than could be covered by the over-allotment option, a naked short position, the position can only be closed out by buying shares in the open market. A naked short position is more likely to be created if the underwriters are concerned that there could be downward pressure on the price of the shares in the open market after pricing that could adversely affect investors who purchase in the offering; and |
| • | penalty bids permit Maxim to reclaim a selling concession from a syndicate member when the common stock originally sold by the syndicate member is purchased in a stabilizing or syndicate covering transaction to cover syndicate short positions. |
These stabilizing transactions, syndicate covering transactions, and penalty bids may have the effect of raising or maintaining the market price of our shares or preventing or retarding a decline in the market price of our securities. As a result, the price of our shares may be higher than the price that might otherwise exist in the open market. Neither we nor the underwriters make any representation or prediction as to the direction or magnitude of any effect that the transactions described above may have on the price of our securities. In addition, neither we nor the underwriters make any representations that the underwriters will engage in these stabilizing transactions or that any transaction, once commenced, will not be discontinued without notice.
-123-
Table of Contents
No Prior Public Market
Prior to this offering, there has been no public market for our securities and the public offering price for our securities will be determined through negotiations between us and Maxim. Among the factors to be considered in these negotiations will be prevailing market conditions, our financial information, market valuations of other companies that we and Maxim believe to be comparable to us, estimates of our business potential, the present state of our development, and other factors deemed relevant.
We offer no assurances that the initial public offering price will correspond to the price at which our shares will trade in the public market subsequent to this offering or that an active trading market for our shares will develop and continue after this offering.
Offers Outside the United States
Other than in the United States, no action has been taken by us or the underwriters that would permit a public offering of the shares offered by this prospectus in any jurisdiction where action for that purpose is required. The shares offered by this prospectus may not be offered or sold, directly or indirectly, nor may this prospectus or any other offering material or advertisements in connection with the offer and sale of any such shares be distributed or published in any jurisdiction, except under circumstances that will result in compliance with the applicable rules and regulations of that jurisdiction. Persons into whose possession this prospectus comes are advised to inform themselves about and to observe any restrictions relating to the offering and the distribution of this prospectus. This prospectus does not constitute an offer to sell or a solicitation of an offer to buy any shares offered by this prospectus in any jurisdiction in which such an offer or a solicitation is unlawful.
European Economic Area
In relation to each Member State of the European Economic Area, or EEA, which has implemented the Prospectus Directive, or a Relevant Member State, an offer to the public of any securities which are the subject of the offering contemplated by this prospectus may not be made in that Relevant Member State, except that an offer to the public in that Relevant Member State of any securities may be made at any time under the following exemptions under the Prospectus Directive, if they have been implemented in that Relevant Member State:
| (a) | to legal entities which are authorized or regulated to operate in the financial markets or, if not so authorized or regulated, whose corporate purpose is solely to invest in securities; |
| (b) | to any legal entity which has two or more of (i) an average of at least 250 employees during the last financial year; (ii) a total balance sheet of more than €43,000,000; and (iii) an annual net turnover of more than €50,000,000, as shown in its last annual or consolidated accounts; |
| (c) | by the underwriters to fewer than 100 or, if the Relevant Member State has implemented the relevant provision of the 2010 PD Amending Directive, 150, natural or legal persons (other than “qualified investors” as defined in the Prospectus Directive) subject to obtaining the prior consent of the representatives for any such offer; or |
| (d) | in any other circumstances falling within Article 3(2) of the Prospectus Directive, provided that no such offer of securities shall result in a requirement for the publication by us or any representative of a prospectus pursuant to Article 3 of the Prospectus Directive. |
Any person making or intending to make any offer of securities within the EEA should only do so in circumstances in which no obligation arises for us or any of the underwriters to produce a prospectus for such offer.
Neither we nor the underwriters have authorized, nor do they authorize, the making of any offer of securities through any financial intermediary, other than offers made by the underwriters which constitute the final offering of securities contemplated in this prospectus.
-124-
Table of Contents
For the purposes of this provision, and your representation below, the expression an “offer to the public” in relation to any securities in any Relevant Member State means the communication in any form and by any means of sufficient information on the terms of the offer and any securities to be offered so as to enable an investor to decide to purchase any securities, as the same may be varied in that Relevant Member State by any measure implementing the Prospectus Directive in that Relevant Member State and the expression “Prospectus Directive” means Directive 2003/71/EC and includes any relevant implementing measure in each Relevant Member State, including the 2010 PD Amending Directive, to the extent implemented in the Relevant Member State. The expression “2010 PD Amending Directive” means Directive 2010/73/EU.
Each person in a Relevant Member State who receives any communication in respect of, or who acquires any securities under, the offer of securities contemplated by this prospectus will be deemed to have represented, warranted, and agreed to and with us and each underwriter that:
| (a) | it is a “qualified investor” within the meaning of the law in that Relevant Member State implementing Article 2(1)(e) of the Prospectus Directive; and |
| (b) | in the case of any securities acquired by it as a financial intermediary, as that term is used in Article 3(2) of the Prospectus Directive, (i) the securities acquired by it in the offering have not been acquired on behalf of, nor have they been acquired with a view to their offer or resale to, persons in any Relevant Member State other than “qualified investors,” as defined in the Prospectus Directive, or in circumstances in which the prior consent of the representatives has been given to the offer or resale; or (ii) where securities have been acquired by it on behalf of persons in any Relevant Member State other than qualified investors, the offer of those securities to it is not treated under the Prospectus Directive as having been made to such persons. |
In addition, in the United Kingdom, this document is being distributed only to, and is directed only at, and any offer subsequently made may only be directed at persons who are “qualified investors,” as defined in the Prospectus Directive, (i) who have professional experience in matters relating to investments falling within Article 19 (5) of the Financial Services and Markets Act 2000 (Financial Promotion) Order 2005, as amended, or the Order, and/or (ii) who are high net worth companies (or persons to whom it may otherwise be lawfully communicated) falling within Article 49(2)(a) to (d) of the Order (all such persons together being referred to as “relevant persons”). This document must not be acted on or relied on in the United Kingdom by persons who are not relevant persons. In the United Kingdom, any investment or investment activity to which this document relates is only available to, and will be engaged in with, relevant persons.
Israel
In the State of Israel, the securities offered hereby may not be offered to any person or entity other than the following:
| • | a fund for joint investments in trust, i.e., mutual fund, as such term is defined in the Law for Joint Investments in Trust, 5754-1994, or a management company of such a fund; |
| • | a provident fund as defined in the Control of the Financial Services (Provident Funds) Law 5765-2005, or a management company of such a fund; |
| • | an insurer, as defined in the Law for Oversight of Insurance Transactions, 5741-1981; |
| • | a banking entity or satellite entity, as such terms are defined in the Banking Law (Licensing), 5741-1981, other than a joint services company, acting for its own account or for the account of investors of the type listed in Section 15A(b) of the Securities Law, 1968; |
| • | a company that is licensed as a portfolio manager, as such term is defined in Section 8(b) of the Law for the Regulation of Investment Advisors and Portfolio Managers, 5755-1995, acting on its own account or for the account of investors of the type listed in Section 15A(b) of the Securities Law, 1968; |
-125-
Table of Contents
| • | an investment advisor or investment distributer, as such term is defined in Section 7(c) of the Law for the Regulation of Investment Advisors and Portfolio Managers, 5755-1995, acting on its own account; |
| • | a member of the Tel Aviv Stock Exchange, acting on its own account or for the account of investors of the type listed in Section 15A(b) of the Securities Law, 1968; |
| • | an underwriter fulfilling the conditions of Section 56(c) of the Securities Law, 5728-1968, acting on its own account; |
| • | a venture capital fund, defined as an entity primarily involved in investments in companies which, at the time of investment, (i) are primarily engaged in research and development or manufacture of new technological products or processes and (ii) involve above-average risk; |
| • | an entity fully owned by investors of the type listed in Section 15A(b) of the Securities Law, 5728-1968; |
| • | an entity, other than an entity formed for the purpose of purchasing securities in this offering, in which the shareholders’ equity is in excess of NIS 50 million; and |
| • | an individual fulfilling the conditions of Section 9 to the supplement to the Law for the Regulation of Investment Advisors and Portfolio Managers, 5755-1995, acting on its own account (for this matter, Section 9 to the supplement shall be referred to as “as an investor for the meaning of Section 15A(b)(1) of the Securities Law 1968” instead of “as an eligible client for the meaning of this law”). |
Offerees of the shares offered hereby, or the Investors, in the State of Israel shall be required to submit written confirmation that they fall within the scope of one of the above criteria, that they are fully aware of the significance of being an Investor pursuant to such criteria, and that they have given their consent, or the Consent. An appeal to an Investor for the Consent shall not be considered a public offering. This prospectus will not be distributed or directed to investors in the State of Israel who do not fall within one of the above criteria.
In addition, if a purchase of securities is made within an institutional trading system, as that term is defined in the Tel Aviv Stock Exchange regulations, a person giving a stock exchange member his prior Consent before submitting a purchase order to the institutional trading system for the first time will be seen as acting within the provisions the above criteria with respect to the Consent, provided that if such person is an investor pursuant to the sixth, ninth, tenth, eleventh or twelfth bullet points specified above, such person committed in advance that, until the last business day of the third month in each year, he will renew his Consent, and that if he withdraws his Consent, he will notify the stock exchange member immediately and will cease to give purchase orders in such institutional trading institution.
Canada
The shares sold in this offering have not been and will not be qualified for distribution under applicable Canadian securities laws. Shares may be offered to residents of Canada pursuant to exemptions from the prospectus requirements of such laws.
Other Relationships
Maxim and its affiliates may in the future engage in investment banking and other commercial dealings in the ordinary course of business with us or our affiliates. They may in the future receive customary fees and commissions for these transactions. Maxim did not provide any financing, investment and/or advisory services to us during the 180-day period preceding the filing of the registration statement related to this offering, and as of the date of this prospectus, other than as previously disclosed, we do not have any agreement or arrangement with Maxim to provide any of such services during the 90-day period following the effective date of the registration statement related to this offering.
-126-
Table of Contents
The validity of the shares of common stock offered hereby will be passed upon for us by Wilson Sonsini Goodrich & Rosati, Professional Corporation, Palo Alto, California. Lowenstein Sandler LLP, New York, New York, has acted as counsel for the underwriters in connection with certain legal matters related to this offering.
The audited financial statements included in this prospectus and elsewhere in the registration statement have been so included in reliance upon the report of Grant Thornton LLP, independent registered public accountants, upon the authority of said firm as experts in accounting and auditing.
WHERE YOU CAN FIND MORE INFORMATION
We have filed with the SEC a registration statement on Form S-1 under the Securities Act with respect to the shares of common stock offered by this prospectus. This prospectus, which constitutes a part of the registration statement, does not contain all of the information set forth in the registration statement, some of which is contained in exhibits to the registration statement as permitted by the rules and regulations of the SEC. For further information with respect to us and our common stock, we refer you to the registration statement, including the exhibits filed as a part of the registration statement. Statements contained in this prospectus concerning the contents of any contract or any other document is not necessarily complete. If a contract or document has been filed as an exhibit to the registration statement, please see the copy of the contract or document that has been filed. Each statement in this prospectus relating to a contract or document filed as an exhibit is qualified in all respects by the filed exhibit. You may obtain copies of this information by mail from the Public Reference Section of the SEC, 100 F Street, N.E., Room 1580, Washington, D.C. 20549, at prescribed rates. You may obtain information on the operation of the public reference rooms by calling the SEC at 1-800-SEC-0330. The SEC also maintains an Internet website that contains reports, proxy statements, and other information about issuers, like us, that file electronically with the SEC. The address of that website is www.sec.gov.
As a result of this offering, we will become subject to the information and reporting requirements of the Securities Exchange Act of 1934 and, in accordance with this law, will file periodic reports, proxy statements, and other information with the SEC. These periodic reports, proxy statements and other information will be available for inspection and copying at the SEC’s public reference facilities and the website of the SEC referred to above. We also maintain a website at www.dermtech.com. Upon completion of this offering, you may access these materials free of charge as soon as reasonably practicable after they are electronically filed with, or furnished to, the SEC. Information contained on our website is not a part of this prospectus and the inclusion of our website address in this prospectus is an inactive textual reference only.
-127-
Table of Contents
| Page | ||||
| F-2 | ||||
| F-3 | ||||
| F-4 | ||||
| F-5 | ||||
| F-6 | ||||
| F-8 | ||||
F-1
Table of Contents
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
Board of Directors and Stockholders
DermTech International
We have audited the accompanying balance sheets of DermTech International (the “Company”) as of December 31, 2012 and 2013, and the related statements of operations, changes in stockholders’ equity (deficit), and cash flows for each of the two years in the period ended December 31, 2013. These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. We were not engaged to perform an audit of the Company’s internal control over financial reporting. Our audits included consideration of internal control over financial reporting as a basis for designing audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion. An audit also includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the financial statements referred to above present fairly, in all material respects, the financial position of DermTech International as of December 31, 2012 and 2013, and the results of its operations and its cash flows for each of the two years in the period ended December 31, 2013 in conformity with accounting principles generally accepted in the United States of America.
The accompanying financial statements have been prepared assuming that the Company will continue as a going concern. As discussed in Note 2 to the financial statements, the Company has incurred net losses since inception and has an accumulated deficit of $37,997,253 as of December 31, 2013. These conditions, along with other matters as set forth in Note 2, raise substantial doubt about the Company’s ability to continue as a going concern. Management’s plans in regard to these matters are also described in Note 2. The financial statements do not include any adjustments that might result from the outcome of this uncertainty.
| /s/ GRANT THORNTON LLP |
| San Diego, California |
| April 4, 2014 |
F-2
Table of Contents
BALANCE SHEETS
December 31, | March 31, 2014 | Pro Forma Stockholders’ Equity as of March 31, 2014 | ||||||||||||||
| 2012 | 2013 | |||||||||||||||
| (unaudited) | (unaudited) | |||||||||||||||
| ASSETS | ||||||||||||||||
Current assets: | ||||||||||||||||
Cash and cash equivalents | $ | 1,352,770 | $ | 1,105,335 | $ | 1,486,925 | ||||||||||
Prepaid expenses and other current assets | 49,280 | 32,294 | 219,963 | |||||||||||||
|
|
|
|
|
|
|
| |||||||||
Total current assets | 1,402,050 | 1,137,629 | 1,706,888 | |||||||||||||
Property and equipment, net | 22,360 | 20,742 | 22,457 | |||||||||||||
Deposits and other assets | 4,980 | 41,946 | 40,000 | |||||||||||||
|
|
|
|
|
|
|
| |||||||||
Total assets | $ | 1,429,390 | $ | 1,200,317 | $ | 1,769,345 | ||||||||||
|
|
|
|
|
|
|
| |||||||||
| LIABILITIES AND STOCKHOLDERS’ EQUITY (DEFICIT) | ||||||||||||||||
Current liabilities: | ||||||||||||||||
Accounts payable | $ | 508,403 | $ | 363,222 | $ | 490,708 | ||||||||||
Accrued liabilities | 143,102 | 142,570 | 434,122 | |||||||||||||
Accrued compensation | 118,947 | 88,370 | 189,765 | |||||||||||||
Convertible notes payable | 2,469,351 | — | — | |||||||||||||
Deferred revenue | — | 42,528 | 21,635 | |||||||||||||
|
|
|
|
|
|
|
| |||||||||
Total current liabilities | 3,239,803 | 636,690 | 1,136,230 | |||||||||||||
|
|
|
|
|
| |||||||||||
Deferred rent | — | — | 1,657 | |||||||||||||
|
|
|
|
|
| |||||||||||
Total liabilities | 3,239,803 | 636,690 | 1,137,887 | |||||||||||||
Commitments and contingencies | ||||||||||||||||
Stockholders’ equity (deficit): | ||||||||||||||||
Series A-1 convertible preferred stock, $0.0001 par value; 5,731,989 shares authorized; 5,731,989, 4,197,454 and 4,197,454 shares issued and outstanding at December 31, 2012 and 2013, and March 31, 2014 (unaudited), respectively; $4,298,992, $3,148,091 and $3,148,091 liquidation preference at December 31, 2012 and 2013, and March 31, 2014 (unaudited), respectively; no shares authorized and no shares issued and outstanding on a pro forma basis at March 31, 2014 (unaudited) | 573 | 420 | 420 | $ | — | |||||||||||
Series A-2 convertible preferred stock, $0.0001 par value; 4,386,479 shares authorized; 4,386,479, 3,749,961 and 3,749,961 shares issued and outstanding at December 31, 2012 and 2013, and March 31, 2014 (unaudited), respectively; $1,798,456, $1,537,484 and $1,537,484 liquidation preference at December 31, 2012 and 2013 and March 31, 2014 (unaudited), respectively; no shares authorized and no shares issued and outstanding on a pro forma basis at March 31, 2014 (unaudited) | 439 | 375 | 375 | — | ||||||||||||
Series A-3 convertible preferred stock, $0.0001 par value; 4,427,218 shares authorized; 4,427,218, 2,085,645 and 2,085,645 shares issued and outstanding at December 31, 2012 and 2013, and March 31, 2014 (unaudited), respectively; $3,541,774, $1,668,516 and $1,668,516 liquidation preference at December 31, 2012 and 2013, and March 31, 2014 (unaudited), respectively; no shares authorized and no shares issued and outstanding on a pro forma basis at March 31, 2014 (unaudited) | 443 | 209 | 209 | — | ||||||||||||
Series B convertible preferred stock, $0.0001 par value; 154,000,000 shares authorized; 0, 105,623,452 and 123,436,549 shares issued and outstanding at December 31, 2012 and 2013, and March 31, 2014 (unaudited), respectively; $0, $8,449,876 and 9,874,924, liquidation preference at December 31, 2012 and 2013, and March 31, 2014 (unaudited), respectively; no shares authorized and no shares issued and outstanding on a pro forma basis at March 31, 2014 (unaudited) | — | 10,562 | 12,344 | — | ||||||||||||
Common stock, $0.0001 par value; 210,346,407 shares authorized; 3,441,303, 7,953,929 and 7,953,929 shares issued and outstanding at December 31, 2012 and 2013, and March 31, 2014 (unaudited), respectively; 161,174,134 shares issued and outstanding on a pro forma basis at March 31, 2014 (unaudited) | 344 | 795 | 795 | 16,118 | ||||||||||||
Additional paid-in capital | 31,006,662 | 38,548,519 | 39,988,711 | 41,566,784 | ||||||||||||
Accumulated deficit | (32,818,874 | ) | (37,997,253 | ) | (39,371,396 | ) | (39,371,396 | ) | ||||||||
|
|
|
|
|
|
|
| |||||||||
Total stockholders’ equity (deficit) | (1,810,413 | ) | 563,627 | 631,458 | $ | 2,211,506 | ||||||||||
|
|
|
|
|
|
|
| |||||||||
Total liabilities and stockholders’ equity (deficit) | $ | 1,429,390 | $ | 1,200,317 | $ | 1,769,345 | ||||||||||
|
|
|
|
|
| |||||||||||
The accompanying notes are an integral part of these financial statements.
F-3
Table of Contents
STATEMENTS OF OPERATIONS
| Year ended December 31, | Three months ended March 31, | |||||||||||||||
| 2012 | 2013 | 2013 | 2014 | |||||||||||||
| (unaudited) | (unaudited) | |||||||||||||||
Revenues | $ | — | $ | — | $ | — | $ | 30,643 | ||||||||
Cost of revenues | — | — | — | 10,166 | ||||||||||||
|
|
|
|
|
|
|
| |||||||||
Gross profit | — | — | — | 20,477 | ||||||||||||
|
|
|
|
|
|
|
| |||||||||
Operating expenses: | ||||||||||||||||
Sales and marketing | 126,042 | 31,476 | 11,234 | 80,874 | ||||||||||||
Research and development | 975,843 | 1,957,641 | 367,590 | 727,811 | ||||||||||||
General and administrative | 1,064,161 | 1,272,957 | 295,601 | 584,580 | ||||||||||||
|
|
|
|
|
|
|
| |||||||||
Total operating expenses | 2,166,046 | 3,262,074 | 674,425 | 1,393,265 | ||||||||||||
|
|
|
|
|
|
|
| |||||||||
Loss from operations | (2,166,046 | ) | (3,262,074 | ) | (674,425 | ) | (1,372,788 | ) | ||||||||
|
|
|
|
|
|
|
| |||||||||
Other income (expense): | ||||||||||||||||
Interest expense, net | (61,802 | ) | (1,916,305 | ) | (50,040 | ) | (1,355 | ) | ||||||||
Other expense, net | — | — | — | — | ||||||||||||
|
|
|
|
|
|
|
| |||||||||
Total other expense | (61,802 | ) | (1,916,305 | ) | (50,040 | ) | (1,355 | ) | ||||||||
|
|
|
|
|
|
|
| |||||||||
Net loss | $ | (2,227,848 | ) | $ | (5,178,379 | ) | $ | (724,465 | ) | $ | (1,374,143 | ) | ||||
|
|
|
|
|
|
|
| |||||||||
Weighted average shares outstanding used in computing net loss per common share, basic and diluted | 3,441,303 | 4,717,976 | 3,441,303 | 7,953,929 | ||||||||||||
Net loss per common share outstanding, basic and diluted | $ | (0.65 | ) | $ | (1.10 | ) | $ | (0.21 | ) | $ | (0.17 | ) | ||||
Weighted average shares outstanding used in computing pro forma net loss per share, basic and diluted (unaudited) | 67,683,700 | 149,496,659 | ||||||||||||||
Pro forma net loss per share, basic and diluted (unaudited) | $ | (0.08 | ) | $ | (0.01 | ) | ||||||||||
The accompanying notes are an integral part of these financial statements.
F-4
Table of Contents
STATEMENTS OF STOCKHOLDERS’ EQUITY (DEFICIT)
| Convertible Preferred Stock | Additional Paid-in Capital | Accumulated Deficit | Note Receivable | Total Stockholders’ Equity (Deficit) | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Series A-1 | Series A-2 | Series A-3 | Series B | Common Stock | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Shares | Amount | Shares | Amount | Shares | Amount | Shares | Amount | Shares | Amount | |||||||||||||||||||||||||||||||||||||||||||||||
Balance, December 31, 2011 | 5,731,989 | 573 | 4,386,479 | 439 | 3,557,125 | 356 | — | — | 3,441,303 | 344 | 30,288,538 | (30,591,026 | ) | — | (300,776 | ) | ||||||||||||||||||||||||||||||||||||||||
Issuance of Series A-3 preferred stock (at $0.80 per share) | — | — | — | — | 870,093 | 87 | — | — | — | — | 695,987 | — | — | 696,074 | ||||||||||||||||||||||||||||||||||||||||||
Stock based compensation | — | — | — | — | — | — | — | — | — | — | 22,137 | — | — | 22,137 | ||||||||||||||||||||||||||||||||||||||||||
Net loss | — | — | — | — | — | — | — | — | — | — | — | (2,227,848 | ) | — | (2,227,848 | ) | ||||||||||||||||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||||||||||||||||||
Balance, December 31, 2012 | 5,731,989 | 573 | 4,386,479 | 439 | 4,427,218 | 443 | — | — | 3,441,303 | 344 | 31,006,662 | (32,818,874 | ) | — | (1,810,413 | ) | ||||||||||||||||||||||||||||||||||||||||
Issuance of Series B preferred stock for conversion of 2012 notes payable and accrued interest (at $0.04 per share) | — | — | — | — | — | — | 66,122,253 | 6,612 | — | — | 2,638,278 | — | — | 2,644,890 | ||||||||||||||||||||||||||||||||||||||||||
Issuance of Series B preferred stock for conversion of 2013 notes payable and accrued interest at (at $0.08 per share) | — | — | — | — | — | — | 5,938,699 | 594 | — | — | 474,502 | — | — | 475,096 | ||||||||||||||||||||||||||||||||||||||||||
Issuance of Series B preferred stock, net of issuance costs of $66,516 (at $0.08 per share) | — | — | — | — | — | — | 30,750,000 | 3,075 | — | — | 2,390,408 | — | — | 2,393,483 | ||||||||||||||||||||||||||||||||||||||||||
Issuance of Series B preferred stock for conversion of accounts payable and accrued expenses at (at $0.08 per share) | — | — | — | — | — | — | 2,812,500 | 281 | — | — | 224,719 | — | — | 225,000 | ||||||||||||||||||||||||||||||||||||||||||
Issuance of warrants in connection with Series B financing | — | — | — | — | — | — | — | — | — | — | 254,782 | — | — | 254,782 | ||||||||||||||||||||||||||||||||||||||||||
Deemed dividend related to issuance of warrants in connection with Series B financing | — | — | — | — | — | — | — | — | — | — | (254,782 | ) | — | — | (254,782 | ) | ||||||||||||||||||||||||||||||||||||||||
Conversion of Series A-1 preferred stock to common stock | (1,425,000 | ) | (143 | ) | — | — | — | — | — | — | 1,425,000 | 143 | — | — | — | — | ||||||||||||||||||||||||||||||||||||||||
Conversion of Series A-1, A-2 and A-3 preferred stock to common stock | (109,535 | ) | (10 | ) | (636,518 | ) | (64 | ) | (2,341,573 | ) | (234 | ) | — | — | 3,087,626 | 308 | — | — | — | — | ||||||||||||||||||||||||||||||||||||
Beneficial conversion feature on 2012 notes payable | — | — | — | — | — | — | — | — | — | — | 1,791,913 | — | — | 1,791,913 | ||||||||||||||||||||||||||||||||||||||||||
Stock based compensation | — | — | — | — | — | — | — | — | — | — | 22,037 | — | — | 22,037 | ||||||||||||||||||||||||||||||||||||||||||
Net loss | — | — | — | — | — | — | — | — | — | — | — | (5,178,379 | ) | — | (5,178,379 | ) | ||||||||||||||||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||||||||||||||||||
Balance, December 31, 2013 | 4,197,454 | $ | 420 | 3,749,961 | $ | 375 | 2,085,645 | $ | 209 | 105,623,452 | $ | 10,562 | 7,953,929 | $ | 795 | $ | 38,548,519 | $ | (37,997,253 | ) | $ | — | $ | 563,627 | ||||||||||||||||||||||||||||||||
Issuance of Series B preferred stock (at $0.08 per share) upon exercise of warrants (unaudited) | — | — | — | — | — | — | 17,813,097 | 1,782 | — | — | 1,423,267 | — | — | 1,425,049 | ||||||||||||||||||||||||||||||||||||||||||
Issuance of New warrants in connection with exercise of Series B warrants (unaudited) | — | — | — | — | — | — | — | — | — | — | 221,590 | — | — | 221,590 | ||||||||||||||||||||||||||||||||||||||||||
Deemed dividend related to issuance of New warrants (unaudited) | — | — | — | — | — | — | — | — | — | — | (221,590 | ) | — | — | (221,590 | ) | ||||||||||||||||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||||||||||||||||||
Stock based compensation (unaudited) | — | — | — | — | — | — | — | — | — | — | 16,925 | — | — | 16,925 | ||||||||||||||||||||||||||||||||||||||||||
Net loss (unaudited) | — | — | — | — | — | — | — | — | — | — | — | (1,374,143 | ) | — | (1,374,143 | ) | ||||||||||||||||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||||||||||||||||||
Balance, March 31, 2014 (unaudited) | 4,197,454 | $ | 420 | 3,749,961 | $ | 375 | 2,085,645 | $ | 209 | 123,436,549 | $ | 12,344 | 7,953,929 | $ | 795 | $ | 39,988,711 | $ | (39,371,396 | ) | $ | — | $ | 631,458 | ||||||||||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||||||||||||||||||||||||||
The accompanying notes are an integral part of these financial statements.
F-5
Table of Contents
STATEMENTS OF CASH FLOWS
| Year ended December 31, | Three months ended March 31, | |||||||||||||||
| 2012 | 2013 | 2013 | 2014 | |||||||||||||
| (unaudited) | (unaudited) | |||||||||||||||
Cash flows from operating activities: | ||||||||||||||||
Net loss | $ | (2,227,848 | ) | $ | (5,178,379 | ) | $ | (724,465 | ) | $ | (1,374,143 | ) | ||||
Adjustments to reconcile net loss to net cash used in operating activities: | ||||||||||||||||
Depreciation and amortization | 54,062 | 18,755 | 5,034 | 2,305 | ||||||||||||
Stock based compensation | 22,137 | 22,037 | 214 | 16,925 | ||||||||||||
Accrued interest expense | 56,469 | 124,483 | 50,040 | 1,355 | ||||||||||||
Beneficial conversion feature on the convertible notes | — | 1,791,913 | — | — | ||||||||||||
Changes in operating assets and liabilities: | ||||||||||||||||
Prepaid expenses and other current assets | (26,080 | ) | 16,986 | 3,656 | (186,012 | ) | ||||||||||
Deposits and other assets | 6,298 | (36,966 | ) | (44,719 | ) | 1,946 | ||||||||||
Accounts payable and accrued expenses | (44,563 | ) | 99,862 | 70,799 | 519,078 | |||||||||||
Deferred revenue | — | 42,528 | — | (20,893 | ) | |||||||||||
|
|
|
|
|
|
|
| |||||||||
Net cash used in operating activities | (2,159,525 | ) | (3,098,781 | ) | (639,441 | ) | (1,039,439 | ) | ||||||||
|
|
|
|
|
|
|
| |||||||||
Cash flows from investing activities: | ||||||||||||||||
Purchase of property and equipment | (7,147 | ) | (17,137 | ) | — | (4,020 | ) | |||||||||
|
|
|
|
|
|
|
| |||||||||
Net cash used in investing activities | (7,147 | ) | (17,137 | ) | — | (4,020 | ) | |||||||||
|
|
|
|
|
|
|
| |||||||||
Cash flows from financing activities: | ||||||||||||||||
Proceeds from convertible notes payable | 2,469,351 | 475,000 | — | — | ||||||||||||
Proceeds from issuance of preferred stock, net | 696,074 | 2,393,483 | — | — | ||||||||||||
Proceeds from sale or exercise of warrants, net | — | — | — | 1,425,049 | ||||||||||||
|
|
|
|
|
|
|
| |||||||||
Net cash provided by financing activities | 3,165,425 | 2,868,483 | — | 1,425,049 | ||||||||||||
|
|
|
|
|
|
|
| |||||||||
Net increase (decrease) in cash | 998,753 | (247,435 | ) | (639,441 | ) | 381,590 | ||||||||||
Cash and cash equivalents, beginning of period | 354,017 | 1,352,770 | 1,352,770 | 1,105,335 | ||||||||||||
|
|
|
|
|
|
|
| |||||||||
Cash and cash equivalents, end of period | $ | 1,352,770 | $ | 1,105,335 | $ | 713,329 | $ | 1,486,925 | ||||||||
|
|
|
|
|
|
|
| |||||||||
The accompanying notes are an integral part of these financial statements.
F-6
Table of Contents
DERMTECH INTERNATIONAL
STATEMENTS OF CASH FLOWS
| Year ended December 31, | Three months ended March 31, | |||||||||||||||
| 2012 | 2013 | 2013 | 2014 | |||||||||||||
| (unaudited) | (unaudited) | |||||||||||||||
Interest paid | $ | — | $ | — | $ | — | $ | — | ||||||||
Income taxes paid | 880 | 888 | — | — | ||||||||||||
Supplemental schedule of non-cash investing and financing activities: | ||||||||||||||||
Conversion of 2012 notes payable and accrued interest into Series B preferred stock | — | 2,644,890 | — | — | ||||||||||||
Conversion of 2013 notes payable and accrued interest into Series B preferred stock | — | 475,096 | — | — | ||||||||||||
Conversion of accounts payable and accrued expenses into Series B preferred stock | — | 225,000 | — | — | ||||||||||||
Warrants issued in connection with Series B financing | — | 254,782 | — | 221,590 | ||||||||||||
The accompanying notes are an integral part of these financial statements.
F-7
Table of Contents
NOTES TO FINANCIAL STATEMENTS
Years Ended December 31, 2012 and 2013 and Three Months Ended March 2014 (unaudited)
NOTE 1—THE COMPANY AND A SUMMARY OF ITS SIGNIFICANT ACCOUNTING POLICIES
Nature of Operations
DermTech International (the “Company”) was incorporated in California on December 28, 1995. The Company is an emerging growth molecular diagnostic company developing and marketing its CLIA laboratory services including gene expression tests to facilitate the diagnosis of dermatologic conditions. The Company has developed a proprietary, non-invasive technique for sampling the surface layers of the skin using an adhesive patch in order to collect individual biological information for commercial applications in the medical diagnostic field. The Company was in the development stage from June 1, 2004 to December 31, 2013, as defined by the Accounting Standards Codification (ASC) 915,Development Stage Entities. In the three months ended March 31, 2014, the Company exited the development stage upon recording revenues from its planned principal operations. During the three months ended March 31, 2014, the Company’s Clinical Laboratory Improvement Amendments (CLIA) laboratory was approved and the Company sold sample collection kits to third parties and performed other CLIA services for research partners relating to assay development.
Use of Estimates
The preparation of financial statements in conformity with accounting principles generally accepted in the United States of America requires that management make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the amounts of revenues and expenses reported during the period. On an ongoing basis, management evaluates these estimates and judgments, including those related to convertible notes payable, warrants, stock based compensation, expense accruals, the realization of deferred tax assets, and common and preferred stock valuation. Actual results may differ from those estimates.
Unaudited Interim Financial Information
The accompanying interim balance sheet as of March 31, 2014, the interim statements of operations and cash flows for the three months ended March 31, 2013 and 2014, and the interim statements of stockholders’ equity (deficit) for the three months ended March 31, 2014 are unaudited. The unaudited interim financial statements have been prepared on a basis consistent with the annual audited financial statements and, in the opinion of management, reflect all adjustments (consisting of normal recurring adjustments) considered necessary to fairly state the Company’s financial position as of March 31, 2014 and results of operations and cash flows for the three months ended March 31, 2013 and 2014 and the stockholders’ equity (deficit) for the three months ended March 31, 2014. The financial data and other information disclosed in the notes to the financial statements related to March 31, 2014 and the three months ended March 31, 2013 and 2014 are unaudited. The results of operations during the three months ended March 31, 2014 are not indicative of the results to be expected for the entire year ending December 31, 2014 or any other future annual or interim period.
Unaudited Pro Forma Information
The unaudited pro forma balance sheet information as of March 31, 2014 and the unaudited pro forma statement of operations information for the year ended December 31, 2013 and the three months ended March 31, 2014 gives effect to (i) the automatic conversion of all outstanding shares of the Company’s Series A-1 preferred stock, Series A-2 preferred stock, Series A-3 preferred stock, Series B preferred stock into 133,469,609 shares of common stock, and (ii) the conversion of Series B warrants into 19,750,956 shares of the Company’s common stock. Shares of common stock issued in an initial public offering (IPO) and any related net proceeds are excluded from the pro forma information.
F-8
Table of Contents
Cash and Cash Equivalents
The Company considers all highly liquid investments with remaining maturities of three months or less when purchased to be cash equivalents. The Company maintains its cash balances at banks and financial institutions. The balances are insured up to the legal limit. The Company maintains cash balances that may, at times, exceed insured limits.
Property and Equipment
Property and equipment is recorded at cost less accumulated depreciation and amortization. Depreciation is computed using the straight-line method over the estimated useful lives of the assets that range from two to five years. Leasehold improvements are amortized over the shorter of the life of the lease or the asset. The Company recorded depreciation expense of $54,062 and $18,755 for the years ended December 31, 2012 and 2013, respectively. The Company recorded depreciation expense of $5,034 and $2,305 for the three months ended March 31, 2013 and 2014 (unaudited), respectively. In 2013, the Company wrote-off the gross amounts and related accumulated depreciation at the time of the office relocation for assets that were abandoned and no longer in service. The gross amount of the assets was approximately $458,000 with a net book value of zero.
Research and Development
Research and development costs are expensed as incurred.
Segment Reporting
The Company operates in one segment.
Income Taxes
The Company provides for federal and state income taxes on the asset and liability approach which requires deferred tax assets and liabilities to be recognized based on temporary differences between the financial statement and tax bases of assets and liabilities using enacted tax rates in effect for the year in which the temporary differences are expected to reverse.
Deferred tax assets are reduced by a valuation allowance when, in management’s opinion, it is more likely than not that some portion or all of the deferred tax assets will not be realized. The Company considers the scheduled reversal of deferred tax liabilities, projected future taxable income, and tax planning strategies in making this assessment. The Company’s valuation allowance is based on available evidence, including its current year operating loss, evaluation of positive and negative evidence with respect to certain specific deferred tax assets including evaluation sources of future taxable income to support the realization of the deferred tax assets. The Company has established a full valuation allowance on the deferred tax assets as of December 31, 2012 and 2013.
Current and deferred tax assets and liabilities are recognized based on the tax positions taken or expected to be taken in the Company’s income tax returns. U.S. GAAP requires that the tax benefits of an uncertain tax position can only be recognized when it is more likely than not that the tax position will be sustained upon examination by the relevant taxing authority. Tax benefits related to tax positions that do not meet this criterion are not recognized in the financial statements.
The Company recognizes interest and penalties related to income tax matters in income tax expense.
F-9
Table of Contents
Deferred Offering Costs Policy
The Company capitalizes all incremental costs directly related to the IPO as deferred offering costs. At the time of the IPO, the Company will record the deferred offering costs to issuance costs and charge it against the gross proceeds from the IPO. As of December 31, 2012 and 2013, and March 31, 2014 (unaudited), $0 and $0, and $192,000, respectively, of deferred offering costs are recorded in prepaid expenses and other current assets in the accompanying balance sheets.
Revenue Recognition
Revenues are generated primarily from the sale of CLIA laboratory services and adhesive sample collection kits. CLIA laboratory revenues result from providing gene expression tests to facilitate the diagnosis of dermatologic conditions. The provision of gene expression tests may include sample collection using the Company’s patented adhesive patch devices, assay development for research partners, RNA isolation, expression and amplification and data analysis and reporting. The Company also sells its non-invasive adhesive patch biopsy devices to third parties who may or may not use the Company’s CLIA laboratory for gene expression.
Revenues are recognized when persuasive evidence of an arrangement exists, delivery has occurred or services have been rendered, the price is fixed or determinable, and collectability is reasonably assured. The Company assesses whether the fee is fixed or determinable based on the nature of the fee charged for the products or services delivered and whether there are existing contractual arrangements. Revenues are deferred for fees received before the earnings process is complete.
The Company generally sells its non-invasive patch biopsy devices to research partners who are under contract. Revenue related to the sale of the patch biopsy devices is recorded when those devices are shipped on a common carrier and title has passed. The Company’s gene expression processing revenues are recognized when the gene expression processing is complete and a report or other data analysis has been submitted to the customer. Revenue from assay development for research partners is recognized as services are delivered.
Statement of Operations
The Company has no comprehensive income or loss items other than the Net Loss for the periods presented.
Stock Based Compensation
Compensation costs associated with stock option awards and other forms of equity compensation are measured at the grant-date fair value of the awards and recognized over the requisite service period of the awards on a straight-line basis. No related tax benefits of the share-based compensation costs have been recognized since the date the Company re-entered the development stage.
The Company grants stock options to purchase common stock to employees with exercise prices equal to the market value of the underlying stock, as determined by the board of directors and outside valuation experts on the date the equity award was granted. The board of directors determined the value of the underlying stock by considering a number of factors, including historical and projected financial results, the risks the Company faced at the time, the preferences of the Company’s preferred stockholders, and the lack of liquidity of the Company’s common stock.
The fair value of each option award is estimated on the date of grant using the Black-Scholes-Merton valuation model. Such value is recognized as expense over the requisite service period, net of estimated forfeitures, using the straight line method. The expected term of options is based on the simplified method set out in Staff Accounting Bulletin (SAB) No. 110,Share-Based Payment (SAB No. 110). The simplified method defines the life as the average of the contractual term of the options and the weighted-average vesting period for all option tranches. The expected volatility of stock options is based upon the historical volatility of a number of
F-10
Table of Contents
publicly traded companies in similar stages of development. The risk-free interest rate is based on the average yield of U.S. Treasury securities with remaining terms similar to the expected term of the share based awards. The assumed dividend yield was based on the Company’s expectation of not paying dividends in the foreseeable future.
The Company accounts for stock options to non-employees using the fair value approach. The fair value of these options is measured using the Black-Scholes option pricing model, reflecting the same assumptions applied to employee options, other than expected life, which is assumed to be the remaining contractual life of the award.
Equity instruments awarded to nonemployees are periodically re-measured as the underlying awards vest unless the instruments are fully vested, immediately exercisable, and nonforfeitable on the date of grant.
The fair value of each option was estimated on the date of grant using the following assumptions:
| Year Ended December 31, 2012 | Year Ended December 31, 2013 | Three months ended March 31, 2014 (unaudited) | ||||
Assumed risk-free interest rate | 0.77% to 2.05% | 1.34% to 2.67% | 1.02% to 2.75% | |||
Assumed volatility | 76% to 88% | 60% to 63% | 51.58% to 62.78% | |||
Expected option term | 5 years to 10 years | 5 years to 10 years | 3 years to 10 years | |||
Expected dividend yield | 0% | 0% | 0% |
The Company recorded stock based compensation expense of $22,137 and $22,037 for the years ended December 31, 2012 and 2013, respectively. The Company recorded stock based compensation expense of $214 and $16,925 for the three months ended March 31, 2013 and 2014 (unaudited), respectively. The stock based compensation expense for consultants was $9,257 and $3,120 for the years ended December 31, 2012 and 2013 and $0 and $1,745 for the three months ended March 31, 2013 and 2014 (unaudited), respectively. The total compensation cost related to nonvested awards not yet recognized at December 31, 2013 and the three months ended March 31, 2013 and 2014 (unaudited) is $48,099, $3,395, and $134,905, respectively, which is expected to be recognized on a straight-line basis over a weighted-average term of 1.10 years, 1.61 years and 1.76 years, respectively.
Recent Accounting Pronouncements
In July 2013, the Financial Accounting Standards Board (FASB) issued Accounting Standards Update No. 2013-11, Presentation of an Unrecognized Tax Benefit When a Net Operating Loss Carryforward, a Similar Tax Loss, or a Tax Credit Carryforward Exists, which requires an unrecognized tax benefit, or a portion of an unrecognized tax benefit, to be presented in the financial statements as a reduction to a deferred tax asset for a net operating loss, or NOL, carryforward, a similar tax loss, or a tax credit carryforward. If an NOL carryforward, a similar tax loss, or a tax credit carryforward is not available at the reporting date under the tax law of the applicable jurisdiction to settle any additional income taxes that would result from the disallowance of a tax position or the tax law of the applicable jurisdiction does not require the entity to use, and the entity does not intend to use, the deferred tax asset for such purpose, the unrecognized tax benefit should be presented in the financial statements as a liability and should not be combined with deferred tax assets. The new guidance is effective for fiscal years beginning after December 15, 2013 and earlier adoption is permitted. The Company adopted this update in the year ended December 31, 2013 and such adoption did not have a material effect on its financial position or results of operations.
Fair Value Measurements
The Company uses a three-tier fair value hierarchy to prioritize the inputs used in the Company’s fair value measurements. These tiers include: Level 1, defined as observable inputs such as quoted prices in active markets
F-11
Table of Contents
for identical assets; Level 2, defined as inputs other than quoted prices in active markets that are either directly or indirectly observable; and Level 3, defined as unobservable inputs in which little or no market data exists, therefore requiring an entity to develop its own assumptions. The Company believes the carrying amount of cash and cash equivalents, accounts payable and accrued expenses approximate their estimated fair values due to the short-term nature of these accounts.
Net Loss Per Share
Basic and diluted net loss per common share is determined by dividing net loss applicable to common shareholders by the weighted average common shares outstanding during the period. Because there is a net loss attributable to common shareholders for the years ended December 31, 2012 and 2013 and the three months ended March 31, 2013 and 2014, the outstanding shares of Preferred stock, warrants, and Common stock options have been excluded from the calculation of diluted loss per common share because their effect would be anti-dilutive. Therefore, the weighted average shares used to calculate both basic and diluted loss per share are the same. Net loss per common share, diluted, excludes the effect of anti-dilutive equity instruments including 14,335,174, 55,156,069, 14,545,686 and 121,792,134 shares of Common stock issuable upon conversion of our Preferred stock, and 618,060, 32,532,214, 531,543 and 49,342,807 shares of Common stock issuable upon the exercise of outstanding warrants and stock options, for the years ended December 31, 2012 and 2013, and the three months ended March 31, 2013 and 2014 (unaudited), respectively.
The weighted average shares outstanding used in computing pro forma net loss per share assumes the conversion of all outstanding preferred stock and preferred stock warrants into an aggregate of 64,242,397 and 141,542,730 shares of common stock for the year ended December 31, 2013 and the three months ended March 31, 2014 (unaudited), respectively.
The pro forma net loss per share, basic and diluted, is calculated using the net loss, adjusted to eliminate the interest on convertible debt during the period, divided by the pro forma weighted average shares outstanding.
NOTE 2—BASIS OF PRESENTATION AND GOING CONCERN
These financial statements have been prepared in conformity with accounting principles generally accepted in the United States of America, which contemplate continuation of the Company as a going concern. The Company has incurred net losses since the Company’s formation and has an accumulated deficit as of December 31, 2013 and March 31, 2014 (unaudited), of $37,997,253 and 39,371,396, respectively. These conditions raise substantial doubt about the Company’s ability to continue as a going concern.
Management intends to pursue additional equity and debt financing, which it believes will be sufficient to provide the Company with the ability to continue in existence, to support its planned operations and to continue developing and commercializing gene expression tests. In February 2014, warrants to purchase 17,813,097 shares of Series B Preferred stock were exercised for $1,425,049 of cash. As a result, New Warrants to purchase 17,813,097 shares of Series B Preferred stock were issued to such holders which are exercisable by May 31, 2014. There can be no assurances as to the availability of additional financing or the terms upon which additional financing may be available to the Company. If the Company is unable to obtain sufficient funding at acceptable terms, it may be forced to significantly curtail its operations, and the lack of sufficient funding may have a material adverse impact on the Company’s ability to continue as a going concern. The financial statements do not include any adjustments relating to the recoverability and classification of recorded asset amounts or the amounts and classification of liabilities that might be necessary should the Company be unable to continue as a going concern.
F-12
Table of Contents
NOTE 3—BALANCE SHEET DETAILS
Balance sheet details are as follows:
| December 31, 2012 | December 31, 2013 | March 31, 2014 (unaudited) | ||||||||||
Property and equipment, net: | ||||||||||||
Laboratory equipment | $ | 313,669 | $ | 25,212 | $ | 25,212 | ||||||
Computer equipment and software | 120,247 | 37,286 | 37,286 | |||||||||
Furniture and fixtures | 89,758 | 16,637 | 16,637 | |||||||||
Leasehold improvements | 12,771 | 16,250 | 20,271 | |||||||||
|
|
|
|
|
| |||||||
| 536,445 | 95,385 | 99,406 | ||||||||||
Less accumulated depreciation | (514,085 | ) | (74,643 | ) | (76,949 | ) | ||||||
|
|
|
|
|
| |||||||
| $ | 22,360 | $ | 20,742 | $ | 22,457 | |||||||
|
|
|
|
|
| |||||||
Accrued liabilities: | ||||||||||||
Accrued interest expense on convertible notes payable | $ | 56,469 | $ | — | $ | — | ||||||
Accrued legal services | — | 40,145 | 227,730 | |||||||||
Accrued consulting services | 67,362 | 72,680 | 144,635 | |||||||||
Other accrued expenses | 19,271 | 29,745 | 61,757 | |||||||||
|
|
|
|
|
| |||||||
| $ | 143,102 | $ | 142,570 | $ | 434,122 | |||||||
|
|
|
|
|
| |||||||
Accrued compensation: | ||||||||||||
Accrued paid time off | $ | 37,319 | $ | 44,902 | $ | 45,461 | ||||||
Accrued payroll and related costs | 81,628 | 43,468 | 144,304 | |||||||||
|
|
|
|
|
| |||||||
| $ | 118,947 | $ | 88,370 | $ | 189,765 | |||||||
|
|
|
|
|
| |||||||
NOTE 4—DEBT
2012 Convertible Notes
In August 2012, the Company entered into a Convertible Note Purchase Agreement where it sold Convertible Promissory Notes (the Notes), which are convertible into shares of Preferred stock issued in the Next Equity Financing. The Notes were issued in August 2012 and October 2012 in the amount of $1,500,000 and $969,351, respectively.
The Notes become due and payable upon written demand by the holder at any time after the first anniversary of the issuance date and bear interest at 8%. In the event of a Qualified Financing, as defined, the Notes automatically convert into the same securities issued in the Qualified Financing at the lowest price paid per share reduced by 50%. The interest expense for the convertible notes was $56,469 and $119,070 in 2012 and 2013, and $48,710 and $0 for the three months ended March 31, 2013 and 2014 (unaudited), respectively.
The 50% reduction of the per share price creates a beneficial conversion feature (BCF) which is contingent on the ultimate issuance of the next equity financing. The BCF was measured when the Series B financing occurred in August 2013 and $1,791,913 was recorded as non-cash interest expense and additional paid-in capital.
2013 Convertible Notes
In June and July 2013, the Company issued promissory notes for $475,000 that are convertible into Series B Preferred stock. The promissory notes bear interest at 0.18%.
F-13
Table of Contents
NOTE 5—STOCKHOLDERS’ EQUITY
Series A-3
In 2011 and 2012, the Company sold 1,493,750 and 870,093 shares of Series A-3 Preferred stock for $1,195,001 and $696,074, respectively, at $0.80 per share.
Series B Financing
In August 2013, the Company issued 105,248,452 shares of Series B Preferred stock for proceeds of $5,774,985 which included the conversion of principal and interest of $2,944,351 and $175,635, respectively, for the Notes issued in 2012 and 2013. The Series B shareholders who invested new cash in 2013 received warrants (the Warrants) to purchase up to 19,563,096 shares of Series B Preferred stock which was equal to 50% of the number of Series B Preferred stock shares purchased in the Qualified Financing. The warrants were valued at $254,782 based on Black-Scholes Option Pricing model and recorded as a deemed dividend to additional paid-in capital. The expiration date of the warrants is one year from the date of issue and the exercise price is $0.08.
The fair value of the warrants is estimated on the date of issuance using the Black-Scholes-Merton valuation model within Option Pricing Method of equity allocation. The expected term of warrants is based on the contractual term. The expected volatility of the warrants is based upon the historical volatility of a number of publicly traded companies in similar stages of development. The risk-free interest rate is based on the average yield of U.S. Treasury securities with remaining terms similar to the expected term of the warrants. The assumed dividend yield was based on the Company’s expectation of not paying dividends in the foreseeable future. The fair value of the warrants was estimated on the issuance date using the following assumptions of volatility 87% and risk-free rate of 0.29%.
The Series B Preferred terms included a pay-to-play provision. If any of the Series A preferred shareholders failed to purchase the greater of $25,000 or their pro rata share, all their shares of Series A Preferred stock would automatically convert to Common stock. As such, 3,087,626 shares converted from Series A Preferred stock to Common stock in October 2013. In addition, there was a conversion of 1,425,000 shares of Series A Preferred stock to Common stock in August 2013 to meet the voting requirements by class of stock.
In October 2013, the Company issued an additional 375,000 shares of Series B Preferred stock for proceeds of $30,000 and warrants to purchase up to 187,500 shares of Series B Preferred Stock.
In November 2013, there was an amendment to the Warrants. If the holders exercise their Warrants on or before March 1, 2014, they will each receive an additional warrant (the New Warrants) to purchase up to the number of shares of Series B Preferred stock equal to 50% of the number of Series B Preferred stock shares purchased in the Qualified Financing. The expiration date for the New Warrants is May 31, 2014.
The modification of the warrants was calculated as the difference between the valuation of the warrants immediately prior to the amendment and the valuation of the warrants immediately after the amendment. There was no incremental value to record for the modification of the warrants.
As of December 31, 2013, the total number of outstanding warrants to purchase Series B Preferred stock was 19,750,596.
F-14
Table of Contents
The following table summarizes warrant transactions for the years ended December 31, 2012 and 2013, and the three months ended March 31, 2014 (unaudited):
Outstanding at December 31, 2012 | — | |||
Issued | 19,750,596 | |||
Exercised | — | |||
Canceled | — | |||
|
| |||
Outstanding at December 31, 2013 | 19,750,596 | |||
Issued (unaudited) | 17,813,097 | |||
Exercised (unaudited) | (17,813,097 | ) | ||
Canceled (unaudited) | — | |||
|
| |||
Outstanding at March 31, 2014 (unaudited) | 19,750,596 | |||
|
|
Classes of Stock
The Company is authorized to issue 380,761,468 shares with a per share par value of $0.0001. 210,346,407 of these shares are Common stock and 170,415,061 are Convertible Preferred stock. The Convertible Preferred stock consists of 5,731,989 shares of Series A-1 Preferred, 4,386,479 shares of Series A-2 Preferred, 4,427,218 shares of Series A-3 Preferred and 154,000,000 shares of Series B Preferred. The issuance price per share is $0.75 for Series A-1 Preferred, $0.41 for Series A-2 Preferred, $0.80 for Series A-3 Preferred and $0.08 for Series B Preferred.
Preferred Dividends
The Series B preferred shareholders are entitled to non-cumulative dividends at a rate of 6% per share of the Initial Purchase Price when and if declared. After the payment of any dividends to the Series B shareholders, the Series A share holders shall be entitled to non-cumulative dividends at a rate of 6% per share of the Initial Purchase Price when and if declared. Any additional dividends shall be distributed to the common shareholders.
Preferred Liquidation Preference
Preferred stock is entitled to a per share liquidation preference equal to the Initial Purchase Price plus declared but unpaid dividends. In the event the amount to be distributed is insufficient to satisfy all liquidation preferences, the Series B Preferred shareholders shall be entitled to receive prior and in preference to any distribution to the holders of Series A Preferred stock or common stock.
Conversion
Preferred stock is convertible into common stock at a rate calculated by dividing the Initial Purchase Price by the Conversion Price. The initial Conversion Price is equal to the Initial Purchase Price. The preferred stock is automatically converted immediately prior to a public offering where the public offering price is not less than $0.24 per share and has aggregate cash proceeds of at least $50,000,000 or a date specified by vote of the holders of the majority of the outstanding shares of Preferred Stock.
The Conversion Price will be adjusted if common stock is issued under certain circumstances at a per share consideration less than the Conversion Price. The new Conversion Price shall be determined by multiplying the Conversion Price then in effect by a fraction, the numerator of which shall be the number of shares of common stock outstanding immediately prior to such issuance plus the number of shares of common stock that the aggregate consideration received by the Company for such issuance would purchase at such Conversion Price; and the denominator of which shall be the number of shares of outstanding common stock plus the number of shares of such additional stock.
F-15
Table of Contents
Voting Rights
Each holder of common stock is entitled to one vote per share held. Each holder of preferred stock is entitled to the number of votes equal to the number of common shares into which their holdings could be converted. Pursuant to the terms of a voting agreement, Preferred and Common stockholders shall be entitled to select two members of the board of directors and Common stockholders are entitled to each elect one member to the board of directors in addition to directors elected by all voting shareholders.
Stock Based Compensation
The Company adopted the DermTech International 2010 Stock Option Plan (the 2010 Plan) in 2010, which provides for the granting of incentive and non-statutory stock options. Under the 2010 Plan, incentive andnon-statutory stock options may be granted at not less than 100% of the fair market value of the Company’s common stock on the date of grant. For incentive stock options granted to a ten percent shareholder under the Plan, the exercise price shall not be less than 110% of the fair market value of a share of stock on the effective date of grant. The Company has reserved 31,816,886 shares of common stock for issuance to employees, nonemployee directors and consultants of the Company. The contractual term of options granted under the Plan is ten years. Vesting provisions vary based on the specific terms of the individual option awards. In addition, one of the option grants had vesting provisions based on performance milestones.
In August 2013, the stockholders approved an increase to the number of shares of the Company’s Common stock reserved for issuance to 31,816,886 shares. As of December 31, 2012 and 2013, and March 31, 2014 (unaudited), 1,272,832, 17,537,150 and 726,557 options remain available for future grant under the Plan, respectively.
The following table summarizes stock option transactions for the years ended December 31, 2012 and 2013 and the three months ended March 31, 2004 (unaudited).
| Total Options | Weighted Average Exercise Price Per Share | |||||||
Outstanding at December 31, 2011 | 352,247 | $ | 0.14 | |||||
Granted | 345,813 | $ | 0.14 | |||||
Exercised | — | |||||||
Canceled | (80,000 | ) | $ | 0.14 | ||||
|
| |||||||
Outstanding at December 31, 2012 | 618,060 | $ | 0.14 | |||||
Granted | 12,334,948 | $ | 0.01 | |||||
Exercised | — | |||||||
Canceled | (171,390 | ) | $ | 0.14 | ||||
|
| |||||||
Outstanding at December 31, 2013 | 12,781,618 | $ | 0.01 | |||||
Granted (unaudited) | 17,603,498 | $ | 0.01 | |||||
Exercised (unaudited) | — | |||||||
Canceled (unaudited) | (792,905 | ) | $ | 0.08 | ||||
|
| |||||||
Outstanding at March 31, 2014 (unaudited) | 29,592,211 | 0.01 | ||||||
|
| |||||||
In 2012, 2013, and the three months ended March 31, 2013 and 2014 (unaudited), the Company granted options to consultants for 85,860, 822,305, 0 and 550,000 shares of Common stock, respectively.
The weighted-average grant date fair value per share for the years ended December 31, 2012 and 2013 and the three months ended March 31, 2014 (unaudited) was $0.10 and $0.11 for employees and non-employees and $0.01 for employees and non-employees, and $0.01 for employees and non-employees, respectively. There were no grants for the three months ended March 31, 2013 (unaudited). At December 31, 2013 and March 31, 2014
F-16
Table of Contents
(unaudited), 3,811,695 and 4,233,896 options were exercisable at a weighted-average exercise price of $0.02 and $0.01, respectively. The weighted-average remaining contractual life of exercisable options at December 31, 2013 and March 31, 2014 (unaudited) was 9.58 years and 9.36 years, respectively. The total fair value of the shares vested was $19,038, $23,354, $3,617 and $4,236 for the years ended December 31, 2012 and 2013 and the three months ended March 31, 2013 and 2014 (unaudited), respectively. The weighted-average fair value of options outstanding was $0.01 per share, $0.09 per share and $0.01 per share and the intrinsic value of options outstanding was $0 at December 31, 2013 and March 31, 2013 and 2014 (unaudited), respectively. Theweighted-average remaining contractual life of options outstanding at December 31, 2013 and March 31, 2014 (unaudited) was 9.77 years and 9.75 years, respectively. The number of fully vested options and options expected to vest at December 31, 2013 and March 31, 2014 (unaudited) was 12,263,021 and 27,428,854, respectively.
In January 2014, the Company repriced 441,470 options from an exercise price of $0.14 to $0.01 (unaudited). The modification of these options resulted in no incremental value to record. In February 2014, the Company issued 17,162,028 stock options and 2,421,102 warrants to the Company’s board of directors, employees and consultants (unaudited).
The following table summarizes warrant transactions for common stock for the years ended December 31, 2012 and 2013 and the three months ended March 31, 2014 (unaudited):
Outstanding at December 31, 2012 | — | |||
Issued | — | |||
Exercised | — | |||
Canceled | — | |||
|
| |||
Outstanding at December 31, 2013 | — | |||
Issued (unaudited) | 2,421,102 | |||
Exercised (unaudited) | — | |||
Canceled (unaudited) | — | |||
|
| |||
Outstanding at March 31, 2014 (unaudited) | 2,421,102 | |||
|
|
Common Stock Reserved for Future Issuance
Common stock reserved for future issuance consists of the following at December 31, 2013 and March 31, 2014:
| December 31, 2013 | March 31, 2014 (unaudited) | |||||||
Conversion of preferred stock | 115,656,512 | 133,469,609 | ||||||
Warrants for purchase of preferred stock | 19,750,596 | 19,750,596 | ||||||
Warrants for purchase of common stock | — | 2,421,102 | ||||||
Stock options issued and outstanding | 12,781,618 | 29,592,211 | ||||||
Authorized for future option grants | 17,537,150 | 726,557 | ||||||
|
|
|
| |||||
| 165,725,876 | 185,960,075 | |||||||
|
|
|
| |||||
NOTE 6—INCOME TAXES
The Company has reported net losses since inception and therefore, the minimum provision for state income taxes has been recorded.
F-17
Table of Contents
The following table provides a reconciliation between income taxes computed at the federal statutory rate of 34% and the Company’s provision for income taxes.
| Year ended December 31, | ||||||||
| 2012 | 2013 | |||||||
Income tax at statutory rate | 34.0 | % | 34.0 | % | ||||
State tax, net of federal tax benefit | 5.8 | 3.8 | ||||||
Permanent items | (0.3 | ) | (11.9 | ) | ||||
Tax credits | 3.8 | 2.4 | ||||||
Valuation allowance increase | (43.3 | ) | (28.3 | ) | ||||
|
|
|
| |||||
Income tax expense | — | % | — | % | ||||
|
|
|
| |||||
Significant components of the Company’s deferred tax assets and liabilities from federal and state income taxes as of December 31 are shown below:
| Year ended December 31, | ||||||||
| 2012 | 2013 | |||||||
Deferred Tax Assets | ||||||||
Depreciation and amortization | $ | 357,600 | $ | 317,400 | ||||
Stock based compensation | 12,800 | 13,100 | ||||||
Accruals and other | 47,400 | 82,000 | ||||||
|
|
|
| |||||
| 417,800 | 412,500 | |||||||
Less valuation allowance | (417,800 | ) | (412,500 | ) | ||||
|
|
|
| |||||
Net deferred tax assets | $ | — | $ | — | ||||
|
|
|
| |||||
The Company has established a valuation allowance to offset the deferred tax assets as realization of such assets is uncertain.
At December 31, 2012 and 2013, the Company had federal tax net operating loss carryforwards of approximately $26,109,000 and $29,477,000, respectively, as well as state tax net operating loss carryforwards at December 31, 2012 and 2013 of approximately $22,544,000 and $25,391,000, respectively. The Company also had federal income tax research and development and other tax credit carryforwards at December 31, 2012 and 2013 of approximately $393,000 and $456,000, respectively, and state income tax research and development and other tax credits totaling $424,000 and $490,000 at December 31, 2012 and 2013, respectively. The federal tax loss carryforwards will begin to expire in 2018, while the state tax loss carryforwards begin to expire in 2014. The federal credit carryforwards will begin to expire in 2021 and the state credit carryforwards do not expire.
The Company has not completed a formal study to assess whether an ownership change as defined pursuant to Internal Revenue Code Section 382 has occurred or whether there have been multiple ownership changes since its formation, due to the complexity and cost associated with such a study, and the fact that there may be additional ownership changes in the future. Based on a preliminary assessment, the Company believes that an ownership change may have occurred during an earlier year. The Company estimates that if such a change did occur, the federal and state net operating loss carryforwards and research and development credits that can be utilized in the future would be significantly limited.
At December 31, 2012 and 2013, the Company had unrecognized tax benefits of $10,866,000 and $12,415,000, respectively, all of which would impact the tax rate if recognized. However, a valuation allowance would be recorded against these amounts. During 2013, unrecognized tax benefits increased by a net $1,549,000 as a result of the tax positions taken in the current year and expiration of prior year unrecognized tax benefits. No interest and penalties have been provided for with respect to the unrecognized tax benefits.
F-18
Table of Contents
A reconciliation of the beginning and ending amount of unrecognized tax benefits for each year is as follows:
| Year ended December 31, | ||||||||
| 2012 | 2013 | |||||||
Unrecognized tax benefit at December 31, | $ | 9,950,000 | $ | 10,866,000 | ||||
Additions for tax positions related to current year | 942,000 | 1,579,000 | ||||||
Reductions for prior year utilizations | — | — | ||||||
Additions/reductions for tax positions related to prior years | (26,000 | ) | (30,000 | ) | ||||
|
|
|
| |||||
Unrecognized tax benefits at December 31, | $ | 10,866,000 | $ | 12,415,000 | ||||
|
|
|
| |||||
The Company’s federal tax returns for 2010 and later years and the Company’s California tax returns for 2009 and later years were still subject to examination by the taxing authorities as of December 31, 2013.
NOTE 7—COMMITMENTS AND CONTINGENCIES
Operating Leases
In January 2013, the Company entered into a non-cancelable lease agreement for its operating facilities. The commencement date of the lease was April 2013 and the lease expired on December 31, 2013. In January 2014, the Company signed an amendment to the lease to extend the term through January 2017 (See Note 10).
Rent expense totaled $111,812 and $252,868 for the years ended December 31, 2012 and 2013, respectively.
The Company recorded rent expense of $36,595 and $109,899 for the three months ended March 31, 2013 and 2014 (unaudited), respectively.
The Company entered into a three year non-cancelable operating lease for laboratory equipment in November 2012. In addition, the Company entered into a one year operating lease for furniture in March 2013. The rent expense related to the leases of laboratory equipment and office furniture totaled $12,430 and $88,453 for the years ended December 31, 2012 and 2013, respectively.
The rent expense related to the leases of laboratory equipment and office furniture totaled $20,258 and $23,335 for the three months ended March 31, 2013 and 2014 (unaudited), respectively. Future minimum lease payments for the operating leases for the operating facilities, laboratory equipment and office furniture as of December 31, 2013 are:
2014 | $ | 75,208 | ||
2015 | 59,651 | |||
|
| |||
| $ | 134,859 | |||
|
|
Executive Incentive Plan
Under the terms of a certain employment agreement with an executive officer, the Company will incur additional cash compensation expense of $8,333 for each calendar month between August 8, 2013 and the date of the Next Qualified Financing. The Next Qualified Financing is defined as the aggregate gross proceeds of at least $5,000,000 from the sale of debt or equity securities including the exercise of Series B preferred stock warrants.
Following completion of the Next Qualified Financing, the Company shall grant the executive officer a stock option to purchase that number of shares of Common stock to represent 5% of the Company’s outstanding shares of Common stock calculated on a fully-diluted basis.
F-19
Table of Contents
Legal Proceedings
The Company is not involved in any legal proceedings.
NOTE 8—RETIREMENT PLAN
The Company has a Section 401(k) retirement plan (the Plan) covering all employees. The Plan does not provide for Company contributions.
NOTE 9—RELATED PARTY TRANSACTIONS
The Company leased its office space from an entity controlled by a Company shareholder and the chairman of the Company’s board of directors during 2012 and through April 2013. Rent expense on this facility was $51,000 and $14,000 in 2012 and 2013, respectively.
In addition, one of the Company’s software suppliers is an entity controlled by its chief executive officer. Total expense for this supplier in 2012 and 2013 was $0 and $74,300, respectively.
NOTE 10—SUBSEQUENT EVENTS
Series B Warrants
In February 2014, Warrants to purchase 17,813,097 shares of Series B Preferred stock were exercised for $1,425,049 of cash. As a result, New Warrants, expiring on May 31, 2014, to purchase 17,813,097 shares of Series B Preferred stock were issued to such holders.
In April and May 2014, Warrants and New Warrants to purchase 18,031,847 shares of Series B Preferred stock were exercised for $1,442,548 of cash (unaudited).
Lease
In January 2014, the Company signed an amendment to the lease on its operating facilities to extend the term through January 31, 2017. The base rent increased from $14,150 in January 2013 to $27,329 in February 2014 and included additional office space. The base rent increases to $28,149 and $28,995 in February 2015 and February 2016, respectively.
Stock Options
In January 2014, the Company re-priced 441,470 options from an exercise price of $0.14 to $0.01. In February 2014, the Company issued 17,162,028 stock options and 2,421,102 warrants to the Company’s board of directors, employees and consultants.
Management has evaluated subsequent events for accrual and disclosure through April 4, 2014, which is the date that the financial statements were available to be issued.
F-20
Table of Contents
Shares of Common Stock

Sole Booking–Running Manager
Maxim Group LLC
Co-Manager
Feltl and Company
Prospectus dated , 2014.
Table of Contents
PART II
INFORMATION NOT REQUIRED IN THE PROSPECTUS
Item 13. Other Expenses of Issuance and Distribution.
Estimated expenses, other than underwriting discounts and commissions, payable by the Registrant in connection with the sale of the common stock being registered under this registration statement are as follows:
| Amount to be Paid | ||||
SEC registration fee | $ | 3,220 | ||
FINRA filing fee | $ | 3,500 | ||
Exchange listing fee | * | |||
Printing and engraving expenses | * | |||
Legal fees and expenses | * | |||
Accounting fees and expenses | * | |||
Blue Sky fees and expenses (including legal fees) | * | |||
Transfer agent and registrar fees and expenses | * | |||
Miscellaneous | * | |||
Total | $ | * | ||
| * | To be completed by amendment |
Item 14. Indemnification of Directors and Officers.
On completion of this offering, the Registrant’s amended and restated certificate of incorporation will contain provisions that eliminate, to the maximum extent permitted by the General Corporation Law of the State of Delaware, the personal liability of the Registrant’s directors and executive officers for monetary damages for breach of their fiduciary duties as directors or officers. The Registrant’s amended and restated certificate of incorporation and bylaws will provide that the Registrant must indemnify its directors and executive officers and may indemnify its employees and other agents to the fullest extent permitted by the General Corporation Law of the State of Delaware.
Sections 145 and 102(b)(7) of the General Corporation Law of the State of Delaware provide that a corporation may indemnify any person made a party to an action by reason of the fact that he or she was a director, executive officer, employee, or agent of the corporation or is or was serving at the request of a corporation against expenses (including attorneys’ fees), judgments, fines and amounts paid in settlement actually and reasonably incurred by him or her in connection with such action if he or she acted in good faith and in a manner he or she reasonably believed to be in, or not opposed to, the best interests of the corporation and, with respect to any criminal action or proceeding, had no reasonable cause to believe his or her conduct was unlawful, except that, in the case of an action by or in right of the corporation, no indemnification may generally be made in respect of any claim as to which such person is adjudged to be liable to the corporation.
The Registrant has entered into indemnification agreements with its directors and executive officers, in addition to the indemnification provided for in its amended and restated certificate of incorporation and bylaws, and intends to enter into indemnification agreements with any new directors and executive officers in the future.
The Registrant has purchased and currently intends to maintain insurance on behalf of each and any person who is or was a director or officer of the Registrant against any loss arising from any claim asserted against him or her and incurred by him or her in any such capacity, subject to certain exclusions.
II-1
Table of Contents
The Underwriting Agreement (Exhibit 1.1 hereto) provides for indemnification by the underwriters of the Registrant and its executive officers and directors, and by the Registrant of the underwriters, for certain liabilities, including liabilities arising under the Securities Act.
See also the undertakings set out in response to Item 17 herein.
Item 15. Recent Sales of Unregistered Securities.
Since January 1, 2011, the Registrant has issued and sold the following securities:
Between February 17, 2011 and September 10, 2012, the Registrant sold 31,518 shares of its Series A-3 preferred stock in a private placement at a purchase price per share of $60.00 for aggregate gross proceeds of approximately $1.9 million. The securities issued in this private placement were sold exclusively to accredited investors.
Between August 31, 2012 and October 15, 2012, the Registrant raised approximately $2.5 million in a private placement of convertible promissory in a supplemental loan financing. The securities issued in this private placements were sold exclusively to accredited investors.
Between June 10, 2013 and July 15, 2013, the Registrant raised approximately $0.5 million in a private placement of convertible promissory notes in a supplemental loan financing. The securities issued in this private placement were sold exclusively to accredited investors.
On August 8, 2013, the Registrant sold 1,403,313 shares of its Series B preferred stock and warrants to purchase 260,841 shares of its Series B preferred stock in a private placement at a purchase price per share of $6.00 (with no additional consideration for any warrants issued with the Series B purchased) for aggregate gross proceeds of approximately $5.8 million, which amount included the conversion of approximately $3.1 million of outstanding convertible promissory notes and accrued interest. The securities issued in this private placement were sold exclusively to accredited investors.
On October 18, 2013, the Registrant sold 5,000 shares of its Series B preferred stock and warrants to purchase 2,500 shares of its Series B preferred stock in a private placement at a purchase price per share of $6.00 (with no additional consideration for any warrants issued with the Series B purchased) for aggregate gross proceeds of $30,000. The securities issued in this private placement were sold exclusively to accredited investors.
Between February 21, 2014 and March 1, 2014, the Registrant sold 237,508 shares of its Series B preferred stock and warrants to purchase 237,508 shares of its Series B preferred stock in connection with the exercise of outstanding warrants at a per share exercise price of $6.00 (with no additional consideration for any warrants issued with the Series B purchased) for aggregate gross proceeds of approximately $1.4 million. The securities issued in this private placement were sold exclusively to accredited investors.
In April and May, 2014, the Registrant sold 240,425 shares of its Series B preferred stock in connection with the exercise of outstanding warrants at a per share exercise price of $6.00. The securities issued in this private placement were sold exclusively to accredited investors.
Between January 1, 2011 and March 31, 2014, the Registrant granted to its directors, employees, consultants, and other service providers options to purchase an aggregate of 403,790 shares of common stock under the Registrant’s 2010 Stock Plan, or the 2010 Plan, at exercise prices ranging from $0.75 to $10.50 per share.
On February 23, 2014, the Registrant granted to its certain employees warrants to purchase an aggregate of 32,281 shares of common stock at an exercise price of $0.75.
II-2
Table of Contents
Between January 1, 2011 and March 31, 2014, the Registrant issued and sold to its directors, employees, consultants, and other service providers an aggregate of 267 shares of common stock upon the exercise of options under the 2010 Plan at an exercise price of $10.50 per share, for an aggregate exercise price of approximately $2,815.
None of the foregoing transactions involved any underwriters, underwriting discounts or commissions, or any public offering. The Registrant believes these transactions were exempt from registration under the Securities Act in reliance upon Section 4(2) of the Securities Act (or Regulation D or Regulation S promulgated thereunder), or Rule 701 promulgated under Section 3(b) of the Securities Act as transactions by an issuer not involving any public offering or pursuant to benefit plans and contracts relating to compensation as provided under Rule 701. The recipients of the securities in each of these transactions represented their intentions to acquire the securities for investment only and not with a view to or for sale in connection with any distribution thereof, and appropriate legends were placed upon the stock certificates issued in these transactions. All recipients had adequate access, through their relationships with us, to information about the Registrant.
Item 16. Exhibits and Financial Statement Schedules.
(a) Exhibits:
See Exhibit Index immediately following the Signature Pages.
(b) Financial Statement Schedules.
All schedules have been omitted because the information required to be presented in them is not applicable or is shown in the financial statements or related notes.
Item 17. Undertakings.
The Registrant hereby undertakes to provide to the underwriters at the closing as specified in the underwriting agreement certificates in such denominations and registered in such names as required by the underwriters to permit prompt delivery to each purchaser.
Insofar as indemnification for liabilities arising under the Securities Act of 1933, as amended, may be permitted to directors, officers and controlling persons of the Registrant pursuant to the foregoing provisions, or otherwise, the Registrant has been advised that in the opinion of the Securities and Exchange Commission such indemnification is against public policy as expressed in the Securities Act of 1933, as amended, and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities (other than the payment by the Registrant of expenses incurred or paid by a director, officer or controlling person of the Registrant in the successful defense of any action, suit or proceeding) is asserted by such director, officer or controlling person in connection with the securities being registered, the Registrant will, unless in the opinion of its counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question whether such indemnification by it is against public policy as expressed in the Securities Act of 1933, as amended, and will be governed by the final adjudication of such issue.
The Registrant hereby undertakes that:
(1) For purposes of determining any liability under the Securities Act of 1933, as amended, the information omitted from a form of prospectus filed as part of this registration statement in reliance upon Rule 430A and contained in the form of prospectus filed by the Registrant pursuant to Rule 424(b)(1) or (4) or 497(h) under the Securities Act of 1933, as amended, shall be deemed to be part of this registration statement as of the time it was declared effective.
(2) For the purpose of determining any liability under the Securities Act of 1933, as amended, each post-effective amendment that contains a form of prospectus shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof.
II-3
Table of Contents
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, the registrant has duly caused this amendment to registration statement to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of San Diego, State of California, on June 3, 2014.
| DERMTECH INTERNATIONAL | ||
| By: | /s/ John Dobak, M.D. | |
| John Dobak, M.D. | ||
| Chief Executive Officer | ||
Pursuant to the requirements of the Securities Act of 1933, this amendment to registration statement has been signed by the following persons in the capacities indicated below:
Signature | Title | Date | ||
/s/ John Dobak, M.D. John Dobak, M.D. | Chief Executive Officer and Director (Principal Executive Officer) | June 3, 2014 | ||
/s/ Steven Kemper Steven Kemper | Chief Financial Officer (Principal Financial and Accounting Officer) | June 3, 2014 | ||
/s/ Gary Jacobs Gary Jacobs | Director and Chairman of the Board | June 3, 2014 | ||
/s/ Scott Pancoast Scott Pancoast | Director | June 3, 2014 | ||
/s/ Carl Peck, M.D. Carl Peck, M.D. | Director | June 3, 2014 | ||
/s/ Gene Salkind, M.D. Gene Salkind, M.D. | Director | June 3, 2014 | ||
II-4
Table of Contents
EXHIBIT INDEX
Exhibit | Description | |
| 1.1* | Form of Underwriting Agreement. | |
| 3.1^ | Amended and Restated Articles of Incorporation of the Registrant, as currently in effect. | |
| 3.2* | Form of Amended and Restated Certificate of Incorporation, to be in effect upon the completion of this offering. | |
| 3.3^ | Amended and Restated Bylaws of the Registrant, as currently in effect. | |
| 3.4* | Form of Amended and Restated Bylaws of the Registrant, to be in effect upon the completion of this offering. | |
| 4.1^ | Amended and Restated Investors’ Rights Agreement, dated August 8, 2013. | |
| 4.2* | Specimen common stock certificate of the Registrant. | |
| 5.1* | Opinion of Wilson Sonsini Goodrich & Rosati, Professional Corporation. | |
| 10.1*+ | Form of Director and Officer Indemnification Agreement. | |
| 10.2^+ | 2010 Stock Plan, as amended, and forms of agreement thereunder. | |
| 10.3*+ | 2014 Equity Incentive Plan and forms of agreement thereunder. | |
| 10.4*+ | Employment Agreement between the Registrant and John Dobak, dated June 6, 2012, as amended on February 25, 2014. | |
| 10.5*+ | Employment Agreement between the Registrant and Steve Kemper, dated April 1, 2014. | |
| 10.6*+ | Employment Agreement between the Registrant and Michael Willis, dated April 1, 2014. | |
| 10.7*+ | Employment Agreement between the Registrant and John Alsobrook, dated April 1, 2014. | |
| 10.8^ | Lease Agreement between AG/Touchstone TP, LLC and the Registrant, dated January 25, 2013, as amended on January 30, 2014. | |
| 10.9^† | Master Service Agreement between Registrant and Biogen Idec Inc., dated November 13, 2013. | |
| 23.1 | Consent of Grant Thornton LLP, Independent Registered Public Accounting Firm. | |
| 23.2* | Consent of Wilson Sonsini Goodrich & Rosati, Professional Corporation (included in Exhibit 5.1). | |
| 24.1^ | Power of Attorney (included on page II-4 to the original filing of this registration statement). | |
| * | To be filed by amendment. |
| ^ | Previously filed. |
| + | Indicates a management contract or a compensatory plan. |
| † | Portions of this exhibit have been omitted pending a determination by the Securities and Exchange Commission as to whether these portions should be granted confidential treatment. |