
Richard Pops Chief Executive Officer Alkermes: Advancing Key Business Priorities in 2022 January 2022 40th Annual J.P. Morgan Healthcare Conference Exhibit 99.1

Forward-Looking Statements Certain statements set forth in this presentation constitute “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995, as amended, including, but not limited to, statements concerning: the company’s expectations with respect to its current and future financial and operating performance, business plans or prospects, including expected commercial growth drivers and development opportunities, and execution against 2022 financial expectations and long-term profitability goals; the potential therapeutic and commercial value of the company’s marketed products and development candidates, including nemvaleukin alfa (“nemvaleukin”) as a cancer immunotherapy when used as monotherapy or in combination and whether delivered intravenously or subcutaneously, and its potential utility across a range of tumor types, dosing options and potential combinations with other targeted therapies; expectations regarding patent life for nemvaleukin; expectations regarding the effectiveness and potential of the company’s research and development (“R&D”) objective, approach and capabilities, including its molecule design and engineering capabilities; timelines, plans and expectations for development activities relating to the company’s development candidates, including (i) for nemvaleukin, planned and ongoing clinical studies in the ARTISTRY development program, including plans to evaluate potential dosing flexibility, and plans to pursue strategic collaborations, (ii) for ALKS 1140, plans to advance the phase 1 program, (iii) for ALKS 2680, plans to complete IND-enabling activities and prepare for initiation of a first-in-human study, and (iv) for the engineered cytokine program, plans to advance the IL-12 and IL-18 preclinical programs to key decision points; and expectations concerning commercial activities relating to the company’s products, including plans for the ongoing commercial launch of LYBALVI® and the company’s ability to leverage its existing commercial infrastructure. The company cautions that forward-looking statements are inherently uncertain. Actual performance and results may differ materially from those expressed or implied in the forward-looking statements due to various risks, assumptions and uncertainties. These risks, assumptions and uncertainties include, among others: whether LYBALVI will be commercialized successfully; the impacts of the ongoing COVID-19 pandemic and continued efforts to mitigate its spread on the company’s business, results of operations or financial condition; the unfavorable outcome of litigation, including so-called “Paragraph IV” litigation or other patent litigation which may lead to competition from generic drug manufacturers, or other disputes related to the company’s products or products using the company’s proprietary technologies; clinical development activities may not be completed on time or at all; the results of the company’s development activities may not be positive or predictive of real-world results, and preliminary data from ongoing studies may not be predictive of future or final data from such studies, results of future studies or real-world results; the U.S. Food and Drug Administration (“FDA”) or other regulatory authorities may not agree with the company’s regulatory approval strategies or components of the company’s marketing applications, including clinical trial designs, conduct and methodologies, manufacturing processes and facilities, or the adequacy of the data or other information included in the company’s regulatory submissions to support their requirements for approval, and may make adverse decisions regarding the company’s products; the company and its licensees may not be able to successfully commercialize their products or support growth of revenue from such products; there may be a reduction in payment rate or reimbursement for the company’s products or an increase in the company’s financial obligations to governmental payers; the company’s products may prove difficult to manufacture, be precluded from commercialization by the proprietary rights of third parties, or have unintended side effects, adverse reactions or incidents of misuse; and those risks, assumptions and uncertainties described under the heading “Risk Factors” in the company’s Annual Report on Form 10-K for the year ended Dec. 31, 2020 and in subsequent filings made by the company with the U.S. Securities and Exchange Commission (“SEC”), which are available on the SEC’s website at www.sec.gov and on the company’s website at www.alkermes.com in the “Investors—SEC filings” section. Existing and prospective investors are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof. Except as required by law, the company disclaims any intention or responsibility for updating or revising any forward-looking statements contained in this presentation. Note Regarding Trademarks: The company and its affiliates are the owners of various U.S. federal trademark registrations (®) and other trademarks (TM), including ARISTADA®, ARISTADA INITIO®, VIVITROL®, and LYBALVI®. VUMERITY® is a registered trademark of Biogen MA Inc., used by Alkermes under license. Any other trademarks referred to in this presentation are the property of their respective owners. Appearances of such other trademarks herein should not be construed as any indicator that their respective owners will not assert their rights thereto.

Focused on disciplined capital allocation and optimized cost structure Restructured commercial organization to support launch of LYBALVI Initiated nemvaleukin alfa studies in mucosal melanoma and platinum-resistant ovarian cancer to support potential registration Initiated ALKS 1140 phase 1 first-in-human study Advanced ALKS 2680 into IND-enabling activities Development Pipeline Advance pipeline of neuroscience and oncology candidates LYBALVI®: Approved and commercially launched ARISTADA®: Drove TRx growth that outpaced the market VIVITROL®: Redefined alcohol dependence strategy to drive next phase of growth Commercial Grow commercial portfolio of proprietary products Three Strategic Priorities Grounded in Strong Culture of Responsibility Profitability Drive long-term profitability Patient-focused ethos and strong commitment to corporate responsibility and governance
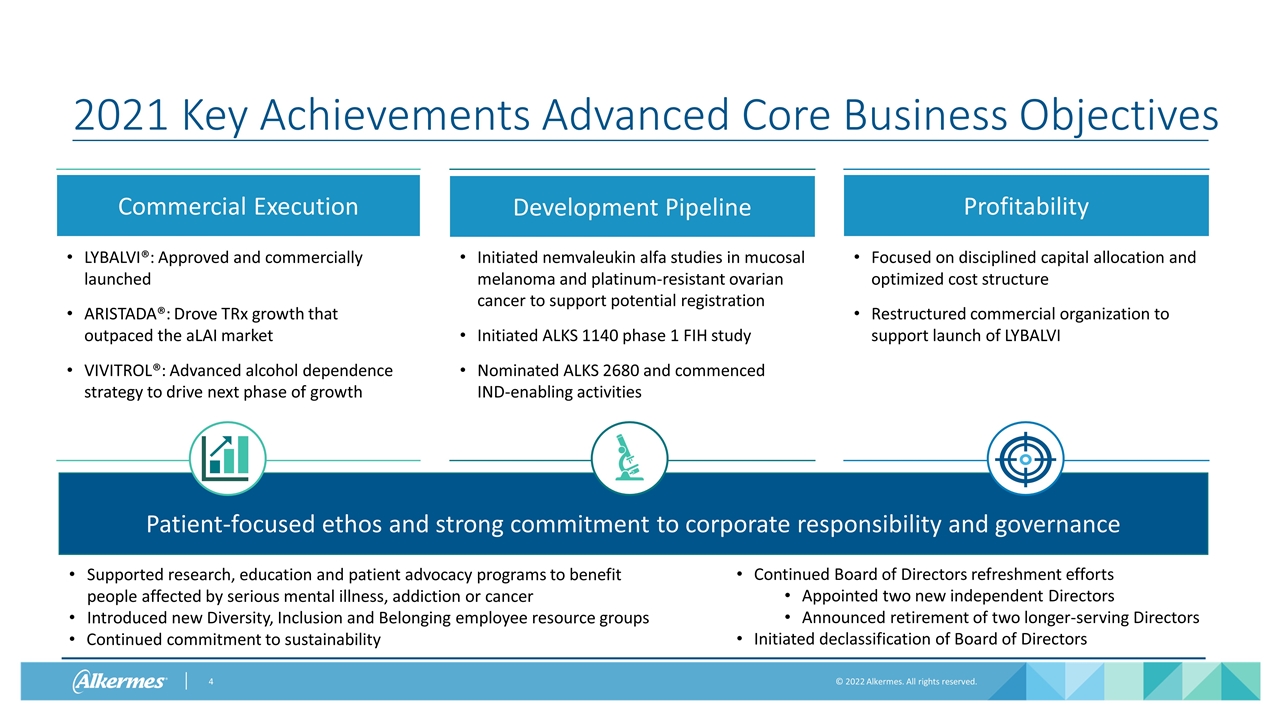
Focused on disciplined capital allocation and optimized cost structure Restructured commercial organization to support launch of LYBALVI Continued Board of Directors refreshment efforts Appointed two new independent Directors Announced retirement of two longer-serving Directors Initiated declassification of Board of Directors Initiated nemvaleukin alfa studies in mucosal melanoma and platinum-resistant ovarian cancer to support potential registration Initiated ALKS 1140 phase 1 FIH study Nominated ALKS 2680 and commenced IND-enabling activities Development Pipeline LYBALVI®: Approved and commercially launched ARISTADA®: Drove TRx growth that outpaced the aLAI market VIVITROL®: Advanced alcohol dependence strategy to drive next phase of growth Commercial Execution 2021 Key Achievements Advanced Core Business Objectives Profitability Supported research, education and patient advocacy programs to benefit people affected by serious mental illness, addiction or cancer Introduced new Diversity, Inclusion and Belonging employee resource groups Continued commitment to sustainability Patient-focused ethos and strong commitment to corporate responsibility and governance
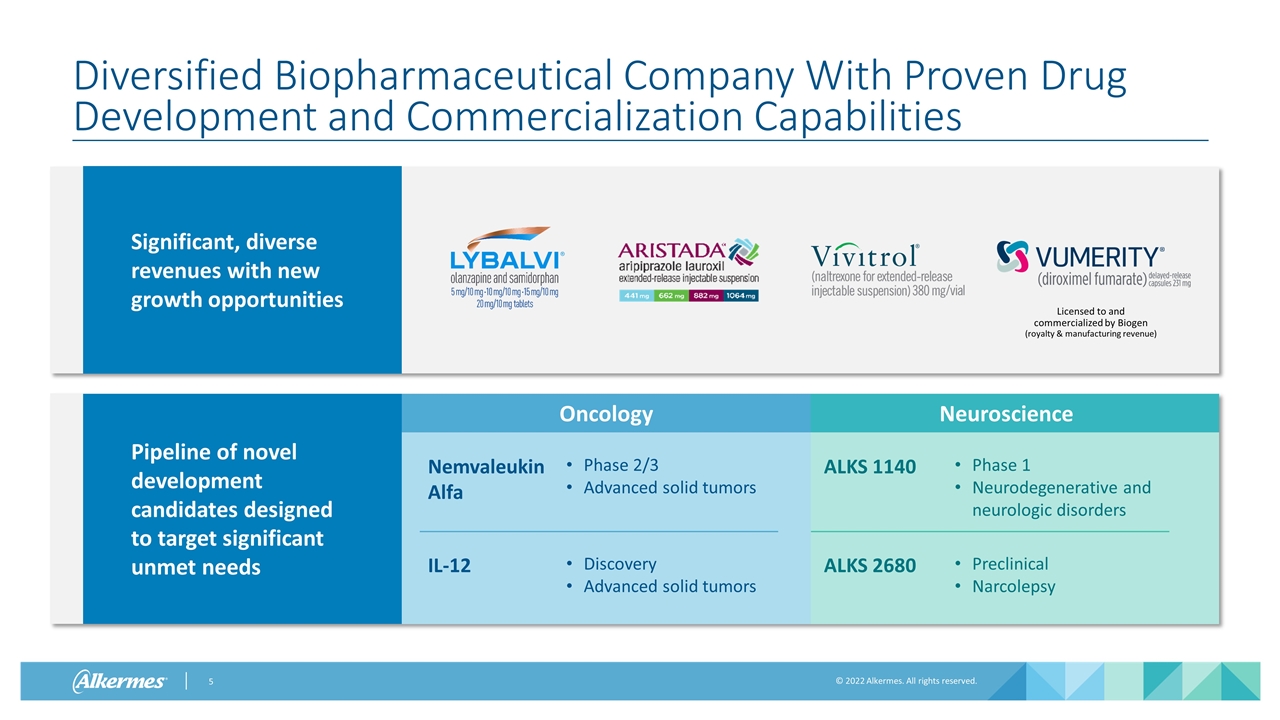
Phase 1 Neurodegenerative and neurologic disorders Neuroscience Oncology Pipeline of novel development candidates designed to target significant unmet needs Diversified Biopharmaceutical Company With Proven Drug Development and Commercialization Capabilities Licensed to and commercialized by Biogen (royalty & manufacturing revenue) Phase 2/3 Advanced solid tumors Nemvaleukin Alfa IL-12 Discovery Advanced solid tumors ALKS 1140 ALKS 2680 Preclinical Narcolepsy Significant, diverse revenues with new growth opportunities

Current Growth Drivers

Commercial Growth Drivers
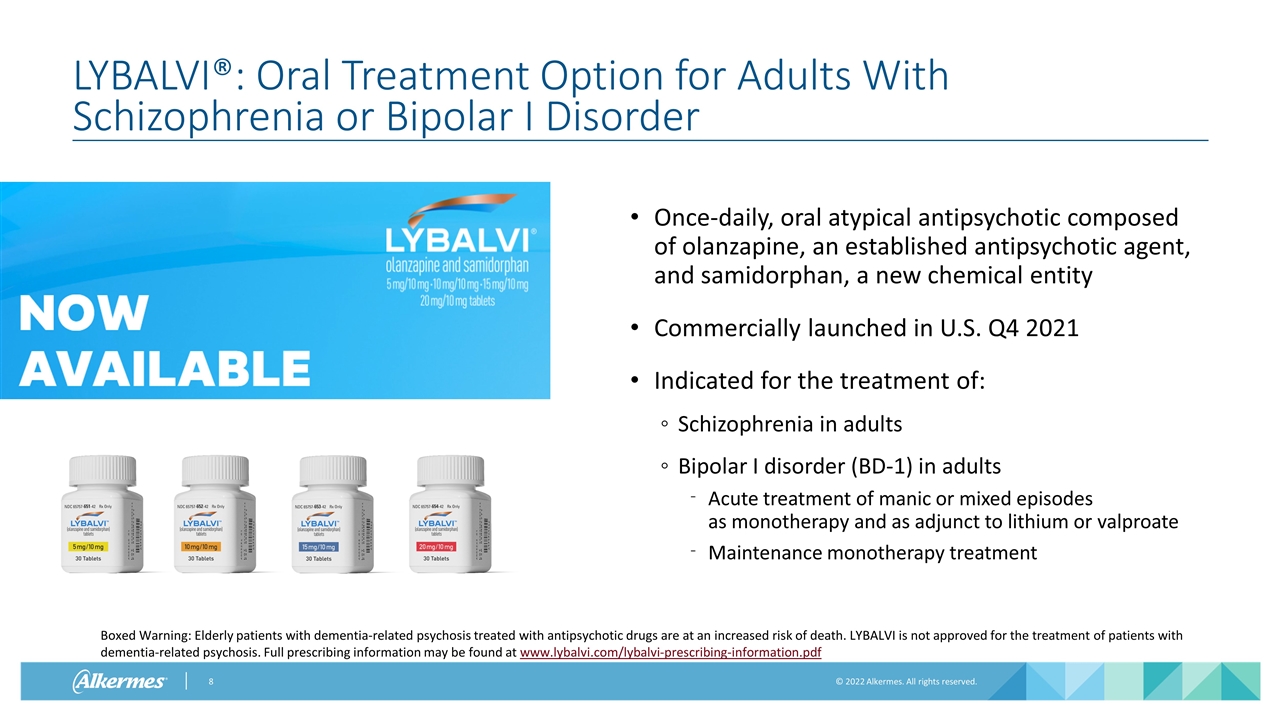
LYBALVI®: Oral Treatment Option for Adults With Schizophrenia or Bipolar I Disorder Once-daily, oral atypical antipsychotic composed of olanzapine, an established antipsychotic agent, and samidorphan, a new chemical entity Commercially launched in U.S. Q4 2021 Indicated for the treatment of: Schizophrenia in adults Bipolar I disorder (BD-1) in adults Acute treatment of manic or mixed episodes as monotherapy and as adjunct to lithium or valproate Maintenance monotherapy treatment Boxed Warning: Elderly patients with dementia-related psychosis treated with antipsychotic drugs are at an increased risk of death. LYBALVI is not approved for the treatment of patients with dementia-related psychosis. Full prescribing information may be found at www.lybalvi.com/lybalvi-prescribing-information.pdf

LYBALVI®: Offers Proven Efficacy With a Differentiated Weight Gain Profile in Adult Patients with Schizophrenia LYBALVI offers proven efficacy* and was associated with less weight gain versus olanzapine in patients with schizophrenia in the ENLIGHTEN-2 clinical trial** ENLIGHTEN-1: LYBALVI demonstrated a statistically significant improvement in the change from baseline in PANSS total score versus placebo in patients with schizophrenia at week 4† ENLIGHTEN-2: LYBALVI was associated with less weight gain versus olanzapine in patients with schizophrenia at week 24† *Inclusion of samidorphan in LYBALVI did not appear to negatively impact the efficacy of olanzapine. **Inclusion of samidorphan in LYBALVI appeared to result in less weight gain than was seen with olanzapine alone.1 † Increased weight was the most common adverse reaction in patients treated with LYBALVI in ENLIGHTEN-1 and ENLIGHTEN-2. Other common adverse reactions were somnolence, dry mouth and headache. Boxed Warning: Elderly patients with dementia-related psychosis treated with antipsychotic drugs are at an increased risk of death. LYBALVI is not approved for the treatment of patients with dementia-related psychosis. Full prescribing information may be found at www.lybalvi.com/lybalvi-prescribing-information.pdf 1Correll CU, Newcomer JW, Silverman B, et al. Effects of olanzapine combined with samidorphan on weight gain in schizophrenia: a 24-week phase 3 study. Am J Psychiatry. 2020. doi.org/10.1176/appi.ajp.2020.19121279.
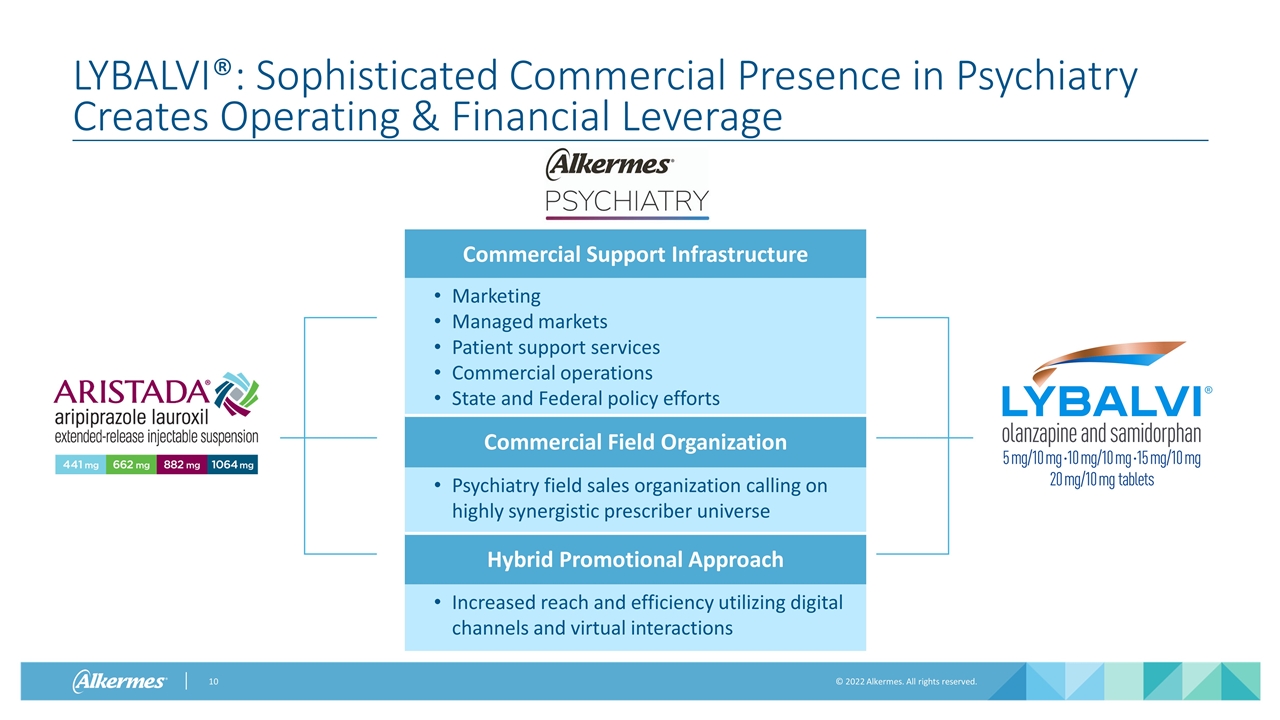
LYBALVI®: Sophisticated Commercial Presence in Psychiatry Creates Operating & Financial Leverage Commercial Support Infrastructure Marketing Managed markets Patient support services Commercial operations State and Federal policy efforts Commercial Field Organization Psychiatry field sales organization calling on highly synergistic prescriber universe Hybrid Promotional Approach Increased reach and efficiency utilizing digital channels and virtual interactions
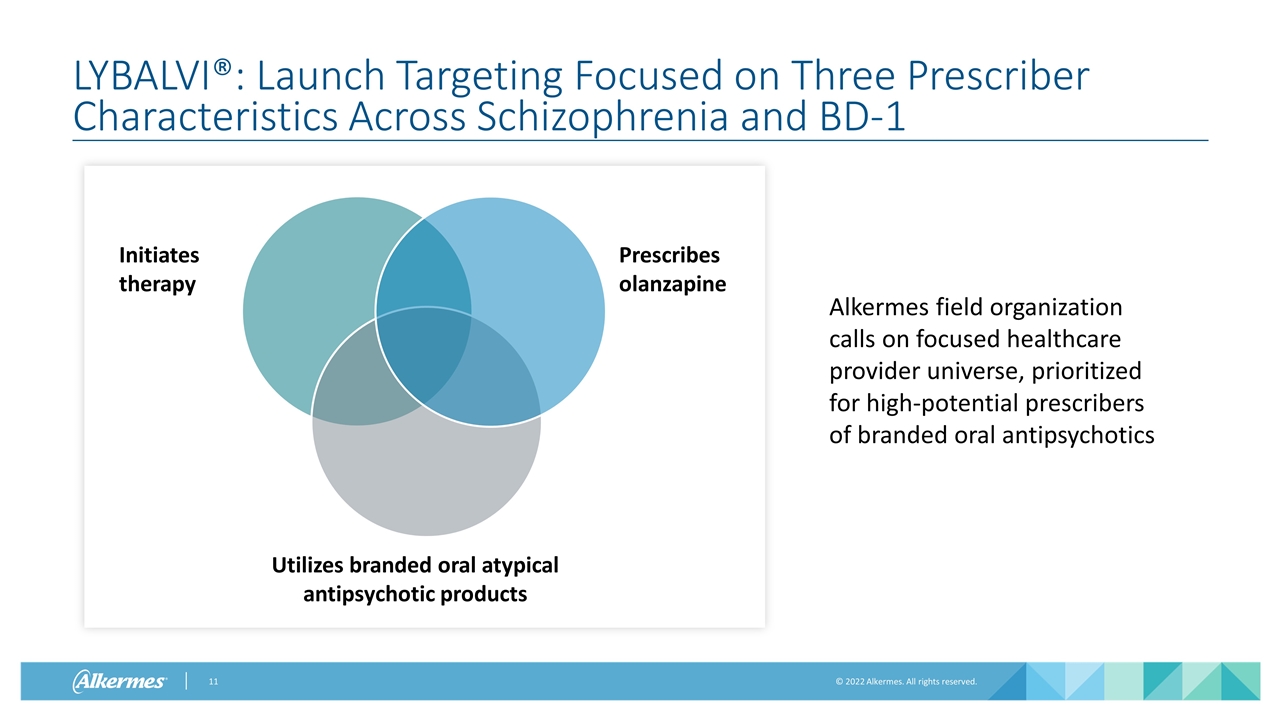
LYBALVI®: Launch Targeting Focused on Three Prescriber Characteristics Across Schizophrenia and BD-1 Alkermes field organization calls on focused healthcare provider universe, prioritized for high-potential prescribers of branded oral antipsychotics Prescribes olanzapine Utilizes branded oral atypical antipsychotic products Initiates therapy Prescribes olanzapine Utilizes branded oral atypical antipsychotic products Initiates therapy Utilizes branded oral atypical antipsychotic products Prescribes olanzapine Initiates therapy
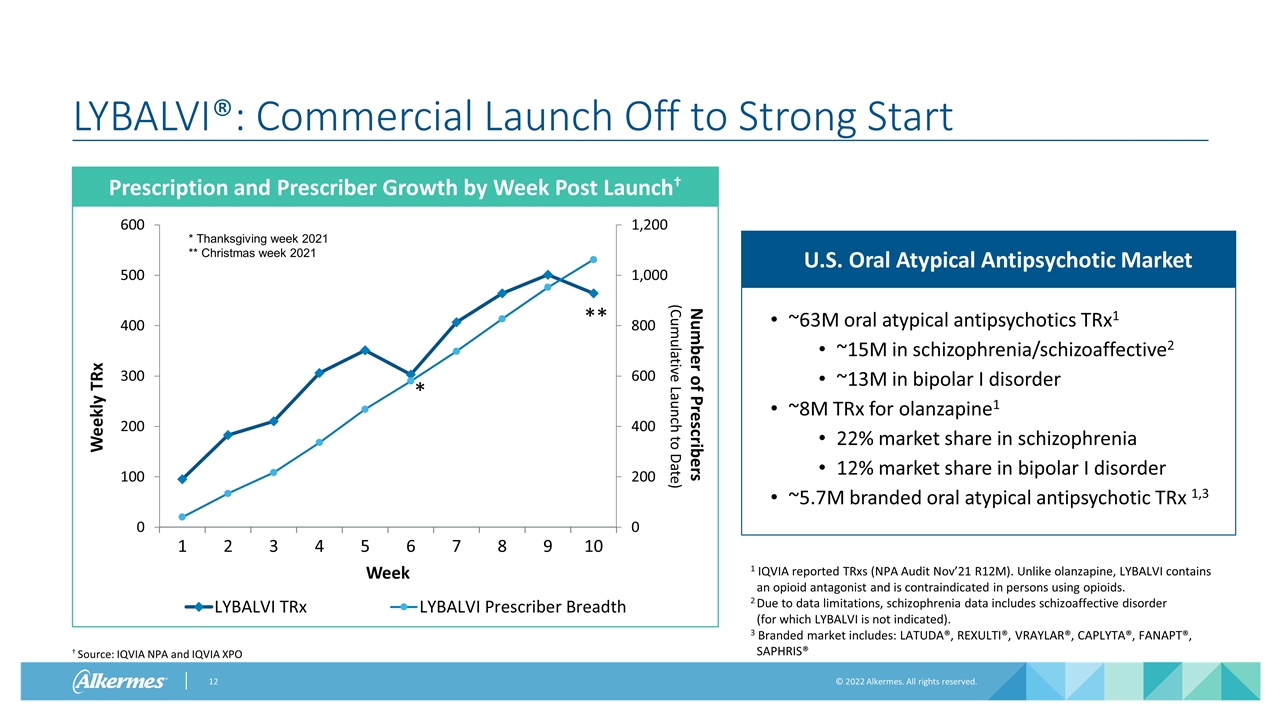
LYBALVI®: Commercial Launch Off to Strong Start Prescription and Prescriber Growth by Week Post Launch† † Source: IQVIA NPA and IQVIA XPO 1 IQVIA reported TRxs (NPA Audit Nov’21 R12M). Unlike olanzapine, LYBALVI contains an opioid antagonist and is contraindicated in persons using opioids. 2Due to data limitations, schizophrenia data includes schizoaffective disorder (for which LYBALVI is not indicated). 3 Branded market includes: LATUDA®, REXULTI®, VRAYLAR®, CAPLYTA®, FANAPT®, SAPHRIS® ~63M oral atypical antipsychotics TRx1 ~15M in schizophrenia/schizoaffective2 ~13M in bipolar I disorder ~8M TRx for olanzapine1 22% market share in schizophrenia 12% market share in bipolar I disorder ~5.7M branded oral atypical antipsychotic TRx 1,3 U.S. Oral Atypical Antipsychotic Market * ** * Thanksgiving week 2021 ** Christmas week 2021

Commercial Growth Drivers

ARISTADA®: LAI for Schizophrenia With Dosing Flexibility Long-acting injectable (LAI) atypical antipsychotic indicated for the treatment of schizophrenia Novel molecular entity designed to address the real-world needs of patients and providers Ability to fully dose on day one for up to two months with ARISTADA INITIO® regimen* *ARISTADA INITIO + single 30 mg oral dose of aripiprazole replaces need for concomitant three weeks of oral aripiprazole for initiation of ARISTADA. The first ARISTADA dose may be administered on the same day as ARISTADA INITIO or up to 10 days thereafter. Full prescribing information for ARISTADA, including Boxed Warning, may be found at www.aristada.com/downloadables/ARISTADA-PI.pdf
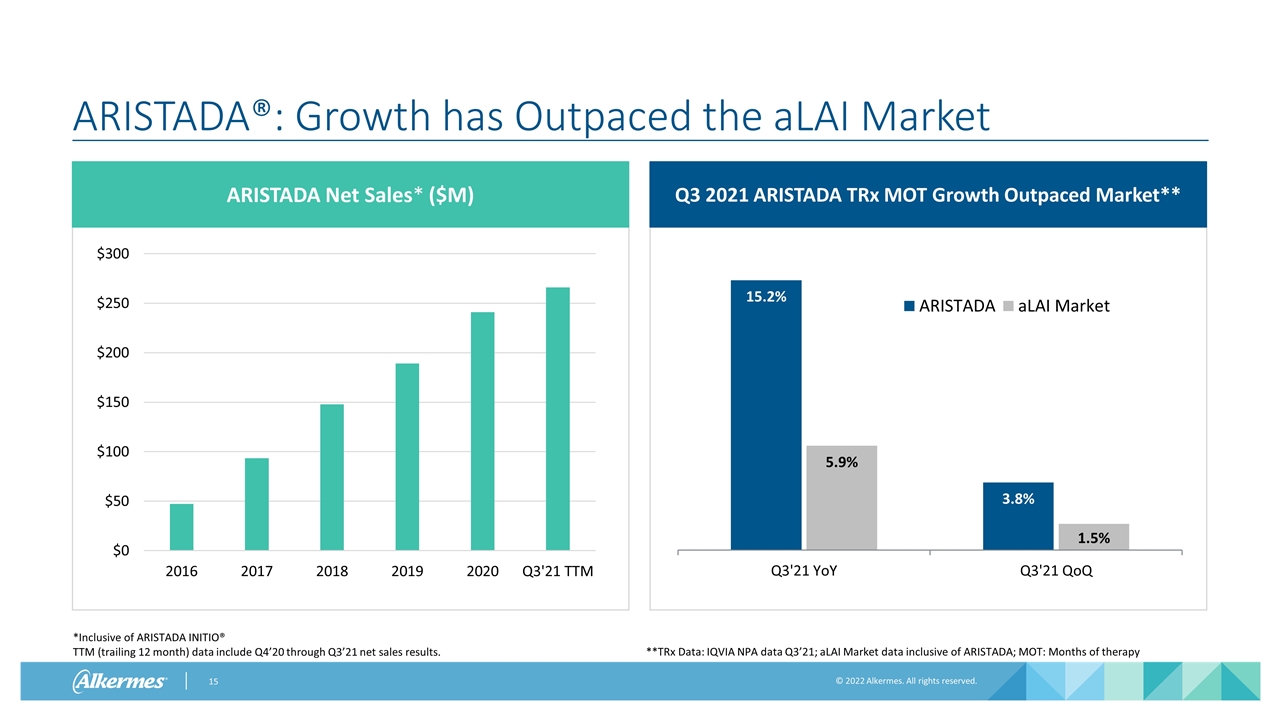
ARISTADA®: Growth has Outpaced the aLAI Market *Inclusive of ARISTADA INITIO® TTM (trailing 12 month) data include Q4’20 through Q3’21 net sales results. ARISTADA Net Sales* ($M) Q3 2021 ARISTADA TRx MOT Growth Outpaced Market** **TRx Data: IQVIA NPA data Q3’21; aLAI Market data inclusive of ARISTADA; MOT: Months of therapy

Commercial Growth Drivers
VIVITROL®: LAI for the Treatment of Opioid Dependence and Alcohol Dependence Extended-release opioid antagonist provides therapeutic levels of naltrexone for a one-month period Indicated for the treatment of alcohol dependence (AD) in patients able to abstain from alcohol in an outpatient setting prior to initiation of treatment with VIVITROL Indicated for the prevention of relapse to opioid dependence (OD), following opioid detoxification Full prescribing information for VIVITROL may be found at www.vivitrol.com/content/pdfs/prescribing-information.pdf. Treatment with VIVITROL should be part of a comprehensive management program that includes psychosocial support.
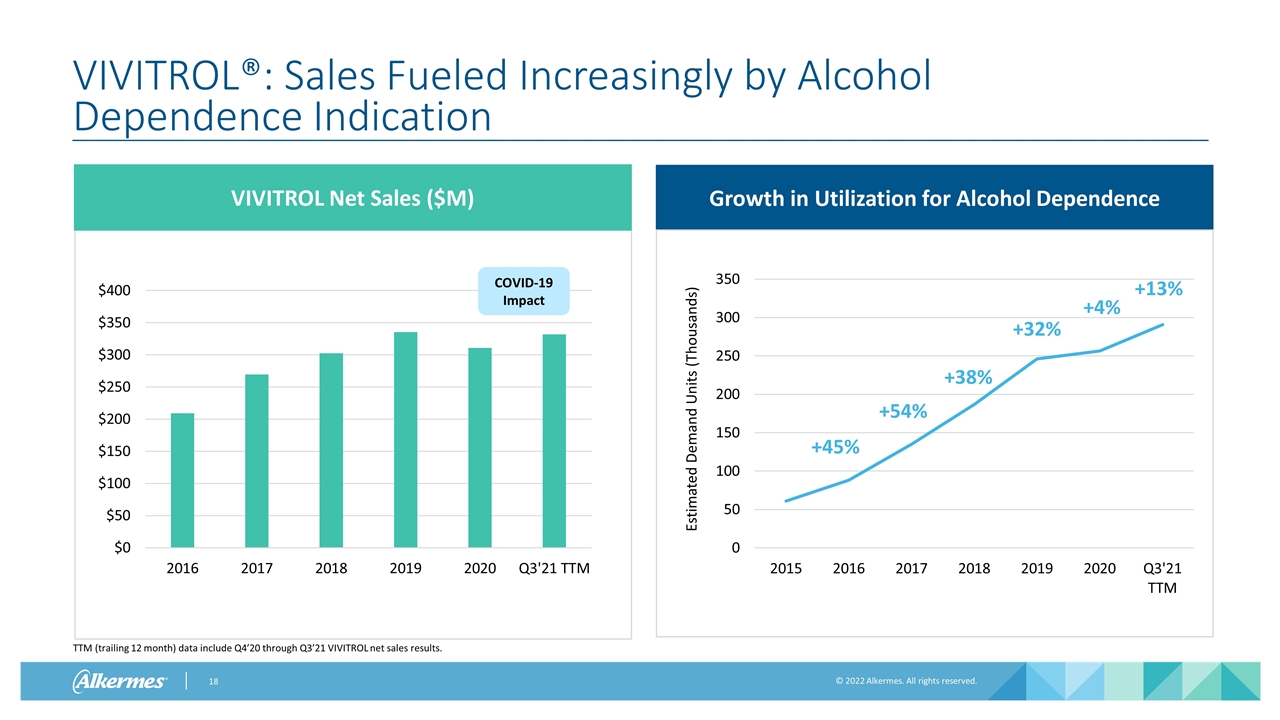
VIVITROL®: Sales Fueled Increasingly by Alcohol Dependence Indication COVID-19 Impact VIVITROL Net Sales ($M) Growth in Utilization for Alcohol Dependence TTM (trailing 12 month) data include Q4’20 through Q3’21 VIVITROL net sales results. +54% +38% +32% +4% +45% +13%

Commercial Growth Drivers

VUMERITY® (Diroximel Fumarate) for Multiple Sclerosis (MS) Novel oral fumarate for the treatment of relapsing forms of multiple sclerosis Discovered and developed by Alkermes Composition of matter patent extends into 2033
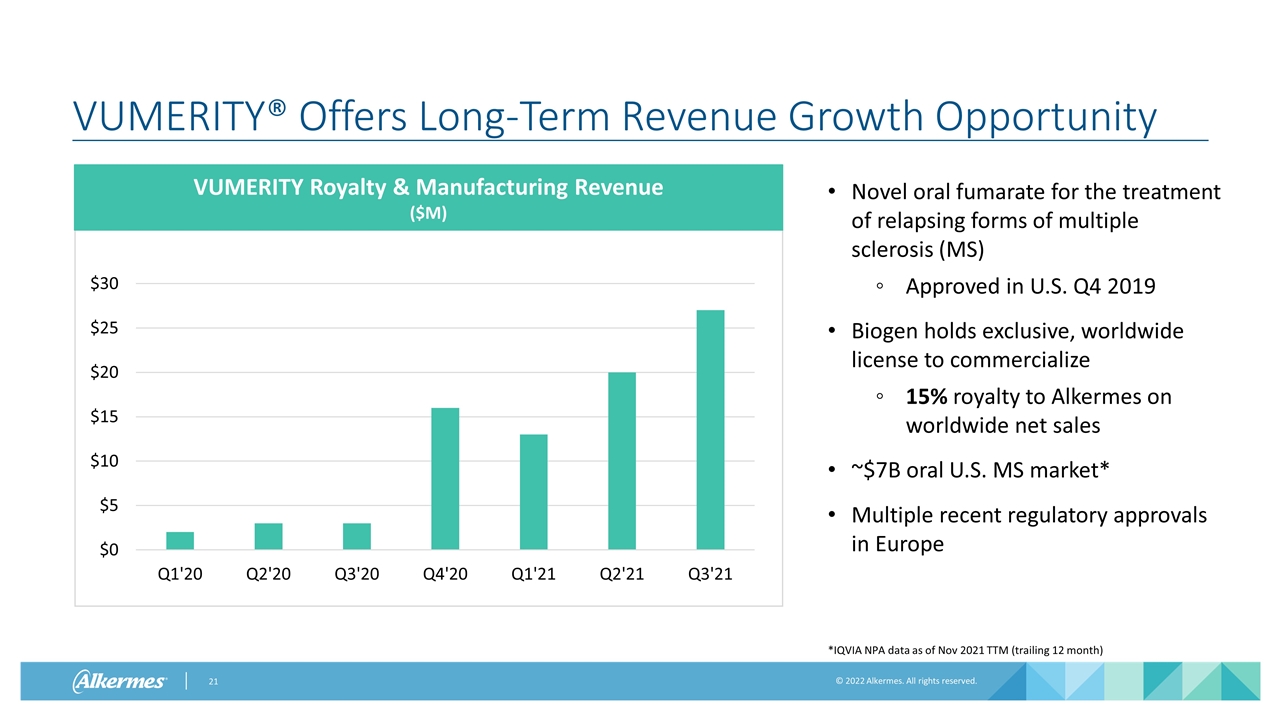
Novel oral fumarate for the treatment of relapsing forms of multiple sclerosis (MS) Approved in U.S. Q4 2019 Biogen holds exclusive, worldwide license to commercialize 15% royalty to Alkermes on worldwide net sales ~$7B oral U.S. MS market* Multiple recent regulatory approvals in Europe VUMERITY® Offers Long-Term Revenue Growth Opportunity VUMERITY Royalty & Manufacturing Revenue ($M) *IQVIA NPA data as of Nov 2021 TTM (trailing 12 month)
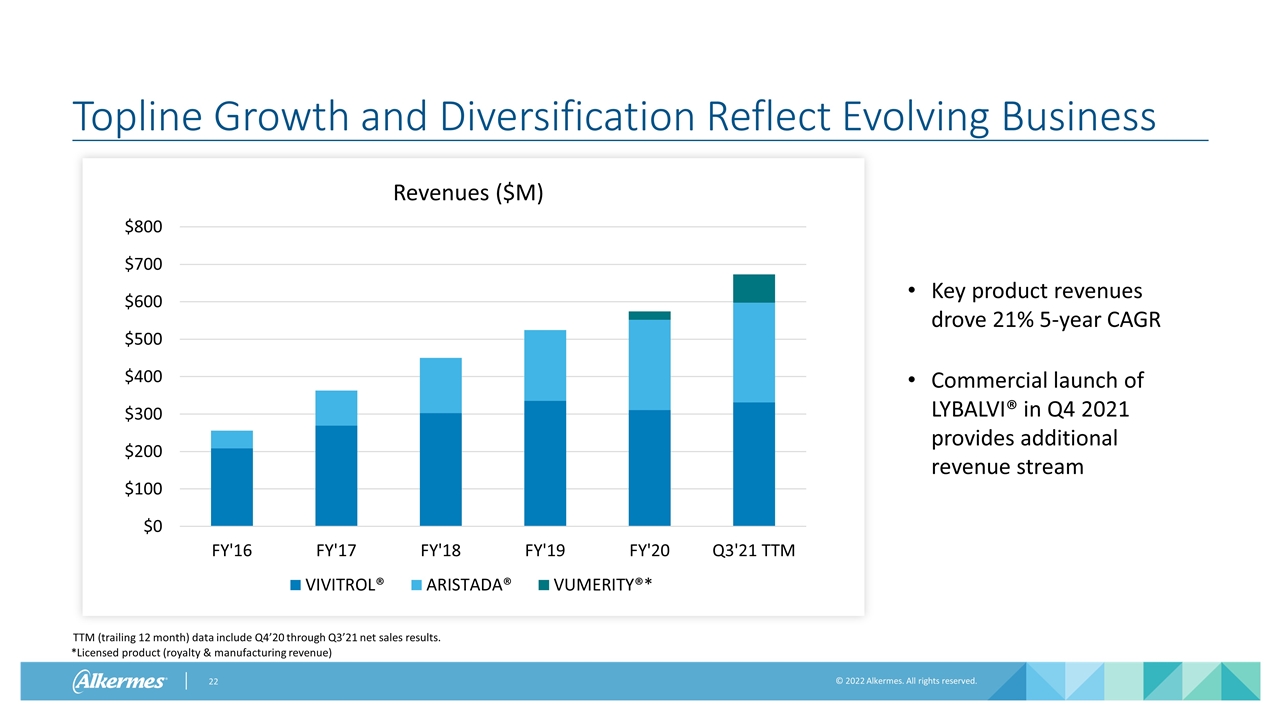
Topline Growth and Diversification Reflect Evolving Business *Licensed product (royalty & manufacturing revenue) Key product revenues drove 21% 5-year CAGR Commercial launch of LYBALVI® in Q4 2021 provides additional revenue stream TTM (trailing 12 month) data include Q4’20 through Q3’21 net sales results.

Innovation Focused on Unmet Patient Need in Neuroscience and Oncology
R&D Objective: Novel Drug Development With Differentiated and Disciplined Approach Leverage advanced medicinal chemistry and protein engineering capabilities to develop novel molecular entities with strong intellectual property protection Employ integrated approach to target selection, development and lifecycle management with continuous evaluation of potential medical and economic value De-risk programs early by accelerating time to data and decision milestones and adhering to clear go/no-go criteria
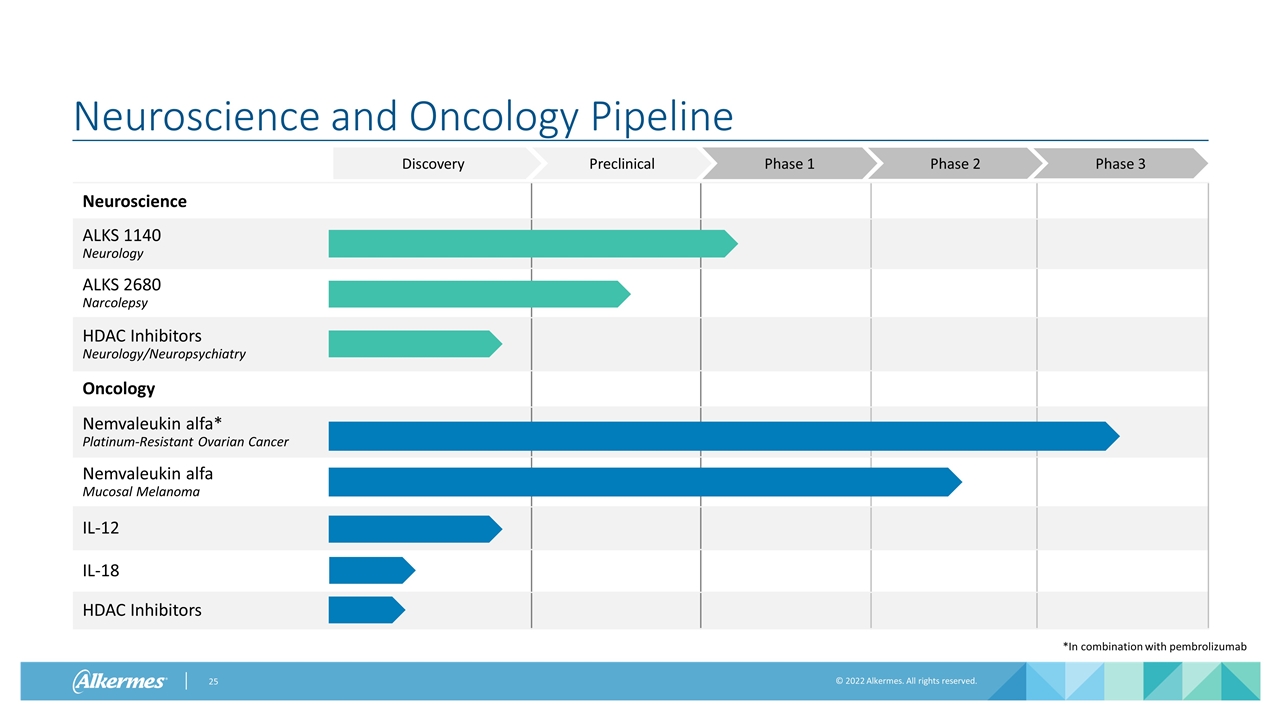
Neuroscience and Oncology Pipeline Neuroscience ALKS 1140 Neurology ALKS 2680 Narcolepsy HDAC Inhibitors Neurology/Neuropsychiatry Oncology Nemvaleukin alfa* Platinum-Resistant Ovarian Cancer Nemvaleukin alfa Mucosal Melanoma IL-12 IL-18 HDAC Inhibitors Preclinical Phase 1 Phase 2 Phase 3 Discovery *In combination with pembrolizumab
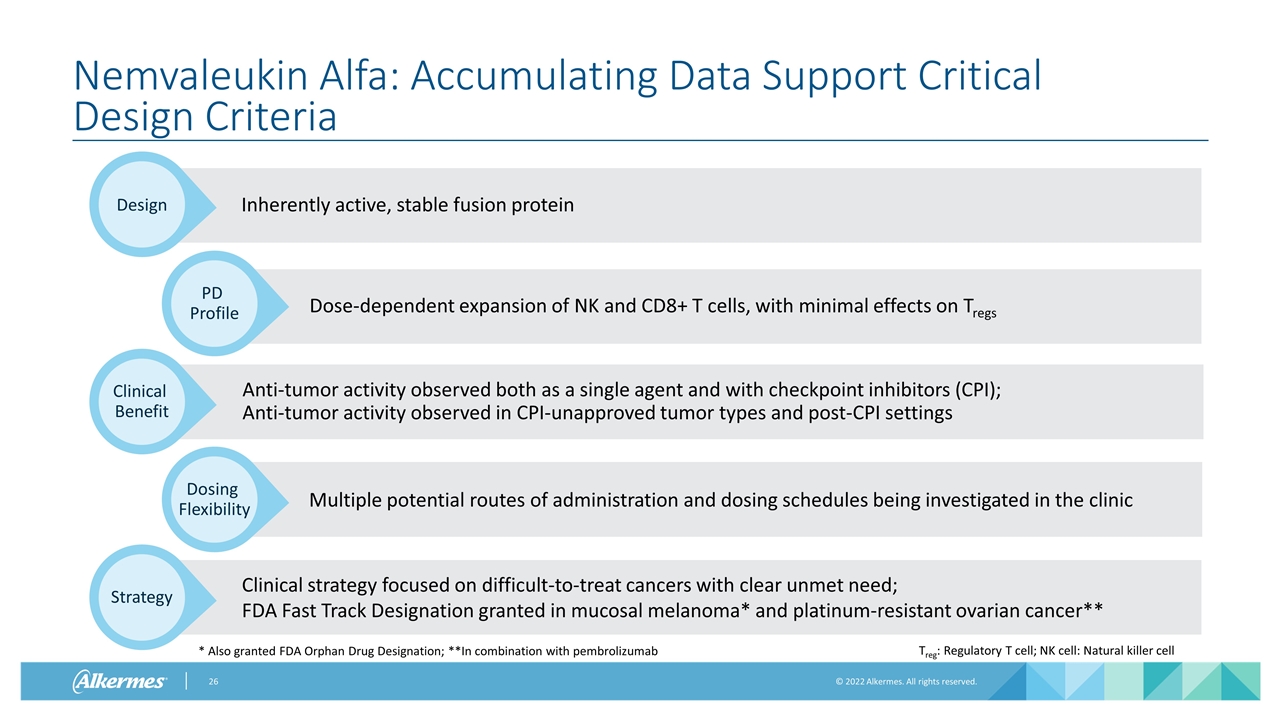
Inherently active, stable fusion protein Nemvaleukin Alfa: Accumulating Data Support Critical Design Criteria Dose-dependent expansion of NK and CD8+ T cells, with minimal effects on Tregs Design Treg: Regulatory T cell; NK cell: Natural killer cell * Also granted FDA Orphan Drug Designation; **In combination with pembrolizumab PD Profile Anti-tumor activity observed both as a single agent and with checkpoint inhibitors (CPI); Anti-tumor activity observed in CPI-unapproved tumor types and post-CPI settings Clinical Benefit Multiple potential routes of administration and dosing schedules being investigated in the clinic Dosing Flexibility Clinical strategy focused on difficult-to-treat cancers with clear unmet need; FDA Fast Track Designation granted in mucosal melanoma* and platinum-resistant ovarian cancer** Strategy
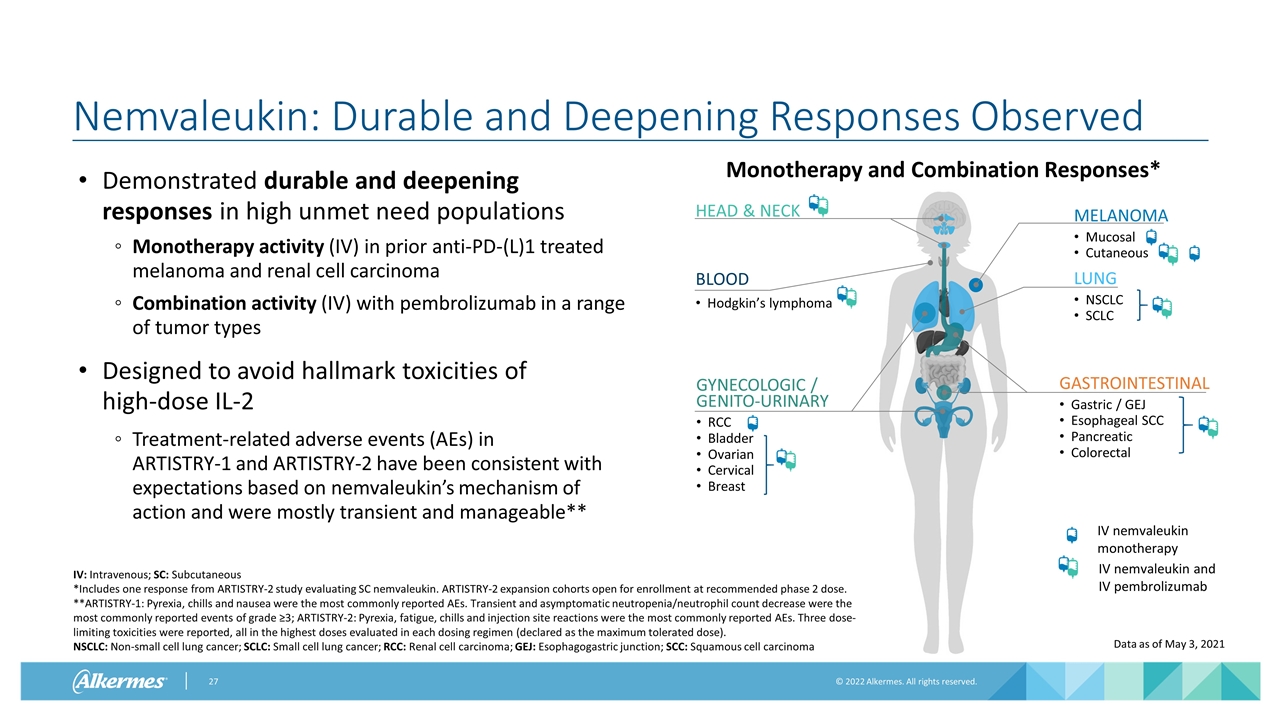
Nemvaleukin: Durable and Deepening Responses Observed Demonstrated durable and deepening responses in high unmet need populations Monotherapy activity (IV) in prior anti-PD-(L)1 treated melanoma and renal cell carcinoma Combination activity (IV) with pembrolizumab in a range of tumor types Designed to avoid hallmark toxicities of high-dose IL-2 Treatment-related adverse events (AEs) in ARTISTRY-1 and ARTISTRY-2 have been consistent with expectations based on nemvaleukin’s mechanism of action and were mostly transient and manageable** IV: Intravenous; SC: Subcutaneous *Includes one response from ARTISTRY-2 study evaluating SC nemvaleukin. ARTISTRY-2 expansion cohorts open for enrollment at recommended phase 2 dose. **ARTISTRY-1: Pyrexia, chills and nausea were the most commonly reported AEs. Transient and asymptomatic neutropenia/neutrophil count decrease were the most commonly reported events of grade ≥3; ARTISTRY-2: Pyrexia, fatigue, chills and injection site reactions were the most commonly reported AEs. Three dose-limiting toxicities were reported, all in the highest doses evaluated in each dosing regimen (declared as the maximum tolerated dose). NSCLC: Non-small cell lung cancer; SCLC: Small cell lung cancer; RCC: Renal cell carcinoma; GEJ: Esophagogastric junction; SCC: Squamous cell carcinoma GYNECOLOGIC / GENITO-URINARY RCC Bladder Ovarian Cervical Breast LUNG NSCLC SCLC MELANOMA Mucosal Cutaneous BLOOD Hodgkin’s lymphoma HEAD & NECK GASTROINTESTINAL Gastric / GEJ Esophageal SCC Pancreatic Colorectal Monotherapy and Combination Responses* IV nemvaleukin monotherapy IV nemvaleukin and IV pembrolizumab Data as of May 3, 2021
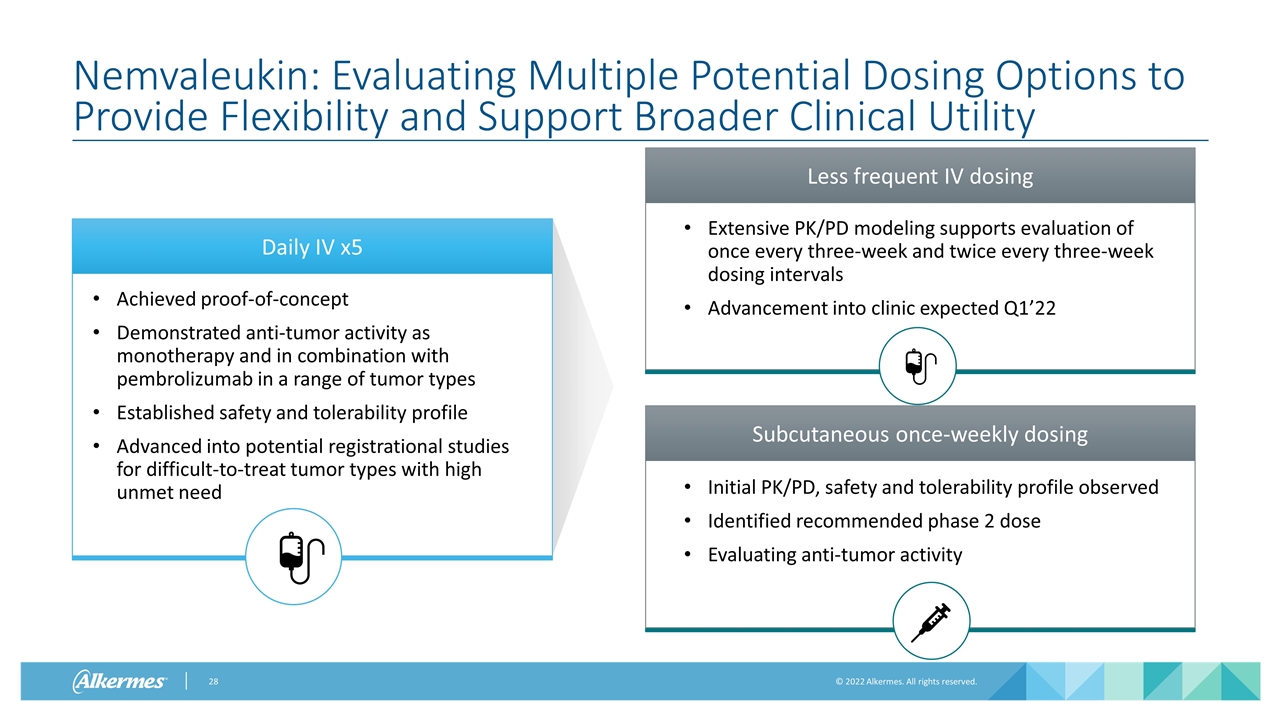
Daily IV x5 Achieved proof-of-concept Demonstrated anti-tumor activity as monotherapy and in combination with pembrolizumab in a range of tumor types Established safety and tolerability profile Advanced into potential registrational studies for difficult-to-treat tumor types with high unmet need Nemvaleukin: Evaluating Multiple Potential Dosing Options to Provide Flexibility and Support Broader Clinical Utility Subcutaneous once-weekly dosing Initial PK/PD, safety and tolerability profile observed Identified recommended phase 2 dose Evaluating anti-tumor activity Less frequent IV dosing Extensive PK/PD modeling supports evaluation of once every three-week and twice every three-week dosing intervals Advancement into clinic expected Q1’22
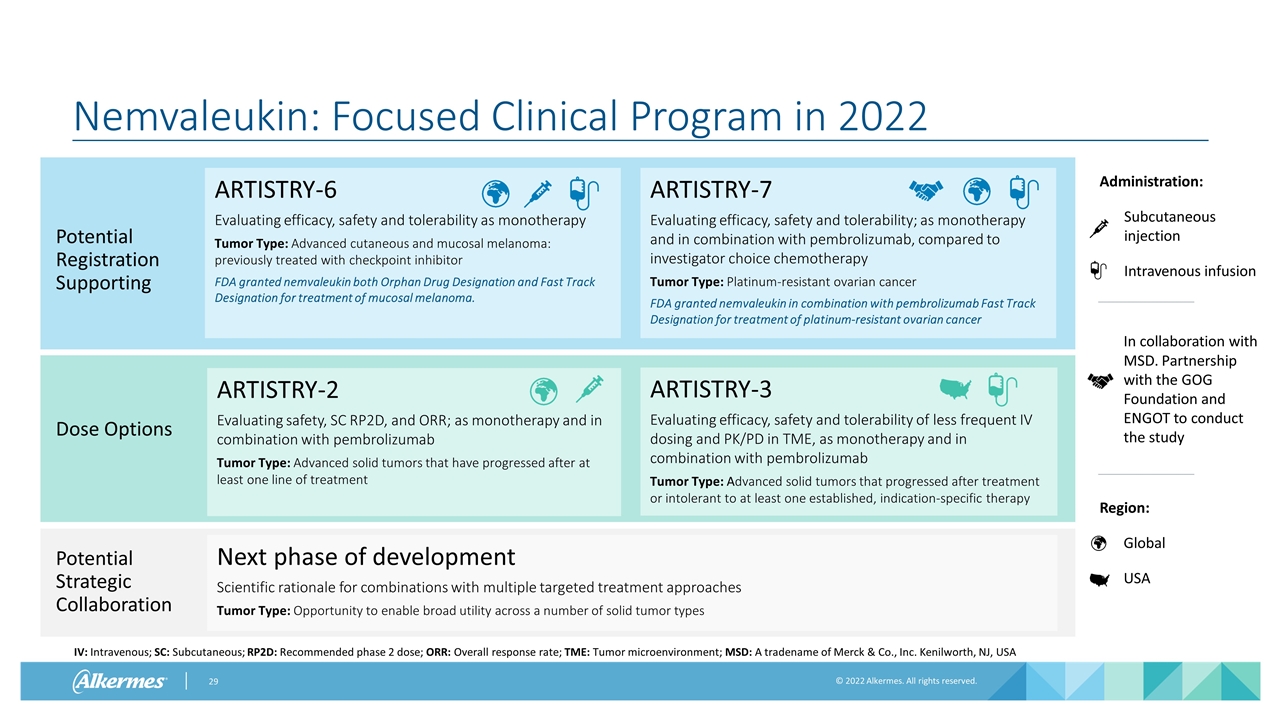
Nemvaleukin: Focused Clinical Program in 2022 IV: Intravenous; SC: Subcutaneous; RP2D: Recommended phase 2 dose; ORR: Overall response rate; TME: Tumor microenvironment; MSD: A tradename of Merck & Co., Inc. Kenilworth, NJ, USA ARTISTRY-3 Evaluating efficacy, safety and tolerability of less frequent IV dosing and PK/PD in TME, as monotherapy and in combination with pembrolizumab Tumor Type: Advanced solid tumors that progressed after treatment or intolerant to at least one established, indication-specific therapy ARTISTRY-2 Evaluating safety, SC RP2D, and ORR; as monotherapy and in combination with pembrolizumab Tumor Type: Advanced solid tumors that have progressed after at least one line of treatment Dose Options ARTISTRY-6 Evaluating efficacy, safety and tolerability as monotherapy Tumor Type: Advanced cutaneous and mucosal melanoma: previously treated with checkpoint inhibitor FDA granted nemvaleukin both Orphan Drug Designation and Fast Track Designation for treatment of mucosal melanoma. ARTISTRY-7 Evaluating efficacy, safety and tolerability; as monotherapy and in combination with pembrolizumab, compared to investigator choice chemotherapy Tumor Type: Platinum-resistant ovarian cancer FDA granted nemvaleukin in combination with pembrolizumab Fast Track Designation for treatment of platinum-resistant ovarian cancer Potential Registration Supporting Next phase of development Scientific rationale for combinations with multiple targeted treatment approaches Tumor Type: Opportunity to enable broad utility across a number of solid tumor types Potential Strategic Collaboration Administration: Subcutaneous injection Intravenous infusion In collaboration with MSD. Partnership with the GOG Foundation and ENGOT to conduct the study Region: Global USA
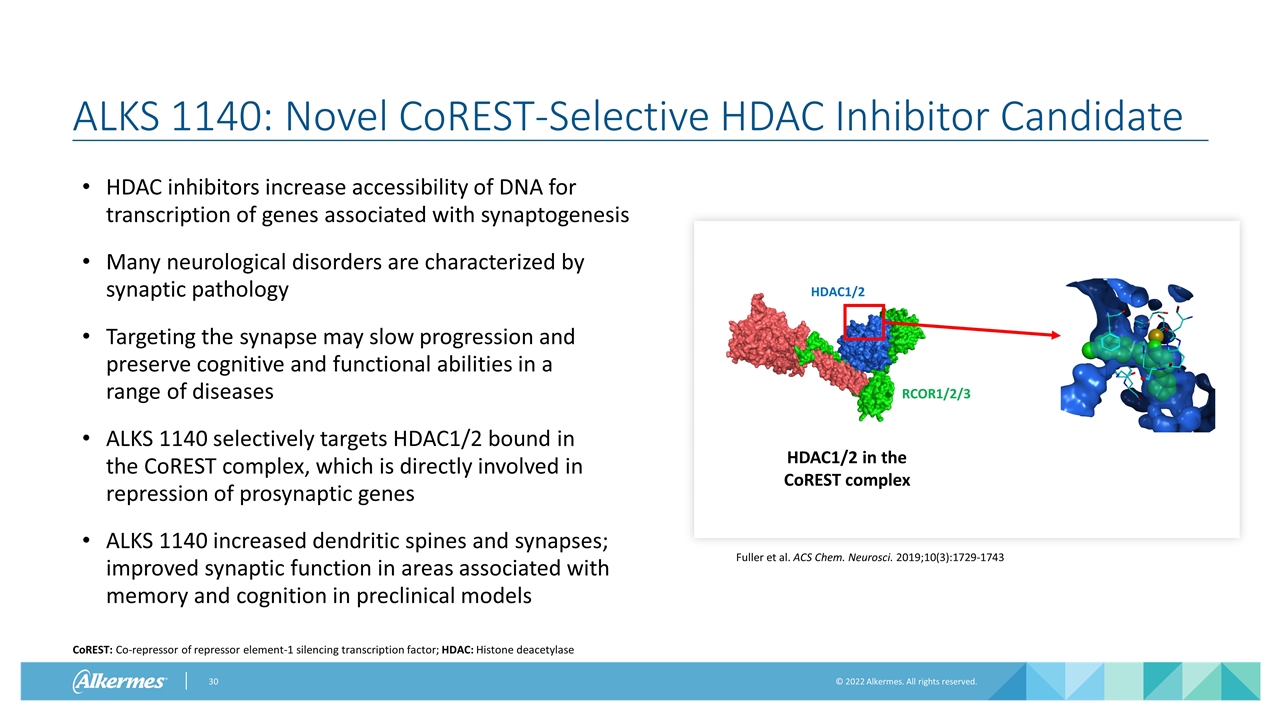
ALKS 1140: Novel CoREST-Selective HDAC Inhibitor Candidate HDAC inhibitors increase accessibility of DNA for transcription of genes associated with synaptogenesis Many neurological disorders are characterized by synaptic pathology Targeting the synapse may slow progression and preserve cognitive and functional abilities in a range of diseases ALKS 1140 selectively targets HDAC1/2 bound in the CoREST complex, which is directly involved in repression of prosynaptic genes ALKS 1140 increased dendritic spines and synapses; improved synaptic function in areas associated with memory and cognition in preclinical models CoREST: Co-repressor of repressor element-1 silencing transcription factor; HDAC: Histone deacetylase HDAC1/2 RCOR1/2/3 HDAC1/2 in the CoREST complex Fuller et al. ACS Chem. Neurosci. 2019;10(3):1729-1743
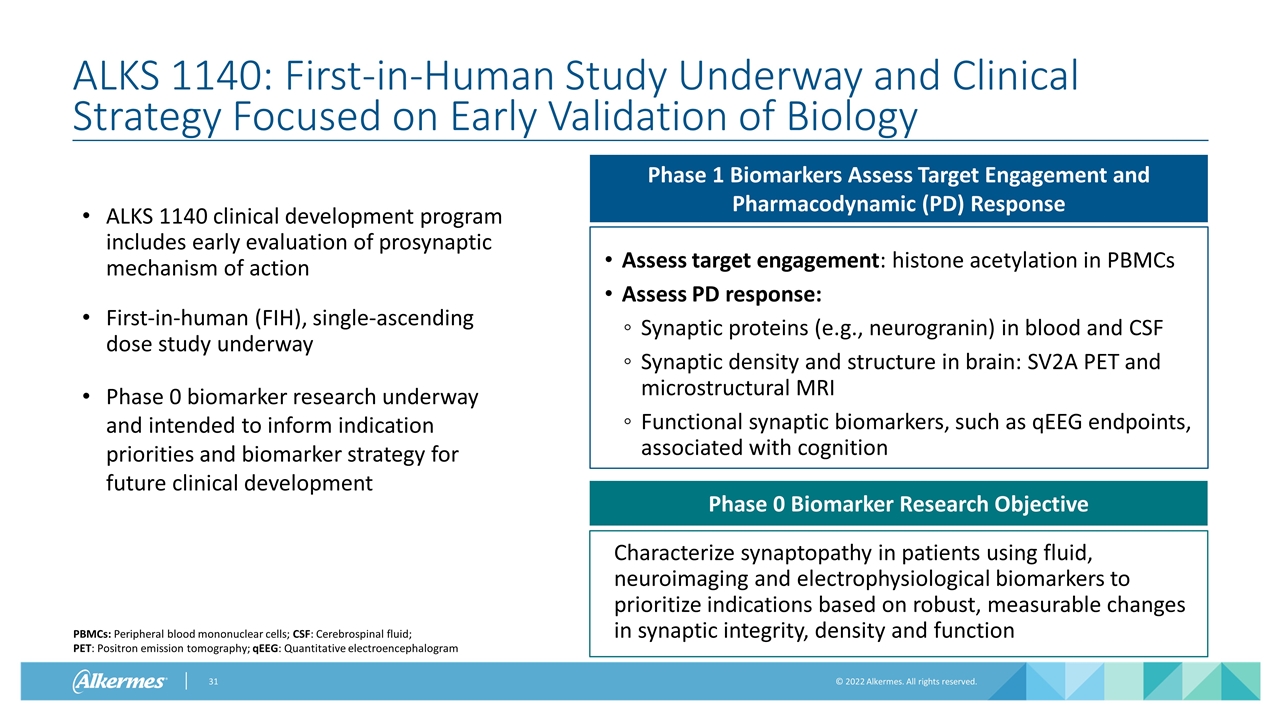
ALKS 1140: First-in-Human Study Underway and Clinical Strategy Focused on Early Validation of Biology ALKS 1140 clinical development program includes early evaluation of prosynaptic mechanism of action First-in-human (FIH), single-ascending dose study underway Phase 0 biomarker research underway and intended to inform indication priorities and biomarker strategy for future clinical development Phase 1 Biomarkers Assess Target Engagement and Pharmacodynamic (PD) Response Assess target engagement: histone acetylation in PBMCs Assess PD response: Synaptic proteins (e.g., neurogranin) in blood and CSF Synaptic density and structure in brain: SV2A PET and microstructural MRI Functional synaptic biomarkers, such as qEEG endpoints, associated with cognition Phase 0 Biomarker Research Objective Characterize synaptopathy in patients using fluid, neuroimaging and electrophysiological biomarkers to prioritize indications based on robust, measurable changes in synaptic integrity, density and function PBMCs: Peripheral blood mononuclear cells; CSF: Cerebrospinal fluid; PET: Positron emission tomography; qEEG: Quantitative electroencephalogram
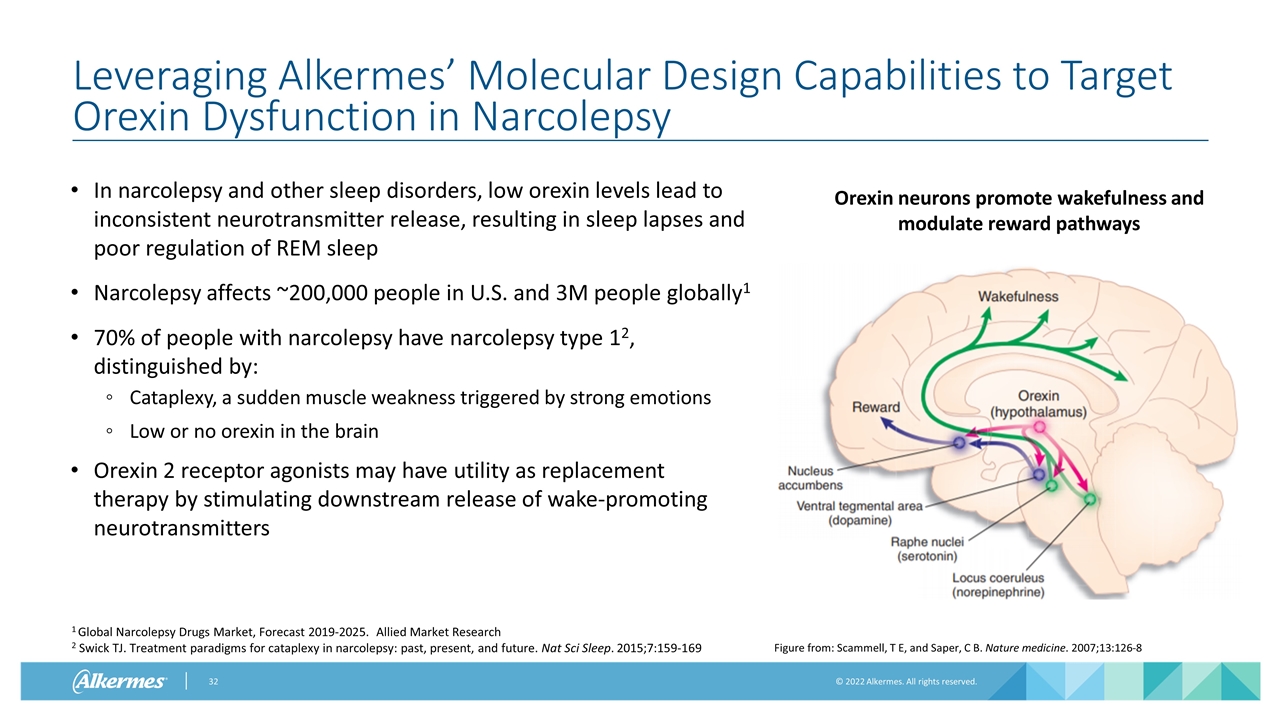
Leveraging Alkermes’ Molecular Design Capabilities to Target Orexin Dysfunction in Narcolepsy In narcolepsy and other sleep disorders, low orexin levels lead to inconsistent neurotransmitter release, resulting in sleep lapses and poor regulation of REM sleep Narcolepsy affects ~200,000 people in U.S. and 3M people globally1 70% of people with narcolepsy have narcolepsy type 12, distinguished by: Cataplexy, a sudden muscle weakness triggered by strong emotions Low or no orexin in the brain Orexin 2 receptor agonists may have utility as replacement therapy by stimulating downstream release of wake-promoting neurotransmitters Figure from: Scammell, T E, and Saper, C B. Nature medicine. 2007;13:126-8 Orexin neurons promote wakefulness and modulate reward pathways 1 Global Narcolepsy Drugs Market, Forecast 2019-2025. Allied Market Research 2 Swick TJ. Treatment paradigms for cataplexy in narcolepsy: past, present, and future. Nat Sci Sleep. 2015;7:159-169

ALKS 2680: Orexin 2 Receptor Agonist ALKS 2680 molecular design objectives for optimization of PK/PD relationship: Mimic potency and performance of endogenous peptide OX2R agonist Increased wakefulness duration Improved cataplexy control Provide convenient dosing Once-daily, oral medication Dose to allow for 8-12 hours wakefulness with no later insomnia Demonstrate favorable tolerability Reduced risk of heart rate and blood pressure effects than seen with stimulants IND-enabling activities underway with potential to initiate first-in-human study by year-end 2022 PK: pharmacokinetic; PD: pharmacodynamic OX2R in Complex with Peptide-Agonist Orexin-B Figure adapted from: Hong, Chuan, et al. Nature communications. 2021:12; 3. PDB ID: 7L1U

Looking Ahead: 2022 Strategic Priorities Execute successful LYBALVI® launch and continue to establish payer access profile Drive growth of VIVITROL® in AD and increase ARISTADA® share of aLAI market Commercial Portfolio Nemvaleukin Early-stage Pipeline Financial Advance enrollment of ARTISTRY-6 & ARTISTRY-7 Execute clinical evaluation of subcutaneous and less frequent IV dosing Pursue strategic collaborations to expand development program ALKS 1140: Advance phase 1 program ALKS 2680: Complete IND-enabling activities and prepare for initiation of FIH study Engineered cytokines: Advance IL-12 and IL-18 preclinical programs to key decision points Provide and execute against 2022 financial expectations and long-term profitability goals

www.alkermes.com