© 2025 Lantheus. All rights reserved. Lantheus to Acquire Life Molecular Imaging Accelerating Innovation for Patients in the Growing Alzheimer’s Disease Radiodiagnostic Market January 13, 2025 Exhibit 99.2

Acquisition Overview & Strategic Rationale Transaction Summary Commercial Infrastructure and R&D Capabilities Growth Strategy Q&A Agenda © 2025 Lantheus. All rights reserved.

Safe Harbor Statements Cautionary Statement Regarding Forward-Looking Statements This document contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995 that are subject to risks and uncertainties and are made pursuant to the safe harbor provisions of Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934, as amended. Such statements are based upon current plans, estimates and expectations that are subject to various risks and uncertainties. The inclusion of forward-looking statements should not be regarded as a representation that such plans, estimates and expectations will be achieved. Words such as “estimate,” “expect,” “may,” “plan,” “potential,” “predict,” “target,” “will,” and words and terms of similar substance used in connection with any discussion of future plans, actions or events identify forward-looking statements. All statements, other than historical facts, including the expected timing of the closing of the transaction; the ability of the parties to complete the transaction considering the various closing conditions; the expected benefits of the transaction, such as efficiencies, cost savings, synergies, revenue growth, creating shareholder value, growth potential, market profile, enhanced competitive position, and financial strength and flexibility; the competitive ability and position of the Company following the transaction; and any assumptions underlying any of the foregoing, are forward-looking statements. Important factors that could cause actual results to differ materially from Lantheus’ plans, estimates or expectations could include, but are not limited to: (i) Life Healthcare Group Holdings may be unable to obtain shareholder approval as required for the transaction; (ii) conditions to the closing of the transaction may not be satisfied; (iii) the transaction may involve unexpected costs, liabilities or delays; (iv) the effect of the announcement of the transaction on the ability of Lantheus or Life Healthcare Group to retain and hire key personnel and maintain relationships with customers, suppliers and others with whom Lantheus or Life Healthcare Group does business, or on Lantheus’ or Life Molecular’s operating results and business generally; (v) Lantheus’ or Life Molecular’s respective businesses may suffer as a result of uncertainty surrounding the transaction and disruption of management’s attention due to the transaction; (vi) the outcome of any legal proceedings related to the transaction; (vii) Lantheus or Life Healthcare Group may be adversely affected by other economic, business, and/or competitive factors; (viii) the occurrence of any event, change or other circumstances that could give rise to the termination of the transaction agreement; (ix) risks that the transaction disrupts current plans and operations and the potential difficulties in employee retention as a result of the transaction; (x) the risk that Lantheus or the Seller may be unable to obtain governmental and regulatory approvals required for the transaction, or that required governmental and regulatory approvals may delay the transaction or result in the imposition of conditions that could reduce the anticipated benefits from the proposed transaction or cause the parties to abandon the proposed transaction; (xi) risks that the anticipated benefits of the transaction or other commercial opportunities may otherwise not be fully realized or may take longer to realize than expected; (xii) the impact of legislative, regulatory, competitive and technological changes; (xiii) expectations for future clinical trials, the timing and potential outcomes of clinical studies and interactions with regulatory authorities; and (xiv) other risks to the consummation of the transaction, including the risk that the transaction will not be consummated within the expected time period or at all. Additional factors that may affect the future results of Lantheus are set forth in its filings with the Securities and Exchange Commission (the “SEC”), including Lantheus’ most recently filed Annual Report on Form 10-K, subsequent Quarterly Reports on Form 10-Q, Current Reports on Form 8-K and other filings with the SEC, which are available on the SEC’s website at www.sec.gov. Readers are urged to consider these factors carefully in evaluating these forward-looking statements, and not to place undue reliance on any forward-looking statements. Readers should also carefully review the risk factors described in other documents that Lantheus files from time to time with the SEC. The forward-looking statements in this document speak only as of the date of these materials. Except as required by law, Lantheus assumes no obligation to update or revise these forward-looking statements for any reason, even if new information becomes available in the future. All trademarks, logos and service marks used in this presentation are the property of their respective owners. Non-GAAP Financial Measures The Company uses non-GAAP financial measures, such as adjusted net income and its line components; adjusted net income per share - fully diluted; adjusted operating income and free cash flow. The Company’s management believes that the presentation of these measures provides useful information to investors. These measures may assist investors in evaluating the Company’s operations, period over period. However, these measures may exclude items that may be highly variable, difficult to predict and of a size that could have a substantial impact on the Company’s reported results of operations for a particular period. Management uses these and other non-GAAP measures internally for evaluation of the performance of the business, including the allocation of resources and the evaluation of results relative to employee performance compensation targets. Investors should consider these non-GAAP measures only as a supplement to, not as a substitute for or as superior to, measures of financial performance prepared in accordance with GAAP. © 2025 Lantheus. All rights reserved.
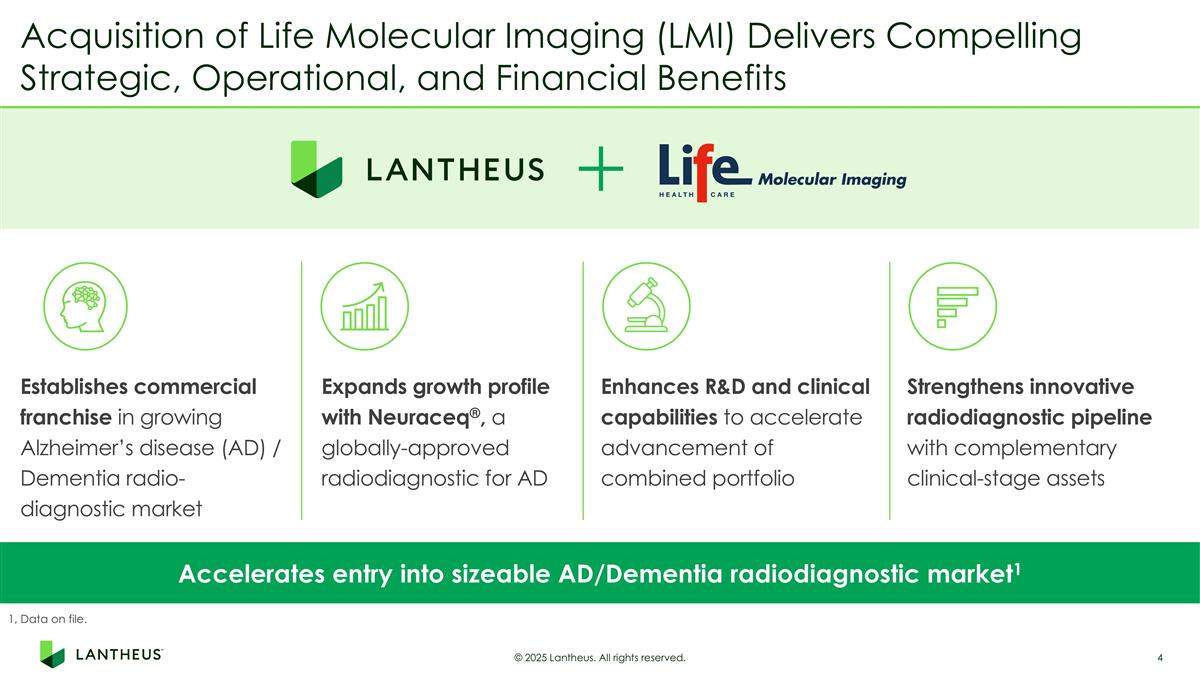
Acquisition of Life Molecular Imaging (LMI) Delivers Compelling Strategic, Operational, and Financial Benefits Accelerates entry into sizeable AD/Dementia radiodiagnostic market1 Establishes commercial franchise in growing Alzheimer’s disease (AD) / Dementia radio-diagnostic market Strengthens innovative radiodiagnostic pipeline with complementary clinical-stage assets Enhances R&D and clinical capabilities to accelerate advancement of combined portfolio Expands growth profile with Neuraceq®, a globally-approved radiodiagnostic for AD © 2025 Lantheus. All rights reserved. 1, Data on file.
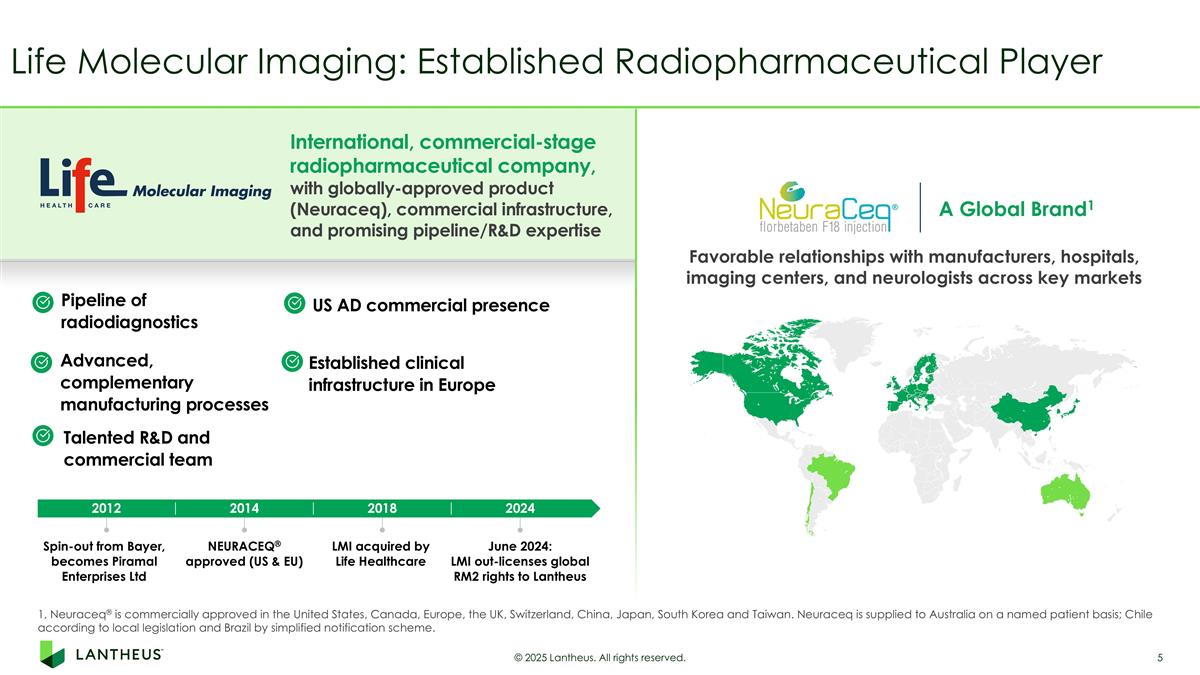
Life Molecular Imaging: Established Radiopharmaceutical Player 1, Neuraceq® is commercially approved in the United States, Canada, Europe, the UK, Switzerland, China, Japan, South Korea and Taiwan. Neuraceq is supplied to Australia on a named patient basis; Chile according to local legislation and Brazil by simplified notification scheme. International, commercial-stage radiopharmaceutical company, with globally-approved product (Neuraceq), commercial infrastructure, and promising pipeline/R&D expertise A Global Brand1 Favorable relationships with manufacturers, hospitals, imaging centers, and neurologists across key markets 2012 Spin-out from Bayer, becomes Piramal Enterprises Ltd NEURACEQ® approved (US & EU) LMI acquired by Life Healthcare June 2024: LMI out-licenses global RM2 rights to Lantheus 2014 2018 2024 Established clinical infrastructure in Europe Pipeline of radiodiagnostics Advanced, complementary manufacturing processes Talented R&D and commercial team © 2025 Lantheus. All rights reserved. US AD commercial presence
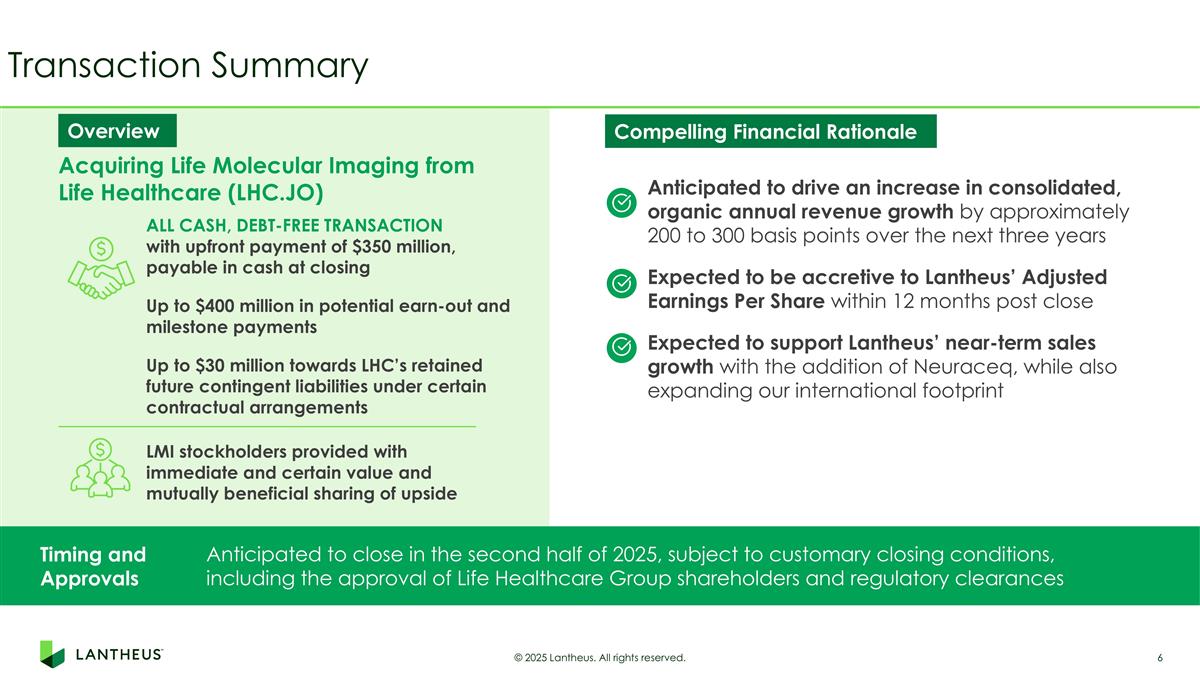
Transaction Summary Timing and Approvals Anticipated to close in the second half of 2025, subject to customary closing conditions, including the approval of Life Healthcare Group shareholders and regulatory clearances Overview Acquiring Life Molecular Imaging from Life Healthcare (LHC.JO) ALL CASH, DEBT-FREE TRANSACTION with upfront payment of $350 million, payable in cash at closing Up to $400 million in potential earn-out and milestone payments Up to $30 million towards LHC’s retained future contingent liabilities under certain contractual arrangements LMI stockholders provided with immediate and certain value and mutually beneficial sharing of upside Compelling Financial Rationale Anticipated to drive an increase in consolidated, organic annual revenue growth by approximately 200 to 300 basis points over the next three years Expected to be accretive to Lantheus’ Adjusted Earnings Per Share within 12 months post close Expected to support Lantheus’ near-term sales growth with the addition of Neuraceq, while also expanding our international footprint © 2025 Lantheus. All rights reserved.
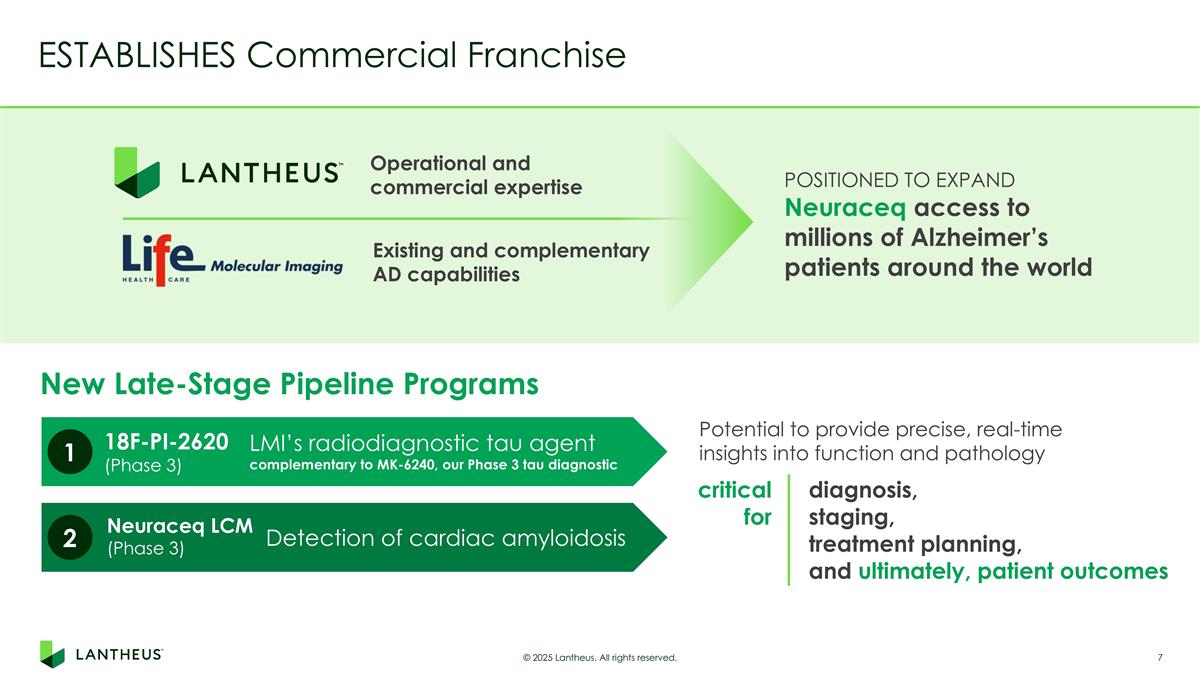
ESTABLISHES Commercial Franchise © 2025 Lantheus. All rights reserved. POSITIONED TO EXPAND Neuraceq access to millions of Alzheimer’s patients around the world New Late-Stage Pipeline Programs Existing and complementary AD capabilities Operational and commercial expertise Potential to provide precise, real-time insights into function and pathology critical for diagnosis, staging, treatment planning, and ultimately, patient outcomes LMI’s radiodiagnostic tau agent complementary to MK-6240, our Phase 3 tau diagnostic 1 18F-PI-2620 (Phase 3) Detection of cardiac amyloidosis 2 Neuraceq LCM (Phase 3)
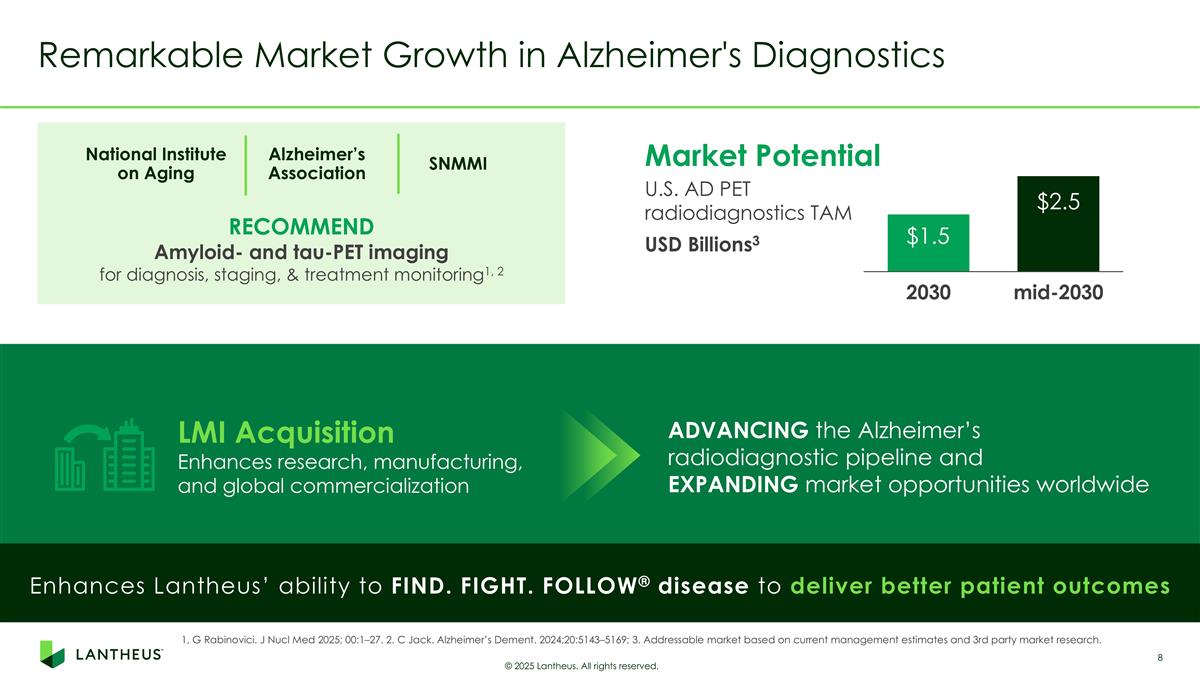
RECOMMEND Amyloid- and tau-PET imaging for diagnosis, staging, & treatment monitoring1, 2 Remarkable Market Growth in Alzheimer's Diagnostics © 2025 Lantheus. All rights reserved. Enhances Lantheus’ ability to FIND. FIGHT. FOLLOW® disease to deliver better patient outcomes ADVANCING the Alzheimer’s radiodiagnostic pipeline and EXPANDING market opportunities worldwide LMI Acquisition Enhances research, manufacturing, and global commercialization Market Potential U.S. AD PET radiodiagnostics TAM USD Billions3 National Institute on Aging Alzheimer’s Association SNMMI 1, G Rabinovici. J Nucl Med 2025; 00:1–27. 2. C Jack. Alzheimer’s Dement. 2024;20:5143–5169; 3. Addressable market based on current management estimates and 3rd party market research.
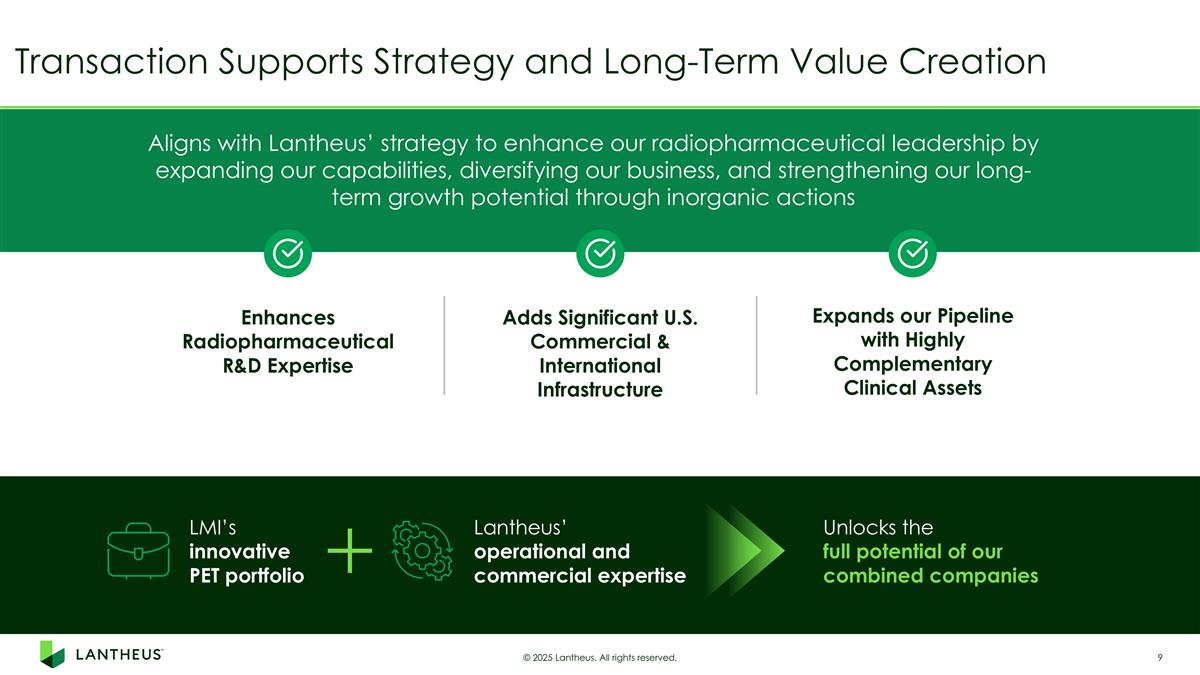
Transaction Supports Strategy and Long-Term Value Creation © 2025 Lantheus. All rights reserved. Aligns with Lantheus’ strategy to enhance our radiopharmaceutical leadership by expanding our capabilities, diversifying our business, and strengthening our long-term growth potential through inorganic actions Lantheus’ operational and commercial expertise LMI’s innovative PET portfolio Unlocks the full potential of our combined companies Enhances Radiopharmaceutical R&D Expertise Adds Significant U.S. Commercial & International Infrastructure Expands our Pipeline with Highly Complementary Clinical Assets

Appendix
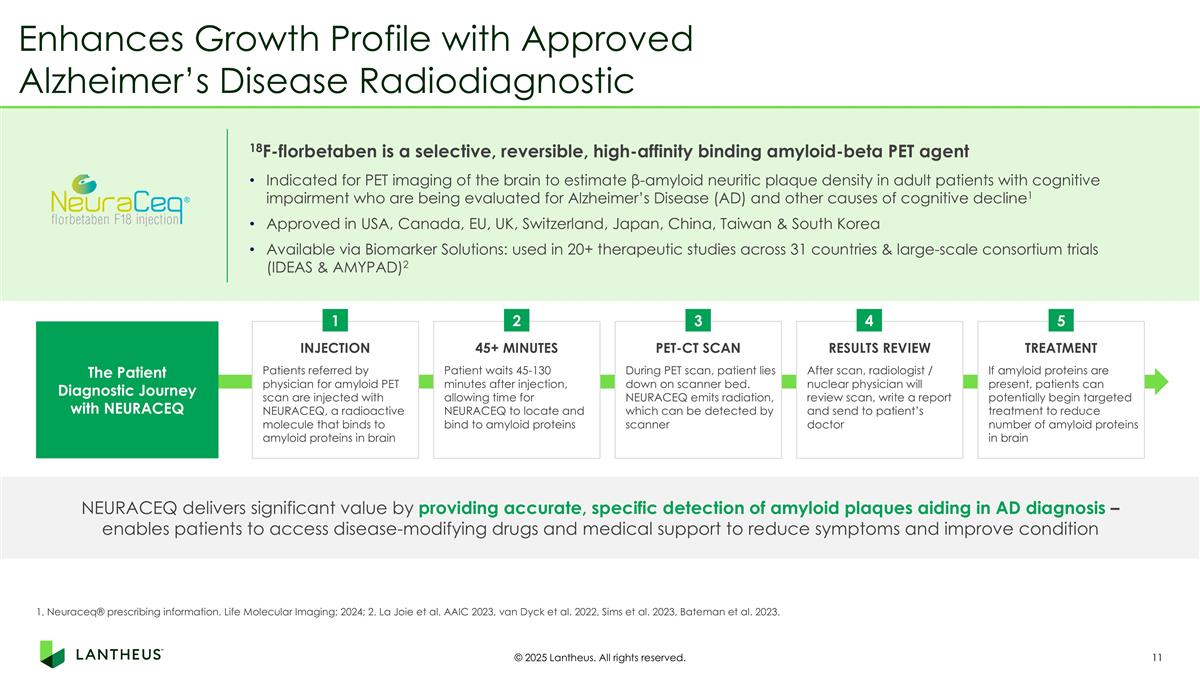
Enhances Growth Profile with Approved Alzheimer’s Disease Radiodiagnostic INJECTION Patients referred by physician for amyloid PET scan are injected with NEURACEQ, a radioactive molecule that binds to amyloid proteins in brain 45+ MINUTES Patient waits 45-130 minutes after injection, allowing time for NEURACEQ to locate and bind to amyloid proteins PET-CT SCAN During PET scan, patient lies down on scanner bed. NEURACEQ emits radiation, which can be detected by scanner RESULTS REVIEW After scan, radiologist / nuclear physician will review scan, write a report and send to patient’s doctor TREATMENT If amyloid proteins are present, patients can potentially begin targeted treatment to reduce number of amyloid proteins in brain The Patient Diagnostic Journey with NEURACEQ 1 2 3 4 5 NEURACEQ delivers significant value by providing accurate, specific detection of amyloid plaques aiding in AD diagnosis – enables patients to access disease-modifying drugs and medical support to reduce symptoms and improve condition 18F-florbetaben is a selective, reversible, high-affinity binding amyloid-beta PET agent © 2025 Lantheus. All rights reserved. Indicated for PET imaging of the brain to estimate β-amyloid neuritic plaque density in adult patients with cognitive impairment who are being evaluated for Alzheimer’s Disease (AD) and other causes of cognitive decline1 Approved in USA, Canada, EU, UK, Switzerland, Japan, China, Taiwan & South Korea Available via Biomarker Solutions: used in 20+ therapeutic studies across 31 countries & large-scale consortium trials (IDEAS & AMYPAD)2 1, Neuraceq® prescribing information. Life Molecular Imaging; 2024; 2. La Joie et al. AAIC 2023, van Dyck et al. 2022, Sims et al. 2023, Bateman et al. 2023.










