Exhibit 99.1

LGSOC Program Update January 24, 2023

2 Today’s Speakers Brian Stuglik Chief Executive Officer, Verastem Oncology Kathleen Moore, MD Assistant Professor at University of Oklahoma Health Sciences Center, Gynecologic Oncologist at University of Oklahoma Health Stephenson Cancer Center and Investigator on RAMP 201 trial Louis Denis, MD Chief Medical Officer, Verastem Oncology Dan Paterson President and Chief Operating Officer, Verastem Oncology

3 Safe Harbor Statement This presentation includes forward - looking statements about, among other things, Verastem Oncology’s programs and product candidates, including anticipated regulatory submissions, approvals, performance and potential benefits of Verastem Oncology’s product candidates, that are subject to substantial risks and uncertainties that could cause actual results to differ materially from those expressed or implied by such statements. Applicable risks and uncertainties include the risks and uncertainties, among other things, regarding: the success in the development and potential commercialization of our product candidates, including defactinib and other compounds in combination with avutometinib (VS - 6766); the occurrence of adverse safety events and/or unexpected concerns that may arise from additional data or analysis or result in unmanageable safety profiles as compared to their levels of efficacy; or our ability to obtain, maintain and enforce patent and other intellectual property protection for our product candidates. Other risks and uncertainties include those identified under the heading “Risk Factors” in the Company’s Annual Report on Form 10 - K for the year ended December 31, 2021, as filed with the Securities and Exchange Commission (SEC) on March 28, 2022, in the Company’s Quarterly Reports on Form 10 - Q for the quarters ended June 30, 2022 and September 30, 2022, as filed with the SEC on August 8, 2022 and November 3, 2022, respectively, and in any subsequent filings with the SEC, which are available at www.sec.gov and www.verastem.com. The forward - looking statements in this presentation speak only as of the original date of this presentation, and we undertake no obligation to update or revise any of these statements.

Brian Stuglik
5 Avutometinib is a Differentiated Agent with the Potential to Serve as the Backbone for Combinations Across RAS Pathway - Driven Cancers • Unique RAF/MEK clamp mechanism of action • Novel intermittent dosing schedule; convenient oral regimen • Breakthrough Therapy Designation in recurrent low - grade serous ovarian cancer • Potential best - in - class safety & tolerability profile relative to marketed MEK inhibitors, with potential for combinability with agents from multiple target classes • Promising signals of clinical activity in various RAS pathway - driven cancers, including in patients whose tumors previously progressed on other MEK inhibitors • Preclinical anti - proliferative activity across multiple MAPK pathway alterations (e.g. KRAS, NRAS, BRAF, NF1 mt) and multiple solid tumor indications • Strong preclinical combination data with other agents targeting RAS pathway and parallel pathways
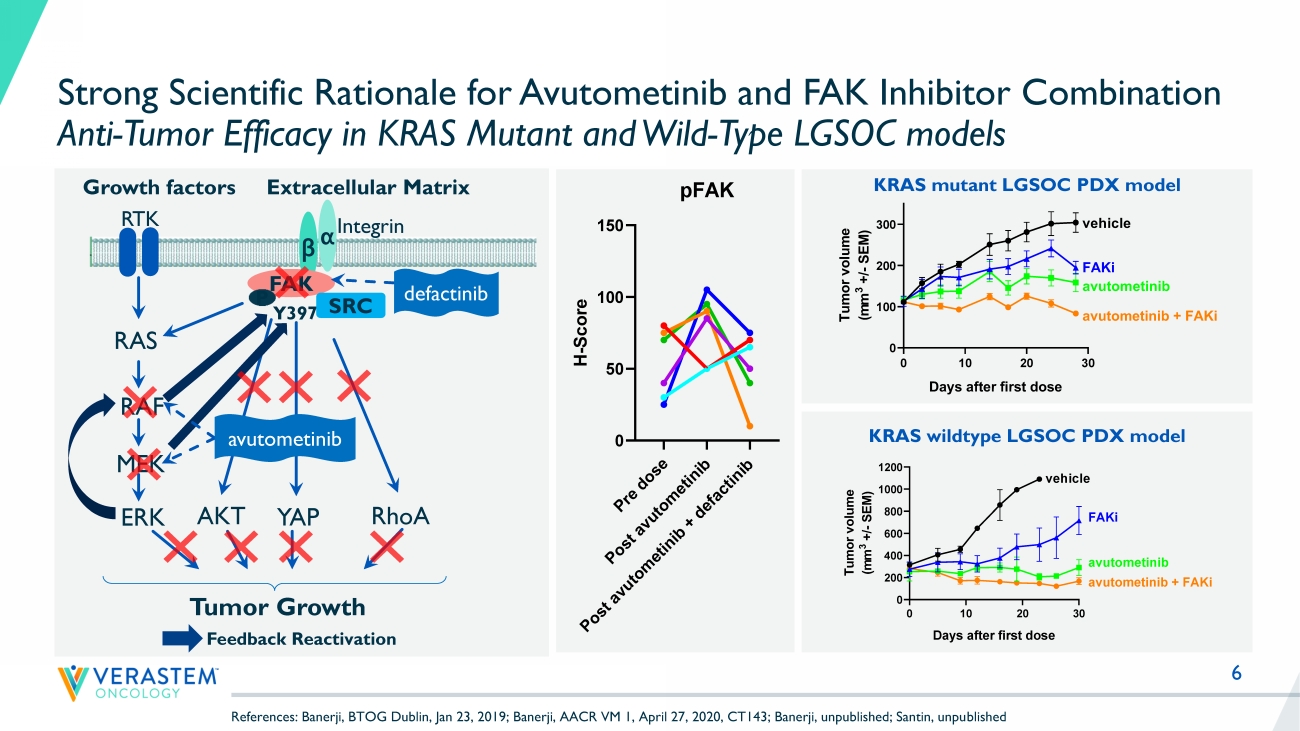
6 Strong Scientific Rationale for Avutometinib and FAK Inhibitor Combination Anti - Tumor Efficacy in KRAS Mutant and Wild - Type LGSOC models KRAS mutant LGSOC PDX model RTK RAS RAF MEK ERK YAP Growth factors β α Y397 Integrin FAK Extracellular Matrix SRC RhoA Tumor Growth P AKT avutometinib defactinib Feedback Reactivation 0 10 20 30 0 100 200 300 Tumor growth Days after first dose T u m o r v o l u m e ( m m 3 + / - S E M ) vehicle FAKi avutometinib avutometinib + FAKi KRAS wildtype LGSOC PDX model P r e d o s e P o s t a v u t o m e t i n i b P o s t a v u t o m e t i n i b + d e f a c t i n i b 0 50 100 150 pFAK H - S c o r e References: Banerji, BTOG Dublin, Jan 23, 2019; Banerji, AACR VM 1, April 27, 2020, CT143; Banerji, unpublished; Santin, unpu bli shed

Kathleen Moore, MD
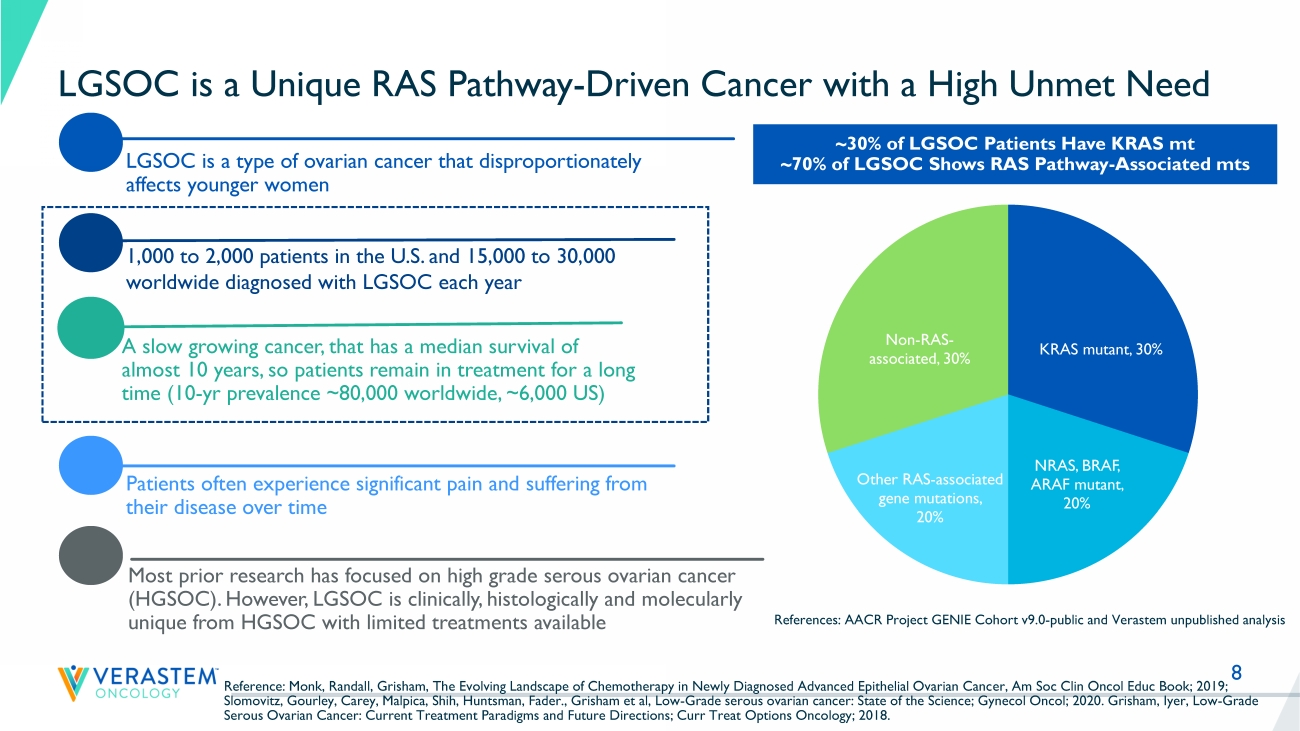
8 LGSOC is a Unique RAS Pathway - Driven Cancer with a High Unmet Need LGSOC is a type of ovarian cancer that disproportionately affects younger women Patients often experience significant pain and suffering from their disease over time A slow growing cancer, that has a median survival of almost 10 years, so patients remain in treatment for a long time (10 - yr prevalence ~80,000 worldwide, ~6,000 US) Most prior research has focused on high grade serous ovarian cancer (HGSOC). However, LGSOC is clinically, histologically and molecularly unique from HGSOC with limited treatments available 1,000 to 2,000 patients in the U.S. and 15,000 to 30,000 worldwide diagnosed with LGSOC each year ~30% of LGSOC Patients Have KRAS mt ~70% of LGSOC Shows RAS Pathway - Associated mts References: AACR Project GENIE Cohort v9.0 - public and Verastem unpublished analysis Reference: Monk, Randall, Grisham, The Evolving Landscape of Chemotherapy in Newly Diagnosed Advanced Epithelial Ovarian Canc er, Am Soc Clin Oncol Educ Book; 2019; Slomovitz, Gourley, Carey, Malpica, Shih, Huntsman, Fader., Grisham et al, Low - Grade serous ovarian cancer: State of the Science ; Gynecol Oncol; 2020. Grisham, Iyer, Low - Grade Serous Ovarian Cancer: Current Treatment Paradigms and Future Directions; Curr Treat Options Oncology; 2018. KRAS mutant , 30% NRAS, BRAF, ARAF mutant , 20% Other RAS - associated gene mutations , 20% Non - RAS - associated , 30%
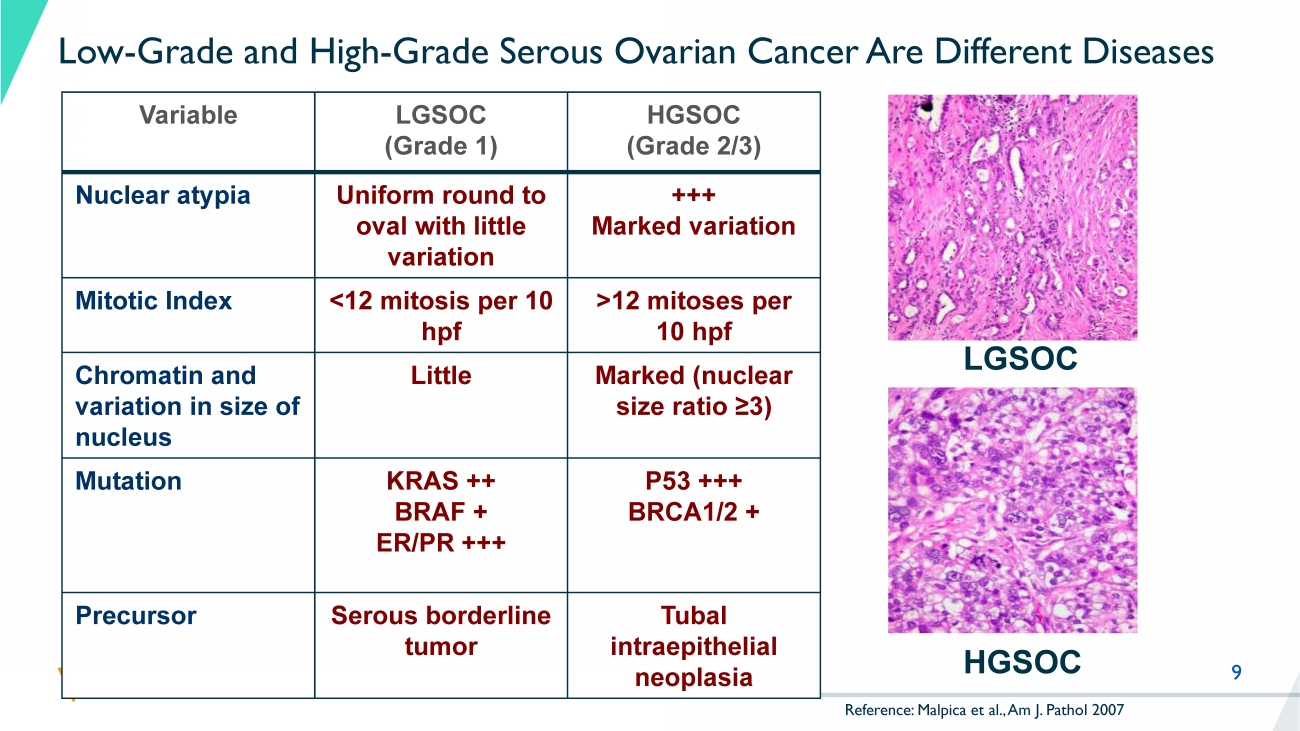
9 Low - Grade and High - Grade Serous Ovarian Cancer Are Different Diseases Variable LGSOC (Grade 1) HGSOC (Grade 2/3) Nuclear atypia Uniform round to oval with little variation +++ Marked variation Mitotic Index <12 mitosis per 10 hpf >12 mitoses per 10 hpf Chromatin and variation in size of nucleus Little Marked (nuclear size ratio ≥3) Mutation KRAS ++ BRAF + ER/PR +++ P53 +++ BRCA1/2 + Precursor Serous borderline tumor Tubal intraepithelial neoplasia LGSOC HGSOC Reference: Malpica et al., Am J. Pathol 2007
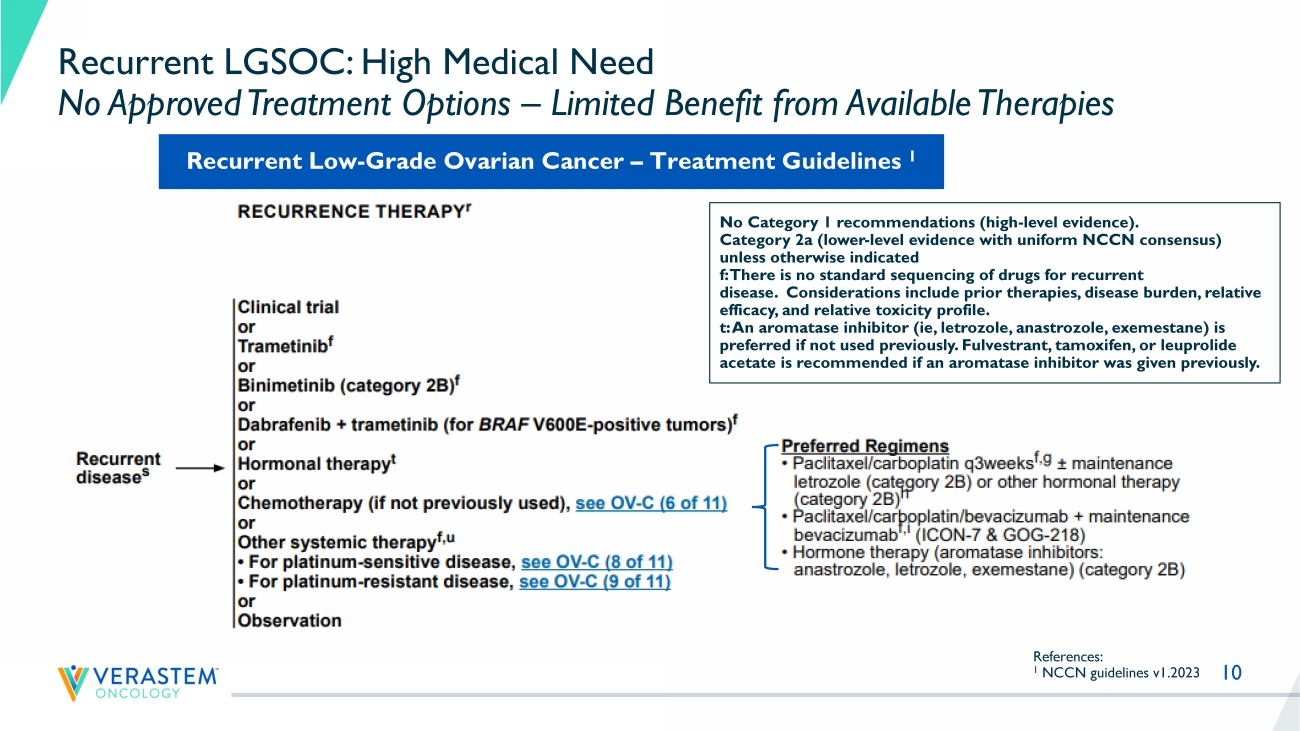
10 Recurrent LGSOC: High Medical Need No Approved Treatment Options – Limited Benefit from Available Therapies References: 1 NCCN guidelines v1.2023 No Category 1 recommendations (high - level evidence). Category 2a (lower - level evidence with uniform NCCN consensus) unless otherwise indicated f: There is no standard sequencing of drugs for recurrent disease. Considerations include prior therapies, disease burden, relative efficacy, and relative toxicity profile. t: An aromatase inhibitor ( ie , letrozole, anastrozole, exemestane) is preferred if not used previously. Fulvestrant , tamoxifen, or leuprolide acetate is recommended if an aromatase inhibitor was given previously. Recurrent Low - Grade Ovarian Cancer – Treatment Guidelines 1

11 Recent LGSOC Trials Highlight High Unmet Need Trial Number of Prior Systemic Therapies Median (range) Prior MEK allowed Prior Bevacizumab Therapy Response Rate ORR Image Assessment Median PFS Months (95% CI) Discontinuation Rate due to AEs GOG 281 1 3 (1 - 10) No * Low % SoC 6% INV 7.2 (5.6 - 9.9) 12 % Trametinib 26% INV 13.0 (9.9 - 15.0) 35% MILO 2 2 (1 - 8) No * Low % SoC 13% BICR 10.6 (9.2 to 14.5) 17% Binimetinib 2 16% BICR 9.1 (7.3 - 11.3) 31% 1 Study GOG 281 trial Gershenson et al., Lancet 2022 2 MILO Study Monk et al., J Clin Oncol 2020. SoC = Standard of Care (endocrine / chemotherapy) INV: Investigator BICR: Blinded independent central review PFS = Progression free survival CI = confidence interval NR = Not reached * Low historical use of bevacizumab during trial conduct. % not reported MILO: no more than 3 lines of prior chemotherapy MILO GOG281 LD0 LB1LB2LB3
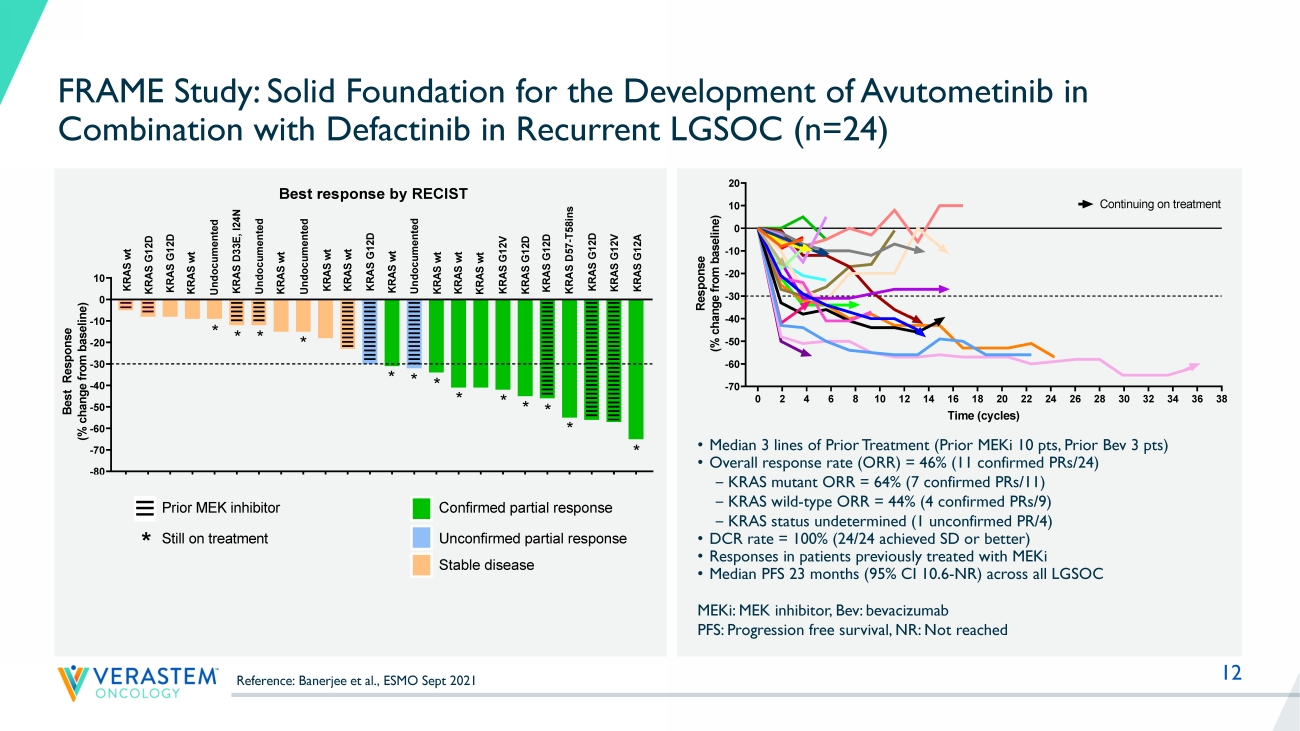
12 0 2 4 6 8 10 12 14 16 18 20 22 24 26 28 30 32 34 36 38 -70 -60 -50 -40 -30 -20 -10 0 10 20 Response by RECIST Time (cycles) R e s p o n s e ( % c h a n g e f r o m b a s e l i n e ) FRA101001 - KRAS G12V FRA101002 - KRAS G12A FRA101009 - KRAS G12D FRA101012 - KRAS WT FRA101007 - KRAS WT FRA101014 - KRAS G12D FRA101015 - KRAS WT FRA101019 - KRAS G12D FRA101024 - KRAS WT FRA101025 - KRAS WT FRA102010 - KRAS WT FRA101028 - undocumented FRA101032 - KRAS D33E, I24N FRA101033 - KRAS G12D FRA101035 - KRAS G12D FRA101037 - KRAS WT FRA101038 - KRAS WT Continuing on treatment FRA101039 - KRAS WT FRA103001 - KRAS G12V FRA104001- KRAS D57-T58ins FRA103002 - undocumented FRA101042 - KRAS G12D FRA103003 - KRAS WT FRA102018 - KRAS WT FRAME Study: Solid Foundation for the Development of Avutometinib in Combination with Defactinib in Recurrent LGSOC (n=24) MEKi : MEK inhibitor, Bev: bevacizumab PFS: Progression free survival, NR: Not reached • Median 3 lines of Prior Treatment (Prior MEKi 10 pts, Prior Bev 3 pts) • Overall response rate (ORR) = 46% (11 confirmed PRs/24) ‒ KRAS mutant ORR = 64% (7 confirmed PRs/11) ‒ KRAS wild - type ORR = 44% (4 confirmed PRs/9) ‒ KRAS status undetermined (1 unconfirmed PR/4) • DCR rate = 100% (24/24 achieved SD or better) • Responses in patients previously treated with MEKi • Median PFS 23 months (95% CI 10.6 - NR) across all LGSOC F R A 1 0 1 0 0 7 F R A 1 0 1 0 1 4 F R A 1 0 1 0 4 2 F R A 1 0 1 0 1 2 F R A 1 0 3 0 0 3 F R A 1 0 1 0 3 2 F R A 1 0 3 0 0 2 F R A 1 0 1 0 3 8 F R A 1 0 2 0 1 8 F R A 1 0 1 0 1 5 F R A 1 0 2 0 1 0 F R A 1 0 1 0 1 9 F R A 1 0 1 0 2 4 F R A 1 0 1 0 2 8 F R A 1 0 1 0 3 9 F R A 1 0 1 0 2 5 F R A 1 0 1 0 3 7 F R A 1 0 3 0 0 1 F R A 1 0 1 0 3 5 F R A 1 0 1 0 3 3 F R A 1 0 4 0 0 1 F R A 1 0 1 0 0 9 F R A 1 0 1 0 0 1 F R A 1 0 1 0 0 2 -80 -70 -60 -50 -40 -30 -20 -10 0 10 B e s t R e s p o n s e ( % c h a n g e f r o m b a s e l i n e ) U n d o c u m e n t e d K R A S w t K R A S w t K R A S G 1 2 D K R A S w t K R A S D 3 3 E , I 2 4 N U n d o c u m e n t e d Prior MEK inhibitor * * K R A S G 1 2 A K R A S G 1 2 V K R A S G 1 2 D K R A S G 1 2 V * K R A S w t K R A S G 1 2 D Confirmed partial response Unconfirmed partial response K R A S w t K R A S w t * * Best response by RECIST K R A S w t K R A S G 1 2 D K R A S w t K R A S w t K R A S G 1 2 D K R A S D 5 7 - T 5 8 i n s * * * * * * Still on treatment * K R A S G 1 2 D U n d o c u m e n t e d * * U n d o c u m e n t e d Stable disease F R A 1 0 1 0 0 7 F R A 1 0 1 0 1 4 F R A 1 0 1 0 4 2 F R A 1 0 1 0 1 2 F R A 1 0 3 0 0 3 F R A 1 0 1 0 3 2 F R A 1 0 3 0 0 2 F R A 1 0 1 0 3 8 F R A 1 0 2 0 1 8 F R A 1 0 1 0 1 5 F R A 1 0 2 0 1 0 F R A 1 0 1 0 1 9 F R A 1 0 1 0 2 4 F R A 1 0 1 0 2 8 F R A 1 0 1 0 3 9 F R A 1 0 1 0 2 5 F R A 1 0 1 0 3 7 F R A 1 0 3 0 0 1 F R A 1 0 1 0 3 5 F R A 1 0 1 0 3 3 F R A 1 0 4 0 0 1 F R A 1 0 1 0 0 9 F R A 1 0 1 0 0 1 F R A 1 0 1 0 0 2 -80 -70 -60 -50 -40 -30 -20 -10 0 10 B e s t R e s p o n s e ( % c h a n g e f r o m b a s e l i n e ) U n d o c u m e n t e d K R A S w t K R A S w t K R A S G 1 2 D K R A S w t K R A S D 3 3 E , I 2 4 N U n d o c u m e n t e d Prior MEK inhibitor * * K R A S G 1 2 A K R A S G 1 2 V K R A S G 1 2 D K R A S G 1 2 V * K R A S w t K R A S G 1 2 D Confirmed partial response Unconfirmed partial response K R A S w t K R A S w t * * Best response by RECIST K R A S w t K R A S G 1 2 D K R A S w t K R A S w t K R A S G 1 2 D K R A S D 5 7 - T 5 8 i n s * * * * * * Still on treatment * K R A S G 1 2 D U n d o c u m e n t e d * * U n d o c u m e n t e d Stable disease Reference: Banerjee et al., ESMO Sept 2021

Louis Denis, MD
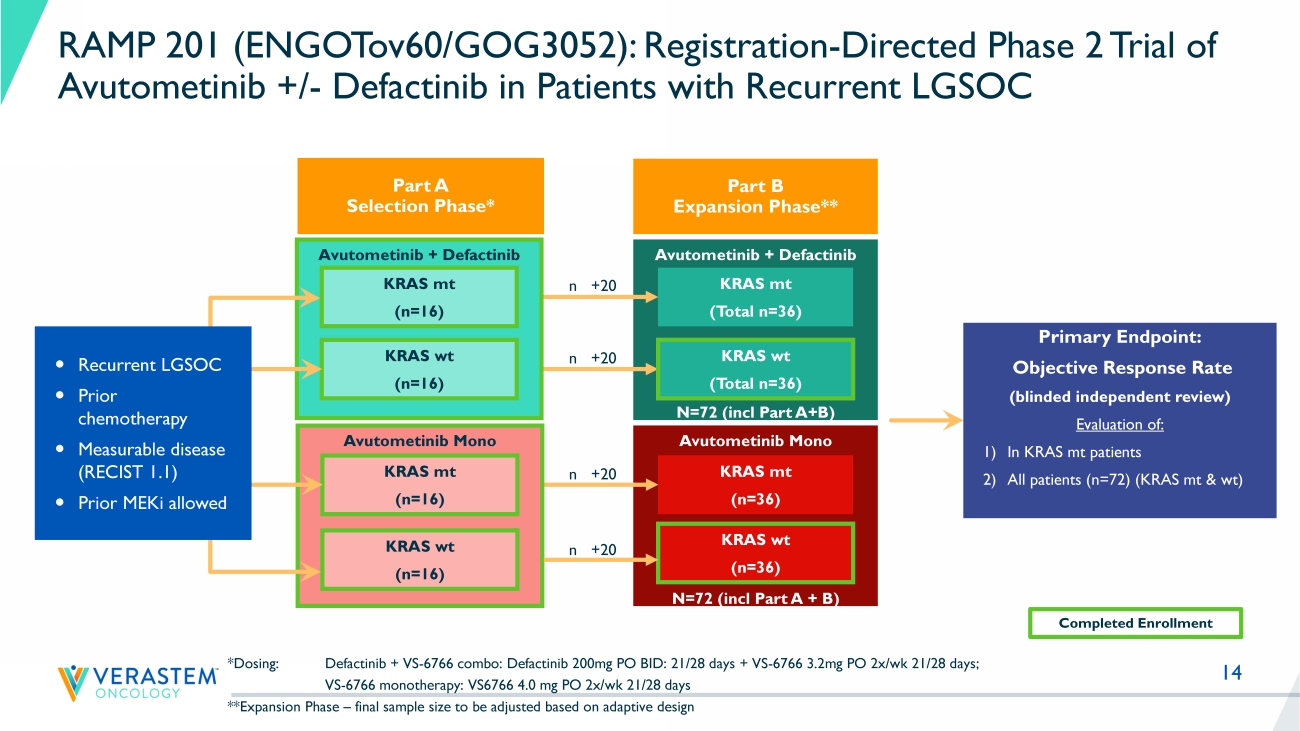
14 Avutometinib Mono N=72 (incl Part A + B) Avutometinib + Defactinib N=72 (incl Part A+B) Avutometinib + Defactinib *Dosing: Defactinib + VS - 6766 combo: Defactinib 200mg PO BID: 21/28 days + VS - 6766 3.2mg PO 2x/ wk 21/28 days; VS - 6766 monotherapy: VS6766 4.0 mg PO 2x/ wk 21/28 days **Expansion Phase – final sample size to be adjusted based on adaptive design KRAS mt (n=16) KRAS wt (n=16) Primary Endpoint: Objective Response Rate (blinded independent review) Evaluation of: 1) In KRAS mt patients 2) All patients (n=72) (KRAS mt & wt ) Avutometinib Mono KRAS mt (n=16) KRAS wt (n=16) KRAS mt (n=36) KRAS wt (n=36) KRAS mt ( Total n =36) KRAS wt ( Total n =36) Recurrent LGSOC Prior chemotherapy Measurable disease (RECIST 1.1) Prior MEKi allowed Completed Enrollment n +20 n +20 n +20 n +20 RAMP 201 (ENGOTov60/GOG3052): Registration - Directed Phase 2 Trial of Avutometinib +/ - Defactinib in Patients with Recurrent LGSOC Part A Selection Phase* Part B Expansion Phase**
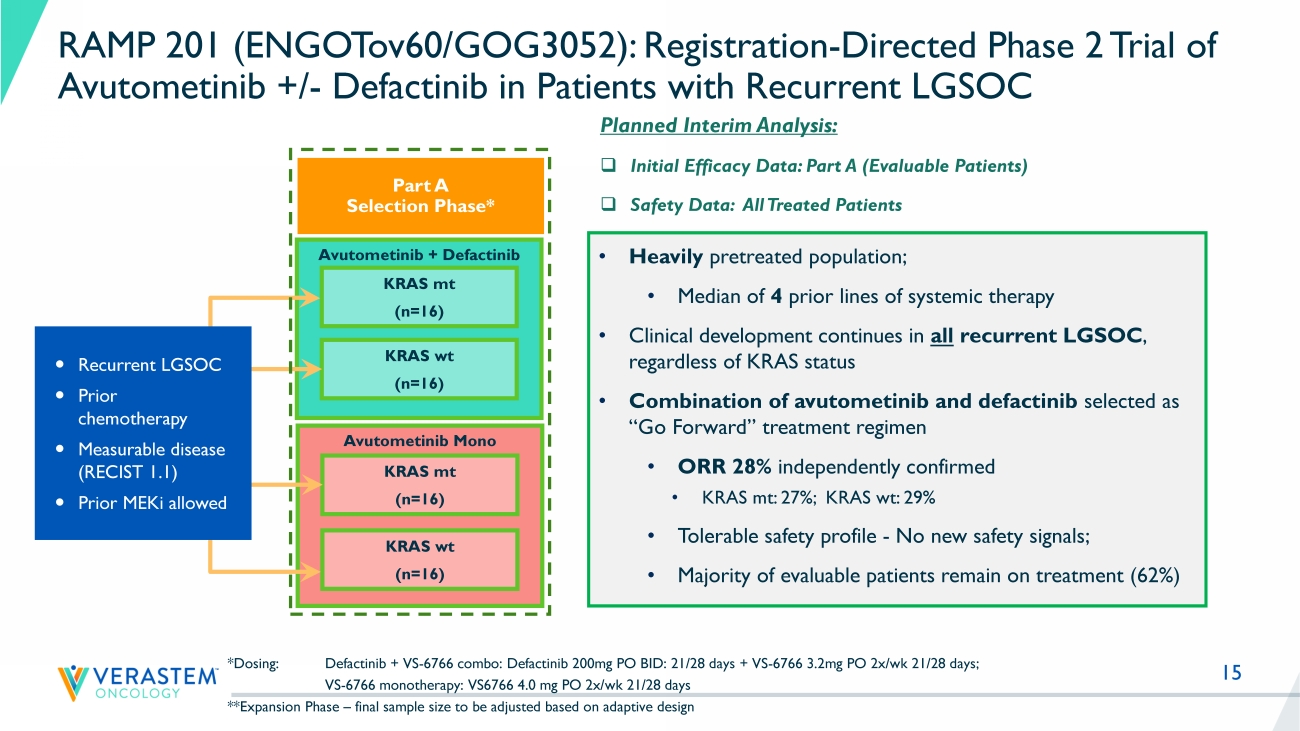
15 Avutometinib + Defactinib *Dosing: Defactinib + VS - 6766 combo: Defactinib 200mg PO BID: 21/28 days + VS - 6766 3.2mg PO 2x/ wk 21/28 days; VS - 6766 monotherapy: VS6766 4.0 mg PO 2x/ wk 21/28 days **Expansion Phase – final sample size to be adjusted based on adaptive design KRAS mt (n=16) KRAS wt (n=16) Avutometinib Mono KRAS mt (n=16) KRAS wt (n=16) Recurrent LGSOC Prior chemotherapy Measurable disease (RECIST 1.1) Prior MEKi allowed RAMP 201 (ENGOTov60/GOG3052): Registration - Directed Phase 2 Trial of Avutometinib +/ - Defactinib in Patients with Recurrent LGSOC Part A Selection Phase* Planned Interim Analysis: □ Initial Efficacy Data: Part A (Evaluable Patients) □ Safety Data: All Treated Patients • Heavily pretreated population; • M edian of 4 prior lines of systemic therapy • Clinical development continues in all recurrent LGSOC , regardless of KRAS status • Combination of avutometinib and defactinib selected as “Go Forward” treatment regimen • ORR 28% independently confirmed • KRAS mt: 27 %; KRAS wt : 29% • Tolerable safety profile - No new safety signals; • Majority of evaluable patients remain on treatment ( 62 %)
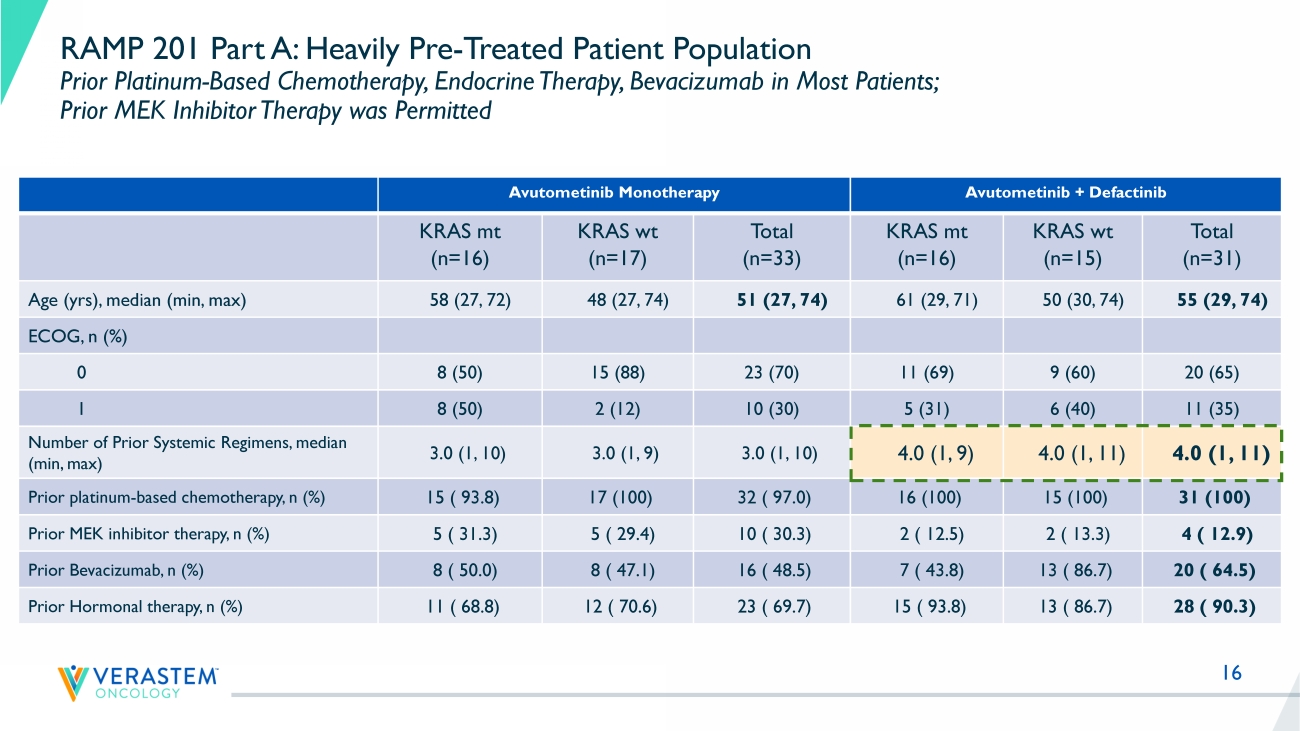
16 RAMP 201 Part A: Heavily Pre - Treated Patient Population Prior Platinum - Based Chemotherapy, Endocrine Therapy, Bevacizumab in Most Patients; Prior MEK Inhibitor Therapy was Permitted Avutometinib Monotherapy Avutometinib + Defactinib KRAS mt (n=16) KRAS wt (n=17) Total (n=33) KRAS mt (n=16) KRAS wt (n=15) Total (n=31) Age ( yrs ), median (min, max) 58 (27, 72) 48 (27, 74) 51 (27, 74) 61 (29, 71) 50 (30, 74) 55 (29, 74) ECOG, n (%) 0 8 (50) 15 (88) 23 (70) 11 (69) 9 (60) 20 (65) 1 8 (50) 2 (12) 10 (30) 5 (31) 6 (40) 11 (35) Number of Prior Systemic Regimens, median (min, max) 3.0 (1, 10) 3.0 (1, 9) 3.0 (1, 10) 4.0 (1, 9) 4.0 (1, 11) 4.0 (1, 11) Prior platinum - based chemotherapy, n (%) 15 ( 93.8) 17 (100) 32 ( 97.0) 16 (100) 15 (100) 31 (100) Prior MEK inhibitor therapy, n (%) 5 ( 31.3) 5 ( 29.4) 10 ( 30.3) 2 ( 12.5) 2 ( 13.3) 4 ( 12.9) Prior Bevacizumab, n (%) 8 ( 50.0) 8 ( 47.1) 16 ( 48.5) 7 ( 43.8) 13 ( 86.7) 20 ( 64.5) Prior Hormonal therapy, n (%) 11 ( 68.8) 12 ( 70.6) 23 ( 69.7) 15 ( 93.8) 13 ( 86.7) 28 ( 90.3)
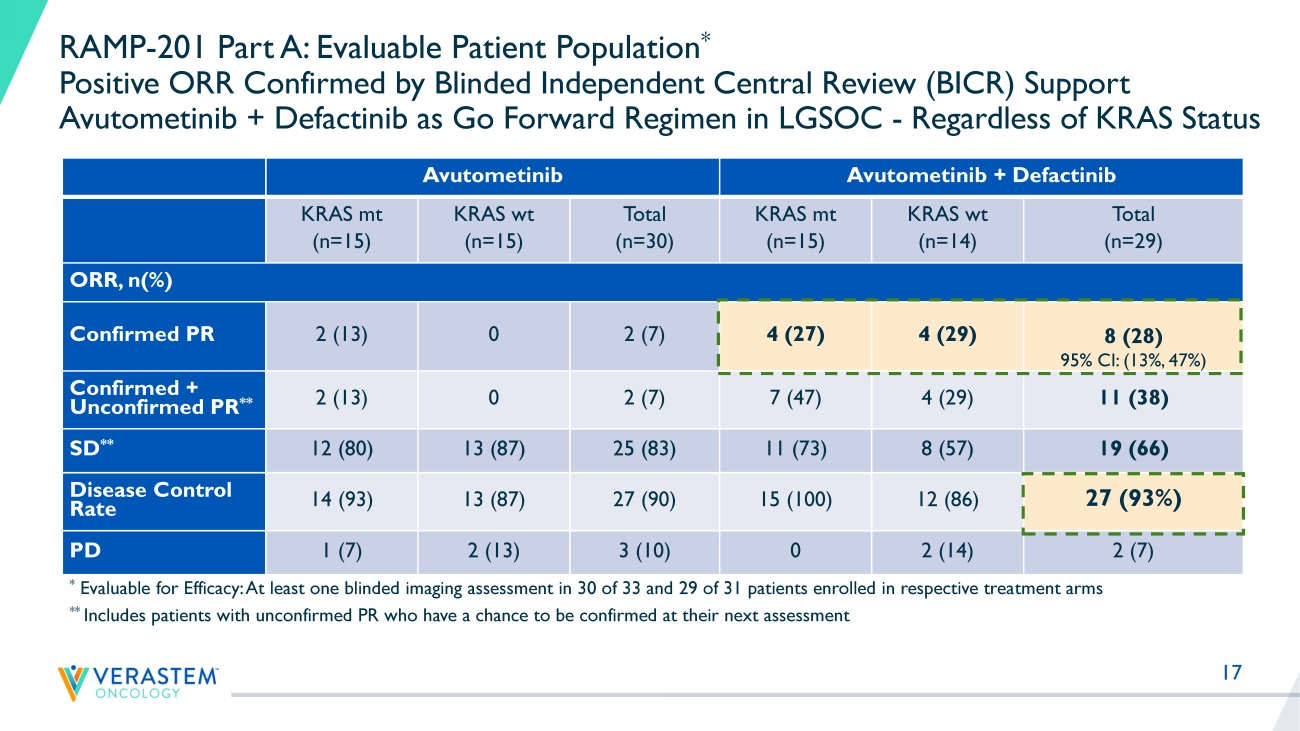
17 RAMP - 201 Part A: Evaluable Patient Population * Positive ORR Confirmed by Blinded Independent Central Review (BICR) Support Avutometinib + Defactinib as Go Forward Regimen in LGSOC - Regardless of KRAS Status Avutometinib Avutometinib + Defactinib KRAS mt (n=15) KRAS wt (n=15) Total (n=30) KRAS mt (n=15) KRAS wt (n=14) Total (n=29) ORR, n(%) Confirmed PR 2 (13) 0 2 (7) 4 (27) 4 (29) 8 (28) 95% CI: (13%, 47%) Confirmed + Unconfirmed PR ** 2 (13) 0 2 (7) 7 (47) 4 (29) 11 (38) SD ** 12 (80) 13 (87) 25 (83) 11 (73) 8 (57) 19 (66) Disease Control Rate 14 (93) 13 (87) 27 (90) 15 (100) 12 (86) 27 (93%) PD 1 (7) 2 (13) 3 (10) 0 2 (14) 2 (7) * Evaluable for Efficacy: At least one blinded imaging assessment in 30 of 33 and 29 of 31 patients enrolled in respective trea tm ent arms ** Includes patients with unconfirmed PR who have a chance to be confirmed at their next assessment
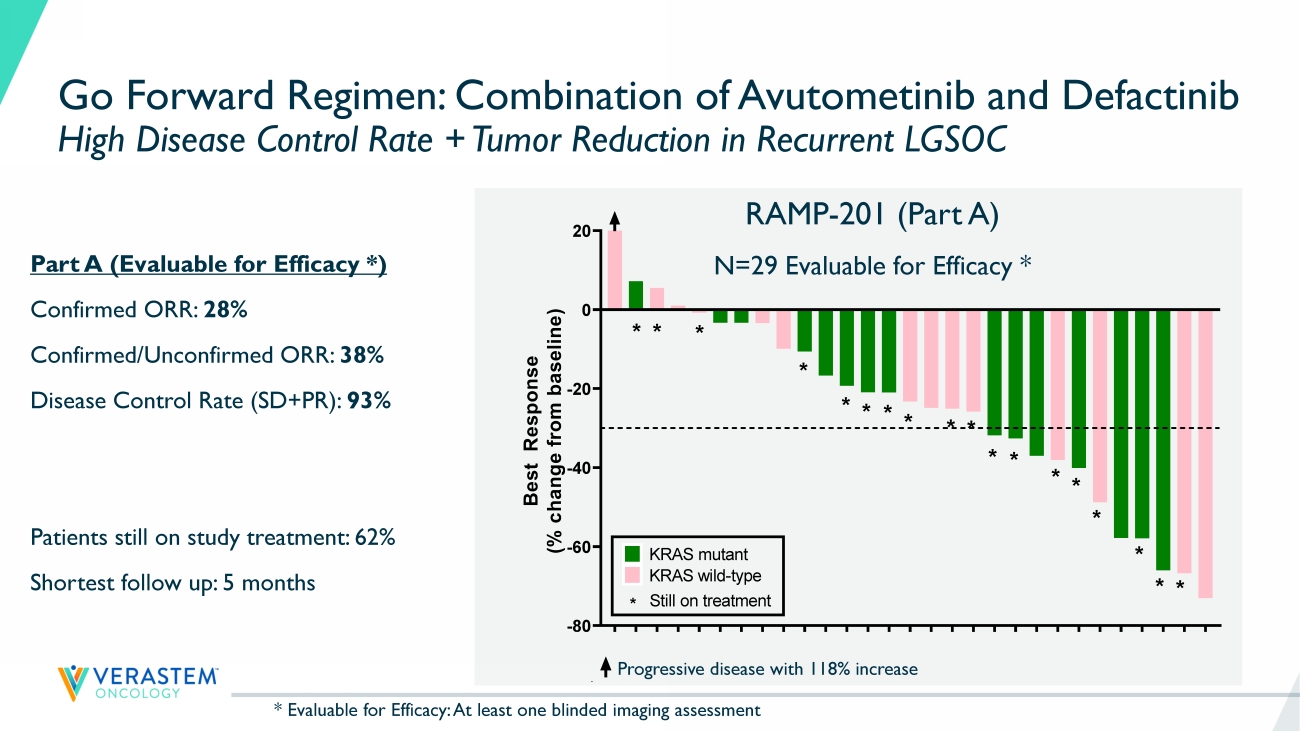
18 K R A S - W T K R A S - M U K R A S - W T K R A S - W T K R A S - W T K R A S - M U K R A S - M U K R A S - W T K R A S - W T K R A S - M U K R A S - M U K R A S - M U K R A S - M U K R A S - M U K R A S - W T K R A S - W T K R A S - W T K R A S - W T K R A S - M U K R A S - M U K R A S - M U K R A S - W T K R A S - M U K R A S - W T K R A S - M U K R A S - M U K R A S - M U K R A S - W T K R A S - W T -80 -60 -40 -20 0 20 B e s t R e s p o n s e ( % c h a n g e f r o m b a s e l i n e ) Best response by RECIST * * * * * * * * * * * * * * * * * * KRAS mutant KRAS wild-type * Still on treatment Go Forward Regimen: Combination of Avutometinib and Defactinib High Disease Control Rate + Tumor Reduction in Recurrent LGSOC Progressive disease with 118% increase Part A (Evaluable for Efficacy *) Confirmed ORR: 28% Confirmed/Unconfirmed ORR: 38% Disease Control Rate (SD+PR): 93% Patients still on study treatment: 62% Shortest follow up: 5 months RAMP - 201 (Part A) N=29 Evaluable for Efficacy * K R A S - W T K R A S - M U K R A S - W T K R A S - W T K R A S - W T K R A S - M U K R A S - M U K R A S - W T K R A S - W T K R A S - M U K R A S - M U K R A S - M U K R A S - M U K R A S - M U K R A S - W T K R A S - W T K R A S - W T K R A S - W T K R A S - M U K R A S - M U K R A S - M U K R A S - W T K R A S - M U K R A S - W T K R A S - M U K R A S - M U K R A S - M U K R A S - W T K R A S - W T -80 -60 -40 -20 0 20 B e s t R e s p o n s e ( % c h a n g e f r o m b a s e l i n e ) Best response by RECIST ** * * * * * * * * * * * * * * * * KRAS mutant KRAS wild-type * Still on treatment * Evaluable for Efficacy: At least one blinded imaging assessment
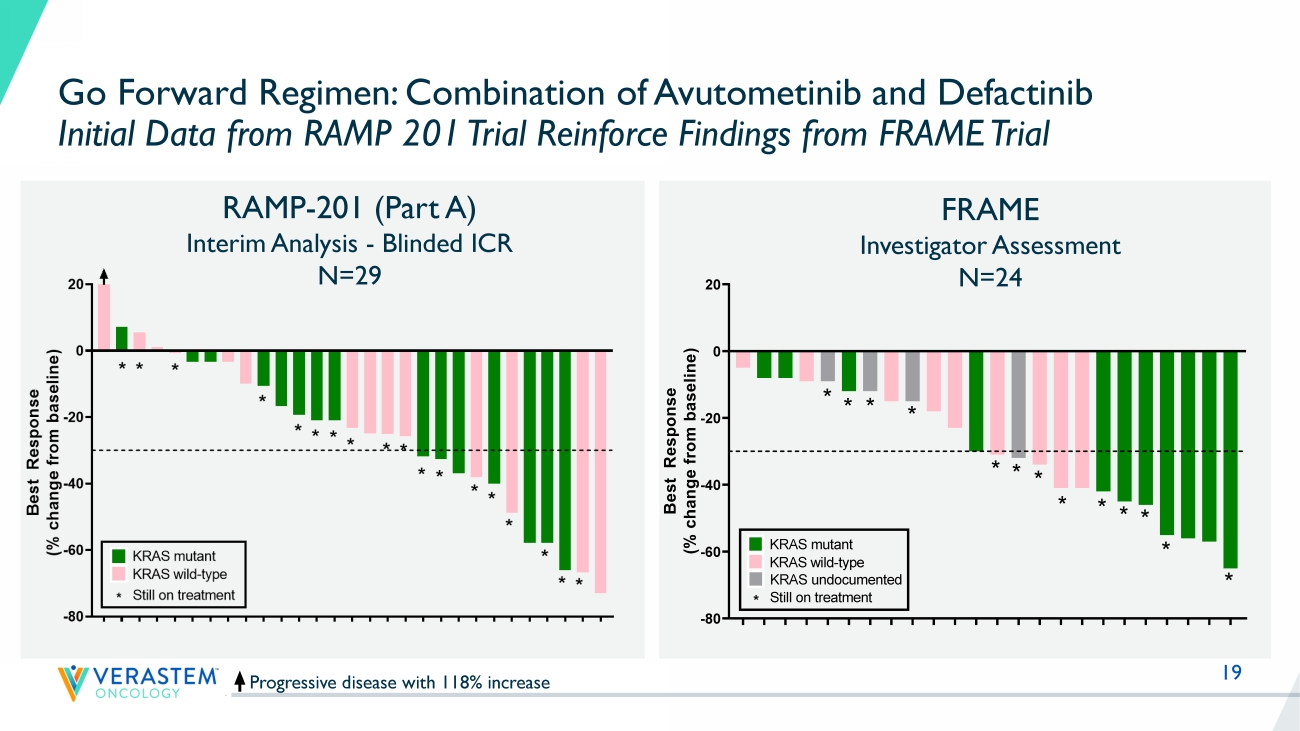
19 Progressive disease with 118% increase RAMP - 201 (Part A) Interim Analysis - Blinded ICR N=29 Go Forward Regimen: Combination of Avutometinib and Defactinib Initial Data from RAMP 201 Trial Reinforce Findings from FRAME Trial Reference: Banerjee et al., ESMO Sept 2021 FRAME Investigator Assessment N=24 K R A S - W T K R A S - M U K R A S - W T K R A S - W T K R A S - W T K R A S - M U K R A S - M U K R A S - W T K R A S - W T K R A S - M U K R A S - M U K R A S - M U K R A S - M U K R A S - M U K R A S - W T K R A S - W T K R A S - W T K R A S - W T K R A S - M U K R A S - M U K R A S - M U K R A S - W T K R A S - M U K R A S - W T K R A S - M U K R A S - M U K R A S - M U K R A S - W T K R A S - W T -80 -60 -40 -20 0 20 B e s t R e s p o n s e ( % c h a n g e f r o m b a s e l i n e ) Best response by RECIST * * * * * * * * * * * * * * * * * * KRAS mutant KRAS wild-type * Still on treatment F R A 1 0 1 0 0 7 F R A 1 0 1 0 1 4 F R A 1 0 1 0 4 2 F R A 1 0 1 0 1 2 F R A 1 0 3 0 0 3 F R A 1 0 1 0 3 2 F R A 1 0 3 0 0 2 F R A 1 0 1 0 3 8 F R A 1 0 2 0 1 8 F R A 1 0 1 0 1 5 F R A 1 0 2 0 1 0 F R A 1 0 1 0 1 9 F R A 1 0 1 0 2 4 F R A 1 0 1 0 2 8 F R A 1 0 1 0 3 9 F R A 1 0 1 0 2 5 F R A 1 0 1 0 3 7 F R A 1 0 3 0 0 1 F R A 1 0 1 0 3 5 F R A 1 0 1 0 3 3 F R A 1 0 4 0 0 1 F R A 1 0 1 0 0 9 F R A 1 0 1 0 0 1 F R A 1 0 1 0 0 2 -80 -60 -40 -20 0 20 B e s t R e s p o n s e ( % c h a n g e f r o m b a s e l i n e ) Best response by RECIST * * * * * * * * * * * * * KRAS mutant KRAS wild-type * Still on treatment KRAS undocumented

20 Any Grade (%) Grade ≥ 3 (%) Avutometinib Monotherapy Avutometinib + Defactinib Avutometinib Monotherapy Avutometinib + Defactinib KRAS mt (n=26) % KRAS wt (n=38) % Total (n=64) % KRAS mt (n=22) % KRAS wt (n=35) % Total (n=57) % KRAS mt (n=26) % KRAS wt (n=38) % Total (n=64) % KRAS mt (n=22) % KRAS wt (n=35) % Total (n=57) % Nausea 23.1 39.5 32.8 45.5 65.7 57.9 0 5.3 3.1 0 0 0 Diarrhea 57.7 55.3 56.3 54.5 51.4 52.6 3.8 0 1.6 4.5 2.9 3.5 Edema peripheral 26.9 28.9 28.1 36.4 48.6 43.9 0 0 0 0 0 0 Fatigue 30.8 31.6 31.3 40.9 40.0 40.4 3.8 0 1.6 9.1 2.9 5.3 Vision blurred 23.1 44.7 35.9 31.8 42.9 38.6 0 2.6 1.6 0 0 0 Vomiting 19.2 26.3 23.4 40.9 37.1 38.6 0 2.6 1.6 0 0 0 Blood creatine phosphokinase increased 26.9 50.0 40.6 40.9 37.1 38.6 7.7 18.4 14.1 18.2 14.3 15.8 Dermatitis acneiform 30.8 36.8 34.4 27.3 45.7 38.6 0 10.5 6.3 4.5 0 1.8 Rash 34.6 28.9 31.3 31.8 37.1 35.1 0 5.3 3.1 0 2.9 1.8 Dry skin 26.9 31.6 29.7 13.6 31.4 24.6 0 0 0 0 0 0 Safety and Tolerability Profile of Avutometinib + Defactinib No New Safety Signals; Few Discontinuation s Due to Adverse Events Most Common Treatment - Related Adverse Events (>20%) in All Treated Patients • Majority of adverse events are mild to moderate • Adverse events are monitorable, manageable and reversible • Few discontinuations due to adverse events (9% in combo n=5 – includes 3 pts for elevated blood CPK laboratory values)
21 Positive Interim Data Supports Selection of Combination of Avutometinib + Defactinib for Continued Development in Recurrent LGSOC – Regardless of KRAS Status Planned Interim Analysis of RAMP - 201 (ENGOTov60/GOG3052) trial • Registration - directed Phase 2 trial of avutometinib +/ - defactinib in recurrent LGSOC Objectives of Part A (“Selection Phase”) achieved • Combination of avutometinib + defactinib declared as ‘Go Forward Treatment Regimen' • Objective response rate by blinded independent review supports continued development in both KRAS mt and KRAS wt LGSOC Positive interim data of the Go Forward Regimen in heavily pretreated recurrent LGSOC • Evidence of disease control and tumor regression in the vast majority of patients • No new safety signals; few discontinuations due to adverse events Initial combination data from RAMP 201 reinforce findings from earlier clinical trial (FRAME) • Majority of patients still on study; mature data with longer follow up to characterize final ORR and duration of response • Enrollment continues for the combination of avutometinib and defactinib

Dan Paterson
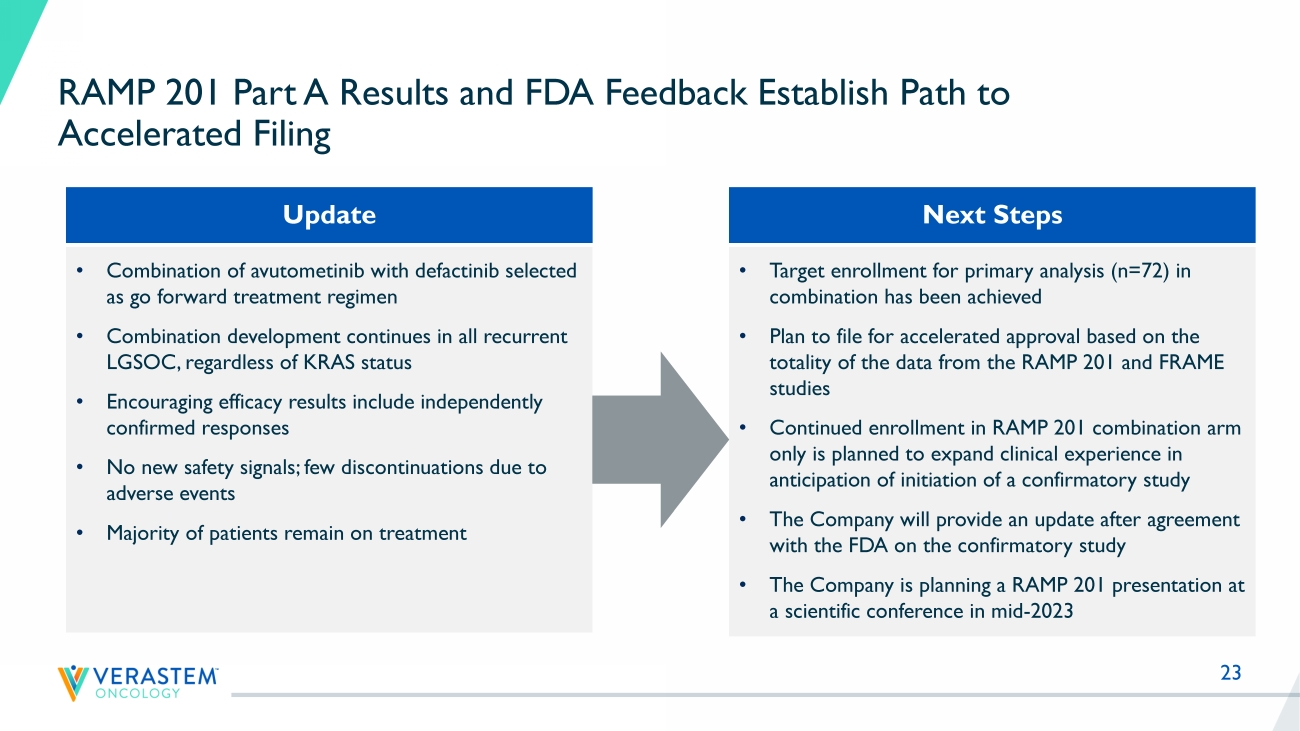
23 RAMP 201 Part A Results and FDA Feedback Establish Path to Accelerated Filing • Target enrollment for primary analysis (n=72) in combination has been achieved • Plan to file for accelerated approval based on the totality of the data from the RAMP 201 and FRAME studies • Continued enrollment in RAMP 201 combination arm only is planned to expand clinical experience in anticipation of initiation of a confirmatory study • The Company will provide an update after agreement with the FDA on the confirmatory study • The Company is planning a RAMP 201 presentation at a scientific conference in mid - 2023 • Combination of avutometinib with defactinib selected as go forward treatment regimen • Combination development continues in all recurrent LGSOC, regardless of KRAS status • Encouraging efficacy results include independently confirmed responses • No new safety signals; few discontinuations due to adverse events • Majority of patients remain on treatment Next Steps Update

24 RAMP 201 Part A Interim Data Support Meaningful Market Potential for All Recurrent LGSOC Regardless of KRAS Status with Long Duration of Therapy NSCLC KRAS G12C 3 Pancreatic Cancer 3 LGSOC 3 Endometrioid Cancer 3 Metastatic uveal melanoma 3 ~6K patients US 1 ~80K patients WW 1 Patient - months of Therapy Per Year 2 (across all 2L+ patients) 1 References : Monk, Randall, Grisham, The Evolving Landscape of Chemotherapy in Newly Diagnosed Advanced Epithelial Ovarian Cancer, Am Soc Cli n Oncol Educ Book; 2019; Slomovitz, Gourley, Carey, Malpica, Shih, Huntsman, Fader., Grisham et al, Low - Grade serous ovarian cancer: State of the S cience; Gynecol Oncol; 2020. Grisham, Iyer, Low - Grade Serous Ovarian Cancer: Current Treatment Paradigms and Future Directions; Curr Treat Options Oncology; 2018; Glo bocan 2020 2 Patient - months of Therapy metric calculated by multiplying relevant incidence/prevalence rate times estimated duration of thera py; represents US market opportunity only; patient population estimates from Globocan 2020, American Cancer Society 2021, AACR Genie Cohort V9.0 public, and scientific pub lications. Duration of therapy estimates from clinical studies and clinician experience. Patient - months on therapy is for 2 nd - line+ patients 3 NSCLC KRAS G12C 2 nd line patients (incidence); Pancreatic RAS/RAF mutant 2 nd - line patients (incidence); LGSOC KRAS mutant and wild - type patients (prevalence); Endometrioid RAS/RAF mutant 2 nd - line patients (incidence); Uveal melanoma RAS/RAF mutant 2nd - line patients (incidence) Prevalence - 50,000 100,000 150,000 ~ 4K ~ 2K KRAS wild type KRAS mutation

25 RAMP 201 Part A Update Advancing Path to Accelerated Filing • LGSOC is a unique RAS pathway - driven cancer with an unacceptably high unmet need • The unique RAF/MEK clamp mechanism of action and novel intermittent dosing schedule of avutometinib make it an attractive combination partner in RAS pathway driven cancers, including LGSOC • Combination of avutometinib with defactinib declared as go forward treatment regimen in LGSOC program • Independently confirmed response rates in both KRAS mutant and KRAS wild - type tumors and favorable safety and tolerability profile in heavily pretreated patient population of RAMP 201 supports continued development in all recurrent LGSOC • Building on Breakthrough Therapy Designation, the Company intends to file for accelerated approval; timing based on mature data from RAMP 201 study and finalization of confirmatory study plans

Q&A

THANK YOU
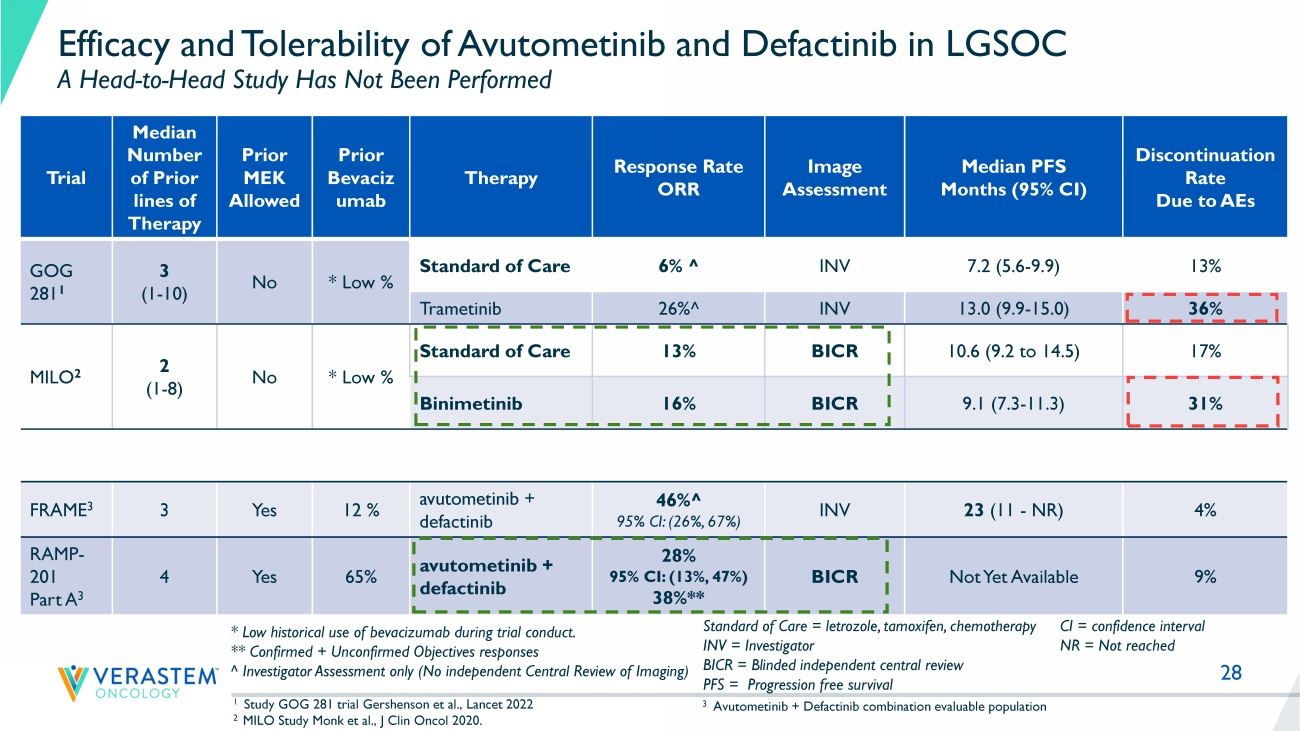
28 Efficacy and Tolerability of Avutometinib and Defactinib in LGSOC A Head - to - Head Study Has Not Been Performed Trial Median Number of Prior lines of Therapy Prior MEK Allowed Prior Bevaciz umab Therapy Response Rate ORR Image Assessment Median PFS Months (95% CI) Discontinuation Rate Due to AEs GOG 281 1 3 (1 - 10) No * Low % Standard of Care 6% ^ INV 7.2 (5.6 - 9.9) 13% Trametinib 26% ^ INV 13.0 (9.9 - 15.0) 36% MILO 2 2 (1 - 8) No * Low % Standard of Care 13% BICR 10.6 (9.2 to 14.5) 17% Binimetinib 16% BICR 9.1 (7.3 - 11.3) 31% FRAME 3 3 Yes 12 % avutometinib + defactinib 46% ^ 95% CI: (26%, 67%) INV 23 (11 - NR) 4% RAMP - 201 Part A 3 4 Yes 65% avutometinib + defactinib 28% 95% CI: (13%, 47%) 38%** BICR Not Yet Available 9% 1 Study GOG 281 trial Gershenson et al., Lancet 2022 2 MILO Study Monk et al., J Clin Oncol 2020. Standard of Care = letrozole, tamoxifen, chemotherapy INV = Investigator BICR = Blinded independent central review PFS = Progression free survival * Low historical use of bevacizumab during trial conduct. ** Confirmed + Unconfirmed Objectives responses ^ Investigator Assessment only (No independent Central Review of Imaging) CI = confidence interval NR = Not reached 3 Avutometinib + Defactinib combination evaluable population
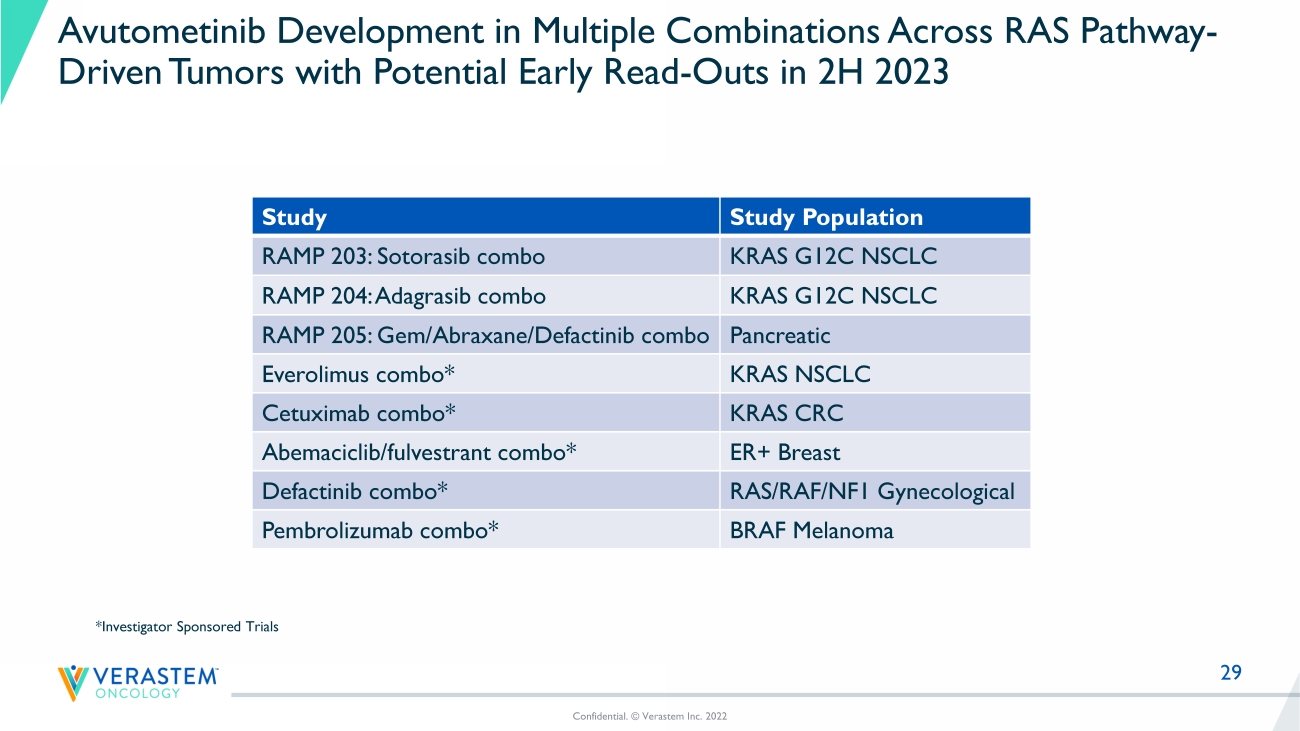
29 Avutometinib Development in Multiple Combinations Across RAS Pathway - Driven Tumors with Potential Early Read - Outs in 2H 2023 Study Study Population RAMP 203: Sotorasib combo KRAS G12C NSCLC RAMP 204: Adagrasib combo KRAS G12C NSCLC RAMP 205: Gem/Abraxane/ Defactinib combo Pancreatic Everolimus combo* KRAS NSCLC Cetuximab combo* KRAS CRC Abemaciclib / fulvestrant combo* ER+ Breast Defactinib combo* RAS/RAF/NF1 Gynecological Pembrolizumab combo* BRAF Melanoma Confidential. © Verastem Inc. 2022 *Investigator Sponsored Trials




























