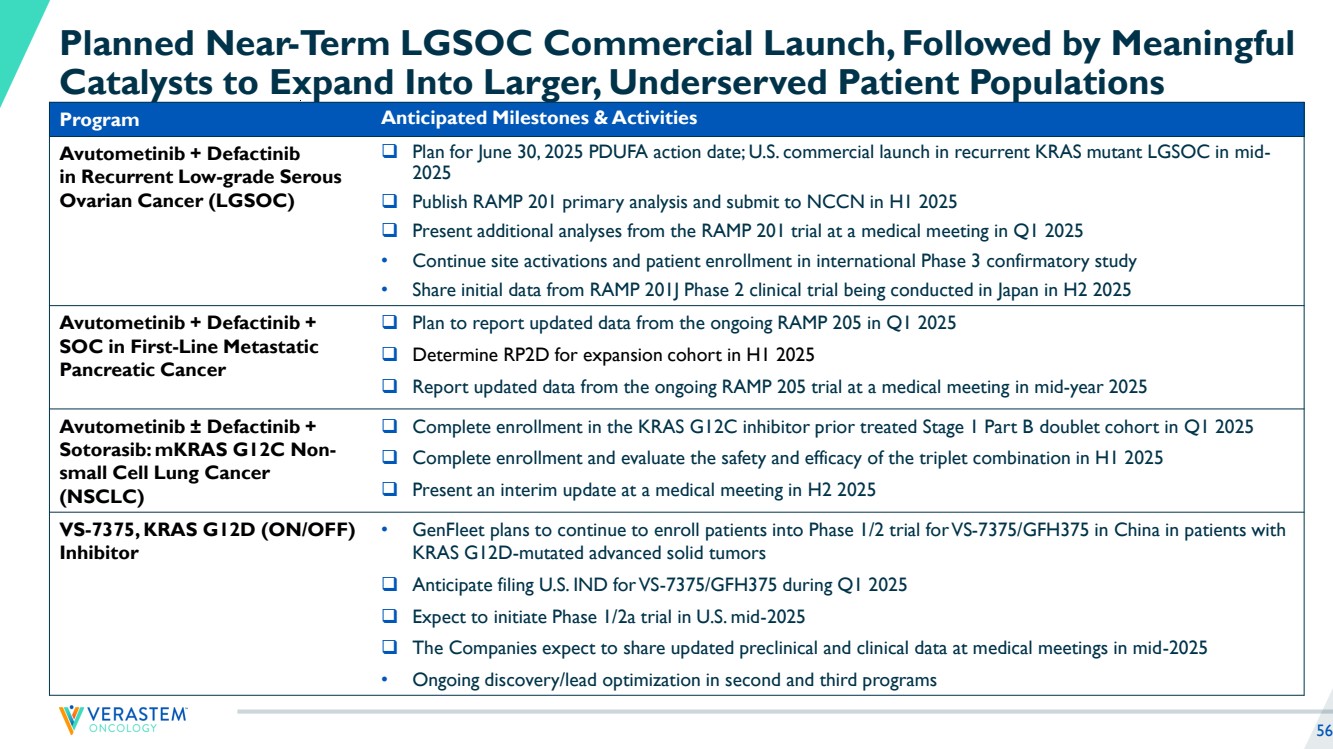
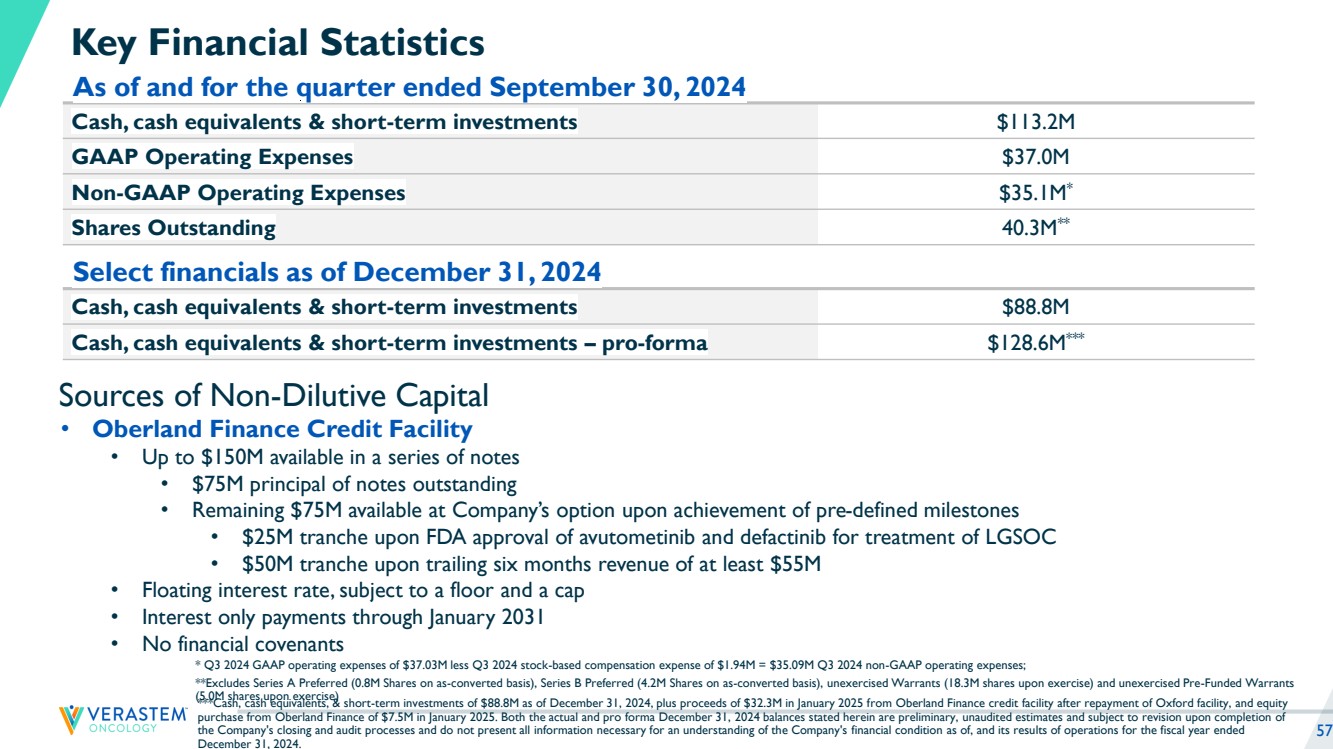
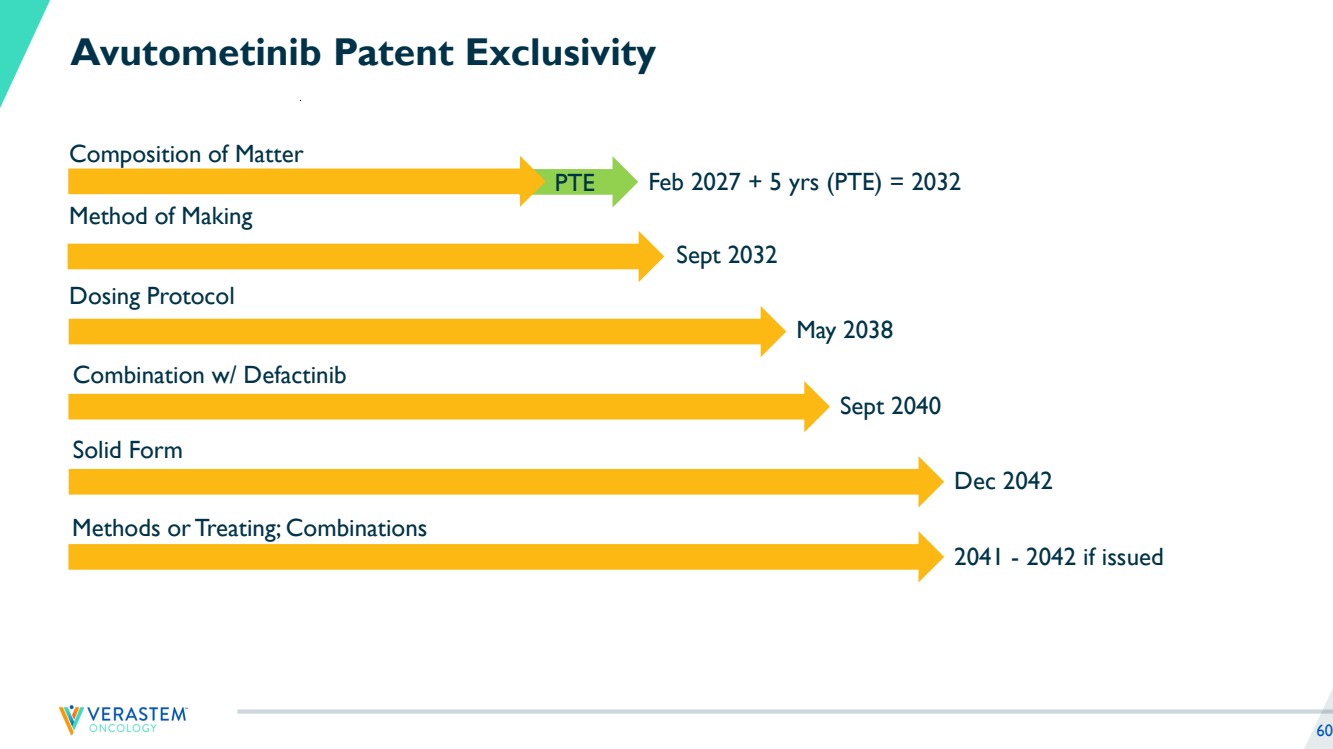
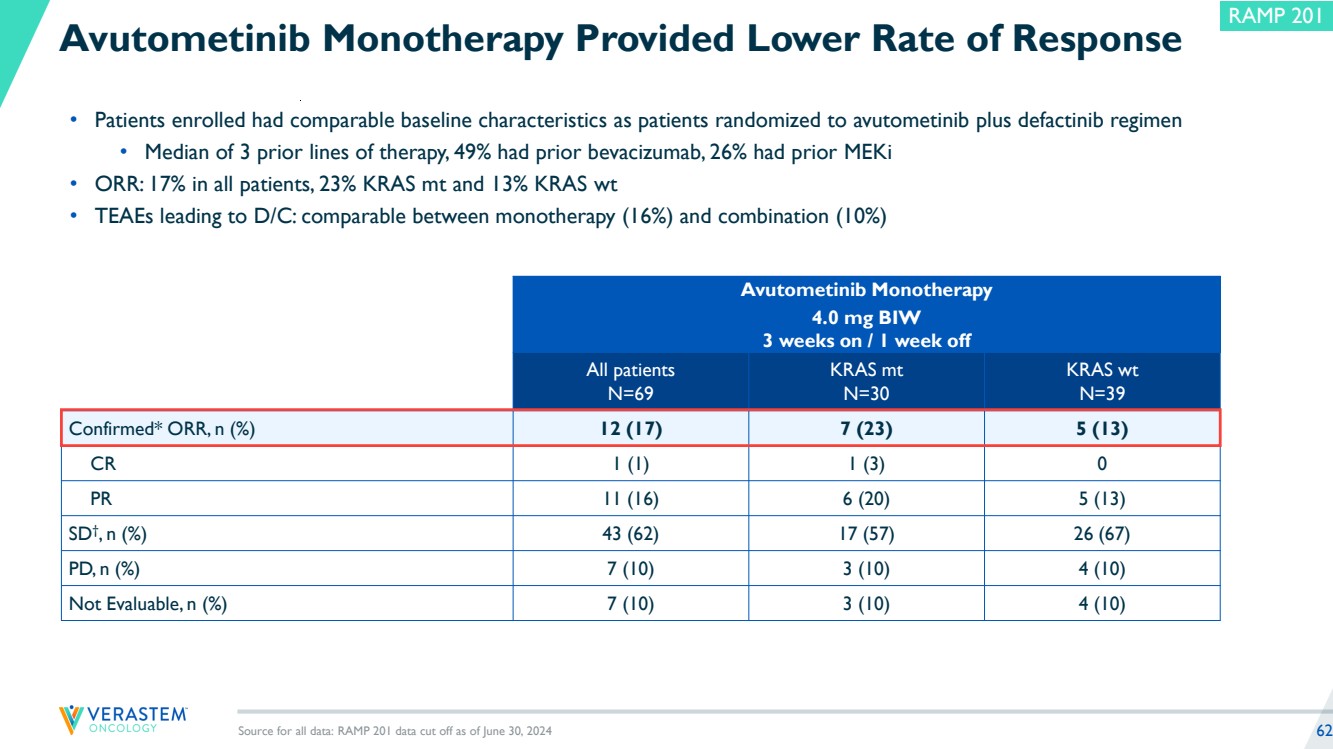
| 2 Disclaimers Forward-Looking Statements This presentation includes forward-looking statements about, among other things, Verastem Oncology’s (the “Company”) programs and product candidates, strategy, future plans and prospects, including statements related to the anticipated timing of a potential launch of avutometinib and defactinib in Low-Grade Serous Ovarian Cancer, the expected outcome and benefits of collaborations, including with GenFleet Therapeutics (Shanghai), Inc. (GenFleet), including the timing of the IND for VS-7375 and the initiation of a Phase 1/2a study with respect to the same, the status of enrollments for and potential of the results of the RAMP 301 Phase 3 trial to expand the indication regardless of KRAS mutation status, the structure of our planned and pending clinical trials, the potential clinical value of various of the Company's clinical trials, including the RAMP 201, RAMP 205 and RAMP 301 trials, the timing of commencing and completing trials, including topline data reports, interactions with regulators, the timeline and indications for clinical development, regulatory submissions, the potential for and timing of commercialization of product candidates and potential for additional development programs involving the Company’s lead compound and the potential market opportunities of, and estimated addressable markets for, our drug candidates. The words "anticipate," "believe," "estimate," "expect," "intend," "may," "plan," "predict," "project," "target," "potential," "will," "would," "could," "should," "continue," “can,” “promising” and similar expressions are intended to identify forward-looking statements, although not all forward-looking statements contain these identifying words. Each forward-looking statement is subject to risks and uncertainties that could cause actual results to differ materially from those expressed or implied in such statement. Applicable risks and uncertainties include the risks and uncertainties, among other things, regarding: the success in the development and potential commercialization of our product candidates, including avutometinib in combination with other compounds, including defactinib, LUMAKRAS and others; the uncertainties inherent in research and development, such as negative or unexpected results of clinical trials, the occurrence or timing of applications for our product candidates that may be filed with regulatory authorities in any jurisdictions; whether and when regulatory authorities in any jurisdictions may approve any such applications that may be filed for our product candidates, and, if approved, whether our product candidates will be commercially successful in such jurisdictions; our ability to obtain, maintain and enforce patent and other intellectual property protection for our product candidates; the scope, timing, and outcome of any legal proceedings; decisions by regulatory authorities regarding trial design, labeling and other matters that could affect the timing, availability or commercial potential of our product candidates; whether preclinical testing of our product candidates and preliminary or interim data from clinical trials will be predictive of the results or success of ongoing or later clinical trials; that the timing, scope and rate of reimbursement for our product candidates is uncertain; that the market opportunities of our drug candidates are based on internal and third-party estimates which may prove to be incorrect; that third-party payors (including government agencies) may not reimburse; that there may be competitive developments affecting our product candidates; that data may not be available when expected; that enrollment of clinical trials may take longer than expected, which may delay our development programs, including delays in review by the FDA of our NDA submission in recurrent KRAS mutant LGSOC if enrollment in our confirmatory trial is not well underway at the time of submission or that the FDA may require the company to enroll additional patients in the Company's ongoing RAMP 301 confirmatory Phase 3 clinical trial prior to Verastem submitting or the FDA taking action on our NDA seeking accelerated approval; risks associated with preliminary and interim data, which may not be representative of more mature data, including with respect to interim duration of therapy data; that our product candidates will cause adverse safety events and/or unexpected concerns may arise from additional data or analysis, or result in unmanageable safety profiles as compared to their levels of efficacy; that we may be unable to successfully validate, develop and obtain regulatory approval for companion diagnostic tests for our product candidates that require or would commercially benefit from such tests, or experience significant delays in doing so; that the mature RAMP 201 data and associated discussions with the FDA may not support the scope of our NDA submission for the avutometinib and defactinib combination in LGSOC, including with respect to KRAS wild type LGSOC; that our product candidates may experience manufacturing or supply interruptions or failures; that any of our third-party contract research organizations, contract manufacturing organizations, clinical sites, or contractors, among others, who we rely on fail to fully perform; that we face substantial competition, which may result in others developing or commercializing products before or more successfully than we do which could result in reduced market share or market potential for our product candidates; that we will be unable to successfully initiate or complete the clinical development and eventual commercialization of our product candidates; that the development and commercialization of our product candidates will take longer or cost more than planned, including as a result of conducting additional studies or our decisions regarding execution of such commercialization; that we may not have sufficient cash to fund our contemplated operations, including certain of our product development programs; that we may not attract and retain high quality personnel; that we or Chugai Pharmaceutical Co., Ltd. will fail to fully perform under the avutometinib license agreement; that our total addressable and target markets for our product candidates might be smaller than we are presently estimating; that we or Secura Bio, Inc. will fail to fully perform under the asset purchase agreement with Secura Bio, Inc., including in relation to milestone payments; that we will not see a return on investment on the payments we have and may continue to make pursuant to the collaboration and option agreement with GenFleet, or that GenFleet will fail to fully perform under the agreement; that we may not be able to establish new or expand on existing collaborations or partnerships, including with respect to in-licensing of our product candidates, on favorable terms, or at all; that we may be unable to obtain adequate financing in the future through product licensing, co-promotional arrangements, public or private equity, debt financing or otherwise; that we will not pursue or submit regulatory filings for our product candidates; and that our product candidates will not receive regulatory approval, become commercially successful products, or result in new treatment options being offered to patients. Other risks and uncertainties include those identified under the heading “Risk Factors” in the Company’s Annual Report on Form 10-K for the year ended December 31, 2023, as filed with the Securities and Exchange Commission (SEC) on March 14, 2024, and in any subsequent filings with the SEC, which are available at www.sec.gov and www.verastem.com. The forward-looking statements in this presentation speak only as of the original date of this presentation, and we undertake no obligation to update or revise any of these statements whether as a result of new information, future events or otherwise, except as required by law. Use of Non-GAAP Financial Measures This presentation contains references to our non-GAAP operating expense, a financial measure that is not calculated in accordance with generally accepted accounting principles in the US (GAAP). This non-GAAP financial measure excludes certain amounts or expenses from the corresponding financial measures determined in accordance with GAAP. Management believes this non-GAAP information is useful for investors, taken in conjunction with the Company’s GAAP financial statements, because it provides greater transparency and period-over-period comparability with respect to the Company’s operating performance and can enhance investors’ ability to identify operating trends in the Company’s business. Management uses this measure, among other factors, to assess and analyze operational results and trends and to make financial and operational decisions. Non-GAAP information is not prepared under a comprehensive set of accounting rules and should only be used to supplement an understanding of the Company’s operating results as reported under GAAP, not in isolation or as a substitute for, or superior to, financial information prepared and presented in accordance with GAAP. In addition, this non-GAAP financial measure is unlikely to be comparable with non-GAAP information provided by other companies. The determination of the amounts that are excluded from non-GAAP financial measures is a matter of management judgment and depends upon, among other factors, the nature of the underlying expense or income amounts. Reconciliations between this non-GAAP financial measure and the most comparable GAAP financial measure are included in the footnotes to the slides in this presentation on which such non-GAAP number appears. Third-Party Sources Certain information contained in this presentation, including industry and market data and other statistical information, relates to or is based on studies, publications, surveys and other data obtained from third-party sources and the Company’s own internal estimates and research. While the Company believes these third-party sources to be reliable as of the date of this presentation, it has not independently verified, and makes no representation as to the adequacy, fairness, accuracy or completeness of, any information obtained from third-party sources. In addition, all of the market data included in this presentation involves a number of assumptions and limitations, and there can be no guarantee as to the accuracy or reliability of such assumptions. |