Table of Contents
Washington, DC 20549
UNDER
THE SECURITIES ACT OF 1933
Delaware | 8071 | 20-4918072 | ||
Delaware | 8071 | 27-4131102 | ||
| (State or Other Jurisdiction of Incorporation or Organization) | (Primary Standard Industrial Classification Code Number) | (I.R.S. Employer Identification No.) |
Palm Beach Gardens, FL 33410
Telephone:(866) 420-5512
(Address, Including Zip Code, and Telephone Number, Including Area Code, of Registrant’s Principal Executive Offices)
Chief Financial Officer
Aurora Diagnostics Holdings, LLC
11025 RCA Center Drive, Suite 300
Palm Beach Gardens, FL 33410
Telephone:(866) 420-5512
(Name, Address, Including Zip Code, and Telephone Number, Including Area Code, of Agent For Service)
Alston & Bird LLP
1201 West Peachtree Street
Atlanta, Georgia30309-3424
Telephone:(404) 881-7000
Facsimile:(404) 881-7777
| Large accelerated filer o | Accelerated filer o | Non-accelerated filer þ | Smaller reporting company o |
| Proposed Maximum | Proposed Maximum | Amount of | ||||||||||
| Title of Each Class of | Amount to be | Offering Price per | Aggregate Offering | Registration | ||||||||
| Securities to be Registered | Registered | Note | Price | Fee(1) | ||||||||
| 10.750% Senior notes due 2018 | $200,000,000 | 100% | $200,000,000 | $23,220 | ||||||||
| Guarantees of the 10.750% Senior notes due 2018(2) | — | — | — | — | ||||||||
| (1) | Calculated in accordance with Rule 457(f)(2) under the Securities Act of 1933, as amended. | |
| (2) | See the Table of Additional Registrants on the following page for a list of the guarantors. No separate consideration will be received for the guarantees, and pursuant to Rule 457(n), no separate fee is payable with regard to the guarantees. |
Table of Contents
| State of | I.R.S. Employer | |||
| Incorporation or | Identification | |||
Exact Name of Registrant as Specified in its Charter* | Organization | Number | ||
| Aurora Diagnostics, LLC | Delaware | 20-4846295 | ||
| Aurora Georgia, LLC | Georgia | 58-2388120 | ||
| Aurora Greensboro, LLC | North Carolina | 26-1150846 | ||
| Aurora LMC, LLC | Nevada | 26-1458503 | ||
| Aurora Massachusetts, LLC | Delaware | 27-1140505 | ||
| Aurora Michigan, LLC | Michigan | 20-5863241 | ||
| Aurora New Hampshire, LLC | New Hampshire | 20-8034990 | ||
| Bernhardt Laboratories, Inc. | Florida | 59-2099699 | ||
| Biopsy Diagnostics, LLC | South Carolina | 55-0796033 | ||
| C R Collections, LLC | Alabama | 63-1214311 | ||
| Covenant Healthcare Lab, LLC | Florida | 26-1476901 | ||
| Cunningham Pathology, L.L.C. | Delaware | 63-1214311 | ||
| DermPath New England, LLC | Massachusetts | 16-1742674 | ||
| Greensboro Pathology, LLC | North Carolina | 56-0948235 | ||
| Hardman Pathology ADX, LLC | Georgia | 20-8658047 | ||
| Laboratory of Dermatopathology ADX, LLC | New York | 20-5292168 | ||
| Mark & Kambour, LLC | Florida | 26-1150913 | ||
| Mark & Kambour Holdings, Inc. | Florida | 27-1102780 | ||
| Pathology Solutions, LLC | New Jersey | 20-2506174 | ||
| Seacoast Pathology, Inc. | New Hampshire | 02-0359182 | ||
| Texas Pathology, LLC | Texas | 20-5081716 | ||
| Twin Cities Dermatopathology, LLC | Minnesota | 41-1656670 | ||
| The LMC Revocable Trust, B.T. | Nevada | 26-6305236 | ||
| The WPC Revocable Trust, B.T. | Nevada | 27-6992795 |
| * | The address for each of the Additional Registrants isc/o Aurora Diagnostics Holdings, LLC, 11025 RCA Center Drive, Suite 300, Palm Beach Gardens, FL 33410, Telephone:(866) 420-5512. The name and address of the agent for service for each Additional Registrant is Gregory A. Marsh, Chief Financial Officer, Aurora Diagnostics Holdings, LLC, 11025 RCA Center Drive, Suite 300, Palm Beach Gardens, FL 33410, Telephone:(866) 420-5512. |
Table of Contents
| The information in this prospectus is not complete and may be changed. We may not sell these securities until the registration statement filed with the Securities and Exchange Commission is effective. This prospectus is not an offer to sell these securities and is not soliciting an offer to buy these securities in any state where the offer or sale is not permitted. |

AURORA DIAGNOSTICS FINANCING, INC.
(as Issuers)
that have been registered under the
10.750% Senior notes due 2018
New York City time, on , 2011, unless extended.
| • | We are offering to exchange $200,000,000 aggregate principal amount of our 10.750% senior notes due 2018, and the guarantees thereof, which have been registered under the Securities Act of 1933, as amended, or the “Securities Act,” and which are referred to in this prospectus as the “new notes,” for all $200,000,000 aggregate principal amount of outstanding unregistered 10.750% senior notes due 2018, and the guarantees thereof, that were issued on December 20, 2010, which are referred to in this prospectus as the “old notes.” We refer to the old notes and the new notes collectively as “notes.” | |
| • | Subject to the terms of this exchange offer, we will exchange the new notes for all old notes that are validly tendered and not withdrawn prior to the expiration of this exchange offer. | |
| • | The new notes will be identical in all material respects to the old notes, except that the new notes will be registered under the Securities Act and will generally not be subject to transfer restrictions or registration rights. The old notes were issued in reliance upon an available exemption from the registration requirements of the Securities Act. | |
| • | We will pay interest on the new notes on each January 15 and July 15. Interest payments under the old notes commenced on July 15, 2011. The next interest payment date is January 15, 2012. | |
| • | The new notes will be our senior unsecured obligations and will rank equally in right of payment with all of our other senior indebtedness, but will be effectively subordinated to our secured indebtedness to the extent of the value of the assets securing that indebtedness. All of our domestic subsidiaries that guarantee our amended senior secured credit facility and, as required by the indenture governing the notes, specified future subsidiaries, will guarantee the new notes on a senior unsecured basis, but such guarantee will be effectively subordinated to secured indebtedness of such subsidiaries to the extent of the value of the assets securing that indebtedness. | |
| • | The exchange of old notes for new notes pursuant to this exchange offer generally should not be a taxable event for U.S. federal income tax purposes. See “Material U.S. Federal Income Tax Considerations.” | |
| • | There is no public market for the new notes. We have not applied, and do not intend to apply, for listing of the new notes on any national securities exchange or automated quotation system. | |
| • | We will not receive any proceeds from this exchange offer. | |
| • | Broker-dealers receiving exchange notes in exchange for outstanding notes acquired for their own account through market-making or other trading activities must deliver a prospectus in any resale of the exchange notes. Each broker-dealer that receives new notes for its own account pursuant to this exchange offer must acknowledge that it will deliver a prospectus in connection with any resale of such new notes. The letter of transmittal states that by so acknowledging and by delivering a prospectus, a broker-dealer will not be deemed to admit that it is an “underwriter” within the meaning of the Securities Act. This prospectus, as it may be amended or supplemented from time to time, may be used by a broker-dealer in connection with resales of new notes received in exchange for old notes where such old notes were acquired by such broker-dealer as a result of market-making activities or other trading activities. We have agreed that, for a period of 180 days after the closing of this exchange offer, or such shorter period during which participating broker-dealers are required by law to deliver such a prospectus, we will make this prospectus available to any broker-dealer for use in connection with any such resale. See “Plan of Distribution.” |
i
Table of Contents
| • | “Laboratory Industry Strategic Outlook: Market Trends and Analysis 2011” prepared by G-2 Intelligence, or the G-2 Report, which is available for purchase athttp://www.g2reports.com; |
ii
Table of Contents
| • | “The U.S. Anatomic Pathology Market Forecast & Trends 2011” prepared by Laboratory Economics, or the Laboratory Economics Report, which is available for purchase athttp://www.laboratoryeconomics.com; | |
| • | “Cancer Facts & Figures 2009” prepared by the American Cancer Society, or the American Cancer Society Report, which is available for download athttp://www.cancer.org/Research/CancerFactsFigures/index; | |
| • | “Trendwatch Chartbook 2000: Trends Affecting Hospitals and Health Systems” prepared by the Lewin Group, Inc. for the American Hospital Association and “Trendwatch Chartbook 2009: Trends Affecting Hospitals and Health Systems” prepared by Avalere Health for the American Hospital Association, or the AHA Reports, which is available for download athttp://www.aha.org/aha/research- and-trends/health-andhospital-trends/2003-or-earlier.html andhttp://www.aha.org/aha/research-and-trends/chartbook/2009chartbook.html, respectively; and | |
| • | “Projections of the Population by Selected Age Groups and Sex for the United States: 2010 to 2050” prepared by the Population Division, U.S. Census Bureau, or the U.S. Census Bureau Report, which is available for download athttp://www.census.gov/population/www/projections/summarytables.html. |
| • | changes in medical treatment or reimbursement rates or utilization for our anatomic pathology markets; |
iii
Table of Contents
| • | competition for our diagnostic services, including the internalization of testing functions and technologies by our clients; | |
| • | changes in payor regulations, policies or payor mix; | |
| • | the anticipated benefits from acquisitions not being fully realized or not being realized within the expected time frames; | |
| • | disruptions or failures of our IT solutions or infrastructure; | |
| • | loss of key executives, physicians and technical personnel; | |
| • | the failure to maintain relationships with clients, including referring physicians and hospitals, and with payors; | |
| • | covenants in our debt agreements; | |
| • | our substantial amount of indebtedness; | |
| • | the protection of our intellectual property; | |
| • | general economic, business or regulatory conditions affecting the health care and diagnostic testing services industries; | |
| • | federal or state health care reform initiatives; | |
| • | violation of, failure to comply with, or changes in federal and state laws and regulations related to, submission of claims for our services, fraud and abuse, patient privacy, and billing arrangements for our services; | |
| • | attainment of licenses required to test patient specimens from certain states or the loss or suspension of licenses; | |
| • | risks related to the notes generally; and | |
| • | the other factors discussed under the heading “Risk Factors” and elsewhere in this prospectus. |
iv
Table of Contents
1
Table of Contents

| (1) | Summit Partners, KRG Capital Partners and Management Equityholders. | |
| (2) | Issuer of the notes offered hereby. | |
| (3) | Borrower under our amended senior secured credit facility. | |
| (4) | Co-Issuer of the Notes offered hereby. | |
| (5) | Guarantors of the notes and our amended senior secured credit facility. | |
| (6) | Non-guarantors of the notes or our amended senior secured credit facility. The affiliated laboratory practices are not owned by us, but are controlled and consolidated through contractual arrangements. See “Business — Corporate Structure” for additional information. |
2
Table of Contents
3
Table of Contents
| Old Notes | $200.0 million aggregate principal amount of 10.750% Senior notes due 2018 that are not registered under the Securities Act and are subject to certain transfer restrictions, registration rights and additional interest upon any failure by us to fulfill our obligations under the registration rights agreement. | |
| New Notes | 10.750% Senior notes due 2018. The terms of the new notes are identical in all material respects to the terms of the old notes, except that the new notes are registered under the Securities Act and generally are not subject to transfer restrictions, registration rights or additional interest penalties. | |
| Exchange Offer | We are offering to exchange $1,000 principal amount of our new notes due January 15, 2018, for each $1,000 principal amount of our old notes due January 15, 2018. Currently, there is $200.0 million in aggregate principal amount of old notes outstanding. | |
| Old notes may be exchanged only in minimum denominations of $2,000 and integral multiples of $1,000 in excess of $2,000. New notes will be issued only in minimum denominations of $2,000 and integral multiples of $1,000 in excess of $2,000. | ||
| Subject to the terms of this exchange offer, we will exchange new notes for all of the old notes that are validly tendered and not validly withdrawn prior to the expiration of this exchange offer. In order to exchange an old note, you must follow the required procedures and we must accept the old note for exchange. We will issue new notes in exchange for corresponding old notes in this exchange offer, if consummated, promptly after the expiration of this exchange offer. | ||
| Expiration Date | This exchange offer will expire at midnight, New York City time, on , 2011, unless we extend it. We may extend the expiration date for any reason. We do not currently intend to extend the expiration date. | |
| Sale of New Notes | Based on interpretive letters of the SEC staff to third parties, we believe that you may offer for resale, resell and otherwise transfer the new notes issued pursuant to the exchange offer without compliance with the registration and prospectus delivery provisions of the Securities Act if you: | |
| • acquire the new notes in the ordinary course of your or any beneficial owner’s business; | ||
| • are not participating, do not intend to participate and have no arrangement or understanding with any person to participate in |
4
Table of Contents
| the distribution (within the meaning of the Securities Act) of the new notes issued in the exchange offer; | ||
| • are not an “affiliate” of ours, as defined in Rule 405 under the Securities Act; and | ||
| • are not a broker-dealer that acquired the old notes from us or in market-making transactions or other trading activities. | ||
| By tendering your notes as described in “The Exchange Offer — Procedures for Tendering,” you will be making representations to this effect. If you fail to satisfy any of these conditions, you cannot rely on the position of the SEC set forth in the interpretive letters referred to above and you must comply with the registration and prospectus delivery requirements of the Securities Act in connection with a sale of the new notes. | ||
| If you are a broker-dealer that acquired old notes as a result of market-making or other trading activities, you must comply with the prospectus delivery requirements of the Securities Act in connection with sales of the new notes, as described in this summary under “Restrictions on Sales by Broker-Dealers” below. If you are an affiliate of ours and hold old notes, you must comply with the registration and prospectus delivery requirements of the Securities Act to the extent applicable to you. | ||
| As noted above, we base our belief on interpretations by the SEC staff in no-action letters issued to other issuers in exchange offers like ours. We cannot guarantee that the SEC would make a similar decision about our exchange offer. | ||
| Restrictions on Sales by Broker-Dealers | If you are a broker-dealer that has received new notes for your own account in exchange for old notes that were acquired as a result of market-making or other trading activities, you must acknowledge that you will deliver a prospectus meeting the requirements of the Securities Act in connection with any sale of the new notes. A broker-dealer may use this prospectus for sales of new notes for a period of 180 days commencing on the day the exchange offer is consummated. See “Plan of Distribution.” | |
| Withdrawal of Tenders | You may withdraw the tender of your old notes at any time prior to midnight, New York City time, on the expiration date. | |
| Taxation | The exchange of old notes for new notes in this exchange offer generally should not be a taxable event for U.S. federal income tax purposes. See “Material U.S. Federal Income Tax Considerations.” | |
| Conditions to the Exchange Offer | This exchange offer is subject to customary conditions, which we may assert or waive. See “The Exchange Offer — Conditions to the Exchange Offer; Waivers.” | |
| Procedures for Tendering | If you wish to accept this exchange offer and your old notes are held by a custodial entity such as a bank, broker, dealer, trust company or other nominee, you must instruct this custodial entity to tender your old notes on your behalf pursuant to the procedures of the custodial entity. If your old notes are registered in your name, you must complete, sign and date the accompanying letter of |
5
Table of Contents
| transmittal, or a facsimile of the letter of transmittal, according to the instructions contained in this prospectus and the letter of transmittal. You must also mail or otherwise deliver the letter of transmittal, or a facsimile of the letter of transmittal, together with the old notes and any other required documents, to the exchange agent at the address set forth on the cover page of the letter of transmittal. | ||
| Custodial entities that are participants in The Depository Trust Company, or “DTC,” may tender old notes through DTC’s Automated Tender Offer Program, or “ATOP,” which enables a custodial entity and the beneficial owner on whose behalf the custodial entity is acting to electronically agree to be bound by the letter of transmittal. A letter of transmittal need not accompany tenders effected through ATOP. | ||
| See “The Exchange Offer — Effect of Surrendering Old Notes.” | ||
| Consequences of Failure to Exchange | If you are eligible to participate in the exchange offer and you do not tender your old notes, you will not have any further registration or exchange rights (subject to certain very limited exceptions) and your old notes will continue to be subject to the existing transfer restrictions. These transfer restrictions and the availability of the new notes could adversely affect the trading market for your notes. | |
| Use of Proceeds | We will not receive any proceeds from the exchange of notes pursuant to the exchange offer. See “Use of Proceeds.” | |
| Exchange Agent | U.S. Bank National Association is the exchange agent for this exchange offer. The address and telephone number of the exchange agent are set forth under “The Exchange Offer — Exchange Agent.” U.S. Bank National Association is also the trustee under the indenture governing the notes. | |
| Risk Factors | Participation in the exchange offer involves substantial risk. See “Risk Factors — Risks Related to the Exchange Offer” for a description of the material risks you should consider before participating in the exchange offer. |
6
Table of Contents
| Issuers | Aurora Diagnostics Holdings, LLC | |
| Co-Issuer | Aurora Diagnostics Financing, Inc., or Aurora Financing, a wholly owned subsidiary of Aurora Holdings, will jointly and severally issue the new notes. Aurora Financing was incorporated in Delaware to serve as a corporate co-issuer of the old notes and the new notes. Aurora Financing does not have any substantial operations, revenues or assets. As a result, holders of the new notes should not expect Aurora Financing to participate in servicing the interest and principal obligations on the new notes. | |
| Notes Offered | $200.0 million aggregate principal amount of 10.750% Senior Notes due 2018. | |
| Maturity Date | January 15, 2018. | |
| Interest Payment Dates | On January 15 and July 15 of each year. Interest payments under the old notes commenced on July 15, 2011. The next interest payment date is January 15, 2012. | |
| Guarantors | The new notes will be unconditionally guaranteed on an unsecured senior basis by all of our current wholly-owned subsidiaries. Aurora Financing, which is a co-issuer of the new notes, and our non-wholly owned subsidiaries are not guarantors of the new notes. | |
| Ranking | The new notes and guarantees will be the unsecured senior obligations of Aurora Holdings, Aurora Financing and the guarantors. Accordingly, they will: | |
| • be effectively subordinated in right of payment to all of the existing and future secured debt of the issuers and the guarantors (including our senior secured credit facilities), to the extent of the value of the assets securing such debt; | ||
| • rank equally with all of the unsecured indebtedness of Aurora Holdings, Aurora Financing and the guarantors; and | ||
| • rank senior in right of payment to all of the subordinated indebtedness of Aurora Holdings, Aurora Financing and the guarantors. | ||
| In addition, the new notes will be structurally subordinated to all of the existing and future liabilities of our subsidiaries that do not guarantee the notes. | ||
| As of June 30, 2011, our total indebtedness was $320.2 million, which excludes $39.8 million of the fair value of contingent consideration for acquisitions completed on or after January 1, 2009. As of June 30, 2011, the new notes and guarantees would have ranked effectively junior to Aurora Holdings’, Aurora Financing’s |
7
Table of Contents
| and the guarantors’ obligations in respect of approximately $114.4 million of our outstanding borrowings pursuant to our amended senior secured credit facility and the guarantors’ guarantees of such debt. | ||
| For the year ended December 31, 2010 and the six months ended June 30, 2011, our subsidiaries, including our affiliated practices, that will not be guarantors of the new notes had revenues of $79.5 million and $53.9 million, respectively, and, as of June 30, 2011, those subsidiaries had debt and other liabilities of $27.6 million (excluding inter-company balances and $13.6 million of the fair value of contingent consideration for acquisitions completed on or after January 1, 2009). | ||
| Optional Redemption | We may redeem some or all of the new notes, at our option, at any time on or after January 15, 2015, at the redemption prices set forth in this prospectus, plus accrued and unpaid interest, if any, to the redemption date. At any time prior to January 15, 2015, we may redeem some or all of the new notes at a price equal to 100% of the aggregate principal amount of the new notes to be redeemed, plus a “make-whole” premium set forth in this prospectus and accrued and unpaid interest, if any, to the redemption date. In addition, at any time prior to January 15, 2014, we may redeem up to 35% of the aggregate principal amount of the new notes with the net cash proceeds of certain equity offerings at the redemption price set forth in this prospectus, plus accrued and unpaid interest, if any, to the redemption date. See “Description of the New Notes — Optional Redemption.” | |
| Change of Control | If we experience specific kinds of change of control events, we must offer to repurchase the new notes at a price of 101% of the principal amount thereof, plus accrued and unpaid interest, if any, to the purchase date. See “Description of the New Notes — Change of Control.” | |
| Certain Covenants | The indenture governing the new notes contains, among other things, certain covenants limiting our ability and the ability of our restricted subsidiaries that are guarantors to: | |
| • incur or guarantee additional indebtedness; | ||
| • pay dividends or make other restricted payments; | ||
| • make certain investments; | ||
| • create or incur certain liens; | ||
| • sell assets and subsidiary stock; | ||
| • transfer all or substantially all of our assets or enter into a merger or consolidation transactions; and | ||
| • enter into transactions with our affiliates. | ||
| However, these limitations are subject to a number of important qualifications and exceptions. See “Description of the New Notes — Certain Covenants.” |
8
Table of Contents
| Transfer Restrictions; Absence of a Public Market | The new notes will generally be freely transferable but will be a new issue of securities for which there will not initially be a market. Accordingly, there can be no assurance as to the development or liquidity of any market for the new notes. We do not intend to apply for a listing of the new notes on any securities exchange or automated dealer quotation system. | |
| Trustee | U.S. Bank National Association | |
| Governing Law | The new notes and the indenture are governed by the laws of the State of New York. | |
| Risk Factors | An investment in the new notes as a result of participation in the exchange involves substantial risk. See “Risk Factors — Risks Related to the New Notes” for a description of the risks you should consider before investing in the new notes through participation in the exchange offer. |
9
Table of Contents
| • | our 2010 acquisitions of Pathology Solutions, LLC and Biopsy Diagnostics, LLC as if these acquisitions occurred January 1, 2010; and | |
| • | our 2011 acquisitions of Austin Pathology Associates and Texas Pathology, LLC, Western Pathology Consultants, Ltd., and DermPath New England, LLC as if these acquisitions occurred January 1, 2010. |
10
Table of Contents
| Pro Forma | ||||||||||||||||||||||||||||
| Pro Forma | Six Months | |||||||||||||||||||||||||||
| Year Ended | Ended | |||||||||||||||||||||||||||
| Year Ended December 31, | December 31, | Six Months Ended June 30, | June 30, | |||||||||||||||||||||||||
| 2008 | 2009 | 2010 | 2010(1) | 2010 | 2011 | 2011(1) | ||||||||||||||||||||||
| (In thousands) | ||||||||||||||||||||||||||||
Consolidated Income Statement Data: | ||||||||||||||||||||||||||||
| Net Revenues | $ | 157,850 | $ | 171,565 | $ | 212,837 | $ | 248,311 | $ | 101,105 | $ | 130,470 | $ | 132,758 | ||||||||||||||
| Operating costs and expenses: | ||||||||||||||||||||||||||||
| Cost of services | 66,382 | 71,778 | 96,868 | 115,573 | 45,904 | 58,846 | 59,726 | |||||||||||||||||||||
| Selling, general and administrative expenses | 33,194 | 36,854 | 49,141 | 54,405 | 23,438 | 30,595 | 30,955 | |||||||||||||||||||||
| Provision for doubtful accounts | 8,037 | 9,488 | 12,393 | 12,420 | 6,028 | 8,862 | 8,862 | |||||||||||||||||||||
| Intangible asset amortization expense | 14,308 | 14,574 | 18,946 | 23,692 | 9,274 | 11,126 | 11,612 | |||||||||||||||||||||
| Management fees | 1,559 | 1,778 | 2,189 | 2,544 | 1,062 | 1,344 | 1,367 | |||||||||||||||||||||
Impairment of goodwill and other intangible assets(3) | — | 8,031 | 4,871 | 4,871 | — | — | — | |||||||||||||||||||||
| Acquisition and business development costs | 676 | 1,074 | 1,032 | 1,032 | 433 | 515 | 515 | |||||||||||||||||||||
| Change in fair value of contingent consideration | — | — | 983 | 983 | 984 | 3,158 | 3,158 | |||||||||||||||||||||
Equity based compensation expense(2) | 1,164 | — | — | — | — | — | — | |||||||||||||||||||||
Total operating costs and expenses | 125,320 | 143,577 | 186,423 | 215,520 | 87,123 | 114,446 | 116,195 | |||||||||||||||||||||
Income from operations | 32,530 | 27,988 | 26,414 | 32,791 | 13,982 | 16,024 | 16,563 | |||||||||||||||||||||
| Other income (expense): | ||||||||||||||||||||||||||||
| Interest expense | (21,577 | ) | (18,969 | ) | (17,041 | ) | (17,070 | ) | (7,587 | ) | (16,268 | ) | (16,268 | ) | ||||||||||||||
Write-off of deferred debt issue costs(4) | — | — | (9,259 | ) | (9,259 | ) | (4,527 | ) | — | — | ||||||||||||||||||
Loss on extinguishment of debt(4) | — | — | (2,296 | ) | (2,296 | ) | (2,296 | ) | — | — | ||||||||||||||||||
| Other income | 125 | 28 | 18 | 38 | 5 | (46 | ) | (46 | ) | |||||||||||||||||||
Total other expense, net | (21,452 | ) | (18,941 | ) | (28,578 | ) | (28,587 | ) | (14,405 | ) | (16,314 | ) | (16,314 | ) | ||||||||||||||
Income (loss) before income taxes | 11,078 | 9,047 | (2,164 | ) | 4,204 | (423 | ) | (290 | ) | (249 | ) | |||||||||||||||||
Provision for income taxes(5) | 408 | 45 | 1,487 | 1,642 | 1,231 | 1,352 | 1,352 | |||||||||||||||||||||
Net income (loss) | $ | 10,670 | $ | 9,002 | $ | (3,651 | ) | $ | 2,562 | $ | (1,654 | ) | $ | (1,642 | ) | $ | (1,103 | ) | ||||||||||
Consolidated Statements of Cash Flow Data: | ||||||||||||||||||||||||||||
| Net cash provided by (used in) | ||||||||||||||||||||||||||||
| Operating activities | $ | 28,970 | $ | 36,363 | $ | 27,001 | $ | 10,380 | $ | 27,780 | ||||||||||||||||||
| Investing activities | (46,272 | ) | (49,261 | ) | (90,885 | ) | (37,334 | ) | (30,156 | ) | ||||||||||||||||||
| Financing activities | 16,022 | 33,044 | 76,401 | 2,148 | (1,077 | ) | ||||||||||||||||||||||
Consolidated Operating Data: | ||||||||||||||||||||||||||||
| Number of accessions | 1,472 | 1,557 | 1,974 | 2,151 | 948 | 1,101 | 1,116 | |||||||||||||||||||||
Other Consolidated Financial Data: | ||||||||||||||||||||||||||||
| Capital expenditures | $ | 2,746 | $ | 2,961 | $ | 3,217 | $ | 1,446 | $ | 1,958 | ||||||||||||||||||
Adjusted EBITDA(6) | 52,066 | 55,931 | 57,747 | 69,276 | 27,339 | 34,109 | 35,157 | |||||||||||||||||||||
11
Table of Contents
| December 31, | June 30, | |||||||||||
| 2009 | 2010 | 2011 | ||||||||||
| (In thousands) | ||||||||||||
Consolidated Balance Sheet Data: | ||||||||||||
| Cash and cash equivalents | $ | 27,424 | $ | 39,941 | $ | 36,488 | ||||||
| Total assets | 463,973 | 588,011 | 624,904 | |||||||||
| Working capital, excluding deferred tax assets, current portion of long-term debt and current portion of fair value of contingent consideration | 29,808 | 51,272 | 35,047 | |||||||||
| Long term debt and fair value of contingent consideration, including current portions | 219,606 | 345,364 | 358,599 | |||||||||
| Members’ equity | 217,064 | 211,343 | 209,701 | |||||||||
| (1) | The unaudited pro forma consolidated financial and operating data for the year ended December 31, 2010 and six months ended June 30, 2011 give effect to: |
| • | our March 12, 2010 acquisition of Pathology Solutions, LLC; | |
| • | our October 8, 2010 acquisition of Biopsy Diagnostics, LLC; | |
| • | our January 1, 2011 acquisitions of Austin Pathology Associates, Texas Pathology, LLC and Western Pathology Consultants, Ltd.; and | |
| • | our June 2, 2011 acquisition of DermPath New England, LLC. |
| (2) | During 2008, we adopted a new equity incentive plan, which we refer to as the 2008 Plan, to replace our original equity incentive plan. The 2008 Plan provided awards of membership interest units in Aurora Holdings that were denominated asClass D-1,Class D-2, andClass D-3 units. During 2008, we authorized and issued 4,000 D-1 units, 3,000 D-2 units and 3,000 D-3 units of Aurora Holdings under the 2008 Plan. All membership interest units in Aurora Holdings issued in 2008 were fully vested as of December 31, 2008. We recorded compensation expense of $1.2 million for these awards. There were no other grants under the 2008 Plan. | |
| (3) | As of September 30, 2010, we tested goodwill and intangible assets for potential impairment and recorded a non-cash impairment expense of $8.0 million resulting from a write down of $6.6 million in the carrying value of goodwill and a write down of $1.4 million in the carrying value of other intangible assets. The write-down of the goodwill and other intangible assets related to one reporting unit. Regarding this reporting unit, we believe events occurred and circumstances changed that more likely than not reduced the fair value of the intangible assets and goodwill below their carrying amounts. These events during 2009 consisted primarily of the loss of significant customers present at the acquisition date, which adversely affected the current year and expected future revenues and operating profit of the reporting unit. | |
| As of September 30, 2010, we tested goodwill and intangible assets for potential impairment and recorded a non-cash impairment expense of $4.9 million resulting from a write down of $2.0 million in the carrying value of goodwill and a write down of $2.9 million in the carrying value of other intangible assets. The write-down of the goodwill and other intangible assets related to one reporting unit. Regarding this reporting unit, we believe events occurred and circumstances changed that more likely than not reduced the fair value of the intangible assets and goodwill below their carrying amounts. These events during 2010 consisted primarily of the loss of significant customers present at the acquisition date, which adversely affected the current year and expected future revenues and operating profit of the reporting unit. | ||
| (4) | In May 2010, we refinanced our then-existing credit facility. As a result, we wrote off unamortized deferred debt issue costs of $4.5 million. In addition, we incurred a $2.3 million prepayment penalty in connection with the May 2010 refinancing. On December 20, 2010 we used a portion of the proceeds from |
12
Table of Contents
| the issuance of the Senior Notes to repay $110.0 million of the $224.4 million principal then owed under our term loan. In connection with the repayment, we recorded a non-cash write-off of the pro rata portion of unamortized original issue discount, prepaid administration fees, and debt issue costs of approximately $4.7 million related to our prior credit facilities. As of June 30, 2011, original issue discount and deferred debt issue costs were approximately $1.4 million and $10.3 million, respectively. | ||
| (5) | Aurora Holdings is a Delaware limited liability company taxed as a partnership for federal and state income tax purposes, in accordance with the applicable provisions of the Internal Revenue Code. Accordingly, Aurora Holdings generally has not been subject to income taxes. The income attributable to Aurora Holdings has been allocated to the members of Aurora Holdings in accordance with the terms of the Aurora Holdings LLC Agreement. However, certain of our subsidiaries are corporations, file separate returns and are subject to federal and state income taxes. The provision for income taxes for these subsidiaries is reflected in our consolidated financial statements and includes federal and state taxes currently payable and changes in deferred tax assets and liabilities excluding the establishment of deferred tax assets and liabilities related to the acquisitions. | |
| (6) | The following is a reconciliation of net income to Adjusted EBITDA: |
| Pro Forma, | ||||||||||||||||||||||||||||
| Pro Forma | Six Months | |||||||||||||||||||||||||||
| Year Ended | Ended | |||||||||||||||||||||||||||
| Year Ended December 31, | December 31, | Six Months Ended June 30, | June 30, | |||||||||||||||||||||||||
| 2008 | 2009 | 2010 | 2010 (F) | 2010 | 2011 | 2011(F) | ||||||||||||||||||||||
| (In thousands) | ||||||||||||||||||||||||||||
Net Income (loss) | $ | 10,670 | $ | 9,002 | $ | (3,651 | ) | $ | 2,562 | $ | (1,654 | ) | $ | (1,642 | ) | $ | (1,103 | ) | ||||||||||
| Interest expense | 21,577 | 18,969 | 17,041 | 17,070 | 7,587 | 16,268 | 16,268 | |||||||||||||||||||||
| Income taxes | 408 | 45 | 1,487 | 1,642 | 1,231 | 1,352 | 1,352 | |||||||||||||||||||||
| Depreciation and amortization | 16,137 | 17,060 | 22,258 | 27,055 | 10,878 | 13,068 | 13,554 | |||||||||||||||||||||
EBITDA | 48,792 | 45,076 | 37,135 | 48,329 | 18,042 | 29,046 | 30,071 | |||||||||||||||||||||
| Management fees(A) | 1,559 | 1,778 | 2,189 | 2,544 | 1,062 | 1,344 | 1,367 | |||||||||||||||||||||
| Equity-based compensation | 1,164 | — | — | — | — | — | — | |||||||||||||||||||||
| Change in fair value of contingent consideration(B) | — | — | 983 | 983 | 984 | 3,158 | 3,158 | |||||||||||||||||||||
| Unusual charges(C)(D)(E) | 676 | 9,105 | 17,458 | 17,458 | 7,256 | 515 | 515 | |||||||||||||||||||||
| Other | (125 | ) | (28 | ) | (18 | ) | (38 | ) | (5 | ) | 46 | 46 | ||||||||||||||||
Adjusted EBITDA | $ | 52,066 | $ | 55,931 | $ | 57,747 | $ | 69,276 | $ | 27,339 | $ | 34,109 | $ | 35,157 | ||||||||||||||
13
Table of Contents
| • | Adjusted EBITDA does not reflect the provision of income tax expense in our various jurisdictions; | |
| • | Adjusted EBITDA does not reflect the interest expense we incur; | |
| • | Adjusted EBITDA does not reflect any attribution of costs to our operations related to our investments and capital expenditures through depreciation and amortization charges; | |
| • | Adjusted EBITDA does not reflect the cost of compensation we provide to our employees in the form of stock option awards; and | |
| • | Adjusted EBITDA excludes expenses that we believe are unusual or non-recurring, but which others may believe are normal expenses for the operation of a business. |
| (A) | In accordance with our amended senior secured credit facility and the indenture governing the notes, management fees payable to affiliates are excluded from Adjusted EBITDA. | |
| (B) | For the year ended December 31, 2010 and the six months ended June 30, 2010 and 2011, we recorded non-cash charges of $1.0 million, $1.0 million and $3.2 million, respectively, related to increases in the estimated fair value of contingent consideration issued in connection with our acquisitions completed after January 1, 2009. The increases related to changes from the original estimate of the fair value, including numerous variables such as the discount rate, remaining pay out period and the projected performance for each acquisition. | |
| (C) | During third quarter of 2009 and 2010, we recorded non-cash impairment charges of $8.0 million and $4.9 million, respectively, related to goodwill and other intangible assets. | |
| (D) | Unusual charges also includes an add-back for acquisition and business development costs as reported in our consolidated statements of operations. | |
| (E) | Net losses for the year ended December 31, 2010 and the six months ended June 30, 2010 reflect $9.3 million and $4.5 million, respectively, of write-offs of deferred debt issue costs and a $2.3 million loss on extinguishment of debt related to the refinancing and repayment of our debt facilities. | |
| (F) | The unaudited pro forma consolidated reconciliation of Adjusted EBITDA as of December 31, 2010 and six months ended June 30, 2011 does not give effect to our August 1, 2011 acquisition of Global Pathology Laboratory Services, Inc., or Global. For purposes of covenant compliance with our credit facility, we estimate the Adjusted EBITDA for Global to be $5.3 million and $2.7 million for the year ended December 31, 2010 and the six months ended June 30, 2011, respectively. |
14
Table of Contents
| Period from | ||||||||||||||||||||||||
| June 2006 | ||||||||||||||||||||||||
| (Inception) to | Six Months | |||||||||||||||||||||||
| December 31, | Year Ended December 31, | Ended June 30, | ||||||||||||||||||||||
| 2006 | 2007 | 2008 | 2009 | 2010 | 2011 | |||||||||||||||||||
Ratio of Earnings to Fixed Charges(A) | — | (B) | 1.3 | 1.5 | 1.5 | — | (C) | 1.1 | ||||||||||||||||
| (A) | For purposes of calculating this ratio, “earnings” consists of income from continuing operations before income taxes plus fixed charges. Fixed charges consist of interest expense, amortization of discount on indebtedness and an appropriate portion of rental expense representative of the interest factor. | |
| (B) | For the period from our inception in June 2006 through December 31, 2006, earnings were insufficient to cover fixed charges by $1,236,000. | |
| (C) | For the year ended December 31, 2010, earnings were insufficient to cover fixed charges by $677,000. |
15
Table of Contents
16
Table of Contents
17
Table of Contents
18
Table of Contents
19
Table of Contents
20
Table of Contents
21
Table of Contents
22
Table of Contents
| • | continue to expand our sales and marketing and research and development organizations; | |
| • | develop or acquire complementary technologies, services, products or businesses; | |
| • | expand operations both organically and through acquisitions; | |
| • | hire, train and retain employees; or | |
| • | respond to competitive pressures or unanticipated working capital requirements. |
23
Table of Contents
| • | execute our business model; | |
| • | create brand recognition; | |
| • | respond effectively to competition; | |
| • | manage growth in our operations; | |
| • | respond to changes in applicable government regulations and legislation; | |
| • | access additional capital when required; and | |
| • | attract and retain key personnel. |
24
Table of Contents
25
Table of Contents
| • | the federal Anti-Kickback Statute, which prohibits persons from knowingly and willfully soliciting, receiving, offering or providing remuneration, directly or indirectly, in cash or in kind, in exchange for or to induce either the referral of an individual for, or the purchase, order or recommendation of, any goods or service for which payment may be made under governmental payor programs such as Medicare and Medicaid; | |
| • | the federal False Claims Act, which prohibits individuals or entities from knowingly presenting to, or causing to be presented to, the federal government, claims for payment that are false or fraudulent; | |
| • | the Health Insurance Portability and Accountability Act, or HIPAA, which established comprehensive federal standards with respect to the use and disclosure of protected health information; | |
| • | the Health Information Technology for Economic and Clinical Health Act, or HITECH Act, which was passed as part of the American Recovery and Reinvestment Act and which strengthens many of the requirements applicable to privacy and security, among other things; | |
| • | the Stark Law, which prohibits a physician from making a referral to an entity for certain designated health services reimbursed by Medicare or Medicaid if the physician (or a member of the physician’s family) has a financial relationship with the entity and which also prohibits the submission of any claim for reimbursement for designated health services furnished pursuant to a prohibited referral; | |
| • | the federal Civil Monetary Penalty Law, which prohibits the offering of remuneration or other inducements to beneficiaries of federal health care programs to influence the beneficiaries’ decisions to seek specific governmentally reimbursable items or services or to choose particular providers; | |
| • | the Clinical Laboratory Improvement Amendments, which requires that laboratories be certified by the federal government or by a federally-approved accreditation agency; | |
| • | the anti-markup rule, which prohibits a physician or supplier billing the Medicare program from marking up the price of a purchased diagnostic service performed by another physician or supplier who does not “share a practice” with the billing physician or supplier; | |
| • | state law equivalents of the above, such as anti-kickback and false claims laws that may apply to items or services reimbursed by any third-party payor, including commercial insurers; | |
| • | state laws that prohibit the splitting or sharing of fees between physicians and non-physicians; | |
| • | state laws that govern the manner in which licensed physicians can be organized to perform and bill for medical services; | |
| • | the reassignment rules, which preclude Medicare payment for covered services to anyone other than the patient, physician, or other person who provided the service, with limited exceptions; and | |
| • | state laws that prohibit other specified practices, such as billing an entity that does not have ultimate financial responsibility for the service, waiving coinsurance or deductibles, billing Medicaid a higher charge than the lowest charge offered to another payor, and placing professionals who draw blood, or phlebotomists, in the offices of referring physicians. |
26
Table of Contents
27
Table of Contents
28
Table of Contents
29
Table of Contents
30
Table of Contents
31
Table of Contents
| • | our operating performance, financial condition and prospects, or the operating performance, financial condition and prospects of companies in our industry generally; | |
| • | the number of holders of the new notes; | |
| • | the interest of securities dealers in making a market for the new notes; | |
| • | prevailing interest rates; | |
| • | the market for similar debt securities; and | |
| • | the securities markets generally. |
32
Table of Contents
| • | increase our vulnerability to general adverse economic and industry positions; | |
| • | subject us to covenants that limit our ability to fund future working capital, capital expenditures, research and development costs and other general corporate requirements; | |
| • | require us to devote a substantial portion of our cash flow from operations to payments on our indebtedness, thereby reducing the availability of our cash flow to fund working capital, capital expenditures, research and development efforts and other general corporate purposes; | |
| • | limit our ability to obtain additional financing to fund future acquisitions; | |
| • | limit our flexibility in planning for, or reacting to, changes in our business and the industry in which we operate; | |
| • | impede our ability to obtain the necessary approvals to operate our business in compliance with the numerous laws and regulations to which we are subject; | |
| • | place us at a competitive disadvantage relative to our competitors that have less debt outstanding; and | |
| • | limit our ability to borrow additional funds for operational or strategic purposes (including to fund future acquisitions), among other things, under the financial and other restrictive covenants in our indebtedness. |
| • | incur additional indebtedness, make guarantees and enter into hedging arrangements; | |
| • | create liens on assets; | |
| • | engage in unrelated businesses; | |
| • | enter into sale and leaseback transactions; |
33
Table of Contents
| • | consolidate or merge with or into other companies or transfer all or substantially all of our assets; | |
| • | sell or dispose of assets; | |
| • | pay dividends on or make distributions in respect of our capital stock or make certain other restricted payments; | |
| • | make investments (including acquisitions), loans and advances; | |
| • | engage in certain transactions with affiliates; and | |
| • | agree to dividend payment and other restrictions affecting our subsidiaries. |
| • | all secured claims against the affected entity have been fully paid; and | |
| • | if the affected entity is a non-guarantor subsidiary, all other claims against that subsidiary, including trade payables, have been fully paid. |
34
Table of Contents
| • | we had outstanding an aggregate of $114.7 million of secured obligations that are effectively senior to the notes; | |
| • | the guarantors had outstanding an aggregate of approximately $114.7 million of secured obligations that are effectively senior to the notes, comprising their guarantee obligations in respect of our outstanding borrowings under our amended senior secured revolving credit facility; and | |
| • | other than their obligations owed to us, the non-guarantor subsidiaries had debt and other liabilities of $27.6 million, which excludes $13.6 million of the fair value of contingent consideration for acquisitions completed on or after January 1, 2009. |
35
Table of Contents
| • | we might not have enough available funds, particularly since a change of control could cause part or all of our other indebtedness to become due; and | |
| • | the agreements governing our amended senior secured credit facility and other secured indebtedness would prohibit us from repurchasing the notes, unless we were able to obtain a waiver or refinance such indebtedness. |
36
Table of Contents
| • | the guarantor intended to hinder, delay or defraud any present or future creditor; or | |
| • | the guarantor received less than reasonably equivalent value or fair consideration for the incurrence of the guarantee; and | |
| • | was insolvent or rendered insolvent by reason of such incurrence; | |
| • | was engaged in a business or transaction for which the guarantor’s remaining assets constituted unreasonably small capital; or | |
| • | intended to incur, or believed that it would incur, debts beyond its ability to pay those debts as they mature. |
| • | the sum of its debts, including contingent liabilities, was greater than the fair saleable value of all of its assets; | |
| • | the present fair saleable value of its assets was less than the amount that would be required to pay its probable liability on its existing debts, including contingent liabilities, as they become absolute and mature; or | |
| • | it could not pay its debts as they become due. |
37
Table of Contents
| • | what standard a court would apply in order to determine whether a guarantor was insolvent as of the date it issued the guarantee or whether, regardless of the method of valuation, a court would determine that the guarantor was insolvent on that date; or | |
| • | whether a court would determine that the payments under the guarantee constituted fraudulent transfers or conveyances on other grounds. |
38
Table of Contents
39
Table of Contents
| June 30, | ||||
| 2011 | ||||
| (Unaudited) | ||||
| (In thousands) | ||||
| Cash and cash equivalents | $ | 36,488 | ||
| Capital lease obligations | 275 | |||
| Guaranteed notes | 5,528 | |||
Fair value of contingent consideration(1) | 39,760 | |||
| Term loan | 114,438 | |||
| Senior notes | 200,000 | |||
| 360,001 | ||||
| Original issue discount, amended senior secured credit facility | (1,402 | ) | ||
| Total debt and fair value of contingent consideration | 358,599 | |||
| Members’ equity | 209,701 | |||
| Total capitalization | $ | 568,300 | ||
| (1) | In connection with the majority of our acquisitions, we have agreed to pay additional consideration in future periods based upon the attainment of stipulated levels of operating earnings by each of the acquired entities, as defined in their respective agreements. The above table does not reflect contingent consideration obligations entered into prior to January 1, 2009. Payments made under the contingent consideration obligations entered into prior to January 1, 2009 are recorded upon the attainment of the objectives, and the amount owed becomes fixed and determinable. As of June 30, 2011, assuming all of the acquired companies through January 1, 2009 achieved the maximum level of stipulated operating earnings, as defined in the agreements, the maximum principal amount of contingent consideration payable over the next five years is approximately $153.1 million, of which we have recorded $39.8 million for the fair value of contingent consideration obligations entered into subsequent to January 1, 2009. The fair value of contingent consideration excludes the amounts payable in connection with our acquisition of Global Pathology Laboratory Services on August 1, 2011. A lesser amount will be paid for earnings below the maximum level of stipulated earnings, and no payments will be made if the acquired companies do not achieve the minimum level of stipulated earnings as outlined in their respective agreements. Generally, the maximum amount payable is two times the amount we would pay based upon the EBITDA at the time we completed the acquisition. |
40
Table of Contents
| • | our 2010 acquisitions of Pathology Solutions, LLC and Biopsy Diagnostics, LLC as if these acquisitions occurred January 1, 2010; and | |
| • | our 2011 acquisitions of Austin Pathology Associates and Texas Pathology, LLC, Western Pathology Consultants, Ltd., and DermPath New England, LLC as if these acquisitions occurred January 1, 2010. |
41
Table of Contents
| Year Ended December 31, 2010 | ||||||||||||||||||||
| Pro | Pro | |||||||||||||||||||
| Forma | Forma | |||||||||||||||||||
| 2010 | 2011 | Acquisition | for | |||||||||||||||||
| Historical(1) | Acquisitions(7) | Acquisitions(3) | Adjustments(3)(7) | Acquisitions | ||||||||||||||||
| (In thousands) | ||||||||||||||||||||
Net Revenues | $ | 212,837 | $ | 8,579 | $ | 26,895 | $ | — | $ | 248,311 | ||||||||||
| Operating costs and expenses: | ||||||||||||||||||||
| Cost of services | 96,868 | 2,845 | 17,278 | (1,418 | )(4) | 115,573 | ||||||||||||||
| Selling, general and administrative expenses | 49,141 | 2,303 | 2,861 | 100 | (4) | 54,405 | ||||||||||||||
| Provision for doubtful accounts | 12,393 | 27 | — | — | 12,420 | |||||||||||||||
| Intangible asset amortization expense | 18,946 | — | — | 4,746 | (5) | 23,692 | ||||||||||||||
| Management fees | 2,189 | — | — | 355 | (6) | 2,544 | ||||||||||||||
| Impairment of goodwill and other intangible assets | 4,871 | — | — | — | 4,871 | |||||||||||||||
| Acquisition and business development costs | 1,032 | — | — | — | 1,032 | |||||||||||||||
| Change in fair value of contingent consideration | 983 | — | — | — | 983 | |||||||||||||||
Total operating costs and expenses | 186,423 | 5,175 | 20,139 | 3,783 | 215,520 | |||||||||||||||
Income from operations | 26,414 | 3,404 | 6,756 | (3,783 | ) | 32,791 | ||||||||||||||
| Other income (expense): | ||||||||||||||||||||
| Interest expense | (17,041 | ) | (9 | ) | (20 | ) | — | (17,070 | ) | |||||||||||
| Write-off of deferred debt issue costs | (9,259 | ) | — | — | — | (9,259 | ) | |||||||||||||
| Loss on extinguishment of debt | (2,296 | ) | — | — | — | (2,296 | ) | |||||||||||||
| Other income | 18 | 12 | 8 | — | 38 | |||||||||||||||
Total other income (expense), net | (28,578 | ) | 3 | (12 | ) | — | (28,587 | ) | ||||||||||||
Income (loss) before income taxes | (2,164 | ) | 3,407 | 6,744 | (3,783 | ) | 4,204 | |||||||||||||
| Provision for income taxes | 1,487 | — | 155 | — | 1,642 | |||||||||||||||
Net income (loss) | $ | (3,651 | ) | $ | 3,407 | $ | 6,589 | $ | (3,783 | ) | $ | 2,562 | ||||||||
42
Table of Contents
| Six Months Ended June 30, 2011 | ||||||||||||||||
| Pro | Pro | |||||||||||||||
| Forma | Forma | |||||||||||||||
| 2011 | Acquisition | for | ||||||||||||||
| Historical(1) | Acquisitions(3) | Adjustments(3) | Acquisitions | |||||||||||||
| (In thousands) | ||||||||||||||||
Net Revenues | $ | 130,470 | $ | 2,288 | $ | — | $ | 132,758 | ||||||||
| Operating costs and expenses: | ||||||||||||||||
| Cost of services | 58,846 | 755 | 125 | (4) | 59,726 | |||||||||||
| Selling, general and administrative expenses | 30,595 | 318 | 42 | 30,955 | ||||||||||||
| Provision for doubtful accounts | 8,862 | — | — | 8,862 | ||||||||||||
| Intangible asset amortization expense | 11,126 | — | 486 | (5) | 11,612 | |||||||||||
| Management fees | 1,344 | — | 23 | (6) | 1,367 | |||||||||||
| Change in fair value of contingent consideration | 3,158 | — | — | 3,158 | ||||||||||||
| Acquisition and business development costs | 515 | — | — | 515 | ||||||||||||
Total operating costs and expenses | 114,446 | 1,073 | 676 | 116,195 | ||||||||||||
Income from operations | 16,024 | 1,215 | (676 | ) | 16,563 | |||||||||||
| Other expense: | ||||||||||||||||
| Interest expense | (16,268 | ) | — | — | (16,268 | ) | ||||||||||
| Other expense | (46 | ) | — | — | (46 | ) | ||||||||||
Total other expense | (16,314 | ) | — | — | (16,314 | ) | ||||||||||
Income (loss) before income taxes | (290 | ) | 1,215 | (676 | ) | 249 | ||||||||||
| Provision for income taxes | 1,352 | — | — | 1,352 | ||||||||||||
Net income (loss) | $ | (1,642 | ) | $ | 1,215 | $ | (676 | ) | $ | (1,103 | ) | |||||
43
Table of Contents
| Twelve Months Ended June 30, 2011 | ||||||||||||||||||||
| Pro | Pro | |||||||||||||||||||
| Forma | Forma | |||||||||||||||||||
| 2010 | 2011 | Acquisition | for | |||||||||||||||||
| Historical(1) | Acquisitions(7) | Acquisitions(3) | Adjustments(3)(7) | Acquisitions | ||||||||||||||||
| (in thousands) | ||||||||||||||||||||
Net Revenues | $ | 242,202 | $ | 2,181 | $ | 16,025 | $ | — | $ | 260,408 | ||||||||||
| Operating costs and expenses: | ||||||||||||||||||||
| Cost of services | 109,810 | 618 | 10,370 | (1,267 | )(4) | 119,531 | ||||||||||||||
| Selling, general and administrative expenses | 56,298 | 788 | 1,838 | 92 | 59,016 | |||||||||||||||
| Provision for doubtful accounts | 15,227 | 36 | — | — | 15,263 | |||||||||||||||
| Intangible asset amortization expense | 20,798 | — | — | 2,569 | (5) | 23,367 | ||||||||||||||
| Management fees | 2,471 | — | — | 183 | (6) | 2,654 | ||||||||||||||
| Impairment of goodwill and other intangible assets | 4,871 | — | — | — | 4,871 | |||||||||||||||
| Change in fair value of contingent consideration | 3,157 | — | — | — | 3,157 | |||||||||||||||
| Acquisition and business development costs | 1,114 | — | — | — | 1,114 | |||||||||||||||
Total operating costs and expenses | 213,746 | 1,442 | 12,208 | 1,577 | 228,973 | |||||||||||||||
Income (loss) from operations | 28,456 | 739 | 3,817 | (1,577 | ) | 31,435 | ||||||||||||||
| Other income (expense): | ||||||||||||||||||||
| Interest expense | (25,722 | ) | (2 | ) | (19 | ) | — | (25,743 | ) | |||||||||||
| Write-off of deferred debt issue costs | (4,732 | ) | — | — | — | (4,732 | ) | |||||||||||||
| Other expense | (33 | ) | — | — | — | (33 | ) | |||||||||||||
Total other expense | (30,487 | ) | (2 | ) | (19 | ) | — | (30,508 | ) | |||||||||||
Income (loss) before income taxes | (2,031 | ) | 737 | 3,798 | (1,577 | ) | 927 | |||||||||||||
| Provision (benefit) for income taxes | 1,608 | — | (67 | ) | — | 1,541 | ||||||||||||||
Net income (loss) | $ | (3,639 | ) | $ | 737 | $ | 3,865 | $ | (1,577 | ) | $ | (614 | ) | |||||||
| (1) | Amounts represent our historical statements of operations for the year ended December 31, 2010 (audited) and the six months ended June 30, 2011 (unaudited) which were derived from the financial statements contained elsewhere in this prospectus. The summary historical unaudited financial information for the twelve months ended June 30, 2011 has been derived by adding our historical unaudited consolidated financial information for the six months ended June 30, 2011 to the historical audited consolidated financial information for the twelve months ended December 31, 2010 and deducting the historical unaudited consolidated financial information for the six months ended June 30, 2010, all included elsewhere in this prospectus, and then reflecting the pro forma adjustments set forth in the related footnotes. | |
| (2) | Amounts represent the combined historical unaudited statements of operations of Pathology Solutions, LLC prior to our acquisition on March 12, 2010, and Biopsy Diagnostics, LLC prior to our acquisition on October 8, 2010, which we refer to as the 2010 Acquisitions with respect to this pro forma information. | |
| (3) | Amounts represent the combined historical unaudited statements of operations of Austin Pathology Associates, Texas Pathology, LLC and Western Pathology Consultants, Ltd. prior to our acquisition on January 1, 2011, and DermPath New England, LLC prior to our acquisition on June 2, 2011, which we refer to as the 2011 Acquisitions, but not our August 1, 2011 acquisition of Global Pathology Laboratory Services, Inc. |
44
Table of Contents
| (4) | The pro forma adjustment reflects the reduction in compensation expense of physicians and former owners of acquired practices, including salary, bonus and other compensation, to the amounts that will be paid to these physicians and former owners in accordance with their post-acquisition employment agreements. | |
| (5) | Represents the additional amortization expense for the identifiable intangible assets, based on our acquisition accounting, as if the 2010 and 2011 Acquisitions had occurred on January 1, 2010. The identifiable intangible assets related to the 2010 and 2011 Acquisitions total approximately $56.0 million and are being amortized over periods ranging from 5 to 15 years. The majority of the identifiable intangible assets relate to healthcare facility agreements and customer relationships. In determining the estimated amortization periods, we considered the operating history and customer stability of the acquired practice and industry information related to customary amortization periods. | |
| (6) | Reflects the management fees payable under a management services agreement with certain of our Principal Equityholders. In accordance with the management services agreement, these fees are calculated as 1 percent of the net revenue of our 2010 and 2011 acquisitions. | |
| (7) | Amounts represent the historical unaudited statements of operations of Biopsy Diagnostics, LLC prior to our acquisition on October 8, 2010. |
45
Table of Contents
46
Table of Contents
Selected Consolidated Statements of Operations
Period from June 2006 (inception) to December 31, 2006,
Years ended December 31, 2007, 2008, 2009 and 2010 and
Six months ended June 30, 2010 and 2011
| Period from | ||||||||||||||||||||||||||||
| June 2006 | ||||||||||||||||||||||||||||
| (Inception) to | Six Months | |||||||||||||||||||||||||||
| December 31, | Year Ended December 31, | Ended June 30, | ||||||||||||||||||||||||||
| 2006 | 2007 | 2008 | 2009 | 2010 | 2010 | 2011 | ||||||||||||||||||||||
| (In thousands) | ||||||||||||||||||||||||||||
| Net Revenues | $ | 3,487 | $ | 63,451 | $ | 157,850 | $ | 171,565 | $ | 212,837 | $ | 101,105 | $ | 130,470 | ||||||||||||||
| Operating costs and expenses: | ||||||||||||||||||||||||||||
| Cost of services | 1,045 | 27,480 | 66,382 | 71,778 | 96,868 | 45,904 | 58,846 | |||||||||||||||||||||
| Selling, general and administrative expenses | 3,035 | 15,172 | 33,194 | 36,854 | 49,141 | 23,438 | 30,595 | |||||||||||||||||||||
| Provision for doubtful accounts | 69 | 2,378 | 8,037 | 9,488 | 12,393 | 6,028 | 8,862 | |||||||||||||||||||||
| Intangible asset amortization expense | 470 | 5,721 | 14,308 | 14,574 | 18,946 | 9,274 | 11,126 | |||||||||||||||||||||
| Management fees | 35 | 644 | 1,559 | 1,778 | 2,189 | 1,062 | 1,344 | |||||||||||||||||||||
| Impairment of goodwill and other intangible assets | — | — | — | 8,031 | (2) | 4,871 | (2) | — | — | |||||||||||||||||||
| Change in fair value of contingent consideration | — | — | — | — | 983 | 984 | 3,158 | |||||||||||||||||||||
| Acquisition and business development costs | — | 374 | 676 | 1,074 | 1,032 | 433 | 515 | |||||||||||||||||||||
| Equity based compensation expense | — | — | 1,164 | (1) | — | — | — | — | ||||||||||||||||||||
Total operating costs and expenses | 4,654 | 51,769 | 125,320 | 143,577 | 186,423 | 87,123 | 114,446 | |||||||||||||||||||||
Income (loss) from operations | (1,167 | ) | 11,682 | 32,530 | 27,988 | 26,414 | 13,982 | 16,024 | ||||||||||||||||||||
| Other income (expense): | ||||||||||||||||||||||||||||
| Interest expense | (94 | ) | (7,114 | ) | (21,577 | ) | (18,969 | ) | (17,041 | ) | (7,587 | ) | (16,268 | ) | ||||||||||||||
| Write-off of deferred debt issue costs | — | (3,451 | )(3) | — | — | (9,259 | )(3) | (4,527 | )(3) | — | ||||||||||||||||||
| Loss on extinguishment of debt | — | — | — | — | (2,296 | )(3) | (2,296 | )(3) | — | |||||||||||||||||||
| Other income | 25 | 124 | 125 | 28 | 18 | 5 | (46 | ) | ||||||||||||||||||||
Total other expense, net | (69 | ) | (10,441 | ) | (21,452 | ) | (18,941 | ) | (28,578 | ) | (14,405 | ) | (16,314 | ) | ||||||||||||||
Income (loss) before income taxes | (1,236 | ) | 1,241 | 11,078 | 9,047 | (2,164 | ) | 423 | (290 | ) | ||||||||||||||||||
Provision for income taxes(4) | — | 762 | 408 | 45 | 1,487 | 1,231 | 1,352 | |||||||||||||||||||||
Net income (loss) | $ | (1,236 | ) | $ | 479 | $ | 10,670 | $ | 9,002 | $ | (3,651 | ) | $ | (1,654 | ) | $ | (1,642 | ) | ||||||||||
47
Table of Contents
| December 31, | June 30, | |||||||||||||||||||||||
| 2006 | 2007 | 2008 | 2009 | 2010 | 2011 | |||||||||||||||||||
| (In thousands) | ||||||||||||||||||||||||
Consolidated Balance Sheet Data | ||||||||||||||||||||||||
| Cash and equivalents | $ | 1,744 | $ | 8,558 | $ | 7,278 | $ | 27,424 | $ | 39,941 | $ | 36,488 | ||||||||||||
| Total assets | 40,180 | 388,339 | 415,516 | 463,973 | 588,011 | 624,904 | ||||||||||||||||||
| Working capital, excluding deferred tax assets, current portion of long-term debt and current portion of fair value of contingent consideration | 1,892 | 10,161 | 11,005 | 29,808 | 51,272 | 35,047 | ||||||||||||||||||
| Long term debt and fair value of contingent consideration, including current portion | 7,550 | 215,678 | 227,313 | 219,606 | 345,364 | 358,599 | ||||||||||||||||||
| Members’ equity | 31,334 | 145,077 | 161,176 | 217,064 | 211,343 | 209,701 | ||||||||||||||||||
| (1) | During 2008, we adopted a new equity incentive plan, which we refer to as the 2008 Plan, to replace our original equity incentive plan. The 2008 Plan provided awards of membership interest units in Aurora Holdings that were denominated asClass D-1,Class D-2 andClass D-3 units. During 2008, we authorized and issued 4,000 D-1 units, 3,000 D-2 units and 3,000 D-3 units of Aurora Holdings under the 2008 Plan. All membership interest units in Aurora Holdings issued in 2008 were fully vested as of December 31, 2008. We recorded compensation expense of $1.2 million for these awards. There were no other grants under the 2008 Plan. | |
| (2) | As of September 30, 2009, we tested goodwill and intangible assets for potential impairment and recorded a non-cash impairment expense of $8.0 million resulting from a write down of $6.6 million in the carrying value of goodwill and a write down of $1.4 million in the carrying value of other intangible assets. The write-down of the goodwill and other intangible assets related to one reporting unit. Regarding this reporting unit, we believe events occurred and circumstances changed that more likely than not reduced the fair value of the intangible assets and goodwill below their carrying amounts. These events during 2009 consisted primarily of the loss of significant customers present at the acquisition date, which adversely affected the current year and expected future revenues and operating profit of the reporting unit. | |
| As of September 30, 2010, we tested goodwill and intangible assets for potential impairment and recorded a non-cash impairment expense of $4.9 million resulting from a write down of $2.0 million in the carrying value of goodwill and a write down of $2.9 million in the carrying value of other intangible assets. The write-down of the goodwill and other intangible assets related to one reporting unit. Regarding this reporting unit, we believe events occurred and circumstances changed that more likely than not reduced the fair value of the intangible assets and goodwill below their carrying amounts. These events during 2010 consisted primarily of the loss of significant customers present at the acquisition date, which adversely affected the current year and expected future revenues and operating profit of the reporting unit. | ||
| (3) | In December 2007 and again in May 2010, we refinanced our then-existing credit facilities. As a result, we wrote off unamortized deferred debt issue costs of $3.5 million in 2007 and $4.5 million in the six months ended June 30, 2010. In addition, we incurred a $2.3 million prepayment penalty in connection with the May 2010 refinancing. On December 20, 2010 we used a portion of the proceeds from the issuance of the Senior Notes to repay $110.0 million of the $224.4 million principal then owed under our term loan. In connection with the repayment, we recorded a non-cash write-off of the pro rata portion of |
48
Table of Contents
| unamortized original issue discount, prepaid administration fees, and debt issue costs of approximately $4.7 million related to our prior credit facilities. | ||
| (4) | Aurora Holdings is a Delaware limited liability company taxed as a partnership for federal and state income tax purposes, in accordance with the applicable provisions of the Internal Revenue Code. Accordingly, Aurora Holdings generally has not been subject to income taxes. The income attributable to Aurora Holdings has been allocated to the members of Aurora Holdings in accordance with the terms of the Aurora Holdings LLC Agreement. However, certain of our subsidiaries are corporations, file separate returns and are subject to federal and state income taxes. The provision for income taxes for these subsidiaries is reflected in our consolidated financial statements and includes federal and state taxes currently payable and changes in deferred tax assets and liabilities, excluding the establishment of deferred tax assets and liabilities related to the acquisitions. |
49
Table of Contents
FINANCIAL CONDITION AND RESULTS OF OPERATIONS
50
Table of Contents
51
Table of Contents
| • | verification of complete insurance information and patient demographics, | |
| • | active management and follow up on denials, | |
| • | delivery of scheduled statements to patients, and/or | |
| • | forwarding significant past due accounts to outside collection agencies. |
52
Table of Contents
| Cash Paid | ||||
| 2008 Acquisition | $ | 27,301 | ||
| 2009 Acquisition | $ | 15,340 | ||
| 2010 Acquisitions | $ | 53,435 | ||
| 2011 Acquisitions | $ | 78,056 | ||
53
Table of Contents
| Year Ended | Six Months Ended | |||||||||||||||||||
| December 31, | June 30, | |||||||||||||||||||
| 2008 | 2009 | 2010 | 2010 | 2011 | ||||||||||||||||
| Net revenues | 100.0 | % | 100.0 | % | 100.0 | % | 100.0 | % | 100.0 | % | ||||||||||
| Operating costs and expenses: | ||||||||||||||||||||
| Cost of services | 42.1 | 41.8 | 45.5 | 45.4 | 45.1 | |||||||||||||||
| Selling, general and administrative expenses | 21.0 | 21.5 | 23.1 | 23.2 | 23.4 | |||||||||||||||
| Provision for doubtful accounts | 5.1 | 5.5 | 5.8 | 6.0 | 6.8 | |||||||||||||||
| Intangible asset amortization expense | 9.1 | 8.5 | 8.9 | 9.2 | 8.5 | |||||||||||||||
| Management fees | 1.0 | 1.0 | 1.0 | 1.1 | 1.0 | |||||||||||||||
| Impairment of goodwill and other intangible assets | — | 4.7 | 2.3 | — | — | |||||||||||||||
| Change in fair value of contingent consideration | — | — | 0.5 | 1.0 | 2.4 | |||||||||||||||
| Acquisition and business development costs | 0.4 | 0.6 | 0.5 | 0.4 | 0.4 | |||||||||||||||
| Equity-based compensation expense | 0.7 | — | — | — | — | |||||||||||||||
Total operating costs and expenses | 79.4 | 83.6 | 87.6 | 86.2 | 87.7 | |||||||||||||||
Income from operations | 20.6 | 16.4 | 12.4 | 13.8 | 12.3 | |||||||||||||||
| Other income (expense): | ||||||||||||||||||||
| Interest expense | (13.7 | ) | (11.1 | ) | (8.0 | ) | (7.5 | ) | (12.5 | ) | ||||||||||
| Write-off of deferred debt issue costs | — | — | (4.4 | ) | (4.5 | ) | — | |||||||||||||
| Loss on extinguishment of debt | — | — | (1.1 | ) | (2.3 | ) | — | |||||||||||||
| Other income | 0.1 | — | — | — | — | |||||||||||||||
Total other expense, net | (13.6 | ) | (11.1 | ) | (13.5 | ) | (14.2 | ) | (12.5 | ) | ||||||||||
Income (loss) before income taxes | 7.0 | 5.3 | (1.0 | ) | (0.4 | ) | (0.2 | ) | ||||||||||||
| Provision for income taxes | 0.3 | — | 0.7 | 1.2 | 1.0 | |||||||||||||||
Net income (loss) | 6.7 | % | 5.3 | % | (1.7 | )% | (1.6 | )% | (1.3 | )% | ||||||||||
54
Table of Contents
55
Table of Contents
56
Table of Contents
57
Table of Contents
58
Table of Contents
59
Table of Contents
60
Table of Contents
61
Table of Contents
62
Table of Contents
63
Table of Contents
64
Table of Contents
| June 30, 2011 | ||||
| Term Loan | $ | 114,438 | ||
| Subordinated unsecured contingent note, dated April 30, 2007 | 5,528 | |||
| Senior Notes | 200,000 | |||
| Capital lease obligations | 275 | |||
| Total: | 320,241 | |||
| Less: | ||||
| Original issue discount, net | (1,402 | ) | ||
| Current portion | (2,753 | ) | ||
| Long-term debt, net of current portion | $ | 316,086 | ||
Years Ending December 31, | ||||
| 2011 | $ | 2,720 | ||
| 2012 | 2,910 | |||
| 2013 | 79 | |||
| 2014 | 67 | |||
| 2015 | 18 | |||
| 2016 | 114,447 | |||
| Thereafter | 200,000 | |||
| $ | 320,241 | |||
65
Table of Contents
66
Table of Contents
67
Table of Contents
| Payments Due by Period | ||||||||||||||||||||||||||||
Contractual Obligations | 2011 | 2012 | 2013 | 2014 | 2015 | Thereafter | Total | |||||||||||||||||||||
| Term loans | $ | — | $ | — | $ | — | $ | — | $ | — | $ | 114,437 | $ | 114,437 | ||||||||||||||
| Senior unsecured notes | — | — | — | — | — | 200,000 | 200,000 | |||||||||||||||||||||
| Capital lease obligations | 82 | 90 | 99 | 87 | 31 | 4 | 393 | |||||||||||||||||||||
Estimated interest on term loans(1) | 7,152 | 7,152 | 7,152 | 7,152 | 7,152 | 10,033 | 45,793 | |||||||||||||||||||||
| Estimated interest on senior notes | — | 21,500 | 21,500 | 21,500 | 21,500 | 64,500 | 150,500 | |||||||||||||||||||||
| Subordinated unsecured contingent notes | 2,689 | 2,840 | — | — | — | — | 5,529 | |||||||||||||||||||||
| Real estate leases | 3,060 | 2,521 | 2,388 | 2,019 | 1,830 | 5,643 | 17,461 | |||||||||||||||||||||
| Total | $ | 12,983 | $ | 34,103 | $ | 31,139 | $ | 30,758 | $ | 30,513 | $ | 394,617 | $ | 534,113 | ||||||||||||||
| (1) | Estimated interest payments on our term loans was calculated using the current effective interest rates (LIBOR (subject to a floor of 2.00 percent), plus 4.25 percent per annum) multiplied by the pro forma outstanding balances as of December 31, 2010. |
68
Table of Contents
69
Table of Contents
70
Table of Contents
71
Table of Contents
72
Table of Contents
73
Table of Contents
| • | Anatomic Pathology. Anatomic pathology services involve the diagnosis of cancer and other medical conditions through the examination of tissues (histology) and the analysis of cells (cytology). Volume is measured by the number of accessions, or the number of patient cases, where each accession may then include multiple specimens. Generally, the anatomic pathology process involves the preparation of slides by trained histotechnologists or cytotechnologists and the review of those slides by anatomic |
74
Table of Contents
| pathologists. Although anatomic pathologists do not treat patients, they establish a definitive diagnosis and may also consult with the referring physician. The anatomic pathology market was $14.3 billion, or 24 percent, of 2009 total diagnostic testing industry revenues according to the Laboratory Economics Report with $8.2 billion derived from the non-hospital anatomic pathology channel. Generally, anatomic pathology services command higher reimbursement rates, on a per specimen basis, than clinical pathology services. According to the G-2 Report the anatomic pathology market has expanded more rapidly than the overall industry, with revenues growing 4.9 percent on a compound annual basis between 2006 and 2010, compared to 4.8 percent for the rest of the industry. Excluding growth in esoteric testing, the remainder of the industry grew at a compound annual rate of only 1.8 percent over the same period. |
| • | Clinical Pathology. Clinical pathology services generally involve chemical testing and analysis of body fluids using standardized laboratory tests. These tests usually do not require the expertise of a pathologist and are frequently routine, automated and performed by large national or regional clinical laboratory companies and hospital laboratories. This is the largest diagnostic testing market representing $44.8 billion, or 76 percent, of 2009 total industry revenues according to the Laboratory Economics Report. | |
| • | Gene-Based and Other Esoteric Testing. Esoteric testing services typically involve unique and complex genetic and molecular diagnostics performed by skilled personnel using sophisticated instruments. As a result, the esoteric testing market is dominated by a limited number of commercial laboratories. Compound annual growth rate for the total esoteric testing market was 19.6 percent from 2006 to 2010, according to the G-2 Report. We believe the esoteric testing market will continue to demonstrate the fastest growth of any market in the U.S. diagnostic testing industry. |
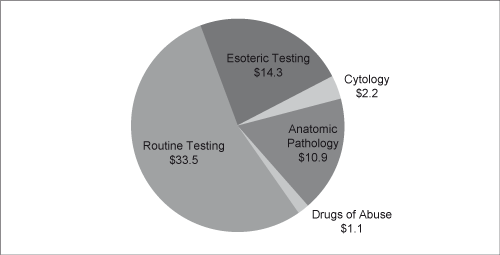
| • | Outpatient Channel. The outpatient channel of the anatomic pathology market involves diagnostic testing performed on tissues and cells of patients who do not reside in a medical facility during their diagnosis and treatment. The outpatient channel is divided into the hospital outpatient channel, which |
75
Table of Contents
| involves patients diagnosed and treated at hospitals on an outpatient basis, and the non-hospital outpatient channel, which involves patients diagnosed and treated at medical facilities other than hospitals on an outpatient basis. The non-hospital outpatient channel is the largest component of the anatomic pathology market and has grown more rapidly than other channels. This channel accounted for $8.2 billion, or 57 percent, of anatomic pathology revenues for the year ended December 31, 2009, representing 10 percent growth according to the Laboratory Economics Report. The hospital outpatient channel accounted for $2.1 billion, or 15 percent, of anatomic pathology revenues, representing 4 percent growth in 2009. |
| • | Inpatient Channel. The inpatient channel of the anatomic pathology market involves diagnostic testing performed on tissues and cells of patients who reside in a hospital during their diagnosis and treatment. Typically, the hospital operates its own clinical and histology laboratories but contracts on an exclusive basis with an independent pathology group. In 2009, according to the Laboratory Economics Report, the hospital inpatient channel accounted for $4.0 billion, or 28 percent, of the anatomic pathology revenues. |
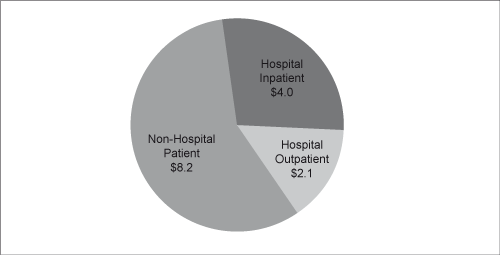
| • | Women’s Health Pathology. The women’s health pathology subspecialty, which includes gynecological pathology and cytopathology, principally involves diagnostic tests performed on samples such as Pap smears and cervical biopsies that are primarily provided by referring physicians in the obstetrics and gynecology areas. In 2009, according to the Laboratory Economics Report, the women’s health pathology subspecialty accounted for $2.7 billion, or 33 percent, of the broader non-hospital outpatient channel. In particular, the cervical cancer screening market in the U.S. has tripled over the past 10 years to $2 billion in 2009, driven by the adoption of more expensive monolayer liquid-based testing technologies, favorable changes in Medicare reimbursement and the introduction of DNA-based human |
76
Table of Contents
| papillomavirus testing for indeterminate Pap tests. From 1998 to 2009, monolayer technology grew from 16 percent to 95 percent of the women’s health pathology subspecialty, according to the Laboratory Economics Report. |
| • | Urologic Pathology. The urologic pathology subspecialty involves diagnostic tests performed on tissue specimens such as prostate biopsies that are provided by referring physicians in the urology area. In particular, the prostate cancer screening market is large and growing and in 2009, according to the Laboratory Economics Report, represented $1.9 billion, or 23 percent, of the broader non-hospital outpatient channel. | |
| • | Dermatopathology. The dermatopathology subspecialty primarily involves diagnostic tests performed on samples such as skin biopsies that are primarily provided by referring physicians in the dermatology or plastic surgery areas. In 2009, according to the Laboratory Economics Report, dermatopathology accounted for $1 billion, or 12 percent, of the broader non-hospital channel, and the number of new skin cancer cases grew 14 percent between 2007 and 2010, according to the Laboratory Economics Report. | |
| • | Other. The non-hospital outpatient channel includes other subspecialty areas such as gastrointestinal pathology, which involves diagnostic tests performed on samples such as endoscopic biopsies that are primarily provided by gastroenterologists, and hematopathology, which involves diagnostic tests performed on samples such as blood, bone marrow and lymph node biopsies that are primarily provided by referring physicians in the hematology and oncology areas. In 2009, according to the Laboratory Economics Report, these other subspecialties accounted for about $2.6 billion, or 32 percent, in aggregate, of the broader non-hospital channel. |
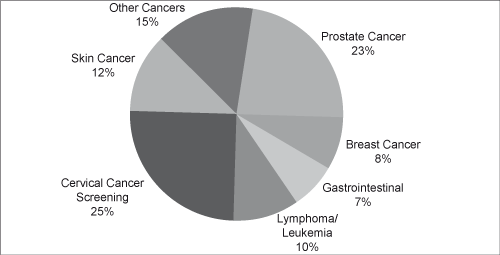
77
Table of Contents
| • | Leading Market Position in Higher-Growth Subspecialties of Expanding Industry. We are a leading specialized diagnostics company, focused on the faster-growing non-hospital outpatient channel within the anatomic pathology market with leading market positions in two of the three higher-growth subspecialties of the market: dermatopathology and women’s health pathology. | |
| • | Locally-Focused Business Model with National Scale. Our business model centers on achieving significant local market share, which yields operating efficiencies and national scale when consolidated across all of our operations. The diagnostic services we provide are designed specifically to meet the needs of the local markets we serve. Our national infrastructure enables us to more efficiently manage our operations, improve productivity and deliver a more extensive menu of diagnostic services to our local clients. As a result of our strong local presence and high-quality diagnostic services, we have established significant loyalty with referring physicians and key payors in our local markets. In 2010, we derived more than 85 percent of our revenues from locally-focused,in-network payor contracts. | |
| • | Experienced, Specialized Pathologists Focused on Client Service. We believe our pathologists have long-standing client relationships and provide high-quality service within their respective local communities. Over one-third of our pathologists are specialized in dermatopathology, with the remainder focused on women’s health pathology, urologic pathology, hematopathology and general surgical pathology. This alignment of our pathologists’ specialties with those of the referring physicians is critical to our ability to retain existing and attract new clients. Our clinical expertise and frequent interactions with clients on patient diagnoses enables us to establish effective consultative and long-term relationships with referring physicians. | |
| • | Professional Sales, Marketing and Client Service Team. We maintain a sales, marketing and client service team of over 100 professionals who are highly-trained and organized by subspecialty to better meet the needs of our referring physicians and their patients. Our sales representatives are incentivized through compensation plans to not only secure new physician clients, but also to maintain and enhance relationships with existing physician clients. As a result, they have enabled us to expand our geographic market presence to approximately 30 states and increase market penetration and market share in our local markets. | |
| • | Proprietary IT Solutions. Delivery of clinical information is essential to our business and a critical aspect of the differentiated service that we provide to our clients. We have developed scalable IT solutions that maximize the flexibility,ease-of-use and speed of delivery of our diagnostic reports, which has enabled us to rapidly grow our accession volume and meet the increasing physician demand for our diagnostic services. We also monitor referral patterns on a daily basis using our IT infrastructure, which allows us to respond quickly to referring physicians through our sales and marketing teams. We achieved this through the development of a proprietary suite of IT solutions calledConnectDXthat is compatible with most electronic medical record, or EMR, systems.ConnectDXincorporates customized interface solutions, low cost and efficient printer capabilities, compliant web portal capacities, and proprietary software, all resulting in efficient and reliable onsite client connections. |
78
Table of Contents
| • | Proven Acquisition, Integration and Development Capabilities. We have significant expertise and a proven track record of identifying, acquiring and integrating pathology practices into our diagnostic laboratory network. Our management team successfully expanded our operations through the acquisition of 22 anatomic pathology laboratories and one clinical laboratory and through the development of two de novo diagnostic laboratories. We have improved the performance of the laboratories we have acquired by applying our standard operating procedures, enhancing sales and marketing capabilities, implementing our IT platform and realizing efficiencies from our national operations and management. We believe our operational platform, expertise and value proposition enable us to capitalize on the considerable consolidation opportunities in the highly-fragmented anatomic pathology market. | |
| • | Experienced Senior Leadership. Our senior management team has approximately 80 years of combined experience in the health care industry, including senior management positions with leading diagnostic companies including AmeriPath, DIANON Systems and Laboratory Corporation of America, and, collectively, have successfully completed over 65 acquisitions and built a number of de novo specialized diagnostic laboratories. We believe that our management’s strong reputation, extensive network of industry relationships and experience in building and growing successful companies in the industry help us to drive operating performance, hire and retain talented pathologists and other employees and attract acquisition candidates. |
| • | Continue to Drive Market Penetration through Sales and Marketing. We plan to drive organic growth through our professional sales and marketing organization. Our 70 person sales and marketing team provides us with broad coverage to augment and further penetrate existing physician relationships and to develop new referral relationships. We plan to strategically add sales professionals to laboratories in markets that will most benefit from enhanced outreach, increasing our presence in existing and new markets. | |
| • | Leverage our IT Platform to Increase Operating Efficiencies. We believe our IT platform will allow us to gain market share in our existing subspecialties by improving productivity and reducing turnaround times. We have recently introduced an IT solution calleddoc2MD, a leading EMR system for dermatology practices for which we have an exclusive, long-term license. We intend to continue to develop our internal IT operations into a better-integrated diagnostic platform, which will improve national coordination and provide real time visibility into key performance metrics. In addition, we plan to continue to introduce innovative IT solutions, interface capabilities and market-specific IT solutions that enhance our value proposition to referring physicians. | |
| • | Expand through Targeted Acquisitions. We plan to identify and acquire leading laboratories to augment our organic growth, broaden our geographic presence and enhance our service offering. We intend to continue to build our business and enhance our reputation as a preferred acquiror for independent laboratories. We believe that our recognizable identity and strong reputation make us a preferred partner for independent laboratories. | |
| • | Expand Diagnostic Services Capabilities. We intend to expand our services in the areas of clinical and molecular diagnostics to complement our existing anatomic pathology businesses. We believe we can leverage our depth of experience and physician relationships to sell these new diagnostic services in conjunction with our existing testing services as a comprehensive offering. As a “one-stop” diagnostic services provider, we would not only better serve our current clients, but also position ourselves to attract new business under a more diverse service model. | |
| • | Develop De Novo Diagnostics Laboratories. We plan to continue to selectively develop diagnostic laboratories on a de novo basis, as we have done in certain markets, to expand our market presence, broaden our service offering and leverage the capabilities of our existing laboratories and pathologists. |
79
Table of Contents
| • | Expand Contracts with Hospitals in Target Markets. We intend to continue to develop additional contracts with hospitals in target markets as part of a broader strategy to strengthen and grow our outpatient business and expand our local market share. | |
| • | Further Expand into Growing Long-Term Care Market. We have a growing presence providing clinical diagnostic services to the long-term care markets in Central and Northern Florida. We intend to expand this regional coverage into the large South Florida market and replicate our success in other states with growing long-term care markets. We believe that our IT solutions, and our ability to meet the rapid service requirements for the long term care market, provide us with a significant competitive advantage in these markets. |
80
Table of Contents
| • | DermDX (dermatopathology services for the dermatology market); | |
| • | WomensDX (Womens Health Services for the OB/GYN market); | |
| • | UroDX (for the Urology market); | |
| • | GastroDX (for the Gastroenterology market); | |
| • | HemaDX (for the Hematology and Oncology market); | |
| • | TreatmentDX (drug treatment market); and | |
| • | CareDX (long term and assisted care markets). |
81
Table of Contents
| • | Local and Regional Pathology Groups. Local and regional pathology groups typically provide a relatively narrow menu of test services to community physicians and, in certain cases, to hospital-based pathologists. | |
| • | National Laboratory Companies. National laboratories typically offer a full suite of tests for a variety of medical professionals, including general practitioners, hospitals and pathologists. National laboratories have identified anatomic pathology as a focus area for future growth and will continue to be a competitive challenge going forward. | |
| • | Hospital Pathologists. Pathologists working in hospitals typically provide most of the diagnostic services required for hospital in-patients and, sometimes, hospital outpatients. Hospital pathologists act |
82
Table of Contents
| as medical directors for the hospital’s clinical and histology laboratories. Typically, hospital pathologists provide these services to hospitals under exclusive and long-term contractual arrangements. |
| • | Specialty Physician Groups. An increasing number of specialty physician groups (dermatologists, urologists and gastroenterologists in particular) are building their own laboratories and in-sourcing pathology services. This trend represents a significant source of competition and has impacted the anatomic pathology landscape, and we believe it will continue to do so in the future. |
83
Table of Contents
84
Table of Contents
| • | recruiting, training, employing and managing the technical and support staff; | |
| • | developing and equipping laboratory facilities; | |
| • | establishing and maintaining courier services to transport specimens; | |
| • | negotiating and maintaining contracts with hospitals, managed care organizations and other payors; | |
| • | providing financial reporting and administration, clerical, purchasing, payroll, billing and collection, information systems, sales and marketing, risk management, employee benefits, legal, tax and accounting services; and | |
| • | monitoring compliance with applicable laws and regulations. |
85
Table of Contents
86
Table of Contents
| • | variability in coverage and information requirements among various payors; | |
| • | missing, incomplete or inaccurate billing information provided by ordering physicians; | |
| • | billings to payors with whom we do not have contracts; and | |
| • | disputes with payors as to which party is responsible for payment or as to the appropriate level of reimbursement. |
87
Table of Contents
| • | variability in coverage and information requirements among various payors; | |
| • | increased complexity in our billing due to the additional procedures and processes required by governmental payor programs; | |
| • | training and education of our employees and customers; | |
| • | compliance and legal costs; and | |
| • | costs related to, among other factors, medical necessity denials and the absence of advance beneficiary notices. |
| • | a third party who provides coverage to the patient, such as an insurance company, managed care organization or a governmental payor program; | |
| • | the physician or other authorized party (such as a hospital or another laboratory) who ordered the testing service or otherwise referred the services to us; or | |
| • | the patient. |
88
Table of Contents
89
Table of Contents
90
Table of Contents
91
Table of Contents
| • | provide items or services to the physician or other referral source without charge or for amounts that are less than their fair market value; | |
| • | pay the physician or other referral source amounts that are in excess of the fair market value of items or services that were provided; or | |
| • | enter into an arrangement with a physician or other entity because it is a current or potential referral source. |
92
Table of Contents
| • | employees to furnish valuable services for physicians (other than collecting patient specimens for testing for the laboratory) that are typically the responsibility of the physicians’ staff; | |
| • | free testing to physicians’ managed care patients in situations where the referring physician benefits from the free laboratory tests; | |
| • | freepick-up and disposal of biohazardous waste for physicians for items unrelated to a laboratory’s testing services; | |
| • | general-use facsimile machines or computers to physicians that are not exclusively used in connection with the laboratory services; and | |
| • | free testing for healthcare providers, their families and their employees, known as professional courtesy testing. |
93
Table of Contents
94
Table of Contents
95
Table of Contents
96
Table of Contents
97
Table of Contents
98
Table of Contents
99
Table of Contents
Name | Age | Position(s) | ||||
| Jon L. Hart | 53 | Manager, Chief Executive Officer and President | ||||
| Martin J. Stefanelli | 50 | Chief Operating Officer, Vice President and Secretary | ||||
| Gregory A. Marsh | 50 | Chief Financial Officer, Vice President and Treasurer | ||||
| Fred Ferrara | 44 | Chief Information Officer | ||||
| Michael J. Null | 41 | Vice President, Sales and Marketing | ||||
| James C. New | 66 | Chairman of our Board of Managers | ||||
| Thomas S. Roberts | 48 | Manager | ||||
| Christopher Dean | 37 | Manager | ||||
| Peter J. Connolly | 38 | Manager | ||||
| Christopher J. Bock | 41 | Manager | ||||
| Blair Tikker | 55 | Manager | ||||
| Bennett Thompson | 33 | Manager | ||||
| James Emanuel | 63 | Manager | ||||
| Jon L. Hart Chief Executive Officer and President | Mr. Harthas served as our Chief Executive Officer and President since September 1, 2011. From 2006 until September 2011, he served as the Senior Vice President and head of Genzyme Genetics, which was sold in 2010 by Genzyme Corporation to Laboratory Corporation of America (LabCorp). From 1998 to 2006, he was a Senior Vice President of Quest Diagnostics. Prior to his time at Quest, Mr. Hart was an executive of SmithKline Beecham Clinical Laboratories. The Board has concluded that Mr. Hart should serve as a manager because he is our Chief Executive Officer and President. We and the Board benefit from his extensive experience in managing pathology companies. | |
| Martin J. Stefanelli Chief Operating Officer, Vice President and Secretary | Mr. Stefanellihas served as our Chief Operating Officer, Vice President and Secretary since 2006. Prior to joining us, Mr. Stefanelli served as the President and Chief Operating Officer of Asterand, a tissue-based research services provider for the pharmaceutical and biotechnology industry, from 2004 to 2006. Mr. Stefanelli served as the Executive Vice President and Chief Operating Officer of AmeriPath, an anatomic pathology laboratory company, from June 2003 to November 2004, and prior to joining AmeriPath, Mr. Stefanelli was employed for thirteen years by DIANON Systems, an anatomic and clinical pathology laboratory company. | |
| Gregory A. Marsh Chief Financial Officer, Vice President and Treasurer | Mr. Marshhas served as our Chief Financial Officer, Vice President and Treasurer since November 2007. Prior to joining us, Mr. Marsh served as an executive officer at CardioNet and PDSHeart, each a cardiovascular diagnostic healthcare provider. He served as the Chief Financial Officer of PDSHeart from 2003 to 2005 and then Chief Operating Officer from 2005 until March 2007, when the company was acquired by CardioNet. Mr. Marsh then served as the Chief Financial Officer of CardioNet until November 2007. From 1996 until 2003, Mr. Marsh was employed by AmeriPath, an anatomic |
100
Table of Contents
| pathology laboratory company, serving as Vice President, Chief Financial Officer and Secretary from 2001 to 2003 and Vice President, Corporate Controller from 1996 to 2001. | ||
| Fred Ferrara Chief Information Officer | Mr. Ferrarahas served as our Chief Information Officer since 2006. Mr. Ferrara served as the Director of Information Technology at LabCorp Inc., an anatomic pathology laboratory company, from 2003 until he joined Aurora in 2006. Mr. Ferrara joined LabCorp upon its acquisition of DIANON Systems, where Mr. Ferrara had been employed since 1997. | |
| Michael J. Null Vice President, Sales and Marketing | Mr. Nullhas served as our Vice President, Sales & Marketing since April 2007. Prior to joining us, Mr. Null served as the Vice President of Sales and Marketing at Asterand, a tissue-based research services provider for the pharmaceutical and biotechnology industry, from 2002 to 2007. He served as a senior account manager and business development manager at Renaissance, a global IT consulting and staffing company, from 1997 to 2002. Prior to joining Renaissance, Mr. Null was employed for four years by DIANON Systems, an anatomic and clinical pathology laboratory company. | |
| James C. New Chairman of the Board of Managers | Mr. Newhas served as our Chairman since 2006. He also served as our Chief Executive Officer and President from 2006 until his retirement on September 1, 2011. Since his retirement, he has served as Special Advisor to the Board of Directors and the Chief Executive Officer. Prior to joining us, Mr. New was a private investor from 2003 to 2006. Mr. New served as the President, Chief Executive Officer and Chairman of AmeriPath, an anatomic pathology laboratory company, from January 1996 through 2003. Prior to joining AmeriPath, Mr. New served as the President, Chief Executive Officer, and a director of RehabClinics, an outpatient rehabilitation company. The Board has concluded that Mr. New should serve as a manager and our Chairman because of his extensive experience in managing anatomic pathology companies, including his experience as our Chief Executive Officer and President. | |
| Thomas S. Roberts Manager | Mr. Robertshas served as one of our managers since 2006 and currently serves as a Managing Director of Summit Partners, a growth equity firm. Mr. Roberts joined Summit Partners in 1989. Mr. Roberts also served in the past as the Chairman and Director of AmeriPath, an anatomic pathology laboratory company. The Board has concluded that Mr. Roberts should serve as a manager because of his significant executive experience as well as the fact that his relationship with Summit Partners, which has a substantial ownership interest in us, aligns his interests with those of our other stakeholders. | |
| Christopher Dean Manager | Mr. Deanhas served as one of our managers since 2006 and currently serves as a Managing Director of Summit Partners, a growth equity firm. Mr. Dean joined Summit Partners in 2001. The Board has concluded that Mr. Dean should serve as a manager because of his significant executive experience as well as the fact that his relationship with Summit Partners, which has a substantial ownership interest in us, aligns his interests with those of our other stakeholders. | |
| Peter J. Connolly Manager | Mr. Connollyhas served as one of our managers since 2006 and currently serves as a Principal at Summit Partners, a growth equity firm. |
101
Table of Contents
| Prior to joining Summit Partners in 2004, Mr. Connolly was employed by Goldman, Sachs & Co., an investment banking firm and Deloitte LLP, an accounting and consulting firm. The Board has concluded that Mr. Connolly should serve as a manager because of his significant executive experience as well as the fact that his relationship with Summit Partners, which has a substantial ownership interest in us, aligns his interests with those of our other stakeholders. | ||
| Christopher J. Bock Manager | Mr. Bockhas served as one of our managers since June 2009 and is a Managing Director of KRG Capital Partners, a private equity investment firm. Mr. Bock joined KRG Capital Partners in 1997. The Board has concluded that Mr. Bock should serve as a manager because of his significant executive experience as well as the fact that his relationship with KRG Capital Partners, which has a substantial ownership interest in us, aligns his interests with those of our other stakeholders. | |
| Blair Tikker Manager | Mr. Tikkerhas served as one of our managers since June 2009 and is a Managing Director of KRG Capital Partners, a private equity investment firm. Mr. Tikker joined KRG Capital Partners in 2007. Prior to joining KRG Capital Partners, Mr. Tikker was employed by a number of hospital systems, physician groups and managed care companies. Mr. Tikker served as the CEO of HMS Healthcare, a hospital information systems provider, from 2001 until 2005. The Board has concluded that Mr. Tikker should serve as a manager because of his significant executive experience as well as the fact that his relationship with KRG Capital Partners, which has a substantial ownership interest in us, aligns his interests with those of our other stakeholders. | |
| Bennett R. Thompson Manager | Mr. Thompsonhas served as one of our managers since August 2011 and has been a regular board observer since June 2009. Mr. Thompson is a Vice President of KRG Capital Partners, a private equity investment firm. Mr. Thompson joined KRG Capital Partners in 2007. Prior to joining KRG Capital Partners in 2007, Mr. Thompson was employed by Heritage Partners, a private equity investment firm and Harris Williams & Co., an investment banking firm. The Board has concluded that Mr. Thompson should serve as a manager because of his significant executive experience as well as the fact that his relationship with KRG Capital Partners, which has a substantial ownership interest in us, aligns his interests with those of our other stakeholders. | |
| James Emanuel Manager | Mr. Emanuelhas served as one of our managers since June 2011. Mr. Emanuel has engaged in consulting and private investment activities since his retirement from Lincare, Inc., a national provider of respiratory therapy services for patients with pulmonary disorders, where he served as Chief Financial Officer from January 1984 to June 1997. Mr. Emanuel also served as Chief Financial Officer and a director of Lincare Holdings Inc. from November 1990 to June 1997. Mr. Emanuel has served as a director of SRI/Surgical Express Inc. since 1996 in addition to serving on private company boards. The Board has concluded that Mr. Emanuel should serve as a manager because of his significant executive experience. |
102
Table of Contents
103
Table of Contents
| • | James C. New, Chairman of our Board of Managers, and our former Chief Executive Officer and President; | |
| • | Gregory A. Marsh, our Chief Financial Officer, Vice President and Treasurer; | |
| • | Fred Ferrara, our Chief Information Officer; | |
| • | Michael J. Null, our Vice President, Sales and Marketing; and | |
| • | Martin J. Stefanelli, our Chief Operating Officer, Vice President and Secretary. |
104
Table of Contents
| • | net revenue and EBITDA to focus the executive on supporting, improving and growing our business; | |
| • | acquisitions of companies with annualized EBITDA at pre-determined levels focuses the executive on identifying acquisitions which meet our financial goals and positively affect our covenant position; and | |
| • | all other performance goals are initiatives deemed by the Board of Managers and executive management to be critical to the future success of the Company. |
105
Table of Contents
Name | Performance Objective and Weight | |||
| Mr. New | • | Net revenue (15)% | ||
| • | EBITDA (40)% | |||
| • | Acquisition Management (15%) | |||
| • | Completion of Initial Public Offering (15%) | |||
| • | Completion of Debt Refinancing (15)% | |||
| Mr. Stefanelli | • | Net revenue (20)% | ||
| • | EBITDA (40)% | |||
| • | Completion of Initial Public Offering (10)% | |||
| • | Completion of Debt Refinancing (10%) | |||
| • | Corporate Budget Management (10%) | |||
| • | LIS Implementation (10)% | |||
| Mr. Marsh | • | Net revenue (20)% | ||
| • | EBITDA (40)% | |||
| • | Completion of Initial Public Offering (15)% | |||
| • | Completion of Debt Refinancing (15%) | |||
| • | Sarbanes-Oxley Compliance (10)% | |||
| Mr. Ferrara | • | Net revenue (20)% | ||
| • | EBITDA (40)% | |||
| • | HIPAA Compliance (10%) | |||
| • | LIS Implementation (20%) | |||
| • | Sarbanes-Oxley Compliance (10)% | |||
| Mr. Null | • | Net revenue (45)% | ||
| • | EBITDA (10)% | |||
| • | Product Launches (45%) | |||
Objective | Target Goal | FY 2010 Actual | ||
| Net Revenue | $227.8 | $212.8 | ||
| EBITDA | $ 69.2 | $ 58.8 | ||
| Acquisition Management | Targets’ Annualized | Targets’ Annualized | ||
| EBITDA of $10.0 | EBITDA of $16.2 | |||
| Completion of Initial Public Offering | N/A | 0% | ||
| Completion of Debt Refinancing | N/A | 100% | ||
| Corporate Budget | $(11.0) | $(10.4) | ||
| LIS Implementation | N/A | 50% | ||
| Sarbanes-Oxley Compliance | N/A | 70-75% | ||
| Product Launches | N/A | 20-200% | ||
| HIPAA Compliance | N/A | 100% |
106
Table of Contents
| Target | Target Bonus | Actual Bonus | Actual Bonus Earned | |||||||||||||
Name | Bonus ($) | (% of Base Salary) | Earned ($) | (% of Base Salary) | ||||||||||||
| Mr. New | $ | 400,000 | 100 | % | $ | 200,500 | 50 | % | ||||||||
| Mr. Stefanelli | $ | 236,250 | 75 | % | $ | 88,100 | 37 | % | ||||||||
| Mr. Marsh | $ | 147,000 | 50 | % | $ | 42,400 | 29 | % | ||||||||
| Mr. Ferrara | $ | 89,040 | 40 | % | $ | 30,600 | 34 | % | ||||||||
| Mr. Null | $ | 79,013 | 35 | % | $ | 40,600 | 51 | % | ||||||||
107
Table of Contents
108
Table of Contents
| Non-Equity | ||||||||||||||||||||||||
| Incentive Plan | All Other | |||||||||||||||||||||||
Name and Principal Position | Year | Salary ($) | Bonus ($) | Compensation ($)(2) | Compensation ($)(3) | Total ($) | ||||||||||||||||||
James C. New(1) | 2010 | 400,000 | — | 200,500 | 40,840 | 641,340 | ||||||||||||||||||
| Chairman of our Board of Managers, Chief Executive Officer and President | ||||||||||||||||||||||||
Martin J. Stefanelli | 2010 | 315,000 | — | 88,100 | 630 | 403,730 | ||||||||||||||||||
| Chief Operating Officer, | ||||||||||||||||||||||||
| Vice President and Secretary | ||||||||||||||||||||||||
Gregory A. Marsh | 2010 | 294,000 | 107,500 | (4) | 42,400 | 966 | 444,866 | |||||||||||||||||
| Chief Financial Officer, | ||||||||||||||||||||||||
| Vice President and Treasurer | ||||||||||||||||||||||||
Fred Ferrara | 2010 | 225,169 | — | 30,600 | 287 | 256,056 | ||||||||||||||||||
| Chief Information Officer | ||||||||||||||||||||||||
Michael J. Null | 2010 | 228,355 | — | 40,600 | 56,006 | 324,961 | ||||||||||||||||||
| Vice President, Sales and Marketing | ||||||||||||||||||||||||
| (1) | Effective September 1, 2011, Mr. New retired as our Chief Executive Officer and President. | |
| (2) | Reflects the dollar amount of annual performance-based bonuses earned by our named executive officers in 2010. | |
| (3) | Reflects premiums paid in accordance with the executive-level life insurance plan. Also includes (i) for Mr. New, reimbursement of $15,970 related to country club memberships, $23,970 in premiums paid for a separate life insurance policy, and $900 for tax preparation services; and (ii) for Mr. Null, $55,700 relating to our forgiveness of a note extended to him in connection with his relocation to Florida. See “Certain Relationships and Related Party Transactions”. | |
| (4) | Reflects the bonus Mr. Marsh received in recognition of his role in connection with our bank refinancing and initial public offering efforts. For more information regarding the annual bonuses, see “— 2010 Elements of Compensation;Annual Cash Bonuses.” |
| Estimated Future Payouts Under | ||||||||||||
| Non-Equity Incentive Plan Awards(1) | ||||||||||||
| Threshold | Target | Maximum | ||||||||||
Name | ($) | ($) | ($) | |||||||||
| Mr. New | — | 400,000 | 800,000 | |||||||||
| Mr. Stefanelli | — | 236,250 | 472,500 | |||||||||
| Mr. Marsh | — | 147,000 | 294,000 | |||||||||
| Mr. Ferrara | — | 89,040 | 178,080 | |||||||||
| Mr. Null | — | 79,013 | 158,026 | |||||||||
109
Table of Contents
| (1) | Reflects potential payout opportunities under the annual bonus plan. The actual amount earned by each named executive officer is reflected in the “Non-Equity Incentive Plan Compensation” column of the Summary Compensation table. |
| • | his base salary for a specified period (in the case of Mr. New, 24 months, in the case of Messrs. Stefanelli, Ferrara and Null, 12 months, and in the case of Mr. Marsh, 12 months if his termination occurs prior to a “qualifying transaction” (as defined below under “— Potential Payments Upon Change in Control”) or 18 months if his termination occurs within one year following a “qualifying transaction”), payable in equal installments in accordance with our regular payroll practices; | |
| • | in the case of Mr. New, an amount equal to two times the average of his previous three annual bonuses, payable in installments in accordance with our regular payroll practices; | |
| • | any unpaid bonus for the previous fiscal year and a pro rata portion of his bonus for the then-current fiscal year; and | |
| • | in the case of Mr. New, continued health care coverage for a period of 24 months. |
110
Table of Contents
| In Connection | ||||||||||||||||||||||
| with a | ||||||||||||||||||||||
| Before Change | Change in | |||||||||||||||||||||
| in Control | Control | |||||||||||||||||||||
| Termination | Termination | For Cause/ | ||||||||||||||||||||
| w/o Cause or for | w/o Cause or for | Voluntary | ||||||||||||||||||||
Name | Benefit | Good Reason ($) | Good Reason ($) | Termination ($) | Death ($) | Disability ($) | ||||||||||||||||
Mr. New(1) | Continued Base Salary(2) | 800,000 | 800,000 | — | — | — | ||||||||||||||||
| Continued Health and Dental Coverage(3) | 27,328 | 27,328 | — | — | — | |||||||||||||||||
| 2x Average Bonus(4) | 808,667 | 808,667 | — | — | — | |||||||||||||||||
| Pro-Rated Bonus(5) | 200,500 | 200,500 | — | — | — | |||||||||||||||||
| Transaction Bonus(6) | — | 400,000 | ||||||||||||||||||||
Total | 1,836,495 | 2,236,495 | — | — | — | |||||||||||||||||
| Mr. Stefanelli | Continued Base Salary(2) | 315,000 | 315,000 | — | — | — | ||||||||||||||||
| Pro-Rated Bonus(5) | 88,100 | 88,100 | — | — | — | |||||||||||||||||
Total | 403,100 | 403,100 | — | — | — | |||||||||||||||||
| Mr. Marsh | Continued Base Salary(2) | 294,000 | 441,000 | — | — | — | ||||||||||||||||
| Pro-Rated Bonus(5) | 42,400 | 42,400 | — | — | — | |||||||||||||||||
| Transaction Bonus(6) | — | 220,500 | — | — | — | |||||||||||||||||
Total | 336,400 | 703,900 | — | — | — | |||||||||||||||||
| Mr. Ferrara | Continued Base Salary(2) | 233,730 | 233,730 | — | — | — | ||||||||||||||||
| Pro-Rated Bonus(5) | 30,600 | 30,600 | — | — | — | |||||||||||||||||
Total | 264,330 | 264,330 | — | — | — | |||||||||||||||||
| Mr. Null | Continued Base Salary(2) | 228,355 | 228,355 | — | — | — | ||||||||||||||||
| Pro-Rated Bonus(5) | 40,600 | 40,600 | — | — | — | |||||||||||||||||
Total | 268,955 | 268,955 | — | — | — | |||||||||||||||||
111
Table of Contents
| (1) | As discussed earlier in this prospectus, effective September 1, 2011, Mr. New retired as our Chief Executive Officer and President. We entered into an agreement with Mr. New, pursuant to which he will receive a one-time payment of $500,000 on or before October 15, 2011. Mr. New will continue to be eligible for all health and welfare, severance, separation and retirement benefits pursuant to his current employment agreement with the Company through December 31, 2011. Beginning January 1, 2012, and continuing through December 31, 2012, Mr. New will provide professional consulting and transition services as a consultant to the Company. For a description of Mr. New’s consulting agreement, see “Compensation Discussion and Analysis — Material Changes to Compensation Program During 2011.” | |
| (2) | Reflects an amount equal to the applicable multiple of the executive’s then-current base salary, payable in installments over 24 months, in the case of Mr. New, or 12 months, in the case of Messrs. Marsh, Stefanelli, Ferrara and Null. Mr. Marsh’s multiple of salary is 1x, in the event of his termination of employment prior to a “qualifying transaction”, or 1.5x, in the event of his termination of employment within one year following the effective date of a “qualifying transaction.” | |
| (3) | Reflects Consolidated Omnibus Budget Reconciliation Act of 1986, or COBRA, payments by us for medical and dental coverage based on 2010 rates for 24 months. | |
| (4) | Reflects an amount equal to two times the average of the bonuses Mr. New received in 2007, 2008 and 2009, payable in installments over 24 months. | |
| (5) | Reflects a pro-rated bonus for the year in which the executive terminates employment. The pro-ration is based on the executive’s and our performance relative to the pre-approved objectives. | |
| (6) | Reflects 100 percent of Mr. New’s annual base salary and 50 percent of the sum of Mr. Marsh’s current annual base salary plus his current target bonus, payable in a lump sum. |
112
Table of Contents
| • | KRG Capital Partners has the right to appoint up to three managers, subject to the conditions provided in the Aurora Holdings LLC Agreement; | |
| • | the Chief Executive Officer of our subsidiary Aurora Diagnostics, LLC is a manager; | |
| • | Summit Partners and KRG Capital Partners have the right to appoint mutually one independent manager; and | |
| • | Summit Partners has the right to appoint the other managers (which, as of the date hereof, is three managers). |
| • | the dissolution or liquidation of Aurora Holdings or Aurora Diagnostics, LLC, except in connection with corporate conversions and reorganizations or a sale of the enterprise described below; | |
| • | the sale of Aurora Holdings or Aurora Diagnostics, LLC, unless the transaction results in specified returns on investment for Summit Partners and KRG Capital Partners; | |
| • | the acquisition, by Aurora Holdings or any subsidiary, of any business for consideration in excess of $20 million or any businesses for aggregate consideration in excess of $60 million; | |
| • | the issuance of any equity except (i) for issuances pursuant to an equity incentive plan, (ii) in connection with a public offering of equity otherwise permitted by the Aurora Holdings LLC Agreement and (iii) for issuances to acquisition targets (or their equityholders) in connection with or related to acquisitions; | |
| • | the incurrence, by Aurora Holdings or any subsidiary, of any new indebtedness or the refinancing of any existing indebtedness, except (i) for amounts of less than $5 million in the aggregate and (ii) to acquisition targets (or their equityholders) in connection with or related to acquisitions; | |
| • | the sale, transfer, termination, assignment, or other disposal of, by Aurora Holdings or any subsidiary of any (i) equity interest of any subsidiary, or (ii) right to vote the equity interests of any affiliated |
113
Table of Contents
| physician-owned professional organization, except in connection with an initial public offering of equity or a sale of the enterprise described above; |
| • | the hiring, firing, material reduction of the employment responsibilities of, or taking of any other action that could give rise to a termination for “Good Reason” or other similar term under any employment agreement or equity agreement between Aurora Holdings and, its Chief Executive Officer, Chief Operating Officer or Chief Financial Officer; | |
| • | the increase of the number of managers serving on the board of managers of Aurora Holdings at any time when KRG Capital Partners has a right to appoint three managers to the board of managers of Aurora Holdings; or | |
| • | the development or implementation of any strategic plan that would materially affect Aurora Holdings’ or Aurora Diagnostics, LLC’s business and business activities ancillary thereto, or materially alter Aurora Holdings’ or Aurora Diagnostics, LLC’s business tactics. |
114
Table of Contents
115
Table of Contents
| • | a $225.0 million senior secured term loan facility, of which $114.4 million was outstanding as of June 30, 2011; and | |
| • | a $110.0 million senior secured revolving credit facility, which includes a $15.0 million letter of creditsub-facility and a $5.0 million swing linesub-facility that is available uponsame-day notice. |
116
Table of Contents
| • | Base Rate Loans, at a rate per annum equal to: |
| • | the highest of: |
| • | Barclays Bank PLC’s prime rate, | |
| • | 0.50% plus Federal Funds Effective Rate, and | |
| • | the LIBOR rate, as adjusted for applicable reserve requirements (subject to a 2.00% floor) for an interest period of one month plus 1.00%;plus |
| • | 3.25%. |
| • | LIBOR Rate Loans, at a rate per annum equal to: |
| • | the LIBOR rate, as adjusted for applicable reserve requirements (subject to a 2.00% floor) determined for the applicable interest period;plus | |
| • | 4.25%. |
117
Table of Contents
| Minimum Interest | ||||
Periods Ending | Coverage Ratio | |||
| September 30, 2011 | 2.00:1.00 | |||
| December 31, 2011 | 2.00:1.00 | |||
| March 31, 2012 | 2.00:1.00 | |||
| June 30, 2012 | 2.00:1.00 | |||
| September 30, 2012 | 2.25:1.00 | |||
| December 31, 2012 | 2.25:1.00 | |||
| March 31, 2013 | 2.25:1.00 | |||
| June 30, 2013 | 2.25:1.00 | |||
| September 30, 2013 | 2.25:1.00 | |||
| December 31, 2013 | 2.50:1.00 | |||
| March 31, 2014 | 2.50:1.00 | |||
| June 30, 2014 | 2.50:1.00 | |||
| September 30, 2014 | 2.50:1.00 | |||
| December 31, 2014 | 2.75:1.00 | |||
| March 31, 2015 | 2.75:1.00 | |||
| June 30, 2015 | 2.75:1.00 | |||
| September 30, 2015 | 2.75:1.00 | |||
| December 31, 2015 and thereafter | 3.00:1.00 | |||
| • | incur or allow to exist certain liens; | |
| • | incur additional indebtedness; | |
| • | guarantee indebtedness; | |
| • | engage in unrelated businesses; | |
| • | make investments or acquisitions or enter into mergers; | |
| • | sell or dispose of assets or acquire, merge with or consolidate with certain businesses; | |
| • | engage in certain transactions with affiliates; | |
| • | make certain payments under the Tax Receivable Agreement that we may enter with our members; | |
| • | make certain changes to the documents related to certain contemplated reorganization transactions; | |
| • | make redemptions or repurchases of, or pay dividends on, equity interests; | |
| • | make voluntary prepayments of or repurchase certain other subordinated indebtedness; |
118
Table of Contents
| • | enter into agreements restricting or prohibiting any subsidiaries from incurring or allowing to exist certain liens; | |
| • | enter into agreements restricting or prohibiting any subsidiaries from paying or declaring certain dividends or distributions; | |
| • | change our organizational documents or material agreements; and | |
| • | enter into sale and leaseback transactions. |
| • | failure to make payments when due; | |
| • | defaults under certain other indebtedness; | |
| • | noncompliance with covenants; | |
| • | incorrectness of representations and warranties in any material respect when made; | |
| • | bankruptcy or certain insolvency events; | |
| • | certain events related to ERISA; | |
| • | material judgments; | |
| • | certain criminal proceedings; | |
| • | failure of certain other indebtedness in excess of specified amounts to be subordinated to obligations under our amended senior secured credit facility; | |
| • | incurrence of indebtedness by affiliated practices in excess of specified amounts; | |
| • | invalidity of loan documentation or material portion of collateral; and | |
| • | occurrence of a “change of control” as defined therein. |
119
Table of Contents
120
Table of Contents
| • | the new notes will be registered under the Securities Act and will not bear legends restricting the transfer of the new notes; | |
| • | holders of the new notes will not be entitled to any of the registration rights of holders of old notes under the registration rights agreement; | |
| • | the new notes will not contain provisions for payment of additional interest in case of non-registration; and | |
| • | the new notes will have a different CUSIP number from the old notes. |
121
Table of Contents
| • | any state, statute, rule or regulation shall have been proposed, adopted or enacted, or interpreted in a manner, which, in our reasonable judgment, would impair our ability to proceed with the exchange offer; | |
| • | any action or proceeding is instituted or threatened in any court or by or before the SEC or any other governmental agency with respect to the exchange offer which, in our reasonable judgment, would impair our ability to proceed with the exchange offer; | |
| • | we have not obtained any governmental approval which we, in our reasonable judgment, consider necessary for the completion of the exchange offer as contemplated by this prospectus; | |
| • | any change, or any condition, event or development involving a prospective change, shall have occurred or be threatened in the general economic, financial, currency exchange or market conditions in the United States or elsewhere that, in our reasonable judgment, would impair our ability to proceed with the exchange offer; | |
| • | any other change or development, including a prospective change or development, that, in our reasonable judgment, has or may have a material adverse effect on us, the market price of the new notes or the old notes or the value of the exchange offer to us; or | |
| • | there shall have occurred (i) any suspension or limitation of trading in securities generally on the new York Stock Exchange or theover-the-counter market; (ii) a declaration of a banking moratorium by United States Federal or New York authorities; or (iii) a commencement or escalation of a war or armed hostilities involving or relating to a country where we do business or other international or national emergency or crisis directly or indirectly involving the United States. |
| • | refuse to accept any old notes and return all tendered old notes to the tendering holders and terminate the exchange offer; | |
| • | extend the exchange offer and retain all old notes tendered before the expiration of the exchange offer, subject, however, to the rights of holders to withdraw these old notes; or | |
| • | waive unsatisfied conditions relating to the exchange offer and accept all properly tendered old notes which have not been withdrawn. If this waiver constitutes a material change to the exchange offer, we will disclose this change by means of a prospectus supplement that will be distributed to the registered holders of the old notes. If the exchange offer would otherwise expire, we will extend the exchange offer for 5-10 business days, depending on how significant the waiver is and the manner of disclosure to registered holders. |
122
Table of Contents
| • | prior to the expiration date, delay accepting any old notes; | |
| • | extend this exchange offer; | |
| • | terminate this exchange offer upon the occurrence of any of the events set forth in “— Conditions of the Exchange Offer”; or | |
| • | waive any conditions or otherwise amend this exchange offer in any respect. |
| • | you must acquire the new notes in the ordinary course of your or any beneficial owner’s business; | |
| • | you must not be participating, not intend to participate and not have an arrangement or understanding with any person to participate in the distribution of the new notes within the meaning of the Securities Act; | |
| • | you must not be an affiliate of ours, as defined under Rule 405 of the Securities Act; and | |
| • | you must not be a broker-dealer that acquired the old notes from us or in market-making transactions or other trading activities. |
123
Table of Contents
| • | you cannot rely on the position of the SEC set forth in the no-action letters referred to above; and | |
| • | you must comply with the registration and prospectus delivery requirements of the Securities Act in connection with a sale of the new notes. |
| • | to us; | |
| • | under a registration statement that has been declared effective under the Securities Act; | |
| • | to a person the seller reasonably believes is a qualified institutional buyer that is purchasing for its own account or for the account of another qualified institutional buyer; | |
| • | through offers and sales that occur outside the United States within the meaning of Regulation S under the Securities Act; or | |
| • | under any other available exemption from the registration requirements of the Securities Act. |
| • | you are acquiring the new notes in the ordinary course of your or any beneficial owner’s business; | |
| • | you are not participating, do not intend to participate and have no arrangement or understanding with any person to participate in the distribution (within the meaning of the Securities Act) of the new notes in violation of the provisions of the Securities Act; |
124
Table of Contents
| • | you are not our “affiliate” (within the meaning of Rule 405 under the Securities Act), or if you are our affiliate, you will comply with the registration and prospectus delivery requirements of the Securities Act; | |
| • | you have full power and authority to tender, exchange, assign and transfer the old notes tendered; | |
| • | we will acquire good, marketable and unencumbered title to the old notes being tendered, free and clear of all security interests, liens, restrictions, charges, encumbrances, or other obligations relating to their sale or transfer, and the old notes will not be subject to any adverse claim, when the old notes are accepted by us; | |
| • | if you are a broker-dealer registered under the Exchange Act or you are participating in the exchange offer for the purposes of distributing the new notes, you must comply with the registration and prospectus delivery requirements of the Securities Act in connection with a secondary sale of the new notes, and you cannot rely on the position of the SEC’s staff in their no-action letters; and | |
| • | we may rely upon these representations for purposes of this exchange offer. |
125
Table of Contents
126
Table of Contents
127
Table of Contents
| • | specify the name of the person who tendered the old notes to be withdrawn; | |
| • | must contain a description of the old notes to be withdrawn, the certificate numbers shown on the particular certificates evidencing such old notes and the aggregate principal amount represented by such old notes; and | |
| • | must be signed by the holder of those old notes in the same manner as the original signature on the letter of transmittal, including any required signature guarantees, or be accompanied by evidence satisfactory to us that the person withdrawing the tender has succeeded to the beneficial ownership of the old notes. In addition, the notice of withdrawal must specify, in the case of old notes tendered by delivery of certificates for such old notes, the name of the registered holder, if different from that of the tendering holder or, in the case of old notes tendered by book-entry transfer, the name and number of the account at DTC to be credited with the withdrawn old notes. The signature on the notice of withdrawal must be guaranteed by an eligible institution unless the old notes have been tendered for the account of an eligible institution. |
| By Mail, Hand Delivery or Overnight Courier: | U.S. Bank National Association Corporate Trust Services Attention: Specialized Finance 60 Livingston Avenue St. Paul, Minnesota 55107-2292 | |
| By Facsimile Transmission: | U.S. Bank National Association Corporate Trust Services Attention: Specialized Finance Facsimile: (651) 495-8158 | |
| For Information or Confirmation by Telephone: | U.S. Bank National Association Corporate Trust Services Attention: Specialized Finance Telephone: (800) 934-6802 |
128
Table of Contents
129
Table of Contents
130
Table of Contents
| • | are general unsecured senior obligations of the Issuers; | |
| • | will rankpari passuin right of payment with all existing and future Senior Indebtedness (including the Credit Agreement) of the Issuers; | |
| • | will be effectively subordinated to all secured Indebtedness of the Issuers (including the Credit Agreement) to the extent of the value of the assets securing such Indebtedness; | |
| • | will be structurally subordinated to all existing and future Indebtedness, claims of holders of Preferred Stock and other liabilities of the Company’s Subsidiaries that are not guaranteeing the new notes; | |
| • | will rank senior in right of payment to any future Subordinated Obligations of the Issuers; and | |
| • | are guaranteed on an unsecured senior basis by each Subsidiary Guarantor as described under “— Guarantees.” |
| Redemption | ||||
Period | Price | |||
| 2015 | 105.375 | % | ||
| 2016 | 102.688 | % | ||
| 2017 and thereafter | 100.000 | % | ||
131
Table of Contents
132
Table of Contents
133
Table of Contents
134
Table of Contents
135
Table of Contents
136
Table of Contents
137
Table of Contents
138
Table of Contents
139
Table of Contents
140
Table of Contents
141
Table of Contents
142
Table of Contents
143
Table of Contents
144
Table of Contents
145
Table of Contents
146
Table of Contents
147
Table of Contents
148
Table of Contents
149
Table of Contents
150
Table of Contents
151
Table of Contents
152
Table of Contents
153
Table of Contents
154
Table of Contents
155
Table of Contents
156
Table of Contents
157
Table of Contents
158
Table of Contents
159
Table of Contents
160
Table of Contents
161
Table of Contents
162
Table of Contents
163
Table of Contents
164
Table of Contents
165
Table of Contents
166
Table of Contents
167
Table of Contents
168
Table of Contents
169
Table of Contents
170
Table of Contents
171
Table of Contents
172
Table of Contents
173
Table of Contents
174
Table of Contents
175
Table of Contents
176
Table of Contents
177
Table of Contents
178
Table of Contents
179
Table of Contents
180
Table of Contents
181
Table of Contents
182
Table of Contents
183
Consolidated Financial Statements
As of December 31, 2009 and 2010 and for the years ended December 31, 2008, 2009 and 2010
Page | ||||
| F-2 | ||||
| F-3 | ||||
| F-4 | ||||
| F-5 | ||||
| F-6 | ||||
| F-7 | ||||
Aurora Diagnostics Holdings, LLC Unaudited Consolidated Financial Statements As of June 30, 2011 and for the six months ended June 30, 2010 and 2011 | ||||
| F-35 | ||||
| F-36 | ||||
| F-37 | ||||
| F-38 | ||||
| F-39 | ||||
Pathology Solutions, LLC Financial Statements | ||||
| F-56 | ||||
| F-57 | ||||
| F-58 | ||||
| F-59 | ||||
| F-60 |
Financial Statements
| F-65 | ||||
| F-66 | ||||
| F-67 | ||||
| F-68 | ||||
| F-69 | ||||
| F-70 | ||||
| F-75 |
F-1
Table of Contents
F-2
Table of Contents
| December 31, | ||||||||
| 2009 | 2010 | |||||||
| ($ In thousands) | ||||||||
ASSETS | ||||||||
| Current Assets | ||||||||
| Cash and cash equivalents | $ | 27,424 | $ | 39,941 | ||||
| Accounts receivable, net | 16,106 | 25,448 | ||||||
| Prepaid expenses and other assets | 1,883 | 1,949 | ||||||
| Prepaid income taxes | 133 | 1,397 | ||||||
| Deferred tax assets | 2,026 | 2,063 | ||||||
Total current assets | 47,572 | 70,798 | ||||||
| Property and Equipment, net | 7,580 | 8,906 | ||||||
| Other Assets: | ||||||||
| Deferred debt issue costs, net | 3,932 | 11,065 | ||||||
| Deposits and other noncurrent assets | 17,297 | 41,087 | ||||||
| Goodwill | 268,911 | 329,199 | ||||||
| Intangible assets, net | 118,681 | 126,956 | ||||||
| 408,821 | 508,307 | |||||||
| $ | 463,973 | $ | 588,011 | |||||
LIABILITIES AND MEMBERS’ EQUITY | ||||||||
| Current Liabilities | ||||||||
| Current portion of long-term debt | $ | 11,596 | $ | 2,770 | ||||
| Current portion of fair value of contingent consideration | 804 | 8,085 | ||||||
| Accounts payable and accrued expenses | 4,850 | 8,387 | ||||||
| Accrued compensation | 7,124 | 8,213 | ||||||
| Accrued interest | 3,047 | 863 | ||||||
| Acquisition related liability | 592 | — | ||||||
| Fair value of derivative | 125 | — | ||||||
Total current liabilities | 28,138 | 28,318 | ||||||
| Long-term debt, net of current portion | 205,056 | 316,044 | ||||||
| Deferred tax liabilities, net | 11,565 | 13,841 | ||||||
| Fair value of contingent consideration, net of current portion | 2,150 | 18,465 | ||||||
| Commitments and Contingencies | ||||||||
Members’ Equity | ||||||||
| Member Contributions | ||||||||
| Class A member units, 85,000 units issued and outstanding | 146,250 | 146,250 | ||||||
Class A-1 member units, 21,382 units issued and outstanding | 50,282 | 50,282 | ||||||
| Class B member units, 10,000 units issued and outstanding | (2,333 | ) | (2,333 | ) | ||||
| Class C member units, 5,000 units issued and outstanding | 1,870 | 1,870 | ||||||
| Class D member units, 10,000 issued and outstanding | 316 | (1,663 | ) | |||||
| Class X, no member units authorized under LLC agreement | 6,708 | 6,708 | ||||||
| Accumulated Other Comprehensive Loss | (125 | ) | — | |||||
| Equity Transaction Costs | (4,825 | ) | (4,825 | ) | ||||
| Retained Earnings | 18,921 | 15,054 | ||||||
Total Members’ Equity | 217,064 | 211,343 | ||||||
| $ | 463,973 | $ | 588,011 | |||||
F-3
Table of Contents
| 2008 | 2009 | 2010 | ||||||||||
| ($ In thousands) | ||||||||||||
| Net Revenues | $ | 157,850 | $ | 171,565 | $ | 212,837 | ||||||
| Operating costs and expenses: | ||||||||||||
| Cost of services | 66,382 | 71,778 | 96,868 | |||||||||
| Selling, general and administrative expenses | 33,194 | 36,854 | 49,141 | |||||||||
| Provision for doubtful accounts | 8,037 | 9,488 | 12,393 | |||||||||
| Intangible asset amortization expense | 14,308 | 14,574 | 18,946 | |||||||||
| Management fees | 1,559 | 1,778 | 2,189 | |||||||||
| Impairment of goodwill and other intangible assets | — | 8,031 | 4,871 | |||||||||
| Acquisition and business development costs | 676 | 1,074 | 1,032 | |||||||||
| Change in fair value of contingent consideration | — | — | 983 | |||||||||
| Equity based compensation expense | 1,164 | — | — | |||||||||
Total operating costs and expenses | 125,320 | 143,577 | 186,423 | |||||||||
Income from operations | 32,530 | 27,988 | 26,414 | |||||||||
| Other income (expense): | ||||||||||||
| Interest expense | (21,577 | ) | (18,969 | ) | (17,041 | ) | ||||||
| Write-off of deferred debt issue costs | — | — | (9,259 | ) | ||||||||
| Loss on extinguishment of debt | — | — | (2,296 | ) | ||||||||
| Other income | 125 | 28 | 18 | |||||||||
Total other expense, net | (21,452 | ) | (18,941 | ) | (28,578 | ) | ||||||
Income (loss) before income taxes | 11,078 | 9,047 | (2,164 | ) | ||||||||
| Provision for income taxes | 408 | 45 | 1,487 | |||||||||
Net income (loss) | $ | 10,670 | $ | 9,002 | $ | (3,651 | ) | |||||
F-4
Table of Contents
| Accumulated | ||||||||||||||||||||||||
| Other | ||||||||||||||||||||||||
| Member | Comprehensive | Equity | Retained | Total | ||||||||||||||||||||
| Member | Contributions | Income | Transaction | (Deficit) | Members’ | |||||||||||||||||||
| Units | (Distributions) | (Loss) | Costs | Earnings | Equity | |||||||||||||||||||
| ($ In thousands) | ||||||||||||||||||||||||
| Balance, January 1, 2008 | 100,000 | $ | 147,828 | $ | — | $ | (2,000 | ) | $ | (751 | ) | $ | 145,077 | |||||||||||
| Components of comprehensive income | ||||||||||||||||||||||||
| Net income | — | — | — | — | 10,670 | 10,670 | ||||||||||||||||||
| Fair value of derivative | — | — | (2,573 | ) | — | — | (2,573 | ) | ||||||||||||||||
| Total comprehensive income | — | — | (2,573 | ) | — | 10,670 | 8,097 | |||||||||||||||||
| Member contributions | — | 7,379 | — | — | — | 7,379 | ||||||||||||||||||
| Adjustment to equity transaction costs | — | — | — | 250 | — | 250 | ||||||||||||||||||
| Equity compensation | 10,000 | 1,164 | — | — | — | 1,164 | ||||||||||||||||||
| Member notes receivable | — | (379 | ) | — | — | — | (379 | ) | ||||||||||||||||
| Tax distributions to members | — | (412 | ) | — | — | — | (412 | ) | ||||||||||||||||
| Balance, December 31, 2008 | 110,000 | 155,580 | (2,573 | ) | (1,750 | ) | 9,919 | 161,176 | ||||||||||||||||
| Components of comprehensive income | ||||||||||||||||||||||||
| Net income | — | — | — | — | 9,002 | 9,002 | ||||||||||||||||||
| Fair value of derivative | — | — | 2,448 | — | — | 2,448 | ||||||||||||||||||
| Total comprehensive income | — | — | 2,448 | — | 9,002 | 11,450 | ||||||||||||||||||
| Contributions from members | 21,382 | 50,322 | — | — | — | 50,322 | ||||||||||||||||||
| Equity transaction costs | — | — | — | (3,075 | ) | — | (3,075 | ) | ||||||||||||||||
| Tax distributions to members | — | (2,809 | ) | — | — | — | (2,809 | ) | ||||||||||||||||
| Balance, December 31, 2009 | 131,382 | 203,093 | (125 | ) | (4,825 | ) | 18,921 | 217,064 | ||||||||||||||||
| Components of comprehensive income | ||||||||||||||||||||||||
| Net loss | — | — | — | — | (3,651 | ) | (3,651 | ) | ||||||||||||||||
| Fair value of derivative | — | — | 125 | — | — | 125 | ||||||||||||||||||
| Total comprehensive income | — | — | 125 | — | (3,651 | ) | (3,526 | ) | ||||||||||||||||
| Contributions from members | — | 8,500 | — | — | — | 8,500 | ||||||||||||||||||
| Distributions to members | — | (8,500 | ) | — | — | — | (8,500 | ) | ||||||||||||||||
| Tax distributions to members | — | (1,979 | ) | — | — | — | (1,979 | ) | ||||||||||||||||
| Special distribution | — | (2,535 | ) | — | — | — | (2,535 | ) | ||||||||||||||||
| Repayment of member notes receivable | — | 2,535 | — | — | — | 2,535 | ||||||||||||||||||
| Dividends | — | — | — | — | (216 | ) | (216 | ) | ||||||||||||||||
| Balance, December 31, 2010 | 131,382 | $ | 201,114 | $ | — | $ | (4,825 | ) | $ | 15,054 | $ | 211,343 | ||||||||||||
F-5
Table of Contents
| 2008 | 2009 | 2010 | ||||||||||
| ($ In thousands) | ||||||||||||
| Cash Flows From Operating Activities | ||||||||||||
| Net income (loss) | $ | 10,670 | $ | 9,002 | $ | (3,651 | ) | |||||
| Adjustments to reconcile net income (loss) to net cash provided by operating activities: | ||||||||||||
| Depreciation and amortization | 16,137 | 17,060 | 22,258 | |||||||||
| Amortization of deferred debt issue costs | 978 | 1,090 | 1,365 | |||||||||
| Amortization of original issue discount on debt | 274 | 305 | 468 | |||||||||
| Deferred income taxes | (1,094 | ) | (1,568 | ) | (1,920 | ) | ||||||
| Change in fair value of contingent consideration | — | — | 983 | |||||||||
| Equity based compensation | 1,164 | — | — | |||||||||
| Write-off of deferred debt issue costs | — | — | 9,259 | |||||||||
| Loss on extinguishment of debt | — | — | 2,296 | |||||||||
| Impairment of goodwill and other intangible assets | — | 8,031 | 4,871 | |||||||||
| Changes in assets and liabilities, net of working capital acquired in business combinations: | ||||||||||||
| (Increase) decrease in: | ||||||||||||
| Accounts receivable | (1,048 | ) | (287 | ) | (5,751 | ) | ||||||
| Prepaid expenses | (256 | ) | 64 | (159 | ) | |||||||
| Prepaid income taxes | — | — | (1,264 | ) | ||||||||
| Increase (decrease) in: | ||||||||||||
| Accounts payable and accrued expenses | 401 | 1,815 | (321 | ) | ||||||||
| Accrued compensation | 1,445 | 1,544 | 670 | |||||||||
| Accrued interest | 1,566 | (395 | ) | (2,184 | ) | |||||||
| Taxes payable | (1,267 | ) | (298 | ) | 81 | |||||||
Net cash provided by operating activities | 28,970 | 36,363 | 27,001 | |||||||||
| Cash Flows From Investing Activities | ||||||||||||
| Purchase of property and equipment | (2,746 | ) | (2,961 | ) | (3,217 | ) | ||||||
| Increase in deposits and other noncurrent assets | 31 | (16,934 | ) | (36,990 | ) | |||||||
| Payment of contingent notes | (12,531 | ) | (12,668 | ) | (16,979 | ) | ||||||
| Businesses acquired, net of cash acquired | (31,026 | ) | (16,698 | ) | (33,699 | ) | ||||||
Net cash used in investing activities | (46,272 | ) | (49,261 | ) | (90,885 | ) | ||||||
| Cash Flows From Financing Activities | ||||||||||||
| Borrowings under term loan facility | 22,100 | — | 225,000 | |||||||||
| Repayments under former term loan facility | (7,800 | ) | (8,200 | ) | (209,100 | ) | ||||||
| Repayments under current term loan facility | — | — | (110,563 | ) | ||||||||
| Issuance of senior notes | — | — | 200,000 | |||||||||
| Repayments of subordinated notes payable | (2,916 | ) | (3,045 | ) | (2,860 | ) | ||||||
| Net borrowings under revolver | (24 | ) | (1 | ) | — | |||||||
| Equity transaction costs | (1,750 | ) | (3,075 | ) | — | |||||||
| Payments under capitalized lease obligations | — | — | (53 | ) | ||||||||
| Payment of debt issuance costs | (176 | ) | (148 | ) | (20,161 | ) | ||||||
| Payment of public offering costs | — | — | (3,667 | ) | ||||||||
| Contributions from members, net of tax distributions | 6,588 | 47,513 | (2,195 | ) | ||||||||
Net cash provided by financing activities | 16,022 | 33,044 | 76,401 | |||||||||
Net increase (decrease) in cash | (1,280 | ) | 20,146 | 12,517 | ||||||||
| Cash and cash equivalents, beginning | 8,558 | 7,278 | 27,424 | |||||||||
| Cash and cash equivalents, ending | $ | 7,278 | $ | 27,424 | $ | 39,941 | ||||||
| Supplemental Disclosures of Cash Flow Information | ||||||||||||
| Cash interest payments | $ | 20,736 | $ | 17,857 | $ | 17,392 | ||||||
| Cash tax payments, including member tax distributions | $ | 3,325 | $ | 4,577 | $ | 6,550 | ||||||
| Supplemental Schedule of Noncash Investing and Financing Activities | ||||||||||||
| Fair value of contingent consideration issued in acquisitions | $ | — | $ | 2,954 | $ | 22,613 | ||||||
| Notes receivable for membership interests | $ | 379 | $ | — | $ | — | ||||||
| Capital lease obligations | $ | — | $ | 280 | $ | 183 | ||||||
F-6
Table of Contents
| Note 1. | Nature of Business and Significant Accounting Policies |
F-7
Table of Contents
F-8
Table of Contents
F-9
Table of Contents
| 2008 | ||||
| Expected life | 3 years | |||
| Volatility percentage | 20.2 | % | ||
| Interest rate | 3.1 | % | ||
| Dividends | — | |||
| Forfeiture rate | — | |||
| 2009 | 2010 | |||||||
| Deferred debt issue costs | $ | 6,080 | $ | 11,617 | ||||
| Less accumulated amortization | (2,148 | ) | (552 | ) | ||||
| Deferred debt issue costs, net | $ | 3,932 | $ | 11,065 | ||||
F-10
Table of Contents
F-11
Table of Contents
F-12
Table of Contents
| Note 2. | Acquisitions |
F-13
Table of Contents
F-14
Table of Contents
Location | Date Acquired | Cash Paid | ||||
| Minnesota | March 7, 2008 | 27,301 | ||||
Total 2008 Acquisition | $ | 27,301 | ||||
| Texas | November 20, 2009 | 15,340 | ||||
Total 2009 Acquisition | $ | 15,340 | ||||
| Florida | January 1, 2010 | 7,976 | ||||
| Michigan | January 1, 2010 | 9,000 | ||||
| New Jersey | March 12, 2010 | 22,500 | ||||
| South Carolina | October 8, 2010 | 13,960 | ||||
Total 2010 Acquisitions | $ | 53,436 | ||||
F-15
Table of Contents
| 2009 | 2010 | |||||||
| Cash | $ | 162 | $ | 3,353 | ||||
| Accounts receivable | 562 | 3,591 | ||||||
| Other assets | 73 | 335 | ||||||
| Property and equipment | 125 | 1,236 | ||||||
| Intangible assets | 7,967 | 30,116 | ||||||
| Goodwill | 12,513 | 44,752 | ||||||
| Assets acquired | 21,402 | 83,383 | ||||||
| Accounts payable and accrued expenses | 12 | 3,080 | ||||||
| Accrued compensation | 264 | 412 | ||||||
| Fair value of contingent consideration | 2,954 | 22,613 | ||||||
| Deferred tax liabilities | 2,832 | 3,842 | ||||||
| Liabilities assumed | 6,062 | 29,947 | ||||||
| Net assets acquired | $ | 15,340 | $ | 53,436 | ||||
| Net assets acquired | $ | 15,340 | $ | 53,436 | ||||
| Less: | ||||||||
| Cash acquired | (162 | ) | (3,353 | ) | ||||
| Deposits on acquisitions | — | (16,976 | ) | |||||
| Cash paid for acquisitions, net of cash acquired | 15,178 | 33,107 | ||||||
| Acquisition costs and acquisition-related liability paid | 1,520 | 592 | ||||||
| Total businesses acquired and related costs, net of cash acquired | $ | 16,698 | $ | 33,699 | ||||
F-16
Table of Contents
| Pro Forma | ||||
| December 31, 2009 | ||||
| Net Revenues | $ | 178,893 | ||
| Net Income | $ | 10,214 | ||
| Pro Forma December 31, | ||||||||
| 2009 | 2010 | |||||||
| Net Revenues | $ | 212,103 | $ | 221,260 | ||||
| Net Income | $ | 18,454 | $ | (2,421 | ) | |||
| Note 3. | Accounts Receivable |
| 2009 | 2010 | |||||||
| Accounts receivable | $ | 24,659 | $ | 37,172 | ||||
| Less: Allowance for doubtful accounts | (8,553 | ) | (11,724 | ) | ||||
| Accounts receivable, net | $ | 16,106 | $ | 25,448 | ||||
F-17
Table of Contents
| Note 4. | Property and Equipment |
| Estimated Useful | ||||||||||
| Life (years) | 2009 | 2010 | ||||||||
| Laboratory, office and data processing equipment | 3 — 5 | $ | 5,524 | $ | 8,374 | |||||
| Building and leasehold improvements | 5 — 15 | 3,009 | 3,337 | |||||||
| Furniture and fixtures | 5 | 550 | 844 | |||||||
| Software | 3 — 5 | 2,504 | 3,420 | |||||||
| Vehicles | 3 — 5 | 431 | 623 | |||||||
| 12,018 | 16,598 | |||||||||
| Less accumulated depreciation | (4,993 | ) | (8,244 | ) | ||||||
| 7,025 | 8,354 | |||||||||
| Construction in progress | 555 | 552 | ||||||||
| $ | 7,580 | $ | 8,906 | |||||||
| Note 5. | Goodwill and Intangible Assets |
| 2009 | 2010 | |||||||
| Goodwill, beginning of period | $ | 250,340 | $ | 268,911 | ||||
| Acquisitions | 12,513 | 44,752 | ||||||
| Contingent notes* | 12,668 | 16,979 | ||||||
| Goodwill impairment | (6,610 | ) | (1,976 | ) | ||||
| Other acquisition costs | — | 533 | ||||||
| Goodwill, end of period | $ | 268,911 | $ | 329,199 | ||||
| * | Related to acquisitions completed prior to January 1, 2009. |
| Weighted Average | December 31, 2009 | |||||||||||||||
| Range | Amortization Period | Accumulated | ||||||||||||||
| (Years) | (Years) | Cost | Amortization | Net | ||||||||||||
| Amortizing intangible assets: | ||||||||||||||||
| Customer relationships | 7 — 10 | 9 | $ | 106,860 | $ | (23,414 | ) | $ | 83,446 | |||||||
| Health care facility agreements | 4 — 18 | 14 | 41,370 | (7,951 | ) | 33,419 | ||||||||||
| Noncompete agreements | 4 — 5 | 5 | 3,370 | (1,554 | ) | 1,816 | ||||||||||
| Total intangible assets | $ | 151,600 | $ | (32,919 | ) | $ | 118,681 | |||||||||
F-18
Table of Contents
| Weighted Average | December 31, 2010 | |||||||||||||||
| Range | Amortization Period | Accumulated | ||||||||||||||
| (Years) | (Years) | Cost | Amortization | Net | ||||||||||||
| Amortizing intangible assets: | ||||||||||||||||
| Customer relationships | 7 — 10 | 8 | $ | 130,533 | $ | (35,825 | ) | $ | 94,708 | |||||||
| Health care facility agreements | 4 — 18 | 14 | 41,370 | (11,260 | ) | 30,110 | ||||||||||
| Noncompete agreements | 2 — 5 | 5 | 4,441 | (2,303 | ) | 2,138 | ||||||||||
| Total intangible assets | $ | 176,344 | $ | (49,388 | ) | $ | 126,956 | |||||||||
F-19
Table of Contents
Year Ending December 31, | ||||
| 2011 | $ | 19,356 | ||
| 2012 | 18,521 | |||
| 2013 | 18,103 | |||
| 2014 | 17,117 | |||
| 2015 | 16,164 | |||
| Thereafter | 37,695 | |||
| $ | 126,956 | |||
| Note 6. | Accounts Payable and Accrued Expenses |
| 2009 | 2010 | |||||||
| Accounts payable | $ | 2,945 | $ | 3,514 | ||||
| Due to predecessor pension plan | — | 1,241 | ||||||
| Accrued management fees | 430 | 562 | ||||||
| Other accrued expenses | 1,475 | 3,070 | ||||||
| $ | 4,850 | $ | 8,387 | |||||
F-20
Table of Contents
| Note 7. | Long-Term Debt |
F-21
Table of Contents
| 2009 | 2010 | |||||||
| Former term loan, first lien | $ | 132,566 | — | |||||
| Former term loan, second lien | 76,534 | — | ||||||
| New term loan | — | 114,438 | ||||||
| Subordinated unsecured contingent note dated March 21, 2007 | 316 | — | ||||||
| Subordinated unsecured contingent note dated April 30, 2007 | 8,072 | 5,528 | ||||||
| Senior Notes | — | 200,000 | ||||||
| Capital lease obligations | 262 | 393 | ||||||
| 217,750 | 320,359 | |||||||
| Less: | ||||||||
| Original issue discount, net | (1,098 | ) | (1,545 | ) | ||||
| Current portion | (11,596 | ) | (2,770 | ) | ||||
| Long-term debt, net of current portion | $ | 205,056 | $ | 316,044 | ||||
Year Ending December 31, | ||||
| 2011 | $ | 2,770 | ||
| 2012 | 2,930 | |||
| 2013 | 99 | |||
| 2014 | 87 | |||
| 2015 | 31 | |||
| Thereafter | 314,442 | |||
| $ | 320,359 | |||
F-22
Table of Contents
| Note 8. | Related Party Transactions |
F-23
Table of Contents
| Note 9. | Members’ Equity |
F-24
Table of Contents
| Members’ | ||||||||||||||||||||||||||||||||
| Class A | Class A-1 | Class B | Class C | Class D | Class X | Class Z | Equity | |||||||||||||||||||||||||
| Balance, January 1, 2008 | $ | 145,985 | $ | — | $ | — | $ | 1,843 | $ | — | $ | — | $ | — | $ | 147,828 | ||||||||||||||||
| Member contributions | 265 | — | — | 54 | 1,164 | 7,060 | 8,543 | |||||||||||||||||||||||||
| Member notes receivable | — | — | — | (27 | ) | — | (352 | ) | (379 | ) | ||||||||||||||||||||||
| Tax distributions | — | — | (412 | ) | — | — | — | (412 | ) | |||||||||||||||||||||||
| Balance, December 31, 2008 | 146,250 | — | (412 | ) | 1,870 | 1,164 | 6,708 | — | 155,580 | |||||||||||||||||||||||
| Contributions from members | — | 50,322 | — | — | — | — | — | 50,322 | ||||||||||||||||||||||||
| Tax distributions | — | (40 | ) | (1,921 | ) | — | (848 | ) | — | — | (2,809 | ) | ||||||||||||||||||||
| Balance, December 31, 2009 | 146,250 | 50,282 | (2,333 | ) | 1,870 | 316 | 6,708 | — | 203,093 | |||||||||||||||||||||||
| Contributions from members | — | — | — | — | — | — | 8,500 | 8,500 | ||||||||||||||||||||||||
| Return of member contibutions | — | — | — | — | — | — | (8,500 | ) | (8,500 | ) | ||||||||||||||||||||||
| Special distribution | — | — | — | (2,145 | ) | — | (390 | ) | — | (2,535 | ) | |||||||||||||||||||||
| Repayment of member notes receivable(1) | — | — | — | 2,145 | — | 390 | — | 2,535 | ||||||||||||||||||||||||
| Tax distributions | — | — | — | — | (1,979 | ) | — | — | (1,979 | ) | ||||||||||||||||||||||
| Balance, December 31, 2010 | $ | 146,250 | $ | 50,282 | $ | (2,333 | ) | $ | 1,870 | $ | (1,663 | ) | $ | 6,708 | $ | — | $ | 201,114 | ||||||||||||||
| (1) | The repayment of member notes receivable included principal of $2,227 and accrued interest of $308. |
| Note 10. | Commitments and Contingencies |
F-25
Table of Contents
F-26
Table of Contents
Year Ending December 31, | ||||
| 2011 | $ | 3,060 | ||
| 2012 | 2,521 | |||
| 2013 | 2,388 | |||
| 2014 | 2,019 | |||
| 2015 | 1,830 | |||
| Thereafter | 5,643 | |||
| $ | 17,461 | |||
| Note 11. | Fair Value of Financial Instruments |
F-27
Table of Contents
| Note 12. | Income Taxes |
| 2008 | 2009 | 2010 | ||||||||||
| Current: | ||||||||||||
| Federal | $ | 1,143 | $ | 1,074 | $ | 2,872 | ||||||
| State | 359 | 539 | 600 | |||||||||
| Total current provision | 1,502 | 1,613 | 3,472 | |||||||||
| Deferred: | ||||||||||||
| Federal | (1,053 | ) | (1,458 | ) | (1,904 | ) | ||||||
| State | (41 | ) | (110 | ) | (81 | ) | ||||||
| Total deferred benefit | (1,094 | ) | (1,568 | ) | (1,985 | ) | ||||||
| Total provision for income taxes | $ | 408 | $ | 45 | $ | 1,487 | ||||||
| 2009 | 2010 | |||||||
| Allowance for doubtful accounts | $ | 1,809 | $ | 1,976 | ||||
| Accrued wages | 25 | 87 | ||||||
| Acquisition related liability | 192 | — | ||||||
| Current deferred tax assets: | $ | 2,026 | $ | 2,063 | ||||
| Noncurrent deferred tax assets: | ||||||||
| Acquisition related liability | 9 | — | ||||||
| Noncurrent deferred tax liabilities: | ||||||||
| Intangible assets acquired | (9,457 | ) | (12,961 | ) | ||||
| Change from cash to accrual basis of accounting by the businesses acquired | (517 | ) | (479 | ) | ||||
| Property and equipment | (225 | ) | (402 | ) | ||||
| Noncurrent deferred tax liabilities, net | $ | (10,190 | ) | $ | (13,842 | ) | ||
| Total deferred tax liabilities, net | $ | (8,164 | ) | $ | (11,779 | ) | ||
| Note 13. | Equity Based Compensation |
| Note 14. | Defined Contribution Plan |
F-28
Table of Contents
| Note 15. | Subsequent Events |
| Note 16. | Guarantor Subsidiaries |
F-29
Table of Contents
| Aurora | ||||||||||||||||
| Diagnostics | Subsidiary | Non-Guarantor | Consolidated | |||||||||||||
December 31, 2009 | Holdings, LLC | Guarantors | Subsidiaries | Total | ||||||||||||
| (In thousands) | ||||||||||||||||
ASSETS | ||||||||||||||||
| Current Assets | ||||||||||||||||
| Cash and cash equivalents | $ | 27,150 | $ | — | $ | 274 | $ | 27,424 | ||||||||
| Accounts receivable, net | — | 7,549 | 8,557 | 16,106 | ||||||||||||
| Prepaid expenses and other assets | 774 | 821 | 288 | 1,883 | ||||||||||||
| Prepaid income taxes | 15 | (105 | ) | 223 | 133 | |||||||||||
| Deferred tax assets | — | 190 | 1,836 | 2,026 | ||||||||||||
Total current assets | 27,939 | 8,455 | 11,178 | 47,572 | ||||||||||||
| Property and equipment, net | 1,353 | 6,227 | — | 7,580 | ||||||||||||
| Other Assets: | ||||||||||||||||
| Deferred debt issue costs, net | 3,932 | — | — | 3,932 | ||||||||||||
| Deposits and other noncurrent assets | 17,224 | 56 | 17 | 17,297 | ||||||||||||
| Goodwill | — | 170,721 | 98,190 | 268,911 | ||||||||||||
| Intangible assets, net | — | 73,950 | 44,731 | 118,681 | ||||||||||||
| 21,156 | 244,727 | 142,938 | 408,821 | |||||||||||||
| $ | 50,448 | $ | 259,409 | $ | 154,116 | $ | 463,973 | |||||||||
LIABILITIES AND MEMBERS’ EQUITY | ||||||||||||||||
| Current Liabilities | ||||||||||||||||
| Current portion of long-term debt | $ | 11,551 | $ | 45 | $ | — | $ | 11,596 | ||||||||
| Current portion of fair value of contingent consideration | — | — | 804 | 804 | ||||||||||||
| Accounts payable and accrued expenses | 2,079 | 1,393 | 1,378 | 4,850 | ||||||||||||
| Accrued compensation | 2,477 | 3,069 | 1,578 | 7,124 | ||||||||||||
| Accrued interest | 3,047 | — | — | 3,047 | ||||||||||||
| Acquisition related liability | — | — | 592 | 592 | ||||||||||||
| Fair value of derivative | 125 | — | — | 125 | ||||||||||||
Total current liabilities | 19,279 | 4,507 | 4,352 | 28,138 | ||||||||||||
| Intercompany payable (receivable) | (328,091 | ) | 191,120 | 136,971 | — | |||||||||||
| Long-term debt, net of current portion | 204,838 | 218 | — | 205,056 | ||||||||||||
| Deferred tax liabilities, net | — | 922 | 10,643 | 11,565 | ||||||||||||
| Fair value of contingent consideration, net of current portion | — | — | 2,150 | 2,150 | ||||||||||||
| Members’ Equity | 154,422 | 62,642 | — | 217,064 | ||||||||||||
| $ | 50,448 | $ | 259,409 | $ | 154,116 | $ | 463,973 | |||||||||
F-30
Table of Contents
| Aurora | ||||||||||||||||
| Diagnostics | Subsidiary | Non-Guarantor | Consolidated | |||||||||||||
December 31, 2010 | Holdings, LLC | Guarantors | Subsidiaries | Total | ||||||||||||
| (In thousands) | ||||||||||||||||
ASSETS | ||||||||||||||||
| Current Assets | ||||||||||||||||
| Cash and cash equivalents | $ | 38,513 | $ | 228 | $ | 1,200 | $ | 39,941 | ||||||||
| Accounts receivable, net | — | 14,397 | 11,051 | 25,448 | ||||||||||||
| Prepaid expenses and other assets | 582 | 893 | 474 | 1,949 | ||||||||||||
| Prepaid income taxes | 96 | 558 | 743 | 1,397 | ||||||||||||
| Deferred tax assets | — | 231 | 1,832 | 2,063 | ||||||||||||
Total current assets | 39,191 | 16,307 | 15,300 | 70,798 | ||||||||||||
| Property and equipment, net | 1,790 | 7,116 | — | 8,906 | ||||||||||||
| Other Assets: | ||||||||||||||||
| Deferred debt issue costs, net | 11,065 | — | — | 11,065 | ||||||||||||
| Deposits and other noncurrent assets | 41,013 | 56 | 18 | 41,087 | ||||||||||||
| Goodwill | — | 218,380 | 110,819 | 329,199 | ||||||||||||
| Intangible assets, net | — | 86,630 | 40,326 | 126,956 | ||||||||||||
| 52,078 | 305,066 | 151,163 | 508,307 | |||||||||||||
| $ | 93,059 | $ | 328,489 | $ | 166,463 | $ | 588,011 | |||||||||
LIABILITIES AND MEMBERS’ EQUITY | ||||||||||||||||
| Current Liabilities | ||||||||||||||||
| Current portion of long-term debt | $ | 2,694 | $ | 76 | $ | — | $ | 2,770 | ||||||||
| Current portion of fair value of contingent consideration | — | 6,753 | 1,332 | 8,085 | ||||||||||||
| Accounts payable and accrued expenses | 2,964 | 2,722 | 2,701 | 8,387 | ||||||||||||
| Accrued compensation | 2,667 | 3,408 | 2,138 | 8,213 | ||||||||||||
| Accrued interest | 863 | — | — | 863 | ||||||||||||
Total current liabilities | 9,188 | 12,959 | 6,171 | 28,318 | ||||||||||||
| Intercompany payable (receivable) | (358,203 | ) | 214,123 | 144,080 | — | |||||||||||
| Long-term debt, net of current portion | 315,766 | 278 | — | 316,044 | ||||||||||||
| Deferred tax liabilities, net | — | 2,747 | 11,094 | 13,841 | ||||||||||||
| Fair value of contingent consideration, net of current portion | — | 13,347 | 5,118 | 18,465 | ||||||||||||
| Members’ Equity | 126,308 | 85,035 | — | 211,343 | ||||||||||||
| $ | 93,059 | $ | 328,489 | $ | 166,463 | $ | 588,011 | |||||||||
F-31
Table of Contents
| Aurora | ||||||||||||||||
| Diagnostics | Subsidiary | Non-Guarantor | Consolidated | |||||||||||||
For the Year Ended December 31, 2008 | Holdings, LLC | Guarantors | Subsidiaries | Total | ||||||||||||
| (In thousands) | ||||||||||||||||
| Net Revenues | $ | — | $ | 97,765 | $ | 60,085 | $ | 157,850 | ||||||||
| Operating costs and expenses: | ||||||||||||||||
| Cost of services | — | 31,250 | 35,132 | 66,382 | ||||||||||||
| Selling, general and administrative expenses | 7,439 | 15,434 | 10,321 | 33,194 | ||||||||||||
| Provision for doubtful accounts | — | 3,890 | 4,147 | 8,037 | ||||||||||||
| Intangible asset amortization expense | — | 8,987 | 5,321 | 14,308 | ||||||||||||
| Management fees | (8,818 | ) | 9,930 | 447 | 1,559 | |||||||||||
| Acquisition and business development costs | 676 | — | — | 676 | ||||||||||||
| Equity based competition expense | 1,164 | — | — | 1,164 | ||||||||||||
Total operating costs and expenses | 461 | 69,491 | 55,368 | 125,320 | ||||||||||||
Income (loss) from operations | (461 | ) | 28,274 | 4,717 | 32,530 | |||||||||||
| Other income (expense): | ||||||||||||||||
| Interest expense | (16,827 | ) | — | (4,750 | ) | (21,577 | ) | |||||||||
| Other income/(expense) | 109 | 19 | (3 | ) | 125 | |||||||||||
Total other income (expense), net | (16,718 | ) | 19 | (4,753 | ) | (21,452 | ) | |||||||||
Income (loss) before income taxes | (17,179 | ) | 28,293 | (36 | ) | 11,078 | ||||||||||
| Provision for income taxes | 87 | 357 | (36 | ) | 408 | |||||||||||
Net income (loss) | $ | (17,266 | ) | $ | 27,936 | $ | — | $ | 10,670 | |||||||
| Aurora | ||||||||||||||||
| Diagnostics | Subsidiary | Non-Guarantor | Consolidated | |||||||||||||
For the Year Ended December 31, 2009 | Holdings, LLC | Guarantors | Subsidiaries | Total | ||||||||||||
| Net Revenues | $ | — | $ | 104,840 | $ | 66,725 | $ | 171,565 | ||||||||
| Operating costs and expenses: | ||||||||||||||||
| Cost of services | — | 33,117 | 38,661 | 71,778 | ||||||||||||
| Selling, general and administrative expenses | 9,281 | 16,482 | 11,091 | 36,854 | ||||||||||||
| Provision for doubtful accounts | — | 4,533 | 4,955 | 9,488 | ||||||||||||
| Intangible asset amortization expense | — | 9,188 | 5,386 | 14,574 | ||||||||||||
| Management fees | (11,275 | ) | 17,877 | (4,824 | ) | 1,778 | ||||||||||
| Impairment of goodwill and other intangible assets | — | — | 8,031 | 8,031 | ||||||||||||
| Acquisition and business development costs | 1,074 | 1,074 | ||||||||||||||
Total operating costs and expenses | (920 | ) | 81,197 | 63,300 | 143,577 | |||||||||||
Income from operations | 920 | 23,643 | 3,425 | 27,988 | ||||||||||||
| Other income (expense): | ||||||||||||||||
| Interest expense | (15,188 | ) | 2 | (3,783 | ) | (18,969 | ) | |||||||||
| Other income/(expense) | (7 | ) | 25 | 10 | 28 | |||||||||||
Total other income (expense), net | (15,195 | ) | 27 | (3,773 | ) | (18,941 | ) | |||||||||
Income (loss) before income taxes | (14,275 | ) | 23,670 | (348 | ) | 9,047 | ||||||||||
| Provision for income taxes | 196 | 197 | (348 | ) | 45 | |||||||||||
Net income (loss) | $ | (14,471 | ) | $ | 23,473 | $ | — | $ | 9,002 | |||||||
F-32
Table of Contents
| Aurora | ||||||||||||||||
| Diagnostics | Subsidiary | Non-Guarantor | Consolidated | |||||||||||||
For the Year Ended December 31, 2010 | Holdings, LLC | Guarantors | Subsidiaries | Total | ||||||||||||
| (In thousands) | ||||||||||||||||
| Net Revenues | $ | — | $ | 133,299 | $ | 79,538 | $ | 212,837 | ||||||||
| Operating costs and expenses: | ||||||||||||||||
| Cost of services | — | 47,069 | 49,799 | 96,868 | ||||||||||||
| Selling, general and administrative expenses | 11,076 | 23,646 | 14,419 | 49,141 | ||||||||||||
| Provision for doubtful accounts | — | 7,067 | 5,326 | 12,393 | ||||||||||||
| Intangible asset amortization expense | — | 11,570 | 7,376 | 18,946 | ||||||||||||
| Management fees | (9,534 | ) | 20,446 | (8,723 | ) | 2,189 | ||||||||||
| Impairment of goodwill and other intangible assets | — | — | 4,871 | 4,871 | ||||||||||||
| Acquisition and business development costs | 1,032 | — | — | 1,032 | ||||||||||||
| Change in fair value of contingent consideration | — | 277 | 706 | 983 | ||||||||||||
Total operating costs and expenses | 2,574 | 110,075 | 73,774 | 186,423 | ||||||||||||
Income from operations | (2,574 | ) | 23,224 | 5,764 | 26,414 | |||||||||||
| Other income (expense): | ||||||||||||||||
| Interest expense | (11,849 | ) | (411 | ) | (4,781 | ) | (17,041 | ) | ||||||||
| Write-off of deferred debt issue costs | (9,259 | ) | — | — | (9,259 | ) | ||||||||||
| Loss on extinguishment of debt | (2,296 | ) | — | — | (2,296 | ) | ||||||||||
| Other income/(expense) | 9 | 4 | 5 | 18 | ||||||||||||
Total other expense, net | (23,395 | ) | (407 | ) | (4,776 | ) | (28,578 | ) | ||||||||
Income (loss) before income taxes | (25,969 | ) | 22,817 | 988 | (2,164 | ) | ||||||||||
| Provision for income taxes | 75 | 424 | 988 | 1,487 | ||||||||||||
Net income (loss) | $ | (26,044 | ) | $ | 22,393 | $ | — | $ | (3,651 | ) | ||||||
F-33
Table of Contents
| Aurora | ||||||||||||||||
| Diagnostics | Subsidiary | Non-Guarantor | Consolidated | |||||||||||||
For the Year Ended December 31, 2008 | Holdings, LLC | Guarantors | Subsidiaries | Total | ||||||||||||
| (In thousands) | ||||||||||||||||
| Cash Flows From Operating Activities: | ||||||||||||||||
| Net income (loss) | $ | (17,266 | ) | $ | 27,936 | $ | — | $ | 10,670 | |||||||
| Adjustments to reconcile net income (loss) to net cash provided by operating activities | 2,934 | 10,145 | 4,380 | 17,459 | ||||||||||||
| Changes in assets and liabilities, net of effects of acquisitions | 4,467 | 1,192 | (4,818 | ) | 841 | |||||||||||
| Net cash (used in) provided by operating activities | (9,865 | ) | 39,273 | (438 | ) | 28,970 | ||||||||||
| Net cash used in investing activities | (1,204 | ) | (41,645 | ) | (3,423 | ) | (46,272 | ) | ||||||||
| Net cash provided by financing activities | 16,022 | — | — | 16,022 | ||||||||||||
| Net increase (decrease) in cash | 4,953 | (2,372 | ) | (3,861 | ) | (1,280 | ) | |||||||||
| Cash and cash equivalents, beginning of period | 2,000 | 2,537 | 4,021 | 8,558 | ||||||||||||
| Cash and cash equivalents, end of period | $ | 6,953 | $ | 165 | $ | 160 | $ | 7,278 | ||||||||
| Aurora | ||||||||||||||||
| Diagnostics | Subsidiary | Non-Guarantor | Consolidated | |||||||||||||
For the Year Ended December 31, 2009 | Holdings, LLC | Guarantors | Subsidiaries | Total | ||||||||||||
| (In thousands) | ||||||||||||||||
| Cash Flows From Operating Activities: | ||||||||||||||||
| Net income (loss) | $ | (14,471 | ) | $ | 23,473 | $ | — | $ | 9,002 | |||||||
| Adjustments to reconcile net income (loss) to net cash provided by operating activities | 2,128 | 10,735 | 12,055 | 24,918 | ||||||||||||
| Changes in assets and liabilities, net of effects of acquisitions | 16,805 | (27,221 | ) | 12,859 | 2,443 | |||||||||||
| Net cash provided by operating activities | 4,462 | 6,987 | 24,914 | 36,363 | ||||||||||||
| Net cash used in investing activities | (17,309 | ) | (7,152 | ) | (24,800 | ) | (49,261 | ) | ||||||||
| Net cash provided by financing activities | 33,044 | — | — | 33,044 | ||||||||||||
| Net increase (decrease) in cash | 20,197 | (165 | ) | 114 | 20,146 | |||||||||||
| Cash and cash equivalents, beginning of period | 6,953 | 165 | 160 | 7,278 | ||||||||||||
| Cash and cash equivalents, end of period | $ | 27,150 | $ | — | $ | 274 | $ | 27,424 | ||||||||
| Aurora | ||||||||||||||||
| Diagnostics | Subsidiary | Non-Guarantor | Consolidated | |||||||||||||
For the Year Ended December 31, 2010 | Holdings, LLC | Guarantors | Subsidiaries | Total | ||||||||||||
| (In thousands) | ||||||||||||||||
| Cash Flows From Operating Activities: | ||||||||||||||||
| Net income (loss) | $ | (26,044 | ) | $ | 22,393 | $ | — | $ | (3,651 | ) | ||||||
| Adjustments to reconcile net income (loss) to net cash provided by operating activities | 19,003 | 13,916 | 6,661 | 39,580 | ||||||||||||
| Changes in assets and liabilities, net of effects of acquisitions | (19,288 | ) | 9,168 | 1,192 | (8,928 | ) | ||||||||||
| Net cash (used in) provided by operating activities | (26,329 | ) | 45,477 | 7,853 | 27,001 | |||||||||||
| Net cash used in investing activities | (38,762 | ) | (45,196 | ) | (6,927 | ) | (90,885 | ) | ||||||||
| Net cash (used in) provided by financing activities | 76,454 | (53 | ) | — | 76,401 | |||||||||||
| Net increase in cash | 11,363 | 228 | 926 | 12,517 | ||||||||||||
| Cash and cash equivalents, beginning of period | 27,150 | — | 274 | 27,424 | ||||||||||||
| Cash and cash equivalents, end of period | $ | 38,513 | $ | 228 | $ | 1,200 | $ | 39,941 | ||||||||
F-34
Table of Contents
| June 30, | December 31, | |||||||
| 2011 | 2010 | |||||||
| (Unaudited) | ||||||||
| (In thousands) | ||||||||
ASSETS | ||||||||
| Current Assets | ||||||||
| Cash and cash equivalents | $ | 36,488 | $ | 39,941 | ||||
| Accounts receivable, net | 28,277 | 25,448 | ||||||
| Prepaid expenses and other assets | 2,366 | 1,949 | ||||||
| Prepaid income taxes | — | 1,397 | ||||||
| Deferred tax assets | 3,191 | 2,063 | ||||||
Total current assets | 70,322 | 70,798 | ||||||
| Property and equipment, net | 10,393 | 8,906 | ||||||
| Other Assets: | ||||||||
| Deferred debt issue costs, net | 10,279 | 11,065 | ||||||
| Deposits and other noncurrent assets | 4,610 | 41,087 | ||||||
| Goodwill | 375,900 | 329,199 | ||||||
| Intangible assets, net | 153,400 | 126,956 | ||||||
| 544,189 | 508,307 | |||||||
| $ | 624,904 | $ | 588,011 | |||||
LIABILITIES AND MEMBERS’ EQUITY | ||||||||
| Current Liabilities | ||||||||
| Current portion of long-term debt | $ | 2,753 | $ | 2,770 | ||||
| Current portion of fair value of contingent consideration | 14,353 | 8,085 | ||||||
| Accounts payable and accrued expenses | 10,573 | 8,387 | ||||||
| Accrued compensation | 9,392 | 8,213 | ||||||
| Accrued interest | 11,805 | 863 | ||||||
| Income taxes payable | 314 | — | ||||||
Total current liabilities | 49,190 | 28,318 | ||||||
| Long-term debt, net of current portion | 316,086 | 316,044 | ||||||
| Deferred tax liabilities, net | 23,155 | 13,841 | ||||||
| Fair value of contingent consideration, net of current portion | 25,407 | 18,465 | ||||||
| Other liabilities | 1,365 | — | ||||||
| Members’ Equity | 209,701 | 211,343 | ||||||
| $ | 624,904 | $ | 588,011 | |||||
F-35
Table of Contents
| 2011 | 2010 | |||||||
| Unaudited | ||||||||
| (In thousands) | ||||||||
| Net Revenues | $ | 130,470 | $ | 101,105 | ||||
| Operating costs and expenses: | ||||||||
| Cost of services | 58,846 | 45,904 | ||||||
| Selling, general and administrative expenses | 30,595 | 23,438 | ||||||
| Provision for doubtful accounts | 8,862 | 6,028 | ||||||
| Intangible asset amortization expense | 11,126 | 9,274 | ||||||
| Management fees | 1,344 | 1,062 | ||||||
| Acquisition and business development costs | 515 | 433 | ||||||
| Change in fair value of contingent consideration | 3,158 | 984 | ||||||
Total operating costs and expenses | 114,446 | 87,123 | ||||||
Income from operations | 16,024 | 13,982 | ||||||
| Other income (expense): | ||||||||
| Interest expense | (16,268 | ) | (7,587 | ) | ||||
| Write-off of deferred debt issue costs | — | (4,527 | ) | |||||
| Loss on extinguishment of debt | — | (2,296 | ) | |||||
| Other income/(expense) | (46 | ) | 5 | |||||
Total other expense, net | (16,314 | ) | (14,405 | ) | ||||
Income (loss) before income taxes | (290 | ) | (423 | ) | ||||
| Provision for income taxes | 1,352 | 1,231 | ||||||
Net income (loss) | $ | (1,642 | ) | $ | (1,654 | ) | ||
F-36
Table of Contents
| Member | Equity | Total | ||||||||||||||||||
| Member | Contributions | Transaction | Retained | Members’ | ||||||||||||||||
| Units | (Distributions) | Costs | Earnings | Equity | ||||||||||||||||
| Unaudited | ||||||||||||||||||||
| (In thousands) | ||||||||||||||||||||
| Balance, December 31, 2010 | 131,382 | $ | 201,114 | $ | (4,825 | ) | $ | 15,054 | $ | 211,343 | ||||||||||
| Net loss | — | — | — | (1,642 | ) | (1,642 | ) | |||||||||||||
| Balance, June 30, 2011 | 131,382 | $ | 201,114 | $ | (4,825 | ) | $ | 13,412 | $ | 209,701 | ||||||||||
F-37
Table of Contents
| 2011 | 2010 | |||||||
| Unaudited | ||||||||
| (In thousands) | ||||||||
| Cash Flows From Operating Activities | ||||||||
| Net loss | $ | (1,642 | ) | $ | (1,654 | ) | ||
| Adjustments to reconcile net loss to net cash provided by operating activities: | ||||||||
| Depreciation and amortization | 13,068 | 10,877 | ||||||
| Amortization of deferred debt issue costs | 951 | 596 | ||||||
| Amortization of original issue discount on debt | 143 | 181 | ||||||
| Deferred income taxes | (2,320 | ) | (1,018 | ) | ||||
| Change in fair value of contingent consideration | 3,158 | 984 | ||||||
| Write-off of deferred debt issue costs | — | 4,527 | ||||||
| Loss on extinguishment of debt | — | 2,296 | ||||||
| Loss on disposal of property | 46 | — | ||||||
| Changes in assets and liabilities, net of working capital acquired in business combinations: | ||||||||
| (Increase) decrease in: | ||||||||
| Accounts receivable | 16 | (4,818 | ) | |||||
| Prepaid income taxes | 1,397 | — | ||||||
| Prepaid expenses | (389 | ) | (523 | ) | ||||
| Increase (decrease) in: | ||||||||
| Accounts payable and accrued expenses | 946 | 777 | ||||||
| Accrued compensation | 1,150 | (789 | ) | |||||
| Accrued interest | 10,942 | (2,463 | ) | |||||
| Taxes payable | 314 | 1,407 | ||||||
Net cash provided by operating activities | 27,780 | 10,380 | ||||||
| Cash Flows From Investing Activities | ||||||||
| Purchase of property and equipment | (1,958 | ) | (1,446 | ) | ||||
| Increase in deposits and other noncurrent assets | 282 | 42 | ||||||
| Payment of contingent notes | (14,523 | ) | (14,701 | ) | ||||
| Businesses acquired, net of cash acquired | (13,957 | ) | (21,229 | ) | ||||
Net cash used in investing activities | (30,156 | ) | (37,334 | ) | ||||
| Cash Flows From Financing Activities | ||||||||
| Payments of capitalized lease obligations | (37 | ) | — | |||||
| Repayments under former term loan facility | — | (209,100 | ) | |||||
| Repayments of subordinated notes payable | — | (337 | ) | |||||
| Net borrowings under revolver | — | 5,000 | ||||||
| Borrowings under new term loan facility | — | 225,000 | ||||||
| Payment of debt issuance costs | (701 | ) | (13,801 | ) | ||||
| Payment of public offering costs | (339 | ) | (2,431 | ) | ||||
| Contributions from members, net of tax distributions | — | (2,183 | ) | |||||
Net cash (used in) provided by financing activities | (1,077 | ) | 2,148 | |||||
Net decrease in cash | (3,453 | ) | (24,806 | ) | ||||
| Cash and cash equivalents, beginning | 39,941 | 27,424 | ||||||
| Cash and cash equivalents, ending | $ | 36,488 | $ | 2,618 | ||||
| Supplemental Disclosures of Cash Flow Information | ||||||||
| Cash interest payments | $ | 4,028 | $ | 9,174 | ||||
| Cash tax payments, including member tax distributions | $ | 1,963 | $ | 2,868 | ||||
| Supplemental Schedule of Noncash Investing and Financing Activities | ||||||||
| Fair value of contingent consideration issued in acquisitions | $ | 12,510 | $ | 18,983 | ||||
| Capital lease obligations | $ | 47 | $ | — | ||||
F-38
Table of Contents
| Note 1. | Nature of Business and Significant Accounting Policies |
F-39
Table of Contents
| Note 2. | Acquisitions |
F-40
Table of Contents
| Cash | ||||||
Location | Date Acquired | Paid | ||||
| Texas | January 1, 2011 | $ | 29,856 | |||
| Nevada | January 1, 2011 | 7,000 | ||||
| Massachusetts | June 2, 2011 | 14,700 | ||||
| Total 2011 Acquisitions | $ | 51,556 | ||||
F-41
Table of Contents
| 2011 | ||||
| Cash | $ | 743 | ||
| Accounts receivable(1) | 2,845 | |||
| Other assets | 28 | |||
| Property and equipment | 153 | |||
| Intangible assets | 37,570 | |||
| Goodwill | 34,047 | |||
| Assets acquired | 75,386 | |||
| Accounts payable and accrued expenses | 785 | |||
| Accrued compensation | 29 | |||
| Fair value of contingent consideration | 12,510 | |||
| Deferred tax liabilities | 10,506 | |||
| Liabilities assumed | 23,830 | |||
| Net assets acquired | $ | 51,556 | ||
| Net assets acquired | $ | 51,556 | ||
| Less: | ||||
| Cash acquired | (743 | ) | ||
| Deposits and other noncurrent assets(2) | (36,856 | ) | ||
| Net cash paid for acquisitions, net of cash acquired | $ | 13,957 | ||
| (1) | Acquired Accounts receivable is net of $1.7 million Allowance for doubtful accounts. | |
| (2) | Cash payments made on December 31, 2010. |
| Pro Forma | ||||||||
| Six Months Ended June 30, | ||||||||
| 2010 | 2011 | |||||||
| Net Revenues | $ | 120,866 | $ | 132,758 | ||||
| Net Income (Loss) | $ | 1,945 | $ | (1,103 | ) | |||
F-42
Table of Contents
| Note 3. | Accounts Receivable |
| June 30, | December 31, | |||||||
| 2011 | 2010 | |||||||
| Accounts receivable | $ | 44,484 | $ | 37,172 | ||||
| Less: Allowance for doubtful accounts | (16,207 | ) | (11,724 | ) | ||||
| Accounts receivable, net | $ | 28,277 | $ | 25,448 | ||||
| Note 4. | Goodwill and Intangible Assets |
| June 30, | December 31, | |||||||
| 2011 | 2010 | |||||||
| Goodwill, beginning of period | $ | 329,199 | $ | 268,911 | ||||
| Acquisitions | 34,047 | 44,752 | ||||||
| Contingent notes* | 12,654 | 16,979 | ||||||
| Goodwill impairment | — | (1,976 | ) | |||||
| Other acquisition costs | — | 533 | ||||||
| Goodwill, end of period | $ | 375,900 | $ | 329,199 | ||||
| * | Related to acquisitions completed prior to January 1, 2009. |
| Weighted Average | June 30, 2011 | |||||||||||||||||
| Range | Amortization Period | Accumulated | ||||||||||||||||
| (Years) | (Years) | Cost | Amortization | Net | ||||||||||||||
| Amortizing intangible assets: | ||||||||||||||||||
| Customer relationships | 5 — 10 | 9 | $ | 142,923 | $ | (43,941 | ) | $ | 98,982 | |||||||||
| Health care facility agreements | 4 — 18 | 15 | 65,560 | (13,721 | ) | 51,839 | ||||||||||||
| Noncompete agreements | 2 — 5 | 5 | 5,431 | (2,852 | ) | 2,579 | ||||||||||||
| Total intangible assets | $ | 213,914 | $ | (60,514 | ) | $ | 153,400 | |||||||||||
| Weighted Average | December 31, 2010 | |||||||||||||||||
| Range | Amortization Period | Accumulated | ||||||||||||||||
| (Years) | (Years) | Cost | Amortization | Net | ||||||||||||||
| Amortizing intangible assets: | ||||||||||||||||||
| Customer relationships | 5 — 10 | 8 | $ | 130,533 | $ | (35,825 | ) | $ | 94,708 | |||||||||
| Health care facility agreements | 4 — 18 | 14 | 41,370 | (11,260 | ) | 30,110 | ||||||||||||
| Noncompete agreements | 2 — 5 | 5 | 4,441 | (2,303 | ) | 2,138 | ||||||||||||
| Total intangible assets | $ | 176,344 | $ | (49,388 | ) | $ | 126,956 | |||||||||||
F-43
Table of Contents
Year Ending December 31, | ||||
| Remainder of 2011 | $ | 11,586 | ||
| 2012 | 22,102 | |||
| 2013 | 21,684 | |||
| 2014 | 20,434 | |||
| 2015 | 19,745 | |||
| 2016 | 19,272 | |||
| Thereafter | 38,577 | |||
| $ | 153,400 | |||
| Note 5. | Accounts Payable and Accrued Expenses |
| June 30, | December 31, | |||||||
| 2011 | 2010 | |||||||
| Accounts payable | $ | 3,143 | $ | 3,514 | ||||
| Due to predecessor pension plan | 1,235 | 1,241 | ||||||
| Accrued management fees | 1,054 | 562 | ||||||
| Other accrued expenses | 5,141 | 3,070 | ||||||
| $ | 10,573 | $ | 8,387 | |||||
| Note 6. | Long-Term Debt |
F-44
Table of Contents
F-45
Table of Contents
| June 30, | December 31, | |||||||
| 2011 | 2010 | |||||||
| Term loan | $ | 114,438 | $ | 114,438 | ||||
| Subordinated unsecured contingent note dated April 30, 2007 | 5,528 | 5,528 | ||||||
| Senior Notes | 200,000 | 200,000 | ||||||
| Capital lease obligations | 275 | 393 | ||||||
| 320,241 | 320,359 | |||||||
| Less: | ||||||||
| Original issue discount, net | (1,402 | ) | (1,545 | ) | ||||
| Current portion | (2,753 | ) | (2,770 | ) | ||||
| Long-term debt, net of current portion | $ | 316,086 | $ | 316,044 | ||||
Year Ending December 31, | ||||
| Remainder of 2011 | $ | 2,720 | ||
| 2012 | 2,910 | |||
| 2013 | 79 | |||
| 2014 | 67 | |||
| 2015 | 18 | |||
| 2016 | 114,447 | |||
| Thereafter | 200,000 | |||
| $ | 320,241 | |||
| Note 7. | Related Party Transactions |
F-46
Table of Contents
| Note 8. | Members’ Equity |
| Note 9. | Commitments and Contingencies |
F-47
Table of Contents
| Note 10. | Fair Value of Financial Instruments |
F-48
Table of Contents
| Significant Other | Significant | |||||||||||||||
| Quoted Prices | Observable | Unobservable | ||||||||||||||
| in Active Markets | Inputs | Inputs | ||||||||||||||
| Fair Value | Level 1 | Level 2 | Level 3 | |||||||||||||
| Assets: | ||||||||||||||||
| Deposits and other non-current assets | ||||||||||||||||
| Interest rate cap | $ | 17 | $ | — | $ | 17 | $ | — | ||||||||
| Liabilities: | ||||||||||||||||
| Current portion of fair value of contingent consideration | $ | 14,353 | $ | — | $ | — | $ | 14,353 | ||||||||
| Fair value of contingent consideration, net of current portion | $ | 25,407 | $ | — | $ | — | $ | 25,407 | ||||||||
| Beginning | Ending | |||||||||||||||||||
| Balance | Total (Gains)/ | Balance | ||||||||||||||||||
| January 1, | Losses Realized | June 30, | ||||||||||||||||||
| 2011 | and Unrealized | Issuances | Settlements | 2011 | ||||||||||||||||
| Contingent consideration | $ | 26,550 | $ | 3,158 | $ | 12,510 | $ | (2,458 | ) | $ | 39,760 | |||||||||
| Note 11. | Income Taxes |
F-49
Table of Contents
| Note 12. | Subsequent Events |
| Note 13. | Guarantor Subsidiaries |
F-50
Table of Contents
F-51
Table of Contents
| Aurora | ||||||||||||||||
| Diagnostics | Subsidiary | Non-Guarantor | Consolidated | |||||||||||||
June 30, 2011 | Holdings, LLC | Guarantors | Subsidiaries | Total | ||||||||||||
| (In thousands) | ||||||||||||||||
ASSETS | ||||||||||||||||
| Current Assets | ||||||||||||||||
| Cash and cash equivalents | $ | 34,261 | $ | 659 | $ | 1,568 | $ | 36,488 | ||||||||
| Accounts receivable, net | — | 15,319 | 12,958 | 28,277 | ||||||||||||
| Prepaid expenses and other assets | 977 | 922 | 467 | 2,366 | ||||||||||||
| Deferred tax assets | — | 417 | 2,774 | 3,191 | ||||||||||||
Total current assets | 35,238 | 17,317 | 17,767 | 70,322 | ||||||||||||
| Property and equipment, net | 1,847 | 8,546 | — | 10,393 | ||||||||||||
| Other Assets: | ||||||||||||||||
| Deferred debt issue costs, net | 10,279 | — | — | 10,279 | ||||||||||||
| Deposits and other noncurrent assets | 4,440 | 152 | 18 | 4,610 | ||||||||||||
| Goodwill | — | 236,703 | 139,197 | 375,900 | ||||||||||||
| Intangible assets, net | — | 88,307 | 65,093 | 153,400 | ||||||||||||
| 14,719 | 325,162 | 204,308 | 544,189 | |||||||||||||
| $ | 51,804 | $ | 351,025 | $ | 222,075 | $ | 624,904 | |||||||||
LIABILITIES AND MEMBERS’ EQUITY | ||||||||||||||||
| Current Liabilities | ||||||||||||||||
| Current portion of long-term debt | $ | 2,694 | $ | 59 | $ | — | $ | 2,753 | ||||||||
| Current portion of fair value of contingent consideration | — | 9,538 | 4,815 | 14,353 | ||||||||||||
| Accounts payable and accrued expenses | 3,525 | 3,028 | 4,020 | 10,573 | ||||||||||||
| Accrued compensation | 3,125 | 3,608 | 2,659 | 9,392 | ||||||||||||
| Accrued interest | 11,805 | — | — | 11,805 | ||||||||||||
| Income taxes payable | (87 | ) | 62 | 339 | 314 | |||||||||||
Total current liabilities | 21,062 | 16,295 | 11,833 | 49,190 | ||||||||||||
| Intercompany payable (receivable) | (398,275 | ) | 217,357 | 180,918 | — | |||||||||||
| Long-term debt, net of current portion | 315,906 | 180 | — | 316,086 | ||||||||||||
| Deferred tax liabilities, net | — | 2,616 | 20,539 | 23,155 | ||||||||||||
| Fair value of contingent consideration, net of current portion | — | 16,622 | 8,785 | 25,407 | ||||||||||||
| Other liabilities | 1,365 | — | — | 1,365 | ||||||||||||
| Members’ Equity | 111,746 | 97,955 | — | 209,701 | ||||||||||||
| $ | 51,804 | $ | 351,025 | $ | 222,075 | $ | 624,904 | |||||||||
F-52
Table of Contents
| Aurora | ||||||||||||||||
| Diagnostics | Subsidiary | Non-Guarantor | Consolidated | |||||||||||||
December 31, 2010 | Holdings, LLC | Guarantors | Subsidiaries | Total | ||||||||||||
| (In thousands) | ||||||||||||||||
ASSETS | ||||||||||||||||
| Current Assets | ||||||||||||||||
| Cash and cash equivalents | $ | 38,513 | $ | 228 | $ | 1,200 | $ | 39,941 | ||||||||
| Accounts receivable, net | — | 14,397 | 11,051 | 25,448 | ||||||||||||
| Prepaid expenses and other assets | 582 | 893 | 474 | 1,949 | ||||||||||||
| Prepaid income taxes | 96 | 558 | 743 | 1,397 | ||||||||||||
| Deferred tax assets | — | 231 | 1,832 | 2,063 | ||||||||||||
Total current assets | 39,191 | 16,307 | 15,300 | 70,798 | ||||||||||||
| Property and equipment, net | 1,790 | 7,116 | — | 8,906 | ||||||||||||
| Other Assets: | ||||||||||||||||
| Deferred debt issue costs, net | 11,065 | — | — | 11,065 | ||||||||||||
| Deposits and other noncurrent assets | 41,013 | 56 | 18 | 41,087 | ||||||||||||
| Goodwill | — | 218,380 | 110,819 | 329,199 | ||||||||||||
| Intangible assets, net | — | 86,630 | 40,326 | 126,956 | ||||||||||||
| 52,078 | 305,066 | 151,163 | 508,307 | |||||||||||||
| $ | 93,059 | $ | 328,489 | $ | 166,463 | $ | 588,011 | |||||||||
LIABILITIES AND MEMBERS’ EQUITY | ||||||||||||||||
| Current Liabilities | ||||||||||||||||
| Current portion of long-term debt | $ | 2,694 | $ | 76 | $ | — | $ | 2,770 | ||||||||
| Current portion of fair value of contingent consideration | — | 6,753 | 1,332 | 8,085 | ||||||||||||
| Accounts payable and accrued expenses | 2,964 | 2,722 | 2,701 | 8,387 | ||||||||||||
| Accrued compensation | 2,667 | 3,408 | 2,138 | 8,213 | ||||||||||||
| Accrued interest | 863 | — | — | 863 | ||||||||||||
Total current liabilities | 9,188 | 12,959 | 6,171 | 28,318 | ||||||||||||
| Intercompany payable (receivable) | (358,203 | ) | 214,123 | 144,080 | — | |||||||||||
| Long-term debt, net of current portion | 315,766 | 278 | — | 316,044 | ||||||||||||
| Deferred tax liabilities, net | — | 2,747 | 11,094 | 13,841 | ||||||||||||
| Fair value of contingent consideration, net of current portion | — | 13,347 | 5,118 | 18,465 | ||||||||||||
| Members’ Equity | 126,308 | 85,035 | — | 211,343 | ||||||||||||
| $ | 93,059 | $ | 328,489 | $ | 166,463 | $ | 588,011 | |||||||||
F-53
Table of Contents
| Aurora | ||||||||||||||||
| Diagnostics | Subsidiary | Non-Guarantor | Consolidated | |||||||||||||
For the Six Months Ended June 30, 2011 | Holdings, LLC | Guarantors | Subsidiaries | Total | ||||||||||||
| (In thousands) | ||||||||||||||||
| Net Revenues | $ | — | $ | 76,555 | $ | 53,915 | $ | 130,470 | ||||||||
| Operating costs and expenses: | ||||||||||||||||
| Cost of services | — | 26,447 | 32,399 | 58,846 | ||||||||||||
| Selling, general and administrative expenses | 7,769 | 14,065 | 8,761 | 30,595 | ||||||||||||
| Provision for doubtful accounts | — | 4,879 | 3,983 | 8,862 | ||||||||||||
| Intangible asset amortization expense | — | 6,452 | 4,674 | 11,126 | ||||||||||||
| Management fees | (6,769 | ) | 8,584 | (471 | ) | 1,344 | ||||||||||
| Acquisition and business development costs | 515 | — | — | 515 | ||||||||||||
| Change in fair value of contingent consideration | — | 2,214 | 944 | 3,158 | ||||||||||||
Total operating costs and expenses | 1,515 | 62,641 | 50,290 | 114,446 | ||||||||||||
Income (loss) from operations | (1,515 | ) | 13,914 | 3,625 | 16,024 | |||||||||||
| Other expense: | ||||||||||||||||
| Interest expense | (13,003 | ) | (206 | ) | (3,059 | ) | (16,268 | ) | ||||||||
| Other expense | (2 | ) | (24 | ) | (20 | ) | (46 | ) | ||||||||
Total other expense | (13,005 | ) | (230 | ) | (3,079 | ) | (16,314 | ) | ||||||||
Income (loss) before income taxes | (14,520 | ) | 13,684 | 546 | (290 | ) | ||||||||||
| Provision for income taxes | 42 | 764 | 546 | 1,352 | ||||||||||||
Net income (loss) | $ | (14,562 | ) | $ | 12,920 | $ | — | $ | (1,642 | ) | ||||||
| Aurora | ||||||||||||||||
| Diagnostics | Subsidiary | Non-Guarantor | Consolidated | |||||||||||||
For the Six Months Ended June 30, 2010 | Holdings, LLC | Guarantors | Subsidiaries | Total | ||||||||||||
| (In thousands) | ||||||||||||||||
| Net Revenues | $ | — | $ | 61,805 | $ | 39,300 | $ | 101,105 | ||||||||
| Operating costs and expenses: | ||||||||||||||||
| Cost of services | — | 21,415 | 24,489 | 45,904 | ||||||||||||
| Selling, general and administrative expenses | 5,402 | 10,931 | 7,105 | 23,438 | ||||||||||||
| Provision for doubtful accounts | — | 3,364 | 2,664 | 6,028 | ||||||||||||
| Intangible asset amortization expense | — | 5,484 | 3,790 | 9,274 | ||||||||||||
| Management fees | (4,732 | ) | 8,187 | (2,393 | ) | 1,062 | ||||||||||
| Acquisition and business development costs | 433 | — | — | 433 | ||||||||||||
| Change in fair value of contingent consideration | — | 487 | 497 | 984 | ||||||||||||
Total operating costs and expenses | 1,103 | 49,868 | 36,152 | 87,123 | ||||||||||||
Income (loss) from operations | (1,103 | ) | 11,937 | 3,148 | 13,982 | |||||||||||
| Other income (expense): | ||||||||||||||||
| Interest expense | (4,992 | ) | (205 | ) | (2,390 | ) | (7,587 | ) | ||||||||
| Write-off of deferred debt issue costs | (4,527 | ) | — | — | (4,527 | ) | ||||||||||
| Loss on extinguishment of debt | (2,296 | ) | — | — | (2,296 | ) | ||||||||||
| Other income | — | 1 | 4 | 5 | ||||||||||||
Total other expense, net | (11,815 | ) | (204 | ) | (2,386 | ) | (14,405 | ) | ||||||||
Income (loss) before income taxes | (12,918 | ) | 11,733 | 762 | (423 | ) | ||||||||||
| Provision for income taxes | 87 | 382 | 762 | 1,231 | ||||||||||||
Net income (loss) | $ | (13,005 | ) | $ | 11,351 | $ | — | $ | (1,654 | ) | ||||||
F-54
Table of Contents
| Aurora | ||||||||||||||||
| Diagnostics | Subsidiary | Non-Guarantor | Consolidated | |||||||||||||
For the Six Months Ended June 30, 2011 | Holdings, LLC | Guarantors | Subsidiaries | Total | ||||||||||||
| (In thousands) | ||||||||||||||||
| Cash Flows From Operating Activities: | ||||||||||||||||
| Net (loss) income | $ | (14,562 | ) | $ | 12,920 | $ | — | $ | (1,642 | ) | ||||||
| Adjustments to reconcile net (loss) income to net cash provided by operating activities | 1,506 | 9,925 | 3,615 | 15,046 | ||||||||||||
| Changes in assets and liabilities, net of effects of acquisitions | 9,903 | 1,989 | 2,484 | 14,376 | ||||||||||||
| Net cash (used in) provided by operating activities | (3,153 | ) | 24,834 | 6,099 | 27,780 | |||||||||||
| Net cash used in investing activities | (59 | ) | (24,366 | ) | (5,731 | ) | (30,156 | ) | ||||||||
| Net cash used in financing activities | (1,040 | ) | (37 | ) | — | (1,077 | ) | |||||||||
| Net (decrease) increase in cash | (4,252 | ) | 431 | 368 | (3,453 | ) | ||||||||||
| Cash and cash equivalents, beginning of period | 38,513 | 228 | 1,200 | 39,941 | ||||||||||||
| Cash and cash equivalents, end of period | $ | 34,261 | $ | 659 | $ | 1,568 | $ | 36,488 | ||||||||
| Aurora | ||||||||||||||||
| Diagnostics | Subsidiary | Non-Guarantor | Consolidated | |||||||||||||
For the Six Months Ended June 30, 2010 | Holdings, LLC | Guarantors | Subsidiaries | Total | ||||||||||||
| (In thousands) | ||||||||||||||||
| Cash Flows From Operating Activities: | ||||||||||||||||
| Net (loss) income | $ | (13,005 | ) | $ | 11,351 | $ | — | $ | (1,654 | ) | ||||||
| Adjustments to reconcile net (loss) income to net cash provided by operating activities | 7,971 | 7,006 | 3,466 | 18,443 | ||||||||||||
| Changes in assets and liabilities, net of effects of acquisitions | (22,778 | ) | 13,759 | 2,610 | (6,409 | ) | ||||||||||
| Net cash (used in) provided by operating activities | (27,812 | ) | 32,116 | 6,076 | 10,380 | |||||||||||
| Net cash provided by (used in) investing activities | 418 | (31,431 | ) | (6,321 | ) | (37,334 | ) | |||||||||
| Net cash provided by (used in) financing activities | 2,485 | (337 | ) | — | 2,148 | |||||||||||
| Net (decrease) increase in cash | (24,909 | ) | 348 | (245 | ) | (24,806 | ) | |||||||||
| Cash and cash equivalents, beginning of period | 27,150 | — | 274 | 27,424 | ||||||||||||
| Cash and cash equivalents, end of period | $ | 2,241 | $ | 348 | $ | 29 | $ | 2,618 | ||||||||
F-55
Table of Contents
F-56
Table of Contents
| March 12, | December 31, | December 31, | December 31, | |||||||||||||
| 2010 | 2009 | 2008 | 2007 | |||||||||||||
| ($ in thousands) | ||||||||||||||||
ASSETS | ||||||||||||||||
| Current Assets | ||||||||||||||||
| Cash | $ | 487 | $ | 675 | $ | 2,368 | $ | 165 | ||||||||
| Investments in trading securities | — | 616 | 459 | — | ||||||||||||
| Accounts receivable, net | 2,525 | 2,586 | 2,162 | 1,384 | ||||||||||||
| Other current assets | 30 | 33 | 10 | 16 | ||||||||||||
Total current assets | 3,042 | 3,910 | 4,999 | 1,565 | ||||||||||||
| Property and Equipment, net | 712 | 693 | 371 | 213 | ||||||||||||
| $ | 3,754 | $ | 4,603 | $ | 5,370 | $ | 1,778 | |||||||||
LIABILITIES AND MEMBERS’ EQUITY | ||||||||||||||||
| Current Liabilities | ||||||||||||||||
| Accounts payable | $ | 463 | $ | 346 | $ | 502 | $ | 112 | ||||||||
| Accrued compensation | 103 | 44 | 17 | 41 | ||||||||||||
| Other accrued expenses | 30 | 10 | 296 | 235 | ||||||||||||
Total current liabilities | 596 | 400 | 815 | 387 | ||||||||||||
| Members’ Equity | 3,158 | 4,203 | 4,555 | 1,391 | ||||||||||||
| $ | 3,754 | $ | 4,603 | $ | 5,370 | $ | 1,778 | |||||||||
F-57
Table of Contents
Statements of Operations and Members’ Equity and Comprehensive Income
Period from January 1, 2010 through March 12, 2010
and For the Years Ended December 31, 2009, 2008 and 2007
| Period | ||||||||||||||||
| January 1, 2010 | ||||||||||||||||
| through | Year Ended | Year Ended | Year Ended | |||||||||||||
| March 12, 2010 | December 31, 2009 | December 31, 2008 | December 31, 2007 | |||||||||||||
| ($ in thousands) | ||||||||||||||||
| Net Revenues | $ | 3,198 | $ | 16,464 | $ | 8,191 | $ | 4,444 | ||||||||
| Operating costs and expenses: | ||||||||||||||||
| Cost of services | 1,177 | 6,923 | 2,925 | 1,923 | ||||||||||||
| Selling, general and administrative expenses | 299 | 2,500 | 1,062 | 702 | ||||||||||||
| Depreciation expense | 53 | 176 | 95 | 50 | ||||||||||||
Total operating costs and expenses | 1,529 | 9,599 | 4,082 | 2,675 | ||||||||||||
Income from operations | 1,669 | 6,865 | 4,109 | 1,769 | ||||||||||||
| Nonoperating income (expense): | ||||||||||||||||
| Interest income | — | 29 | 19 | 4 | ||||||||||||
| Other income (expense) | 12 | 323 | 7 | (8 | ) | |||||||||||
Other income | 12 | 352 | 26 | (4 | ) | |||||||||||
Net income | $ | 1,681 | $ | 7,217 | $ | 4,135 | $ | 1,765 | ||||||||
Statements of Members’ Equity and Comprehensive Income | ||||||||||||||||
| Balance, beginning | $ | 4,203 | $ | 4,555 | $ | 1,391 | $ | 77 | ||||||||
| Contributions | — | — | — | 50 | ||||||||||||
| Distributions | (2,726 | ) | (7,569 | ) | (911 | ) | (505 | ) | ||||||||
| Net income | 1,681 | 7,217 | 4,135 | 1,765 | ||||||||||||
| Accumulated other comprehensive income | — | — | (60 | ) | 4 | |||||||||||
| Balance, ending | $ | 3,158 | $ | 4,203 | $ | 4,555 | $ | 1,391 | ||||||||
F-58
Table of Contents
Statements of Cash Flows
Period from January 1, 2010 through March 12, 2010
and For the Years Ended December 31, 2009, 2008 and 2007
| Period | ||||||||||||||||
| January 1, 2010 | ||||||||||||||||
| through | Year Ended | Year Ended | Year Ended | |||||||||||||
| March 12, | December 31, | December 31, | December 31, | |||||||||||||
| 2010 | 2009 | 2008 | 2007 | |||||||||||||
| ($ in thousands) | ||||||||||||||||
| Cash Flows From Operating Activities | ||||||||||||||||
| Net income | $ | 1,681 | 7,217 | 4,135 | 1,765 | |||||||||||
| Adjustments to reconcile net income to net cash provided by operating activities: | ||||||||||||||||
| Depreciation | 53 | 176 | 95 | 50 | ||||||||||||
| Net unrealized loss (gain) on trading securities | 34 | (67 | ) | 21 | — | |||||||||||
| Allowance for bad debt | (128 | ) | (56 | ) | 230 | 213 | ||||||||||
| Changes in assets and liabilities: | ||||||||||||||||
| (Increase) Decrease in: | ||||||||||||||||
| Accounts receivable | 189 | (369 | ) | (1,007 | ) | (1,364 | ) | |||||||||
| Other assets | 2 | (23 | ) | 6 | (16 | ) | ||||||||||
| Increase (Decrease) in: | ||||||||||||||||
| Accounts payable | 117 | (155 | ) | 390 | 100 | |||||||||||
| Accrued compensation | 59 | 28 | (25 | ) | 22 | |||||||||||
| Other accrued expenses | 20 | (287 | ) | 2 | 192 | |||||||||||
Net cash provided by operating activities | 2,027 | 6,464 | 3,847 | 962 | ||||||||||||
| Cash Flows From Investing Activities | ||||||||||||||||
| Net trading securities | 582 | (89 | ) | (480 | ) | — | ||||||||||
| Purchase of property and equipment | (71 | ) | (499 | ) | (253 | ) | (165 | ) | ||||||||
Net cash provided by (used in) investing activities | 511 | (588 | ) | (733 | ) | (165 | ) | |||||||||
| Cash Flows From Financing Activities | ||||||||||||||||
| Payments on line of credit | — | — | — | (180 | ) | |||||||||||
| Capital contributions | — | — | — | 50 | ||||||||||||
| Distributions to members | (2,726 | ) | (7,569 | ) | (911 | ) | (505 | ) | ||||||||
Net cash used in financing activities | (2,726 | ) | (7,569 | ) | (911 | ) | (635 | ) | ||||||||
Net increase (decrease) in cash | (188 | ) | (1,693 | ) | 2,203 | 162 | ||||||||||
| Cash, beginning | 675 | 2,368 | 165 | 3 | ||||||||||||
| Cash, ending | $ | 487 | 675 | 2,368 | 165 | |||||||||||
F-59
Table of Contents
Notes to Financial Statements
| Note 1. | Nature of Business and Significant Accounting Policies |
F-60
Table of Contents
| March 12, | December 31, | December 31, | December 31, | |||||||||||||
| 2010 | 2009 | 2008 | 2007 | |||||||||||||
| Blue Cross/Blue Shield | 33 | % | 27 | % | 29 | % | 28 | % | ||||||||
| Medicare | 13 | % | 31 | % | 28 | % | 21 | % | ||||||||
F-61
Table of Contents
| Note 2. | Accounts Receivable |
| March 12, | December 31, | December 31, | December 31, | |||||||||||||
| 2010 | 2009 | 2008 | 2007 | |||||||||||||
| Accounts receivable and unbilled receivables | $ | 2,805 | $ | 2,994 | $ | 2,625 | $ | 1,618 | ||||||||
| Less: allowance for doubtful accounts | (280 | ) | (408 | ) | (463 | ) | (234 | ) | ||||||||
| Accounts receivable, net | $ | 2,525 | $ | 2,586 | $ | 2,162 | $ | 1,384 | ||||||||
| Note 3. | Property and Equipment |
| Estimated | ||||||||||||||||||||
| Useful Life | March 12, | December 31, | December 31, | December 31, | ||||||||||||||||
| (Years) | 2010 | 2009 | 2008 | 2007 | ||||||||||||||||
| Transportation equipment | 5 | $ | 142 | $ | 127 | $ | 35 | $ | 17 | |||||||||||
| Leasehold improvements | 5 | 73 | 69 | — | — | |||||||||||||||
| Furniture and fixtures | 7 | 42 | 42 | 7 | 3 | |||||||||||||||
| Equipment | 3 - 5 | 801 | 762 | 481 | 253 | |||||||||||||||
| Software | 3 | 56 | 42 | 21 | 19 | |||||||||||||||
| 1,114 | 1,042 | 544 | 292 | |||||||||||||||||
| Less accumulated depreciation | (402 | ) | (349 | ) | (173 | ) | (79 | ) | ||||||||||||
| $ | 712 | $ | 693 | $ | 371 | $ | 213 | |||||||||||||
| Note 4. | Commitments and Contingencies |
F-62
Table of Contents
Year Ending December 31, | ||||
| 2010 | $ | 68 | ||
| 2011 | 72 | |||
| 2012 | 56 | |||
| Thereafter | 0 | |||
| $ | 196 | |||
| Note 5. | Related Party Transactions |
| Note 6. | Employee Benefit Plans |
| December 31, | December 31, | December 31, | ||||||||||
| 2009 | 2008 | 2007 | ||||||||||
| Fair value of plan assets | $ | — | $ | 524,210 | $ | 284,700 | ||||||
| Projected benefit obligations | — | 580,660 | 281,000 | |||||||||
| Funded status at end of years | $ | — | $ | (56,450 | ) | $ | 3,700 | |||||
F-63
Table of Contents
| December 31, | December 31, | December 31, | ||||||||||
| 2009 | 2008 | 2007 | ||||||||||
| Current assets | $ | — | $ | — | $ | 3,700 | ||||||
| Current liabilities | — | 56,450 | — | |||||||||
| $ | — | $ | 56,450 | $ | 3,700 | |||||||
| Note 7. | Subsequent Events |
F-64
Table of Contents
F-65
Table of Contents
December 31, 2010 and 2009
| 2010 | 2009 | |||||||
| ($ in thousands) | ||||||||
ASSETS | ||||||||
| Current Assets | ||||||||
| Cash | $ | 382 | $ | 181 | ||||
| Accounts receivable, net | 1,630 | 1,579 | ||||||
| Income tax receivable | — | 63 | ||||||
| Other assets | 109 | 31 | ||||||
Total current assets | 2,121 | 1,854 | ||||||
| Property and Equipment, net | 45 | 56 | ||||||
| Investment | — | 1,000 | ||||||
| Other Assets | 61 | 58 | ||||||
| $ | 2,227 | $ | 2,968 | |||||
LIABILITIES AND MEMBERS’ EQUITY | ||||||||
| Current Liabilities | ||||||||
| Accounts payable | $ | 292 | $ | 82 | ||||
| Accrued compensation | 88 | 60 | ||||||
| Income tax payable | 46 | 7 | ||||||
| Note payable, current portion | — | 250 | ||||||
| Deferred tax liabilities | 477 | 501 | ||||||
| Other accrued expenses | 49 | 25 | ||||||
Total current liabilities | 952 | 925 | ||||||
| Deferred Tax Liabilities, net of current portion | 16 | 19 | ||||||
| Note Payable, net of current portion | — | 389 | ||||||
Total liabilities | 968 | 1,333 | ||||||
| Commitments and Contingencies | ||||||||
| Members’ equity | ||||||||
| Members’ equity | 1,259 | 1,635 | ||||||
Total equity | 1,259 | 1,635 | ||||||
| $ | 2,227 | $ | 2,968 | |||||
F-66
Table of Contents
| 2010 | 2009 | |||||||
| ($ in thousands) | ||||||||
| Net Revenues | $ | 14,436 | $ | 14,701 | ||||
| Operating costs and expenses: | ||||||||
| Cost of services | 10,710 | 10,277 | ||||||
| Selling, general and administrative expenses | 628 | 1,523 | ||||||
| Depreciation expense | 16 | 16 | ||||||
Total operating costs and expenses | 11,354 | 11,816 | ||||||
Income from operations | 3,082 | 2,885 | ||||||
| Interest expense | 20 | 32 | ||||||
Income before income taxes | 3,062 | 2,853 | ||||||
| Provision (benefit) for income taxes | 154 | (126 | ) | |||||
Net income | $ | 2,908 | $ | 2,979 | ||||
F-67
Table of Contents
Consolidated Statements of Members’ Equity
Years Ended December 31, 2010 and 2009
| ($ in thousands) | ||||
| Balance, January 1, 2009 | $ | 1,854 | ||
| Net income | 2,979 | |||
| Distributions | (3,198 | ) | ||
| Balance, December 31, 2009 | 1,635 | |||
| Net income | 2,908 | |||
| Distributions | (3,284 | ) | ||
| Balance, December 31, 2010 | $ | 1,259 | ||
F-68
Table of Contents
| 2010 | 2009 | |||||||
| ($ in thousands) | ||||||||
| Cash Flows From Operating Activities | ||||||||
| Net income | $ | 2,908 | $ | 2,979 | ||||
| Adjustments to reconcile net income to net cash provided by operating activities: | ||||||||
| Depreciation | 16 | 16 | ||||||
| Deferred income taxes | (27 | ) | (264 | ) | ||||
| Allowance for doubtful accounts | (369 | ) | 567 | |||||
| Changes in assets and liabilities: | ||||||||
| (Increase) Decrease in: | ||||||||
| Accounts receivable | 318 | 221 | ||||||
| Income tax receivable | 63 | (63 | ) | |||||
| Other assets | (82 | ) | 39 | |||||
| Income tax payable | 39 | 8 | ||||||
| Accounts payable | 210 | (16 | ) | |||||
| Accrued compensation | 28 | — | ||||||
| Other accrued expenses | 24 | 20 | ||||||
Net cash provided by operating activities | 3,128 | 3,507 | ||||||
| Cash Flows From Investing Activities | ||||||||
| Purchase of property and equipment | (4 | ) | (33 | ) | ||||
Net cash used in investing activities | (4 | ) | (33 | ) | ||||
| Cash Flows From Financing Activities | ||||||||
| Repayment of debt | (639 | ) | (359 | ) | ||||
| Distributions to members | (2,284 | ) | (3,198 | ) | ||||
Net cash used in financing activities | (2,923 | ) | (3,557 | ) | ||||
Net increase (decrease) in cash | 201 | (83 | ) | |||||
| Cash: | ||||||||
| Beginning | 181 | 264 | ||||||
| Ending | $ | 382 | $ | 181 | ||||
| Supplemental Disclosures of Cash Flow Information | ||||||||
| Cash interest payments | $ | 16 | $ | 25 | ||||
| Cash tax payments | $ | 100 | $ | 175 | ||||
| Supplemental Schedule of Noncash Investing and Financing Activities | ||||||||
| Distribution of investment in non-marketable equity securities | $ | (1,000 | ) | $ | — | |||
F-69
Table of Contents
F-70
Table of Contents
F-71
Table of Contents
| 2010 | 2009 | |||||||
| Accounts receivable and unbilled receivables | $ | 2,652 | $ | 2,970 | ||||
| Less: allowance for bad debt | (1,022 | ) | (1,391 | ) | ||||
| Accounts receivable, net | $ | 1,630 | $ | 1,579 | ||||
| Estimated Useful | ||||||||||
| Life (Years) | 2010 | 2009 | ||||||||
| Equipment | 5 — 7 | $ | 224 | $ | 223 | |||||
| Furniture and fixtures | 7 | 13 | 9 | |||||||
| 237 | 232 | |||||||||
| Less accumulated depreciation | (192 | ) | (176 | ) | ||||||
| $ | 45 | $ | 56 | |||||||
F-72
Table of Contents
| 2010 | 2009 | |||||||
| Current: | ||||||||
| Federal | $ | 103 | $ | 72 | ||||
| State | 78 | 67 | ||||||
Total current provision | 181 | 139 | ||||||
| Deferred: | ||||||||
| Federal | (27 | ) | (265 | ) | ||||
| State | — | — | ||||||
Total deferred benefit | (27 | ) | (265 | ) | ||||
Total provision (benefit) for income taxes | $ | 154 | $ | (126 | ) | |||
| 2010 | 2009 | |||||||
| Accrual to cash basis adjustments | $ | (477 | ) | $ | (501 | ) | ||
Current deferred tax liabilities | (477 | ) | (501 | ) | ||||
| Depreciation | (16 | ) | (19 | ) | ||||
Noncurrent deferred tax liabilities | (16 | ) | (19 | ) | ||||
Total deferred tax liabilities | $ | (493 | ) | $ | (520 | ) | ||
F-73
Table of Contents
F-74
Table of Contents
Schedule II — Valuation and Qualifying Accounts
Years Ended December 31, 2008, 2009 and 2010
| Charged to | Net Write-offs | |||||||||||||||||||
| Beginning | Statement of | and Other | Ending | |||||||||||||||||
Description | Balance | Operations | Other(1) | Adjustments | Balance | |||||||||||||||
| (In thousands) | ||||||||||||||||||||
| Allowance for Doubtful Accounts: | ||||||||||||||||||||
| Year ended December 31, 2008 | $ | 6,286 | $ | 8,037 | $ | 81 | $ | (6,207 | ) | $ | 8,197 | |||||||||
| Year ended December 31, 2009 | $ | 8,197 | $ | 9,488 | $ | 240 | $ | (9,372 | ) | $ | 8,553 | |||||||||
| Year ended December 31, 2010 | $ | 8,553 | $ | 12,393 | $ | 875 | $ | (10,097 | ) | $ | 11,724 | |||||||||
| (1) | Represents the Allowance for Doubtful Accounts recorded in connection with the application of acquisition accounting for the 2008, 2009 and 2010 acquisitions. |
F-75
Table of Contents

AURORA DIAGNOSTICS FINANCING, INC.
which have been registered under the
Securities Act of 1933
10.750% Senior notes due 2018
Table of Contents
| Item 20. | Indemnification of Directors and Officers |
II-1
Table of Contents
II-2
Table of Contents
II-3
Table of Contents
II-4
Table of Contents
II-5
Table of Contents
II-6
Table of Contents
II-7
Table of Contents
II-8
Table of Contents
II-9
Table of Contents
II-10
Table of Contents
II-11
Table of Contents
II-12
Table of Contents
| Item 21. | Exhibits and Financial Statement Schedules |
| Item 22. | Undertakings |
II-13
Table of Contents
II-14
Table of Contents
| By: | /s/ Gregory A. Marsh |
Name | Title | |||
/s/ Jon L. Hart Jon L. Hart | Chief Executive Officer and Manager (principal executive officer) | |||
/s/ Gregory A. Marsh Gregory A. Marsh | Chief Financial Officer (principal financial and accounting officer) | |||
/s/ James C. New James C. New | Manager | |||
/s/ Thomas S. Roberts Thomas S. Roberts | Manager | |||
/s/ Christopher Dean Christopher Dean | Manager | |||
/s/ Peter J. Connolly Peter J. Connolly | Manager | |||
II-15
Table of Contents
Name | Title | |||
/s/ Christopher J. Bock Christopher J. Bock | Manager | |||
/s/ Blair Tikker Blair Tikker | Manager | |||
/s/ Bennett Thompson Bennett Thompson | Manager | |||
/s/ James Emanuel James Emanuel | Manager | |||
II-16
Table of Contents
| By: | /s/ Gregory A. Marsh |
Name | Title | |||
/s/ Jon L. Hart Jon L. Hart | Chief Executive Officer and Director (principal executive officer) | |||
/s/ Gregory A. Marsh Gregory A. Marsh | Chief Financial Officer and Director (principal financial and accounting officer) | |||
/s/ Martin J. Stefanelli Martin J. Stefanelli | Director | |||
II-17
Table of Contents
| By: | /s/ Gregory A. Marsh |
Name | Title | |||
/s/ Jon L. Hart Jon L. Hart | Chief Executive Officer and Manager (principal executive officer) | |||
/s/ Gregory A. Marsh Gregory A. Marsh | Chief Financial Officer (principal financial and accounting officer) | |||
/s/ James C. New James C. New | Manager | |||
/s/ Thomas S. Roberts Thomas S. Roberts | Manager | |||
/s/ Christopher Dean Christopher Dean | Manager | |||
/s/ Peter J. Connolly Peter J. Connolly | Manager | |||
/s/ Christopher J. Bock Christopher J. Bock | Manager | |||
II-18
Table of Contents
Name | Title | |||
/s/ Blair Tikker Blair Tikker | Manager | |||
/s/ Bennett Thompson Bennett Thompson | Manager | |||
/s/ James Emanuel James Emanuel | Manager | |||
II-19
Table of Contents
| By: | /s/ Gregory A. Marsh |
Name | Title | |||
/s/ Jon L. Hart Jon L. Hart | Chief Executive Officer (principal executive officer) | |||
/s/ Gregory A. Marsh Gregory A. Marsh | Chief Financial Officer (principal financial and accounting officer) | |||
/s/ Gregory A. Marsh Aurora Diagnostics, LLC | Member | |||
| By: Gregory A. Marsh Title: Chief Financial Officer | ||||
II-20
Table of Contents
| By: | /s/ Gregory A. Marsh |
Name | Title | |||
/s/ Jon L. Hart Jon L. Hart | Chief Executive Officer (principal executive officer) | |||
/s/ Gregory A. Marsh Gregory A. Marsh | Chief Financial Officer (principal financial and accounting officer) | |||
/s/ Gregory A. Marsh Aurora Diagnostics, LLC | Member | |||
| By: Gregory A. Marsh Title: Chief Financial Officer | ||||
II-21
Table of Contents
| By: | /s/ Gregory A. Marsh |
Name | Title | |||
/s/ Jon L. Hart Jon L. Hart | Chief Executive Officer (principal executive officer) | |||
/s/ Gregory A. Marsh Gregory A. Marsh | Chief Financial Officer (principal financial and accounting officer) | |||
/s/ Gregory A. Marsh Aurora Diagnostics, LLC | Member | |||
| By: Gregory A. Marsh Title: Chief Financial Officer | ||||
II-22
Table of Contents
| By: | /s/ Gregory A. Marsh |
Name | Title | |||
/s/ Jon L. Hart Jon L. Hart | Chief Executive Officer (principal executive officer) | |||
/s/ Gregory A. Marsh Gregory A. Marsh | Chief Financial Officer (principal financial and accounting officer) | |||
/s/ Gregory A. Marsh Aurora Diagnostics, LLC | Member | |||
| By: Gregory A. Marsh Title: Chief Financial Officer | ||||
II-23
Table of Contents
| By: | /s/ Gregory A. Marsh |
Name | Title | |||
/s/ Jon L. Hart Jon L. Hart | Chief Executive Officer (principal executive officer) | |||
/s/ Gregory A. Marsh Gregory A. Marsh | Chief Financial Officer (principal financial and accounting officer) | |||
/s/ Gregory A. Marsh Aurora Diagnostics, LLC | Member | |||
| By: Gregory A. Marsh Title: Chief Financial Officer | ||||
II-24
Table of Contents
| By: | /s/ Gregory A. Marsh |
Name | Title | |||
/s/ Jon L. Hart Jon L. Hart | Chief Executive Officer (principal executive officer) | |||
/s/ Gregory A. Marsh Gregory A. Marsh | Chief Financial Officer (principal financial and accounting officer) | |||
/s/ Gregory A. Marsh Aurora Diagnostics, LLC | Member | |||
| By: Gregory A. Marsh Title: Chief Financial Officer | ||||
II-25
Table of Contents
| By: | /s/ Gregory A. Marsh |
Name | Title | |||
/s/ Jon L. Hart Jon L. Hart | Chief Executive Officer and Director (principal executive officer) | |||
/s/ Gregory A. Marsh Gregory A. Marsh | Chief Financial Officer and Director (principal financial and accounting officer) | |||
/s/ Martin J. Stefanelli Martin J. Stefanelli | Director | |||
II-26
Table of Contents
| By: | /s/ Gregory A. Marsh |
Name | Title | |||
/s/ Jon L. Hart Jon L. Hart | Chief Executive Officer (principal executive officer) | |||
/s/ Gregory A. Marsh Gregory A. Marsh | Chief Financial Officer (principal financial and accounting officer) | |||
/s/ Gregory A. Marsh Aurora Diagnostics, LLC | Member | |||
| By: Gregory A. Marsh Title: Chief Financial Officer | ||||
II-27
Table of Contents
| By: | /s/ Gregory A. Marsh |
Name | Title | |||
/s/ Jon L. Hart Jon L. Hart | Chief Executive Officer (principal executive officer) | |||
/s/ Gregory A. Marsh Gregory A. Marsh | Chief Financial Officer (principal financial and accounting officer) | |||
/s/ Gregory A. Marsh Cunningham Pathology, L.L.C. | Member | |||
| By: Gregory A. Marsh Title: Chief Financial Officer | ||||
II-28
Table of Contents
| By: | /s/ Gregory A. Marsh |
Name | Title | |||
/s/ Jon L. Hart Jon L. Hart | Chief Executive Officer (principal executive officer) | |||
/s/ Gregory A. Marsh Gregory A. Marsh | Chief Financial Officer (principal financial and accounting officer) | |||
/s/ Gregory A. Marsh Aurora Diagnostics, LLC | Member | |||
| By: Gregory A. Marsh Title: Chief Financial Officer | ||||
II-29
Table of Contents
| By: | /s/ Gregory A. Marsh |
Name | Title | |||
/s/ Jon L. Hart Jon L. Hart | Chief Executive Officer (principal executive officer) | |||
/s/ Gregory A. Marsh Gregory A. Marsh | Chief Financial Officer (principal financial and accounting officer) | |||
/s/ Gregory A. Marsh Aurora Diagnostics, LLC | Member | |||
| By: Gregory A. Marsh Title: Chief Financial Officer | ||||
II-30
Table of Contents
| By: | /s/ Gregory A. Marsh |
Name | Title | |||
/s/ Jon L. Hart Jon L. Hart | Chief Executive Officer (principal executive officer) | |||
/s/ Gregory A. Marsh Gregory A. Marsh | Chief Financial Officer (principal financial and accounting officer) | |||
/s/ Gregory A. Marsh Aurora Diagnostics, LLC | Member | |||
| By: Gregory A. Marsh Title: Chief Financial Officer | ||||
II-31
Table of Contents
| By: | /s/ Gregory A. Marsh |
Name | Title | |||
/s/ Jon L. Hart Jon L. Hart | Chief Executive Officer (principal executive officer) | |||
/s/ Gregory A. Marsh Gregory A. Marsh | Chief Financial Officer (principal financial and accounting officer) | |||
/s/ Gregory A. Marsh Aurora Greensboro, LLC | Member | |||
| By: Gregory A. Marsh Title: Chief Financial Officer | ||||
II-32
Table of Contents
| By: | /s/ Gregory A. Marsh |
Name | Title | |||
/s/ Jon L. Hart Jon L. Hart | Chief Executive Officer (principal executive officer) | |||
/s/ Gregory A. Marsh Gregory A. Marsh | Chief Financial Officer (principal financial and accounting officer) | |||
/s/ Gregory A. Marsh Aurora Diagnostics, LLC | Member | |||
| By: Gregory A. Marsh Title: Chief Financial Officer | ||||
II-33
Table of Contents
| By: | /s/ Gregory A. Marsh |
Name | Title | |||
/s/ Jon L. Hart Jon L. Hart | Chief Executive Officer (principal executive officer) | |||
/s/ Gregory A. Marsh Gregory A. Marsh | Chief Financial Officer (principal financial and accounting officer) | |||
/s/ Gregory A. Marsh Aurora Diagnostics, LLC | Member | |||
| By: Gregory A. Marsh Title: Chief Financial Officer | ||||
II-34
Table of Contents
| By: | /s/ Gregory A. Marsh |
Name | Title | |||
/s/ Jon L. Hart Jon L. Hart | Chief Executive Officer (principal executive officer) | |||
/s/ Gregory A. Marsh Gregory A. Marsh | Chief Financial Officer (principal financial and accounting officer) | |||
/s/ Gregory A. Marsh Aurora Diagnostics, LLC | Member | |||
| By: Gregory A. Marsh Title: Chief Financial Officer | ||||
II-35
Table of Contents
| By: | /s/ Gregory A. Marsh |
Name | Title | |||
/s/ Jon L. Hart Jon L. Hart | Chief Executive Officer and Director (principal executive officer) | |||
/s/ Gregory A. Marsh Gregory A. Marsh | Chief Financial Officer and Director (principal financial and accounting officer) | |||
/s/ Martin J. Stefanelli Martin J. Stefanelli | Director | |||
II-36
Table of Contents
| By: | /s/ Gregory A. Marsh |
Name | Title | |||
/s/ Jon L. Hart Jon L. Hart | Chief Executive Officer (principal executive officer) | |||
/s/ Gregory A. Marsh Gregory A. Marsh | Chief Financial Officer (principal financial and accounting officer) | |||
/s/ Gregory A. Marsh Aurora Diagnostics, LLC | Member | |||
| By: Gregory A. Marsh Title: Chief Financial Officer | ||||
II-37
Table of Contents
| By: | /s/ Gregory A. Marsh |
Name | Title | |||
/s/ Jon L. Hart Jon L. Hart | Chief Executive Officer and Director (principal executive officer) | |||
/s/ Gregory A. Marsh Gregory A. Marsh | Chief Financial Officer and Director (principal financial and accounting officer) | |||
/s/ Martin J. Stefanelli Martin J. Stefanelli | Director | |||
II-38
Table of Contents
| By: | /s/ Gregory A. Marsh |
Name | Title | |||
/s/ Jon L. Hart Jon L. Hart | Chief Executive Officer (principal executive officer) | |||
/s/ Gregory A. Marsh Gregory A. Marsh | Chief Financial Officer (principal financial and accounting officer) | |||
/s/ Gregory A. Marsh Aurora Diagnostics, LLC | Member | |||
| By: Gregory A. Marsh Title: Chief Financial Officer | ||||
II-39
Table of Contents
| By: | /s/ Gregory A. Marsh |
Name | Title | |||
/s/ Jon L. Hart Jon L. Hart | Chief Executive Officer (principal executive officer) | |||
/s/ Gregory A. Marsh Gregory A. Marsh | Chief Financial Officer (principal financial and accounting officer) | |||
/s/ Gregory A. Marsh Aurora Diagnostics, LLC | Member | |||
| By: Gregory A. Marsh Title: Chief Financial Officer | ||||
II-40
Table of Contents
| By: | /s/ Jon L. Hart |
| By: | /s/ Martin J. Stefanelli |
Name | Title | |||
/s/ Jon L. Hart Jon L. Hart | Trustee | |||
/s/ Martin J. Stefanelli Martin J. Stefanelli | Trustee | |||
/s/ Gregory A. Marsh Aurora LMC, LLC | Trustor and Sole Beneficiary | |||
| By: Gregory A. Marsh Title: Chief Financial Officer | ||||
II-41
Table of Contents
| By: | /s/ Jon L. Hart |
| By: | /s/ Martin J. Stefanelli |
Name | Title | |||
/s/ Jon L. Hart Jon L. Hart | Trustee | |||
/s/ Martin J. Stefanelli Martin J. Stefanelli | Trustee | |||
/s/ Gregory A. Marsh Aurora LMC, LLC | Trustor and Sole Beneficiary | |||
| By: Gregory A. Marsh Title: Chief Financial Officer | ||||
II-42
Table of Contents
| Exhibit | ||||
No. | Description of Exhibit | |||
| 3 | .1 | Certificate of Formation of Aurora Diagnostics Holdings, LLC | ||
| 3 | .2 | Second Amended and Restated Limited Liability Company Agreement of Aurora Diagnostics Holdings, LLC | ||
| 3 | .3 | Certificate of Incorporation of Aurora Diagnostics Financing, Inc. | ||
| 3 | .4 | Bylaws of Aurora Diagnostics Financing, Inc. | ||
| 3 | .5 | Certificate of Formation of Aurora Diagnostics, LLC | ||
| 3 | .6 | Limited Liability Company Agreement of Aurora Diagnostics, LLC | ||
| 3 | .7 | First Amendment to the Limited Liability Company Agreement of Aurora Diagnostics, LLC | ||
| 3 | .8 | Articles of Organization of Aurora Georgia, LLC | ||
| 3 | .9 | Limited Liability Company Operating Agreement of Aurora Georgia, LLC | ||
| 3 | .10 | Articles of Organization of Aurora Greensboro LLC | ||
| 3 | .11 | Limited Liability Company Operating Agreement of Aurora Greensboro, LLC | ||
| 3 | .12 | First Amendment to the Limited Liability Company Operating Agreement of Aurora Greensboro, LLC | ||
| 3 | .13 | Articles of Organization of Aurora LMC, LLC | ||
| 3 | .14 | Limited Liability Company Operating Agreement of Aurora LMC, LLC | ||
| 3 | .15 | Certificate of Formation of Aurora Massachusetts, LLC | ||
| 3 | .16 | Limited Liability Company Operating Agreement of Aurora Massachusetts, LLC | ||
| 3 | .17 | Articles of Organization of Aurora Michigan, LLC | ||
| 3 | .18 | Operating Agreement of Aurora Michigan, LLC | ||
| 3 | .19 | Certificate of Formation of Aurora New Hampshire, LLC | ||
| 3 | .20 | Limited Liability Company Agreement of Aurora New Hampshire, LLC | ||
| 3 | .21 | Amended and Restated Articles of Incorporation of Bernhardt Laboratories, Inc. | ||
| 3 | .22 | Amended and Restated Bylaws of Bernhardt Laboratories, Inc. | ||
| 3 | .23 | Articles of Organization of Biopsy Diagnostics, LLC | ||
| 3 | .24 | Amended and Restated Limited Liability Company Operating Agreement of Biopsy Diagnostics, LLC | ||
| 3 | .25 | Articles of Organization of C R Collections, LLC | ||
| 3 | .26 | Limited Liability Company Operating Agreement of C R Collections, LLC | ||
| 3 | .27 | Articles of Organization of Covenant Healthcare Lab, LLC | ||
| 3 | .28 | Limited Liability Company Operating Agreement of Covenant Healthcare Lab, LLC | ||
| 3 | .29 | Certificate of Formation of Cunningham Pathology, L.L.C. | ||
| 3 | .30 | Second Amended and Restated Operating Agreement of Cunningham Pathology, L.L.C. | ||
| 3 | .31 | Certificate of Organization of DermPath New England, LLC | ||
| 3 | .32 | Limited Liability Company Operating Agreement of DermPath New England, LLC | ||
| 3 | .33 | Articles of Organization of Greensboro Pathology, LLC | ||
| 3 | .34 | Amendment of Articles of Organization of Greensboro Pathology, LLC | ||
| 3 | .35 | Amended and Restated Operating Agreement of Greensboro Pathology, LLC | ||
| 3 | .36 | Articles of Organization of Hardman Pathology ADX, LLC | ||
| 3 | .37 | Articles of Amendment to the Articles of Organization of Hardman Pathology ADX, LLC | ||
| 3 | .38 | Operating Agreement of Hardman Pathology ADX, LLC | ||
| 3 | .39 | Articles of Organization of Laboratory of Dermatopathology ADX, LLC | ||
| 3 | .40 | Amendment to Articles of Organization of Laboratory of Dermatopathology ADX, LLC | ||
| 3 | .41 | Operating Agreement of Laboratory of Dermatopathology ADX, LLC | ||
| 3 | .42 | Articles of Organization of Mark & Kambour, LLC | ||
| 3 | .43 | Limited Liability Company Operating Agreement of Mark & Kambour, LLC | ||
| 3 | .44 | Articles of Incorporation of Mark & Kambour Holdings, Inc. | ||
II-43
Table of Contents
| Exhibit | ||||
No. | Description of Exhibit | |||
| 3 | .45 | Bylaws of Mark & Kambour Holdings, Inc. | ||
| 3 | .46 | Certificate of Formation of Pathology Solutions, LLC | ||
| 3 | .47 | Amendment to Certificate of Formation of Pathology Solutions, LLC | ||
| 3 | .48 | Amended and Restated Limited Liability Company Operating Agreement of Pathology Solutions, LLC | ||
| 3 | .49 | Restated Articles of Incorporation of Seacoast Pathology, Inc. | ||
| 3 | .50 | Amended and Restated Bylaws of Seacoast Pathology, Inc. | ||
| 3 | .51 | Certificate of Formation of Texas Pathology, LLC | ||
| 3 | .52 | Amended and Restated Limited Liability Company Operating Agreement of Texas Pathology, LLC | ||
| 3 | .53 | Articles of Organization of Twin Cities Dermatopathology, LLC | ||
| 3 | .54 | Amended and Restated Limited Liability Company Operating Agreement of Twin Cities Dermatopathology, LLC | ||
| 3 | .55 | Certificate of Business Trust of The LMC Revocable Trust, B.T. | ||
| 3 | .56 | Revocable Trust Agreement of The LMC Revocable Trust, B.T. | ||
| 3 | .57 | First Amendment to Revocable Trust Agreement of The LMC Revocable Trust, B.T. | ||
| 3 | .58 | Certificate of Business Trust of The WPC Revocable Trust, B.T. | ||
| 3 | .59 | Revocable Trust Agreement of The WPC Revocable Trust, B.T. | ||
| 4 | .1 | Indenture, dated December 20, 2010, by and among Aurora Diagnostics Holdings, LLC, Aurora Diagnostics Financing, Inc., the guarantors named therein and U.S. Bank National Association | ||
| 4 | .2 | First Supplemental Indenture, dated December 31, 2011, by and among Aurora Diagnostics Holdings, LLC, Aurora Diagnostics Financing, Inc., the guarantors named therein and U.S. Bank National Association | ||
| 4 | .3 | Second Supplemental Indenture, dated December 31, 2011, by and among Aurora Diagnostics Holdings, LLC, Aurora Diagnostics Financing, Inc., the guarantors named therein and U.S. Bank National Association | ||
| 4 | .4 | Third Supplemental Indenture, dated June 2, 2011, by and among Aurora Diagnostics Holdings, LLC, Aurora Diagnostics Financing, Inc., the guarantors named therein and U.S. Bank National Association | ||
| 4 | .5 | Fourth Supplemental Indenture, dated August 12, 2011, by and among Aurora Diagnostics Holdings, LLC, Aurora Diagnostics Financing, Inc., the guarantors named therein and U.S. Bank National Association | ||
| 4 | .6 | Form of 10.750% senior notes due 2018 (included as Exhibit A to Exhibit 4.1) | ||
| 4 | .7 | Registration Rights Agreement, dated December 20, 2010, by and among Aurora Diagnostics Holdings, LLC, Aurora Diagnostics Financing, Inc., the guarantors named therein, Morgan Stanley & Co. Incorporated, Barclays Capital, Inc. and UBS Securities LLC | ||
| 4 | .8 | Amended and Restated Registration Rights Agreement, dated June 12, 2009, by and among Aurora Diagnostics Holdings, LLC and each of the signatories thereto | ||
| 5 | .1 | Opinion of Alston & Bird LLP | ||
| 10 | .1 | Credit and Guaranty Agreement, by and among Aurora Diagnostics LLC as Borrower, Aurora Diagnostics Holdings, LLC and certain subsidiaries and affiliates of Aurora Diagnostics, LLC as Guarantors, the various lenders party thereto, Barclays Bank PLC as Administrative Agent and Collateral Agent, Barclays Capital, Morgan Stanley Senior Funding, Inc. and UBS Securities LLC as Joint Lead Arrangers and Joint Bookrunners, Morgan Stanley Senior Funding, Inc. as Syndication Agent and UBS Securities LLC as Documentation Agent | ||
| 10 | .2 | Senior Management Agreement, dated June 2, 2006, by and among Aurora Diagnostics Holdings, LLC and James C. New* | ||
| 10 | .3 | Senior Management Agreement, dated October 2006, by and among Aurora Diagnostics Holdings, LLC, Aurora Diagnostics, LLC, James C. New and Martin J. Stefanelli* | ||
| 10 | .4 | First Amendment to Senior Management Agreement, dated April 2010, by and among Aurora Diagnostics Holdings, LLC, Aurora Diagnostics, LLC and Martin J. Stefanelli* | ||
II-44
Table of Contents
| Exhibit | ||||
No. | Description of Exhibit | |||
| 10 | .5 | Senior Management Agreement, dated November 5, 2007, by and among Aurora Diagnostics Holdings, LLC, Aurora Diagnostics, LLC and Greg Marsh* | ||
| 10 | .6 | Letter Agreement, dated August 11, 2008, by and among Aurora Diagnostics Holdings, LLC, Aurora Diagnostics, LLC and Greg Marsh* | ||
| 10 | .7 | Senior Management Agreement, dated October 2006, by and among Aurora Diagnostics Holdings, LLC, Aurora Diagnostics, LLC, James C. New and Fred Ferrara* | ||
| 10 | .8 | First Amendment to Senior Management Agreement, dated October 2006, by and among Aurora Diagnostics Holdings, LLC, Aurora Diagnostics, LLC, James C. New and Fred Ferrara* | ||
| 10 | .9 | Senior Management Agreement, dated April 2007, by and among Aurora Diagnostics Holdings, LLC, Aurora Diagnostics, LLC, James C. New and Michael Null* | ||
| 10 | .10 | Employment Agreement by and between Aurora Diagnostics Holdings, LLC and Jon L. Hart* | ||
| 10 | .11 | Aurora Diagnostics Holdings, LLC 2011 Equity Incentive Plan* | ||
| 10 | .12 | First Amendment to the Aurora Diagnostics Holdings, LLC 2011 Equity Incentive Plan* | ||
| 10 | .13 | Form of Unit Option Award Certificate under the Aurora Diagnostics Holdings, LLC 2011 Equity Incentive Plan* | ||
| 10 | .14 | Consulting Agreement by and between Aurora Diagnostics, LLC and James C. New* | ||
| 10 | .15 | Purchase Agreement, dated December 14, 2010, by and among Aurora Diagnostics Holdings, LLC, Aurora Diagnostics Financing, Inc., the Guarantors party thereto, Morgan Stanley & Co. Incorporated, Barclays Capital, Inc. and UBS Securities LLC | ||
| 10 | .16 | Management Rights Agreement, dated June 2, 2006, by and among Aurora Diagnostics Holdings, LLC and each of the signatories thereto | ||
| 10 | .17 | Management Rights Agreement, dated June 12, 2009, by and among KRG Capital Partners, L.L.C., KRG Aurora Blocker, Inc. and Aurora Diagnostics Holdings, LLC | ||
| 10 | .18 | Amended and Restated Management Services Agreement, dated June 12, 2009, by and among Summit Partners, L.P., KRG Capital Management, L.P., and Aurora Diagnostics, LLC | ||
| 10 | .19 | First Amendment to Amended and Restated Management Services Agreement, dated May 20, 2010, by and among Summit Partners, L.P., KRG Capital Management, L.P., and Aurora Diagnostics, LLC | ||
| 10 | .20 | Form of Management Agreement by and between Aurora Diagnostics, LLC and certain of our affiliated practice subsidiaries | ||
| 10 | .21 | Form of Nominee Agreement by and between Aurora Diagnostics, LLC and certain of our affiliated practice subsidiaries | ||
| 10 | .22 | Form of Non-Alienation Agreement by and between Aurora Diagnostics, LLC and certain of our affiliated practice subsidiaries | ||
| 10 | .23 | Form of Services Agreement by and between Aurora Diagnostics, LLC and certain of our affiliated practice subsidiaries | ||
| 12 | .1 | Statement Regarding Computation of Ratio of Earnings to Fixed Charges | ||
| 21 | .1 | List of subsidiaries of Aurora Diagnostics Holdings, LLC | ||
| 23 | .1 | Consent of McGladrey & Pullen, LLP | ||
| 23 | .2 | Consent of McGladrey & Pullen, LLP | ||
| 23 | .3 | Consent of Alston & Bird LLP (included in Exhibit 5.1) | ||
| 24 | .1 | Powers of Attorney (contained on the signature pages of this registration statement) | ||
| 25 | .1 | Statement of Eligibility onForm T-1 of U.S. Bank National Association, as the Trustee under the Indenture | ||
| 99 | .1 | Form of Letter of Transmittal | ||
| 99 | .2 | Form of Notice of Guaranteed Delivery | ||
| 99 | .3 | Form of Instruction to Registered Holder and/or Book-Entry Transfer Facility Participant from Beneficial Owner | ||
| * | Management contract or compensatory plan or arrangement required to be filed as an exhibit to this report. |
II-45