Table of Contents
Washington, D.C. 20549
| Spain | 2834 | Not applicable | ||
| (Jurisdiction of incorporation or organization) | (Primary Standard Industrial Classification Code Number) | (I.R.S. Employer Identification Number) |
| Julie M. Allen, Esq. Proskauer Rose LLP Eleven Times Square New York, New York 10036 Telephone:(212) 969-3000 Facsimile:(212) 969-2900 | Tomás Dagá Raimon Grifols Osborne Clarke S.L.P. Avenida Diagonal, 477 Planta 20, 08036 Barcelona, Spain Tel: +34 93 419 1818 |
| Large accelerated filer o | Accelerated filer o | Non-accelerated filer þ | Smaller reporting company o |
| Proposed Maximum | Proposed Maximum | Amount of | ||||||||||||||||||
| Title of Each Class of | Amount to be | Offering | Aggregate | Registration | ||||||||||||||||
| Securities to be Registered | Registered(1) | Price per Note(1) | Offering Price(1) | Fee | ||||||||||||||||
| 8.25% Senior Notes due 2018 | $ | 1,100,000,000 | 100 | % | $ | 1,100,000,000 | $ | 126,060 | ||||||||||||
| Guarantee of 8.25% Senior Notes due 2018(2) | — | — | — | — | ||||||||||||||||
| (1) | Estimated solely for the purpose of calculating the registration fee pursuant to Rule 457 under the Securities Act of 1933, as amended (the “Securities Act”). | |
| (2) | Pursuant to Rule 457(n) under the Securities Act, no separate fee is payable with respect to the guarantee. |
Table of Contents
| Address, Including Zip | ||||||||
| State or Other | Code and Telephone | |||||||
| Jurisdiction of | Primary Standard | I.R.S. Employer | Number, Including Area | |||||
| Incorporation or | Industrial | Identification | Code, of Principal | |||||
| Exact Name as Specified in Its Charter | Organization | Classification Number | Number | Executive Offices | ||||
Grifols Inc.(1) | Virginia, United States | 2834 | 20-2533768 | 2410 Lillyvale Ave., Los Angeles, CA 90032, (323) 225-2221 | ||||
| Grifols Biologicals Inc. | Delaware, United States | 2834 | 13-4253630 | 5555 Valley Boulevard, Los Angeles, CA 90032, (323) 225-2221 | ||||
| Biomat USA, Inc. | Delaware, United States | 8099 | 95-4343492 | 2410 Lillyvale Ave., Los Angeles, CA 90032, (323) 225-2221 | ||||
| Grifols Therapeutics Inc. | Delaware, United States | 2834 | 34-2032472 | 4101 Research Commons 79 T.W. Alexander Drive, Research Triangle Park, North Carolina 27709 (919) 316-6300 | ||||
| Talecris Plasma Resources, Inc. | Delaware, United States | 8099 | 20-5444433 | 4101 Research Commons 79 T.W. Alexander Drive, Research Triangle Park, North Carolina 27709 (919) 316-6300 | ||||
| Instituto Grifols, S.A. | Spain | 2834 | N/A | Polígono Levante, calle Can Guasch s/n 08150 Parets del Vallés, Barcelona, Spain (34) 93 5710200 | ||||
| Diagnostic Grifols, S.A. | Spain | 3826 | N/A | Polígono Levante, calle Can Guasch s/n, 08150 Parets del Vallés, Barcelona, Spain (34) 93 5710400 | ||||
| Movaco, S.A. | Spain | 3826 | N/A | Polígono Levante, calle Can Guasch s/n, 08150 Parets del Vallés, Barcelona, Spain (34) 935710200 | ||||
| Laboratorios Grifols, S.A. | Spain | 5122 | N/A | Polígono Levante, calle Can Guasch s/n, 08150 Parets del Vallés, Barcelona, Spain (34) 93 5710100 | ||||
| Grifols Italia, S.p.A. | Italy | 2834 | N/A | Via Carducci, 62D, Loc. La Fontina 56010 San Giuliano Terme (PI) Frazione Ghezzano, Italy (39) 050 8798341 | ||||
| Grifols Deutschland GmbH | Germany | 2834 | N/A | Lyoner Strasse 15 60528 Frankfurt am Main, Germany (49) 6103 75020 |
| (1) | Grifols Inc. is the Issuer of the new notes offered hereby. The other listed registrants, including Grifols, S.A., are Guarantors of the new notes. |
Table of Contents
| The information in this prospectus is not complete and may be changed. We may not sell these securities until the registration statement filed with the Securities and Exchange Commission is effective. This prospectus is not an offer to sell these securities and it is not soliciting an offer to buy these securities in any state or jurisdiction where the offer or sale is not permitted. |

| • | The existing notes were originally issued by Giant Funding Corp., an escrow company formed solely for the purpose of issuing the existing notes, on January 21, 2011. Giant Funding Corp. merged into Grifols Inc., our wholly owned subsidiary, and Grifols Inc. assumed its obligations under the existing notes and the related indenture on June 1, 2011. The exchange notes will represent the same debt as the existing notes and Grifols Inc. will issue the exchange notes under the same indenture. | |
| • | The terms of the exchange notes are substantially identical to the existing notes, except that the transfer restrictions and registration rights relating to the existing notes will not apply to the exchange notes and the exchange notes will not provide for the payment of special interest under circumstances related to the timing and completion of the exchange offer. | |
| • | We are making the exchange offer to satisfy your registration rights, as a holder of existing notes. | |
| • | The exchange offer expires at 5:00 p.m., New York City time, on , 2011, unless extended. | |
| • | Subject to the satisfaction or waiver of specified conditions, we will exchange your validly tendered unregistered existing notes that have not been withdrawn prior to the expiration of the exchange offer for an equal principal amount of exchange notes that have been registered under the Securities Act of 1933, as amended, or the Securities Act. | |
| • | The exchange offer is not subject to any condition other than that the exchange offer not violate applicable law or any applicable interpretation of the staff of the Securities and Exchange Commission, or the SEC, and other customary conditions. | |
| • | You may withdraw your tender of notes at any time before the exchange offer expires. | |
| • | The exchange of notes should not be a taxable exchange for U.S. federal income tax purposes. | |
| • | We will not receive any proceeds from the exchange offer. | |
| • | Any outstanding existing notes not validly tendered will remain subject to existing transfer restrictions. | |
| • | The exchange notes will not be traded on any national securities exchange and, therefore, we do not anticipate that an active public market in the exchange notes will develop. |
| ii | ||||||||
| iii | ||||||||
| iv | ||||||||
| 1 | ||||||||
| 10 | ||||||||
| 13 | ||||||||
| 25 | ||||||||
| 27 | ||||||||
| 28 | ||||||||
| 32 | ||||||||
| 34 | ||||||||
| 71 | ||||||||
| 72 | ||||||||
| 73 | ||||||||
| 74 | ||||||||
| 83 | ||||||||
| 113 | ||||||||
| 143 | ||||||||
| 151 | ||||||||
| 161 | ||||||||
| 171 | ||||||||
| 173 | ||||||||
| 175 | ||||||||
| 177 | ||||||||
| 225 | ||||||||
| 227 | ||||||||
| 232 | ||||||||
| 233 | ||||||||
| 234 | ||||||||
| 234 | ||||||||
| 234 | ||||||||
| F-1 | ||||||||
| EX-3.1.1 | ||||||||
| EX-3.1.2 | ||||||||
| EX-3.2.1 | ||||||||
| EX-3.2.2 | ||||||||
| EX-3.3.1 | ||||||||
| EX-3.3.2 | ||||||||
| EX-3.3.3 | ||||||||
| EX-3.4.1 | ||||||||
| EX-3.4.2 | ||||||||
| EX-3.5.1 | ||||||||
| EX-3.5.2 | ||||||||
| EX-3.5.3 | ||||||||
| EX-3.5.4 | ||||||||
| EX-3.6.1 | ||||||||
| EX-3.6.2 | ||||||||
| EX-3.6.3 | ||||||||
| EX-3.7.1 | ||||||||
| EX-3.7.2 | ||||||||
| EX-3.8.1 | ||||||||
| EX-3.8.2 | ||||||||
| EX-3.9.1 | ||||||||
| EX-3.9.2 | ||||||||
| EX-3.10.1 | ||||||||
| EX-3.10.2 | ||||||||
| EX-3.11.1 | ||||||||
| EX-3.11.2 | ||||||||
| EX-3.12 | ||||||||
| EX-4.1 | ||||||||
| EX-4.3 | ||||||||
| EX-4.4 | ||||||||
| EX-4.5 | ||||||||
| EX-4.6 | ||||||||
| EX-5.1 | ||||||||
| EX-5.2 | ||||||||
| EX-5.3 | ||||||||
| EX-5.4 | ||||||||
| EX-5.5 | ||||||||
| EX-10.1 | ||||||||
| EX-10.2 | ||||||||
| EX-10.3 | ||||||||
| EX-10.4 | ||||||||
| EX-10.5 | ||||||||
| EX-10.6 | ||||||||
| EX-12.1 | ||||||||
| EX-21.1 | ||||||||
| EX-23.6 | ||||||||
| EX-23.7 | ||||||||
| EX-25.1 | ||||||||
| EX-99.1 | ||||||||
| EX-99.2 | ||||||||
| EX-99.3 | ||||||||
| EX-99.4 | ||||||||
i
Table of Contents
| • | all references in this document to “Grifols,” the “Company,” “we,” “us,” and “our” refer to Grifols, S.A., a company (sociedad anónima) organized under the laws of Spain, and our consolidated subsidiaries; for periods after the acquisition these terms include the acquired Talecris operations; | |
| • | all references to “the Issuer” refer to Grifols Inc., a Virginia corporation and wholly owned subsidiary of Grifols, S.A.; | |
| • | all references to “Talecris” refer to Talecris Biotherapeutics Holdings Corp., a Delaware corporation, and its consolidated subsidiaries, as existing prior to the closing of the acquisition; and | |
| • | all references to the “acquisition” refer to our acquisition of Talecris, consummated on June 1, 2011. |
ii
Table of Contents
iii
Table of Contents
| • | risks related to the notes and the guarantees; | |
| • | our significant indebtedness; | |
| • | our ability to service our indebtedness, which in turn depends on our ability to generate cash; | |
| • | the subordinated nature of the notes and guarantees; | |
| • | the restrictive covenants governing our debt; | |
| • | Federal and state statutes permitting courts to void guarantees under certain circumstances; | |
| • | Bankruptcy law limitations on the amounts payable to note holders; | |
| • | limitations on the enforcement of civil liabilities under U.S. securities laws; | |
| • | extensive environmental, health and safety laws and regulations; | |
| • | implementation of health care reform law in the U.S.; | |
| • | potential decreases or limitations on reimbursement for purchasers of our products; | |
| • | our ability to maintain compliance with government regulations and licenses, including those related to plasma collection, production, and marketing; | |
| • | regulatory actions or lawsuits brought under federal or state laws; | |
| • | risks related to compliance with the Sarbanes-Oxley Act of 2002 and our internal controls over financial reporting; | |
| • | product concentration risk; |
iv
Table of Contents
| • | the risks associated with the potential damage or contamination of plasma, our main raw material; | |
| • | side effects associated with our products; | |
| • | our adherence to cGMP; | |
| • | our ability to procure adequate quantities of plasma and other materials which are acceptable for use in our manufacturing processes; | |
| • | increased competition in our industry; | |
| • | the impact of competitive products and pricing and actions of competitors; | |
| • | fluctuations in the balance between supply and demand with respect to the market for plasma-derived products; | |
| • | potential product liability claims or product recalls involving our products; | |
| • | the impact of our substantial capital expenditures; | |
| • | market risks, such as interest rate risk and foreign exchange rate risk; | |
| • | the unprecedented volatility in the global economy and fluctuations in the financial markets; | |
| • | unexpected shut-downs of our manufacturing and storage facilities or delays in opening new facilities; | |
| • | disruptions in our distribution channels; | |
| • | our ability to identify growth opportunities for existing products and to identify and develop new product candidates through our research and development activities; | |
| • | our ability to protect our intellectual property rights and defend against allegations of infringement by others; | |
| • | our ability to resume or replace sales to countries affected by the ongoing Foreign Corrupt Practices Act (“FCPA”) investigation; | |
| • | potential sanctions, if any, that the Department of Justice (“DOJ”) or other federal agencies, may impose on us as a result of the ongoing FCPA investigation; | |
| • | the impact of the pending investigation into compliance with the Pharmaceutical Pricing Agreement (“PPA”) under the Public Health Service Program; and | |
| • | other factors that are set forth below under the section entitled “Risk Factors.” |
v
Table of Contents
1
Table of Contents
2
Table of Contents
3
Table of Contents
4
Table of Contents
5
Table of Contents
6
Table of Contents
7
Table of Contents
8
Table of Contents
9
Table of Contents
| As of December 31, | As of June 30, | |||||||||||||||||||||||
| 2006 | 2007 | 2008 | 2009 | 2010 | 2011 | |||||||||||||||||||
| (In thousands of Euros) | ||||||||||||||||||||||||
ASSETS | ||||||||||||||||||||||||
Non-current assets | ||||||||||||||||||||||||
| Intangible assets | ||||||||||||||||||||||||
| Goodwill | 150,820 | 150,243 | 158,567 | 174,000 | 189,448 | 2,281,696 | ||||||||||||||||||
| Other intangible assets | 60,850 | 57,223 | 57,756 | 69,385 | 78,299 | 128,474 | ||||||||||||||||||
| Total intangible assets | 211,670 | 207,466 | 216,323 | 243,385 | 267,747 | 2,410,170 | ||||||||||||||||||
| Property, plant and equipment | 184,993 | 201,332 | 301,009 | 371,705 | 434,131 | 639,735 | ||||||||||||||||||
| Investments in equity accounted investees | 253 | 243 | 374 | 383 | 598 | 3,546 | ||||||||||||||||||
| Non-current financial assets | 2,012 | 891 | 1,636 | 3,731 | 7,535 | 41,667 | ||||||||||||||||||
| Deferred tax assets | 41,452 | 34,110 | 34,297 | 33,395 | 34,889 | 139,435 | ||||||||||||||||||
| Total non-current assets | 440,380 | 444,042 | 553,639 | 652,599 | 744,900 | 3,234,553 | ||||||||||||||||||
Current assets | ||||||||||||||||||||||||
| Inventories | 235,475 | 270,659 | 373,098 | 484,462 | 527,865 | 997,826 | ||||||||||||||||||
| Trade and other receivables | ||||||||||||||||||||||||
| Trade receivables | 173,053 | 174,351 | 186,324 | 207,840 | 224,355 | 405,450 | ||||||||||||||||||
| Other receivables | 22,588 | 28,624 | 43,443 | 39,540 | 44,032 | 48,971 | ||||||||||||||||||
| Current income tax assets | 3,710 | 2,402 | 5,428 | 7,802 | 14,607 | 41,029 | ||||||||||||||||||
| Trade and other receivables | 199,351 | 205,377 | 235,195 | 255,182 | 282,994 | 495,450 | ||||||||||||||||||
| Other current financial assets | 6,232 | 7,600 | 6,680 | 8,217 | 12,946 | 19,254 | ||||||||||||||||||
| Other current assets | 5,353 | 6,201 | 5,259 | 7,345 | 80,628 | 13,344 | ||||||||||||||||||
| Cash and cash equivalents | 26,883 | 5,690 | 6,368 | 249,372 | 239,649 | 583,792 | ||||||||||||||||||
| Total current assets | 473,294 | 495,527 | 626,600 | 1,004,578 | 1,144,082 | 2,109,666 | ||||||||||||||||||
Total Assets | 913,674 | 939,569 | 1,180,239 | 1,657,177 | 1,888,982 | 5,344,219 | ||||||||||||||||||
10
Table of Contents
| As of December 31, | As of June 30, | |||||||||||||||||||||||
| 2006 | 2007 | 2008 | 2009 | 2010 | 2011 | |||||||||||||||||||
| (In thousands of Euros) | ||||||||||||||||||||||||
EQUITY AND LIABILITIES | ||||||||||||||||||||||||
Equity | ||||||||||||||||||||||||
| Share capital | 106,532 | 106,532 | 106,532 | 106,532 | 106,532 | 114,914 | ||||||||||||||||||
| Reserves | 284,092 | 316,440 | 369,471 | 436,705 | 525,406 | 1,460,037 | ||||||||||||||||||
| Own shares | — | (28,893 | ) | (33,087 | ) | (677 | ) | (1,927 | ) | (1,927 | ) | |||||||||||||
| Interim dividend | — | — | — | (31,960 | ) | — | — | |||||||||||||||||
| Profit for the year attributable to the Parent | 45,394 | 87,774 | 121,728 | 147,972 | 115,513 | 19,269 | ||||||||||||||||||
| Total equity | 436,018 | 481,853 | 564,644 | 658,572 | 745,524 | 1,592,293 | ||||||||||||||||||
Available-for-sale financial assets | (52 | ) | (152 | ) | (158 | ) | — | — | (575 | ) | ||||||||||||||
| Cash flow hedges | — | — | — | (1,948 | ) | (1,751 | ) | (2,331 | ) | |||||||||||||||
| Translation differences | (68,022 | ) | (98,516 | ) | (84,457 | ) | (90,253 | ) | (50,733 | ) | (88,734 | ) | ||||||||||||
| Other comprehensive income | (68,074 | ) | (98,668 | ) | (84,615 | ) | (92,201 | ) | (52,484 | ) | (91,640 | ) | ||||||||||||
| Equity attributable to the Parent | 367,944 | 383,185 | 480,029 | 566,371 | 693,040 | 1,500,653 | ||||||||||||||||||
| Minority interest | 408 | 981 | 1,250 | 12,157 | 14,350 | 12,941 | ||||||||||||||||||
Total Equity | 368,352 | 384,166 | 481,279 | 578,528 | 707,390 | 1,513,594 | ||||||||||||||||||
Non-current liabilities | ||||||||||||||||||||||||
| Grants | 4,819 | 4,545 | 2,353 | 2,311 | 2,088 | 1,815 | ||||||||||||||||||
| Provisions | 902 | 999 | 3,045 | 1,232 | 1,378 | 10,461 | ||||||||||||||||||
| Non-current financial liabilities | ||||||||||||||||||||||||
| Loans and borrowings, bonds and other marketable securities | 198,329 | 178,425 | 311,513 | 703,186 | 665,385 | 2,642,944 | ||||||||||||||||||
| Other financial liabilities | 12,646 | 11,064 | 12,542 | 12,552 | 10,474 | 72,400 | ||||||||||||||||||
| Total non-current financial liabilities | 210,975 | 189,489 | 324,055 | 715,738 | 675,859 | 2,715,344 | ||||||||||||||||||
| Deferred tax liabilities | 45,862 | 43,794 | 51,969 | 60,325 | 79,141 | 140,075 | ||||||||||||||||||
| Total non-current liabilities | 262,558 | 238,827 | 381,422 | 779,606 | 758,466 | 2,867,695 | ||||||||||||||||||
Current liabilities | ||||||||||||||||||||||||
| Provisions | 3,890 | 3,957 | 3,830 | 4,702 | 4,365 | 35,828 | ||||||||||||||||||
| Current financial liabilities | ||||||||||||||||||||||||
| Loans and borrowings, bonds and other marketable securities | 138,123 | 177,540 | 147,547 | 113,991 | 191,635 | 507,374 | ||||||||||||||||||
| Other financial liabilities | 31,583 | 9,555 | 9,685 | 12,230 | 18,236 | 17,336 | ||||||||||||||||||
| Total current financial liabilities | 169,706 | 187,095 | 157,232 | 126,221 | 209,871 | 524,710 | ||||||||||||||||||
| Debts with associates | — | — | — | — | 1,162 | 2,352 | ||||||||||||||||||
| Trade and other payables | ||||||||||||||||||||||||
| Suppliers | 67,401 | 90,790 | 107,613 | 120,909 | 160,678 | 266,393 | ||||||||||||||||||
| Other payables | 23,282 | 11,396 | 9,068 | 17,832 | 11,928 | 25,618 | ||||||||||||||||||
| Current income tax liabilities | 4,345 | 3,770 | 16,362 | 3,258 | 4,172 | 27,227 | ||||||||||||||||||
| Total trade and other payables | 95,028 | 105,956 | 133,043 | 141,999 | 176,778 | 319,238 | ||||||||||||||||||
| Other current liabilities | 14,140 | 19,568 | 23,433 | 26,121 | 30,950 | 80,802 | ||||||||||||||||||
| Total current liabilities | 282,764 | 316,576 | 317,538 | 299,043 | 423,126 | 962,930 | ||||||||||||||||||
Total Liabilities | 545,322 | 555,403 | 698,960 | 1,078,649 | 1,181,592 | 3,830,625 | ||||||||||||||||||
Total Equity and Liabilities | 913,674 | 939,569 | 1,180,239 | 1,657,177 | 1,888,982 | 5,344,219 | ||||||||||||||||||
11
Table of Contents
| For the Six Months | ||||||||||||||||||||||||||||
| For the Year Ended December 31, | Ended June 30, | |||||||||||||||||||||||||||
| 2006 | 2007 | 2008 | 2009 | 2010 | 2010 | 2011 | ||||||||||||||||||||||
| (In thousands of Euros, except share amounts) | ||||||||||||||||||||||||||||
| Revenues | 648,417 | 703,291 | 814,311 | 913,186 | 990,730 | 487,809 | 635,341 | |||||||||||||||||||||
| Changes in inventories of finished goods and work in progress | (21,631 | ) | 16,882 | 31,058 | 73,093 | 45,749 | 41,209 | 2,757 | ||||||||||||||||||||
| Self-constructed non-current assets | 12,472 | 19,860 | 25,794 | 41,142 | 33,513 | 16,051 | 32,346 | |||||||||||||||||||||
| Supplies | (181,541 | ) | (196,308 | ) | (206,738 | ) | (286,274 | ) | (304,818 | ) | (157,107 | ) | (175,142 | ) | ||||||||||||||
| Other operating income | 380 | 2,322 | 1,289 | 1,443 | 1,196 | 631 | 1,009 | |||||||||||||||||||||
| Personnel expenses | (184,730 | ) | (209,049 | ) | (238,159 | ) | (273,168 | ) | (289,008 | ) | (141,972 | ) | (183,727 | ) | ||||||||||||||
| Other operating expenses | (143,477 | ) | (158,273 | ) | (192,288 | ) | (203,381 | ) | (205,260 | ) | (98,279 | ) | (155,532 | ) | ||||||||||||||
| Amortization and depreciation | (29,357 | ) | (31,528 | ) | (33,256 | ) | (39,554 | ) | (45,776 | ) | (21,434 | ) | (28,156 | ) | ||||||||||||||
| Transaction costs of Talecris business combination | — | — | — | — | (16,999 | ) | (2,019 | ) | (38,607 | ) | ||||||||||||||||||
| Non-financial and other capital grants | 531 | 282 | 2,941 | 1,188 | 728 | 550 | 742 | |||||||||||||||||||||
| Impairment and gains/(losses) on disposal of fixed assets | (574 | ) | (1,125 | ) | (1,991 | ) | (1,147 | ) | (372 | ) | 681 | (22,302 | ) | |||||||||||||||
Results from operating activities | 100,490 | 146,354 | 202,961 | 226,528 | 209,683 | 126,120 | 68,729 | |||||||||||||||||||||
| Finance income | 4,667 | 4,526 | 2,682 | 7,067 | 4,526 | 2,179 | 1,761 | |||||||||||||||||||||
| Finance expenses | (42,140 | ) | (23,523 | ) | (29,305 | ) | (27,087 | ) | (49,660 | ) | (25,285 | ) | (55,546 | ) | ||||||||||||||
| Change in fair value of financial instruments | 1,480 | 829 | (1,268 | ) | (587 | ) | (7,593 | ) | (15,404 | ) | 13,945 | |||||||||||||||||
| Impairment of gains/(losses) on disposal of financial instruments | — | — | — | (245 | ) | 91 | — | — | ||||||||||||||||||||
| Exchange losses | (1,064 | ) | (4,618 | ) | (2,825 | ) | (1,733 | ) | 1,616 | 1,970 | (2,122 | ) | ||||||||||||||||
Finance income and expense | (37,057 | ) | (22,786 | ) | (30,716 | ) | (22,585 | ) | (51,020 | ) | (36,540 | ) | (41,962 | ) | ||||||||||||||
| Share of profit of equity accounted investees | 76 | 19 | 24 | 51 | (879 | ) | (728 | ) | (807 | ) | ||||||||||||||||||
Profit before income tax from continuing operations | 63,509 | 123,587 | 172,269 | 203,994 | 157,784 | 88,852 | 25,960 | |||||||||||||||||||||
| Income tax expense | (17,824 | ) | (35,239 | ) | (50,153 | ) | (56,424 | ) | (42,517 | ) | (23,022 | ) | (7,347 | ) | ||||||||||||||
Profit after income tax from continuing operations | 45,685 | 88,348 | 122,116 | 147,570 | 115,267 | 65,830 | 18,613 | |||||||||||||||||||||
| Profit attributable to equity holders of the Parent | 45,394 | 87,774 | 121,728 | 147,972 | 115,513 | 66,408 | 19,269 | |||||||||||||||||||||
| Profit attributable to minority interest | 291 | 574 | 388 | (402 | ) | (246 | ) | (578 | ) | (656 | ) | |||||||||||||||||
Consolidated profit for the year | 45,685 | 88,348 | 122,116 | 147,570 | 115,267 | 65,830 | 18,613 | |||||||||||||||||||||
Basic earnings per ordinary share (Euros) | 0.24 | 0.41 | 0.58 | 0.71 | 0.54 | 0.31 | 0.09 | |||||||||||||||||||||
Diluted earnings per ordinary share (Euros) | 0.25 | 0.41 | 0.58 | 0.71 | 0.54 | 0.31 | 0.09 | |||||||||||||||||||||
Cash dividend per ordinary share (Euros) | 0.06 | 0.06 | 0.17 | 0.38 | 0.13 | — | — | |||||||||||||||||||||
12
Table of Contents
| • | The audited consolidated financial statements of Grifols as of and for the year ended December 31, 2010, which have been prepared in accordance with IFRS as issued by the IASB and included elsewhere in this prospectus; | |
| • | The unaudited condensed consolidated interim financial statements of Grifols as of and for the six month period ended June 30, 2011, which have been prepared in accordance with IAS 34,Interim Financial Reportingand included elsewhere in this prospectus; | |
| • | The audited consolidated financial statements of Talecris as of and for the year ended December 31, 2010, which have been prepared in accordance with U.S. GAAP and are included elsewhere in this prospectus. These consolidated financial statements have been adjusted to IFRS and translated to euros for purposes of presentation in the unaudited pro forma condensed combined financial information. | |
| • | The unaudited consolidated financial information of Talecris as of and for the five month period ended May 31, 2011. This consolidated financial information has been adjusted to IFRS and translated to euros for purposes of presentation in the unaudited pro forma condensed combined financial information. |
13
Table of Contents
14
Table of Contents
Six Month Period Ended June 30, 2011
| Historical | Historical | ||||||||||||||||
| GRIFOLS IFRS | TALECRIS IFRS | ||||||||||||||||
| Six Month Period | Five Month Period | ||||||||||||||||
| Ended June 30 | Ended May 31 | Pro forma | Pro Forma | ||||||||||||||
| 2011 | 2011 | Adjustments | Combined | ||||||||||||||
| (Note 1) | (Note 2 ) | (Note 3) | GRIFOLS | ||||||||||||||
| (In thousands of Euros, except share amounts) | |||||||||||||||||
| Revenues | 635,341 | 504,051 | 1,139,392 | ||||||||||||||
| Changes in inventories of finished goods and work in | 2,757 | (21,862 | ) | (19,105 | ) | ||||||||||||
| Self-constructed non-current assets | 32,346 | 6,192 | 38,538 | ||||||||||||||
| Supplies | (175,142 | ) | (91,731 | ) | (266,873 | ) | |||||||||||
| Other operating income | 1,009 | — | 1,009 | ||||||||||||||
| Personnel expenses | (183,727 | ) | (149,571 | ) | 14,810 | (c) | (318,488 | ) | |||||||||
| Other operating expenses | (155,532 | ) | (125,456 | ) | (280,988 | ) | |||||||||||
| Amortisation and depreciation | (28,156 | ) | (16,387 | ) | (44,543 | ) | |||||||||||
| Transition costs of Talecris business combination | (38,607 | ) | (16,756 | ) | 55,363 | (b) | — | ||||||||||
| Non-financial and other capital grants | 742 | — | 742 | ||||||||||||||
| Impairment and gains/(losses) on disposal of fixed assets | (22,302 | ) | — | (22,302 | ) | ||||||||||||
Results from operating activities | 68,729 | 88,480 | 70,173 | 227,382 | |||||||||||||
| Finance income | 1,761 | 146 | 1,907 | ||||||||||||||
| Finance expenses | (55,546 | ) | (13,163 | ) | (45,898 | )(a) | (114,607 | ) | |||||||||
| Change in fair value of financial instruments | 13,945 | 1,880 | 15,825 | ||||||||||||||
| Exchange gains/(losses) | (2,122 | ) | (2,989 | ) | (5,111 | ) | |||||||||||
Financial income and expense | (41,962 | ) | (14,126 | ) | (45,898 | ) | (101,986 | ) | |||||||||
| Share of (loss) profit of equity accounted investees | (807 | ) | 234 | (573 | ) | ||||||||||||
Profit before income tax from continuing Operations | 25,960 | 74,588 | 24,275 | 124,823 | |||||||||||||
| Income tax (expense) benefit | (7,347 | ) | (24,991 | ) | (9,152 | )(d) | (41,490 | ) | |||||||||
Profit after income tax from continuing operations | 18,613 | 49,597 | 15,123 | 83,333 | |||||||||||||
| Profit attributable to equity holders of the Parent | 19,269 | 49,597 | 15,123 | 83,989 | |||||||||||||
| Profit attributable to minority interest | (656 | ) | — | (656 | ) | ||||||||||||
Consolidated profit for the three month period | 18,613 | 49,597 | 15,123 | 83,333 | |||||||||||||
| Basic earnings per share | 0.09 | 0.39 | |||||||||||||||
| Weighted average number of share in issue(B) | 213,064,899 | 213,064,899 | |||||||||||||||
| Diluted earnings per share | 0.09 | 0.39 | |||||||||||||||
| Weighted average number of share on fully diluted basis | 213,064,899 | 213,064,899 | |||||||||||||||
| (B) | The weighted average number of shares outstanding during the period has been adjusted to give effect to shares issued as consideration for the transaction as if the acquisition had taken place as of January 1, 2010. |
15
Table of Contents
Year Ended December 31, 2010
| Pro forma | |||||||||||||||||
| GRIFOLS IFRS | TALECRIS IFRS | Adjustments | Pro Forma | ||||||||||||||
| (Note 1) | (Note 2) | (Note 3) | GRIFOLS | ||||||||||||||
| (In thousands of Euros, except share amounts) | |||||||||||||||||
| Revenues | 990,730 | 1,207,766 | 2,198,496 | ||||||||||||||
| Changes in inventories of finished goods and work in | 45,749 | 27,932 | 73,681 | ||||||||||||||
| Self-constructed non-current assets | 33,513 | 15,292 | 48,805 | ||||||||||||||
| Supplies | (306,859 | ) | (319,563 | ) | (626,422 | ) | |||||||||||
| Other operating income | 1,196 | — | 1,196 | ||||||||||||||
| Personnel expenses | (289,008 | ) | (352,803 | ) | 12,422 | (c) | (629,389 | ) | |||||||||
| Other operating expenses | (220,218 | ) | (292,504 | ) | 37,911 | (b) | (474,811 | ) | |||||||||
| Amortisation and depreciation | (45,776 | ) | (39,235 | ) | (85,011 | ) | |||||||||||
| Non-financial and other capital grants | 728 | — | 728 | ||||||||||||||
| Impairment and gains/(losses) on disposal of fixed assets | (372 | ) | (1,124 | ) | (1,496 | ) | |||||||||||
Results from operating activities | 209,683 | 245,761 | 50,333 | 505,777 | |||||||||||||
| PCA Adjustment | (32,946 | ) | (32,946 | ) | |||||||||||||
| Finance income | 4,526 | 255 | 4,781 | ||||||||||||||
| Finance expenses | (49,660 | ) | (34,301 | ) | (113,978 | )(a) | (197,939 | ) | |||||||||
| Change in fair value of financial instruments | (7,593 | ) | — | (7,593 | ) | ||||||||||||
| Impairment of gains/(losses) on disposal of financial instruments | 91 | — | 91 | ||||||||||||||
| Exchange gains | 1,616 | 3,565 | 5,181 | ||||||||||||||
Financial income and expense | (51,020 | ) | (63,427 | ) | (113,978 | ) | (228,425 | ) | |||||||||
| Share of (loss) profit of equity accounted investees | (879 | ) | 747 | (132 | ) | ||||||||||||
Profit before income tax from continuing operations | 157,784 | 183,081 | (63,645 | ) | 277,220 | ||||||||||||
| Income tax (expense) benefit | (42,517 | ) | (59,543 | ) | 23,994 | (d) | (78,066 | ) | |||||||||
Profit after income tax from continuing operations | 115,267 | 123,538 | (39,651 | ) | 199,154 | ||||||||||||
| Profit attributable to equity holders of the Parent | 115,513 | 123,538 | (39,651 | ) | 199,400 | ||||||||||||
| Profit attributable to minority interest | (246 | ) | — | (246 | ) | ||||||||||||
Consolidated profit for the year | 115,267 | 123,538 | (39,651 | ) | 199,154 | ||||||||||||
| Basic earnings per share | 0.54 | 0.94 | |||||||||||||||
| Weighted average number of share in issue(B) | 212,909,162 | 212,909,162 | |||||||||||||||
| Diluted earnings per share | 0.54 | 0.94 | |||||||||||||||
| Weighted average number of share on fully diluted basis | 212,909,162 | 212,909,162 | |||||||||||||||
| (B) | The weighed averaged number of shares outstanding during the period has been adjusted to give effect to shares issued as consideration for the transaction as if the acquisition had taken place as of January 1, 2010. |
16
Table of Contents
| 1. | Historical Grifols Information |
| • | unaudited condensed consolidated interim financial statements as of and for the six month period ended June 30, 2011; and | |
| • | audited consolidated financial statements as of and for the year ended December 31, 2010. |
| 2. | Talecris Reconciliation to IFRS in Euros |
17
Table of Contents
| Statement of Income Five Month Period Ended May 31, 2011 | |||||||||||||||||||||||||||||||||||||||
| Historical | Historical | Ex. | Historical | ||||||||||||||||||||||||||||||||||||
| TALECRIS | Reclassifications | TALECRIS | Inventory | Capitalize | Income | TALECRIS | Rate | TALECRIS | |||||||||||||||||||||||||||||||
| US GAAP Cost | to Cost | US GAAP | Recovery | R&D Cost | Taxes | IFRS Cost | (h) | IFRS as Shown | |||||||||||||||||||||||||||||||
| by Function | by Nature(f) | Cost by Nature | (b) | (c) | (e) | by Nature | 1.3721 | in the Pro forma | |||||||||||||||||||||||||||||||
| A | B | A+B | |||||||||||||||||||||||||||||||||||||
| (in thousands of $) | (in thousands of $) | ||||||||||||||||||||||||||||||||||||||
| (in thousands of €) | |||||||||||||||||||||||||||||||||||||||
| Net revenue | |||||||||||||||||||||||||||||||||||||||
| Product | 691,609 | ||||||||||||||||||||||||||||||||||||||
| Other | |||||||||||||||||||||||||||||||||||||||
Total | 691,609 | Revenues | 691,609 | 691,609 | 504,051 | ||||||||||||||||||||||||||||||||||
| Cost of goods sold | (399,219 | ) | 399,219 | ||||||||||||||||||||||||||||||||||||
Gross profit | 292,390 | ||||||||||||||||||||||||||||||||||||||
| Operating expenses | |||||||||||||||||||||||||||||||||||||||
| Selling, general and administrative | (135,617 | ) | 135,617 | ||||||||||||||||||||||||||||||||||||
| Research and development | (32,208 | ) | 32,208 | ||||||||||||||||||||||||||||||||||||
Total | (167,375 | ) | |||||||||||||||||||||||||||||||||||||
Income from operations | 125,015 | ||||||||||||||||||||||||||||||||||||||
| (29,635 | ) | Changes in inventories of finished goods and work in progress | (29,635 | ) | (362 | ) | (29,997 | ) | (21,862 | ) | |||||||||||||||||||||||||||||
| 4,199 | Self-constructed non-current assets | 4,199 | 4,297 | 8,496 | 6,192 | ||||||||||||||||||||||||||||||||||
| (125,864 | ) | Supplies | (125,864 | ) | (125,864 | ) | (91,731 | ) | |||||||||||||||||||||||||||||||
| (205,227 | ) | Personnel expenses | (205,227 | ) | (205,227 | ) | (149,571 | ) | |||||||||||||||||||||||||||||||
| (172,138 | ) | Other operating expenses | (172,138 | ) | (172,138 | ) | (124,456 | ) | |||||||||||||||||||||||||||||||
| (13,417 | ) | Amortization and depreciation | (13,417 | ) | (9,067 | ) | (22,484 | ) | (16,387 | ) | |||||||||||||||||||||||||||||
| (22,991 | ) | Transition costs of Talecris business combin | (22,991 | ) | (22,991 | ) | (16,756 | ) | |||||||||||||||||||||||||||||||
Results from operating activities | 126,536 | (362 | ) | (4,770 | ) | — | 144,395 | 88,480 | |||||||||||||||||||||||||||||||
| Other non-operating (expense) income | |||||||||||||||||||||||||||||||||||||||
| Interest expense, net | (17,859 | ) | 17,859 | ||||||||||||||||||||||||||||||||||||
| Equity in earnings of affiliate | 320 | (320 | ) | ||||||||||||||||||||||||||||||||||||
Total | (17,539 | ) | |||||||||||||||||||||||||||||||||||||
| (825 | ) | PCA judgment | (825 | ) | (825 | ) | (601 | ) | |||||||||||||||||||||||||||||||
| 202 | Finance income | 201 | 201 | 146 | |||||||||||||||||||||||||||||||||||
| (17,236 | ) | Finance expenses | (17,236 | ) | (17,236 | ) | (12,562 | ) | |||||||||||||||||||||||||||||||
| 2,580 | Changes in FV of financial instruments | 2,580 | 2,580 | 1,880 | |||||||||||||||||||||||||||||||||||
| (4,101 | ) | Exchange gains | (4,101 | ) | (4,101 | ) | (2,989 | ) | |||||||||||||||||||||||||||||||
Financial income and expense | (19,381 | ) | — | — | — | (19,381 | ) | (14,126 | ) | ||||||||||||||||||||||||||||||
| 321 | Share of profit of equity accounted investees | 321 | 321 | 234 | |||||||||||||||||||||||||||||||||||
Income before income taxes | 107,476 | Profit before income tax from continuing operations | 107,476 | (362 | ) | (4,770 | ) | — | 125,335 | 74,588 | |||||||||||||||||||||||||||||
| (Provision) benefit for income taxes | (35,882 | ) | 0 | Income tax (expense) benefit | (35,882 | ) | 1,593 | (34,289 | ) | (24,991 | ) | ||||||||||||||||||||||||||||
Profit after income tax from | |||||||||||||||||||||||||||||||||||||||
Net income | 71,594 | continuing operations | 71,594 | (362 | ) | (4,770 | ) | 1,593 | 91,046 | 49,597 | |||||||||||||||||||||||||||||
18
Table of Contents
| Statement of Income of the year ended December 31, 2010 | |||||||||||||||||||||||||||||||||||||||||||||||
| Historical | Historical | Adjustments to IFRS | Ex. | Historical | |||||||||||||||||||||||||||||||||||||||||||
| TALECRIS | Reclassifications | TALECRIS | Debt | Inventory | Capitalize | Shared Based | Income | TALECRIS | Rate | TALECRIS | |||||||||||||||||||||||||||||||||||||
| US GAAP Cost | to Cost | US GAAP | Issuance | Recovery | R&D Cost | Payments | Taxes | IFRS Cost | (g) | IFRS as Shown | |||||||||||||||||||||||||||||||||||||
| by Function | by Nature(f) | Cost by Nature | Costs(a) | (b) | (c) | (d) | (e) | by Nature | 1.3261 | in the Pro forma | |||||||||||||||||||||||||||||||||||||
| A | B | A+B | |||||||||||||||||||||||||||||||||||||||||||||
| (In thousands of $) | (In thousands of $) | ||||||||||||||||||||||||||||||||||||||||||||||
| (In thousands of €) | |||||||||||||||||||||||||||||||||||||||||||||||
| Net revenue | |||||||||||||||||||||||||||||||||||||||||||||||
| Product | 1,601,619 | ||||||||||||||||||||||||||||||||||||||||||||||
| Other | |||||||||||||||||||||||||||||||||||||||||||||||
Total | 1,601,619 | Revenues | 1,601,619 | 1,601,619 | 1,207,766 | ||||||||||||||||||||||||||||||||||||||||||
| Cost of goods sold | (911,976 | ) | 911,976 | ||||||||||||||||||||||||||||||||||||||||||||
Gross profit | 689,643 | ||||||||||||||||||||||||||||||||||||||||||||||
| Operating expenses | |||||||||||||||||||||||||||||||||||||||||||||||
| Selling, general and administrative | (287,011 | ) | 287,011 | ||||||||||||||||||||||||||||||||||||||||||||
| Research and development | (69,649 | ) | 69,649 | ||||||||||||||||||||||||||||||||||||||||||||
Total | (356,660 | ) | |||||||||||||||||||||||||||||||||||||||||||||
Income from operations | 332,983 | ||||||||||||||||||||||||||||||||||||||||||||||
| 37,647 | Changes in inventories of finished goods and work in progress | 37,647 | (606 | ) | 37,041 | 27,932 | |||||||||||||||||||||||||||||||||||||||||
| 8,610 | Self-constructed non-current assets | 8,610 | 11,669 | 20,279 | 15,292 | ||||||||||||||||||||||||||||||||||||||||||
| (423,772 | ) | Supplies | (423,772 | ) | (423,772 | ) | (319,563 | ) | |||||||||||||||||||||||||||||||||||||||
| (470,437 | ) | Personnel expenses | (470,437 | ) | 2,585 | (467,852 | ) | (352,803 | ) | ||||||||||||||||||||||||||||||||||||||
| (387,890 | ) | Other operating expenses | (387,890 | ) | (387,890 | ) | (292,504 | ) | |||||||||||||||||||||||||||||||||||||||
| (36,030 | ) | Amortization and depreciation | (36,030 | ) | (16,000 | ) | (52,030 | ) | (39,235 | ) | |||||||||||||||||||||||||||||||||||||
| (1,491 | ) | Impairment and gains/(losses) on disposal of fixed assets | (1,491 | ) | (1,491 | ) | (1,124 | ) | |||||||||||||||||||||||||||||||||||||||
Results from operating activities | 328,256 | — | (606 | ) | (4,331 | ) | 2,585 | — | 325,904 | 245,761 | |||||||||||||||||||||||||||||||||||||
| Other non-operating (expense) income | |||||||||||||||||||||||||||||||||||||||||||||||
| Interest expense, net | (45,837 | ) | 45,837 | ||||||||||||||||||||||||||||||||||||||||||||
| PCA judgment | (43,690 | ) | 43,690 | PCA judgment | (43,690 | ) | (43,690 | ) | (32,946 | ) | |||||||||||||||||||||||||||||||||||||
| Equity in earnings of affiliate | 991 | (991 | ) | ||||||||||||||||||||||||||||||||||||||||||||
| Total | (88,536 | ) | |||||||||||||||||||||||||||||||||||||||||||||
| 338 | Finance income | 338 | 338 | 255 | |||||||||||||||||||||||||||||||||||||||||||
| (46,175 | ) | Finance expenses | (46,175 | ) | 688 | (45,487 | ) | (34,301 | ) | ||||||||||||||||||||||||||||||||||||||
| 4,727 | Exchange gains | 4,727 | 4,727 | 3,565 | |||||||||||||||||||||||||||||||||||||||||||
expense | (84,800 | ) | 688 | — | — | — | — | (84,112 | ) | (63,427 | ) | ||||||||||||||||||||||||||||||||||||
| 991 | Share of profit of equity accounted investees | 991 | 991 | 747 | |||||||||||||||||||||||||||||||||||||||||||
Income before income taxes | 244,447 | Profit before income tax from continuing operations | 244,447 | 688 | (606 | ) | (4,331 | ) | 2,585 | — | 242,783 | 183,081 | |||||||||||||||||||||||||||||||||||
| (Provision) benefit for income taxes | (78,379 | ) | 0 | Income tax (expense) benefit | (78,379 | ) | (581 | ) | (78,960 | ) | (59,543 | ) | |||||||||||||||||||||||||||||||||||
Profit after income tax from | |||||||||||||||||||||||||||||||||||||||||||||||
Net income | 166,068 | continuing operations | 166,068 | 688 | (606 | ) | (4,331 | ) | 2,585 | (581 | ) | 163,823 | 123,538 | ||||||||||||||||||||||||||||||||||
19
Table of Contents
| (a) | Debt Issuance Costs |
| (b) | Inventory Impairment Recoveries |
| (c) | Capitalized Research and Development Costs |
| (d) | Share-Based Payments |
| (e) | Income Taxes |
20
Table of Contents
| (f) | Reclassifications |
| 3. | Pro forma Adjustments |
| (a) | Debt interest expenses |
| (In thousands of Euros) | ||||
| Purchase price: | ||||
| Cash(i) | 1,763,601 | |||
| Fair value of shares issued(ii) | 829,799 | |||
| 2,593,400 | ||||
| (i) | Cash |
Cash Consideration per Talecris Share($)(1) | 19 | |||||||
| Total Talecris Shares Outstanding | 126,107,617 | |||||||
Cash for Talecris Shares Outstanding($) | 2,396,044,723 | |||||||
| Cash for Talecris Options / Awards (Net Method)($) | 76,327,737 | |||||||
| Cash in Lieu for Fractional Talecris Options /Awards (Net Method)($) | 4,525 | |||||||
Cash for Talecris Options / Awards (Net Method)($) | 76,332,262 | |||||||
| Additional Cash Consideration per Fractional Share($) | 19.693 | |||||||
| Fractional Shares (from Talecris Shares Outstanding) | 21.9095 | |||||||
Cash for Fractional Shares (Talecris Shares Outstanding)($) | 431 | |||||||
Cash($) | 2,472,377,417 | |||||||
| Taxes Withheld on Cash Consideration (Options / Awards)($) | 41,142,748 | |||||||
| Taxes Withheld on Stock Consideration (Options / Awards)($) | 27,476,748 | |||||||
Cash for Tax Payments (Options / Awards)($) | 68,619,495 | |||||||
Total Cash($) | 2,540,996,912 | |||||||
Exchange rate $/Euros(2) | 1.4408 | |||||||
Cash in thousands of euros | 1,763,601 |
21
Table of Contents
| (1) | Based on the Merger Agreement | |
| (2) | Exchange rate $/Euro at May 31, 2011 based on the exchange rate included elsewhere in this prospectus. |
| (ii) | Estimated fair value of shares issued |
| Talecris Shares Outstanding (Affiliated Shareholders) | 62,517,962 | |||||||
Exchange Ratio (Talecris Affiliated Shareholders)(1) | 0.6410 | |||||||
| Grifols Class B Shares (Talecris Affiliated Shareholders) | 40,074,014 | |||||||
| Fractional Shares (Talecris Affiliated Shares Outstanding) | (3.6420 | ) | ||||||
Grifols Class B Shares to be Issued (Talecris Affiliated Shareholders) | 40,074,010 | |||||||
| Talecris Shares Outstanding (Unaffiliated Shareholders) | 63,589,655 | |||||||
Exchange Ratio (Talecris Unaffiliated Shareholders)(2) | 0.6485 | |||||||
| Grifols Class B Shares (Talecris Unaffiliated Shareholders) | 41,237,891 | |||||||
| Fractional Shares (Talecris Unaffiliated Shares Outst.) | (18.2675 | ) | ||||||
Grifols Class B Shares to be Issued (Talecris Unaffiliated Shareholders) | 41,237,873 | |||||||
Grifols Class B Shares for Talecris Shares Outstanding (included Affiliated/Unaffil.) | 81,311,883 | |||||||
| Grifols Class B Shares for Talecris Options/Awards (Net Method) | 2,499,805 | |||||||
Total Grifols Class B Shares (to be Issued by Grifols) | 83,811,688 | |||||||
Price of Grifols Class B Shares($)(3) | 14.2650 | |||||||
Estimated fair value of Class B Shares issued in thousands of $ | 1,195,574 | |||||||
Exchange rate $/Euros(4) | 1.4408 | |||||||
Estimated fair value of Class B Shares issued in thousands of Euros | 829,799 |
| (1) | Based on the Merger Agreement | |
| (2) | Based on Amendment No. 1 to the Merger Agreement | |
| (3) | The fair value of the Class B shares has been determined based on the average of the daily median trading price of the ADSs on the NASDAQ Global Select Market from June 6 through June 28, 2011. The Class B shares started quotation on June 2, 2011. | |
| (4) | Exchange rate $/Euro at May 31, 2011 based on the exchange rate included elsewhere in this prospectus. |
| (iii) | Preliminary Goodwill |
| (In thousands of Euros) | ||||
| Net assets acquired provisional (book value) as at May 31, 2011 | 469,318 | |||
| Purchase price | 2,593,400 | |||
Preliminary goodwill(1)(2) | 2,124,082 | |||
| (1) | As of the date of the preparation of this unaudited pro forma condensed combined financial information, a purchase price allocation, in accordance with IFRS 3 (revised), could not be completed. When the purchase price allocation is completed, other intangible assets may be identified (i.e. licenses, in process research and development, customer relationships, etc.), which could result in a reduction of goodwill. The identification of such intangible assets could generate an amortization charge, which could have an impact on the unaudited pro forma condensed combined statement of income. | |
| Based on preliminary assumptions for the fair value adjustments of tangible and intangible assets in connection with the purchase price allocation, such allocation represents approximately 40% of the estimated goodwill of €2,124 million as of June 30, 2011. On this basis the amount of the annual depreciation and amortization expense net of the tax effect (calculated based on statutory rates of 37.7%) would be approximately between €50 million and €60 million. |
22
Table of Contents
| For purposes of a potential income statement adjustment, the depreciation expense of assets was estimated based on the following residual lives, approximately 20 to 25 years for buildings, 5 to 8 years for machinery and equipment and 20 to 30 years for intangible assets. Those estimated useful lives are management’s best estimate based on the available information. | ||
| (2) | Goodwill will be denominated in U.S. Dollars and will be approximately $3.060 billion. The effect of future currency fluctuations between the Euro, which is the functional currency of Grifols, and the U.S. Dollar will be accounted for as a currency translation adjustment. |
| (iv) | Cash required |
| (In thousands of Euros) | ||||
| Cash and cash equivalents at Grifols | 149,693 | |||
| Incremental debts | 1,613,908 | |||
| Purchase price in cash in connection with the transaction | 1,763,601 | |||
| (v) | Interest expenses on the incremental debt in relation to the acquisition |
| Calculation of interest | ||||||||||||
| expenses on the incremental debt in | Exchange | Euros | ||||||||||
| relation to the acquisition | $ Thousands | rate $/Euros | Thousands | |||||||||
| Purchase price in cash in connection with the transaction | 2,540,997 | 1.4408 | 1,763,601 | |||||||||
| Cash and cash equivalents at Talecris | (215,678 | ) | 1.4408 | (149,693 | ) | |||||||
| Incremental debt to be issued in relation to the acquisition | 2,325,319 | 1,613,908 | ||||||||||
Average interest rate(1) | 6.50 | % | ||||||||||
| Period of five months ended May 31, 2011 finance expenses | 62,977 | 1.3721 | 45,898 | |||||||||
| 2010 Annual finance expenses | 151,146 | 1.3261 | 113,978 | |||||||||
| (1) | Assumed weighted average interest rate on incremental debt. A 25 basis point increase in the interest rate on the incremental debt would increase interest expense on an annual basis by €4.4 million. |
| Grifols | Talecris | Total | ||||||||||
| (In thousands of Euros) | ||||||||||||
Acquisition related costs 2010(2) | 17,000 | 20,911 | 37,911 | |||||||||
Acquisition related costs 2011(2) | 38,607 | 16,756 | 55,363 | |||||||||
Related with debt issuance(1) | 152,880 | 152,880 | ||||||||||
| Related with capital increase and transaction related expenses | 2,264 | 2,264 | ||||||||||
| Total acquisition related costs considered in the pro formas | 210,751 | 37,667 | 248,418 | |||||||||
| The acquisition costs already incurred in the year 2010 and in the six month period ended June 30, 2011 amounts to €37.911 million (Grifols €17.000 and Talecris $27.730 million (€20.911 million)) and €55.363 million (Grifols €38.607 and Talecris $22.991 million (€16.756 million)) respectively were adjusted in the unaudited pro forma |
23
Table of Contents
| condensed combined statement of income for the year 2010 and six month period ended June 30, 2011 to give the effect that the transaction occurred on January 1, 2010. | ||
| (1) | The acquisition costs related to debt issuance are estimated based on the incremental debt | |
| (2) | These acquisition costs may relate to the income statement; however, as they could represent material non-recurring charges that result directly from the transaction, these expenses have not been included in the unaudited condensed combined pro forma statement of income. |
| (c) | Personnel expenses: |
| • | Non-cash equity compensation expense of €10.845 million in 2010 and €11.252 million for the five month period ended May 31, 2011. The Merger Agreement contemplated that, upon completion of the acquisition, all the Talecris stock based awards would become fully vested and be cancelled. As this is a material nonrecurring charge that results directly from the transaction, these expenses have not been included in the corresponding unaudited condensed combined pro forma statements of income for the periods presented. | |
| • | Compensation expense associated with special recognition bonus awards and retention related costs including fringe benefits granted to certain Talecris employees and senior executives of €1.577 million in 2010 and €3.558 million for the five-month period ended May 31, 2011 paid upon the transaction closing. As this is a material nonrecurring charge that results directly from the transaction, this expense has not been included in the unaudited pro forma condensed combined statement of income for the periods presented. |
| (d) | Tax effect: |
24
Table of Contents
| Class A Shares | ||||||||
| High | Low | |||||||
| (Euros) | ||||||||
Fiscal Year 2006 | ||||||||
| Annual (from May 17, 2006) | 10.08 | 4.99 | ||||||
Fiscal Year 2007 | ||||||||
| Annual | 18.25 | 10.11 | ||||||
Fiscal Year 2008 | ||||||||
| First Quarter | 16.63 | 13.28 | ||||||
| Second Quarter | 20.53 | 15.77 | ||||||
| Third Quarter | 20.53 | 17.67 | ||||||
| Fourth Quarter | 17.69 | 11.76 | ||||||
Fiscal Year 2009 | ||||||||
| First Quarter | 14.29 | 10.30 | ||||||
| Second Quarter | 13.45 | 11.07 | ||||||
| Third Quarter | 13.19 | 11.92 | ||||||
| Fourth Quarter | 12.88 | 11.01 | ||||||
Fiscal Year 2010 | ||||||||
| First Quarter | 12.44 | 10.12 | ||||||
| Second Quarter | 11.60 | 8.44 | ||||||
| Third Quarter | 10.93 | 8.32 | ||||||
| Fourth Quarter | 12.45 | 8.11 | ||||||
Fiscal Year 2011 | ||||||||
| First Quarter | 12.49 | 9.85 | ||||||
| April | 13.48 | 12.12 | ||||||
| May | 14.44 | 13.18 | ||||||
| June | 14.49 | 12.89 | ||||||
| July | 15.30 | 13.81 | ||||||
| August | 15.80 | 12.70 | ||||||
| September | 14.61 | 13.24 | ||||||
| October (through October 21) | 14.50 | 13.44 | ||||||
25
Table of Contents
| Class B | ||||||||
| ADSs | ||||||||
| High | Low | |||||||
| (U.S. Dollars) | ||||||||
Fiscal Year 2011 | ||||||||
June (from June 2nd) | 8.50 | 6.85 | ||||||
| July | 8.16 | 7.24 | ||||||
| August | 8.07 | 6.33 | ||||||
| September | 6.99 | 5.30 | ||||||
| October (through October 21) | 6.75 | 6.00 | ||||||
| Gross per Share | Net per Share | |||||||||||
Year | Type | Payment Date | Amount | Amount(1) | ||||||||
| (Euros) | (Euros) | |||||||||||
| 2009 | Ordinary | July 1, 2010 | 0.12789208 | 0.10359258 | ||||||||
| Interim | December 18, 2009 | 0.15305538 | 0.12550541 | |||||||||
| 2008 | Ordinary | July 2, 2009 | 0.23209553 | 0.19031833 | ||||||||
| (1) | Net of Spanish withholding tax. The Spanish withholding tax rate was 18% in 2008 and 2009 and 19% in 2010. |
26
Table of Contents
| Period | Average | |||||||||||||||
Annual Data (Year Ended December 31,) | End ($) | Rate ($)(1) | High ($) | Low ($) | ||||||||||||
| 2006 | 1.3197 | 1.2661 | 1.3327 | 1.1860 | ||||||||||||
| 2007 | 1.4603 | 1.3797 | 1.4862 | 1.2904 | ||||||||||||
| 2008 | 1.3919 | 1.4698 | 1.6010 | 1.2446 | ||||||||||||
| 2009 | 1.4332 | 1.3955 | 1.5100 | 1.2547 | ||||||||||||
| 2010 | 1.3269 | 1.3261 | 1.4536 | 1.1959 | ||||||||||||
Interim Data (Six Months Ended June 30,) | ||||||||||||||||
| 2010 | 1.2291 | 1.3183 | 1.4536 | 1.1959 | ||||||||||||
| 2011 | 1.4523 | 1.4211 | 1.4875 | 1.2944 | ||||||||||||
| (1) | The average of the Noon Buying Rates for the euro on the last day reported of each month during the relevant period. |
Recent Monthly Data | High ($) | Low ($) | ||||||
| March 2011 | 1.4212 | 1.3813 | ||||||
| April 2011 | 1.4821 | 1.4211 | ||||||
| May 2011 | 1.4875 | 1.4015 | ||||||
| June 2011 | 1.4675 | 1.4155 | ||||||
| July 2011 | 1.4508 | 1.4014 | ||||||
| August 2011 | 1.4510 | 1.4158 | ||||||
| September 2011 | 1.4283 | 1.3518 | ||||||
| October 2011 (through October 14) | 1.3861 | 1.3281 | ||||||
27
Table of Contents
| The Offering of the Exchange Notes | The existing notes were originally issued by Giant Funding Corp. (the “Escrow Corp.”), an escrow company formed solely for the purpose of issuing the existing notes, on January 21, 2011 in an offering not registered under the Securities Act. On June 1, 2011, upon consummation of the acquisition, Escrow Corp. was merged with and into the Issuer, and the Issuer assumed all of Escrow Corp.’s obligations under the existing notes and the indenture governing the existing notes. In addition, upon consummation of the acquisition, the Issuer, the Parent Guarantor (as defined below) and the initial Subsidiary Guarantors (as defined below) executed joinders to a registration rights agreement that had been entered into by Escrow Corp. and the initial purchasers of the existing notes on January 21, 2011. Pursuant to the registration rights agreement, the Issuer, the Parent Guarantor and the Subsidiary Guarantors party thereto agreed, for the benefit of the holders of the existing notes, at our cost, to file the registration statement of which this prospectus forms a part and to complete an exchange offer for the existing notes. This exchange offer is intended to satisfy that obligation. | |
| The Exchange Offer | We are offering to exchange the exchange notes that have been registered under the Securities Act for the existing notes. As of this date, there is an aggregate principal amount of $1,100,000,000 of our existing notes outstanding. | |
| Required Representations | In order to participate in this exchange offer, you will be required to make certain representations to us in a letter of transmittal, including that: | |
| • any exchange notes will be acquired by you in the ordinary course of your business; | ||
| • you have not engaged in and do not intend to engage in, and do not have an arrangement or understanding with any person to participate in, a distribution of the exchange notes; and | ||
| • you are not an “affiliate” of our company or any of our subsidiaries, as that term is defined in Rule 405 of the Securities Act. | ||
| If our belief is inaccurate and you transfer any exchange note issued to you in the exchange offer without delivering a prospectus meeting the requirements of the Securities Act or without an exemption from registration of your exchange notes from such requirements, you may incur liability under the Securities Act. We do not assume, or indemnify you against, any such liability. The SEC has not considered this exchange offer in the context of a no-action letter, and we cannot be sure that the SEC would make the |
28
Table of Contents
| same determination with respect to this exchange offer as it has in other circumstances. | ||
| Each broker-dealer that is issued exchange notes for its own account in exchange for existing notes that were acquired by such broker-dealer as a result of market-making or other trading activities also must acknowledge that it has not entered into any arrangement or understanding with us or any of our affiliates to distribute the exchange notes and will deliver a prospectus meeting the requirements of the Securities Act in connection with any resale of the exchange notes issued in the exchange offer. | ||
| We have agreed in the registration rights agreement that a broker-dealer may use this prospectus for an offer to resell, resale or other retransfer of the exchange notes issued to it in the exchange offer. | ||
| Expiration Date | The exchange offer will expire at 5:00 p.m., New York City time, on , 2011, unless extended, in which case the term “expiration date” shall mean the latest date and time to which we extend the exchange offer. | |
| Conditions to the Exchange Offer | The exchange offer is subject to certain customary conditions, which may be waived by us. The exchange offer is not conditioned upon any minimum principal amount of existing notes being tendered. | |
| Procedures for Tendering Existing Notes | If you wish to tender existing notes, you must (a)(1) complete, sign and date the letter of transmittal, or a facsimile of it, according to its instructions and (2) send the letter of transmittal, together with your existing notes to be exchanged and other required documentation, to the Exchange Agent (as defined below) at the address provided in the letter of transmittal; or (b) tender through the Depository Trust Company (“DTC”) pursuant to DTC’s Automated Tender Offer Program, or ATOP system. The letter of transmittal or a valid agent’s message through ATOP must be received by the Exchange Agent by 5:00 p.m., New York City time, on the expiration date. See “The Exchange Offer — Procedures for Tendering,” and “— Book-Entry Tender.” By executing the letter of transmittal, you are representing to us that you are acquiring the exchange notes in the ordinary course of your business, that you are not participating, do not intend to participate and have no arrangement or understanding with any person to participate in the distribution of exchange notes, and that you are not an “affiliate” of ours. See “The Exchange Offer — Procedures for Tendering,” and “— Book-Entry Tender.” | |
| Do not send letters of transmittal and certificates representing existing notes to us. Send these documents only to the Exchange Agent. See “The Exchange Offer — Procedures for Tendering” for more information. | ||
| Special Procedures for Beneficial Owners | If you are the beneficial owner of book-entry interests and your name does not appear on a security position listing of DTC as the holder of the book-entry interests or if you are a beneficial owner whose existing notes are registered in the name of a broker, dealer, |
29
Table of Contents
| commercial bank, trust company or other nominee, and you wish to tender your existing notes in the exchange offer, you should contact the registered holder promptly and instruct the registered holder to tender on your behalf. If you are a beneficial owner and wish to tender on your own behalf, you must, before completing and executing the letter of transmittal and delivering your existing notes, either make appropriate arrangements to register ownership of the existing notes in your name or obtain a properly completed bond power from the registered holder. See “The Exchange Offer — Procedure if the Existing Notes Are Not Registered in Your Name,” and “— Beneficial Owner Instructions to Holders of Existing Notes.” The transfer of registered ownership may take considerable time and may not be possible to complete before the expiration date. | ||
| Guaranteed Delivery Procedures | If you wish to tender existing notes and time will not permit the documents required by the letter of transmittal to reach the exchange agent prior to the expiration date, or the procedure for book-entry transfer cannot be completed on a timely basis, you must tender your existing notes according to the guaranteed delivery procedures described under “The Exchange Offer — Guaranteed Delivery Procedures.” | |
| Acceptance of Existing Notes and Delivery of Exchange Notes | Subject to the conditions described under “The Exchange Offer — Conditions,” we will accept for exchange any and all existing notes which are validly tendered in the exchange offer and not withdrawn, prior to 5:00 p.m., New York City time, on the expiration date. | |
| Interest on Existing Notes | Interest will not be paid on existing notes that are tendered and accepted for exchange in the exchange offer. | |
| Withdrawal Rights | You may withdraw your tender of existing notes at any time prior to 5:00 p.m., New York City time, on the expiration date, subject to compliance with the procedures for withdrawal described in this prospectus under the heading “The Exchange Offer — Withdrawal of Tenders.” | |
| Federal Income Tax Consequences | For a discussion of the material federal income tax considerations relating to the exchange of existing notes for the exchange notes as well as the ownership of the exchange notes, see “Certain Material United States Federal Income Tax Considerations.” | |
| Exchange Agent | The Bank of New York Mellon Trust Company, N.A. is serving as the exchange agent (the “Exchange Agent”). The address, telephone number and facsimile number of the exchange agent are set forth in this prospectus under the heading “The Exchange Offer — Exchange Agent.” The Bank of New York Mellon Trust Company, N.A. is also the trustee under the indenture, as supplemented, among the Issuer, the Parent Guarantor, the Subsidiary Guarantors party thereto and The Bank of New York Mellon Trust Company, N.A. governing the notes, as described under “Description of the Notes.” |
30
Table of Contents
| Consequences of Failure to Exchange the Existing Notes | If you do not exchange existing notes for exchange notes, you will continue to be subject to the restrictions on transfer provided in the existing notes and in the indenture governing the existing notes. In general, the unregistered existing notes may not be offered or sold, unless they are registered under the Securities Act, except pursuant to an exemption from, or in a transaction not subject to, the Securities Act and applicable state securities laws. | |
| In addition, after the consummation of the exchange offer, it is anticipated that the outstanding principal amount of the existing notes available for trading will be significantly reduced. The reduced float will adversely affect the liquidity and market price of the existing notes. A smaller outstanding principal amount at maturity of existing notes available for trading may also tend to make the price more volatile. | ||
| Use of Proceeds | We will not receive any proceeds from the issuance of the exchange notes in exchange for the existing notes. | |
| Fees and Expenses | We will pay all fees and expenses related to this exchange offer. |
31
Table of Contents
| Issuer | Grifols Inc., a Virginia corporation (the “Issuer”). | |
| Securities Offered | $1,100,000,000 aggregate principal amount of 8.25% senior notes due 2018. | |
| Maturity Date | February 1, 2018. | |
| Interest Rate | 8.25% per year. | |
| Interest Payment Dates | February 1 and August 1, beginning on February 1, 2012. | |
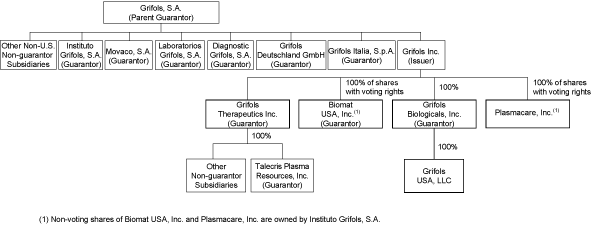
| Guarantees | The exchange notes will be fully and unconditionally guaranteed on a joint and several senior unsecured basis by Grifols, S.A., a company incorporated under the laws of the Kingdom of Spain (the “Parent Guarantor”) and each of the Parent Guarantor’s existing and future direct or indirect subsidiaries that guarantee our Senior Credit Facilities (other than any foreign subsidiary of Grifols Inc.) (each a “Subsidiary Guarantor” and together with the Parent Guarantor, the “Guarantors”). On the issue date of the exchange notes, the Subsidiary Guarantors will consist of: Grifols Biologicals Inc., Biomat USA, Inc., Grifols Therapeutics Inc., Talecris Plasma Resources, Inc., Instituto Grifols, S.A., Diagnostic Grifols, S.A., Movaco, S.A., Laboratorios Grifols, S.A., Grifols Italia, S.p.A., and Grifols Deutschland GmbH. | |
| Ranking | The exchange notes will be senior unsecured obligations of the Issuer and will: | |
| • rank equally in right of payment to all of the Issuer’s existing and future senior indebtedness; | ||
| • be effectively subordinated in right of payment to the Issuer’s secured indebtedness (including its obligations under our Senior Credit Facilities), to the extent of the value of the collateral securing such indebtedness; and | ||
| • be structurally subordinated to all existing and future liabilities of each non-guarantor subsidiary of the Parent Guarantor. | ||
| The guarantees will be the senior unsecured obligations of the Guarantors and will: | ||
| • rank equally in right of payment to all existing and future senior indebtedness of the Guarantors; | ||
| • be effectively subordinated in right of payment to the Guarantors’ secured indebtedness (including their obligations under our Senior Credit Facilities), to the extent of the value of the collateral securing such indebtedness; and | ||
| • be structurally subordinated to all existing and future liabilities of each non-guarantor subsidiary of Parent Guarantor. |
32
Table of Contents
| Optional Redemption | The Issuer may redeem some or all of the exchange notes at any time prior to February 1, 2014 at a price equal to 100% of the principal amount of the exchange notes plus a “make-whole” premium as set forth under “Description of the Notes — Optional Redemption.” Additionally, the Issuer may redeem the exchange notes, in whole or in part, at any time on and after February 1, 2014 at the redemption prices set forth under “Description of the Notes — Optional Redemption.” | |
| Optional Redemption After Equity Offerings | The Issuer may redeem up to 35% of the notes with money raised in one or more equity offerings by the Parent Guarantor at any time (which may be more than once) prior to February 1, 2014, as long as at least 65% of the aggregate principal amount of notes issued under the indenture remains outstanding immediately following any such offerings. See “Description of the Notes — Optional Redemption.” | |
| Change of Control Offer | If we experience a change of control, the Issuer must give holders of the exchange notes the opportunity to sell the Issuer their exchange notes at 101% of their face amount, plus accrued and unpaid interest. See “Description of the Notes — Repurchase at the Option of Holders — Change of Control.” | |
| Certain Indenture Provisions | The exchange notes will be, and the existing notes are, governed by an indenture containing covenants that, among other things, restrict the Parent Guarantor’s ability and the ability of its restricted subsidiaries, including the Issuer, to: | |
| • pay dividends or make certain other restricted payments or investments; | ||
| • incur additional indebtedness or provide guarantees of indebtedness and issue disqualified stock; | ||
| • create liens on assets; | ||
| • merge, consolidate or sell all or substantially all of our and our restricted subsidiaries’ assets; | ||
| • enter into certain transactions with affiliates; | ||
| • create restrictions on dividends or other payments by our restricted subsidiaries; and | ||
| • create guarantees of indebtedness by restricted subsidiaries. | ||
| These covenants are subject to a number of important limitations and exceptions. See “Description of the Notes — Certain Covenants.” | ||
| No Prior Market for Exchange Notes | The exchange notes will be new securities for which there is no market. We cannot assure you a liquid market for the exchange notes will develop or be maintained. |
33
Table of Contents
34
Table of Contents
| • | our high level of indebtedness makes it more difficult for us to satisfy our obligations with respect to the notes; | |
| • | our high level of indebtedness makes us more vulnerable to economic downturns and adverse developments in our business; | |
| • | our ability to obtain additional financing for working capital, capital expenditures, acquisitions or general corporate purposes may be impaired; | |
| • | we must use a substantial portion of our cash flow from operations to pay interest on the notes and our other indebtedness, which will reduce the funds available to us for operations and other purposes; | |
| • | all of the indebtedness outstanding under our purchase money indebtedness, equipment financing and real estate mortgages will have a prior ranking claim on the underlying assets; | |
| • | our ability to fund a change of control offer may be limited; |
35
Table of Contents
| • | our high level of indebtedness could place us at a competitive disadvantage compared to our competitors that may have proportionately less debt; | |
| • | our flexibility in planning for, or reacting to, changes in our business and the industry in which we operate may be limited; and | |
| • | we may be restricted from making strategic acquisitions or exploiting other business opportunities. |
36
Table of Contents
37
Table of Contents
| • | incur or assume liens or additional debt or provide guarantees in respect of obligations of other persons; | |
| • | issue redeemable stock and preferred equity; | |
| • | pay dividends or distributions or redeem or repurchase capital stock; | |
| • | prepay, redeem or repurchase debt; | |
| • | make loans, investments and capital expenditures; | |
| • | enter into agreements that restrict distributions from our subsidiaries; | |
| • | sell assets and capital stock of our subsidiaries; | |
| • | enter into certain transactions with affiliates; and | |
| • | consolidate or merge with or into, or sell substantially all of our assets to, another person. |
38
Table of Contents
| • | it was insolvent or rendered insolvent by reason of issuing the guarantee; |
39
Table of Contents
| • | it was engaged, or about to engage, in a business or transaction for which its remaining unencumbered assets constituted unreasonably small capital to carry on its business; | |
| • | it intended to incur, or believed that it would incur, debts beyond its ability to pay as they mature; or | |
| • | it was a defendant in an action for money damages, or had a judgment for money damages docketed against it if, in either case, after final judgment, the judgment is unsatisfied, |
40
Table of Contents
41
Table of Contents
42
Table of Contents
43
Table of Contents
44
Table of Contents
45
Table of Contents
46
Table of Contents
47
Table of Contents
48
Table of Contents
49
Table of Contents
50
Table of Contents
51
Table of Contents
52
Table of Contents
53
Table of Contents
| • | A reduction in the donor pool. Regulators in most of the largest markets for plasma derivative products, including the United States, restrict the use of plasma collected from specific countries and regions in the manufacture of plasma derivative products. For example, the appearance of the variant Creutzfeldt-Jakob disease, commonly referred to as “mad cow” disease (which resulted in the suspension of the use of plasma collected from U.K. residents), and concern over the safety of blood products (which has led to increased domestic and foreign regulatory control over the collection and testing of plasma and the disqualification of certain segments of the population from the donor pool) have significantly reduced the potential donor pool. The appearance of new viral strains could further reduce the potential donor pool. Also, improvements in socio-economic conditions in the areas where |
54
Table of Contents
| our and our suppliers’ collection centers are located can reduce the attractiveness of financial incentives for donors, resulting in increased donor feesand/or a reduction in the number of donors. |
| • | Regulatory requirements. The collection of plasma is heavily regulated, and our ability to collect plasma (or to increase plasma collection) through our collection centers, or to obtain plasma from other suppliers, may be limited or disrupted by the inability to obtain or maintain necessary regulatory licenses to operate plasma collection centers in a timely manner or at all, or by the temporary or permanent shutdown of our or our suppliers’ plasma collection centers as a result of regulatory violations. | |
| • | Plasma supply sources. In recent years, there has been vertical integration in the industry as plasma derivatives manufacturers have been acquiring plasma collectors. Plasma availability in the United States grew from approximately 13.7 million liters in 2002 to approximately 18.3 million liters in 2010, while the number of plasma collection centers declined from 407 to 396 during the same period. Any significant disruption in supply of plasma or an increased demand for plasma may require plasma from alternative sources, which may not be available on a timely basis. |
55
Table of Contents
56
Table of Contents
57
Table of Contents
| • | decreased demand for our products and any product candidates that we may develop; | |
| • | injury to our reputation; | |
| • | withdrawal of clinical trial participants; | |
| • | costs to defend the related litigation; | |
| • | substantial monetary awards to trial participants or patients; | |
| • | loss of revenue; and | |
| • | the inability to commercialize any products that we may develop. |
58
Table of Contents
59
Table of Contents
60
Table of Contents
61
Table of Contents
62
Table of Contents
63
Table of Contents
| • | regulators or institutional review boards may not authorize us to commence a clinical trial or conduct a clinical trial within a country or at a prospective trial site respectively; | |
| • | the regulatory requirements for product approval may not be explicit, may evolve over time and may diverge by jurisdiction; |
64
Table of Contents
| • | our preclinical tests or clinical trials may produce negative or inconclusive results, and we may decide, or regulators may require us, to conduct additional preclinical testing or clinical trials or we may abandon projects that we had expected to be promising; | |
| • | the number of patients required for our clinical trials may be larger than we anticipate, enrollment in our clinical trials may be slower than we currently anticipate, or participants may drop out of our clinical trials at a higher rate than we anticipate, any of which would result in significant delays; | |
| • | our third-party contractors may fail to comply with regulatory requirements or meet their contractual obligations to us in a timely manner; | |
| • | we might have to suspend or terminate our clinical trials if the participants are being exposed to unacceptable health risks or if any participant experiences an unexpected serious adverse event; | |
| • | regulators or institutional review boards may require that we hold, suspend or terminate clinical research for various reasons, including noncompliance with regulatory requirements; | |
| • | undetected or concealed fraudulent activity by a clinical researcher, if discovered, could preclude the submission of clinical data prepared by that researcher, lead to the suspension or substantive scientific review of one or more of our marketing applications by regulatory agencies, and result in the recall of any approved product distributed pursuant to data determined to be fraudulent; | |
| • | the cost of our clinical trials may be greater than we anticipate; | |
| • | the supply or quality of our product candidates or other materials necessary to conduct our clinical trials may be insufficient or inadequate because we do not currently have any agreements with third-party manufacturers for the long-term commercial supply of any of our product candidates; | |
| • | an audit of preclinical or clinical studies by the FDA or other regulatory authority may reveal noncompliance with applicable regulations, which could lead to disqualification of the results and the need to perform additional studies; and | |
| • | the effects of our product candidates may not achieve the desired clinical benefits or may cause undesirable side effects or the product candidates may have other unexpected characteristics. |
| • | be delayed in obtaining marketing approval for our product candidates; | |
| • | not be able to obtain marketing approval; | |
| • | not be able to obtain reimbursement for our products in some countries; | |
| • | obtain approval for indications that are not as broad as intended; or | |
| • | have the product removed from the market after obtaining marketing approval. |
65
Table of Contents
| • | the prevalence and severity of any side effects; | |
| • | the efficacy and potential advantages over alternative treatments; | |
| • | the ability to offer our product candidates for sale at competitive prices; | |
| • | relative convenience and ease of administration; | |
| • | the willingness of the target patient population to try new therapies and of physicians to prescribe these therapies; | |
| • | the strength of marketing and distribution support; and | |
| • | sufficient third-party coverage or reimbursement. |
66
Table of Contents
67
Table of Contents
68
Table of Contents
69
Table of Contents
70
Table of Contents
71
Table of Contents
| As of | ||||
| June 30, 2011 | ||||
| Actual | ||||
| € in thousands | ||||
Cash and cash equivalents(1) | 583,792 | |||
Long-term debt: | ||||
The notes(2) | 761,088 | |||
| Senior secured credit facilities: | ||||
Revolving Credit Facilities(3) | — | |||
| Senior Term Loan — Tranche A (USD) | 830,277 | |||
| Senior Term Loan — Tranche A (EUR) | 213,125 | |||
| Senior Term Loan — Tranche B (USD) | 892,721 | |||
| Senior Term Loan — Tranche B (EUR) | 217,800 | |||
Other bank loans(4) | 18,391 | |||
Capital lease obligations(5) | 28,947 | |||
Total long-term debt(6) | 2,962,349 | |||
Short-term debt: | ||||
Talecris Old Notes(7) | 427,691 | |||
Promissory Notes(8) | 9,990 | |||
| Senior secured credit facilities: | ||||
| Senior Term Loan — Tranche A (USD) | 6,746 | |||
| Senior Term Loan — Tranche A (EUR) | 6,875 | |||
| Senior Term Loan — Tranche B (USD) | 0 | |||
| Senior Term Loan — Tranche B (EUR) | 2,200 | |||
Other bank loans(4) | 74,673 | |||
Capital lease obligations(5) | 8,657 | |||
Total short-term debt(9) | 536,832 | |||
| Total equity | 1,513,594 | |||
Total capitalization | 5,012,775 | |||
| (1) | At June 30, 2011, cash and cash equivalents included €428 million held in a restricted cash account in connection with the redemption of the 7.75% senior unsecured notes due 2016 (the “Talecris Old Notes”) issued by Talecris in October 2009, which were subsequently redeemed on July 1, 2011. We paid a €78 million make-whole premium payment in connection with the redemption of the Talecris Old Notes. We extinguished this existing debt in connection with the acquisition and related refinancing and in accordance with the terms of our Senior Credit Facilities. | |
| (2) | Represents the $1.1 billion aggregate principal amount of 8.25% senior notes due 2018. | |
| (3) | Represents our Revolving Credit Facilities in the amount of $50 million, €36.7 million and the $200 million equivalent in multicurrencies. As of June 30, 2011, there were no drawings for cash or letters of credit outstanding against the facilities. See the section in this prospectus entitled “Management’s Discussion and Analysis of Financial Condition and Results of Operations— Liquidity and Capital Resources” for a more detailed discussion of the terms of the Revolving Credit Facilities. | |
| (4) | Represents short and long-term credit facilities in various currencies with various lenders of both us and our subsidiaries. We have €189,476,000 of aggregate availability under our short-term credit facilities. | |
| (5) | Represents short and long-term capital lease obligations in various currencies of both us and our subsidiaries. | |
| (6) | Excludes amortized deferred expenses in the amount of €299,840,000. | |
| (7) | All of the outstanding Talecris Old Notes were subsequently redeemed on July 1, 2011. | |
| (8) | Represents multiple one-year promissory notes issued by our subsidiary Instituto Grifols, S.A. to certain of its employees in 2011. These promissory notes bear interest at a rate of 5.00% per year. | |
| (9) | Excludes amortized deferred expenses and accrued interest in the amount of €29,458,000. |
72
Table of Contents
| Six Months | ||||||||||||||||||||||
| Ended | ||||||||||||||||||||||
| June 30, | Year Ended December 31, | |||||||||||||||||||||
2011 | 2010 | 2009 | 2008 | 2007 | 2006 | |||||||||||||||||
| 2.6x | 3.9x | 7.8x | 6.6x | 6.0x | 2.5x | |||||||||||||||||
73
Table of Contents
| • | file a registration statement for the exchange offer with the SEC and cause the registration statement to become effective under the Securities Act within 365 days after the issue date of the outstanding notes; | |
| • | consummate the exchange offer within 60 days after the effectiveness date of the registration statement; and | |
| • | file a shelf registration statement for the resale of the outstanding notes under certain circumstances and cause such registration statement to become effective under the Securities Act within the later of (a) 365 days after the issue date of the outstanding notes and (b) 90 days following a request to file such shelf registration statement by an initial purchaser or certain other holder. |
74
Table of Contents
| • | will not be able to tender its existing notes in the exchange offer; | |
| • | will not be able to rely on the interpretations of the staff of the SEC; and | |
| • | must comply with the registration and prospectus delivery requirements of the Securities Act in connection with any sale or transfer of the existing notes unless such sale or transfer is made pursuant to an exemption from these requirements. |
| • | any exchange notes to be received by you will be acquired in the ordinary course of your business; | |
| • | you have no arrangement or understanding with any person to participate in the distribution of the exchange notes in violation of the provisions of the Securities Act; | |
| • | you are not our “affiliate” (within the meaning of Rule 405 promulgated under the Securities Act); | |
| • | if you are not a broker-dealer, you are not engaged in, and do not intend to engage in, a distribution of exchange notes; and | |
| • | if you are a broker-dealer, you acquired the existing notes for your own account as a result of market-making or other trading activities (and as such, you are a “participating broker-dealer”), you have not entered into any arrangement or understanding with us or any of our affiliates to distribute the exchange notes and you will deliver a prospectus meeting the requirements of the Securities Act in connection with any resale of the exchange notes. |
75
Table of Contents
| • | the offering of the exchange notes has been registered under the Securities Act; | |
| • | the exchange notes generally will not be subject to transfer restrictions or have registration rights; and | |
| • | certain provisions relating to special interest on the existing notes provided for under certain circumstances will be eliminated. |
| • | notify the exchange agent of any extension orally (confirmed in writing) or in writing; and | |
| • | notify the registered holders of the existing notes by means of a press release or other public announcement, each before 9:00 a.m., New York City time, on the next business day after the previously scheduled expiration date. |
| • | to delay accepting any existing notes; | |
| • | to extend the exchange offer; or | |
| • | if any conditions listed below under “— Conditions” are not satisfied, to terminate the exchange offer by giving oral or written notice of the delay, extension or termination to the exchange agent. |
76
Table of Contents
| • | complete, sign and date the enclosed letter of transmittal, or a copy of it; | |
| • | have the signature on the letter of transmittal guaranteed if required by the letter of transmittal or transmit an agent’s message in connection with a book-entry transfer; and | |
| • | mail, fax or otherwise deliver the letter of transmittal or copy to the exchange agent before the expiration date. |
| • | the exchange agent must receive a timely confirmation of a book-entry transfer of your existing notes, if that procedure is available, into the account of the exchange agent at DTC, the “book-entry transfer facility,” under the procedure for book-entry transfer described below before the expiration date; | |
| • | the exchange agent must receive certificates for your existing notes, the letter of transmittal and other required documents before the expiration date; or | |
| • | you must comply with the guaranteed delivery procedures described below. |
| • | to participate in ATOP; | |
| • | to be bound by the terms of the letter of transmittal; and | |
| • | that we may enforce the agreement against the participant. |
77
Table of Contents
78
Table of Contents
| • | the tender is made through an Eligible Institution (as defined in the Letter of Transmittal), | |
| • | prior to the expiration date, the exchange agent receives from such Eligible Institution a properly completed and duly executed notice of guaranteed delivery, by facsimile transmittal, mail or hand delivery, | |
| • | stating the name and address of the holder, the certificate number or numbers of such holder’s existing notes and the principal amount of such existing notes tendered; | |
| • | stating that the tender is being made thereby; | |
| • | guaranteeing that, within three New York Stock Exchange trading days after the expiration date, the letter of transmittal, or a facsimile thereof, together with the certificate(s) representing the existing notes to be tendered in proper form for transfer, or an agent’s message and confirmation of a book-entry transfer into the exchange agent’s account at DTC of existing notes delivered electronically, and any other documents required by the letter of transmittal, will be deposited by the Eligible Institution with the exchange agent; and | |
| • | such properly completed and executed letter of transmittal, or a facsimile thereof, together with the certificate(s) representing all tendered existing notes in proper form for transfer, or an agent’s message and confirmation of a book-entry transfer into the exchange agent’s account at DTC of existing notes delivered electronically and all other documents required by the letter of transmittal are received by the exchange agent within three New York Stock Exchange trading days after the expiration date. |
79
Table of Contents
| • | specify the name of the person who deposited the existing notes to be withdrawn; | |
| • | identify the existing notes to be withdrawn, including the certificate number or number and principal amount of such existing notes or, in the case of existing notes transferred by book-entry transfer, the name and number of the account at DTC to be credited; and | |
| • | be signed in the same manner as the original signature on the letter of transmittal by which such existing notes were tendered, including any required signature guarantee. |
| • | the exchange offer violates applicable law, rules or regulations or an applicable interpretation of the staff of the SEC; | |
| • | an action or proceeding has been instituted or threatened in any court or by any governmental agency that might materially impair our ability to proceed with the exchange offer; | |
| • | there has been proposed, adopted or enacted any law, rule or regulation that, in our reasonable judgment, would impair materially our ability to consummate the exchange offer; or | |
| • | all governmental approvals that we deem necessary for the completion of the exchange offer have not been obtained. |
| • | refuse to accept any existing notes and return all tendered existing notes to you; | |
| • | extend the exchange offer and retain all existing notes tendered before the exchange offer expires, subject, however, to your rights to withdraw the existing notes; or | |
| • | waive the unsatisfied conditions with respect to the exchange offer and accept all properly tendered existing notes that have not been withdrawn. |
80
Table of Contents
81
Table of Contents
| • | to a person whom the seller reasonably believes is a qualified institutional buyer in a transaction meeting the requirements of Rule 144A promulgated under the Securities Act; | |
| • | in a transaction meeting the requirements of Rule 144 promulgated under the Securities Act; | |
| • | outside the United States to a foreign person in a transaction meeting the requirements of Rule 903 or 904 of Regulation S promulgated under the Securities Act; | |
| • | in accordance with another exemption from the registration requirements of the Securities Act and based upon an opinion of counsel if we so request; | |
| • | to us; or | |
| • | pursuant to an effective registration statement. |
82
Table of Contents
83
Table of Contents
84
Table of Contents
85
Table of Contents
86
Table of Contents
87
Table of Contents
| Depreciation | ||||
| Method | Rates | |||
| Buildings | Straight line | 1%-3% | ||
| Plant and machinery | Straight line | 8%-10% | ||
| Other installations, equipment and furniture | Straight line | 10%-30% | ||
| Other property, plant and equipment | Straight line | 16%-25% |
| Amortization | Estimated Years of | |||
| Method | Useful Life | |||
| Development expenses | Straight line | 3 - 5 | ||
| Concessions, patents, licenses, trademarks and similar | Straight line | 5 - 15 | ||
| Software | Straight line | 3 - 6 |
| • | we have technical studies justifying the feasibility of the production process; | |
| • | we have undertaken a commitment to complete production of the asset whereby it is in condition for sale or internal use; | |
| • | the asset will generate sufficient future economic benefits; | |
| • | we have sufficient financial and technical resources to complete development of the asset and have developed budget and cost accounting control systems that allow budgeted costs, introduced changes and costs actually assigned to different projects to be monitored. |
88
Table of Contents
| Balances at | Balances at | |||||||||||||||||||
| December 31, | Business | Translation | June 30, | |||||||||||||||||
| 2010 | combinations | Impairment | Differences | 2011 | ||||||||||||||||
Net value | ||||||||||||||||||||
| Grifols UK, Ltd. | 7,982 | 0 | 0 | (370 | ) | 7,612 | ||||||||||||||
| Grifols Italia, S.p.A. | 6,118 | 0 | 0 | 0 | 6,118 | |||||||||||||||
| Biomat USA, Inc. | 113,052 | 0 | 0 | (8,534 | ) | 104,518 | ||||||||||||||
| Plasmacare, Inc. | 38,464 | 0 | 0 | (2,903 | ) | 35,561 | ||||||||||||||
| Woolloomooloo Holdings Pty Ltd. (Australia) | 23,832 | 0 | (13,000 | ) | (415 | ) | 10,417 | |||||||||||||
Talecris Biotherapeutics, Inc.(1) | 0 | 2,124,082 | 0 | (6,612 | ) | 2,117,470 | ||||||||||||||
| 189,448 | 2,124,082 | (13,000 | ) | (18,834 | ) | 2,281,696 | ||||||||||||||
| (1) | The name of Talecris Biotherapeutics, Inc. was changed to Grifols Therapeutics Inc. in August 2011. |
89
Table of Contents
| 12/31/2010 | ||||
| Growth rate | Pre-tax discount rate | |||
| Bioscience | 2.0% - 3.0% | 10.5% - 10.9% | ||
| Diagnostic | 2.0% | 10.4% | ||
| 06/30/2011 | ||||
| Growth rate | Pre-tax discount rate | |||
| Bioscience | N/A | N/A | ||
| Diagnostic | 2.0% | 11.5% | ||
90
Table of Contents
| • | Raw materials and other supplies: replacement cost. Nevertheless, raw materials are not written down below cost if the finished goods into which they will be incorporated are expected to be sold at or above cost of production. | |
| • | Goods for resale and finished goods: estimated selling price, less costs to sell. | |
| • | Work in progress: the estimated selling price of related finished goods, less the estimated costs of completion and the estimated costs necessary to make the sale. |
91
Table of Contents
| Six Months Ended | ||||||||||||||||||||
| June 30, | Years Ended December 31, | |||||||||||||||||||
| 2011 | 2010 | 2010 | 2009 | 2008 | ||||||||||||||||
| Revenues | 635,341 | 487,809 | 990,730 | 913,186 | 814,311 | |||||||||||||||
| Changes in inventories of finished goods and work in progress | 2,757 | 41,209 | 45,749 | 73,093 | 31,058 | |||||||||||||||
| Self-constructed non-current assets | 32,346 | 16,051 | 33,513 | 41,142 | 25,794 | |||||||||||||||
| Supplies | (175,142 | ) | (157,107 | ) | (304,818 | ) | (286,274 | ) | (206,738 | ) | ||||||||||
| Other operating income | 1,009 | 631 | 1,196 | 1,443 | 1,289 | |||||||||||||||
| Personnel expenses | (183,727 | ) | (141,972 | ) | (289,008 | ) | (273,168 | ) | (238,159 | ) | ||||||||||
| Other operating expenses | (155,532 | ) | (98,279 | ) | (205,260 | ) | (203,381 | ) | (192,288 | ) | ||||||||||
| Amortisation and depreciation | (28,156 | ) | (21,434 | ) | (45,776 | ) | (39,554 | ) | (33,256 | ) | ||||||||||
| Transaction costs of Talecris business combination | (38,607 | ) | (2,019 | ) | (16,999 | ) | — | — | ||||||||||||
| Non-financial and other capital grants | 742 | 550 | 728 | 1,188 | 2,941 | |||||||||||||||
| Impairment and gains/(losses) on disposal of fixed assets | (22,302 | ) | 681 | (372 | ) | (1,147 | ) | (1,991 | ) | |||||||||||
Results from operating activities | 68,729 | 126,120 | 209,683 | 226,528 | 202,961 | |||||||||||||||
| Finance income | 1,761 | 2,179 | 4,526 | 7,067 | 2,682 | |||||||||||||||
| Finance expenses | (55,546 | ) | (25,285 | ) | (49,660 | ) | (27,087 | ) | (29,305 | ) | ||||||||||
| Change in fair value of financial instruments | 13,945 | (15,404 | ) | (7,593 | ) | (587 | ) | (l,268 | ) | |||||||||||
| Gains/(losses) on disposal of financial instruments | — | — | 91 | (245 | ) | — | ||||||||||||||
| Exchange gains/(losses) | (2,122 | ) | 1,970 | 1,616 | (1,733 | ) | (2,825 | ) | ||||||||||||
Finance expense | (41,962 | ) | (36,540 | ) | (51,020 | ) | (22,585 | ) | (30,716 | ) | ||||||||||
| Share of loss of equity accounted investees | (807 | ) | (728 | ) | (879 | ) | 51 | 24 | ||||||||||||
Profit before income tax | 25,960 | 88,852 | 157,784 | 203,994 | 172,269 | |||||||||||||||
| Income tax expense | (7,347 | ) | (23,022 | ) | (42,517 | ) | (56,424 | ) | (50,153 | ) | ||||||||||
Consolidated profit for the period | 18,613 | 65,830 | 115,267 | 147,570 | 122,116 | |||||||||||||||
| Six Months Ended | ||||||||||||||||
| June 30, | Change | |||||||||||||||
| 2011 | 2010 | € | % | |||||||||||||
| Revenues | 635,341 | 487,809 | 147,532 | 30.2 | % | |||||||||||
| Changes in inventories of finished goods and work in progress | 2,757 | 41,209 | (38,452 | ) | (93.3 | )% | ||||||||||
| Self-constructed non-current assets | 32,346 | 16,051 | 16,295 | 101.5 | % | |||||||||||
| Supplies | (175,142 | ) | (157,107 | ) | (18,035 | ) | 11.5 | % | ||||||||
| Other operating income | 1,009 | 631 | 378 | 59.9 | % | |||||||||||
| Personnel expenses | (183,727 | ) | (141,972 | ) | (41,755 | ) | 29.4 | % | ||||||||
| Other operating expenses | (155,532 | ) | (98,279 | ) | (57,253 | ) | 58.3 | % | ||||||||
| Amortisation and depreciation | (28,156 | ) | (21,434 | ) | (6,722 | ) | 31.4 | % | ||||||||
| Transaction costs of Talecris business combination | (38,607 | ) | (2,019 | ) | (36,588 | ) | 1812.2 | % | ||||||||
| Non-financial and other capital grants | 742 | 550 | 192 | 34.9 | % | |||||||||||
| Impairment and gains/(losses) on disposal of fixed assets | (22,302 | ) | 681 | (22,983 | ) | (3374.9 | )% | |||||||||
Results from operating activities | 68,729 | 126,120 | (57,391 | ) | (45.5 | )% | ||||||||||
92
Table of Contents
| Six Months Ended | ||||||||||||||||
| June 30, | Change | |||||||||||||||
| 2011 | 2010 | € | % | |||||||||||||
| Finance income | 1,761 | 2,179 | (418 | ) | (19.2 | )% | ||||||||||
| Finance expenses | (55,546 | ) | (25,285 | ) | (30,261 | ) | 119.7 | % | ||||||||
| Change in fair value of financial instruments | 13,945 | (15,404 | ) | 29,349 | 190.5 | % | ||||||||||
| Exchange gains/(losses) | (2,122 | ) | 1,970 | (4,092 | ) | (207.7 | )% | |||||||||
Finance expense | (41,962 | ) | (36,540 | ) | (5,422 | ) | 14.8 | % | ||||||||
| Share of loss of equity accounted investees | (807 | ) | (728 | ) | (79 | ) | 10.9 | % | ||||||||
Profit before income tax | 25,960 | 88,852 | (62,892 | ) | (70.8 | )% | ||||||||||
| Income tax expense | (7,347 | ) | (23,022 | ) | 15,675 | (68.1 | )% | |||||||||
Consolidated profit for the period | 18,613 | 65,830 | (47,217 | ) | (71.7 | )% | ||||||||||
| Six Months | Six Months | |||||||||||||||||||||||
| Ended | Ended | |||||||||||||||||||||||
| June 30, | % of total | June 30, | % of total | |||||||||||||||||||||
| 2011 | revenues | 2010 | revenues | % var | % var CC(a) | |||||||||||||||||||
| Bioscience | 521,538 | 82.1 | 380,081 | 77.9 | 37.2 | 40.9 | ||||||||||||||||||
| Diagnostic | 56,831 | 8.9 | 54,413 | 11.2 | 4.4 | 3.8 | ||||||||||||||||||
| Hospital | 49,289 | 7.8 | 45,146 | 9.3 | 9.2 | 8.8 | ||||||||||||||||||
| Raw Materials | 1,658 | 0.3 | 1,835 | 0.4 | (9.6 | ) | (6.8 | ) | ||||||||||||||||
| Others | 6,025 | 1.0 | 6,334 | 1.3 | (4.9 | ) | (4.9 | ) | ||||||||||||||||
Total | 635,341 | 100.0 | 487,809 | 100.0 | 30.2 | 33.0 | ||||||||||||||||||
93
Table of Contents
| (a) | Revenue variance in constant currency is determined by comparing adjusted current period revenue, calculated using prior period monthly average exchange rates, to the prior period revenue. This measure is considered to be a non-IFRS financial measure. Revenue variance in constant currency calculates revenue variance without the impact of foreign exchange fluctuations. We believe that constant currency revenue variance is an important measure of our operations because it neutralizes foreign exchange impact and better illustrates the underlying change in revenues from one year to the next. We believe that this presentation provides a more usefulperiod-over-period comparison as changes due solely to changes in exchange rates are eliminated. Revenue variance in constant currency, as defined and presented by us, may not be comparable to similar measures reported by other companies. Revenue variance in constant currency has limitations, particularly because the currency effects that are eliminated constitute a significant element of our revenue and expenses and could impact our performance significantly. We do not evaluate our results and performance without considering revenue variances in constant currency on the one hand and changes in revenue prepared in accordance with IFRS on the other. We caution the readers of this prospectus to follow a similar approach by considering data regarding constant currencyperiod-over-period revenue variance only in addition to, and not as a substitute for or superior to, other measures of financial performance prepared in accordance with IFRS. |
| Six Months | Six Months | |||||||||||||||||||||||
| Ended | Ended | |||||||||||||||||||||||
| June 30, | % of total | June 30, | % of total | |||||||||||||||||||||
| 2011 | revenues | 2010 | revenues | % var | % var CC* | |||||||||||||||||||
| European Union | 246,144 | 38.7 | 223,019 | 45.7 | 10.4 | 10.1 | ||||||||||||||||||
| United States and Canada | 267,124 | 42.0 | 157,948 | 32.4 | 69.1 | 79.3 | ||||||||||||||||||
| Rest of World | 122,073 | 19.0 | 106,842 | 21.9 | 14.3 | 12.4 | ||||||||||||||||||
Total | 635,341 | 100.0 | 487,809 | 100.0 | 30.2 | 33.0 | ||||||||||||||||||
| * | Please see footnote (a) to the table above. |
94
Table of Contents
95
Table of Contents
| Years Ended December 31, | Change | |||||||||||||||
| 2010 | 2009 | € | % | |||||||||||||
| Revenues | 990,730 | 913,186 | 77,544 | 8.5 | % | |||||||||||
| Changes in inventories of finished goods and work in progress | 45,749 | 73,093 | (27,344 | ) | (37.4 | )% | ||||||||||
| Self-constructed non-current assets | 33,513 | 41,142 | (7,629 | ) | (18.5 | )% | ||||||||||
| Supplies | (304,818 | ) | (286,274 | ) | (18,544 | ) | 6.5 | % | ||||||||
| Other operating income | 1,196 | 1,443 | (247 | ) | (17.1 | )% | ||||||||||
| Personnel expenses | (289,008 | ) | (273,168 | ) | (15,840 | ) | 5.8 | % | ||||||||
| Other operating expenses | (205,260 | ) | (203,381 | ) | (1,879 | ) | 0.9 | % | ||||||||
| Amortisation and depreciation | (45,776 | ) | (39,554 | ) | (6,222 | ) | 15.7 | % | ||||||||
| Transaction costs of Talecris business combination | (16,999 | ) | 0 | (16,999 | ) | 100 | % | |||||||||
| Non-financial and other capital grants | 728 | 1,188 | (460 | ) | (38.7 | )% | ||||||||||
| Impairment and gains/(losses) on disposal of fixed assets | (372 | ) | (1,147 | ) | 775 | (67.6 | )% | |||||||||
Results from operating activities | 209,683 | 226,528 | (16,845 | ) | (7.4 | )% | ||||||||||
| Finance income | 4,526 | 7,067 | (2,541 | ) | (36.0 | )% | ||||||||||
| Finance expenses | (49,660 | ) | (27,087 | ) | (22,573 | ) | 83.3 | % | ||||||||
| Change in fair value of financial instruments | (7,593 | ) | (587 | ) | (7,006 | ) | 1193.5 | % | ||||||||
| Gains/(losses) on disposal of financial instruments | 91 | (245 | ) | 336 | 137.1 | % | ||||||||||
| Exchange gains/(losses) | 1,616 | (1,733 | ) | 3,349 | 193.2 | % | ||||||||||
Finance expense | (51,020 | ) | (22,585 | ) | (28,435 | ) | 125.9 | % | ||||||||
| Share of loss of equity accounted investees | (879 | ) | 51 | (930 | ) | (1823.5 | )% | |||||||||
Profit before income tax | 157,784 | 203,994 | (46,210 | ) | (22.7 | )% | ||||||||||
| Income tax expense | (42,517 | ) | (56,424 | ) | 13,907 | (24.6 | )% | |||||||||
Consolidated profit for the year | 115,267 | 147,570 | (32,303 | ) | (21.9 | )% | ||||||||||
96
Table of Contents
| Year Ended | Year Ended | |||||||||||||||||||||||
| December 31, | % of total | December 31, | % of total | |||||||||||||||||||||
| 2010 | revenues | 2009 | revenues | % var | % var CC* | |||||||||||||||||||
| Bioscience | 773,372 | 78.1 | 694,969 | 76.1 | 11.3 | 7.7 | ||||||||||||||||||
| Diagnostic | 109,088 | 11.0 | 103,091 | 11.3 | 5.8 | 3.4 | ||||||||||||||||||
| Hospital | 89,552 | 9.0 | 86,328 | 9.5 | 3.7 | 2.9 | ||||||||||||||||||
| Raw Materials | 4,815 | 0.5 | 22,665 | 2.5 | (78.8 | ) | (79.8 | ) | ||||||||||||||||
| Others | 13,903 | 1.4 | 6,133 | 0.7 | 126.7 | 126.6 | ||||||||||||||||||
Total | 990,730 | 100.0 | 913,186 | 100.0 | 9.6 | 6.5 | ||||||||||||||||||
| * | Please see footnote (a) to the table on page 93. |
| Year Ended | Year Ended | |||||||||||||||||||||||
| December 31, | % of total | December 31, | % of total | |||||||||||||||||||||
| 2010 | revenues | 2009 | revenues | % var | % var CC* | |||||||||||||||||||
| European Union | 432,191 | 43.6 | 424,591 | 46.5 | 1.8 | 1.1 | ||||||||||||||||||
| United States and Canada | 339,018 | 34.2 | 296,659 | 32.5 | 14.3 | 10.0 | ||||||||||||||||||
| Rest of World | 219,521 | 22.2 | 191,936 | 21.0 | 14.4 | 7.7 | ||||||||||||||||||
Total | 990,730 | 100.0 | 913,186 | 100.0 | 8.5 | 5.4 | ||||||||||||||||||
| * | Please see footnote (a) to the table on page 93. |
97
Table of Contents
98
Table of Contents
| Years Ended December 31, | Change | |||||||||||||||
| 2009 | 2008 | € | % | |||||||||||||
| Revenues | 913,186 | 814,311 | 98,875 | 12.1 | % | |||||||||||
| Changes in inventories of finished goods and work in progress | 73,093 | 31,058 | 42,035 | 135.3 | % | |||||||||||
| Self-constructed non-current assets | 41,142 | 25,794 | 15,348 | 59.5 | % | |||||||||||
| Supplies | (286,274 | ) | (206,738 | ) | (79,536 | ) | 38.5 | % | ||||||||
| Other operating income | 1,443 | 1,289 | 154 | 11.9 | % | |||||||||||
| Personnel expenses | (273,168 | ) | (238,159 | ) | (35,009 | ) | 14.7 | % | ||||||||
| Other operating expenses | (203,381 | ) | (192,288 | ) | (11,093 | ) | 5.8 | % | ||||||||
| Amortisation and depreciation | (39,554 | ) | (33,256 | ) | (6,298 | ) | 18.9 | % | ||||||||
| Non-financial and other capital grants | 1,188 | 2,941 | (1,753 | ) | (59.6 | )% | ||||||||||
| Impairment and net losses on disposal of fixed assets | (1,147 | ) | (1,991 | ) | 844 | (42.4 | )% | |||||||||
Results from operating activities | 226,528 | 202,961 | 23,567 | 11.6 | % | |||||||||||
| Finance income | 7,067 | 2,682 | 4,385 | 163.5 | % | |||||||||||
| Finance expenses | (27,087 | ) | (29,305 | ) | 2,218 | (7.6 | )% | |||||||||
| Change in fair value of financial instruments | (587 | ) | (1,268 | ) | 681 | (53.7 | )% | |||||||||
| Gains/(losses) on disposal of financial instruments | (245 | ) | — | (245 | ) | — | ||||||||||
| Exchange gains/(losses) | (1,733 | ) | (2,825 | ) | 1,092 | (38.7 | )% | |||||||||
Net Finance expense | (22,585 | ) | (30,716 | ) | 8,131 | (26.5 | )% | |||||||||
| Share of (profit)/loss of equity accounted investees | 51 | 24 | 27 | 112.5 | % | |||||||||||
Profit before income tax | 203,994 | 172,269 | 31,725 | 18.4 | % | |||||||||||
| Income tax expense | (56,424 | ) | (50,153 | ) | (6,271 | ) | 12.5 | % | ||||||||
Consolidated profit for the year | 147,570 | 122,116 | 25,454 | 20.8 | % | |||||||||||
| Year Ended | Year Ended | |||||||||||||||||||||||
| December 31, | % of total | December 31, | % of total | |||||||||||||||||||||
| 2009 | revenues | 2008 | revenues | % var | % var CC* | |||||||||||||||||||
| Bioscience | 694,969 | 76.1 | 617,918 | 78.6 | 12.5 | 11.9 | ||||||||||||||||||
| Diagnostic | 103,091 | 11.3 | 85,705 | 10.9 | 20.3 | 20.9 | ||||||||||||||||||
| Hospital | 86,328 | 9.5 | 82,566 | 10.5 | 4.6 | 5.0 | ||||||||||||||||||
| Raw Materials | 22,665 | 2.5 | 22,794 | 2.9 | 4.6 | (14.0 | ) | |||||||||||||||||
| Others | 6,133 | 0.7 | 5,328 | 0.7 | (0.6 | ) | 15.3 | |||||||||||||||||
Total | 913,186 | 100.0 | 814,311 | 100.0 | 12.5 | 12.1 | ||||||||||||||||||
| * | Please see footnote (a) to the table on page 93. |
99
Table of Contents
| Year Ended | Year Ended | |||||||||||||||||||||||
| December 31, | % of total | December 31, | % of total | |||||||||||||||||||||
| 2009 | revenues | 2008 | revenues | % var | % var CC* | |||||||||||||||||||
| European Union | 424,591 | 46.5 | 404,099 | 49.6 | 5.1 | 7.3 | ||||||||||||||||||
| United States and Canada | 296,659 | 32.5 | 290,666 | 35.7 | 2.1 | (4.9 | ) | |||||||||||||||||
| Rest of World | 191,936 | 21.0 | 119,546 | 14.7 | 60.6 | 65 | ||||||||||||||||||
Total | 913,186 | 100.0 | 814,311 | 100.0 | 12.1 | 11.4 | ||||||||||||||||||
| * | Please see footnote (a) to the table on page 93. |
100
Table of Contents
| • | costs and expenses relating to the operation of our business, including working capital for inventory purchases and accounts receivable financing; | |
| • | capital expenditures for existing and new operations; and | |
| • | debt service requirements relating to our existing and future debt. |
101
Table of Contents
For the Years Ended December 31, 2010, 2009, and 2008 and the Six Months Ended
June 30, 2011 and 2010
(In thousands of Euros)
| 6/30/11 | 6/30/10 | 12/31/10 | 12/31/09 | 12/31/08 | ||||||||||||||||
Cash flows from operating activities | ||||||||||||||||||||
Profit before tax | 25,960 | 88,852 | 157,784 | 203,994 | 172,269 | |||||||||||||||
Adjustments for: | 92,638 | 53,782 | 92,351 | 61,800 | 66,034 | |||||||||||||||
| Amortisation and depreciation | 28,156 | 21,434 | 45,776 | 39,554 | 33,256 | |||||||||||||||
| Other adjustments: | 64,482 | 32,348 | 46,575 | 22,246 | 32,778 | |||||||||||||||
| (Profit) /losses on equity accounted investments | 807 | 728 | 879 | (51 | ) | (24 | ) | |||||||||||||
| Exchange differences | 2,122 | (1,970 | ) | (1,616 | ) | 1,733 | 2,825 | |||||||||||||
| Net provision charges | 14,454 | 129 | 913 | 53 | 1,994 | |||||||||||||||
| (Profit) /loss on disposal of fixed assets | 9,416 | (681 | ) | (276 | ) | 1,147 | 2,001 | |||||||||||||
| Government grants taken to income | (742 | ) | (550 | ) | (728 | ) | (1,188 | ) | (2,943 | ) | ||||||||||
| Finance expense/income | 37,130 | 33,386 | 47,442 | 17,551 | 27,891 | |||||||||||||||
| Other adjustments | 1,295 | 1,306 | (39 | ) | 3,001 | 1,034 | ||||||||||||||
Changes in capital and assets | (65,159 | ) | 13,700 | (78,767 | ) | (104,127 | ) | (86,550 | ) | |||||||||||
| Change in inventories | 752 | (11,982 | ) | (18,306 | ) | (113,104 | ) | (98,520 | ) | |||||||||||
| Change in trade and other receivables | (66,961 | ) | 20,239 | (23,546 | ) | (12,549 | ) | (7,951 | ) | |||||||||||
| Change in current financial assets and other current assets | (451 | ) | (3,875 | ) | (73,022 | ) | (1,287 | ) | 405 | |||||||||||
| Change in current trade and other payables | 1,501 | 9,318 | 36,107 | 22,813 | 19,516 | |||||||||||||||
Other cash flows from operating activities | (36,745 | ) | (34,465 | ) | (67,116 | ) | (73,487 | ) | (77,310 | ) | ||||||||||
| Interest paid | (34,021 | ) | (19,801 | ) | (40,129 | ) | (14,719 | ) | (25,972 | ) | ||||||||||
| Interest recovered | 999 | 3,861 | 5,436 | 2,509 | 2,213 | |||||||||||||||
| Income tax paid/(recovered) | (3,723 | ) | (18,525 | ) | (32,423 | ) | (61,277 | ) | (53,551 | ) | ||||||||||
Net cash from operating activities | 16,694 | 121,869 | 104,252 | 88,180 | 74,443 | |||||||||||||||
Cash flows from investing activities | ||||||||||||||||||||
| Payments for investments | (1,669,390 | ) | (56,997 | ) | (108,588 | ) | (136,626 | ) | (130,923 | ) | ||||||||||
| Group companies and business units | (1,615,417 | ) | (3,727 | ) | (1,474 | ) | (15,385 | ) | (632 | ) | ||||||||||
| Property, plant and equipment and intangible assets | (52,838 | ) | (49,151 | ) | (103,402 | ) | (118,770 | ) | (129,568 | ) | ||||||||||
| Property, plant and equipment | (42,841 | ) | (43,146 | ) | (86,800 | ) | (103,415 | ) | (119,824 | ) | ||||||||||
| Intangible assets | (9,997 | ) | (6,005 | ) | (16,602 | ) | (15,355 | ) | (9,744 | ) | ||||||||||
| Other financial assets | (1,135 | ) | (4,119 | ) | (3,712 | ) | (2,471 | ) | (723 | ) | ||||||||||
| Proceeds from the sale of investments | 69,151 | 2,863 | 4,532 | 673 | 157 | |||||||||||||||
| Property, plant and equipment | 69,151 | 2,863 | 3,911 | 673 | 157 | |||||||||||||||
| Associates | 0 | 0 | 621 | 0 | 0 | |||||||||||||||
Net cash used in investing activities | (1,600,239 | ) | (54,134 | ) | (104,056 | ) | (135,953 | ) | (130,766 | ) | ||||||||||
Cash flows from financing activities | ||||||||||||||||||||
| Proceeds from and payments for equity instruments | (2,264 | ) | (1,250 | ) | (1,250 | ) | 26,655 | (4,212 | ) | |||||||||||
| Issue | (2,264 | ) | (1,250 | ) | 0 | (76 | ) | 0 | ||||||||||||
| Acquisition of treasury shares | 0 | 0 | (1,250 | ) | (25,186 | ) | (4,880 | ) | ||||||||||||
| Disposal of treasury shares | 0 | 0 | 0 | 51,917 | 668 | |||||||||||||||
| Proceeds from and payments for financial liability instruments | 2,235,339 | (8,671 | ) | (1,066 | ) | 344,413 | 96,349 | |||||||||||||
| Issue | 2,982,877 | 51,067 | 118,238 | 525,078 | 394,109 | |||||||||||||||
| Redemption and repayment | (747,538 | ) | (59,738 | ) | (119,304 | ) | (180,665 | ) | (297,760 | ) | ||||||||||
| Dividends and interest on other equity instruments paid | 0 | (53 | ) | (27,282 | ) | (80,913 | ) | (34,792 | ) | |||||||||||
| Other cash flows from financing activities | (287,203 | ) | 323 | 323 | 741 | 0 | ||||||||||||||
| Transaction costs of financial instruments issued in the acquisition of Talecris | (287,550 | ) | 0 | 0 | 0 | 0 | ||||||||||||||
| Other amounts received from financing activities | 347 | 323 | 323 | 741 | 0 | |||||||||||||||
Net cash (used in)/from financing activities | 1,945,872 | (9,651 | ) | (29,275 | ) | 290,896 | 57,345 | |||||||||||||
Effect of exchange rate fluctuations on cash | (18,184 | ) | 42,684 | 19,356 | (119 | ) | (344 | ) | ||||||||||||
Net increase in cash and cash equivalents | 344,143 | 100,768 | (9,723 | ) | 243,004 | 678 | ||||||||||||||
Cash and cash equivalents at beginning of the period | 239,649 | 249,372 | 249,372 | 6,368 | 5,690 | |||||||||||||||
Cash and cash equivalents at end of the period | 583,792 | 350,140 | 239,649 | 249,372 | 6,368 | |||||||||||||||
102
Table of Contents
| • | a €12.5 million increase in receivables, with the days sales outstanding ratio remaining at 83 days, which was similar to 2008; | |
| • | a €113.1 million increase in inventories, which represented a significant increase from 2008, with the days sales outstanding ratio at 377 days, due to increased plasma collection activity andslower-than-budgeted sales, which resulted in remaining inventory at the stage of intermediate or finished goods; and | |
| • | a €22.8 million increase in current liabilities, with a day payable outstanding ratio of 64 days. |
| • | a €23.5 million increase in receivables, with the days sales outstanding ratio at 83 days, which was similar to 2009; | |
| • | a €18.3 million increase in inventories with the stocks turnover ratio at 364 days, which is lower than 2009; | |
| • | a €73.0 million increase in current financial assets and other current assets due to the costs incurred related to the issue of debt and equity that are expected in connection with the acquisition of Talecris included in other current assets; and | |
| • | a €36.1 million increase in current trade and other payables, with a day payable outstanding ratio of 76 days. |
| • | a decrease in profit before tax due to the transaction costs incurred during the six month period ended June 30, 2011 amounting to €39 million (€2 million for the six months ended at June 30, 2010) that have been paid in this period; and | |
| • | the change in current trade and other payables includes €20 million corresponding to business combination costs accrued by Talecris companies prior to acquisition date and paid during June 2011. |
103
Table of Contents
| December 31, | June 30, | |||||||||||||||||||||||||||||||
| 2008 | 2009 | 2010 | 2011 | |||||||||||||||||||||||||||||
| € | Days(1) | € | Days(1) | € | Days(1) | € | Days(1) | |||||||||||||||||||||||||
Inventory(2) | 373,098 | 327 | 484,462 | 377 | 527,865 | 364 | 997,826 | 300 | ||||||||||||||||||||||||
Receivables(3) | 186,324 | 84 | 207,840 | 83 | 224,355 | 83 | 390,979 | 63 | ||||||||||||||||||||||||
Payables(4) | 95,396 | 65 | 108,274 | 64 | 120,326 | 76 | — | (5) | — | (5) | ||||||||||||||||||||||
| (1) | At the last day of the period. | |
| (2) | Aging of inventory is calculated by dividing total inventories, as the case may be, at the end of each period by the total cost of sales for such period and multiplying the result by 365. | |
| (3) | We have calculated the average age of receivables by Total Receivables * 365/Sales (last 12 months). | |
| (4) | We have calculated the average age of payables by Total Payables * 365/Purchases + External services + Acquisitions fixed assets third parties (last 12 months). Payables include only the concepts that are also included as purchases, external services and acquisitions fixed assets. | |
| (5) | Due to the timing of the acquisition, at June 30, 2011, we were unable to determine accounts payable reflecting Talecris operations. |
104
Table of Contents
| Six Months Ended | Years Ended | |||||||||||||||
| June 30, | December 31, | |||||||||||||||
| 2011 | 2010 | 2009 | 2008 | |||||||||||||
Facilities(1) | 30,027 | 62,287 | 65,076 | 62,292 | ||||||||||||
Development cost(2) | 1,675 | 3,057 | 5,626 | 3,662 | ||||||||||||
Bioscience division | 31,702 | 65,344 | 70,702 | 65,954 | ||||||||||||
Facilities(1) | 8,597 | 12,559 | 7,524 | 9,266 | ||||||||||||
Development cost(2) | — | 573 | — | — | ||||||||||||
Hospital division | 8,597 | 13,132 | 7,524 | 9,266 | ||||||||||||
Facilities(1) | 4,153 | 12,914 | 11,547 | 12,485 | ||||||||||||
Development cost(2) | 1,885 | 2,983 | 2,520 | 1,593 | ||||||||||||
Diagnostic division | 6,038 | 15,897 | 14,067 | 14,078 | ||||||||||||
Raw materials division | — | — | — | 516 | ||||||||||||
Shared infrastructure | 6,762 | 11,424 | 26,477 | 39,879 | ||||||||||||
Total | 53,098 | 105,797 | 118,770 | 129,693 | ||||||||||||
| (1) | Facilities include manufacturing and other facilities. | |
| (2) | Development cost includes the capitalized portion only. Development expenses are capitalized only when the conditions of IAS 38 for such capitalization are met and are subsequently depreciated over an estimated useful life, as permitted under IFRS. Otherwise, research and development expenses are expensed as they are incurred. For 2008, 2009, 2010 and the six months ended June 30, 2011, Grifols had total development expenses of €25.6 million, €35.2 million, €36.6 million and €25.7 million, respectively, and had amortizations on development cost of €4.6 million, €5.6 million, €3.7 million and €4.8 million, respectively. |
| • | the acquisition of Novartis’ former industrial facilities in Parets del Vallès in Barcelona, Spain for €17.5 million; |
105
Table of Contents
| • | the acquisition of our headquarters for €35 million in 2008; | |
| • | the opening of a new building in Barcelona to house the raw material storage unit for €2.5 million in 2008; | |
| • | the establishment of research and development quality control laboratories and the installation of new manufacturing lines for parenteral nutrition products for the Hospital division for €2.5 million in 2008; | |
| • | the establishment of a new preparation area to increase the production of DG Gel cards completed in 2008 for €1.7 million; | |
| • | investment at the Los Angeles plant to fund its expansion, improvements and specialized machinery for €5 million; | |
| • | capital expenditures for the Los Angeles plant in the amount of €29.2 million in 2009; | |
| • | refurbishment work at our headquarters for €14.2 million in 2009; | |
| • | investments in the Los Angeles IVIG plant of approximately €18.1 million in 2010; | |
| • | refurbishment costs and information technology-related expenditures at our headquarters of approximately €7.7 million in 2010; | |
| • | costs related to the expansion of the new fractionation plant in Parets del Vallès of approximately €9.4 million in 2010; | |
| • | investments for the new laboratories in San Marcos, Texas of approximately €10.2 million in 2010; and | |
| • | investments for new plants and machinery in our Hospital division of approximately €10.0 million in 2010. |
| • | completion of the FDA licensing process for the sale of Flebogamma DIF IVIG in the United States in early 2007, which consists of a new method for obtaining IVIG that could significantly increase the protein yield and would provide increased safety through the use of a new method of viral elimination known as nanofiltration; | |
| • | completion of the development of Erytra in early 2010, which is a fully automated instrument with a high processing capacity for pre-transfusion compatibility tests using the gel agglutination technique; | |
| • | improvements to the Triturus and Wadiana automated analyzers; | |
| • | pursuit of Albumin usage in the treatment of Alzheimer’s disease; | |
| • | conducting clinical trials for the sale of Flebogamma DIF 10% in ITP in the European Union; and | |
| • | continuation of clinical trials for the sale of fibrin glue, which is a fibrin clot preparation to control bleeding during surgery, in the United States. |
| • | investments related to the Parets del Vallès plant in Spain of approximately €1.3 million in 2011 and €11.9 million in 2012, mainly related to fractionation capacity expansion; |
106
Table of Contents
| • | incur further headquarters-related expenses and IT projects of approximately €11.7 million in 2011 and €22.2 million in 2012; | |
| • | investments related to the Clayton, North Carolina plant of approximately €134.2 million in 2011 and €50.6 million in 2012, mainly related to fractionation capacity expansion; and | |
| • | further expenditures at our new plant in Murcia, Spain of approximately €8.9 million in 2011 and €2.1 million in 2012. |
107
Table of Contents
Fiscal Year | Percentage | |||
| 2014 | 106.188 | % | ||
| 2015 | 104.125 | % | ||
| 2016 | 102.063 | % | ||
| 2017 and thereafter | 100.000 | % | ||
108
Table of Contents
109
Table of Contents
| Payments Due by Period | ||||||||||||||||||||||||
| After | ||||||||||||||||||||||||
| Total | 2011 | 2012 | 2013 | 2014 | 2014 | |||||||||||||||||||
Operating leases(1) | 52,628 | 13,769 | 10,897 | 8,427 | 7,201 | 12,334 | ||||||||||||||||||
Financial debt obligations(2) | 857,021 | 191,635 | 85,171 | 51,582 | 17,936 | 510,697 | ||||||||||||||||||
Interest — financial debt obligations(3) | 300,669 | 34,895 | 36,300 | 34,497 | 33,603 | 161,374 | ||||||||||||||||||
Licenses and royalties(4) | 7,188 | 3,289 | 3,215 | 684 | 0 | 0 | ||||||||||||||||||
| Total | 1,217,506 | 243,588 | 135,583 | 95,190 | 58,740 | 684,405 | ||||||||||||||||||
| (1) | Operating leases include primarily leases for our plasma collection centers and marketing offices worldwide. These amounts reflect only our contractual obligations as of December 31, 2010, and therefore assume that these operating leases will not be renewed or replaced with new operating leases upon expiration. Investors are cautioned that our operating lease expenses will likely be substantially higher than the amounts provided in this table because our operations will require us to either renew or replace our operating leases. | |
| (2) | Includes principal amortization for short and long-term debt including, among other things, capitalized lease obligations. The financial debt primarily relates to €857 million outstanding as of December 31, 2010 including $600.0 million of the Grifols Old Notes, a €165.7 million syndicated loan facility that bore interest at an annual rate of EURIBOR plus 0.80% for Euro-denominated debt and LIBOR plus 0.80% for United States Dollar-denominated debt. The remaining financial debt was made up largely of working capital facilities that bore interest at market rate. | |
| (3) | Computed using interest rates in effect as of December 31, 2010 | |
| (4) | License and royalty payment formulas are generally based on volume of sales. The amounts presented in the table are calculated based on the net sales of 2011 without assuming any growth in sales. Additionally, the columns “After 2014” and “Total” only include one year of payments under the license agreement with Marca Grifols, S.L. which expires in January 2092. See “Certain Relationships and Related Party Transactions.” |
110
Table of Contents
111
Table of Contents
112
Table of Contents
113
Table of Contents
114
Table of Contents
115
Table of Contents
116
Table of Contents
117
Table of Contents
118
Table of Contents
119
Table of Contents
120
Table of Contents
| • | Plasma collected through our 147 plasma collection centers in the United States; and | |
| • | approximately 800,000 liters of plasma per year to be purchased from third-party suppliers for the next three years pursuant to multiple plasma purchase agreements assumed from Talecris in connection with the acquisition. |
121
Table of Contents
122
Table of Contents
123
Table of Contents
| Product Description | Main Applications | |
| Flebogamma IVIG. Human intravenous immunoglobulin, liquid, pasteurized and solvent detergent inactivation. Flebogamma DIF IVIG. Human intravenous immunoglobulin, liquid, pasteurized, solvent detergent inactivation and nanofiltration. Gamunex IVIG.Human intravenous immunoglobulin, liquid | IVIG assists in the treatment of primary and secondary immunological deficiencies, the treatment of immune-mediated idiopathic thrombocytopenic purpura (“ITP”), Guillain Barré syndrome, Kawasaki disease, allogeneic bone marrow transplants, CPI, and on an off-label application basis, multiple sclerosis, skin disease, asthma and CIDP, a neurological indication. IVIG is also currently being investigated for use in the treatment of Alzheimer’s disease and other neurological conditions. | |
| Gamunex-C.Human immunoglobulin, liquid, injection |
124
Table of Contents
| Product Description | Main Applications | |
| Our IVIG products have a 5% and 10% concentration. | ||
| Prolastin/Prolastin-C A1PI. Human proteinase inhibitor | Used to treat congenital A1PI deficiency-related emphysema. | |
| Trypsone A1PI. Human proteinase inhibitor | ||
| Fanhdi Factor VIII and Alphanate Factor VIII. High purity anti-hemophilic Factor VIII containing von Willebrand factor. | Used for the prevention and control of bleeding in Factor VIII deficiency (hemophilia A), and indication in the United States for von Willebrand congenital hemorrhagic disease. | |
| Koate DVI.Anti-hemophilic Factor VIII | ||
| Grifols Albumin. Pasteurized sterile aqueous solutions containing 25%, 20% or 5% human serum albumin. This albumin has a low aluminum content, a requirement in Europe but not in the United States, that makes it particularly attractive for biotechnology companies. We also offerAlbutein Albumin, a product containing 25%, 20% or 5% human serum that we obtained in our acquisition of Alpha and Talecris’Plasbumina product containing 25%, 20% or 5% human serum | Used to re-establish and maintain circulation volume in the treatment of traumatic or hemorrhagic shock and severe burns. Also used for liver disease and increasingly by biotechnology companies as a stabilizer. |
| • | Flebogamma/Gamunex IVIG. We have 93 licenses for marketing and sale of one or both of these IVIG products: 32 in the European Union, 4 in the United States, 26 in Latin America, 12 in Asia and 19 in the rest of the world; | |
| • | Fanhdi/Alphanate/Koate Factor VIII. We have 97 licenses for the marketing and sale of one or more of these Factor VIII products: 22 in the European Union, 31 in Latin America, 2 in the United States, 22 in Asia and 20 in the rest of the world; | |
| • | Albumin Grifols/Albutein/Plasbumin Albumin. We have 195 licenses for the marketing and sale of one or more of these albumin products in its various concentrations: 37 in the European Union, 13 in the United States and Canada, 52 in Latin America, 67 in Asia and 26 in the rest of the world; and | |
| • | Prolastin/Trypsone A1PI. We have 23 licenses for the marketing and sale of one or both of these A1PI products: 16 in the European Union, 5 in Latin America, and 2 in the United States. |
125
Table of Contents
126
Table of Contents
| Product Description | Main Applications | |
| Intravenous therapy: | ||
| Intravenous fluid and electrolyte solutions. Main product groups include hypotonic solutions, isotonic solutions, hypertonic solutions and plasma volume expander solutions. | Fluid and electrolyte replacement and conduit for the administration of medicines. | |
| Intravenous washing solutions. Washing solutions in specially designed containers. | Cleaning of injury and operation areas and urological irrigation. | |
| Intravenous mixtures.Ready-to-use intravenous mixtures of potassium, antibiotics, gastroprotective agents, levofloxacine and paracetamol for various purposes. We complement this product by offering Grifill, a system for the preparation of intravenous mixtures at in-hospital pharmacies using the principle of sterile filtration. We obtained a product license for Grifill in December 2003 from the FDA, which allows us to market and sell this product in the United States. | Increases safety and efficiency by rendering unnecessary the mixing of solutions at in-hospital pharmacies. | |
| Nutrition: | ||
| Soyacal fat emulsion. Fat emulsion at 10% and 20%, administered intravenously. | The fat emulsion is the main energy source for the patient in parenteral nutrition, providing calories and acid fats. | |
| Glucose solution. High glucose concentrate (5%, 15%, 30% and 50%). | Offers carbohydrate support for a patient’s diet that is administered intravenously. | |
| Dietgrif enteral liquid diets. Oral diets with all the requirements for balanced nutrition. Different diets include standard, standard fiber, polypeptidic, hyperproteic and energetic. | For patients who are unable to eat enough to maintain a nutritious diet, administered through feeding tubes as well as orally. | |
| Amino acid solutions. Solutions at 8% and 10% and hyper-nitrogenated amino acid solutions. | Offers amino acid support for a patient’s diet. | |
| Medical devices. Disposable sterile therapeutic medical products and radiological diagnosis instruments. | The products are used in uruology, radiology, cardiology, neurology hemodynamics, anesthesia, urodynamics and lithotripsy. | |
| Hospital logistics. Products, some manufactured by third-parties, like furniture, transport carts, bottling instruments and software programs, including our own Pyxis system. | Used in the logistical organization of the pharmacy and general warehouse of hospitals as well as in hospital management, admissions and accounting. |
127
Table of Contents
| Product Description | Main Applications | |
| Immunohematology: | ||
| Wadiana/Erytra analyzers. Automated immunohematology analyzers which use gel technology. | Used to perform routine pre-transfusion testing and immunohematology tests in general. | |
| Immunology: | ||
| Triturus analyzers. Fully automated analyzer with open system for any ELISA test offering multi-test/multi-batch capability. | Allows hospitals to automate the enzyme immunoassays in microtiter plate format and to process several batches of samples simultaneously. | |
| Reagents, instrumentation and software. Instruments, reagents and software for coagulation testing. | Used to establish the coagulation status of patients and to handle the corresponding results. | |
| Hemostasis: | ||
| Q-Coagulometer analyzers. Fully automated hemostasis analyzer which uses reagents to measure coagulation levels. | Used to diagnose and measure coagulation status of patients with coagulation-related and hemorrhagic disorders. | |
| Blood banks: | ||
| Leucored and Standard Blood bags. Blood bags configured according to all blood bank separation protocols. Leucored blood bags incorporate an in-line filtration system. | Used for collection and transfusion of blood. |
128
Table of Contents
| Six Months | ||||||||||||||||
| Ended | ||||||||||||||||
| June 30, | Years Ended December 31, | |||||||||||||||
| 2011 | 2010 | 2009 | 2008 | |||||||||||||
| (In thousands of Euros) | ||||||||||||||||
| European Union | 246,144 | 432,191 | 424,591 | 404,099 | ||||||||||||
| United States and Canada | 267,124 | 339,018 | 296,659 | 290,666 | ||||||||||||
| Rest of the World | 122,073 | 219,521 | 191,936 | 119,546 | ||||||||||||
| Total | 635,341 | 990,730 | 913,186 | 814,311 | ||||||||||||
| • | Developing new products. We constantly obtain, purify and inactivate proteins whose therapeutic purpose is known, such as its use as a fibrin sealant. Fibrin sealant is a combination of two plasma proteins (fibrogen and thrombin) that is being developed as an adjunct to surgical hemostasis. When locally applied, both proteins mix and coagulate producing a biological adhesive that mimics natural fibrin blood clot. We are currently carrying out clinical trials for this protein in vascular, organ and soft-tissue surgery and we estimate launch of this product in 2014. A Phase I safety study regarding the safety and efficacy of Plasmin for the treatment of aPAO was completed. A Phase II study is currently ongoing. We are also preparing a new catheter design for improved administration of Plasmin. We are thereby using the experience of our Hospital and Diagnostic divisions to deliver potential synergies. Among the additional products under development, the most relevant are: a topical Thrombin to help stop bleeding during surgery; Prothrombin Complex with potential indications on reversal of warfarin overdose in anticoagulated patients or treatment of Factor VIII inhibitors in hemophilia A; Intravenous fibrinogen, with indication in congenital and acquired deficiency and, potentially, in massive bleedings; and a human derived supplement for cell culture for use in research and, potentially, for cGMP applications related to, for example: biotechnology (recombinant protein production), stem cell based regenerative medicine or gene therapy; |
129
Table of Contents
| • | Researching new applications for existing products. We have been conducting clinical trials for the use of Fanhdi and Alphanate, anti-hemophilic products that consist of the Factor VIII protein and use the von Willebrand coagulation factor, on patients who have a von Willebrand factor deficiency. We have launched this product in Italy and in the United States and we expect to seek its license in other countries. We have undertaken several studies to explore alternative uses for albumin. We began a line of research focused on the systematic practice of therapeutic plasmapheresis with albumin in the treatment of Alzheimer’s disease in 2005 with the participation of the ACE Foundation (Catalan Institute of Applied Neuroscience) and Hospital Vall d’Hebron in Barcelona, Hospital Gregorio Marañón in Madrid, Howard University in Washington, D.C., and the Mid Atlantic Geriatric Association in New Jersey. The positive interim results obtained from the first studies were published in 2009 and led to the design of a further medical study combining plasmapheresis and intravenous immunoglobulins that began in 2011. In addition to the Alzheimer’s research, we signed a collaboration agreement with the Fundacio Clinic per a la Recerca Biomedica (the Clinic Foundation for Biomedical Research) to finance two additional lines of albumin research. The first is an ongoing multicenter trial that uses albumin on patients with liver cirrhosis and ascites to prevent the complications inherent to this illness. The second trial includes conducting plasma replacements in patients withacute-on-chronic liver failure and it is expected that the first patient will be treated during 2010. We are also conducting a pilot randomized clinical trial with Anbinex (antithrombin) in cardiac surgery with cardiopulmonary bypass, as well as a study of the efficacy and safety of Anbinex treatment in patients suffering from severe burns. We plan to include Talecris’ Thrombate to these programs. |
| • | Improving our manufacturing processes to improve yields, safety and efficiency. Among the projects related to improving yields, safety and efficiency, we are developing a nanofiltered Factor VIII/VWF concentrate that will increase the already excellent safety margin of our current products while simplifying process steps, which will improve efficiency without significantly impacting yields. Research is being done also in liquid formulations for Alpha-1 Antitrypsin, which would represent an advantage for the user and reduce production costs associated with freeze-drying the product. Alternative packaging materials are being developed for albumin and IVIG, which would represent an advantage to the user because of ease of use. Also in development is a nanofiltered albumin preparation that would improve the already well established safety profile of albumin and might be of interest for certain end users, like vaccine manufacturers. |
130
Table of Contents
| • | the continued development of technology associated with the classification of blood types through the use of gel technology; | |
| • | a research project to enable us to work with frozen blood bags to improve the availability and quality of the reagent red cells manufactured therefrom is ongoing, as diluted red cells are an important reagent in the screening process of antibodies and antibody identification. It is estimated the project will be completed by the end of 2011; | |
| • | a new stand-alone digital reader will be launched during the last quarter of 2011 in order to automate the reading and interpretation process of the gel cards we manufacture and sell; | |
| • | a new auto analyzer to perform ELISA techniques in microplates. This analyzer is set to replace the current Triturus, of which over one thousand units have been sold and placed worldwide. This new instrument will increase the output and sample and reagents capacity in comparison to the Triturus, adding some new features such as continuous loading and unloading of samples and an innovative simplified fluidic system; | |
| • | a new Hemostasis Analyzer, complementary to the Q-Coagulometer, that will be able to serve medium to large laboratories. It will have about three times the capacity and output of the Q-Coagulometer; | |
| • | a newly formulated thromboplastin reagent is close to completion. Additionally, a new liquid Thrombin reagent of human origin is in the final stage of development. We are also working on a new activated cephaline reagent based on synthetic phospholipids; and | |
| • | an instrument called Stat, which will be able to read the results shown by the multicards (rapid blood typing test), is being developed by our Swiss subsidiary acquired in 2009. |
131
Table of Contents
132
Table of Contents
| • | Process for the production of virus-inactivated human Gammaglobulin G. We have patents for this process in Argentina, Austria, Belgium, Chile, Czech Republic, Finland, France, Germany, Greece, Hong Kong, Hungary, Ireland, Italy, Japan, Mexico, Netherlands, Portugal, Slovakia, Spain, Sweden, Switzerland/Liechtenstein, Turkey, United Kingdom, Uruguay and the United States which are either pending registration or are set to expire between 2022 and 2024; |
133
Table of Contents
| • | Use of Therapeutic Human Albumin for the preparation of a drug for the treatment of patients suffering from cognitive disorders. We have patents for this process in Argentina, Australia, Brazil, Canada, Chile, China, the European Union, Hong Kong, Japan, Mexico, New Zealand, Russia, Spain, Uruguay and the United States which are either pending registration or are set to expire between 2027 and 2028; and | |
| • | Process for removing viruses in fibrinogen solutions. We have patents for this process in Argentina, Australia, Austria, Belgium, Brazil, Canada, Chile, Czech Republic, Finland, France, Germany, Greece, Hungary, Ireland, Italy, Japan, Mexico, Netherlands, New Zealand, Poland, Portugal, Slovakia, Spain, Sweden, Switzerland/Liechtenstein, Turkey, United Kingdom and the United States which are either pending registration or are set to expire in 2024. |
| • | Process for the sterile filling of flexible material bags (Grifill). We have patents for this apparatus in Austria, Belgium, Chile, Denmark, France, Germany, Italy, Japan, Mexico, Netherlands, Portugal, Spain, Sweden, Switzerland/Liechtenstein, United Kingdom and the United States which are set to expire between 2014 and 2022; | |
| • | Bags with filtration system (Gribag). We have patents for this vessel in France, Germany, Italy, Japan, Portugal, Spain, Switzerland/Liechtenstein and the United Kingdom which are set to expire between 2012 and 2014; | |
| • | Instrument for the precise low rate administration of parenteral solutions without the need for mechanical equipment (Griflow). We have patents for this process in Austria, Belgium, Brazil, France, Germany, Italy, Japan, Mexico, Portugal, Switzerland/Liechtenstein, United Kingdom and the United States which are set to expire between 2011 and 2014; | |
| • | Wadiana machine for clinical analysis. We have patents for this apparatus in Argentina, Austria, Belgium, Brazil, Chile, France, Germany, Italy, Japan, Mexico, Netherlands, Portugal, Slovakia, Spain, Switzerland/Liechtenstein, United Kingdom and the United States which are set to expire between 2018 and 2023; | |
| • | Triturus machine for automated laboratory tests. We have patents for this apparatus in Argentina, France, Germany, Italy, Japan, Mexico, Spain, United Kingdom and the United States which are set to expire in 2018; | |
| • | Centrifuge machine for clinical analysis, which we have a patent for in Spain and which is set to expire in 2018; | |
| • | Blister handling machine (Blispack). We have patents for this apparatus in Argentina, Brazil, Chile, Spain, the European Union and Mexico which are either pending registration or are set to expire in 2028; | |
| • | Q Coagulometer. We have patents for this apparatus in Spain, the European Union, Japan and the United States which are either pending registration or are set to expire in 2025; and | |
| • | Erytra apparatus for the automatic analysis of samples on gel cards. We have patents for this apparatus in Argentina, Australia, Brazil, Canada, Chile, China, Spain, the European Union, Hong Kong, Japan, Mexico and the United States which are either pending registration or are set to expire in 2029. |
134
Table of Contents
| 2010 Actual | Current Installed | |||||||
| Complex | Location and Size | Products(1) | Production(2) | Annual Capacity(2) | ||||
| Industrial Complex One Parets | Barcelona, Spain 58,285 square meters of which we own 45,647 square meters | Fanhdi Factor VIII Albumin Grifols Anbin - Antithrombin III Flebogamma IVIG Intramuscular Gammaglobulins Trypsone - A1PI Novix Factor IX | 1,933 liters 1,888 liters 70 liters 2,585 liters 54 liters 283 liters 40 liters | 2,450 liters 2,200 liters 400 liters 4,100 liters 450 liters 450 liters 60 liters | ||||
| Industrial Complex Two Parets | Barcelona, Spain 35,525 square meters of which we own 19,853 square meters | Parenteral Solutions Gel Cards | 21.3 million rigid bottles 12 million units | 45 million rigid bottles 18 million units | ||||
| Industrial Complex Three Parets | Barcelona, Spain 40,113 square meters which we rent | Plasma Storage | 0.6 million liters | 0.8 million liters | ||||
| Industrial Complex USA | Los Angeles, California 93,078 square meters which we own | Albutein Albumin Alphanate Factor VIII Alphanine IX Profilnine | 1,340 liters 1,223 liters 125 liters 51 liters | 1,500 liters 2,000 liters 500 liters 100 liters | ||||
| Clayton Facility | Clayton, North Carolina 69,203 square meters of which we own 60,771 square meters | Koate Factor VIII Plasbumin/Plasmanate-Fraction V Antithrombin III Gamunex-C IVIG Intramuscular Gammaglobulins Prolastin/ Prolastin-C A1PI | 2,200 liters 2,376 liters 220 liters 3,381 liters 60 liters 3,012 liters | 2,700 liters 2,400 liters 1,700 liters 5,200 liters 350 liters 3,503 liters |
135
Table of Contents
| 2010 Actual | Current Installed | |||||||
| Complex | Location and Size | Products(1) | Production(2) | Annual Capacity(2) | ||||
| City of Industry USA | Temple, California 5,000 square meters which we rent | Plasma storage | 400 liters | 1,200 liters | ||||
| Industrial Complex One Murcia | Murcia, Spain 10,285 square meters which we rent | Blood bags | 6 million equivalent units | 8 million equivalent units | ||||
| Industrial Complex Two Murcia | Murcia, Spain 26,873 square meters which we own | Parenteral and intravenous solutions | 21.5 million flexible containers | 27 million flexible containers | ||||
| Industrial Complex Switzerland | Fribourg, Switzerland 12,000 square meters which we rent | Multicards | 0.2 million units | 2.5 million units | ||||
| Industrial Complex Australia | Melbourne, Australia 3,838 square meters which we own | Gel Cards | 1 million units | 7.0 million units | ||||
| Plasma Testing Lab Austin, TX | Austin, TX, USA 2,235 square meters which we rent | Plasma testing | N/A | N/A | ||||
| Plasma Testing Lab San Marcos, TX | San Marcos, TX, USA 7,670 square meters which we own | Plasma testing | N/A | N/A | ||||
| Headquarters Sant Cugat Barcelona | Sant Cugat, Spain 32,211 square meters which we rent | Headquarters | N/A | N/A | ||||
| Plasma Testing Lab Raleigh, North Carolina | Raleigh, North Carolina 7,061 square meters which we rent | Plasma testing | N/A | N/A | ||||
| Melville Facility | Melville, New York 9,562 square meters which we rent | Plasma fractionation | 1,286 liters | 1,550 liters |
136
Table of Contents
| 2010 Actual | Current Installed | |||||||
| Complex | Location and Size | Products(1) | Production(2) | Annual Capacity(2) | ||||
| Benson | Benson, North Carolina 3,642 square meters which we rent | Plasma storage | 758 liters | 1,200 liters |
| (1) | For diagnostic products, other than blood bags, there is not a meaningful installed capacity measurement as each unit is built and assembled individually. | |
| (2) | In thousands of liters, except as indicated. |
137
Table of Contents
| Number of | Marketing | R&D and | Senior | |||||||||||||||||||||
Location | Employees | and Sales | Production | Technical | Administrative | Management | ||||||||||||||||||
| Spain | 2,383 | 255 | 1,466 | 246 | 374 | 42 | ||||||||||||||||||
| Rest of European Union | 275 | 151 | 28 | 27 | 53 | 17 | ||||||||||||||||||
| Total European Union | 2,658 | 406 | 1,493 | 273 | 427 | 59 | ||||||||||||||||||
| United States | 8,282 | 306 | 7,032 | 420 | 452 | 71 | ||||||||||||||||||
| Rest of the World | 234 | 143 | 23 | 13 | 43 | 11 | ||||||||||||||||||
Total | 11,174 | 855 | 8,548 | 706 | 922 | 142 | ||||||||||||||||||
138
Table of Contents
139
Table of Contents
140
Table of Contents
141
Table of Contents
142
Table of Contents
143
Table of Contents
144
Table of Contents
| • | IVIGis the part of the plasma that contains antibodies. IVIG assists in the treatment of primary and secondary immunological deficiencies, idiopathic thrombocytopenic purpura (“ITP”), Guillain-Barré syndrome, Kawasaki disease, Allogeneic bone marrow transplant, and Chronic Inflammatory Demyelinating Polyneuropathy (“CIDP”). In addition, physicians prescribe IVIG for a variety of diseases, including multiple sclerosis, skin disease and asthma, even though these uses are not described in the product’s labeling and differ from those tested in clinical studies and approved by the FDA or similar regulatory authorities in other countries. These unapproved, or “off-label,” uses are common across medical specialties, and physicians may believe such off-label uses constitute the preferred standard of care or treatment of last resort for many patients in varied circumstances. IVIG is also currently being investigated for use in the treatment of Alzheimer’s disease and other neurological conditions. Industry participants believe that, because IVIG is a complex mixture of antibody molecules, it is unlikely that a recombinant (or synthetic) alternative will be developed within the foreseeable future. IVIG has global sales of $5.1 billion, which represents 43.4% of the total plasma derivatives sales. IVIG sales experienced significant growth in recent years driven by improving usages, physician awareness and a strong reimbursement environment, and it now represents the largest plasma-derived product by sales value. It is one of the key growth drivers of the industry largely due to the increasing number of medical conditions for which IVIG is used; | |
| • | Factor VIIIis a blood coagulation factor which ensures that blood coagulates correctly after hemorrhage. Persons born with Factor VIII deficiency or who acquire this deficiency over time through the formation of antibodies that inactivate it, require administration of Factor VIII in determined situations (before surgery or after injury or serious hemorrhage). Factor VIII is also often used for the treatment of hemophilia A, a disease that is suffered by one out of every 10,000 men (women are not susceptible to this disease). Factor VIII used in these cases is either extracted from human plasma or is genetically modified into a recombinant substitute from bovine, mouse or hamster cells. Recombinant products account for most sales in the Factor VIII market. In 2008, worldwide plasma-derived Factor VIII and von Willebrand Factor annual sales were approximately $1.8 billion, comprising 15.5% of total plasma derivatives sales. Plasma-derived Factor VIII and von Willebrand Factor had a compound annual growth rate of 8.7% over the past ten years. Growth in Factor VIII is being driven by increased patient identification and treatment in developing countries. The current per capita Factor VIII utilization is significantly higher in the United States and Europe than in developing countries; | |
| • | Albuminis the most commonly found protein in plasma and represents the biggest product by volume but has low unitary prices given its commoditized nature. One of albumin’s main functions is to carry and store a wide variety of small molecules such as bilirubin, cortisol, sex hormones, free fatty acids and some medicines. Albumin is used in the treatment of burns, severe hemorrhage, sepsis, hemodialyzed patients with hypotension, nephritic syndrome and necrotizing pancreatitis, among others. Biotechnology companies also use high-purity albumin as a stabilizer for their products. Clinical trials are currently underway for new applications for this product, including, among others, for the treatment of stroke and liver cirrhosis. Albumin has global sales of $1.7 billion, comprising 14.4% of the total plasma derivatives industry. The demand for albumin has increased since 2000 and is projected to grow moderately over the next few years; and | |
| • | A1PIis a naturally occurring, self-defensive protein produced in the liver. A1PI is used to treat congenital A1PI deficiency-related emphysema. This deficiency may predispose an individual to several illnesses but most commonly appears as emphysema in adults. U.S. sales of A1PI have experienced a compound annual growth rate of 16% between 1997 and 2010. We believe there are approximately 11,000 individuals currently identified with A1PI deficiency in North America and Europe with 5,500 of those individuals currently undergoing A1PI treatment, based on internal estimates. There are an estimated 200,000 individuals with A1PI deficiency at high risk for development of emphysema in North America and Europe. Many individuals with symptoms are misdiagnosed before receiving a diagnosis of A1PI deficiency-related emphysema. Based on patient registries in many European countries, we believe that severe A1PI deficiency is also prevalent in Europe, and that European |
145
Table of Contents
| patients may represent approximately 30% of potential global sales. Epidemiological surveys have demonstrated that there is significant latent demand for A1PI as only approximately 10% of all patients in need of treatment have been identified (source: Alpha-1-antitrypsin deficiency. High prevalence in the St. Louis area determined by direct population screening. Silverman EK, et al.Am Rev Respir Dis.1989; 140:961-966). Even fewer patients are being treated using an A1PI product due to the limited number of countries with licensed product. |
| Percentage of | ||||
Product | Global Sales | |||
| IVIG | 43.4 | % | ||
Factor VIII(1) | 15.5 | % | ||
| Albumin | 14.4 | % | ||
| Factor IX | 2.7 | % | ||
| Hyperimmunes | 7.6 | % | ||
| Alpha 1 Proteinase Inhibitor (“A1PI”) | 3.6 | % | ||
| Fibrin glue | 3.7 | % | ||
| Antithrombin III | 2.9 | % | ||
| Others | 6.2 | % | ||
| (1) | Including sales of von Willebrand Factor |
| Percentage of | ||||
Region | Global Sales | |||
| North America | 36.7 | % | ||
| Europe | 36.2 | % | ||
| Asia Pacific | 15.3 | % | ||
| Latin America | 5.7 | % | ||
| Middle East | 2.6 | % | ||
| Others | 3.5 | % | ||
| • | IVIG. According to the MRB, worldwide sales for IVIG have grown at a 12.5% compound annual rate between 1994 and 2008, although current growth is materially lower. This growth has been driven by increased evidence that IVIG is effective in treating a broader universe of ailments than previously considered and increased incidence of acquired autoimmune and other ailments due to an increase in life expectancy. | |
| • | Factor VIII. According to the MRB, the worldwide sales of plasma-derived Factor VIII, including von Willebrand factor sales,have grown at a 5.1% compound annual rate between 1994 and 2008, and |
146
Table of Contents
| Grifols and Talecris believe that demand growth will continue. The United States Factor VIII market is supplied primarily by recombinant products. We believe that continued plasma-derived Factor VIII growth worldwide will be driven by the following therapeutic indications: |
| • | Treatment of von Willebrand disease. The treatment of von Willebrand disease requires a Factor VIII product containing von Willebrand factor. Von Willebrand factor is not present in recombinant and monoclonal Factor VIII products; and | |
| • | Immune Tolerance Therapy (“ITT”). Plasma-derived ITT is used principally as a second attempt at treatment when an initial course of recombinant ITT has failed. The daily administration of a high dose of either recombinant or plasma-derived Factor VIII for six months to a year is an increasingly popular treatment to combat inhibitors, which are substances that restrict the activity of Factor VIII. Doses in the second attempt at ITT tend to be significantly higher than in the initial course of treatment. |
| • | Albumin. According to MRB, the worldwide sales demand for albumin has grown at a 0.6% compound annual rate between 1994 and 2008. This slow growth is due to a perception that less expensive alternatives such as saline are as effective as albumin in the treatment of traumatic or hemorrhagic shock and severe burns. | |
| • | Alpha 1 Proteinase Inhibitor (“A1PI”). A1PI is a fourth protein that is growing in sales. According to the MRB, the worldwide sales demand for Alpha-1 has grown at a 14.6% compound annual rate between 1994 and 2008. |
147
Table of Contents
| • | “Group Purchasing Organizations,” which are referred to as GPOs, which are umbrella buying groups representing inpatient and outpatient hospitals and non-acute members who benefit through consolidated |
148
Table of Contents
| supply contracts. GPOs do not purchase products directly, rather, they select authorized distributors which purchase inventory and handle all product logistics for their members; |
| • | Wholesalers/Distributors either provide product directly to, or enter into distribution agreements with, hospitals, GPOs, and physician offices. The distributor is generally paid service fees for “encumbered” products on a GPO contract, or they purchase “unencumbered” products directly from manufacturers which are not part of a GPO contract; | |
| • | Homecare and specialty pharmacy providers are a growing segment which provides patient treatment in the home, either through self-medication or with the assistance of a nurse. These providers either purchase products direct from manufacturers or through GPOs; and | |
| • | Manufacturer Direct programs distribute products directly to a physician’s office or a patient’s home. |
| • | According to the MRB, it is estimated that 45% of the IVIG sold in the United States in 2010 was purchased by hospitals; alternate infusion sites, including physician offices represented about 20% of IVIG volume; and homecare companies represented 30% of the IVIG volume; | |
| • | A1PI is generally distributed by homecare companies and specialty pharmacies and administered by a nurse at home or at a hospital infusion suite; | |
| • | Albumin is generally used in surgical and trauma settings and is generally sold to hospital groups; and | |
| • | Clotting factors, such as Factor VIII, generally are self-administered by patients and are mainly channeled from manufacturers to patients through home care companies and similar agencies. |
| • | immunohematology, which is the diagnosis of blood type and the screening of antibodies, accounting for 2.6%, or $1.11 million, of the 2010 world market for in vitro diagnostic products according to the business information company Global Data; | |
| • | immunology, which is the study of defense mechanisms against antigens, accounting for 47.9%, or $20.4 million, of the 2010 world market; and | |
| • | hemostasis, which is the analysis of processes related to blood coagulation, accounting for 4.0%, or $1.70 million, of the 2010 world market. |
149
Table of Contents
150
Table of Contents
| • | completion of preclinical laboratory tests, animal studies and formulation studies under the FDA’s good laboratory practices regulations; | |
| • | submission to the FDA of an Investigational New Drug Application (“IND”), for human clinical testing, which must become effective before human clinical trials may begin and which must include approval by an independent Institutional Review Board, which is referred to as an IRB, at each clinical site before the trials may be initiated; | |
| • | performance of adequate and well-controlled clinical trials in accordance with Good Clinical Practices to establish the safety and efficacy of the product for each indication; | |
| • | submission to the FDA of a BLA, which contains detailed information about the chemistry, manufacturing and controls for the product, reports of the outcomes and full data sets of the clinical trials and proposed labeling and packaging for the product; | |
| • | satisfactory review of the contents of the BLA by the FDA, including the satisfactory resolution of any questions raised during the review; | |
| • | satisfactory completion of an FDA Advisory Committee review, if applicable; | |
| • | satisfactory completion of an FDA inspection of the manufacturing facility or facilities at which the product is produced to assess compliance with cGMP to assure that the facilities, methods and controls are adequate to ensure the product’s identity, strength, quality and purity; and | |
| • | FDA approval of the BLA including agreement on post-marketing commitments, if applicable. |
151
Table of Contents
| • | evaluate dosage tolerance and appropriate dosage; | |
| • | identify possible adverse effects and safety risks; and | |
| • | provide a preliminary evaluation of the efficacy of the drug for specific indications. |
152
Table of Contents
153
Table of Contents
154
Table of Contents
| • | derived from biotechnology processes, such as genetic engineering; | |
| • | advanced-therapy medicines, such as gene-therapy, somatic cell-therapy or tissue-engineered medicines; |
155
Table of Contents
| • | intended for the treatment of HIV/Aids, cancer, diabetes, neurodegenerative disorders or autoimmune diseases and other immune dysfunctions; and | |
| • | officially designated “orphan medicines” (medicines used for rare diseases). |
| • | the medicinal product is intended for the diagnosis, prevention or treatment of a life-threatening or chronically debilitating condition affecting no more than five in 10,000 persons in the European Union at the time of submission of the designation application (prevalence criterion); or | |
| • | the medicinal product is intended for the diagnosis, prevention or treatment of a life-threatening, seriously debilitating or serious and chronic condition and without incentives it is unlikely that the revenue after marketing of the medicinal product would cover the investment in its development; and | |
| • | either no satisfactory method of diagnosis, prevention or treatment of the condition concerned is authorized, or, if such method exists, the medicinal product will be of significant benefit to those affected by the condition. |
| • | protocol assistance (scientific advice for orphan medicines during the product-development phase); | |
| • | direct access to centralized marketing authorization and10-year marketing exclusivity; | |
| • | financial incentives (fee reductions or exemptions); and | |
| • | national incentives detailed in an inventory made available by the European Commission. |
156
Table of Contents
| • | full (100%) reduction for protocol assistance andfollow-up; | |
| • | full (100%) reduction for pre-authorization inspections 50% reduction for new applications for marketing authorization to applicants other than small and medium-sized enterprises; | |
| • | full (100%) reduction for new applications for marketing authorization only to small and medium-sized enterprises; and | |
| • | full (100%) reduction for post authorization activities including annual fees only to small and medium sized enterprises in the first year after granting a marketing authorization. |
157
Table of Contents
158
Table of Contents
159
Table of Contents
160
Table of Contents
161
Table of Contents
162
Table of Contents
Name | Title | Type | Director Since | |||
| Víctor Grifols Roura | Director, Chairman of the Board and Chief Executive Officer (Consejero Delegado) | Executive | July 1991(1) | |||
| Juan Ignacio Twose Roura | Director | Executive | April 2000(2) | |||
| Ramón Riera Roca | Director | Executive | April 2000(3) | |||
| Tomás Dagá Gelabert | Director | Other External | April 2000 | |||
| Thorthol Holdings B.V. (represented by Mr. José Antonio Grifols Gras) | Director | Proprietary | January 2000(4) | |||
| Thomas H. Glanzmann | Director | Other External | April 2006 | |||
| Edgar Dalzell Jannotta | Director | Independent | December 2006 | |||
| Anna Veiga Lluch | Director | Independent | December 2008 | |||
| William Brett Ingersoll | Director | Independent | June 2011 | |||
| Luis Isasi Fernandez De Bobadilla | Director | Independent | May 2011 | |||
| Steven Francis Mayer | Director | Independent | June 2011 | |||
| Raimon Grifols Roura | Secretary non-member | — | July 2001 | |||
| Nuria Martín Barnés | Vice Secretary non-member | — | July 2001 |
| (1) | Between July 8, 1991 and May 30, 2002, Mr. Víctor Grifols Roura was not a director but sat on the Board as representative of our then director Deria, S.A. | |
| (2) | Between May 25, 2001 and May 30, 2002, Mr. Juan Ignacio Twose Roura was not a director but sat on the Board as representative of our then director Grifols Engineering, S.A. | |
| (3) | Between May 25, 2001 and May 30, 2002, Mr. Ramón Riera Roca was not a director but sat on the Board as representative of our then director Grifols International, S.A. | |
| (4) | Thorthol Holdings B.V. is represented on the Board of Directors by Mr. José Antonio Grifols Gras. Between January 20, 2000 and June 1, 2002 Thorthol Holdings B.V. was not a director but its current representative on the Board, Mr. José Antonio Grifols Gras, sat on the Board as director. |
163
Table of Contents
164
Table of Contents
165
Table of Contents
Name | Title | Since | ||||
| Víctor Grifols Roura | President and Chief Executive Officer | 1985 | ||||
| Juan Ignacio Twose Roura | President of Global Industrial Division | 1988 | ||||
| Ramón Riera Roca | President of Global Commercial Division | 1988 | ||||
| Alfredo Arroyo Guerra | Chief Financial Officer | 2007 | ||||
| Montserrat Lloveras Calvo | Director of Corporate Accounting and Reporting | 1991 | ||||
| Antonio Viñes Páres | Director of Corporate Planning and Control | 1994 | ||||
| Eva Bastida Tubau | Director of Scientific Affairs | 2007 | ||||
| Vicente Blanquer Torre | Technical Director of Biological Industrial Group | 1993 | ||||
| Mateo Florencio Borrás Humbert | Director of Global Human Resources | 2008 | ||||
| Carlos Roura Fernández | Co-President of Global Industrial Division | 1987 | ||||
| Francisco Javier Jorba Ribes | President of Biological Industrial Group | 1995 | ||||
| Gregory Gene Rich | President and Chief Executive Officer of Grifols Inc. | 2001 | ||||
| David Ian Bell | Vice President of Grifols Inc. | 2003 | ||||
| Albert Grifols Roura | Co-President of Instituto Grifols, S.A. | 1999 | ||||
| Nuria Pascual Lapeña | Director of Corporate Investor Relations Office | 1997 | ||||
| Shinji Wada | President of Plasma Centers of Grifols Inc. | 2003 | ||||
| Mary Kuhn | Executive Vice President of Grifols Inc. | 2011 | ||||
| Joel Abelson | President of North America Commercial Division | 2011 | ||||
166
Table of Contents
167
Table of Contents
168
Table of Contents
| • | Report to the shareholders at general shareholders meetings regarding matters for which the Audit Committee is responsible; | |
| • | Have sole authority to recommend to the Board the appointment, hiring and replacement of the external auditor regardless of the faculties vested in the general shareholders’ meeting and the Board with regard to the approval of such resolutions under Spanish law; | |
| • | Monitor the internal audit services and propose the selection, appointment, reelection and resignation of the manager of the Internal Audit Department; propose the budget for the Internal Audit Department; receive periodic information on the Internal Audit Department’s activities (including the annual work plan and annual activities reports prepared by the manager); and verify that the top management takes the conclusions and recommendations of their reports into account; | |
| • | Set up and supervise procedures for the receipt, retention and treatment of complaints regarding accounting, internal controls or auditing matters, as well as the confidential and anonymous submission by employees of concerns regarding questionable accounting or auditing matters; | |
| • | Know the process for gathering financial information and the internal control system; review the annual accounts and the periodic financial statements that should be submitted to the securities regulatory authorities and make sure that the appropriate accounting standards are followed; report to the Board on any change in the accounting standards and on balance sheet and off balance sheet risks; | |
| • | Receive information from the auditors regarding matters that could impair their independence, or any other matters relating to conduct of audits of the financial statements as well as any other communications provided for in the legislation governing audits of financial statements and in technical auditing regulations; | |
| • | Supervise any transactions entered into with significant shareholders as set forth in the Board Regulations; and | |
| • | Ensure compliance with the Internal Code of Conduct for Securities Market Issues, the Board Regulations, the rules of conduct set out in the “Code of Ethics for Grifols Executives” and the “Code of Conduct for Grifols’ Employees” and, in general, any other corporate regulations; Make the necessary proposals to improve such regulations. |
169
Table of Contents
| • | assisting in the nomination of directors, including evaluating potential nominees in light of the level of knowledge, competence and experience necessary to serve on the Board; | |
| • | reporting and making proposals to the Board on the appointment of members to the various committees of the Board and on the persons who should hold the office of Secretary and Vice-secretary of the Board; | |
| • | making proposals for the orderly and planned succession of the Chairman of the Board and the Chief Executive Officer; | |
| • | reporting on proposals for the appointment and removal of any members of senior management made by the Chief Executive Officer; | |
| • | making proposals on the remuneration plans for the Board and senior management; | |
| • | periodically reviewing the remuneration plans of senior management, including considering their suitability and performance; and | |
| • | reporting on transactions in which directors may have a conflict of interest. |
170
Table of Contents
171
Table of Contents
| Amount Paid | ||||
Component | in 2010 | |||
| (In thousands of Euros) | ||||
| Salaries | 3,779 | |||
| Variable Compensation | 893 | |||
| Stock options and/or other securities | N/A | |||
| Other — e.g., life and health insurance | N/A | |||
| Other — e.g., pensions/savings | N/A | |||
172
Table of Contents
EXECUTIVE OFFICERS
| Number of | Percentage of | |||||||
Name of Beneficial Owner | Class A Shares | Class A Shares | ||||||
Major Shareholders | ||||||||
Capital Research and Management Company(1) | 31,995,474 | 15.017 | ||||||
Deria S.A.(2) | 18,706,988 | 8.771 | ||||||
Scranton Enterprises B.V.(3) | 16,149,937 | 7.580 | ||||||
Thorthol Holdings B.V.(4) | 15,042,766 | 7.060 | ||||||
Víctor Grifols Lucas(5) | 13,112,187 | 6.154 | ||||||
American Funds Insurance Series Growth Fund(6) | 6,400,370 | 3.004 | ||||||
Directors | ||||||||
José Antonio Grifols Gras(4) | 15,042,766 | 7.060 | ||||||
| Víctor Grifols Roura | 440,450 | * | ||||||
| Edgar Dalzell Jannotta | 254,127 | * | ||||||
Ramón Riera Roca(7) | 169,085 | * | ||||||
| Juan Ignacio Twose Roura | 119,274 | * | ||||||
Thomas H. Glanzmann(8) | 79,561 | * | ||||||
| Tomás Dagá Gelabert | 51,898 | * | ||||||
| Anna Veiga Lluch | 100 | * | ||||||
| Luis Isasi Fernandez De Bobadilla | 100 | * | ||||||
| Steven F. Mayer | 0 | |||||||
| W. Brett Ingersoll | 0 | * | ||||||
Executive Officers | ||||||||
| Antonio Viñes Parés | 111,115 | * | ||||||
| Gregory Gene Rich | 71,598 | * | ||||||
| Carlos Roura Fernández | 48,314 | * | ||||||
| Francisco Javier Jorba Ribes | 47,364 | * | ||||||
| Montserrat Lloveras Calvo | 35,309 | * | ||||||
| Vicente Blanquer Torre | 22,377 | * | ||||||
| Alberto Grifols Roura | 13,000 | * | ||||||
| David Ian Bell | 10,000 | * | ||||||
| Nuria Pascual Lapeña | 9,796 | * | ||||||
| Mateo Florencio Borrás Humbert | 491 | * | ||||||
| Alfredo Arroyo Guerra | — | — | ||||||
| Eva Bastida Tubau | — | — | ||||||
| Shinji Wada | — | — | ||||||
| Mary Kuhn | — | — | ||||||
| Joel Abelson | — | — | ||||||
| * | Less than 1%. |
173
Table of Contents
| (1) | Capital Research and Management Company has indirect voting rights over all 31,995,474 our Class A shares. American Funds Insurance Series Growth Fund has direct voting rights over 8,771,882 of our Class A shares reported by Capital Research and Management Company. | |
| (2) | The various members of the Grifols Roura family hold their respective shares indirectly through Deria S.A. | |
| (3) | Scranton Enterprises B.V. is a corporation whose shares are owned by certain of our directors and by William Blair & Co. L.L.C. Some Grifols family members who are directors or executive officers hold part of their shares indirectly through Scranton Enterprises B.V. | |
| (4) | The various members of the Grifols Gras family hold their respective shares indirectly through Thorthol Holdings B.V., which is represented on the Board of Directors by José Antonio Grifols Gras. | |
| (5) | 13,112,187 Class A shares are held directly by Rodellar Amsterdam B.V., through which Víctor Grifols Lucas exercises indirect voting rights. | |
| (6) | American Funds Insurance Series Growth Fund has direct voting rights over all 6,400,370 of our Class A shares. American Funds Insurance Series Growth Fund has delegated the right to vote its proxies to Capital Research and Management Company, its investment advisor. | |
| (7) | 8,000 Class A shares are held indirectly. | |
| (8) | 61,000 Class A shares are held indirectly through Kolholmen Investments AB. |
174
Table of Contents
| • | Compulsory initial term of five years; | |
| • | Initial rent established at market prices, to be reviewed annually, based on the percentage variation in the Spanish Consumer Price Index; | |
| • | Automatic extensions for five-year periods that can be terminated by either party by advance six month notice; and | |
| • | Upon vacating the premises, the lessor will reimburse us for the remaining value of any leasehold improvements which we have made. |
175
Table of Contents
176
Table of Contents
| • | general unsecured obligations of the Company; | |
| • | senior in right of payment to all of the Company’s existing and any future Subordinated Indebtedness; | |
| • | pari passuin right of payment with all of the Company’s existing and any future unsecured Indebtedness that is not by its terms expressly subordinated to the Notes; | |
| • | effectively junior in right of payment to the Company’s existing and future secured Indebtedness, including the Company’s Obligations under the Credit Agreement, to the extent of the value of the collateral securing such Indebtedness; | |
| • | unconditionally guaranteed by Parent and its Restricted Subsidiaries that Guarantee the Obligations under the Credit Agreement (other than any Foreign Subsidiary of the Company); and | |
| • | structurally subordinated to Indebtedness of Subsidiaries of Parent that are not Guarantors. |
177
Table of Contents
| • | a senior unsecured obligation of each Guarantor; | |
| • | senior in right of payment to all existing and any future subordinated Indebtedness of such Guarantor; | |
| • | pari passuin right of payment with all existing and any future Indebtedness of that Guarantor that is not by its terms expressly subordinated to its Guarantee of the Notes; | |
| • | effectively junior in right of payment to the existing and future secured Indebtedness of that Guarantor, including such Guarantor’s obligations under the Credit Agreement, to the extent of the value of the collateral securing such Indebtedness; and | |
| • | structurally subordinated to Indebtedness of any Subsidiaries of that Guarantor that are not Guarantors. |
178
Table of Contents
| • | the acquisition of Talecris Biotherapeutics Holdings Corp. by the Parent and its Subsidiaries had been consummated in accordance with the Merger Agreement without any waiver or amendment thereof or any consent thereunder, in any case which was materially adverse to the Holders of Notes; | |
| • | (a) Parent and its other Subsidiaries party thereto had entered into the Credit Agreement on the terms described in the offering memorandum with such changes as were not in the aggregate materially adverse to the Holders of Notes and (b) no Default or Event of Default shall have occurred and be continuing thereunder (after giving effect to the Transactions) that shall not have been waived; | |
| • | the Escrow Issuer had merged with and into the Company (with the Company continuing as the surviving corporation), the Company had assumed all of the obligations of the Escrow Issuer under the Notes and the Indenture and the Guarantors had become parties to the Indenture; and | |
| • | no Default of Event of Default had occurred or was continuing under the Indenture. |
179
Table of Contents
180
Table of Contents
Fiscal Year | Percentage | |||
| 2014 | 106.188 | % | ||
| 2015 | 104.125 | % | ||
| 2016 | 102.063 | % | ||
| 2017 and thereafter | 100.000 | % | ||
181
Table of Contents
182
Table of Contents
183
Table of Contents
184
Table of Contents
185
Table of Contents
186
Table of Contents
187
Table of Contents
188
Table of Contents
189
Table of Contents
190
Table of Contents
191
Table of Contents
192
Table of Contents
193
Table of Contents
194
Table of Contents
195
Table of Contents
196
Table of Contents
197
Table of Contents
198
Table of Contents
199
Table of Contents
200
Table of Contents
201
Table of Contents
202
Table of Contents
203
Table of Contents
204
Table of Contents
205
Table of Contents
206
Table of Contents
207
Table of Contents
208
Table of Contents
209
Table of Contents
210
Table of Contents
211
Table of Contents
212
Table of Contents
213
Table of Contents
214
Table of Contents
215
Table of Contents
216
Table of Contents
217
Table of Contents
218
Table of Contents
219
Table of Contents
220
Table of Contents
221
Table of Contents
222
Table of Contents
223
Table of Contents
224
Table of Contents
| • | upon deposit of each global note with DTC’s custodian, DTC will credit portions of the principal amount of each global note to the accounts of the DTC Participants; and | |
| • | ownership of beneficial interests in each global note will be shown on, and transfer of ownership of those interests will be effected only through, records maintained by DTC (with respect to interests of DTC Participants) and the records of DTC Participants (with respect to other owners of beneficial interests in any of the global notes). |
| • | a limited purpose trust company organized under the laws of the State of New York; | |
| • | a “banking organization” within the meaning of the New York State Banking Law; | |
| • | a member of the Federal Reserve System; | |
| • | a “clearing corporation” within the meaning of the Uniform Commercial Code; and | |
| • | a “clearing agency” registered under Section 17A of the Exchange Act. |
| • | will not be entitled to have notes represented by the global note registered in their names; | |
| • | will not receive or be entitled to receive physical, certificated notes; and |
| • | will not be considered the owners or holders of the notes under the Indentures for any purpose, including with respect to the giving of any direction, instruction or approval to the trustee under the Indentures. |
225
Table of Contents
| • | DTC notifies us at any time that it is unwilling or unable to continue as depositary for the global notes and a successor depositary is not appointed within 90 days; | |
| • | DTC ceases to be registered as a clearing agency under the Exchange Act and a successor depositary is not appointed within 90 days; | |
| • | we, at our option, notify the trustee that we elect to cause the issuance of certificated notes; or | |
| • | certain other events provided in the indenture should occur. |
226
Table of Contents
227
Table of Contents
228
Table of Contents
| • | does not actually (or constructively) own 10% or more of the total combined voting power of all classes of our voting stock within the meaning of the Code and the Treasury Regulations; | |
| • | is not a controlled foreign corporation that is related, directly or indirectly, to us through stock ownership; | |
| • | is not a bank whose receipt of interest on the exchange notes is pursuant to a loan agreement entered into in the ordinary course of business; and | |
| • | has fulfilled the certification requirements set forth below. |
229
Table of Contents
| • | that gain is effectively connected with the conduct of a trade or business in the United States by thenon-U.S. holder, (and, if a tax treaty applies, such gain is attributable to a permanent establishment in the United States); or | |
| • | thenon-U.S. holder is an individual who is present in the United States for 183 days or more in the taxable year of that disposition, and certain other conditions are met. |
230
Table of Contents
231
Table of Contents
232
Table of Contents
233
Table of Contents
Grifols, S.A. Avinguda de la Generalitat,152-158 Parc de Negocis Can Sant Joan 08174 Sant Cugat del Vallès, 08174, Barcelona, Spain |
Attention: Investor Relations Telephone: (+34)935-710-500 |
234
Table of Contents
| F-2 | ||||
| F-4 | ||||
| F-5 | ||||
| F-6 | ||||
| F-7 | ||||
| F-8 | ||||
| F-29 | ||||
Audited Consolidated Financial Statements: | ||||
| F-37 | ||||
| F-38 | ||||
| F-40 | ||||
| F-41 | ||||
| F-42 | ||||
| F-43 | ||||
| F-45 | ||||
| F-127 | ||||
| F-129 | ||||
| F-132 | ||||
| F-135 | ||||
| F-138 | ||||
Talecris Biotherapeutics Holdings Corp. | ||||
Audited Consolidated Financial Statements: | ||||
| F-148 | ||||
| F-149 | ||||
| F-150 | ||||
| F-151 | ||||
| F-152 | ||||
| F-153 | ||||
| F-206 |
F-1
Table of Contents
At 30 June 2011 and 31 December 2010
| 30/06/11 | 31/12/10 | |||||||
| (Unaudited) | ||||||||
| (Expressed in thousands of Euros) | ||||||||
ASSETS | ||||||||
Non-current assets | ||||||||
| Intangible assets | ||||||||
| Goodwill (note 6) | 2,281,696 | 189,448 | ||||||
| Other intangible assets (note 7) | 128,474 | 78,299 | ||||||
| Total intangible assets | 2,410,170 | 267,747 | ||||||
| Property, plant and equipment (note 7) | 639,735 | 434,131 | ||||||
| Investments in equity accounted investees | 3,546 | 598 | ||||||
| Non-current financial assets (note 12) | 41,667 | 7,535 | ||||||
| Deferred tax assets | 139,435 | 34,889 | ||||||
| Total non-current assets | 3,234,553 | 744,900 | ||||||
Current assets | ||||||||
| Inventories | 997,826 | 527,865 | ||||||
| Trade and other receivables | ||||||||
| Trade receivables (note 8) | 405,450 | 224,355 | ||||||
| Other receivables | 48,971 | 44,032 | ||||||
| Current income tax assets | 41,029 | 14,607 | ||||||
| Trade and other receivables | 495,450 | 282,994 | ||||||
| Other current financial assets | 19,254 | 12,946 | ||||||
| Other current assets (note 9) | 13,344 | 80,628 | ||||||
| Cash and cash equivalents (note 10) | 583,792 | 239,649 | ||||||
| Total current assets | 2,109,666 | 1,144,082 | ||||||
Total assets | 5,344,219 | 1,888,982 | ||||||
F-2
Table of Contents
| 30/06/11 | 31/12/10 | |||||||
| (Unaudited) | ||||||||
| (Expressed in thousands of Euros) | ||||||||
EQUITY AND LIABILITIES | ||||||||
Equity | ||||||||
| Share capital (note 11) | 114,914 | 106,532 | ||||||
| Share premium (note 11) | 890,355 | 121,802 | ||||||
| Reserves (note 11) | 569,682 | 403,604 | ||||||
| Own shares (note 11) | (1,927 | ) | (1,927 | ) | ||||
| Profit for the period/year attributable to the Parent | 19,269 | 115,513 | ||||||
| Total | 1,592,293 | 745,524 | ||||||
Available-for-sale financial assets | (575 | ) | — | |||||
| Cash flow hedges | (2,331 | ) | (1,751 | ) | ||||
| Translation differences | (88,734 | ) | (50,733 | ) | ||||
| Other comprehensive income | (91,640 | ) | (52,484 | ) | ||||
Equity attributable to the Parent | 1,500,653 | 693,040 | ||||||
| Non-controlling interests | 12,941 | 14,350 | ||||||
Total equity | 1,513,594 | 707,390 | ||||||
LIABILITIES | ||||||||
Non-current liabilities | ||||||||
| Grants | 1,815 | 2,088 | ||||||
| Provisions | 10,461 | 1,378 | ||||||
| Non-current financial liabilities | ||||||||
| Loans and borrowings, bonds and other marketable securities | 2,642,944 | 665,385 | ||||||
| Other financial liabilities | 72,400 | 10,474 | ||||||
| Total non-current financial liabilities (note 12) | 2,715,344 | 675,859 | ||||||
| Deferred tax liabilities | 140,075 | 79,141 | ||||||
| Total non-current liabilities | 2,867,695 | 758,466 | ||||||
Current liabilities | ||||||||
| Provisions | 35,828 | 4,365 | ||||||
| Current financial liabilities | ||||||||
| Loans and borrowings, bonds and other marketable securities | 507,374 | 191,635 | ||||||
| Other financial liabilities | 17,336 | 18,236 | ||||||
| Total current financial liabilities (note 12) | 524,710 | 209,871 | ||||||
| Debts with associates | 2,352 | 1,162 | ||||||
| Trade and other payables | ||||||||
| Suppliers | 266,393 | 160,678 | ||||||
| Other payables | 25,618 | 11,928 | ||||||
| Current income tax liabilities | 27,227 | 4,172 | ||||||
| Total trade and other payables | 319,238 | 176,778 | ||||||
| Other current liabilities | 80,802 | 30,950 | ||||||
| Total current liabilities | 962,930 | 423,126 | ||||||
Total liabilities | 3,830,625 | 1,181,592 | ||||||
Total equity and liabilities | 5,344,219 | 1,888,982 | ||||||
F-3
Table of Contents
For the Six Month Period Ended 30 June 2011 and 2010
| 30/06/11 | 30/06/10 | |||||||
| (Unaudited) | ||||||||
| (Expressed in thousands of Euros) | ||||||||
Continuing Operations | ||||||||
| Revenues (note 5) | 635,341 | 487,809 | ||||||
| Changes in inventories of finished goods and work in progress | 2,757 | 41,209 | ||||||
| Self-constructed non-current assets | 32,346 | 16,051 | ||||||
| Supplies | (175,142 | ) | (157,107 | ) | ||||
| Other operating income | 1,009 | 631 | ||||||
| Personnel expenses | (183,727 | ) | (141,972 | ) | ||||
| Other operating expenses | (155,532 | ) | (98,279 | ) | ||||
| Amortisation and depreciation (note 7) | (28,156 | ) | (21,434 | ) | ||||
| Transaction costs of Talecris business combination (note 3 & 9) | (38,607 | ) | (2,019 | ) | ||||
| Non-financial and other capital grants | 742 | 550 | ||||||
| Impairment and gains/(losses) on disposal of fixed assets (notes 6 & 7) | (22,302 | ) | 681 | |||||
Results from operating activities | 68,729 | 126,120 | ||||||
| Finance income | 1,761 | 2,179 | ||||||
| Finance expenses (notes 8 & 13) | (55,546 | ) | (25,285 | ) | ||||
| Change in fair value of financial instruments (note 13) | 13,945 | (15,404 | ) | |||||
| Exchange gains/(losses) | (2,122 | ) | 1,970 | |||||
Finance expense | (41,962 | ) | (36,540 | ) | ||||
| Share of loss of equity accounted investees | (807 | ) | (728 | ) | ||||
Profit before income tax | 25,960 | 88,852 | ||||||
| Income tax expense (note 14) | (7,347 | ) | (23,022 | ) | ||||
Consolidated profit for the period | 18,613 | 65,830 | ||||||
| Profit attributable to equity holders of the Parent | 19,269 | 66,408 | ||||||
| Loss attributable to non-controlling interests | (656 | ) | (578 | ) | ||||
Basic earnings per share (Euros) | 0.09 | 0.31 | ||||||
Diluted earnings per share (Euros) | 0.09 | 0.31 | ||||||
F-4
Table of Contents
For the Six Month Period Ended 30 June 2011 and 2010
| 30/06/11 | 30/06/10 | |||||||
| (Unaudited) | ||||||||
| (Expressed in | ||||||||
| thousands of Euros) | ||||||||
Consolidated profit for the period | 18,613 | 65,830 | ||||||
Other comprehensive income | ||||||||
Income and expenses generated during the period | ||||||||
| Measurement of financial instruments | (575 | ) | 0 | |||||
Available-for-sale financial assets | (822 | ) | 0 | |||||
| Tax effect | 247 | 0 | ||||||
| Cash flow hedges | (2,331 | ) | 0 | |||||
| Cash flow hedges | (3,829 | ) | 0 | |||||
| Tax effect | 1,498 | 0 | ||||||
| Translation differences | (38,541 | ) | 74,874 | |||||
Income and expenses generated during the period | (41,447 | ) | 74,874 | |||||
Income and expense recognised in the income statement: | ||||||||
| Cash flow hedges | 1,751 | 99 | ||||||
| Cash flow hedges | 2,870 | 159 | ||||||
| Tax effect | (1,119 | ) | (60 | ) | ||||
Income and expense recognised in the income statement: | 1,751 | 99 | ||||||
Other comprehensive income and expenses for the period | (39,697 | ) | 74,973 | |||||
Total comprehensive income and expenses for the period | (21,083 | ) | 140,803 | |||||
| Total comprehensive income/(losses) attributable to the Parent | (19,887 | ) | 139,935 | |||||
| Total comprehensive income/(losses) attributable to non-controlling interests | (1,196 | ) | 868 | |||||
Total comprehensive income for the period | (21,083 | ) | 140,803 | |||||
F-5
Table of Contents
For the Six Month Period Ended 30 June 2011
| Attributable to Equity Holders of the Parent | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Other Comprehensive Income | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Profit | Available-for | Equity | ||||||||||||||||||||||||||||||||||||||||||||||||||
| Attributable | Sale | Attributable | ||||||||||||||||||||||||||||||||||||||||||||||||||
| Share | Share | to | Interim | Own | Translation | Cash Flow | Financial | to | Non-Controlling | |||||||||||||||||||||||||||||||||||||||||||
| Capital | Premium | Reserves (*) | Parent | Dividend | Shares | Differences | Hedges | Assets | Parent | Interests | Equity | |||||||||||||||||||||||||||||||||||||||||
| (Unaudited) | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| (Expressed in thousands of Euros) | ||||||||||||||||||||||||||||||||||||||||||||||||||||
Balances at 31 December 2009 | 106,532 | 121,802 | 314,903 | 147,972 | (31,960 | ) | (677 | ) | (90,253 | ) | (1,948 | ) | 0 | 566,371 | 12,157 | 578,528 | ||||||||||||||||||||||||||||||||||||
| Translation differences | — | — | — | — | — | — | 73,428 | — | — | 73,428 | 1,446 | 74,874 | ||||||||||||||||||||||||||||||||||||||||
| Cash flow hedges | — | — | — | — | — | — | — | 99 | — | 99 | — | 99 | ||||||||||||||||||||||||||||||||||||||||
Other comprehensive income for the period | 0 | 0 | 0 | 0 | 0 | 0 | 73,428 | 99 | 0 | 73,527 | 1,446 | 74,973 | ||||||||||||||||||||||||||||||||||||||||
| Profit/(loss) for the period | — | — | — | 66,408 | — | — | — | — | — | 66,408 | (578 | ) | 65,830 | |||||||||||||||||||||||||||||||||||||||
Total comprehensive income for the period | 0 | 0 | 0 | 66,408 | 0 | 0 | 73,428 | 99 | 0 | 139,935 | 868 | 140,803 | ||||||||||||||||||||||||||||||||||||||||
| Operations with own shares | — | — | — | — | — | (1,250 | ) | — | — | — | (1,250 | ) | — | (1,250 | ) | |||||||||||||||||||||||||||||||||||||
| Other changes | — | — | 1 | — | — | — | — | — | — | 1 | — | 1 | ||||||||||||||||||||||||||||||||||||||||
| Distribution of 2009 profit | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Reserves | — | — | 88,783 | (88,783 | ) | — | — | — | — | — | 0 | — | 0 | |||||||||||||||||||||||||||||||||||||||
| Dividends | — | — | — | (27,229 | ) | — | — | — | — | — | (27,229 | ) | (53 | ) | (27,282 | ) | ||||||||||||||||||||||||||||||||||||
| Interim dividend | — | — | — | (31,960 | ) | 31,960 | — | — | — | — | 0 | — | 0 | |||||||||||||||||||||||||||||||||||||||
Operations with equity holders or owners | 0 | 0 | 88,784 | (147,972 | ) | 31,960 | (1,250 | ) | 0 | 0 | 0 | (28,478 | ) | (53 | ) | (28,531 | ) | |||||||||||||||||||||||||||||||||||
Balances at 30 June 2010 | 106,532 | 121,802 | 403,687 | 66,408 | 0 | (1,927 | ) | (16,825 | ) | (1,849 | ) | 0 | 677,828 | 12,972 | 690,800 | |||||||||||||||||||||||||||||||||||||
Balances at 31 December 2010 | 106,532 | 121,802 | 403,604 | 115,513 | 0 | (1,927 | ) | (50,733 | ) | (1,751 | ) | 0 | 693,040 | 14,350 | 707,390 | |||||||||||||||||||||||||||||||||||||
| Translation differences | — | — | — | — | — | — | (38,001 | ) | — | (38,001 | ) | (540 | ) | (38,541 | ) | |||||||||||||||||||||||||||||||||||||
| Cash flow hedges | — | — | — | — | — | — | — | (580 | ) | — | (580 | ) | — | (580 | ) | |||||||||||||||||||||||||||||||||||||
Available-for-sale financial assets Gains/(losses) | — | — | — | — | — | — | — | — | (575 | ) | (575 | ) | — | (575 | ) | |||||||||||||||||||||||||||||||||||||
Other comprehensive income for the period | 0 | 0 | 0 | 0 | 0 | 0 | (38,001 | ) | (580 | ) | (575 | ) | (39,156 | ) | (540 | ) | (39,696 | ) | ||||||||||||||||||||||||||||||||||
| Profit/(loss) for the period | — | — | — | 19,269 | — | — | — | — | — | 19,269 | (656 | ) | 18,613 | |||||||||||||||||||||||||||||||||||||||
Total comprehensive income for the period | 0 | 0 | 0 | 19,269 | 0 | 0 | (38,001 | ) | (580 | ) | (575 | ) | (19,887 | ) | (1,196 | ) | (21,083 | ) | ||||||||||||||||||||||||||||||||||
| Other changes | — | — | (35 | ) | — | — | — | — | — | — | (35 | ) | (213 | ) | (248 | ) | ||||||||||||||||||||||||||||||||||||
| Capital Increase (note 11) | 8,382 | 768,553 | (2,264 | ) | — | — | — | — | — | — | 774,671 | — | 774,671 | |||||||||||||||||||||||||||||||||||||||
| Other movements (note 11) | — | — | 52,864 | — | — | — | — | — | — | 52,864 | — | 52,864 | ||||||||||||||||||||||||||||||||||||||||
| Distribution of 2010 profit | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Reserves | — | — | 115,513 | (115,513 | ) | — | — | — | — | — | 0 | — | 0 | |||||||||||||||||||||||||||||||||||||||
Operations with equity holders or owners | 8,382 | 768,553 | 166,078 | (115,513 | ) | 0 | 0 | 0 | 0 | 0 | 827,500 | (213 | ) | 827,287 | ||||||||||||||||||||||||||||||||||||||
Balance at 30 June 2011 | 114,914 | 890,355 | 569,682 | 19,269 | 0 | (1,927 | ) | (88,734 | ) | (2,331 | ) | (575 | ) | 1,500,653 | 12,941 | 1,513,594 | ||||||||||||||||||||||||||||||||||||
| (*) | Reserves include accumulated earnings and other reserves |
F-6
Table of Contents
For the Six Month Period Ended 30 June 2011 and 2010
| 30/06/11 | 30/06/10 | |||||||
| (Unaudited) | ||||||||
| (Expressed in thousands of Euros) | ||||||||
Cash flows from operating activities | ||||||||
Profit before tax | 25,960 | 88,852 | ||||||
Adjustments for: | 92,638 | 53,782 | ||||||
| Amortisation and depreciation | 28,156 | 21,434 | ||||||
| Other adjustments: | 64,482 | 32,348 | ||||||
| Losses on equity accounted investments | 807 | 728 | ||||||
| Exchange differences | 2,122 | (1,970 | ) | |||||
| Net provision charges | 14,454 | 129 | ||||||
| (Profit)/loss on disposal of fixed assets | 9,416 | (681 | ) | |||||
| Government grants taken to income | (742 | ) | (550 | ) | ||||
| Finance expense/income | 37,130 | 33,386 | ||||||
| Other adjustments | 1,295 | 1,306 | ||||||
Changes in capital and assets | (65,159 | ) | 13,700 | |||||
| Change in inventories | 752 | (11,982 | ) | |||||
| Change in trade and other receivables | (66,961 | ) | 20,239 | |||||
| Change in current financial assets and other current assets | (451 | ) | (3,875 | ) | ||||
| Change in current trade and other payables | 1,501 | 9,318 | ||||||
Other cash flows from operating activities | (36,745 | ) | (34,465 | ) | ||||
| Interest paid | (34,021 | ) | (19,801 | ) | ||||
| Interest recovered | 999 | 3,861 | ||||||
| Income tax recovered | (3,723 | ) | (18,525 | ) | ||||
Net cash from operating activities | 16,694 | 121,869 | ||||||
Cash flows from investing activities | ||||||||
| Payments for investments | (1,669,390 | ) | (56,997 | ) | ||||
| Group companies and business units (note 3) | (1,615,417 | ) | (3,727 | ) | ||||
| Property, plant and equipment and intangible assets | (52,838 | ) | (49,151 | ) | ||||
| Property, plant and equipment | (42,841 | ) | (43,146 | ) | ||||
| Intangible assets | (9,997 | ) | (6,005 | ) | ||||
| Other financial assets | (1,135 | ) | (4,119 | ) | ||||
| Proceeds from the sale of property, plant and equipment | 69,151 | 2,863 | ||||||
| Property, plant and equipment | 69,151 | 2,863 | ||||||
Net cash used in investing activities | (1,600,239 | ) | (54,134 | ) | ||||
Cash flows from financing activities | ||||||||
| Proceeds from and payments for equity instruments | (2,264 | ) | (1,250 | ) | ||||
| Issue | (2,264 | ) | (1,250 | ) | ||||
| Proceeds from and payments for financial liability instruments | 2,235,339 | (8,671 | ) | |||||
| Issue | 2,982,877 | 51,067 | ||||||
| Redemption and repayment | (747,538 | ) | (59,738 | ) | ||||
| Dividends and interest on other equity instruments paid | 0 | (53 | ) | |||||
| Other cash flows from financing activities | (287,203 | ) | 323 | |||||
| Transaction costs of financial instruments issued in the acquisition of Talecris | (287,550 | ) | 0 | |||||
| Other amounts received from financing activities | 347 | 323 | ||||||
Net cash from/(used in) financing activities | 1,945,872 | (9,651 | ) | |||||
Effect of exchange rate fluctuations on cash | (18,184 | ) | 42,684 | |||||
Net increase in cash and cash equivalents | 344,143 | 100,768 | ||||||
Cash and cash equivalents at beginning of the period | 239,649 | 249,372 | ||||||
Cash and cash equivalents at end of period | 583,792 | 350,140 | ||||||
F-7
Table of Contents
| (1) | General Information |
| (2) | Basis of Presentation and Accounting Principles Applied |
| • | IAS 24 Revised Related Party Disclosures (effective date: 1 January 2011). | |
| • | Amendment to IFRIC 14: Prepayment of a minimum funding requirement (effective date: 1 January 2011). | |
| • | IFRS 7 Amendments resulting from May 2010 Annual Improvements (effective date: 1 January 2011). | |
| • | Amendment to IFRIC 13 Customer Loyalty Programmes (effective date: 1 January 2011). | |
| • | IAS 34 Amendments resulting from May 2010 Annual Improvements (effective date: 1 January 2011). | |
| • | IAS 1 Amendments resulting from May 2010 Annual Improvements (effective date: 1 January 2011). |
F-8
Table of Contents
| • | Amendment to IAS 12 Deferred tax: recovery of underlying assets (effective date: 1 January 2012) | |
| • | Amendment to IFRS 1 Severe Hyperinflation and Removal of Fixed Dates for First-time Adopters (effective date: 1 July 2011) | |
| • | Amendment to IFRS 7 Financial Instrument: Disclosures — Transfer of Financial Assets (effective date: 1 July 2011) | |
| • | IFRS 9 Financial instruments (effective date: 1 January 2013) | |
| • | IFRS 10 Consolidated Financial Statements (effective date: 1 January 2013) | |
| • | IFRS 11 Joint Arrangements (effective date: 1 January 2013) | |
| • | IFRS 12 Disclosures of Interests in Other Entities (effective date: 1 January 2013) | |
| • | IFRS 13 Fair Value Measurement (effective date: 1 January 2013) | |
| • | IAS 27 Separate Financial Statements (effective date: 1 January 2013) | |
| • | IAS 28 Investments in Associates and Joint Ventures (effective date: 1 January 2013) | |
| • | IAS 19 Employee Benefits (effective date: 1 January 2013) |
| • | The corporate tax expense which, according to IAS 34, is recognised in interim periods based on the best estimate of the average tax rate that the Group expects for the annual period. | |
| • | The useful lives of property, plant, and equipment and intangible assets. | |
| • | Measurement of assets and goodwill to determine any related impairment losses. | |
| • | Evaluation of the capitalisation of development costs. | |
| • | Evaluation of provisions and contingencies. | |
| • | The assumptions used for calculation of the fair value of financial instruments. | |
| • | Evaluation of the effectiveness of hedging. | |
| • | Evaluation of the nature of leases (operating or financial). |
F-9
Table of Contents
| (3) | Changes in the composition of the Group |
| • | Kedrion and Grifols entered into a contract manufacturing agreement to fractionate and purify Kedrion’s plasma to deliver IVIG and Albumin under Kedrion’s private label, and Factor VIII under the trade name Koate, all of them for sale only in the United States. |
F-10
Table of Contents
| • | Grifols is committed to sell to Kedrion the Melville fractionation facility. Grifols lease from Kedrion the Melville fractionation facility being the lease term 3 years with an optional extension of up to 1 year at Grifols request. | |
| • | Grifols transfer to Kedrion all Koate (factor VIII) technology and commercial agreements for the US market. Grifols will produce Koate for Kedrion up to a period of 7 years. | |
| • | Grifols is committed to sell to Kedrion two plasma collection centers. In addition Grifols committed to sell 200.000 liters of source plasma to Kedrion at a fixed price. | |
| • | Grifols authorizes Kedrion to market and sell in the US, IVIG and albumin manufactured by Grifols for Kedrion. |
| Thousands of | Thousands | |||||||
| Euros | of USD | |||||||
| Cost of business combination (valuation of Class B Shares) | 829,799 | 1,195,574 | ||||||
| Cash paid (19 USD per share) | 1,763,601 | 2,540,997 | ||||||
| Total cost of business combination | 2,593,400 | 3,736,571 | ||||||
| Book value of net assets acquired (provisional) | 469,318 | 676,193 | ||||||
| Goodwill (excess of the cost of the business combination over the fair value of identifiable net assets acquired) | 2,124,082 | 3,060,378 | ||||||
| (see note 6 | ) | |||||||
| Cash paid | 1,763,601 | 2,540,996 | ||||||
| Cash and cash equivalents of the acquired company | (149,693 | ) | (215,678 | ) | ||||
| Cash outflow for the acquisition | 1,613,908 | 2,325,319 | ||||||
F-11
Table of Contents
| Book Value | ||||||||
| Thousands of | Thousands of | |||||||
| Euros | USD | |||||||
| Intangible assets (note 7) | 50,621 | 72,936 | ||||||
| Property, plant and equipment (note 7) | 306,401 | 441,462 | ||||||
| Non — current financial assets | 3,720 | 5,359 | ||||||
| Deferred tax assets | 80,115 | 115,429 | ||||||
| Inventories | 490,976 | 707,398 | ||||||
| Trade and other receivables | 126,772 | 182,653 | ||||||
| Other assets | 3,683 | 5,307 | ||||||
| Cash and cash equivalents | 149,693 | 215,678 | ||||||
| Total assets | 1,211,981 | 1,746,222 | ||||||
| Non — current provisions | 9,250 | 13,327 | ||||||
| Non — current financial liabilities | 6,289 | 9,061 | ||||||
| Current financial liabilities | 473,085 | 681,621 | ||||||
| Current provisions | 31,180 | 44,924 | ||||||
| Trade and other payables | 158,113 | 227,809 | ||||||
| Other current liabilities | 44,055 | 63,475 | ||||||
| Deferred tax liabilities | 20,691 | 29,812 | ||||||
| Total liabilities and contingent liabilities | 742,663 | 1,070,029 | ||||||
| Total net assets acquired | 469,318 | 676,193 | ||||||
| (4) | Financial Risk Management Policy |
F-12
Table of Contents
| (5) | Segment Reporting |
| Net Revenues | ||||||||
| Six Months Ended | Six Months Ended | |||||||
Segments | 30 June 2011 | 30 June 2010 | ||||||
| (Thousands of Euros) | ||||||||
| Bioscience | 521,538 | 380,081 | ||||||
| Hospital | 49,289 | 45,146 | ||||||
| Diagnostic | 56,831 | 54,413 | ||||||
| Raw materials + Other | 7,683 | 8,169 | ||||||
TOTAL | 635,341 | 487,809 | ||||||
| Consolidated | ||||||||
| Income/(loss) | ||||||||
| Six Months Ended | Six Months Ended | |||||||
Segments | 30 June 2011 | 30 June 2010 | ||||||
| (Thousands of Euros) | ||||||||
| Bioscience | 186,521 | 162,938 | ||||||
| Hospital | 4,786 | 5,196 | ||||||
| Diagnostic | (11,264 | ) | 4,798 | |||||
| Raw materials + Other | 3,694 | 4,763 | ||||||
Total income of reported segments | 183,737 | 177,695 | ||||||
| Unallocated expenses plus net financial result | (157,777 | ) | (88,843 | ) | ||||
Profit before income tax from continuing operations | 25,960 | 88,852 | ||||||
F-13
Table of Contents
| (6) | Goodwill |
| Balance at | Business | Translation | Balance at | |||||||||||||||||
| 31/12/10 | Combination | Impairment | Differences | 30/06/11 | ||||||||||||||||
| Thousand Euros | ||||||||||||||||||||
Net value | ||||||||||||||||||||
| Grifols UK,Ltd. (UK) | 7,982 | 0 | 0 | (370 | ) | 7,612 | ||||||||||||||
| Grifols Italia,S.p.A. (Italy) | 6,118 | 0 | 0 | 0 | 6,118 | |||||||||||||||
| Biomat USA, Inc. (USA) | 113,052 | 0 | 0 | (8,534 | ) | 104,518 | ||||||||||||||
| Plasmacare, Inc. (USA) | 38,464 | 0 | 0 | (2,903 | ) | 35,561 | ||||||||||||||
| Woolloomooloo Holdings Pty Ltd. (Australia) | 23,832 | 0 | (13,000 | ) | (415 | ) | 10,417 | |||||||||||||
| Talecris Biotherapeutics | ||||||||||||||||||||
| (USA) | 0 | 2,124,082 | 0 | (6,612 | ) | 2,117,470 | ||||||||||||||
| 189,448 | 2,124,082 | (13,000 | ) | (18,834 | ) | 2,281,696 | ||||||||||||||
| (note 3 | ) | |||||||||||||||||||
| • | UK: bioscience segment | |
| • | Italy: bioscience segment | |
| • | USA: bioscience segment | |
| • | Australia: mainly to diagnostic segment. |
F-14
Table of Contents
| 31/12/2010 | ||||
| Growth Rate | Pre- Tax Discount Rate | |||
| Bioscience | 2.0% - 3.0% | 10.5% - 10.9% | ||
| Diagnostic | 2.0% | 10.4% | ||
| 30/06/2011 | ||||
| Growth Rate | Pre - Tax Discount Rate | |||
| Bioscience | N/A | N/A | ||
| Diagnostic | 2.0% | 11.5% | ||
| (7) | Other Intangible Assets and Property, Plant, and Equipment |
| Other | ||||||||||||
| Intangible | Property, Plant | |||||||||||
| Assets | and Equipment | Total | ||||||||||
Total Cost at 31/12/2010 | 151,861 | 656,295 | 808,156 | |||||||||
Total dep. & amort. At 31/12/2010 | (73,562 | ) | (221,515 | ) | (295,077 | ) | ||||||
Impairment at 31/12/2010 | 0 | (649 | ) | (649 | ) | |||||||
Balance at 31/12/2010 | 78,299 | 434,131 | 512,430 | |||||||||
Cost | ||||||||||||
| Additions | 9,997 | 43,101 | 53,098 | |||||||||
| Business Combination | 50,621 | 306,401 | 357,022 | |||||||||
| Disposals | (588 | ) | (123,965 | ) | (124,553 | ) | ||||||
| Transfers | (126 | ) | (885 | ) | (1,011 | ) | ||||||
| Translation differences | (2,515 | ) | (19,327 | ) | (21,842 | ) | ||||||
Total Cost at 30/06/2011 | 209,250 | 861,620 | 1,070,870 | |||||||||
Depreciation & amortization | ||||||||||||
| Additions | (8,630 | ) | (19,526 | ) | (28,156 | ) | ||||||
| Disposals | 0 | 13,727 | 13,727 | |||||||||
| Transfers | 600 | 411 | 1,011 | |||||||||
| Translation differences | 816 | 5,553 | 6,369 | |||||||||
Total dep. & amort. At 30/06/2011 | (80,776 | ) | (221,350 | ) | (302,126 | ) | ||||||
Impairment | ||||||||||||
| Additions | 0 | 114 | 114 | |||||||||
Impairment at 30/06/2011 | 0 | (535 | ) | (535 | ) | |||||||
Balance at 30/06/2011 | 128,474 | 639,735 | 768,209 | |||||||||
F-15
Table of Contents
| (a) | Sale of Spanish properties and lease back |
| • | Compulsory initial term of five years, | |
| • | Initial rent established at market prices and will be reviewed annually, based on the percentage variation in the Spanish Consumer Price Index (CPI), | |
| • | Automatic extensions of five-year periods that can be avoided by both parties by a six month anticipated notice. | |
| • | Upon vacating the premises, the lessor will reimburse Grifols for the remaining value of leasehold improvements Grifols made. |
| (b) | Sale of properties and equipment in the USA and lease back |
F-16
Table of Contents
| • | Compulsory initial term of 20 years. | |
| • | Initial rent has been established at market prices and will be reviewed annually with a 3% increase. On the first day of the sixth year, the remaining rents until year twenty will be paid in advance in a lump sum. | |
| • | Renewal option to extend for a ten-year period at Grifols Group election. | |
| • | Purchase options granted during the sixth year and in year twenty (20) at market value, to be estimated by independent appraisers. |
| 30/06/11 | ||||
| Thousand Euros | ||||
| Maturity: | ||||
| Up to 1 year | 20,990 | |||
| Between 1 and 5 years | 87,742 | |||
| More than 5 years | 17,293 | |||
| Total future minimum payments | 126,025 | |||
| 30/06/11 | ||||||||
| Current | Non-current | |||||||
| Thousand Euros | ||||||||
| Minimum payments | 4,659 | 17,295 | ||||||
| Interest | (1,932 | ) | (3,553 | ) | ||||
| Present value | 2,727 | 13,742 | ||||||
| 30/06/11 | ||||||||||||
| Minimum | ||||||||||||
| Payments | Interest | Present Value | ||||||||||
| Thousand Euros | ||||||||||||
| Maturity at: | ||||||||||||
| Less than one year | 4,659 | 1,932 | 2,727 | |||||||||
| Two years | 4,391 | 1,486 | 2,905 | |||||||||
| Three years | 4,391 | 1,119 | 3,272 | |||||||||
| Four years | 4,391 | 706 | 3,685 | |||||||||
| Five years | 4,122 | 242 | 3,880 | |||||||||
| Total | 21,954 | 5,485 | 16,469 | |||||||||
F-17
Table of Contents
| (8) | Trade Receivables |
| (9) | Other current assets |
| (10) | Cash and Cash equivalents |
| • | The Group has sold properties in Spain amounting to Euros 80.4 million which together with its related mortgage loan of Euros 53.5 million resulted in a net cash inflow of Euros 26.9 million (see note 7). | |
| • | Part of the consideration paid in the acquisition of Talecris Group has been realized by delivery of Class B shares (see note 3). The issue of Class B shares has had no cash impacts. |
| • | This amount includes a decrease in profit before tax due to the transaction costs incurred by the Group during the six month period ended 30 June 2011 amounting to Euros 38,607 thousand (2,019 thousand for the six months ended at 30 June 2010) that have been paid in this period. | |
| • | Change in current trade and other payables includes Euros 19,516 thousand corresponding to business combination costs accrued by Talecris companies prior to acquisition date and paid during June 2011. |
| (11) | Capital and Reserves |
F-18
Table of Contents
| (a) | Share Capital and Share Premium |
| • | Class A Shares: 213,064,899 ordinary shares of 0.50 Euros nominal value each, fully subscribed and paid up, of the same class and series being the ordinary shares of the Company. | |
| • | Class B Shares: 83,811,688 preference non-voting shares of 0.10 Euros nominal value each, of the same class and series, and with the preferential rights set forth in the Company’s by laws. |
| • | Each Class B share entitles its holder to receive a minimum annual preferred dividend out of the distributable profits at the end of each fiscal year equal to a 0.01 Euros per Class B share if the aggregate preferred dividend does not exceed the distributable profits of that fiscal year. This preferred dividend is not cumulative if no sufficient distributable profits are obtained in the year. | |
| • | Each Class B share is entitled to receive, in addition to the preferred dividend referred to above, the same dividends and other distributions as one Grifols ordinary share. | |
| • | Each Class B share entitles its holder to have it redeemed under certain circumstances, if a tender offer for all or part of the shares in the Company is made and settled except if holders of Class B shares have been entitled to participate in such offer and have their shares acquired in such offer equally and on the same terms as holders of Class A shares. Terms and conditions of redemption incorporated in by laws limit the amounts to be redeemed to the existence of distributable reserves and limit the percentage of shares to be redeemed to a relation to the ordinary shares to which the offer is addressed. | |
| • | Each Class B shares has the right to receive prior to the ordinary shares, upon thewinding-up and liquidation of Grifols, an amount equal to the sum of (i) the nominal value of each Class B share, and (ii) the share premiumpaid-up for such Class B share when it was subscribed for. Each holder is entitled to receive, in addition to the Class B liquidation amount, the same liquidation amount that is paid to each Grifols ordinary share. |
| (b) | Reserves |
F-19
Table of Contents
| (c) | Own Shares |
| Num. of Shares | Thousand Euros | |||||||
| Balance at 1 January 2010 | 53,326 | 677 | ||||||
| Acquisitions | 105,000 | 1,250 | ||||||
Balance at 30 June 2010 and 30 June 2011 | 158,326 | 1,927 | ||||||
| (d) | Dividends |
F-20
Table of Contents
| (12) | Financial Liabilities |
Non-current Financial Liabilities | 30/06/11 | 31/12/10 | ||||||
| Thousand Euros | ||||||||
| Issue of Corporate bonds(a) | 0 | 446,918 | ||||||
| Issue High Yield Bonds(a) | 761,088 | 0 | ||||||
| Transaction costs on bonds | (110,542 | ) | (5,715 | ) | ||||
| Non-current promissory notes(a) | 650,546 | 441,203 | ||||||
| Tranche A (USD) | 830,277 | 0 | ||||||
| Tranche B (USD) | 892,721 | 0 | ||||||
| Tranche A (EUR) | 213,125 | 0 | ||||||
| Tranche B (EUR) | 217,800 | 0 | ||||||
| Implicit Floor and swap floor | (19,565 | ) | 0 | |||||
| Transaction costs on loans and borrowings | (185,314 | ) | (1,365 | ) | ||||
| Club Deal | 0 | 100,000 | ||||||
| Other loans | 18,391 | 120,813 | ||||||
| Finance lease liabilities | 24,963 | 4,734 | ||||||
| Loans and borrowings(b) | 1,992,398 | 224,182 | ||||||
| Loans and borrowings and bonds or other non current marketable securities | 2,642,944 | 665,385 | ||||||
| Financial derivatives | 61,685 | 0 | ||||||
| Other non-current financial liabilities | 10,715 | 10,474 | ||||||
| 2,715,344 | 675,859 | |||||||
| (a) | High Yield Senior Unsecured Notes |
F-21
Table of Contents
| (b) | Loans and borrowings |
| • | Non-current syndicated financing Tranche A: Senior Debt Loan repayable in five years divided into two tranches: U.S. Tranche A and Foreign Tranche A. |
| • | U.S. Tranche A: |
| • | Aggregate Principal Amount of US 1,200 million. | |
| • | Applicable margin of 375 basic points (bp) linked to US Libor. | |
| • | Floor over US Libor of 1.75% |
| • | Foreign Tranche A : |
| • | Aggregate Principal Amount of EUR 220 million. | |
| • | Applicable margin of 400 basic points (bp) linked to Euribor. | |
| • | Floor over Euribor of 1.75% |
| US Tranche A | Foreign Tranche A | |||||||||||||||||||
| Amortization in | Amortization in | |||||||||||||||||||
| Thousands of US | Thousands of | Amortization in | ||||||||||||||||||
| Currency | Dollar | Euros | Currency | Thousands of Euros | ||||||||||||||||
| Maturity | ||||||||||||||||||||
| 2012 | USD | 112,500 | 77,839 | EUR | 20,625 | |||||||||||||||
| 2013 | USD | 127,500 | 88,217 | EUR | 23,375 | |||||||||||||||
| 2014 | USD | 180,000 | 124,542 | EUR | 33,000 | |||||||||||||||
| 2015 | USD | 585,000 | 404,760 | EUR | 107,250 | |||||||||||||||
| 2016 | USD | 195,000 | 134,920 | EUR | 35,750 | |||||||||||||||
Total | USD | 1,200,000 | 830,277 | EUR | 220,000 | |||||||||||||||
| • | Non-current syndicated financing Tranche B: six year loan (payment of whole principal upon maturity) divided into two tranches: U.S. Tranche B and Foreign Tranche B. |
| • | U.S. Tranche B : |
| • | Aggregate Principal Amount of US 1,300 million. | |
| • | Applicable margin of 425 basic points (bp) linked to US Libor. | |
| • | Floor over US Libor of 1.75% |
| • | Foreign Tranche B : |
| • | Aggregate Principal Amount of EUR 220 million. | |
| • | Applicable margin of 450 basic points (bp) linked to Euribor. Floor over Euribor of 1.75% |
F-22
Table of Contents
| US Tranche B | Foreign Tranche B | |||||||||||||||||||
| Amortization in | Amortization in | |||||||||||||||||||
| Thousands of US | Thousands of | Amortization in | ||||||||||||||||||
| Currency | Dollar | Euros | Currency | Thousands of Euros | ||||||||||||||||
| Maturity | ||||||||||||||||||||
| 2011 | USD | 6,500 | 4,497 | EUR | 1,100 | |||||||||||||||
| 2012 | USD | 13,000 | 8,995 | EUR | 2,200 | |||||||||||||||
| 2013 | USD | 13,000 | 8,995 | EUR | 2,200 | |||||||||||||||
| 2014 | USD | 13,000 | 8,995 | EUR | 2,200 | |||||||||||||||
| 2015 | USD | 13,000 | 8,995 | EUR | 2,200 | |||||||||||||||
| 2016 | USD | 9,750 | 6,746 | EUR | 1,650 | |||||||||||||||
| 2017 | USD | 1,231,750 | 852,245 | EUR | 208,450 | |||||||||||||||
Total | USD | 1,300,000 | 899,467 | EUR | 220,000 | |||||||||||||||
| • | Senior revolving credit facilityamounting to US Dollars 300 million. No amounts have been drawn against the credit facility as of 30 June 2011. |
| • | U.S. Revolving Credit Facility: |
| • | Committed Amount : US 50 million | |
| • | Applicable margin of 375 basis point (bp). |
| • | U.S. Multicurrency Revolving Credit Facility: |
| • | Committed Amount : US 200 million | |
| • | Applicable margin of 375 basis point (bp) |
| • | Foreign Revolving Credit Facility : |
| • | Committed Amount : US 50 million. | |
| • | Applicable margin of 400 basis point (bp). |
| Tranche A and B Senior | ||||||||
| High Yield Bond | Loan | |||||||
| Thousands of Euros | ||||||||
| Maturity | ||||||||
| 2011 | 59,301 | 80,474 | ||||||
| 2012 | 62,790 | 235,792 | ||||||
| 2013 | 62,790 | 241,228 | ||||||
| 2014 | 62,790 | 279,372 | ||||||
| 2015 | 62,790 | 613,129 | ||||||
| 2016 | 62,790 | 248,557 | ||||||
| 2017 | 62,790 | 1,087,608 | ||||||
| 2018 | 763,994 | 0 | ||||||
| Total | 1,200,034 | 2,786,160 | ||||||
F-23
Table of Contents
| (c) | Derivatives |
| Notional at | Notional at | Value at | Value at | |||||||||||||
Financial Derivatives | 30/06/11 | 31/12/10 | 30/06/11 | 31/12/10 | ||||||||||||
| Thousands of Euros | ||||||||||||||||
| Interest Rate Swap | 50,000 | 50,000 | (1,146 | ) | (1,809 | ) | ||||||||||
| Interest Rate Swap (Cash flow hedge) | 1,072,442 | — | (3,829 | ) | — | |||||||||||
| Implicit Floor | 3,113,540 | — | (54,364 | ) | — | |||||||||||
| Currency Rate Swap | 47,800 | — | (2,346 | ) | — | |||||||||||
| Liability | 4,283,782 | 50,000 | (61,685 | ) | (1,809 | ) | ||||||||||
| Unquoted future | 17,416 | 23,221 | 3,344 | (2,821 | ) | |||||||||||
| Unquoted future | 26,370 | 26,370 | 4,078 | (3,930 | ) | |||||||||||
| Swap floor | 1,072,442 | — | 32,558 | — | ||||||||||||
| Assets | 1,116,227 | 49,591 | 39,980 | (6,751 | ) | |||||||||||
F-24
Table of Contents
Current Financial Liabilities | 30/06/11 | 31/12/10 | ||||||
| Thousand Euros | ||||||||
| Talecris bonds (note 10) | 427,691 | 0 | ||||||
| Transaction costs High Yield Bonds | (18,032 | ) | 0 | |||||
| Interest accrued on bonds | 27,907 | 7,207 | ||||||
| Promisory notes | 9,586 | 8,235 | ||||||
| Bonds | 447,152 | 15,442 | ||||||
| Tranche A (USD) | 0 | 0 | ||||||
| Tranche B (USD) | 6,746 | 0 | ||||||
| Tranche A (EUR) | 6,875 | 0 | ||||||
| Tranche B (EUR) | 2,200 | 0 | ||||||
| Transaction costs on loans and borrowings | (37,216 | ) | (708 | ) | ||||
| Club Deal | 0 | 66,667 | ||||||
| Other loans | 75,079 | 106,954 | ||||||
| Finance lease liabilities | 6,538 | 3,280 | ||||||
| Loans and borrowings | 60,222 | 176,193 | ||||||
| Loans and borrowings and bonds or other current marketeable securities | 507,374 | 191,635 | ||||||
| Financial derivatives | 7,320 | 8,560 | ||||||
| Other current financial liabilities | 10,016 | 9,676 | ||||||
| Other current financial liabilities | 17,336 | 18,236 | ||||||
| 524,710 | 209,871 | |||||||
| (13) | Financial Income and Expenses |
| (14) | Income Tax |
| (15) | Discontinued Operations |
F-25
Table of Contents
| (a) | Capital Commitments |
| (b) | Plasma Centers of America, LLC and G&M Crandall Limited Family Partnership |
| (c) | Foreign Corrupt Practices Act |
F-26
Table of Contents
| (d) | Compliance with Pharmaceutical Pricing Agreement |
| (17) | Related Parties |
| Key Management | Other Related | Board of Directors | ||||||||||||||
| Associates | Personnel | Parties | of the Company | |||||||||||||
| Thousand Euros | ||||||||||||||||
| Net sales | 21 | — | — | — | ||||||||||||
| Other service expenses | (1,690 | ) | (15,045 | ) | (120 | ) | ||||||||||
| Personnel expenses | — | (3,250 | ) | — | (1,168 | ) | ||||||||||
| Sales of Property | ||||||||||||||||
| Plant and Equipment | — | — | 80,393 | — | ||||||||||||
| (1,669 | ) | (3,250 | ) | 65,348 | (1,288 | ) | ||||||||||
F-27
Table of Contents
| Key Management | Other Related | Board of Directors | ||||||||||
| Personnel | Parties | of the company | ||||||||||
| Thousand Euros | ||||||||||||
| Other service expenses | — | (5,912 | ) | (90 | ) | |||||||
| Personnel expenses | (2,931 | ) | — | (1,033 | ) | |||||||
| (2,931 | ) | (5,912 | ) | (1,123 | ) | |||||||
| (18) | Condensed Consolidating Financial Information |
| (19) | Subsequent events |
F-28
Table of Contents
| Guarantor | Non-Guarantor | Consolidating | ||||||||||||||||||||||
Assets | Parent | Issuer | Subsidiaries | Subsidiaries | Adjustments | Consolidated | ||||||||||||||||||
| (Expressed in thousands of Euros) | ||||||||||||||||||||||||
Non-current assets | ||||||||||||||||||||||||
| Intangible assets | ||||||||||||||||||||||||
| Goodwill | 0 | 0 | 29,895 | 15,123 | 144,430 | 189,448 | ||||||||||||||||||
| Other intangible assets | 5,731 | 3,162 | 49,120 | 4,725 | 15,561 | 78,299 | ||||||||||||||||||
| Total intangible assets | 5,731 | 3,162 | 79,015 | 19,848 | 159,991 | 267,747 | ||||||||||||||||||
| Property, plant and equipment | 95,452 | 28,150 | 231,728 | 78,801 | 0 | 434,131 | ||||||||||||||||||
| Investments in Subsidiaries | 345,025 | 222,273 | 2,532 | 938 | (570,768 | ) | 0 | |||||||||||||||||
| Advances and notes between parent and subsidiaries | 0 | 21,005 | 0 | 0 | (21,005 | ) | 0 | |||||||||||||||||
| Investments in equity accounted investees | 0 | 0 | 0 | 0 | 598 | 598 | ||||||||||||||||||
| Non-current financial assets | 709 | 5,804 | 734 | 288 | 0 | 7,535 | ||||||||||||||||||
| Deferred tax assets | 1,091 | 2,076 | 9,534 | 2,932 | 19,256 | 34,889 | ||||||||||||||||||
| Total non-current assets | 448,008 | 282,470 | 323,543 | 102,807 | (411,928 | ) | 744,900 | |||||||||||||||||
Current assets | ||||||||||||||||||||||||
| Inventories | 796 | 0 | 538,311 | 59,401 | (70,643 | ) | 527,865 | |||||||||||||||||
| Trade and other receivables | ||||||||||||||||||||||||
| Trade receivables | 8,946 | 11,561 | 209,758 | 100,719 | (106,629 | ) | 224,355 | |||||||||||||||||
| Other receivables | 3,157 | 201 | 27,612 | 11,971 | 1,091 | 44,032 | ||||||||||||||||||
| Current income tax assets | 6,168 | 6,071 | 297 | 2,071 | 0 | 14,607 | ||||||||||||||||||
| Trade and other receivables | 18,271 | 17,833 | 237,667 | 114,761 | (105,538 | ) | 282,994 | |||||||||||||||||
| Advances and notes between parent and subsidiaries | 238,262 | (1,311 | ) | 14,699 | 22,860 | (274,510 | ) | 0 | ||||||||||||||||
| Other current financial assets | 267 | 224 | 8 | 12,447 | 0 | 12,946 | ||||||||||||||||||
| Other current assets | 13,460 | 60,568 | 5,150 | 1,450 | 0 | 80,628 | ||||||||||||||||||
| Cash and cash equivalents | 25 | 227,456 | 1,444 | 10,724 | 0 | 239,649 | ||||||||||||||||||
| Total current assets | 271,081 | 304,770 | 797,279 | 221,643 | (450,691 | ) | 1,144,082 | |||||||||||||||||
Total assets | 719,089 | 587,240 | 1,120,822 | 324,450 | (862,619 | ) | 1,888,982 | |||||||||||||||||
F-29
Table of Contents
| Guarantor | Non-Guarantor | Consolidating | ||||||||||||||||||||||
Equity and Liabilities | Parent | Issuer | Subsidiaries | Subsidiaries | Adjustments | Consolidated | ||||||||||||||||||
| (Expressed in thousands of Euros) | ||||||||||||||||||||||||
Equity | ||||||||||||||||||||||||
| Share capital | 106,532 | 0 | 21,497 | 36,340 | (57,837 | ) | 106,532 | |||||||||||||||||
| Share premium | 121,802 | 72,932 | 106,854 | 5,703 | (185,489 | ) | 121,802 | |||||||||||||||||
| Reserves | 73,076 | 190,844 | 175,221 | 59,636 | (95,173 | ) | 403,604 | |||||||||||||||||
| Own shares | (1,927 | ) | 0 | 0 | 0 | 0 | (1,927 | ) | ||||||||||||||||
| Interim dividend | 0 | 0 | 0 | (152 | ) | 152 | 0 | |||||||||||||||||
| Profit for the year attributable to the Parent | 63,226 | (10,726 | ) | 112,853 | 23,906 | (73,746 | ) | 115,513 | ||||||||||||||||
| Total equity | 362,709 | 253,050 | 416,425 | 125,433 | (412,093 | ) | 745,524 | |||||||||||||||||
| Cash flow hedges | 0 | (1,751 | ) | 0 | 0 | 0 | (1,751 | ) | ||||||||||||||||
| Translation differences | 0 | 20,449 | (18,344 | ) | 11,123 | (63,961 | ) | (50,733 | ) | |||||||||||||||
| Accumulated other comprehensive income | 0 | 18,698 | (18,344 | ) | 11,123 | (63,961 | ) | (52,484 | ) | |||||||||||||||
Equity attributable to the Parent | 362,709 | 271,748 | 398,081 | 136,556 | (476,054 | ) | 693,040 | |||||||||||||||||
| Non-controlling interests | 0 | 0 | 0 | 0 | 14,350 | 14,350 | ||||||||||||||||||
Total equity | 362,709 | 271,748 | 398,081 | 136,556 | (461,704 | ) | 707,390 | |||||||||||||||||
Liabilities | ||||||||||||||||||||||||
Non-current liabilities | ||||||||||||||||||||||||
| Grants | 142 | 187 | 1,683 | 76 | 0 | 2,088 | ||||||||||||||||||
| Provisions | 0 | 0 | 1,127 | 251 | 0 | 1,378 | ||||||||||||||||||
| Non-current financial liabilities | ||||||||||||||||||||||||
| Loans and borrowings, bonds and other marketable securities | 133,982 | 441,203 | 40,350 | 49,695 | 155 | 665,385 | ||||||||||||||||||
| Advances and notes between parent and subsidiaries | 15,875 | 0 | 0 | 5,130 | (21,005 | ) | 0 | |||||||||||||||||
| Other financial liabilities | 200 | 0 | 9,596 | 678 | 0 | 10,474 | ||||||||||||||||||
| Total non-current financial liabilities | 150,057 | 441,203 | 49,946 | 55,503 | (20,850 | ) | 675,859 | |||||||||||||||||
| Deferred tax liabilities | 11,907 | 2,860 | 62,718 | 1,519 | 137 | 79,141 | ||||||||||||||||||
| Total non-current liabilities | 162,106 | 444,250 | 115,474 | 57,349 | (20,713 | ) | 758,466 | |||||||||||||||||
Current liabilities | ||||||||||||||||||||||||
| Provisions | 257 | 0 | 30 | 4,078 | 0 | 4,365 | ||||||||||||||||||
| Current financial liabilities | ||||||||||||||||||||||||
| Loans and borrowings, bonds and | ||||||||||||||||||||||||
| other marketable securities | 103,131 | 7,364 | 41,433 | 39,862 | (155 | ) | 191,635 | |||||||||||||||||
| Advances and notes between parent and subsidiaries | 42,863 | (162,772 | ) | 385,947 | 9,017 | (275,055 | ) | 0 | ||||||||||||||||
| Other financial liabilities | 8,830 | 0 | 9,316 | 90 | 0 | 18,236 | ||||||||||||||||||
| Total current financial liabilities | 154,824 | (155,408 | ) | 436,696 | 48,969 | (275,210 | ) | 209,871 | ||||||||||||||||
| Debts with associates | 1,162 | 0 | 0 | 0 | 0 | 1,162 | ||||||||||||||||||
| Trade and other payables | ||||||||||||||||||||||||
| Suppliers | 33,426 | 24,766 | 146,861 | 60,617 | (104,992 | ) | 160,678 | |||||||||||||||||
| Other payables | 1,141 | 12 | 5,288 | 3,627 | 1,860 | 11,928 | ||||||||||||||||||
| Current income tax liabilities | 0 | 0 | 2,369 | 3,663 | (1,860 | ) | 4,172 | |||||||||||||||||
| Total trade and other payables | 34,567 | 24,778 | 154,518 | 67,907 | (104,992 | ) | 176,778 | |||||||||||||||||
| Other current liabilities | 3,464 | 1,872 | 16,023 | 9,591 | 0 | 30,950 | ||||||||||||||||||
| Total current liabilities | 194,274 | (128,758 | ) | 607,267 | 130,545 | (380,202 | ) | 423,126 | ||||||||||||||||
Total liabilities | 356,380 | 315,492 | 722,741 | 187,894 | (400,915 | ) | 1,181,592 | |||||||||||||||||
Total equity and liabilities | 719,089 | 587,240 | 1,120,822 | 324,450 | (862,619 | ) | 1,888,982 | |||||||||||||||||
F-30
Table of Contents
| Guarantor | Non-Guarantor | Consolidating | ||||||||||||||||||||||
Assets | Parent | Issuer | Subsidiaries | Subsidiaries | Adjustments | Consolidated | ||||||||||||||||||
| (Expressed in thousands of euros) | ||||||||||||||||||||||||
Non-current assets | ||||||||||||||||||||||||
| Intangible assets | ||||||||||||||||||||||||
| Goodwill | 0 | 0 | 147,240 | 14,884 | 2,119,572 | 2,281,696 | ||||||||||||||||||
| Other intangible assets | 4,856 | 4,668 | 97,924 | 5,638 | 15,388 | 128,474 | ||||||||||||||||||
| Total intangible assets | 4,856 | 4,668 | 245,164 | 20,522 | 2,134,960 | 2,410,170 | ||||||||||||||||||
| Property, plant and equipment | 60,159 | 25,515 | 517,262 | 36,799 | 0 | 639,735 | ||||||||||||||||||
| Investments in Subsidiaries | 1,132,109 | 2,790,820 | 19,856 | 1,654 | (3,944,439 | ) | 0 | |||||||||||||||||
| Advances and notes between parent and subsidiaries | 0 | 0 | 92,597 | 0 | (92,597 | ) | 0 | |||||||||||||||||
| Investments in equity accounted investees | 0 | 0 | 2,283 | 0 | 1,263 | 3,546 | ||||||||||||||||||
| Non-current financial assets | 756 | 37,830 | 2,261 | 820 | 0 | 41,667 | ||||||||||||||||||
| Deferred tax assets | 1,538 | 28,162 | 91,897 | 3,149 | 14,689 | 139,435 | ||||||||||||||||||
| Total non-current assets | 1,199,418 | 2,886,995 | 971,320 | 62,944 | (1,886,124 | ) | 3,234,553 | |||||||||||||||||
Current assets | ||||||||||||||||||||||||
| Inventories | 861 | 0 | 992,913 | 72,362 | (68,310 | ) | 997,826 | |||||||||||||||||
| Trade and other receivables | ||||||||||||||||||||||||
| Trade receivables | 22,331 | 38,346 | 485,460 | 105,548 | (246,235 | ) | 405,450 | |||||||||||||||||
| Other receivables | 1,452 | 171 | 32,683 | 14,665 | 0 | 48,971 | ||||||||||||||||||
| Current income tax assets | 7,054 | 29,059 | 2,340 | 2,576 | 0 | 41,029 | ||||||||||||||||||
| Trade and other receivables | 30,837 | 67,576 | 520,483 | 122,789 | (246,235 | ) | 495,450 | |||||||||||||||||
| Advances and notes between parent and subsidiaries | 457,222 | 384,790 | 15,364 | 21,536 | (878,912 | ) | 0 | |||||||||||||||||
| Other current financial assets | 7,422 | 211 | 10 | 11,611 | 0 | 19,254 | ||||||||||||||||||
| Other current assets | 1,929 | 235 | 12,201 | 2,154 | (3,175 | ) | 13,344 | |||||||||||||||||
| Cash and cash equivalents | 46,027 | 40,056 | 486,482 | 11,228 | (1 | ) | 583,792 | |||||||||||||||||
| Total current assets | 544,298 | 492,868 | 2,027,453 | 241,680 | (1,196,633 | ) | 2,109,666 | |||||||||||||||||
Total assets | 1,743,716 | 3,379,863 | 2,998,773 | 304,624 | (3,082,757 | ) | 5,344,219 | |||||||||||||||||
F-31
Table of Contents
| Guarantor | Non-Guarantor | Consolidating | ||||||||||||||||||||||
Equity and liabilities | Parent | Issuer | Subsidiaries | Subsidiaries | Adjustments | Consolidated | ||||||||||||||||||
| (Expressed in thousands of euros) | ||||||||||||||||||||||||
Equity | ||||||||||||||||||||||||
| Share capital | 114,914 | 0 | 45,570 | 36,340 | (81,910 | ) | 114,914 | |||||||||||||||||
| Share premium | 890,355 | 849,867 | 623,197 | 6,125 | (1,479,189 | ) | 890,355 | |||||||||||||||||
| Reserves | 134,038 | 232,983 | 310,167 | 77,088 | (184,594 | ) | 569,682 | |||||||||||||||||
| Own shares | (1,927 | ) | 0 | 0 | 0 | 0 | (1,927 | ) | ||||||||||||||||
| Profit for the period/ year attributable to the Parent | 31,802 | (22,950 | ) | 81,268 | (505 | ) | (70,346 | ) | 19,269 | |||||||||||||||
Total equity | 1,169,182 | 1,059,900 | 1,060,202 | 119,048 | (1,816,039 | ) | 1,592,293 | |||||||||||||||||
Available-for-sale financial assets | (575 | ) | 0 | 0 | 0 | 0 | (575 | ) | ||||||||||||||||
| Cash flow hedges | 0 | (2,331 | ) | 0 | 0 | 0 | (2,331 | ) | ||||||||||||||||
| Translation differences | 0 | (2,219 | ) | (40,085 | ) | 5,838 | (52,268 | ) | (88,734 | ) | ||||||||||||||
Other comprehensive income | (575 | ) | (4,550 | ) | (40,085 | ) | 5,838 | (52,268 | ) | (91,640 | ) | |||||||||||||
Equity attributable to the Parent | 1,168,607 | 1,055,350 | 1,020,117 | 124,886 | (1,868,307 | ) | 1,500,653 | |||||||||||||||||
| Non-controlling interests | 0 | 0 | 0 | 0 | 12,941 | 12,941 | ||||||||||||||||||
Total equity | 1,168,607 | 1,055,350 | 1,020,117 | 124,886 | (1,855,366 | ) | 1,513,594 | |||||||||||||||||
Liabilities | ||||||||||||||||||||||||
Non-current liabilities | ||||||||||||||||||||||||
| Grants | 159 | 170 | 1,408 | 78 | 0 | 1,815 | ||||||||||||||||||
| Provisions | 0 | 0 | 10,211 | 250 | 0 | 10,461 | ||||||||||||||||||
| Non-current financial liabilities | ||||||||||||||||||||||||
| Loans and borrowings, bonds and | ||||||||||||||||||||||||
| other marketable securities | 405,262 | 2,200,801 | 36,728 | 279 | (126 | ) | 2,642,944 | |||||||||||||||||
| Advances and notes between parent and subsidiaries | 0 | 0 | 479,210 | 0 | (479,210 | ) | 0 | |||||||||||||||||
| Other financial liabilities | 4,869 | 57,016 | 9,792 | 725 | (2 | ) | 72,400 | |||||||||||||||||
| Total non-current financial liabilities | 410,131 | 2,257,817 | 525,730 | 1,004 | (479,338 | ) | 2,715,344 | |||||||||||||||||
| Deferred tax liabilities | 8,625 | 45,540 | 83,551 | 2,218 | 141 | 140,075 | ||||||||||||||||||
| Total non-current liabilities | 418,915 | 2,303,527 | 620,900 | 3,550 | (479,197 | ) | 2,867,695 | |||||||||||||||||
Current liabilities | ||||||||||||||||||||||||
| Provisions | 341 | 0 | 30 | 4,204 | 31,253 | 35,828 | ||||||||||||||||||
| Current financial liabilities | ||||||||||||||||||||||||
| Loans and borrowings, bonds and other marketable securities | 13,330 | (20,002 | ) | 469,601 | 44,445 | 0 | 507,374 | |||||||||||||||||
| Advances and notes between parent and subsidiaries | 36,901 | 22,625 | 390,957 | 41,816 | (492,299 | ) | 0 | |||||||||||||||||
| Other financial liabilities | 1,330 | 3,829 | 11,937 | 240 | 0 | 17,336 | ||||||||||||||||||
| Total current financial liabilities | 51,561 | 6,452 | 872,495 | 86,501 | (492,299 | ) | 524,710 | |||||||||||||||||
| Debts with associates | 2,352 | 0 | 0 | 0 | 0 | 2,352 | ||||||||||||||||||
Trade and other payables | ||||||||||||||||||||||||
| Suppliers | 82,788 | 14,222 | 357,739 | 67,540 | (255,896 | ) | 266,393 | |||||||||||||||||
| Other payables | 13,511 | 10 | 6,911 | 3,466 | 1,720 | 25,618 | ||||||||||||||||||
| Current income tax liabilities | 2,327 | (976 | ) | 24,144 | 3,452 | (1,720 | ) | 27,227 | ||||||||||||||||
| Total trade and other payables | 98,626 | 13,256 | 388,794 | 74,458 | (255,896 | ) | 319,238 | |||||||||||||||||
| Other current liabilities | 3,314 | 1,278 | 96,437 | 11,025 | (31,252 | ) | 80,802 | |||||||||||||||||
| Total current liabilities | 156,194 | 20,986 | 1,357,756 | 176,188 | (748,194 | ) | 962,930 | |||||||||||||||||
Total liabilities | 575,109 | 2,324,513 | 1,978,656 | 179,738 | (1,227,391 | ) | 3,830,625 | |||||||||||||||||
Total equity and liabilities | 1,743,716 | 3,379,863 | 2,998,773 | 304,624 | (3,082,757 | ) | 5,344,219 | |||||||||||||||||
F-32
Table of Contents
| Guarantor | Non-Guarantor | Consolidating | ||||||||||||||||||||||
Profit and loss | Parent | Issuer | Subsidiaries | Subsidiaries | Adjustments | Consolidated | ||||||||||||||||||
| (Expressed in thousands of Euros) | ||||||||||||||||||||||||
| Revenues | 34,477 | 10,887 | 684,918 | 218,344 | (460,817 | ) | 487,809 | |||||||||||||||||
| Changes in inventories of finished goods and work in progress | 0 | 0 | 16,172 | (1,200 | ) | 26,237 | 41,209 | |||||||||||||||||
| Self-constructed non-current assets | 259 | 529 | 4,340 | (500 | ) | 11,423 | 16,051 | |||||||||||||||||
| Supplies | (80 | ) | 0 | (369,205 | ) | (123,511 | ) | 335,689 | (157,107 | ) | ||||||||||||||
| Other operating income | 41 | 0 | 535 | 55 | 0 | 631 | ||||||||||||||||||
| Personnel expenses | (11,439 | ) | (6,398 | ) | (93,855 | ) | (30,280 | ) | 0 | (141,972 | ) | |||||||||||||
| Other operating expenses | (15,026 | ) | (3,470 | ) | (132,985 | ) | (37,136 | ) | 90,337 | (98,279 | ) | |||||||||||||
| Amortisation and depreciation | (3,634 | ) | (475 | ) | (13,231 | ) | (3,917 | ) | (177 | ) | (21,434 | ) | ||||||||||||
| Transaction costs of Talecris business combination | (322 | ) | (1,678 | ) | (14 | ) | (4 | ) | 0 | (2,019 | ) | |||||||||||||
| Non-financial and other capital grants | 323 | 0 | 227 | 0 | 0 | 550 | ||||||||||||||||||
| Impairment and net losses on disposal of fixed assets | 10 | (1 | ) | (68 | ) | (362 | ) | 1,102 | 681 | |||||||||||||||
Results from operating activities | 4,609 | (606 | ) | 96,834 | 21,489 | 3,794 | 126,120 | |||||||||||||||||
| Finance income | 1,875 | 7,158 | 1,468 | 355 | (8,677 | ) | 2,179 | |||||||||||||||||
| Dividends | 56,774 | 0 | 0 | 159 | (56,933 | ) | 0 | |||||||||||||||||
| Finance expense | (3,868 | ) | (15,610 | ) | (11,955 | ) | (2,534 | ) | 8,682 | (25,285 | ) | |||||||||||||
| Change in fair value of financial instruments | (15,540 | ) | 0 | 0 | 3 | 133 | (15,404 | ) | ||||||||||||||||
| Gains/ (losses) on disposal of financial instruments | 0 | 0 | 0 | (720 | ) | 720 | 0 | |||||||||||||||||
| Exchange gains/ (losses) | 120 | (910 | ) | 37 | 2,723 | 0 | 1,970 | |||||||||||||||||
Net Finance expense | 39,361 | (9,362 | ) | (10,450 | ) | (14 | ) | (56,075 | ) | (36,540 | ) | |||||||||||||
| Share of loss of equity accounted investees | 0 | 0 | 0 | 0 | (728 | ) | (728 | ) | ||||||||||||||||
Profit before income tax | 43,970 | (9,968 | ) | 86,384 | 21,475 | (53,009 | ) | 88,852 | ||||||||||||||||
| Income tax expense | 4,510 | 3,820 | (22,296 | ) | (5,699 | ) | (3,357 | ) | (23,022 | ) | ||||||||||||||
Consolidated profit for the year | 48,480 | (6,148 | ) | 64,088 | 15,776 | (56,366 | ) | 65,830 | ||||||||||||||||
| Profit attributable to equity holders of the Parent | 48,480 | (6,148 | ) | 64,175 | 15,776 | (55,875 | ) | 66,408 | ||||||||||||||||
| Loss attributable to non-controlling interests | 0 | 0 | (87 | ) | 0 | (491 | ) | (578 | ) | |||||||||||||||
F-33
Table of Contents
| Guarantor | Non-Guarantor | Consolidating | ||||||||||||||||||||||
Profit and loss | Parent | Issuer | Subsidiaries | Subsidiaries | Adjustments | Consolidated | ||||||||||||||||||
| (Expressed in thousands of Euros) | ||||||||||||||||||||||||
| Revenues | 28,108 | 11,547 | 872,666 | 218,750 | (495,730 | ) | 635,341 | |||||||||||||||||
| Changes in inventories of finished goods and work in progress | 0 | 0 | (907 | ) | 2,598 | 1,066 | 2,757 | |||||||||||||||||
| Self-constructed non-current assets | 288 | 792 | 23,826 | 2,492 | 4,948 | 32,346 | ||||||||||||||||||
| Supplies | (234 | ) | 0 | (438,787 | ) | (136,825 | ) | 400,704 | (175,142 | ) | ||||||||||||||
| Other operating income | 39 | 0 | 911 | 59 | 0 | 1,009 | ||||||||||||||||||
| Personnel expenses | (13,045 | ) | (7,390 | ) | (130,255 | ) | (33,037 | ) | 0 | (183,727 | ) | |||||||||||||
| Other operating expenses | (17,211 | ) | (4,642 | ) | (176,397 | ) | (38,666 | ) | 81,384 | (155,532 | ) | |||||||||||||
| Amortisation and depreciation | (3,417 | ) | (506 | ) | (19,737 | ) | (4,129 | ) | (367 | ) | (28,156 | ) | ||||||||||||
| Transaction costs of Talecris business combination | (38,462 | ) | (145 | ) | 0 | 0 | 0 | (38,607 | ) | |||||||||||||||
| Non-financial and other capital grants | 333 | 0 | 409 | 0 | 0 | 742 | ||||||||||||||||||
| Impairment and net losses on disposal of fixed assets | 574 | 0 | (4,379 | ) | (5,497 | ) | (13,000 | ) | (22,302 | ) | ||||||||||||||
Results from operating activities | (43,027 | ) | (344 | ) | 127,350 | 5,745 | (20,995 | ) | 68,729 | |||||||||||||||
| Finance income | 3,446 | 7,804 | 1,407 | 697 | (11,593 | ) | 1,761 | |||||||||||||||||
| Dividends | 53,352 | 0 | 0 | (357 | ) | (52,995 | ) | 0 | ||||||||||||||||
| Finance expense | (8,949 | ) | (40,034 | ) | (14,223 | ) | (3,408 | ) | 11,068 | (55,546 | ) | |||||||||||||
| Change in fair value of financial instruments | 16,023 | (2,492 | ) | 414 | 0 | 0 | 13,945 | |||||||||||||||||
| Gains/ (losses) on disposal of financial instruments | 0 | 0 | 0 | (772 | ) | 772 | 0 | |||||||||||||||||
| Exchange gains/ (losses) | 824 | (1,155 | ) | 402 | (2,193 | ) | 0 | (2,122 | ) | |||||||||||||||
Net Finance expense | 64,696 | (35,877 | ) | (12,000 | ) | (6,033 | ) | (52,748 | ) | (41,962 | ) | |||||||||||||
| Share of loss of equity accounted investees | 0 | 0 | 37 | 0 | (844 | ) | (807 | ) | ||||||||||||||||
Profit before income tax | 21,669 | (36,221 | ) | 115,387 | (288 | ) | (74,587 | ) | 25,960 | |||||||||||||||
| Income tax expense | 10,133 | 13,271 | (34,119 | ) | (217 | ) | 3,585 | (7,347 | ) | |||||||||||||||
Consolidated profit for the year | 31,802 | (22,950 | ) | 81,268 | (505 | ) | (71,002 | ) | 18,613 | |||||||||||||||
| Profit attributable to equity holders of the Parent | 31,802 | (22,950 | ) | 81,268 | (505 | ) | (70,346 | ) | 19,269 | |||||||||||||||
| Loss attributable to non-controlling interests | 0 | 0 | 0 | 0 | (656 | ) | (656 | ) | ||||||||||||||||
F-34
Table of Contents
| Guarantor | Non-Guarantor | Consolidating | ||||||||||||||||||||||
| Parent | Issuer | Subsidiaries | Subsidiaries | Adjustments | Consolidated | |||||||||||||||||||
| (Expressed in thousands of Euros) | ||||||||||||||||||||||||
Cash flows from operating activities | ||||||||||||||||||||||||
Profit before tax | 43,970 | (9,968 | ) | 86,384 | 21,475 | (53,009 | ) | 88,852 | ||||||||||||||||
Adjustments for: | (36,258 | ) | 53,117 | (14,924 | ) | 992 | 50,855 | 53,782 | ||||||||||||||||
| Amortisation and depreciation | 3,634 | 475 | 13,231 | 3,917 | 177 | 21,434 | ||||||||||||||||||
| Other adjustments: | (39,892 | ) | 52,642 | (28,155 | ) | (2,925 | ) | 50,678 | 32,348 | |||||||||||||||
| Losses on equity accounted investments | 0 | 0 | 0 | 0 | 728 | 728 | ||||||||||||||||||
| Exchange differences | (120 | ) | 910 | (37 | ) | (2,723 | ) | 0 | (1,970 | ) | ||||||||||||||
| Net provision charges | 0 | 0 | 222 | 611 | (704 | ) | 129 | |||||||||||||||||
| (Profit) / loss on disposal of fixed assets | (10 | ) | 1 | 68 | 362 | (1,102 | ) | (681 | ) | |||||||||||||||
| Government grants taken to income | (323 | ) | 0 | (227 | ) | 0 | 0 | (550 | ) | |||||||||||||||
| Finance expense / income | (39,453 | ) | 15,292 | (1,275 | ) | 1,924 | 56,898 | 33,386 | ||||||||||||||||
| Other adjustments | 14 | 36,439 | (26,906 | ) | (3,099 | ) | (5,142 | ) | 1,306 | |||||||||||||||
Changes in capital and assets | (10,057 | ) | (5,934 | ) | (153,089 | ) | (5,655 | ) | 188,435 | 13,700 | ||||||||||||||
| Change in inventories | (185 | ) | 0 | (15,656 | ) | 1,417 | 2,442 | (11,982 | ) | |||||||||||||||
| Change in trade and other receivables | 1,174 | 178,830 | 84,421 | 13,813 | (257,999 | ) | 20,239 | |||||||||||||||||
| Change in current financial assets and other current assets | (14,769 | ) | (154,124 | ) | (6,038 | ) | (15,249 | ) | 186,305 | (3,875 | ) | |||||||||||||
| Change in current trade and other payables | 3,723 | (30,640 | ) | (215,816 | ) | (5,636 | ) | 257,687 | 9,318 | |||||||||||||||
Other cash flows from operating activities | 59,972 | (10,669 | ) | (18,577 | ) | (7,085 | ) | (58,106 | ) | (34,465 | ) | |||||||||||||
| Interest paid | (2,978 | ) | (15,528 | ) | (686 | ) | (1,153 | ) | 544 | (19,801 | ) | |||||||||||||
| Interest recovered | 1,876 | 540 | 3,301 | 20 | (1,876 | ) | 3,861 | |||||||||||||||||
| Dividends received | 56,774 | 0 | 0 | 0 | (56,774 | ) | 0 | |||||||||||||||||
| Income tax recovered | 4,300 | 4,319 | (21,192 | ) | (5,952 | ) | 0 | (18,525 | ) | |||||||||||||||
Net cash from operating activities | 57,627 | 26,546 | (100,206 | ) | 9,727 | 128,175 | 121,869 | |||||||||||||||||
Cash flows from investing activities | ||||||||||||||||||||||||
| Payments for investments | (5,294 | ) | (10,008 | ) | (33,253 | ) | (10,198 | ) | 1,757 | (56,997 | ) | |||||||||||||
| Group companies and business units | (2,263 | ) | 0 | (535 | ) | (1,472 | ) | 543 | (3,727 | ) | ||||||||||||||
| Property, plant and equipment and intangible assets | (3,013 | ) | (5,915 | ) | (32,734 | ) | (8,702 | ) | 1,214 | (49,151 | ) | |||||||||||||
| Property, plant and equipment | (2,014 | ) | (4,997 | ) | (29,785 | ) | (7,519 | ) | 1,169 | (43,146 | ) | |||||||||||||
| Intangible assets | (999 | ) | (918 | ) | (2,950 | ) | (1,183 | ) | 45 | (6,005 | ) | |||||||||||||
| Other financial assets | (18 | ) | (4,093 | ) | 16 | (24 | ) | 0 | (4,119 | ) | ||||||||||||||
| Proceeds from the sale of property, plant and equipment | 382 | 0 | (1,467 | ) | 3,980 | (32 | ) | 2,863 | ||||||||||||||||
| Group companies and business units | 0 | 0 | (1,464 | ) | 1,423 | 41 | 0 | |||||||||||||||||
| Property, plant and equipment | 382 | 0 | (3 | ) | 2,557 | (73 | ) | 2,863 | ||||||||||||||||
Net cash used in investing activities | (4,912 | ) | (10,008 | ) | (34,720 | ) | (6,218 | ) | 1,725 | (54,134 | ) | |||||||||||||
Cash flows from financing activities | ||||||||||||||||||||||||
| Proceeds from and payments for equity instruments | (1,250 | ) | 0 | 0 | 540 | (540 | ) | (1,250 | ) | |||||||||||||||
| Issue | 0 | 0 | 0 | 540 | (540 | ) | 0 | |||||||||||||||||
| Acquisition of own shares | (1,250 | ) | 0 | 0 | 0 | 0 | (1,250 | ) | ||||||||||||||||
| Proceeds from and payments for financial liability instruments | (26,358 | ) | 3,364 | 191,273 | 9,182 | (186,132 | ) | (8,671 | ) | |||||||||||||||
| Issue | (299 | ) | (184 | ) | 29,580 | 21,970 | 0 | 51,067 | ||||||||||||||||
| Redemption and repayment | (28,016 | ) | (5,417 | ) | (24,265 | ) | (2,040 | ) | 0 | (59,738 | ) | |||||||||||||
| Debts with group companies | 1,957 | 8,965 | 185,958 | (10,748 | ) | (186,132 | ) | 0 | ||||||||||||||||
| Dividends and interest on other equity instruments paid | 0 | 0 | (51,079 | ) | (5,748 | ) | 56,774 | (53 | ) | |||||||||||||||
| Other cash flows from financing activities | 324 | 0 | (1 | ) | 0 | 0 | 323 | |||||||||||||||||
| Transaction costs of financial instruments issued in the acquisition of Talecris | 0 | 0 | 0 | 0 | 0 | 0 | ||||||||||||||||||
| Other amounts received from financing activities | 324 | 0 | (1 | ) | 0 | 0 | 323 | |||||||||||||||||
Net cash from / (used in) financing activities | (27,284 | ) | 3,364 | 140,193 | 3,974 | (129,898 | ) | (9,651 | ) | |||||||||||||||
Effect of exchange rate fluctuations on cash | 0 | 41,374 | 928 | 384 | (2 | ) | 42,684 | |||||||||||||||||
Net increase in cash and cash equivalents | 25,431 | 61,276 | 6,195 | 7,866 | 0 | 100,768 | ||||||||||||||||||
Cash and cash equivalents at beginning of the period | 144 | 237,804 | 7,191 | 4,233 | 0 | 249,372 | ||||||||||||||||||
Cash and cash equivalents at end of period | 25,575 | 299,080 | 13,386 | 12,099 | 0 | 350,140 | ||||||||||||||||||
F-35
Table of Contents
| Guarantor | Non-Guarantor | Consolidating | ||||||||||||||||||||||
| Parent | Issuer | Subsidiaries | Subsidiaries | Adjustments | Consolidated | |||||||||||||||||||
| (Expressed in thousands of Euros) | ||||||||||||||||||||||||
Cash flows from operating activities | ||||||||||||||||||||||||
Profit before tax | 21,669 | (36,221 | ) | 115,387 | (288 | ) | (74,587 | ) | 25,960 | |||||||||||||||
Adjustments for: | (64,381 | ) | 43,007 | (5,099 | ) | 29,999 | 89,112 | 92,638 | ||||||||||||||||
| Amortisation and depreciation | 3,417 | 506 | 19,737 | 4,129 | 367 | 28,156 | ||||||||||||||||||
| Other adjustments: | (67,798 | ) | 42,501 | (24,836 | ) | 25,870 | 88,745 | 64,482 | ||||||||||||||||
| Losses / (Profit) on equity accounted investments | 0 | 0 | (36 | ) | 0 | 843 | 807 | |||||||||||||||||
| Exchange differences | (824 | ) | 1,155 | (402 | ) | 2,193 | 0 | 2,122 | ||||||||||||||||
| Net provision charges | 0 | 0 | 1,121 | 333 | 13,000 | 14,454 | ||||||||||||||||||
| (Profit) / loss on disposal of fixed assets | (574 | ) | 0 | 10,006 | (16 | ) | 0 | 9,416 | ||||||||||||||||
| Government grants taken to income | (333 | ) | 0 | (409 | ) | 0 | 0 | (742 | ) | |||||||||||||||
| Finance expense / income | (66,067 | ) | 36,497 | (32,416 | ) | 24,542 | 74,574 | 37,130 | ||||||||||||||||
| Other adjustments | 0 | 4,849 | (2,700 | ) | (1,182 | ) | 328 | 1,295 | ||||||||||||||||
Changes in capital and assets | (173,540 | ) | (392,604 | ) | (58,615 | ) | (17,028 | ) | 576,628 | (65,159 | ) | |||||||||||||
| Change in inventories | (63 | ) | 0 | 29,076 | (14,981 | ) | (13,280 | ) | 752 | |||||||||||||||
| Change in trade and other receivables | (5,535 | ) | (27,638 | ) | (62,348 | ) | (16,166 | ) | 44,726 | (66,961 | ) | |||||||||||||
| Change in current financial assets and other current assets | (222,035 | ) | (373,208 | ) | (729 | ) | 5,604 | 589,917 | (451 | ) | ||||||||||||||
| Change in current trade and other payables | 54,094 | 8,242 | (24,614 | ) | 8,515 | (44,735 | ) | 1,501 | ||||||||||||||||
Other cash flows from operating activities | 58,291 | (13,635 | ) | (26,294 | ) | (420 | ) | (54,687 | ) | (36,745 | ) | |||||||||||||
| Interest paid | (5,498 | ) | (26,733 | ) | (2,727 | ) | (124 | ) | 1,061 | (34,021 | ) | |||||||||||||
| Interest recovered | 2,395 | 126 | 874 | 0 | (2,396 | ) | 999 | |||||||||||||||||
| Dividends received | 53,352 | 0 | 0 | 0 | (53,352 | ) | 0 | |||||||||||||||||
| Income tax recovered / (paid) | 8,042 | 12,972 | (24,441 | ) | (296 | ) | (0 | ) | (3,723 | ) | ||||||||||||||
Net cash from operating activities | (157,961 | ) | (399,453 | ) | 25,378 | 12,263 | 536,467 | 16,694 | ||||||||||||||||
Cash flows from investing activities | ||||||||||||||||||||||||
| Payments for investments | (4,366 | ) | (1,765,855 | ) | 132,900 | (31,298 | ) | (771 | ) | (1,669,390 | ) | |||||||||||||
| Group companies and business units | (411 | ) | (1,763,601 | ) | 149,693 | (327 | ) | (771 | ) | (1,615,417 | ) | |||||||||||||
| Property, plant and equipment and intangible assets | (3,374 | ) | (2,342 | ) | (16,696 | ) | (30,426 | ) | (0 | ) | (52,838 | ) | ||||||||||||
| Property, plant and equipment | (2,345 | ) | (576 | ) | (10,876 | ) | (29,044 | ) | (0 | ) | (42,841 | ) | ||||||||||||
| Intangible assets | (1,029 | ) | (1,766 | ) | (5,820 | ) | (1,382 | ) | 0 | (9,997 | ) | |||||||||||||
| Other financial assets | (581 | ) | 88 | (97 | ) | (545 | ) | 0 | (1,135 | ) | ||||||||||||||
| Proceeds from the sale of investments | 26,472 | (1 | ) | 41,069 | 1,611 | 0 | 69,151 | |||||||||||||||||
| Property, plant and equipment | 26,472 | (1 | ) | 41,069 | 1,611 | 0 | 69,151 | |||||||||||||||||
Net cash used in investing activities | 22,106 | (1,765,856 | ) | 173,969 | (29,687 | ) | (771 | ) | (1,600,239 | ) | ||||||||||||||
Cash flows from financing activities | ||||||||||||||||||||||||
| Proceeds from and payments for equity instruments | (2,264 | ) | 0 | 0 | 0 | 0 | (2,264 | ) | ||||||||||||||||
| Issue | (2,264 | ) | 0 | 0 | 0 | 0 | (2,264 | ) | ||||||||||||||||
| Proceeds from and payments for financial liability instruments | 206,299 | 2,259,181 | 333,225 | 25,217 | (588,583 | ) | 2,235,339 | |||||||||||||||||
| Issue | 465,722 | 2,517,783 | 9,907 | (10,535 | ) | (0 | ) | 2,982,877 | ||||||||||||||||
| Redemption and repayment | (243,297 | ) | (431,704 | ) | (80,975 | ) | 8,438 | (0 | ) | (747,538 | ) | |||||||||||||
| Debts with group companies | (16,126 | ) | 173,102 | 404,293 | 27,314 | (588,583 | ) | 0 | ||||||||||||||||
| Dividends and interest on other equity instruments paid | 0 | 0 | (47,421 | ) | (5,931 | ) | 53,352 | 0 | ||||||||||||||||
| Other cash flows from financing activities | (22,178 | ) | (264,102 | ) | (486 | ) | (437 | ) | 0 | (287,203 | ) | |||||||||||||
| Deferred acquisition costs of financial instruments related to acquisition of Talecris | (22,540 | ) | (264,099 | ) | (486 | ) | (425 | ) | 0 | (287,550 | ) | |||||||||||||
| Other amounts received from financing activities | 362 | (3 | ) | 0 | (12 | ) | 0 | 347 | ||||||||||||||||
Net cash from financing activities | 181,857 | 1,995,079 | 285,318 | 18,849 | (535,231 | ) | 1,945,872 | |||||||||||||||||
Effect of exchange rate fluctuations on cash | 0 | (17,170 | ) | 373 | (921 | ) | (466 | ) | (18,184 | ) | ||||||||||||||
Net increase / (decrease) in cash and cash equivalents | 46,002 | (187,400 | ) | 485,038 | 504 | (1 | ) | 344,143 | ||||||||||||||||
Cash and cash equivalents at beginning of the period | 25 | 227,456 | 1,444 | 10,724 | 0 | 239,649 | ||||||||||||||||||
Cash and cash equivalents at end of period | 46,027 | 40,056 | 486,482 | 11,228 | (1 | ) | 583,792 | |||||||||||||||||
F-36
Table of Contents
F-37
Table of Contents
| 31/12/10 | 31/12/09 | |||||||
| (Expressed in thousands of Euros) | ||||||||
ASSETS | ||||||||
Non-current assets | ||||||||
| Intangible assets Goodwill (note 7) | 189,448 | 174,000 | ||||||
| Other intangible assets (note 8) | 78,299 | 69,385 | ||||||
| Total intangible assets | 267,747 | 243,385 | ||||||
| Property, plant and equipment (note 9) | 434,131 | 371,705 | ||||||
| Investments in equity accounted investees (note 10) | 598 | 383 | ||||||
| Non-current financial assets (note 11) | 7,535 | 3,731 | ||||||
| Deferred tax assets (note 29) | 34,889 | 33,395 | ||||||
| Total non-current assets | 744,900 | 652,599 | ||||||
Current assets | ||||||||
| Inventories (note 12) | 527,865 | 484,462 | ||||||
| Trade and other receivables Trade receivables | 224,355 | 207,840 | ||||||
| Other receivables | 44,032 | 39,540 | ||||||
| Current income tax assets | 14,607 | 7,802 | ||||||
| Trade and other receivables (note 13) | 282,994 | 255,182 | ||||||
| Other current financial assets (note 14) | 12,946 | 8,217 | ||||||
| Other current assets (note 15) | 80,628 | 7,345 | ||||||
| Cash and cash equivalents (note 16) | 239,649 | 249,372 | ||||||
| Total current assets | 1,144,082 | 1,004,578 | ||||||
Total assets | 1,888,982 | 1,657,177 | ||||||
F-38
Table of Contents
| 31/12/10 | 31/12/09 | |||||||
| (Expressed in thousands of Euros) | ||||||||
EQUITY AND LIABILITIES | ||||||||
Equity | ||||||||
| Share capital | 106,532 | 106,532 | ||||||
| Share premium | 121,802 | 121,802 | ||||||
| Reserves | 403,604 | 314,903 | ||||||
| Own shares | (1,927 | ) | (677 | ) | ||||
| Interim dividend | 0 | (31,960 | ) | |||||
| Profit for the year attributable to the Parent | 115,513 | 147,972 | ||||||
| Total equity | 745,524 | 658,572 | ||||||
| Cash flow hedges | (1,751 | ) | (1,948 | ) | ||||
| Translation differences | (50,733 | ) | (90,253 | ) | ||||
| Accumulated other comprehensive income | (52,484 | ) | (92,201 | ) | ||||
Equity attributable to the Parent (note 17) | 693,040 | 566,371 | ||||||
Non-controlling interests(note 19) | 14,350 | 12,157 | ||||||
Total equity | 707,390 | 578,528 | ||||||
LIABILITIES | ||||||||
Non-current liabilities | ||||||||
| Grants (note 20) | 2,088 | 2,311 | ||||||
| Provisions (note 21) | 1,378 | 1,232 | ||||||
| Non-current financial liabilities Loans and borrowings, bonds and other marketable securities | 665,385 | 703,186 | ||||||
| Other financial liabilities | 10,474 | 12,552 | ||||||
| Total non-current financial liabilities (note 22) | 675,859 | 715,738 | ||||||
| Deferred tax liabilities (note 29) | 79,141 | 60,325 | ||||||
| Total non-current liabilities | 758,466 | 779,606 | ||||||
Current liabilities | ||||||||
| Provisions (note 21) | 4,365 | 4,702 | ||||||
| Current financial liabilities | ||||||||
| Loans and borrowings, bonds and other marketable securities | 191,635 | 113,991 | ||||||
| Other financial liabilities | 18,236 | 12,230 | ||||||
| Total current financial liabilities (note 22) | 209,871 | 126,221 | ||||||
| Debts with associates (note 33) | 1,162 | 0 | ||||||
| Trade and other payables | ||||||||
| Suppliers | 160,678 | 120,909 | ||||||
| Other payables | 11,928 | 17,832 | ||||||
| Current income tax liabilities | 4,172 | 3,258 | ||||||
| Total trade and other payables (note 23) | 176,778 | 141,999 | ||||||
| Other current liabilities (note 24) | 30,950 | 26,121 | ||||||
| Total current liabilities | 423,126 | 299,043 | ||||||
Total liabilities | 1,181,592 | 1,078,649 | ||||||
Total equity and liabilities | 1,888,982 | 1,657,177 | ||||||
F-39
Table of Contents
| 31/12/10 | 31/12/09 | 31/12/08 | ||||||||||
| (Expressed in thousands of Euros) | ||||||||||||
| Revenues (note 25) | 990,730 | 913,186 | 814,311 | |||||||||
| Changes in inventories of finished goods and work in progress (note 12) | 45,749 | 73,093 | 31,058 | |||||||||
| Self-constructed non-current assets (notes 8 and 9) | 33,513 | 41,142 | 25,794 | |||||||||
| Supplies (note 12) | (306,859 | ) | (286,274 | ) | (206,738 | ) | ||||||
| Other operating income (note 27) | 1,196 | 1,443 | 1,289 | |||||||||
| Personnel expenses (note 26) | (289,008 | ) | (273,168 | ) | (238,159 | ) | ||||||
| Other operating expenses (note 27) | (220,218 | ) | (203,381 | ) | (192,288 | ) | ||||||
| Amortisation and depreciation (notes 8 and 9) | (45,776 | ) | (39,554 | ) | (33,256 | ) | ||||||
| Non-financial and other capital grants (note 20) | 728 | 1,188 | 2,941 | |||||||||
| Impairment and net losses on disposal of fixed assets | (372 | ) | (1,147 | ) | (1,991 | ) | ||||||
Results from operating activities | 209,683 | 226,528 | 202,961 | |||||||||
| Finance income | 4,526 | 7,067 | 2,682 | |||||||||
| Finance expenses | (49,660 | ) | (27,087 | ) | (29,305 | ) | ||||||
| Change in fair value of financial instruments (note 32) | (7,593 | ) | (587 | ) | (1,268 | ) | ||||||
| Gains/(losses) on disposal of financial instruments | 91 | (245 | ) | — | ||||||||
| Exchange gains/(losses) | 1,616 | (1,733 | ) | (2,825 | ) | |||||||
Net finance expense(note 28) | (51,020 | ) | (22,585 | ) | (30,716 | ) | ||||||
| Share of profit/(loss) of equity accounted investees (note 10) | (879 | ) | 51 | 24 | ||||||||
Profit before income tax from continuing operations | 157,784 | 203,994 | 172,269 | |||||||||
| Income tax expense (note 29) | (42,517 | ) | (56,424 | ) | (50,153 | ) | ||||||
Profit after income tax from continuing operations | 115,267 | 147,570 | 122,116 | |||||||||
Consolidated profit for the year | 115,267 | 147,570 | 122,116 | |||||||||
| Profit attributable to equity holders of the Parent | 115,513 | 147,972 | 121,728 | |||||||||
| Profit/(loss) attributable to non-controlling interests (note 19) | (246 | ) | (402 | ) | 388 | |||||||
Basic earnings per share (Euros) (note 18) | 0.54 | 0.71 | 0.58 | |||||||||
Diluted earnings per share (Euros) (note 18) | 0.54 | 0.71 | 0.58 | |||||||||
F-40
Table of Contents
| 31/12/10 | 31/12/09 | 31/12/08 | ||||||||||
| (Expressed in thousands of Euros) | ||||||||||||
Consolidated profit for the year | 115,267 | 147,570 | 122,116 | |||||||||
Income and expenses generated during the year | ||||||||||||
| Measurement of financial instruments (note 11) | 0 | (14 | ) | (6 | ) | |||||||
Available-for-sale financial assets | 0 | (18 | ) | (9 | ) | |||||||
| Tax effect | 0 | 4 | 3 | |||||||||
| Cash flow hedges (note 17 (f)) | 0 | (1,998 | ) | 0 | ||||||||
| Cash flow hedges | 0 | (3,275 | ) | 0 | ||||||||
| Tax effect | 0 | 1,277 | 0 | |||||||||
| Translation differences | 42,225 | (4,145 | ) | 13,955 | ||||||||
Income and expenses generated during the year | 42,225 | (6,157 | ) | 13,949 | ||||||||
Income and expense recognised in the income statement: | ||||||||||||
| Measurement of financial instruments (note 11) | 0 | 172 | 0 | |||||||||
Available-for-sale financial assets | 0 | 245 | 0 | |||||||||
| Tax effect | 0 | (73 | ) | 0 | ||||||||
| Cash flow hedges (note 17 (f)) | 197 | 50 | 0 | |||||||||
| Cash flow hedges | 324 | 80 | 0 | |||||||||
| Tax effect | (127 | ) | (30 | ) | 0 | |||||||
Income and expense recognised in the income statement: | 197 | 222 | 0 | |||||||||
Total comprehensive income for the year | 157,689 | 141,635 | 136,065 | |||||||||
| Total comprehensive income attributable to the Parent | 155,230 | 140,386 | 135,781 | |||||||||
| Total comprehensive income attributable to non-controlling interests | 2,459 | 1,249 | 284 | |||||||||
Total comprehensive income for the year | 157,689 | 141,635 | 136,065 | |||||||||
F-41
Table of Contents
| 31/12/10 | 31/12/09 | 31/12/08 | ||||||||||
| (Expressed in thousands of Euros) | ||||||||||||
Cash flows from/(used in) operating activities | ||||||||||||
Profit before income tax | 157,784 | 203,994 | 172,269 | |||||||||
Adjustments for: | 92,351 | 61,800 | 66,034 | |||||||||
| Amortisation and depreciation (notes 8 and 9) | 45,776 | 39,554 | 33,256 | |||||||||
| Other adjustments: | 46,575 | 22,246 | 32,778 | |||||||||
| (Profit)/losses on equity accounted investments (note 10) | 879 | (51 | ) | (24 | ) | |||||||
| Exchange differences | (1,616 | ) | 1,733 | 2,825 | ||||||||
| Impairment of assets and net provision charges | 913 | 53 | 1,994 | |||||||||
| (Profits)/losses on disposal of fixed assets | (276 | ) | 1,147 | 2,001 | ||||||||
| Government grants taken to income (note 20) | (728 | ) | (1,188 | ) | (2,943 | ) | ||||||
| Net finance expense | 47,442 | 17,551 | 27,891 | |||||||||
| Other adjustments | (39 | ) | 3,001 | 1,034 | ||||||||
Change in operating assets and liabilities | (78,767 | ) | (104,127 | ) | (86,550 | ) | ||||||
| Change in inventories | (18,306 | ) | (113,104 | ) | (98,520 | ) | ||||||
| Change in trade and other receivables | (23,546 | ) | (12,549 | ) | (7,951 | ) | ||||||
| Change in current financial assets and other current assets | (73,022 | ) | (1,287 | ) | 405 | |||||||
| Change in current trade and other payables | 36,107 | 22,813 | 19,516 | |||||||||
Other cash flows used in operating activities | (67,116 | ) | (73,487 | ) | (77,310 | ) | ||||||
| Interest paid | (40,129 | ) | (14,719 | ) | (25,972 | ) | ||||||
| Interest received | 5,436 | 2,509 | 2,213 | |||||||||
| Income tax paid | (32,423 | ) | (61,277 | ) | (53,551 | ) | ||||||
Net cash from operating activities | 104,252 | 88,180 | 74,443 | |||||||||
Cash flows from/(used in) investing activities | ||||||||||||
| Payments for investments | (108,588 | ) | (136,626 | ) | (130,923 | ) | ||||||
| Group companies and business units | (1,474 | ) | (15,385 | ) | (632 | ) | ||||||
| Property, plant and equipment and intangible assets | (103,402 | ) | (118,770 | ) | (129,568 | ) | ||||||
| Property, plant and equipment | (86,800 | ) | (103,415 | ) | (119,824 | ) | ||||||
| Intangible assets | (16,602 | ) | (15,355 | ) | (9,744 | ) | ||||||
| Other financial assets | (3,712 | ) | (2,471 | ) | (723 | ) | ||||||
| Proceeds from the sale of investments | 4,532 | 673 | 157 | |||||||||
| Property, plant and equipment | 3,911 | 673 | 157 | |||||||||
| Associates (note 2 (c)) | 621 | 0 | 0 | |||||||||
Net cash used in investing activities | (104,056 | ) | (135,953 | ) | (130,766 | ) | ||||||
Cash flows from/(used in) financing activities | ||||||||||||
| Proceeds from and payments for equity instruments | (1,250 | ) | 26,655 | (4,212 | ) | |||||||
| Issue | 0 | (76 | ) | 0 | ||||||||
| Acquisition of own shares (note 17 (d)) | (1,250 | ) | (25,186 | ) | (4,880 | ) | ||||||
| Disposal of treasury shares | 0 | 51,917 | 668 | |||||||||
| Proceeds from and payments for financial liability instruments | (1,066 | ) | 344,413 | 96,349 | ||||||||
| Issue | 118,238 | 525,078 | 394,109 | |||||||||
| Redemption and repayment | (119,304 | ) | (180,665 | ) | (297,760 | ) | ||||||
| Dividends and interest on other equity instruments paid | (27,282 | ) | (80,913 | ) | (34,792 | ) | ||||||
| Other cash flows from financing activities | 323 | 741 | 0 | |||||||||
| Other amounts received from financing activities | 323 | 741 | 0 | |||||||||
Net cash from/(used in) financing activities | (29,275 | ) | 290,896 | 57,345 | ||||||||
Effect of exchange rate fluctuations on cash | 19,356 | (119 | ) | (344 | ) | |||||||
Net increase/(decrease) in cash and cash equivalents | (9,723 | ) | 243,004 | 678 | ||||||||
Cash and cash equivalents at beginning of the year | 249,372 | 6,368 | 5,690 | |||||||||
Cash and cash equivalents at end of year | 239,649 | 249,372 | 6,368 | |||||||||
F-42
Table of Contents
| Attributable to Equity Holders of the Parent | ||||||||||||||||||||||||||||||||||||||||||||||||
| Accumulated Other Comprehensive Income | ||||||||||||||||||||||||||||||||||||||||||||||||
| Profit | Available-for | Equity | ||||||||||||||||||||||||||||||||||||||||||||||
| Attributable | Sale | Attributable | Non- | |||||||||||||||||||||||||||||||||||||||||||||
| Share | Share | to | Interim | Own | Translation | Cash Flow | Financial | to | Controlling | |||||||||||||||||||||||||||||||||||||||
| Capital | Premium | Reserves* | Parent | Dividend | Shares | Differences | Hedges | Assets | Parent | Interests | Equity | |||||||||||||||||||||||||||||||||||||
| (Expressed in thousands of Euros) | ||||||||||||||||||||||||||||||||||||||||||||||||
Balances at 31 December 2007 | 106,532 | 131,832 | 184,608 | 87,774 | 0 | (28,893 | ) | (98,516 | ) | 0 | (152 | ) | 383,185 | 981 | 384,166 | |||||||||||||||||||||||||||||||||
| Translation differences | — | — | — | — | — | — | 14,059 | — | — | 14,059 | (104 | ) | 13,955 | |||||||||||||||||||||||||||||||||||
Available-for-sale financial assets Gains/(losses) | — | — | — | — | — | — | — | (6 | ) | (6 | ) | — | (6 | ) | ||||||||||||||||||||||||||||||||||
Other comprehensive income for the year | 0 | 0 | 0 | 0 | 0 | 0 | 14,059 | 0 | (6 | ) | 14,053 | (104 | ) | 13,949 | ||||||||||||||||||||||||||||||||||
| Profit for the year | — | — | — | 121,728 | — | — | — | — | — | 121,728 | 388 | 122,116 | ||||||||||||||||||||||||||||||||||||
Total comprehensive income for the year | 0 | 0 | 0 | 121,728 | 0 | 0 | 14,059 | 0 | (6 | ) | 135,781 | 284 | 136,065 | |||||||||||||||||||||||||||||||||||
| Net movement in own shares | — | — | 24 | — | — | (4,194 | ) | — | — | (4,170 | ) | — | (4,170 | ) | ||||||||||||||||||||||||||||||||||
| Other changes | — | — | — | — | — | — | — | — | 0 | (15 | ) | (15 | ) | |||||||||||||||||||||||||||||||||||
| Distribution of 2007 profit | ||||||||||||||||||||||||||||||||||||||||||||||||
| Reserves | — | — | 63,037 | (63,037 | ) | — | — | — | — | — | 0 | — | 0 | |||||||||||||||||||||||||||||||||||
| Dividends | — | (10,030 | ) | — | (24,737 | ) | — | — | — | — | — | (34,767 | ) | — | (34,767 | ) | ||||||||||||||||||||||||||||||||
Transaction with owners of the Company | 0 | (10,030 | ) | 63,061 | (87,774 | ) | 0 | (4,194 | ) | 0 | 0 | 0 | (38,937 | ) | (15 | ) | (38,952 | ) | ||||||||||||||||||||||||||||||
Balances at 31 December 2008 | 106,532 | 121,802 | 247,669 | 121,728 | 0 | (33,087 | ) | (84,457 | ) | 0 | (158 | ) | 480,029 | 1,250 | 481,279 | |||||||||||||||||||||||||||||||||
| Translation differences | — | — | — | — | — | — | (5,796 | ) | — | — | (5,796 | ) | 1,651 | (4,145 | ) | |||||||||||||||||||||||||||||||||
| Cash flow hedges | — | — | — | — | — | — | — | (1,948 | ) | — | (1,948 | ) | — | (1,948 | ) | |||||||||||||||||||||||||||||||||
Gains/(Losses) onavailable-for-sale financial assets | — | — | — | — | — | — | — | — | 158 | 158 | — | 158 | ||||||||||||||||||||||||||||||||||||
Other comprehensive income for the year | 0 | 0 | 0 | 0 | 0 | 0 | (5,796 | ) | (1,948 | ) | 158 | (7,586 | ) | 1,651 | (5,935 | ) | ||||||||||||||||||||||||||||||||
| Profit/(loss) for the year | — | — | — | 147,972 | 0 | — | — | — | — | 147,972 | (402 | ) | 147,570 | |||||||||||||||||||||||||||||||||||
Total comprehensive income for the year | 0 | 0 | 0 | 147,972 | 0 | 0 | (5,796 | ) | (1,948 | ) | 158 | 140,386 | 1,249 | 141,635 | ||||||||||||||||||||||||||||||||||
F-43
Table of Contents
| Attributable to Equity Holders of the Parent | ||||||||||||||||||||||||||||||||||||||||||||||||
| Accumulated Other Comprehensive Income | ||||||||||||||||||||||||||||||||||||||||||||||||
| Profit | Available-for | Equity | ||||||||||||||||||||||||||||||||||||||||||||||
| Attributable | Sale | Attributable | Non- | |||||||||||||||||||||||||||||||||||||||||||||
| Share | Share | to | Interim | Own | Translation | Cash Flow | Financial | to | Controlling | |||||||||||||||||||||||||||||||||||||||
| Capital | Premium | Reserves* | Parent | Dividend | Shares | Differences | Hedges | Assets | Parent | Interests | Equity | |||||||||||||||||||||||||||||||||||||
| (Expressed in thousands of Euros) | ||||||||||||||||||||||||||||||||||||||||||||||||
| Net movement in own shares | — | — | (5,679 | ) | — | 32,410 | — | — | — | 26,731 | — | 26,731 | ||||||||||||||||||||||||||||||||||||
| Other changes | — | — | (124 | ) | — | — | — | — | — | (124 | ) | 44 | (80 | ) | ||||||||||||||||||||||||||||||||||
| Business combinations | — | — | — | — | — | — | — | — | 0 | 9,876 | 9,876 | |||||||||||||||||||||||||||||||||||||
| Distribution of 2008 profit | ||||||||||||||||||||||||||||||||||||||||||||||||
| Reserves | — | — | 73,037 | (73,037 | ) | — | — | — | — | — | 0 | — | 0 | |||||||||||||||||||||||||||||||||||
| Dividends | — | — | — | (48,691 | ) | — | — | — | — | — | (48,691 | ) | (54 | ) | (48,745 | ) | ||||||||||||||||||||||||||||||||
| Interim dividend | — | — | — | — | (31,960 | ) | — | — | — | — | (31,960 | ) | (208 | ) | (32,168 | ) | ||||||||||||||||||||||||||||||||
Transaction with owners of the Company | 0 | 0 | 67,235 | (121,728 | ) | (31,960 | ) | 32,410 | 0 | 0 | 0 | (54,044 | ) | 9,658 | (44,386 | ) | ||||||||||||||||||||||||||||||||
Balance at 31 December 2009 | 106,532 | 121,802 | 314,903 | 147,972 | (31,960 | ) | (677 | ) | (90,253 | ) | (1,948 | ) | 0 | 566,371 | 12,157 | 578,528 | ||||||||||||||||||||||||||||||||
| Translation differences | 0 | 0 | 0 | 0 | 0 | 0 | 39,520 | 0 | 0 | 39,520 | 2,705 | 42,225 | ||||||||||||||||||||||||||||||||||||
| Cash flow hedges | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 197 | 0 | 197 | 0 | 197 | ||||||||||||||||||||||||||||||||||||
Other comprehensive income for the year | 0 | 0 | 0 | 0 | 0 | 0 | 39,520 | 197 | 0 | 39,717 | 2,705 | 42,422 | ||||||||||||||||||||||||||||||||||||
| Profit/(loss) for the year | 0 | 0 | 0 | 115,513 | 0 | 0 | 0 | 0 | 0 | 115,513 | (246 | ) | 115,267 | |||||||||||||||||||||||||||||||||||
Total comprehensive income for the year | 0 | 0 | 0 | 115,513 | 0 | 0 | 39,520 | 197 | 0 | 155,230 | 2,459 | 157,689 | ||||||||||||||||||||||||||||||||||||
| Net movement in own shares | 0 | 0 | 0 | 0 | 0 | (1,250 | ) | 0 | 0 | 0 | (1,250 | ) | (1,250 | ) | ||||||||||||||||||||||||||||||||||
| Other changes | 0 | 0 | (82 | ) | 0 | 0 | 0 | 0 | 0 | 0 | (82 | ) | (213 | ) | (295 | ) | ||||||||||||||||||||||||||||||||
| Distribution of 2009 profit | ||||||||||||||||||||||||||||||||||||||||||||||||
| Reserves | 0 | 0 | 88,783 | (88,783 | ) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||||||||||||||||||||||||||||||||||||
| Dividends | 0 | 0 | 0 | (27,229 | ) | 0 | 0 | 0 | 0 | 0 | (27,229 | ) | (53 | ) | (27,282 | ) | ||||||||||||||||||||||||||||||||
| Interim dividend | 0 | 0 | 0 | (31,960 | ) | 31,960 | 0 | 0 | 0 | 0 | 0 | 0 | ||||||||||||||||||||||||||||||||||||
Transaction with owners of the Company | 0 | 0 | 88,701 | (147,972 | ) | 31,960 | (1,250 | ) | 0 | 0 | 0 | (28,561 | ) | (266 | ) | (28,827 | ) | |||||||||||||||||||||||||||||||
Balance at 31 December 2010 | 106,532 | 121,802 | 403,604 | 115,513 | 0 | (1,927 | ) | (50,733 | ) | (1,751 | ) | 0 | 693,040 | 14,350 | 707,390 | |||||||||||||||||||||||||||||||||
| * | Reserves include accumulated earnings, legal reserves and other reserves |
F-44
Table of Contents
| (1) | Nature, Principal Activities and Subsidiaries |
| (a) | Grifols, S.A. |
| (b) | Subsidiaries |
| • | Industrial area |
F-45
Table of Contents
| • | Commercial area |
F-46
Table of Contents
F-47
Table of Contents
F-48
Table of Contents
| • | Services area |
| (c) | Associates and other participations |
F-49
Table of Contents
| (2) | Basis of Presentation of the Consolidated Financial Statements |
| (a) | Changes to IFRS in 2010, 2009 and 2008 |
| • | Amendment to IAS 39 Reclassification of Financial Assets: Effective Date and Transition (effective date 1 July 2008). | |
| • | IFRIC 16 Hedges of a Net Investment in a Foreign Operation (effective date 1 October 2008). | |
| • | IFRIC 12 Service Concession Arrangements (effective date 1 January 2008). | |
| • | IFRIC 14 IAS 19 The Limit of a Defined Benefit Asset, Minimum Funding Requirements and their Interaction (effective date 1 January 2008) |
F-50
Table of Contents
| • | Amendments to IAS 39 and IFRS 7: Reclassification of Financial Instruments (effective date 1 July 2008). |
| a) | Standards effective as of 1 January 2009 that have required changes to accounting policies and presentation |
| • | IAS 1 Presentation of Financial Statements (revised 2007) (annual periods beginning on or after 1 January 2009). This standard modifies the requirements for presentation of the financial statements, introducing the statement of comprehensive income, which comprises income and other comprehensive income. Entities may also present two separate statements, an income statement showing profit or loss for the year and a statement of other comprehensive income presenting profit or loss for the year and other comprehensive income. When an entity changes an accounting policy retrospectively or makes a retrospective reclassification of items in its financial statements, it must also present a statement of financial position (balance sheet) as at the beginning of the earliest comparative period. | |
| • | IFRS 8 Operating Segments (annual periods beginning on or after 1 January 2009). The impact of this standard mainly relates to the disclosure of financial information by segment. See note 6. | |
| • | IAS 23 Borrowing Costs (revised 2007) (annual periods beginning on or after 1 January 2009). This is a change in accounting policy. The Group applies this standard to borrowing costs related to qualifying assets capitalised on or subsequent to the date this standard became effective. The standard eliminates the possibility of recognising these borrowing costs as an expense, stipulating that borrowing costs that are directly attributable to the acquisition, construction or production of a qualifying asset form part of the cost of that asset. Since 1 January 2009 the Group has capitalised interest amounting to Euros 1,278 thousand (see note 26). | |
| • | Amendments to IFRS 7: “Improving Disclosures about Financial Instruments” (applicable for years beginning on or after 1 January 2009). |
| • | IFRIC 13 Customer Loyalty Programmes (annual periods beginning after 31 December 2008). | |
| • | IFRS 2 Share-Based Payment: Modifications to vesting conditions and cancellations (applied retrospectively to annual periods beginning on or after 1 January 2009). | |
| • | IAS 32 Financial Instruments: Presentation and IAS 1: Presentation of Financial Statements: Changes to puttable financial instruments and obligations arising on liquidation (effective as of 1 January 2009). | |
| • | Improvements to IFRSs. This document modifies various standards and is effective for years beginning on or after 1 July 2009. | |
| • | IFRS 1 First-time Adoption of International Financial Reporting Standards and IAS 27 Consolidated and Separate Financial Statements: These changes relate to the measurement of investments in separate financial statements. This standard is applied prospectively for years started on or after 1 January 2009. | |
| • | Embedded derivatives: Amendments to IFRIC 9 and IAS 39 (applicable for years started after 31 December 2008). | |
| • | IFRS 1 First-time Adoption of International Financial Reporting Standards (applicable to annual periods beginning after 31 December 2009). This change does not affect the Group. | |
| • | IFRIC 15 Agreements for the Construction of Real Estate. |
F-51
Table of Contents
| • | IFRIC 17 Distribution of Non-Cash Assets to Owners (effective date: 1 July 2009). | |
| • | IFRIC 18 Transfers of Assets from Customers (effective date: 1 July 2009). | |
| • | IAS 39 Financial Instruments: Recognition and Measurement. Changes to the items that can be classified as hedged. The amendment clarifies the types of risks that can be classified as hedged when applying hedge accounting (effective date: 1 July 2009). |
| • | Amendment to IFRS 2 Group Cash-settled Share Based Payment Transactions (effective date: 1 January 2010). | |
| • | 2009 improvements to IFRSs (effective date: 1 January 2010). | |
| • | Amendment to IAS 32 Financial Instruments: Presentation, Classification of Rights Issues (effective date: 1 February 2010). | |
| • | IFRIC 19 Extinguishing financial liabilities with equity instruments (effective date: 1 July 2010). | |
| • | Amendment to IFRS 1 Limited Exemption from Comparative IFRS 7 Disclosures for First-time Adopters (effective date: 1 July 2010). | |
| • | IFRS 3 Amendments resulting from May 2010 Annual Improvements (effective date: 1 July 2010). | |
| • | IAS 27 Amendments resulting from May 2010 Annual Improvements (effective date: 1 July 2010). |
| • | IAS 24 Revised Related Party Disclosures | |
| • | Amendment to IFRIC 14: Prepayment of a minimum funding requirement (effective date: 1 January 2011). | |
| • | IFRS 7 Amendments resulting from May 2010 Annual Improvements (effective date: 1 January 2011). | |
| • | Amendment to IFRIC 13 Customer Loyalty Programmes (effective date: 1 January 2011). | |
| • | IAS 34 Amendments resulting from May 2010 Annual Improvements (effective date: 1 January 2011). | |
| • | IAS 1 Amendments resulting from May 2010 Annual Improvements (effective date: 1 January 2011). |
| (b) | Relevant accounting estimates, assumptions and judgements used when applying accounting principles |
F-52
Table of Contents
| • | The assumptions used for calculation of the fair value of financial instruments (see note 4 (k)). | |
| • | Measurement of assets and goodwill to determine any related impairment losses (see note 4(i)). | |
| • | Useful lives of property, plant and equipment and intangible assets (see notes 4(g) and 4(h)). | |
| • | Evaluation of the capitalisation of development costs (see note 4(h)). | |
| • | Evaluation of provisions and contingencies (see note 4(r)). | |
| • | Evaluation of the effectiveness of hedging (see note 4(l) and 17 (f)). | |
| • | The application of the definition of a business (see note 4(b)). |
F-53
Table of Contents
| (c) | Consolidation |
| 31/12/10 | 31/12/09 | 31/12/08 | ||||||||||||||||||||||
| Percentage Ownership | Percentage Ownership | Percentage Ownership | ||||||||||||||||||||||
| Direct | Indirect | Direct | Indirect | Direct | Indirect | |||||||||||||||||||
Parent | ||||||||||||||||||||||||
| Grifols, S.A. | ||||||||||||||||||||||||
Fully-consolidated companies | ||||||||||||||||||||||||
| Laboratorios Grifols, S.A. | 99.998 | 0.002 | 99.998 | 0.002 | 99.998 | 0.002 | ||||||||||||||||||
| Instituto Grifols, S.A. | 99.998 | 0.002 | 99.998 | 0.002 | 99.998 | 0.002 | ||||||||||||||||||
| Movaco, S.A. | 99.999 | 0.001 | 99.999 | 0.001 | 99.999 | 0.001 | ||||||||||||||||||
| Grifols Portugal Productos Farmacéuticos e Hospitalares, Lda. | 0.010 | 99.990 | 0.015 | 99.985 | 0.015 | 99.985 | ||||||||||||||||||
| Diagnostic Grifols, S.A. | 99.998 | 0.002 | 99.998 | 0.002 | 99.998 | 0.002 | ||||||||||||||||||
| Logister, S.A. | — | 100.000 | — | 100.000 | — | 100.000 | ||||||||||||||||||
| Grifols Chile, S.A. | 99.000 | — | 99.000 | — | 99.000 | — | ||||||||||||||||||
| Biomat, S.A. | 99.900 | 0.100 | 99.900 | 0.100 | 99.900 | 0.100 | ||||||||||||||||||
| Grifols Argentina, S.A. | 99.260 | 0.740 | 100.000 | — | 100.000 | — | ||||||||||||||||||
| Grifols, s.r.o. | 100.000 | — | 100.000 | — | 100.000 | — | ||||||||||||||||||
| Logistica Grifols S.A. de C.V. | 99.990 | 0.010 | 100.000 | — | 100.000 | — | ||||||||||||||||||
| Grifols México, S.A. de C.V. | 99.990 | 0.010 | 100.000 | — | 100.000 | — | ||||||||||||||||||
| Grifols Viajes, S.A. | 99.900 | 0.100 | 99.900 | 0.100 | 99.900 | 0.100 | ||||||||||||||||||
| Grifols USA, LLC. | — | 100.000 | — | 100.000 | — | 100.000 | ||||||||||||||||||
| Grifols International, S.A. | 99.900 | 0.100 | 99.900 | 0.100 | 99.900 | 0.100 | ||||||||||||||||||
| Grifols Italia, S.p.A. | 100.000 | — | 100.000 | — | 100.000 | — | ||||||||||||||||||
| Grifols UK, Ltd. | 100.000 | — | 100.000 | — | 100.000 | — | ||||||||||||||||||
| Grifols Deutschland, GmbH | 100.000 | — | 100.000 | — | 100.000 | — | ||||||||||||||||||
| Grifols Brasil, Ltda. | 100.000 | — | 100.000 | — | 100.000 | — | ||||||||||||||||||
| Grifols France, S.A.R.L. | 99.000 | 1.000 | 99.000 | 1.000 | 99.000 | 1.000 | ||||||||||||||||||
| Grifols Engineering, S.A. | 99.950 | 0.050 | 99.950 | 0.050 | 99.950 | 0.050 | ||||||||||||||||||
| Biomat USA, Inc. | — | 100.000 | — | 100.000 | — | 100.000 | ||||||||||||||||||
| Squadron Reinsurance Ltd. | 100.000 | — | 100.000 | — | 100.000 | — | ||||||||||||||||||
| Grifols Inc. | 100.000 | — | 100.000 | — | 100.000 | — | ||||||||||||||||||
| Grifols Biologicals Inc. | — | 100.000 | — | 100.000 | — | 100.000 | ||||||||||||||||||
| Alpha Therapeutic Italia, S.p.A. | 100.000 | — | 100.000 | — | 100.000 | — | ||||||||||||||||||
| Grifols Asia Pacific Pte., Ltd. | 100.000 | — | 100.000 | — | 100.000 | — | ||||||||||||||||||
F-54
Table of Contents
| 31/12/10 | 31/12/09 | 31/12/08 | ||||||||||||||||||||||
| Percentage Ownership | Percentage Ownership | Percentage Ownership | ||||||||||||||||||||||
| Direct | Indirect | Direct | Indirect | Direct | Indirect | |||||||||||||||||||
| Grifols Malaysia Sdn Bhd | — | 30.000 | — | 30.000 | — | 30.000 | ||||||||||||||||||
| Grifols (Thailand) Ltd. | — | 48.000 | — | 48.000 | — | 48.000 | ||||||||||||||||||
| Grifols Polska Sp.z.o.o. | 100.000 | — | 100.000 | — | 100.000 | — | ||||||||||||||||||
| Plasmacare, Inc. | — | 100.000 | — | 100.000 | — | 100.000 | ||||||||||||||||||
| Plasma Collection Centers, Inc. | — | — | — | 100.000 | — | 100.000 | ||||||||||||||||||
| Arrahona Optimus S.L. | 99.995 | 0.005 | 100.000 | — | 100.000 | — | ||||||||||||||||||
| Woolloomooloo Holdings Pty Ltd. | 49.000 | — | 49.000 | — | — | — | ||||||||||||||||||
| Lateral Grifols Pty Ltd. | — | 49.000 | — | 49.000 | — | — | ||||||||||||||||||
| Australian Corporate Number 073 272 830 Pty Ltd | — | 49.000 | — | 49.000 | — | — | ||||||||||||||||||
| Saturn Australia Pty Ltd. | — | 49.000 | — | 49.000 | — | — | ||||||||||||||||||
| Saturn Investments AG | — | 49.000 | — | 49.000 | — | — | ||||||||||||||||||
| Medion Grifols Diagnostic AG | — | 39.200 | — | 39.200 | — | — | ||||||||||||||||||
| Medion Diagnostics GmbH | — | 39.200 | — | 39.200 | — | — | ||||||||||||||||||
| Gri-Cel, S.A. | 0.001 | 99.999 | 0.001 | 99.999 | — | — | ||||||||||||||||||
| Grifols Colombia, Ltda. | 99.000 | 1.000 | — | — | — | — | ||||||||||||||||||
| Grifols Nordic AB | 100.000 | — | — | — | — | — | ||||||||||||||||||
| 31/12/10 | 31/12/09 | 31/12/08 | ||||||||||||||||||||||
| Percentage Ownership | Percentage Ownership | Percentage Ownership | ||||||||||||||||||||||
| Direct | Indirect | Direct | Indirect | Direct | Indirect | |||||||||||||||||||
Companies accounted for using the equity method | ||||||||||||||||||||||||
| Quest International, Inc. | — | — | — | 35.000 | — | 35.000 | ||||||||||||||||||
| Nanotherapix, S.L | — | 51.000 | — | — | — | — | ||||||||||||||||||
F-55
Table of Contents
| (3) | Business Combinations |
| (a) | Acquisition of plasma collection centre from AmeriHealth Plasma LLC. |
| 2009 | 2008 | |||||||
| Thousands of Euros | ||||||||
| Cost of the business combination | ||||||||
| Cash paid | 632 | 632 | ||||||
| Fair value of deferred payment | 1,968 | 1,743 | ||||||
| 2,600 | 2,375 | |||||||
| Fair value of net assets acquired | 3 | 3 | ||||||
| Goodwill | 2,597 | 2,372 | ||||||
| (see note 7 | ) | (see note 7 | ) | |||||
F-56
Table of Contents
| (b) | Acquisition of Australian-Swiss group |
| Thousands of Euros | ||||
| Cost of the business combination | ||||
| Cash paid | 25,000 | |||
| Fair value of deferred payment | 497 | |||
| Total cost of the business combination | 25,497 | |||
| Fair value of net assets acquired | 9,307 | |||
| Goodwill | 16,190 | |||
| (see note 7 | ) | |||
F-57
Table of Contents
| Fair Value | Book Value | |||||||
| Thousands of Euros | ||||||||
| Intangible assets (note 8) | 6,525 | 476 | ||||||
| Property, plant and equipment (note 9) | 2,307 | 3,113 | ||||||
| Deferred tax assets (note 29) | 500 | 258 | ||||||
| Inventories (note 12) | 3,549 | 3,549 | ||||||
| Trade and other receivables | 2,096 | 2,096 | ||||||
| Other assets | 293 | 293 | ||||||
| Cash and cash equivalents | 10,112 | 10,112 | ||||||
| Total assets | 25,382 | 19,897 | ||||||
| Trade and other payables | 3,165 | 3,165 | ||||||
| Other liabilities | 1,273 | 1,272 | ||||||
| Deferred tax liabilities (note 29) | 1,761 | 551 | ||||||
| Total liabilities and contingent liabilities | 6,199 | 4,988 | ||||||
| Total net assets | 19,183 | 14,909 | ||||||
| Non-controlling interests (note 19) | (9,876 | ) | ||||||
| Total net assets acquired | 9,307 | |||||||
| Goodwill (note 7) | 16,190 | |||||||
| Cash paid | 25,497 | |||||||
| Cash and cash equivalents of the acquired company | (10,112 | ) | ||||||
| Cash outflow for the acquisition | 15,385 | |||||||
| (4) | Accounting and Valuation Principles Applied |
| (a) | Subsidiaries |
F-58
Table of Contents
| (b) | Business combinations |
F-59
Table of Contents
| (c) | Non-controlling interests |
F-60
Table of Contents
| (d) | Associates and joint ventures |
F-61
Table of Contents
| (e) | Foreign currency transactions |
| (i) | Functional currency and presentation currency |
| (ii) | Transactions, balances and cash flows in foreign currency |
| (iii) | Translation of foreign operations |
| • | Assets and liabilities, including goodwill and net asset adjustments derived from the acquisition of the operations, including comparative amounts, are translated at the closing rate at each balance sheet date. | |
| • | Income and expenses, including comparative amounts, are translated into thousands of Euros using the previous month’s exchange rate for all transactions performed during the current month. This method does not differ significantly from using the exchange rate at the date of the transaction; | |
| • | All resulting exchange differences are recognised as translation differences in equity. |
F-62
Table of Contents
| (f) | Borrowing costs |
| (g) | Property, plant and equipment |
| (i) | Initial recognition |
| (ii) | Depreciation |
| Depreciation | ||||||||
| Method | Rates | |||||||
| Buildings | Straight line | 1% — 3% | ||||||
| Plant and machinery | Straight line | 8% — 10% | ||||||
| Other installations, equipment and furniture | Straight line | 10% — 30% | ||||||
| Other property, plant and equipment | Straight line | 16% — 25% | ||||||
F-63
Table of Contents
| (h) | Intangible assets |
| (i) | Goodwill |
| (ii) | Internally generated intangible assets |
| • | The Group has technical studies justifying the feasibility of the production process. | |
| • | The Group has undertaken a commitment to complete production of the asset whereby it is in condition for sale or internal use. | |
| • | The asset will generate sufficient future economic benefits. | |
| • | The Group has sufficient financial and technical resources to complete development of the asset and has developed budget and cost accounting control systems which allow budgeted costs, introduced changes and costs actually assigned to different projects to be monitored. |
F-64
Table of Contents
| (iii) | Other intangible assets |
| (iv) | Emission rights |
| (v) | Useful life and amortisation rates |
| Estimated | ||||||
| Amortisation | Years of | |||||
| Method | Useful Life | |||||
| Development expenses | Straight line | 3 — 5 | ||||
| Concessions, patents, licences, trademarks and similar | Straight line | 5 — 15 | ||||
| Software | Straight line | 3 — 6 | ||||
F-65
Table of Contents
| (i) | Impairment of non-financial assets subject to depreciation or amortisation |
| (i) | Lessee accounting records |
F-66
Table of Contents
| (i) | Classification of financial instruments |
| • | it is acquired or incurred principally for the purpose of selling or repurchasing it in the near term | |
| • | it forms part of a portfolio of identified financial instruments that are managed together and for which there is evidence of a recent pattern of short-term profit-taking, or | |
| • | it is a derivative, except for a derivative which has been designated as a hedging instrument and complies with conditions for effectiveness or a derivative that is a financial guarantee contract. |
F-67
Table of Contents
| • | Firstly, the Group applies the quoted prices of the most advantageous active market to which the entity has immediate access, adjusted where appropriate to reflect any differences in counterparty credit risk between instruments traded in that market and the one being valued. The quoted market price for an asset held or liability to be issued is the current bid price and, for an asset to be acquired or liability held, the asking price. If the Group has assets and liabilities with offsetting market risks, it uses mid-market prices as a basis for establishing fair values for the offsetting risk positions and applies the bid or asking price to the net open position as appropriate. | |
| • | When current bid and asking prices are unavailable, the price of the most recent transactions is used, adjusted to reflect changes in economic circumstances. | |
| • | Otherwise, the Group applies generally accepted measurement techniques using, insofar as is possible, market data and, to a lesser extent, specific Group data. |
F-68
Table of Contents
| • | Payment of the cash flows is conditional on their prior collection. | |
| • | The Group is unable to sell or pledge the financial asset. | |
| • | The cash flows collected on behalf of the eventual recipients are remitted without material delay and the Group is not entitled to reinvest the cash flows. This criterion is not applicable to investments in |
F-69
Table of Contents
| cash or cash equivalents made by the Group during the settlement period from the collection date to the date of required remittance to the eventual recipients, provided that interest earned on such investments is passed on to the eventual recipients. |
| • | If the Group has not retained control, it derecognises the financial asset and recognises separately as assets or liabilities any rights and obligations created or retained in the transfer. | |
| • | If the Group has retained control, it continues to recognise the financial asset to the extent of its continuing involvement in the financial asset and recognises an associated liability. The extent of the Group’s continuing involvement in the transferred asset is the extent to which it is exposed to changes in the value of the transferred asset. The transferred asset and the associated liability are measured on a basis that reflects the rights and obligations that the Group has retained. The associated liability is measured in such a way that the carrying amount of the transferred asset and the associated liability is equal to the amortised cost of the rights and obligations retained by the Group, if the transferred asset is measured at amortised cost, or to the fair value of the rights and obligations retained by the Group, if the transferred asset is measured at fair value. The Group continues to recognise any income arising on the transferred asset to the extent of its continuing involvement and recognises any expense incurred on the associated liability. Recognised changes in the fair value of the transferred asset and the associated liability are accounted for consistently with each other in profit and loss or equity, following the general recognition criteria described previously, and are not offset. |
| (l) | Hedge accounting |
F-70
Table of Contents
| (m) | Company own shares |
| (n) | Inventories |
| • | Raw materials and other supplies: replacement cost. Nevertheless, raw materials are not written down below cost if the finished goods into which they will be incorporated are expected to be sold at or above cost of production. | |
| • | Goods for resale and finished goods: estimated selling price, less costs to sell. | |
| • | Work in progress: the estimated selling price of related finished goods, less the estimated costs of completion and the estimated costs necessary to make the sale. |
F-71
Table of Contents
| (o) | Cash and cash equivalents |
| (p) | Government grants |
| (i) | Capital grants |
| (ii) | Operating grants |
| (iii) | Interest rate grants |
| (q) | Employee benefits |
| (i) | Defined contribution plans |
| (ii) | Termination benefits |
F-72
Table of Contents
| (iii) | Short-term employee benefits |
| (r) | Provisions |
| (s) | Revenue recognition |
| (i) | Sale of goods |
| • | the Group has transferred to the buyer the significant risks and rewards of ownership of the goods. | |
| • | the Group retains neither continuing managerial involvement to the degree usually associated with ownership nor effective control over the goods sold; | |
| • | the amount of revenue can be measured reliably; | |
| • | it is probable that the economic benefits associated with the transaction will flow to the Group; and | |
| • | the costs incurred or to be incurred in respect of the transaction can be measured reliably. |
| (ii) | Rendering of services |
F-73
Table of Contents
| (iii) | Revenue from dividends |
| (iv) | Revenue from interest on delayed collections |
| (t) | Income taxes |
| (i) | Taxable temporary differences |
| • | They arise from the initial recognition of goodwill or an asset or liability in a transaction which is not a business combination and at the time of the transaction, affects neither accounting profit nor taxable income; | |
| • | They are associated with investments in subsidiaries over which the Group is able to control the timing of the reversal of the temporary difference and it is not probable that the temporary difference will reverse in the foreseeable future. |
| (ii) | Deductible temporary differences |
| • | It is probable that taxable profit will be available against which the deductible temporary difference can be utilised, unless the differences arise from the initial recognition of an asset or liability in a transaction which is not a business combination and at the time of the transaction, affects neither accounting profit nor taxable profit. |
F-74
Table of Contents
| • | The temporary differences are associated with investments in subsidiaries to the extent that the difference will reverse in the foreseeable future and sufficient taxable income is expected to be generated against which the temporary difference can be offset. |
| (iii) | Measurement |
| (iv) | Offset and recognition |
| (u) | Segment reporting |
| (v) | Classification of assets and liabilities as current and non-current |
| • | Assets are classified as current when they are expected to be realised, or are intended for sale or consumption in the Group’s normal operating cycle within twelve months after the balance sheet date and they are held primarily for the purpose of trading. Cash and cash equivalents are also classified as current, except where they may not be exchanged or used to settle a liability, at least within twelve months after the balance sheet date. |
F-75
Table of Contents
| • | Liabilities are classified as current when they are expected to be settled in the Group’s normal operating cycle within 12 months after the balance sheet date and they are held primarily for the purpose of trading, or where the Group does not have an unconditional right to defer settlement of the liability for at least 12 months after the balance sheet date. | |
| • | Financial liabilities are classified as current when they are due to be settled within twelve months after the reporting period, even if the original term was for a period longer than twelve months, and an agreement to refinance, or to reschedule payments, on a long-term basis is completed after the reporting period and before the consolidated financial statements are authorised for issue. |
| (5) | Financial Risk Management Policy |
| (a) | General |
| • | Credit risk | |
| • | Liquidity risk | |
| • | Market risk |
F-76
Table of Contents
F-77
Table of Contents
| (b) | Capital management |
| (6) | Segment Reporting |
F-78
Table of Contents
| • | Balance sheet: cash and cash equivalents, receivables, public entities, deferred tax assets and liabilities, loans and borrowings and certain payables. | |
| • | Income statement: general administration expenses, other operating income/expenses, finance income/expense and income tax. |
| (a) | Operating segments |
| • | Bioscience: including all activities related with products deriving from human plasma for therapeutic use. | |
| • | Hospital: comprising all non-biological pharmaceutical products and medical supplies manufactured by Group companies earmarked for hospital pharmacy. Products related with this business which the Group does not manufacture but markets as supplementary to its own products are also included. | |
| • | Diagnostic: including the marketing of diagnostic testing equipment, reagents and other equipment, manufactured by Group or other companies. | |
| • | Raw materials: including sales of intermediate biological products and the rendering of manufacturing services to third party companies. |
| % of Sales | ||||||||||||
| 2010 | 2009 | 2008 | ||||||||||
| Hemoderivatives | 77.9 | % | 76.0 | % | 75.6 | % | ||||||
| Other hemoderivatives | 0.2 | % | 0.2 | % | 0.3 | % | ||||||
| Transfusional medicine | 7.9 | % | 8.2 | % | 7.3 | % | ||||||
| In vitro diagnosis | 3.1 | % | 3.1 | % | 3.2 | % | ||||||
| Fluid therapy and nutrition | 5.0 | % | 5.2 | % | 5.7 | % | ||||||
| Hospital supplies | 4.1 | % | 4.2 | % | 4.4 | % | ||||||
| Raw materials | 0.5 | % | 2.5 | % | 2.8 | % | ||||||
| Other | 1.3 | % | 0.6 | % | 0.7 | % | ||||||
| 100 | % | 100 | % | 100 | % | |||||||
| (b) | Geographical information |
| • | Spain | |
| • | Rest of the European Union |
F-79
Table of Contents
| • | United States of America | |
| • | Rest of the world |
| (c) | Main customer |
| (7) | Goodwill |
| Balances at | Business | Translation | Balances at | ||||||||||||||
| 31/12/07 | Combinations | Differences | 31/12/08 | ||||||||||||||
| Thousands of Euros | |||||||||||||||||
Net value | |||||||||||||||||
| Grifols UK, Ltd. | 9,369 | — | (2,156 | ) | 7,213 | ||||||||||||
| Grifols Italia,S.p.A. | 6,118 | — | — | 6,118 | |||||||||||||
| Biomat USA, Inc. | 85,390 | 2,372 | 5,256 | 93,018 | |||||||||||||
| Plasmacare, Inc. | 34,912 | — | 2,017 | 36,929 | |||||||||||||
| Plasma Collection Centers, Inc. | 14,454 | — | 835 | 15,289 | |||||||||||||
| 150,243 | 2,372 | 5,952 | 158,567 | ||||||||||||||
| (note 3(a)) | |||||||||||||||||
| Balances at | Business | Translation | Balances at | |||||||||||||
| 31/12/08 | Combinations | Differences | 31/12/09 | |||||||||||||
| Thousands of Euros | ||||||||||||||||
Net value | ||||||||||||||||
| Grifols UK, Ltd. | 7,213 | — | 523 | 7,736 | ||||||||||||
| Grifols Italia, S.p.A. | 6,118 | — | — | 6,118 | ||||||||||||
| Biomat USA, Inc. | 93,018 | 225 | (3,154 | ) | 90,089 | |||||||||||
| Plasmacare, Inc. | 36,929 | — | (1,253 | ) | 35,676 | |||||||||||
| Plasma Collection Centers, Inc. | 15.289 | — | (519 | ) | 14,770 | |||||||||||
| Woolloomooloo Holdings Pty Ltd. | — | 16,190 | 3,421 | 19,611 | ||||||||||||
| 158,567 | 16,415 | (982 | ) | 174,000 | ||||||||||||
| (note 3(a) | and 3(b)) | |||||||||||||||
F-80
Table of Contents
| Balances at | Translation | Balances at | ||||||||||||||
| 31/12/09 | Transfers | Differences | 31/12/10 | |||||||||||||
| Thousands of Euros | ||||||||||||||||
Net value | ||||||||||||||||
| Grifols UK, Ltd. | 7,736 | — | 246 | 7,982 | ||||||||||||
| Grifols Italia,S.p.A. | 6,118 | — | — | 6,118 | ||||||||||||
| Biomat USA, Inc. | 90,089 | 14,770 | 8,193 | 113,052 | ||||||||||||
| Plasmacare, Inc. | 35,676 | — | 2,788 | 38,464 | ||||||||||||
| Plasma Collection Centers, Inc. | 14,770 | (14,770 | ) | — | — | |||||||||||
| Woolloomooloo Holdings Pty Ltd. | 19,611 | — | 4,221 | 23,832 | ||||||||||||
| 174,000 | — | 15,448 | 189,448 | |||||||||||||
| • | UK: bioscience segment | |
| • | Italy: bioscience segment | |
| • | USA: bioscience segment | |
| • | Australia: mainly to the Diagnostics segment. |
| Growth Rate | Discount Rate After Tax | |||||||||||||||
| 2010 | 2009 | 2010 | 2009 | |||||||||||||
| Bioscience | 2% — 3% | 3% | 8% — 8.5% | 8% | ||||||||||||
| Diagnostic | 2% | 2% | 8.30% | 8.7% | ||||||||||||
F-81
Table of Contents
| Discount Rate Before Tax | ||||||||
| 2010 | 2009 | |||||||
| Bioscience | 10.5% — 10.9% | 9.5% — 13% | ||||||
| Diagnostic | 10.40% | 9.8% | ||||||
| (8) | Other Intangible Assets |
| Discount Rate After Tax | ||||||||
| 2010 | 2009 | |||||||
| Growth rate used to extrapolate projections | 3% | 3% | ||||||
| Discount rate after tax | 8.5% | 8% | ||||||
F-82
Table of Contents
| (9) | Property, Plant and Equipment |
| • | Purchase of land and buildings in Parets del Vallès, Barcelona with a value of Euros 19.4 million, financed through a mortgage loan from Caixa Catalunya. | |
| • | Purchase of land and buildings under construction in Sant Cugat del Vallès, Barcelona through the acquisition of the real estate company Arrahona Optimus, S.L. for Euros 33 million at 31 December 2008, financed through a mortgage loan from BBVA. |
| a) | Mortgaged property, plant and equipment |
| b) | Official capital grants received |
| c) | Insurance |
| d) | Revalued assets |
F-83
Table of Contents
| e) | Assets under finance lease |
| Accumulated | ||||||||||||
Asset | Cost | Depreciation | Net value | |||||||||
| Thousands of Euros | ||||||||||||
| Technical installations and other property, plant and equipment | 19,641 | (5,507 | ) | 14,134 | ||||||||
| Accumulated | ||||||||||||
Asset | Cost | Depreciation | Net value | |||||||||
| Thousands of Euros | ||||||||||||
| Technical installations and other property, plant and equipment | 15,264 | (4,782 | ) | 10,482 | ||||||||
| f) | Fully-depreciated assets |
| g) | Self-constructed property, plant and equipment |
| (10) | Investments Accounted for Using the Equity Method |
| Balances at | Translation | Balances at | ||||||||||||||
| 31/12/07 | Gains | Differences | 31/12/08 | |||||||||||||
| Thousands of Euros | ||||||||||||||||
| Equity accounted investments | 243 | 24 | 107 | 374 | ||||||||||||
F-84
Table of Contents
| Balances at | Translation | Balances at | ||||||||||||||
| 31/12/08 | Gains | Differences | 31/12/09 | |||||||||||||
| Thousands of Euros | ||||||||||||||||
| Equity accounted investments | 374 | 51 | (42 | ) | 383 | |||||||||||
| Balances at | Gain/(Losses & | Translation | Balances at | |||||||||||||||||||||
| 31/12/09 | Impairment) | Disposals | Acquisitions | Differences | 31/12/10 | |||||||||||||||||||
| Thousands of Euros | ||||||||||||||||||||||||
| Equity accounted investments | 383 | (879 | ) | (463 | ) | 1,472 | 85 | 598 | ||||||||||||||||
| Percentage | ||||||||||||||||||||||||
| Country | Ownership | Assets | Liabilities | Equity | Result | |||||||||||||||||||
| Thousands of Euros | ||||||||||||||||||||||||
31/12/2008 | ||||||||||||||||||||||||
| Quest International, Inc | USA | 35 | % | 1,736 | 667 | 1,069 | 69 | |||||||||||||||||
31/12/2009 | ||||||||||||||||||||||||
| Quest International, Inc | USA | 35 | % | 1,664 | 580 | 1,084 | 145 | |||||||||||||||||
31/12/2010 | ||||||||||||||||||||||||
| Nanotherapix, S.L. | Spain | 51 | % | 2,375 | 1,212 | 1,163 | (312 | ) | ||||||||||||||||
| (11) | Non-Current Financial Assets |
| 31/12/10 | 31/12/09 | 31/12/08 | ||||||||||
| Thousands of Euros | ||||||||||||
| Non-current guarantee deposits | 1,217 | 1,142 | 1,113 | |||||||||
| Assets available for sale | 535 | 501 | 523 | |||||||||
| Loans to third parties | 5,783 | 2,088 | — | |||||||||
Non-current financial assets | 7,535 | 3,731 | 1,636 | |||||||||
| • | The interest of less than 1% that the Group holds in Northfield Laboratories, Inc. (USA). At 31 December 2010, 2009 and 2008 provision has been made for the full amount of this investment, based on its fair value. |
F-85
Table of Contents
| • | The interest of less than 2% in the share capital of biotechnology company, Cardio 3 Bioscience (with registered offices in Belgium) acquired by Grifols, S.A. in December 2008 for an amount of Euros 500 thousand. The activity of this company involves research into and the development of biological therapies using stem cells for the treatment of cardiovascular diseases. The Group has measured this asset at cost, as its fair value cannot be reliably determined. |
| (12) | Inventories |
| 2010 | 2009 | |||||||
| Thousands of Euros | ||||||||
| Goods for resale | 63,050 | 65,718 | ||||||
| Raw materials and other supplies | 160,326 | 170,987 | ||||||
| Work in progress and semi-finished goods | 203,971 | 146,612 | ||||||
| Finished goods | 100,518 | 101,145 | ||||||
| 527,865 | 484,462 | |||||||
| 31/12/10 | 31/12/09 | 31/12/08 | ||||||||||
| * | * | * | ||||||||||
| Thousands of Euros | ||||||||||||
| Inventories of goods for resale | ||||||||||||
| Net purchases | 56,542 | 50,886 | 79,902 | |||||||||
| Changes in inventories | 6,911 | (9,201 | ) | (22,700 | ) | |||||||
| 63,453 | 41,685 | 57,202 | ||||||||||
| Raw materials and supplies | ||||||||||||
| Net purchases | 225,994 | 274,537 | 190,667 | |||||||||
| Changes in inventories | 17,412 | (29,948 | ) | (41,131 | ) | |||||||
| 243,406 | 244,589 | 149,536 | ||||||||||
| Materials consumed | 306,859 | 286,274 | 206,738 | |||||||||
| Changes in inventories of finished products and work in progress | (45,749 | ) | (73,093 | ) | (31,058 | ) | ||||||
| Changes in inventories of finished products , work in progress and materials consumed | 261,110 | 213,181 | 175,680 | |||||||||
| * | Expenses/(Income) |
F-86
Table of Contents
| 2010 | 2009 | 2008 | ||||||||||
| Thousands of Euros | ||||||||||||
| Inventories of goods for resale at 1 January | 65,718 | 54,509 | 37,138 | |||||||||
| Business combinations | — | 158 | — | |||||||||
| Net cancellations for the year | — | (568 | ) | (515 | ) | |||||||
| Increase/(decrease) of goods for resale | (6,911 | ) | 9,201 | 22,700 | ||||||||
| Translation differences | 4,243 | 2,418 | (4,814 | ) | ||||||||
| Inventories of goods for resale at 31 December | 63,050 | 65,718 | 54,509 | |||||||||
| 2010 | 2009 | 2008 | ||||||||||
| Thousands of Euros | ||||||||||||
| Inventories of raw materials at 1 January | �� | 170,987 | 142,209 | 96,044 | ||||||||
| Business combinations | — | 824 | — | |||||||||
| Increase/(decrease) in raw materials | (17,412 | ) | 29,948 | 41,131 | ||||||||
| Translation differences | 6,751 | (1,994 | ) | 5,034 | ||||||||
| Inventories of raw materials at 31 December | 160,326 | 170,987 | 142,209 | |||||||||
| 2010 | 2009 | 2008 | ||||||||||
| Thousands of Euros | ||||||||||||
| Inventories of finished goods and work in progress at 1 January | 247,757 | 176,939 | 138,226 | |||||||||
| Business combinations | — | 2,567 | — | |||||||||
| Increase in inventories of finished goods and work in progress | 45,749 | 73,093 | 31,058 | |||||||||
| Translation differences | 10,983 | (4,842 | ) | 7.655 | ||||||||
| Inventories of finished goods and work in progress at 31 December | 304,489 | 247,757 | 176,939 | |||||||||
| 31/12/10 | 31/12/09 | 31/12/08 | ||||||||||
| Thousands of Euros | ||||||||||||
Currency | ||||||||||||
| US Dollar | 145,584 | 196,936 | 168,037 | |||||||||
| Other currencies | 6,569 | 4,498 | 7,315 | |||||||||
F-87
Table of Contents
| (13) | Trade and Other Receivables |
| 31/12/10 | 31/12/09 | |||||||
| Thousands of Euros | ||||||||
Trade receivables | 224,355 | 207,840 | ||||||
| Other receivables | 31,012 | 27,210 | ||||||
| Associates | 5 | 812 | ||||||
| Personnel | 366 | 395 | ||||||
| Advances for fixed assets | 494 | 1,103 | ||||||
| Other advances | 3,265 | 1,844 | ||||||
| Public entities, other receivables | 8,890 | 8,176 | ||||||
Other receivables | 44,032 | 39,540 | ||||||
Current income tax assets | 14,607 | 7,802 | ||||||
| 282,994 | 255,182 | |||||||
| 31/12/10 | 31/12/09 | |||||||
| Thousands of Euros | ||||||||
Currency | ||||||||
| US Dollar | 52,466 | 45,297 | ||||||
| Chilean Peso | 17,008 | 12,778 | ||||||
| Mexican Peso | 10,583 | 7,986 | ||||||
| Argentinean Peso | 4,075 | 3,404 | ||||||
| Brazilian Real | 4,616 | 3,225 | ||||||
| Czech Crown | 3,030 | 3,217 | ||||||
| Pound Sterling | 3,116 | 2,849 | ||||||
| Thai Baht | 1,842 | 1,366 | ||||||
| Polish Zloty | 2,379 | 1,292 | ||||||
| Australian Dollar | 3,769 | 1,101 | ||||||
| Other currencies | 2,412 | 1,644 | ||||||
F-88
Table of Contents
| 31/12/10 | 31/12/09 | |||||||
| Thousands of Euros | ||||||||
| Taxation authorities, VAT | 8,191 | 7,451 | ||||||
| Social Security | 85 | 107 | ||||||
| Other public entities | 614 | 618 | ||||||
| Public entities, other receivables | 8,890 | 8,176 | ||||||
| 31/12/10 | 31/12/09 | |||||||
| Thousands of Euros | ||||||||
| Recoverable income tax: | ||||||||
| Current year | 9,352 | 7,188 | ||||||
| Prior years | 5,255 | 614 | ||||||
| Current tax assets | 14,607 | 7,802 | ||||||
| (14) | Other Current Financial Assets |
| 31/12/10 | 31/12/09 | |||||||
| Thousands of Euros | ||||||||
| Current investments | 12,387 | 5,943 | ||||||
| Guarantee deposits | 44 | 209 | ||||||
| Current loans to third parties | 515 | 395 | ||||||
| Financial derivatives (note 32) | — | 1,670 | ||||||
| Total other current financial assets | 12,946 | 8,217 | ||||||
F-89
Table of Contents
| (15) | Other Current Assets |
| 31/12/10 | 31/12/09 | |||||||
| Thousands of Euros | ||||||||
| Prepaid expenses — professional services | 72,983 | 1,703 | ||||||
| Prepaid expenses — insurance | 3,508 | 3,403 | ||||||
| Royalties and rentals | 2,589 | 611 | ||||||
| Other prepaid expenses | 1,548 | 1,628 | ||||||
| Total other current assets | 80,628 | 7,345 | ||||||
| (16) | Cash and Cash Equivalents |
| 31/12/10 | 31/12/09 | |||||||
| Thousands of Euros | ||||||||
| Current deposits | 211,564 | 237,801 | ||||||
| Cash at banks | 28,085 | 11,571 | ||||||
| �� | ||||||||
| Total cash and cash equivalents | 239,649 | 249,372 | ||||||
| 31/12/10 | 31/12/09 | |||||||
| Thousands of Euros | ||||||||
Currency | ||||||||
| Euro | 4,268 | 2,153 | ||||||
| US Dollar | 202,942 | 208,800 | ||||||
| Other currency | 32,439 | 38,419 | ||||||
| 239,649 | 249,372 | |||||||
| (17) | Equity |
F-90
Table of Contents
| (a) | Share capital |
| Percentage Ownership | ||||||||
| 31/12/10 | 31/12/09 | |||||||
| Scranton Enterprises,B.V. | 7.58 | % | 10.65 | % | ||||
| Capital Research and Management Company | 10.02 | % | — | |||||
| Other | 82.40 | % | 89.35 | % | ||||
| 100.00 | % | 100.00 | % | |||||
| (b) | Share premium |
| (c) | Reserves |
F-91
Table of Contents
| (d) | Own shares |
| No. of | Thousands of | |||||||
| Shares | Euros | |||||||
| Balance at 1 January 2009 | 2,411,622 | 33,087 | ||||||
| Acquisitions | 2,176,929 | 25,186 | ||||||
| Disposals | (4,535,225 | ) | (57,596 | ) | ||||
| Balance at 31 December 2009 | 53,326 | 677 | ||||||
| No. of | Thousands of | |||||||
| Shares | Euros | |||||||
| Balance at 1 January 2010 | 53,326 | 677 | ||||||
| Acquisitions | 105,000 | 1,250 | ||||||
| Balance at 31 December 2010 | 158,326 | 1,927 | ||||||
| (e) | Distribution of profits |
| 30/06/2009 | ||||||||||||
| Amount | ||||||||||||
| % of par | Euro per | (Thousands of | ||||||||||
| Value | Share | Euros) | ||||||||||
| Ordinary shares | 46 | 0.23 | 48,691 | |||||||||
Total dividends paid in June 2009 | 46 | 0.23 | 48,691 | |||||||||
| Dividends with a charge to profits | 46 | 0.23 | 48,691 | |||||||||
Total dividends paid in June 2009 | 46 | 0.23 | 48,691 | |||||||||
F-92
Table of Contents
| 31/12/2009 | ||||||||||||
| Amount | ||||||||||||
| % of par | Euro per | (Thousands of | ||||||||||
| Value | Share | Euros) | ||||||||||
| Ordinary shares | 30 | 0.15 | 31,960 | |||||||||
Total dividends paid in December 2009 | 30 | 0.15 | 31,960 | |||||||||
| Interim dividend | 30 | 0.15 | 31,960 | |||||||||
Total dividends paid in December 2009 | 30 | 0.15 | 31,960 | |||||||||
| 31/07/2010 | ||||||||||||
| Amount | ||||||||||||
| % of par | Euro per | (Thousands of | ||||||||||
| Value | Share | Euros) | ||||||||||
Total dividends paid in July 2010(ordinary shares) | 26 | 0.13 | 27,229 | |||||||||
| (f) | Cash flow hedges |
| (18) | Earnings per Share |
| 2010 | 2009 | 2008 | ||||||||||
| Profit for the year attributable to equity holders of the Company (thousands of Euros) | 115,513 | 147,972 | 121,728 | |||||||||
| Weighted average number of ordinary shares in circulation | 212,909,162 | 209,451,806 | 210,707,597 | |||||||||
| Basic earnings per share (Euros per share) | 0.54 | 0.71 | 0.58 | |||||||||
| Number of Shares | ||||||||||||
| 2010 | 2009 | 2008 | ||||||||||
| Issued ordinary shares at 1 January | 213,011,573 | 210,653,277 | 210,964,436 | |||||||||
| Effect of own shares | (102,411 | ) | (1,201,471 | ) | (256,839 | ) | ||||||
| 212,909,162 | 209,451,806 | 210,707,597 | ||||||||||
F-93
Table of Contents
| (19) | Non-controlling Interests |
| Balances at | Business | Translation | Balances at | |||||||||||||||||||||
| 31/12/08 | Additions | Combinations | Dividends | Differences | 31/12/09 | |||||||||||||||||||
| Thousands of Euros | ||||||||||||||||||||||||
| Grifols (Thailand) Pte Ltd | 977 | 308 | — | (112 | ) | 30 | 1,203 | |||||||||||||||||
| Grifols Malaysia Sdn Bhd | 273 | 35 | — | — | (5 | ) | 303 | |||||||||||||||||
| Woolloomooloo Holdings Pty Ltd. | — | (745 | ) | 9,876 | (106 | ) | 1,626 | 10,651 | ||||||||||||||||
| 1,250 | (402 | ) | 9,876 | (218 | ) | 1,651 | 12,157 | |||||||||||||||||
| (note 3 | (b)) | |||||||||||||||||||||||
| Balances at | Translation | Balances at | ||||||||||||||||||
| 31/12/09 | Additions | Dividends | Differences | 31/12/10 | ||||||||||||||||
| Thousands of Euros | ||||||||||||||||||||
| Grifols (Thailand) Pte Ltd | 1,203 | 367 | (108 | ) | 255 | 1,717 | ||||||||||||||
| Grifols Malaysia Sdn Bhd | 303 | 302 | — | 76 | 681 | |||||||||||||||
| Woolloomooloo Holdings Pty Ltd. | 10,651 | (915 | ) | (158 | ) | 2,374 | 11,952 | |||||||||||||
| 12,157 | (246 | ) | (266 | ) | 2,705 | 14,350 | ||||||||||||||
| (20) | Grants |
| 31/12/10 | 31/12/09 | |||||||
| Thousands of Euros | ||||||||
| Capital grants | 1,830 | 2,025 | ||||||
| Interest-rate grants (preference loans) | 258 | 286 | ||||||
Grants | 2,088 | 2,311 | ||||||
F-94
Table of Contents
| 31/12/10 | 31/12/09 | |||||||
| Thousands of Euros | ||||||||
| Total amount of capital grant: | ||||||||
| Prior to 1995 | 330 | 330 | ||||||
| 1995 | 627 | 627 | ||||||
| 1996 | 54 | 54 | ||||||
| 1997 | 426 | 426 | ||||||
| 1998 | 65 | 65 | ||||||
| 1999 | 42 | 42 | ||||||
| 2000 | 181 | 181 | ||||||
| 2001 | 214 | 214 | ||||||
| 2002 | 626 | 626 | ||||||
| 2004 | 1,940 | 1,940 | ||||||
| 2005 | 35 | 35 | ||||||
| 2006 | 35 | 35 | ||||||
| 2007 | 33 | 33 | ||||||
| 2008 | 124 | 124 | ||||||
| 2009 | 742 | 742 | ||||||
| Current period | 323 | — | ||||||
| 5,797 | 5,474 | |||||||
| Less, revenues recognised: | ||||||||
| Prior years | (3,140 | ) | (2,444 | ) | ||||
| Current year | (612 | ) | (696 | ) | ||||
| (3,752 | ) | (3,140 | ) | |||||
| Translation differences | (215 | ) | (309 | ) | ||||
| Net value of capital grants | 1,830 | 2,025 | ||||||
| Balances at | Transfers to | Balances at | ||||||||||||||
| 31/12/07 | Additions | Profit or Loss | 31/12/08 | |||||||||||||
| Interest-rate grants (preference loans) | 2,463 | 561 | (2,686 | ) | 338 | |||||||||||
| Balances at | Transfers to | Balances at | ||||||||||||||
| 31/12/08 | Additions | Profit or Loss | 31/12/09 | |||||||||||||
| Interest-rate grants (preference loans) | 338 | 440 | (492 | ) | 286 | |||||||||||
F-95
Table of Contents
| Balances at | Transfers to | Balances at | ||||||||||||||
| 31/12/09 | Additions | Profit or Loss | 31/12/10 | |||||||||||||
| Interest-rate grants (preference loans) | 286 | 88 | (116 | ) | 258 | |||||||||||
| (21) | Provisions |
Non-Current Provisions(a) | 31/12/10 | 31/12/09 | ||||||
| Thousands of Euros | ||||||||
| Provisions for pensions and similar obligations | 787 | 595 | ||||||
| Other provisions | 591 | 637 | ||||||
| Non-current provisions | 1,378 | 1,232 | ||||||
Current Provisions(b) | 31/12/10 | 31/12/09 | ||||||
| Thousands of Euros | ||||||||
| Trade provisions | 4,365 | 4,702 | ||||||
| (a) | Non-current provisions |
| Balances at | Business | Translation | Balances at | |||||||||||||||||||||
| 31/12/08 | Combination | Reversal | Cancellation | Differences | 31/12/09 | |||||||||||||||||||
| Thousands of Euros | ||||||||||||||||||||||||
| Non-current provisions | 3,045 | 102 | (1,411 | ) | (457 | ) | (47 | ) | 1,232 | |||||||||||||||
| Balances at | Translation | Balances at | ||||||||||||||||||
| 31/12/09 | Charge | Cancellation | Differences | 31/12/10 | ||||||||||||||||
| Thousands of Euros | ||||||||||||||||||||
| Non-current provisions | 1,232 | 140 | (71 | ) | 77 | 1,378 | ||||||||||||||
| (b) | Current provisions |
| Balances at | Business | Translation | Balances at | |||||||||||||||||
| 31/12/08 | Combination | Charge | Differences | 31/12/09 | ||||||||||||||||
| Thousands of Euros | ||||||||||||||||||||
| Trade provisions | 3,830 | 198 | 636 | 38 | 4,702 | |||||||||||||||
| Balances at | Translation | Balances at | ||||||||||||||||||
| 31/12/09 | Charge | Cancellation | Differences | 31/12/10 | ||||||||||||||||
| Thousands of Euros | ||||||||||||||||||||
| Trade provisions | 4,702 | 41 | (414 | ) | 36 | 4,365 | ||||||||||||||
F-96
Table of Contents
| (22) | Financial Liabilities |
| a) | Non-current financial liabilities |
Non-Current Financial Liabilities | 31/12/10 | 31/12/09 | 31/12/08 | |||||||||
| Thousands of Euros | ||||||||||||
| Corporate bonds (a.1.1) | 441,203 | 410,552 | — | |||||||||
| Bonds | 441,203 | 410,552 | — | |||||||||
| Club Deal (a.1.2) | 99,408 | 195,471 | 225,320 | |||||||||
| Other loans (a.1.2) | 120,040 | 90,961 | 79,069 | |||||||||
| Finance lease liabilities (a.1.3) | 4,734 | 6,202 | 7,124 | |||||||||
| Loans and borrowings | 224,182 | 292,634 | 311,513 | |||||||||
Loans and borrowings and bonds or other non-current marketable securities (a.1) | 665,385 | 703,186 | 311,513 | |||||||||
| Preference loans extended by the Spanish Ministry of Science and Technology (a.2) | 9,744 | 11,135 | 10,685 | |||||||||
| Debt on the acquisition of the plasma centre (a.2) | 530 | 1,050 | 1,098 | |||||||||
| Other | 200 | 367 | 759 | |||||||||
Other non-current financial liabilities (a.2) | 10,474 | 12,552 | 12,542 | |||||||||
| 675,859 | 715,738 | 324,055 | ||||||||||
| 31/12/10 | 31/12/09 | 31/12/08 | ||||||||||
| Thousands of Euros | ||||||||||||
| Loan arrangement expenses | 1,365 | 2,105 | 2,245 | |||||||||
| (a.1) | Loans and borrowings and bonds or other non-current marketable securities |
F-97
Table of Contents
| Fixed | ||||||||||||
| Duration | Interest | |||||||||||
Amount | (Years) | Rate | ||||||||||
| 100,000 | Thousands of USD | 7 | 6.42 | % | ||||||||
| 245,000 | Thousands of USD | 10 | 6.94 | % | ||||||||
| 200,000 | Thousands of USD | 12 | 7.14 | % | ||||||||
| 10,000 | Thousands of EUR | 10 | 6.94 | % | ||||||||
| 25,000 | Thousands of GBP | 10 | 6.94 | % | ||||||||
| 31/12/10 | 31/12/09 | |||||||
| Thousands of Euros | ||||||||
Opening balance | ||||||||
| Issuance of corporate bonds in the USA | 416,465 | 409,411 | ||||||
| Transaction costs | (5,913 | ) | (5,967 | ) | ||||
| 410,552 | 403,444 | |||||||
Movements | ||||||||
| Transferred to profit and loss | 660 | 150 | ||||||
| Corporate bonds issued in the USA, exchange differences | (1,772 | ) | 338 | |||||
| Translation differences | 31,763 | 6,620 | ||||||
Closing balance | ||||||||
| Corporate bonds issued in the USA | 446,918 | 416,465 | ||||||
| Transaction costs | (5,715 | ) | (5,913 | ) | ||||
| 441,203 | 410,552 | |||||||
F-98
Table of Contents
| 31/12/10 | 31/12/09 | |||||||||||||||
| Current | Non-Current | Current | Non-Current | |||||||||||||
| Thousands of Euros | ||||||||||||||||
| Minimum payments | 3,552 | 5,089 | 5,088 | 6,675 | ||||||||||||
| Interest | (272 | ) | (355 | ) | (354 | ) | (473 | ) | ||||||||
| Present value | 3,280 | 4,734 | 4,734 | 6,202 | ||||||||||||
| 31/12/10 | 31/12/09 | |||||||||||||||||||||||
| Minimum | Present | Minimum | Present | |||||||||||||||||||||
| Payments | Interest | Value | Payments | Interest | Value | |||||||||||||||||||
| Thousands of Euros | ||||||||||||||||||||||||
| Maturity at: | ||||||||||||||||||||||||
| Less than one year | 3,552 | 272 | 3,280 | 5,088 | 354 | 4,734 | ||||||||||||||||||
| Two years | 2,411 | 161 | 2,250 | 3,364 | 200 | 3,164 | ||||||||||||||||||
| Three years | 1,271 | 96 | 1,175 | 1,382 | 114 | 1,268 | ||||||||||||||||||
| Four years | 763 | 50 | 713 | 831 | 72 | 759 | ||||||||||||||||||
| Five years | 314 | 23 | 291 | 577 | 41 | 536 | ||||||||||||||||||
| More than five years | 330 | 25 | 305 | 521 | 46 | 475 | ||||||||||||||||||
| Total | 8,641 | 627 | 8,014 | 11,763 | 827 | 10,936 | ||||||||||||||||||
F-99
Table of Contents
| 31/12/10 | 31/12/09 | |||||||
| Thousands of Euros | ||||||||
| Maturity at: | ||||||||
| Two years | 85,171 | 81,388 | ||||||
| Three years | 51,582 | 79,696 | ||||||
| Four years | 17,936 | 75,905 | ||||||
| Five years | 17,548 | 12,506 | ||||||
| More than five years | 493,148 | 453,691 | ||||||
| 665,385 | 703,186 | |||||||
| 31/12/10 | 31/12/09 | |||||||||||||||||||||||
| Date | Amount | Non- | Non- | |||||||||||||||||||||
Company | Awarded | Awarded | Current | Current | Current | Current | ||||||||||||||||||
| Thousands of Euros | ||||||||||||||||||||||||
| Instituto Grifols S.A. | 31/01/2001 | 637 | — | — | — | 86 | ||||||||||||||||||
| Instituto Grifols S.A. | 13/02/2002 | 691 | — | 94 | 89 | 94 | ||||||||||||||||||
| Instituto Grifols S.A. | 17/01/2003 | 1,200 | 157 | 165 | 307 | 165 | ||||||||||||||||||
| Instituto Grifols S.A. | 13/11/2003 | 2,000 | 520 | 279 | 762 | 279 | ||||||||||||||||||
| Instituto Grifols S.A. | 17/01/2005 | 2,680 | 1,031 | 375 | 1,345 | 375 | ||||||||||||||||||
| Instituto Grifols S.A. | 29/12/2005 | 2,100 | 1,025 | 288 | 1,253 | 288 | ||||||||||||||||||
| Instituto Grifols S.A. | 29/12/2006 | 1,700 | 1,015 | 234 | 1,190 | 234 | ||||||||||||||||||
| Instituto Grifols S.A. | 27/12/2007 | 1,700 | 1,164 | 232 | 1,324 | — | ||||||||||||||||||
| Instituto Grifols S.A. | 31/12/2008 | 1,419 | 1,175 | — | 1,131 | — | ||||||||||||||||||
| Instituto Grifols S.A. | 16/01/2009 | 1,540 | 1,294 | — | 1,249 | — | ||||||||||||||||||
| Laboratorios Grifols, S.A. | 20/03/2001 | 219 | — | — | — | 30 | ||||||||||||||||||
| Laboratorios Grifols, S.A. | 29/01/2002 | 210 | — | 29 | 27 | 29 | ||||||||||||||||||
| Laboratorios Grifols, S.A. | 15/01/2003 | 220 | 29 | 30 | 56 | 30 | ||||||||||||||||||
| Laboratorios Grifols, S.A. | 26/09/2003 | 300 | 76 | 41 | 111 | 41 | ||||||||||||||||||
| Laboratorios Grifols, S.A. | 22/10/2004 | 200 | 77 | 28 | 100 | 28 | ||||||||||||||||||
| Laboratorios Grifols, S.A. | 20/12/2005 | 180 | 88 | 25 | 107 | 25 | ||||||||||||||||||
| Laboratorios Grifols, S.A. | 29/12/2006 | 400 | 233 | 54 | 273 | 54 | ||||||||||||||||||
| Laboratorios Grifols, S.A. | 27/12/2007 | 360 | 212 | 42 | 242 | — | ||||||||||||||||||
| Laboratorios Grifols, S.A. | 31/12/2008 | 600 | 497 | — | 478 | — | ||||||||||||||||||
| Diagnostic Grifols, S.A. | 27/11/2008 | 857 | 358 | 129 | 468 | 129 | ||||||||||||||||||
| Diagnostic Grifols, S.A. | 25/05/2010 | 203 | 116 | 31 | — | — | ||||||||||||||||||
| Grifols Engineering, S.A. | 21/04/2009 | 524 | 427 | 34 | 447 | — | ||||||||||||||||||
| Grifols Engineering, S.A. | 21/04/2009 | 203 | 165 | 13 | 176 | — | ||||||||||||||||||
| Grifols Engineering, S.A. | 28/01/2010 | 100 | 85 | — | — | — | ||||||||||||||||||
| 20,243 | 9,744 | 2,123 | 11,135 | 1,887 | ||||||||||||||||||||
F-100
Table of Contents
| 31/12/10 | 31/12/09 | |||||||
| Thousands of Euros | ||||||||
| Maturity at: | ||||||||
| Two years | 2,964 | 2,632 | ||||||
| Three years | 2,159 | 2,883 | ||||||
| Four years | 1,989 | 2,026 | ||||||
| Five years | 1,266 | 1,867 | ||||||
| More than five years | 2,096 | 3,144 | ||||||
| 10,474 | 12,552 | |||||||
| b) | Current Financial Liabilities |
Current financial liabilities | 31/12/10 | 31/12/09 | ||||||
| Thousands of Euros | ||||||||
| Bonds (b.1.1) | 8,235 | 6,407 | ||||||
| Interest of issue corporate bonds in the USA (b.1.1) | 7,207 | 6,716 | ||||||
| Bonds | 15,442 | 13,123 | ||||||
| Club Deal (b.1.2) | 66,250 | 33,014 | ||||||
| Other loans (b.1.2) | 106,663 | 63,120 | ||||||
| Finance lease liabilities (a.1.3) | 3,280 | 4,734 | ||||||
| Loans and borrowings | 176,193 | 100,868 | ||||||
Loans and borrowings and bonds and other marketable securities (b.1) | 191,635 | 113,991 | ||||||
| Financial derivatives (note 32) | 8,560 | 3,333 | ||||||
| Preference loans extended by the Spanish Ministry of Science and Technology (a.2) | 2,123 | 1,887 | ||||||
| Receivables from social security affiliated entities transferred to a financial institution (b.2) | 6,503 | 5,459 | ||||||
| Debt on the acquisition of the plasma centre (a.2) | 637 | 442 | ||||||
| Debt with Novartis (b.2) | — | 779 | ||||||
| Guarantee deposits received | 149 | 59 | ||||||
| Other current financial liabilities | 264 | 271 | ||||||
Other current financial liabilities (b.2) | 18,236 | 12,230 | ||||||
| 209,871 | 126,221 | |||||||
F-101
Table of Contents
| 31/12/10 | 31/12/09 | |||||||
| Thousands of Euros | ||||||||
| Loan arrangement expenses | 707 | 825 | ||||||
| (b.1.1) | Bonds |
| 31/12/10 | 31/12/09 | |||||||
| Thousands of Euros | ||||||||
| Promissory notes issued to bearer | 8,373 | 6,510 | ||||||
| Interest pending accrual on promissory notes issued to bearer | (138 | ) | (103 | ) | ||||
| Interest accrued on corporate bonds | 7,207 | 6,716 | ||||||
| 15,442 | 13,123 | |||||||
| 31/12/09 | ||||||||||||||||||||||||
| Promissory | Interest | |||||||||||||||||||||||
| Nominal | Notes | Pending | ||||||||||||||||||||||
| Amount | Subscribed | Accrual | ||||||||||||||||||||||
| Maturity | (Thousands | Interest | (Thousands | (Thousands | ||||||||||||||||||||
| Issue Date | Date | of Euros) | Rate | of Euros) | of Euros) | |||||||||||||||||||
| Issue of bearer promissory notes | 05/05/09 | 05/05/10 | 3.000 | 4.75 | % | 6,510 | (103 | ) | ||||||||||||||||
| 31/12/10 | ||||||||||||||||||||||||
| Promissory | Interest | |||||||||||||||||||||||
| Nominal | Notes | Pending | ||||||||||||||||||||||
| Amount | Subscribed | Accrual | ||||||||||||||||||||||
| Maturity | (Thousands | Interest | (Thousands | (Thousands | ||||||||||||||||||||
| Issue Date | Date | of Euros) | Rate | of Euros) | of Euros) | |||||||||||||||||||
| Issue of bearer promissory notes | 05/05/10 | 05/05/11 | 3.000 | 5.00 | % | 8,373 | (138 | ) | ||||||||||||||||
F-102
Table of Contents
| (b.1.2) | Other current loans and borrowings |
| Interest Rate (*) | Drawn Down | |||||||||
| Min - Max | 31/12/10 | 31/12/09 | ||||||||
| Thousands of Euros | ||||||||||
| Loans in: | ||||||||||
| US Dollars | 5.00% | 1,384 | 3,010 | |||||||
| Euros | 1.17% — 6% | 143,990 | 73,664 | |||||||
| Other currencies | TIIE+2% — 15% | 26,368 | 18,449 | |||||||
| 171,742 | 95,123 | |||||||||
| Discounted trade notes (note 13) | 1.4-4.69% | 1,396 | 1,298 | |||||||
| Current interest on loans and borrowings | 483 | 538 | ||||||||
| Finance lease payables | 3,552 | 5,088 | ||||||||
| 177,173 | 102,047 | |||||||||
| Less, current portion of deferred finance expenses for leasing | (272 | ) | (354 | ) | ||||||
| Less, current portion of loan arrangement expenses | (708 | ) | (825 | ) | ||||||
| 176,193 | 100,868 | |||||||||
| (*) | Loans accrue variable interest rates. |
F-103
Table of Contents
| (23) | Trade and Other Payables |
| 31/12/10 | 31/12/09 | |||||||
| Thousands of Euros | ||||||||
| Suppliers and trade payables | 160,678 | 120,887 | ||||||
| Other | — | 22 | ||||||
Suppliers | 160,678 | 120,909 | ||||||
| Public entities, other payables | 11,928 | 17,832 | ||||||
Other trade payables | 11,928 | 17,832 | ||||||
Current income tax liabilities | 4,172 | 3,258 | ||||||
| 176,778 | 141,999 | |||||||
| 31/12/10 | 31/12/09 | |||||||
| Thousands of Euros | ||||||||
Currency | ||||||||
| US Dollar | 58,932 | 31,377 | ||||||
| Chilean Peso | 1,490 | 894 | ||||||
| Swiss Franc | 897 | 686 | ||||||
| Czech Crown | 568 | 380 | ||||||
| Brazilian Real | 428 | 621 | ||||||
| Pound Sterling | 405 | 266 | ||||||
| Other currencies | 665 | 1,419 | ||||||
| 31/12/10 | 31/12/09 | |||||||
| Thousands of Euros | ||||||||
| Taxation authorities, VAT/Canary Islands Tax | 3,472 | 3,292 | ||||||
| Taxation authorities, withholdings | 3,119 | 8,184 | ||||||
| Social Security | 3,246 | 3,027 | ||||||
| Other public entities | 2,091 | 3,329 | ||||||
| Public entities, other payables | 11,928 | 17,832 | ||||||
F-104
Table of Contents
| 31/12/10 | 31/12/09 | |||||||
| Thousands of Euros | ||||||||
| Taxation authorities, income tax: | ||||||||
| Current year | 4,161 | 3,185 | ||||||
| Prior years | 11 | 73 | ||||||
| Current tax liabilities | 4,172 | 3,258 | ||||||
| (24) | Other Current Liabilities |
| 31/12/10 | 31/12/09 | |||||||
| Thousands of Euros | ||||||||
| Salaries payable | 28,321 | 24,367 | ||||||
| Other payables | 2,629 | 1,754 | ||||||
Other current liabilities | 30,950 | 26,121 | ||||||
| (25) | Revenues |
| % | ||||||||||||
| 31/12/10 | 31/12/09 | 31/12/08 | ||||||||||
| Bioscience | 78 | % | 76 | % | 76 | % | ||||||
| Diagnostics | 11 | % | 10 | % | 10 | % | ||||||
| Hospital | 9 | % | 10 | % | 10 | % | ||||||
| Raw materials | 1 | % | 3 | % | 3 | % | ||||||
| Others | 1 | % | 1 | % | 1 | % | ||||||
| 100 | % | 100 | % | 100 | % | |||||||
| % | ||||||||||||
| 31/12/10 | 31/12/09 | 31/12/08 | ||||||||||
| Spain | 23 | % | 25 | % | 24 | % | ||||||
| European Union | 21 | % | 22 | % | 26 | % | ||||||
| United States | 34 | % | 32 | % | 36 | % | ||||||
| Rest of the world | 22 | % | 21 | % | 14 | % | ||||||
| 100 | % | 100 | % | 100 | % | |||||||
F-105
Table of Contents
| 31/12/10 | 31/12/09 | 31/12/08 | ||||||||||
| Thousands of Euros | ||||||||||||
Currency | ||||||||||||
| US Dollar | 405,439 | 349,064 | 304,445 | |||||||||
| Pound Sterling | 36,199 | 33,668 | 36,668 | |||||||||
| Chilean Peso | 28,760 | 21,083 | 16,047 | |||||||||
| Mexican Peso | 25,652 | 36,472 | 29,182 | |||||||||
| Brazilian Real | 21,949 | 21,262 | 15,916 | |||||||||
| Australian Dollar | 13,950 | 6,387 | — | |||||||||
| Czech Crown | 13,698 | 12,863 | 12,568 | |||||||||
| Argentinean Peso | 13,122 | 11,323 | 9,145 | |||||||||
| Polish Zloty | 11,668 | 13,525 | — | |||||||||
| Other currency | 18,989 | 18,013 | 13,062 | |||||||||
| (26) | Personnel Expenses |
| 31/12/10 | 31/12/09 | 31/12/08 | ||||||||||
| Thousands of Euros | ||||||||||||
| Wages and salaries | 232,174 | 219,803 | 191,644 | |||||||||
| Contributions to pension plans (note 31) | 1,615 | 1,571 | 1,365 | |||||||||
| Other social charges | 8,615 | 8,072 | 6,310 | |||||||||
| Social Security | 46,604 | 43,722 | 38,840 | |||||||||
| 289,008 | 273,168 | 238,159 | ||||||||||
| (27) | Other Operating Income and Expenses |
| 31/12/10 | 31/12/09 | 31/12/08 | ||||||||||
| Thousands of Euros | ||||||||||||
| Changes in trade provisions (notes 21 (b) and 32) | 398 | 1,348 | 561 | |||||||||
| Professional services (note 15) | 40,530 | �� | 25,266 | 22,874 | ||||||||
| Commissions | 8,038 | 7,711 | 7,075 | |||||||||
| Supplies and other materials | 30,544 | 28,859 | 26,874 | |||||||||
| Operating leases (note 30 (a)) | 19,272 | 17,364 | 16,583 | |||||||||
| Freight | 20,956 | 20,518 | 19,485 | |||||||||
| Repairs and maintenance costs | 22,480 | 21,365 | 17,642 | |||||||||
| Advertising | 14,708 | 15,580 | 16,872 | |||||||||
| Insurance | 10,807 | 10,803 | 10,367 | |||||||||
| Royalties and service charges | 884 | 4,954 | 8,760 | |||||||||
| Travel expenses | 12,742 | 11,935 | 14,210 | |||||||||
| External services | 24,603 | 25,024 | 21,891 | |||||||||
| Others | 14,256 | 12,654 | 9,094 | |||||||||
Other operating expenses | 220,218 | 203,381 | 192,288 | |||||||||
F-106
Table of Contents
| 31/12/10 | 31/12/09 | 31/12/08 | ||||||||||
| Thousands of Euros | ||||||||||||
| Income from insurance claims | 771 | 807 | 584 | |||||||||
| Grants | 307 | 378 | 497 | |||||||||
| Other income | 118 | 258 | 208 | |||||||||
Other operating income | 1,196 | 1,443 | 1,289 | |||||||||
| (28) | Finance Income and Expense |
| 31/12/10 | 31/12/09 | 31/12/08 | ||||||||||
| Thousands of Euros | ||||||||||||
| Interest from Social Security | 2,876 | 6,510 | 2,212 | |||||||||
| Other finance income | 1,650 | 557 | 470 | |||||||||
| Finance income | 4,526 | 7,067 | 2,682 | |||||||||
| Syndicated loan (other finance expenses) | (1,172 | ) | (747 | ) | (1,849 | ) | ||||||
| Syndicated loan (interest) | (3,303 | ) | (6,289 | ) | (12,152 | |||||||
| Finance expenses from sale of receivables (note 13) | (5,378 | ) | (2,531 | ) | (2,128 | ) | ||||||
| Interests costs of Corporate bonds issued in the USA (note 22) | (31,923 | ) | (6,766 | ) | — | |||||||
| Implicit interest on preference loans (note 22 (a2)) | (567 | ) | (616 | ) | (516 | ) | ||||||
| Capitalised interest | 2,399 | 1,278 | — | |||||||||
| Other finance expenses | (9,716 | ) | (11,416 | ) | (12,660 | ) | ||||||
| Finance expenses | (49,660 | ) | (27,087 | ) | (29,305 | ) | ||||||
| Change in fair value of financial derivatives (note 32) | (7,593 | ) | (587 | ) | (1,268 | ) | ||||||
| Impairment and profit/(losses) on disposal of financial instruments | 91 | (245 | ) | — | ||||||||
| Exchange differences | 1,616 | (1,733 | ) | (2,825 | ) | |||||||
| Net finance income and expense | (51,020 | ) | (22,585 | ) | (30,716 | ) | ||||||
| (29) | Taxation |
F-107
Table of Contents
| a) | Reconciliation of accounting and taxable income |
| 31/12/10 | 31/12/09 | 31/12/08 | ||||||||||
| Thousands of Euros | ||||||||||||
| Profit for the year before income tax | 157,784 | 203,994 | 172,269 | |||||||||
| Tax at 30% | 47,335 | 61,198 | 51,680 | |||||||||
| Permanent differences | 2,300 | 1,935 | 2,678 | |||||||||
| Effect of different tax rates | 3,346 | 5,159 | 4,366 | |||||||||
| Tax credits for research and development | (7,281 | ) | (8,106 | ) | (5,403 | ) | ||||||
| Other tax credits (deductions) | (3,516 | ) | (4,548 | ) | (4,199 | ) | ||||||
| Other income tax expenses | 333 | 786 | 1,031 | |||||||||
| Total income tax expense | 42,517 | 56,424 | 50,153 | |||||||||
| Deferred tax expenses | 15,547 | 8,832 | 6,987 | |||||||||
| Current income tax | 26,970 | 47,592 | 43,166 | |||||||||
| Total | 42,517 | 56,424 | 50,153 | |||||||||
| Tax Effect | ||||||||||||
| 31/12/10 | 31/12/09 | 31/12/08 | ||||||||||
| Thousands of Euros | ||||||||||||
Assets | ||||||||||||
| Tax credits (deductions) | 4,830 | 5,992 | 13,215 | |||||||||
| Tax loss carryforwards | 1,233 | 88 | 163 | |||||||||
| Fixed assets, amortisation and depreciation | 998 | 728 | 299 | |||||||||
| Unrealised margins on inventories | 19,256 | 19,814 | 17,222 | |||||||||
| Provision for bad debts | 395 | 444 | 281 | |||||||||
| Inventories | 235 | 225 | 1,004 | |||||||||
| Cash flow hedges | 1,120 | 1,247 | — | |||||||||
| Other provisions | 4,297 | 2,439 | 825 | |||||||||
| Others | 2,525 | 2,418 | 1,187 | |||||||||
| 34,889 | 33,395 | 34,297 | ||||||||||
Liabilities | ||||||||||||
| Goodwill | 17,948 | 15,186 | 12,423 | |||||||||
| Revaluations of assets | 15,210 | 15,011 | 15,345 | |||||||||
| Fixed assets, amortisation and depreciation | 40,520 | 23,873 | 14,028 | |||||||||
| Finance leases | 3,396 | 3,634 | 3,647 | |||||||||
| Inventories | — | — | 2,041 | |||||||||
| Provision for investments | 696 | 873 | 2,322 | |||||||||
| Others | 1,371 | 1,748 | 2,163 | |||||||||
| 79,141 | 60,325 | 51,969 | ||||||||||
F-108
Table of Contents
Deferred Tax Assets | 2010 | 2009 | 2008 | |||||||||
| Thousands of Euros | ||||||||||||
| Balance at 1 January | 33,395 | 34,297 | 34,110 | |||||||||
| Movements during the year | 865 | (1,478 | ) | 687 | ||||||||
| Business combinations (note 3) | — | 500 | — | |||||||||
| Adjustments for changes in tax rate through profit and loss | — | 69 | (514 | ) | ||||||||
| Translation differences | 629 | 7 | 14 | |||||||||
| Balance at 31 December | 34,889 | 33,395 | 34,297 | |||||||||
Deferred Tax Liabilities | 2010 | 2009 | 2008 | |||||||||
| Thousands of Euros | ||||||||||||
| Balance at 1 January | 60,325 | 51,969 | 43,794 | |||||||||
| Movements during the year | 16,537 | 7,423 | 6,721 | |||||||||
| Business combinations (note 3) | — | 1,761 | — | |||||||||
| Adjustments for changes in tax rate through profit and loss | — | — | 439 | |||||||||
| Translation differences | 2,279 | (828 | ) | 1,015 | ||||||||
| Balance at 31 December | 79,141 | 60,325 | 51,969 | |||||||||
| Tax Effect | ||||||||||||
| 31/12/10 | 31/12/09 | 31/12/08 | ||||||||||
| Thousands of Euros | ||||||||||||
Available-for-sale financial assets | — | (69 | ) | 3 | ||||||||
| Cash flow hedges (note 17 (g)) | 127 | 1,247 | — | |||||||||
| 127 | 1,178 | 3 | ||||||||||
| Applicable | ||||||||||||
Year of Origin | 2010 | 2009 | Through | |||||||||
| 2008 | 101 | 417 | 2023 | |||||||||
| 2009 | 500 | 5,575 | 2024 | |||||||||
| 2010 | 4,229 | — | 2025 | |||||||||
| 4,830 | 5,992 | |||||||||||
F-109
Table of Contents
| c) | Years open to inspection |
| • | On 30 June 2010, in relation to the inspection underway on Grifols, S.A., Instituto Grifols, S.A., Laboratorios Grifols, S.A. and Movaco, S.A., the Group has received assessments for income tax, value added tax (VAT), personal income tax and withholding tax on investment income. The total amount settled was Euros 586 thousand and the income tax expense amounts to Euros 1,257 thousand. | |
| • | During 2010 the tax inspection on the income tax, VAT and withholdings for 2006 of Grifols Italia, S.p.A. was concluded, implying no significant payment for the Group. | |
| • | Logística Grifols, S.A. de CV: Tax ruling on the financial statements for 2005 and 2006. Group management does not expect any significant liabilities to arise as a result of this inspection. | |
| • | At 30 June 2010 Grifols, Inc. and subsidiaries received notification of income tax inspection for the years closed 31 December 2006, 2007 and 2008. Due to, among other reasons, differences in the interpretation of prevailing tax legislation, the Group’s directors have set up a provision of Euros 1,860 thousand, which is recognised under “income tax” in the income statement and under “public entities, other” in the balance sheet (see note 23). | |
| • | Grifols Brasil, Lda.: Tax on circulation of goods and services (ICMS) for 2006 to 2010. Group management does not expect that any significant liability will arise from this inspection. |
F-110
Table of Contents
| • | Notification of the completion of the inspection of Biomat USA, Inc., resulting in a favourable conclusion. |
| • | Notification of the favourable completion of the inspection of Grifols Deutschland, except for Euros 150 thousand which was taken to profit and loss in 2008. | |
| • | Notification of the favourable completion of the inspection of Grifols, Inc., Grifols Biologicals, Inc., Grifols USA, Inc. and Plasmacare, Inc. |
| (30) | Operating Leases |
| (a) | Operating leases (as lessee) |
| 31/12/10 | 31/12/09 | 31/12/08 | ||||||||||
| Thousands of Euros | ||||||||||||
| Maturity: | ||||||||||||
| Up to 1 year | 13,769 | 10,098 | 9,575 | |||||||||
| Between 1 and 5 years | 31,003 | 25,943 | 24,919 | |||||||||
| More than 5 years | 7,856 | 8,084 | 7,192 | |||||||||
| Total future minimum payments | 52,628 | 44,125 | 41,686 | |||||||||
| (b) | Operating leases (as lessor) |
| 31/12/10 | 31/12/09 | 31/12/08 | ||||||||||
| Thousands of Euros | ||||||||||||
| Maturity: | ||||||||||||
| Up to 1 year | 64 | 91 | 69 | |||||||||
| Between 1 and 5 years | 21 | 56 | 50 | |||||||||
| More than 5 years | — | 10 | — | |||||||||
| Total future minimum payments | 85 | 157 | 119 | |||||||||
F-111
Table of Contents
| (31) | Other Commitments with Third Parties and Other Contingent Liabilities |
| (a) | Guarantees |
| (b) | Obligations with personnel |
| (c) | Judicial procedures and arbitration |
| • | Litigation was initiated in February 2000. Proceedings have been brought jointly against the Company and another plasma fractioning company. |
| • | A claim brought against the Health Board of Castilla y León in February 2005. |
| • | The Company was notified in 2007 of a claim for maximum damages of Euros 12,960 thousand filed by a group of 100 Catalan haemophiliacs against all plasma fractionation companies. During 2008 this claim was rejected by the Courts, and the ruling appealed by the group of haemophiliacs. Notification was published on 21 January 2011 that on 18 January 2011 the Barcelona Provincial Court had rejected the haemophiliacs’ claim. This ruling is pending confirmation, unless it is appealed in the Spanish High Court. |
F-112
Table of Contents
| • | Legal proceedings (consent decree) which were brought against the plasma fractioning centre in Los Angeles. |
| The blood plasma fractioning centre in Los Angeles is managed through consent decree which was applied for in January 1998 to the Courts by the FDA and US Department of Justice as a result of an infringement of FDA regulations committed by the former owner of the centre (Alpha Therapeutic Corporation, hereinafter ATC). As a result of this consent decree, the Los Angeles centre is subject to strict FDA audits and may only sell products manufactured in the centre subsequent to prior authorisation. | ||
| The Company cannot guarantee if or when the consent decree will be lifted. | ||
| In March 2004 as a result of improvements to the centre made by the Group, the FDA awarded several free sales certificates for the former ATC products manufactured in this centre. | ||
| Based on the current level of compliance, there are no commercial activities that are prohibited or limited by the consent decree. |
| (d) | Long-term materials supply contract |
| (e) | Agreement for the acquisition of Talecris Biotherapeutics Holdings Corp (Talecris) |
F-113
Table of Contents
| • | Non-current syndicated financing with financial institutions: Loan repayable in 5 years totalling US Dollars 1,500 million. Margin of 375 basis points (bp) linked to US Libor and 400 bp linked to Euribor. BB and Ba3 rating. | |
| • | Non-current syndicated financing with institutional investors: 6 year bullet loan (payment of whole principal upon maturity) amounting to US Dollars 1,600 million. Margin of 425 bp linked to US Libor and 450 bp linked to Euribor. BB and Ba3 rating. | |
| • | Senior revolving credit facility amounting to US Dollars 300 million. BB and Ba3 rating. |
| (32) | Financial Instruments |
| 31/12/09 | ||||||||||||||||
| Financial | ||||||||||||||||
| Available-for- | Assets/ | |||||||||||||||
| Sale Financial | Loans and | (Liabilities) Held | Debts and | |||||||||||||
| Assets | Receivables | for Trading | Payables | |||||||||||||
| Thousands of Euros | ||||||||||||||||
| Non-current financial assets | 501 | 3,230 | — | — | ||||||||||||
| Other current financial assets | — | 6,547 | — | — | ||||||||||||
| Interest-rate swap | — | — | (3,333 | ) | — | |||||||||||
| Unquoted futures | — | — | 1,670 | — | ||||||||||||
| Trade and other receivables | 239,204 | — | — | |||||||||||||
| Bank loans | — | — | — | (382,566 | ) | |||||||||||
| Other financial liabilities | — | — | — | (21,449 | ) | |||||||||||
| Bonds and other securities | — | — | — | (423,675 | ) | |||||||||||
| Finance lease liabilities | — | — | — | (10,936 | ) | |||||||||||
| Trade and other payables | — | — | — | (120,909 | ) | |||||||||||
| Other current liabilities | — | — | — | (1,754 | ) | |||||||||||
| 501 | 248,981 | (1,663 | ) | (961,289 | ) | |||||||||||
F-114
Table of Contents
| 31/12/10 | ||||||||||||||||
| Financial | ||||||||||||||||
| Available-for- | Assets/ | |||||||||||||||
| Sale Financial | Loans and | (Liabilities) Held | Debts and | |||||||||||||
| Assets | Receivables | for Trading | Payables | |||||||||||||
| Thousands of Euros | ||||||||||||||||
| Non-current financial assets | 535 | 7,000 | — | — | ||||||||||||
| Other current financial assets | — | 12,946 | — | — | ||||||||||||
| Interest-rate swap | — | — | (1,809 | ) | — | |||||||||||
| Unquoted futures | — | — | (6,751 | ) | — | |||||||||||
| Trade and other receivables | — | 259,497 | — | — | ||||||||||||
| Bank loans | — | — | — | (392,361 | ) | |||||||||||
| Other financial liabilities | — | — | — | (20,150 | ) | |||||||||||
| Bonds and other securities | — | — | — | (456,645 | ) | |||||||||||
| Finance lease liabilities | — | — | — | (8,014 | ) | |||||||||||
| Trade and other payables | — | — | — | (160,678 | ) | |||||||||||
| Payables for Group companies | — | — | — | (1,162 | ) | |||||||||||
| Other current liabilities | — | — | — | (2,629 | ) | |||||||||||
| 535 | 279,443 | (8,560 | ) | (1,041,639 | ) | |||||||||||
| 31/12/09 | ||||||||||||||||
| Available- | ||||||||||||||||
| Assets at Fair | for-Sale | |||||||||||||||
| Value through | Loans And | Financial | ||||||||||||||
| Profit or Loss | Receivables | Assets | Total | |||||||||||||
| Thousands of Euros | ||||||||||||||||
| Finance income at amortised cost | — | 7,067 | — | 7,067 | ||||||||||||
| Change in fair value | 2,015 | — | — | 2,015 | ||||||||||||
| Reclassification of equity to profit or loss | — | — | (172 | ) | (172 | ) | ||||||||||
| Net gains/(losses) in profit and loss | 2,015 | 7,067 | (172 | ) | 8,910 | |||||||||||
| Change in fair value | — | — | 14 | 14 | ||||||||||||
| Net gains in equity | — | — | 14 | 14 | ||||||||||||
Total | 2,015 | 7,067 | (158 | ) | 8,924 | |||||||||||
F-115
Table of Contents
| 31/12/10 | ||||||||||||||||
| Available- | ||||||||||||||||
| Assets at Fair | for-Sale | |||||||||||||||
| Value through | Loans and | Financial | ||||||||||||||
| Profit or Loss | Receivables | Assets | Total | |||||||||||||
| Thousands of Euros | ||||||||||||||||
| Finance income at amortised cost | — | 4,526 | — | 4,526 | ||||||||||||
| Change in fair value | 1,601 | — | — | 1,601 | ||||||||||||
| Net gains in profit and loss | 1,601 | 4,526 | — | 6,127 | ||||||||||||
Total | 1,601 | 4,526 | — | 6,127 | ||||||||||||
| 31/12/09 | ||||||||||||||||
| Liabilities at | ||||||||||||||||
| Fair Value | ||||||||||||||||
| through Profit | Debts and | Hedging | ||||||||||||||
| or Loss | Payables | Derivatives | Total | |||||||||||||
| Thousands of Euros | ||||||||||||||||
| Finance expenses at amortised cost | — | (27,087 | ) | — | (27,087 | ) | ||||||||||
| Change in fair value | (2,602 | ) | — | — | (2,602 | ) | ||||||||||
| Reclassification of equity to profit or loss | — | — | (50 | ) | (50 | ) | ||||||||||
| Net losses in profit and loss | (2,602 | ) | (27,087 | ) | (50 | ) | (29,739 | ) | ||||||||
| Change in fair value | — | — | 1,998 | 1,998 | ||||||||||||
| Net gains in equity | — | — | 1,998 | 1,998 | ||||||||||||
Total | (2,602 | ) | (27,087 | ) | 1,948 | (27,741 | ) | |||||||||
| 31/12/10 | ||||||||||||||||
| Liabilities at | ||||||||||||||||
| Fair Value | ||||||||||||||||
| through Profit | Debts and | Hedging | ||||||||||||||
| or Loss | Payables | Derivatives | Total | |||||||||||||
| Thousands of Euros | ||||||||||||||||
| Finance expenses at amortised cost | — | (49,660 | ) | — | (49,660 | ) | ||||||||||
| Change in fair value | (9,194 | ) | — | — | (9,194 | ) | ||||||||||
| Reclassification of equity to profit or loss | — | — | (197 | ) | (197 | ) | ||||||||||
| Net losses in profit and loss | (9,194 | ) | (49,660 | ) | (197 | ) | (59,051 | ) | ||||||||
Total | (9,194 | ) | (49,660 | ) | (197 | ) | (59,051 | ) | ||||||||
F-116
Table of Contents
| a) | Derivative financial instruments at fair value through profit or loss |
| Value at | ||||||||||||
Derivatives | Par | 31/12/09 | Maturity | |||||||||
| Thousands of Euros | ||||||||||||
| Interest rate swap | 50,000 | (3,333 | ) | 26/07/2013 | ||||||||
| (note 22 | ) | |||||||||||
| Unquoted future | 23,221 | 1,189 | 30/12/2010 | |||||||||
| Unquoted future | 26,370 | 481 | 30/12/2010 | |||||||||
| 49,591 | 1,670 | |||||||||||
| (note 14 | ) | |||||||||||
| Value at | ||||||||||||
Derivatives | Par | 31/12/10 | Maturity | |||||||||
| Thousands of Euros | ||||||||||||
| Interest rate swap | 50,000 | (1,809 | ) | 26/07/2013 | ||||||||
| Unquoted future | 23,221 | (2,821 | ) | 31/03/2011 | ||||||||
| Unquoted future | 26,370 | (3,930 | ) | 31/03/2011 | ||||||||
| 49,591 | (6,751 | ) | ||||||||||
| Total | 8,560 | |||||||||||
| (note 22 | ) | |||||||||||
| b) | Bond issue hedging derivative financial instruments |
F-117
Table of Contents
Carrying Amount | Note | 31/12/10 | 31/12/09 | |||||||||
| Thousands of Euros | ||||||||||||
| Non-current financial assets | 11 | 7,535 | 3,731 | |||||||||
| Other current financial assets | 14 | 12,946 | 6,547 | |||||||||
| Financial derivatives | 14 | — | 1,670 | |||||||||
| Trade receivables | 13 | 224,355 | 207,840 | |||||||||
| Other receivables | 13 | 35,142 | 31,364 | |||||||||
| Cash and cash equivalents | 16 | 239,649 | 249,372 | |||||||||
| 519,627 | 500,524 | |||||||||||
Carrying Amount | 31/12/10 | 31/12/09 | ||||||
| Thousands of Euros | ||||||||
| Domestic | 70,517 | 70,521 | ||||||
| EU countries | 46,787 | 47,755 | ||||||
| United States of America | 43,833 | 29,130 | ||||||
| United Kingdom | 3,423 | 3,054 | ||||||
| Other European countries | 3,162 | 5,454 | ||||||
| Other regions | 56,633 | 51,926 | ||||||
| 224,355 | 207,840 | |||||||
| 31/12/10 | 31/12/09 | |||||||
| Thousands of Euros | ||||||||
| Not due | 148,838 | 120,339 | ||||||
| Overdue less than 1 month | 21,860 | 38,278 | ||||||
| Overdue from 1 to 4 months | 32,729 | 25,597 | ||||||
| Overdue from 4 months to 1 year | 14,812 | 17,357 | ||||||
| Overdue more than a year | 6,116 | 6,269 | ||||||
| 224,355 | 207,840 | |||||||
F-118
Table of Contents
| 31/12/10 | 31/12/09 | 31/12/08 | ||||||||||
| Thousands of Euros | ||||||||||||
| Opening balance | 4,038 | 3,172 | 3,285 | |||||||||
| Net provisions for the year | 357 | 712 | 317 | |||||||||
| Net cancellations for the year | (796 | ) | (42 | ) | (249 | ) | ||||||
| Translation differences | 178 | 196 | (181 | ) | ||||||||
| Closing balance | 3,777 | 4,038 | 3,172 | |||||||||
| Carrying | More | |||||||||||||||||||||||||||||||
| Amount at | Contractual | 6 Months | 6-12 | 1-2 | 2-5 | Than | ||||||||||||||||||||||||||
Carrying Amount | Note | 31/12/09 | Flows | or Less | Months | Years | Years | 5 Years | ||||||||||||||||||||||||
| Thousands of Euros | ||||||||||||||||||||||||||||||||
Non-derivative financial liabilities | ||||||||||||||||||||||||||||||||
| Bank loans | 22 | 382,566 | 412,390 | 88,707 | 15,087 | 83,772 | 177,413 | 47,411 | ||||||||||||||||||||||||
| Other financial liabilities | 22 | 21,449 | 27,420 | 6,927 | 2,582 | 4,417 | 10,076 | 3,418 | ||||||||||||||||||||||||
| Bonds and other securities | 22 | 423,675 | 687,798 | 27,440 | 14,317 | 28,634 | 85,903 | 531,504 | ||||||||||||||||||||||||
| Finance lease liabilities | 22 | 10,936 | 11,334 | 230 | 4,751 | 3,294 | 2,586 | 473 | ||||||||||||||||||||||||
| Suppliers | 23 | 120,909 | 120,909 | 120,550 | 359 | — | — | — | ||||||||||||||||||||||||
| Other current liabilities | 24 | 1,754 | 1,754 | 1,754 | — | — | — | — | ||||||||||||||||||||||||
Derivative financial liabilities | ||||||||||||||||||||||||||||||||
| Interest rate swap | 22 | 3,333 | 3,333 | — | — | — | 3,333 | — | ||||||||||||||||||||||||
| Unquoted futures | 14 | (1,670 | ) | (1,670 | ) | — | (1,670 | ) | — | — | — | |||||||||||||||||||||
| Total | 962,952 | 1,263,268 | 245,608 | 35,426 | 120,117 | 279,311 | 582,806 | |||||||||||||||||||||||||
| Carrying | More | |||||||||||||||||||||||||||||||
| Amount at | Contractual | 6 Months | 6-12 | 1-2 | 2-5 | Than | ||||||||||||||||||||||||||
Carrying Amount | Note | 31/12/10 | Flows | or Less | Months | Years | Years | 5 Years | ||||||||||||||||||||||||
| Thousands of Euros | ||||||||||||||||||||||||||||||||
Non-derivative financial liabilities | ||||||||||||||||||||||||||||||||
| Bank loans | 22 | 392,361 | 420,168 | 117,256 | 66,428 | 87,986 | 92,561 | 55,937 | ||||||||||||||||||||||||
| Other financial liabilities | 22 | 20,150 | 22,361 | 8,150 | 2,134 | 3,467 | 6,283 | 2,327 | ||||||||||||||||||||||||
| Bonds and other securities | 22 | 456,645 | 728,893 | 23,771 | 15,537 | 31,073 | 93,220 | 565,292 | ||||||||||||||||||||||||
| Finance lease liabilities | 22 | 8,014 | 8,629 | 2,034 | 1,505 | 2,412 | 2,348 | 330 | ||||||||||||||||||||||||
| Debts with associates | 33 | 1,162 | 1,162 | 1,162 | — | — | — | — | ||||||||||||||||||||||||
| Suppliers | 23 | 160,678 | 160,678 | 160,657 | 21 | — | — | — | ||||||||||||||||||||||||
| Other current liabilities | 24 | 2,629 | 2,629 | 2,629 | — | — | — | — | ||||||||||||||||||||||||
Derivative financial liabilities | ||||||||||||||||||||||||||||||||
| Interest rate swap | 22 | 1,809 | 1,809 | — | — | — | 1,809 | — | ||||||||||||||||||||||||
| Unquoted futures | 22 | 6,751 | 6,751 | 6,751 | — | — | — | — | ||||||||||||||||||||||||
| Total | 1,050,199 | 1,353,080 | 322,410 | 85,625 | 124,938 | 196,221 | 623,886 | |||||||||||||||||||||||||
F-119
Table of Contents
| 31/12/09 | ||||||||
| EUR (*) | USD (**) | |||||||
| Thousands of Euros | ||||||||
| Trade receivables | 1,839 | 7,308 | ||||||
| Loans to Group companies | 16,854 | — | ||||||
| Trade payables | (252 | ) | (2,339 | ) | ||||
| Payables to Group companies | (10,365 | ) | (17,946 | ) | ||||
| Loans with Group companies | — | (10,431 | ) | |||||
| Non-current bank loans | (6,854 | ) | — | |||||
| Non-current bonds | (10,000 | ) | — | |||||
| Balance sheet exposure | (8,778 | ) | (23,408 | ) | ||||
| (*) | balances in Euros in subsidiaries with USD local currency | |
| (**) | Balances in USD in subsidiaries with Euro local currency |
| 31/12/10 | ||||||||
| EUR (*) | USD (**) | |||||||
| Thousands of Euros | ||||||||
| Trade receivables | 67 | 3,938 | ||||||
| Receivables from Group companies | 12 | — | ||||||
| Loans to Group companies | 16,852 | — | ||||||
| Cash | 415 | 45 | ||||||
| Trade payables | (533 | ) | (6,200 | ) | ||||
| Payables for Group companies | (6,828 | ) | (21,455 | ) | ||||
| Current bank loans | (5,875 | ) | — | |||||
| Non-current bank loans | (979 | ) | (262 | ) | ||||
| Non-current bonds | (9,860 | ) | — | |||||
| Balance sheet exposure | (6,729 | ) | (23,934 | ) | ||||
| (*) | balances in Euros in subsidiaries with USD local currency | |
| (**) | Balances in USD in subsidiaries with Euro local currency |
| Average Exchange Rate | Closing Exchange Rate | |||||||||||||||
Euro | 2010 | 2009 | 31/12/10 | 31/12/09 | ||||||||||||
| USD | 1.34 | 1.38 | 1.34 | 1.44 | ||||||||||||
F-120
Table of Contents
| 31/12/10 | 31/12/09 | |||||||
| Thousands of Euros | ||||||||
| Fixed-interest financial instruments | ||||||||
| Financial assets | 19,220 | 9,674 | ||||||
| Financial liabilities | (457,521 | ) | (423,675 | ) | ||||
| (438,301 | ) | (414,001 | ) | |||||
| Variable-interest financial instruments Financial liabilities | (399,499 | ) | (393,502 | ) | ||||
| (399,499 | ) | (393,502 | ) | |||||
| (837,800 | ) | (807,503 | ) | |||||
| (33) | Balances and Transactions with Related Parties |
| 31/12/10 | 31/12/09 | |||||||
| Thousands of Euros | ||||||||
| Receivables from associates | 5 | 812 | ||||||
| Debts with associates | (1,162 | ) | — | |||||
| Payables to associates | — | (22 | ) | |||||
| Payables to members of the board of directors | (62 | ) | (121 | ) | ||||
| Payables to other related parties | (4,641 | ) | (3,322 | ) | ||||
| (5,860 | ) | (2,653 | ) | |||||
F-121
Table of Contents
| Key | Board of | |||||||||||||||
| Management | Other Related | Directors of | ||||||||||||||
| Associates | Personnel | Parties | the Company | |||||||||||||
| Thousands of Euros | ||||||||||||||||
| Net purchases | 125 | — | — | — | ||||||||||||
| Other service expenses | — | — | 4,981 | 180 | ||||||||||||
| Personnel expenses | — | 4.253 | — | 1,995 | ||||||||||||
| 125 | 4,253 | 4,981 | 2,175 | |||||||||||||
| Key | Other | Board of | ||||||||||||||
| Management | Related | Directors of | ||||||||||||||
| Associates | Personnel | Parties | the Company | |||||||||||||
| Thousands of Euros | ||||||||||||||||
| Net purchases | 86 | — | — | — | ||||||||||||
| Net sales | (700 | ) | — | — | — | |||||||||||
| Other service expenses | — | — | 7,257 | 240 | ||||||||||||
| Personnel expenses | — | 5,849 | — | 2,148 | ||||||||||||
| (614 | ) | 5,849 | 7,257 | 2,388 | ||||||||||||
| Key | Other | Board of | ||||||||||||||
| Management | Related | Directors of | ||||||||||||||
| Associates | Personnel | Parties | the Company | |||||||||||||
| Thousands of Euros | ||||||||||||||||
| Net purchases | 505 | — | — | — | ||||||||||||
| Net sales | (14 | ) | — | — | — | |||||||||||
| Other service expenses | — | — | 12,506 | 180 | ||||||||||||
| Personnel expenses | — | 5,839 | — | 2,066 | ||||||||||||
| 491 | 5,839 | 12,506 | 2,246 | |||||||||||||
F-122
Table of Contents
| (34) | Subsequent Events |
| (35) | Subsequent Events (2) |
| • | Kedrion and Grifols entered into a contract manufacturing agreement to fractionate and purify Kedrion’s plasma to deliver IVIG and Albumin under Kedrion’s private label, and Factor VIII under the trade name Koate, all of them for sale only in the United States. | |
| • | Grifols is committed to sell to Kedrion the Melville fractionation facility. Grifols lease from Kedrion the Melville fractionation facility being the lease term 3 years with an optional extension of up to 1 year at Grifols request. | |
| • | Grifols transfer to Kedrion all Koate (factor VIII) technology and commercial agreements for the US market. Grifols will produce Koate for Kedrion up to a period of 7 years. | |
| • | Grifols is committed to sell to Kedrion two plasma collection centers. In addition Grifols committed to sell 200.000 liters of source plasma to Kedrion at a fixed price. | |
| • | Grifols authorizes Kedrion to market and sell in the US, IVIG and albumin manufactured by Grifols for Kedrion. |
F-123
Table of Contents
| Thousands of | Thousands | |||||||
| Euros | of USD | |||||||
| Cost of business combination (valuation of Class B Shares) | 829,799 | 1,195,574 | ||||||
| Cash paid (19 USD per share) | 1,763,601 | 2,540,997 | ||||||
| Total cost of business combination | 2,593,400 | 3,736,571 | ||||||
| Book value of net assets acquired (provisional) | 469,318 | 676,193 | ||||||
| Goodwill (excess of the cost of the business combination over the fair value of identifiable net assets acquired) | 2,124,082 | 3,060,378 | ||||||
| (see note 6 | ) | |||||||
| Cash paid | 1,763,601 | 2,540,996 | ||||||
| Cash and cash equivalents of the acquired company | (149,693 | ) | (215,678 | ) | ||||
| Cash outflow for the acquisition | 1,613,908 | 2,325,319 | ||||||
F-124
Table of Contents
| • | Compulsory initial term of five years, | |
| • | Initial rent established at market prices and will be reviewed annually, based on the percentage variation in the Spanish Consumer Price Index (CPI), | |
| • | Automatic extensions of five-year periods that can be avoided by both parties by a six month anticipated notice. | |
| • | Upon vacating the premises, the lessor will reimburse Grifols for the remaining value of leasehold improvements Grifols made. |
| • | Compulsory initial term of 20 years. | |
| • | Initial rent has been established at market prices and will be reviewed annually with a 3% increase. On the first day of the sixth year, the remaining rents until year twenty will be paid in advance in a lump sum. | |
| • | Renewal option to extend for a ten-year period at Grifols Group election. | |
| • | Purchase options granted during the sixth year and in year twenty (20) at market value, to be estimated by independent appraisers. |
F-125
Table of Contents
| (36) | Condensed Consolidated Financial Information |
F-126
Table of Contents
| Bioscience | Hospital | Diagnostics | Raw Materials | Others/Unallocated | Consolidated | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 2010 | 2009 | 2008 | 2010 | 2009 | 2008 | 2010 | 2009 | 2008 | 2010 | 2009 | 2008 | 2010 | 2009 | 2008 | 2010 | 2009 | 2008 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| (Expressed in thousands of Euros) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Revenues | 773,372 | 694,969 | 617,918 | 89,552 | 86,328 | 82,566 | 109,088 | 103,091 | 85,705 | 4,815 | 22,665 | 22,794 | 13,903 | 6,133 | 5,328 | 990,730 | 913,186 | 814,311 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
Total revenues | 773,372 | 694,969 | 617,918 | 89,552 | 86,328 | 82,566 | 109,088 | 103,091 | 85,705 | 4,815 | 22,665 | 22,794 | 13,903 | 6,133 | 5,328 | 990,730 | 913,186 | 814,311 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
Profit/(Loss) for the segment | 306,091 | 297,584 | 262,229 | 7,401 | 8,374 | 8,534 | 6,793 | 12,136 | 13,603 | 2,110 | 3,850 | 7,369 | 7,785 | 6,133 | 5,328 | 330,180 | 328,077 | 297,063 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Unallocated expense | (120,497 | ) | (101,549 | ) | (94,102 | ) | (120,497 | ) | (101,549 | ) | (94,102 | ) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Operating profit | 209,683 | 226,528 | 202,961 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Finance income/expenses | (51,020 | ) | (22,585 | ) | (30,716 | ) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Share of profit/(loss) of equity accounted investees | (879 | ) | 0 | 0 | 0 | 0 | 0 | 0 | 51 | 24 | 0 | 0 | 0 | 0 | 0 | 0 | (879 | ) | 51 | 24 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Income tax expense | (42,517 | ) | (56,424 | ) | (50,153 | ) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Profit for the year after tax | 115,267 | 147,570 | 122,116 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Segment assets | 1,062,464 | 994,245 | 798,843 | 85,992 | 68,214 | 63,660 | 129,824 | 82,202 | 67,087 | 954 | 1,312 | 4,379 | 0 | 0 | 0 | 1,279,234 | 1,145,973 | 933,969 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Equity accounted investments | 516 | 0 | 0 | 0 | 0 | 0 | 0 | 383 | 374 | 0 | 0 | 0 | 0 | 0 | 0 | 516 | 383 | 374 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Unallocated assets | 609,232 | 510,821 | 245,896 | 609,232 | 510,821 | 245,896 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Total assets | 1,888,982 | 1,657,177 | 1,180,239 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Segment liabilities | 74,489 | 79,988 | 75,120 | 14,486 | 12,579 | 11,909 | 12,573 | 10,763 | 9,066 | 0 | 0 | 0 | 0 | 0 | 0 | 101,548 | 103,330 | 96,095 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Unallocated liabilities | 1,080,044 | 975,319 | 602,865 | 1,080,044 | 975,319 | 602,865 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Total liabilities | 1,181,592 | 1,078,649 | 698,960 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Other information: | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Amortisation and depreciation | 21,630 | 21,893 | 21,644 | 4,719 | 3,808 | 3,725 | 8,265 | 5,261 | 5,000 | 0 | 0 | 67 | 11,162 | 8,592 | 2,820 | 45,776 | 39,554 | 33,256 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Expenses that do not require cash payments | (526 | ) | (2,059 | ) | (1,744 | ) | (12 | ) | (70 | ) | 32 | 0 | (1 | ) | 15 | 0 | 0 | (7 | ) | 0 | (26 | ) | (275 | ) | (538 | ) | (2,156 | ) | (1,979 | ) | ||||||||||||||||||||||||||||||||||||||||||
| Additions for the year of property, plant & equipment and intangible assets | 65,344 | 70,702 | 65,954 | 13,132 | 7,524 | 9,266 | 15,897 | 14,067 | 14,078 | 0 | 0 | 516 | 11,424 | 26,477 | 39,879 | 105,797 | 118,770 | 129,693 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
F-127
Table of Contents
| Spain | European Union | United States | Rest of the World | Consolidated | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 2010 | 2009 | 2008 | 2010 | 2009 | 2008 | 2010 | 2009 | 2008 | 2010 | 2009 | 2008 | 2010 | 2009 | 2008 | ||||||||||||||||||||||||||||||||||||||||||||||
| (Expressed in thousands of Euros) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Revenues | 227,947 | 225,759 | 190,809 | 204,244 | 198,832 | 213,290 | 339,018 | 296,659 | 290,666 | 219,521 | 191,936 | 119,546 | 990,730 | 913,186 | 814,311 | |||||||||||||||||||||||||||||||||||||||||||||
Assets by geographic areas | 682,473 | 632,537 | 532,392 | 117,706 | 82,245 | 96,845 | 936,030 | 821,641 | 502,797 | 152,773 | 120,754 | 48,205 | 1,888,982 | 1,657,177 | 1,180,239 | |||||||||||||||||||||||||||||||||||||||||||||
Other information: | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Additions for the year of property, plant & equipment and intangible assets | 50,319 | 65,046 | 93,005 | 3,972 | 2,341 | 999 | 43,847 | 43,726 | 33,475 | 7,659 | 7,657 | 2,214 | 105,797 | 118,770 | 129,693 | |||||||||||||||||||||||||||||||||||||||||||||
F-128
Table of Contents
| Balances at | Translation | Balances at | ||||||||||||||||||||||
| 31/12/2009 | Additions | Transfers | Disposals | Differences | 31/12/2010 | |||||||||||||||||||
| (Expressed in thousands of Euros) | ||||||||||||||||||||||||
| Development costs | 55,414 | 6,614 | 0 | (26 | ) | 69 | 62,071 | |||||||||||||||||
| Concessions, patents, licenses brands & similar | 46,259 | 2,410 | 847 | 0 | 3,227 | 52,743 | ||||||||||||||||||
| Software | 28,597 | 5,455 | 318 | (20 | ) | 352 | 34,702 | |||||||||||||||||
| Other intangible assets | 513 | 2,121 | 0 | (299 | ) | 10 | 2,345 | |||||||||||||||||
| Total cost of intangible assets | 130,783 | 16,600 | 1,165 | (345 | ) | 3,658 | 151,861 | |||||||||||||||||
| Accum. amort. of development costs | (29,427 | ) | (3,699 | ) | 0 | 0 | (69 | ) | (33,195 | ) | ||||||||||||||
| Accum. amort of concessions, patents, licenses, brands & similar | (15,526 | ) | (1,603 | ) | (845 | ) | 0 | (654 | ) | (18,628 | ) | |||||||||||||
| Accum. amort. of software | (16,430 | ) | (4,965 | ) | 1 | 20 | (172 | ) | (21,546 | ) | ||||||||||||||
| Accum. amort. of other intangible assets | 0 | (189 | ) | (4 | ) | 0 | 0 | (193 | ) | |||||||||||||||
| Total accum. amort intangible assets | (61,383 | ) | (10,456 | ) | (848 | ) | 20 | (895 | ) | (73,562 | ) | |||||||||||||
| Impairment of other intangible assets | (15 | ) | 0 | 0 | 15 | 0 | 0 | |||||||||||||||||
Carrying amount of intangible assets | 69,385 | 6,144 | 317 | (310 | ) | 2,763 | 78,299 | |||||||||||||||||
F-129
Table of Contents
| Balances at | Business | Translation | Balances at | |||||||||||||||||||||||||
| 31/12/2008 | Additions | Combinations | Transfers | Disposals | Differences | 31/12/2009 | ||||||||||||||||||||||
| (Expressed in thousands of Euros) | ||||||||||||||||||||||||||||
| Development costs | 47,299 | 8,146 | 0 | 0 | 0 | (31 | ) | 55,414 | ||||||||||||||||||||
| Concessions, patents, licenses brands & similar | 40,461 | 1 | 6,525 | (5 | ) | 0 | (723 | ) | 46,259 | |||||||||||||||||||
| Software | 22,272 | 6,700 | 0 | 1 | (240 | ) | (136 | ) | 28,597 | |||||||||||||||||||
| Other intangible assets | 0 | 508 | 0 | 5 | 0 | 0 | 513 | |||||||||||||||||||||
| Total cost of intangible assets | 110,032 | 15,355 | 6,525 | 1 | (240 | ) | (890 | ) | 130,783 | |||||||||||||||||||
| Accum. amort. of development costs | (23,878 | ) | (5,580 | ) | 0 | 0 | 0 | 31 | (29,427 | ) | ||||||||||||||||||
| Accum. amort of concessions, patents, licenses, brands & similar | (14,881 | ) | (806 | ) | 0 | 0 | 0 | 161 | (15,526 | ) | ||||||||||||||||||
| Accum. amort. of software | (13,517 | ) | (3,097 | ) | 0 | 0 | 132 | 52 | (16,430 | ) | ||||||||||||||||||
| Total accum. amort intangible assets | (52,276 | ) | (9,483 | ) | 0 | 0 | 132 | 244 | (61,383 | ) | ||||||||||||||||||
| Impairment of other intangible assets | 0 | (15 | ) | 0 | 0 | 0 | 0 | (15 | ) | |||||||||||||||||||
Carrying amount of intangible assets | 57,756 | 5,857 | 6,525 | 1 | (108 | ) | (646 | ) | 69,385 | |||||||||||||||||||
| (note 3 | ) | |||||||||||||||||||||||||||
F-130
Table of Contents
| Balances at | Translation | Balances at | ||||||||||||||||||||||
| 31/12/2007 | Additions | Transfers | Disposals | Differences | 31/12/2008 | |||||||||||||||||||
| (Expressed in thousands of Euros) | ||||||||||||||||||||||||
Intangible assets | ||||||||||||||||||||||||
| Development costs | 43,141 | 5,255 | 0 | (1,146 | ) | 49 | 47,299 | |||||||||||||||||
| Concessions, patents, licenses brands and similar | 40,790 | 0 | 0 | (1,618 | ) | 1,289 | 40,461 | |||||||||||||||||
| Software | 17,704 | 4,489 | (59 | ) | (8 | ) | 146 | 22,272 | ||||||||||||||||
| Total cost of intangible assets | 101,635 | 9,744 | (59 | ) | (2,772 | ) | 1,484 | 110,032 | ||||||||||||||||
| Accum. amort. of development costs | (18,916 | ) | (4,634 | ) | (287 | ) | 0 | (41 | ) | (23,878 | ) | |||||||||||||
| Accum. amort of concessions, patents, licenses, brands & similar | (14,110 | ) | (2,322 | ) | 287 | 1,616 | (352 | ) | (14,881 | ) | ||||||||||||||
| Accum. amort. of software | (11,386 | ) | (2,124 | ) | 59 | 10 | (76 | ) | (13,517 | ) | ||||||||||||||
| Total Accum. amort intangible assets | (44,412 | ) | (9,080 | ) | 59 | 1,626 | (469 | ) | (52,276 | ) | ||||||||||||||
Carrying amount of intangible assets | 57,223 | 664 | 0 | (1,146 | ) | 1,015 | 57,756 | |||||||||||||||||
F-131
Table of Contents
| Balances at | Translation | Balances at | ||||||||||||||||||||||
| 31/12/09 | Additions | Transfers | Disposals | Differences | 31/12/10 | |||||||||||||||||||
| (Expressed in thousands of Euros) | ||||||||||||||||||||||||
Cost: | ||||||||||||||||||||||||
| Land and buildings | 142,600 | 10,594 | 28,930 | (1,085 | ) | 3,703 | 184,742 | |||||||||||||||||
| Plant and machinery | 344,030 | 35,356 | 21,857 | (8,242 | ) | 12,268 | 405,269 | |||||||||||||||||
| Under construction | 70,781 | 43,247 | (49,694 | ) | 0 | 1,950 | 66,284 | |||||||||||||||||
| 557,411 | 89,197 | 1,093 | (9,327 | ) | 17,921 | 656,295 | ||||||||||||||||||
Accumulated depreciation: | ||||||||||||||||||||||||
| Buildings | (9,502 | ) | (1,890 | ) | (16 | ) | 0 | (139 | ) | (11,547 | ) | |||||||||||||
| Plant and machinery | (176,204 | ) | (33,430 | ) | (1,394 | ) | 6,016 | (4,956 | ) | (209,968 | ) | |||||||||||||
| (185,706 | ) | (35,320 | ) | (1,410 | ) | 6,016 | (5,095 | ) | (221,515 | ) | ||||||||||||||
| Impairment of other property, plant and equipment | 0 | (649 | ) | 0 | 0 | 0 | (649 | ) | ||||||||||||||||
Carrying amount | 371,705 | 53,228 | (317 | ) | (3,311 | ) | 12,826 | 434,131 | ||||||||||||||||
F-132
Table of Contents
| Balances at | Business | Translation | Balances at | |||||||||||||||||||||||||
| 31/12/08 | Additions | Combinations | Transfers | Disposals | Differences | 31/12/09 | ||||||||||||||||||||||
| (Expressed in thousands of Euros) | ||||||||||||||||||||||||||||
Cost: | ||||||||||||||||||||||||||||
| Land and buildings | 111,067 | 9,729 | 0 | 22,905 | 0 | (1,101 | ) | 142,600 | ||||||||||||||||||||
| Plant and machinery | 287,761 | 33,994 | 2,307 | 27,784 | (5,881 | ) | (1,935 | ) | 344,030 | |||||||||||||||||||
| Under construction | 63,620 | 59,692 | 0 | (50,882 | ) | (757 | ) | (892 | ) | 70,781 | ||||||||||||||||||
| 462,448 | 103,415 | 2,307 | (193 | ) | (6,638 | ) | (3,928 | ) | 557,411 | |||||||||||||||||||
Accumulated depreciation: | ||||||||||||||||||||||||||||
| Buildings | (8,049 | ) | (1,514 | ) | 0 | 0 | 0 | 61 | (9,502 | ) | ||||||||||||||||||
| Plant and machinery | (153,390 | ) | (28,557 | ) | 0 | 192 | 4,942 | 609 | (176,204 | ) | ||||||||||||||||||
| (161,439 | ) | (30,071 | ) | 0 | 192 | 4,942 | 670 | (185,706 | ) | |||||||||||||||||||
Carrying amount | 301,009 | 73,344 | 2,307 | (1 | ) | (1,696 | ) | (3,258 | ) | 371,705 | ||||||||||||||||||
| (note 3 | ) | |||||||||||||||||||||||||||
F-133
Table of Contents
| Balances at | Business | Translation | Balances at | |||||||||||||||||||||||||
| 31/12/07 | Additions | Combinations | Transfers | Disposals | Differences | 31/12/08 | ||||||||||||||||||||||
| (Expressed in thousands of Euros) | ||||||||||||||||||||||||||||
Cost: | ||||||||||||||||||||||||||||
| Land and buildings | 79,845 | 29,142 | 0 | 641 | 0 | 1,439 | 111,067 | |||||||||||||||||||||
| Plant and machinery | 233,812 | 35,408 | 3 | 22,423 | (5,939 | ) | 2,054 | 287,761 | ||||||||||||||||||||
| Under construction | 30,079 | 55,399 | 0 | (23,948 | ) | (128 | ) | 2,218 | 63,620 | |||||||||||||||||||
| 343,736 | 119,949 | 3 | (884 | ) | (6,067 | ) | 5,711 | 462,448 | ||||||||||||||||||||
Accumulated depreciation: | ||||||||||||||||||||||||||||
| Buildings | (6,735 | ) | (1,234 | ) | 0 | (39 | ) | 29 | (70 | ) | (8,049 | ) | ||||||||||||||||
| Plant and machinery | (135,669 | ) | (22,942 | ) | 0 | 923 | 5,027 | (729 | ) | (153,390 | ) | |||||||||||||||||
| (142,404 | ) | (24,176 | ) | 0 | 884 | 5,056 | (799 | ) | (161,439 | ) | ||||||||||||||||||
Carrying amount | 201,332 | 95,773 | 3 | 0 | (1,011 | ) | 4,912 | 301,009 | ||||||||||||||||||||
| (note 3 | ) | |||||||||||||||||||||||||||
F-134
Table of Contents
| Initial Loan | ||||||||||||||||||||||||||
| Interest | Concession | Maturity | Face | Arrangement | Carrying | |||||||||||||||||||||
Loan | Currency | Rate | Date | Date | Amount | Expenses | Amount | |||||||||||||||||||
| Thousands of Euros | ||||||||||||||||||||||||||
| (Expressed in thousands of Euros) | ||||||||||||||||||||||||||
| Syndicated loan — Club deal | EUR | Euribor + 0,8% | 01/05/2008 | 26/05/2013 | 350,000 | (2,427 | ) | 99,408 | ||||||||||||||||||
| Instituto de crédito Oficial | EUR | Euribor + 1% | 01/06/2006 | 26/05/2016 | 30,000 | (210 | ) | 17,955 | ||||||||||||||||||
| Caixa Catalunya — Mortgage loan | EUR | Euribor + 0,9% | 01/02/2008 | 01/02/2018 | 14,000 | (294 | ) | 10,115 | ||||||||||||||||||
| Banco Santander | EUR | ICO + 1,8% | 01/06/2009 | 01/06/2016 | 6,000 | — | 5,400 | |||||||||||||||||||
| Caja de Madrid | EUR | Euribor + 1% | 05/06/2009 | 05/06/2016 | 6,000 | — | 5,400 | |||||||||||||||||||
| Banco Guipuzcoano | EUR | Euribor + 1% | 25/03/2010 | 25/03/2020 | 8,500 | — | 8,500 | |||||||||||||||||||
| Banco Sabadell | EUR | Euribor + 1% | 11/06/2010 | 30/06/2012 | 1,465 | — | 1,413 | |||||||||||||||||||
| SCH | EUR | 1.75% | 13/10/2010 | 13/10/2017 | 900 | — | 876 | |||||||||||||||||||
| Ibercaja | EUR | Euribor + 1,99% | 30/07/2009 | 31/07/2016 | 1,800 | — | 1,664 | |||||||||||||||||||
| Caja de Madrid | Euribor + 2% | 09/03/2010 | 25/03/2020 | 10,000 | — | 10,000 | ||||||||||||||||||||
| SCH | Euribor + 1% | 18/11/2010 | 31/01/2012 | 169 | — | 169 | ||||||||||||||||||||
| BBVA — Mortgage loan | EUR | Euribor + 1,2% | 21/10/2008 | 31/12/2024 | 45,000 | (676 | ) | 39,201 | ||||||||||||||||||
| Caixa Catalunya | EUR | ICO + 1,99% | 30/07/2009 | 25/08/2016 | 1,440 | — | 1,353 | |||||||||||||||||||
| Caixa Galicia | EUR | Euribor + 1% | 11/06/2010 | 25/06/2020 | 1,180 | — | 1,003 | |||||||||||||||||||
| Banca Toscana | EUR | 6 months Euribor + 1% | 08/05/2008 | 30/06/2013 | 3,000 | — | 939 | |||||||||||||||||||
| Cofides | EUR | 6 months Euribor + 0,45% | 01/08/2008 | 20/08/2017 | 6,854 | — | 5,875 | |||||||||||||||||||
| Cofides | EUR | Euribor + 2% | 20/09/2011 | 20/03/2017 | 10,745 | — | 10,177 | |||||||||||||||||||
| 497,053 | (3,607 | ) | 219,448 | |||||||||||||||||||||||
| Non-current finance lease creditors (see note 22) | — | — | 4,734 | |||||||||||||||||||||||
| 497,053 | (3,607 | ) | 224,182 | |||||||||||||||||||||||
F-135
Table of Contents
GRIFOLS, S.A. AND SUBSIDIARIES
Non-current Loans and Borrowings
For the Year Ended 31 December 2009
| Initial Loan | ||||||||||||||||||||||||
| Interest | Concession | Maturity | Face | Arrangement | Carrying | |||||||||||||||||||
Loan | Currency | Rate | Date | Date | Amount | Expenses | Amount | |||||||||||||||||
| Thousands of Euros | ||||||||||||||||||||||||
| (Expressed in thousands of Euros) | ||||||||||||||||||||||||
| Syndicated loan — Club deal | EUR | Euribor + 0,8% | 01/05/2008 | 26/05/2013 | 350,000 | (2,427 | ) | 195,471 | ||||||||||||||||
| Instituto de crédito Oficial | EUR | Euribor + 1% | 01/06/2006 | 26/05/2016 | 30,000 | (210 | ) | 21,933 | ||||||||||||||||
| Caixa Catalunya — Mortgage loan | EUR | Euribor + 0,9% | 01/02/2008 | 01/02/2018 | 14,000 | (294 | ) | 11,733 | ||||||||||||||||
| Banco Santander | EUR | ICO + 1,8% | 01/06/2009 | 01/06/2016 | 6,000 | — | 6,000 | |||||||||||||||||
| Caja de Madrid | EUR | Euribor + 1% | 05/06/2009 | 05/06/2016 | 6,000 | — | 6,000 | |||||||||||||||||
| Ibercaja | EUR | Euribor + 1,9% | 30/07/2009 | 31/07/2016 | 1,800 | — | 1,800 | |||||||||||||||||
| BBVA — Mortgage loan | EUR | Euribor + 1,2% | 21/10/2008 | 31/12/2024 | 45,000 | (676 | ) | 33,649 | ||||||||||||||||
| Caixa Catalunya | EUR | ICO + 1,99% | 30/07/2009 | 25/08/2016 | 1,440 | — | 1,440 | |||||||||||||||||
| Banca Toscana | EUR | 6 months Euribor + 1% | 08/05/2008 | 30/06/2013 | 3,000 | — | 1,552 | |||||||||||||||||
| Cofides | EUR | 6 months Euribor + 0,45% | 01/08/2008 | 20/08/2017 | 6,854 | — | 6,854 | |||||||||||||||||
| 464,094 | (3,607 | ) | 286,432 | |||||||||||||||||||||
| Non-current finance lease creditors (see note 22) | — | — | 6,202 | |||||||||||||||||||||
| 464,094 | (3,607 | ) | 292,634 | |||||||||||||||||||||
F-136
Table of Contents
| Initial Loan | ||||||||||||||||||||||||
| Interest | Concession | Maturity | Face | Arrangement | Carrying | |||||||||||||||||||
Loan | Currency | Rate | Date | Date | Amount | Expenses | Amount | |||||||||||||||||
| Thousands of Euros | ||||||||||||||||||||||||
| (Expressed in thousands of Euros) | ||||||||||||||||||||||||
| Syndicated loan — Club deal | EUR/USD | Euribor + 0,8% | 01/05/2008 | 26/05/2013 | 350,000 | (1,984 | ) | 225,320 | ||||||||||||||||
| Institut Catalá de Finances | EUR | 5.70% | 27/01/2005 | 28/02/2010 | 6,247 | (62 | ) | 312 | ||||||||||||||||
| Instituto de Crédito Oficial | EUR | 4.94% | 01/06/2006 | 26/05/2016 | 30,000 | (210 | ) | 25,907 | ||||||||||||||||
| Caixa Catalunya — Mortgage loan | EUR | 5.25% | 01/02/2008 | 01/02/2018 | 14,000 | (294 | ) | 13,350 | ||||||||||||||||
| BBVA — Mortgage loan | EUR | 6.50% | 21/10/2008 | 31/12/2024 | 45,000 | (676 | ) | 30,463 | ||||||||||||||||
| Banca Toscana | EUR | 5.33% | 08/05/2008 | 30/06/2013 | 3,000 | — | 2,183 | |||||||||||||||||
| Cofides | EUR | 5.61% | 01/08/2008 | 20/08/2017 | 6,854 | — | 6,854 | |||||||||||||||||
| 448,247 | (3,226 | ) | 304,389 | |||||||||||||||||||||
| Non-current finance lease creditors (see note 22) | — | — | 7,124 | |||||||||||||||||||||
| 448,247 | (3,226 | ) | 311,513 | |||||||||||||||||||||
F-137
Table of Contents
GRIFOLS, S.A. AND SUBSIDIARIES
Condensed Consolidated Balance Sheets
at 31 December 2009
| Guarantor | Non-Guarantor | Consolidating | ||||||||||||||||||||||
Assets | Parent | Issuer | Subsidiaries | Subsidiaries | Adjustments | Consolidated | ||||||||||||||||||
| (Unaudited) | ||||||||||||||||||||||||
| (Expressed in thousands of euros) | ||||||||||||||||||||||||
Non-current assets | ||||||||||||||||||||||||
| Intangible assets | ||||||||||||||||||||||||
| Goodwill | 0 | 0 | 27,165 | 12,465 | 134,370 | 174,000 | ||||||||||||||||||
| Other intangible assets | 8,111 | 950 | 44,251 | 3,405 | 12,668 | 69,385 | ||||||||||||||||||
| Total intangible assets | 8,111 | 950 | 71,416 | 15,870 | 147,038 | 243,385 | ||||||||||||||||||
| Property, plant and equipment | 94,683 | 18,508 | 187,213 | 72,299 | (998 | ) | 371,705 | |||||||||||||||||
| Investments in Subsidiaries | 342,810 | 206,165 | 16,356 | 273 | (565,604 | ) | 0 | |||||||||||||||||
| Advances and notes between parent and subsidiaries | 0 | 21,748 | 0 | 0 | (21,748 | ) | 0 | |||||||||||||||||
| Investments in equity accounted investees | 0 | 0 | 0 | 0 | 383 | 383 | ||||||||||||||||||
| Non-current financial assets | 650 | 2,125 | 727 | 229 | 0 | 3,731 | ||||||||||||||||||
| Deferred tax assets | 953 | 1,774 | 9,218 | 1,604 | 19,846 | 33,395 | ||||||||||||||||||
| Total non-current assets | 447,207 | 251,270 | 284,930 | 90,275 | (421,083 | ) | 652,599 | |||||||||||||||||
Current assets | ||||||||||||||||||||||||
| Inventories | 704 | 0 | 495,542 | 62,006 | (73,790 | ) | 484,462 | |||||||||||||||||
| Trade and other receivables | ||||||||||||||||||||||||
| Trade receivables | 9,419 | 182,073 | 270,117 | 118,660 | (372,429 | ) | 207,840 | |||||||||||||||||
| Other receivables | 8,344 | 51 | 23,989 | 7,156 | 0 | 39,540 | ||||||||||||||||||
| Current income tax assets | 4,178 | 2,139 | 508 | 2,476 | (1,499 | ) | 7,802 | |||||||||||||||||
| Trade and other receivables | 21,940 | 184,263 | 294,613 | 128,292 | (373,926 | ) | 255,182 | |||||||||||||||||
| Advances and notes between parent and subsidiaries | 222,829 | 44,522 | 13,522 | 21,840 | (302,713 | ) | 0 | |||||||||||||||||
| Other current financial assets | 1,921 | 123 | 1 | 6,172 | 0 | 8,217 | ||||||||||||||||||
| Other current assets | 2,707 | 318 | 3,156 | 1,164 | 0 | 7,345 | ||||||||||||||||||
| Cash and cash equivalents | 144 | 237,804 | 7,191 | 4,233 | 0 | 249,372 | ||||||||||||||||||
| Total current assets | 250,245 | 467,030 | 814,025 | 223,707 | (750,429 | ) | 1,004,578 | |||||||||||||||||
Total assets | 697,452 | 718,300 | 1,098,955 | 313,982 | (1,171,512 | ) | 1,657,177 | |||||||||||||||||
F-138
Table of Contents
| Guarantor | Non-Guarantor | Consolidating | ||||||||||||||||||||||
Equity and liabilities | Parent | Issuer | Subsidiaries | Subsidiaries | Adjustments | Consolidated | ||||||||||||||||||
| (Unaudited) | ||||||||||||||||||||||||
| (Expressed in thousands of euros) | ||||||||||||||||||||||||
Equity | ||||||||||||||||||||||||
| Share capital | 106,532 | 0 | 21,497 | 36,080 | (57,577 | ) | 106,532 | |||||||||||||||||
| Share premium | 121,802 | 72,932 | 123,317 | 5,703 | (201,952 | ) | 121,802 | |||||||||||||||||
| Reserves | 59,342 | 193,822 | 92,666 | 52,597 | (83,524 | ) | 314,903 | |||||||||||||||||
| Own shares | (677 | ) | 0 | 0 | 0 | 0 | (677 | ) | ||||||||||||||||
| Interim dividend | (31,960 | ) | 0 | 0 | (208 | ) | 208 | (31,960 | ) | |||||||||||||||
| Profit for the year attributable to the Parent | 72,923 | (2,976 | ) | 137,853 | 26,664 | (86,492 | ) | 147,972 | ||||||||||||||||
| Total equity | 327,962 | 263,778 | 375,333 | 120,836 | (429,337 | ) | 658,572 | |||||||||||||||||
| Cash flow hedges | 0 | (1,948 | ) | 0 | 0 | 0 | (1,948 | ) | ||||||||||||||||
| Translation differences | 0 | (44 | ) | (34,796 | ) | (478 | ) | (54,935 | ) | (90,253 | ) | |||||||||||||
| Accumulated other comprehensive income | 0 | (1,992 | ) | (34,796 | ) | (478 | ) | (54,935 | ) | (92,201 | ) | |||||||||||||
Equity attributable to the Parent | 327,962 | 261,786 | 340,537 | 120,358 | (484,272 | ) | 566,371 | |||||||||||||||||
| Non-controlling interests | 0 | 0 | 0 | 86 | 12,071 | 12,157 | ||||||||||||||||||
Total equity | 327,962 | 261,786 | 340,537 | 120,444 | (472,201 | ) | 578,528 | |||||||||||||||||
Liabilities | ||||||||||||||||||||||||
Non-current liabilities | ||||||||||||||||||||||||
| Grants | 77 | 174 | 2,000 | 60 | 0 | 2,311 | ||||||||||||||||||
| Provisions | 0 | 0 | 989 | 243 | 0 | 1,232 | ||||||||||||||||||
| Non-current financial liabilities | ||||||||||||||||||||||||
| Loans and borrowings, bonds and other marketable securities | 236,733 | 410,550 | 21,577 | 34,327 | 0 | 703,187 | ||||||||||||||||||
| Advances and notes between parent and subsidiaries | 16,854 | 0 | 0 | 7,089 | (23,943 | ) | 0 | |||||||||||||||||
| Other financial liabilities | 367 | 0 | 11,565 | 620 | 0 | 12,552 | ||||||||||||||||||
| Total non-current financial liabilities | 253,954 | 410,550 | 33,142 | 42,036 | (23,943 | ) | 715,739 | |||||||||||||||||
| Deferred tax liabilities | 11,231 | 1,349 | 44,503 | 4,172 | (931 | ) | 60,324 | |||||||||||||||||
| Total non-current liabilities | 265,262 | 412,073 | 80,634 | 46,511 | (24,874 | ) | 779,606 | |||||||||||||||||
Current liabilities | ||||||||||||||||||||||||
| Provisions | 291 | 0 | 30 | 4,381 | 0 | 4,702 | ||||||||||||||||||
| Current financial liabilities | ||||||||||||||||||||||||
| Loans and borrowings, bonds and other marketable securities | 35,579 | 6,907 | 42,988 | 28,517 | 0 | 113,991 | ||||||||||||||||||
| Advances and notes between parent and subsidiaries | 43,768 | 0 | 234,177 | 22,576 | (300,521 | ) | 0 | |||||||||||||||||
| Other financial liabilities | 3,594 | 0 | 7,496 | 1,140 | 0 | 12,230 | ||||||||||||||||||
| Total current financial liabilities | 82,941 | 6,907 | 284,661 | 52,233 | (300,521 | ) | 126,221 | |||||||||||||||||
| Debts with associates | 0 | 0 | 0 | 0 | 0 | 0 | ||||||||||||||||||
| Trade and other payables | ||||||||||||||||||||||||
| Suppliers | 10,583 | 35,919 | 371,086 | 75,744 | (372,423 | ) | 120,909 | |||||||||||||||||
| Other payables | 7,464 | 8 | 7,116 | 3,244 | 0 | 17,832 | ||||||||||||||||||
| Current income tax liabilities | 0 | 0 | 946 | 3,805 | (1,493 | ) | 3,258 | |||||||||||||||||
| Total trade and other payables | 18,047 | 35,927 | 379,148 | 82,793 | (373,916 | ) | 141,999 | |||||||||||||||||
| Other current liabilities | 2,949 | 1,607 | 13,945 | 7,620 | 0 | 26,121 | ||||||||||||||||||
| Total current liabilities | 104,228 | 44,441 | 677,784 | 147,027 | (674,437 | ) | 299,043 | |||||||||||||||||
Total liabilities | 369,490 | 456,514 | 758,418 | 193,538 | (699,311 | ) | 1,078,649 | |||||||||||||||||
Total equity and liabilities | 697,452 | 718,300 | 1,098,955 | 313,982 | (1,171,512 | ) | 1,657,177 | |||||||||||||||||
F-139
Table of Contents
| Guarantor | Non-Guarantor | Consolidating | ||||||||||||||||||||||
Assets | Parent | Issuer | Subsidiaries | Subsidiaries | Adjustments | Consolidated | ||||||||||||||||||
| (Expressed in thousands of Euros) | ||||||||||||||||||||||||
Non-current assets | ||||||||||||||||||||||||
| Intangible assets | ||||||||||||||||||||||||
| Goodwill | 0 | 0 | 29,895 | 15,123 | 144,430 | 189,448 | ||||||||||||||||||
| Other intangible assets | 5,731 | 3,162 | 49,120 | 4,725 | 15,561 | 78,299 | ||||||||||||||||||
| Total intangible assets | 5,731 | 3,162 | 79,015 | 19,848 | 159,991 | 267,747 | ||||||||||||||||||
| Property, plant and equipment | 95,452 | 28,150 | 231,728 | 78,801 | 0 | 434,131 | ||||||||||||||||||
| Investments in Subsidiaries | 345,025 | 222,273 | 2,532 | 938 | (570,768 | ) | 0 | |||||||||||||||||
| Advances and notes between parent and subsidiaries | 0 | 21,005 | 0 | 0 | (21,005 | ) | 0 | |||||||||||||||||
| Investments in equity accounted investees | 0 | 0 | 0 | 0 | 598 | 598 | ||||||||||||||||||
| Non-current financial assets | 709 | 5,804 | 734 | 288 | 0 | 7,535 | ||||||||||||||||||
| Deferred tax assets | 1,091 | 2,076 | 9,534 | 2,932 | 19,256 | 34,889 | ||||||||||||||||||
| Total non-current assets | 448,008 | 282,470 | 323,543 | 102,807 | (411,928 | ) | 744,900 | |||||||||||||||||
Current assets | ||||||||||||||||||||||||
| Inventories | 796 | 0 | 538,311 | 59,401 | (70,643 | ) | 527,865 | |||||||||||||||||
| Trade and other receivables | ||||||||||||||||||||||||
| Trade receivables | 8,946 | 11,561 | 209,758 | 100,719 | (106,629 | ) | 224,355 | |||||||||||||||||
| Other receivables | 3,157 | 201 | 27,612 | 11,971 | 1,091 | 44,032 | ||||||||||||||||||
| Current income tax assets | 6,168 | 6,071 | 297 | 2,071 | 0 | 14,607 | ||||||||||||||||||
| Trade and other receivables | 18,271 | 17,833 | 237,667 | 114,761 | (105,538 | ) | 282,994 | |||||||||||||||||
| Advances and notes between parent and subsidiaries | 238,262 | (1,311 | ) | 14,699 | 22,860 | (274,510 | ) | 0 | ||||||||||||||||
| Other current financial assets | 267 | 224 | 8 | 12,447 | 0 | 12,946 | ||||||||||||||||||
| Other current assets | 13,460 | 60,568 | 5,150 | 1,450 | 0 | 80,628 | ||||||||||||||||||
| Cash and cash equivalents | 25 | 227,456 | 1,444 | 10,724 | 0 | 239,649 | ||||||||||||||||||
| Total current assets | 271,081 | 304,770 | 797,279 | 221,643 | (450,691 | ) | 1,144,082 | |||||||||||||||||
Total assets | 719,089 | 587,240 | 1,120,822 | 324,450 | (862,619 | ) | 1,888,982 | |||||||||||||||||
F-140
Table of Contents
| Guarantor | Non-Guarantor | Consolidating | ||||||||||||||||||||||
Equity and Liabilities | Parent | Issuer | Subsidiaries | Subsidiaries | Adjustments | Consolidated | ||||||||||||||||||
| (Expressed in thousands of Euros) | ||||||||||||||||||||||||
Equity | ||||||||||||||||||||||||
| Share capital | 106,532 | 0 | 21,497 | 36,340 | (57,837 | ) | 106,532 | |||||||||||||||||
| Share premium | 121,802 | 72,932 | 106,854 | 5,703 | (185,489 | ) | 121,802 | |||||||||||||||||
| Reserves | 73,076 | 190,844 | 175,221 | 59,636 | (95,173 | ) | 403,604 | |||||||||||||||||
| Own shares | (1,927 | ) | 0 | 0 | 0 | 0 | (1,927 | ) | ||||||||||||||||
| Interim dividend | 0 | 0 | 0 | (152 | ) | 152 | 0 | |||||||||||||||||
| Profit for the year attributable to the Parent | 63,226 | (10,726 | ) | 112,853 | 23,906 | (73,746 | ) | 115,513 | ||||||||||||||||
| Total equity | 362,709 | 253,050 | 416,425 | 125,433 | (412,093 | ) | 745,524 | |||||||||||||||||
| Cash flow hedges | 0 | (1,751 | ) | 0 | 0 | 0 | (1,751 | ) | ||||||||||||||||
| Translation differences | 0 | 20,449 | (18,344 | ) | 11,123 | (63,961 | ) | (50,733 | ) | |||||||||||||||
| Accumulated other comprehensive income | 0 | 18,698 | (18,344 | ) | 11,123 | (63,961 | ) | (52,484 | ) | |||||||||||||||
Equity attributable to the Parent | 362,709 | 271,748 | 398,081 | 136,556 | (476,054 | ) | 693,040 | |||||||||||||||||
| Non-controlling interests | 0 | 0 | 0 | 0 | 14,350 | 14,350 | ||||||||||||||||||
Total equity | 362,709 | 271,748 | 398,081 | 136,556 | (461,704 | ) | 707,390 | |||||||||||||||||
Liabilities | ||||||||||||||||||||||||
Non-current liabilities | ||||||||||||||||||||||||
| Grants | 142 | 187 | 1,683 | 76 | 0 | 2,088 | ||||||||||||||||||
| Provisions | 0 | 0 | 1,127 | 251 | 0 | 1,378 | ||||||||||||||||||
| Non-current financial liabilities | ||||||||||||||||||||||||
| Loans and borrowings, bonds and other marketable securities | 133,982 | 441,203 | 40,350 | 49,695 | 155 | 665,385 | ||||||||||||||||||
| Advances and notes between parent and subsidiaries | 15,875 | 0 | 0 | 5,130 | (21,005 | ) | 0 | |||||||||||||||||
| Other financial liabilities | 200 | 0 | 9,596 | 678 | 0 | 10,474 | ||||||||||||||||||
| Total non-current financial liabilities | 150,057 | 441,203 | 49,946 | 55,503 | (20,850 | ) | 675,859 | |||||||||||||||||
| Deferred tax liabilities | 11,907 | 2,860 | 62,718 | 1,519 | 137 | 79,141 | ||||||||||||||||||
| Total non-current liabilities | 162,106 | 444,250 | 115,474 | 57,349 | (20,713 | ) | 758,466 | |||||||||||||||||
Current liabilities | ||||||||||||||||||||||||
| Provisions | 257 | 0 | 30 | 4,078 | 0 | 4,365 | ||||||||||||||||||
| Current financial liabilities | ||||||||||||||||||||||||
| Loans and borrowings, bonds and | ||||||||||||||||||||||||
| other marketable securities | 103,131 | 7,364 | 41,433 | 39,862 | (155 | ) | 191,635 | |||||||||||||||||
| Advances and notes between parent and subsidiaries | 42,863 | (162,772 | ) | 385,947 | 9,017 | (275,055 | ) | 0 | ||||||||||||||||
| Other financial liabilities | 8,830 | 0 | 9,316 | 90 | 0 | 18,236 | ||||||||||||||||||
| Total current financial liabilities | 154,824 | (155,408 | ) | 436,696 | 48,969 | (275,210 | ) | 209,871 | ||||||||||||||||
| Debts with associates | 1,162 | 0 | 0 | 0 | 0 | 1,162 | ||||||||||||||||||
| Trade and other payables | ||||||||||||||||||||||||
| Suppliers | 33,426 | 24,766 | 146,861 | 60,617 | (104,992 | ) | 160,678 | |||||||||||||||||
| Other payables | 1,141 | 12 | 5,288 | 3,627 | 1,860 | 11,928 | ||||||||||||||||||
| Current income tax liabilities | 0 | 0 | 2,369 | 3,663 | (1,860 | ) | 4,172 | |||||||||||||||||
| Total trade and other payables | 34,567 | 24,778 | 154,518 | 67,907 | (104,992 | ) | 176,778 | |||||||||||||||||
| Other current liabilities | 3,464 | 1,872 | 16,023 | 9,591 | 0 | 30,950 | ||||||||||||||||||
| Total current liabilities | 194,274 | (128,758 | ) | 607,267 | 130,545 | (380,202 | ) | 423,126 | ||||||||||||||||
Total liabilities | 356,380 | 315,492 | 722,741 | 187,894 | (400,915 | ) | 1,181,592 | |||||||||||||||||
Total equity and liabilities | 719,089 | 587,240 | 1,120,822 | 324,450 | (862,619 | ) | 1,888,982 | |||||||||||||||||
F-141
Table of Contents
| Guarantor | Non-Guarantor | Consolidating | ||||||||||||||||||||||
Profit and Loss | Parent | Issuer | Subsidiaries | Subsidiaries | Adjustments | Consolidated | ||||||||||||||||||
| (Expressed in thousands of Euros) | ||||||||||||||||||||||||
| Revenues | 54,725 | 17,424 | 1,211,130 | 320,481 | (789,449 | ) | 814,311 | |||||||||||||||||
| Changes in inventories of finished goods and work in progress | 0 | 0 | 64,328 | 482 | (33,752 | ) | 31,058 | |||||||||||||||||
| Self-constructed non-current assets | 1,731 | 0 | 9,823 | 2,395 | 11,845 | 25,794 | ||||||||||||||||||
| Supplies | (288 | ) | 0 | (670,916 | ) | (177,875 | ) | 642,341 | (206,738 | ) | ||||||||||||||
| Other operating income | 68 | 0 | 1,171 | 50 | 0 | 1,289 | ||||||||||||||||||
| Personnel expenses | (20,483 | ) | (8,708 | ) | (162,901 | ) | (46,067 | ) | 0 | (238,159 | ) | |||||||||||||
| Other operating expenses | (31,786 | ) | (8,305 | ) | (243,732 | ) | (53,113 | ) | 144,648 | (192,288 | ) | |||||||||||||
| Amortisation and depreciation | (4,245 | ) | (497 | ) | (24,178 | ) | (4,270 | ) | (66 | ) | (33,256 | ) | ||||||||||||
| Non-financial and other capital grants | 0 | 0 | 2,941 | 0 | 0 | 2,941 | ||||||||||||||||||
| Impairment and net losses on disposal of fixed assets | (31 | ) | 0 | (1,960 | ) | 0 | 0 | (1,991 | ) | |||||||||||||||
Results from operating activities | (309 | ) | (86 | ) | 185,706 | 42,083 | (24,433 | ) | 202,961 | |||||||||||||||
| Finance income | 11,184 | 1 | 2,411 | 1,151 | (12,065 | ) | 2,682 | |||||||||||||||||
| Dividends | 72,172 | 25,035 | 10,382 | 48 | (107,637 | ) | 0 | |||||||||||||||||
| Finance expense | (18,626 | ) | (1,892 | ) | (18,443 | ) | (2,597 | ) | 12,253 | (29,305 | ) | |||||||||||||
| Change in fair value of financial instruments | (1,195 | ) | 0 | (73 | ) | 0 | 0 | (1,268 | ) | |||||||||||||||
| Exchange gains/(losses) | (1,335 | ) | 752 | 2,395 | (4,637 | ) | 0 | (2,825 | ) | |||||||||||||||
Net Finance expense | 62,200 | 23,896 | (3,328 | ) | (6,035 | ) | (107,449 | ) | (30,716 | ) | ||||||||||||||
| Share of profit/(loss) of equity accounted investees | 0 | 0 | 0 | 0 | 24 | 24 | ||||||||||||||||||
Profit before income tax from continuing operations | 61,891 | 23,810 | 182,378 | 36,048 | (131,858 | ) | 172,269 | |||||||||||||||||
| Income tax expense | 2,755 | 448 | (50,673 | ) | (10,295 | ) | 7,612 | (50,153 | ) | |||||||||||||||
Profit after income tax from continuing operations | 64,646 | 24,258 | 131,705 | 25,753 | (124,246 | ) | 122,116 | |||||||||||||||||
Consolidated profit for the year | 64,646 | 24,258 | 131,705 | 25,753 | (124,246 | ) | 122,116 | |||||||||||||||||
| Profit attributable to equity holders of the Parent | 64,646 | 24,258 | 131,705 | 25,753 | (124,634 | ) | 121,728 | |||||||||||||||||
| Profit/(loss) attributable to non-controlling interests | 0 | 0 | 0 | 0 | 388 | 388 | ||||||||||||||||||
F-142
Table of Contents
| Guarantor | Non-Guarantor | Consolidating | ||||||||||||||||||||||
Profit and Loss | Parent | Issuer | Subsidiaries | Subsidiaries | Adjustments | Consolidated | ||||||||||||||||||
| (Expressed in thousands of Euros) | ||||||||||||||||||||||||
| Revenues | 64,981 | 19,074 | 1,367,208 | 373,372 | (911,449 | ) | 913,186 | |||||||||||||||||
| Changes in inventories of finished goods and work in progress | 0 | 0 | 103,193 | 989 | (31,089 | ) | 73,093 | |||||||||||||||||
| Self-constructed non-current assets | 2,406 | 571 | 11,605 | 6,973 | 19,587 | 41,142 | ||||||||||||||||||
| Supplies | (449 | ) | 0 | (809,640 | ) | (215,708 | ) | 739,523 | (286,274 | ) | ||||||||||||||
| Other operating income | 59 | 0 | 678 | 706 | 0 | 1,443 | ||||||||||||||||||
| Personnel expenses | (23,337 | ) | (12,781 | ) | (181,298 | ) | (55,763 | ) | 11 | (273,168 | ) | |||||||||||||
| Other operating expenses | (31,633 | ) | (5,727 | ) | (258,789 | ) | (67,898 | ) | 160,666 | (203,381 | ) | |||||||||||||
| Amortisation and depreciation | (5,659 | ) | (850 | ) | (27,319 | ) | (5,726 | ) | 0 | (39,554 | ) | |||||||||||||
| Non-financial and other capital grants | 431 | 0 | 637 | 120 | 0 | 1,188 | ||||||||||||||||||
| Impairment and net losses on disposal of fixed assets | (148 | ) | (2 | ) | (843 | ) | (154 | ) | 0 | (1,147 | ) | |||||||||||||
Results from operating activities | 6,651 | 285 | 205,432 | 36,911 | (22,751 | ) | 226,528 | |||||||||||||||||
| Finance income | 9,395 | 6,054 | 3,699 | 1,384 | (13,465 | ) | 7,067 | |||||||||||||||||
| Dividends | 72,226 | 0 | 0 | 165 | (72,391 | ) | 0 | |||||||||||||||||
| Finance expense | (10,660 | ) | (10,267 | ) | (16,787 | ) | (3,762 | ) | 14,389 | (27,087 | ) | |||||||||||||
| Change in fair value of financial instruments | (979 | ) | 0 | 146 | 0 | 246 | (587 | ) | ||||||||||||||||
| Gains/(losses) on disposal of financial instruments | 0 | 0 | (1,482 | ) | 0 | 1,237 | (245 | ) | ||||||||||||||||
| Exchange gains/(losses) | (723 | ) | (858 | ) | (242 | ) | 90 | 0 | (1,733 | ) | ||||||||||||||
Net Finance expense | 69,259 | (5,071 | ) | (14,666 | ) | (2,123 | ) | (69,984 | ) | (22,585 | ) | |||||||||||||
| Share of profit/(loss) of equity accounted investees | 0 | 0 | 0 | 0 | 51 | 51 | ||||||||||||||||||
Profit before income tax from continuing operations | 75,910 | (4,786 | ) | 190,766 | 34,788 | (92,684 | ) | 203,994 | ||||||||||||||||
| Income tax expense | (2,987 | ) | 1,810 | (52,913 | ) | (8,124 | ) | 5,790 | (56,424 | ) | ||||||||||||||
Profit after income tax from continuing operations | 72,923 | (2,976 | ) | 137,853 | 26,664 | (86,894 | ) | 147,570 | ||||||||||||||||
Consolidated profit for the year | 72,923 | (2,976 | ) | 137,853 | 26,664 | (86,894 | ) | 147,570 | ||||||||||||||||
| Profit attributable to equity holders of the Parent | 72,923 | (2,976 | ) | 137,853 | 26,664 | (86,492 | ) | 147,972 | ||||||||||||||||
| Profit/(loss) attributable to non-controlling interests | 0 | 0 | 0 | 0 | (402 | ) | (402 | ) | ||||||||||||||||
F-143
Table of Contents
| Guarantor | Non-Guarantor | Consolidating | ||||||||||||||||||||||
Profit and Loss | Parent | Issuer | Subsidiaries | Subsidiaries | Adjustments | Consolidated | ||||||||||||||||||
| (Expressed in thousands of Euros) | ||||||||||||||||||||||||
| Revenues | 66,966 | 21,313 | 1,351,397 | 428,832 | (877,778 | ) | 990,730 | |||||||||||||||||
| Changes in inventories of finished goods and work in progress | 0 | 0 | 25,189 | (1,872 | ) | 22,432 | 45,749 | |||||||||||||||||
| Self-constructed non-current assets | 580 | 1,208 | 10,165 | 2,672 | 18,888 | 33,513 | ||||||||||||||||||
| Supplies | (464 | ) | 0 | (732,316 | ) | (247,988 | ) | 673,909 | (306,859 | ) | ||||||||||||||
| Other operating income | 93 | 0 | 1,014 | 89 | 0 | 1,196 | ||||||||||||||||||
| Personnel expenses | (23,931 | ) | (13,651 | ) | (189,100 | ) | (62,326 | ) | 0 | (289,008 | ) | |||||||||||||
| Other operating expenses | (43,753 | ) | (7,417 | ) | (260,686 | ) | (77,041 | ) | 168,679 | (220,218 | ) | |||||||||||||
| Amortisation and depreciation | (7,384 | ) | (992 | ) | (28,623 | ) | (8,222 | ) | (555 | ) | (45,776 | ) | ||||||||||||
| Non-financial and other capital grants | 259 | 0 | 470 | (1 | ) | 0 | 728 | |||||||||||||||||
| Impairment and net losses on disposal of fixed assets | (2 | ) | (139 | ) | (756 | ) | (578 | ) | 1,103 | (372 | ) | |||||||||||||
Results from operating activities | (7,636 | ) | 322 | 176,754 | 33,565 | 6,678 | 209,683 | |||||||||||||||||
| Finance income | 4,054 | 15,172 | 2,856 | 1,243 | (18,799 | ) | 4,526 | |||||||||||||||||
| Dividends | 76,491 | 0 | 0 | 160 | (76,651 | ) | 0 | |||||||||||||||||
| Finance expense | (8,124 | ) | (32,274 | ) | (22,759 | ) | (5,304 | ) | 18,801 | (49,660 | ) | |||||||||||||
| Change in fair value of financial instruments | (7,670 | ) | 0 | 0 | 77 | 0 | (7,593 | ) | ||||||||||||||||
| Gains/ (losses) on disposal of financial instruments | 0 | 0 | 1,573 | (879 | ) | (603 | ) | 91 | ||||||||||||||||
| Exchange gains/(losses) | 75 | (455 | ) | (389 | ) | 2,385 | 0 | 1,616 | ||||||||||||||||
Net Finance expense | 64,826 | (17,557 | ) | (18,719 | ) | (2,318 | ) | (77,252 | ) | (51,020 | ) | |||||||||||||
| Share of profit/(loss) of equity accounted investees | 0 | 0 | 0 | 0 | (879 | ) | (879 | ) | ||||||||||||||||
Profit before income tax from continuing operations | 57,190 | (17,235 | ) | 158,035 | 31,247 | (71,453 | ) | 157,784 | ||||||||||||||||
| Income tax expense | 6,036 | 6,509 | (45,182 | ) | (7,445 | ) | (2,435 | ) | (42,517 | ) | ||||||||||||||
Profit after income tax from continuing operations | 63,226 | (10,726 | ) | 112,853 | 23,802 | (73,888 | ) | 115,267 | ||||||||||||||||
Consolidated profit for the year | 63,226 | (10,726 | ) | 112,853 | 23,802 | (73,888 | ) | 115,267 | ||||||||||||||||
| Profit attributable to equity holders of the Parent | 63,226 | (10,726 | ) | 112,853 | 23,906 | (73,746 | ) | 115,513 | ||||||||||||||||
| Profit/(loss) attributable to non-controlling interests | 0 | 0 | 0 | (104 | ) | (142 | ) | (246 | ) | |||||||||||||||
F-144
Table of Contents
| Guarantor | Non-Guarantor | Consolidating | ||||||||||||||||||||||
| Parent | Issuer | Subsidiaries | Subsidiaries | Adjustments | Consolidated | |||||||||||||||||||
| (Expressed in thousands of Euros) | ||||||||||||||||||||||||
Cash flows from/(used in) operating activities | ||||||||||||||||||||||||
Profit before income tax | 61,891 | 23,810 | 182,378 | 36,048 | (131,858 | ) | 172,269 | |||||||||||||||||
Adjustments for: | (56,619 | ) | (16,796 | ) | 35,898 | 13,009 | 90,542 | 66,034 | ||||||||||||||||
| Amortisation and depreciation | 4,245 | 497 | 24,178 | 4,270 | 66 | 33,256 | ||||||||||||||||||
| Other adjustments: | (60,864 | ) | (17,293 | ) | 11,720 | 8,739 | 90,476 | 32,778 | ||||||||||||||||
| (Profit)/losses on equity accounted investments | 0 | 0 | 0 | 0 | (24 | ) | (24 | ) | ||||||||||||||||
| Exchange differences | 1,335 | (752 | ) | (2,395 | ) | 4,637 | 0 | 2,825 | ||||||||||||||||
| Impairment of assets and net provision charges | 0 | 0 | 1,800 | 194 | 0 | 1,994 | ||||||||||||||||||
| (Profits)/losses on disposal of fixed assets | 31 | 0 | 1,960 | 10 | 0 | 2,001 | ||||||||||||||||||
| Government grants taken to income | 0 | 0 | (2,941 | ) | (2 | ) | 0 | (2,943 | ) | |||||||||||||||
| Net finance expense | (62,437 | ) | (23,139 | ) | 4,416 | 1,587 | 107,464 | 27,891 | ||||||||||||||||
| Other adjustments | 207 | 6,598 | 8,880 | 2,313 | (16,964 | ) | 1,034 | |||||||||||||||||
Change in operating assets and liabilities | (112,558 | ) | (138,087 | ) | (15,842 | ) | (4,178 | ) | 184,115 | (86,550 | ) | |||||||||||||
| Change in inventories | (117 | ) | 0 | (120,261 | ) | (18,933 | ) | 40,791 | (98,520 | ) | ||||||||||||||
| Change in trade and other receivables | 4,579 | (133,431 | ) | 36,498 | (12,003 | ) | 96,406 | (7,951 | ) | |||||||||||||||
| Change in current financial assets and other current assets | (123,479 | ) | 27,683 | (49,701 | ) | 2,913 | 142,989 | 405 | ||||||||||||||||
| Change in current trade and other payables | 6,459 | (32,339 | ) | 117,622 | 23,845 | (96,071 | ) | 19,516 | ||||||||||||||||
Other cash flows used in operating activities | 66,654 | 22,420 | (40,647 | ) | (18,107 | ) | (107,630 | ) | (77,310 | ) | ||||||||||||||
| Interest paid | (17,206 | ) | (1,818 | ) | (16,641 | ) | (1,478 | ) | 11,171 | (25,972 | ) | |||||||||||||
| Interest received | 11,171 | 0 | 2,213 | 0 | (11,171 | ) | 2,213 | |||||||||||||||||
| Dividends received | 72,172 | 25,035 | 10,382 | 41 | (107,630 | ) | 0 | |||||||||||||||||
| Income tax paid | 517 | (797 | ) | (36,601 | ) | (16,670 | ) | 0 | (53,551 | ) | ||||||||||||||
Net cash from operating activities | (40,632 | ) | (108,653 | ) | 161,787 | 26,772 | 35,169 | 74,443 | ||||||||||||||||
Cash flows from/(used in) investing activities | ||||||||||||||||||||||||
| Payments for investments | (36,693 | ) | (8,282 | ) | (48,123 | ) | (39,752 | ) | 1,927 | (130,923 | ) | |||||||||||||
| Group companies and business units | (1,927 | ) | 0 | (632 | ) | 0 | 1,927 | (632 | ) | |||||||||||||||
| Property, plant and equipment and intangible assets | (34,212 | ) | (8,250 | ) | (47,434 | ) | (39,672 | ) | 0 | (129,568 | ) | |||||||||||||
| Other financial assets | (554 | ) | (32 | ) | (57 | ) | (80 | ) | 0 | (723 | ) | |||||||||||||
| Proceeds from the sale of investments | 1,740 | 0 | (6 | ) | 102 | (1,679 | ) | 157 | ||||||||||||||||
| Property, plant and equipment | 61 | 0 | (6 | ) | 102 | 0 | 157 | |||||||||||||||||
| Other financial assets | 0 | 0 | 0 | 0 | 0 | 0 | ||||||||||||||||||
| Associates | 1,679 | 0 | 0 | 0 | (1,679 | ) | 0 | |||||||||||||||||
Net cash used in investing activities | (34,953 | ) | (8,282 | ) | (48,129 | ) | (39,650 | ) | 248 | (130,766 | ) | |||||||||||||
Cash flows from/(used in) financing activities | ||||||||||||||||||||||||
| Proceeds from and payments for equity instruments | (4,212 | ) | 41,179 | (1,619 | ) | 1,926 | (41,486 | ) | (4,212 | ) | ||||||||||||||
| Issue | 0 | 41,179 | (1,619 | ) | 1,926 | (41,486 | ) | 0 | ||||||||||||||||
| Acquisition of own shares | (4,880 | ) | 0 | 0 | 0 | 0 | (4,880 | ) | ||||||||||||||||
| Disposal of treasury shares | 668 | 0 | 0 | 0 | 0 | 668 | ||||||||||||||||||
| Proceeds from and payments for financial liability instruments | 114,565 | 76,084 | (29,551 | ) | 38,775 | (103,524 | ) | 96,349 | ||||||||||||||||
| Issue | 306,325 | 27,058 | 21,262 | 39,223 | 241 | 394,109 | ||||||||||||||||||
| Redemption and repayment | (250,641 | ) | 0 | (41,516 | ) | (5,603 | ) | 0 | (297,760 | ) | ||||||||||||||
| Debts with group companies | 58,881 | 49,026 | (9,297 | ) | 5,155 | (103,765 | ) | 0 | ||||||||||||||||
| Dividends and interest on other equity instruments paid | (34,767 | ) | 0 | (81,473 | ) | (28,141 | ) | 109,589 | (34,792 | ) | ||||||||||||||
| Other cash flows from financing activities | 0 | 0 | 0 | 0 | 0 | 0 | ||||||||||||||||||
| Other amounts received from financing activities | 0 | 0 | 0 | 0 | 0 | 0 | ||||||||||||||||||
Net cash from/(used in) financing activities | 75,586 | 117,263 | (112,643 | ) | 12,560 | (35,421 | ) | 57,345 | ||||||||||||||||
Effect of exchange rate fluctuations on cash | 0 | (18 | ) | (90 | ) | (236 | ) | 0 | (344 | ) | ||||||||||||||
Net increase/(decrease) in cash and cash equivalents | 1 | 310 | 925 | (554 | ) | (4 | ) | 678 | ||||||||||||||||
Cash and cash equivalents at beginning of the year | 72 | (310 | ) | 991 | 4,938 | 0 | 5,691 | |||||||||||||||||
Cash and cash equivalents at end of year | 73 | 0 | 1,916 | 4,384 | (5 | ) | 6,368 | |||||||||||||||||
F-145
Table of Contents
| Guarantor | Non-Guarantor | Consolidating | ||||||||||||||||||||||
| Parent | Issuer | Subsidiaries | Subsidiaries | Adjustments | Consolidated | |||||||||||||||||||
| (Expressed in thousands of Euros) | ||||||||||||||||||||||||
Cash flows from/(used in) operating activities | ||||||||||||||||||||||||
Profit before income tax | 75,910 | (4,786 | ) | 190,766 | 34,788 | (92,684 | ) | 203,994 | ||||||||||||||||
Adjustments for: | (64,395 | ) | 6,960 | 34,101 | 15,848 | 69,287 | 61,800 | |||||||||||||||||
| Amortisation and depreciation | 5,659 | 850 | 27,319 | 5,726 | 0 | 39,554 | ||||||||||||||||||
| Other adjustments: | (70,054 | ) | 6,110 | 6,782 | 10,122 | 69,287 | 22,246 | |||||||||||||||||
| (Profit) /losses on equity accounted investments | 0 | 0 | 0 | 0 | (51 | ) | (51 | ) | ||||||||||||||||
| Exchange differences | 723 | 858 | 242 | (90 | ) | (0 | ) | 1,733 | ||||||||||||||||
| Impairment of assets and net provision charges | 15 | 0 | (378 | ) | 857 | (441 | ) | 53 | ||||||||||||||||
| (Profits) / losses on disposal of fixed assets | 148 | 2 | 843 | 154 | 0 | 1,147 | ||||||||||||||||||
| Government grants taken to income | (431 | ) | 0 | (637 | ) | (120 | ) | 0 | (1,188 | ) | ||||||||||||||
| Net finance expense | (71,094 | ) | 9,104 | 2,801 | 7,650 | 69,091 | 17,552 | |||||||||||||||||
| Other adjustments | 585 | (3,854 | ) | 3,911 | 1,671 | 688 | 3,001 | |||||||||||||||||
Change in operating assets and liabilities | 64,484 | (70,131 | ) | (42,265 | ) | (21,021 | ) | (35,195 | ) | (104,127 | ) | |||||||||||||
| Change in inventories | 31 | 0 | (119,451 | ) | (14,745 | ) | 21,061 | (113,104 | ) | |||||||||||||||
| Change in trade and other receivables | 14,276 | (44,627 | ) | (71,598 | ) | (20,188 | ) | 109,587 | (12,549 | ) | ||||||||||||||
| Change in current financial assets and other current assets | 57,822 | (52,577 | ) | 57,541 | (6,128 | ) | (57,945 | ) | (1,287 | ) | ||||||||||||||
| Change in current trade and other payables | (7,645 | ) | 27,073 | 91,243 | 20,040 | (107,898 | ) | 22,813 | ||||||||||||||||
Other cash flows used in operating activities | 51,198 | (1,187 | ) | (39,676 | ) | (11,597 | ) | (72,225 | ) | (73,487 | ) | |||||||||||||
| Interest paid | (9,889 | ) | (2,362 | ) | (2,265 | ) | (7,135 | ) | 6,932 | (14,719 | ) | |||||||||||||
| Interest received | 6,932 | 0 | 2,256 | 253 | (6,932 | ) | 2,509 | |||||||||||||||||
| Dividends received | 72,226 | 0 | 0 | 0 | (72,226 | ) | 0 | |||||||||||||||||
| Income tax paid | (18,070 | ) | 1,175 | (39,667 | ) | (4,715 | ) | 0 | (61,277 | ) | ||||||||||||||
Net cash from operating activities | 127,197 | (69,144 | ) | 142,926 | 18,018 | (130,817 | ) | 88,180 | ||||||||||||||||
Cash flows from/(used in) investing activities | ||||||||||||||||||||||||
| Payments for investments | (45,238 | ) | (6,092 | ) | (72,920 | ) | (19,379 | ) | 7,003 | (136,626 | ) | |||||||||||||
| Group companies and business units | (32,497 | ) | 0 | 0 | 10,109 | 7,003 | (15,385 | ) | ||||||||||||||||
| Property, plant and equipment and intangible assets | (12,490 | ) | (4,081 | ) | (72,770 | ) | (29,429 | ) | (0 | ) | (118,770 | ) | ||||||||||||
| Other financial assets | (251 | ) | (2,011 | ) | (150 | ) | (59 | ) | (0 | ) | (2,471 | ) | ||||||||||||
| Proceeds from the sale of investments | 39 | (1 | ) | 182 | 453 | (0 | ) | 673 | ||||||||||||||||
| Property, plant and equipment | 39 | (1 | ) | 182 | 453 | 0 | 673 | |||||||||||||||||
| Other financial assets | 0 | 0 | 0 | 0 | (0 | ) | 0 | |||||||||||||||||
| Associates (note 2 (c)) | 0 | 0 | 0 | 0 | 0 | 0 | ||||||||||||||||||
Net cash used in investing activities | (45,199 | ) | (6,093 | ) | (72,738 | ) | (18,926 | ) | 7,003 | (135,953 | ) | |||||||||||||
Cash flows from/(used in) financing activities | ||||||||||||||||||||||||
| Proceeds from and payments for equity instruments | 26,730 | 0 | 6,923 | 60 | (7,058 | ) | 26,655 | |||||||||||||||||
| Issue | 0 | 0 | 6,923 | 60 | (7,059 | ) | (76 | ) | ||||||||||||||||
| Acquisition of own shares | (25,186 | ) | 0 | 0 | 0 | 0 | (25,186 | ) | ||||||||||||||||
| Disposal of treasury shares | 51,917 | 0 | 0 | 0 | 0 | 51,917 | ||||||||||||||||||
| Proceeds from and payments for financial liability instruments | (28,061 | ) | 318,419 | (14,808 | ) | 10,136 | 58,727 | 344,413 | ||||||||||||||||
| Issue | 78,933 | 406,807 | 22,614 | 16,724 | (0 | ) | 525,078 | |||||||||||||||||
| Redemption and repayment | (95,331 | ) | (39,510 | ) | (34,533 | ) | (11,291 | ) | 0 | (180,665 | ) | |||||||||||||
| Debts with group companies | (11,662 | ) | (48,878 | ) | (2,889 | ) | 4,703 | 58,726 | 0 | |||||||||||||||
| Dividends and interest on other equity instruments paid | (80,651 | ) | (5,552 | ) | (57,404 | ) | (9,456 | ) | 72,150 | (80,913 | ) | |||||||||||||
| Other cash flows from financing activities | 54 | 174 | 431 | 82 | 0 | 741 | ||||||||||||||||||
| Other amounts received from financing activities | 54 | 174 | 431 | 82 | 0 | 741 | ||||||||||||||||||
Net cash from/(used in) financing activities | (81,928 | ) | 313,041 | (64,858 | ) | 822 | 123,818 | 290,896 | ||||||||||||||||
Effect of exchange rate fluctuations on cash | 0 | 0 | (54 | ) | (65 | ) | 0 | (119 | ) | |||||||||||||||
Net increase / (decrease) in cash and cash equivalents | 71 | 237,804 | 5,276 | (151 | ) | 4 | 243,004 | |||||||||||||||||
Cash and cash equivalents at beginning of the year | 73 | 0 | 1,915 | 4,384 | (4 | ) | 6,368 | |||||||||||||||||
Cash and cash equivalents at end of year | 144 | 237,804 | 7,191 | 4,233 | 0 | 249,372 | ||||||||||||||||||
F-146
Table of Contents
| Guarantor | Non-Guarantor | Consolidating | ||||||||||||||||||||||
| Parent | Issuer | Subsidiaries | Subsidiaries | Adjustments | Consolidated | |||||||||||||||||||
| (Expressed in thousands of Euros) | ||||||||||||||||||||||||
Cash flows from/(used in) operating activities | ||||||||||||||||||||||||
Profit before income tax | 57,190 | (17,235 | ) | 158,035 | 31,247 | (71,453 | ) | 157,784 | ||||||||||||||||
Adjustments for: | (59,557 | ) | 49,580 | 23,077 | 6,508 | 72,743 | 92,351 | |||||||||||||||||
| Amortisation and depreciation | 7,384 | 992 | 28,623 | 8,222 | 555 | 45,776 | ||||||||||||||||||
| Other adjustments: | (66,941 | ) | 48,588 | (5,546 | ) | (1,714 | ) | 72,188 | 46,575 | |||||||||||||||
| (Profit)/losses on equity accounted investments | 0 | 0 | 0 | 0 | 879 | 879 | ||||||||||||||||||
| Exchange differences | (75 | ) | 455 | 389 | (2,385 | ) | 0 | (1,616 | ) | |||||||||||||||
| Impairment of assets and net provision charges | (1,097 | ) | 0 | (160 | ) | 518 | 1,652 | 913 | ||||||||||||||||
| (Profits)/losses on disposal of fixed assets | 2 | 139 | 756 | 578 | (1,751 | ) | (276 | ) | ||||||||||||||||
| Government grants taken to income | (259 | ) | 0 | (470 | ) | 1 | 0 | (728 | ) | |||||||||||||||
| Net finance expense | (65,578 | ) | 30,875 | 2,513 | 2,731 | 76,901 | 47,442 | |||||||||||||||||
| Other adjustments | 66 | 17,119 | (8,574 | ) | (3,157 | ) | (5,493 | ) | (39 | ) | ||||||||||||||
Change in operating assets and liabilities | 2,233 | 145,612 | (199,272 | ) | 7,902 | (35,242 | ) | (78,767 | ) | |||||||||||||||
| Change in inventories | (92 | ) | 0 | (23,919 | ) | 8,854 | (3,149 | ) | (18,306 | ) | ||||||||||||||
| Change in trade and other receivables | 4,915 | 170,362 | 54,428 | 13,110 | (266,361 | ) | (23,546 | ) | ||||||||||||||||
| Change in current financial assets and other current assets | (20,490 | ) | (13,649 | ) | (2,979 | ) | (1,263 | ) | (34,641 | ) | (73,022 | ) | ||||||||||||
| Change in current trade and other payables | 17,900 | (11,101 | ) | (226,802 | ) | (12,799 | ) | 268,909 | 36,107 | |||||||||||||||
Other cash flows used in operating activities | 77,492 | (26,404 | ) | (28,009 | ) | (13,704 | ) | (76,491 | ) | (67,116 | ) | |||||||||||||
| Interest paid | (5,891 | ) | (31,361 | ) | (4,404 | ) | (2,525 | ) | 4,052 | (40,129 | ) | |||||||||||||
| Interest received | 4,052 | 1,110 | 4,326 | 0 | (4,052 | ) | 5,436 | |||||||||||||||||
| Dividends received | 76,491 | 0 | 0 | 0 | (76,491 | ) | 0 | |||||||||||||||||
| Income tax paid | 2,840 | 3,847 | (27,931 | ) | (11,179 | ) | 0 | (32,423 | ) | |||||||||||||||
Net cash from operating activities | 77,358 | 151,553 | (46,169 | ) | 31,953 | (110,443 | ) | 104,252 | ||||||||||||||||
Cash flows from/(used in) investing activities | ||||||||||||||||||||||||
| Payments for investments | (7,854 | ) | (16,042 | ) | (67,863 | ) | (19,020 | ) | 2,191 | (108,588 | ) | |||||||||||||
| Group companies and business units | (8 | ) | 0 | (884 | ) | (1,523 | ) | 941 | (1,474 | ) | ||||||||||||||
| Property, plant and equipment and intangible assets | (7,788 | ) | (12,405 | ) | (67,006 | ) | (17,453 | ) | 1,250 | (103,402 | ) | |||||||||||||
| Other financial assets | (58 | ) | (3,637 | ) | 27 | (44 | ) | 0 | (3,712 | ) | ||||||||||||||
| Proceeds from the sale of investments | 109 | 946 | (2,169 | ) | 5,555 | 91 | 4,532 | |||||||||||||||||
| Property, plant and equipment | 109 | 946 | (1,289 | ) | 4,054 | 91 | 3,911 | |||||||||||||||||
| Other financial assets | 0 | 0 | (880 | ) | 1,501 | 0 | 621 | |||||||||||||||||
| Associates | 0 | 0 | 0 | 0 | 0 | 0 | ||||||||||||||||||
Net cash used in investing activities | (7,745 | ) | (15,096 | ) | (70,032 | ) | (13,465 | ) | 2,282 | (104,056 | ) | |||||||||||||
Cash flows from/(used in) financing activities | ||||||||||||||||||||||||
| Proceeds from and payments for equity instruments | (1,250 | ) | 0 | 0 | 890 | (890 | ) | (1,250 | ) | |||||||||||||||
| Issue | 0 | 0 | 0 | 890 | (890 | ) | 0 | |||||||||||||||||
| Acquisition of own shares | (1,250 | ) | 0 | 0 | 0 | 0 | (1,250 | ) | ||||||||||||||||
| Disposal of treasury shares | 0 | 0 | 0 | 0 | 0 | 0 | ||||||||||||||||||
| Proceeds from and payments for financial liability instruments | (41,495 | ) | (165,388 | ) | 165,287 | 8,437 | 32,093 | (1,066 | ) | |||||||||||||||
| Issue | 63,226 | (2,616 | ) | 30,546 | 27,082 | 0 | 118,238 | |||||||||||||||||
| Redemption and repayment | (99,505 | ) | 0 | (16,672 | ) | (3,127 | ) | 0 | (119,304 | ) | ||||||||||||||
| Debts with group companies | (5,216 | ) | (162,772 | ) | 151,413 | (15,518 | ) | 32,093 | 0 | |||||||||||||||
| Dividends and interest on other equity instruments paid | (27,229 | ) | 0 | (55,298 | ) | (21,713 | ) | 76,958 | (27,282 | ) | ||||||||||||||
| Other cash flows from financing activities | 242 | 0 | 56 | 25 | 0 | 323 | ||||||||||||||||||
| Other amounts received from financing activities | 242 | 0 | 81 | 0 | 0 | 323 | ||||||||||||||||||
Net cash from/(used in) financing activities | (69,732 | ) | (165,388 | ) | 110,045 | (12,361 | ) | 108,161 | (29,275 | ) | ||||||||||||||
Effect of exchange rate fluctuations on cash | 0 | 18,583 | 409 | 364 | 0 | 19,356 | ||||||||||||||||||
Net increase/(decrease) in cash and cash equivalents | (119 | ) | (10,348 | ) | (5,747 | ) | 6,491 | 0 | (9,723 | ) | ||||||||||||||
Cash and cash equivalents at beginning of the year | 144 | 237,804 | 7,191 | 4,233 | 0 | 249,372 | ||||||||||||||||||
Cash and cash equivalents at end of year | 25 | 227,456 | 1,444 | 10,724 | 0 | 239,649 | ||||||||||||||||||
F-147
Table of Contents
F-148
Table of Contents
| December 31, | ||||||||
| 2010 | 2009 | |||||||
| (In thousands, except share and per share amounts) | ||||||||
ASSETS | ||||||||
| Current assets: | ||||||||
| Cash and cash equivalents | $ | 197,876 | $ | 65,239 | ||||
| Accounts receivable, net of allowances of $3,253 and $3,461, respectively | 134,842 | 136,978 | ||||||
| Inventories | 694,499 | 644,054 | ||||||
| Deferred income taxes | 96,593 | 88,652 | ||||||
| Prepaid expenses and other | 29,662 | 31,466 | ||||||
| Total current assets | 1,153,472 | 966,389 | ||||||
| Property, plant, and equipment, net | 382,793 | 267,199 | ||||||
| Investment in affiliate | 2,926 | 1,935 | ||||||
| Intangible assets | 10,880 | 10,880 | ||||||
| Goodwill | 172,860 | 172,860 | ||||||
| Deferred income taxes | — | 5,848 | ||||||
| Other | 15,522 | 19,894 | ||||||
| Total assets | $ | 1,738,453 | $ | 1,445,005 | ||||
LIABILITIES AND STOCKHOLDERS’ EQUITY | ||||||||
| Current liabilities: | ||||||||
| Accounts payable | $ | 59,975 | $ | 71,046 | ||||
| Accrued expenses and other liabilities | 251,726 | 170,533 | ||||||
| Current portion of capital lease obligations | 860 | 740 | ||||||
| Total current liabilities | 312,561 | 242,319 | ||||||
| Long-term debt and capital lease obligations | 605,301 | 605,267 | ||||||
| Deferred income taxes | 14,432 | — | ||||||
| Other | 11,795 | 15,265 | ||||||
| Total liabilities | 944,089 | 862,851 | ||||||
| Commitments and contingencies (Note 14) | ||||||||
| Stockholders’ equity: | ||||||||
| Common stock, $0.01 par value; 400,000,000 shares authorized; 125,577,877 and 122,173,274 shares issued and outstanding, respectively | 1,253 | 1,212 | ||||||
| Additional paid-in capital | 813,783 | 767,032 | ||||||
| Accumulated deficit | (20,378 | ) | (186,446 | ) | ||||
| Accumulated other comprehensive (loss) income, net of tax | (294 | ) | 356 | |||||
| Total stockholders’ equity | 794,364 | 582,154 | ||||||
| Total liabilities and stockholders’ equity | $ | 1,738,453 | $ | 1,445,005 | ||||
F-149
Table of Contents
| Years Ended December 31, | ||||||||||||
| 2010 | 2009 | 2008 | ||||||||||
| (In thousands, except per share amounts) | ||||||||||||
Net revenue: | ||||||||||||
| Product | $ | 1,576,936 | $ | 1,507,754 | $ | 1,334,550 | ||||||
| Other | 24,683 | 25,455 | 39,742 | |||||||||
| Total | 1,601,619 | 1,533,209 | 1,374,292 | |||||||||
| Cost of goods sold | 911,976 | 901,077 | 882,157 | |||||||||
Gross profit | 689,643 | 632,132 | 492,135 | |||||||||
| Operating expenses: | ||||||||||||
| Selling, general, and administrative | 287,011 | 289,929 | 227,524 | |||||||||
| Research and development | 69,649 | 71,223 | 66,006 | |||||||||
| Total | 356,660 | 361,152 | 293,530 | |||||||||
Income from operations | 332,983 | 270,980 | 198,605 | |||||||||
| Other non-operating (expense) income | ||||||||||||
| Interest expense, net | (45,837 | ) | (74,491 | ) | (96,640 | ) | ||||||
| PCA judgment | (43,690 | ) | — | — | ||||||||
| CSL merger termination fee | — | 75,000 | — | |||||||||
| Loss on extinguishment of debt | — | (43,033 | ) | — | ||||||||
| Equity in earnings of affiliate | 991 | 441 | 426 | |||||||||
| Total | (88,536 | ) | (42,083 | ) | (96,214 | ) | ||||||
| Income before income taxes | 244,447 | 228,897 | 102,391 | |||||||||
| Provision for income taxes | (78,379 | ) | (75,008 | ) | (36,594 | ) | ||||||
Net income | 166,068 | 153,889 | 65,797 | |||||||||
| Less dividends to preferred stockholders and other non-common stockholders’ charges | — | (11,744 | ) | (14,672 | ) | |||||||
Net income available to common stockholders | $ | 166,068 | $ | 142,145 | $ | 51,125 | ||||||
Net income per common share: | ||||||||||||
| Basic | $ | 1.35 | $ | 4.56 | $ | 39.01 | ||||||
| Diluted | $ | 1.29 | $ | 1.50 | $ | 0.71 | ||||||
F-150
Table of Contents
| Years Ended December 31, | ||||||||||||
| 2010 | 2009 | 2008 | ||||||||||
| (In thousands) | ||||||||||||
Cash flows from operating activities: | ||||||||||||
| Net income | $ | 166,068 | $ | 153,889 | $ | 65,797 | ||||||
| Adjustments to reconcile net income to net cash provided by operating activities: | ||||||||||||
| Depreciation and amortization | 36,030 | 28,936 | 20,269 | |||||||||
| Amortization of deferred loan fees and debt discount | 4,262 | 3,785 | 3,764 | |||||||||
| Share-based compensation expense | 16,966 | 47,546 | 38,707 | |||||||||
| Amortization of deferred compensation | 1,983 | 5,714 | 5,922 | |||||||||
| Write-off of unamortized debt issuance costs | — | 12,141 | — | |||||||||
| Asset impairment | 595 | 3,061 | 4,282 | |||||||||
| Provision for doubtful receivables and advances | 3,519 | 2,858 | 4,978 | |||||||||
| Recognition of previously deferred revenue | (230 | ) | (230 | ) | (4,784 | ) | ||||||
| Equity in earnings of affiliate | (991 | ) | (441 | ) | (426 | ) | ||||||
| Loss on disposal of property, plant, and equipment | 896 | 1,196 | 48 | |||||||||
| Decrease (increase) in deferred tax assets | 12,339 | 1,215 | (5,488 | ) | ||||||||
| Excess tax benefits from share-based payment arrangements | (13,481 | ) | (13,406 | ) | — | |||||||
| Changes in assets and liabilities, excluding the effects of business acquisitions | 27,526 | (12,109 | ) | (100,055 | ) | |||||||
| Net cash provided by operating activities | 255,482 | 234,155 | 33,014 | |||||||||
Cash flows from investing activities: | ||||||||||||
| Purchases of property, plant, and equipment | (152,849 | ) | (75,163 | ) | (86,212 | ) | ||||||
| Business acquisitions, net of cash acquired | — | (30,431 | ) | (10,272 | ) | |||||||
| Financing arrangements with third party suppliers, net of repayments | — | — | (16,335 | ) | ||||||||
| Other | 765 | 976 | 880 | |||||||||
| Net cash used in investing activities | (152,084 | ) | (104,618 | ) | (111,939 | ) | ||||||
Cash flows from financing activities: | ||||||||||||
| Borrowings under revolving credit facility | 915 | 1,201,749 | 1,430,092 | |||||||||
| Repayments of borrowings under revolving credit facility | (915 | ) | (1,381,690 | ) | (1,363,188 | ) | ||||||
| Repayments of borrowings under term loans | — | (1,016,000 | ) | (7,000 | ) | |||||||
| Repayments of capital lease obligations | (751 | ) | (574 | ) | (1,192 | ) | ||||||
| Proceeds from issuance of 7.75% Notes | — | 600,000 | — | |||||||||
| Discount on 7.75% Notes | — | (4,074 | ) | — | ||||||||
| Financing transaction costs | (394 | ) | (14,879 | ) | — | |||||||
| Proceeds from initial public offering, net of issuance costs | — | 519,749 | — | |||||||||
| Costs related to initial public offering | — | (2,557 | ) | — | ||||||||
| Repurchases of common stock | (4,917 | ) | (4,183 | ) | (36,118 | ) | ||||||
| Proceeds from exercises of stock options | 22,333 | 7,581 | — | |||||||||
| Excess tax benefits from share-based payment arrangements | 13,481 | 13,406 | — | |||||||||
| Net cash provided by (used in) financing activities | 29,752 | (81,472 | ) | 22,594 | ||||||||
| Effect of exchange rate changes on cash and cash equivalents | (513 | ) | 195 | (157 | ) | |||||||
Net increase (decrease) in cash and cash equivalents | 132,637 | 48,260 | (56,488 | ) | ||||||||
Cash and cash equivalents at beginning of year | 65,239 | 16,979 | 73,467 | |||||||||
Cash and cash equivalents at end of year | $ | 197,876 | $ | 65,239 | $ | 16,979 | ||||||
F-151
Table of Contents
| Accumulated | ||||||||||||||||||||||||
| Additional | Other | |||||||||||||||||||||||
| Common Stock | Paid-in | Accumulated | Comprehensive | |||||||||||||||||||||
| Shares | Amount | Capital | Deficit | Income (Loss) | Total | |||||||||||||||||||
| (In thousands, except share amounts) | ||||||||||||||||||||||||
| Balance at December 31, 2007 | 5,317,232 | $ | — | $ | 27,010 | $ | (406,132 | ) | $ | (11,635 | ) | $ | (390,757 | ) | ||||||||||
| Net income | — | — | — | 65,797 | — | 65,797 | ||||||||||||||||||
| Other comprehensive loss | — | — | — | — | (11,772 | ) | (11,772 | ) | ||||||||||||||||
| Comprehensive income | — | — | — | — | — | 54,025 | ||||||||||||||||||
| Share-based compensation cost | — | — | 29,258 | — | — | 29,258 | ||||||||||||||||||
| Issuance of restricted stock | 42,720 | — | — | — | — | — | ||||||||||||||||||
| Forfeitures of restricted stock | (287,784 | ) | — | — | — | — | — | |||||||||||||||||
| Repurchases and retirement of common stock | (2,215,880 | ) | — | — | — | — | — | |||||||||||||||||
| Fair value adjustment on common stock with put/call feature | — | — | (8,942 | ) | — | — | (8,942 | ) | ||||||||||||||||
| Interest accretion on IBR put option | — | — | (309 | ) | — | — | (309 | ) | ||||||||||||||||
| Balance at December 31, 2008 | 2,856,288 | — | 47,017 | (340,335 | ) | (23,407 | ) | (316,725 | ) | |||||||||||||||
| Net income | — | — | — | 153,889 | — | 153,889 | ||||||||||||||||||
| Other comprehensive income | — | — | — | — | 476 | 476 | ||||||||||||||||||
| Reclassification of unrealized loss on derivatives to earnings | — | — | — | — | 23,287 | 23,287 | ||||||||||||||||||
| Comprehensive income | — | — | — | — | — | 177,652 | ||||||||||||||||||
| Share-based compensation cost | — | — | 39,206 | — | — | 39,206 | ||||||||||||||||||
| Issuance of restricted stock | 14,464 | — | — | — | — | — | ||||||||||||||||||
| Forfeitures of restricted stock | (16,368 | ) | — | — | — | — | — | |||||||||||||||||
| Repurchases and retirement of common stock | (251,108 | ) | — | (51 | ) | — | — | (51 | ) | |||||||||||||||
| Series A and B preferred stock dividends declared | — | — | (45,250 | ) | — | — | (45,250 | ) | ||||||||||||||||
| Conversion of Series A and B preferred stock to common stock | 88,227,868 | 882 | 154,903 | — | — | 155,785 | ||||||||||||||||||
| Initial public offering | 28,947,368 | 289 | 519,460 | — | — | 519,749 | ||||||||||||||||||
| Costs related to initial public offering | — | — | (2,557 | ) | — | — | (2,557 | ) | ||||||||||||||||
| Fair value adjustment on common stock with put/call feature | — | — | (6,585 | ) | — | — | (6,585 | ) | ||||||||||||||||
| Reclassification of mezzanine equity to permanent equity upon cancellation of common stock put/call feature | — | 17 | 39,926 | — | — | 39,943 | ||||||||||||||||||
| Stock option exercises | 2,394,762 | 24 | 7,557 | — | — | 7,581 | ||||||||||||||||||
| Excess tax benefit from share-based compensation | — | — | 13,406 | — | — | 13,406 | ||||||||||||||||||
| Balance at December 31, 2009 | 122,173,274 | 1,212 | 767,032 | (186,446 | ) | 356 | 582,154 | |||||||||||||||||
| Net income | — | — | — | 166,068 | — | 166,068 | ||||||||||||||||||
| Other comprehensive loss | — | — | — | — | (650 | ) | (650 | ) | ||||||||||||||||
| Comprehensive income | — | — | — | — | — | 165,418 | ||||||||||||||||||
| Share-based compensation cost | — | — | 15,895 | — | — | 15,895 | ||||||||||||||||||
| Repurchases and retirement of common stock | (246,823 | ) | (3 | ) | (4,914 | ) | — | — | (4,917 | ) | ||||||||||||||
| Stock option exercises | 3,650,579 | 37 | 22,296 | — | — | 22,333 | ||||||||||||||||||
| Excess tax benefit from share-based compensation | — | — | 13,481 | — | — | 13,481 | ||||||||||||||||||
| Shares issued upon RSU vesting | 847 | — | — | — | — | — | ||||||||||||||||||
| Vesting of restricted common stock | — | 7 | (7 | ) | — | — | — | |||||||||||||||||
| Balance at December 31, 2010 | 125,577,877 | $ | 1,253 | $ | 813,783 | $ | (20,378 | ) | $ | (294 | ) | $ | 794,364 | |||||||||||
F-152
Table of Contents
| 1. | Description of Business |
F-153
Table of Contents
| 2. | Summary of Significant Accounting Policies |
F-154
Table of Contents
| • | At December 31, 2010: Amerisource Bergen- 12.8% | |
| • | At December 31, 2009: FFF Enterprise, Inc.- 14.6% |
| Years Ended December 31, | ||||||||||||
| 2010 | 2009 | 2008 | ||||||||||
| FFF Enterprise, Inc. | 13.9 | % | 14.4 | % | 12.8 | % | ||||||
| Amerisource Bergen | 13.1 | % | 12.3 | % | 12.0 | % | ||||||
| Canadian Blood Services | <10 | % | <10 | % | 10.6 | % | ||||||
F-155
Table of Contents
Asset Type | Useful Life (Years) | |
| Buildings | 10 to 45 | |
| Building improvements | 10 to 20 | |
| Machinery and equipment | 3 to 20 | |
| Furniture and fixtures | 5 to 10 | |
| Computer hardware and software | 3 to 7 | |
| Leasehold improvements | the estimated useful life of the improvement or, if shorter, the life of the lease |
F-156
Table of Contents
F-157
Table of Contents
F-158
Table of Contents
F-159
Table of Contents
F-160
Table of Contents
F-161
Table of Contents
F-162
Table of Contents
| Gross | Tax | Net | ||||||||||
| Amount | Effect | Amount | ||||||||||
Year ended December 31, 2010 | ||||||||||||
| Foreign currency translation adjustments | $ | (576 | ) | $ | — | $ | (576 | ) | ||||
| Additional minimum pension liability | (74 | ) | — | (74 | ) | |||||||
| Other comprehensive loss | $ | (650 | ) | $ | — | $ | (650 | ) | ||||
Year ended December 31, 2009 | ||||||||||||
| Foreign currency translation adjustments | $ | 232 | $ | — | $ | 232 | ||||||
| Additional minimum pension liability | 244 | — | 244 | |||||||||
| Reclassification of unrealized loss on derivative financial instruments | 37,513 | (14,226 | ) | 23,287 | ||||||||
| Other comprehensive income | $ | 37,989 | $ | (14,226 | ) | $ | 23,763 | |||||
Year ended December 31, 2008 | ||||||||||||
| Foreign currency translation adjustments | $ | (216 | ) | $ | — | $ | (216 | ) | ||||
| Net unrealized loss on derivative financial instruments | (18,477 | ) | 6,973 | (11,504 | ) | |||||||
| Additional minimum pension liability | (52 | ) | — | (52 | ) | |||||||
| Other comprehensive loss | $ | (18,475 | ) | $ | 6,973 | $ | (11,772 | ) | ||||
F-163
Table of Contents
| December 31, | ||||||||
| 2010 | 2009 | |||||||
| Foreign currency translation adjustments | $ | (412 | ) | $ | 164 | |||
| Additional minimum pension liability | 118 | 192 | ||||||
| Accumulated other comprehensive (loss) income | $ | (294 | ) | $ | 356 | |||
F-164
Table of Contents
| 3. | Definitive Merger Agreement with Grifols S.A. and Grifols, Inc. (Grifols) |
F-165
Table of Contents
| 4. | Initial Public Offering and Use of Proceeds |
F-166
Table of Contents
| 5. | Definitive Merger Agreement with CSL Limited (CSL) |
| 6. | Business Acquisitions |
F-167
Table of Contents
| Years Ended December 31, | ||||||||
| 2009 | 2008 | |||||||
| Payments at closing | $ | 5,181 | $ | 2,147 | ||||
| Notes receivable and other advances | 44,540 | 10,430 | ||||||
| Performance incentive payments | 837 | 843 | ||||||
| Allocable portion of accelerated contingent consideration | 6,020 | 2,580 | ||||||
| Transaction costs | — | 56 | ||||||
| Total purchase price | $ | 56,578 | $ | 16,056 | ||||
| Cash and cash equivalents | $ | 62 | $ | 21 | ||||
| Inventory | 5,416 | 1,778 | ||||||
| Other current assets | 183 | — | ||||||
| Property, plant, and equipment | 10,181 | 1,814 | ||||||
| Intangible assets- regulatory licenses | 3,860 | 840 | ||||||
| Goodwill | 37,060 | 11,643 | ||||||
| Total assets acquired | 56,762 | 16,096 | ||||||
| Current liabilities assumed | (184 | ) | (40 | ) | ||||
| Total purchase price | $ | 56,578 | $ | 16,056 | ||||
| Number of plasma collection centers acquired | 12 | 3 | ||||||
| 7. | Goodwill and Intangible Assets |
| Balance at December 31, 2008 | $ | 135,800 | ||
| Acquisitions of plasma collection centers from IBR | 37,060 | |||
| Balance at December 31, 2009 | $ | 172,860 | ||
F-168
Table of Contents
| 8. | Collaborative and Other Agreements |
F-169
Table of Contents
| 9. | Inventories and Cost of Goods Sold |
| December 31, | ||||||||
| 2010 | 2009 | |||||||
| Raw material | $ | 184,664 | $ | 171,866 | ||||
| Work-in-process | 346,086 | 312,178 | ||||||
| Finished goods | 163,749 | 160,010 | ||||||
| Total inventories | $ | 694,499 | $ | 644,054 | ||||
F-170
Table of Contents
| 10. | Property, Plant, and Equipment, net |
| December 31, | ||||||||
| 2010 | 2009 | |||||||
| Land | $ | 4,136 | $ | 4,136 | ||||
| Buildings and improvements | 88,652 | 68,417 | ||||||
| Machinery and equipment | 143,813 | 102,887 | ||||||
| Furniture and fixtures | 7,377 | 5,492 | ||||||
| Computer hardware and software | 64,729 | 54,761 | ||||||
| Capital leases of buildings | 8,704 | 8,374 | ||||||
| 317,411 | 244,067 | |||||||
| Less: accumulated depreciation and amortization | (95,319 | ) | (62,463 | ) | ||||
| 222,092 | 181,604 | |||||||
| Construction in progress | 160,701 | 85,595 | ||||||
| Total property, plant, and equipment, net | $ | 382,793 | $ | 267,199 | ||||
| 11. | Investment in Affiliate |
F-171
Table of Contents
| 12. | Long-Term Debt and Capital Lease Obligations |
| December 31, | ||||||||
| 2010 | 2009 | |||||||
| 7.75% Notes | $ | 600,000 | $ | 600,000 | ||||
| Discount on 7.75% Notes | (3,379 | ) | (3,954 | ) | ||||
| Revolving credit facility | — | — | ||||||
| Capital lease obligations | 9,540 | 9,961 | ||||||
| Total debt and capital lease obligations | 606,161 | 606,007 | ||||||
| Less: current maturities | (860 | ) | (740 | ) | ||||
| Long-term debt and capital lease obligations, net of current maturities | $ | 605,301 | $ | 605,267 | ||||
Fiscal Year | Percentage | |||
| 2012 | 103.875 | % | ||
| 2013 | 102.583 | % | ||
| 2014 | 101.292 | % | ||
| 2015 and thereafter | 100.000 | % | ||
F-172
Table of Contents
F-173
Table of Contents
F-174
Table of Contents
| October 21, | ||||||||||||||||||||||||
| December 31, | 2009 Net | October 6, | 2009 | December 31, | ||||||||||||||||||||
| 2008 | Repayments | 2009 IPO | Refinancing | Amortization | 2009 | |||||||||||||||||||
| Revolving Credit Facility | $ | 179,941 | $ | (124,348 | ) | $ | — | $ | (55,593 | ) | $ | — | $ | — | ||||||||||
| First Lien Term Loan | 686,000 | (5,250 | ) | (389,812 | ) | (290,938 | ) | — | — | |||||||||||||||
| Second Lien Term Loan | 330,000 | — | (129,937 | ) | (200,063 | ) | — | — | ||||||||||||||||
| 7.75% Notes | — | — | — | 600,000 | — | 600,000 | ||||||||||||||||||
| Discount on 7.75% Notes | — | — | — | (4,074 | ) | 120 | (3,954 | ) | ||||||||||||||||
| Total indebtedness | $ | 1,195,941 | $ | (129,598 | ) | $ | (519,749 | ) | $ | 49,332 | $ | 120 | $ | 596,046 | ||||||||||
| Newly | ||||||||||||||||||||
| Capitalized | ||||||||||||||||||||
| December 31, | Debt Issuance | December 31, | ||||||||||||||||||
| 2008 | Charges | Costs | Amortization | 2009 | ||||||||||||||||
| Revolving Credit Facility | $ | 3,014 | $ | — | $ | 1,545 | $ | (1,041 | ) | $ | 3,518 | |||||||||
| First Lien Term Loan | 9,629 | (8,054 | ) | — | (1,575 | ) | — | |||||||||||||
| Second Lien Term Loan | 4,744 | (4,087 | ) | — | (657 | ) | — | |||||||||||||
| 7.75% Notes | — | — | 13,334 | (392 | ) | 12,942 | ||||||||||||||
| Total deferred debt issuance costs | $ | 17,387 | $ | (12,141 | ) | $ | 14,879 | $ | (3,665 | ) | $ | 16,460 | ||||||||
F-175
Table of Contents
| • | Write offs, write-downs, asset revaluations and other non-cash charges, losses, and expenses, including non-cash equity compensation expense; | |
| • | Impairments of intangibles and goodwill; | |
| • | Extraordinary gains and losses; | |
| • | Fees paid pursuant to our Management Agreement, as amended, with Talecris Holdings, LLC, which was terminated in connection with our IPO; | |
| • | Fees and expenses incurred in connection with transactions and permitted acquisitions and investments; | |
| • | Extraordinary, unusual, or non-recurring charges and expenses including transition, restructuring, and “carve-out” expenses; | |
| • | Legal, accounting, consulting, and other expenses relating to potential or actual issuances of equity interests, including an initial public offering of common stock; | |
| • | Costs associated with our Special Recognition Bonuses; and | |
| • | Other items. |
F-176
Table of Contents
| 13. | Income Taxes |
| Years Ended December 31, | ||||||||||||
| 2010 | 2009 | 2008 | ||||||||||
| Current provision: | ||||||||||||
| Federal | $ | 58,907 | $ | 68,960 | $ | 28,639 | ||||||
| State and local | 5,418 | 3,421 | 4,590 | |||||||||
| Foreign | 1,793 | 1,348 | 1,776 | |||||||||
| Total current provision | 66,118 | 73,729 | 35,005 | |||||||||
| Deferred provision: | ||||||||||||
| Federal | 12,366 | 69 | 7 | |||||||||
| State and local | (105 | ) | 1,210 | 1,582 | ||||||||
| Total deferred provision | 12,261 | 1,279 | 1,589 | |||||||||
| Provision for income taxes | $ | 78,379 | $ | 75,008 | $ | 36,594 | ||||||
F-177
Table of Contents
| Years Ended December 31, | ||||||||||||
| 2010 | 2009 | 2008 | ||||||||||
| Amount computed at statutory rate | $ | 85,556 | $ | 80,114 | $ | 35,837 | ||||||
| State income taxes (net of Federal benefit) | 7,040 | 4,291 | 4,059 | |||||||||
| Research and development credits | (11,426 | ) | (7,732 | ) | (4,052 | ) | ||||||
| State tax credits (net of Federal benefit) | (1,607 | ) | (871 | ) | (600 | ) | ||||||
| Federal benefit of tax deductions for qualified production activities | (6,080 | ) | (2,764 | ) | (2,037 | ) | ||||||
| Capitalized transaction costs | 6,800 | (2,352 | ) | 584 | ||||||||
| Nondeductible meals and entertainment expenses | 671 | 504 | 425 | |||||||||
| Other | (2,575 | ) | 3,818 | 2,378 | ||||||||
| Provision for income taxes | $ | 78,379 | $ | 75,008 | $ | 36,594 | ||||||
| December 31, | ||||||||
| 2010 | 2009 | |||||||
| Current: | ||||||||
| Deferred income tax assets: | ||||||||
| Allowances on accounts receivable | $ | 8,074 | $ | 11,020 | ||||
| Inventories | 32,026 | 23,928 | ||||||
| Revenue recognition | 1,455 | 7,857 | ||||||
| Stock-based compensation | 23,855 | 30,952 | ||||||
| Deferred bonuses | 4,200 | 4,617 | ||||||
| Accrued expenses | 21,994 | 4,568 | ||||||
| State tax credit carry-forward | 3,335 | 3,195 | ||||||
| Other | 2,341 | 3,543 | ||||||
| Total deferred income tax assets | 97,280 | 89,680 | ||||||
| Deferred income tax liabilities: | ||||||||
| Other liabilities | (687 | ) | (1,028 | ) | ||||
| Total deferred income tax liabilities | (687 | ) | (1,028 | ) | ||||
| Net current deferred income tax assets | $ | 96,593 | $ | 88,652 | ||||
F-178
Table of Contents
| December 31, | ||||||||
| 2010 | 2009 | |||||||
| Non-current: | ||||||||
| Deferred income tax assets: | ||||||||
| Property, plant, and equipment | $ | — | $ | 14,170 | ||||
| Other | 459 | 252 | ||||||
| Total deferred income tax assets | 459 | 14,422 | ||||||
| Deferred income tax liabilities: | ||||||||
| Property, plant, and equipment | (1,292 | ) | — | |||||
| Intangibles | (13,599 | ) | (8,574 | ) | ||||
| Total deferred income tax liabilities | (14,891 | ) | (8,574 | ) | ||||
| Net non-current deferred income tax (liabilities) assets | $ | (14,432 | ) | $ | 5,848 | |||
| Net deferred income tax assets | $ | 82,161 | $ | 94,500 | ||||
| Unrecognized tax benefits at December 31, 2007 | $ | 6,885 | ||
| Additions for tax positions taken in the current year | 3,626 | |||
| Reductions for tax positions taken in a prior year | (521 | ) | ||
| Unrecognized tax benefits at December 31, 2008 | 9,990 | |||
| Additions for tax positions taken during a prior year | 3,899 | |||
| Reductions for tax positions taken in a prior year | (1,642 | ) | ||
| Unrecognized tax benefits at December 31, 2009 | 12,247 | |||
| Additions for tax positions taken in the current year | 2,485 | |||
| Reductions for tax positions taken in a prior year | (5,908 | ) | ||
| Unrecognized tax benefits at December 31, 2010 | $ | 8,824 | ||
F-179
Table of Contents
| 14. | Commitments and Contingencies |
| Non-Cancellable | ||||||||
| Operating | ||||||||
| Capital | Leases | |||||||
| 2011 | $ | 1,814 | $ | 17,427 | ||||
| 2012 | 1,836 | 14,614 | ||||||
| 2013 | 1,862 | 10,246 | ||||||
| 2014 | 1,830 | 8,921 | ||||||
| 2015 | 1,729 | 7,016 | ||||||
| Thereafter | 4,845 | 27,198 | ||||||
| Total future minimum lease payments | 13,916 | $ | 85,422 | |||||
| Less: amounts representing interest | (4,376 | ) | ||||||
| Present value of net minimum lease payments | 9,540 | |||||||
| Less: current portion of capital lease obligations | (860 | ) | ||||||
| Total | $ | 8,680 | ||||||
F-180
Table of Contents
F-181
Table of Contents
F-182
Table of Contents
| 2011 | $ | 201,875 | ||
| 2012 | 179,850 | |||
| 2013 | 167,637 | |||
| 2014 | 127,080 | |||
| 2015 | 121,308 | |||
| Thereafter | 55,595 | |||
| Total | $ | 853,345 | ||
F-183
Table of Contents
| 15. | Related Party Transactions |
F-184
Table of Contents
| Years Ended December 31, | ||||||||||||
Expenses | 2010 | 2009 | 2008 | |||||||||
| Centric (product distribution and other services) | $ | 22,865 | $ | 20,306 | $ | 17,508 | ||||||
| Cerberus/Ampersand (management fees) | $ | — | $ | 5,715 | $ | 6,871 | ||||||
| Cerberus (operational support) | $ | — | $ | 608 | $ | 4,184 | ||||||
| December 31, | ||||||||
Payable | 2010 | 2009 | ||||||
| Centric (product distribution and other services) | $ | 6,406 | $ | 5,537 | ||||
| Cerberus (operational support) | $ | — | $ | 349 | ||||
| 16. | Equity Transactions |
| 17. | Redeemable Series A and B Senior Convertible Preferred Stock |
F-185
Table of Contents
| 18. | Share-Based Compensation |
| Years Ended December 31, | ||||||||||||
| 2010 | 2009 | 2008 | ||||||||||
| SG&A | $ | 12,606 | $ | 40,968 | $ | 33,780 | ||||||
| R&D | 1,024 | 2,303 | 2,361 | |||||||||
| Cost of goods sold | 3,336 | 4,275 | 2,566 | |||||||||
| Total expense | $ | 16,966 | $ | 47,546 | $ | 38,707 | ||||||
| Capitalized in inventory | $ | 2,265 | $ | 3,574 | $ | 3,233 | ||||||
| Weighted- | ||||||||
| Unrecognized | Average | |||||||
| Compensation | Period | |||||||
| Cost | (Years) | |||||||
| Stock options | $ | 3,965 | 2.10 | |||||
| Restricted share awards | 721 | 0.25 | ||||||
| RSU’s | 6,589 | 2.10 | ||||||
| Performance share awards | 1,138 | 1.25 | ||||||
| Total | $ | 12,413 | ||||||
F-186
Table of Contents
| Weighted | ||||||||||||||||
| Average | ||||||||||||||||
| Weighted | Remaining | |||||||||||||||
| Average | Contractual | Aggregate | ||||||||||||||
| Exercise | Term | Intrinsic | ||||||||||||||
| Shares | Price | (Years) | Value | |||||||||||||
| Outstanding at December 31, 2007 | 12,932,344 | $ | 6.42 | |||||||||||||
| Granted | 2,291,304 | $ | 10.98 | |||||||||||||
| Forfeited | (945,232 | ) | $ | 2.98 | ||||||||||||
| Outstanding at December 31, 2008 | 14,278,416 | $ | 6.96 | |||||||||||||
| Granted | 638,472 | $ | 18.91 | |||||||||||||
| Forfeited | (392,688 | ) | $ | 4.89 | ||||||||||||
| Exercised | (2,394,762 | ) | $ | 3.17 | ||||||||||||
| Outstanding at December 31, 2009 | 12,129,438 | $ | 8.40 | 6.7 | $ | 168,235 | ||||||||||
| Granted | 73,593 | $ | 20.39 | |||||||||||||
| Forfeited | (24,950 | ) | $ | 19.05 | ||||||||||||
| Exercised | (3,650,579 | ) | $ | 6.12 | ||||||||||||
| Outstanding at December 31, 2010 | 8,527,502 | $ | 9.45 | 5.7 | $ | 118,090 | ||||||||||
| Exercisable at December 31, 2010 | 7,882,452 | $ | 8.66 | 4.8 | $ | 115,419 | ||||||||||
| Vested and expected to vest at December 31, 2010 | 8,488,004 | $ | 9.36 | 5.7 | $ | 118,296 | ||||||||||
F-187
Table of Contents
| Years Ended December 31, | ||||||||||||
| 2010 | 2009 | 2008 | ||||||||||
| Risk-free interest rate | 2.66 | % | 2.66 | % | 2.65 | % | ||||||
| Expected term (years) | 5.66 | 5.97 | 5.20 | |||||||||
| Expected volatility | 50 | % | 50 | % | 50 | % | ||||||
| Expected dividend yield | 0 | % | 0 | % | 0 | % | ||||||
F-188
Table of Contents
| Weighted | ||||||||||||||||
| Average | ||||||||||||||||
| Weighted | Remaining | |||||||||||||||
| Average | Contractual | Aggregate | ||||||||||||||
| Grant Date | Term | Intrinsic | ||||||||||||||
| Shares | Fair Value | (Years) | Value | |||||||||||||
| Outstanding at December 31, 2008 | — | — | ||||||||||||||
| Granted | 483,100 | $ | 19.00 | |||||||||||||
| Forfeited | (3,076 | ) | $ | 19.00 | ||||||||||||
| Outstanding at December 31, 2009 | 480,024 | $ | 19.00 | |||||||||||||
| Granted | 36,015 | $ | 20.40 | |||||||||||||
| Forfeited | (30,299 | ) | $ | 19.04 | ||||||||||||
| Vested | (1,182 | ) | $ | 19.00 | ||||||||||||
| Outstanding at December 31, 2010 | 484,558 | $ | 19.10 | 2.16 | $ | 11,290 | ||||||||||
| Weighted | ||||||||
| Average | ||||||||
| Grant Date | ||||||||
| Shares | Fair Value | |||||||
| December 31, 2007 unvested shares outstanding | 2,811,000 | $ | 13.78 | |||||
| Granted | 42,720 | $ | 9.88 | |||||
| Forfeited | (287,784 | ) | $ | 11.00 | ||||
| Vested | (870,432 | ) | $ | 13.45 | ||||
| December 31, 2008 unvested shares outstanding | 1,695,504 | $ | 14.40 | |||||
| Granted | 14,464 | $ | 16.63 | |||||
| Forfeited | (16,368 | ) | $ | 11.00 | ||||
| Vested | (779,744 | ) | $ | 13.50 | ||||
| December 31, 2009 unvested shares outstanding | 913,856 | $ | 15.27 | |||||
| Vested | (727,256 | ) | $ | 13.74 | ||||
| December 31, 2010 unvested shares outstanding | 186,600 | $ | 21.25 | |||||
F-189
Table of Contents
| Weighted | ||||||||
| Average | ||||||||
| Grant Date | ||||||||
| Shares | Fair Value | |||||||
| Outstanding PSU’s outstanding at December 31, 2009 | — | $ | — | |||||
| Granted | 261,327 | $ | 21.51 | |||||
| Forfeited | (1,627 | ) | $ | 21.51 | ||||
| Unvested PSU’s outstanding at December 31, 2010 | 259,700 | $ | 21.51 | |||||
| 19. | Employee Benefit Plans |
F-190
Table of Contents
| 20. | Deferred Compensation |
F-191
Table of Contents
| 21. | Segment Reporting |
| Years Ended December 31, | ||||||||||||
| 2010 | 2009 | 2008 | ||||||||||
| Product net revenue: | ||||||||||||
| Immunology/Neurology | $ | 964,145 | $ | 928,054 | $ | 781,408 | ||||||
| Pulmonology | 351,499 | 319,080 | 316,495 | |||||||||
| Critical Care/Hemostasis | 175,071 | 167,469 | 134,216 | |||||||||
| Other | 86,221 | 93,151 | 102,431 | |||||||||
| Total product net revenue | 1,576,936 | 1,507,754 | 1,334,550 | |||||||||
| Other revenue | 24,683 | 25,455 | 39,742 | |||||||||
| Total net revenue | $ | 1,601,619 | $ | 1,533,209 | $ | 1,374,292 | ||||||
| Years Ended December 31, | ||||||||||||
| 2010 | 2009 | 2008 | ||||||||||
| United States | $ | 1,098,969 | $ | 1,011,468 | $ | 906,376 | ||||||
| Canada | 195,092 | 214,883 | 215,964 | |||||||||
| Europe | 196,571 | 185,297 | 168,081 | |||||||||
| Other | 110,987 | 121,561 | 83,871 | |||||||||
| Total net revenue | $ | 1,601,619 | $ | 1,533,209 | $ | 1,374,292 | ||||||
F-192
Table of Contents
| 22. | Earnings per Share |
| Years Ended December 31, | ||||||||||||
| 2010 | 2009 | 2008 | ||||||||||
| Net income | $ | 166,068 | $ | 153,889 | $ | 65,797 | ||||||
| Less: | ||||||||||||
| Series A preferred stock undeclared dividends | — | (9,602 | ) | (11,745 | ) | |||||||
| Series B preferred stock undeclared dividends | — | (2,142 | ) | (2,619 | ) | |||||||
| Accretion of common stock put option | — | — | (308 | ) | ||||||||
| Net income available to common stockholders | $ | 166,068 | $ | 142,145 | $ | 51,125 | ||||||
| Weighted average common shares outstanding | 123,323,722 | 31,166,613 | 1,310,448 | |||||||||
| Basic net income per common share | $ | 1.35 | $ | 4.56 | $ | 39.01 | ||||||
| Years Ended December 31, | ||||||||||||
| 2010 | 2009 | 2008 | ||||||||||
| Net income | $ | 166,068 | $ | 153,889 | $ | 65,797 | ||||||
| Less accretion of common stock put option | — | — | (308 | ) | ||||||||
| Net income available to common stockholders | $ | 166,068 | $ | 153,889 | $ | 65,489 | ||||||
| Weighted average common shares outstanding | 123,323,722 | 31,166,613 | 1,310,448 | |||||||||
| Plus incremental shares from assumed conversions: | ||||||||||||
| Series A preferred stock | — | 53,654,795 | 72,000,000 | |||||||||
| Series B preferred stock | — | 10,318,354 | 13,846,320 | |||||||||
| Stock options and restricted shares | 5,603,331 | 7,374,601 | 5,605,032 | |||||||||
| Dilutive potential common shares | 128,927,053 | 102,514,363 | 92,761,800 | |||||||||
| Diluted net income per common share | $ | 1.29 | $ | 1.50 | $ | 0.71 | ||||||
F-193
Table of Contents
| 23. | Other Consolidated Balance Sheet Information |
| December 31, | ||||||||
| 2010 | 2009 | |||||||
| Accrued expenses and other liabilities: | ||||||||
| Accrued goods and services | $ | 80,769 | $ | 45,044 | ||||
| Accrued payroll, bonuses, and employee benefits | 80,317 | 73,983 | ||||||
| Medicaid, commercial rebates, and chargebacks | 29,744 | 30,771 | ||||||
| Interest payable | 6,011 | 9,111 | ||||||
| PCA judgment | 43,690 | — | ||||||
| Other | 11,195 | 11,624 | ||||||
| Total accrued expenses and other liabilities | $ | 251,726 | $ | 170,533 | ||||
| 24. | Cash Flow Supplemental Disclosures |
| Years Ended December 31, | ||||||||||||
| 2010 | 2009 | 2008 | ||||||||||
| Interest, net of amounts capitalized(1) | $ | 44,546 | $ | 55,131 | $ | 87,213 | ||||||
| Income taxes | $ | 63,025 | $ | 56,849 | $ | 48,910 | ||||||
| (1) | Interest paid in the table above excludes payments related to our interest rate swap contracts, which amounted to $17.0 million and $9.2 million for the years ended December 31, 2009 and 2008, respectively. The interest rate swap contracts were terminated and settled during the fourth quarter of 2009 as discussed in Note 12, “Long-Term Debt and Capital Lease Obligations.” |
| Years Ended December 31, | ||||||||||||
| 2010 | 2009 | 2008 | ||||||||||
| Changes in: | ||||||||||||
| Accounts receivable | $ | (1,382 | ) | $ | 8,575 | $ | (26,894 | ) | ||||
| Inventories | (52,034 | ) | (57,452 | ) | (92,856 | ) | ||||||
| Prepaid expenses and other assets | 653 | 7,987 | (15,823 | ) | ||||||||
| Accounts payable | (11,071 | ) | 16,143 | 16,594 | ||||||||
| Interest payable | (3,100 | ) | (2,239 | ) | (1,957 | ) | ||||||
| Accrued expenses and other liabilities | 94,460 | 14,877 | 20,881 | |||||||||
| Total | $ | 27,526 | $ | (12,109 | ) | $ | (100,055 | ) | ||||
F-194
Table of Contents
F-195
Table of Contents
| 25. | Selected Unaudited Quarterly Financial Data |
| 2010 Quarter Ended | ||||||||||||||||
| March 31 | June 30 | September 30 | December 31 | |||||||||||||
| Net revenue | $ | 380,961 | $ | 402,826 | $ | 407,001 | $ | 410,831 | ||||||||
| Cost of goods sold | 217,351 | 223,217 | 229,908 | 241,500 | ||||||||||||
| Gross profit | 163,610 | 179,609 | 177,093 | 169,331 | ||||||||||||
| Operating expenses | 83,891 | 91,892 | 80,056 | 100,821 | ||||||||||||
| Operating income | 79,719 | 87,717 | 97,037 | 68,510 | ||||||||||||
| Total other non-operating expense, net | (11,116 | ) | (11,944 | ) | (11,256 | ) | (54,220 | ) | ||||||||
| Income before income taxes | 68,603 | 75,773 | 85,781 | 14,290 | ||||||||||||
| (Provision) benefit for income taxes | (23,264 | ) | (28,150 | ) | (29,729 | ) | 2,764 | |||||||||
| Net income | $ | 45,339 | $ | 47,623 | $ | 56,052 | $ | 17,054 | ||||||||
| Earnings per share: | ||||||||||||||||
| Basic | $ | 0.37 | $ | 0.39 | $ | 0.45 | $ | 0.14 | ||||||||
| Diluted | $ | 0.35 | $ | 0.37 | $ | 0.43 | $ | 0.13 | ||||||||
| 2009 Quarter Ended | ||||||||||||||||
| March 31 | June 30 | September 30 | December 31 | |||||||||||||
| Net revenue | $ | 371,795 | $ | 375,570 | $ | 395,731 | $ | 390,113 | ||||||||
| Cost of goods sold | 209,201 | 224,008 | 230,666 | 237,202 | ||||||||||||
| Gross profit | 162,594 | 151,562 | 165,065 | 152,911 | ||||||||||||
| Operating expenses | 88,963 | 81,023 | 95,655 | 95,511 | ||||||||||||
| Operating income | 73,631 | 70,539 | 69,410 | 57,400 | ||||||||||||
| Total other non-operating (expense) income, net | (21,256 | ) | 54,582 | (19,475 | ) | (55,934 | ) | |||||||||
| Income before income taxes | 52,375 | 125,121 | 49,935 | 1,466 | ||||||||||||
| Provision for income taxes | (18,940 | ) | (41,849 | ) | (14,125 | ) | (94 | ) | ||||||||
| Net income | $ | 33,435 | $ | 83,272 | $ | 35,810 | $ | 1,372 | ||||||||
| Earnings per share: | ||||||||||||||||
| Basic | $ | 25.09 | $ | 47.42 | $ | 12.01 | $ | 0.01 | ||||||||
| Diluted | $ | 0.36 | $ | 0.89 | $ | 0.38 | $ | 0.01 | ||||||||
F-196
Table of Contents
| 26. | Condensed Consolidating Financial Information |
F-197
Table of Contents
| Parent/ | Guarantor | Non-Guarantor | Consolidating | |||||||||||||||||
| Issuer | Subsidiaries | Subsidiaries | Adjustments | Consolidated | ||||||||||||||||
Assets: | ||||||||||||||||||||
| Current assets: | ||||||||||||||||||||
| Cash | $ | — | $ | 183,737 | $ | 14,139 | $ | — | $ | 197,876 | ||||||||||
| Accounts receivable, net | — | 248,124 | 37,793 | (151,075 | ) | 134,842 | ||||||||||||||
| Inventories | — | 657,493 | 37,006 | — | 694,499 | |||||||||||||||
| Other | — | 126,684 | (429 | ) | — | 126,255 | ||||||||||||||
| Total current assets | — | 1,216,038 | 88,509 | (151,075 | ) | 1,153,472 | ||||||||||||||
| Property, plant, and equipment, net | — | 381,707 | 1,086 | — | 382,793 | |||||||||||||||
| Intangible assets | — | 10,880 | — | — | 10,880 | |||||||||||||||
| Goodwill | — | 172,860 | — | — | 172,860 | |||||||||||||||
| Investment in Subsidiaries | 826,782 | (36,573 | ) | — | (790,209 | ) | — | |||||||||||||
| Advances and notes between Parent and Subsidiaries | 1,397,133 | 826,919 | — | (2,224,052 | ) | — | ||||||||||||||
| Other | — | 18,167 | 281 | — | 18,448 | |||||||||||||||
| Total assets | $ | 2,223,915 | $ | 2,589,998 | $ | 89,876 | $ | (3,165,336 | ) | $ | 1,738,453 | |||||||||
Liabilities and Stockholders’ Equity (Deficit): | ||||||||||||||||||||
| Current liabilities: | ||||||||||||||||||||
| Accounts payable | $ | — | $ | 96,503 | $ | 114,547 | $ | (151,075 | ) | $ | 59,975 | |||||||||
| Accrued expenses and other liabilities | 6,011 | 234,730 | 10,985 | — | 251,726 | |||||||||||||||
| Current portion of capital lease obligations | — | 860 | — | — | 860 | |||||||||||||||
| Total current liabilities | 6,011 | 332,093 | 125,532 | (151,075 | ) | 312,561 | ||||||||||||||
| Long-term debt and capital lease obligations | 596,621 | 8,680 | — | — | 605,301 | |||||||||||||||
| Advances and notes between Parent and Subsidiaries | 826,919 | 1,397,133 | — | (2,224,052 | ) | — | ||||||||||||||
| Other | — | 25,310 | 917 | — | 26,227 | |||||||||||||||
| Total liabilities | 1,429,551 | 1,763,216 | 126,449 | (2,375,127 | ) | 944,089 | ||||||||||||||
| Stockholders’ equity (deficit) | 794,364 | 826,782 | (36,573 | ) | (790,209 | ) | 794,364 | |||||||||||||
| Total liabilities and stockholders’ equity (deficit) | $ | 2,223,915 | $ | 2,589,998 | $ | 89,876 | $ | (3,165,336 | ) | $ | 1,738,453 | |||||||||
F-198
Table of Contents
| Parent/ | Guarantor | Non-Guarantor | Consolidating | |||||||||||||||||
| Issuer | Subsidiaries | Subsidiaries | Adjustments | Consolidated | ||||||||||||||||
Assets: | ||||||||||||||||||||
| Current assets: | ||||||||||||||||||||
| Cash | $ | — | $ | 58,320 | $ | 6,919 | $ | — | $ | 65,239 | ||||||||||
| Accounts receivable, net | — | 222,007 | 64,454 | (149,483 | ) | 136,978 | ||||||||||||||
| Inventories | — | 605,324 | 38,730 | — | 644,054 | |||||||||||||||
| Other | — | 117,670 | 2,448 | — | 120,118 | |||||||||||||||
| Total current assets | — | 1,003,321 | 112,551 | (149,483 | ) | 966,389 | ||||||||||||||
| Property, plant, and equipment, net | — | 266,067 | 1,132 | — | 267,199 | |||||||||||||||
| Intangible assets | — | 10,880 | — | — | 10,880 | |||||||||||||||
| Goodwill | — | 172,860 | — | — | 172,860 | |||||||||||||||
| Investment in Subsidiaries | 680,459 | (27,925 | ) | — | (652,534 | ) | — | |||||||||||||
| Advances and notes between Parent and Subsidiaries | 1,355,631 | 862,406 | — | (2,218,037 | ) | — | ||||||||||||||
| Other | — | 27,054 | 623 | — | 27,677 | |||||||||||||||
| Total assets | $ | 2,036,090 | $ | 2,314,663 | $ | 114,306 | $ | (3,020,054 | ) | $ | 1,445,005 | |||||||||
Liabilities and Stockholders’ Equity (Deficit): | ||||||||||||||||||||
| Current liabilities: | ||||||||||||||||||||
| Accounts payable | $ | — | $ | 103,460 | $ | 117,069 | $ | (149,483 | ) | $ | 71,046 | |||||||||
| Accrued expenses and other liabilities | 9,111 | 150,936 | 10,486 | — | 170,533 | |||||||||||||||
| Current portion of capital lease obligations | — | 740 | — | — | 740 | |||||||||||||||
| Total current liabilities | 9,111 | 255,136 | 127,555 | (149,483 | ) | 242,319 | ||||||||||||||
| Long-term debt and capital lease obligations | 596,046 | 9,221 | — | — | 605,267 | |||||||||||||||
| Advances and notes between Parent and Subsidiaries | 848,779 | 1,355,626 | 13,632 | (2,218,037 | ) | — | ||||||||||||||
| Other | — | 14,221 | 1,044 | — | 15,265 | |||||||||||||||
| Total liabilities | 1,453,936 | 1,634,204 | 142,231 | (2,367,520 | ) | 862,851 | ||||||||||||||
| Stockholders’ equity (deficit) | 582,154 | 680,459 | (27,925 | ) | (652,534 | ) | 582,154 | |||||||||||||
| Total liabilities and stockholders’ equity (deficit) | $ | 2,036,090 | $ | 2,314,663 | $ | 114,306 | $ | (3,020,054 | ) | $ | 1,445,005 | |||||||||
F-199
Table of Contents
| Parent/ | Guarantor | Non-Guarantor | Consolidating | |||||||||||||||||
| Issuer | Subsidiaries | Subsidiaries | Adjustments | Consolidated | ||||||||||||||||
| Net revenue | $ | — | $ | 1,438,162 | $ | 163,457 | $ | — | $ | 1,601,619 | ||||||||||
| Cost of goods sold | — | 783,710 | 128,266 | — | 911,976 | |||||||||||||||
| Gross profit | — | 654,452 | 35,191 | — | 689,643 | |||||||||||||||
| Operating expenses | — | 315,350 | 41,310 | — | 356,660 | |||||||||||||||
| Income from operations | — | 339,102 | (6,119 | ) | — | 332,983 | ||||||||||||||
| Equity in earnings (losses) of Subsidiaries | 166,068 | (7,998 | ) | — | (158,070 | ) | — | |||||||||||||
| Other non-operating (expense) income, net | — | (88,555 | ) | 19 | — | (88,536 | ) | |||||||||||||
| Income (loss) before income taxes | 166,068 | 242,549 | (6,100 | ) | (158,070 | ) | 244,447 | |||||||||||||
| Provision for income taxes | — | (76,481 | ) | (1,898 | ) | — | (78,379 | ) | ||||||||||||
| Net income (loss) | $ | 166,068 | $ | 166,068 | $ | (7,998 | ) | $ | (158,070 | ) | $ | 166,068 | ||||||||
| Parent/ | Guarantor | Non-Guarantor | Consolidating | |||||||||||||||||
| Issuer | Subsidiaries | Subsidiaries | Adjustments | Consolidated | ||||||||||||||||
| Net revenue | $ | — | $ | 1,389,172 | $ | 144,037 | $ | — | $ | 1,533,209 | ||||||||||
| Cost of goods sold | — | 784,635 | 116,442 | — | 901,077 | |||||||||||||||
| Gross profit | — | 604,537 | 27,595 | — | 632,132 | |||||||||||||||
| Operating expenses | 5,715 | 321,525 | 33,912 | — | 361,152 | |||||||||||||||
| Income (loss) from operations | (5,715 | ) | 283,012 | (6,317 | ) | — | 270,980 | |||||||||||||
| Equity in earnings (losses) of Subsidiaries | 108,854 | (7,466 | ) | — | (101,388 | ) | — | |||||||||||||
| Other non-operating (expense) income, net | 75,000 | (117,106 | ) | 23 | — | (42,083 | ) | |||||||||||||
| Income (loss) before income taxes | 178,139 | 158,440 | (6,294 | ) | (101,388 | ) | 228,897 | |||||||||||||
| (Provision) benefit for income taxes | (24,250 | ) | (49,586 | ) | (1,172 | ) | — | (75,008 | ) | |||||||||||
| Net income (loss) | $ | 153,889 | $ | 108,854 | $ | (7,466 | ) | $ | (101,388 | ) | $ | 153,889 | ||||||||
F-200
Table of Contents
| Parent/ | Guarantor | Non-Guarantor | Consolidating | |||||||||||||||||
| Issuer | Subsidiaries | Subsidiaries | Adjustments | Consolidated | ||||||||||||||||
| Net revenue | $ | — | $ | 1,232,366 | $ | 141,926 | $ | — | $ | 1,374,292 | ||||||||||
| Cost of goods sold | — | 764,952 | 117,205 | — | 882,157 | |||||||||||||||
| Gross profit | — | 467,414 | 24,721 | — | 492,135 | |||||||||||||||
| Operating expenses | 6,871 | 257,281 | 29,378 | — | 293,530 | |||||||||||||||
| Income (loss) from operations | (6,871 | ) | 210,133 | (4,657 | ) | — | 198,605 | |||||||||||||
| Equity in earnings (losses) of Subsidiaries | 70,263 | (5,590 | ) | — | (64,673 | ) | — | |||||||||||||
| Other non-operating (expense) income, net | — | (96,832 | ) | 618 | — | (96,214 | ) | |||||||||||||
| Income (loss) before income taxes | 63,392 | 107,711 | (4,039 | ) | (64,673 | ) | 102,391 | |||||||||||||
| (Provision) benefit for income taxes | 2,405 | (37,448 | ) | (1,551 | ) | — | (36,594 | ) | ||||||||||||
| Net income (loss) | $ | 65,797 | $ | 70,263 | $ | (5,590 | ) | $ | (64,673 | ) | $ | 65,797 | ||||||||
F-201
Table of Contents
| Parent/ | Guarantor | Non-Guarantor | Consolidating | |||||||||||||||||
| Issuer | Subsidiaries | Subsidiaries | Adjustments | Consolidated | ||||||||||||||||
Cash flows from operating activities: | ||||||||||||||||||||
| Net income (loss) | $ | 166,068 | $ | 166,068 | $ | (7,998 | ) | $ | (158,070 | ) | $ | 166,068 | ||||||||
| Undistributed equity in (earnings) losses of Subsidiaries | (166,068 | ) | 7,998 | — | 158,070 | — | ||||||||||||||
| Adjustments to reconcile net income (loss) to net cash flows provided by (used in) operating activities | — | 39,946 | 29,728 | 19,740 | 89,414 | |||||||||||||||
Net cash provided by (used in) operating activities | — | 214,012 | 21,730 | 19,740 | 255,482 | |||||||||||||||
Cash flows from investing activities: | ||||||||||||||||||||
| Purchases of property, plant, and equipment | — | (152,484 | ) | (365 | ) | — | (152,849 | ) | ||||||||||||
| Net advances and notes between Parent and Subsidiaries | (30,897 | ) | — | — | 30,897 | — | ||||||||||||||
| Other | — | 14,397 | (13,632 | ) | — | 765 | ||||||||||||||
Net cash (used in) provided by investing activities | (30,897 | ) | (138,087 | ) | (13,997 | ) | 30,897 | (152,084 | ) | |||||||||||
Cash flows from financing activities: | ||||||||||||||||||||
| Net advances and notes between Parent and Subsidiaries | — | 50,637 | — | (50,637 | ) | — | ||||||||||||||
| Other | 30,897 | (1,145 | ) | — | — | 29,752 | ||||||||||||||
Net cash provided by (used in) financing activities | 30,897 | 49,492 | — | (50,637 | ) | 29,752 | ||||||||||||||
| Effect of exchange rate changes on cash and cash equivalents | — | — | (513 | ) | — | (513 | ) | |||||||||||||
| Net increase in cash and cash equivalents | — | 125,417 | 7,220 | — | 132,637 | |||||||||||||||
| Cash and cash equivalents at beginning of year | — | 58,320 | 6,919 | — | 65,239 | |||||||||||||||
| Cash and cash equivalents at end of year | $ | — | $ | 183,737 | $ | 14,139 | $ | — | $ | 197,876 | ||||||||||
F-202
Table of Contents
| Parent/ | Guarantor | Non-Guarantor | Consolidating | |||||||||||||||||
| Issuer | Subsidiaries | Subsidiaries | Adjustments | Consolidated | ||||||||||||||||
Cash flows from operating activities: | ||||||||||||||||||||
| Net income (loss) | $ | 153,889 | $ | 108,854 | $ | (7,466 | ) | $ | (101,388 | ) | $ | 153,889 | ||||||||
| Undistributed equity in (earnings) losses of Subsidiaries | (108,854 | ) | 7,466 | — | 101,388 | — | ||||||||||||||
| Adjustments to reconcile net income (loss) to net cash flows provided by (used in) operating activities | (2,007 | ) | 64,009 | 4,759 | 13,505 | 80,266 | ||||||||||||||
Net cash provided by (used in) operating activities | 43,028 | 180,329 | (2,707 | ) | 13,505 | 234,155 | ||||||||||||||
Cash flows from investing activities: | ||||||||||||||||||||
| Purchases of property, plant, and equipment | — | (74,576 | ) | (587 | ) | — | (75,163 | ) | ||||||||||||
| Business acquisitions, net of cash acquired | — | (30,431 | ) | — | — | (30,431 | ) | |||||||||||||
| Net advances and notes between Parent and Subsidiaries | (1,172,950 | ) | — | — | 1,172,950 | — | ||||||||||||||
| Other | — | (2,788 | ) | 3,764 | — | 976 | ||||||||||||||
Net cash (used in) provided by investing activities | (1,172,950 | ) | (107,795 | ) | 3,177 | 1,172,950 | (104,618 | ) | ||||||||||||
Cash flows from financing activities: | ||||||||||||||||||||
| Net repaments of borrowings | — | (1,196,515 | ) | — | — | (1,196,515 | ) | |||||||||||||
| Issuance of 7.75% Notes, net of discount | 595,926 | — | — | — | 595,926 | |||||||||||||||
| Proceeds from initial public offering, net of issuance costs | 517,192 | — | — | — | 517,192 | |||||||||||||||
| Net advances and notes between Parent and Subsidiaries | — | 1,186,455 | — | (1,186,455 | ) | — | ||||||||||||||
| Other | 16,804 | (14,879 | ) | — | — | 1,925 | ||||||||||||||
Net cash provided by (used in) financing activities | 1,129,922 | (24,939 | ) | — | (1,186,455 | ) | (81,472 | ) | ||||||||||||
| Effect of exchange rate changes on cash and cash equivalents | — | — | 195 | — | 195 | |||||||||||||||
| Net increase in cash and cash equivalents | — | 47,595 | 665 | — | 48,260 | |||||||||||||||
| Cash and cash equivalents at beginning of year | — | 10,726 | 6,253 | — | 16,979 | |||||||||||||||
| Cash and cash equivalents at end of year | $ | — | $ | 58,321 | $ | 6,918 | $ | — | $ | 65,239 | ||||||||||
F-203
Table of Contents
| Parent/ | Guarantor | Non-Guarantor | Consolidating | |||||||||||||||||
| Issuer | Subsidiaries | Subsidiaries | Adjustments | Consolidated | ||||||||||||||||
Cash flows from operating activities: | ||||||||||||||||||||
| Net income (loss) | $ | 65,797 | $ | 70,263 | $ | (5,590 | ) | $ | (64,673 | ) | $ | 65,797 | ||||||||
| Undistributed equity in (earnings) losses of Subsidiaries | (70,263 | ) | 5,590 | — | 64,673 | — | ||||||||||||||
| Adjustments to reconcile net income (loss) to net cash flows provided by (used in) operating activities | 424 | 14,776 | (63,115 | ) | 15,132 | (32,783 | ) | |||||||||||||
Net cash provided by (used in) operating activities | (4,042 | ) | 90,629 | (68,705 | ) | 15,132 | 33,014 | |||||||||||||
Cash flows from investing activities: | ||||||||||||||||||||
| Purchases of property, plant, and equipment | — | (86,051 | ) | (161 | ) | — | (86,212 | ) | ||||||||||||
| Business acquisitions, net of cash acquired | — | (10,272 | ) | — | — | (10,272 | ) | |||||||||||||
| Net advances and notes between Parent and Subsidiaries | 40,160 | — | — | (40,160 | ) | — | ||||||||||||||
| Other | — | (15,455 | ) | — | — | (15,455 | ) | |||||||||||||
Net cash (used in) provided by investing activities | 40,160 | (111,778 | ) | (161 | ) | (40,160 | ) | (111,939 | ) | |||||||||||
Cash flows from financing activities: | ||||||||||||||||||||
| Net borrowings | — | 58,712 | — | — | 58,712 | |||||||||||||||
| Net advances and notes between Parent and Subsidiaries | — | (34,896 | ) | 9,868 | 25,028 | — | ||||||||||||||
| Other | (36,118 | ) | — | — | — | (36,118 | ) | |||||||||||||
Net cash provided by (used in) financing activities | (36,118 | ) | 23,816 | 9,868 | 25,028 | 22,594 | ||||||||||||||
| Effect of exchange rate changes on cash and cash equivalents | — | 91 | (248 | ) | — | (157 | ) | |||||||||||||
| Net increase in cash and cash equivalents | — | 2,758 | (59,246 | ) | — | (56,488 | ) | |||||||||||||
| Cash and cash equivalents at beginning of year | — | 7,968 | 65,499 | — | 73,467 | |||||||||||||||
| Cash and cash equivalents at end of year | $ | — | $ | 10,726 | $ | 6,253 | $ | — | $ | 16,979 | ||||||||||
F-204
Table of Contents
| 27. | Subsequent Events |
F-205
Table of Contents
| Balance at | Charges to | Charges to | Balance at | |||||||||||||||||
| Beginning of | Costs and | Other | End of | |||||||||||||||||
| Period | Expenses | Accounts | Deductions | Period | ||||||||||||||||
| (In thousands) | ||||||||||||||||||||
Reserve for doubtful accounts receivable: | ||||||||||||||||||||
| Year ended December 31, 2010 | $ | 3,461 | $ | 3,519 | $ | — | $ | (3,727 | )(1) | $ | 3,253 | |||||||||
| Year ended December 31, 2009 | $ | 2,020 | $ | 2,858 | $ | — | $ | (1,417 | )(1) | $ | 3,461 | |||||||||
| Year ended December 31, 2008 | $ | 2,631 | $ | 728 | $ | — | $ | (1,339 | )(1) | $ | 2,020 | |||||||||
Reserve for doubtful notes receivable and other advances: | ||||||||||||||||||||
| Year ended December 31, 2010 | $ | 4,250 | $ | — | $ | — | $ | — | $ | 4,250 | ||||||||||
| Year ended December 31, 2009 | $ | 4,250 | $ | — | $ | — | $ | — | $ | 4,250 | ||||||||||
| Year ended December 31, 2008 | $ | — | $ | 4,250 | (3) | $ | — | $ | 4,250 | |||||||||||
Inventory reserves: | ||||||||||||||||||||
| Year ended December 31, 2010 | $ | 44,377 | $ | 55,419 | $ | — | $ | (39,796 | )(2) | $ | 60,000 | |||||||||
| Year ended December 31, 2009 | $ | 49,766 | $ | 21,758 | $ | — | $ | (27,147 | )(2) | $ | 44,377 | |||||||||
| Year ended December 31, 2008 | $ | 47,534 | $ | 36,840 | $ | — | $ | (34,608 | )(2) | $ | 49,766 | |||||||||
| (1) | Includes write-offs of uncollectible accounts receivable and the effects of foreign exchange. | |
| (2) | Includes the net of write-offs, reversals of reserved inventory that was sold or recoverable for other purposes such as testing, and the effects of foreign exchange. | |
| (3) | Includes a provision for $3.2 million related to notes receivables and $1.0 million related to advances for unlicensed plasma from a then existing third party supplier due to uncertainty regarding collection. |
F-206
Table of Contents
| Item 20. | Indemnification of Directors and Officers |
II-1
Table of Contents
II-2
Table of Contents
| Item 21. | Exhibits |
| Exhibit | ||||
Number | Description of Exhibit | |||
| 2 | .1 | Agreement and Plan of Merger, dated as of June 6, 2010, by and among Grifols, S.A., Grifols, Inc. and Talecris Biotherapeutics Holdings Corp. (incorporated by reference to Exhibit 2.1 of our Registration Statement onForm F-4 (FileNo. 333-168701) filed on August 10, 2010) | ||
| 2 | .2 | Amendment No. 1 to the Agreement and Plan of Merger, dated as of November 4, 2010, by and among Grifols, S.A., Grifols, Inc. and Talecris Biotherapeutics Holdings Corp. (incorporated by reference to Exhibit 2.2 of Amendment No. 2 to our Registration Statement onForm F-4 (FileNo. 333-168701) filed on November 5, 2010) | ||
| 3 | .1.1* | By-Laws (Estatutos) of Grifols, S.A. | ||
| 3 | .1.2* | By-Laws (Estatutos) of Grifols, S.A. (English translation) | ||
| 3 | .2.1* | Amended and Restated Articles of Incorporation of Grifols Inc. | ||
| 3 | .2.2* | Amended and Restated By-laws of Grifols Inc. | ||
| 3 | .3.1* | Certificate of Incorporation of Grifols Biologicals Inc. | ||
| 3 | .3.2* | Certificate of Correction of the Certificate of Incorporation of Grifols Biologicals Inc. | ||
| 3 | .3.3* | By-laws of Grifols Biologicals Inc. | ||
| 3 | .4.1* | Amended and Restated Certificate of Incorporation of Biomat USA, Inc. | ||
| 3 | .4.2* | By-laws of Biomat USA, Inc. | ||
| 3 | .5.1* | Certificate of Incorporation of Grifols Therapeutics, Inc. | ||
| 3 | .5.2* | Certificate of Amendment to the Certificate of Incorporation of Grifols Therapeutics, Inc. | ||
| 3 | .5.3* | Certificate of Amendment to the Certificate of Incorporation of Grifols Therapeutics, Inc. | ||
II-3
Table of Contents
| Exhibit | ||||
Number | Description of Exhibit | |||
| 3 | .5.4* | By-laws of Grifols Therapeutics, Inc. | ||
| 3 | .6.1* | Certificate of Incorporation of Talecris Plasma Resources, Inc. | ||
| 3 | .6.2* | Certificate of Amendment of Certificate of Incorporation of Talecris Plasma Resources, Inc. | ||
| 3 | .6.3* | Amended and Restated By-laws of Talecris Plasma Resources, Inc. | ||
| 3 | .7.1* | By-laws (Estatutos) of Instituto Grifols, S.A. | ||
| 3 | .7.2* | By-laws (Estatutos) of Instituto Grifols, S.A. (English translation) | ||
| 3 | .8.1* | By-laws (Estatutos) of Diagnostic Grifols, S.A. | ||
| 3 | .8.2* | By-laws (Estatutos) of Diagnostic Grifols, S.A. (English translation) | ||
| 3 | .9.1* | By-laws (Estatutos) of Movaco, S.A. | ||
| 3 | .9.2* | By-laws (Estatutos) of Movaco, S.A. (English translation) | ||
| 3 | .10.1* | By-laws (Estatutos) of Laboratorios Grifols, S.A. | ||
| 3 | .10.2* | By-laws (Estatutos) of Laboratorios Grifols, S.A. (English translation) | ||
| 3 | .11.1* | By-laws of Grifols Italia, S.p.A. | ||
| 3 | .11.2* | By-laws of Grifols Italia, S.p.A. (English translation) | ||
| 3 | .12* | By-laws of Grifols Deutschland GmbH | ||
| 4 | .1* | Senior Notes Indenture, dated as of January 21, 2011, relating to the 8.25% Senior Notes due 2018, among Giant Funding Corp. and The Bank of New York Mellon Trust Company, N.A., as trustee. | ||
| 4 | .2* | Form of 8.25% Senior Note (included as Exhibit A to Exhibit 4.1) | ||
| 4 | .3* | Supplemental Indenture, dated June 1, 2011, by and among Grifols Inc., Grifols, S.A., the subsidiary guarantors party thereto and The Bank of New York Mellon Trust Company, N.A., as trustee. | ||
| 4 | .4* | Second Supplemental Indenture, dated as of October 4, 2011, between Grifols Deutschland GmbH and The Bank of New York Mellon Trust Company, N.A., as trustee. | ||
| 4 | .5* | Registration Rights Agreement, dated January 21, 2011, by and among Giant Funding Corp. and Deutsche Bank Securities Inc., as representative of the several initial purchasers. | ||
| 4 | .6* | Registration Rights Agreement Joinder, dated as of June 1, 2011, by and among Grifols Inc., Grifols, S.A. and the subsidiary guarantors party thereto. | ||
| 5 | .1* | Opinion of Proskauer Rose LLP, New York, NY, United States of America | ||
| 5 | .2* | Opinion of Hunton & Williams LLP, Richmond, VA, United States of America | ||
| 5 | .3* | Opinion of Osborne Clarke, S.L.P., Barcelona, Spain | ||
| 5 | .4* | Opinion of SLA Studio Legale Associato, Milan, Italy with respect to Grifols Italia, S.p.A. | ||
| 5 | .5* | Opinion of Osborne Clarke, Munich, Germany with respect to Grifols Deutschland GmbH | ||
| 10 | .1* | Credit and Guaranty Agreement, dated as of November 23, 2010, among Grifols Inc., Grifols, S.A., certain subsidiaries of Grifols, S.A., the lenders party thereto, Deutsche Bank Securities, Inc., Nomura International PLC, Banco Bilbao Vizcaya Argentaria, S.A., BNP Paribas, HSBC Securities (USA) Inc. and Morgan Stanley Senior Funding, Inc., as joint lead arrangers and joint bookrunners and Deutsche Bank AG New York branch, as administrative agent and collateral agent. | ||
| 10 | .2* | First Amendment to Credit and Guaranty Agreement, dated as of March 3, 2011, by and among Grifols, Inc., Deutsche Bank AG New York Branch, as administrative agent. | ||
| 10 | .3* | Second Amendment to Credit and Guaranty Agreement, dated as of May 31, 2011, by and among Grifols, Inc. and Deutsche Bank AG New York Branch, as administrative agent. | ||
| 10 | .4* | Counterpart Agreement, dated June 1, 2011, by and among Grifols Inc., Grifols, S.A. and certain of its subsidiaries, the Lenders party thereto and Deutsche Bank AG New York Branch, as administrative agent and collateral agent. | ||
| 10 | .5* | U.S. Pledge and Security Agreement, dated as of June 1, 2011, among the grantors party thereto and Deutsche Bank AG New York Branch, as collateral agent. | ||
II-4
Table of Contents
| Exhibit | ||||
Number | Description of Exhibit | |||
| 10 | .6* | Assumption Agreement, dated as of June 1, 2011, by and between Grifols, Inc. and Deutsche Bank AG New York Branch, as administrative agent. | ||
| 10 | .7 | Voting Agreement between Grifols, S.A. and Talecris Holdings, LLC (incorporated by reference to Exhibit 10.1 of our Registration Statement onForm F-4 (FileNo. 333-168701) filed on August 10, 2010) | ||
| 10 | .8 | Form of Voting Agreement between certain holders of ordinary shares of Grifols, S.A. and Talecris Biotherapeutics Holdings Corp. (incorporated by reference to Exhibit 10.2 of our Registration Statement onForm F-4 (FileNo. 333-168701) filed on August 10, 2010) | ||
| 10 | .9 | Lock-up Agreement between Grifols, S.A. and Talecris Holdings, LLC (incorporated by reference to Exhibit 10.3 of our Registration Statement onForm F-4 (FileNo. 333-168701) filed on August 10, 2010) | ||
| 10 | .10 | Appraisal Indemnity Agreement, dated as of November 4, 2010, by and among Talecris Biotherapeutics Holdings Corp., Grifols, S.A., and Talecris Holdings, LLC, and solely with respect to the provisions of Section 9, Cerberus Capital Management, L.P. (incorporated by reference to Exhibit 10.4 of Amendment No. 2 to our Registration Statement onForm F-4 (FileNo. 333-168701) filed on November 5, 2010) | ||
| 10 | .11 | Toll Manufacturing Agreement for Testing and Packaging, dated April 4, 2008, by and between Talecris Biotherapeutics, GmbH and Catalent France Limoges SAS (incorporated by reference to Exhibit 10.35 of Amendment No. 8 to Talecris’ Registration Statement onForm S-1 (FileNo. 333-144941) filed on August 19, 2009)+ | ||
| 10 | .12 | Retained Intellectual Property License Agreement, dated as of March 31, 2005, by and between Bayer Healthcare LLC and Talecris Biotherapeutics, Inc. (f/k/a NPS Biotherapeutics, Inc.) (incorporated by reference to Exhibit 10.31.1 of Amendment No. 1 to Talecris’ Registration Statement onForm S-1 (FileNo. 333-144941) filed on September 24, 2007) | ||
| 10 | .13 | Amendment to Retained Intellectual Property Licensing Agreement, entered into as of August 10, 2007, by and between Bayer Healthcare LLC and Talecris Biotherapeutics, Inc. (incorporated by reference to Exhibit 10.31.2 of Amendment No. 1 to Talecris’ Registration Statement onForm S-1 (FileNo. 333-144941) filed on September 24, 2007) | ||
| 10 | .14 | Fractionation Services and Commercial Products Agreement, dated as of April 1, 2008, between and amongst Canadian Blood Services/Societe Canadienne Du Sang, Talecris Biotherapeutics, Inc. and Talecris Biotherapeutics, Ltd. (incorporated by reference to Exhibit 10.29 of Amendment No. 9 to Talecris’ Registration Statement onForm S-1 (FileNo. 333-144941) filed on September 11, 2009)+ | ||
| 10 | .15 | Fractionation Services and Commercial Products Agreement, dated as of April 1, 2008, between and amongst Héma-Québec, Talecris Biotherapeutics, Ltd. and Talecris Biotherapeutics, Inc. (incorporated by reference to Exhibit 10.30.1 of Amendment No. 9 to Talecris’ Registration Statement onForm S-1 (FileNo. 333-144941) filed on September 11, 2009)+ | ||
| 10 | .16 | Amending Agreement No. 1, effective as of May 26, 2008, to Fractionation Services and Commercial Products, dated as of April 1, 2008, by and among Héma-Québec, Talecris Biotherapeutics, Ltd. and Talecris Biotherapeutics, Inc. (incorporated by reference to Exhibit 10.30.2 of Amendment No. 6 to Talecris’ Registration Statement onForm S-1 (FileNo. 333-144941) filed on July 2, 2009)+ | ||
| 12 | .1* | Statement of Computation of Ratio of Earnings to Fixed Charges | ||
| 21 | .1* | Subsidiaries of the Registrants | ||
| 23 | .1* | Consent of Proskauer Rose LLP, New York, NY, United States of America (included in Exhibit 5.1) | ||
| 23 | .2* | Consent of Hunton & Williams LLP, Richmond, VA, United States of America (included in Exhibit 5.2) | ||
| 23 | .3* | Consent of Osborne Clarke Spain, Barcelona, Spain (included in Exhibit 5.3) | ||
| 23 | .4* | Consent of SLA Studio Legale Associato, Milan, Italy with respect to Grifols Italia, S.p.A. (included in Exhibit 5.4) | ||
II-5
Table of Contents
| Exhibit | ||||
Number | Description of Exhibit | |||
| 23 | .5* | Consent of Osborne Clarke Germany, Munich, Germany with respect to Grifols Deutschland GmbH (included in Exhibit 5.5) | ||
| 23 | .6* | Consent of KPMG Auditores, S.L., Independent Registered Public Accounting Firm | ||
| 23 | .7* | Consent of PriceWaterhouseCoopers LLP, Independent Registered Public Accounting Firm | ||
| 24 | .1* | Power of Attorney (included on the signature pages hereto) | ||
| 25 | .1* | Statement of Eligibility onForm T-1 of The Bank of New York Mellon Trust Company, N.A., as trustee under the indenture | ||
| 99 | .1* | Form of Letter of Transmittal | ||
| 99 | .2* | Form of Notice of Guaranteed Delivery | ||
| 99 | .3* | Form of Letter to Registered Holders and Depository Trust Company Participants | ||
| 99 | .4* | Form of Letter to Clients | ||
| * | Filed herewith. | |
| + | Portions of the exhibit have been omitted pursuant to an order granting confidential treatment dated September 30, 2009 by the Securities and Exchange Commission. |
| Item 22. | Undertakings |
II-6
Table of Contents
II-7
Table of Contents
II-8
Table of Contents
| By: | /s/ David I. Bell |
| Title: | Director |
Signature | Title | |||
/s/ Gregory G. Rich Gregory G. Rich | Director and Chief Executive Officer (principal executive officer) | |||
/s/ Max de Brouwer Max de Brouwer | Chief Financial Officer (principal financial officer and principal accounting officer) | |||
/s/ Victor Grifols Roura Victor Grifols Roura | Director | |||
/s/ David I. Bell David I. Bell | Director | |||
/s/ Ramón Riera Ramón Riera | Director | |||
/s/ Juan Ignacio Twose Roura Juan Ignacio Twose Roura | Director | |||
/s/ Alfredo Arroyo Alfredo Arroyo | Director | |||
Tomás Dagá Gelabert | Director | |||
Thomas Glanzmann | Chairman of the Board of Directors | |||
II-9
Table of Contents
| By: | /s/ Victor Grifols Roura |
| Title: | Chairman and Chief Executive Officer |
Signature | Title | |||
/s/ Victor Grifols Roura Victor Grifols Roura | Director, Chairman of the Board of Directors and Chief Executive Officer (principal executive officer) | |||
/s/ Alfredo Arroyo Guerra Alfredo Arroyo Guerra | Vice President and Chief Financial Officer (principal financial officer) | |||
/s/ Montserrat Lloveras Calvo Montserrat Lloveras Calvo | Administrative Director and Controller (principal accounting officer) | |||
/s/ Juan Ignacio Twose Roura Juan Ignacio Twose Roura | Director and Vice President | |||
/s/ Ramón Riera Roca Ramón Riera Roca | Director and Vice President | |||
/s/ Tomás Dagá Gelabert Tomás Dagá Gelabert | Director | |||
II-10
Table of Contents
Signature | Title | |||
/s/ José Antonio Grifols Gras Thorthol Holdings B.V. (represented by José Antonio Grifols Gras) | Director | |||
/s/ Edgar Dalzell Jannotta Edgar Dalzell Jannotta | Director | |||
/s/ Anna Veiga Lluch Anna Veiga Lluch | Director | |||
/s/ William Brett Ingersoll William Brett Ingersoll | Director | |||
Luis Isasi Fernández de Bobadilla | Director | |||
/s/ Steven Francis Mayer Steven Francis Mayer | Director | |||
/s/ Thomas Glanzmann Thomas Glanzmann | Director | |||
/s/ David I. Bell David I. Bell | Authorized Representative in the United States | |||
II-11
Table of Contents
| By: | /s/ David I. Bell |
| Title: | Vice President |
Signature | Title | |||
/s/ Gregory G. Rich Gregory G. Rich | Director, Chairman of the Board of Directors and the principal executive officer | |||
/s/ David I. Bell David I. Bell | Director | |||
/s/ Willie Zuniga Willie Zuniga | Director | |||
/s/ Max de Brouwer Max de Brouwer | Principal financial officer and principal accounting officer | |||
II-12
Table of Contents
| By: | /s/ David I. Bell |
| Title: | Chairman |
Signature | Title | |||
/s/ David I. Bell David I. Bell | Director and Chairman of the Board of Directors | |||
/s/ Gregory Gene Rich Gregory Gene Rich | Director and the principal executive officer | |||
/s/ Victor Grifols Roura Victor Grifols Roura | Director | |||
/s/ Juan Ignacio Twose Roura Juan Ignacio Twose Roura | Director | |||
Tomás Dagá Gelabert | Director | |||
/s/ Ramón Riera Roca Ramón Riera Roca | Director | |||
/s/ Shinji Wada Shinji Wada | Director | |||
/s/ Javier Jorba Javier Jorba | Director | |||
/s/ Max de Brouwer Max de Brouwer | Principal financial officer and principal accounting officer | |||
II-13
Table of Contents
| By: | /s/ David I. Bell |
| Title: | Director |
Signature | Title | |||
/s/ Gregory Gene Rich Gregory Gene Rich | Director, Chairman of the Board of Directors and the principal executive officer | |||
/s/ David I. Bell David I. Bell | Director | |||
Mary J. Kuhn | Director | |||
/s/ Max de Brouwer Max de Brouwer | Principal financial officer and principal accounting officer | |||
II-14
Table of Contents
| By: | /s/ David I. Bell |
| Title: | Director |
Signature | Title | |||||
/s/ Gregory G. Rich Gregory G. Rich | Director and the principal executive officer | |||||
Mary J. Kuhn | Director | |||||
/s/ David I. Bell David I. Bell | Director | |||||
/s/ Shinji Wada Shinji Wada | Director | |||||
/s/ Max de Brouwer Max de Brouwer | principal financial officer and principal accounting officer | |||||
II-15
Table of Contents
| By: | /s/ Victor Grifols Roura |
| Title: | Chairman and Chief Executive Officer |
Signature | Title | |||||
/s/ Victor Grifols Roura Victor Grifols Roura | Director, Chairman and Chief Executive Officer | |||||
/s/ Javier Jorba Ribes Biomat, S.A. (represented by Javier Jorba Ribes) | Director and Chief Executive Officer (principal executive officer) | |||||
José Antonio Grifols Gras | Director | |||||
Edgar Dalzell Jannotta | Director | |||||
Thomas Glanzmann | Director | |||||
/s/ Juan Ignacio Twose Roura Juan Ignacio Twose Roura | Director | |||||
II-16
Table of Contents
Signature | Title | |||||
/s/ Ramón Riera Roca Ramón Riera Roca | Director | |||||
/s/ Alfredo Arroyo Guerra Alfredo Arroyo Guerra | Chief Financial Officer (principal financial officer) | |||||
/s/ Montserrat Lloveras Calvo Montserrat Lloveras Calvo | Controller (principal accounting officer) | |||||
/s/ David I. Bell David I. Bell | Authorized Representative in the United States | |||||
II-17
Table of Contents
| By: | /s/ Victor Grifols Roura |
| Title: | Director |
Signature | Title | |||||
/s/ Victor Grifols Roura Victor Grifols Roura | Director (Administrador Solidario) | |||||
/s/ Oriol Duñach Fulla Oriol Duñach Fulla | Director (Administrador Solidario) and the principal executive officer | |||||
/s/ Alfredo Arroyo Guerra Alfredo Arroyo Guerra | Chief Financial Officer (principal financial officer) | |||||
/s/ Montserrat Lloveras Calvo Montserrat Lloveras Calvo | Controller (principal accounting officer) | |||||
/s/ David I. Bell David I. Bell | Authorized Representative in the United States | |||||
II-18
Table of Contents
| By: | /s/ Victor Grifols Roura |
| Title: | Director |
Signature | Title | |||||
/s/ Victor Grifols Roura Victor Grifols Roura | Director (Administrador Solidario) | |||||
/s/ Santiago González Orti Santiago González Orti | Director (Administrador Solidario) and the principal executive officer | |||||
/s/ Miquel Pascual Montblanch Miquel Pascual Montblanch | Director (Administrador Solidario) | |||||
/s/ Alfredo Arroyo Guerra Alfredo Arroyo Guerra | Chief Financial Officer (principal financial officer) | |||||
/s/ Montserrat Lloveras Calvo Montserrat Lloveras Calvo | Controller (principal accounting officer) | |||||
/s/ David I. Bell David I. Bell | Authorized Representative in the United States | |||||
II-19
Table of Contents
| By: | /s/ Victor Grifols Roura |
| Title: | Director |
Signature | Title | |||||
/s/ Victor Grifols Roura Victor Grifols Roura | Director (Administrador Solidario) | |||||
/s/ Alberto Grifols Roura Alberto Grifols Roura | Director (Administrador Solidario) and the principal executive officer | |||||
/s/ Alfredo Arroyo Guerra Alfredo Arroyo Guerra | Chief Financial Officer (principal financial officer) | |||||
/s/ Montserrat Lloveras Calvo Montserrat Lloveras Calvo | Controller (principal accounting officer) | |||||
/s/ David I. Bell David I. Bell | Authorized Representative in the United States | |||||
II-20
Table of Contents
| By: | /s/ Victor Grifols Roura |
| Title: | Attorney-in-fact |
Signature | Title | |||||
/s/ Riccardo Vanni Riccardo Vanni | Chief Executive Officer (principal executive officer) | |||||
/s/ Montserrat Lloveras Calvo Montserrat Lloveras Calvo | Director | |||||
/s/ Ramón Riera Roca Ramón Riera Roca | Director, Chairman of the Board of Directors | |||||
/s/ Alfredo Arroyo Guerra Alfredo Arroyo Guerra | Director | |||||
/s/ Andrea Guidi Andrea Guidi | Chief Financial Officer (principal financial officer and principal accounting officer) | |||||
/s/ David I. Bell David I. Bell | Authorized Representative in the United States | |||||
II-21
Table of Contents
| By: | /s/ Ramon Riera Roca |
| Title: | Managing Director |
| By: | /s/ Alfredo Arroyo Guerra |
| Title: | Managing Director |
Signature | Title | |||||
/s/ Ramón Riera Roca Ramón Riera Roca | Managing Director | |||||
/s/ Alfredo Arroyo Guerra Alfredo Arroyo Guerra | Managing Director and the principal executive officer | |||||
/s/ Thierry Heirich Thierry Heirich | Managing Director | |||||
/s/ Montserrat Lloveras Calvo Montserrat Lloveras Calvo | Managing Director | |||||
/s/ Ainhoa Mendizabal Ainhoa Mendizabal | Chief Financial Officer (principal financial officer and principal accounting officer) | |||||
/s/ David I. Bell David I. Bell | Authorized Representative in the United States | |||||
II-22
Table of Contents
| Exhibit | ||||
Number | Description of Exhibit | |||
| 2 | .1 | Agreement and Plan of Merger, dated as of June 6, 2010, by and among Grifols, S.A., Grifols, Inc. and Talecris Biotherapeutics Holdings Corp. (incorporated by reference to Exhibit 2.1 of our Registration Statement onForm F-4 (FileNo. 333-168701) filed on August 10, 2010) | ||
| 2 | .2 | Amendment No. 1 to the Agreement and Plan of Merger, dated as of November 4, 2010, by and among Grifols, S.A., Grifols, Inc. and Talecris Biotherapeutics Holdings Corp. (incorporated by reference to Exhibit 2.2 of Amendment No. 2 to our Registration Statement onForm F-4 (FileNo. 333-168701) filed on November 5, 2010) | ||
| 3 | .1.1* | By-Laws (Estatutos) of Grifols, S.A. | ||
| 3 | .1.2* | By-Laws (Estatutos) of Grifols, S.A. (English translation) | ||
| 3 | .2.1* | Amended and Restated Articles of Incorporation of Grifols Inc. | ||
| 3 | .2.2* | Amended and Restated By-laws of Grifols Inc. | ||
| 3 | .3.1* | Certificate of Incorporation of Grifols Biologicals Inc. | ||
| 3 | .3.2* | Certificate of Correction of the Certificate of Incorporation of Grifols Biologicals Inc. | ||
| 3 | .3.3* | By-laws of Grifols Biologicals Inc. | ||
| 3 | .4.1* | Amended and Restated Certificate of Incorporation of Biomat USA, Inc. | ||
| 3 | .4.2* | By-laws of Biomat USA, Inc. | ||
| 3 | .5.1* | Certificate of Incorporation of Grifols Therapeutics, Inc. | ||
| 3 | .5.2* | Certificate of Amendment to the Certificate of Incorporation of Grifols Therapeutics, Inc. | ||
| 3 | .5.3* | Certificate of Amendment to the Certificate of Incorporation of Grifols Therapeutics, Inc. | ||
| 3 | .5.4* | By-laws of Grifols Therapeutics, Inc. | ||
| 3 | .6.1* | Certificate of Incorporation of Talecris Plasma Resources, Inc. | ||
| 3 | .6.2* | Certificate of Amendment of Certificate of Incorporation of Talecris Plasma Resources, Inc. | ||
| 3 | .6.3* | Amended and Restated By-laws of Talecris Plasma Resources, Inc. | ||
| 3 | .7.1* | By-laws (Estatutos) of Instituto Grifols, S.A. | ||
| 3 | .7.2* | By-laws (Estatutos) of Instituto Grifols, S.A. (English translation) | ||
| 3 | .8.1* | By-laws (Estatutos) of Diagnostic Grifols, S.A. | ||
| 3 | .8.2* | By-laws (Estatutos) of Diagnostic Grifols, S.A. (English translation) | ||
| 3 | .9.1* | By-laws (Estatutos) of Movaco, S.A. | ||
| 3 | .9.2* | By-laws (Estatutos) of Movaco, S.A. (English translation) | ||
| 3 | .10.1* | By-laws (Estatutos) of Laboratorios Grifols, S.A. | ||
| 3 | .10.2* | By-laws (Estatutos) of Laboratorios Grifols, S.A. (English translation) | ||
| 3 | .11.1* | By-laws of Grifols Italia, S.p.A. | ||
| 3 | .11.2* | By-laws of Grifols Italia, S.p.A. (English translation) | ||
| 3 | .12* | By-laws of Grifols Deutschland GmbH | ||
| 4 | .1* | Senior Notes Indenture, dated as of January 21, 2011, relating to the 8.25% Senior Notes due 2018, among Giant Funding Corp. and The Bank of New York Mellon Trust Company, N.A., as trustee. | ||
| 4 | .2* | Form of 8.25% Senior Note (included as Exhibit A to Exhibit 4.1) | ||
| 4 | .3* | Supplemental Indenture, dated June 1, 2011, by and among Grifols Inc., Grifols, S.A., the subsidiary guarantors party thereto and The Bank of New York Mellon Trust Company, N.A., as trustee. | ||
| 4 | .4* | Second Supplemental Indenture, dated as of October 4, 2011, between Grifols Deutschland GmbH and The Bank of New York Mellon Trust Company, N.A., as trustee. | ||
| 4 | .5* | Registration Rights Agreement, dated January 21, 2011, by and among Giant Funding Corp. and Deutsche Bank Securities Inc., as representative of the several initial purchasers. | ||
| 4 | .6* | Registration Rights Agreement Joinder, dated as of June 1, 2011, by and among Grifols Inc., Grifols, S.A. and the subsidiary guarantors party thereto. | ||
| 5 | .1* | Opinion of Proskauer Rose LLP, New York, NY, United States of America | ||
Table of Contents
| Exhibit | ||||
Number | Description of Exhibit | |||
| 5 | .2* | Opinion of Hunton & Williams LLP, Richmond, VA, United States of America | ||
| 5 | .3* | Opinion of Osborne Clarke, S.L.P., Barcelona, Spain | ||
| 5 | .4* | Opinion of SLA Studio Legale Associato, Milan, Italy with respect to Grifols Italia, S.p.A. | ||
| 5 | .5* | Opinion of Osborne Clarke, Munich, Germany with respect to Grifols Deutschland GmbH | ||
| 10 | .1* | Credit and Guaranty Agreement, dated as of November 23, 2010, among Grifols Inc., Grifols, S.A., certain subsidiaries of Grifols, S.A., the lenders party thereto, Deutsche Bank Securities, Inc., Nomura International PLC, Banco Bilbao Vizcaya Argentaria, S.A., BNP Paribas, HSBC Securities (USA) Inc. and Morgan Stanley Senior Funding, Inc., as joint lead arrangers and joint bookrunners and Deutsche Bank AG New York branch, as administrative agent and collateral agent. | ||
| 10 | .2* | First Amendment to Credit and Guaranty Agreement, dated as of March 3, 2011, by and among Grifols, Inc., Deutsche Bank AG New York Branch, as administrative agent. | ||
| 10 | .3* | Second Amendment to Credit and Guaranty Agreement, dated as of May 31, 2011, by and among Grifols, Inc. and Deutsche Bank AG New York Branch, as administrative agent. | ||
| 10 | .4* | Counterpart Agreement, dated June 1, 2011, by and among Grifols Inc., Grifols, S.A. and certain of its subsidiaries, the Lenders party thereto and Deutsche Bank AG New York Branch, as administrative agent and collateral agent. | ||
| 10 | .5* | U.S. Pledge and Security Agreement, dated as of June 1, 2011, among the grantors party thereto and Deutsche Bank AG New York Branch, as collateral agent. | ||
| 10 | .6* | Assumption Agreement, dated as of June 1, 2011, by and between Grifols, Inc. and Deutsche Bank AG New York Branch, as administrative agent. | ||
| 10 | .7 | Voting Agreement between Grifols, S.A. and Talecris Holdings, LLC (incorporated by reference to Exhibit 10.1 of our Registration Statement onForm F-4 (FileNo. 333-168701) filed on August 10, 2010) | ||
| 10 | .8 | Form of Voting Agreement between certain holders of ordinary shares of Grifols, S.A. and Talecris Biotherapeutics Holdings Corp. (incorporated by reference to Exhibit 10.2 of our Registration Statement onForm F-4 (FileNo. 333-168701) filed on August 10, 2010) | ||
| 10 | .9 | Lock-up Agreement between Grifols, S.A. and Talecris Holdings, LLC (incorporated by reference to Exhibit 10.3 of our Registration Statement onForm F-4 (FileNo. 333-168701) filed on August 10, 2010) | ||
| 10 | .10 | Appraisal Indemnity Agreement, dated as of November 4, 2010, by and among Talecris Biotherapeutics Holdings Corp., Grifols, S.A., and Talecris Holdings, LLC, and solely with respect to the provisions of Section 9, Cerberus Capital Management, L.P. (incorporated by reference to Exhibit 10.4 of Amendment No. 2 to our Registration Statement onForm F-4 (FileNo. 333-168701) filed on November 5, 2010) | ||
| 10 | .11 | Toll Manufacturing Agreement for Testing and Packaging, dated April 4, 2008, by and between Talecris Biotherapeutics, GmbH and Catalent France Limoges SAS (incorporated by reference to Exhibit 10.35 of Amendment No. 8 to Talecris’ Registration Statement onForm S-1 (FileNo. 333-144941) filed on August 19, 2009)+ | ||
| 10 | .12 | Retained Intellectual Property License Agreement, dated as of March 31, 2005, by and between Bayer Healthcare LLC and Talecris Biotherapeutics, Inc. (f/k/a NPS Biotherapeutics, Inc.) (incorporated by reference to Exhibit 10.31.1 of Amendment No. 1 to Talecris’ Registration Statement onForm S-1 (FileNo. 333-144941) filed on September 24, 2007) | ||
| 10 | .13 | Amendment to Retained Intellectual Property Licensing Agreement, entered into as of August 10, 2007, by and between Bayer Healthcare LLC and Talecris Biotherapeutics, Inc. (incorporated by reference to Exhibit 10.31.2 of Amendment No. 1 to Talecris’ Registration Statement onForm S-1 (FileNo. 333-144941) filed on September 24, 2007) | ||
| 10 | .14 | Fractionation Services and Commercial Products Agreement, dated as of April 1, 2008, between and amongst Canadian Blood Services/Societe Canadienne Du Sang, Talecris Biotherapeutics, Inc. and Talecris Biotherapeutics, Ltd. (incorporated by reference to Exhibit 10.29 of Amendment No. 9 to Talecris’ Registration Statement onForm S-1 (FileNo. 333-144941) filed on September 11, 2009)+ | ||
Table of Contents
| Exhibit | ||||
Number | Description of Exhibit | |||
| 10 | .15 | Fractionation Services and Commercial Products Agreement, dated as of April 1, 2008, between and amongst Héma-Québec, Talecris Biotherapeutics, Ltd. and Talecris Biotherapeutics, Inc. (incorporated by reference to Exhibit 10.30.1 of Amendment No. 9 to Talecris’ Registration Statement onForm S-1 (FileNo. 333-144941) filed on September 11, 2009)+ | ||
| 10 | .16 | Amending Agreement No. 1, effective as of May 26, 2008, to Fractionation Services and Commercial Products, dated as of April 1, 2008, by and among Héma-Québec, Talecris Biotherapeutics, Ltd. and Talecris Biotherapeutics, Inc. (incorporated by reference to Exhibit 10.30.2 of Amendment No. 6 to Talecris’ Registration Statement onForm S-1 (FileNo. 333-144941) filed on July 2, 2009)+ | ||
| 12 | .1* | Statement of Computation of Ratio of Earnings to Fixed Charges | ||
| 21 | .1* | Subsidiaries of the Registrants | ||
| 23 | .1* | Consent of Proskauer Rose LLP, New York, NY, United States of America (included in Exhibit 5.1) | ||
| 23 | .2* | Consent of Hunton & Williams LLP, Richmond, VA, United States of America (included in Exhibit 5.2) | ||
| 23 | .3* | Consent of Osborne Clarke Spain, Barcelona, Spain (included in Exhibit 5.3) | ||
| 23 | .4* | Consent of SLA Studio Legale Associato, Milan, Italy with respect to Grifols Italia, S.p.A. (included in Exhibit 5.4) | ||
| 23 | .5* | Consent of Osborne Clarke Germany, Munich, Germany with respect to Grifols Deutschland GmbH (included in Exhibit 5.5) | ||
| 23 | .6* | Consent of KPMG Auditores, S.L., Independent Registered Public Accounting Firm | ||
| 23 | .7* | Consent of PriceWaterhouseCoopers LLP, Independent Registered Public Accounting Firm | ||
| 24 | .1* | Power of Attorney (included on the signature pages hereto) | ||
| 25 | .1* | Statement of Eligibility onForm T-1 of The Bank of New York Mellon Trust Company, N.A., as trustee under the indenture | ||
| 99 | .1* | Form of Letter of Transmittal | ||
| 99 | .2* | Form of Notice of Guaranteed Delivery | ||
| 99 | .3* | Form of Letter to Registered Holders and Depository Trust Company Participants | ||
| 99 | .4* | Form of Letter to Clients | ||
| * | Filed herewith. | |
| + | Portions of the exhibit have been omitted pursuant to an order granting confidential treatment dated September 30, 2009 by the Securities and Exchange Commission. |