 RXi Pharmaceuticals NASDAQ: RXII 15th Annual Biotech in Europe Forum for Global Partnering and Investment Conference September 29, 2015 RXi Pharmaceuticals NASDAQ: RXII 15th Annual Biotech in Europe Forum for Global Partnering and Investment Conference September 29, 2015 ® Exhibit 99.1 |
 2 Property of RXi Pharmaceuticals Forward Looking Statements Forward Looking Statements This presentation contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. Words such as “believes,” “anticipates,” “plans,” “expects,” “indicates,” “will,” “intends,” “potential,” “suggests” and similar expressions are intended to identify forward-looking statements. These statements are based on RXi Pharmaceuticals Corporation’s (the “Company”) current beliefs and expectations. Such statements include, but are not limited to, statements about the future development of the Company’s products (including timing of clinical trials and related matters associated therewith), the expected timing of certain developmental milestones, the reporting of unblinded data, potential partnership opportunities, the Company’s competition and market opportunity and pro forma estimates. The inclusion of forward-looking statements should not be regarded as a representation by the Company that any of its plans will be achieved. Actual results may differ from those set forth in this presentation due to risks and uncertainties in the Company’s business, including those identified under “Risk Factors” in the Company’s most recently filed Quarterly Report on Form 10-Q and in other filings the Company periodically makes with the U.S. Securities and Exchange Commission. The Company does not undertake to update any of these forward-looking statements to reflect a change in its views or events or circumstances that occur after the date of this presentation. |
 3 Property of RXi Pharmaceuticals Corporate Highlights Corporate Highlights Leader in development of advanced RNAi-based therapeutics Novel, self-delivering RNAi (sd-rxRNA ® ) platform provides improved cellular uptake, safety, potency and selectivity over classic siRNA Developing innovative therapeutics in dermatology and ophthalmology Discovery and clinical development programs based on proprietary RNAi technology and immunotherapy agents RXI-109: sd-rxRNA ® compound initially being developed to reduce or inhibit scar formation in the skin and retina Samcyprone ™ : immunomodulator initially being developed for the treatment of alopecia areata, warts, and cutaneous metastases of melanoma Numerous clinical and business development opportunities Extensive patent portfolio covering sd-rxRNA platform and additional compounds Discovery and clinical pipeline with substantial market potential |
 4 Property of RXi Pharmaceuticals Leader in the Development of Advanced RNAi-based Therapeutics Leader in the Development of Advanced RNAi-based Therapeutics The sd-rxRNA Platform The sd-rxRNA Advantage ‘Self-delivering’ therapeutic compounds with drug-like properties Single compound incorporates activity and delivery Structural diversity = novel intellectual property Robust uptake & silencing in multiple preclinical models Combines many positives of RNAi & antisense, while avoiding many negatives Provides for broad pipeline of RNAi drugs for unmet medical needs sd-rxRNA |
 5 Property of RXi Pharmaceuticals Dermatology Franchise Dermatology Franchise Clinical Multiple Development Opportunities Program Discovery Preclinical RXI-109 (Hypertrophic Scars & Keloids) Samcyprone™ (Alopecia Areata, Warts, Cutaneous Metastases Melanoma) Anti-Collagenase (Aging, Chronic wounds) Anti-Tyrosinase (Melasma, PIH, Lentigines) Phase 1 Phase 2 Phase 3 |
 6 Property of RXi Pharmaceuticals Dermal Scarring: RXI-109 Unmet Medical Need with Large Market Potential Dermal Scarring: RXI-109 Unmet Medical Need with Large Market Potential Targeting Connective Tissue Growth Factor (CTGF) Unmet need with limited competition for truly effective therapies Trials initiated with RXi’s first clinical candidate: RXI-109 RXI-109 is an sd-rxRNA compound developed to reduce dermal and retinal scarring Large market in scar prevention/revision Approximately 45M surgical procedures per year in the United States* Depending on price point, annual market potential between $1-3B Dermatology Franchise *US anti-scarring market – The Nemetz Group – December 2009 |
 7 Property of RXi Pharmaceuticals RXI-109 Dermal Clinical Program RXI-109 Dermal Clinical Program Dermatology Franchise Phase 1 RXI-109-1201 - Single dose Complete – n=15 RXI-109-1202 - Multi-dose Complete – n=15 Phase 2a RXI-109-1301 - Hypertrophic scars Enrollment and treatment complete. Three doses over a 2-week period. Data collection ongoing. n=22 RXI-109-1401 - Keloids Enrollment and treatment complete. Four doses over a 1-month period. Data collection ongoing. n=16 RXI-109-1402 - Hypertrophic scars Extended dosing based on previous clinical results. Six doses over a 3-month period. n=20 Phase 2b Projected initiation 2016 Phase 3 Projected initiation 2018 |
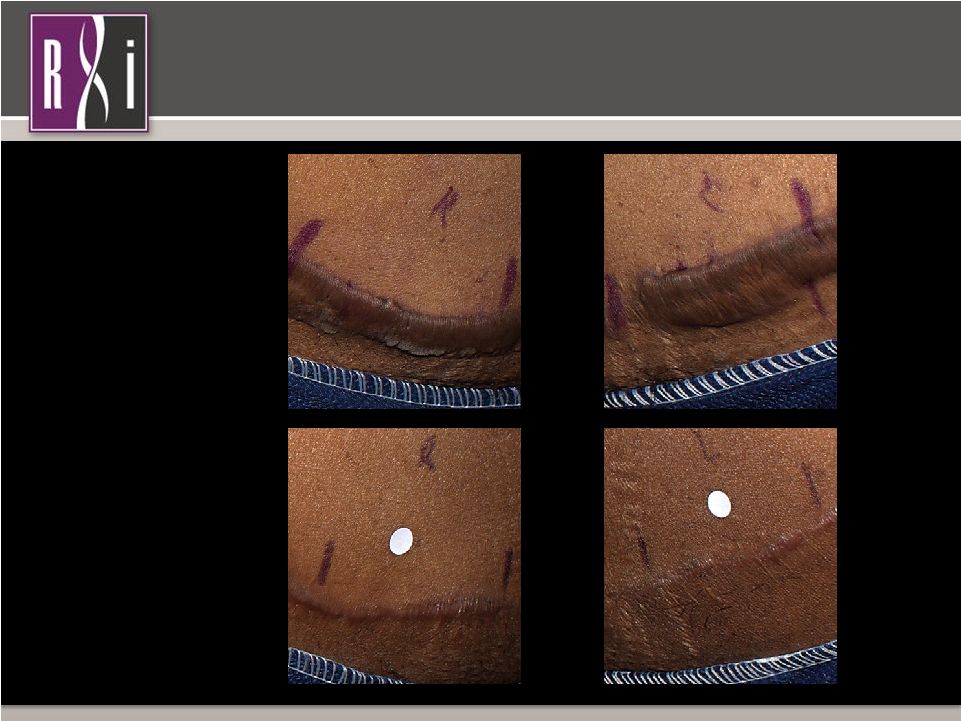 8 Property of RXi Pharmaceuticals Phase 2a: Scar Comparison at 3 Months Treatment with RXI-109 Initiated Two Weeks Post Scar Revision Surgery Phase 2a: Scar Comparison at 3 Months Treatment with RXI-109 Initiated Two Weeks Post Scar Revision Surgery Phase 2a: RXI-109-1301 Pre-scar revision surgery 3 months post-scar revision surgery PLACEBO RXI-109 *White dots are markers used in clinical photography. |
 9 Property of RXi Pharmaceuticals Topical Immunotherapy: Samcyprone ™ Topical Immunotherapy: Samcyprone ™ Samcyprone ™ - RXi’s second clinical candidate A proprietary topical formulation of diphenylcyclopropenone (DPCP) DPCP is a topical immunomodulator that works by eliciting a T-cell response Efficacy in the three target indications, alopecia areata, warts, and cutaneous metastasis of melanoma, reported in peer-reviewed journals with DPCP Orphan-drug designation of Samcyprone ™ for malignant melanoma stage IIb to IV Combined market for three indications estimated >$1B Major competitors for treatment of warts: Aldara ™ (Imiquimod) and Picato ® (Ingenol mebutate) Dermatology Franchise |
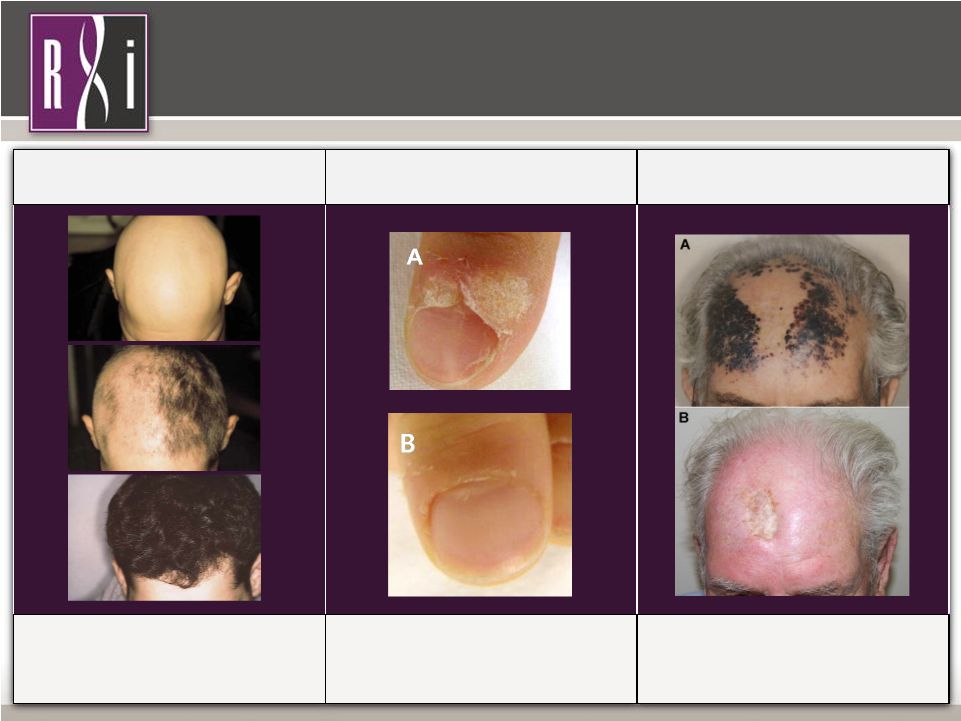          10 Property of RXi Pharmaceuticals Treatment with Diphencyprone (DPCP) DPCP is the Active Ingredient in Samcyprone ™ Treatment with Diphencyprone (DPCP) DPCP is the Active Ingredient in Samcyprone ™ Alopecia Areata Cotellessa, C et al. JAAD 2001;44:73-6 • >200 patients treated for several months • Response rates of 67-78% Warts (refractory plantar & periungual) Choi, Y et al. Ann Dermatol. 2013, 25 4:434-439 • >350 patients treated for 12 – 24 weeks • Response rates of 80 - 85% Cutaneous Metastases of Melanoma (refractory) Damian, DL et al. J Surg Oncol 2014, 109:308-313 • 50 patients with mean of 15 months treatment • 46% complete clearance and 38% partial clearance Dermatology Franchise |
 11 Property of RXi Pharmaceuticals Ophthalmology Franchise Ophthalmology Franchise Program Discovery Preclinical Phase 1 Phase 2 Phase 3 Clinical Multiple Development Opportunities Retinal scarring (Macular Degeneration) Corneal scarring (Topical) “Self-Delivering” (“sd”) Adaptation to acquired OPKO estate New targets |
 Property of RXi Pharmaceuticals sd-rxRNA Stabilized Placebo siRNA Mouse immediately post-dose Mouse at 24 hours post-dose Mouse at 24 hours post-dose Rabbit at 24 hours post-dose • Dosing by intravitreal injection to mouse or rabbit eye • Dy547-labeled sd-rxRNA, stabilized siRNA or placebo sd-rxRNA: In Ophthalmology Improved Delivery vs. Stabilized RNAi sd-rxRNA chemistry is required for robust uptake to the cells of the eye No overt toxicity observed after sd-rxRNA treatment to the eye Byrne et al., JOPT. December 2013, 29(10): 855-864. Ophthalmology Franchise 12 |
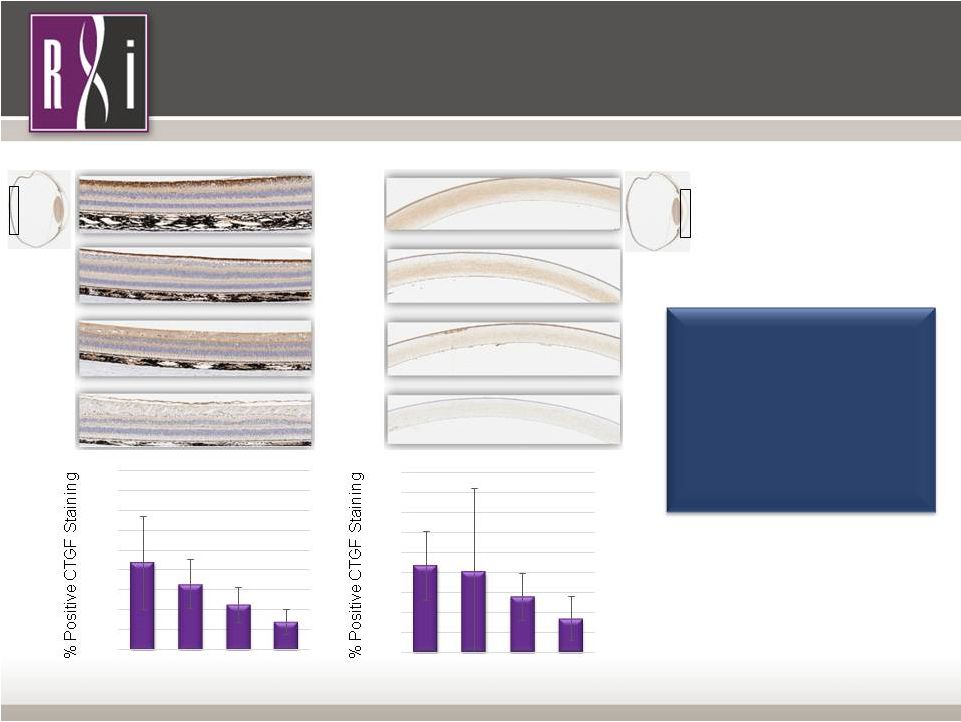 13 Property of RXi Pharmaceuticals CTGF Protein Levels in the NHP Retina and Cornea Decrease Following Treatment with RXI-109 Retina Cornea CTGF protein levels are reduced in a dose dependent manner 7-days following single intravitreal injection of RXI-109 Treatment Dose Treatment Dose PBS 0.1 mg 0.33 mg 1 mg * ** * * p < 0.05, ** p < 0.01 Ophthalmology Franchise 0 5 10 15 20 25 30 35 40 45 PBS 0.1 mg 0.33 mg 1 mg 0 5 10 15 20 25 30 35 40 45 PBS 0.1 mg 0.33 mg 1 mg |
 14 Property of RXi Pharmaceuticals Research and Development Programs New Targets for Potential Preclinical Development MMP1 enzyme involved in breakdown of extracellular matrix Collagenase (MMP1) • Potent MMP1 sd-rxRNAs identified for potential for preclinical development such as skin aging disorders including photo aging TYR key enzyme in synthesis of melanin Tyrosinase (TYR) • Potent TYR sd-rxRNAs identified for potential discovery and preclinical development. Potential indications include cutaneous hyperpigmentation disorders, lentigines (age spots, liver spots, freckles) Acquired estate provides multiple development opportunities OPKO RNAi Assets • Novel potent sd-rxRNA compounds targeting VEGF for potential intra-ocular therapy for age-related macular degeneration • Other targets - discovery stage efforts ongoing Combined multi-billion dollar global market potential Market Potential Multiple Development Opportunities |
 15 Property of RXi Pharmaceuticals Research and Development Programs New Targets for Potential Preclinical Development Research and Development Programs New Targets for Potential Preclinical Development MMP1 TYR VEGF DISCOVERY Target Selection In Progress Bioinformatics Pending Compound Synthesis Pending In vitro Screening Pending File IP Pending Chemical Optimization Pending Pending Test in Relevant Models (in vitro and in vivo) In Progress In Progress Pending Pending Lead Candidate Identification In Progress Q4 2015 In Progress Q4 2015 Pending Pending Multiple Development Opportunities |
 16 Property of RXi Pharmaceuticals NTC UTC MMP-26038 0 h 24 h 48 h 72 h MMP1 – Targeting sd-rxRNA Compounds Reduced Migration Rate of A549 Lung Carcinoma Cells in vitro 0 100 200 300 400 500 600 700 800 900 1000 Comparison of A549 Cell Migration after Treatment with MMP1 Targeting sd-rxRNA 48 h 24 h 72 h 48 h 24 h 72 h • Scratch migration assay - A549 non-small cell lung carcinoma cell line • Cells in culture treated with MMP1 – targeting sd-rxRNA, non-targeting control (NTC), or untreated control (UTC) • Reduced migration in the scratch assay may indicate a reduction in the invasive nature of the cancer cell due to MMP1 reduction as a result of sd-rxRNA treatment |
 17 Property of RXi Pharmaceuticals TYR – Targeting sd-rxRNA Compounds Lead to a Visible Reduction of Pigmentation in vitro TYR – Targeting sd-rxRNA Compounds Lead to a Visible Reduction of Pigmentation in vitro NTC TYR - 21698 TYR - 26231 • MelanoDerm™ 3-dimensional epidermal culture model containing melanocytes • Fourteen day culture with TYR-targeting sd-rxRNA added to culture media Light microscopy Multiple Development Opportunities Histological cross section with staining for melanin |
 18 Property of RXi Pharmaceuticals RXII: Driving Growth and Innovation RXII: Driving Growth and Innovation Research & Development Communicate preliminary read-outs for RXI-109-1402 Phase 2a for Hypertrophic Scars Q4 2015 Initiate Phase 1/2 trial in ophthalmology with RXI-109 Q4 2015 Initiate Phase 2 trial for cutaneous warts with Samcyrone ™ Q4 2015 Identify lead sd-rxRNA candidates targeting Collagenase (MMP1) and Tyrosinase Q4 2015 7 Initiatives for Value Creation – Next 12 Months Business Development Partner/Collaborate with other companies create proprietary sd-rxRNA compounds against targets of their choice Out-license/Create spin-out outside of RXi’s core strategic areas Maximize potential of sd-rxRNA platform in exchange for equity in the receiving company (e.g. MirImmune license) Generate larger deals with equity investment by strategic regional partners |