
RXi Pharmaceuticals Corporation (NASDAQ:RXII) “The discovery of RNA interference (RNAi) is considered one of the greatest scientific breakthroughs since the discovery of DNA”1 Investor Presentation 1Wong, J. The Nobel for RNAi: From Petunias to Potential Cure. Oxbridge Biotech Roundtable Review; 2013, June 14. Exhibit 99.1

Forward Looking Statements This presentation contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. Words such as “believes,” “anticipates,” “plans,” “expects,” “indicates,” “will,” “intends,” “potential,” “suggests” and similar expressions are intended to identify forward-looking statements. These statements are based on RXi Pharmaceuticals Corporation’s (the “Company”) current beliefs and expectations. Such statements include, but are not limited to, statements about the future development of the Company’s products (including timing of clinical trials and related matters associated therewith), the expected timing of certain developmental milestones, the reporting of unblinded data, potential partnership opportunities, risks that the potential acquisition of MirImmune may not proceed; the actual realization of anticipated benefits of the purchase of MirImmune; risks related to our ability to control the timing of the purchase of MirImmune; the Company’s competition and market opportunity and pro forma estimates. The inclusion of forward-looking statements should not be regarded as a representation by the Company that any of its plans will be achieved. Actual results may differ from those set forth in this presentation due to risks and uncertainties in the Company’s business, including those identified under “Risk Factors” in the Company’s most recently filed Quarterly Report on Form 10-Q and in other filings the Company periodically makes with the U.S. Securities and Exchange Commission. The Company does not undertake to update any of these forward-looking statements to reflect a change in its views or events or circumstances that occur after the date of this presentation.

Risk Factors Investing in the Company involves a high degree of risk. Before investing, you should consider carefully the risks described below, together with the other information contained in the Company’s annual report 10-K. Risk factors include, but are not limited to: RXII is dependent on the success of its lead drug candidates, which may not receive regulatory approval or be successfully monetized. RXII has no commercial products and currently generates no revenue from commercial sales or collaborations and may never be able to develop marketable products. A number of different factors could prevent RXII from obtaining regulatory approval or commercializing RXII’s product candidates on a timely basis, or at all. Factors could include delays in filing or acceptance of initial drug applications, difficulty in securing centers to conduct clinical trials, conditions imposed on RXII by the FDA or comparable foreign authorities regarding the scope or design of its clinical trials, and others. The approach RXII is taking to discover and develop novel technologies using RNAi is unproven and may never lead to marketable products. The FDA could impose a unique regulatory regime for RXII’s therapeutics. The substances RXII intends to develop may represent a new class of drug, and the FDA has not yet established any definitive policies, practices or guidelines in relation to these drugs. RXII may decide not to exercise its option to acquire MirImmune Inc. and if RXII does acquire MirImmune Inc., the acquisition may be unsuccessful and could dilute the Company’s shareholders’ ownership interests in the Company. If RXII exercises this option, it may not be able to integrate the acquisition successfully into its existing business or may not realize the anticipated benefits from the acquisitions.
Company Overview Option to Acquire MirImmune RXi Pharmaceuticals’ Pipeline Upcoming Milestones
Company Overview sd-rxRNA® RXi has developed a robust proprietary RNAi therapeutic platform that includes self-delivering RNAi (sd-rxRNA®) compounds. RNAi is a powerful molecular tool that has the ability to “silence” or down-regulate the expression of a specific gene that may be over-expressed in a disease condition. RXi Pharmaceuticals Corporation is a clinical-stage RNAi company developing innovative therapeutics based on its proprietary technology platform.
Company Overview (continued..) Selection of six lead sd-rxRNA compounds against six different extracellular and intracellular immune check points; Preclinical data that demonstrate silencing of all six targets in vitro, singly and in combinations; Efficient and long-lasting knockdown of immune checkpoints in vivo; Applicability of sd-rxRNA transfection in cell therapy to solid tumors; Filing of intellectual property that covers the use of RNAi compounds for use in cell therapy. MirImmune: Demonstrated Effectiveness & Results In 2015, MirImmune licensed RXi’s sd-rxRNA technology for use in development of innovative cell-based cancer immunotherapies.
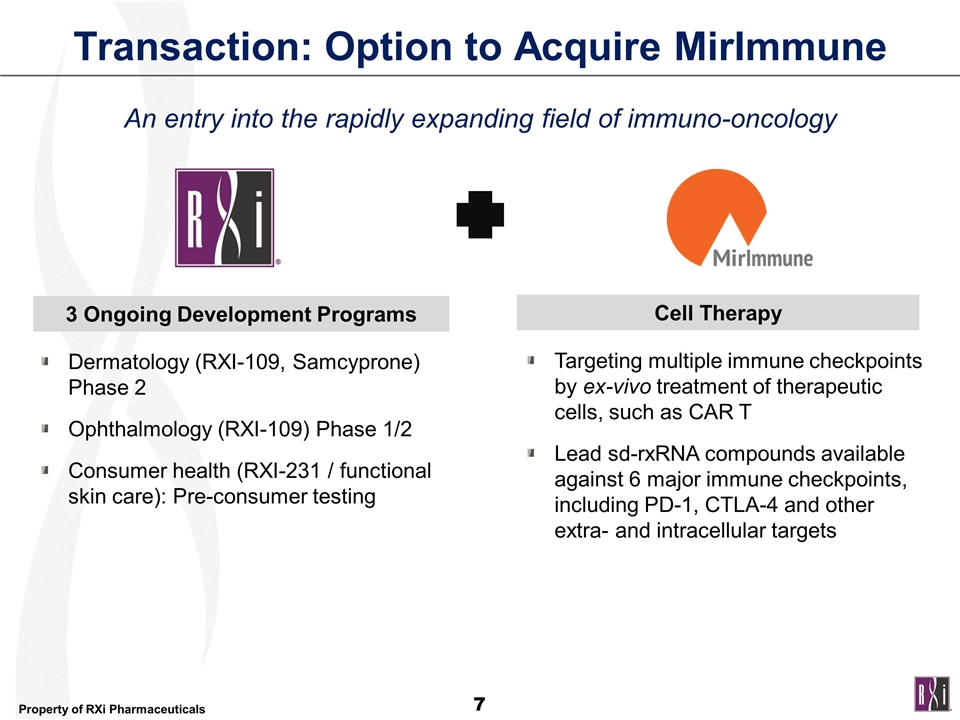
Transaction: Option to Acquire MirImmune Dermatology (RXI-109, Samcyprone) Phase 2 Ophthalmology (RXI-109) Phase 1/2 Consumer health (RXI-231 / functional skin care): Pre-consumer testing 3 Ongoing Development Programs Targeting multiple immune checkpoints by ex-vivo treatment of therapeutic cells, such as CAR T Lead sd-rxRNA compounds available against 6 major immune checkpoints, including PD-1, CTLA-4 and other extra- and intracellular targets Cell Therapy An entry into the rapidly expanding field of immuno-oncology
Terms of the Option to Acquire MirImmune Acquire all outstanding capital stock of MirImmune Consideration for a number of shares equal to 19.99% of the then outstanding common stock of RXi Additional potential consideration contingent on MirImmune reaching certain milestones
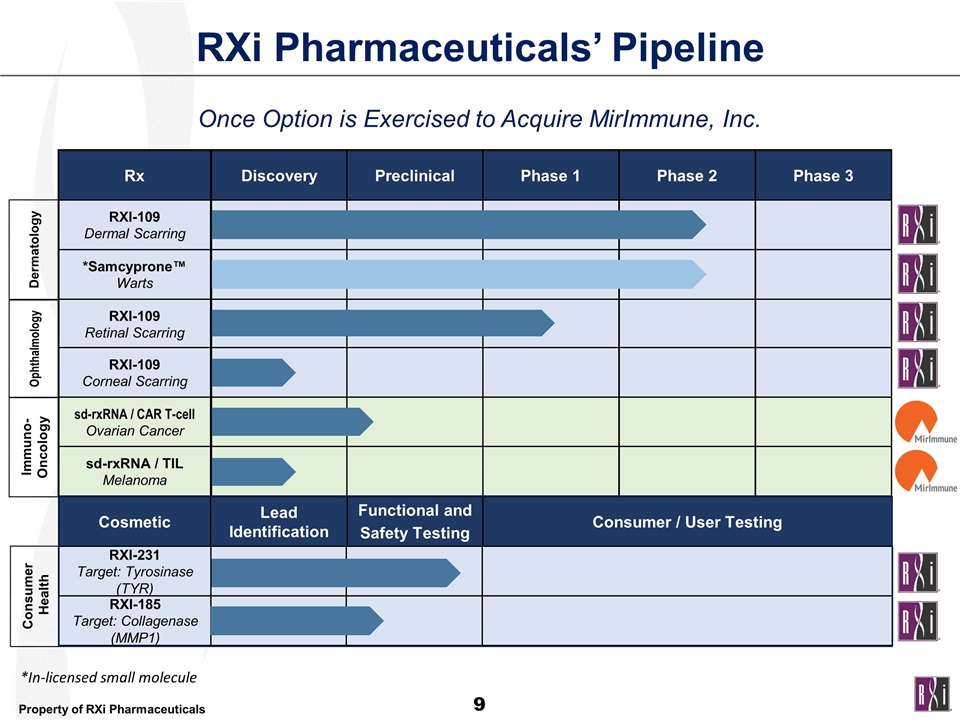
Rx Discovery Preclinical Phase 1 Phase 2 RXI-109 Dermal Scarring *Samcyprone™ Warts RXI-109 Retinal Scarring RXI-109 Corneal Scarring Phase 3 sd-rxRNA / CAR T-cell Ovarian Cancer sd-rxRNA / TIL Melanoma Consumer Health Cosmetic Lead Identification Functional and Safety Testing Consumer / User Testing RXI-185 Target: Collagenase (MMP1) RXI-231 Target: Tyrosinase (TYR) Immuno-Oncology Ophthalmology Dermatology RXi Pharmaceuticals’ Pipeline Once Option is Exercised to Acquire MirImmune, Inc. *In-licensed small molecule
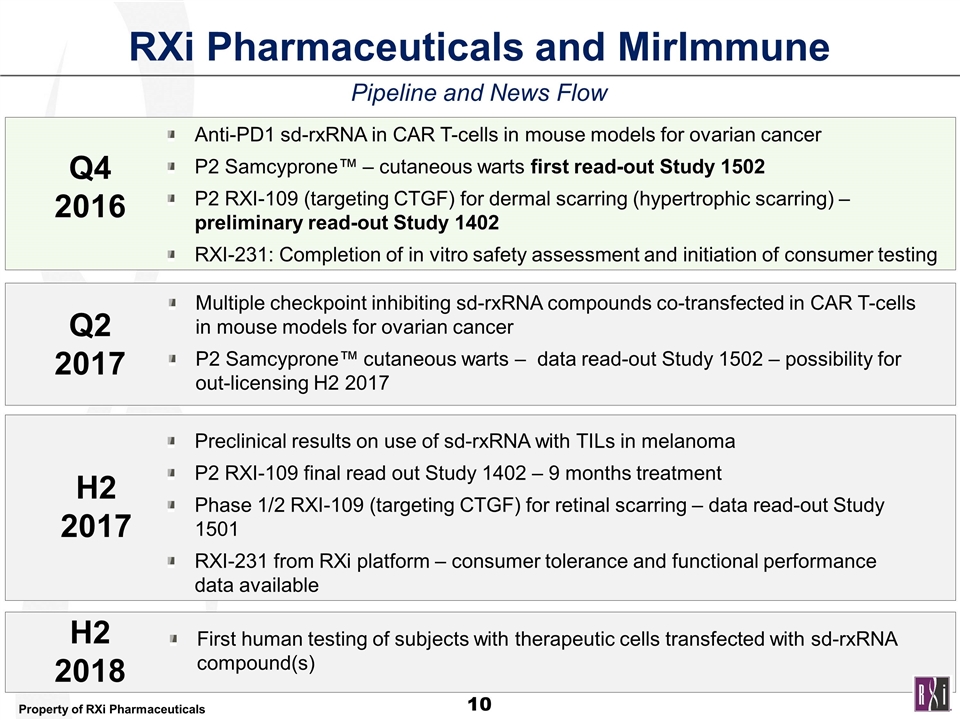
Pipeline and News Flow RXi Pharmaceuticals and MirImmune Anti-PD1 sd-rxRNA in CAR T-cells in mouse models for ovarian cancer P2 Samcyprone™ – cutaneous warts first read-out Study 1502 P2 RXI-109 (targeting CTGF) for dermal scarring (hypertrophic scarring) – preliminary read-out Study 1402 RXI-231: Completion of in vitro safety assessment and initiation of consumer testing Multiple checkpoint inhibiting sd-rxRNA compounds co-transfected in CAR T-cells in mouse models for ovarian cancer P2 Samcyprone™ cutaneous warts – data read-out Study 1502 – possibility for out-licensing H2 2017 Preclinical results on use of sd-rxRNA with TILs in melanoma P2 RXI-109 final read out Study 1402 – 9 months treatment Phase 1/2 RXI-109 (targeting CTGF) for retinal scarring – data read-out Study 1501 RXI-231 from RXi platform – consumer tolerance and functional performance data available First human testing of subjects with therapeutic cells transfected with sd-rxRNA compound(s) Q4 2016 Q2 2017 H2 2017 H2 2018

RXi’s Proprietary sd-rxRNA Platform
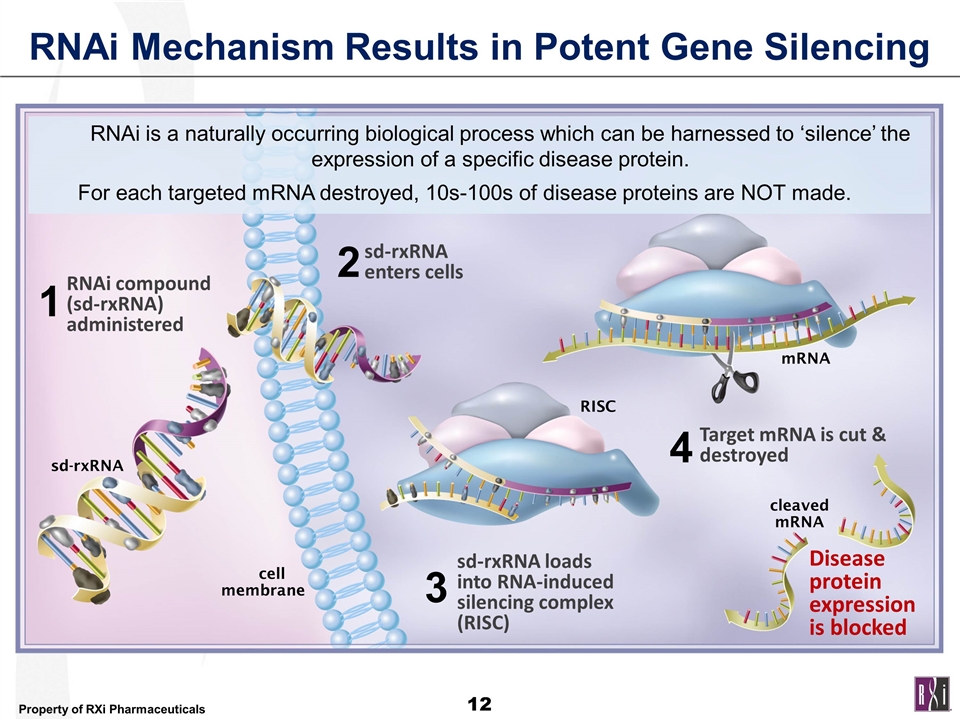
RNAi Mechanism Results in Potent Gene Silencing cell membrane RISC mRNA cleaved mRNA 1 2 3 4 RNAi compound (sd-rxRNA) administered sd-rxRNA enters cells sd-rxRNA loads into RNA-induced silencing complex (RISC) Target mRNA is cut & destroyed Disease protein expression is blocked sd-rxRNA RNAi is a naturally occurring biological process which can be harnessed to ‘silence’ the expression of a specific disease protein. For each targeted mRNA destroyed, 10s-100s of disease proteins are NOT made.
The sd-rxRNA Advantage Novel, self-delivering RNAi therapeutic compounds Single compound incorporates gene silencing activity & cellular uptake Robust uptake & silencing in multiple preclinical models Demonstration of safety and clinical activity in human Best RNAi technology for enhancement of cell-based therapeutics Potential to expand CAR T-cell therapy to solid tumors Provides for broad pipeline of RNAi drugs for unmet medical needs sd-rxRNA Technology Platform Self-delivering RNAi Platform (sd-rxRNA®)
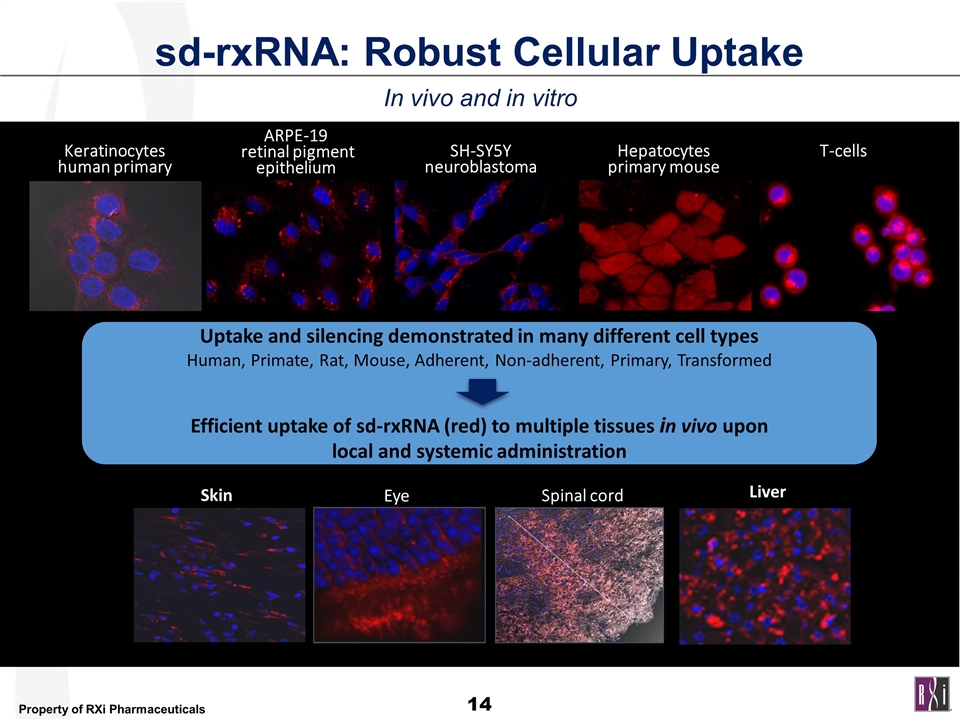
sd-rxRNA: Robust Cellular Uptake In vivo and in vitro T-cells ARPE-19 retinal pigment epithelium Hepatocytes primary mouse Keratinocytes human primary SH-SY5Y neuroblastoma Skin Eye Spinal cord Uptake and silencing demonstrated in many different cell types Human, Primate, Rat, Mouse, Adherent, Non-adherent, Primary, Transformed Efficient uptake of sd-rxRNA (red) to multiple tissues in vivo upon local and systemic administration Liver
RXi’s Advantages Over Competitors Proprietary Platform Delivery Efficacy / Safety Compatibility Single chemically-modified RNA compound with improved drug-like properties Potent, stable, specific Efficient cellular uptake and gene silencing Rapid lead identification and optimization (4 months) No delivery formulation required for local therapeutic applications Delivery not limited to a specific cell type Robust, long lasting in vivo efficacy in multiple tissues Demonstrated safety and efficacy in human clinical trials Compatible with various systemic delivery systems Highlights of sd-rxRNA compounds

Clinical Programs

Dermal Clinical Program: RXI-109 An sd-rxRNA in Development to Reduce Dermal Scarring Phase 2a: Current trial RXI-109-1402 3rd and 4th cohorts initiated Q4 2015 Objective: Reduce the formation of hypertrophic scars following scar revision surgery Market Potential: $1-3B Approximately 45M surgical procedures per year in the U.S. No FDA-approved targeted therapies in the U.S. for the treatment and prevention of scars Validated Target: CTGF RXI-109: sd-rxRNA silences CTGF Regulatory component of fibrosis and scar formation, plays a key role in tissue regeneration and repair Hypertrophic Scars are raised and may appear darker than the existing skin
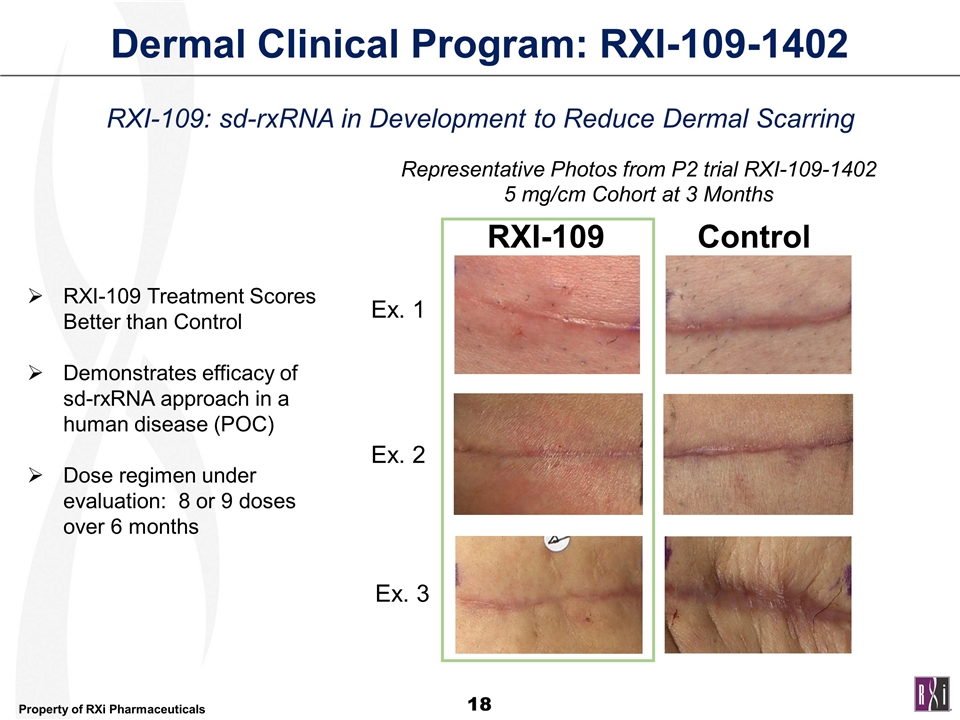
Dermal Clinical Program: RXI-109-1402 RXI-109 Control Ex. 1 Ex. 3 Ex. 2 Representative Photos from P2 trial RXI-109-1402 5 mg/cm Cohort at 3 Months RXI-109: sd-rxRNA in Development to Reduce Dermal Scarring RXI-109 Treatment Scores Better than Control Demonstrates efficacy of sd-rxRNA approach in a human disease (POC) Dose regimen under evaluation: 8 or 9 doses over 6 months

Ophthalmology Clinical Program: RXI-109-1501 RXI-109: sd-rxRNA in Development to Reduce Retinal Scarring The objective is to block the formation of subretinal scarring in patients with advanced wet age-related macular degeneration (AMD) Goal: to maintain vision for a longer period of time than current standard of care (i.e. anti-VEGF treatments) alone Ongoing clinical trial being conducted at Johns Hopkins Enrolling ahead of schedule (2 of 3 cohorts completely enrolled) No safety issues to date Fundus angiograph image showing retinal scarring Day 1
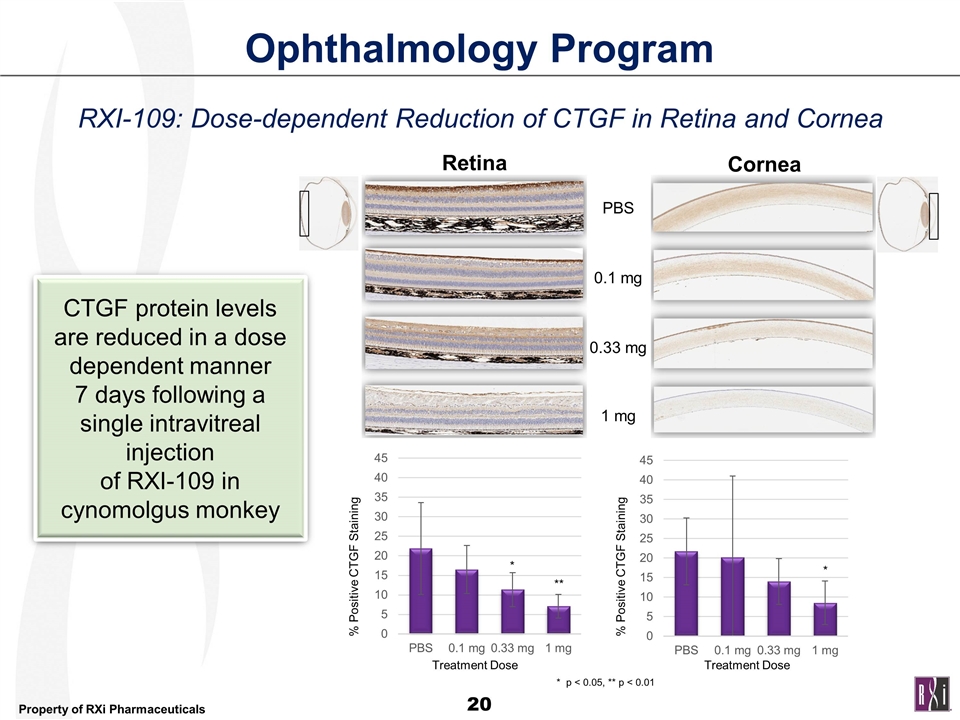
CTGF protein levels are reduced in a dose dependent manner 7 days following a single intravitreal injection of RXI-109 in cynomolgus monkey Retina Cornea % Positive CTGF Staining % Positive CTGF Staining Treatment Dose Treatment Dose PBS 0.1 mg 0.33 mg 1 mg * ** * * p < 0.05, ** p < 0.01 Ophthalmology Program RXI-109: Dose-dependent Reduction of CTGF in Retina and Cornea

Overview of Phase 2 Clinical Trial RXI-SCP-1502 Phase 2a: Subjects with up to four cutaneous warts on the hands, feet, limbs or trunk Subjects are treated with a sensitization dose on the inner arm and on one preselected wart lesion After a confirmed sensitization response, subjects will be treated with one treatment dose, weekly for 10 weeks Next Steps: Fully enroll by end of 2016 Early read-outs expected H2 2016
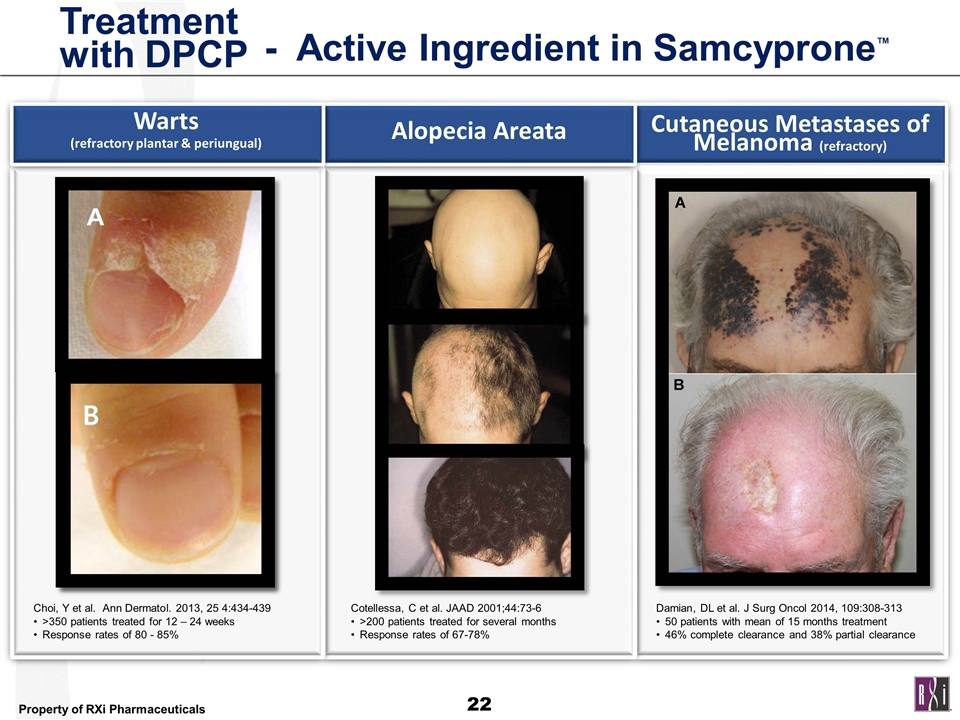
Treatment with DPCP Cutaneous Metastases of Melanoma (refractory) Cotellessa, C et al. JAAD 2001;44:73-6 >200 patients treated for several months Response rates of 67-78% Damian, DL et al. J Surg Oncol 2014, 109:308-313 50 patients with mean of 15 months treatment 46% complete clearance and 38% partial clearance Choi, Y et al. Ann Dermatol. 2013, 25 4:434-439 >350 patients treated for 12 – 24 weeks Response rates of 80 - 85% Alopecia Areata Warts (refractory plantar & periungual) - Active Ingredient in Samcyprone™
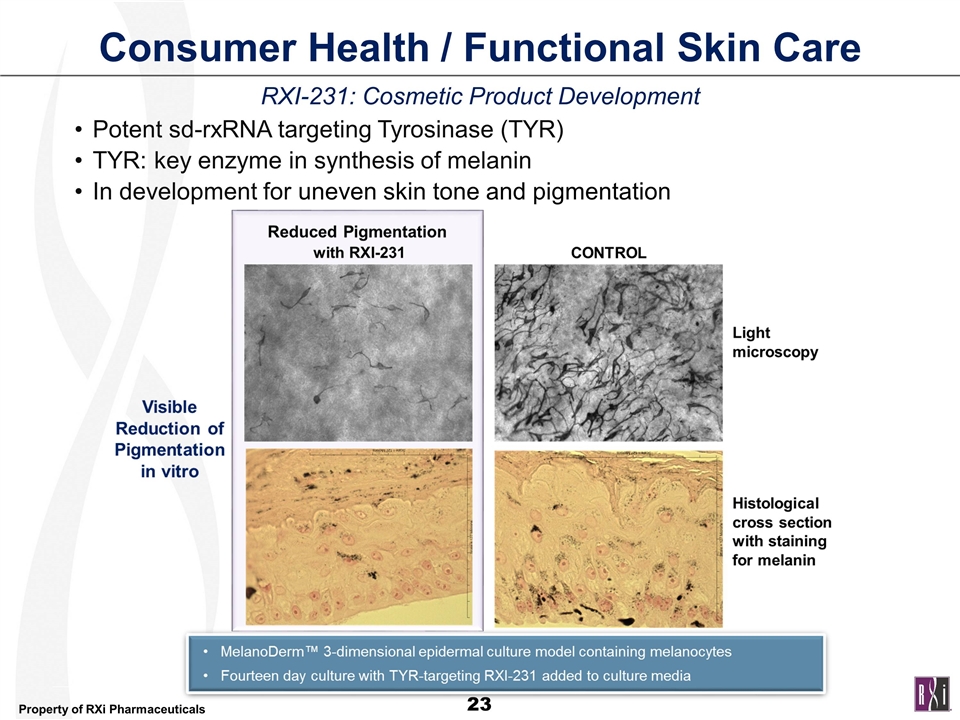
Consumer Health / Functional Skin Care RXI-231: Cosmetic Product Development Potent sd-rxRNA targeting Tyrosinase (TYR) TYR: key enzyme in synthesis of melanin In development for uneven skin tone and pigmentation
MirImmune Overview
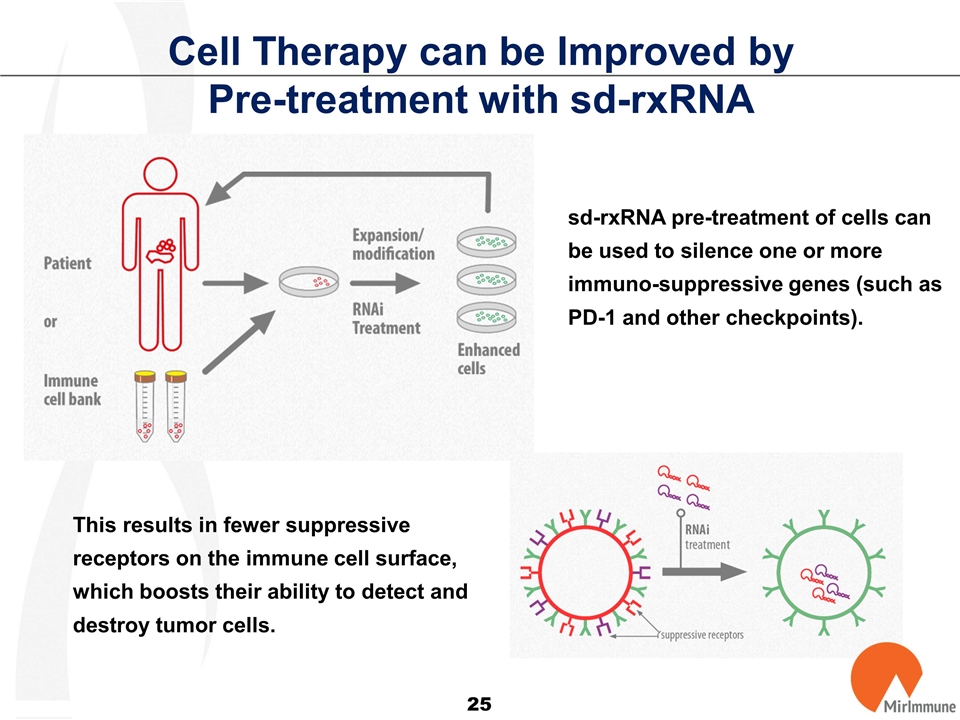
Cell Therapy can be Improved by Pre-treatment with sd-rxRNA sd-rxRNA pre-treatment of cells can be used to silence one or more immuno-suppressive genes (such as PD-1 and other checkpoints). This results in fewer suppressive receptors on the immune cell surface, which boosts their ability to detect and destroy tumor cells.
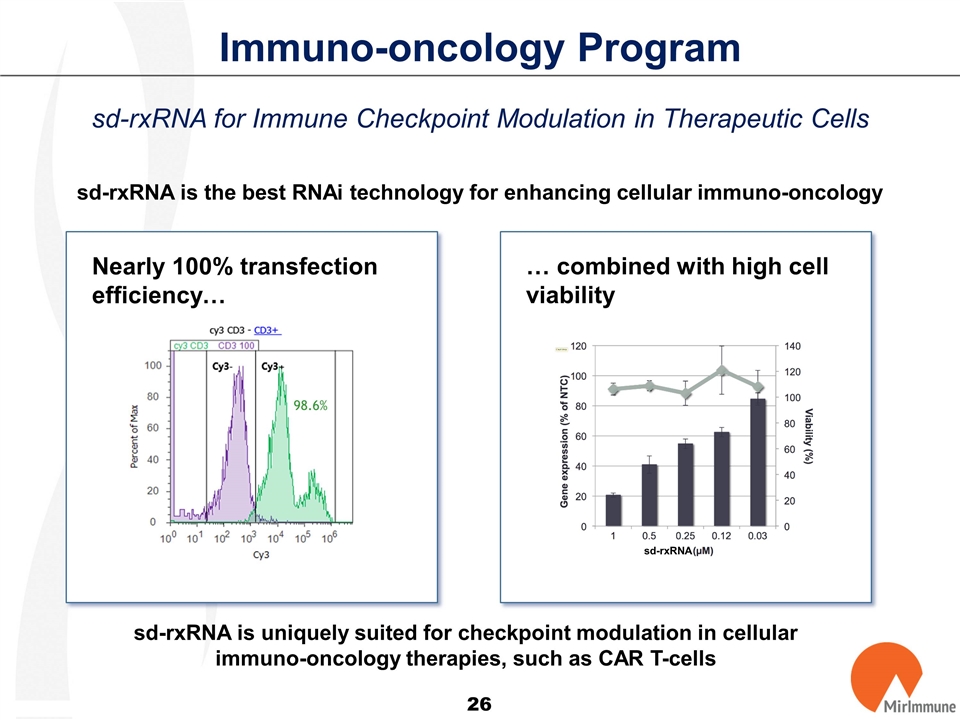
Immuno-oncology Program sd-rxRNA is the best RNAi technology for enhancing cellular immuno-oncology Nearly 100% transfection efficiency… sd-rxRNA is uniquely suited for checkpoint modulation in cellular immuno-oncology therapies, such as CAR T-cells 98.6% … combined with high cell viability sd-rxRNA sd-rxRNA for Immune Checkpoint Modulation in Therapeutic Cells
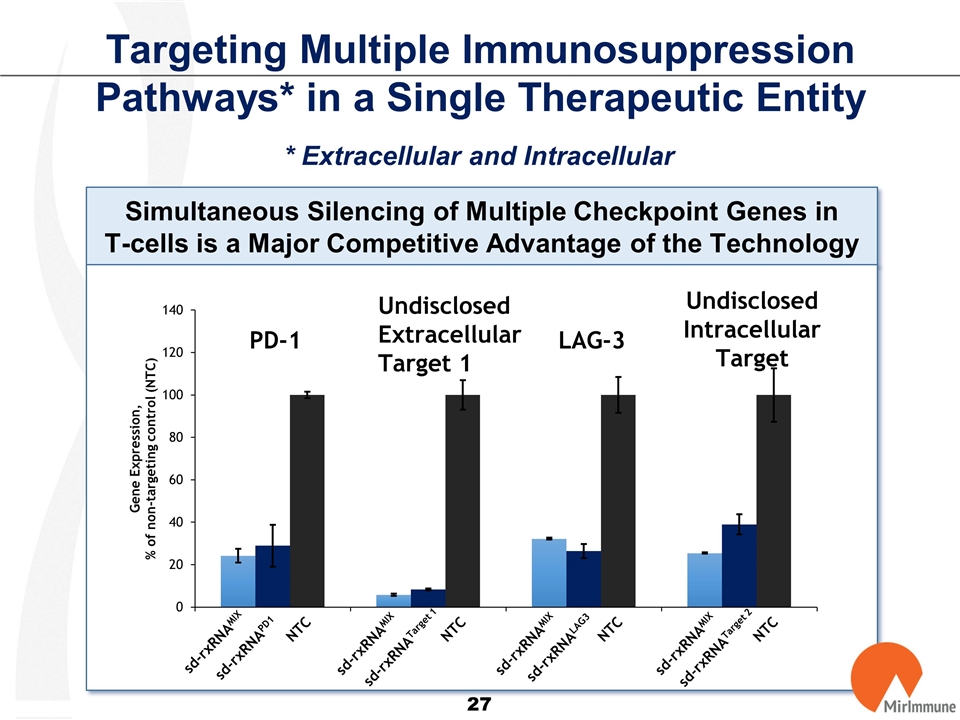
Targeting Multiple Immunosuppression Pathways* in a Single Therapeutic Entity * Extracellular and Intracellular Simultaneous Silencing of Multiple Checkpoint Genes in T-cells is a Major Competitive Advantage of the Technology sd-rxRNAMIX sd-rxRNAPD1 sd-rxRNATarget 1 sd-rxRNALAG3 sd-rxRNATarget 2 NTC NTC NTC NTC PD-1 Undisclosed Extracellular Target 1 LAG-3 UndisclosedIntracellular Target sd-rxRNAMIX sd-rxRNAMIX sd-rxRNAMIX
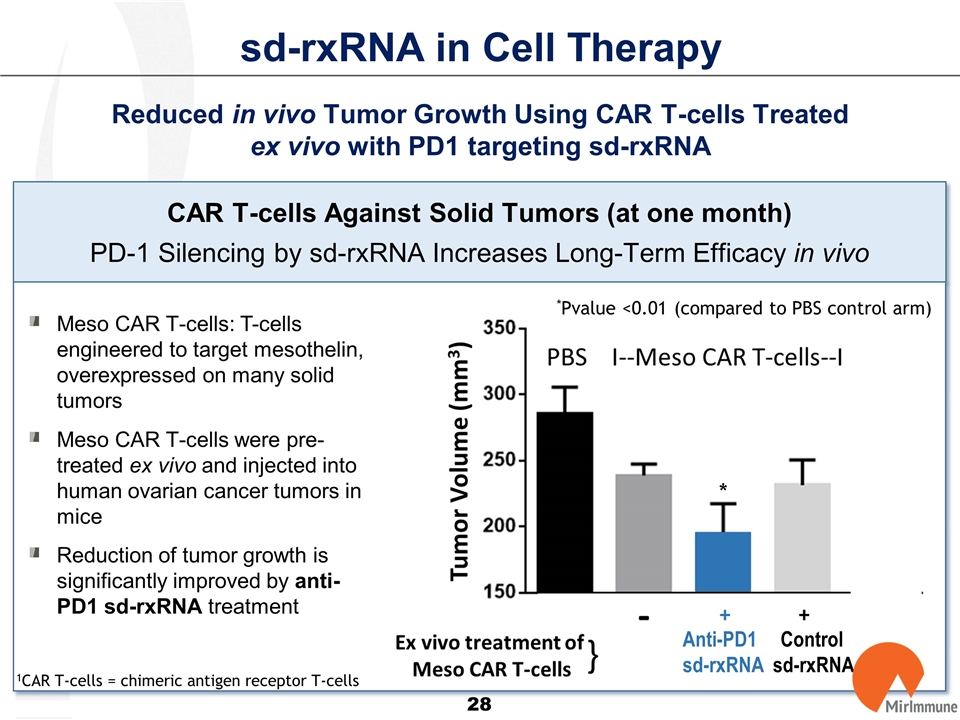
sd-rxRNA in Cell Therapy Reduced in vivo Tumor Growth Using CAR T-cells Treated ex vivo with PD1 targeting sd-rxRNA CAR T-cells Against Solid Tumors (at one month) PD-1 Silencing by sd-rxRNA Increases Long-Term Efficacy in vivo Meso CAR T-cells: T-cells engineered to target mesothelin, overexpressed on many solid tumors Meso CAR T-cells were pre-treated ex vivo and injected into human ovarian cancer tumors in mice Reduction of tumor growth is significantly improved by anti-PD1 sd-rxRNA treatment 1CAR T-cells = chimeric antigen receptor T-cells Anti-PD1 Control sd-rxRNA sd-rxRNA * *Pvalue <0.01 (compared to PBS control arm)
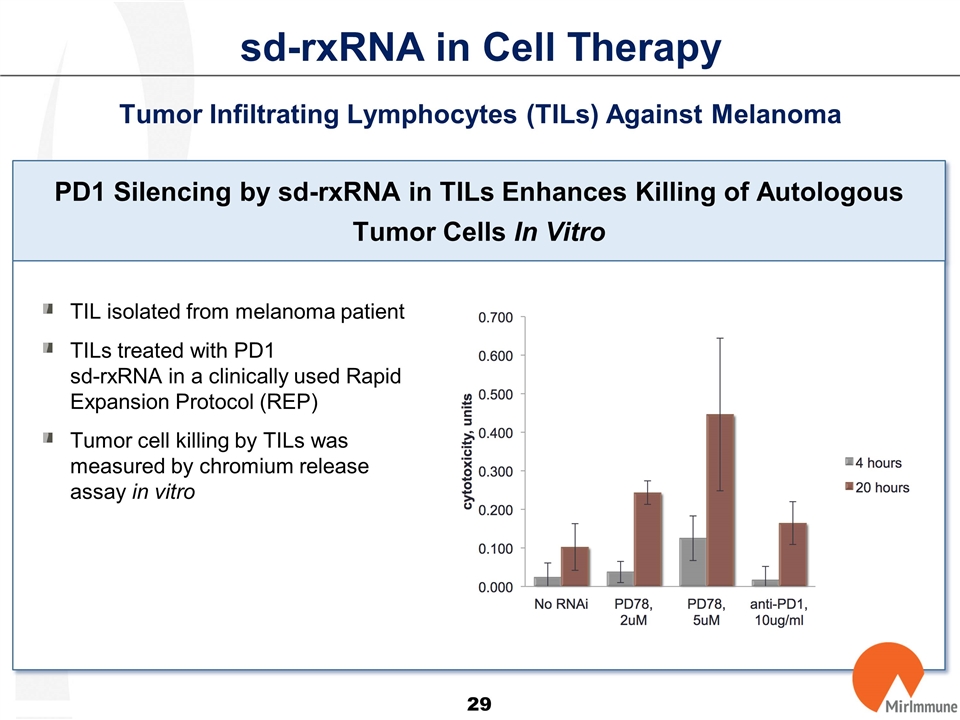
sd-rxRNA in Cell Therapy Tumor Infiltrating Lymphocytes (TILs) Against Melanoma PD1 Silencing by sd-rxRNA in TILs Enhances Killing of Autologous Tumor Cells In Vitro TIL isolated from melanoma patient TILs treated with PD1 sd-rxRNA in a clinically used Rapid Expansion Protocol (REP) Tumor cell killing by TILs was measured by chromium release assay in vitro

Management Team

Management Team Dr. Geert Cauwenbergh – President and Chief Executive Officer Served as Chief Executive Officer of Barrier Therapeutics, Inc. (formerly NASDAQ:BTRX) a biopharmaceutical company that he founded in 2001 and where he also served as Chairman. Barrier, which focused on dermatology drug development and commercialization, was acquired by Stiefel Laboratories, Inc. for $156 million. Held a number of ascending senior management positions at Johnson & Johnson (NYSE:JNJ), where he was employed for 23 years. As Vice President of Research and Development for Johnson & Johnson’s Skin Research Center, he was responsible for the worldwide research and development of all skin care products for the Johnson & Johnson consumer companies. Doctorate in Medical Sciences from the Catholic University of Leuven, Faculty of Medicine (Belgium), where he also completed his masters and undergraduate work. Dr. Alexey Eliseev – Chief Business Officer (post transaction) Founder and CEO of MirImmune, Inc. with over twenty years of experience in academia, biotechnology industry and venture capital. Co-founded the Therascope, later Alantos Pharmaceuticals, with a number of prominent founders including French Nobel Laureate Jean-Marie Lehn. He then became CTO of the company and President of its US division. Alantos was acquired by Amgen in 2007. Dr. Eliseev was also among the founders of AC Immune (Switzerland) and Boston BioCom LLC. Over the recent years he has worked with Maxwell Biotech Venture Fund as its Managing Director and ran the investment activity of the fund in the United States. PhD in Bioorganic Chemistry from Moscow State University and MBA from the MIT Sloan School of Management. Alexey has been working in the United States and Europe since 1992. Following three years of postdoctoral research in Germany and in the US, he joined the faculty at SUNY Buffalo in 1995 where he was awarded tenure in 2000.

Management Team Pamela Pavco, PhD – Chief Development Officer Served as the Senior Vice President of Pharmaceutical Development of Galena Biopharma, Inc. from March 2007 until April 2012. Over 20 years of research and development experience in oligonucleotides. Senior Director, R & D Project Management at Sirna Therapeutics, Inc., from 2002 until 2006, when it was acquired by Merck & Co., Inc. for $1.1 billion. Responsible for the discovery research and development of Sirna-027, the first chemically modified siRNA to enter clinical trials. Dr. Pavco also managed Sirna’s alliance with Allergan, Inc. that was initiated to continue discovery research in the area of ophthalmology and take Sirna-027 forward into Phase 2 clinical studies. Ph.D. in Biochemistry from Virginia Commonwealth University in 1983 and post-doctoral work at Duke University. Karen Bulock, PhD – Vice President of Research Served as the Associate Director of Research of Galena Biopharma, Inc. from October 2007 until April 2012. Dr. Bulock has over twenty years of experience in assay development and discovery project management. Managed several key programs, including the discovery and preclinical development of RXI-109, RXi’s first clinical candidate. Spent several years leading assay development and screening projects to support small molecule drug discovery programs in the fields of metabolic disease and anti-infectives at CytRx Corporation and Essential Therapeutics, Inc. Ph.D. in Pharmacology from Yale University.
Capitalization
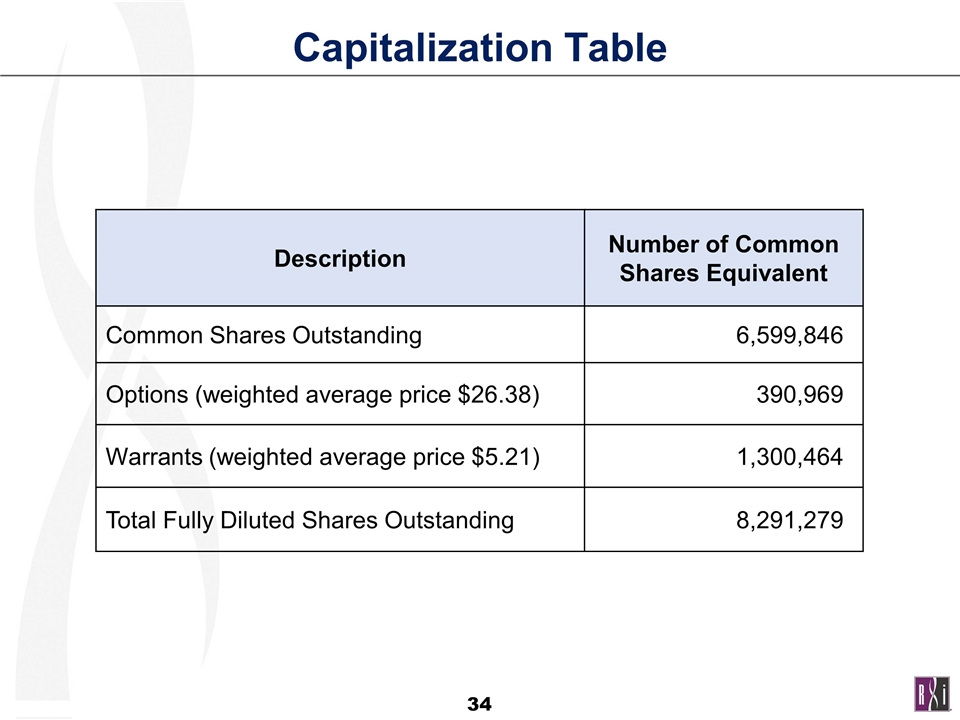
Capitalization Table Description Number of Common Shares Equivalent Common Shares Outstanding 6,599,846 Options (weighted average price $26.38) 390,969 Warrants (weighted average price $5.21) 1,300,464 Total Fully Diluted Shares Outstanding 8,291,279

RXi Pharmaceuticals NASDAQ: RXII Developing Innovative Therapeutics For a Better Life www.rxipharma.com


































