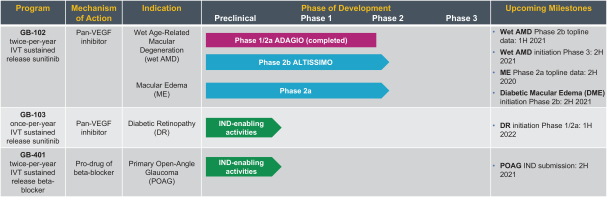
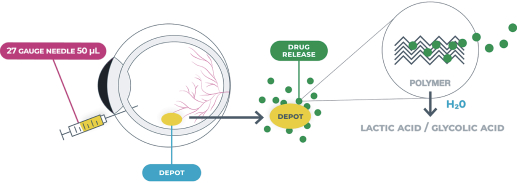
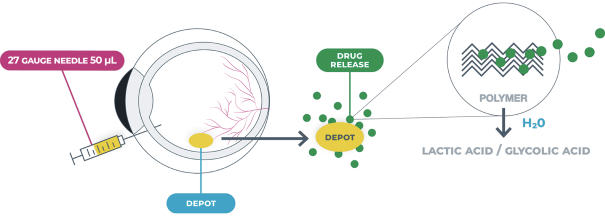
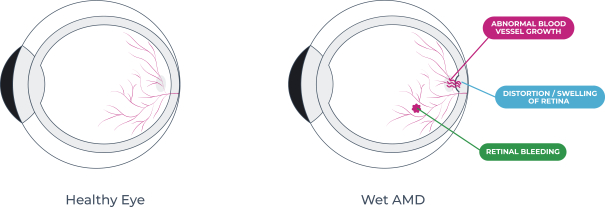
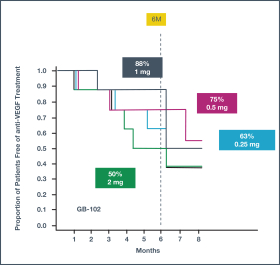
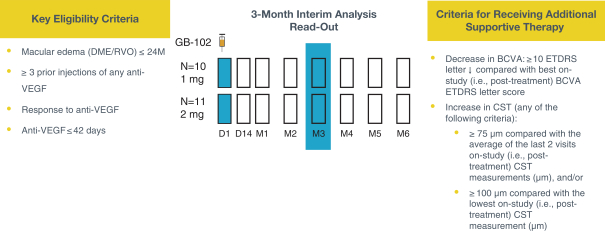
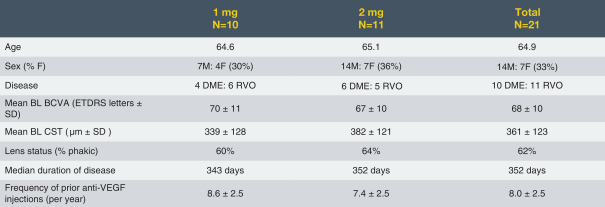
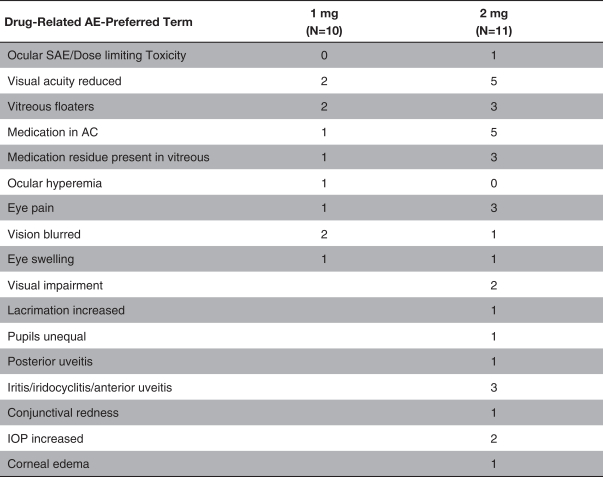
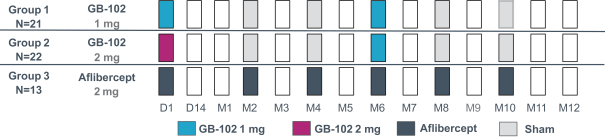
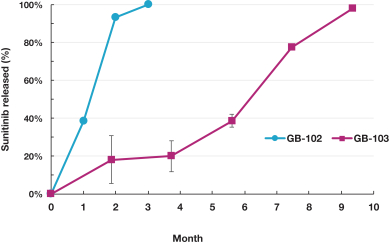
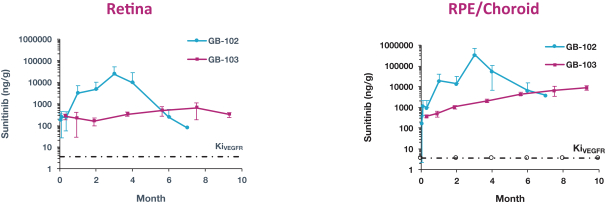
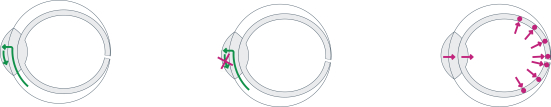
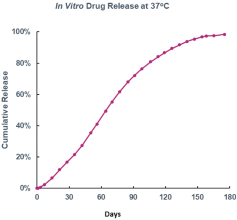
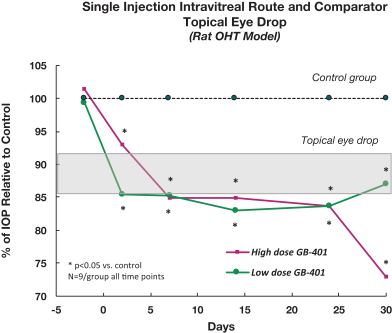
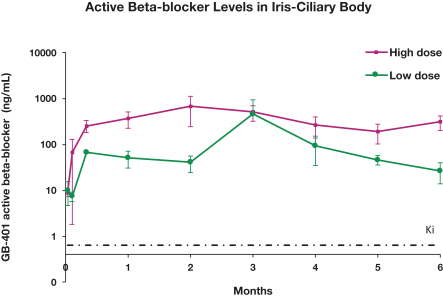
microparticles, and methods of use. Therefore, we have six patent families that cover GB-401 as a compound and in an aggregating microparticle, and their uses and manufacture.
In addition, the first patent family exclusively licensed from JHU described above that describes polymeric microparticles has claims that cover GB-401, pharmaceutical compositions of these microparticles, and methods of use.
Patent filings that cover additional novel prodrugs, compositions, uses and manufacture
Patent filings solely owned by us
We also own several patent families that cover additional novel prodrugs, pharmaceutical compositions and methods of use in the area of ocular therapy. We may or may not develop these inventions to commercial products, and we have the possibility to license undeveloped technologies to other entities where advantageous to us.
The seventh patent family owned by us covers an extensive number of novel prodrugs, including prodrugs of sunitinib, timolol, brinzolamide and dorzolamide, pharmaceutical compositions, and methods of use for ocular therapy. The family includes seven granted U.S. Patents (US 9,808,531; US 9,956,302; US 10,098,965; US 10,111,964; US 10,117,950; US 10,159,747; and US 10,485,876) and is currently pending in Australia, Brazil, Canada, China, Eurasia, Europe, Hong Kong, Israel, India, Japan and Mexico. The expected year of expiration for these composition-of-matter patents, where issued, valid and enforceable, is 2036, without regard to any extensions, adjustments or restorations of term that may be available under national law.
The eighth patent family covers prodrug derivatives of ethacrynic acid, timolol, brinzolamide and pharmaceutical compositions, and methods of use for ocular disorders, including the lowering of intraocular pressure. The patent family includes one U.S. pending application and corresponding applications in Australia, Canada, China, Europe, Japan and Russia. The expected year of expiration for these composition-of-matter patents, where issued, valid and enforceable, is 2038, without regard to any extensions, adjustments or restorations of term that may be available under national law.
The ninth patent family covers novel prodrugs of loop diuretics, pharmaceutical compositions, and methods of use for ocular disorders, including the lowering of intraocular pressure. This family includes one international application filed under the Patent Cooperation Treaty, PCT US 2019/029416 (which we intend to file in the United States and other selected foreign countries on or before the deadline to do so). The expected year of expiration for these composition-of-matter patents, where issued, valid and enforceable, is 2039, without regard to any extensions, adjustments or restorations of term that may be available under national law.
Our tenth patent family includes one international application filed under the Patent Cooperation Treaty, PCT/US19/53513 (which we intend to file in the United States and other selected foreign countries on or before the deadline to do so), and one patent application filed in Taiwan that covers additional novel prodrugs of sunitinib, brinzolamide, and dorzolamide, pharmaceutical compositions, and methods of use for ocular disorders. The expected year of expiration for these composition-of-matter patents, where issued, valid and enforceable, is 2039, without regard to any extensions, adjustments or restorations of term that may be available under national law.
Patent filings exclusively licensed from JHU
We have exclusively licensed a fourth patent family from JHU that covers hydrophobic-hydrophilic copolymers of HIF-1 inhibitors, pharmaceutical compositions, and method of use for ocular therapeutics. This patent family includes two granted U.S. Patents (US 8,962,577 and US 9,950,072) and corresponding applications in Australia, Canada, China, Eurasia, Europe, Hong Kong and Japan. The expected year of expiration for these composition-of-matter patents, where issued, valid and enforceable, is 2033, without regard to any extensions, adjustments or restorations of term that may be available under national law.
118