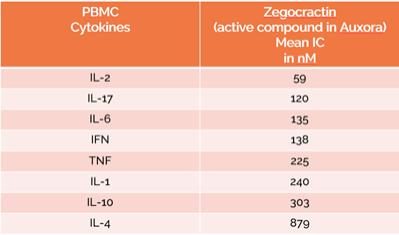
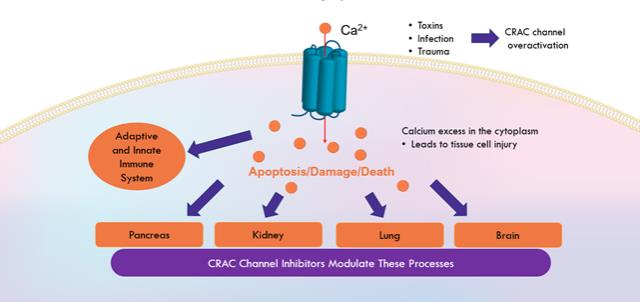
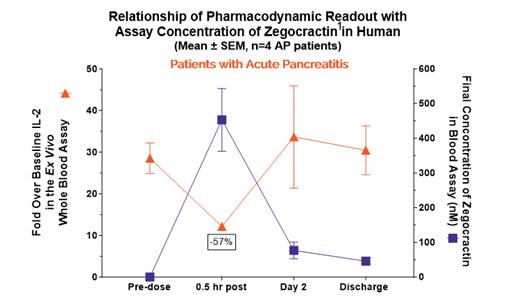
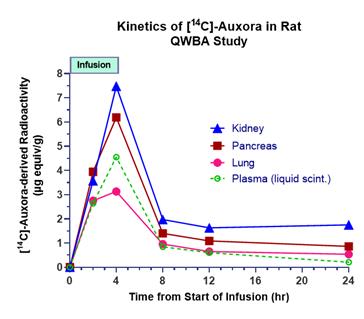
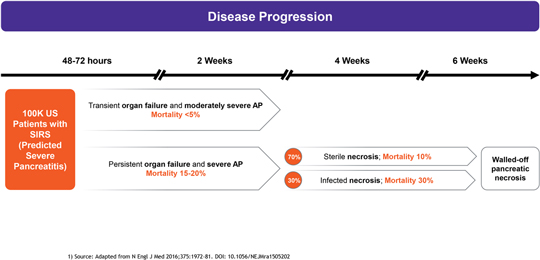
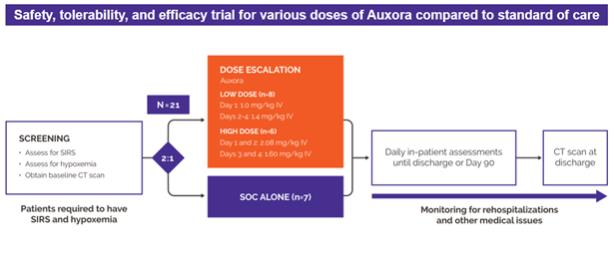
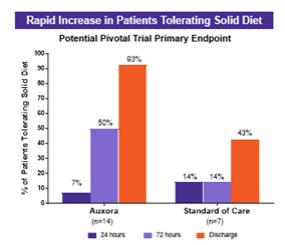
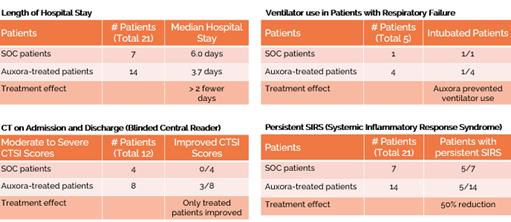
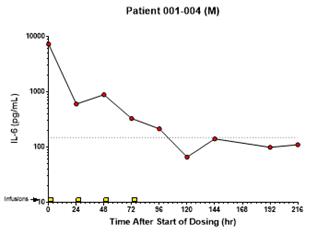
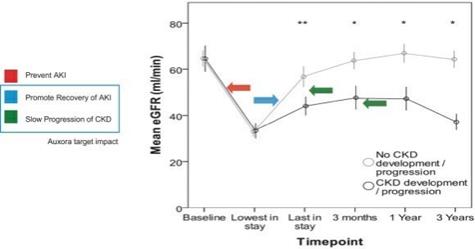
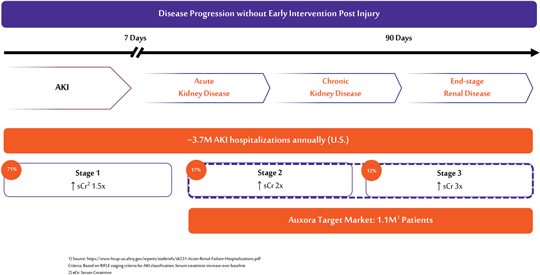
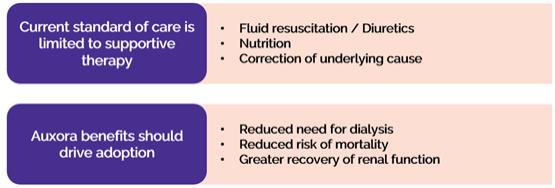
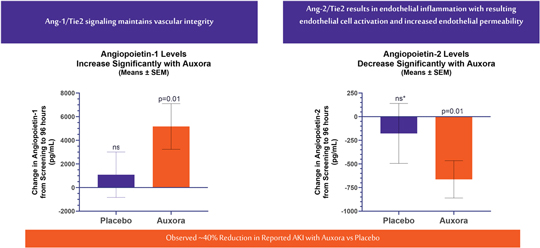
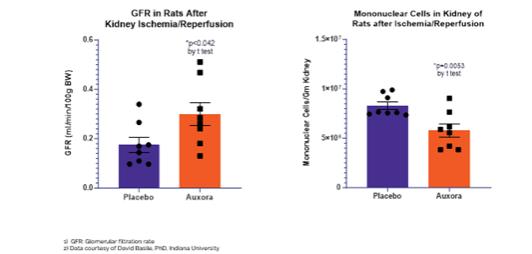
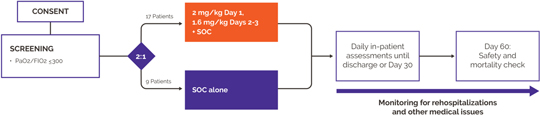
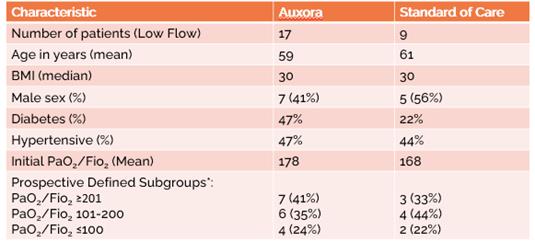
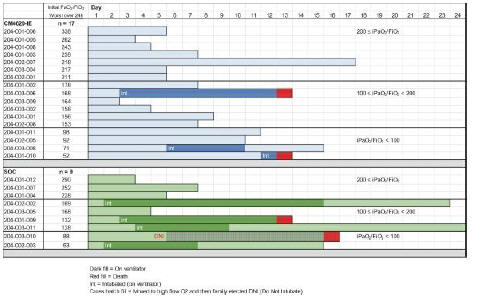
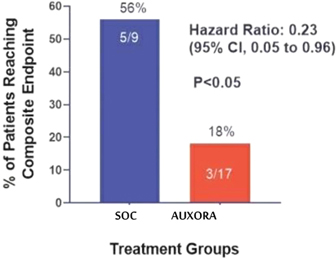
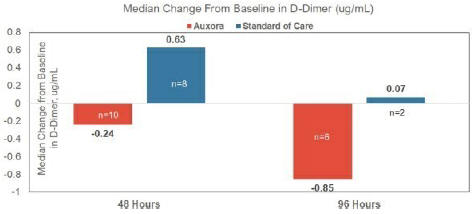
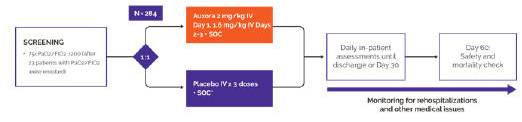
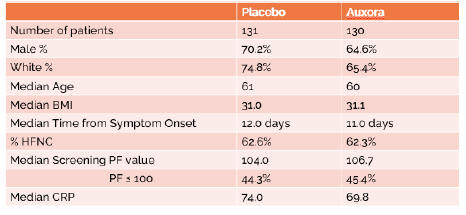
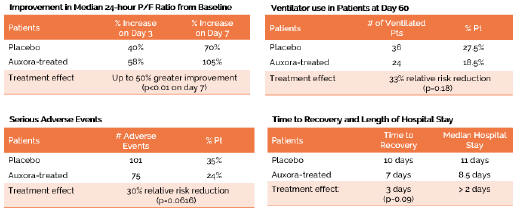
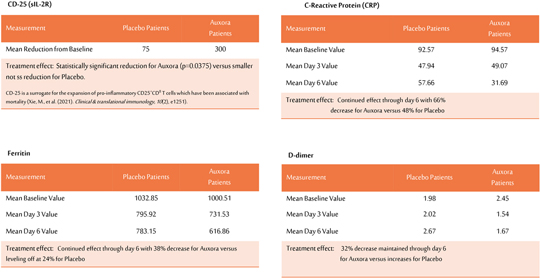
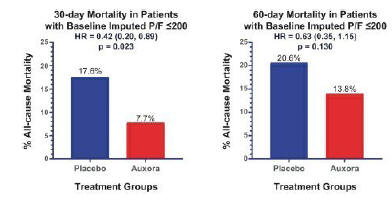
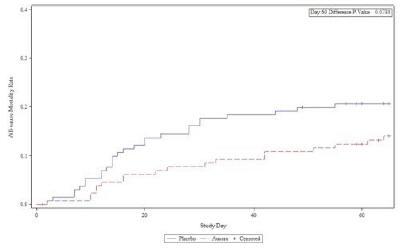
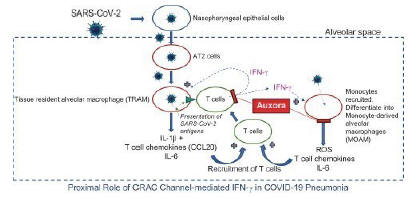
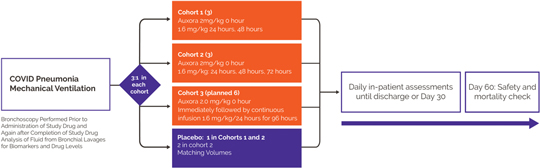
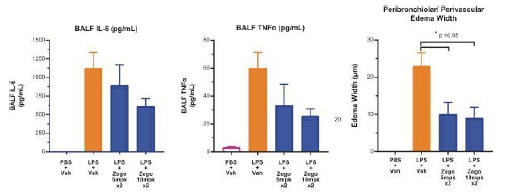
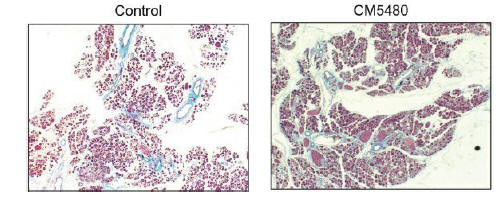
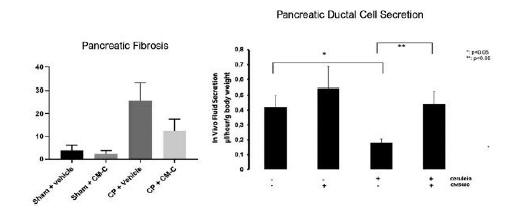
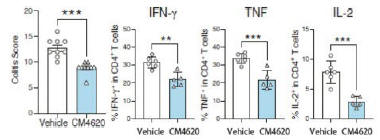
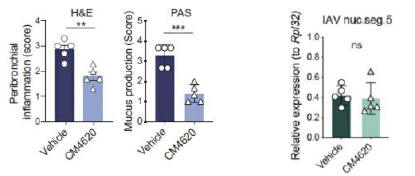
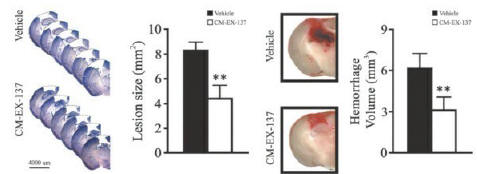
anakinra and dexamethasone are all approved under EUAs to treat severe COVID-19 pneumonia patients on oxygen. Sanofi’s sarilumab which has the same mechanism of action as tocilizumab has also been recommended for use in severe COVID-19 pneumonia by WHO. Some of these drugs as well as others currently in development for COVID-19 pneumonia may prove efficacious in broader respiratory failure, particularly respiratory failure caused by viral pneumonias.
Our commercial opportunity could be substantially limited in the event that our competitors develop and commercialize products that are more effective, safer, more convenient or cheaper than our product candidates. In geographies that are critical to our commercial success, competitors may also obtain regulatory approvals before us, resulting in our competitors building a strong market position in advance of our product’s entry. We believe the competitive factors that will determine the success of our programs will be the efficacy, safety, pricing and reimbursement, and convenience of our future product candidates.
Intellectual Property
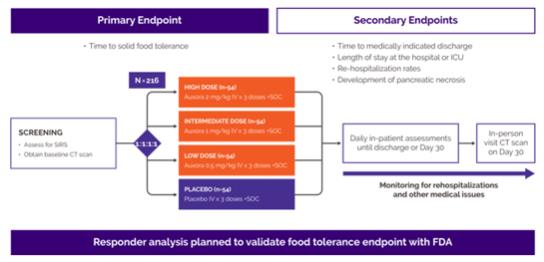
We have developed and continue to expand our patent portfolio for Auxora. As of July 31, 2023, we have issued patents and pending patent applications in the United States and other countries throughout the world directed to compositions of matter, various methods of use, formulations, and synthetic processes. For patents directed to compositions covering Auxora, we own three issued U.S. patents and 59 issued patents in the following jurisdictions: Argentina, Australia, Austria, Belgium, Bulgaria, Brazil, Canada, Czech Republic, Denmark, Finland, France, Germany, Greece, Hungary, Ireland, Israel, Italy, Japan, Luxembourg, Monaco, Netherlands, Norway, Poland, Portugal, Romania, Spain, Sweden, Slovakia, Switzerland, Turkey, United Kingdom, Eurasian Patent Organization, and Taiwan. We also have one pending U.S. patent application and seven pending patent applications in the following jurisdictions: Japan, Canada, China, India, and Korea directed to compositions covering Auxora. Composition of matter patents for our drug compound portfolio have expirations ranging from 2031 to 2036 with Auxora and other pre-clinical drugs having world-wide composition of matter patents to 2036, not including any patent term adjustment or any patent term extension.
For patents and patent applications directed to methods of using Auxora for the treatment of AP, as of July 31, 2023, we own four issued U.S. patents and 33 issued patents in the following jurisdictions: China, Japan, Australia the Eurasian Patent Organization, Austria, Belgium, Bulgaria, Canada, Czech Republic, Denmark, Finland, France, Germany, Greece, Hungary, Ireland, Italy, Japan, Luxembourg, Monaco, Netherlands, Norway, Poland, Portugal, Romania, Spain, Sweden, Slovakia, Switzerland, Turkey, United Kingdom, and Taiwan. We also own two U.S. patent applications, and fourteen pending patent applications in the following jurisdictions: Argentina, Australia, Canada, China, Europe, Hong Kong, Japan, and Taiwan for other indications. These issued patents and any patents issuing from pending U.S. and ex-U.S. applications are expected to expire between 2031-2041, not including any patent term adjustment or any patent term extension. We have also filed one international application directed to using a subject’s P/F ratio (the ratio of arterial oxygen pressure to fractional inspired oxygen) as a biomarker when treating acute lung injury and acute respiratory distress syndrome with Auxora. Any patents ultimately issuing from this international application are expected to expire around 2043, not including any patent term adjustment or patent term extension.
Additionally, we jointly own one issued U.S. patent, 13 issued patents in the following jurisdictions: Australia, Germany, France, United Kingdom, Belgium, Switzerland, Denmark, Ireland, Italy, Luxembourg, Netherlands, Sweden, and Japan directed to treatment for stroke and traumatic brain injury. We also own one pending patent application in Canada. These patents and any patents issuing from applications are expected to expire around 2036, not including any patent term adjustment or patent term extension. Also, we have filed one provisional application directed to treatment of non-alcoholic fatty liver disease using Auxora. Any patents ultimately issuing from this provision alapplication are expected to expire around 2044, not including any patent term adjustment or patent term extension.
Moreover, for patent protection directed to formulations and crystalline forms of Auxora, we have filed one U.S. patent application, one granted patent in Mexico, and eight pending applications in the following jurisdictions: Australia, Brazil, Canada, China, Europe, Japan, Korea, and Mexico. Any patents that ultimately issue from these patent applications are expected to expire around 2038, not including any patent term adjustment or patent term extension.
With respect to synthetic processes of Auxora, we have filed one U.S. patent application, and pending applications in the following jurisdictions: Canada, China, Europe, Japan, and Korea. Any patents ultimately issuing from these PCT applications are expected to expire around 2040, not including any patent term adjustment or any patent term extension.
Beyond patent coverage for Auxora, we have 22 issued U.S. patents, 39 issued ex-U.S. patents, one pending U.S. patent application, and four pending ex-U.S. patent applications directed to CRAC channel inhibitors and their uses.
In addition to patent protection, we rely on trade secret protection and know-how to expand our proprietary position around our chemistry, technology, and other discoveries and inventions that we consider important to our business.
Government Regulation and Product Approval
As a pharmaceutical company that operates in the United States, we are subject to extensive regulation. Government authorities in the United States (at the federal, state and local level) and in other countries extensively regulate, among other things, the research, development, testing, manufacturing, quality control, approval, labeling, packaging, storage, record-keeping, promotion, advertising, distribution, post-approval monitoring and reporting, marketing and export and import of drug products such as those we are developing. Product candidates that we develop must be approved by the FDA, before they may be legally marketed in the United States and by the appropriate foreign regulatory agency before they may be legally marketed in a foreign country. Generally, our activities in other countries will be subject to regulation that is similar in nature and scope as that imposed in the United States, although there can be important differences. Additionally, some significant aspects of regulation in Europe are addressed in a centralized way, but country-specific regulation remains essential in many respects.
U.S. Drug Development Process
In the United States, the FDA regulates drugs under the Federal Food, Drug and Cosmetic Act (“FDCA”), and its implementing regulations. A new drug must be approved by the FDA pursuant to a new drug application (“NDA”) before it may be legally marketed in the United States. Drugs are also subject to other federal, state and local statutes and regulations. The process of obtaining regulatory approvals and the subsequent compliance with appropriate federal, state, and local statutes and regulations require the expenditure of substantial time and financial resources. Failure to comply with the applicable U.S. requirements at any time during the product development process, approval process or after approval, may subject an applicant to administrative or judicial sanctions. Sanctions brought by the FDA and the Department of Justice (“DOJ”), or other governmental entities, could include, among other actions, refusal to approve pending applications, withdrawal of an approval, a clinical hold, warning letters, product recalls or withdrawals from the market, product seizures, total or partial suspension of production or distribution injunctions, fines, refusals of government contracts, restitution, disgorgement or civil or criminal penalties. Any agency or judicial enforcement action could have a material adverse effect on us.
27