
Exhibit 99.1 Developing Novel Therapies for Acute Inflammatory and Immunologic Diseases February 2024 1

Forward-Looking Statements This presentation contains forward-looking statements which include, but are not limited to, statements regarding CalciMedica’s business strategy and clinical development plans; the design and potential benefits of CalciMedica’s product candidates; CalciMedica’s ongoing and planned clinical trials; the timing for CalciMedica’s receipt and announcement of data from its clinical trials; the estimated patient populations and addressable market for CalciMedica’s product candidates; and expectations regarding CalciMedica’s cash runway. These forward-looking statements are subject to the safe harbor provisions under the Private Securities Litigation Reform Act of 1995. CalciMedica’s expectations and beliefs regarding these matters may not materialize. Actual outcomes and results may differ materially from those contemplated by these forward-looking statements as a result of uncertainties, risks, and changes in circumstances, including but not limited to risks and uncertainties related to: the impact of fluctuations in global financial markets on CalciMedica’s business and the actions it may take in response thereto; CalciMedica’s ability to execute its plans and strategies; the ability to obtain and maintain regulatory approval for CalciMedica’s product candidates; results from clinical trials may not be indicative of results that may be observed in the future; potential safety and other complications from CalciMedica’s product candidates; economic, business, competitive, and/or regulatory factors affecting the business of CalciMedica generally; CalciMedica’s ability to protect its intellectual property position; and the impact of government laws and regulations. Additional risks and uncertainties that could cause actual outcomes and results to differ materially from those contemplated by the forward-looking statements are included under the caption “Risk Factors” in CalciMedica’s most recently filed periodic report, and subsequent periodic reports filed by CalciMedica, under the Securities Exchange Act of 1934, as amended, from time to time and available at www.sec.gov. These documents can be accessed on CalciMedica’s web page at calcimedica.com. These forward-looking statements are based on information available to, and expectations of, CalciMedica of the date of this presentation. CalciMedica disclaims any obligation to update these forward-looking statements, except as may be required by law. 2
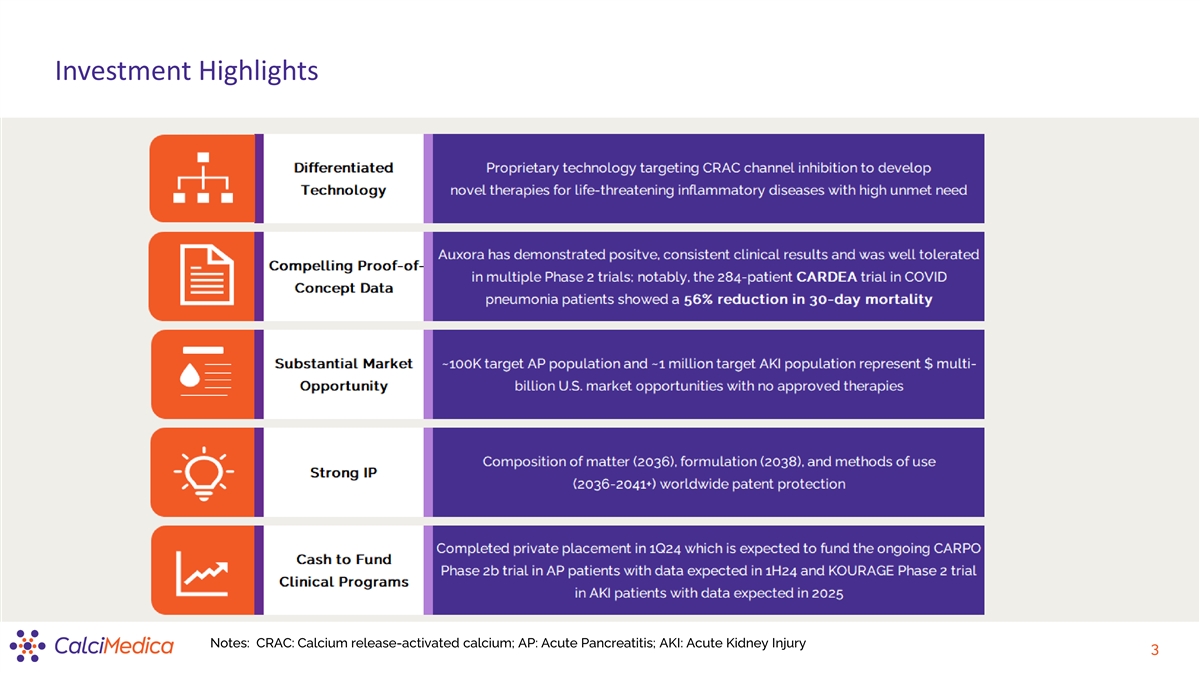
Investment Highlights Notes: CRAC: Calcium release-activated calcium; AP: Acute Pancreatitis; AKI: Acute Kidney Injury 3
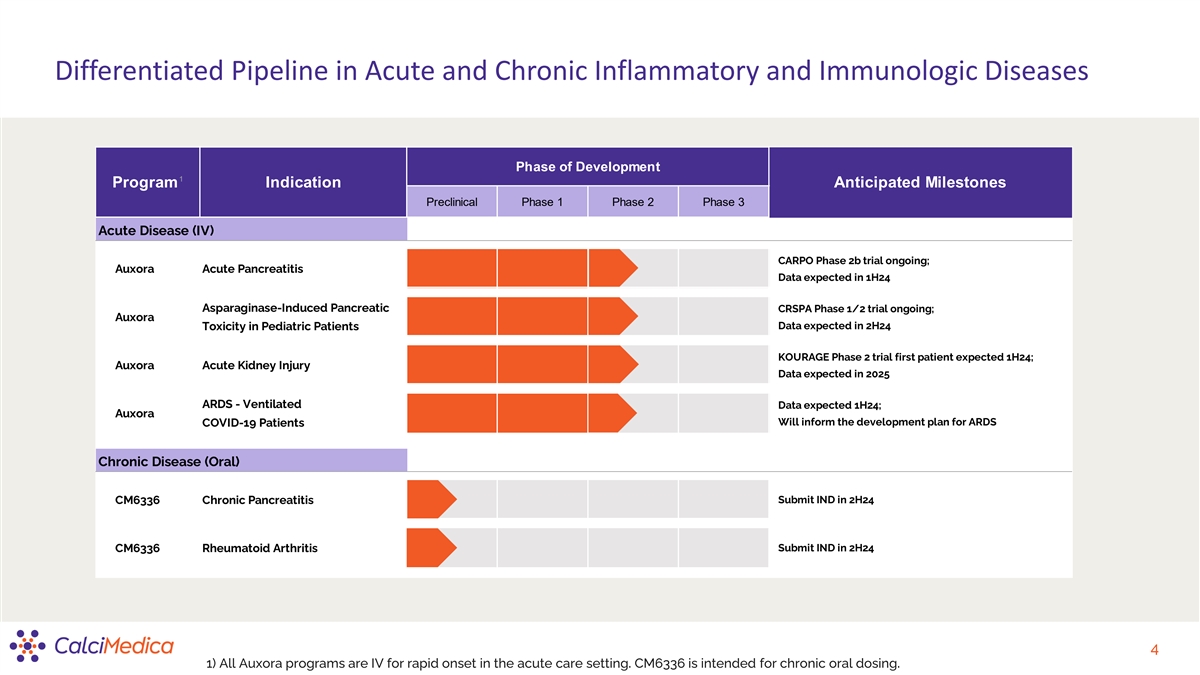
Differentiated Pipeline in Acute and Chronic Inflammatory and Immunologic Diseases Phase of Development 1 Program Indication Anticipated Milestones Preclinical Phase 1 Phase 2 Phase 3 Acute Disease (IV) CARPO Phase 2b trial ongoing; Auxora Acute Pancreatitis Data expected in 1H24 Asparaginase-Induced Pancreatic CRSPA Phase 1/2 trial ongoing; Auxora Toxicity in Pediatric Patients Data expected in 2H24 KOURAGE Phase 2 trial first patient expected 1H24; Auxora Acute Kidney Injury Data expected in 2025 ARDS - Ventilated Data expected 1H24; Auxora Will inform the development plan for ARDS COVID-19 Patients Chronic Disease (Oral) CM6336 Chronic Pancreatitis Submit IND in 2H24 Submit IND in 2H24 CM6336 Rheumatoid Arthritis 4 1) All Auxora programs are IV for rapid onset in the acute care setting. CM6336 is intended for chronic oral dosing.
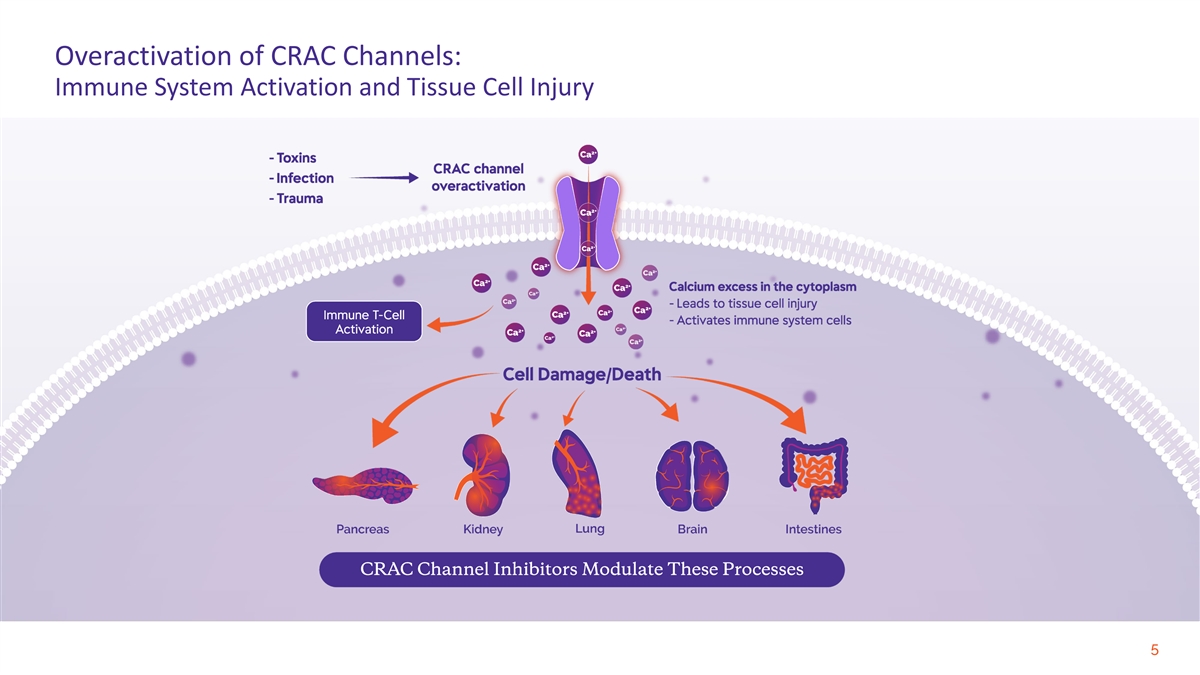
Overactivation of CRAC Channels: Immune System Activation and Tissue Cell Injury 5
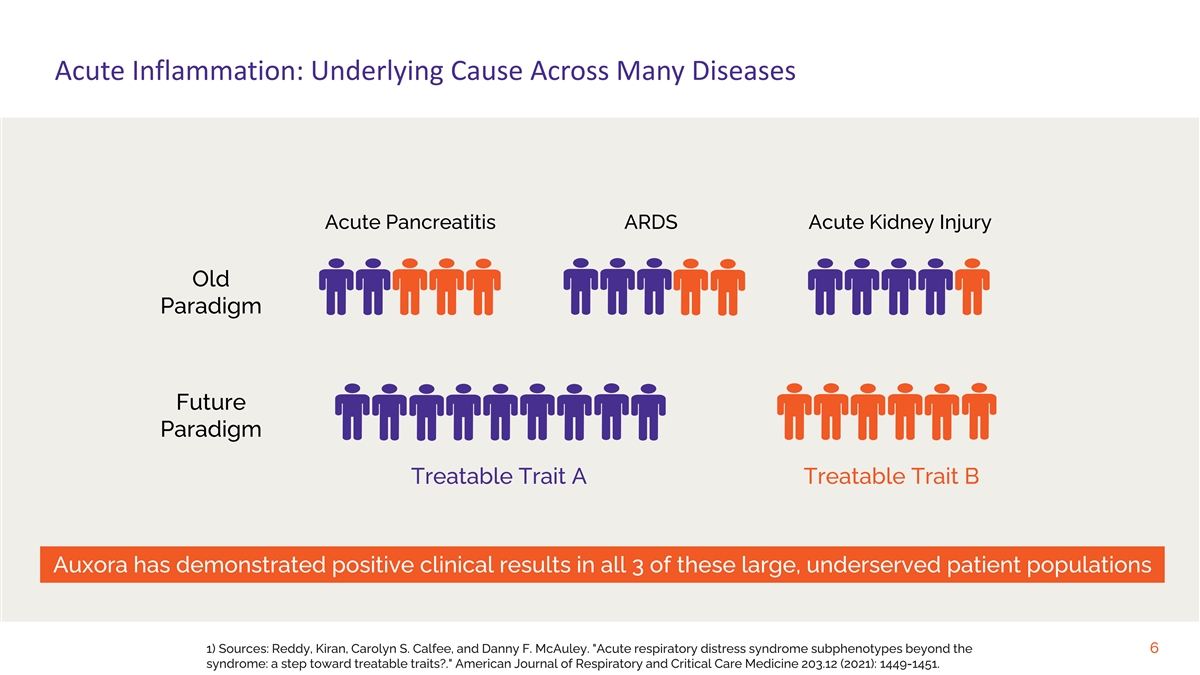
Acute Inflammation: Underlying Cause Across Many Diseases Acute Pancreatitis ARDS Acute Kidney Injury Old Paradigm Future Paradigm Treatable Trait A Treatable Trait B Auxora has demonstrated positive clinical results in all 3 of these large, underserved patient populations 1) Sources: Reddy, Kiran, Carolyn S. Calfee, and Danny F. McAuley. Acute respiratory distress syndrome subphenotypes beyond the 6 syndrome: a step toward treatable traits?. American Journal of Respiratory and Critical Care Medicine 203.12 (2021): 1449-1451.
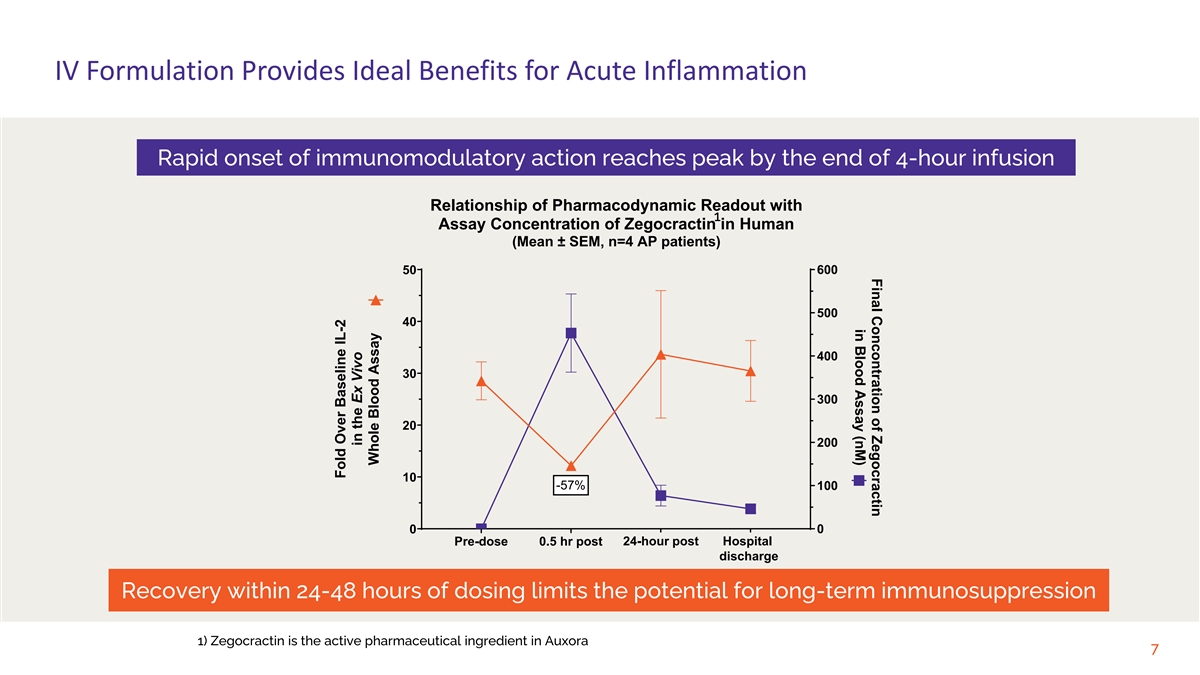
Final Concontration of Zegocractin in Blood Assay (nM) IV Formulation Provides Ideal Benefits for Acute Inflammation Rapid onset of immunomodulatory action reaches peak by the end of 4-hour infusion Relationship of Pharmacodynamic Readout with 1 Assay Concentration of Zegocractin in Human (Mean ± SEM, n=4 AP patients) 600 50 500 40 400 30 300 20 200 10 -57% 100 0 0 24-hour post Hospital Pre-dose 0.5 hr post Day 2 Discharge discharge Recovery within 24-48 hours of dosing limits the potential for long-term immunosuppression 1) Zegocractin is the active pharmaceutical ingredient in Auxora 7 Fold Over Baseline IL-2 in the Ex Vivo Whole Blood Assay
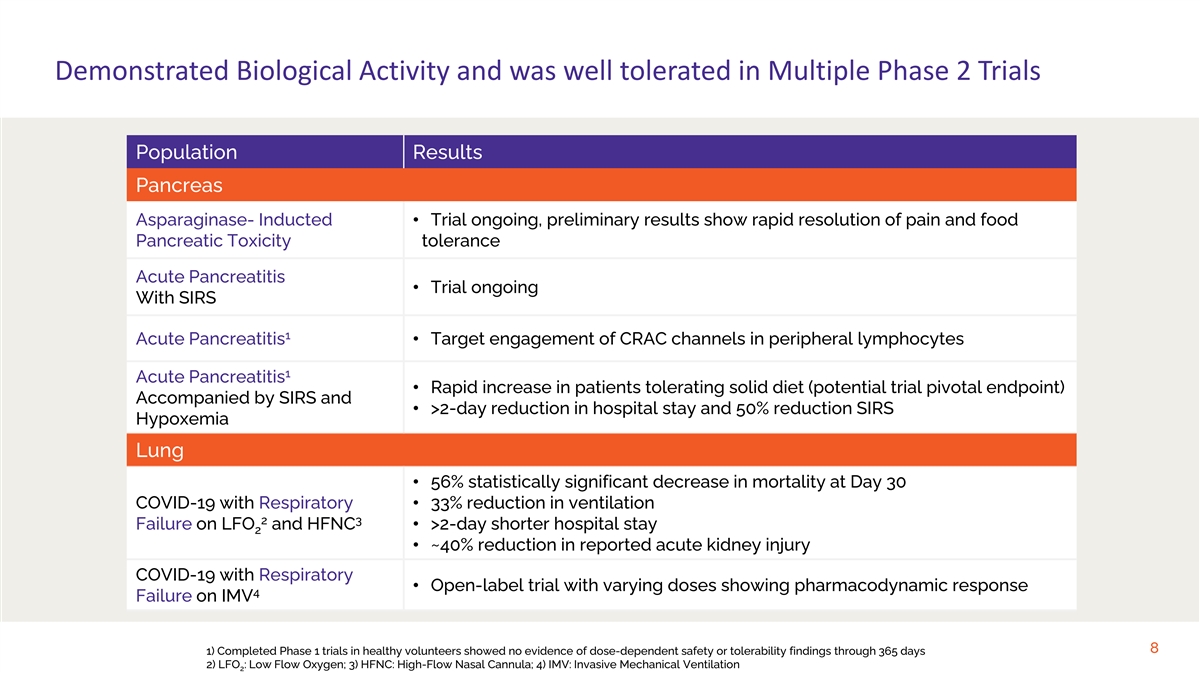
Demonstrated Biological Activity and was well tolerated in Multiple Phase 2 Trials Population Results Pancreas Asparaginase- Inducted • Trial ongoing, preliminary results show rapid resolution of pain and food Pancreatic Toxicity tolerance Acute Pancreatitis • Trial ongoing With SIRS 1 Acute Pancreatitis • Target engagement of CRAC channels in peripheral lymphocytes 1 Acute Pancreatitis • Rapid increase in patients tolerating solid diet (potential trial pivotal endpoint) Accompanied by SIRS and • >2-day reduction in hospital stay and 50% reduction SIRS Hypoxemia Lung • 56% statistically significant decrease in mortality at Day 30 COVID-19 with Respiratory • 33% reduction in ventilation 2 3 Failure on LFO and HFNC • >2-day shorter hospital stay 2 • ~40% reduction in reported acute kidney injury COVID-19 with Respiratory • Open-label trial with varying doses showing pharmacodynamic response 4 Failure on IMV 8 1) Completed Phase 1 trials in healthy volunteers showed no evidence of dose-dependent safety or tolerability findings through 365 days 2) LFO : Low Flow Oxygen; 3) HFNC: High-Flow Nasal Cannula; 4) IMV: Invasive Mechanical Ventilation 2
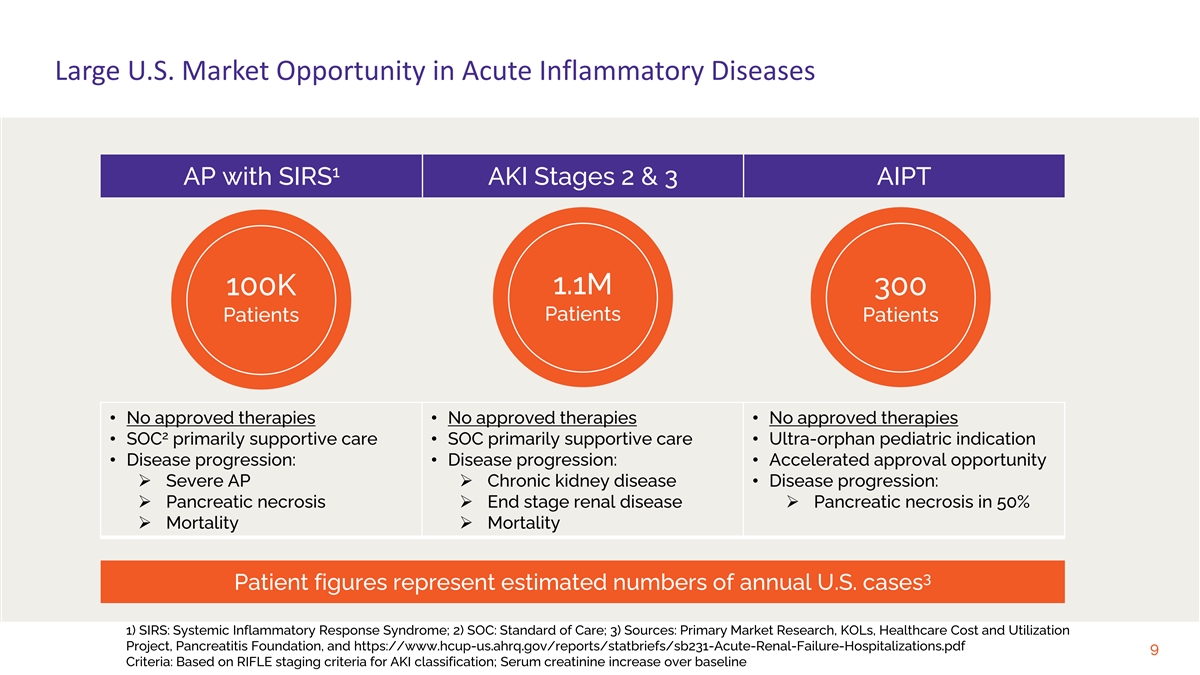
Large U.S. Market Opportunity in Acute Inflammatory Diseases 1 AP with SIRS AKI Stages 2 & 3 AIPT 100K 1.1M 300 Patients Patients Patients • No approved therapies • No approved therapies • No approved therapies 2 • SOC primarily supportive care • SOC primarily supportive care • Ultra-orphan pediatric indication • Disease progression: • Disease progression: • Accelerated approval opportunity Ø Severe APØ Chronic kidney disease • Disease progression: Ø Pancreatic necrosisØ End stage renal diseaseØ Pancreatic necrosis in 50% Ø MortalityØ Mortality 3 Patient figures represent estimated numbers of annual U.S. cases 1) SIRS: Systemic Inflammatory Response Syndrome; 2) SOC: Standard of Care; 3) Sources: Primary Market Research, KOLs, Healthcare Cost and Utilization Project, Pancreatitis Foundation, and https://www.hcup-us.ahrq.gov/reports/statbriefs/sb231-Acute-Renal-Failure-Hospitalizations.pdf 9 Criteria: Based on RIFLE staging criteria for AKI classification; Serum creatinine increase over baseline

Auxora for Acute Pancreatitis (AP) 10

AP Population: Significant Unmet Need U.S. Hospitalizations per Year from AP: ~275,000 ~40% of patients have SIRS at presentation High risk for moderate to severe disease Patients with SIRS+: ~110,000 Small percentage of patients missed Misdiagnosis, timing constraint, or other Target Patients: ~100,000 Target population is in-hospital patients with SIRS; currently no approved therapy 11 1) Source: Fletcher Spaght Market Research Report (2016)
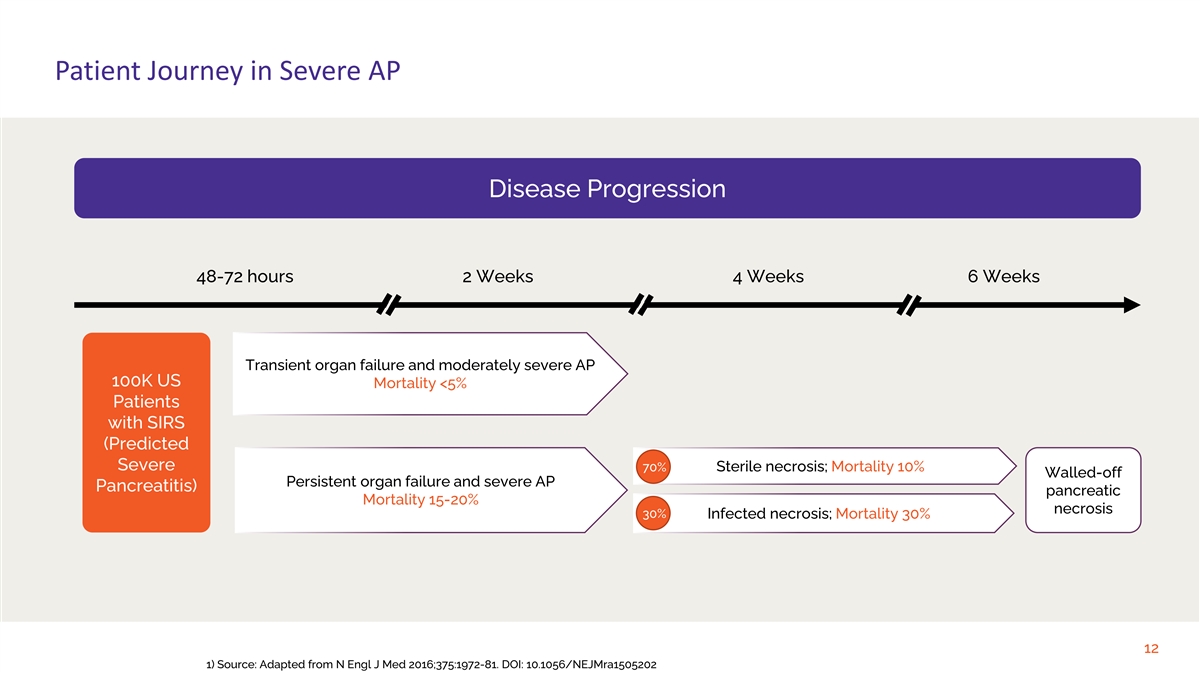
Patient Journey in Severe AP Disease Progression 48-72 hours 2 Weeks 4 Weeks 6 Weeks Transient organ failure and moderately severe AP 100K US Mortality <5% Patients with SIRS (Predicted Severe 70% Sterile necrosis; Mortality 10% Walled-off Persistent organ failure and severe AP Pancreatitis) pancreatic Mortality 15-20% necrosis 30% Infected necrosis; Mortality 30% 12 1) Source: Adapted from N Engl J Med 2016;375:1972-81. DOI: 10.1056/NEJMra1505202
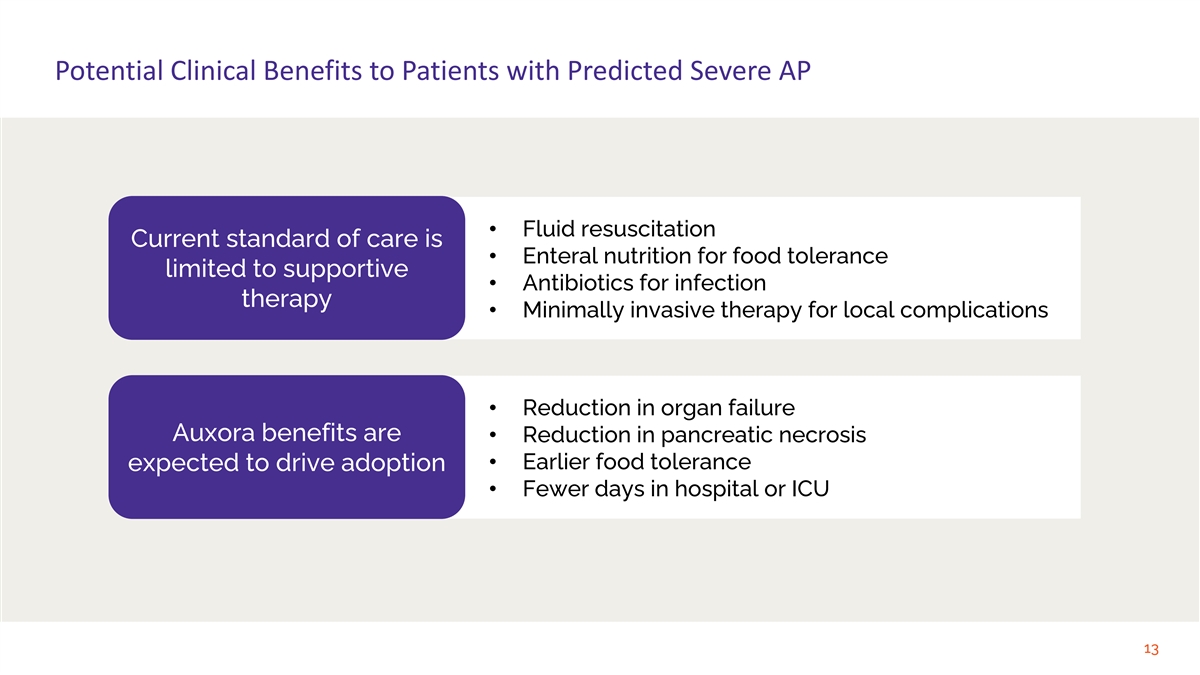
Potential Clinical Benefits to Patients with Predicted Severe AP • Fluid resuscitation Current standard of care is • Enteral nutrition for food tolerance limited to supportive • Antibiotics for infection therapy • Minimally invasive therapy for local complications • Reduction in organ failure Auxora benefits are • Reduction in pancreatic necrosis • Earlier food tolerance expected to drive adoption • Fewer days in hospital or ICU 13

AP Phase 2a Clinical Trial Safety, tolerability, and efficacy trial for various doses of Auxora compared to standard of care 14

Positive Phase 2a Results on Potential Pivotal Trial Primary Endpoints Rapid Increase in Patients Tolerating Solid Diet >2 Fewer Days Spent in Hospital Potential Pivotal Trial Primary Endpoint Median Hospital Stay SOC patients (n=7) 6.0 days 100 93% Auxora-treated patients (n=14) 3.7 days 90 80 1 Only Auxora Patients Improved on CTSI Scores 70 1 Moderate to Severe CTSI Scores 60 SOC patients (n=4) 0/4 (0%) 50% 50 43% Auxora-treated patients (n=8) 3/8 (38%) 40 30 50% Reduction in Persistent SIRS 20 14% 14% 7% Patients with Persistent SIRS 10 SOC patients (n=7) 5/7 (71%) 0 Auxora Standard of Care Auxora-treated patients (n=14) 5/14 (36%) (n=14) (n=7) 24 hours 72 hours Discharge 1) CTSI: CT Severity Index 15 % of Patients Tolerating Solid Diet
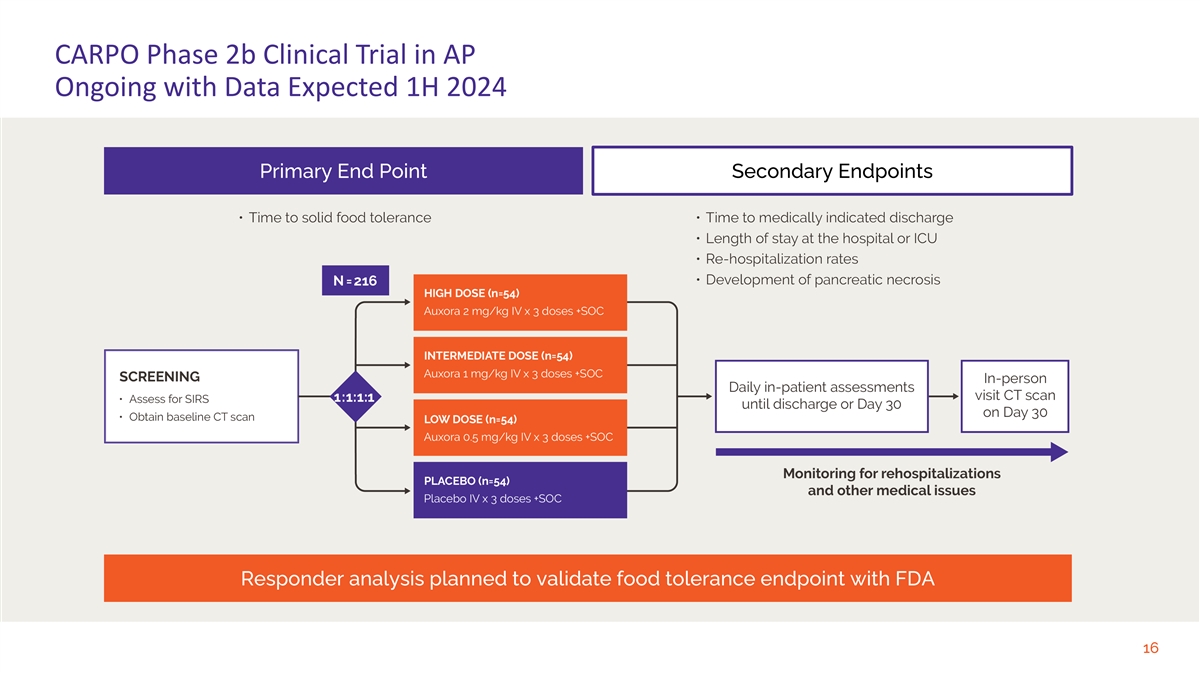
CARPO Phase 2b Clinical Trial in AP Ongoing with Data Expected 1H 2024 Primary End Point Secondary Endpoints Responder analysis planned to validate food tolerance endpoint with FDA 16
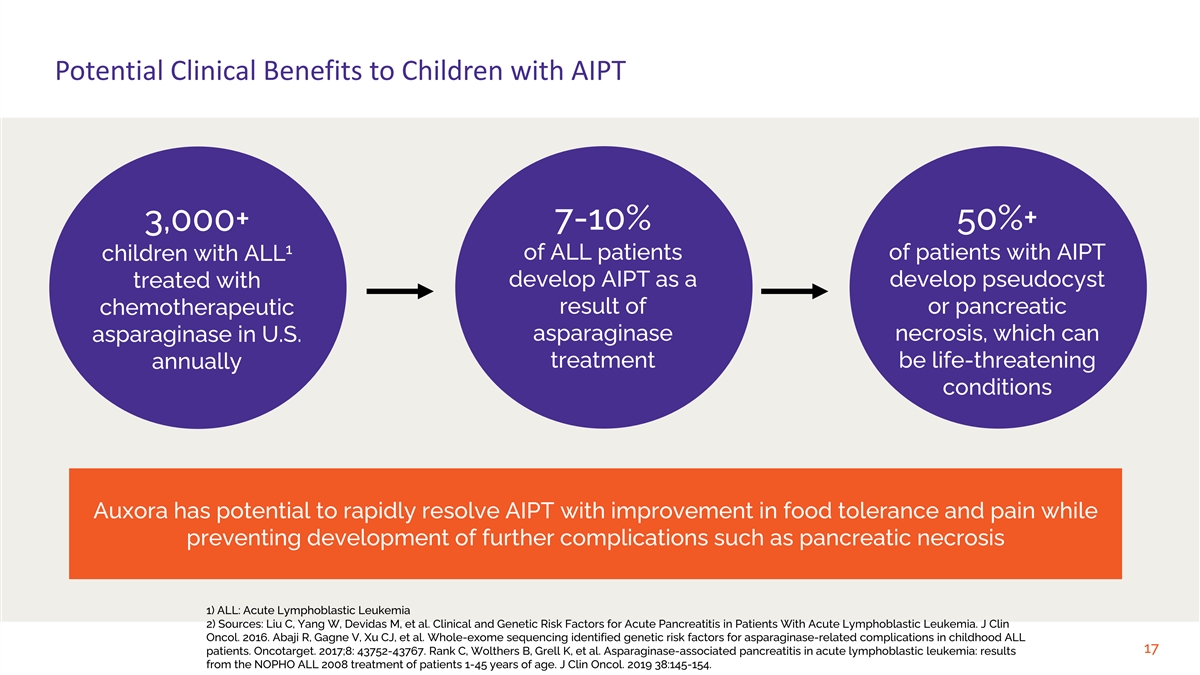
Potential Clinical Benefits to Children with AIPT 7-10% 50%+ 3,000+ 1 of ALL patients of patients with AIPT children with ALL treated with develop AIPT as a develop pseudocyst result of or pancreatic chemotherapeutic asparaginase necrosis, which can asparaginase in U.S. treatment be life-threatening annually conditions Auxora has potential to rapidly resolve AIPT with improvement in food tolerance and pain while preventing development of further complications such as pancreatic necrosis 1) ALL: Acute Lymphoblastic Leukemia 2) Sources: Liu C, Yang W, Devidas M, et al. Clinical and Genetic Risk Factors for Acute Pancreatitis in Patients With Acute Lymphoblastic Leukemia. J Clin Oncol. 2016. Abaji R, Gagne V, Xu CJ, et al. Whole-exome sequencing identified genetic risk factors for asparaginase-related complications in childhood ALL 17 patients. Oncotarget. 2017;8: 43752-43767. Rank C, Wolthers B, Grell K, et al. Asparaginase-associated pancreatitis in acute lymphoblastic leukemia: results from the NOPHO ALL 2008 treatment of patients 1-45 years of age. J Clin Oncol. 2019 38:145-154.
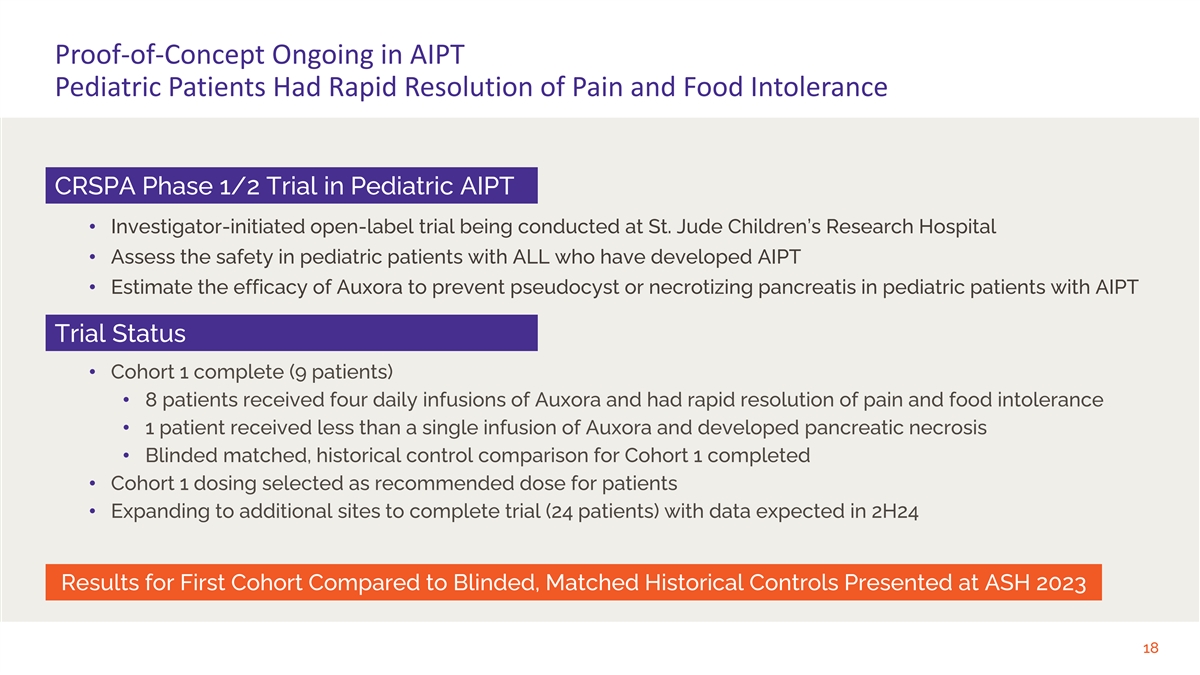
Proof-of-Concept Ongoing in AIPT Pediatric Patients Had Rapid Resolution of Pain and Food Intolerance CRSPA Phase 1/2 Trial in Pediatric AIPT • Investigator-initiated open-label trial being conducted at St. Jude Children’s Research Hospital • Assess the safety in pediatric patients with ALL who have developed AIPT • Estimate the efficacy of Auxora to prevent pseudocyst or necrotizing pancreatis in pediatric patients with AIPT Trial Status • Cohort 1 complete (9 patients) • 8 patients received four daily infusions of Auxora and had rapid resolution of pain and food intolerance • 1 patient received less than a single infusion of Auxora and developed pancreatic necrosis • Blinded matched, historical control comparison for Cohort 1 completed • Cohort 1 dosing selected as recommended dose for patients • Expanding to additional sites to complete trial (24 patients) with data expected in 2H24 Results for First Cohort Compared to Blinded, Matched Historical Controls Presented at ASH 2023 18

CRSPA First Cohort Data: Presented at ASH 2023 Total 16 (T16): All AIPT Matched T16 AIPT cohort CRSPA evaluable for efficacy Patients with AIPT 51 16 8 Age: mean (range) 10.3 (2.2-19.4) 9 (2.2-18.4) 8.2 (3.1-17.6) Female (%) 17 (33.3%) 5 (31.3%) 3 (37.5%) Low-risk therapy (%) 9 (17.6%) 1 (6.3%) 2 (25%) Hospital days (range) 12.1 (2-70) 13.4 (2-27) 6.3 (5-8) ICU needed (%) 11 (21.6%) 3 (18.8%) 1 (12.5%) ICU days mean (range) 5.1 (1-9) 5 (3-7) 3 TPN needed (%) 27 (52.9%) 11 (68.8%) 0 TPN days mean (range) 37.7 (3-153) 27.2 (4-63) NA ≥30% pancreatic necrosis (%) NA 4 (26.7%) * 0 CTSI mean (range) NA 5.4 (0-10) * 2.4 (0-4) CTSI ≥ 7 (%) NA 4 (26.7%) * 0 *One patient in matched T16 cohort was unable to be evaluated for pancreatic necrosis or a CTSI score CTSI score definitions: 0-3 mild acute pancreatitis, 4-6 moderately severe acute pancreatitis, ≥7 severe acute pancreatitis Source: Seth Karol et al., Zegocractin to reduce the severity of asparagine-associated pancreatitis in children with acute lymphoblastic leukemia: results of 19 the Phase 1 portion of the CRSPA study, ASH Poster #2837, December 2023.

Auxora for Acute Kidney Injury (AKI) 20
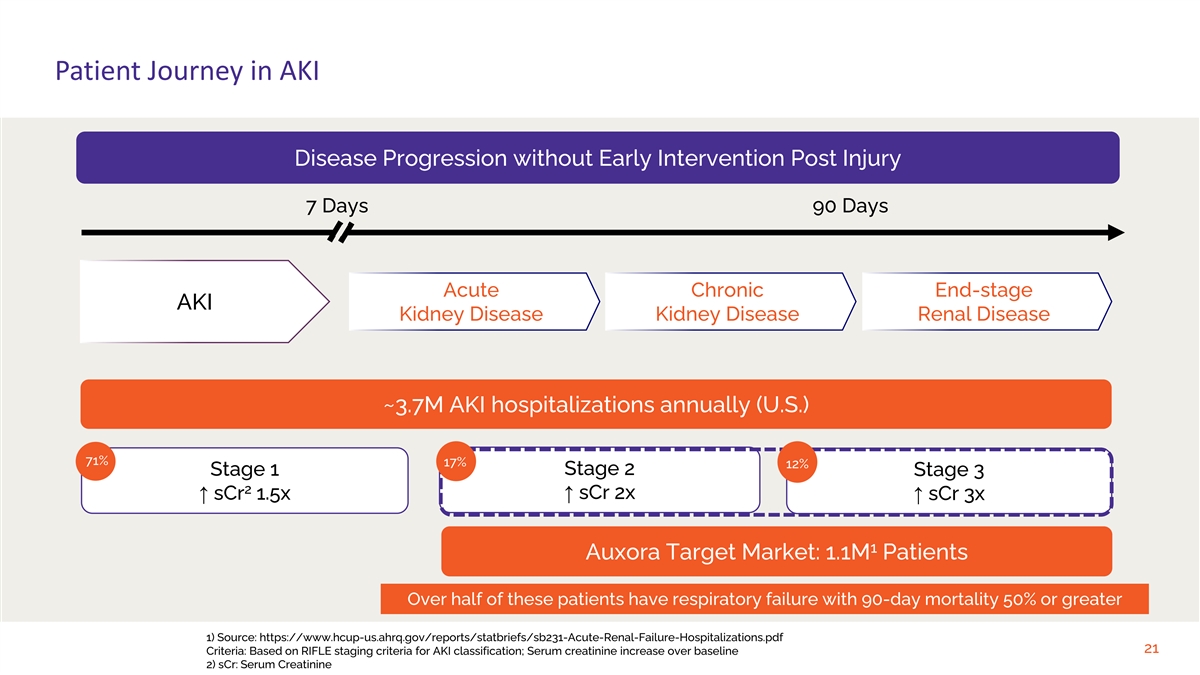
Patient Journey in AKI Disease Progression without Early Intervention Post Injury 7 Days 90 Days Acute Chronic End-stage AKI Kidney Disease Kidney Disease Renal Disease ~3.7M AKI hospitalizations annually (U.S.) 71% 17% 12% Stage 2 Stage 1 Stage 3 2 ↑ sCr 2x ↑ sCr 1.5x↑ sCr 3x 1 Auxora Target Market: 1.1M Patients Over half of these patients have respiratory failure with 90-day mortality 50% or greater 1) Source: https://www.hcup-us.ahrq.gov/reports/statbriefs/sb231-Acute-Renal-Failure-Hospitalizations.pdf 21 Criteria: Based on RIFLE staging criteria for AKI classification; Serum creatinine increase over baseline 2) sCr: Serum Creatinine
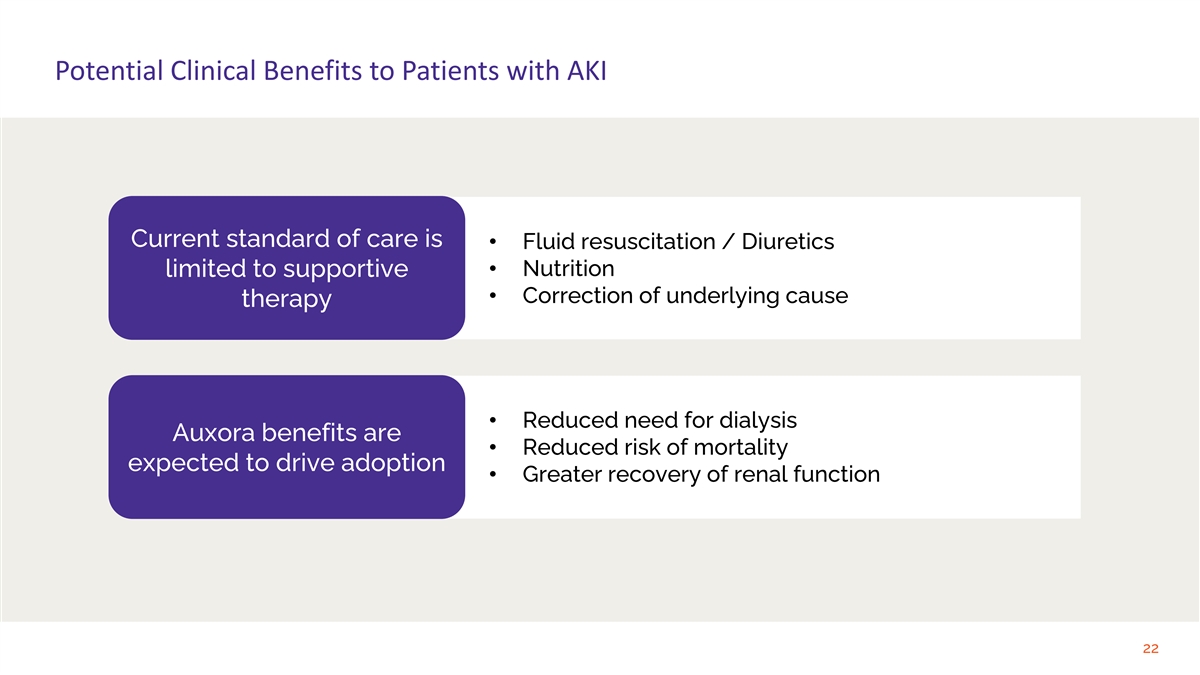
Potential Clinical Benefits to Patients with AKI Current standard of care is • Fluid resuscitation / Diuretics • Nutrition limited to supportive • Correction of underlying cause therapy • Reduced need for dialysis Auxora benefits are • Reduced risk of mortality expected to drive adoption • Greater recovery of renal function 22
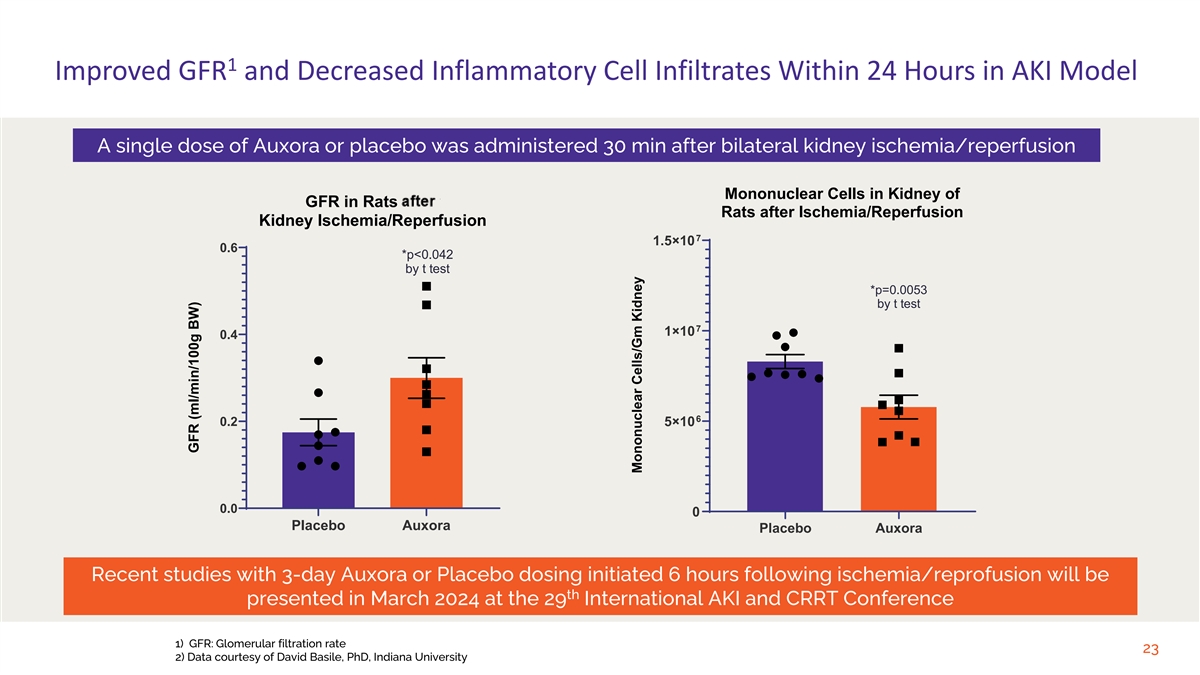
1 Improved GFR and Decreased Inflammatory Cell Infiltrates Within 24 Hours in AKI Model A single dose of Auxora or placebo was administered 30 min after bilateral kidney ischemia/reperfusion Mononuclear Cells in Kidney of GFR in Rats After Rats after Ischemia/Reperfusion Kidney Ischemia/Reperfusion 7 1.5×10 0.6 *p<0.042 by t test *p=0.0053 by t test 7 1×10 0.4 6 0.2 5×10 0.0 0 Placebo Auxora Placebo Auxora Recent studies with 3-day Auxora or Placebo dosing initiated 6 hours following ischemia/reprofusion will be th presented in March 2024 at the 29 International AKI and CRRT Conference 1) GFR: Glomerular filtration rate 23 2) Data courtesy of David Basile, PhD, Indiana University GFR (ml/min/100g BW) Mononuclear Cells/Gm Kidney
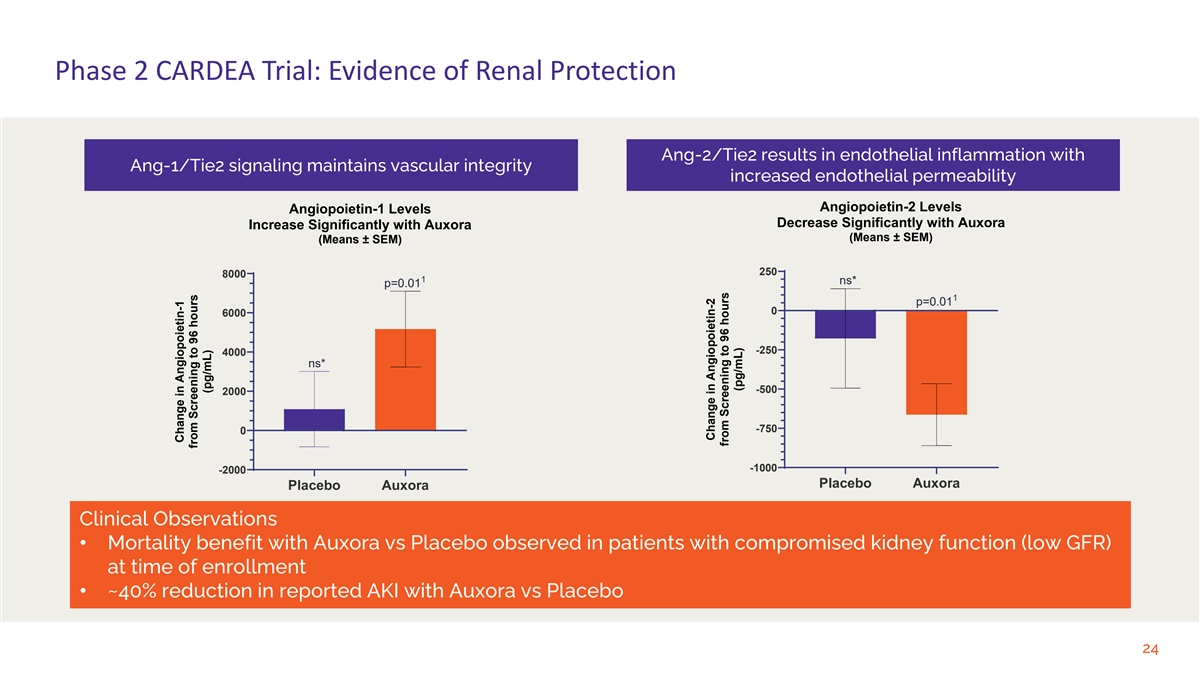
Phase 2 CARDEA Trial: Evidence of Renal Protection Ang-2/Tie2 results in endothelial inflammation with Ang-1/Tie2 signaling maintains vascular integrity increased endothelial permeability Angiopoietin-2 Levels Angiopoietin-1 Levels Decrease Significantly with Auxora Increase Significantly with Auxora (Means ± SEM) (Means ± SEM) 250 8000 1 ns* p=0.01 1 p=0.01 0 6000 -250 4000 ns* -500 2000 -750 0 -1000 -2000 Placebo Auxora Placebo Auxora Clinical Observations • Mortality benefit with Auxora vs Placebo observed in patients with compromised kidney function (low GFR) at time of enrollment • ~40% reduction in reported AKI with Auxora vs Placebo 24 Change in Angiopoietin-1 from Screening to 96 hours (pg/mL) Change in Angiopoietin-2 from Screening to 96 hours (pg/mL)
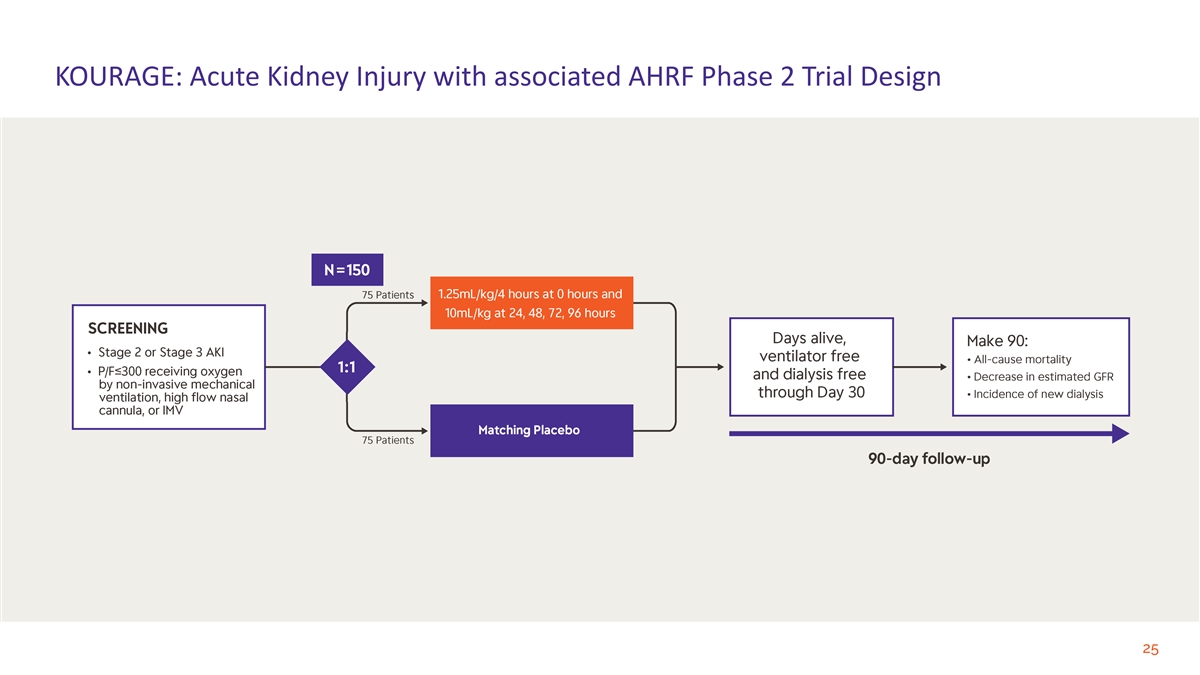
KOURAGE: Acute Kidney Injury with associated AHRF Phase 2 Trial Design 25

Auxora for Acute Respiratory Distress Syndrome (ARDS) 26

Promising Phase 2 Data from Trials in COVID-19 Pneumonia and in Ventilated Patients with Respiratory Failure CARDEA Phase 2 Trial Complete • 56% reduction in mortality at Day 30 (p=0.0165) Severe and Critical COVID-19 • 33% reduction ventilation (p=0.18) Pneumonia Patients • Three-day shorter hospital stay (p=0.09) N=284 Trial Ongoing; Data Analysis Underway Phase 2 • Reduction in inflammatory cell-type gene expression by COVID-19 Ventilated macrophages in lungs Patients N=9 • No reduction in mitochondrial and ribosomal gene expression Data Analysis of Biomarker and Mechanism-of-Action in Ventilated Patients to Inform Development Plan for ARDS expected in 1H24 27

Platform Application for CRAC Channel Inhibition 28
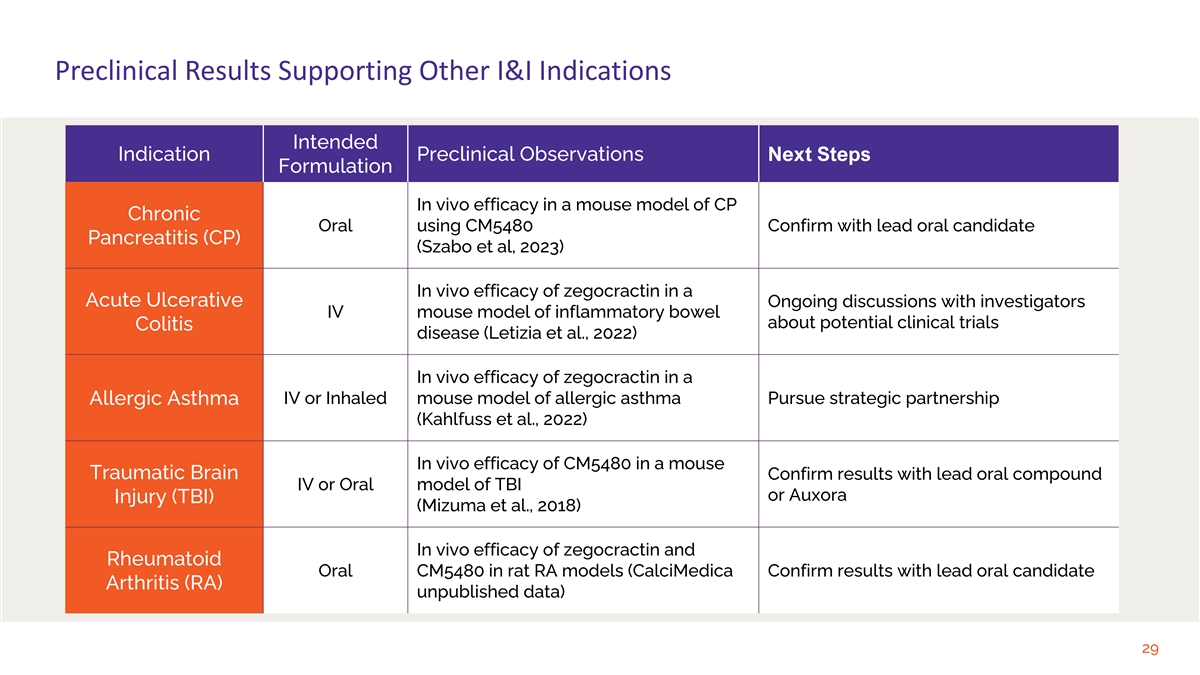
Preclinical Results Supporting Other I&I Indications Intended Indication Preclinical Observations Next Steps Formulation In vivo efficacy in a mouse model of CP Chronic Oral using CM5480 Confirm with lead oral candidate Pancreatitis (CP) (Szabo et al, 2023) In vivo efficacy of zegocractin in a Acute Ulcerative Ongoing discussions with investigators IV mouse model of inflammatory bowel about potential clinical trials Colitis disease (Letizia et al., 2022) In vivo efficacy of zegocractin in a IV or Inhaled mouse model of allergic asthma Pursue strategic partnership Allergic Asthma (Kahlfuss et al., 2022) In vivo efficacy of CM5480 in a mouse Traumatic Brain Confirm results with lead oral compound IV or Oral model of TBI or Auxora Injury (TBI) (Mizuma et al., 2018) In vivo efficacy of zegocractin and Rheumatoid Oral CM5480 in rat RA models (CalciMedica Confirm results with lead oral candidate Arthritis (RA) unpublished data) 29

Anticipated Milestones 30
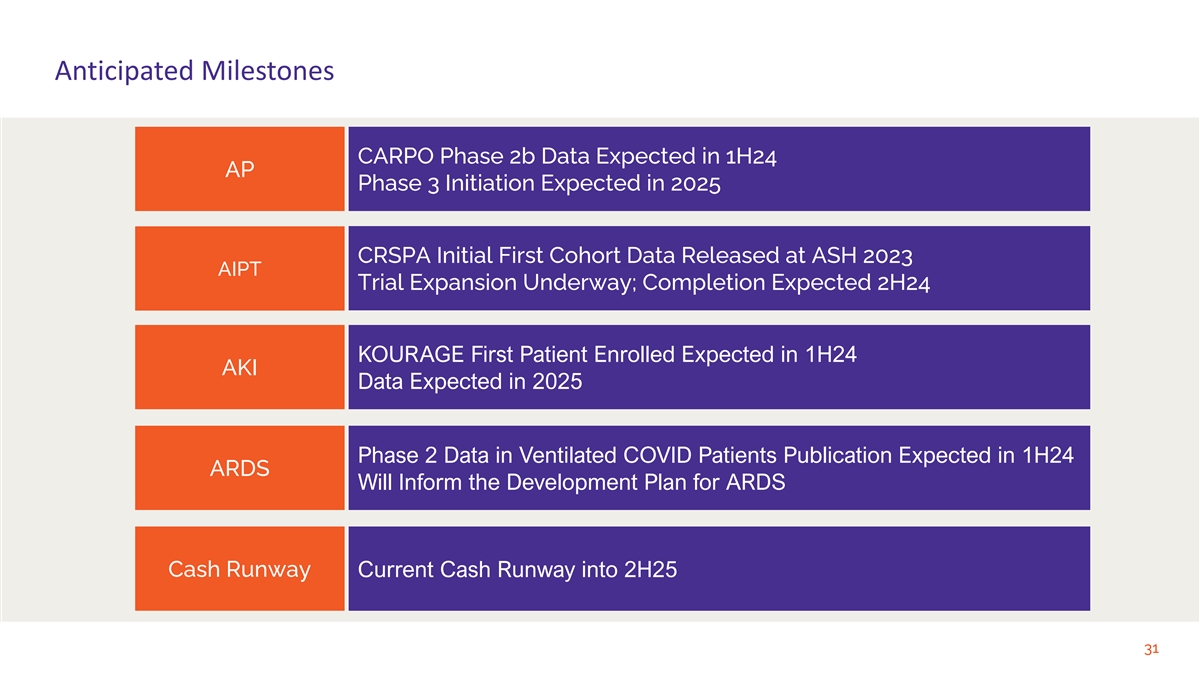
Anticipated Milestones CARPO Phase 2b Data Expected in 1H24 AP Phase 3 Initiation Expected in 2025 CRSPA Initial First Cohort Data Released at ASH 2023 AIPT Trial Expansion Underway; Completion Expected 2H24 KOURAGE First Patient Enrolled Expected in 1H24 AKI Data Expected in 2025 Phase 2 Data in Ventilated COVID Patients Publication Expected in 1H24 ARDS Will Inform the Development Plan for ARDS Cash Runway Current Cash Runway into 2H25 31






























