Exhibit 99.1

Ladenburg Thalmann 2015 Healthcare Conference September 29, 2015 Exhibit 99.1

Forward Looking Statements 2 This presentation contains forward - looking statements about Lipocine Inc. (the “Company”). These forward - looking statements are made pursuant to the safe harbor provisions of the Private Securities Litigation Reform Act of 1995. These forward - looking stat ements relate to the Company’s product candidates, clinical and regulatory processes and objectives, potential benefits of the Compa ny’ s product candidates, intellectual property and related matters, all of which involve known and unknown risks and uncertainties . Actual results may differ materially from the forward - looking statements discussed in this presentation . Accordingly, the Company cautions investors not to place undue reliance on the forward - looking statements contained in, or made in connection with, this presentation . Several factors may affect the initiation and completion of clinical trials, the potential advantages of the Company’s product candidates and the Company’s capital needs. Among other things, the projected commencement and completion of the Company’s clinical trials may be affected by difficulties or delays. In addition, the Company’s results ma y b e affected by its ability to manage its financial resources, difficulties or delays in developing manufacturing processes for its produc t c andidates, preclinical and toxicology testing and regulatory developments. Delays in clinical programs, whether caused by competitive developments, adverse events, patient enrollment rates, regulatory issues or other factors, could adversely affect the Compan y’s financial position and prospects. Prior clinical trial program designs and results are not necessarily predictive of future cli nical trial designs or results. If the Company’s product candidates do not meet safety or efficacy endpoints in clinical evaluations, th ey will not receive regulatory approval and the Company will not be able to market them. The Company may not be able to enter into any strategic partnership agreements. Operating expense and cash flow projections involve a high degree of uncertainty, including variances in future spending rates due to changes in corporate priorities, the timing and outcomes of clinical trials, compet iti ve developments and the impact on expenditures and available capital from licensing and strategic collaboration opportunities. If the Company is unable to raise additional capital when required or on acceptable terms, it may have to significantly delay, scale ba ck or discontinue one or more of its drug development or discovery research programs. The Company is at an early stage of developm ent and may not ever have any products that generate significant revenue. The forward - looking statements contained in this presentation are further qualified by the detailed discussion of risks and uncertainties set forth in the documents filed by the Company w ith the Securities and Exchange Commission, all of which can be obtained on the Company’s website at www.lipocine.com or on the SEC website at www.sec.gov . The forward - looking statements contained in this document represent the Company’s estimates and assumptions only as of the date of this presentation and the Company undertakes no duty or obligation to update or revise publicly any forward - looking statements contained in this presentation as a result of new information, future events or changes in the Company’s expectations.

Lipocine Investment Highlights ▪ First oral testosterone replacement option – Targeting ~ $2.0 Billion TRT market – NDA submitted August 2015 – Differentiated from existing products ▪ Orphan designated oral alternative for the prevention of preterm birth – A voids painful injections of the only FDA approved intra - muscular drug – Multi - dose PK study expected to start 4Q 2015 ▪ Strong Balance Sheet – Over $53M in Cash as of June 30th – Sufficient to last past LPCN 1021 anticipated PDUFA date – No Debt 3
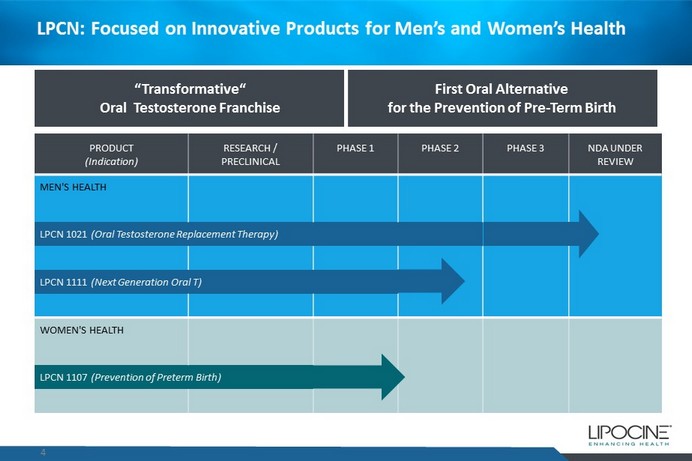
LPCN: Focused on Innovative Products for Men’s and Women’s Health 4 “Transformative“ Oral Testosterone Franchise First Oral Alternative for the Prevention of Pre - Term Birth PRODUCT (Indication) RESEARCH / PRECLINICAL PHASE 1 PHASE 2 PHASE 3 NDA UNDER REVIEW MEN'S HEALTH LPCN 1021 (Oral Testosterone Replacement Therapy ) LPCN 1111 (Next Generation Oral T) WOMEN'S HEALTH LPCN 1107 (Prevention of Preterm Birth)

LPCN 1021 - First Oral TRT Option (NDA Submitted) ▪ Robust efficacy established in pivotal P hase 3 clinical study – Met FDA primary efficacy endpoint targets ▪ Completed the 52 week safety extension arm – Well tolerated ▪ Overall AE profile comparable to active control, Androgel ® 1.62% (market leader ) – No cardiac, hepatic, gastrointestinal or drug related SAEs ▪ Completed food effect study – Consistent and predictable T levels not sensitive to meal fat content 5
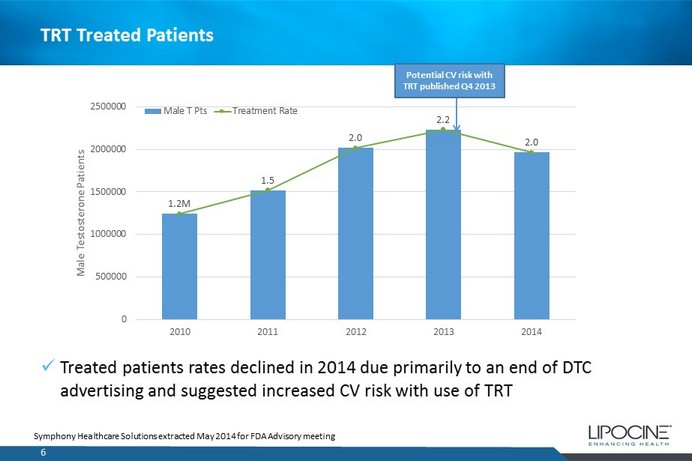
TRT Treated Patients 6 Symphony Healthcare Solutions extracted May 2014 for FDA Advisory meeting 1.2M 1.5 2.0 2.2 2.0 0 500000 1000000 1500000 2000000 2500000 2010 2011 2012 2013 2014 Male Testosterone Patients Male T Pts Treatment Rate x Treated patients rates declined in 2014 due primarily to an end of DTC advertising and suggested increased CV risk with use of TRT Potential CV risk with TRT published Q4 2013

Dollar Sales in the TRT Market Remain Dominated by Gels x Total TRx’s are stabilizing in 2015 with injectable products’ TRx volumes continuing to increase IMS Health Sept 2015 8 $0.0 $0.5 $1.0 $1.5 $2.0 $2.5 2013 2014 2015 Sales by administration (in billions, $) 0 1 2 3 4 5 6 7 8 9 2013 2014 2015 TRx by administration (in millions) Oral Injectable Transdermal $ 2.36 bn $ 2.11 bn 7.64 M 6.68 M $ 1.86 bn* 5.73 M* *2015 are actual numbers through July 31, 2015 and IMS forecasts for balance of the year. 7
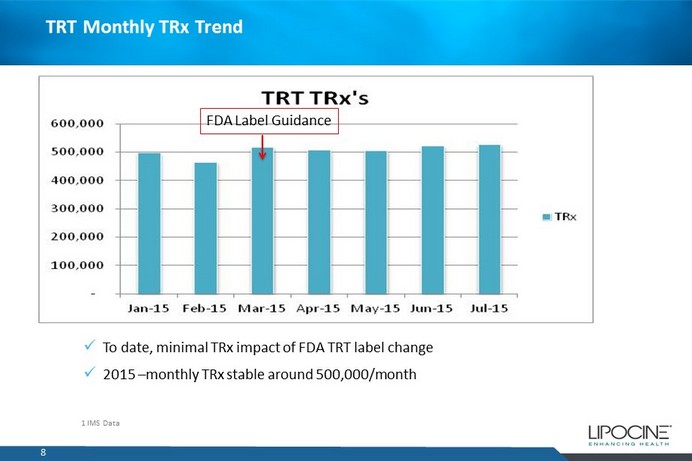
TRT Monthly TRx Trend 8 x To date, minimal TRx impact of FDA TRT label change x 2015 – monthly TRx stable around 500,000/month 1 IMS Data FDA Label Guidance
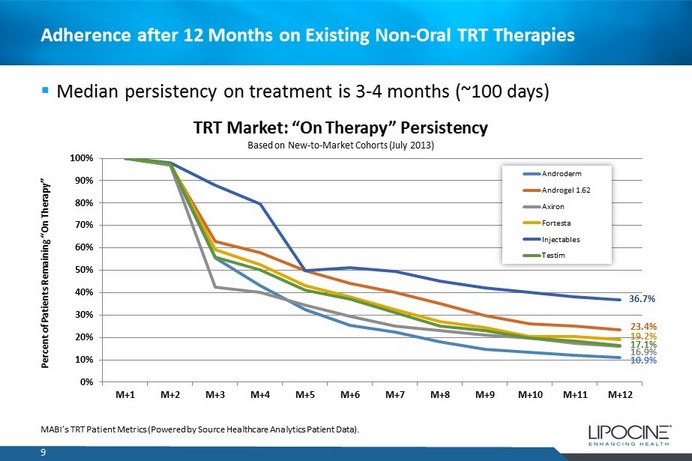
Adherence after 12 Months on Existing Non - Oral TRT Therapies 0% 10% 20% 30% 40% 50% 60% 70% 80% 90% 100% M+1 M+2 M+3 M+4 M+5 M+6 M+7 M+8 M+9 M+10 M+11 M+12 Percent of Patients Remaining “On Therapy” TRT Market: “On Therapy” Persistency Based on New - to - Market Cohorts (July 2013) Androderm Androgel 1.62 Axiron Fortesta Injectables Testim 9 MABI’s TRT Patient Metrics (Powered by Source Healthcare Analytics Patient Data). ▪ Median persistency on treatment is 3 - 4 months (~100 days) 36.7% 23.4% 19.2% 17.1% 16.9% 10.9%
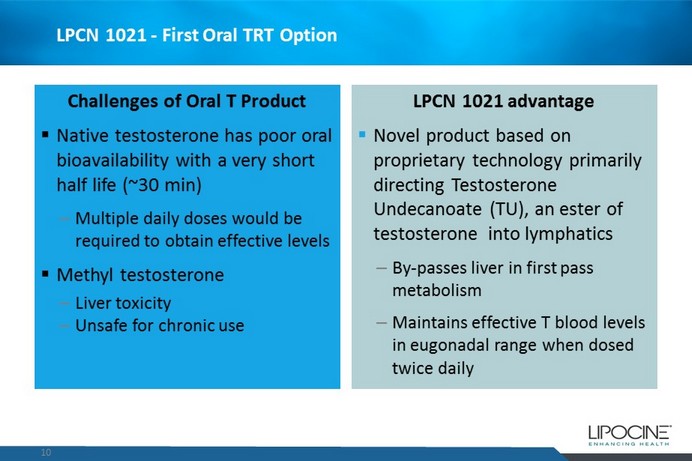
LPCN 1021 - First Oral TRT Option Challenges of Oral T Product ▪ Native testosterone has poor oral bioavailability with a very short half life (~ 30 min) – Multiple daily doses would be required to obtain effective levels ▪ Methyl testosterone – Liver toxicity – Unsafe for chronic use LPCN 1021 advantage ▪ Novel product based on proprietary technology primarily directing Testosterone Undecanoate (TU), an ester of testosterone into lymphatics – By - passes liver in first pass metabolism – Maintains effective T blood levels in eugonadal range when dosed twice daily 10
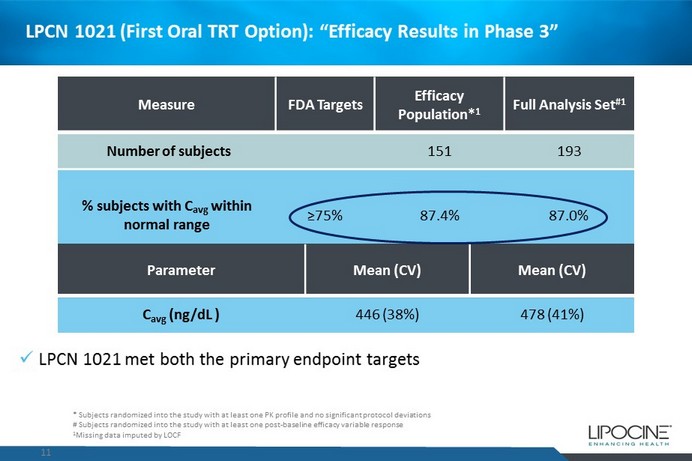
LPCN 1021 (First Oral TRT Option): “Efficacy Results in Phase 3” 11 Measure FDA Targets Efficacy Population* 1 Full Analysis Set #1 Number of subjects 151 193 % subjects with C avg w ithin normal range ≥75% 87.4% 87.0% 95 % CI lower bound ≥ 65% 81.9% 82.0% x LPCN 1021 met both the primary endpoint targets Parameter Mean (CV) Mean (CV) C avg ( ng / dL ) 446 (38%) 478 (41%) * Subjects randomized into the study with at least one PK profile and no significant protocol deviations # Subjects randomized into the study with at least one post - baseline efficacy variable response 1 Missing data imputed by LOCF
12 LPCN 1021 (First Oral TRT Option): 52 Week Safety Results ▪ LPCN 1021 was well tolerated during 52 weeks of dosing ▪ No reported cardiac, hepatic, gastrointestinal or drug related serious adverse events (SAEs) ▪ Overall adverse event (AE) profile for LPCN 1021 was comparable to the active control, Androgel ® 1.62% ▪ None of the cardiac AEs occurred in greater than 1% of the subjects in the LPCN 1021 arm and none were classified as severe ▪ All observed adverse drug reactions (ADRs) were classified as mild or moderate in severity and no serious ADRs occurred during the 52 - week treatment period
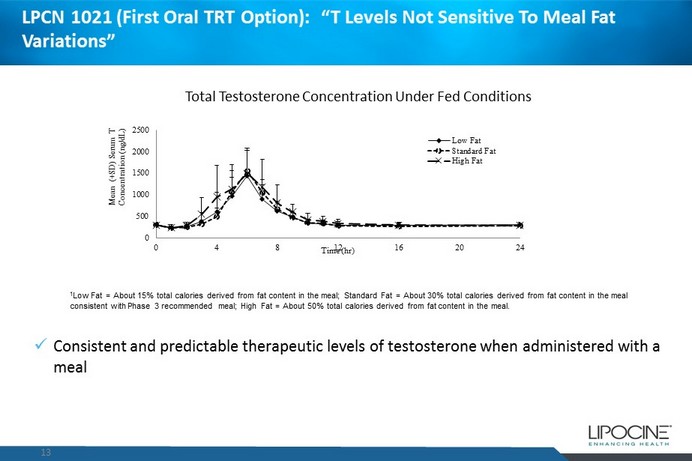
LPCN 1021 (First Oral TRT Option): “ T Levels N ot S ensitive T o M eal F at Variations” 13 0 500 1000 1500 2000 2500 0 4 8 12 16 20 24 Mean (+SD) Serum T Concentration (ng/dL) Time (hr) Low Fat Standard Fat High Fat 1 Low Fat = About 15% total calories derived from fat content in the meal; Standard Fat = About 30% total calories derived from fat content in the meal consistent with Phase 3 recommended meal; High Fat = About 50% total calories derived from fat content in the meal. x Consistent and predictable therapeutic levels of testosterone when administered with a meal Total Testosterone Concentration Under Fed Conditions
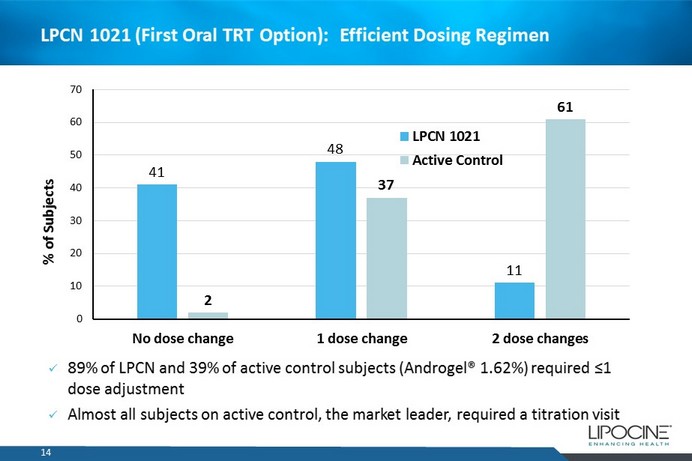
14 41 48 11 2 37 61 0 10 20 30 40 50 60 70 No dose change 1 dose change 2 dose changes % of Subjects LPCN 1021 Active Control x 89% of LPCN and 39% of active control subjects (Androgel® 1.62%) required ≤1 dose adjustment x Almost all subjects on active control, the market leader, required a titration visit LPCN 1021 (First Oral TRT Option): Efficient Dosing Regimen
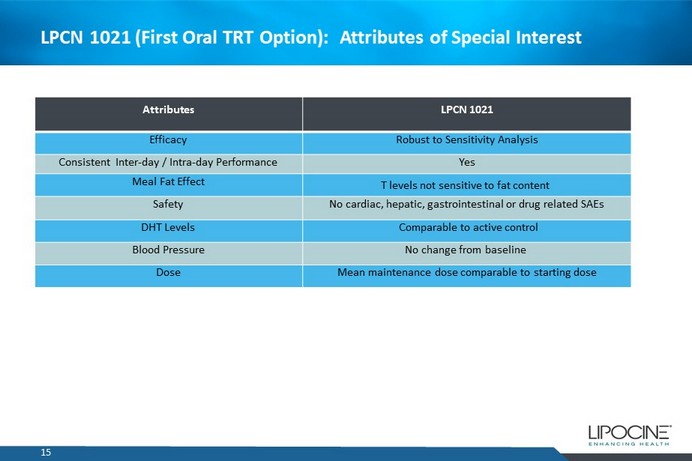
LPCN 1021 (First Oral TRT Option): Attributes of Special Interest Attributes LPCN 1021 Efficacy Robust to Sensitivity Analysis Consistent Inter - day / Intra - day Performance Yes Meal Fat E ffect T levels not sensitive to fat content Safety No cardiac, hepatic, gastrointestinal or drug related SAEs DHT Levels Comparable to active control Blood Pressure No change from baseline Dose Mean maintenance dose comparable to starting dose 15
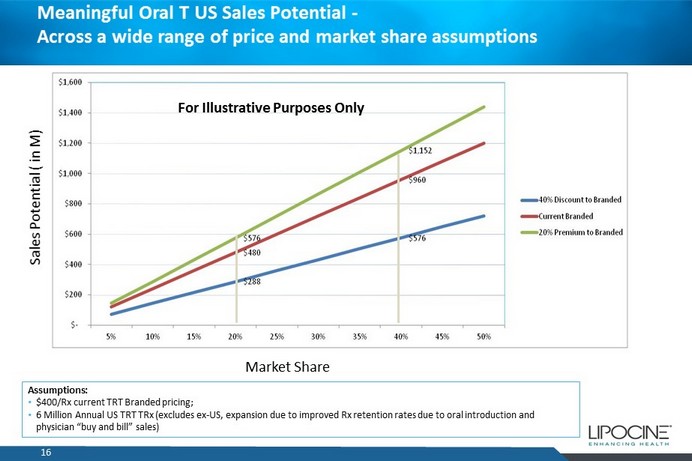
Meaningful Oral T US Sales Potential - Across a wide range of price and market share assumptions 16 Market Share Sales Potential ( in M) Assumptions: • $400/Rx current TRT Branded pricing; • 6 Million Annual US TRT TRx (excludes ex - US, expansion due to improved Rx retention rates due to oral introduction and physician “buy and bill” sales) For Illustrative Purposes Only
LPCN 1111 (Next Generation Oral TRT Option): “Once Daily” ▪ Novel prodrug of testosterone for oral delivery through proprietary drug delivery technology ▪ Once daily is expected to improve and sustain market share or oral T franchise ▪ Once daily feasibility established – Positive Phase 2a study results in hypogonadal men ▪ Single daily oral dose provides T levels in the eugonadal range ▪ No subject exceeded peak T levels of 1500 ng/ dL ▪ Expect to Initiate Phase 2b PK dose finding study – 4Q 2015 17

Preterm Birth (PTB) Represents a Significant Unmet Medical Need ▪ 11.7% of all US pregnancies 2 result in PTB (< 37 weeks) - a leading cause of neonatal mortality and morbidity 3 ▪ ~10x more first year medical costs are for PTB infants than for full term infants 4 ▪ ≥ $26 billion economic impact: 4 $1 billion market opportunity 5 18 1 Pediatric Research (2006) 60, 775 – 776 2 CDC (2010) 3 J . Maternal - Fetal and Neonatal Medicine, Dec. 2006, 19(12), 773 – 782 4 Institute of Medicine of the National Academies. Jul.2006 5 AMAG Pharmaceuticals presentation 09/29/2014 One preterm infant per minute in the U.S. 1
LPCN 1107 (First Oral PTB Candidate): “Addresses Unmet Need” ▪ The only FDA approved product for the prevention of PTB is an injectable hydroxyprogesterone caproate (HPC) ▪ Issues with the injectable HPC: – Weekly injection in doctor’s office or home – Injection site pain (35%), swelling (17%) • LPCN 1107: An oral HPC alternative • Non - invasive x No injection site reactions x No potential for pulmonary embolism – Self - administered – Life - style friendly ▪ Reduced travel burden 19
LPCN 1107 (First Oral PTB Candidate): “Status” 20 ▪ Potential to be the first oral standard - of care therapy • Elimination of 18 - 22 injections ▪ Orphan drug designation • A major contribution to patient care ▪ Oral feasibility established • Successful Phase 1 studies in healthy non - pregnant women and pregnant women ▪ Development path identified • Expect to initiate multi - dose PK dose finding study - 4Q 2015
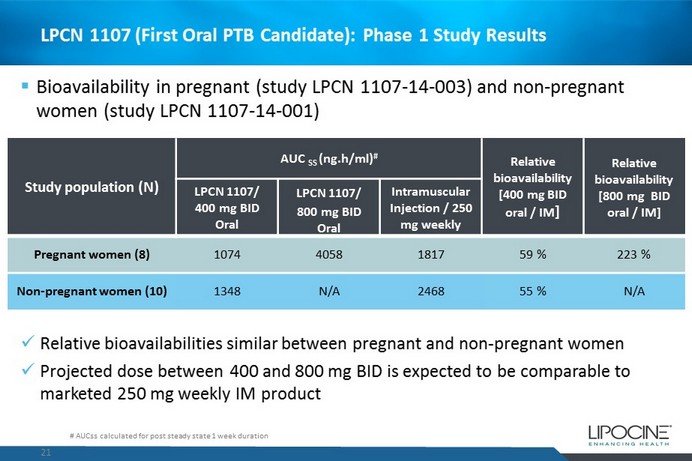
LPCN 1107 (First Oral PTB Candidate): Phase 1 Study Results 21 # AUCss calculated for post steady state 1 week duration Study population (N) AUC SS ( ng.h /ml) # Relative bioavailability [400 mg BID oral / IM ] Relative bioavailability [800 mg BID oral / IM] LPCN 1107/ 400 mg BID Oral LPCN 1107/ 800 mg BID Oral Intramuscular Injection / 250 mg weekly Pregnant women (8) 1074 4058 1817 59 % 223 % Non - pregnant women (10) 1348 N/A 2468 55 % N/A x Relative bioavailabilities similar between pregnant and non - pregnant women x Projected dose between 400 and 800 mg BID is expected to be comparable to marketed 250 mg weekly IM product ▪ Bioavailability in pregnant (study LPCN 1107 - 14 - 003) and non - pregnant women (study LPCN 1107 - 14 - 001)
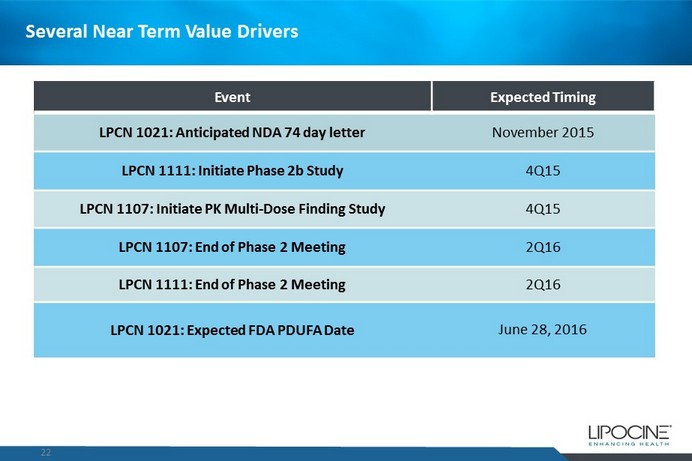
Several Near Term Value Drivers 22 Event Expected Timing LPCN 1021: Anticipated NDA 74 day letter November 2015 LPCN 1111: Initiate Phase 2b Study 4Q15 LPCN 1107: Initiate PK Multi - Dose Finding Study 4Q15 LPCN 1107: End of Phase 2 Meeting 2Q16 LPCN 1111: End of Phase 2 Meeting 2Q16 LPCN 1021: Expected FDA PDUFA Date June 28, 2016
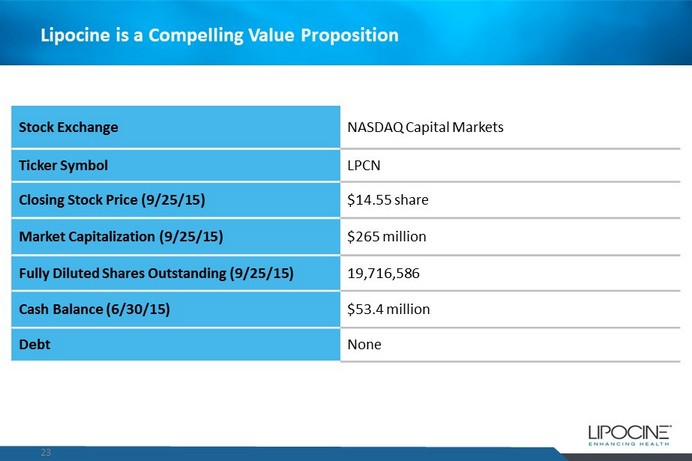
Lipocine is a Compelling Value Proposition 23 Stock Exchange NASDAQ Capital Markets Ticker Symbol LPCN Closing Stock Price (9/25/15) $14.55 share Market Capitalization (9/25/15) $265 million Fully Diluted Shares Outstanding (9/25/15) 19,716,586 Cash Balance (6/30/15) $53.4 million Debt None

Lipocine Investment Highlights ▪ First oral testosterone replacement option – Targeting ~ $2.0 Billion TRT market – NDA submitted August 2015 – Differentiated from existing products ▪ Orphan designated oral alternative for the prevention of preterm birth – A voids painful injections of the only FDA approved intra - muscular drug – Multi - dose PK study expected to start 4Q 2015 ▪ Strong Balance Sheet – Over $53M in Cash as of June 30th – Sufficient to last past LPCN 1021 anticipated PDUFA date – No Debt 24























