
1 Exhibit 99.2

Enabling Oral Drug Delivery to Improve Patient Compliance NASH - TAG Conference Jan 2019 LPCN 1144 Improves NAFLD/NASH Biomarkers in Subjects at Risk of NAFLD/NASH Jonathan Baker, Ph.D.

Forward - Looking Statements This presentation contains forward - looking statements about Lipocine Inc. (the “Company”). These forward - looking statements are made pursuant to the safe harbor provisions of the Private Securities Litigation Reform Act of 1995. These forward - looking statements relate to the Company’s product candidates, the expected timing of the FDA review process related to our resubmitted NDA for TLANDO™, clinical and regulatory pr ocesses and objectives, potential benefits of the Company’s product candidates, intellectual property and related matters, all of which i nvo lve known and unknown risks and uncertainties. Actual results may differ materially from the forward - looking statements discussed in this pre sentation . Accordingly, the Company cautions investors not to place undue reliance on the forward - looking statements contained in, or made in connection with, this presentation . Several factors may affect the initiation and completion of clinical trials, the potential advantages of the Company’s prod uct candidates and the Company’s capital needs. Among other things, the projected commencement and completion of the Company’s c lin ical trials may be affected by difficulties or delays. We may encounter delays or other issues in the FDA approval process, including that th e F DA may determine there are deficiencies in our resubmitted NDA. We are also subject to risks related to the possibility of an advisory committ ee meeting related to TLANDO™. In addition, the Company’s results may be affected by its ability to manage its financial resources, difficulties or de lays in developing manufacturing processes for its product candidates, preclinical and toxicology testing and regulatory developments. Delays i n c linical programs, whether caused by competitive developments, adverse events, patient enrollment rates, regulatory issues or other factors, cou ld adversely affect the Company’s financial position and prospects. Prior clinical trial program designs and results are not necessarily predictive of future clinical trial designs or results. If the Company’s product candidates do not meet safety or efficacy endpoints in clinical evaluations, they will not receive regulatory approval and the Company will not be able to market them. The Company may not be able to enter into any strategic partnership ag reements. The Company’s commercial success depends on its ability to manufacture, market and sell products without infringing the proprieta ry rights of third parties. Operating expense and cash flow projections involve a high degree of uncertainty, including variances in future spen din g rates due to changes in corporate priorities, the timing and outcomes of clinical trials, competitive developments and the impact on expen dit ures and available capital from licensing and strategic collaboration opportunities. If the Company is unable to raise additional capital when req uired or on acceptable terms, it may have to significantly delay, scale back or discontinue one or more of its drug development or discovery researc h p rograms. The Company is at an early stage of development and may not ever have any products that generate significant revenue. The forward - looking statements contained in this presentation are further qualified by the detailed discussion of risks and uncertainties set forth in the C omp any’s annual report on Form 10 - K and other periodic reports filed by the Company with the Securities and Exchange Commission, all of which can be obtai ned on the Company’s website at www.lipocine.com or on the SEC website at www.sec.gov . The forward - looking statements contained in this document represent the Company’s estimates and assumptions only as of the date of this presentation and the Company undertakes no duty or obligation to update or revise publicly any forward - looking statements contained in this presentation as a result of new information, future events or changes in the Company’s expectations. 3
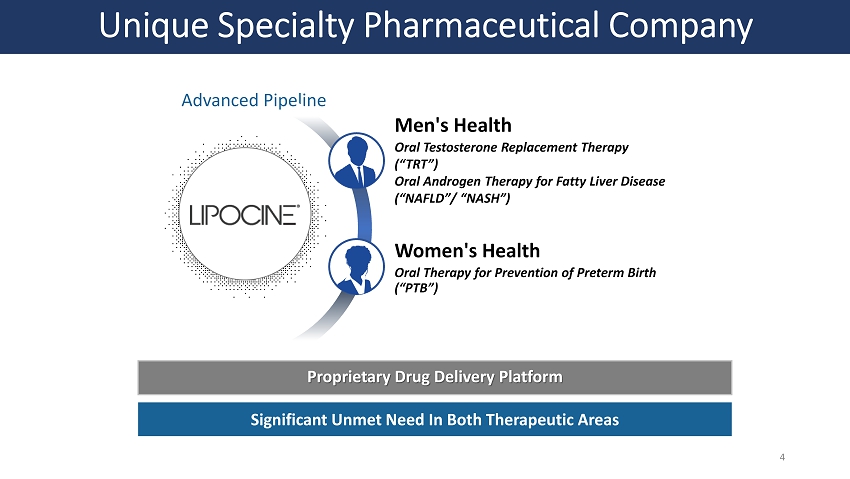
Unique Specialty Pharmaceutical Company Advanced Pipeline Men's Health Oral Testosterone Replacement Therapy (“TRT”) Oral Androgen Therapy for Fatty Liver Disease (“NAFLD”/ “NASH”) Women's Health Oral Therapy for Prevention of Preterm Birth (“PTB”) Proprietary Drug Delivery Platform Significant Unmet Need In Both Therapeutic Areas 4
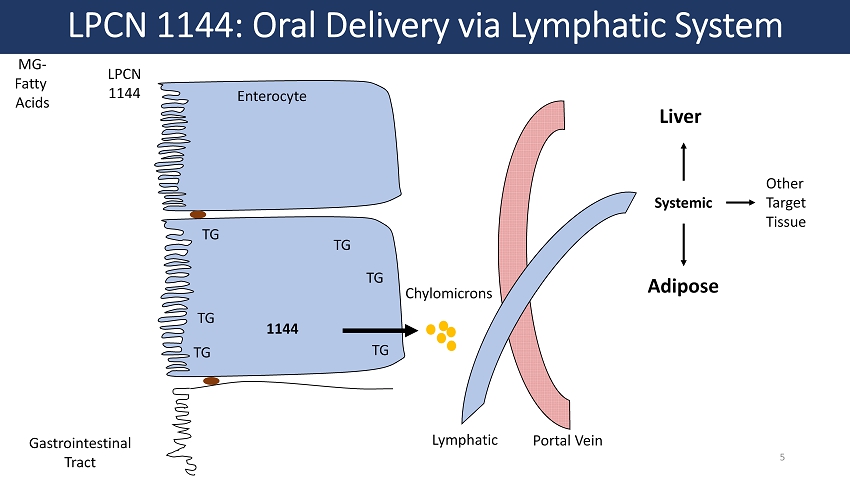
LPCN 1144 Lymphatic Systemic Adipose Liver Other Target Tissue LPCN 1144: Oral Delivery via Lymphatic System 1144 MG - Fatty Acids Enterocyte Chylomicrons Gastrointestinal Tract TG TG TG TG TG TG Portal Vein 5
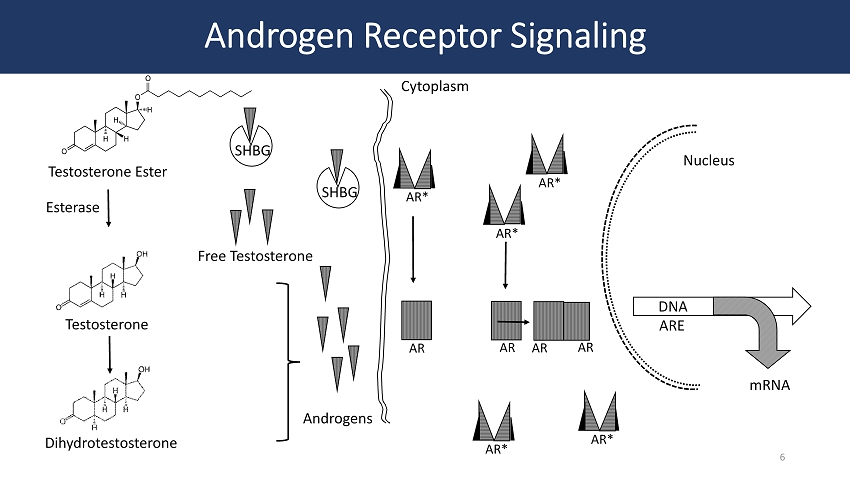
Androgen Receptor Signaling Testosterone Dihydrotestosterone Androgens Nucleus Cytoplasm Testosterone Ester Esterase AR* AR* AR* AR* AR* AR AR DNA mRNA AR AR SHBG SHBG Free Testosterone ARE 6
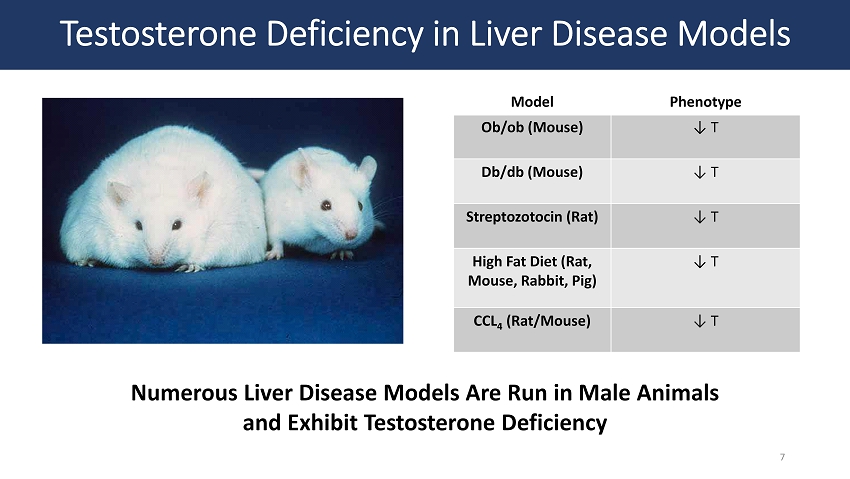
Testosterone Deficiency in Liver Disease Models Numerous Liver Disease Models Are Run in Male Animals and Exhibit Testosterone Deficiency Model Phenotype Ob/ ob (Mouse) ↓ T Db/ db (Mouse) ↓ T Streptozotocin (Rat) ↓ T High Fat Diet (Rat, Mouse, Rabbit, Pig) ↓ T CCL 4 (Rat/Mouse) ↓ T 7
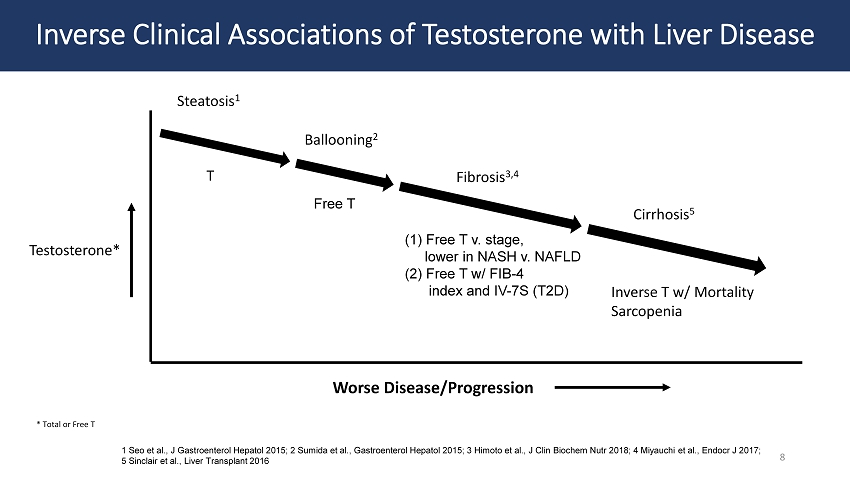
Inverse Clinical Associations of Testosterone with Liver Disease Worse Disease/Progression Testosterone* * Total or Free T Steatosis 1 Ballooning 2 Fibrosis 3,4 T (1) Free T v. stage, lower in NASH v. NAFLD (2) Free T w/ FIB - 4 index and IV - 7S (T2D) Free T Cirrhosis 5 Inverse T w/ Mortality Sarcopenia 1 Seo et al., J Gastroenterol Hepatol 2015; 2 Sumida et al., Gastroenterol Hepatol 2015; 3 Himoto et al., J Clin Biochem Nutr 2018; 4 Miyauchi et al., Endocr J 2017; 5 Sinclair et al., Liver Transplant 2016 8
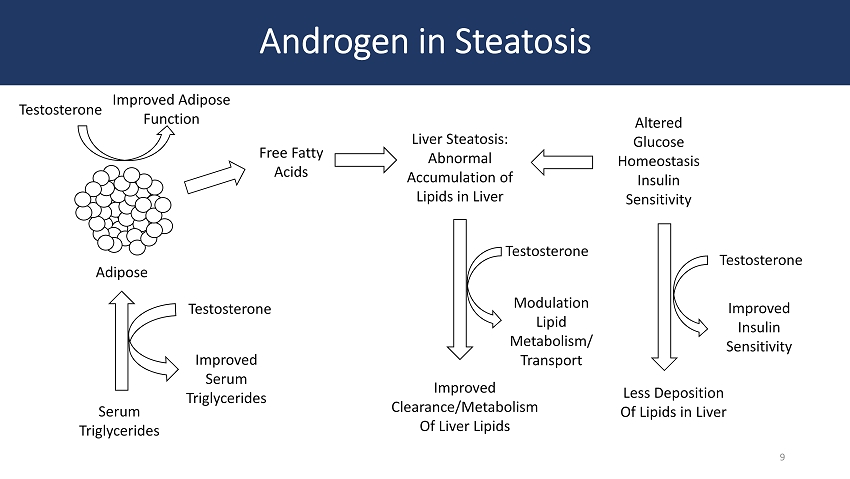
Androgen in Steatosis Adipose Free Fatty Acids Liver Steatosis: Abnormal Accumulation of Lipids in Liver Altered Glucose Homeostasis Insulin Sensitivity Serum Triglycerides Testosterone Improved Insulin Sensitivity Testosterone Less Deposition Of Lipids in Liver Modulation Lipid Metabolism/ Transport Improved Clearance/Metabolism Of Liver Lipids Testosterone Improved Serum Triglycerides Testosterone Improved Adipose Function 9
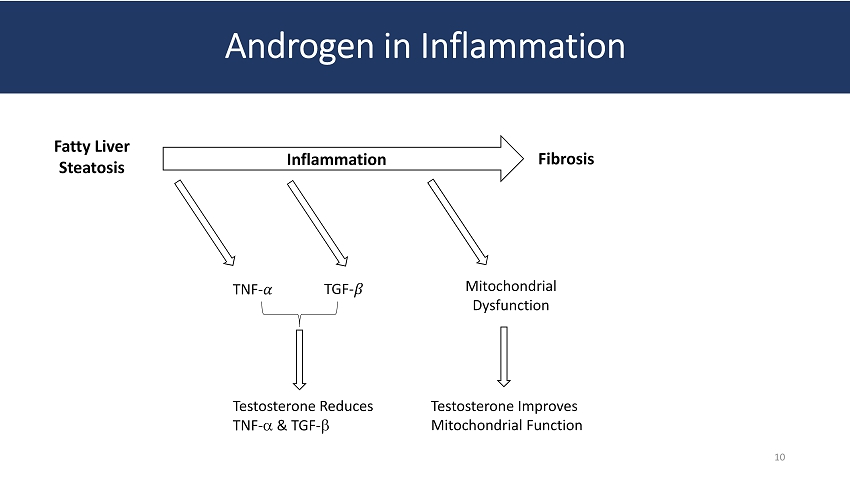
Androgen in Inflammation Fatty Liver Steatosis Inflammation Fibrosis TNF - ߙ TGF - ߚ Mitochondrial Dysfunction Testosterone Reduces TNF - a & TGF - b Testosterone Improves Mitochondrial Function 10
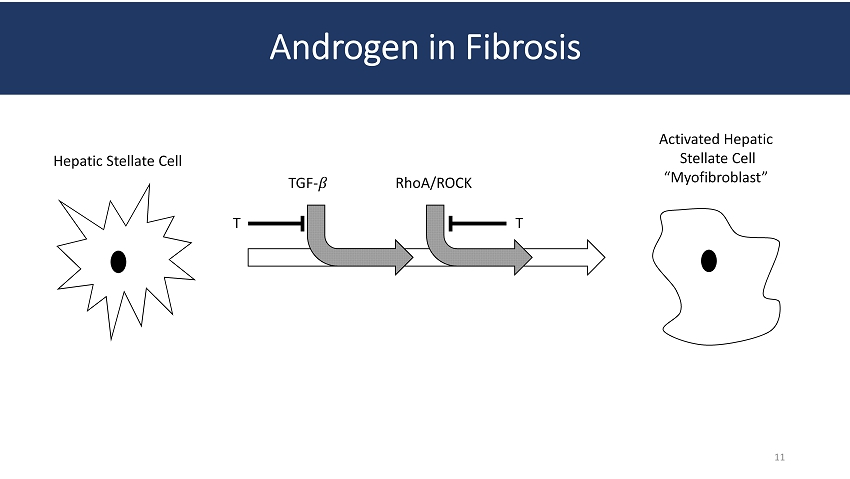
Androgen in Fibrosis Hepatic Stellate Cell Activated Hepatic Stellate Cell “Myofibroblast” TGF - ߚ T RhoA /ROCK T 11
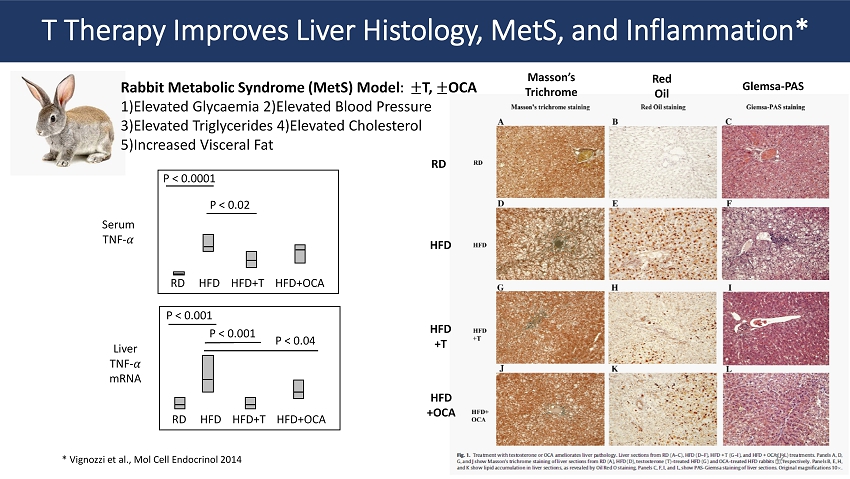
T Therapy Improves Liver Histology, MetS , and Inflammation* Rabbit Metabolic Syndrome ( MetS ) Model ǣ � േ T, േ OCA 1)Elevated Glycaemia 2)Elevated Blood Pressure 3)Elevated Triglycerides 4)Elevated Cholesterol 5)Increased Visceral Fat RD HFD HFD+T HFD+OCA RD HFD HFD+T HFD+OCA Serum TNF - ߙ Liver TNF - ߙ mRNA P < 0.0001 P < 0.02 P < 0.001 P < 0.001 P < 0.04 Masson’s Trichrome Red Oil Glemsa - PAS RD HFD HFD +T HFD +OCA * Vignozzi et al., Mol Cell Endocrinol 2014 12

LPCN 1144: Well Characterized Safety □ LPCN 1144 is a Prodrug of Bioidentical Sex Hormone □ Extensive Clinical Safety Database with LPCN 1144 ▪ 591 subjects in 12 studies with up to 52 week exposure ▪ Well tolerated with no deaths or MACE events; no drug related SAEs ▪ Good gastrointestinal tolerability ▪ No signs of skeletal fragility or nephrotoxicity 13

LPCN 1144: Liver Specific Safety □ No Adverse Drug Reactions Reported in Hepatic System Organ Class with LPCN 1144 ▪ The safety of LPCN 1144 administered twice daily in hypogonadal subjects who continued to receive treatment for up to 52 weeks was evaluated in SOAR Trial. No adverse drug reactions (i.e., drug - related adverse events) were reported in the hepatic system organ class. ▪ None of the subjects during the study reported adverse events including pruritis, peliosis hepatitis, hepatic neoplasms, cholestatic hepatitis and jaundice. ▪ Observed Improvements in Liver Function Tests 14

LPCN 1144: Significant Reductions in Liver Enzyme Levels □ Placebo Controlled 4 Week Study (M12 - 778) 15 - 47.4% - 47.3% - 11.5% - 21.0% - 11.0% - 6.6% - 15.8% - 16.7% -60% -50% -40% -30% -20% -10% 0% LPCN 1144 225mg BID LPCN 1144 300mg BID Placebo - adjusted Mean Change ALT AST ALP GGT
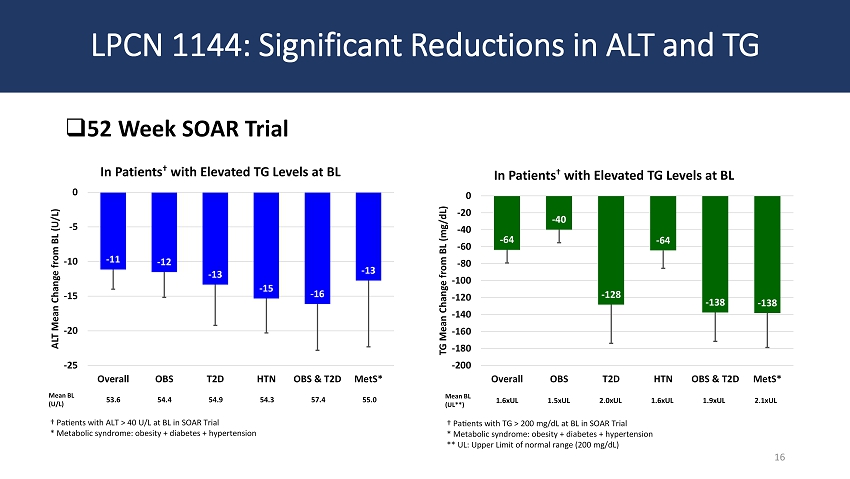
LPCN 1144: Significant Reductions in ALT and TG □ 52 Week SOAR Trial 16 Mean BL (U/L) 53.6 54.4 54.9 54.3 57.4 55.0 † Patients with ALT > 40 U/L at BL in SOAR Trial * Metabolic syndrome: obesity + diabetes + hypertension Mean BL (UL**) 1.6xUL 1.5xUL 2.0xUL 1.6xUL 1.9xUL 2.1xUL † Patients with TG > 200 mg/dL at BL in SOAR Trial * Metabolic syndrome: obesity + diabetes + hypertension ** UL: Upper Limit of normal range (200 mg/dL) - 11 - 12 - 13 - 15 - 16 - 13 -25 -20 -15 -10 -5 0 Overall OBS T2D HTN OBS & T2D MetS* ALT Mean Change from BL (U/L) In Patients † with Elevated TG Levels at BL - 64 - 40 - 128 - 64 - 138 - 138 -200 -180 -160 -140 -120 -100 -80 -60 -40 -20 0 Overall OBS T2D HTN OBS & T2D MetS* TG Mean Change from BL (mg/dL) In Patients † with Elevated TG Levels at BL
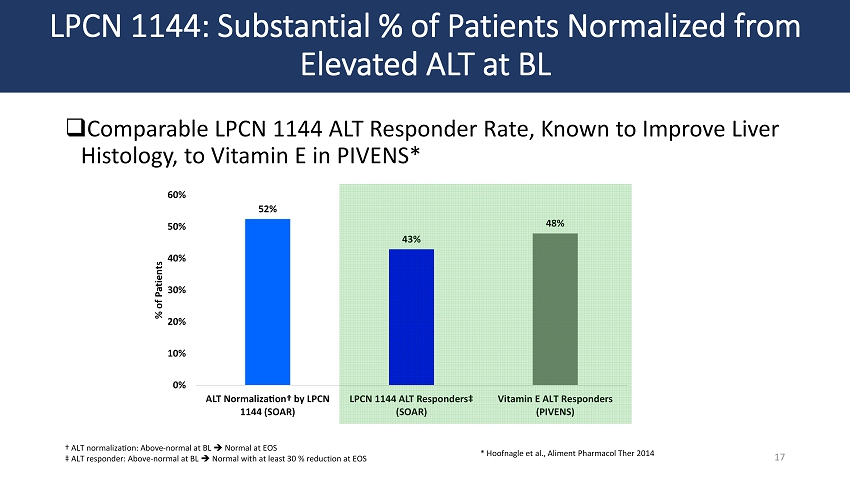
LPCN 1144: Substantial % of Patients Normalized from Elevated ALT at BL □ Comparable LPCN 1144 ALT Responder Rate, Known to Improve Liver Histology, to Vitamin E in PIVENS* 17 52% 43% 48% 0% 10% 20% 30% 40% 50% 60% ALT Normalization† by LPCN 1144 (SOAR) LPCN 1144 ALT Responders‡ (SOAR) Vitamin E ALT Responders (PIVENS) % of Patients † ALT normalization: Above - normal at BL Normal at EOS ‡ ALT responder: Above - normal at BL Normal with at least 30 % reduction at EOS * Hoofnagle et al., Aliment Pharmacol Ther 2014

□ 52 Week SOAR Trial LPCN 1144: Improves Mental and Sexual PROs Mental Component Summary Mental Health Role Emotional Maintained Erection Sexual Activity Negative Mood Positive Mood Overall Sexual Desire -4 -2 0 2 4 6 8 Mean Change ( “ 95% CI) from Baseline SF - 36 PDQ SF - 36, Short Form - 36 (0 - 100); PDQ, Psychosexual Daily Questionnaire (0 - 7); * ALT > 40 U/L at BL (N=33) 18
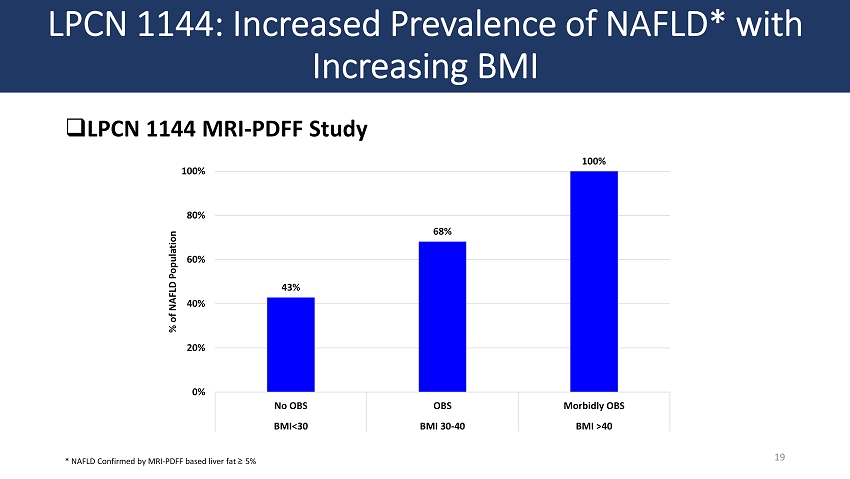
□ LPCN 1144 MRI - PDFF Study LPCN 1144: Increased Prevalence of NAFLD* with Increasing BMI 19 43% 68% 100% 0% 20% 40% 60% 80% 100% No OBS OBS Morbidly OBS BMI<30 BMI 30-40 BMI >40 % of NAFLD Population * NAFLD Confirmed by MRI - PDFF based liver fat ≥ 5%
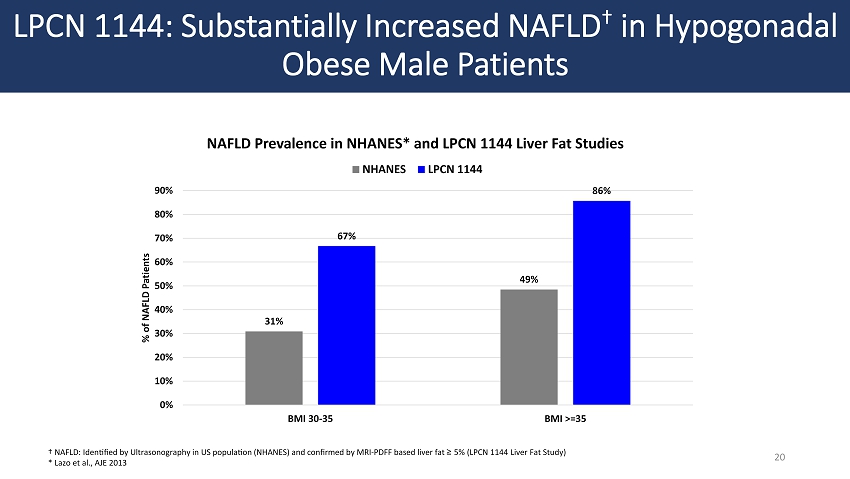
LPCN 1144: Substantially Increased NAFLD † in Hypogonadal Obese Male Patients 20 † NAFLD: Identified by Ultrasonography in US population (NHANES) and confirmed by MRI - PDFF based liver fat ≥ 5% (LPCN 1144 Liver Fat Study) * Lazo et al., AJE 2013 31% 49% 67% 86% 0% 10% 20% 30% 40% 50% 60% 70% 80% 90% BMI 30-35 BMI >=35 % of NAFLD Patients NAFLD Prevalence in NHANES* and LPCN 1144 Liver Fat Studies NHANES LPCN 1144

Conclusion □ Strong Rationale for Androgen Therapy in NAFLD/NASH Patients ▪ MRI - PDFF confirmed High (~70%) Prevalence of Fatty Liver in Hypogonadal men ▪ LPCN 1144 Improves Liver Function Tests & Serum Triglycerides in Subjects at Risk of NAFLD/NASH □ Well Tolerated LPCN 1144 Therapy with Potential for Improvements in Bone and Muscle Function □ Potential Improvements in Sexual and Mental Domains □ Further Study of LPCN 1144 in Liver Disease is On - Going 21




















