
4Q & FY 2019 Conference Call May 29, 2019 NASDAQ: TYME Exhibit 99.2

TYME Conference Call Participants Business Highlights and 2019 Milestones Steve Hoffman, Chairman & Chief Executive Officer Clinical Development Progress Michele Korfin, R.Ph., M.B.A., Chief Operating Officer Giuseppe Del Priore, M.D., M.P.H., Chief Medical Officer Corporate and Financial Update Ben R. Taylor, President & Chief Financial Officer

TYME Technologies TYME is an emerging biotechnology company focused on exploring novel therapeutic approaches designed to target cancer’s unique metabolism TYME is advancing proprietary cancer metabolism-based therapies (CMBTs™) for difficult-to-treat cancers

Business Updates and 2019 Milestones Steve Hoffman, Chairman & Chief Executive Officer
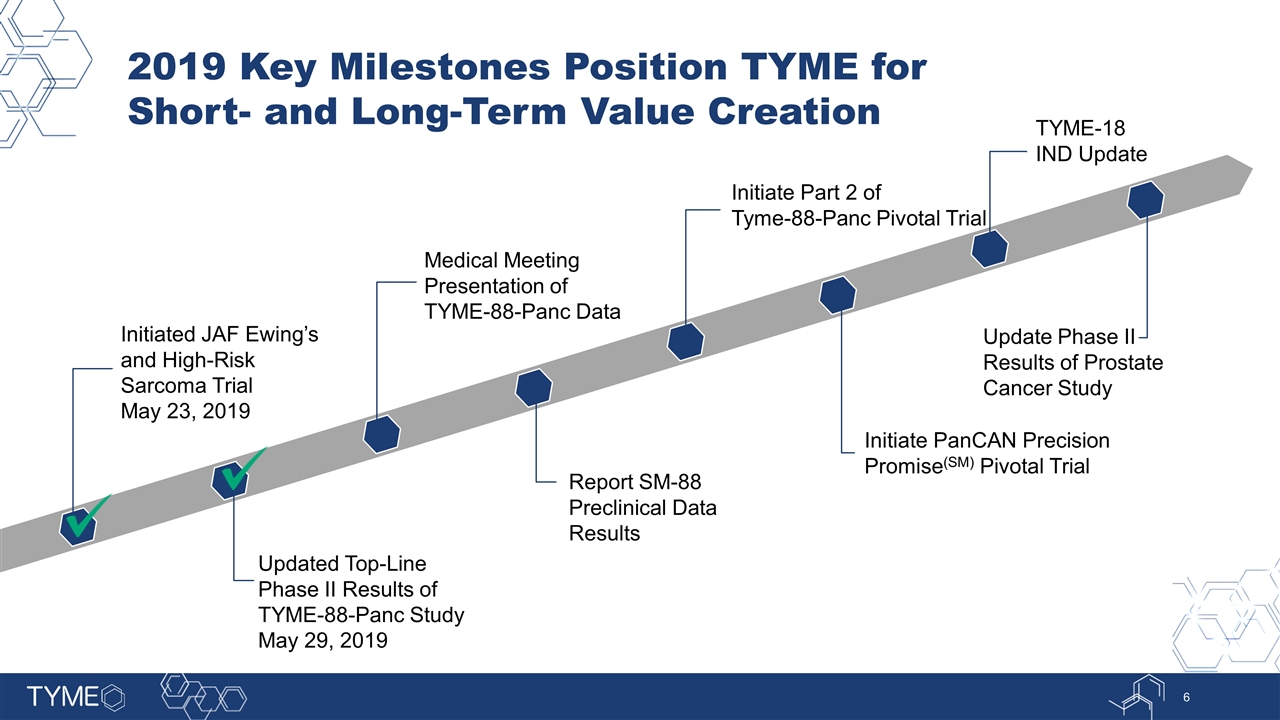
2019 Key Milestones Position TYME for Short- and Long-Term Value Creation Updated Top-Line Phase II Results of TYME-88-Panc Study May 29, 2019 Initiate Part 2 of Tyme-88-Panc Pivotal Trial Report SM-88 Preclinical Data Results Initiate PanCAN Precision Promise(SM) Pivotal Trial Initiated JAF Ewing’s and High-Risk Sarcoma Trial May 23, 2019 TYME-18 IND Update Update Phase II Results of Prostate Cancer Study Medical Meeting Presentation of TYME-88-Panc Data

Clinical DEVELOPMENT PROGRESS Michele Korfin, R.Ph., M.B.A., Chief Operating Officer Giuseppe Del Priore, M.D., M.P.H., Chief Medical Officer
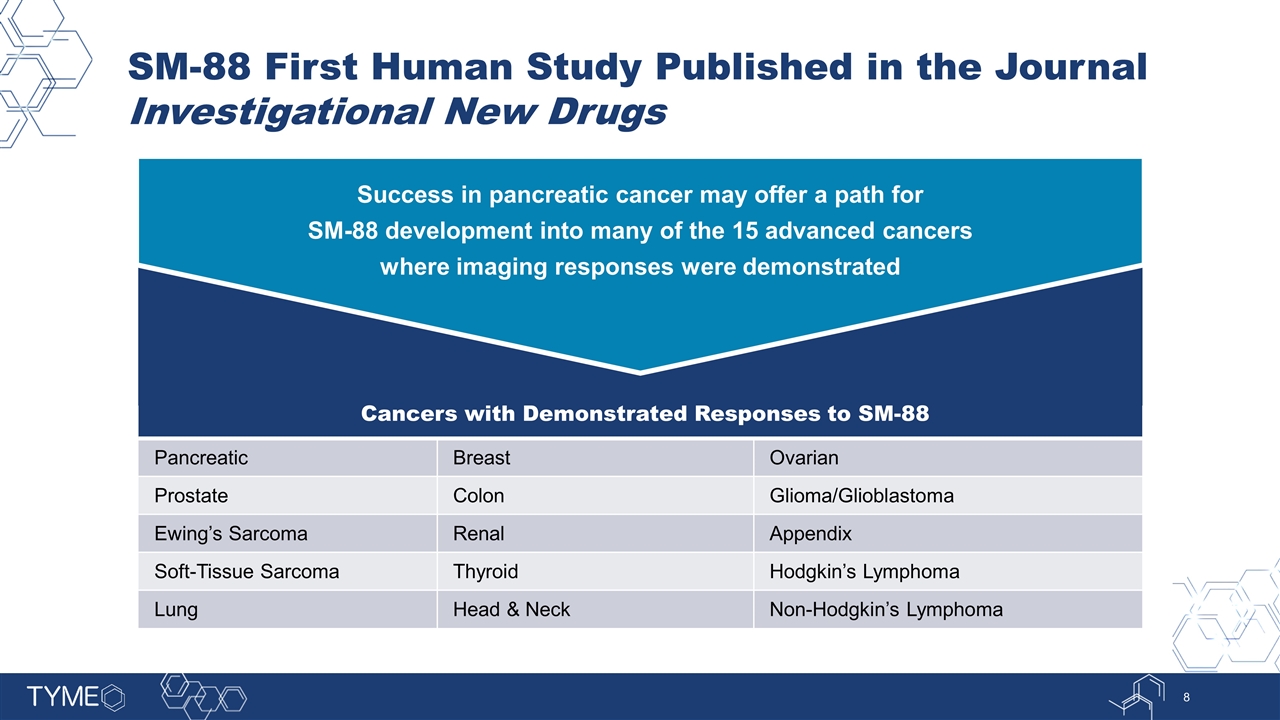
SM-88 First Human Study Published in the Journal Investigational New Drugs Success in pancreatic cancer may offer a path for SM-88 development into many of the 15 advanced cancers where imaging responses were demonstrated Cancers with Demonstrated Responses to SM-88 Pancreatic Breast Ovarian Prostate Colon Glioma/Glioblastoma Ewing’s Sarcoma Renal Appendix Soft-Tissue Sarcoma Thyroid Hodgkin’s Lymphoma Lung Head & Neck Non-Hodgkin’s Lymphoma
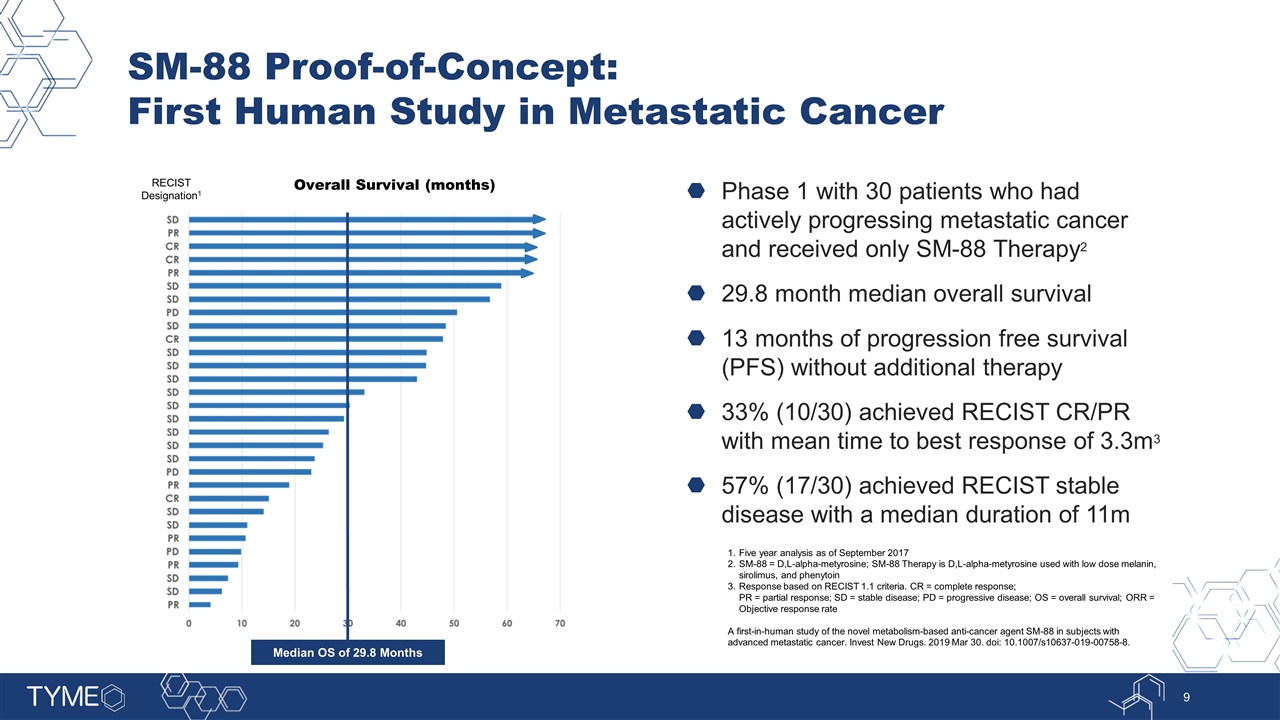
Overall Survival (months) RECIST Designation1 Median OS of 29.8 Months SM-88 Proof-of-Concept: First Human Study in Metastatic Cancer Phase 1 with 30 patients who had actively progressing metastatic cancer and received only SM-88 Therapy2 29.8 month median overall survival 13 months of progression free survival (PFS) without additional therapy 33% (10/30) achieved RECIST CR/PR with mean time to best response of 3.3m3 57% (17/30) achieved RECIST stable disease with a median duration of 11m Five year analysis as of September 2017 SM-88 = D,L-alpha-metyrosine; SM-88 Therapy is D,L-alpha-metyrosine used with low dose melanin, sirolimus, and phenytoin Response based on RECIST 1.1 criteria. CR = complete response; PR = partial response; SD = stable disease; PD = progressive disease; OS = overall survival; ORR = Objective response rate A first-in-human study of the novel metabolism-based anti-cancer agent SM-88 in subjects with advanced metastatic cancer. Invest New Drugs. 2019 Mar 30. doi: 10.1007/s10637-019-00758-8.
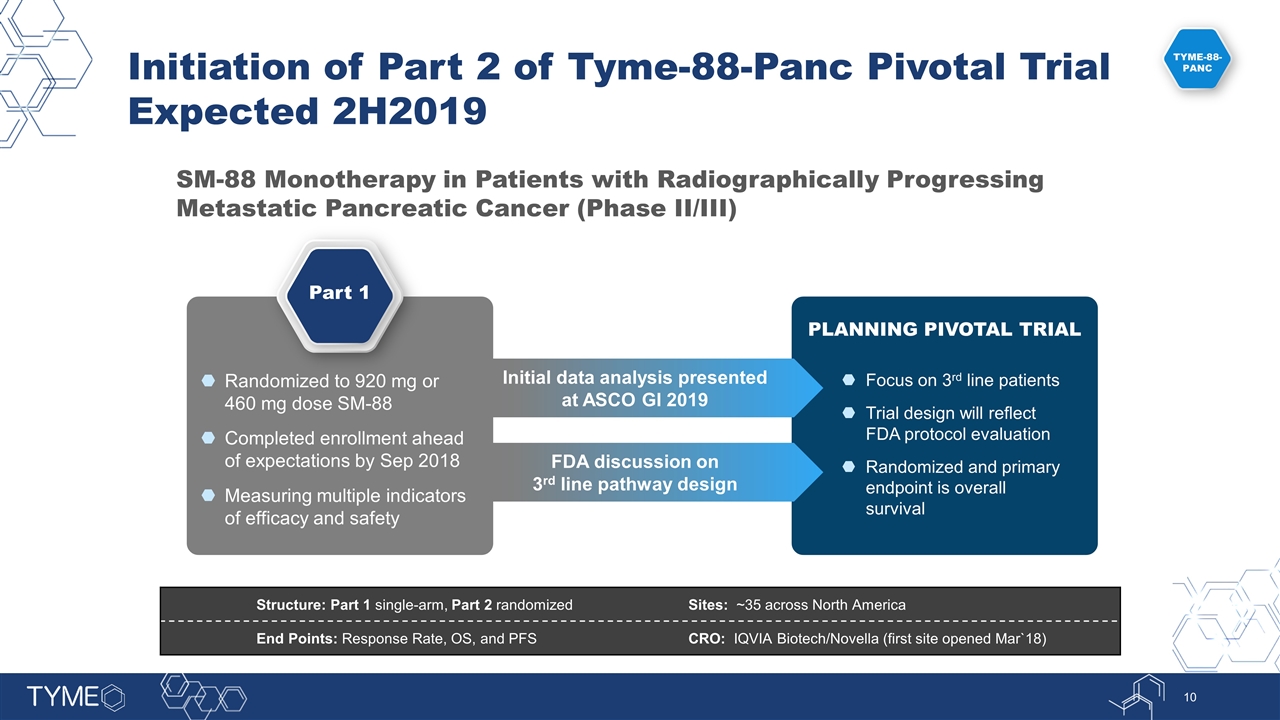
Focus on 3rd line patients Trial design will reflect FDA protocol evaluation Randomized and primary endpoint is overall survival SM-88 Monotherapy in Patients with Radiographically Progressing Metastatic Pancreatic Cancer (Phase II/III) Structure: Part 1 single-arm, Part 2 randomized Sites: ~35 across North America End Points: Response Rate, OS, and PFS CRO: IQVIA Biotech/Novella (first site opened Mar`18) Part 1 Randomized to 920 mg or 460 mg dose SM-88 Completed enrollment ahead of expectations by Sep 2018 Measuring multiple indicators of efficacy and safety Initial data analysis presented at ASCO GI 2019 FDA discussion on 3rd line pathway design PLANNING PIVOTAL TRIAL TYME-88-PANC Initiation of Part 2 of Tyme-88-Panc Pivotal Trial Expected 2H2019
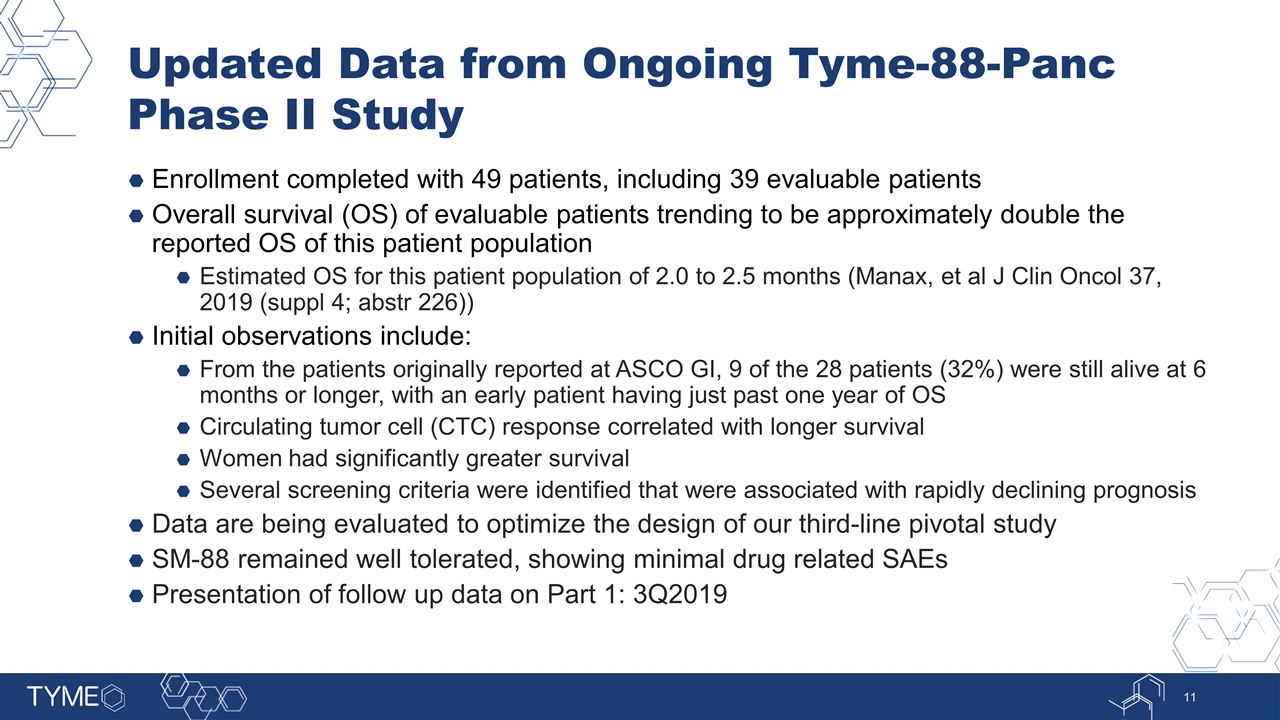
Updated Data from Ongoing Tyme-88-Panc Phase II Study Enrollment completed with 49 patients, including 39 evaluable patients Overall survival (OS) of evaluable patients trending to be approximately double the reported OS of this patient population Estimated OS for this patient population of 2.0 to 2.5 months (Manax, et al J Clin Oncol 37, 2019 (suppl 4; abstr 226)) Initial observations include: From the patients originally reported at ASCO GI, 9 of the 28 patients (32%) were still alive at 6 months or longer, with an early patient having just past one year of OS Circulating tumor cell (CTC) response correlated with longer survival Women had significantly greater survival Several screening criteria were identified that were associated with rapidly declining prognosis Data are being evaluated to optimize the design of our third-line pivotal study SM-88 remained well tolerated, showing minimal drug related SAEs Presentation of follow up data on Part 1: 3Q2019
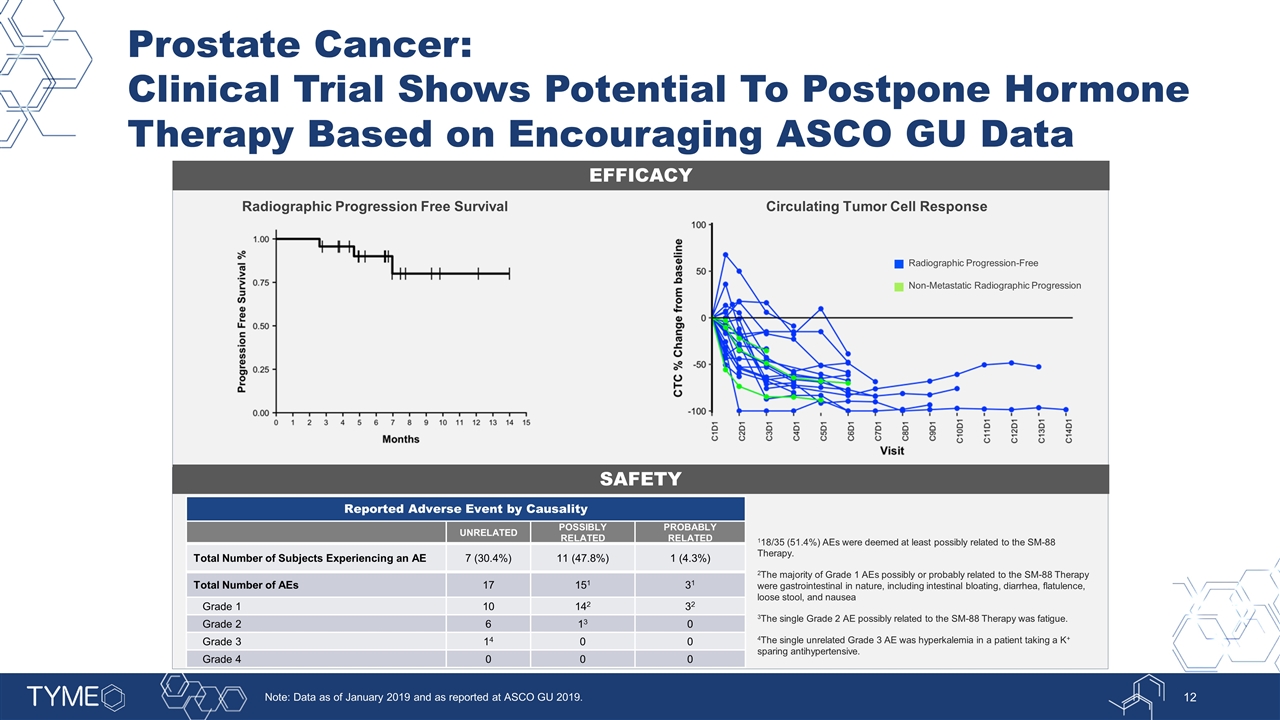
Prostate Cancer: Clinical Trial Shows Potential To Postpone Hormone Therapy Based on Encouraging ASCO GU Data EFFICACY SAFETY Circulating Tumor Cell Response Radiographic Progression Free Survival Radiographic Progression-Free Non-Metastatic Radiographic Progression Reported Adverse Event by Causality UNRELATED POSSIBLY RELATED PROBABLY RELATED Total Number of Subjects Experiencing an AE 7 (30.4%) 11 (47.8%) 1 (4.3%) Total Number of AEs 17 151 31 Grade 1 10 142 32 Grade 2 6 13 0 Grade 3 14 0 0 Grade 4 0 0 0 118/35 (51.4%) AEs were deemed at least possibly related to the SM-88 Therapy. 2The majority of Grade 1 AEs possibly or probably related to the SM-88 Therapy were gastrointestinal in nature, including intestinal bloating, diarrhea, flatulence, loose stool, and nausea 3The single Grade 2 AE possibly related to the SM-88 Therapy was fatigue. 4The single unrelated Grade 3 AE was hyperkalemia in a patient taking a K+ sparing antihypertensive. Note: Data as of January 2019 and as reported at ASCO GU 2019.

Advocacy Partners Michele Korfin, R.Ph., M.B.A., Chief Operating Officer
PanCAN Precision PromiseSM Clinical Trial Consortium Sites PanCAN: World’s Largest Advocate Committed to Curing Pancreatic Cancer Precision Promise is the first response-adaptive randomized clinical trial platform for pancreatic cancer patients in the world and the Pancreatic Cancer Action Network’s groundbreaking initiative to dramatically improve outcomes for pancreatic cancer patients and advance the organization’s goal to double survival. “ ” – Pancreatic Cancer Action Network
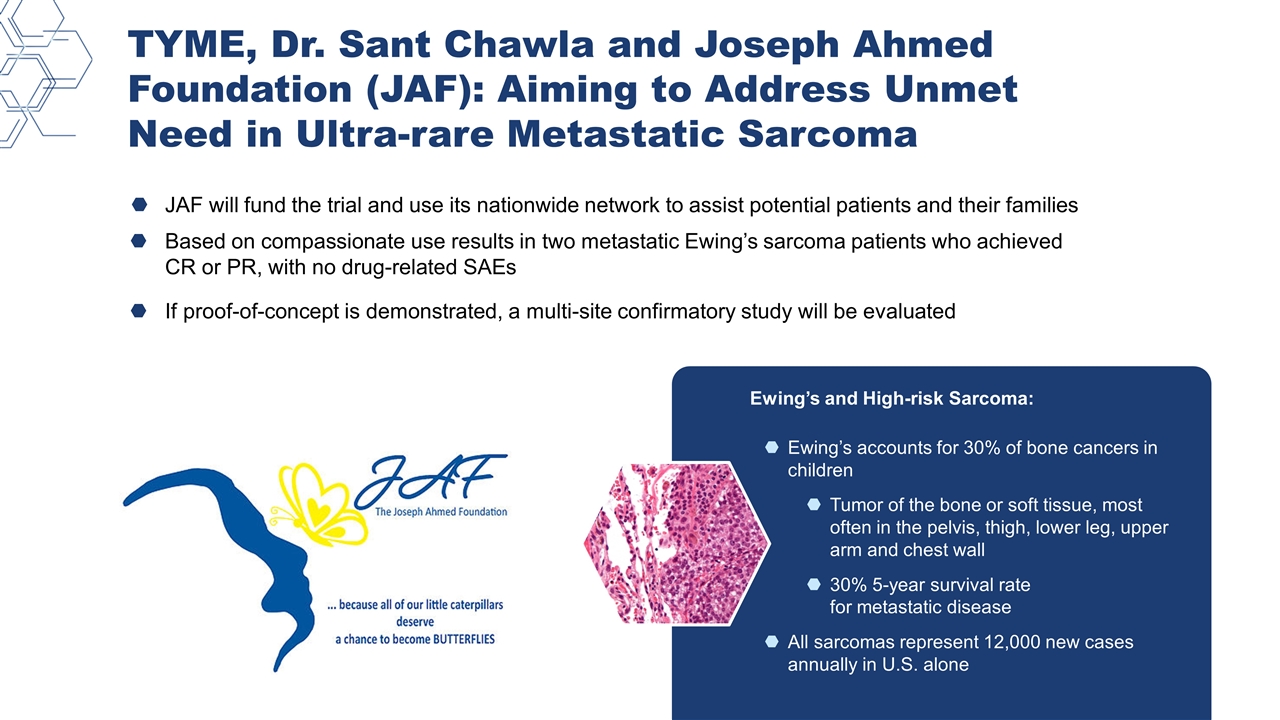
Ewing’s accounts for 30% of bone cancers in children Tumor of the bone or soft tissue, most often in the pelvis, thigh, lower leg, upper arm and chest wall 30% 5-year survival rate for metastatic disease All sarcomas represent 12,000 new cases annually in U.S. alone JAF will fund the trial and use its nationwide network to assist potential patients and their families Based on compassionate use results in two metastatic Ewing’s sarcoma patients who achieved CR or PR, with no drug-related SAEs If proof-of-concept is demonstrated, a multi-site confirmatory study will be evaluated Ewing’s and High-risk Sarcoma: TYME, Dr. Sant Chawla and Joseph Ahmed Foundation (JAF): Aiming to Address Unmet Need in Ultra-rare Metastatic Sarcoma

positioned for success Ben R. Taylor, President & Chief Financial Officer
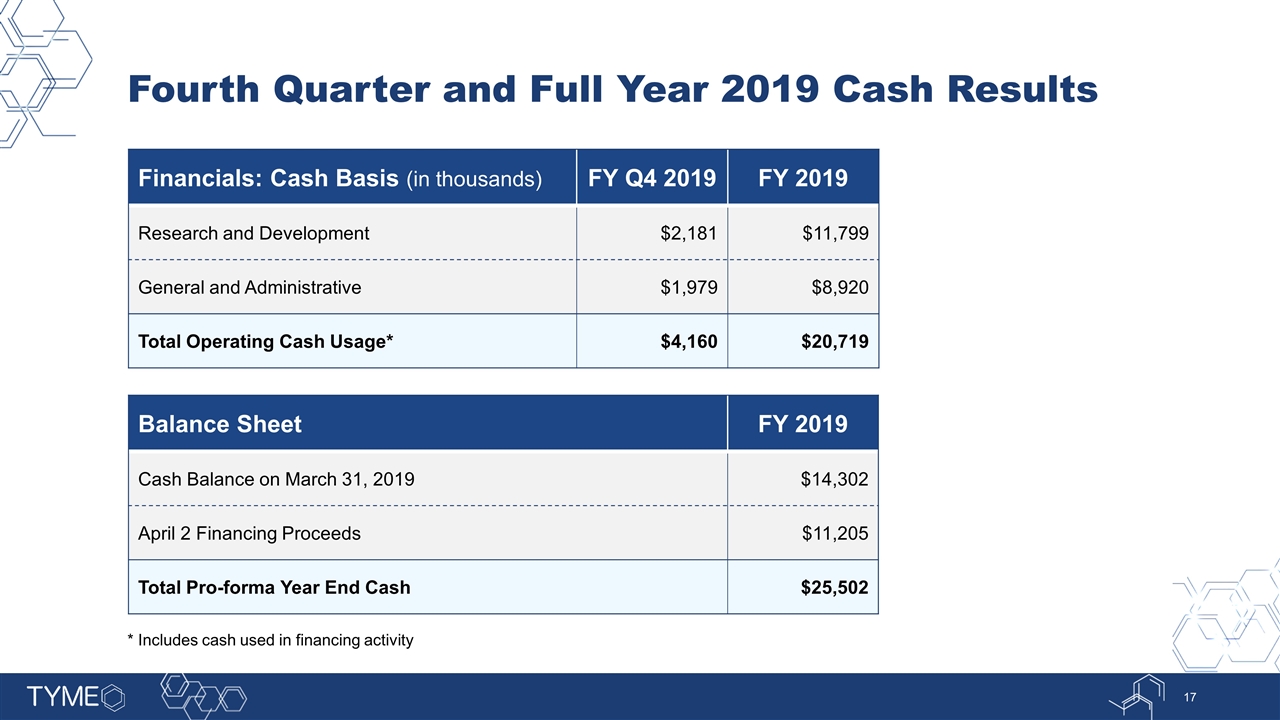
Fourth Quarter and Full Year 2019 Cash Results Financials: Cash Basis (in thousands) FY Q4 2019 FY 2019 Research and Development $2,181 $11,799 General and Administrative $1,979 $8,920 Total Operating Cash Usage* $4,160 $20,719 Balance Sheet FY 2019 Cash Balance on March 31, 2019 $14,302 April 2 Financing Proceeds $11,205 Total Pro-forma Year End Cash $25,502 * Includes cash used in financing activity
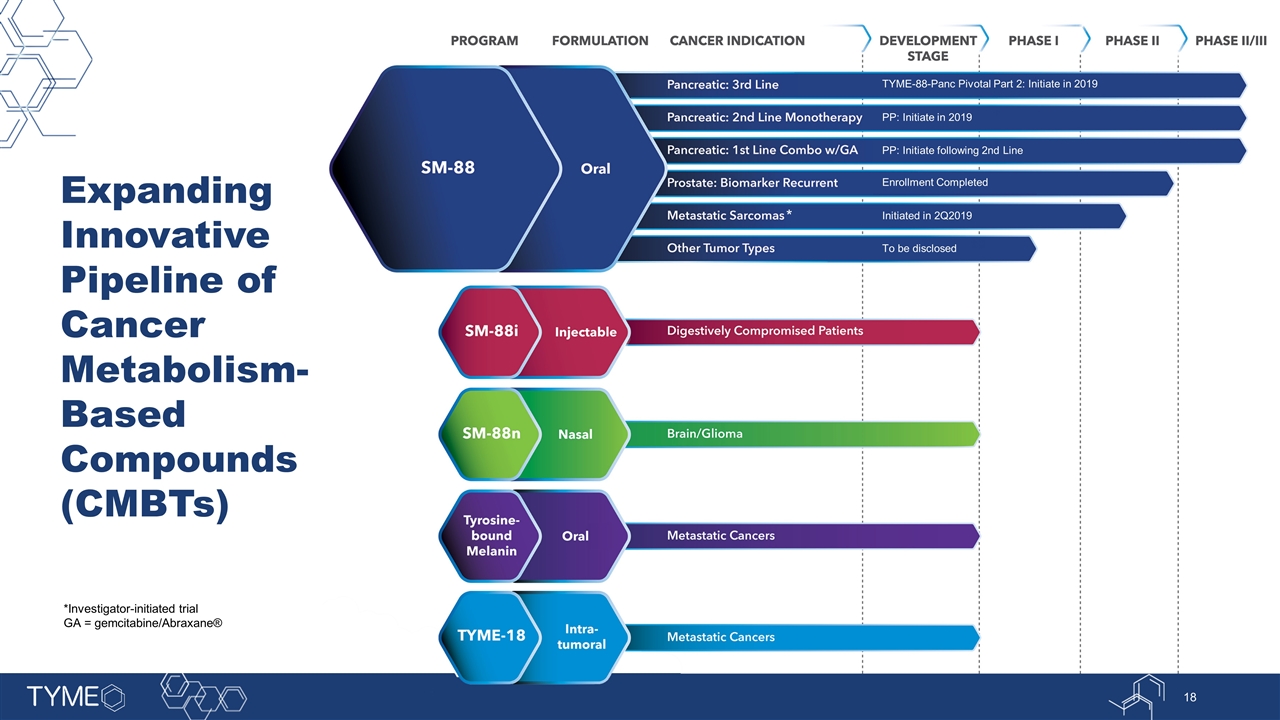
Expanding Innovative Pipeline of Cancer Metabolism-Based Compounds (CMBTs) *Investigator-initiated trial GA = gemcitabine/Abraxane® * TYME-88-Panc Pivotal Part 2: Initiate in 2019 PP: Initiate in 2019 PP: Initiate following 2nd Line Enrollment Completed Initiated in 2Q2019 To be disclosed
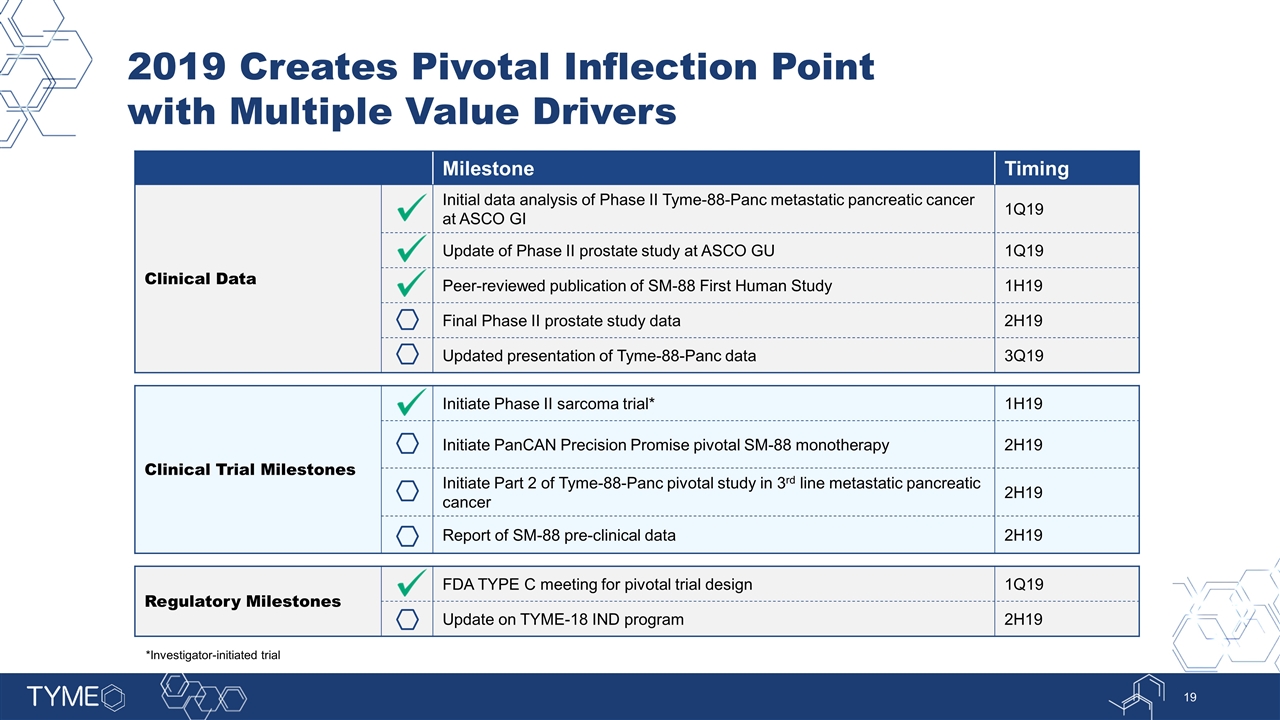
Milestone Timing Clinical Data Initial data analysis of Phase II Tyme-88-Panc metastatic pancreatic cancer at ASCO GI 1Q19 Update of Phase II prostate study at ASCO GU 1Q19 Peer-reviewed publication of SM-88 First Human Study 1H19 Final Phase II prostate study data 2H19 Updated presentation of Tyme-88-Panc data 3Q19 Clinical Trial Milestones Initiate Phase II sarcoma trial* 1H19 Initiate PanCAN Precision Promise pivotal SM-88 monotherapy 2H19 Initiate Part 2 of Tyme-88-Panc pivotal study in 3rd line metastatic pancreatic cancer 2H19 Report of SM-88 pre-clinical data 2H19 Regulatory Milestones FDA TYPE C meeting for pivotal trial design 1Q19 Update on TYME-18 IND program 2H19 2019 Creates Pivotal Inflection Point with Multiple Value Drivers *Investigator-initiated trial




















