
OASIS Phase 1/2a Clinical Trial Safety Results December 21, 2021 Exhibit 99.1

Forward-Looking Statements This presentation contains forward-looking statements as defined in the Private Securities Litigation Reform Act of 1995, as amended. The words “may,” “will,” “could,” “would,” “should,” “expect,” “plan,” “anticipate,” “intend,” “believe,” “estimate,” “predict,” “project,” “potential,” “continue,” “target” and similar expressions are intended to identify forward-looking statements, although not all forward-looking statements contain these identifying words. Clearside Biomedical, Inc.’s views as of the date of this presentation about future events and are subject to risks, uncertainties, assumptions, and changes in circumstances that may cause Clearside’s actual results, performance, or achievements to differ significantly from those expressed or implied in any forward-looking statement. Although Clearside believes that the expectations reflected in the forward-looking statements are reasonable, Clearside cannot guarantee future events, results, performance, or achievements. Some of the key factors that could cause actual results to differ from Clearside’s expectations include its plans to develop and potentially commercialize its product candidates; Clearside’s planned clinical trials and preclinical studies for its product candidates; the timing of and Clearside’s ability to obtain and maintain regulatory approvals for its product candidates; the extent of clinical trials potentially required for Clearside’s product candidates; the clinical utility and market acceptance of Clearside’s product candidates; Clearside’s commercialization, marketing and manufacturing capabilities and strategy; Clearside’s intellectual property position; and Clearside’s ability to identify additional product candidates with significant commercial potential that are consistent with its commercial objectives. For further information regarding these risks, uncertainties and other factors you should read the “Risk Factors” section of Clearside’s Annual Report on Form 10-K for the year ended December 31, 2020, filed with the SEC on March 15, 2021, and Clearside’s other Periodic Reports filed with the SEC. Clearside expressly disclaims any obligation to update or revise the information herein, including the forward-looking statements, except as required by law. This presentation also contains estimates and other statistical data made by independent parties and by Clearside relating to market size and growth and other data about its industry. This data involves a number of assumptions and limitations, and you are cautioned not to give undue weight to such estimates. In addition, projections, assumptions and estimates of Clearside’s future performance and the future performance of the markets in which Clearside operates are necessarily subject to a high degree of uncertainty and risk. 2
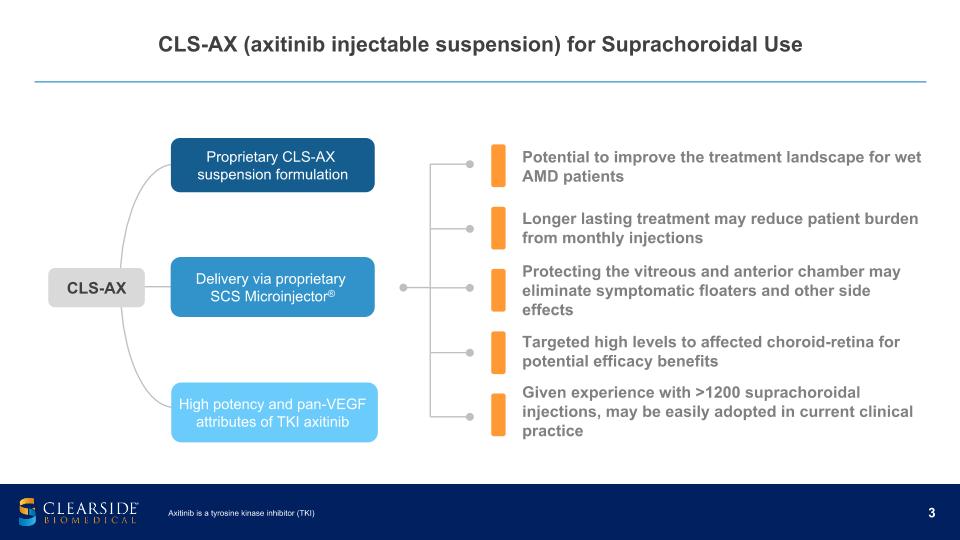
CLS-AX (axitinib injectable suspension) for Suprachoroidal Use Potential to improve the treatment landscape for wet AMD patients Longer lasting treatment may reduce patient burden from monthly injections Protecting the vitreous and anterior chamber may eliminate symptomatic floaters and other side effects Targeted high levels to affected choroid-retina for potential efficacy benefits Given experience with >1200 suprachoroidal injections, may be easily adopted in current clinical practice Axitinib is a tyrosine kinase inhibitor (TKI) Proprietary CLS-AX suspension formulation Delivery via proprietary SCS Microinjector® High potency and pan-VEGF attributes of TKI axitinib CLS-AX
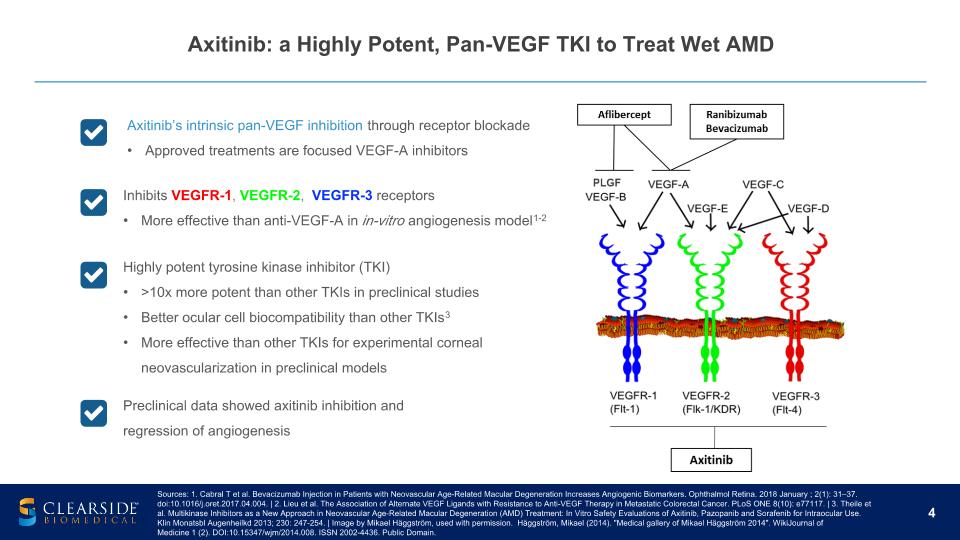
Axitinib: a Highly Potent, Pan-VEGF TKI to Treat Wet AMD Sources: 1. Cabral T et al. Bevacizumab Injection in Patients with Neovascular Age-Related Macular Degeneration Increases Angiogenic Biomarkers. Ophthalmol Retina. 2018 January ; 2(1): 31–37. doi:10.1016/j.oret.2017.04.004. | 2. Lieu et al. The Association of Alternate VEGF Ligands with Resistance to Anti-VEGF Therapy in Metastatic Colorectal Cancer. PLoS ONE 8(10): e77117. | 3. Theile et al. Multikinase Inhibitors as a New Approach in Neovascular Age-Related Macular Degeneration (AMD) Treatment: In Vitro Safety Evaluations of Axitinib, Pazopanib and Sorafenib for Intraocular Use. Klin Monatsbl Augenheilkd 2013; 230: 247-254. | Image by Mikael Häggström, used with permission. Häggström, Mikael (2014). "Medical gallery of Mikael Häggström 2014". WikiJournal of Medicine 1 (2). DOI:10.15347/wjm/2014.008. ISSN 2002-4436. Public Domain. Axitinib’s intrinsic pan-VEGF inhibition through receptor blockade Approved treatments are focused VEGF-A inhibitors Inhibits VEGFR-1, VEGFR-2, VEGFR-3 receptors More effective than anti-VEGF-A in in-vitro angiogenesis model1-2 Highly potent tyrosine kinase inhibitor (TKI) >10x more potent than other TKIs in preclinical studies Better ocular cell biocompatibility than other TKIs3 More effective than other TKIs for experimental corneal neovascularization in preclinical models Preclinical data showed axitinib inhibition and regression of angiogenesis
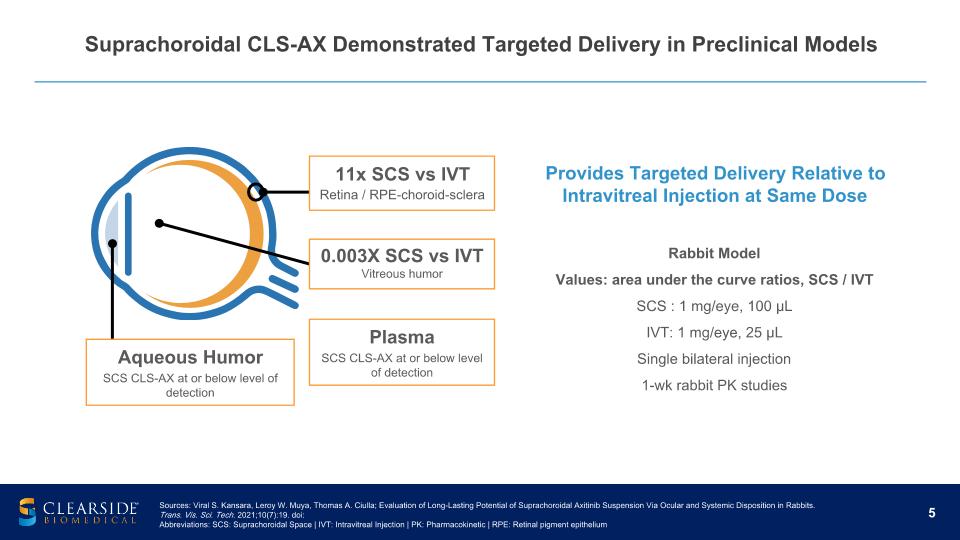
Suprachoroidal CLS-AX Demonstrated Targeted Delivery in Preclinical Models Plasma SCS CLS-AX at or below level of detection Rabbit Model Values: area under the curve ratios, SCS / IVT SCS : 1 mg/eye, 100 µL IVT: 1 mg/eye, 25 µL Single bilateral injection 1-wk rabbit PK studies 11x SCS vs IVT Retina / RPE-choroid-sclera 0.003X SCS vs IVT Vitreous humor Aqueous Humor SCS CLS-AX at or below level of detection Sources: Viral S. Kansara, Leroy W. Muya, Thomas A. Ciulla; Evaluation of Long-Lasting Potential of Suprachoroidal Axitinib Suspension Via Ocular and Systemic Disposition in Rabbits. Trans. Vis. Sci. Tech. 2021;10(7):19. doi: Abbreviations: SCS: Suprachoroidal Space | IVT: Intravitreal Injection | PK: Pharmacokinetic | RPE: Retinal pigment epithelium Provides Targeted Delivery Relative to Intravitreal Injection at Same Dose
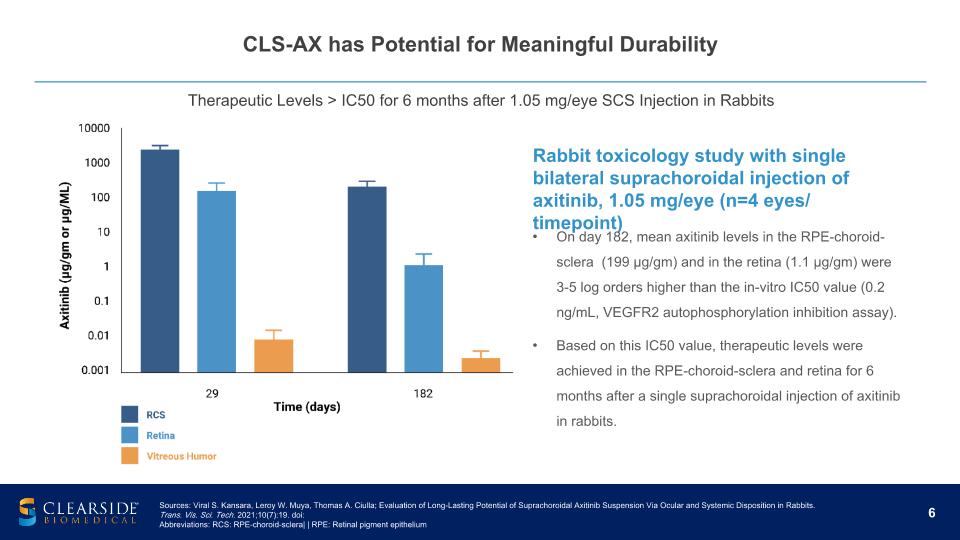
CLS-AX has Potential for Meaningful Durability Therapeutic Levels > IC50 for 6 months after 1.05 mg/eye SCS Injection in Rabbits Rabbit toxicology study with single bilateral suprachoroidal injection of axitinib, 1.05 mg/eye (n=4 eyes/ timepoint) On day 182, mean axitinib levels in the RPE-choroid-sclera (199 µg/gm) and in the retina (1.1 µg/gm) were 3-5 log orders higher than the in-vitro IC50 value (0.2 ng/mL, VEGFR2 autophosphorylation inhibition assay). Based on this IC50 value, therapeutic levels were achieved in the RPE-choroid-sclera and retina for 6 months after a single suprachoroidal injection of axitinib in rabbits. Sources: Viral S. Kansara, Leroy W. Muya, Thomas A. Ciulla; Evaluation of Long-Lasting Potential of Suprachoroidal Axitinib Suspension Via Ocular and Systemic Disposition in Rabbits. Trans. Vis. Sci. Tech. 2021;10(7):19. doi: Abbreviations: RCS: RPE-choroid-sclera| | RPE: Retinal pigment epithelium
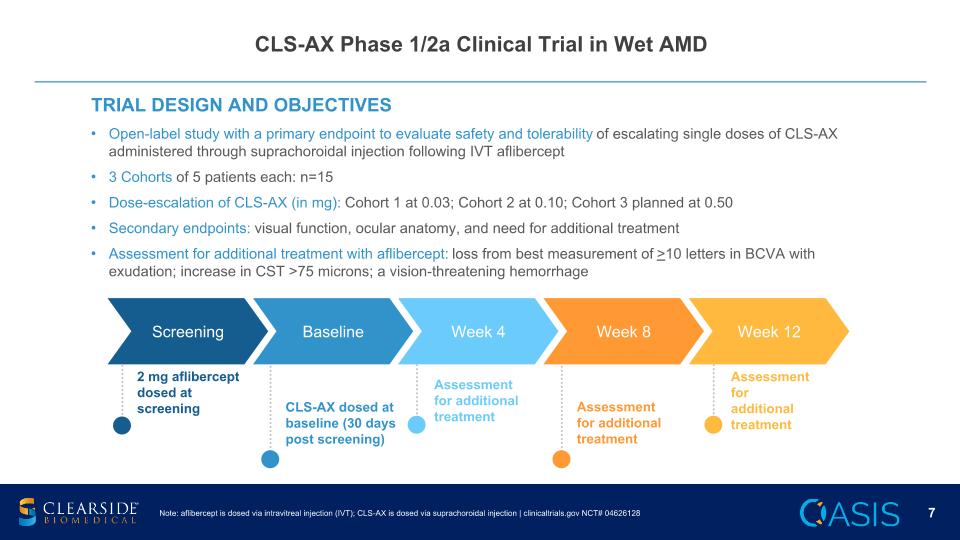
CLS-AX Phase 1/2a Clinical Trial in Wet AMD TRIAL DESIGN AND OBJECTIVES Open-label study with a primary endpoint to evaluate safety and tolerability of escalating single doses of CLS-AX administered through suprachoroidal injection following IVT aflibercept 3 Cohorts of 5 patients each: n=15 Dose-escalation of CLS-AX (in mg): Cohort 1 at 0.03; Cohort 2 at 0.10; Cohort 3 planned at 0.50 Secondary endpoints: visual function, ocular anatomy, and need for additional treatment Assessment for additional treatment with aflibercept: loss from best measurement of >10 letters in BCVA with exudation; increase in CST >75 microns; a vision-threatening hemorrhage Screening Baseline Week 4 Week 8 Week 12 2 mg aflibercept dosed at screening Assessment for additional treatment CLS-AX dosed at baseline (30 days post screening) Assessment for additional treatment Assessment for additional treatment Note: aflibercept is dosed via intravitreal injection (IVT); CLS-AX is dosed via suprachoroidal injection | clinicaltrials.gov NCT# 04626128
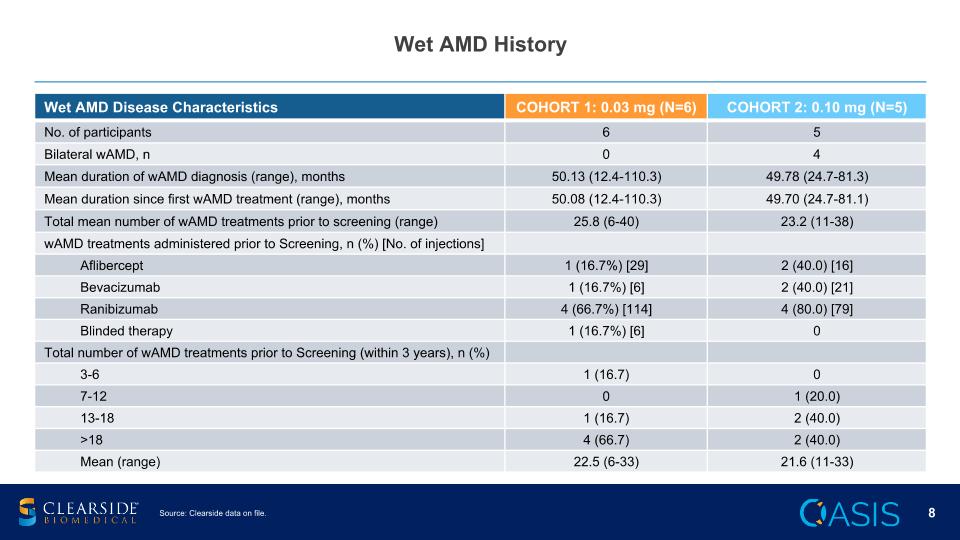
Wet AMD History Wet AMD Disease Characteristics COHORT 1: 0.03 mg (N=6) COHORT 2: 0.10 mg (N=5) No. of participants 6 5 Bilateral wAMD, n 0 4 Mean duration of wAMD diagnosis (range), months 50.13 (12.4-110.3) 49.78 (24.7-81.3) Mean duration since first wAMD treatment (range), months 50.08 (12.4-110.3) 49.70 (24.7-81.1) Total mean number of wAMD treatments prior to screening (range) 25.8 (6-40) 23.2 (11-38) wAMD treatments administered prior to Screening, n (%) [No. of injections] Aflibercept 1 (16.7%) [29] 2 (40.0) [16] Bevacizumab 1 (16.7%) [6] 2 (40.0) [21] Ranibizumab 4 (66.7%) [114] 4 (80.0) [79] Blinded therapy 1 (16.7%) [6] 0 Total number of wAMD treatments prior to Screening (within 3 years), n (%) 3-6 1 (16.7) 0 7-12 0 1 (20.0) 13-18 1 (16.7) 2 (40.0) >18 4 (66.7) 2 (40.0) Mean (range) 22.5 (6-33) 21.6 (11-33) Source: Clearside data on file.
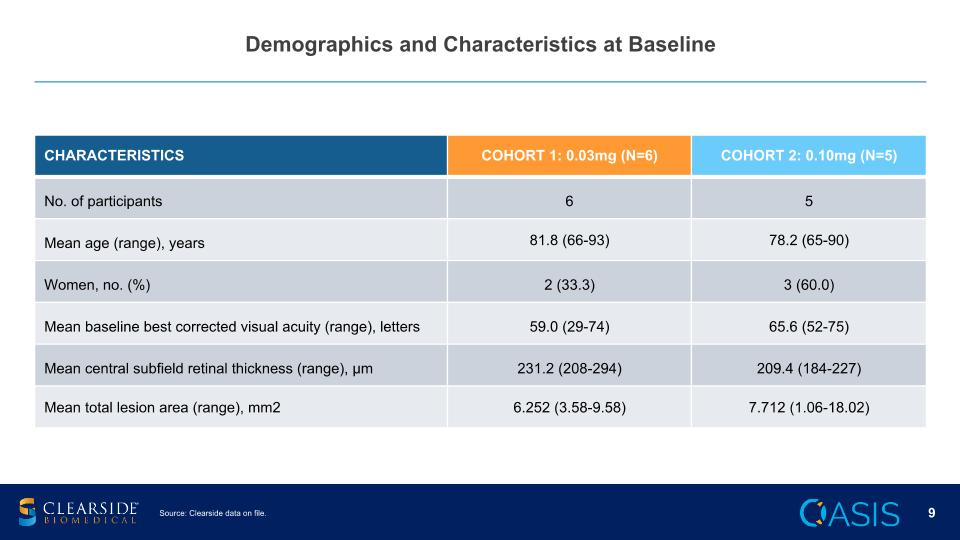
Demographics and Characteristics at Baseline CHARACTERISTICS COHORT 1: 0.03mg (N=6) COHORT 2: 0.10mg (N=5) No. of participants 6 5 Mean age (range), years 81.8 (66-93) 78.2 (65-90) Women, no. (%) 2 (33.3) 3 (60.0) Mean baseline best corrected visual acuity (range), letters 59.0 (29-74) 65.6 (52-75) Mean central subfield retinal thickness (range), µm 231.2 (208-294) 209.4 (184-227) Mean total lesion area (range), mm2 6.252 (3.58-9.58) 7.712 (1.06-18.02) Source: Clearside data on file.
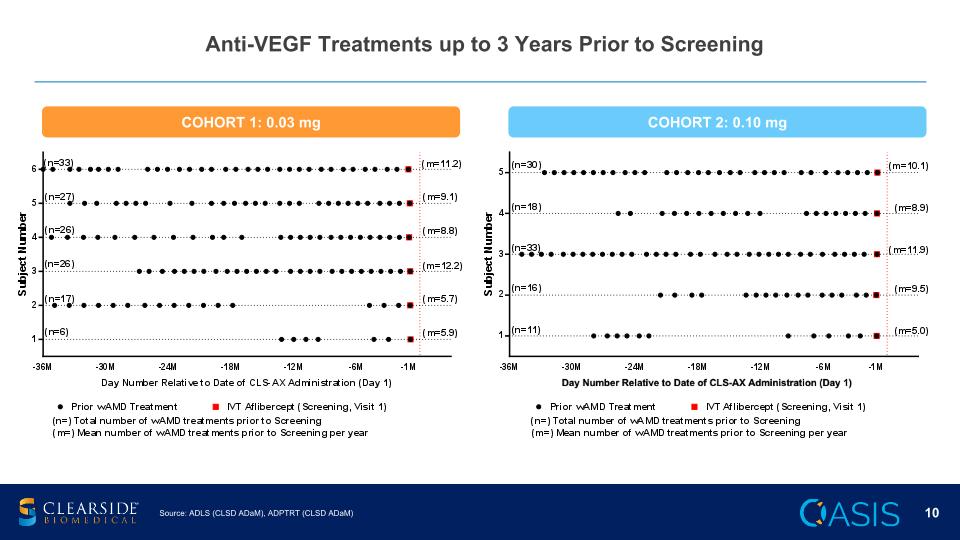
Anti-VEGF Treatments up to 3 Years Prior to Screening Source: ADLS (CLSD ADaM), ADPTRT (CLSD ADaM) COHORT 1: 0.03 mg COHORT 2: 0.10 mg
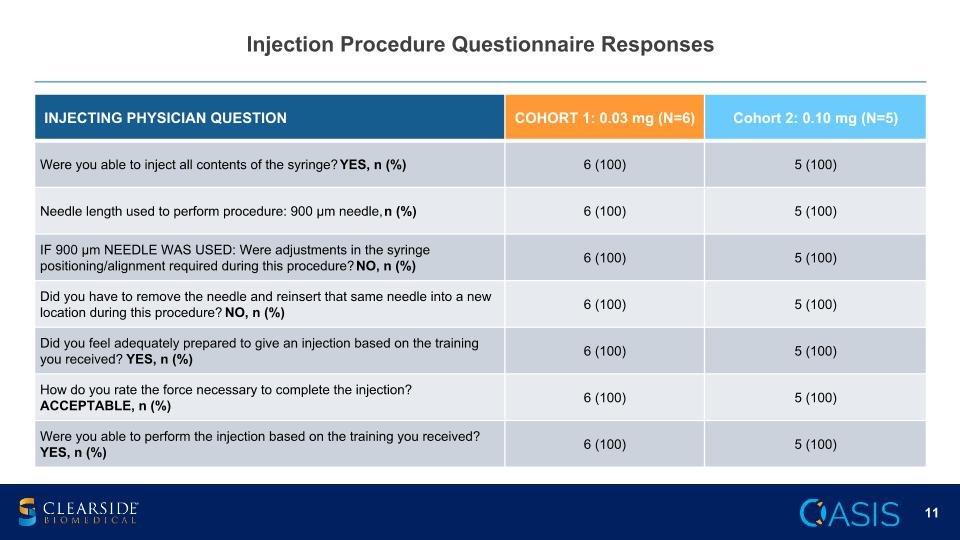
Injection Procedure Questionnaire Responses INJECTING PHYSICIAN QUESTION COHORT 1: 0.03 mg (N=6) Cohort 2: 0.10 mg (N=5) Were you able to inject all contents of the syringe? YES, n (%) 6 (100) 5 (100) Needle length used to perform procedure: 900 µm needle, n (%) 6 (100) 5 (100) IF 900 µm NEEDLE WAS USED: Were adjustments in the syringe positioning/alignment required during this procedure? NO, n (%) 6 (100) 5 (100) Did you have to remove the needle and reinsert that same needle into a new location during this procedure? NO, n (%) 6 (100) 5 (100) Did you feel adequately prepared to give an injection based on the training you received? YES, n (%) 6 (100) 5 (100) How do you rate the force necessary to complete the injection? ACCEPTABLE, n (%) 6 (100) 5 (100) Were you able to perform the injection based on the training you received? YES, n (%) 6 (100) 5 (100)
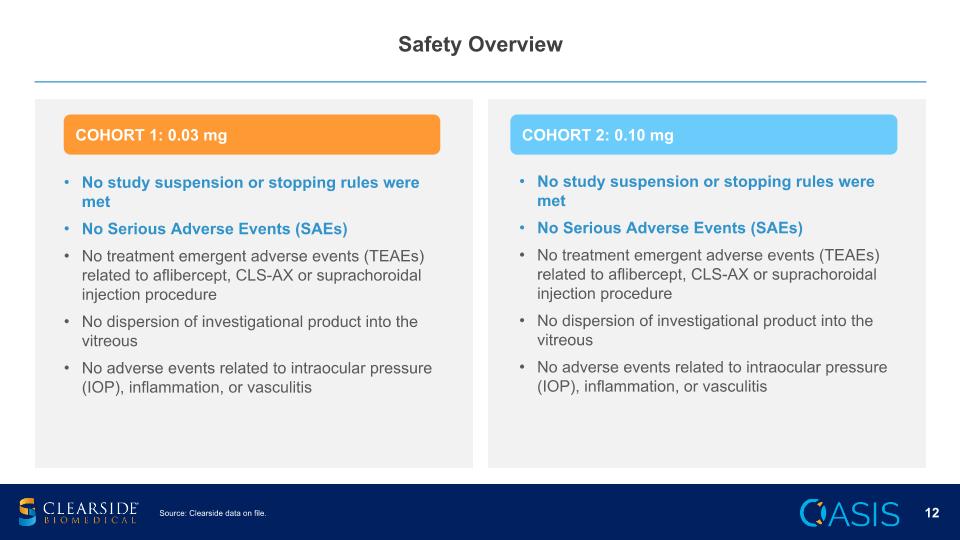
Safety Overview No study suspension or stopping rules were met No Serious Adverse Events (SAEs) No treatment emergent adverse events (TEAEs) related to aflibercept, CLS-AX or suprachoroidal injection procedure No dispersion of investigational product into the vitreous No adverse events related to intraocular pressure (IOP), inflammation, or vasculitis COHORT 1: 0.03 mg COHORT 2: 0.10 mg Source: Clearside data on file. No study suspension or stopping rules were met No Serious Adverse Events (SAEs) No treatment emergent adverse events (TEAEs) related to aflibercept, CLS-AX or suprachoroidal injection procedure No dispersion of investigational product into the vitreous No adverse events related to intraocular pressure (IOP), inflammation, or vasculitis
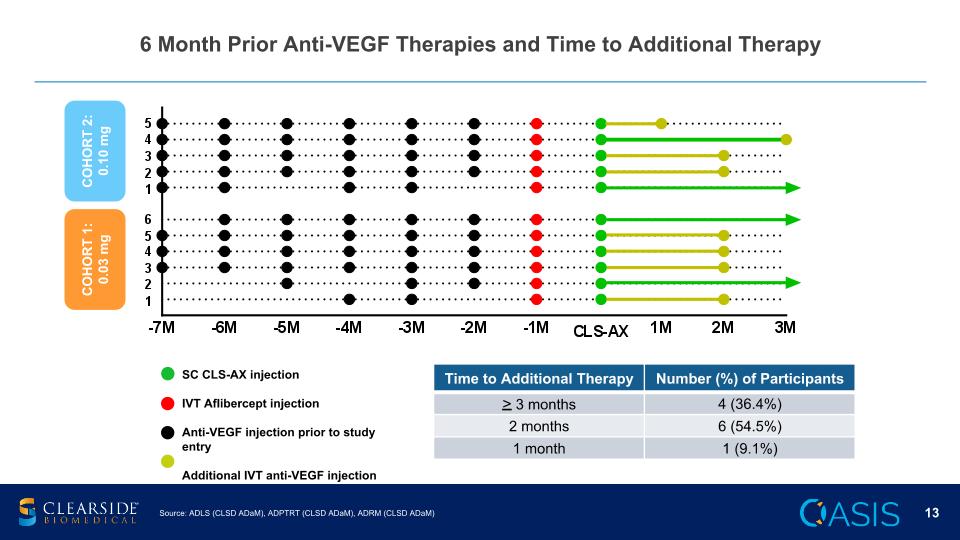
6 Month Prior Anti-VEGF Therapies and Time to Additional Therapy Source: ADLS (CLSD ADaM), ADPTRT (CLSD ADaM), ADRM (CLSD ADaM) Time to Additional Therapy Number (%) of Participants > 3 months 4 (36.4%) 2 months 6 (54.5%) 1 month 1 (9.1%) SC CLS-AX injection IVT Aflibercept injection Anti-VEGF injection prior to study entry Additional IVT anti-VEGF injection COHORT 1: 0.03 mg COHORT 2: 0.10 mg
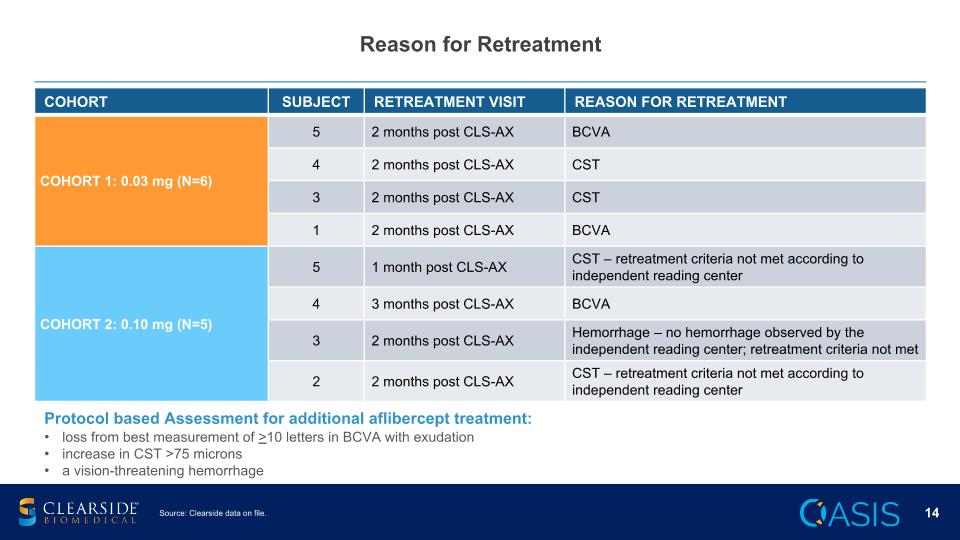
Reason for Retreatment COHORT SUBJECT RETREATMENT VISIT REASON FOR RETREATMENT COHORT 1: 0.03 mg (N=6) 5 2 months post CLS-AX BCVA 4 2 months post CLS-AX CST 3 2 months post CLS-AX CST 1 2 months post CLS-AX BCVA COHORT 2: 0.10 mg (N=5) 5 1 month post CLS-AX CST – retreatment criteria not met according to independent reading center Cohort 2: 0.10 mg (N=5) 4 3 months post CLS-AX BCVA 3 2 months post CLS-AX Hemorrhage – no hemorrhage observed by the independent reading center; retreatment criteria not met 2 2 months post CLS-AX CST – retreatment criteria not met according to independent reading center Protocol based Assessment for additional aflibercept treatment: loss from best measurement of >10 letters in BCVA with exudation increase in CST >75 microns a vision-threatening hemorrhage Source: Clearside data on file.
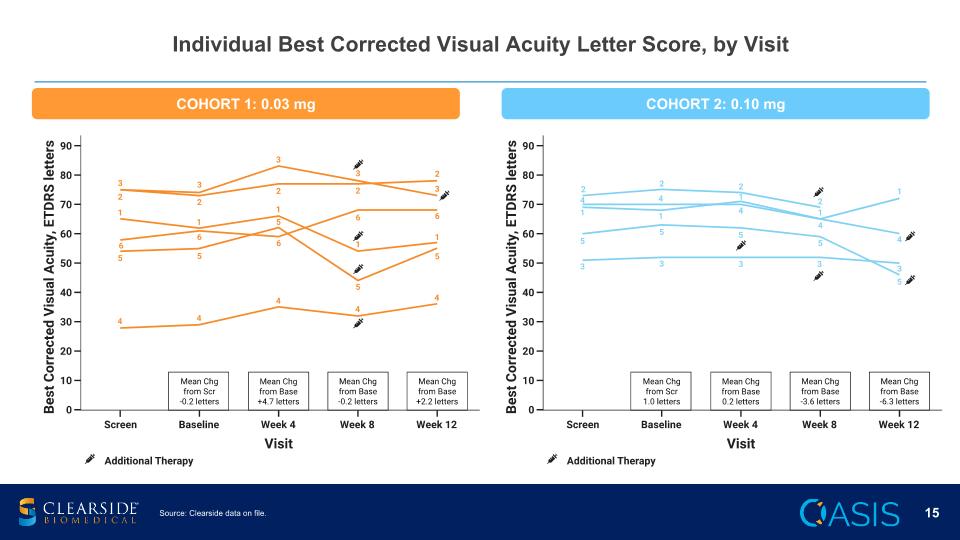
Individual Best Corrected Visual Acuity Letter Score, by Visit COHORT 1: 0.03 mg COHORT 2: 0.10 mg Source: Clearside data on file.
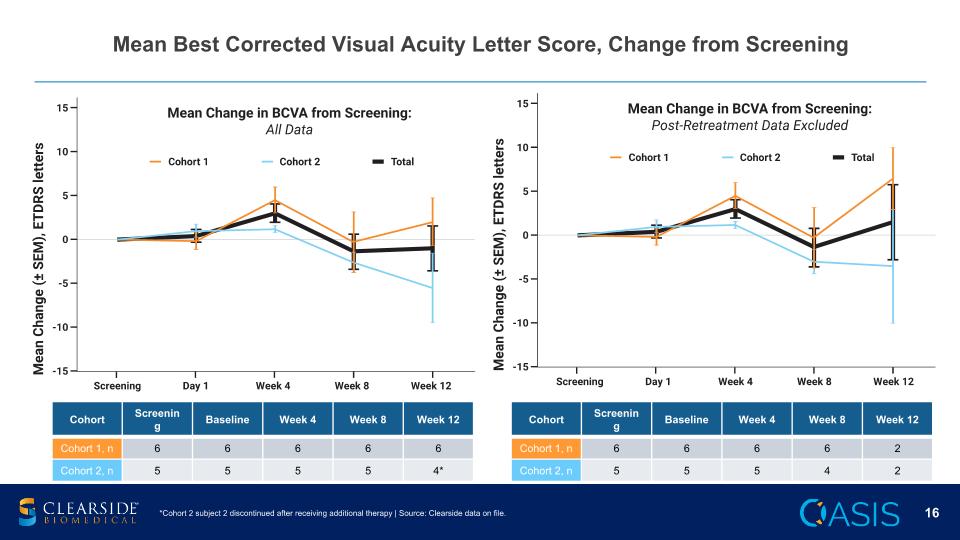
Mean Best Corrected Visual Acuity Letter Score, Change from Screening *Cohort 2 subject 2 discontinued after receiving additional therapy | Source: Clearside data on file. Cohort Screening Baseline Week 4 Week 8 Week 12 Cohort 1, n 6 6 6 6 6 Cohort 2, n 5 5 5 5 4* Cohort Screening Baseline Week 4 Week 8 Week 12 Cohort 1, n 6 6 6 6 2 Cohort 2, n 5 5 5 4 2
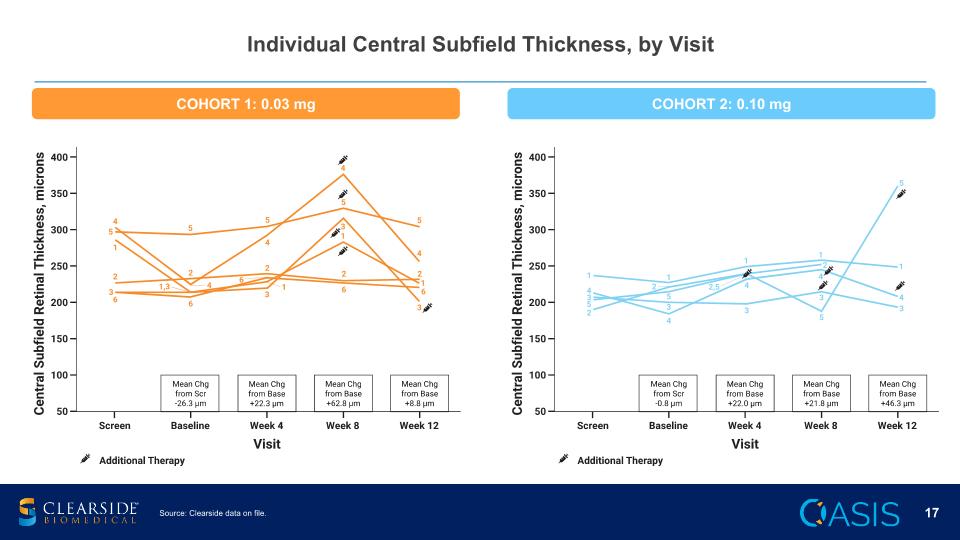
Individual Central Subfield Thickness, by Visit Source: Clearside data on file. COHORT 1: 0.03 mg COHORT 2: 0.10 mg
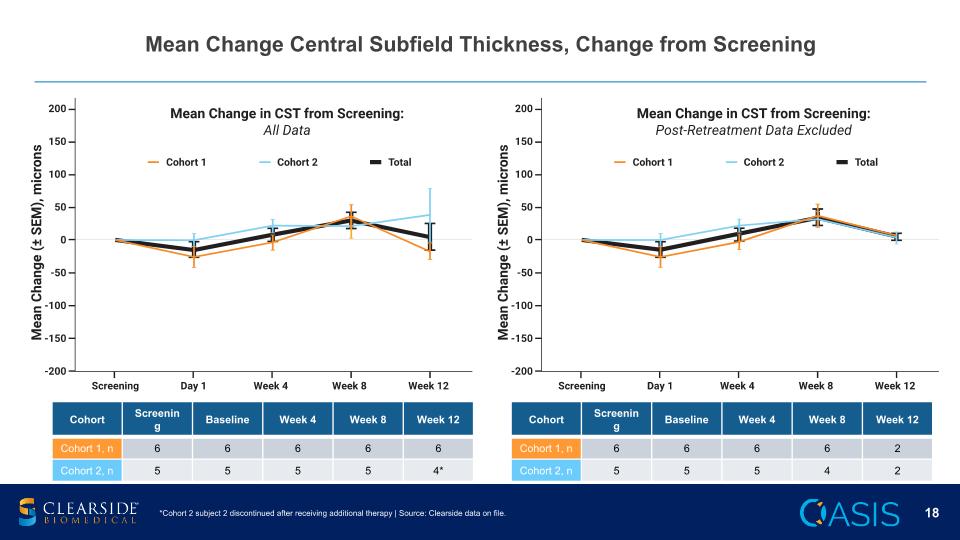
Mean Change Central Subfield Thickness, Change from Screening Cohort Screening Baseline Week 4 Week 8 Week 12 Cohort 1, n 6 6 6 6 6 Cohort 2, n 5 5 5 5 4* Cohort Screening Baseline Week 4 Week 8 Week 12 Cohort 1, n 6 6 6 6 2 Cohort 2, n 5 5 5 4 2 *Cohort 2 subject 2 discontinued after receiving additional therapy | Source: Clearside data on file.

OASIS Cohort 1 & 2 Results Support Advancing to Cohort 3 DURABILITY POST CLS-AX IN HEAVILY PRE-TREATED PATIENTS 4/11 (36%) of patients did not require additional therapy for > 3 months 6/11 (55%) of patients did not require additional therapy for 2 months 1/11 (9%) patient was retreated at 1 month VISUAL ACUITY Stable visual acuity, on average, over three months even after excluding patients who were retreated ANATOMIC EFFECTS Stable disease activity (based on CST), on average, over three months even after excluding patients who were retreated SAFETY CLS-AX well tolerated with no dose limiting toxicities No dispersion of investigational product into the vitreous No adverse events related to intraocular pressure (IOP), inflammation, or vasculitis Source: Clearside data on file. COHORT 1 & 2 RESULTS
Cohort 1 0.03 mg Cohort 2 0.1 mg Cohort 3 0.5 mg Given that this is the first time a tyrosine kinase inhibitor has been injected suprachoroidally in humans, we initiated OASIS with a low dose to establish a foundation for safety. The lack of dose limiting toxicities in Cohorts 1 and 2, along with preclinical toxicology studies, support greater dose escalation than previously planned. Protocol Dosing Change for Cohort 3

Nasdaq: CLSD




















