
ODYSSEY Phase 2b Clinical Trial Results October 9, 2024 Exhibit 99.1

Forward-Looking Statements This presentation contains forward-looking statements as defined in the Private Securities Litigation Reform Act of 1995, as amended. The words “may,” “will,” “could,” “would,” “should,” “expect,” “plan,” “anticipate,” “intend,” “believe,” “estimate,” “predict,” “project,” “potential,” “continue,” “target” or the negative of these terms and other similar words or expressions are intended to identify forward-looking statements, although not all forward-looking statements contain these identifying words. Clearside Biomedical, Inc.’s views as of the date of this presentation about future events and are subject to risks, uncertainties, assumptions, and changes in circumstances that may cause Clearside’s actual results, performance, or achievements to differ significantly from those expressed or implied in any forward-looking statement. These forward looking statements include statements regarding the clinical development of CLS-AX, the timing of correspondence with regulatory authorities, and the trial design features of a potential Phase 3 trial. Although Clearside believes that the expectations reflected in the forward-looking statements are reasonable, new risks and uncertainties may emerge from time to time, and Clearside cannot guarantee future events, results, performance, or achievements. Some of the key factors that could cause actual results to differ from Clearside’s expectations include its plans to develop and potentially commercialize its product candidates; adverse differences between preliminary or interim data and final data; Clearside’s planned clinical trials and preclinical studies for its product candidates; the timing of and Clearside’s ability to obtain and maintain regulatory approvals for its product candidates; the extent of clinical trials potentially required for Clearside’s product candidates; the clinical utility and market acceptance of Clearside’s product candidates; Clearside’s commercialization, marketing and manufacturing capabilities and strategy; Clearside’s intellectual property position; Clearside's ability to expand its pipeline; developments and projections relating to Clearside's competitors and its industry; the impact of government laws and regulations; the timing and anticipated results of Clearside's preclinical studies and clinical trials and the risk that the results of Clearside's preclinical studies and clinical trials may not be predictive of future results in connection with future studies or clinical trials and may not support further development and marketing approval; findings from investigational review boards at clinical trial sites and publication review bodies; Clearside's estimates regarding future revenue, expenses, capital requirements and need for additional financing; and Clearside’s ability to identify additional product candidates with significant commercial potential that are consistent with its commercial objectives. For further information regarding these risks, uncertainties and other factors you should read the “Risk Factors” section of Clearside’s Annual Report on Form 10-K for the year ended December 31, 2023, filed with the U.S. Securities and Exchange Commission (SEC) on March 12, 2024, , Clearside’s Quarterly Report on Form 10-Q filed with the SEC on August 12, 2024, and Clearside's subsequent filings with the SEC. Clearside expressly disclaims any obligation to update or revise the information herein, including the forward-looking statements, except as required by law. In addition, projections, assumptions and estimates of Clearside’s future performance and the future performance of the markets in which Clearside operates are necessarily subject to a high degree of uncertainty and risk. 2
CLS-AX Now Phase 3 Ready Based on Positive ODYSSEY Data in Wet AMD Enrolled Only Difficult-to-Treat Participants with Active Disease Achieved Primary Outcome Maintaining Stable BCVA with Repeat Dosing Compelling Intervention-Free Rates Abbreviation: Wet AMD = neovascular age-related macular degeneration; BCVA = Best Corrected Visual Acuity Positive Safety Profile with Repeat Dosing Preliminary Topline Results Subject to Change

Delivering on the Potential of the Suprachoroidal Space Proven Leader in Suprachoroidal Delivery with Thousands of Injections Performed in the Clinic Validated Suprachoroidal Space (SCS) Delivery with Approved Product, Multiple Collaborations, and Comprehensive IP Portfolio Differentiated SCS Clinical Program Targeting Multi-Billion Dollar Wet AMD Market 4
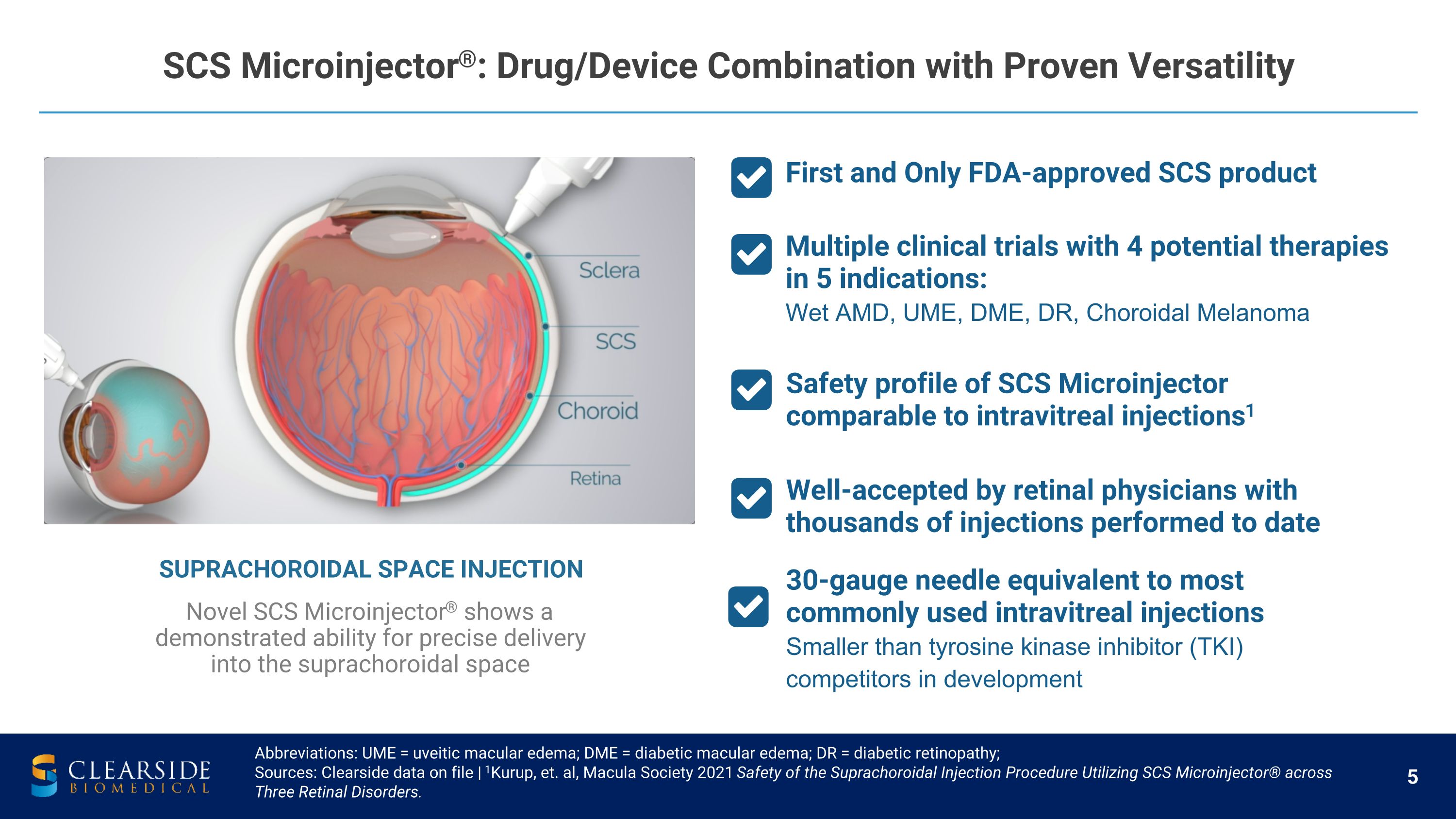
Abbreviations: UME = uveitic macular edema; DME = diabetic macular edema; DR = diabetic retinopathy; Sources: Clearside data on file | 1Kurup, et. al, Macula Society 2021 Safety of the Suprachoroidal Injection Procedure Utilizing SCS Microinjector® across Three Retinal Disorders. Well-accepted by retinal physicians with thousands of injections performed to date Multiple clinical trials with 4 potential therapies in 5 indications: Wet AMD, UME, DME, DR, Choroidal Melanoma Safety profile of SCS Microinjector comparable to intravitreal injections1 SUPRACHOROIDAL SPACE INJECTION Novel SCS Microinjector® shows a demonstrated ability for precise delivery into the suprachoroidal space 30-gauge needle equivalent to most commonly used intravitreal injections Smaller than tyrosine kinase inhibitor (TKI) competitors in development SCS Microinjector®: Drug/Device Combination with Proven Versatility First and Only FDA-approved SCS product

CLS-AX for the Treatment of Wet AMD 6
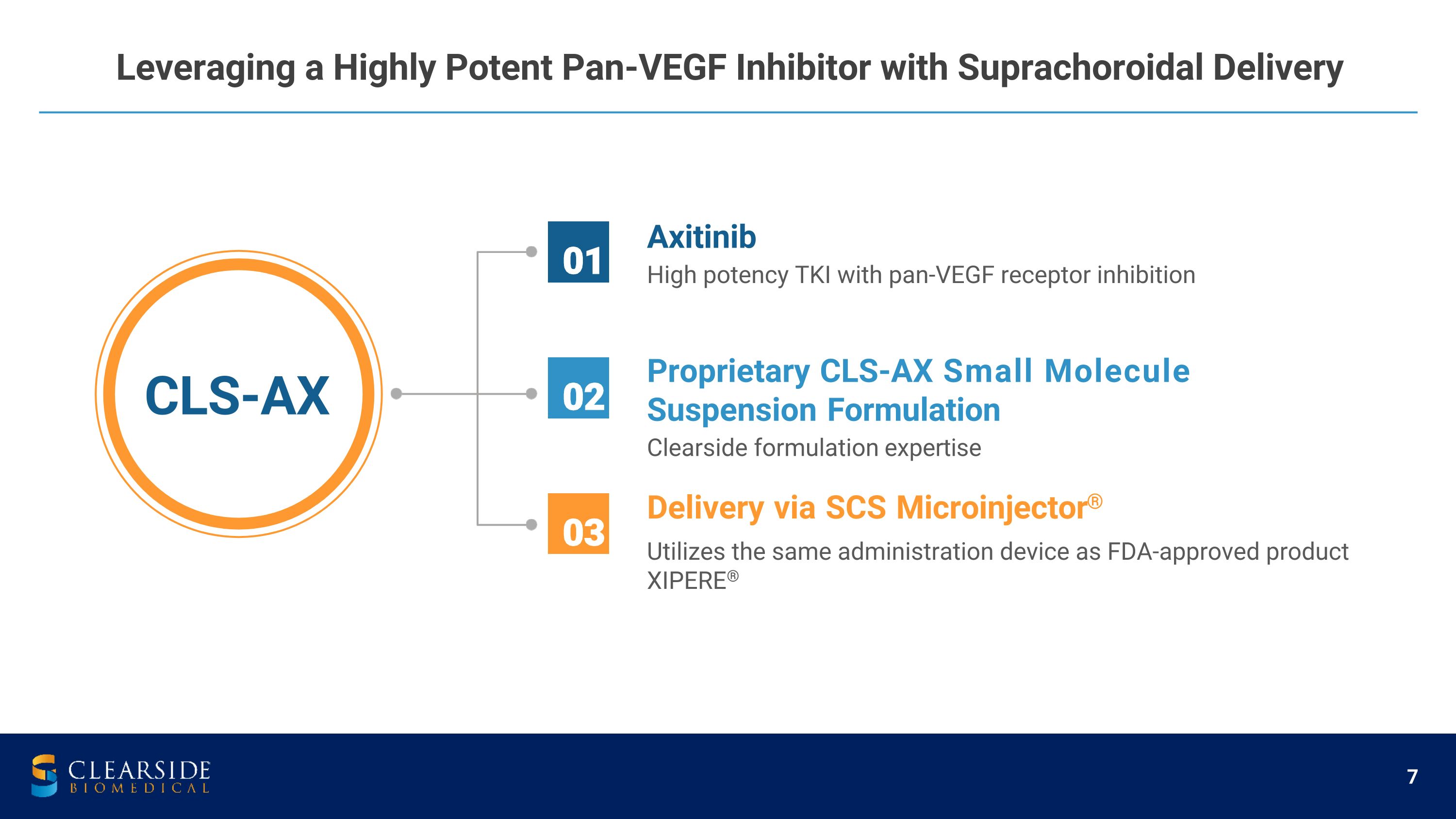
Leveraging a Highly Potent Pan-VEGF Inhibitor with Suprachoroidal Delivery Axitinib High potency TKI with pan-VEGF receptor inhibition Proprietary CLS-AX Small Molecule Suspension Formulation Clearside formulation expertise Delivery via SCS Microinjector® Utilizes the same administration device as FDA-approved product XIPERE® 01 02 03 CLS-AX
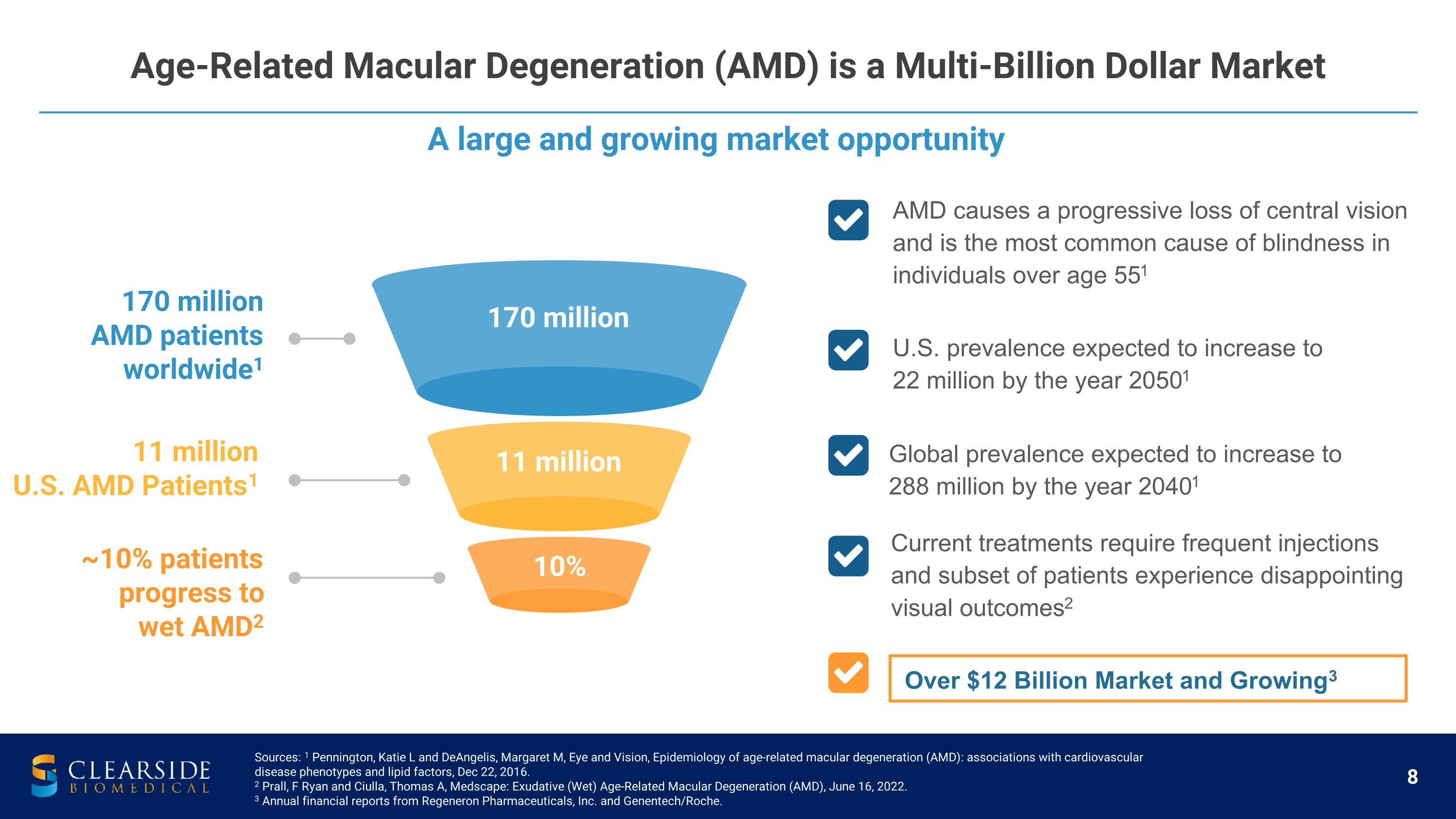
A large and growing market opportunity Age-Related Macular Degeneration (AMD) is a Multi-Billion Dollar Market Sources: 1 Pennington, Katie L and DeAngelis, Margaret M, Eye and Vision, Epidemiology of age-related macular degeneration (AMD): associations with cardiovascular disease phenotypes and lipid factors, Dec 22, 2016. 2 Prall, F Ryan and Ciulla, Thomas A, Medscape: Exudative (Wet) Age-Related Macular Degeneration (AMD), June 16, 2022. 3 Annual financial reports from Regeneron Pharmaceuticals, Inc. and Genentech/Roche. ~10% patients�progress to �wet AMD2 11 million U.S. AMD Patients1 170 million AMD patients worldwide1 170 million 11 million 10% AMD causes a progressive loss of central vision and is the most common cause of blindness in individuals over age 551 U.S. prevalence expected to increase to 22 million by the year 20501 Global prevalence expected to increase to 288 million by the year 20401 Current treatments require frequent injections and subset of patients experience disappointing �visual outcomes2 Over $12 Billion Market and Growing3
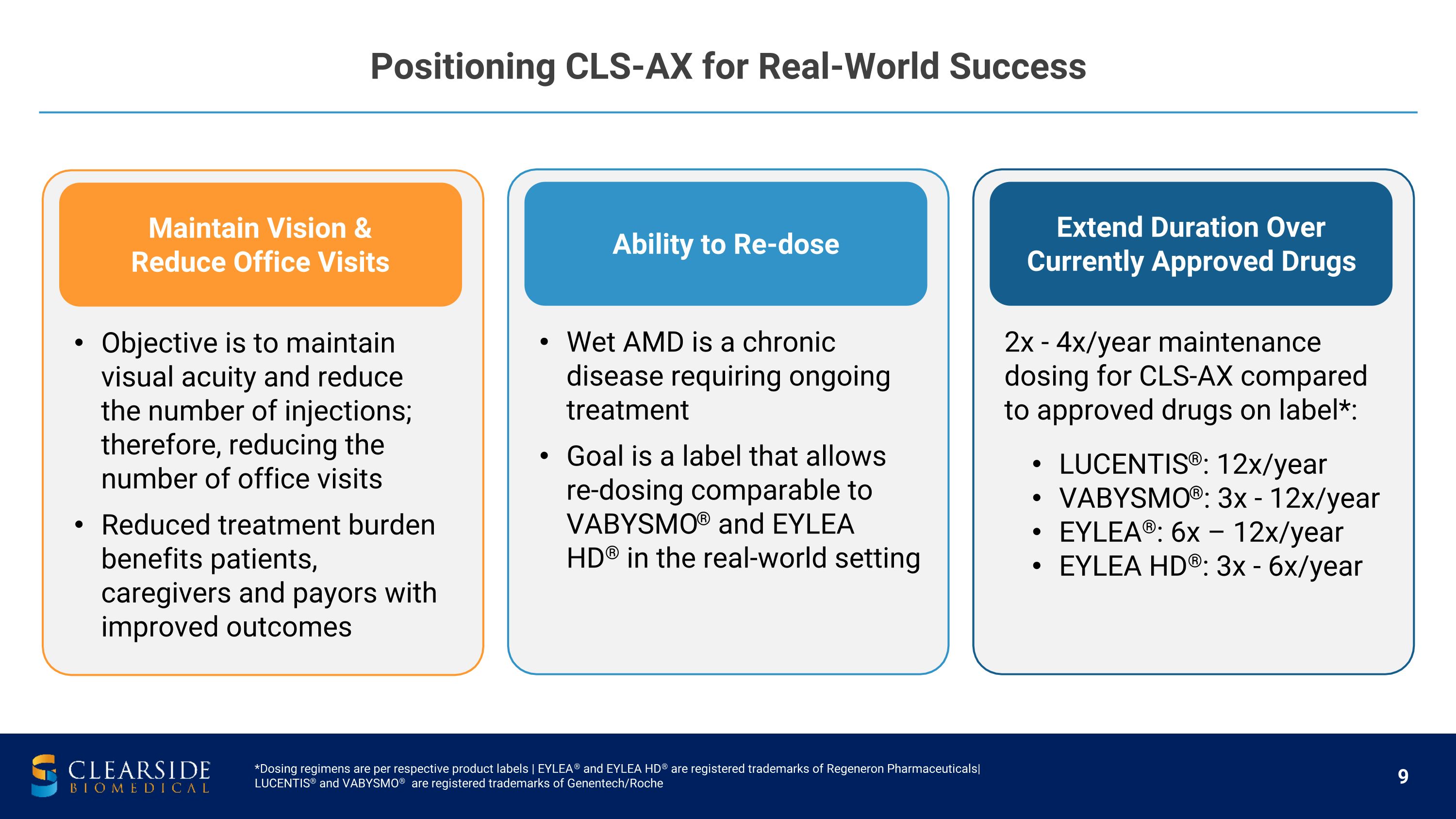
Positioning CLS-AX for Real-World Success *Dosing regimens are per respective product labels | EYLEA® and EYLEA HD® are registered trademarks of Regeneron Pharmaceuticals| LUCENTIS® and VABYSMO® are registered trademarks of Genentech/Roche Maintain Vision & Reduce Office Visits Objective is to maintain visual acuity and reduce the number of injections; therefore, reducing the number of office visits Reduced treatment burden benefits patients, caregivers and payors with improved outcomes Ability to Re-dose Wet AMD is a chronic disease requiring ongoing treatment Goal is a label that allows re-dosing comparable to VABYSMO® and EYLEA HD® in the real-world setting Extend Duration Over Currently Approved Drugs 2x - 4x/year maintenance dosing for CLS-AX compared to approved drugs on label*: LUCENTIS®: 12x/year VABYSMO®: 3x - 12x/year EYLEA®: 6x – 12x/year EYLEA HD®: 3x - 6x/year

Phase 2b Topline Data Summary 10
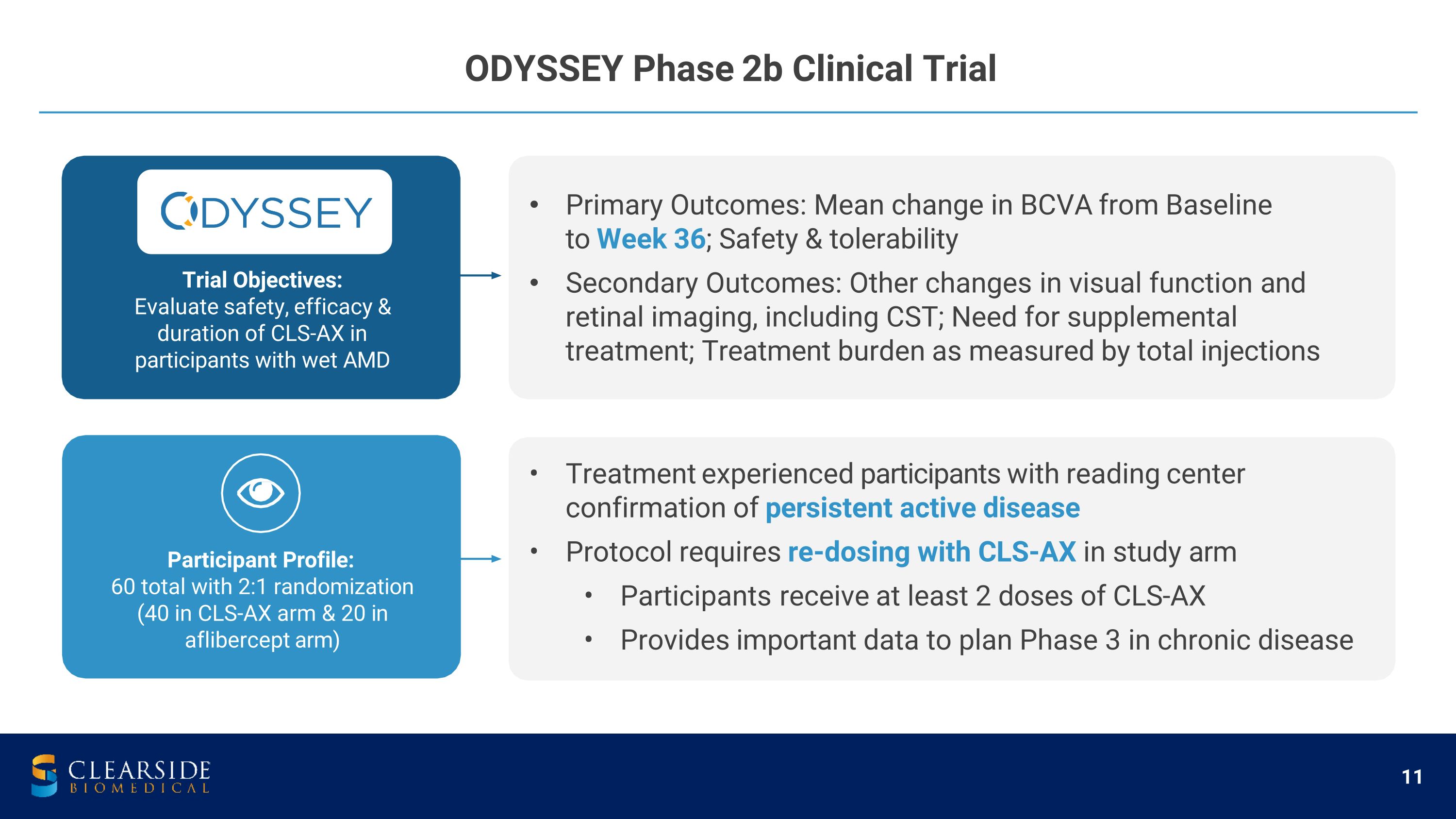
ODYSSEY Phase 2b Clinical Trial Trial Objectives: Evaluate safety, efficacy & duration of CLS-AX in participants with wet AMD Primary Outcomes: Mean change in BCVA from Baseline to Week 36; Safety & tolerability Secondary Outcomes: Other changes in visual function and retinal imaging, including CST; Need for supplemental treatment; Treatment burden as measured by total injections Treatment experienced participants with reading center confirmation of persistent active disease Protocol requires re-dosing with CLS-AX in study arm Participants receive at least 2 doses of CLS-AX Provides important data to plan Phase 3 in chronic disease Participant Profile: 60 total with 2:1 randomization (40 in CLS-AX arm & 20 in aflibercept arm)
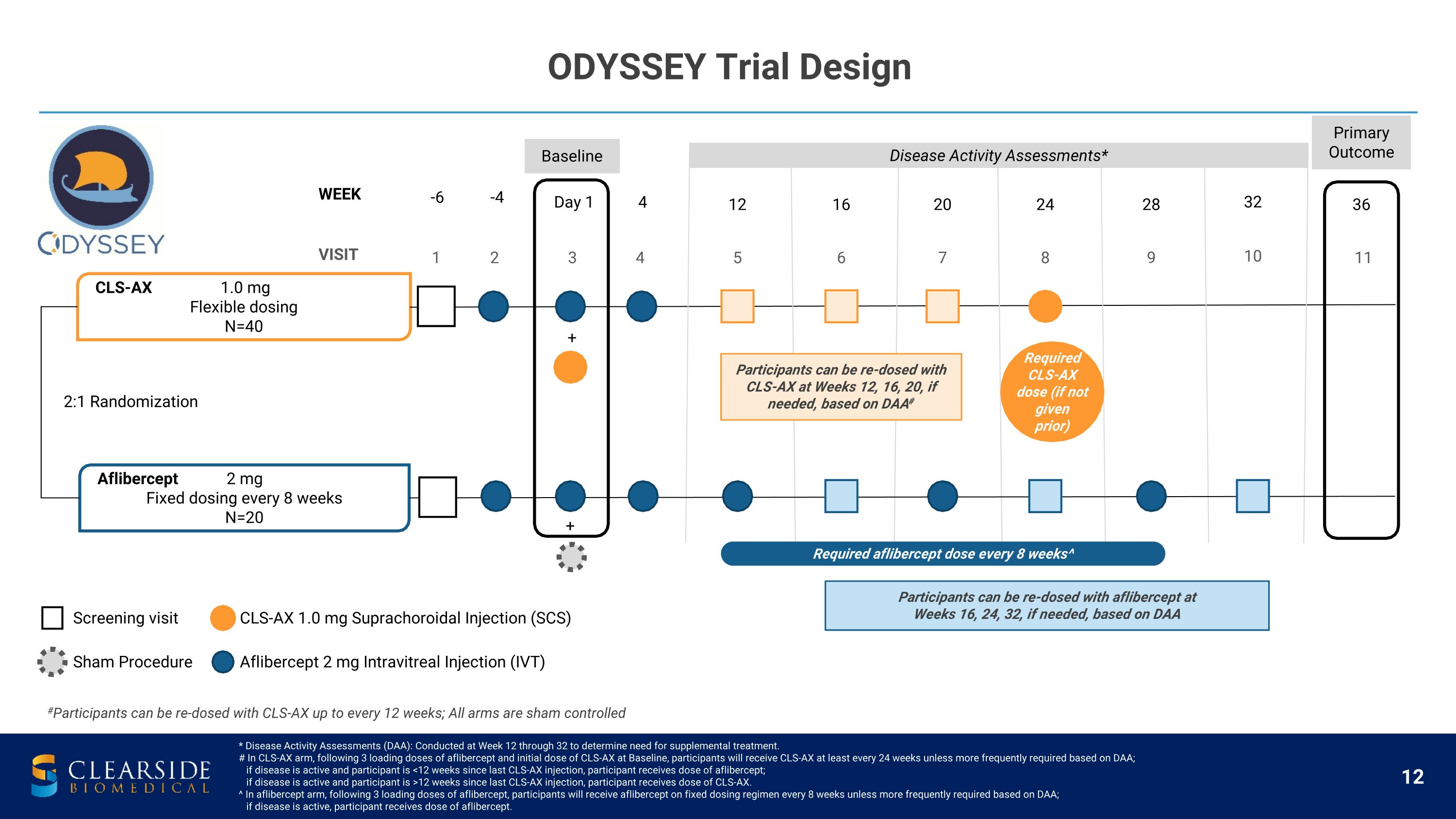
* Disease Activity Assessments (DAA): Conducted at Week 12 through 32 to determine need for supplemental treatment.�# In CLS-AX arm, following 3 loading doses of aflibercept and initial dose of CLS-AX at Baseline, participants will receive CLS-AX at least every 24 weeks unless more frequently required based on DAA; � if disease is active and participant is <12 weeks since last CLS-AX injection, participant receives dose of aflibercept; � if disease is active and participant is >12 weeks since last CLS-AX injection, participant receives dose of CLS-AX. ^ In aflibercept arm, following 3 loading doses of aflibercept, participants will receive aflibercept on fixed dosing regimen every 8 weeks unless more frequently required based on DAA; � if disease is active, participant receives dose of aflibercept. CLS-AX 1.0 mg Flexible dosing N=40 Aflibercept 2 mg Fixed dosing every 8 weeks N=20 VISIT 1 2 3 4 5 6 7 8 9 10 11 WEEK -6 -4 Day 1 4 12 16 20 24 28 32 36 + + Primary Outcome Disease Activity Assessments* Baseline 2:1 Randomization Sham Procedure CLS-AX 1.0 mg Suprachoroidal Injection (SCS) Aflibercept 2 mg Intravitreal Injection (IVT) Screening visit Required aflibercept dose every 8 weeks^ ODYSSEY Trial Design Required CLS-AX dose (if not given prior) #Participants can be re-dosed with CLS-AX up to every 12 weeks; All arms are sham controlled Participants can be re-dosed with CLS-AX at Weeks 12, 16, 20, if needed, based on DAA# Participants can be re-dosed with aflibercept at Weeks 16, 24, 32, if needed, based on DAA
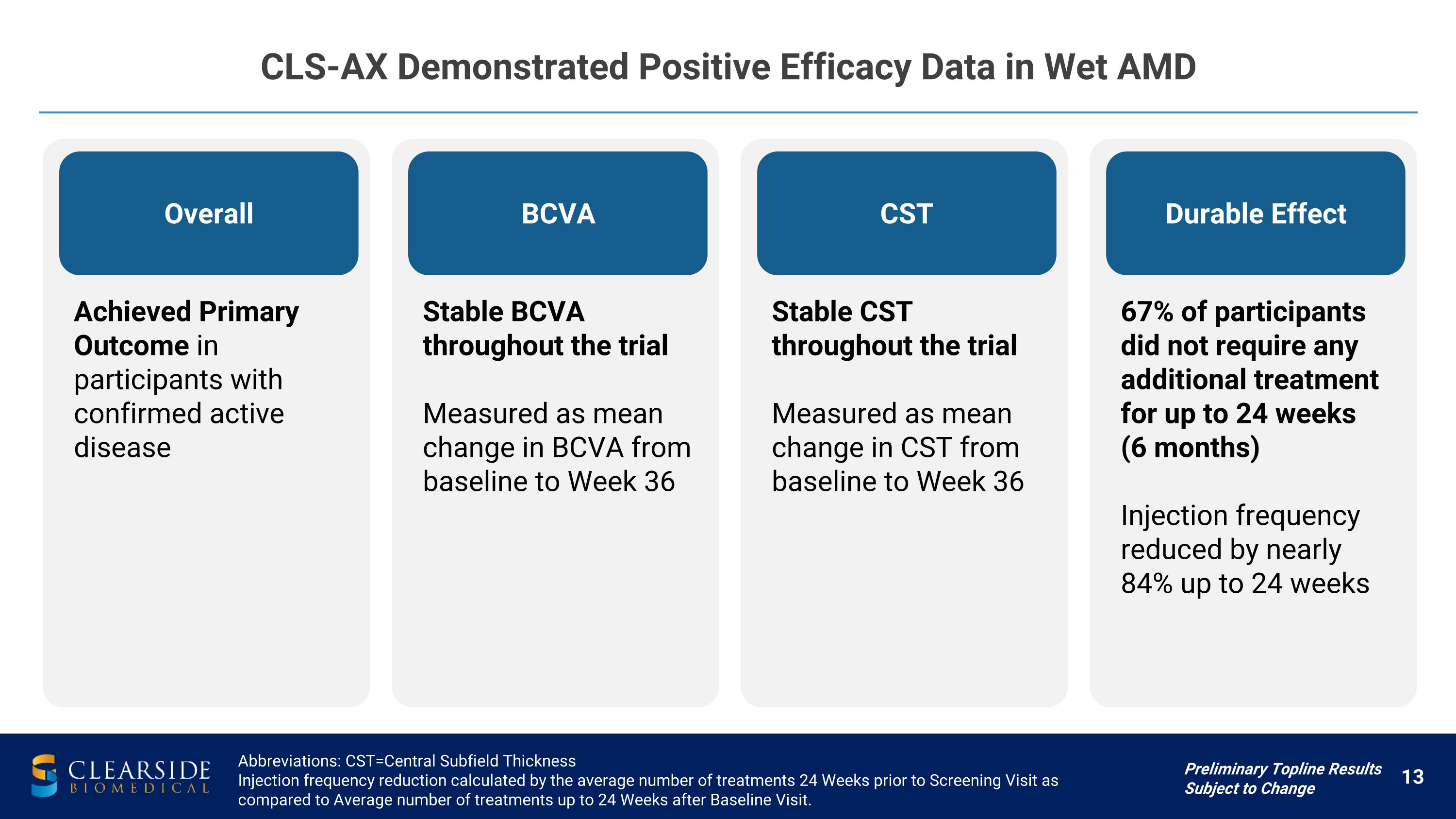
CLS-AX Demonstrated Positive Efficacy Data in Wet AMD Preliminary Topline Results Subject to Change Abbreviations: CST=Central Subfield Thickness Injection frequency reduction calculated by the average number of treatments 24 Weeks prior to Screening Visit as compared to Average number of treatments up to 24 Weeks after Baseline Visit. Overall Achieved Primary Outcome in participants with confirmed active disease BCVA Stable BCVA throughout the trial Measured as mean change in BCVA from baseline to Week 36 CST Stable CST throughout the trial Measured as mean change in CST from baseline to Week 36 Durable Effect 67% of participants did not require any additional treatment for up to 24 weeks �(6 months) Injection frequency reduced by nearly 84% up to 24 weeks
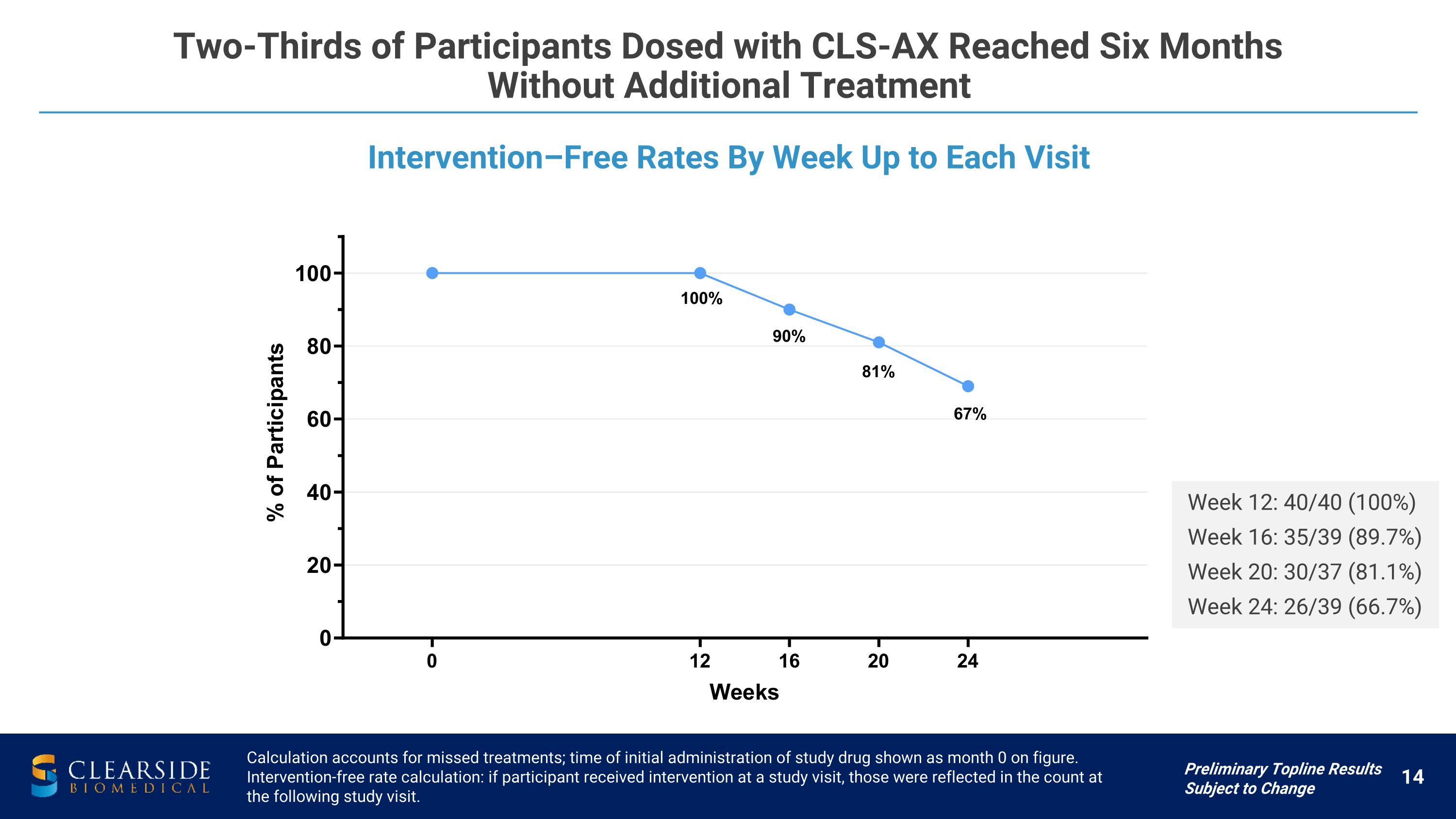
Intervention–Free Rates By Week Up to Each Visit Two-Thirds of Participants Dosed with CLS-AX Reached Six Months �Without Additional Treatment Calculation accounts for missed treatments; time of initial administration of study drug shown as month 0 on figure. Intervention-free rate calculation: if participant received intervention at a study visit, those were reflected in the count at the following study visit. Week 12: 40/40 (100%) Week 16: 35/39 (89.7%) Week 20: 30/37 (81.1%) Week 24: 26/39 (66.7%) Preliminary Topline Results Subject to Change
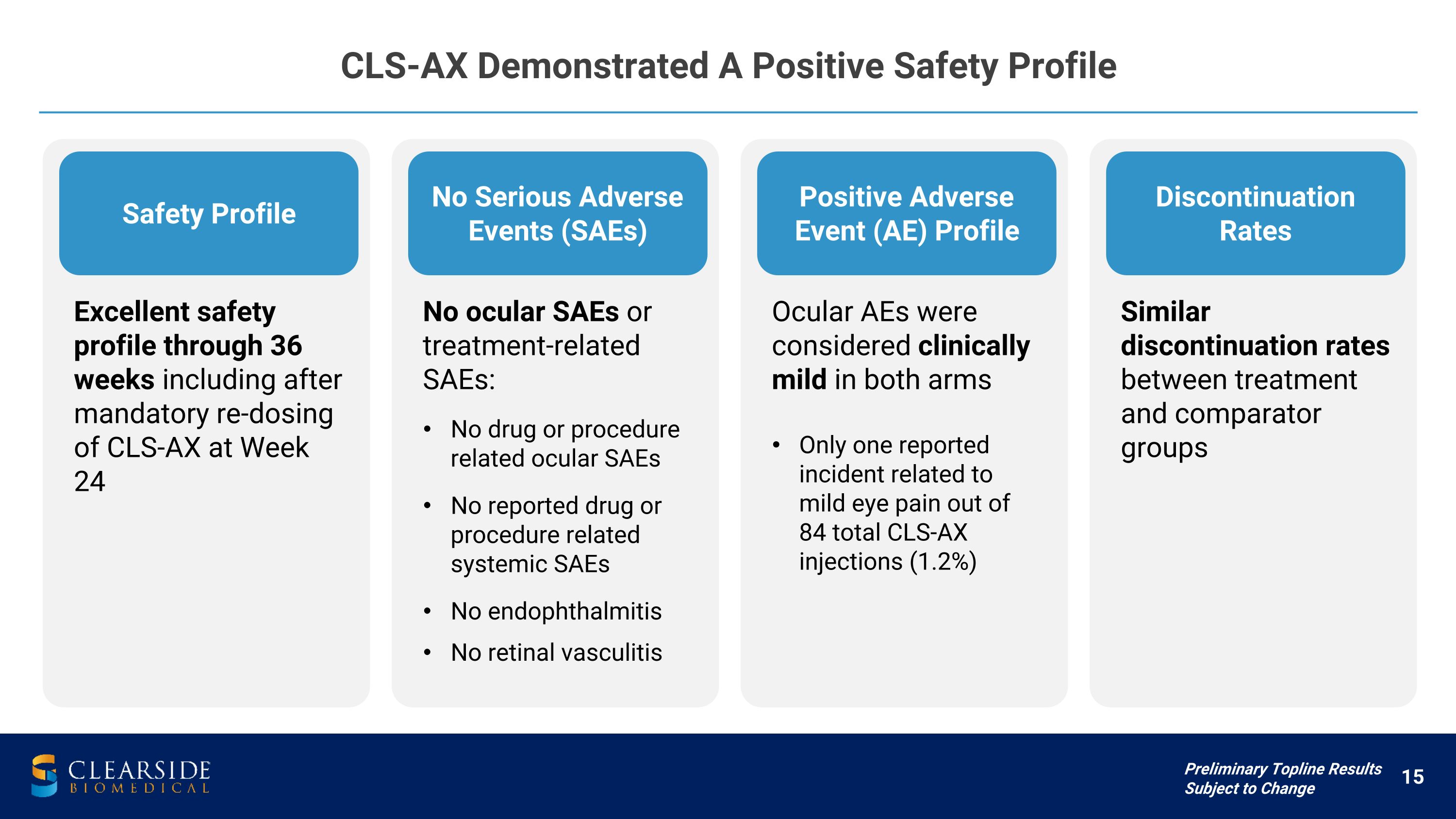
CLS-AX Demonstrated A Positive Safety Profile Preliminary Topline Results Subject to Change Safety Profile Excellent safety profile through 36 weeks including after mandatory re-dosing of CLS-AX at Week 24 No Serious Adverse Events (SAEs) No ocular SAEs or treatment-related SAEs: No drug or procedure related ocular SAEs
No reported drug or procedure related systemic SAEs No endophthalmitis No retinal vasculitis Positive Adverse Event (AE) Profile Ocular AEs were considered clinically mild in both arms Only one reported incident related to �mild eye pain out of �84 total CLS-AX injections (1.2%) Discontinuation Rates Similar discontinuation rates between treatment and comparator groups

Phase 2b Trial Participant Characteristics 16

ODYSSEY Trial Focused on Participants with Active Disease Criteria for Supplemental Treatment Dosing Regimen Key Inclusion Criteria Disease Activity Assessments (DAA) Diagnosed with neovascular AMD (wet AMD) within 36 months of screening History of 2 to 4 anti-VEGF treatments in 6 months before screening and response to prior anti-VEGF treatment for wet AMD Reading center confirmation of persistent active disease; BCVA of 20 to 80 letters# Monthly DAA: Weeks 12 through 32 in both arms to determine if there is need for supplemental treatment Supplemental treatment criteria: Decrease in BCVA, increase in CST, or new or worsening vision-threatening hemorrhage due to wet AMD BCVA reduction of >10 letters from Baseline measurement Increase in CST of >100 microns on SD-OCT from Baseline measurement BCVA reduction of > 5 letters from Baseline measurement AND increase in CST of >75 microns on SD-OCT from Baseline measurement Presence of new or worsening vision-threatening hemorrhage Aflibercept is dosed via intravitreal injection (IVT); CLS-AX is dosed via suprachoroidal injection. �# Using the Early Treatment Diabetic Retinopathy Study (ETDRS) measurement. Abbreviations: SD-OCT (Spectral Domain Optical Coherence Tomography). Participants in both arms received 3 aflibercept (2 mg) loading doses (2nd dose = Baseline visit) CLS-AX arm received one dose of CLS-AX (1.0 mg) at Baseline visit Unless DAA required more frequent dosing, CLS-AX arm dosed at least every 24 weeks & aflibercept arm dosed every 8 weeks
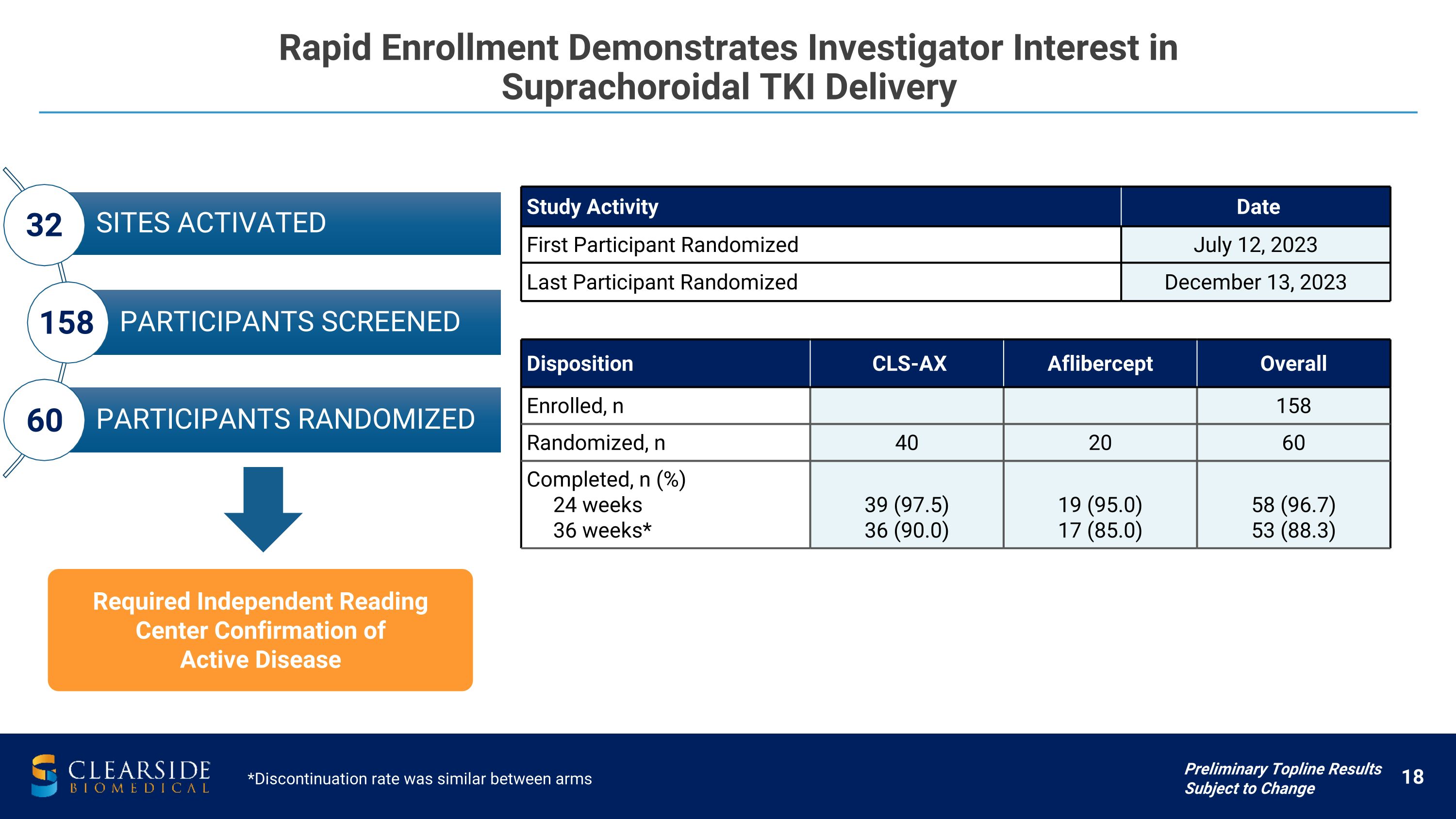
Rapid Enrollment Demonstrates Investigator Interest in �Suprachoroidal TKI Delivery Study Activity Date First Participant Randomized July 12, 2023 Last Participant Randomized December 13, 2023 SITES ACTIVATED PARTICIPANTS SCREENED PARTICIPANTS RANDOMIZED 32 60 158 Preliminary Topline Results Subject to Change Disposition CLS-AX Aflibercept Overall Enrolled, n 158 Randomized, n 40 20 60 Completed, n (%) 24 weeks 36 weeks* 39 (97.5) 36 (90.0) 19 (95.0) 17 (85.0) 58 (96.7) 53 (88.3) Required Independent Reading Center Confirmation of Active Disease *Discontinuation rate was similar between arms
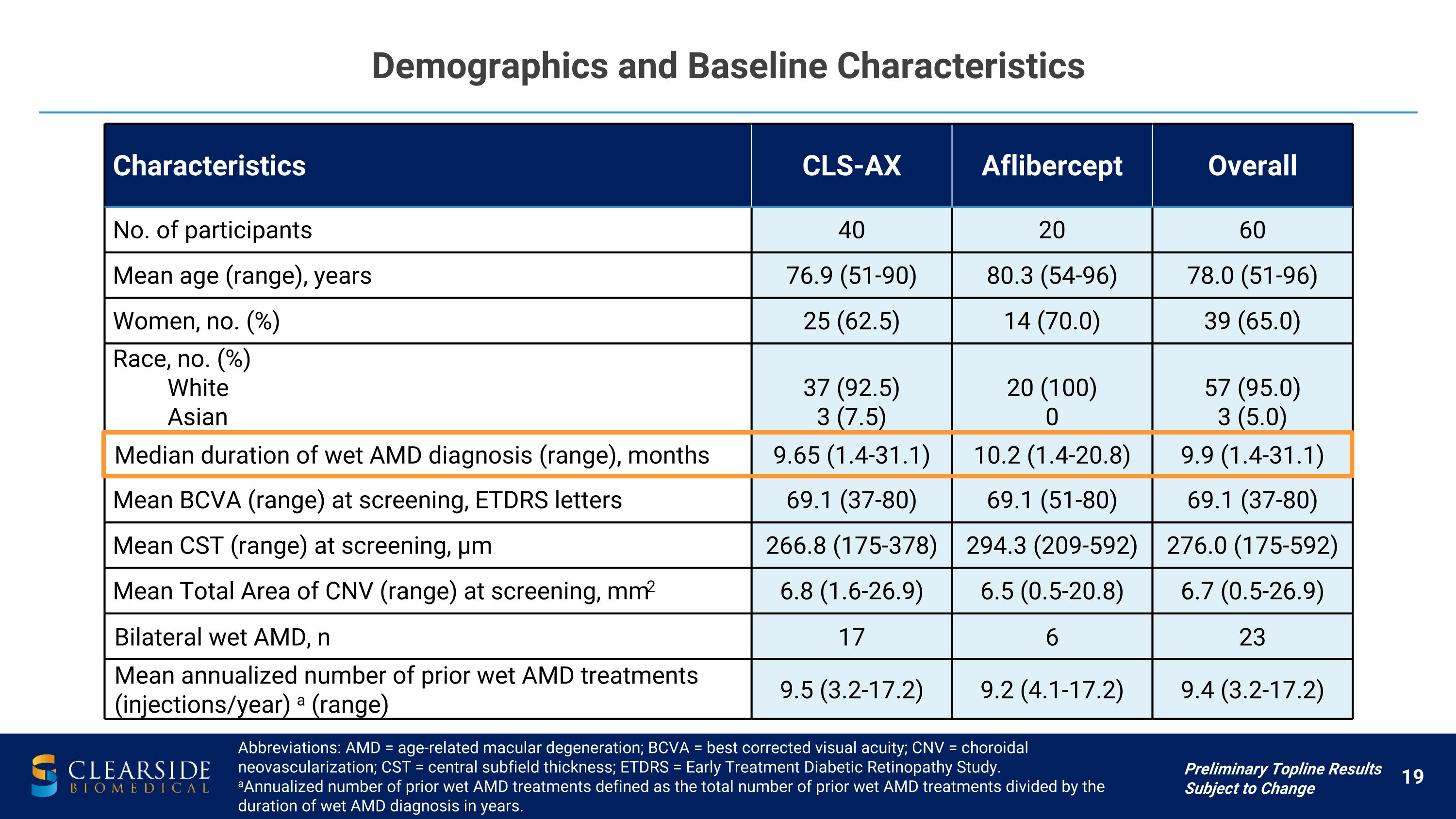
Demographics and Baseline Characteristics Characteristics CLS-AX Aflibercept Overall No. of participants 40 20 60 Mean age (range), years 76.9 (51-90) 80.3 (54-96) 78.0 (51-96) Women, no. (%) 25 (62.5) 14 (70.0) 39 (65.0) Race, no. (%) White Asian 37 (92.5) 3 (7.5) 20 (100) 0 57 (95.0) 3 (5.0) Median duration of wet AMD diagnosis (range), months 9.65 (1.4-31.1) 10.2 (1.4-20.8) 9.9 (1.4-31.1) Mean BCVA (range) at screening, ETDRS letters 69.1 (37-80) 69.1 (51-80) 69.1 (37-80) Mean CST (range) at screening, µm 266.8 (175-378) 294.3 (209-592) 276.0 (175-592) Mean Total Area of CNV (range) at screening, mm2 6.8 (1.6-26.9) 6.5 (0.5-20.8) 6.7 (0.5-26.9) Bilateral wet AMD, n 17 6 23 Mean annualized number of prior wet AMD treatments�(injections/year) a (range) 9.5 (3.2-17.2) 9.2 (4.1-17.2) 9.4 (3.2-17.2) Abbreviations: AMD = age-related macular degeneration; BCVA = best corrected visual acuity; CNV = choroidal neovascularization; CST = central subfield thickness; ETDRS = Early Treatment Diabetic Retinopathy Study. aAnnualized number of prior wet AMD treatments defined as the total number of prior wet AMD treatments divided by the duration of wet AMD diagnosis in years. Preliminary Topline Results Subject to Change

Phase 2b Topline Data Results 20 Preliminary Topline Results Subject to Change
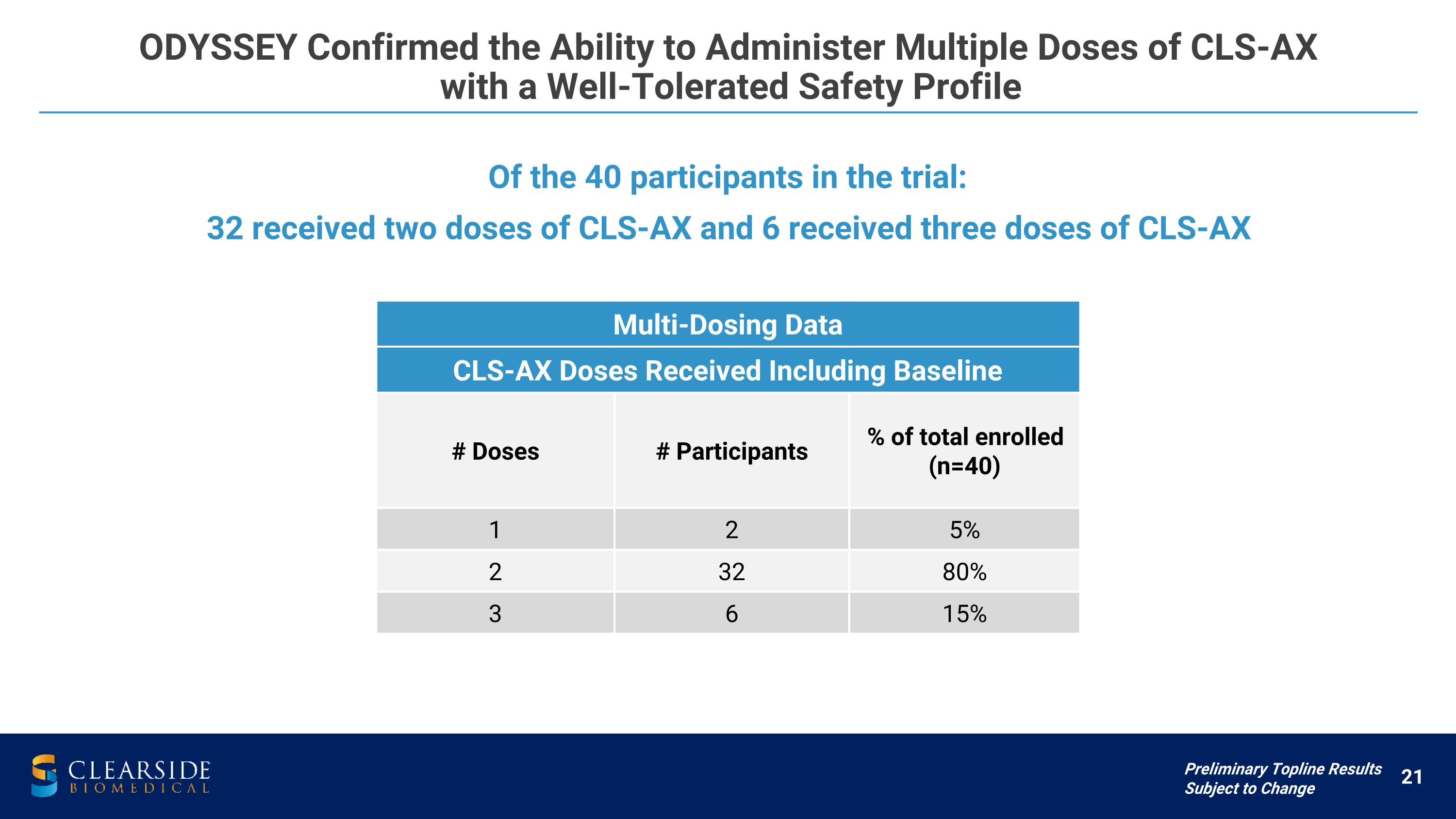
ODYSSEY Confirmed the Ability to Administer Multiple Doses of CLS-AX �with a Well-Tolerated Safety Profile Multi-Dosing Data CLS-AX Doses Received Including Baseline # Doses # Participants % of total enrolled (n=40) 1 2 5% 2 32 80% 3 6 15% Of the 40 participants in the trial: 32 received two doses of CLS-AX and 6 received three doses of CLS-AX Preliminary Topline Results Subject to Change
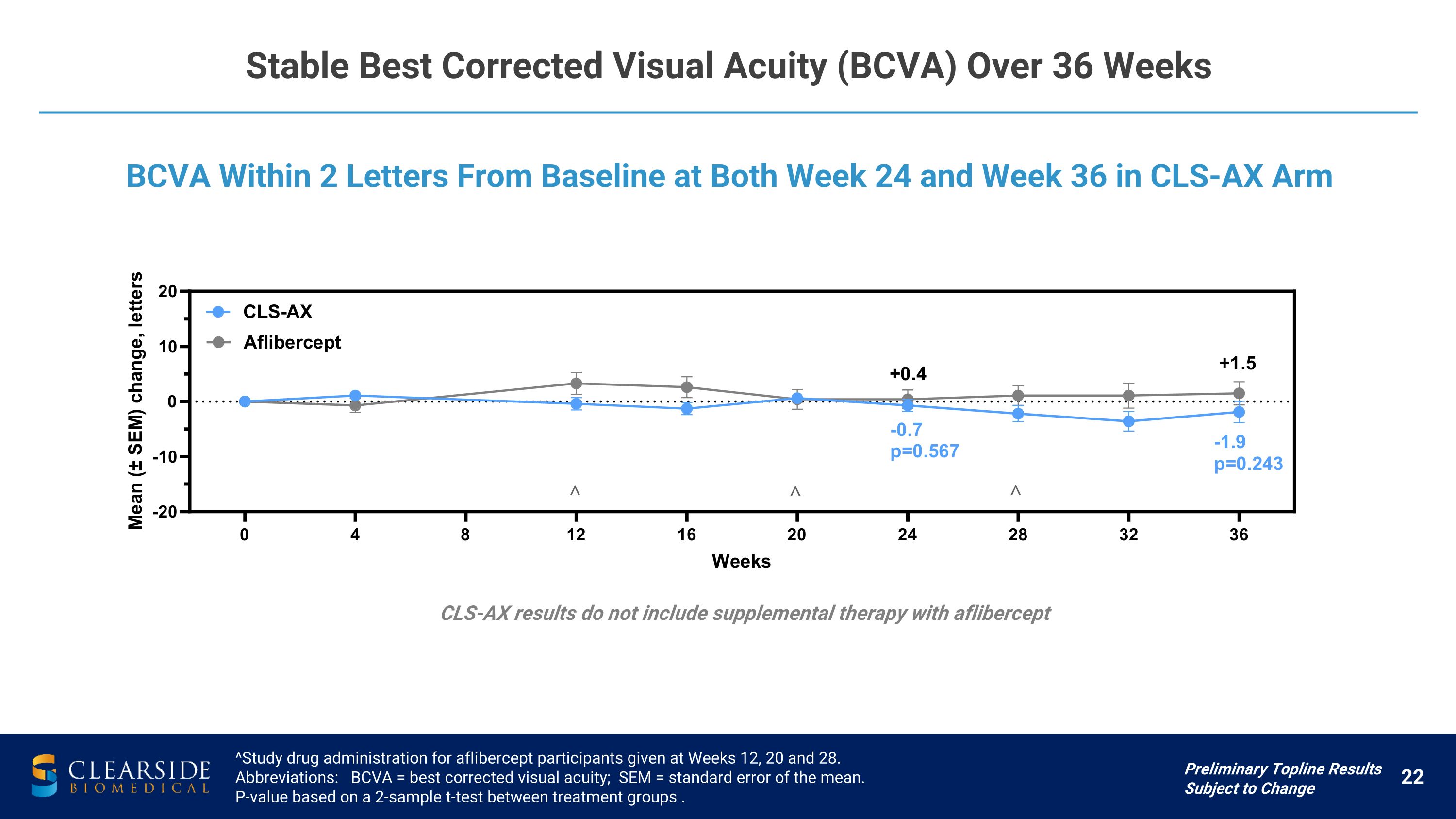
Stable Best Corrected Visual Acuity (BCVA) Over 36 Weeks ^Study drug administration for aflibercept participants given at Weeks 12, 20 and 28. Abbreviations: BCVA = best corrected visual acuity; SEM = standard error of the mean. P-value based on a 2-sample t-test between treatment groups . BCVA Within 2 Letters From Baseline at Both Week 24 and Week 36 in CLS-AX Arm Preliminary Topline Results Subject to Change CLS-AX results do not include supplemental therapy with aflibercept
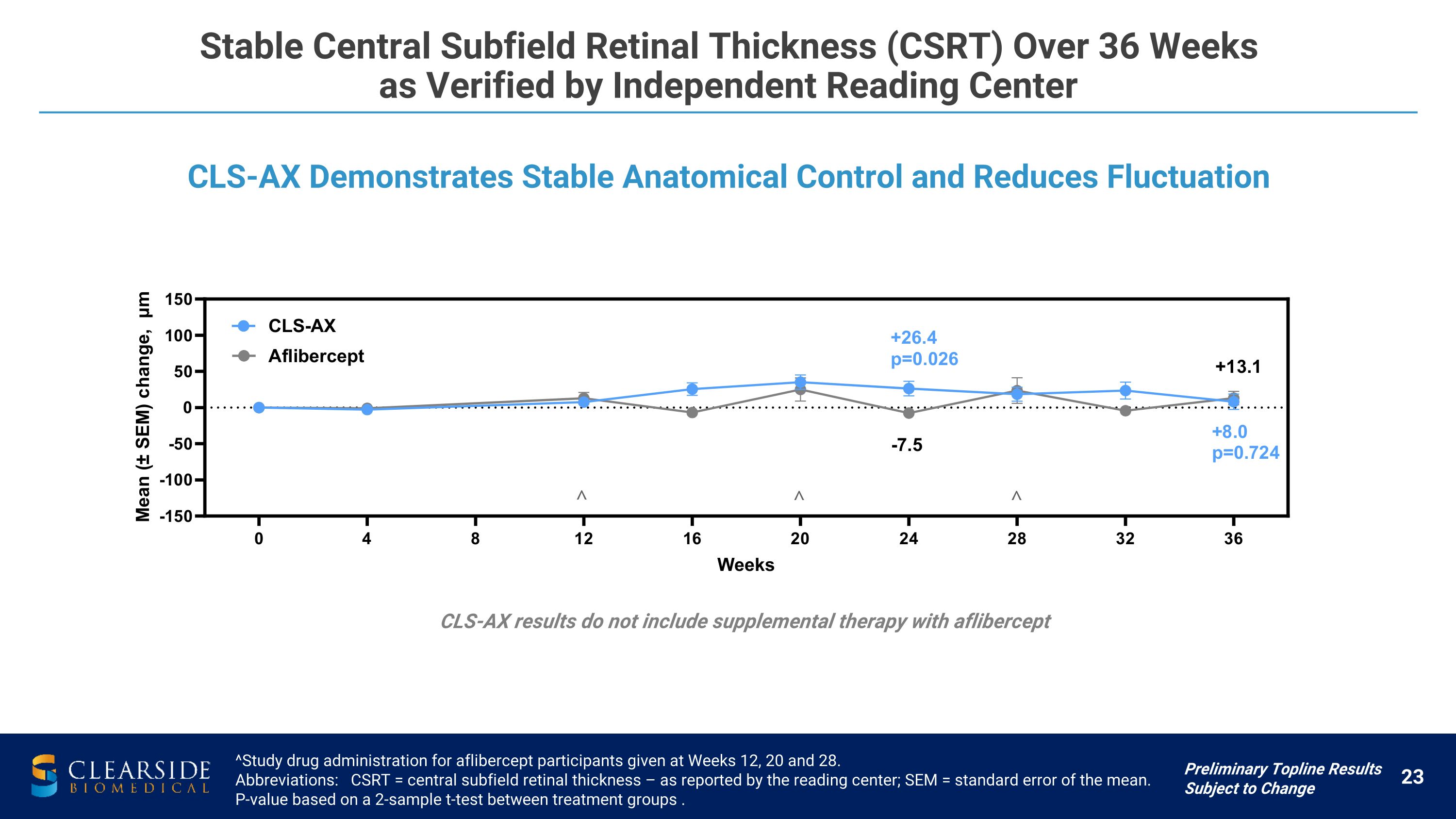
Stable Central Subfield Retinal Thickness (CSRT) Over 36 Weeks �as Verified by Independent Reading Center ^Study drug administration for aflibercept participants given at Weeks 12, 20 and 28. Abbreviations: CSRT = central subfield retinal thickness – as reported by the reading center; SEM = standard error of the mean. P-value based on a 2-sample t-test between treatment groups . CLS-AX Demonstrates Stable Anatomical Control and Reduces Fluctuation Preliminary Topline Results Subject to Change CLS-AX results do not include supplemental therapy with aflibercept
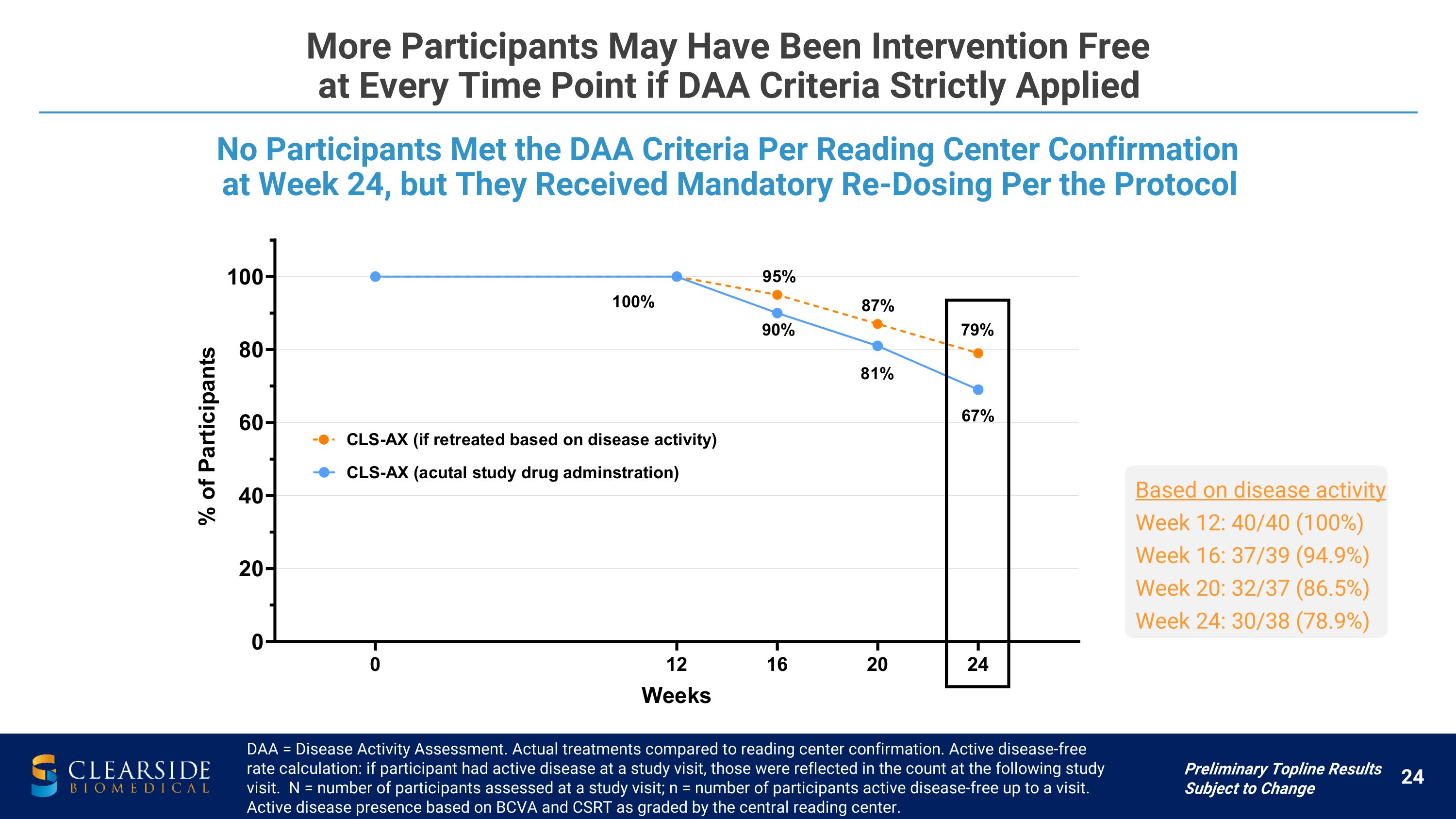
More Participants May Have Been Intervention Free �at Every Time Point if DAA Criteria Strictly Applied DAA = Disease Activity Assessment. Actual treatments compared to reading center confirmation. Active disease-free rate calculation: if participant had active disease at a study visit, those were reflected in the count at the following study visit. N = number of participants assessed at a study visit; n = number of participants active disease-free up to a visit. Active disease presence based on BCVA and CSRT as graded by the central reading center. No Participants Met the DAA Criteria Per Reading Center Confirmation at Week 24, but They Received Mandatory Re-Dosing Per the Protocol Based on disease activity Week 12: 40/40 (100%) Week 16: 37/39 (94.9%) Week 20: 32/37 (86.5%) Week 24: 30/38 (78.9%) Preliminary Topline Results Subject to Change
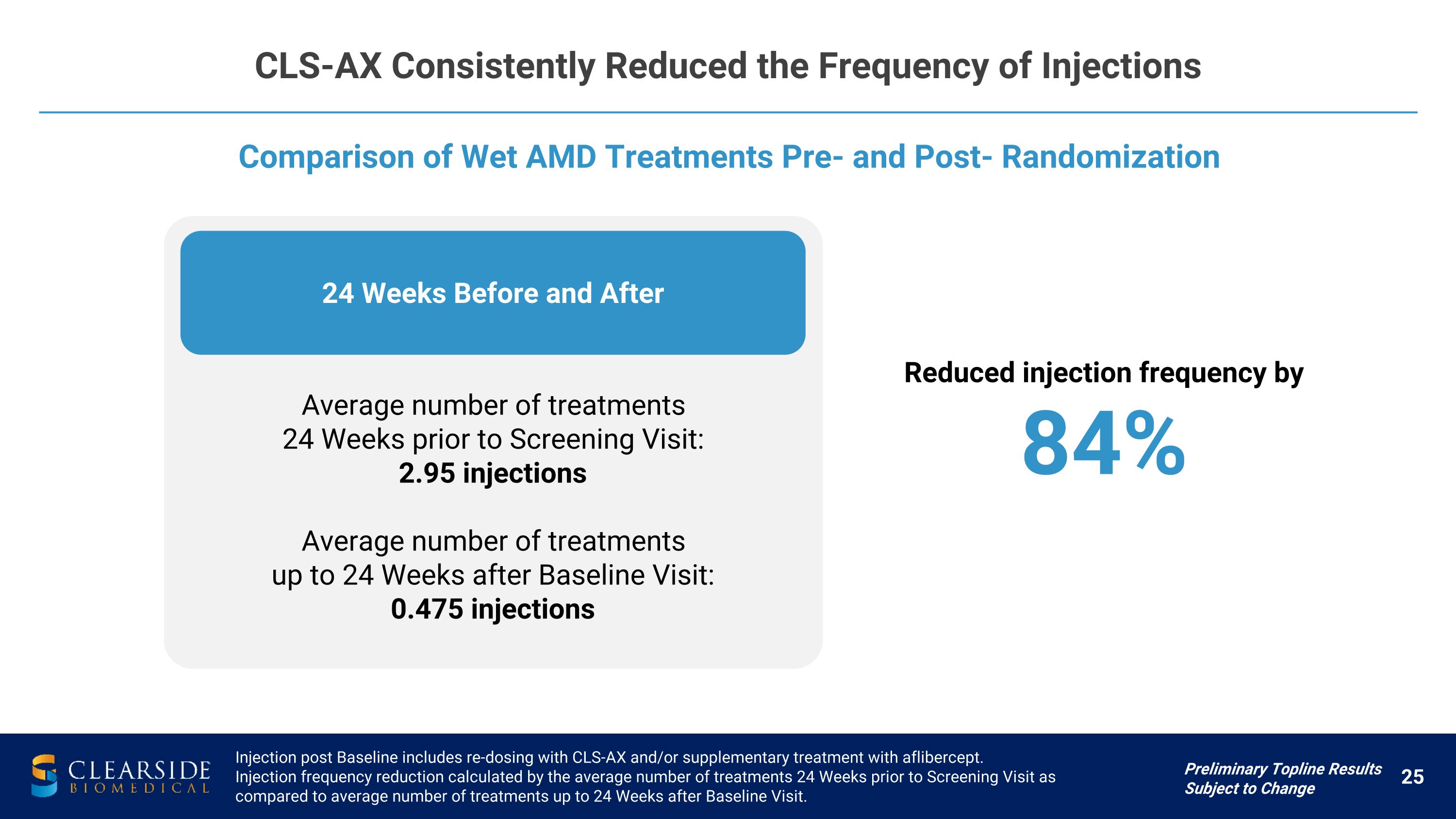
24 Weeks Before and After CLS-AX Consistently Reduced the Frequency of Injections Comparison of Wet AMD Treatments Pre- and Post- Randomization Injection post Baseline includes re-dosing with CLS-AX and/or supplementary treatment with aflibercept. Injection frequency reduction calculated by the average number of treatments 24 Weeks prior to Screening Visit as compared to average number of treatments up to 24 Weeks after Baseline Visit. Preliminary Topline Results Subject to Change Average number of treatments 24 Weeks prior to Screening Visit: 2.95 injections Average number of treatments up to 24 Weeks after Baseline Visit: 0.475 injections Reduced injection frequency by 84%
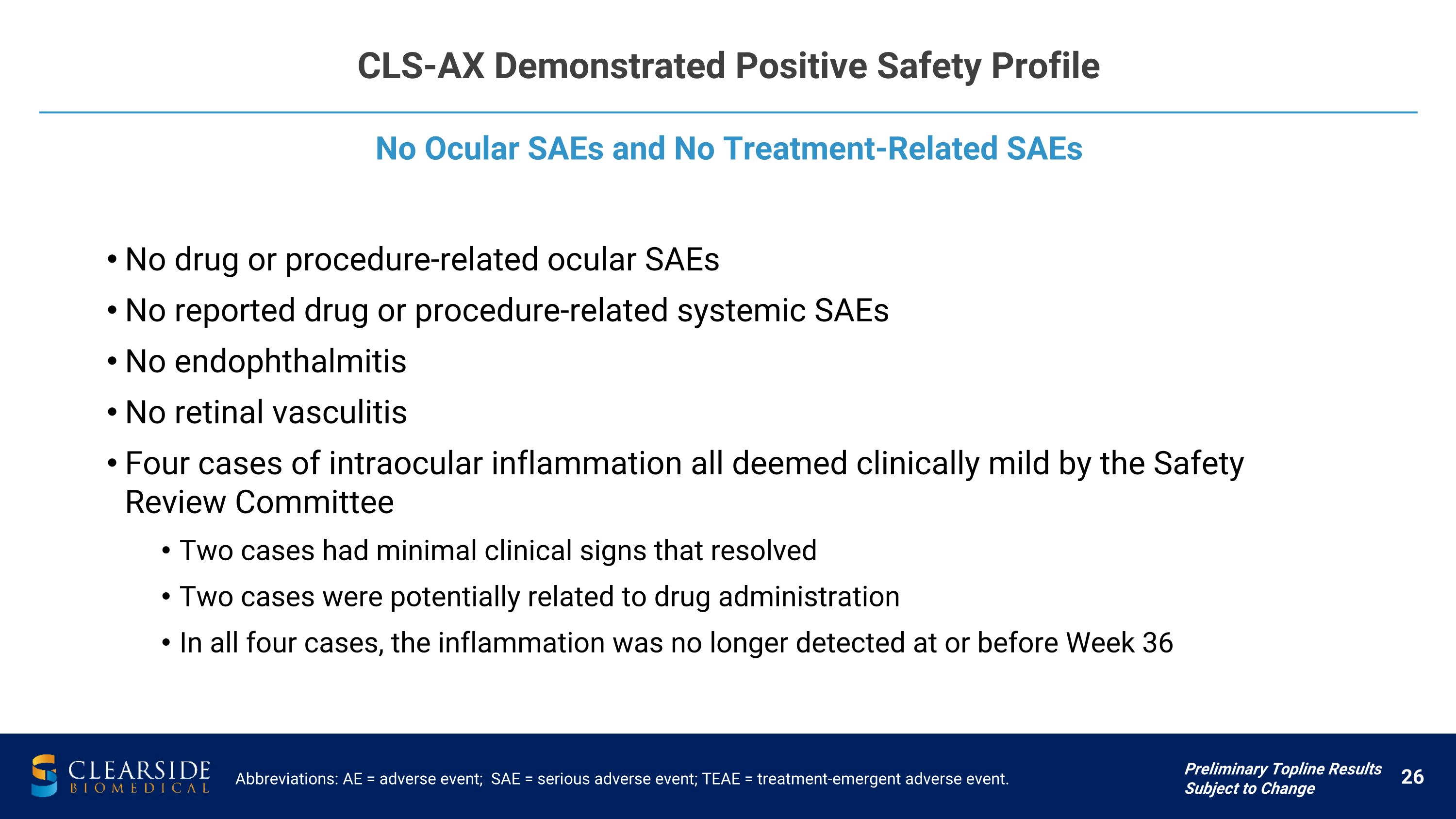
CLS-AX Demonstrated Positive Safety Profile Abbreviations: AE = adverse event; SAE = serious adverse event; TEAE = treatment-emergent adverse event. No Ocular SAEs and No Treatment-Related SAEs Preliminary Topline Results Subject to Change No drug or procedure-related ocular SAEs No reported drug or procedure-related systemic SAEs No endophthalmitis No retinal vasculitis Four cases of intraocular inflammation all deemed clinically mild by the Safety Review Committee Two cases had minimal clinical signs that resolved Two cases were potentially related to drug administration In all four cases, the inflammation was no longer detected at or before Week 36

Phase 3 Planning 27 Phase 3 plans are in development and subject to change
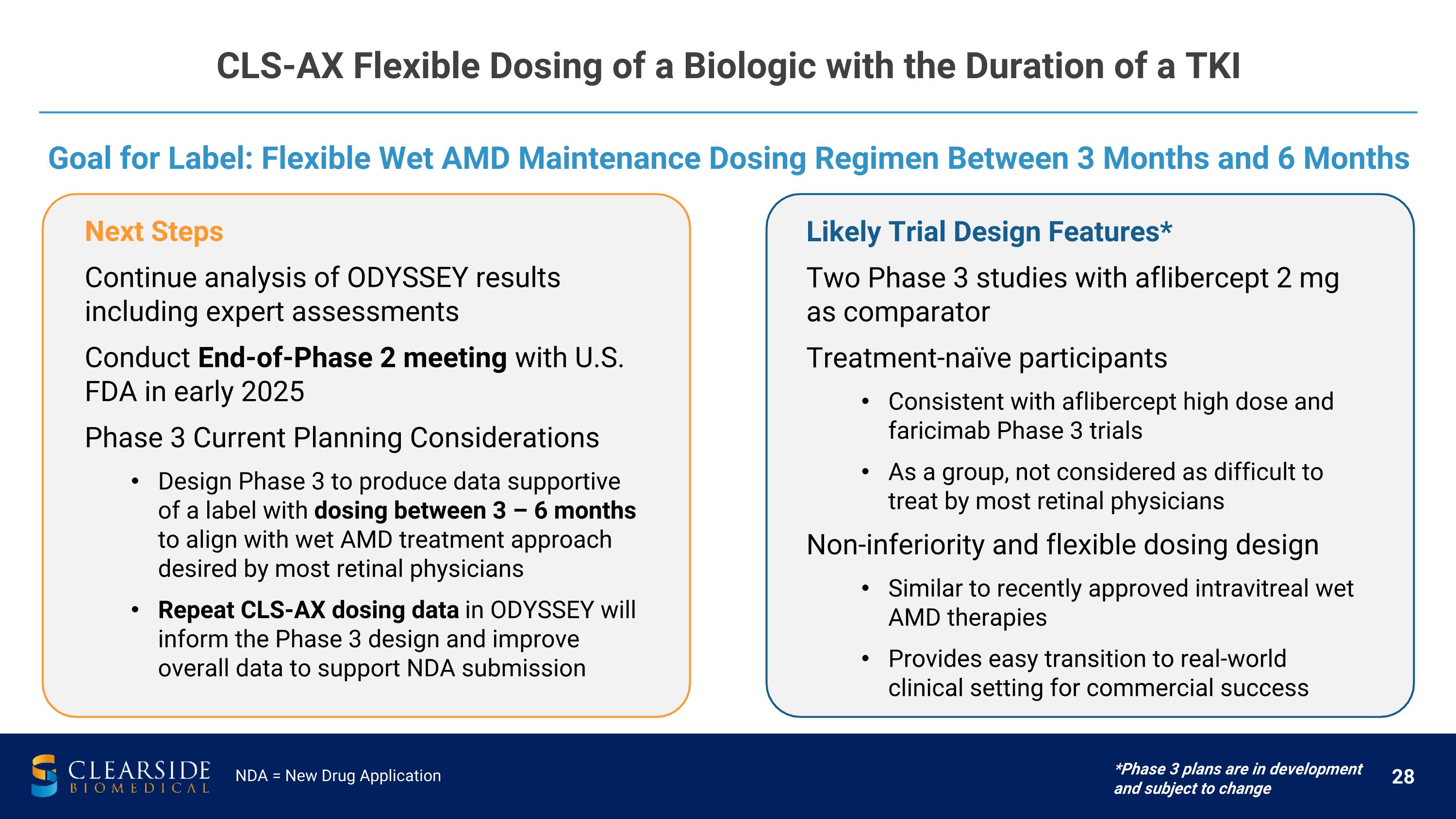
CLS-AX Flexible Dosing of a Biologic with the Duration of a TKI NDA = New Drug Application Goal for Label: Flexible Wet AMD Maintenance Dosing Regimen Between 3 Months and 6 Months *Phase 3 plans are in development and subject to change Next Steps Continue analysis of ODYSSEY results including expert assessments Conduct End-of-Phase 2 meeting with U.S. FDA in early 2025 Phase 3 Current Planning Considerations Design Phase 3 to produce data supportive of a label with dosing between 3 – 6 months to align with wet AMD treatment approach desired by most retinal physicians Repeat CLS-AX dosing data in ODYSSEY will inform the Phase 3 design and improve overall data to support NDA submission Likely Trial Design Features* Two Phase 3 studies with aflibercept 2 mg as comparator Treatment-naïve participants Consistent with aflibercept high dose and faricimab Phase 3 trials As a group, not considered as difficult to treat by most retinal physicians Non-inferiority and flexible dosing design Similar to recently approved intravitreal wet AMD therapies Provides easy transition to real-world clinical setting for commercial success

Results Summary 29

CLS-AX Now Phase 3 Ready Based on Positive ODYSSEY Data Preliminary Topline Results Subject to Change ✔︎ ✔︎ ✔︎ ✔︎ Achieved Primary Objective: Stable BCVA to Week 36 Difficult-to-treat Wet AMD participants with confirmed activity Compelling injection free rates up to 6 months Injection frequency reduced by nearly 84% Positive safety profile No ocular SAEs or treatment-related SAEs CLS-AX was well-tolerated after re-dosing Only Phase 2 trial in wet AMD with repeat TKI dosing data to better inform and potentially de-risk Phase 3 design

Roger A. Goldberg, MD, MBA Bay Area Retina Associates

Nasdaq: CLSD TM































