
Corporate Presentation | June 2021 Exhibit 99.1

Forward-Looking Statements This presentation contains forward-looking statements as defined in the Private Securities Litigation Reform Act of 1995, as amended. The words “may,” “will,” “could,” “would,” “should,” “expect,” “plan,” “anticipate,” “intend,” “believe,” “estimate,” “predict,” “project,” “potential,” “continue,” “target” and similar expressions are intended to identify forward-looking statements, although not all forward-looking statements contain these identifying words. Clearside Biomedical, Inc.’s views as of the date of this presentation about future events and are subject to risks, uncertainties, assumptions, and changes in circumstances that may cause Clearside’s actual results, performance, or achievements to differ significantly from those expressed or implied in any forward-looking statement. Although Clearside believes that the expectations reflected in the forward-looking statements are reasonable, Clearside cannot guarantee future events, results, performance, or achievements. Some of the key factors that could cause actual results to differ from Clearside’s expectations include its plans to develop and potentially commercialize its product candidates; Clearside’s planned clinical trials and preclinical studies for its product candidates; the timing of and Clearside’s ability to obtain and maintain regulatory approvals for its product candidates; the extent of clinical trials potentially required for Clearside’s product candidates; the clinical utility and market acceptance of Clearside’s product candidates; Clearside’s commercialization, marketing and manufacturing capabilities and strategy; Clearside’s intellectual property position; and Clearside’s ability to identify additional product candidates with significant commercial potential that are consistent with its commercial objectives. For further information regarding these risks, uncertainties and other factors you should read the “Risk Factors” section of Clearside’s Annual Report on Form 10-K for the year ended December 31, 2020, filed with the SEC on March 15, 2021, and Clearside’s other Periodic Reports filed with the SEC. Clearside expressly disclaims any obligation to update or revise the information herein, including the forward-looking statements, except as required by law. This presentation also contains estimates and other statistical data made by independent parties and by Clearside relating to market size and growth and other data about its industry. This data involves a number of assumptions and limitations, and you are cautioned not to give undue weight to such estimates. In addition, projections, assumptions and estimates of Clearside’s future performance and the future performance of the markets in which Clearside operates are necessarily subject to a high degree of uncertainty and risk.
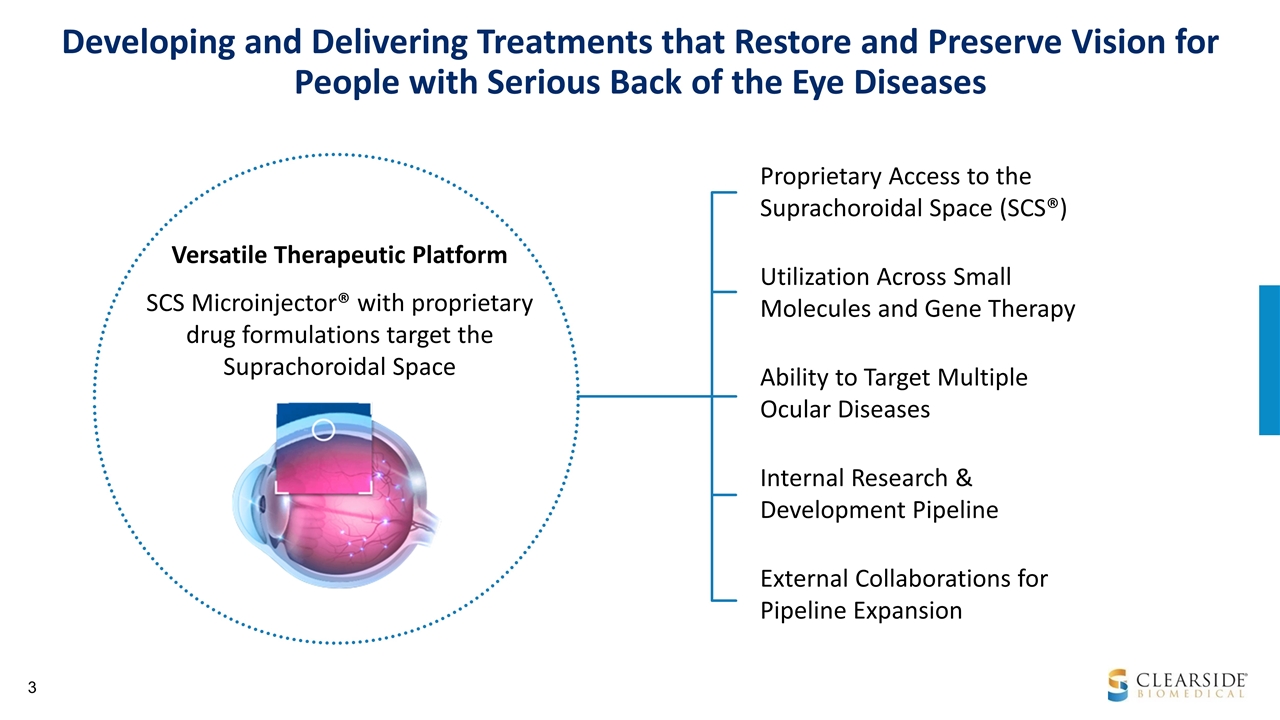
Versatile Therapeutic Platform SCS Microinjector® with proprietary drug formulations target the Suprachoroidal Space Proprietary Access to the Suprachoroidal Space (SCS®) Utilization Across Small Molecules and Gene Therapy Ability to Target Multiple Ocular Diseases Internal Research & Development Pipeline External Collaborations for Pipeline Expansion Developing and Delivering Treatments that Restore and Preserve Vision for People with Serious Back of the Eye Diseases
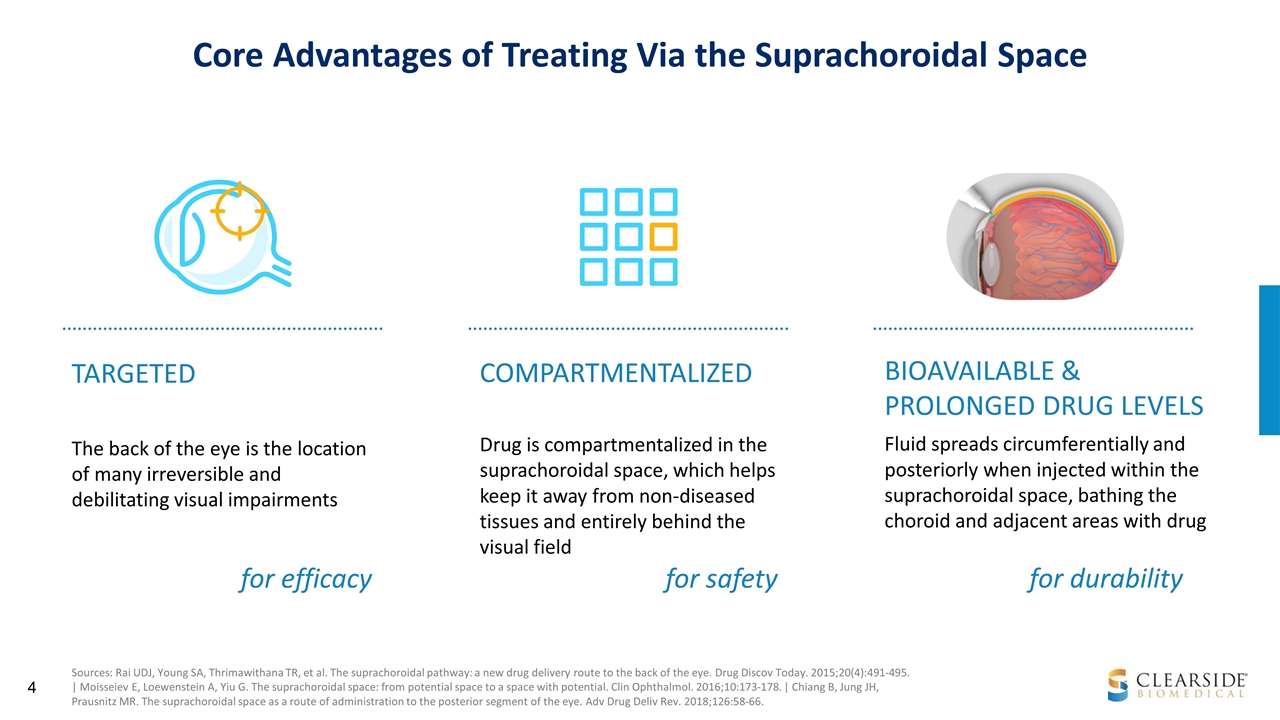
Core Advantages of Treating Via the Suprachoroidal Space Sources: Rai UDJ, Young SA, Thrimawithana TR, et al. The suprachoroidal pathway: a new drug delivery route to the back of the eye. Drug Discov Today. 2015;20(4):491-495. | Moisseiev E, Loewenstein A, Yiu G. The suprachoroidal space: from potential space to a space with potential. Clin Ophthalmol. 2016;10:173-178. | Chiang B, Jung JH, Prausnitz MR. The suprachoroidal space as a route of administration to the posterior segment of the eye. Adv Drug Deliv Rev. 2018;126:58-66. TARGETED The back of the eye is the location of many irreversible and debilitating visual impairments BIOAVAILABLE & PROLONGED DRUG LEVELS Fluid spreads circumferentially and posteriorly when injected within the suprachoroidal space, bathing the choroid and adjacent areas with drug COMPARTMENTALIZED Drug is compartmentalized in the suprachoroidal space, which helps keep it away from non-diseased tissues and entirely behind the visual field for efficacy for safety for durability
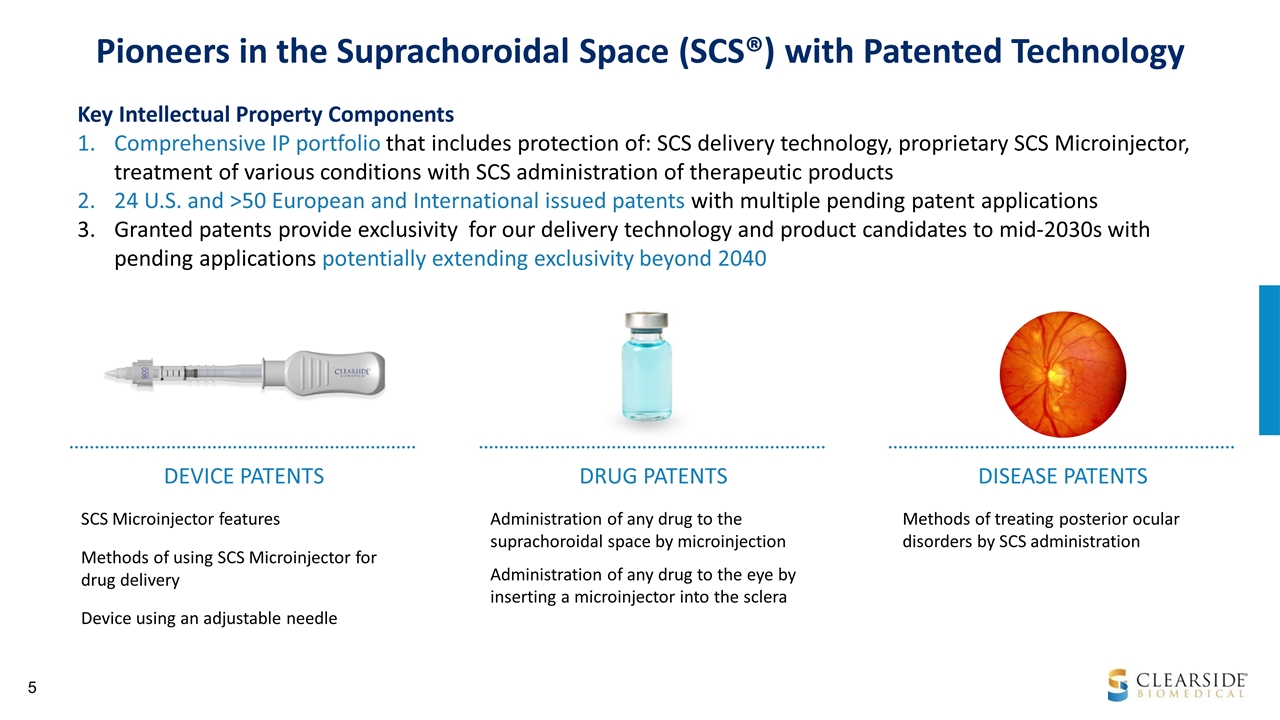
Administration of any drug to the suprachoroidal space by microinjection Administration of any drug to the eye by inserting a microinjector into the sclera SCS Microinjector features Methods of using SCS Microinjector for drug delivery Device using an adjustable needle Pioneers in the Suprachoroidal Space (SCS®) with Patented Technology DEVICE PATENTS DRUG PATENTS Methods of treating posterior ocular disorders by SCS administration DISEASE PATENTS Key Intellectual Property Components Comprehensive IP portfolio that includes protection of: SCS delivery technology, proprietary SCS Microinjector, treatment of various conditions with SCS administration of therapeutic products 24 U.S. and >50 European and International issued patents with multiple pending patent applications Granted patents provide exclusivity for our delivery technology and product candidates to mid-2030s with pending applications potentially extending exclusivity beyond 2040
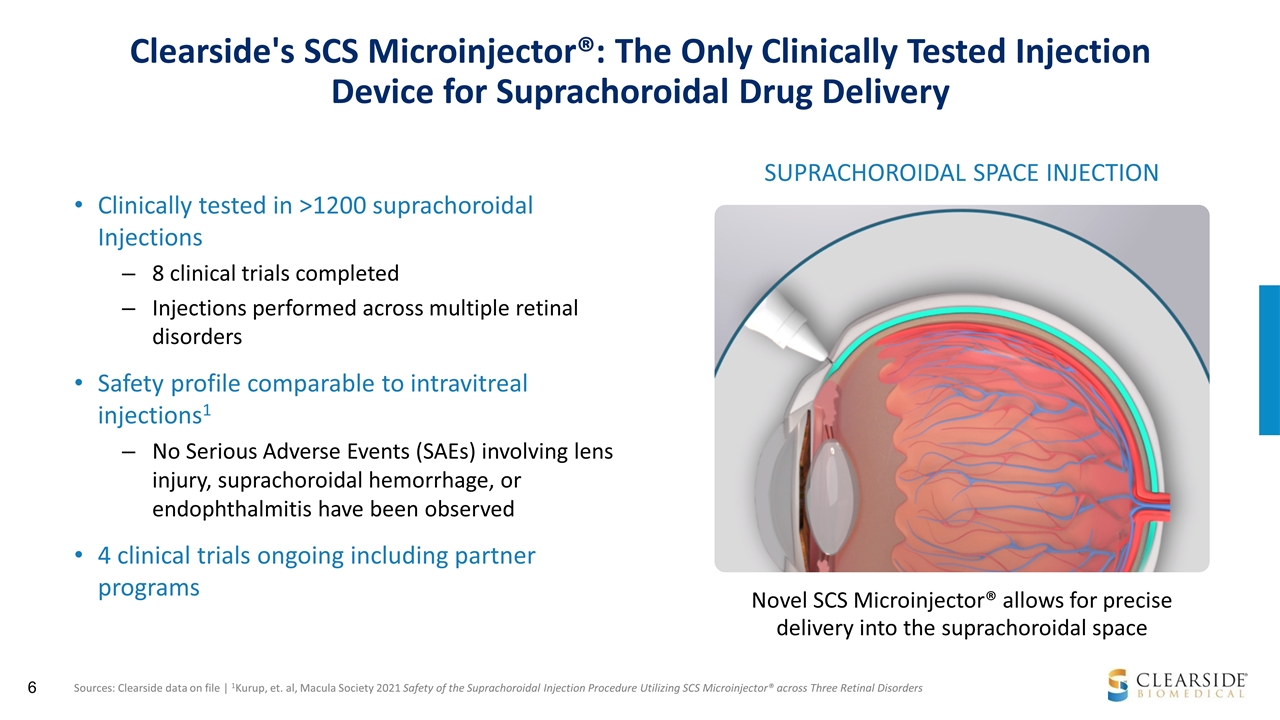
Clearside's SCS Microinjector®: The Only Clinically Tested Injection Device for Suprachoroidal Drug Delivery Clinically tested in >1200 suprachoroidal Injections 8 clinical trials completed Injections performed across multiple retinal disorders Safety profile comparable to intravitreal injections1 No Serious Adverse Events (SAEs) involving lens injury, suprachoroidal hemorrhage, or endophthalmitis have been observed 4 clinical trials ongoing including partner programs SUPRACHOROIDAL SPACE INJECTION Novel SCS Microinjector® allows for precise delivery into the suprachoroidal space Sources: Clearside data on file | 1Kurup, et. al, Macula Society 2021 Safety of the Suprachoroidal Injection Procedure Utilizing SCS Microinjector® across Three Retinal Disorders
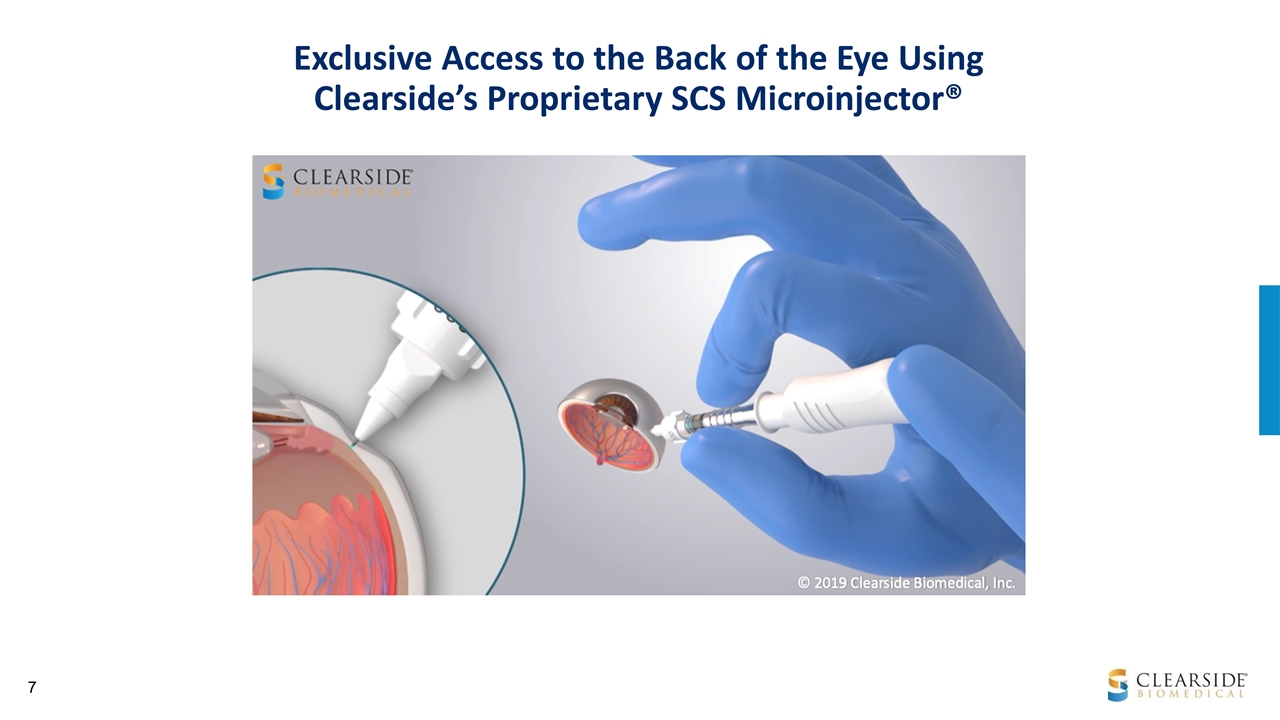
Exclusive Access to the Back of the Eye Using Clearside’s Proprietary SCS Microinjector®

CLS-AX Delivered with SCS Microinjector® for Wet AMD
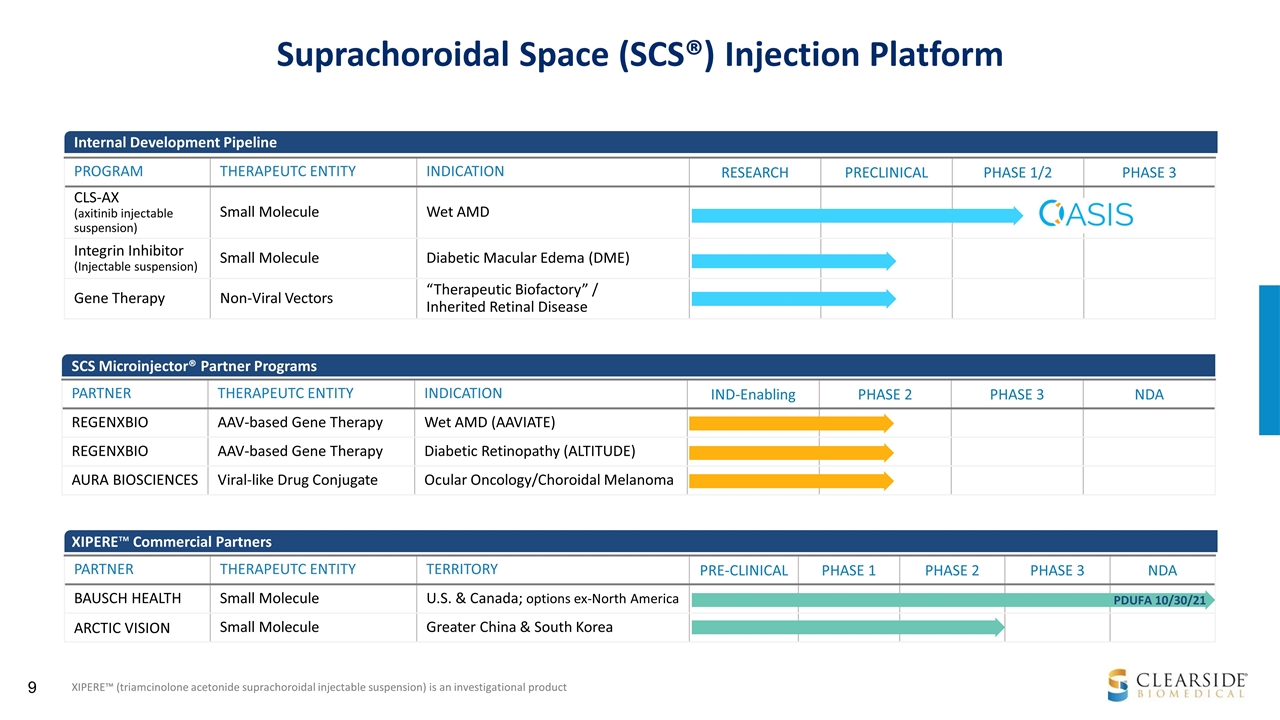
Suprachoroidal Space (SCS®) Injection Platform PARTNER THERAPEUTC ENTITY INDICATION IND-Enabling PHASE 2 PHASE 3 NDA REGENXBIO AAV-based Gene Therapy Wet AMD (AAVIATE) REGENXBIO AAV-based Gene Therapy Diabetic Retinopathy (ALTITUDE) AURA BIOSCIENCES Viral-like Drug Conjugate Ocular Oncology/Choroidal Melanoma XIPERE™ (triamcinolone acetonide suprachoroidal injectable suspension) is an investigational product SCS Microinjector® Partner Programs XIPERE™ Commercial Partners PARTNER THERAPEUTC ENTITY TERRITORY PRE-CLINICAL PHASE 1 PHASE 2 PHASE 3 NDA BAUSCH HEALTH Small Molecule U.S. & Canada; options ex-North America ARCTIC VISION Small Molecule Greater China & South Korea PROGRAM THERAPEUTC ENTITY INDICATION RESEARCH PRECLINICAL PHASE 1/2 PHASE 3 CLS-AX (axitinib injectable suspension) Small Molecule Wet AMD Integrin Inhibitor (Injectable suspension) Small Molecule Diabetic Macular Edema (DME) Gene Therapy Non-Viral Vectors “Therapeutic Biofactory” / Inherited Retinal Disease Internal Development Pipeline PDUFA 10/30/21

Macular edema is the leading cause of vision loss in patients with non-infectious uveitis NDA was resubmitted and accepted for review with PDUFA goal date of October 30, 2021 Commercialization and development partnerships to enhance value and expand patient access XIPERE™: Potential Suprachoroidal Approach to Treating Uveitic Macular Edema XIPERE™ is an investigational product If approved, XIPERE would represent the FIRST therapy for macular edema associated with uveitis FIRST uveitis trial using visual acuity change as a primary endpoint (Phase 3 PEACHTREE) FIRST approved therapeutic delivered into the suprachoroidal space FIRST commercial product for Clearside
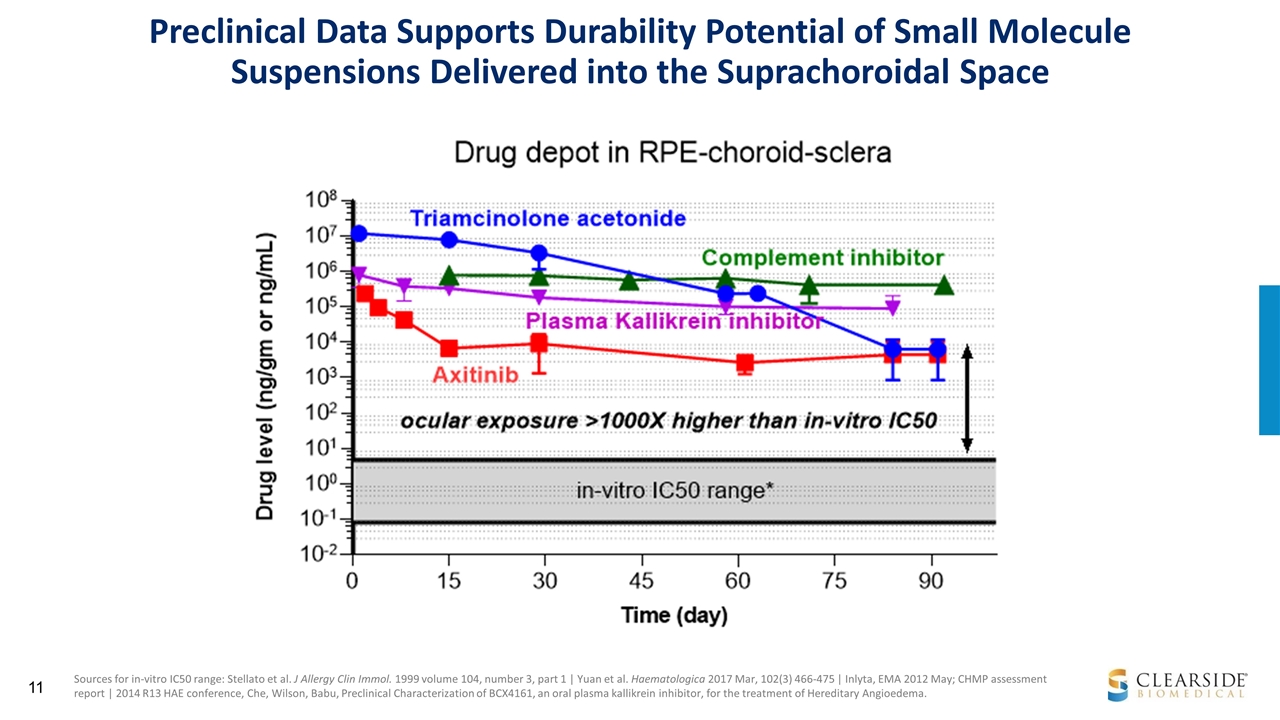
Sources for in-vitro IC50 range: Stellato et al. J Allergy Clin Immol. 1999 volume 104, number 3, part 1 | Yuan et al. Haematologica 2017 Mar, 102(3) 466-475 | Inlyta, EMA 2012 May; CHMP assessment report | 2014 R13 HAE conference, Che, Wilson, Babu, Preclinical Characterization of BCX4161, an oral plasma kallikrein inhibitor, for the treatment of Hereditary Angioedema. Preclinical Data Supports Durability Potential of Small Molecule Suspensions Delivered into the Suprachoroidal Space

CLS-AX (axitinib injectable suspension) for Suprachoroidal Injection

AMD causes a progressive loss of central vision and is the most common cause of blindness in individuals over age 55 Neovascular or Wet AMD accounts for the majority of blindness U.S. prevalence expected to increase to 22 million by the year 2050 Global prevalence expected to increase to 288 million by the year 2040 Current treatments require frequent injections causing reduced compliance Under-treatment contributes to limited outcomes Age-Related Macular Degeneration (AMD) A large and growing market opportunity Sources: Medscape: F Ryan Prall, MD, et al Assistant Professor of Ophthalmology, Indiana University School of Medicine | Pennington, Katie L and DeAngelis, Margaret M Eye and Vision, Epidemiology of age-related macular degeneration (AMD): associations with cardiovascular disease phenotypes and lipid factors , Dec 22, 2016. 170 million patients worldwide 10-20% patients wet AMD 11 million U.S. Patients
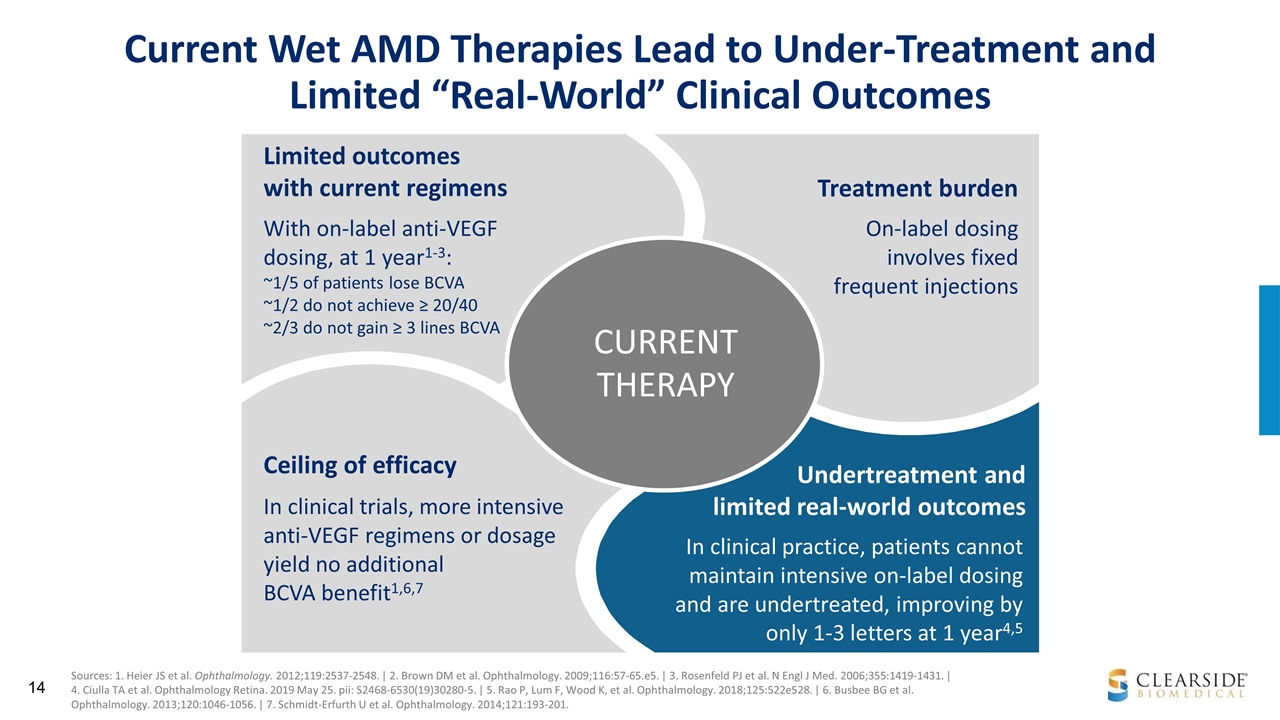
Ceiling of efficacy Treatment burden Limited outcomes with current regimens CURRENT THERAPY On-label dosing involves fixed frequent injections With on-label anti-VEGF dosing, at 1 year1-3: ~1/5 of patients lose BCVA ~1/2 do not achieve ≥ 20/40 ~2/3 do not gain ≥ 3 lines BCVA In clinical trials, more intensive anti-VEGF regimens or dosage yield no additional BCVA benefit1,6,7 In clinical practice, patients cannot maintain intensive on-label dosing and are undertreated, improving by only 1-3 letters at 1 year4,5 Undertreatment and limited real-world outcomes Current Wet AMD Therapies Lead to Under-Treatment and Limited “Real-World” Clinical Outcomes Sources: 1. Heier JS et al. Ophthalmology. 2012;119:2537-2548. | 2. Brown DM et al. Ophthalmology. 2009;116:57-65.e5. | 3. Rosenfeld PJ et al. N Engl J Med. 2006;355:1419-1431. | 4. Ciulla TA et al. Ophthalmology Retina. 2019 May 25. pii: S2468-6530(19)30280-5. | 5. Rao P, Lum F, Wood K, et al. Ophthalmology. 2018;125:522e528. | 6. Busbee BG et al. Ophthalmology. 2013;120:1046-1056. | 7. Schmidt-Erfurth U et al. Ophthalmology. 2014;121:193-201.
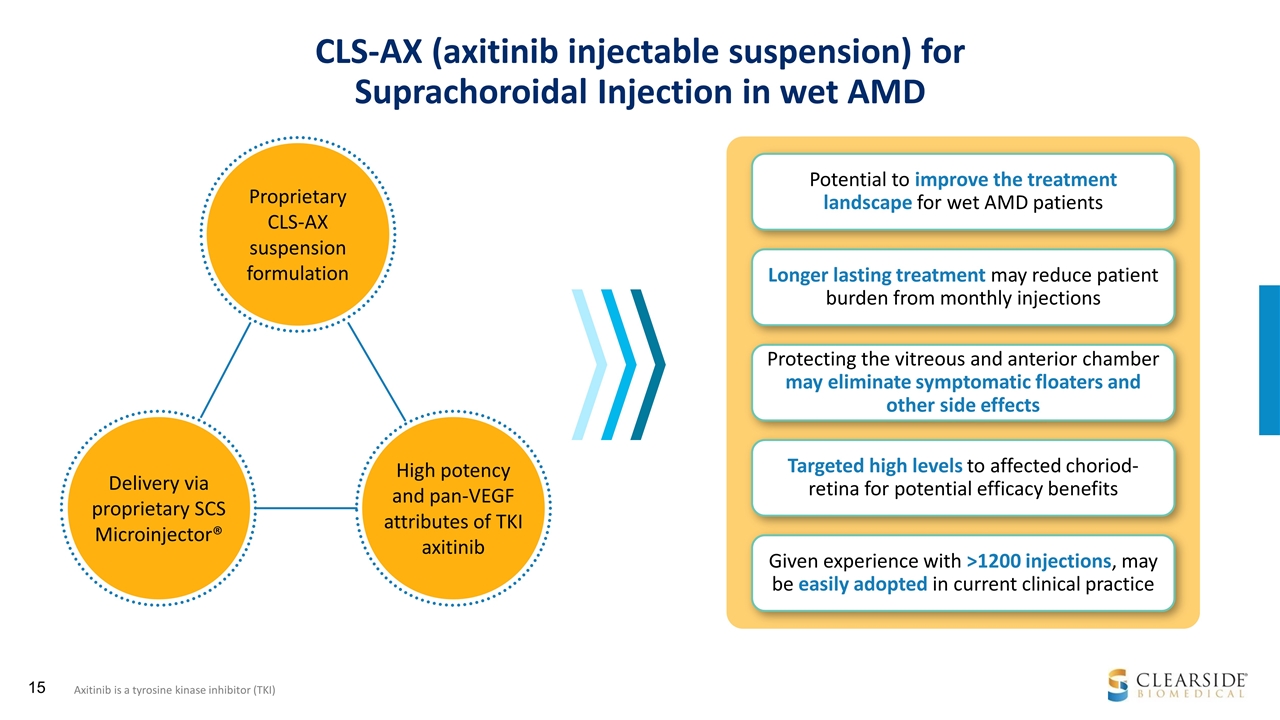
CLS-AX (axitinib injectable suspension) for Suprachoroidal Injection in wet AMD Proprietary CLS-AX suspension formulation Potential to improve the treatment landscape for wet AMD patients Longer lasting treatment may reduce patient burden from monthly injections Targeted high levels to affected choriod-retina for potential efficacy benefits Protecting the vitreous and anterior chamber may eliminate symptomatic floaters and other side effects Given experience with >1200 injections, may be easily adopted in current clinical practice Axitinib is a tyrosine kinase inhibitor (TKI) Delivery via proprietary SCS Microinjector® High potency and pan-VEGF attributes of TKI axitinib
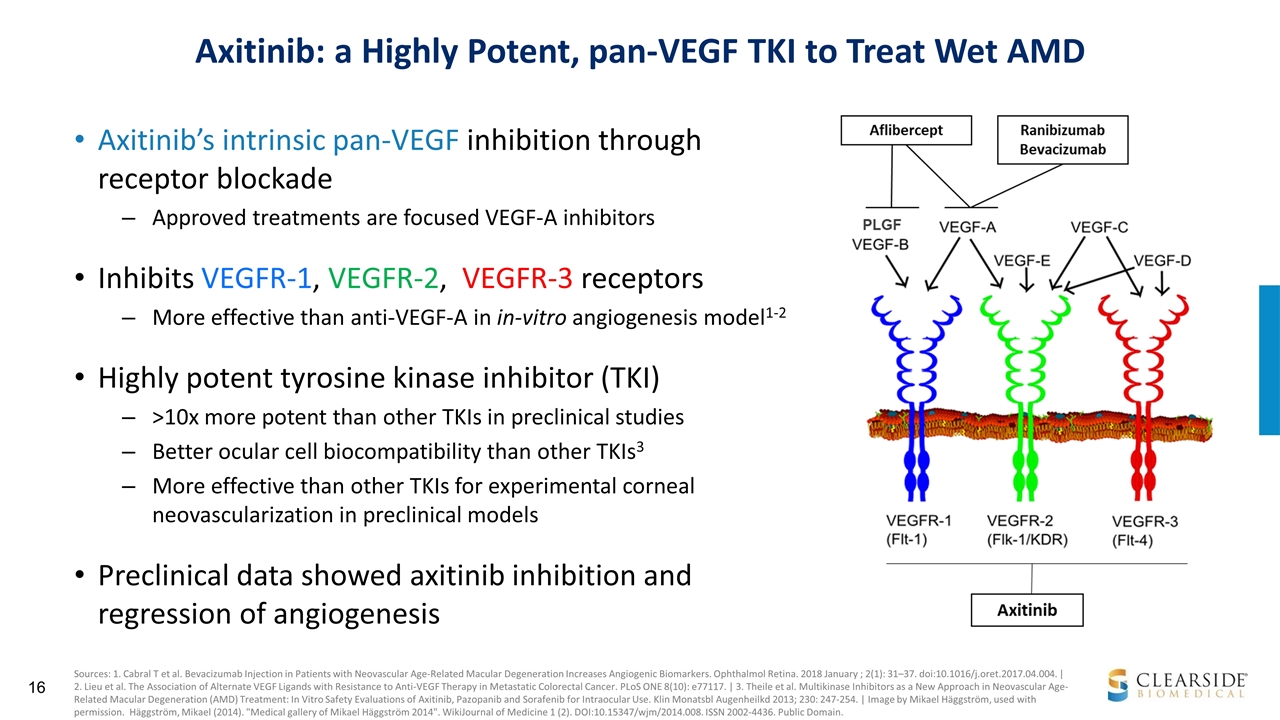
Axitinib: a Highly Potent, pan-VEGF TKI to Treat Wet AMD Axitinib’s intrinsic pan-VEGF inhibition through receptor blockade Approved treatments are focused VEGF-A inhibitors Inhibits VEGFR-1, VEGFR-2, VEGFR-3 receptors More effective than anti-VEGF-A in in-vitro angiogenesis model1-2 Highly potent tyrosine kinase inhibitor (TKI) >10x more potent than other TKIs in preclinical studies Better ocular cell biocompatibility than other TKIs3 More effective than other TKIs for experimental corneal neovascularization in preclinical models Preclinical data showed axitinib inhibition and regression of angiogenesis Sources: 1. Cabral T et al. Bevacizumab Injection in Patients with Neovascular Age-Related Macular Degeneration Increases Angiogenic Biomarkers. Ophthalmol Retina. 2018 January ; 2(1): 31–37. doi:10.1016/j.oret.2017.04.004. | 2. Lieu et al. The Association of Alternate VEGF Ligands with Resistance to Anti-VEGF Therapy in Metastatic Colorectal Cancer. PLoS ONE 8(10): e77117. | 3. Theile et al. Multikinase Inhibitors as a New Approach in Neovascular Age-Related Macular Degeneration (AMD) Treatment: In Vitro Safety Evaluations of Axitinib, Pazopanib and Sorafenib for Intraocular Use. Klin Monatsbl Augenheilkd 2013; 230: 247-254. | Image by Mikael Häggström, used with permission. Häggström, Mikael (2014). "Medical gallery of Mikael Häggström 2014". WikiJournal of Medicine 1 (2). DOI:10.15347/wjm/2014.008. ISSN 2002-4436. Public Domain.
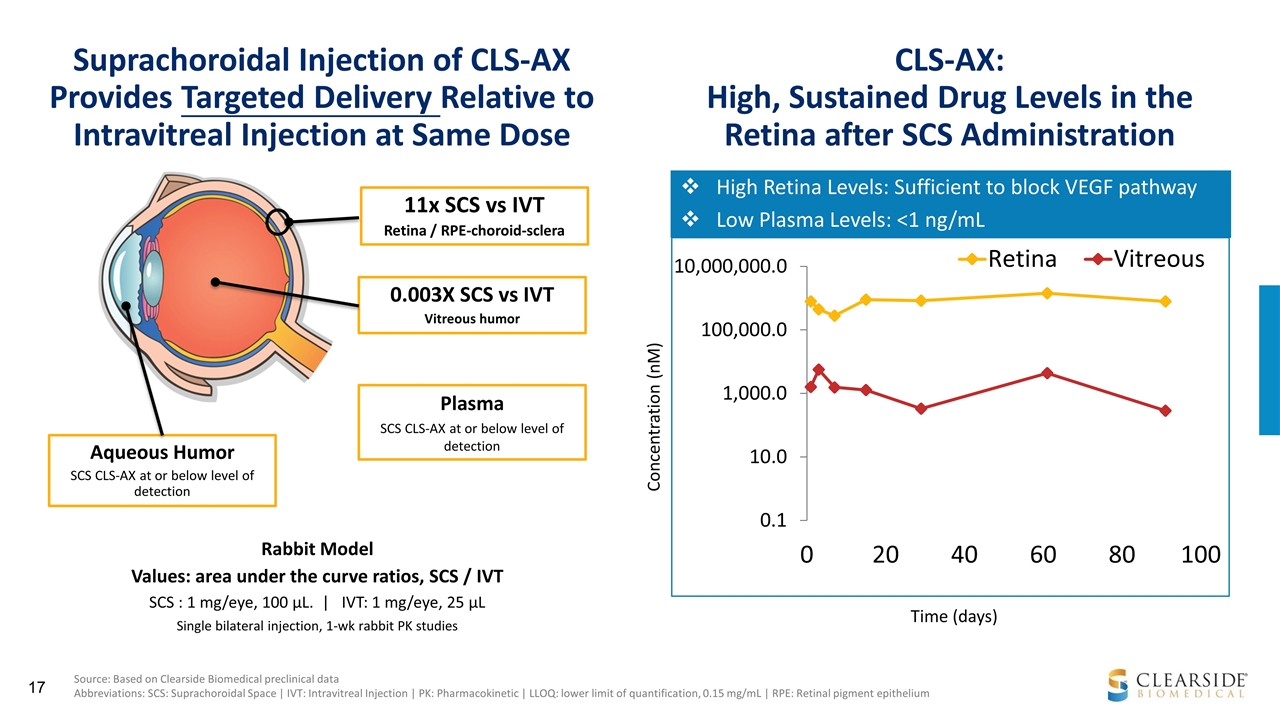
Suprachoroidal Injection of CLS-AX Provides Targeted Delivery Relative to Intravitreal Injection at Same Dose 11x SCS vs IVT Retina / RPE-choroid-sclera 0.003X SCS vs IVT Vitreous humor Aqueous Humor SCS CLS-AX at or below level of detection Rabbit Model Values: area under the curve ratios, SCS / IVT SCS : 1 mg/eye, 100 µL. | IVT: 1 mg/eye, 25 µL Single bilateral injection, 1-wk rabbit PK studies Plasma SCS CLS-AX at or below level of detection Source: Based on Clearside Biomedical preclinical data Abbreviations: SCS: Suprachoroidal Space | IVT: Intravitreal Injection | PK: Pharmacokinetic | LLOQ: lower limit of quantification, 0.15 mg/mL | RPE: Retinal pigment epithelium Concentration (nM) High Retina Levels: Sufficient to block VEGF pathway Low Plasma Levels: <1 ng/mL Time (days) CLS-AX: High, Sustained Drug Levels in the Retina after SCS Administration
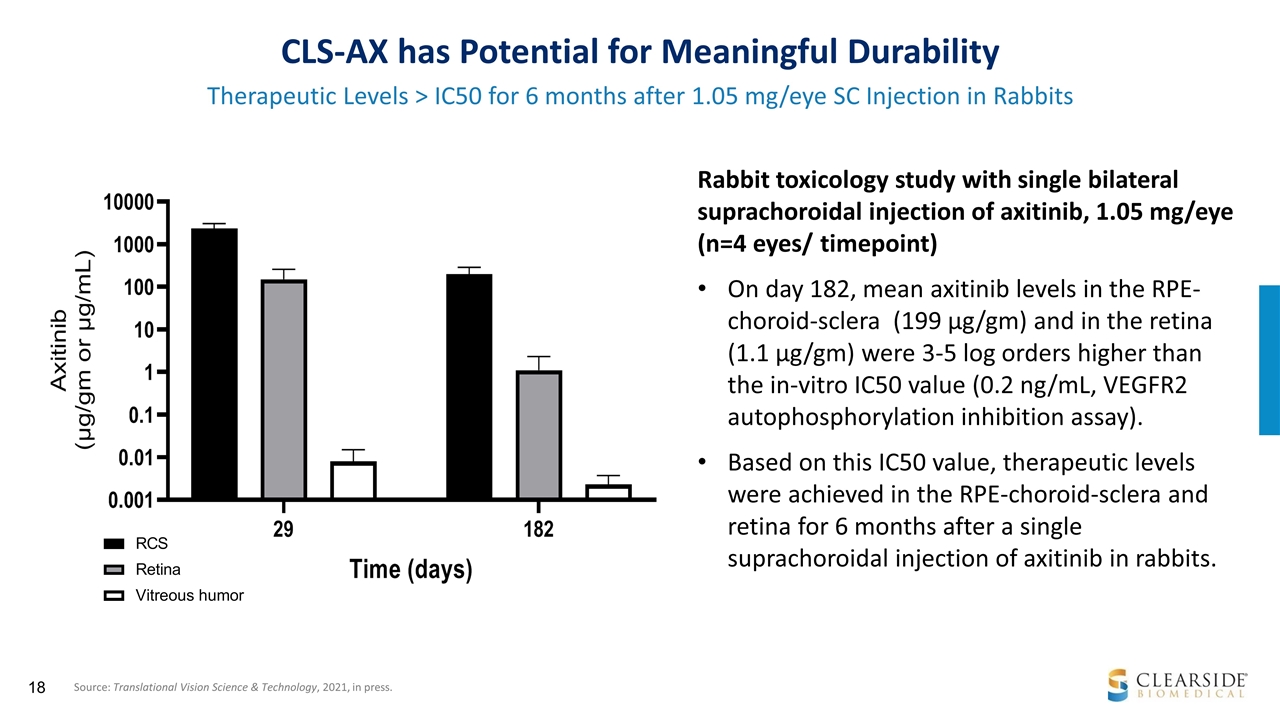
CLS-AX has Potential for Meaningful Durability Therapeutic Levels > IC50 for 6 months after 1.05 mg/eye SC Injection in Rabbits Rabbit toxicology study with single bilateral suprachoroidal injection of axitinib, 1.05 mg/eye (n=4 eyes/ timepoint) On day 182, mean axitinib levels in the RPE-choroid-sclera (199 µg/gm) and in the retina (1.1 µg/gm) were 3-5 log orders higher than the in-vitro IC50 value (0.2 ng/mL, VEGFR2 autophosphorylation inhibition assay). Based on this IC50 value, therapeutic levels were achieved in the RPE-choroid-sclera and retina for 6 months after a single suprachoroidal injection of axitinib in rabbits. Source: Translational Vision Science & Technology, 2021, in press.
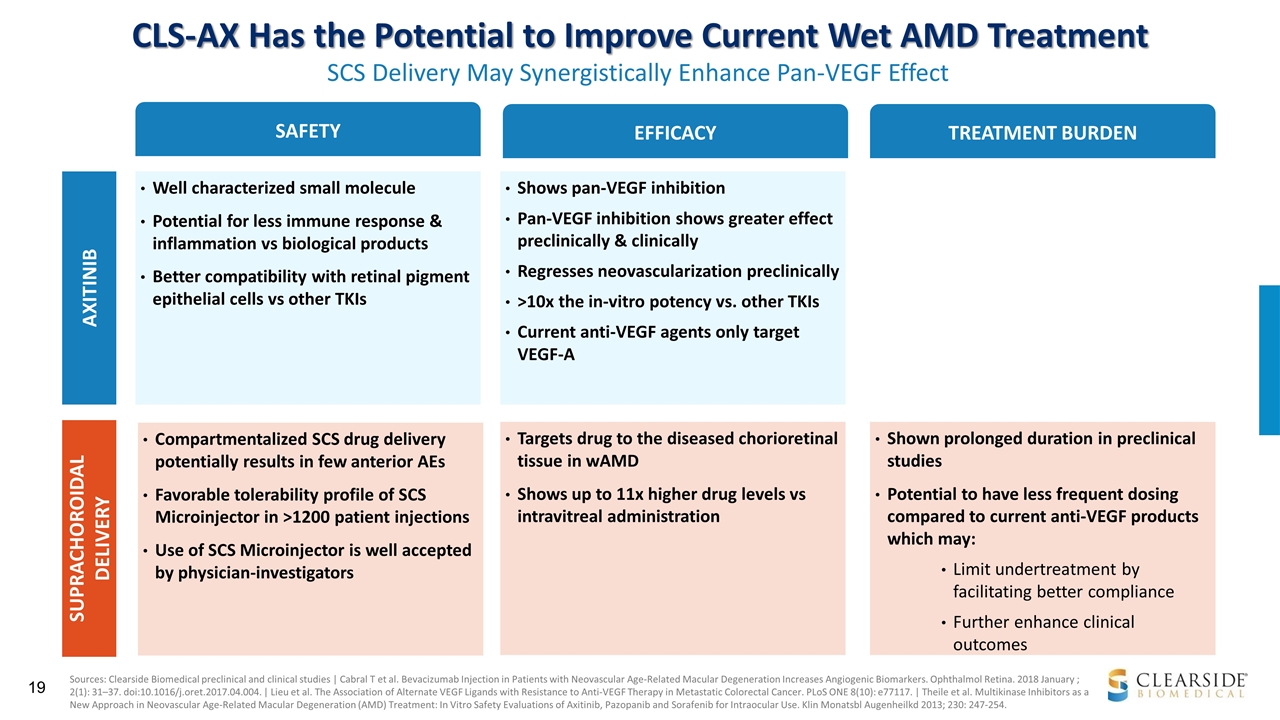
CLS-AX Has the Potential to Improve Current Wet AMD Treatment SCS Delivery May Synergistically Enhance Pan-VEGF Effect SAFETY EFFICACY TREATMENT BURDEN Well characterized small molecule Potential for less immune response & inflammation vs biological products Better compatibility with retinal pigment epithelial cells vs other TKIs AXITINIB SUPRACHOROIDAL DELIVERY Compartmentalized SCS drug delivery potentially results in few anterior AEs Favorable tolerability profile of SCS Microinjector in >1200 patient injections Use of SCS Microinjector is well accepted by physician-investigators Shows pan-VEGF inhibition Pan-VEGF inhibition shows greater effect preclinically & clinically Regresses neovascularization preclinically >10x the in-vitro potency vs. other TKIs Current anti-VEGF agents only target VEGF-A Targets drug to the diseased chorioretinal tissue in wAMD Shows up to 11x higher drug levels vs intravitreal administration Shown prolonged duration in preclinical studies Potential to have less frequent dosing compared to current anti-VEGF products which may: Limit undertreatment by facilitating better compliance Further enhance clinical outcomes Sources: Clearside Biomedical preclinical and clinical studies | Cabral T et al. Bevacizumab Injection in Patients with Neovascular Age-Related Macular Degeneration Increases Angiogenic Biomarkers. Ophthalmol Retina. 2018 January ; 2(1): 31–37. doi:10.1016/j.oret.2017.04.004. | Lieu et al. The Association of Alternate VEGF Ligands with Resistance to Anti-VEGF Therapy in Metastatic Colorectal Cancer. PLoS ONE 8(10): e77117. | Theile et al. Multikinase Inhibitors as a New Approach in Neovascular Age-Related Macular Degeneration (AMD) Treatment: In Vitro Safety Evaluations of Axitinib, Pazopanib and Sorafenib for Intraocular Use. Klin Monatsbl Augenheilkd 2013; 230: 247-254.
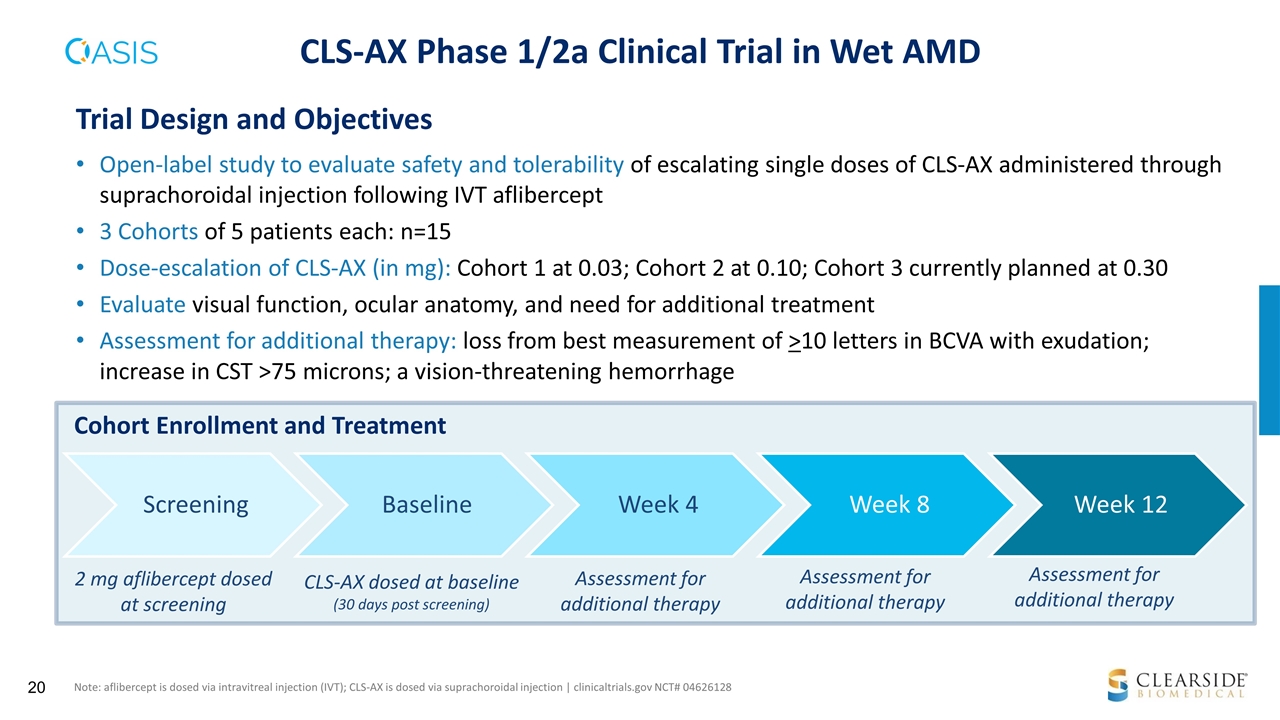
CLS-AX Phase 1/2a Clinical Trial in Wet AMD 2 mg aflibercept dosed at screening CLS-AX dosed at baseline (30 days post screening) Cohort Enrollment and Treatment Open-label study to evaluate safety and tolerability of escalating single doses of CLS-AX administered through suprachoroidal injection following IVT aflibercept 3 Cohorts of 5 patients each: n=15 Dose-escalation of CLS-AX (in mg): Cohort 1 at 0.03; Cohort 2 at 0.10; Cohort 3 currently planned at 0.30 Evaluate visual function, ocular anatomy, and need for additional treatment Assessment for additional therapy: loss from best measurement of >10 letters in BCVA with exudation; increase in CST >75 microns; a vision-threatening hemorrhage Trial Design and Objectives Note: aflibercept is dosed via intravitreal injection (IVT); CLS-AX is dosed via suprachoroidal injection | clinicaltrials.gov NCT# 04626128 Assessment for additional therapy Assessment for additional therapy Assessment for additional therapy Screening Baseline Week 4 Week 8 Week 12

Cohort 1 Objective: To establish a floor of safety in this first-in-human trial with low dose CLS-AX (0.03 mg dose) Patients: Highly treatment-experienced Total number prior anti-VEGF treatments: mean = 25.8, median = 28.0 Total number prior anti-VEGF treatments within the last 12 months: mean = 9.0, median = 11.0 Conclusion Cohort 1 supports progression to Cohort 2 Cohort 1: Encouraging Results Support Progression to Cohort 2 Source: Clearside data on file.
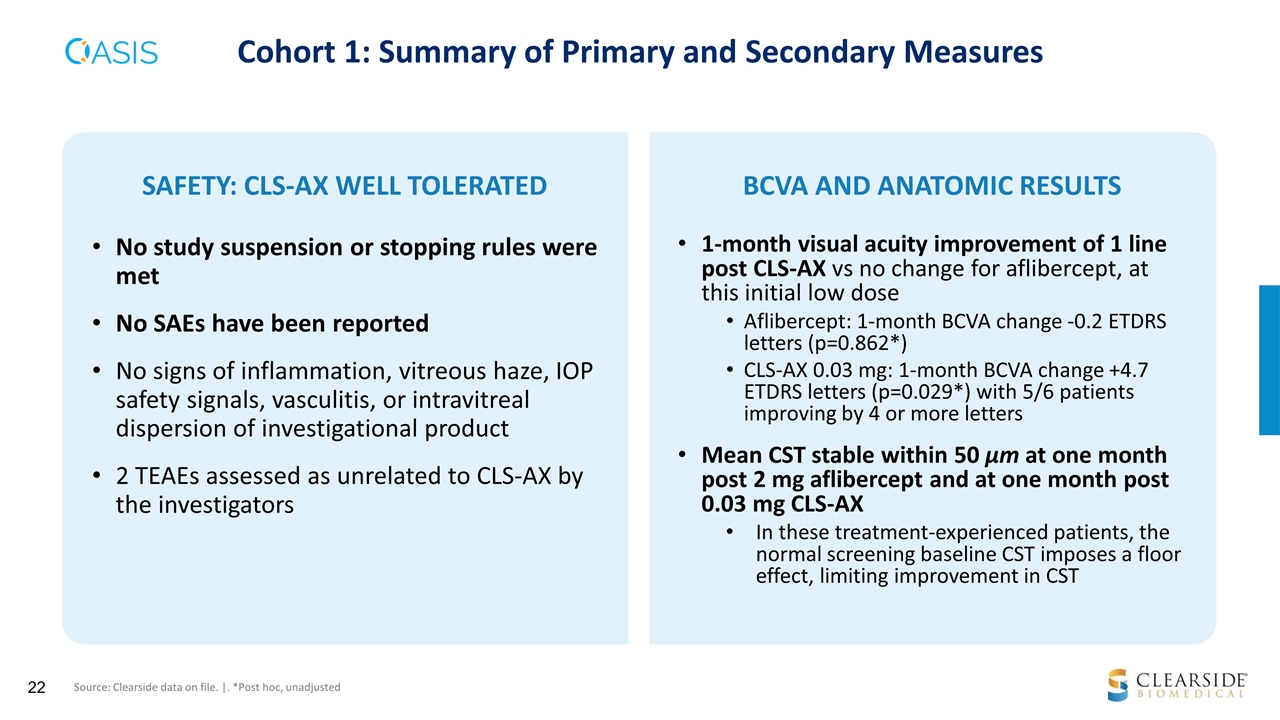
1-month visual acuity improvement of 1 line post CLS-AX vs no change for aflibercept, at this initial low dose Aflibercept: 1-month BCVA change -0.2 ETDRS letters (p=0.862*) CLS-AX 0.03 mg: 1-month BCVA change +4.7 ETDRS letters (p=0.029*) with 5/6 patients improving by 4 or more letters Mean CST stable within 50 µm at one month post 2 mg aflibercept and at one month post 0.03 mg CLS-AX In these treatment-experienced patients, the normal screening baseline CST imposes a floor effect, limiting improvement in CST SAFETY: CLS-AX WELL TOLERATED No study suspension or stopping rules were met No SAEs have been reported No signs of inflammation, vitreous haze, IOP safety signals, vasculitis, or intravitreal dispersion of investigational product 2 TEAEs assessed as unrelated to CLS-AX by the investigators BCVA AND ANATOMIC RESULTS Cohort 1: Summary of Primary and Secondary Measures Source: Clearside data on file. |. *Post hoc, unadjusted
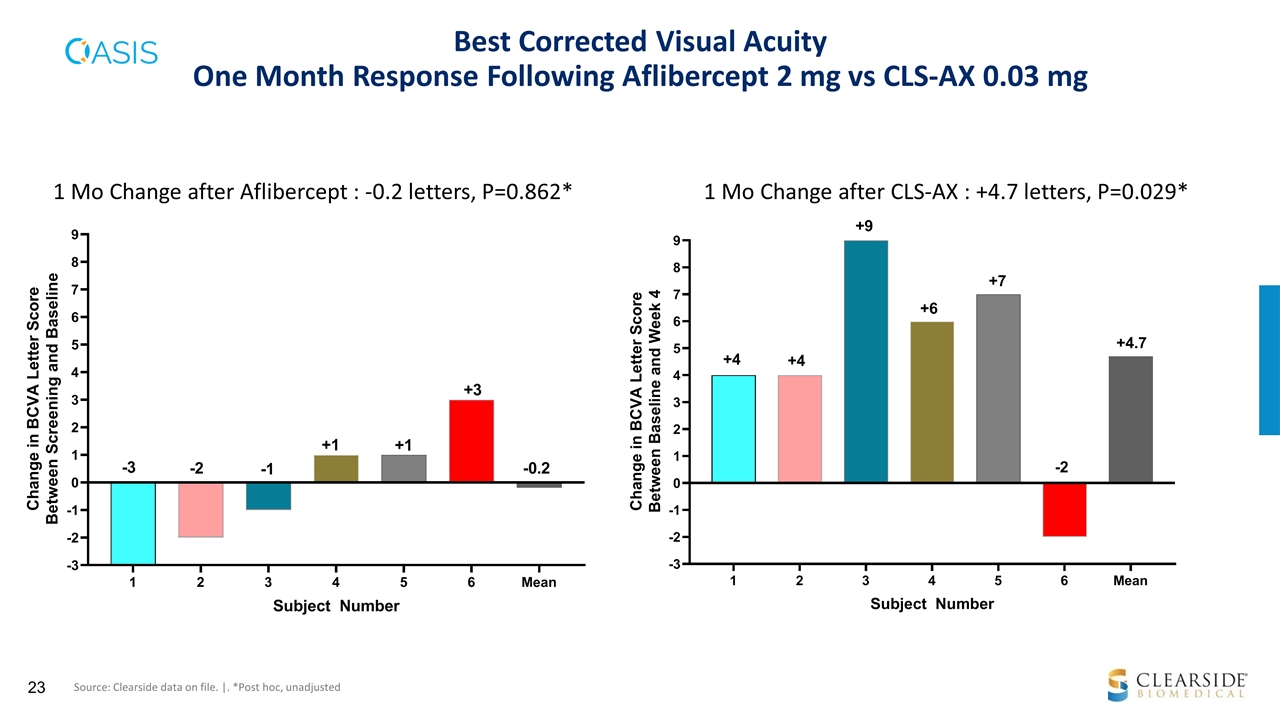
Best Corrected Visual Acuity One Month Response Following Aflibercept 2 mg vs CLS-AX 0.03 mg 1 Mo Change after Aflibercept : -0.2 letters, P=0.862* 1 Mo Change after CLS-AX : +4.7 letters, P=0.029* Source: Clearside data on file. |. *Post hoc, unadjusted
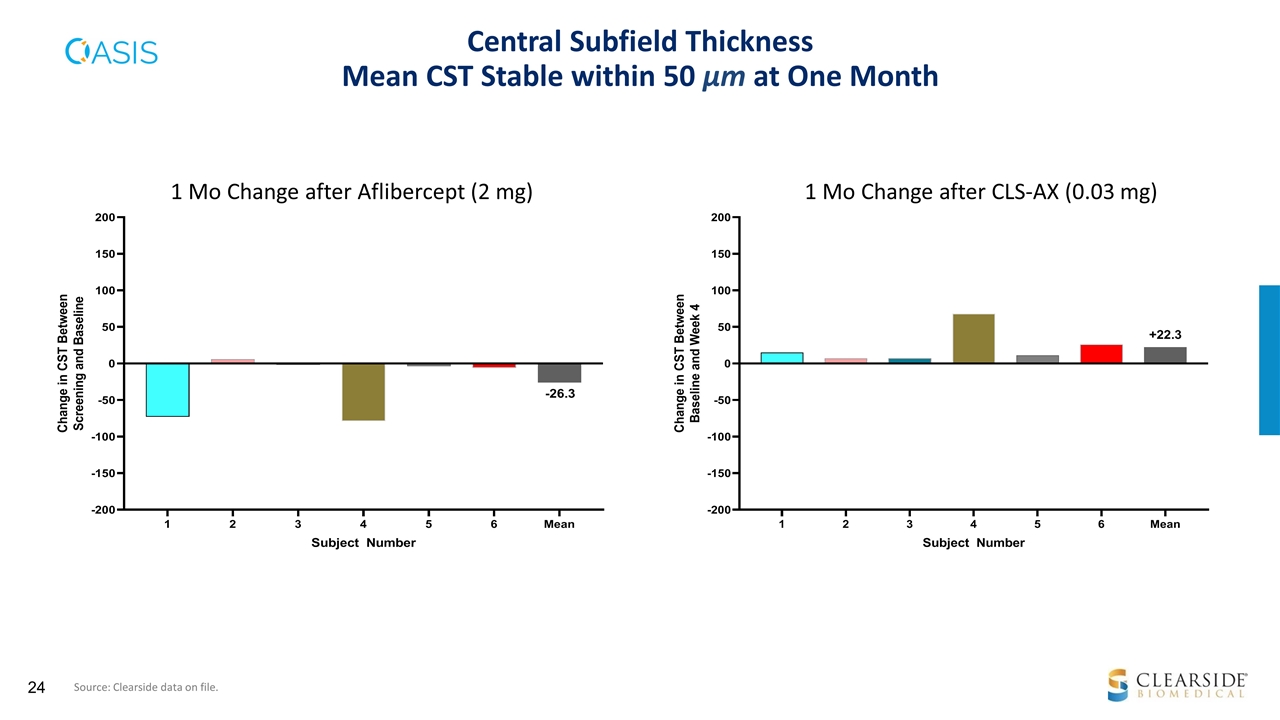
Central Subfield Thickness Mean CST Stable within 50 µm at One Month 1 Mo Change after Aflibercept (2 mg) 1 Mo Change after CLS-AX (0.03 mg) Source: Clearside data on file.
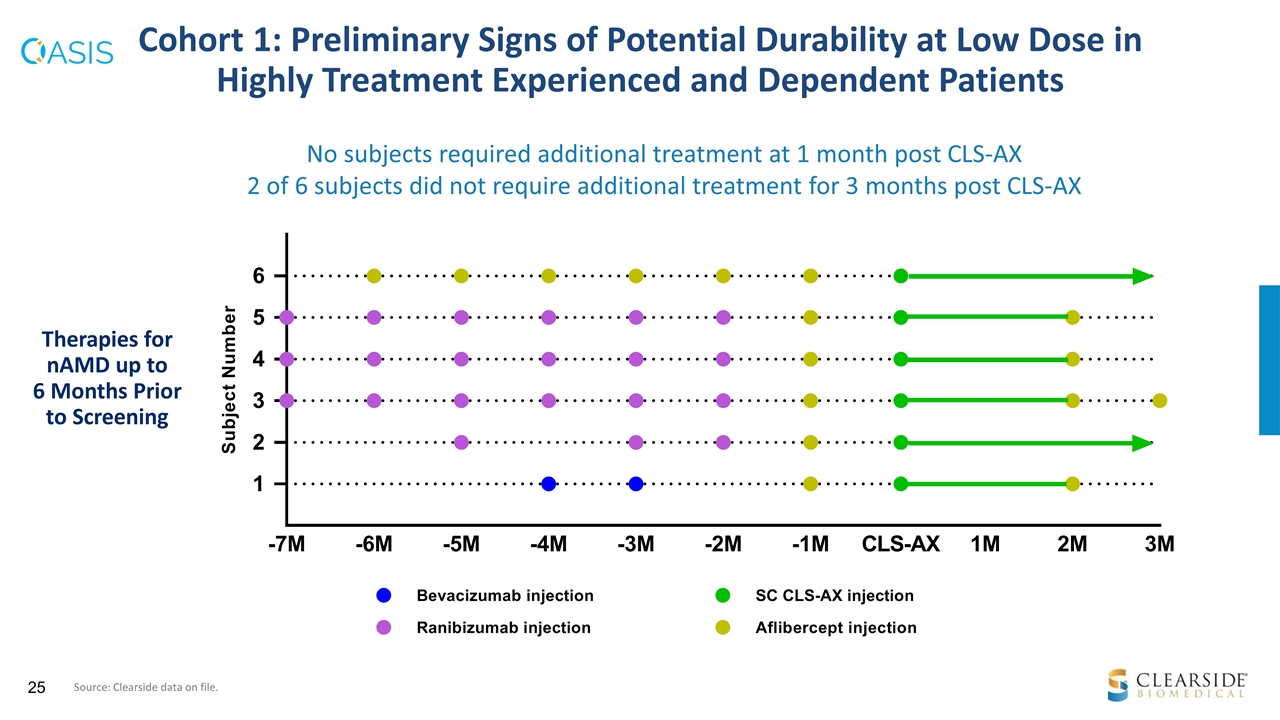
No subjects required additional treatment at 1 month post CLS-AX 2 of 6 subjects did not require additional treatment for 3 months post CLS-AX Cohort 1: Preliminary Signs of Potential Durability at Low Dose in Highly Treatment Experienced and Dependent Patients Therapies for nAMD up to 6 Months Prior to Screening Source: Clearside data on file.
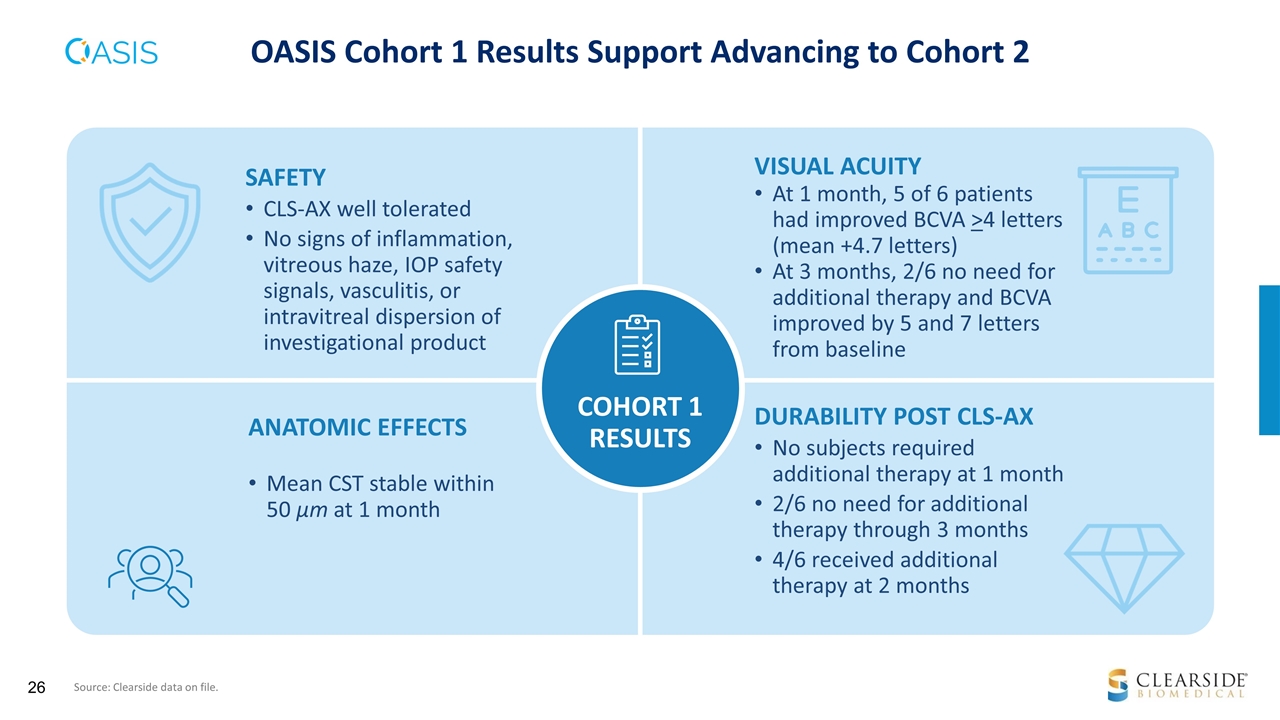
OASIS Cohort 1 Results Support Advancing to Cohort 2 SAFETY CLS-AX well tolerated No signs of inflammation, vitreous haze, IOP safety signals, vasculitis, or intravitreal dispersion of investigational product VISUAL ACUITY At 1 month, 5 of 6 patients had improved BCVA >4 letters (mean +4.7 letters) At 3 months, 2/6 no need for additional therapy and BCVA improved by 5 and 7 letters from baseline ANATOMIC EFFECTS Mean CST stable within 50 µm at 1 month DURABILITY POST CLS-AX No subjects required additional therapy at 1 month 2/6 no need for additional therapy through 3 months 4/6 received additional therapy at 2 months COHORT 1 RESULTS Source: Clearside data on file.
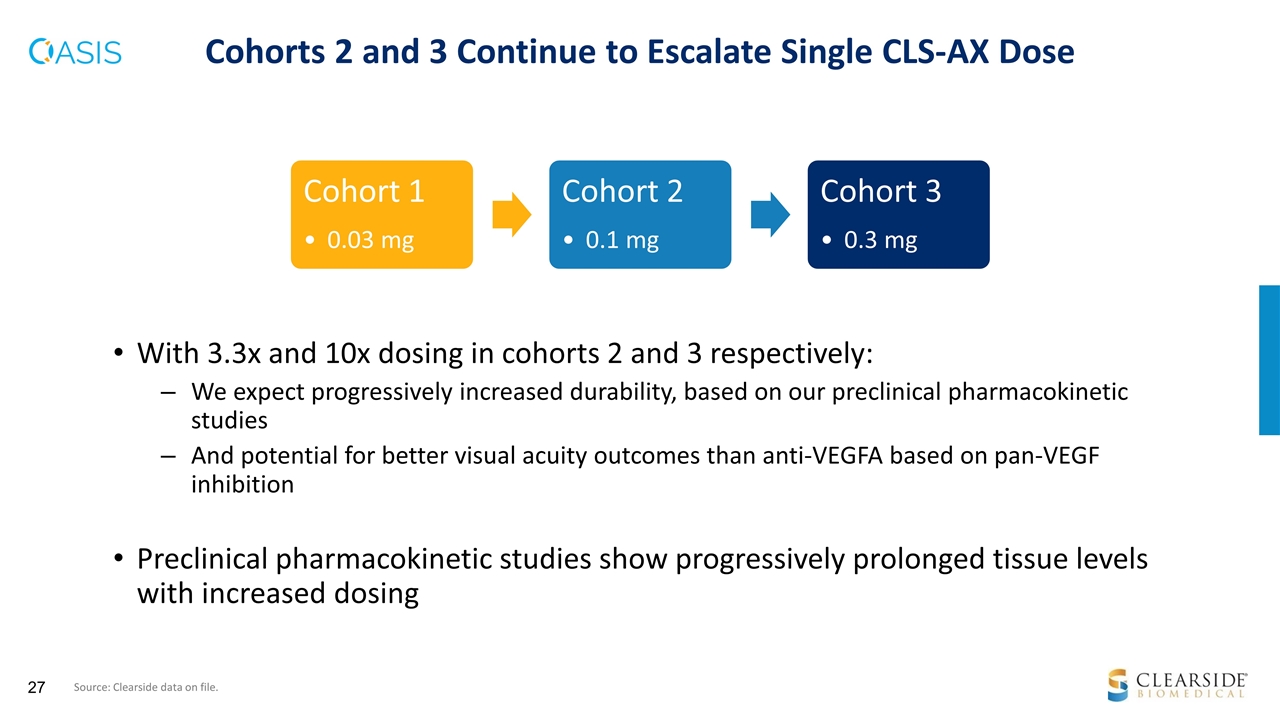
With 3.3x and 10x dosing in cohorts 2 and 3 respectively: We expect progressively increased durability, based on our preclinical pharmacokinetic studies And potential for better visual acuity outcomes than anti-VEGFA based on pan-VEGF inhibition Preclinical pharmacokinetic studies show progressively prolonged tissue levels with increased dosing Cohorts 2 and 3 Continue to Escalate Single CLS-AX Dose Source: Clearside data on file. Cohort 1 0.03 mg Cohort 2 0.1 mg Cohort 3 0.3 mg

Early-Stage Pipeline
The Opportunity Beyond the VEGF pathway Novel target Early industry validation in DME and AMD Advantages of targeted suprachoroidal administration with potential for: Improved safety profile, through compartmentalization in SCS Enhanced efficacy, through drug levels at affected tissues Extended durability Limited potential competition in the non-VEGF approach to treatment Primary Need Targeted delivery addressing disease-modifying pathways beyond anti-VEGF therapy SCS Injection Platform and Integrin Inhibition
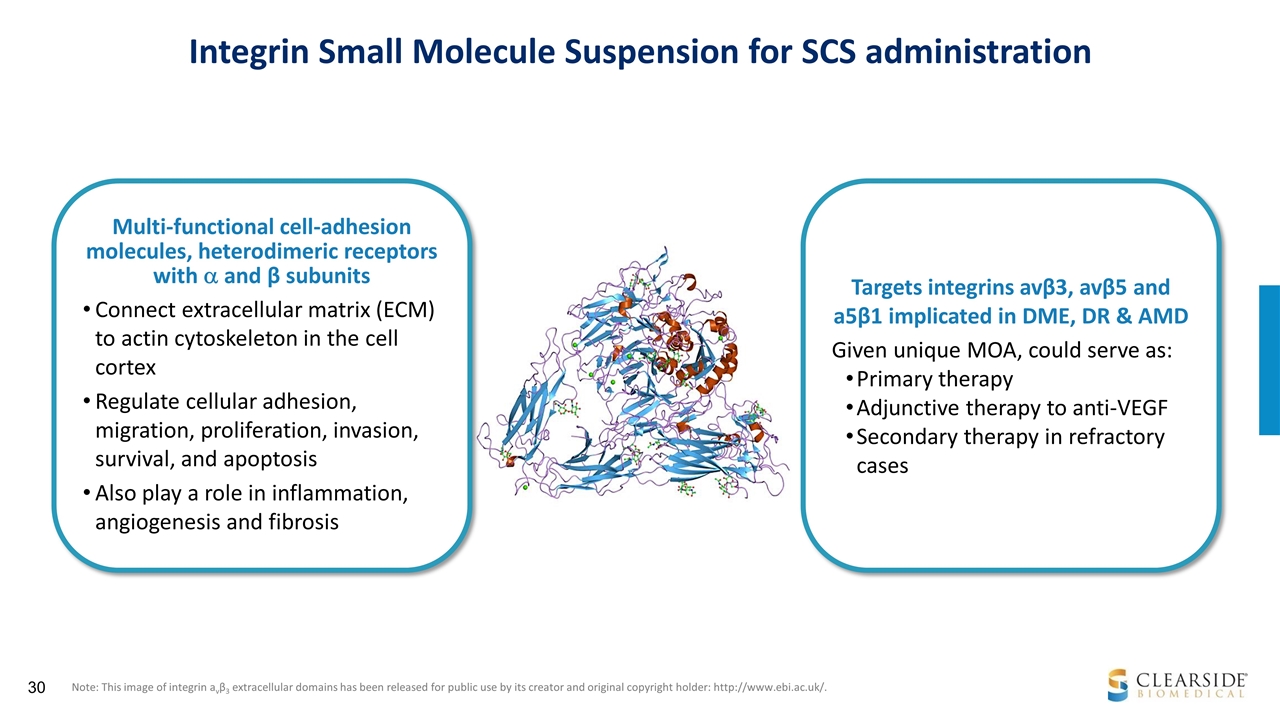
Integrin Small Molecule Suspension for SCS administration Note: This image of integrin avβ3 extracellular domains has been released for public use by its creator and original copyright holder: http://www.ebi.ac.uk/. Multi-functional cell-adhesion molecules, heterodimeric receptors with a and β subunits Connect extracellular matrix (ECM) to actin cytoskeleton in the cell cortex Regulate cellular adhesion, migration, proliferation, invasion, survival, and apoptosis Also play a role in inflammation, angiogenesis and fibrosis Targets integrins avβ3, avβ5 and a5β1 implicated in DME, DR & AMD Given unique MOA, could serve as: Primary therapy Adjunctive therapy to anti-VEGF Secondary therapy in refractory cases
Strategic Priority: Seeking a clinical development partner to advance SCS delivery of current gene therapy assets Suprachoroidal Injection of Gene Therapy May Offer Potential for Safe and Efficient Delivery Sources: Clearside data on file. | Campochiaro, P. A. (2019). AAV8-vectored suprachoroidal gene transfer produces widespread ocular transgene expression. Journal of Clinical Investigation. doi: 10.1172/jci129085 The Opportunity Convert gene therapy into an office-based procedure Avoid risks of vitrectomy (surgery) Avoid risks of retinotomy, subretinal injection, and macular detachment Enhance patient access Equivalent expression for subretinal and suprachoroidal administration preclinically Potential for broader retinal coverage & repeat dosing of suprachoroidal vs subretinal injection Delivery of viral and non-viral vectors Preclinical studies with AAV show transfection of photoreceptors

Corporate Partnerships & Milestones
Enabling SCS Delivery of AAV Gene Therapy for Retinal Disease The Opportunity: Gene Therapy Exclusive worldwide rights to our SCS Microinjector for delivery of adeno-associated virus (AAV)-based therapeutics to the suprachoroidal space to treat wet AMD, diabetic retinopathy and other conditions for which anti-VEGF treatment is the standard of care Delivery of gene therapy through the SCS may provide a targeted, in-office, non-surgical treatment approach option Encouraging preclinical results delivering RGX-314 into the SCS SCS delivery of AAV gene therapy well tolerated to date The Terms: Up to an additional $34M in development milestones across multiple indications Up to $102M in sales milestones Mid single digit royalties on net sales of products using SCS Microinjector
REGENXBIO: Two Phase 2 Trials Using SCS Microinjector® Two multi-center, open-label, randomized, controlled, dose-escalation studies evaluating the efficacy, safety and tolerability of suprachoroidal delivery of RGX-314 RGX-314 for Treatment of Wet Age-Related Macular Degeneration (wet AMD) Phase 2 AAVIATE trial of suprachoroidal delivery of RGX-314 using SCS Microinjector is ongoing Patient population: severe wet AMD patients who are responsive to anti-VEGF treatment Interim efficacy data from Cohort 1 expected in Q3 2021 Interim data from Cohort 2 expected in H2 2021 Enrolling Cohort 3 in patients who are positive for neutralizing antibodies RGX-314 for Treatment of Diabetic Retinopathy (DR) Phase 2 ALTITUDE trial of suprachoroidal delivery of RGX-314 using SCS Microinjector is ongoing Initial data expected in 2021
Aura Bioscience: Phase 2 Ocular Oncology trial using SCS Microinjector® The Opportunity: Ocular Oncology Worldwide licensing agreement for the use of our SCS Microinjector to deliver their proprietary drug candidates into the SCS for the potential treatment of certain ocular cancers, including choroidal melanoma Non-surgical alternative to intravitreal delivery of Aura’s oncology drug candidates via our SCS Microinjector Choroidal melanoma is the most common, primary intraocular tumor in adults Aura’s Phase 2 clinical trial is ongoing using SCS Microinjector SCS delivery clinically well tolerated to date The Terms: Potential future financial upside for Clearside from pre-specified development and sales milestones Royalties on net sales of products using SCS Microinjector
XIPERE: Two Global Commercialization & Development Partners XIPERE™ is an investigational product License for the U.S. and Canada; options for other territories Received $5M upfront payment Up to $15M in FDA approval and pre-launch milestones Up to $57.3M in milestone payments; tiered royalties from the high-teens to 20% License for Greater China & South Korea Received $4M upfront payment $4M at U.S. approval Up to $31.5M in approval, development and sales milestones Tiered royalties of 10% to 12%
1 2 3 4 Maximizes the commercial and development opportunities for XIPERE in multiple geographic markets Validates our investment in suprachoroidal delivery using our SCS Microinjector Expands our overall internal and collaborative product development pipeline Eligible to receive >$230M from the four partnerships in potential development and sales milestones, and potential royalties to fund our internal R&D pipeline Four Validating Partnerships to Drive Growth
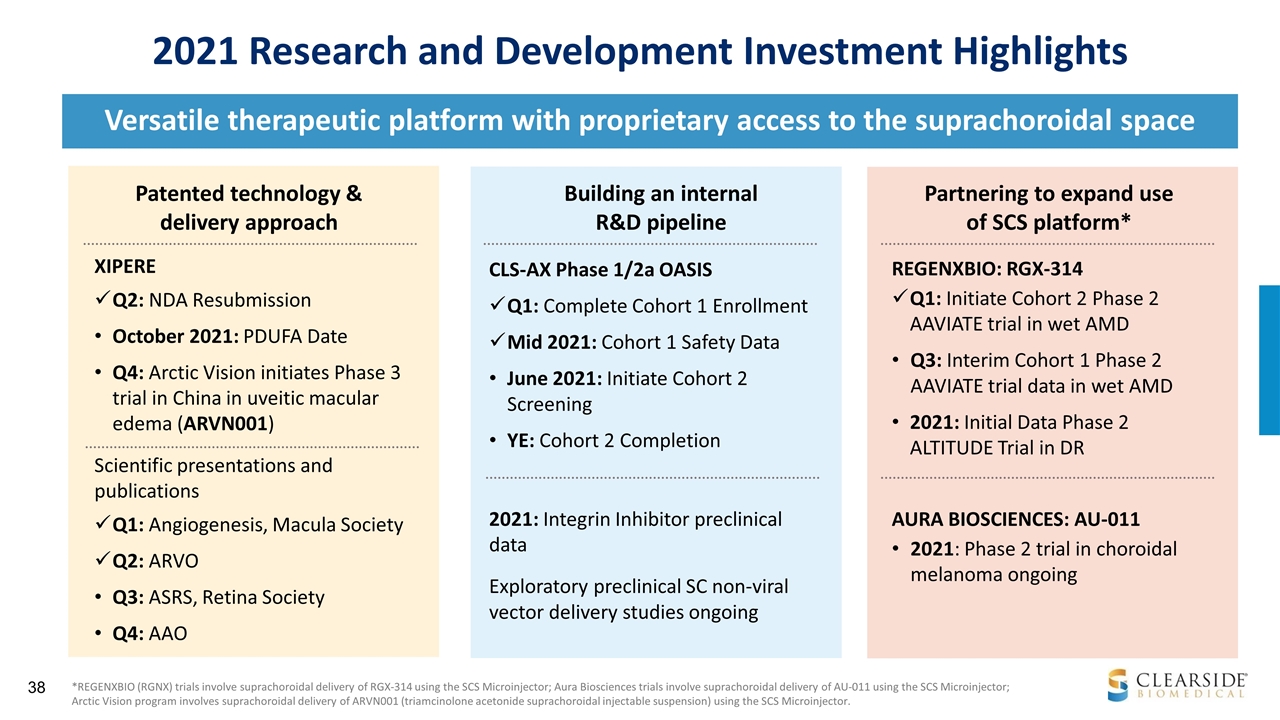
2021 Research and Development Investment Highlights *REGENXBIO (RGNX) trials involve suprachoroidal delivery of RGX-314 using the SCS Microinjector; Aura Biosciences trials involve suprachoroidal delivery of AU-011 using the SCS Microinjector; Arctic Vision program involves suprachoroidal delivery of ARVN001 (triamcinolone acetonide suprachoroidal injectable suspension) using the SCS Microinjector. Versatile therapeutic platform with proprietary access to the suprachoroidal space Scientific presentations and publications Q1: Angiogenesis, Macula Society Q2: ARVO Q3: ASRS, Retina Society Q4: AAO REGENXBIO: RGX-314 Q1: Initiate Cohort 2 Phase 2 AAVIATE trial in wet AMD Q3: Interim Cohort 1 Phase 2 AAVIATE trial data in wet AMD 2021: Initial Data Phase 2 ALTITUDE Trial in DR AURA BIOSCIENCES: AU-011 2021: Phase 2 trial in choroidal melanoma ongoing Patented technology & delivery approach Partnering to expand use of SCS platform* CLS-AX Phase 1/2a OASIS Q1: Complete Cohort 1 Enrollment Mid 2021: Cohort 1 Safety Data June 2021: Initiate Cohort 2 Screening YE: Cohort 2 Completion Building an internal R&D pipeline XIPERE Q2: NDA Resubmission October 2021: PDUFA Date Q4: Arctic Vision initiates Phase 3 trial in China in uveitic macular edema (ARVN001) 2021: Integrin Inhibitor preclinical data Exploratory preclinical SC non-viral vector delivery studies ongoing

Nasdaq: CLSD






































