
TM OASIS Phase 1/2a Clinical Trial 6-Month Extension Study Results February 2, 2023 Exhibit 99.1

Forward-Looking Statements This presentation contains forward-looking statements as defined in the Private Securities Litigation Reform Act of 1995, as amended. The words “may,” “will,” “could,” “would,” “should,” “expect,” “plan,” “anticipate,” “intend,” “believe,” “estimate,” “predict,” “project,” “potential,” “continue,” “target” or the negative of these terms and other similar words or expressions are intended to identify forward-looking statements, although not all forward-looking statements contain these identifying words. Clearside Biomedical, Inc.’s views as of the date of this presentation about future events and are subject to risks, uncertainties, assumptions, and changes in circumstances that may cause Clearside’s actual results, performance, or achievements to differ significantly from those expressed or implied in any forward-looking statement. Although Clearside believes that the expectations reflected in the forward-looking statements are reasonable, new risks and uncertainties may emerge from time to time, and Clearside cannot guarantee future events, results, performance, or achievements. Some of the key factors that could cause actual results to differ from Clearside’s expectations include its plans to develop and potentially commercialize its product candidates; adverse differences between preliminary or interim data and final data; Clearside’s planned clinical trials and preclinical studies for its product candidates; the timing of and Clearside’s ability to obtain and maintain regulatory approvals for its product candidates; the extent of clinical trials potentially required for Clearside’s product candidates; the clinical utility and market acceptance of Clearside’s product candidates; Clearside’s commercialization, marketing and manufacturing capabilities and strategy; Clearside’s intellectual property position; Clearside's ability to expand its pipeline; developments and projections relating to Clearside's competitors and its industry; the impact of government laws and regulations; the impact of public health epidemics affecting countries or regions in which Clearside has operations or does business, such as the COVID-19 pandemic; the timing and anticipated results of Clearside's preclinical studies and clinical trials and the risk that the results of Clearside's preclinical studies and clinical trials may not be predictive of future results in connection with future studies or clinical trials and may not support further development and marketing approval; findings from investigational review boards at clinical trial sites and publication review bodies; Clearside's estimates regarding future revenue, expenses, capital requirements and need for additional financing; and Clearside’s ability to identify additional product candidates with significant commercial potential that are consistent with its commercial objectives. For further information regarding these risks, uncertainties and other factors you should read the “Risk Factors” section of Clearside’s Annual Report on Form 10-K for the year ended December 30, 2021, filed with the SEC on March 11, 2022, Clearside's Form 10-Q for the quarter ended September 30, 2022, filed with the SEC on November 9, 2022, and Clearside’s other Periodic Reports filed with the SEC. Clearside expressly disclaims any obligation to update or revise the information herein, including the forward-looking statements, except as required by law. This presentation also contains estimates and other statistical data made by independent parties and by Clearside relating to market size and growth and other data about its industry. This data involves a number of assumptions and limitations, and you are cautioned not to give undue weight to such estimates. In addition, projections, assumptions and estimates of Clearside’s future performance and the future performance of the markets in which Clearside operates are necessarily subject to a high degree of uncertainty and risk.
Developing and Delivering Treatments that Restore and Preserve Vision for Serious Back of the Eye Diseases First FDA Approved Product: XIPERE® Proprietary CLS-AX Phase 1/2 data in Wet AMD shows significant reduction in treatment burden and demonstrated a strong duration profile Proprietary Access to the Suprachoroidal Space (SCS®) Internal Research & Development Pipeline External Collaborations for Pipeline Expansion XIPERE® (triamcinolone acetonide injectable suspension), for suprachoroidal use Versatile Therapeutic Platform SCS Microinjector® with proprietary drug formulations target the suprachoroidal space
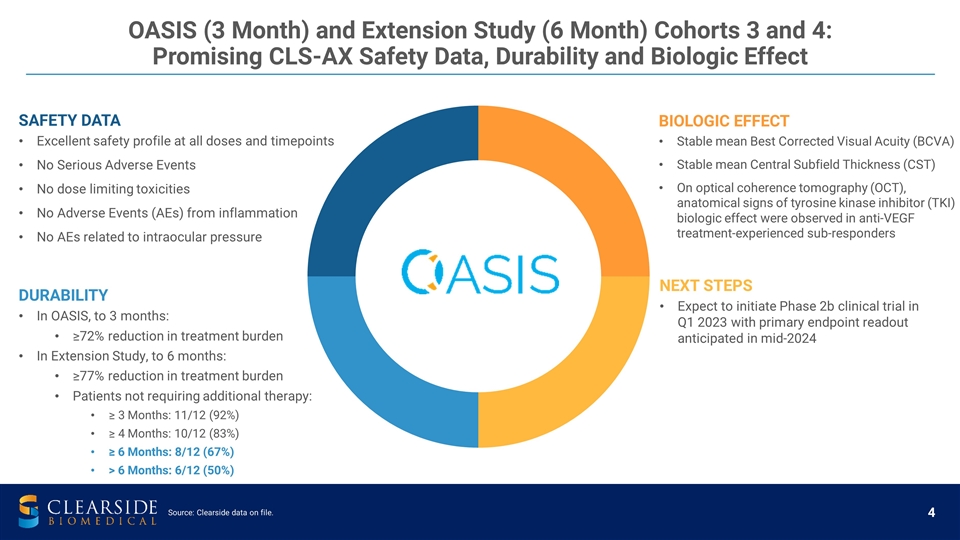
SAFETY DATA Excellent safety profile at all doses and timepoints No Serious Adverse Events No dose limiting toxicities No Adverse Events (AEs) from inflammation No AEs related to intraocular pressure BIOLOGIC EFFECT Stable mean Best Corrected Visual Acuity (BCVA) Stable mean Central Subfield Thickness (CST) On optical coherence tomography (OCT), anatomical signs of tyrosine kinase inhibitor (TKI) biologic effect were observed in anti-VEGF treatment-experienced sub-responders NEXT STEPS Expect to initiate Phase 2b clinical trial in Q1 2023 with primary endpoint readout anticipated in mid-2024 OASIS (3 Month) and Extension Study (6 Month) Cohorts 3 and 4: Promising CLS-AX Safety Data, Durability and Biologic Effect Source: Clearside data on file. DURABILITY In OASIS, to 3 months: ≥72% reduction in treatment burden In Extension Study, to 6 months: ≥77% reduction in treatment burden Patients not requiring additional therapy: ≥ 3 Months: 11/12 (92%) ≥ 4 Months: 10/12 (83%) ≥ 6 Months: 8/12 (67%) > 6 Months: 6/12 (50%)

CLS-AX (axitinib injectable suspension) for Suprachoroidal Injection
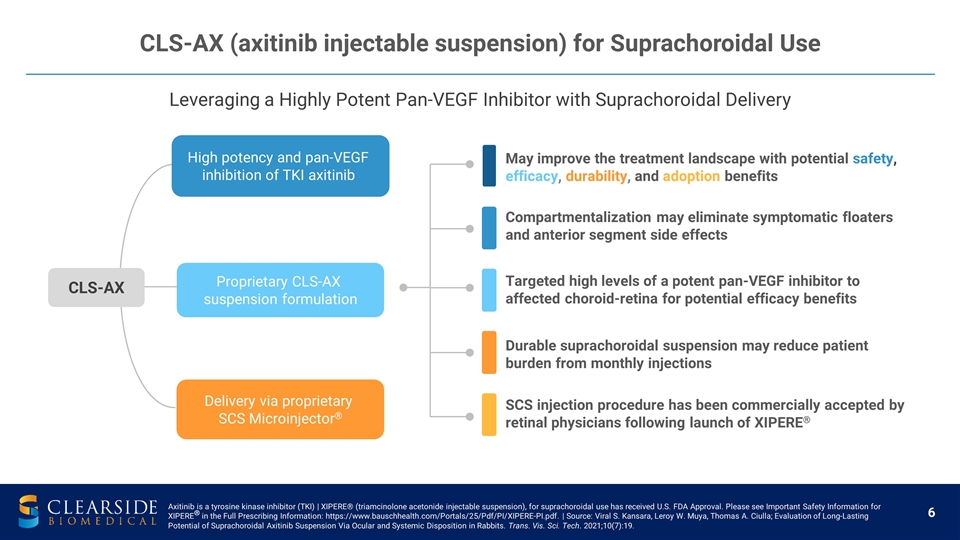
Leveraging a Highly Potent Pan-VEGF Inhibitor with Suprachoroidal Delivery CLS-AX (axitinib injectable suspension) for Suprachoroidal Use May improve the treatment landscape with potential safety, efficacy, durability, and adoption benefits Durable suprachoroidal suspension may reduce patient burden from monthly injections Compartmentalization may eliminate symptomatic floaters and anterior segment side effects Targeted high levels of a potent pan-VEGF inhibitor to affected choroid-retina for potential efficacy benefits SCS injection procedure has been commercially accepted by retinal physicians following launch of XIPERE® Proprietary CLS-AX suspension formulation Delivery via proprietary SCS Microinjector® High potency and pan-VEGF inhibition of TKI axitinib CLS-AX Axitinib is a tyrosine kinase inhibitor (TKI) | XIPERE® (triamcinolone acetonide injectable suspension), for suprachoroidal use has received U.S. FDA Approval. Please see Important Safety Information for XIPERE® in the Full Prescribing Information: https://www.bauschhealth.com/Portals/25/Pdf/PI/XIPERE-PI.pdf. | Source: Viral S. Kansara, Leroy W. Muya, Thomas A. Ciulla; Evaluation of Long-Lasting Potential of Suprachoroidal Axitinib Suspension Via Ocular and Systemic Disposition in Rabbits. Trans. Vis. Sci. Tech. 2021;10(7):19.
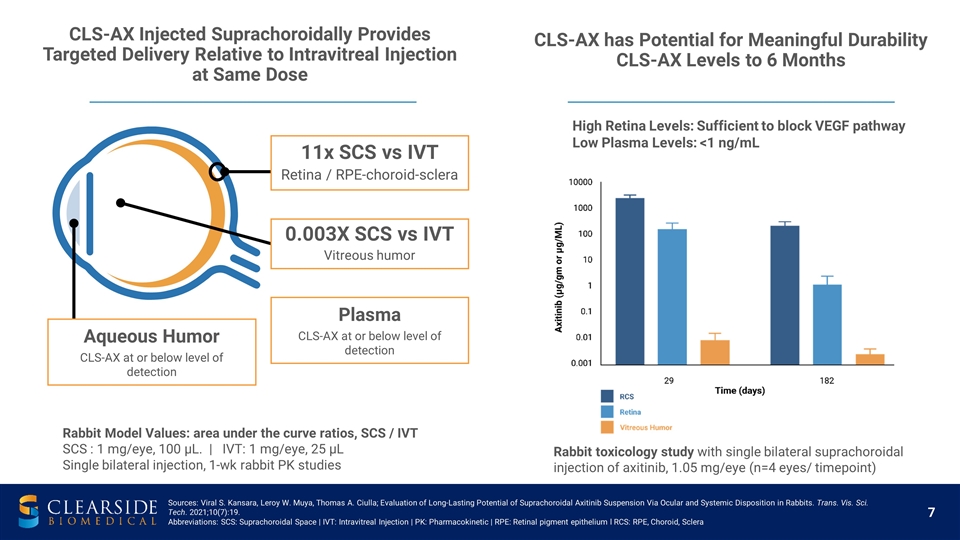
Plasma CLS-AX at or below level of detection Rabbit Model Values: area under the curve ratios, SCS / IVT SCS : 1 mg/eye, 100 µL. | IVT: 1 mg/eye, 25 µL Single bilateral injection, 1-wk rabbit PK studies High Retina Levels: Sufficient to block VEGF pathway Low Plasma Levels: <1 ng/mL 11x SCS vs IVT Retina / RPE-choroid-sclera 0.003X SCS vs IVT Vitreous humor Aqueous Humor CLS-AX at or below level of detection Rabbit toxicology study with single bilateral suprachoroidal injection of axitinib, 1.05 mg/eye (n=4 eyes/ timepoint) CLS-AX has Potential for Meaningful Durability CLS-AX Levels to 6 Months CLS-AX Injected Suprachoroidally Provides Targeted Delivery Relative to Intravitreal Injection at Same Dose Sources: Viral S. Kansara, Leroy W. Muya, Thomas A. Ciulla; Evaluation of Long-Lasting Potential of Suprachoroidal Axitinib Suspension Via Ocular and Systemic Disposition in Rabbits. Trans. Vis. Sci. Tech. 2021;10(7):19. Abbreviations: SCS: Suprachoroidal Space | IVT: Intravitreal Injection | PK: Pharmacokinetic | RPE: Retinal pigment epithelium l RCS: RPE, Choroid, Sclera
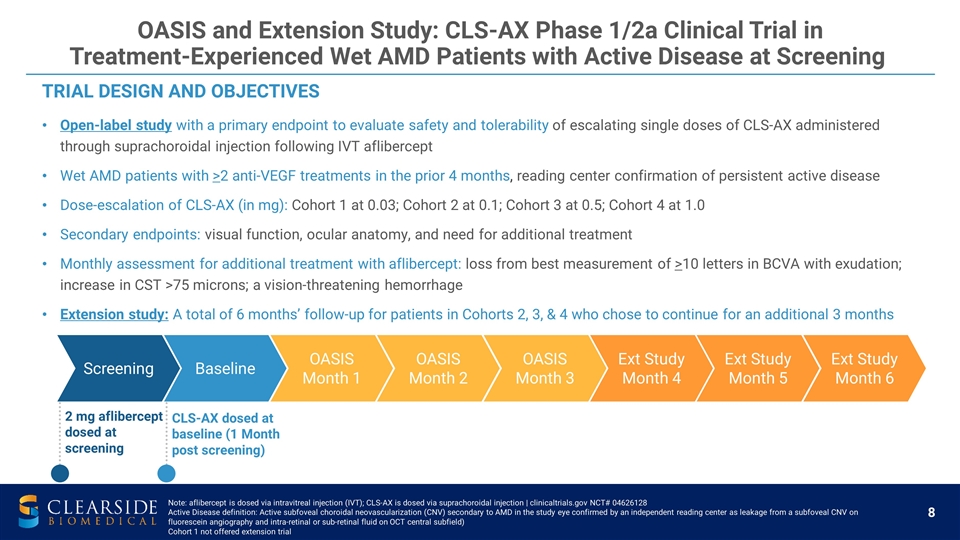
TRIAL DESIGN AND OBJECTIVES Open-label study with a primary endpoint to evaluate safety and tolerability of escalating single doses of CLS-AX administered through suprachoroidal injection following IVT aflibercept Wet AMD patients with >2 anti-VEGF treatments in the prior 4 months, reading center confirmation of persistent active disease Dose-escalation of CLS-AX (in mg): Cohort 1 at 0.03; Cohort 2 at 0.1; Cohort 3 at 0.5; Cohort 4 at 1.0 Secondary endpoints: visual function, ocular anatomy, and need for additional treatment Monthly assessment for additional treatment with aflibercept: loss from best measurement of >10 letters in BCVA with exudation; increase in CST >75 microns; a vision-threatening hemorrhage Extension study: A total of 6 months’ follow-up for patients in Cohorts 2, 3, & 4 who chose to continue for an additional 3 months Screening Baseline 2 mg aflibercept dosed at screening CLS-AX dosed at baseline (1 Month post screening) OASIS Month 1 OASIS Month 2 Ext Study Month 4 OASIS and Extension Study: CLS-AX Phase 1/2a Clinical Trial in Treatment-Experienced Wet AMD Patients with Active Disease at Screening OASIS Month 3 Ext Study Month 5 Ext Study Month 6 Note: aflibercept is dosed via intravitreal injection (IVT); CLS-AX is dosed via suprachoroidal injection | clinicaltrials.gov NCT# 04626128 Active Disease definition: Active subfoveal choroidal neovascularization (CNV) secondary to AMD in the study eye confirmed by an independent reading center as leakage from a subfoveal CNV on fluorescein angiography and intra-retinal or sub-retinal fluid on OCT central subfield) Cohort 1 not offered extension trial
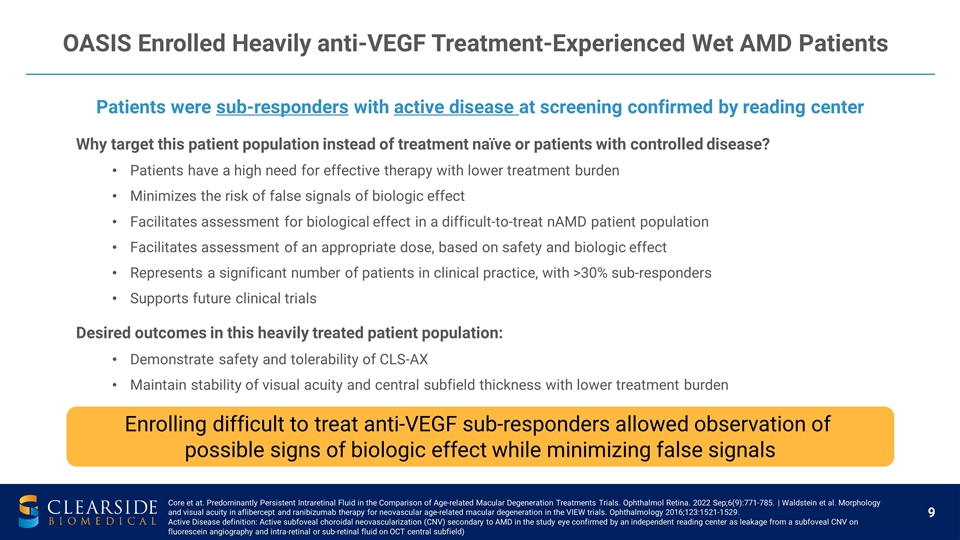
Why target this patient population instead of treatment naïve or patients with controlled disease? Patients have a high need for effective therapy with lower treatment burden Minimizes the risk of false signals of biologic effect Facilitates assessment for biological effect in a difficult-to-treat nAMD patient population Facilitates assessment of an appropriate dose, based on safety and biologic effect Represents a significant number of patients in clinical practice, with >30% sub-responders Supports future clinical trials Desired outcomes in this heavily treated patient population: Demonstrate safety and tolerability of CLS-AX Maintain stability of visual acuity and central subfield thickness with lower treatment burden OASIS Enrolled Heavily anti-VEGF Treatment-Experienced Wet AMD Patients Patients were sub-responders with active disease at screening confirmed by reading center Enrolling difficult to treat anti-VEGF sub-responders allowed observation of possible signs of biologic effect while minimizing false signals Core et at. Predominantly Persistent Intraretinal Fluid in the Comparison of Age-related Macular Degeneration Treatments Trials. Ophthalmol Retina. 2022 Sep;6(9):771-785. | Waldstein et al. Morphology and visual acuity in aflibercept and ranibizumab therapy for neovascular age-related macular degeneration in the VIEW trials. Ophthalmology 2016;123:1521-1529. Active Disease definition: Active subfoveal choroidal neovascularization (CNV) secondary to AMD in the study eye confirmed by an independent reading center as leakage from a subfoveal CNV on fluorescein angiography and intra-retinal or sub-retinal fluid on OCT central subfield)
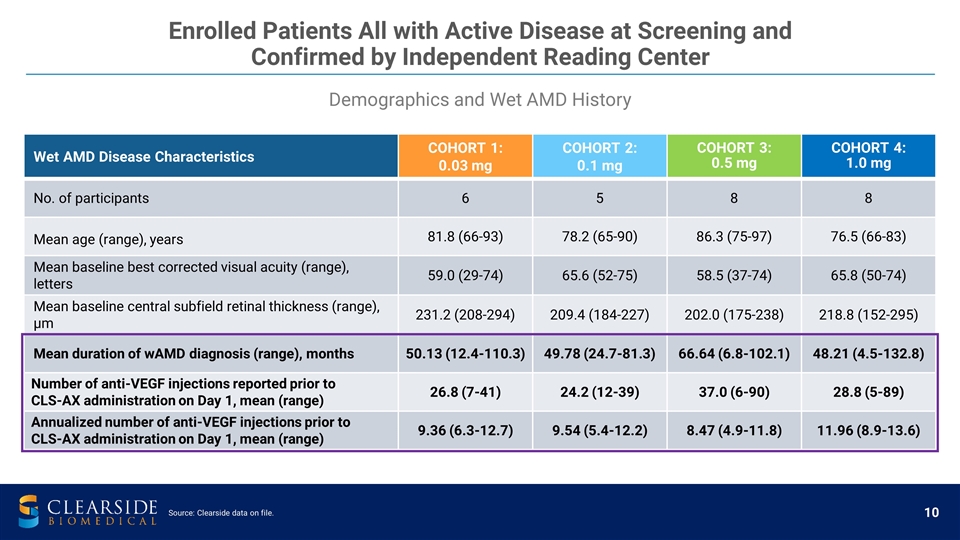
Wet AMD Disease Characteristics COHORT 1: 0.03 mg COHORT 2: 0.1 mg COHORT 3: 0.5 mg COHORT 4: 1.0 mg No. of participants 6 5 8 8 Mean age (range), years 81.8 (66-93) 78.2 (65-90) 86.3 (75-97) 76.5 (66-83) Mean baseline best corrected visual acuity (range), letters 59.0 (29-74) 65.6 (52-75) 58.5 (37-74) 65.8 (50-74) Mean baseline central subfield retinal thickness (range), µm 231.2 (208-294) 209.4 (184-227) 202.0 (175-238) 218.8 (152-295) Mean duration of wAMD diagnosis (range), months 50.13 (12.4-110.3) 49.78 (24.7-81.3) 66.64 (6.8-102.1) 48.21 (4.5-132.8) Number of anti-VEGF injections reported prior to CLS-AX administration on Day 1, mean (range) 26.8 (7-41) 24.2 (12-39) 37.0 (6-90) 28.8 (5-89) Annualized number of anti-VEGF injections prior to CLS-AX administration on Day 1, mean (range) 9.36 (6.3-12.7) 9.54 (5.4-12.2) 8.47 (4.9-11.8) 11.96 (8.9-13.6) Demographics and Wet AMD History Enrolled Patients All with Active Disease at Screening and Confirmed by Independent Reading Center Source: Clearside data on file.
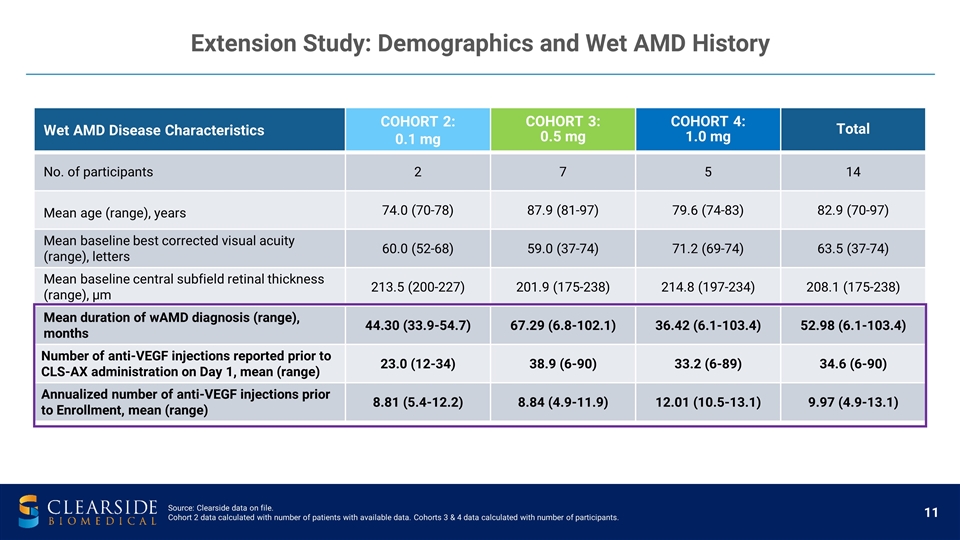
Wet AMD Disease Characteristics COHORT 2: 0.1 mg COHORT 3: 0.5 mg COHORT 4: 1.0 mg Total No. of participants 2 7 5 14 Mean age (range), years 74.0 (70-78) 87.9 (81-97) 79.6 (74-83) 82.9 (70-97) Mean baseline best corrected visual acuity (range), letters 60.0 (52-68) 59.0 (37-74) 71.2 (69-74) 63.5 (37-74) Mean baseline central subfield retinal thickness (range), µm 213.5 (200-227) 201.9 (175-238) 214.8 (197-234) 208.1 (175-238) Mean duration of wAMD diagnosis (range), months 44.30 (33.9-54.7) 67.29 (6.8-102.1) 36.42 (6.1-103.4) 52.98 (6.1-103.4) Number of anti-VEGF injections reported prior to CLS-AX administration on Day 1, mean (range) 23.0 (12-34) 38.9 (6-90) 33.2 (6-89) 34.6 (6-90) Annualized number of anti-VEGF injections prior to Enrollment, mean (range) 8.81 (5.4-12.2) 8.84 (4.9-11.9) 12.01 (10.5-13.1) 9.97 (4.9-13.1) Extension Study: Demographics and Wet AMD History Source: Clearside data on file. Cohort 2 data calculated with number of patients with available data. Cohorts 3 & 4 data calculated with number of participants.

OASIS Results: Safety, Durability, & Treatment Burden Reduction
3-Month & 6-Month Extension Study Data CLS-AX Demonstrated a Positive Safety Profile in All Four Cohorts Excellent Safety Profile at all doses and timepoints No serious adverse events (SAEs) No treatment emergent adverse events (TEAEs) related to study treatment No dose limiting toxicities No adverse events related to inflammation, vasculitis or vascular occlusion No vitreous “floaters” or dispersion of CLS-AX into the vitreous No retinal detachment No endophthalmitis No adverse events related to intraocular pressure SAFETY DATA Source: Clearside data on file.
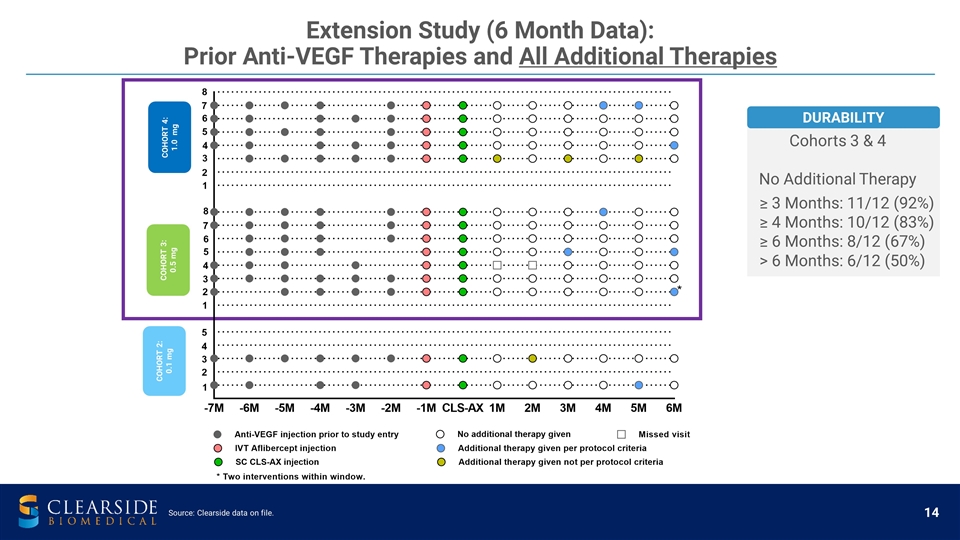
Extension Study (6 Month Data): Prior Anti-VEGF Therapies and All Additional Therapies COHORT 2: 0.1 mg COHORT 3: 0.5 mg COHORT 4: 1.0 mg Cohorts 3 & 4 No Additional Therapy ≥ 3 Months: 11/12 (92%) ≥ 4 Months: 10/12 (83%) ≥ 6 Months: 8/12 (67%) > 6 Months: 6/12 (50%) DURABILITY Source: Clearside data on file.
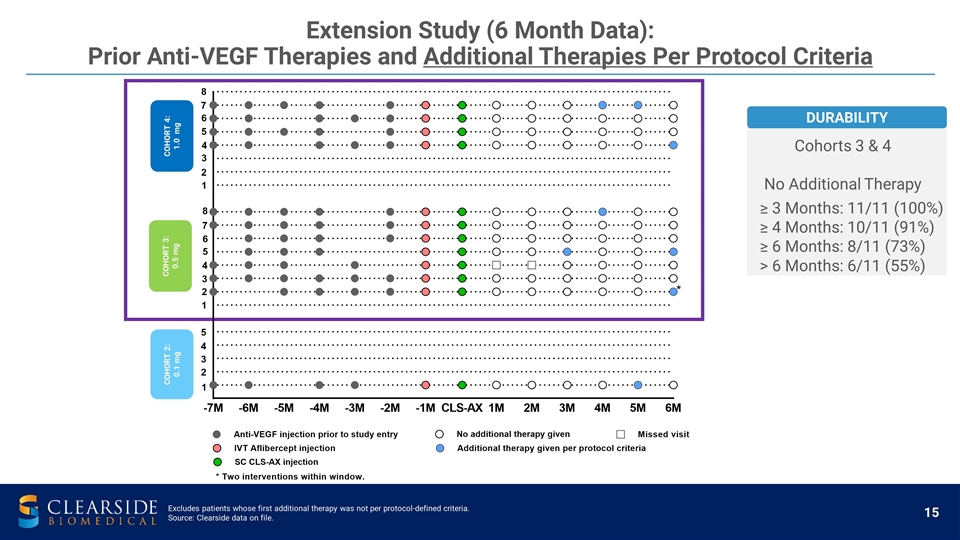
Extension Study (6 Month Data): Prior Anti-VEGF Therapies and Additional Therapies Per Protocol Criteria COHORT 2: 0.1 mg COHORT 3: 0.5 mg COHORT 4: 1.0 mg DURABILITY Cohorts 3 & 4 No Additional Therapy ≥ 3 Months: 11/11 (100%) ≥ 4 Months: 10/11 (91%) ≥ 6 Months: 8/11 (73%) > 6 Months: 6/11 (55%) Excludes patients whose first additional therapy was not per protocol-defined criteria. Source: Clearside data on file.
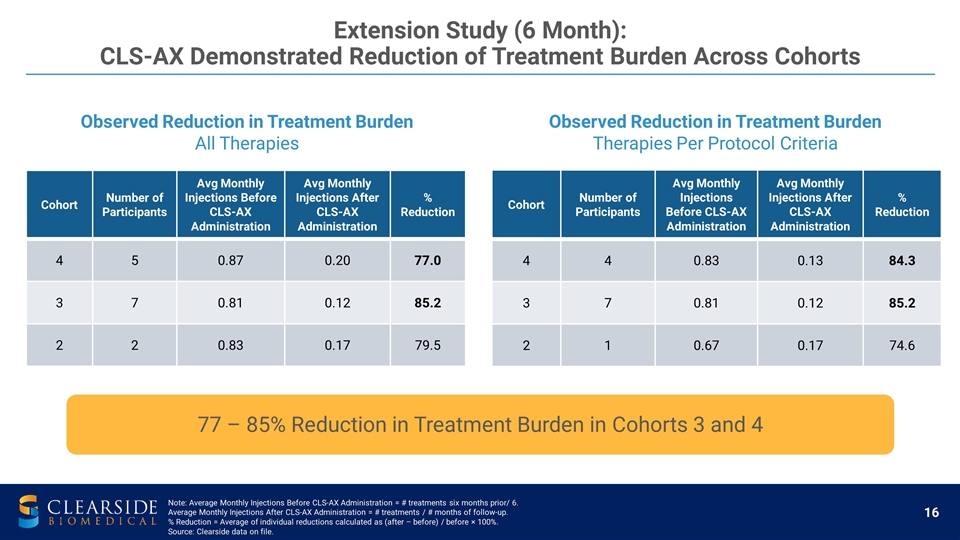
Extension Study (6 Month): CLS-AX Demonstrated Reduction of Treatment Burden Across Cohorts Observed Reduction in Treatment Burden All Therapies Observed Reduction in Treatment Burden Therapies Per Protocol Criteria Cohort Number of Participants Avg Monthly Injections Before CLS-AX Administration Avg Monthly Injections After CLS-AX Administration % Reduction 4 4 0.83 0.13 84.3 3 7 0.81 0.12 85.2 2 1 0.67 0.17 74.6 77 – 85% Reduction in Treatment Burden in Cohorts 3 and 4 Cohort Number of Participants Avg Monthly Injections Before CLS-AX Administration Avg Monthly Injections After CLS-AX Administration % Reduction 4 5 0.87 0.20 77.0 3 7 0.81 0.12 85.2 2 2 0.83 0.17 79.5 Note: Average Monthly Injections Before CLS-AX Administration = # treatments six months prior/ 6. Average Monthly Injections After CLS-AX Administration = # treatments / # months of follow-up. % Reduction = Average of individual reductions calculated as (after – before) / before × 100%. Source: Clearside data on file.
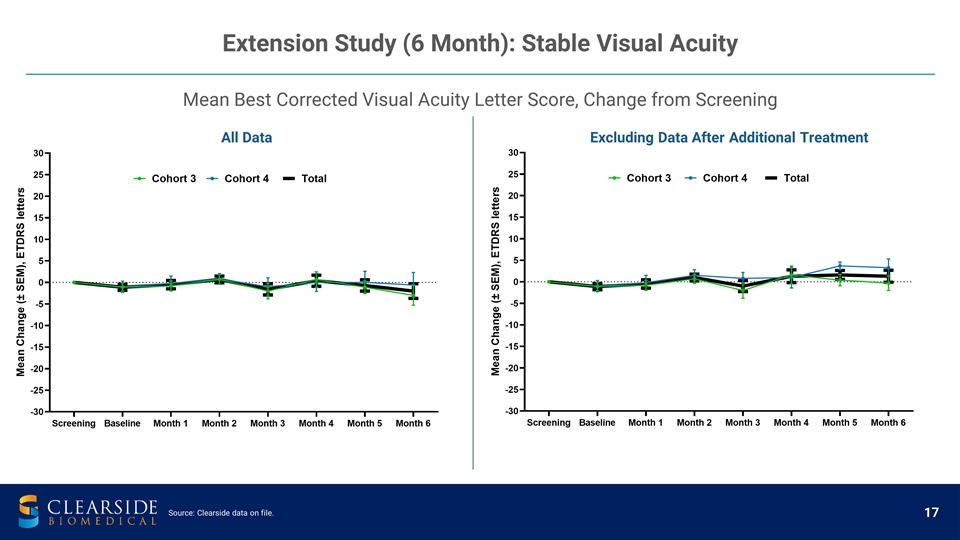
Mean Best Corrected Visual Acuity Letter Score, Change from Screening Extension Study (6 Month): Stable Visual Acuity All Data Excluding Data After Additional Treatment Source: Clearside data on file.
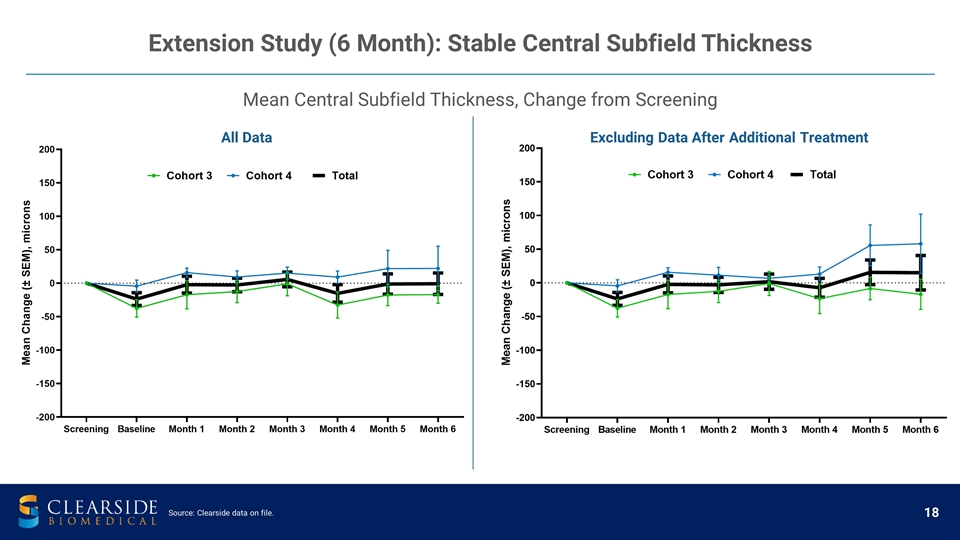
Mean Central Subfield Thickness, Change from Screening Extension Study (6 Month): Stable Central Subfield Thickness All Data Excluding Data After Additional Treatment Source: Clearside data on file.
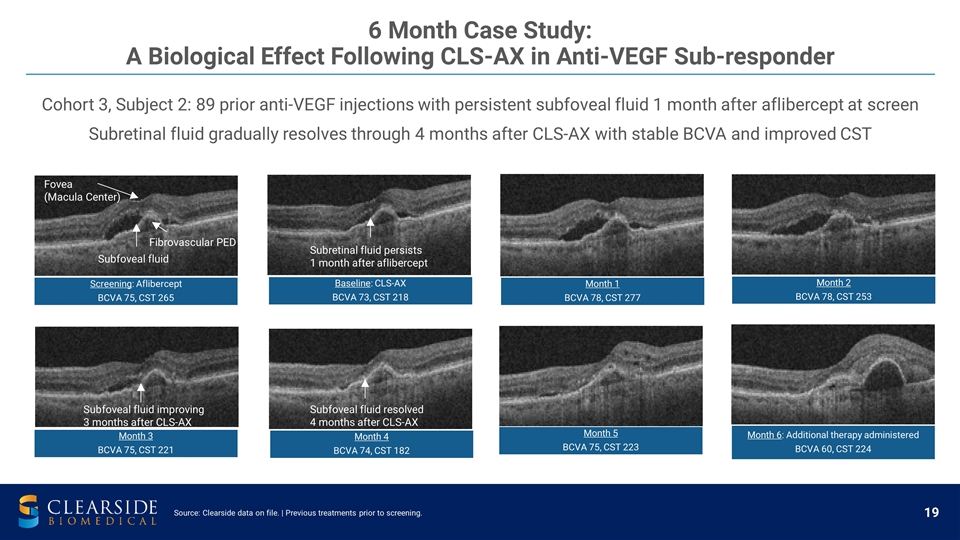
Cohort 3, Subject 2: 89 prior anti-VEGF injections with persistent subfoveal fluid 1 month after aflibercept at screen Subretinal fluid gradually resolves through 4 months after CLS-AX with stable BCVA and improved CST 6 Month Case Study: A Biological Effect Following CLS-AX in Anti-VEGF Sub-responder Subretinal fluid persists 1 month after aflibercept Subfoveal fluid resolved 4 months after CLS-AX Subfoveal fluid improving 3 months after CLS-AX Subfoveal fluid Fibrovascular PED Fovea (Macula Center) Source: Clearside data on file. | Previous treatments prior to screening. Month 1 BCVA 78, CST 277 Screening : Aflibercept BCVA 75, CST 265 Month 2 BCVA 78, CST 253 Month 3 BCVA 75, CST 221 Month 4 BCVA 74, CST 182 Month 5 BCVA 75, CST 223 Month 6 : Additional therapy administered BCVA 60 , CST 224 Baseline : CLS-AX BCVA 73, CST 218
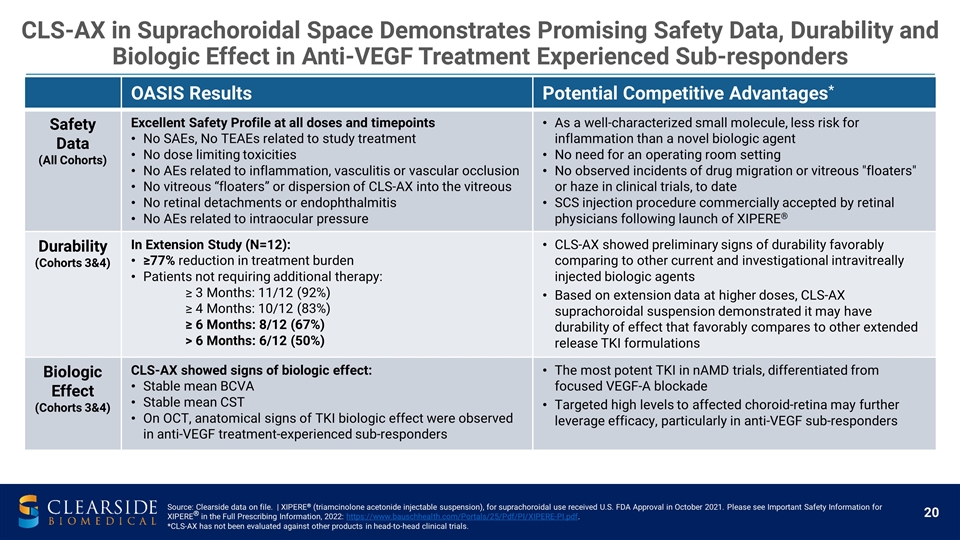
OASIS Results Potential Competitive Advantages* Safety Data (All Cohorts) Excellent Safety Profile at all doses and timepoints No SAEs, No TEAEs related to study treatment No dose limiting toxicities No AEs related to inflammation, vasculitis or vascular occlusion No vitreous “floaters” or dispersion of CLS-AX into the vitreous No retinal detachments or endophthalmitis No AEs related to intraocular pressure As a well-characterized small molecule, less risk for inflammation than a novel biologic agent No need for an operating room setting No observed incidents of drug migration or vitreous "floaters" or haze in clinical trials, to date SCS injection procedure commercially accepted by retinal physicians following launch of XIPERE® Durability (Cohorts 3&4) In Extension Study (N=12): ≥77% reduction in treatment burden Patients not requiring additional therapy: ≥ 3 Months: 11/12 (92%) ≥ 4 Months: 10/12 (83%) ≥ 6 Months: 8/12 (67%) > 6 Months: 6/12 (50%) CLS-AX showed preliminary signs of durability favorably comparing to other current and investigational intravitreally injected biologic agents Based on extension data at higher doses, CLS-AX suprachoroidal suspension demonstrated it may have durability of effect that favorably compares to other extended release TKI formulations Biologic Effect (Cohorts 3&4) CLS-AX showed signs of biologic effect: Stable mean BCVA Stable mean CST On OCT, anatomical signs of TKI biologic effect were observed in anti-VEGF treatment-experienced sub-responders The most potent TKI in nAMD trials, differentiated from focused VEGF-A blockade Targeted high levels to affected choroid-retina may further leverage efficacy, particularly in anti-VEGF sub-responders CLS-AX in Suprachoroidal Space Demonstrates Promising Safety Data, Durability and Biologic Effect in Anti-VEGF Treatment Experienced Sub-responders Source: Clearside data on file. | XIPERE® (triamcinolone acetonide injectable suspension), for suprachoroidal use received U.S. FDA Approval in October 2021. Please see Important Safety Information for XIPERE® in the Full Prescribing Information, 2022: https://www.bauschhealth.com/Portals/25/Pdf/PI/XIPERE-PI.pdf. *CLS-AX has not been evaluated against other products in head-to-head clinical trials.

ODYSSEY CLS-AX Phase 2b Clinical Trial
Key inclusion criteria: Treatment naïve wet AMD participants Subfoveal CNV secondary to wet AMD Best Corrected Visual Acuity (BCVA) of 78–24 letters* Primary endpoint: Mean change in BCVA Key secondary endpoints: Mean change in Central Subfield Thickness (CST) Treatment burden reduction as measured by total anti-VEGF injections over trial duration Monthly disease activity assessments: Beginning 2 months after last faricimab loading dose to determine if retreatment is needed Retreatment criteria: Decrease in BCVA, increase in CST, or new macular hemorrhage (per faricimab Phase 3 trial retreatment criteria#) * Inclusive (20/32–20/320 approximate Snellen equivalent) ODYSSEY Phase 2b Trial in Treatment-Naïve Wet AMD Participants Randomized, Double-Masked, CLS-AX Maintenance vs Faricimab Maintenance Trial Objectives: Stable visual acuity with reduced treatment burden/better durability Number of Participants: Total of 110 patients (55 in each arm) # Increase ≥75 μm in CST compared with the lowest CST value recorded at either of the previous 2 scheduled visits, or Increase >50 μm in CST compared with the average CST value over the previous 2 scheduled visits, or Decrease ≥5 letters in BCVA compared with the average BCVA value over the previous 2 scheduled visits, owing to nAMD disease activity (as determined by the Investigator), or Decrease ≥10 letters in BCVA compared with the highest BCVA value recorded at either of the previous 2 scheduled visits, owing to nAMD disease activity (as determined by the Investigator), or Presence of new macular hemorrhage (as determined by the Investigator), owing to nAMD disease activity.

Potential to Demonstrate Better Durability and Reduced Treatment Burden ODYSSEY Wet AMD Phase 2b Trial – Clinical Rationale Mechanism of Action: Pan-VEGF receptor inhibitor delivered by SCS Microinjector® CLS-AX OASIS Phase 1/2a clinical trial data in treatment-experienced anti-VEGF sub-responders: 83% went ≥ 4 months without additional treatment 67% went ≥ 6 months without additional treatment 50% did not require additional treatment for more than 6 months Mechanism of Action: VEGF & angiopoietin 2 (Ang-2) inhibitor delivered by intravitreal injection Phase 3 clinical trial data in treatment naïve participants1: 55% required additional treatment ≤ 3 months after four monthly loading doses 2 months after loading doses: 22% required retreatment 3 months after loading doses: 33% required retreatment Faricimab 1Refer to Prescribing Information for VABYSMO® (faricimab-svoa) injection, for intravitreal use.
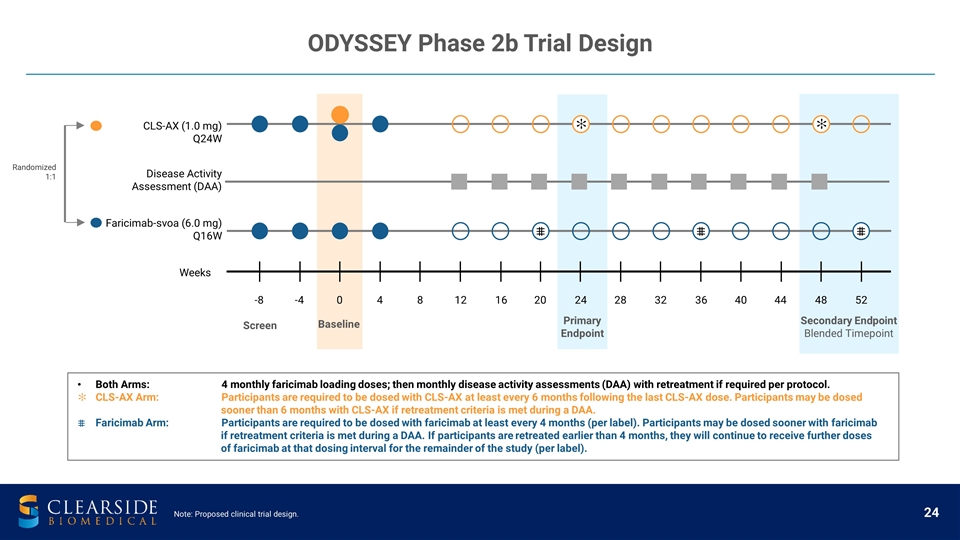
ODYSSEY Phase 2b Trial Design -8 -4 0 4 8 12 16 20 24 28 32 36 40 44 48 52 CLS-AX (1.0 mg) Q24W Faricimab-svoa (6.0 mg) Q16W Weeks Baseline Screen Primary Endpoint Secondary Endpoint Blended Timepoint Disease Activity Assessment (DAA) Randomized 1:1 Both Arms: 4 monthly faricimab loading doses; then monthly disease activity assessments (DAA) with retreatment if required per protocol. CLS-AX Arm: Participants are required to be dosed with CLS-AX at least every 6 months following the last CLS-AX dose. Participants may be dosed sooner than 6 months with CLS-AX if retreatment criteria is met during a DAA. Faricimab Arm: Participants are required to be dosed with faricimab at least every 4 months (per label). Participants may be dosed sooner with faricimab if retreatment criteria is met during a DAA. If participants are retreated earlier than 4 months, they will continue to receive further doses of faricimab at that dosing interval for the remainder of the study (per label). ✻ ✻ Note: Proposed clinical trial design.
ODYSSEY Wet AMD Phase 2b Trial Summary Comparator Treatment-Naïve Participants Maintenance Dosing Regimen Trial Size and Timeline VABYSMO® (faricimab-svoa) is the most recently approved product for wAMD Selected based on KOL input anticipating VABYSMO could become the future branded standard of care More likely to respond to treatment and show similar visual stability to standard of care than treatment resistant participants Same population as faricimab Phase 3 trials Designed to demonstrate reduced treatment burden and better durability of CLS-AX versus on-label faricimab dosing; Same disease activity assessment design as faricimab Phase 3 trials CLS-AX has potential for 2-3x/year maintenance dosing compared to on-label maintenance dosing for approved drugs: LUCENTIS®: 12x/year, EYLEA®: 6x/year, VABYSMO®: up to 6x/year 6-month primary endpoint and 12-month secondary endpoints expected to produce comparable visual acuity results with lower treatment burden Balanced to meet objectives, recruit in timely manner and to produce meaningful results in a reasonable time, with anticipated data readout in mid-2024 VABYSMO ® (faricimab-svoa) and LUCENTIS® (ranibizumab injection) are registered trademarks of Genentech; EYLEA ® (aflibercept) is a registered trademark of Regeneron.

Director of Retinal Research Institute, Retinal Consultants of Arizona Clinical Assistant Professor of Ophthalmology, University of Arizona College of Medicine - Phoenix Mark R. Barakat, M.D.

NASDAQ: CLSD

OASIS Case Studies
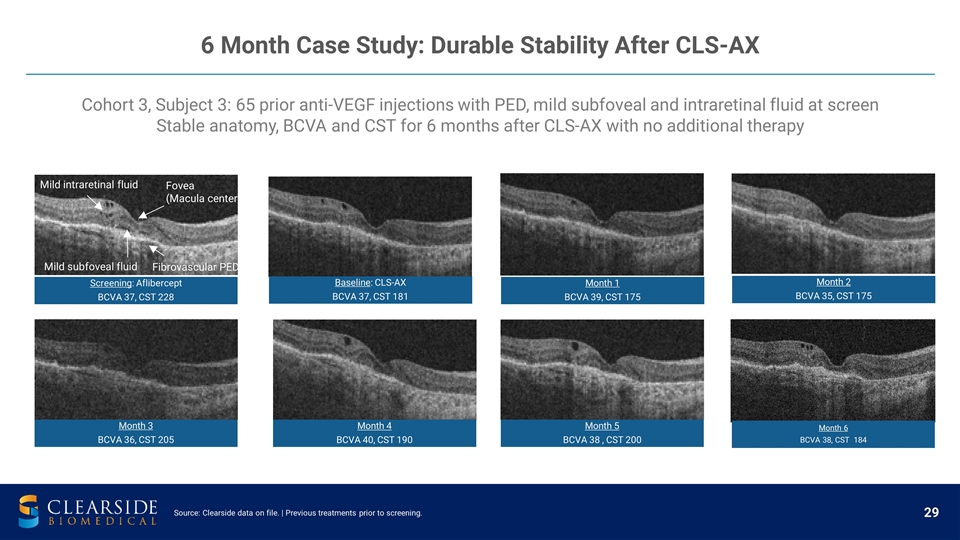
Cohort 3, Subject 3: 65 prior anti-VEGF injections with PED, mild subfoveal and intraretinal fluid at screen Stable anatomy, BCVA and CST for 6 months after CLS-AX with no additional therapy 6 Month Case Study: Durable Stability After CLS-AX Mild subfoveal fluid Fibrovascular PED Fovea (Macula center) Mild intraretinal fluid Source: Clearside data on file. | Previous treatments prior to screening. Screening : Aflibercept BCVA 37, CST 228 Baseline : CLS-AX BCVA 37, CST 181 Month 1 BCVA 39, CST 175 Month 2 BCVA 35, CST 175 Month 5 BCVA 38 , CST 200 Month 4 BCVA 40, CST 190 Month 3 BCVA 36, CST 205 Month 6 BCVA 38, CST 184
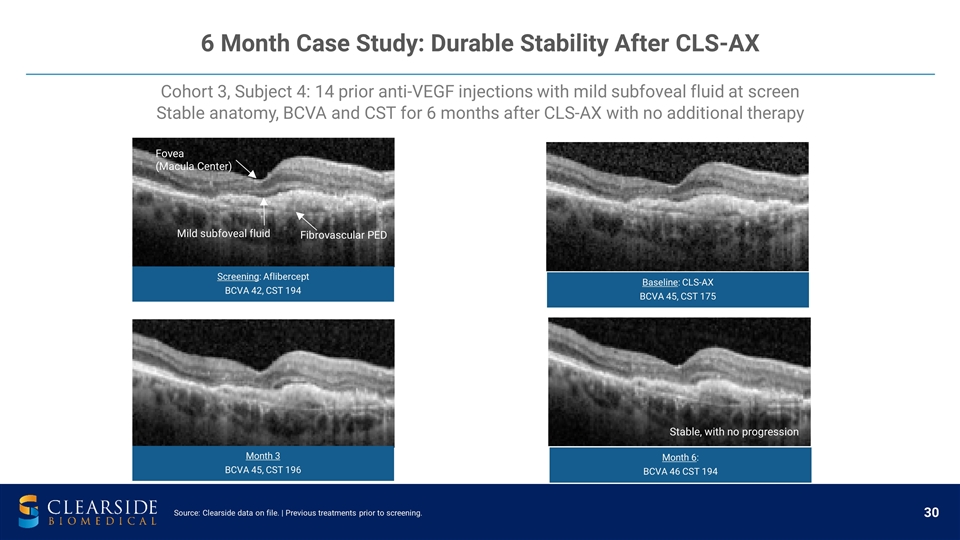
6 Month Case Study: Durable Stability After CLS-AX Cohort 3, Subject 4: 14 prior anti-VEGF injections with mild subfoveal fluid at screen Stable anatomy, BCVA and CST for 6 months after CLS-AX with no additional therapy Fovea (Macula Center) Stable, with no progression Mild subfoveal fluid Fibrovascular PED Fovea (Macula Center) Stable, with no progression Source: Clearside data on file. | Previous treatments prior to screening. Screening : Aflibercept BCVA 42, CST 194 Baseline : CLS-AX BCVA 45, CST 175 Month 6 : BCVA 46 CST 194 Month 3 BCVA 45, CST 196
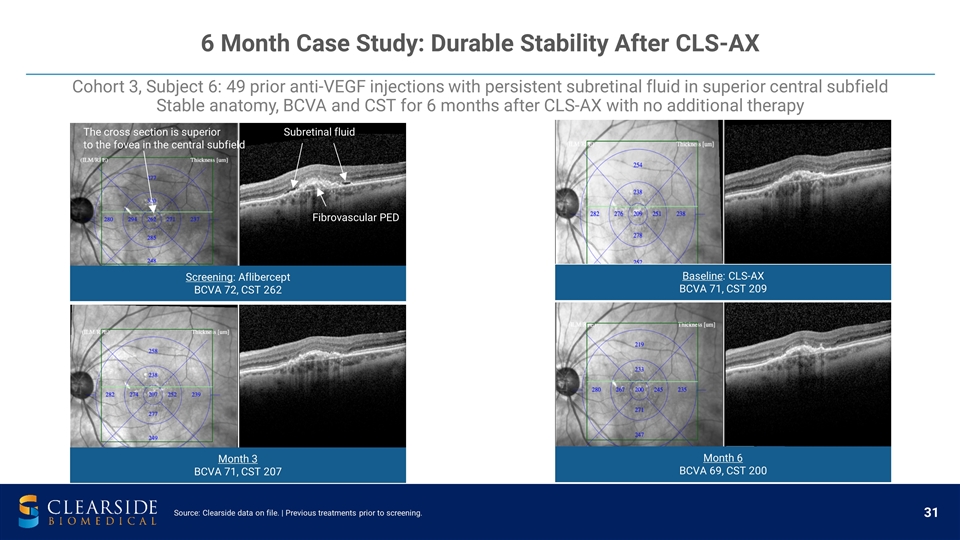
6 Month Case Study: Durable Stability After CLS-AX Screening: Aflibercept BCVA 72, CST 262 Month 3 BCVA 71, CST 207 Baseline: CLS-AX BCVA 71, CST 209 Month 6 BCVA 69, CST 200 Subretinal fluid Fibrovascular PED The cross section is superior to the fovea in the central subfield Cohort 3, Subject 6: 49 prior anti-VEGF injections with persistent subretinal fluid in superior central subfield Stable anatomy, BCVA and CST for 6 months after CLS-AX with no additional therapy Source: Clearside data on file. | Previous treatments prior to screening.

Screening: Aflibercept BCVA 70, CST 244 Month 3 BCVA 69, CST 249 Baseline: CLS-AX BCVA 70, CST 234 Month 6 BCVA 73, CST 246 Intraretinal fluid Shallow fibrovascular PED The cross section is superior to the fovea in the central subfield Cohort 4, Subject 5: 29 prior anti-VEGF injections with persistent PED and intraretinal fluid in superior central subfield Stable anatomy, BCVA and CST for 6 months after CLS-AX with no additional therapy Fovea (Macula center) Source: Clearside data on file. | Previous treatments prior to screening. 6 Month Case Study: Durable Stability After CLS-AX
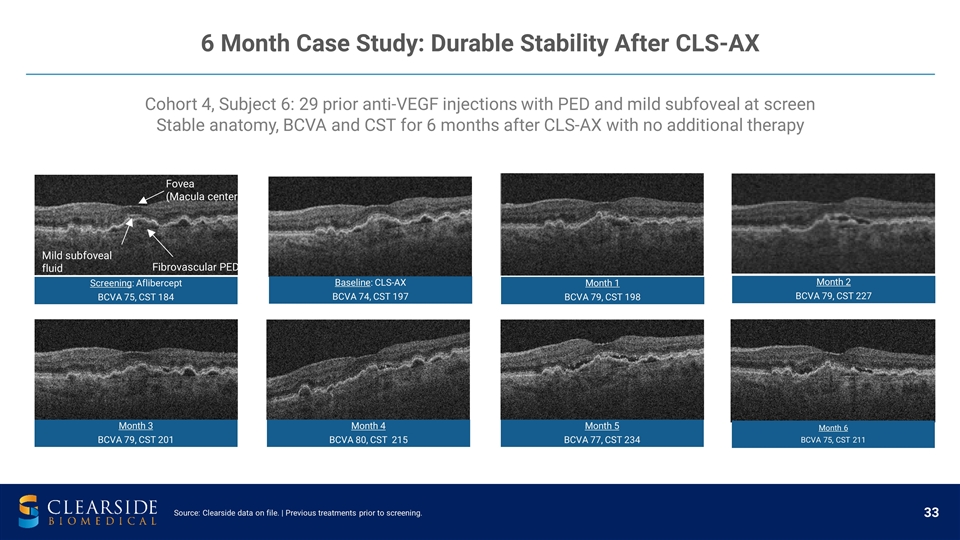
Cohort 4, Subject 6: 29 prior anti-VEGF injections with PED and mild subfoveal at screen Stable anatomy, BCVA and CST for 6 months after CLS-AX with no additional therapy Fibrovascular PED Fovea (Macula center) Mild subfoveal fluid Source: Clearside data on file. | Previous treatments prior to screening. 6 Month Case Study: Durable Stability After CLS-AX Screening : Aflibercept BCVA 75, CST 184 Baseline : CLS-AX BCVA 74, CST 197 Month 1 BCVA 79, CST 198 Month 2 BCVA 79, CST 227 Month 5 BCVA 77, CST 234 Month 4 BCVA 80, CST 215 Month 3 BCVA 79, CST 201 Month 6 BCVA 75, CST 211

OASIS Individual Patient Data
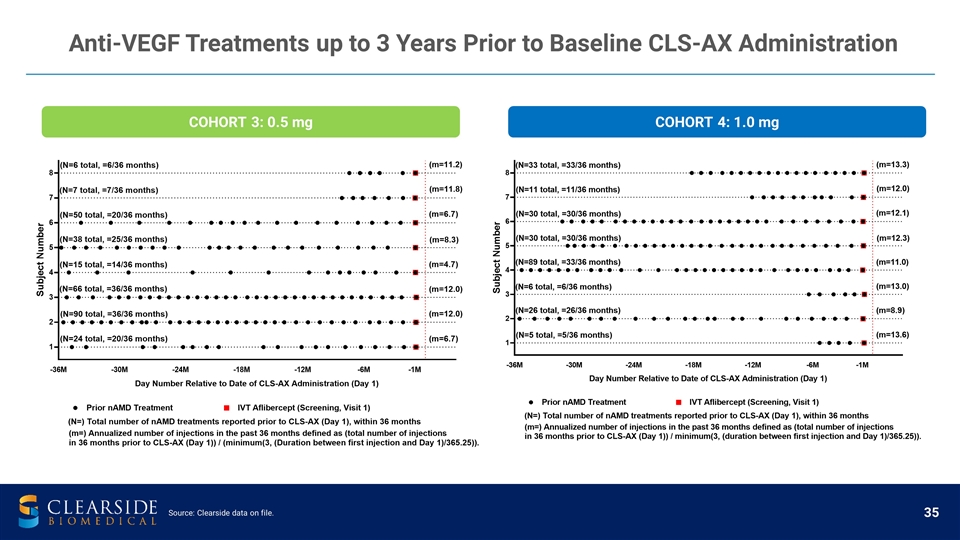
COHORT 3: 0.5 mg COHORT 4: 1.0 mg Source: Clearside data on file. Anti-VEGF Treatments up to 3 Years Prior to Baseline CLS-AX Administration
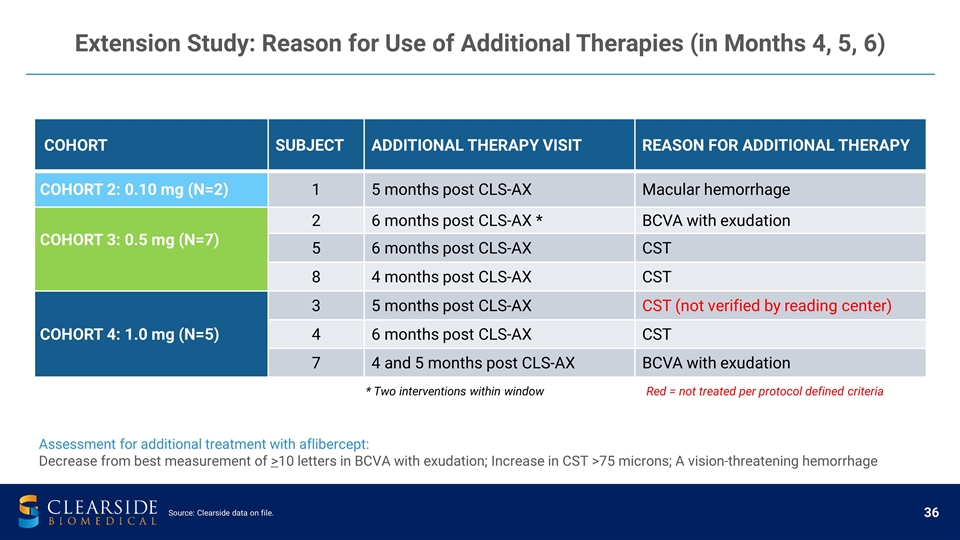
Extension Study: Reason for Use of Additional Therapies (in Months 4, 5, 6) COHORT SUBJECT ADDITIONAL THERAPY VISIT REASON FOR ADDITIONAL THERAPY COHORT 2: 0.10 mg (N=2) 1 5 months post CLS-AX Macular hemorrhage COHORT 3: 0.5 mg (N=7) 2 6 months post CLS-AX * BCVA with exudation 5 6 months post CLS-AX CST 8 4 months post CLS-AX CST COHORT 4: 1.0 mg (N=5) 3 5 months post CLS-AX CST (not verified by reading center) 4 6 months post CLS-AX CST 7 4 and 5 months post CLS-AX BCVA with exudation Assessment for additional treatment with aflibercept: Decrease from best measurement of >10 letters in BCVA with exudation; Increase in CST >75 microns; A vision-threatening hemorrhage * Two interventions within window Red = not treated per protocol defined criteria Source: Clearside data on file.
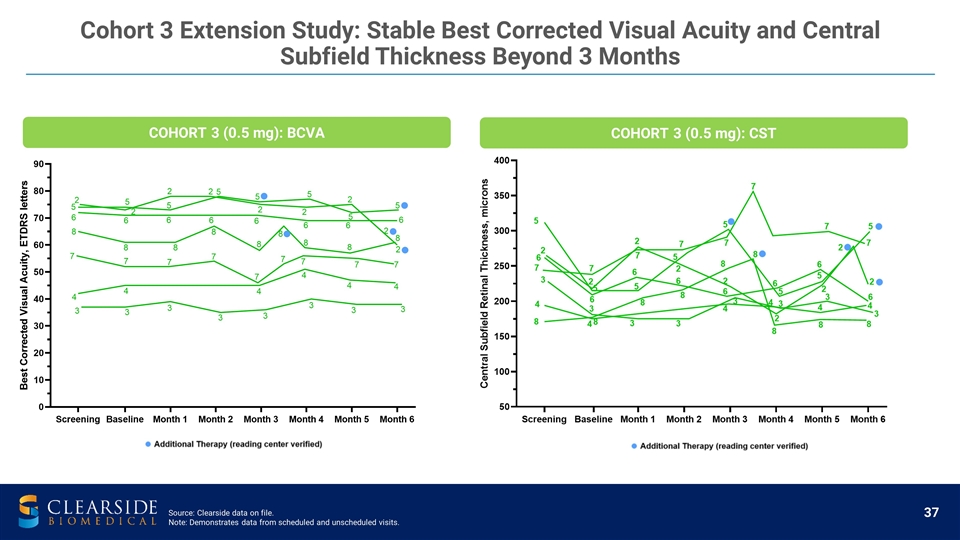
Cohort 3 Extension Study: Stable Best Corrected Visual Acuity and Central Subfield Thickness Beyond 3 Months COHORT 3 (0.5 mg): BCVA COHORT 3 (0.5 mg): CST Source: Clearside data on file. Note: Demonstrates data from scheduled and unscheduled visits.
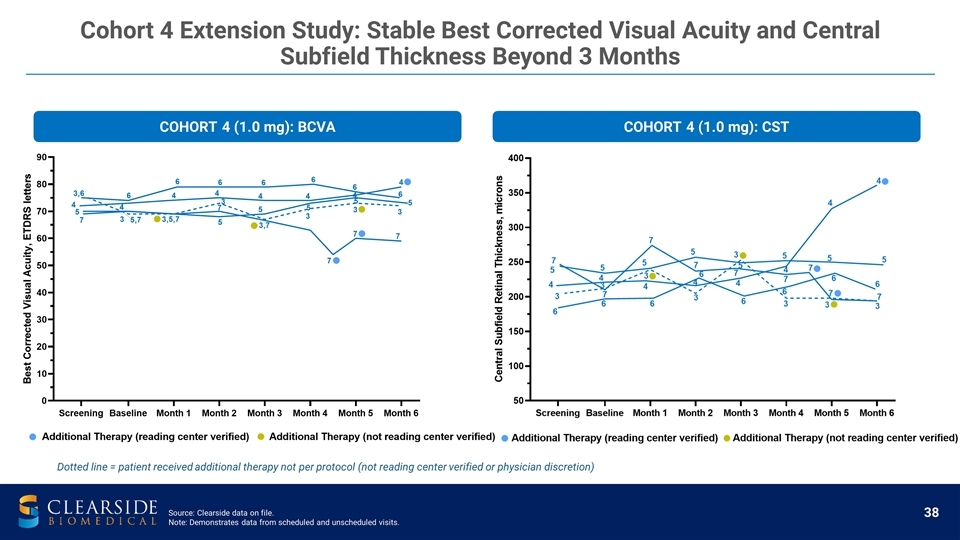
COHORT 4 (1.0 mg): CST COHORT 4 (1.0 mg): BCVA Dotted line = patient received additional therapy not per protocol (not reading center verified or physician discretion) Cohort 4 Extension Study: Stable Best Corrected Visual Acuity and Central Subfield Thickness Beyond 3 Months Source: Clearside data on file. Note: Demonstrates data from scheduled and unscheduled visits.

OASIS Appendix
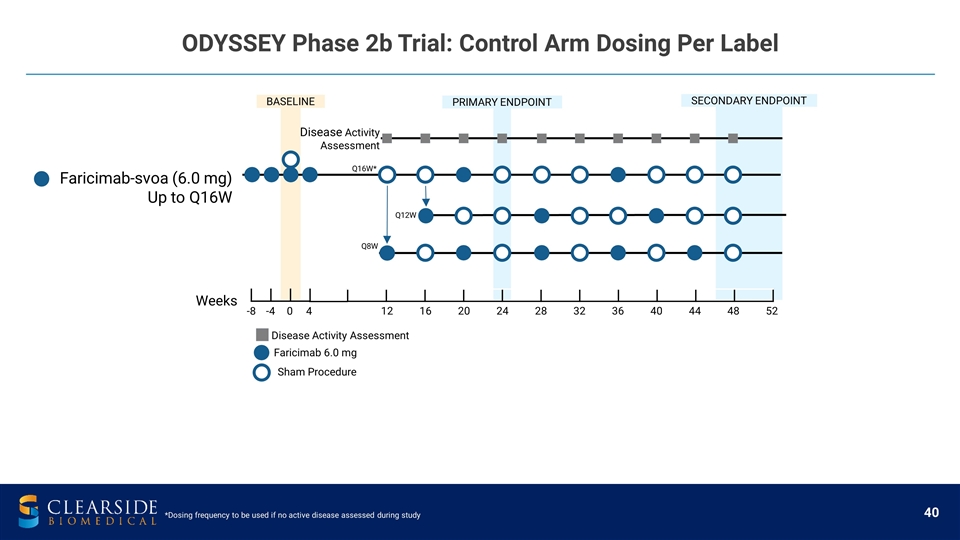
ODYSSEY Phase 2b Trial: Control Arm Dosing Per Label PRIMARY ENDPOINT 12 16 20 24 28 32 36 40 44 48 52 Weeks -8 -4 0 4 Faricimab 6.0 mg Sham Procedure Disease Activity Assessment Disease Activity Assessment Faricimab-svoa (6.0 mg) Up to Q16W Q12W Q16W* BASELINE SECONDARY ENDPOINT Q8W *Dosing frequency to be used if no active disease assessed during study

NASDAQ: CLSD








































