Exhibit 99.1
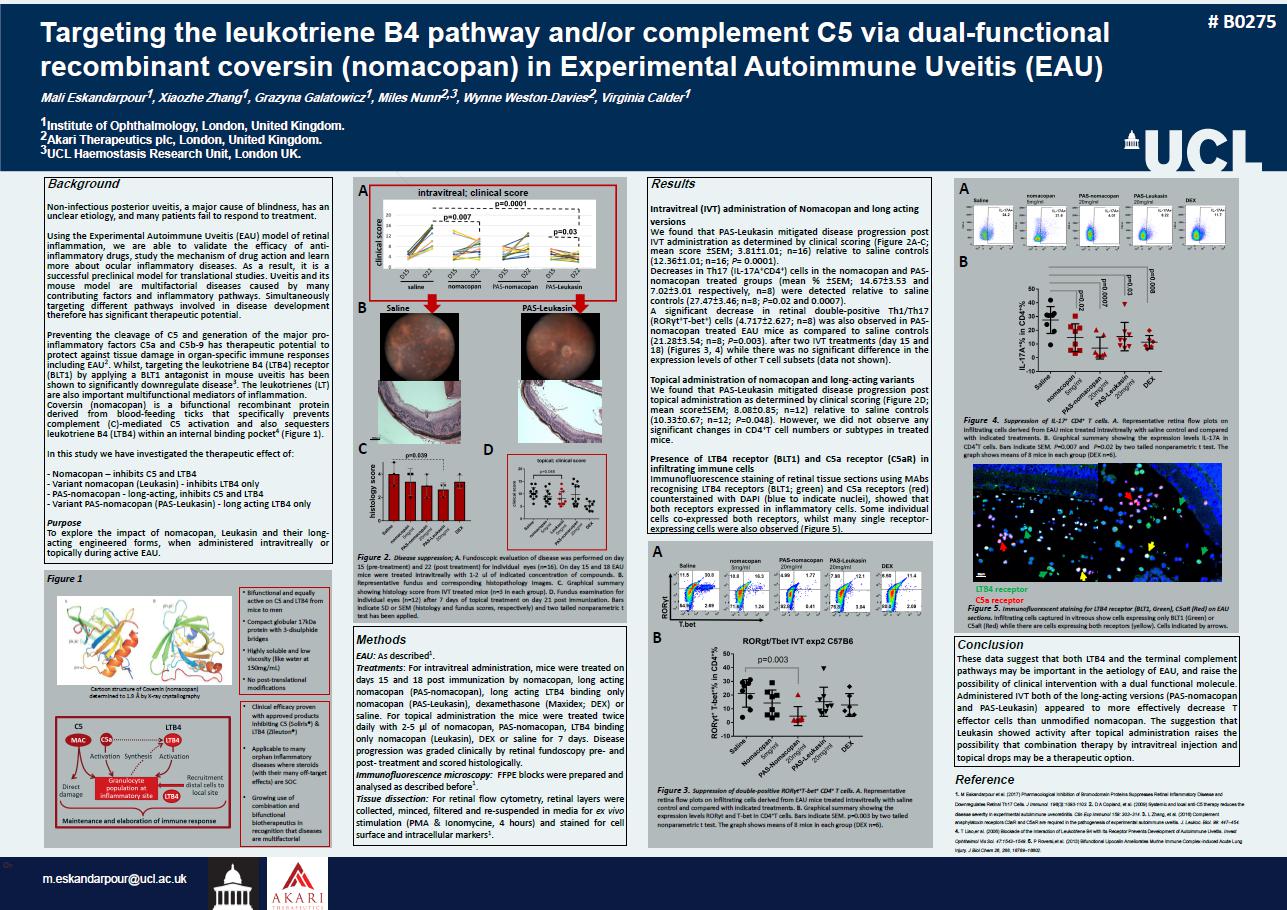
Or Targeting the leukotriene B4 pathway and/or complement C5 via dual-functional recombinant coversin(nomacopan) in Experimental Autoimmune Uveitis (EAU) Mali Eskandarpour1, XiaozheZhang1, GrazynaGalatowicz1, Miles Nunn2,3, Wynne Weston-Davies2, Virginia Calder1 1Institute of Ophthalmology, London, United Kingdom. 2Akari Therapeutics plc, London, United Kingdom. 3UCL Haemostasis Research Unit, London UK. m.eskandarpour@ucl.ac.uk Methods EAU:Asdescribed1. Treatments:Forintravitrealadministration,miceweretreatedondays15and18postimmunizationbynomacopan,longactingnomacopan(PAS-nomacopan),longactingLTB4bindingonlynomacopan(PAS-Leukasin),dexamethasone(Maxidex;DEX)orsaline.Fortopicaladministrationthemiceweretreatedtwicedailywith2-5µlofnomacopan,PAS-nomacopan,LTB4bindingonlynomacopan(Leukasin),DEXorsalinefor7days.Diseaseprogressionwasgradedclinicallybyretinalfundoscopypre-andpost-treatmentandscoredhistologically. Immunofluorescence microscopy:FFPE blocks were prepared and analysed as described before1. Tissuedissection:Forretinalflowcytometry,retinallayerswerecollected,minced,filteredandre-suspendedinmediaforexvivostimulation(PMA&Ionomycine,4hours)andstainedforcellsurfaceandintracellularmarkers1. Results Intravitreal (IVT) administration of Nomacopanand long acting versions WefoundthatPAS-LeukasinmitigateddiseaseprogressionpostIVTadministrationasdeterminedbyclinicalscoring(Figure2A-C;meanscore±SEM;3.81±1.01;n=16)relativetosalinecontrols(12.36±1.01;n=16;P=0.0001). DecreasesinTh17(IL-17A+CD4+)cellsinthenomacopanandPAS-nomacopantreatedgroups(mean%±SEM;14.67±3.53and7.02±3.01respectively,n=8)weredetectedrelativetosalinecontrols(27.47±3.46;n=8;P=0.02and0.0007). Asignificantdecreaseinretinaldouble-positiveTh1/Th17(ROR.t+T-bet+)cells(4.717±2.627;n=8)wasalsoobservedinPAS-nomacopantreatedEAUmiceascomparedtosalinecontrols(21.28±3.54;n=8;P=0.003).aftertwoIVTtreatments(day15and18)(Figures3,4)whiletherewasnosignificantdifferenceintheexpressionlevelsofotherTcellsubsets(datanotshown). Topicaladministrationofnomacopanandlong-actingvariants WefoundthatPAS-Leukasinmitigateddiseaseprogressionposttopicaladministrationasdeterminedbyclinicalscoring(Figure2D;meanscore±SEM;8.08±0.85;n=12)relativetosalinecontrols(10.33±0.67;n=12;P=0.048).However,wedidnotobserveanysignificantchangesinCD4+Tcellnumbersorsubtypesintreatedmice. PresenceofLTB4receptor(BLT1)andC5areceptor(C5aR)ininfiltratingimmunecells ImmunofluorescencestainingofretinaltissuesectionsusingMAbsrecognisingLTB4receptors(BLT1;green)andC5areceptors(red)counterstainedwithDAPI(bluetoindicatenuclei),showedthatbothreceptorsexpressedininflammatorycells.Someindividualcellsco-expressedbothreceptors,whilstmanysinglereceptor-expressingcellswerealsoobserved(Figure5). Cartoon structure of Coversin(nomacopan) determined to 1.9 Åby X-ray crystallography •Bifunctional and equally active on C5 and LTB4 from mice to men •Compact globular 17kDa protein with 3-disulphide bridges •Highly soluble and low viscosity (like water at 150mg/mL) •No post-translational modifications •Clinical efficacy proven with approved products inhibiting C5 (Soliris®) & LTB4 (Zileuton®) •Applicable to many orphan inflammatory diseases where steroids (with their many off-target effects) are SOC •Growing use of combination and bifunctional biotherapeutics in recognition that diseases are multifactorial Figure2.Diseasesuppression;A.Fundoscopicevaluationofdiseasewasperformedonday15(pre-treatment)and22(posttreatment)forindividualeyes(n=16).Onday15and18EAUmiceweretreatedintravitreallywith1-2ulofindicatedconcentrationofcompounds.B.Representativefundusandcorrespondinghistopathologyimages.C.GraphicalsummaryshowinghistologyscorefromIVTtreatedmice(n=3ineachgroup).D.Fundusexaminationforindividualeyes(n=12)after7daysoftopicaltreatmentonday21postimmunization.BarsindicateSDorSEM(histologyandfundusscores,respectively)andtwotailednonparametricttesthasbeenapplied. Reference 1. M Eskandarpour et al. (2017) Pharmacological Inhibition of BromodomainProteins Suppresses Retinal Inflammatory Disease and Downregulates Retinal Th17 Cells. J Immunol. 198(3):1093-1103.2. D A Copland, et al. (2009) Systemic and local anti-C5 therapy reduces the disease severity in experimental autoimmune uveoretinitis. ClinExpImmunol159: 303–314. 3. L Zhang, et al. (2016) Complement anaphylatoxinreceptors C3aR and C5aR are required in the pathogenesis of experimental autoimmune uveitis. J. Leukoc. Biol. 99: 447–454.4. T Liao,eral. (2006) Blockade of the Interaction of Leukotriene B4 with Its Receptor Prevents Development of Autoimmune Uveitis. Invest OphthalmolVis Sci. 47:1543–1549. 5. P Roversi,etal. (2013) BifunctionalLipocalinAmeliorates Murine Immune Complex-induced Acute Lung Injury. J BiolChem26, 288, 18789–18802. Figure 3. Suppression of double-positive ROR.t+T-bet+CD4+T cells. A. Representative retina flow plots on infiltrating cells derived from EAU mice treated intravitreallywith saline control and compared with indicated treatments. B.Graphical summary showing the expression levels ROR.t and T-bet in CD4+T cells. Bars indicate SEM. p=0.003 by two tailed nonparametric t test. The graph shows means of 8 mice in each group (DEX n=6). A B C D A B B A Figure4.SuppressionofIL-17+CD4+Tcells.A.RepresentativeretinaflowplotsoninfiltratingcellsderivedfromEAUmicetreatedintravitreallywithsalinecontrolandcomparedwithindicatedtreatments.B.GraphicalsummaryshowingtheexpressionlevelsIL-17AinCD4+Tcells.BarsindicateSEM.P=0.007andP=0.02bytwotailednonparametricttest.Thegraphshowsmeansof8miceineachgroup(DEXn=6). Figure 5. Immunofluorescentstaining for LTB4 receptor (BLT1, Green), C5aR (Red) on EAU sections. Infiltrating cells captured in vitreous show cells expressing only BLT1 (Green) or C5aR (Red) while there are cells expressing both receptors (yellow). Cells indicated by arrows. LTB4 receptor C5a receptor 20µm 100µm Figure 1 intravitreal;clinicalscore Saline PAS-Leukasin Conclusion ThesedatasuggestthatbothLTB4andtheterminalcomplementpathwaysmaybeimportantintheaetiologyofEAU,andraisethepossibilityofclinicalinterventionwithadualfunctionalmolecule.AdministeredIVTbothofthelong-actingversions(PAS-nomacopanandPAS-Leukasin)appearedtomoreeffectivelydecreaseTeffectorcellsthanunmodifiednomacopan.ThesuggestionthatLeukasinshowedactivityaftertopicaladministrationraisesthepossibilitythatcombinationtherapybyintravitrealinjectionandtopicaldropsmaybeatherapeuticoption. # B0275 Background Non-infectiousposterioruveitis,amajorcauseofblindness,hasanunclearetiology,andmanypatientsfailtorespondtotreatment. UsingtheExperimentalAutoimmuneUveitis(EAU)modelofretinalinflammation,weareabletovalidatetheefficacyofanti-inflammatorydrugs,studythemechanismofdrugactionandlearnmoreaboutocularinflammatorydiseases.Asaresult,itisasuccessfulpreclinicalmodelfortranslationalstudies.Uveitisanditsmousemodelaremultifactorialdiseasescausedbymanycontributingfactorsandinflammatorypathways.Simultaneouslytargetingdifferentpathwaysinvolvedindiseasedevelopmentthereforehassignificanttherapeuticpotential. PreventingthecleavageofC5andgenerationofthemajorpro-inflammatoryfactorsC5aandC5b-9hastherapeuticpotentialtoprotectagainsttissuedamageinorgan-specificimmuneresponsesincludingEAU2.Whilst,targetingtheleukotrieneB4(LTB4)receptor(BLT1)byapplyingaBLT1antagonistinmouseuveitishasbeenshowntosignificantlydownregulatedisease3.Theleukotrienes(LT)arealsoimportantmultifunctionalmediatorsofinflammation. Coversin(nomacopan)isabifunctionalrecombinantproteinderivedfromblood-feedingticksthatspecificallypreventscomplement(C)-mediatedC5activationandalsosequestersleukotrieneB4(LTB4)withinaninternalbindingpocket4(Figure1). Inthisstudywehaveinvestigatedthetherapeuticeffectof: -Nomacopan–inhibitsC5andLTB4 -Variantnomacopan(Leukasin)-inhibitsLTB4only -PAS-nomacopan-long-acting,inhibitsC5andLTB4 -VariantPAS-nomacopan(PAS-Leukasin)-longactingLTB4only Purpose Toexploretheimpactofnomacopan,Leukasinandtheirlong-actingengineeredforms,whenadministeredintravitreallyortopicallyduringactiveEAU.
