Forward-Looking Statements This presentation includes forward-looking statements about the Company's plans for the OvaPrimeSM treatment, the OvaTureSM treatment and the AUGMENTSM treatment, including forward looking statements relating to: the potential market for the Company’s treatments; future milestones for the OvaPrime treatment, including anticipated timing for data readouts, embryo transfers, patient enrollment and initiation of the Phase 1b/2a OvaPrime clinical trial; the Company’s anticipated cash runway; plans to work with the U.S. Food and Drug Administration under its available procedures to determine the most appropriate regulatory pathway for the Company’s treatments; next steps for the bovine OvaTure program, including plans relating to fertilization, embryo transfer and live birth; and next steps for the human OvaTure program, including those related to the maturation, pursuit of authorization to fertilize, fertilization, determination of regulatory pathway and submission for regulatory approval of a potential future clinical study. Actual results may differ materially from those indicated by these forward-looking statements as a result of various important factors, including risks related to: challenges implicit in the discovery and development process of seeking to mature and fertilize bovine and human EggPCSM cell-derived eggs; challenges associated with enrolling, collecting data from and completing clinical trials; the science underlying our treatments (including the OvaPrime, OvaTure and AUGMENT treatments), which is unproven; risks associated with reliance on third parties, including our commercial and academic partners; our ability to obtain regulatory approval or licenses where necessary for our treatments; our ability to develop our treatments on the timelines we expect, if at all; , as well as those risks more fully discussed in the "Risk Factors" section of our most recently filed Quarterly Report on Form 10-Q and/or Annual Report on Form 10-K as filed with the Securities and Exchange Commission. The forward-looking statements contained in the presentation reflect our current views with respect to future events. We anticipate that subsequent events and developments will cause our views to change. However, while we may elect to update these forward-looking statements in the future, we specifically disclaim any obligation to do so. These forward-looking statements should not be relied upon as representing our view as of any date subsequent to the date hereof. 2

A company pioneering breakthrough, first-in-class science Poised to revolutionize traditional in vitro fertilization (IVF) treatment by removing the need for hormone stimulation And developing fertility solutions for women for whom traditional IVF offers little hope for their own genetic child OvaScience 3
OvaScience: Transforming Female Infertility Therapy 4 $75.7 million in cash and short-term investments as of September 30, 2017 Cash runway into 2020 Cost efficient operating structure Patent protection into 2035 65 issued patents in 44 countries 170+ pending applications in more than 130 countries 163 million women worldwide are struggling with infertility1 1.9 million undergo in vitro fertilization per year2 Average cost of $14,000 per IVF cycle in U.S.; largely self-pay3 Large and Growing Market Strong Financial Position Robust IP Portfolio Sources: 1Centers for Disease Control 2CDC, 2015 ART National Summary Report; Assisted Reproductive Technologies in Canada: Results from Canadian ART Register; Takeshima, J Assist Reprod Genet (2014) 31:477-484; Japan Registry, 2014, Assisted Reproductive Technology in Europe: Results from European Registers by ESHRE; Assisted Reproductive Technology in Italy, 2013; Assisted Reproductive Technology Fact Sheet by ESHRE, Europe; Germany IVF-Registry (2013-2015); Luke et al. NEJM vol.366 (26):2483-91 and supplement, 2012; Aptis Partners; Germany D.I.R. Annual Report; HFEA Fertility Treatment 2014 Trends and Figures; Italian Assisted Reproductive Technology Register 2013 3Clinic websites, RESOLVE Breakthrough Technology in Reproductive Medicine Discovery of egg precursor (EggPCSM) cells unlocks innovative opportunities to improve female fertility through novel treatments OvaPrimeSM, in clinical development, potential fertility treatment that restores egg production OvaTureSM, in preclinical development, potential fertility treatment that eliminates need for hormone stimulation AUGMENTSM, available at select clinics in Canada and Japan, designed to improve traditional IVF success
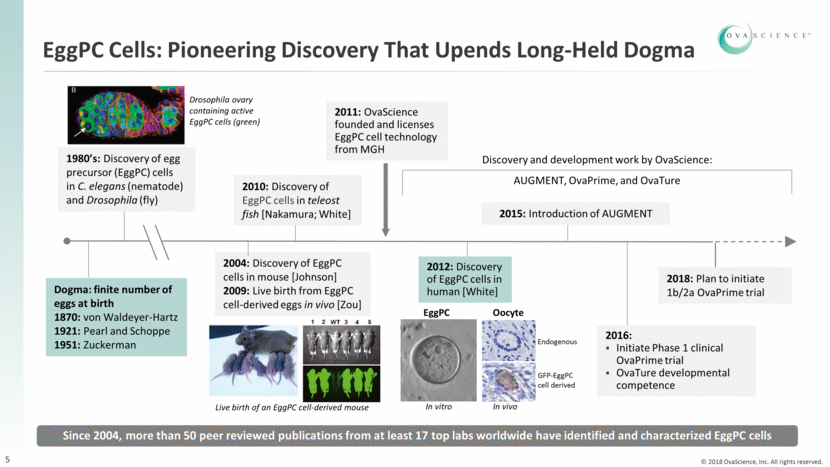
EggPC Cells: Pioneering Discovery That Upends Long-Held Dogma 2011: OvaScience founded and licenses EggPC cell technology from MGH Discovery and development work by OvaScience: AUGMENT, OvaPrime, and OvaTure Drosophila ovary containing active EggPC cells (green) 2004: Discovery of EggPC cells in mouse [Johnson] 2009: Live birth from EggPC cell-derived eggs in vivo [Zou] 1980’s: Discovery of egg precursor (EggPC) cells in C. elegans (nematode) and Drosophila (fly) 2010: Discovery of EggPC cells in teleost fish [Nakamura; White] 2015: Introduction of AUGMENT 2016: Initiate Phase 1 clinical OvaPrime trial OvaTure developmental competence 2018: Plan to initiate 1b/2a OvaPrime trial Since 2004, more than 50 peer reviewed publications from at least 17 top labs worldwide have identified and characterized EggPC cells Dogma: finite number of eggs at birth 1870: von Waldeyer-Hartz 1921: Pearl and Schoppe 1951: Zuckerman 2012: Discovery of EggPC cells in human [White] 5 Live birth of an EggPC cell-derived mouse In vitro Endogenous GFP-EggPC cell derived In vivo EggPC Oocyte
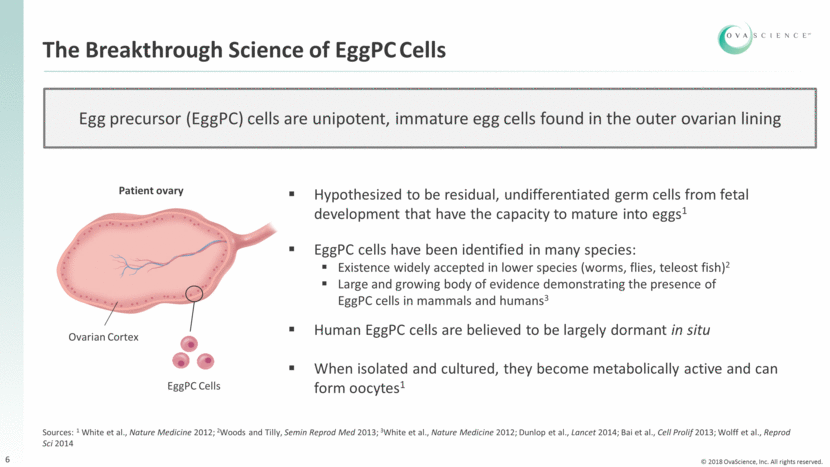
The Breakthrough Science of EggPC Cells 6 Egg precursor (EggPC) cells are unipotent, immature egg cells found in the outer ovarian lining Patient ovary Ovarian Cortex EggPC Cells Hypothesized to be residual, undifferentiated germ cells from fetal development that have the capacity to mature into eggs1 EggPC cells have been identified in many species: Existence widely accepted in lower species (worms, flies, teleost fish)2 Large and growing body of evidence demonstrating the presence of EggPC cells in mammals and humans3 Human EggPC cells are believed to be largely dormant in situ When isolated and cultured, they become metabolically active and can form oocytes1 Sources: 1 White et al., Nature Medicine 2012; 2Woods and Tilly, Semin Reprod Med 2013; 3White et al., Nature Medicine 2012; Dunlop et al., Lancet 2014; Bai et al., Cell Prolif 2013; Wolff et al., Reprod Sci 2014
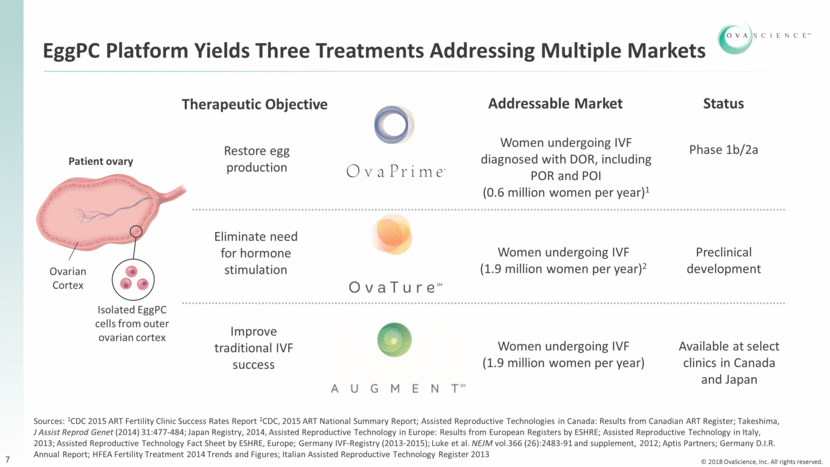
EggPC Platform Yields Three Treatments Addressing Multiple Markets Addressable Market Status Phase 1b/2a Women undergoing IVF diagnosed with DOR, including POR and POI (0.6 million women per year)1 Preclinical development Women undergoing IVF (1.9 million women per year)2 Women undergoing IVF (1.9 million women per year) Available at select clinics in Canada and Japan Therapeutic Objective Patient ovary Isolated EggPC cells from outer ovarian cortex Ovarian Cortex 7 Sources: 1CDC 2015 ART Fertility Clinic Success Rates Report 2CDC, 2015 ART National Summary Report; Assisted Reproductive Technologies in Canada: Results from Canadian ART Register; Takeshima, J Assist Reprod Genet (2014) 31:477-484; Japan Registry, 2014, Assisted Reproductive Technology in Europe: Results from European Registers by ESHRE; Assisted Reproductive Technology in Italy, 2013; Assisted Reproductive Technology Fact Sheet by ESHRE, Europe; Germany IVF-Registry (2013-2015); Luke et al. NEJM vol.366 (26):2483-91 and supplement, 2012; Aptis Partners; Germany D.I.R. Annual Report; HFEA Fertility Treatment 2014 Trends and Figures; Italian Assisted Reproductive Technology Register 2013 Eliminate need for hormone stimulation Restore egg production Improve traditional IVF success
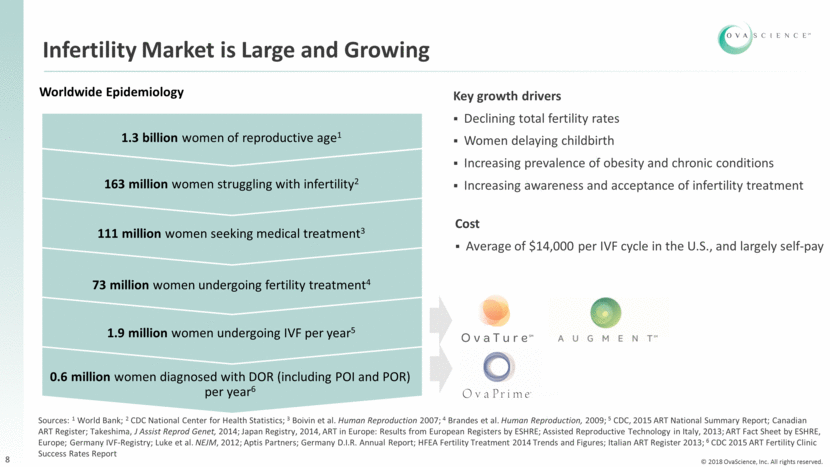
Infertility Market is Large and Growing 8 1.3 billion women of reproductive age1 163 million women struggling with infertility2 111 million women seeking medical treatment3 73 million women undergoing fertility treatment4 1.9 million women undergoing IVF per year5 0.6 million women diagnosed with DOR (including POI and POR) per year6 Worldwide Epidemiology Sources: 1 World Bank; 2 CDC National Center for Health Statistics; 3 Boivin et al. Human Reproduction 2007; 4 Brandes et al. Human Reproduction, 2009; 5 CDC, 2015 ART National Summary Report; Canadian ART Register; Takeshima, J Assist Reprod Genet, 2014; Japan Registry, 2014, ART in Europe: Results from European Registers by ESHRE; Assisted Reproductive Technology in Italy, 2013; ART Fact Sheet by ESHRE, Europe; Germany IVF-Registry; Luke et al. NEJM, 2012; Aptis Partners; Germany D.I.R. Annual Report; HFEA Fertility Treatment 2014 Trends and Figures; Italian ART Register 2013; 6 CDC 2015 ART Fertility Clinic Success Rates Report Key growth drivers Declining total fertility rates Women delaying childbirth Increasing prevalence of obesity and chronic conditions Increasing awareness and acceptance of infertility treatment Cost Average of $14,000 per IVF cycle in the U.S., and largely self-pay
Our Treatments 9

OvaPrime Potential Fertility Treatment that Restores Egg Production 10

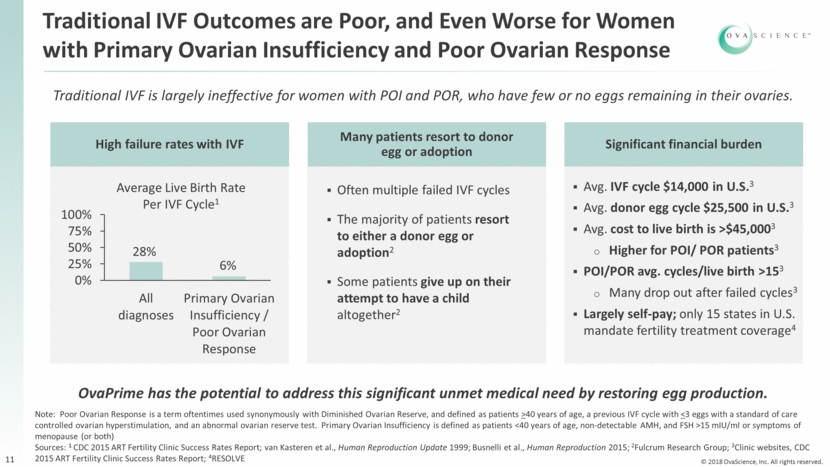
11 Traditional IVF is largely ineffective for women with POI and POR, who have few or no eggs remaining in their ovaries. High failure rates with IVF Many patients resort to donor egg or adoption Often multiple failed IVF cycles The majority of patients resort to either a donor egg or adoption2 Some patients give up on their attempt to have a child altogether2 Average Live Birth Rate Per IVF Cycle1 Note: Poor Ovarian Response is a term oftentimes used synonymously with Diminished Ovarian Reserve, and defined as patients >40 years of age, a previous IVF cycle with <3 eggs with a standard of care controlled ovarian hyperstimulation, and an abnormal ovarian reserve test. Primary Ovarian Insufficiency is defined as patients <40 years of age, non-detectable AMH, and FSH >15 mIU/ml or symptoms of menopause (or both) Sources: 1 CDC 2015 ART Fertility Clinic Success Rates Report; van Kasteren et al., Human Reproduction Update 1999; Busnelli et al., Human Reproduction 2015; 2Fulcrum Research Group; 3Clinic websites, CDC 2015 ART Fertility Clinic Success Rates Report; 4RESOLVE Significant financial burden Avg. IVF cycle $14,000 in U.S.3 Avg. donor egg cycle $25,500 in U.S.3 Avg. cost to live birth is >$45,0003 Higher for POI/ POR patients3 POI/POR avg. cycles/live birth >153 Many drop out after failed cycles3 Largely self-pay; only 15 states in U.S. mandate fertility treatment coverage4 28% 6% Traditional IVF Outcomes are Poor, and Even Worse for Women with Primary Ovarian Insufficiency and Poor Ovarian Response OvaPrime has the potential to address this significant unmet medical need by restoring egg production. 0% 25% 50% 75% 100% All diagnoses Primary Ovarian Insufficiency / Poor Ovarian Response
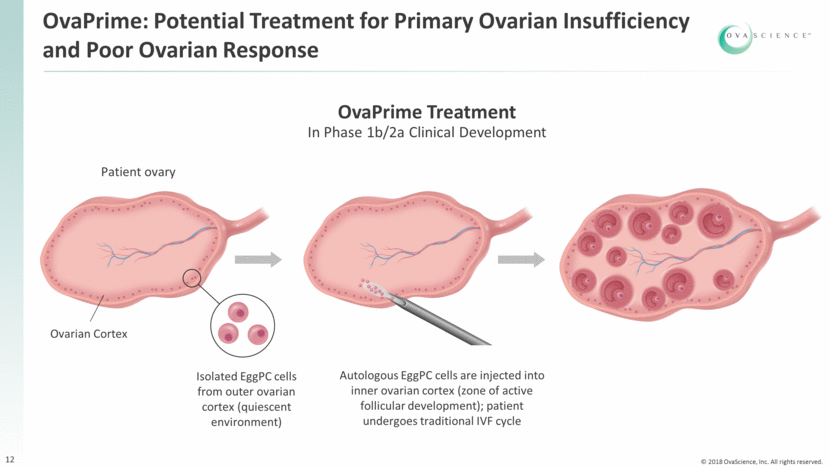
OvaPrime: Potential Treatment for Primary Ovarian Insufficiency and Poor Ovarian Response 12 Patient ovary Isolated EggPC cells from outer ovarian cortex (quiescent environment) Ovarian Cortex Autologous EggPC cells are injected into inner ovarian cortex (zone of active follicular development); patient undergoes traditional IVF cycle OvaPrime Treatment In Phase 1b/2a Clinical Development
OvaPrime Preclinical Data: Animal Proof-of-Concept Zou et al., 2009 Transplanted EggPC cells matured into oocytes which were fertilized after natural mating producing offspring expressing GFP Zhang et al., 2011 Transplanted EggPC cells matured into oocytes which were fertilized producing heterozygous offspring after mating with wild-type male Wu et al., 2017 Transplanted EggPC cells migrated to the inner ovarian cortex, increased in size, and expressed meiosis- and oocyte- specific genes as they matured into oocytes; fertilized producing heterozygous offspring after mating with wild-type males 13 Sources: 1Zou et al., Nature Cell Biology 2009 2Zhang et al., Journal of Molecular Cell Biology 2011 3Wu C et al., Molecular Therapy 2017
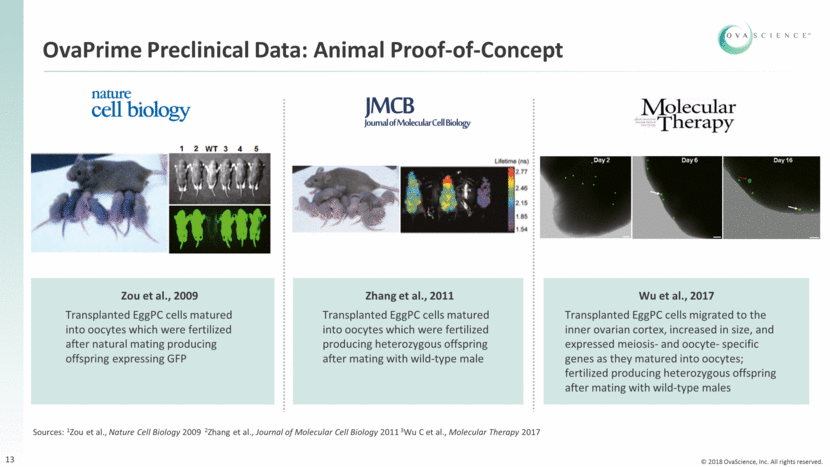
OvaPrime Preclinical Data: Proof-of-Concept Using Human EggPC Cells 14 Human EggPC cells engineered to express GFP Micro-injected GFP-EggPC cells into human ovarian cortical strips Xenografted tissue into NOD/SCID* mice In Vivo (xenografts) Endogenous 14 days post transplant Source: 1White et al., Nature Medicine 2012 *Non-obese diabetic mice with severe combined immunodeficiency disease
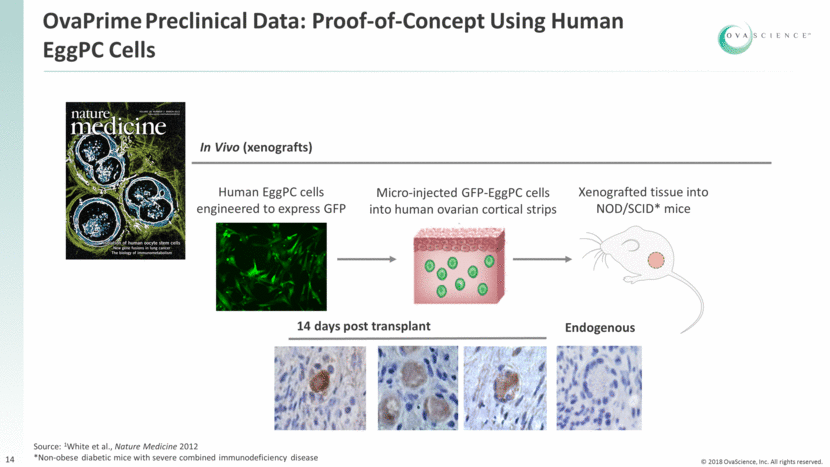
2017 2018 2019 2020 Phase 1 Study Single center, prospective, blinded, randomized, controlled study of 60 patients with POI and POR Initial readout of first 20 patients: No serious adverse events (SAEs) or adverse events (AEs) related to EggPC cells 7 mild AEs; 4 unrelated; 3 related to laparoscopic reintroduction No patients discontinued treatment due to AE Followed for nine months Enrollment closed Initial safety readout in 20 patients Complete six month safety readout in all patients Multi-center, prospective, controlled study of 40 patients with POI and POR Rationale for Phase 1a/2b Broader experience with multi-center study Recent improvements to cell processing and thaw techniques allow for consistently higher number of EggPC cells at reintroduction Refined patient population Embryo transfer readout Begin enrollment OvaPrime Clinical Development Program 15 Phase 1b/2a Study ✓ Complete six month safety readout in all patients
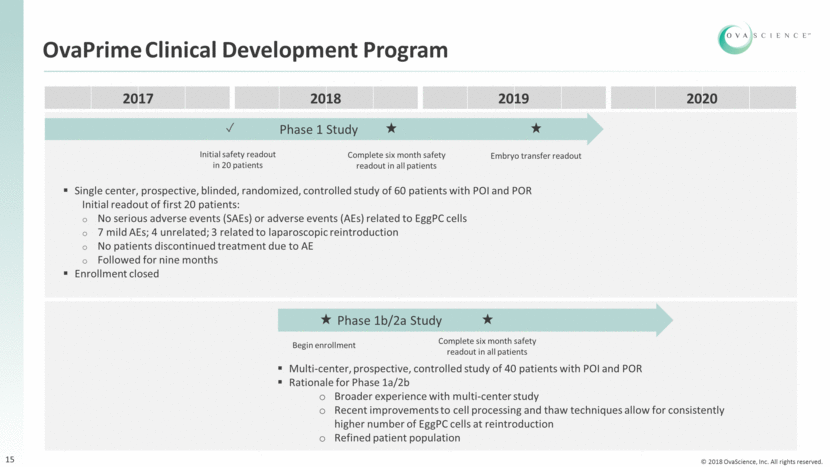
Key OvaPrime Milestones 16 Phase 1 study Readout: 6 months post-EggPCSM cell reintroduction safety for first 20 patients Completed Readout: 6 months post-EggPC cell reintroduction safety for all patients Q4 2018 Readout: Embryo transfers Q3 2019 Readout: Complete data set 2020 Phase 1b/2a study Begin enrollment H1 2018 Readout: 6 months post-EggPC cell reintroduction safety for all patients Q2 2019 Readout: Initial secondary endpoint data (indications of efficacy) 2019 Readout: Complete data set 2020 Regulatory Initiate regulatory pathway determination 2018
OvaTure Potential Fertility Treatment that Eliminates Hormone Stimulation 17
Traditional IVF is Associated with Risk of Serious Maternal Morbidity 18 Hormone injections are a required component of traditional IVF. Sources: 1Kumar et al., Jrnl of Human Reproductive Sciences, 2011; Nastri CO et al., Ultrasound in Obstetrics & Gynecology, 2015; 2RESOLVE 3Luke et al. NEJM 2012 Risk of severe ovarian hyperstimulation syndrome (OHSS) Significant discomfort, emotional distress and inconvenience Symptoms include enlarged ovaries, abdominal pain, bloating, breast tenderness, nausea, vomiting, diarrhea, and depression 1 Requires the inconvenience of multiple clinician visits and self-injections2 High treatment drop out rates3 1 in 4 patients discontinue after first IVF failure3 Severe OHSS affects 1-3% of IVF patients (and up to 20% of high responders, such as those with Polycystic Ovary Syndrome) 1 Complications include multiorgan dysfunction, and potentially, death1 OvaTure has the potential to address this significant unmet medical need by eliminating hormone stimulation.
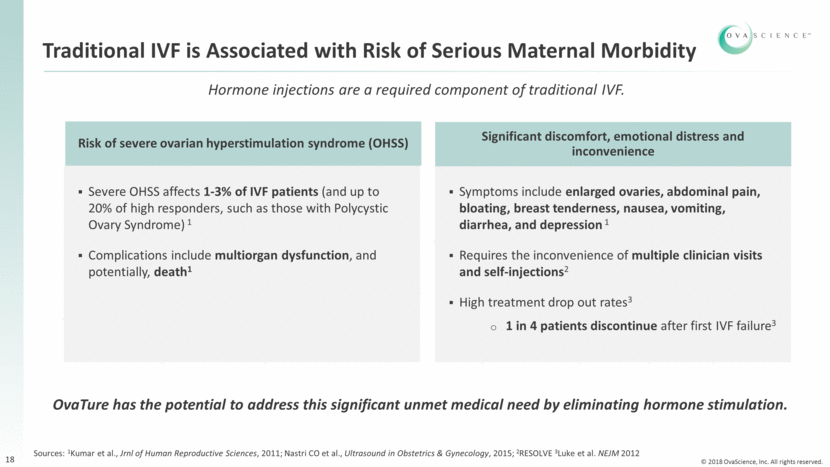
OvaTure: Potential Fertility Treatment that Eliminates Hormone Stimulation 19 In vitro maturation Sperm Patient ovary Isolated EggPC cells from outer ovarian cortex (quiescent environment) Ovarian Cortex OvaTure Treatment In Preclinical Development *ICSI: intracytoplasmic sperm injection Fertilization by ICSI*
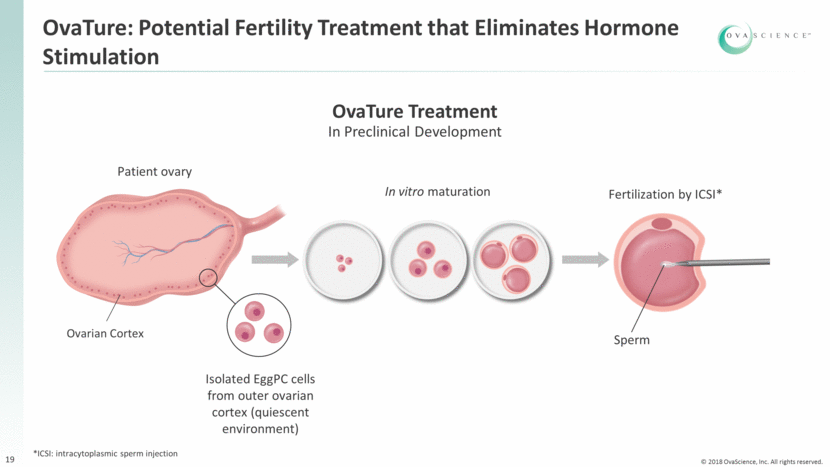
Substantial Progress Toward in vitro Development of a Mature Egg from an EggPC Cell Optimized FACS sorting techniques Developed proprietary mAb with high specificity and selectivity Optimized Aggregate formation techniques Seeding density Media components Timing of dissection Optimized Addition of media components Follicle subculture techniques EggPC isolation Aggregate culture strategy Subculture strategy 50µm Germinal Vesicle (GV) enlarged nucleus of an oocyte before meiotic division is completed Polar Body (PB) one of the small cells produced during the two meiotic divisions of developing oocytes Zona Pellucida (ZP) Thickened outer membrane 50µm 50µm Oocyte surrounded by multiple supporting cells ZP morphology and diameter comparable to endogenous eggs 20 Fertilization 50µm 50µm Sources: Internal OVAS data 2016/2017 (representative examples)
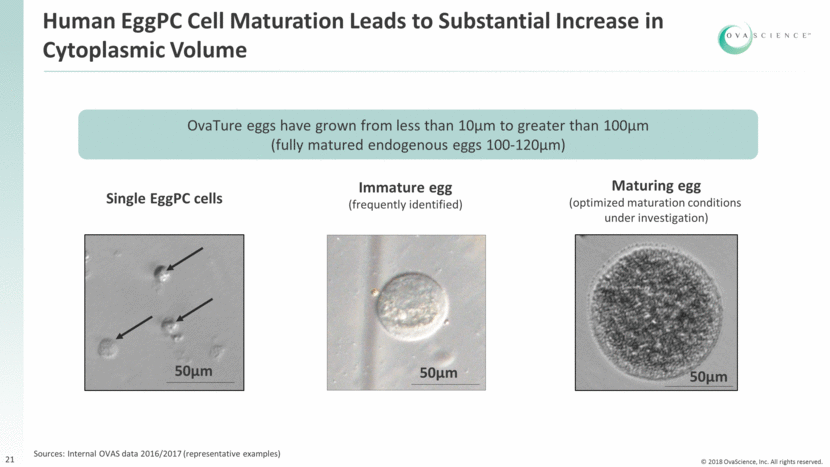
Human EggPC Cell Maturation Leads to Substantial Increase in Cytoplasmic Volume 21 OvaTure eggs have grown from less than 10µm to greater than 100µm (fully matured endogenous eggs 100-120µm) Single EggPC cells 50µm 50µm 50µm Immature egg (frequently identified) Maturing egg (optimized maturation conditions under investigation) Sources: Internal OVAS data 2016/2017 (representative examples)
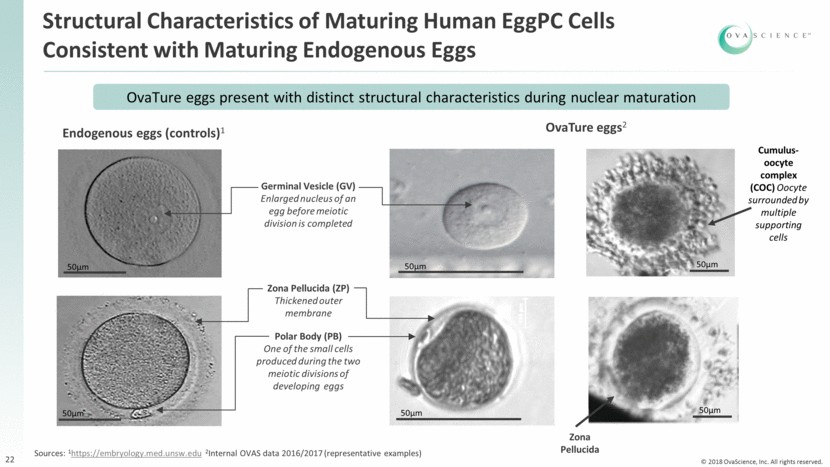
Endogenous eggs (controls)1 50µm 50µm Germinal Vesicle (GV) Enlarged nucleus of an egg before meiotic division is completed Polar Body (PB) One of the small cells produced during the two meiotic divisions of developing eggs 50µm 50µm Zona Pellucida (ZP) Thickened outer membrane Structural Characteristics of Maturing Human EggPC Cells Consistent with Maturing Endogenous Eggs 22 OvaTure eggs2 OvaTure eggs present with distinct structural characteristics during nuclear maturation Sources: 1https://embryology.med.unsw.edu 2Internal OVAS data 2016/2017 (representative examples) Cumulus-oocyte complex (COC) Oocyte surrounded by multiple supporting cells Zona Pellucida 50µm 50µm
23 OvaTure Next Steps Bovine Fertilization of bovine EggPCSM cell-derived egg Perform embryo transfer Bovine live birth from EggPC cell-derived egg Human Mature human EggPC cell-derived egg Where necessary, seek authorization to fertilize EggPC cell-derived egg (for research) Initiate regulatory pathway (clinical study) Submit for regulatory approval (clinical study)

AUGMENT Designed to Improve Traditional IVF Success 24

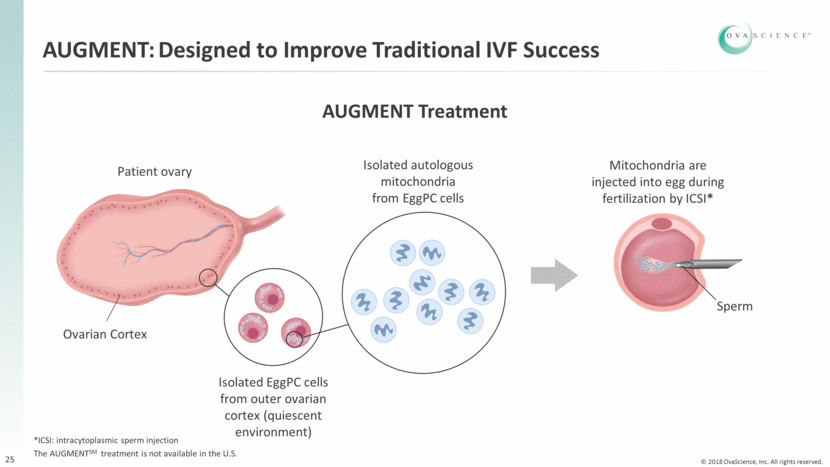
Patient ovary Ovarian Cortex AUGMENT: Designed to Improve Traditional IVF Success Isolated autologous mitochondria from EggPC cells Sperm AUGMENT Treatment Mitochondria are injected into egg during fertilization by ICSI* Isolated EggPC cells from outer ovarian cortex (quiescent environment) 25 *ICSI: intracytoplasmic sperm injection The AUGMENTSM treatment is not available in the U.S.
26 AUGMENT Status Offered at select clinics in Canada and Japan Signed exclusive licensing agreement with IVF Japan Group, a clinic network with extensive AUGMENT experience Opportunity to generate additional registry database and refine target patient population Minimal expense to OvaScience OvaScience retains worldwide commercialization rights for AUGMENT outside of Japan Continuing to work with the FDA under its available procedures to discuss regulatory status

Corporate 27
Financial Summary Cash, cash equivalents and short term investments: $75.7 million as of September 30, 2017 Shares outstanding: 35,705,722 as of September 30, 2017 Strong financial position with runway through key value inflection points 28
OvaScience: Transforming Female Infertility Therapy 29 $75.7 million in cash and short-term investments as of September 30, 2017 Cash runway into 2020 Cost efficient operating structure Patent protection into 2035 65 issued patents in 44 countries 170+ pending applications in more than 130 countries 163 million women worldwide are estimated to be infertile1 1.9 million undergo in vitro fertilization per year2 Average cost of $14,000 per IVF cycle in U.S.; largely self-pay3 Large and Growing Market Strong Financial Position Robust IP Portfolio Sources: 1Centers for Disease Control 2CDC, 2015 ART National Summary Report; Assisted Reproductive Technologies in Canada: Results from Canadian ART Register; Takeshima, J Assist Reprod Genet (2014) 31:477-484; Japan Registry, 2014, Assisted Reproductive Technology in Europe: Results from European Registers by ESHRE; Assisted Reproductive Technology in Italy, 2013; Assisted Reproductive Technology Fact Sheet by ESHRE, Europe; Germany IVF-Registry (2013-2015); Luke et al. NEJM vol.366 (26):2483-91 and supplement, 2012; Aptis Partners; Germany D.I.R. Annual Report; HFEA Fertility Treatment 2014 Trends and Figures; Italian Assisted Reproductive Technology Register 2013 3Clinic websites, RESOLVE Breakthrough Technology in Reproductive Medicine Discovery of egg precursor (EggPCSM) cells unlocks innovative opportunities to improve female fertility through novel treatments OvaPrime, in clinical development, potential fertility treatment that restores egg production OvaTure, in preclinical development, potential fertility treatment that eliminates need for hormone stimulation AUGMENT, available in Canada and Japan, designed to improve IVF success
[LOGO]

