
Medicines to Outmatch Disease Investor Presentation January 13, 2025 Exhibit 99.2

Important Notice and Disclaimers This presentation contains statements that relate to future events and expectations and as such constitute forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. When or if used in this presentation, the words “anticipate,” “believe,” “could,” “estimate,” “expect,” “intend,” “may,” “outlook,” “plan,” “predict,” “should,” “will,” and similar expressions and their variants, as they relate to Nurix Therapeutics, Inc. (“Nurix”, the “Company,” “we,” “us” or “our”), may identify forward-looking statements. All statements that reflect Nurix’s expectations, assumptions or projections about the future, other than statements of historical fact, are forward-looking statements, including, without limitation, statements regarding our future financial or business plans; our future performance, prospects and strategies; future conditions, trends, and other financial and business matters; our current and prospective drug candidates; the planned timing and conduct of the clinical trial programs for our drug candidates; the planned timing for the provision of clinical updates and initial findings from our clinical studies; the potential benefits of our collaborations, including potential milestone and sales-related payments; the potential advantages of DEL-AI and our drug candidates; the extent to which our scientific approach, our drug discovery engine, targeted protein degradation, and Degrader Antibody Conjugates may potentially address a broad range of diseases; the extent animal model data predicts human efficacy; the timing and success of the development and commercialization of our current and anticipated drug candidates; our estimated, unaudited cash and investment position; and our ability to fund our operations into the first half of 2027. Forward-looking statements reflect Nurix’s current beliefs, expectations, and assumptions. Although Nurix believes the expectations and assumptions reflected in such forward-looking statements are reasonable, Nurix can give no assurance that they will prove to be correct. Forward-looking statements are not guarantees of future performance and are subject to risks, uncertainties and changes in circumstances that are difficult to predict, which could cause Nurix’s actual activities and results to differ materially from those expressed in any forward-looking statement. Such risks and uncertainties include, but are not limited to: (i) risks and uncertainties related to Nurix’s ability to advance its drug candidates, obtain regulatory approval of and ultimately commercialize its drug candidates; (ii) the timing and results of clinical trials; (iii) Nurix’s ability to fund development activities and achieve development goals; (iv) risks and uncertainties relating to the timing and receipt of payments from Nurix's collaboration partners, including milestone payments and royalties on future potential product sales; (v) the impact of macroeconomic events and conditions, including increasing financial market volatility and uncertainty, inflation, interest rate fluctuations, instability in the global banking system, uncertainty with respect to the federal budget and debt ceiling, the impact of war, military or regional conflicts, and global health pandemics, on Nurix’s clinical trials and operations; (vi) Nurix’s ability to protect intellectual property and (vii) other risks and uncertainties described under the heading “Risk Factors” in Nurix’s Quarterly Report on Form 10-Q for the fiscal quarter ended August 31, 2024, and other SEC filings. Accordingly, readers are cautioned not to place undue reliance on these forward-looking statements. The statements in this presentation speak only as of the date of this presentation, even if subsequently made available by Nurix on its website or otherwise. Nurix disclaims any intention or obligation to update publicly any forward-looking statements, whether in response to new information, future events, or otherwise, except as required by applicable law. Certain information contained in this presentation relates to or is based on studies, publications, surveys and other data obtained from third-party sources and the Company’s own internal estimates and research. While the Company believes these third-party sources to be reliable as of the date of this presentation, it has not independently verified, and makes no representation as to the adequacy, fairness, accuracy or completeness of, any information obtained from third-party sources. In addition, all of the market data included in this presentation involves a number of assumptions and limitations, and there can be no guarantee as to the accuracy or reliability of such assumptions. Furthermore, while we believe our own internal estimates and research are reliable, such estimates and research have not been verified by any independent source. The estimated cash and investments amount included in this presentation is a preliminary, unaudited estimate based upon information available to Nurix's management as of the date of this presentation. It is subject to the completion of financial closing procedures, including the completion of audit procedures by Nurix's independent public accounting firm, and therefore is subject to adjustment. The amount does not present all information necessary for a complete understanding of Nurix’s financial condition as of or for the year ended November 30, 2024. Nurix’s audited results as of and for the year ended November 30, 2024, will be included in Nurix’s Annual Report on Form 10-K for the year ended November 30, 2024. 2

Key accomplishments in 2024 Another Great Year at Nurix 3 Demonstrated clear clinical proof of concept for BTK degradation with NX-5948 • Completed Phase 1a dose escalation • Demonstrated robust efficacy in CLL and WM with favorable safety profile • Oral presentations at ASH and EHA Established unmet medical need with key regulatory agencies • Fast Track Designation for CLL and WM from the U.S. Food and Drug Administration (FDA) • PRIME designation from the European Medicines Agency (EMA) Advanced two other wholly owned assets in the clinic • NX-2127, a dual degrader of BTK and IKZF1/3 • NX-1607, an inhibitor of CBL-B Progressed three major partnerships with Sanofi, Gilead, and Pfizer • Extension of the STAT6 degrader program with Sanofi • Presented first preclinical data for IRAK4 degrader clinical candidate and advanced toward IND submission • Achieved potent cell-based activity of degrader antibody conjugates Ended fiscal year in a strong cash position with $609.6M* NX-5948 BTK Degrader Pipeline BTK, Bruton’s tyrosine kinase; CLL chronic lymphocytic leukemia, CBL-B, casitas B lymphoma-b; WM, Waldenstrom’s macroglobulinemia * This estimated cash and investment amount is a preliminary, unaudited estimate based upon information available to Nurix's management as of the date of this presentation. The amount is subject to the completion of financial closing procedures, including the completion of audit procedures by Nurix’s independent registered public accounting firm, and therefore is subject to adjustment.

Meeting the needs of patients with breakthrough therapies Drug Discovery Pipeline Strategy 4 Clinically validated targets where inhibitors fail to address resistance and scaffolding Unmet medical need where inhibitors are not sufficient to drive efficacy "Undruggable" targets Kinase targets in cancer BTK – B-cell malignancies and I&I Signaling proteins with scaffolding function IRAK4 – rheumatoid arthritis STAT6 – T2 inflammatory diseases Transcriptions factors; fusion proteins; E3 ligases BTK, Bruton’s tyrosine kinase; I&I, inflammation and immunology; IRAK4, interleukin-1 receptor-associated kinase 4; STAT6, signal transducer and activator of transcription 6
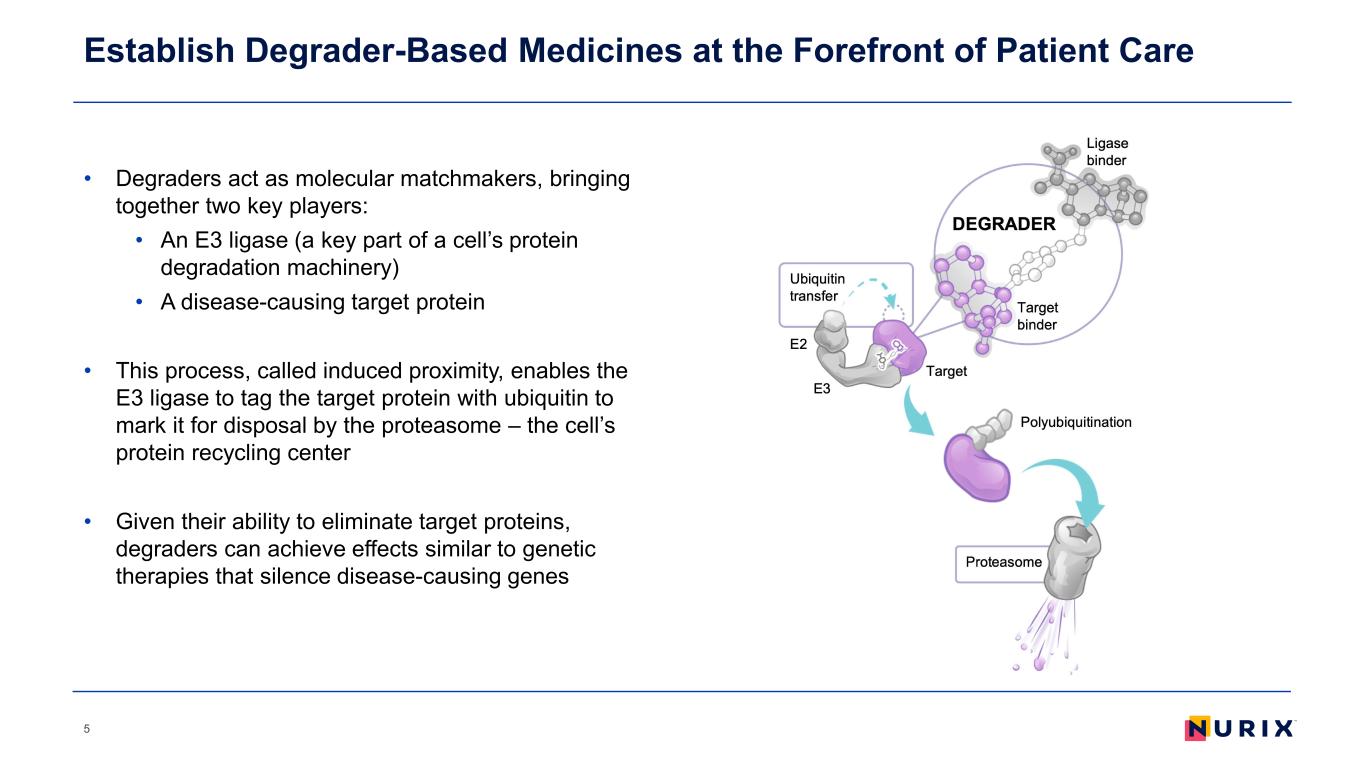
Establish Degrader-Based Medicines at the Forefront of Patient Care • Degraders act as molecular matchmakers, bringing together two key players: • An E3 ligase (a key part of a cell’s protein degradation machinery) • A disease-causing target protein • This process, called induced proximity, enables the E3 ligase to tag the target protein with ubiquitin to mark it for disposal by the proteasome – the cell’s protein recycling center • Given their ability to eliminate target proteins, degraders can achieve effects similar to genetic therapies that silence disease-causing genes 5

Industry Leading DEL-AI Discovery Engine for TPD and DAC Drug Discovery Our DEL-AI discovery engine leverages the combined power of our data-rich DEL capabilities, automated chemistry, and advanced machine learning to accelerate drug discovery 6 TPD, targeted protein degradation; DAC, degrader antibody conjugate; DEL, DNA encoded library

Nurix Is Advancing a Pipeline of Proprietary and Partnered Programs in Oncology and Inflammation & Immunology 7 Program Target Modality Therapeutic area Discovery IND-Enabling Phase 1A Phase 1B/2 NX-5948 BTK Degrader B-cell malignancies NX-2127 BTK-IKZF Degrader B-cell malignancies NX-1607 CBL-B Inhibitor of degradation Immuno-oncology BRAF degrader Pan-mutant BRAF Degrader Solid tumors Multiple Undisclosed Degrader Undisclosed Multiple Undisclosed Degrader Undisclosed Undisclosed Undisclosed Degrader Undisclosed Multiple Undisclosed DAC Undisclosed Program Target Modality Therapeutic area Discovery IND-Enabling Phase 1A Phase 1B/2 NX-5948 BTK Degrader Autoimmune cytopenia NX-0479 / GS-6791 IRAK4 Degrader Rheumatoid arthritis and other inflammatory diseases STAT6 degrader STAT6 Degrader Type 2 inflammatory diseases Undisclosed Undisclosed Degrader Inflammation / autoimmune Multiple Undisclosed DAC Inflammation / autoimmune O nc ol og y In fla m m at io n & Im m un ol og y
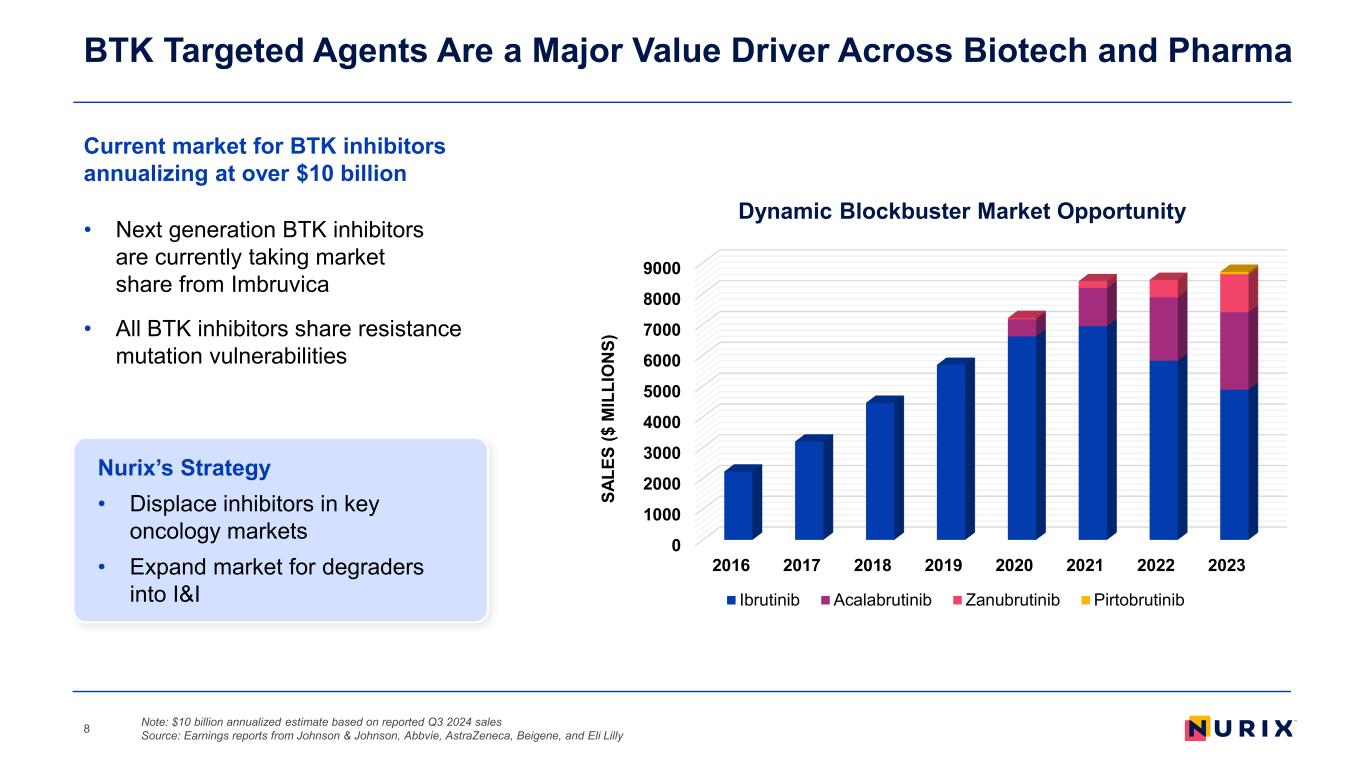
• Next generation BTK inhibitors are currently taking market share from Imbruvica • All BTK inhibitors share resistance mutation vulnerabilities Current market for BTK inhibitors annualizing at over $10 billion BTK Targeted Agents Are a Major Value Driver Across Biotech and Pharma 8 0 1000 2000 3000 4000 5000 6000 7000 8000 9000 2016 2017 2018 2019 2020 2021 2022 2023 SA LE S ($ M IL LI O N S) Dynamic Blockbuster Market Opportunity Ibrutinib Acalabrutinib Zanubrutinib Pirtobrutinib Nurix’s Strategy • Displace inhibitors in key oncology markets • Expand market for degraders into I&I Note: $10 billion annualized estimate based on reported Q3 2024 sales Source: Earnings reports from Johnson & Johnson, Abbvie, AstraZeneca, Beigene, and Eli Lilly
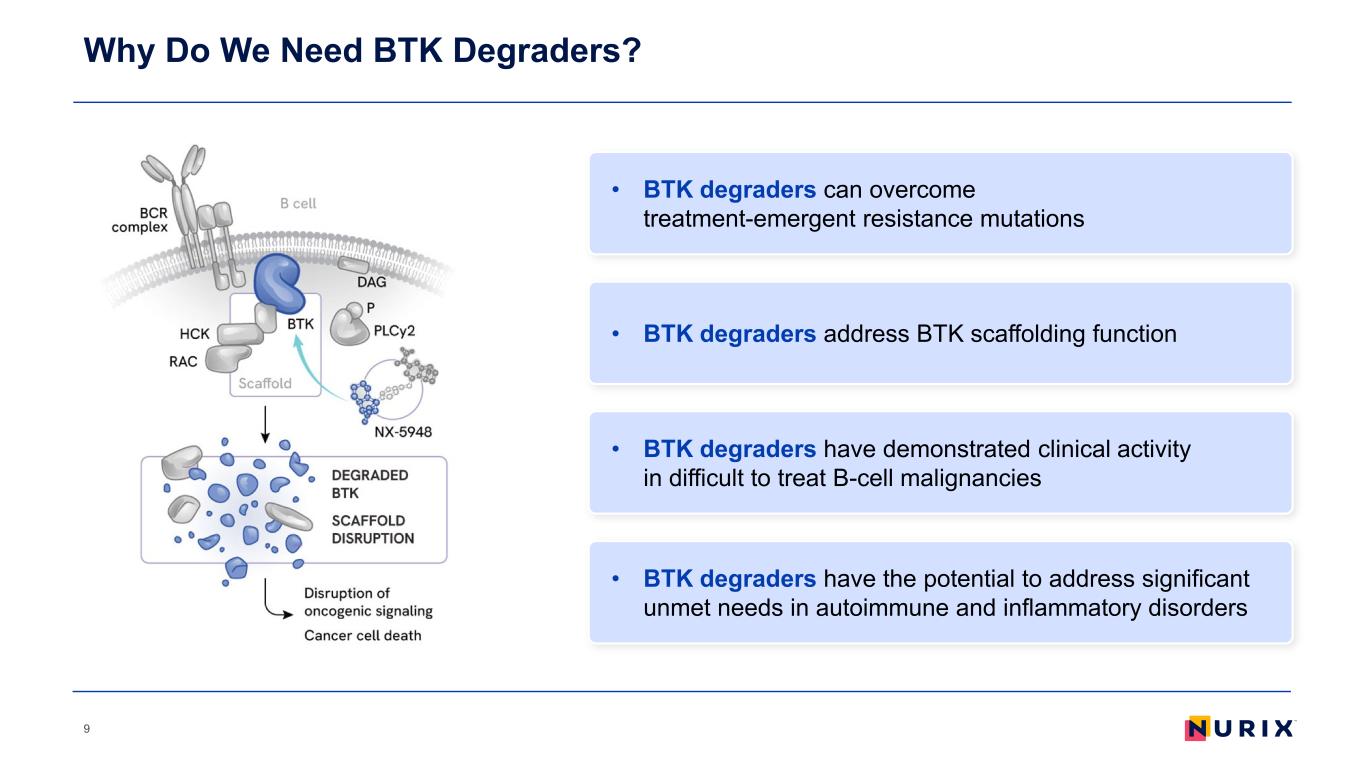
Why Do We Need BTK Degraders? 9 • BTK degraders address BTK scaffolding function • BTK degraders have demonstrated clinical activity in difficult to treat B-cell malignancies • BTK degraders have the potential to address significant unmet needs in autoimmune and inflammatory disorders • BTK degraders can overcome treatment-emergent resistance mutations

NX-5948-301 Trial Design Phase 1a/b Trial in Adults with Relapsed/Refractory B-cell Malignancies CLL, chronic lymphocytic leukemia; SLL, small lymphocytic lymphoma; NHL, non-Hodgkin’s lymphoma; WM, Waldenström macroglobulinemia; BTKi, BTK inhibitor; BCL2i, Bcl-2 inhibitor; QD, once daily Phase 1a dose escalation (fully enrolled) Ongoing CLL Phase 1b expansion cohorts 50 mg QD 100 mg QD 200 mg QD 300 mg QD 450 mg QD 600 mg QDNHL/WM CLL/SLL 50 mg QD 100 mg QD 200 mg QD 300 mg QD 450 mg QD 600 mg QD CLL/SLL (200 mg) Prior BTKi and BCL2i CLL/SLL (600 mg) Prior BTKi and BCL2i Randomized Fast Track Designation WM Waldenstrom’s macroglobulinemia MZL Marginal zone lymphoma FL Follicular lymphoma Ongoing NHL/WM Phase 1b expansion cohorts 10

Baseline disease characteristics Heavily Pre-Treated Patients With a High Prevalence of Baseline Mutations aBaseline disease characteristics in CLL cohort were comparable to those in the overall population; bPatients could have received multiple prior treatments; cAll patients who received ncBTKi have also previously received cBTKi; dMutations presented here were centrally sequenced. BCL2, B-cell lymphoma 2; BCL2i, BCL2 inhibitor; BTK, Bruton’s tyrosine kinase; BTKi, BTK inhibitor; cBTKi, covalent BTKi; CAR-T, chimeric antigen receptor T-cell; CLL, chronic lymphocytic leukemia; CNS, central nervous system; ECOG PS, Eastern Cooperative Oncology Group (ECOG) performance status; ncBTKi, non-covalent BTKi; PI3Ki, phosphoinositide 3-kinase inhibitor; PLCG2, phospholipase C gamma 2; SLL, small lymphocytic lymphoma Characteristics Patients with CLL/SLLa (n=60) ECOG PS, n (%) 0 1 24 (40.0) 36 (60.0) CNS involvement, n (%) 5 (8.3) Median prior lines of therapy (range) 4.0 (1–12) Previous treatmentsb, n (%) BTKi cBTKi ncBTKic BCL2i BTKi and BCL2i CAR-T therapy Bispecific antibody PI3Ki Chemo/chemo-immunotherapies (CIT) 59 (98.3) 59 (98.3) 17 (28.3) 50 (83.3) 49 (81.7) 3 (5.0) 4 (6.7) 18 (30.0) 43 (71.7) Mutation statusd (n=57), n (%) TP53 BTK PLCG2 BCL2 23 (40.4) 22 (38.6) 7 (12.3) 6 (10.5) Data cutoff: 10 Oct 2024 11

NX-5948 degrades gatekeeper, kinase-proficient and kinase-dead BTK mutations NX-5948 Degrades Wild-Type and Mutated BTK Reference 1. Montoya et al. Science 2024;383 Patients with CLL/SLL (n=57)c Baseline mutation status, n (%) BTK mutations1,a,b 22 (38.6) C481S 12 (21.1) C481R 2 (3.5) L528W 4 (7.0) L528S 1 (1.8) T474I 5 (8.8) T474F 1 (1.8) V416M 1 (1.8) V416L 1 (1.8) G541V 1 (1.8) aPatients could have multiple prior treatments and BTK mutations; BTK mutations were tested at baseline by next-generation sequencing centrally; ≥5% allelic frequency is reported bPatients can have more than one resistance mutation cPatients with available mutation status Note: Some patients have multiple BTK mutations BTK degradation BTK, Bruton’s tyrosine kinase; CLL, chronic lymphocytic leukemia; MFI, mean fluorescence intensity; SLL, small lymphocytic lymphoma Data cutoff: 10 Oct 2024 12

NX-5948 overall response assessment Response Rate Deepens with Longer Time on Treatment CLL, chronic lymphocytic leukemia; CR, complete response; iwCLL, International Workshop on CLL; ORR, objective response rate; PD, progressive disease; PR, partial response; PR-L, partial response with rebound lymphocytosis; SD, stable disease CLL response-evaluable patients Primary ORR analysisb ≥1 response assessment(s) at 8 weeks Exploratory ORR analysisb ≥2 response assessments at 16 weeks n=49c n=38c Objective response rate (ORR),a % (95% CI) 75.5 (61.1–86.7) 84.2 (68.7–94.0) Best response, n (%) CR 0 (0.0) 0 (0.0) PR 36 (73.5) 32 (84.2) PR-L 1 (2.0) 0 (0.0) SD 10 (20.4) 4 (10.5) PD 2 (4.1) 2 (5.3) aObjective response rate includes CR + PR + PR-L bPatients who progressed prior to their first response assessment and patients who discontinued for any reason after their first response assessment are included in the denominators cPatients without identified target lesion(s) at baseline are evaluated as disease-evaluable per iwCLL criteria, while they may not be represented in waterfall plot Data cutoff: 10 Oct 2024 13
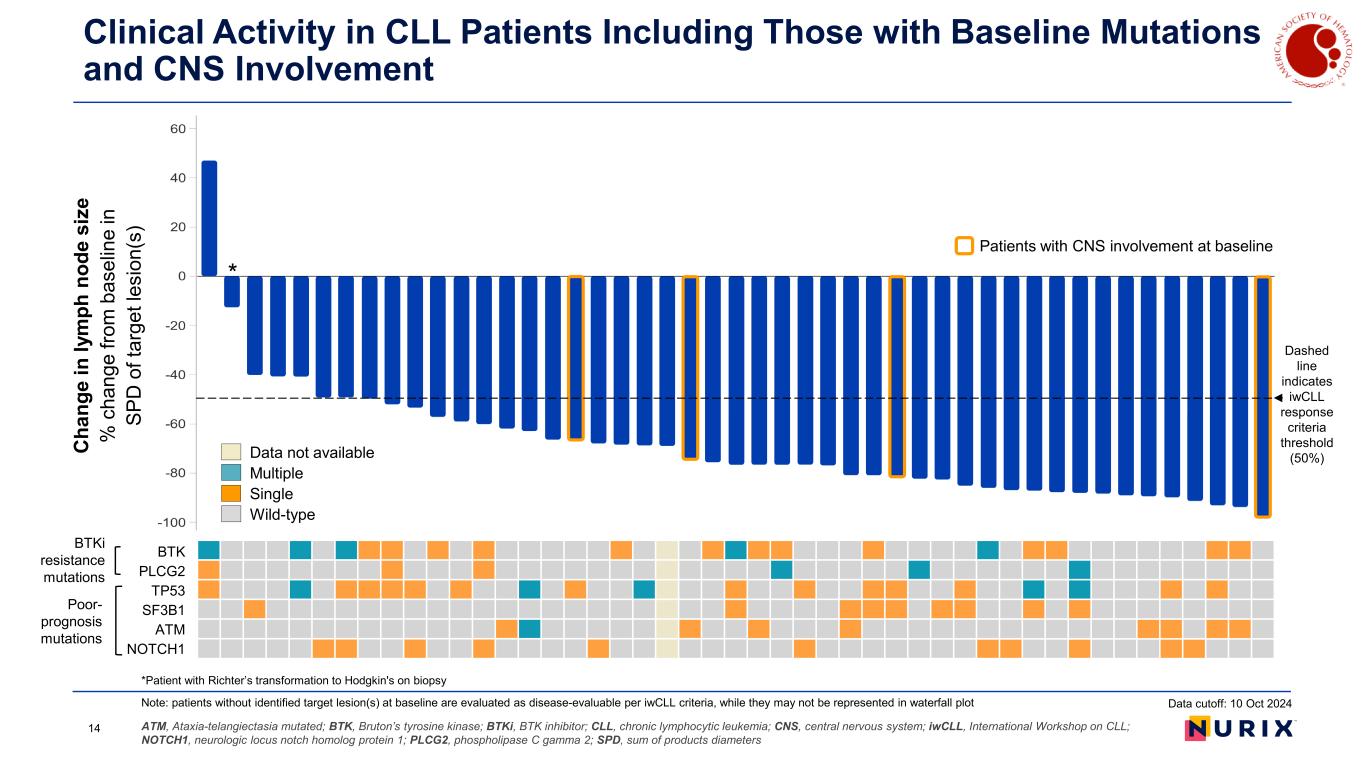
Clinical Activity in CLL Patients Including Those with Baseline Mutations and CNS Involvement ATM, Ataxia-telangiectasia mutated; BTK, Bruton’s tyrosine kinase; BTKi, BTK inhibitor; CLL, chronic lymphocytic leukemia; CNS, central nervous system; iwCLL, International Workshop on CLL; NOTCH1, neurologic locus notch homolog protein 1; PLCG2, phospholipase C gamma 2; SPD, sum of products diameters *Patient with Richter’s transformation to Hodgkin's on biopsy Note: patients without identified target lesion(s) at baseline are evaluated as disease-evaluable per iwCLL criteria, while they may not be represented in waterfall plot Patients with CNS involvement at baseline BTK PLCG2 TP53 SF3B1 ATM NOTCH1 BTKi resistance mutations Poor- prognosis mutations BT PLCG TP5 SF3B AT NOTCH * Data not available Multiple Single Wild-type C ha ng e in ly m ph n od e si ze % c ha ng e fro m b as el in e in SP D o f t ar ge t l es io n( s) Patients with CNS involvement at baseline Dashed line indicates iwCLL response criteria threshold (50%) Data cutoff: 10 Oct 2024 14
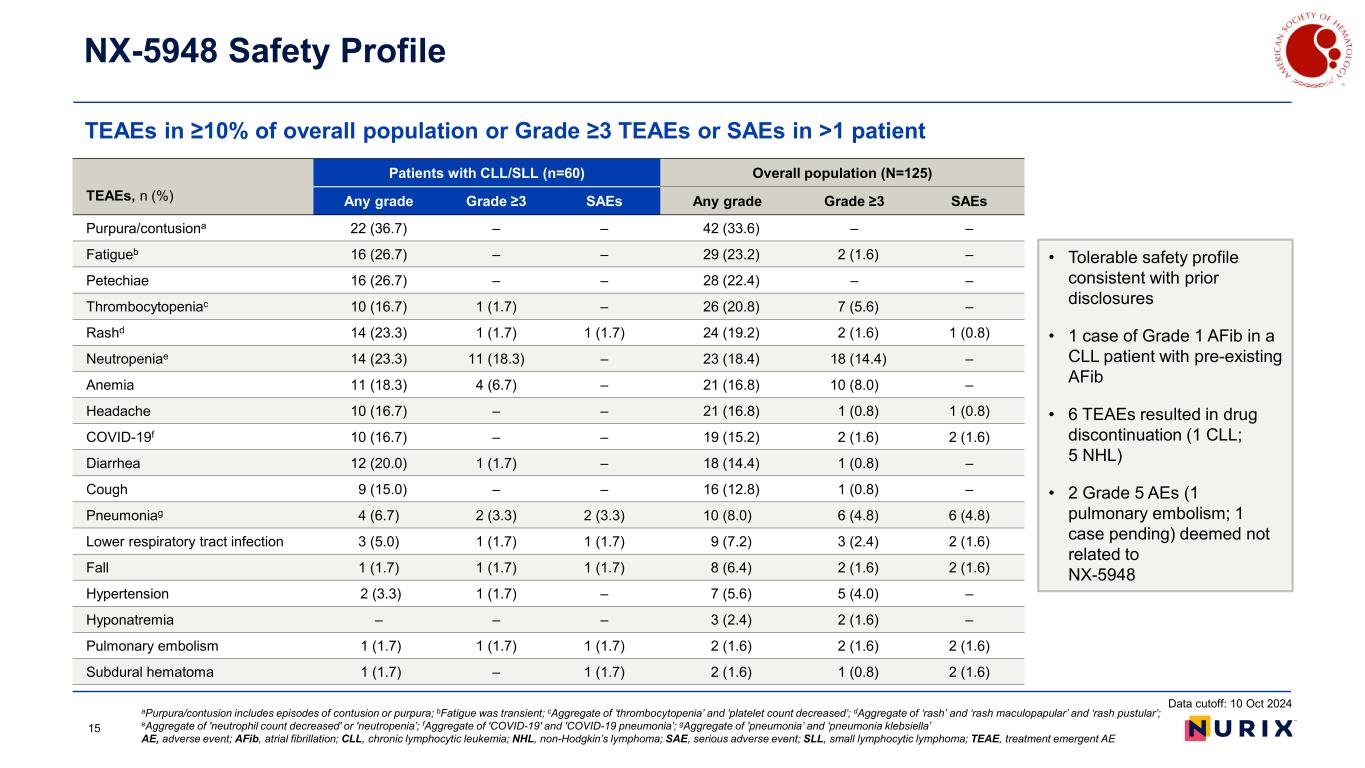
TEAEs in ≥10% of overall population or Grade ≥3 TEAEs or SAEs in >1 patient NX-5948 Safety Profile aPurpura/contusion includes episodes of contusion or purpura; bFatigue was transient; cAggregate of ‘thrombocytopenia’ and ‘platelet count decreased’; dAggregate of ‘rash’ and ‘rash maculopapular’ and ‘rash pustular’; eAggregate of 'neutrophil count decreased' or 'neutropenia’; fAggregate of 'COVID-19' and 'COVID-19 pneumonia’; gAggregate of 'pneumonia’ and ‘pneumonia klebsiella’ AE, adverse event; AFib, atrial fibrillation; CLL, chronic lymphocytic leukemia; NHL, non-Hodgkin’s lymphoma; SAE, serious adverse event; SLL, small lymphocytic lymphoma; TEAE, treatment emergent AE TEAEs, n (%) Patients with CLL/SLL (n=60) Overall population (N=125) Any grade Grade ≥3 SAEs Any grade Grade ≥3 SAEs Purpura/contusiona 22 (36.7) – – 42 (33.6) – – Fatigueb 16 (26.7) – – 29 (23.2) 2 (1.6) – Petechiae 16 (26.7) – – 28 (22.4) – – Thrombocytopeniac 10 (16.7) 1 (1.7) – 26 (20.8) 7 (5.6) – Rashd 14 (23.3) 1 (1.7) 1 (1.7) 24 (19.2) 2 (1.6) 1 (0.8) Neutropeniae 14 (23.3) 11 (18.3) – 23 (18.4) 18 (14.4) – Anemia 11 (18.3) 4 (6.7) – 21 (16.8) 10 (8.0) – Headache 10 (16.7) – – 21 (16.8) 1 (0.8) 1 (0.8) COVID-19f 10 (16.7) – – 19 (15.2) 2 (1.6) 2 (1.6) Diarrhea 12 (20.0) 1 (1.7) – 18 (14.4) 1 (0.8) – Cough 9 (15.0) – – 16 (12.8) 1 (0.8) – Pneumoniag 4 (6.7) 2 (3.3) 2 (3.3) 10 (8.0) 6 (4.8) 6 (4.8) Lower respiratory tract infection 3 (5.0) 1 (1.7) 1 (1.7) 9 (7.2) 3 (2.4) 2 (1.6) Fall 1 (1.7) 1 (1.7) 1 (1.7) 8 (6.4) 2 (1.6) 2 (1.6) Hypertension 2 (3.3) 1 (1.7) – 7 (5.6) 5 (4.0) – Hyponatremia – – – 3 (2.4) 2 (1.6) – Pulmonary embolism 1 (1.7) 1 (1.7) 1 (1.7) 2 (1.6) 2 (1.6) 2 (1.6) Subdural hematoma 1 (1.7) – 1 (1.7) 2 (1.6) 1 (0.8) 2 (1.6) • Tolerable safety profile consistent with prior disclosures • 1 case of Grade 1 AFib in a CLL patient with pre-existing AFib • 6 TEAEs resulted in drug discontinuation (1 CLL; 5 NHL) • 2 Grade 5 AEs (1 pulmonary embolism; 1 case pending) deemed not related to NX-5948 Data cutoff: 10 Oct 2024 15
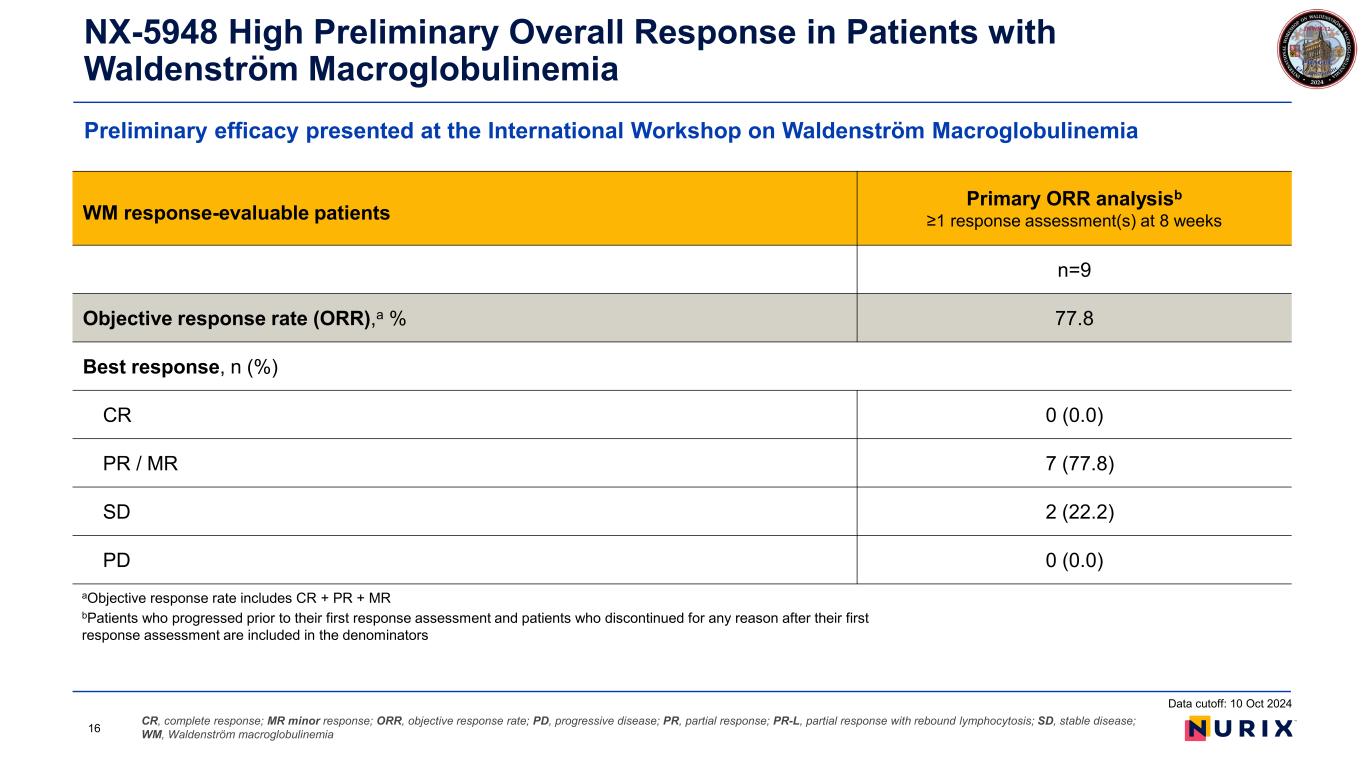
Preliminary efficacy presented at the International Workshop on Waldenström Macroglobulinemia NX-5948 High Preliminary Overall Response in Patients with Waldenström Macroglobulinemia CR, complete response; MR minor response; ORR, objective response rate; PD, progressive disease; PR, partial response; PR-L, partial response with rebound lymphocytosis; SD, stable disease; WM, Waldenström macroglobulinemia aObjective response rate includes CR + PR + MR bPatients who progressed prior to their first response assessment and patients who discontinued for any reason after their first response assessment are included in the denominators Data cutoff: 10 Oct 2024 16 WM response-evaluable patients Primary ORR analysisb ≥1 response assessment(s) at 8 weeks n=9 Objective response rate (ORR),a % 77.8 Best response, n (%) CR 0 (0.0) PR / MR 7 (77.8) SD 2 (22.2) PD 0 (0.0)
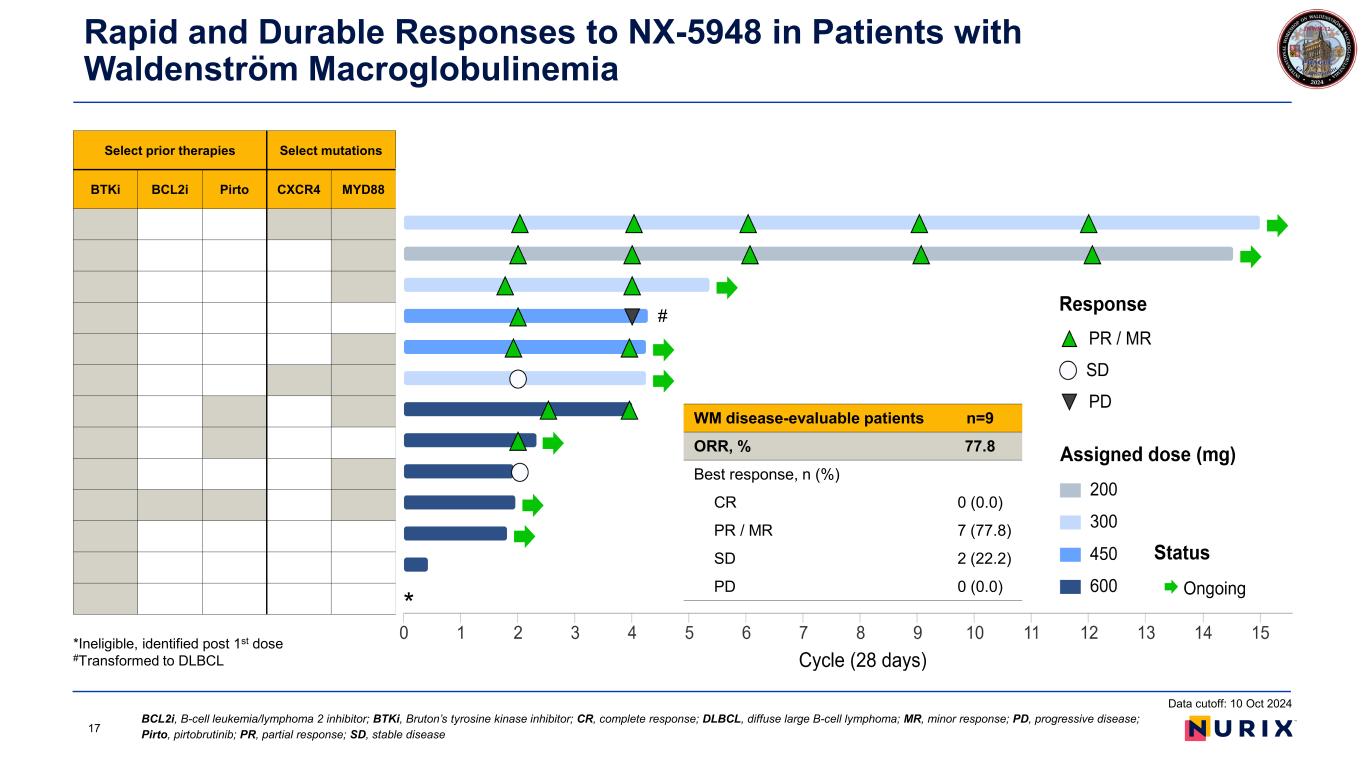
Rapid and Durable Responses to NX-5948 in Patients with Waldenström Macroglobulinemia Data cutoff: 10 Oct 2024 Select prior therapies Select mutations BTKi BCL2i Pirto CXCR4 MYD88 *Ineligible, identified post 1st dose #Transformed to DLBCL * # WM disease-evaluable patients n=9 ORR, % 77.8 Best response, n (%) CR 0 (0.0) PR / MR 7 (77.8) SD 2 (22.2) PD 0 (0.0) 17 BCL2i, B-cell leukemia/lymphoma 2 inhibitor; BTKi, Bruton’s tyrosine kinase inhibitor; CR, complete response; DLBCL, diffuse large B-cell lymphoma; MR, minor response; PD, progressive disease; Pirto, pirtobrutinib; PR, partial response; SD, stable disease

NX-5948 Regulatory Milestones 18 CLL WM Advancing NX-5948 program globally in CLL and WM • U.S. Fast Track Designation from the FDA in January 2024 • Type B End of Phase 1 meeting held with the FDA, key takeaways: • Reviewed dose levels of 200 mg QD and 600 mg QD in the context of Project Optimus • Feedback on principles of pivotal trial designs including Fast Track population and considerations for randomized controlled trials • Nurix plans future interactions in 2025 as sufficient data is accumulated • EU expansion of enrollment into France, Poland, Italy and Spain approved in Q3 2024 • EU PRIME designation from EMA in November 2024 • U.S. Fast Track Designation from the FDA in December 2024 CLL, chronic lymphocytic leukemia; WM, Waldenström macroglobulinemia
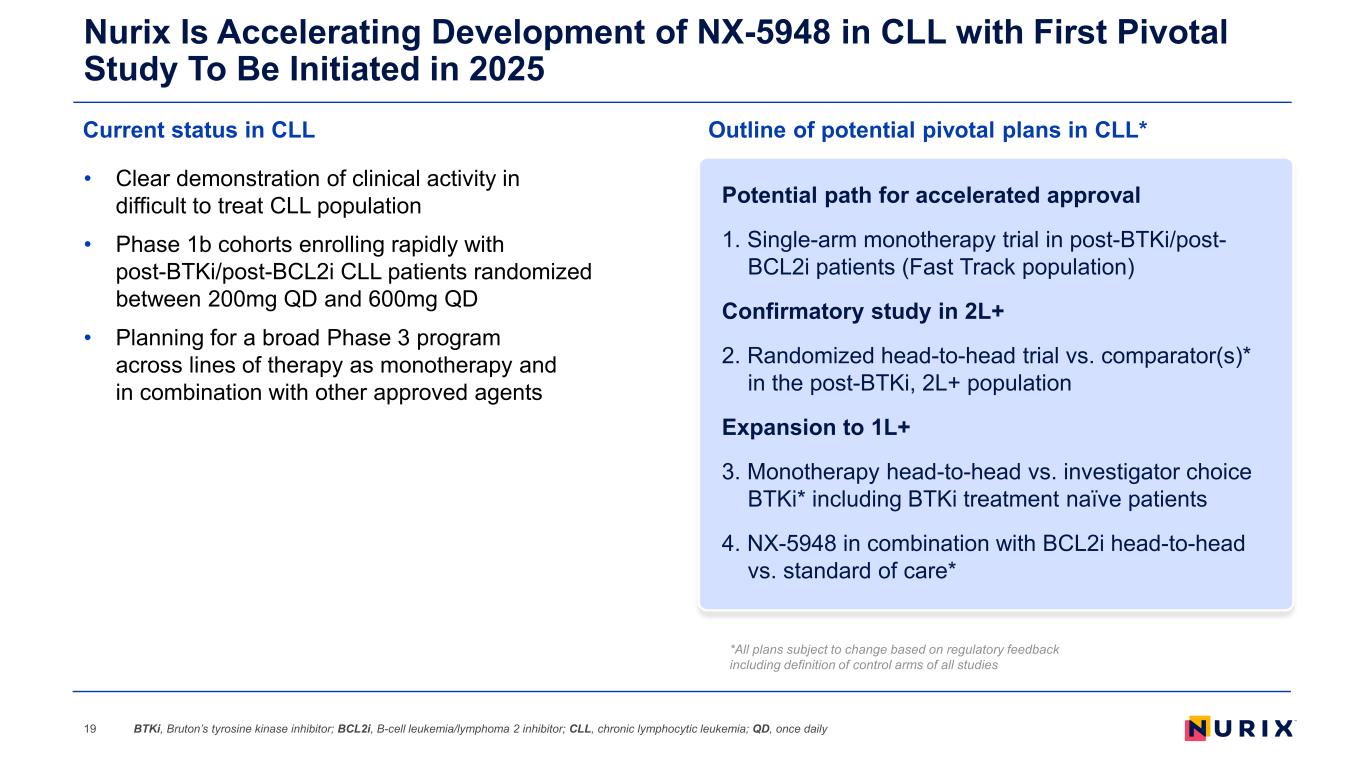
Nurix Is Accelerating Development of NX-5948 in CLL with First Pivotal Study To Be Initiated in 2025 • Clear demonstration of clinical activity in difficult to treat CLL population • Phase 1b cohorts enrolling rapidly with post-BTKi/post-BCL2i CLL patients randomized between 200mg QD and 600mg QD • Planning for a broad Phase 3 program across lines of therapy as monotherapy and in combination with other approved agents Current status in CLL Outline of potential pivotal plans in CLL* 19 Potential path for accelerated approval 1. Single-arm monotherapy trial in post-BTKi/post- BCL2i patients (Fast Track population) Confirmatory study in 2L+ 2. Randomized head-to-head trial vs. comparator(s)* in the post-BTKi, 2L+ population Expansion to 1L+ 3. Monotherapy head-to-head vs. investigator choice BTKi* including BTKi treatment naïve patients 4. NX-5948 in combination with BCL2i head-to-head vs. standard of care* *All plans subject to change based on regulatory feedback including definition of control arms of all studies BTKi, Bruton’s tyrosine kinase inhibitor; BCL2i, B-cell leukemia/lymphoma 2 inhibitor; CLL, chronic lymphocytic leukemia; QD, once daily

Inflammation & Immunology 20 Leveraging our expertise in B- and T-cell biology, Nurix is building a pipeline of innovative degrader drugs to address the unmet medical need for patients living with inflammation and autoimmune disorders

Advancing NX-5948 in Immunology and Inflammation The genetics of BTK are compelling: highly specific with potent biology • Human and mouse knockouts are associated with reduced immune function yet have otherwise normal physiology Positive clinical experience • BTK inhibitors have shown positive clinical results across a wide range of I&I diseases in hematology, dermatology, and neurology Inhibitors leave room for improvement • The same scaffolding functions that limit efficacy of inhibitors in oncology may also be limiting their efficacy in autoimmune disease settings Key observations underpinning Nurix’s NX-5948 I&I strategy 21 BTK, Bruton’s tyrosine kinase; I&I, inflammation and immunology; LPS, lipopolysaccharide; TLR, toll-like receptor; BCR, B cell receptor; ssRNA/DNA, single-stranded RNA/DNA

Potent Suppression of Myeloid Cell Stimulation NX-5948 More Potently Suppresses Activation of Stimulated B Cells and Myeloid Cells Compared to a Range of BTK Inhibitors 22 Potent Suppression of B Cell Stimulation 0.001 0.01 0.1 1 10 100 1000 -75 -50 -25 0 25 50 75 100 125 Compound (nM) N or m al iz ed C D 69 NX-5948 Rilzabrutinib Remibrutinib Acalabrutinib Fenebrutinib Bead-bound anti-IgM stimulation 0.001 0.01 0.1 1 10 100 1000 0.00 0.25 0.50 0.75 1.00 1.25 Compound (nM) IL -8 F ol d C ha ng e NX-5948 Rilzabrutinib Remibrutinib Acalabrutinib Fenebrutinib Plate-bound IgG2 stimulation Source: Noviski et al., ACR 2024, November 2024
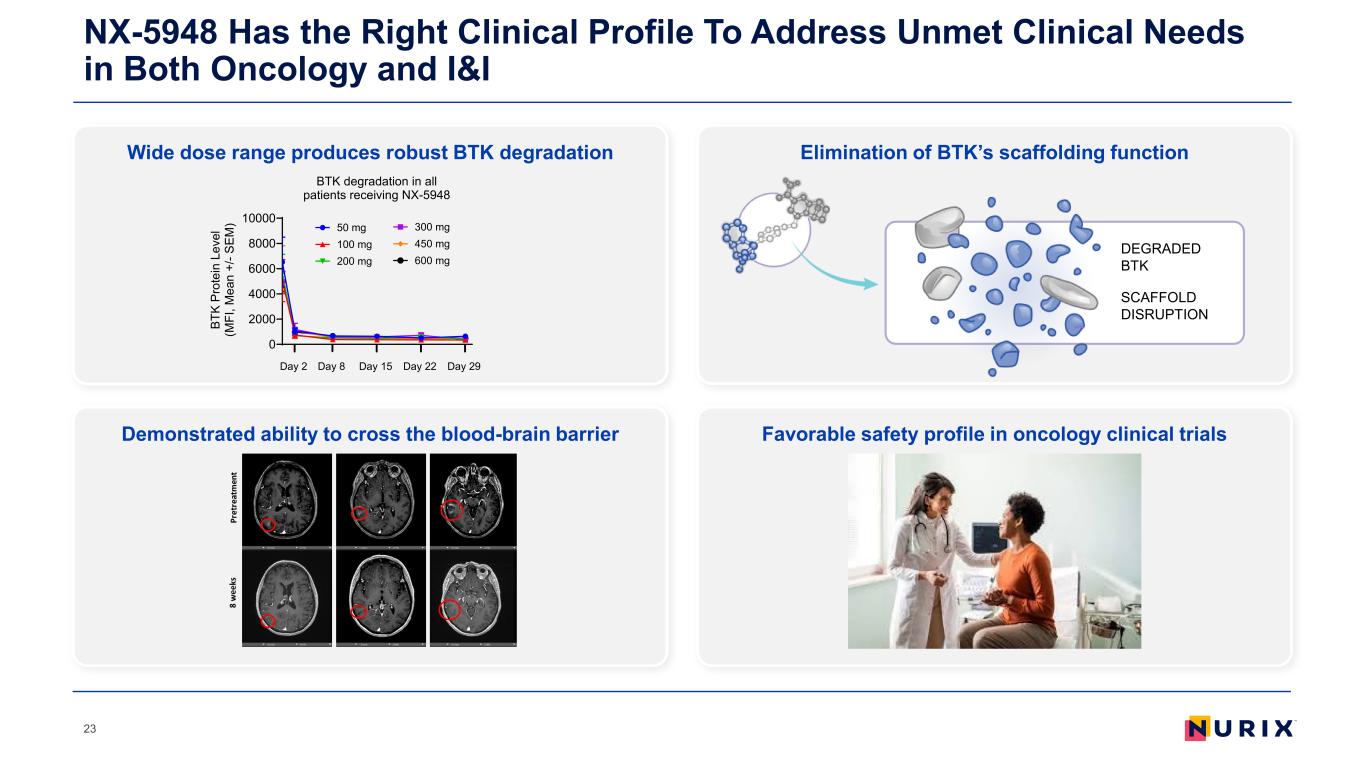
Wide dose range produces robust BTK degradation NX-5948 Has the Right Clinical Profile To Address Unmet Clinical Needs in Both Oncology and I&I 23 Elimination of BTK’s scaffolding function Demonstrated ability to cross the blood-brain barrier Favorable safety profile in oncology clinical trials 0 2000 4000 6000 8000 10000 BTK degradation in all patients receiving NX-5948 BT K Pr ot ei n Le ve l (M FI , M ea n +/ - S EM ) Day 2 Day 15 Day 22 Day 29Day 8 50 mg 100 mg 200 mg 300 mg 450 mg 600 mg DEGRADED BTK SCAFFOLD DISRUPTION

Nurix’s Systematic Approach To Expand Development of NX-5948 Across Multiple I&I Indications Next Steps: 1. Plan to open a new Phase 1b cohort for patients with CLL and associated autoimmune hemolytic anemia in H1 2025 2. Plan non-malignant hematology IND in 2025 for autoimmune cytopenias (e.g., wAIHA) 3. Conduct a healthy volunteer study of a new formulation to address potential need for broader range of doses and dose regimens for I&I indications (study underway) 4. Explore potential for additional indications in other organ systems based on evolving data (e.g., dermatology and neurology) 24

IRAK4 Degrader NX-0479/GS-6791 for the Potential Treatment of Rheumatoid Arthritis and Other Inflammatory Diseases Source: Teng et al., ACR 2024, November 2024 .25 IND anticipated in 2025; Nurix has a co-development and 50/50 profit share option in the United States • IRAK4 is a master regulator of the Toll-like Receptor (TLR) and Interleukin-1 Receptor (IL-1R) signaling pathways • Inappropriate activation of these receptors promotes inflammation and autoimmunity through the release of inflammatory cytokines and chemokines • IRAK4 exhibits both kinase and scaffolding functions • Degradation of IRAK4 achieves more complete blockade of the TLR/IL-14 signaling pathways and yields broader anti-inflammatory effects than inhibition alone PBMC, peripheral blood mononuclear cell; TNF, tumor necrosis factor
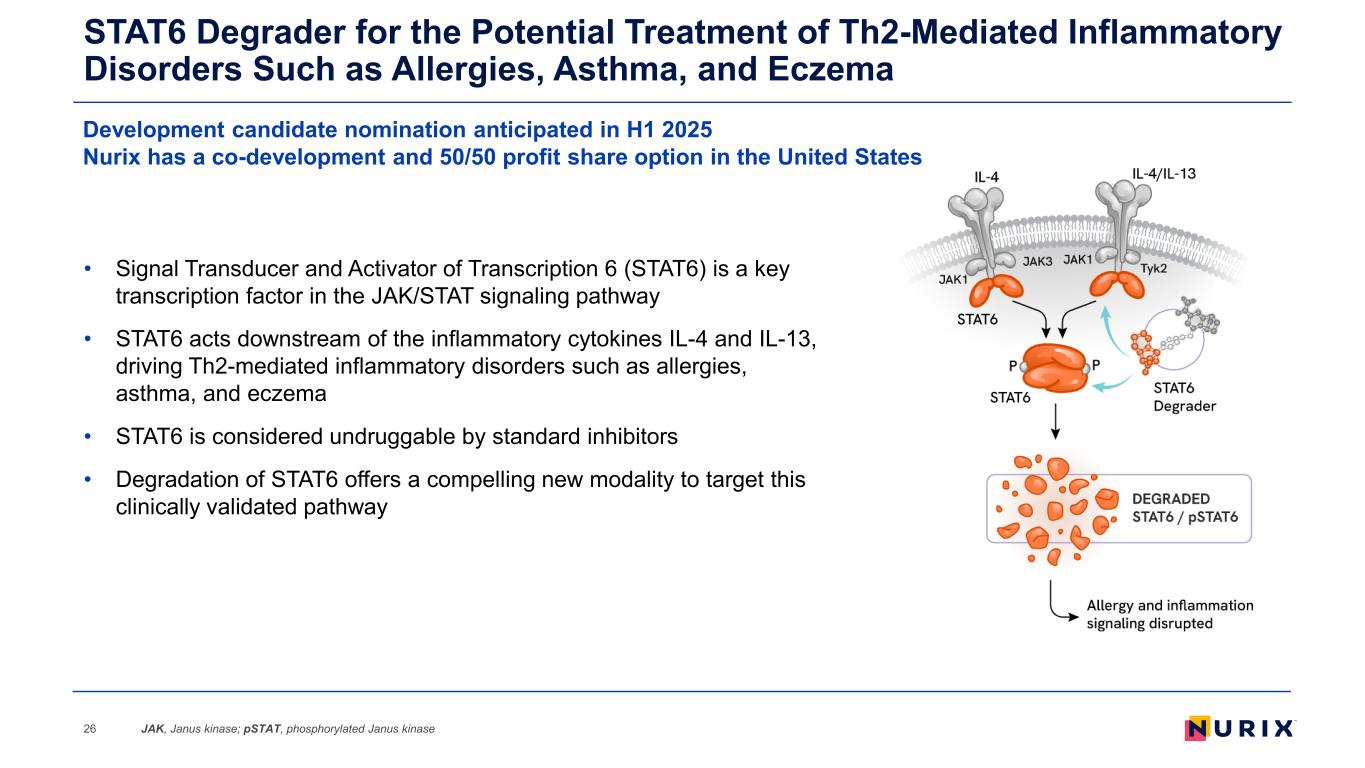
STAT6 Degrader for the Potential Treatment of Th2-Mediated Inflammatory Disorders Such as Allergies, Asthma, and Eczema JAK, Janus kinase; pSTAT, phosphorylated Janus kinase26 • Signal Transducer and Activator of Transcription 6 (STAT6) is a key transcription factor in the JAK/STAT signaling pathway • STAT6 acts downstream of the inflammatory cytokines IL-4 and IL-13, driving Th2-mediated inflammatory disorders such as allergies, asthma, and eczema • STAT6 is considered undruggable by standard inhibitors • Degradation of STAT6 offers a compelling new modality to target this clinically validated pathway Development candidate nomination anticipated in H1 2025 Nurix has a co-development and 50/50 profit share option in the United States

27 DACs represent the next evolution in targeted protein degradation, combining the highly potent and catalytic activity of degraders with the cell and tissue specificity of antibodies Degrader Antibody Conjugates (DACs)

Advancing a New Therapeutic Class • DACs combine the catalytic activity of a degrader with the specificity of an antibody • DACs represent the next generation of antibody drug conjugates (ADCs) Degrader Antibody Conjugates (DACs) 28 Seagen* Deal Terms • $60 million upfront cash payment • $3.4 billion in potential research, development, regulatory and commercial milestone payments • Mid-single to low double-digit percentage tiered royalties on future product sales • Option for U.S. profit sharing and co-promotion on up to two products arising from the collaboration DEGRADER DAC * Seagen is now part of Pfizer
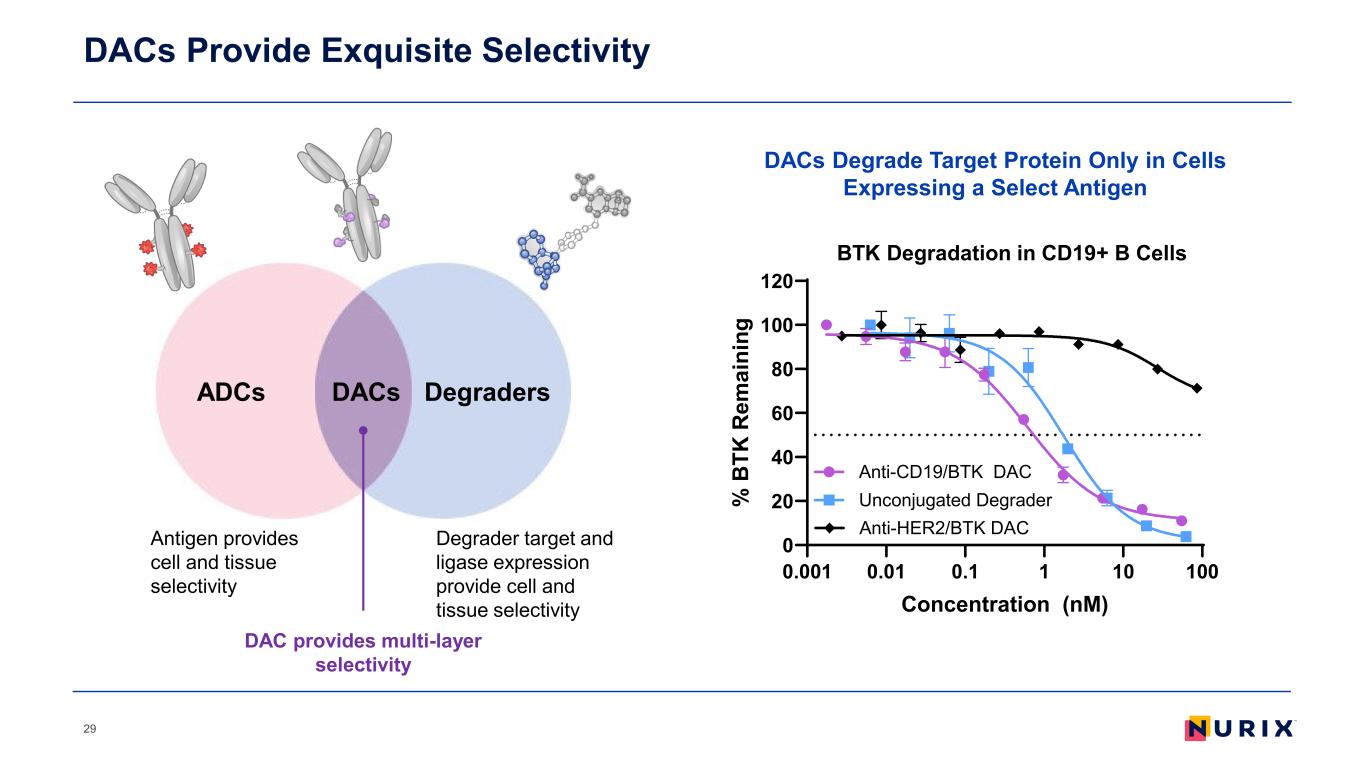
DACs Provide Exquisite Selectivity 29 ADCs DegradersDACs Antigen provides cell and tissue selectivity Degrader target and ligase expression provide cell and tissue selectivity DAC provides multi-layer selectivity DACs Degrade Target Protein Only in Cells Expressing a Select Antigen 0.001 0.01 0.1 1 10 100 0 20 40 60 80 100 120 Concentration (nM) % B TK R em ai ni ng Anti-HER2/BTK DAC Unconjugated Degrader Anti-CD19/BTK DAC BTK Degradation in CD19+ B Cells

Estimated $609.6 million in cash and investments at fiscal year-end 2024, November 30, 2024* Well Funded to Execute on Our Strategy Key initiatives for 2025 • Initiate a suite of clinical trials in 2025 intended to support global registration of NX-5948 for the treatment of patients with chronic lymphocytic leukemia • Expand the development of NX-5948 in additional cancer indications and inflammatory diseases • Advance our portfolio of partnered programs in inflammation and immunology, including degraders of IRAK4 and STAT6 • Advance Nurix’s two other wholly owned clinical-stage assets, NX-2127 and NX-1607 • Invest in our highly productive DEL-AI discovery engine to create and advance novel degrader-based treatments in our wholly owned and partnered portfolios • Maintain a strong cash position with the ability to fund and control our most valuable programs and co-development options 30 Cash runway to fund operations into H1 2027** * The estimated cash and investment amount is a preliminary, unaudited estimate based upon information available to Nurix's management as of the date of this presentation. The amount is subject to the completion of financial closing procedures, including the completion of audit procedures by Nurix’s independent registered public accounting firm, and therefore is subject to adjustment. ** Cash runway guidance is based on Nurix’s current operating plan































