
A New Champion in GI With A One Tract Mind Focused on Rare, Orphan & Unmet Needs April 2020

Forward Looking Statements This presentation includes forward-looking statements including, but not limited to, statements related to our operations and business strategy and the development of drug candidates. The forward-looking statements contained in this press release are based on management’s current expectations and are subject to substantial risks, uncertainty and changes in circumstances. Actual results may differ materially from those expressed by these expectations due to risks and uncertainties, including, among others, those related to our ability to obtain additional capital on favorable terms to us, or at all, the success, timing and cost of our drug development program and our ongoing or future clinical trials, the lengthy and unpredictable nature of the drug approval process, our ability to commercialize our product candidates if approved, statements about the structure, timing and completion of the proposed Merger, the Company’s ability to complete the concurrent capital raise with the merger and the ability of the Company to close the merger and integrate the two companies. These risks and uncertainties include, but may not be limited to, those described in our Form 10-K for the year ended December 31, 2018, our Form 10-Q for the quarter ended September 30, 2019, and in any subsequent filings with the SEC. Forward-looking statements speak only as of the date of this presentation and we undertakenoobligationtoreviewor update any forward-looking statement except as may be required by applicable law. 2
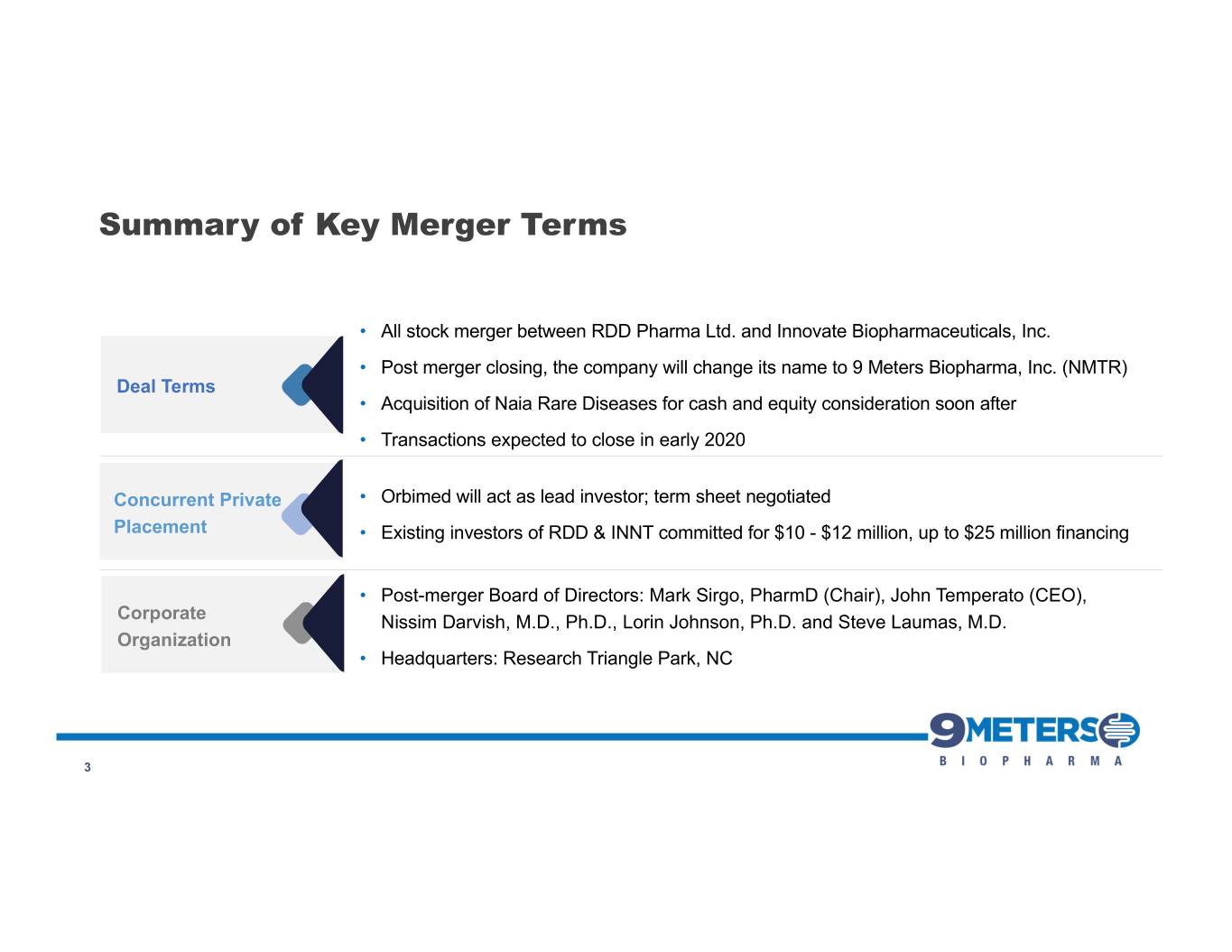
Summary of Key Merger Terms • All stock merger between RDD Pharma Ltd. and Innovate Biopharmaceuticals, Inc. • Post merger closing, the company will change its name to 9 Meters Biopharma, Inc. (NMTR) Deal Terms • Acquisition of Naia Rare Diseases for cash and equity consideration soon after • Transactions expected to close in early 2020 Concurrent Private • Orbimed will act as lead investor; term sheet negotiated Placement • Existing investors of RDD & INNT committed for $10 - $12 million, up to $25 million financing • Post-merger Board of Directors: Mark Sirgo, PharmD (Chair), John Temperato (CEO), Corporate Nissim Darvish, M.D., Ph.D., Lorin Johnson, Ph.D. and Steve Laumas, M.D. Organization • Headquarters: Research Triangle Park, NC 3

Investment Highlights: A Powerful Combination NASDAQ listed company focused on rare, orphan and unmet needs in gastrointestinal disorders NM-002: Novel therapeutic approach for Short Bowel Syndrome, an underserved, debilitating orphan disease with multiple near-term data read-outs Larazotide: First drug to move to into a Phase 3 trial in Celiac Disease with readout in 2021 Focused on increasing shareholder value by creating solutions for GI diseases with high unmet needs Leading institutional investor support 4

Board of Directors* Mark Sirgo, PharmD Chairman John Temperato CEO & Director Nissim Darvish, MD, PhD Director Lorin Johnson, PhD Director Steve Laumas, MD Director * *Ongoing Board after merger close 5

Diverse Pipeline Led by Programs in Celiac Disease and Short Bowel Syndrome Program Class (Route) Indication Type R & D Phase 1 Phase 2 Phase 3 Rights Next Update Tight Junction Topline readout Celiac Larazotide Regulator NCEPhase 3 Ongoing Global Phase 3 CeD study Disease (Oral; Gut Restricted) 2H’21 NM-002 Initiate Phase 1b/2a Long-Acting GLP-1 Short Bowel (Formerly NCEPhase 1b/2a Orphan (US) Global SBS Study (Injectable) Syndrome NB-1001) 2H’20 NM-003 Indication selection of Long-Acting GLP-2 TBD Entering (Formerly NCE Global GLP-2 (Injectable) Post Merger Phase 1 NB-1002) 2H’20 NM-004 Global (excludes Indication selection of Immunomodulator TBD Orphan (US) (Formerly NCE Phase 2a Ready Asia; includes Immunomodulator (Oral; Gut Restricted) Post Merger Pediatric UC INN-108) Japan) 2H’20 Fecal NM-005 ⍺1/⍺2 Receptor Incontinence Complex Phase 1 (US)/ Orphan (EU) Phase 1 FI study (Formerly Agonist Global in Spinal 505(b)(2) Entering Phase 2b (EU) Fast Track (US) 1H’20 RDD-0315) (Intra-anal gel) Cord Injury 6

Multiple Inflection Points Over Next 24 Months 1H 2020 2H 2020 1H 2021 2H 2021 9 Meters Biopharma, Inc. Initiate Phase 1/2 SBS Top-line results Phase 1/2 Start Phase 3 SBS fully integrated post Study (NM-002) SBS (NM-002) Study (NM-002) mergers with Innovate and Initiate monetization of Interim look: Phase 3 Top-line larazotide Naia Rare Diseases non-core assets larazotide in Celiac Phase 3 readout Celiac R & D Investor Day Disease Disease Indication selection of GLP-2 agonist (NM-003) Initiate RoW partnering Indication selection of process in SBS (NM-002) Immunomodulator Non-dilutive financing for (NM-004) 2nd Celiac Disease Phase 3 7

Proforma Cap Structure Post-Merger • Shares outstanding ‒ INNT (incl. all options): ~46M ‒ RDD (newly issued shares): ~44M ‒ INNT warrants (as exercised): ~15M Fully Diluted shares: ~105M* *Does not include concurrent financing; additionally $2.85 M distributed to NAIA as part of the upfront for the acquisition not included 8

Short Bowel Syndrome (SBS) Novel approach using a Long-Acting GLP-1 Agonist (NM-002; Orphan Designation)

Short Bowel Syndrome (SBS) is a Debilitating Orphan Disease Orphan disease (orphan designation granted) with a large underserved market Affects up to 20,000 people in the U.S. with similar prevalence in Europe1,2 Normal SBS patient Length of gastrointestinal tract Length of gastrointestinal tract5 Severe disease with life changing consequences ~ 9.0 m /~ 30ft < 2.0 m /~ 6.5 ft Impaired intestinal absorption, diarrhea & metabolic complications3 Life-long dependency on Total Parenteral Nutrition (TPN) Complex parenteral support to survive with risk of life-threatening infections & extra-organimpairment4 Takeda/Shire’s GATTEX® TTM sales of $590 million Safety concerns administered under REMS program Shire acquired NPS Pharma for $5.2 billion in 2015 Sold via specialty pharmacy distribution model 1Jeppesen P. Expert Opin Orphan Drugs; 1:515-25; 2Transparency Market Research; Short Bowel Syndrome Market, 2017; 3Amiot A et al. Clin Nutr 2013;32:368–74; Boland E et al. Am J Surg 2010;200:690–3; 4Torres C. Current Paediatr 2006;16:291–7; Bielawska B. Nutrients 2017;9:466–79; Pironi L et al. Clin Nutr 2016;352:247–307; Hofstetter S et al. Curr Med Res Opin 2013;29:495–504 10

New Approach to SBS with Long-Acting GLP-1 Agonist Long Acting GLP-1 (exenatide) licensed from Amunix • Developed specifically for SBS patients & gastric effects • Dosing twice monthly; potentially monthly • Different from GLP-1s used in diabetes & pancreatic effects • Potentially lowers drug exposure Companies using Amunix long-acting technology in development: Lilly, Janssen, Roche, Biogen Phase 1b Study: 70 patients with diabetes; Placebo controlled, single dose ascending study – 12.5 mg to 200 mg showed no safety concerns Amunix patent portfolio covers product out to mid 2030s • GLP-1 agonist therapy slowed gut transit • Significantly reduced bowel movements • Some patients discontinued total parenteral nutrition (TPN)1 • Existing treatments including parenteral nutrition, surgical techniques and growth factors (Zorptive® (somatropin); Gattex® (teduglutide)) all have key limitations 1Kunkel, D., Basseri, B., Low, K., Lezcano, S., Soffer, E.E., Conklin, J.L., Mathur, R. and Pimentel, M., 2011. Efficacy of the glucagon-like peptide-1 agonist exenatide in the treatment of short bowel syndrome. Neurogastroenterology & Motility, 23(8), pp.739-e328. 11

Key Advantages of GLP-1 vs GLP-2 in SBS Long-acting GLP-1 (NM-002) GLP-2 Analogues Frequency of Dose Twice monthly; potentiallymonthly Daily injection to once-weekly forms GLP-1 class AEs mild and tolerable; potentially Safety REMS: Neoplastic growth warning (trophic) less due to lower exposure • Predicted decrease of TPN volume by > 50% • Decrease TPN volume by > ~20% Efficacy • ≥ 50% discontinuation of TPN • ~5% d/c of TPNper trials Benefits potentially within first week b/c of Onset of Action Benefits in weeks to months (GATTEX® ~ 8 weeks) GLP-1 mechanism NM-002 is a long-acting GLP-1 NCE uniquely designed to take advantage of a gastric effect in SBS patients; other GLP-1 agonists have sequence changes optimized for pancreatic effects in diabetic populations. 12

NM-002 GI Safety in Diabetic Patients (P1 Single-Dose Study) Gastrointestinal Side Effect 12.5 mg 25 mg 50 mg 100 mg 150 mg 200 mg PBO Overall Reported (n=8) (n=10) (n=9) (n=8) (n=9) (n=8) (n=18) (n=70) Nausea 0 1 (10%) 1 (11%) 2 (25%) 8 (89%) 5 (63%) 2 (11%) 19 (27%) Dyspepsia 0 0 0 1 (13%) 1 (11%) 1 (13%) 0 3 (4%) Vomiting 0 0 0 1 (13%) 4 (44%) 4 (50%) 1 (6%) 10 (14%) Constipation 0 0 0 0 3 (33%) 0 0 3 (4%) Eructation (Belching) 0 0 0 0 2 (22%) 1 (13%) 0 3 (4%) Diarrhea 0 0 0 0 1 (11%) 0 3 (17%) 4 (6%) Epigastric Discomfort 0 0 0 0 1 (11%) 2 (25%) 0 3 (4%) There were no serious adverse events reported and no patients were withdrawn from the study due to adverse events. The majority of adverse events (173 of 227 [76%]) were mild (Grade 1) in severity. No adverse events of Grade 3 or greater (severe or above) were reported during the study. Source: Cleleand, J et. al. Diabetologia (2012) 55:[Suppl1]S1–S538; Abstract #821. pp. s338-339; Data on file 13

Proof-of-Concept of Exenatide (GLP-1 agonist) in SBS Patients • Five patients with SBS based on <90 cm of small bowel and clinical evidence of nutritional deprivation selected • Each patient started on 5µg dose of exenatide BID and continued over the following month, baseline parameters repeated • The subjects consisted of four males and one female, aged 46–69 years • At baseline, all had severe diarrhea (from 6 to 15 bowel movements per day) often within minutes of eating • Post exenatide, all patients had improvement in bowel frequency and form and no longer meal-related • Total parenteral nutrition was stopped successfully in three patients • Antroduodenal manometry revealed continuous low amplitude gastric contractions during fasting which completely normalized with exenatide 14

Phase 1b/2a SBS Proposed Clinical Study Design 7 Day Safety 7 Day Safety 7 Day Safety Assessment Assessment Cohort 2 Assessment Cohort 1 (50 mg) (100 mg) n= 2 to 5 n=2 to 5 Cohort 1 (50 mg) Total n = 12 to 30 Screening n= 2 to 5 Cohort 2 (100 mg) n=2 to 5 Cohort 3 Cohort 3 (150 mg) (150 mg) n=2 to 5 n=2 to 5 The primary objectives of the study are: • To assess the safety and tolerability of three doses of NM-002 in patients with SBS • Determine whether there is any change in urine output over two 48-hour periods post-dose (days 2-3, 4-5 and days 16-17, 18-19) compared to baseline 15

Comparable Deals/Companies in SBS Two companies have been acquired with GLP-2 analogues for SBS; a third public company, Zealand (NASDAQ:ZEAL), is developing a GLP-2 analogue for SBS October 2015 May 2019 August 2017 Acquires for $340 million upfront+ $470 Approved for SBS in 2012 million CVRs (2019); GLP-2 asset for Raised $78 million by August 2017 SBS in spin-off (VectivBio) Acquired NPS for $5.2 Billion Acquires post $60 million mezzanine Current Market Cap* financing in 2018 led by Novo Ventures ~$1.2 Billion (Apraglutide (GLP-2): Phase 1 complete for SBS) (Glepaglutide (GLP-2)in Phase 3 for SBS) *Mkt cap as of April 30, 2020 16

Celiac Disease Larazotide: Oral, Non-Absorbable, Tight-Junction Regulator

Celiac Disease: Autoimmune Disorder with a Genetic Link Triggered by dietary gluten • Intestinal epithelia barrier leakiness leads to “Intestinal- Inflammatory Loop” • Eventually, intestinal surface (villi) become atrophied Genetic Link • Worldwide prevalence of around 1% and on the rise1 GI Abdominal Domain Symptoms ‒ Celiac patients have a specific HLA class II gene variant2 . HLA-DQ2 (~95%) or, Abdominal Pain Abdominal Cramps . HLA-DQ8 (5%)2 Bloating Gas • Genome-Wide Assoc. Studies (GWAS) link disease to four genes involved with regulation of tight junctions3 US & EU ~1% Gluten Free Diet (GFD) is the only therapy Prevalence US ~3.2 million • Nutritional imbalances EU ~3.5 million • Cost burden to patients ROW ~15 million 1Schuppan and Dieterich, UpTo Date (2018) 2Fasano, A. Genetics of Celiac Disease ( Nov 2016) https://emedicine.medscape.com/article/1790189-overview 3 Withoff, S., Li, Y., Jonkers, I. and Wijmenga, C., 2016. Trends in Genetics, 32(5), pp.295-308 18

Larazotide Normalizes Intestinal Barrier in Celiac Disease Preventing Antigen Trafficking Larazotide is an 8 natural amino acid peptide which normalizes disrupted tight junctions (IFN α, IFN γ, TNF, IL 4, IL 5 and IL 21) Adapted from: Withoff, S., Li, Y., Jonkers, I. and Wijmenga, C., 2016. Understanding celiac disease by genomics. Trends in Genetics, 32(5), pp.295-308 19

Lack of Absorption → Unique Pharmacology as Cleaved Fragment Accumulates, it Inhibits Larazotide Brush Border Peptidase Cleaves Larazotide to a Translates to Clinically Relevant Doses 6-mer (Fragment 2) in Clinical Trials Porcine Intestine Ussing Chamber studies showed larazotide normalizes tight junctions while the addition of the 6-mer fragment inhibited larazotide activity 1 μM → 0.25 mg 2 μM → 0.5 mg Rationale For Doses Used in Phase 3 trial Hernandez L, Carlson et al.. Digestive Diseases Week 2018; SA1183 20
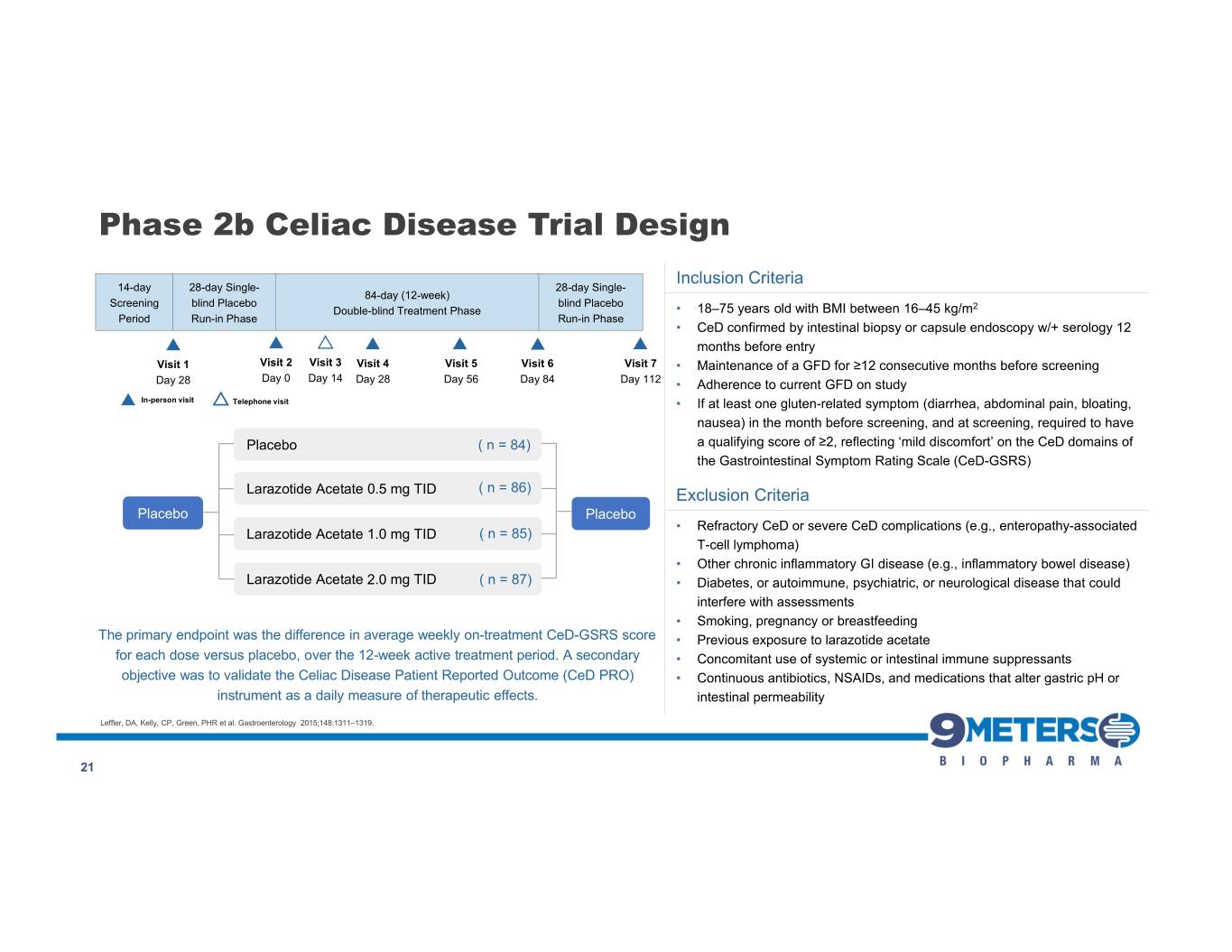
Phase 2b Celiac Disease Trial Design Inclusion Criteria 14-day 28-day Single- 28-day Single- 84-day (12-week) Screening blind Placebo blind Placebo Double-blind Treatment Phase • 18–75 years old with BMI between 16–45 kg/m2 Period Run-in Phase Run-in Phase • CeD confirmed by intestinal biopsy or capsule endoscopy w/+ serology 12 months before entry Visit 1 Visit 2 Visit 3 Visit 4 Visit 5 Visit 6 Visit 7 • Maintenance of a GFD for ≥12 consecutive months before screening Day 28 Day 0 Day 14 Day 28 Day 56 Day 84 Day 112 • Adherence to current GFD on study In-person visit Telephone visit • If at least one gluten-related symptom (diarrhea, abdominal pain, bloating, nausea) in the month before screening, and at screening, required to have Placebo ( n = 84) a qualifying score of ≥2, reflecting ‘mild discomfort’ on the CeD domains of the Gastrointestinal Symptom Rating Scale (CeD-GSRS) Larazotide Acetate 0.5 mg TID ( n = 86) Exclusion Criteria Placebo Placebo • Refractory CeD or severe CeD complications (e.g., enteropathy-associated Larazotide Acetate 1.0 mg TID ( n = 85) T-cell lymphoma) • Other chronic inflammatory GI disease (e.g., inflammatory bowel disease) Larazotide Acetate 2.0 mg TID ( n = 87) • Diabetes, or autoimmune, psychiatric, or neurological disease that could interfere with assessments • Smoking, pregnancy or breastfeeding The primary endpoint was the difference in average weekly on-treatment CeD-GSRS score • Previous exposure to larazotide acetate for each dose versus placebo, over the 12-week active treatment period. A secondary • Concomitant use of systemic or intestinal immune suppressants objective was to validate the Celiac Disease Patient Reported Outcome (CeD PRO) • Continuous antibiotics, NSAIDs, and medications that alter gastric pH or instrument as a daily measure of therapeutic effects. intestinal permeability Leffler, DA, Kelly, CP, Green, PHR et al. Gastroenterology 2015;148:1311–1319. 21

PRO Endpoints Show Robust Treatment Effect CeD-GSRS: CeD PRO1 Responder2 Analysis P2b Trial Primary Endpoint for Phase 2b (‘012) Primary Endpoint for Phase 3 p = 0.022 30.0% 28.6% 20.0% 14.3% ____ Treatment Effect 14.3% 10.0% % Responders* 0.0% Placebo 0.50mg Dose 0.5mg 1mg 2mg n=84 for Placebo and n=84 for 0.5 mg dose CeD GSRS1 p values 0.022 0.900 0.590 Positive Phase 2b with Statistically Significant Treatment effect > than approved IBS brands with Phase 3 PROs p Value at Therapeutic Dose (Xifaxan®, Viberzi®, Linzess®, Amitiza®, and Trulance®) 1CeD PRO Abdominal Domain is the agreed upon endpoint for phase 3 with the FDA. The CeD PRO was pre-specified & an exploratory endpoint in the Phase 2b Study 2Responder ≈Subject has 50% improvement vs. baseline CeD-PRO abdominal score (6/12 weeks) Leffler, DA, Kelly, CP, Green, PHR et al. Gastroenterology 2015;148:1311–1319 FDA Drug Labels for Xifaxan® (Salix/Bausch), Viberzi® (Allergan), Linzess® (Allergan/Ironwood), Amitiza® (Takeda/Sucampo) and Trulance® (Synergy/Salix/Bausch) 22

GI Safety of Larazotide in Celiac Patients (Phase 2b Study) Gastrointestinal Side Effect Reported PBO (n=84) 0.5 mg (n=85) 1 mg (n=84) 2 mg (n=87) Overall (n=256) Diarrhea 7 (8.3%) 7 (8.2%) 5 (6.0%) 8 (9.2%) 20 (7.8%) Nausea 9 (10.7%) 4 (4.7%) 7 (8.3%) 7 (8.0%) 18 (7.0%) Constipation 2 (2.4%) 3 (3.5%) 7 (8.3%) 5 (5.7%) 15 (5.9%) Abdominal pain 4 (4.8%) 1 (1.2%) 5 (6.0%) 6 (6.9%) 12 (4.7%) Abdominal pain upper 2 (2.4%) 3 (3.5%) 4 (4.8%) 2 (2.3%) 9 (3.5%) Flatulence 0 2 (2.4%) 4 (4.8%) 3 (3.4%) 9 (3.5%) Gastroesophageal reflux disease 0 2 (2.4%) 1 (1.2%) 6 (6.9%) 9 (3.5%) Abdominal distension 1 (1.2%) 2 (2.4%) 5 (6.0%) 1 (1.1%) 8 (3.1%) Vomiting 6 (7.1%) 2 (2.4%) 2 (2.4%) 3 (3.4%) 7 (2.7%) Dyspepsia 3 (3.6%) 1 (1.2%) 2 (2.4%) 2 (2.3%) 5 (2.0%) Source: Larazotide phase 2b study report; Data on file 23
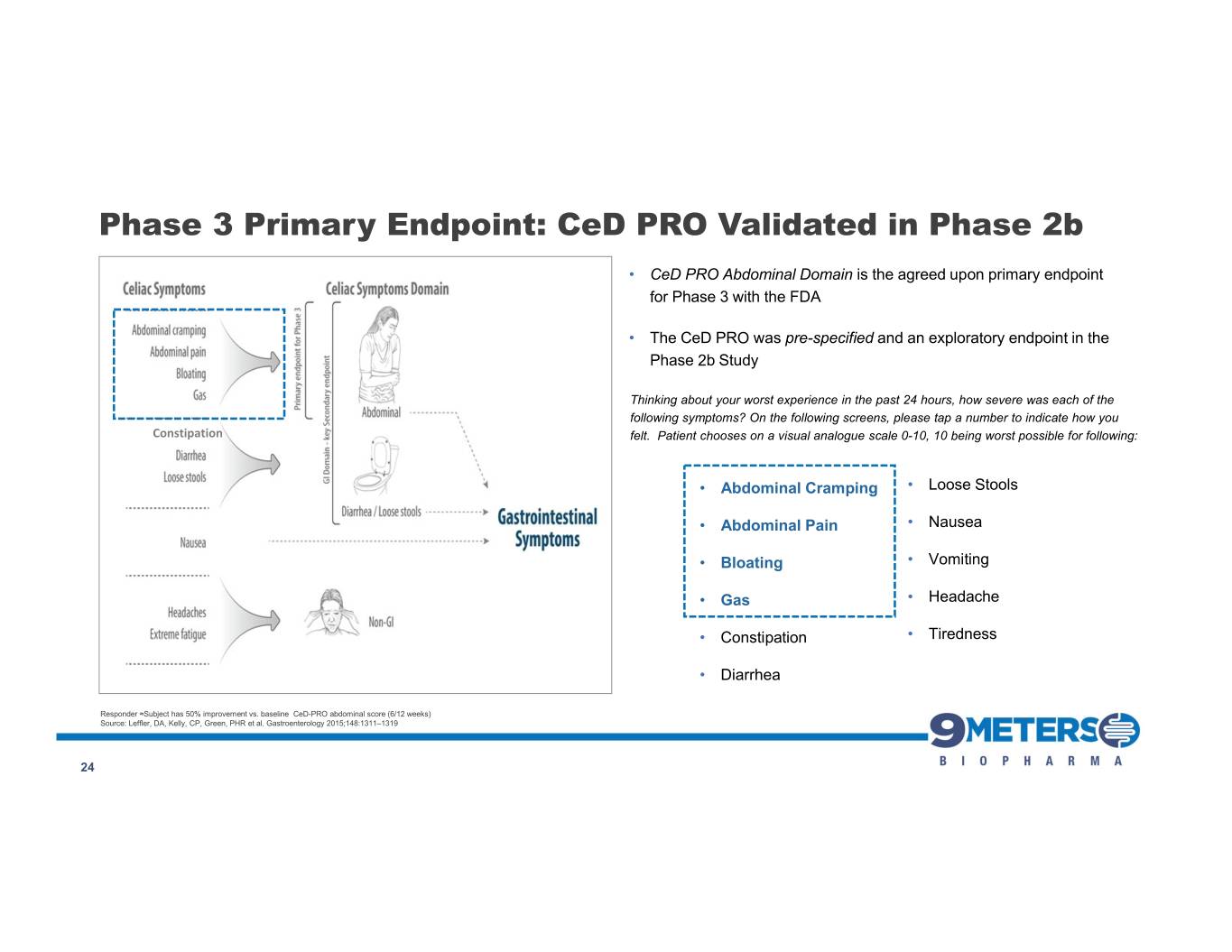
Phase 3 Primary Endpoint: CeD PRO Validated in Phase 2b • CeD PRO Abdominal Domain is the agreed upon primary endpoint for Phase 3 with the FDA • The CeD PRO was pre-specified and an exploratory endpoint in the Phase 2b Study Thinking about your worst experience in the past 24 hours, how severe was each of the following symptoms? On the following screens, please tap a number to indicate how you Constipation felt. Patient chooses on a visual analogue scale 0-10, 10 being worst possible for following: • Abdominal Cramping • Loose Stools • Abdominal Pain • Nausea • Bloating • Vomiting • Gas • Headache • Constipation • Tiredness • Diarrhea Responder ≈Subject has 50% improvement vs. baseline CeD-PRO abdominal score (6/12 weeks) Source: Leffler, DA, Kelly, CP, Green, PHR et al. Gastroenterology 2015;148:1311–1319 24
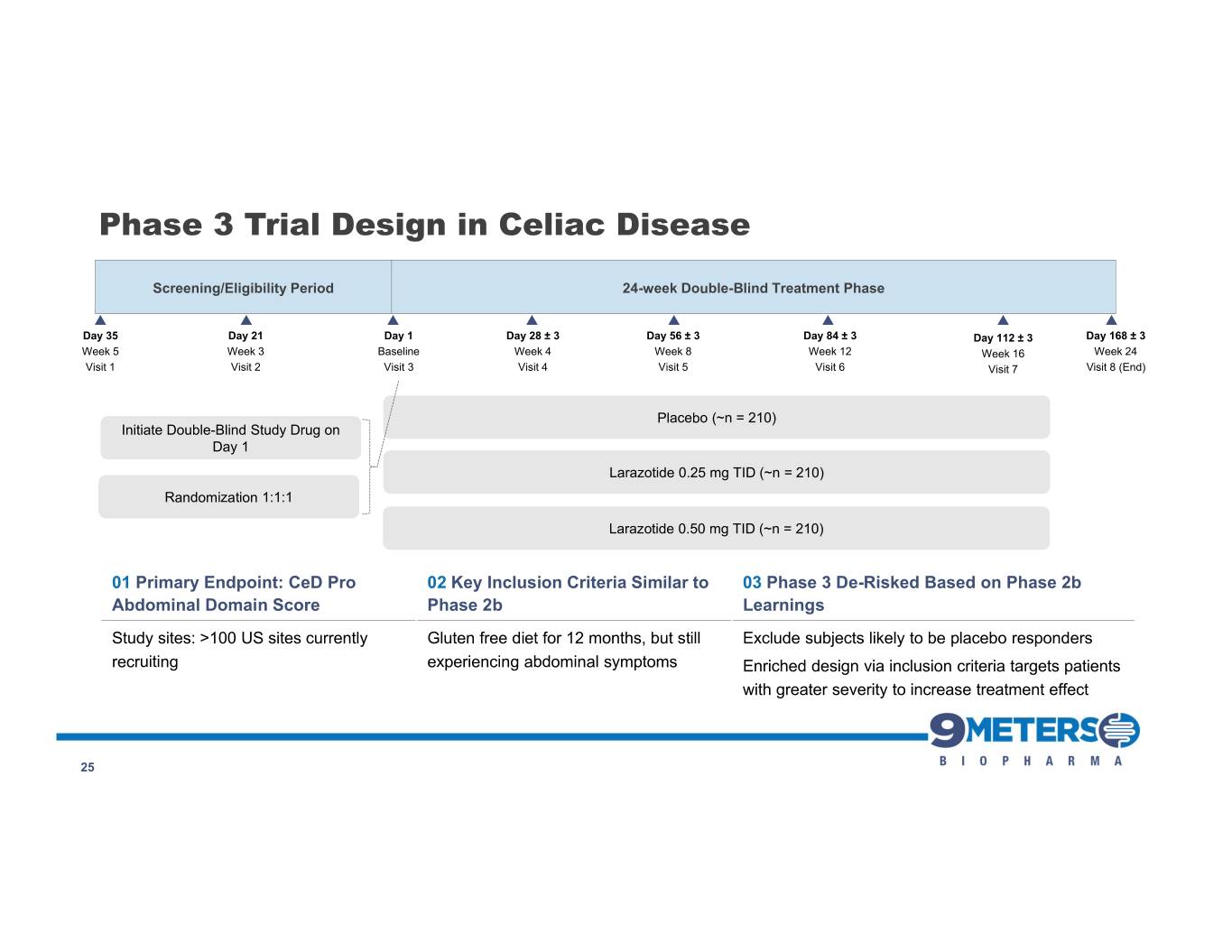
Phase 3 Trial Design in Celiac Disease Screening/Eligibility Period 24-week Double-Blind Treatment Phase Day 35 Day 21 Day 1 Day 28 ± 3 Day 56 ± 3 Day 84 ± 3 Day 112 ± 3 Day 168 ± 3 Week 5 Week 3 Baseline Week 4 Week 8 Week 12 Week 16 Week 24 Visit 1 Visit 2 Visit 3 Visit 4 Visit 5 Visit 6 Visit 7 Visit 8 (End) Placebo (~n = 210) Initiate Double-Blind Study Drug on Day 1 Larazotide 0.25 mg TID (~n = 210) Randomization 1:1:1 Larazotide 0.50 mg TID (~n = 210) 01 Primary Endpoint: CeD Pro 02 Key Inclusion Criteria Similar to 03 Phase 3 De-Risked Based on Phase 2b Abdominal Domain Score Phase 2b Learnings Study sites: >100 US sites currently Gluten free diet for 12 months, but still Exclude subjects likely to be placebo responders recruiting experiencing abdominal symptoms Enriched design via inclusion criteria targets patients with greater severity to increase treatment effect 25

Investment Highlights: A Powerful Combination NASDAQ listed company focused on rare, orphan and unmet needs in gastrointestinal disorders NM-002: Novel therapeutic approach for Short Bowel Syndrome, an underserved, debilitating orphan disease with multiple near-term data read-outs Larazotide: First drug to move to into a Phase 3 trial in Celiac Disease with readout in 2021 Focused on increasing shareholder value by creating solutions for GI diseases with high unmet needs Leading institutional investor support 26

Thank You John Temperato President & CEO jtemperato@9meters.com Edward Sitar Chief Financial Officer esitar@9meters.com Sireesh Appajosyula, PharmD SVP, Corporate Development & Operations sireesh@9meters.com


























