
Positive Phase 1b/2a Topline Data in Short Bowel Syndrome -Rapid onset and sustained clinical effect following first dose in all 9 patients in total stool output (TSO) volume -Clinically relevant improvements in TSO volume and bowel movement frequency -Twice-monthly fixed-dosing regimen exhibited an excellent safety and tolerability profile -Plan to meet with FDA as soon as possible in the first quarter of 2021 to discuss next steps for clinical development of NM-002 Conference Call December 7, 2020

Forward Looking Statements This presentation includes forward-looking statements based upon the Company's current expectations. Forward-looking statements involve risks and uncertainties, and include, but are not limited to, the development and commercial potential and potential benefits of any product candidates of the Company; anticipated preclinical and clinical drug development activities, including initiation, enrollment, completion and related timelines and the expected timing for data and other clinical and preclinical results; the potential effects of the ongoing coronavirus outbreak and related mitigation efforts on the Company's clinical, financial and operational activities; the Company's continued listing on Nasdaq; expectations regarding future financings; the future operations of the Company; the nature, strategy and focus of the Company; the Company having sufficient resources to advance its pipeline; and any other statements that are not historical fact. Actual results and the timing of events could differ materially from those anticipated in such forward-looking statements as a result of these risks and uncertainties, which include, without limitation: (i) uncertainties associated with the clinical development and regulatory approval of product candidates; (ii) risks related to the inability of the Company to obtain sufficient additional capital to continue to advance these product candidates and its preclinical programs; (iii) uncertainties in obtaining successful clinical results for product candidates and unexpected costs that may result therefrom; (iv) risks related to the failure to realize any value from product candidates and preclinical programs being developed and anticipated to be developed in light of inherent risks and difficulties involved in successfully bringing product candidates to market; (v) the impact of COVID-19 on our operations, clinical trials or future financings and (vi) risks associated with the possible failure to realize certain anticipated benefits of the Company's recent merger and the Naia acquisition, including with respect to future financial and operating results. Actual results and the timing of events could differ materially from those anticipated in such forward-looking statements because of these risks and uncertainties. These and other risks and uncertainties are more fully described in periodic filings with the SEC, including the factors described in the section entitled "Risk Factors" in the Company's. Annual Report on Form 10-K for the year ended December 31, 2019, Form 10-Q for the quarter ended September 30, 2020 and in other filings that the Company has made and future filings the Company will make with the SEC. You should not place undue reliance on these forward-looking statements, which are made only as of the date hereof or as of the dates indicated in the forward-looking statements. The Company expressly disclaims any obligation or undertaking to release publicly any updates or revisions to any forward-looking statements contained herein to reflect any change in its expectations with regard thereto or any change in events, conditions or circumstances on which any such statements are based. 2

Short Bowel Syndrome Background & Current Paradigm

4 1Jeppesen P. Expert Opin Orphan Drugs; 1:515-25; 2Transparency Market Research; Short Bowel Syndrome Market, 2019; 3Amiot A et al. Clin Nutr 2013;32:368–74; Boland E et al. Am J Surg 2010;200:690–3; 4Bielawska B. Nutrients 2017;9:466–79; Pironi L et al. Clin Nutr 2016;352:247–307; Hofstetter S et al. Curr Med Res Opin 2013;29:495–504; 5. https://www.beckershospitalreview.com/pharmacy/20-most-expensive-drugs-in-the-us.html accessed Nov. 30, 2020 Normal Length of GI Tract ~ 9.0 m / ~ 30 ft SBS Patient Length of GI Tract Significantly Shortened Short Bowel Syndrome (SBS) is a Debilitating Orphan Disease • Orphan disease (orphan designation granted) with an underserved market • Affects up to 20,000 people in the US with similar prevalence in EU1,2 • Severe disease characterized by a lack of gut motility with significant impact on quality of life • Impaired intestinal absorption, diarrhea & metabolic complications3 • Limited treatment options with dependency on parenteral support (PS) • Complex and costly parenteral nutritional support to survive; risk of life- threatening infections & extra-organ impairment4 • Gattex® (teduglutide) is a GLP-2 analogue approved in US in 2012 • ~1,400 patients under management WW • ~$600M in global sales in 2019/2020 • One of top 10 most expensive medicine in US in 2020 ~~$40,000/month)5
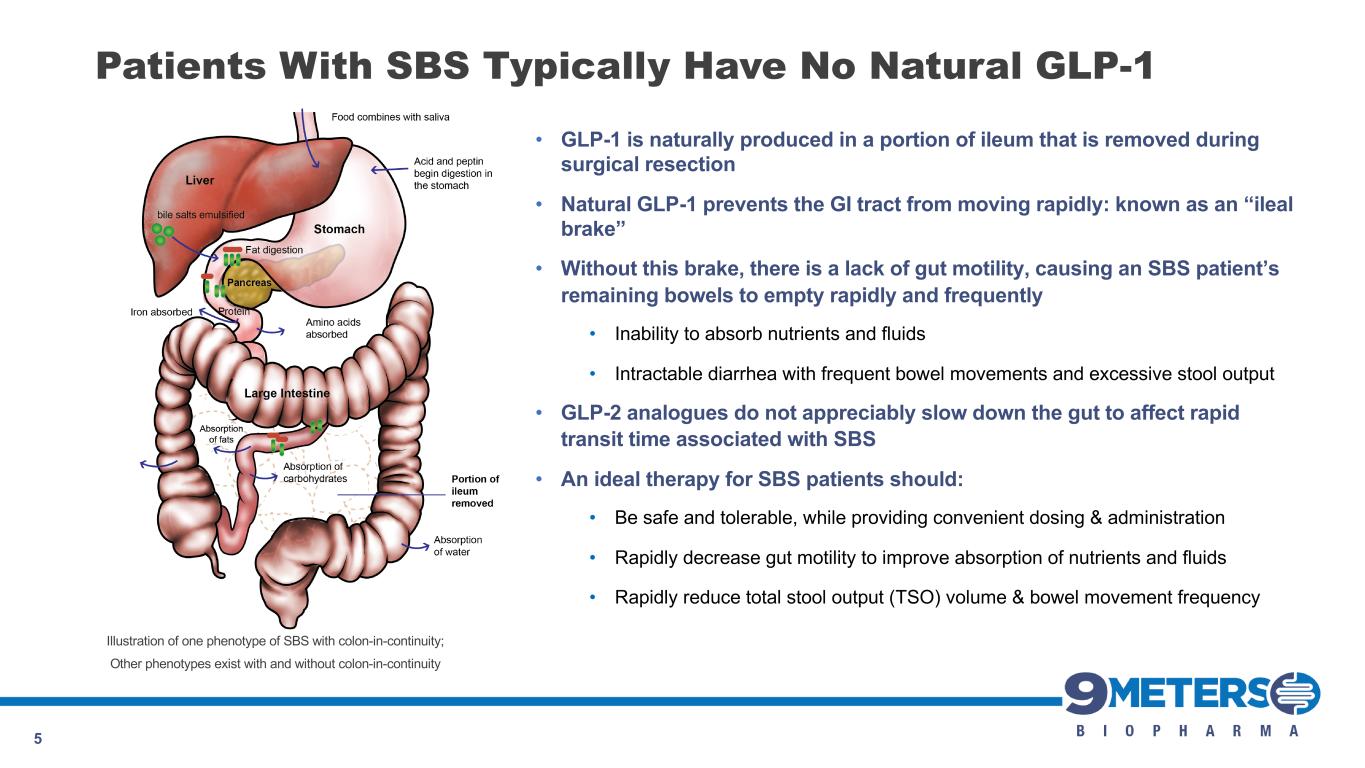
5 Patients With SBS Typically Have No Natural GLP-1 • GLP-1 is naturally produced in a portion of ileum that is removed during surgical resection • Natural GLP-1 prevents the GI tract from moving rapidly: known as an “ileal brake” • Without this brake, there is a lack of gut motility, causing an SBS patient’s remaining bowels to empty rapidly and frequently • Inability to absorb nutrients and fluids • Intractable diarrhea with frequent bowel movements and excessive stool output • GLP-2 analogues do not appreciably slow down the gut to affect rapid transit time associated with SBS • An ideal therapy for SBS patients should: • Be safe and tolerable, while providing convenient dosing & administration • Rapidly decrease gut motility to improve absorption of nutrients and fluids • Rapidly reduce total stool output (TSO) volume & bowel movement frequency Illustration of one phenotype of SBS with colon-in-continuity; Other phenotypes exist with and without colon-in-continuity

SBS Patients’ Perspectives on Living with the Disease 6 Patient quotes from NPS market research; Source: SEC 8-k April 2010 NPS Pharmaceuticals Inc. If you want a one-word description [of living with Short-Bowel Syndrome], it’s ’hell’. It changes your whole life, it ruins your life. There are ups and downs – mostly downs. It’s very difficult to manage, very difficult. I’m going so much I have no life. I can’t go anywhere – you go out anywhere you keep going to the bathroom. The bathroom is probably the most annoying thing… I always have plastic bags with me and because my bag will fill up really fast… I’ve emptied it on the subway... If you do it really fast, people have no idea what’s going on, but it’s still a pain to do.

NM-002: Long-Acting GLP-1 Agonist Phase 1b/2a Topline Results
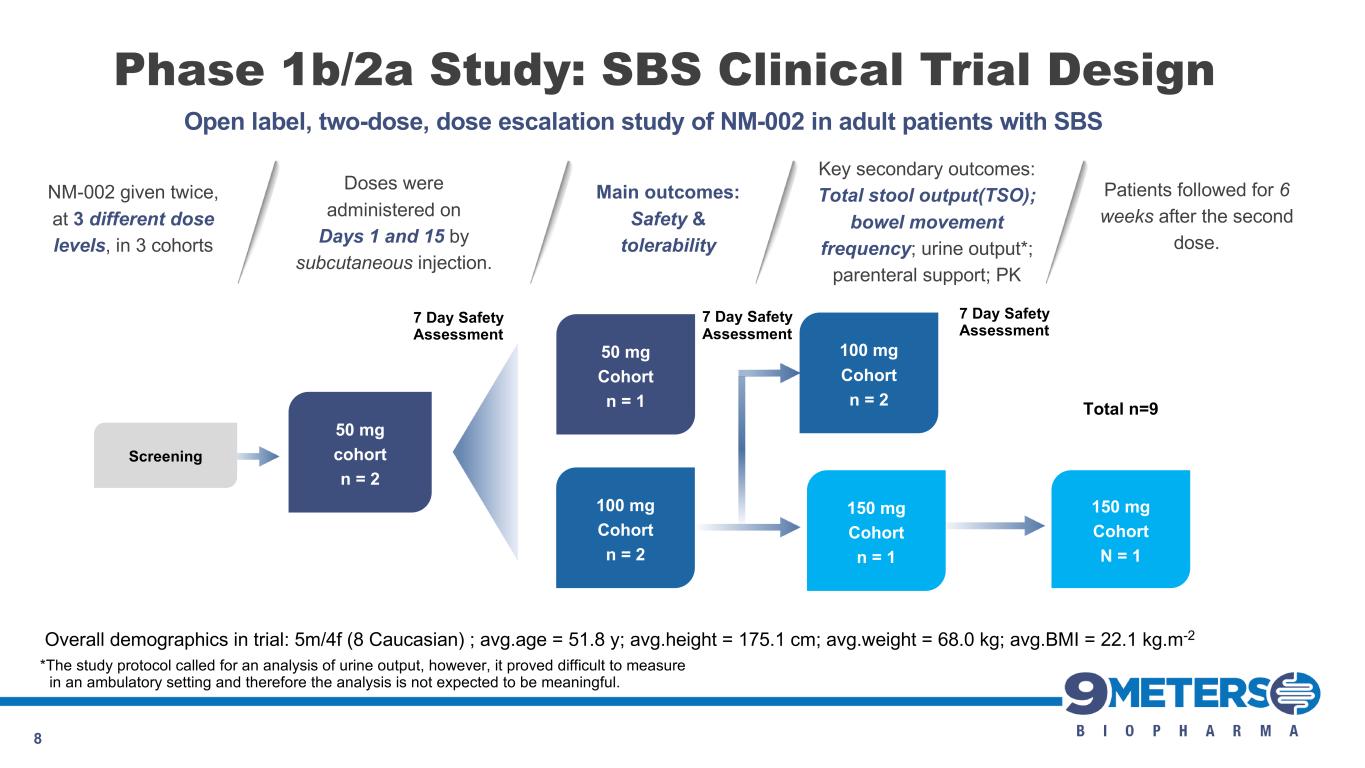
Phase 1b/2a Study: SBS Clinical Trial Design 8 Screening 50 mg cohort n = 2 100 mg Cohort n = 2 50 mg Cohort n = 1 100 mg Cohort n = 2 Total n=9 7 Day Safety Assessment 150 mg Cohort n = 1 150 mg Cohort N = 1 7 Day Safety Assessment 7 Day Safety Assessment Open label, two-dose, dose escalation study of NM-002 in adult patients with SBS NM-002 given twice, at 3 different dose levels, in 3 cohorts Doses were administered on Days 1 and 15 by subcutaneous injection. Patients followed for 6 weeks after the second dose. Main outcomes: Safety & tolerability Key secondary outcomes: Total stool output(TSO); bowel movement frequency; urine output*; parenteral support; PK *The study protocol called for an analysis of urine output, however, it proved difficult to measure in an ambulatory setting and therefore the analysis is not expected to be meaningful. Overall demographics in trial: 5m/4f (8 Caucasian) ; avg.age = 51.8 y; avg.height = 175.1 cm; avg.weight = 68.0 kg; avg.BMI = 22.1 kg.m-2
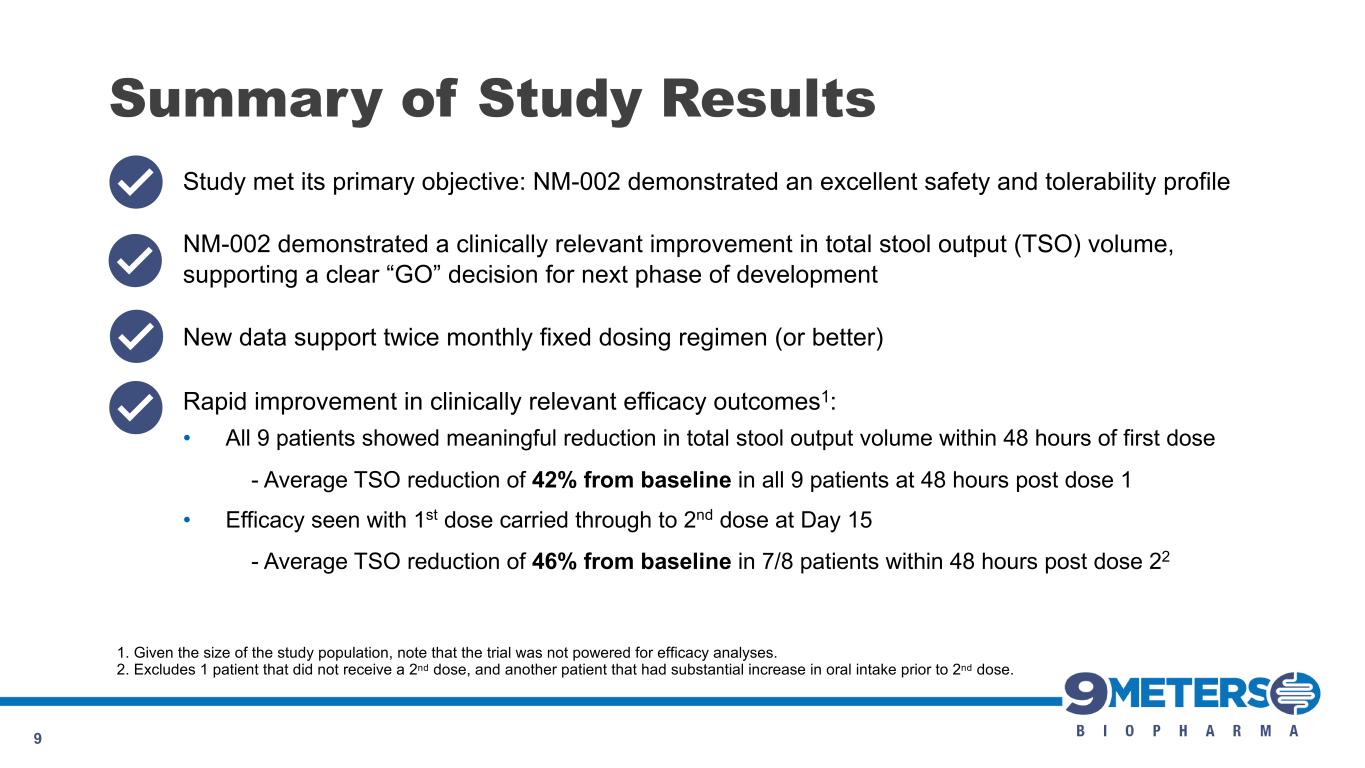
Summary of Study Results 9 Study met its primary objective: NM-002 demonstrated an excellent safety and tolerability profile NM-002 demonstrated a clinically relevant improvement in total stool output (TSO) volume, supporting a clear “GO” decision for next phase of development New data support twice monthly fixed dosing regimen (or better) Rapid improvement in clinically relevant efficacy outcomes1: • All 9 patients showed meaningful reduction in total stool output volume within 48 hours of first dose - Average TSO reduction of 42% from baseline in all 9 patients at 48 hours post dose 1 • Efficacy seen with 1st dose carried through to 2nd dose at Day 15 - Average TSO reduction of 46% from baseline in 7/8 patients within 48 hours post dose 22 1. Given the size of the study population, note that the trial was not powered for efficacy analyses. 2. Excludes 1 patient that did not receive a 2nd dose, and another patient that had substantial increase in oral intake prior to 2nd dose.
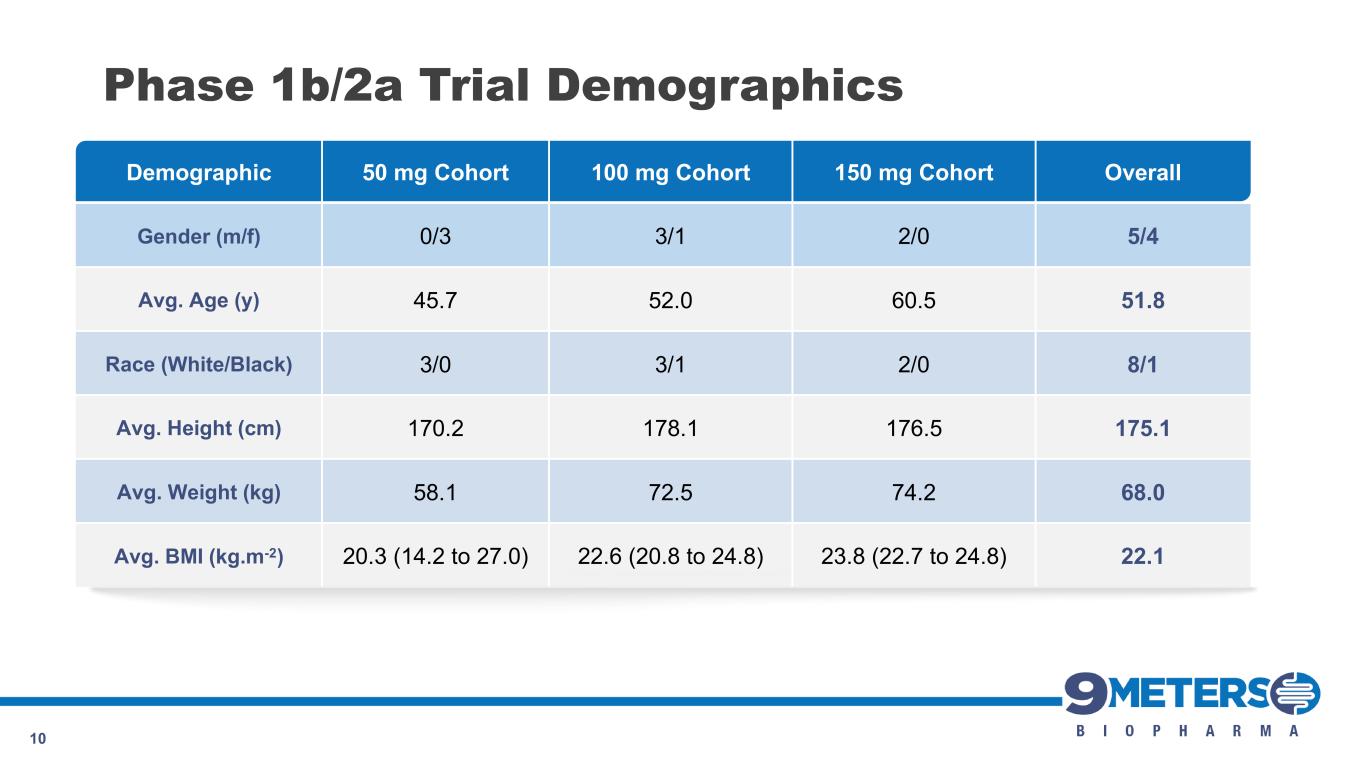
Demographic 50 mg Cohort 100 mg Cohort 150 mg Cohort Overall Gender (m/f) 0/3 3/1 2/0 5/4 Avg. Age (y) 45.7 52.0 60.5 51.8 Race (White/Black) 3/0 3/1 2/0 8/1 Avg. Height (cm) 170.2 178.1 176.5 175.1 Avg. Weight (kg) 58.1 72.5 74.2 68.0 Avg. BMI (kg.m-2) 20.3 (14.2 to 27.0) 22.6 (20.8 to 24.8) 23.8 (22.7 to 24.8) 22.1 10 Phase 1b/2a Trial Demographics
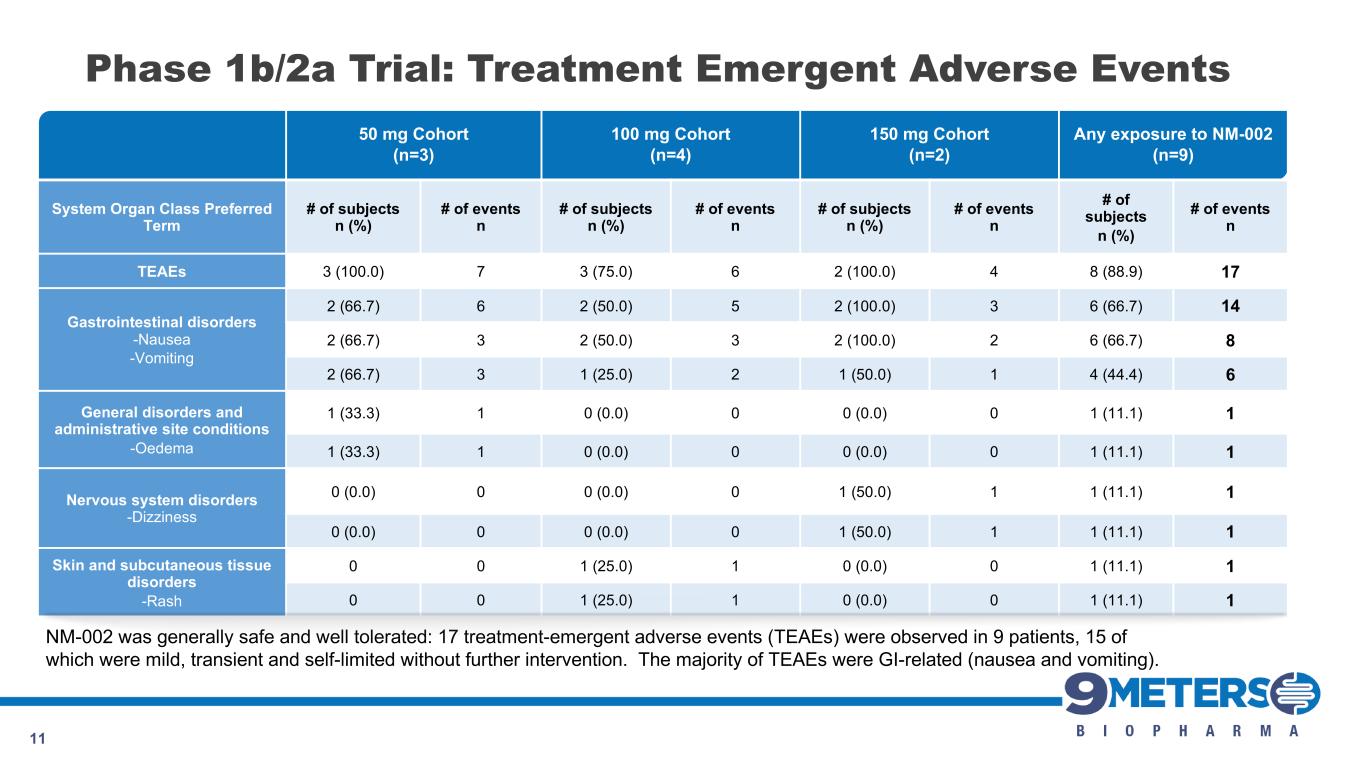
50 mg Cohort (n=3) 100 mg Cohort (n=4) 150 mg Cohort (n=2) Any exposure to NM-002 (n=9) System Organ Class Preferred Term # of subjects n (%) # of events n # of subjects n (%) # of events n # of subjects n (%) # of events n # of subjects n (%) # of events n TEAEs 3 (100.0) 7 3 (75.0) 6 2 (100.0) 4 8 (88.9) 17 Gastrointestinal disorders -Nausea -Vomiting 2 (66.7) 6 2 (50.0) 5 2 (100.0) 3 6 (66.7) 14 2 (66.7) 3 2 (50.0) 3 2 (100.0) 2 6 (66.7) 8 2 (66.7) 3 1 (25.0) 2 1 (50.0) 1 4 (44.4) 6 General disorders and administrative site conditions -Oedema 1 (33.3) 1 0 (0.0) 0 0 (0.0) 0 1 (11.1) 1 1 (33.3) 1 0 (0.0) 0 0 (0.0) 0 1 (11.1) 1 Nervous system disorders -Dizziness 0 (0.0) 0 0 (0.0) 0 1 (50.0) 1 1 (11.1) 1 0 (0.0) 0 0 (0.0) 0 1 (50.0) 1 1 (11.1) 1 Skin and subcutaneous tissue disorders -Rash 0 0 1 (25.0) 1 0 (0.0) 0 1 (11.1) 1 0 0 1 (25.0) 1 0 (0.0) 0 1 (11.1) 1 11 Phase 1b/2a Trial: Treatment Emergent Adverse Events NM-002 was generally safe and well tolerated: 17 treatment-emergent adverse events (TEAEs) were observed in 9 patients, 15 of which were mild, transient and self-limited without further intervention. The majority of TEAEs were GI-related (nausea and vomiting).

Patient NM-002 Dose (mg) Baseline (mL) First 48 Hours Post Dose 1 (mL) Change from Baseline (mL) First 48 Hours Post Dose 2 (mL) Change from Baseline (mL) 1 50 22508 12550 −9958 13950 −8558 2 50 1900 200 −1700 300 −1600 61 50 1175 350 −825 - - 3 100 720 615 −105 325 −395 4 100 1285 420 -865 24502 1165 5 100 2280 2000 −280 1940 −340 7 100 5390 3010 -2380 2600 -2790 8 150 2570 2150 -420 1480 -1090 9 150 4850 3900 -950 3000 -1850 12 Phase 1b/2a Efficacy: Total Stool Output (TSO) Lasting effect seen in patients, confirming single ascending dose T2DM study data (Cleland, et. al.) 1. Patient 06 did not receive a 2nd dose. 2. The baseline prior to the second dose in this patient was substantially higher than the original baseline volume due to rapid increase in oral intake.

Patients 1 had an end jejunostomy and 3 had an ileostomy therefore missing from the bowel frequency table; stool output (previous slide) more relevant in these patients 13 Phase 1b/2a Efficacy Measure: Bowel Frequency Patient NM-002 Dose (mg) Baseline (#) First 48 Hours Post Dose 1 (#) Change from Baseline (#) First 48 Hours Post Dose 2 (#) Change from Baseline (#) 2 50 22 1 −21 4 −18 61 50 5 8 3 - - 4 100 20 20 0 10 −10 5 100 8 11 3 13 5 7 100 22 17 -5 10 -12 8 150 12 9 -3 9 -3 9 150 13 9 -4 8 -5 1. Patient 06 did not receive a 2nd dose

14 Day Parameter 50 mg Cohort (n=3) 100 mg Cohort (n=3)2 150 mg Cohort (n=1)3,4 1 Mean AUC0-t (h*ng/m L) 1481465.77 2941663.33 3087281.46 Mean Cmax (ng/mL) 13893.92 25502.68 26704.34 Mean Tmax (h) 144.00 144.00 168.00 Day Parameter 50 mg Cohort (n=2)1 100 mg Cohort (n=3)2 150 mg Cohort3,4 15 Mean AUC0-t (h*ng/m L) 5151279.93 11646344.56 - Mean Cmax (ng/mL) 19067.26 43303.06 - Mean Tmax (h) 84.00 112.00 - All parameters are mean values. 1. One 50 mg dose patient did not receive a 2nd dose; 2. One 100 mg dose patient pending both day 1 and day 15 PK analysis; 3. One 150 mg dose patient pending day 15 PK analysis; 4. One 150 mg patient pending both day 1 and day 15 PK analysis Preliminary Pharmacokinetic Analysis (n=7)
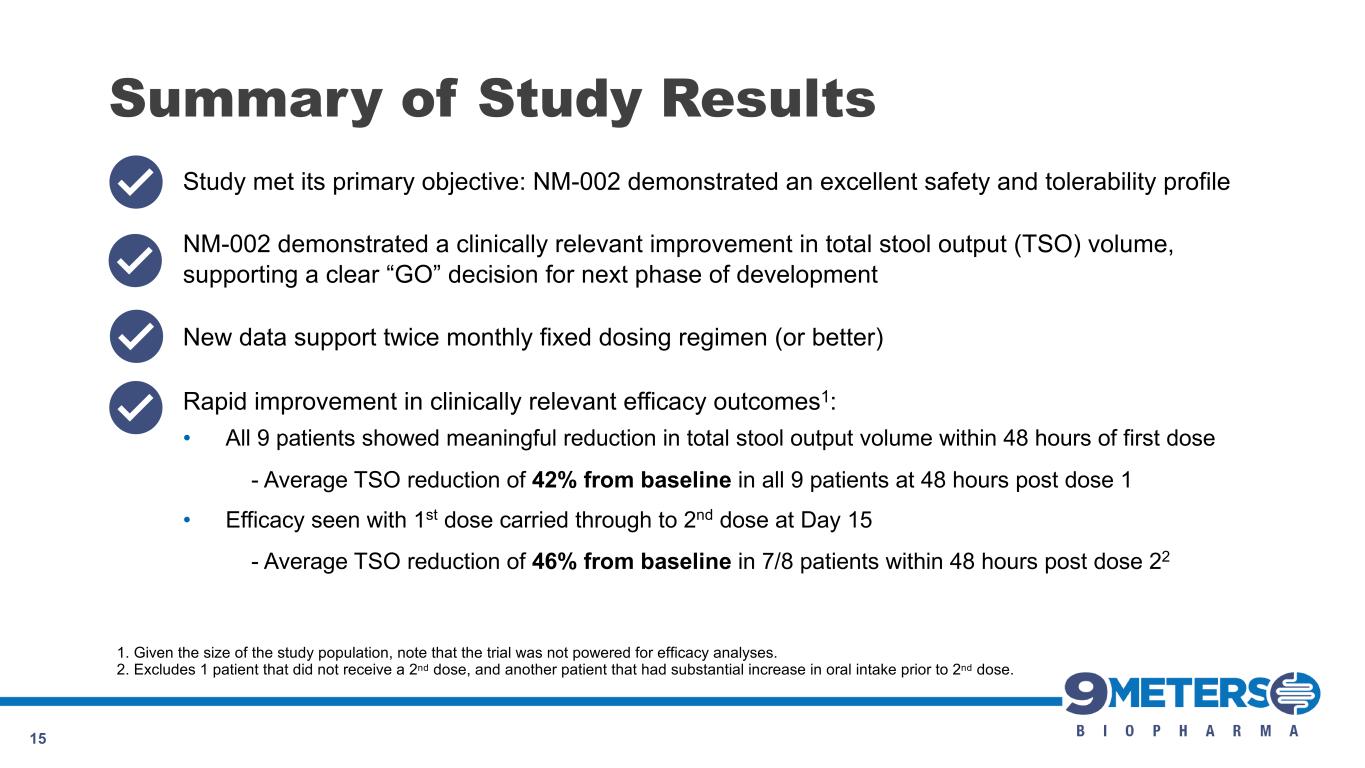
Summary of Study Results 15 Study met its primary objective: NM-002 demonstrated an excellent safety and tolerability profile NM-002 demonstrated a clinically relevant improvement in total stool output (TSO) volume, supporting a clear “GO” decision for next phase of development New data support twice monthly fixed dosing regimen (or better) Rapid improvement in clinically relevant efficacy outcomes1: • All 9 patients showed meaningful reduction in total stool output volume within 48 hours of first dose - Average TSO reduction of 42% from baseline in all 9 patients at 48 hours post dose 1 • Efficacy seen with 1st dose carried through to 2nd dose at Day 15 - Average TSO reduction of 46% from baseline in 7/8 patients within 48 hours post dose 22 1. Given the size of the study population, note that the trial was not powered for efficacy analyses. 2. Excludes 1 patient that did not receive a 2nd dose, and another patient that had substantial increase in oral intake prior to 2nd dose.
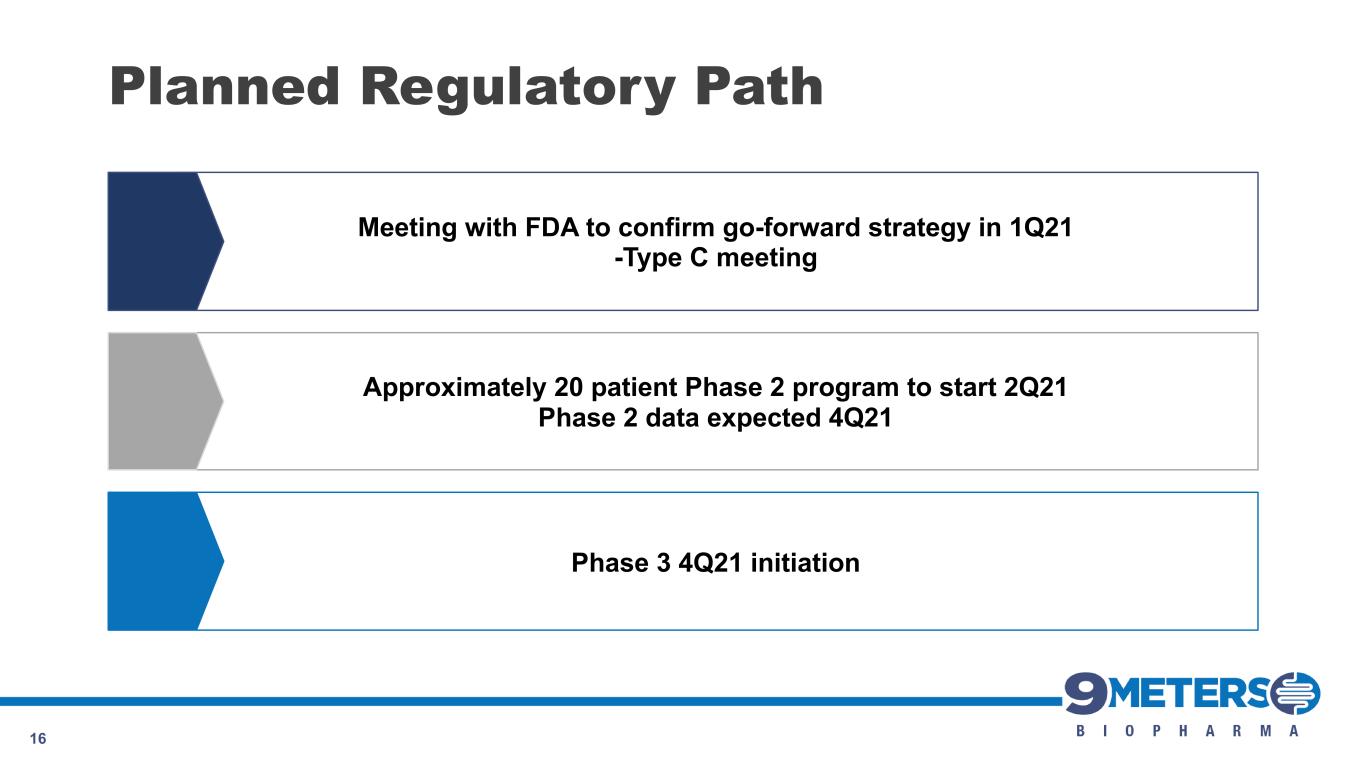
Planned Regulatory Path 16 Phase 3 4Q21 initiation Meeting with FDA to confirm go-forward strategy in 1Q21 -Type C meeting Approximately 20 patient Phase 2 program to start 2Q21 Phase 2 data expected 4Q21
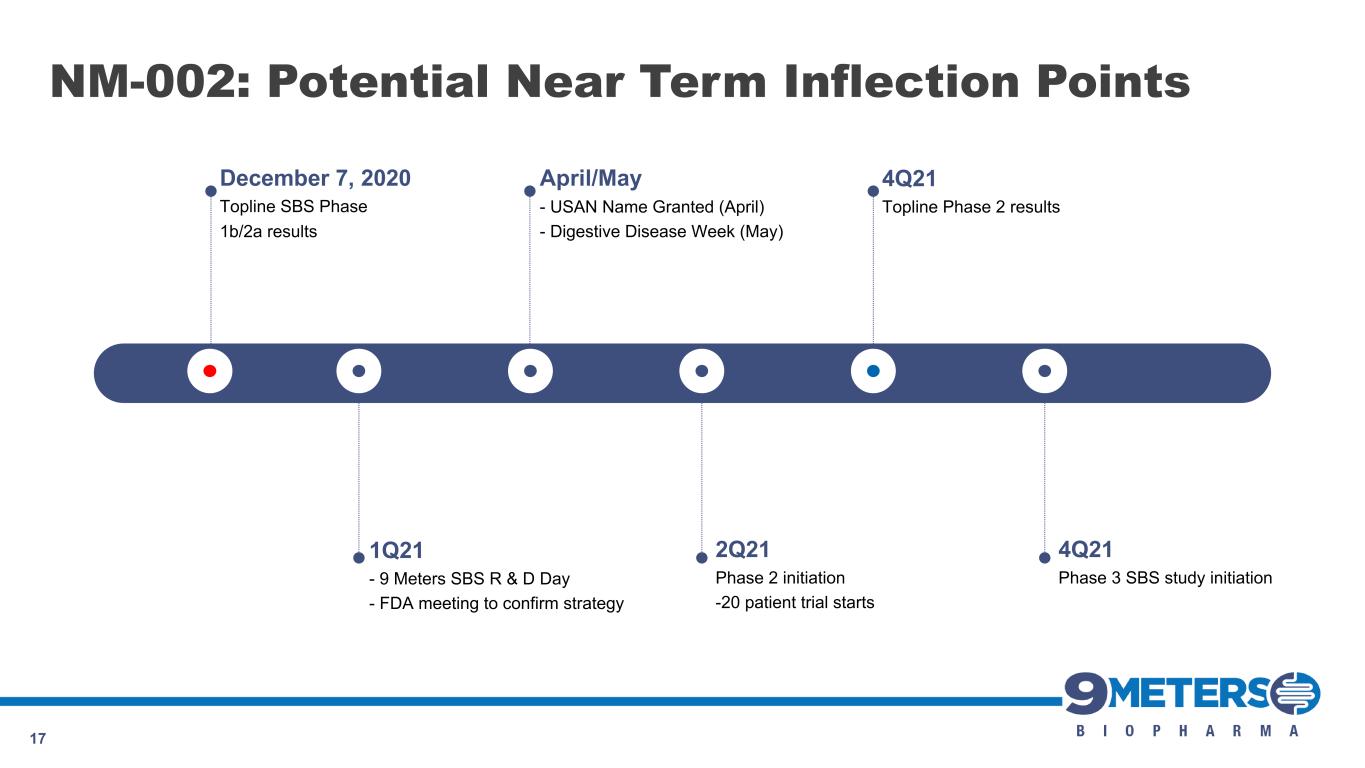
April/May - USAN Name Granted (April) - Digestive Disease Week (May) NM-002: Potential Near Term Inflection Points 17 1Q21 - 9 Meters SBS R & D Day - FDA meeting to confirm strategy 2Q21 Phase 2 initiation -20 patient trial starts 4Q21 Topline Phase 2 results 4Q21 Phase 3 SBS study initiation December 7, 2020 Topline SBS Phase 1b/2a results

18 NM-002 (proprietary long-acting GLP-1)* GLP-2 Class Profile ü Long acting GLP-1 receptor agonist ü Slows gut motility ü Increases time for absorption of key nutrients – GLP-2 analogue – Expand intestinal mucosa / villous growth – Limited effect on gut motility Efficacy ü Improvements in total stool output volume ü Improvements in bowel movement frequency ü Diarrhea no longer meal-related ü Reduction in nocturnal diarrhea – Statistically significant reductions in PS volume – Very low rates of patients weaned off Onset of Action ü Within hours-to-days of dosing – Weeks to months (2 to 6 months) Safety ü Known target ü Transient side effects ü Molecule has over 15 patient years of use – REMS program; safety concerns include: – Acceleration of neoplastic growth – Intestinal obstruction – Biliary and pancreatic disease Dosing ü Twice-monthly (potentially monthly) ü Fixed-dosing – QD injections; newer versions once- or twice- weekly NM-002 Target Product Profile *NM-002 remains investigational and under development

Thank You John Temperato President & CEO jtemperato@9meters.com Edward Sitar Chief Financial Officer esitar@9meters.com 8480 Honeycutt Road, Suite 120 Raleigh, NC 27615 Telephone: 1-919-275-1933 info@9meters.com www.9meters.com Twitter LinkedIn


















