As filed with the Securities and Exchange Commission on April 13, 2016.
Registration No. 333-205135
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
Post-Effective Amendment No. 1
to
FORM F-1
REGISTRATION STATEMENT
UNDER
THE SECURITIES ACT OF 1933
Benitec Biopharma Limited
(Exact name of registrant as specified in its charter)
| | | | |
| Australia | | 2834 | | Not Applicable |
(State or other jurisdiction of
incorporation or organization) | | (Primary Standard Industrial
Classification Code Number) | | (I.R.S. Employer
Identification Number) |
F6/1-15 Barr Street
Balmain, NSW, 2041, Australia
Tel: +61 2 9555 6986
(Address, including zip code, and telephone number, including area code, of registrant’s principal executive offices)
Tacere Therapeutics, Inc.
3940 Trust Way
Hayward, CA 94545
Tel: (510) 780-0819
(Name, address, including zip code, and telephone number, including area code, of agent for service)
Copies to:
| | |
Andrew S. Reilly
Baker & McKenzie
50 Bridge Street, Level 27
Sydney, NSW 2000, Australia
Tel: +61 2 9225 0200
Fax: +61 2 9225 1595 | | Marc R. Paul
Baker & McKenzie LLP
815 Connecticut Avenue, N.W.
Washington, DC 20006
Tel: (202) 452-7000
Fax: (202) 416-7035 |
Approximate date of commencement of proposed sale to the public:
As soon as practicable after the effective date of this registration statement.
If any of the securities being registered on this form are to be offered on a delayed or continuous basis pursuant to Rule 415 under the Securities Act of 1933, check the following box. x
If this Form is filed to register additional securities for an offering pursuant to Rule 462(b) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ¨
If this Form is a post-effective amendment filed pursuant to Rule 462(c) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ¨
If this Form is a post-effective amendment filed pursuant to Rule 462(d) under the Securities Act, check the following box and list the Securities Act registration statement number of the earliest effective registration statement for the same offering. ¨
The registrant hereby amends this registration statement on such date or dates as may be necessary to delay its effective date until the registrant shall file a further amendment which specifically states that this registration statement shall thereafter become effective in accordance with Section 8(a) of the Securities Act of 1933, as amended, or until the registration statement shall become effective on such date as the Securities and Exchange Commission, acting pursuant to said Section 8(a), may determine.
EXPLANATORY NOTE
This Post-Effective Amendment No. 1 to Benitec Biopharma Limited’s Registration Statement on Form F-1, SEC File No. 333-205135 is being filed pursuant to the undertaking in the Warrant Agent Agreement to update and supplement the information contained in the Registration Statement, which was originally declared effective by the Securities and Exchange Commission, or SEC, on August 17, 2015.
The information included in this filing updates and supplements the Registration Statement and the prospectus contained therein. No additional securities are being registered under this Post-Effective Amendment No. 1. Accordingly, this Post Effective Amendment No. 1 concerns only the exercise of the warrants issued in connection with our initial public offering in the United States, which warrants were registered under the Registration Statement that the SEC declared effective. All applicable registration fees were paid at the time of the original filing of the Registration Statement.
The information in this prospectus may be changed. We may not sell these securities until this Post-Effective Amendment No. 1 to the Registration Statement filed with the Securities and Exchange Commission is declared effective. This prospectus is not an offer to sell these securities and we are not soliciting offers to buy these securities in any state where the offer or sale is not permitted.
| | |
| PRELIMINARY PROSPECTUS | | SUBJECT TO COMPLETION, DATED APRIL 13, 2016. |
1,500,000 ADSs
Representing 30,000,000 Ordinary Shares
Warrants to Purchase 575,000 ADSs
Representing 11,500,000 Ordinary Shares
Benitec Biopharma Limited
US$13,820,000
In connection with this prospectus, we previously offered 1,500,000 American Depositary Shares, or ADSs, together with warrants to purchase 500,000 ADSs. Each ADS was sold together with one third of a warrant. Each full warrant is exercisable for one ADS at an exercise price of US$5.50 per ADS, exercisable from the date of issuance until five years thereafter. We refer to these warrants as the Warrants. Each ADS represents 20 ordinary shares. We have not issued and do not intend to issue fractional ADSs or Warrants.
The initial public offering price of our ADSs was US$9.21 per ADS. The warrants were sold for US$0.01 per warrant. On April 8, 2016, the last reported price of our ADSs on The NASDAQ Capital Market was $1.68 per ADS and the last reported price of our Warrants was $0.95 per Warrant.
Prior to our initial public offering in the United States, neither the ADSs nor the Warrants were listed on any stock exchange. The ADSs and Warrants are now listed on The NASDAQ Capital Market under the symbols “BNTC” and “BNTCW.” Each of the ADSs and Warrants trade separately.
Our ordinary shares are listed on the Australian Securities Exchange under the symbol “BLT.”
We are an “emerging growth company,” as that term is used in the Jumpstart Our Business Startups Act of 2012, and, as such, we have elected to comply with certain reduced public company reporting requirements.
Investing in the ADSs involves risks. See “Risk Factors” beginning on page 11 of this prospectus.
| | | | | | | | | | | | |
| | | Per ADS | | | Per Warrant(1) | | | Total | |
Public offering price | | US$ | 9.21 | | | US$ | 0.01 | | | US$ | 13,820,000 | |
| | | |
Underwriting discounts and commissions | | US$ | 0.6447 | | | US$ | 0.0007 | | | US$ | 967,400 | |
| | | |
Proceeds, before expenses, to us(2) | | US$ | 8.5653 | | | US$ | 0.0093 | | | US$ | 12,852,600 | |
| (1) | | Each ADS was sold together with one third of a Warrant. Each full Warrant is exercisable for one ADS at an exercise price of US$5.50 per ADS. |
| (2) | | We reimbursed the underwriter for certain expenses. See “Underwriting.” |
We granted the underwriter an option, exercisable for 45 days from the date of our initial public offering in the United States, to purchase up to an additional 225,000 ADSs and/or 75,000 Warrants from us at the public offering price less the underwriting discounts and commissions. The underwriter partially exercised its option and purchased 75,000 Warrants from us.
Neither the Securities and Exchange Commission nor any other regulatory body has approved or disapproved of these securities or determined if this prospectus is truthful or complete. Any representation to the contrary is a criminal offense.
The underwriter delivered the ADSs and Warrants against payment in New York, New York, on or about August 21, 2015.
Prospectus dated April , 2016.
TABLE OF CONTENTS
You may rely only on the information contained in this prospectus. Neither we nor the underwriter have authorized anyone to provide information different from that contained in this prospectus. When you make a decision about whether to invest in the ADSs and Warrants, you should not rely upon any information other than the information in this prospectus. Neither the delivery of this prospectus nor the sale of the ADSs or Warrants means that information contained in this prospectus is correct after the date of this prospectus. This prospectus is not an offer to sell or solicitation of an offer to buy the ADSs or Warrants in any circumstances under which the offer of solicitation is unlawful.
We have not taken any action to permit a public offering of the ADSs and Warrants outside the United States or to permit the possession or distribution of this prospectus outside the United States. Persons outside the United States who come into possession of this prospectus must inform themselves about and observe any restrictions relating to the offering of the ADSs and Warrants and the distribution of this prospectus outside of the United States.
i
CONVENTIONS THAT APPLY TO THIS PROSPECTUS
Unless otherwise indicated or the context implies otherwise:
| | • | | “we,” “us,” “our” or “Benitec” refers to Benitec Biopharma Limited, an Australian corporation, and its subsidiaries; |
| | • | | “shares” or “ordinary shares” refers to our ordinary shares; |
| | • | | “ADSs” refers to American Depositary Shares, each of which represents 20 ordinary shares; |
| | • | | “ADRs” refers to American Depositary Receipts, which evidence the ADSs; and |
| | • | | “Warrant” refers to a warrant to purchase one ADS at an exercise price of US$5.50 per ADS, exercisable from the date of issuance until five years thereafter. |
Our reporting and functional currency is the Australian dollar. Solely for the convenience of the reader, this prospectus contains translations of some Australian dollar amounts into U.S. dollars at specified rates. Except as otherwise stated in this prospectus, all translations from Australian dollars to U.S. dollars are based on the rate published by the Reserve Bank of Australia on the date indicated. See “Exchange Rate Information.” No representation is made that the Australian dollar amounts referred to in this prospectus could have been or could be converted into U.S. dollars at such rate.
Unless otherwise noted, all industry and market data in this prospectus, including information provided by independent industry analysts, is presented in U.S. dollars. Unless otherwise noted, all other financial and other data related to Benitec Biopharma Limited in this prospectus is presented in Australian dollars. All references to “$” in this prospectus refer to Australian dollars or U.S. dollars, as the context requires based on the foregoing. All references to “A$” in this prospectus mean Australian dollars. All references to “US$” in this prospectus mean U.S. dollars.
Our fiscal year end is June 30. References to a particular “fiscal year” are to our fiscal year ended June 30 of that calendar year.
Unless otherwise indicated, the consolidated financial statements and related notes included in this prospectus have been prepared in accordance with International Accounting Standards and also comply with International Financial Reporting Standards, or IFRS, and interpretations issued by the International Accounting Standards Board, or IASB, which differ in certain significant respects from Generally Accepted Accounting Principles in the United States, or GAAP. See “Management’s Discussion and Analysis of Financial Condition and Results of Operations-Certain Differences Between IFRS and GAAP.”
INDUSTRY AND MARKET DATA
This prospectus includes information with respect to market and industry conditions and market share from third-party sources or based upon estimates using such sources when available. We believe that such information and estimates are reasonable and reliable. We also believe the information extracted from publications of third-party sources has been accurately reproduced. However, we have not independently verified any of the data from third-party sources. Similarly, our internal research is based upon our understanding of industry conditions, and such information has not been verified by any independent sources.
ii
TRADEMARKS AND TRADENAMES
We have proprietary and licensed rights to trademarks used in this prospectus which are important to our business, many of which are registered under applicable intellectual property laws. These trademarks are as follows:
| | • | | SILENCING GENES FOR LIFE® |
Solely for convenience, trademarks and trade names referred to in this prospectus appear without the “®” or “™” symbols, but such references are not intended to indicate, in any way, that we will not assert, to the fullest extent possible under applicable law, our rights or the rights of the applicable licensor to these trademarks and trade names. We do not intend our use or display of other companies’ trade names, trademarks or service marks to imply a relationship with, or endorsement or sponsorship of us by, any other companies. Each trademark, trade name or service mark of any other company appearing in this prospectus is the property of its respective holder.
iii
PROSPECTUS SUMMARY
This summary provides a brief overview of information contained elsewhere in this prospectus and is qualified in its entirety by the more detailed information and the financial statements and notes thereto included elsewhere in this prospectus. This summary does not contain all of the information that you should consider before investing in the ADSs and Warrants. You should read the entire prospectus carefully before making an investment decision, including the information presented under the headings “Risk Factors,” “Cautionary Note Regarding Forward-Looking Statements” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and the historical consolidated financial statements and the related notes to those financial statements included elsewhere in this prospectus.
BUSINESS
Overview
We are a biotechnology company developing a novel, proprietary therapeutic technology platform that combines gene silencing and gene therapy with a goal of providing sustained, long-lasting silencing of disease-causing genes from a single administration. We believe our technology has the potential to be a “one shot” cure for a wide range of diseases that are currently addressed by strict ongoing treatment regimens or that have no effective treatment or only palliative care options. We are using our technology, called DNA-directed RNA interference, or ddRNAi, to develop our pipeline of product candidates for the treatment of several chronic and life-threatening human diseases, such as hepatitis B, age-related macular degeneration, or AMD and oculopharyngeal muscular dystrophy, or OPMD. We will require additional financing to conduct clinical trials for our product candidates for hepatitis B, AMD and OPMD. These diseases have large patient populations, with the exception of OPMD which is a rare disease. In addition, we have licensed our ddRNAi technology to other biopharmaceutical companies whose pipeline programs are progressing towards, or are in, clinical development for applications including HIV/AIDS, retinitis pigmentosa, Huntington’s disease, cancer immunotherapy and intractable neuropathic pain.
Our Technology
Standard RNAi approach
Many diseases are known to be caused by the inappropriate expression of a gene or multiple genes. It has been observed since 1998 that RNA interference, or RNAi, is a potential mechanism to specifically turn off, or silence, genes whose sequences are known. Thus, RNAi can potentially be used to treat or cure diseases with a genetic basis by targeting a specific region of the molecular sequence of the disease-causing gene. RNAi is potentially applicable to over 20,000 human genes and a large number of disease-causing microorganism-specific genes. The mechanism of action of RNAi involves the introduction of short interfering RNA, or siRNA, into a cell. The siRNA’s sequence is constructed to match a short region of the target gene. The siRNA is processed by the cell’s own enzymes to destroy the target gene’s messenger RNA, or mRNA, thus preventing the disease-causing gene from being expressed. This occurs as long as the siRNA remains prevalent in the cell.
Our ddRNAi approach
Our approach differs from the standard RNAi approach, which is commonly referred to as siRNA, which is being developed by several other companies, including Alnylam Pharmaceuticals, Inc., or Alnylam, Arbutus Biopharma Corporation, or Arbutus (formerly known as Tekmira Pharmaceuticals Corporation before its name change and integration with OnCore BioPharma), and Dicerna Pharmaceuticals, Inc., or Dicerna. In this standard RNAi approach, double-stranded siRNA is produced synthetically and subsequently introduced into the target
1
cell either by chemical modification of the RNA or by a range of other delivery methods. While clinical efficacy has been demonstrated for a number of indications utilizing this approach, it has a number of limitations, including:
| | • | | treatment requires repeat administration for multiple cycles in order to maintain its efficacy; |
| | • | | patient adherence challenges due to dosing frequency and treatment duration; |
| | • | | therapeutic concentrations of siRNA are not stably maintained because the levels of synthetic siRNA in the cells decrease over time; |
| | • | | novel chemical modifications or novel delivery materials are typically required to introduce the siRNA into the target cells, making it complicated to develop therapeutics; |
| | • | | limited ability to target diseases of tissues other than the liver; |
| | • | | can have an adverse immune response, or interferon response, potentially resulting in serious adverse effects; |
| | • | | requirement for specialized delivery formulations for those diseases caused by multiple disease-causing genes; and |
| | • | | siRNA only acts to silence genes, but cannot be used to replace defective genes with normally functioning genes. |
Our ddRNAi technology is designed to utilize the specificity and gene silencing effect of RNA interference while overcoming many of the limitations associated with the ongoing administration of siRNA. Our ddRNAi approach combines RNA interference with gene therapy. Unlike siRNA, our ddRNAi technology starts with a DNA construct. Gene therapy vectors, which are carrier molecules, often viruses, that deliver genetic material into the cell, are used to deliver the DNA construct to the nucleus of the targeted cells. The DNA construct then generates double-stranded short hairpin RNAs, or shRNAs, which are processed by the cell into siRNAs, which in turn silence the disease-associated genes. Advantages of our ddRNAi approach include:
| | • | | ddRNAi is designed to produce sustained, long-lasting silencing of the disease-causing gene, following a single administration, leading to the potential for “one shot” cures for a wide range of diseases, which could eliminate the requirement for patient compliance to take regular doses of medicine for long-term management of their disease; |
| | • | | ddRNAi technology can target a wide range of tissues, including, but not limited to, the liver; |
| | • | | ddRNAi uses the cell’s own transcriptional mechanisms to produce a constant level of shRNA so that intracellular levels of siRNA do not fall below threshold levels required for disease suppression; |
| | • | | the level of shRNA in the cells can potentially be fine-tuned to achieve optimal concentrations; |
| | • | | off-tissue effects can be minimized; |
| | • | | the DNA constructs are shielded in gene therapy vectors that are designed to avoid activating the interferon response; |
| | • | | ddRNAi provides the option to both silence the defective gene and replace the defective gene with a normal version; |
| | • | | ddRNAi can be designed to express multiple siRNAs in the same cell, targeting either a single gene at several different sites to minimize the risk of viral resistance, or multiple genes in distinct cellular pathways, potentially enabling treatment of complex genetic diseases such as cancer, diabetes and heart disease; and |
| | • | | ddRNAi can elicit long-term response by continued expression of siRNA from a single administration, potentially preventing viral reinfection. |
2
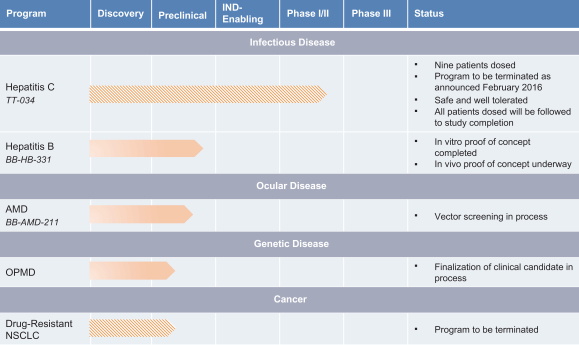
Our Pipeline
The following tables set forth our product candidates and out-licensed product candidates and their development status.
In-House Programs
3
Licensed Programs
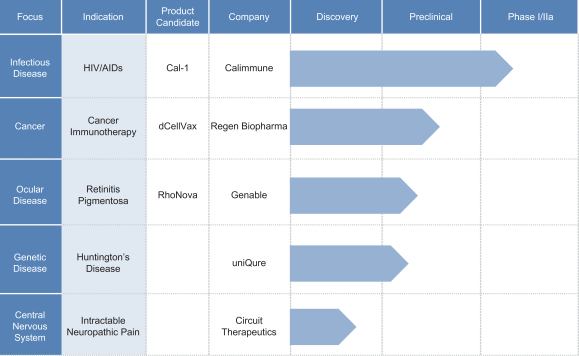
Proceeds from our initial public offering in the United States, with our pre-existing cash and cash equivalents, will advance our product candidates for hepatitis B, AMD and OPMD. Additional financing will be needed to advance our product candidates for hepatitis B, AMD and OPMD through clinical trials.
We are developing Hepbarna, or BB-HB-331, which currently is in preclinical studies, for the treatment of the hepatitis B virus, or HBV. We plan to file an investigational new drug, or IND, application in the third quarter of 2017. HBV is a small DNA virus that, according to the WHO, infects up to 240 million people worldwide, resulting in up to 780,000 deaths per year. Infection with HBV occurs in phases ranging from a silent, acute phase that can be resolved by the immune system, to a persistent chronic infection requiring life-long therapy. In the case of a chronic HBV infection, the presence of viral particles and proteins, particularly the s-antigen, causes hepatic inflammation leading to liver dysfunction, acute hepatic failure, cirrhosis or hepatocellular carcinoma. Patients suffering from HBV have limited treatment options from therapies consisting of antivirals and, less commonly, interferon therapy. These treatments require adherence to strict recurrent treatment regimens, may cause the hepatitis B virus to mutate and develop antiviral drug resistance, and may only provide viral suppression through the course of administration, and not a cure. The long-term use of interferon, particularly in high doses, may also be associated with significant side effects, including nausea, vomiting, shortness of breath, dizziness and fatigue, that can cause patients to deviate from the course of treatment. BB-HB-331 is designed to be a single administration ddRNAi-based monotherapy that is delivered using a gene therapy vector that targets the liver and inhibits viral replication and s-antigen production on a long-term basis.
In March 2016, we announced results of our recentin vivo study of BB-HB-331. Key findings of thein vivo study indicate that a single BB-HB-331 treatment in the PhoenixBio (PXB) mouse model can result in suppression of HBV. Thein vivo experiment supports the BB-HB-331in vitro findings previously observed in human hepatocytes isolated from the PXB mouse model, announced in December 2015. We believe these results
4
demonstrate the potential utility of an approach that combines RNAi with gene therapy to treat HBV, and we intend to advance the HBV program towards the clinic. Our hepatitis B program continues to attract considerable interest from pharmaceutical companies.
We are in the process of winding down for commercial reasons a partially completed Phase I/IIa clinical trial for our now discontinued product candidate, TT-034, which we were developing to treat patients chronically infected with the most common genotypte of the hepatitis C virus, or HCV. Early stages of the clinical trial of TT-034 indicated that TT-034 caused no treatment-related serious adverse events among the nine patients dosed to date. Of the nine patients, seven were biopsied and indicated a relationship between the amount of the compound administered and hepatic tissue transduction. However, as a result of the increasingly competitive landscape among available HCV therapies, in February 2016 we concluded that the TT-034 program no longer offered the commercial value necessary to attract a worthwhile commercial partnership deal and, as a result, did not warrant additional expenditure or focus of our resources beyond completion of the existing patients. We believe the early stage TT-034 clinical trial results provide support for the safety of our platform technology.
In addition to BB-HB-331 for HBV, we are focusing on developing product candidates to treat AMD and OPMD. We will require additional financing to conduct clinical trials for our product candidates for HBV, AMD and OPMD. For selected product candidates, at the appropriate stage, we may collaborate with large pharmaceutical companies to further develop and, if approved, commercialize them to achieve broad product distribution. For certain products we deem to be outside of our immediate focus, we will continue to out-license, where appropriate, applications of our ddRNAi technology for the development of a range of therapeutics, which we believe could provide further validation of our technology’s potential to address numerous diseases. We anticipate completingin vivo preclinical proof of concept studies for AMD and OPMD in the fourth quarter of 2016.
Our Strategy
Our objective is to become the leader in discovering, developing, clinically validating and commercializing ddRNAi-based therapeutics for a range of human diseases with high unmet clinical need or large patient populations, and to thereby provide a better life for patients with these diseases. Our strategy to accomplish this goal is to:
| | • | | Progress our pipeline of proprietary ddRNAi-based therapeutics. We are pursuing early preclinical research in HBV, AMD and OPMD and plan to submit IND applications for those product candidates. We expect to complete preclinicalin vivo proof-of-concept studies for our HBV product candidate in the second quarter of 2016, and for our AMD and OPMD product candidates in the fourth quarter of 2016. Based on the potential market opportunity and considerable interest from pharmaceutical companies, we plan to prioritize the HBV program as our next candidate for clinical development. |
| | • | | Continue our leadership position in ddRNAi-based therapeutics. We believe we are the only company to date to have advanced into a clinical trial an RNAi therapeutic for systemic administration by gene therapy vectors. |
| | • | | Further develop and improve our ddRNAi platform technology and its associated intellectual property position. In addition to progressing our pipeline of product candidates, we will further develop and improve our ddRNAi platform technology and its associated intellectual property through in-house development and in-licensing of complementary technologies. |
| | • | | Develop drugs in our core disease areas and partner selectively to commercialize and expand our pipeline. The adaptability of our platform also presents an opportunity for us to selectively form collaborations to expand our capabilities and product offerings into a range of diseases and potentially to accelerate the development and commercialization of ddRNAi therapeutics more broadly. We will continue to expand our franchise of ddRNAi-based therapeutics by out-licensing, where appropriate, |
5
| | applications of ddRNAi for the development of a range of therapeutics outside of our immediate focus. Once clinical proof of concept has been achieved for a product candidate, we plan, where appropriate, to enter into collaborations with pharmaceutical companies to develop that product candidate and ultimately commercialize it if it receives regulatory approval. |
| | • | | Pursue indications with high unmet medical need or large patient populations. Each of our three current core indications are severe diseases with high unmet medical need or large patient populations. We believe there is a strong rationale for treating these diseases and other diseases that have well-characterized gene targets that can be silenced, thus preventing the disease-causing gene from being expressed. We also intend to develop ddRNAi applications in novel technologies including immunotherapy, such as chimeric antigen receptor T cells, or CAR T, for a range of additional disease areas, subject to our ability to obtain additional financing apart from our initial public offering in the United States. |
Our development team has significant experience in designing and developing ddRNAi therapeutics and includes founding scientists in the ddRNAi field. Additionally, we have rights to intellectual property that includes a patent portfolio protecting our ddRNAi technology platform in numerous jurisdictions through 2019, and a growing portfolio of patents protecting improvements to our ddRNAi technology and product candidates in numerous jurisdictions through at least 2025.
RISK FACTORS
You should carefully consider the risks described under the “Risk Factors” section beginning on page 10ie. Some of these risks are:
| | • | | We have incurred significant net losses and anticipate that we will continue to incur significant net losses for the foreseeable future. We may never achieve or maintain profitability. |
| | • | | We have never generated any revenue from product sales and may never be profitable. |
| | • | | We will need to raise additional funding, which may not be available on acceptable terms, or at all. Failure to obtain this necessary capital when needed may force us to delay, limit or discontinue our product development efforts or other operations. |
| | • | | Currently, no product candidates utilizing ddRNAi technology have been approved for commercial sale, and our approach to the development of ddRNAi technology may not result in safe, effective or marketable products. |
| | • | | We are early in our product development efforts and all of our current product candidates are still in preclinical development. We may not be able to obtain regulatory approvals for the commercialization of some or all of our product candidates. |
| | • | | Issues which may impact ddRNAi delivery into the cell could limit our ability to develop and commercialize product candidates. |
| | • | | If other companies develop technologies or product candidates for our target disease indications more rapidly than we do or if their technologies, including delivery technologies, are more effective, our ability to develop and successfully commercialize product candidates may be compromised. |
| | • | | If we are unable to obtain or protect intellectual property rights related to our product candidates, we may not be able to obtain exclusivity for our product candidates or prevent others from developing similar competitive products. |
| | • | | If we are classified as a “passive foreign investment company,” our U.S. shareholders could suffer adverse tax consequences as a result. |
| | • | | Australian takeover laws may discourage takeover offers being made for us or may discourage the acquisition of a significant position in our ordinary shares or ADSs. |
6
These and other risks described in this prospectus could materially and adversely impact our business, financial condition, operating results and cash flow, which could cause the trading price of our ADSs to decline and could result in a loss of your investment.
CORPORATE INFORMATION
Benitec Biopharma Limited was incorporated under the laws of Australia in 1995 and has been listed on the Australian Securities Exchange, or ASX, since 1997.
Our headquarters are located at F6/1-15 Barr St, Balmain, New South Wales, Australia. Our telephone number is +61 2 9555 6986. Our website address is www.benitec.com. Information on our website and the websites linked to it do not constitute part of this prospectus or the registration statement to which this prospectus forms a part. Our agent for service of process in the United States is Tacere Therapeutics, Inc., 3940 Trust Way, Hayward, CA 94545.
IMPLICATIONS OF BEING AN EMERGING GROWTH COMPANY
As a company with less than US$1.0 billion in revenue during our last fiscal year, with less than US$1.0 billion in non-convertible debt securities issued in the past three years, and that has recently completed our first registered equity offering in the United States, we qualify as an “emerging growth company” as defined in the Jumpstart Our Business Startups Act of 2012, or the JOBS Act. An emerging growth company may avail itself of certain exemptions from various reporting requirements that are applicable to other public companies that are not emerging growth companies. For example, we have elected to rely on an exemption from the auditor attestation requirements of Section 404 of the Sarbanes-Oxley Act of 2002, or the Sarbanes-Oxley Act, relating to internal control over financial reporting, and we will not provide such an attestation from our auditors for as long as we qualify as an emerging growth company.
We will remain an emerging growth company until the earliest of:
| | • | | the end of the fiscal year in which the fifth anniversary of the completion of our initial public offering in the United States occurs, or June 30, 2021; |
| | • | | the end of the first fiscal year in which the market value of our ordinary shares held by non-affiliates exceeds US$700 million as of the end of the second quarter of such fiscal year; |
| | • | | the end of the first fiscal year in which we have total annual gross revenues of at least US$1.0 billion; and |
| | • | | the date on which we have issued more than US$1.0 billion in non-convertible debt securities in any rolling three-year period. |
Once we cease to be an emerging growth company, we will not be entitled to the exemptions provided for by the JOBS Act.
IMPLICATIONS OF BEING A FOREIGN PRIVATE ISSUER
We are also considered a “foreign private issuer” pursuant to Rule 405 under the Securities Act of 1933, as amended. In our capacity as a foreign private issuer, we are exempt from certain rules under the Securities Exchange Act of 1934, as amended, or the Exchange Act, that impose certain disclosure obligations and procedural requirements for proxy solicitations under Section 14 of the Exchange Act. In addition, our officers, directors and principal shareholders are exempt from the reporting and “short-swing” profit recovery provisions of Section 16 of the Exchange Act and the rules under the Exchange Act with respect to their purchases and sales
7
of our common shares. Moreover, we are not required to file periodic reports and financial statements with the U.S. Securities and Exchange Commission, or SEC, as frequently or as promptly as U.S. companies whose securities are registered under the Exchange Act. In addition, we are not required to comply with Regulation FD, which restricts the selective disclosure of material information.
We may take advantage of these exemptions until such time as we are no longer a foreign private issuer. We would cease to be a foreign private issuer at such time as more than 50% of our outstanding voting securities are held by U.S. residents and any of the following three circumstances applies: (1) the majority of our executive officers or directors are U.S. citizens or residents; (2) more than 50% of our assets are located in the United States; or (3) our business is administered principally in the United States. We are required to determine our status as a foreign private issuer on an annual basis at the end of our second fiscal quarter.
8
THE OFFERING
Ordinary shares outstanding as of December 31, 2015 | 146,529,096 |
Securities issued by us in our initial public offering in the United States | 1,500,000 ADSs, together with Warrants to purchase 500,000 ADSs (excluding any exercise of underwriter’s option) were offered in our initial public offering in the United States. Each ADS was sold together with one third of a Warrant. |
Warrants issued pursuant to the underwriter’s option in connection with our initial public offering in the United States | 75,000 Warrants |
The ADSs | Each ADS represents 20 ordinary shares. |
| | The depositary (as identified below) is the holder of the ordinary shares underlying the ADSs and ADS holders have the rights provided in the deposit agreement among us, the depositary and holders and beneficial owners of ADSs from time to time. |
| | ADS holders may surrender ADSs to the depositary to withdraw the ordinary shares underlying the ADSs. The depositary will charge ADS holders a fee for such an exchange. |
| | We may amend or terminate the deposit agreement for any reason without the consent of ADS holders. Any amendment that imposes or increases fees or charges or which materially prejudices any substantial existing right ADS holders have will not become effective as to outstanding ADSs until 30 days after notice of the amendment is given to ADS holders. If an amendment becomes effective, an ADS holder will be bound by the deposit agreement as amended if the ADS holder continues to hold ADSs. |
| | To better understand the terms of the ADSs, ADS holders should carefully read the section in this prospectus entitled “Description of Securities.” We also encourage ADS holders to read the deposit agreement, which is an exhibit to the registration statement to which this prospectus forms a part. |
The Warrants | Each full warrant has a per ADS exercise price of US$5.50, which is exercisable upon issuance and expires five years from the date of issuance. The ADSs issuable upon exercise of the Warrants are subject to anti-dilution upon the occurrence of certain stock dividends and distributions, stock split, stock subdivision and combinations, reclassifications or similar events affecting our ADSs or ordinary shares, or upon the occurrence of a change in ADS ratio. To better understand the terms of the Warrants, Warrant holders should carefully read the section in this prospectus entitled “Description of Securities.” |
9
Depositary | The Bank of New York Mellon. |
Use of proceeds | The net proceeds to us from our initial public offering in the United States, including the Underwriter’s partial exercise of its over-allotment option, after deducting the estimated underwriting discounts and commissions and estimated offering expenses payable by us, was approximately US$11.3 million, at US$9.21 per ADS and US$0.01 per Warrant. If all of the Warrants are exercised (excluding those already issued pursuant to the underwriter’s option), the additional net proceeds to us will be approximately US$2.8 million. There can be no assurance that the any of the Warrants will be exercised. We have begun to use, and expect to continue to use, the net proceeds from our initial public offering to advance the programs for our therapies for hepatitis B, AMD and OPMD, for discovery, development and acquisition of complementary targets and technologies and for working capital and for general corporate purposes. We intend to use any proceeds from the exercise of the Warrants for the same purposes. See “Use of Proceeds.” |
Risk factors | You should carefully read and consider the information in this prospectus under the heading “Risk Factors” and all other information included in this prospectus before deciding to invest in our securities. |
Listing and trading symbol | The ADSs and Warrants are listed on The NASDAQ Capital Market under the symbols “BNTC” and “BNTCW.” |
10
RISK FACTORS
An investment in the ADSs involves significant risks. You should carefully consider the risks described below and the other information in this prospectus, including our consolidated financial statements and related notes included elsewhere in this prospectus, before you decide to invest in the ADSs. If any of the following risks actually occurs, our business, prospects, financial condition and results of operations could be materially and adversely affected, the trading price of the ADSs could decline and you could lose all or part of your investment.
Risks Related to Our Financial Condition and Capital Requirements
We have incurred significant net losses. We anticipate that we will continue to incur significant net losses for the foreseeable future and we may never achieve or maintain profitability.
We are a biotechnology company and have not yet generated significant revenue. We have incurred losses of A$3.5 million, A$7.0 million and A$11.5 million for the fiscal years ended June 30, 2013, 2014 and 2015, respectively. We have not generated any revenues from sales of any of our product candidates.
As of December 31, 2015, we had accumulated losses of A$123.0 million. We have devoted most of our financial resources to research and development, including our clinical and preclinical development activities. To date, we have financed our operations primarily through the issuance of equity securities, research and development grants from the Australian government and payments from our collaboration partners. We have not generated, and do not expect to generate, any significant revenue for the foreseeable future, and we expect to continue to incur significant operating losses for the foreseeable future due to the cost of research and development, preclinical studies and clinical trials and the regulatory approval process for product candidates. The amount of our future net losses is uncertain and will depend, in part, on the rate of our future expenditures. Our ability to continue operations will depend on, among other things, our ability to obtain funding through equity or debt financings, strategic collaborations or additional grants.
We expect to continue to incur significant expenses and increasing operating losses for the foreseeable future. We anticipate that our expenses will increase substantially if and as we:
| | • | | continue our research and preclinical development of our product candidates; |
| | • | | expand the scope of our current preclinical studies for our product candidates or initiate additional preclinical, clinical or other studies for product candidates; |
| | • | | seek regulatory and marketing approvals for any of our product candidates that successfully complete clinical trials; |
| | • | | further develop the manufacturing process for our product candidates; |
| | • | | change or add additional manufacturers or suppliers; |
| | • | | seek to identify and validate additional product candidates; |
| | • | | acquire or in-license other product candidates and technologies, which may or may not include those related to our ddRNAi technology and delivery vectors for our therapeutic candidates; |
| | • | | maintain, protect and expand our intellectual property portfolio; |
| | • | | create additional infrastructure to support our operations as a public company in the United States and our product development and future commercialization efforts; and |
| | • | | experience any delays or encounter issues with any of the above. |
The net losses we incur may fluctuate significantly from quarter to quarter and year to year, such that a period-to-period comparison of our results of operations may not be a good indication of our future performance. In any particular quarter or quarters, our operating results could be below the expectations of securities analysts or investors, which could cause the price of the ADSs to decline.
11
We have never generated any revenue from product sales and may never be profitable.
Our ability to generate significant revenue and achieve profitability depends on our ability to, alone or with strategic collaboration partners, successfully complete the development of and obtain the regulatory approvals for our product candidates, to manufacture sufficient supply of our product candidates, to establish a sales and marketing organization or suitable third-party alternative for the marketing of any approved products and to successfully commercialize any approved products on commercially reasonable terms. All of these activities will require us to raise sufficient funds to finance business activities. We do not expect any milestone payments from our collaborative partners to be significant in the foreseeable future. In addition, we do not anticipate generating revenue from commercializing product candidates for the foreseeable future, if ever. Our ability to generate future revenues from commercializing product candidates depends heavily on our success in:
| | • | | establishing proof of concept in preclinical studies and clinical trials for our product candidates; |
| | • | | successfully completing clinical trials of our product candidates; |
| | • | | obtaining regulatory and marketing approvals for product candidates for which we complete clinical trials; |
| | • | | maintaining, protecting and expanding our intellectual property portfolio, and avoiding infringing on intellectual property of third parties; |
| | • | | establishing and maintaining successful licenses, collaborations and alliances with third parties; |
| | • | | developing a sustainable, scalable, reproducible and transferable manufacturing process for our product candidates; |
| | • | | establishing and maintaining supply and manufacturing relationships with third parties that can provide products and services adequate, in amount and quality, to support clinical development and commercialization of our product candidates, if approved; |
| | • | | launching and commercializing any product candidates for which we obtain regulatory and marketing approval, either by collaborating with a partner or, if launched independently, by establishing a sales, marketing and distribution infrastructure; |
| | • | | obtaining market acceptance of any product candidates that receive regulatory approval as viable treatment options; |
| | • | | obtaining favorable coverage and reimbursement rates for our products from third-party payors; |
| | • | | addressing any competing technological and market developments; |
| | • | | identifying and validating new product candidates; and |
| | • | | negotiating favorable terms in any collaboration, licensing or other arrangements into which we may enter. |
The process of developing product candidates for ddRNAi-based therapeutics contains a number of inherent risks and uncertainties. For example, it may not be possible to identify a target region of a disease-associated gene that has not been previously identified and/or patented by others, resulting in restrictions on freedom to operate for that target sequence. Silencing the target gene may not ultimately result in curing the disease as there may be more factors contributing to the development of the disease than the target gene. Silencing the target gene using ddRNAi may lead to short-term or long-term adverse effects that were not predicted or observed in preclinical studies. The delivery of the DNA construct to the target cells may not be possible, or complete or adequate to provide sufficient therapeutic benefit.
Even if one or more of our product candidates is approved for commercial sale, we may incur significant costs associated with commercializing any approved product candidate. As one example, our expenses could increase beyond expectations if we are required by the Food and Drug Administration, or FDA, or other regulatory agencies, domestic or foreign, to perform clinical and other studies in addition to those that we currently anticipate. Even if we are able to generate revenues from the sale of any approved products, we may not become profitable and may need to obtain additional funding to continue operations, which could have an adverse effect on our business, financial condition, results of operations and prospects.
12
We will need to continue our efforts to raise additional funding, which may not be available on acceptable terms, or at all. Failure to obtain this necessary capital when needed may force us to delay, limit or discontinue our product development efforts or other operations.
Developing ddRNAi products is expensive, and we expect our research and development expenses to increase substantially in connection with our ongoing activities, particularly as we advance our product candidates in preclinical studies and in future clinical trials and as we undertake preclinical studies of new product candidates.
As of December 31, 2015, our cash and cash equivalents were A$22.8 million. We estimate that our cash and cash equivalents will be sufficient to fund our operations until approximately August 2017. However, our operating plan may change as a result of many factors currently unknown to us, and we may need to seek additional funds sooner than planned, through public or private equity or debt financings, government grants or other third-party funding, strategic alliances and licensing arrangements or a combination of these approaches. In addition, because the length of time and activities associated with successful development of our product candidates is highly uncertain, we are unable to estimate the actual funds we will require for development and any approved marketing and commercialization activities. In any event, we will require additional capital to obtain regulatory approval for our product candidates and to commercialize any product candidates that receive regulatory approval.
Any additional fundraising efforts may divert our management from their day-to-day activities, which may compromise our ability to develop and commercialize our product candidates. In addition, we cannot guarantee that future financing will be available in sufficient amounts or on terms acceptable to us, if at all. Moreover, the terms of any financing may adversely affect the holdings or the rights of our shareholders, and the issuance of additional securities, whether equity or debt, by us, or the possibility of such issuance, may cause the market price of our ordinary shares, ADSs and Warrants to decline. If we incur indebtedness we may be required to agree to restrictive covenants, such as limitations on our ability to incur additional debt, limitations on our ability to acquire, sell or license intellectual property rights and other operating restrictions that could compromise our ability to conduct our business. We could also seek financing through arrangements with collaborative partners at an earlier stage than would otherwise be desirable and we may be required to relinquish rights to some or all of our technologies or product candidates or otherwise agree to terms unfavorable to us.
If we are unable to obtain funding on a timely basis or on acceptable terms, we may be required to significantly curtail, delay or discontinue one or more of our research or development programs or the commercialization of any approved product candidates.
We will be unable to conclude clinical trials for our product candidates for hepatitis B, AMD and OPMD if we are unable to raise additional financing.
We plan to develop our pipeline of product candidates using our ddRNAi technology to deliver therapeutics for a number of life-threatening conditions. We intend to advance our product candidates for hepatitis B, AMD and OPMD through clinical trials, but we will need additional financing to do so. If we are unable to raise capital when needed or on attractive terms, we could be forced to delay, reduce or eliminate our research and development programs for these product candidates.
We receive Australian government research and development grants. If we lose funding from these research and development grants, we may encounter difficulties in the funding of future research and development projects, which could harm our operating results.
We have historically received, and expect to continue to receive, grants through the Australian federal government’s Research and Development Tax Incentive program, under which the government provides a cash refund for the 45% of eligible research and development expenditures by small Australian entities, which are defined as Australian entities with less than A$20 million in revenue, having a tax loss. The Research and Development Tax Incentive grant is made by the Australian federal government for eligible research and
13
development purposes based on the filing of an annual application. We received Research and Development Tax Incentive grants in the fiscal years ended June 30, 2013, 2014 and 2015 of A$0.82, A$0.78 and A$2.32 million, respectively. This grant is available for our research and development activities in Australia, as well as activities in the United States to the extent such U.S.-based expenses relate to our activities in Australia, do not exceed half the expenses for the relevant activities and are approved by the Australian government. To the extent our research and development expenditures are deemed to be “ineligible,” then our grants would decrease. In addition, the Australian government may in the future modify the requirements of, reduce the amounts of the grants available under, or discontinue the Research and Development Tax Incentive program. Any such change in the Research and Development Tax Incentive program would have a negative effect on our future cash flows.
Risks Related to the Product Development and Regulatory Approval of Our Product Candidates
Our product candidates are based on ddRNAi technology. Currently, no product candidates utilizing ddRNAi technology have been approved for commercial sale and our approach to the development of ddRNAi technology may not result in safe, effective or marketable products.
We have concentrated our product research and development efforts on our ddRNAi technology, and our future success depends on successful clinical development of this technology. We plan to develop a pipeline of product candidates using our ddRNAi technology and deliver therapeutics for a number of chronic and life-threatening conditions, including hepatitis B, age-related macular degeneration and OPMD.
The scientific research that forms the basis of our efforts to develop product candidates is based on the therapeutic use of ddRNAi, and the identification, optimization and delivery of ddRNAi-based product candidates is relatively new. The scientific evidence to support the feasibility of successfully developing therapeutic treatments based on ddRNAi is preliminary and limited. There can be no assurance that any development and technical problems we experience in the future will not cause significant delays or unanticipated costs, or that such development problems can be solved. We may be unable to reach agreement on favorable terms, or at all, with providers of vectors needed to optimize delivery of our product candidates to target disease cells and we may also experience unanticipated problems or delays in expanding our manufacturing capacity or transferring our manufacturing process to commercial partners, any of which may prevent us from completing our clinical trials or commercializing our products on a timely or profitable basis, if at all.
Only a few product candidates based on RNAi or ddRNAi have been tested in either animals or humans, and a number of clinical trials conducted by other companies using other forms of RNAi technologies have not been successful. We may discover that application of ddRNAi does not possess properties required for a therapeutic benefit, such as the ability to continually express shRNAs for the period of time required to be maximally effective or the ability of viral vectors or other technologies to effectively deliver ddRNAi constructs to target cells in therapeutically relevant concentrations. In addition, application of ddRNAi-based products in humans may result in safety problems. We currently have only limited data, and no conclusive evidence, to suggest that we can effectively produce effective therapeutic treatments using our ddRNAi technology.
We are early in our product development efforts and all of our current product candidates are still in preclinical development. We may not be able to obtain regulatory approvals for the commercialization of some or all of our product candidates.
The research, testing, manufacturing, labeling, approval, selling, marketing and distribution of biologics is subject to extensive regulation by the FDA and other regulatory authorities, and these regulations differ from country to country. We do not have any products on the market and are early in our development efforts. All of our product candidates are in preclinical development. All of our current and future product candidates are subject to the risks of failure typical for development of biologics. The development and approval process is expensive and can take many years to complete, and its outcome is inherently uncertain. In addition, the outcome of preclinical testing and early clinical trials may not be predictive of the success of later clinical trials, and interim results of a clinical trial do not necessarily predict final results.
14
We have not submitted an application, or received marketing approval, for any of our product candidates and will not submit any applications for marketing approval for several years. We have limited experience in conducting and managing clinical trials necessary to obtain regulatory approvals, including approval by the FDA. To receive approval, we must, among other things, demonstrate with evidence from clinical trials that the product candidate is both safe and effective for each indication for which approval is sought, and failure can occur in any stage of development. Satisfaction of the approval requirements typically takes several years and the time needed to satisfy them may vary substantially, based on the type, complexity and novelty of the pharmaceutical product. We cannot predict if or when we might receive regulatory approvals for any of our product candidates currently under development.
The FDA and foreign regulatory authorities also have substantial discretion in the pharmaceutical product approval process. The numbers, types and sizes of preclinical studies and clinical trials that will be required for regulatory approval varies depending on the product candidate, the disease or condition that the product candidate is designed to address and the regulations applicable to any particular product candidate. Approval policies, regulations or the type and amount of clinical data necessary to gain approval may change during the course of a product candidate’s clinical development and may vary among jurisdictions, and there may be varying interpretations of data obtained from preclinical studies or clinical trials, any of which may cause delays or limitations in the approval or the decision not to approve an application. Regulatory agencies can delay, limit or deny approval of a product candidate for many reasons, including:
| | • | | the FDA or comparable foreign regulatory authorities may disagree with the design or implementation of any future clinical trials; |
| | • | | we may be unable to demonstrate to the satisfaction of the FDA or comparable foreign regulatory authorities that a product candidate is safe and effective for its proposed indication; |
| | • | | the results of any future clinical trials may not meet the level of statistical or clinical significance required by the FDA or comparable foreign regulatory authorities for approval; |
| | • | | the patients recruited for a particular clinical program may not be sufficiently broad or representative to assure safety in the full population for which we seek approval; |
| | • | | the results of any future clinical trials may not confirm the positive results from earlier preclinical studies or clinical trials; |
| | • | | we may be unable to demonstrate that a product candidate’s clinical and other benefits outweigh its safety risks; |
| | • | | the FDA or comparable foreign regulatory authorities may disagree with our interpretation of data from preclinical studies or clinical trials; |
| | • | | the data collected from any future clinical trials of our product candidates may not be sufficient to the satisfaction of FDA or comparable foreign regulatory authorities to support the submission of a biologics license application, or BLA, or other comparable submission in foreign jurisdictions or to obtain regulatory approval in the United States or elsewhere; |
| | • | | the FDA or comparable foreign regulatory authorities may only agree to approve a product candidate under conditions that are so restrictive that the product is not commercially viable; |
| | • | | regulatory agencies might not approve or might require changes to our manufacturing processes or facilities; or |
| | • | | regulatory agencies may change their approval policies or adopt new regulations in a manner rendering our clinical data insufficient for approval. |
Any delay in obtaining or failure to obtain required approvals could materially and adversely affect our ability to generate revenue from the particular product candidate, which likely would result in significant harm to
15
our financial position and adversely impact the price of the ADSs and Warrants. Furthermore, any regulatory approval to market a product may be subject to limitations on the indicated uses for which we may market the product. These limitations may limit the size of the market for the product.
We are not permitted to market our product candidates in the United States or in other countries until we receive approval of a BLA from the FDA or marketing approval from applicable regulatory authorities outside the United States. Obtaining approval of a BLA can be a lengthy, expensive and uncertain process. If we fail to obtain FDA approval to market our product candidates, we will be unable to sell our product candidates in the United States, which will significantly impair our ability to generate any revenues. In addition, failure to comply with FDA and non-U.S. regulatory requirements may, either before or after product approval, if any, subject us to administrative or judicially imposed sanctions, including:
| | • | | restrictions on our ability to conduct clinical trials, including full or partial clinical holds on ongoing or planned trials; |
| | • | | restrictions on the products, manufacturers or manufacturing process; |
| | • | | civil and criminal penalties; |
| | • | | suspension or withdrawal of regulatory approvals; |
| | • | | product seizures, detentions or import bans; |
| | • | | voluntary or mandatory product recalls and publicity requirements; |
| | • | | total or partial suspension of production; |
| | • | | imposition of restrictions on operations, including costly new manufacturing requirements; and |
| | • | | refusal to approve pending BLAs or supplements to approved BLAs. |
Even if we do receive regulatory approval to market a product candidate, any such approval may be subject to limitations on the indicated uses for which we may market the product. It is possible that none of our existing product candidates or any product candidates we may seek to develop in the future will ever obtain the appropriate regulatory approvals necessary for us or our collaborators to commence product sales. Any delay in obtaining, or an inability to obtain, applicable regulatory approvals would prevent us from commercializing our product candidates, generating revenues and achieving and sustaining profitability.
Because our product candidates are based on a novel technology, it is difficult to predict the time and cost of product candidate development and subsequently obtaining regulatory approval.
The clinical trial requirements of the FDA and comparable foreign regulatory authorities and the criteria these regulators use to determine the safety and efficacy of a product candidate vary substantially according to the type, complexity, novelty and intended use and market of such product candidates. The regulatory approval process for novel product candidates such as ours can be more expensive and take longer than for other pharmaceutical product candidates. The FDA and comparable foreign regulatory authorities have relatively limited experience with ddRNAi-based therapeutics, which makes it difficult to determine how long it will take or how much it will cost to obtain regulatory approvals for our product candidates in the United States or other countries. We and our current collaborators, or any future collaborators, may never receive approval to market and commercialize any product candidate. Even if we or a collaborator obtain regulatory approval, the approval may be for disease indications or patient populations that are not as broad as we intended or desired and may require labeling that includes significant use or distribution restrictions or safety warnings.
Regulatory requirements governing gene and cell therapy products have changed frequently and may continue to change in the future. For example, the FDA has established the Office of Cellular, Tissue and Gene
16
Therapies within its Center for Biologics Evaluation and Research, or CBER, to consolidate the review of gene therapy and related products, and the Cellular, Tissue and Gene Therapies Advisory Committee to advise CBER on its review. Gene therapy clinical trials conducted at institutions that receive funding for recombinant DNA research from the United States National Institutes of Health, or the NIH, are also subject to review by the NIH Office of Biotechnology Activities’ Recombinant DNA Advisory Committee, or the RAC. Although the FDA decides whether individual gene therapy protocols may proceed, the RAC review process can delay the initiation of a clinical trial, even if the FDA has reviewed the trial design and details and approved its initiation. Conversely, the FDA can put a proposed biological product on clinical hold even if the RAC has provided a favorable review of the product. Also, before a clinical trial can begin at any institution, that institution’s institutional review board, or IRB, and its institutional biosafety committee, or IBC, if it has one, have to review the proposed clinical trial to assess the safety of the trial. In addition, adverse developments in clinical trials of gene therapy products conducted by others may cause the FDA or comparable foreign regulatory bodies to change the requirements for approval of any of our product candidates.
These committees and advisory groups and the new guidelines they promulgate and new requirements they may impose may lengthen the clinical development and regulatory review process, require us to perform additional studies, increase our development costs, lead to changes in regulatory positions and interpretations, delay or prevent approval and commercialization of these product candidates or lead to significant post-approval limitations or restrictions. As we advance our product candidates, we will be required to consult with these regulatory committees and advisory groups, and comply with applicable guidelines and requirements as they may change from time to time. If we fail to do so, we may be required to delay or discontinue development of our product candidates. These additional processes may result in a development, review and approval process that is longer than we otherwise would have expected for our product candidates. Delay or failure to obtain, or unexpected costs in obtaining, the regulatory approval necessary to bring a potential product to market would delay or prevent us from commercializing our product candidates, generating revenues and achieving and sustaining profitability.
Preliminary positive results from the clinical trial of TT-034, our now discontiuued product candidate that advanced to early stage clinical trials, are not necessarily predictive of the results of any future clinical trials for any other product candidates.
As of March 2016, nine patients had been dosed in our Phase I/IIa clinical trial for TT-034. In the seven biopsied patients for whom we reported results, we have observed dose-dependent transduction of TT-034 into hepatic tissues and increases in shRNA. The preliminary results from an early stage clinical trial are not necessarily predictive of the final results of the trial, and the clinical trial for TT-034 was discontinued prior to completion for commercial reasons.
The biological effect reported for these TT-034 patients is not statistically significant and might not be observed in any other patients treated with TT-034. It is also important to note that in these dose cohorts, the biological response was designed to be, and was observed to be, lower than the level required to achieve a therapeutic effect in those patients.
Positive results from preclinical studies of our product candidates are not necessarily predictive of the results of our planned clinical trials of our product candidates.
Positive results in preclinical proof-of-concept and animal studies of our product candidates may not result in positive results in clinical trials in humans. Many companies in the pharmaceutical and biotechnology industries have suffered significant setbacks in clinical trials after achieving positive results in preclinical development or early stage clinical trials, and we cannot be certain that we will not face similar setbacks. These setbacks have been caused by, among other things, preclinical findings made while clinical trials were underway or safety or efficacy observations made in clinical trials, including adverse events. Moreover, preclinical and clinical data are often susceptible to varying interpretations and analyses, and many companies that believed their product candidates performed satisfactorily in preclinical studies and clinical trials nonetheless failed to obtain
17
FDA or other regulatory authority approval. If we fail to produce positive results in our clinical trials of our product candidates, the development timeline and regulatory approval and commercialization prospects for our product candidates, and, correspondingly, our business and financial prospects, would be negatively impacted.
Issues that may impact ddRNAi delivery to the cell could adversely affect or limit our ability to develop and commercialize product candidates.
Successful clinical development of ddRNAi-based therapeutics is largely dependent on using the appropriate vectors to obtain therapeutically relevant concentrations of the DNA constructs that express the shRNAs in the appropriate target cells. To develop effective product candidates, we will need to license delivery technologies from third parties or develop delivery technologies with research collaborators. Although delivery technologies, including AAV vectors, have been identified and are well defined for specific tissue types, we continue to seek vectors with ideal delivery properties for other indications we are pursuing, including OPMD. The tissue tropism and other physical properties of AAV vectors are limited and may not be effective for other product candidates or delivery into a wide array of tissues types. AAV vectors can also trigger immune responses in some patients, and those patients will not derive clinical benefit from administration of a product candidate unless steps are taken to clinically address the issue. If we or our collaborators are not successful in identifying effective vectors for our product candidates, we may not succeed in developing marketable products. In addition, if we are unable to reach agreement on favorable terms, or at all, with providers of any effective vectors that we do identify, we may not succeed in completing our clinical trials or commercializing our products on a timely or profitable basis, if at all. We have only one such agreement in place that allows us to use a vector both for clinical trials and for commercialization, and that agreement is with respect to our program for the treatment of AMD.
We use AAV vectors as part of our ddRNAi approach for several indications. As such, we require licenses and the ability to manufacture large quantities of AAV particles under the FDA’s current good manufacturing practices, or cGMP, requirements and those of comparable foreign regulatory authorities in order to commercialize a product candidate using an AAV vector.
We may find it difficult to enroll patients in our clinical trials, and patients could discontinue their participation in any future clinical trials, which could delay or prevent any future clinical trials of our product candidates and make those trials more expensive to undertake.
Identifying and qualifying patients to participate in any future clinical trials of our product candidates is critical to our success. The timing of our clinical trials depends on the speed at which we can recruit patients to participate in testing our product candidates. Patients may be unwilling to participate in any future clinical trials because of negative publicity from adverse events in the biotechnology, RNAi or gene therapy industries. Patients may be unavailable for other reasons, including competitive clinical trials for similar patient populations, and the timeline for recruiting patients, conducting trials and obtaining regulatory approval of potential products may be delayed. If we have difficulty enrolling a sufficient number of patients to conduct any future clinical trials as planned, we may need to delay, limit or discontinue those clinical trials. Clinical trial delays could result in increased costs, slower product development, setbacks in testing the effectiveness of our technology or discontinuation of the clinical trials altogether.
We may not be able to identify, recruit and enroll a sufficient number of patients, or those with required or desired characteristics to achieve diversity in a trial, to complete any future clinical trials in a timely manner. Patient enrollment is affected by factors including:
| | • | | finding and diagnosing patients; |
| | • | | severity of the disease under investigation; |
| | • | | design of the clinical trial protocol; |
| | • | | size and nature of the patient population; |
18
| | • | | eligibility criteria for the trial in question; |
| | • | | perceived risks and benefits of the product candidate under study; |
| | • | | proximity and availability of clinical trial sites for prospective patients; |
| | • | | availability of competing therapies and clinical trials; |
| | • | | clinicians’ and patients’ perceptions of the potential advantages of the product being studied in relation to other available therapies, including any new products that may be approved for the indications we are investigating; |
| | • | | patient referral practices of physicians; and |
| | • | | ability to monitor patients adequately during and after treatment. |
For example, we experienced some difficulties in enrolling patients in our clinical trial of TT-034, which was discontinued for commercial reasons. These difficulties were due to several factors, including sudden changes in patients’ viral load, liver enzymes and other clinical parameters immediately prior to their dosing, as well as late withdrawal due to personal reasons. We believe the increased availability of new and effective therapies such as Sovaldi and Harvoni, which were recently approved for treatment of the hepatitis C virus, and the fact that the early lower-dose cohort patients receive a sub-therapeutic dose of TT-034, may also have been contributing factors. We may face similar difficulties enrolling patients in clinical trials for other product candidates for these and other reasons.
We or our collaborators plan to seek initial marketing approval for our product candidates in the United States, Australia and Europe. We may not be able to initiate or continue any future clinical trials in a timely manner if we cannot enroll a sufficient number of eligible patients to participate in the clinical trials required by the FDA or comparable foreign regulatory agencies. Our ability to successfully initiate, enroll and complete a clinical trial in any foreign country is subject to numerous risks unique to conducting business in foreign countries, including:
| | • | | difficulty in establishing or managing relationships with contract research organizations, or CROs, clinical sites and physicians; |
| | • | | different standards for the conduct of clinical trials; |
| | • | | our inability to locate and engage qualified local consultants, physicians and partners; and |
| | • | | the potential burden of complying with a variety of foreign laws, medical standards and regulatory requirements, including the regulation of pharmaceutical and biotechnology products and treatments. |
In addition, patients enrolled in any future clinical trials may discontinue their participation at any time during the trial as a result of a number of factors, including experiencing adverse events, which may or may not be judged related to our product candidates under evaluation. The discontinuation of patients in any one of our trials may cause us to delay or discontinue our clinical trial, or cause the results from that trial not to be positive or sufficient to support either partnering with a pharmaceutical company to further develop and commercialize the product candidate or filing for regulatory approval of the product candidate.
We may encounter substantial delays in any future clinical trials or we may fail to demonstrate safety and efficacy to the satisfaction of applicable regulatory authorities.
None of our proprietary product candidates are currently in clinical trials. Before obtaining marketing approval from regulatory authorities for the sale of any of our product candidates, we must conduct extensive clinical trials to demonstrate the safety and efficacy of the product candidates in humans. Clinical trials are expensive and time-consuming, and their outcome is uncertain. We cannot guarantee that any clinical trials will be initiated or conducted as planned or completed on schedule, if at all. A failure of one or more clinical trials
19
can occur at any stage of testing. Events that may prevent successful or timely completion of clinical development include:
| | • | | inability to generate sufficient preclinical, toxicology or other data to support the initiation of human clinical trials; |
| | • | | delays in reaching consensus with regulatory agencies on trial design; |
| | • | | identifying, recruiting and training suitable clinical investigators; |
| | • | | delays in reaching agreement on acceptable terms with prospective CROs and clinical trial sites; |
| | • | | delays in obtaining required IRB or IBC approval at each clinical trial site; |
| | • | | delays in recruiting suitable patients to participate in our clinical trials; |
| | • | | imposition of a clinical hold by regulatory agencies, including after an inspection of our clinical trial operations or trial sites; |
| | • | | failure by our CROs, other third parties or us to adhere to clinical trial requirements; |
| | • | | failure to perform in accordance with the FDA’s good clinical practices, or GCP, or applicable regulatory requirements in other countries; |
| | • | | inability to manufacture, test, release, import or export for use sufficient quantities of our product candidates for use in clinical trials; |
| | • | | failure to manufacture our product candidate in accordance with cGMP requirements or applicable regulatory guidelines in other countries; |
| | • | | delays in the testing, validation and manufacturing of our product candidates; |
| | • | | delays in the delivery of our product candidates to the clinical trial sites; |
| | • | | delays in having patients complete participation in a trial or return for post-treatment follow-up; |
| | • | | clinical trial sites dropping out of a trial; |
| | • | | occurrence of serious adverse events associated with the product candidate that are viewed to outweigh its potential benefits; |
| | • | | changes in regulatory requirements and guidance that require amending or submitting new clinical trial protocols; or |
| | • | | clinical trials of our product candidates may produce negative or inconclusive results, and we may decide, or regulators may require us, to conduct additional clinical trials or discontinue product development programs. |
Further, a clinical trial may be suspended or discontinued by us, our collaborators, the IRBs or the IBCs at the sites in which such trials are being conducted, the data safety monitoring board, or DSMB, for such trial, or by the FDA or comparable foreign regulatory authorities due to a number of factors, including the imposition of a clinical hold or termination of a trial due to failure to conduct the clinical trial in accordance with regulatory requirements or our clinical protocols, unforeseen safety issues or adverse side effects of our product candidate, or a product candidate from another company that shares similar properties, failure to demonstrate adequate benefit from using a product candidate, changes in governmental regulations or administrative actions or lack of adequate funding to continue the clinical trial. If we experience discontinuation of, or delays in the completion of, any clinical trial of our product candidates, the commercial prospects of our product candidates may be harmed, and our ability to generate product revenues from any of these product candidates may be eliminated or delayed. Furthermore, many of the factors that lead to a delay in the commencement or completion of clinical trials may also ultimately lead to the denial of regulatory approval of our product candidates.
20
In addition, if we or our third-party collaborators make significant manufacturing or formulation changes to our product candidates, we or they may need to conduct additional studies to bridge the modified product candidates to earlier versions to ensure comparability and safety of the two different product candidates.
Any inability to successfully complete preclinical and clinical development could result in additional costs to us or impair our ability to commercialize our programs and product candidates. Clinical trial delays could also shorten any periods during which we may have the exclusive right to commercialize our product candidates or allow our competitors to bring products to market before we do, which could impair our ability to successfully commercialize our product candidates.
If the results of any future clinical trials are inconclusive or if there are safety concerns or adverse events associated with our product candidates, we may:
| | • | | fail to obtain, or be delayed in obtaining, marketing approval for our product candidates; |
| | • | | obtain approval for indications or patient populations that are not as broad as intended or desired; |
| | • | | obtain approval with labeling that includes significant use or distribution restrictions or safety warnings; |
| | • | | need to change the way the product is administered; |
| | • | | be required to perform additional clinical trials to support approval or be subject to additional post-marketing testing requirements; |
| | • | | have regulatory authorities withdraw their marketing approval of the product after granting it; |
| | • | | have regulatory authorities impose restrictions on distribution of the product in the form of a risk evaluation and mitigation strategy, or REMS, or modified REMS, that limit our ability to commercialize the product; |
| | • | | be subject to the addition of labeling statements, such as warnings or contraindications; |
| | • | | be sued and held liable for harm caused to patients; or |
| | • | | experience damage to our reputation. |
Any of these events could prevent us from achieving or maintaining market acceptance of our product candidates and impair our ability to commercialize our product candidates.
In addition, principal investigators for our clinical trials may serve as scientific advisors or consultants to us from time to time and may receive cash or equity compensation in connection with such services. If these relationships and any related compensation result in perceived or actual conflicts of interest, or the FDA concludes that the financial relationship may have affected the interpretation of any particular study, the integrity of the data generated at the applicable clinical trial site may be questioned and the utility of the clinical trial itself may be jeopardized, which could result in the delay or rejection of any BLA we submit to the FDA or any comparable foreign regulating authorities. Any such delay or rejection could prevent us from commercializing our product candidates.
Our product candidates and the process for administering our product candidates may cause undesirable side effects or have other properties that could delay or prevent their regulatory approval, limit the commercial profile of an approved label, or result in significant negative consequences following any potential marketing approval.
Treatment with our product candidates may produce undesirable side effects or adverse reactions or events. For example, the AAV vector and related capsid protein, which we are currently using to deliver most of our ddRNAi product candidates, could cause adverse immunological side effects due to preexisting and/or recall responses to the naturally occurring virus from which the vector is engineered, or to the DNA construct product
21
itself. These responses may also interfere with therapeutic efficacy if not identified and managed optimally. Preexisting immune responses to AAV manifesting as neutralizing antibodies are common within the general population and may be a limitation to the enrollment of patients in gene therapy clinical trials using AAV vectors, the successful use of AAV vectors in gene therapy clinical trials and the market acceptance of product candidates, if approved, that are delivered using AAV vectors. Patients with neutralizing antibodies to AAV will not derive clinical benefit from administration of such a product candidate unless steps are taken to clinically address the issue and those treatments themselves may cause adverse effects. In previous clinical trials undertaken by other companies involving systemic administration of AAV viral vectors for gene therapy, some subjects experienced adverse events, including the development of a negative T cell response against the AAV capsid protein. If our vectors cause similar adverse events, we may be required to delay or discontinue further clinical development of our product candidates. It is also possible that we may discover new adverse events related to AAV or other vectors, which could potentially enhance the risk to patients who use our product candidates delivered with that vector.
If any such adverse events occur, any future clinical trials could be suspended or discontinued and the FDA or comparable foreign regulatory authorities could order us to cease further development or deny approval of our product candidates for any or all targeted indications. The product-related side effects could affect patient recruitment or the ability of enrolled patients to complete the trial. If we elect or are required to delay, suspend or discontinue any clinical trial of any of our product candidates, the commercial prospects of such product candidates will be harmed and our ability to generate product revenues from any of these product candidates will be delayed or eliminated. Any of these occurrences may harm our business, financial condition and prospects significantly.
Additionally, if any of our product candidates receive marketing approval, the FDA could require us to adopt a REMS to ensure that the benefits of the product outweigh its risks, which may include, among other things, a medication guide outlining the risks of gene therapies for distribution to patients and a communication plan to patients and healthcare practitioners. Other elements to assure safe use in a mandated REMS could include, but are not limited to, restrictions upon distribution and prescribing, additional prescriber training, establishment of patient registries and other measures that could limit commercialization of the product. Comparable foreign regulating authorities might require adoption of measures similar to a REMS. Furthermore, if we or others later identify undesirable side effects caused by our product candidate, a number of potentially significant negative consequences could result, including:
| | • | | regulatory authorities may withdraw approvals of such product candidate; |
| | • | | regulatory authorities may require additional warnings on the label; |
| | • | | we may be required to create a medication guide outlining the risks of such side effects for distribution to patients; |
| | • | | we may be required to change the way a product candidate is administered or conduct additional clinical trials; |
| | • | | we could be sued and held liable for harm caused to patients; and |
| | • | | our reputation may suffer. |
Any of these events could prevent us from achieving or maintaining market acceptance of our product candidates and could significantly harm our business, prospects, financial condition and results of operations.
If we are unable to successfully develop related diagnostics for our therapeutic product candidates, or experience significant delays in doing so, we may not achieve marketing approval or realize the full commercial potential of our therapeutic product candidates.
We plan to develop related diagnostics for some of our therapeutic product candidates. Such related diagnostics are subject to regulation by the FDA and comparable foreign regulatory authorities as medical
22
devices and require separate regulatory approval or clearance prior to commercialization. Marketing approval or clearance of the diagnostic will require sufficient data to support its safety and efficacy. In addition, at least in some cases, the FDA and comparable foreign regulatory authorities may require the development and regulatory approval or clearance of a related diagnostic as a condition to approving our therapeutic product candidates. While we have some, limited experience in developing diagnostics, we plan to rely in large part on third parties to perform these functions. We may seek to enter into arrangements with one or more third parties to create a related diagnostic for use with our current or future product candidates.
If we, or any third parties that we engage to assist us, are unable to successfully develop or obtain marketing approval or clearance for related diagnostics for our therapeutic product candidates, or experience delays in doing so:
| | • | | the development of relevant product candidates may be delayed or impaired altogether if we are unable to appropriately select patients for enrollment in our clinical trials; |
| | • | | our relevant therapeutic product candidate may not receive marketing approval if its effective use depends on a related diagnostic in the regulatory authority’s judgment; and |
| | • | | we may not realize the full commercial potential of any therapeutic product candidates that receive marketing approval if, among other reasons, we are unable to appropriately identify patients with the specific genetic alterations targeted by our therapeutic product candidates. |
If any of these events were to occur, our business would be harmed.
Even if we complete the necessary preclinical studies and clinical trials, we cannot predict when or if we will obtain regulatory approval to commercialize a product candidate or the approval may be for a more narrow indication than we expect.
We cannot commercialize a product until the appropriate regulatory authorities have reviewed and approved the product candidate. Even if our product candidates demonstrate safety and efficacy in clinical trials, the regulatory agencies may not complete their review processes in a timely manner, or we may not be able to obtain regulatory approval. Additional delays may result if an FDA Advisory Committee or comparable foreign regulatory authority recommends non-approval or restrictions on approval. In addition, we may experience delays or rejections based upon additional government regulation from future legislation or administrative action, or changes in regulatory agency policy during the period of product development, clinical trials and the review process. Regulatory agencies also may approve a treatment candidate for fewer or more limited indications than requested, may not approve the price we intend to charge for our product candidate, may limit our ability to promote the product, may impose significant limitations upon the approval of the product, including, but not limited to, narrow indications, significant warnings, precautions or contraindications with respect to conditions of use, or may grant approval subject to the performance of post-marketing studies. In addition, regulatory agencies may not approve the labeling claims that are necessary or desirable for the successful commercialization of our product candidates. The FDA or comparable foreign regulatory authorities may impose a REMS or other conditions upon our approval that limit our ability to commercialize the product candidate.
Even if we obtain regulatory approval for a product candidate, our products will remain subject to regulatory scrutiny.
Even if we obtain regulatory approval in a jurisdiction, the regulatory authority may still impose significant restrictions on the indicated uses or marketing of our product candidates, or impose ongoing requirements for potentially costly post-approval studies or post-market surveillance. For example, the holder of an approved BLA is obligated to monitor and report to the FDA adverse events and any failure of a product to meet the specifications in the BLA. FDA guidance advises that patients treated with some types of gene therapy undergo follow-up observations for potential adverse events for as long as 15 years. The holder of an approved BLA must
23
also submit new or supplemental applications and obtain FDA approval for certain changes to the approved product, product labeling or manufacturing process. Advertising and promotional materials must comply with FDA rules and are subject to FDA review, in addition to other potentially applicable foreign, federal and state laws.
In addition, product manufacturers and their establishments, products and applications are subject to payment of user fees and continual review and periodic inspections by the FDA and comparable foreign regulatory authorities for compliance with cGMP and comparable foreign requirements, and adherence to commitments made in the BLA. If we or a regulatory agency discover previously unknown problems with a product such as adverse events of unanticipated severity or frequency, or problems with the facility where the product is manufactured, a regulatory agency may impose restrictions relative to that product or the manufacturing facility, including requiring recall or withdrawal of the product from the market or suspension of manufacturing.
If we fail to comply with applicable regulatory requirements following approval of any of our product candidates, a regulatory agency may:
| | • | | issue a warning letter asserting that we are in violation of the law; |
| | • | | seek an injunction or impose civil or criminal penalties or monetary fines; |
| | • | | suspend or withdraw regulatory approval; |
| | • | | suspend any ongoing clinical trials; |
| | • | | refuse to permit government reimbursement of our product by government-sponsored third-party payors; |
| | • | | refuse to approve a pending BLA or supplements to a BLA submitted by us for other indications or new product candidates; |
| | • | | refuse to allow us to enter into or continue supply contracts, including government contracts. |
Any government investigation of alleged violations of law could require us to expend significant time and resources in response and could generate negative publicity. The occurrence of any event or penalty described above may inhibit our ability to commercialize our product candidates and generate revenues.
Even if we obtain and maintain approval for our product candidates from the FDA, we may never obtain approval for our product candidates outside of the United States, which would limit our market opportunities and adversely affect our business.
Approval of a product candidate in the United States by the FDA does not ensure approval of such product candidate by regulatory authorities in other countries or jurisdictions, and approval by one foreign regulatory authority does not ensure approval by regulatory authorities in other foreign countries or by the FDA. Sales of our product candidates outside of the United States will be subject to foreign regulatory requirements governing clinical trials and marketing approval. Even if the FDA grants marketing approval for a product candidate, comparable regulatory authorities of foreign countries must also approve the manufacturing and marketing of the product candidates in those countries. Approval procedures vary among jurisdictions and can involve requirements and administrative review periods different from, and greater than, those in the United States, including additional preclinical studies or clinical trials. In many countries outside the United States, a product candidate must be approved for reimbursement before it can be approved for sale in that country. In some cases, the price that we intend to charge for our products, if approved, is also subject to approval. Even if a product candidate is approved, the FDA or comparable regulatory authorities in other countries, as the case may be, may limit the indications for which the product may be marketed, require extensive warnings on the product labeling or require expensive and time-consuming clinical trials or reporting as conditions of approval. Regulatory authorities in countries outside of the United States also have requirements for approval of product candidates
24
with which we must comply prior to marketing in those countries. Obtaining foreign regulatory approvals and compliance with foreign regulatory requirements could result in significant delays, difficulties and costs for us and could delay or prevent the introduction of our product candidates in certain countries.
Further, clinical trials conducted in one country may not be accepted by regulatory authorities in other countries. Regulatory approval of a product candidate in one country does not ensure approval in any other country, but a failure or delay in obtaining regulatory approval in one country may have a negative effect on the regulatory approval process in other countries. Also, regulatory approval for any of our product candidates may be withdrawn based on adverse events reported or regulatory decisions made in other countries. If we fail to comply with the regulatory requirements in international markets and/or fail to receive applicable marketing approvals, our target market will be reduced and our ability to realize the full market potential of our product candidates will be compromised and our business may be adversely affected.
Our future prospects are also dependent on our or our collaborators’ ability to successfully develop a pipeline of additional product candidates, and we and our collaborators may not be successful in efforts to use our platform technologies to identify or discover additional product candidates.
The success of our business depends primarily upon our ability to identify, develop and commercialize products based on our platform technology. We do not have any products on the market and are early in our development efforts. All of our product candidates are in preclinical development. Our product candidates derived from our platform technology may not successfully complete IND-enabling studies, and our research programs may fail to identify other potential product candidates for clinical development for a number of reasons. Our and our collaborators’ research methodology may be unsuccessful in identifying potential product candidates, our potential product candidates may not demonstrate the necessary preclinical outcomes to progress to clinical studies, or our product candidates may be shown to have harmful side effects or may have other characteristics that may make the products unmarketable or unlikely to receive marketing approval.
If any of these events occur, we may be forced to discontinue our development efforts for a program or programs. Research programs to identify new product candidates require substantial technical, financial and human resources. We may focus our efforts and resources on potential programs or product candidates that ultimately prove to be unsuccessful.
We may not be able to obtain orphan drug exclusivity for our product candidates.
Of our current product candidates, the only one designed for treatment of an indication that would likely qualify for rare disease status is Pabparna for the treatment of OPMD. Regulatory authorities in some jurisdictions, including the United States and the European Union, may designate drugs or biological products for relatively small patient populations as orphan drugs. Under the Orphan Drug Act, the FDA may designate a product as an orphan drug if it is a product intended to treat a rare disease or condition, which is generally defined as a patient population of fewer than 200,000 individuals annually in the United States. The FDA may also designate a product as an orphan drug if it is intended to treat a disease or condition of more than 200,000 individuals in the United States and there is no reasonable expectation that the cost of developing and making a drug or biological product available in the United States for this type of disease or condition will be recovered from sales of the product candidate. Under the European Union orphan drug legislation, a rare disease or condition means a disease or condition which affects not more than five in ten thousand persons in the European Union at the time of the orphan drug designation application.
Generally, if a product with an orphan drug designation subsequently receives the first marketing approval for the indication for which it has such designation, the product is entitled to a period of marketing exclusivity, which precludes the FDA from approving another marketing application for the same drug for such indication for that time period. During the marketing exclusivity period, in the European Union, the European Medicines Agency, or the EMA, is precluded from approving a similar drug with an identical therapeutic indication. The
25
applicable period is seven years in the United States and ten years in the European Union. The European Union exclusivity period can be reduced to six years if a drug no longer meets the criteria for orphan drug designation or if the drug is sufficiently profitable so that market exclusivity is no longer justified. Orphan drug exclusivity may be lost if the FDA or EMA determines that the request for designation was materially defective or if the manufacturer is unable to assure sufficient quantity of the drug to meet the needs of patients with the rare disease or condition.
Even if we obtain orphan drug exclusivity for a product, that exclusivity may not effectively protect the product from competition because different drugs can be approved for the same condition, and the same drug could be approved for a different condition. Even after an orphan drug is approved, the FDA can subsequently approve the same drug, made by a competitor, for the same condition if the FDA concludes that the competitive product is clinically superior in that it is shown to be safer, more effective or makes a major contribution to patient care. In the European Union, the EMA can approve a competitive product if the orphan drug no longer meets the criteria for orphan designation (including sufficient profitability), if the competitive product is safer, more effective or otherwise clinically superior, or if the orphan drug cannot be supplied in sufficient quantities.
Risks Related to Our Reliance on Third Parties
Our prospects for successful development and commercialization of our products are dependent to varying degrees upon the research, development, commercialization, and marketing efforts of our collaborators.
We rely on third parties for certain aspects of the research, development, commercialization and marketing of our current and any future product candidates. Other than as provided for in our collaboration agreements, we have no control over the resources, time and effort that our collaborators may devote to the development of product candidates using our ddRNAi technology. We are dependent on our collaborators to conduct some aspects of the research and development of each of our product candidates, and expect to need access to them to facilitate and/or to complete the regulatory process. We will likely rely on a pharmaceutical company for the successful marketing and commercialization of any such product candidates for which they/we receive approval, if any. There can be no guarantee at this stage that we will conclude a partnership with such a company on favorable terms, or at all, nor even if we do so, that success will be achieved.
Our ability to recognize revenues from successful collaborations may be impaired by multiple factors including:
| | • | | a collaborator may shift its priorities and resources away from our programs due to a change in business strategies, or a merger, acquisition, sale or downsizing of its company or business unit; |
| | • | | a collaborator may cease development in an area that is the subject of a collaboration agreement; |
| | • | | a collaborator may change the success criteria for a particular program or product candidate in development, thereby delaying or ceasing development of such program or product candidate in development; |
| | • | | a collaborator with commercialization obligations may not commit sufficient financial or human resources to the marketing, distribution or sale of a product; |
| | • | | a collaborator with manufacturing responsibilities may encounter regulatory, resource or quality issues and be unable to meet demand requirements; |
| | • | | a collaborator may exercise its rights under the agreement to discontinue our collaboration; |
| | • | | a dispute may arise between us and a collaborator concerning the development and commercialization of a product candidate in development, resulting in a delay in milestones, royalty payments, or discontinuation of a program and possibly resulting in costly litigation or arbitration that may divert management attention and resources; |
26
| | • | | a collaborator may not adequately protect the intellectual property rights associated with a product candidate; and |
| | • | | a collaborator may use our proprietary information or intellectual property in such a way as to expose us to litigation from a third party. |
If our collaborators do not perform in the manner we expect or fulfill their responsibilities in a timely manner, or at all, the development, regulatory and commercialization process could be delayed or discontinued or otherwise be unsuccessful. Conflicts between us and our collaborators may arise. In the event of discontinuation of one or more of our collaboration agreements, it may become necessary for us to assume the responsibility for any such product candidates at our own expense or seek new collaborators. In that event, we likely would be required to limit the size and scope of one or more of our independent programs or increase our expenditures and seek additional funding, which may not be available on acceptable terms or at all, and our business may be harmed.
We rely on third parties to conduct our preclinical studies and expect to rely on third parties to conduct any future clinical trials. If these third parties do not meet our deadlines or otherwise conduct the studies as required, we may be delayed in progressing, or ultimately may not be able to progress, product candidates to clinical trials, our clinical development programs could be delayed or unsuccessful, and we may not be able to commercialize or obtain regulatory approval for our product candidates when expected, or at all.
We do not have the ability to conduct all aspects of our preclinical testing or any future clinical trials ourselves. We are dependent on third parties to conduct the preclinical studies for our product candidates and will depend on third parties to conduct any future clinical trials for our product candidates, and therefore the timing of the initiation and completion of these trials and studies is reliant on third parties and may occur at times substantially different from our estimates or expectations.
In the case of clinical trials, we expect to rely on CROs and third-party collaborators to conduct any future clinical trials in accordance with our clinical protocols and regulatory requirements. We expect our CROs, investigators and third-party collaborators will play a significant role in the conduct of these trials and subsequent collection and analysis of data. There is no guarantee that any CROs, investigators or the other third-party collaborators on which we rely for administration and conduct of any future clinical trials will devote adequate time and resources to such trials or perform as contractually required. If any of these third parties fails to meet expected deadlines, fails to adhere to our clinical protocols, fails to meet regulatory requirements or otherwise performs in a substandard manner, any future clinical trials may be extended, delayed or terminated. If any of our future clinical trial sites terminates for any reason, we may lose all of the information on subjects enrolled in any then clinical trials.
If we cannot contract with acceptable third parties on commercially reasonable terms, or if these third parties do not carry out their contractual duties, satisfy legal and regulatory requirements for the conduct of preclinical studies or clinical trials or meet expected deadlines, our clinical development programs could be delayed or discontinued.
In all events, we are responsible for ensuring that each of our preclinical studies and any future clinical trials is conducted in accordance with the general investigational plan and protocols for the trial. The FDA requires clinical trials to be conducted in accordance with current GCP, including for conducting, recording and reporting the results of preclinical studies and clinical trials to assure that data and reported results are credible and accurate and that the rights and confidentiality of clinical trial participants are protected. Our reliance on third parties that we do not control does not relieve us of these responsibilities and requirements, and any failure to satisfy these responsibilities and requirements, whether caused by us or by third parties upon whom we rely, could have a material adverse effect on our business, financial condition, results of operations and prospects.
27
Because we rely on third-party manufacturing and supply partners, our supply of research and development, preclinical and clinical development materials may become limited or interrupted or may not be of satisfactory quantity or quality.
We rely on third-party supply and manufacturing partners to manufacture and supply the materials for our research and development and preclinical and clinical study supplies. We do not own manufacturing facilities or supply sources for such materials.
There can be no assurance that our supply of research and development, preclinical and clinical development biologics and other materials will not be limited, interrupted or restricted in certain geographic regions, be of satisfactory quality or continue to be available at acceptable prices. Replacement of a third-party manufacturer could require significant effort, cost and expertise because there may be a limited number of qualified replacements.
The manufacturing process for a product candidate is subject to FDA and foreign regulatory authority review. Suppliers and manufacturers must meet applicable manufacturing requirements and undergo rigorous facility and process validation tests required by regulatory authorities in order to comply with regulatory standards, such as cGMP. In the event that any of our suppliers or manufacturers fails to comply with such requirements or to perform its obligations to us in relation to quality, timing or otherwise, or if our supply of components or other materials becomes limited or interrupted for other reasons, we may be forced to manufacture the materials ourselves or enter into an agreement with another third party, which would be costly and delay any future clinical trials.
In some cases, the technical skills or technology required to manufacture our product candidates may be unique or proprietary to the original manufacturer and we may have difficulty, or there may be contractual restrictions prohibiting us from, transferring such skills or technology to another third party. These factors increase our reliance on our manufacturers and may require us to obtain a license from a manufacturer in order to have another third-party manufacture our product candidates. If we are required to change manufacturers for any reason, we will be required to verify that the new manufacturer maintains facilities and procedures that comply with quality standards and with all applicable regulations and guidelines of the FDA and comparable foreign regulatory authorities. The delays and costs associated with the verification of a new manufacturer could increase our costs and delay the development of our product candidates.
We expect to continue to rely on third-party manufacturers for preclinical and clinical grade product candidates and if we receive regulatory approval for any product candidate. To the extent that we have existing, or enter into future, manufacturing arrangements with third parties, we will depend on these third parties to perform their obligations in a timely manner consistent with contractual and regulatory requirements, including those related to quality control and assurance. If we are unable to obtain or maintain third-party manufacturing for product candidates, or to do so on commercially reasonable terms, we may not be able to develop and commercialize our product candidates successfully. Our or a third party’s failure to execute on our manufacturing requirements could adversely affect our business in a number of ways, including:
| | • | | an inability to conduct necessary preclinical studies to progress our product candidates to clinical trials; |
| | • | | an inability to initiate or continue any future clinical trials of product candidates under development; |
| | • | | delay in submitting regulatory applications, or receiving regulatory approvals, for product candidates; |
| | • | | loss of the cooperation of a collaborator; |
| | • | | subjecting our product candidates to additional inspections by regulatory authorities; |
| | • | | requirements to cease distribution or to recall batches of our product candidates; and |
| | • | | in the event of approval to market and commercialize a product candidate, an inability to meet commercial demands for our products. |
28
We and our collaborators may disagree over our right to receive payments under our collaboration agreements, potentially resulting in costly litigation and loss of reputation.
Our ability to receive payments under our collaboration agreements depends on our ability to clearly delineate our rights under those agreements. We have out-licensed portions of our intellectual property to our collaborators with the intent that our collaborators will develop product candidates based on our ddRNAi technology to address specific conditions, including HIV/AIDS, certain cancers, ocular diseases and genetic diseases and intractable neuropathic pain. However, a collaborator may use our intellectual property without our permission, dispute our ownership of intellectual property rights, or argue that our intellectual property does not cover, or add value to, any product candidates they develop. If a dispute arises, it may result in costly patent office procedures and litigation, and our collaborator may refuse to pay us while the dispute is ongoing. Furthermore, regardless of any resort to legal action, a dispute with a collaborator over intellectual property rights may damage our relationship with that collaborator and may also harm our reputation in the industry. Even if we are entitled to payments from our collaborators, we may not actually receive these payments, or we may experience difficulties in collecting the payments to which we believe we are entitled. After our collaborators launch commercial products containing our licensed traits, we will need to rely on the good faith of our collaborators to report to us the sales they earn from these products and to accurately calculate the payments we are entitled to, a process that will involve complicated and difficult calculations. Although we seek to address these concerns in our collaboration agreements by reserving our right to audit financial records, such provisions may not be effective.
We have only limited experience in regulatory affairs and intend to rely on consultants and other third parties for regulatory matters, which may affect our ability or the time we require to obtain necessary regulatory approvals.
We have limited experience in filing and prosecuting the applications necessary to gain regulatory approvals for gene therapy or ddRNAi product candidates. Moreover, the product candidates that are likely to result from our development programs are based on novel technologies that have not been extensively tested in humans. The regulatory requirements governing these types of product candidates may be less well defined or more rigorous than for conventional products. As a result, we may experience a longer regulatory process in connection with obtaining regulatory approvals of any products that we develop. We intend to rely on independent consultants for purposes of our regulatory compliance and product development and approvals in the United States and elsewhere. Any failure by our consultants to properly advise us regarding, or properly perform tasks related to, regulatory compliance requirements could compromise our ability to develop and seek regulatory approval of our product candidates.
Our reliance on third parties requires us to share our trade secrets, which increases the possibility that a competitor will discover them or that our trade secrets will be misappropriated or disclosed.
Because we rely on third parties to manufacture our product candidates, and because we collaborate with various organizations and academic institutions on the advancement of our technology and product candidates, we may, at times, share trade secrets with them. We seek to protect our proprietary technology in part by entering into confidentiality agreements and, if applicable, material transfer agreements, collaborative research agreements, consulting agreements or other similar agreements with our collaborators, advisors, employees and consultants prior to beginning research or disclosing proprietary information. These agreements typically limit the rights of the third parties to use or disclose our confidential information, such as trade secrets. Despite these contractual provisions, the need to share trade secrets and other confidential information increases the risk that such trade secrets become known by potential competitors, are inadvertently incorporated into the technology of others, or are disclosed or used in violation of these agreements. Given that our proprietary position is based, in part, on our know-how and trade secrets, discovery by a third party of our trade secrets or other unauthorized use or disclosure would impair our intellectual property rights and protections in our product candidates.
29
In addition, these agreements typically restrict the ability of our collaborators, advisors, employees and consultants to publish data potentially relating to our trade secrets. Our academic collaborators typically have rights to publish data, provided that we are notified in advance and may delay publication for a specified time in order to secure our intellectual property rights arising from the collaboration. In other cases, publication rights are controlled exclusively by us. In other cases we may share these rights with other parties. Despite our efforts to protect our trade secrets, our competitors may discover our trade secrets, either through breach of these agreements, independent development or publication of information including our trade secrets in cases where we do not have proprietary or otherwise protected rights at the time of publication.
Risks Related to Commercialization of Our Product Candidates
We have not entered into agreements with any third-party manufacturers to support commercialization of our product candidates. Additionally, no manufacturers have experience producing our product candidates at commercial levels, and any manufacturer that we work with may not achieve the necessary regulatory approvals or produce our product candidates at the quality, quantities, locations and timing needed to support commercialization.
We have not yet secured manufacturing capabilities for commercial quantities of our product candidates or established facilities in the desired locations to support commercialization of our product candidates.We intend to rely on third-party manufacturers for commercialization, but have not entered into any agreements with such manufacturers to support our product candidates currently in development. We may be unable to negotiate agreements with third-party manufacturers to support our commercialization activities on commercially reasonable terms.
We may encounter technical or scientific issues related to manufacturing or development that we may be unable to resolve in a timely manner or with available funds. Currently, we do not have the capacity to manufacture our product candidates on a commercial scale. In addition, our product candidates are novel, and no manufacturer currently has experience producing our product candidates on a large scale. If we are unable to engage manufacturing partners to produce our product candidates on a larger scale on reasonable terms, our commercialization efforts will be harmed.
Even if we timely develop a manufacturing process and successfully transfer it to the third-party manufacturers of our product candidates, if such third-party manufacturers are unable to produce the necessary quantities of our product candidates, or do so in compliance with cGMP or with pertinent foreign regulatory requirements, and within our planned time frame and cost parameters, the development and sales of our product candidates, if approved, may be impaired.
If we are unable to enter into agreements with third parties to commercialize our product candidates or establish sales and marketing capabilities to market and sell our product candidates, we may be unable to generate any revenues.
We currently have no sales and marketing organization and have no experience selling and marketing pharmaceutical products. To successfully commercialize any product candidates that may be approved, we will need to develop these capabilities, either through our relationships with collaborators or our own. We may seek to enter into collaborations with other entities to utilize their marketing and distribution capabilities, but we may be unable to enter into marketing agreements on favorable terms, if at all. If our future collaborators do not commit sufficient resources to commercialize our future products, if any, and we are unable to develop the necessary marketing capabilities on our own, we will be unable to generate sufficient product revenue to sustain our business. The establishment and development of our own sales force or the establishment of a contract sales force to market any products we may develop will be expensive and time-consuming and could delay any product launch. Moreover, we cannot be certain that we will be able to successfully develop this capability. We will be competing with many companies that currently have extensive and well-funded marketing and sales
30
operations to recruit, hire, train and retain marketing and sales personnel. We also face competition in our search for third parties to assist us with the sales and marketing efforts of our product candidates. Without an internal team or the support of a third party to perform marketing and sales functions, we may be unable to compete successfully against these more established companies.
Physicians, patients, third-party payors or others in the medical community may not be receptive to our product candidates, and we may not generate any future revenue from the sale or licensing of our product candidates.
Even if we obtain approval for a product candidate, we may not generate or sustain revenue from sales of the product if the product cannot be sold at a competitive cost or if it fails to achieve market acceptance by physicians, patients, third-party payors or others in the medical community. These market participants may be hesitant to adopt a novel treatment based on ddRNAi technology, and we may not be able to convince the medical community and third-party payors to accept and use, or to provide favorable reimbursement for, any product candidates developed by us or our existing or future collaborators. Market acceptance of our product candidates will depend on, among other factors:
| | • | | the safety and efficacy of our product candidates; |
| | • | | our ability to offer our products for sale at competitive prices; |
| | • | | the relative convenience and ease of administration of our product candidates; |
| | • | | the prevalence and severity of any adverse side effects associated with our product candidates; |
| | • | | the terms of any approvals and the countries in which approvals are obtained; |
| | • | | limitations or warnings contained in any labeling approved by the FDA or comparable foreign regulatory authorities; |
| | • | | conditions upon the approval imposed by FDA or comparable foreign regulatory authorities, including, but not limited to, a REMS; |
| | • | | the willingness of patients to try new treatments and of physicians to prescribe these treatments; |
| | • | | the availability of government and other third-party payor coverage and adequate reimbursement; and |
| | • | | availability of alternative effective treatments for the disease indications our product candidates are intended to treat and the relative risks, benefits and costs of those treatments. |
Since we are focused on the emerging therapeutic modality of ddRNAi, these risks may increase if new competitors are able to market ddRNAi-based therapeutics or if these treatments become less favored in the commercial marketplace. In addition, we believe that one of the benefits of our ddRNAi technology is the expected length of time of its effect. If our treatments do not have a long-term effect after administration, such a development would likely significantly and adversely affect market acceptance of our product candidates, if approved.
Additional risks apply in relation to any disease indications we pursue which are classified as rare diseases and allow for orphan drug designation by regulatory agencies in major commercial markets, such as the United States or European Union. If pricing is not approved or accepted in the market at an appropriate level for any approved product for which we pursue and receive an orphan drug designation, such product may not generate enough revenue to offset costs of development, manufacturing, marketing and commercialization despite any benefits received from the orphan drug designation, such as market exclusivity, for a period of time. Orphan exclusivity could temporarily delay or block approval of one of our products if a competitor obtains orphan drug designation for its product first. However, even if we obtain orphan exclusivity for one of our products upon approval, our exclusivity may not block the subsequent approval of a competitive product that is shown to be clinically superior to our product.
31
Market size is also a variable in disease indications not classified as rare. Our estimates regarding potential market size for any indication may be materially different from what we discover to exist at the time we commence commercialization, if any, for a product, which could result in significant changes in our business plan and have a material adverse effect on our business, financial condition, results of operations and prospects.
We face competition from entities that have developed or may develop product candidates for our target disease indications, including companies developing novel treatments and technology platforms based on modalities and technology similar to ours. If these companies develop technologies or product candidates more rapidly than we do or their technologies, including delivery technologies, are more effective, our ability to develop and successfully commercialize product candidates may be compromised.
The development and commercialization of pharmaceutical products is highly competitive. We compete with a variety of multinational pharmaceutical companies and specialized biotechnology companies, as well as technology being developed at universities and other research institutions. Our competitors have developed, are developing or could develop product candidates and processes competitive with our product candidates. Competitive therapeutic treatments include those that have already been approved and accepted by the medical community, patients and third-party payors, and any new treatments that enter the market.
We believe that a significant number of products are currently under development, and may become commercially available in the future, for the treatment of conditions for which we are developing, and may in the future try to develop, product candidates.
This increasingly competitive landscape may compromise the development of our product candidates. For example, improvements in the efficacy, delivery and success rates of competitors’ product candidates, in conjunction with a reduction in the price and duration of their treatments, diminished partnering interest from pharmaceutical companies in our product candidate TT-034 for the treatment of HCV. This caused us to announce in February 2016 the discontinuation of our program to develop TT-034 before the conclusion of its clinical trial, despite the promising clinical results regarding both the safety of that product candidate and hepatic tissue transduction achieved to date.
We are aware of multiple companies that are working in the field of RNAi therapeutics, including Alnylam, Arbutus, Arrowhead, Silence Therapeutics plc, RXi Pharmaceuticals Corporation, Quark Pharmaceuticals, Inc., Marina Biotech, Inc. and Dicerna. Arrowhead, Arbutus and Alnylam are all developing siRNA-based therapeutics for HBV. All of our current product candidates, if approved, would compete with approved and currently marketed treatments.
In addition, our product candidates would compete with antisense and other RNA-based pharmaceutical products currently under development. Like RNAi therapeutics, antisense products target mRNA with the objective of suppressing the activity of specific genes. The development of antisense products is more advanced than that of RNAi therapeutics, and antisense technology may become the preferred technology for products that target mRNAs.
Many of our competitors have significantly greater financial, technical, manufacturing, marketing, sales and supply resources and experience than we have. If we successfully obtain approval for any product candidate, we will face competition based on many different factors, including the safety and effectiveness of our products, the ease with which our products can be administered and the extent to which patients accept relatively new routes of administration, the timing and scope of regulatory approvals for these products, the availability and cost of manufacturing, marketing and sales capabilities, price, reimbursement coverage and patent position.
Competing products could present superior treatment alternatives, including by being more effective, safer, less expensive or marketed and sold more effectively than any products we may develop. Competitive products may make any products we develop obsolete or non-competitive before we recover the expense of developing
32
and commercializing our product candidates. Such competitors could also recruit our employees, which could negatively impact our level of expertise and our ability to execute our business plan.
A variety of risks associated with international operations could hurt our business.
If any of our product candidates are approved for commercialization, it is our current intention to market them on a worldwide basis, either alone or in collaboration with others. In addition, we conduct development activities in various jurisdictions throughout the world. We expect that we will be subject to additional risks related to engaging in international operations, including:
| | • | | different regulatory requirements for approval of pharmaceutical products in foreign countries; |
| | • | | reduced protection for intellectual property rights; |
| | • | | unexpected changes in tariffs, trade barriers and regulatory requirements; |
| | • | | economic weakness, including inflation, or political instability in particular foreign economies and markets; |
| | • | | compliance with tax, employment, immigration and labor laws for employees living or traveling abroad; |
| | • | | foreign currency fluctuations, which could result in increased operating expenses and reduced revenue, and other obligations incident to doing business in another country; |
| | • | | workforce uncertainty in countries where labor unrest is more common than in Australia or the United States; |
| | • | | production shortages resulting from any events affecting raw material supply or manufacturing capabilities abroad; and |
| | • | | business interruptions resulting from geopolitical actions, including war and terrorism, or natural disasters including earthquakes, typhoons, floods and fires. |
The insurance coverage and reimbursement status of newly approved products is uncertain. Failure to obtain or maintain coverage and adequate reimbursement for any of our product candidates that are approved could limit our ability to market those products and compromise our ability to generate revenue.
The availability of coverage and adequate reimbursement by governmental and private payors is essential for most patients to be able to afford expensive treatments. Sales of our product candidates will depend substantially, both in the United States and abroad, on the extent to which the costs of our product candidates will be paid by health maintenance, managed care, pharmacy benefit and similar healthcare management organizations, or reimbursed by third-party payors. If reimbursement is not available, or is available only to limited levels, we may not be able to successfully commercialize our product candidates. Even if coverage is provided, the reimbursement amounts approved by third-party payors may not be high enough to allow us to establish or maintain pricing sufficient to realize a return on our investment.
There is significant uncertainty related to the insurance coverage and reimbursement of newly approved products. In the United States, the principal decisions about reimbursement for new medicines are typically made by the Centers for Medicare & Medicaid Services, or CMS, an agency within the U.S. Department of Health and Human Services, as CMS decides whether and to what extent a new medicine will be covered and reimbursed under Medicare. Private payors tend to follow CMS to a substantial degree. It is difficult to predict what CMS will decide with respect to reimbursement for fundamentally novel products such as ours, as there is no body of established practices and precedents for these new products.
The intended use of a pharmaceutical product by a physician can also affect pricing. For example, CMS could initiate a National Coverage Determination administrative procedure, by which the agency determines
33
which uses of a therapeutic product would and would not be reimbursable under Medicare. This determination process can be lengthy, thereby creating a long period during which the future reimbursement for a particular product may be uncertain.
Outside the United States, international operations are generally subject to extensive governmental price controls and other market regulations, and we believe the increasing emphasis on cost-containment initiatives in Europe, Canada and other countries is likely to put pressure on the pricing and usage of any of our product candidates that may be approved for marketing in the future. In many countries, the prices of medical products are subject to varying price control mechanisms as part of national health systems. In general, the prices of medicines under such systems can be substantially lower than in the United States. Other countries allow companies to fix their own prices for medicines, but monitor and control company profits. In some countries, we or our collaborators may be required to conduct a clinical trial or other studies that compare the cost-effectiveness of our product candidates to other available therapies in order to obtain or maintain reimbursement or pricing approval. Additional foreign price controls or other changes in pricing regulation could restrict the amount that we are able to charge for our product candidates. Accordingly, in markets outside the United States, the reimbursement for our products may be reduced compared with the United States and may be insufficient to generate commercially reasonable revenue and profits.
Moreover, increasing efforts by governmental and other third-party payors, in the United States and abroad, to cap or reduce healthcare costs, resulting in legislation and reforms such as, in the United States, the Patient Protection and Affordable Care Act of 2010, as amended by the Health Care Education Reconciliation Act, or the ACA. The ACA may cause such organizations to limit both coverage and level of reimbursement for new products approved and, as a result, they may not cover or provide adequate payment for our product candidates. In addition, other legislative changes have been adopted since the ACA was enacted. These changes included aggregate reductions to Medicare payments to providers of up to 2% per fiscal year effective April 2013 that, due to subsequent legislative amendments to the statute, will stay in effect through 2024 unless additional Congressional action is taken. Additionally, in January 2013, President Obama signed into law the American Taxpayer Relief Act of 2012, which, among other things, reduced Medicare payments to several providers, and increased the statute of limitations period for the government to recover overpayments to providers from three to five years. These new laws may result in additional reductions in Medicare and other healthcare funding, which could negatively affect coverage or reduce the reimbursement for any of our product candidates that receive regulatory approval.
We expect to experience pricing pressures in connection with the sale of any of our product candidates, due to the trend toward managed healthcare, the increasing influence of health maintenance organizations and additional legislative changes. The downward pressure on healthcare costs in general, particularly prescription pharmaceutical products and surgical procedures and other treatments, has become very intense. As a result, increasingly high barriers are being erected to the entry of new products. We cannot predict what healthcare reform initiatives may be adopted in the future. Further federal, state and foreign legislative and regulatory developments are likely, and we expect ongoing initiatives to increase pressure on pharmaceutical product pricing. Such reforms could depress pricing for any product candidates that we may successfully develop and for which we may obtain regulatory approval and may negatively affect our overall financial condition and ability to develop additional product candidates.
Our relationships with third-party payors, healthcare professionals and customers in the United States and elsewhere may be subject, directly or indirectly, to applicable anti-kickback, fraud and abuse, false claims, transparency, health information privacy and security and other healthcare laws and regulations, which could expose us to significant penalties.
Our relationships with third-party payors, healthcare professionals and customers may expose us to broadly applicable fraud and abuse and other healthcare laws and regulations, including, without limitation, the federal Anti-Kickback Statute and the federal False Claims Act, that may constrain the business or financial arrangements and
34
relationships through which we sell, market and distribute any pharmaceutical products for which we obtain marketing approval. In addition, we may be subject to transparency laws and patient privacy regulation by the federal government and by the U.S. states and foreign jurisdictions in which we conduct our business. The applicable federal, state and foreign healthcare laws and regulations that may affect our ability to operate include, but are not limited to, the following:
| | • | | the federal Anti-Kickback Statute, which prohibits, among other things, persons and entities from knowingly and willfully soliciting, offering, receiving or providing remuneration, directly or indirectly, in cash or in kind, to induce or reward, or in return for, either the referral of an individual for, or the purchase, order or recommendation of, any good or service, for which payment may be made under federal and state healthcare programs such as Medicare and Medicaid; |
| | • | | federal civil and criminal false claims laws and civil monetary penalty laws, including the federal False Claims Act, which impose criminal and civil penalties, including civil whistleblower or qui tam actions, against individuals or entities for knowingly presenting, or causing to be presented, to the federal government, including the Medicare and Medicaid programs, claims for payment that are false or fraudulent or making a false statement to avoid, decrease or conceal an obligation to pay money to the federal government; |
| | • | | the federal Health Insurance Portability and Accountability Act of 1996, or HIPAA, which imposes criminal and civil liability for, among other things, executing a scheme to defraud any healthcare benefit program or making false statements relating to healthcare matters; |
| | • | | HIPAA, as amended by the Health Information Technology for Economic and Clinical Health Act of 2009, or HITECH, and their respective implementing regulations, which impose obligations on covered healthcare providers, health plans and healthcare clearinghouses, as well as their business associates that create, receive, maintain or transmit individually identifiable health information for or on behalf of a covered entity, with respect to safeguarding the privacy, security and transmission of individually identifiable health information; |
| | • | | the federal Open Payments program, created under the ACA, and its implementing regulations, which requires certain manufacturers of drugs, devices, biologics and medical supplies for which payment is available under Medicare, Medicaid or the Children’s Health Insurance Program, with specific exceptions, to report annually to the CMS information related to payments or other transfers of value made to physicians and applicable group purchasing organizations to report annually to CMS ownership and investment interests held by the physicians and their immediate family members by the 90th day of each subsequent calendar year, and disclosure of such information will be made by CMS on a publicly available website; and |
| | • | | analogous state and foreign laws and regulations, such as state anti-kickback and false claims laws, which may apply to sales or marketing arrangements and claims involving healthcare items or services reimbursed by non-governmental third-party payors, including private insurers; state and foreign laws that require pharmaceutical companies to comply with the pharmaceutical industry’s voluntary compliance guidelines and the relevant compliance guidance promulgated by the federal government or otherwise restrict payments that may be made to healthcare providers; state and foreign laws that require pharmaceutical manufacturers to report information related to payments and other transfers of value to physicians and other healthcare providers or marketing expenditures; and state and foreign laws governing the privacy and security of health information in certain circumstances, many of which differ from each other in significant ways and often are not preempted by HIPAA, thus complicating compliance efforts. |
Efforts to ensure that our business arrangements with third parties will comply with applicable healthcare laws and regulations may involve substantial costs. It is possible that governmental authorities will conclude that our business practices may not comply with current or future statutes, regulations or case law involving applicable fraud and abuse or other healthcare laws and regulations. If our operations are found to be in violation of any of these laws or any other governmental regulations that may apply to us, we may be subject to significant
35
civil, criminal and administrative penalties, including, without limitation, damages, fines, disgorgement, imprisonment, exclusion from participation in government healthcare programs, such as Medicare and Medicaid, and the curtailment or restructuring of our operations, which could have a material adverse effect on our business. If any of the physicians or other healthcare providers or entities with whom we expect to do business is found not to be in compliance with applicable laws, they may be subject to criminal, civil or administrative sanctions, including exclusions from participation in government healthcare programs, which could also materially affect our business.
Negative public opinion and increased regulatory scrutiny of gene therapy and genetic research may damage public perception of our product candidates or compromise our ability to conduct our business or obtain regulatory approvals for our product candidates.
Gene therapy remains a novel technology and no gene therapy product utilizing ddRNAi has been approved to date in the United States. Public perception may be influenced by claims that gene therapy is unsafe, and gene therapy may not gain the acceptance of the public or the medical community. In particular, our success will depend upon physicians specializing in the treatment of those diseases that our product candidates target prescribing treatments that involve the use of our product candidates in lieu of, or in addition to, existing treatments they are already familiar with and for which greater clinical data may be available. More restrictive government regulations or negative public opinion would have a negative effect on our business or financial condition and may delay or impair the development and commercialization of our product candidates or demand for any products we may develop. Our product candidates, including our viral delivery systems, could produce adverse events. Adverse events in our clinical trials or following approval of any of our product candidates, even if not ultimately attributable to our product candidates, could result in increased governmental regulation, unfavorable public perception, potential regulatory delays in the testing or approval of our product candidates, stricter labeling requirements for those product candidates that are approved and a decrease in demand for any such product candidates.
Risks Related to Our Business Operations
We may not successfully engage in strategic transactions or enter into new collaborations, which could adversely affect our ability to develop and commercialize product candidates, impact our cash position, increase our expenses and present significant distractions to our management.
From time to time, we may consider additional strategic transactions, such as collaborations, acquisitions, asset purchases or sales and out- or in-licensing of product candidates or technologies. In particular, we will evaluate and, if strategically attractive, seek to enter into additional collaborations, including with major biotechnology or pharmaceutical companies. The competition for collaborators is significant, and the negotiation process is time-consuming and complex. Any new collaboration may be on terms that are not optimal for us, and we may not be able to maintain any new or existing collaboration if, for example, development or approval of a product candidate is delayed, sales of an approved product candidate do not meet expectations or the collaborator discontinues the collaboration. Any such collaboration, or other strategic transaction, may require us to incur non-recurring or other charges, increase our expenditures, pose significant integration or implementation challenges or disrupt our management or business.
These transactions would entail numerous operational and financial risks, including exposure to unknown liabilities, incurrence of substantial debt or dilutive issuances of equity securities to pay transaction consideration or costs, higher than expected collaboration, acquisition or integration costs, write-downs of assets or goodwill or impairment charges, increased amortization expenses, difficulty and cost in facilitating the collaboration or combining the operations and personnel of any acquired business, impairment of relationships with key suppliers, manufacturers or customers of any acquired business due to changes in management and ownership and the inability to retain key employees of any acquired business.
36
Accordingly, although there can be no assurance that we will undertake or successfully complete any additional transactions of the nature described above, any transactions that we do complete may be subject to the foregoing or other risks and have a material adverse effect on our business, results of operations, financial condition and prospects. Conversely, any failure to enter any collaboration or other strategic transaction that would be beneficial to us could delay and make more expensive the development and potential commercialization of our product candidates and have a negative impact on the competitiveness of any product candidate that reaches market.
Any inability to attract and retain qualified key management and technical personnel would impair our ability to implement our business plan.
Our success largely depends on the continued service of key management and other specialized personnel, including Mr. Gregory West, our Chief Financial Officer and interim Chief Executive Officer, Dr. David Suhy, PhD, our Chief Scientist, Mr. Carl Stubbings, our Chief Business Officer, and Ms. Georgina Kilfoil, our Chief Clinical Officer. We are currently engaged in a search for a permanent Chief Executive Officer. The loss of one or more members of our management team or other key employees or advisors could delay or increase the cost of our research and development programs and materially harm our business, financial condition, results of operations and prospects. The relationships that our key managers have cultivated within our industry make us particularly dependent upon their continued employment with us. We are dependent on the continued service of our technical personnel because of the highly technical nature of our product candidates and the specialized nature of the regulatory approval process for our product candidates. Because our management team and key employees are not obligated to provide us with continued service, they could terminate their employment with us at any time without penalty. We do not maintain key person life insurance policies on any of our management team members or key employees. Our future success will depend in large part on our continued ability to attract and retain other highly qualified scientific, technical and management personnel, as well as personnel with expertise in clinical testing, manufacturing, governmental regulation and commercialization. We face competition for personnel from other companies, universities, public and private research institutions, government entities and other organizations.
Our collaborations with outside scientists and consultants may be subject to restriction and change.
We work with medical experts, chemists, biologists and other scientists at academic and other institutions, and consultants who assist us in our research, development and regulatory efforts, including the members of our scientific advisory board. In addition, these scientists and consultants have provided, and we expect that they will continue to provide, valuable advice regarding our programs and regulatory approval processes. These scientists and consultants are not our employees and may have other commitments that would limit their future availability to us. If a conflict of interest arises between their work for us and their work for another entity, we may lose their services. In addition, we are limited in our ability to prevent them from establishing competing businesses or developing competing products. For example, if a key scientist acting as a principal investigator in any of our future clinical trials identifies a potential product or compound that is more scientifically interesting to his or her professional interests, his or her availability to remain involved in any future clinical trials could be restricted or eliminated.
We may experience difficulties in managing our growth and expanding our operations.
We have limited experience in development and commercialization of pharmaceutical products. As our product candidates continue to advance through preclinical studies and any future clinical trials and potentially toward regulatory approval and commercial sale, we will need to expand our development, regulatory, manufacturing and sales capabilities or contract with other organizations to provide these capabilities for us. In the future, we expect to have to manage additional relationships with collaborators or partners, suppliers and other organizations. Our ability to manage our operations and future growth will require us to continue to improve our operational, financial and management controls, reporting systems and procedures. We may not be
37
able to implement improvements to our management information and control systems in an efficient or timely manner and may discover deficiencies in existing systems and controls.
Our employees, independent contractors, principal investigators, CROs, consultants and vendors may engage in misconduct or other improper activities, including noncompliance with regulatory standards and insider trading.
We are exposed to the risk of fraud or other misconduct by our employees, independent contractors, principal investigators, CROs, consultants, commercial partners and vendors. Misconduct by these parties could include intentional failures to comply with the regulations of the FDA and comparable foreign regulators, provide accurate information to the FDA and comparable foreign regulators, comply with healthcare fraud and abuse laws and regulations in the United States and abroad, report financial information or data accurately or disclose unauthorized activities to us. In particular, sales, marketing and business arrangements in the healthcare industry are subject to extensive laws and regulations intended to prevent fraud, misconduct, kickbacks, self-dealing and other abusive practices. These laws and regulations may restrict or prohibit a wide range of pricing, discounting, marketing and promotion, sales commission, customer incentive programs and other business arrangements. Such misconduct could also involve the improper use of information obtained in the course of any future clinical trials, which could result in regulatory sanctions and cause serious harm to our reputation. We have adopted a code of conduct applicable to all of our employees, but it is not always possible to identify and deter employee misconduct, and the precautions we take to detect and prevent this activity may not be effective in controlling unknown or unmanaged risks or losses or in protecting us from governmental investigations or other actions or lawsuits stemming from a failure to comply with these laws or regulations. If any such actions are instituted against us, and we are not successful in defending ourselves or asserting our rights, those actions could have a significant impact on our business, including the imposition of civil, criminal and administrative penalties, damages, monetary fines, possible exclusion from participation in Medicare, Medicaid and other federal healthcare programs, contractual damages, reputational harm, diminished profits and future earnings, and curtailment of our operations, any of which could adversely affect our ability to operate our business and our results of operations.
We face potential product liability, and, if successful claims are brought against us, we may incur substantial liability and costs. If the use of our product candidates harms patients, or is perceived to harm patients even when such harm is unrelated to our product candidates, our regulatory approvals could be revoked or otherwise negatively impacted and we could be subject to costly and damaging product liability claims.
The use of our product candidates in clinical trials and the sale of any products for which we may in the future obtain marketing approval exposes us to the risk of product liability claims. Product liability claims might be brought against us by consumers, healthcare providers, pharmaceutical companies or others selling or otherwise coming into contact with our product candidates. There is a risk that our product candidates may induce adverse events. If we cannot successfully defend against product liability claims, we could incur substantial liability and costs. In addition, regardless of merit or eventual outcome, product liability claims may result in:
| | • | | impairment of our business reputation; |
| | • | | withdrawal of clinical trial participants; |
| | • | | costs due to related litigation; |
| | • | | distraction of management’s attention from our primary business; |
| | • | | substantial monetary awards to patients or other claimants; |
| | • | | the inability to commercialize our product candidates; |
| | • | | decreased demand for our product candidates, if approved for commercial sale; and |
| | • | | increased cost, or impairment of our ability, to obtain or maintain product liability insurance coverage. |
38
We carry combined public and products liability (including human clinical trials extension) insurance of A$10 million per occurrence with a A$10 million aggregate limit. We believe our product liability insurance coverage is sufficient in light of our current clinical programs. However, we may not be able to maintain insurance coverage at a reasonable cost or in sufficient amounts to protect us against losses due to liability. If we obtain marketing approval for any product candidates, we intend to expand our insurance coverage to include the sale of commercial products, but we may not be able to obtain or maintain product liability insurance on commercially reasonable terms or in adequate amounts. On occasion, large judgments have been awarded against other pharmaceutical companies in class action lawsuits based on pharmaceutical products, or medical treatments that had unanticipated adverse effects. A successful product liability claim or series of claims brought against us could cause the price of the ADSs and Warrants to decline and, if judgments exceed our insurance coverage, could materially and adversely affect our financial position.
During the course of treatment, patients may suffer adverse events, including death, for reasons that may be related to our product candidates. Such events could subject us to costly litigation, require us to pay substantial amounts of money to injured patients, delay, negatively impact or end our opportunity to receive or maintain regulatory approval to market our products, or require us to suspend or discontinue our commercialization efforts. Even in a circumstance in which we do not believe that an adverse event is related to our product candidate, the investigation into the circumstance may be time-consuming or inconclusive. These investigations may harm our reputation, delay our regulatory approval process, limit the type of regulatory approvals our product candidates receive or maintain, and compromise the market acceptance of any of our product candidates that may in the future receive regulatory approval. As a result of these factors, a product liability claim, even if successfully defended, could hurt our business and impair our ability to generate revenue.
We and our development partners, third-party manufacturers and suppliers use biological materials and may use hazardous materials, and any claims relating to improper handling, storage or disposal of these materials could be time consuming or costly.
We and our development partners, third-party manufacturers and suppliers may use hazardous materials, including chemicals and biological agents and compounds that could be dangerous to human health and safety or the environment. Our operations and the operations of our third-party manufacturers and suppliers also produce hazardous waste products. National, state and local laws and regulations in the United States, Australia and other countries govern the use, generation, manufacture, storage, handling and disposal of these materials and wastes. Compliance with applicable environmental laws and regulations may be expensive, and current or future environmental laws and regulations may impair our product development and commercialization efforts. In addition, we cannot entirely eliminate the risk of accidental injury or contamination from these materials or wastes. We do not carry specific biological or hazardous waste insurance coverage, and our property, casualty and general liability insurance policies specifically exclude coverage for damages and fines arising from biological or hazardous waste exposure or contamination. Accordingly, in the event of contamination or injury, we could be held liable for damages or be penalized with fines in an amount exceeding our resources, and any future clinical trials, regulatory approvals or product commercialization progress could be suspended.
We may use our limited financial and human resources to pursue a particular research program or product candidate and fail to capitalize on programs or product candidates that may be more profitable or for which there is a greater likelihood of success.
Because we have limited resources, we may forego or delay pursuit of opportunities with certain programs or product candidates or for indications that later prove to have greater commercial potential. Our resource allocation decisions may cause us to fail to capitalize on viable commercial products or profitable market opportunities. Our spending on current and future research and development programs for product candidates may not yield any commercially viable products. If we do not accurately evaluate the commercial potential or target market for a particular product candidate, we may relinquish valuable rights to that product candidate through strategic collaboration, licensing or other royalty arrangements in cases in which it would have been
39
more advantageous for us to retain sole development and commercialization rights to such product candidate, or we may allocate internal resources to a product candidate in a therapeutic area in which it would have been more advantageous to enter into a collaboration arrangement.
Our internal computer and information technology systems, or those of our collaborators and other development partners, third-party CROs or other contractors or consultants, may fail or suffer security breaches, which could result in a disruption of our product development programs.
Despite the implementation of security measures, our internal computer and information technology systems and those of our current and any future CROs and other contractors, consultants and collaborators are vulnerable to damage from computer viruses, cyber-attacks, unauthorized access, natural disasters, terrorism, war and telecommunication and electrical failures. Such events could cause interruptions of our operations. While we have not experienced any material system failure, accident or security breach to date, if such an event were to occur and cause interruptions in our operations, it could result in a disruption of our development programs and our business operations, whether due to a loss of our trade secrets or other similar disruptions. For example, the loss of clinical trial data from ongoing or future clinical trials or data from preclinical studies could result in delays in our regulatory approval efforts and significantly increase our costs to recover or reproduce the data. Likewise, we rely on third parties to manufacture our product candidates and will rely on third parties to conduct future clinical trials, and similar events relating to their computer systems could also have similar consequences to our business. To the extent that any disruption or security breach were to result in a loss of, or damage to, our data or applications, or inappropriate disclosure of confidential or proprietary information, we could incur liability and the further development and commercialization of our product candidates could be delayed and become more expensive.
Our current laboratory operations are concentrated in one location and any events affecting this location may seriously compromise our ability to operate our business and continue the development of our product candidates.
Our current laboratory operations are located in our facility situated in Hayward, California. Any unplanned event, such as flood, fire, explosion, earthquake, extreme weather condition, medical epidemics, power shortage, telecommunication failure or other natural or manmade accidents or incidents that result in us being unable to fully utilize the facility, may compromise our ability to operate our business, particularly on a daily basis, cause us financial losses and inhibit or delay our continued development of our product candidates. Loss of access to this facility may result in increased costs, delays in the development of our product candidates or interruption of our business operations. As part of our risk management policy, we maintain insurance coverage at levels that we believe are appropriate for our business. However, in the event of an accident or incident at this facility, we cannot assure you that the amounts of insurance will be sufficient to satisfy any damages and losses. If our facility is unable to operate because of an accident or incident or for any other reason, even for a short period of time, any or all of our research and development programs may be harmed. Any business interruption may have a material adverse effect on our business, financial position, results of operations and prospects.
The investment of our cash and cash equivalents is subject to risks which may cause losses and affect the liquidity of these investments.
As of December 31, 2015, we had A$22.8 million in cash and cash equivalents. We historically have invested substantially all of our available cash and cash equivalents, including the net proceeds of our initial public offering, in cash deposits meeting the criteria of our investment policy, which is focused on the preservation of our capital. These investments are subject to general credit, liquidity, market and interest rate risks. We may realize losses in the fair value of these investments, which would have a negative effect on our financial results. In addition, should our investments cease paying or reduce the amount of interest paid to us, our interest income would suffer. The market risks associated with our investment portfolio may have an adverse effect on our results of operations, liquidity and financial condition.
40
Our ability to utilize our net operating losses and certain other tax attributes may be limited.
We have substantial carried forward tax losses which may not be available to offset any future assessable income. In order for an Australian corporate taxpayer to carry forward and utilize tax losses, the taxpayer must pass either the continuity of ownership test, or COT, or, if it fails the COT, the same business test, or SBT, in respect of relevant tax losses.
We have not carried out any analysis as to whether we have met the COT or, failing the COT, the SBT over relevant periods. In addition, future shareholding changes may result in a significant ownership change for us. It is therefore uncertain as to whether any of our tax losses carried forward as of December 31, 2015 will be available to be carried forward and available to offset our assessable income, if any, in future periods.
Risks Related to Our Intellectual Property
If we are unable to obtain or protect intellectual property rights related to our product candidates, we may not be able to obtain exclusivity for our product candidates or prevent others from developing similar competitive products.
We rely upon a combination of patents, know-how, trade secret protection and confidentiality agreements to protect the intellectual property related to our product candidates.
The patent application process, also known as patent prosecution, is expensive and time-consuming, and we and our current or future licensors and licensees may not be able to prepare, file, and prosecute all necessary or desirable patent applications at a reasonable cost or in a timely manner. It is also possible that we or our current licensors or licensees, or any future licensors or licensees, will fail to identify patentable aspects of inventions made in the course of development and commercialization activities before it is too late to obtain patent protection on them. Therefore, our patents and patent applications may not be prosecuted and enforced in a manner consistent with the best interests of our business. It is possible that defects of form in the preparation or filing of our patents or patent applications may exist, or may arise in the future, for example with respect to proper priority claims, inventorship, claim scope, patent term adjustments, etc., although we are unaware of any such defects. If we or our current licensors or licensees, or any future licensors or licensees, fail to establish, maintain or protect such patents and other intellectual property rights, such rights may be reduced or eliminated. If our current licensors or licensees, or any future licensors or licensees, are not fully cooperative or disagree with us as to the prosecution, maintenance or enforcement of any patent rights, such patent rights could be compromised. If there are material defects in the form or preparation of our patents or patent applications, such patents or applications may be invalid and unenforceable. Any of these outcomes could impair our ability to prevent competition from third parties, which may have an adverse impact on our business.
The strength of patents in the biotechnology and pharmaceutical field involves complex legal and scientific questions and can be uncertain. The patent applications that we own or in-license may fail to result in issued patents with claims that cover our product candidates in the United States or other jurisdictions. In addition, we cannot guarantee that any patents will issue from any pending or future patent applications owned by or licensed to us. There is no assurance that all of the potentially relevant prior art relating to our patents and patent applications has been found. If such prior art exists, it can invalidate a patent or prevent a patent from issuing from a pending patent application. Even if patents do successfully issue and even if such patents cover our product candidates, third parties may initiate opposition, interference, re-examination, post-grant review, inter partes review, nullification or derivation action in court or before patent offices or similar proceedings challenging the validity, enforceability or scope of such patents, which may result in the patent claims being narrowed or invalidated. For example, three of our patents licensed from CSIRO, that expire in 2019, have been subject to oppositions, and these oppositions have thus far resulted in appealable decisions to revoke two of those patents in Europe. CSIRO has appealed both of these revocations but we cannot know whether these appeals, if carried through, will be decided favorably for us. Furthermore, even if our patents and patent applications are unchallenged, they may not adequately protect our intellectual property, provide exclusivity for our product
41
candidates or prevent others from designing around our claims. Any of these outcomes could impair our ability to prevent competition from third parties.
If the patent applications we hold or have in-licensed with respect to our programs or product candidates fail to issue, or are revoked, if the breadth or strength of our patent protection is threatened, or if our patent portfolio fails to provide meaningful exclusivity for our product candidates, it could dissuade companies from collaborating with us to develop product candidates and threaten our ability to commercialize future products. Any successful opposition to any patents owned by or licensed to us could deprive us of rights necessary for the successful commercialization of any product candidates that we may develop. Further, if we encounter delays in regulatory approvals, the period of time during which we could market a product candidate under patent protection could be reduced. Since patent applications in the United States and most other countries are confidential for a period of time after filing, and some remain so until issued, we cannot be certain that we or our licensors were the first to file any patent application related to a product candidate. Furthermore, if third parties have filed such patent applications before March 16, 2013, an interference proceeding in the United States can be initiated by such third parties to determine who was the first to invent any of the subject matter covered by the patent claims of our applications. If third parties have filed such applications on or after March 16, 2013, a derivation proceeding in the United States can be initiated by such third parties to determine whether our invention was derived from theirs. Even where we have a valid and enforceable patent, we may not be able to exclude others from practicing our invention where the other party can show that they used the invention in commerce before our filing date or the other party benefits from a compulsory license. In addition, patents have a limited lifespan. In the United States, the natural expiration of a patent is generally 20 years after it is filed. Various extensions may be available, but the life of a patent, and the protection it affords, is limited. Even if patents covering our product candidates are obtained, once the patent life has expired for a product, we may be open to competition from competitive medications, including biosimilar or generic medications. This risk is material in light of the length of the development process of our products and lifespan of our current patent portfolio.
In addition to the protection afforded by patents, we rely on trade secret protection and confidentiality agreements to protect proprietary know-how that is not patentable or that we elect not to patent, processes for which patents are difficult to enforce and any other elements of our product candidate discovery and development processes that involve proprietary know-how, information or technology that is not covered by patents. However, trade secrets can be difficult to protect. What constitutes a trade secret and what protections are available for trade secrets varies from state to state in the United States and country by country worldwide. We seek to protect our proprietary technology and processes, in part, by entering into confidentiality agreements with our employees, consultants, scientific advisors and contractors. We also seek to preserve the integrity and confidentiality of our data and trade secrets by maintaining physical security of our premises and physical and electronic security of our information technology systems. Security measures may be breached, and we may not have adequate remedies for any breach. In addition, our trade secrets may otherwise become known or be independently discovered by competitors. Although we expect all of our employees and consultants to assign their inventions to us, and all of our employees, consultants, advisors and any third parties who have access to our proprietary know-how, information or technology to enter into confidentiality agreements, we cannot provide any assurances that all such agreements have been duly executed or that our trade secrets and other confidential proprietary information will not be disclosed or that competitors will not otherwise gain access to our trade secrets or independently develop substantially equivalent information and techniques. Misappropriation or unauthorized disclosure of our trade secrets can be difficult to detect, could impair our competitive position and may have a material adverse effect on our business. Additionally, if the steps taken to maintain our trade secrets are deemed inadequate, we may have insufficient recourse against third parties for misappropriating the trade secret. In addition, others may independently discover our trade secrets and proprietary information. For example, the FDA, as part of its Transparency Initiative, is currently considering whether to make additional information publicly available on a routine basis, including information that we may consider to be trade secrets or other proprietary information, and it is not clear at the present time how the FDA’s disclosure policies may change in the future, if at all.
42
Enforcing a claim that a party illegally disclosed or misappropriated a trade secret is difficult, expensive and time-consuming, and the outcome is unpredictable. Further, the laws of some foreign countries such as India and China do not protect proprietary rights to the same extent or in the same manner as the laws of the United States. As a result, we may encounter significant problems in protecting and defending our intellectual property both in the United States and abroad. If we are unable to prevent material disclosure of the non-patented intellectual property related to our technologies to third parties, and there is no guarantee that we will have any such enforceable trade secret protection, we may not be able to establish or maintain a competitive advantage in our markets.
We rely on license relationships with a number of third parties for portions of our intellectual property, including platform technology patents relating to our ddRNAi technology. This arrangement could restrict the scope and enforcement of our intellectual property rights and limit our ability to successfully commercialize current and future product candidates.
We have in-licensed certain ddRNAi-related intellectual property from third-party licensors. We rely on some of these third parties to file and prosecute patent applications and maintain patents and otherwise protect the intellectual property we license. We have not had and do not have primary control over these activities for certain of our patents or patent applications and other intellectual property rights we license, and therefore cannot guarantee that these patents and applications will be prosecuted or enforced in a manner consistent with the best interests of our business. We cannot be certain that such activities by third parties have been or will be conducted in compliance with applicable laws and regulations or will result in valid and enforceable patents and other intellectual property rights. Additionally, we may not be able to control the publication or other disclosures of research carried out by our licensors relating to technology that could otherwise prove patentable. Pursuant to the terms of some of our license agreements with third parties, some of our third-party licensors have the right, but not the obligation, to control enforcement of our licensed patents or defense of any claims asserting the invalidity of these patents. Even if we are permitted to pursue such enforcement or defense, we will require the cooperation of our licensors, and cannot guarantee that we would receive it and on what terms. We cannot be certain that our licensors will allocate sufficient resources or prioritize their or our enforcement of such patents or defense of such claims to protect our interests in the licensed patents. If we cannot obtain patent protection, or enforce existing or future patents against third parties, our competitive position and our financial condition could suffer.
Third-party claims of intellectual property infringement may prevent or delay our development and commercialization efforts.
Our commercial success depends in part on our avoiding infringement of the patents and proprietary rights of third parties. There is a substantial amount of litigation, both within and outside the United States, involving patent and other intellectual property rights in the biotechnology and pharmaceutical industries, including patent infringement lawsuits, interferences, oppositions and inter partes review proceedings before the USPTO, and corresponding foreign patent offices. Numerous U.S. and foreign issued patents and pending patent applications, which are owned by third parties, exist in the fields in which we are pursuing development candidates. As the biotechnology and pharmaceutical industries expand and more patents are issued, the risk increases that our product candidates may be subject to claims of infringement of the patent rights of third parties.
Third parties may assert that we are employing their proprietary technology without authorization. There may be third-party patents or patent applications with claims to materials, formulations, methods of manufacture or methods for treatment related to the use or manufacture of our product candidates. Because patent applications can take many years to issue, there may be currently pending patent applications which may later result in issued patents that our product candidates may be accused of infringing. In addition, third parties may obtain patents in the future and claim that use of our technologies infringes upon these patents. If any third-party patents were held by a court of competent jurisdiction to cover the manufacturing process of any of our product candidates, any DNA constructs formed during the manufacturing process or any final product itself, the holders of any such patents may be able to block our ability to commercialize such product candidate unless we obtained a license
43
under the applicable patents, or until such patents expire. Similarly, if any third-party patents were held by a court of competent jurisdiction to cover aspects of our formulations, processes for manufacture or methods of use, the holders of any such patents may be able to block our ability to develop and commercialize the applicable product candidate unless we obtained a license or until such patent expires. In either case, such a license may not be available on commercially reasonable terms or at all.
Parties making claims against us may obtain injunctive or other equitable relief, which could effectively block our ability to further develop and commercialize one or more of our product candidates. Defense of these claims, regardless of their merit, would involve substantial litigation expense and would be a substantial diversion of management and employee resources from our business. In the event of a successful claim of infringement against us, we may have to pay substantial damages, including treble damages and attorneys’ fees for willful infringement, pay royalties, redesign our infringing products or obtain one or more licenses from third parties, which may be impossible or require substantial time and monetary expenditure. Even if a license can be obtained on acceptable terms, the rights may be non-exclusive, which could give our competitors access to the same technology or intellectual property rights licensed to us. If we fail to obtain a required license, we may be unable to effectively market product candidates based on our technology, which could limit our ability to generate revenue or achieve profitability and possibly prevent us from generating revenue sufficient to sustain our operations.
We may not be successful in obtaining or maintaining necessary rights to gene therapy product components and processes for our development pipeline through acquisitions and in-licenses.
Presently we have rights to intellectual property to develop our current gene therapy product candidates. However, our product candidates may require specific formulations to work effectively and efficiently and rights to such formulations may be held by others. In addition, we may need additional intellectual property rights as we develop future therapy product candidates. We may be unable to acquire or in-license any compositions, methods of use, processes or other third-party intellectual property rights from third parties that we identify on terms that we find acceptable, or at all. The licensing and acquisition of third-party intellectual property rights is a competitive area, and a number of more established companies are also pursuing strategies to license or acquire third-party intellectual property rights that we may consider attractive. These established companies may have a competitive advantage over us due to their size, cash resources and greater clinical development and commercialization capabilities.
For example, we sometimes collaborate with U.S. and foreign academic institutions to accelerate our preclinical research or development under written agreements with these institutions. Typically, these institutions provide us with an option to negotiate a license to any of the institution’s rights in technology resulting from the collaboration. Regardless of such right of first negotiation for intellectual property, we may be unable to negotiate a license within the specified time frame or under terms that are acceptable to us. If we are unable to do so, the institution may offer the intellectual property rights to other parties, potentially blocking our ability to pursue our program.
In addition, companies that perceive us to be a competitor may be unwilling to assign or license rights to us. We also may be unable to license or acquire third-party intellectual property rights on terms that would allow us to make an appropriate return on our investment. If we are unable to successfully obtain rights to required third-party intellectual property rights, our business, financial condition and prospects for growth could suffer.
We may enter into license agreements with third parties, and if we fail to comply with our obligations in such agreements under which we license intellectual property rights from third parties or otherwise experience disruptions to our business relationships with our licensors, we could lose license rights that are important to our business.
We may need to obtain licenses from third parties to advance our research or allow commercialization of our product candidates, and we have done so from time to time. We may fail to obtain any of these licenses at a
44
reasonable cost or on reasonable terms, if at all. In that event, we may be required to expend significant time and resources to develop or license replacement technology. If we are unable to do so, we may be unable to develop or commercialize the affected product candidates.
In many cases, patent prosecution of our in-licensed technology is controlled solely by the licensor. If our licensors fail to obtain and maintain patent or other protection for the proprietary intellectual property we license from them, we could lose our rights to the intellectual property or our exclusivity with respect to those rights, and our competitors could market competing products using the intellectual property. In some cases, we control the prosecution of patents resulting from licensed technology. In the event we breach any of our obligations related to such prosecution, we may incur significant liability to our licensing partners. Licensing of intellectual property is of critical importance to our business and involves complex legal, business and scientific issues and is complicated by the rapid pace of scientific discovery in our industry. Disputes may arise regarding intellectual property subject to a licensing agreement, including:
| | • | | the scope of rights granted under the license agreement and other interpretation-related issues; |
| | • | | the extent to which our technology and processes infringe on intellectual property of the licensor that is not subject to the licensing agreement; |
| | • | | the sublicensing of patent and other rights under any collaboration relationships we might enter into in the future; |
| | • | | our diligence obligations under the license agreement and what activities satisfy those diligence obligations; |
| | • | | the ownership of inventions and know-how resulting from the joint creation or use of intellectual property by our licensors and us and our partners; and |
| | • | | the priority of invention of patented technology. |
If disputes over intellectual property that we have licensed prevent or impair our ability to maintain our current licensing arrangements on acceptable terms, we may be unable to successfully develop and commercialize the affected product candidates.
We may be involved in lawsuits to protect or enforce our patents or the patents of our licensors, which could be expensive, time-consuming and unsuccessful, and issued patents covering our product candidates could be found invalid or unenforceable if challenged in court.
Competitors may infringe our patents or the patents of our licensors. To counter infringement or unauthorized use, we may be required to file infringement claims, which can be expensive and time-consuming. In addition, in an infringement proceeding, a court may decide that a patent of ours or our licensors is not valid, is unenforceable or is not infringed, or may refuse to stop the other party from using the technology at issue on the grounds that our patents do not cover the technology in question. An adverse result in any litigation or defense proceedings could put one or more of our patents at risk of being invalidated or interpreted narrowly and could put our patent applications at risk of not issuing.
For example, in patent litigation in the United States, defendant counterclaims alleging invalidity or unenforceability are commonplace. Grounds for a validity challenge could be an alleged failure to meet any of several statutory requirements, for example, lack of novelty, obviousness or non-enablement. Grounds for an unenforceability assertion could be an allegation that someone connected with prosecution of the patent withheld relevant information from the USPTO, or made a misleading statement, during prosecution. The outcome following legal assertions of invalidity and unenforceability during patent litigation is unpredictable. With respect to the validity question, for example, we cannot be certain that there is no invalidating prior art, of which we and the patent examiner were unaware during prosecution. Third parties may also raise similar claims before administrative bodies in the United States or abroad, even outside the context of litigation. Such mechanisms
45
include re-examination, post grant review, and equivalent proceedings in foreign jurisdictions, such as opposition proceedings. Such proceedings could result in revocation, amendments to our patent claims or statements being made on the record such that our claims may no longer be construed to cover our product candidates. For example, three of our patents licensed from CSIRO have been subject to oppositions, and these oppositions have thus far resulted in appealable decisions to revoke two of those patents in Europe. Outcomes or statements on the record in one country could have a disadvantageous effect on prosecution or enforcement of a patent or patent application in another country. The outcome following legal assertions of invalidity and unenforceability is unpredictable. With respect to the validity question, for example, we cannot be certain that no invalidating prior art exists or that the patent examiner was aware of all material prior art during prosecution. Moreover, in some circumstances, we do not have the right to control the preparation, filing and prosecution of patent applications, or to maintain, enforce or defend the patents, covering technology that we license from third parties. Therefore, these patents and applications may not be prosecuted, enforced and defended in a manner consistent with the best interests of our business. If a defendant were to prevail on a legal assertion of invalidity or unenforceability, we could lose at least part, and perhaps all, of the patent protection on one or more of our products or certain aspects of our platform technology. Such a loss of patent protection could have a material adverse impact on our business. Patents and other intellectual property rights also will not protect our technology if competitors design around our protected technology without legally infringing our patents or other intellectual property rights. Even if resolved in our favor, litigation or other legal proceedings relating to intellectual property claims may cause us to incur significant expenses, and could distract our technical and management personnel from their normal responsibilities. In addition, enforcement of a favorable decision by a court can depend on cooperation of a governmental authority which may or may not be available in every jurisdiction. There could be public announcements of the results of hearings, motions or other interim proceedings or developments, and if securities analysts or investors perceive these results to be negative, it could depress the market price of our ADSs and Warrants. Such litigation or proceedings could substantially increase our operating losses and reduce the resources available for development activities or any future sales, marketing or distribution activities.
For our patents and patent applications filed in the United States before March 16, 2013, interference proceedings provoked by third parties or brought by us may be necessary to determine the priority of inventions with respect to our patents or patent applications or those of our licensors. An unfavorable outcome could require us to cease using the related technology or to attempt to license rights to it from the prevailing party. Our business could be harmed if the prevailing party does not offer us a license on commercially reasonable terms. Litigation or interference proceedings may result in a decision adverse to our interests and, even if successful, may result in substantial costs and distract our management and other employees. We may not be able to prevent, alone or with our licensors, misappropriation of our intellectual property rights, particularly in countries where the laws may not protect those rights as fully as in the United States.
Furthermore, because of the substantial amount of discovery required in connection with intellectual property litigation, there is a risk that some of our confidential information could be compromised by disclosure during this type of litigation. There could also be public announcements of the results of hearings, motions or other interim proceedings or developments. If securities analysts or investors perceive these results to be negative, it could cause the trading price of our ADSs and Warrants to fall.
Our results of operations will be affected by the level of royalty payments that we are required to pay to third parties.
We are a party to license agreements that require us to remit royalty payments and other payments related to in-licensed intellectual property. Under our in-license agreements, we may pay up-front fees and milestone payments and be subject to future royalties. We cannot precisely predict the amount, if any, of royalties we will owe in the future, and if our calculations of royalty payments are incorrect, we may owe additional royalties, which could negatively affect our results of operations. As our product sales increase, we may, from time to time, disagree with our third-party collaborators as to the appropriate royalties owed, and the resolution of such disputes may be costly, may consume management’s time, and may damage our relationship with our
46
collaborators. Furthermore, we may enter into additional license agreements in the future, which may also include royalty, milestone and other payments.
The licenses we grant to our collaborators to use our ddRNAi technology are exclusive to the development of product candidates for certain conditions.
Some of the out-licenses we grant to our collaborators to use our ddRNAi technology are exclusive to the development of product candidates for certain conditions, so long as our collaborators comply with certain requirements. That means that once our ddRNAi technology is licensed to a collaborator for a specified condition, we are generally prohibited from developing product candidates for that condition and from licensing the ddRNAi to any third party for that condition. The limitations imposed by these exclusive licenses could prevent us from expanding our business and increasing our development of product candidates with new collaborators, both of which could adversely affect our business and results of operations.
We may be subject to claims that our employees, consultants or independent contractors have wrongfully used or disclosed confidential information of third parties or that our employees have wrongfully used or disclosed alleged trade secrets of their former employers.
Certain of our key employees and personnel are or were previously employed at universities, medical institutions or other biotechnology or pharmaceutical companies, including our competitors or potential competitors. Although we try to ensure that our employees, consultants and independent contractors do not use the proprietary information or know-how of others in their work for us, we may be subject to claims that we or our employees, consultants or independent contractors have inadvertently or otherwise used or disclosed intellectual property, including trade secrets or other proprietary information, of any of our employee’s former employer or other third parties. Litigation may be necessary to defend against these claims. Furthermore, universities or medical institutions who employ some of our key employees and personnel in parallel to their engagement by us may claim that intellectual property developed by such person is owned by the respective academic or medical institution under the respective institution intellectual property policy or applicable law. If we fail in defending any such claims, in addition to paying monetary damages, we may lose valuable intellectual property rights or personnel. Even if we are successful in defending against such claims, litigation could result in substantial costs, be a distraction to management and other employees, and damage our relationships with the academic and medical institutions.
We may be subject to claims challenging the inventorship or ownership of our patents and other intellectual property.
We may be subject to claims that former employees, collaborators or other third parties have an ownership interest in our patents or other intellectual property. We may in the future have ownership disputes arising, for example, from conflicting obligations of consultants or others who are involved in developing our product candidates. Litigation may be necessary to defend against these and other claims challenging inventorship or ownership. If we fail in defending any such claims, in addition to paying monetary damages, we may lose valuable intellectual property rights, such as exclusive ownership of, or right to use, valuable intellectual property. Such an outcome could have a material adverse effect on our business. Even if we are successful in defending against such claims, litigation could result in substantial costs and be a distraction to management and other employees.
Obtaining and maintaining our patent protection depends on compliance with various procedural, document submission, fee payment and other requirements imposed by governmental patent agencies, and our patent protection could be reduced or eliminated for non-compliance with these requirements.
Periodic maintenance fees, renewal fees, annuity fees and various other governmental fees on patents and applications are required to be paid to the USPTO and various governmental patent agencies outside of the United States in several stages over the lifetime of the patents and applications. The USPTO and various
47
corresponding governmental patent agencies outside of the United States require compliance with a number of procedural, documentary, fee payment and other similar provisions during the patent application process and after a patent has issued. There are situations in which non-compliance can result in abandonment or lapse of the patent or patent application, resulting in partial or complete loss of patent rights in the relevant jurisdiction.
Changes in U.S. patent law could diminish the value of patents in general, thereby impairing our ability to protect our products.
As is the case with other biotechnology companies, our success is heavily dependent on intellectual property, particularly patents. Obtaining and enforcing patents in the biotechnology industry involve both technological and legal complexity, and it therefore is costly, time-consuming and inherently uncertain. In addition, on September 16, 2011, the Leahy-Smith America Invents Act, or the Leahy-Smith Act, was signed into law. The Leahy-Smith Act includes a number of significant changes to U.S. patent law, including provisions that affect the way patent applications will be prosecuted and may also affect patent litigation.
An important change introduced by the Leahy-Smith Act is that, as of March 16, 2013, the United States transitioned to a “first-to-file” system for deciding which party should be granted a patent when two or more patent applications are filed by different parties claiming the same invention. A third party that files a patent application in the USPTO after that date but before us could therefore be awarded a patent covering an invention of ours even if we had made the invention before it was made by the third party. This will require us to be cognizant going forward of the time from invention to filing of a patent application. It is not clear what, if any, impact the Leahy-Smith Act will have on the operation of our business. However, the Leahy-Smith Act and its implementation could increase the uncertainties and costs surrounding the prosecution of our patent applications and the enforcement or defense of our issued patents.
Among some of the other changes introduced by the Leahy-Smith Act are changes that limit where a patentee may file a patent infringement suit and providing opportunities for third parties to challenge any issued patent in the USPTO. This applies to all of our U.S. patents, even those issued before March 16, 2013. Because of a lower evidentiary standard in USPTO proceedings compared to the evidentiary standard in U.S. federal court necessary to invalidate a patent claim, a third party could potentially provide evidence in a USPTO proceeding sufficient for the USPTO to hold a claim invalid even though the same evidence would be insufficient to invalidate the claim if first presented in a district court action. Accordingly, a third party may attempt to use the USPTO procedures to invalidate our patent claims that would not have been invalidated if first challenged by the third party as a defendant in a district court action.
Recent U.S. Supreme Court rulings such as Association for Molecular Pathology v. Myriad Genetics, Inc. (Myriad I); BRCA1- &BRCA2-Based Hereditary Cancer Test Patent Litig. (Myriad II); and Promega Corp. v. Life Technologies Corp. have also narrowed the scope of patent protection available in certain circumstances and weakened the rights of patent owners in some situations. In addition to increasing uncertainty with regard to our ability to obtain patents in the future, this combination of events has created uncertainty with respect to the value of patents, once obtained. Depending on decisions by the U.S. Congress, the federal courts, and the USPTO, the laws and regulations governing patents could change in unpredictable ways that would weaken our ability to obtain new patents or to enforce our existing patents and patents that we might obtain in the future.
Our success depends, in part, on our ability to protect our intellectual property and our technologies outside the United States. We may not be able to protect our intellectual property rights throughout the world.
Our commercial success depends, in part, on our ability to obtain and maintain patent and trade secret protection for our technologies, our traits, and their uses, as well as our ability to operate without infringing upon the proprietary rights of others outside the United States. If we do not adequately protect our intellectual property, competitors may be able to use our technologies and erode or negate any competitive advantage we may have, which could harm our business and ability to achieve profitability.
48
Filing, prosecuting and defending patents on product candidates in all countries around the world would be prohibitively expensive, and our intellectual property rights in some countries outside the United States can be less extensive than those in the United States. In addition, the laws of some foreign countries do not protect intellectual property rights to the same extent as federal and state laws in the United States. In addition, we may at times in-license third-party technologies for which limited international patent protection exists and for which the time period for filing international patent applications has passed. Consequently, we may not be able to prevent third parties from practicing our inventions in all countries outside the United States, or from selling or importing products made using our inventions in and into the United States or other jurisdictions. Potential competitors may use our technologies in jurisdictions where we have not obtained patent protection to develop their own products and further, may export otherwise infringing products to territories where we have patent protection, but enforcement is not as strong as that in the United States. These products may compete with our product candidates, if approved, and our patents or other intellectual property rights may not be effective or sufficient to prevent them from competing.
Many companies have encountered significant problems in protecting and defending intellectual property rights in foreign jurisdictions. The legal systems of certain countries, particularly certain developing countries, do not favor the enforcement of patents, trade secrets and other intellectual property protection, particularly those relating to biotechnology products, which could make it difficult for us to stop the infringement of our patents or marketing of competing products in violation of our proprietary rights generally. Proceedings to enforce our patent rights in foreign jurisdictions could result in substantial costs and divert our efforts and attention from other aspects of our business, could put our patents at risk of being invalidated or interpreted narrowly and our patent applications at risk of not issuing and could provoke third parties to assert claims against us. We may not prevail in any lawsuits that we initiate and the damages or other remedies awarded, if any, may not be commercially meaningful. Accordingly, our efforts to enforce our intellectual property rights around the world may be inadequate to obtain a significant commercial advantage from the intellectual property that we develop or license.
Risks Related to the ADSs, Warrants and this Offering
The market price and trading volume of the ADSs and Warrants may be volatile and may be affected by economic conditions beyond our control.
The market price of the ADSs and Warrants may be highly volatile and subject to wide fluctuations. In addition, the trading volume of the ADSs and Warrants may fluctuate and cause significant price variations to occur. If the market price of the ADSs or Warrants declines significantly, you may be unable to resell your ADSs or Warrants at or above your purchase price, if at all. We cannot assure you that the market price of the ADSs or Warrants will not fluctuate or significantly decline in the future.
Some specific factors that could negatively affect the price of the ADSs and Warrants or result in fluctuations in their price and trading volume include:
| | • | | actual or expected fluctuations in our operating results; |
| | • | | changes in market valuations of similar companies; |
| | • | | changes in our key personnel; |
| | • | | changes in financial estimates or recommendations by securities analysts; |
| | • | | trading prices of our ordinary shares on the ASX; |
| | • | | changes in trading volume of ADSs or Warrants on The NASDAQ Capital Market, or NASDAQ, and of our ordinary shares on the ASX; |
| | • | | sales of the ADSs or Warrants or ordinary shares by us, our executive officers or our shareholders in the future; and |
| | • | | conditions in the financial markets or changes in general economic conditions. |
49
An active trading market for the ADSs or Warrants may not continue to develop or may not be liquid enough for you to sell your ADSs or Warrants quickly or at market price.
Prior to our recent U.S. initial public offering, our securities were not listed on any U.S. stock exchange, we were not a reporting company under the Exchange Act and there had been only a limited public market in the United States for the ADSs and no public market in the United States for the Warrants. Although the ADSs and Warrants are listed on The NASDAQ Capital Market, if an active public market in the United States for the ADSs and Warrants does not continue to develop, the market price and liquidity of the ADSs and Warrants may be adversely affected. The initial public offering price for the ADSs and Warrants was determined by negotiation between us and the underwriter. The price at which the ADSs are traded has declined below the initial public offering price, and the price at which the Warrants are traded has not exceeded the Warrant exercise price. The ADS price and/or the Warrant price may continue to decline, which means you may experience a decrease in the value of your ADSs and Warrants regardless of our operating performance or prospects. In the past, following periods of volatility in the market price of a company’s securities, shareholders often instituted securities class action litigation against that company. If we were involved in a class action suit, it could divert the attention of senior management and, if adversely determined, could cause us significant financial harm.
Investors purchasing the ADSs may suffer immediate and substantial dilution.
The exercise price for the Warrants is substantially higher than the net tangible book value per ADS of the underlying ordinary shares. If you exercise your Warrants, you may incur substantial and immediate dilution in the net tangible book value of your investment. Net tangible book value per represents the amount of total tangible assets less total liabilities, divided by the number of ordinary shares then outstanding, multiplied by 20, the number of ordinary shares underlying each ADS. To the extent that options that are currently outstanding are exercised, including the Warrants, there will be further dilution to your investment. We may also issue additional ordinary shares, ADSs, warrants, performance rights, options and other securities in the future that may result in further dilution of your ADSs. See “Dilution” for a calculation of the extent to which your investment will be diluted.
The dual listing of our ordinary shares and the ADSs and Warrants may adversely affect the liquidity and value of the ADSs and Warrants.
Our ADSs and Warrants are listed on NASDAQ, and our ordinary shares are listed on the ASX. We cannot predict the continued effect of this dual listing on the value of our ordinary shares, ADSs and Warrants. However, the dual listing of our ordinary shares, ADSs and Warrants may dilute the liquidity of these securities in one or both markets and may impair the development of an active trading market for the ADSs and Warrants in the United States. The trading price of the ADSs and Warrants could also be adversely affected by trading in our ordinary shares on the ASX.
As a foreign private issuer, we are permitted and we expect to follow certain home country corporate governance practices in lieu of certain NASDAQ requirements applicable to domestic issuers. This may afford less protection to holders of the ADSs and Warrants.
As a foreign private issuer whose ADSs and Warrants are listed on NASDAQ, we will be permitted to follow certain home country corporate governance practices in lieu of certain NASDAQ requirements. For example, we may follow home country practice with regard to the composition of the board of directors and quorum requirements applicable to shareholder meetings. A foreign private issuer must disclose in its annual reports filed with the SEC the requirements with which it does not comply followed by a description of its applicable home country practice. The Australian home country practices described above may afford less protection to holders of the ADSs and Warrants than that provided under NASDAQ rules. See “Description of Share Capital—Exemptions from Certain NASDAQ Corporate Governance Rules.”
50
As a foreign private issuer, we are permitted to file less information with the SEC than a company incorporated in the United States. Accordingly, there may be less publicly available information concerning us than there is for companies incorporated in the United States.
As a foreign private issuer, we are exempt from certain rules under the Exchange Act, that impose disclosure requirements as well as procedural requirements for proxy solicitations under Section 14 of the Exchange Act. In addition, our officers, directors and principal shareholders are exempt from the reporting and “short-swing” profit recovery provisions of Section 16 of the Exchange Act. Moreover, we are not required to file periodic reports and financial statements with the SEC as frequently or as promptly as a company that files as a U.S. company whose securities are registered under the Exchange Act, nor are we required to comply with the SEC’s Regulation FD, which restricts the selective disclosure of material non-public information. Accordingly, there may be less information publicly available concerning us than there is for a company that files as a domestic issuer.
We are an emerging growth company as defined in the JOBS Act and the reduced disclosure requirements applicable to emerging growth companies may make the ADSs or Warrants less attractive to investors and, as a result, adversely affect the price of the ADSs or Warrants and result in a less active trading market for the ADSs or Warrants.
We are an emerging growth company as defined in the JOBS Act, and we may take advantage of certain exemptions from various reporting requirements that are applicable to other public companies that are not emerging growth companies. For example, we have elected to rely on an exemption from the auditor attestation requirements of Section 404 of the Sarbanes-Oxley Act relating to internal control over financial reporting, and we will not provide such an attestation from our auditors for so long as we qualify as an emerging growth company.
We may avail ourselves of these disclosure exemptions until we are no longer an emerging growth company. We cannot predict whether investors will find the ADSs or Warrants less attractive because of our reliance on some or all of these exemptions. If investors find the ADSs or Warrants less attractive, it may cause the trading price of the ADSs or Warrants to decline and there may be a less active trading market for the ADSs or Warrants.
We will cease to be an emerging growth company upon the earliest of:
| | • | | the end of the fiscal year in which the fifth anniversary of completion of our initial public offering in the United States occurs, or June 30, 2021; |
| | • | | the end of the first fiscal year in which the market value of our ordinary shares held by non-affiliates exceeds US$700 million as of the end of the second quarter of such fiscal year; |
| | • | | the end of the first fiscal year in which we have total annual gross revenues of at least US$1.0 billion; and |
| | • | | the date on which we have issued more than US$1.0 billion in non-convertible debt securities in any rolling three-year period. |
If we fail to establish and maintain proper internal financial reporting controls, our ability to produce accurate financial statements or comply with applicable regulations could be impaired.
Section 404(a) of the Sarbanes-Oxley Act requires that, beginning with our second annual report after the completion of our initial public offering in the United States, our management assess and report annually on the effectiveness of our internal controls over financial reporting and identify any material weaknesses in our internal controls over financial reporting. Although Section 404(b) of the Sarbanes-Oxley Act requires our independent registered public accounting firm to issue an annual report that addresses the effectiveness of our internal controls over financial reporting, we have opted to rely on the exemption provided in the JOBS Act, and consequently will not be required to comply with SEC rules that implement Section 404(b) of the Sarbanes-Oxley Act until such time as we are no longer an emerging growth company.
51
Our first Section 404(a) assessment will take place beginning with our second annual report after the completion of our initial public offering in the United States (i.e., our annual report for the year ending June 30, 2016). The presence of material weaknesses could result in financial statement errors which, in turn, could lead to errors in our financial reports and/or delays in our financial reporting, which could require us to restate our operating results. We might not identify one or more material weaknesses in our internal controls in connection with evaluating our compliance with Section 404(a) of the Sarbanes-Oxley Act. In order to maintain and improve the effectiveness of our disclosure controls and procedures and internal controls over financial reporting, we will need to expend significant resources and provide significant management oversight. Implementing any appropriate changes to our internal controls may require specific compliance training of our directors and employees, entail substantial costs in order to modify our existing accounting systems, take a significant period of time to complete and divert management’s attention from other business concerns. These changes may not, however, be effective in maintaining the adequacy of our internal control.
If we are unable to conclude that we have effective internal controls over financial reporting, investors may lose confidence in our operating results, the price of the ADSs or Warrants could decline and we may be subject to litigation or regulatory enforcement actions. In addition, if we are unable to meet the requirements of Section 404 of the Sarbanes-Oxley Act, the ADSs and Warrants may not be able to remain listed on NASDAQ.
ADS holders and Warrant holders may be subject to additional risks related to holding ADSs and Warrants rather than ordinary shares.
ADS holders and Warrant holders do not hold ordinary shares directly and, as such, are subject to, among others, the following additional risks:
| | • | | As an ADS holder, we will not treat you as one of our shareholders and you will not be able to exercise shareholder rights, except through the ADR depositary as permitted by the deposit agreement. As a Warrant holder, you will have no rights as an ADS holder until you exercise your Warrant. |
| | • | | Distributions on the ordinary shares represented by your ADSs will be paid to the ADR depositary, and before the ADR depositary makes a distribution to you on behalf of your ADSs, any withholding taxes that must be paid will be deducted. Additionally, if the exchange rate fluctuates during a time when the ADR depositary cannot convert the foreign currency, you may lose some or all of the value of the distribution. |
| | • | | We and the ADR depositary may amend or terminate the deposit agreement without the ADS holders’ consent in a manner that could prejudice ADS holders. We may also amend the ADS Warrant Agent Agreement without the consent of any Warrant holder for the purpose of curing any ambiguity, or curing, correcting or supplementing any defective provision contained in the ADS Warrant Agent Agreement or adding or changing any other provisions with respect to matters or questions arising under that agreement as we and the Warrant Agent deem shall not adversely affect the interest of the Warrant holders. We may also modify or amend the ADS Warrant Agent Agreement in other respects with the vote or written consent of the holders of at least 65% of the then outstanding Warrants. |
The Warrants are a risky investment. You may be unable to exercise your Warrants for a profit.
The value of the Warrants will depend on the value of our ADSs, which will depend on factors related and unrelated to the success of our commercialization and product development activities, and cannot be predicted at this time, as well as the factors described herein that may affect the value of our ordinary shares. The Warrants have an exercise period of 5 years.
If the price of our ADSs does not increase to an amount sufficiently above the exercise price of the Warrants during the exercise period of the Warrants, you may be unable to recover any of your investment in the Warrants. In addition, because we are an Australian corporation whose ordinary shares are listed on the ASX, the anti-dilution adjustments included in the Warrants are limited to those permitted by the rules of the ASX. As a result,
52
the Warrants do not include any value-weighted average price or similar adjustment provision for issuances of ADSs at a price below the exercise price of the Warrants or the market price of our ADSs or ordinary shares. There can be no assurance that any of the factors that could impact the trading price of our ADSs will result in the trading price increasing to an amount that will exceed the exercise price or the price required for you to achieve a positive return on your investment in the Warrants.
You must act through the ADR depositary to exercise your voting rights and, as a result, you may be unable to exercise your voting rights on a timely basis. Holders of Warrants will have no rights as ADS holders until they acquire ADSs.
As a holder of ADSs (and not the ordinary shares underlying your ADSs), we will not treat you as one of our shareholders, and you will not be able to exercise shareholder rights. The ADR depositary will be the holder of the ordinary shares underlying your ADSs, and ADS holders will be able to exercise voting rights with respect to the ordinary shares represented by the ADSs only in accordance with the deposit agreement relating to the ADSs. There are practical limitations on the ability of ADS holders to exercise their voting rights due to the additional procedural steps involved in communicating with these holders. For example, holders of our ordinary shares will receive notice of shareholders’ meetings by mail and will be able to exercise their voting rights by either attending the shareholders meeting in person or voting by proxy. ADS holders, by comparison, will not receive notice directly from us. Instead, in accordance with the deposit agreement, we will provide notice to the ADR depositary of any such shareholders meeting and details concerning the matters to be voted upon at least 30 days in advance of the meeting date. If we so instruct, the ADR depositary will mail to holders of ADSs the notice of the meeting and a statement as to the manner in which voting instructions may be given by holders as soon as practicable after receiving notice from us of any such meeting. To exercise their voting rights, ADS holders must then instruct the ADR depositary as to voting the ordinary shares represented by their ADSs. Due to these procedural steps involving the ADR depositary, the process for exercising voting rights may take longer for ADS holders than for holders of ordinary shares. The ordinary shares represented by ADSs for which the ADR depositary fails to receive timely voting instructions will not be voted.
Until you acquire ADSs upon exercise of the Warrants, you will have no rights with respect to our ADSs or the ordinary shares underlying the ADSs, including the right the receive dividend payments, vote or respond to tender offers. Upon exercise of your Warrants, you will be entitled to exercise the rights of ADS holders as to matters for which the record date occurs after the exercise date. See “Description of Securities—Warrants.”
Although we are required to use our reasonable best efforts to have an effective registration statement covering the issuance of the ADSs underlying the Warrants at the time that holders of our Warrants exercise their Warrants, we cannot guarantee that a registration statement will be effective, in which case holders of our Warrants may not be able to receive freely tradable ADSs upon exercise of the Warrants.
Holders of our Warrants will be able to exercise the Warrants and receive freely tradable shares only if (i) a current registration statement under the Securities Act relating to the ADSs underlying the Warrants is then effective, or an exemption from such registration is available, and (ii) such ADSs are qualified for sale or exempt from qualification under the applicable securities laws of the states in which the various holders of Warrants reside, as further described in the ADS Warrant Agent Agreement. Although we have undertaken in the ADS Warrant Agent Agreement, and therefore have a contractual obligation, to use our reasonable best efforts to maintain a current registration statement covering the ADSs underlying the Warrants to the extent required by federal securities laws, we may not be able to do so. If we are not able to do so, holders will not be able to exercise their Warrants and receive freely tradable ADSs but rather will have the exercise price for the Warrants returned to them. The value of the Warrants may be greatly reduced if a registration statement covering the ADSs issuable upon exercise of the Warrants is not kept current.
53
The Warrants may not have any value.
The Warrants will expire on August 21, 2020, the 5 year anniversary of the closing of our U.S. initial public offering. In the event the price of the ADSs does not exceed the exercise price of the Warrants during the period in which the Warrants are exercisable, the Warrants may not have any value.
If we are classified as a “passive foreign investment company,” then our U.S. shareholders could suffer adverse tax consequences as a result.
Generally, if, for any taxable year, at least 75% of our gross income is passive income or at least 50% of the average quarterly value of our gross assets is attributable to assets that produce passive income or are held for the production of passive income, including cash, we would be characterized as a passive foreign investment company, or PFIC, for U.S. federal income tax purposes. For purposes of these tests, passive income includes dividends, interest, and gains from the sale or exchange of investment property and rents and royalties other than rents and royalties which are received from unrelated parties in connection with the active conduct of a trade or business. If we are characterized as a PFIC, a U.S. holder of our ordinary shares or ADSs may suffer adverse tax consequences, including having gains realized on the sale of our ordinary shares or ADSs treated as ordinary income, rather than capital gain, the loss of the preferential rate applicable to dividends received on our ordinary shares or ADSs by individuals who are U.S. holders, and having interest charges added to their tax on distributions from us and on gains from the sale of our ordinary shares or ADSs. See “Taxation—U.S. Federal Income Tax Considerations—Passive Foreign Investment Company.”
Since PFIC status depends on the composition of our income and the composition and value of our assets, which may be determined in large part by reference to the market value of our ordinary shares or ADSs, which may be volatile, there can be no assurance that we will not be a PFIC for any taxable year. While we believe that we were not a PFIC for fiscal 2014 or 2015, since the PFIC tests are applied only at the end of a taxable year no assurance of our PFIC status can be provided for fiscal 2016 or future years. Prospective U.S. investors should discuss the issue of our possible status as a PFIC with their tax advisors.
Currency fluctuations may adversely affect the price of our ordinary shares, ADSs and Warrants.
Our ordinary shares are quoted in Australian dollars on the ASX and the ADSs and Warrants will be quoted in U.S. dollars on NASDAQ. Movements in the Australian dollar/U.S. dollar exchange rate may adversely affect the U.S. dollar price of the ADSs and Warrants. In the past year the Australian dollar has generally weakened against the U.S. dollar. However, this trend may not continue and may be reversed. If the Australian dollar weakens against the U.S. dollar, the U.S. dollar price of the ADSs and Warrants could decline, even if the price of our ordinary shares in Australian dollars increases or remains unchanged.
We have never declared or paid dividends on our ordinary shares and we do not anticipate paying dividends in the foreseeable future.
We have never declared or paid cash dividends on our ordinary shares. For the foreseeable future, we currently intend to retain all available funds and any future earnings to support our operations and to finance the growth and development of our business. Any future determination to declare cash dividends will be made at the discretion of our board of directors, subject to compliance with applicable laws and covenants under current or future credit facilities, which may restrict or limit our ability to pay dividends, and will depend on our financial condition, operating results, capital requirements, general business conditions and other factors that our board of directors may deem relevant. We do not anticipate paying any cash dividends on our ordinary shares in the foreseeable future. As a result, a return on your investment in the ADS and the Warrants will only occur if our ADS price appreciates.
54
You may not receive distributions on our ordinary shares represented by the ADSs or any value for such distribution if it is illegal or impractical to make them available to holders of ADSs.
While we do not anticipate paying any dividends on our ordinary shares in the foreseeable future, if such a dividend is declared, the depositary for the ADSs has agreed to pay to you the cash dividends or other distributions it or the custodian receives on our ordinary shares or other deposited securities after deducting its fees and expenses. You will receive these distributions in proportion to the number of our ordinary shares your ADSs represent. However, in accordance with the limitations set forth in the deposit agreement, it may be unlawful or impractical to make a distribution available to holders of ADSs. We have no obligation to take any other action to permit the distribution of the ADSs, ordinary shares, rights or anything else to holders of the ADSs. This means that you may not receive the distributions we make on our ordinary shares or any value from them if it is unlawful or impractical to make them available to you. These restrictions may have a material adverse effect on the value of your ADSs.
Australian takeover laws may discourage takeover offers being made for us or may discourage the acquisition of a significant position in our ordinary shares, ADSs or Warrants.
We are incorporated in Australia and are subject to the takeover laws of Australia. Among other things, we are subject to the Australian Corporations Act 2001, or the Corporations Act. Subject to a range of exceptions, the Corporations Act prohibits the acquisition of a direct or indirect interest in our issued voting shares if the acquisition of that interest will lead to a person’s voting power in us increasing to more than 20%, or increasing from a starting point that is above 20% and below 90%. Australian takeover laws may discourage takeover offers being made for us or may discourage the acquisition of a significant position in our ordinary shares. This may have the ancillary effect of entrenching our board of directors and may deprive or limit our shareholders’, ADS holders’ or Warrant holders’ opportunity to sell their ordinary shares, ADSs or Warrants and may further restrict the ability of our shareholders, ADS holders and Warrants holders to obtain a premium from such transactions. See “Description of Share Capital—Change of Control.”
Our Constitution and Australian laws and regulations applicable to us may adversely affect our ability to take actions that could be beneficial to our shareholders.
As an Australian company, we are subject to different corporate requirements than a corporation organized under the laws of the states of the United States. Our Constitution, as well as the Australian Corporations Act, set forth various rights and obligations that are unique to us as an Australian company. These requirements may operate differently than those of many U.S. companies. You should carefully review the summary of these matters set forth under the section entitled, “Description of Share Capital” as well as our Constitution, which is included as an exhibit to this registration statement to which this prospectus forms a part, prior to investing in the ADSs.
55
CAUTIONARY NOTE REGARDING FORWARD-LOOKING STATEMENTS
This prospectus contains forward looking statements that are subject to a number of risks and uncertainties, many of which are beyond our control. All statements, other than statements of historical fact included in this prospectus, regarding our strategy, future operations, financial position, projected costs, prospects, plans and objectives of management are forward looking statements. When used in this prospectus, the words “could,” “believe,” “anticipate,” “intend,” “estimate,” “expect,” “may,” “continue,” “predict,” “potential,” “project,” or the negative of these terms, and similar expressions are intended to identify forward looking statements, although not all forward looking statements contain such identifying words. These statements involve known and unknown risks, uncertainties and other important factors that may cause our actual results, levels of activity, performance or achievements to be materially different from the information expressed or implied by these forward-looking statements. Although we believe that we have a reasonable basis for each forward-looking statement contained in this prospectus, we caution you that these statements are based on a combination of facts and important factors currently known by us and our expectations of the future, about which we cannot be certain.
Forward-looking statements may include statements about:
| | • | | our plans to develop and potentially commercialize our product candidates; |
| | • | | the timing of the initiation and completion of preclinical studies and clinical trials; |
| | • | | the timing of patient enrollment and dosing in any future clinical trials; |
| | • | | the timing of the availability of data from clinical trials; |
| | • | | the timing of expected regulatory filings; |
| | • | | the development of novel AAV vectors; |
| | • | | expectations about the plans of licensees of our technology; |
| | • | | the clinical utility and potential attributes and benefits of ddRNAi and our product candidates, including the potential duration of treatment effects and the potential for a “one shot” cure; |
| | • | | potential future out-licenses and collaborations; |
| | • | | our expectations regarding expenses, ongoing losses, future revenue, capital needs and needs for additional financing; |
| | • | | our use of proceeds from this offering; |
| | • | | the length of time over which we expect our cash and cash equivalents and the proceeds from our U.S. initial public offering to be sufficient; and |
| | • | | our intellectual property position and the duration of our patent portfolio. |
All forward-looking statements speak only as of the date of this prospectus. You should not place undue reliance on these forward-looking statements. Although we believe that our plans, objectives, expectations and intentions reflected in or suggested by the forward-looking statements we make in this prospectus are reasonable, we can give no assurance that these plans, objectives, expectations or intentions will be achieved. We disclose important factors that could cause our actual results to differ materially from our expectations under “Risk Factors” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and elsewhere in this prospectus.
The forward-looking statements made in this prospectus relate only to events or information as of the date on which the statements are made in this prospectus. Except as required by law, we undertake no obligation to update or revise publicly any forward-looking statements, whether as a result of new information, future events or otherwise, after the date on which the statements are made or to reflect the occurrence of unanticipated events.
56
USE OF PROCEEDS
The net proceeds from the sale of the ADSs and Warrants in this offering was approximately US$11.3 million, including the partial exercise of the underwriter’s option to purchase additional ADSs and/or Warrants, based on our initial U.S. public offering price of US$9.21 per ADS, after deducting estimated underwriting discounts and commissions and estimated offering expenses payable by us. If Warrant holders exercise their Warrants for cash, we will also receive proceeds from such exercise at the time of such exercise. If all of the Warrants (excluding those already issued pursuant to the underwriter’s option) are exercised, the additional net proceeds to us will be approximately US$2.8 million. We cannot predict when or if the Warrants will be exercised. It is possible that the Warrants may expire and may never be exercised, in which case we will not receive any additional proceeds.
We have begun to use and expect to continue to use the net proceeds from this offering as follows:
| | • | | approximately US$5.5 million for development of BB-HB-331 for hepatitis B, for preclinical studies intended to establish in vivo proof of concept and through to submission of an IND application; |
| | • | | approximately US$3.5 million for preclinical studies intended to establish in vivo proof of concept of the AMD program and through to submission of an IND application and initiation of a clinical trial; |
| | • | | approximately US$2.0 million for development of the OPMD program, for preclinical studies intended to establish in vivo proof of concept and through to submission of an IND application; and |
| | • | | the remainder (including any proceeds from the exercise of the Warrants, if any) for the discovery, development and acquisition of complementary targets and technologies, and for working capital and general corporate purposes. |
We believe that the proceeds from this offering, together with our pre-existing cash and cash equivalents, will be sufficient to advance our product candidates for hepatitis B and OPMD through completion of preclinical proof of concept trials and to submission of IND applications and to advance our product candidate for AMD through completion of preclinical trials, submission of an IND application and initiation of a clinical trial. Advancing our product candidates for hepatitis B and OPMD into clinical trial would require additional financing. As of December 31, 2015, our cash and cash equivalents were A$22.8 million. As of the date of this prospectus, we cannot specify with certainty all of the particular uses for the net proceeds from this offering. The amounts and timing of our actual expenditures may vary significantly from our expectations depending upon numerous factors, including the progress of our research and development efforts, the progress of our clinical trials, our operating costs and factors described under “Risk Factors” in this prospectus. Accordingly, we retain the discretion to allocate the net proceeds of this offering and we reserve the right to change the allocation of the net proceeds described above.
Pending these uses, we have invested the net proceeds from this offering in investment grade, interest-bearing instruments, securities or certificates of deposit.
57
PRICE RANGE OF ORDINARY SHARES, ADSS AND WARRANTS
Our Ordinary Shares
The following table presents, for the periods indicated, the high and low market prices for our ordinary shares reported on the ASX, under the symbol BLT. All prices are in Australian dollars.
| | | | | | | | |
| | | High | | | Low | |
| | | A$ | | | A$ | |
Annual: | | | | | | | | |
Fiscal year ended June 30, | | | | | | | | |
2015 | | | 1.32 | | | | 0.52 | |
2014 | | | 2.38 | | | | 0.28 | |
2013 | | | 0.50 | (1) | | | 0.25 | (1) |
2012 | | | 0.75 | (1) | | | 0.25 | (1) |
2011 | | | 1.00 | (1) | | | 0.50 | (1) |
| | |
Quarterly: | | | | | | | | |
Fiscal year ended June 30, 2016 | | | | | | | | |
Fourth quarter (through April 8, 2016) | | | 0.12 | | | | 0.12 | |
Third quarter | | | 0.33 | | | | 0.12 | |
Second quarter | | | 0.56 | | | | 0.27 | |
First quarter | | | 0.96 | | | | 0.69 | |
Fiscal year ended June 30, 2015 | | | | | | | | |
Fourth quarter | | | 0.89 | | | | 0.67 | |
Third quarter | | | 1.08 | | | | 0.71 | |
Second quarter | | | 1.07 | | | | 0.52 | |
First quarter | | | 1.32 | | | | 0.87 | |
Fiscal year ended June 30, 2014 | | | | | | | | |
Fourth quarter | | | 1.84 | | | | 0.86 | |
Third quarter | | | 2.38 | | | | 0.56 | |
Second quarter | | | 0.75 | | | | 0.35 | |
First quarter | | | 0.41 | | | | 0.25 | (1) |
Fiscal year ended June 30, 2013 | | | | | | | | |
Fourth quarter | | | 0.50 | (1) | | | 0.25 | (1) |
Third quarter | | | 0.50 | (1) | | | 0.25 | (1) |
Second quarter | | | 0.50 | (1) | | | 0.25 | (1) |
First quarter | | | 0.50 | (1) | | | 0.25 | (1) |
| | |
Most Recent Six Months: | | | | | | | | |
March 2016 | | | 0.14 | | | | 0.12 | |
February 2016 | | | 0.28 | | | | 0.12 | |
January 2016 | | | 0.33 | | | | 0.26 | |
December 2015 | | | 0.89 | | | | 0.29 | |
November 2015 | | | 0.43 | | | | 0.33 | |
October 2015 | | | 0.56 | | | | 0.43 | |
| (1) | | Takes into account a 25:1 share consolidation that became effective in July 2013. |
On April 8, 2016, the closing price of our ordinary shares as traded on the ASX was A$0.12 per ordinary share (US$0.09 per share based on the foreign exchange rate of A$1.00 to US$0.7535 as published by the Reserve Bank of Australia as of such date).
58
Based on information known to us, as of December 31, 2015, we had 146,529,096 ordinary shares outstanding, with 39,869,071 of our ordinary shares being held (directly or through ADSs) in the United States by 60 holders and 106,660,025 of our ordinary shares being held in Australia by 4,246 holders. A large number of our ordinary shares are held in nominee companies so we cannot be certain of the identity of those beneficial owners.
Our ADSs
Since August 18, 2015, our ordinary shares in the form of ADSs and Warrants have been trading on The NASDAQ Capital Market under the symbols “BNTC” and “BNTCW”, respectively. The following table sets forth the high and low sales for our ADSs and Warrants for the periods indicated as reported on The NASDAQ Capital Market:
| | | | | | | | |
Quarter | | High | | | Low | |
ADSs | | | | | | | | |
August 2015 | | $ | 9.00 | | | $ | 7.60 | |
September 2015 | | $ | 8.10 | | | $ | 6.00 | |
October 2015 | | $ | 7.30 | | | $ | 5.89 | |
First Quarter 2016 (ended September 30, 2015) | | $ | 9.00 | | | $ | 6.00 | |
Second Quarter 2016 (ended December 31, 2015) | | $ | 7.30 | | | $ | 3.95 | |
Third Quarter 2016 (ended March 31, 2016) | | $ | 4.34 | | | $ | 1.22 | |
Fourth Quarter 2016 (through April 8, 2016) | | $ | 1.75 | | | $ | 1.67 | |
| | |
Warrants | | | | | | | | |
August 2015 | | $ | 3.99 | | | $ | 2.06 | |
September 2015 | | $ | 3.88 | | | $ | 2.01 | |
October 2015 | | $ | 3.50 | | | $ | 2.00 | |
First Quarter (ended September 30, 2015) | | $ | 3.99 | | | $ | 2.01 | |
Second Quarter (ended December 31, 2015) | | $ | 3.50 | | | $ | 1.75 | |
Third Quarter 2016 (ended March 31, 2016) | | $ | 2.50 | | | $ | 0.31 | |
Fourth Quarter 2016 (through April 8, 2016) | | $ | 0.95 | | | $ | 0.95 | |
59
DIVIDEND POLICY
We have not declared or paid any dividends on our ordinary shares, and we do not anticipate paying any dividends in the foreseeable future. Our board of directors presently intends to reinvest all earnings in the continued development and operation of our business.
Payment of dividends in the future, if any, will be at the discretion of our board of directors. If our board of directors elects to pay dividends, the form, frequency and amount will depend upon our future operations and earnings, capital requirements and surplus, general financial conditions, contractual restrictions and other factors that our board of directors may deem relevant.
60
EXCHANGE RATE INFORMATION
The Australian dollar is convertible into U.S. dollars at freely floating rates. There are no legal restrictions on the flow of Australian dollars between Australia and the United States. Any remittances of dividends or other payments by us to persons in the United States are not and will not be subject to any exchange controls.
Our financial statements are prepared and presented in Australian dollars.
The table below sets forth for the periods identified the number of U.S. dollars per Australian dollar as published by the Reserve Bank of Australia. We make no representation that any Australian dollar or U.S. dollar amounts could have been, or could be, converted into U.S. dollars or Australian dollars, as the case may be, at any particular rate, the rates stated below, or at all.
| | | | | | | | | | | | | | | | |
| | | At Period
End(1) | | | Average
Rate(2) | | | High(3) | | | Low(4) | |
Fiscal year ended June 30, | | | | | | | | | | | | | | | | |
2015 | | | 0.7680 | | | | 0.8293 | | | | 0.9458 | | | | 0.7590 | |
2014 | | | 0.9420 | | | | 0.9187 | | | | 0.9672 | | | | 0.8716 | |
2013 | | | 0.9275 | | | | 1.0271 | | | | 1.0593 | | | | 0.9202 | |
| | | | |
Month ended: | | | | | | | | | | | | | | | | |
March 31, 2016 | | | 0.7657 | | | | 0.7483 | | | | 0.7657 | | | | 0.7132 | |
February 29, 2016 | | | 0.7140 | | | | 0.7128 | | | | 0.7240 | | | | 0.7015 | |
January 31, 2016 | | | 0.7100 | | | | 0.7015 | | | | 0.7223 | | | | 0.6867 | |
December 31, 2015 | | | 0.7306 | | | | 0.7248 | | | | 0.7332 | | | | 0.7134 | |
November 30, 2015 | | | 0.7189 | | | | 0.7155 | | | | 0.7265 | | | | 0.7047 | |
October 31, 2015 | | | 0.7099 | | | | 0.7210 | | | | 0.7332 | | | | 0.7038 | |
| (1) | | Determined by the published rate on the last trading day of the month. |
| (2) | | Determined by averaging the published rate on the last day of each full month during the fiscal year indicated or by averaging the published rates for all trading days during the month indicated. |
| (3) | | Determined by the highest published rate during the month. |
| (4) | | Determined by the lowest published rate during the month. |
61
CAPITALIZATION
The following table sets forth our cash and cash equivalents and our capitalization as of December 31, 2015, presented both in Australian dollars and U.S. dollars:
| | • | | on an actual basis; and |
| | • | | on an as adjusted basis to give effect to the exercise of 575,000 Warrants sold in this offering that remain outstanding at December 31, 2015 at an exercise price of US$5.50 per warrant, which would result in 11,500,000 ordinary shares being issued represented by 575,000 ADSs. |
| | | | | | | | | | | | | | | | |
| (in thousands) | | As of December 31, 2015 | |
| | | Actual | | | Actual | | | As Adjusted | | | As Adjusted | |
| | | (A$) | | | (US$)(1) | | | (A$) | | | (US$) | |
Cash and cash equivalents | | $ | 22,764 | | | $ | 16,631 | | | $ | 27,093 | | | $ | 19,794 | |
| | | | | | | | | | | | | | | | |
Equity: | | | | | | | | | | | | | | | | |
Issued capital | | $ | 147,641 | | | $ | 107,867 | | | $ | 151,970 | | | $ | 111,030 | |
Reserves | | | 2,607 | | | | 1,905 | | | | 2,607 | | | | 1,905 | |
Accumulated losses | | | (122,963 | ) | | | (89,837 | ) | | | (122,963 | ) | | | (89,837 | ) |
| | | | | | | | | | | | | | | | |
Total equity | | | 27,285 | | | | 19,934 | | | | 30,614 | | | | 23,098 | |
| | | | | | | | | | | | | | | | |
Total capitalization | | $ | 27,285 | | | $ | 19,934 | | | $ | 30,614 | | | $ | 23,098 | |
| | | | | | | | | | | | | | | | |
| (1) | | The amounts have been translated into U.S. dollars from Australian dollars based upon the exchange rate as published by the Reserve Bank of Australia as of December 31, 2015. These translations are merely for the convenience of the reader and should not be construed as representations that the Australian dollar amounts actually represent such U.S. dollar amounts or could be converted into U.S. dollars at the rate indicated. |
62
DILUTION
If you exercise Warrants sold in this offering, your interest will be diluted to the extent of the difference between the exercise price of the Warrants and our net tangible book value per ADS. Dilution results from the fact that the exercise price per Warrant is substantially in excess of the net tangible book value per 20 ordinary shares underlying the ADSs. Our net tangible book value as at December 31, 2015 was A$27.3 million (US$19.9 million), or A$0.19 (US$0.14) per ordinary share, equivalent to A$3.72 (US$2.72) per ADS. Net tangible book value per ADS represents the amount of total tangible assets, minus the amount of total liabilities, divided by the total number of ordinary shares outstanding and multiplied by 20, the number of ordinary shares underlying each ADS. Dilution is determined by subtracting net tangible book value per ADS from the initial public offering price per ADS.
Without taking into account any other changes in our net tangible book value after December 31, 2015, other than to give effect to the exercise of the 575,000 Warrants sold in this offering that remain outstanding at December 31, 2015 at an exercise price of US$5.50 per Warrant, which would result in 11,500,000 ordinary shares being issued represented by 575,000 ADSs, our adjusted net tangible book value as at December 31, 2015 would have been US$23.1 million, or US$2.92 per ADS. This represents an immediate increase in net tangible book value of US$0.20 per ADS to existing shareholders and an immediate dilution in net tangible book value of US$2.58 per ADS to holders of Warrants exercised in this offering. The following table presents this dilution to warrants exercised from this offering:
| | | | | | | | |
Exercise price per Warrant | | | | | | | US$5.50 | |
Net tangible book value as at December 31, 2015 | | US$ | 2.72 | | | | | |
| | | | | | | | |
Increase in net tangible book value attributable to exercise of all outstanding Warrants | | | 0.20 | | | | | |
| | | | | | | | |
As-adjusted net tangible book value immediately after the exercise of all outstanding Warrants | | | | | | | 2.92 | |
| | | | | | | | |
Dilution to holders of Warrants exercised in this offering | | | | | | | US$2.58 | |
| | | | | | | | |
The following table summarizes, as of December 31, 2015, on the as-adjusted basis described above, the differences between the existing shareholders as of December 31, 2015 and the holders of Warrants exercised in this offering with respect to the number of ADSs, or equivalent number of ordinary shares, purchased from us, the total consideration paid to us and the average price per ADS, or equivalent number of ordinary shares, based on an exercise price of US$5.50 per Warrant.
| | | | | | | | | | | | | | | | | | |
| | | ADS (or
Equivalent
Ordinary
Shares
Purchased(1)) | | | Total
Consideration
| | | Average
Price Per
ADS or
Equivalent
Ordinary
Shares(1) | |
| | | Number | | % | | | Amount | | | % | | |
Existing shareholders | | 7,278,181 | | | 93 | % | | | US$107,866,515 | | | | 97 | % | | US$ | 14.82 | |
Holders of Warrants | | 575,000 | | | 7 | % | | | 3,162,500 | | | | 3 | % | | | 5.50 | |
| | | | | | | | | | | | | | | | | | |
Total | | 7,853,181 | | | 100 | % | | | US$111,029,015 | | | | 100 | % | | | | |
| | | | | | | | | | | | | | | | | | |
| (1) | | Reflects 20 ordinary shares as equivalent to each ADS. |
To the extent that we grant options or other equity awards to our employees or members of our management in the future, and those options or other equity awards are exercised or become vested or other issuance of our ordinary shares are made, there will be further dilution to new investors.
63
The share information above:
| | • | | is based on 146,529,096 ordinary shares outstanding as of December 31, 2015; and |
| | • | | excludes an aggregate of 27,066,203 ordinary shares issuable upon the exercise of options outstanding at December 31, 2015. |
64
SELECTED HISTORICAL CONSOLIDATED FINANCIAL DATA
The following tables set forth summary historical financial data for the periods indicated.
The consolidated statement of profit or loss and other comprehensive income data and consolidated statement of financial position data for and as of the fiscal years ended June 30, 2013, 2014 and 2015 are derived from the audited consolidated financial statements included in this prospectus. The consolidated statement of profit or loss and other comprehensive income data for the six-month periods ended December 31, 2014 and 2015 and the consolidated statement of financial position data as of December 31, 2015 and 2014 are derived from the unaudited consolidated financial statements that are included in this prospectus. In our management’s opinion, these financial statements include all adjustments necessary for the fair presentation of our financial condition as of such dates and our results of operations for such periods.
Our financial statements have been prepared in Australian dollars and in accordance with International Accounting Standards. Our financial statements comply with IFRS, as issued by the IASB.
You should read the selected consolidated financial data in conjunction with our consolidated financial statements and related notes beginning on page F-1 of this prospectus, and “Management’s Discussion and Analysis of Financial Condition and Results of Operations” included elsewhere in this prospectus. Our historical results do not necessarily indicate our expected results for any future periods.
| | | | | | | | | | | | | | | | | | | | |
| (in thousands, except per share data) | | For the year ended
June 30, | | | For the six months ended
December 31, | |
| | | 2015 | | | 2014 | | | 2013 | | | 2015 | | | 2014 | |
Statement of Profit or Loss and Other Comprehensive Income Data: | | | | | | | | | | | | | | | | | | | | |
Revenue: | | | | | | | | | | | | | | | | | | | | |
Revenue | | A$ | 1,081 | | | A$ | 598 | | | A$ | 640 | | | A$ | 322 | | | A$ | 639 | |
Other income | | | 2,891 | | | | 776 | | | | 2,350 | | | | — | | | | 392 | |
| | | | | | | | | | | | | | | | | | | | |
Total revenue | | | 3,972 | | | | 1,374 | | | | 2,990 | | | | 322 | | | | 1031 | |
| | | | | | | | | | | | | | | | | | | | |
Costs and expenses: | | | | | | | | | | | | | | | | | | | | |
Royalties and license fees | | | (40 | ) | | | (193 | ) | | | (30 | ) | | | (49 | ) | | | (40 | ) |
Research and development | | | (6,228 | ) | | | (3,758 | ) | | | (1,280 | ) | | | (8,151 | ) | | | (2,210 | ) |
Employment related | | | (3,425 | ) | | | (2,444 | ) | | | (1,832 | ) | | | (3,357 | ) | | | (1,502 | ) |
Share based expenses | | | (1,503 | ) | | | (355 | ) | | | (519 | ) | | | (1,475 | ) | | | (842 | ) |
Impairment costs | | | — | | | | — | | | | (1,503 | ) | | | — | | | | — | |
Travel related expenses | | | (1,039 | ) | | | (585 | ) | | | (346 | ) | | | (725 | ) | | | (517 | ) |
Consultants costs | | | (882 | ) | | | (653 | ) | | | (337 | ) | | | (561 | ) | | | (400 | ) |
Occupancy costs | | | (275 | ) | | | (122 | ) | | | (100 | ) | | | (286 | ) | | | (131 | ) |
Corporate expenses | | | (1,018 | ) | | | (646 | ) | | | (532 | ) | | | (591 | ) | | | (436 | ) |
Foreign exchange translation | | | — | | | | (111 | ) | | | — | | | | (241 | ) | | | — | |
IPO Costs | | | (1,071 | ) | | | — | | | | — | | | | (960 | ) | | | — | |
| | | | | | | | | | | | | | | | | | | | |
Total costs and expenses | | | (15,481 | ) | | | (8,867 | ) | | | (4,953 | ) | | | (16,396 | ) | | | (6,078 | ) |
| | | | | | | | | | | | | | | | | | | | |
Loss before income tax | | | (11,509 | ) | | | (7,493 | ) | | | (3,489 | ) | | | (16,074 | ) | | | (5,047 | ) |
Income tax benefit | | | — | | | | 454 | | | | — | | | | — | | | | — | |
| | | | | | | | | | | | | | | | | | | | |
Loss for the period | | A$ | (11,509 | ) | | A$ | (7,039 | ) | | A$ | (3,489 | ) | | A$ | (16,074 | ) | | A$ | (5,047 | ) |
| | | | | | | | | | | | | | | | | | | | |
| | | | | |
Loss per share, basic and diluted | | A$ | (0.0996 | ) | | A$ | (0.0778 | ) | | A$ | (0.0825 | ) | | A$ | (0.1160 | ) | | A$ | (0.044 | ) |
Weighted-average shares outstanding, basic and diluted | | | 115,507 | | | | 90,432 | | | | 41,689 | | | | 138,120 | | | | 115,218 | |
65
| | | | | | | | | | | | | | | | | | | | |
| (in thousands) | | As of June 30, | | | As of December 31, | |
| | | 2015 | | | 2014 | | | 2013 | | | 2015 | | | 2014 | |
Statement of Financial Position Data: | | | | | | | | | | | | | | | | | | | | |
Cash and cash equivalents | | A$ | 21,787 | | | A$ | 31,359 | | | A$ | 1,587 | | | A$ | 22,764 | | | A$ | 26,827 | |
Total current assets | | | 25,064 | | | | 34,448 | | | | 1,723 | | | | 27,802 | | | | 29,918 | |
Total assets | | | 25,520 | | | | 34,496 | | | | 1,751 | | | | 28,288 | | | | 30,336 | |
Total current liabilities | | | 1,642 | | | | 955 | | | | 1,110 | | | | 1,003 | | | | 719 | |
Total liabilities | | | 1,642 | | | | 955 | | | | 1,110 | | | | 1,003 | | | | 719 | |
Total equity | | | 23,878 | | | | 33,541 | | | | 640 | | | | 27,285 | | | | 29,617 | |
66
MANAGEMENT’S DISCUSSION AND ANALYSIS
OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
You should read the following discussion and analysis of our financial condition and results of operations in conjunction with the section entitled “Selected Historical Consolidated Financial Data” and our consolidated financial statements and related notes included elsewhere in this prospectus. This discussion contains forward-looking statements that involve risks and uncertainties. Our actual results and the timing of selected events could differ materially from those anticipated in these forward-looking statements as a result of various factors, including, but not limited to, those set forth under “Risk Factors” and elsewhere in this prospectus.
Our financial statements have been prepared in Australian dollars and in accordance with International Accounting Standards. Our financial statements comply with IFRS, as issued by the IASB. Our fiscal year end is June 30. References to a particular “fiscal year” are to our fiscal year ended June 30 of that year.
Overview
We are a biotechnology company with a pipeline of in-house and partnered therapeutic programs based on our patented gene-silencing technology, ddRNAi. We are developing treatments for chronic and life-threatening human diseases such as hepatitis B, age-related macular degeneration and oculopharyngeal muscular dystrophy based on this technology. In addition, we have licensed ddRNAi technology to other biopharmaceutical companies that are progressing their programs towards, or are in, clinical development for applications, including HIV/AIDS, retinitis pigmentosa, Huntington’s disease, cancer immunotherapy and intractable neuropathic pain.
Our focus is to validate that ddRNAi is safe and efficacious in a clinical setting. With this goal in mind, we are developing BB-HB-331, which is currently in preclinical studies, as a therapy for hepatitis B. We plan to file an IND application for BB-HB-331 in the third quarter of 2017. Based on the potential market opportunity and considerable interest from pharmaceutical companies, we plan to prioritize the HBV program as our next candidate for clinical development.
We are in the process of winding down for commercial reasons a partially completed Phase I/IIa “first-in-human” clinical trial for our now discontinued product candidate, TT-034. Early stages of the clinical trial ofTT-034 indicated that TT-034 caused no treatment-related serious adverse events among the nine patients dosed to date. Of the nine patients, seven were biopsied and indicated a relationship between the amount of the compound administered and hepatic tissue transduction. However, as a result of the increasingly competitive landscape among available HCV therapies, in February 2016 we concluded that the TT-034 program no longer offered the commercial value necessary to attract a worthwhile commercial partnership deal and, as a result, did not warrant additional expenditure or focus of our resources beyond completion of the existing patients. We believe the early stage TT-034 clinical trial results provide support for the safety of our platform technology.
The success of preclinical studies and advancement to and completion of a clinical trial would be a key step in validating ddRNAi for therapeutic use, seeking regulatory approval of a product candidate based on ddRNAi and ultimately commercializing the product if it achieves approval. In the future, we expect to earn revenue primarily from licensing programs, strategic alliances and collaboration arrangements with pharmaceutical companies. There can be no assurance, however, as to whether we will enter into any additional such arrangement or what the terms of any such arrangement could be.
Since we were incorporated in Australia in 1995, we have devoted the majority of our resources to development efforts relating to ddRNAi. While we have established some licensing arrangements, we do not have any products approved for sale and have not generated any revenue from product sales. We have funded our operations primarily from private placements of ordinary shares, including A$31.5 million of gross proceeds raised in February 2014. We have also been awarded research and development grants from the Australian federal government, totaling A$2.3 million in fiscal 2015 and A$0.8 million in fiscal 2014. We have earned
67
licensing revenue from licensing our ddRNAi technology to five biopharmaceutical companies, totaling A$0.3 million in fiscal 2015 and A$0.3 million in fiscal 2014.
In October 2012, we acquired Tacere Therapeutics, Inc., or Tacere, an RNA interference therapeutics company based in California with a development program focused on hepatitis C. As consideration for the acquisition, we issued a total of 4,092,854 ordinary shares (taking into account a 25:1 share consolidation that became effective in July 2013), representing 9.8% of our issued capital immediately after the transaction, having an aggregate value of A$1.5 million.
In August 2015, we completed our U.S. initial public offering in which we issued 30,000,000 ordinary shares (represented by 1,500,000 ADSs) and 575,000 Warrants. The ADSs and Warrants are listed on The NASDAQ Capital Market.
We have incurred losses from operations in each year since inception. Our net losses were A$11.5 million, A$7.0 million and A$3.5 million for the fiscal years ended June 30, 2015, 2014 and 2013, respectively. The majority of our net losses resulted from costs incurred in connection with our research and development programs and from general and administrative costs associated with our operations. We expect to continue to incur significant expenses and increasing operating losses for at least the next several years. We expect our expenses will increase substantially in connection with our ongoing activities as we:
| | • | | pursue clinical proof of concept across our programs, including treatments for hepatitis B, AMD and OPMD; |
| | • | | continue preclinical development of immunotherapy programs and non-viral delivery of DNA constructs through preclinical proof of concept; |
| | • | | continue our research and development efforts of ddRNAi-based technology; |
| | • | | seek regulatory approval for our product candidates; and |
| | • | | add personnel and resources to support our product development and commercialization efforts. |
As of December 31, 2015, we had cash and cash equivalents of A$22.8 million.
We may generate revenue from licensing programs, strategic alliances or collaboration arrangement with pharmaceutical companies. These arrangements are likely to be more appealing to them when our pipeline is more advanced. We do not expect to generate revenue from product sales unless and until we successfully complete clinical development and obtain regulatory approval for one or more of our product candidates, which we expect will take a number of years, is subject to significant uncertainty and may never occur.
We expect that the net proceeds from our initial public offering in the United States and our pre-existing cash and cash equivalents will be sufficient to enable us to advance planned preclinical programs for certain of our current product candidates until approximately August 2017. See “Use of Proceeds.”
We will continue to pursue licensing programs, strategic alliances and collaboration arrangements with pharmaceutical companies and we regard this as our key value creation opportunity unless and until we are able to gain regulatory approval for one of our product candidates and decide to commercialize it ourselves. If we were to decide to take one or more product candidates to commercialization on our own, the process of obtaining regulatory approval for the selected programs and building the commercial infrastructure that would be necessary to commercialize them, if approved, would require substantial additional funding.
Our current operating plan may change as a result of many factors currently unknown to us, and we may need to seek additional funds sooner than planned. These additional funds could be raised through public or private equity or debt financings (although debt financings are unlikely to be available until we have significant revenue and cash flow to service debt we may incur), government or other third-party funding, strategic alliances
68
and licensing arrangements or a combination of these approaches. However, we may be unable to raise additional funds or enter into such other arrangements when needed on favorable terms or at all. Our failure to raise capital or enter into such other arrangements as and when needed would have a negative impact on our financial condition and compromise our ability to develop our product candidates and pursue our strategy.
We expect to incur losses for the foreseeable future, and we expect these losses to increase as we continue our development of, and seek regulatory approvals for, our product candidates. Because of the numerous risks and uncertainties associated with product development in our field, we are unable to predict the timing or amount of increased expenses, or when or if we will be able to generate product revenue or achieve or maintain profitability. Our ability to generate revenue from licensing, strategic alliances and collaboration arrangements and product sales will depend on a number of factors, including, among others, obtaining and maintaining adequate coverage and reimbursement from third-party payors for any of our product candidates that may receive regulatory approval. Even if we are able to generate revenues from licensing programs, strategic alliances or collaboration arrangements or commercial sale of our products, we may not become profitable. If we fail to become profitable or are unable to sustain profitability on a continuing basis, then we may be unable to continue our operations at planned levels and we could be forced to reduce our operations.
Financial operations overview
Revenue
To date, we have derived revenues from licensing fees, the Australian federal government’s Research and Development Tax Incentive program and interest income. We have not generated any revenues from the sales of products. Revenues from licensing fees are included in the revenue line item on our statements of profit or loss.
Our licensing fees have been generated through the licensing of our ddRNAi technology to biopharmaceutical companies.
Our grant revenue is generated through the Australian federal government’s Research and Development Tax Incentive program, under which the government provides a cash refund for the 45% of eligible research and development expenditures, including salaries, by small Australian entities having a tax loss. For this purpose, small Australian entities are defined as those with less than A$20 million in revenue. This grant is available for our research and development activities in Australia, as well as activities in the United States to the extent such U.S.-based expenses relate to our activities in Australia, do not exceed half the expenses for the relevant activities and are approved by the Australian government. Because the grants are determined by the Australian government following the completion of a fiscal year based upon eligible research and development expenditures, grants are recorded in the fiscal year received rather than the fiscal year to which they relate.
We also record interest and other financial income earned from bank accounts, term deposits and short-term investments as other revenues in our statements of profit or loss.
Research and development expenses
Research and development expenses consist primarily of costs incurred for the development of our product candidates, which include:
| | • | | expenses incurred under agreements with academic research centers, clinical research organizations and investigative sites that conduct our clinical trials; and |
| | • | | the cost of acquiring, developing, and manufacturing clinical trial materials. |
Research and development expenses do not include employment related expenses, which are included in our Statement of Profit or Loss and Other Comprehensive Income as a separate line item.
69
Research and development costs are expensed as incurred. Costs for certain development activities are recognized based on an evaluation of the progress to completion of specific tasks using information and data provided to us by our vendors and our clinical sites.
We cannot determine with certainty the duration and completion costs of the current or future product development, preclinical studies or clinical trials of our product candidates. The duration, costs, and timing of clinical trials and development of our product candidates will depend on a variety of factors, including:
| | • | | the scope, rate of progress, and expense of our ongoing as well as any additional clinical trials and other research and development activities; |
| | • | | the countries in which trials are conducted; |
| | • | | future clinical trial results; |
| | • | | uncertainties in clinical trial enrollment rates or drop-out or discontinuation rates of patients; |
| | • | | potential additional safety monitoring or other studies requested by regulatory agencies; |
| | • | | significant and changing government regulation; and |
| | • | | the timing and receipt of any regulatory approvals. |
A change in the outcome of any of these variables with respect to the development of a product candidate could mean a significant change in the costs and timing associated with the development of that product candidate. For example, if the FDA, or another regulatory authority were to require us to conduct clinical trials beyond those that we anticipate will be required to complete clinical development of a product candidate or if we experience significant delays in enrollment in any of our clinical trials, we could be required to expend significant additional financial resources and time on the completion of clinical development.
Our research and development expenses, categorized by product candidate or program, in fiscal 2015, fiscal 2014 and the six months ended December 31, 2015 were as follows:
| | | | | | | | | | | | |
| | | For the year ended
June 30, | | | For the six
months ended
December 31,
2015 | |
| Product candidate or program | | 2015 | | | 2014 | | |
TT-034 for the treatment of HCV | | A$ | 3,171,886 | | | A$ | 2,475,086 | | | A$ | 2,205,864 | |
BB-HB-331 for the treatment of HBV | | | 491,878 | | | | — | | | | 3,255,292 | |
BB-AMD-211 and BB-AMD-231 for the treatment of AMD* | | | 1,179,269 | | | | 793 | | | | 790,378 | |
Tribetarna for the treatment of drug-resistant NSCLC | | | 761,047 | | | | 752,683 | | | | 272,672 | |
Intractable neuropathic pain | | | — | | | | 1,688 | | | | — | |
Pabparna for treatment of OPMD | | | — | | | | 40,299 | | | | 444,920 | |
Other project related research and development costs | | | 624,211 | | | | 487,320 | | | | 1,180,839 | |
| | | | | | | | | | | | |
Total | | A$ | 6,228,291 | | | A$ | 3,757,869 | | | A$ | 8,149,965 | |
| | | | | | | | | | | | |
| * | | formerly known as TT-211 and TT-231, respectively. |
We plan to increase our research and development expenses for the foreseeable future as we continue the development of ddRNAi product candidates and explore further potential applications of our technology.
Employment related costs
Employment related costs include salaries for all our employees and related benefits, including the grant of share options, which are valued and included in the statements of profit or loss and other comprehensive income as share based expenses.
70
Impairment
We assess at the end of each fiscal year and half year whether there is an indication that an asset may be impaired. If any such indication exists, or when annual impairment testing is required for an asset, such as goodwill, intangible assets with indefinite useful lives and intangible assets not yet available for use, we make an estimate of the asset’s recoverable amount. An asset’s recoverable amount is the higher of its fair value less costs to sell and its value in use and is determined for an individual asset, unless the asset does not generate cash inflows that are largely independent of those from other assets or groups of assets and the asset’s value in use cannot be estimated to be close to its fair value. In such cases, the asset is tested for impairment as part of the cash generating unit to which it belongs. When the carrying amount of an asset or cash-generating unit exceeds its recoverable amount, the asset or cash-generating unit is considered impaired and is written down to its recoverable amount. No impairment was recorded in fiscal years 2015 or 2014.
In assessing value in use, the estimated future cash flows are discounted to their present value using a pre-tax discount rate that reflects current market assessments of the time value of money and the risks specific to the asset. Impairment losses relating to continuing operations are recognized in those expense categories consistent with the function of the impaired asset unless the asset is carried at revalued amount (in which case the impairment loss is treated as a revaluation decrease).
Royalties and license fees
We pay royalties and license fees in connection with our licensing of intellectual property from third parties. In connection with our acquisition of Tacere in 2012, we agreed to pay to the former shareholders of Tacere royalties on certain licensing revenue earned by us through the license of certain products, including TT-034, covered by a patent controlled by Tacere in October 2012. Any such royalties would be calculated as follows: 15% if the license is entered into prior to commencement of a Phase III clinical study and 2.5% if the license is entered into after commencement of a Phase III clinical study. Also, if we were to directly sell these products, then we would pay a royalty of 2.5% on net sales to the former shareholders of Tacere.
In August 2009, we entered into a collaborative agreement with Biomics Biotech Co., Ltd., or Biomics, pursuant to which we agreed to share any revenue generated from commercializing our jointly filed patents which relate to single-stranded RNA and shRNA sequences for treatment of hepatitis B. In July 2015, we entered into an earn-out agreement with Biomics pursuant to which we acquired all rights, title and interest in these patents in exchange for upfront and milestone payments. At the time of signing the agreement, we paid Biomics A$2.5 million consisting of A$2.0 million in cash and 647,333 ordinary shares (having a value of A$500,000 at the time the agreement was entered into). These shares could not be traded until October 1, 2015 and thereafter Biomics may only sell up to A$100,000 in value of those shares in any calendar month. Upon out-licensing a patent in this patent family we will also pay Biomics 50% of the initial licensing revenue received by us up to a maximum of A$3.5 million and, in the event we receive licensing revenue greater than A$6.0 million, we would pay Biomics 1.5% of licensing revenue on any such additional amounts.
In August 2013, we entered into a commercial license arrangement with NewSouth Innovations Pty Limited, or NSi, of University of New South Wales for the patent portfolio relating to our to be discontinued therapeutic product candidate for NSCLC. The license provides for modest up-front and ongoing license fees, and also milestone and single digit percentage royalty payments on net sales. A percentage of sub-licensing revenue is also payable to NSi. We may terminate the license at will, and in the event of certain breaches by NSi. NSi may terminate the license in the event of certain breaches by us.
Foreign exchange translation
The foreign currency translation reserve represents the currency translation movements of subsidiary company balances denominated in foreign currencies at year end. Foreign currency monetary items are translated
71
at the year-end exchange rate. Non-monetary items measured at historical cost continue to be carried at the exchange rate at the date of the transaction. Non-monetary items measured at fair value are reported at the exchange rate at the date when fair values were determined. Movements in the foreign currency translation reserve are shown in our Statement of Profit or Loss and Other Comprehensive Income.
Foreign currency transactions are translated into functional currency using the exchange rates prevailing at the date of the transactions. Exchange rate differences are recognized in the Statement of Profit or Loss and Other Comprehensive Income.
The significant movement in the foreign currency translation reserve and foreign exchange transactional gain or loss in the fiscal year ended June 30, 2013 is due to historic intercompany loan balances between Benitec and its foreign subsidiaries that are eliminated on consolidation which were deemed to be non-repayable and were accordingly transferred to equity during fiscal 2014. As a result of the transfer of historical intercompany receivable and payable balances to equity, these balances are translated at a historical rate and no further foreign exchange translational reserve or transactional gain or loss on these balances was required at June 30, 2014. The significant appreciation of the U.S. dollar against the Australian dollar, along with a high U.S. dollar cash balance following an equity placement in 2014, contributed to the net foreign exchange gain of A$0.573 in fiscal 2015.
Critical Accounting Policies and Estimates
The preparation of our financial statements requires us to make estimates and judgments that can affect the reported amounts of assets, liabilities, revenues and expenses, as well as the disclosure of contingent assets and liabilities at the date of our financial statements. We analyze our estimates and judgments and we base our estimates and judgments on historical experience and various other assumptions that we believe to be reasonable under the circumstances. Actual results may vary from our estimates. Our significant accounting policies are detailed in Note 1 to our consolidated financial statements for the fiscal year ended June 30, 2015 appearing elsewhere in this prospectus. We have summarized below the accounting policies of particular importance to the portrayal of our financial position and results of operations and that require the application of significant judgment or estimates by our management.
Share-based payments transactions. We measure the cost of equity-settled transactions with employees by reference to the fair value of the equity instruments at the date at which they are granted. The fair value is determined using a Black-Scholes model.
Tax losses. Given our history of recent losses, we have not recognized a deferred tax asset with regard to unused tax losses and other temporary differences, as it has not been determined whether we or our subsidiaries will generate sufficient taxable income against which the unused tax losses and other temporary differences can be utilized. We note that the availability of tax losses is subject to an Australian continuity of ownership test or, if we fail that test, the same business test. If we continue to obtain funding from new shareholders, then we may not comply with the continuity of ownership test.
Certain Differences Between IFRS and GAAP
IFRS differs from GAAP in a few respects. While we have not assessed the materiality of differences between IFRS and GAAP, we note in particular that IFRS permits the recording of finance income and research and development grants as revenue, unlike GAAP, under which interest and other finance income would not be recorded as revenue but instead as net finance income and research and development grants would be recorded as an offsetting reduction to research and development expenses. In addition, under IFRS, all employment-related expenses are reported in their own line item in our Statement of Profit or Loss and Other Comprehensive Income, unlike GAAP, under which employment-related expenses are generally allocated to line items such as research and development expense or general and administrative expense based on the functions performed by each applicable employee.
72
Results of Operation
The following discussion relates to our consolidated results of operations, financial condition and capital resources. You should read this discussion in conjunction with our consolidated financial statements and the notes thereto contained elsewhere in this prospectus.
Comparison of the six months ended December 31, 2015 and 2014
Revenue
| | | | | | | | | | | | |
| (in thousands) | | For the six months ended
December 31, | | | Increase
(Decrease) | |
| | | 2015 | | | 2014 | | |
Revenue: | | | | | | | | | | | | |
Licensing revenue and royalties | | A$ | 188 | | | A$ | 187 | | | | 1 | |
Finance income—interest | | | 134 | | | | 452 | | | | (318 | ) |
| | | |
Other income: | | | | | | | | | | | | |
Net foreign exchange fluctuation* | | | — | | | | 392 | | | | (392 | ) |
| | | | | | | | | | | | |
Total revenue and other income | | A$ | 322 | | | A$ | 1,031 | | | | (709 | ) |
| | | | | | | | | | | | |
| * | | The net foreign exchange fluctuation is due to our significant U.S. dollar cash balances (following an equity placement in April 2014 and its U.S. initial public offering in August 2015) and currency moves between the U.S. dollar and the Australian dollar, creating a loss in translation of U.S. dollar cash balances compared to the previous period. |
Licensing revenue and royalties increased very slightly from the six months ended December 31, 2014 to the six months ended December 31, 2015 primarily due to timing differences in the recognition of such revenue.
Finance income decreased from A$0.5 million in the six months ended December 31, 2014 to A$0.1 million in the six months ended December 31, 2015, as a result of holding cash in low yielding U.S. dollar bank accounts.
Expenses
Research and development expense. Research and development expense increased by A$5.9 million, from A$2.2 million in the six months ended December 31, 2014 to A$8.2 million in the six months ended December 31, 2015, primarily due to:
| | • | | A$2.5 million as an initial payment to acquire the full rights to the patents underlying our preclinical ddRNAi-based hepatitis B therapeutic program that were jointly filed by Biomics and us; |
| | • | | higher research and development activity in 2015, including the dosing of patients in our Phase I/IIa clinical trial for TT-034; and |
| | • | | work under our agreement with 4D Molecular Therapeutics LLC to develop vectors. |
Employment related expenses. Employment-related expenses increased by A$1.9 million in the six months ended December 31, 2015 compared to the six months ended December 31, 2014, due to increased staff levels, particularly at Tacere’s laboratory, in fiscal 2015.
Share based expenses. Share based expenses increased by A$0.7 million, from A$0.8 million in the six months ended December 31, 2014 to A$1.5 million in the six months ended December 31, 2015, largely due to the share based expense costs for options granted to our directors in November 2015. Share based expenses are calculated using a Black-Scholes model. The share based expense model uses a data set that includes share price and exercise price, exercise probability, volatility, exercise time and interest rates. Variation in these factors and
73
an increased level of option grants to our directors were the major contributors to this expense increase. We recognize share based expenses over the service period in which the employee earns the award, which is the vesting period of the award.
Travel related costs. Travel related costs increased by A$0.2 million from A$0.5 million in the six months ended December 31, 2014 to A$0.7 million in the six months ended December 31, 2015 due to travel related to our U.S. initial public offering in August 2015, an increase in staff levels and more participation in international conferences, in addition to meetings with pharmaceutical companies.
Consultants costs. Consultants costs increased by A$0.2 million from A$0.4 million in the six months ended December 31, 2014 to A$0.6 million in the six months ended December 31, 2015. We retained specialist advisers in relation to our key product candidate programs and for media and shareholder relations capabilities.
Occupancy costs. Occupancy costs increased by A$0.2 million from A$0.1 million in the six months ended December 31, 2014 to A$0.3 million in the six months ended December 31, 2015 due to a new expanded lease for the laboratory in California and increased space under lease in Australia.
Corporate expenses. Corporate expenses increased by A$0.2 million from A$0.4 million in the six months ended December 31, 2014 to A$0.6 million in the six months ended December 31, 2015 due to an increase in the size of our company and consequent expenses.
IPO costs. We incurred legal, accounting and other costs of A$1.0 million during the six months ended December 31, 2015 in relation to our U.S. initial public offering that was completed in August 2015.
Loss for the period
As a result of the foregoing, our loss for the period after income tax benefit increased by A$11.1 million from A$5.0 million in the six months ended December 31, 2014 to A$16.1 million in the six months ended December 31, 2015.
Given our and our subsidiaries’ history of recent losses, we have not recognized a deferred tax asset with regard to unused tax losses and other temporary differences, as it has not been determined whether we or our subsidiaries will generate sufficient taxable income against which the unused tax losses and other temporary differences can be utilized.
Comparison of the fiscal years ended June 30, 2015 and 2014
Our initial public offering in the United States was completed after the end of fiscal 2015. The capital we raised in fiscal 2014 continued to allow us to progress our research and development efforts in fiscal 2015.
Revenue
| | | | | | | | | | | | |
| (in thousands) | | For the fiscal year ended
June 30, | | | Increase
(Decrease) | |
| | | 2015 | | | 2014 | | |
Revenue: | | | | | | | | | | | | |
Licensing revenue and royalties | | A$ | 307 | | | A$ | 277 | | | | 30 | |
Finance income—interest | | | 774 | | | | 321 | | | | 453 | |
Other income: | | | | | | | | | | | | |
Australian government research and development grants | | | 2,318 | | | | 776 | | | | 1,542 | |
Net foreign exchange gain | | | 573 | | | | — | | | | 573 | |
| | | | | | | | | | | | |
Total revenue and other income | | A$ | 3,972 | | | A$ | 1,374 | | | | 2,598 | |
| | | | | | | | | | | | |
74
Licensing revenue and royalties increased very slightly from fiscal 2014 to fiscal 2015 primarily due to timing differences in the recognition of such revenue.
Grants from the Australian government increased A$1.5 million, from A$0.8 million in fiscal 2014 to A$2.3 million in fiscal 2015 due to a higher level of eligible research and development expense in fiscal 2015.
Finance income increased by A$0.5 million from A$0.3 million in fiscal 2014 to A$0.8 million in fiscal 2015, as a result of a private placement of ordinary shares to institutional investors and a shareholder purchase plan in February 2014 that raised A$39.6 million, thus providing for higher interest returns on increased bank account cash balances in fiscal 2015.
Net foreign exchange gain in fiscal 2015 was due to a high U.S. dollar cash balance following an equity placement in 2014, coupled with a significant appreciation of the U.S. dollar against the Australian dollar during fiscal 2015. There was no such gain in fiscal 2014.
Expenses
Royalties and licence fees. Royalties and licence fees decreased from approximately A$193,000 in fiscal 2014 to A$40,000 in fiscal 2015 due primarily to a one-off payment related to certain intellectual property in fiscal 2014.
Research and development expense. Research and development expense increased by A$2.4 million, from A$3.8 million in fiscal 2014 to A$6.2 million in fiscal 2015, primarily due to higher research and development activity in fiscal 2015, including the dosing of patients in our now discontinued Phase I/IIa clinical trial for TT-034 and the execution of an agreement with 4D Molecular Therapeutics LLC, or 4DMT, to develop vectors.
Employment related expenses. Employment-related expenses increased by A$1.0 million, or 40%, in fiscal 2015 compared to fiscal 2014, due to increased staff levels, particularly at Tacere’s laboratory, in fiscal 2015.
Share based expenses. Share based expenses increased by A$1.1 million, from A$0.4 million in fiscal 2014 to A$1.5 million in fiscal 2015. Share based expenses are calculated using a Black-Scholes model. The share based expense model uses a data set that includes share price and exercise price, exercise probability, volatility, exercise time and interest rates. Variation in these factors and an increased level of option grants to staff were the major contributors to this expense increase. We recognize share based expenses over the service period in which the employee earns the award, which is the vesting period of the award.
Travel related costs. Travel related costs increased by A$0.5 million from A$0.6 million in fiscal 2014 to A$1.0 million in fiscal 2015 due to an increase in staff levels and more participation in international conferences, in addition to meetings with pharmaceutical companies.
Consultants costs. Consultants costs increased slightly from A$0.7 million in fiscal 2014 to A$0.9 million in fiscal 2015, as we retained specialist advisers in relation to our key product candidate programs and built up our shareholder relations capabilities.
Occupancy costs. Occupancy costs increased slightly from A$0.1 million in fiscal 2014 to A$0.3 million in fiscal 2015 due to increased lease costs for the Tacere laboratory in California and increased space under lease in Australia.
Corporate expenses. Corporate expenses increased by A$0.4 million, or 58%, from A$0.6 million in fiscal 2014 to A$1.0 million in fiscal 2015 due to an increase in the size of our company and increases in consequent expenses.
75
IPO costs. We incurred legal, accounting and other costs of A$1.8 million in fiscal 2015 in relation to our U.S. initial public offering that was completed in August 2015, while no such costs had been incurred in fiscal 2014.
Loss for the period
As a result of the foregoing, our loss for the period after income tax benefit increased by A$4.5 million, or 64%, from A$7.0 million in fiscal 2014 to A$11.5 million in fiscal 2015.
Given our and our subsidiaries’ history of recent losses, we have not recognized a deferred tax asset with regard to unused tax losses and other temporary differences, as it has not been determined whether we or our subsidiaries will generate sufficient taxable income against which the unused tax losses and other temporary differences can be utilized.
Comparison of the fiscal years ended June 30, 2014 and 2013
We raised significant capital in fiscal 2014 that enabled us to accelerate our research and development efforts. In particular, we raised A$39.6 million, net of transaction costs, in private placements of ordinary shares to institutional investors and a shareholder purchase plan to shareholders resident in Australia and New Zealand. With these funds available, we were able to advance the programs in our pipeline, including our hepatitis B and AMD programs. We were also able to advance our now discontinued TT-034 program for hepatitis C, as well as our program for drug-resistant non-small cell lung cancer, which we have recently decided to terminate. With respect to TT-034, we submitted an IND application to the FDA in December 2013 and, with the application approved in January 2014, we were able to commence clinical trials with TT-034.
Revenue
| | | | | | | | | | | | |
| (in thousands) | | For the fiscal year ended
June 30, | | | Increase
(Decrease) | |
| | | 2014 | | | 2013 | | |
Revenue: | | | | | | | | | | | | |
Licensing revenue and royalties | | A$ | 277 | | | A$ | 521 | | | A$ | (244 | ) |
Australian government research and development grants | | | 776 | | | | 824 | | | | (48 | ) |
| | | |
Other income: | | | | | | | | | | | | |
Finance income—interest | | | 321 | | | | 119 | | | | 202 | |
| | | | | | | | | | | | |
Total revenue | | A$ | 1,374 | | | A$ | 1,464 | | | A$ | (90 | ) |
| | | | | | | | | | | | |
Licensing revenue and royalties decreased by A$0.2 million, or 47%, from A$0.5 million in fiscal 2013 to A$0.3 million in fiscal 2014 due to amounts earned in fiscal 2013 that were not repeated in fiscal 2014.
Grants from the Australian government decreased A$48,500, or 6%, from fiscal 2013 to fiscal 2014 because we undertook less research and development activity on approved “eligible expenditure” in fiscal 2013, and thus received and recorded less grant revenue in fiscal 2014, compared to activities in fiscal 2012.
Finance income more than doubled in fiscal 2014 from fiscal 2013 as a result of a private placement of ordinary shares to institutional investors and a shareholder purchase plan in 2014 that raised proceeds of A$39.6 million, thus providing for higher bank deposit and short-term investment balances in fiscal 2014 compared to fiscal 2013.
76
Expenses
Royalties and licence fees. Royalties and licence fees decreased by A$0.2 million from fiscal 2013 to fiscal 2014 due primarily to licences being established in fiscal 2013, with upfront payments representing most of the change in revenue.
Research and development expense. Research and development expense more than doubled to A$3.8 million in fiscal 2014 compared to fiscal 2013, primarily due to (i) fiscal 2014 including a full 12 months of research and development activity by Tacere compared to only eight months of research and development activity in fiscal 2013 following our acquisition of Tacere on October 30, 2012 and (ii) the acceleration of our activities in connection with the now discontinued TT-034 program for the treatment of hepatitis C. In addition, the completion of a private placement of ordinary shares in February 2014 enabled us to accelerate other research and development activity in the latter half of fiscal 2014.
Employment related expenses. Employment-related expenses increased by A$0.6 million, or 33%, in fiscal 2014 compared to fiscal 2013, due in large part to the strategy of building our staff capabilities to match our anticipated longer-term requirements, such as the appointment of senior scientific, executive and support staff, including staff acquired through the acquisition of Tacere.
Share based expenses. Share based expenses decreased by A$0.2 million, or 32%, from fiscal 2013 to fiscal 2014. Share based expenses are calculated using a Black-Scholes model. The share based expense model uses a data set that includes share price and exercise price, exercise probability, volatility, exercise time and interest rates. Variation in these factors and a reduced level of option grants to staff were the major contributors to this expense decrease. We recognize share based expenses over the service period in which the employee earns the award, which is the vesting period of the award.
Impairment costs. Impairment costs of A$1.5 million relating to identifiable intangibles on the acquisition of Tacere were recognized in fiscal 2013. The Tacere acquisition in-process research reflected the excess of the consideration we paid over the fair value of the identifiable assets we acquired, less liabilities we assumed. The write-off of the identifiable intangibles was recorded following a review for impairment, and was considered to be the most appropriate accounting treatment as the intellectual property was a preclinical asset and, hence, the future economic benefit was uncertain.
Travel related costs. Travel related costs increased by A$0.2 million, or 69%, from fiscal 2013 to fiscal 2014 due to increased staff levels and participation in international conferences, in addition to meetings with pharmaceutical companies. Travel also increased as a result of the acquisition of Tacere and the progression of the now discontinued HCV program to a clinical trial.
Consultants costs. Consultants costs increased by A$0.3 million, or 94%, from fiscal 2013 to fiscal 2014, as we retained specialist advisers in relation to our key programs and built appropriate shareholder relations capabilities.
Occupancy costs. Occupancy costs increased by A$21,429, or 21%, from fiscal 2013 to fiscal 2014 due to the increased lease costs arising from the acquisition of Tacere and increased space under lease in Australia.
Corporate expenses. Corporate expenses increased by A$0.1 million, or 22%, from fiscal 2013 to fiscal 2014 due to an increase in the size of the business and increases in consequent expenses.
Foreign exchange translation. A foreign exchange loss of A$0.1 million was recorded in fiscal 2014 compared to a gain of A$1.5 million in fiscal 2013 reflecting the historic intercompany loan balances between Benitec and its foreign subsidiaries that were transferred to equity during fiscal 2014.
77
Loss for the period
As a result of the foregoing, our loss for the period after income tax benefit increased by A$3.5 million, or 102%, from A$3.5 million in fiscal 2013 to A$7.0 million in fiscal 2014.
Given our and our subsidiaries’ history of recent losses, we have not recognized a deferred tax asset with regard to unused tax losses and other temporary differences, as it has not been determined whether we or our subsidiaries will generate sufficient taxable income against which the unused tax losses and other temporary differences can be utilized.
Liquidity and capital resources
We have incurred cumulative losses and negative cash flows from operations since our inception in 1995 and, as of June 30, 2015, we had accumulated losses of A$107.8 million and as of December 31, 2015 we had accumulated losses of A$123.0 million. We anticipate that we will continue to incur losses for at least the next several years. We expect that our research and development and general and administrative expenses will continue to increase and, as a result, we will need additional capital to fund our operations, which we may raise through a combination of equity offerings, debt financings, other third-party funding and other collaborations, strategic alliances and licensing arrangements.
We had no borrowings in fiscal 2013, fiscal 2014, fiscal 2015 or the six months ended December 31, 2015 and do not currently have a credit facility.
As of December 31, 2015, we had cash and cash equivalents of A$22.8 million. Cash in excess of immediate requirements is invested in accordance with our investment policy, primarily with a view to liquidity and capital preservation. Currently, our cash and cash equivalents are held in bank accounts. Our short-term investments consist of term deposits with maturity within 180 days.
Cash flows
The following table sets forth the sources and uses of cash for the six months ended December 31, 2015 and 2014:
| | | | | | | | |
| (in thousands) | | For the six months ended
December 31, | |
| | | 2015 | | | 2014 | |
Net cash used in operating activities | | A$ | (14,487 | ) | | A$ | (4,787 | ) |
Net cash provided by (used in) investing activities | | | (2,071 | ) | | | (394 | ) |
Net cash provided by financing activities | | | 17,510 | | | | 257 | |
Operating activities. Net cash used in operating activities for the six months ended December 31, 2015 was A$14.5 million. The net cashed used in operating activities for the six months ended December 31, 2014 was A$4.8 million. The use of net cash in both periods resulted from our net losses.
Investing activities. Net cash used in investing activities for the six months ended December 31, 2015 was A$2.1 million, which related to investments in short term deposits. Net cash used in investing activities in the six months ended December 31, 2014 was A$0.4 million, which related to purchases of equipment.
Financing activities. Net cash provided by financing activities was A$17.5 million for the six months ended December 31, 2015. Net cash provided by financing activities was A$0.3 million for the six months ended December 31, 2014. All such cash from financing activities related to the issuance of ordinary shares, ADRs and Warrants, particularly our initial public offering in the United States during the six months ended December 31, 2015 with gross proceeds of A$18.8 million (US$13.8 million).
78
The following table set forth the sources and uses of cash for the past three fiscal years:
| | | | | | | | | | | | |
| (in thousands) | | For the fiscal year ended
June 30, | |
| | | 2015 | | | 2014 | | | 2013 | |
Net cash used in operating activities | | A$ | (9,692 | ) | | A$ | (9,271 | ) | | A$ | (2,733 | ) |
Net cash provided by (used in) investing activities | | | (505 | ) | | | (32 | ) | | | 134 | |
Net cash provided by financing activities | | | 52 | | | | 39,076 | | | | 1,086 | |
Operating activities. Net cash used in operating activities for fiscal 2015, fiscal 2014 and fiscal 2013 was A$9.7 million, A$9.3 million and A$2.7 million, respectively. The use of net cash in all periods resulted from our net losses.
Investing activities. Net cash used in investing activities for fiscal 2015 was A$0.5 million, which related to purchases for equipment. Net cash used in investing activities in fiscal 2014 was A$0.3 million, which related to purchases of equipment. Net cash provided by investing activities in fiscal 2013 was A$0.1 million, which primarily related to costs associated with the acquisition of Tacere in October 2012.
Financing activities. Net cash provided by financing activities was A$0.05 million, A$39.1 million and A$1.1 million for fiscal 2015, fiscal 2014 and fiscal 2013. All such cash from financing activities related to the issuance of ordinary shares. In addition to issuances from the exercise of options to purchase ordinary shares, in fiscal 2014, A$39.5 million was raised from private placements and A$2.8 million was raised from a share purchase plan.
Operating capital requirements
To date, our sources of liquidity have been licensing revenue and royalties, Australian government research and development grants, interest on invested cash in excess of immediate requirements and proceeds of the issuance of equity securities.
In the future, we expect our revenue stream will be generated mostly from licensing, strategic alliances and collaboration arrangements with pharmaceutical companies. While we continue to progress discussions and advance opportunities to engage with pharmaceutical companies and continue to seek licensing partners for ddRNAi in disease areas that are not our focus, there can be no assurance as to whether we will enter into such arrangements or what the terms of any such arrangement could be.
While we have established some licensing arrangements, we do not have any products approved for sale and have not generated any revenue from product sales. We do not know when, or if, we will generate any revenue from product sales. We do not expect to generate significant revenue from product sales unless and until we obtain regulatory approval of and commercialize one of our current or future product candidates.
Unless and until we establish significant revenues from licensing programs, strategic alliances or collaboration arrangements with pharmaceutical companies, or from product sales, we anticipate that we will continue to generate losses for the foreseeable future, and we expect the losses to increase as we continue the development of product candidates and begin to prepare to commercialize any product that receives regulatory approval. We are subject to the risks inherent in the development of new gene therapy products, and we may encounter unforeseen expenses, difficulties, complications, delays and other unknown factors that may adversely affect our business.
We expect that the net proceeds from our initial public offering in the United States together with our pre-existing cash and cash equivalents will be sufficient to enable us to complete planned preclinical proof-of-concept studies for certain of our product candidates until approximately August 2017. In order to complete the planned preclinical proof-of-concept studies for our lead product candidates and to build the infrastructure that we believe will be necessary to commercialize our lead product candidates, we will require substantial additional funding.
79
We have based our projections of operating capital requirements on assumptions that may prove to be incorrect and we may use all of our available capital resources sooner than we expect. Because of the numerous risks and uncertainties associated with research, development and commercialization of pharmaceutical products, we are unable to estimate the exact amount of our operating capital requirements. Our future funding requirements will depend on many factors, including, but not limited to:
| | • | | the timing and costs of our planned clinical trials for our product candidates; |
| | • | | the timing and costs of our planned preclinical studies for our product candidates; |
| | • | | the number and characteristics of product candidates that we pursue; |
| | • | | the outcome, timing and costs of seeking regulatory approvals; |
| | • | | revenue received from commercial sales of any of our product candidates that may receive regulatory approval; |
| | • | | the terms and timing of any future collaborations, licensing, consulting or other arrangements that we may establish; |
| | • | | the amount and timing of any payments we may be required to make, or that we may receive, in connection with the licensing, filing, prosecution, defense and enforcement of any patents or other intellectual property rights; |
| | • | | the costs of preparing, filing and prosecuting patent applications, maintaining and protecting our intellectual property rights and defending against intellectual property related claims; and |
| | • | | the extent to which we need to in-license or acquire other products and technologies. |
Contractual obligations and commitments
The following table summarizes our contractual obligations as of December 31, 2015:
| | | | | | | | | | | | | | | | | | | | |
| | | Payments due by period (A$) | |
| | | Total | | | Less than
1 year | | | 1 - 3 years | | | 3 - 5 years | | | More
than
5 years | |
Operating lease obligations | | A$ | 993,340 | | | A$ | 219,230 | | | A$ | 447,688 | | | A$ | 326,422 | | | A$ | — | |
Obligations to clinical research organizations | | | 780,948 | | | | 723,100 | | | | 28,924 | | | | 28,924 | | | | — | |
| | | | | | | | | | | | | | | | | | | | |
Total | | A$ | 1,774,288 | | | A$ | 942,330 | | | A$ | 476,612 | | | A$ | 355,346 | | | A$ | — | |
| | | | | | | | | | | | | | | | | | | | |
We have obligations under contracts with clinical research organizations relating to their work on our clinical trials. These obligations, which are subject to termination under certain circumstances, include:
| | • | | In 2012, we appointed Synteract Inc. as a clinical research organization responsible for the progression of TT-034 into Phase I/II clinical trials in the United States. |
| | • | | In 2014, we appointed European-based Clinical Trials Group as a clinical research organization responsible for the progression of our non-small cell lung cancer therapeutic Tribetarna into Phase II clinical trials. We have recently decided to discontinue developing Tribetarna due to commercial reasons. |
Off-balance sheet arrangements
We did not have over the past three fiscal years, and we currently do not have, any off-balance sheet arrangements as defined in the rules and regulations of the Securities and Exchange Commission.
80
Quantitative and qualitative disclosures about market risks
We are exposed to market risk related to changes in interest rates and exchange rates.
As of June 30, 2015, 2014 and 2013 and December 31, 2015, we had cash and cash equivalents of A$21.8 million, A$31.4 million, A$1.6 million and A$22.8 million, respectively, primarily held in bank accounts and term deposits. Our primary exposure to market risk is interest rate sensitivity, which is affected primarily by changes in the general level of Australian interest rates. Our available for sale securities are subject to interest rate risk and will fall in value if market interest rates increase. Due to the short-term duration of our investment portfolio and the low risk profile of our investments, an immediate 10% increase in interest rates would not have a material effect on the fair market value of our portfolio.
We are exposed to fluctuations in foreign currencies that arise from foreign currencies held in bank accounts and the translation of results from our operations outside Australia. Our foreign exchange exposure is primarily the U.S. dollar. Foreign currency risks arising from commitments in foreign currencies are managed by holding cash in that currency. Foreign currency translation risk is not hedged.
See Note 18 of our June 30, 2015 financial statements included in this prospectus for more detailed information on our financial risk management.
81
BUSINESS
Overview
We are a biotechnology company developing a novel, proprietary therapeutic technology platform that combines gene silencing and gene therapy with a goal of providing sustained, long-lasting silencing of disease-causing genes from a single administration. We believe our technology has the potential to be a “one shot” cure for a wide range of diseases that are currently addressed by strict ongoing treatment regimens or that have no effective treatment or only palliative care options. We are using our technology, called DNA-directed RNA interference, or ddRNAi, to develop our pipeline of product candidates for the treatment of several chronic and life-threatening human diseases, such as hepatitis B, age-related macular degeneration, or AMD and oculopharyngeal muscular dystrophy, or OPMD. We will require additional financing to conduct clinical trials for our product candidates for hepatitis B, AMD and OPMD. These diseases have large patient populations, with the exception of OPMD which is a rare disease. In addition, we have licensed our ddRNAi technology to other biopharmaceutical companies whose pipeline programs are progressing towards, or are in, clinical development for applications including HIV/AIDS, retinitis pigmentosa, Huntington’s disease, cancer immunotherapy and intractable neuropathic pain.
Many diseases are known to be caused by the inappropriate expression of a gene or multiple genes. It has been observed since 1998 that RNA interference, or RNAi, is a mechanism that can potentially be used to specifically turn off, or silence, genes whose sequences are known. Thus, RNAi can potentially be used to treat or cure diseases with a genetic basis by targeting a specific region of the molecular sequence of the disease-causing gene. RNAi is potentially applicable to over 20,000 human genes and a large number of disease-causing microorganism-specific genes. The mechanism of action of RNAi involves the introduction of short interfering RNA, or siRNA, into a cell. The siRNA’s sequence is constructed to match a short region of the target gene. The siRNA is processed by the cell’s own enzymes to destroy the target gene’s messenger RNA, or mRNA, thus preventing the disease-causing gene from being expressed. This occurs as long as the siRNA remains prevalent in the cell.
Our approach differs from the standard RNAi approach, which is commonly referred to as siRNA and is being developed by a number of other companies, including Alnylam Pharmaceuticals, Inc., or Alnylam, Arbutus Biopharma Corporation, or Arbutus (formerly known as Tekmira Pharmaceuticals Corporation before its name change and integration with OnCore BioPharma), and Dicerna Pharmaceuticals, Inc., or Dicerna. In this standard RNAi approach, double-stranded siRNA is produced synthetically and subsequently introduced into the target cell either by chemical modification of the RNA or by a range of other delivery methods. While clinical efficacy has been demonstrated for a number of indications utilizing this approach, it has a number of limitations, which include:
| | • | | Once administered, siRNA levels within cells are finite and limited to the initial amount delivered. Extended therapeutic benefit requires repeated administration for multiple cycles. |
| | • | | Patient adherence challenges due to dosing frequency and treatment duration. |
| | • | | Therapeutic concentrations of siRNA are not stably maintained because the levels of synthetic siRNA in the cells decrease over time. |
| | • | | Delivery of siRNA into the appropriate target cells has been a considerable challenge. Unmodified siRNA is unstable in the bloodstream, can cause an adverse immune response and does not readily cross membranes to enter cells. Therefore, novel chemical modifications or the use of novel delivery materials are required to introduce the siRNA into the target cells, making it complicated to develop therapeutics. |
| | • | | Because the liver acts to clear foreign contaminants from the bloodstream, current systemic delivery of siRNA molecules is limited primarily to targeting the liver. As a result, the majority of siRNA pipeline drugs have been restricted to liver diseases. |
82
| | • | | When injected into the body, siRNA is recognized as a foreign contaminant and can cause an adverse immune response, or interferon response, potentially resulting in serious adverse effects. |
| | • | | There are multiple target disease-causing genes in some diseases, including certain viral infections and certain types of cancer. siRNA-based applications typically involve multiple siRNA molecules, one for each gene desired to be silenced, and may require specialized delivery formulations to ensure distribution of each molecule in the target cells. This delivery challenge makes it more difficult to develop treatments for diseases that implicate multiple genes. |
| | • | | siRNA only acts to silence genes, but cannot be used to replace defective genes with normally functioning genes. |
Our ddRNAi technology is designed to utilize the specificity and gene silencing effect of RNA interference while overcoming many of the limitations associated with the ongoing administration of siRNA. Our ddRNAi approach combines RNA interference with gene therapy. Unlike siRNA, our ddRNAi technology starts with a DNA construct. Gene therapy vectors, which are carrier molecules, often viruses, that deliver genetic material into the cell, are used to deliver the DNA construct to the nucleus of the targeted cells. The DNA construct then generates double-stranded short hairpin RNAs, or shRNAs, which are processed by the cell into siRNAs, which in turn silence the disease-associated genes. Advantages of our ddRNAi approach include:
| | • | | ddRNAi is designed to produce sustained, long-lasting silencing of the disease-causing gene, following a single administration, leading to the potential for “one shot” cures for a wide range of diseases, which could eliminate the requirement for patient compliance to take regular doses of medicine for long-term management of their disease. |
| | • | | ddRNAi technology can potentially use any clinically validated gene therapy vector, enabling it to target a wide range of tissues, including, but not limited to, the liver. |
| | • | | Because ddRNAi uses the cell’s own transcriptional mechanisms to produce shRNA, a constant level of shRNA can potentially be produced so that intracellular levels of siRNA do not fall below threshold levels required for disease suppression. |
| | • | | The level of shRNA in the cells can potentially be fine-tuned to achieve optimal concentrations. |
| | • | | Off-tissue effects can be minimized by using tissue specific promoters that are designed to restrict expression of shRNA to only the target tissue. |
| | • | | The DNA constructs are shielded in gene therapy vectors that are designed to avoid activating the interferon response. |
| | • | | ddRNAi provides the option to both silence the defective gene and replace the defective gene with a normal version, using the same gene therapy vector. Thus silencing and replacement of the mutant gene occurs in the same cell. We believe this “silence and replace” strategy is ideally suited to developing therapeutics for a number of genetic disorders. |
| | • | | ddRNAi can be designed to express multiple siRNAs in the same cell, targeting either a single gene at several different sites to minimize the risk of viral resistance, or multiple genes in distinct cellular pathways, potentially enabling treatment of complex genetic diseases such as cancer, diabetes and heart disease. |
| | • | | ddRNAi can elicit long-term response by continued expression of siRNA from a single administration, potentially preventing viral reinfection. |
Our strategy is to discover, develop and commercialize treatments that leverage the capabilities of ddRNAi. We intend to do so by progressing our pipeline of ddRNAi-based therapeutics designed to treat and cure a number of human diseases, thereby demonstrating the broad clinical application of ddRNAi.
83
Proceeds from our initial public offering in the United States, with our pre-existing cash and cash equivalents, will advance our product candidates for hepatitis B, AMD and OPMD. Additional financing will be needed to advance our product candidates for hepatitis B, AMD and OPMD through clinical trials.
We are developing BB-HB-331, which currently is in preclinical studies, for the treatment of the hepatitis B virus, or HBV. We plan to file an investigational new drug, or IND, application in the third quarter of 2017. HBV is a small DNA virus that, according to the WHO, infects up to 240 million people worldwide, resulting in up to 780,000 deaths per year. Infection with HBV occurs in phases ranging from a silent, acute phase that can be resolved by the immune system to a persistent chronic infection requiring life-long therapy. In the case of a chronic HBV infection, the presence of viral particles and proteins, particularly the s-antigen, causes hepatic inflammation leading to liver dysfunction, acute hepatic failure, cirrhosis or hepatocellular carcinoma. Patients suffering from HBV have limited treatment options from therapies consisting of antivirals and, less commonly, interferon therapy. These treatments require adherence to strict recurrent treatment regimens, may cause the hepatitis B virus to mutate and develop antiviral drug resistance, and may only provide viral suppression through the course of administration, and not a cure. The long-term use of interferon, particularly in high doses, may also be associated with significant side effects, including nausea, vomiting, shortness of breath, dizziness and fatigue, that can cause patients to deviate from the course of treatment. BB-HB-331 is designed to be a single administration ddRNAi-based monotherapy that is delivered using a gene therapy vector that targets the liver and inhibits viral replication and s-antigen production on a long-term basis.
In March 2016, Benitec announced results of its recentin vivo study of BB-HB-331. Key findings of thein vivo study indicate that a single BB-HB-331 treatment in the PhoenixBio (PXB) mouse model can result in suppression of HBV. Thein vivo experiment supports the BB-HB-331in vitro findings previously observed in human hepatocytes isolated from the PXB mouse model, announced in December 2015. We believe these results demonstrate the potential utility of an approach that combines RNAi with gene therapy to treat HBV, and we intend to advance the HBV program towards the clinic. Our hepatitis B program continues to attract considerable interest from pharmaceutical companies.
In addition to BB-HB-331 for HBV, we are focusing on developing product candidates to treat AMD and OPMD. We will require additional financing to conduct clinical trials for our product candidates for HBV, AMD and OPMD. For selected product candidates, at the appropriate stage, we may collaborate with large pharmaceutical companies to further develop and, if approved, commercialize them to achieve broad product distribution. For certain products we deem to be outside of our immediate focus, we will continue to out-license, where appropriate, applications of our ddRNAi technology for the development of a range of therapeutics. We anticipate completingin vivo preclinical proof of concept studies for AMD and OPMD in the fourth quarter of 2016.
We are in the process of winding down for commercial reasons a partially completed Phase I/IIa clinical trial for our now discontinued product candidate, TT-034, which we were developing to treat patients chronically infected with the most common genotype of the hepatits C virus, or HCV. We believe the early stage clinical trial results for TT-034 provide support for the safety of our platform technology.
Our Strengths
We believe that the combination of our proprietary ddRNAi technology and our deep expertise and know-how in designing and clinical development of ddRNAi-based therapeutics will enable us to achieve and maintain a leading position in gene silencing for treatment of human disease. Our key strengths include:
| | • | | A first mover advantage for ddRNAi-based therapeutics; |
| | • | | Exclusive rights to a novel, proprietary ddRNAi technology platform that is potentially the basis of single-administration therapies with sustained, long-term silencing of disease-causing genes; |
84
| | • | | A pipeline of programs focused on life threatening or chronic diseases with either large patient populations, including hepatitis B and AMD, or rare disease status potentially supporting an orphan drug classification, including OPMD; |
| | • | | Collaborations with third parties to expand the technology platform and develop additional expertise in DNA delivery technologies, in scalable manufacturing, in DNA construct design and in developing related diagnostics to help identify the most appropriate patient populations to benefit from these novel treatments; |
| | • | | Out-licensing agreements with third parties utilizing our ddRNAi technology to develop therapies outside of our core research areas, which we believe could provide further validation of our technology’s potential to address numerous diseases; |
| | • | | Our development team has significant experience in designing and developing ddRNAi therapeutics and includes founding scientists in the ddRNAi field; and |
| | • | | Rights to intellectual property that includes a patent portfolio protecting our ddRNAi technology platform in numerous jurisdictions through 2019, and a growing portfolio of patents protecting improvements to our ddRNAi technology and product candidates in numerous jurisdictions through at least 2025. |
Our Strategy
Our objective is to become the leader in discovering, developing, clinically validating and commercializing ddRNAi-based therapeutics for a range of human diseases with high unmet clinical need or large patient populations, and to thereby provide a better life for patients with these diseases. Our strategy to accomplish this goal is to:
| | • | | Progress our pipeline of proprietary ddRNAi-based therapeutics. We are pursuing early preclinical research in HBV, AMD and OPMD and plan to submit IND applications for those product candidates. We expect to complete preclinicalin vivo proof-of-concept studies for our HBV product candidate in the second quarter of 2016, and for our AMD and OPMD product candidates in the fourth quarter of 2016. |
| | • | | Continue our leadership position in ddRNAi-based therapeutics. We believe we are the only company to date to have advanced into a clinical trial an RNAi therapeutic for systemic administration by gene therapy vectors. Early stages of the clinical trial of our now discontinued TT-034 product candidate indicated that TT-034 caused no treatment-related serious adverse events among the nine patients dosed to date. Of the nine patients, seven were biopsied and indicated a relationship between the amount of the compound administered and hepatic tissue transduction. We have developed significant experience in ddRNAi through our work on HCV, HBV, AMD and OPMD. We have strong relationships with key opinion leaders in the field and will continue to engage with the medical and scientific community to communicate the potential therapeutic value of ddRNAi. |
| | • | | Further develop and improve our ddRNAi platform technology and its associated intellectual property position. In addition to progressing our pipeline of product candidates, we will further develop and improve our ddRNAi platform technology and its associated intellectual property through in-house development and in-licensing of complementary technologies. One such example is our relationship with 4D Molecular Therapeutics LLC, or 4DMT, a company that is developing a vector to deliver our ddRNAi constructs to the retinal cells of the eye from an intravitreal injection to treat patients with AMD. |
| | • | | Develop drugs in our core disease areas and partner selectively to commercialize and expand our pipeline. The adaptability of our platform also presents an opportunity for us to selectively form collaborations to expand our capabilities and product offerings into a range of diseases and potentially to accelerate the development and commercialization of ddRNAi therapeutics more broadly. We will continue to expand our franchise of ddRNAi-based therapeutics by out-licensing, where appropriate, applications of ddRNAi for the development of a range of therapeutics outside of our immediate focus. Once clinical proof of concept has been achieved for a product candidate, we plan, where appropriate, to |
85
| | enter into collaborations with pharmaceutical companies to develop that product candidate and ultimately commercialize it if it receives regulatory approval. |
| | • | | Pursue indications with high unmet medical need or large patient populations. Each of our three current core indications are severe diseases with high unmet medical need or large patient populations. We believe there is a strong rationale for treating these diseases and other diseases that have well-characterized gene targets that can be silenced, thus preventing the disease-causing gene from being expressed. Given the poor prognosis and limited treatment options for most of these diseases, we believe our ddRNAi-based product candidates may offer a potential single-treatment alternative for these patients. Our ddRNAi-based product candidates, if successful, may offer a potentially superior long-term value proposition for our patients and the healthcare system more broadly, which we believe will allow us to derive premium value while delivering patients life-altering treatments. We also intend to develop ddRNAi applications in novel technologies including immunotherapy, such as chimeric antigen receptor T cells, or CAR T, for a range of additional disease areas, subject to our ability to obtain additional financing apart from our initial public offering in the United States. |
Our Technology—ddRNAi
Our proprietary technology platform is called DNA-directed RNA interference, or ddRNAi, which is designed to produce long-term silencing of disease-causing genes, by combining RNA interference, or RNAi, with delivery agents typically associated with gene therapy.
Standard gene therapy is normally used to compensate for abnormal or malfunctioning genes or to make a beneficial protein to address a defect. If a mutated gene causes a necessary protein to be faulty or missing, gene therapy is used to introduce a wild type, or normal, copy of the gene to restore the function of the protein. This is the approach to gene therapy taken by a number of other companies to date.
With our ddRNAi approach, gene therapy vectors are used to deliver a DNA construct that produces shRNAs, which are processed by the cell into siRNAs, which then silence the disease-associated genes.
Overview of RNAi and siRNA approach
Many diseases are known to be caused by the inappropriate expression of a gene or multiple genes. These disease-associated genes can be turned off, or silenced, by the use of RNAi, resulting in a treatment or cure of the disease. Thus, RNAi provides the ability to develop therapeutics against diseases caused by inappropriate gene expression, by targeting a specific region of the molecular sequence of the disease-causing gene. RNAi is potentially applicable to over 20,000 human genes and a large number of disease-causing microorganism-specific genes.
The mechanism of action of RNAi involves the introduction of siRNA into a cell. The siRNA’s sequence is constructed to match a short region of the target gene. The siRNA is processed by the cell’s own enzymes to destroy the target gene’s mRNA, thus preventing the disease-causing gene from being expressed. This occurs as long as the siRNA remains prevalent in the cell. In the standard RNAi approach, siRNA is produced synthetically in the laboratory and introduced into the target cell either by chemical modification of the RNA or by a range of delivery materials. A number of other companies, including Alnylam, Arbutus, and Dicerna, utilize this approach in their RNAi product candidates.
86
Figure 1. The siRNA approach.
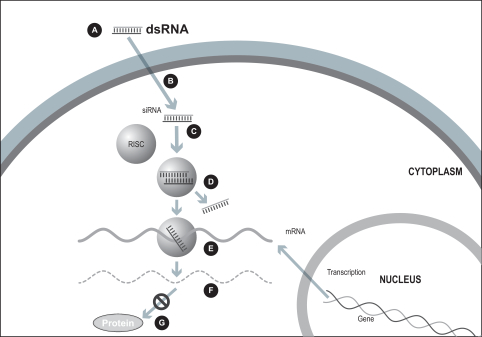
A small double stranded RNA, or dsRNA, molecule (A), comprising one strand known as the sense strand and another strand known as the antisense strand, which are complementary to each other, is synthesized in the laboratory. These small dsRNAs are called small interfering RNAs, or siRNAs. The sequence of the sense strand corresponds to a short region of the target gene mRNA. The siRNA is delivered to the target cell (B), where a group of enzymes, referred to as the RNA Interference Specificity Complex, or RISC, process the siRNA (C), where one of the strands (usually the sense strand) is released (D). RISC uses the antisense strand to find the mRNA that has a complementary sequence (E) leading to the cleavage of the target mRNA (F). As a consequence, the output of the mRNA (protein production) does not occur (G).
Our Approach to Gene Silencing—ddRNAi
Our ddRNAi technology is designed to utilize the specificity and gene silencing effect of RNAi while overcoming many of the limitations of siRNA. Our ddRNAi approach combines RNA interference with gene therapy vectors to deliver a DNA compound to the target diseased tissue in order to silence the disease-associated genes.
Gene therapy
Gene therapy is designed to introduce genetic material into cells, usually to compensate for abnormal or malfunctioning genes or to make a beneficial protein to address a defect. If a mutated gene causes a necessary protein to be faulty or missing, gene therapy is used typically to introduce a normal copy of the gene to restore the function of the protein. Genetic material that is inserted directly into a cell usually does not function. Instead, a carrier called a vector is genetically engineered to deliver the gene. Certain viruses are often used as vectors because they can deliver the new gene by infecting the cell. The vector viruses are designed not to cause disease when used in people. Some types of vector viruses, such as lentivirus, integrate their genetic material, including the new gene, into a chromosome in the human cell. Other vector viruses, such as adenoviruses and adeno-associated viruses, or AAV, introduce their DNA into the nucleus of the cell, but the DNA of the vector virus is not integrated into a chromosome; only the therapeutic gene is integrated. Most of our ddRNAi programs utilize AAV as the delivery vector. A number of viral vectors can produce gene expression for months or years following a single administration, depending on the target tissue.
87
The vector can be given intravenously or injected directly into a specific tissue in the body, where it enters individual cells. Alternatively, a sample of the patient’s cells can be removed and exposed to the vector in a laboratory setting. The cells containing the vector are then returned to the patient where they produce the expressed RNA or protein.
ddRNAi
Our ddRNAi technology utilizes DNA as the therapeutic molecule designed to generate shRNAs continuously in the target cell. A range of viral and non-viral gene therapy vectors can be used to deliver the DNA construct into the cell’s nucleus. Once delivered, the DNA sequence codes for specific shRNAs, which are then processed by the cell’s endogenous machinery into siRNA. The siRNA created by the cell then completes the RNAi cycle described above by cleaving the mRNA of the target gene thus preventing the disease-causing gene from being expressed.
Figure 2. The ddRNAi approach.
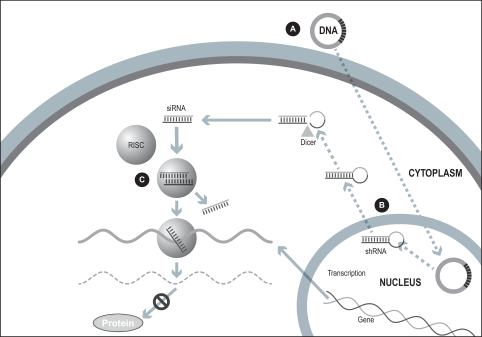
A DNA construct is delivered to the target cell’s nucleus by a gene therapy vector (A) such as an AAV. Once in the nucleus, the DNA construct continuously produces shRNAs (B) which are processed by an enzyme called Dicer into siRNAs (C). The processed siRNA is incorporated into RISC and silences the target gene using the same mechanism shown in Figure 1.
88
Our Pipeline
We are developing a portfolio of product candidates based on our proprietary ddRNAi gene silencing technology focused on chronic and life threatening conditions with disease-associated genes.
In-House Programs

Proceeds from our initial public offering in the United States, with our pre-existing cash and cash equivalents, will advance our product candidates for hepatitis B, AMD and OPMD. Additional financing will be needed to advance our product candidates for hepatitis B, AMD and OPMD through clinical trials.
BB-HB-331 for the Treatment of Hepatitis B
We are developing BB-HB-331 for the treatment of HBV. We are currently conducting preclinical studies of BB-HB-331 and are targeting completion of thein vivoproof-of-concept preclinical studies in the second quarter of 2016. Results of recentin vivo andin vitro studies, from March 2016 and December 2015, respectively, have, we believe, demonstrated the potential utility of an approach that combines RNAi with gene therapy to treat HBV, and we intend to advance the HBV program towards the clinic. However, we will need to seek additional financing to proceed with clinical trials for BB-HB-331.
We are in the process of winding down for commercial reasons a partially completed Phase I/IIa clinical trial for our now discontinued product candidate, TT-034, which we were developing to treat patients chronically infected with the most common genotype of HCV. Early stages of the clinical trial of TT-034 indicated thatTT-034 caused no treatment-related serious adverse events among the nine patients dosed to date. Of the nine patients, seven were biopsied and indicated a relationship between the amount of the compound administered and hepatic tissue transduction. We believe that our experience with the TT-034 clinical trial will be of value in designing and implementing our other programs, in particular HBV, as both HBV and HCV replicate in the liver. We have designed BB-HB-331 to mimic the design elements of TT-034.
89
The human hepatitis B virus is a small DNA virus that, according to the WHO, infects up to 240 million people worldwide, resulting in up to 780,000 deaths per year. Infection with HBV occurs in phases ranging from a silent, acute phase that can be resolved by the immune system to a persistent chronic infection requiring life-long therapy. In the case of a chronic HBV infection, the presence of viral proteins, particularly the s-antigen, causes hepatic inflammation leading to liver dysfunction, acute hepatic failure, cirrhosis or hepatocellular carcinoma.
Current Hepatitis B Treatments
HBV predominantly exists as eight genotypes, designated A through H, with distinct geographic distribution. We believe the keys to developing a successful HBV therapeutic are to stop viral replication and to prevent production of or clear specific antigens generated by the virus, in particular the HBV surface antigen, or HBsAg.
According to GlobalData, a market research firm, the global hepatitis B therapeutics market was $3 billion in 2011, and is expected to grow to $4.4 billion by 2019. The current therapies used as standard of care for HBV consist of antivirals composed of nucleotide and nucleoside analogues, or NUCs, and, less commonly, interferon therapy.
The most common anti-viral medications are taken as tablets each day for a year or longer and primarily act to inhibit viral replication:
| | • | | Lamivudine (Zeffix). There are almost no side effects to Lamivudine, however a significant concern is the possible development of hepatitis B virus mutations and antiviral drug resistance after long-term use. |
| | • | | Adefovir (Hepsera). Adefovir is often used for people who have developed a hepatitis B virus mutation after taking Lamivudine. There are almost no side effects except for the possibility of developing hepatitis B virus mutations and antiviral drug resistance. |
| | • | | Entecavir (Baraclude). Entecavir has potent activity against chronic hepatitis B. There are almost no side effects except for the possibility of developing hepatitis B virus mutations and antiviral drug resistance. |
| | • | | Tenofovir (Viread). Tenofovir has potent activity against chronic hepatitis B. It is particularly useful in patients who have developed drug resistance to other medications. |
| | • | | Pegylated Interferon (Pegasys). Interferon is given by injection once a week, usually for six months to a year. The drug has many potential side effects, such as flu symptoms and depression, but is reported to control the hepatitis B virus in a third of patients without need for long-term medication. |
Most of these therapies can provide long-term viral load suppression but have low cure rates and have the additional risk of drug-resistant mutations. The long-term use of interferon, particularly in high doses, may also be associated with significant side effects, including nausea, vomiting, shortness of breath, dizziness and fatigue, adding to issues with patient compliance for the course of treatment. We believe that there is significant unmet medical need for HBV treatment due to the following factors:
| | • | | Inability of existing therapies to address the risk of recurrence of the infection, once an antiviral therapeutic is removed, due to the persistence of HBV covalently closed circular DNA, or cccDNA. cccDNA is a supercoiled DNA molecule that is present in the nucleus of HBV-infected cells and acts as a reservoir for further HBV infectivity. cccDNA is responsible for persistence of infection in the natural course of chronic HBV infection and during prolonged antiviral therapy. |
| | • | | Mutations in the HBV genome conferring resistance to existing therapies. |
| | • | | Long treatment regimens and, in some cases, significant debilitating side effects associated with current therapies, which lead to a risk of patient non-compliance. |
90
A problem inherent to all of the current HBV antiviral treatment approaches is their inability to achieve a curative outcome. According to a study published in theJournal of Viral Hepatitis in 2014, HBsAg clearance occurs in only 3% of patients treated with NUCs and 7.8% of patients treated with interferon.Sustained suppression of HBV cccDNA by treatment with NUCs is only possible with continued treatment for many years, and clear-cut guidelines on when to stop treatment are not yet available. The authors concluded that there is a need for alternative therapeutic approaches such as drugs that can preferentially target stages in the life cycle of HBV, referred to as direct acting anti-virals, or DAAs, as well as new immunotherapeutic approaches. We believe that our ddRNAi-based therapeutic for HBV has characteristics of both of these approaches.
Hepatitis B Treatments in Development
DAAs
DAAs are chemicals that have been used to inhibit HBV replication by targeting one of the various stages of the viral life cycle. Use of DAAs may prove ineffective in clearing infected hepatocytes, and thus elimination of the cccDNA pool may be problematic. Accordingly, we believe combination treatments involving immunotherapeutic approaches may be necessary. Immunotherapeutic approaches that are being developed include DNA-based vaccines and molecules that are designed to activate immune responses to the hepatitis B virus. These approaches are not yet in clinical development.
Cytotoxics
TetraLogic Pharmaceuticals Corporation, or TetraLogic, has conducted preclinical studies to evaluate the potential development of the chemotherapeutic drug birinapant as a hepatitis B therapeutic to stimulate apoptosis, or programmed cell death, in HBV-infected liver cells. Using a mouse model of HBV infection, TetraLogic reported that birinapant was observed to have activity in the clearance of liver cells infected with HBV. The clearance was additive when given in combination with entecavir. Birinapant caused a decline in HBsAg, whereas entecavir alone did not, implying that birinapant exerts its effect on apoptosis via a different mechanism of action.
siRNA
Several therapeutics based on siRNA are in late preclinical stages or clinical stages of development at companies such as Arbutus, Arrowhead and Alnylam. The most advanced of these therapies is Arrowhead’s drug candidate, ARC-520, which consists of two siRNAs targeting HBV mRNAs. Using transient and transgenic mouse models, it was observed that a single injection of ARC-520 resulted in a 3–4 log reduction in HBV-DNA and viral antigens. In addition, 95% reduction in HBV-DNA levels and approximately 90% reduction in HBsAg levels were observed after two doses of ARC-520 in a chimpanzee chronically infected with HBV. Arrowhead is currently testing ARC-520 in hepatitis B patients in a Phase II clinical study.
In addition, ARB-1467, an Arbutus drug candidate, has progressed to a Phase II multiple-dosing study initiated in December 2015. ARB-1467 uses a three-trigger siRNA design with a goal to facilitate the loss of HBsAg expression in chronic HBV patients. Using chimeric mouse models, ARB-1467 showed inhibition of HBsAg and HBeAg, as well as a reduction in viral DNA and cccDNA.
We believe these results highlight the potential of RNAi to have a therapeutic effect on chronic HBV infection. However, like NUCs and DAAs, siRNA-based therapeutics for HBV rely on ongoing treatment to be effective and they are subject to the other limitations of siRNA described above. Some of these limitations may have contributed to the modest efficacy of ARC-520 in their Phase I/IIa clinical trial announced by Arrowhead in 2014.
91
Our ddRNAi-Based Hepatitis B Therapeutic—BB-HB-331
We are developing BB-HB-331 to address many of the limitations of therapeutics for HBV currently on the market and those in development. BB-HB-331 is expected to be a single administration ddRNAi-based monotherapy that is delivered using an AAV vector that targets the liver and expresses three shRNAs that target highly conserved regions on the HBV genome to inhibit both viral replication and viral protein, includings-antigen, production on a long-term basis.
We have designed BB-HB-331 to mimic our now discontinued product candidate for HCV, TT-034, as both HBV and HCV replicate in the liver. The same AAV vector is used in both therapeutic candidates, designed to achieve the same biodistribution and liver transduction properties. The AAV vector is expected to be delivered by a single administration monotherapy. The AAV vector will target the liver and express three shRNAs that target three separate conserved regions of the genome. The most significant change is the replacement of the three anti-HCV shRNAs of TT-034 with three anti-HBV shRNAs in BB-HB-331. Though we have discontinued our clinical trial for TT-034 for commercial reasons, we believe the early stage clinical results of TT-034 provide support for the safety of our platform technology.
Despite the discontinuation of the TT-034 Phase I/IIa trial and the need for further analysis of the final trial data once available, we believe useful information was obtained from the trial which should be beneficial forBB-HB-331’s clinical development, its IND-enabling studies, the design and regulatory approval pathway for its initial clinical trials, and potentially its starting clinical dose, in light of the similarities between TT-034 andBB-HB-331.
BB-HB-331—Design and Mechanism of Action
The design of the BB-HB-331 DNA construct takes advantage of the structure of the HBV genome. The hepatitis B virus is a small DNA virus with four overlapping open reading frames, meaning several genes are produced from the same DNA sequence by shifting the starting point of the translation process(Figure 3). These four genes are known as the core, surface, X and polymerase genes. The core gene encodes the core nucleocapsid protein, which is important in viral packaging and thought to help stabilize cccDNA, and hepatitis B e-antigen. The surface gene encodes proteins, including s-antigen. The X gene encodes the X protein, which has properties that may be important in liver carcinogenesis. The polymerase gene encodes a large protein with functions critical for viral packaging and replication. Although HBV is a DNA virus, it replicates through an RNA intermediate. BB-HB-331 targets the viral mRNA at three overlapping regions of the genome(Figure 3), simultaneously silencing the surface, X, core and polymerase genes. As a result, we believe that the long-term suppression of HBV viral replication, through silencing of the polymerase gene and targeting the HBV RNA used for replication, the inhibition of HBV viral proteins production, including the s-antigen production, through silencing of the surface gene, and the inhibition of the cccDNA, through silencing of the core protein gene, could lead to eradication of HBV infection in patients by a single administration of BB-HB-331.
In vitro Development Highlights
Our bioinformatics analysis of the major HBV genotypes, A through H, has identified several well-conserved regions of the genome for targeting with ddRNAi therapeutics. We have designed numerous shRNAs to target these regions, and we have identified lead candidates based on several factors, including activity, hyperfunctionality, meaning the highest activity at lowest levels, and the ability of the shRNA strand to be incorporated into the cell’s natural RNA processing mechanism. The final clinical construct we have chosen is illustrated in Figure 3.
92
Figure 3. The design for BB-HB-331
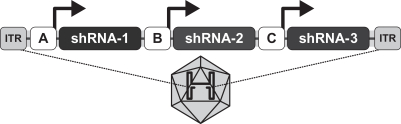
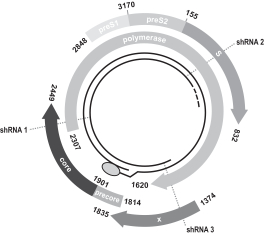
The design for BB-HB-331 is based on the design of TT-034, utilizing an AAV capsid and a triple construct targeting three separate conserved regions on the HBV mRNA (A); The shRNAs target regions on the overlapping reading frames of the HBV genome (B), allowing simultaneous targeting of the mRNAs that express viral proteins including DNA polymerase, s-antigen and core proteins.
93
Figure 4. The mechanism of action of BB-HB-331
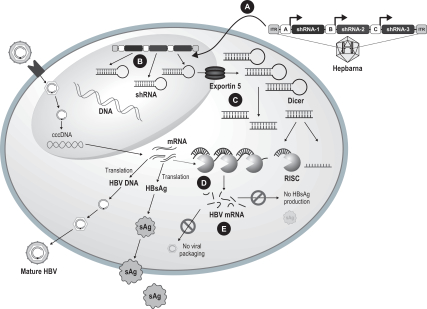
The DNA construct is delivered to the nucleus of hepatocytes via an AAV vector (A). Upon reaching the nucleus the construct expresses three shRNAs (B) that are processed by the cell’s endogenous machinery to produce siRNAs (C) that cleave the HBV mRNA (D) and prevent the virus from replicating and producing HBsAg (E).
In vivo Development Highlights and Ongoing Development Plan for BB-HB-331
Our development plan for BB-HB-331 is focused on demonstrating clinical proof of concept in a Phase I/IIa clinical trial. We expect to completein vivo proof-of-concept studies in the second quarter of 2016. Our planned activities include:
| | • | | In vitro testing of BB-HB-331 in an HBV human hepatocyte cell culture model to assess effects on HBV and mRNA levels, suppression of viral protein production and cccDNA elimination. |
| | • | | In vivo testing of BB-HB-331 in a HBV mouse model to assess parameters such as effects on HBV mRNA levels, suppression of viral protein production and cccDNA elimination. |
Initialin vitrodata from preclinical results in December 2015 demonstrated the efficacy of BB-HB-331 to suppress multiple aspects of HBV in infected human liver cells. Thein vitrodata was supported byin vivo experiment results reported in March 2016, which demonstrated suppression of HBVin vivo in a mouse model following a single administration. Key findings from thein vivo study indicated that a single BB-HB-331 treatment:
| | • | | reduced serum HBV DNA by 1.83 logs, equivalent to 98.5% elimination of circulating HBV; |
| | • | | reduced intracellular liver HBV DNA by 94.9%; |
| | • | | suppressed serum antigens, HBsAg and HBV enveleope antigen (HBeAg), by 97.6% and 92.5%, respectively; and |
| | • | | decreased levels of HBV viral RNA and cccDNA. |
94
BB-AMD-211 and BB-AMD-231 for the Treatment of Age-Related Macular Degeneration
Overview
We are developing two ddRNAi-based therapies, one for the treatment of wet AMD, which is designated BB-AMD-211, and the other potentially for both wet and dry AMD, which is designated BB-AMD-231. The delivery vector for both BB-AMD-211 and BB-AMD-231 is being developed in collaboration with 4DMT. The aim of this program is to develop a therapeutic that provides long-term treatment of AMD from a single intravitreal injection. We believe this could replace the need for regular injections of therapeutics into the eye, which is the current standard of care.
AMD is the deterioration of the eye’s macula. The macula is a small area in the retina that is responsible for central vision. AMD is the leading cause of blindness and visual impairment in older adults, often involving blood vessel overgrowth and damage to the retina resulting in the loss of vision in the central visual field. The vascular endothelial growth factor, or VEGF-A, is responsible for stimulating the new blood vessel growth. The disease occurs in two forms, wet and dry. Dry AMD is the most common type of macular degeneration and affects 85% to 90% of the people with AMD. Dry AMD often develops into wet AMD.
In the dry form, there is a breakdown of retinal pigment epithelial cells in the macula. These cells support the light-sensitive photoreceptor cells that are critical for vision. Generally, the damage caused by the dry form is not as severe or rapid as that of the wet form. However, over time, it can cause profound vision loss. There are currently no approved treatments for dry AMD.
Wet AMD is the more advanced type of AMD. In wet AMD, which is also called exudative, or neovascular, AMD, the Bruch’s membrane underlying the retina thickens, then breaks. The oxygen supply to the macula is disrupted and, as a result, new abnormal blood vessels grow through the subretinal membrane towards the macula, often raising the retina. The blood vessels are fragile, and often leak fluid that damages the macula. VEGF-A is responsible for stimulating the new blood vessel growth in wet AMD. Although it affects only 10% to 15% of those who have AMD, wet AMD accounts for 90% of the severe vision loss caused by macular degeneration.
According to a study published in JAMA Ophthalmology, AMD is the leading cause of irreversible vision loss in the United States, affecting an estimated 1.75 million people. It is estimated that 196 million people will be affected by AMD worldwide by 2020 according to a study published in Lancet Global Health.
There are a number of treatments currently available for wet AMD. According to GlobalData, the annual wet AMD treatment market across the United States, the United Kingdom, Germany, France, Spain, Italy and Japan will almost double from US$5.1 billion in 2013 to US$10.1 billion by 2023.
Current AMD Treatments
According to GlobalData, the global AMD treatment market is dominated by anti-VEGF drugs, including Lucentis, Avastin and Eylea, which together accounted for 98% of sales for AMD in 2013.
| | • | | Lucentis (ranibizumab) is an antibody fragment, or macromolecule, that directly inhibits VEGF-A by binding to it and thus preventing its binding to the corresponding receptor in the retina. The main challenge for Lucentis is that it requires frequent administration, typically monthly or bimonthly, via intravitreal injections. |
| | • | | Avastin (bevacizumab) is a monoclonal antibody that also binds to VEGF-A. Although approved only for use in colon cancer, it is used off label for AMD. It is also injected intravitreally and requires ongoing regular injections to maintain its effect. |
95
| | • | | Eylea (aflibercept) is a recombinant fusion protein consisting of portions of human VEGF receptors 1 and 2 extracellular domains fused to the Fc portion of human IgG1. It is recommended to be injected intravitreally every four weeks for the first three months and every eight weeks thereafter. |
| | • | | Macugen (pegaptanib) is an RNA aptamer that is directed against VEGF-A. It is recommended to be injected intravitreally every six weeks. |
All four treatments have similar risks and potential for adverse events, due primarily to their use of frequent intravitreal injection. Risks of intravitreal injections include increase in intra-ocular pressure, retinal detachment and endophthalmitis, or inflammation of the internal chambers of the eye. Patients and doctors dislike ocular injections and tend to prefer treatments that require these injections less frequently. The use of VEGF inhibitors can also cause blood clots.
A number of companies are developing gene therapy-based treatments for AMD. In general, these approaches involve the delivery of genes expressing proteins that are designed to inhibit new blood vessel formation, which is one of the hallmarks of the disease. The genes that are being developed include genes that express VEGF inhibitors in addition to other factors that activate new blood vessel formation. In contrast, our approach is designed to directly silence the gene responsible for producing VEGF-A. We believe this could be more effective than other gene therapy approaches as our approach is to design ddRNAi-based therapeutics to prevent the production of VEGF-A rather than to deliver a new gene that expresses a new protein to inhibit VEGF-A after it has been produced. Furthermore, we believe that most of the other gene therapy approaches are delivered using subretinal injections. This route of administration presents challenges, including the requirement for hospitalization as a result of general anesthesia and the length of time required to complete the complicated surgical procedure.
Our ddRNAi-Based AMD Therapeutics—BB-AMD-211 and BB-AMD-231
There are several challenges in the development of AMD therapeutic market and we believe that our ddRNAi technology has the potential to address and overcome a number of these challenges, including:
| | • | | The relatively short half-life of current standard-of-care therapies results in the need for regular administration by intravitreal injection every 4 to 8 weeks. We believe our ddRNAi-based therapies have the potential for sustained inhibition of VEGF-A, possibly for months or years, from a single intravitreal injection. |
| | • | | AMD therapeutic programs under development at a number of other gene therapy companies focus on administering the product to target cells by subretinal injection. We are co-developing with 4DMT AAV vectors to target the subretinal cells following intravitreal injection, which we believe is a more commercially viable and less invasive route of administration and is the route used in most current anti-VEGF therapies. |
| | • | | There are no approved treatments for dry AMD. We have designed and tested a ddRNAi construct that we believe has potential to address this unmet market need. |
BB-AMD-211 and BB-AMD-231- Design and Mechanism of Action
We are developing two ddRNAi-based product candidates, one for wet AMD, called BB-AMD-211, and one for both wet and dry AMD, called BB-AMD-231, that are designed to address many of the limitations for therapeutics for AMD currently on the market or under development.
BB-AMD-211 is a ddRNAi construct expressing a single shRNA targeting the VEGF-A gene (Figure 5A). VEGF-A is responsible for stimulating the new blood vessel growth in wet AMD.
BB-AMD-231 is our second generation product candidate designed to express three shRNAs, which target three different genes, VEGF receptor 2, PDGF-beta and human complement factor B, which all play a role in the
96
progression of AMD(Figure 5B). VEGFR2 is the receptor known to bind VEGF-A, so silencing that receptor should prevent it from functioning to stimulate new blood vessel growth. PDGF-beta has a known role in recruiting cells that stabilize newly formed blood vessels for long term-persistence. Human complement factor B is a known component of ocular drusen.
Figure 5. Our AMD ddRNAi constructs

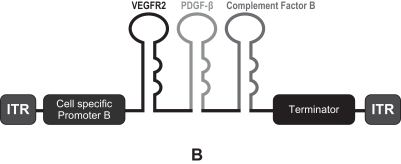
BB-AMD-211 is a single construct targeting VEGF-A for wet AMD (A); and BB-AMD-231 is a ddRNAi construct targeting three genes shown to have a role in both wet and dry AMD (B).
97
We have observed inin vitro studies that both BB-AMD-211 (Figure 6) and BB-AMD-231 (Figure 7) are effective at silencing the target genes.
Figure 6. Effects of BB-AMD-211 on VEGF-A.
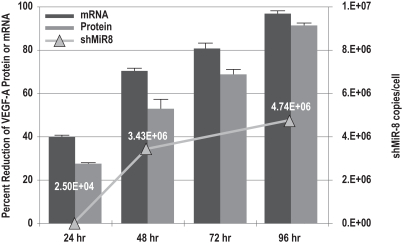
A retinal pigment epithelial cell line culturedin vitro was treated with the BB-AMD-211 expression construct and monitored for up to 96 hours for inhibition of VEGF-A protein (light gray bars) and mRNA (dark grey bars) expression as well as expression of the shRNA produced (triangles). The production of anti-VEGF-A shRNAs inside of the cells correlated with significant silencing of VEGF-A mRNA and protein levels. By 96 hours, greater than 90% inhibition was observed.
Figure 7. Effects of BB-AMD-231.
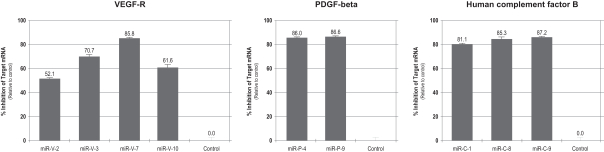
Inin vitro studies in retinal pigment epithelial cells, BB-AMD-231 inhibited target mRNA levels of VEGF-receptor, PDGF-beta and human complement factor B.
Ongoing Development Plan for AMD – Development of Novel Delivery Vector
We have engaged 4DMT to develop a vector that will deliver our ddRNAi constructs to the retinal cells of the eye from an intravitreal injection. 4DMT has developed novel AAV vectors with desirable physical properties, such as enhanced tissue attractions, or tropisms, and reduced immunogenicity using a technique called directed evolution. This involves sequential passaging of a starting AAV libraryin vivo to isolate those variants that have the highest affinity for the target tissue. The sequential passaging for our AMD program is being conducted in non-human primates to enable identification of vectors expected to be suitable for the complex human eye structure. Preliminary results have evidenced the presence of AAV particles in the retina from intravitreal administration of the starting AAV library. We expect that we will select an AAV vector candidate by the second quarter of 2016 for use in ourin vivo studies of BB-AMD-211 in a primate model of wet AMD. Upon production of the vector, we will initiatein vivo proof-of-concept studies in which the 4DMT vector will be used
98
to deliver the BB-AMD-211 DNA construct that expresses an shRNA designed to silence VEGF-A intravitreally in a non-human primate model of AMD. We believe that the construct will silence the VEGF-A gene in the retinal cells by the mechanism shown in Figure 8.
Figure 8. The expected mechanism of action of BB-AMD-211
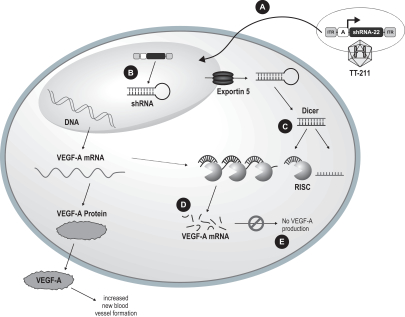
Following an intravitreal injection, the DNA construct is delivered to the nucleus of retinal cells via an AAV vector (A). Upon reaching the nucleus, the construct expresses three shRNAs (B) that are processed by the cell’s endogenous machinery to produce siRNAs (C) that cleave the VEGF-A mRNA (D) and prevent the VEGF-A protein from being expressed (E) thus preventing it from stimulating the growth of new blood vessels.
We expect to completein vivo preclinical proof-of-concept studies in the fourth quarter of 2016. However, we will need to seek additional financing to proceed with clinical trials for our product candidates for AMD.
Pabparna for Treatment of Oculopharyngeal Muscular Dystrophy
We are developing Pabparna for the treatment of OPMD, an autosomal-dominant inherited, slow-progressing, late-onset degenerative muscle disorder that usually starts in patients during their 40s or 50s. The disease is manifested by progressive swallowing difficulties, or dysphagia, and eyelid drooping, or ptosis, due to specific effects on the pharyngeal and cricopharyngeal muscle, which is located at the top of the esophagus. The disease is caused by a specific mutation in the poly(A)-binding protein nuclear 1, or PABPN1, gene. The main pathological characteristic of OPMD is the presence of dense intranuclear inclusions of mutated PABPN1 protein.
Pabparna utilizes a “silence and replace” approach designed to silence the mutant PABPN1 gene with a ddRNAi construct and replace the mutant gene with the normal PABPN1 gene, delivered with an AAV vector.
Results fromin vivo studies in an animal model of OPMD support proof of concept of this approach in Pabparna’s individual components. In conjunction with collaborators, we are working to optimize thein vivo delivery of Pabparna and, assuming successful results, we plan to develop Pabparna through IND-enabling studies, and subsequent submission of an IND application.
99
OPMD is a rare disease and has been reported in at least 33 countries. Patients suffering with OPMD are well identified and are aggregated in particular regions, which we believe should simplify clinical development and commercialization of Pabparna, if it is approved. The largest OPMD cluster is in the French-Canadian population, with estimated prevalence of one in every one thousand people, and its highest prevalence is among Bukhara Jews living in Israel, where it affects one in six hundred people. In Europe, the estimated prevalence is one in one hundred thousand people. The relatively low abundance of patients afflicted by this disease allows this indication to be characterized as a rare disease, potentially supporting an orphan drug designation.
Current OPMD Treatments and Products in Development
The therapies for OPMD currently available and under development consist of a symptomatic surgical intervention called cricopharyngeal myotomy, an intravenous trehalose injection, Cabaletta, and cell transplantation. Each of these therapies has treatment limitations.
Cricopharyngeal myotomy is used to address ptosis and improve swallowing in moderate-to-severely affected individuals. It is the only current treatment to improve swallowing in OPMD patients but does not correct the progressive degradation of the pharyngeal musculature, which often leads to death from swallowing difficulties and choking.
Bioblast Pharma Ltd., an Israeli biotechnology company, is developing Cabaletta. It is currently being tested in Phase II/III clinical trials in Israel and Canada. Cabaletta is a solution of trehalose administered intravenously and we believe that it will require ongoing re-administration to remain effective.
The Institut de Myologie in Paris is currently undertaking a Phase I/II trial of a cell transplantation therapy, grafting autologous myoblasts isolated from spared muscles into the pharyngeal muscle. This is a significantly invasive procedure requiring surgery in two different sites of a patient’s body.
Our ddRNAi-Based OPMD Therapeutic—Pabparna
We are developing Pabparna, a single administration ddRNAi-based gene therapy, to correct the gene defect which causes the disease and to address many of the limitations of therapeutic approaches currently available and those in development for OPMD. Pabparna is a monotherapy delivered using an AAV vector and is designed to silence the expression of the mutant PABPN1 gene in esophageal muscle cells of OPMD patients while simultaneously introducing a silencing-resistant normal form of the gene. We believe OPMD is well suited for this “silence and replace” approach since the genetic mutation is well characterized and the target tissue is relatively small. Once validated, we believe a similar approach could be applied to other inherited disorders.
Pabparna—Design and Mechanism of Action
Pabparna is designed to target three distinct regions of the PABPN1 mRNA, by generating three separate shRNAs from a single DNA construct, and to express a silencing-resistant version of the normal PABPN1 gene(Figure 9).
100
Figure 9. Pabparna AAV “silence and replace” combination vector
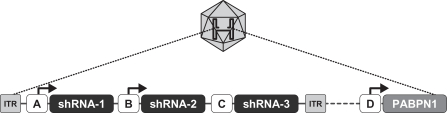
Pabparna comprises a ddRNAi DNA construct expressing three separate shRNAs targeting three separate regions of the PABPN1 gene, designed to silence the defective PABPN1 gene in OPMD patients, combined with a gene expression construct that produces a silencing-resistant version of the normal PABPN1 gene, delivered using an AAV vector.
In collaboration with researchers at the Royal Holloway University of London and the Institut de Myologie in Paris we have observed effective silencing of the PABPN1 genein vitro by the ddRNAi construct. Furthermore, we have generated a gene expression construct that produces a silencing-resistant version of the normal PABPN1 gene. The mechanism of action of Pabparna is shown in Figure 10.
Figure 10. The mechanism of action of Pabparna
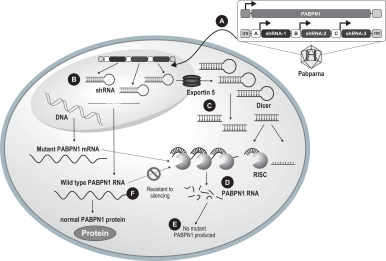
The DNA construct is delivered to the affected muscle cells via an AAV vector (A). Upon reaching the nucleus, the construct expresses three shRNAs (B) that are processed by the cell’s endogenous machinery to produce siRNAs (C) that cleave the mutant PABPN1 mRNA (D) and silence the expression of the mutant gene (E). In addition, the PABPN1 gene expression construct expresses a silencing-resistant version of the normal PABPN1 gene (F), which we believe may promote restoration of muscle function to the cell.
Inin vivo studies using a transgenic mouse model of OPMD at the Royal Holloway University of London and the Institut de Myologie, we observed normalization of muscle strength following administration of the ddRNAi and gene expression constructs (Figure 11).
101
Figure 11. Restoration of muscle functionin vivo following gene silencing and replacement
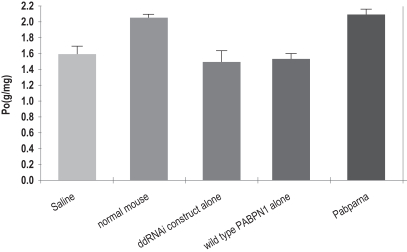
Restoration of muscle function in vivofollowing suppression of the mutant PABPN1 and replacement with the normal PABPN1 gene, with muscle function measured by specific tetanic force (Po). Neither the expression of a triple hairpin construct to down-regulate the mutant form of the PABPN1 protein, nor the expression of the normal protein, was sufficient by itself to restore specific force levels. The combination of the silencing of the mutant gene with the triple hairpin construct and replacement with the normal gene was observed to restore specific force capacity to healthy levels.
Ongoing Development Plans for Pabparna
Our development plan for Pabparna is focused on investigating options for the optimal delivery of the combined constructs that comprise Pabparna. The options include combining the two constructs into a single AAV vector (Figure 9) and testing that combination vectorin vivo in the OPMD mouse model, and generating and testing lentivirus-based combination vectors and testing their ability to produce gene modified autologous muscle stem cellsin vitro.
Successful results from these studies may inform the design of later translational studies in dogs moving towards a clinical trial in OPMD patients initially via local administration of AAV vectors. We plan to have completed preclinical proof-of-concept studies using the chosen clinical construct in the fourth quarter of 2016.
TT-034 for the Treatment of Hepatitis C
Hepatitis C is a complex public health problem, characterized by a high prevalence of chronic infection by an RNA virus, an increasing burden of HCV-associated disease, low rates of testing and treatment, and the prospect of increasing incidence associated with injectable drug abuse. According to the WHO, over 170 million individuals worldwide have chronic hepatitis C. Chronic infection can result in cirrhosis and death in 20% of patients due to end-stage liver disease or hepatocellular carcinoma. We have been developing a ddRNAi-based therapeutic, TT-034, for the treatment of the most common genotype of the human hepatitis C virus. We began a Phase I/IIa first-in-human clinical trial of TT-034 in January 2014.
The primary endpoint for this study was safety, measured by incidence of serious adverse events and changes in clinical parameters. The secondary endpoints were efficacy-based, measured by shRNA expression in the liver, and sustained reduction in HCV viral load in the blood. As of March 2016, nine patients had been dosed in connection with the Phase I/IIa clinical trial and there have been no treatment-related serious adverse events observed in the study to date. Of the seven patients biopsied to date, a dose-dependent transduction of TT-034 in hepatic tissues and an increase in shRNA has been observed.
102
Although early stages of the clinical trial of TT-034 indicated that TT-034 caused no treatment-related serious adverse events among the nine patients dosed to date, and results from the seven biopsied patients also indicated a relationship between the amount of the compound administered and hepatic tissue transduction, in February 2016 we decided to discontinue the HCV program as a result of feedback from potential commercial partners.
Following the commencement of the clinical trial of TT-034, a number of new and effective therapeutics were approved for the treatment of HCV. In recent months before the date of this prospectus, competitors’ products showed improvements in the efficacy, delivery and success rate of treatments for HCV while, at the same time, reducing the price and duration of their treatments.
As a result of the increasingly competitive landscape, in February 2016 we concluded that the TT-034 program no longer offered the commercial value necessary to attract a worthwhile commercial partnership deal and, as a result, did not warrant additional expenditure or focus of our resources beyond completion of the existing patients. We are committed to completing the collection of trial data and monitoring patients through the required long-term safety follow-up period. Final data will be reported in the fourth quarter of 2016 when the study is completed. We believe that completing this work will provide us with valuable data that supports our ddRNAi technology platform and other pipeline programs.
Although we will have certain FDA reporting obligations following the discontinuance of our hepatitis C program, no significant financial obligation will arise from the discontinuance, and we believe that our experience with the TT-034 clinical trial will be of value in designing and implementing our other programs, in particular HBV, as both HBV and HCV replicate in the liver.
Tribetarna for the Treatment of Drug-Resistant Non-Small Cell Lung Cancer
Lung cancer, including drug-resistant non-small cell lung cancer, or NSCLC, and small cell lung carcinoma, or SCLC, is the most common form of human cancer, and about 80% of diagnosed lung cancer cases are categorized as NSCLC. We were developing Tribetarna, our ddRNAi therapeutic, to target drug-resistant NSCLC and re-sensitize the tumors to chemotherapy by silencing the TUBB3 gene. We undertook preclinical proof-of-concept studies for Tribetarna in collaboration with researchers at the University of New South Wales, or UNSW, who were one of the first groups to demonstrate a link between TUBB3 expression and chemoresistance in NSCLC cells.
As a result of feedback from potential commercial partners, we have recently decided to terminate the NSCLC program, allowing us to focus our resources on developing other preclinical programs which have attracted stronger interest from potential commercial partners and investors. We are in discussions with the UNSW to determine the best use of the data gathered to date. The program has provided insights into optimizing ddRNAi design and delivery.
Our Out-Licensed Programs
We have licensed our ddRNAi technology to companies who are developing therapeutic programs in five disease areas. These licenses expand the areas and number of ddRNAi-based therapeutics being developed. Each of them provides a potential opportunity for further clinical validation of the technology and potential revenue opportunity. These licenses have been granted to small early-stage biotechnology companies with modest upfront and early development milestone payments and greater milestone payments due upon later-stage program success. Under these agreements, we have received aggregate payments of A$276,824 and A$307,310 for the fiscal years ended June 30, 2014 and June 30, 2015, respectively. We do not expect that any milestone payments we may receive in the future will be significant to our business.
103
The following table sets forth our out-licensed product candidates and their development status.

HIV/AIDS
In March 2012, we granted a non-exclusive, royalty-bearing, worldwide license to a U.S.-based biotechnology company, Calimmune, Inc., or Calimmune. Under the agreement, Calimmune can develop, use and commercialize ddRNAi to silence up to three targets for the treatment or prevention of HIV/AIDS. Calimmune’s approach was developed with core technology from the laboratory of Dr. David Baltimore, a Nobel Laureate in the area of HIV/AIDS, and involves silencing the gene that codes for a receptor protein known as CCR5. Calimmune’s HIV/AIDS treatment is known as Cal-1.
The license provides for modest upfront and milestone payments and single-digit percentage royalty payments on net sales. In addition, we receive a percentage of any sub-licensing revenues received. Unless terminated at an earlier date, the license agreement continues until the expiration or termination of all patents subject to the license. We may terminate the license agreement in the event of certain breaches by Calimmune or if Calimmune commences an action or proceeding with respect to the patent rights that are the subject of the license. Calimmune may terminate the license agreement at will.
In 2014, Calimmune commenced a Phase I/IIa clinical trial of Cal-1. The goal of the trial is to assess the safety of the therapy, to determine the ease of use and feasibility of the approach for HIV/AIDS patients and to evaluate what, if any, side effects there may be. Calimmune has reported that, following review by the DSMB of the first cohort of patients for the trial, a second patient cohort was dosed, consisting of four patients, who received a preconditioning regimen designed to make the treatment more effective.
Retinitis Pigmentosa
In July 2012, we granted an exclusive, royalty-bearing, worldwide license to an Ireland-based biotechnology company, Genable Technologies Limited, or Genable, to use, develop or commercialize RNAi for treatment or prevention of retinitis pigmentosa. Genable’s treatment involves suppression of the mutant and normal genes,
104
and replacement with a normal RHO gene that has been modified to be resistant to ddRNAi gene silencing. Genable has reported that it established proof of concept in anin vivo model of the disease. Genable’s treatment for retinitis pigmentosa, GT308, is named RhoNova.
The license provides for modest upfront and milestone payments and single-digit percentage royalty payments on net sales. In addition, we receive a percentage of any sub-licensing revenues received. Unless terminated at an earlier date, the license agreement continues until the expiration or termination of all patents subject to the license. We may terminate the license agreement in the event of certain breaches or if Genable commences an action or proceeding with respect to the patent rights that are the subject of the license. Genable may terminate the license agreement at will.
In October 2014, the European Medicines Agency, or EMA, granted RhoNova Advanced Therapy Medicinal Product classification. The classification enables Genable to procure centralized scientific advice and guidance from EMA regulators on RhoNova’s ongoing development. In 2013, the FDA granted Genable orphan drug designation for RhoNova. In March 2016, Genable announced that Spark Therapeutics, or Spark, had acquired the company for a combination of cash and common stock. Spark has indicated support for continuing the development of RhoNova.
Huntington’s Disease
In December 2012, we granted a non-exclusive, royalty-bearing, worldwide license to a Netherlands-based biotechnology company, uniQure biopharma B.V., or uniQure, to use, develop or commercialize RNAi for treatment of Huntington’s disease. Our license grants to uniQure rights to develop, use and commercialize an AAV vector with a ddRNAi cassette targeting the gene associated with Huntington’s disease, or the Htt gene, or an AAV-RNAi-based product for Huntington’s disease directed to up to three gene targets specific to Huntington’s disease.
The license provides for modest upfront and milestone payments and single-digit percentage royalty payments on net sales. In addition, we receive a percentage of any sub-licensing revenues received. Under the agreement, uniQure has an option to convert the license to an exclusive license depending upon achievement of certain preclinical milestones, and also to acquire additional licenses to our ddRNAi technology for other specific diseases. Unless terminated at an earlier date, the license agreement continues until the expiration of either all patents subject to the license or regulatory exclusivity, whichever is longer. We may terminate the license agreement in the event of certain breaches or if uniQure has not met a defined sales milestone or commences an action or proceeding with respect to the patent rights that are the subject of the license. uniQure may terminate the license agreement at will. In addition, certain rights and licenses granted to uniQure pursuant to the license agreement will automatically terminate in the event that our license of technology from Galapagos NV expires or is terminated.
In May 2013, uniQure announced that it, along with its partners in a pan-European consortium devoted to finding a gene therapy cure for Huntington’s disease, were awarded a 2.5 million Euros grant for use in the development of a RNAi-based approach. uniQure has reported that it is using RNAi to non-specifically knock down all expression of the Htt gene and to specifically inhibit the mutant allele of the Htt gene. Evaluation of these two approaches is in progress.
Cancer Immunotherapy
In August 2013, we granted an exclusive, royalty-bearing, worldwide license to a U.S.-based biotechnology company, Regen Biopharma Inc., or Regen, to use ddRNAi for silencing expression of indoleamine 2,3—dioxygenase, or IDO, in dendritic cells. Regen is developing a cancer immunotherapy using the licensed technology. IDO is associated with immune-suppression and is overexpressed in some cancers. Regen has reported preclinical evidence that modification of these cells using ddRNAi targeting the silencing of IDO may
105
significantly enhance their efficacy in cancer immunotherapy. Regen’s first treatment, which is for breast cancer, is called dCellVax.
The license provides for modest upfront and milestone payments, payable in cash or stock of Regen’s parent company at Regen’s discretion, and single-digit percentage royalty payments on net sales. In addition, we receive a percentage of any sub-licensing revenues received. Unless terminated at an earlier date, the license agreement continues until the expiration or termination of all patents subject to the license. We may terminate the license agreement in the event of certain breaches or if Regen has not met a defined sales milestone or commences an action or proceeding with respect to the patent rights. Regen may terminate the license agreement, in whole or in part, at will.
In November 2014, Regen announced the FDA had issued an IND number for a proposed Phase I/II clinical trial assessing safety with signals of efficacy for dCellVax.
Intractable Neuropathic Pain
In November 2014, we granted an exclusive, royalty-bearing, worldwide license to a U.S.-based biotechnology company, Circuit Therapeutics, Inc., or Circuit Therapeutics, to use ddRNAi for the development of treatments for and the prevention of pain. Under the licensing agreement, Circuit Therapeutics has rights to develop, use and commercialize treatments that use ddRNAi to silence Nav1.7, a sodium ion channel that is exclusively expressed in certain sensory nerves and is critical for generation of pain.
The license provides for modest upfront and milestone payments and single-digit percentage royalty payments on net sales. In addition, we receive a percentage of any sub-licensing revenues received. Unless terminated at an earlier date, the license agreement continues until the expiration or termination of all patents subject to the license. We may terminate the license agreement in the event of certain breaches or if Circuit Therapeutics commences an action or proceeding with respect to the patent rights. We may also terminate the license agreement if Circuit Therapeutics has not met certain sales and development milestones. Circuit Therapeutics may terminate the license agreement at will.
New Areas for ddRNAi Application
We believe the applicability of ddRNAi to disease treatment could be further expanded using a number of strategies that are in the early stages of development. These strategies include:
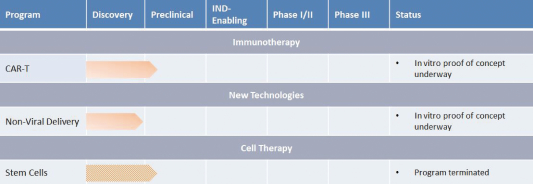
Immunotherapies
One approach to immunotherapy involves engineering patients’ own immune cells to recognize and attack their tumors. This approach is called adoptive cell transfer, or ACT. ACT utilizes T cells. After isolation from a patient’s blood, the T cells are genetically engineered to produce special receptors on their surface called
106
chimeric antigen receptors, or CARs. CARs are proteins that enable T cells to recognize a specific protein, or antigen on tumor cells. These engineered CAR T cells are cultured and expanded in the laboratory and then infused into the patient. After the infusion, the technology relies on the T cells multiplying in the patient’s body and, with guidance from their engineered receptor, recognize and kill cancer cells that harbor the antigen on their surfaces.
CAR T cell therapy can cause side effects, the most common being cytokine-release syndrome. The infused T cells release cytokines, chemical messengers that help the T cells function. With cytokine-release syndrome, there is a rapid and massive release of cytokines into the bloodstream, which can lead to dangerously high fevers and precipitous drops in blood pressure. In addition, the reliance on patient-derived stem cells requires a non-scalable manufacturing process that is complex, time consuming and expensive.
An important aim for next generation of CAR T therapies is to silence multiple genes known to be associated with cytokine release syndrome, including the T cell receptor, or TCR, and major histocompatibility complex, or MHC. We believe that ddRNAi technology could potentially be used to achieve this goal.
Most of the current CAR applications use lentivirus to deliver gene constructs expressing CARs. The packaging capacity of this vector allows the expression of several shRNA constructs along with the CAR in a single construct. Thus, the same cell population that is transduced to express the CAR can also be modified by the activity of shRNA expressed from the same vector.
In addition to silencing the TCR, we believe ddRNAi technology could potentially be used to enhance other properties of CAR T cells.
Non-viral delivery of DNA constructs
Efficient delivery of DNA constructs to tissues remains a principal challenge to the development of gene therapy based therapeutics. Most gene therapy strategies employ viral vectors. We are using AAV based vectors in our HBV program to deliver therapeutic ddRNAi constructs to hepatocytes. While AAV vectors can transduce livers with high efficiency and drive sustained expression of shRNAs, there are certain disadvantages with their use.
A proportion of patients possess neutralizing antibodies to AAV, and efficient liver transduction could consequently be inhibited in such patients. Treatment with AAV vectors could also induce an immune response in some patients, potentially limiting the ability to readminister therapeutic viral vectors to those patients. While strategies such as transient immune suppression might circumvent these issues, we believe the use of non-viral DNA delivery vectors, designed to be non-immunogenic, could offer intrinsic advantages for gene therapy. We are consequently investigating strategies to develop non-viral vectors for the delivery of therapeutic ddRNAi constructs.
Our initial work on the development of non-viral vectors is being undertaken in the context of the HBV program. We have prepared DNA constructs that show persistence of expressionin vivo and have also demonstrated that ddRNAi constructs prepared in this manner can express HBV therapeutic shRNAs. We are attempting to complex these DNA constructs in nanoparticles to achieve efficient transduction of hepatocytesin vivo and achieve sustained expression of shRNAs.
Ocular diseases
We believe a number of ocular diseases beyond AMD, such as diabetic retinopathy, could be targeted by ddRNAi therapeutics, assuming the AAV vector selection program with 4DMT is successful. This program aims to produce an AAV vector that can target a broad range of ocular cells from an intravitreal injection. We have exclusive access to modified AAV vectors 4DMT for all ocular indications using ddRNAi.
107
Cell Therapies
ddRNAi could potentially be used to produce modified stem cells that express shRNA for enhanced therapeutic benefit. We had been researching this approach for some of our pipeline programs but have recently decided to ceased further development.
Intellectual Property
We actively seek to protect the intellectual property and proprietary technology that we believe are important to our business, which includes seeking and maintaining patents claiming our ddRNAi technology, and other inventions relating to our products in development, or otherwise commercially and/or strategically important to the development of our business. We also rely on know-how and trade secrets that may be important to the development of our business and actively seek to protect the confidentiality of such know-how and trade secrets.
Our success will depend on our ability to obtain and maintain patent and other proprietary protection for commercially important technology, inventions and know-how related to our business, defend and enforce our patents, preserve the confidentiality of our trade secrets and operate without infringing the valid and enforceable patents and proprietary rights of third parties. For more information, please see “Risk Factors—Risks Related to Our Intellectual Property.”
As of March 2016, we own or co-own a total of eleven patent families (10 owned and 1 co-owned), of which two are in the provisional phase and others have progressed to national prosecution. These families include granted patents in the United States (9), Europe (2), Australia (3), Canada (2) and New Zealand (3). We have more than 20 pending national/regional applications in a total of 12 jurisdictions (counting as one jurisdiction the member states of the European Patent Convention). This intellectual property portfolio for our ddRNAi technology, improvements to the technology and product-specific patents can be commercialized collectively or in individual product candidate programs.
We also have a portfolio of in-licensed patents relating to the ddRNAi platform technology. The license from Commonwealth Scientific & Industrial Research Organization, or CSIRO, includes irrevocable, exclusive rights in the field of human therapeutics to CSIRO’s patents claiming ddRNAi. The license includes two patent families with patents in the United States (9), Europe (3), the United Kingdom (1), Australia (4) and Canada (1). The license also includes nine (9) pending national/regional applications in a total of four jurisdictions.
The patent portfolios for the ddRNAi platform and our product candidate pipeline are summarized below. The expected expiration dates included in the summary below do not give effect to patent term extensions that may be available due to delays in the patent office or due to steps taken to obtain regulatory approvals.
ddRNAi Platform Technology
The two patent families exclusively licensed from CSIRO include different aspects of the ddRNAi technology. The first of these patent families relates to DNA constructs and methods for using DNA to deliver RNA molecules, particularly shRNA, directed to the target gene. The granted U.S. patents in this family include claims to the structure and design of the DNA constructs, as well as human, animal and plant cells containing such DNA constructs and methods of using these constructs to reduce expression of a target gene. We expect any patents granted in this patent family to expire in March 2019.
The second patent family is an extension of the first patent family, and relates to chimeric DNA and methods for using DNA to deliver RNA molecules, particularly shRNA. The granted U.S. patent in this family claims DNA constructs and methods for reducing expression of a target gene in a plant as well as plant cells, subject matter that is not within our current field of use. We expect any patents granted in this patent family to expire in April 2019.
108
In December 2009, we entered into a commercial license arrangement with CSIRO for these two patent families relating to ddRNAi technology. This worldwide license in the field of human therapeutics is exclusive and irrevocable. In exchange for the license, we issued ordinary shares to CSIRO, and we are required to pay CSIRO approximately $300,000 in the event of corporate transactions such as a merger, sale, change of control, capital reconstruction or insolvency event relating to Benitec. Under the license agreement, following notice to us and receipt of our comments, if any, CSIRO has control over prosecution of patent applications and litigation, if any.
Two of the CSIRO patents in the first family are in European Patent Office opposition proceedings. The European Patent Office Opposition Board published decisions in these proceedings in July 2014 and July 2015.
In its July 2014 decision, the Board revoked the first of these two related European patents and, in its July 2015 decision, it revoked the second. CSIRO has appealed both decisions. The U.S. patent corresponding to the two currently revoked European patents was upheld, with modified claims, upon re-examination at the USPTO in 2011, but we cannot know whether the appeals regarding the revoked European patents, if undertaken and carried through, will be decided favorably for us. The Company believes that even if the two currently revoked European patents, which are scheduled to expire in 2019, are not restored upon appeal, such revocation should have no materially adverse effect upon our other owned and licensed patents and patent applications described below.
In the second patent family, one of the CSIRO patents is subject to opposition proceedings in the European Patent Office and one of the patent applications was subject to USPTO interference proceedings. In February 2015, the European Patent Office Opposition Board published its decision to uphold CSIRO’s European patent in the second family. The opponents have appealed this decision. In October 2012, the USPTO issued its decision to refuse to grant CSIRO’s claims involved in the interference. CSIRO appealed this decision to the United States Court of Appeals for the Federal Circuit which reversed the USPTO’s decision and granted the relevant claims to CSIRO. On remand, the Patent Trial and Appeal Board cancelled the other party’s involved claims. The other party had until the deadline of March 28, 2016 to petition the U.S. Supreme Court to review the Federal Circuit’s decision but did not file such an appeal.
Technology Improvements
We own two patent families that relate to improvements to ddRNAi technology. The first patent family relates to compositions of matter and methods for delivering shRNA molecules to animal cells for a variety of target genes. As of March 2016, this patent family included patents granted in the United Kingdom, Singapore and South Africa. We expect any patents granted in this patent family to expire in March 2021.
The second patent family relates to nucleic acid constructs and methods for using DNA to produce hairpin RNA molecules that can target multiple genes within one molecule. As of March 2016, this patent family included patents granted in Australia, New Zealand, Singapore and South Africa. We expect any patents granted in this patent family to expire in June 2024.
Targeting the Hepatitis Virus
We own two patents in the United States that relate to liver-specific promoters or enhancers. The first claims an expression cassette with a synthetic enhancer and a particular liver-specific promoter that may be used to express a variety of genes in liver. The second claims ddRNAi expression constructs that include a liver-specific promoter and one or more RNAi constructs that provide RNAi agents that target hepatitis virus genes. These granted U.S. patents provide options for promoter and construct design based on the target regions in the hepatitis gene of interest. The first of these two patents is expected to expire in February 2026, and the second is expected to expire in March 2027.
109
Our ddRNAi-based treatment for hepatitis B
Our patent portfolio related to our ddRNAi-based treatment for hepatitis B includes one patent family relating to single-stranded RNA and shRNA sequences to a range of target regions of the hepatitis B viral genome. This patent family was jointly filed with Biomics Biotech Co., Ltd., or Biomics, and Biomics subsequently assigned its ownership interests in the patent to us. As of March 2016, this patent family included a patent granted in the United States and pending applications in the United States, Europe, Australia, Brazil, Canada, China, India, Korea and Russia. We expect any patents granted in this patent family to have expired by October 2031.
This patent family is the outcome of a collaborative research agreement between us and Biomics which was commenced in August 2009. The arrangement provided for both parties to receive a share of any revenue generated from commercializing this patent family commensurate with our respective contributions to the intellectual property subject to the agreement, which contributions were equal. In July 2015, we entered into an earn-out agreement with Biomics pursuant to which we acquired all rights, title and interest in this patent family in exchange for upfront and milestone payments. At the time of signing the agreement, we paid Biomics A$2.5 million consisting of A$2.0 million in cash and 647,333 ordinary shares (having a value of A$500,000 at the time the agreement was entered into). These shares could not be traded until October 1, 2015 and thereafter Biomics may only sell up to A$100,000 in value of those shares in any calendar month. Upon licensing a patent in this patent family we will also pay Biomics 50% of the initial licensing revenue received by us up to a maximum of A$3.5 million and, in the event we receive licensing revenue greater than A$6.0 million, we would pay Biomics 1.5% of licensing revenue on any such additional amounts.
A U.S. priority document has been filed by us to claim composition of matter and methods of using a range of RNA molecules in the treatment of hepatitis B. This new patent filing claims the shRNA sequences that are under development as the lead candidates for BB-HB-331, and also include other target and RNA sequences to different regions of the HBV genome. This new patent application names Benitec as the sole applicant.
Our ddRNAi-based therapeutic candidates for AMD
Our patent portfolio for AMD includes one patent family relating to the target genes for AMD. This patent family relates to the target gene sequences for our two ddRNAi-based therapeutic candidates for AMD. As of March 2016, this patent family included pending applications in the United States, Europe, Australia, Canada, China, India, Israel, Mexico, Singapore, Japan, South Africa, Korea and Russia. We expect any patents to grant in this patent family to expire in January 2034.
TT-034—ddRNAi-based treatment for hepatitis C
Our patent portfolio related to our now discontinued product candidate TT-034 includes two patent families relating primarily to the shRNA sequences of TT-034 and the expression cassette design of the therapeutic. The first patent family has claims for methods and genetic constructs that use the shRNA sequences of TT-034 in the treatment of hepatitis C. The patent family relates to a range of different shRNA sequences, and includes the three candidate shRNA sequences incorporated in TT-034, as well as using ddRNAi to deliver the shRNA. The design of the expression cassette in this patent family is for three shRNA sequences with independent promoters driving the expression of each shRNA. As of March 2016, this patent family included patents granted in the United States (3), Europe, Australia, Canada, China, Israel, Japan and Korea. We expect any patents granted in this patent family to expire in March 2025.
The second patent family also includes claims for methods and genetic constructs using the shRNA sequences of TT-034 in the treatment of hepatitis C. The design of the expression in this patent family is for a single promoter to drive the expression of multiple shRNA sequences. As of March 2016, this patent family included patents granted in the United States (3), Australia, China, Europe, Canada, New Zealand and Hong Kong. We expect any patents granted in this patent family to expire in February 2026.
110
Tribetarna—our ddRNAi-based treatment for lung cancer
Our patent portfolio related to our lung cancer program, to-be-discontinued, includes one patent family relating to methods of increasing the sensitivity of a tumor cell to DNA damaging agents (cisplatin) or tubulin-binding agents (paclitaxel) using nucleic acid constructs encoding a shRNA or siRNA directed to regions of the TUBB2A, TUBB2C, or TUBB3 tubulin genes and conjugated to polyethylenimine. This patent family is licensed exclusively from the University of New South Wales. As of March 2016, this patent family included patents granted in Australia, China, Hong Kong, Israel, Japan and Singapore. We expect any patents granted in this patent family to expire in March 2028.
In August 2013, we entered into a commercial license arrangement with NewSouth Innovations Pty Limited, or NSi, of University of New South Wales for this patent portfolio. The license provides for modest up-front and ongoing license fees, and also milestone and single digit percentage royalty payments on net sales. A percentage of sub-licensing revenue is also payable to NSi. We may terminate the license at will, and in the event of certain breaches by NSi. NSi may terminate the license in the event of certain breaches by us.
We have filed a U.S. priority document to claim composition of matter and methods of using the shRNA sequences of Tribetarna in the treatment of lung cancer. This new provisional patent filing claims the expression cassette with the triple shRNA sequences of Tribetarna. This new patent application names us as the sole applicant.
Know-How
In addition to patent protection of ddRNAi technology and our product candidates, we also rely on proprietary know-how that is not patentable or that we elect not to patent, as valuable intellectual property for our business. This know-how is related to the areas of, among others, identifying nucleic acid targets for ddRNAi technology and designing ddRNAi constructs for targeting preferred genes. We have implemented a number of security measures to safeguard our know-how including limiting access to our research facilities, databases and networks. We also protect know-how by way of confidentiality agreements when engaging with external providers for progressing our pipeline of therapeutic candidates.
Laws and Regulations Regarding Patent Terms
The term of individual patents depends upon the legal terms of the patents in the countries in which they are obtained. In most countries in which we file, the patent term is 20 years from the earliest date of filing a non-provisional patent application. In the United States, a patent term may be shortened if a patent is terminally disclaimed over another patent or as a result of delays in patent prosecution by the patentee. A patent’s term may be lengthened by a patent term adjustment, which compensates a patentee for administrative delays by the USPTO in granting a patent. The patent term of a European patent is 20 years from its filing date, which, unlike in the United States, is not subject to patent term adjustments.
The term of a patent that covers an FDA-approved biologic may also be eligible for patent term extension, which permits patent term restoration as compensation for the patent term lost during the FDA regulatory review process. The Drug Price Competition and Patent Term Restoration Act of 1984, or the Hatch-Waxman Act, permits a patent term extension of up to five years beyond the expiration of the patent. The length of the patent term extension is related to the length of time the biologic is under clinical testing regulatory review. Patent extension cannot extend the remaining term of a patent beyond a total of 14 years from the date of product approval and only one patent applicable to an approved biologic may be extended. Similar provisions are available in Europe and other jurisdictions to extend the term of a patent that covers an approved biologic although the eligibility requirements for any duration of such extension vary. In the future, if and when our products receive FDA approval, we expect to apply for patent term extensions on patents covering those products.
111
Trademarks
Our trademarks include registrations for company branding and product names for our pipeline in development.
| | | | | | | | |
Trade Mark (program) | | Trade Mark
Number | | Filing date | | Jurisdiction | | Status |
BENITEC® | | 1103049 | | 10 Mar 2006 | | Australia | | Registered |
BENITEC™ | | NA (Appln: 86795296) | | 21 Oct 2015 | | United States | | Pending |
BENITEC™ | | 1728797 | | 16 Sep 2015 | | Australia | | Pending |
BENITEC™ | | 14680003 | | 16 Oct 2015 | | Europe | | Pending |
BENITEC BIOPHARMA® | | 1448046 | | 13 Sep 2011 | | Australia | | Registered |
BENITEC BIOPHARMA® | | 4636053 | | 11 Feb 2014 | | United States | | Registered |
SILENCING GENES FOR LIFE® | | 1448041 | | 13 Sep 2011 | | Australia | | Registered |
SILENCING GENES FOR LIFE® | | 4807242 | | 22 Dec 2014 | | United States | | Registered |
Tribetarna® (Lung cancer) | | 1526479 | | 19 Nov 2012 | | Australia | | Registered |
Hepbarna® (Hepatitis B) | | 1526483 | | 19 Nov 2012 | | Australia | | Registered |
Nervarna® (Pain) | | 1526478 | | 19 Nov 2012 | | Australia | | Registered |
Manufacturing
The manufacture of the complex biological products required for gene therapy is complex and difficult. We do not currently own or operate manufacturing facilities for the production of preclinical, clinical or commercial quantities of any of our product candidates. We are also exploring long-term manufacturing alliances with a number of potential partners to investigate manufacturing processes in order to produce materials at reasonable scale and cost of goods to support future commercialization efforts. We do not have a long-term agreement with any third-party manufacturer, but we plan to establish such a relationship with an appropriate manufacturer to serve our long-term needs.
Manufacturing is subject to extensive regulations that impose various procedural and documentation requirements, which govern record keeping, manufacturing processes and controls, personnel, quality control and quality assurance, among others. Our contract manufacturing organizations manufacture our product candidates under cGMP, conditions. cGMP is a regulatory standard for the production of pharmaceuticals that will be used in humans.
Sales and Marketing
We have not yet established sales, marketing or product distribution operations because our product candidates are in preclinical or clinical development. If we receive marketing and commercialization approval for any of our product candidates, we intend to market the product through strategic alliances and distribution agreements with third parties. In certain cases we may market an approved product directly worldwide or in selected geographical segments. The ultimate implementation of our strategy for realizing the financial value of our product candidates is dependent on the results of clinical trials for our product candidates, the availability of funds and the ability to negotiate acceptable commercial terms with third parties.
Competition
The biopharmaceutical industry is characterized by intense and dynamic competition to develop new technologies and proprietary therapies.
Any product candidates that we successfully develop and commercialize will have to compete with existing therapies and new therapies that may become available in the future. While we believe that our proprietary technology estate and scientific expertise in gene silencing using ddRNAi provide us with competitive advantages, we face potential competition from many different sources, including larger and better-funded
112
pharmaceutical, specialty pharmaceutical and biotechnology companies, as well as from academic institutions and governmental agencies and public and private research institutions that may develop potentially competitive products or technologies. We are aware of several companies focused on developing gene therapy or gene silencing product candidates, including:
| | • | | Alnylam, Arbutus and Arrowhead—developing siRNA-based therapeutics for hepatitis B; |
| | • | | Avalanche Biotechnologies, Inc., Applied Genetic Technologies Corporation and Oxford Biomedica—developing gene therapies for wet AMD |
We are not aware of any companies developing a gene therapy or gene silencing approach for OPMD. There are other therapies either being marketed or currently in development for all of these diseases, some of which already have significant market share. Our product candidates, if approved, would also compete with treatments that have already been approved and accepted by the medical community, patients and third-party payors.
Many of our competitors and potential competitors, alone or with their strategic partners, have substantially greater financial, technical and human resources than we do and significantly greater experience in the discovery and development of product candidates, obtaining FDA and other regulatory approvals of treatments and the commercialization of those treatments. Mergers and acquisitions in the biotechnology and pharmaceutical industries may result in even more resources being concentrated among a smaller number of our competitors.
Our commercial opportunity could be reduced or eliminated if our competitors develop and commercialize products that are safer, more effective, have fewer or less severe side effects, are more convenient or are less expensive than any products that we may develop. Our competitors also may obtain FDA or other regulatory approval for their products more rapidly than we may obtain approval for ours, which could result in our competitors establishing a strong market position before we are able to enter the market.
Mergers and acquisitions in the biotechnology and pharmaceutical industries may result in even more resources being concentrated among a smaller number of our competitors. These competitors also compete with us in recruiting and retaining qualified scientific and management personnel and establishing clinical study sites and subject registration for clinical studies, as well as in acquiring technologies complementary to, or necessary for, our programs. Smaller or early-stage companies may also prove to be significant competitors, particularly through collaborative arrangements with large and established companies.
We anticipate that we will face intense and increasing competition as new products enter the market and advanced technologies become available. We expect any treatments that we develop and commercialize to compete on the basis of, among other things, efficacy, safety, convenience of administration and delivery, price, the level of competition and the availability of reimbursement from government and other third-party payors.
Our commercial opportunity could be reduced or eliminated if our competitors develop and commercialize products that are safer, more effective, have fewer or less severe side effects, are more convenient or are less expensive than any products that we may develop. Our competitors also may obtain FDA or other regulatory approval for their products more rapidly than we may obtain approval for ours, which could result in our competitors establishing a strong market position before we are able to enter the market. In addition, we expect that our therapeutic products, if approved, will be priced at a significant premium over competitive products and our ability to compete may be affected in many cases by insurers or other third-party payors seeking to encourage the use of competitive products including biosimilar or generic products.
This increasingly competitive landscape may compromise the development of our product candidates. For example, improvements in the efficacy, delivery and success rates of competitors’ product candidates, in conjunction with a reduction in the price and duration of their treatments, diminished partnering interest from pharmaceutical companies in our product candidate TT-034 for the treatment of HCV. This caused us to announce in February 2016 the discontinuation of our program to develop TT-034 before the conclusion of its clinical trial, despite the promising clinical results regarding the safety of that product candidate achieved to date.
113
Government Regulation
As a pharmaceutical and biological product company that wishes to conduct clinical trials and ultimately obtain marketing approval in the United States, we are subject to extensive regulation by the FDA, and other federal, state, and local regulatory agencies. The Federal Food, Drug, and Cosmetic Act, or the FDC Act, the Public Health Service Act, or PHS Act, and their implementing regulations set forth, among other things, requirements for the research, testing, development, manufacture, quality control, safety, effectiveness, approval, labeling, storage, record keeping, reporting, distribution, import, export, advertising and promotion of our products. A failure to comply explicitly with any requirements during the product development, approval, or post-approval periods, may lead to administrative or judicial sanctions. These sanctions could include the imposition by the FDA or an IRB, of a suspension on clinical trials, refusal to approve pending marketing applications or supplements, withdrawal of approval, warning letters, product recalls, product seizures, total or partial suspension of production or distribution, injunctions, fines, civil penalties or criminal prosecution.
Although the discussion below focuses on regulation in the United States, we anticipate seeking approval for the testing and marketing of our products in other countries. Generally, our activities in other countries will be subject to regulation that is similar in nature and scope as that imposed in the United States, although there can be important differences. Additionally, some significant aspects of regulation in the European Union are addressed in a centralized way through the EMA, but country-specific regulation remains essential in many respects.
Government regulation may delay or prevent testing or marketing of our products and impose costly procedures upon our activities. The testing and approval process, and the subsequent compliance with appropriate statutes and regulations, requires substantial time, effort, and financial resources, and we cannot be certain that the FDA or any other regulatory agency will grant approvals for our products or any future products on a timely basis, if at all. The FDA’s or any other regulatory agency’s policies may change and additional governmental regulations may be enacted that could prevent or delay regulatory approval of our products or any future products or approval of new indications or label changes. We cannot predict the likelihood, nature or extent of adverse governmental regulation that might arise from future legislative, judicial, or administrative action, either in the United States or abroad.
Recent Developments in Regulation of Gene Therapy
Although the FDA has not yet approved any human gene therapy product for sale, it has provided guidance for the development of gene therapy products. For example, the FDA has established the Office of Cellular, Tissue and Gene Therapies within CBER, to consolidate the review of gene therapy and related products, and the Cellular, Tissue and Gene Therapies Advisory Committee to advise CBER on its reviews. In addition, the FDA has issued a growing body of clinical guidelines, chemical, manufacturing and control, or CMC, guidelines and other guidelines, all of which are intended to facilitate industry’s development of gene therapy products.
In 2012, the EMA approved a gene therapy product called Glybera, which is the first gene therapy product approved by regulatory authorities in the United States or the European Union.
Marketing Approval
In the United States, for premarket approval purposes, the FDA regulates gene products as biologics under the FDC Act, the PHS Act and related regulations.
The steps required before a new biologic may be marketed in the United States generally include:
| | • | | nonclinical pharmacology and toxicology laboratory and animal tests according to good laboratory practices, or GLPs, and applicable requirements for the humane use of laboratory animals or other applicable regulations; |
114
| | • | | submission of an IND application which must become effective before human clinical trials may begin; |
| | • | | adequate and well-controlled human clinical trials according to GCPs and any additional requirements for the protection of human research subjects and their health information to establish the safety and efficacy of the investigational product for each targeted indication; |
| | • | | submission of a biologics license application, or BLA, to the FDA; |
| | • | | FDA’s pre-approval inspection of manufacturing facilities to assess compliance with cGMPs and, if applicable, the FDA’s good tissue practices, or GTPs, for the use of human cellular and tissue products to prevent the introduction, transmission, or spread of communicable diseases; |
| | • | | FDA’s audit of clinical trial sites that generated data in support of the BLA; and |
| | • | | FDA approval of a BLA, which must occur before a product can be marketed or sold. |
Product Development Process
Before testing any biologic in humans, the product enters the nonclinical, or preclinical, testing stage. Nonclinical tests include laboratory evaluations of product chemistry, toxicity, and formulation, as well as animal studies to assess the potential safety and activity of the product. The conduct of nonclinical tests must comply with federal regulations and requirements including GLPs.
Where a gene therapy trial is conducted at, or sponsored by, institutions receiving NIH funding for recombinant DNA research, prior to the submission of an IND to the FDA, a protocol and related documentation is submitted to and the trial is registered with the NIH Office of Biotechnology Activities, or OBA, pursuant to the NIH Guidelines for Research Involving Recombinant or Synthetic Nucleic Acid Molecules, or NIH.
Guidelines. Compliance with the NIH Guidelines is mandatory for investigators at institutions receiving NIH funds for research involving recombinant DNA, however many companies and other institutions not otherwise subject to the NIH Guidelines voluntarily follow them. The NIH is responsible for convening the RAC, a federal advisory committee, which discusses protocols that raise novel or particularly important scientific, safety or ethical considerations at one of its quarterly public meetings. The OBA will notify the FDA of the RAC’s decision regarding the necessity for full public review of a gene therapy protocol. RAC proceedings and reports are posted to the OBA web site and may be accessed by the public.
The product sponsor then submits the results of the nonclinical testing, together with manufacturing information, analytical data, any available clinical data or literature, and a proposed clinical protocol, to the FDA in an IND, which is a request for authorization from the FDA to administer an investigational product to humans. Some nonclinical testing may continue even after the IND application is submitted. IND authorization is required before interstate shipping and administration of any new product to humans that is not the subject of an approved BLA. The IND automatically becomes effective 30 days after receipt by the FDA unless the FDA, within the30-day time period, raises concerns or questions about the conduct of the clinical trial and places the clinical trial on a clinical hold. In such case, the IND sponsor must resolve any outstanding concerns with the FDA before the clinical trial may begin. Further, an IRB for each site proposing to conduct the clinical trial must review and approve the plan for any clinical trial before it commences at that site. If the site has an IBC, it may also have to review and approve the proposed clinical trial. Clinical trials involve the administration of the investigational product to patients under the supervision of qualified investigators following GCPs, requirements meant to protect the rights and health of patients and to define the roles of clinical trial sponsors, investigators, and monitors. Clinical trials are conducted under protocols that detail, among other things, the objectives of the clinical trial, dosing procedures, subject selection and exclusion criteria, the parameters to be used in monitoring safety, including stopping rules that assure a clinical trial will be stopped if certain adverse events should occur, and the efficacy criteria to be evaluated. Each protocol involving testing on U.S. patients and subsequent protocol amendments must be submitted to the FDA as part of the IND. The informed written consent of each participating subject is required and the form and content of the informed consent must be approved by each IRB.
115
The clinical investigation of an investigational product is generally divided into three phases. Although the phases are usually conducted sequentially, they may overlap or be combined. The three phases of an investigation are as follows:
| | • | | Phase I includes the initial introduction of an investigational product into humans. Phase I clinical trials may be conducted in patients with the target disease or condition or on healthy volunteers. These studies are designed to evaluate the safety, metabolism, pharmacokinetics and pharmacologic actions of the investigational product in humans, the side effects associated with increasing doses, and if possible, to gain early evidence on effectiveness. During Phase I clinical trials, sufficient information about the investigational product’s pharmacokinetics and pharmacological effects may be obtained to permit the design of Phase II clinical trials. The total number of participants included in Phase I clinical trials varies, but is generally in the range of 20 to 80. |
| | • | | Phase II includes the controlled clinical trials conducted to evaluate the effectiveness of the investigational product for a particular indication(s) in patients with the disease or condition under study, to determine dosage tolerance and optimal dosage, and to identify possible adverse side effects and safety risks associated with the product. Phase II clinical trials are typically well-controlled, closely monitored, and conducted in a limited patient population, usually involving no more than several hundred participants. Phase IIa trials provide information on the impact of dose ranging on safety, biomarkers and proof of concept, while Phase IIb trials are patient dose-ranging efficacy trials. |
| | • | | Phase III clinical trials are controlled clinical trials conducted in an expanded patient population at geographically dispersed clinical trial sites. They are performed after preliminary evidence suggesting effectiveness of the investigational product has been obtained, and are intended to further evaluate dosage, clinical effectiveness and safety, to establish the overall benefit-risk relationship of the product, and to provide an adequate basis for product approval. Phase III clinical trials usually involve several hundred to several thousand participants. In most cases, the FDA requires two adequate and well controlled Phase III clinical trials to demonstrate the efficacy of the product. FDA may accept a single Phase III trial with other confirmatory evidence in rare instances where the trial is a large multicenter trial demonstrating internal consistency and a statistically very persuasive finding of a clinically meaningful effect on mortality, irreversible morbidity or prevention of a disease with a potentially serious outcome and confirmation of the result in a second trial would be practically or ethically impossible. |
Annual progress reports detailing the results of the clinical trials must be submitted to the FDA. Written IND safety reports must be promptly submitted to the FDA and the investigators for serious and unexpected adverse events; any findings from other studies, tests in laboratory animals orin vitro testing that suggest a significant risk for human subjects; or any clinically important increase in the rate of a serious suspected adverse reaction over that listed in the protocol or investigator brochure. The sponsor must submit an IND safety report within 15 calendar days after the sponsor determines that the information qualifies for reporting. The sponsor also must notify the FDA of any unexpected fatal or life-threatening suspected adverse reaction within seven calendar days after the sponsor’s initial receipt of the information. The FDA typically recommends that sponsors observe subjects for potential gene therapy-related delayed adverse events for a 15-year period, including a minimum of five years of annual examinations followed by 10 years of annual queries, either in person or by questionnaire, of trial subjects.
The decision to terminate a clinical trial of an investigational biologic may be made by the FDA or other regulatory authority, an IRB, an IBC, or institutional ethics committee, or by a company for various reasons. The FDA may place a clinical hold and order the temporary, or permanent, discontinuation of a clinical trial at any time, or impose other sanctions, if it believes that the clinical trial either is not being conducted in accordance with FDA requirements or presents an unacceptable risk to the clinical trial patients. If the FDA imposes a clinical hold, trials may not recommence without FDA and IRB authorization and then only under terms authorized by the FDA and IRB. In some cases, clinical trials are overseen by an independent group of qualified experts organized by the trial sponsor, or the clinical monitoring board or DSMB. This group provides authorization for whether or not a trial may move forward at designated check points. These decisions are based
116
on the limited access to data from the ongoing trial. The suspension or termination of a clinical trial can occur during any phase of clinical trials if it is determined that the participants or patients are being exposed to an unacceptable health risk. In addition, there are requirements for the registration of ongoing clinical trials of drugs and biologics on public registries and the disclosure of certain information pertaining to the trials as well as clinical trial results after completion.
Assuming successful completion of all required testing in accordance with all applicable regulatory requirements, detailed investigational product information is submitted to the FDA in the form of a BLA for a biologic to request marketing approval for the product in specified indications.
Human gene therapy products are a new category of therapeutics. Because this is a relatively new and expanding area of novel therapeutic interventions, there can be no assurance as to the length of the trial period, the number of patients the FDA will require to be enrolled in the trials in order to establish the safety, efficacy, purity and potency of human gene therapy products, or that the data generated in these trials will be acceptable to the FDA to support marketing approval. The NIH and the FDA have a publicly accessible database, the Genetic Modification Clinical Research Information System, which includes information on gene transfer trials and serves as an electronic tool to facilitate the reporting and analysis of adverse events on these trials. Over the last several years the FDA has issued helpful guidance on development of gene therapy products and shown a willingness to work closely with developers, especially with those working in orphan disease areas.
Biologics License Application Approval Process
In order to obtain approval to market a biologic in the United States, a BLA must be submitted to the FDA that provides data from nonclinical studies and clinical trials and manufacturing information establishing to the FDA’s satisfaction the safety, purity, and potency or efficacy of the investigational product for the proposed indication. The BLA must be accompanied by a substantial user fee payment unless a waiver or exemption applies.
The FDA will initially review the BLA for completeness before it accepts it for filing. Under the FDA’s procedures, the agency has 60 days from its receipt of a BLA to determine whether the application will be accepted for filing based on the agency’s threshold determination that the application is sufficiently complete to permit substantive review. After the BLA submission is accepted for filing, the FDA reviews the BLA to determine, among other things, whether the proposed product is safe, pure and potent, which includes determining whether it is effective for its intended use, and whether the product is being manufactured in accordance with cGMP, to assure and preserve the product’s identity, safety, strength, quality, potency and purity, and in accordance with biological product standards. The FDA will inspect the facilities at which the product is manufactured to ensure the manufacturing processes and facilities are in compliance with cGMP requirements and are adequate to assure consistent production of the product within required specifications. For a human cellular or tissue product, the FDA also will not approve the product if the manufacturer is not in compliance with the GTPs. These are FDA regulations that govern the methods used in, and the facilities and controls used for, the manufacture of human cells, tissues, and cellular and tissue based products, which are human cells or tissue intended for implantation, transplant, infusion, or transfer into a human recipient. The primary intent of the GTP requirements is to ensure that cell and tissue based products are manufactured in a manner designed to prevent the introduction, transmission, and spread of communicable disease. Additionally, before approving a BLA, the FDA may inspect one or more clinical sites to assure compliance with GCP.
If the FDA determines the application, manufacturing process or manufacturing facilities are not acceptable, it typically will outline the deficiencies and often will request additional testing or information, or corrective action for a manufacturing facility. This may significantly delay further review of the application. If the FDA finds that a clinical site did not conduct the clinical trial in accordance with GCP, the FDA may determine the data generated by the clinical site should be excluded from the primary efficacy analyses provided in the BLA. Additionally, notwithstanding the submission of any requested additional information, the FDA ultimately may decide that the application does not satisfy the regulatory criteria for approval.
117
The FDA may refer applications for novel products or products that present difficult questions of safety or efficacy to an advisory committee. The FDA also may determine a REMS is necessary to assure the safe use of the biologic, in which case the BLA sponsor must submit a proposed REMS. The REMS may include, but is not limited to, a Medication Guide, a communications plan, and other elements to assure safe use, such as restrictions on distribution, prescribing, and dispensing.
After the FDA completes its initial review of a BLA, it will either license, or approve, the product, or issue a complete response letter to communicate that it will not approve the BLA in its current form and to inform the sponsor of changes that the sponsor must make or additional clinical, nonclinical or manufacturing data that must be received before the FDA can approve the application, with no implication regarding the ultimate approvability of the application. If a complete response letter is issued, the sponsor may either resubmit the BLA, addressing all of the deficiencies identified in the letter, or withdraw the application.
The testing and approval process for both a drug and biologic requires substantial time, effort and financial resources and this process may take several years to complete. Data obtained from clinical trials are not always conclusive and may be susceptible to varying interpretations, which could delay, limit or prevent regulatory approval. The FDA may not grant approval on a timely basis, or at all. We may encounter difficulties or unanticipated costs in our efforts to secure necessary governmental approvals, which could delay or preclude us from marketing our products.
Orphan Drug Designation
Under the Orphan Drug Act, the FDA may grant orphan designation to a drug or biological product candidate intended to treat a rare disease or condition, which is generally a disease or condition that affects fewer than 200,000 individuals in the United States, or more than 200,000 individuals in the United States and for which there is no reasonable expectation that the cost of developing and making a drug or biological product available in the United States for this type of disease or condition will be recovered from sales of the product candidate. Orphan product designation must be requested before submitting a BLA. After the FDA grants orphan product designation, the identity of the therapeutic agent and its potential orphan use are disclosed publicly by the FDA. Orphan product designation does not convey any advantage in or shorten the duration of the regulatory review and approval process.
If a product candidate that has orphan designation subsequently receives the first FDA approval for the disease or condition for which it has such designation, the product is entitled to orphan product exclusivity, which means that the FDA may not approve any other applications to market the same drug or biological product for the same indication for seven years, except in limited circumstances, such as a showing of clinical superiority to the product with orphan exclusivity. Competitors, however, may receive approval of different products for the indication for which the orphan product has exclusivity or obtain approval for the same product but for a different indication for which the orphan product has exclusivity. Orphan product exclusivity also could block the approval of one of our products for seven years if a competitor obtains approval of the same biological product as defined by the FDA or if our product candidate is determined to be contained within the competitor’s product for the same indication or disease. If a drug or biological product designated as an orphan product receives marketing approval for an indication broader than what is designated, it may not be entitled to orphan product exclusivity. Orphan drug status in the European Union has similar, but not identical, benefits.
Expedited Development and Review Programs
The FDA has a fast track program that is intended to expedite or facilitate the process for reviewing new drugs and biological products that meet certain criteria. Specifically, new drugs and biological products are eligible for fast track designation if they are intended to treat a serious or life-threatening condition and demonstrate the potential to address unmet medical needs for the condition. Fast track designation applies to the combination of the product and the specific indication for which it is being studied. The sponsor of a new
118
biologic or drug may request the FDA to designate the biologic or drug as a fast track product at any time during the clinical development of the product. Unique to a fast track product, the FDA may consider for review sections of the marketing application on a rolling basis before the complete application is submitted, if the sponsor provides a schedule for the submission of the sections of the application, the FDA agrees to accept sections of the application and determines that the schedule is acceptable, and the sponsor pays any required user fees upon submission of the first section of the application.
Any product submitted to the FDA for marketing, including under a fast track program, may be eligible for other types of FDA programs intended to expedite development and review, such as priority review and accelerated approval. Any product is eligible for priority review if it has the potential to provide safe and effective therapy where no satisfactory alternative therapy exists or a significant improvement in the treatment, diagnosis or prevention of a disease compared to marketed products. The FDA will attempt to direct additional resources to the evaluation of an application for a new biological or drug product designated for priority review in an effort to facilitate the review. Additionally, a product may be eligible for accelerated approval. Biological or drug products studied for their safety and effectiveness in treating serious or life-threatening illnesses and that provide meaningful therapeutic benefit over existing treatments may receive accelerated approval, which means that they may be approved on the basis of adequate and well-controlled clinical trials establishing that the product has an effect on a surrogate endpoint that is reasonably likely to predict a clinical benefit, or on the basis of an effect on a clinical endpoint other than survival or irreversible morbidity. As a condition of approval, the FDA may require that a sponsor of a biological or drug product receiving accelerated approval perform adequate and well-controlled post-marketing clinical trials. In addition, the FDA currently requires as a condition for accelerated approval pre-approval of promotional materials, which could adversely impact the timing of the commercial launch of the product. Lastly, under the provisions of the new Food and Drug Administration Safety and Innovation Act, enacted in 2012, a sponsor can request designation of a product candidate as a “breakthrough therapy.” A breakthrough therapy is defined as a drug or biological that is intended, alone or in combination with one or more other drugs or biological, to treat a serious or life-threatening disease or condition, and preliminary clinical evidence indicates that the drug or biological may demonstrate substantial improvement over existing therapies on one or more clinically significant endpoints, such as substantial treatment effects observed early in clinical development. Drugs and biologicals designated as breakthrough therapies are also eligible for accelerated approval and receive the same benefits as drugs and biologicals with Fast Track designation. The FDA must take certain actions, such as holding timely meetings and providing advice, intended to expedite the development and review of an application for approval of a breakthrough therapy.
Fast Track designation, and priority review may expedite the product approval process, but do not change the standards for approval. Accelerated approval and breakthrough therapy designation do change the standards for product approval and thus may expedite the development and/or approval process.
FDA Additional Requirements
The FDA may require, or companies may pursue, additional clinical trials after a product is approved. These so-called Phase 4 clinical trials may be made a condition to be satisfied for continuing drug and biologic approval. The results of Phase 4 clinical trials can confirm the efficacy of a product candidate and can provide important safety information. In addition, the FDA has express statutory authority to require sponsors to conduct post-market studies to specifically address safety issues identified by the agency.
Even if a product candidate receives regulatory approval, the approval may be limited to specific disease states, patient populations and dosages, or might contain significant limitations on use in the form of warnings, precautions or contraindications, or in the form of an onerous REMS, restrictions on distribution, orpost-marketing study requirements. Further, even after regulatory approval is obtained, later discovery of previously unknown problems with a product may result in restrictions on the product or even complete withdrawal of the product from the market. In addition, we cannot predict what adverse governmental regulations may arise from future U.S. or foreign governmental action.
119
Medical device requirements
Our contemplated diagnostics, for use with certain of our therapeutic products, are regulated by FDA asin vitro diagnostic, or IVD, medical devices. Such IVD devices must comply with applicable FDA IVD-specific regulations as well as FDA regulations applicable more broadly to medical devices. These FDA regulations include requirements for registering establishments with FDA; listing IVD devices with FDA; reporting certain adverse events related to IVD devices to FDA; complying with the Quality System Regulation (current good manufacturing practices for devices); labeling IVD devices; and obtaining premarket approval or clearance prior to marketing IVD devices (unless exempt). There are also regulations covering the requirements for investigational devices and the conduct of clinical investigations of devices. Like drugs and biologics, failure to comply with applicable device/IVD requirements can result in legal or administrative enforcement actions against an IVD device firm, its officers or employees, and/or its products.
FDA Post-Approval Requirements
Any products manufactured or distributed by us or on our behalf pursuant to FDA approvals are subject to continuing regulation by the FDA, including requirements for record-keeping, reporting of adverse experiences with the biologic or drug, and submitting biological product deviation reports to notify the FDA of unanticipated changes in distributed products. Manufacturers are required to register their facilities with the FDA and certain state agencies, and are subject to periodic announced or unannounced inspections by the FDA and certain state agencies for compliance with cGMP requirements, which impose certain quality processes, manufacturing controls and documentation requirements upon us and our third-party manufacturers in order to ensure that the product is safe, has the identity and strength, and meets the quality, purity and potency characteristics that it purports to have. In November 2013, the Drug Quality and Security Act, or DQSA, became law and establishes requirements to facilitate the tracing of prescription drug and biological products through the pharmaceutical supply distribution chain. This law includes a number of new requirements that will be implemented over time and will require us to devote additional resources to satisfy these requirements, including serializing the product and using new technology and data storage to electronically trace the product from manufacturer to dispenser. If our products are not covered by the serialization and tracing requirements of the DQSA, they may be subject to state pedigree and traceability requirements. We cannot be certain that we or our present or future suppliers will be able to comply with the cGMP and other FDA regulatory requirements. If our present or future suppliers are not able to comply with these requirements, the FDA may halt our clinical trials, refuse to approve any BLA, force us to recall a product from distribution, shut down manufacturing operations or withdraw approval of the applicable BLA. Noncompliance with cGMP or other requirements can result in issuance of warning or untitled letters, civil and criminal penalties, seizures, and injunctive action.
The FDA and other federal and state agencies closely regulate the labeling, marketing and promotion of drugs and biologics. Government regulators, including the Department of Justice and the Office of the Inspector General of the Department of Health and Human Services, as well as state authorities, recently have increased their scrutiny of the promotion and marketing of drugs and biologics. While doctors are free to prescribe any product approved by the FDA for any use, a company can only make claims relating to safety and efficacy of a product that are consistent with FDA approval, and the company is allowed to market a product only for the particular use and treatment approved by the FDA. In addition, any claims we make for our products in advertising or promotion must, among other things, be appropriately balanced with important safety information and otherwise be adequately substantiated. Failure to comply with these requirements can result in adverse publicity, warning or untitled letters, corrective advertising, injunctions, potential civil and criminal penalties, criminal prosecution, and agreements with governmental agencies that materially restrict the manner in which a company promotes or distributes products.
Pediatric Research Equity Act
Under the Pediatric Research Equity Act, or PREA, as amended, a BLA or supplement must contain data to assess the safety and efficacy of the product for the claimed indications in all relevant pediatric subpopulations
120
and to support dosing and administration for each pediatric subpopulation for which the product is safe and effective. Manufacturers must submit a pediatric study plan to the IND not later than 60 days after the end-of-phase 2 meeting with the FDA; if there is no such meeting, before the initiation of any phase 3 studies or a combined phase 2 and phase 3 study; or if no such study will be conducted, no later than 210 days before a marketing application or supplement is submitted. The intent of PREA is to compel sponsors whose products have pediatric applicability to study those products in pediatric populations, rather than ignoring pediatric indications for adult indications that could be more economically desirable. The FDA may grant deferrals for submission of data or full or partial waivers. By its terms, PREA does not apply to any product for an indication for which orphan designation has been granted, unless the FDA issues regulations stating otherwise. Because the FDA has not issued any such regulations, submission of a pediatric assessment is not required for an application to market a product for an orphan-designated indication.
Patent Term Restoration and Marketing Exclusivity
Depending on the timing, duration and specifics of FDA marketing approval of our product candidates, some of our U.S. patents may be eligible for limited patent term extension under the Hatch-Waxman Amendments. The Hatch-Waxman Amendments permit a patent restoration term of up to five years as compensation for patent term lost during product development and the FDA regulatory review process. However, patent term restoration cannot extend the remaining term of a patent beyond a total of 14 years from the product’s approval date. The patent term restoration period is generally one-half the time between the effective date of an IND and the submission date of a BLA plus the time between the submission date of a BLA and the approval of that application. Only one patent applicable to an approved biological is eligible for the extension and the application for the extension must be submitted prior to the expiration of the patent and within sixty days of approval of the biological. The USPTO, in consultation with the FDA, reviews and approves the application for any patent term extension or restoration.
The Biologics Price Competition and Innovation Act of 2009, which was included within the Patient Protection and Affordable Care Act, created an abbreviated approval pathway for biological products shown to be similar to, or interchangeable with, an FDA-licensed reference biological product, and grants a reference biologic twelve years of exclusivity from the time of first licensure. Biosimilarity, which requires that there be no clinically meaningful differences between the biological product and the reference product in terms of safety, purity, and potency, can be shown through analytical studies, animal studies, and a clinical study or studies. Interchangeability requires that a product is biosimilar to the reference product and the product must demonstrate that it can be expected to produce the same clinical results as the reference product and, for products administered multiple times, the biologic and the reference biologic may be switched after one has been previously administered without increasing safety risks or risks of diminished efficacy relative to exclusive use of the reference biologic. However, complexities associated with the larger, and often more complex, structure of biological products, as well as the process by which such products are manufactured, pose significant hurdles to implementation that are still being worked out by the FDA.
Pediatric exclusivity is another type of exclusivity in the United States. Pediatric exclusivity, if granted, provides an additional six months of exclusivity to be attached to any existing exclusivity, e.g., twelve year exclusivity, or patent protection for a drug. This six month exclusivity, which runs from the end of other exclusivity protection or patent delay, may be granted based on the voluntary completion of a pediatric trial in accordance with an FDA-issued “Written Request” for such a trial.
Government Regulation Outside the United States
In addition to regulations in the United States, we will be subject to a variety of regulations in other jurisdictions governing, among other things, clinical trials and any commercial sales and distribution of our products.
121
Whether or not we obtain FDA approval for a product, we must obtain the requisite approvals from regulatory authorities in foreign countries prior to the commencement of clinical trials or marketing of the product in those countries. Certain countries outside of the United States have a similar process that requires the submission of a clinical trial application much like the IND prior to the commencement of human clinical trials. In the European Union, for example, a request for a clinical trial authorization, or CTA, must be submitted to each country’s national health authority and an independent ethics committee, much like the FDA and the IRB, respectively. Once the CTA is approved in accordance with a country’s requirements, clinical trial development may proceed.
The requirements and process governing the conduct of clinical trials, product approval or licensing, pricing and reimbursement vary from country to country. In all cases, the clinical trials are conducted in accordance with GCP and the applicable regulatory requirements and the ethical principles that have their origin in the Declaration of Helsinki.
To obtain regulatory approval of a biological product under European Union regulatory systems, we must submit a marketing authorization application. The application required in the European Union is similar to a BLA in the United States, with the exception of, among other things, country-specific document requirements. The European Union also provides opportunities for market exclusivity. For example, in the European Union, upon receiving marketing authorization, a new biological generally receives eight years of data exclusivity and an additional two years of market exclusivity. If granted, data exclusivity prevents regulatory authorities in the European Union from referencing the innovator’s data to assess a biosimilar application. During the additional two-year period of market exclusivity, a biosimilar marketing authorization can be submitted, and the innovator’s data may be referenced, but no biosimilar product can be marketed until the expiration of the market exclusivity. The innovator may obtain an additional one year of market exclusivity if the innovator obtains an additional authorization during the initial eight year period for one or more new indications that demonstrate significant clinical benefit over existing therapies. This data and market exclusivity regime in the European Union of a total of 10 or 11 years protects against generic competition, but does not protect against the launch of a competing product if the competitor, rather than referencing the clinical data of the originator, has conducted its own clinical trials to support its marketing authorization application.
Orphan drugs in the European Union are eligible for 10-year market exclusivity. This 10-year market exclusivity may be reduced to six years if, at the end of the fifth year, it is established that the product no longer meets the criteria for orphan designation, for example, if the product is sufficiently profitable not to justify maintenance of market exclusivity. Additionally, marketing authorization may be granted to a similar product for the same indication at any time if:
| | • | | the second applicant can establish that its product, although similar, is safer, more effective or otherwise clinically superior; |
| | • | | the applicant consents to a second orphan medicinal product application; or |
| | • | | the applicant cannot supply enough orphan medicinal product. |
If we fail to comply with applicable foreign regulatory requirements, we may be subject to, among other things, fines, suspension or withdrawal of regulatory approvals, product recalls, seizure of products, operating restrictions and criminal prosecution.
Pharmaceutical Coverage, Pricing and Reimbursement
Sales of our products, when and if approved for marketing, will depend, in part, on the extent to which our products will be covered by third-party payors, such as federal, state, and foreign government healthcare programs, commercial insurance and managed healthcare organizations. These third-party payors are increasingly reducing reimbursements for medical products, biologicals, drugs and services. In addition, the U.S.
122
government, state legislatures and foreign governments have continued implementing cost containment programs, including price controls, restrictions on coverage and reimbursement and requirements for substitution of interchangeable products. Adoption of price controls and cost containment measures, and adoption of more restrictive policies in jurisdictions with existing controls and measures, could further limit our net revenue and results. Decreases in third-party reimbursement for our product candidates or a decision by a third-party payor not to cover our product candidates could reduce physician usage of our products once approved and have a material adverse effect on our sales, results of operations and financial condition.
The containment of healthcare costs has become a priority of federal, state and foreign governments. Third-party payors are increasingly challenging the prices charged for drug products and medical services, examining the medical necessity and reviewing the cost effectiveness of drug products and medical services, in addition to questioning safety and efficacy. If these third-party payors do not consider our products to be cost-effective compared to other available therapies, they may not cover our products after FDA approval or, if they do, the level of payment may not be sufficient to allow us to sell our products at a profit.
In the United States and some foreign jurisdictions, there have been, and likely will continue to be, a number of legislative and regulatory changes and proposed changes regarding the healthcare system directed at broadening the availability of healthcare and containing or lowering the cost of healthcare. In March 2010, the Patient Protection and Affordable Care Act, as amended by the Health Care Education Reconciliation Act, or the ACA, was enacted. The ACA includes measures that have significantly changed, and are expected to continue to significantly change, the way healthcare is financed by both governmental and private insurers. Other legislative changes have been proposed and adopted in the United States since the ACA was enacted. In August 2011, the Budget Control Act of 2011, among other things, created measures for spending reductions by Congress. A Joint Select Committee on Deficit Reduction, tasked with recommending a targeted deficit reduction of at least $1.2 trillion for the years 2013 through 2021, was unable to reach required goals, thereby triggering the legislation’s automatic reduction to several government programs. This includes aggregate reductions of Medicare payments to providers up to 2% per fiscal year, which went into effect in April 2013 and will remain in effect through 2024 unless additional Congressional action is taken. In January 2013, President Obama signed into law the American Taxpayer Relief Act of 2012, which, among other things, further reduced Medicare payments to several providers, including hospitals, imaging centers and cancer treatment centers, and increased the statute of limitations period for the government to recover overpayments to providers from three to five years.
Other Healthcare Laws
Although we currently do not have any products on the market, we may be subject to additional healthcare regulation and enforcement by the federal government and by authorities in the states and other countries in which we conduct our business. Such laws include, without limitation, state and federal anti-kickback, fraud and abuse, false claims, privacy and security and physician sunshine and open payment laws and regulations, many of which may become more applicable to us if our product candidates are approved and we begin commercialization. If our operations are found to be in violation of any of such laws or any other governmental regulations that apply to us, we may be subject to penalties, including, without limitation, administrative, civil and criminal penalties, damages, fines, disgorgement, the curtailment or restructuring of our operations, exclusion from participation in federal and state healthcare programs and imprisonment, any of which could adversely affect our ability to operate our business and our financial results.
Facilities
Our corporate headquarters are located in Sydney, Australia and consist of approximately 1,700 square feet of leased office space under a lease that expires in August 2016. Our research and development facility is located in Hayward, California, and consists of approximately 4,750 square feet of leased office space under a lease that expires in May 2018.
We believe that our existing facilities are adequate for our current needs.
123
Employees
As of December 31, 2015, we had 20 full-time employees, 12 of whom have a PhD or other post-graduate degrees. Of these full-time employees, 14 are engaged in research and development activities and 6 are engaged in finance, legal, human resources, facilities and general management. Our employees are located in Sydney, Australia and at our research and development facility located in Hayward, California.
Legal Proceedings
We are not currently a party to any material legal proceedings. From time to time, we may be subject to various legal proceedings and claims that arise in the ordinary course of our business activities. Although the results of litigation and claims cannot be predicted with certainty, any such future litigation could have an adverse impact on us because of defense and settlement costs, diversion of management resources and other factors.
124
MANAGEMENT
Directors and Executive Officers
The following table sets forth information covering our current directors and executive officers.
| | |
Name | | Position |
Greg West | | Chief Financial Officer, Company Secretary and Interim Chief Executive Officer |
Peter Francis | | Chairman of the Board of Directors |
David Suhy | | Chief Scientific Officer |
Carl Stubbings | | Chief Business Officer |
Georgina Kilfoil | | Chief Clinical Officer |
John Chiplin | | Director |
Iain Ross | | Director |
J. Kevin Buchi | | Director |
Greg Westhas been our Chief Financial Officer and Company Secretary since May 2011, and since December 2015 he has also been serving as our Interim Chief Executive Officer. From May 2010 to January 2011, he was Chief Financial Officer at Immune Systems Therapeutics, Ltd., a medical diagnostic company. Mr. West is a Chartered Accountant. He is a Director and Audit Committee Chairman of each of UOWE Limited (a business arm of Wollongong University) and IDP Education Pty Ltd and Education Australia Limited. He worked at PricewaterhouseCoopers and has held finance executive roles with financial institutions, including BT Financial Group, Deutsche Bank AG and IAG New Zealand Limited.
Peter Francishas been Chairman of our Board since February 2006. Since 1993, Mr. Francis has been a partner at Francis Abourizk Lightowlers, a firm of commercial and technology lawyers with offices in Melbourne, Australia. He is a legal specialist in the areas of intellectual property and licensing and provides legal advice to corporations and research bodies. Mr. Francis completed his studies in law and jurisprudence at Monash University.
Dr. David Suhywas appointed as our Chief Scientific Officer in April 2015, prior to which he served as our Senior Vice President, Research & Development since October 2012. In April 2008, he joined Tacere Therapeutics, Inc. as Director of Research & Development and continued in that role until our acquisition of Tacere in October 2012. Previous roles include Associate Director of R&D at Clontech Laboratories, Inc. and Principal Scientist at Antara Biosciences Inc. He also led the Target Validation Group at PPD Discovery, a company with proprietary technology surrounding the use of genetic suppressor elements to identify “druggable” genomic targets for large pharmaceutical companies.
Carl Stubbingshas been our Chief Business Officer since July 2012. Prior to joining our company, Mr. Stubbings was Vice President of Sales & Marketing for Focus Diagnostics, Inc., a subsidiary of Quest Diagnostics Inc. from February 2008 to June 2012.
Georgina Kilfoilwas appointed as our Chief Clinical Officer in April 2015 prior to which she served as Vice President, Clinical Operations since February 2015. Ms. Kilfoil initially provided services to Benitec in October 2014 in the role of Senior Drug Development Consultant. Prior to joining Benitec, Ms. Kilfoil was a business consultant from March 2013 to September 2014 and prior to that was Senior Vice President, Management and Development at Anthera Pharmaceuticals, Inc. from March 2010 to February 2013. Previous to this, Ms. Kilfoil served as Project Management Consultant at InClin, Inc. and Vice President of Project Management at Peninsula Pharmaceuticals, Inc. Ms. Kilfoil is a certified Project Management Professional, has a Bachelor of Science Degree in Pharmacology from the University of Bristol, United Kingdom, and a Masters of Business Administration from the Australian Graduate School of Management, Sydney, Australia.
125
Dr. John Chiplinhas been a Director since February 2010. Dr. Chiplin is a founder of and has served as a Managing Director of investment company, Newstar Ventures Ltd., since 1998. More recently, he has served as a director of Medistem, Inc. through its acquisition by Intrexon Corporation in 2014, as founding Chief Executive Officer of Arana Therapeutics Limited from 2006 through its acquisition by Cephalon, Inc. in 2009, as director of Domantis Ltd through its acquisition by GlaxoSmithKline plc in 2006, and as Managing Director of ITI Life Sciences Fund from 2003 to 2005. Dr. Chiplin currently serves on the board of directors of Adalta Pty Ltd, ScienceMedia Inc., Prophecy Inc., Batu Biologics Inc., The Coma Research Institute and Cynata Therapeutics Limited which is traded on the ASX. Dr. Chiplin’s Pharmacy and PhD degrees are from the University of Nottingham, Nottingham, United Kingdom.
Iain Rosshas been a Director since June 2010. Following a career with Sandoz, Fisons, Hoffman La Roche and Celltech, Mr. Ross is a Qualified Chartered Director in the United Kingdom and currently he is Chairman of the Board of Premier Veterinary Group plc, formerly Ark Therapeutics Group plc which is traded on the London Stock Exchange, and Biomer Technology Limited; and a non-executive director of Amarantus Bioscience Inc which is traded on the OTCQB, and Tissue Therapies Limited and Anatara Lifesciences Limited, which are both traded on the ASX. Mr. Ross served as the Executive Chairman for Ark Therapeutics Group plc from September 2010 to March 2013. He is also Vice Chairman of the Council of Royal Holloway, University of London.
J. Kevin Buchihas been a Director since April 2013. Since August 2013, he has served as Chief Executive Officer of TetraLogic Pharmaceuticals Corporation. Mr. Buchi served as Chief Executive Officer of Cephalon, Inc., or Cephalon, from December 2010 through its acquisition by Teva Pharmaceutical Industries Ltd., or Teva Pharmaceuticals, in October 2011. After the acquisition Mr. Buchi served as Corporate Vice President, Global Branded Products of Teva Pharmaceuticals. Mr. Buchi joined Cephalon in 1991 and held various positions, including Chief Operating Officer, from January 2010 to December 2010, Chief Financial Officer and Head of Business Development prior to being appointed Chief Executive Officer. Mr. Buchi currently serves as President and Chief Executive Officer and a member of the Board of Directors of TetraLogic Pharmaceuticals Corp. Mr. Buchi is also on the Board of Directors of Stemline Therapeutics, Inc., Forward Pharma A/S, Alexza Pharmaceuticals, Inc. and Epirus Biopharmaceuticals Inc. Mr. Buchi has a B.A. in chemistry from Cornell University and a Masters in Management from Kellogg Graduate School of Management at Northwestern University. He is also a Certified Public Accountant.
There are no family relationships among any of our directors or executive officers. The business addresses for each of our directors and executive officers is Benitec Biopharma Limited, F6/1-15 Barr Street, Balmain, New South Wales, Australia.
Board of Directors
Our board of directors currently consists of five members, including our interim Chief Executive Officer. Our directors are elected at each annual general meeting of our shareholders and serve until their successors are elected or appointed, unless their office is earlier vacated. We believe that each of our directors has relevant industry experience. The membership of our board of directors is directed by the following requirements:
| | • | | our Constitution specifies that there must be a minimum of three directors and a maximum of 10, and our board of directors may determine the number of directors within those limits; |
| | • | | as set forth in our Board Charter, the membership of the board of directors should consist of a majority of independent directors who satisfy the criteria recommended by the ASX Corporate Governance Principles and Recommendations; |
| | • | | the Chairman of our Board should be an independent director who satisfies the criteria for independence recommended by the ASX Corporate Governance Principles and Recommendations; and |
| | • | | our board of directors should, collectively, have the appropriate level of personal qualities, skills, experience, and time commitment to properly fulfill its responsibilities or have ready access to such skills where they are not available. |
126
Our board of directors has delegated responsibility for the conduct of our businesses to the Chief Executive Officer, but remains responsible for overseeing the performance of management. Our board of directors has established delegated limits of authority, which define the matters that are delegated to management and those that require board of directors approval. Under the Corporations Act, at least two of our directors must be resident Australians. None of our directors have any service contracts with Benitec that provide for benefits upon termination of employment.
Committees
To assist our board of directors with the effective discharge of its duties, it has established a Remuneration and Nomination Committee and an Audit and Risk Committee, which committees operate under a specific charter approved by our board of directors.
Remuneration and Nomination Committee. The members of our Remuneration and Nomination Committee are Peter Francis, Kevin Buchi, John Chiplin and Iain Ross, each of whom our board of directors has determined meets the criteria for independence under Nasdaq Listing Rule 5605(a)(2). Dr. John Chiplin acts as chairman of the committee. The committee’s role involves:
| | • | | identifying, evaluating and recommending qualified nominees to serve on our board of directors; |
| | • | | developing and overseeing our internal corporate governance processes; |
| | • | | maintaining a management succession plan; |
| | • | | evaluating, adopting and administering our compensation plans and similar programs advisable for us, as well as modifying or terminating existing plans and programs; |
| | • | | establishing policies with respect to equity compensation arrangements; and |
| | • | | overseeing, reviewing and reporting on various remuneration matters to our board of directors. |
Audit and Risk Committee. The members of our Audit and Risk Committee are Peter Francis, Kevin Buchi, and Iain Ross. Our board of directors has determined that Kevin Buchi and Iain Ross meet the criteria for independence of audit committee members set forth in Rule 10A-3(b)(1) under the Securities Exchange Act of 1934, as amended, or the Exchange Act, and the applicable rules of The NASDAQ Capital Market and that Peter Francis will meet both such independence criteria in one year from August 17, 2015, the effective date of the registration statement for our initial public offering in the United States, pursuant to the requirements of the rules of The NASDAQ Capital Market and Rule 10A-3(b)(1)(iv)(2) under the Exchange Act. Each member of our audit committee meets the financial literacy requirements of the listing standards of The NASDAQ Capital Market. Iain Ross acts as the chairman of the audit committee and our board of directors has determined that Iain Ross is an audit committee “financial expert,” as defined by Item 407(d) of Regulation S-K under the Securities Act. The principal duties and responsibilities of our audit committee include, among other things:
| | • | | overseeing and reporting on various auditing and accounting matters to our board of directors, including the selection of our independent accountants, the scope of our annual audits, fees to be paid to the independent accountants, the performance of our independent accountants and our accounting practices; |
| | • | | overseeing and reporting on various risk management matters to our board of directors. |
| | • | | considering and approving or disapproving all related-party transactions; |
| | • | | reviewing our annual and semi-annual financial statements and reports and discussing the statements and reports with our independent registered public accounting firm and management; |
| | • | | reviewing and pre-approving the engagement of our independent registered public accounting firm to perform audit services and any permissible non-audit services; |
127
| | • | | evaluating the performance of our independent registered public accounting firm and deciding whether to retain their services; and |
| | • | | establishing procedures for the receipt, retention and treatment of complaints received by us regarding financial controls, accounting or auditing matters. |
Code of Conduct
We have established a Code of Conduct, which sets out the standards of behavior that apply to every aspect of our dealings and relationships, both within and outside Benitec. The following standards of behavior apply to all directors, executive officers and employees of Benitec:
| | • | | comply with all laws that govern us and our operations; |
| | • | | act honestly and with integrity and fairness in all dealings with others and each other; |
| | • | | avoid or manage conflicts of interest; |
| | • | | use our assets responsibly and in the best interests of Benitec; and |
| | • | | be responsible and accountable for our actions. |
The Code of Conduct is available on our website at www.benitec.com.
Remuneration
The remuneration policy of Benitec is to align director and executive objectives with shareholder and business objectives by providing a fixed remuneration component and typically offering long-term incentives based on key performance areas. Our board of directors believes the remuneration policy to be appropriate and effective in its ability to attract and retain the best executives and directors to run and manage the consolidated entity, as well as create goal congruence between directors, executives, and shareholders.
Our board of directors is responsible for determining the appropriate remuneration package for our Chief Executive Officer, and our Chief Executive Officer is in turn responsible for determining the appropriate remuneration packages for senior management.
Executives typically receive a base salary based on factors such as experience and comparable industry information, options and performance incentives. Our board of directors reviews our Chief Executive Officer’s remuneration package, and our Chief Executive Officer reviews the other senior executives’ remuneration packages, annually by reference to the consolidated entity’s performance, executive performance, and comparable information within the industry.
The performance of executives is measured against criteria agreed annually with each executive and is based predominantly on the overall success of Benitec in achieving its broader corporate goals. Bonuses and incentives are linked to predetermined performance criteria. Our board of directors may, however, exercise its discretion in relation to approving incentives, bonuses, and options, and can recommend changes to our Chief Executive Officer’s recommendations. The policy is designed to attract the highest caliber of executives and reward them for performance that results in long-term growth in shareholder wealth.
Executives may be invited to participate in the employee share option plan.
Australian executives or directors receive a superannuation guarantee contribution required by the government and do not receive any other retirement benefits.
All remuneration paid to directors and executives is valued at the cost to us and expensed. Options are valued using the Black-Scholes methodology. The board of directors’ policy is to remunerate non-executive
128
directors at market rates for comparable companies for time, commitment, and responsibilities. Our board of directors as a whole determines payments to the non-executive directors and reviews their remuneration annually, based on market practice, duties and accountability. The maximum aggregate amount of fees that can be paid to non-executive directors is subject to approval by shareholders at our annual general meeting. Fees for non-executive directors are not linked to the performance of the consolidated entity. However, to align directors’ interests with shareholder interests, the directors are encouraged to hold our shares.
Performance Based Remuneration
Each executive’s remuneration package typically has a performance-based component. The intention of this approach is to facilitate goal congruence between executives with the business and shareholders. Generally, the executive’s performance based remuneration is tied to Benitec’s successful achievement of certain key milestones relating to its operating activities, as well as Benitec’s overall financial position.
The remuneration policy has been tailored to align the goals of shareholders, directors, and executives. Two methods are applied in achieving this aim, the first being a performance-based bonus linked to achievement of key corporate milestones, and the second being the issuance of options to the majority of directors and executives to encourage the alignment of personal and shareholder interests.
Details of Remuneration for fiscal 2015
| | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | Short Term Benefits | | | Post-
Employment
Benefits | | | Long
Term
Benefits | | | Share-
Based
Payments | | | | |
2015 | | Cash
Salary
and Fees
A$ | | | Cash
Bonus
A$ | | | Non-
Monetary
A$ | | | Super
Annuation
A$ | | | Employee
Leave
A$ | | | Options
A$ | | | Total
A$ | |
Non-Executive Directors: | | | | | | | | | | | | | | | | | | | | |
Peter Francis | | | 113,328 | | | | — | | | | — | | | | — | | | | — | | | | — | | | | 113,328 | |
Kevin Buchi | | | 56,000 | �� | | | — | | | | — | | | | — | | | | — | | | | 64,783 | | | | 120,783 | |
John Chiplin | | | 56,000 | | | | — | | | | — | | | | — | | | | — | | | | — | | | | 56,000 | |
Iain Ross | | | 62,000 | | | | — | | | | — | | | | — | | | | — | | | | — | | | | 62,000 | |
| | | | | |
Executive Officers: | | | | | | | | | | | | | | | | | | | | |
Peter French* | | | 400,000 | | | | — | | | | — | | | | 18,783 | | | | — | | | | 90,847 | | | | 509,630 | |
Greg West | | | 230,000 | | | | — | | | | — | | | | 18,783 | | | | — | | | | 220,622 | | | | 469,405 | |
David Suhy | | | 298,936 | | | | — | | | | — | | | | — | | | | — | | | | 224,361 | | | | 523,297 | |
Carl Stubbings | | | 275,000 | | | | — | | | | — | | | | 18,783 | | | | — | | | | 152,718 | | | | 446,501 | |
Georgina Kilfoil | | | 83,333 | | | | — | | | | — | | | | 7,826 | | | | — | | | | 185,077 | | | | 276,236 | |
Michael Graham** | | | 161,250 | | | | — | | | | — | | | | 32,178 | | | | — | | | | 97,715 | | | | 291,143 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| | | 1,735,847 | | | | — | | | | — | | | | 96,353 | | | | — | | | | 1,036,123 | | | | 2,868,323 | |
| | | | | | | | | | | | | | | | | | | | | | | | | | | | |
| * | | No longer an executive officer since December 9, 2015 |
| ** | | No longer an executive officer since March 31, 2015 |
129
The proportion of remuneration at risk and the fixed proportion are as follows:
| | | | | | | | | | | | | | | | | | | | | | | | |
| | | Fixed
remuneration | | | At risk – STI
(bonus) | | | At risk – LTI
(options) | |
Name | | 2015 | | | 2014 | | | 2015 | | | 2014 | | | 2015 | | | 2014 | |
Non-Executive Directors: | | | | | | | | | | | | |
Peter Francis | | | 100 | % | | | 80 | % | | | — | | | | — | | | | — | | | | 20 | % |
Kevin Buchi | | | 46 | % | | | 34 | % | | | — | | | | — | | | | 54 | % | | | 66 | % |
John Chiplin | | | 100 | % | | | 88 | % | | | — | | | | — | | | | — | | | | 12 | % |
Iain Ross | | | 100 | % | | | 89 | % | | | — | | | | — | | | | — | | | | 11 | % |
Mel Bridges | | | — | | | | 89 | % | | | — | | | | — | | | | — | | | | 11 | % |
| | | |
Executive Officers: | | | | | | | | | | | | |
Peter French* | | | 82 | % | | | 54 | % | | | — | | | | 25 | % | | | 18 | % | | | 21 | % |
Greg West | | | 53 | % | | | 78 | % | | | — | | | | 17 | % | | | 47 | % | | | 5 | % |
David Suhy | | | 57 | % | | | 66 | % | | | — | | | | 26 | % | | | 43 | % | | | 8 | % |
Carl Stubbings | | | 66 | % | | | 79 | % | | | — | | | | 15 | % | | | 34 | % | | | 6 | % |
Georgina Kilfoil | | | 33 | % | | | — | | | | — | | | | — | | | | 67 | % | | | — | |
Michael Graham** | | | 66 | % | | | 84 | % | | | — | | | | 12 | % | | | 34 | % | | | 4 | % |
| * | | No longer an executive officer since December 9, 2015 |
| ** | | No longer an executive officer since March 31, 2015 |
The proportion of the cash bonus paid/payable or forfeited is as follows:
| | | | | | | | | | | | | | | | |
| | | Cash bonus paid/payable | | | Cash bonus forfeited | |
Name | | 2015 | | | 2014 | | | 2015 | | | 2014 | |
Executive Officers: | | | | | | | | | | | | | | | | |
Peter French* | | | — | % | | | 100 | % | | | — | % | | | — | % |
Greg West | | | — | % | | | 100 | % | | | — | % | | | — | % |
David Suhy | | | — | % | | | 100 | % | | | — | % | | | — | % |
Carl Stubbings | | | — | % | | | 100 | % | | | — | % | | | — | % |
Michael Graham** | | | — | % | | | 100 | % | | | — | % | | | — | % |
| * | | No longer an executive officer since December 9, 2015 |
| ** | | No longer an executive officer since March 31, 2015 |
130
There were no shares issued to directors and executive officers as part of compensation during the year ended June 30, 2015. The terms and conditions of each grant of options over ordinary shares affecting remuneration of directors and executive officers in this financial year or future reporting years is as follows:
| | | | | | | | | | | | | | | | |
Grant date | | No. granted | | | Expiry date | | | Exercise price | | | Fair value per
option at
grant date | |
12/17/2014 | | | 2,300,000 | | | | 12/17/2019 | | | A$ | 1.250 | | | $ | 0.563 | |
05/06/2015 | | | 300,000 | | | | 05/06/2020 | | | A$ | 1.250 | | | $ | 0.529 | |
Details of options over ordinary shares granted, vested and lapsed for directors and executive officers as part of compensation during the year ended June 30, 2015 are set out below:
| | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | | |
Name of executive officer | | Number of
options
granted | | | Grant date | | | Value per
option at
grant
date
(A$) | | | Value of
options at
grant date
(A$) | | | Number
vested | | | Exercise
price
(A$) | | | Vested
and first
exercise
date | | | Last exercise
date | |
Greg West | | | 600,000 | | | | 12/17/2014 | | | | 0.56 | | | | 337,800 | | | | 200,000 | | | A$ | 1.25 | | | | 12/17/2014 | | | | 12/17/2019 | |
David Suhy | | | 600,000 | | | | 12/17/2014 | | | | 0.56 | | | | 337,800 | | | | 200,000 | | | A$ | 1.25 | | | | 12/17/2014 | | | | 12/17/2019 | |
Carl Stubbings | | | 400,000 | | | | 12/17/2014 | | | | 0.56 | | | | 225,200 | | | | 133,333 | | | A$ | 1.25 | | | | 12/17/2014 | | | | 12/17/2019 | |
Georgina Kilfoil | | | 300,000 | | | | 12/17/2014 | | | | 0.56 | | | | 168,900 | | | | 100,000 | | | A$ | 1.25 | | | | 17/12/2014 | | | | 17/12/2019 | |
Michael Graham** | | | 400,000 | | | | 12/17/2014 | | | | 0.56 | | | | 225,200 | | | | 133,333 | | | A$ | 1.25 | | | | 17/12/2014 | | | | 17/12/2019 | |
Georgina Kilfoil | | | 300,000 | | | | 05/06/2015 | | | | 0.53 | | | | 158,610 | | | | 100,000 | | | A$ | 1.25 | | | | 06/05/2015 | | | | 06/05/2020 | |
| ** | | No longer an executive officer since March 31, 2015 |
Employment Agreements with Executive Officers
The key provisions of the employment agreements (other than remuneration) are set out below for each of our executive officers. None of these employment agreements have termination dates.
| | | | | | |
Name of executive officer | | Title of executive officer | | Date employment
agreement commenced | | Notice period required to
terminate without cause by either
party |
Greg West | | Chief Financial Officer, Company Secretary and Chief Executive Officer | | August 23, 2011 | | Three months |
| | | |
Carl Stubbings | | Chief Business Officer | | May 28, 2012 | | Three months |
| | | |
David Suhy | | Chief Scientific Officer | | August 28, 2012 | | At will |
| | | |
Georgina Kilfoil | | Chief Clinical Officer | | September 19, 2014 | | Three months |
131
Additional Disclosures Relating to Key Management Personnel
The number of shares in Benitec held during the financial year ended June 30, 2015 by each director and executive officer, including their personally related parties, is set out below:
| | | | | | | | | | | | | | | | | | | | |
| | | Balance at
the start of
the year | | | Received as
part of
remuneration | | | Exercise of
options ** | | | Disposables /
other | | | Balance at
the end of
the year | |
Ordinary shares | | | | | | | | | | | | | | | | | | | | |
Peter Francis | | | 327,250 | | | | — | | | | 96,924 | | | | — | | | | 424,174 | |
Peter French* | | | 342,554 | | | | — | | | | 249,231 | | | | — | | | | 591,785 | |
Kevin Buchi | | | 615,385 | | | | — | | | | 246,154 | | | | — | | | | 861,539 | |
John Chiplin | | | 263,020 | | | | — | | | | — | | | | (63,020 | ) | | | 200,000 | |
Iaian Ross | | | 66,364 | | | | — | | | | — | | | | — | | | | 66,364 | |
Mel Bridges* | | | 391,744 | | | | — | | | | — | | | | (391,744 | ) | | | — | |
Carl Stubbings | | | 124,479 | | | | — | | | | 12,308 | | | | — | | | | 136,787 | |
Michael Graham* | | | 47,448 | | | | — | | | | — | | | | (47,448 | ) | | | — | |
| | | | | | | | | | | | | | | | | | | | |
| | | 2,178,244 | | | | — | | | | 604,617 | | | | (502,212 | ) | | | 2,280,649 | |
| | | | | | | | | | | | | | | | | | | | |
| * | | other—where relevant, may represent the option holding of the individual at the time of cessation of being classified as an executive officer of the consolidated entity. |
| ** | | options exercised relate to those either granted as remuneration or purchased as part of company financing. |
Employee Share Option Plan
We had an employee share option plan that was approved by our shareholders in November 2009 and was in effect until November 2014. Our shareholders approved a new Officers’ and Employees’ Option Plan at our 2015 Annual General Meeting held November 12, 2015.
The following ordinary share options of Benitec Biopharma Limited were outstanding as at December 31, 2015:
| | | | | | | | | | |
| | | | | Share options
outstanding at
December 31, 2015 | |
Grant date | | Expiry date | | Exercise
price | | | Number
under option | |
September 26, 2011* | | September 26, 2016 | | $ | 1.25 | | | | 2,800,000 | |
November 17, 2011** | | November 17, 2016 | | $ | 1.25 | | | | 600,000 | |
February 7, 2012** | | February 7, 2017 | | $ | 1.25 | | | | 156,000 | |
July 18, 2012** | | July 18, 2017 | | $ | 1.25 | | | | 400,000 | |
November 16, 2012** | | November 16, 2017 | | $ | 1.25 | | | | 400,000 | |
10 November 2013* | | May 18, 2018 | | $ | 0.62 | | | | 400,000 | |
August 22, 2013** | | August 22, 2018 | | $ | 1.25 | | | | 680,000 | |
May 15, 2014** | | May 15, 2019 | | $ | 1.50 | | | | 180,000 | |
December 17, 2014** | | December 17, 2019 | | $ | 1.25 | | | | 3,334,000 | |
May 6, 2015** | | May 6, 2020 | | $ | 1.25 | | | | 950,000 | |
November 12, 2015* | | November 12, 2020 | | $ | 0.77 | | | | 3,920,000 | |
| | | | | | | | | | |
| | | | 13,820,000 | |
| | | | | | | | | | |
| * | | Non-Executive Directors options |
No employee options were exercised during the six months ended December 31, 2015.
132
PRINCIPAL SHAREHOLDERS
The following table and accompanying footnotes present certain information regarding the beneficial ownership of our ordinary shares based on 146,529,096 ordinary shares outstanding as of December 31, 2015 by:
| | • | | each person known by us to be the beneficial owner of more than 5% of our ordinary shares; |
| | • | | each of our directors and executive officers individually; and |
| | • | | all of our directors and executive officers as a group. |
Beneficial ownership is determined according to the rules of the SEC and generally means that a person has beneficial ownership of a security if he, she or it possesses sole or shared voting or investment power of that security, including options that are exercisable within 60 days of December 31, 2015. Information with respect to beneficial ownership has been furnished to us by each director, executive officer, or 5% or more shareholder, as the case may be. Ordinary shares subject to options currently exercisable or exercisable within 60 days of December 31, 2015 are deemed to be outstanding for computing the percentage ownership of the person holding these options and the percentage ownership of any group of which the holder is a member, but are not deemed outstanding for computing the percentage of any other person.
Based on information known to us, as of December 31, 2015, we had 60 shareholders in the United States. These shareholders held an aggregate of 39,869,071 of our outstanding ordinary shares, or approximately 27.21% of our outstanding ordinary shares. A large number of our ordinary shares are held by nominee companies so we cannot be certain of the identity of those beneficial owners.
Unless otherwise indicated, to our knowledge each shareholder possesses sole voting and investment power over the ordinary shares listed subject to community property laws, where applicable. None of our shareholders has different voting rights from other shareholders. Unless otherwise indicated, the address for each of the persons listed in the table below is Benitec Biopharma Limited, F6/1-15 Barr Street, Balmain, New South Wales, Australia.
| | | | | | | | |
| | | Ordinary Shares
Beneficially
Owned | |
Shareholder | | Number | | | Percent | |
5% Shareholders | | | | | | | | |
Dr. Christopher Bremner | | | 8,133,547 | | | | 5.5 | % |
Sabby Management, LLC | | | 12,697,331 | | | | 8.7 | |
Officers and Directors | | | | | | | | |
Peter Francis | | | 2,490,840 | (1) | | | 1.7 | |
Greg West | | | 706,666 | (2) | | | * | |
Carl Stubbings | | | 936,787 | (3) | | | * | |
David Suhy | | | 933,334 | (4) | | | * | |
Georgina Kilfoil | | | 400,000 | (5) | | | * | |
John Chiplin | | | 880,000 | (6) | | | * | |
Iain Ross | | | 746,364 | (7) | | | * | |
J. Kevin Buchi | | | 1,541,539 | (8) | | | 1.1 | |
| | | | | | | | |
Officers and Directors as a group (8 persons) | | | 29,466,408 | | | | 5.9 | % |
| * | | Represents beneficial ownership of less than 1% of the outstanding ordinary shares of Benitec. |
| (1) | | Includes (i) 424,174 shares and (ii) 2,066,666 shares that Mr. Francis has the right to acquire pursuant to options that are exercisable as of December 31, 2015 or will become exercisable within 60 days of such date. |
| (2) | | Includes 706,666 shares that Mr. West has the right to acquire pursuant to options that are exercisable as of December 31, 2015 or will become exercisable within 60 days of such date. |
133
| (3) | | Includes (i) 136,787 shares and (ii) 800,000 shares that Mr. Stubbings has the right to acquire pursuant to options that are exercisable as of December 31, 2015 or will become exercisable within 60 days of such date. |
| (4) | | Includes 933,334 shares that Dr. Suhy has the right to acquire pursuant to options that are exercisable as of December 31, 2015 or will become exercisable within 60 days of such date. |
| (5) | | Includes 400,000 shares that Ms. Kilfoil has the right to acquire pursuant to options that are exercisable as of December 31, 2015 or will become exercisable within 60 days of such date. |
| (6) | | Includes (i) 200,000 shares and (ii) 680,000 shares that Dr. Chiplin has the right to acquire pursuant to options that are exercisable as of December 31, 2015 or will become exercisable within 60 days of such date. |
| (7) | | Includes (i) 66,364 shares and (ii) 680,000 shares that Mr. Ross has the right to acquire pursuant to options that are exercisable as of December 31, 2015 or will become exercisable within 60 days of such date. |
| (8) | | Includes (i) 861,539 shares and (ii) 680,000 shares that Mr. Buchi has the right to acquire pursuant to options that are exercisable as of December 31, 2015 or will become exercisable within 60 days of such date. |
To our knowledge, there have not been any significant changes in the ownership of our ordinary shares by major shareholders over the past three years, except as follows (which is based upon substantial shareholder notices filed with the ASX):
| | • | | Sabby Management, LLC increased its interest by 1.2%, from 7.54% in August 2015 to 8.67% of the total voting power as at December 31, 2015. Sabby Management, LLC beneficially owned an aggregate of 12,697,331 ordinary shares as of December 31, 2015, up from 11,000,000 aggregate ordinary shares acquired in August 2015 in connection with our initial public offering in the United States. Sabby Management, LLC controls the Sabby Healthcare Master Fund and the Sabby Volatility Warrant Master Fund, which beneficially owned 10,781,061 and 1,916,270 ordinary shares, respectively, as of December 31, 2015. Hal Mintz is the Manager of Sabby Mangement, LLC. In April 2016, Sabby Management, LLC reported that in March 2016 it sold 5,345,860 ordinary shares and, as at April 5, 2016, beneficially held an aggregate of 7,351,471 ordinary shares, or 5.02% of the total voting power. |
| | • | | RA Capital Management, LLC, or RA Capital, and its associates became a substantial shareholder on February 28, 2014, when it reported that it held 7,009,345 ordinary shares, or 7.0%, of the total voting power as of that date. Between April 2014 and July 2015, RA Capital acquired an aggregate of 7,009,346 ordinary shares for A$7,500,000 and sold an aggregate of 3,589,366 ordinary shares for A$3,602,867. On July 1, 2015, RA Capital reported that it held 10,429,325 ordinary shares, or 9.00% of the total voting power, as of that date. In August 2015, RA Capital reported that it sold 5,454,582 ordinary shares and ceased to own more than 5% of Benitec’s ordinary shares. |
| | • | | Dalit Pty Ltd, or Dalit, became a substantial shareholder on July 23, 2013, when it reported that it held 4,545,455 ordinary shares, or 6.17%, of the total voting power as of that date. As a result of a capital raising in February 2014, it ceased to be a substantial shareholder. |
| | • | | Irwin Biotech Nominees Pty Ltd, as trustee for BIOA Trust, became a substantial shareholder on July 23, 2013 when it reported that it held 4,769,091 ordinary shares, or 6.47%, of the total voting power as of that date. On February 28, 2014, Irwin reported that it ceased to be a substantial shareholder (as a result of share dilution due to a capital raising). |
| | • | | MJGD Nominees Pty Ltd, as trustee for BSMI Trust, became a substantial shareholder on July 23, 2013 when it reported that it held 4,769,091 ordinary shares, or 6.47%, of the total voting power as of that date. On February 28, 2014, MJGD reported that it ceased to be a substantial shareholder (as a result of share dilution due to a capital raising). |
| | • | | Commonwealth Scientific & Industrial Research Organisation, or CSIRO, became a substantial shareholder on January 11, 2010, when it reported that it held 40,097,026 ordinary shares, or 10%, of the total voting power as of that date. On February 28, 2014, CSIRO reported that it ceased to be a substantial shareholder (as a result of share dilution due to a capital raising). |
134
RELATED PARTY TRANSACTIONS
Other than as disclosed below, since July 1, 2012, we did not enter into any transactions or loans with any: (i) enterprises that directly or indirectly, through one or more intermediaries, control, are controlled by or are under common control with us; (ii) associates; (iii) individuals owning, directly or indirectly, an interest in our voting power that gives them significant influence over us, and close members of any such individual’s family; (iv) executive officers and close members of such individuals’ families; or (v) enterprises in which a substantial interest in our voting power is owned, directly or indirectly, by any person described in (iii) or (iv) or over which such person is able to exercise significant influence.
| | • | | Legal services at normal commercial rates totaling A$59,102 for the six months ended December 31, 2015, A$143,684 for fiscal 2015, A$108,913 for fiscal 2014 and A$103,492 for fiscal 2013 were provided by Francis Abourizk Lightowlers, a law firm in which Mr. Peter Francis is a partner and has a beneficial interest. In addition, Benitec temporarily rented office space in Melbourne from Francis Abourizk Lightowlers and the associated rental cost was A$11,102 during the six months ended December 31, 2015. |
| | • | | Consultancy fees were paid for services totaling A$85,113 for the six months ended December 31, 2015, A$118,013 for fiscal 2015, A$40,000 for fiscal 2014 and A$40,000 for fiscal 2013 provided by NewStar Ventures Ltd, a corporation in which Dr. John Chiplin is a director and has a beneficial interest. |
| | • | | Annabel West, the wife of Greg West, our Chief Financial Officer, was employed by us as a part-time clerical and administrative assistant. Annabel West was paid wages of A$49,117 and A$24,683, respectively, for fiscal 2015 and the six months ended December 31, 2015. |
| | • | | Hannah Stubbings, daughter of our Chief Business Officer, Carl Stubbings, was employed by us as a part-time intern from September 2013 until April 2014 and was paid wages of A$19,864. |
| | • | | Genevieve French, daughter of our former Chief Executive Officer, Peter French, was employed by us as a part-time intern. Genevieve French was paid wages of A$4,125 and A$4,950, repectively, for fiscal 2015 and the six months ended December 31, 2015. |
Transactions between related parties are on normal commercial terms and the conditions no more favorable than those available to other non-related parties.
135
DESCRIPTION OF SHARE CAPITAL
General
The following description of our ordinary shares is only a summary. We encourage you to read our Constitution, which is included as an exhibit to this registration statement, of which this prospectus forms a part.
We are a public company limited by shares registered under the Corporations Act by the Australian Securities and Investments Commission, or ASIC. Our corporate affairs are principally governed by our Constitution, the Corporations Act and the ASX Listing Rules. Our ordinary shares trade on the ASX, and the ADSs and Warrants trade on The NASDAQ Capital Market.
The Australian law applicable to our Constitution is not significantly different than a U.S. company’s charter documents except we do not have a limit on our authorized share capital, the concept of par value is not recognized under Australian law and as further discussed under “—Our Constitution.”
Subject to restrictions on the issue of securities in our Constitution, the Corporations Act and the ASX Listing Rules and any other applicable law, we may at any time issue shares and grant options or warrants on any terms, with the rights and restrictions and for the consideration that our board of directors determine.
The rights and restrictions attaching to ordinary shares are derived through a combination of our Constitution, the common law applicable to Australia, the ASX Listing Rules, the Corporations Act and other applicable law. A general summary of some of the rights and restrictions attaching to our ordinary shares are summarized below. Each ordinary shareholder is entitled to receive notice of, and to be present, vote and speak at, general meetings.
Changes to Our Share Capital
As of December 31, 2015, we had (i) 146,529,096 ordinary shares outstanding and (ii) 38,566,203 outstanding options and warrants to purchase an aggregate of 38,566,203 ordinary shares.
During the last three years, the following changes have been made to our ordinary share capital:
| | 1. | | On October 30, 2012, we issued 78,446,306 ordinary shares to the vendors of Tacere Therapeutics, Inc. as part of the consideration under an acquisition agreement, at an issue price of A$0.01496 per share. |
| | 2. | | On March 11, 2013, we issued 43,846,155 ordinary shares as part of a private placement at A$0.013 per share to institutional and professional investors outside the United States. Participants in the placement received two free unlisted options for every five shares subscribed for in the placement and we therefore also issued 17,538,462 unlisted options with an exercise price of A$0.013 per share. |
| | 3. | | On March 28, 2013, we issued 13,846,154 ordinary shares as part of a private placement at A$0.013 per share to institutional and professional investors outside the United States. Participants in the placement received two free unlisted options for every five shares subscribed for in the placement and we therefore also issued 5,538,462 unlisted options with an exercise price of A$0.013 per share. |
| | 4. | | On May 28, 2013, we issued 7,692,308 ordinary shares as part of a private placement at A$0.013 per share to institutional and professional investors outside the United States. Participants in the placement received two free unlisted options for every five shares subscribed for in the placement and we therefore also issued 3,076,923 unlisted options with an exercise price of A$0.013 per share. |
| | 5. | | On June 14, 2013, we issued 37,454,591 ordinary shares as part of a private placement at A$0.011 per share to institutional and professional investors outside the United States. |
| | 6. | | On July 23, 2013, we issued 27,229,089 ordinary shares as part of a private placement at A$0.275 per share to institutional and professional investors outside the United States. |
136
| | 7. | | On July 23, 2013, we issued 400,000 ordinary shares at A$0.325 per share to directors resident outside the United States. Participants in the placement received two free unlisted options for every five shares subscribed for in the placement and we therefore also issued 160,000 unlisted options with an exercise price of A$0.013 per share. |
| | 8. | | On August 6, 2013, we issued 10,254,696 ordinary shares at A$0.275 per share to shareholders resident in Australia or New Zealand under a share purchase plan. |
| | 9. | | On October 30, 2013, we issued 955,002 ordinary shares to the vendors of Tacere Therapeutics, Inc. as part of the consideration under an acquisition agreement. The consideration was A$350,000. |
| | 10. | | On February 28, 2014, we issued 14,717,995 ordinary shares and 6,623,098 unlisted options, as the first tranche of a private placement transacted over two tranches to institutional investors in Australia and the United States. Consideration received from the issue of the ordinary shares was A$15,748,255. |
| | 11. | | On April 15, 2014, we issued 14,717,999 ordinary shares and 6,623,105 unlisted options, as the second tranche of a private placement transacted over two tranches to institutional investors in Australia and the United States. Consideration received from the issue of the ordinary shares was A$15,748,259. |
| | 12. | | On August 18, 2015, we issued 30,000,000 ordinary shares (in the form of American Depositary Shares) and warrants to purchase 11,500,000 ordinary shares as part of our initial public offering in the United States. Consideration received from the issue of the ordinary shares and warrants was approximately US$13,820,000, before deducting underwriting discount and commissions and expenses. |
In addition, we issued the following ordinary shares upon exercise of options (excluding the warrants discussed in the immediately preceding paragraph) over the past three and one-half fiscal years:
| | • | | 3,920,000 ordinary shares during the six months ended December 31, 2015; |
| | • | | 982,767 ordinary shares in fiscal 2015; |
| | • | | 547,088 ordinary shares in fiscal 2014; and |
| | • | | no ordinary shares in fiscal 2013. |
Our Constitution
Our Constitution is similar in nature to the bylaws of a U.S. corporation. It does not provide for or prescribe any specific objectives or purposes of Benitec. Our Constitution is subject to the terms of the ASX Listing Rules and the Corporations Act. It may be amended or repealed and replaced by special resolution of shareholders, which is a resolution passed by at least 75% of the votes cast by shareholders entitled to vote on the resolution.
Under Australian law, a company has the legal capacity and powers of an individual both within and outside Australia. The material provisions of our Constitution are summarized below. This summary is not intended to be complete nor to constitute a definitive statement of the rights and liabilities of our shareholders. Our Constitution is filed as an exhibit to the registration statement, of which this prospectus forms a part.
Interested Directors
A director may not vote in respect of any contract or arrangement in which the director has, directly or indirectly, any material interest according to our Constitution. Such director must not be counted in a quorum, must not vote on the matter and must not be present at the meeting while the matter is being considered. However, that director may execute or otherwise act in respect of that contract or arrangement notwithstanding any material personal interest.
Unless a relevant exception applies, the Corporations Act requires our directors to provide disclosure of certain interests or conflicts of interests and prohibits directors from voting on matters in which they have a
137
material personal interest and from being present at the meeting while the matter is being considered. In addition, the Corporations Act and the ASX Listing Rules require shareholder approval of any provision of related party benefits to our directors.
Directors’ Compensation
Our directors are paid remuneration for their services as directors (but excluding any remuneration payable to a director under any executive services contract with us or one of our related bodies corporate) which is determined in a general meeting of shareholders. The aggregate, fixed sum for directors’ remuneration is to be divided among the directors in such proportion as the directors themselves agree and in accordance with our Constitution. The fixed sum remuneration for directors may not be increased except at a general meeting of shareholders and the particulars of the proposed increase are required to have been provided to shareholders in the notice convening the meeting. In addition, executive directors may be paid remuneration as employees of Benitec.
Fees payable to our non-executive directors must be by way of a fixed sum and not by way of a commission on or a percentage of profits or operating revenue. Remuneration paid to our executive directors must also not include a commission or percentage of operating revenue.
Pursuant to our Constitution, any director who performs services that in the opinion of our board of directors, are outside the scope of the ordinary duties of a director may be paid extra remuneration, which is determined by our board of directors.
In addition to other remuneration provided in our Constitution, all of our directors are entitled to be paid by us for reasonable travel accommodation and other expenses incurred by the directors in attending general meetings, board meetings, committee meetings or otherwise in connection with our business.
In addition, in accordance with our Constitution, a director may be paid a retirement benefit as determined by our board of directors subject to the limits set out in the Corporations Act and the ASX Listing Rules which broadly restrict our ability to pay our officers a termination benefit in the event of a change of control of the Company or our subsidiaries as well as impose requirements for shareholder approval to be obtained to pay certain retirement benefits to our officers.
Borrowing Powers Exercisable by Directors
Pursuant to our Constitution, the management and control of our business affairs are vested in our board of directors. Our board of directors has the power to raise or borrow money, and charge any of our property or business or any uncalled capital, and may issue debentures or give any other security for any of our debts, liabilities or obligations or of any other person, in each case, in the manner and on terms it deems fit.
Retirement of Directors
Pursuant to our Constitution and the ASX Listing Rules, one-third of our directors, other than the managing director, must retire from office at every annual general meeting. If the number of directors is not a multiple of three, then the number nearest, to but not exceeding, one-third must retire from office. The directors who retire in this manner are required to be the directors or director longest in office since last being elected. A director, other than the director who is the Chief Executive Officer, must retire from office at the conclusion of the third annual general meeting after which the director was elected. Retired directors are eligible for a re-election to the board of directors unless disqualified from acting as a director under the Corporations Act or our Constitution.
Rights and Restrictions on Classes of Shares
The rights attaching to our ordinary shares are detailed in our Constitution. Our Constitution provides that our directors may issue shares with preferred, deferred or other special rights, whether in relation to dividends,
138
voting, return of share capital, or otherwise as our board of directors may determine. Subject to any approval which is required from our shareholders under the Corporations Act and the ASX Listing Rules (see “—Exemptions from Certain NASDAQ Corporate Governance Rules” and “—Change of Control”), any rights and restrictions attached to a class of shares, we may issue further shares on such terms and conditions as our board of directors resolve. Currently, our outstanding share capital consists of only one class of ordinary shares.
Dividend Rights
Our board of directors may from time to time determine to pay dividends to shareholders. All dividends unclaimed for one year after having been declared may be invested or otherwise made use of by our board of directors for our benefit until claimed or otherwise disposed of in accordance with our Constitution.
Voting Rights
Under our Constitution, and subject to any voting exclusions imposed under the ASX Listing Rules (which typically exclude parties from voting on resolutions in which they have an interest), the rights and restrictions attaching to a class of shares, each shareholder has one vote on a show of hands at a meeting of the shareholders unless a poll is demanded under the Constitution or the Corporations Act. On a poll vote, each shareholder shall have one vote for each fully paid share and a fractional vote for each share held by that shareholder that is not fully paid, such fraction being equivalent to the proportion of the amount that has been paid to such date on that share. Shareholders may vote in person or by proxy, attorney or representative. Under Australian law, shareholders of a public company are not permitted to approve corporate matters by written consent. Our Constitution does not provide for cumulative voting.
Note that ADS holders may not directly vote at a meeting of the shareholders but may instruct the depositary to vote the number of deposited ordinary shares their ADSs represent.
Right To Share in Our Profits
Pursuant to our Constitution, our shareholders are entitled to participate in our profits only by payment of dividends. Our board of directors may from time to time determine to pay dividends to the shareholders; however, no dividend is payable except in accordance with the thresholds set out in the Corporations Act.
Rights to Share in the Surplus in the Event of Liquidation
Our Constitution provides for the right of shareholders to participate in a surplus in the event of our liquidation, subject to the rights attaching to a class of shares.
No Redemption Provision for Ordinary Shares
There are no redemption provisions in our Constitution in relation to ordinary shares. Under our Constitution, any preference shares may be issued on the terms that they are, or may at our option be, liable to be redeemed.
Variation or Cancellation of Share Rights
Subject to the terms of issue of shares of that class, the rights attached to shares in a class of shares may only be varied or cancelled by a special resolution of Benitec, together with either:
| | • | | a special resolution passed by members holding shares in the class; or |
| | • | | the written consent of members with at least 75% of the shares in the class. |
139
Directors May Make Calls
Our Constitution provides that subject to the terms on which the shares have been issued directors may make calls on a shareholder for amounts unpaid on shares held by that shareholder, other than monies payable at fixed times under the conditions of allotment. Shares represented by the ADSs issued in this offering will be fully paid and will not be subject to calls by directors.
General Meetings of Shareholders
General meetings of shareholders may be called by our board of directors. Except as permitted under the Corporations Act, shareholders may not convene a meeting. The Corporations Act requires the directors to call and arrange to hold a general meeting on the request of shareholders with at least 5% of the votes that may be cast at a general meeting or at least 100 shareholders who are entitled to vote at the general meeting. Notice of the proposed meeting of our shareholders is required at least 28 days prior to such meeting under the Corporations Act.
Foreign Ownership Regulation
There are no limitations on the rights to own securities imposed by our Constitution. However, acquisitions and proposed acquisitions of securities in Australian companies may be subject to review and approval by the Australian Federal Treasurer under the Foreign Acquisitions and Takeovers Act 1975, or the FATA, which generally applies to acquisitions or proposed acquisitions:
| | • | | by a foreign person (as defined in the FATA) or associated foreign persons that would result in such persons having an interest in 15% or more of the issued shares of, or control of 15% or more of the voting power in, an Australian company; and |
| | • | | by non-associated foreign persons that would result in such foreign person having an interest in 40% or more of the issued shares of, or control of 40% or more of the voting power in, an Australian company, where the Australian company is valued above the monetary threshold prescribed by FATA. |
However, no such review or approval under the FATA is required if the foreign acquirer is a U.S. entity and the value of the target is less than A$1,094 million.
The Australian Federal Treasurer may prevent a proposed acquisition in the above categories or impose conditions on such acquisition if the Treasurer is satisfied that the acquisition would be contrary to the national interest. If a foreign person acquires shares or an interest in shares in an Australian company in contravention of the FATA, the Australian Federal Treasurer may order the divestiture of such person’s shares or interest in shares in that Australian company.
Ownership Threshold
There are no provisions in our Constitution that require a shareholder to disclose ownership above a certain threshold. The Corporations Act, however, requires a shareholder to notify us and the ASX once it, together with its associates, acquires a 5% interest in our ordinary shares, at which point the shareholder will be considered to be a “substantial” shareholder. Further, once a shareholder owns a 5% interest in us, such shareholder must notify us and the ASX of any increase or decrease of 1% or more in its holding of our ordinary shares, and must also notify us and the ASX on its ceasing to be a “substantial” shareholder. Following our initial public offering in the United States, our shareholders are also subject to disclosure requirements under U.S. securities laws.
Issues of Shares and Change in Capital
Subject to our Constitution, the Corporations Act, the ASX Listing Rules and any other applicable law, we may at any time issue shares and grant options or warrants on any terms, with preferred, deferred or other special rights and restrictions and for the consideration and other terms that the directors determine.
140
Subject to the requirements of our Constitution, the Corporations Act, the ASX Listing Rules and any other applicable law, including relevant shareholder approvals, we may consolidate or divide our share capital into a larger or smaller number by resolution, reduce our share capital (provided that the reduction is fair and reasonable to our shareholders as a whole and does not materially prejudice our ability to pay creditors) or buy back our ordinary shares whether under an equal access buy-back or on a selective basis.
Change of Control
Takeovers of listed Australian public companies, such as Benitec, are regulated by the Corporations Act, which prohibits the acquisition of a “relevant interest” in issued voting shares in a listed company if the acquisition will lead to that person’s or someone else’s voting power in Benitec increasing from 20% or below to more than 20% or increasing from a starting point that is above 20% and below 90%, subject to a range of exceptions.
Generally, a person will have a relevant interest in securities if the person:
| | • | | is the holder of the securities; |
| | • | | has power to exercise, or control the exercise of, a right to vote attached to the securities; or |
| | • | | has the power to dispose of, or control the exercise of a power to dispose of, the securities, including any indirect or direct power or control. |
If, at a particular time, a person has a relevant interest in issued securities and the person:
| | • | | has entered or enters into an agreement with another person with respect to the securities; |
| | • | | has given or gives another person an enforceable right, or has been or is given an enforceable right by another person, in relation to the securities (whether the right is enforceable presently or in the future and whether or not on the fulfillment of a condition); |
| | • | | has granted or grants an option to, or has been or is granted an option by, another person with respect to the securities; or |
| | • | | the other person would have a relevant interest in the securities if the agreement were performed, the right enforced or the option exercised; |
the other person is taken to already have a relevant interest in the securities.
There are a number of exceptions to the above prohibition on acquiring a relevant interest in issued voting shares above 20%. In general terms, some of the more significant exceptions include:
| | • | | when the acquisition results from the acceptance of an offer under a formal takeover bid; |
| | • | | when the acquisition is conducted on market by or on behalf of the bidder under a takeover bid, the acquisition occurs during the bid period, the bid is for all the voting shares in a bid class and the bid is unconditional or only conditioned on prescribed matters set out in the Corporations Act; |
| | • | | when shareholders of Benitec approve the takeover by resolution passed at general meeting; |
| | • | | an acquisition by a person if, throughout the six months before the acquisition, that person or any other person has had voting power in Benitec of at least 19% and, as a result of the acquisition, none of the relevant persons would have voting power in Benitec more than three percentage points higher than they had six months before the acquisition; |
| | • | | when the acquisition results from the issue of securities under a rights issue; |
| | • | | when the acquisition results from the issue of securities under dividend reinvestment schemes; |
| | • | | when the acquisition results from the issue of securities under underwriting arrangements; |
141
| | • | | when the acquisition results from the issue of securities through operation of law; |
| | • | | an acquisition that arises through the acquisition of a relevant interest in another listed company which is listed on a prescribed financial market or a financial market approved by ASIC; |
| | • | | an acquisition arising from an auction of forfeited shares conducted on-market; or |
| | • | | an acquisition arising through a compromise, arrangement, liquidation or buy-back. |
Breaches of the takeovers provisions of the Corporations Act are criminal offenses. ASIC and the Australian Takeover Panel have a wide range of powers relating to breaches of takeover provisions, including the ability to make orders canceling contracts, freezing transfers of, and rights attached to, securities, and forcing a party to dispose of securities. There are certain defenses to breaches of the takeover provisions provided in the Corporations Act.
Access to and Inspection of Documents
Inspection of our records is governed by the Corporations Act. Any member of the public has the right to inspect or obtain copies of our registers on the payment of a prescribed fee. Shareholders are not required to pay a fee for inspection of our registers or minute books of the meetings of shareholders. Other corporate records, including minutes of directors’ meetings, financial records and other documents, are not open for inspection by shareholders. Where a shareholder is acting in good faith and an inspection is deemed to be made for a proper purpose, a shareholder may apply to the court to make an order for inspection of our books.
Exemptions from Certain NASDAQ Corporate Governance Rules
The NASDAQ listing rules allow for a foreign private issuer, such as Benitec, to follow its home country practices in lieu of certain of the NASDAQ’s corporate governance standards. In connection with our NASDAQ Listing Application, we have relied on and expect to continue to rely on exemptions from certain corporate governance standards that are contrary to the laws, rules, regulations or generally accepted business practices in Australia. These exemptions are described below:
| | • | | Although the majority of our directors currently qualify as independent under the NASDAQ Listing Rules, we have relied on and expect in the future to continue to rely on an exemption from these independence requirements for a majority of our board of directors as prescribed by NASDAQ Listing Rules. The ASX Listing Rules do not require us to have a majority of independent directors although ASX Corporate Governance Principles and Recommendations do recommend a majority of independent directors. During fiscal 2015, we did, however, have a majority of directors who were “independent” as defined in the ASX Corporate Governance Principles and Recommendations, which definition differs from NASDAQ’s definition. Accordingly, because Australian law and generally accepted business practices in Australia regarding director independence differ from the independence requirements under NASDAQ Listing Rules, we may seek to claim this exemption in the future. |
| | • | | We have relied on and expect to continue to rely on an exemption from the requirement that our independent directors meet regularly in executive sessions under NASDAQ Listing Rules. The ASX Listing Rules and the Corporations Act do not require the independent directors of an Australian company to have such executive sessions and, accordingly, we seek to claim this exemption. |
| | • | | We have relied on and expect to continue to rely on an exemption from the quorum requirements applicable to meetings of shareholders under NASDAQ Listing Rules. In compliance with Australian law, our Constitution provides that three shareholders present, in person or by proxy, attorney or a representative, shall constitute a quorum for a general meeting. NASDAQ Listing Rules require that an issuer provide for a quorum as specified in its by-laws for any meeting of the holders of ordinary shares, which quorum may not be less than 331/3% of the outstanding shares of an issuer’s voting ordinary shares. Accordingly, because applicable Australian law and rules governing quorums at shareholder meetings differ from NASDAQ’s quorum requirements, we seek to claim this exemption. |
142
| | • | | We have relied on and expect to continue to rely on an exemption from the requirement prescribed by NASDAQ Listing Rules that issuers obtain shareholder approval prior to the issuance of securities in connection with certain acquisitions, private placements of securities, or the establishment or amendment of certain stock option, purchase or other compensation plans. Applicable Australian law and the ASX Listing Rules differ from NASDAQ requirements, with the ASX Listing Rules providing generally for prior shareholder approval in numerous circumstances, including (i) issuance of equity securities exceeding 15% of our issued share capital in any 12-month period (but, in determining the 15% limit, securities issued under an exception to the rule or with shareholder approval are not counted), (ii) issuance of equity securities to related parties (as defined in the ASX Listing Rules) and (iii) issuances of securities to directors or their associates under an employee incentive plan. Due to differences between Australian law and rules and the NASDAQ shareholder approval requirements, we seek to claim this exemption. |
143
DESCRIPTION OF SECURITIES
American Depositary Shares
The Bank of New York Mellon, as depositary, has registered and delivered American Depositary Shares, also referred to as ADSs. Each ADS represents 20 ordinary shares (or a right to receive 20 ordinary shares) deposited with National Australia Bank Limited, as custodian for the depositary. Each ADS may also represent any other securities, cash or other property which may be held by the depositary. The depositary’s office at which the ADSs are administered is located at 101 Barclay Street, New York, New York 10286. The Bank of New York Mellon’s principal executive office is located at One Wall Street, New York, New York 10286.
You may hold ADSs either (A) directly (i) by having an American Depositary Receipt, also referred to as an ADR, which is a certificate evidencing a specific number of ADSs, registered in your name, or (ii) by having ADSs registered in your name in the Direct Registration System, or (B) indirectly by holding a security entitlement in ADSs through your broker or other financial institution. If you hold ADSs directly, you are a registered ADS holder, or ADS holder. This description assumes you are an ADS holder. If you hold the ADSs indirectly, you must rely on the procedures of your broker or other financial institution to assert the rights of ADS holders described in this section. You should consult with your broker or financial institution to find out what those procedures are.
The Direct Registration System, or DRS, is a system administered by The Depository Trust Company, also referred to as DTC, pursuant to which the depositary may register the ownership of uncertificated ADSs, which ownership is confirmed by periodic statements sent by the depositary to the registered holders of uncertificated ADSs.
As an ADS holder, we will not treat you as one of our shareholders and you will not have shareholder rights. Australian law governs shareholder rights. The depositary is the holder of the shares underlying your ADSs. As a registered holder of ADSs, you will have ADS holder rights. A deposit agreement among us, the depositary and you, as an ADS holder, and all other persons directly or indirectly holding ADSs sets out ADS holder rights as well as the rights and obligations of the depositary. New York law governs the deposit agreement and the ADSs.
The following is a summary of the material provisions of the deposit agreement. Because it is a summary, it does not contain all the information that may be important to you. For more complete information, you should read the entire deposit agreement and the form of ADR which summarizes certain terms of your ADSs. A copy of the deposit agreement is filed as an exhibit to the registration statement of which this prospectus forms a part. You may also obtain a copy of the deposit agreement at the SEC’s Public Reference Room which is located at 100 F Street, NE, Washington, DC 20549. You may obtain information on the operation of the Public Reference Room by calling the SEC at 1-800-732-0330. You may also find the registration statement and the deposit agreement on the SEC’s website at http://www.sec.gov.
Dividends and Other Distributions
How will you receive dividends and other distributions on the shares?
The depositary has agreed to pay to you the cash dividends or other distributions it or the custodian receives on shares or other deposited securities, after deducting its fees and expenses. You will receive these distributions in proportion to the number of ordinary shares your ADSs represent.
| | • | | Cash. The depositary will convert any cash dividend or other cash distribution we pay on the shares into U.S. dollars, if it can do so on a reasonable basis and can transfer the U.S. dollars to the United States. If that is not possible or if any government approval is needed and can not be obtained, the deposit agreement allows the depositary to distribute the foreign currency only to those ADS holders to whom it is possible to do so. It will hold the foreign currency it cannot convert for the account of the ADS holders who have not been paid. It will not invest the foreign currency and it will not be liable for any interest. |
144
Before making a distribution, any withholding taxes, or other governmental charges that must be paid will be deducted. See “Taxation.” It will distribute only whole U.S. dollars and cents and will round fractional cents to the nearest whole cent. If the exchange rates fluctuate during a time when the depositary cannot convert the foreign currency, you may lose some or all of the value of the distribution.
| | • | | Shares. The depositary may distribute additional ADSs representing any shares we distribute as a dividend or free distribution to the extent reasonably practicable and permitted under law. The depositary will only distribute whole ADSs. It will try to sell shares which would require it to deliver a fractional ADS and distribute the net proceeds in the same way as it does with cash. If the depositary does not distribute additional ADSs, the outstanding ADSs will also represent the new shares. The depositary may sell a portion of the distributed shares sufficient to pay its fees and expenses in connection with that distribution. |
| | • | | Rights to purchase additional shares. If we offer holders of our securities any rights to subscribe for additional shares or any other rights, the depositary may make these rights available to you. If the depositary decides it is not legal and practical to make the rights available but that it is practical to sell the rights, the depositary will use reasonable efforts to sell the rights and distribute the proceeds in the same way as it does with cash. The depositary will allow rights that are not distributed or sold to lapse. In that case, you will receive no value for such rights. |
If the depositary makes rights available to ADS holders, it will exercise the rights and purchase the shares on your behalf all in accordance with your instructions. The depositary will then deposit the shares and deliver ADSs to you. It will only exercise rights if you pay the exercise price and any other charges the rights require you to pay and comply with other applicable instructions.
U.S. securities laws may restrict transfers and cancellation of the ADSs representing shares purchased upon exercise of rights. For example, you may not be able to trade such ADSs freely in the United States. In this case, the depositary may deliver restricted depositary shares that have the same terms as the ADSs described in this section except for changes needed to put the necessary restrictions in place.
| | • | | Other Distributions. The depositary will send to you anything else we distribute on deposited securities by any means it determines is legal, fair and practical. If it cannot make the distribution in that way, the depositary may adopt another legal, fair and practical method. It may decide to sell what we distributed and distribute the net proceeds in the same way as it does with cash. Or, it may decide to hold what we distributed, in which case ADSs will also represent the newly distributed property. However, the depositary is not required to distribute any securities (other than ADSs) to ADS holders unless it receives reasonably satisfactory evidence from us that it is legal to make that distribution. The depositary may sell a portion of the distributed securities or property sufficient to pay its fees and expenses in connection with that distribution. |
The depositary is not responsible if it decides that it is unlawful or impractical to make a distribution available to any ADS holders. We have no obligation to register ADSs, shares, rights or other securities under the Securities Act. We also have no obligation to take any other action to permit the distribution of ADSs, shares, rights or any other property to ADS holders. This means that you may not receive the distributions we make on our ordinary shares or any value for them if it is illegal or impractical for us to make them available to you.
Deposit, Withdrawal and Cancellation
How are ADSs issued?
The depositary will deliver ADSs if you or your broker deposit shares or evidence of rights to receive shares with the custodian. Upon payment of its fees and expenses and of any taxes or charges, such as stamp taxes or share transfer taxes or fees, the depositary will register the appropriate number of ADSs in the names you request and will deliver the ADSs to or upon the order of the person or persons that made the deposit.
145
How can ADS holders withdraw the deposited securities?
You may surrender your ADSs at the depositary’s office. Upon payment of its fees and expenses and of any taxes or charges, such as stamp taxes or share transfer taxes or fees, the depositary will deliver the shares and any other deposited securities underlying the ADSs to the ADS holder or a person designated by you at the office of the custodian. In the alternative, at your request, risk and expense, the depositary will deliver the deposited securities at its office, if feasible.
How do ADS holders interchange between certificated ADSs and uncertificated ADSs?
You may surrender your ADR to the depositary for the purpose of exchanging your ADR for uncertificated ADSs. The depositary will cancel that ADR and will send to you a statement confirming that you are the registered holder of uncertificated ADSs. Alternatively, upon receipt by the depositary of a proper instruction from a registered holder of uncertificated ADSs requesting the exchange of uncertificated ADSs for certificated ADSs, the depositary will execute and deliver to you an ADR evidencing those ADSs.
Voting Rights
How do you vote?
You may instruct the depositary to vote the number of deposited ordinary shares your ADSs represent. The depositary will notify you of shareholders’ meetings and arrange to deliver our voting materials to you upon our request. Those materials will describe the matters to be voted on and explain how ADS holders may instruct the depositary how to vote. For instructions to be valid, they must reach the depositary by a date established by the depositary.
Otherwise, you won’t be able to exercise your right to vote unless you withdraw the shares underlying the ADSs. However, you may not know about the meeting with a sufficient amount of advance notice to withdraw the shares.
The depositary will attempt, as far as practical, subject to the laws of Australia and of our Constitution or similar documents, to vote or to have its agents vote the shares or other deposited securities represented by your ADSs as instructed by ADS holders. The depositary will only vote or attempt to vote as instructed.
We cannot assure you that you will receive the voting materials in time to ensure that you can instruct the depositary to vote your ordinary shares. In addition, the depositary and its agents are not responsible for failing to carry out voting instructions or for the manner of carrying out voting instructions. This means that you may not be able to exercise your right to vote and there may be nothing you can do if your ordinary shares are not voted as you requested.
In order to give you a reasonable opportunity to instruct the depositary as to the exercise of voting rights relating to deposited securities, if we request the depositary to act, we agree to give the depositary notice of any such meeting and details concerning the matters to be voted upon at least 30 days in advance of the meeting date.
146
Fees and Expenses
| | |
Persons depositing or withdrawing ordinary shares or ADS
holders must pay the depositary: | | For: |
| $5.00 (or less) per 100 ADSs (or portion of 100 ADSs) | | • Issuance of ADSs, including issuances resulting from a distribution of shares or rights or other property • Cancellation of ADSs for the purpose of withdrawal, including if the deposit agreement terminates |
| |
| $.05 (or less) per ADS | | • Any cash distribution to you |
| |
| A fee equivalent to the fee that would be payable if securities distributed to you had been shares and the shares had been deposited for issuance of ADSs | | • Distribution of securities distributed to holders of deposited securities which are distributed by the depositary to you |
| |
| $.05 (or less) per ADS per calendar year | | • Depositary services |
| |
| Registration or transfer fees | | • Transfer and registration of shares on our share register to or from the name of the depositary or its agent when you deposit or withdraw shares |
| |
| Expenses of the depositary | | • Cable, telex and facsimile transmissions (when expressly provided in the deposit agreement) • Converting foreign currency to U.S. dollars |
| |
| Taxes and other governmental charges the depositary or the custodian have to pay on any ADS or share underlying an ADS, for example, stock transfer taxes, stamp duty or withholding taxes | | • As necessary |
| |
| Any charges incurred by the depositary or its agents for servicing the deposited securities | | • As necessary |
The depositary collects its fees for delivery and surrender of ADSs directly from investors depositing shares or surrendering ADSs for the purpose of withdrawal or from intermediaries acting for them. The depositary collects fees for making distributions to investors by deducting those fees from the amounts distributed or by selling a portion of distributable property to pay the fees. The depositary may collect its annual fee for depositary services by deduction from cash distributions or by directly billing investors or by charging the book-entry system accounts of participants acting for them. The depositary may generally refuse to provide fee-attracting services until its fees for those services are paid. The depositary may collect any of its fees by deduction from any cash distribution payable to you that are obligated to pay those fees.
From time to time, the depositary may make payments to us to reimburse or share revenue from the fees collected from you, or waive fees and expenses for services provided, generally relating to costs and expenses arising out of establishment and maintenance of the ADS program. In performing its duties under the deposit agreement, the depositary may use brokers, dealers or other service providers that are affiliates of the depositary and that may earn or share fees or commissions.
Payment of Taxes
You will be responsible for any taxes or other governmental charges payable on your ADSs or on the deposited securities represented by any of your ADSs. The depositary may refuse to register any transfer of your ADSs or allow you to withdraw the deposited securities represented by your ADSs until such taxes or other
147
charges are paid. It may apply payments owed to you or sell deposited securities represented by your ADSs to pay any taxes owed and you will remain liable for any deficiency. If the depositary sells deposited securities, it will, if appropriate, reduce the number of ADSs to reflect the sale and pay to you any proceeds, or send to ADS holders any property, remaining after it has paid the taxes.
Reclassifications, Recapitalizations and Mergers
| | |
If we: | | Then: |
• Reclassify, split up or consolidate any of the deposited securities • Distribute securities in respect of deposited shares that are not distributed to you • Recapitalize, reorganize, merge, liquidate, sell all or substantially all of our assets, or take any similar action | | The cash, shares or other securities received by the depositary will become deposited securities. Each ADS will automatically represent its equal share of the new deposited securities. The depositary may distribute some or all of the cash, shares or other securities it received. It may also ask you to surrender your outstanding ADRs in exchange for new ADRs identifying the new deposited securities. |
Amendment and Termination
How may the deposit agreement be amended?
We may agree with the depositary to amend the deposit agreement and the ADRs without your consent for any reason. If an amendment adds or increases fees or charges, except for taxes and other governmental charges or expenses of the depositary for registration fees, facsimile costs, delivery charges or similar items, or prejudices a substantial right of ADS holders, it will not become effective for outstanding ADSs until 30 days after the depositary notifies ADS holders of the amendment. At the time an amendment becomes effective, you are considered, by continuing to hold your ADSs, to agree to the amendment and to be bound by the ADRs and the deposit agreement as amended.
How may the deposit agreement be terminated?
The depositary will terminate the deposit agreement at our direction by mailing notice of termination to the ADS holders then outstanding at least 30 days prior to the date fixed in such notice for such termination. The depositary may also terminate the deposit agreement by mailing notice of termination to us and the ADS holders if 60 days have passed since the depositary told us it wants to resign but a successor depositary has not been appointed and accepted its appointment.
After termination, the depositary and its agents will do the following under the deposit agreement (but nothing else):
| | • | | collect distributions on the deposited securities; |
| | • | | sell rights and other property; and |
| | • | | deliver shares and other deposited securities upon cancellation of ADSs. |
Four months after termination, the depositary may sell any remaining deposited securities by public or private sale. After that, the depositary will hold the money it received on the sale, as well as any other cash it is holding under the deposit agreement for the pro rata benefit of the ADS holders that have not surrendered their ADSs. It will not invest the money and has no liability for interest. The depositary’s only obligations will be to account for the money and other cash. After termination our only obligations will be to indemnify the depositary and to pay fees and expenses of the depositary that we agreed to pay.
148
Limitations on Obligations and Liability
Limits on our Obligations and the Obligations of the Depositary; Limits on Liability to Holders of ADSs
The deposit agreement expressly limits our obligations and the obligations of the depositary. It also limits our liability and the liability of the depositary. We and the depositary:
| | • | | are only obligated to take the actions specifically set forth in the deposit agreement; |
| | • | | are not liable if we are or it is prevented or delayed by law or circumstances beyond our control from performing our or its obligations under the deposit agreement; |
| | • | | are not liable if we or it exercises discretion permitted under the deposit agreement; |
| | • | | are not liable for the inability of any holder of ADSs to benefit from any distribution on deposited securities that is not made available to holders of ADSs under the terms of the deposit agreement, or for any special, consequential or punitive damages for any breach of the terms of the deposit agreement; |
| | • | | have no obligation to become involved in a lawsuit or other proceeding related to the ADSs or the deposit agreement on your behalf or on behalf of any other person; |
| | • | | may rely upon any documents we believe or it believes in good faith to be genuine and to have been signed or presented by the proper person. |
In the deposit agreement, we and the depositary agree to indemnify each other under certain circumstances.
Requirements for Depositary Actions
Before the depositary will deliver or register a transfer of an ADS, make a distribution on an ADS, or permit withdrawal of shares, the depositary may require:
| | • | | payment of stock transfer or other taxes or other governmental charges and transfer or registration fees charged by third parties for the transfer of any shares or other deposited securities; |
| | • | | satisfactory proof of the identity and genuineness of any signature or other information it deems necessary; and |
| | • | | compliance with regulations it may establish, from time to time, consistent with the deposit agreement, including presentation of transfer documents. |
The depositary may refuse to deliver ADSs or register transfers of ADSs generally when the transfer books of the depositary or our transfer books are closed or at any time if the depositary or we think it advisable to do so.
Your Right to Receive the Shares Underlying your ADSs
You have the right to cancel their ADSs and withdraw the underlying shares at any time except:
| | • | | when temporary delays arise because: (i) the depositary has closed its transfer books or we have closed our transfer books; (ii) the transfer of shares is blocked to permit voting at a shareholders’ meeting; or (iii) we are paying a dividend on our ordinary shares; |
| | • | | when you owe money to pay fees, taxes and similar charges; and |
| | • | | when it is necessary to prohibit withdrawals in order to comply with any laws or governmental regulations that apply to ADSs or to the withdrawal of ordinary shares or other deposited securities. |
This right of withdrawal may not be limited by any other provision of the deposit agreement.
149
Pre-Release of ADSs
The deposit agreement permits the depositary to deliver ADSs before deposit of the underlying ordinary shares. This is called a pre-release of the ADSs. The depositary may also deliver shares upon cancellation of pre-released ADSs (even if the ADSs are canceled before the pre-release transaction has been closed out). A pre-release is closed out as soon as the underlying shares are delivered to the depositary. The depositary may receive ADSs instead of shares to close out a pre-release. The depositary may pre-release ADSs only under the following conditions:
| | • | | before or at the time of the pre-release, the person to whom the pre-release is being made represents to the depositary in writing that it or its customer owns the shares or ADSs to be deposited; |
| | • | | the pre-release is fully collateralized with cash, U.S. government securities or other collateral that the depositary considers appropriate; and |
| | • | | the depositary must be able to close out the pre-release on not more than five business days’ notice. |
In addition, the depositary has agreed to limit the number of ADSs that may be outstanding at any time as a result of pre-release to 30% of the shares deposited under the deposit agreement, although the depositary may disregard the limit from time to time, if it thinks it is reasonably appropriate to do so.
Direct Registration System
In the deposit agreement, all parties to the deposit agreement acknowledge that the DRS and Profile Modification System, or Profile, will apply to uncertificated ADSs upon acceptance thereof to DRS by DTC. DRS is the system administered by DTC under which the depositary may register the ownership of uncertificated ADSs, which ownership will be evidenced by periodic statements sent by the depositary to the registered holders of uncertificated ADSs. Profile is a required feature of DRS that allows a DTC participant, claiming to act on behalf of a registered holder of ADSs, to direct the depositary to register a transfer of those ADSs to DTC or its nominee and to deliver those ADSs to the DTC account of that DTC participant without receipt by the depositary of prior authorization from the ADS holder to register that transfer.
In connection with and in accordance with the arrangements and procedures relating to DRS/Profile, the parties to the deposit agreement understand that the depositary will not determine whether the DTC participant that is claiming to be acting on behalf of an ADS holder in requesting registration of transfer and delivery described in the paragraph above has the actual authority to act on behalf of the ADS holder (notwithstanding any requirements under the Uniform Commercial Code). In the deposit agreement, the parties agree that the depositary’s reliance on and compliance with instructions received by the depositary through the DRS/Profile System and in accordance with the deposit agreement will not constitute negligence or bad faith on the part of the depositary.
Shareholder Communications; Inspection of Register of Holders of ADSs
The depositary will make available for your inspection at its office all communications that it receives from us as a holder of deposited securities that we make generally available to holders of deposited securities. The depositary will send you copies of those communications if we ask it to. You have a right to inspect the register of holders of ADSs, but not for the purpose of contacting those holders about a matter unrelated to our business or the ADSs.
Disclosure of Interests
We may from time to time request ADS holders to provide information as to the capacity in they own or owned ADSs and regarding the identity of any other persons then or previously interested in such ADSs and the nature of such interest. Each ADS holder agrees to provide any information of that kind that is requested by us or
150
the depositary. To the extent that provisions of or governing the deposited securities or the rules or regulations of any governmental authority or securities exchange or automated quotation system may require the disclosure of beneficial or other ownership of deposited securities, other shares and other securities to us or other persons and may provide for blocking transfer and voting or other rights to enforce such disclosure or limit such ownership, the depositary has agreed to use its reasonable efforts to comply with our written instructions in respect of any such enforcement or limitation.
Warrants
The following summary of certain terms of the Warrants is not complete and is subject to, and qualified in its entirety by the provisions of the ADS Warrant Agent Agreement and Form of Global Warrant to Purchase ADSs, which are filed as exhibits to the registration statement of which this prospectus is a part. Prospective investors should carefully review the terms set forth in the ADS Warrant Agent Agreement and Form of Global Warrant to Purchase ADSs. Warrants issued in connection with our initial public offering in the United States are administered by The Bank of New York Mellon as Warrant Agent.
| | • | | Global Certificates, Book-entry Interests. The Warrants are represented by one or more global certificates in registered form. The global certificate was deposited with the Warrant Agent as custodian for The Depository Trust Company (“DTC”) and registered in the name of Cede & Co., as nominee of DTC. Ownership of interests in the global warrant certificate is limited to persons that have accounts with DTC or persons that have accounts with DTC participants. Book-entry interests in the Warrants will be shown on, and transfers of such interests will be effected only through records maintained by DTC and its participants. So long as the Warrants are held in global form, DTC will be considered the sole holder of the Warrants. Beneficial owners must rely on the procedures of the participants through which they own book-entry interests to exercise their Warrants or transfer their Warrants. |
| | • | | Exercisability. The Warrants are exercisable immediately upon issuance and at any time up to the date that is five years from the date of issuance. The Warrants are exercisable, at the option of each holder, in whole or in part by delivering to us a duly executed exercise notice accompanied by payment in full for the number of ADSs purchased upon such exercise. The Company will pay the ADS issuance fee of US$0.05 per ADS and any other applicable charges and taxes in connection with any such exercise. However, holders of our Warrants will be able to exercise the Warrants and receive freely tradable shares only if (i) a current registration statement under the Securities Act relating to the ADSs underlying the Warrants is then effective, or an exemption from such registration is available, and (ii) such ADSs are qualified for sale or exempt from qualification under the applicable securities laws of the states in which the various holders of Warrants reside, as further described in the ADS Warrant Agent Agreement. If these conditions are not met, holders will not be able to exercise their Warrants and receive freely tradable ADSs but rather will have the exercise price for the Warrants returned to them. In this event, the Company will pay in cash to each such Warrant holder the difference between the product of the number of Warrant ADSs set forth in the Warrant holder’s election to purchase and the closing sale price of the Warrant ADSs on The NASDAQ Capital Market and the aggregate exercise price payable to exercise Warrants to purchase the number of Warrant ADSs referenced in such election to purchase. |
| | • | | Maximum Percentage. A holder of a Warrant shall not have the right to exercise such Warrant, to the extent that after giving effect to such exercise, such person (together with such person’s affiliates and certain other persons), would beneficially own in excess of 4.99% (the “Maximum Percentage”) of the ordinary shares outstanding immediately after giving effect to such exercise. Subject to certain exceptions, “beneficial ownership” for purposes of determining the Maximum Percentage is calculated in accordance with Section 13(d) of the Exchange Act and the regulations of the SEC thereunder. Upon request by a Warrant holder, we will provide current information regarding the number of our outstanding ordinary shares. |
| | • | | Exercise Price. The initial exercise price per ADS purchasable upon exercise of the Warrants is equal to US$5.50. The Warrants may not be exercised on a “cashless” or “net exercise” basis. |
151
| | • | | Restrictive Legend Events. We will notify the Warrant Agent and each holder if we are unable to deliver ADSs via DTC transfer or otherwise (without restrictive legend), because (a) the SEC has issued a stop order with respect to the registration statement relating to the ADSs, (b) the SEC otherwise has suspended or withdrawn the effectiveness of such registration statement, either temporarily or permanently, (c) we have suspended or withdrawn the effectiveness of the registration statement, either temporarily or permanently, or (d) otherwise (each a “Restrictive Legend Event”). If a Restrictive Legend Event occurs after a Warrant holder has exercised a Warrant in accordance with its terms but prior to the delivery of the ADSs, or if we do not cause the depositary to timely deliver ADSs to a Warrant holder upon exercise of the Warrants, we will be obligated to pay a cash buy-in amount to the holder of the Warrants who did not receive ADSs upon such holder’s exercise of Warrants. |
| | • | | Anti-Dilution Provisions.The exercise price per Warrant and the numbers of Warrants shall be subject to adjustment from time to time in accordance with the ASX Listing Rules upon the occurrence of certain stock dividends and distributions, stock splits, stock subdivisions and combinations, reclassifications, rights issues, or similar events affecting our ADSs or ordinary shares, or upon the occurrence of a change in ADS ratio. |
| | • | | Transferability. Subject to applicable laws, the Warrants may be transferred at the option of the holders upon surrender of the Warrants to us together with the appropriate instruments of transfer. |
| | • | | Warrant Agent and Exchange Listing. The Warrants are issued in registered form under an ADS Warrant Agent Agreement between The Bank of New York Mellon, as warrant agent and us. |
| | • | | Rights as a Shareholder. Except as otherwise provided in the ADS Warrant Agent Agreement or by virtue of such holder’s ownership of ADSs or ordinary shares, the holder of Warrants does not have rights or privileges of a holder of ADSs or ordinary shares, including any voting rights, until the holder exercises the Warrant. |
152
SHARES ELIGIBLE FOR FUTURE SALE
As of December 31, 2015, we had:
| | • | | 146,529,096 ordinary shares outstanding, including shares underlying the ADSs; and |
| | • | | 38,566,203 ordinary shares issuable upon exercise of warrants and options. |
Future sales of substantial amounts of our ordinary shares or ADSs in the public market in the United States or in Australia, including ordinary shares issued upon exercise of outstanding options, or the possibility of such sales, could negatively affect the market price in the United States of the ADSs or Warrants and our ability to raise equity capital in the future.
All of the ADSs and Warrants sold in our initial public offering in the United States will be freely transferable in the United States by persons other than our “affiliates,” as that term is defined in Rule 144 under the Securities Act. As defined in Rule 144, an “affiliate” of an issuer is a person that directly, or indirectly through one or more intermediaries, controls, or is controlled by, or is under common control with, the issuer. ADSs or Warrants purchased by one of our affiliates may not be resold, except pursuant to an effective registration statement or an exemption from registration, including Rule 144 under the Securities Act (as described below).
Rule 144
In general, under Rule 144 of the Securities Act and beginning 90 days after the date of this prospectus, a person who is not deemed to have been our affiliate at any time during the three months preceding a sale and who has beneficially owned “restricted securities” within the meaning of Rule 144 for more than six months would be entitled to sell an unlimited number of shares, subject only to the availability of current public information about us. A non-affiliate who has beneficially owned “restricted securities” for at least one year from the later of the date these shares were acquired from us or from our affiliate would be entitled to freely sell those shares.
A person who is deemed to be an affiliate of ours and who has beneficially owned “restricted securities” for at least six months would be entitled to sell, within any three-month period, a number of shares that is not more than the greater of:
| | • | | 1.0% of the number of our ordinary shares then outstanding; or |
| | • | | the average weekly reported trading volume of our ordinary shares on NASDAQ during the four calendar weeks preceding the date on which a notice of the sale on Form 144 is filed with the SEC by such person. |
Sales under Rule 144 of the Securities Act by persons who are deemed to be our affiliates are also subject to manner-of-sale provisions, notice requirements and the availability of current public information about us as specified in Rule 144.
Regulation S
Regulation S provides generally that sales made in offshore transactions are not subject to the registration or prospectus delivery requirements of the Securities Act.
Rule 701
In general, under Rule 701 of the Securities Act, each of our employees, consultants or advisors who purchased our ordinary shares from us in connection with a compensatory stock plan or other written agreement executed prior to the completion of our initial public offering in the United States is eligible to resell such ordinary shares in reliance on Rule 144, but without compliance with some of the restrictions, including the holding period, contained in Rule 144.
153
Equity Incentive Plans
We have filed with the SEC a registration statement on Form S-8 under the Securities Act covering the ordinary shares reserved for issuance under our equity incentive plans. Accordingly, shares registered under the Form S-8 registration statement will be available for sale in the open market following the registration statement’s effective date, subject to Rule 144 volume limitations described above, if applicable.
154
TAXATION
The following is a summary of material U.S. federal and Australian income tax considerations to U.S. holders, as defined below, of the acquisition, ownership and disposition of ordinary shares, ADSs or Warrants. This discussion is based on the laws in force as of the date of this registration statement, and is subject to changes in the relevant income tax law, including changes that could have retroactive effect. The following summary does not take into account or discuss the tax laws of any country or other taxing jurisdiction other than the United States and Australia. Holders are advised to consult their tax advisors concerning the overall tax consequences of the acquisition, ownership and disposition of ordinary shares, ADSs or Warrants in their particular circumstances. This discussion is not intended, and should not be construed, as legal or professional tax advice.
This summary does not address the 3.8% U.S. Federal Medicare Tax on net investment income, the effects of U.S. federal estate and gift tax laws, the alternative minimum tax, or any state and local tax considerations within the United States, and is not a comprehensive description of all U.S. federal or Australian income tax considerations that may be relevant to a decision to acquire or dispose of ordinary shares, ADSs or Warrants. Furthermore, this summary does not address U.S. federal or Australian income tax considerations relevant to holders subject to taxing jurisdictions other than, or in addition to, the United States and Australia, and does not address all possible categories of holders, some of which may be subject to special tax rules.
U.S. Federal Income Tax Considerations
The following summary describes the material U.S. federal income tax consequences to U.S. holders (as defined below) of the acquisition, ownership and disposition of our ordinary shares and ADSs or Warrants as of the date hereof. Subject to the qualifications, assumptions and limitations set forth herein, this discussion of the material U.S. federal income tax consequences to U.S. holders of our ordinary shares and ADSs or Warrants represents the opinion of Baker & McKenzie LLP, our U.S. counsel. Except where noted, this summary deals only with ordinary shares or ADSs or Warrants acquired in the initial offering and held as capital assets within the meaning of Section 1221 of the Internal Revenue Code of 1986, as amended (the “Code”). This section does not discuss the tax consequences to any particular holder, nor any tax considerations that may apply to holders subject to special tax rules, such as:
| | • | | financial institutions; |
| | • | | individual retirement and other tax-deferred accounts; |
| | • | | regulated investment companies; |
| | • | | real estate investment trusts; |
| | • | | individuals who are former U.S. citizens or former long-term U.S. residents; |
| | • | | brokers or dealers in securities or currencies; |
| | • | | traders that elect to use a mark-to-market method of accounting; |
| | • | | investors in pass-through entities for U.S. federal income tax purposes; |
| | • | | persons that hold ordinary shares or ADSs or Warrants as a position in a straddle or as part of a hedging, constructive sale or conversion transaction for U.S. federal income tax purposes; |
| | • | | persons that have a functional currency other than the U.S. dollar; |
| | • | | persons that are not U.S. holders (as defined below); |
| | • | | persons who are resident in or have a permanent establishment in Australia. |
155
In this section, a “U.S. holder” means a beneficial owner of ordinary shares or ADSs or Warrants that is, for U.S. federal income tax purposes:
| | • | | an individual who is a citizen or resident of the United States; |
| | • | | a corporation created or organized in or under the laws of the United States or any state thereof or the District of Columbia; |
| | • | | an estate the income of which is subject to U.S. federal income taxation regardless of its source; or |
| | • | | a trust (i) the administration of which is subject to the primary supervision of a court in the United States and for which one or more U.S. persons have the authority to control all substantial decisions or (ii) that has an election in effect under applicable income tax regulations to be treated as a U.S. person. |
As used in this section, a “non-U.S. holder” is a beneficial owner of ordinary shares or ADSs or Warrants that is not a U.S. holder or an entity or arrangement treated as a partnership for U.S. federal income tax purposes.
The discussion below is based upon the provisions of the Code, and the U.S. Treasury regulations, rulings and judicial decisions thereunder as of the date hereof, and such authorities may be replaced, revoked or modified, possibly with retroactive effect, so as to result in U.S. federal income tax consequences different from those discussed below. In addition, this summary is based, in part, upon representations made by the depositary to us and assumes that the deposit agreement, and all other related agreements, will be performed in accordance with their terms.
If an entity or arrangement treated as a partnership for U.S. federal income tax purposes acquires, owns or disposes of ordinary shares or ADSs or Warrants, the U.S. federal income tax treatment of a partner generally will depend on the status of the partner and the activities of the partnership. Partners of partnerships that acquire, own or dispose of ordinary shares or ADSs or Warrants should consult their tax advisors.
You are urged to consult your own tax advisor with respect to the U.S. federal, as well as state, local and non-U.S., tax consequences to you of acquiring, owning and disposing of ordinary shares or ADSs or Warrants in light of your particular circumstances, including the possible effects of changes in U.S. federal and other tax laws.
Allocation of Purchase Price
A U.S. holder will allocate the amount paid for ordinary shares or ADSs and Warrants based on their relative fair market values in computing the holder’s basis in the ordinary shares or ADSs and Warrants for U.S. federal income tax purposes.
ADSs
If you hold ADSs, you generally will be treated, for U.S. federal income tax purposes, as the owner of the underlying ordinary shares that are represented by such ADSs. Accordingly, deposits or withdrawals of ordinary shares for ADSs will not be subject to U.S. federal income tax.
Distributions
Subject to the passive foreign investment company rules discussed below, U.S. holders generally will include as dividend income the U.S. dollar value of the gross amount of any distributions of cash or property (without deduction for any withholding tax), other than certain pro rata distributions of ordinary shares, with respect to ordinary shares to the extent the distributions are made from our current or accumulated earnings and profits, as determined for U.S. federal income tax purposes. A U.S. holder will include the dividend income on the day actually or constructively received by the holder, in the case of ordinary shares, or by the depositary, in the case of ADSs. To the extent, if any, that the amount of any distribution by us exceeds our current and accumulated earnings
156
and profits, as so determined, the excess will be treated first as a tax-free return of the U.S. holder’s tax basis in the ordinary shares or ADSs and thereafter as capital gain. Notwithstanding the foregoing, we do not intend to maintain calculations of earnings and profits, as determined for U.S. federal income tax purposes. Consequently, any distributions generally will be reported as dividend income for U.S. information reporting purposes. See “Backup Withholding Tax and Information Reporting Requirements” below. Dividends paid by us will not be eligible for the dividends-received deduction generally allowed to U.S. corporate shareholders.
Subject to certain exceptions for short-term and hedged positions, the U.S. dollar amount of dividends received by an individual, trust or estate with respect to the ordinary shares or ADSs will be subject to taxation at a maximum rate of 20% if the dividends are “qualified dividends.” Dividends will be treated as qualified dividends (a) if certain holding period requirements are satisfied, (b) if the Treaty is a qualified treaty and we are eligible for benefits under the Agreement between the Government of the United States of America and the Government of Australia for the Avoidance of Double Taxation and the Prevention of Fiscal Evasion with Respect to Taxes on Income (the “Treaty”) or our ordinary shares or ADSs are readily tradable on a U.S. securities market, and (c) provided that we were not, in the taxable year prior to the year in which the dividend was paid, and are not, in the taxable year in which the dividend is paid, a PFIC. The Treaty has been approved for the purposes of the qualified dividend rules and the ADSs are listed on NASDAQ. We do not believe we were a PFIC in 2015 and do not expect to be a PFIC for 2016. However, our status in the current year and future years will depend in part upon our use of the funds from our initial public offering in the United States, as well as our income and assets (which for this purpose depends in part on the market value of our shares) in those years. See the discussion below under “—Passive Foreign Investment Company”. You should consult your tax adviser regarding the availability of the reduced tax rate on qualified dividends.
Includible distributions paid in Australian dollars, including any Australian withholding taxes, will be included in the gross income of a U.S. holder in a U.S. dollar amount calculated by reference to the spot exchange rate in effect on the date of actual or constructive receipt, regardless of whether the Australian dollars are converted into U.S. dollars at that time. If Australian dollars are converted into U.S. dollars on the date of actual or constructive receipt, the tax basis of the U.S. holder in those Australian dollars will be equal to their U.S. dollar value on that date and, as a result, a U.S. holder generally should not be required to recognize any foreign exchange gain or loss.
If Australian dollars so received are not converted into U.S. dollars on the date of receipt, the U.S. holder will have a basis in the Australian dollars equal to their U.S. dollar value on the date of receipt. Any gain or loss on a subsequent conversion or other disposition of the Australian dollars generally will be treated as ordinary income or loss to such U.S. holder and generally will be income or loss from sources within the United States for foreign tax credit limitation purposes.
Dividends received by a U.S. holder with respect to ordinary shares or ADSs will be treated as foreign source income, which may be relevant in calculating the holder’s foreign tax credit limitation. The limitation on foreign taxes eligible for credit is calculated separately with respect to specific classes of income. For these purposes, dividends generally will be categorized as “passive” or “general” income depending on a U.S. holder’s circumstance.
Subject to certain complex limitations, a U.S. holder generally will be entitled, at its option, to claim either a credit against its U.S. federal income tax liability or a deduction in computing its U.S. federal taxable income in respect of any Australian taxes withheld. If a U.S. holder elects to claim a deduction, rather than a foreign tax credit, for Australian taxes withheld for a particular taxable year, the election will apply to all foreign taxes paid or accrued by or on behalf of the U.S. holder in the particular taxable year.
You may not be able to claim a foreign tax credit (and instead may claim a deduction) for non-U.S. taxes imposed on dividends paid on the ordinary shares or ADSs if you (i) have held the ordinary shares or ADSs for less than a specified minimum period during which you are not protected from risk of loss with respect to such shares, or (ii) are obligated to make payments related to the dividends (for example, pursuant to a short sale).
157
The availability of the foreign tax credit and the application of the limitations on its availability are fact specific and are subject to complex rules. You are urged to consult your own tax advisor as to the consequences of Australian withholding taxes and the availability of a foreign tax credit or deduction. See “Australian Tax Considerations—Taxation of Dividends.”
Exercise, Expiration and Disposition of Warrants
A U.S. holder will not recognize gain or loss upon exercise of a Warrant (except with respect to any cash received in lieu of a fractional ordinary share or ADS). A U.S. holder will have a tax basis in the ADSs received upon the exercise of a Warrant equal to the sum of its tax basis in the Warrant and the aggregate cash exercise price paid in respect of such exercise, less any amount attributable to any fractional ordinary share or ADS. The holding period of the ordinary shares or ADSs received upon the exercise of a Warrant will commence on the day after the Warrant is exercised. If a Warrant expires without being exercised, a U.S. holder will recognize a capital loss in an amount equal to its tax basis in the Warrant.
Subject to the passive foreign investment company rules discussed below, upon the sale, exchange or redemption of a Warrant, a U.S. holder will recognize a gain or loss equal to the difference between the amount realized on the sale, exchange or redemption of the Warrant and the U.S. holder’s tax basis in such Warrant. Such gain or loss will be long-term capital gain or loss if, at the time of such sale, exchange, or redemption, the Warrant has been held for more than one year. Long term capital gains of individuals (as well as certain trusts and estates) are subject to U.S. federal income tax at preferential rates. The deductibility of capital losses is subject to significant limitations. A U.S. holder’s gain or loss on the sale, exchange, or redemption of a Warrant will be treated as U.S. source income or loss for U.S. foreign tax credit limitation purposes.
In the event we are a passive foreign investment company, a U.S. holder will be taxed upon the sale, exchange, redemption or other taxable disposition of a Warrant in the same manner that such U.S. holder would be taxed upon the sale, exchange, redemption or other taxable disposition of shares in a passive foreign investment company, except that the Warrants will not be eligible for the mark-to-market election. See the discussion under “—Passive Foreign Investment Company” below.
Sale, Exchange or other Disposition of Ordinary Shares or ADSs
Subject to the passive foreign investment company rules discussed below, a U.S. holder generally will, for U.S. federal income tax purposes, recognize capital gain or loss on a sale, exchange or other disposition of ordinary shares or ADSs equal to the difference between the amount realized on the disposition and the U.S. holder’s tax basis (in U.S. dollars) in the ordinary shares or ADSs. This recognized gain or loss will generally be long-term capital gain or loss if the U.S. holder has held the ordinary shares or ADSs for more than one year. Generally, for U.S. holders who are individuals (as well as certain trusts and estates), long-term capital gains are subject to U.S. federal income tax at preferential rates. For foreign tax credit limitation purposes, gain or loss recognized upon a disposition generally will be treated as from sources within the United States. However, in limited circumstances, the Treaty can re-source U.S. source income as Australian source income. The deductibility of capital losses is subject to limitations for U.S. federal income tax purposes.
You should consult your own tax advisor regarding the availability of a foreign tax credit or deduction in respect of any Australian tax imposed on a sale or other disposition of ordinary shares or ADSs. See “Australian Tax Considerations—Tax on Sales or other Dispositions of Shares.”
Passive Foreign Investment Company
The Code provides special, generally adverse, rules regarding certain distributions received by U.S. holders with respect to, and sales, exchanges and other dispositions, including pledges, of, shares of stock of a PFIC. A foreign corporation will be a PFIC for any taxable year if at least 75% of its gross income for the taxable year is passive income or at least 50% of its gross assets during the taxable year, based on a quarterly average and generally
158
by value, produce or are held for the production of passive income. Passive income for this purpose generally includes, among other things, dividends, interest, rents, royalties, gains from commodities and securities transactions and gains from the disposition of assets that produce or are held for the production of passive income. In determining whether a foreign corporation is a PFIC, a pro-rata portion of the income and assets of each corporation in which it owns, directly or indirectly, at least a 25% interest (by value) is taken into account.
Based on our business results for the last fiscal year and the composition of our assets, we believe that we were not a PFIC for U.S. federal income tax purposes for the taxable year ended June 30, 2015. Similarly, based on our business projections and the anticipated composition of our assets for the current and future years, we expect that we will also not be a PFIC for the taxable year ending June 30, 2016. However, the determination of PFIC status is a factual determination that must be made annually at the close of each taxable year and therefore, there can be no certainty as to our status for a taxable year until the close of that taxable year. Our status could change depending, among other things, upon a decrease in the trading price of our ordinary shares or ADSs, how quickly we make use of the proceeds from our initial public offering in the United States, as well as changes in the composition and relative values of our assets and the composition of our income. Moreover, the rules governing whether certain assets are active or passive are complex and in some cases their application can be uncertain. If we were a PFIC in any year during a U.S. holder’s holding period for the ordinary shares or ADSs, we generally would continue to be treated as a PFIC for each subsequent year during which the U.S. holder owned the ordinary shares or ADSs.
If we are a PFIC for any taxable year during which a U.S. holder holds ordinary shares or ADSs, any “excess distribution” that the holder receives and any gain realized from a sale or other disposition (including a pledge) of such ordinary shares or ADSs will be subject to special tax rules, unless the holder makes a mark-to-market election or qualified electing fund election, as discussed below. Any distribution in a taxable year that is greater than 125% of the average annual distribution received by a U.S. holder during the shorter of the three preceding taxable years or such holder’s holding period for the ordinary shares or ADSs will be treated as an excess distribution. Under these special tax rules:
| | • | | the excess distribution or gain will be allocated ratably over the U.S. holder’s holding period for the ordinary shares or ADSs; |
| | • | | the amount allocated to the current taxable year, and any taxable year prior to the first taxable year in which we were a PFIC in the U.S. holder’s holding period, will be treated as ordinary income arising in the current taxable year; and |
| | • | | the amount allocated to each other year will be subject to income tax at the highest rate in effect for that year and the interest charge generally applicable to underpayments of tax will be imposed on the resulting tax attributable to each such year. |
The tax liability for amounts allocated to years prior to the year of disposition or excess distribution cannot be offset by any net operating loss, and gains (but not losses) realized on the transfer of the ordinary shares or ADSs cannot be treated as capital gains, even if the ordinary shares or ADSs are held as capital assets. In addition, non-corporate U.S. holders will not be eligible for reduced rates of taxation on any dividends that we pay if we are a PFIC for either the taxable year in which the dividend is paid or the preceding year. Furthermore, unless otherwise provided by the U.S. Treasury Department, each U.S. holder of a PFIC is required to file an annual report containing such information as the U.S. Treasury Department may require.
If we are a PFIC for any taxable year during which any of our non-U.S. subsidiaries is also a PFIC, a U.S. holder of ordinary shares or ADSs during such year would be treated as owning a proportionate amount (by value) of the shares of the lower-tier PFIC for purposes of the application of these rules to such subsidiary. You should consult your tax advisors regarding the tax consequences if the PFIC rules apply to any of our subsidiaries.
159
In certain circumstances, in lieu of being subject to the special tax rules discussed above, you may make an election to include gain on the stock of a PFIC as ordinary income under a mark-to-market method, provided that such stock is regularly traded on a qualified exchange. Under current law, the mark-to-market election may be available to U.S. holders of ADSs if the ADSs are listed on NASDAQ, which constitutes a qualified exchange, although there can be no assurance that the ADSs will be “regularly traded” for purposes of the mark-to-market election. It should also be noted that it is intended that only the ADSs and not the ordinary shares are listed on NASDAQ. While we would expect the Australian Stock Exchange, on which the ordinary shares are listed, to be considered a qualified exchange, no assurance can be given as to whether the Australian Stock Exchange is a qualified exchange, or that the ordinary shares would be traded in sufficient frequency to be considered regularly traded for these purposes. Additionally, because a mark-to-market election cannot be made for equity interests in any lower-tier PFIC that we may own, a U.S. holder that makes a mark-to-mark election with respect to us may continue to be subject to the PFIC rules with respect to any indirect investments held by us that are treated as an equity interest in a PFIC for U.S. federal income tax purposes. If you make an effective mark-to-market election, you will include in each year that we are a PFIC as ordinary income the excess of the fair market value of your ordinary shares or ADSs at the end of your taxable year over your adjusted tax basis in the ordinary shares or ADSs. You will be entitled to deduct as an ordinary loss in each such year the excess of your adjusted tax basis in the ordinary shares or ADSs over their fair market value at the end of the year, but only to the extent of the net amount previously included in income as a result of the mark-to-market election. If you make an effective mark-to-market election, any gain you recognize upon the sale or other disposition of your ordinary shares or ADSs in a year that we are a PFIC will be treated as ordinary income and any loss will be treated as ordinary loss, but only to the extent of the net amount previously included in income as a result of the mark-to-market election. Any gain or loss you recognize upon the sale or other disposition of your ordinary shares or ADSs in a year when we are not a PFIC will be a capital gain or loss. See “—Sale, Exchange or other Disposition of Ordinary shares or ADSs” above for the treatment of capital gains and losses.
Your adjusted tax basis in the ordinary shares or ADSs will be increased by the amount of any income inclusion and decreased by the amount of any deductions under the mark-to-market rules. If you make a mark-to-market election it will be effective for the taxable year for which the election is made and all subsequent taxable years unless the ordinary shares or ADSs are no longer regularly traded on a qualified exchange or the IRS consents to the revocation of the election. You are urged to consult your tax advisors about the availability of the mark-to-market election, and whether making the election would be advisable in your particular circumstances. In the case of a valid mark-to-market election, any distributions we make would generally be subject to the rules discussed above under “—Taxation of Dividends,” except the reduced rates of taxation on any dividends received from us would not apply.
Alternatively, you can sometimes avoid the PFIC rules described above by electing to treat us as a “qualified electing fund” under Section 1295 of the Code. However, this option will not be available to you because we do not intend to comply with the requirements necessary to permit you to make this election.
U.S. holders are urged to contact their own tax advisors regarding the determination of whether we are a PFIC and the tax consequences of such status.
Backup Withholding Tax and Information Reporting Requirements
Payments of dividends with respect to the ordinary shares or ADSs and proceeds from the sale, exchange or other disposition of the ordinary shares or ADSs or Warrants, by a U.S. paying agent or other U.S. intermediary, or made into the United States, will be reported to the IRS and to the U.S. holder as may be required under applicable Treasury regulations. Backup withholding may apply to these payments if the U.S. holder fails to provide an accurate taxpayer identification number or certification of exempt status or fails to comply with applicable certification requirements. Certain U.S. holders (including, among others, corporations) are not subject to backup withholding and information reporting. Backup withholding is not an additional tax. Any amounts withheld under the backup withholding rules from a payment to a U.S. holder will be refunded (or
160
credited against such U.S. holder’s U.S. federal income tax liability, if any), provided the required information is timely furnished to the IRS. Prospective investors should consult their own tax advisors as to their qualification for exemption from backup withholding and the procedure for establishing an exemption.
Certain individual U.S. holders (and under proposed Treasury regulations, certain entities) may be required to report to the IRS information with respect to their investment in the ordinary shares or ADSs or Warrants not held through an account with a U.S. financial institution. U.S. holders who fail to report required information could become subject to substantial penalties. U.S. holders are encouraged to consult with their own tax advisors regarding foreign financial asset reporting requirements with respect to their investment in the ordinary shares or ADSs.
U.S. holders who acquire any of the ordinary shares or ADSs or Warrants for cash may be required to file an IRS Form 926 (Return by a U.S. Transferor of Property to a Foreign Corporation) with the IRS and to supply certain additional information to the IRS if (i) immediately after the transfer, the U.S. holder owns directly or indirectly (or by attribution) at least 10% of our total voting power or value or (ii) the amount of cash transferred to us in exchange for the ordinary shares or ADSs or Warrants when aggregated with all related transfers under applicable regulations, exceeds US$100,000. Substantial penalties may be imposed on a U.S. holder that fails to comply with this reporting requirement. Each U.S. holder is urged to consult with its own tax advisor regarding this reporting obligation.
The discussion above is not intended to constitute a complete analysis of all tax considerations applicable to an investment in ordinary shares or ADSs or Warrants. You should consult with your own tax advisor concerning the tax consequences to you in your particular situation.
Australian Tax Considerations
In this section, we discuss the material Australian income tax, stamp duty and goods and services tax considerations related to the acquisition, ownership and disposal by the absolute beneficial owners of the ordinary shares, ADSs or Warrants. This discussion represents the opinion of Baker & McKenzie, Australian counsel to Benitec.
It is based upon existing Australian tax law as of the date of this registration statement, which is subject to change, possibly retrospectively. This discussion does not address all aspects of Australian tax law which may be important to particular investors in light of their individual investment circumstances, such as shares held by investors subject to special tax rules (for example, financial institutions, insurance companies or tax exempt organizations). In addition, this summary does not discuss any foreign or state tax considerations, other than stamp duty and goods and services tax.
Prospective investors are urged to consult their tax advisors regarding the Australian and foreign income and other tax considerations of the acquisition, ownership and disposition of the shares or warrants. As used in this summary a “Non-Australian Shareholder” is a holder that is not an Australian tax resident and is not carrying on business in Australia through a permanent establishment.
Nature of ADSs for Australian Taxation Purposes
Ordinary shares represented by ADSs held by a U.S. holder will be treated for Australian taxation purposes as held under a “bare trust” for such holder. Consequently, the underlying ordinary shares will be regarded as owned by the ADS holder for Australian income tax and capital gains tax purposes. Dividends paid on the underlying ordinary shares will also be treated as dividends paid to the ADS holder, as the person beneficially entitled to those dividends. Therefore, in the following analysis we discuss the tax consequences to Non-Australian Shareholders of ordinary shares for Australian taxation purposes. We note that the holder of an ADS will be treated for Australian tax purposes as the owner of the underlying ordinary shares that are represented by such ADSs.
161
Taxation of Dividends
Australia operates a dividend imputation system under which dividends may be declared to be “franked” to the extent of tax paid on company profits. Fully franked dividends are not subject to dividend withholding tax. An exemption for dividend withholding tax can also apply to unfranked dividends that are declared to be conduit foreign income, or CFI, and paid to Non-Australian Shareholders. Dividend withholding tax will be imposed at 30%, unless a shareholder is a resident of a country with which Australia has a double taxation agreement and qualifies for the benefits of the treaty. Under the provisions of the current Double Taxation Convention between Australia and the United States, the Australian tax withheld on unfranked dividends that are not declared to be CFI paid by us to a resident of the United States which is beneficially entitled to that dividend is limited to 15% where that resident is a qualified person for the purposes of the Double Taxation Convention between Australia and the United States.
If a Non-Australian Shareholder is a company and owns a 10% or more interest, the Australian tax withheld on dividends paid by us to which a resident of the United States is beneficially entitled is limited to 5%. In limited circumstances the rate of withholding can be reduced to zero.
Exercise of Warrants
Any capital gain or loss on exercise of a warrant is disregarded. The amount paid to acquire the warrant, and the amount paid to exercise the warrant, are both included in the cost base and reduced cost base of the ordinary shares underlying the Warrants.
Tax on Sales or other Dispositions of Shares or Warrants—Capital gains tax
Non-Australian Shareholders will not be subject to Australian capital gains tax on the gain made on a sale or other disposal of ordinary shares or warrants (or recognise a capital loss on the lapse of a warrant), unless they, together with associates, hold 10% or more of our issued capital, at the time of disposal or for 12 months of the last 2 years prior to disposal.
Non-Australian Shareholders who own a 10% or more interest would be subject to Australian capital gains tax if more than 50% of our direct or indirect assets, determined by reference to market value, consists of Australian land, leasehold interests or Australian mining, quarrying or prospecting rights. The Double Taxation Convention between the United States and Australia is unlikely to limit Australia’s right to tax any gain in these circumstances. Net capital gains are calculated after reduction for capital losses, which may only be offset against capital gains.
Tax on Sales or other Dispositions of Shares or Warrants—Shareholders Holding Shares and Warrants on Revenue Account
Some Non-Australian Shareholders may hold shares on revenue rather than on capital account for example, share traders. These shareholders may have the gains made on the sale or other disposal of the shares and/or warrants included in their assessable income under the ordinary income taxing provisions of the income tax law, if the gains are sourced in Australia.
Non-Australian Shareholders assessable under these ordinary income provisions in respect of gains made on shares and/or warrants held on revenue account would be assessed for such gains at the Australian tax rates for non-Australian residents, which start at a marginal rate of 32.5%. This rate does not include the Temporary Budget Repair Levy of 2% that applies in certain circumstances. Some relief from Australian income tax may be available to Non-Australian Shareholders under the Double Taxation Convention between the United States and Australia.
162
To the extent an amount would be included in a Non-Australian Shareholder’s assessable income under both the capital gains tax provisions and the ordinary income provisions, the capital gain amount would generally be reduced, so that the shareholder would not be subject to double tax on any part of the income gain or capital gain.
Dual Residency
If a shareholder is a resident of both Australia and the United States under those countries’ domestic taxation laws, that shareholder may be subject to tax as an Australian resident. If, however, the shareholder is determined to be a U.S. resident for the purposes of the Double Taxation Convention between the United States and Australia, the Australian tax would be subject to limitation by the Double Taxation Convention. Shareholders should obtain specialist taxation advice in these circumstances.
Stamp Duty
No stamp duty is payable by Australian residents or non-Australian residents on the issue and trading of shares or warrants that are quoted on the ASX or NASDAQ at all relevant times and the shares do not represent 90% or more of all of our issued shares.
Australian Death Duty
Australia does not have estate or death duties. As a general rule, no capital gains tax liability is realized upon the inheritance of a deceased person’s shares. The disposal of inherited shares by beneficiaries may, however, give rise to a capital gains tax liability if the gain falls within the scope of Australia’s jurisdiction to tax.
Goods and Services Tax
The issue or transfer of shares or warrants to a non-Australian resident investor will not incur Australian goods and services tax.
163
UNDERWRITING
We and the underwriter named below entered into an underwriting agreement, dated August 17, 2015, with respect to the ADSs and Warrants being offered in our initial public offering in the United States. Subject to certain conditions, the underwriter agreed to purchase the number of ADSs and Warrants indicated in the following table.
| | | | | | | | |
Underwriter | | Number of
Warrants | | | Number of
ADSs | |
Maxim Group LLC | | | 500,000 | | | | 1,500,000 | |
| | | | | | | | |
Total | | | 500,000 | | | | 1,500,000 | |
| | | | | | | | |
The underwriter took and paid for all of the ADSs and Warrants offered in our initial public offering in the United States.
The underwriter was granted a 45-day option to buy up to an additional 225,000 ADSs and/or 75,000 additional Warrants from us to cover sales by the underwriter of a greater number of ADSs than the total number set forth in the table above. The underwriter partially exercised its option and purchased 75,000 Warrants, which the underwriter offered on the same terms as those on which the Warrants were offered in connection with our U.S. initial public offering.
The underwriter offered the ADSs and Warrants representing an interest in our ordinary shares directly to the public at the initial public offering price of $9.21 per ADS and $0.01 per Warrant and to certain dealers at such offering price less a concession not in excess of US$0.6447 per ADS and US$0.0007 per Warrant. After the initial public offering of the ADSs and Warrants in the United States, the underwriter was permitted to change the offering price and the selling concession.
All of the Warrants to purchase ADSs which are covered by this prospectus are already outstanding and not additional Warrants will be issued. We will deliver the ADSs underlying the Warrants directly to the Warrant holders upon exercise of the Warrants.
The following table shows the initial public offering price per ADS and Warrant, the total underwriting discounts and commissions to be paid by us to the underwriter and the proceeds, before expenses, to us, with and without the underwriter’s partial exercise of its option.
| | | | | | | | | | | | | | | | |
| | | Per ADS | | | Per Warrant | | | Total Without
Over-Allotment | | | Total With
Partial Over-
Allotment | |
Public offering price | | US$ | 9.21 | | | US$ | 0.01 | | | US$ | 13,820,000 | | | US$ | 13,820,750 | |
Underwriter discount and commission | | US$ | 0.6447 | | | US$ | 0.0007 | | | US$ | 967,400 | | | US$ | 967,453 | |
Proceeds, before expenses, to us | | US$ | 8.5653 | | | US$ | 0.0093 | | | US$ | 12,852,600 | | | US$ | 12,853,297 | |
We paid Maxim Group LLC an advance of US$30,000 to be applied towards its anticipated out-of-pocket expenses to be incurred in connection with our initial public offering in the United States.
The total expenses of our U.S. initial public offering, including registration, filing and listing fees, printing fees and legal and accounting expenses, but excluding underwriting discounts and commissions, were US$1.8 million, all of which were paid by us. We also reimbursed the underwriter for certain of their expenses incurred in connection with the clearance of our initial public offering in the United States with the Financial Industry Regulatory Authority, Inc.
Prior to our initial public offering in the United States, neither the ADSs nor the Warrants were listed on any stock exchange in the United States. Our ordinary shares are listed on the Australian Securities Exchange, or
164
ASX, under the symbol “BLT.” The public offering price of the ADSs was determined by negotiations between us and the underwriter taking into account the most recent closing price of our ordinary shares on the ASX prior to the pricing date as well as other factors, including: prevailing market conditions, our historical performance, estimates of our business potential and earnings prospects, an assessment of our management and the consideration of the above factors in relation to market valuation of companies in related businesses.
The ADSs and Warrants are listed on The NASDAQ Capital Market under the symbols “BNTC” and “BNTCW.”
In connection with the offering, the underwriter may purchase and sell ADSs and Warrants in the open market. These transactions may include short sales, stabilizing transactions and purchases to cover positions created by short sales. Short sales involve the sale by the underwriter of a greater number of ADSs and Warrants than they are required to purchase in the offering, and a short position represents the amount of such sales that have not been covered by subsequent purchases. A “covered short position” is a short position that is not greater than the amount of additional ADSs for which the underwriter’s option described above may be exercised. The underwriter may cover any covered short position by either exercising their option to purchase additional ADSs or purchasing ADSs in the open market. In determining the source of ADSs to cover the covered short position, the underwriter will consider, among other things, the price of ADSs available for purchase in the open market as compared to the price at which they may purchase additional ADSs pursuant to the option described above. “Naked” short sales are any short sales that create a short position greater than the amount of additional ADSs for which the option described above may be exercised. The underwriter must cover any such naked short position by purchasing ADSs in the open market. A naked short position is more likely to be created if the underwriter is concerned that there may be downward pressure on the price of the ADSs in the open market after pricing that could adversely affect investors who purchase in the offering. Stabilizing transactions consist of various bids for or purchases of ADSs made by the underwriter in the open market prior to the completion of the offering.
Purchases to cover a short position and stabilizing transactions, as well as other purchases by the underwriter for its own account, may have the effect of preventing or retarding a decline in the market price of the ADSs, and together with the imposition of the penalty bid, may stabilize, maintain or otherwise affect the market price of the ADSs. As a result, the price of our ADSs may be higher than the price that otherwise might exist in the open market. The underwriter is not required to engage in these activities and may end any of these activities at any time. These transactions may be effected on The NASDAQ Capital Market, in the over-the-counter market or otherwise.
In connection with this offering, the underwriter may engage in passive market making transactions in the ADSs on The NASDAQ Capital Market in accordance with Rule 103 of Regulation M under the Exchange Act during a period before the commencement of offers or sales of ADSs and extending through the completion of distribution. A passive market maker must display its bid at a price not in excess of the highest independent bid of that security. However, if all independent bids are lowered below the passive market maker’s bid, that bid must then be lowered when specified purchase limits are exceeded. Passive market making may cause the price of our ADSs to be higher than the price that otherwise would exist in the open market in the absence of those transactions. The underwriter is not required to engage in passive market making and may end passive market making activities at any time.
We have agreed to indemnify the underwriter against certain liabilities, including liabilities under the Securities Act and to contribute to payments that the underwriter may be required to make for these liabilities.
A prospectus in electronic format may be made available on websites maintained by the underwriter, or selling group members, if any, participating in this offering. The underwriter may agree to allocate a number of ADSs to one or more selling group members for sale to their online brokerage account holders. Internet distributions will be allocated by the underwriter on the same basis as other allocations.
165
The underwriter and its affiliates are full service financial institutions engaged in various activities, which may include sales and trading, commercial and investment banking, advisory, investment management, investment research, principal investment, hedging, market making, brokerage and other financial and non-financial activities and services. The underwriter and its affiliates have provided, and may in the future provide, a variety of these services to the issuer and to persons and entities with relationships with the issuer, for which they received or will receive customary fees and expenses.
In the ordinary course of their various business activities, the underwriter and its affiliates, officers, directors and employees may purchase, sell or hold a broad array of investments and actively trade securities, derivatives, loans, commodities, currencies, credit default swaps and other financial instruments for their own account and for the accounts of their customers, and such investment and trading activities may involve or relate to assets, securities and/or instruments of the issuer (directly, as collateral securing other obligations or otherwise) and/or persons and entities with relationships with the issuer. The underwriter and its affiliates may also communicate independent investment recommendations, market color or trading ideas and/or publish or express independent research views in respect of such assets, securities or instruments and may at any time hold, or recommend to clients that they should acquire, long and/or short positions in such assets, securities and instruments.
Offer Restrictions Outside the United States
Other than in the United States, no action has been taken by us or the underwriter that would permit a public offering of the ADSs or Warrants offered by this prospectus in any jurisdiction where action for that purpose is required. The ADSs and Warrants offered by this prospectus may not be offered or sold, directly or indirectly, nor may this prospectus or any other offering material or advertisements in connection with the offer and sale of any such ADSs or Warrants be distributed or published in any jurisdiction, except under circumstances that will result in compliance with the applicable rules and regulations of that jurisdiction. Persons into whose possession this prospectus comes are advised to inform themselves about and to observe any restrictions relating to the offering and the distribution of this prospectus. This prospectus does not constitute an offer to sell or a solicitation of an offer to buy any ADSs or Warrants offered by this prospectus in any jurisdiction in which such an offer or a solicitation is unlawful.
166
EXPENSES RELATING TO OUR INITIAL PUBLIC OFFERING
Set forth below is an itemization of the estimated expenses, excluding underwriting discounts, that were incurred in connection with our initial public offering and sale of the ADSs and Warrants in the United States. Expenses for our initial public offering in the United States were borne by us.
| | | | |
SEC registration fee | | $ | 9,000 | |
NASDAQ listing fee | | | 125,000 | |
Financial Industry Regulatory Authority Inc. filing fee | | | 27,000 | |
Printing expenses | | | 150,000 | |
Legal fees and expenses | | | 1,200,000 | |
Accounting fees and expenses | | | 220,000 | |
Roadshow expenses | | | 60,000 | |
Other fees and expenses | | | 19,000 | |
| | | | |
Total | | $ | 1,810,000 | |
| | | | |
LEGAL MATTERS
The validity of the ordinary shares represented by the ADSs and the ordinary shares underlying the Warrants issued in our initial public offering in the United States was passed upon for us by Baker & McKenzie, our Australian counsel. The validity of the Warrants and certain other matters as to U.S. federal law and New York state law was passed upon for us by Baker & McKenzie LLP, our U.S. counsel.
EXPERTS
The audited financial statements included in this prospectus and elsewhere in the registration statement have been so included in reliance upon the report of Grant Thornton Audit Pty Ltd., independent registered public accountants, upon the authority of said firm as experts in accounting and auditing.
ENFORCEABILITY OF CIVIL LIABILITIES
We are a public limited company incorporated under the laws of Australia. Certain of our directors are non-residents of the United States and substantially all of their assets are located outside the United States. As a result, it may not be possible for you to:
| | • | | effect service of process within the United States upon our non-U.S. resident directors or on us; |
| | • | | enforce in U.S. courts judgments obtained against our non-U.S. resident directors or us in the United States courts in any action, including actions under the civil liability provisions of U.S. securities laws; |
| | • | | enforce in U.S. courts judgments obtained against our non-U.S. resident directors or us in courts of jurisdictions outside the United States in any action, including actions under the civil liability provisions of U.S. securities laws; or |
| | • | | bring an original action in an Australian court to enforce liabilities against our non-U.S. resident directors or us based solely upon U.S. securities laws. |
You may also have difficulties enforcing in courts outside the United States judgments that are obtained in U.S. courts against any of our non-U.S. resident directors or us, including actions under the civil liability provisions of the U.S. securities laws.
167
With that noted, there are no treaties between Australia and the United States that would affect the recognition or enforcement of foreign judgments in Australia. We also note that investors may be able to bring an original action in an Australian court against us to enforce liabilities based in part upon U.S. federal securities laws.
The disclosure in this section is not based on the opinion of counsel.
We have appointed Tacere Therapeutics, Inc., our wholly owned U.S. subsidiary, as our agent to receive service of process with respect to any action brought against us in the U.S. District Court for the Southern District of New York under the federal securities laws of the United States or any action brought against us in the Supreme Court of the State of New York in the County of New York under the securities laws of the State of New York.
WHERE YOU CAN FIND ADDITIONAL INFORMATION
We have filed with the SEC a registration statement on Form F-1, including relevant exhibits and schedules, under the Securities Act with respect to the ordinary shares to be sold in this offering. This prospectus, which constitutes a part of the registration statement, summarizes material provisions of contracts and other documents that we refer to in this prospectus. Since this prospectus does not contain all of the information contained in the registration statement, you should read the registration statement and its exhibits and schedules for further information with respect to us and our ordinary shares represented by ADSs and Warrants. Statements contained in this prospectus regarding the contents of any agreement, contract or other document referred to are not necessarily complete and reference is made in each instance to the copy of the contract or document filed as an exhibit to the registration statement. All information we file with the SEC is available through the SEC’s Electronic Data Gathering, Analysis and Retrieval system, which may be accessed through the SEC’s website at www.sec.gov. Information filed with the SEC may also be inspected and copied at the public reference room maintained by the SEC at 100 F Street, N.E., Washington, D.C. 20549. You can request copies of these documents upon payment of a duplicating fee, by writing to the SEC. Please visit the SEC’s website at www.sec.gov for further information on the SEC’s public reference room.
We are subject to periodic reporting and other informational requirements of the Exchange Act as applicable to foreign private issuers. Our annual report on Form 20-F for the year ending June 30, 2015 has been filed with the SEC and an annual report on Form-20-F for subsequent years will be due within four months following the fiscal year end. We are not required to disclose certain other information that is required from U.S. domestic issuers. As a foreign private issuer, we are exempt under the Exchange Act from, among other things, the rules prescribing the furnishing and content of proxy statements, and our executive officers, directors and principal shareholders are exempt from the reporting and short-swing profit recovery provisions contained in Section 16 of the Exchange Act and Regulation FD (Fair Disclosure), which was adopted to ensure that select groups of investors are not privy to specific information about an issuer before other investors.
We are, however, still subject to the anti-fraud and anti-manipulation rules of the SEC, such as Rule 10b-5. Since many of the disclosure obligations required of us as a foreign private issuer are different than those required by companies filing as a domestic issuer, our shareholders, potential shareholders and the investing public in general should not expect to receive information about us in the same amount and at the same time as information is received from, or provided by, companies filing as a domestic issuer. We are liable for violations of the rules and regulations of the SEC, which do apply to us as a foreign private issuer.
168
INDEX TO FINANCIAL STATEMENTS
F-1
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
BOARD OF DIRECTORS AND SHAREHOLDERS
BENITEC BIOPHARMA LIMITED
We have audited the accompanying consolidated statements of financial position of Benitec Biopharma Limited and subsidiaries (the “Group”) as of June 30, 2015 and 2014, and the related consolidated statements of profit or loss and other comprehensive income, changes in equity, and cash flows for each of the two years in the period ended June 30, 2015. These financial statements are the responsibility of the Group’s management. Our responsibility is to express an opinion on these financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. We were not engaged to perform an audit of the Group’s internal control over financial reporting. Our audits included consideration of internal control over financial reporting as a basis for designing audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the Group’s internal control over financial reporting. Accordingly, we express no such opinion. An audit also includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the consolidated financial statements referred to above present fairly, in all material respects, the financial position of Benitec Biopharma Limited and subsidiaries as of June 30, 2015 and 2014, and the results of their operations and their cash flows for each of the two years in the period ended June 30, 2015 in conformity with International Financial Reporting Standards as issued by the International Accounting Standards Board.
GRANT THORNTON AUDIT PTY LTD
Chartered Accountants
Sydney, NSW, Australia
November 16, 2015
F-2
|
| Benitec Biopharma Limited |
| Statement of profit or loss and other comprehensive income |
| For the years ended 30 June 2015 and 2014 |
| | | | | | | | | | | | |
| | | | | | Consolidated | |
| | | Note | | | 2015 | | | 2014 | |
| | | | | | $‘000 | | | $‘000 | |
Revenue | | | 4 | | | | 1,081 | | | | 598 | |
Other income | | | 5 | | | | 2,891 | | | | 776 | |
| | | |
Expenses | | | | | | | | | | | | |
Royalties and licence fees | | | | | | | (40 | ) | | | (193 | ) |
Research and development | | | 6 | | | | (6,228 | ) | | | (3,758 | ) |
Employee benefits expense | | | | | | | (3,425 | ) | | | (2,444 | ) |
Share-based expense | | | | | | | (1,503 | ) | | | (355 | ) |
Travel related costs | | | | | | | (1,039 | ) | | | (585 | ) |
Consultants costs | | | | | | | (882 | ) | | | (653 | ) |
Occupancy costs | | | | | | | (275 | ) | | | (122 | ) |
Corporate expenses | | | | | | | (1,018 | ) | | | (646 | ) |
Net loss foreign exchange | | | | | | | — | | | | (111 | ) |
IPO costs | | | | | | | (1,071 | ) | | | — | |
| | | | | | | | | | | | |
Loss before income tax benefit | | | | | | | (11,509 | ) | | | (7,493 | ) |
Income tax benefit | | | 7 | | | | — | | | | 454 | |
| | | | | | | | | | | | |
Loss after income tax benefit for the year attributable to the owners of Benitec Biopharma Limited | | | 16 | | | | (11,509 | ) | | | (7,039 | ) |
| | | | | | | | | | | | |
| | | |
Other comprehensive income | | | | | | | | | | | | |
Items that may be reclassified subsequently to profit or loss | | | | | | | | | | | | |
Foreign currency translation | | | | | | | 6 | | | | 8 | |
| | | | | | | | | | | | |
Other comprehensive income for the year, net of tax | | | | | | | 6 | | | | 8 | |
| | | | | | | | | | | | |
Total comprehensive income for the year attributable to the owners of Benitec Biopharma Limited | | | | | | | (11,503 | ) | | | (7,031 | ) |
| | | | | | | | | | | | |
| | | | | | | Cents | | | | Cents | |
Basic earnings per share | | | 28 | | | | (9.96 | ) | | | (7.78 | ) |
Diluted earnings per share | | | 28 | | | | (9.96 | ) | | | (7.78 | ) |
The above statement of profit or loss and other comprehensive income should be read in conjunction with the accompanying notes
F-3
|
| Benitec Biopharma Limited |
| Statement of financial position |
| As at 30 June 2015 and 2014 |
| | | | | | | | | | |
| | | | | Consolidated | |
| | | Note | | 2015 | | | 2014 | |
| | | | | $‘000 | | | $‘000 | |
Assets | | | | | | | | | | |
Current assets | | | | | | | | | | |
Cash and cash equivalents | | 8 | | | 21,787 | | | | 31,359 | |
Trade and other receivables | | 9 | | | 123 | | | | 122 | |
Other | | 10 | | | 3,154 | | | | 2,967 | |
| | | | | | | | | | |
Total current assets | | | | | 25,064 | | | | 34,448 | |
| | | | | | | | | | |
Non-current assets | | | | | | | | | | |
Property, plant and equipment | | 11 | | | 456 | | | | 48 | |
| | | | | | | | | | |
Total non-current assets | | | | | 456 | | | | 48 | |
| | | | | | | | | | |
Total assets | | | | | 25,520 | | | | 34,496 | |
| | | | | | | | | | |
Liabilities | | | | | | | | | | |
Current liabilities | | | | | | | | | | |
Trade and other payables | | 12 | | | 1,449 | | | | 788 | |
Provisions | | 13 | | | 193 | | | | 167 | |
| | | | | | | | | | |
Total current liabilities | | | | | 1,642 | | | | 955 | |
| | | | | | | | | | |
Total liabilities | | | | | 1,642 | | | | 955 | |
| | | | | | | | | | |
Net assets | | | | | 23,878 | | | | 33,541 | |
| | | | | | | | | | |
Equity | | | | | | | | | | |
Issued capital | | 14 | | | 129,631 | | | | 129,186 | |
Reserves | | 15 | | | 2,038 | | | | 641 | |
Accumulated losses | | 16 | | | (107,791 | ) | | | (96,286 | ) |
| | | | | | | | | | |
Total equity | | | | | 23,878 | | | | 33,541 | |
| | | | | | | | | | |
The above statement of financial position should be read in conjunction with the accompanying notes
F-4
|
| Benitec Biopharma Limited |
| Statement of changes in equity |
| For the years ended 30 June 2015 and 2014 |
| | | | | | | | | | | | | | | | |
Consolidated | | Issued
capital | | | Reserves | | | Accumulated
losses | | | Total
equity | |
| | | $‘000 | | | $‘000 | | | $‘000 | | | $‘000 | |
Balance at 1 July 2013 | | | 89,609 | | | | 278 | | | | (89,247 | ) | | | 640 | |
Loss after income tax benefit for the year | | | — | | | | — | | | | (7,039 | ) | | | (7,039 | ) |
Other comprehensive income for the year, net of tax | | | — | | | | 8 | | | | — | | | | 8 | |
| | | | | | | | | | | | | | | | |
Total comprehensive income for the year | | | — | | | | 8 | | | | (7,039 | ) | | | (7,031 | ) |
| | | | |
Transactions with owners in their capacity as owners: | | | | | | | | | | | | | | | | |
Contributions of equity, net of transaction costs (note 14) | | | 39,577 | | | | — | | | | — | | | | 39,577 | |
Share-based payments (note 29) | | | — | | | | 355 | | | | — | | | | 355 | |
| | | | | | | | | | | | | | | | |
Balance at 30 June 2014 | | | 129,186 | | | | 641 | | | | (96,286 | ) | | | 33,541 | |
| | | | | | | | | | | | | | | | |
| | | | |
Consolidated | | Issued
capital | | | Reserves | | | Accumulated
losses | | | Total
equity | |
| | | $‘000 | | | $‘000 | | | $‘000 | | | $‘000 | |
Balance at 1 July 2014 | | | 129,186 | | | | 641 | | | | (96,286 | ) | | | 33,541 | |
Loss after income tax benefit for the year | | | — | | | | — | | | | (11,509 | ) | | | (11,509 | ) |
Other comprehensive income for the year, net of tax | | | — | | | | 6 | | | | — | | | | 6 | |
| | | | | | | | | | | | | | | | |
Total comprehensive income for the year | | | — | | | | 6 | | | | (11,509 | ) | | | (11,503 | ) |
| | | | |
Transactions with owners in their capacity as owners: | | | | | | | | | | | | | | | | |
Contributions of equity, net of transaction costs (note 14) | | | 337 | | | | — | | | | — | | | | 337 | |
Share-based payments (note 29) | | | — | | | | 1,503 | | | | — | | | | 1,503 | |
Transfer of expired share-based payments | | | — | | | | (4 | ) | | | 4 | | | | — | |
Transfer to share capital for options exercised | | | 108 | | | | (108 | ) | | | — | | | | — | |
| | | | | | | | | | | | | | | | |
Balance at 30 June 2015 | | | 129,631 | | | | 2,038 | | | | (107,791 | ) | | | 23,878 | |
| | | | | | | | | | | | | | | | |
The above statement of changes in equity should be read in conjunction with the accompanying notes
F-5
|
| Benitec Biopharma Limited |
| Statement of cash flows |
| For the years ended 30 June 2015 and 2014 |
| | | | | | | | | | | | |
| | | | | | Consolidated | |
| | | Note | | | 2015 | | | 2014 | |
| | | | | | $‘000 | | | $‘000 | |
Cash flows from operating activities | | | | | | | | | | | | |
Receipts from customers (inclusive of GST) | | | | | | | 307 | | | | 260 | |
Research and development grants | | | | | | | 2,318 | | | | 776 | |
Interest received | | | | | | | 774 | | | | 321 | |
Income taxes refunded (paid) | | | | | | | — | | | | 454 | |
Payments to suppliers and employees (inclusive of GST) | | | | | | | (13,091 | ) | | | (11,082 | ) |
| | | | | | | | | | | | |
Net cash used in operating activities | | | 27 | | | | (9,692 | ) | | | (9,271 | ) |
| | | | | | | | | | | | |
Cash flows from investing activities | | | | | | | | | | | | |
Payments for property, plant and equipment | | | 11 | | | | (505 | ) | | | (32 | ) |
| | | | | | | | | | | | |
Net cash used in investing activities | | | | | | | (505 | ) | | | (32 | ) |
| | | | | | | | | | | | |
Cash flows from financing activities | | | | | | | | | | | | |
Proceeds from issue of shares | | | | | | | 385 | | | | 39,076 | |
IPO and share issue transaction costs | | | | | | | (333 | ) | | | — | |
| | | | | | | | | | | | |
Net cash from financing activities | | | | | | | 52 | | | | 39,076 | |
| | | | | | | | | | | | |
Net (decrease)/increase in cash and cash equivalents | | | | | | | (10,145 | ) | | | 29,773 | |
Cash and cash equivalents at the beginning of the financial year | | | | | | | 31,359 | | | | 1,587 | |
Effects of exchange rate changes on cash and cash equivalents | | | | | | | 573 | | | | (1 | ) |
| | | | | | | | | | | | |
Cash and cash equivalents at the end of the financial year | | | 8 | | | | 21,787 | | | | 31,359 | |
| | | | | | | | | | | | |
The above statement of cash flows should be read in conjunction with the accompanying notes
F-6
|
| Benitec Biopharma Limited |
| Notes to the financial statements |
| 30 June 2015 and 2014 |
Note 1. Significant accounting policies
The principal accounting policies adopted in the preparation of the financial statements are set out below. These policies have been consistently applied to all the years presented, unless otherwise stated.
New, revised or amending Accounting Standards and Interpretations adopted
The Group has adopted all of the new, revised or amending Accounting Standards and Interpretations issued by the International Accounting Standards Board (‘IASB’) that are mandatory for the current reporting period. The adoption of these Accounting Standards and Interpretations did not have any significant impact on the financial performance or position of the Group.
Any new, revised or amending Accounting Standards or Interpretations that are not yet mandatory have not been early adopted.
The following Accounting Standards and Interpretations are most relevant to the Group:
| | • | | Amendments to IAS 32: Offsetting Financial Assets and Financial Liabilities; |
| | • | | Amendments to IAS 36: Recoverable Amount Disclosures for Non-Financial Assets; and |
| | • | | Annual improvements to IFRS: 2010-2012 cycle. |
Going concern
The directors have prepared the financial statements on a going concern basis after taking into consideration the net loss for the year of $11,509,000 (2014: $7,039,000) and the cash and cash equivalents balance of $21,787,000 (2014: $31,359,000).
The Company announced the closing of its U.S. initial public offering of 1,500,000 American Depositary Shares (ADSs)1, representing 30,000,000 fully paid ordinary shares of Benitec, together with warrants to purchase 500,000 ADSs, representing 10,000,000 fully paid ordinary shares. Each ADS represents 20 ordinary shares of Benitec. At the time of pricing, Benitec granted the underwriter a45-day option to purchase up to an additional 225,000 ADSs and/or 75,000 warrants to purchase ADSs to cover over-allotments, if any. This was not ultimately exercised. Simultaneously with the closing, Benitec issued and sold 75,000 warrants in connection with the underwriter’s partial exercise of such option. The gross proceeds from the offering were $18,844,000 (US$13,820,000), before deducting underwriting discounts and commissions and other offering expenses. Net proceeds from the offering will be used primarily to advance Benitec’s therapeutic programs.
Basis of preparation
The financial report covers Benitec Biopharma Limited and its subsidiaries as a consolidated entity (“Group”). Benitec Biopharma Limited is a listed public company, incorporated and domiciled in Australia. All amounts are stated in Australian dollars.
The consolidated general purpose financial statements of the Group have been prepared in accordance with the requirements of theCorporations Act 2001, International Accounting Standards and other authoritative pronouncements of the International Accounting Standards Board. Compliance with International Accounting Standards results in full compliance with the International Financial Reporting Standards (IFRS) as issued by the
F-7
|
| Benitec Biopharma Limited |
| Notes to the financial statements |
| 30 June 2015 and 2014 |
International Accounting Standards Board (IASB). Benitec Biopharma Limited is a for-profit entity for the purpose of preparing financial statements.
The consolidated financial statements have been prepared using the measurement bases specified by International Accounting Standards for each type of asset, liability, income and expense. The measurement bases are more fully described in the accounting policies below.
The consolidated financial statements for the year ended 30 June 2015 were approved and authorized for issue by the Board of Directors on 16 November 2015.
Historical cost convention
The financial statements have been prepared on an accrual basis on historical costs modified by the revaluation of selected non-current assets and financial instruments for which the fair value basis of accounting has been applied.
Critical accounting estimates
The preparation of the financial statements requires the use of certain critical accounting estimates. It also requires management to exercise its judgement in the process of applying the Group’s accounting policies. The areas involving a higher degree of judgement or complexity, or areas where assumptions and estimates are significant to the financial statements, are disclosed in note 2.
Parent entity information
In accordance with the Corporations Act 2001, these financial statements present the results of the Group only. Supplementary information about the parent entity is disclosed in note 24.
Principles of consolidation
The consolidated financial statements incorporate the assets and liabilities of all subsidiaries of Benitec Biopharma Limited (‘Company’ or ‘parent entity’) as at 30 June 2015 and the results of all subsidiaries for the year then ended. Benitec Biopharma Limited and its subsidiaries together are referred to in these financial statements as the ‘Group’.
Subsidiaries are all those entities over which the Group has control. The Group controls an entity when it is exposed to, or has rights to, variable returns from its involvement with the entity and has the ability to affect those returns through its power to direct the activities of the entity. Subsidiaries are fully consolidated from the date on which control is transferred to the Group. They are de-consolidated from the date that control ceases.
Intercompany transactions, balances and unrealised gains on transactions between entities in the Group are eliminated. Unrealised losses are also eliminated unless the transaction provides evidence of the impairment of the asset transferred. Accounting policies of subsidiaries have been changed where necessary to ensure consistency with the policies adopted by the Group.
The acquisition of subsidiaries is accounted for using the acquisition method of accounting. A change in ownership interest, without the loss of control, is accounted for as an equity transaction, where the difference between the consideration transferred and the book value of the share of the non-controlling interest acquired is recognised directly in equity attributable to the parent.
F-8
|
| Benitec Biopharma Limited |
| Notes to the financial statements |
| 30 June 2015 and 2014 |
Where the Group loses control over a subsidiary, it derecognises the assets including goodwill, liabilities and non-controlling interest in the subsidiary together with any cumulative translation differences recognised in equity. The Group recognises the fair value of the consideration received and the fair value of any investment retained together with any gain or loss in profit or loss.
Operating segments
Operating segments are presented using the ‘management approach’, where the information presented is on the same basis as the internal reports provided to the Chief Operating Decision Makers (‘CODM’). The CODM is responsible for the allocation of resources to operating segments and assessing their performance.
Foreign currency translation
The financial statements are presented in Australian dollars, which is Benitec Biopharma Limited’s functional and presentation currency.
Foreign currency transactions
Foreign currency transactions are translated into Australian dollars using the exchange rates prevailing at the dates of the transactions. Foreign exchange gains and losses resulting from the settlement of such transactions and from the translation at financial year-end exchange rates of monetary assets and liabilities denominated in foreign currencies are recognised in profit or loss.
Foreign operations
The assets and liabilities of foreign operations are translated into Australian dollars using the exchange rates at the reporting date. The revenues and expenses of foreign operations are translated into Australian dollars using the average exchange rates, which approximate the rates at the dates of the transactions, for the period. All resulting foreign exchange differences are recognised in other comprehensive income through the foreign currency reserve in equity.
The foreign currency reserve is recognised in profit or loss when the foreign operation or net investment is disposed of.
Revenue recognition
Revenue is recognised when it is probable that the economic benefit will flow to the Group and the revenue can be reliably measured. Revenue is measured at the fair value of the consideration received or receivable.
Licensing revenue and royalties
Revenue from the granting of licenses is recognised in accordance with the terms of the relevant agreements and is usually recognised on an accruals basis, unless the substance of the agreement provides evidence that it is more appropriate to recognise revenue on some other systematic rational basis.
Interest
Interest revenue is recognised as interest accrues using the effective interest method. This is a method of calculating the amortised cost of a financial asset and allocating the interest income over the relevant period using the effective interest rate, which is the rate that exactly discounts estimated future cash receipts through the expected life of the financial asset to the net carrying amount of the financial asset.
F-9
|
| Benitec Biopharma Limited |
| Notes to the financial statements |
| 30 June 2015 and 2014 |
Government research and development grants
Government grants are recognised at fair value where there is reasonable assurance that the grant will be received and all grant conditions will be met. Grants relating to expense items are recognised as income over the periods necessary to match the grant costs they are compensating. Grants relating to assets are credited to deferred income at fair value and are credited to income over the expected useful life of the asset on a straight-line basis.
Research and development grant revenue is recognised as income when it is received.
Income tax
The income tax expense or benefit for the period is the tax payable on that period’s taxable income based on the applicable income tax rate for each jurisdiction, adjusted by the changes in deferred tax assets and liabilities attributable to temporary differences, unused tax losses and the adjustment recognised for prior periods, where applicable.
Deferred tax assets and liabilities are recognised for temporary differences at the tax rates expected to be applied when the assets are recovered or liabilities are settled, based on those tax rates that are enacted or substantively enacted, except for:
| | • | | When the deferred income tax asset or liability arises from the initial recognition of goodwill or an asset or liability in a transaction that is not a business combination and that, at the time of the transaction, affects neither the accounting nor taxable profits; or |
| | • | | When the taxable temporary difference is associated with interests in subsidiaries, associates or joint ventures, and the timing of the reversal can be controlled and it is probable that the temporary difference will not reverse in the foreseeable future. |
Deferred tax assets are recognised for deductible temporary differences and unused tax losses only if it is probable that future taxable amounts will be available to utilise those temporary differences and losses.
The carrying amount of recognised and unrecognised deferred tax assets are reviewed at each reporting date. Deferred tax assets recognised are reduced to the extent that it is no longer probable that future taxable profits will be available for the carrying amount to be recovered. Previously unrecognised deferred tax assets are recognised to the extent that it is probable that there are future taxable profits available to recover the asset.
Deferred tax assets and liabilities are offset only where there is a legally enforceable right to offset current tax assets against current tax liabilities and deferred tax assets against deferred tax liabilities; and they relate to the same taxable authority on either the same taxable entity or different taxable entities which intend to settle simultaneously.
Benitec Biopharma Limited (the ‘head entity’) and its wholly-owned Australian subsidiaries have formed an income tax consolidated group under the tax consolidation regime. The head entity and each subsidiary in the tax consolidated group continue to account for their own current and deferred tax amounts. The tax consolidated group has applied the ‘separate taxpayer within group’ approach in determining the appropriate amount of taxes to allocate to members of the tax consolidated group. No tax sharing agreement has been entered between entities in the tax consolidated group.
In addition to its own current and deferred tax amounts, the head entity also recognises the current tax liabilities (or assets) and the deferred tax assets arising from unused tax losses and unused tax credits assumed from each subsidiary in the tax consolidated group.
F-10
|
| Benitec Biopharma Limited |
| Notes to the financial statements |
| 30 June 2015 and 2014 |
Current and non-current classification
Assets and liabilities are presented in the statement of financial position based on current and non-current classification.
An asset is classified as current when: it is either expected to be realised or intended to be sold or consumed in normal operating cycle; it is held primarily for the purpose of trading; it is expected to be realised within 12 months after the reporting period; or the asset is cash or cash equivalent unless restricted from being exchanged or used to settle a liability for at least 12 months after the reporting period. All other assets are classified as non-current.
A liability is classified as current when: it is either expected to be settled in normal operating cycle; it is held primarily for the purpose of trading; it is due to be settled within 12 months after the reporting period; or there is no unconditional right to defer the settlement of the liability for at least 12 months after the reporting period. All other liabilities are classified as non-current.
Cash and cash equivalents
Cash and cash equivalents includes cash on hand, deposits held at call with financial institutions, other short-term, highly liquid investments with original maturities of three months or less that are readily convertible to known amounts of cash and which are subject to an insignificant risk of changes in value.
Trade and other receivables
Other receivables are recognised at amortised cost, less any provision for impairment.
Investments and other financial assets
Investments and other financial assets are initially measured at fair value. Transaction costs are included as part of the initial measurement, except for financial assets at fair value through profit or loss. They are subsequently measured at either amortised cost or fair value depending on their classification. Classification is determined based on the purpose of the acquisition and subsequent reclassification to other categories is restricted.
Financial assets are derecognised when the rights to receive cash flows from the financial assets have expired or have been transferred and the Group has transferred substantially all the risks and rewards of ownership.
Loans and receivables
Loans and receivables are non-derivative financial assets with fixed or determinable payments that are not quoted in an active market. They are carried at amortised cost using the effective interest rate method. Gains and losses are recognised in profit or loss when the asset is derecognised or impaired.
Impairment of financial assets
The Group assesses at the end of each reporting period whether there is any objective evidence that a financial asset or group of financial assets is impaired. Objective evidence includes significant financial difficulty of the issuer or obligor; a breach of contract such as default or delinquency in payments; the lender granting to a borrower concessions due to economic or legal reasons that the lender would not otherwise do; it becomes probable that the borrower will enter bankruptcy or other financial reorganisation; the disappearance of an active market for the financial asset; or observable data indicating that there is a measurable decrease in estimated future cash flows.
F-11
|
| Benitec Biopharma Limited |
| Notes to the financial statements |
| 30 June 2015 and 2014 |
The amount of the impairment allowance for loans and receivables carried at amortised cost is the difference between the asset’s carrying amount and the present value of estimated future cash flows, discounted at the original effective interest rate. If there is a reversal of impairment, the reversal cannot exceed the amortised cost that would have been recognised had the impairment not been made and is reversed to profit or loss.
Property, plant and equipment
Plant and equipment is stated at historical cost less accumulated depreciation and impairment. Historical cost includes expenditure that is directly attributable to the acquisition of the items.
Depreciation is calculated on a straight-line basis to write off the net cost of each item of property, plant and equipment (excluding land) over their expected useful lives as follows:
| | |
Leasehold improvements | | 3-10 years |
Plant and equipment | | 3-7 years |
The residual values, useful lives and depreciation methods are reviewed, and adjusted if appropriate, at each reporting date.
An item of property, plant and equipment is derecognised upon disposal or when there is no future economic benefit to the Group. Gains and losses between the carrying amount and the disposal proceeds are taken to profit or loss.
Leases
The determination of whether an arrangement is or contains a lease is based on the substance of the arrangement and requires an assessment of whether the fulfilment of the arrangement is dependent on the use of a specific asset or assets and the arrangement conveys a right to use the asset.
A distinction is made between finance leases, which effectively transfer from the lessor to the lessee substantially all the risks and benefits incidental to the ownership of leased assets, and operating leases, under which the lessor effectively retains substantially all such risks and benefits.
Finance leases are capitalised. A lease asset and liability are established at the fair value of the leased assets, or if lower, the present value of minimum lease payments. Lease payments are allocated between the principal component of the lease liability and the finance costs, so as to achieve a constant rate of interest on the remaining balance of the liability.
Leased assets acquired under a finance lease are depreciated over the asset’s useful life or over the shorter of the asset’s useful life and the lease term if there is no reasonable certainty that the Group will obtain ownership at the end of the lease term.
Operating lease payments, net of any incentives received from the lessor, are charged to profit or loss on a straight-line basis over the term of the lease.
Impairment of non-financial assets
Other intangible assets that have an indefinite useful life are not subject to amortisation and are tested annually for impairment, or more frequently if events or changes in circumstances indicate that they might be
F-12
|
| Benitec Biopharma Limited |
| Notes to the financial statements |
| 30 June 2015 and 2014 |
impaired. Other non-financial assets are reviewed for impairment whenever events or changes in circumstances indicate that the carrying amount may not be recoverable. An impairment loss is recognised for the amount by which the asset’s carrying amount exceeds its recoverable amount.
Recoverable amount is the higher of an asset’s fair value less costs of disposal and value-in-use. The value-in-use is the present value of the estimated future cash flows relating to the asset using a pre-tax discount rate specific to the asset or cash-generating unit to which the asset belongs. Assets that do not have independent cash flows are grouped together to form a cash-generating unit.
Trade and other payables
These amounts represent liabilities for goods and services provided to the Group prior to the end of the financial year and which are unpaid. Due to their short-term nature they are measured at amortised cost and are not discounted. The amounts are unsecured and are usually paid within 30 days of recognition.
Employee benefits
Short-term employee benefits
Liabilities for wages and salaries and other employee benefits expected to be settled within 12 months of the reporting date are measured at the amounts expected to be paid when the liabilities are settled.
Other long-term employee benefits
Employee benefits not expected to be settled within 12 months of the reporting date are measured as the present value of expected future payments to be made in respect of services provided by employees up to the reporting date using the projected unit credit method. Consideration is given to expected future wage and salary levels, experience of employee departures and periods of service. Expected future payments are discounted using market yields at the reporting date on corporate bonds with terms to maturity and currency that match, as closely as possible, the estimated future cash outflows.
Defined contribution superannuation expense
Contributions to defined contribution superannuation plans are expensed in the period in which they are incurred.
Share-based payments
Equity-settled share-based compensation benefits are provided to directors and senior executives. The plan currently in place to provide these benefits is the Employee Share Option Plan (‘ESOP’).
Equity-settled transactions are awards of shares, or options over shares, that are provided to employees in exchange for the rendering of services.
The cost of equity-settled transactions are measured at fair value on grant date. Fair value is independently determined usingBlack-Scholes option pricing model that takes into account the exercise price, the term of the option, the impact of dilution, the share price at grant date and expected price volatility of the underlying share, the expected dividend yield and the risk free interest rate for the term of the option, together with non-vesting conditions that do not determine whether the Group receives the services that entitle the employees to receive payment. No account is taken of any other vesting conditions.
F-13
|
| Benitec Biopharma Limited |
| Notes to the financial statements |
| 30 June 2015 and 2014 |
The cost of equity-settled transactions are recognised as an expense with a corresponding increase in equity over the vesting period. The cumulative charge to profit or loss is calculated based on the grant date fair value of the award, the best estimate of the number of awards that are likely to vest and the expired portion of the vesting period. The amount recognised in profit or loss for the period is the cumulative amount calculated at each reporting date less amounts already recognised in previous periods.
Market conditions are taken into consideration in determining fair value. Therefore any awards subject to market conditions are considered to vest irrespective of whether or not that market condition has been met, provided all other conditions are satisfied.
If equity-settled awards are modified, as a minimum an expense is recognised as if the modification has not been made. An additional expense is recognised, over the remaining vesting period, for any modification that increases the total fair value of the share-based compensation benefit as at the date of modification.
If the non-vesting condition is within the control of the Group or employee, the failure to satisfy the condition is treated as a cancellation. If the condition is not within the control of the Group or employee and is not satisfied during the vesting period, any remaining expense for the award is recognised over the remaining vesting period, unless the award is forfeited.
If equity-settled awards are cancelled, it is treated as if it has vested on the date of cancellation, and any remaining expense is recognised immediately. If a new replacement award is substituted for the cancelled award, the cancelled and new award is treated as if they were a modification.
The dilutive effect, if any, of outstanding options is reflected as additional share dilution in the computation of earnings per share.
Fair value measurement
When an asset or liability, financial or non-financial, is measured at fair value for recognition or disclosure purposes, the fair value is based on the price that would be received to sell an asset or paid to transfer a liability in an orderly transaction between market participants at the measurement date; and assumes that the transaction will take place either: in the principal market; or in the absence of a principal market, in the most advantageous market.
Fair value is measured using the assumptions that market participants would use when pricing the asset or liability, assuming they act in their economic best interests. For non-financial assets, the fair value measurement is based on its highest and best use. Valuation techniques that are appropriate in the circumstances and for which sufficient data are available to measure fair value, are used, maximising the use of relevant observable inputs and minimising the use of unobservable inputs.
Issued capital
Ordinary shares are classified as equity.
Incremental costs directly attributable to the issue of new shares or options are shown in equity as a deduction, net of tax, from the proceeds.
Costs related to an initial offering are expensed in the statement of profit or loss and other comprehensive income.
F-14
|
| Benitec Biopharma Limited |
| Notes to the financial statements |
| 30 June 2015 and 2014 |
Earnings per share
Basic earnings per share
Basic earnings per share is calculated by dividing the profit attributable to the owners of Benitec Biopharma Limited, excluding any costs of servicing equity other than ordinary shares, by the weighted average number of ordinary shares outstanding during the financial year, adjusted for bonus elements in ordinary shares issued during the financial year.
Diluted earnings per share
Diluted earnings per share adjusts the figures used in the determination of basic earnings per share to take into account the after income tax effect of interest and other financing costs associated with dilutive potential ordinary shares and the weighted average number of shares assumed to have been issued for no consideration in relation to dilutive potential ordinary shares.
Goods and Services Tax (‘GST’) and other similar taxes
Revenues, expenses and assets are recognised net of the amount of associated GST, unless the GST incurred is not recoverable from the tax authority. In this case it is recognised as part of the cost of the acquisition of the asset or as part of the expense.
Receivables and payables are stated inclusive of the amount of GST receivable or payable. The net amount of GST recoverable from, or payable to, the tax authority is included in other receivables or other payables in the statement of financial position.
Cash flows are presented on a gross basis. The GST components of cash flows arising from investing or financing activities which are recoverable from, or payable to the tax authority, are presented as operating cash flows.
Commitments and contingencies are disclosed net of the amount of GST recoverable from, or payable to, the tax authority.
Comparative figures
When required by accounting standards, comparative figures have been adjusted to conform to changes in the presentation for the current financial year.
Rounding of amounts
Amounts in this report have been rounded to the nearest thousand dollars, or in certain cases, the nearest dollar.
New Accounting Standards and Interpretations not yet mandatory or early adopted
Accounting Standards and Interpretations that have recently been issued or amended but are not yet mandatory, have not been early adopted by the Group for the annual reporting period ended 30 June 2015. The Group’s assessment of the impact of these new or amended Accounting Standards and Interpretations, most relevant to the Group, are set out below.
IFRS 9 Financial Instruments
This standard is applicable to annual reporting periods beginning on or after 1 January 2018. The standard replaces all previous versions of IFRS 9 and completes the project to replace IAS 39 ‘Financial Instruments:
F-15
|
| Benitec Biopharma Limited |
| Notes to the financial statements |
| 30 June 2015 and 2014 |
Recognition and Measurement’. IFRS 9 introduces new classification and measurement models for financial assets. New simpler hedge accounting requirements are intended to more closely align the accounting treatment with the risk management activities of the entity. New impairment requirements will use an ‘expected credit loss’ (‘ECL’) model to recognise an allowance. The Group will adopt this standard from 1 July 2018 but the impact of its adoption is yet to be assessed. The impact on the Group is however likely to be immaterial.
IFRS 15 Revenue from Contracts with Customers
This standard is currently applicable to annual reporting periods beginning on or after 1 January 2018. The standard provides a single standard for revenue recognition. The core principle of the standard is that an entity will recognise revenue to depict the transfer of promised goods or services to customers in an amount that reflects the consideration to which the entity expects to be entitled in exchange for those goods or services). It is expected that the Group will adopt this standard from 1 July 2018. The impact of adoption is likely to be immaterial, however a full impact assessment has yet to be undertaken.
Note 2. Critical accounting judgements, estimates and assumptions
The preparation of the financial statements requires management to make judgements, estimates and assumptions that affect the reported amounts in the financial statements. Management continually evaluates its judgements and estimates in relation to assets, liabilities, contingent liabilities, revenue and expenses. Management bases its judgements, estimates and assumptions on historical experience and on other various factors, including expectations of future events, management believes to be reasonable under the circumstances. The resulting accounting judgements and estimates will seldom equal the related actual results. The judgements, estimates and assumptions that have a significant risk of causing a material adjustment to the carrying amounts of assets and liabilities (refer to the respective notes) within the next financial year are discussed below.
Research and development expenses
Management does not consider the development programs to be sufficiently advanced to reliably determine the economic benefits and technical feasibility to justify capitalisation of development costs. These costs have been recognised as an expense when incurred.
Research and development expenses relate primarily to the cost of conducting clinical and pre-clinical trials. Clinical development costs are a significant component of research and development expenses. Estimates have been used in determining the expense liability under certain clinical trial contracts where services have been performed but not yet invoiced. Generally the costs, and therefore estimates, associated with clinical trial contracts are based on the number of patients, drug administration cycles, the type of treatment and the outcome being measured. The length of time before actual amounts can be determined will vary depending on length of the patient cycles and the timing of the invoices by the clinical trial partners.
The Group accounts for the federal government research and development grants tax incentive on cash basis due to the difficulty in making a reasonable estimation as at year end.
Share-based payment transactions
The Group measures the cost of equity-settled transactions with employees by reference to the fair value of the equity instruments at the date at which they are granted. The fair value is determined by using either the Black-Scholes model taking into account the terms and conditions upon which the instruments were granted. The accounting estimates and assumptions relating to equity-settled share-based payments would have no impact on the carrying amounts of assets and liabilities within the next annual reporting period but may impact profit or loss and equity.
F-16
|
| Benitec Biopharma Limited |
| Notes to the financial statements |
| 30 June 2015 and 2014 |
Recovery of deferred tax assets
Deferred tax assets are recognised for deductible temporary differences only if the Group considers it is probable that future taxable amounts will be available to utilise those temporary differences and losses. Given the Company’s and each individual entities’ history of recent losses, the Group has not recognised a deferred tax asset with regard to unused tax losses and other temporary differences, as it has not been determined whether the Company or its subsidiaries will generate sufficient taxable income against which the unused tax losses and other temporary differences can be utilised.
Costs of capital raising
Costs directly attributable to an equity transaction are held in the statement of financial position until the completion of the transaction. On completion, the costs will be applied against issued capital.
Costs associated with an initial public offering or abandoned or sub-optimal equity transactions are expensed to profit or loss in the year the transaction is determined to no longer be viable under existing conditions.
Note 3. Operating segments
Identification of reportable operating segments
The Group has only one operating segment during the financial year, being the global commercialisation by licensing and partnering of patents and licences in biotechnology, more specifically in functional genomics, with applications in biomedical research and human therapeutics. This operating segment is based on the internal reports that are reviewed and used by the Board of Directors (who are identified as the Chief Operating Decision Makers (‘CODM’)) in assessing performance and in determining the allocation of resources.
Geographical information
| | | | | | | | | | | | | | | | |
| | | Sales to external
customers | | | Geographical
non-current
assets | |
| | | 2015 | | | 2014 | | | 2015 | | | 2014 | |
| | | $‘000 | | | $‘000 | | | $‘000 | | | $‘000 | |
Australia | | | 307 | | | | 274 | | | | 456 | | | | 48 | |
United States of America | | | — | | | | 3 | | | | — | | | | — | |
| | | | | | | | | | | | | | | | |
| | | 307 | | | | 277 | | | | 456 | | | | 48 | |
| | | | | | | | | | | | | | | | |
Note 4. Revenue
| | | | | | | | |
| | | Consolidated | |
| | | 2015 | | | 2014 | |
| | | $‘000 | | | $‘000 | |
Sales revenue | | | | | | | | |
Licensing revenue and royalties | | | 307 | | | | 277 | |
| | |
Other revenue | | | | | | | | |
Interest | | | 774 | | | | 321 | |
| | | | | | | | |
Revenue | | | 1,081 | | | | 598 | |
| | | | | | | | |
F-17
|
| Benitec Biopharma Limited |
| Notes to the financial statements |
| 30 June 2015 and 2014 |
Note 5. Other income
| | | | | | | | |
| | | Consolidated | |
| | | 2015 | | | 2014 | |
| | | $‘000 | | | $‘000 | |
Net foreign exchange gain | | | 573 | | | | — | |
Federal government research and development grants | | | 2,318 | | | | 776 | |
| | | | | | | | |
Other income | | | 2,891 | | | | 776 | |
| | | | | | | | |
Note 6. Expenses
| | | | | | | | |
| | | Consolidated | |
| | | 2015 | | | 2014 | |
| | | $‘000 | | | $‘000 | |
Loss before income tax includes the following specific expenses: | | | | | | | | |
| | |
Depreciation | | | | | | | | |
Leasehold improvements | | | 10 | | | | 2 | |
Plant and equipment | | | 87 | | | | 11 | |
| | | | | | | | |
Total depreciation | | | 97 | | | | 13 | |
| | | | | | | | |
Research and development | | | | | | | | |
Project expenses | | | 4,983 | | | | 3,310 | |
Other IP related expenses | | | 1,245 | | | | 448 | |
| | | | | | | | |
Total research and development | | | 6,228 | | | | 3,758 | |
| | | | | | | | |
Rental expense relating to operating leases | | | | | | | | |
Minimum lease payments | | | 179 | | | | 59 | |
| | | | | | | | |
Superannuation expense | | | | | | | | |
Defined contribution superannuation expense | | | 128 | | | | 89 | |
| | | | | | | | |
Employee benefits expense excluding superannuation | | | | | | | | |
Employee benefits expense excluding superannuation | | | 3,297 | | | | 2,355 | |
| | | | | | | | |
F-18
|
| Benitec Biopharma Limited |
| Notes to the financial statements |
| 30 June 2015 and 2014 |
Note 7. Income tax benefit
| | | | | | | | |
| | | Consolidated | |
| | | 2015 | | | 2014 | |
| | | $‘000 | | | $‘000 | |
Income tax benefit | | | | | | | | |
Current tax | | | — | | | | (454 | ) |
| | | | | | | | |
Aggregate income tax benefit | | | — | | | | (454 | ) |
| | | | | | | | |
Numerical reconciliation of income tax benefit and tax at the statutory rate | | | | | | | | |
Loss before income tax benefit | | | (11,509 | ) | | | (7,493 | ) |
| | | | | | | | |
Tax at the statutory tax rate of 30% | | | (3,453 | ) | | | (2,248 | ) |
Tax effect amounts which are not deductible/(taxable) in calculating taxable income: | | | | | | | | |
Legal expenses | | | 15 | | | | 16 | |
Share-based payments | | | 451 | | | | 107 | |
Non-assessable foreign currency translation provision | | | — | | | | (2 | ) |
Capital items deductible | | | (487 | ) | | | (232 | ) |
Sundry items | | | 472 | | | | 24 | |
| | | | | | | | |
| | | (3,002 | ) | | | (2,335 | ) |
Deferred tax asset not brought to account | | | 3,002 | | | | 2,335 | |
Income tax paid/(refund) from an overseas subsidiary | | | — | | | | (454 | ) |
| | | | | | | | |
Income tax benefit | | | — | | | | (454 | ) |
| | | | | | | | |
| | | | | | | | |
| | | Consolidated | |
| | | 2015 | | | 2014 | |
| | | $‘000 | | | $‘000 | |
Tax losses not recognised | | | | | | | | |
Unused tax losses for which no deferred tax asset has been recognised | | | 53,866 | | | | 43,677 | |
| | | | | | | | |
Potential tax benefit @ 30% | | | 16,160 | | | | 13,103 | |
| | | | | | | | |
Capital unused tax losses for which no deferred tax asset has been recognised | | | 1,272 | | | | 1,272 | |
| | | | | | | | |
Potential tax benefit at statutory tax rates | | | 382 | | | | 382 | |
| | | | | | | | |
The above potential tax benefit have not been recognised in the statement of financial position. These tax losses are recognised only if the consolidated entity considers it is probable that future taxable amounts will be available to utilise those temporary differences and losses.
| | | | | | | | |
| | | Consolidated | |
| | | 2015 | | | 2014 | |
| | | $‘000 | | | $‘000 | |
Deferred tax assets not recognised | | | | | | | | |
Deferred tax assets not recognised comprises temporary differences attributable to: | | | | | | | | |
Others | | | 58 | | | | 50 | |
| | | | | | | | |
Total deferred tax assets not recognised | | | 58 | | | | 50 | |
| | | | | | | | |
The above potential tax benefit, which excludes tax losses, for deductible temporary differences has not been recognised in the statement of financial position as the recovery of this benefit is uncertain.
F-19
|
| Benitec Biopharma Limited |
| Notes to the financial statements |
| 30 June 2015 and 2014 |
Note 8. Current assets—cash and cash equivalents
| | | | | | | | |
| | | Consolidated | |
| | | 2015 | | | 2014 | |
| | | $‘000 | | | $‘000 | |
Cash at bank | | | 916 | | | | 289 | |
Cash on deposit | | | 20,871 | | | | 31,070 | |
| | | | | | | | |
| | | 21,787 | | | | 31,359 | |
| | | | | | | | |
Note 9. Current assets—trade and other receivables
| | | | | | | | |
| | | Consolidated | |
| | | 2015 | | | 2014 | |
| | | $‘000 | | | $‘000 | |
Other receivables | | | — | | | | 28 | |
BAS receivable | | | 123 | | | | 94 | |
| | | | | | | | |
| | | 123 | | | | 122 | |
| | | | | | | | |
There is no receivable balance that is either past due or impaired.
Note 10. Current assets—other
| | | | | | | | |
| | | Consolidated | |
| | | 2015 | | | 2014 | |
| | | $‘000 | | | $‘000 | |
Prepayments | | | 74 | | | | 27 | |
Prepaid clinical trials * | | | 2,700 | | | | 2,700 | |
IPO costs ** | | | 285 | | | | — | |
Other current assets | | | 95 | | | | 240 | |
| | | | | | | | |
| | | 3,154 | | | | 2,967 | |
| | | | | | | | |
| * | | The Group announced on 3 June 2013 that it had committed to moving its non-small cell lung cancer therapeutic, into clinical development. The Group is using European-based clinical research organisation Clinical Trials Group (‘CTGCRO’) to manage both the initial clinical development and trials. The prepayment was made to secure favourable commercial terms with CTGCRO for the conduct of the trials. As at the 30 June 2015 the trials had still no commenced. |
| ** | | IPO costs were incurred during the year for the public offer in the United States and the associated listing on the NASDAQ Global Select Market. Refer to note 26 for further details. |
F-20
|
| Benitec Biopharma Limited |
| Notes to the financial statements |
| 30 June 2015 and 2014 |
Note 11. Non-current assets—property, plant and equipment
| | | | | | | | |
| | | Consolidated | |
| | | 2015 | | | 2014 | |
| | | $‘000 | | | $‘000 | |
Leasehold improvements—at cost | | | 252 | | | | 13 | |
Less: Accumulated depreciation | | | (15 | ) | | | (5 | ) |
| | | | | | | | |
| | | 237 | | | | 8 | |
| | | | | | | | |
Plant and equipment—at cost | | | 544 | | | | 278 | |
Less: Accumulated depreciation | | | (325 | ) | | | (238 | ) |
| | | | | | | | |
| | | 219 | | | | 40 | |
| | | | | | | | |
| | | 456 | | | | 48 | |
| | | | | | | | |
Reconciliations
Reconciliations of the written down values at the beginning and end of the current and previous financial year are set out below:
| | | | | | | | | | | | |
Consolidated | | Leasehold
improvement | | | Plant and
equipment | | | Total | |
| | | $‘000 | | | $‘000 | | | $‘000 | |
Balance at 1 July 2013 | | | 10 | | | | 18 | | | | 28 | |
Additions | | | — | | | | 33 | | | | 33 | |
Depreciation expense | | | (2 | ) | | | (11 | ) | | | (13 | ) |
| | | | | | | | | | | | |
Balance at 30 June 2014 | | | 8 | | | | 40 | | | | 48 | |
Additions | | | 239 | | | | 266 | | | | 505 | |
Depreciation expense | | | (10 | ) | | | (87 | ) | | | (97 | ) |
| | | | | | | | | | | | |
Balance at 30 June 2015 | | | 237 | | | | 219 | | | | 456 | |
| | | | | | | | | | | | |
Note 12. Current liabilities—trade and other payables
| | | | | | | | |
| | | Consolidated | |
| | | 2015 | | | 2014 | |
| | | $‘000 | | | $‘000 | |
Trade payables | | | 760 | | | | 573 | |
Other payables | | | 689 | | | | 215 | |
| | | | | | | | |
| | | 1,449 | | | | 788 | |
| | | | | | | | |
Refer to note 18 for further information on financial instruments.
Note 13. Current liabilities—provisions
| | | | | | | | |
| | | Consolidated | |
| | | 2015 | | | 2014 | |
| | | $‘000 | | | $‘000 | |
Employee benefits | | | 193 | | | | 167 | |
| | | | | | | | |
F-21
|
| Benitec Biopharma Limited |
| Notes to the financial statements |
| 30 June 2015 and 2014 |
Note 14. Equity—issued capital
| | | | | | | | | | | | | | | | |
| | | Consolidated | |
| | | 2015 | | | 2014 | | | 2015 | | | 2014 | |
| | | Shares | | | Shares | | | $‘000 | | | $‘000 | |
Ordinary shares—fully paid | | | 115,881,763 | | | | 114,898,993 | | | | 129,631 | | | | 129,186 | |
| | | | | | | | | | | | | | | | |
Movements in ordinary share capital
| | | | | | | | | | | | | | |
Details | | Date | | Shares | | | Issue price | | | $‘000 | |
Balance | | 1 July 2013 | | | 46,076,562 | | | | | | | | 89,609 | |
Placement of shares | | 23 July 2013 | | | 27,629,089 | | | $ | 0.280 | | | | 7,618 | |
Share Purchase Plan issue | | 6 August 2013 | | | 10,254,696 | | | $ | 0.280 | | | | 2,820 | |
Release of Tacere escrow shares | | 30 October 2013 | | | 955,002 | | | $ | 0.370 | | | | 357 | |
Placement of shares | | 28 February 2014 | | | 14,717,995 | | | $ | 1.070 | | | | 15,748 | |
Placement of shares | | 15 April 2014 | | | 14,717,999 | | | $ | 1.070 | | | | 15,749 | |
Options exercised | | 13 October 2013 | | | 197,540 | | | $ | 0.325 | | | | 64 | |
Options exercised | | 14 January 2014 | | | 43,077 | | | $ | 0.325 | | | | 14 | |
Options exercised | | 29 January 2014 | | | 49,464 | | | $ | 0.325 | | | | 16 | |
Options exercised | | 10 February 2014 | | | 160,000 | | | $ | 0.325 | | | | 52 | |
Options exercised | | 27 February 2014 | | | 32,000 | | | $ | 0.325 | | | | 11 | |
Options exercised | | 20 March 2014 | | | 61,539 | | | $ | 0.325 | | | | 20 | |
Options exercised | | 15 April 2014 | | | 3,468 | | | $ | 2.500 | | | | 9 | |
Remaining consolidation of shares on a 25:1 basis | | | | | 562 | | | $ | 0.000 | | | | — | |
Transaction costs | | | | | — | | | $ | 0.000 | | | | (2,901 | ) |
| | | | | | | | | | | | | | |
Balance | | 30 June 2014 | | | 114,898,993 | | | | | | | | 129,186 | |
Transfer from share based payments for options exercised | | | | | — | | | $ | 0.000 | | | | 108 | |
Options exercised | | 30 July 2014 | | | 200,000 | | | $ | 0.510 | | | | 102 | |
Options exercised | | 30 July 2014 | | | 60,000 | | | $ | 0.570 | | | | 34 | |
Options exercised | | 11 August 2014 | | | 60,000 | | | $ | 0.570 | | | | 34 | |
Options exercised | | 28 November 2014 | | | 258,462 | | | $ | 0.325 | | | | 84 | |
Options exercised | | 23 December 2014 | | | 86,155 | | | $ | 0.325 | | | | 28 | |
Options exercised | | 13 January 2015 | | | 40,000 | | | $ | 0.325 | | | | 13 | |
Options exercised | | 9 February 2015 | | | 61,538 | | | $ | 0.325 | | | | 20 | |
Options exercised | | 17 February 2015 | | | 93,538 | | | $ | 0.325 | | | | 30 | |
Options exercised | | 19 February 2015 | | | 123,077 | | | $ | 0.325 | | | | 40 | |
IPO and transaction costs | | | | | — | | | $ | 0.000 | | | | (48 | ) |
| | | | | | | | | | | | | | |
Balance | | 30 June 2015 | | | 115,881,763 | | | | | | | | 129,631 | |
| | | | | | | | | | | | | | |
Ordinary shares
Ordinary shares entitle the holder to participate in dividends and the proceeds on the winding up of the Company in proportion to the number of and amounts paid on the shares held. The fully paid ordinary shares have no par value and the Company does not have a limited amount of authorised capital.
On a show of hands every member present at a meeting in person or by proxy shall have one vote and upon a poll each share shall have one vote.
F-22
|
| Benitec Biopharma Limited |
| Notes to the financial statements |
| 30 June 2015 and 2014 |
Share buy-back
There is no current on-market share buy-back.
Capital risk management
The Group’s objectives when managing capital is to safeguard its ability to continue as a going concern, so that it can provide returns for shareholders and benefits for other stakeholders and to maintain an optimum capital structure to reduce the cost of capital.
The capital structure of the Group consists of cash and cash equivalents and equity attributable to equity holders. Operating globally, the Group develops speciality pharmaceutical products. The overall strategy of the Group is to continue its drug development programs, which depends on selling assets and raising additional equity to fund the activities.
The capital risk management policy remains unchanged from the 2014 Annual Report.
Note 15. Equity—reserves
| | | | | | | | |
| | | Consolidated | |
| | | 2015 | | | 2014 | |
| | | $‘000 | | | $‘000 | |
Foreign currency reserve | | | (1,300 | ) | | | (1,306 | ) |
Share-based payments reserve | | | 3,338 | | | | 1,947 | |
| | | | | | | | |
| | | 2,038 | | | | 641 | |
| | | | | | | | |
Foreign currency reserve
The reserve is used to recognise exchange differences arising from the translation of the financial statements of foreign operations to Australian dollars.
Share-based payments reserve
The reserve is used to recognise the value of equity benefits provided to employees and directors as part of their remuneration, and other parties as part of their compensation for services.
Movements in reserves
Movements in each class of reserve during the current and previous financial year are set out below:
| | | | | | | | | | | | |
Consolidated | | Foreign
currency
| | | Share-
based
payments
| | | Total
| |
| | | $‘000 | | | $‘000 | | | $‘000 | |
Balance at 1 July 2013 | | | (1,314 | ) | | | 1,592 | | | | 278 | |
Foreign currency translation | | | 8 | | | | — | | | | 8 | |
Share-based payments | | | — | | | | 355 | | | | 355 | |
| | | | | | | | | | | | |
Balance at 30 June 2014 | | | (1,306 | ) | | | 1,947 | | | | 641 | |
Foreign currency translation | | | 6 | | | | — | | | | 6 | |
Share-based payments | | | — | | | | 1,503 | | | | 1,503 | |
Transfer of expired share-based payments | | | — | | | | (4 | ) | | | (4 | ) |
Transfer to share capital for options exercised | | | — | | | | (108 | ) | | | (108 | ) |
| | | | | | | | | | | | |
Balance at 30 June 2015 | | | (1,300 | ) | | | 3,338 | | | | 2,038 | |
| | | | | | | | | | | | |
F-23
|
| Benitec Biopharma Limited |
| Notes to the financial statements |
| 30 June 2015 and 2014 |
Note 16. Equity—accumulated losses
| | | | | | | | |
| | | Consolidated | |
| | | 2015 | | | 2014 | |
| | | $‘000 | | | $‘000 | |
Accumulated losses at the beginning of the financial year | | | (96,286 | ) | | | (89,247 | ) |
Loss after income tax benefit for the year | | | (11,509 | ) | | | (7,039 | ) |
Transfer from share-based payment reserve | | | 4 | | | | — | |
| | | | | | | | |
Accumulated losses at the end of the financial year | | | (107,791 | ) | | | (96,286 | ) |
| | | | | | | | |
Note 17. Equity—dividends
There were no dividends paid, recommended or declared during the current or previous financial year.
Note 18. Financial instruments
Financial risk management objectives
The Group’s activities expose it to a variety of financial risks: market risk (including foreign currency risk and interest rate risk) and liquidity risk. The Group’s principal financial instruments comprise receivables, payables, cash and short-term deposits. The Group manages its exposure to key financial risks, including interest rate and currency risk in accordance with the Company financial risk management policy. The objective of the policy is to protect the assets and provide a solid return.
Market risk
Foreign currency risk
The Group undertakes certain transactions denominated in foreign currency and is exposed to foreign currency risk through foreign exchange rate fluctuations.
Foreign exchange risk arises from future commercial transactions and recognised financial assets and financial liabilities denominated in a currency that is not the entity’s functional currency. The risk is measured using sensitivity analysis and cash flow forecasting.
Interest rate risk
The Group generates income from interest on surplus funds. At reporting date, the Group had the following assets exposed to Australian variable interest rate risk that are not designated in cash flow hedges:
As at the reporting date, the Group had the following variable rate cash and cash equivalents outstanding:
| | | | | | | | | | | | | | | | |
| | | 2015 | | | 2014 | |
Consolidated | | Weighted
average
interest
rate | | | Balance | | | Weighted
average
interest
rate | | | Balance | |
| | | % | | | $‘000 | | | % | | | $‘000 | |
Cash and cash equivalents | | | 3.26 | % | | | 21,787 | | | | 3.67 | % | | | 31,359 | |
| | | | | | | | | | | | | | | | |
Net exposure to cash flow interest rate risk | | | | | | | 21,787 | | | | | | | | 31,359 | |
| | | | | | | | | | | | | | | | |
An analysis by remaining contractual maturities in shown in ‘liquidity and interest rate risk management’ below.
F-24
|
| Benitec Biopharma Limited |
| Notes to the financial statements |
| 30 June 2015 and 2014 |
Credit risk
Credit risk refers to the risk that a counterparty will default on its contractual obligations resulting in financial loss to the Group. The maximum exposure to credit risk at the reporting date to recognised financial assets is the carrying amount, net of any provisions for impairment of those assets, as disclosed in the statement of financial position and notes to the financial statements. The Group does not hold any collateral.
Liquidity risk
Vigilant liquidity risk management requires the Group to maintain sufficient liquid assets (mainly cash and cash equivalents) and available borrowing facilities to be able to pay debts as and when they become due and payable.
The Group manages liquidity risk by maintaining adequate cash reserves and available borrowing facilities by continuously monitoring actual and forecast cash flows and matching the maturity profiles of financial assets and liabilities.
Remaining contractual maturities
The following tables detail the Group’s remaining contractual maturity for its financial instrument liabilities. The tables have been drawn up based on the undiscounted cash flows of financial liabilities based on the earliest date on which the financial liabilities are required to be paid.
| | | | | | | | | | | | | | | | | | | | | | | | |
Consolidated - 2015 | | Weighted
average
interest
rate | | | 1 year
or less
| | | Between
1 and 2
years
| | | Between
2 and 5
years
| | | Over
5 years
| | | Remaining
contractual
maturities
| |
| | | % | | | $‘000 | | | $‘000 | | | $‘000 | | | $‘000 | | | $‘000 | |
Non-derivatives | | | | | | | | | | | | | | | | | | | | | | | | |
Non-interest bearing | | | | | | | | | | | | | | | | | | | | | | | | |
Trade payables | | | — | % | | | 760 | | | | — | | | | — | | | | — | | | | 760 | |
Other payables | | | — | % | | | 688 | | | | — | | | | — | | | | — | | | | 688 | |
| | | | | | | | | | | | | | | | | | | | | | | | |
Total non-derivatives | | | | | | | 1,448 | | | | — | | | | — | | | | — | | | | 1,448 | |
| | | | | | | | | | | | | | | | | | | | | | | | |
| | | | | | |
Consolidated - 2014 | | Weighted
average
interest
rate | | | 1 year
or less
| | | Between
1 and 2
years
| | | Between
2 and 5
years
| | | Over
5 years
| | | Remaining
contractual
maturities
| |
| | | % | | | $‘000 | | | $‘000 | | | $‘000 | | | $‘000 | | | $‘000 | |
Non-derivatives | | | | | | | | | | | | | | | | | | | | | | | | |
Non-interest bearing | | | | | | | | | | | | | | | | | | | | | | | | |
Trade payables | | | — | % | | | 573 | | | | — | | | | — | | | | — | | | | 573 | |
Other payables | | | — | % | | | 215 | | | | — | | | | — | | | | — | | | | 215 | |
| | | | | | | | | | | | | | | | | | | | | | | | |
Total non-derivatives | | | | | | | 788 | | | | — | | | | — | | | | — | | | | 788 | |
| | | | | | | | | | | | | | | | | | | | | | | | |
The cash flows in the maturity analysis above are not expected to occur significantly earlier than contractually disclosed above.
Fair value of financial instruments
Unless otherwise stated, the carrying amounts of financial instruments reflect their fair value.
F-25
|
| Benitec Biopharma Limited |
| Notes to the financial statements |
| 30 June 2015 and 2014 |
Note 19. Key management personnel disclosures
Compensation
The aggregate compensation made to directors and other members of key management personnel of the Group is set out below:
| | | | | | | | |
| | | Consolidated | |
| | | 2015 | | | 2014 | |
| | | $ | | | $ | |
Short-term employee benefits | | | 1,735,847 | | | | 1,884,781 | |
Post-employment benefits | | | 96,353 | | | | 71,100 | |
Share-based payments | | | 1,036,123 | | | | 348,014 | |
| | | | | | | | |
| | | 2,868,323 | | | | 2,303,895 | |
| | | | | | | | |
Note 20. Remuneration of auditors
During the financial year the following fees were paid or payable for services provided by Grant Thornton Audit Pty Ltd, the auditor of the Company:
| | | | | | | | |
| | | Consolidated | |
| | | 2015 | | | 2014 | |
| | | $ | | | $ | |
Audit services—Grant Thornton Audit Pty Ltd | | | | | | | | |
Audit or review of the financial statements | | | 95,000 | | | | 73,238 | |
| | | | | | | | |
Other services—Grant Thornton Audit Pty Ltd | | | | | | | | |
Tax compliance and corporate advisory services | | | 20,050 | | | | 24,000 | |
IPO services | | | 180,000 | | | | — | |
| | | | | | | | |
| | | 200,050 | | | | 24,000 | |
| | | | | | | | |
| | | 295,050 | | | | 97,238 | |
| | | | | | | | |
Note 21. Contingent liabilities and commitments
In January 2010, the Group reached a settlement with the CSIRO to replace the existing Licence Agreement and Commercial Agreement with a new exclusive Licence Agreement for the use of intellectual property and the Capital Growth Agreement with the issue of ordinary shares. As part of the settlement, a Transition Agreement was put in place in order to facilitate the change from the old agreements to the new agreement and to deal with a number of other matters.
Under the terms of the Transition Agreement, the Group agreed to pay CSIRO an amount of $297,000 for past patent costs only in the event of a trigger event, being either a corporate transaction or an insolvency event.
Scientific work on the therapeutic programs
On 18 December 2012, the Group announced the appointment of Synteract, Inc. as its Clinical Research Organisation responsible for the progression of TT-034 into Phase I/IIa clinical trials in the USA. The Group has negotiated a contract with favourable commercial terms, in some instances requiring prepayment, for Synteract to continue to manage the clinical trials throughout 2014, 2015 and 2016.
F-26
|
| Benitec Biopharma Limited |
| Notes to the financial statements |
| 30 June 2015 and 2014 |
On 3 June 2014, the Group announced plans to progress its non-small cell lung cancer (‘NSCLC’) therapeutic candidate, Tribetarna advising it had reached agreement to use European-based clinical research organisation CTGCRO to manage clinical trials, and subsequently negotiated favourable commercial terms, which included prepayments covering the clinical trial and consulting services.
On 11 November 2014, the Group entered into a Collaborative Research and License Agreement with 4D Molecular Therapeutics (4DMT) to identify and develop adeno-associated virus (“AAV”) vector variants optimized for gene delivery to tissues within the eye using 4D technology and products combining such optimized AAV vector variants with Benitec’s ddRNAi technology, for further development and commercialization by Benitec under license from 4D Molecular. Under this agreement the Group shall fund 4DMT for the studies to be carried out by 4DMT according to the research plan that was agreed between the parties.
The Group has contracted for scientific work on the therapeutic programs, as described above, and payments due within the next 12 months total approximately $2,892,000.
Note 22. Commitments
| | | | | | | | |
| | | Consolidated | |
| | | 2015 | | | 2014 | |
| | | $‘000 | | | $‘000 | |
Lease commitments—operating | | | | | | | | |
Committed at the reporting date but not recognised as liabilities, payable: | | | | | | | | |
Within one year | | | 118 | | | | 116 | |
One to five years | | | 378 | | | | 109 | |
| | | | | | | | |
| | | 496 | | | | 225 | |
| | | | | | | | |
Operating lease commitments includes contracted amounts for offices under non-cancellable operating leases expiring within 3 years with, in some cases, options to extend. The leases have various escalation clauses. On renewal, the terms of the leases are renegotiated.
Note 23. Related party transactions
Parent entity
Benitec Biopharma Limited is the parent entity.
Subsidiaries
Interests in subsidiaries are set out in note 25.
Key management personnel
Disclosures relating to key management personnel are set out in note 19 and the remuneration report in the directors’ report.
F-27
|
| Benitec Biopharma Limited |
| Notes to the financial statements |
| 30 June 2015 and 2014 |
Transactions with related parties
The following transactions occurred with related parties:
| | | | | | | | |
| | | Consolidated | |
| | | 2015 | | | 2014 | |
| | | $ | | | $ | |
Payment for other expenses: | | | | | | | | |
Legal services paid / payable to Francis Abourizk Lightowlers, a law firm in which Mr Peter Francis is a partner and has a beneficial interest. | | | 143,684 | | | | 108,913 | |
Consultancy fees for executive duties paid/payable to NewStar Ventures Ltd, a corporation in which Dr John Chiplin is a director and has a beneficial interest. | | | 118,013 | | | | 40,000 | |
Receivable from and payable to related parties
There were no trade receivables from or trade payables to related parties at the current and previous reporting date.
Loans to/from related parties
There were no loans to or from related parties at the current and previous reporting date.
Terms and conditions
All transactions were made on normal commercial terms and conditions and at market rates.
Note 24. Parent entity information
Set out below is the supplementary information about the parent entity.
Statement of profit or loss and other comprehensive income
| | | | | | | | |
| | | Parent | |
| | | 2015 | | | 2014 | |
| | | $‘000 | | | $‘000 | |
Loss after income tax | | | (9,562 | ) | | | (7,037 | ) |
| | | | | | | | |
Total comprehensive income | | | (9,562 | ) | | | (7,037 | ) |
| | | | | | | | |
F-28
|
| Benitec Biopharma Limited |
| Notes to the financial statements |
| 30 June 2015 and 2014 |
Statement of financial position
| | | | | | | | |
| | | Parent | |
| | | 2015 | | | 2014 | |
| | | $‘000 | | | $‘000 | |
Total current assets | | | 26,763 | | | | 34,386 | |
| | | | | | | | |
Total assets | | | 27,108 | | | | 34,568 | |
| | | | | | | | |
Total current liabilities | | | 1,574 | | | | 1,312 | |
| | | | | | | | |
Total liabilities | | | 1,574 | | | | 1,312 | |
| | | | | | | | |
Equity | | | | | | | | |
Issued capital | | | 129,631 | | | | 129,186 | |
Share-based payments reserve | | | 3,338 | | | | 1,947 | |
Accumulated losses | | | (107,435 | ) | | | (97,877 | ) |
| | | | | | | | |
Total equity | | | 25,534 | | | | 33,256 | |
| | | | | | | | |
Guarantees entered into by the parent entity in relation to the debts of its subsidiaries
The parent entity had no guarantees in relation to the debts of its subsidiaries as at 30 June 2015 and 30 June 2014.
Contingent liabilities
The parent entity had no contingent liabilities as at 30 June 2015 (2014: nil), other than the contingent liabilities described in note 21.
Capital commitments—Property, plant and equipment
The parent entity had no capital commitments for property, plant and equipment as at 30 June 2015 and 30 June 2014.
Significant accounting policies
The accounting policies of the parent entity are consistent with those of the Group, as disclosed in note 1, except for the following:
| | • | | Investments in subsidiaries are accounted for at cost, less any impairment, in the parent entity. |
| | • | | Dividends received from subsidiaries are recognised as other income by the parent entity and its receipt may be an indicator of an impairment of the investment. |
F-29
|
| Benitec Biopharma Limited |
| Notes to the financial statements |
| 30 June 2015 and 2014 |
Note 25. Interests in subsidiaries
The consolidated financial statements incorporate the assets, liabilities and results of the following subsidiaries in accordance with the accounting policy described in note 1:
| | | | | | |
| | | | | Ownership interest |
Name | | Principal place
of business /
Country of
incorporation | | 2015
% | | 2014
% |
Benitec Australia Limited | | Australia | | 100.00% | | 100.00% |
Benitec Biopharma Limited | | United Kingdom | | 100.00% | | 100.00% |
Benitec, Inc. | | USA | | 100.00% | | 100.00% |
Benitec LLC | | USA | | 100.00% | | 100.00% |
RNAi Therapeutics, Inc. | | USA | | 100.00% | | 100.00% |
Tacere Therapeutics, Inc. | | USA | | 100.00% | | 100.00% |
Note 26. Events after the reporting period
On 9 July 2015, the Group announced that it had acquired the full rights to the pre-clinical ddRNAi-based hepatitis B (HBV) therapeutic program, Hepbarna® from Biomics Biotechnologies, which was previously under development as a joint venture between the two companies. The Company will pay $2,500,000 upfront with a further $3,500,000 upon successful commercialisation of the program and right to royalty on net sales. 647,333 ordinary shares in the Company were issued on 22 July 2015 as consideration.
On 22 July 2015, the Company’s shareholders at a General Meeting passed a resolution to issue up to 115,000,000 new shares through an initial public offer, which would be represented by American Depositary Shares for trading on Nasdaq.
On 20 August 2015, the Company has successfully completed an initial public offer in the United States and the associated listing on the NASDAQ Global Select Market. Benitec issued 30,000,000 ordinary shares (converted to 1,500,000 NASDAQ ADS: BNTC) and 10,000,000 options (converted to 500,000 NASDAQ warrants: BNTCW representing 20 options for each warrant) through the initial public offer and raised $18,844,000 (US$13,820,000) under the IPO. Benitec intends to use the net proceeds of the IPO to advance the programs for its therapies, for working capital and for general corporate purposes.
No other matter or circumstance has arisen since 30 June 2015 that has significantly affected, or may significantly affect the Group’s operations, the results of those operations, or the Group’s state of affairs in future financial years.
F-30
|
| Benitec Biopharma Limited |
| Notes to the financial statements |
| 30 June 2015 and 2014 |
Note 27. Reconciliation of loss after income tax to net cash used in operating activities
| | | | | | | | |
| | | Consolidated | |
| | | 2015 | | | 2014 | |
| | | $‘000 | | | $‘000 | |
Loss after income tax benefit for the year | | | (11,509 | ) | | | (7,039 | ) |
| | |
Adjustments for: | | | | | | | | |
Depreciation and amortisation | | | 97 | | | | 13 | |
Share-based payments | | | 1,503 | | | | 355 | |
Foreign exchange differences | | | (567 | ) | | | 9 | |
| | |
Change in operating assets and liabilities: | | | | | | | | |
Increase in trade and other receivables | | | (1 | ) | | | (17 | ) |
Decrease/(increase) in other operating assets | | | 98 | | | | (2,937 | ) |
Increase in trade and other payables | | | 661 | | | | 277 | |
Increase in employee benefits | | | 26 | | | | 68 | |
| | | | | | | | |
Net cash used in operating activities | | | (9,692 | ) | | | (9,271 | ) |
| | | | | | | | |
Note 28. Earnings per share
| | | | | | | | |
| | | Consolidated | |
| | | 2015 | | | 2014 | |
| | | $‘000 | | | $‘000 | |
Loss after income tax attributable to the owners of Benitec Biopharma Limited | | | (11,509 | ) | | | (7,039 | ) |
| | | | | | | | |
| | |
| | | Number | | | Number | |
Weighted average number of ordinary shares used in calculating basic earnings per share | | | 115,507,308 | | | | 90,432,177 | |
| | | | | | | | |
Weighted average number of ordinary shares used in calculating diluted earnings per share | | | 115,507,308 | | | | 90,432,177 | |
| | | | | | | | |
| | |
| | | Cents | | | Cents | |
Basic earnings per share | | | (9.96 | ) | | | (7.78 | ) |
Diluted earnings per share | | | (9.96 | ) | | | (7.78 | ) |
Outstanding options to acquire ordinary shares are not considered dilutive for the years ended 30 June 2015 and 30 June 2014.
On 20 August 2015, the Company issued 30,000,000 ordinary shares and 10,000,000 options as detailed on note 25 events after the reporting period.
F-31
|
| Benitec Biopharma Limited |
| Notes to the financial statements |
| 30 June 2015 and 2014 |
Note 29. Share-based payments
Benitec Biopharma Limited Employees Share Option Plan (ESOP):
Description of plan
The Group may from time to time issue employees options to acquire shares in the parent at a fixed price. Each option when exercised entitles the option holder to one share in the Company. Options are exercisable on or before an expiry date, do not carry any voting or dividend rights and are not transferable except on death of the option holder.
The following table shows the number and weighted average exercise price (WAEP) of share options issued under the ESOP:
| | | | | | | | | | | | | | | | |
| | | 2015
Number | | | 2015
WAEP | | | 2014
Number | | | 2014
WAEP | |
Outstanding at the beginning of the year | | | 5,288,000 | | | | 1.229 | | | | 3,028,000 | | | | 1.200 | |
Granted during the year | | | 4,284,000 | | | | 1.250 | | | | 2,260,000 | | | | 1.270 | |
Exercised during the year | | | (200,000 | ) | | | 0.510 | | | | — | | | | — | |
Lapsed or forfeited during the year | | | (72,000 | ) | | | 1.250 | | | | — | | | | — | |
| | | | | | | | | | | | | | | | |
Outstanding at the end of the year | | | 9,300,000 | | | | 1.250 | | | | 5,288,000 | | | | 1.229 | |
| | | | | | | | | | | | | | | | |
Options exercisable at the end of the year | | | 4,670,667 | | | | | | | | 2,178,667 | | | | | |
| | | | | | | | | | | | | | | | |
Details of ESOP share options outstanding as at end of year:
| | | | | | | | | | | | | | |
Grant date | | Expiry date | | Exercise
price | | | 2015
Number | | | 2014
Number | |
13/07/2010 | | 19/08/2014 | | | 0.51 | | | | — | | | | 260,000 | |
17/11/2011 | | 17/11/2016 | | | 1.25 | | | | 1,800,000 | | | | 1,800,000 | |
07/02/2012 | | 07/02/2017 | | | 1.25 | | | | 156,000 | | | | 168,000 | |
18/07/2012 | | 18/07/2017 | | | 1.25 | | | | 400,000 | | | | 400,000 | |
16/11/2012 | | 16/11/2017 | | | 1.25 | | | | 400,000 | | | | 400,000 | |
22/08/2013 | | 22/08/2018 | | | 1.25 | | | | 2,080,000 | | | | 2,080,000 | |
15/05/2014 | | 15/05/2019 | | | 1.50 | | | | 180,000 | | | | 180,000 | |
17/12/2014 | | 17/12/2019 | | | 1.25 | | | | 3,334,000 | | | | — | |
06/05/2015 | | 06/05/2020 | | | 1.25 | | | | 950,000 | | | | — | |
| | | | | | | | | | | | | | |
| | | | | | | | | 9,300,000 | | | | 5,288,000 | |
| | | | | | | | | | | | | | |
The weighted average remaining life of the options issued under the ESOP at 30 June 2015 was 3 years and 4 months (2014: 3 years and 3 months).
For the options granted during the current financial year, the valuation model inputs used to determine the fair value at the grant date, are as follows:
| | | | | | | | | | | | | | | | | | | | | | | | | | |
Grant date | | Expiry date | | Share price
at grant
date | | | Exercise
price | | | Expected*
volatility | | | Dividend
yield | | | Risk-free
interest
rate | | | Fair value
at grant
date | |
17/12/2014 | | 17/12/2019 | | $ | 0.840 | | | $ | 1.250 | | | | 95.00 | % | | | — | % | | | 2.35 | % | | $ | 0.563 | |
06/05/2015 | | 06/05/2020 | | $ | 0.810 | | | $ | 1.250 | | | | 95.00 | % | | | — | % | | | 2.35 | % | | $ | 0.534 | |
| * | | expected volatility was determined by reference to Bloomberg for the Benitec share price based on historical volatility |
Total expenses arising from share-based payment transactions recognised during the period as part of employee benefit expense were $1,502,726 (2014: $355,116).
F-32
|
| REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM |
BOARD OF DIRECTORS AND SHAREHOLDERS
BENITEC BIOPHARMA LIMITED
We have audited the accompanying consolidated statements of financial position of Benitec Biopharma Limited and its controlled entities (the “Group”) as of 30 June 2014 and 2013, and the related consolidated statements of profit or loss and other comprehensive income, statements of changes in equity, and statements of cash flows for each of the two years in the period ended 30 June 2014. These financial statements are the responsibility of the Group’s management. Our responsibility is to express an opinion on these financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. We were not engaged to perform an audit of the Group’s internal control over financial reporting. Our audit included consideration of internal control over financial reporting as a basis for designing audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the Group’s internal control over financial reporting. Accordingly, we express no such opinion. An audit also includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the consolidated financial statements referred to above present fairly, in all material respects, the financial position of Benitec Biopharma Limited and its’ controlled entities as of 30 June 2014 and 2013 and the results of their operations and their cash flows for each of the two years in the period ended 30 June 2014 and 2013 in conformity with International Financial Reporting Standards, as issued by the International Accounting Standards Board.
GRANT THORNTON AUDIT PTY LTD
Chartered Accountants
Sydney, NSW, Australia
1 May 2015
F-33
Consolidated Statement of Profit or Loss and Other Comprehensive Income
For the Year Ended 30 June 2014
| | | | | | | | | | | | |
| | | Note | | | 2014 | | | 2013 | |
| | | | | | $ | | | $ | |
Continuing Operations | | | | | | | | | | | | |
Revenue | | | 2 | | | | 597,940 | | | | 639,849 | |
Other income | | | 2 | | | | 775,833 | | | | 824,333 | |
| | | | | | | | | | | | |
| | | | | | | 1,373,773 | | | | 1,464,182 | |
| | | | | | | | | | | | |
Royalties & licence fees | | | | | | | (192,753 | ) | | | (30,000 | ) |
Research and development | | | | | | | (3,757,869 | ) | | | (1,280,012 | ) |
Employment related | | | | | | | (2,444,015 | ) | | | (1,832,065 | ) |
Share based expense | | | | | | | (355,116 | ) | | | (518,749 | ) |
Impairment costs | | | | | | | — | | | | (1,503,296 | ) |
Travel related costs | | | | | | | (585,359 | ) | | | (345,826 | ) |
Consultants costs | | | | | | | (652,839 | ) | | | (336,570 | ) |
Occupancy costs | | | | | | | (121,582 | ) | | | (100,153 | ) |
Corporate expenses | | | | | | | (646,315 | ) | | | (531,686 | ) |
Foreign exchange translation | | | | | | | (111,399 | ) | | | 1,526,215 | |
| | | | | | | | | | | | |
Total expenses | | | | | | | (8,867,247 | ) | | | (4,952,142 | ) |
| | | | | | | | | | | | |
Loss before income tax | | | | | | | (7,493,474 | ) | | | (3,487,960 | ) |
Income tax benefit | | | 4 | | | | 454,365 | | | | — | |
| | | | | | | | | | | | |
Loss for the year attributable to members of the parent entity | | | | | | | (7,039,109 | ) | | | (3,487,960 | ) |
| | | | | | | | | | | | |
Other Comprehensive Income: | | | | | | | | | | | | |
Items that may be reclassified subsequently to profit and loss | | | | | | | | | | | | |
Other Comprehensive Income for the year, Foreign exchange translation, net of tax | | | | | | | 7,747 | | | | (1,313,792 | ) |
| | | | | | | | | | | | |
Total Comprehensive Income for the year | | | | | | | (7,031,362 | ) | | | (4,801,752 | ) |
| | | | | | | | | | | | |
Total Comprehensive Income attributable to members of the parent entity | | | | | | | (7,031,362 | ) | | | (4,801,752 | ) |
| | | | | | | | | | | | |
Earnings per share (cents per share): | | | | | | | | |
Basic and diluted loss for the year attributable to ordinary equity holders of the parent entity | | | | | | | | | | | | |
Cents per share, with the comparative adjusted for the share consolidation at 25:1 in July 2013 | | | 6 | | | | (7.78 | ) | | | (8.25 | ) |
This statement should be read in conjunction with the notes to the financial statements.
F-34
Consolidated Statement of Financial Position
As at 30 June 2014
| | | | | | | | | | |
| | | Note | | 2014 | | | 2013 | |
| | | | | $ | | | $ | |
CURRENT ASSETS | | | | | | | | | | |
Cash and cash equivalents | | 8 | | | 31,359,199 | | | | 1,587,299 | |
Trade and other receivables | | 9 | | | 121,587 | | | | 105,073 | |
Other current assets | | 10 | | | 2,966,739 | | | | 30,218 | |
| | | | | | | | | | |
TOTAL CURRENT ASSETS | | | | | 34,447,525 | | | | 1,722,590 | |
| | | | | | | | | | |
NON-CURRENT ASSETS | | | | | | | | | | |
Property, plant and equipment | | 12 | | | 47,677 | | | | 28,120 | |
| | | | | | | | | | |
TOTAL NON-CURRENT ASSETS | | | | | 47,677 | | | | 28,120 | |
| | | | | | | | | | |
TOTAL ASSETS | | | | | 34,495,202 | | | | 1,750,710 | |
| | | | | | | | | | |
CURRENT LIABILITIES | | | | | | | | | | |
Trade and other payables | | 14 | | | 788,169 | | | | 1,011,733 | |
Provisions | | 15 | | | 166,511 | | | | 98,637 | |
TOTAL CURRENT LIABILITIES | | | | | 954,680 | | | | 1,110,370 | |
| | | | | | | | | | |
TOTAL LIABILITIES | | | | | 954,680 | | | | 1,110,370 | |
| | | | | | | | | | |
NET ASSETS | | | | | 33,540,522 | | | | 640,340 | |
| | | | | | | | | | |
EQUITY | | | | | | | | | | |
Contributed equity | | 16 | | | 129,185,676 | | | | 89,609,248 | |
Reserves | | 17 | | | 640,773 | | | | 277,910 | |
Accumulated losses | | | | | (96,285,927 | ) | | | (89,246,818 | ) |
| | | | | | | | | | |
TOTAL EQUITY | | | | | 33,540,522 | | | | 640,340 | |
| | | | | | | | | | |
This statement should be read in conjunction with the notes to the financial statements.
F-35
Consolidated Statement of Changes in Equity
For the year ended 30 June 2014
| | | | | | | | | | | | | | | | | | | | |
| | | Contributed
Equity | | | Share-based
Payments
Reserve | | | Foreign
exchange
translation
reserve | | | Accumulated
Losses | | | Total | |
| | | $ | | | $ | | | $ | | | $ | | | $ | |
Balance at 1 July 2012 | | | 87,348,819 | | | | 1,394,142 | | | | — | | | | (86,080,047 | ) | | | 2,662,914 | |
| | | | | | | | | | | | | | | | | | | | |
Loss for the year | | | — | | | | — | | | | — | | | | (3,487,960 | ) | | | (3,487,960 | ) |
Other comprehensive income for year | | | — | | | | — | | | | (1,313,792 | ) | | | — | | | | (1,313,792 | ) |
| | | | | | | | | | | | | | | | | | | | |
Total comprehensive income for year | | | — | | | | — | | | | (1,313,792 | ) | | | (3,487,960 | ) | | | (4,801,752 | ) |
| | | | | | | | | | | | | | | | | | | | |
Share issue to Tacere on business acquisition | | | 1,173,585 | | | | — | | | | — | | | | — | | | | 1,173,585 | |
Transfer to Accumulated Losses the Share Based Payments Reserve no longer required | | | — | | | | (321,189 | ) | | | — | | | | 321,189 | | | | — | |
Share Based Payments | | | — | | | | 518,749 | | | | — | | | | — | | | | 518,749 | |
Share issues, net of transaction costs | | | 1,086,844 | | | | — | | | | — | | | | — | | | | 1,086,844 | |
| | | | | | | | | | | | | | | | | | | | |
Transactions with owners | | | 2,260,429 | | | | 197,560 | | | | — | | | | 321,189 | | | | 2,779,178 | |
| | | | | | | | | | | | | | | | | | | | |
Balance 30 June 2013 | | | 89,609,248 | | | | 1,591,702 | | | | (1,313,792 | ) | | | (89,246,818 | ) | | | 640,340 | |
| | | | | | | | | | | | | | | | | | | | |
Loss for the year | | | — | | | | — | | | | — | | | | (7,039,109 | ) | | | (7,039,109 | ) |
Other comprehensive income for year | | | — | | | | — | | | | 7,747 | | | | — | | | | 7,747 | |
| | | | | | | | | | | | | | | | | | | | |
Total comprehensive income for year | | | | | | | | | | | 7,747 | | | | (7,039,109 | ) | | | (7,031,362 | ) |
| | | | | | | | | | | | | | | | | | | | |
Share Based Payments | | | — | | | | 355,116 | | | | — | | | | — | | | | 355,116 | |
Share issues, net of transaction costs | | | 39,576,428 | | | | — | | | | — | | | | — | | | | 39,576,428 | |
| | | | | | | | | | | | | | | | | | | | |
Transactions with owners | | | 39,576,428 | | | | 355,116 | | | | — | | | | — | | | | 39,931,544 | |
| | | | | | | | | | | | | | | | | | | | |
Balance 30 June 2014 | | | 129,185,676 | | | | 1,946,818 | | | | (1,306,045 | ) | | | (96,285,927 | ) | | | 33,540,522 | |
| | | | | | | | | | | | | | | | | | | | |
This statement should be read in conjunction with the notes to the financial statements.
F-36
Consolidated Statement of Cash Flows
For the year ended 30 June 2014
| | | | | | | | | | |
| | | Note | | 2014 | | | 2013 | |
| | | | | $ | | | $ | |
CASH FLOWS FROM OPERATING ACTIVITIES | | | | | | | | | | |
Receipts from customers | | | | | 260,310 | | | | 566,754 | |
Research and development grants | | | | | 775,833 | | | | 824,333 | |
Interest received | | | | | 321,116 | | | | 133,011 | |
Income tax benefit | | | | | 454,365 | | | | — | |
Payments to suppliers and employees | | | | | (11,081,963 | ) | | | (4,256,694 | ) |
| | | | | | | | | | |
Net cash used in operating activities | | 8 | | | (9,270,339 | ) | | | (2,732,596 | ) |
| | | | | | | | | | |
CASH FLOWS FROM INVESTING ACTIVITIES | | | | | | | | | | |
Business acquisition | | 26 | | | — | | | | 143,603 | |
Purchase of property, plant and equipment | | | | | (32,365 | ) | | | (9,889 | ) |
| | | | | | | | | | |
Net cash provided/(used) by investing activities | | | | | (32,365 | ) | | | 133,714 | |
| | | | | | | | | | |
CASH FLOWS FROM FINANCING ACTIVITIES | | | | | | | | | | |
Net proceeds from issue of shares | | | | | 39,075,618 | | | | 1,086,844 | |
| | | | | | | | | | |
Net cash provided by financing activities | | | | | 39,075,618 | | | | 1,086,844 | |
| | | | | | | | | | |
Net decrease in cash held | | | | | 29,772,914 | | | | (1,512,038 | ) |
Exchange differences on cash and cash equivalents | | | | | (1,014 | ) | | | 23,457 | |
Cash and cash equivalents, beginning of year | | | | | 1,587,299 | | | | 3,075,880 | |
| | | | | | | | | | |
Cash and cash equivalents, end of year | | 8 | | | 31,359,199 | | | | 1,587,299 | |
| | | | | | | | | | |
This statement should be read in conjunction with the notes to the financial statements.
F-37
Notes to the Consolidated Financial Statements for the Year Ended 30 June 2014
Note 1: Summary of significant accounting policies
(a) Basis of Preparation
The financial report covers Benitec Biopharma Limited and its controlled entities as a consolidated entity (“Group”). Benitec Biopharma Limited is a listed public company, incorporated and domiciled in Australia. All amounts are stated in Australian dollars.
The consolidated general purpose financial statements of the Group have been prepared in accordance with the requirements of theCorporations Act 2001, International Accounting Standards and other authoritative pronouncements of the International Accounting Standards Board. Compliance with International Accounting Standards results in full compliance with the International Financial Reporting Standards (IFRS) as issued by the International Accounting Standards Board (IASB). Benitec Biopharma Limited is a for-profit entity for the purpose of preparing financial statements.
The consolidated financial statements for the year ended 30 June 2014 (including comparatives) were approved and authorised for issue by the board of directors on 1 May 2015.
The consolidated financial statements have been prepared using the measurement bases specified by International Accounting Standards for each type of asset, liability, income and expense. The measurement bases are more fully described in the accounting policies below.
(b) Principles of Consolidation
A controlled entity is any entity controlled by Benitec Biopharma Limited whereby Benitec Biopharma Limited has the power to control the financial and operating policies of an entity so as to obtain benefits from its activities.
All inter-company balances and transactions between entities in the consolidated entity, including any unrealised profits or losses, have been eliminated on consolidation. Accounting policies of controlled entities have been changed where necessary to ensure consistencies with those policies applied by the parent entity.
Where controlled entities have entered or left the consolidated entity during the year, their operating results have been included/excluded from the date control was obtained or until the date control ceased.
A list of controlled entities is contained in note 11 to the financial statements. All controlled entities have a June financial year-end except for Benitec Ltd (UK) which has a December year-end.
(c) Accounting Standards
New and revised standards that are effective for these financial statements
A number of new and revised standards are effective for annual periods beginning on or after 1 July 2013. Information on these new standards is presented below.
Amendments to IFRS 8 Operating Segments
The 2012 amendment to IFRS 8 Operating Segments requires the disclosure of judgments made by management in applying the aggregation criteria.
IFRS 10 Consolidated Financial Statements
IFRS 10 supersedes IAS 27Consolidated and Separate Financial Statements IFRS 10. IFRS 10 revises the definition of control and provides extensive new guidance on its application. These new requirements have the
F-38
Notes to the Consolidated Financial Statements for the Year Ended 30 June 2014
potential to affect which of the Group’s investees are considered to be subsidiaries and therefore to change the scope of consolidation. The requirements on consolidation procedures, accounting for changes in non-controlling interests and accounting for loss of control of a subsidiary are unchanged.
Management has reviewed its control assessments in accordance with IFRS 10 and has concluded that there is no effect on the classification (as subsidiaries or otherwise) of any of the Group’s investees held during the period or comparative periods covered by these financial statements.
IFRS 11 Joint Arrangements
IFRS 11 supersedes IAS 31Interests in Joint Ventures. IFRS 11 revises the categories of joint arrangement, and the criteria for classification into the categories, with the objective of more closely aligning the accounting with the investor’s rights and obligations relating to the arrangement. In addition, IAS 31’s option of using proportionate consolidation for arrangements classified as jointly controlled entities under that Standard has been eliminated. IFRS 11 now requires the use of the equity method for arrangements classified as joint ventures (as for investments in associates).
IFRS 13 Fair Value Measurement
IFRS 13 clarifies the definition of fair value and provides related guidance and enhanced disclosures about fair value measurements. It does not affect which items are required to be fair-valued. The scope of IFRS 13 is broad and it applies for both financial and non-financial items for which other International Accounting Standards require or permit fair value measurements or disclosures about fair value measurements, except in certain circumstances.
IFRS 13 applies prospectively for annual periods beginning on or after 1 January 2013. Its disclosure requirements need not be applied to comparative information in the first year of application. The Group has however included as comparative information the IFRS 13 disclosures that were required previously by IFRS 7Financial Instruments: Disclosures.
Amendments to IAS 19 Employee Benefits
The 2011 amendments to IAS 19 made a number of changes to the accounting for employee benefits. The amendments which impact the Group related to the following:
| | • | | Under the amendments, employee benefits ‘expected to be settled wholly’ (as opposed to ‘due to be settled’ under the superseded version of IFRS 19) within 12 months after the end of the reporting period are short-term benefits, and are therefore not discounted when calculating leave liabilities. As the Group does not expect all annual leave for all employees to be used wholly within 12 months of the end of reporting period, annual leave is included in ‘other long-term benefit’ and discounted when calculating the leave liability. This change has had no impact on the presentation of annual leave as a current liability in accordance with IAS 1Presentation of Financial Statements. |
Management have assessed the impact of this change and noted that it is not material to the Group for the years ended 30 June 2013 and 30 June 2014.
Accounting Standards issued but not yet effective and not been adopted early by the Group
At the date of authorisation of these financial statements, certain new standards, amendments and interpretations to existing standards have been published but are not yet effective, and have not been adopted
F-39
Notes to the Consolidated Financial Statements for the Year Ended 30 June 2014
early by the Group. Management anticipates that all of the relevant pronouncements will be adopted in the Group’s accounting policies for the first period beginning after the effective date of the pronouncement. Information on new standards, amendments and interpretations that are expected to be relevant to the Group’s financial statements is provided below. Certain other new standards and interpretations have been issued but are not expected to have a material impact on the Group’s financial statements.
Accounting for Acquisitions of Interests in Joint Operations
The amendments to IFRS 11 state that an acquirer of an interest in a joint operation in which the activity of the joint operation constitutes a ‘business’, as defined in IFRS 3 Business Combinations, should:
| | • | | apply all of the principles on business combinations accounting in IFRS 3 and other IFRSs except principles that conflict with the guidance of IFRS 11. This requirement also applies to the acquisition of additional interests in an existing joint operation that results in the acquirer retaining joint control of the joint operation (note that this requirement applies to the additional interest only, i.e. the existing interest is not remeasured) and to the formation of a joint operation when an existing business is contributed to the joint operation by one of the parties that participate in the joint operation; and |
| | • | | provide disclosures for business combinations as required by IFRS 3 and other IFRSs. |
IFRS 9 Financial Instruments
IFRS 9 introduces new requirements for the classification and measurement of financial assets and liabilities. These requirements improve and simplify the approach for classification and measurement of financial assets compared with the requirements of IAS 39. The main changes are:
(a) Financial assets that are debt instruments will be classified based on (1) the objective of the entity’s business model for managing the financial assets; and (2) the characteristics of the contractual cash flows.
(b) Allows an irrevocable election on initial recognition to present gains and losses on investments in equity instruments that are not held for trading in other comprehensive income (instead of in profit or loss). Dividends in respect of these investments that are a return on investment can be recognised in profit or loss and there is no impairment or recycling on disposal of the instrument.
(c) Financial assets can be designated and measured at fair value through profit or loss at initial recognition if doing so eliminates or significantly reduces a measurement or recognition inconsistency that would arise from measuring assets or liabilities, or recognising the gains and losses on them, on different bases.
(d) Where the fair value option is used for financial liabilities the change in fair value is to be accounted for as follows:
| | • | | The change attributable to changes in credit risk are presented in other comprehensive income (OCI); and |
| | • | | The remaining change is presented in profit or loss. |
If this approach creates or enlarges an accounting mismatch in the profit or loss, the effect of the changes in credit risk are also presented in profit or loss.
(d) Revenue
Revenue from the granting of licenses is recognised in accordance with the terms of the relevant agreements and is usually recognised on an accruals basis, unless the substance of the agreement provides evidence that it is
F-40
Notes to the Consolidated Financial Statements for the Year Ended 30 June 2014
more appropriate to recognise revenue on some other systematic rational basis. Interest revenue is recognised on a proportional basis taking into account the interest rates applicable to the financial assets. Revenue from the rendering of a service is recognised upon the delivery of the service to the customers. All revenue is stated net of the amount of goods and services tax (GST).
Government grants are recognised at fair value where there is reasonable assurance that the grant will be received and all grant conditions will be met. Grants relating to expense items are recognised as income over the periods necessary to match the grant costs they are compensating. Grants relating to assets are credited to deferred income at fair value and are credited to income over the expected useful life of the asset on a straight line basis.
Research and Development Grant revenue is recognised as income when it is received.
(e) Income Tax
The charge for current income tax expense is based on the loss for the year adjusted for any non-assessable or disallowed items. It is calculated using tax rates that have been enacted or are substantially enacted by reporting date.
Deferred tax is accounted for using the liability method in respect of temporary differences arising between the tax bases of assets and liabilities and their carrying amounts in the financial statements. No deferred income tax will be recognised from the initial recognition of an asset or liability, excluding a business combination, where there is no effect on accounting or taxable profit or loss.
Deferred tax is calculated at the tax rates that are expected to apply to the period when the asset is realised or liability is settled. Deferred tax is credited in the statement of comprehensive income except where it relates to items that may be credited directly to equity, in which case the deferred tax is adjusted directly against equity. Deferred income tax assets are recognised to the extent that it is probable that future tax profits will be available against which deductible temporary differences can be utilised.
The amount of benefits brought to account or which may be realised in the future is based on the assumption that no adverse change will occur in income taxation legislation and the anticipation that the consolidated entity will derive sufficient future assessable income to enable the benefit to be realised and comply with the conditions of deductibility imposed by the law.
Benitec Biopharma Limited and its wholly-owned Australian subsidiary has formed an income tax consolidated group under the Tax Consolidation Regime. Benitec Biopharma Limited is responsible for recognising the current and deferred tax assets and liabilities for the tax consolidated group. The Group notified the ATO on 12 February 2004 that it had formed an income tax consolidated group to apply from 1 July 2002. No tax sharing agreement has been entered between entities in the tax consolidated group.
(f) Critical Accounting Estimates and Judgments
The Directors evaluate estimates and judgments incorporated into the financial report based on historical knowledge and best available current information. Estimates assume a reasonable expectation of future events and are based on current trends and economic data, obtained both externally and within the Group.
Key estimates—share-based payments transactions
The Group measures the cost of equity-settled transactions with employees by reference to the fair value of the equity instruments at the date at which they are granted. The fair value is determined using a Black-Scholes model, using the assumptions detailed in note 21.
F-41
Notes to the Consolidated Financial Statements for the Year Ended 30 June 2014
Key judgements—tax losses
Given the company’s and each individual entities’ history of recent losses, the Group has not recognised a deferred tax asset with regard to unused tax losses and other temporary differences, as it has not been determined whether the company or its subsidiaries will generate sufficient taxable income against which the unused tax losses and other temporary differences can be utilised.
Key judgements—compound financial instruments
The Group measures the fair value of the liability component using the prevailing market interest rate for similar convertible instruments.
(g) Impairment of Non-Financial Assets
The Group assesses at each reporting date whether there is an indication that an asset may be impaired. If any such indication exists, or when annual impairment testing for an asset is required (i.e. Goodwill, intangible assets with indefinite useful lives and intangible assets not yet available for use), the Group makes an estimate of the asset’s recoverable amount. An asset’s recoverable amount is the higher of its fair value less costs to sell and its value in use and is determined for an individual asset, unless the asset does not generate cash inflows that are largely independent of those from other assets or groups of assets and the asset’s value in use cannot be estimated to be close to its fair value. In such cases the asset is tested for impairment as part of the cash generating unit to which it belongs. When the carrying amount of an asset or cash-generating unit exceeds its recoverable amount, the asset or cash-generating unit is considered impaired and is written down to its recoverable amount.
In assessing value in use, the estimated future cash flows are discounted to their present value using a pre-tax discount rate that reflects current market assessments of the time value of money and the risks specific to the asset. Impairment losses relating to continuing operations are recognised in those expense categories consistent with the function of the impaired asset unless the asset is carried at revalued amount (in which case the impairment loss is treated as a revaluation decrease).
(h) Cash and Cash Equivalents
Cash and cash equivalents includes cash on hand, deposits held at call with banks, other short-term highly liquid investments with original maturities of three months or less, and bank overdrafts. Bank overdrafts are shown within short term borrowings in current liabilities on the statement of financial position.
(i) Trade and Other Receivables
Trade receivables, which generally have 30 day terms, are recognised and carried at original invoice amount less an allowance for any uncollectible amounts. An estimate for doubtful debts is made when collection of the full amount is no longer probable. Bad debts are written off when identified.
(j) Property, Plant and Equipment
Each class of property, plant and equipment is carried at cost or fair value less, where applicable, any accumulated depreciation and impairment losses.
Plant and equipment
Plant and equipment are measured on the cost basis less depreciation and impairment losses. The carrying amount of plant and equipment is reviewed annually by directors to ensure it is not in excess of the recoverable
F-42
Notes to the Consolidated Financial Statements for the Year Ended 30 June 2014
amount from these assets. The recoverable amount is assessed on the basis of the expected net cash flows that will be received from the assets employment and subsequent disposal. The expected net cash flows have been discounted to their present values in determining recoverable amounts.
Subsequent costs are included in the asset’s carrying amount or recognised as a separate asset, as appropriate, only when it is probable that future economic benefits associated with the item will flow to the Group and the cost of the item can be measured reliably. All other repairs and maintenance are charged to the statement of comprehensive income during the financial period in which they are incurred.
Depreciation
The depreciable amount of all fixed assets including capitalised lease assets is depreciated on a straight line basis over their useful lives to the consolidated entity commencing from the time the asset is held ready for use. Leasehold improvements are depreciated over the shorter of either the unexpired period of the lease or the estimated useful lives of the improvements.
The depreciation rates used for plant and equipment were 20-33%. The assets’ residual values and useful lives are reviewed, and adjusted if appropriate, at each reporting date. An asset’s carrying amount is written down immediately to its recoverable amount if the asset’s carrying amount is greater than its estimated recoverable amount.
Gains and losses on disposals are determined by comparing proceeds with the carrying amount. These gains and losses are included in the statement of comprehensive income. When assets which have been revalued are sold, amounts included in the revaluation reserve relating to that asset are transferred to retained earnings.
(k) Leases
Leases of fixed assets are classified as finance leases where the Group has substantially all the risks and benefits incidental to the ownership of the asset, but not the legal ownership.
Finance leases are capitalised by recording an asset and a liability at the lower of the amounts equal to the fair value of the leased property or the present value of the minimum lease payments, including any guaranteed residual values. Lease payments are allocated between the reduction of the lease liability and the lease interest expense for the period. Leased assets are depreciated on a straight-line basis over their estimated useful lives where it is likely that the consolidated entity will obtain ownership of the asset or over the term of the lease. Lease payments for operating leases, where substantially all the risks and benefits remain with the lessor, are charged as expenses in the periods in which they are incurred.
Lease incentives under operating leases are recognised as a liability and amortised on a straight-line basis over the life of the lease term.
(l) Financial Instruments
Recognition
Financial instruments are initially measured at cost on trade date, which includes transaction costs, when the related contractual rights or obligations exist. Subsequent to initial recognition these instruments are measured as set out below.
Loans and receivables
Loans and receivables are non-derivative financial assets with fixed or determinable payments that are not quoted in an active market and are stated at amortised cost using the effective interest rate method.
F-43
Notes to the Consolidated Financial Statements for the Year Ended 30 June 2014
Financial liabilities
Non-derivative financial liabilities are recognised at amortised cost, comprising original debt less principal payments and amortisation.
Compound instruments
The component parts of compound instruments issued by the Group are classified separately as financial liabilities and equity in accordance with the substance of the contractual arrangement. The liability component is recorded on an amortised cost basis using the effective interest method until extinguished upon conversion or at the instrument’s maturity date. The equity component is determined by deducting the amount of the liability component from the fair value of the compound instrument as a whole. This is recognised and included in equity, net of income tax effects, and is not subsequently remeasured.
Fair value
Fair value is determined based on current bid prices for all quoted investments. Valuation techniques are applied to determine the fair value for all unlisted securities, including recent arm’s length transactions, reference to similar instruments and option pricing models.
Impairment
At each reporting date, the Group assess whether there is objective evidence that a financial instrument has been impaired. In the case of available-for-sale financial instruments, a prolonged or significant decline in the value of the instrument is considered to determine whether impairment has arisen. Impairment losses are recognised in the statement of comprehensive income.
(m) Intangibles
Research and development
Expenditure during the research phase of a project is recognised as an expense when incurred. Development costs are capitalised only when technical feasibility studies identify that the project will deliver future economic benefits and these benefits can be measured reliably.
Development costs have a finite life and are amortised on a systematic basis matched to the future economic benefits over the useful life of the project.
Goodwill
Goodwill, representing the excess of the cost of acquisition over the fair value of the identifiable assets, liabilities and contingent liabilities acquired, is recognised as an asset and not amortised, but tested at least annually for impairment and whenever there is an indication that the goodwill may be impaired. Any impairment is recognised immediately in profit or loss and is not subsequently reversed.
Refer to Note 1 (g) for a description of impairment testing procedures.
(n) Trade and Other Payables
Trade payables and other payables are carried at amortised costs and represent liabilities for goods and services provided to the Group prior to the end of the financial year that are unpaid and arise when the Group becomes obliged to make future payments in respect of the purchase of these goods and services.
F-44
Notes to the Consolidated Financial Statements for the Year Ended 30 June 2014
(o) Employee Benefits
Provision is made for the Group’s liability for employee benefits arising from services rendered by employees to reporting date. Employee benefits that are expected to be settled within one year have been measured at the amounts expected to be paid when the liability is settled, plus related on-costs. Employee benefits payable later than one year have been measured at the present value of the estimated future cash outflows to be made for those benefits.
(p) Provisions
Provisions are recognised when the Group has a legal or constructive obligation, as a result of past events, for which it is probable that an outflow of economic benefits will results and that outflow can be reliably measured.
(q) Contributed Equity
Ordinary shares are classified as equity. Incremental costs directly attributable to the issue of new shares or options are shown in equity as a deduction, net of tax, from the proceeds.
(r) Share-based Payment Transactions
Benefits are provided to employees of the Group in the form of share-based payment transactions, whereby employees render services in exchange for shares or rights over shares (‘equity-settled transactions’). The plan currently in place to provide these benefits is the Employee Share Option Plan (ESOP), which provides benefits to senior executives.
The cost of these equity-settled transactions with employees is measured by reference to the fair value at the date at which they are granted. The fair value is determined using a Black-Scholes model. In valuing equity-settled transactions, no account is taken of any performance conditions, other than conditions linked to the price of the shares of Benitec Biopharma Limited (‘market conditions’).
The cost of equity-settled transactions is recognised, together with a corresponding increase in equity, over the period in which the performance conditions are fulfilled, ending on the date on which the relevant employees become fully entitled to the award (‘vesting date’).
The cumulative expense recognised for equity-settled transactions at each reporting date until vesting date reflects (i) the extent to which the vesting period has expired and (ii) the number of awards that, in the opinion of the directors of the Group, will ultimately vest. This opinion is formed based on the best available information at reporting date. No adjustment is made for the likelihood of market performance conditions being met as the effect of these conditions is included in the determination of fair value at grant date.
No expense is recognised for awards that do not ultimately vest, except for awards where vesting is conditional upon a market condition.
Where the terms of an equity-settled award are modified, as a minimum an expense is recognised as if the terms had not been modified. In addition, an expense is recognised for any increase in the value of the transaction as a result of the modification, as measured at the date of modification. Where an equity-settled award is cancelled, it is treated as if it had vested on the date of cancellation, and any expense not yet recognised for the award is recognised immediately. However, if a new award is substituted for the cancelled award, and designated as a replacement award on the date that it is granted, the cancelled and new award are treated as if they were a modification of the original award, as described in the previous paragraph.
F-45
Notes to the Consolidated Financial Statements for the Year Ended 30 June 2014
The dilutive effect, if any, of outstanding options is reflected as additional share dilution in the computation of earnings per share.
(s) Earnings per Share
Basic earnings per share is calculated as net profit attributable to members of the parent, adjusted to exclude any costs of servicing equity (other than dividends) and preference share dividends, divided by the weighted average number of ordinary shares, adjusted for any bonus element.
Diluted earnings per share is calculated as net profit attributable to members of the parent, adjusted for:
| | • | | costs of servicing equity (other than dividends) and preference share dividends; |
| | • | | the after tax effect of dividends and interest associated with dilutive potential ordinary shares that have been recognised as expenses; and |
| | • | | other non-discretionary changes in revenues or expenses during the period that would result from the dilution of potential ordinary shares; |
divided by the weighted average number of ordinary shares and dilutive potential ordinary shares, adjusted for any bonus element.
(t) Foreign Currency Transactions and Balances
Functional and presentation currency
The functional currency of each of the Group’s entities is measured using the currency of the primary economic environment in which that entity operates. The consolidated financial statements are presented in Australian dollars which is the parent entity’s functional and presentation currency.
Transaction and balances
Foreign currency transactions are translated into functional currency using the exchange rates prevailing at the date of the transaction. Foreign currency monetary items are translated at the year-end exchange rate. Non-monetary items measured at historical cost continue to be carried at the exchange rate at the date of the transaction. Non-monetary items measured at fair value are reported at the exchange rate at the date when fair values were determined.
Exchange differences arising on the translation of monetary items are recognised in the statement of comprehensive income, except where deferred in equity as a qualifying cash flow or net investment hedge. Exchange differences arising on the translation of non-monetary items are recognised directly in equity to the extent that the gain or loss is directly recognised in equity, otherwise the exchange difference is recognised in the statement of comprehensive income.
Group companies
The financial results and position of foreign operations whose functional currency is different from the Group’s presentation currency are translated as follows:
| | • | | Assets and liabilities are translated at year-end exchange rates prevailing at that reporting date. |
| | • | | Income and expenses are translated at average exchange rates for the period. |
| | • | | Retained profits are translated at the exchange rates prevailing at the date of the transaction. |
F-46
Notes to the Consolidated Financial Statements for the Year Ended 30 June 2014
(u) Goods and Services Tax (GST)
Revenues, expenses and assets are recognised net of the amount of GST, except where the amount of GST incurred is not recoverable from the Australian Tax Office. In these circumstances the GST is recognised as part of the cost of acquisition of the asset or as part of an item of the expense. Receivables and payables in the statement of financial position are shown inclusive of GST.
Cash flows are presented in the statement of cash flows on a gross basis, except for the GST component of investing and financing activities, which are disclosed as operating cash flows.
(v) Comparative Figures
When required by Accounting Standards, comparative figures have been adjusted to conform to changes in presentation for the current financial year.
(w) Going Concern
The directors have prepared the financial statements on a going concern basis after taking into consideration the net loss for the year of $7,039,109 and the cash and cash equivalents balance of $31,359,199, The directors have recognised the capital raisings in 2013 and 2014, performed a review of the cash flow forecasts, considered the cash flow needs of the Group, and believe that the strategies in place are appropriate to generate funding which will be sufficient to maintain the going concern status of the Group. If these strategies are unsuccessful then the Group may need to realise its assets and extinguish liabilities other than in the ordinary course of business and at amounts different to those disclosed in the financial report.
Note 2: Revenue from continuing operations
| | | | | | | | |
| | | 2014 | | | 2013 | |
| | | $ | | | $ | |
Revenue | | | | |
Licensing revenue and royalties | | | 276,824 | | | | 521,140 | |
Finance income—interest received | | | 321,116 | | | | 118,709 | |
| | | | | | | | |
| | | 597,940 | | | | 639,849 | |
Other income | | | | |
Federal Government Research and Development Grants | | | 775,833 | | | | 824,333 | |
| | | | | | | | |
Total revenue and other income | | | 1,373,773 | | | | 1,464,182 | |
| | | | | | | | |
F-47
Notes to the Consolidated Financial Statements for the Year Ended 30 June 2014
Note 3: Loss for the year
(a) Expenses incurred by continuing operations
Items included in Statement of Comprehensive Income
| | | | | | | | |
| | | 2014 | | | 2013 | |
| | | $ | | | $ | |
Depreciation | | | | | | | | |
Included in Occupancy expenses | | | | | | | | |
Depreciation of plant and equipment | | | 12,808 | | | | 29,794 | |
Employee benefits expense | | | | | | | | |
Included in Employment related expenses | | | | | | | | |
Wages and salaries | | | 2,109,860 | | | | 1,759,745 | |
Superannuation costs | | | 89,090 | | | | 72,320 | |
(b) Expenses
Research and development expenses costs consist of:
| | | | | | | | |
| | | 2014 | | | 2013 | |
| | | $ | | | $ | |
Project expenses | | | 3,310,014 | | | | 1,075,844 | |
Other IP related expenses | | | 447,855 | | | | 204,168 | |
| | | | | | | | |
| | | 3,757,869 | | | | 1,280,012 | |
| | | | | | | | |
Note 4: Income tax expense
| (a) | The prima facie tax on loss from ordinary activities before income tax is reconciled to the income tax as follows: |
| | | | | | | | |
| | | 2014 | | | 2013 | |
| | | $ | | | $ | |
Prima facie tax payable on loss from ordinary activities before income tax at 30% (2013: 30%) | | | (2,248,042 | ) | | | (1,046,388 | ) |
Add Tax effect of: | | | | | | | | |
Non-deductible share-based payment expense | | | 106,535 | | | | 155,625 | |
Non-assessable foreign currency translation provision | | | (2,324 | ) | | | 457,865 | |
Non-deductible legal fees | | | 15,906 | | | | 9,326 | |
Capital items deductible | | | (231,942 | ) | | | (58,863 | ) |
Other non-deductible items | | | 19,500 | | | | 46,842 | |
Deductible items not included in operating result | | | 4,800 | | | | (48,354 | ) |
Deferred tax asset not brought to account | | | 2,335,567 | | | | 483,947 | |
| | | | | | | | |
Income tax benefit | | | — | | | | — | |
| | | | | | | | |
Income tax benefit reported in the income statement | | | 454,365 | | | | — | |
| | | | | | | | |
The income tax benefit was a cash refund of income tax in the US in Tacere Therapeutics Inc. (a wholly owned subsidiary) not previously recorded.
| (b) | | The parent entity, acting as the Head Entity, notified the Australian Taxation Office on 12 February 2004 that it had formed a Tax Consolidated Group applicable as from 1 July 2002. No tax sharing agreement has been entered between entities in the tax consolidated group. |
F-48
Notes to the Consolidated Financial Statements for the Year Ended 30 June 2014
| (c) | | As at 30 June 2014, the Tax Consolidated Group has estimated carry-forward tax losses of $13,103,412 (2013: $11,751,713) calculated at 30% of the accumulated annual Australian tax losses. The tax losses have not been recognised in the financial statements and the capacity of the Tax Consolidated Group to use the tax losses will be subject to conforming with regulatory tests. The deferred tax asset relating to temporary differences (calculated at 30%) was $49,953 (2013: $29,591). |
The Tax Consolidated Group also has Australian capital tax losses for which no deferred tax asset is recognised in the financial statements of $381,588 (2013: $381,588). The capacity of the Tax Consolidated Group to use the capital tax losses will be subject to conforming with regulatory tests.
The recoupment of available tax losses as at 30 June 2014 is contingent upon the following:
| | (i) | | the Consolidated Group deriving future assessable income of a nature and of an amount sufficient to enable the benefit from the losses to be realised; |
| | (ii) | | the conditions for deductibility imposed by tax legislation continuing to be complied with; and |
| | (iii) | | there being no changes in tax legislation which would adversely affect the Tax Consolidated Group from realising the benefit from the losses. |
Note 5: Auditor’s remuneration
| | | | | | | | |
| | | 2014 | | | 2013 | |
| | | $ | | | $ | |
Audit Services | | | | | | | | |
Remuneration of Grant Thornton Audit Pty Ltd for: | | | | | | | | |
—auditing or reviewing the financial report | | | 73,238 | | | | 54,000 | |
Other Services | | | | | | | | |
Remuneration of Grant Thornton Australia Limited for: | | | | | | | | |
—taxation compliance and corporate advisory services | | | 24,000 | | | | 43,230 | |
Note 6: Earnings per share
Basic earnings per share is calculated by dividing the net loss for the year attributable to ordinary shareholders by the weighted average number of ordinary shares on issue during the year.
Diluted earnings per share amounts are calculated by dividing the net loss attributable to ordinary shareholders by the weighted average number of ordinary shares on issue during the year (adjusted for the effects of dilutive options) and the weighted average number of ordinary shares that would be issued on conversion of all dilutive potential ordinary shares.
| | | | | | | | |
| | | 2014 | | | 2013 | |
| | | $ | | | $ | |
Loss after income tax used in the calculation of basic EPS and dilutive EPS | | | (7,039,109 | ) | | | (3,487,960 | ) |
| | | | | | | | |
| | | Number | | | Number | |
Weighted average number of ordinary shares for basic and diluted earnings per share | | | 90,432,177 | | | | 41,688,975 | |
Weighted average number of converted, lapsed or cancelled potential ordinary shares included in diluted earnings per share | | | — | | | | — | |
Outstanding options to acquire ordinary shares are not considered dilutive for the years ended 30 June 2014 and 30 June 2013.
F-49
Notes to the Consolidated Financial Statements for the Year Ended 30 June 2014
Classification of securities
No securities were classified as potential ordinary shares under IAS33 and therefore have not been included in determination of dilutive EPS due to their anti-dilutive nature.
Note 7: Key management personnel
(a) Details of Key Management Personnel
(i) Non-Executive Directors
| | | | |
| Mr Peter Francis | | Chairman—Non-Executive | | Appointed on 23 February 2006 |
| Dr John Chiplin | | Director—Non-Executive | | Appointed on 1 February 2010 |
| Mr Iain Ross | | Director—Non-Executive | | Appointed on 1 June 2010 |
| Mr Kevin Buchi | | Director—Non-Executive | | Appointed on 11 April 2014 |
| Dr Mel Bridges | | Director—Non-Executive | | Appointed on 12 October 2007 Resigned 18 June 2014 |
(ii) Specified Executives
| | | | |
| Dr Peter French | | Managing Director and Chief Executive Officer | | Appointed as Managing Director on 26 August 2014 Appointed Chief Executive Officer on 4 June 2010 Appointed Chief Scientific Officer on 4 August 2009 |
| Dr Michael Graham | | Chief Scientific Officer | | Appointed on 1 January 2012 |
| Mr Greg West | | Company Secretary | | Appointed on 26 May 2011 |
| Mr Carl Stubbings | | Chief Business Officer | | Appointed on 2 July 2012 |
| Dr David Suhy | | Senior VP Research and Development | | Appointed on 1 October 2012 |
(b) Key management personnel remuneration includes the following expenses:
| | | | | | | | |
| | | 2014 | | | 2013 | |
| | | $ | | | $ | |
Short term employee benefits | | | | | | | | |
Salaries including bonuses | | | 1,859,719 | | | | 1,252,095 | |
Post-employment benefits | | | | | | | | |
Superannuation | | | 71,100 | | | | 61,935 | |
Share-based payments | | | 348,013 | | | | 492,976 | |
| | | | | | | | |
Total Remuneration | | | 2,278,832 | | | | 1,807,006 | |
| | | | | | | | |
During the year no key management personnel exercised options which were granted either under ESOP or by a General Meeting of Members to Non-Executive Directors
Note 8: Cash and cash equivalents
| | | | | | | | |
| | | 2014 | | | 2013 | |
| | | $ | | | $ | |
Cash at bank | | | 288,945 | | | | 614,746 | |
Deposits at call | | | 31,070,254 | | | | 972,553 | |
| | | | | | | | |
| | | 31,359,199 | | | | 1,587,299 | |
| | | | | | | | |
F-50
Notes to the Consolidated Financial Statements for the Year Ended 30 June 2014
Reconciliation of Cash Flow from Operations with Loss after Income Tax
| | | | | | | | |
| | | 2014 | | | 2013 | |
| | | $ | | | $ | |
Loss after Income Tax | | | (7,039,109 | ) | | | (3,487,960 | ) |
| |
Non-cash flows included in operating loss: | | | | |
Impairment | | | — | | | | 1,503,296 | |
Foreign exchange on intercompany balances | | | — | | | | (1,526,215 | ) |
Depreciation | | | 12,808 | | | | 29,794 | |
Share-based payments | | | 355,116 | | | | 518,749 | |
Foreign currency translation unrealised | | | 8,761 | | | | (23,457 | ) |
| |
Changes in assets and liabilities: | | | | |
(Increase)/decrease in other assets | | | (2,936,521 | ) | | | (13,163 | ) |
Decrease in receivables | | | (16,514 | ) | | | 22,393 | |
Decrease/(increase) in payables | | | 277,246 | | | | 200,452 | |
Increase/(decrease) in employee provisions | | | 67,874 | | | | 43,515 | |
| | | | | | | | |
Net cash flows from operations | | | (9,270,339 | ) | | | (2,732,596 | ) |
| | | | | | | | |
Note 9: Trade and other receivables
| | | | | | | | |
| | | 2014 | | | 2013 | |
| | | $ | | | $ | |
CURRENT | | | | | | | | |
Sundry Debtors | | | 121,587 | | | | 105,073 | |
| | | | | | | | |
Note 10: Other assets
| | | | | | | | |
| | | 2014 | | | 2013 | |
| | | $ | | | $ | |
CURRENT | | | | | | | | |
Prepayments | | | 26,679 | | | | 14,190 | |
Prepaid clinical trials * | | | 2,700,000 | | | | — | |
Other current assets | | | 240,060 | | | | 16,028 | |
| | | | | | | | |
| | | 2,966,739 | | | | 30,218 | |
| | | | | | | | |
| * | | Prepaid clinical trials—The Company announced on 3 June 2013 that it had committed to moving its non-small cell lung cancer therapeutic into clinical development. The Company is using European-based clinical research organisation Clinical Trials Group (CTGCRO) to manage both the initial clinical development and trials. The Company made prepayments in the September quarter 2013 in order to secure favourable commercial terms with CTGCRO for the conduct of the trials. |
F-51
Notes to the Consolidated Financial Statements for the Year Ended 30 June 2014
Note 11: Controlled entities
(a) Controlled entities:
| | | | | | | | | | |
| | | Country of
Incorporation | | Percentage Owned | |
| | | | | 2014 | | | 2013 | |
Parent Entity: | | | | | | | | | | |
Benitec Biopharma Limited | | Australia | | | | | | | | |
| | | |
Controlled entities of Benitec Biopharma Limited: | | | | | | | | | | |
Benitec Australia Limited | | Australia | | | 100 | % | | | 100 | % |
Benitec Biopharma Limited | | United Kingdom | | | 100 | % | | | 100 | % |
Benitec, Inc. | | USA | | | 100 | % | | | 100 | % |
Benitec LLC | | USA | | | 100 | % | | | 100 | % |
RNAi Therapeutics, Inc. | | USA | | | 100 | % | | | 100 | % |
Tacere Therapeutics, Inc. | | USA | | | 100 | % | | | 100 | % |
(b) Controlled entities acquired or disposed:
No controlled entities were acquired or disposed during the 2014 financial year.
Note 12: Property, plant and equipment
| | | | | | | | |
| | | 2014 | | | 2013 | |
| | | $ | | | $ | |
At cost | | | 127,795 | | | | 95,431 | |
Accumulated depreciation | | | (80,118 | ) | | | (67,311 | ) |
| | | | | | | | |
Total Property, Plant and Equipment | | | 47,677 | | | | 28,120 | |
| | | | | | | | |
Movements in Carrying Amounts
Movement in the carrying amounts for each class of property, plant and equipment between the beginning and the end of the current financial year.
| | | | | | | | | | | | |
| | | Leasehold
Improvement | | | Plant and
Equipment | | | Total | |
| | | $ | | | $ | | | $ | |
Balance at 30 June 2012 | | | 11,760 | | | | 19,043 | | | | 30,803 | |
Additions | | | — | | | | 27,111 | | | | 27,111 | |
Less Disposals | | | — | | | | — | | | | — | |
Depreciation expense | | | (1,550 | ) | | | (28,244 | ) | | | (29,794 | ) |
| | | | | | | | | | | | |
Balance at 30 June 2013 | | | 10,210 | | | | 17,910 | | | | 28,120 | |
| | | | | | | | | | | | |
Additions | | | — | | | | 32,365 | | | | 32,365 | |
Less Disposals | | | — | | | | — | | | | — | |
Depreciation expense | | | (1,550 | ) | | | (11,258 | ) | | | (12,808 | ) |
| | | | | | | | | | | | |
Balance at 30 June 2014 | | | 8,660 | | | | 39,017 | | | | 47,677 | |
| | | | | | | | | | | | |
F-52
Notes to the Consolidated Financial Statements for the Year Ended 30 June 2014
Note 13: Intangibles
The net carrying amount of intangibles can be analysed as follows:
| | | | |
| | | $ | |
Gross carrying amount | | | | |
Balance at 30 June 2012 | | | — | |
Acquired through business combination | | | 1,503,296 | |
| | | | |
Balance at 30 June 2013 | | | 1,503,296 | |
| | | | |
Acquired through business combination | | | — | |
| | | | |
Balance at 30 June 2014 | | | 1,503,296 | |
| | | | |
Accumulated impairment | | | | |
Balance at 30 June 2012 | | | — | |
Impairment loss recognised in the year | | | (1,503,296 | ) |
| | | | |
Balance at 30 June 2013 | | | (1,503,296 | ) |
| | | | |
Impairment loss recognised in the year | | | — | |
| | | | |
Balance at 30 June 2014 | | | (1,503,296 | ) |
| | | | |
Net book value | | | | |
At 30 June 2013 | | | — | |
| | | | |
As at 30 June 2014 | | | — | |
| | | | |
Impairment
A review of the carrying value of thein-process research which arose on the acquisition of Tacere Therapeutics Inc. was undertaken in the 2013 financial year. The recoverable amounts of the cash generating units to which thein-process research was allocated were determined based on value-in-use calculations. This review identified that the full value of thein-process research should be impaired and as such a non-cash impairment charge of $1,503,296 was booked in the 2013 financial year.
Note 14: Trade and other payables
| | | | | | | | |
| | | 2014 | | | 2013 | |
| | | $ | | | $ | |
CURRENT | | | | | | | | |
Unsecured liabilities | | | | | | | | |
Trade creditors | | | 572,557 | | | | 279,994 | |
Sundry creditors and accrued expenses | | | 215,612 | | | | 374,560 | |
Deferred consideration—Tacere vendors | | | — | | | | 357,179 | |
| | | | | | | | |
| | | 788,169 | | | | 1,011,733 | |
| | | | | | | | |
Note 15: Provisions
| | | | | | | | |
CURRENT | | | | | | | | |
Provision for employee benefits | | | 166,511 | | | | 98,637 | |
| | | | | | | | |
Note 16: Contributed equity
The share capital of the Company consists only of fully paid ordinary shares; the shares do not have a par value. All shares are equally eligible to receive dividends and the repayment of capital and represent one vote at
F-53
Notes to the Consolidated Financial Statements for the Year Ended 30 June 2014
the shareholders’ meeting of the Company. The Board monitors capital funding requirements in its competitive landscape and continues to actively manage its cash requirements as part of a broader capital management program to ensure adequate capital is in place to fund the company’s operations.
(a) Ordinary Shares, reported in post consolidation share numbers for 2014 and 2013
114,898,992 (2013: 46,076,562) issued and fully paid ordinary shares
| | | | | | | | |
| | | 2014 | | | 2013 | |
| | | $ | | | $ | |
Contributed Equity at the beginning of the reporting period, (after applying the 25:1 consolidation) | | | 89,609,248 | | | | 87,348,819 | |
Placements in March to June 2013 | | | — | | | | 1,262,000 | |
Placement in July 2013 | | | 7,618,326 | | | | — | |
Share Purchase Plan August 2013 | | | 2,820,000 | | | | — | |
Issued to Tacere shareholders on purchase of Tacere | | | — | | | | 1,173,585 | |
Tacere escrow shares released October 2013 | | | 357,190 | | | | — | |
Options exercised during the year | | | 185,503 | | | | — | |
Placements in February and April 2014 | | | 31,496,504 | | | | — | |
Transaction costs relating to share issues during the year | | | (2,901,095 | ) | | | (175,156 | ) |
| | | | | | | | |
Contributed Equity at the close of the reporting period | | | 129,185,676 | | | | 89,609,248 | |
| | | | | | | | |
| | | | | | | | |
| | | Number | | | Number | |
At the beginning of reporting period | | | 46,076,562 | | | | 38,825,141 | |
Shares issued during the year | | | 68,822,430 | | | | 7,251,421 | |
| | | | | | | | |
| | | 114,898,992 | | | | 46,076,562 | |
| | | | | | | | |
(b) Share options
At the end of the financial year, there were 23,320,173 unissued ordinary shares (2013: 17,594,313) over which options were outstanding.
| | | | | | | | | | |
| | | Expiry Date | | Exercise Price | | | | |
Strategic Advisor warrants | | 4 August 2014 | | | 22.500 | | | | 245,078 | |
ESOP Options | | 19 August 2014 | | | 0.510 | | | | 260,000 | |
NED Options | | 19 August 2014 | | | 0.570 | | | | 120,000 | |
Unlisted Options—placement | | 18 February 2015 | | | 0.325 | | | | 662,767 | |
Unlisted other options | | 10 April 2015 | | | 2.500 | | | | 480,000 | |
Unlisted other options | | 23 October 2015 | | | 4.250 | | | | 78,125 | |
NED Options | | 26 September 2016 | | | 1.250 | | | | 1,600,000 | |
ESOP Options | | 17 November 2016 | | | 1.250 | | | | 1,800,000 | |
NED Options | | 26 September 2016 | | | 1.250 | | | | 1,200,000 | |
ESOP Options | | 7 February 2017 | | | 1.250 | | | | 168,000 | |
ESOP Options | | 18 July 2017 | | | 1.250 | | | | 400,000 | |
ESOP Options | | 16 November 2017 | | | 1.250 | | | | 400,000 | |
NED Options | | 18 May 2018 | | | 0.625 | | | | 400,000 | |
ESOP Options | | 22 August 2018 | | | 1.250 | | | | 2,080,000 | |
Unlisted options—placement | | 28 February 2019 | | | 1.260 | | | | 13,246,203 | |
ESOP Options | | 15-May-19 | | | 1.500 | | | | 180,000 | |
| | | | | | | | | | |
| | | | | | | | | 23,320,173 | |
| | | | | | | | | | |
F-54
Notes to the Consolidated Financial Statements for the Year Ended 30 June 2014
Since 30 June 2013, the following options were issued under the ESOP:
| | | | | | | | | | | | | | | | | | | | | | |
Expiry date | | Exercise
price | | | Issue date | | Number | | | Weighted
average
share
price | | | Volatility | | | Risk
free
rate | |
22 August 2018 | | | 1.250 | | | 22 August 2013 | | | 2,080,000 | | | $ | 0.29 | | | | 112 | % | | | 3.55 | % |
15 May 2019 | | | 1.500 | | | 15 May 2014 | | | 180,000 | | | $ | 0.30 | | | | 100 | % | | | 2.60 | % |
| | | | | | | | | | | | | | | | | | | | | | |
| | | | | | | | | 2,260,000 | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | |
Rights over shares are provided to employees under the Employee Share Option Plan (ESOP). The cost of these equity-settled transactions with employees is measured by reference to the fair value at the date at which they are granted. The fair value is determined using a Black-Scholes model. In valuing equity-settled transactions, no account is taken of any performance conditions, other than conditions linked to the price of the shares of Benitec Biopharma Limited (‘market conditions’).
The following information was factored in to the Black-Scholes model for the options issued under ESOP this year:
| | i. | | weighted average share price as shown above |
| | ii. | | exercise prices were as shown above |
| | iii. | | expected volatility was as shown above and was determined by reference to Bloomberg for the Benitec share price based on historical volatility |
| | iv. | | option life is 5 years |
| | v. | | The risk-free interest rate used was as shown above |
There were no options issued to staff or directors in the period from 30 June 2014 to the date this report was issued.
Note 17: Reserves
| | | | | | | | |
| | | 2014 | | | 2013 | |
| | | $ | | | $ | |
Share-based payments reserve | | | | | | | | |
At the beginning of the reporting period | | | 1,591,702 | | | | 1,394,142 | |
Share based payments | | | 355,116 | | | | 518,749 | |
Transferred to Accumulated Losses Reserve no longer required | | | — | | | | (321,189 | ) |
| | | | | | | | |
| | | 1,946,818 | | | | 1,591,702 | |
| | | | | | | | |
Foreign currency translation reserve | | | | | | | | |
At the beginning of the reporting period | | | (1,313,792 | ) | | | — | |
Foreign currency translation | | | 7,747 | | | | (1,313,792 | ) |
| | | | | | | | |
| | | (1,306,045 | ) | | | (1,313,792 | ) |
| | | | | | | | |
Total Reserves | | | 640,773 | | | | 277,910 | |
| | | | | | | | |
Nature and purpose of Reserves
Share Based Payments Reserve
The Share-based Payments Reserve represents the expense attributed to options based on a Black Scholes valuation method for vested options.
F-55
Notes to the Consolidated Financial Statements for the Year Ended 30 June 2014
Foreign currency translation reserve
The Foreign currency translation reserve represents the currency translation movements of subsidiary company balances denominated in foreign currencies at year end.
Note 18: Operating segments
Business Segments
The Group had only one business segment during the financial year, being the global commercialisation by licensing and partnering of patents and licences in biotechnology, more specifically in functional genomics, with applications in biomedical research and human therapeutics.
Geographical Segments
Business operations are principally conducted in Australia, with laboratory and other activities in the USA.
| | | | | | | | | | | | | | | | | | | | | | | | |
Geographical location | | Segment Revenues | | | Segment Results | | | Carrying Amount of
Segment Assets | |
| | 2014 | | | 2013 | | | 2014 | | | 2013 | | | 2014 | | | 2013 | |
| | | $ | | | $ | | | $ | | | $ | | | $ | | | $ | |
Australia | | | | | | | | | | | (7,495,377 | ) | | | (3,220,240 | ) | | | 34,433,803 | | | | 1,507,350 | |
| | | | | | | | | | | | | | | | | | | | | | | | |
External customers | | | 274,413 | | | | 521,140 | | | | | | | | | | | | | | | | | |
Interest revenue | | | 321,116 | | | | 118,709 | | | | | | | | | | | | | | | | | |
Other income | | | 775,833 | | | | 823,354 | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | |
| | | 1,371,362 | | | | 1,463,203 | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | |
United States of America | | | | | | | | | | | 1,903 | | | | (267,720 | ) | | | 61,399 | | | | 243,360 | |
| | | | | | | | | | | | | | | | | | | | | | | | |
External customers | | | 2,411 | | | | — | | | | | | | | | | | | | | | | | |
Interest revenue | | | — | | | | — | | | | | | | | | | | | | | | | | |
Other income | | | — | | | | 979 | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | |
| | | 2,411 | | | | 979 | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | | | | | | | | | | | |
| | | 1,373,773 | | | | 1,464,182 | | | | (7,493,474 | ) | | | (3,487,960 | ) | | | 34,495,202 | | | | 1,750,710 | |
| | | | | | | | | | | | | | | | | | | | | | | | |
Accounting Policies
Segment revenues and expenses are directly attributable to the identified segments and include joint venture revenue and expenses where a reasonable allocation basis exists. Segment assets include all assets used by a segment and consist mainly of cash, receivables, inventories, intangibles and property, plant and equipment, net of any allowances, accumulated depreciation and amortisation. Where joint assets correspond to two or more segments, allocation of the net carrying amount has been made on a reasonable basis to a particular segment. Segment liabilities include mainly accounts payable, employee entitlements, accrued expenses, provisions and borrowings. Deferred income tax provisions are not included in segment assets and liabilities.
Note 19: Financial risk management objectives and policies
The Group’s principal financial instruments comprise receivables, payables, cash and short-term deposits. The Group manages its exposure to key financial risks, including interest rate and currency risk in accordance with the Company financial risk management policy. The objective of the policy is to protect the assets and provide a solid return.
F-56
Notes to the Consolidated Financial Statements for the Year Ended 30 June 2014
The main risks arising from the financial instruments are interest rate risk, liquidity risk, foreign currency risk and credit risk. The Board reviews and agrees policies for managing each of these risks and they are summarised below.
Risk Exposures and Responses
Interest rate risk
The Group generates income from interest on surplus funds. At reporting date, the Group had the following mix of financial assets and liabilities exposed to Australian variable interest rate risk that are not designated in cash flow hedges:
| | | | | | | | |
| | | 2014 | | | 2013 | |
| | | $ | | | $ | |
Financial Assets | | | | | | | | |
Cash and cash equivalents | | | 31,359,199 | | | | 1,587,299 | |
Financial Liabilities | | | — | | | | — | |
| | | | | | | | |
Net Exposure | | | 31,359,199 | | | | 1,587,299 | |
| | | | | | | | |
The policy is to analyse the Company’s interest rate exposure across the Group’s financial assets and liabilities. Consideration is given to the return on funds invested, alternative financing, the mix of fixed and variable interest rates and hedging positions. The Group currently has short term deposits at variable interest rates. The average interest rate applying to cash deposits in the year was 3.67% (2013 4.00%).
The following sensitivity analysis is based on the interest rate risk exposures in existence at the reporting date:
At 30 June 2014, if interest rates had moved, as illustrated in the table below, with all other variables held constant, the judgment of reasonably possible movements in post-tax profit and equity would have been as follows:
| | | | | | | | | | | | | | | | |
| | | Post Tax Result
Higher/ (Lower) | | | Equity
Higher/ (Lower) | |
| | | 2014 | | | 2013 | | | 2014 | | | 2013 | |
| | | $ | | | $ | | | $ | | | $ | |
+1% (100 basis points) | | | 143,625 | | | | 12,797 | | | | 143,625 | | | | 12,797 | |
-0.5% (50 basis points) | | | (71,812 | ) | | | (6,399 | ) | | | (71,812 | ) | | | (6,399 | ) |
Liquidity risk
The Group’s objective is to obtain revenue from commercialisation and to continue to access funding markets. The Group has a pipeline of programs to take its research and development to the clinic and potentially originate licensing transactions with pharmaceutical companies. Trade payables and other financial liabilities originate from the financing of the ongoing research and development programs in addition to the operations of the business generally.
The table below reflects all contractually fixed pay-offs and receivables for settlement, repayments and interest resulting from recognised financial assets and liabilities as at 30 June 2014. Cash flows for financial assets and liabilities with fixed amount or timing are presented with their respective discounted cash flows for the respective upcoming fiscal years.
F-57
Notes to the Consolidated Financial Statements for the Year Ended 30 June 2014
The remaining contractual maturities of the Group’s financial liabilities are:
| | | | | | | | |
| | | 2014 | | | 2013 | |
| | | $ | | | $ | |
6 months or less | | | 788,169 | | | | 1,011,733 | |
6-12 months | | | — | | | | — | |
1-5 years | | | — | | | | — | |
Over 5 years | | | — | | | | — | |
| | | | | | | | |
| | | 788,169 | | | | 1,011,733 | |
| | | | | | | | |
Maturity analysis of financial assets and liabilities based on management’s expectation
The table below reflects management’s expectation of the maturity of financial assets and liabilities.
These assets are considered in the context of the Group’s overall liquidity risk. The Group has established a risk reporting process overseen by the board which monitors existing financial assets and liabilities and provides information to enable effective risk management. The Board regularly evaluates managements rolling forecasts of liquidity which includes assessments of cash income and outgoings.
| | | | | | | | | | | | | | | | | | | | |
| | | < 6 months | | | 6-12 months | | | 1-5 years | | | >5 years | | | Total | |
| | | $ | | | $ | | | $ | | | $ | | | $ | |
Financial assets | | | | | | | | | | | | | | | | | | | | |
Cash and cash equivalents | | | 23,359,199 | | | | 8,000,000 | | | | — | | | | — | | | | 31,359,199 | |
Trade and other receivables | | | 121,587 | | | | — | | | | — | | | | — | | | | 121,587 | |
| | | | | |
Financial Liabilities | | | | | | | | | | | | | | | | | | | | |
Trade and other payables | | | (788,169 | ) | | | — | | | | — | | | | — | | | | (788,169 | ) |
| | | | | | | | | | | | | | | | | | | | |
Net Maturity | | | 22,692,617 | | | | 8,000,000 | | | | — | | | | — | | | | 30,692,617 | |
| | | | | | | | | | | | | | | | | | | | |
Foreign currency risk
The Group has transactional currency exposures. Such exposure arises from licensing fees and royalties as well as expenditure by the Group in currencies other than the unit’s measurement currency. With the exception of unrealised movements on intercompany loans, foreign currency income and expenditure accounts for less than 15% of the Groups transactions and therefore management have assessed that movements in foreign exchange would not materially impact the financial statements.
Credit risk
Credit risk arises from the financial assets of the Group, which comprise cash and cash equivalents, and trade and other receivables. The Group’s exposure to credit risk arises from potential counter party payment default, with a maximum exposure equal to the carrying amount. Exposures at each reporting date are assessed and disclosed in the financial statements.
The Group does not hold any credit derivatives to offset its credit exposure. The Group trades only with recognised, creditworthy third parties and as such collateral is not requested. The Group does not securitise its trade and other receivables.
Customers who wish to trade on credit terms are subject to credit assessment procedures which may include an assessment of their independent credit rating, financial position, past experience and industry reputation. Receivable balances are regularly monitored. There are no significant concentrations of credit risk within the Group.
F-58
Notes to the Consolidated Financial Statements for the Year Ended 30 June 2014
Note 20: Financial instruments
Fair values
Fair values of financial assets and liabilities are equivalent to carrying values due their short term to maturity.
Note 21: Share based payments
Benitec Biopharma Limited Employees Share Option Plan (ESOP):
Description of plan
The Group may from time to time issue employees options to acquire shares in the parent at a fixed price. Each option when exercised entitles the option holder to one share in the Company. Options are exercisable on or before an expiry date, do not carry any voting or dividend rights and are not transferable except on death of the option holder.
Share Options granted during the year
The following options were issued to executives by Benitec Biopharma Limited under its ESOP and are unlisted.
| | | | | | | | | | |
Executive | | Grant Date | | Number | | | Exercise Price | | Expiry Date |
Peter French | | 22 August 2013 | | | 1,400,000 | | | $1.250 | | 22 August 2018 |
David Suhy | | 22 August 2013 | | | 200,000 | | | $1.250 | | 22 August 2018 |
Greg West | | 22 August 2013 | | | 280,000 | | | $1.250 | | 22 August 2018 |
Carl Stubbings | | 22 August 2013 | | | 200,000 | | | $1.250 | | 22 August 2018 |
Tin Mao | | 15 May 2014 | | | 90,000 | | | $1.500 | | 15 May 2019 |
Shin-chu Kao | | 15 May 2014 | | | 90,000 | | | $1.500 | | 15 May 2019 |
| | | | | | | | | | |
| | | | | 2,260,000 | | | |
| | | | | | | | | | |
There were no options issued to directors in the year to 30 June 2014. The closing market price of an ordinary share of Benitec Biopharma Limited (ASX Code: BLT) on the Australian Securities Exchange at 30 June 2014 was $1.15 (30 June 2013: $0.375, after adjusting for the securities consolidation in July 2013)
The following table shows the number and weighted average exercise price (WAEP) of share options issued under the ESOP:
| | | | | | | | | | | | | | | | |
| | | 2014
Number | | | 2014
WAEP | | | 2013
Number | | | 2013
WAEP | |
Outstanding at the beginning of the year | | | 3,028,000 | | | | 1.200 | | | | 2,440,000 | | | | 1.147 | |
Granted during the year | | | 2,260,000 | | | | 1.270 | | | | 800,000 | | | | 1.250 | |
Exercised during the year | | | — | | | | — | | | | — | | | | — | |
Lapsed or forfeited during the year | | | | | | | (212,000 | ) | | | 0.792 | |
| | | | | | | | | | | | | | | | |
Outstanding at the end of the year | | | 5,288,000 | | | | 1.229 | | | | 3,028,000 | | | | 1.200 | |
| | | | | | | | | | | | | | | | |
Options exercisable at the end of the year | | | 2,178,667 | | | | | | | | 1,456,000 | | | | | |
| | | | | | | | | | | | | | | | |
F-59
Notes to the Consolidated Financial Statements for the Year Ended 30 June 2014
Details of ESOP share options outstanding as at end of year:
| | | | | | | | | | | | |
Grant Date | | Expiry Date | | Exercise
Price | | 2014
Number | | | 2013
Number | |
13 July 2010 | | 19 August 2014 | | $0.510 | | | 260,000 | | | | 260,000 | |
17 November 2011 | | 17 November 2016 | | $1.250 | | | 1,800,000 | | | | 1,800,000 | |
7 February 2012 | | 7 February 2017 | | $1.250 | | | 168,000 | | | | 168,000 | |
18 July 2012 | | 18 July 2017 | | $1.250 | | | 400,000 | | | | 400,000 | |
16 November 2012 | | 16 November 2017 | | $1.250 | | | 400,000 | | | | 400,000 | |
22 August 2013 | | 22 August 2018 | | $1.250 | | | 2,080,000 | | | | — | |
28 May 2014 | | 18 May 2019 | | $1.500 | | | 180,000 | | | | — | |
| | | | | | | | | | | | |
| | | | | | | 5,288,000 | | | | 3,028,000 | |
| | | | | | | | | | | | |
The weighted average remaining life of the options issued under the ESOP at 30 June 2014 was 3 years and 3 months.( 2013: 3 years and 4 months)
Note 22: Events subsequent to reporting date
No matters or circumstances have arisen since 30 June 2014 which have significantly affected or may significantly affect the operations of the Group, the results of those operations or the state of affairs of the Group, in subsequent financial years.
Note 23: Contingent liabilities
In January 2010, the Company reached a settlement with the CSIRO to replace the existing Licence Agreement and Commercial Agreement with a new exclusive Licence Agreement for the use of intellectual property and the Capital Growth Agreement with the issue of ordinary shares. As part of the settlement, a Transition Agreement was put in place in order to facilitate the change from the old agreements to the new agreement and to deal with a number of other matters.
Under the terms of the Transition Agreement, the Company agreed to pay CSIRO an amount of $297,293 for past patent costs only in the event of a trigger event, being either a corporate transaction or an insolvency event.
Scientific work on the therapeutic programs
On 18 December 2012 Benitec announced the appointment of Synteract Inc. as the Company’s Clinical Research Organisation responsible for the progression of TT-034 into Phase I/II (a) Clinical Trials in the USA. Benitec has negotiated a contract with favourable commercial terms, in some instances requiring prepayment, for Synteract to continue to manage the Clinical Trials throughout 2014 and 2014.
Benitec announced plans on 3 June 2014 to progress its non-small cell lung cancer (NSCLC) therapeutic Tribetarna™ into Phase II clinical trials in late 2014 calendar year. The Company had reached agreement to use European-based clinical research organisation Clinical Trials Group (CTGCRO) to manage the trial, and subsequently negotiated favourable commercial terms which included prepayments covering the clinical trial and consulting services.
The Company has contracted for scientific work on the therapeutic programs, as described above, and payments due within the next twelve months total approximately $2,092,500 (2013: $4,178,261).
F-60
Notes to the Consolidated Financial Statements for the Year Ended 30 June 2014
Note 24: Capital management policies and procedures
The Group’s capital management objectives are to ensure the Group has the ability to fund its activities, to continue as a going concern; and to provide value to shareholders.
The Group’s capital management plan targets appropriate cash levels to service expected future cash flow needs based on the forecasts for current and future programs and business running costs. Management assesses the Group’s capital requirements in order to maintain an efficient overall financing structure. The Group manages the capital structure and makes adjustments to it in the light of changes in access to funding, business conditions and the risk characteristics of the business. In order to maintain appropriate funding the Group may issue new shares. The amounts managed as capital by the Group for the reporting periods under review are as shown in the statement of financial position.
Note 25: Related party transactions
| | | | | | | | |
| | | 2014
| | | 2013
| |
| | | $ | | | $ | |
Transactions with Directors and Director-related Entities: | | | | | | | | |
Legal services paid / payable to Francis Abourizk Lightowlers, a law firm in which Mr Peter Francis is a partner and has a beneficial interest. | | | 108,913 | | | | 103,492 | |
Consultancy fees for executive duties paid/payable to NewStar Ventures Ltd, a corporation in which Dr John Chiplin is a director and has a beneficial interest. | | | 40,000 | | | | 40,000 | |
Transactions between related parties are on normal commercial terms and the conditions no more favourable than those available to other non-related parties. There are no outstanding balances as at 30 June 2014 (2013: nil)
Note 26: Business combination—Tacere Therapeutics Inc. acquisition in October 2012
Benitec announced an agreement to acquire the US-based RNA interference (RNAi) therapeutics company Tacere Therapeutics Inc. (‘Tacere’) on 11 October 2012. The acquisition was completed on 30 October 2012 when Benitec acquired 100% of the issued share capital and voting rights of Tacere, a company based in the United States. Tacere was a privately held drug development company with a Phase I/II ready program in hepatitis C (HCV) that uses Benitec’s novel gene silencing technology.
Benitec acquired Tacere’s extensive HCV program data and materials, as well as an advanced preclinical program for the eye disease macular degeneration, The Tacere acquisition provided Benitec with the opportunity to commence Phase I/II clinical trials in 2014.
The consideration for the acquisition was an issue of shares in Benitec Biopharma Limited for USD $1,530,765 plus a potential cash royalty on future licensing revenue. The shares issued as consideration represented 9.5% of the issued capital at the time of the acquisition.
Further, the agreements with the Tacere vendors provided for AUD $357,179 Benitec Biopharma Limited shares (included in the acquisition consideration) be treated as reserve shares and not issued to the Tacere vendors for a period of 12 months from acquisition. The reserve shares are accounted for as a creditor in 2013 (refer to note 6). The reserve shares were established by an agreement with the Tacere vendors for the purposes of satisfying indemnities to Benitec, if required. The Tacere Vendors also provided a cash escrow of USD $360,000 to provide Benitec with additional security should certain pre-acquisition liabilities emerge.
Impairment costs, relating to thein-process research on the acquisition of Tacere of $1,503,296 were recognised in the 2013 financial year. The Tacere acquisitionin-process research is deemed to be the excess of the consideration over the fair value of the identifiable assets acquired less liabilities assumed. The immediate
F-61
Notes to the Consolidated Financial Statements for the Year Ended 30 June 2014
write off of thein-process research, following the impairment review, was considered to be the most appropriate accounting treatment as the intellectual property is a preclinical trial and hence the future economic benefit is uncertain.
Financial details of the business combination made in the previous financial year (year ended 30 June 2013) were:
| | | | |
| | | $ | |
Fair value of consideration transferred | | | | |
Consideration for the acquisition in October 2012 was the issue of 102,321,345 (pre-consolidation) shares in Benitec Biopharma Limited, plus a potential cash royalty on future licensing revenue | | | 1,530,765 | |
| | | | |
Recognised amounts of identifiable net assets | | | | |
Property, plant and equipment | | | 17,567 | |
Cash and cash equivalents | | | 138,760 | |
Amount owing to Benitec Biopharma Limited | | | (126,882 | ) |
Other liabilities | | | (1,976 | ) |
| | | | |
Identifiable net assets | | | 27,469 | |
| | | | |
Goodwill on acquisition impaired in the June 2013 financial statements | | | 1,503,296 | |
| | | | |
Net cash inflow on acquisition | | | 143,603 | |
Acquisition related costs recognised as an expense in the Group corporate expenses | | | 77,104 | |
Post-acquisition loss of Tacere in the period to 30 June 2013 | | | 267,720 | |
Post-acquisition loss of Tacere in the period to 30 June 2014 | | | 1,903 | |
Note 27: Benitec Biopharma Limited parent company information
| | | | | | | | |
| | | Parent Entity | |
| | | 2014 | | | 2013 | |
| | | $ | | | $ | |
ASSETS | | | | | | | | |
Current assets | | | 34,386,167 | | | | 1,478,422 | |
Non-current assets | | | 181,547 | | | | 48,999 | |
| | | | | | | | |
TOTAL ASSETS | | | 34,567,714 | | | | 1,527,421 | |
| | | | | | | | |
LIABILITIES | | | | | | | | |
Current liabilities | | | 1,311,608 | | | | 1,165,652 | |
Non-current liabilities | | | — | | | | — | |
| | | | | | | | |
TOTAL LIABILITIES | | | 1,311,608 | | | | 1,165,652 | |
| | | | | | | | |
NET ASSETS | | | 33,256,106 | | | | 361,769 | |
| | | | | | | | |
EQUITY | | | | | | | | |
Contributed equity | | | 129,185,675 | | | | 89,609,248 | |
Share based payments reserve | | | 2,096,818 | | | | 1,591,702 | |
Accumulated losses | | | (98,026,387 | ) | | | (90,839,181 | ) |
| | | | | | | | |
TOTAL EQUITY | | | 33,256,106 | | | | 361,769 | |
| | | | | | | | |
FINANCIAL PERFORMANCE | | | | | | | | |
Loss for the year | | | (7,037,206 | ) | | | (4,885,852 | ) |
Other comprehensive income | | | — | | | | — | |
| | | | | | | | |
TOTAL COMPREHENSIVE INCOME | | | (7,037,206 | ) | | | (4,885,852 | ) |
| | | | | | | | |
F-62
Notes to the Consolidated Financial Statements for the Year Ended 30 June 2014
Contingent liabilities
The parent entity had no contingent liabilities as at 30 June 2014 (2013: nil), other than the contingent liabilities described in note 22.
Capital commitments
The parent entity has no capital commitments as at 30 June 2014 (2013: nil).
Significant accounting policies
The accounting policies of the parent are consistent with those of the consolidated entity (Note 1)
F-63
Consolidated Statement of Profit or Loss and Other Comprehensive Income
For the Half-Year Ended December 31, 2015 (unaudited)
| | | | | | | | | | | | |
| | | | | | Half-Year | |
| (in thousands) | | Notes | | | December
2015 | | | December
2014 | |
| | | | | | A$ | | | A$ | |
Revenue | | | 2 | | | | 322 | | | | 639 | |
| | | | | | | | | | | | |
Other income | | | 2 | | | | — | | | | 392 | |
Royalties and licence fees | | | | | | | (49 | ) | | | (40 | ) |
Research and development costs | | | | | | | (8,151 | ) | | | (2,210 | ) |
Employee benefits expense | | | | | | | (3,357 | ) | | | (1,502 | ) |
Share based expense | | | | | | | (1,475 | ) | | | (842 | ) |
Travel related costs | | | | | | | (725 | ) | | | (517 | ) |
Consultants costs | | | | | | | (561 | ) | | | (400 | ) |
Occupancy costs | | | | | | | (286 | ) | | | (131 | ) |
Corporate expenses | | | | | | | (591 | ) | | | (436 | ) |
Foreign exchange translation | | | | | | | (241 | ) | | | — | |
IPO costs | | | | | | | (960 | ) | | | — | |
| | | | | | | | | | | | |
Loss before income tax | | | | | | | (16,074 | ) | | | (5,047 | ) |
Income tax benefit | | | | | | | — | | | | — | |
| | | | | | | | | | | | |
Loss after income tax for the period attributable to the owners of Benitec Biopharma Limited | | | | | | | (16,074 | ) | | | (5,047 | ) |
Other comprehensive income | | | | | | | (4 | ) | | | 24 | |
| | | | | | | | | | | | |
Other comprehensive income for the period, net of tax | | | | | | | (4 | ) | | | 24 | |
| | | | | | | | | | | | |
Total comprehensive loss for the half year attributable to members of Benitec Biopharma Limited | | | | | | | (16,078 | ) | | | (5,023 | ) |
| | | | | | | | | | | | |
Basic earnings (loss) for the half-year | | | | | | | (11.6 | ) | | | (4.4 | ) |
Diluted earnings (loss) for the half-year | | | | | | | (11.6 | ) | | | (4.4 | ) |
The above consolidated statement of profit or loss and other comprehensive income should be read in conjunction with the accompanying notes.
F-64
Consolidated Statement of Financial Position
For the Half-Year Ended December 31, 2015 (unaudited)
| | | | | | | | | | | | |
| (in thousands) | | Notes | | | December
2015 | | | June
2015 | |
| | | | | | A$ | | | A$ | |
ASSETS | | | | | | | | | | | | |
Current Assets | | | | | | | | | | | | |
Cash and cash equivalents | | | | | | | 22,764 | | | | 21,787 | |
Other financial assets | | | | | | | 2,000 | | | | — | |
Trade and other receivables | | | | | | | 39 | | | | 123 | |
Other | | | 5 | | | | 3,000 | | | | 3,154 | |
| | | | | | | | | | | | |
Total Current Assets | | | | | | | 27,802 | | | | 25,064 | |
| | | | | | | | | | | | |
Non-current Assets | | | | | | | | | | | | |
Plant and equipment | | | | | | | 486 | | | | 456 | |
| | | | | | | | | | | | |
Total Non-current Assets | | | | | | | 486 | | | | 456 | |
| | | | | | | | | | | | |
TOTAL ASSETS | | | | | | | 28,288 | | | | 25,520 | |
| | | | | | | | | | | | |
LIABILITIES | | | | | | | | | | | | |
Current Liabilities | | | | | | | | | | | | |
Trade and other payables | | | 6 | | | | 891 | | | | 1,449 | |
Provisions | | | | | | | 112 | | | | 193 | |
| | | | | | | | | | | | |
Total Current Liabilities | | | | | | | 1,003 | | | | 1,642 | |
| | | | | | | | | | | | |
TOTAL LIABILITIES | | | | | | | 1,003 | | | | 1,642 | |
| | | | | | | | | | | | |
NET ASSETS | | | | | | | 27,285 | | | | 23,878 | |
| | | | | | | | | | | | |
EQUITY | | | | | | | | | | | | |
Issued capital | | | 7 | | | | 147,641 | | | | 129,631 | |
Reserves | | | | | | | 2,607 | | | | 2,038 | |
Accumulated losses | | | | | | | (122,963 | ) | | | (107,791 | ) |
| | | | | | | | | | | | |
TOTAL EQUITY | | | | | | | 27,285 | | | | 23,878 | |
| | | | | | | | | | | | |
The above consolidated statement of financial position should be read in conjunction with the accompanying notes.
F-65
Consolidated Statement of Changes in Equity
For the Half-Year Ended December 31, 2015 (unaudited)
| | | | | | | | | | | | | | | | |
| (in thousands) | | Issued
capital | | | Reserves | | | Accumulated
Losses | | | Total
equity | |
| | | A$ | | | A$ | | | A$ | | | A$ | |
Balance at June 30, 2014 | | | 129,186 | | | | 641 | | | | (96,286 | ) | | | 33,541 | |
| | | | | | | | | | | | | | | | |
Loss for the period | | | — | | | | — | | | | (5,047 | ) | | | (5,047 | ) |
Other comprehensive income | | | — | | | | 24 | | | | — | | | | 24 | |
| | | | | | | | | | | | | | | | |
Total comprehensive income | | | — | | | | 24 | | | | (5,047 | ) | | | (5,023 | ) |
Share issues, net of transaction costs | | | 257 | | | | — | | | | — | | | | 257 | |
Share based payments | | | — | | | | 842 | | | | — | | | | 842 | |
Transfer of expired share-based payments | | | — | | | | (4 | ) | | | 4 | | | | — | |
| | | | | | | | | | | | | | | | |
Transfer to share capital for options exercised | | | 108 | | | | (108 | ) | | | — | | | | — | |
At December 31, 2014 | | | 129,551 | | | | 1,395 | | | | (101,329 | ) | | | 29,617 | |
| | | | | | | | | | | | | | | | |
Loss for the period | | | — | | | | — | | | | (6,462 | ) | | | (6,462 | ) |
Other comprehensive income | | | — | | | | (18 | ) | | | — | | | | (18 | ) |
| | | | | | | | | | | | | | | | |
Total comprehensive income | | | — | | | | (18 | ) | | | (6,462 | ) | | | (6,480 | ) |
Contributions of equity, net of transaction costs | | | 80 | | | | — | | | | — | | | | 80 | |
Share based payments | | | — | | | | 661 | | | | — | | | | 661 | |
| | | | | | | | | | | | | | | | |
At June 30, 2015 | | | 129,631 | | | | 2,038 | | | | (107,791 | ) | | | 23,878 | |
| | | | | | | | | | | | | | | | |
Loss for the period | | | — | | | | — | | | | (16,074 | ) | | | (16,074 | ) |
Other comprehensive income | | | — | | | | (4 | ) | | | — | | | | (4 | ) |
| | | | | | | | | | | | | | | | |
Total comprehensive income | | | — | | | | (4 | ) | | | (16,074 | ) | | | (16,078 | ) |
| | | | | | | | | | | | | | | | |
Share issues, net of transaction costs | | | 18,010 | | | | — | | | | — | | | | 18,010 | |
Share based payments | | | — | | | | 1,475 | | | | — | | | | 1,475 | |
Transfer of forfeited share based payments | | | — | | | | (902 | ) | | | 902 | | | | — | |
| | | | | | | | | | | | | | | | |
At December 31, 2015 | | | 147,641 | | | | 2,607 | | | | (122,963 | ) | | | 27,285 | |
| | | | | | | | | | | | | | | | |
The above consolidated statement of changes in equity should be read in conjunction with the accompanying notes.
F-66
Consolidated Statement of Cash Flows
For the Half-Year Ended December 31, 2015 (unaudited)
| | | | | | | | |
| | | Half-Year | |
| (in thousands) | | December
2015 | | | December
2014 | |
| | | A$ | | | A$ | |
Cash flows from operating activities | | | | | | | | |
Receipts from customers | | | 272 | | | | 255 | |
Interest received | | | 134 | | | | 452 | |
Payments to suppliers and employees | | | (14,893 | ) | | | (5,494 | ) |
| | | | | | | | |
Net cash used in operating activities | | | (14,487 | ) | | | (4,787 | ) |
| | | | | | | | |
Cash flows from investing activities | | | | | | | | |
Payments for property, plant and equipment | | | (71 | ) | | | (394 | ) |
| | | | | | | | |
Investments/purchase of short term deposits | | | (2,000 | ) | | | — | |
| | | | | | | | |
Net cash used in investing activities | | | (2,071 | ) | | | (394 | ) |
| | | | | | | | |
Cash flows from financing activities | | | | | | | | |
Proceeds from issue of shares | | | 19,462 | | | | 257 | |
IPO and share issue transaction costs | | | (1,952 | ) | | | — | |
| | | | | | | | |
Net cash from financing activities | | | 17,510 | | | | 257 | |
| | | | | | | | |
Net increase/(decrease) in cash and cash equivalents | | | 952 | | | | (4,924 | ) |
Cash and cash equivalents at beginning of the period | | | 21,788 | | | | 31,359 | |
Effects of exchange rate changes on cash and cash equivalents | | | 24 | | | | 392 | |
| | | | | | | | |
Cash and cash equivalents at end of half-year | | | 22,764 | | | | 26,827 | |
| | | | | | | | |
The above consolidated statement of cash flows should be read in conjunction with the accompanying notes.
F-67
Notes to the Consolidated Financial Statements
For the Half-Year Ended December 31, 2015 (unaudited)
| 1. | | BASIS OF PREPARATION OF THE CONSOLIDATED FINANCIAL REPORT |
The condensed interim consolidated financial statements (the interim financial statements) of the Group are for the six months ended December 31, 2015 and are presented in Australian dollars (A$), which is the functional currency of the parent company. These general purpose interim financial statements have been prepared in accordance with the requirements of theCorporations Act 2001andAASB 134 Interim Financial Reporting (which is consistent with IAS 34 Interim Financial Reporting). They do not include all of the information required in annual financial statements in accordance with International Accounting Standards, and should be read in conjunction with the consolidated financial statements of the Group for the year ended June 30, 2015 and any public announcements made by the Group during the half-year in accordance with continuous disclosure requirements arising under the Australian Stock Exchange Listing Rules and theCorporations Act 2001.
The interim financial statements have been approved and authorised for issue by the Board of Directors on February 26, 2016.
(a) Basis of accounting
The half-year financial report is a general-purpose financial report, which has been prepared in accordance with the requirements of the Corporations Act 2001, applicable Accounting Standards including AASB 134 “Interim Financial Reporting” (which is consistent with IAS 34 Interim Financial Reporting) and other mandatory professional reporting requirements.
This financial report has been prepared on a going concern basis.
During the six months ended December 31, 2015, the consolidated entity incurred a loss of A$16,074,519 (2014 comparative period: loss A$5,046,975) and had net operating cash outflows of A$14,486,796 (2014 comparative period A$4,786,947)
The ability of the Group to continue as a going concern has been determined by directors on the following basis:
| | i. | | consistent with start-up biotechnology companies, the Group’s operations are subject to considerable risks, primarily due to the nature of program development and commercialization being undertaken; and |
| | ii. | | to allow the Group to execute its long-term plans, it may be necessary to raise additional capital, and generate further income from commercializing the consolidated entity’s intellectual property. |
The financial report does not contain any adjustments to the amounts or classifications of recorded assets or liabilities that might be necessary if the Group does not continue as a going concern.
The financial statements take no account of the consequences, if any, of the effects of unsuccessful product development or commercialisation, nor of the inability of the Group to obtain adequate funding in the future.
The financial report has been prepared in accordance with the historical convention. For the purpose of preparing the financial report, the half-year has been treated as a discrete reporting period.
(b) Summary of significant accounting policies
The interim financial statements have been prepared in accordance with the accounting policies adopted in the Group’s last annual financial statements for the year ended June 30, 2015.
F-68
(c) Estimates
When preparing the interim financial statements, management undertakes a number of judgements, estimates and assumptions about recognition and measurement of assets, liabilities, income and expenses. The actual results may differ from the judgements, estimates and assumptions made by management, and will seldom equal the estimated results.
The judgements, estimates and assumptions applied in the interim financial statements, including the key sources of estimation uncertainty were the same as those applied in the consolidated entity’s last annual financial statements for the year ended June 30, 2015.
(d) Significant events and transactions
Key highlights of the interim reporting period to December 31, 2015 include the following:
| | • | | Closed an Initial Public Offering in the United States with listing on the NASDAQ |
In August, Benitec closed its U.S. initial public offering. The gross proceeds from the offering were A$18.8 million (US$13.8 million), before deducting underwriting discounts and commissions and other offering expenses. Net proceeds from the offering will be used primarily to advance Benitec’s therapeutic programs.
| | • | | Acquired full rights to hepatitis B program and announced positive in vitro data with HB-BB-331 |
In July, Benitec acquired the full rights to its pre-clinical ddRNAi-based hepatitis B therapeutic program that was previously a joint development effort between Benitec and Biomics. The initial collaboration allowed Benitec to make significant advances in the program. Based on promisingin vitro data, Benitec made the decision to develop BB-HB-331 as a wholly owned pipeline program by acquiring Biomics’ share in exchange for ordinary shares and cash consideration. Since acquiring full rights, Benitec has continued to progress the program.
| | • | | Entered into a Manufacturing Services Agreement with Lonza |
In October, Benitec entered into a Manufacturing Services Agreement with Lonza Houston, Inc. to develop a scalable manufacturing process for Benitec’s ddRNAi-based, Adeno-Associated Virus (AAV)-delivered products intended for therapeutic use in humans.
| | • | | Ceased further development of TT-034 for the treatment of hepatitis C |
On Feburary 26, 2016 the Company announced that it will wind-down its hepatitis C program and terminate the program upon completion of patients in Cohort 4 in its Phase I/IIa clinical trial for TT-034. Further detail on the termination of the program is included in ‘in house Programs’ earlier in this report.
Benitec is committed to completing the collection of trial data and monitoring patients through the required long-term safety follow-up period. Final data supporting the primary and secondary endpoints of the study will be reported in Q4 2016 when the study is completed. Although the hepatitis C program is being discontinued, it is important to note that TT-034 has been shown to be safe and well tolerated, meeting the primary endpoint of the study and, as such, will assist in other programs.
F-69
| | | | | | | | | | |
| | | | | HALF-YEAR | |
| | | (in thousands) | | December
2015 | | | December
2014 | |
| | | | | A$ | | | A$ | |
(a) | | Revenue | | | | | | | | |
| | (i) Sales revenue | | | | | | | | |
| | Licensing revenue and royalties | | | 188 | | | | 187 | |
| | | | | | | | | | |
| | (ii) Other revenue | | | | | | | | |
| | Interest | | | 134 | | | | 452 | |
| | | | | | | | | | |
| | | | �� | 322 | | | | 639 | |
| | | | | | | | | | |
(b) | | Otherincome | | | | | | | | |
| | Foreign exchange translation | | | — | | | | 392 | |
(c) | | Expenses | | | | | | | | |
| | Depreciation | | | 47 | | | | 24 | |
| | Share based payments | | | 1,475 | | | | 842 | |
| | Foreign exchange fluctuation | | | 241 | | | | — | |
(d) Seasonality of operations
There is no discernible seasonality in the operations of the consolidated entity.
Business Segments
The Group had only one business segment during the period, being the global commercialisation by licensing and partnering of patents and licences in biotechnology, with applications in biomedical research and human therapeutics.
Geographical Segments
Business operations are conducted in Australia. However there are controlled entities based in the USA and United Kingdom. The United Kingdom entity has no segment revenues, results or assets.
| | | | | | | | | | | | | | | | | | | | | | | | |
| (in thousands) | | | | | | | | | | | | | | | | | | |
Geographical location | | Segment Revenues from
External Customers | | | Segment Results | | | Carrying Amount of
Segment Assets | |
| | | Dec 2015 | | | Dec 2014 | | | Dec 2015 | | | Dec 2014 | | | Dec 2015 | | | June 2015 | |
| | | A$ | | | A$ | | | A$ | | | A$ | | | A$ | | | A$ | |
Australia | | | 322 | | | | 639 | | | | (13,891 | ) | | | (4,334 | ) | | | 27,881 | | | | 24,894 | |
United States of America | | | — | | | | — | | | | (2,183 | ) | | | (713 | ) | | | 407 | | | | 626 | |
| | | | | | | | | | | | | | | | | | | | | | | | |
| | | 322 | | | | 639 | | | | (16,074 | ) | | | (5,047 | ) | | | 28,288 | | | | 25,520 | |
| | | | | | | | | | | | | | | | | | | | | | | | |
Accounting Policies
Segment revenues and expenses are directly attributable to the identified segments and include joint venture revenue and expenses where a reasonable allocation basis exists. Segment assets include all assets used by a segment and consist mainly of cash, receivables, inventories, intangibles and property, plant and equipment, net of any allowances, accumulated depreciation and amortisation. Where joint assets correspond to two or more segments, allocation of the net carrying amount has been made on a reasonable basis to a particular segment.
F-70
Segment liabilities include mainly accounts payable, employee entitlements, accrued expenses, provisions and borrowings. Deferred income tax provisions are not included in segment assets and liabilities.
| 4. | | EVENTS AFTER THE BALANCE SHEET DATE |
On February 26, 2016 the Company announced that it will wind-down its hepatitis C program and terminate the program upon completion of patients in Cohort 4 in its Phase I/IIa clinical trial for TT-034. Other than this, there were no other significant events after balance date.
| | | | | | | | |
| | | Consolidated | |
| (in thousands) | | Dec 2015 | | | June 2015 | |
| | | A$ | | | A$ | |
Prepayments | | | 288 | | | | 74 | |
Prepaid clinical trials * | | | 2,700 | | | | 2,700 | |
IPO costs ** | | | — | | | | 285 | |
Other current assets | | | 12 | | | | 95 | |
| | | | | | | | |
| | | 3,000 | | | | 3,154 | |
| | | | | | | | |
| * | | Benitec announced on June 3, 2013 its intention to progress its non-small cell lung cancer therapeutic into clinical development. Benitec advised it had reached agreement to use European-based clinical research organisation CTGCRO to manage clinical trials and negotiated favourable commercial terms which included prepayment for the clinical trial and consulting services. As a result of feedback from potential commercial partners, the Company has recently decided to terminate the non-small cell lung cancer program, allowing resources to be focused on developing the other preclinical programs. The non-small cell lung cancer program provided information into optimising ddRNAi design and delivery. The Company is currently negotiating with CTGCRO to apply the prepayment against other programs. |
| ** | | IPO costs were incurred during the year for the public offer in the United States and the associated listing on the NASDAQ Global Select Market. These were held as a prepayment at June 30, 2015. They were subsequently transferred to equity once the capital raise to which they related was finalised. |
| 6. | | TRADE AND OTHER PAYABLES |
| | | | | | | | |
| (in thousands) | | December
2015 | | | June
2015 | |
| | | A$ | | | A$ | |
Trade creditors | | | 527 | | | | 760 | |
Sundry creditors and accrued expenses | | | 364 | | | | 689 | |
| | | | | | | | |
| | | 891 | | | | 1,449 | |
| | | | | | | | |
| | | | | | | | | | | | | | |
Details | | Date | | No. of Shares | | | Issue price
(net of costs) | | | A$ | |
Balance (net of transaction costs) | | June 30, 2015 | | | 115,881,763 | | | | | | | | 129,631,140 | |
Biomics issue | | July 15, 2015 | | | 647,333 | | | | 0.7724 | | | | 500,000 | |
| | | | | | | | | | | | | | |
IPO issue | | August 15, 2015 | | | 30,000,000 | | | | 0.6488 | | | | 19,462,643 | |
IPO and share issue transaction costs | | | | | — | | | | | | | | (1,952,886 | ) |
Balance | | December 31, 2015 | | | 146,529,096 | | | | | | | | 147,640,897 | |
| | | | | | | | | | | | | | |
The weighted average number of shares on issue during the period was | | | | | 138,118,918 | | | | | | | | | |
| | | | | | | | | | | | | | |
F-71
Ordinary Shares
Ordinary shares entitle the holder to participate in dividends and the proceeds on the winding up of the Company in proportion to the number of and amounts paid on the shares held. The fully paid ordinary shares have no par value and the Company does not have a limited amount of authorised capital.
On a show of hands every member present at a meeting in person or by proxy shall have one vote and upon a poll each share shall have one vote.
Share buy-back
There is no current on-market share buy-back.
| | | | | | | | | | |
Share options outstanding at December 31, 2015 | |
Grant date | | Expiry date | | Exercise price | | | Number
under option | |
September 26, 2011 * | | September 26, 2016 | | | A$1.25 | | | | 2,800,000 | |
November 17, 2011 ** | | November 17, 2016 | | | A$1.25 | | | | 600,000 | |
February 7, 2012 ** | | February 7, 2017 | | | A$1.25 | | | | 156,000 | |
July 18, 2012 ** | | July 18, 2017 | | | A$1.25 | | | | 400,000 | |
November 16, 2012 ** | | November 16, 2017 | | | A$1.25 | | | | 400,000 | |
November 10, 2013 * | | May 18, 2018 | | | A$0.62 | | | | 400,000 | |
August 22, 2013 ** | | August 22, 2018 | | | A$1.25 | | | | 680,000 | |
February 28, 2014 *** | | February 28, 2019 | | | A$1.26 | | | | 13,246,203 | |
May 15, 2014 ** | | May 15, 2019 | | | A$1.50 | | | | 180,000 | |
December 17, 2014 ** | | December 17, 2019 | | | A$1.25 | | | | 3,334,000 | |
May 6, 2015 ** | | May 6, 2020 | | | A$1.25 | | | | 950,000 | |
August 20, 2015 **** | | August 21, 2020 | | | US $ 0.275 | | | | 11,500,000 | |
November 12, 2015 * | | November 12, 2020 | | | A$0.77 | | | | 3,920,000 | |
| | | | | | | | | | |
| | | | | | | | | 38,566,203 | |
| | | | | | | | | | |
| * | | Non-Executive Directors options |
| **** | | Options converted to listed NASDAQ warrants, with one NASDAQ warrant representing 20 ordinary shares. ‘Warrant’ refers to a warrant to purchase one ADS at an exercise price of US$5.50 per ADS (the equivalent of 20 options over ordinary shares at US $0.275), exercisable from the date of issuance until five years thereafter. |
| 8. | | CONTINGENT LIABILITIES AND COMMITMENTS |
On December 18, 2012, the Group announced the appointment of Synteract, Inc. as its Clinical Research Organisation responsible for the progression of TT-034 into Phase I/IIa clinical trials in the USA. The Group has negotiated a contract with favourable commercial terms, in some instances requiring prepayment, for Synteract to continue to manage the Phase I/IIa clinical trial and the follow-up clinical trial through 2016 and beyond.
Benitec announced on June 3, 2013 its intention to progress its non-small cell lung cancer therapeutic into clinical development. Benitec advised it had reached agreement to use European-based clinical research organisation CTGCRO to manage clinical trials and negotiated favourable commercial terms which included prepayment for the clinical trial and consulting services. As a result of feedback from potential commercial partners, the Company has recently decided to terminate the non-small cell lung cancer program, allowing resources to be focused on developing the other preclinical programs. The non-small cell lung cancer program
F-72
provided information into optimising ddRNAi design and delivery. The Company is currently negotiating with CTGCRO to apply the prepayment against other programs.
On November 11, 2014, the Group entered into a Collaborative Research and License Agreement with 4D Molecular Therapeutics (4DMT) to identify and develop adeno-associated virus (“AAV”) vector variants optimized for gene delivery to tissues within the eye using 4D technology and products combining such optimized AAV vector variants with Benitec’s ddRNAi technology, for further development and commercialization by Benitec under license from 4D Molecular. Under this agreement the Group shall fund 4DMT for the studies to be carried out by 4DMT according to the research plan that was agreed between the parties.
The Group has contracted for scientific work on the therapeutic programs, as described above, and payments due within the next 12 months total approximately A$2,320,000 (June 30, 2015: A$2,892,000).
| 9. | | RELATED PARTY TRANSACTIONS |
Parent entity
Benitec Biopharma Limited is the parent entity.
Key management personnel
Disclosures relating to key management personnel are set out in June 30, 2015 Annual Report in the remuneration report.
Other transactions with key management personnel and their related parties
Legal services at normal commercial rates totalling A$59,102 (half year ended December 31, 2014: A$52,961) were provided by Francis Abourizk Lightowlers, a law firm in which Peter Francis is a partner and has a beneficial interest. In addition, Benitec has rented office space in Melbourne from Francis Abourizk Lightowlers and the rental cost for the period was A$11,102.
Consultancy fees were paid for executive duties totalling A$85,113 (half year ended December 31, 2014: A$29,516) provided by Newstar Ventures Ltd, a corporation in which John Chiplin is a Director and has a beneficial interest.
Receivable from and payable to related parties
There were no trade receivables from or trade payables to related parties at the current and previous reporting date.
Loans to/from related parties
There were no loans to or from related parties at the current and previous reporting date.
Terms and conditions
All transactions were made on normal commercial terms and conditions and at market rates.
F-73
1,500,000 ADSs
Representing 30,000,000 Ordinary Shares
Warrants to Purchase 575,000 ADSs
Representing 11,500,000 Ordinary Shares
Benitec Biopharma Limited
PRELIMINARY PROSPECTUS
, 2016
PART II
INFORMATION NOT REQUIRED IN PROSPECTUS
Item 6. Indemnification of Directors and Officers
Australian law. Australian law provides that a company or a related body corporate of the company may provide for indemnification of officers and directors, except to the extent of any of the following liabilities incurred as an officer or director of the company:
| | • | | a liability owed to the company or a related body corporate of the company; |
| | • | | a liability for a pecuniary penalty order made under section 1317G or a compensation order under section 961M, 1317H, 1317HA or 1317HB of the Australian Corporations Act 2001; |
| | • | | a liability that is owed to someone other than the company or a related body corporate of the company and did not arise out of conduct in good faith; or |
| | • | | legal costs incurred in defending an action for a liability incurred as an officer or director of the company if the costs are incurred: |
| | • | | in defending or resisting proceedings in which the officer or director is found to have a liability for which they cannot be indemnified as set out above; |
| | • | | in defending or resisting criminal proceedings in which the officer or director is found guilty; |
| | • | | in defending or resisting proceedings brought by the Australian Securities & Investments Commission or a liquidator for a court order if the grounds for making the order are found by the court to have been established (except costs incurred in responding to actions taken by the Australian Securities & Investments Commission or a liquidator as part of an investigation before commencing proceedings for a court order); or |
| | • | | in connection with proceedings for relief to the officer or a director under the Corporations Act, in which the court denies the relief. |
Constitution. Our Constitution provides, except to the extent prohibited by the law and the Corporations Act, for the indemnification of every person who is or has been an officer or a director of the company against liability (other than legal costs that are unreasonable) incurred by that person as an officer or director. This includes any liability incurred by that person in their capacity as an officer or director of a subsidiary of the company where the company requested that person to accept that appointment.
Indemnification Agreements. Pursuant to Deeds of Access, Insurance and Indemnity, the form of which is filed as Exhibit 10.9 to this registration statement, we have agreed to indemnify our directors against certain liabilities and expenses incurred by such persons in connection with claims made by reason of their being such a director.
SEC Position. Insofar as indemnification for liabilities arising under the Securities Act may be permitted to directors, officers or persons controlling us pursuant to the foregoing provisions, we have been informed that in the opinion of the SEC such indemnification is against public policy as expressed in the Securities Act and is therefore unenforceable.
Pursuant to the underwriting agreement for this offering, the form of which is filed as Exhibit 1.1 to this registration statement, the underwriter will agree to indemnify our directors and officers and persons controlling us, within the meaning of the Securities Act, against certain liabilities that might arise out of or are based upon certain information furnished to us by such underwriter.
II-1
Item 7. Recent Sales of Unregistered Securities
Over the past three years, we have issued and sold to third parties the securities listed below without registering the securities under the Securities Act of 1933, as amended (the “Securities Act”). None of these transactions involved any public offering. All our securities were sold through private placement either (i) outside the United States or (ii) in the United States to a limited number of investors in transactions not involving any public offering. As discussed below, we believe that each issuance of these securities was exempt from, or not subject to, registration under the Securities Act.
| 1. | | On October 30, 2012, we issued 78,446,306 ordinary shares to the vendors of Tacere Therapeutics Inc. as part of the consideration under an acquisition agreement, at an issue price of A$0.01496 per share. This issuance was exempt from registration under the Securities Act in reliance on Section 4(a)(2). |
| 2. | | On March 11, 2013, we issued 43,846,155 ordinary shares as part of a private placement at A$0.013 per share to institutional and professional investors outside the United States. Participants in the placement received two free options for every five shares subscribed for in the placement and, as a result, we issued 17,538,462 options with an exercise price of A$0.013 per share. These issuances were exempt from registration under the Securities Act in reliance on Regulation S. |
| 3. | | On March 28, 2013, we issued 13,846,154 ordinary shares as part of a private placement at A$0.013 per share to institutional and professional investors outside the United States. Participants in the placement received two free options for every five shares subscribed for in the placement and, as a result, we issued 5,538,462 options with an exercise price of A$0.013 per share. These issuances were exempt from registration under the Securities Act in reliance on Regulation S. |
| 4. | | On May 28, 2013, we issued 7,692,308 ordinary shares as part of a private placement at A$0.013 per share to institutional and professional investors outside the United States. Participants in the placement received two free options for every five shares subscribed for in the placement and, as a result, we issued 3,076,923 options with an exercise price of A$0.013 per share. These issuances were exempt from registration under the Securities Act in reliance on Regulation S. |
| 5. | | On June 14, 2013, we issued 37,454,591 ordinary shares as part of a private placement at A$0.011 per share to institutional and professional investors outside the United States. This issuance was exempt from registration under the Securities Act in reliance on Regulation S. |
| 6. | | On July 23, 2013, we issued 27,229,089 ordinary shares as part of a private placement at A$0.275 per share to institutional and professional investors outside the United States. This issuance was exempt from registration under the Securities Act in reliance on Regulation S. |
| 7. | | On July 23, 2013, we issued 400,000 ordinary shares at A$0.325 per share to directors resident outside the United States. Participants in the placement received two free options for every five shares subscribed for in the placement and, as a result, we issued 160,000 unlisted options with an exercise price of A$0.013 per share. These issuances were exempt from registration under the Securities Act in reliance on Regulation S. |
| 8. | | On August 6, 2013, we issued 10,254,696 ordinary shares at A$0.275 per share to shareholders resident in Australia or New Zealand under a share purchase plan. This issuance was exempt from registration under the Securities Act in reliance on Regulation S. |
| 9. | | On October 30, 2013, we issued 955,002 ordinary shares to the vendors of Tacere Therapeutics Inc. as part of the consideration under an acquisition agreement. The consideration was A$350,000. This issuance was exempt from registration under the Securities Act in reliance on Section 4(a)(2). |
| 10. | | On February 28, 2014, we issued 14,717,995 ordinary shares and 6,623,098 unlisted options, as the first tranche of a private placement transacted over two tranches to institutional investors in Australia and the United States. Consideration received from the issue of the ordinary shares was A$15,748,255. Maxim Group LLC acted as U.S. placement agent. This issuance was exempt from registration under the Securities Act in reliance on Section 4(a)(2) and Regulation S. |
II-2
| 11. | | On April 15, 2014, we issued 14,717,999 ordinary shares and 6,623,105 unlisted options, as the second tranche of a private placement transacted over two tranches to institutional investors in Australia and the United States. Consideration received from the issue of the ordinary shares was A$15,748,259. Maxim Group LLC acted as U.S. placement agent. This issuance was exempt from registration under the Securities Act in reliance on Section 4(a)(2) and Regulation S. |
Since July 1, 2012, we have granted options to employees, directors and consultants under our Employee Share Option Plan covering an aggregate of 12,542,000 ordinary shares, with exercise prices ranging from A$0.51 to A$1.50 per share. As of December 31, 2015, 60,000 of these options have been exercised and 5,400,000 of these options have been forfeited/expired without being exercised. We believe that the issuance of these securities were exempt from registration under the Securities Act in reliance upon Regulation S or Rule 701 of the Securities Act as transactions pursuant to written compensatory plans or pursuant to a written contract relating to compensation. No underwriters were employed in connection with the foregoing option grants.
Item 8. Exhibits and Financial Statement Schedules
| | | | See Exhibit Index beginning on page II-6 of this registration statement. |
| | (b) | | Financial Statement Schedules |
Schedules have been omitted because the information required to be set forth therein is not applicable or is shown in the Consolidated Financial Statements or the Notes thereto.
Item 9. Undertakings
The undersigned registrant hereby undertakes to provide to the underwriters at the closing specified in the underwriting agreements, certificates in such denominations and registered in such names as required by the underwriters to permit prompt delivery to each purchaser.
Insofar as indemnification for liabilities arising under the Securities Act may be permitted to directors, officers and controlling persons of the registrant pursuant to the foregoing provisions, or otherwise, the registrant has been advised that in the opinion of the SEC such indemnification is against public policy as expressed in the Securities Act and is, therefore, unenforceable. In the event that a claim for indemnification against such liabilities (other than payment by a registrant of expenses incurred or paid by a director, officer or controlling person of the registrant in the successful defense of any action, suit or proceeding) is asserted by such director, officer or controlling person in connection with the securities being registered, the registrant will, unless in the opinion of its counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question of whether such indemnification by it is against public policy as expressed in the Securities Act and will be governed by the final adjudication of such issue.
The undersigned registrant hereby undertakes that:
| | (1) | | For purposes of determining any liability under the Securities Act, the information omitted from the form of prospectus filed as part of this registration statement in reliance upon Rule 430A and contained in a form of prospectus filed by the registrant pursuant to Rule 424(b)(1) or (4) or 497(h) under the Securities Act shall be deemed to be part of this registration statement as of the time it was declared effective. |
| | (2) | | For the purpose of determining any liability under the Securities Act, each post-effective amendment that contains a form of prospectus shall be deemed to be a new registration statement relating to the securities offered therein, and this offering of such securities at that time shall be deemed to be the initial bona fide offering thereof. |
II-3
| | (3) | | to file, during any period in which offers or sales are being made, a post-effective amendment to this registration statement to: |
| | (i) | | include any prospectus required by section 10(a)(3) of the Securities Act; |
| | (ii) | | reflect in the prospectus any facts or events which, individually or together, represent a fundamental change in the information in the registration statement. Notwithstanding the foregoing, any increase or decrease in volume of securities offered (if the total dollar value of securities offered would not exceed that which was registered) and any deviation from the low or high end of the estimated maximum offering range may be reflected in the form of prospectus filed with the SEC pursuant to Rule 424(b) if, in the aggregate, the changes in volume and price represent no more than a 20% change in the maximum aggregate offering price set forth in the “Calculation of Registration Fee” table in the effective registration statement; and |
| | (iii) | | include any material information with respect to the plan of distribution not previously disclosed in the registration statement or any material change to such information in the registration statement; |
| | (4) | | that, for the purpose of determining any liability under the Securities Act of 1933, each post-effective amendment shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof. |
| | (5) | | to remove from registration by means of a post-effective amendment any of the securities being registered which remain unsold at the termination of the offering. |
| | (6) | | to file a post-effective amendment to the registration statement to include any financial statements required by “Item 8.A. of Form 20-F” at the start of any delayed offering or throughout a continuous offering. Financial statements and information otherwise required by Section 10(a)(3) of the Act need not be furnished, provided that the registrant includes in the prospectus, by means of a post-effective amendment, financial statements required pursuant to this paragraph (a)(d) and other information necessary to ensure that all other information in the prospectus is at least as current as the date of those financial statements. Notwithstanding the foregoing, with respect to registration statements on Form F-3, a post-effective amendment need not be filed to include financial statements and information required by Section 10(a)(3) of the Act if such financial statements and information are contained in periodic reports filed with or furnished to the Commission by the registrant pursuant to section 13 or section 15(d) of the Securities Exchange Act of 1934 that are incorporated by reference in the FormF-3. |
| | (7) | | that, for the purpose of determining liability of the registrant under the Securities Act of 1933 to any purchaser in the initial distribution of the securities: |
The undersigned registrant undertakes that in a primary offering of securities of the undersigned registrant pursuant to this registration statement, regardless of the underwriting method used to sell the securities to the purchaser, if the securities are offered or sold to such purchaser by means of any of the following communications, the undersigned registrant will be a seller to the purchaser and will be considered to offer or sell such securities to such purchaser:
| | (i) | | any preliminary prospectus or prospectus of the undersigned registrant relating to the offering required to be filed pursuant to Rule 424; |
| | (ii) | | any free writing prospectus relating to the offering prepared by or on behalf of the undersigned registrant or used or referred to by the undersigned registrant; |
| | (iii) | | the portion of any other free writing prospectus relating to the offering containing material information about the undersigned registrant or its securities provided by or on behalf of the undersigned registrant; and. |
| | (iv) | | any other communication that is an offer in the offering made by the undersigned registrant to the purchaser. |
II-4
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, the registrant certifies that it has reasonable grounds to believe that it meets all of the requirements for filing on Form F-1 and has duly caused this amendment to the registration statement to be signed on its behalf by the undersigned, thereunto duly authorized, in Sydney, Australia on April 13, 2016.
| | | | |
| Benitec Biopharma Limited |
| |
By: | | /s/ Greg West |
| | Name: Greg West |
| | Title: Interim Chief Executive Officer and Chief Financial Officer and Company Secretary |
Pursuant to the requirements of the Securities Act, this amendment to the registration statement has been signed by the following persons in the capacities and on the dates indicated.
| | | | |
Signature | | Title | | Date |
| | |
* Name: Peter Francis | | Chairman | | April 13, 2016 |
| | |
/s/ Greg West Name: Greg West | | Chief Executive Officer and Chief Financial Officer and Company Secretary (principal executive officer) | | April 13, 2016 |
| | |
* Name: John Chiplin | | Director | | April 13, 2016 |
| | |
* Name: Iain Ross | | Director | | April 13, 2016 |
| | |
* Name: J. Kevin Buchi | | Director | | April 13, 2016 |
| | |
| |
| * By | | /s/ Greg West |
| | Greg West Attorney-in-fact |
II-5
SIGNATURE OF AUTHORIZED REPRESENTATIVE IN THE UNITED STATES
Pursuant to the Securities Act of 1933, as amended, the undersigned, the duly authorized representative in the United States of Benitec Biopharma Limited, has signed this registration statement or amendment thereto in Hayward, California, on April 13, 2016.
| | |
| Authorized U.S. Representative |
|
| TACERE THERAPEUTICS, INC. |
| |
By: | | /s/ John Chiplin |
| | Name: John Chiplin |
| | Title: Director |
II-6
EXHIBIT INDEX
| | | | |
Exhibits | | | Description |
| |
| | 1.1 | | | Form of Underwriting Agreement* |
| |
| | 3.1 | | | Constitution of Benitec Biopharma Limited* |
| |
| | 4.1 | | | Deposit Agreement, dated May 30, 2014, between Benitec Biopharma Limited and The Bank of New York Mellon, as depositary, and Owners and Holders of the American Depositary Shares* |
| |
| | 4.2 | | | Form of Amendment to Deposit Agreement between Benitec Biopharma Limited and The Bank of New York Mellon, as depositary, and Owners and Holders of the American Depositary Shares* |
| |
| | 4.3 | | | Form of American Depositary Receipt evidencing American Depositary Shares (included in Exhibit 4.2)* |
| |
| | 4.4 | | | Form of Global Warrant to Purchase ADSs (included in Exhibit 4.5) |
| |
| | 4.5 | | | Form of ADS Warrant Agent Agreement, between Benitec Biopharma Limited and The Bank of New York Mellon, as warrant agent* |
| |
| | 4.6 | | | Benitec Officers’ and Employees Share Option Plan** |
| |
| | 5.1 | | | Opinion of Baker & McKenzie regarding the validity of the ordinary shares being issued and the ordinary shares underlying the warrants* |
| |
| | 5.2 | | | Opinion of Baker & McKenzie LLP regarding validity of Warrants* |
| |
| | 8.1 | | | Opinion of Baker & McKenzie LLP regarding material U.S. tax matters* |
| |
| | 8.2 | | | Opinion of Baker & McKenzie regarding material Australian tax matters* |
| |
| | 10.1 | | | License Agreement, dated December 23, 2009, between Commonwealth Scientific and Industrial Research Organisation (“CSIRO”) and Benitec* |
| |
| | 10.2 | | | Research and Collaboration Agreement–Overall Project, dated July 11, 2014, between Biomics Biotech Co., Ltd and Benitec* |
| |
| | 10.3 | | | Commercial License Agreement–Amended and Restated, dated December 3, 2014, between NewSouth Innovations Pty Limited and Benitec Australia Limited* |
| |
| | 10.4 | | | Collaborative Research and License Agreement, dated November 11, 2014, between 4D Molecular Therapeutics, LLC and Benitec*† |
| |
| | 10.5 | | | Lease Agreement, dated May 12, 2014, between Hayward Point Eden 1 Limited Partnership and Benitec* |
| |
| | 10.6 | | | First Amendment to Lease Agreement, dated May 7, 2015, between Hayward Point Eden 1 Limited Partnership and Benitec* |
| |
| | 10.7 | | | Commercial Lease Agreement, dated August 5, 2014, between Mary Montague and Janice Leong and Benitec* |
| |
| | 10.8 | | | Form of Executive Employment Agreement for executive officers* |
| |
| | 10.9 | | | Form of Deed of Access, Insurance and Indemnity for Directors and Officers* |
| |
| | 10.10 | | | Earn-Out Agreement, dated July 9, 2015, between Biomics Biotech Co., Ltd and Benitec* |
| |
| | 21.1 | | | List of significant subsidiaries of Benitec Biopharma Limited* |
| |
| | 23.1 | | | Consent of Baker & McKenzie (see Exhibit 5.1)* |
II-7
| | | | |
Exhibits | | | Description |
| |
| | 23.2 | | | Consent of Baker & McKenzie LLP (see Exhibit 5.2)* |
| |
| | 23.3 | | | Consent of Baker & McKenzie LLP (see Exhibit 8.1)* |
| |
| | 23.4 | | | Consent of Baker & McKenzie (see Exhibit 8.2)* |
| |
| | 23.5 | | | Consent of Grant Thornton Audit Pty Ltd |
| |
| | 24.1 | | | Power of Attorney* |
| * | | Previously filed with Form F-1, filed with the SEC on June 22, 2015, or amendments to Form F-1, filed with the SEC on July 27, 2015, August 10, 2015 and August 13, 2015 |
| ** | | Previously filed with Form S-8, filed with the SEC on February 4, 2016 |
| † | | Confidential treatment has been requested with respect to portions of this Exhibit. Omitted portions have been submitted separately with the Securities and Exchange Commission. |
II-8