
Benitec Corporate Overview Ladenburg Thalmann September 2019 Exhibit 99.1
Benitec Biopharma: General Financial and Capital Markets Overview Benitec Biopharma: Gene therapy-focused biotechnology company developing novel genetic medicines derived from the proprietary DNA-directed RNA interference (“ddRNAi”) platform Market Cap: USD$8.6 million Net Cash (June 30, 2019): USD$15.5 million Public Listing Exchanges: NASDAQ (Listed in 2015): BNTC ASX (Listed in 2002): BLT
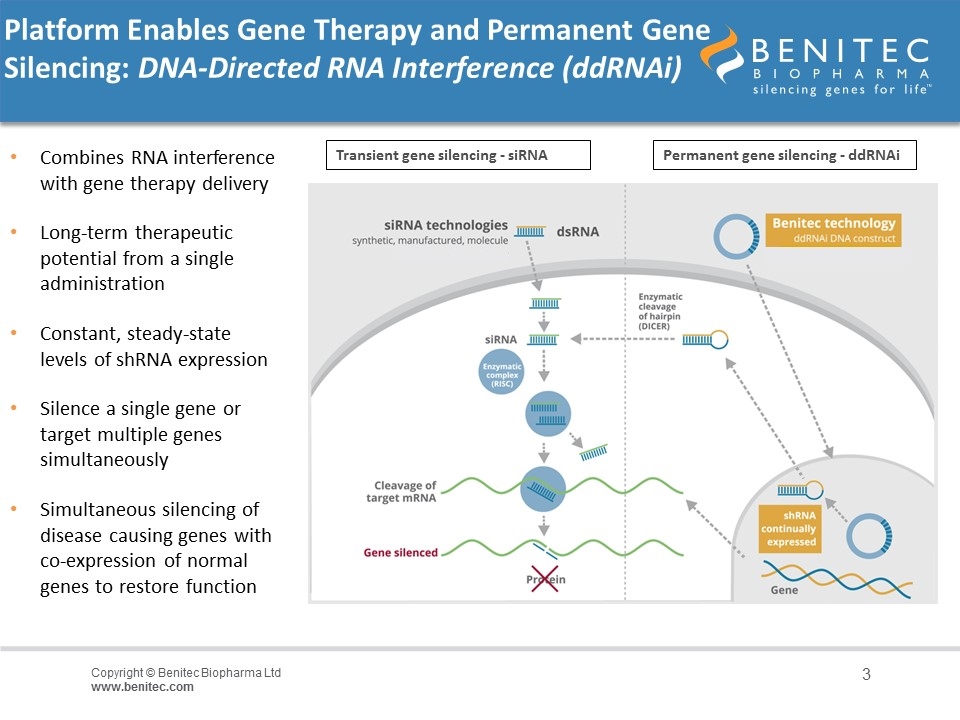
Platform Enables Gene Therapy and Permanent Gene Silencing: DNA-Directed RNA Interference (ddRNAi) Combines RNA interference with gene therapy delivery Long-term therapeutic potential from a single administration Constant, steady-state levels of shRNA expression Silence a single gene or target multiple genes simultaneously Simultaneous silencing of disease causing genes with co-expression of normal genes to restore function Transient gene silencing - siRNA Permanent gene silencing - ddRNAi
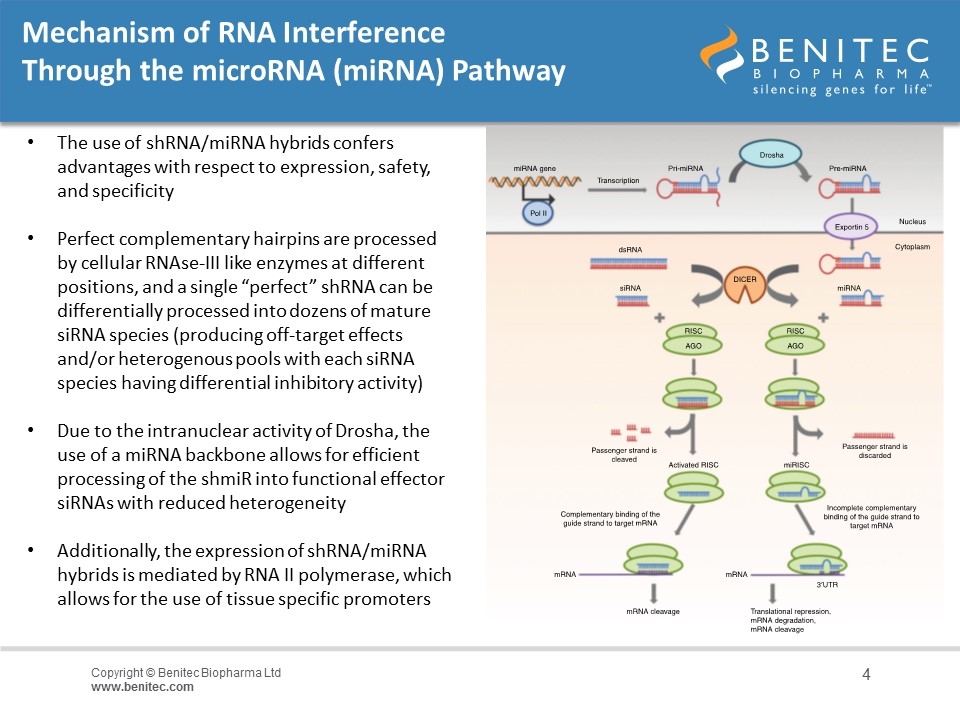
Mechanism of RNA Interference Through the microRNA (miRNA) Pathway The use of shRNA/miRNA hybrids confers advantages with respect to expression, safety, and specificity Perfect complementary hairpins are processed by cellular RNAse-III like enzymes at different positions, and a single “perfect” shRNA can be differentially processed into dozens of mature siRNA species (producing off-target effects and/or heterogenous pools with each siRNA species having differential inhibitory activity) Due to the intranuclear activity of Drosha, the use of a miRNA backbone allows for efficient processing of the shmiR into functional effector siRNAs with reduced heterogeneity Additionally, the expression of shRNA/miRNA hybrids is mediated by RNA II polymerase, which allows for the use of tissue specific promoters

Corporate and Scientific Team Deep expertise in gene therapy Scientific operations in Hayward, California Internal manufacturing expertise Demonstrated capabilities for optimization of complex, scalable, AAV-based manufacturing processes Management team with background in operations and capital allocation within the biopharmaceutical sector

Executive Team Jerel Banks, M.D., Ph.D. CEO and Executive Chairman Healthcare investment professional with over 13 years of experience Former vice president and co-portfolio manager at Franklin Templeton Investments Earned an M.D. and Ph.D. at Brown University, and holds an A.B. in Chemistry from Princeton University Megan Boston Executive Director, Head of Operations Australia CEO and Managing Director of ASX listed entities Chartered Accountant with over 20 years of experience Held senior executive roles at various banking institutions in the area of risk and compliance, as well as PricewaterhouseCoopers Claudia Kloth, Ph.D. SVP of Manufacturing Over 20 years of cGMP manufacturing and process development experience in therapeutics Led Process Development group at Lonza Viral Therapeutics Developed, optimized and transferred robust viral-based products (Ad5, AAV, lentivirus) to cGMP manufacturing Guided process transfer and process validation activities of Yervoy (Bristol-Myers Squibb) Peter Roelvink, D.Sc.Ag. Senior Director Research First to demonstrate delivery of a targeted vector from its native receptor to an artificial receptor Co-inventor on 19 issued US patents that cover RNAi vectors, targeted delivery of adenoviruses, and tissue specific expression using AAV
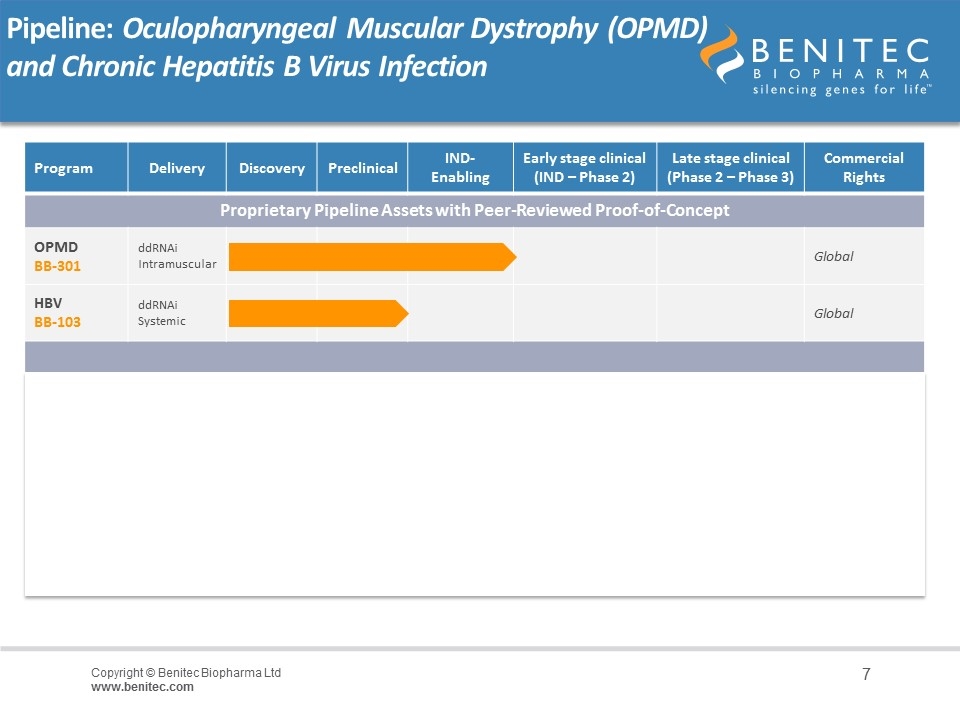
Pipeline: Oculopharyngeal Muscular Dystrophy (OPMD) and Chronic Hepatitis B Virus Infection Program Delivery Discovery Preclinical IND-Enabling Early stage clinical (IND – Phase 2) Late stage clinical (Phase 2 – Phase 3) Commercial Rights Proprietary Pipeline Assets with Peer-Reviewed Proof-of-Concept OPMD BB-301 ddRNAi Intramuscular Global HBV BB-103 ddRNAi Systemic Global Proprietary Orphan CNS/Neuromuscular Assets with Proof-of-Concept Anticipated over Next 18-to-24 Months Undisclosed BB-601 AAV Local Delivery Global Proprietary Orphan CNS/Neuromuscular Assets with Proof-of-Concept Anticipated over Next 18-to-24 Months Undisclosed BB-701 AAV Local Delivery Global
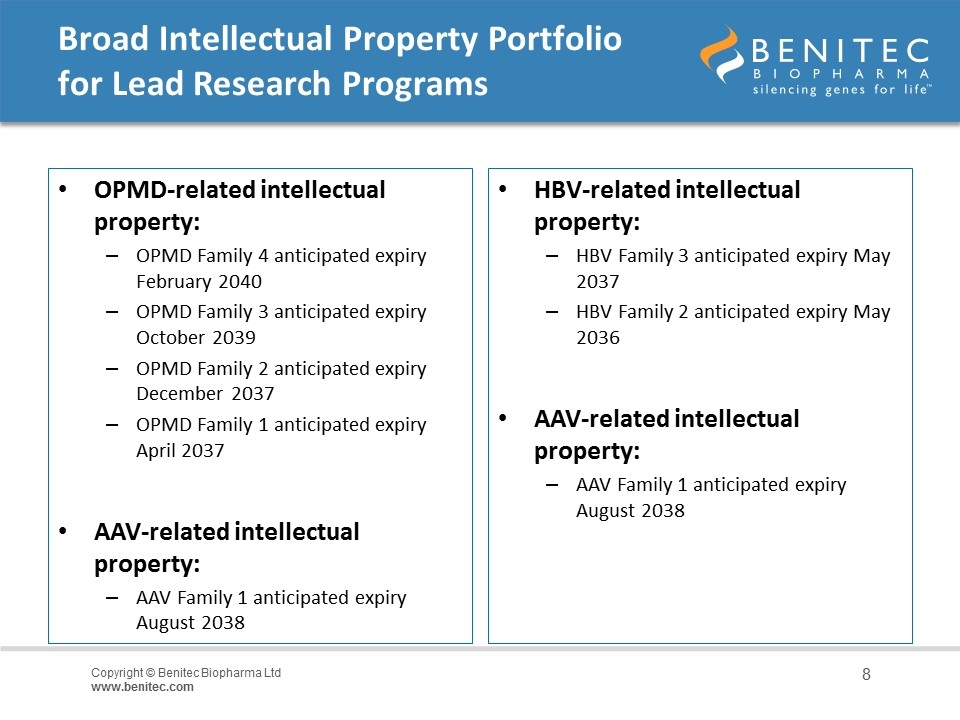
Broad Intellectual Property Portfolio for Lead Research Programs OPMD-related intellectual property: OPMD Family 4 anticipated expiry February 2040 OPMD Family 3 anticipated expiry October 2039 OPMD Family 2 anticipated expiry December 2037 OPMD Family 1 anticipated expiry April 2037 AAV-related intellectual property: AAV Family 1 anticipated expiry August 2038 HBV-related intellectual property: HBV Family 3 anticipated expiry May 2037 HBV Family 2 anticipated expiry May 2036 AAV-related intellectual property: AAV Family 1 anticipated expiry August 2038

BB-301 Program Overview BB-301 is an internally optimized, AAV-based gene therapy agent that can both silence the expression of the mutated, disease-causing PABPN1 gene (to slow, or halt, the underlying mechanism of disease progression in OPMD) and replace the mutant PABPN1 gene with a normal, “wild type” PABPN1 gene (to drive restoration of function in diseased cells) The biological efficacy and safety profiles observed for BB-301 in prior published non-clinical studies remain unchanged (BB-301 fully corrects the OPMD disease phenotype in the A17 mouse model), and upcoming publications will further outline the efficacy of BB-301 in this animal model The Benitec manufacturing team has optimized and reproduced the BB-301 commercial-scale manufacturing process, which removes key risks associated with production for clinical studies

BB-301 Program Overview Over the preceding 12-months, an incomplete set of BB-301-focused non-clinical experiments were conducted in large animal subjects, the results of which were inconclusive As these non-clinical studies require repetition, Benitec will complete three non-clinical studies for BB-301 that will facilitate the filing of an Investigational New Drug (IND) application and the formal initiation of a Phase I clinical trial in patients suffering from OPMD The three non-clinical BB-301 studies will be conducted in canine subjects and will support the optimization of the methods of administration, confirm the efficiency of vector transduction in the key tissue compartments underlying the disease phenotype, confirm the optimal drug doses in advance of initiation of human clinical studies, and finalize experiments designed to characterize any toxicological data-points
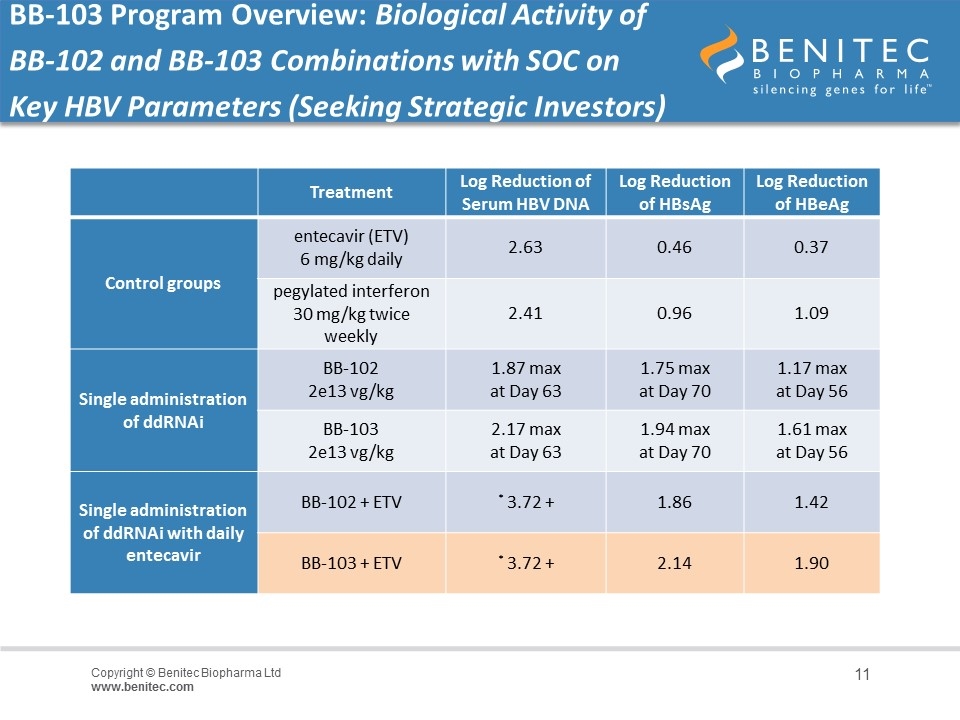
BB-103 Program Overview: Biological Activity of BB-102 and BB-103 Combinations with SOC on Key HBV Parameters (Seeking Strategic Investors) Treatment Log Reduction of Serum HBV DNA Log Reduction of HBsAg Log Reduction of HBeAg Control groups entecavir (ETV) 6 mg/kg daily 2.63 0.46 0.37 pegylated interferon 30 mg/kg twice weekly 2.41 0.96 1.09 Single administration of ddRNAi BB-102 2e13 vg/kg 1.87 max at Day 63 1.75 max at Day 70 1.17 max at Day 56 BB-103 2e13 vg/kg 2.17 max at Day 63 1.94 max at Day 70 1.61 max at Day 56 Single administration of ddRNAi with daily entecavir BB-102 + ETV * 3.72 + 1.86 1.42 BB-103 + ETV * 3.72 + 2.14 1.90

Late-Stage Non-clinical Asset with Category-leading biological efficacy BB-301 for Oculopharyngeal Muscular Dystrophy Global prevalence of OPMD exceeds 15,000 patients and commercial opportunity exceeds USD$1 billion
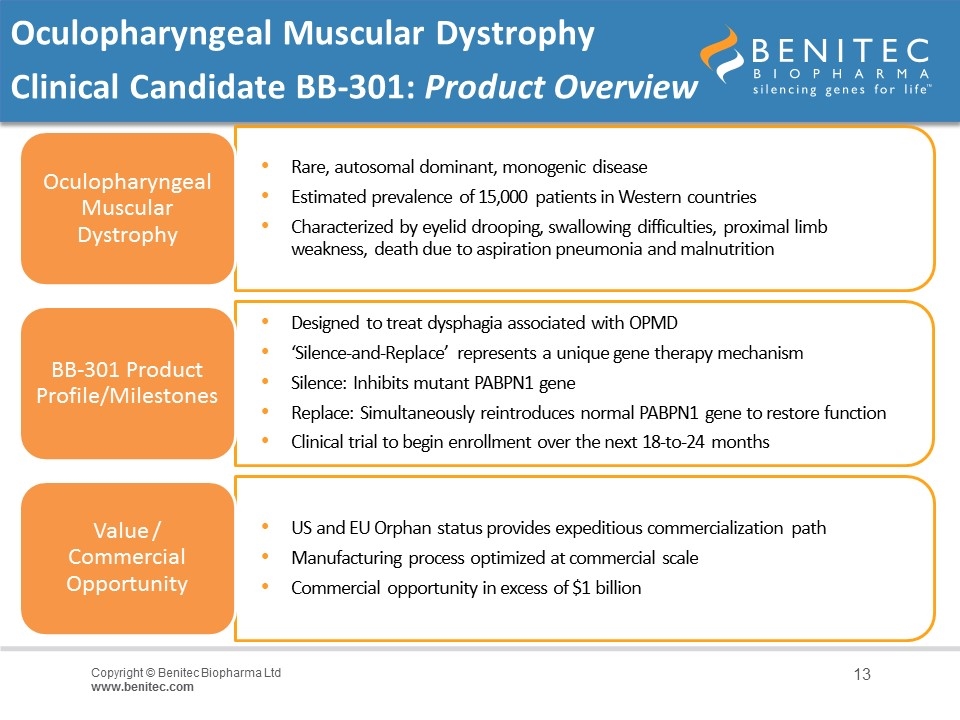
Oculopharyngeal Muscular Dystrophy Clinical Candidate BB-301: Product Overview Oculopharyngeal Muscular Dystrophy BB-301 Product Profile/Milestones Designed to treat dysphagia associated with OPMD Value / Commercial Opportunity US and EU Orphan status provides expeditious commercialization path ‘Silence-and-Replace’ represents a unique gene therapy mechanism Silence: Inhibits mutant PABPN1 gene Replace: Simultaneously reintroduces normal PABPN1 gene to restore function Rare, autosomal dominant, monogenic disease Estimated prevalence of 15,000 patients in Western countries Characterized by eyelid drooping, swallowing difficulties, proximal limb weakness, death due to aspiration pneumonia and malnutrition Commercial opportunity in excess of $1 billion Clinical trial to begin enrollment over the next 18-to-24 months Manufacturing process optimized at commercial scale
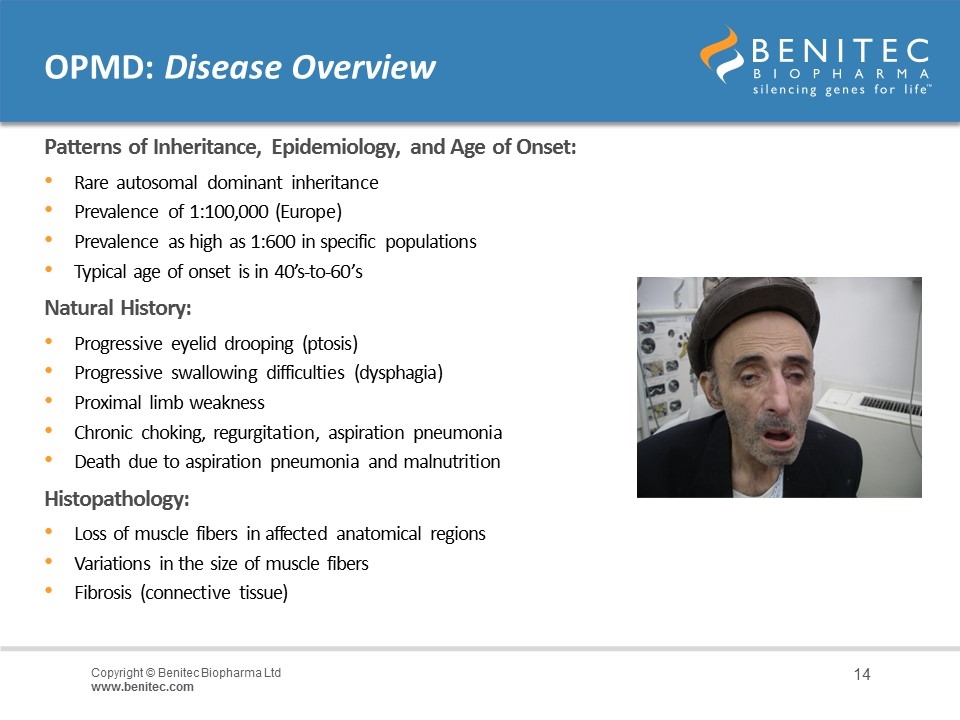
OPMD: Disease Overview Patterns of Inheritance, Epidemiology, and Age of Onset: Rare autosomal dominant inheritance Prevalence of 1:100,000 (Europe) Prevalence as high as 1:600 in specific populations Typical age of onset is in 40’s-to-60’s Natural History: Progressive eyelid drooping (ptosis) Progressive swallowing difficulties (dysphagia) Proximal limb weakness Chronic choking, regurgitation, aspiration pneumonia Death due to aspiration pneumonia and malnutrition Histopathology: Loss of muscle fibers in affected anatomical regions Variations in the size of muscle fibers Fibrosis (connective tissue)
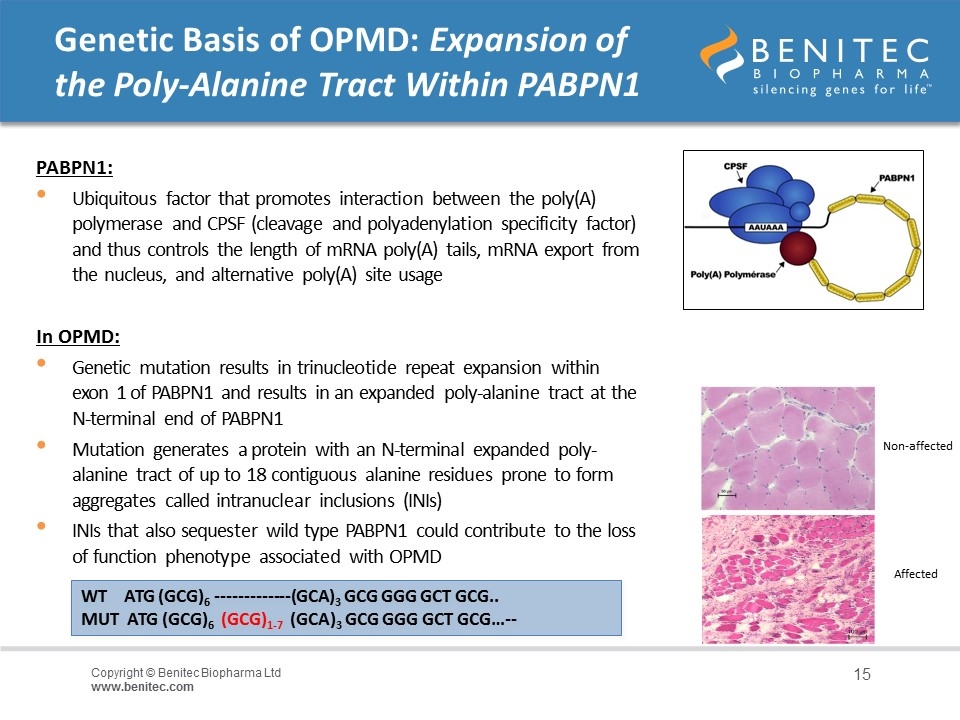
Genetic Basis of OPMD: Expansion of the Poly-Alanine Tract Within PABPN1 PABPN1: Ubiquitous factor that promotes interaction between the poly(A) polymerase and CPSF (cleavage and polyadenylation specificity factor) and thus controls the length of mRNA poly(A) tails, mRNA export from the nucleus, and alternative poly(A) site usage In OPMD: Genetic mutation results in trinucleotide repeat expansion within exon 1 of PABPN1 and results in an expanded poly-alanine tract at the N-terminal end of PABPN1 Mutation generates a protein with an N-terminal expanded poly-alanine tract of up to 18 contiguous alanine residues prone to form aggregates called intranuclear inclusions (INIs) INIs that also sequester wild type PABPN1 could contribute to the loss of function phenotype associated with OPMD WT ATG (GCG)6 -------------(GCA)3 GCG GGG GCT GCG.. MUT ATG (GCG)6 (GCG)1-7 (GCA)3 GCG GGG GCT GCG…-- Non-affected Affected
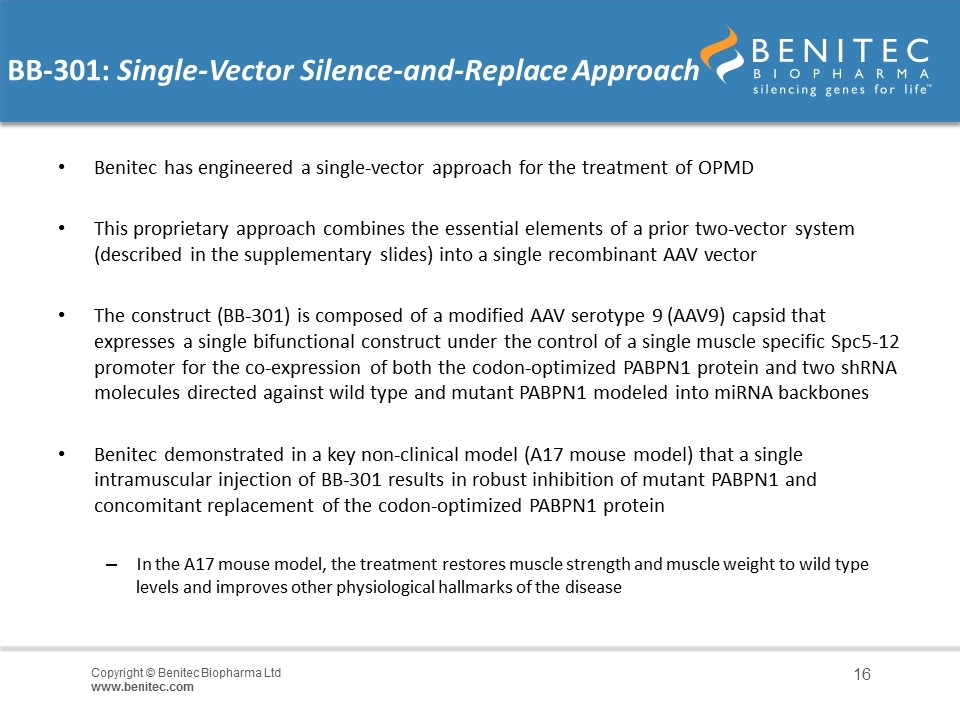
BB-301: Single-Vector Silence-and-Replace Approach Benitec has engineered a single-vector approach for the treatment of OPMD This proprietary approach combines the essential elements of a prior two-vector system (described in the supplementary slides) into a single recombinant AAV vector The construct (BB-301) is composed of a modified AAV serotype 9 (AAV9) capsid that expresses a single bifunctional construct under the control of a single muscle specific Spc5-12 promoter for the co-expression of both the codon-optimized PABPN1 protein and two shRNA molecules directed against wild type and mutant PABPN1 modeled into miRNA backbones Benitec demonstrated in a key non-clinical model (A17 mouse model) that a single intramuscular injection of BB-301 results in robust inhibition of mutant PABPN1 and concomitant replacement of the codon-optimized PABPN1 protein In the A17 mouse model, the treatment restores muscle strength and muscle weight to wild type levels and improves other physiological hallmarks of the disease
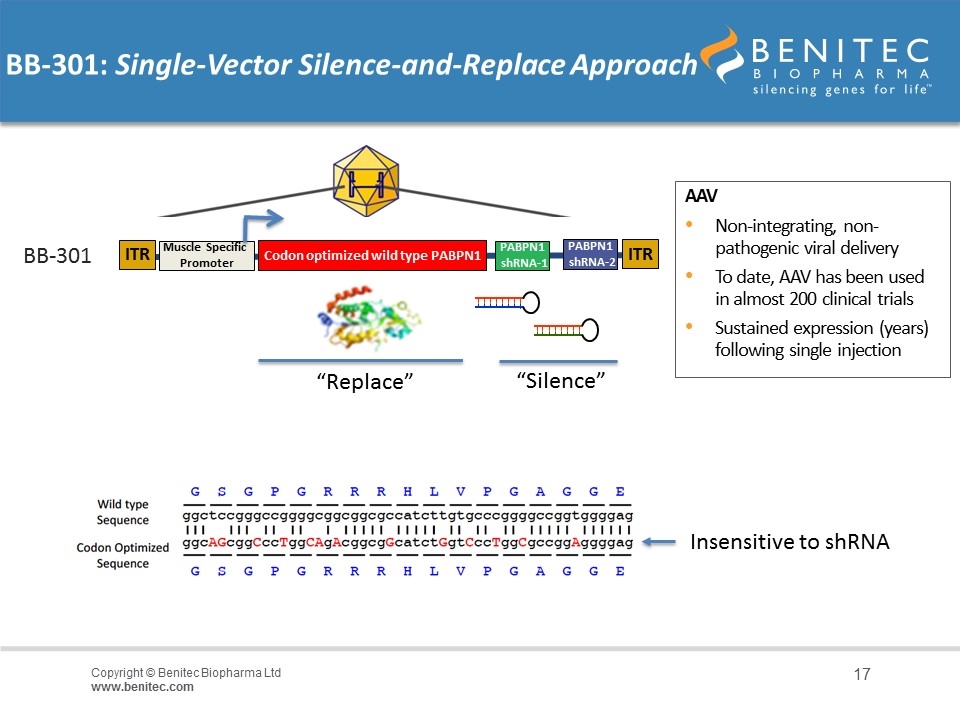
REP/CAP removed and replaced with expression cassette AAV Non-integrating, non-pathogenic viral delivery To date, AAV has been used in almost 200 clinical trials Sustained expression (years) following single injection BB-301: Single-Vector Silence-and-Replace Approach “Silence” “Replace” BB-301 ITR Muscle Specific Promoter PABPN1 shRNA-2 PABPN1 shRNA-1 Codon optimized wild type PABPN1 ITR Insensitive to shRNA
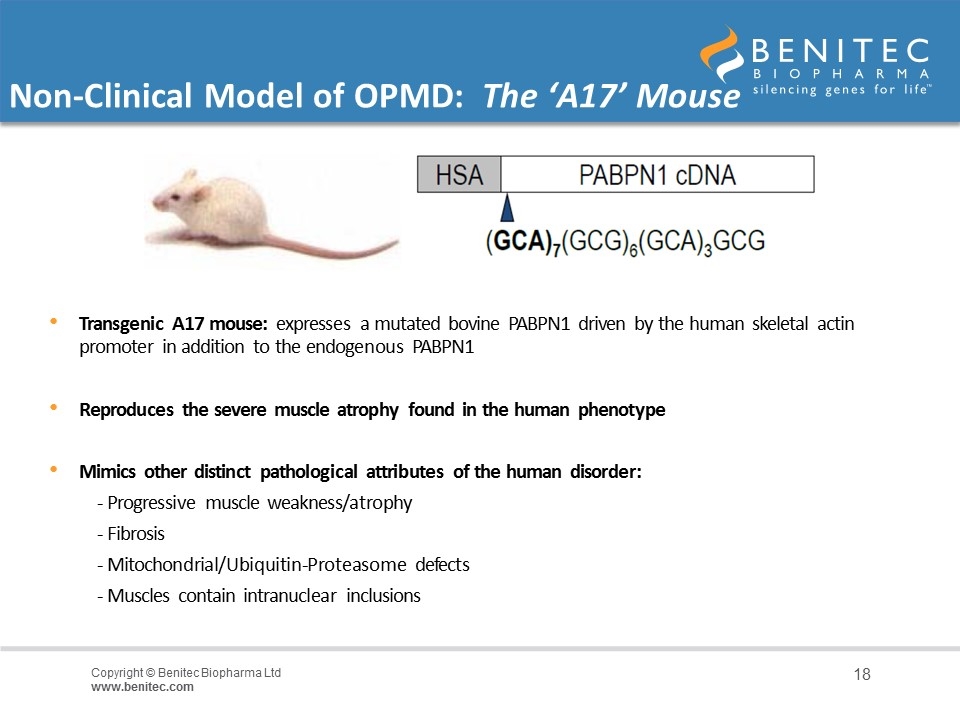
Non-Clinical Model of OPMD: The ‘A17’ Mouse Transgenic A17 mouse: expresses a mutated bovine PABPN1 driven by the human skeletal actin promoter in addition to the endogenous PABPN1 Reproduces the severe muscle atrophy found in the human phenotype Mimics other distinct pathological attributes of the human disorder: - Progressive muscle weakness/atrophy - Fibrosis - Mitochondrial/Ubiquitin-Proteasome defects - Muscles contain intranuclear inclusions
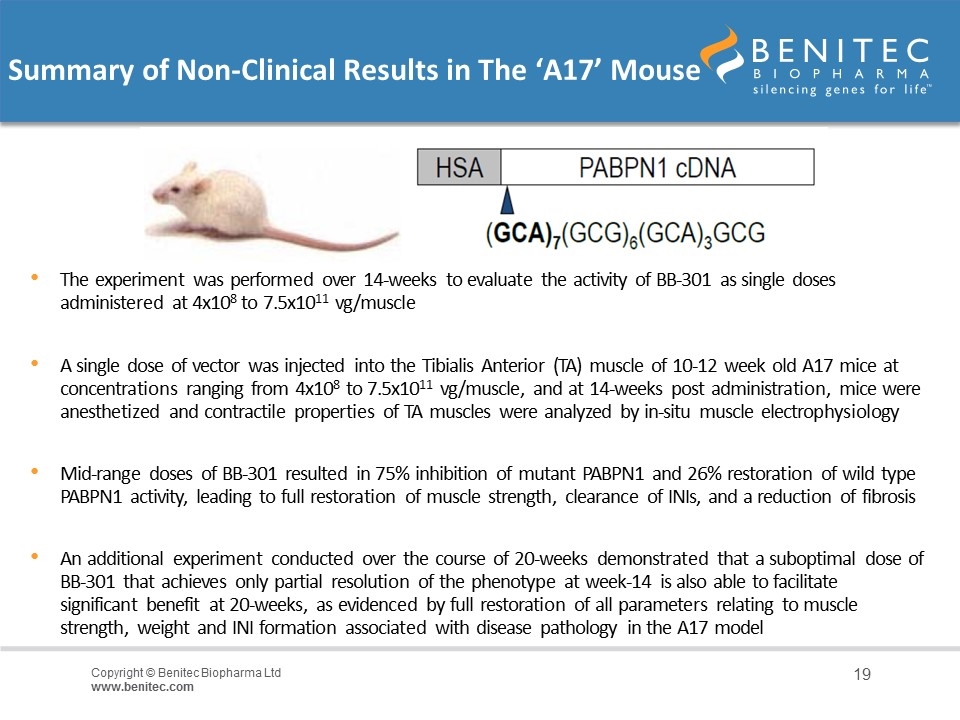
Summary of Non-Clinical Results in The ‘A17’ Mouse The experiment was performed over 14-weeks to evaluate the activity of BB-301 as single doses administered at 4x108 to 7.5x1011 vg/muscle A single dose of vector was injected into the Tibialis Anterior (TA) muscle of 10-12 week old A17 mice at concentrations ranging from 4x108 to 7.5x1011 vg/muscle, and at 14-weeks post administration, mice were anesthetized and contractile properties of TA muscles were analyzed by in-situ muscle electrophysiology Mid-range doses of BB-301 resulted in 75% inhibition of mutant PABPN1 and 26% restoration of wild type PABPN1 activity, leading to full restoration of muscle strength, clearance of INIs, and a reduction of fibrosis An additional experiment conducted over the course of 20-weeks demonstrated that a suboptimal dose of BB-301 that achieves only partial resolution of the phenotype at week-14 is also able to facilitate significant benefit at 20-weeks, as evidenced by full restoration of all parameters relating to muscle strength, weight and INI formation associated with disease pathology in the A17 model
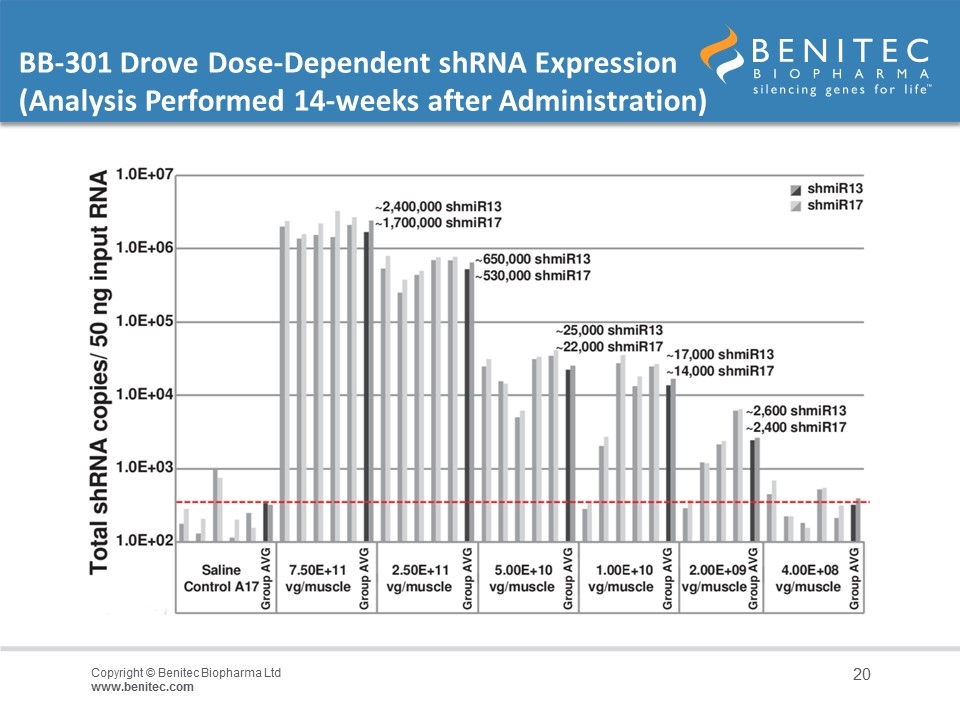
BB-301 Drove Dose-Dependent shRNA Expression (Analysis Performed 14-weeks after Administration)
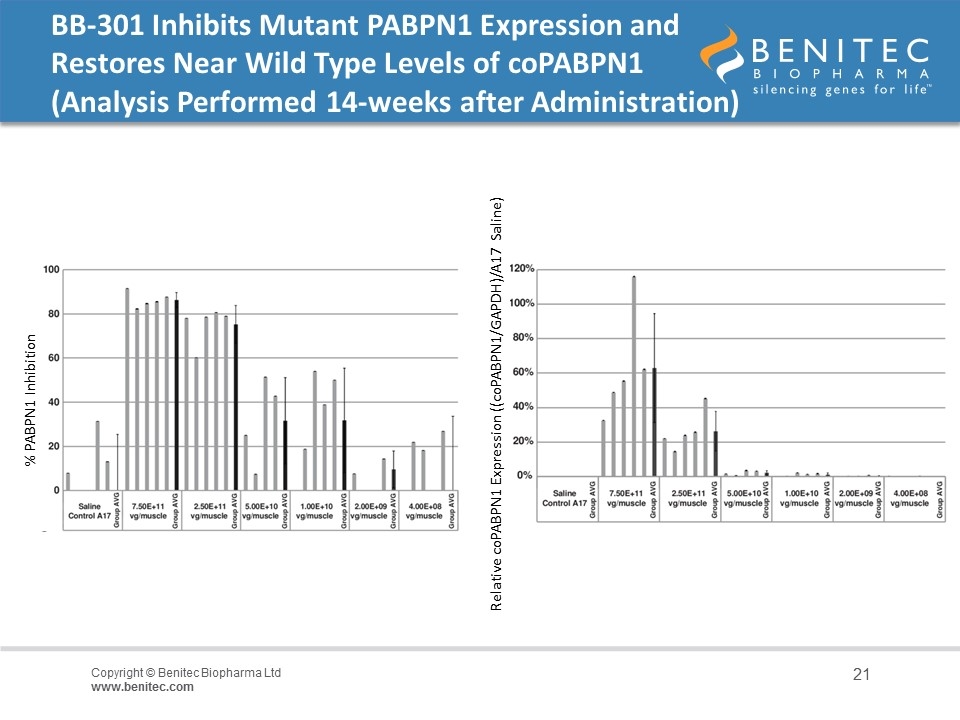
BB-301 Inhibits Mutant PABPN1 Expression and Restores Near Wild Type Levels of coPABPN1 (Analysis Performed 14-weeks after Administration) % PABPN1 Inhibition Relative coPABPN1 Expression ((coPABPN1/GAPDH)/A17 Saline)
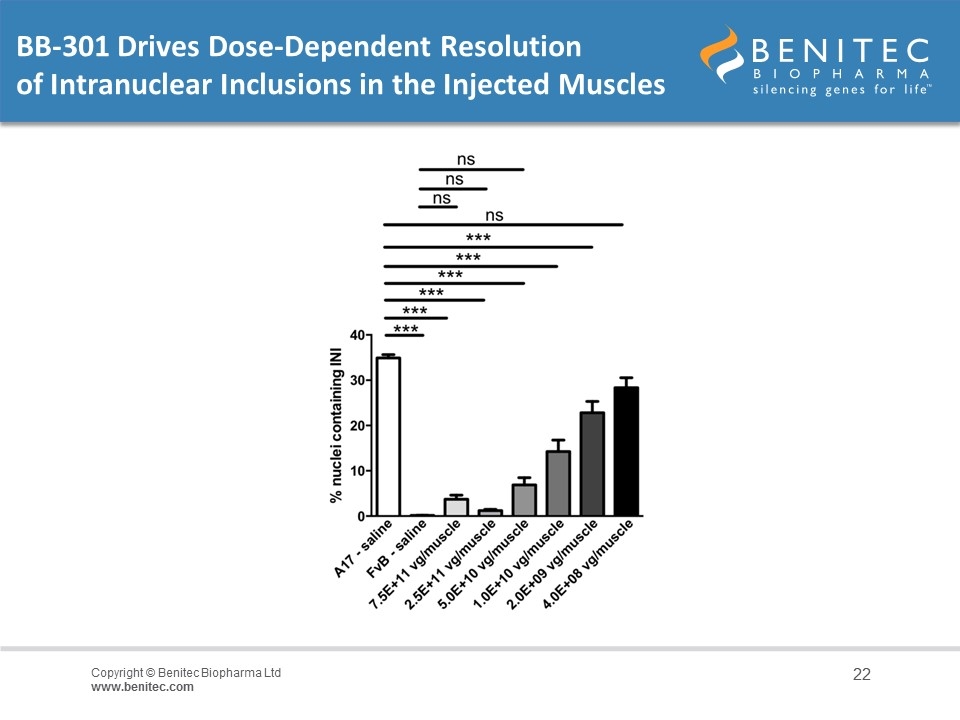
BB-301 Drives Dose-Dependent Resolution of Intranuclear Inclusions in the Injected Muscles
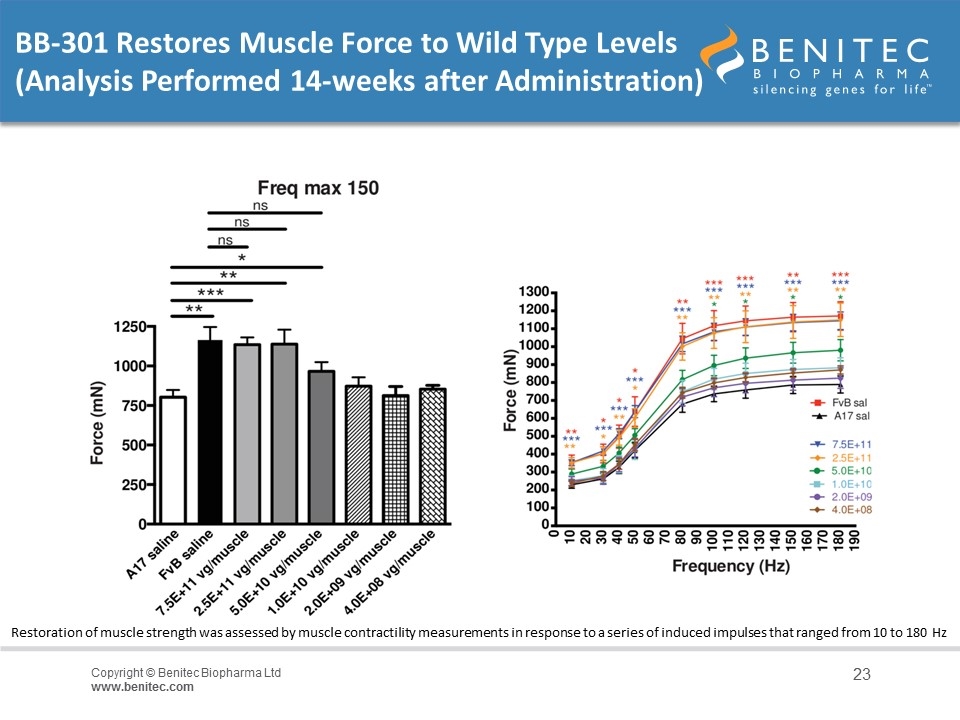
BB-301 Restores Muscle Force to Wild Type Levels (Analysis Performed 14-weeks after Administration) Restoration of muscle strength was assessed by muscle contractility measurements in response to a series of induced impulses that ranged from 10 to 180 Hz
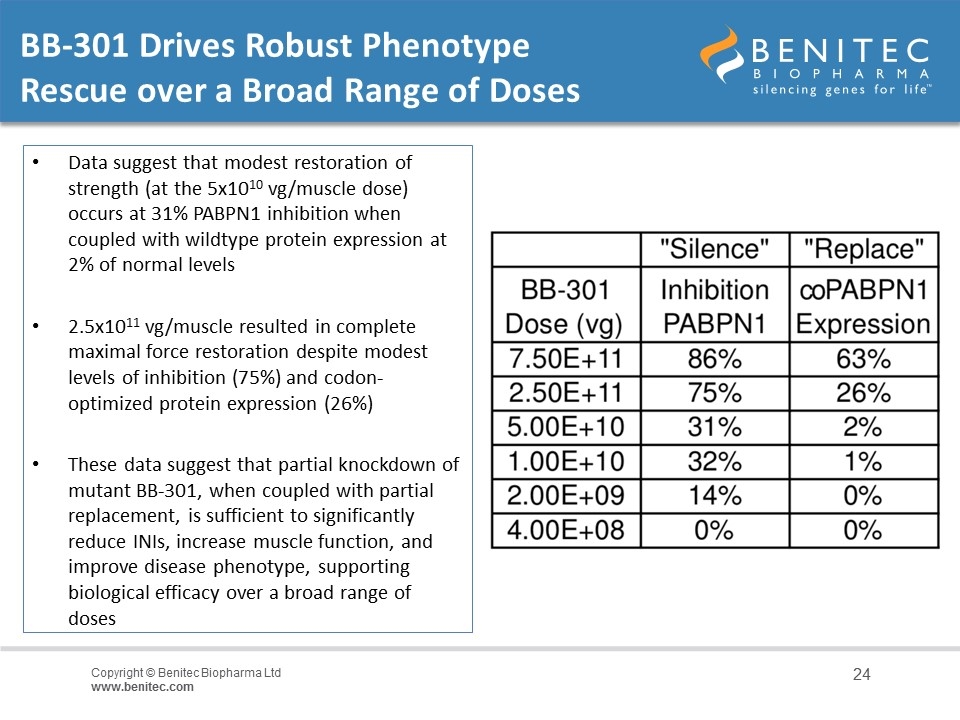
BB-301 Drives Robust Phenotype Rescue over a Broad Range of Doses Data suggest that modest restoration of strength (at the 5x1010 vg/muscle dose) occurs at 31% PABPN1 inhibition when coupled with wildtype protein expression at 2% of normal levels 2.5x1011 vg/muscle resulted in complete maximal force restoration despite modest levels of inhibition (75%) and codon-optimized protein expression (26%) These data suggest that partial knockdown of mutant BB-301, when coupled with partial replacement, is sufficient to significantly reduce INIs, increase muscle function, and improve disease phenotype, supporting biological efficacy over a broad range of doses
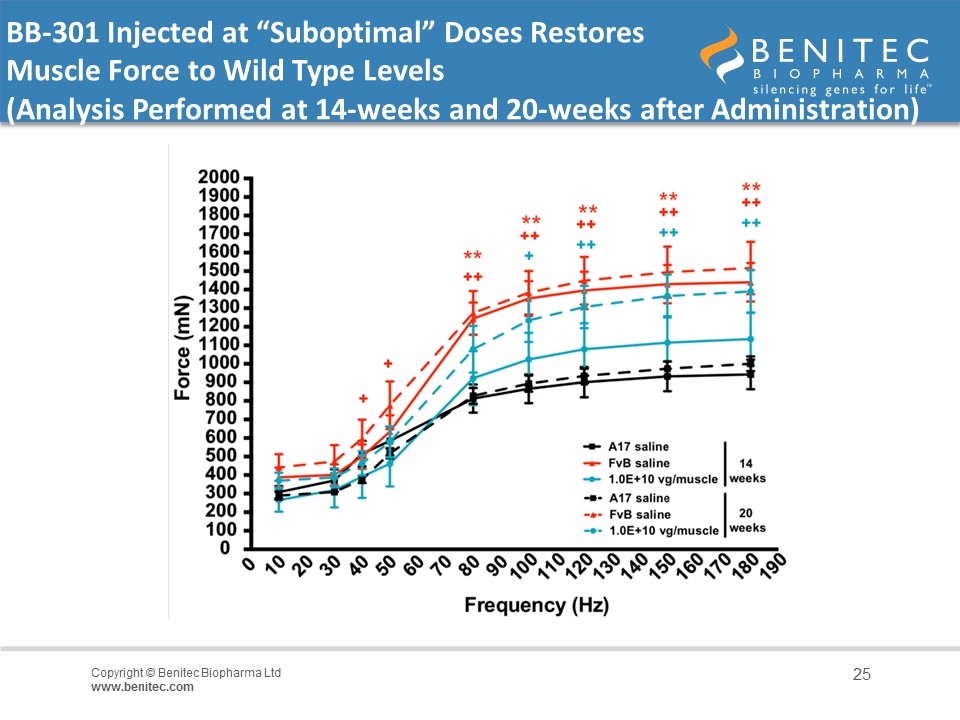
BB-301 Injected at “Suboptimal” Doses Restores Muscle Force to Wild Type Levels (Analysis Performed at 14-weeks and 20-weeks after Administration)
Non-Clinical Experiments Conducted During the Past 12-months in Canines Employed Inconsistent Dosing Methods and Tissue-Processing Techniques An endoscopic procedure was employed, without the aid of fluoroscopy or electromyography, to directly inject BB-301 into the muscles of interest in canines This method of administration led to significant variability with respect to the vector genomes of BB-301 delivered to the targeted anatomical sites (e.g. contralateral sides of the same target muscle contained vector copy numbers that could vary by 3-fold to 8-fold) Additionally, a chemical dye-based tissue marking procedure was employed to facilitate analyses of the tissues near the BB-301 injection sites in the muscles of each animal, however, as the dye was not present at the time of necropsy, this method did not support accurate characterization of the vector copy numbers achieved following BB-301 administration (i.e. BB-301 tissue transduction) Several concentrations and volumes of dye were injected with BB-301 or adjacent to the dosing site, however, at necropsy, no dye was found in the target muscles

New BB-301 Non-Clinical Studies Will be Conducted Over the Next 12-to-18 Months The IND-enabling non-clinical studies will be carried out under the guidance of the scientific team at Benitec in close collaboration with a team of Thought Leaders in both medicine and surgery that have been deeply engaged in the treatment of OPMD patients for several decades 6-week study in Beagle dogs to confirm the transduction efficiency of BB-301 upon administration via direct intramuscular injection into the pharyngeal muscles following an open surgical approach Direct injection of BB-301 into the tibialis anterior muscle of A17 mice demonstrated that AAV9PL achieved robust transduction of the targeted skeletal muscle cells This follow-up study will be conducted to optimize the dosing and surgical administration procedures for BB-301 injection into the pharyngeal muscle tissues of interest in Beagle dogs and, ultimately, in human subjects 20-week dose-range finding study in Beagle dogs This study will be conducted to further bolster our understanding of the transduction efficiency of BB-301 in addition to providing a more fulsome characterization of the level of shRNA and codon-optimized PABPN1 expression observed across distinct drug doses administered into the targeted pharyngeal muscles (and will facilitate BB-301 dose selection for the Phase I/II clinical trial) 12-week regulatory toxicology study in Beagle dogs
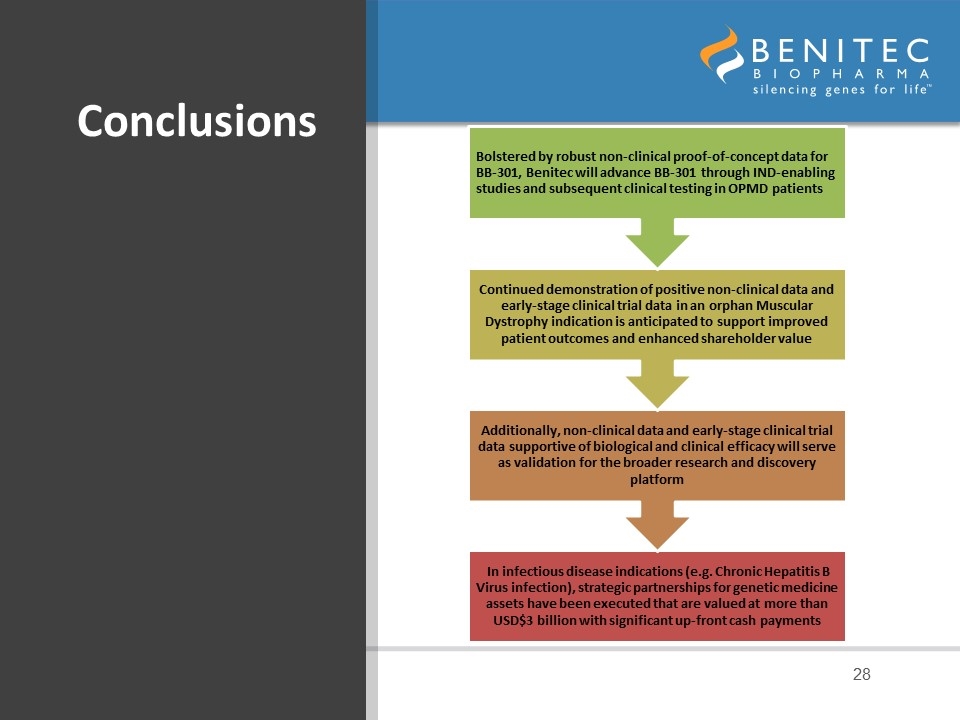
Conclusions Bolstered by robust non-clinical proof-of-concept data for BB-301, Benitec will advance BB-301 through IND-enabling studies and subsequent clinical testing in OPMD patients Continued demonstration of positive non-clinical data and early-stage clinical trial data in an orphan Muscular Dystrophy indication is anticipated to support improved patient outcomes and enhanced shareholder value In infectious disease indications (e.g. Chronic Hepatitis B Virus infection), strategic partnerships for genetic medicine assets have been executed that are valued at more than USD$3 billion with significant up-front cash payments Additionally, non-clinical data and early-stage clinical trial data supportive of biological and clinical efficacy will serve as validation for the broader research and discovery platform

Supplementary Slides September 2019

Late-Stage Non-clinical Asset with Category-leading biological efficacy BB-301 for Oculopharyngeal Muscular Dystrophy Global prevalence of OPMD exceeds 15,000 patients and commercial opportunity exceeds USD$1 billion
Two-Vector “Silence-and-Replace” Approach for the Treatment of OPMD Benitec previously designed a two-vector gene therapy strategy that significantly ameliorated the OPMD-derived pathology in the A17 mouse model of the disease (the A17 mouse model is described on Slide 20) This initial approach was based on the intramuscular injection of two recombinant AAVs, one expressing three short hairpin RNAs (shRNAs) to silence both mutant and wildtype PABPN1 and one expressing a codon-optimized, shRNA-insensitive version of PABPN1 The two recombinant AAV vectors were comprised of (1) a recombinant AAV8 producing short hairpin RNA (shRNAs) to silence endogenous (mutant and wildtype) PABPN1 and (2) an AAV9 vector expressing a codon-optimized version of wildtype PABPN1 (coPABPN1) that takes advantage of amino acid codon degeneracy to produce the wildtype PABPN1 protein from a mRNA that is not targeted by the anti-PABPN1 shRNAs Single administration of either of the two vectors resulted only in partial improvement of the mutant phenotype, however, simultaneous administration of both vectors led to restoration of muscle strength to wildtype levels A two-vector approach for human therapeutic applications is, however, undesirable, as it would require two independent manufacturing processes, which can significantly increase costs, technical risk and introduce potential regulatory challenges as well
Late-Stage Non-clinical Asset for Licensure to Potential Partners BB-103 for Chronic Hepatitis B Virus Infection Category-Leading Biological Efficacy Achieved in Validated animal model
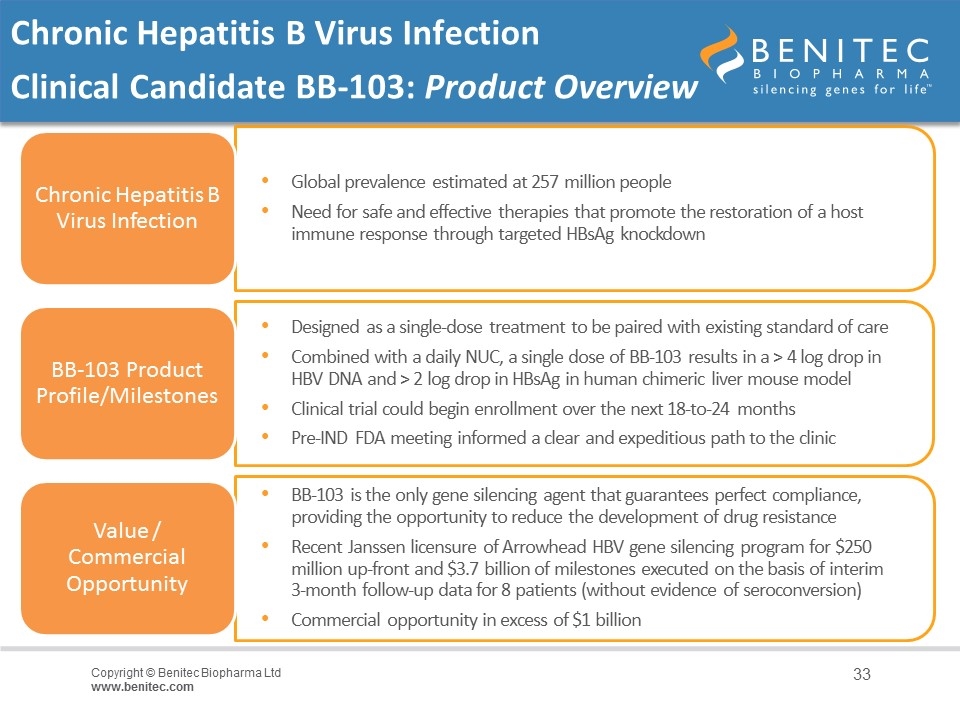
Chronic Hepatitis B Virus Infection Clinical Candidate BB-103: Product Overview Chronic Hepatitis B Virus Infection BB-103 Product Profile/Milestones Designed as a single-dose treatment to be paired with existing standard of care Value / Commercial Opportunity BB-103 is the only gene silencing agent that guarantees perfect compliance, providing the opportunity to reduce the development of drug resistance Global prevalence estimated at 257 million people Commercial opportunity in excess of $1 billion Clinical trial could begin enrollment over the next 18-to-24 months Need for safe and effective therapies that promote the restoration of a host immune response through targeted HBsAg knockdown Combined with a daily NUC, a single dose of BB-103 results in a > 4 log drop in HBV DNA and > 2 log drop in HBsAg in human chimeric liver mouse model Pre-IND FDA meeting informed a clear and expeditious path to the clinic Recent Janssen licensure of Arrowhead HBV gene silencing program for $250 million up-front and $3.7 billion of milestones executed on the basis of interim 3-month follow-up data for 8 patients (without evidence of seroconversion)
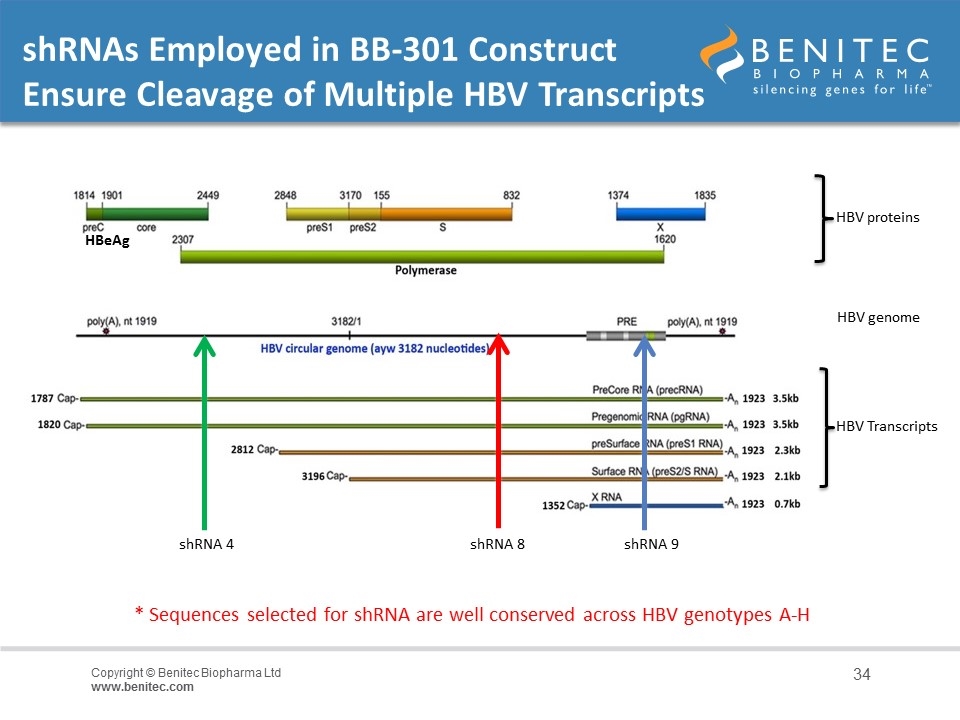
shRNAs Employed in BB-301 Construct Ensure Cleavage of Multiple HBV Transcripts shRNA 9 shRNA 4 HBV proteins HBV Transcripts HBV genome shRNA 8 * Sequences selected for shRNA are well conserved across HBV genotypes A-H HBeAg
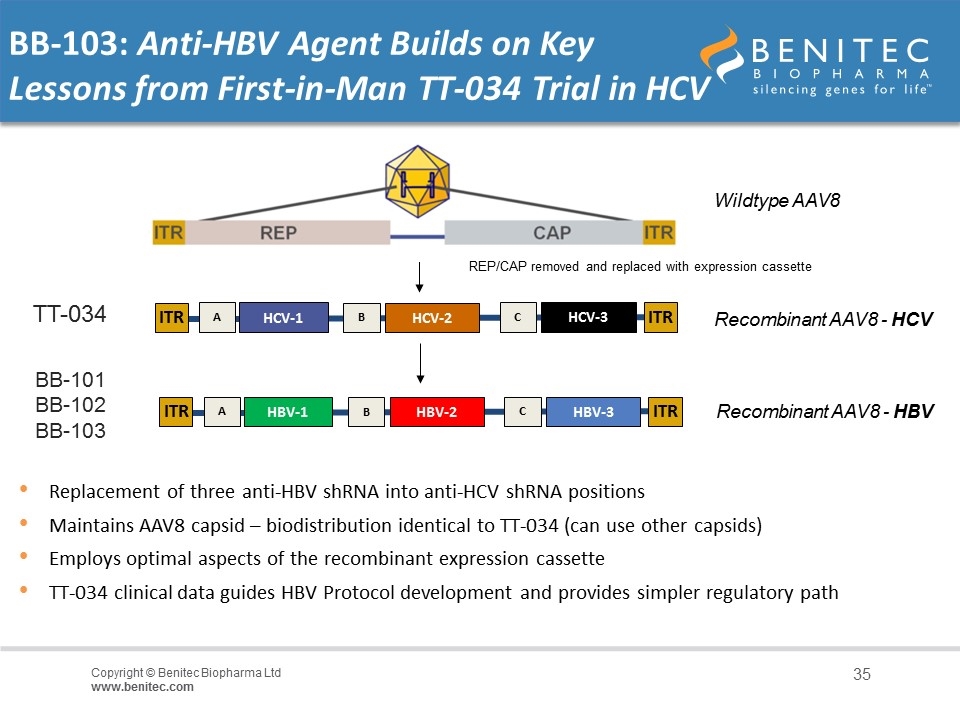
Replacement of three anti-HBV shRNA into anti-HCV shRNA positions Maintains AAV8 capsid – biodistribution identical to TT-034 (can use other capsids) Employs optimal aspects of the recombinant expression cassette TT-034 clinical data guides HBV Protocol development and provides simpler regulatory path BB-101 BB-102 BB-103 ITR ITR A HBV-1 B HBV-2 C HBV-3 Recombinant AAV8 - HBV BB-103: Anti-HBV Agent Builds on Key Lessons from First-in-Man TT-034 Trial in HCV ITR ITR A HCV-1 B HCV-2 C HCV-3 TT-034 Wildtype AAV8 Recombinant AAV8 - HCV REP/CAP removed and replaced with expression cassette
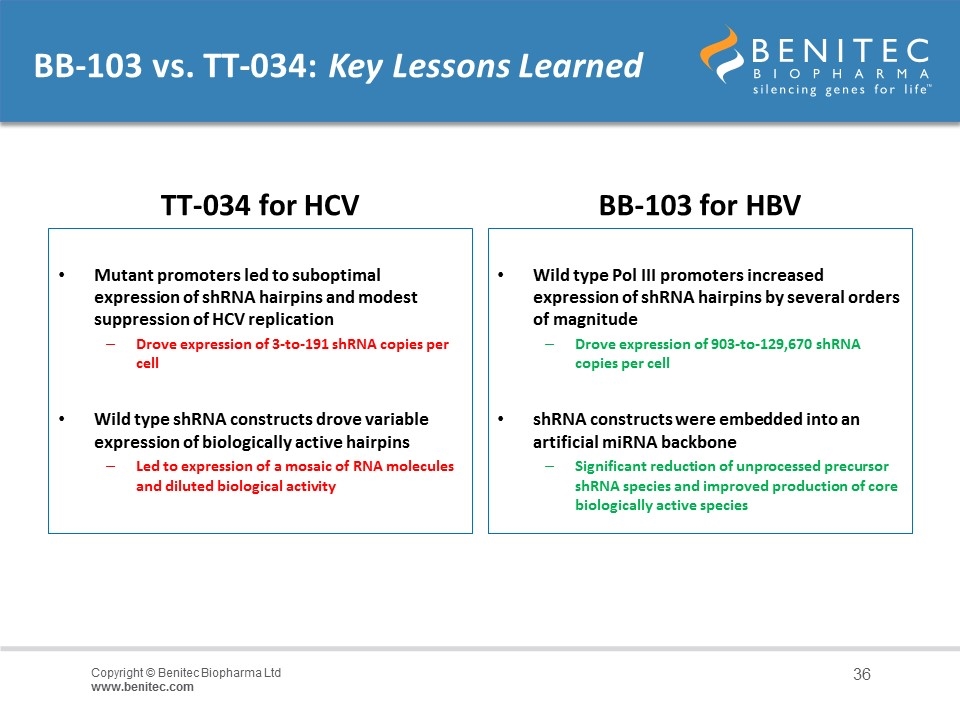
BB-103 vs. TT-034: Key Lessons Learned TT-034 for HCV Mutant promoters led to suboptimal expression of shRNA hairpins and modest suppression of HCV replication Drove expression of 3-to-191 shRNA copies per cell Wild type shRNA constructs drove variable expression of biologically active hairpins Led to expression of a mosaic of RNA molecules and diluted biological activity BB-103 for HBV Wild type Pol III promoters increased expression of shRNA hairpins by several orders of magnitude Drove expression of 903-to-129,670 shRNA copies per cell shRNA constructs were embedded into an artificial miRNA backbone Significant reduction of unprocessed precursor shRNA species and improved production of core biologically active species
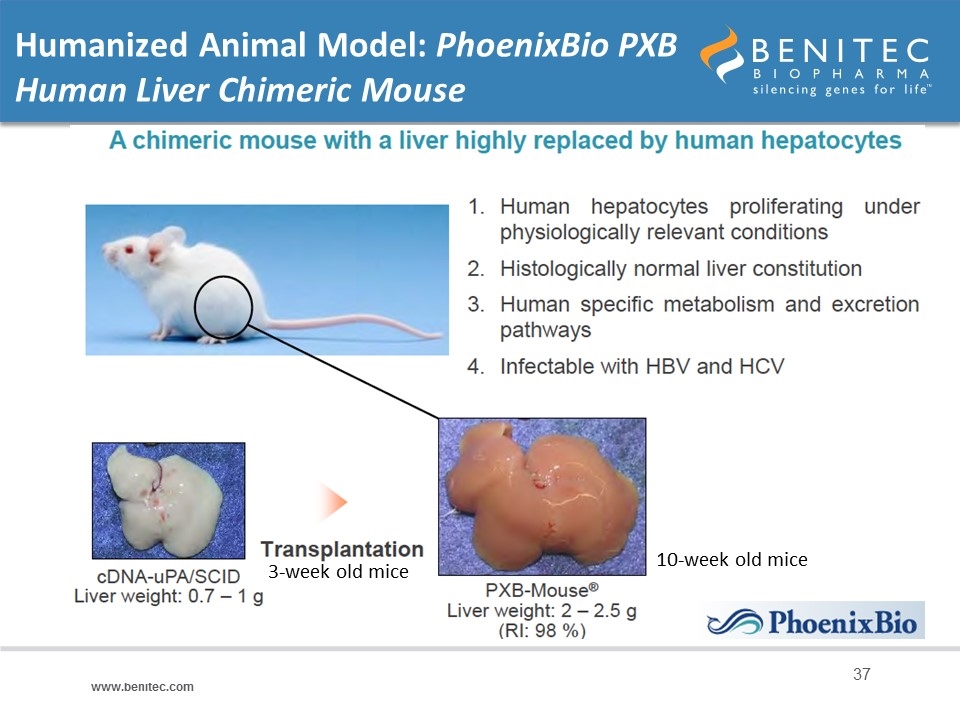
Humanized Animal Model: PhoenixBio PXB Human Liver Chimeric Mouse 3-week old mice 10-week old mice
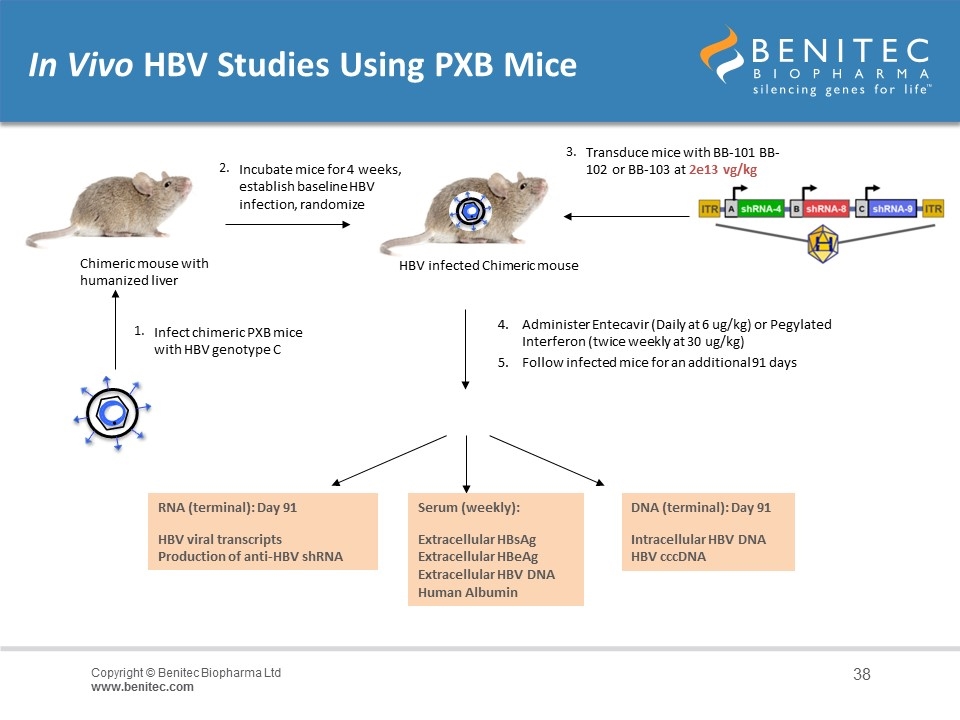
In Vivo HBV Studies Using PXB Mice Chimeric mouse with humanized liver 1. Infect chimeric PXB mice with HBV genotype C 2. Transduce mice with BB-101 BB-102 or BB-103 at 2e13 vg/kg Administer Entecavir (Daily at 6 ug/kg) or Pegylated Interferon (twice weekly at 30 ug/kg) Serum (weekly): Extracellular HBsAg Extracellular HBeAg Extracellular HBV DNA Human Albumin DNA (terminal): Day 91 Intracellular HBV DNA HBV cccDNA RNA (terminal): Day 91 HBV viral transcripts Production of anti-HBV shRNA Incubate mice for 4 weeks, establish baseline HBV infection, randomize HBV infected Chimeric mouse 5. Follow infected mice for an additional 91 days 3.
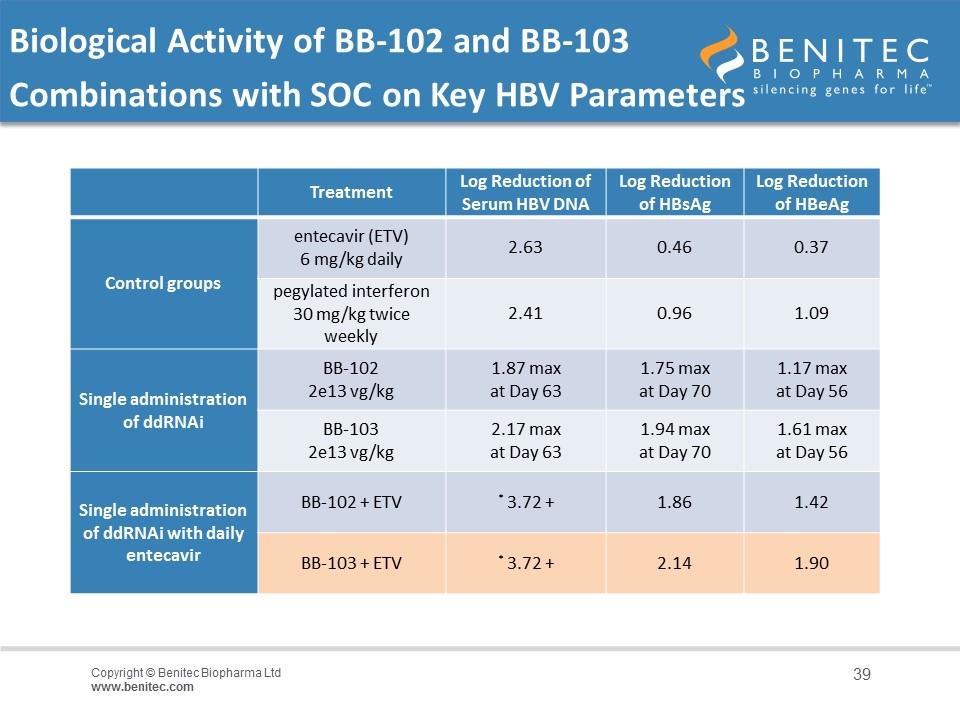
Biological Activity of BB-102 and BB-103 Combinations with SOC on Key HBV Parameters Treatment Log Reduction of Serum HBV DNA Log Reduction of HBsAg Log Reduction of HBeAg Control groups entecavir (ETV) 6 mg/kg daily 2.63 0.46 0.37 pegylated interferon 30 mg/kg twice weekly 2.41 0.96 1.09 Single administration of ddRNAi BB-102 2e13 vg/kg 1.87 max at Day 63 1.75 max at Day 70 1.17 max at Day 56 BB-103 2e13 vg/kg 2.17 max at Day 63 1.94 max at Day 70 1.61 max at Day 56 Single administration of ddRNAi with daily entecavir BB-102 + ETV * 3.72 + 1.86 1.42 BB-103 + ETV * 3.72 + 2.14 1.90






































