balance between advancing near-term opportunities and exploring additional markets for our technology. However, due to the significant resources required for the development of workflows for new markets, we must make decisions regarding which markets to pursue and the amount of resources to allocate to each. Our decisions concerning the allocation of research, development, collaboration, management and financial resources toward particular markets or workflows may not lead to the development of any viable product and may divert resources away from better opportunities. Similarly, our potential decisions to delay, terminate or collaborate with third parties in respect of certain markets may subsequently also prove to be suboptimal and could cause us to miss valuable opportunities. In particular, if we are unable to develop additional relevant products and applications for markets such as antibody therapeutics, cell therapy or the synthetic biology market, it could slow or stop our business growth and negatively impact our business, financial condition, results of operations, and prospects.
If we market our products for clinical or diagnostic purposes, our products could become subject to onerous regulation by the U.S. Food and Drug Administration, or FDA, or other regulatory agencies in the future, which could increase our costs and delay or prevent commercialization of our products, thereby materially and adversely affecting our business, financial condition, results of operations, and prospects.
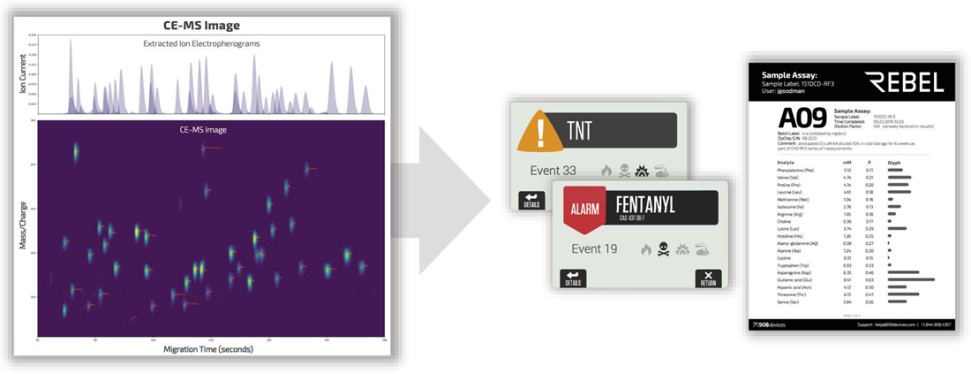
We make our platform and devices, including our MX908, Rebel, and ZipChip Interface, available to customers as research-use-only, or RUO, products. Products that are labeled as RUO are exempt from compliance with most FDA requirements, including premarket clearance or approval, manufacturing requirements, and others. A product labeled RUO but which is actually intended for clinical diagnostic use may be viewed by the FDA as adulterated and misbranded under the Federal Food, Drug, and Cosmetic Act, or FDCA, and subject to FDA enforcement action. The FDA has indicated that when determining the intended use of a product labeled RUO, the FDA will consider the totality of the circumstances surrounding distribution and use of the product, including how the product is marketed and to whom. The FDA could disagree with our assessment that our products are properly marketed as RUOs, or could conclude that products labeled as RUO are actually intended for clinical diagnostic use, and could take enforcement action against us, including requiring us to stop distribution of our products until we are in compliance with applicable regulations, which would reduce our revenue, increase our costs and adversely affect our business, prospects, results of operations and financial condition. In the event that the FDA requires us to obtain marketing authorization of our RUO products in the future, there can be no assurance that the FDA will grant any clearance or approval requested by us in a timely manner, or at all. Furthermore, although we currently market our products as RUO, we may in the future make the decision to market them for clinical or diagnostic purposes, or may develop other different products intended for clinical or diagnostic purposes, which would result in the application of a more onerous set of regulatory requirements.
We depend on our key personnel and other highly qualified personnel, and if we are unable to recruit, train and retain our personnel, we may not achieve our goals.
Our future success depends on our ability to recruit, train, retain and motivate key personnel, including our senior management, research and development, manufacturing and sales, customer service and marketing personnel. In particular, Dr. Knopp, our Chief Executive Officer and one of our co-founders, and Dr. Brown, our Chief Technology Officer and one of our co-founders, are critical to our vision, strategic direction, culture and products. Each of our employees may terminate his or her relationship with us at any time and the loss of the services of such persons could have an adverse effect on our business. We rely on our senior management to manage our existing business operations and to identify and pursue new growth opportunities. The loss of any member of senior management could significantly delay or prevent the achievement of our business objectives and their replacement would likely involve significant time and expense.
As we continue to scale our business, we may find that certain of our products, certain customers or certain markets, including the biopharmaceutical market, may require a dedicated sales force or sales personnel with different experience than those whom we currently employ. Our continued growth will depend, in part, on attracting, retaining and motivating highly-trained sales personnel with the necessary scientific background and technical ability to understand our systems and effectively identify and sell to potential new customers. Identifying, recruiting and training additional qualified personnel will require significant time, expense and attention. In addition, the continued development of