UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-K
(Mark One)
| x | ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 | |
| For the fiscal year ended December 31, 2017 | ||
| or | ||
| o | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 | |
| For the transition period from __________ to __________ | ||
Commission File Number: 001-35797
| Zoetis Inc. |
| (Exact name of registrant as specified in its charter) |
| Delaware | 46-0696167 | |
| (State or other jurisdiction of | (I.R.S. Employer Identification No.) | |
| incorporation or organization) | ||
| 10 Sylvan Way, Parsippany, New Jersey | 07054 | |
| (Address of principal executive offices) | (Zip Code) | |
| (973) 822-7000 |
| (Registrant’s telephone number, including area code) |
Securities registered pursuant to Section 12(b) of the Act:
| Title of each class | Name of each exchange on which registered | ||
| Common Stock, $0.01 par value per share | New York Stock Exchange | ||
Securities registered pursuant to Section 12(g) of the Act: None
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of Securities Act. Yes x No ¨
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes ¨ No x
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes x No ¨
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Website, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). Yes x No ¨
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K (§229.405 of this chapter) is not contained herein, and will not be contained, to the best of the registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. ¨
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company,” and "emerging growth company" in Rule 12b-2 of the Exchange Act. (Check one):
Large accelerated filer x | Accelerated filer ¨ | Non-accelerated filer ¨ | Smaller reporting company ¨ | Emerging growth company ¨ |
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ¨
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes ¨ No x
The aggregate market value of the voting stock held by nonaffiliates of the registrant as of June 30, 2017, the last business day of the registrant's most recently completed second fiscal quarter, was $30,545 million. The registrant has no non-voting common stock.
The number of shares outstanding of the registrant's common stock as of February 9, 2018 was 485,253,713 shares.
DOCUMENTS INCORPORATED BY REFERENCE:
Portions of the registrant’s Proxy Statement for the 2018 Annual Meeting of Shareholders (hereinafter referred to as the “2018 Proxy Statement”) are incorporated into Part III of this Form 10-K.
TABLE OF CONTENTS
| Page | ||||
| Item 1. | ||||
| Item 1A. | ||||
| Item 1B. | ||||
| Item 2. | ||||
| Item 3. | ||||
| Item 4. | ||||
| Item 5. | ||||
| Item 6. | ||||
| Item 7. | ||||
| Item 7A. | ||||
| Item 8. | ||||
| Item 9. | ||||
| Item 9A. | ||||
| Item 9B. | ||||
| Item 10. | ||||
| Item 11. | ||||
| Item 12. | ||||
| Item 13. | ||||
| Item 14. | ||||
| Item 15. | ||||
| Item 16. | ||||
PART I
Item 1. Business.
Overview
Zoetis Inc. is a global leader in the discovery, development, manufacture and commercialization of animal health medicines and vaccines, with a focus on both livestock and companion animals. We have a diversified business, commercializing products across eight core species: cattle, swine, poultry, fish and sheep (collectively, livestock) and dogs, cats and horses (collectively, companion animals); and within five major product categories: anti-infectives, vaccines, parasiticides, medicated feed additives and other pharmaceuticals. For more than 60 years, we have been committed to enhancing the health of animals and bringing solutions to our customers who raise and care for them.
We were incorporated in Delaware in July 2012 and prior to that the company was a business unit of Pfizer Inc. (Pfizer). The address of our principal executive offices is 10 Sylvan Way, Parsippany, New Jersey 07054. Unless the context requires otherwise, references to “Zoetis,” “the company,” “we,” “us” or “our” in this Annual Report on Form 10-K for the fiscal year ended December 31, 2017 (2017 Annual Report) refer to Zoetis Inc., a Delaware corporation, and its subsidiaries. In addition, unless the context requires otherwise, references to “Pfizer” in this 2017 Annual Report refer to Pfizer Inc., a Delaware corporation, and its subsidiaries.
Operating Segments
The animal health medicines and vaccines market is characterized by meaningful differences in customer needs across different regions. This is due to a variety of factors, including:
| • | economic differences, such as standards of living in developed markets as compared to emerging markets; |
| • | cultural differences, such as dietary preferences for different animal proteins, pet ownership preferences and pet care standards; |
| • | epidemiological differences, such as the prevalence of certain bacterial and viral strains and disease dynamics; |
| • | treatment differences, such as utilization of different types of medicines and vaccines, as well as the pace of adoption of new technologies; |
| • | environmental differences, such as seasonality, climate and the availability of arable land and fresh water; and |
| • | regulatory differences, such as standards for product approval and manufacturing. |
As a result of these differences, among other things, we organize and operate our business in two segments:
| • | United States with revenue of $2,620 million, or 49% of total revenue for the year ended December 31, 2017; and |
| • | International with revenue of $2,643 million, or 50% of total revenue for the year ended December 31, 2017. |
Within each of these operating segments, we offer a diversified product portfolio for both livestock and companion animal customers so that we can capitalize on local trends and customer needs.
In addition, our Client Supply Services (CSS) organization provides contract manufacturing services to third parties and represented approximately 1% of our total revenue for the year ended December 31, 2017.
1 |
Our 2017 revenue for the United States and key international markets, together with the percentage of revenue attributable to livestock and companion animal products in those markets, is as follows:
| (MILLIONS OF DOLLARS) | Revenue | Livestock | Companion Animal |
| United States | $2,620 | 47% | 53% |
| Australia | $176 | 62% | 38% |
| Brazil | $300 | 80% | 20% |
| Canada | $184 | 59% | 41% |
| China | $174 | 67% | 33% |
| France | $121 | 60% | 40% |
| Germany | $137 | 50% | 50% |
| Italy | $89 | 49% | 51% |
| Japan | $138 | 46% | 54% |
| Mexico | $86 | 84% | 16% |
| Spain | $93 | 71% | 29% |
| United Kingdom | $149 | 43% | 57% |
| Other Developed | $339 | 66% | 34% |
| Other Emerging | $657 | 83% | 17% |
For additional information regarding our performance in each of these operating segments and the impact of foreign exchange rates, see Item 7. Management's Discussion and Analysis of Financial Condition and Results of Operations and Item 8. Financial Statements and Supplementary Data:
Notes to Consolidated Financial Statements— Note 18A. Segment, Geographic and Other Revenue Information—Segment Information. Our 2017 reported revenue for each segment, by species, is as follows:
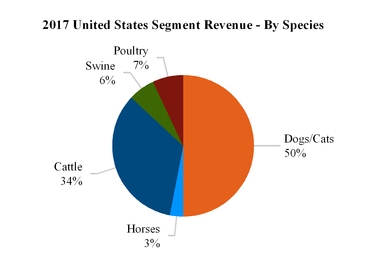

2 |
Products
Over the course of our history, we have focused on developing a diverse portfolio of animal health products, including medicines and vaccines, complemented by biodevices, diagnostics, and genetics. We refer to a single product in all brands, or its dosage forms for all species, as a product line. We have approximately 300 comprehensive product lines, including products for both livestock and companion animals across each of our major product categories.
Our livestock products primarily help prevent or treat diseases and conditions to enable the cost-effective production of safe, high-quality animal protein. Human population growth and increasing standards of living are important long-term growth drivers for our livestock products in three major ways. First, population growth and increasing standards of living drive increased demand for improved nutrition, particularly animal protein. Second, population growth leads to increased natural resource constraints driving a need for enhanced productivity. Finally, as standards of living improve, there is increased focus on food quality and safety. Livestock products represented approximately 57% of our revenue for the year ended December 31, 2017.
Our companion animal products help extend and improve the quality of life for pets; increase convenience and compliance for pet owners; and help veterinarians improve the quality of their care and the efficiency of their businesses. Growth in the companion animal medicines and vaccines sector is driven by economic development, related increases in disposable income and increases in pet ownership and spending on pet care. Companion animals are also living longer, receiving increased medical treatment and benefiting from advances in animal health medicines and vaccines. Companion animal products represented approximately 42% of our revenue for the year ended December 31, 2017.
In addition, our CSS organization provides contract manufacturing services to third parties and represented 1% of our total revenue for the year ended December 31, 2017.
Our major product categories are:
| • | anti-infectives: products that prevent, kill or slow the growth of bacteria, fungi or protozoa; |
• | vaccines: biological preparations that help prevent diseases of the respiratory, gastrointestinal and reproductive tracts or induce a specific immune response; |
| • | parasiticides: products that prevent or eliminate external and internal parasites such as fleas, ticks and worms; |
| • | medicated feed additives: products added to animal feed that provide medicines to livestock; and |
| • | other pharmaceutical products: allergy and dermatology, pain and sedation, antiemetic, reproductive, and oncology products. |
Our remaining revenue is derived from other product categories, such as nutritionals and agribusiness, as well as products and services in complementary areas, including biodevices, diagnostics and genetics.
As part of our growth strategy, through our research and development (R&D) group, we focus on the discovery and development of new chemical and biological entities, as well as product lifecycle innovation. Historically, a substantial portion of our products and revenue has been the result of product lifecycle innovation where we actively work to broaden the value of existing products by developing claims in additional species, more convenient formulations and combinations, and by expanding usage into more countries. For example, the first product in our ceftiofur line was an anti-infective approved for treating bovine respiratory disease (BRD) in cattle that was administered via intramuscular injection. Through follow-on studies and reformulations, we have expanded the product line into additional cattle claims and administration routes, as well as other species and regions. The ceftiofur product line currently includes the brands Excede®, Excenel®, Naxcel® and Spectramast®.
Examples of our first-in-class and/or best-in-class products that we have launched in recent years and products that we believe may represent platforms for future product lifecycle innovation include (listed alphabetically):
| • | Apoquel®, the first Janus kinase inhibitor for use in veterinary medicine, was approved for the control of pruritus associated with allergic dermatitis and the control of atopic dermatitis in dogs at least 12 months of age. Since January 2014, we launched Apoquel in all key markets including the United States, Europe, Japan, Brazil, and Australia; |
| • | Cerenia®, the first and only product on the market to prevent vomiting due to motion sickness in dogs, was first launched in Europe in 2006, followed by the United States in 2007; it was approved to prevent vomiting in cats in 2012 in the United States and European countries. In January 2016, it was approved in the United States for intravenous administration in dogs and cats four months of age and older and for the prevention of vomiting caused by emetogenic or chemotherapeutic agents in dogs four months of age or older; |
| • | Convenia®, the first single-injection anti-infective for common bacterial skin infections in cats and dogs, launched in 2006; |
| • | Cytopoint®, the first canine monoclonal antibody to help reduce the clinical signs such as itching of atopic dermatitis in dogs of any age, licensed in the United States in 2016 and Canada, the European Union and New Zealand in 2017. An injection given once every four to eight weeks, Cytopoint neutralizes interleukin - 31, a protein that has been demonstrated to trigger itching in dogs. |
| • | Fostera® PCV MH was introduced in November 2013 in the United States and approved in the European Union in 2015 and Australia in 2017. It was developed to help protect pigs from porcine circovirus-associated disease (PCVAD) and enzootic pneumonia caused by M. hyopneumoniae (M. hyo). The one-bottle formulation of Fostera PCV MH allows the convenience of a one-dose program or the flexibility of a two-dose program. The Fostera franchise also includes Fostera/Suvaxyn® PRRS, which was approved in the United States in 2015 and in Taiwan, Vietnam and European Union countries in 2017. This vaccine offers protection against both the respiratory and reproductive forms of disease caused by porcine reproductive and respiratory syndrome (PRRS) virus. |
| • | Improvac®/Improvest®/Vivax®, a protein product that works like an immunization, is currently the only product that provides a safe and effective alternative to physical castration to manage unpleasant aromas that can occur when cooking pork; launched in Australia and New Zealand in 2004, in Brazil in 2007, in certain European countries beginning in 2008, and in the United States in 2011; |
3 |
| • | Inforce®3, the first vaccine for cattle that prevents respiratory disease caused by bovine respiratory syncytial virus (BRSV) while also aiding in the prevention of infectious bovine rhinotracheitis (IBR) and parainfluenza 3 (PI 3), launched in 2010; |
| • | Simparica® (sarolaner) Chewables, a monthly chewable tablet for dogs to control fleas and ticks, was approved in the European Union and New Zealand in 2015, the United States, Canada, Australia, and Brazil (Simparic) in 2016, and Japan along with multiple additional European, Latin American and Asia Pacific markets in 2017. Building on this franchise, in 2017, Zoetis received European Commission approval for Stronghold® Plus (selamectin/sarolaner), a topical combination product that treats ticks, fleas, ear mites, lice and gastrointestinal worms and prevents heartworm disease in cats; |
| • | Vanguard® is a market leading vaccine line for dogs intended to help prevent a range of diseases. In recent years, Zoetis has added new and innovative enhancements to its Vanguard line with Vanguard crLyme, Vanguard Rapid Resp Intranasal, Vanguard B Oral, and Vanguard CIV H3N2/H3N8. |
We pursue the development of new vaccines for emerging infectious diseases, with an operating philosophy of “first to know and fast to market.” Examples of the successful execution of this strategy include the first equine vaccine for West Nile virus in the United States and European Union; the first swine vaccine for pandemic H1N1 influenza virus in the United States; the first fully licensed vaccine to help reduce disease caused by the Georgia 08 variant of infectious bronchitis virus (IBV) in poultry; a conditionally licensed vaccine to help fight porcine epidemic diarrhea virus (PEDv) in the United States, and the first conditionally licensed vaccine to help prevent the H3N2 type of canine influenza that emerged in the United States. Examples also include the first and only vaccine to aid in the prevention of clinical symptoms of the disease caused by Hendra virus in horses, a serious zoonotic disease identified in Australia that can be fatal to horses and people; a conditionally licensed vaccine in the United States for use in poultry as an aid in the prevention of avian influenza virus H5N1; the first centrally-authorized vaccine in the European Union to reduce viremia associated with Schmallenberg virus infection in cattle and sheep; and the first live recombinant marker vaccine in the European Union and United States to prevent mortality and reduce infection caused by Classical Swine Fever in pigs. Additionally, the Pharmaq business of Zoetis is the global leader in vaccines and innovation for health products in aquaculture. In 2017, Pharmaq added to its leading Alpha Ject® vaccine line with Alpha Ject® micro 1 PD vaccine, an efficacious and safe monovalent vaccine against Pancreas Disease (PD), the most economically damaging viral disease for the Norwegian, Scottish and Irish salmon farming industries, proven to be suitable for co-injection with other Pharmaq vaccines.
Our diverse portfolio also includes diagnostics products such as the Witness® and Serelisa® lines of immunodiagnostic kits. In 2017, we expanded both product lines with the launch of Witness Lepto (canine) in the United States and the launch of Witness FeLV FIV Heartworm (feline) globally. We also expanded our Serelisa® laboratory kit line with Serelisa PEDv (swine) for porcine epidemic diarrhea virus and Serelisa BVDv (cattle), which provides additional disease detection capabilities for bovine viral diarrhea virus.
In 2017, our top two selling products, Apoquel® and the ceftiofur line, each contributed approximately 7% of our revenue, and combined with our next two top selling products, Draxxin® and Revolution®, these four contributed approximately 25% of our revenue. Our top ten product lines contributed 39% of our revenue.
4 |
Our product lines and products that represented approximately 1% or more of our revenue in 2017, which comprise 57% of our total revenue, are as follows (listed alphabetically):
Livestock products
| Product line / product | Description | Primary species | ||
| Anti-infectives | ||||
| Ceftiofur injectable line | Broad-spectrum cephalosporin antibiotic active against gram-positive and gram-negative bacteria, including ß-lactamase-producing strains, with some formulations producing a single course of therapy in one injection | Cattle, sheep, swine | ||
Draxxin® | Single-dose low-volume antibiotic for the treatment and prevention of bovine and swine respiratory disease, infectious bovine keratoconjunctivitis and bovine foot rot | Cattle, swine, sheep | ||
Spectramast® | Treatment of subclinical or clinical mastitis in dry or lactating dairy cattle, delivered via intramammary infusion; same active ingredient as the ceftiofur line | Cattle | ||
Terramycin® line | Antibiotic for the treatment of susceptible infections | Cattle, poultry, sheep, swine | ||
| Vaccines | ||||
Bovi-Shield® line | Aids in preventing diseases, including infectious bovine rhinotracheitis (IBR), bovine viral diarrhea (BVD) Types 1 and 2, parainfluenza 3 (PI 3), bovine respiratory syncytial virus (BRSV), and leptospirosis caused by Leptospira borgpetersenii, L.canicola, L grippotyphosa, L. hardjo, L. icterohaemorrhagiae, and L. pamona, depending on formulation | Cattle | ||
| Improvac / Improvest / Vivax | Reduces boar taint, as an alternative to surgical castration | Swine | ||
Rispoval® line | Aids in preventing three key viruses involved in cattle pneumonia-BRSV, PI 3 virus and BVD-viruses as well as other respiratory diseases, depending on formulation | Cattle | ||
Suvaxyn® / Fostera® | Aids in preventing or controlling disease associated with major pathogens in swine such as porcine circovirus type 2 (PCV2), porcine reproductive and respiratory syndrome virus (PRRSv) and Mycoplasma hyopneumoniae, depending on formulation | Swine | ||
| Parasiticides | ||||
Cydectin® | Injectable or pour-on endectocide to treat and control internal and external cattle parasites, including gastrointestinal roundworms, lungworms, cattle grubs, mites and lice | Cattle, sheep | ||
Dectomax® | Injectable or pour-on endectocide, characterized by extended duration of activity, for the treatment and control of internal and external parasite infections | Cattle, swine | ||
| Medicated Feed Additives | ||||
Aureomycin® | Provides livestock producers control, treatment and convenience against a wide range of respiratory, enteric and reproductive diseases | Cattle, poultry, sheep, swine | ||
BMD® | Aids in preventing and controlling enteritis; and increases rate of weight gain and improves feed efficiency in poultry and swine | Poultry, swine | ||
| Lasalocid line | Controls coccidiosis in poultry (Avatec®) and cattle (Bovatec®) and for increased rate of weight gain and improved feed efficiency in cattle | Poultry, cattle | ||
| Lincomycin line | Controls necrotic enteritis; treatment of dysentery (bloody scours), control of ileitis and treatment/reduction in severity of mycoplasmal pneumonia | Swine, poultry | ||
| Other | ||||
Embrex® devices | Devices for enhancing hatchery operations' efficiency through in ovo detection and vaccination | Poultry | ||
Lutalyse® | For estrus control or in the induction of parturition or abortion | Cattle, swine | ||
5 |
Companion animal products
| Product line / product | Description | Primary species | ||
| Anti-infectives | ||||
Clavamox® / Synulox® | A broad-spectrum antibiotic and the first and only potentiated penicillin approved for use in dogs and cats | Cats, dogs | ||
Convenia® | Anti-infective for the treatment of common bacterial skin infections that provides a course of treatment in a single injection | Cats, dogs | ||
| Vaccines | ||||
Vanguard® L4 (4-way Lepto) | Compatible with the Vanguard line and helps protect against leptospirosis caused by Leptospira canicola, L. grippotyphosa, L. icterohaemorrhagiae and L. pomona | Dogs | ||
Vanguard® line | Aids in preventing canine distemper caused by canine distemper virus; infectious canine hepatitis caused by canine adenovirus type 1; respiratory disease caused by canine adenovirus type 2; canine parainfluenza caused by canine parainfluenza virus; canine parvoviral enteritis caused by canine parvovirus; Lyme disease and subclinical arthritis associated with Borrelia burgdorferi, the causative agent of Lyme disease; and Rapid Resp - a group of three vaccines combating infections in dogs caused by Bordetella bronchiseptica, canine parainfluenza and canine adenovirus; canine influenza vaccines; and an oral vaccine for Bordatella bronchiseptica | Dogs | ||
| Parasiticides | ||||
ProHeart® | Prevents heartworm infestation; also for treatment of existing larval and adult hookworm infections | Dogs | ||
Revolution® / Stronghold® | An antiparasitic for protection against fleas, heartworm disease and ear mites in cats and dogs; sarcoptic mites and American dog tick in dogs and roundworms and hookworms for cats | Cats, dogs | ||
Simparica® | A monthly chewable tablet for dogs to control fleas and ticks | Dogs | ||
| Other | ||||
Apoquel® | A selective inhibitor of the Janus Kinase 1 enzyme that controls pruritus associated with allergic dermatitis and control of atopic dermatitis in dogs at least 12 months of age | Dogs | ||
Cerenia® | A medication that prevents and treats acute vomiting in dogs, treats acute vomiting in cats and prevents vomiting due to motion sickness in dogs | Cats, dogs | ||
Cytopoint® | An injectable to help reduce the clinical signs such as itching of atopic dermatitis in dogs of any age | Dogs | ||
Rimadyl® | For the relief of pain and inflammation associated with osteoarthritis and for the control of postoperative pain associated with soft tissue and orthopedic surgeries | Dogs | ||
International Operations
We directly market our products in approximately 45 countries across North America, Europe, Africa, Asia, Australia and South America, and our products are sold in more than 100 countries. Operations outside the United States accounted for 50% of our total revenue for the year ended December 31, 2017. Through our efforts to establish an early and direct presence in many emerging markets, such as Brazil, China and Mexico, emerging markets contributed 23% of our revenue for the year ended December 31, 2017.
Our international businesses are subject, in varying degrees, to a number of risks inherent in carrying on business in other countries. These include, among other things, currency fluctuations, capital and exchange control regulations, expropriation and other restrictive government actions. See Item 1A. Risk Factors— Risks related to our international operations.
Sales and Marketing
Our sales organization includes sales representatives and technical and veterinary operations specialists. In markets where we do not have a direct commercial presence, we generally contract with distributors that provide logistics and sales and marketing support for our products.
Our sales representatives visit our customers, including veterinarians and livestock producers, to provide information and to promote and sell our products and services. Our technical and veterinary operations specialists, who generally have advanced veterinary medicine degrees, provide scientific consulting focused on disease management and herd management, training and education on diverse topics, including responsible product use. These direct relationships with customers allow us to understand the needs of our customers. Additionally, our sales representatives and technical and veterinary operations specialists partner with customers to provide training and support in areas of disease awareness and treatment protocols, including through the use of our products. As a result of these relationships, our sales and consulting visits are typically longer, more meaningful and provide us with better access to customer decision makers as compared to human health. As of December 31, 2017, our sales organization consisted of approximately 2,900 employees.
Our livestock and companion animal products are primarily available by prescription through a veterinarian. On a more limited basis, in certain markets, we sell certain products through local agricultural and farming retail outlets, pharmacies and pet stores. We also market our products by advertising to veterinarians, livestock producers and pet owners.
6 |
Customers
We sell our livestock products directly to a diverse set of livestock producers, including beef and dairy farmers as well as pork and poultry operations, and to veterinarians, third-party veterinary distributors and retail outlets that then typically sell the products to livestock producers. We primarily sell our companion animal products to veterinarians or to third-party veterinary distributors that typically then sell our products to veterinarians, and in each case veterinarians then typically sell our products to pet owners. Our two largest customers, both distributors, represented approximately 14% and 7%, respectively, of our revenue for the year ended December 31, 2017, and no other customer represented more than 6% of our revenue for the same period.
Research and Development
Our research and development (R&D) operations are comprised of a dedicated veterinary medicine R&D organization, research alliances and other operations focused on the development, registration and regulatory maintenance of our products. We incurred R&D expense of $382 million in 2017, $376 million in 2016 and $364 million in 2015.
Our R&D efforts are comprised of more than 200 programs and reflect our commitment to develop better solutions. We create new insights for preventing and treating disease, and maximizing healthy performance, that result in the development of new platforms of knowledge which become the basis for continuous innovation. Leveraging internal discoveries, complemented by diverse external research collaborations, results in the delivery of novel vaccine, pharmaceutical and biopharmaceutical products to help our customers face their toughest challenges. While the development of new chemical and biological entities through new product R&D plays a critical role in our growth strategies, a significant share of our R&D investment (including regulatory functions) is focused on product lifecycle innovation. A commitment to continuous innovation, based on customer need, ensures we actively work to broaden the value of existing products by developing claims in additional species, more convenient formulations and combinations, and by expanding usage into more countries. We also create opportunities to optimize solutions through our extensive capabilities in diagnostics and genetics research, ensuring we can help our customers predict, prevent, detect and treat a variety of conditions.
We prioritize our R&D spending on an annual basis with the goal of aligning our research and business objectives, and do not disaggregate our R&D operations by research stage or by therapeutic area for purposes of managing our business. We make our strategic investments in R&D based on four criteria: strategic fit and importance to our current portfolio; technical feasibility of development and manufacture; return on investment; and the needs of customers and the market. A centralized portfolio management function links development plans with financial systems to build a comprehensive view of the status of project progression and spend. This view facilitates our ability to set targets for project timing and goals for investment efficiency. The allocation of our R&D investment between product lifecycle innovation and new product development, in addition to our ability to leverage the discoveries of our existing R&D and other industries, supports a cost-effective, efficient, sustainable and relatively predictable R&D process.
We regularly enter into agreements with external parties that enable us to collaborate on research programs or gain access to substrates and technologies. Some of our external partnerships involve funding from a non-governmental organization or a government grant. We are generally responsible for providing technical direction and supplemental expertise for, as well as investment in, such external partnerships. Depending on the nature of the agreement, we may act as the commercialization partner for discoveries that originate during the period of collaborative research, or we may own or have exclusive rights to any intellectual property that enables the development of proprietary products or models.
As of December 31, 2017, we employed approximately 1,000 employees in our global R&D operations. Our R&D headquarters is located in Kalamazoo, Michigan. We have R&D operations co-located with manufacturing sites in Louvain-la-Neuve, Belgium; Campinas, Brazil; Olot, Spain; Kalamazoo, Michigan; and Lincoln, Nebraska, United States. We co-locate R&D operations with manufacturing sites to facilitate the efficient transfer of production processes from our laboratories to manufacturing. In addition, we maintain R&D operations in Sydney, Australia; Zaventem, Belgium; São Paulo, Brazil; Beijing, China; Navi Mumbai, India; and Durham, North Carolina, United States. We also maintain R&D operations in Farum, Denmark, Suzhou, China; Thanh Binh, Vietnam; Hong Ngu, Vietnam; and Oslo, Norway, related to our acquisitions of Pharmaq and Scandinavian Micro Biodevices. Each site is designed to meet the regulatory requirements for working with chemical or infectious disease agents.
Manufacturing and Supply Chain
Our products are manufactured at both sites operated by us and sites operated by third-party contract manufacturing organizations, which we refer to as CMOs. We have a global manufacturing network of 25 sites.
7 |
Our global manufacturing network is comprised of the following sites:
| Site | Location | Site | Location | |||
| Campinas | Brazil | Melbourne | Australia | |||
| Catania | Italy | Olot | Spain | |||
| Charles City | Iowa, U.S. | Oslo | Norway | |||
| Chicago Heights | Illinois, U.S. | Overhalla | Norway | |||
| Durham | North Carolina, U.S. | Rathdrum(b) | Ireland | |||
| Eagle Grove | Iowa, U.S. | Salisbury | Maryland, U.S. | |||
| Farum | Denmark | San Diego | California, U.S. | |||
Jilin(a) | China | Suzhou | China | |||
| Kalamazoo | Michigan, U.S. | Tullamore(c) | Ireland | |||
| Lincoln | Nebraska, U.S. | Wellington | New Zealand | |||
| London | Ontario, Canada | White Hall | Illinois, U.S. | |||
| Louvain-la-Neuve | Belgium | Willow Island | West Virginia, U.S. | |||
| Medolla | Italy | |||||
(a) | In September 2017, Zoetis acquired the remaining noncontrolling interest in our China joint venture, Jilin Zoetis Guoyan Animal Health Company, Ltd. |
(b) | In September 2017, Zoetis completed the purchase of a manufacturing facility in Rathdrum, Ireland. We are investing in this facility and expect it to be ready for commercial production in 2020. |
(c) | In July 2017, Zoetis acquired a biologic therapeutics company in Ireland. We will be investing in this facility to prepare it for commercial production. |
We own all of these sites, with the exception of our facilities in Medolla (Italy), Melbourne (Australia), San Diego, California (U.S.) and Tullamore (Ireland), which are leased sites.
In addition to our global manufacturing network and our CMOs, Pfizer continues to manufacture products for us at four Pfizer sites pursuant to a master manufacturing and supply agreement.
Our global manufacturing and supply chain is supported by a network of CMOs. As of December 31, 2017, this network was comprised of approximately 180 CMOs, including those centrally managed as well as local CMOs.
We select CMOs based on several factors: (i) their ability to reliably supply products or materials that meet our quality standards at an optimized cost; (ii) their access to niche products and technologies; (iii) capacity; and (iv) financial efficiency analyses. Our regional and global manufacturing teams seek to ensure that all of the CMOs we use adhere to our standards of manufacturing quality.
We purchase certain raw materials necessary for the commercial production of our products from a variety of third-party suppliers. We utilize logistics service providers as a part of our global supply chain, primarily for shipping and logistics support.
We intend to continue our efficiency improvement programs in our manufacturing and supply chain organization, including Six Sigma and Lean capabilities, which are processes intended to improve manufacturing efficiency. We have strong globally managed and coordinated quality control and quality assurance programs in place at our global manufacturing network sites, and we regularly inspect and audit our global manufacturing network and CMO sites. As a result of a review of our global manufacturing and supply network, we have announced plans to exit or sell certain sites and have exited eight manufacturing sites since 2015, including Yantai (China) and Guarulhos (Brazil) in 2017. See Operational Efficiency Program and Supply Network Strategy.
Competition
Although our business is the largest based on revenue in the animal health medicines and vaccines industry, we face competition in the regions in which we compete. Principal drivers of competition vary depending on the particular region, species, product category and individual product, and include new product development, quality, price, service and promotion to veterinary professionals, pet owners and livestock producers.
Our primary competitors include animal health medicines and vaccines companies such as Boehringer Ingelheim Animal Health Inc., the animal health division of Boehringer Ingelheim GmbH, which acquired Merial, former animal health division of Sanofi S.A., in January 2017; Merck Animal Health, the animal health division of Merck & Co., Inc.; Elanco, the animal health division of Eli Lilly and Company; and Bayer Animal Health, the animal health division of Bayer AG. There are also several new start-up companies working in the animal health area. In addition, we compete with hundreds of other producers of animal health products throughout the world.
The level of competition from generic products varies from market to market. For example, the level of generic competition is higher in Europe and certain emerging markets than in the United States. Unlike in the human health market, there is no large, well-capitalized company focused on generic animal health products that exists as a global competitor in the industry. The reasons for this include the relatively smaller average market size of each product opportunity, the importance of direct distribution and education to veterinarians and livestock producers and the primarily self-pay nature of the business. In addition, companion animal health products are often directly prescribed and dispensed by veterinarians.
The importance of quality and safety concerns to pet owners, veterinarians and livestock producers also contributes to animal health brand loyalty. As a result, we believe that significant brand loyalty to products often continues after the loss of patent-based and regulatory exclusivity.
8 |
Intellectual Property
Our technology, brands and other intellectual property are important elements of our business. We rely on patent, trademark, copyright and trade secret laws, as well as regulatory exclusivity periods and non-disclosure agreements to protect our intellectual property rights. Our policy is to vigorously protect, enforce and defend our rights to our intellectual property, as appropriate.
Our product portfolio enjoys the protection of approximately 5,200 granted patents and 1,700 pending patent applications, filed in more than 60 countries, with a focus on our major markets, including Australia, Brazil, Canada, China, Europe, Japan and the United States, as well as other countries with strong patent systems. Many of the patents and patent applications in our portfolio are the result of our in-house research and development, while other patents and patent applications in our portfolio were wholly or partially developed by third parties and are licensed to Zoetis.
Patents for individual products expire at different times based on the date of the patent filing (or sometimes the date of patent grant) and the legal term of patents in the countries where such patents are obtained. The active ingredient of Draxxin, tulathromycin, is covered by both compound and formulation patents in the United States, Europe, Canada, Australia and other key markets, with terms that expire between May 2019 and January 2021 in the United States, between November 2018 and November 2020 in Europe, and between May 2018 and November 2020 in Canada and Australia. Several patents covering the ceftiofur antibiotic product line (Excede) began expiring in the United States in 2015. However, various formulation and use patents relevant to the product line extend through to 2024. The compound patent for selamectin, the active ingredient in our parasiticide Revolution, expired in 2014. Again, we have process and formulation patents covering this product which expire in important markets in 2018 and 2019, respectively. The patent for the active ingredient of Convenia has expired, however, there are formulation patents relevant to the product line which expire between November 2022 and October 2023. The patent for the active ingredient of Cerenia has expired, however, there are formulation patents relevant to the product line which expire between May 2020 and January 2027. The patent relating to the formulation of Orbeseal expired in December 2017. Zoetis typically enforces all of its patents.
Additionally, many of our vaccine products are based on proprietary master seeds and proprietary or patented adjuvant formulations. We actively seek to protect our proprietary information, including our trade secrets and proprietary know-how, including by seeking to require our employees, consultants, advisors and partners to enter into confidentiality agreements and other arrangements upon the commencement of their employment or engagement.
As a result of our separation from Pfizer, where necessary Pfizer has licensed to us the right to use certain intellectual property rights in the animal health field. We license to Pfizer the right to use certain of our trademarks and substantially all of our other intellectual property rights in the human health field and all other fields outside of animal health. In addition, Pfizer granted us a perpetual license to use certain of Pfizer's product name trademarks.
We seek to file and maintain trademarks around the world based on commercial activities in most regions where we have, or desire to have, a business presence for a particular product or service. We currently maintain more than 10,000 trademark applications and registrations in major regions, identifying goods and services dedicated to the care of livestock and companion animals.
Operational Efficiency Program and Supply Network Strategy
During 2015, we launched a comprehensive operational efficiency program, which was incremental to the previously announced supply network strategy. These initiatives have focused on reducing complexity in our product portfolios through the elimination of approximately 5,000 product stock keeping units (SKUs), changing our selling approach in certain markets, reducing our presence in certain countries, and planning to sell or exit 10 manufacturing sites over the long term. As of December 31, 2017, we divested or exited three U.S. manufacturing sites, four international manufacturing sites, and our 55 percent ownership share of a Taiwan joint venture, inclusive of its related manufacturing site. We are also continuing to optimize our resource allocation and efficiency by reducing resources associated with non-customer facing activities and operating more efficiently as a result of less internal complexity and more standardization of processes.
As part of these initiatives, we planned to reduce certain positions through divestitures, normal attrition and involuntary terminations by approximately 2,000 to 2,500, subject to consultations with works councils and unions in certain countries. In 2016, the operations of the Guarulhos, Brazil manufacturing site, including approximately 300 employees, were transferred to us from Pfizer, which increased our range of planned reduction in certain positions to 2,300 to 2,800. Including divestitures, as of December 31, 2017, approximately 2,600 positions have been eliminated. The comprehensive operational efficiency program is substantially complete, however in the fourth quarter of 2017, we expanded the supply network strategy initiative which increases our planned reductions in certain positions by 40. We expect these additional reductions related to our supply network strategy to take place over the next twelve months, and the remainder of the reductions from the initial plan to take place through divestitures over the next several years.
Regulatory
The sale of animal health products is governed by the laws and regulations specific to each country in which we sell our products. To maintain compliance with these regulatory requirements, we have established processes, systems and dedicated resources with end-to-end involvement from product concept to launch and maintenance in the market. Our regulatory function actively seeks to engage in dialogue with various global agencies regarding their policies that relate to animal health products. In the majority of our markets, the relevant animal health authority is separate from those governing human medicinal products.
United States
United States Food and Drug Administration (FDA). The regulatory body that is responsible for the regulation of animal health pharmaceuticals in the United States is the Center for Veterinary Medicine (CVM), housed within the FDA. All manufacturers of animal health pharmaceuticals must show their products to be safe, effective and produced by a consistent method of manufacture as defined under the Federal Food, Drug and Cosmetic
9 |
Act. The FDA's basis for approving a drug application is documented in a Freedom of Information Summary. Post-approval monitoring of products is required by law, with reports being provided to the CVM's Surveillance and Compliance group. Reports of product quality defects, adverse events or unexpected results are produced in accordance with the law. Additionally, we are required to submit all new information for a product, regardless of the source.
United States Department of Agriculture (USDA). The regulatory body in the United States for veterinary vaccines is the USDA. The USDA's Center for Veterinary Biologics is responsible for the regulation of animal health vaccines, including immunotherapeutics. All manufacturers of animal health biologicals must show their products to be pure, safe, effective and produced by a consistent method of manufacture as defined under the Virus Serum Toxin Act. Post-approval monitoring of products is required. Reports of product quality defects, adverse events or unexpected results are produced in accordance with the agency requirements.
Environmental Protection Agency (EPA). The main regulatory body in the United States for veterinary pesticides is the EPA. The EPA's Office of Pesticide Programs is responsible for the regulation of pesticide products applied to animals. All manufacturers of animal health pesticides must show their products will not cause “unreasonable adverse effects to man or the environment” as stated in the Federal Insecticide, Fungicide, and Rodenticide Act. Within the United States, pesticide products that are approved by the EPA must also be approved by individual state pesticide authorities before distribution in that state. Post-approval monitoring of products is required, with reports provided to the EPA and some state regulatory agencies.
In addition, the U.S. Foreign Corrupt Practices Act (FCPA) prohibits U.S. corporations and their representatives from offering, promising, authorizing or making payments to any foreign government official, government staff member, political party or political candidate in an attempt to obtain or retain business abroad. The scope of the FCPA includes interactions with certain healthcare professionals in many countries. Other countries have enacted similar anti-corruption laws and/or regulations.
Outside the United States
European Union (EU). The European Medicines Agency (EMA) is the centralized regulatory agency of the EU, located in London. The agency is responsible for the scientific evaluation of medicines developed by healthcare companies seeking centralized approval for use in the EU. The agency has a veterinary review section distinct from the medical review section. The Committee for Veterinary Medicinal Products (CVMP) is responsible for scientific and technical review of the submissions for innovative pharmaceuticals, biopharmaceuticals and vaccines. After the CVMP issues a positive opinion on the approvability of a product, the EU commission reviews the opinion and, if they agree with the CVMP, they grant the product market authorization. Once granted by the European Commission, a centralized marketing authorization is valid in all EU and European Economic Area-European Free Trade Association states. Products can also be registered in the EU via a decentralized route under the supervision of the Co-ordination Group for Mutual Recognition and Decentralized Procedures - Veterinary (CMDv). This co-ordination group is composed of one representative per member state from each national regulatory agency, including Norway, Iceland and Liechtenstein. The CMDv reviews submissions of pharmaceuticals and vaccines for authorization of a veterinary product in two or more member states in accordance with the mutual recognition or the decentralized procedure. A series of Regulations, Directives, Guidelines and EU Pharmacopeia Monographs provide the requirements for product approval in the EU. In general, these requirements are similar to those in the United States, requiring demonstrated evidence of, safety, efficacy, and quality/consistency of manufacturing processes.
Brazil. The Ministry of Agriculture, Livestock Production and Supply (MAPA) is the regulatory body in Brazil that is responsible for the regulation and control of pharmaceuticals, biologicals and medicated feed additives for animal use. MAPA's regulatory activities are conducted through the Secretary of Agricultural Defense and its Livestock Products Inspection Department. In addition, regulatory activities are conducted at a local level through the Federal Agriculture Superintendence. These activities include the inspection and licensing of both manufacturing and commercial establishments for veterinary products, as well as the submission, review and approval of pharmaceuticals, biologicals and medicated feed additives. MAPA is one of the most active regulatory agencies in Latin America, having permanent seats at several international animal health forums, such as Codex Alimentarius, World Organization for Animal Health and Committee of Veterinary Medicines for the Americas. MAPA was also invited to be a Latin American representative at meetings of the International Cooperation on Harmonisation of Technical Requirements for Registration of Veterinary Medicinal Products (VICH). Several normative instructions issued by MAPA have set regulatory trends in Latin America.
Australia. The Australian Pesticides and Veterinary Medicines Authority (APVMA) is an Australian government statutory authority established in 1993 to centralize the registration of all agricultural and veterinary products into the Australian marketplace. Previously each State and Territory government had its own system of registration. The APVMA assesses applications from companies and individuals seeking registration so they can supply their product to the marketplace. Applications undergo rigorous assessment using the expertise of the APVMA's scientific staff and drawing on the technical knowledge of other relevant scientific organizations, Commonwealth government departments and state agriculture departments. If the product works as intended and the scientific data confirms that when used as directed on the product label it will have no harmful or unintended effects on people, animals, the environment or international trade, the APVMA will register the product. As well as registering new agricultural and veterinary products, the APVMA reviews older products that have been on the market for a substantial period of time to ensure they still do the job users expect and are safe to use. The APVMA also reviews registered products when particular concerns are raised about their safety and effectiveness. The review of a product may result in confirmation of its registration, or it may see registration continue with some changes to the way the product can be used. In some cases the review may result in the registration of a product being canceled and the product taken off the market.
Rest of world. Country-specific regulatory laws have provisions that include requirements for certain labeling, safety, efficacy and manufacturers' quality control procedures (to assure the consistency of the products), as well as company records and reports. With the exception of the EU, most other countries' regulatory agencies will generally refer to the FDA, USDA, EU and other international animal health entities, including the World Organization for Animal Health, Codex Alimentarius, in establishing standards and regulations for veterinary pharmaceuticals and vaccines.
Global policy and guidance
Joint FAO/WHO Expert Committee on Food Additives. The Joint FAO/WHO Expert Committee on Food Additives is an international expert scientific committee that is administered jointly by the Food and Agriculture Organization of the United Nations (FAO) and the World Health
10 |
Organization (WHO). They provide a risk assessment/safety evaluation of residues of veterinary drugs in animal products, exposure and residue definition and maximum residue limit proposals for veterinary drugs. We work with them to establish acceptable safe levels of residual product in food-producing animals after treatment. This in turn enables the calculation of appropriate withdrawal times for our products prior to an animal entering the food chain.
Advertising and promotion review. Promotion of prescription animal health products is controlled by regulations in many countries. These rules generally restrict advertising and promotion to those claims and uses that have been reviewed and endorsed by the applicable agency. We conduct a review of promotion materials for compliance with the local and regional requirements in the markets where we sell animal health products.
Food Safety Inspection Service/generally recognized as safe. The FDA is authorized to determine the safety of substances (including “generally recognized as safe” substances, food additives and color additives), as well as prescribing safe conditions of use. However, although the FDA has the responsibility for determining the safety of substances, the Food Safety and Inspection Service, the public health agency in the USDA, still retains, under the tenets of the Federal Meat Inspection Act and the Poultry Products Inspection Act and their implementing regulations, the authority to determine that new substances and new uses of previously approved substances are suitable for use in meat and poultry products.
International Cooperation on Harmonisation of Technical Requirements for Registration of Veterinary Medicinal Products (VICH). VICH is a trilateral (EU-Japan-USA) program aimed at harmonizing technical requirements for veterinary product registration. The objectives of the VICH are as follows:
| • | Establish and implement harmonized technical requirements for the registration of veterinary medicinal products in the VICH regions, which meet high quality, safety and efficacy standards and minimize the use of test animals and costs of product development. |
| • | Provide a basis for wider international harmonization of registration requirements through the VICH Outreach Forum. |
| • | Monitor and maintain existing VICH guidelines, taking particular note of the ICH work program and, where necessary, update these VICH guidelines. |
| • | Ensure efficient processes for maintaining and monitoring consistent interpretation of data requirements following the implementation of VICH guidelines. |
| • | By means of a constructive dialogue between regulatory authorities and industry, provide technical guidance enabling response to significant emerging global issues and science that impact on regulatory requirements within the VICH regions. |
Employees
As of December 31, 2017, we had approximately 9,200 employees worldwide, which included approximately 3,950 employees in the United States and approximately 5,250 in other jurisdictions. Some of these employees are members of unions, works councils, trade associations or are otherwise subject to collective bargaining agreements, including approximately 50 union employees in the United States.
Environmental, Health and Safety
We are subject to various federal, state, local and foreign environmental, health and safety laws and regulations. These laws and regulations govern matters such as the emission and discharge of hazardous materials into the ground, air or water; the generation, use, storage, handling, treatment, packaging, transportation, exposure to, and disposal of hazardous and biological materials, including recordkeeping, reporting and registration requirements; and the health and safety of our employees. Due to our operations, these laws and regulations also require us to obtain, and comply with, permits, registrations or other authorizations issued by governmental authorities. These authorities can modify or revoke our permits, registrations or other authorizations and can enforce compliance through fines and injunctions.
Certain environmental laws, such as the U.S. Comprehensive Environmental Response, Compensation and Liability Act of 1980, as amended (CERCLA), impose joint and several liability, without regard to fault, for cleanup costs on persons who disposed of or released hazardous substances into the environment, including at third-party sites or offsite disposal locations, or that currently own or operate (or formerly owned or operated) sites where such a release occurred. In addition to clean-up actions brought by federal, state, local and foreign governmental entities, private parties could raise personal injury or other claims against us due to the presence of, or exposure to, hazardous materials on, from or otherwise relating to such a property.
We have made, and intend to continue to make, necessary expenditures for compliance with applicable environmental, health and safety laws and regulations. We are also a party to proceedings in which the primary relief sought is the cost of past and/or future remediation, or remedial measures to mitigate or remediate pollution. In connection with such proceedings, and otherwise, we are investigating and cleaning up environmental contamination from past industrial activity at certain sites, or financing other parties' completion of such activities. As a result, we incurred capital and operational expenditures in 2017 for environmental compliance purposes and for the clean-up of certain past industrial activities as follows:
• | environmental-related capital expenditures - approximately $6 million; and |
• | other environmental-related expenditures - approximately $9 million. |
However, we may not have identified all of the potential environmental liabilities relating to our current and former properties, or those liabilities associated with off-site disposal locations. Such liability could have a material adverse effect on our operating results and financial condition. Furthermore, regulatory agencies are showing increasing concern over the impact of animal health products and livestock operations on the environment. This increased regulatory scrutiny may necessitate that additional time and resources be spent to address these concerns in both new and existing products.
In connection with past acquisitions and divestitures, we have undertaken certain indemnification obligations that require us, or may require us in the future, to conduct or finance environmental cleanups at sites that we no longer own or operate. We have also entered into indemnification agreements
11 |
in which we are being indemnified for various environmental cleanups; however, such indemnities are limited in both time and scope and may be further limited in the presence of new information, or may not be available at all.
While we cannot predict with certainty our future capital expenditures or operating costs for environmental compliance or remediation of contaminated sites, we have no reason to believe that they will have a material adverse effect on our operating results or financial condition.
Available Information
The company's Internet website address is www.zoetis.com. On our website, the company makes available, free of charge, its annual, quarterly and current reports, including amendments to such reports, as soon as reasonably practicable after the company electronically files such material with, or furnishes such material to, the Securities and Exchange Commission (SEC).
Also available on our website is information relating to corporate governance at Zoetis and our Board of Directors, including as follows: our Corporate Governance Principles; Director Qualification Standards; Zoetis Code of Conduct (for all of our employees, including our Chief Executive Officer, Chief Financial Officer, Principal Accounting Officer, and Controller); Code of Business Conduct and Ethics for our Directors; Board Committees and Committee Charters; and ways to communicate by email with our Directors. We will provide any of the foregoing information without charge upon written request to our Corporate Secretary, Zoetis Inc., 10 Sylvan Way, Parsippany, New Jersey 07054. Information relating to shareholder services is also available on our website. We will disclose any future amendments to, or waivers from, provisions of these ethics policies and standards affecting our Chief Executive Officer, Chief Financial Officer, Principal Accounting Officer, and Controller on our website as promptly as practicable, as may be required under applicable SEC and NYSE rules.
We use our website (www.zoetis.com) as a means of disclosing material non-public information and for complying with our disclosure obligations under Regulation Fair Disclosure promulgated by the SEC. These disclosures are included in the “Investors” and “News & Media” sections of our website. Accordingly, investors should monitor these portions of our website, in addition to following our press releases, SEC filings and public conference calls and webcasts.
The information contained on our website does not constitute, and shall not be deemed to constitute, a part of this 2017 Annual Report, or any other report we file with, or furnish to, the SEC. Our references to the URLs for websites are intended to be inactive textual references only.
Item 1A. Risk Factors.
In addition to the other information set forth in this 2017 Annual Report, any of the factors described below could materially adversely affect our operating results, financial condition and liquidity, which could cause the trading price of our securities to decline.
This report contains “forward-looking” statements within the meaning of the Private Securities Litigation Reform Act of 1995. Forward-looking statements reflect our current views with respect to, among other things, future events and performance. We generally identify forward-looking statements by words such as “anticipate,” “estimate,” “could,” “expect,” “intend,” “project,” “plan,” “predict,” “believe,” “seek,” “continue,” “outlook,” "objective," "target," “may,” “might,” “will,” “should,” “can have,” “likely” or the negative version of these words or comparable words or by using future dates in connection with any discussion of future performance, actions or events. Forward-looking statements are based on beliefs and assumptions made by management using currently available information. These statements are not guarantees of future performance, actions or events.
In particular, forward-looking statements include statements relating to our 2018 financial guidance, future actions, business plans or prospects, prospective products, product approvals or products under development, product supply disruptions, R&D costs, timing and likelihood of success, future operating or financial performance, future results of current and anticipated products and services, strategies, sales efforts, expenses, production efficiencies, production margins, integration of acquired businesses, interest rates, tax rates, changes in tax regimes and laws, foreign exchange rates, growth in emerging markets, the outcome of contingencies, such as legal proceedings, plans related to share repurchases and dividends, our agreements with Pfizer, government regulation and financial results. Forward-looking statements are subject to risks and uncertainties, many of which are beyond our control, and are potentially inaccurate assumptions. However, there may also be other risks that we are unable to predict at this time. If one or more of these risks or uncertainties materialize, or if management's underlying beliefs and assumptions prove to be incorrect, actual results may differ materially from those contemplated by a forward-looking statement. You should not put undue reliance on forward-looking statements. Forward-looking statements speak only as of the date on which they are made.
We undertake no obligation to publicly update forward-looking statements, whether as a result of new information, future events or otherwise, except as required by law or by the rules and regulations of the SEC. You are advised, however, to consult any further disclosures we make on related subjects in our Form 10-Q and 8-K reports and our other filings with the SEC. We note these factors for investors as permitted by the Private Securities Litigation Reform Act of 1995. You should understand that it is not possible to predict or identify all such factors. Consequently, you should not consider the following to be a complete discussion of all potential risks or uncertainties.
Risks related to our business and industry
Restrictions and bans on the use of and consumer preferences regarding antibacterials in food-producing animals may become more prevalent.
The issue of the potential transfer of increased antibacterial resistance in bacteria from food-producing animals to human pathogens, and the causality of that transfer, continue to be the subject of global scientific and regulatory discussion. Antibacterials refer to small molecules that can be used to treat or prevent bacterial infections and are a sub-categorization of the products that make up our anti-infectives and medicated feed additives portfolios. In some countries, this issue has led to government restrictions and bans on the use of specific antibacterials in some food-producing animals, regardless of the route of administration (in feed or injectable). These restrictions are more prevalent in countries where animal protein is plentiful and governments are willing to take action even when there is scientific uncertainty. Our total revenue attributable to antibacterials for livestock was approximately $1.2 billion for the year ended December 31, 2017.
12 |
For example, in December 2013, the FDA announced final guidance establishing procedures for the voluntary phase-out in the United States over a three-year period of the use of medically important antibacterials in animal feed for growth promotion in food production animals (medically important antibacterials include classes that are prescribed in animal and human health). The guidance provides for continued use of antibacterials in food producing animals for treatment, control and under certain circumstances for prevention of disease, all under the supervision of a veterinarian. The FDA indicated that it took this action to help preserve the efficacy of medically important antibacterials to treat infections in humans. As part of those efforts, stricter regulations governing the administration of medically important antibiotics have recently come into effect. As of January 1, 2017, the use of medically important antibiotics in the water or feed of food production animals now requires written authorization by a licensed veterinarian under the FDA guidance and the related rule known as the Veterinary Feed Directive. As a result of the implementation by livestock producers of the FDA guidance and the Veterinary Feed Directive, we have seen a negative impact on revenue in the U.S. on certain medicated feed additive products for both cattle and swine in 2017. If these regulations continue to negatively affect our U.S. cattle and swine medicated feed additive revenue, our future operating results could be negatively impacted.
In addition, other countries, such as France and Vietnam, have passed restrictions or bans on antibiotic use.
In certain markets, there has been an increase in consumer preference towards proteins produced without the use of antibiotics.
We cannot predict whether antibacterial resistance concerns will result in additional restrictions or bans, expanded regulations, public pressure to discontinue or reduce use of antibacterials in food-producing animals or increased consumer preference for antibiotic-free protein, any of which could materially adversely affect our operating results and financial condition.
Perceived adverse effects on human health linked to the consumption of food derived from animals that utilize our products could cause a decline in the sales of such products.
Our livestock business depends heavily on a healthy and growing livestock industry. If the public perceives a risk to human health from the consumption of the food derived from animals that utilize our products, there may be a decline in the production of such food products and, in turn, demand for our products. For example, livestock producers may experience decreased demand for their products or reputational harm as a result of evolving consumer views of animal rights, nutrition, health-related or other concerns. Any reputational harm to the livestock industry may also extend to companies in related industries, including our company. Adverse consumer views related to the use of one or more of our products in livestock also may result in a decrease in the use of such products and could have a material adverse effect on our operating results and financial condition.
Animal health products are subject to unanticipated safety, quality or efficacy concerns, which may harm our reputation.
Unanticipated safety, quality or efficacy concerns can arise with respect to animal health products, whether or not scientifically or clinically supported, leading to product recalls, withdrawals or suspended or declining sales, as well as product liability and other claims.
Regulatory actions based on these types of safety, quality or efficacy concerns could impact all or a significant portion of a product’s sales and could, depending on the circumstances, materially adversely affect our operating results.
In addition, since we depend on positive perceptions of the safety, quality and efficacy of our products, and animal health products generally, by our customers, veterinarians and end-users, any concerns as to the safety, qualify or efficacy of our products, whether actual or perceived, may harm our reputation. These concerns and the related harm to our reputation could materially adversely affect our operating results and financial condition, regardless of whether such reports are accurate.
Increased regulation or decreased governmental financial support relating to the raising, processing or consumption of food-producing animals could reduce demand for our livestock products.
Companies in the livestock industries are subject to extensive and increasingly stringent regulations. If livestock producers are adversely affected by new regulations or changes to existing regulations, they may reduce herd sizes or become less profitable and, as a result, they may reduce their use of our products, which may materially adversely affect our operating results and financial condition. Furthermore, new or more stringent regulations could, directly or indirectly, impact the use of one or more of our products. More stringent regulation of the livestock industry or our products could have a material adverse effect on our operating results and financial condition. Also, many food-producing companies, including livestock producers, benefit from governmental subsidies, and if such subsidies were to be reduced or eliminated, these companies may become less profitable and, as a result, may reduce their use of our products.
An outbreak of infectious disease carried by animals could negatively affect the sale and production of our products.
Sales of our livestock products could be materially adversely affected by the outbreak of disease carried by animals, which could lead to the widespread death or precautionary destruction of animals as well as the reduced consumption and demand for animal protein. In addition, outbreaks of disease carried by animals may reduce regional or global sales of particular animal-derived food products or result in reduced exports of such products, either due to heightened export restrictions or import prohibitions, which may reduce demand for our products due to reduced herd or flock sizes. In recent years, outbreaks of various diseases, including avian influenza, foot-and-mouth disease, bovine spongiform encephalopathy (otherwise known as BSE or mad cow disease) and porcine epidemic diarrhea virus (otherwise known as PEDv), have impacted the animal health business. The discovery of additional cases of any of these, or new, diseases may result in additional restrictions on animal proteins, reduced herd sizes, or reduced demand for, animal protein, which may have a material adverse effect on our operating results and financial condition. Also, the outbreak of any highly contagious disease near our main production sites could require us to immediately halt production of our products at such sites or force us to incur substantial expenses in procuring raw materials or products elsewhere.
Consolidation of our customers and distributors could negatively affect the pricing of our products.
Veterinarians and livestock producers are our primary customers. In recent years, there has been a trend towards the concentration of veterinarians in large clinics and hospitals. In addition, livestock producers, particularly swine and poultry producers, and our distributors, have seen recent consolidation in their industries. Furthermore, we have seen the expansion of larger cross-border corporate customers and an increase in the
13 |
consolidation of buying groups (cooperatives of veterinary practices that leverage volume to pursue discounts from manufacturers). The pace of consolidation and structure of markets varies greatly across geographies. If these trends towards consolidation continue, these customers and distributors could attempt to improve their profitability by leveraging their buying power to obtain favorable pricing. The resulting decrease in our prices could have a material adverse effect on our operating results and financial condition.
Our business may be negatively affected by weather conditions and the availability of natural resources.
The animal health industry and demand for many of our animal health products in particular regions are affected by weather conditions, as usage of our products follows varying weather patterns and weather-related pressures from pests, such as ticks. As a result, we may experience regional and seasonal fluctuations in our results of operations.
In addition, veterinary hospitals and practitioners depend on visits from and access to animals under their care. Veterinarians’ patient volume and ability to operate could be adversely affected if they experience prolonged snow, ice or other weather conditions, particularly in regions not accustomed to sustained inclement weather. Furthermore, livestock producers depend on the availability of natural resources, including large supplies of fresh water. Their animals' health and their ability to operate could be adversely affected if they experience a shortage of fresh water due to human population growth or floods, droughts or other weather conditions. In the event of adverse weather conditions or a shortage of fresh water, veterinarians or livestock producers may purchase less of our products.
For example, the widespread drought that impacted the United States in 2011, 2012 and in some regions in 2013 was considered the worst in many years, resulting in a reduction in the total cow herd in 2013. Droughts such as this one can lead to a decrease in harvested corn and higher corn prices, which may impact the profitability of livestock producers of cattle, pork and poultry. Higher corn prices may contribute to reductions in herd or flock sizes that may result in reduced spending on animal health products. In addition, droughts can lead to reduced availability of grazing pastures, forcing cattle producers to cull their herds. Fewer heads of cattle could result in reduced demand for our products. A prolonged drought could have a material adverse effect on our operating results and financial condition.
Our business is subject to risk based on global economic conditions.
Macroeconomic, business and financial disruptions could have a material adverse effect on our operating results, financial condition and liquidity. Certain of our customers and suppliers could be affected directly by an economic downturn and could face credit issues or cash flow problems that could give rise to payment delays, increased credit risk, bankruptcies and other financial hardships that could decrease the demand for our products or hinder our ability to collect amounts due from customers. If one or more of our large customers, including distributors, discontinue their relationship with us as a result of economic conditions or otherwise, our operating results and financial condition may be materially adversely affected. In addition, economic concerns may cause some pet owners to forgo or defer visits to veterinary practices or could reduce their willingness to treat pet health conditions or even to continue to own a pet. Furthermore, our exposure to credit and collectability risk is higher in certain international markets and our ability to mitigate such risks may be limited. While we have procedures to monitor and limit exposure to credit and collectability risk, there can be no assurances such procedures will effectively limit such risk and avoid losses.
Our results of operations are dependent upon the success of our top products.
If any of our top products experience issues, such as loss of patent protection, material product liability litigation, new or unexpected side effects, regulatory proceedings, labeling changes, negative publicity, changes to veterinarian or customer preferences, and/or disruptive innovations or the introduction of more effective products, our revenues could be negatively impacted, perhaps significantly. Our top four products, Apoquel, the ceftiofur product line, Draxxin and Revolution, contributed approximately 25% of our revenue in 2017. Any issues with these top products would have a more significant impact to our results of operations.
Modification of U.S. foreign trade policy may harm our U.S. livestock product customers.
Changes in U.S. laws, agreements and policies governing foreign trade in the territories and countries where our customers do business could negatively impact such customers’ businesses and adversely affect our operating results. A number of our customers, particularly U.S.-based livestock producers, benefit from free trade agreements such as the North American Free Trade Agreement (NAFTA). The current President of the United States has initiated negotiations with Canada and Mexico aimed at re-negotiating the terms of NAFTA. Efforts by the United States to withdraw from or materially modify NAFTA or other international trade agreements to which it is a party could harm our customers, and as a result, negatively impact our financial condition and results of operations.
Our business is subject to risk based on customer exposure to rising costs and reduced customer income.
Feed, fuel and transportation and other key costs for livestock producers may increase or animal protein prices or sales may decrease. Either of these trends could cause deterioration in the financial condition of our livestock product customers, potentially inhibiting their ability to purchase our products or pay us for products delivered. Our livestock product customers may offset rising costs by reducing spending on our products, including by switching to lower-cost alternatives to our products. In addition, concerns about the financial resources of pet owners also could cause veterinarians to alter their treatment recommendations in favor of lower-cost alternatives to our products. These shifts could result in a decrease in sales of our companion animal products, especially in developed countries where there is a higher rate of pet ownership.
Changes in distribution channels for companion animal products could negatively impact our market share, margins and distribution of our products.
In most markets, companion animal owners typically purchase their animal health products directly from veterinarians. Companion animal owners increasingly have the option to purchase animal health products from sources other than veterinarians, such as Internet-based retailers, “big-box” retail stores or other over-the-counter distribution channels. This trend has been demonstrated by the significant shift away from the veterinarian distribution channel in the sale of flea and tick products in recent years. Companion animal owners also could decrease their reliance on, and visits to, veterinarians as they rely more on Internet-based animal health information. Because we market our companion animal prescription products through the veterinarian distribution channel, any decrease in visits to veterinarians by companion animal owners could reduce our market share for such
14 |
products and materially adversely affect our operating results and financial condition. In addition, companion animal owners may substitute human health products for animal health products if human health products are deemed to be lower-cost alternatives.
Legislation has also been proposed in the United States, and may be proposed in the United States or abroad in the future, that could impact the distribution channels for our companion animal products. For example, such legislation may require veterinarians to provide pet owners with written prescriptions and disclosure that the pet owner may fill prescriptions through a third party, which may further reduce the number of pet owners who purchase their animal health products directly from veterinarians. Such requirements may lead to increased use of generic alternatives to our products or the increased substitution of our products with other animal health products or human health products if such other products are deemed to be lower-cost alternatives. Many states already have regulations requiring veterinarians to provide prescriptions to pet owners upon request and the American Veterinary Medical Association has long-standing policies in place to encourage this practice.
Over time, these and other competitive conditions may increase our reliance on Internet-based retailers, “big-box” retail stores or other over-the-counter distribution channels to sell our companion animal products. We may be unable to sustain our current margins and we may not be adequately prepared or able to distribute our products if an increased portion of our sales is through these channels. Any of these events could materially adversely affect our operating results and financial condition.
The animal health industry is highly competitive.
The animal health industry is highly competitive. We believe many of our competitors are conducting R&D activities in areas served by our products and in areas in which we are developing products. Our competitors include the animal health businesses of large pharmaceutical companies and specialty animal health businesses. There are also several new start-up companies working in the animal health area. These competitors may have access to greater financial, marketing, technical and other resources. As a result, they may be able to devote more resources to developing, manufacturing, marketing and selling their products, initiating or withstanding substantial price competition or more readily taking advantage of acquisitions or other opportunities. In addition to competition from established market participants, new entrants to the animal health medicines and vaccines industry could substantially reduce our market share or render our products obsolete.
To the extent that any of our competitors are more successful with respect to any key competitive factor or we are forced to reduce, or are unable to raise, the price of any of our products in order to remain competitive, our operating results and financial condition could be materially adversely affected. Competitive pressure could arise from, among other things, safety and efficacy concerns, limited demand growth or a significant number of additional competitive products being introduced into a particular market, price reductions by competitors, the ability of competitors to capitalize on their economies of scale, the ability of competitors to produce or otherwise procure animal health products at lower costs than us and the ability of competitors to access more or newer technology than us.
Generic products may be viewed as more cost-effective than our products.
We face competition from products produced by other companies, including generic alternatives to our products. We depend on patents and regulatory data exclusivity periods to provide us with exclusive marketing rights for some of our products. Patents for individual products expire at different times based on the date of the patent filing (or sometimes the date of patent grant) and the legal term of patents in the countries where such patents are obtained. The extent of protection afforded by our patents varies from country to country and is limited by the scope of the claimed subject matter of our patents, the term of the patent and the availability and enforcement of legal remedies in the applicable country. As a result, we may face competition from lower-priced generic alternatives to many of our products. Generic competitors are becoming more aggressive in terms of launching at risk before patent rights expire and, because of their pricing, are an increasing percentage of overall animal health sales in certain regions. For example, several companies have launched generic versions of our Rimadyl chewable product. As a result, sales of our Rimadyl chewable product in the U.S. have continued to decline, decreasing by approximately 8% in 2017 compared to the prior year. If animal health customers increase their use of new or existing generic products, our operating results and financial condition could be materially adversely affected.
Over the next several years, several of our products' patents will expire. The active ingredient of Draxxin, tulathromycin, is covered by both compound and formulation patents in the United States, Europe, Canada, Australia and other key markets, with terms that expire between May 2019 and January 2021 in the United States, between November 2018 and November 2020 in Europe, and between May 2018 and November 2020 in Canada and Australia. Several patents covering the ceftiofur antibiotic product line (Excede) began expiring in the United States in 2015. However, various formulation and use patents relevant to the product line extend through to 2024. The compound patent for selamectin, the active ingredient in our parasiticide Revolution, expired in 2014. Again, we have process and formulation patents covering this product which expire in important markets in 2018 and 2019, respectively. The ceftiofur product line, Draxxin and Revolution, contributed approximately 18% of our revenue in 2017. In addition, the patent for the active ingredient of Convenia® has expired, however, there are formulation patents relevant to the product line which expire between November 2022 and October 2023. The patent for the active ingredient of Cerenia has expired, however, there are formulation patents relevant to the product line which expire between May 2020 and January 2027. A generic version of Cerenia has recently been registered in Europe and is marketed in the Netherlands and France. The patent relating to the formulation of Orbeseal expired in December 2017. Zoetis typically enforces all of its patents.
We may not successfully acquire and integrate other businesses, license rights to technologies or products, form and manage alliances or divest businesses.
We pursue acquisitions, technology licensing arrangements, strategic alliances or divestitures of some of our businesses as part of our business strategy. We may not complete these transactions in a timely manner, on a cost-effective basis or at all. In addition, we may be subject to regulatory constraints or limitations or other unforeseen factors that prevent us from realizing the expected benefits. Even if we are successful in making an acquisition, the products and technologies that are acquired may not be successful or may require significantly greater resources and investments than originally anticipated. We may be unable to integrate acquisitions successfully into our existing business, and we may be unable to achieve expected gross margin improvements or efficiencies. We also could incur or assume significant debt and unknown or contingent liabilities. Our reported results of operations could be negatively affected by acquisition or disposition-related charges, amortization of expenses related to intangibles and charges for impairment of long-term assets. We may be subject to litigation in connection with, or as a result of, acquisitions, dispositions, licenses or other
15 |
alliances, including claims from terminated employees, customers or third parties, and we may be liable for future or existing litigation and claims related to the acquired business, disposition, license or other alliance because either we are not indemnified for such claims or the indemnification is insufficient. These effects could cause us to incur significant expenses and could materially adversely affect our operating results and financial condition.
We may not successfully implement our business strategies.
We are pursuing, and will continue to pursue, strategic initiatives that management considers critical to our long-term success, including, but not limited to, increasing sales in emerging markets; operational revenue growth through new product development and value-added product lifecycle innovation; using cash flow from operations to service debt; and expanding our complementary products and services. We also have acquired or partnered with a number of smaller animal health businesses, and we intend to continue to do so in the future. There are significant risks involved with the execution of these initiatives, including significant business, economic and competitive uncertainties, many of which are outside of our control. Accordingly, we cannot predict whether we will succeed in implementing these strategic initiatives. It could take several years to realize the anticipated benefits from these initiatives, if any benefits are achieved at all. Additionally, our business strategy may change from time to time, which could delay our ability to implement initiatives that we believe are important to our business.
Our business could be adversely affected by labor disputes, strikes or work stoppages.
Some of our employees are members of unions, works councils, trade associations or are otherwise subject to collective bargaining agreements in certain jurisdictions, including the United States. As a result, we are subject to the risk of labor disputes, strikes, work stoppages and other labor-relations matters. We may be unable to negotiate new collective bargaining agreements on similar or more favorable terms and may experience work stoppages or other labor problems in the future at our sites. We could experience a disruption of our operations or higher ongoing labor costs, which could have a material adverse effect on our operating results and financial condition, potentially resulting in canceled orders by customers, unanticipated inventory accumulation or shortages and reduced revenue and net income. We may also experience difficulty or delays in implementing changes to our workforce in certain markets. In addition, labor problems at our suppliers or CMOs could have a material adverse effect on our operating results and financial condition.
Loss of our executive officers or other key personnel could disrupt our operations.
We depend on the efforts of our executive officers and certain key personnel. Our executive officers and other key personnel are not currently, and are not expected to be, subject to non-compete provisions. In addition, we generally do not enter into employment agreements with our executive officers and other key personnel. Any unplanned turnover or our failure to develop an adequate succession plan for one or more of our executive officer or other key positions could deplete our institutional knowledge base and erode our competitive advantage. The loss or limited availability of the services of one or more of our executive officers or other key personnel, or our inability to recruit and retain qualified executive officers or other key personnel in the future, could, at least temporarily, have a material adverse effect on our operating results and financial condition.
We may be required to write down goodwill or identifiable intangible assets.
Under accounting principles generally accepted in the United States of America (U.S. GAAP), if we determine goodwill or identifiable intangible assets are impaired, we will be required to write down these assets and record a non-cash impairment charge. As of December 31, 2017, we had goodwill of $1.5 billion and identifiable intangible assets, less accumulated amortization, of $1.3 billion. Identifiable intangible assets consist primarily of developed technology rights, brands, trademarks, license agreements, patents, acquired customer relationships and in-process R&D.
Determining whether an impairment exists and the amount of the potential impairment involves quantitative data and qualitative criteria that are based on estimates and assumptions requiring significant management judgment. Future events or new information may change management's valuation of an intangible asset in a short amount of time. The timing and amount of impairment charges recorded in our consolidated statements of income and write-downs recorded in our consolidated balance sheets could vary if management's conclusions change. Any impairment of goodwill or identifiable intangible assets could have a material adverse effect on our operating results and financial position.
Risks related to research and development
Our R&D, acquisition and licensing efforts may fail to generate new products and product lifecycle innovations.
Our future success depends on both our existing product portfolio and our pipeline of new products, including new products that we may develop through joint ventures and products that we are able to obtain through license or acquisition. We commit substantial effort, funds and other resources to R&D, both through our own dedicated resources and through collaborations with third parties.
We may be unable to determine with accuracy when or whether any of our products now under development will be approved or launched, or we may be unable to develop, license or otherwise acquire product candidates or products. In addition, we cannot predict whether any products, once launched, will be commercially successful or will achieve sales and revenue that are consistent with our expectations. The animal health industry is subject to regional and local trends and regulations and, as a result, products that are successful in some of our markets may not achieve similar success when introduced into new markets. Furthermore, the timing and cost of our R&D may increase, and our R&D may become less predictable. For example, changes in regulations applicable to our industry may make it more time-consuming and/or costly to research, develop and register products.
Products in the animal health industry are sometimes derived from molecules and compounds discovered or developed as part of human health research. We have and expect to continue to enter into collaboration or licensing arrangements with third parties, including Pfizer, to provide us with access to compounds and other technology for purposes of our business. Such agreements are typically complex and require time to negotiate and implement. If we enter into these arrangements, we may not be able to maintain these relationships or establish new ones in the future on acceptable terms or at all. In addition, any collaboration that we enter into may not be successful, and the success may depend on the efforts and actions of our collaborators, which we may not be able to control. If we are unable to access human health-generated molecules and compounds to conduct R&D on cost-effective terms, our ability to develop some types of new products could be limited.
16 |
Disruptive innovations and advances in veterinary medical practices and animal health technologies could negatively affect the market for our products.
The market for our products could be impacted negatively by the introduction and/or broad market acceptance of newly-developed or alternative products that address the diseases and conditions for which we sell products, including “green” or “holistic” health products or specially bred disease-resistant animals. In addition, technological breakthroughs by others may obviate our technology and reduce or eliminate the market for our products. Introduction or acceptance of such products or technologies could materially adversely affect our operating results and financial condition.
Our R&D relies on evaluations in animals, which may become subject to bans or additional restrictive regulations.
As an animal health medicines and vaccines business, the evaluation of our existing and new products in animals is required to register our products. Animal testing in certain industries has been the subject of controversy and adverse publicity. Some organizations and individuals have attempted to ban animal testing or encourage the adoption of additional regulations applicable to animal testing. To the extent that the activities of such organizations and individuals are successful, our R&D, and by extension our operating results and financial condition, could be materially adversely affected. In addition, negative publicity about us or our industry could harm our reputation.
Risks related to manufacturing
Manufacturing problems and capacity imbalances may cause product launch delays, inventory shortages, recalls or unanticipated costs.
In order to sell our products, we must be able to produce and ship our products in sufficient quantities. On December 31, 2017, we had a global manufacturing network consisting of 25 manufacturing sites located in 12 countries. We also employ a network of approximately 180 third party CMOs, including a number owned by Pfizer. Many of our products involve complex manufacturing processes and are sole-sourced from certain manufacturing sites.
Minor deviations in our manufacturing or logistical processes, such as temperature excursions or improper package sealing, could result in delays, inventory shortages, unanticipated costs, product recalls, product liability and/or regulatory action. In addition, a number of factors could cause production interruptions, including:
| • | the failure of us or any of our vendors or suppliers, including logistical service providers, to comply with applicable regulations and quality assurance guidelines; |
| • | construction delays; |
| • | equipment malfunctions; |
| • | shortages of materials; |
| • | labor problems; |
| • | natural disasters; |
| • | power outages; |
| • | criminal and terrorist activities; |
| • | changes in manufacturing production sites and limits to manufacturing capacity due to regulatory requirements, changes in types of products produced, shipping distributions or physical limitations; and |
| • | the outbreak of any highly contagious diseases near our production sites. |
These interruptions could result in launch delays, inventory shortages, recalls, unanticipated costs or issues with our agreements under which we supply third parties, which may adversely affect our operating results and financial condition. For example, our manufacturing site in Medolla, Italy was damaged in an earthquake in May 2012, which resulted in production interruptions at that site. In addition, we experienced challenges in manufacturing Apoquel when it was initially launched in 2015 that impacted our ability to meet customer demand. As a result, we had to place limits on the amounts of this product veterinarians could purchase and delayed the launch of the product in certain markets.
Our manufacturing network may be unable to meet the demand for our products or we may have excess capacity if demand for our products changes. The unpredictability of a product's regulatory or commercial success or failure, the lead time necessary to construct highly technical and complex manufacturing sites, and shifting customer demand (including as a result of market conditions or entry of branded or generic competition) increase the potential for capacity imbalances. In addition, construction of sites is expensive, and our ability to recover costs will depend on the market acceptance and success of the products produced at the new sites, which is uncertain.
We rely on third parties to provide us with materials and services, and are subject to increased labor and material costs and potential disruptions in supply.
The materials used to manufacture our products may be subject to availability constraints and price volatility caused by changes in demand, weather conditions, supply conditions, government regulations, economic climate and other factors. In addition, labor costs may be subject to volatility caused by the supply of labor, governmental regulations, economic climate and other factors. Increases in the demand for, availability or the price of, materials used to manufacture our products and increases in labor costs could increase the costs to manufacture our products. We may not be able to pass all or a material portion of any higher material or labor costs on to our customers, which could materially adversely affect our operating results and financial condition.
In addition, certain third-party suppliers are the sole or exclusive source of certain materials and services necessary for production of our products. We may be unable to meet demand for certain of our products if any of our third-party suppliers cease or interrupt operations, fail to renew contracts with us or otherwise fail to meet their obligations to us.
17 |
There may be delays and additional costs due to changes to our existing manufacturing facilities and the construction of new manufacturing plants.
As part of our supply network strategy, we have invested and will continue to invest in improvements to our existing manufacturing facilities and in new manufacturing plants. We are currently investing in two new plants, one in Rathdrum, Ireland for the production of active ingredients for some of our key products and one in Suzhou, China for the research and production of vaccines in China. These types of projects are subject to risks of delay or cost overruns inherent in any large construction project, and will require licensure by various regulatory authorities. Significant cost overruns or delays in completing these projects could have an adverse effect on the Company’s return on investment.
Risks related to legal matters and regulation
We may incur substantial costs and receive adverse outcomes in litigation and other legal matters.
Our operating results, financial condition and liquidity could be materially adversely affected by unfavorable results in pending or future litigation matters. These matters include, among other things, allegations of violation of United States and foreign competition laws, labor laws, consumer protection laws, and environmental laws and regulations, as well as claims or litigations relating to product liability, intellectual property, securities, breach of contract and tort. In addition, changes in the interpretations of laws and regulations to which we are subject, or in legal standards in one or more of the jurisdictions in which we operate, could increase our exposure to liability. For example, in the United States, attempts have been made to allow damages for emotional distress and pain and suffering in connection with the loss of, or injury to, a companion animal. If such attempts were successful, our exposure with respect to product liability claims could increase materially.
Litigation matters, regardless of their merits or their ultimate outcomes, are costly, divert management's attention and may materially adversely affect our reputation and demand for our products. We cannot predict with certainty the eventual outcome of pending or future litigation matters. An adverse outcome of litigation or legal matters could result in our being responsible for significant damages. Any of these negative effects resulting from litigation matters could materially adversely affect our operating results and financial condition.
The misuse or off-label use of our products may harm our reputation or result in financial or other damages.
Our products have been approved for use under specific circumstances for the treatment of certain diseases and conditions in specific species. There may be increased risk of product liability claims if veterinarians, livestock producers, pet owners or others attempt to use our products off-label, including the use of our products in species (including humans) for which they have not been approved. For example, Ketamine, the active pharmaceutical ingredient in our Ketaset product (a nonnarcotic agent for anesthetic use in cats), is abused by humans as a hallucinogen. Furthermore, the use of our products for indications other than those indications for which our products have been approved may not be effective, which could harm our reputation and lead to an increased risk of litigation. If we are deemed by a governmental or regulatory agency to have engaged in the promotion of any of our products for off-label use, such agency could request that we modify our training or promotional materials and practices and we could be subject to significant fines and penalties, and the imposition of these sanctions could also affect our reputation and position within the industry. Any of these events could materially adversely affect our operating results and financial condition.
The illegal distribution and sale by third parties of counterfeit or illegally compounded versions of our products or of stolen, diverted or relabeled products could have a negative impact on our reputation and business.
Third parties may illegally distribute and sell counterfeit or illegally compounded versions of our products that do not meet the exacting standards of our development, manufacturing and distribution processes. Counterfeit or illegally compounded medicines pose a significant risk to animal health and safety because of the conditions under which they are manufactured and the lack of regulation of their contents. Counterfeit or illegally compounded products are frequently unsafe or ineffective and can be potentially life-threatening to animals. Our reputation and business could suffer harm as a result of counterfeit or illegally compounded products which are alleged to be equivalent and/or which are sold under our brand name. We are aware of at least one pharmacy in Brazil that may be engaged in the practice of illegally compounding oclacitinib, the active pharmaceutical ingredient in our Apoquel product. In addition, products stolen or unlawfully diverted from inventory, warehouses, plants or while in transit, which are not properly stored or which have an expired shelf life and which have been repackaged or relabeled and which are sold through unauthorized channels, could adversely impact animal health and safety, our reputation and our business. Public loss of confidence in the integrity of vaccines and/or pharmaceutical products as a result of counterfeiting, illegally compounding or theft could have a material adverse effect on our product sales, business and results of operations.
Our business is subject to substantial regulation.
As a global company, we are subject to various state, federal and international laws and regulations, including regulations relating to the development, quality assurance, manufacturing, importation, distribution, marketing and sale of our products. In addition, our manufacturing facilities are subject to periodic inspections by regulatory agencies. An inspection may report conditions or practices that indicate possible violations of regulatory requirements. Our failure to comply with these regulatory requirements, allegations of such non-compliance or the discovery of previously unknown problems with a product or manufacturer could result in, among other things, inspection observation notices, warning letters or similar regulatory correspondence, fines, a partial or total shutdown of production in one or more of our facilities while an alleged violation is remediated, withdrawals or suspensions of current products from the market, and civil or criminal prosecution, as well as decreased sales as a result of negative publicity and product liability claims. Any one of these consequences could materially adversely affect our operating results and financial condition.
In addition, we will not be able to market new products unless and until we have obtained all required regulatory approvals in each jurisdiction where we propose to market those products. Even after a product reaches market, it may be subject to re-review and may lose its approvals. We have changed, and may in the future change, the locations of where certain of our products are manufactured and, because of these changes, we may be required to obtain new regulatory approvals. Our failure to obtain approvals, delays in the approval process, or our failure to maintain approvals in any jurisdiction, may prevent us from selling products in that jurisdiction until approval or reapproval is obtained, if ever.
18 |
Furthermore, we cannot predict the nature of future laws, regulations, or changes in tax laws, challenges brought against our incentive tax rulings, and tariffs, nor can we determine the effect that additional laws or regulations or changes in existing laws or regulations could have on our business when and if promulgated, or the impact of changes in the interpretation of these laws and regulations, or of disparate federal, state, local and foreign regulatory schemes. Changes to such laws or regulations may include, among other things, changes to taxation requirements, such as tax-rate changes and changes affecting the taxation by the United States of income earned outside the United States.
Changes in applicable federal, state, local and foreign laws and regulations could have a material adverse effect on our operating results and financial condition.
We are subject to complex environmental, health and safety laws and regulations.
We are subject to various federal, state, local and foreign environmental, health and safety laws and regulations. These laws and regulations govern matters such as the emission and discharge of hazardous materials into the ground, air or water; the generation, use, storage, handling, treatment, packaging, transportation, exposure to, and disposal of hazardous and biological materials, including recordkeeping, reporting and registration requirements; and the health and safety of our employees. Due to our operations, these laws and regulations also require us to obtain, and comply with, permits, registrations or other authorizations issued by governmental authorities. These authorities can modify or revoke our permits, registrations or other authorizations and can enforce compliance through fines and injunctions.
Given the nature of our business, we have incurred, are currently incurring and may in the future incur, liabilities under CERCLA or under other federal, state, local and foreign environmental cleanup laws, with respect to our current or former sites, adjacent or nearby third-party sites, or offsite disposal locations. See Item 1. Business—Environmental, Health and Safety. The costs associated with future cleanup activities that we may be required to conduct or finance could be material. Additionally, we may become liable to third parties for damages, including personal injury and property damage, resulting from the disposal or release of hazardous materials into the environment. Such liability could materially adversely affect our operating results and financial condition. Furthermore, regulatory agencies are showing increasing concern over the impact of animal health products and livestock operations on the environment. This increased regulatory scrutiny may necessitate that additional time and resources be spent to address these concerns in both new and existing products.
A failure to comply with the environmental, health and safety laws and regulations to which we are subject, including any permits issued thereunder, may result in environmental remediation costs, loss of permits, fines, penalties or other adverse governmental or private actions, including regulatory or judicial orders enjoining or curtailing operations or requiring corrective measures, installation of pollution control equipment or remedial measures. We could also be held liable for any and all consequences arising out of human exposure to hazardous materials or environmental damage. Environmental laws and regulations are complex, change frequently, have tended to become more stringent and stringently enforced over time and may be subject to new interpretation. We cannot assure you that our costs of complying with current and future environmental, health and safety laws, and our liabilities arising from past or future releases of, or exposure to, hazardous materials will not materially adversely affect our business, results of operations or financial condition.
Risks related to our international operations
A significant portion of our operations are conducted in foreign jurisdictions and are subject to the economic, political, legal and business environments of the countries in which we do business.
Our international operations could be limited or disrupted by any of the following:
| • | volatility in the international financial markets; |
| • | compliance with governmental controls; |
| • | difficulties enforcing contractual and intellectual property rights; |
| • | parallel trade in our products (importation of our products from European Union countries where our products are sold at lower prices into European Union countries where the products are sold at higher prices); |
| • | compliance with a wide variety of laws and regulations, such as the FCPA and similar non-U.S. laws and regulations; |
| • | compliance with foreign labor laws; |
| • | burdens to comply with multiple and potentially conflicting foreign laws and regulations, including those relating to environmental, health and safety requirements; |
| • | changes in laws, regulations, government controls or enforcement practices with respect to our business and the businesses of our customers, including the imposition of limits on our profitability; |
| • | political and social instability, including crime, civil disturbance, terrorist activities and armed conflicts; |
| • | trade restrictions and restrictions on direct investments by foreign entities, including restrictions administered by the Office of Foreign Assets Control of the U.S. Department of Treasury (OFAC) and the European Union, in relation to our products or the products of farmers and other customers (e.g., restrictions on the importation of agricultural products from the European Union to Russia); |
| • | government limitations on foreign ownership; |
| • | government takeover or nationalization of business; |
| • | changes in tax laws, challenges brought against our incentive tax rulings, and tariffs; |
| • | imposition of anti-dumping and countervailing duties or other trade-related sanctions; |
| • | costs and difficulties in staffing, managing and monitoring international operations; |
| • | longer payment cycles and increased exposure to counterparty risk; and |
19 |
| • | additional limitations on transferring personal information between countries or other restrictions on the processing of personal information. |
In addition, international transactions may involve increased financial and legal risks due to differing legal systems and customs. Compliance with these requirements may prohibit the import or export of certain products and technologies or may require us to obtain a license before importing or exporting certain products or technology. A failure to comply with any of these laws, regulations or requirements could result in civil or criminal legal proceedings, monetary or non-monetary penalties, or both, disruptions to our business, limitations on our ability to import and export products and services, and damage to our reputation. In addition, variations in the pricing of our products between jurisdictions may result in the unauthorized importation or unauthorized re-importation of our products between jurisdictions and may also result in the imposition of anti-dumping and countervailing duties or other trade-related sanctions. While the impact of these factors is difficult to predict, any of them could materially adversely affect our operating results and financial condition. Changes in any of these laws, regulations or requirements, or the political environment in a particular country, may affect our ability to engage in business transactions in certain markets, including investment, procurement and repatriation of earnings.
In June 2016, voters in the United Kingdom approved an advisory referendum to withdraw from the European Union, commonly referred to as "Brexit." This referendum has created political and economic uncertainty, particularly in the United Kingdom and the European Union, and this uncertainty may persist for years. A withdrawal could significantly disrupt the free movement of goods, services, and people between the United Kingdom and the European Union, and result in increased legal and regulatory complexities, as well as potential higher costs of conducting business in Europe. The United Kingdom's vote to exit the European Union could also result in similar referendums or votes in other European countries in which we do business. On March 29, 2017, the United Kingdom Prime Minister formally notified the European Council of the United Kingdom’s intention to withdraw from the European Union under Article 50 of the Treaty of Lisbon. The notice begins the two-year negotiation period to establish the withdrawal terms. If no agreement is reached after two years, the United Kingdom’s separation still becomes effective, unless the remaining European Union members unanimously agree to an extension. The uncertainty surrounding the terms of the United Kingdom's withdrawal and its consequences could adversely impact consumer and investor confidence, and could affect sales or regulation of our products. Any of these effects, among others, could materially and adversely affect our business, results of operations, and financial condition.
Finally, there has been recent political instability in Catalonia, which depending on the outcome could impact our R&D and manufacturing operations in Olot, Spain.
Foreign exchange rate fluctuations and potential currency controls affect our results of operations, as reported in our financial statements.
We conduct operations in many areas of the world, involving transactions denominated in a variety of currencies. In 2017, we generated approximately 47% of our revenue in currencies other than the U.S. dollar, principally the euro, Brazilian real and Canadian dollar. We are subject to currency exchange rate risk to the extent that our costs are denominated in currencies other than those in which we earn revenue. In addition, because our financial statements are reported in U.S. dollars, changes in currency exchange rates between the U.S. dollar and other currencies have had, and will continue to have, an impact on our results of operations.
We also face risks arising from currency devaluations and the imposition of cash repatriation restrictions and exchange controls. Currency devaluations result in a diminished value of funds denominated in the currency of the country instituting the devaluation. Cash repatriation restrictions and exchange controls may limit our ability to convert foreign currencies into U.S. dollars or to remit dividends and other payments by our foreign subsidiaries or businesses located in or conducted within a country imposing restrictions or controls. While we currently have no need, and do not intend, to repatriate or convert cash held in countries that have significant restrictions or controls in place, should we need to do so to fund our operations, we may be unable to repatriate or convert such cash, or be unable to do so without incurring substantial costs. We currently have substantial operations in countries that have cash repatriation restrictions or exchange controls in place, including China, and, if we were to need to repatriate or convert such cash, these controls and restrictions may have a material adverse effect on our operating results and financial condition.
In 2015, we recorded a net remeasurement loss of $89 million on bolivar-denominated net monetary assets, primarily related to cash deposits in Venezuela. This loss was recorded as a result of our evaluation of evolving economic conditions in Venezuela, including the devaluation of the Venezuelan bolivar in 2013 and the subsequent changes to Venezuela's foreign currency exchange mechanisms, in addition to our expectation of Venezuela's responses to changes in its economy, and continued volatility.
We may not be able to realize the expected benefits of our investments in emerging markets and are subject to certain risks due to our presence in emerging markets, including political or economic instability and failure to adequately comply with legal and regulatory requirements.
We have been taking steps to increase our presence in emerging markets. Failure to continue to maintain and expand our business in emerging markets could materially adversely affect our operating results and financial condition.
Some countries within emerging markets may be especially vulnerable to periods of local, regional or global economic, political or social instability or crisis. For example, our sales in certain emerging markets have suffered from extended periods of disruption due to natural disasters. Furthermore, we have also experienced lower than expected sales in certain emerging markets due to local, regional and global restrictions on banking and commercial activities in those countries. In addition, certain emerging markets have currencies that fluctuate substantially, which may impact our financial performance. For example, in the past, our revenue in certain emerging markets in Latin America has been adversely impacted by currency fluctuations and devaluations.
In addition, certain emerging markets have legal systems that are less developed or familiar to us. Other jurisdictions in which we conduct business may have legal and regulatory regimes that differ materially from United States laws and regulations, are continuously evolving or do not include sufficient judicial or administrative guidance to interpret such laws and regulations. Compliance with diverse legal requirements is costly and time-consuming and requires significant resources. In the event we believe or have reason to believe our employees have or may have violated applicable laws or regulations, we may be subject to investigation costs, potential penalties and other related costs which in turn could negatively affect our reputation and our results of operations.
20 |
For all these and other reasons, doing business within emerging markets carries significant risks.
Risks related to tax matters
The Company could be subject to changes in its tax rates, the adoption of new U.S. or foreign tax legislation or exposure to additional tax liabilities.
The multinational nature of our business subjects us to taxation in the United States and numerous foreign jurisdictions. Due to economic and political conditions, tax rates in various jurisdictions may be subject to significant change. The company’s future effective tax rates could be affected by changes in the mix of earnings in countries with differing statutory tax rates, changes in the valuation of deferred tax assets and liabilities, or changes in tax laws or their interpretation.
For example, the European Commission opened formal investigations to examine whether decisions by the tax authorities in certain European countries, including Belgium, comply with European Union rules on state aid. In the case of Belgium, the European Commission concluded on January 11, 2016, that the excess profits ruling violates the European Union’s state aid rules. The impact of this conclusion was a net tax charge of approximately $35 million recorded in 2016. This net charge relates to the Belgium government's recovery of benefits for the periods 2013 through 2015 offset by the remeasurement of the company’s deferred tax assets and liabilities using the rates expected to be in place at the time of the reversal and without consideration of implementation of any future operational changes, and does not include any benefits associated with a successful appeal of the decision.
In addition, on June 20, 2016, the Member States of the European Union adopted the anti-tax-avoidance directive proposed on January 28, 2016, which is designed to provide uniform implementation of Base Erosion and Profits Shifting measures and other minimum taxation standards across Member States. The Member States are required to implement all components of the directive by January 1, 2020. Once enacted by the Member States, the results of the directive could have an impact on our effective tax rate. In October 2016, the European Union also introduced a proposal to impose a uniform set of rules on taxing corporate profits, known as the Common Consolidated Corporate Tax Base. This proposal is in its early stages but may have an impact to our effective tax rate.
On December 22, 2017, President Trump signed into law the Tax Cuts and Jobs Act (the Tax Act) effective January 1, 2018. Some notable provisions of the Tax Act include a reduction of the corporate income tax rate from 35% to 21%, and a change from a worldwide system with deferral to a territorial tax system, which includes a one-time mandatory deemed repatriation tax, payable over eight years, on certain undistributed earnings of non-U.S. subsidiaries. As of December 31, 2017, the cumulative amount of non-U.S. undistributed earnings was approximately $4.5 billion, which includes an allocation of non-U.S. undistributed earnings as a result of the separation from Pfizer on June 24, 2013. Pursuant to the Staff Accounting Bulletin published by the Securities and Exchange Commission on December 22, 2017, addressing the challenges in accounting for the effects of the Tax Act in the period of enactment, companies must report provisional amounts for those specific income tax effects of the Tax Act for which the accounting is incomplete but a reasonable estimate can be determined. Those provisional amounts will be subject to adjustment during a measurement period of up to one year from the enactment date. The company is currently in the process of evaluating the full impact of this new legislation on its consolidated financial statements, and in the fourth quarter of 2017 has recorded a provisional net charge of $212 million related to the one-time mandatory deemed repatriation tax, partially offset by the remeasurement of the deferred tax assets and liabilities, as of the date of enactment, due to the reduction in the U.S. federal corporate tax rate. At this time, we are properly reflecting the provision for taxes on income using all current enacted global tax laws in every jurisdiction in which we operate.
On March 29, 2017, United Kingdom (UK) Prime Minister Theresa May formally notified the European Council of the UK’s intention to withdraw from the European Union, commonly referred to as “Brexit”, under Article 50 of the Treaty of Lisbon. The notice begins the two-year negotiation period to establish the withdrawal terms. If no agreement is reached after two years, the UK’s separation still becomes effective, unless the remaining European Union members unanimously agree to an extension. At this time, the impact of Brexit to our effective tax rate is uncertain.
In addition, our effective tax rate is subject to potential risks that various taxing authorities may challenge the pricing of our cross-border arrangements and subject us to additional tax, adversely impacting our effective tax rate and our tax liability. The company is also subject to the examination of its tax returns and other tax matters by the Internal Revenue Service and other tax authorities and governmental bodies. The company regularly assesses the likelihood of an adverse outcome resulting from these examinations to determine the adequacy of its provision for taxes. There can be no assurance as to the outcome of these examinations. If the company’s effective tax rates were to increase, particularly in the United States or other material foreign jurisdictions, or if the ultimate determination of the company’s taxes owed is for an amount in excess of amounts previously accrued, the company’s operating results, cash flows and financial condition could be adversely affected.
Risks related to intellectual property
The alleged intellectual property rights of third parties may negatively affect our business.
A third party may sue us or otherwise make a claim, alleging infringement or other violation of the third-party's patents, trademarks, trade dress, copyrights, trade secrets, domain names or other intellectual property rights. If we do not prevail in this type of dispute, we may be required to:
| • | pay monetary damages; |
| • | obtain a license in order to continue manufacturing or marketing the affected products, which may not be available on commercially reasonable terms, or at all; or |
| • | stop activities, including any commercial activities, relating to the affected products, which could include a recall of the affected products and/or a cessation of sales in the future. |
The costs of defending an intellectual property action are often substantial and could materially adversely affect our operating results and financial condition, even if we successfully defend such action. The intellectual property positions of animal health medicines and vaccines businesses frequently involve complex legal and factual questions, and an issued patent does not provide the right to practice the patented technology or develop, manufacture or commercialize the patented product. We cannot guarantee that a competitor or other third party does not have or will not
21 |
obtain rights to intellectual property that, in the absence of a license, may prevent us from manufacturing, developing or marketing certain of our products, regardless of whether we believe such intellectual property rights are valid and enforceable, which may harm our operating results and financial condition.
If our intellectual property rights are challenged or circumvented, competitors may be able to take advantage of our research and development efforts.
Our long-term success largely depends on our ability to market technologically competitive products. We rely and expect to continue to rely on a combination of intellectual property, including patent, trademark, trade dress, copyright, trade secret, data protection, and domain name protection laws, as well as confidentiality and license agreements with our employees and others, to protect our intellectual property and proprietary rights. If we fail to obtain and maintain adequate intellectual property protection, we may not be able to prevent third parties from using our proprietary technologies or from marketing products that are very similar or identical to ours. Our currently pending or future patent applications may not result in issued patents, or be approved on a timely basis, or at all. Similarly, any term extensions that we seek may not be approved on a timely basis, if at all. In addition, our issued patents may not contain claims sufficiently broad to protect us against third parties with similar technologies or products or provide us with any competitive advantage, including exclusivity in a particular product area. The scope of our patent claims also may vary between countries, as individual countries have their own patent laws. For example, some countries only permit the issuance of patents covering a novel chemical compound itself, and its first use, and thus further methods of use for the same compound, may not be patentable. We may be subject to challenges by third parties regarding our intellectual property, including claims regarding validity, enforceability, scope and effective term. The validity, enforceability, scope and effective term of patents can be highly uncertain and often involve complex legal and factual questions and proceedings. Our ability to enforce our patents also depends on the laws of individual countries and each country's practice with respect to enforcement of intellectual property rights. In addition, if we are unable to maintain our existing license agreements or other agreements pursuant to which third parties grant us rights to intellectual property, including because such agreements expire or are terminated, our operating results and financial condition could be materially adversely affected.
Patent law reform in the United States and other countries may also weaken our ability to enforce our patent rights, or make such enforcement financially unattractive. For instance, U.S. court decisions in the recent years have led to U.S. Patent and Trademark Office Guidelines regarding inventions in the field of products isolated from nature and diagnostic methods which may influence future patenting strategy in these areas. A similar court decision in Australia was issued recently with regard to the patentability of nucleic acids. Such reforms could result in increased costs to protect our intellectual property and/or limit our ability to patent our products in these jurisdictions.
Additionally, certain foreign governments have indicated that compulsory licenses to patents may be granted in the case of national emergencies, which could diminish or eliminate sales and profits from those regions and materially adversely affect our operating results and financial condition.
Likewise, in the United States and other countries, we currently hold issued trademark registrations and have trademark applications pending, any of which may be the subject of a governmental or third-party objection, which could prevent the maintenance or issuance of the same and thus create the potential need to rebrand or re-label a product. As our products mature, our reliance on our trademarks to differentiate us from our competitors increases and as a result, if we are unable to prevent third parties from adopting, registering or using trademarks and trade dress that infringe, dilute or otherwise violate our trademark rights, our business could be materially adversely affected.
Many of our vaccine products and other products are based on or incorporate proprietary information, including proprietary master seeds and proprietary or patented adjuvant formulations. We actively seek to protect our proprietary information, including our trade secrets and proprietary know-how, by requiring our employees, consultants, other advisors and other third parties to execute proprietary information and confidentiality agreements upon the commencement of their employment, engagement or other relationship. Despite these efforts and precautions, we may be unable to prevent a third party from copying or otherwise obtaining and using our trade secrets or our other intellectual property without authorization and legal remedies may not adequately compensate us for the damages caused by such unauthorized use. Further, others may independently and lawfully develop substantially similar or identical products that circumvent our intellectual property by means of alternative designs or processes or otherwise.
The misappropriation and infringement of our intellectual property, particularly in foreign countries where the laws may not protect our proprietary rights as fully as in the United States, may occur even when we take steps to prevent it. We are currently, and expect to be in the future, party to patent lawsuits and other intellectual property rights claims that are expensive and time consuming, and if resolved adversely, could have a significant impact on our business and financial condition. In the future, we may not be able to enforce intellectual property that relates to our products for various reasons, including licensor restrictions and other restrictions imposed by third parties, or the cost of enforcing our intellectual property may outweigh the value of doing so; either of which could have a material adverse impact on our business and financial condition.
Risks related to information technology
We depend on sophisticated information technology and infrastructure.
We rely on the efficient and uninterrupted operation of complex information technology systems to manage our operations, to process, transmit and store electronic and financial information, and to comply with regulatory, legal and tax requirements. We also depend on our information technology infrastructure for digital marketing activities and for electronic communications among our personnel, customers and suppliers around the world. System failures or outages could compromise our ability to perform these functions in a timely manner, which could harm our ability to conduct business, hurt our relationships with our customers, or delay our financial reporting. Such failures could materially adversely affect our operating results and financial condition.
In addition, we depend on third parties and applications on virtualized (cloud) infrastructure to operate and support our information systems. These third parties include large established vendors, as well as many small, privately owned companies. Failure by these providers to adequately support our operations or a change in control or insolvency of these providers could have an adverse effect on our business, which in turn may materially adversely affect our operating results and financial condition.
22 |
Assuming we are able to implement new systems successfully, all information systems, despite implementation of security measures, are vulnerable to disability, failures or unauthorized access. If our information systems were to fail or be breached, such failure or breach could materially adversely affect our ability to perform critical business functions and sensitive and confidential data could be compromised.
We may be unable to successfully manage our online ordering sites.
In many markets around the world, such as the United States and Brazil, we provide online ordering sites to customers, often relying on third parties to host and support the application. The operation of our online business depends on our ability to maintain the efficient and uninterrupted operation of our online order-taking and fulfillment operations. Risks associated with our online business include: disruptions in telephone or internet service or power outages; failures of the information systems that support our website, including inadequate system capacity, computer viruses, human error, changes in programming, security breaches, system upgrades or migration of these services to new systems; reliance on third parties for computer hardware and software as well as delivery of merchandise to our customers; rapid technology changes; credit card fraud; natural disasters or adverse weather conditions; power and network outages; changes in applicable federal and state regulations; liability for online content; and consumer privacy concerns. Problems in any one or more of these areas could have a material adverse effect on our operating results and financial condition and could damage our reputation.
We may be unable to adequately protect our information technology systems from cyber-attacks, breaches of security or misappropriation of data, which could result in the disclosure of confidential information, damage our reputation, and subject us to significant financial and legal exposure.
Our reputation as a global leader in animal health and our reliance on complex information systems make us inherently vulnerable to malicious cyber intrusion and attack. Cyber-attacks are increasing in their frequency, sophistication and intensity, and have become increasingly difficult to detect. Cyber-attacks could include wrongful conduct by hostile foreign governments, industrial espionage, the deployment of harmful malware, ransomware, denial-of-service attacks, and other means to threaten data confidentiality, integrity and availability. In addition, despite our efforts to protect sensitive, confidential or personal data or information, we may be vulnerable to material security breaches, theft, misplaced or lost data, programming errors, employee errors and/or malfeasance that could potentially lead to the compromise of sensitive, confidential or personal data or information, improper use of our systems or networks, unauthorized access, use, disclosure, modification or destruction of information (including confidential business information, trade secrets and corporate strategic plans), defective products, production downtimes and operational disruptions.
Like other global companies, we have experienced threats to our data and information technology systems. To date, those threats have not had a material impact on our business operations or financial condition. However, although we devote resources to protect our information technology systems, we expect cyber-attacks to continue, and there can be no assurance that our efforts will prevent information security breaches that would result in business, legal or reputational harm to us, or would have a material adverse effect on our operating results and financial condition.
If hackers or cyberthieves gain improper access to our technology systems, networks, or infrastructure, they may be able to access, steal, publish, delete, misappropriate, modify or otherwise disrupt access to confidential data. Moreover, additional harm to customers could be perpetrated by third parties who are given access to the confidential data. A network disruption (including one resulting from a cyberattack) could cause an interruption or degradation of service as well as permit access, theft, publishing, deletion, misappropriation, or modification to or of confidential data. Due to the evolving techniques used in cyberattacks to disrupt or gain unauthorized access to technology networks, we may not be able to anticipate or prevent such disruption or unauthorized access.
The costs imposed on us as a result of a cyberattack or network disruption could be significant. Among others, such costs could include increased expenditures on cyber security measures, litigation, fines, and sanctions, lost revenues from business interruption, and damage to the public’s perception regarding our ability to keep our information secure. As a result, a cyberattack or network disruption could have a material adverse effect on our business, financial condition, and operating results.
We may be unable to adequately protect our stakeholders' privacy or we may fail to comply with privacy laws.
The protection of customer, employee, supplier and company data is critical and the regulatory environment surrounding information security, storage, use, processing, disclosure and privacy is demanding, with the frequent imposition of new and changing requirements. In addition, our customers, employees and suppliers expect that we will adequately protect their personal information. Any actual or perceived significant breakdown, intrusion, interruption, cyber-attack or corruption of customer, employee or company data or our failure to comply with federal, state, local and foreign privacy laws, including the EU General Data Protection Regulation (GDPR), could result in lost sales, remediation costs, and legal liability including severe penalties, regulatory action and reputational harm. For example, the EU’s GDPR becomes effective May 25, 2018 and requires companies to meet new and enhanced requirements regarding the handling of personal data, including its use, protection and the rights of data subjects to request correction or deletion of their personal data. Failure to meet GDPR requirements could result in penalties of up to 4% of worldwide revenue.
Despite our considerable efforts and investments in technology to secure our computer network, security could be compromised, confidential information could be misappropriated or system disruptions could occur. Failure to comply with the security requirements or rectify a security issue may result in fines and the imposition of restrictions on our ability to accept payment by credit or debit cards. In addition, the payment card industry (PCI) is controlled by a limited number of vendors that have the ability to impose changes in PCI's fee structure and operational requirements on us without negotiation. Such changes in fees and operational requirements may result in our failure to comply with PCI security standards, as well as significant unanticipated expenses. Such failures could materially adversely affect our operating results and financial condition.
Risks related to our indebtedness
We have substantial indebtedness.
We have a significant amount of indebtedness, which could materially adversely affect our operating results, financial condition and liquidity. As of December 31, 2017, we had approximately $5.0 billion of total unsecured indebtedness outstanding. In addition, we have entered into an agreement
23 |
for a five-year revolving credit facility and a commercial paper program each with a capacity of up to $1.0 billion. While we currently do not have any amounts drawn under the credit facility nor any commercial paper issued under the commercial paper program, we may incur indebtedness under these arrangements in the future.
We may incur substantial additional debt from time to time to finance working capital, capital expenditures, investments or acquisitions, or for other purposes. If we do so, the risks related to our high level of debt could intensify. Specifically, our high level of debt could have important consequences, including:
• | making it more difficult for us to satisfy our obligations with respect to our debt; |
• | limiting our ability to obtain additional financing to fund future working capital, capital expenditures, business development or other general corporate requirements, including dividends; |
• | increasing our vulnerability to general adverse economic and industry conditions; |
• | exposing us to the risk of increased interest rates as certain of our borrowings are and may in the future be at variable rates of interest; |
• | limiting our flexibility in planning for and reacting to changes in the animal health industry; |
• | placing us at a competitive disadvantage to other, less leveraged competitors; |
• | impacting our effective tax rate; and |
| • | increasing our cost of borrowing. |
In addition, the instruments governing our indebtedness contain restrictive covenants that will limit our ability to engage in activities that may be in our long-term best interest. For example, our credit facility contains a financial covenant requiring us to not exceed a maximum total leverage ratio and covenants that, among other things, limit or restrict our and our subsidiaries' ability, subject to certain exceptions, to incur liens, merge, consolidate or sell, transfer or lease assets, transact with affiliates and incur priority indebtedness. Our failure to comply with such covenants could result in an event of default which, if not cured or waived, could result in the acceleration of all our debt.
We may not be able to generate sufficient cash to service all of our indebtedness and may be forced to take other actions to satisfy our obligations under our indebtedness, which may not be successful.
Our ability to make scheduled payments on or refinance our debt obligations depends on our financial condition and operating performance, which are subject to prevailing economic and competitive conditions and to certain financial, business, legislative, regulatory and other factors beyond our control. We may be unable to maintain a level of cash flows from operating activities sufficient to permit us to pay the principal and interest on our indebtedness.
If our cash flows and capital resources are insufficient to fund our debt service obligations, we could face substantial liquidity problems and could be forced to reduce or delay investments and capital expenditures, or to dispose of material assets or operations, alter our dividend policy, seek additional debt or equity capital or restructure or refinance our indebtedness. We may not be able to effect any such alternative measures on commercially reasonable terms or at all and, even if successful, those alternative actions may not allow us to meet our scheduled debt service obligations. The instruments that will govern our indebtedness may restrict our ability to dispose of assets and may restrict the use of proceeds from those dispositions and may also restrict our ability to raise debt or equity capital to be used to repay other indebtedness when it becomes due. We may not be able to consummate those dispositions or to obtain proceeds in an amount sufficient to meet any debt service obligations when due.
In addition, we conduct our operations through our subsidiaries. Accordingly, repayment of our indebtedness will depend on the generation of cash flow by our subsidiaries, including certain international subsidiaries, and their ability to make such cash available to us, by dividend, debt repayment or otherwise. Our subsidiaries may not have any obligation to pay amounts due on our indebtedness or to make funds available for that purpose. Our subsidiaries may not be able to, or may not be permitted to, make distributions to enable us to make payments in respect of our indebtedness. Each subsidiary is a distinct legal entity, and under certain circumstances, legal, tax and contractual restrictions may limit our ability to obtain cash from our subsidiaries. In the event that we do not receive distributions from our subsidiaries, we may be unable to make required principal and interest payments on our indebtedness.
Our inability to generate sufficient cash flows to satisfy our debt obligations, or to refinance our indebtedness on commercially reasonable terms or at all, may materially adversely affect our operating results, financial condition and liquidity and our ability to satisfy our obligations under our indebtedness or pay dividends on our common stock.
We may not have the funds necessary to finance the change of control offer required by the indenture governing our senior notes.
Upon the occurrence of a change of control of Zoetis and a downgrade below investment grade by Moody's Investor Services, Inc. and Standard & Poor's Rating Services, we will be required to offer to repurchase all of our outstanding senior notes. However, we may not have sufficient funds available at the time of the change of control to finance the required change of control offer or restrictions in our then-existing debt instruments will not allow such repurchases. Our failure to purchase the senior notes as required under the indenture would result in a default under the indenture, which could have material adverse consequences for us and the holders of the senior notes.
Our credit ratings may not reflect all risks of an investment in our senior notes.
The credit ratings assigned to our senior notes are limited in scope, and do not address all material risks relating to an investment in our senior notes, but rather reflect only the view of each rating agency at the time the rating is issued. There can be no assurance that such credit ratings will remain in effect for any given period of time or that a rating will not be lowered, suspended or withdrawn entirely by the applicable rating agencies, if, in such rating agency's judgment, circumstances so warrant. Credit ratings are not a recommendation to buy, sell or hold any security. Each agency's rating should be evaluated independently of any other agency's rating. Actual or anticipated changes or downgrades in our credit ratings, including any announcement that our ratings are under further review for a downgrade, could affect the market prices of our securities and increase our borrowing costs.
24 |
Risks related to our relationship with Pfizer
Certain of our directors may have actual or potential conflicts of interest because of their positions with Pfizer.
Certain of our directors are employed or have been employed by Pfizer or may own Pfizer common stock, options to purchase Pfizer common stock or other Pfizer equity awards. Certain of these holdings may be individually significant to these directors as compared with such director's total assets. These directors' positions at Pfizer and the ownership of any Pfizer equity or equity awards may create, or may create the appearance of, conflicts of interest when these directors are faced with decisions that could have different implications for Pfizer than for us.
To preserve the tax-free treatment to Pfizer and/or its stockholders of the Exchange Offer and certain related transactions, we may not be able to engage in certain transactions.
On May 22, 2013, Pfizer announced an exchange offer (the Exchange Offer) whereby Pfizer shareholders could exchange a portion of Pfizer common stock for Zoetis common stock. The Exchange Offer was completed on June 24, 2013, resulting in the full separation of Zoetis and the disposal of Pfizer's entire ownership and voting interest in Zoetis. To preserve the tax-free treatment to Pfizer and/or its stockholders of the Exchange Offer and certain related transactions, under the tax matters agreement, we are restricted from taking any action that prevents such transactions from being tax-free for U.S. federal, state, local and foreign income tax purposes. These restrictions may limit our ability to engage in certain transactions, including taking certain actions with respect to our 3.250% Senior Notes due 2023.
Pfizer's rights as licensor under the patent and know-how license could limit our ability to develop and commercialize certain products.
Under the patent and know-how license agreement (Pfizer as licensor) (the Patent and Know-How License Agreement), Pfizer licenses to us certain of its intellectual property. If we fail to comply with our obligations under this license agreement and Pfizer exercises its right to terminate it, our ability to continue to research, develop and commercialize products incorporating that intellectual property will be limited. In addition, in circumstances where Pfizer has an interest in the licensed intellectual property in connection with its human health development programs, our rights to use the licensed intellectual property are restricted and/or, in limited instances, subject to Pfizer's right to terminate such license at will. These limitations and termination rights may make it more difficult, time-consuming or expensive for us to develop and commercialize certain new products, or may result in our products being later to market than those of our competitors.
We are dependent on Pfizer to prosecute, maintain and enforce certain intellectual property.
Under the Patent and Know-How License Agreement, Pfizer is responsible for filing, prosecuting and maintaining patents that Pfizer licenses to us. In the animal health field, Pfizer has the first right, and in some cases the sole right, to enforce such licensed patents, and in the human health field, subject to certain exceptions, Pfizer has the sole right to enforce the licensed patents. If Pfizer fails to fulfill its obligations or chooses to not enforce the licensed patents under this agreement, we may not be able to prevent competitors from making, using and selling competitive products, which could have an adverse effect on our business.
If there is a later determination that the Exchange Offer or certain related transactions are taxable for U.S. federal income tax purposes because the facts, assumptions, representations or undertakings underlying the IRS private letter ruling and/or any tax opinion are incorrect or for any other reason, we could incur significant liabilities.
Pfizer has received a private letter ruling from the IRS substantially to the effect that, among other things, the Exchange Offer will qualify as a transaction that is tax-free for U.S. federal income tax purposes under Sections 355 and 368(a)(1)(D) of the U.S. Internal Revenue Code of 1986 (the Code). Completion by Pfizer of the Exchange Offer was conditioned on, among other things, the continuing application of Pfizer's private letter ruling from the IRS and the receipt of an opinion of tax counsel, to the effect that, among other things, the Exchange Offer will qualify as a transaction that is tax-free for U.S. federal income tax purposes under Sections 355 and 368(a)(1)(D) of the Code. The ruling and the opinion rely on certain facts, assumptions, representations and undertakings from Pfizer and us regarding the past and future conduct of the companies' respective businesses and other matters. If any of these facts, assumptions, representations or undertakings are incorrect or not otherwise satisfied, Pfizer and its stockholders may not be able to rely on the ruling or the opinion of tax counsel and could be subject to significant tax liabilities. Notwithstanding the private letter ruling and opinion of tax counsel, the IRS could determine on audit that the Exchange Offer or certain related transactions are taxable if it determines that any of these facts, assumptions, representations or undertakings are not correct or have been violated or if it disagrees with the conclusions in the opinion that are not covered by the private letter ruling, or for other reasons, including as a result of certain significant changes in the stock ownership of Pfizer or us after the Exchange Offer. If the Exchange Offer or certain related transactions are determined to be taxable for U.S. federal income tax purposes, we could incur significant liabilities under applicable law or under the tax matters agreement.
Risks related to our common stock
The price of our common stock may fluctuate substantially, and you could lose all or part of your investment in Zoetis common stock as a result.
Our common stock has a limited trading history and there may be wide fluctuations in the market value of our common stock as a result of many factors. From our IPO through December 31, 2017, the sales price of our common stock as reported by the NYSE has ranged from a low sales price of $28.14 on April 15, 2014 to a high sales price of $73.58 on December 19, 2017. Some factors that may cause the market price of our common stock to fluctuate, in addition to the other risks mentioned in this section and in our 2017 Annual Report, are:
| • | our operating performance and the performance of our competitors; |
| • | our or our competitors' press releases, other public announcements and filings with the SEC regarding new products or services, enhancements, significant contracts, acquisitions or strategic investments; |
| • | changes in earnings estimates or recommendations by securities analysts, if any, who cover our common stock; |
| • | changes in our investor base; |
| • | failures to meet external expectations or management guidance; |
25 |
| • | fluctuations in our financial results or the financial results of companies perceived to be similar to us; |
| • | changes in our capital structure or dividend policy, future issuances of securities, sales of large blocks of common stock by our stockholders or the incurrence of additional debt; |
| • | reputational issues; |
| • | changes in general economic and market conditions in any of the regions in which we conduct our business; |
| • | the arrival or departure of key personnel; |
| • | the actions of speculators and financial arbitrageurs (such as hedge funds); |
| • | changes in applicable laws, rules or regulations and other dynamics; and |
| • | other developments or changes affecting us, our industry or our competitors. |
In addition, if the market for stocks in our industry or industries related to our industry, or the stock market in general, experiences a loss of investor confidence, the trading price of our common stock could decline for reasons unrelated to our business, financial condition and results of operations. If any of the foregoing occurs, it could cause our stock price to fall and may expose us to lawsuits that, even if unsuccessful, could be costly to defend and a distraction to management.
While we currently pay a quarterly cash dividend to our common stockholders, we may change our dividend policy at any time.
On December 11, 2017, our Board of Directors declared the 2018 first quarter dividend of $0.126 per share to be paid on March 1, 2018, to holders of record on January 19, 2018; and on February 13, 2018, our Board of Directors declared the 2018 second quarter dividend of $0.126 per share to be paid on June 1, 2018, to holders of record on April 20, 2018. Although we currently pay a quarterly cash dividend to our common stockholders, we have no obligation to do so, and our dividend policy may change at any time without notice to our stockholders. Returns on stockholders' investments will primarily depend on the appreciation, if any, in the price of our common stock. We anticipate that we will retain most of our future earnings, if any, for use in the development and expansion of our business, repayment of indebtedness and for general corporate purposes. The declaration and payment of dividends is at the discretion of our Board of Directors in accordance with applicable law after taking into account various factors, including our financial condition, operating results, current and anticipated cash needs, cash flows available in the United States, impact on our effective tax rate, indebtedness, legal requirements and other factors that our Board of Directors deems relevant.
Provisions in our restated certificate of incorporation, amended and restated by-laws, and Delaware law may prevent or delay an acquisition of us, which could decrease the trading price of our common stock.
Our amended and restated certificate of incorporation, which we refer to as “our certificate of incorporation,” and our amended and restated by-laws, which we refer to as “our by-laws,” contain provisions that are intended to deter coercive takeover practices and inadequate takeover bids and to encourage prospective acquirers to negotiate with our Board of Directors rather than to attempt a hostile takeover. These provisions include:
| • | a Board of Directors that is divided into three classes with staggered terms; |
| • | rules regarding how our stockholders may present proposals or nominate directors for election at stockholder meetings; |
| • | the right of our Board of Directors to issue preferred stock without stockholder approval; and |
| • | limitations on the right of stockholders to remove directors. |
In addition, Delaware law also imposes some restrictions on mergers and other business combinations between us and any holder of 15% or more of our outstanding common stock. These provisions apply even if the offer may be considered beneficial by some stockholders and could delay or prevent an acquisition that our Board of Directors determines is not in our and our stockholders' best interests.
Item 1B. Unresolved Staff Comments.
None.
Item 2. Properties.
We have 146 owned and leased properties, amounting to approximately 10.2 million square feet, around the world for sales and marketing, customer service, regulatory compliance, R&D, manufacturing and distribution, and administrative support functions. In many locations, operations are co-located to achieve synergies and operational efficiencies. Our largest R&D facility is our owned U.S. research and development site located in Kalamazoo, Michigan, which represents approximately 1.5 million square feet. None of our other non-manufacturing sites are more than 0.2 million square feet. The largest manufacturing site in our global manufacturing network is our manufacturing site located in Kalamazoo, Michigan, which represents approximately 0.6 million square feet. No other site in our global manufacturing network is more than 0.6 million square feet. In addition, our global manufacturing network will continue to be supplemented by approximately 180 CMOs.
Our corporate headquarters are located at 10 Sylvan Way, Parsippany, New Jersey 07054. Our operations extend internationally to approximately 60 countries. Under the transitional services agreement we entered into with Pfizer, Pfizer granted us continued access to certain of its premises occupied by our employees prior to the IPO. We currently lease space from Pfizer in 7 different locations globally, mainly in Europe.
We believe that our existing properties, as supplemented by sites operated by CMOs, including Pfizer, and access to Pfizer facilities provided under the transitional services agreement are adequate for our current requirements and for our operations in the foreseeable future.
26 |
Item 3. Legal Proceedings.
We are from time to time subject to claims and litigation arising in the ordinary course of business. These claims and litigation may include, among other things, allegations of violation of U.S. and foreign competition law, labor laws, consumer protection laws, and environmental laws and regulations, as well as claims or litigation relating to product liability, intellectual property, securities, breach of contract and tort. We operate in multiple jurisdictions and, as a result, a claim in one jurisdiction may lead to claims or regulatory penalties in other jurisdictions. We intend to defend vigorously against any pending or future claims and litigation.
At this time, in the opinion of management, the likelihood is remote that the impact of such proceedings, either individually or in the aggregate, would have a material adverse effect on our consolidated results of operations, financial condition or cash flows. However, one or more unfavorable outcomes in any claim or litigation against us could have a material adverse effect for the period in which they are resolved. In addition, regardless of their merits or their ultimate outcomes, such matters are costly, divert management's attention and may materially adversely affect our reputation, even if resolved in our favor.
Certain legal proceedings in which we are involved are discussed in Notes to Consolidated Financial Statements— Note 17. Commitments and Contingencies, and are incorporated by reference from such discussion.
Item 4. Mine Safety Disclosures.
Not applicable.
27 |
PART II
| Item 5. | Market for Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities. |
Our shares of common stock have been listed on the NYSE (symbol ZTS) since February 1, 2013. Prior to that time, there was no public market for our stock.
The following table sets forth the high and low sales price of our common stock for each quarter presented below:
| High | Low | |
| 2016 | ||
| First Quarter | $48.35 | $38.26 |
| Second Quarter | $49.10 | $45.01 |
| Third Quarter | $52.64 | $46.84 |
| Fourth Quarter | $54.15 | $46.86 |
| 2017 | ||
| First Quarter | $56.50 | $52.00 |
| Second Quarter | $63.85 | $52.25 |
| Third Quarter | $65.83 | $59.50 |
| Fourth Quarter | $73.58 | $63.03 |
As of February 9, 2018, there were 485,253,713 shares of our common stock outstanding, held by 1,889 shareholders of record.
Additional information relating to our common stock is included in this Annual Report on Form 10-K in Notes to Consolidated Financial Statements— Note 15. Stockholders’ Equity.
Purchases of Equity Securities by the Issuer
On November 18, 2014, we announced that our Board of Directors authorized the repurchase of up to $500 million of our outstanding common stock. This program was substantially completed as of December 31, 2016. On December 6, 2016, we announced that our Board of Directors authorized the repurchase of an additional $1.5 billion of our outstanding common stock. These programs do not have a stated expiration date. Purchases of Zoetis shares may be made at the discretion of management, depending on market conditions and business needs. We repurchase shares pursuant to Rules 10b5-1 and 10b-18 under the Securities Exchange Act of 1934, as amended, through repurchase agreements established with several brokers.
Issuer purchases of equity securities for the three months ended December 31, 2017 were as follows:
| Issuer Purchases of Equity Securities | ||||
Total Number of Shares Purchased(a) | Average Price Paid Per Share | Total Number of Shares Purchased as Part of Publicly Announced Programs | Approximate Dollar Value of Shares that May Yet Be Purchased Under Plans or Programs | |
| October 2 - October 29, 2017 | 630,182 | $64.62 | 626,898 | $1,084,681,628 |
| October 30 - November 30, 2017 | 599,007 | $68.38 | 598,493 | $1,043,755,651 |
| December 1 - December 31, 2017 | 605,653 | $72.02 | 604,762 | $1,000,123,816 |
| Total | 1,834,842 | $68.29 | 1,830,153 | $1,000,123,816 |
(a) The company repurchased 4,689 shares during the three-month period ended December 31, 2017, that were not part of the publicly announced share repurchase authorization. These shares were purchased from employees to satisfy tax withholding requirements on the vesting of restricted shares from equity-based awards.
Dividend Policy, Declaration and Payment
During the years ended December 31, 2017 and 2016, we paid the following quarterly cash dividends per share on our common stock:
| 2017 | 2016 | |
| First Quarter | $0.105 | $0.095 |
| Second Quarter | $0.105 | $0.095 |
| Third Quarter | $0.105 | $0.095 |
| Fourth Quarter | $0.105 | $0.095 |
On December 11, 2017, our Board of Directors declared the 2018 first quarter dividend of $0.126 per share to be paid on March 1, 2018, to holders of record on January 19, 2018. On February 13, 2018, our Board of Directors declared the 2018 second quarter dividend of $0.126 per share to be paid on June 1, 2018, to holders of record on April 20, 2018.
28 |
The declaration and payment of dividends to holders of our common stock will be at the discretion of our Board of Directors in accordance with applicable law after taking into account various factors, including our financial condition, operating results, current and anticipated cash needs, cash flows available in the United States, impact on our effective tax rate, indebtedness, legal requirements and other factors that our Board of Directors deems relevant. In addition, the instruments governing our indebtedness may limit our ability to pay dividends. Therefore, no assurance is given that we will pay any dividends to our common stockholders or as to the amount of any such dividends if our Board of Directors determines to do so.
Because we are a holding company, our ability to pay cash dividends on our common stock will depend on the receipt of dividends or other distributions from certain of our subsidiaries.
Stock Performance Graph(a)
The graph below compares the cumulative total shareholder return on an investment in our common stock, the S&P 500 Index and the S&P 500 Pharmaceuticals Index for the period from our initial public offering through the year ended December 31, 2017. The shareholder return shown on the graph is not necessarily indicative of future performance, and we do not make or endorse any predictions as to future shareholder returns.
The graph assumes the investment of $100 on February 1, 2013, in our common stock, the S&P 500 Index and the S&P 500 Pharmaceuticals Index and assumes dividends, if any, are reinvested.
COMPARISON OF CUMULATIVE TOTAL RETURN
Among Zoetis Inc., the S&P 500 Index and the S&P 500 Pharmaceuticals Index

| February 1, 2013 | June 30, 2013 | December 31, 2013 | June 29, 2014 | December 31, 2014 | June 28, 2015 | December 31, 2015 | July 3, 2016 | December 31, 2016 | July 2, 2017 | December 31, 2017 | |
| Zoetis Inc. | $100 | $99.81 | $106.07 | $105.56 | $140.84 | $159.73 | $157.98 | $157.42 | $177.95 | $208.55 | $241.21 |
| S&P 500 | $100 | $107.14 | $124.61 | $133.55 | $141.67 | $146.06 | $143.63 | $149.46 | $160.81 | $175.83 | $195.92 |
| S&P 500 Pharmaceuticals Index | $100 | $109.67 | $125.16 | $140.83 | $152.97 | $166.53 | $161.82 | $169.39 | $159.29 | $175.62 | $179.31 |
(a) This section is not “soliciting material,” is not deemed “filed” with the SEC and is not to be incorporated by reference in any filing of Zoetis under the Securities Act of 1933, as amended, or the Exchange Act whether made before or after the date hereof and irrespective of any general incorporation language contained in any such filing.
29 |
Item 6. Selected Financial Data.
The following table sets forth our selected historical consolidated and combined financial data for the periods indicated.
The selected consolidated statements of income data for the years ended December 31, 2017, 2016 and 2015, and the selected consolidated balance sheet data as of December 31, 2017 and 2016 presented below have been derived from our audited consolidated financial statements included in Item 8. Financial Statements and Supplementary Data. The selected historical consolidated statements of income data for the years ended December 31, 2014 and 2013, and the selected historical consolidated balance sheet data as of December 31, 2015, 2014 and 2013 presented below has been derived from our audited financial statements not included in this 2017 Annual Report.
You should read the selected historical consolidated and combined financial data set forth below in conjunction with Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations and our consolidated financial statements and notes thereto included in Item 8. Financial Statements and Supplementary Data.
Year Ended December 31,(a) | ||||||||||||||||||||
| (MILLIONS, EXCEPT PER SHARE AMOUNTS) | 2017 | 2016 | 2015 | 2014 | 2013 | |||||||||||||||
| Statement of income data: | ||||||||||||||||||||
| Revenue | $ | 5,307 | $ | 4,888 | $ | 4,765 | $ | 4,785 | $ | 4,561 | ||||||||||
| Net income attributable to Zoetis | 864 | 821 | 339 | 583 | 504 | |||||||||||||||
| Balance sheet data: | ||||||||||||||||||||
| Total assets | $ | 8,586 | $ | 7,649 | $ | 7,913 | $ | 6,588 | $ | 6,536 | ||||||||||
| Long-term obligations | 4,953 | 4,468 | 4,463 | 3,624 | 3,620 | |||||||||||||||
| Other data (unaudited): | ||||||||||||||||||||
Adjusted net income(b) | $ | 1,185 | $ | 975 | $ | 889 | $ | 790 | $ | 709 | ||||||||||
| Earnings per share attributable to Zoetis Inc. stockholders: | ||||||||||||||||||||
| Basic | $ | 1.76 | $ | 1.66 | $ | 0.68 | $ | 1.16 | $ | 1.01 | ||||||||||
| Diluted | $ | 1.75 | $ | 1.65 | $ | 0.68 | $ | 1.16 | $ | 1.01 | ||||||||||
| Dividends declared per common share | $ | 0.441 | $ | 0.390 | $ | 0.344 | $ | 0.299 | $ | 0.267 | ||||||||||
| Weighted average shares outstanding (in thousands): | ||||||||||||||||||||
| Basic | 489,918 | 495,715 | 499,707 | 501,055 | 500,002 | |||||||||||||||
| Diluted | 493,161 | 498,225 | 502,019 | 502,025 | 500,317 | |||||||||||||||
Certain amounts may reflect rounding adjustments.
(a) | Starting in 2015, includes the acquisitions of Pharmaq and certain assets from Abbott Animal Health. |
(b) | Adjusted net income (a non-GAAP financial measure) is defined as reported net income attributable to Zoetis excluding purchase accounting adjustments, acquisition-related costs and certain significant items. Management uses adjusted net income, among other factors, to set performance goals and to measure the performance of the overall company, as described in Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations—Adjusted net income. We believe that investors’ understanding of our performance is enhanced by disclosing this performance measure. Reconciliations of U.S. GAAP reported net income attributable to Zoetis to non-GAAP adjusted net income for the years ended December 31, 2017, 2016 and 2015 are provided in Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations—Adjusted net income. The adjusted net income measure is not, and should not be viewed as, a substitute for U.S. GAAP reported net income attributable to Zoetis. |
30 |
Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations.
Introduction
Our management’s discussion and analysis of financial condition and results of operations (MD&A) is provided to assist readers in understanding our performance, as reflected in the results of our operations, our financial condition and our cash flows. This MD&A should be read in conjunction with our consolidated financial statements and notes to consolidated financial statements included in Item 8. Financial Statements and Supplementary Data. The discussion in this MD&A contains forward-looking statements that involve substantial risks and uncertainties. Our future results could differ materially from historical performance and from those anticipated in the forward-looking statements as a result of various factors such as those discussed in Item 1A. Risk Factors and Forward-looking statements and factors that may affect future results sections of this MD&A.
Overview of our business
We are a global leader in the discovery, development, manufacture and commercialization of animal health medicines and vaccines, with a focus on both livestock and companion animals. For more than 60 years we have been committed to enhancing the health of animals and bringing solutions to our customers who raise and care for them.
We manage our operations through two geographic operating segments: the United States (U.S.) and International. Within each of these operating segments, we offer a diversified product portfolio for both livestock and companion animal customers in order to capitalize on local and regional trends and customer needs. See Notes to Consolidated Financial Statements—Note 18. Segment, Geographic and Other Revenue Information.
We directly market our products to veterinarians and livestock producers located in approximately 45 countries across North America, Europe, Africa, Asia, Australia and South America, and are a market leader in nearly all of the major regions in which we operate. Through our efforts to establish an early and direct presence in many emerging markets, such as Brazil, China and Mexico, we believe we are the largest animal health medicines and vaccines business as measured by revenue across emerging markets as a whole. In markets where we do not have a direct commercial presence, we generally contract with distributors that provide logistics and sales and marketing support for our products.
We believe our investments in one of the industry’s largest sales organizations, including our extensive network of technical and veterinary operations specialists, our high-quality manufacturing and reliability of supply, and our long track record of developing products that meet customer needs, has led to enduring and valued relationships with our customers. Our research and development (R&D) efforts enable us to deliver innovative products to address unmet needs and evolve our product lines so they remain relevant for our customers. Additionally, our management team’s focus on improving operational and cost efficiencies increases the likelihood of achieving our core growth strategies and enhancing long-term value for our shareholders.
A summary of our 2017 performance compared with the comparable 2016 and 2015 periods follows:
| Years Ended December 31, | % Change | ||||||||||||||||
| (MILLIONS OF DOLLARS) | 2017 | 2016 | 2015 | 17/16 | 16/15 | ||||||||||||
| Revenue | $ | 5,307 | $ | 4,888 | $ | 4,765 | 9 | 3 | |||||||||
| Net income attributable to Zoetis | 864 | 821 | 339 | 5 | * | ||||||||||||
Adjusted net income(a) | 1,185 | 975 | 889 | 22 | 10 | ||||||||||||
* Calculation not meaningful.
(a) | Adjusted net income is a non-GAAP financial measure. See the Adjusted net income section of this MD&A for more information. |
Our operating environment
Industry
The animal health industry, which focuses on both livestock and companion animals, is a growing industry that impacts billions of people worldwide. The primary livestock species for the production of animal protein are cattle (both beef and dairy), swine, poultry, fish and sheep. Livestock health and production are essential to meeting the growing demand for animal protein of a global population. Factors influencing growth in demand for livestock medicines and vaccines include:
• | human population growth and increasing standards of living, particularly in many emerging markets; |
• | increasing demand for improved nutrition, particularly animal protein; |
• | natural resource constraints, such as scarcity of arable land, fresh water and increased competition for cultivated land, resulting in fewer resources that will be available to meet this increased demand for animal protein; |
| • | increasing urbanization; and |
• | increased focus on food safety and food security. |
The primary companion animal species are dogs, cats and horses. Factors influencing growth in demand for companion animal medicines and vaccines include:
• | economic development and related increases in disposable income, particularly in many emerging markets; |
• | increasing pet ownership; and |
31 |
• | companion animals living longer, increasing medical treatment of companion animals and advances in companion animal medicines and vaccines. |
Product development initiatives
Our future success depends on both our existing product portfolio and our pipeline of new products, including new products that we may develop through joint ventures and products that we are able to obtain through license or acquisition. We believe we are an industry leader in animal health R&D, with a track record of generating new products and product lifecycle innovation. The majority of our R&D programs focus on product lifecycle innovation, which is defined as R&D programs that leverage existing animal health products by adding new species or claims, achieving approvals in new markets or creating new combinations and reformulations.
Perceptions of product quality, safety and reliability
We believe that animal health customers value high-quality manufacturing and reliability of supply. The importance of quality and safety concerns to pet owners, veterinarians and livestock producers also contributes to animal health brand loyalty, which we believe often continues after the loss of patent-based and regulatory exclusivity. We depend on positive perceptions of the safety and quality of our products by our customers, veterinarians and end-users.
In addition, negative beliefs about animal health products generally could impact demand for our products. For example, the issue of the potential transfer of increased antibacterial resistance in bacteria from food-producing animals to human pathogens, and the causality of that transfer, continue to be the subject of global scientific and regulatory discussion. Antibacterials refer to small molecules that can be used to treat or prevent bacterial infections and are a sub-categorization of the products that make up our anti-infectives and medicated feed additives portfolios. In some countries, this issue has led to government restrictions and bans on the use of specific antibacterials in some food-producing animals, regardless of the route of administration (in feed or injectable). These restrictions are more prevalent in countries where animal protein is plentiful and governments are willing to take action even when there is scientific uncertainty. Our total revenue attributable to antibacterials for livestock was approximately $1.2 billion for the year ended December 31, 2017.
We cannot predict whether antibacterial resistance concerns will result in additional restrictions or bans, expanded regulations, public pressure to discontinue or reduce use of antibacterials in food-producing animals or increased consumer preference for antibiotic-free protein.
The overall economic environment
In addition to industry-specific factors, we, like other businesses, face challenges related to global economic conditions. Growth in both the livestock and companion animal sectors is driven by overall economic development and related growth, particularly in many emerging markets. In recent years, certain of our customers and suppliers have been affected directly by economic downturns, which decreased the demand for our products and, in some cases, hindered our ability to collect amounts due from customers.
The cost of medicines and vaccines to our livestock producer customers is small relative to other production costs, including feed, and the use of these products is intended to improve livestock producers’ economic outcomes. As a result, demand for our products has historically been more stable than demand for other production inputs. Similarly, industry sources have reported that pet owners indicated a preference for reducing spending on other aspects of their lifestyle, including entertainment, clothing and household goods, before reducing spending on pet care. While these factors have mitigated the impact of recent downturns in the global economy, further economic challenges could increase cost sensitivity among our customers, which may result in reduced demand for our products, which could have a material adverse effect on our operating results and financial condition.
Competition
The animal health industry is competitive. Although our business is the largest by revenue in the animal health medicines and vaccines industry, we face competition in the regions in which we operate. Principal methods of competition vary depending on the particular region, species, product category or individual product. Some of these methods include new product development, quality, price, service and promotion to veterinary professionals, pet owners and livestock producers. Our competitors include the animal health businesses of large pharmaceutical companies and specialty animal health businesses. In recent years, there has been an increase in consolidation in the animal health industry. There are also several new start-up companies working in the animal health area. In addition to competition from established market participants, there could be new entrants to the animal health medicines and vaccines industry in the future. In certain markets, we also compete with companies that produce generic products, but the level of competition from generic products varies from market to market. For example, the level of generic competition is higher in Europe and certain emerging markets than in the United States.
Weather conditions and the availability of natural resources
The animal health industry and demand for many of our animal health products in a particular region are affected by weather conditions, as usage of our products follows varying weather patterns and weather-related pressures from pests, such as ticks. As a result, we may experience regional and seasonal fluctuations in our results of operations.
In addition, veterinary hospitals and practitioners depend on visits from and access to the animals under their care. Veterinarians’ patient volume and ability to operate could be adversely affected if they experience prolonged snow, ice or other severe weather conditions, particularly in regions not accustomed to sustained inclement weather. Furthermore, livestock producers depend on the availability of natural resources, including large supplies of fresh water. Their animals’ health and their ability to operate could be adversely affected if they experience a shortage of fresh water due to human population growth or floods, droughts or other weather conditions. In the event of adverse weather conditions or a shortage of fresh water, veterinarians and livestock producers may purchase less of our products.
For example, drought conditions could negatively impact, among other things, the supply of corn and the availability of grazing pastures. A decrease in harvested corn results in higher corn prices, which could negatively impact the profitability of livestock producers of cattle, pork and poultry. Higher corn prices and reduced availability of grazing pastures contribute to reductions in herd or flock sizes that in turn result in less spending on animal health products. As such, a prolonged drought could have a material adverse impact on our operating results and financial condition. Factors
32 |
influencing the magnitude and timing of effects of a drought on our performance include, but may not be limited to, weather patterns and herd management decisions.
Disease outbreaks
Sales of our livestock products could be adversely affected by the outbreak of disease carried by animals. Outbreaks of disease may reduce regional or global sales of particular animal-derived food products or result in reduced exports of such products, either due to heightened export restrictions or import prohibitions, which may reduce demand for our products. Also, the outbreak of any highly contagious disease near our main production sites could require us to immediately halt production of our products at such sites or force us to incur substantial expenses in procuring raw materials or products elsewhere. Alternatively, sales of products that treat specific disease outbreaks may increase.
Manufacturing and supply
In order to sell our products, we must be able to produce and ship our products in sufficient quantities. Many of our products involve complex manufacturing processes and are sole-sourced from certain manufacturing sites. Minor deviations in our manufacturing or logistical processes, such as temperature excursions or improper package sealing, could result in delays, inventory shortages, unanticipated costs, product recalls, product liability and/or regulatory action. In addition, a number of factors could cause production interruptions that could result in launch delays, inventory shortages, recalls, unanticipated costs or issues with our agreements under which we supply third parties.
Our manufacturing network may be unable to meet the demand for our products or we may have excess capacity if demand for our products changes. The unpredictability of a product's regulatory or commercial success or failure, the lead time necessary to construct highly technical and complex manufacturing sites, and shifting customer demand increase the potential for capacity imbalances.
Foreign exchange rates
Significant portions of our revenue and costs are exposed to changes in foreign exchange rates. Our products are sold in more than 100 countries and, as a result, our revenue is influenced by changes in foreign exchange rates. For the year ended December 31, 2017, approximately 47% of our revenue was denominated in foreign currencies. We seek to manage our foreign exchange risk, in part, through operational means, including managing same-currency revenue in relation to same-currency costs and same-currency assets in relation to same-currency liabilities. As we operate in multiple foreign currencies, including the Australian dollar, Brazilian real, Canadian dollar, Chinese yuan, euro, U.K. pound and other currencies, changes in those currencies relative to the U.S. dollar will impact our revenue, cost of goods and expenses, and consequently, net income. Exchange rate fluctuations may also have an impact beyond our reported financial results and directly impact operations. These fluctuations may affect the ability to buy and sell our goods and services between markets impacted by significant exchange rate variances. For the year ended December 31, 2017, approximately 53% of our total revenue was in U.S. dollars. Our year-over-year revenue growth was favorably impacted by 1% from changes in foreign currency values relative to the U.S. dollar.
In 2015, we recorded a net remeasurement loss of $89 million on bolivar-denominated net monetary assets, primarily related to cash deposits in Venezuela. This loss was recorded as a result of our evaluation of evolving economic conditions in Venezuela, including the devaluation of the Venezuelan bolivar in 2013 and the subsequent changes to Venezuela's foreign currency exchange mechanisms, in addition to our expectation of Venezuela's responses to changes in its economy, and continued volatility.
Operational efficiency program and supply network strategy
During 2015, we launched a comprehensive operational efficiency program, which was incremental to the previously announced supply network strategy. These initiatives have focused on reducing complexity in our product portfolios through the elimination of approximately 5,000 product stock keeping units (SKUs), changing our selling approach in certain markets, reducing our presence in certain countries, and planning to sell or exit 10 manufacturing sites over a long term period. As of December 31, 2017, we divested or exited three U.S. manufacturing sites, four international manufacturing sites, and our 55 percent ownership share of a Taiwan joint venture, inclusive of its related manufacturing site. We are also continuing to optimize our resource allocation and efficiency by reducing resources associated with non-customer facing activities and operating more efficiently as a result of less internal complexity and more standardization of processes.
As part of these initiatives, we planned to reduce certain positions through divestitures, normal attrition and involuntary terminations by approximately 2,000 to 2,500, subject to consultations with works councils and unions in certain countries. In 2016, the operations of the Guarulhos, Brazil manufacturing site, including approximately 300 employees, were transferred to us from Pfizer, which increased our range of planned reduction in certain positions to 2,300 to 2,800. Including divestitures, as of December 31, 2017, approximately 2,600 positions have been eliminated and the comprehensive operational efficiency program is substantially complete, however in the fourth quarter of 2017, we expanded the supply network strategy initiative which increases our planned reductions in certain positions by 40. We expect these additional reductions related to our supply network strategy to take place over the next twelve months, and the remainder of the reductions from the initial plan to take place through divestitures over the next several years.
For additional information, see Notes to Consolidated Financial Statements— Note 4B. Acquisitions and Divestitures: Divestitures and Note 5. Restructuring Charges and Other Costs Associated with Acquisitions and Cost-Reduction/Productivity Initiatives.
Our growth strategies
We seek to enhance the health of animals and to bring solutions to our customers who raise and care for them. We have a global presence in both developed and emerging markets and we intend to grow our business by pursuing the following core strategies:
| • | leverage our direct local presence and strong customer relationships—Through our direct selling commercial model, we can deepen our understanding of our customers’ businesses and can encourage the adoption of more sophisticated animal health products; |
33 |
| • | further penetrate emerging markets—We seek to maximize our presence where economic development is driving increased demand for animal protein and increased demand for and spending on companion animals; |
| • | pursue new product research and development and value-added product lifecycle innovation to extend our product portfolio—New product R&D and product lifecycle innovation enable us to deliver products to address unmet needs and evolve our product lines so they remain relevant for our customers. We leverage our strong direct presence in many regions and cost-effectively develop new products; |
| • | remain the partner of choice for access to new products and technologies—We support cutting-edge research and secure the right to develop and commercialize new products and technologies; |
| • | continue to provide high-quality products and improve manufacturing production margins—We believe our manufacturing and supply chain provides us with a global platform for continued expansion, including in emerging markets, and that our quality and reliability differentiate us from our competitors; and |
| • | expand into complementary businesses to become a more complete, trusted partner in providing solutions—We believe we have the potential to generate incremental and complementary revenue, in the areas of diagnostics, genetics, devices, dairy data management, e-learning and professional consulting, which could also enhance the loyalty of our customer base and may lead to increased product sales. |
Components of revenue and costs and expenses
Our revenue, costs and expenses are reported for the year ended December 31 for each year presented, except for operations outside the United States, for which the financial information is included in our consolidated financial statements for the fiscal year ended November 30 for each year presented.
Revenue
Our revenue is primarily derived from our diversified product portfolio of medicines and vaccines used to treat and protect livestock and companion animals. Generally, our products are promoted to veterinarians and livestock producers by our sales organization which includes sales representatives and technical and veterinary operations specialists, and then sold directly by us or through distributors. The depth of our product portfolio enables us to address the varying needs of customers in different species and geographies. In 2017, our top two selling products, Apoquel and the ceftiofur line, each contributed approximately 7% of our revenue, and combined with our next two top selling products, Draxxin and Revolution, these four contributed approximately 25% of our revenue. Our top ten product lines contributed 39% of our revenue. For additional information regarding our products, including descriptions of our product lines that each represented approximately 1% or more of our revenue in 2017, see Item 1. Business—Products.
Costs and expenses
Costs of sales consist primarily of cost of materials, facilities and other infrastructure used to manufacture our medicine and vaccine products and royalty expenses associated with the intellectual property of our products, when relevant.
Selling, general and administrative (SG&A) expenses consist of, among other things, the internal and external costs of marketing, promotion, advertising and shipping and handling as well as certain costs related to business technology, facilities, legal, finance, human resources, business development, public affairs and procurement.
Research and development (R&D) expenses consist primarily of project costs specific to new product R&D and product lifecycle innovation, overhead costs associated with R&D operations and investments that support local market clinical trials for approved indications and expenses related to regulatory approvals for our products. We do not disaggregate R&D expenses by research stage or by therapeutic area for purposes of managing our business.
Amortization of intangible assets consists primarily of the amortization expense for identifiable finite-lived intangible assets that have been acquired through business combinations. These assets consist of, but are not limited to, developed technology, brands and trademarks.
Restructuring charges and certain acquisition-related costs consist of all restructuring charges (those associated with acquisition activity and those associated with cost reduction/productivity initiatives), as well as costs associated with acquiring and integrating businesses. Restructuring charges are associated with employees, assets and activities that will not continue in the company. Acquisition-related costs are associated with acquiring and integrating acquired businesses, such as Pharmaq Holding AS and Abbott Animal Health (AAH) both acquired in 2015, and may include transaction costs and expenditures for consulting and the integration of systems and processes.
Other (income)/deductions—net consist primarily of various items including net (gains)/losses on asset disposals, royalty-related income, foreign exchange translation (gains)/losses and certain asset impairment charges.
Significant accounting policies and application of critical accounting estimates
In presenting our financial statements in conformity with U.S. GAAP, we are required to make estimates and assumptions that affect the reported amounts of assets, liabilities, revenue, costs and expenses and related disclosures. For a description of our significant accounting policies, see Notes to Consolidated Financial Statements— Note 3. Significant Accounting Policies.
We believe that the following accounting policies are critical to an understanding of our consolidated financial statements as they require the application of the most difficult, subjective and complex judgments and, therefore, could have the greatest impact on our financial statements: (i) fair value; (ii) revenue; (iii) asset impairment reviews; and (iv) contingencies.
34 |
Below are some of our more critical accounting estimates. See also Notes to Consolidated Financial Statements— Note 3. Significant Accounting Policies— Estimates and Assumptions for a discussion about the risks associated with estimates and assumptions.
Fair value
For a discussion about the application of fair value to our long-term debt and financial instruments, see Notes to Consolidated Financial Statements—Note 9. Financial Instruments.
For a discussion about the application of fair value to our business combinations, see Notes to Consolidated Financial Statements— Note 3. Significant Accounting Policies: Fair Value.
For a discussion about the application of fair value to our asset impairment reviews, see Asset impairment reviews below.
Revenue
Our gross product revenue is subject to deductions that are generally estimated and recorded in the same period that the revenue is recognized and primarily represents sales returns and revenue incentives. For example:
• | for sales returns, we perform calculations in each market that incorporate the following, as appropriate: local returns policies and practices; returns as a percentage of revenue; an understanding of the reasons for past returns; estimated shelf life by product; an estimate of the amount of time between shipment and return or lag time; and any other factors that could impact the estimate of future returns, product recalls, discontinuation of products or a changing competitive environment; and |
• | for revenue incentives, we use our historical experience with similar incentives programs to estimate the impact of such programs on revenue. |
If any of our ratios, factors, assessments, experiences or judgments are not indicative or accurate predictors of our future experience, our results could be materially affected. Although the amounts recorded for these revenue deductions are dependent on estimates and assumptions, historically our adjustments to actual results have not been material. The sensitivity of our estimates can vary by program, type of customer and geographic location.
Amounts recorded for revenue deductions can result from a complex series of judgments about future events and uncertainties and can rely on estimates and assumptions. For further information about the risks associated with estimates and assumptions, see Notes to Consolidated Financial Statements— Note 3. Significant Accounting Policies: Estimates and Assumptions.
Asset impairment reviews
We review all of our long-lived assets for impairment indicators throughout the year and we perform detailed testing whenever impairment indicators are present. In addition, we perform impairment testing for goodwill and indefinite-lived intangible assets at least annually. When necessary, we record charges for impairments of long-lived assets for the amount by which the fair value is less than the carrying value of these assets.
Our impairment review processes are described below and in Notes to Consolidated Financial Statements— Note 3. Significant Accounting Policies: Amortization of Intangible Assets, Depreciation and Certain Long-Lived Assets and, for deferred tax assets, in Note 3. Significant Accounting Policies: Deferred Tax Assets and Liabilities and Income Tax Contingencies.
Examples of events or circumstances that may be indicative of impairment include:
• | a significant adverse change in the extent or manner in which an asset is used. For example, restrictions imposed by the regulatory authorities could affect our ability to manufacture or sell a product, and |
• | a projection or forecast that demonstrates losses or reduced profits associated with an asset. This could result, for example, from the introduction of a competitor’s product that results in a significant loss of market share or the inability to achieve the previously projected revenue growth, or from the lack of acceptance of a product by customers. |
Our impairment reviews of most of our long-lived assets depend on the determination of fair value, as defined by U.S. GAAP, and these judgments can materially impact our results of operations. A single estimate of fair value can result from a complex series of judgments about future events and uncertainties and can rely on estimates and assumptions. For information about the risks associated with estimates and assumptions, see Notes to Consolidated Financial Statements—Note 3. Significant Accounting Policies: Estimates and Assumptions.
Intangible assets other than goodwill
We test indefinite-lived intangible assets for impairment at least annually, or more frequently if impairment indicators exist, by first assessing qualitative factors to determine whether it is more likely than not that the fair value of the indefinite-lived intangible asset is less than its carrying amount. If we conclude it is more likely than not that the fair value is less than the carrying amount, a quantitative test that compares the fair value of the indefinite-lived intangible asset with its carrying value is performed. If the fair value is less than the carrying amount, an impairment loss is recognized.
As a result of our overall intangible asset impairment reviews, we recorded the following impairments of identifiable intangible assets other than goodwill, in Restructuring charges and certain acquisition-related costs and Other (income)/deductions—net, as applicable:
| • | In 2017, we did not have any significant intangible asset impairment charges. |
| • | In 2016, the intangible asset impairment charges reflect approximately $1 million of finite-lived trademarks related to a canine pain management product that is no longer marketed. |
35 |
| • | In 2015, the intangible asset impairment charges reflect (i) approximately $27 million of developed technology rights due to product rationalization decisions associated with our operational efficiency initiative; and (ii) approximately $2 million of acquired in-process research and development (IPR&D) assets related to the termination of a canine oncology project. |
When we are required to determine the fair value of intangible assets other than goodwill, we use an income approach, specifically the multi-period excess earnings method, also known as the discounted cash flow method. We start with a forecast of all the expected net cash flows associated with the asset, which includes the application of a terminal value for indefinite-lived assets, and then we apply an asset-specific discount rate to arrive at a net present value amount. Some of the more significant estimates and assumptions inherent in this approach include: the amount and timing of the projected net cash flows, which includes the expected impact of competitive, legal and/or regulatory forces on the projections, the impact of technological risk associated with IPR&D assets, as well as the selection of a long-term growth rate; the discount rate, which seeks to reflect the risks inherent in the projected cash flows; foreign currency fluctuations; and the tax rate, which seeks to incorporate the geographic diversity of the projected cash flows.
While all identifiable intangible assets can be impacted by events and thus lead to impairment, in general, identifiable intangible assets that are at the highest risk of impairment include IPR&D assets (approximately $224 million as of December 31, 2017). IPR&D assets are higher-risk assets because R&D is an inherently risky activity.
For a description of our accounting policy, see Notes to Consolidated Financial Statements—Note 3. Significant Accounting Policies: Amortization of Intangible Assets, Depreciation and Certain Long-Lived Assets.
Goodwill
Goodwill represents the excess of the consideration transferred over the fair value of net assets of businesses purchased and is assigned to reporting units. We test goodwill for impairment on at least an annual basis, or more frequently if impairment indicators exist, either by assessing qualitative factors to determine whether it is more likely than not that the fair value of a reporting unit is less than its carrying amount or by performing a quantitative assessment.
Factors considered in the qualitative assessment include general macroeconomic conditions, conditions specific to the industry and market, cost factors which could have a significant effect on earnings or cash flows, the overall financial performance of the reporting unit and whether there have been sustained declines in our share price. Additionally, we evaluate the extent to which the fair value exceeded the carrying value of the reporting unit at the date of the last quantitative assessment performed.
When performing a quantitative assessment to test for goodwill impairment we utilize the income approach, which is forward-looking, and relies primarily on internal forecasts. Within the income approach, the method that we use is the discounted cash flow method. We start with a forecast of all the expected net cash flows associated with the reporting unit, which includes the application of a terminal value, and then apply a reporting unit-specific discount rate to arrive at a net present value. Some of the more significant estimates and assumptions inherent in this approach include: the amount and timing of the projected net cash flows, which includes the expected impact of technological risk and competitive, legal and/or regulatory forces on the projections, as well as the selection of a long-term growth rate; the discount rate, which seeks to reflect the various risks inherent in the projected cash flows; and the tax rate, which seeks to incorporate the geographic diversity of the projected cash flows.
In 2017, we performed both qualitative and select quantitative impairment assessments as of October 1, 2017, which did not result in the impairment of goodwill associated with any of our reporting units.
In 2016, we performed a qualitative impairment assessment as of October 2, 2016, determined that is it not more likely than not that the fair value of our reporting units are less than the carrying amount, and therefore concluded that a quantitative fair value test was not required.
For all of our reporting units, there are a number of future events and factors that may impact future results and that could potentially have an impact on the outcome of subsequent goodwill impairment testing. For a list of these factors, see Forward-looking statements and factors that may affect future results.
For a description of our accounting policy, see Notes to Consolidated Financial Statements— Note 3. Significant Accounting Policies: Amortization of Intangible Assets, Depreciation and Certain Long-Lived Assets.
Contingencies
For a discussion about income tax contingencies, see Notes to Consolidated Financial Statements— Note 8D. Tax Matters: Tax Contingencies.
For a discussion about legal contingencies, guarantees and indemnifications, see Notes to Consolidated Financial Statement— Note 17. Commitments and Contingencies.
Analysis of the consolidated statements of income
The following discussion and analysis of our consolidated statements of income should be read along with our consolidated financial statements, and the notes thereto.
36 |
| Year Ended December 31, | % Change | |||||||||||||||||
| (MILLIONS OF DOLLARS) | 2017 | 2016 | 2015 | 17/16 | 16/15 | |||||||||||||
| Revenue | $ | 5,307 | $ | 4,888 | $ | 4,765 | 9 | 3 | ||||||||||
| Costs and expenses: | ||||||||||||||||||
Cost of sales(a) | 1,775 | 1,666 | 1,738 | 7 | (4 | ) | ||||||||||||
| % of revenue | 33 | % | 34 | % | 36 | % | ||||||||||||
Selling, general and administrative expenses(a) | 1,334 | 1,364 | 1,532 | (2 | ) | (11 | ) | |||||||||||
| % of revenue | 25 | % | 28 | % | 32 | % | ||||||||||||
Research and development expenses(a) | 382 | 376 | 364 | 2 | 3 | |||||||||||||
| % of revenue | 7 | % | 8 | % | 8 | % | ||||||||||||
Amortization of intangible assets(a) | 91 | 85 | 61 | 7 | 39 | |||||||||||||
| Restructuring charges and certain acquisition-related costs | 19 | 5 | 320 | * | (98 | ) | ||||||||||||
| Interest expense, net of capitalized interest | 175 | 166 | 124 | 5 | 34 | |||||||||||||
| Other (income)/deductions—net | 6 | (2 | ) | 81 | * | * | ||||||||||||
| Income before provision for taxes on income | 1,525 | 1,228 | 545 | 24 | * | |||||||||||||
| % of revenue | 29 | % | 25 | % | 11 | % | ||||||||||||
| Provision for taxes on income | 663 | 409 | 206 | 62 | 99 | |||||||||||||
| Effective tax rate | 43.5 | % | 33.3 | % | 37.8 | % | ||||||||||||
| Net income before allocation to noncontrolling interests | 862 | 819 | 339 | 5 | * | |||||||||||||
| Less: Net income attributable to noncontrolling interests | (2 | ) | (2 | ) | — | — | * | |||||||||||
| Net income attributable to Zoetis | $ | 864 | $ | 821 | $ | 339 | 5 | * | ||||||||||
| % of revenue | 16 | % | 17 | % | 7 | % | ||||||||||||
* Calculation not meaningful.
Certain amounts and percentages may reflect rounding adjustments.
(a) | Amortization expense related to finite-lived acquired intangible assets that contribute to our ability to sell, manufacture, research, market and distribute products, compounds and intellectual property is included in Amortization of intangible assets as these intangible assets benefit multiple business functions. Amortization expense related to finite-lived acquired intangible assets that are associated with a single function is included in Cost of sales, Selling, general and administrative expenses or Research and development expenses, as appropriate. |
Revenue
Total revenue by operating segment was as follows:
| Year Ended December 31, | % Change | ||||||||||||||||
| (MILLIONS OF DOLLARS) | 2017 | 2016 | 2015 | 17/16 | 16/15 | ||||||||||||
| U.S. | $ | 2,620 | $ | 2,447 | $ | 2,328 | 7 | 5 | |||||||||
| International | 2,643 | 2,390 | 2,386 | 11 | — | ||||||||||||
| Total operating segments | 5,263 | 4,837 | 4,714 | 9 | 3 | ||||||||||||
| Contract manufacturing | 44 | 51 | 51 | (14 | ) | — | |||||||||||
| Total Revenue | $ | 5,307 | $ | 4,888 | $ | 4,765 | 9 | 3 | |||||||||
Certain amounts and percentages may reflect rounding adjustments.
On a global basis, the mix of revenue between livestock and companion animal products was as follows:
| Year Ended December 31, | % Change | |||||||||||||||||
| (MILLIONS OF DOLLARS) | 2017 | 2016 | 2015 | 17/16 | 16/15 | |||||||||||||
| Livestock | $ | 3,037 | $ | 2,881 | $ | 2,958 | 5 | (3 | ) | |||||||||
| Companion animal | 2,226 | 1,956 | 1,756 | 14 | 11 | |||||||||||||
| Contract manufacturing | 44 | 51 | 51 | (14 | ) | — | ||||||||||||
| Total Revenue | $ | 5,307 | $ | 4,888 | $ | 4,765 | 9 | 3 | ||||||||||
Certain amounts and percentages may reflect rounding adjustments.
37 |
2017 vs. 2016
Total revenue increased by $419 million in 2017, compared with 2016 reflecting operational revenue growth of $406 million, or 8%. Operational revenue growth (a non-GAAP financial measure) is defined as revenue growth excluding the impact of foreign exchange. Operational revenue growth was comprised primarily of the following:
| • | increased sales of our dermatology portfolio and new product launches, which contributed approximately 7%; and |
| • | growth of our in-line products, which contributed approximately 2%, of which volume comprised 1% and price comprised 1%, |
partially offset by:
| • | product rationalizations as part of the operational efficiency initiative, which resulted in a decline of approximately 1%. |
Foreign exchange increased our reported revenue growth by approximately 1%.
2016 vs. 2015
Total revenue increased by $123 million in 2016, compared with 2015, reflecting operational revenue growth of $250 million, or 5%. Operational revenue growth was comprised primarily of the following:
| • | increased sales of Apoquel® and new product launches, which contributed approximately 5%; |
| • | growth of our in-line products, which contributed approximately 3%, of which price comprised 2% and volume comprised 1%; and |
| • | recent acquisitions, primarily Pharmaq and the acquisition of certain assets of Abbott Animal Health, which contributed approximately 2%, |
partially offset by:
| • | our product and market rationalization as part of the operational efficiency initiative, which resulted in a decline of approximately 5%. |
Foreign exchange reduced our reported revenue growth by approximately 2%.
Costs and Expenses
Cost of sales
| Year Ended December 31, | % Change | ||||||||||||||||
| (MILLIONS OF DOLLARS) | 2017 | 2016 | 2015 | 17/16 | 16/15 | ||||||||||||
| Cost of sales | $ | 1,775 | $ | 1,666 | $ | 1,738 | 7 | (4 | ) | ||||||||
| % of revenue | 33 | % | 34 | % | 36 | % | |||||||||||
Certain amounts and percentages may reflect rounding adjustments.
2017 vs. 2016
Cost of sales increased $109 million, or 7%, in 2017 compared with 2016, primarily as a result of:
| • | an increase in sales volume; |
| • | an increase in manufacturing and supply costs; and |
| • | unfavorable foreign exchange, |
partially offset by:
| • | a decrease in inventory obsolescence, scrap and other charges; |
| • | the nonrecurrence of charges reflecting fair value adjustments to inventory related to the acquisition of Pharmaq; and |
| • | favorable product mix. |
2016 vs. 2015
Cost of sales decreased $72 million, or 4%, in 2016 compared with 2015, primarily as a result of:
| • | favorable product mix; |
| • | favorable foreign exchange; |
| • | a reduction in the amount of costs related to becoming an independent public company; |
| • | lower global manufacturing and supply costs; and |
| • | business model changes in Venezuela, |
partially offset by:
| • | the inclusion of the cost of products for Pharmaq, as well as charges reflecting fair value adjustments to inventory related to the acquisition of Pharmaq; |
| • | an increase in sales volume; and |
| • | an increase in inventory obsolescence, scrap and other charges. |
38 |
Selling, general and administrative expenses
| Year Ended December 31, | % Change | |||||||||||||||||
| (MILLIONS OF DOLLARS) | 2017 | 2016 | 2015 | 17/16 | 16/15 | |||||||||||||
| Selling, general and administrative expenses | $ | 1,334 | $ | 1,364 | $ | 1,532 | (2 | ) | (11 | ) | ||||||||
| % of revenue | 25 | % | 28 | % | 32 | % | ||||||||||||
Certain amounts and percentages may reflect rounding adjustments.
2017 vs. 2016
SG&A expenses decreased $30 million, or 2%, in 2017 compared with 2016, primarily as a result of:
| • | the nonrecurrence of additional costs related to becoming an independent public company; |
| • | a reduction in marketing and general and administrative expense driven by our operational efficiency initiative; and |
| • | a reduction in consulting charges relating to our operational efficiency initiative, |
partially offset by:
| • | higher advertising and promotional spending associated with new products and Apoquel®. |
2016 vs. 2015
SG&A expenses decreased $168 million, or 11%, in 2016 compared with 2015, primarily as a result of:
| • | a reduction in marketing and general and administrative expense driven by our operational efficiency initiative; |
| • | a reduction in the amount of additional costs related to becoming an independent public company; |
| • | favorable foreign exchange; and |
| • | a reduction in consulting charges relating to our operational efficiency initiative, |
partially offset by:
| • | higher advertising and promotional spending associated with new products; |
| • | the inclusion of Pharmaq; and |
| • | an increase in depreciation associated with the implementation of our enterprise resource planning system. |
Research and development expenses
| Year Ended December 31, | % Change | |||||||||||||||
| (MILLIONS OF DOLLARS) | 2017 | 2016 | 2015 | 17/16 | 16/15 | |||||||||||
| Research and development expenses | $ | 382 | $ | 376 | $ | 364 | 2 | 3 | ||||||||
| % of revenue | 7 | % | 8 | % | 8 | % | ||||||||||
Certain amounts and percentages may reflect rounding adjustments.
2017 vs. 2016
R&D expenses increased $6 million, or 2%, in 2017 compared with 2016, primarily as a result of:
| • | the inclusion of a veterinary diagnostics business acquired in 2016 and an Irish biologic therapeutics company in 2017, |
partially offset by:
| • | a reduction in spending driven by our operational efficiency initiative. |
2016 vs. 2015
R&D expenses increased $12 million, or 3%, in 2016 compared with 2015, primarily as a result of:
| • | higher development expenses for late-stage projects; and |
| • | the inclusion of Pharmaq; |
partially offset by:
| • | a reduction in spending driven by our operational efficiency initiative. |
39 |
Amortization of intangible assets
| Year Ended December 31, | % Change | |||||||||||||||
| (MILLIONS OF DOLLARS) | 2017 | 2016 | 2015 | 17/16 | 16/15 | |||||||||||
| Amortization of intangible assets | $ | 91 | $ | 85 | $ | 61 | 7 | 39 | ||||||||
Certain amounts and percentages may reflect rounding adjustments.
2017 vs. 2016
Amortization of intangible assets increased $6 million, or 7%, in 2017 compared with 2016, primarily as a result of certain intangible assets, acquired in November 2015 as part of the Pharmaq acquisition, being placed into service during the first quarter of 2017.
2016 vs. 2015
Amortization of intangible assets increased $24 million, or 39%, in 2016 compared with 2015, primarily as a result of certain intangible assets acquired in the Pharmaq acquisition in November 2015.
Restructuring charges and certain acquisition-related costs
| Year Ended December 31, | % Change | ||||||||||||||||
| (MILLIONS OF DOLLARS) | 2017 | 2016 | 2015 | 17/16 | 16/15 | ||||||||||||
| Restructuring charges and certain acquisition-related costs | $ | 19 | $ | 5 | $ | 320 | * | (98 | ) | ||||||||
* Calculation not meaningful.
Certain amounts and percentages may reflect rounding adjustments.
During 2015, we launched a comprehensive operational efficiency program, which was incremental to the previously announced supply network strategy. These initiatives have focused on reducing complexity in our product portfolios through the elimination of approximately 5,000 product stock keeping units (SKUs), changing our selling approach in certain markets, reducing our presence in certain countries, and planning to sell or exit 10 manufacturing sites over a long term period. As of December 31, 2017, we divested or exited three U.S. manufacturing sites, four international manufacturing sites, and our 55 percent ownership share of a Taiwan joint venture, inclusive of its related manufacturing site. We are also continuing to optimize our resource allocation and efficiency by reducing resources associated with non-customer facing activities and operating more efficiently as a result of less internal complexity and more standardization of processes. As part of these initiatives, we planned to reduce certain positions through divestitures, normal attrition and involuntary terminations by approximately 2,000 to 2,500, subject to consultations with works councils and unions in certain countries. In 2016, the operations of the Guarulhos, Brazil manufacturing site, including approximately 300 employees, were transferred to us from Pfizer, which increased our range of planned reduction in certain positions to 2,300 to 2,800. Including divestitures, as of December 31, 2017, approximately 2,600 positions have been eliminated and the comprehensive operational efficiency program is substantially complete, however in the fourth quarter of 2017, we expanded the supply network strategy initiative which increases our planned reductions in certain positions by 40. We expect these additional reductions related to our supply network strategy to take place over the next twelve months, and the remainder of the reductions from the initial plan to take place through divestitures over the next several years.
Our acquisition-related costs primarily relate to restructuring charges for employees, assets and activities that will not continue in the future, as well as integration costs. The majority of these net restructuring charges are related to termination costs, but we also exited a number of distributor and other contracts and performed certain facility rationalization efforts. Our integration costs are generally comprised of consulting costs related to the integration of systems and processes, as well as product transfer costs.
For additional information regarding restructuring charges and acquisition-related costs, see Notes to Consolidated Financial Statements— Note 5. Restructuring Charges and Other Costs Associated with Acquisitions and Cost-Reduction/Productivity Initiatives.
2017 vs. 2016
Restructuring charges and certain acquisition-related costs increased by $14 million in 2017 compared with 2016, primarily as a result of:
| • | higher employee termination costs a result of the acquisition of an Irish biologic therapeutics company in the third quarter of 2017, initiatives to better align our organizational structure in Europe, and our operational efficiency initiative and supply network strategy, and |
| • | higher integration costs in 2017 as a result of the acquisition of an Irish biologic therapeutics company in the third quarter of 2017, and the acquisition of Pharmaq in 2015. |
2016 vs. 2015
Restructuring charges and certain acquisition-related costs decreased by $315 million, or 98%, in 2016 compared with 2015, primarily as a result of:
| • | higher employee termination costs and higher asset impairment charges incurred in 2015 as a result of the launch of our operational efficiency initiative and supply network strategy, and |
| • | lower transaction costs in 2016. |
40 |
Interest expense, net of capitalized interest
| Year Ended December 31, | % Change | |||||||||||||||
| (MILLIONS OF DOLLARS) | 2017 | 2016 | 2015 | 17/16 | 16/15 | |||||||||||
| Interest expense, net of capitalized interest | $ | 175 | $ | 166 | $ | 124 | 5 | 34 | ||||||||
Certain amounts and percentages may reflect rounding adjustments.
2017 vs. 2016
Interest expense, net of capitalized interest, increased by $9 million, or 5%, in 2017 compared with 2016, as a result of the issuance of $1.25 billion of our senior notes in September 2017.
2016 vs. 2015
Interest expense, net of capitalized interest, increased by $42 million, or 34%, in 2016 compared with 2015, as a result of the issuance of $1.25 billion of our senior notes in November 2015.
Other (income)/deductions—net
| Year Ended December 31, | % Change | |||||||||||||||
| (MILLIONS OF DOLLARS) | 2017 | 2016 | 2015 | 17/16 | 16/15 | |||||||||||
| Other (income)/deductions—net | $ | 6 | $ | (2 | ) | $ | 81 | * | * | |||||||
* Calculation not meaningful.
Certain amounts and percentages may reflect rounding adjustments.
2017 vs. 2016
The change in Other (income)/deductions—net reflects an unfavorable impact of $8 million in 2017 compared with 2016, primarily as a result of:
| • | a net loss of $11 million in 2017 related to sales of certain manufacturing sites and products, including the disposal of our manufacturing site in Guarulhos, Brazil, in the fourth quarter of 2017, compared with a net gain of $26 million in 2016 related to sales of certain manufacturing sites and products, and |
| • | lower royalty income, |
partially offset by:
| • | lower foreign currency losses, primarily driven by costs related to hedging and exposures to certain emerging market currencies, and |
| • | income of a $8 million in 2017 due to an insurance recovery related to commercial settlements in Mexico, as well as a favorable outcome on a patent infringement settlement, compared with a charge of $14 million in 2016 related to a commercial settlement in Mexico. |
2016 vs. 2015
The change in Other (income)/deductions—net reflects a favorable impact of $83 million on income in 2016 compared with 2015, primarily as a result of:
| • | charges of $89 million in 2015 related to the revaluation of the net monetary assets in Venezuela; and |
| • | a net gain of $26 million in 2016 related to sales of certain manufacturing sites and products, |
partially offset by:
| • | a charge of $14 million in 2016 related to a commercial settlement in Mexico; and |
| • | a charge of $15 million related to the devaluation of the Egyptian pound in November 2016. |
Provision for taxes on income
| Year Ended December 31, | % Change | |||||||||||||||
| (MILLIONS OF DOLLARS) | 2017 | 2016 | 2015 | 17/16 | 16/15 | |||||||||||
| Provision for taxes on income | $ | 663 | $ | 409 | $ | 206 | 62 | 99 | ||||||||
| Effective tax rate | 43.5 | % | 33.3 | % | 37.8 | % | ||||||||||
Certain amounts and percentages may reflect rounding adjustments.
The income tax provision in the consolidated statements of income includes tax costs and benefits, such as uncertain tax positions, repatriation decisions and audit settlements, among others.
On December 22, 2017, the Tax Cuts and Jobs Act (the Tax Act) was enacted which, among other changes, reduces the U.S. federal corporate tax rate from 35% to 21% effective January 1, 2018. The Tax Act makes broad and complex changes to the U.S. tax code and it will take time to fully analyze the impact of the changes. Based on the information available, and the current interpretation of the Tax Act, the company was able to make a reasonable estimate and recorded a provisional tax expense related to the one-time mandatory deemed repatriation tax, partially offset by the remeasurement of the deferred tax assets and liabilities, as of the date of enactment, due to the reduction in the U.S. federal corporate tax rate. Pursuant to the Staff Accounting Bulletin published by the Securities and Exchange Commission on December 22, 2017, addressing the challenges in accounting for the effects of the Tax Act in the period of enactment, companies must report provisional amounts for those specific income tax effects of the Tax Act for which the accounting is incomplete but a reasonable estimate can be determined. Those provisional amounts will be subject to adjustment during a measurement period of up to one year from the enactment date. Pursuant to this guidance, the estimated impact of the Tax Act is
41 |
based on a preliminary review of the new law and projected future financial results and is subject to revision based upon further analysis and interpretation of the Tax Act and to the extent that future results differ from currently available projections.
On January 11, 2016, the European Commission concluded that the Belgium “excess profits tax scheme” constitutes illegal state aid and ordered the Belgian authorities to recover benefits from taxpayers who are parties to an Excess Profits Ruling. As a result, the 2017 and 2016 effective tax rates do not reflect any benefit of the excess profits ruling and does incorporate the Belgium government's recovery of benefits for the periods 2013 through 2015 offset by the remeasurement of the company’s deferred tax assets and liabilities using the rates expected to be in place at the time of the reversal and without consideration of implementation of any future operational changes.
For more information, see Notes to Consolidated Financial Statements— Note 8A. Tax Matters: Taxes on Income.
2017 vs. 2016
The higher effective tax rate in 2017 compared with 2016 is primarily due to the following components:
| • | a $212 million net discrete provisional tax expense recorded in the fourth quarter of 2017, related to the impact of the Tax Act enacted on December 22, 2017, including a one-time mandatory deemed repatriation tax, partially offset by a net tax benefit related to the remeasurement of the deferred tax assets and liabilities, as of the date of enactment, due to the reduction in the U.S. federal corporate tax rate; |
| • | changes in valuation allowances and resolution of other tax items; |
| • | tax expense related to changes in uncertain tax positions, see Notes to Consolidated Financial Statements— Note 8D. Tax Matters: Tax Contingencies, |
partially offset by:
| • | changes in the jurisdictional mix of earnings, which includes the impact of the location of earnings from operations and repatriation costs. The jurisdictional mix of earnings can vary as a result of repatriation decisions and operating fluctuations in the normal course of business and the impact of non-deductible items; |
| • | a $15 million discrete tax benefit recorded in the fourth quarter of 2017 related to the effective settlement of certain issues with U.S. and non-U.S. tax authorities; |
| • | a $9 million discrete tax benefit recorded in 2017 related to the excess tax benefits for share-based payments; and |
| • | a $3 million discrete tax benefit recorded in the first quarter of 2017 related to a remeasurement of deferred tax assets and liabilities as a result of a change in non-U.S. statutory tax rates. |
2016 vs. 2015
The lower effective tax rate in 2016 compared with 2015 is primarily due to the following components:
| • | the impact of the extent and location of restructuring charges related to the operational efficiency initiative, supply network strategy, asset impairments and gains and losses on asset divestitures; |
| • | a $15 million discrete tax benefit recorded in the fourth quarter of 2016 related to prior period tax adjustments; |
| • | a $10 million discrete tax benefit recorded in the first quarter of 2016 related to a remeasurement of deferred taxes as a result of a change in statutory tax rates; |
| • | a $7 million discrete tax benefit recorded in 2016 related to the excess tax benefits for share-based payments; and |
| • | a $2 million discrete tax benefit recorded in the second half of 2016 related to a remeasurement of the company’s deferred tax assets and liabilities using the tax rates expected to be in place going forward as a result of the implementation of operational changes, |
partially offset by:
| • | changes in the jurisdictional mix of earnings, which includes the impact of the location of earnings from operations and repatriation costs. The jurisdictional mix of earnings can vary as a result of repatriation decisions and operating fluctuations in the normal course of business and the impact of non-deductible items; |
| • | a net tax expense of approximately $35 million mainly recorded in the first half of 2016 related to the impact of the European Commission’s negative decision on the excess profits rulings in Belgium. This net charge represents the recovery of prior tax benefits for the periods 2013 through 2015 offset by the remeasurement of the company’s deferred tax assets and liabilities using the rates expected to be in place at the time of the reversal and without consideration of implementation of any future operational changes, and does not include any benefits associated with a successful appeal of the decision; |
| • | changes in valuation allowances and resolution of other tax items; and |
| • | tax expense related to changes in uncertain tax positions, see Notes to Consolidated Financial Statements— Note 8D. Tax Matters: Tax Contingencies. |
42 |
Operating Segment Results
We believe that it is important to not only understand overall revenue and earnings growth, but also “operational growth.” Operational growth is defined as revenue or earnings growth excluding the impact of foreign exchange.
On a global basis, the mix of revenue between livestock and companion animal products was as follows:
| % Change | |||||||||||||||||||||||||||
| 17/16 | 16/15 | ||||||||||||||||||||||||||
| Related to | Related to | ||||||||||||||||||||||||||
| Year Ended December 31, | Foreign | Foreign | |||||||||||||||||||||||||
| (MILLIONS OF DOLLARS) | 2017 | 2016 | 2015 | Total | Exchange | Operational | Total | Exchange | Operational | ||||||||||||||||||
| U.S. | |||||||||||||||||||||||||||
| Livestock | $ | 1,244 | $ | 1,227 | $ | 1,251 | 1 | — | 1 | (2 | ) | — | (2 | ) | |||||||||||||
| Companion animal | 1,376 | 1,220 | 1,077 | 13 | — | 13 | 13 | — | 13 | ||||||||||||||||||
| 2,620 | 2,447 | 2,328 | 7 | — | 7 | 5 | — | 5 | |||||||||||||||||||
| International | |||||||||||||||||||||||||||
| Livestock | 1,793 | 1,654 | 1,707 | 8 | 1 | 7 | (3 | ) | (6 | ) | 3 | ||||||||||||||||
| Companion animal | 850 | 736 | 679 | 15 | (1 | ) | 16 | 8 | (5 | ) | 13 | ||||||||||||||||
| 2,643 | 2,390 | 2,386 | 11 | 1 | 10 | — | (5 | ) | 5 | ||||||||||||||||||
| Total | |||||||||||||||||||||||||||
| Livestock | 3,037 | 2,881 | 2,958 | 5 | — | 5 | (3 | ) | (4 | ) | 1 | ||||||||||||||||
| Companion animal | 2,226 | 1,956 | 1,756 | 14 | — | 14 | 11 | (2 | ) | 13 | |||||||||||||||||
| Contract manufacturing | 44 | 51 | 51 | (14 | ) | 1 | (15 | ) | — | (3 | ) | 3 | |||||||||||||||
| $ | 5,307 | $ | 4,888 | $ | 4,765 | 9 | 1 | 8 | 3 | (2 | ) | 5 | |||||||||||||||
Certain amounts and percentages may reflect rounding adjustments.
Earnings by segment and the operational and foreign exchange changes versus the comparable prior year period were as follows:
| % Change | ||||||||||||||||||||
| 17/16 | 16/15 | |||||||||||||||||||
| Related to | Related to | |||||||||||||||||||
| Year Ended December 31, | Foreign | Foreign | ||||||||||||||||||
| (MILLIONS OF DOLLARS) | 2017 | 2016 | 2015 | Total | Exchange | Operational | Total | Exchange | Operational | |||||||||||
| U.S. | $ | 1,637 | $ | 1,508 | $ | 1,390 | 9 | — | 9 | 8 | — | 8 | ||||||||
| International | 1,240 | 1,054 | 941 | 18 | 1 | 17 | 12 | (5 | ) | 17 | ||||||||||
| Total reportable segments | 2,877 | 2,562 | 2,331 | 12 | — | 12 | 10 | (2 | ) | 12 | ||||||||||
| Other business activities | (313 | ) | (309 | ) | (293 | ) | 1 | 5 | ||||||||||||
| Reconciling Items: | ||||||||||||||||||||
| Corporate | (625 | ) | (684 | ) | (606 | ) | (9 | ) | 13 | |||||||||||
| Purchase accounting adjustments | (88 | ) | (99 | ) | (57 | ) | (11 | ) | 74 | |||||||||||
| Acquisition-related costs | (10 | ) | (4 | ) | (21 | ) | * | (81 | ) | |||||||||||
| Certain significant items | (25 | ) | (57 | ) | (592 | ) | (56 | ) | (90 | ) | ||||||||||
| Other unallocated | (291 | ) | (181 | ) | (217 | ) | 61 | (17 | ) | |||||||||||
| Income before income taxes | $ | 1,525 | $ | 1,228 | $ | 545 | 24 | * | ||||||||||||
* Calculation not meaningful.
Certain amounts and percentages may reflect rounding adjustments.
2017 vs. 2016
U.S. operating segment
U.S. segment revenue increased by $173 million, or 7%, in 2017 compared with 2016, of which approximately $17 million resulted from growth in livestock products and approximately $156 million resulted from growth in companion animal products.
| • | Livestock revenue increased primarily due to cattle and poultry products, partly offset by swine products. Cattle experienced growth across our portfolio, while poultry growth was due to increased sales of medicated feed additives. Certain medicated feed additive products for both cattle and swine were negatively impacted by livestock producers’ implementation of the Veterinary Feed Directive. In addition, swine declined due to vaccine competition. |
43 |
| • | Companion animal revenue growth was driven primarily by our dermatology portfolio, in addition to new products, particularly Simparica®. Growth was tempered by the prior year’s initial sales of certain products into expanded distribution relationships, as well as lower sales due to competition for our pain and anti-infective products. |
U.S. segment earnings increased by $129 million, or 9%, in 2017 compared with 2016, primarily due to revenue growth and improved gross margin, partially offset by higher operating expenses related to promotional activity for new products and Apoquel®.
International operating segment
International segment revenue increased by $253 million, or 11%, in 2017 compared with 2016. Operational revenue increased $240 million, or 10%, reflecting growth of approximately $125 million in livestock products and growth of approximately $115 million in companion animal products.
| • | Livestock growth was driven primarily by increased sales of cattle, swine and fish products. Growth of cattle products was driven by Latin American markets, while swine was driven by new product launches primarily in Europe and Asia, as well as growth in China. Growth of fish products was driven by new products and in-line product growth across various markets. Livestock growth was partially offset by product rationalizations, primarily impacting poultry and swine product sales. |
| • | Companion animal revenue growth resulted primarily from increased sales of our dermatology portfolio, in addition to new products, primarily Simparica®. Sales also benefited from increased demand in China due to field force expansions and increasing medicalization rates. |
Segment revenue was favorably impacted by foreign exchange, which increased revenue by approximately 1%, primarily driven by the appreciation of the Brazilian real, partly offset by the depreciation of the U.K. pound and Egyptian Pound.
International segment earnings increased by $186 million, or 18%, in 2017 compared with 2016. Operational earnings growth was $183 million, or 17%, primarily due to higher revenue and improved gross margin.
2016 vs. 2015
U.S. operating segment
U.S. segment revenue increased by $119 million, or 5%, in 2016 compared with 2015, of which approximately $24 million resulted from declines in livestock products and approximately $143 million resulted from growth in companion animal products.
| • | Livestock revenue declined primarily due to product rationalizations as part of the company’s operational efficiency initiative, which impacted both poultry and swine. Additionally, sales of cattle products were impacted by unfavorable market conditions, while swine was impacted by increased competition. |
| • | Companion animal revenue growth was driven by increased sales of Apoquel®, new product launches, and initial sales of products into expanded distribution relationships. Partially offsetting growth was a decline in the company’s surgical fluid products. |
U.S. segment earnings increased by $118 million, or 8%, in 2016 compared with 2015, primarily due to revenue growth and improved gross margin.
International operating segment
International segment revenue increased by $4 million, in 2016 compared with 2015. Segment revenue was unfavorably impacted by foreign exchange, which decreased revenue by approximately 5%, primarily driven by the depreciation of the Brazilian real, Argentine peso and U.K. pound.
Operational revenue increased $130 million, or 5%, reflecting growth of approximately $43 million in livestock products and growth of approximately $87 million in companion animal products.
| • | Livestock revenue growth was driven primarily by the acquisition of Pharmaq, with sales primarily in Chile and Norway. Growth also benefited from swine performance in China, as well as cattle performance in certain emerging markets. Growth was partially offset by our operational efficiency initiative, which includes product rationalization and the impact of our business decisions in Venezuela and India. |
| • | Companion animal revenue growth resulted from increased sales of Apoquel®, other new product launches, and demand for our vaccines portfolio in China, due to increased field force expansions and positive medicalization rates. |
International segment earnings increased by $113 million, or 12%, in 2016 compared with 2015. Operational earnings growth was $159 million, or 17%, primarily due to higher revenue, improved gross margin, and lower operating expenses.
Other business activities
Other business activities includes our CSS contract manufacturing results, as well as expenses associated with our dedicated veterinary medicine R&D organization, research alliances, U.S. regulatory affairs and other operations focused on the development of our products. Other R&D-related costs associated with non-U.S. market and regulatory activities are generally included in the international segment.
2017 vs. 2016
Other business activities net loss increased by $4 million, or 1%, in 2017 compared with 2016, reflecting the inclusion of the veterinary diagnostics business acquired in 2016 and the Irish biologic therapeutics company acquired in 2017.
2016 vs. 2015
Other business activities net loss increased by $16 million, or 5%, in 2016 compared with 2015, reflecting an increase in R&D spending driven by higher development expenses for late-stage projects and the addition of Pharmaq R&D expenses, partially offset by a decrease in R&D spending driven by our operational efficiency initiative
44 |
Reconciling items
Reconciling items include certain costs that are not allocated to our operating segments results, such as costs associated with the following:
| • | Corporate, which includes certain costs associated with information technology, facilities, legal, finance, human resources, business development, and communications, among others. These costs also include certain compensation costs and other miscellaneous operating expenses that are not charged to our operating segments, as well as interest income and expense; |
| • | Certain transactions and events such as (i) Purchase accounting adjustments, which includes expenses associated with the amortization of fair value adjustments to inventory, intangible assets and property, plant and equipment; (ii) Acquisition-related activities, which includes costs for acquisition and integration; and (iii) Certain significant items, which includes non-acquisition-related restructuring charges, certain asset impairment charges, stand-up costs, certain legal and commercial settlements, and costs associated with cost reduction/productivity initiatives; and |
| • | Other unallocated, which includes (i) certain overhead expenses associated with our global manufacturing operations not charged to our operating segments; (ii) certain costs associated with information technology and finance that specifically support our global manufacturing operations; (iii) certain supply chain and global logistics costs; and (iv) procurement costs. |
2017 vs. 2016
Corporate expenses decreased by $59 million, or 9%, in 2017 compared with 2016, primarily due to the favorable impact of foreign exchange, a reduction in expenses driven by our operational efficiency initiative and a decrease in certain compensation costs not allocated to our operating segments, partially offset by higher interest expense, net of capitalized interest associated with the additional debt issued in September 2017.
Other unallocated expenses increased by $110 million, or 61%, in 2017 compared with 2016, primarily due to higher global manufacturing and supply costs and unfavorable foreign exchange, partially offset by a decrease in inventory obsolescence, scrap and other charges.
See Notes to Consolidated Financial Statements— Note 18. Segment, Geographic and Other Revenue Information for further information.
2016 vs. 2015
Corporate expenses increased by $78 million, or 13%, in 2016 compared with 2015, primarily due to the unfavorable impact of foreign exchange, which includes exposures to certain emerging market currencies and costs related to hedging, and higher interest expense, net of capitalized interest, associated with the additional debt issued in November 2015, partly offset by a decrease in certain compensation costs not charged to our operating segments.
Other unallocated expenses decreased by $36 million, or 17%, in 2016 compared with 2015, primarily due to lower global manufacturing and supply costs and favorable foreign exchange, partially offset by an increase in inventory obsolescence, scrap and other charges.
See Notes to Consolidated Financial Statements— Note 18. Segment, Geographic and Other Revenue Information for further information.
Adjusted net income
General description of adjusted net income (a non-GAAP financial measure)
Adjusted net income is an alternative view of performance used by management, and we believe that investors’ understanding of our performance is enhanced by disclosing this performance measure. We report adjusted net income to portray the results of our major operations, the discovery, development, manufacture and commercialization of our products, prior to considering certain income statement elements. We have defined adjusted net income as net income attributable to Zoetis before the impact of purchase accounting adjustments, acquisition-related costs and certain significant items described below. The adjusted net income measure is not, and should not be viewed as, a substitute for U.S. GAAP reported net income attributable to Zoetis.
The adjusted net income measure is an important internal measurement for us. We measure our overall performance on this basis in conjunction with other performance metrics. The following are examples of how the adjusted net income measure is utilized:
| • | senior management receives a monthly analysis of our operating results that is prepared on an adjusted net income basis; |
| • | our annual budgets are prepared on an adjusted net income basis; and |
| • | other goal setting and performance measurements. |
Despite the importance of this measure to management in goal setting and performance measurement, adjusted net income is a non-GAAP financial measure that has no standardized meaning prescribed by U.S. GAAP and, therefore, may not be comparable to the calculation of similar measures of other companies. Adjusted net income is presented to permit investors to more fully understand how management assesses performance.
We also recognize that, as an internal measure of performance, the adjusted net income measure has limitations, and we do not restrict our performance management process solely to this metric. A limitation of the adjusted net income measure is that it provides a view of our operations without including all events during a period, such as the effects of an acquisition or amortization of purchased intangibles, and does not provide a comparable view of our performance to other companies. We also use other specifically tailored metrics designed to achieve the highest levels of performance.
45 |
Purchase accounting adjustments
Adjusted net income is calculated prior to considering certain significant purchase accounting impacts that result from business combinations and net asset acquisitions. These impacts, primarily associated with the acquisition of the Pharmaq business (acquired in November 2015), certain assets of Abbott Animal Health (acquired in February 2015), KAH (acquired in 2011), FDAH (acquired in 2009), and Pharmacia Animal Health business (acquired in 2003), include amortization related to the increase in fair value of the acquired finite-lived intangible assets and depreciation related to the increase/decrease to fair value of the acquired fixed assets. Therefore, the adjusted net income measure includes the revenue earned upon the sale of the acquired products without considering the aforementioned significant charges.
While certain purchase accounting adjustments can occur through 20 or more years, this presentation provides an alternative view of our performance that is used by management to internally assess business performance. We believe the elimination of amortization attributable to acquired intangible assets provides management and investors an alternative view of our business results by providing a degree of parity to internally developed intangible assets for which R&D costs previously have been expensed.
A completely accurate comparison of internally developed intangible assets and acquired intangible assets cannot be achieved through adjusted net income. These components of adjusted net income are derived solely from the impact of the items listed above. We have not factored in the impact of any other differences in experience that might have occurred if we had discovered and developed those intangible assets on our own, and this approach does not intend to be representative of the results that would have occurred in those circumstances. For example, our R&D costs in total, and in the periods presented, may have been different; our speed to commercialization and resulting revenue, if any, may have been different; or our costs to manufacture may have been different. In addition, our marketing efforts may have been received differently by our customers. As such, in total, there can be no assurance that our adjusted net income amounts would have been the same as presented had we discovered and developed the acquired intangible assets.
Acquisition-related costs
Adjusted net income is calculated prior to considering transaction and integration costs associated with significant business combinations or net asset acquisitions because these costs are unique to each transaction and represent costs that were incurred to acquire and integrate certain businesses as a result of the acquisition decision. We have made no adjustments for the resulting synergies.
We believe that viewing income prior to considering these charges provides investors with a useful additional perspective because the significant costs incurred in a business combination result primarily from the need to eliminate duplicate assets, activities or employees––a natural result of acquiring a fully integrated set of activities. For this reason, we believe that the costs incurred to convert disparate systems, to close duplicative facilities or to eliminate duplicate positions (for example, in the context of a business combination) can be viewed differently from those costs incurred in the ordinary course of business.
The integration costs associated with a business combination may occur over several years, with the more significant impacts generally ending within three years of the transaction. Because of the need for certain external approvals for some actions, the span of time needed to achieve certain restructuring and integration activities can be lengthy. For example, due to the regulated nature of the animal health medicines and vaccines business, the closure of excess facilities can take several years, as all manufacturing changes are subject to extensive validation and testing and must be approved by the FDA and/or other regulatory authorities.
Certain significant items
Adjusted net income is calculated prior to considering certain significant items. Certain significant items represent substantive, unusual items that are evaluated on an individual basis. Such evaluation considers both the quantitative and the qualitative aspect of their unusual nature. Unusual, in this context, may represent items that are not part of our ongoing business; items that, either as a result of their nature or size, we would not expect to occur as part of our normal business on a regular basis; items that would be nonrecurring; or items that relate to products that we no longer sell. While not all-inclusive, examples of items that could be included as certain significant items would be costs related to becoming an independent public company; a major non-acquisition-related restructuring charge and associated implementation costs for a program that is specific in nature with a defined term, such as those related to our non-acquisition-related cost-reduction and productivity initiatives; amounts related to disposals of products or facilities that do not qualify as discontinued operations as defined by U.S. GAAP; certain intangible asset impairments; adjustments related to the resolution of certain tax positions; significant currency devaluation; the impact of adopting certain significant, event-driven tax legislation; or charges related to legal matters. See Notes to Consolidated Financial Statements— Note 17. Commitments and Contingencies. Our normal, ongoing defense costs or settlements of and accruals on legal matters made in the normal course of our business would not be considered certain significant items.
Reconciliation
A reconciliation of net income attributable to Zoetis, as reported under U.S. GAAP, to adjusted net income follows:
| Year Ended December 31, | % Change | |||||||||||||||||
| (MILLIONS OF DOLLARS) | 2017 | 2016 | 2015 | 17/16 | 16/15 | |||||||||||||
| GAAP reported net income attributable to Zoetis | $ | 864 | $ | 821 | $ | 339 | 5 | * | ||||||||||
| Purchase accounting adjustments—net of tax | 51 | 60 | 39 | (15 | ) | 54 | ||||||||||||
| Acquisition-related costs—net of tax | 7 | 4 | 22 | 75 | (82 | ) | ||||||||||||
| Certain significant items—net of tax | 263 | 90 | 489 | * | (82 | ) | ||||||||||||
Non-GAAP adjusted net income(a)(b) | $ | 1,185 | $ | 975 | $ | 889 | 22 | 10 | ||||||||||
* Calculation not meaningful.
Certain amounts and percentages may reflect rounding adjustments.
46 |
(a) | The effective tax rate on adjusted pretax income is 28.2%, 29.9% and 26.8% for full year 2017, 2016 and 2015, respectively. The lower effective tax rate in 2017 compared to 2016 is primarily due to changes in the jurisdictional mix of earnings, which includes the impact of the location of earnings as well as repatriation costs, a $15 million discrete tax benefit recorded in the fourth quarter of 2017 related to the effective settlement of certain issues with U.S. and non-U.S. tax authorities, and a $9 million discrete tax benefit recorded in 2017 related to the excess tax benefits for share-based payments. The higher effective tax rate in 2016 compared to 2015 is primarily due to changes in the jurisdictional mix of earnings, which includes the impact of the location of earnings as well as repatriation costs, offset by a $15 million discrete tax benefit related to prior period tax adjustments, a $7 million discrete tax benefit recorded in 2016 related to the excess tax benefits for share-based payments, and a $4 million discrete tax benefit recorded in the first quarter of 2015 related to prior period deferred tax adjustments. |
(b) | The impact of the incentive tax rulings in Belgium and Singapore were a component of the 2015 effective tax rate, but are no longer a component of the 2017 and 2016 effective tax rates. For additional information on the impact of the European Commission’s negative decision on the Belgium excess profits ruling on January 11, 2016, see Notes to Consolidated Financial Statements— Note 8A. Tax Matters: Taxes on Income. The U.S. Research and Development Tax Credit which was permanently extended on December 18, 2015, is a component of the 2017, 2016 and 2015 effective tax rates. |
A reconciliation of reported diluted earnings per share (EPS), as reported under U.S. GAAP, to non-GAAP adjusted diluted EPS follows:
| Year Ended December 31, | % Change | |||||||||||||||||
| 2017 | 2016 | 2015 | 17/16 | 16/15 | ||||||||||||||
Earnings per share—diluted(a)(b): | ||||||||||||||||||
| GAAP reported EPS attributable to Zoetis—diluted | $ | 1.75 | $ | 1.65 | $ | 0.68 | 6 | * | ||||||||||
| Purchase accounting adjustments—net of tax | 0.10 | 0.12 | 0.08 | (17 | ) | 50 | ||||||||||||
| Acquisition-related costs—net of tax | 0.01 | 0.01 | 0.04 | — | (75 | ) | ||||||||||||
| Certain significant items—net of tax | 0.54 | 0.18 | 0.97 | * | (81 | ) | ||||||||||||
| Non-GAAP adjusted EPS—diluted | $ | 2.40 | $ | 1.96 | $ | 1.77 | 22 | 11 | ||||||||||
* Calculation not meaningful.
Certain amounts and percentages may reflect rounding adjustments.
(a) | Diluted earnings per share was computed using the weighted-average common shares outstanding during the period plus the common stock equivalents related to stock options, restricted stock units, performance-vesting restricted stock units and deferred stock units. |
(b) | EPS amounts may not add due to rounding. |
Adjusted net income includes the following charges for each of the periods presented:
| Year Ended December 31, | ||||||||||||
| (MILLIONS OF DOLLARS) | 2017 | 2016 | 2015 | |||||||||
| Interest expense, net of capitalized interest | $ | 175 | $ | 166 | $ | 124 | ||||||
| Interest income | 13 | 8 | 6 | |||||||||
| Income taxes | 465 | 415 | 326 | |||||||||
| Depreciation | 136 | 133 | 124 | |||||||||
| Amortization | 18 | 16 | 16 | |||||||||
47 |
Adjusted net income, as shown above, excludes the following items:
| Year Ended December 31, | ||||||||||||
| (MILLIONS OF DOLLARS) | 2017 | 2016 | 2015 | |||||||||
| Purchase accounting adjustments: | ||||||||||||
Amortization and depreciation(a) | $ | 81 | $ | 76 | $ | 48 | ||||||
Cost of sales(b) | 7 | 23 | 9 | |||||||||
| Total purchase accounting adjustments—pre-tax | 88 | 99 | 57 | |||||||||
Income taxes(c) | 37 | 39 | 18 | |||||||||
| Total purchase accounting adjustments—net of tax | 51 | 60 | 39 | |||||||||
| Acquisition-related costs: | ||||||||||||
| Integration costs | 6 | 3 | 10 | |||||||||
| Transaction costs | — | — | 9 | |||||||||
Restructuring charges(d) | 4 | — | — | |||||||||
| Other | — | 1 | 2 | |||||||||
| Total acquisition-related costs—pre-tax | 10 | 4 | 21 | |||||||||
Income taxes(c) | 3 | — | (1 | ) | ||||||||
| Total acquisition-related costs—net of tax | 7 | 4 | 22 | |||||||||
| Certain significant items: | ||||||||||||
Operational efficiency initiative(e) | 5 | (9 | ) | 346 | ||||||||
Supply network strategy(f) | 15 | 19 | 27 | |||||||||
Other restructuring charges and cost-reduction/productivity initiatives(g) | 4 | (1 | ) | — | ||||||||
Certain asset impairment charges(h) | — | 1 | 5 | |||||||||
Stand-up costs(i) | 3 | 23 | 118 | |||||||||
Foreign currency loss related to Venezuela revaluation(j) | — | — | 93 | |||||||||
Other(k) | (2 | ) | 24 | 3 | ||||||||
| Total certain significant items—pre-tax | 25 | 57 | 592 | |||||||||
Income taxes(c) | (238 | ) | (33 | ) | 103 | |||||||
| Total certain significant items—net of tax | 263 | 90 | 489 | |||||||||
| Total purchase accounting adjustments, acquisition-related costs, and certain significant items—net of tax | $ | 321 | $ | 154 | $ | 550 | ||||||
Certain amounts may reflect rounding adjustments.
(a) | Amortization and depreciation expenses related to Purchase accounting adjustments with respect to identifiable intangible assets and property, plant and equipment. |
(b) | Amortization and depreciation expense, as well as fair value adjustments to acquired inventory. |
(c) | Income taxes include the tax effect of the associated pre-tax amounts, calculated by determining the jurisdictional location of the pre-tax amounts and applying that jurisdiction's applicable tax rate. |
Income taxes in Purchase accounting adjustments for 2017, also includes (i) a provisional tax benefit of approximately $17 million related to the remeasurement of the company’s deferred taxes due to the reduction in the U.S. federal corporate tax rate as provided by the Tax Act enacted on December 22, 2017, (ii) remeasurement of deferred taxes as a result of a change in non-U.S. statutory tax rates, and (iii) a net tax charge related to prior period tax adjustments. |
Income taxes in Purchase accounting adjustments for 2016, also includes a tax benefit related to the remeasurement of deferred taxes as a result of a change in tax rates. |
Income taxes in Acquisition-related costs for 2016, also includes a tax charge related to the acquisition of certain assets of Abbott Animal Health. |
Income taxes in Certain significant items for 2017, also includes (i) a provisional net tax charge of approximately $229 million related to the impact of the Tax Act enacted on December 22, 2017, including a one-time mandatory deemed repatriation tax on the company’s undistributed non-U.S. earnings, partially offset by a net tax benefit related to the remeasurement of the company’s deferred tax assets and liabilities, as of the date of enactment, due to the reduction in the U.S. federal corporate tax rate, (ii) a net tax charge of approximately $3 million as a result of the implementation of certain operational changes, and (iii) a tax charge of approximately $2 million related to the disposal of certain assets. |
Income taxes in Certain significant items for 2016, also includes (i) a net tax charge of approximately $20 million recorded in the second half of 2016, as a result of the implementation of certain operational changes, which represents an increase to current income tax expense of approximately $22 million, offset by a $2 million tax benefit related to a remeasurement of the company’s deferred tax assets and liabilities using the tax rates expected to be in place going forward, and (ii) a net tax charge of approximately $35 million mainly recorded in the first half of 2016, related to the impact of the European Commission’s negative decision on the excess profits rulings in Belgium which represents the recovery of prior tax benefits for the periods 2013 through 2015, offset by the remeasurement of the company’s deferred tax assets and liabilities, using the rates expected to be in place at the time of the reversal and without consideration of implementation of any future operational changes, and does not include any benefits associated with a successful appeal of the decision. |
(d) | For 2017, represents employee termination costs related to the acquisition of an Irish biologic therapeutics company in the third quarter of 2017. |
(e) | For 2017, includes an adjustment to inventory reserves of $2 million, consulting fees of $2 million, restructuring charges of $4 million related to employee termination costs ($1 million) and exit costs ($3 million), and a net loss related to sales of certain manufacturing sites and products of $1 million. |
48 |
| For 2016, includes inventory write-offs of $5 million, consulting fees of $14 million, accelerated depreciation of $1 million, a reversal of employee termination costs of $8 million, an increase in exit costs of $5 million, and a $26 million net gain related to divestitures. |
| For 2015, includes restructuring charges of $291 million related to employee termination costs ($253 million) and asset impairments ($38 million), inventory write-offs of $13 million, accelerated depreciation of $2 million, and $40 million primarily related to consulting fees. |
(f) | For 2017, includes accelerated depreciation of $2 million, an adjustment of $2 million related to discontinuing the depreciation of assets located at the manufacturing site in Guarulhos, Brazil that was sold in the fourth quarter of 2017, consulting fees of $5 million, restructuring charges of $1 million related to a net increase in employee termination costs, and a net loss of $9 million, related to sales of certain manufacturing sites and products, including our manufacturing site in Guarulhos, Brazil. |
For 2016, represents restructuring charges of $6 million related to employee termination costs, accelerated depreciation of $6 million, inventory write-offs of $1 million and consulting fees of $6 million. |
| For 2015, represents restructuring charges of $10 million related to employee termination costs ($9 million) and asset impairments ($1 million), accelerated depreciation of $1 million, and $16 million primarily related to consulting fees. |
(g) | Represents charges incurred for restructuring and cost-reduction/productivity initiatives. For 2017, charges are related to employee termination costs in Europe as a result of initiatives to better align our organizational structure. |
(h) | For 2016, represents an impairment of finite-lived trademarks related to a canine pain management product. For 2015, primarily represents impairment charges related to assets held by our joint venture in Taiwan, which was subsequently sold in 2016, and an impairment of IPR&D assets related to the termination of a canine oncology project. |
(i) | Represents certain non-recurring costs related to becoming an independent public company, such as the creation of standalone systems and infrastructure, site separation, new branding (including changes to the manufacturing process for required new packaging),and certain legal registration and patent assignment costs. |
(j) | For 2015, represents charges primarily related to the foreign currency losses associated with our Venezuela business. For additional information, see Notes to Consolidated Financial Statements— Note 7. Foreign Currency Losses Related to Venezuela Revaluation. |
(k) | For 2017, primarily represents costs associated with changes to our operating model of $3 million, and income of $5 million related to an insurance recovery from commercial settlements in Mexico recorded in 2014 and 2016. |
| For 2016, represents costs associated with changes to our operating model of $10 million and a charge associated with a commercial settlement in Mexico of $14 million. |
| For 2015, represents charges due to unusual investor-related activities. |
49 |
The classification of the above items excluded from adjusted net income are as follows:
| Year Ended December 31, | ||||||||||||
| (MILLIONS OF DOLLARS) | 2017 | 2016 | 2015 | |||||||||
| Cost of sales: | ||||||||||||
| Purchase accounting adjustments | $ | 7 | $ | 23 | $ | 9 | ||||||
| Accelerated depreciation | 2 | 6 | 1 | |||||||||
| Inventory write-offs | (2 | ) | 5 | 13 | ||||||||
| Consulting fees | 6 | 6 | 16 | |||||||||
| Stand-up costs | 3 | 1 | 27 | |||||||||
| Other | (2 | ) | 1 | — | ||||||||
| Total Cost of sales | 14 | 42 | 66 | |||||||||
| Selling, general & administrative expenses: | ||||||||||||
| Purchase accounting adjustments | 5 | 5 | — | |||||||||
| Accelerated depreciation | — | 1 | — | |||||||||
| Consulting fees | 2 | 14 | 40 | |||||||||
| Stand-up costs | — | 22 | 90 | |||||||||
| Other | 2 | 10 | — | |||||||||
| Total Selling, general & administrative expenses | 9 | 52 | 130 | |||||||||
| Research & development expenses: | ||||||||||||
| Purchase accounting adjustments | 2 | 2 | 2 | |||||||||
| Accelerated depreciation | — | — | 2 | |||||||||
| Total Research & development expenses | 2 | 2 | 4 | |||||||||
| Amortization of intangible assets: | ||||||||||||
| Purchase accounting adjustments | 74 | 69 | 46 | |||||||||
| Total Amortization of intangible assets | 74 | 69 | 46 | |||||||||
| Restructuring (benefits)/charges and certain acquisition-related costs: | ||||||||||||
| Integration costs | 6 | 3 | 10 | |||||||||
| Transaction costs | — | — | 9 | |||||||||
| Employee termination costs | 10 | (2 | ) | 262 | ||||||||
| Asset impairments | — | — | 39 | |||||||||
| Exit costs | 3 | 4 | — | |||||||||
| Total Restructuring (benefits)/charges and certain acquisition-related costs | 19 | 5 | 320 | |||||||||
| Other (income)/deductions—net: | ||||||||||||
| Net loss/(gain) on sale of assets | 10 | (26 | ) | — | ||||||||
| Acquisition-related costs | — | 1 | — | |||||||||
| Asset impairments | — | 1 | 5 | |||||||||
| Stand-up costs | — | — | 1 | |||||||||
| Foreign currency loss related to Venezuela revaluation | — | — | 93 | |||||||||
| Other | (5 | ) | 14 | 5 | ||||||||
| Total Other (income)/deductions—net | 5 | (10 | ) | 104 | ||||||||
| Provision for taxes on income | (198 | ) | 6 | 120 | ||||||||
| Total purchase accounting adjustments, acquisition-related costs, and certain significant items—net of tax | $ | 321 | $ | 154 | $ | 550 | ||||||
Certain amounts may reflect rounding adjustments.
Analysis of the consolidated statements of comprehensive income
Substantially all changes in other comprehensive income for the periods presented are related to foreign currency translation adjustments. These changes result from the strengthening or weakening of the U.S. dollar as compared to the currencies in the countries in which we do business. The gains and losses associated with these changes are deferred on the balance sheet in Accumulated other comprehensive loss until realized.
50 |
Analysis of the consolidated balance sheets
December 31, 2017 vs. December 31, 2016
For a discussion about the changes in Cash and cash equivalents, Short-term borrowings, Current portion of long term debt, and Long-term debt, net of discount and issuance costs, see "Analysis of financial condition, liquidity and capital resources" below.
Accounts receivable, less allowance for doubtful accounts increased due to higher net sales and the impact of foreign exchange.
Inventories decreased primarily as a result of our inventory reduction initiative and the sale of our Guarulhos, Brazil manufacturing site, partially offset by the impact of foreign exchange. See Notes to Consolidated Financial Statements— Note 10. Inventories.
Other current assets decreased primarily as a result of lower receivables due to value-added tax for our international markets.
Property, plant and equipment, less accumulated depreciation increased primarily as a result of capital spending, partially offset by depreciation expense and the sale of assets associated with the disposal of our manufacturing site in Guarulhos, Brazil.
Goodwill increased primarily due to the 2017 acquisition of an Irish biologic therapeutics company and the consolidation of a European livestock monitoring company, a variable interest entity of which Zoetis is the primary beneficiary, in addition to the impact of foreign exchange. See Notes to Consolidated Financial Statements— Note 4. Acquisitions and Divestitures and Note 12. Goodwill and Other Intangible Assets.
Identifiable intangible assets, less accumulated amortization increased primarily due to the 2017 acquisitions of an Irish biologic therapeutics company and a Norwegian fish vaccination company, in addition to the consolidation of a European livestock monitoring company, a variable interest entity of which Zoetis is the primary beneficiary. This was partially offset by amortization expense. See Notes to Consolidated Financial Statements— Note 4. Acquisitions and Divestitures and Note 12. Goodwill and Other Intangible Assets.
The net changes in Noncurrent deferred tax assets, Noncurrent deferred tax liabilities, Income taxes payable and Other taxes payable primarily reflect adjustments to the accrual for the income tax provision for the year ended December 31, 2017, as well as the impact of the Tax Act related to the provisional tax liability recorded for the one-time mandatory deemed repatriation tax on the company's undistributed non-U.S. earnings, and the remeasurement of the deferred tax assets and liabilities, as of the date of enactment, due to the reduction in the U.S. federal corporate tax rate. See Notes to Consolidated Financial Statements— Note 8. Tax Matters.
Accrued expenses and Other current liabilities decreased primarily as a result of payment of employee termination costs associated with operational efficiency initiatives. See Notes to Consolidated Financial Statements— Note 5. Restructuring Charges and Other Costs Associated with Acquisitions and Cost-Reduction/Productivity Initiatives.
For an analysis of the changes in Total Equity, see the Consolidated Statements of Equity and Notes to Consolidated Financial Statements— Note 15. Stockholders' Equity.
Analysis of the consolidated statements of cash flows
| Year Ended December 31, | % Change | |||||||||||||||||
| (MILLIONS OF DOLLARS) | 2017 | 2016 | 2015 | 17/16 | 16/15 | |||||||||||||
| Net cash provided by (used in): | ||||||||||||||||||
| Operating activities | $ | 1,346 | $ | 713 | $ | 664 | 89 | 7 | ||||||||||
| Investing activities | (270 | ) | (214 | ) | (1,115 | ) | 26 | (81 | ) | |||||||||
| Financing activities | (251 | ) | (903 | ) | 755 | (72 | ) | * | ||||||||||
| Effect of exchange-rate changes on cash and cash equivalents | 12 | (23 | ) | (32 | ) | * | (28 | ) | ||||||||||
| Net increase/(decrease) in cash and cash equivalents | $ | 837 | $ | (427 | ) | $ | 272 | * | * | |||||||||
* Calculation not meaningful.
Certain amounts and percentages may reflect rounding adjustments.
Operating activities
2017 vs. 2016
Net cash provided by operating activities was $1,346 million in 2017 compared with $713 million in 2016. The increase in operating cash flows was primarily attributable to higher income before allocation of noncontrolling interests, lower employee termination payments related to our operational efficiency initiative and supply network strategy, the timing of receipts and payments in the ordinary course of business, and lower inventory levels.
2016 vs. 2015
Net cash provided by operating activities was $713 million in 2016 compared with $664 million in 2015. The increase in operating cash flows was primarily attributable to higher income before allocation of noncontrolling interests, and the timing of receipts and payments in the ordinary course of business, partially offset by employee termination payments related to our operational efficiency initiative and supply network strategy and higher inventory levels.
51 |
Investing activities
2017 vs. 2016
Net cash used in investing activities was $270 million in 2017 compared with $214 million in 2016. The net cash used in investing activities for 2017 was due primarily to capital expenditures and the acquisitions of an Irish biologic therapeutics company and a Norwegian fish vaccination company, partially offset by the proceeds from the sale of our manufacturing site in Guarulhos, Brazil. The net cash used in investing activities in 2016 was due primarily to capital expenditures, the acquisition of a veterinary diagnostics business in Denmark, partially offset by proceeds from the sales of certain manufacturing sites and products as part of the operational efficiency initiative.
2016 vs. 2015
Net cash used in investing activities was $214 million in 2016 compared with $1,115 million in 2015. The net cash used in investing activities in 2016 was due primarily to capital expenditures, the acquisition of a veterinary diagnostics business in Denmark, partially offset by proceeds from the sales of certain manufacturing sites and products as part of the operational efficiency initiative. The net cash used in investing activities in 2015 was primarily attributable to the acquisitions of Pharmaq and of certain assets of Abbott Animal Health.
Financing activities
2017 vs. 2016
Net cash used in financing activities was $251 million in 2017 compared with $903 million in 2016. The net cash used in financing activities for 2017 was primarily attributable to the senior note payment in October 2017, the purchase of treasury shares and the payment of dividends, partially offset by the proceeds received from the issuance of senior notes in September 2017. The net cash used in financing activities for 2016 was due primarily to the senior note payment in February 2016, the purchase of treasury shares and the payment of dividends.
2016 vs. 2015
Net cash used in financing activities was $903 million in 2016 compared with net cash provided by financing activities of $755 million in 2015. The net cash used in financing activities for 2016 was due primarily to the senior note payment in February 2016, the purchase of treasury shares, the acquisition of the remaining noncontrolling interest in a variable interest entity previously consolidated by Zoetis as the primary beneficiary, and the payment of dividends. The net cash provided by financing activities in 2015 was primarily attributable to proceeds received from the November 2015 issuance of senior notes, partially offset by the purchase of treasury shares and the payment of dividends.
Analysis of financial condition, liquidity and capital resources
While we believe our cash and cash equivalents on hand, our operating cash flows and our existing financing arrangements will be sufficient to support our future cash needs, this may be subject to the environment in which we operate. Risks to our meeting future funding requirements include global economic conditions described in the following paragraph.
Global financial markets may be impacted by macroeconomic, business and financial volatility. As markets change, we will continue to monitor our liquidity position, but there can be no assurance that a challenging economic environment or an economic downturn will not impact our liquidity or our ability to obtain future financing.
Selected measures of liquidity and capital resources
Certain relevant measures of our liquidity and capital resources follow:
| December 31, | December 31, | ||||||
| (MILLIONS OF DOLLARS) | 2017 | 2016 | |||||
| Cash and cash equivalents | $ | 1,564 | $ | 727 | |||
Accounts receivable, net(a) | 998 | 913 | |||||
| Long-term debt | 4,953 | 4,468 | |||||
| Working capital | 3,123 | 2,273 | |||||
| Ratio of current assets to current liabilities | 3.85:1 | 3.03:1 | |||||
(a) | Accounts receivable are usually collected over a period of 60 to 90 days. For the year ended December 31, 2017, compared to the year ended December 31, 2016, the number of days that accounts receivables are outstanding remained approximately the same. We regularly monitor our accounts receivable for collectability, particularly in markets where economic conditions remain uncertain. We believe that our allowance for doubtful accounts is appropriate. Our assessment is based on such factors as past due aging, historical and expected collection patterns, the financial condition of our customers, the robust nature of our credit and collection practices and the economic environment. |
For additional information about the sources and uses of our funds, see the Analysis of the consolidated balance sheets and Analysis of the consolidated statements of cash flows sections of this MD&A.
Credit facility and other lines of credit
In December 2016, we entered into an amended and restated revolving credit agreement with a syndicate of banks providing for a five-year $1.0 billion senior unsecured revolving credit facility (the credit facility). In December 2017, the maturity for the amended and restated revolving credit agreement was extended through December 2022. Subject to certain conditions, we have the right to increase the credit facility to up to $1.5 billion. The credit facility contains a financial covenant requiring us to not exceed a maximum total leverage ratio (the ratio of consolidated net debt as of the end of the period to consolidated Earnings Before Interest, Income Taxes, Depreciation and Amortization (EBITDA) for such period) of 3.50:1. Upon
52 |
entering into a material acquisition, the maximum total leverage ratio increases to 4.00:1, and extends until the fourth full consecutive fiscal quarter ended immediately following the consummation of a material acquisition.
The credit facility also contains a clause which adds back to Adjusted Consolidated EBITDA, any operational efficiency restructuring charge (defined as charges recorded by the company during the period commencing on October 1, 2016 and ending December 31, 2019, related to operational efficiency initiatives), provided that for any twelve month period such charges added back to Adjusted Consolidated EBITDA shall not exceed $100 million in the aggregate.
The credit facility also contains a financial covenant requiring that we maintain a minimum interest coverage ratio (the ratio of EBITDA at the end of the period to interest expense for such period) of 3.50:1. In addition, the credit facility contains other customary covenants.
We were in compliance with all financial covenants as of December 31, 2017 and December 31, 2016. There were no amounts drawn under the credit facility as of December 31, 2017 or December 31, 2016.
We have additional lines of credit and other credit arrangements with a group of banks and other financial intermediaries for general corporate purposes. We maintain cash and cash equivalent balances in excess of our outstanding short-term borrowings. As of December 31, 2017, we had access to $75 million of lines of credit which expire at various times throughout 2017 and 2018, and are generally renewed annually. We did not have any borrowings outstanding related to these facilities as of December 31, 2017 and December 31, 2016.
Domestic and international short-term funds
Many of our operations are conducted outside the United States. The amount of funds held in the United States will fluctuate due to the timing of receipts and payments in the ordinary course of business and due to other reasons, such as business development activities. As part of our ongoing liquidity assessments, we regularly monitor the mix of U.S. and international cash flows (both inflows and outflows). Repatriation of overseas funds can result in additional United States, federal, state and local income tax payments. We record U.S. deferred tax liabilities for certain unremitted earnings, but when amounts earned overseas are expected to be indefinitely reinvested outside the United States, no accrual for U.S. taxes is provided. See Notes to Consolidated Financial Statements— Note 8. Tax Matters.
Global economic conditions
The challenging economic environment has not had, nor do we anticipate that it will have, a significant impact on our liquidity. Due to our operating cash flows, financial assets, access to capital markets and available lines of credit and revolving credit agreements, we continue to believe that we have the ability to meet our liquidity needs for the foreseeable future. As markets change, we continue to monitor our liquidity position. There can be no assurance that a challenging economic environment or a further economic downturn would not impact our ability to obtain financing in the future.
Contractual obligations
Payments due under contractual obligations as of December 31, 2017, are set forth below:
| 2019- | 2021- | There- | ||||||||||||||||||
| (MILLIONS OF DOLLARS) | Total | 2018 | 2020 | 2022 | after | |||||||||||||||
Long-term debt, including current portion and interest obligations(a) | $ | 7,759 | $ | 191 | $ | 882 | $ | 348 | $ | 6,338 | ||||||||||
Other liabilities reflected on our consolidated balance sheets under U.S. GAAP(b) | 139 | 79 | 21 | 11 | 28 | |||||||||||||||
| Operating lease commitments | 168 | 35 | 49 | 31 | 53 | |||||||||||||||
Purchase obligations and other(c) | 442 | 119 | 215 | 106 | 2 | |||||||||||||||
Benefit plans - continuing service credit obligations(d) | 19 | 4 | 8 | 7 | — | |||||||||||||||
Uncertain tax positions(e) | — | — | — | — | — | |||||||||||||||
Certain amounts may reflect rounding adjustments.
(a) | Long-term debt consists of senior notes and other notes. Our calculations of expected interest payments incorporate only current period assumptions for interest rates, foreign currency translation rates and Zoetis hedging strategies. See Notes to Consolidated Financial Statements— Note 9A. Financial Instruments: Debt. |
(b) | Includes expected employee termination payments that represent contractual obligations, expected payments related to our unfunded U.S. supplemental (non-qualified) savings plans, deferred compensation and expected payments relating to our future benefit payments net of plan assets (included in the determination of the projected benefit obligation) for pension plans that are dedicated to Zoetis employees and those transferred to us from Pfizer. See Notes to Consolidated Financial Statements— Note 4. Acquisitions and Divestitures, Note 5. Restructuring Charges and Other Costs Associated with Acquisitions and Cost-Reduction/Productivity Initiatives and Note 13. Benefit Plans. Excludes approximately $62 million of noncurrent liabilities related to legal and environmental accruals, certain employee termination and exit costs, deferred income and other accruals, most of which do not represent contractual obligations. See Notes to Consolidated Financial Statements— Note 5. Restructuring Charges and Other Costs Associated with Acquisitions and Cost-Reduction/Productivity Initiatives and Note 17. Commitments and Contingencies. |
(c) | Includes agreements to purchase goods and services that are enforceable and legally binding and includes amounts relating to advertising, contract manufacturing, information technology services and potential milestone payments deemed reasonably likely to occur. |
(d) | Includes the cost of service credit continuation for certain Zoetis employees in the Pfizer U.S. qualified defined benefit pension and U.S. retiree medical plans, in accordance with the employee matters agreement. See Notes to Consolidated Financial Statements— Note 13. Benefit Plans. |
(e) | Except for amounts reflected in Income taxes payable, we are unable to predict the timing of tax settlements, as tax audits can involve complex issues and the resolution of those issues may span multiple years, particularly if subject to negotiation or litigation. |
The table above excludes amounts for potential milestone payments unless the payments are deemed reasonably likely to occur. Payments under these agreements generally become due and payable only upon the achievement of certain development, regulatory and/or commercialization milestones, which may span several years and/or which may never occur. Our contractual obligations in the table above are not necessarily indicative of our contractual obligations in the future.
53 |
Debt
On September 12, 2017, we issued $1.25 billion aggregate principal amount of our senior notes (2017 senior notes), with an original issue discount of $7 million. These notes are comprised of $750 million aggregate principal amount of 3.000% senior notes due 2027 and $500 million aggregate principal amount of 3.950% senior notes due 2047. Net proceeds from this offering were partially used in October 2017 to repay, prior to maturity, the aggregate principal amount of $750 million, and a make-whole amount and accrued interest of $4 million, of our 1.875% senior notes due 2018. The remainder of the net proceeds will be used for general corporate purposes.
On November 13, 2015, we issued $1.25 billion aggregate principal amount of our senior notes (2015 senior notes), with an original issue discount of $2 million. On January 28, 2013, we issued $3.65 billion aggregate principal amount of our senior notes (2013 senior notes) in a private placement, with an original issue discount of $10 million.
The 2013, 2015 and 2017 senior notes are governed by an indenture and supplemental indenture (collectively, the indenture) between us and Deutsche Bank Trust Company Americas, as trustee. The indenture contains certain covenants, including limitations on our and certain of our subsidiaries' ability to incur liens or engage in sale lease-back transactions. The indenture also contains restrictions on our ability to consolidate, merge or sell substantially all of our assets. In addition, the indenture contains other customary terms, including certain events of default, upon the occurrence of which (if not cured or waived), the 2013, 2015 and 2017 senior notes may be declared immediately due and payable.
Pursuant to the indenture, we are able to redeem the 2013, 2015 and 2017 senior notes of any series, in whole or in part, at any time by paying a “make whole” premium, plus accrued and unpaid interest to, but excluding, the date of redemption. Pursuant to our tax matters agreement with Pfizer, we will not be permitted to redeem the 2013 senior notes due 2023 pursuant to this optional redemption provision, except under limited circumstances. Upon the occurrence of a change of control of us and a downgrade of the 2013, 2015 and 2017 senior notes below an investment grade rating by each of Moody's Investors Service, Inc. and Standard & Poor's Ratings Services, we are, in certain circumstances, required to make an offer to repurchase all of the outstanding 2013, 2015 and 2017 senior notes at a price equal to 101% of the aggregate principal amount of the 2013, 2015 and 2017 senior notes together with accrued and unpaid interest to, but excluding, the date of repurchase.
The components of our long-term debt follow:
| Description | Principal Amount | Interest Rate | Terms |
| 2015 Senior Note due 2020 | $500 million | 3.450% | Interest due semi annually, not subject to amortization, aggregate principal due on November 13, 2020 |
| 2013 Senior Note due 2023 | $1,350 million | 3.250% | Interest due semi annually, not subject to amortization, aggregate principal due on February 1, 2023 |
| 2015 Senior Note due 2025 | $750 million | 4.500% | Interest due semi annually, not subject to amortization, aggregate principal due on November 13, 2025 |
| 2017 Senior Note due 2027 | $750 million | 3.000% | Interest due semi annually, not subject to amortization, aggregate principal due on September 12, 2027 |
| 2013 Senior Note due 2043 | $1,150 million | 4.700% | Interest due semi annually, not subject to amortization, aggregate principal due on February 1, 2043 |
| 2017 Senior Note due 2047 | $500 million | 3.950% | Interest due semi annually, not subject to amortization, aggregate principal due on September 12, 2047 |
Credit ratings
Two major corporate debt-rating organizations, Moody's and S&P, assign ratings to our short-term and long-term debt. A security rating is not a recommendation to buy, sell or hold securities and the rating is subject to revision or withdrawal at any time by the rating organization. Each rating should be evaluated independently of any other rating.
The following table provides the current ratings assigned by these rating agencies to our commercial paper and senior unsecured non-credit-enhanced long-term debt:
| Commercial | ||||||||
| Paper | Long-term Debt | Date of | ||||||
| Name of Rating Agency | Rating | Rating | Outlook | Last Action | ||||
| Moody’s | P-2 | Baa1 | Stable | August 2017 | ||||
| S&P | A-2 | BBB | Stable | December 2016 | ||||
Pension obligations
Our employees ceased to participate in the Pfizer U.S. qualified defined benefit and U.S. retiree medical plans effective December 31, 2012, and liabilities associated with our employees under these plans were retained by Pfizer. As part of the separation from Pfizer, Pfizer is continuing to credit certain employees' service with Zoetis generally through December 31, 2017 (or termination of employment from Zoetis, if earlier), for certain early retirement benefits with respect to Pfizer's U.S. defined benefit pension and retiree medical plans. In connection with the employee matters agreement, Zoetis will be responsible for payment of three-fifths of the total cost of the service credit continuation (approximately $38 million) for these plans. The amount of the service cost continuation payment to be paid by Zoetis to Pfizer was determined and fixed based on an actuarial assessment of the value of the grow-in benefits and will be paid in equal installments over a period of 10 years. As of December 31, 2017, the remaining payments due to Pfizer (approximately $19 million in the aggregate) are to be paid over the next five years.
As part of the separation from Pfizer, Pfizer transferred to us the net pension obligations associated with certain international defined benefit plans. We expect to contribute a total of approximately $6 million to these plans in 2018.
As of December 31, 2017, the supplemental savings plan liability was approximately $31 million.
54 |
For additional information, see Notes to Consolidated Financial Statements— Note 13. Benefit Plans.
Share repurchase program
In November 2014, the company's Board of Directors authorized a $500 million share repurchase program. This program was substantially completed as of December 31, 2016. In December 2016, the company's Board of Directors authorized an additional $1.5 billion share repurchase program. Purchases of Zoetis shares may be made at the discretion of management, depending on market conditions and business needs. Share repurchases may be executed through various means, including open market or privately negotiated transactions. During 2017, approximately 8 million shares were repurchased. As of December 31, 2017, there was approximately $1 billion remaining under this authorization.
Off-balance sheet arrangements
In the ordinary course of business and in connection with the sale of assets and businesses, we may indemnify our counterparties against certain liabilities that may arise in connection with a transaction or that are related to activities prior to a transaction. These indemnifications typically pertain to environmental, tax, employee and/or product-related matters, and patent-infringement claims. If the indemnified party were to make a successful claim pursuant to the terms of the indemnification, we would be required to reimburse the loss. These indemnifications are generally subject to threshold amounts, specified claim periods and other restrictions and limitations. Historically, we have not paid significant amounts under these provisions and, as of December 31, 2017 and December 31, 2016, recorded amounts for the estimated fair value of these indemnifications are not significant.
New accounting standards
For discussion of our new accounting standards, see Notes to Consolidated Financial Statements—Note 3. Significant Accounting Policies—New Accounting Standards.
Recently Issued Accounting Standards, Not Adopted as of December 31, 2017
In August 2017, the FASB issued an accounting standards update which amends the hedge accounting recognition and presentation requirements and allows for more hedging strategies to be eligible for hedge accounting. Recognition of periodic hedge effectiveness will no longer be required for cash flow and net investment hedges and companies may elect to perform subsequent hedge effectiveness assessments qualitatively. The update also clarifies that the change in fair value of a derivative must be recorded in the same income statement line item as the earnings effect of the hedged item and introduces additional disclosure requirements including cumulative basis adjustments for fair value hedges and the effect of hedging on individual income statement line items. The provisions of the update are effective beginning January 1, 2019 for interim and annual periods with early adoption permitted for any interim period after issuance of the update. We are currently assessing the timing of our adoption as well as the potential impact that the standard will have on our consolidated financial statements.
In March 2017, the FASB issued an accounting standards update to simplify and improve the reporting of net periodic pension benefit cost by requiring only present service cost to be presented in the same line item as other current employee compensation costs while remaining components of net periodic benefit cost would be presented within Other (income)/deductions—net outside of operations. We will adopt this guidance as of January 1, 2018, the required effective data, and do not expect that the new standard will have a significant impact on our consolidated financial statements.
In October 2016, the FASB issued an accounting standards update that will require the recognition of the income tax consequences of an intra-entity asset transfer, other than inventory, when the transfer occurs as opposed to when the asset is sold to an outside third party. The provisions of the new standard are effective beginning January 1, 2018, for annual and interim reporting periods. We will adopt this guidance as of January 1, 2018, the required effective date, and do not expect that the new standard will have a significant impact on our consolidated financial statements.
In February 2016, the FASB issued an accounting standards update which requires lessees to recognize most leases on the balance sheet with a corresponding right of use asset. Leases will be classified as financing or operating which will drive the expense recognition pattern. For lessees, the income statement presentation and expense recognition pattern for financing and operating leases is similar to the current model for capital and operating leases, respectively. Companies may elect to exclude short-term leases. The update also requires additional disclosures that will better enable users of financial statements to assess the amount, timing, and uncertainty of cash flows arising from leases. We plan to adopt this guidance as of January 1, 2019, the required effective date, for annual and interim reporting periods. The new standard requires a modified retrospective adoption approach, at the beginning of the earliest comparative period presented in the financial statements. We are currently evaluating the impact that adopting this new guidance will have on our consolidated financial statements.
In May 2014, the FASB issued an accounting standards update that outlines a new, single comprehensive model for companies to use in accounting for revenue arising from contracts with customers. This update supersedes most current revenue recognition guidance under U.S. GAAP. The core principle of the new guidance is that an entity should recognize revenue to depict the transfer of goods or services to customers in an amount that reflects the consideration to which the entity expects to be entitled in exchange for those goods or services. The guidance includes a five-step model for determining how, when and how much revenue should be recognized. This update also requires additional disclosure about the nature, amount, timing and uncertainty of revenue and cash flows arising from contracts with customers. We will adopt this guidance as of January 1, 2018, the required effective date, using the modified retrospective transition method. Under the modified retrospective method, the cumulative effect of applying the new standard will be recognized as of the date of initial application with disclosure of results under both the new and prior standards. The new standard will not have a significant impact on our consolidated financial statements upon adoption or on an ongoing basis.
55 |
Forward-looking statements and factors that may affect future results
This report contains “forward-looking” statements. We generally identify forward-looking statements by using words such as “anticipate,” “estimate,” “could,” “expect,” “intend,” “project,” “plan,” “predict,” “believe,” “seek,” “continue,” “outlook,” "objective," "target," “may,” “might,” “will,” “should,” “can have,” “likely” or the negative version of these words or comparable words or by using future dates in connection with any discussion of future performance, actions or events.
In particular, forward-looking statements include statements relating to our 2018 financial guidance, future actions, business plans or prospects, prospective products, product approvals or products under development, product supply disruptions, R&D costs, timing and likelihood of success, future operating or financial performance, future results of current and anticipated products and services, strategies, sales efforts, expenses, production efficiencies, production margins, integration of acquired businesses, interest rates, tax rates, changes in tax regimes and laws, foreign exchange rates, growth in emerging markets, the outcome of contingencies, such as legal proceedings, plans related to share repurchases and dividends, our agreements with Pfizer, government regulation and financial results. These statements are not guarantees of future performance, actions or events. Forward-looking statements are subject to risks and uncertainties, many of which are beyond our control, and are based on potentially inaccurate assumptions. Among the factors that could cause actual results to differ materially from past results and future plans and projected future results are the following:
| • | emerging restrictions and bans on the use of antibacterials in food-producing animals; |
| • | perceived adverse effects on human health linked to the consumption of food derived from animals that utilize our products; |
| • | unanticipated safety, quality or efficacy concerns about our products; |
| • | increased regulation or decreased governmental support relating to the raising, processing or consumption of food-producing animals; |
| • | fluctuations in foreign exchange rates and potential currency controls; |
| • | changes in tax laws and regulations; |
| • | legal factors, including product liability claims, antitrust litigation and governmental investigations, including tax disputes, environmental concerns, commercial disputes and patent disputes with branded and generic competitors, any of which could preclude commercialization of products or negatively affect the profitability of existing products; |
| • | failure to protect our intellectual property rights or to operate our business without infringing the intellectual property rights of others; |
| • | an outbreak of infectious disease carried by animals; |
| • | adverse weather conditions and the availability of natural resources; |
| • | adverse global economic conditions; |
| • | failure of our R&D, acquisition and licensing efforts to generate new products; |
| • | the possible impact of competing products, including generic alternatives, on our products and our ability to compete against such products; |
| • | quarterly fluctuations in demand and costs; |
| • | governmental laws and regulations affecting domestic and foreign operations, including without limitation, tax obligations and changes affecting the tax treatment by the United States of income earned outside the United States that may result from pending and possible future proposals; and |
| • | governmental laws and regulations affecting our interactions with veterinary healthcare providers. |
However, there may also be other risks that we are unable to predict at this time. These risks or uncertainties may cause actual results to differ materially from those contemplated by a forward-looking statement. You should not put undue reliance on forward-looking statements. Forward-looking statements speak only as of the date on which they are made. We undertake no obligation to publicly update forward-looking statements, whether as a result of new information, future events or otherwise, except as required by law or by the rules and regulations of the SEC. You are advised, however, to consult any further disclosures we make on related subjects in our Form 10-Q and 8-K reports and our other filings with the SEC. You should understand that it is not possible to predict or identify all such factors. Consequently, you should not consider the above to be a complete discussion of all potential risks or uncertainties.
| Item 7A. | Quantitative and Qualitative Disclosures About Market Risk. |
A significant portion of our revenue and costs are exposed to changes in foreign exchange rates. In addition, our outstanding borrowings may be subject to risk from changes in interest rates and foreign exchange rates. The overall objective of our financial risk management program is to seek to minimize the impact of foreign exchange rate movements and interest rate movements on our earnings. We manage these financial exposures through operational means and by using certain financial instruments. These practices may change as economic conditions change.
Foreign exchange risk
Our primary net foreign currency translation exposures are the Australian dollar, Brazilian real, Canadian dollar, Chinese yuan, euro, and U.K. pound. We seek to manage our foreign exchange risk, in part, through operational means, including managing same-currency revenue in relation to same-currency costs and same-currency assets in relation to same-currency liabilities.
56 |
Foreign exchange risk is also managed through the use of foreign currency forward-exchange contracts. These contracts are used to offset the potential earnings effects from mostly intercompany short-term foreign currency assets and liabilities that arise from operations.
Our financial instruments at December 31, 2017, were analyzed to determine their sensitivity to foreign exchange rate changes. The fair values of these instruments were determined using Level 2 inputs. For additional details, see Notes to Consolidated Financial Statements— Note 3. Significant Accounting Policies: Fair Value. The sensitivity analysis of changes in the fair value of all foreign currency forward-exchange contracts at December 31, 2017, indicates that if the U.S. dollar were to appreciate against all other currencies by 10%, the fair value of these contracts would increase by $24 million, and if the U.S. dollar were to weaken against all other currencies by 10%, the fair value of these contracts would decrease by $32 million. For additional details, see Notes to Consolidated Financial Statements— Note 9B. Financial Instruments: Derivative Financial Instruments.
Interest rate risk
Our outstanding debt balances are fixed rate debt. While changes in interest rates will have no impact on the interest we pay on our fixed rate debt, interest on our revolving credit facility will be exposed to interest rate fluctuations. At December 31, 2017, we had no outstanding principal balance under our revolving credit facility. See Notes to Consolidated Financial Statements— Note 9. Financial Instruments.
57 |
Item 8. Financial Statements and Supplementary Data.
INDEX TO FINANCIAL STATEMENTS
| Page | |
| Audited Consolidated Financial Statements of Zoetis Inc. and Subsidiaries: | |
| Consolidated Statements of Income for the Years Ended December 31, 2017, 2016 and 2015 | |
| Consolidated Statements of Comprehensive Income for the Years Ended December 31, 2017, 2016 and 2015 | |
| Consolidated Balance Sheets as of December 31, 2017 and 2016 | |
| Consolidated Statements of Equity for the Years Ended December 31, 2017, 2016 and 2015 | |
| Consolidated Statements of Cash Flows for the Years Ended December 31, 2017, 2016 and 2015 | |
| Schedule II—Valuation and Qualifying Accounts | |
58 |
Report of Independent Registered Public Accounting Firm
To the Stockholders and Board of Directors
Zoetis, Inc.:
Opinion on the Consolidated Financial Statements
We have audited the accompanying consolidated balance sheets of Zoetis, Inc. and subsidiaries (the “Company”) as of December 31, 2017 and 2016, the related consolidated statements of income, comprehensive income, equity, and cash flows for each of the years in the three‑year period ended December 31, 2017, and the related notes and financial statement schedule II - Valuation and Qualifying Accounts (collectively, the “consolidated financial statements”). In our opinion, the consolidated financial statements present fairly, in all material respects, the financial position of the Company as of December 31, 2017 and 2016, and the results of its operations and its cash flows for each of the years in the three‑year period ended December 31, 2017, in conformity with U.S. generally accepted accounting principles.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States) (“PCAOB”), the Company’s internal control over financial reporting as of December 31, 2017, based on criteria established in Internal Control - Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission, and our report dated February 15, 2018 expressed an unqualified opinion on the effectiveness of the Company’s internal control over financial reporting.
Basis for Opinion
These consolidated financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these consolidated financial statements based on our audits. We are a public accounting firm registered with the PCAOB and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the consolidated financial statements are free of material misstatement, whether due to error or fraud. Our audits included performing procedures to assess the risks of material misstatement of the consolidated financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the consolidated financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the consolidated financial statements. We believe that our audits provide a reasonable basis for our opinion.
We have served as the Company’s auditor since 2011.
/s/ KPMG LLP
Short Hills, New Jersey
February 15, 2018
59 |
Report of Independent Registered Public Accounting Firm
To the Stockholders and Board of Directors
Zoetis, Inc.:
Opinion on Internal Control Over Financial Reporting
We have audited Zoetis, Inc. and subsidiaries’ (the “Company”) internal control over financial reporting as of December 31, 2017, based on criteria established in Internal Control - Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission. In our opinion, the Company maintained, in all material respects, effective internal control over financial reporting as of December 31, 2017, based on criteria established in Internal Control - Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States) (“PCAOB”), the consolidated balance sheets of the Company as of December 31, 2017 and 2016, the related consolidated statements of income, comprehensive income, equity, and cash flows for each of the years in the three-year period ended December 31, 2017, and the related notes and financial statement schedule II - Valuation and Qualifying Accounts (collectively, the “consolidated financial statements”), and our report dated February 15, 2018 expressed an unqualified opinion on those consolidated financial statements.
Basis for Opinion
The Company’s management is responsible for maintaining effective internal control over financial reporting and for its assessment of the effectiveness of internal control over financial reporting, included in the accompanying Management’s Report on Internal Control Over Financial Reporting. Our responsibility is to express an opinion on the Company’s internal control over financial reporting based on our audit. We are a public accounting firm registered with the PCAOB and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audit in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether effective internal control over financial reporting was maintained in all material respects. Our audit of internal control over financial reporting included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, and testing and evaluating the design and operating effectiveness of internal control based on the assessed risk. Our audit also included performing such other procedures as we considered necessary in the circumstances. We believe that our audit provides a reasonable basis for our opinion.
Definition and Limitations of Internal Control Over Financial Reporting
A company’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company’s internal control over financial reporting includes those policies and procedures that (1) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (3) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company’s assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
/s/ KPMG LLP
Short Hills, New Jersey
February 15, 2018
60 |
ZOETIS INC. AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF INCOME
| Year Ended December 31, | ||||||||||||
| (MILLIONS OF DOLLARS AND SHARES, EXCEPT PER SHARE DATA) | 2017 | 2016 | 2015 | |||||||||
| Revenue | $ | 5,307 | $ | 4,888 | $ | 4,765 | ||||||
| Costs and expenses: | ||||||||||||
Cost of sales(a) | 1,775 | 1,666 | 1,738 | |||||||||
Selling, general and administrative expenses(a) | 1,334 | 1,364 | 1,532 | |||||||||
Research and development expenses(a) | 382 | 376 | 364 | |||||||||
| Amortization of intangible assets | 91 | 85 | 61 | |||||||||
| Restructuring charges and certain acquisition-related costs | 19 | 5 | 320 | |||||||||
| Interest expense, net of capitalized interest | 175 | 166 | 124 | |||||||||
| Other (income)/deductions––net | 6 | (2 | ) | 81 | ||||||||
| Income before provision for taxes on income | 1,525 | 1,228 | 545 | |||||||||
| Provision for taxes on income | 663 | 409 | 206 | |||||||||
| Net income before allocation to noncontrolling interests | 862 | 819 | 339 | |||||||||
| Less: Net loss attributable to noncontrolling interests | (2 | ) | (2 | ) | — | |||||||
| Net income attributable to Zoetis | $ | 864 | $ | 821 | $ | 339 | ||||||
| Earnings per share attributable to Zoetis Inc. stockholders: | ||||||||||||
| Basic | $ | 1.76 | $ | 1.66 | $ | 0.68 | ||||||
| Diluted | $ | 1.75 | $ | 1.65 | $ | 0.68 | ||||||
| Weighted-average common shares outstanding: | ||||||||||||
| Basic | 489.918 | 495.715 | 499.707 | |||||||||
| Diluted | 493.161 | 498.225 | 502.019 | |||||||||
| Dividends declared per common share | $ | 0.441 | $ | 0.390 | $ | 0.344 | ||||||
(a) | Exclusive of amortization of intangible assets, except as disclosed in Note 3. Significant Accounting Policies—Amortization of Intangible Assets, Depreciation and Certain Long-Lived Assets. |
See Notes to Consolidated Financial Statements, which are an integral part of these statements.
61 |
ZOETIS INC. AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF COMPREHENSIVE INCOME
| Year Ended December 31, | ||||||||||||
| (MILLIONS OF DOLLARS) | 2017 | 2016 | 2015 | |||||||||
| Net income before allocation to noncontrolling interests | $ | 862 | $ | 819 | $ | 339 | ||||||
| Other comprehensive income/(loss), net of tax and reclassification adjustments: | ||||||||||||
Unrealized (loss)/gain on derivatives, net(a) | (11 | ) | 10 | (2 | ) | |||||||
| Foreign currency translation adjustments, net | 98 | 17 | (269 | ) | ||||||||
Benefit plans: Actuarial gain/(loss), net(a) | 8 | (6 | ) | 9 | ||||||||
| Total other comprehensive income/(loss), net of tax | 95 | 21 | (262 | ) | ||||||||
| Comprehensive income before allocation to noncontrolling interests | 957 | 840 | 77 | |||||||||
| Comprehensive loss attributable to noncontrolling interests | — | (3 | ) | (1 | ) | |||||||
| Comprehensive income attributable to Zoetis | $ | 957 | $ | 843 | $ | 78 | ||||||
(a) | Presented net of reclassification adjustments and tax impacts, which are not significant in any period presented. Reclassification adjustments related to benefit plans are generally reclassified, as part of net periodic pension cost, into Cost of sales, Selling, general and administrative expenses, and/or Research and development expenses, as appropriate, in the consolidated statements of income. |
See Notes to Consolidated Financial Statements, which are an integral part of these statements.
62 |
ZOETIS INC. AND SUBSIDIARIES
CONSOLIDATED BALANCE SHEETS
| December 31, | December 31, | |||||||
| (MILLIONS OF DOLLARS, EXCEPT PER SHARE DATA) | 2017 | 2016 | ||||||
| Assets | ||||||||
Cash and cash equivalents(a) | $ | 1,564 | $ | 727 | ||||
| Accounts receivable, less allowance for doubtful accounts of $25 in 2017 and $30 in 2016 | 998 | 913 | ||||||
| Inventories | 1,427 | 1,502 | ||||||
| Other current assets | 228 | 248 | ||||||
| Total current assets | 4,217 | 3,390 | ||||||
| Property, plant and equipment, less accumulated depreciation of $1,471 in 2017 and $1,358 in 2016 | 1,435 | 1,381 | ||||||
| Goodwill | 1,510 | 1,481 | ||||||
| Identifiable intangible assets, less accumulated amortization | 1,269 | 1,228 | ||||||
| Noncurrent deferred tax assets | 80 | 96 | ||||||
| Other noncurrent assets | 75 | 73 | ||||||
| Total assets | $ | 8,586 | $ | 7,649 | ||||
| Liabilities and Equity | ||||||||
| Accounts payable | $ | 261 | $ | 265 | ||||
| Dividends payable | 61 | 52 | ||||||
| Accrued expenses | 432 | 464 | ||||||
| Accrued compensation and related items | 236 | 224 | ||||||
| Income taxes payable | 60 | 71 | ||||||
| Other current liabilities | 44 | 41 | ||||||
| Total current liabilities | 1,094 | 1,117 | ||||||
| Long-term debt, net of discount and issuance costs | 4,953 | 4,468 | ||||||
| Noncurrent deferred tax liabilities | 380 | 244 | ||||||
| Other taxes payable | 172 | 73 | ||||||
| Other noncurrent liabilities | 201 | 248 | ||||||
| Total liabilities | 6,800 | 6,150 | ||||||
Commitments and contingencies (Note 17) | ||||||||
| Stockholders' equity: | ||||||||
| Preferred stock, $0.01 par value; 1,000,000,000 authorized, none issued | — | — | ||||||
Common stock, $0.01 par value: 6,000,000,000 authorized, 501,891,243 and 501,891,243 shares issued; 486,130,461 and 492,855,297 shares outstanding at December 31, 2017 and 2016, respectively | 5 | 5 | ||||||
| Treasury stock, at cost, 15,760,782 and 9,035,946 shares of common stock at December 31, 2017 and 2016, respectively | (852 | ) | (421 | ) | ||||
| Additional paid-in capital | 1,013 | 1,024 | ||||||
| Retained earnings | 2,109 | 1,477 | ||||||
| Accumulated other comprehensive loss | (505 | ) | (598 | ) | ||||
| Total Zoetis Inc. equity | 1,770 | 1,487 | ||||||
| Equity attributable to noncontrolling interests | 16 | 12 | ||||||
| Total equity | 1,786 | 1,499 | ||||||
| Total liabilities and equity | $ | 8,586 | $ | 7,649 | ||||
(a) | As of December 31, 2017, includes $6 million of restricted cash. |
See Notes to Consolidated Financial Statements, which are an integral part of these statements.
63 |
ZOETIS INC. AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF EQUITY
| Zoetis | ||||||||||||||||||||||||||||
| Accumulated | Equity | |||||||||||||||||||||||||||
| Additional | Other | Attributable to | ||||||||||||||||||||||||||
| Common | Treasury | Paid-in | Retained | Comprehensive | Noncontrolling | Total | ||||||||||||||||||||||
| (MILLIONS OF DOLLARS) | Stock(a) | Stock(a) | Capital | Earnings | Loss | Interests | Equity | |||||||||||||||||||||
| Balance, December 31, 2014 | $ | 5 | $ | — | $ | 958 | $ | 709 | $ | (361 | ) | $ | 26 | $ | 1,337 | |||||||||||||
| Net income | — | — | — | 339 | — | — | 339 | |||||||||||||||||||||
| Other comprehensive loss | — | — | — | — | (261 | ) | (1 | ) | (262 | ) | ||||||||||||||||||
Share-based compensation awards(b) | — | (4 | ) | 51 | — | — | — | 47 | ||||||||||||||||||||
Treasury stock acquired(c) | — | (199 | ) | — | — | — | — | (199 | ) | |||||||||||||||||||
Employee benefit plan contribution from Pfizer Inc.(d) | — | — | 3 | — | — | — | 3 | |||||||||||||||||||||
| Dividends declared | — | — | — | (172 | ) | — | (2 | ) | (174 | ) | ||||||||||||||||||
| Balance, December 31, 2015 | $ | 5 | $ | (203 | ) | $ | 1,012 | $ | 876 | $ | (622 | ) | $ | 23 | $ | 1,091 | ||||||||||||
| Net income/(loss) | — | — | — | 821 | — | (2 | ) | 819 | ||||||||||||||||||||
| Other comprehensive income/(loss) | — | — | — | — | 22 | (1 | ) | 21 | ||||||||||||||||||||
Share-based compensation awards(b) | — | 82 | 9 | (27 | ) | — | — | 64 | ||||||||||||||||||||
Treasury stock acquired(c) | — | (300 | ) | — | — | — | — | (300 | ) | |||||||||||||||||||
Employee benefit plan contribution from Pfizer Inc.(d) | — | — | 3 | — | — | — | 3 | |||||||||||||||||||||
Divestitures(e) | — | — | — | — | 2 | (8 | ) | (6 | ) | |||||||||||||||||||
| Dividends declared | — | — | — | (193 | ) | — | — | (193 | ) | |||||||||||||||||||
| Balance, December 31, 2016 | $ | 5 | $ | (421 | ) | $ | 1,024 | $ | 1,477 | $ | (598 | ) | $ | 12 | $ | 1,499 | ||||||||||||
| Net income/(loss) | — | — | — | 864 | — | (2 | ) | 862 | ||||||||||||||||||||
| Other comprehensive income | — | — | — | — | 93 | 2 | 95 | |||||||||||||||||||||
Consolidation of a noncontrolling interest(f) | — | — | — | — | — | 18 | 18 | |||||||||||||||||||||
Purchase of shares from noncontrolling interest(g) | — | — | (29 | ) | — | — | (14 | ) | (43 | ) | ||||||||||||||||||
Share-based compensation awards(b) | — | 69 | 15 | (16 | ) | — | — | 68 | ||||||||||||||||||||
Treasury stock acquired(c) | — | (500 | ) | — | — | — | — | (500 | ) | |||||||||||||||||||
Employee benefit plan contribution from Pfizer Inc.(d) | — | — | 3 | — | — | — | 3 | |||||||||||||||||||||
| Dividends declared | — | — | — | (216 | ) | — | — | (216 | ) | |||||||||||||||||||
| Balance, December 31, 2017 | $ | 5 | $ | (852 | ) | $ | 1,013 | $ | 2,109 | $ | (505 | ) | $ | 16 | $ | 1,786 | ||||||||||||
(a) | As of December 31, 2017 and 2016, respectively, there were 486,130,461 and 492,855,297 outstanding shares of common stock and 15,760,782 and 9,035,946 shares or treasury stock. Treasury stock is recognized at the cost to reacquire the shares. For additional information, see Note 15. Stockholders' Equity. |
(b) | Includes the issuance of shares of Zoetis Inc. common stock and the reacquisition of shares of treasury stock associated with exercises of employee share-based awards. Upon reissuance of treasury stock, differences between the proceeds from reissuance and the cost of the treasury stock that result in gains are recorded in Additional paid-in capital. Losses are recorded in Additional paid-in capital to the extent that they can offset previously recorded gains. If no such credit exists, the differences are recorded in Retained earnings. Also includes the reacquisition of shares of treasury stock associated with the vesting of employee share-based awards to satisfy tax withholding requirements. For additional information, see Note 14. Share-Based Payments and Note 15. Stockholders' Equity. |
(c) | Reflects the acquisition of treasury shares in connection with the share repurchase program. For additional information, see Note 15. Stockholders' Equity. |
(d) | Represents contributed capital from Pfizer Inc. associated with service credit continuation for certain Zoetis Inc. employees in Pfizer Inc.'s U.S. qualified defined benefit and U.S. retiree medical plans. See Note 13. Benefit Plans. |
(e) | Reflects the divestiture of our share of our Taiwan joint venture. See Note 4B. Acquisitions and Divestitures: Divestitures. |
(f) | Represents the consolidation of a European livestock monitoring company, a variable interest entity of which Zoetis is the primary beneficiary. |
(g) | Represents the acquisition of the remaining 55 percent noncontrolling interest in Jilin Zoetis Guoyuan Animal Health Co., Ltd., a variable interest entity previously consolidated by Zoetis as the primary beneficiary. |
See Notes to Consolidated Financial Statements, which are an integral part of these statements.
64 |
ZOETIS INC. AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF CASH FLOWS
| Year Ended December 31, | ||||||||||||
| (MILLIONS OF DOLLARS) | 2017 | 2016 | 2015 | |||||||||
| Operating Activities | ||||||||||||
| Net income before allocation to noncontrolling interests | $ | 862 | $ | 819 | $ | 339 | ||||||
| Adjustments to reconcile net income before noncontrolling interests to net cash | ||||||||||||
| provided by operating activities: | ||||||||||||
| Depreciation and amortization expense | 242 | 240 | 199 | |||||||||
| Share-based compensation expense | 44 | 37 | 43 | |||||||||
| Restructuring | 19 | 5 | 203 | |||||||||
| Asset write-offs and asset impairments | 3 | 5 | 60 | |||||||||
| Loss/(gain) on sales of assets | 11 | (26 | ) | — | ||||||||
| Provision for losses on inventory | 54 | 105 | 94 | |||||||||
| Deferred taxes | 127 | (55 | ) | (85 | ) | |||||||
| Foreign currency loss related to Venezuela Revaluation, excluding impact on cash | — | — | 6 | |||||||||
| Employee benefit plan contribution from Pfizer Inc. | 3 | 3 | 3 | |||||||||
| Other non-cash adjustments | 10 | 19 | 10 | |||||||||
| Other changes in assets and liabilities, net of acquisitions and divestitures and transfers with Pfizer Inc. | ||||||||||||
| Accounts receivable | (50 | ) | 15 | (58 | ) | |||||||
| Inventories | 19 | (101 | ) | (262 | ) | |||||||
| Other assets | (16 | ) | (50 | ) | (9 | ) | ||||||
| Accounts payable | (10 | ) | (28 | ) | 17 | |||||||
| Other liabilities | (57 | ) | (295 | ) | 70 | |||||||
| Other tax accounts, net | 85 | 20 | 34 | |||||||||
| Net cash provided by operating activities | 1,346 | 713 | 664 | |||||||||
| Investing Activities | ||||||||||||
| Capital expenditures | (224 | ) | (216 | ) | (224 | ) | ||||||
| Acquisitions | (82 | ) | (88 | ) | (883 | ) | ||||||
| Net proceeds from sales of assets | 37 | 90 | 2 | |||||||||
| Other investing activities | (1 | ) | — | (10 | ) | |||||||
| Net cash used in investing activities | (270 | ) | (214 | ) | (1,115 | ) | ||||||
| Financing Activities | ||||||||||||
| Decrease in short-term borrowings, net | — | (5 | ) | (2 | ) | |||||||
| Principal payments on long-term debt | (750 | ) | (400 | ) | — | |||||||
| Proceeds from issuance of long-term debt—senior notes, net of discount and fees | 1,231 | — | 1,236 | |||||||||
| Payment of contingent consideration related to previously acquired assets | (7 | ) | (32 | ) | — | |||||||
Share-based compensation-related proceeds, net of taxes paid on withholding shares and excess tax benefits(a) | 24 | 25 | 11 | |||||||||
Purchases of treasury stock(b) | (500 | ) | (300 | ) | (203 | ) | ||||||
| Cash dividends paid | (206 | ) | (188 | ) | (168 | ) | ||||||
| Cash paid to settle Pharmaq debt | — | — | (119 | ) | ||||||||
| Acquisition of noncontrolling interest | (43 | ) | — | — | ||||||||
| Payment of debt issuance costs | — | (3 | ) | — | ||||||||
| Net cash (used in)/provided by financing activities | (251 | ) | (903 | ) | 755 | |||||||
| Effect of exchange-rate changes on cash and cash equivalents | 12 | (23 | ) | (32 | ) | |||||||
| Net increase/(decrease) in cash and cash equivalents | 837 | (427 | ) | 272 | ||||||||
| Cash and cash equivalents at beginning of period | 727 | 1,154 | 882 | |||||||||
| Cash and cash equivalents at end of period | $ | 1,564 | $ | 727 | $ | 1,154 | ||||||
See Notes to Consolidated Financial Statements, which are an integral part of these statements.
65 |
| Supplemental cash flow information | ||||||||||||
| Cash paid during the period for: | ||||||||||||
| Income taxes | $ | 455 | $ | 408 | $ | 224 | ||||||
| Interest, net of capitalized interest | 167 | 165 | 117 | |||||||||
| Non-cash transactions: | ||||||||||||
| Capital expenditures | $ | 5 | 8 | 11 | ||||||||
Contingent purchase price consideration(c) | 29 | 27 | 23 | |||||||||
| Dividends declared, not paid | 61 | 52 | 47 | |||||||||
(a) | Effective 2016, excess tax benefits are reflected within operating activities. See Note 3. Significant Accounting Policies for additional information. |
(b) | Reflects the acquisition of treasury shares in connection with the share repurchase program. For additional information, see Note 15. Stockholders' Equity. |
(c) | For 2016, relates primarily to the non-cash portion of the acquisition of a livestock business in South America and a veterinary diagnostics business in Denmark. For 2015, relates primarily to the non-cash portion of the acquisition of certain assets of Abbott Animal Health. |
See Notes to Consolidated Financial Statements, which are an integral part of these statements.
66 |
ZOETIS INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
1. Business Description
Zoetis Inc. (including its subsidiaries, collectively, Zoetis, the company, we, us or our) is a global leader in the discovery, development, manufacture and commercialization of animal health medicines and vaccines, with a focus on both livestock and companion animals. We organize and operate our business in two geographic regions: the United States (U.S.) and International.
We directly market our products in approximately 45 countries across North America, Europe, Africa, Asia, Australia and South America. Our products are sold in more than 100 countries, including developed markets and emerging markets. We have a diversified business, marketing products across eight core species: cattle, swine, poultry, sheep and fish (collectively, livestock) and dogs, cats and horses (collectively, companion animals); and within five major product categories: anti-infectives, vaccines, parasiticides, medicated feed additives and other pharmaceuticals.
We were incorporated in Delaware in July 2012 and prior to that the company was a business unit of Pfizer Inc. (Pfizer).
| 2. | Basis of Presentation |
The consolidated financial statements were prepared in accordance with accounting principles generally accepted in the United States of America (U.S. GAAP). For subsidiaries operating outside the United States, the consolidated financial information is included as of and for the fiscal year ended November 30 for each year presented. All significant intercompany balances and transactions between the legal entities that comprise Zoetis have been eliminated. For those subsidiaries included in these consolidated financial statements where our ownership is less than 100%, including a variable interest entity consolidated by Zoetis as the primary beneficiary, the noncontrolling interests have been shown in equity as Equity attributable to noncontrolling interests.
3. Significant Accounting Policies
Recently Adopted Accounting Standards
In January 2017, the Financial Accounting Standards Board (FASB) issued an accounting standards update which clarifies the definition of a business. Under the new guidance, a set of integrated activities and assets is a business only if it has, at a minimum, an input and substantive process that together significantly contribute to the ability to create outputs. The update also introduces the concept of an initial screening or “Step 1” which requires companies to first determine if substantially all of the fair value of the gross assets acquired is concentrated in a single (or group of similar) identifiable assets. Transactions that pass the Step 1 screening will be considered a business if they contain an input and substantive process and either; (1) an output or (2) an organized workforce with skills critical to the ability to create outputs and inputs that can be utilized to create the outputs. Companies will no longer be required to evaluate whether a market participant could replace any missing inputs or processes, instead focusing on the substance of what was acquired. The provisions of the new standard are effective, on a prospective basis, beginning January 1, 2018, for annual and interim reporting periods and may be adopted early for any transactions not yet reported in issued financial statements. We elected to early adopt the new standard for any new transactions occurring on or after January 1, 2017.
In July 2015, the FASB issued an accounting standards update to simplify the measurement of inventory by requiring that inventory be measured at the lower of cost or net realizable value, rather than at the lower of cost or market, with market being defined as either replacement cost, net realizable value or net realizable value less a normal profit margin. We adopted this guidance as of January 1, 2017. This guidance did not have a significant impact on our consolidated financial statements.
In March 2016, the FASB issued an accounting standards update which simplifies the accounting for employee share-based payments. The new standard requires the immediate recognition of all excess tax benefits and deficiencies in the income statement, and requires classification of excess tax benefits as an operating activity as opposed to a financing activity in the statements of cash flows. The standard also clarifies that all cash payments made to taxing authorities on the employees' behalf for shares withheld should be presented as financing activities on the statements of cash flows and provides for a policy election to either estimate the number of awards that are expected to vest or account for forfeitures as they occur. The provisions of the new standard are effective beginning January 1, 2017, and early adoption is permitted if all amendments are adopted in the same period. We elected to early adopt the new standard effective January 1, 2016. Excess tax benefits of $9 million and $7 million generated during the years ended December 31, 2017, and 2016, respectively, are reflected as a component of Provision for taxes on income as presented in the consolidated statements of income. We have elected to apply the change in cash flow classification for excess tax benefits on a prospective basis. Cash payments made to taxing authorities on the behalf of company employees are reflected as a financing outflow in the consolidated statements of cash flows, consistent with prior years. We continue to include the impact of estimated forfeitures when determining share-based compensation expense.
Recently Issued Accounting Standards
In August 2017, the FASB issued an accounting standards update which amends the hedge accounting recognition and presentation requirements and allows for more hedging strategies to be eligible for hedge accounting. Recognition of periodic hedge effectiveness will no longer be required for cash flow and net investment hedges and companies may elect to perform subsequent hedge effectiveness assessments qualitatively. The update also clarifies that the change in fair value of a derivative must be recorded in the same income statement line item as the earnings effect of the hedged item and introduces additional disclosure requirements including cumulative basis adjustments for fair value hedges and the effect of hedging on individual income statement line items. The provisions of the update are effective beginning January 1, 2019 for interim and annual periods with
67 |
early adoption permitted for any interim period after issuance of the update. We are currently assessing the timing of our adoption as well as the potential impact that the standard will have on our consolidated financial statements.
In March 2017, the FASB issued an accounting standards update to simplify and improve the reporting of net periodic pension benefit cost by requiring only present service cost to be presented in the same line item as other current employee compensation costs while remaining components of net periodic benefit cost would be presented within Other (income)/deductions—net outside of operations. We will adopt this guidance as of January 1, 2018, the required effective date, and do not expect that the new standard will have a significant impact on our consolidated financial statements.
In October 2016, the FASB issued an accounting standards update that will require the recognition of the income tax consequences of an intra-entity asset transfer, other than inventory, when the transfer occurs as opposed to when the asset is sold to an outside third party. The provisions of the new standard are effective beginning January 1, 2018, for annual and interim reporting periods. We will adopt this guidance as of January 1, 2018, the required effective date, and do not expect that the new standard will have a significant impact on our consolidated financial statements.
In February 2016, the FASB issued an accounting standards update which requires lessees to recognize most leases on the balance sheet with a corresponding right of use asset. Leases will be classified as financing or operating which will drive the expense recognition pattern. For lessees, the income statement presentation and expense recognition pattern for financing and operating leases is similar to the current model for capital and operating leases, respectively. Companies may elect to exclude short-term leases. The update also requires additional disclosures that will better enable users of financial statements to assess the amount, timing, and uncertainty of cash flows arising from leases. We plan to adopt this guidance as of January 1, 2019, the required effective date, for annual and interim reporting periods. The new standard requires a modified retrospective adoption approach, at the beginning of the earliest comparative period presented in the financial statements. We are currently evaluating the impact that adopting this new guidance will have on our consolidated financial statements.
In May 2014, the FASB issued an accounting standards update that outlines a new, single comprehensive model for companies to use in accounting for revenue arising from contracts with customers. This update supersedes most current revenue recognition guidance under U.S. GAAP. The core principle of the new guidance is that an entity should recognize revenue to depict the transfer of goods or services to customers in an amount that reflects the consideration to which the entity expects to be entitled in exchange for those goods or services. The guidance includes a five-step model for determining how, when and how much revenue should be recognized. This update also requires additional disclosure about the nature, amount, timing and uncertainty of revenue and cash flows arising from contracts with customers. We will adopt this guidance as of January 1, 2018, the required effective date, using the modified retrospective transition method. Under the modified retrospective method, the cumulative effect of applying the new standard will be recognized as of the date of initial application with disclosure of results under both the new and prior standards. The new standard will not have a material impact on our consolidated financial statements upon adoption or on an ongoing basis.
Estimates and Assumptions
In preparing the consolidated financial statements, we use certain estimates and assumptions that affect reported amounts and disclosures, including amounts recorded in connection with acquisitions. These estimates and underlying assumptions can impact all elements of our consolidated financial statements. For example, in the consolidated statements of income, estimates are used when accounting for deductions from revenue (such as rebates, sales allowances, product returns and discounts), determining cost of sales, allocating cost in the form of depreciation and amortization, and estimating restructuring charges and the impact of contingencies. On the consolidated balance sheets, estimates are used in determining the valuation and recoverability of assets, such as accounts receivables, inventories, fixed assets, goodwill and other identifiable intangible assets, and estimates are used in determining the reported amounts of liabilities, such as taxes payable, benefit obligations, the impact of contingencies, deductions from revenue and restructuring reserves, all of which also impact the consolidated statements of income.
Our estimates are often based on complex judgments, probabilities and assumptions that we believe to be reasonable but that can be inherently uncertain and unpredictable. If our estimates and assumptions are not representative of actual outcomes, our results could be materially impacted.
As future events and their effects cannot be determined with precision, our estimates and assumptions may prove to be incomplete or inaccurate, or unanticipated events and circumstances may occur that might cause us to change those estimates and assumptions. We are subject to risks and uncertainties that may cause actual results to differ from estimated amounts, such as changes in competition, litigation, legislation and regulations. We regularly evaluate our estimates and assumptions using historical experience and expectations about the future. We adjust our estimates and assumptions when facts and circumstances indicate the need for change. Those changes generally will be reflected in our consolidated financial statements on a prospective basis unless they are required to be treated retrospectively under relevant accounting standards. It is possible that others, applying reasonable judgment to the same facts and circumstances, could develop and support a range of alternative estimated amounts.
Acquisitions
Our consolidated financial statements include the operations of acquired businesses from the date of acquisition. We account for acquired businesses using the acquisition method of accounting, which requires, among other things, that most assets acquired and liabilities assumed be recognized at their estimated fair values as of the acquisition date and that the fair value of acquired in-process research and development (IPR&D) be recorded on the balance sheet. Transaction costs are expensed as incurred. Any excess of the consideration transferred over the assigned values of the net assets acquired is recorded as goodwill. When we acquire net assets that do not constitute a business as defined in U.S. GAAP, no goodwill is recognized.
Amounts recorded for acquisitions can result from a complex series of judgments about future events and uncertainties and can rely on estimates and assumptions. For information about the risks associated with estimates and assumptions, see Estimates and Assumptions.
Foreign Currency Translation
For most of our international operations, local currencies have been determined to be the functional currencies. We translate functional currency assets and liabilities to their U.S. dollar equivalents at exchange rates in effect at the balance sheet date and we translate functional currency income and expense amounts to their U.S. dollar equivalents at average exchange rates for the period. The U.S. dollar effects that arise from changing translation rates are recorded in Other comprehensive income/(loss), net of tax. The effects of converting non-functional currency assets and liabilities
68 |
into the functional currency are recorded in Other (income)/deductions––net. For operations in highly inflationary economies, we translate monetary items at rates in effect at the balance sheet date, with translation adjustments recorded in Other (income)/deductions––net, and we translate non-monetary items at historical rates.
Revenue, Deductions from Revenue and the Allowance for Doubtful Accounts
We record revenue from product sales when the goods are shipped and title and risk of loss passes to the customer. At the time of sale, we also record estimates for a variety of deductions from revenue, such as rebates, sales allowances, product returns and discounts. Sales deductions are estimated and recorded at the time that related revenue is recorded except for sales incentives, which are estimated and recorded at the time the related revenue is recorded or when the incentive is offered, whichever is later. As applicable, our estimates are generally based on contractual terms or historical experience, adjusted as necessary to reflect our expectations about the future. Taxes collected from customers relating to product sales and remitted to governmental authorities are excluded from Revenue.
As of December 31, 2017, and 2016, accruals for sales deductions included in Other current liabilities are approximately $141 million and $122 million, respectively.
We also record estimates for bad debts. We periodically assess the adequacy of the allowance for doubtful accounts by evaluating the collectability of outstanding receivables based on factors such as past due history, historical and expected collection patterns, the financial condition of our customers, the robust nature of our credit and collection practices and the economic environment.
Amounts recorded for sales deductions and bad debts can result from a complex series of judgments about future events and uncertainties and can rely on estimates and assumptions. For information about the risks associated with estimates and assumptions, see Estimates and Assumptions.
Cost of Sales and Inventories
Inventories are carried at the lower of cost or net realizable value. The cost of finished goods, work-in-process and raw materials is determined using average actual cost. We regularly review our inventories for impairment and adjustments are recorded when necessary.
Selling, General and Administrative Expenses
Selling, general and administrative costs are expensed as incurred. Among other things, these expenses include the internal and external costs of marketing, advertising, and shipping and handling as well as certain costs related to business technology, facilities, legal, finance, human resources, business development, public affairs and procurement, among others.
Advertising expenses relating to production costs are expensed as incurred, and the costs of space in publications are expensed when the related advertising occurs. Advertising and promotion expenses totaled approximately $154 million in 2017, $119 million in 2016 and $106 million in 2015.
Shipping and handling costs totaled approximately $53 million in 2017, $51 million in 2016 and $59 million in 2015.
Research and Development Expenses
Research and development (R&D) costs are expensed as incurred. Research is the effort associated with the discovery of new knowledge that will be useful in developing a new product or in significantly improving an existing product. Development is the implementation of the research findings. Before a compound receives regulatory approval, we record upfront and milestone payments made by us to third parties under licensing arrangements as expense. Upfront payments are recorded when incurred, and milestone payments are recorded when the specific milestone has been achieved. Once a compound receives regulatory approval in a major market, we record any milestone payments in Identifiable intangible assets, less accumulated amortization and, unless the assets are determined to have an indefinite life, we amortize them on a straight-line basis over the remaining agreement term or the expected product life cycle, whichever is shorter.
Amortization of Intangible Assets, Depreciation and Certain Long-Lived Assets
Long-lived assets include:
| • | Goodwill—goodwill represents the excess of the consideration transferred for an acquired business over the assigned values of its net assets. Goodwill is not amortized. |
| • | Identifiable intangible assets, less accumulated amortization—these acquired assets are recorded at our cost. Identifiable intangible assets with finite lives are amortized on a straight-line basis over their estimated useful lives. Identifiable intangible assets with indefinite lives that are associated with marketed products are not amortized until a useful life can be determined. Identifiable intangible assets associated with IPR&D projects are not amortized until regulatory approval is obtained. The useful life of an amortizing asset generally is determined by identifying the period in which substantially all of the cash flows are expected to be generated. |
| • | Property, plant and equipment, less accumulated depreciation––these assets are recorded at our cost and are increased by the cost of any significant improvements after purchase. Property, plant and equipment assets, other than land and construction-in-progress, are depreciated on a straight-line basis over the estimated useful life of the individual assets. Depreciation begins when the asset is ready for its intended use. For tax purposes, accelerated depreciation methods are used as allowed by tax laws. |
Amortization expense related to finite-lived identifiable intangible assets that contribute to our ability to sell, manufacture, research, market and distribute products, compounds and intellectual property are included in Amortization of intangible assets as they benefit multiple business functions. Amortization expense related to intangible assets that are associated with a single function and depreciation of property, plant and equipment are included in Cost of sales, Selling, general and administrative expenses and Research and development expenses, as appropriate.
We review all of our long-lived assets for impairment indicators throughout the year and we perform detailed testing whenever impairment indicators are present. In addition, we perform impairment testing for goodwill and indefinite-lived assets at least annually. When necessary, we record charges for impairments. Specifically:
69 |
| • | For finite-lived identifiable intangible assets, such as developed technology rights, and for other long-lived assets, such as property, plant and equipment, whenever impairment indicators are present, we calculate the undiscounted value of the projected cash flows associated with the asset, or asset group, and compare this estimated amount to the carrying amount. If the carrying amount is found to be greater, we record an impairment loss for the excess of book value over fair value. In addition, in all cases of an impairment review, we re-evaluate the remaining useful lives of the assets and modify them, as appropriate. |
| • | For indefinite-lived identifiable intangible assets, such as brands and IPR&D assets, we test for impairment at least annually, or more frequently if impairment indicators exist, by first assessing qualitative factors to determine whether it is more likely than not that the fair value of the indefinite-lived intangible asset is less than its carrying amount. If we conclude it is more likely than not that the fair value is less than the carrying amount, a quantitative test that compares the fair value of the indefinite-lived intangible asset with its carrying value is performed. If the fair value is less than the carrying amount, an impairment loss is recognized. We record an impairment loss, if any, for the excess of book value over fair value. In addition, in all cases of an impairment review other than for IPR&D assets, we re-evaluate whether continuing to characterize the asset as indefinite-lived is appropriate. |
| • | For goodwill, we test for impairment on at least an annual basis, or more frequently if impairment indicators exist, either by assessing qualitative factors to determine whether it is more likely than not that the fair value of a reporting unit is less than its carrying amount, or by performing a quantitative assessment. If we choose to perform a qualitative analysis and conclude it is more likely than not that the fair value of a reporting unit is less than its carrying amount, a quantitative fair value test is performed. We determine the implied fair value of goodwill by subtracting the fair value of all the identifiable net assets other than goodwill from the fair value of the reporting unit and record an impairment loss for the excess, if any, of book value of goodwill over the implied fair value. In 2017, we performed both qualitative and select quantitative impairment assessments as of October 1, 2017, which did not result in the impairment of goodwill associated with any of our reporting units. In 2016, we qualitatively assessed, as of October 2, 2016, whether it is more likely than not that the respective fair values of our reporting units are less than their carrying amounts, including goodwill. Based on that assessment, we determined that this condition does not exist for any of our reporting units and therefore concluded that a quantitative fair value test was not required and no impairments were recorded. |
Impairment reviews can involve a complex series of judgments about future events and uncertainties and can rely on estimates and assumptions. For information about the risks associated with estimates and assumptions, see Estimates and Assumptions.
Software Capitalization and Depreciation
We capitalize certain costs incurred in connection with obtaining or developing internal-use software, including payroll and payroll-related costs for employees who are directly associated with the internal-use software project, external direct costs of materials and services and interest costs while developing the software. Capitalized software costs are included in Property, plant and equipment and are amortized using the straight-line method over the estimated useful life of five to ten years. Capitalization of such costs ceases when the project is substantially complete and ready for its intended purpose. Costs incurred during the preliminary project and post-implementation stages, as well as software maintenance and training costs, are expensed in the period in which they are incurred. The company capitalized $8 million and $26 million of internal-use software for the years ended December 31, 2017, and 2016, respectively. Depreciation expense for capitalized software was $21 million in 2017, $19 million in 2016 and $14 million in 2015.
Restructuring Charges and Certain Acquisition-Related Costs
We may incur restructuring charges in connection with acquisitions when we implement plans to restructure and integrate the acquired operations or in connection with cost-reduction and productivity initiatives. Included in Restructuring charges and certain acquisition-related costs are all restructuring charges and certain costs associated with acquiring and integrating an acquired business. Transaction costs and integration costs are expensed as incurred. Termination costs are a significant component of restructuring charges and are generally recorded when the actions are probable and estimable.
Amounts recorded for restructuring charges and other associated costs can result from a complex series of judgments about future events and uncertainties and can rely on estimates and assumptions. For information about the risks associated with estimates and assumptions, see Estimates and Assumptions.
Earnings per Share
Basic earnings per share is computed by dividing net income attributable to Zoetis by the weighted-average number of common shares outstanding during the period. Diluted earnings per share adjusts the weighted-average number of common shares outstanding for the potential dilution that could occur if common stock equivalents (stock options, restricted stock units, and performance-vesting restricted stock units) were exercised or converted into common stock, calculated using the treasury stock method.
Cash Equivalents
Cash equivalents include items almost as liquid as cash, such as certificates of deposit and time deposits with maturity periods of three months or less when purchased.
Fair Value
Certain assets and liabilities are required to be measured at fair value, either upon initial recognition or for subsequent accounting or reporting. For example, we use fair value extensively in the initial recognition of net assets acquired in a business combination. Fair value is estimated using an exit price approach, which requires, among other things, that we determine the price that would be received to sell an asset or paid to transfer a liability in an orderly market. The determination of an exit price is considered from the perspective of market participants, considering the highest and best use of assets and, for liabilities, assuming that the risk of non-performance will be the same before and after the transfer.
When estimating fair value, depending on the nature and complexity of the asset or liability, we may use one or all of the following approaches:
70 |
| • | Income approach, which is based on the present value of a future stream of net cash flows. |
| • | Market approach, which is based on market prices and other information from market transactions involving identical or comparable assets or liabilities. |
| • | Cost approach, which is based on the cost to acquire or construct comparable assets less an allowance for functional and/or economic obsolescence. |
These fair value methodologies depend on the following types of inputs:
| • | Quoted prices for identical assets or liabilities in active markets (Level 1 inputs). |
| • | Quoted prices for similar assets or liabilities in active markets or quoted prices for identical or similar assets or liabilities in markets that are not active or are directly or indirectly observable (Level 2 inputs). |
| • | Unobservable inputs that reflect estimates and assumptions (Level 3 inputs). |
A single estimate of fair value can result from a complex series of judgments about future events and uncertainties and can rely on estimates and assumptions. For information about the risks associated with estimates and assumptions, see Estimates and Assumptions.
Accounts Receivable
The recorded amounts of accounts receivable approximate fair value because of their relatively short-term nature. As of December 31, 2017, and 2016, Accounts receivable, less allowance for doubtful accounts, of $998 million and $913 million, respectively, includes approximately $62 million and $49 million of other receivables, such as trade notes receivable and royalty receivables, among others.
Deferred Tax Assets and Liabilities and Income Tax Contingencies
Deferred tax assets and liabilities are recognized for the expected future tax consequences of differences between the financial reporting and tax bases of assets and liabilities using enacted tax rates and laws. We provide a valuation allowance when we believe that our deferred tax assets are not recoverable based on an assessment of estimated future taxable income that incorporates ongoing, prudent and feasible tax planning strategies.
We account for income tax contingencies using a benefit recognition model. If we consider that a tax position is more likely than not to be sustained upon audit, based solely on the technical merits of the position, we recognize the benefit. We measure the benefit by determining the amount that is greater than 50% likely of being realized upon settlement, presuming that the tax position is examined by the appropriate taxing authority that has full knowledge of all relevant information. Under the benefit recognition model, if the initial assessment fails to result in the recognition of a tax benefit, we regularly monitor our position and subsequently recognize the tax benefit: (i) if there are changes in tax law, analogous case law or there is new information that sufficiently raise the likelihood of prevailing on the technical merits of the position to more likely than not; (ii) if the statute of limitations expires; or (iii) if there is a completion of an audit resulting in a favorable settlement of that tax year with the appropriate agency. We regularly re-evaluate our tax positions based on the results of audits of federal, state and foreign income tax filings, statute of limitations expirations, changes in tax law or receipt of new information that would either increase or decrease the technical merits of a position relative to the “more-likely-than-not” standard. Liabilities associated with uncertain tax positions are classified as current only when we expect to pay cash within the next 12 months. Interest and penalties, if any, are recorded in Provision for taxes on income and are classified on our consolidated balance sheet with the related tax liability.
Amounts recorded for valuation allowances and income tax contingencies can result from a complex series of judgments about future events and uncertainties and can rely on estimates and assumptions. For information about the risks associated with estimates and assumptions, see Estimates and Assumptions.
Benefit Plans
All dedicated benefit plans are pension plans. For our dedicated benefit plans, we recognize the overfunded or underfunded status of defined benefit plans as an asset or liability on the consolidated balance sheets and the obligations generally are measured at the actuarial present value of all benefits attributable to employee service rendered, as provided by the applicable benefit formula. Pension obligations may include assumptions such as long-term rate of return on plan assets, expected employee turnover, participant mortality, and future compensation levels. Plan assets are measured at fair value. Net periodic benefit costs are recognized, as required, into Cost of sales, Selling, general and administrative expenses and Research and development expenses, as appropriate.
Amounts recorded for benefit plans can result from a complex series of judgments about future events and uncertainties and can rely on estimates and assumptions. For information about the risks associated with estimates and assumptions, see Estimates and Assumptions.
Asset Retirement Obligations
We record accruals for the legal obligations associated with the retirement of tangible long-lived assets, including obligations under the doctrine of promissory estoppel and those that are conditioned upon the occurrence of future events. These obligations generally result from the acquisition, construction, development and/or normal operation of long-lived assets. We recognize the fair value of these obligations in the period in which they are incurred by increasing the carrying amount of the related asset. Over time, we recognize expense for the accretion of the liability and for the amortization of the asset.
As of December 31, 2017 and 2016, accruals for asset retirement obligations included in Accrued expenses are $0.1 million for both periods, and included in Other noncurrent liabilities are $19 million and $12 million, respectively.
Amounts recorded for asset retirement obligations can result from a complex series of judgments about future events and uncertainties and can rely on estimates and assumptions. For information about the risks associated with estimates and assumptions, see Estimates and Assumptions.
71 |
Legal and Environmental Contingencies
We are subject to numerous contingencies arising in the ordinary course of business, such as product liability and other product-related litigation, commercial litigation, patent litigation, environmental claims and proceedings, government investigations and guarantees and indemnifications. We record accruals for these contingencies to the extent that we conclude that a loss is both probable and reasonably estimable. If some amount within a range of loss appears to be a better estimate than any other amount within the range, we accrue that amount. Alternatively, when no amount within a range of loss appears to be a better estimate than any other amount, we accrue the lowest amount in the range. We record anticipated recoveries under existing insurance contracts when recovery is assured.
Amounts recorded for contingencies can result from a complex series of judgments about future events and uncertainties and can rely on estimates and assumptions. For information about the risks associated with estimates and assumptions, see Estimates and Assumptions.
Share-Based Payments
Our compensation programs can include share-based payment plans. All grants under share-based payment programs are accounted for at fair value and such amounts generally are amortized on a straight-line basis over the vesting term to Cost of sales, Selling, general and administrative expenses, and Research and development expenses, as appropriate. We include the impact of estimated forfeitures when determining share-based compensation expense.
Amounts recorded for share-based compensation can result from a complex series of judgments about future events and uncertainties and can rely on estimates and assumptions. For information about the risks associated with estimates and assumptions, see Estimates and Assumptions.
4. Acquisitions and Divestitures
| A. | Acquisitions |
During 2017, Zoetis completed the acquisitions of the remaining 55 percent noncontrolling interest in Jilin Zoetis Guoyuan Animal Health Co., Ltd. (a variable interest entity previously consolidated by Zoetis as the primary beneficiary), an Irish biologic therapeutics company, and a Norwegian fish vaccination company. In addition, we also consolidated a European livestock monitoring company, a variable interest entity of which Zoetis is the primary beneficiary. These transactions did not have a significant impact on our consolidated financial statements.
| B. | Divestitures |
On May 5, 2015, in conjunction with the announcement of our comprehensive operational efficiency program and supply network strategy, we announced our intent to sell or exit 10 manufacturing sites over the long term. For additional information, see Note 5. Restructuring Charges and Other Costs Associated with Acquisitions and Cost-Reduction/Productivity Initiatives.
On November 14, 2017, as part of our supply network strategy, we completed the sale of our manufacturing site in Guarulhos, Brazil to Uniao Quimica (UQ), a Brazilian-based pharmaceutical company. In conjunction with the sale, we also entered into a five-year manufacturing and supply agreement with UQ, commencing upon completion of the sale. We received $41 million in cash at closing, and an additional $8 million subsequent to the closing in December 2017. Additionally, we recorded a net pre-tax loss of approximately $9 million, inclusive of charges of $5 million recorded during the third quarter of 2017, within Other (income)/deductions—net.
On May 11, 2017, we completed the sale of our manufacturing site in Shenzhou, China. We had previously exited operations at this site during the second quarter of 2015 as part of our operational efficiency program. We received total cash proceeds of approximately $3 million and recorded a net pre-tax gain of approximately $2 million within Other (income)/deductions—net.
Additionally, in the second quarter of 2017, we recorded a $4 million expense within Other (income)/deductions—net related to the prior year sale of the U.S. manufacturing sites noted below.
On April 28, 2016, we completed the sale of our 55 percent ownership share of a Taiwan joint venture, including a manufacturing site in Hsinchu, Taiwan to Yung Shin Pharmaceutical Industrial Co., Ltd., a pharmaceutical company with an animal health business and headquarters in Taiwan, as part of our operational efficiency program. The sale also included a portfolio of products in conjunction with our comprehensive operational efficiency program. These products include medicated feed additives, anti-infective medicines and nutritional premixes for livestock, sold primarily in Taiwan and in international markets. We received $13 million in cash upon closing.
On February 17, 2016, we completed the sale of our manufacturing site in Haridwar, India to the India-based pharmaceutical company Zydus Cadila (Cadila Healthcare Ltd.). The agreement also included the sale of a portfolio of our products in conjunction with our comprehensive operational efficiency program. These products included medicated feed additives, anti-infectives, parasiticides, and nutritionals for livestock, sold primarily in India.
On February 12, 2016, we completed the sale of two of our manufacturing sites in the United States: Laurinburg, North Carolina, and Longmont, Colorado to Huvepharma NV (Huvepharma), a European animal health company. Huvepharma also assumed the assets and operations and the lease of our manufacturing and distribution site in Van Buren, Arkansas. The agreement included the sale of a portfolio of products in conjunction with our comprehensive operational efficiency program. These products included medicated feed additives, water soluble therapeutics and nutritionals for livestock sold in the U.S. and international markets.
During 2016, we received total cash proceeds of approximately $88 million related to the divestitures of our share of our Taiwan joint venture and the India and U.S. manufacturing sites noted above. During the first quarter of 2016, we recognized a net pre-tax gain of approximately $33 million, partially offset by a net pre-tax loss of approximately $6 million recognized during the second quarter of 2016. Gains and losses related to divestitures are recorded within Other (income)/deductions— net.
72 |
The divestiture transactions required transitional supply and service agreements, including technology transfers, where necessary and appropriate, as well as other customary ancillary agreements.
| 5. | Restructuring Charges and Other Costs Associated with Acquisitions and Cost-Reduction/Productivity Initiatives |
In connection with our cost-reduction/productivity initiatives, we typically incur costs and charges associated with site closings and other facility rationalization actions, workforce reductions and the expansion of shared services, including the development of global systems. In connection with our acquisition activity, we typically incur costs and charges associated with executing the transactions, integrating the acquired operations, which may include expenditures for consulting and the integration of systems and processes, product transfers and restructuring the consolidated company, which may include charges related to employees, assets and activities that will not continue in the consolidated company. All operating functions can be impacted by these actions, including sales and marketing, manufacturing and R&D, as well as functions such as business technology, shared services and corporate operations.
During 2015, we launched a comprehensive operational efficiency program, which was incremental to the previously announced supply network strategy. These initiatives have focused on reducing complexity in our product portfolios through the elimination of approximately 5,000 product stock keeping units (SKUs), changing our selling approach in certain markets, reducing our presence in certain countries, and planning to sell or exit 10 manufacturing sites over a long term period. As of December 31, 2017, we divested or exited three U.S. manufacturing sites, four international manufacturing sites, and our 55 percent ownership share of a Taiwan joint venture, inclusive of its related manufacturing site. See Note 4B. Acquisitions and Divestitures: Divestitures for additional information. We are also continuing to optimize our resource allocation and efficiency by reducing resources associated with non-customer facing activities and operating more efficiently as a result of less internal complexity and more standardization of processes. As part of these initiatives, we planned to reduce certain positions through divestitures, normal attrition and involuntary terminations by approximately 2,000 to 2,500, subject to consultations with works councils and unions in certain countries. In 2016, the operations of the Guarulhos, Brazil manufacturing site, including approximately 300 employees, were transferred to us from Pfizer, which increased our range of planned reduction in certain positions to 2,300 to 2,800. Including divestitures, as of December 31, 2017, approximately 2,600 positions have been eliminated. The comprehensive operational efficiency program is substantially complete, however in the fourth quarter of 2017, we expanded the supply network strategy initiative which increases our planned reductions in certain positions by 40. We expect these additional reductions related to our supply network strategy to take place over the next twelve months, and the remainder of the reductions from the initial plan to take place through divestitures over the next several years.
The components of costs incurred in connection with restructuring initiatives, acquisitions and cost-reduction/productivity initiatives are as follows:
| Year Ended December 31, | ||||||||||||
| (MILLIONS OF DOLLARS) | 2017 | 2016 | 2015 | |||||||||
| Restructuring charges/(reversals) and certain acquisition-related costs: | ||||||||||||
Integration costs(a) | $ | 6 | $ | 3 | $ | 10 | ||||||
Transaction costs(b) | — | — | 9 | |||||||||
Restructuring charges/(reversals)(c)(d): | ||||||||||||
| Employee termination costs/(reversals) | 10 | (2 | ) | 262 | ||||||||
| Asset impairment charges | — | — | 39 | |||||||||
| Exit costs | 3 | 4 | — | |||||||||
Total Restructuring charges and certain acquisition-related costs | $ | 19 | $ | 5 | $ | 320 | ||||||
(a) | Integration costs represent external, incremental costs directly related to integrating acquired businesses and primarily include expenditures for consulting and the integration of systems and processes, as well as product transfer costs. |
(b) | Transaction costs represent external costs directly related to acquiring businesses and primarily include expenditures for banking, legal, accounting and other similar services. |
(c) | The restructuring charges for the year ended December 31, 2017, are primarily related to: |
| • | a net increase in employee termination costs of $2 million related to the operational efficiency initiative and supply network strategy; |
| • | employee termination costs of $4 million related to the acquisition of an Irish biologic therapeutics company in the third quarter of 2017, and |
| • | employee termination costs of $4 million in Europe, as a result of initiatives to better align our organizational structure. |
| The restructuring charges/(reversals) for the years ended December 31, 2016 and 2015 primarily relate to our operational efficiency initiative and supply network strategy. |
(d) | The restructuring charges/(reversals) are associated with the following: |
| • | For the year ended December 31, 2017, International of $2 million and Manufacturing/research/corporate of $11 million. |
| • | For the year ended December 31, 2016, U.S. of $1 million, International of a $13 million reversal and Manufacturing/research/corporate of $14 million. |
| • | For the year ended December 31, 2015, U.S. of $31 million, International of $132 million and Manufacturing/research/corporate of $138 million. |
73 |
Charges/(benefits) related to the operational efficiency initiative and supply network strategy are as follows:
| Year Ended December 31, | ||||||||||||
| (MILLIONS OF DOLLARS) | 2017 | 2016 | 2015 | |||||||||
| Restructuring charges/(reversals) and certain acquisition-related costs: | ||||||||||||
| Operational efficiency initiative | ||||||||||||
Employee termination costs(a) | $ | 1 | $ | (8 | ) | $ | 253 | |||||
| Asset impairment | — | — | 38 | |||||||||
| Exit costs | 3 | 5 | — | |||||||||
| 4 | (3 | ) | 291 | |||||||||
| Supply network strategy: | ||||||||||||
| Employee termination costs | 1 | 6 | 9 | |||||||||
| Asset impairment charges | — | — | 1 | |||||||||
| 1 | 6 | 10 | ||||||||||
| Total restructuring charges related to the operational efficiency initiative and supply network strategy | 5 | 3 | 301 | |||||||||
| Other operational efficiency initiative charges: | ||||||||||||
| Cost of sales: | ||||||||||||
| Inventory write-offs | (2 | ) | 5 | 13 | ||||||||
| Selling, general and administrative expenses: | ||||||||||||
| Accelerated depreciation | — | 1 | — | |||||||||
| Consulting fees | 2 | 14 | 40 | |||||||||
| Research and development expenses: | ||||||||||||
| Accelerated depreciation | — | — | 2 | |||||||||
| Other (income)/deductions: | ||||||||||||
Net loss/(gain) on sale of assets(b) | 1 | (26 | ) | — | ||||||||
| Total other operational efficiency initiative charges | 1 | (6 | ) | 55 | ||||||||
| Other supply network strategy charges: | ||||||||||||
| Cost of sales: | ||||||||||||
| Accelerated depreciation | 2 | 6 | 1 | |||||||||
| Consulting fees | 5 | 6 | 16 | |||||||||
| Inventory write-offs | — | 1 | ||||||||||
Other(c) | (2 | ) | — | |||||||||
| Other (income)/deductions—net: | ||||||||||||
Net loss on sale of assets(d) | 9 | — | ||||||||||
| Total other supply network strategy charges | 14 | 13 | 17 | |||||||||
| Total costs associated with the operational efficiency initiative and supply network strategy | $ | 20 | $ | 10 | $ | 373 | ||||||
(a) | For the year ended December 31, 2016, includes a reduction in employee termination accruals primarily as a result of higher than expected voluntary attrition rates experienced in the first half of 2016. |
(b) | For the year ended December 31, 2016, represents the net gain on the sale of certain manufacturing sites and products, partially offset by the loss on the sale of our share of our Taiwan joint venture, as part of our operational efficiency initiative. |
(c) | For the year ended December 31, 2017, represents an adjustment related to discontinuing the depreciation of assets, located at our manufacturing site in Guarulhos, Brazil, that was sold in the fourth quarter of 2017. |
(d) | For the year ended December 31, 2017, represents the net loss related to the sale our manufacturing site in Guarulhos, Brazil. |
74 |
The components of, and changes in, our restructuring accruals are as follows:
| Employee | Asset | |||||||||||||||
| Termination | Impairment | Exit | ||||||||||||||
| (MILLIONS OF DOLLARS) | Costs | Charges | Costs | Accrual | ||||||||||||
| Balance, December 31, 2014 | 18 | — | 1 | 19 | ||||||||||||
| Provision | 262 | 39 | — | 301 | ||||||||||||
Utilization and other(a) | (59 | ) | — | — | (59 | ) | ||||||||||
| Non-cash activity | — | (39 | ) | — | (39 | ) | ||||||||||
| Balance, December 31, 2015 | 221 | — | 1 | 222 | ||||||||||||
| Provision/(benefit) | (2 | ) | — | 4 | 2 | |||||||||||
Utilization and other(a) | (129 | ) | — | (5 | ) | (134 | ) | |||||||||
Balance, December 31, 2016(b) | $ | 90 | $ | — | $ | — | $ | 90 | ||||||||
| Provision | 10 | — | 3 | 13 | ||||||||||||
Utilization and other(a) | (59 | ) | — | (3 | ) | (62 | ) | |||||||||
Balance, December 31, 2017(b) | 41 | — | — | 41 | ||||||||||||
(a) | Includes adjustments for foreign currency translation. |
(b) | At December 31, 2017 and 2016, included in Accrued Expenses ($19 million and $61 million, respectively) and Other noncurrent liabilities ($22 million and $29 million, respectively). |
| 6. | Other (Income)/Deductions—Net |
The components of Other (income)/deductions—net follow:
| Year Ended December 31, | ||||||||||||
| (MILLIONS OF DOLLARS) | 2017 | 2016 | 2015 | |||||||||
Royalty-related income(a) | $ | (12 | ) | $ | (30 | ) | $ | (24 | ) | |||
Identifiable intangible asset impairment charges(b) | — | 1 | 2 | |||||||||
Other asset impairment charges(c) | — | — | 6 | |||||||||
Net loss/(gain) on sale of assets(d) | 11 | (26 | ) | — | ||||||||
Certain legal and other matters, net(e) | (8 | ) | 14 | — | ||||||||
Foreign currency loss(f) | 29 | 49 | 13 | |||||||||
Foreign currency loss related to Venezuela revaluation(g) | — | — | 89 | |||||||||
Other, net(h) | (14 | ) | (10 | ) | (5 | ) | ||||||
| Other (income)/deductions—net | $ | 6 | $ | (2 | ) | $ | 81 | |||||
(a) | Includes an adjustment to our royalty income. |
(b) | In 2016, the intangible asset impairment charge represents an impairment of finite-lived trademarks related to a canine pain management product. In 2015, charges include the impairment of acquired IPR&D assets related to the termination of a canine oncology project. |
(c) | For 2015, represents impairment charges related to assets held by our joint venture in Taiwan, which was subsequently sold in 2016. See Note 4B. Acquisitions and Divestitures. |
(d) | For 2017, primarily represents the net loss related to sales of certain manufacturing sites and products, including our manufacturing site in Guarulhos, Brazil, as part of our operational efficiency initiative and supply network strategy. For 2016, represents the net gain on the sale of certain manufacturing sites and products, partially offset by the loss on the sale of our share of our Taiwan joint venture, as part of our operational efficiency initiative. |
(e) | In July 2014 and December 2016, we reached commercial settlements with several large poultry customers in Mexico associated with specific lots of a Zoetis poultry vaccine. Although there have been no quality or efficacy issues with the manufacturing of this vaccine, certain shipments from several lots in Mexico may have experienced an issue in storage with a third party in Mexico that could have impacted their efficacy. We issued a recall of these lots in July 2014 and the product is currently unavailable in Mexico. For 2017, includes income associated with an insurance recovery related to these commercial settlements, as well as a favorable outcome on a patent infringement settlement. For 2016, represents a charge related to the commercial settlement in Mexico for these products. |
(f) | Primarily driven by costs related to hedging and exposures to certain emerging market currencies. For 2016, also includes losses related to the depreciation of the Egyptian pound in the fourth quarter of 2016. |
(g) | For additional information, see Note 7. Foreign Currency Loss Related to Venezuela Revaluation. |
(h) | Includes interest income and other miscellaneous income. For 2017, primarily includes a settlement refund and reimbursement of legal fees related to costs incurred by Pharmaq prior to the acquisition in 2015 and income associated with certain state business employment tax incentive credits. For 2016, primarily represents income associated with certain state business employment tax incentive credits, as well as interest income. For 2015, includes inventory losses sustained as result of weather damage at storage facilities in Brazil and Australia. |
75 |
| 7. | Foreign Currency Loss Related to Venezuela Revaluation |
On February 13, 2013, the Venezuelan government devalued its currency from a rate of 4.3 to 6.3 Venezuelan bolivars per U.S. dollar. Our Venezuelan subsidiary's functional currency is the U.S. dollar because of the hyperinflationary status of the Venezuelan economy. In the first quarter of 2014, the Venezuelan government expanded its exchange mechanisms, resulting in three official rates of exchange for the Venezuelan bolivar.
Through the fourth quarter of 2015, we used the CENCOEX official rate of 6.3 to report our Venezuela financial position, results of operations and cash flows. In the fourth quarter of 2015, upon evaluation of evolving economic conditions in Venezuela and our expectation of Venezuela's responses to changes in its economy, continued volatility, and the fact that we have not received any approved payments from Venezuela for transactions at the CENCOEX official rate of 6.3 per U.S. dollar in 2015, we determined that our outstanding Venezuelan bolivar-denominated net monetary assets are no longer expected to be settled at the CENCOEX official rate of 6.3, but rather at the SIMADI rate of 199. On November 30, 2015, we recorded a net remeasurement loss of $89 million on bolivar-denominated net monetary assets, primarily related to cash deposits in Venezuela, using the SIMADI rate of 199 bolivars to the U.S. dollar, and this rate will be used prospectively. We believe this best represented the estimate of the U.S. dollar amount that would ultimately be collected. Additionally, the company recorded a lower of cost or market adjustment to inventory of $4 million, and asset impairment charges of $3 million.
Effective March 10, 2016, the Venezuelan government made the following changes to its foreign currency exchange mechanisms: (i) the three-tier exchange rate system existing in the country changed to a dual system with the elimination of the SICAD rate, (ii) the official CENCOEX rate was replaced with DIPRO and was devalued from 6.3 to 10 Venezuelan bolivars per U.S. dollar, and (iii) the SIMADI rate was replaced with DICOM. As of November 30, 2016, the Venezuelan bolivar to U.S. dollar exchange rates were the DIPRO rate of 10 and the DICOM rate of 663. Beginning in the second quarter of 2016, we use the DICOM rate to report our Venezuela financial position, results of operations and cash flows.
| 8. | Tax Matters |
| A. | Taxes on Income |
The income tax provision in the consolidated statements of income includes tax costs and benefits, such as uncertain tax positions, repatriation decisions and audit settlements, among others.
On December 22, 2017, the Tax Cuts and Jobs Act (the Tax Act) was enacted which, among other changes, reduces the U.S. federal corporate tax rate from 35% to 21% effective January 1, 2018. The Tax Act makes broad and complex changes to the U.S. tax code and it will take time to fully analyze the impact of the changes. Based on the information available, and the current interpretation of the Tax Act, the company was able to make a reasonable estimate and recorded a provisional net tax expense related to the one-time mandatory deemed repatriation tax, payable over eight years, partially offset by the remeasurement of the deferred tax assets and liabilities, as of the date of enactment, due to the reduction in the U.S. federal corporate tax rate. Pursuant to the Staff Accounting Bulletin published by the Securities and Exchange Commission on December 22, 2017, addressing the challenges in accounting for the effects of the Tax Act in the period of enactment, companies must report provisional amounts for those specific income tax effects of the Tax Act for which the accounting is incomplete but a reasonable estimate can be determined. Those provisional amounts will be subject to adjustment during a measurement period of up to one year from the enactment date. Pursuant to this guidance, the estimated impact of the Tax Act is based on a preliminary review of the new tax law and projected future financial results and is subject to revision based upon further analysis and interpretation of the Tax Act and to the extent that future results differ from currently available projections.
Our accounting for the following elements of the Tax Act is incomplete. However, we are able to make reasonable estimates and recorded a provisional net tax expense of $212 million related to the following elements of the Tax Act pursuant to the Staff Accounting Bulletin referred to above:
| • | One-Time Mandatory Deemed Repatriation Tax: As a result of the enactment of the Tax Act, the company will be subject to a one-time mandatory deemed repatriation tax on accumulated non-U.S. earnings. Due to the fact that the non-U.S. subsidiaries are on a fiscal year ending November 30, this tax liability will not become a fixed obligation until 2018. The estimated impact of the Tax Act is based on a preliminary review of the new law and projected future financial results and is subject to revision based upon further analysis and interpretation of the Tax Act and to the extent that future results differ from currently available projections. |
| • | Reduction of U.S. Federal Corporate Tax Rate: The Tax Act reduces the corporate tax rate to 21%, effective January 1, 2018. Consequently, we have recorded a decrease related to deferred tax assets and liabilities with a corresponding net adjustment to deferred income tax benefit for the year ended December 31, 2017. Since, as discussed herein, the company has recorded provisional amounts related to certain portions of the Tax Act, any corresponding deferred tax remeasurement is also provisional. |
| • | Valuation Allowances: The company must assess whether its valuation allowance analyses are affected by various aspects of the Tax Act (e.g., one-time mandatory deemed repatriation of deferred foreign income, global intangible low-taxed income inclusions, and new categories of foreign tax credits). Since, as discussed herein, the company has recorded provisional amounts related to certain portions of the Tax Act, any corresponding determination of the need for or change in a valuation allowance is also provisional. |
| • | Global Intangible Low-Taxed Income (GILTI) Policy Election: The GILTI provisions of the Tax Act do not apply to the company until 2019 and we are still evaluating its impact. The FASB allows companies to adopt an accounting policy to either recognize deferred taxes for GILTI or treat such tax cost as a current-period expense when incurred. We have not yet determined our accounting policy because determining the impact of the GILTI provisions requires analysis of our existing legal entity structure, the reversal of our U.S. GAAP and U.S. tax basis differences in the assets and liabilities of our foreign subsidiaries, and our ability to offset any tax with foreign tax credits. As such, we have not made a policy decision regarding whether to record deferred taxes on GILTI or treat such tax cost as a current-period expense. |
On January 11, 2016, the European Commission concluded that the Belgium “excess profits tax scheme” constitutes illegal state aid and ordered the Belgian authorities to recover benefits from taxpayers who are parties to an Excess Profits Ruling. As a result, the 2017 and 2016 effective tax rates
76 |
do not reflect any benefit of the excess profits ruling and does incorporate the Belgium government's recovery of benefits for the periods 2013 through 2015 offset by the remeasurement of the company’s deferred tax assets and liabilities using the rates expected to be in place at the time of the reversal and without consideration of implementation of any future operational changes. The 2015 effective tax rate includes the benefit of the incentive tax rulings in Belgium and Singapore.
The components of Income before provision for taxes on income follow:
| Year Ended December 31, | ||||||||||||
| (MILLIONS OF DOLLARS) | 2017 | 2016 | 2015 | |||||||||
| United States | $ | 897 | $ | 723 | $ | 469 | ||||||
| International | 628 | 505 | 76 | |||||||||
| Income before provision for taxes on income | $ | 1,525 | $ | 1,228 | $ | 545 | ||||||
The components of Provision for taxes on income based on the location of the taxing authorities follow:
| Year Ended December 31, | ||||||||||||
| (MILLIONS OF DOLLARS) | 2017 | 2016 | 2015 | |||||||||
| United States: | ||||||||||||
| Current income taxes: | ||||||||||||
| Federal | $ | 384 | $ | 281 | $ | 221 | ||||||
| State and local | 25 | 3 | 19 | |||||||||
| Deferred income taxes: | ||||||||||||
| Federal | 113 | (38 | ) | (63 | ) | |||||||
| State and local | 2 | 11 | (15 | ) | ||||||||
| Total U.S. tax provision | 524 | 257 | 162 | |||||||||
| International: | ||||||||||||
| Current income taxes | 126 | 179 | 50 | |||||||||
| Deferred income taxes | 13 | (27 | ) | (6 | ) | |||||||
| Total international tax provision | 139 | 152 | 44 | |||||||||
Provision for taxes on income(a)(b)(c) | $ | 663 | $ | 409 | $ | 206 | ||||||
(a) | In 2017, the Provision for taxes on income reflects the following: |
| • | the change in the jurisdictional mix of earnings, which includes the impact of the location of earnings from (i) operations and (ii) restructuring charges related to the operational efficiency initiative and supply network strategy, as well as repatriation costs. The jurisdictional mix of earnings can vary as a result of repatriation decisions and as a result of operating fluctuations in the normal course of business, the impact of non-deductible items and the extent and location of other income and expense items, such as restructuring charges/(benefits), asset impairments and gains and losses on asset divestitures; |
| • | a $212 million net discrete provisional tax expense recorded in the fourth quarter of 2017, related to the impact of the Tax Act enacted on December 22, 2017, including a one-time mandatory deemed repatriation tax, partially offset by a net tax benefit related to the remeasurement of the deferred tax assets and liabilities, as of the date of enactment, due to the reduction in the U.S. federal corporate tax rate; |
| • | U.S. tax benefit related to U.S. Research and Development Tax Credit and the U.S. Domestic Production Activities deduction; |
| • | a $15 million discrete tax benefit recorded in the fourth quarter of 2017 related to the effective settlement of certain issues with U.S. and non-U.S. tax authorities; |
| • | a $9 million discrete tax benefit recorded in 2017 related to the excess tax benefits for share-based payments; |
| • | a $3 million discrete tax benefit recorded in the first quarter of 2017 related to a remeasurement of the company’s deferred tax assets and liabilities using the tax rates expected to be in place going forward; |
| • | tax expense related to the changes in valuation allowances and the resolution of other tax items; and |
| • | tax expense related to changes in uncertain tax positions (see D. Tax Contingencies). |
(b) | In 2016, the Provision for taxes on income reflects the following: |
| • | the change in the jurisdictional mix of earnings, which includes the impact of the location of earnings from (i) operations and (ii) restructuring charges related to the operational efficiency initiative and supply network strategy, as well as repatriation costs. The jurisdictional mix of earnings can vary as a result of repatriation decisions and as a result of operating fluctuations in the normal course of business, the impact of non-deductible items and the extent and location of other income and expense items, such as restructuring charges/(benefits), asset impairments and gains and losses on asset divestitures; |
| • | U.S. tax benefit related to U.S. Research and Development Tax Credit and the U.S. Domestic Production Activities deduction; |
| • | a $15 million discrete tax benefit recorded in the fourth quarter of 2016 related to prior period tax adjustments; |
| • | a $10 million discrete tax benefit recorded in the first quarter of 2016 related to a remeasurement of deferred taxes as a result of a change in statutory tax rates; |
| • | a $7 million discrete tax benefit recorded in 2016 related to the excess tax benefits for share-based payments; |
| • | a $2 million discrete tax benefit related to a remeasurement of the company’s deferred tax assets and liabilities using the tax rates expected to be in place going forward; |
| • | a net tax expense of approximately $35 million mainly recorded in the first half of 2016 related to the impact of the European Commission’s negative decision on the excess profits rulings in Belgium. This net charge represents the recovery of prior tax benefits for the periods 2013 through 2015 offset by the remeasurement of the company’s deferred tax assets and liabilities using the rates expected to be in place at the time of the reversal and without consideration of implementation of any future operational changes, and does not include any benefits associated with a successful appeal of the decision; |
77 |
| • | tax expense related to the changes in valuation allowances and the resolution of other tax items; and |
| • | tax expense related to changes in uncertain tax positions (see D. Tax Contingencies). |
(c) | In 2015, the Provision for taxes on income reflects the following: |
| • | the change in the jurisdictional mix of earnings, which includes the impact of the location of earnings from (i) operations and (ii) restructuring charges related to the operational efficiency initiative and supply network strategy, as well as repatriation costs. The jurisdictional mix of earnings can vary as a result of repatriation decisions and as a result of operating fluctuations in the normal course of business, the impact of non-deductible items and the extent and location of other income and expense items, such as restructuring charges/(benefits), asset impairments and gains and losses on asset divestitures; |
| • | the tax expense related to the non-deductible revaluation of the net monetary assets in Venezuela to the SIMADI exchange rate recorded in the fourth quarter of 2015; |
| • | tax expense related to the changes in valuation allowances and the resolution of other tax items; |
| • | tax expense related to changes in uncertain tax positions (see D. Tax Contingencies); |
| • | a $9 million discrete tax benefit recorded in the first quarter of 2015 related to a remeasurement of deferred taxes as a result of a change in tax rates; |
| • | a $6 million discrete tax benefit recorded in the second quarter of 2015 related to prior period tax adjustments; and |
| • | U.S. tax benefit related to U.S. Research and Development Tax Credit which was permanently extended on December 18, 2015, and the U.S. Domestic Production Activities deduction. |
Tax Rate Reconciliation
The reconciliation of the U.S. statutory income tax rate to our effective tax rate follows:
| Year Ended December 31, | |||||||||
| 2017 | 2016 | 2015 | |||||||
| U.S. statutory income tax rate | 35.0 | % | 35.0 | % | 35.0 | % | |||
| State and local taxes, net of federal benefits | 0.7 | 0.8 | (1.1 | ) | |||||
Taxation of non-U.S. operations(a)(b) | (3.9 | ) | (3.0 | ) | (3.2 | ) | |||
Unrecognized tax benefits and tax settlements and resolution of certain tax positions(c) | 6.0 | 0.4 | 1.8 | ||||||
Impact of the Tax Act(d) | 7.7 | — | — | ||||||
Venezuela revaluation(e) | — | — | 5.6 | ||||||
Annulment of Belgium Excess Profit Ruling(f) | — | 2.9 | — | ||||||
U.S. Research and Development Tax Credit and U.S. Domestic Production Activities deduction(g) | (1.3 | ) | (1.4 | ) | (2.8 | ) | |||
Stock-based compensation(h) | (0.5 | ) | (0.5 | ) | — | ||||
Non-deductible / non-taxable items(i) | 0.5 | 0.2 | 1.3 | ||||||
| All other—net | (0.7 | ) | (1.1 | ) | 1.2 | ||||
| Effective tax rate | 43.5 | % | 33.3 | % | 37.8 | % | |||
(a) | The rate impact of taxation of non-U.S. operations was a decrease to our effective tax rate in 2015 through 2017 due to (i) the jurisdictional mix of earnings as tax rates outside the United States are generally lower than the U.S. statutory income tax rate; and (ii) incentive tax rulings in Belgium and Singapore in 2015. |
(b) | In all years, the impact to the rate due to increases in uncertain tax positions was more than offset by the jurisdictional mix of earnings and other U.S. tax implications of our foreign operations described in the above footnotes. |
(c) | For a discussion about unrecognized tax benefits and tax settlements and resolution of certain tax positions, see A. Taxes on Income and D. Tax Contingencies. |
(d) | The rate impact related to the Tax Act was an increase to our effective tax rate in 2017. This provisional net tax charge represents the amount related to the one-time mandatory deemed repatriation tax on the company’s undistributed non-U.S. earnings, partially offset by a net tax benefit related to the remeasurement of the company’s deferred tax assets and liabilities, as of the date of enactment, due to the reduction in the U.S. federal corporate tax rate. |
(e) | The rate impact related to the non-deductible revaluation of the net monetary assets in Venezuela to the SIMADI exchange rate was an increase to our effective tax rate in 2015. |
(f) | The rate impact related to the European Commission’s negative decision on the excess profits rulings in Belgium was an increase to our effective tax rate in 2016. This net charge represents the recovery of prior tax benefits for the periods 2013 through 2015 offset by the remeasurement of the company’s deferred tax assets and liabilities using the rates expected to be in place at the time of the reversal and without consideration of implementation of any future operational changes, and does not include any benefits associated with a successful appeal of the decision. |
(g) | In all years, the decrease in the rate was due to the benefit associated with the U.S. Research and Development Tax Credit and the U.S. Domestic Production Activities deduction. |
(h) | The rate impact related to the excess tax benefits for share-based payments was a decrease to our effective tax rate in 2017 and 2016. |
(i) | In all years, non-deductible items include meals and entertainment expenses. |
| B. | Tax Matters Agreement |
In connection with the separation from Pfizer in 2013, we entered into a tax matters agreement with Pfizer that governs the parties' respective rights, responsibilities and obligations with respect to tax liabilities and benefits, tax attributes, the preparation and filing of tax returns, the control of audits and other tax proceedings and other matters regarding taxes.
78 |
In general, under the agreement:
| • | Pfizer will be responsible for any U.S. federal, state, local or foreign income taxes and any U.S. state or local non-income taxes (and any related interest, penalties or audit adjustments and including those taxes attributable to our business) reportable on a consolidated, combined or unitary return that includes Pfizer or any of its subsidiaries (and us and/or any of our subsidiaries) for any periods or portions thereof ending on or prior to December 31, 2012. We will be responsible for the portion of any such taxes for periods or portions thereof beginning on or after January 1, 2013, as would be applicable to us if we filed the relevant tax returns on a standalone basis. |
| • | We will be responsible for any U.S. federal, state, local or foreign income taxes and any U.S. state or local non-income taxes (and any related interest, penalties or audit adjustments) that are reportable on returns that include only us and/or any of our subsidiaries, for all tax periods whether before or after the completion of the separation from Pfizer. |
| • | Pfizer will be responsible for certain specified foreign taxes directly resulting from certain aspects of the separation from Pfizer. |
We will not generally be entitled to receive payment from Pfizer in respect of any of our tax attributes or tax benefits or any reduction of taxes of Pfizer. Neither party's obligations under the agreement will be limited in amount or subject to any cap. The agreement also assigns responsibilities for administrative matters, such as the filing of returns, payment of taxes due, retention of records and conduct of audits, examinations or similar proceedings. In addition, the agreement provides for cooperation and information sharing with respect to tax matters.
Pfizer is primarily responsible for preparing and filing any tax return with respect to the Pfizer affiliated group for U.S. federal income tax purposes and with respect to any consolidated, combined, unitary or similar group for U.S. state or local or foreign income tax purposes or U.S. state or local non-income tax purposes that includes Pfizer or any of its subsidiaries, including those that also include us and/or any of our subsidiaries. We are generally responsible for preparing and filing any tax returns that include only us and/or any of our subsidiaries.
The party responsible for preparing and filing a given tax return will generally have exclusive authority to control tax contests related to any such tax return.
| C. | Deferred Taxes |
Deferred taxes arise as a result of basis differentials between financial statement accounting and tax amounts.
The components of our deferred tax assets and liabilities follow:
| As of December 31, | ||||||||
| 2017 | 2016 | |||||||
| (MILLIONS OF DOLLARS) | Assets (Liabilities) | |||||||
| Prepaid/deferred items | $ | 54 | $ | 52 | ||||
| Inventories | 8 | 47 | ||||||
| Intangibles | (170 | ) | (203 | ) | ||||
| Property, plant and equipment | (80 | ) | (101 | ) | ||||
| Employee benefits | 53 | 63 | ||||||
| Restructuring and other charges | 4 | 9 | ||||||
| Legal and product liability reserves | 14 | 18 | ||||||
| Net operating loss/credit carryforwards | 137 | 94 | ||||||
| Unremitted earnings | (148 | ) | — | |||||
| All other | (2 | ) | (2 | ) | ||||
| Subtotal | (130 | ) | (23 | ) | ||||
| Valuation allowance | (170 | ) | (125 | ) | ||||
Net deferred tax liability(a)(b) | $ | (300 | ) | $ | (148 | ) | ||
(a) | The increase in the total net deferred tax liability from December 31, 2016, to December 31, 2017, is primarily attributable to the provisional liability recorded for the one-time mandatory deemed repatriation tax on the company’s undistributed non-U.S. earnings, partially offset by the remeasurement of all U.S. deferred tax assets and liabilities as of December 22, 2017, based on the provisions of the Tax Act. In addition, the increase in the total net deferred tax liability was also attributable to an increase in valuation allowances representing the amounts determined to be unrecoverable, a decrease in deferred tax assets related to inventory, and employee benefits, partially offset by an increase in deferred tax assets related to net operating loss/credit carryforwards and a decrease in deferred tax liabilities related to intangibles, and property, plant and equipment. |
(b) | In 2017, included in Noncurrent deferred tax assets ($80 million) and Noncurrent deferred tax liabilities ($380 million). In 2016, included in Noncurrent deferred tax assets ($96 million) and Noncurrent deferred tax liabilities ($244 million). |
We have carryforwards, primarily related to net operating losses, which are available to reduce future foreign and U.S. state income taxes payable with either an indefinite life or expiring at various times from 2018 to 2037.
Valuation allowances are provided when we believe that our deferred tax assets are not recoverable based on an assessment of estimated future taxable income that incorporates ongoing, prudent and feasible tax planning strategies. On the basis of this evaluation, as of December 31, 2017, and December 31, 2016, a valuation allowance of $170 million and $125 million, respectively, has been recorded to record only the portion of the deferred tax asset that is more likely than not to be realized. The amount of the deferred tax asset considered realizable, however, could be adjusted if estimates of future taxable income during the carryforward period are reduced or increased or if objective negative evidence in the form of cumulative losses is no longer present and additional weight may be given to subjective evidence such as projections for growth.
79 |
In general, it is our practice and intention to permanently reinvest the majority of the earnings of the company’s non U.S. subsidiaries. As of December 31, 2017, the cumulative amount of such undistributed earnings was approximately $4.5 billion, for which we have not provided taxes for U.S. state income, foreign withholding and currency gains and losses. As these earnings are intended to be indefinitely reinvested overseas, as of December 31, 2017, we cannot predict the time or manner of a potential repatriation. As such, other than the deferred tax liability associated with the one-time mandatory deemed repatriation tax on such undistributed earnings imposed by the Tax Act, it is not practicable to estimate the amount of any additional deferred tax liabilities associated with the actual repatriation of the unremitted earnings due to the complexity of its hypothetical calculation.
| D. | Tax Contingencies |
We are subject to income tax in many jurisdictions, and a certain degree of estimation is required in recording the assets and liabilities related to income taxes. All of our tax positions are subject to audit by the local taxing authorities in each tax jurisdiction. These tax audits can involve complex issues, interpretations and judgments and the resolution of matters may span multiple years, particularly if subject to negotiation or litigation. Our assessments are based on estimates and assumptions that have been deemed reasonable by management, but our estimates of unrecognized tax benefits and potential tax benefits may not be representative of actual outcomes, and variation from such estimates could materially affect our financial statements in the period of settlement or when the statute of limitations expire. We treat these events as discrete items in the period of resolution.
For a description of our accounting policies associated with accounting for income tax contingencies, see Note 3. Significant Accounting Policies: Deferred Tax Assets and Liabilities and Income Tax Contingencies. For a description of the risks associated with estimates and assumptions, see Note 3. Significant Accounting Policies: Estimates and Assumptions.
Uncertain Tax Positions
As tax law is complex and often subject to varied interpretations, it is uncertain whether some of our tax positions will be sustained upon audit. As of December 31, 2017, 2016 and 2015, we had approximately $161 million, $65 million and $60 million, respectively, in net liabilities associated with uncertain tax positions, excluding associated interest:
| • | Tax assets associated with uncertain tax positions primarily represent our estimate of the potential tax benefits in one tax jurisdiction that could result from the payment of income taxes in another tax jurisdiction. These potential benefits generally result from cooperative efforts among taxing authorities, as required by tax treaties to minimize double taxation, commonly referred to as the competent authority process. The recoverability of these assets, which we believe to be more likely than not, is dependent upon the actual payment of taxes in one tax jurisdiction and, in some cases, the successful petition for recovery in another tax jurisdiction. As of December 31, 2017, 2016 and 2015, we had approximately $3 million, $3 million and $1 million, respectively, in assets associated with uncertain tax positions recorded in Other noncurrent assets. |
| • | Tax liabilities associated with uncertain tax positions represent unrecognized tax benefits, which arise when the estimated benefit recorded in our financial statements differs from the amounts taken or expected to be taken in a tax return because of the uncertainties described above. These unrecognized tax benefits relate primarily to issues common among multinational corporations. Substantially all of these unrecognized tax benefits, if recognized, would impact our effective income tax rate. |
The reconciliation of the beginning and ending amounts of gross unrecognized tax benefits follows:
| (MILLIONS OF DOLLARS) | 2017 | 2016 | 2015 | |||||||||
| Balance, January 1 | $ | (68 | ) | $ | (61 | ) | $ | (54 | ) | |||
Increases based on tax positions taken during a prior period(a)(b) | (4 | ) | (48 | ) | — | |||||||
Decreases based on tax positions taken during a prior period(a)(c) | 12 | 2 | 6 | |||||||||
Increases based on tax positions taken during the current period(a)(d) | (107 | ) | (9 | ) | (14 | ) | ||||||
Settlements(e) | — | 46 | — | |||||||||
| Lapse in statute of limitations | 3 | 2 | 1 | |||||||||
Balance, December 31(f) | $ | (164 | ) | $ | (68 | ) | $ | (61 | ) | |||
(a) | Primarily included in Provision for taxes on income. |
(b) | In 2017, the increases are primarily related to movements on prior year positions, including movements in foreign translation adjustments on prior year positions. In 2016, the increases are primarily related to the impact of the European Commission’s negative decision on the excess profits rulings in Belgium. See A. Taxes on Income. |
(c) | In 2017, the decreases are primarily related to movements on prior year positions and effective settlement of certain issues with U.S. and non-U.S. tax authorities. In 2016, the decreases are primarily related to movements on prior year positions. In 2015, the decreases are primarily related to movements in foreign translation adjustments on prior year positions and effective settlement of certain issues with U.S. tax authorities and non-U.S. tax authorities. See A. Taxes on Income. |
(d) | In 2017, the increases are primarily related to the impact of the newly enacted Tax Act. See A. Taxes on Income. |
(e) | In 2016, the decreases are due to cash payments related to the impact of the European Commission’s negative decision on the excess profits rulings in Belgium. See A. Taxes on Income. |
(f) | In 2017, included in Noncurrent deferred tax assets ($3 million) and Other taxes payable ($161 million). In 2016, included in Noncurrent deferred tax assets ($3 million) and Other taxes payable ($65 million). In 2015, included in Noncurrent deferred tax assets ($6 million) and Other taxes payable ($55 million). |
• | Interest related to our unrecognized tax benefits is recorded in accordance with the laws of each jurisdiction and is recorded in Provision for taxes on income in our consolidated statements of income. In 2017, we recorded a net interest expense of $1 million; in 2016, we recorded |
80 |
a net interest expense of $2 million; and in 2015, we recorded a net interest expense of $1 million. Gross accrued interest totaled $7 million, $6 million and $4 million as of December 31, 2017, 2016 and 2015, respectively, and were included in Other taxes payable. Gross accrued penalties totaled $4 million, $4 million and $3 million as of December 31, 2017, 2016 and 2015, respectively, and were included in Other taxes payable.
Status of Tax Audits and Potential Impact on Accruals for Uncertain Tax Positions
We are subject to taxation in the United States including various states, and foreign jurisdictions. The United States is one of our major tax jurisdictions, and we are currently under audit for tax years 2013 and 2014. For U.S. Federal and state tax purposes, the tax years 2013 through 2017 are open for examination (see B. Tax Matters Agreement for years prior to 2013).
In addition to the open audit years in the United States, we have open audit years in other major foreign tax jurisdictions, such as Canada (2013-2017), Asia-Pacific (2011-2017 primarily reflecting Australia, China and Japan), Europe (2012-2017, primarily reflecting France, Germany, Italy, Spain and the United Kingdom) and Latin America (2005-2017, primarily reflecting Brazil and Mexico).
Any settlements or statute of limitations expirations could result in a significant decrease in our uncertain tax positions. We do not expect that within the next twelve months any of our gross unrecognized tax benefits, exclusive of interest, could significantly decrease as a result of settlements with taxing authorities or the expiration of the statutes of limitations. Our assessments are based on estimates and assumptions that have been deemed reasonable by management, but our estimates of unrecognized tax benefits and potential tax benefits may not be representative of actual outcomes, and any variation from such estimates could materially affect our financial statements in the period of settlement or when the statutes of limitations expire, as we treat these events as discrete items in the period of resolution. Finalizing audits with the relevant taxing authorities can include formal administrative and legal proceedings, and, as a result, it is difficult to estimate the timing and range of possible change related to our uncertain tax positions, and such changes could be significant.
| 9. | Financial Instruments |
| A. | Debt |
Credit Facilities
In December 2016, we entered into an amended and restated revolving credit agreement with a syndicate of banks providing for a five-year $1.0 billion senior unsecured revolving credit facility (the credit facility). In December 2017, the maturity for the amended and restated revolving credit agreement was extended through December 2022. Subject to certain conditions, we have the right to increase the credit facility to up to $1.5 billion. The credit facility contains a financial covenant requiring us to not exceed a maximum total leverage ratio (the ratio of consolidated net debt as of the end of the period to consolidated Earnings Before Interest, Income Taxes, Depreciation and Amortization (EBITDA) for such period) of 3.50:1. Upon entering into a material acquisition, the maximum total leverage ratio increases to 4.00:1, and extends until the fourth full consecutive fiscal quarter ended immediately following the consummation of a material acquisition. The credit facility also contains a clause which adds back to Adjusted Consolidated EBITDA, any operational efficiency restructuring charge (defined as charges recorded by the company during the period commencing on October 1, 2016 and ending December 31, 2019, related to operational efficiency initiatives), provided that for any twelve-month period such charges added back to Adjusted Consolidated EBITDA shall not exceed $100 million in the aggregate.
The credit facility also contains a financial covenant requiring that we maintain a minimum interest coverage ratio (the ratio of EBITDA at the end of the period to interest expense for such period) of 3.50:1. In addition, the credit facility contains other customary covenants.
We were in compliance with all financial covenants as of December 31, 2017 and December 31, 2016. There were no amounts drawn under the credit facility as of December 31, 2017 or December 31, 2016.
We have additional lines of credit and other credit arrangements with a group of banks and other financial intermediaries for general corporate purposes. We maintain cash and cash equivalent balances in excess of our outstanding short-term borrowings. As of December 31, 2017, we had access to $75 million of lines of credit which expire at various times through 2018 and are generally renewed annually. We did not have any borrowings outstanding related to these facilities as of December 31, 2017.
Commercial Paper Program and Other Short-Term Borrowings
In February 2013, we entered into a commercial paper program with a capacity of up to $1.0 billion. As of December 31, 2017, and 2016, there was no commercial paper outstanding under this program. As of December 31, 2017 and 2016, we did not have any other short-term borrowings outstanding.
Senior Notes and Other Long-Term Debt
On September 12, 2017, we issued $1.25 billion aggregate principal amount of our senior notes (2017 senior notes), with an original issue discount of $7 million. These notes are comprised of $750 million aggregate principal amount of 3.000% senior notes due 2027 and $500 million aggregate principal amount of 3.950% senior notes due 2047. Net proceeds from this offering were partially used in October 2017 to repay, prior to maturity, the aggregate principal amount of $750 million, and a make-whole amount and accrued interest of $4 million, of our 1.875% senior notes due 2018. The remainder of the net proceeds will be used for general corporate purposes.
On November 13, 2015, we issued $1.25 billion aggregate principal amount of our senior notes (2015 senior notes), with an original issue discount of $2 million. On January 28, 2013, we issued $3.65 billion aggregate principal amount of our senior notes (the 2013 senior notes offering) in a private placement, with an original issue discount of $10 million.
The 2013, 2015 and 2017 senior notes are governed by an indenture and supplemental indenture (collectively, the indenture) between us and Deutsche Bank Trust Company Americas, as trustee. The indenture contains certain covenants, including limitations on our, and certain of our
81 |
subsidiaries' ability to incur liens or engage in sale-leaseback transactions. The indenture also contains restrictions on our ability to consolidate, merge or sell substantially all of our assets. In addition, the indenture contains other customary terms, including certain events of default, upon the occurrence of which the 2013, 2015 and 2017 senior notes may be declared immediately due and payable.
Pursuant to the indenture, we are able to redeem the 2013, 2015 and 2017 senior notes, in whole or in part, at any time by paying a "make whole" premium, plus accrued and unpaid interest to, but excluding, the date of redemption. Pursuant to our tax matters agreement with Pfizer, we will not be permitted to redeem the 2013 senior notes due 2023 pursuant to this optional redemption provision, except under limited circumstances. Upon the occurrence of a change of control of Zoetis and a downgrade of the 2013, 2015 and 2017 senior notes below an investment grade rating by each of Moody's Investors Service, Inc. and Standard & Poor's Ratings Services, we are, in certain circumstances, required to make an offer to repurchase all of the outstanding 2013, 2015 and 2017 senior notes at a price equal to 101% of the aggregate principal amount of the 2013, 2015 and 2017 senior notes together with accrued and unpaid interest to, but excluding, the date of repurchase.
The components of our long-term debt are as follows:
| As of December 31, | ||||||||
| (MILLIONS OF DOLLARS) | 2017 | 2016 | ||||||
| 1.875% 2013 senior notes due 2018 | — | 750 | ||||||
| 3.450% 2015 senior notes due 2020 | 500 | 500 | ||||||
| 3.250% 2013 senior notes due 2023 | 1,350 | 1,350 | ||||||
| 4.500% 2015 senior notes due 2025 | 750 | 750 | ||||||
| 3.000% 2017 senior notes due 2027 | 750 | — | ||||||
| 4.700% 2013 senior notes due 2043 | 1,150 | 1,150 | ||||||
| 3.950% 2017 senior notes due 2047 | 500 | — | ||||||
| 5,000 | 4,500 | |||||||
| Unamortized debt discount / debt issuance costs | (47 | ) | (32 | ) | ||||
| Less current portion of long-term debt | — | — | ||||||
| Long-term debt, net of discount and issuance costs | $ | 4,953 | $ | 4,468 | ||||
The fair value of our long-term debt, including the current portion of long-term debt, was $5,291 million and $4,565 million as of December 31, 2017, and December 31, 2016, respectively, and has been determined using a third-party matrix-pricing model that uses significant inputs derived from, or corroborated by, observable market data and Zoetis’ credit rating (Level 2 inputs). See Note 3. Significant Accounting Policies— Fair Value.
The principal amount of long-term debt outstanding as of December 31, 2017, matures in the following years:
| After | ||||||||||||||||||||||||||||
| (MILLIONS OF DOLLARS) | 2018 | 2019 | 2020 | 2021 | 2022 | 2022 | Total | |||||||||||||||||||||
| Maturities | $ | — | $ | — | $ | 500 | $ | — | $ | — | $ | 4,500 | $ | 5,000 | ||||||||||||||
Interest Expense
Interest expense, net of capitalized interest, was $175 million for 2017, $166 million for 2016 and $124 million for 2015. Capitalized interest expense was $4 million for 2017, $3 million for 2016, and $4 million for 2015.
| B. | Derivative Financial Instruments |
Foreign Exchange Risk
A significant portion of our revenue, earnings and net investment in foreign affiliates is exposed to changes in foreign exchange rates. We seek to manage our foreign exchange risk, in part, through operational means, including managing same-currency revenue in relation to same-currency costs and same-currency assets in relation to same-currency liabilities. Depending on market conditions, foreign exchange risk is also managed through the use of derivative financial instruments. These financial instruments serve to protect net income against the impact of the translation into U.S. dollars of certain foreign exchange-denominated transactions. The aggregate notional amount of foreign exchange derivative financial instruments offsetting foreign currency exposures was $1.4 billion and $1.2 billion as of December 31, 2017, and December 31, 2016, respectively. The derivative financial instruments primarily offset exposures in the Australian dollar, Brazilian real, British pound, Canadian dollar, Chinese yuan, euro, and Norwegian krone. The vast majority of the foreign exchange derivative financial instruments mature within 60 days and all mature within 180 days.
All derivative contracts used to manage foreign currency risk are measured at fair value and are reported as assets or liabilities on the consolidated balance sheet. The company has not designated the foreign currency forward-exchange contracts as hedging instruments. We recognize the gains and losses on forward-exchange contracts that are used to offset the same foreign currency assets or liabilities immediately into earnings along with the earnings impact of the items they generally offset. These contracts essentially take the opposite currency position of that reflected in the month-end balance sheet to counterbalance the effect of any currency movement.
Interest Rate Risk
The company may use interest rate swap contracts on certain investing and borrowing transactions to manage its net exposure to interest rates and to reduce its overall cost of borrowing. In anticipation of issuing fixed-rate debt, we may use forward-starting interest rate swaps that are designated as cash flow hedges to hedge against changes in interest rates that could impact expected future issuances of debt. To the extent these hedges of cash
82 |
flows related to anticipated debt are effective, any unrealized gains or losses on the forward-starting interest rate swaps are reported in Accumulated other comprehensive loss and are recognized in income over the life of the future fixed-rate notes. When the company discontinues hedge accounting because it is no longer probable that an anticipated transaction will occur within the originally expected period of execution, or within an additional two-month period thereafter, changes to fair value accumulated in other comprehensive income are recognized immediately in earnings.
During 2017 and 2016, we entered into forward starting interest rate swaps with an aggregate notional value of $500 million. During the third quarter of 2017, we also entered into treasury lock trades with an aggregate notional value of $500 million. We designated these swaps and treasury locks (contracts) as cash flow hedges against interest rate exposure related principally to the anticipated future issuance of fixed-rate debt to be used primarily to refinance our 1.875% 2013 senior note due in 2018.
Upon issuance of our 2017 senior notes (see A. Debt: Senior Notes and Other Long-Term Debt), we terminated these contracts and paid $3 million in cash to the counterparties for settlement. The settlement amount, which represents the fair value of the contracts at the time of termination, was recorded in Accumulated other comprehensive loss, and will be amortized into income over the life of the 2017 senior notes. There was $0.1 million of ineffectiveness related to the forward swaps through the date of settlement which was immediately recognized as a loss within Interest expense—net of capitalized interest. In addition, in previous years we had entered into various forward-starting interest rate swap contracts that were designated as cash flow hedges and that were terminated upon issuance of fixed-rate notes.
Fair Value of Derivative Instruments
The classification and fair values of derivative instruments are as follows:
| Fair Value of Derivatives | ||||||||||
| As of December 31, | ||||||||||
| (MILLIONS OF DOLLARS) | Balance Sheet Location | 2017 | 2016 | |||||||
| Derivatives Not Designated as Hedging Instruments: | ||||||||||
| Foreign currency forward-exchange contracts | Other current assets | $ | 10 | $ | 12 | |||||
| Foreign currency forward-exchange contracts | Other current liabilities | (9 | ) | (8 | ) | |||||
| Total derivatives not designated as hedging instruments | 1 | 4 | ||||||||
| Derivatives Designated as Hedging Instruments: | ||||||||||
| Interest rate swap contracts | Other current assets | — | 17 | |||||||
| Total derivatives designated as hedging instruments | — | 17 | ||||||||
| Total derivatives | $ | 1 | $ | 21 | ||||||
We use a market approach in valuing financial instruments on a recurring basis. Our derivative financial instruments are measured at fair value on a recurring basis using Level 2 inputs in the calculation of fair value. See Note 3. Significant Accounting Policies— Fair Value.
The amounts of net losses on derivative instruments not designated as hedging instruments, recorded in Other (income)/deductions, are as follows:
| Year Ended December 31, | ||||||||
| (MILLIONS OF DOLLARS) | 2017 | 2016 | ||||||
| Foreign currency forward-exchange contracts | $ | (33 | ) | $ | (24 | ) | ||
These amounts were substantially offset in Other (income)/deductions—net by the effect of changing exchange rates on the underlying foreign currency exposures.
The amounts of unrecognized net gains/(losses) on derivative instruments designated as cash flow hedges, recorded, net of tax, in Other comprehensive income/(loss), are as follows:
| Year Ended December 31, | ||||||||
| (MILLIONS OF DOLLARS) | 2017 | 2016 | ||||||
| Interest rate swaps | $ | (11 | ) | $ | 10 | |||
The net amount of deferred gains/(losses) related to derivative instruments designated as cash flow hedges that is expected to be reclassified from Accumulated other comprehensive loss into earnings over the next 12 months is insignificant.
83 |
| 10. | Inventories |
The components of inventory follow:
| As of December 31, | ||||||||
| (MILLIONS OF DOLLARS) | 2017 | 2016 | ||||||
| Finished goods | $ | 788 | $ | 799 | ||||
| Work-in-process | 484 | 499 | ||||||
| Raw materials and supplies | 155 | 204 | ||||||
| Inventories | $ | 1,427 | $ | 1,502 | ||||
11. Property, Plant and Equipment
The components of property, plant and equipment follow:
| Useful Lives | As of December 31, | |||||||||
| (MILLIONS OF DOLLARS) | (Years) | 2017 | 2016 | |||||||
| Land | — | $ | 22 | $ | 22 | |||||
| Buildings | 33 1/3 - 50 | 934 | 923 | |||||||
| Machinery, equipment and fixtures | 3 - 20 | 1,696 | 1,582 | |||||||
| Construction-in-progress | — | 254 | 212 | |||||||
| 2,906 | 2,739 | |||||||||
| Less: Accumulated depreciation | 1,471 | 1,358 | ||||||||
| Property, plant and equipment | $ | 1,435 | $ | 1,381 | ||||||
Depreciation expense was $142 million in 2017, $145 million in 2016 and $135 million in 2015.
| 12. | Goodwill and Other Intangible Assets |
| A. | Goodwill |
The components of, and changes in, the carrying amount of goodwill follow:
| (MILLIONS OF DOLLARS) | U.S. | International | Total | |||||||||
| Balance, December 31, 2015 | $ | 665 | $ | 790 | $ | 1,455 | ||||||
Additions / Adjustments(a) | (4 | ) | 20 | 16 | ||||||||
Other(b) | — | 10 | 10 | |||||||||
| Balance, December 31, 2016 | $ | 661 | $ | 820 | $ | 1,481 | ||||||
Additions / Adjustments (a) | 10 | 7 | 17 | |||||||||
Other(b) | — | 12 | 12 | |||||||||
| Balance, December 31, 2017 | $ | 671 | $ | 839 | $ | 1,510 | ||||||
(a) | For 2017, primarily represents $9 million related to the acquisition of an Irish biologic therapeutics company in the third quarter of 2017, as well as $10 million related to the consolidation of a European livestock monitoring company, a variable interest entity of which Zoetis is the primary beneficiary, in the first quarter of 2017, partially offset by a $2 million reduction to the consolidation of a European livestock monitoring company, in the third quarter of 2017. |
| For 2016, primarily includes a $16 million purchase price allocation associated with the acquisition of a veterinary diagnostics business in Denmark and a $12 million purchase price allocation associated with the acquisition of a livestock business in South America, offset by a $13 million reduction in the acquisition date fair value of goodwill associated with the acquisition of certain assets of Abbott Animal Health. |
(b) | Includes adjustments for foreign currency translation. For 2017, also includes $3 million related to the sale of our manufacturing site in Guarulhos, Brazil. |
The gross goodwill balance was $2,046 million as of December 31, 2017, and $2,017 million as of December 31, 2016. Accumulated goodwill impairment losses (generated entirely in fiscal 2002) was $536 million as of December 31, 2017, and December 31, 2016.
84 |
| B. | Other Intangible Assets |
The components of identifiable intangible assets follow:
| As of December 31, 2017 | As of December 31, 2016 | |||||||||||||||||||||||
| Identifiable | Identifiable | |||||||||||||||||||||||
| Gross | Intangible Assets, | Gross | Intangible Assets, | |||||||||||||||||||||
| Carrying | Accumulated | Less Accumulated | Carrying | Accumulated | Less Accumulated | |||||||||||||||||||
| (MILLIONS OF DOLLARS) | Amount | Amortization | Amortization | Amount | Amortization | Amortization | ||||||||||||||||||
| Finite-lived intangible assets: | ||||||||||||||||||||||||
Developed technology rights(a)(b) | $ | 1,185 | $ | (428 | ) | $ | 757 | $ | 1,064 | $ | (342 | ) | $ | 722 | ||||||||||
| Brands | 213 | (143 | ) | 70 | 213 | (132 | ) | 81 | ||||||||||||||||
| Trademarks and tradenames | 62 | (47 | ) | 15 | 62 | (44 | ) | 18 | ||||||||||||||||
Other(c) | 234 | (143 | ) | 91 | 222 | (130 | ) | 92 | ||||||||||||||||
| Total finite-lived intangible assets | 1,694 | (761 | ) | 933 | 1,561 | (648 | ) | 913 | ||||||||||||||||
| Indefinite-lived intangible assets: | ||||||||||||||||||||||||
| Brands | 37 | — | 37 | 37 | — | 37 | ||||||||||||||||||
| Trademarks and trade names | 67 | — | 67 | 66 | — | 66 | ||||||||||||||||||
In-process research and development(b)(d) | 224 | — | 224 | 204 | — | 204 | ||||||||||||||||||
| Product rights | 8 | — | 8 | 8 | — | 8 | ||||||||||||||||||
| Total indefinite-lived intangible assets | 336 | — | 336 | 315 | — | 315 | ||||||||||||||||||
| Identifiable intangible assets | $ | 2,030 | $ | (761 | ) | $ | 1,269 | $ | 1,876 | $ | (648 | ) | $ | 1,228 | ||||||||||
(a) | Includes the consolidation of a European livestock monitoring company, a variable interest entity of which Zoetis is the primary beneficiary, and intangible assets associated with the purchase of a Norwegian fish vaccination company, both during the first quarter of 2017. |
(b) | In the first quarter of 2017, certain intangible assets, acquired in 2015 as part of the Pharmaq acquisition, were placed into service. In 2016, includes the acquisition of intangible assets associated with the purchase of a veterinary diagnostics business in Denmark in the third quarter of 2016, the acquisition of intangible assets associated with the purchase of a livestock business in South America in the first quarter of 2016 and an increase in the acquisition date fair value of intangible assets associated with the acquisition of certain assets of Abbott Animal Health, as well as the impact of foreign exchange. |
(c) | Includes the acquisition of land use rights in China in the fourth quarter of 2017 and the acquisition of intangible assets associated with the purchase of a veterinary diagnostics business in Denmark in the third quarter of 2016. |
(d) | Includes the intangible assets related to the acquisition of an Irish biologic therapeutics company in the third quarter of 2017. |
Developed Technology Rights
Developed technology rights represent the amortized cost associated with developed technology, which has been acquired from third parties and which can include the right to develop, use, market, sell and/or offer for sale the product, compounds and intellectual property that we have acquired with respect to products, compounds and/or processes that have been completed. These assets include technologies related to the care and treatment of cattle, swine, poultry, sheep, fish, dogs, cats and horses.
Brands
Brands represent the amortized or unamortized cost associated with product name recognition, as the products themselves do not receive patent protection. The more significant finite-lived brands are Excenel, Lutalyse and Spirovac and the more significant indefinite-lived brands are the Linco family products and Mastitis.
Trademarks and Tradenames
Trademarks and tradenames represent the amortized or unamortized cost associated with legal trademarks and tradenames. The more significant components of indefinite-lived trademarks and tradenames are indefinite-lived trademarks and tradenames acquired from SmithKlineBeecham. The more significant finite-lived trademarks and tradenames are finite-lived trademarks and tradenames for vaccines acquired from CSL Animal Health.
In-Process Research and Development
IPR&D assets represent R&D assets that have not yet received regulatory approval in a major market. The majority of these IPR&D assets were acquired in connection with our acquisition of Pharmaq.
IPR&D assets are required to be classified as indefinite-lived assets until the successful completion or abandonment of the associated R&D effort. Accordingly, during the development period after the date of acquisition, these assets will not be amortized until approval is obtained in a major market, typically either the United States or the EU, or in a series of other countries, subject to certain specified conditions and management judgment. At that time, we will determine the useful life of the asset, reclassify the asset out of IPR&D and begin amortization. If the associated R&D effort is abandoned, the related IPR&D assets will be written-off, and we will record an impairment charge.
For IPR&D assets, there can be no certainty that these assets ultimately will yield a successful product.
Product Rights
Product rights represent product registration and application rights that were acquired from Pfizer in 2014.
85 |
| C. | Amortization |
The weighted average life of our total finite-lived intangible assets is approximately 11 years. Total amortization expense for finite-lived intangible assets was $100 million in 2017, $95 million in 2016, and $64 million in 2015.
The annual amortization expense expected for the years 2018 through 2022 is as follows:
| (MILLIONS OF DOLLARS) | 2018 | 2019 | 2020 | 2021 | 2022 | |||||||||||||||
| Amortization expense | $ | 100 | $ | 97 | $ | 94 | $ | 93 | $ | 84 | ||||||||||
| D. | Impairments |
For information about intangible asset impairments, see Note 6. Other (Income)/Deductions––Net.
13. Benefit Plans
Our employees ceased to participate in the Pfizer U.S. qualified defined benefit and U.S. retiree medical plans effective December 31, 2012, and liabilities associated with our employees under these plans were retained by Pfizer. Pfizer continued to credit certain employees' service with Zoetis generally through December 31, 2017 (or termination of employment from Zoetis, if earlier) for certain early retirement benefits with respect to Pfizer's U.S. defined benefit pension and retiree medical plans. In connection with an employee matters agreement between Pfizer and Zoetis, Zoetis is responsible for payment of three-fifths of the total cost of the service credit continuation (approximately $38 million) for these plans and Pfizer is responsible for the remaining two-fifths of the total cost (approximately $25 million). The $25 million capital contribution from Pfizer and corresponding contra-equity account (which is being reduced as the service credit continuation is incurred) is included in Employee benefit plan contribution from Pfizer Inc. in the consolidated statement of equity. The balance in the contra-equity account was approximately $13 million and $15 million as of December 31, 2017 and 2016, respectively. The amount of the service cost continuation payment to be paid by Zoetis to Pfizer was determined and fixed based on an actuarial assessment of the value of the grow-in benefits and will be paid in equal installments over a period of 10 years. Pension and postretirement benefit expense associated with the extended service for certain employees in the U.S. plans totaled approximately $6 million per year in 2017 and 2016.
Pension expense associated with the U.S. and certain significant international locations (inclusive of service cost grow-in benefits discussed above) totaled approximately $15 million in 2017, $14 million in 2016, and $20 million in 2015.
| A. | International Pension Plans |
During 2015, our pension plan in the Philippines was transferred to us from Pfizer. The net pension obligation (approximately $1 million) and the related accumulated other comprehensive loss (which was less than $1 million, net of tax) associated with this plan were recorded.
Information about the dedicated pension plans, including the plans transferred to us as part of the separation from Pfizer, is provided in the tables below.
Obligations and Funded Status––Dedicated Plans
The following table provides an analysis of the changes in the benefit obligations, plan assets and funded status of our dedicated pension plans (including those transferred to us):
86 |
| As of and for the | ||||||||
| Year Ended December 31, | ||||||||
| (MILLIONS OF DOLLARS) | 2017 | 2016 | ||||||
| Change in benefit obligation: | ||||||||
| Projected benefit obligation, beginning | $ | 130 | $ | 127 | ||||
| Service cost | 7 | 9 | ||||||
| Interest cost | 3 | 3 | ||||||
| Plan combinations | (1 | ) | — | |||||
| Changes in actuarial assumptions and other | (6 | ) | 9 | |||||
Settlements and curtailments(a) | (10 | ) | (12 | ) | ||||
| Benefits paid | (5 | ) | (3 | ) | ||||
Net transfer out related to divestitures(b) | — | (4 | ) | |||||
| Adjustments for foreign currency translation | 11 | 1 | ||||||
| Benefit obligation, ending | 129 | 130 | ||||||
| Change in plan assets: | ||||||||
| Fair value of plan assets, beginning | 68 | 72 | ||||||
| Plan combinations | (1 | ) | — | |||||
| Actual return on plan assets | 5 | (1 | ) | |||||
| Company contributions | 6 | 9 | ||||||
Settlements and curtailments(a) | (10 | ) | (6 | ) | ||||
Divestitures(b) | — | (4 | ) | |||||
| Adjustments for foreign currency translation | 5 | 1 | ||||||
| Other––net | (4 | ) | (3 | ) | ||||
| Fair value of plan assets, ending | 69 | 68 | ||||||
Funded status—Projected benefit obligation in excess of plan assets at end of year(c) | $ | (60 | ) | $ | (62 | ) | ||
(a) | For 2017, reflects the conversion of a defined benefit plan in Norway into a defined contribution plan. For 2016, reflects the impact of employee terminations due to our operational efficiency and supply network strategy initiatives. |
(b) | For 2016, reflects the impact of the sale of our share of our Taiwan joint venture. |
(c) | Included in Other noncurrent liabilities. |
Actuarial losses were approximately $22 million ($14 million net of tax) at December 31, 2017, and $31 million ($23 million net of tax) at December 31, 2016. The actuarial gains and losses primarily represent the cumulative difference between the actuarial assumptions and actual return on plan assets, changes in discount rates and changes in other assumptions used in measuring the benefit obligations. These actuarial gains and losses are recognized in Accumulated other comprehensive income/(loss). The actuarial losses will be amortized into net periodic benefit costs over an average period of 12.9 years.
The estimated net actuarial loss that will be amortized from Accumulated other comprehensive loss into 2018 net periodic benefit cost is approximately $1 million.
Information related to the funded status of selected plans follows:
| As of December 31, | ||||||||
| (MILLIONS OF DOLLARS) | 2017 | 2016 | ||||||
| Pension plans with an accumulated benefit obligation in excess of plan assets: | ||||||||
| Fair value of plan assets | $ | 58 | $ | 49 | ||||
| Accumulated benefit obligation | 97 | 88 | ||||||
| Pension plans with a projected benefit obligation in excess of plan assets: | ||||||||
| Fair value of plan assets | 67 | 58 | ||||||
| Projected benefit obligation | 128 | 120 | ||||||
Net Periodic Benefit Costs––Dedicated Plans
The following table provides the net periodic benefit cost associated with dedicated pension plans (including those transferred to us):
| Year Ended December 31, | ||||||||||||
| (MILLIONS OF DOLLARS) | 2017 | 2016 | 2015 | |||||||||
| Service cost | $ | 7 | $ | 9 | $ | 9 | ||||||
| Interest cost | 3 | 3 | 3 | |||||||||
| Expected return on plan assets | (3 | ) | (3 | ) | (3 | ) | ||||||
| Amortization of net (gains) / losses | 1 | 1 | 1 | |||||||||
| Special termination benefits | — | — | 1 | |||||||||
| Settlement and curtailments (gains) / losses | 1 | (2 | ) | 2 | ||||||||
| Net periodic benefit cost | $ | 9 | $ | 8 | $ | 13 | ||||||
87 |
Actuarial Assumptions––Dedicated Plans
The following table provides the weighted average actuarial assumptions for the dedicated pension plans (including those transferred to us):
| As of December 31, | |||||||||
| (PERCENTAGES) | 2017 | 2016 | 2015 | ||||||
| Weighted average assumptions used to determine benefit obligations: | |||||||||
| Discount rate | 2.2 | % | 2.1 | % | 2.6 | % | |||
| Rate of compensation increase | 3.0 | % | 3.1 | % | 3.0 | % | |||
| Weighted average assumptions used to determine net benefit cost for the year ended December 31: | |||||||||
| Discount rate | 2.1 | % | 2.5 | % | 2.8 | % | |||
| Expected return on plan assets | 3.9 | % | 4.1 | % | 4.3 | % | |||
| Rate of compensation increase | 3.2 | % | 2.9 | % | 3.6 | % | |||
The assumptions above are used to develop the benefit obligations at the end of the year and to develop the net periodic benefit cost for the following year. Therefore, the assumptions used to determine the net periodic benefit cost for each year are established at the end of each previous year, while the assumptions used to determine the benefit obligations are established at each year-end. The net periodic benefit cost and the benefit obligations are based on actuarial assumptions that are reviewed on an annual basis. The assumptions are revised based on an annual evaluation of long-term trends, as well as market conditions that may have an impact on the cost of providing retirement benefits.
Actuarial and other assumptions for pension plans can result from a complex series of judgments about future events and uncertainties and can rely on estimates and assumptions. For a description of the risks associated with estimates and assumptions, see Note 3. Significant Accounting Policies—Estimates and Assumptions.
Plan Assets—Dedicated Plans
The components of plan assets follow:
| As of December 31, | ||||||||
| (MILLIONS OF DOLLARS) | 2017 | 2016 | ||||||
| Cash and cash equivalents | $ | 1 | $ | 1 | ||||
| Equity securities: Equity commingled funds | 26 | 22 | ||||||
| Debt securities: Government bonds | 32 | 27 | ||||||
| Other investments | 10 | 18 | ||||||
Total(a) | $ | 69 | $ | 68 | ||||
(a) | Fair values are determined based on valuation inputs categorized as Level 1, 2 or 3 (see Note 3. Significant Accounting Policies—Fair Value). Investment plan assets are valued using Level 1 or Level 2 inputs. |
A single estimate of fair value can result from a complex series of judgments about future events and uncertainties and can rely on estimates and assumptions. For information about the risks associated with estimates and assumptions, see Note 3. Significant Accounting Policies—Estimates and Assumptions.
Specifically, the following methods and assumptions were used to estimate the fair value of our pension assets:
| • | Equity commingled funds––observable market prices. |
| • | Government bonds and other investments––principally observable market prices. |
The long-term target asset allocations and the percentage of the fair value of plans assets for dedicated benefit plans follow:
| As of December 31, | |||||||||
| Target allocation | |||||||||
| percentage | Percentage of Plan Assets | ||||||||
| (PERCENTAGES) | 2017 | 2017 | 2016 | ||||||
| Cash and cash equivalents | 0-10% | 1.0 | % | 0.8 | % | ||||
| Equity securities | 0-60% | 41.9 | % | 35.5 | % | ||||
| Debt securities | 0-100% | 45.9 | % | 39.5 | % | ||||
| Other investments | 0-100% | 11.2 | % | 24.2 | % | ||||
| Total | 100 | % | 100 | % | 100 | % | |||
Zoetis utilizes long-term asset allocation ranges in the management of our plans’ invested assets. Long-term return expectations are developed with input from outside investment consultants based on the company’s investment strategy, which takes into account historical experience, as well as the impact of portfolio diversification, active portfolio management, and the investment consultant’s view of current and future economic and financial market conditions. As market conditions and other factors change, the targets may be adjusted accordingly and actual asset allocations may vary from the target allocations.
The long-term asset allocation ranges reflect the asset class return expectations and tolerance for investment risk within the context of the respective plans’ long-term benefit obligations. These ranges are supported by an analysis that incorporates historical and expected returns by asset class, as well
88 |
as volatilities and correlations across asset classes and our liability profile. This analysis, referred to as an asset-liability analysis, also provides an estimate of expected returns on plan assets, as well as a forecast of potential future asset and liability balances.
The investment consultants review investment performance with Zoetis on a quarterly basis in total, as well as by asset class, relative to one or more benchmarks.
Cash Flows—Dedicated Plans
Our plans are generally funded in amounts that are at least sufficient to meet the minimum requirements set forth in applicable employee benefit laws and local tax and other laws.
We expect to contribute approximately $6 million to our dedicated pension plans in 2018. Benefit payments are expected to be approximately $3 million for 2018, $3 million for 2019, $5 million for 2020, and $4 million for 2021 and $6 million for 2022. Benefit payments are expected to be approximately $32 million in the aggregate for the five years thereafter. These expected benefit payments reflect the future plan benefits subsequent to 2018 projected to be paid from the plans or from the general assets of Zoetis entities under the current actuarial assumptions used for the calculation of the projected benefit obligation and, therefore, actual benefit payments may differ from projected benefit payments.
| B. | Postretirement Plans |
As discussed above, Pfizer continued to credit certain United States employees' service with Zoetis generally through December 31, 2017 (or termination of employment from Zoetis, if earlier), for certain early retirement benefits with respect to Pfizer's U.S. retiree medical plans. Postretirement benefit expense associated with these U.S. retiree medical plans totaled approximately $4 million per year in 2017, 2016 and 2015 (inclusive of service cost grow-in benefits discussed above). The expected benefit payments for each of the next five years is approximately $4 million per year.
Employees in the United States who meet certain eligibility requirements participate in a supplemental (non-qualified) savings plan sponsored by Zoetis. The cost of the supplemental savings plan was $7 million and $4 million in 2017 and 2016, respectively.
| C. | Defined Contribution Plans |
Zoetis has a voluntary defined contribution plan (Zoetis Savings Plan) that allows participation by substantially all U. S. employees. Zoetis matches 100% of employee contributions, up to a maximum of 5% of each employee’s eligible compensation. The Zoetis Savings Plan also includes a profit-sharing feature that provides for an additional contribution ranging between 0 and 8 percent of each employee’s eligible compensation. All eligible employees receive the profit-sharing contribution regardless of the amount they choose to contribute to the Zoetis Savings Plan. The profit-sharing contribution is a discretionary amount provided by Zoetis and is determined on an annual basis. Employees can direct their contributions and the company's matching and profit-sharing contributions into any of the funds offered. These funds provide participants with a cross section of investing options, including the Zoetis stock fund. The matching and profit-sharing contributions are cash funded.
Employees are permitted to diversify all or any portion of their company matching or profit-sharing contribution. Once the contributions have been paid, Zoetis has no further payment obligations. Contribution expense, associated with the U.S. defined contribution plans, totaled approximately $38 million in 2017, $40 million in 2016 and $43 million in 2015.
| 14. | Share-Based Payments |
The Zoetis 2013 Equity and Incentive Plan (Equity Plan) provides long-term incentives to our employees and non-employee directors. The principal types of share-based awards available under the Equity Plan may include, but are not limited to, stock options, restricted stock and restricted stock units (RSUs), deferred stock units (DSUs), performance-vesting restricted stock units (PSUs), and other equity-based or cash-based awards.
Twenty-five million shares of stock were approved and registered with the Securities and Exchange Commission for grants to participants under the Equity Plan. The shares reserved may be used for any type of award under the Equity Plan. At December 31, 2017, the aggregate number of remaining shares available for future grant under the Equity Plan was approximately 13 million shares.
| A. | Share-Based Compensation Expense |
The components of share-based compensation expense follow:
| Year Ended December 31, | ||||||||||||
| (MILLIONS OF DOLLARS) | 2017 | 2016 | 2015 | |||||||||
| Stock options / stock appreciation rights | $ | 10 | $ | 10 | $ | 20 | ||||||
| RSUs / DSUs | 26 | 22 | 21 | |||||||||
| PSUs | 8 | 5 | 2 | |||||||||
Share-based compensation expense—total(a)(b) | $ | 44 | $ | 37 | $ | 43 | ||||||
| Tax benefit for share-based compensation expense | (13 | ) | (10 | ) | (8 | ) | ||||||
| Share-based compensation expense, net of tax | $ | 31 | $ | 27 | $ | 35 | ||||||
(a) | For each of the years ended December 31, 2017, 2016 and 2015, we capitalized approximately $1 million of share-based compensation expense to inventory. |
(b) | Includes additional share-based compensation expense as a result of accelerated vesting of the outstanding stock options and the settlement, on a pro-rata basis, of other equity awards of terminated employees in connection with our operational efficiency initiative and supply network strategy for the years ended December 31, 2016 and 2015, of approximately $1 million and $2 million, respectively, which is included in Restructuring charges/(benefits) and certain acquisition-related costs. (See G. Accelerated Vesting of Outstanding Equity Awards). These amounts were de minimis in 2017. |
89 |
| B. | Stock Options |
Stock options represent the right to purchase shares of our common stock within a specified period of time at a specified price. The exercise price for a stock option will be not less than 100% of the fair market value of the common stock on the date of grant. Stock options granted may include those intended to be “incentive stock options” within the meaning of Section 422 of the U.S. Internal Revenue Code of 1986 (the Code).
Stock options are accounted for using a fair-value-based method at the date of grant in the consolidated statement of income. The values determined through this fair-value-based method generally are amortized on a straight-line basis over the vesting term into Cost of sales, Selling, general and administrative expenses, or Research and development expenses, as appropriate.
Eligible employees may receive Zoetis stock option awards. Zoetis stock option awards generally vest after three years of continuous service from the date of grant and have a contractual term of 10 years.
The fair-value-based method for valuing each Zoetis stock option grant on the grant date uses the Black-Scholes-Merton option-pricing model, which incorporates a number of valuation assumptions noted in the following table, shown at their weighted-average values:
| Year Ended December 31, | |||||||||
| 2017 | 2016 | 2015 | |||||||
Expected dividend yield(a) | 0.76 | % | 0.89 | % | 0.72 | % | |||
Risk-free interest rate(b) | 2.29 | % | 1.57 | % | 1.79 | % | |||
Expected stock price volatility(c) | 23.26 | % | 26.70 | % | 23.92 | % | |||
Expected term(d) (years) | 6.5 | 6.5 | 6.5 | ||||||
(a) | Determined using a constant dividend yield during the expected term of the Zoetis stock option. |
(b) | Determined using the interpolated yield on U.S. Treasury zero-coupon issues. |
(c) | Determined using an equal weighting between historical volatility of the Zoetis stock price and implied volatility. The selection of the blended historical and implied volatility approach was based on our assessment that this calculation of expected volatility is more representative of future stock price trends. |
(d) | Determined using expected exercise and post-vesting termination patterns. |
The following table provides an analysis of stock option activity for the year ended December 31, 2017:
| Weighted-Average | |||||||||||||
| Weighted-Average | Remaining | Aggregate | |||||||||||
| Exercise Price | Contractual Term | Intrinsic Value(a) | |||||||||||
| Shares | Per Share | (Years) | (MILLIONS) | ||||||||||
| Outstanding, December 31, 2016 | 5,370,784 | $ | 33.23 | ||||||||||
| Granted | 718,278 | 55.17 | |||||||||||
| Exercised | (1,151,726 | ) | 30.18 | ||||||||||
| Forfeited | (31,452 | ) | 42.05 | ||||||||||
| Outstanding, December 31, 2017 | 4,905,884 | $ | 37.10 | 6.8 | $ | 171 | |||||||
| Exercisable, December 31, 2017 | 2,742,526 | $ | 28.73 | 5.7 | $ | 119 | |||||||
(a) | Market price of underlying Zoetis common stock less exercise price. |
As of December 31, 2017, there was approximately $8 million of unrecognized compensation costs related to nonvested stock options, which will be recognized over an expected remaining weighted-average period of 0.5 years.
The following table summarizes data related to stock option activity:
| Year Ended/As of December 31, | ||||||||||||
| (MILLIONS OF DOLLARS, EXCEPT PER STOCK OPTION AMOUNTS) | 2017 | 2016 | 2015 | |||||||||
| Weighted-average grant date fair value per stock option | $ | 14.31 | $ | 11.34 | $ | 11.70 | ||||||
| Aggregate intrinsic value on exercise | 32 | 23 | 5 | |||||||||
| Cash received upon exercise | 35 | 36 | 9 | |||||||||
| Tax benefits realized related to exercise | 16 | 15 | 2 | |||||||||
| C. | Restricted Stock Units (RSUs) |
Restricted stock units represent the right to receive a share of our common stock that is subject to a risk of forfeiture until the restrictions lapse at the end of the vesting period subject to the recipient's continued employment. RSUs accrue dividend equivalent units and are paid in shares of our common stock upon vesting (or cash determined by reference to the value of our common stock).
RSUs are accounted for using a fair-value-based method that utilizes the closing price of Zoetis common stock on the date of grant. Zoetis RSUs generally vest after three years of continuous service from the grant date and the values are amortized on a straight-line basis over the vesting term into Cost of sales, Selling, general and administrative expenses, or Research and development expenses, as appropriate.
90 |
The following table provides an analysis of RSU activity for the year ended December 31, 2017:
| Weighted-Average | |||||||
| Grant Date Fair Value | |||||||
| Shares | Per Share | ||||||
| Nonvested, December 31, 2016 | 1,792,988 | $ | 39.86 | ||||
| Granted | 538,604 | 55.18 | |||||
| Vested | (600,177 | ) | 31.94 | ||||
| Reinvested dividend equivalents | 12,310 | 45.18 | |||||
| Forfeited | (82,225 | ) | 45.45 | ||||
| Nonvested, December 31, 2017 | 1,661,500 | $ | 47.45 | ||||
As of December 31, 2017, there was approximately $32 million of unrecognized compensation costs related to nonvested RSUs, which will be recognized over an expected remaining weighted-average period of 1.2 years.
| D. | Deferred Stock Units (DSUs) |
Deferred stock units, which are granted to non-employee compensated Directors, represent the right to receive shares of our common stock at a future date. The DSU awards will be automatically settled and paid in shares within 60 days following the Director’s separation from service on the Board of Directors.
DSUs are accounted for using a fair-value-based method that utilizes the closing price of Zoetis common stock on the date of grant. DSUs vest immediately as of the grant date and the values are expensed at the time of grant into Selling, general and administrative expenses.
For the years ended December 31, 2017 and 2016, there were no DSUs granted. As of December 31, 2017 and 2016, there were 73,359 and 72,838 DSUs outstanding, respectively, including dividend equivalents.
| E. | Performance-Vesting Restricted Stock Units (PSUs) |
Performance-vesting restricted stock units, which are granted to eligible senior management, represent the right to receive a share of our common stock that is subject to a risk of forfeiture until the restrictions lapse, which include continued employment through the end of the vesting period and the attainment of performance goals. PSUs represent the right to receive shares of our common stock in the future (or cash determined by reference to the value of our common stock).
PSUs are accounted for using a Monte Carlo simulation model. The units underlying the PSUs will be earned and vested over a three-year performance period, based upon the total shareholder return of the company in comparison to the total shareholder return of the companies comprising the S&P 500 index at the start of the performance period, excluding companies that during the performance period are acquired or are no longer publicly traded (Relative TSR). The weighted-average fair value was estimated based on volatility assumptions of Zoetis common stock and an average of peer companies, which were 23.1% and 25.5%, respectively, in 2017, and 23.8% and 25.2%, respectively, in 2016. Depending on the company’s Relative TSR performance at the end of the performance period, the recipient may earn between 0% and 200% of the target number of units. Vested units, including dividend equivalent units, are paid in shares of the company’s common stock. PSU values are amortized on a straight-line basis over the vesting term into Cost of sales, Selling, general and administrative expenses, or Research and development expenses, as appropriate.
The following table provides an analysis of PSU activity for the year ended December 31, 2017:
| Weighted-Average | |||||||
| Grant Date Fair Value | |||||||
| Shares | Per Share | ||||||
| Nonvested, December 31, 2016 | 289,889 | $ | 55.29 | ||||
| Granted | 136,964 | 74.28 | |||||
| Vested | (8,434 | ) | 58.15 | ||||
| Reinvested dividend equivalents | 2,812 | 60.26 | |||||
| Forfeited | (12,489 | ) | 58.53 | ||||
| Nonvested, December 31, 2017 | 408,742 | $ | 61.53 | ||||
As of December 31, 2017, there was approximately $11 million of unrecognized compensation costs related to nonvested PSUs, which will be recognized over an expected remaining weighted-average period of 1.2 years.
| F. | Other Equity-Based or Cash-Based Awards. |
Our Compensation Committee is authorized to grant awards in the form of other equity-based awards or other cash-based awards, as deemed to be consistent with the purposes of the Equity Plan.
| G. | Accelerated Vesting of Outstanding Equity Awards |
As a result of our operational efficiency initiative and supply network strategy, the company accelerated the vesting, and in some cases the settlement on a pro-rata basis, of outstanding RSUs of terminated employees, subject, in each case, to the requirements of Section 409A of the U.S. Internal Revenue Code, the terms of the Equity Plan and the applicable award agreements, and any outstanding deferral elections. Generally, unvested stock
91 |
options previously granted to terminated employees accelerated in full, and employees generally have the ability to exercise the stock options for three months after termination. Zoetis employees who held stock options and were retirement eligible as of their termination date generally have the full term of the stock option to exercise. In addition, outstanding PSUs of terminated employees vested on a pro-rata basis will be settled on or after the third anniversary of the grant date, subject to the achievement of performance goals. The unvested portion of RSUs and PSUs were forfeited.
The accelerated vesting of the outstanding stock options and the settlement, on a pro-rata basis, of other equity awards resulted in the recognition of additional stock-based compensation expense of approximately $1 million and $2 million, in the years ended December 31, 2016 and 2015, respectively, which is included in Restructuring charges and certain acquisition-related costs. These amounts were de minimis in 2017.
| 15. | Stockholders' Equity |
Zoetis is authorized to issue 6,000,000,000 shares of common stock and 1,000,000,000 shares of preferred stock.
In November 2014, the company's Board of Directors authorized a $500 million share repurchase program. This program was substantially completed as of December 31, 2016. In December 2016, the company's Board of Directors authorized an additional $1.5 billion share repurchase program. Purchases of Zoetis shares may be made at the discretion of management, depending on market conditions and business needs.
Changes in common shares and treasury stock were as follows:
| (MILLIONS OF SHARES) | Common Shares Issued(a) | Treasury Stock(a) | ||||
| Balance, December 31, 2014 | 501.34 | 0.01 | ||||
Stock-based compensation(b) | 0.47 | 0.06 | ||||
| Defined contribution plan | — | 4.34 | ||||
| Balance, December 31, 2015 | 501.81 | 4.41 | ||||
Stock-based compensation(b) | 0.08 | (1.72 | ) | |||
| Share repurchase program | — | 6.34 | ||||
| Balance, December 31, 2016 | 501.89 | 9.04 | ||||
Stock-based compensation(b) | — | (1.55 | ) | |||
| Share repurchase program | — | 8.28 | ||||
| Balance, December 31, 2017 | 501.89 | 15.76 | ||||
(a) | Shares may not add due to rounding. |
(b) | Includes the issuance of shares of common stock and, beginning in the first quarter of 2016, the reissuance of shares from treasury stock in connection with the vesting of employee share-based awards. Treasury stock also includes the reacquisition of shares associated with the vesting of employee share-based awards to satisfy tax withholding requirements. For additional information regarding share-based compensation, see Note 14. Share-Based Payments. |
Accumulated other comprehensive income/(loss)
Changes, net of tax, in accumulated other comprehensive loss, excluding noncontrolling interest, follow:
| Currency Translation | Accumulated | |||||||||||||||
| Derivatives | Adjustment | Benefit Plans | Other | |||||||||||||
| Net Unrealized | Net Unrealized | Actuarial | Comprehensive | |||||||||||||
| (MILLIONS OF DOLLARS) | (Losses)/Gains | (Losses)/Gains | (Losses)/Gains | (Loss)/Income | ||||||||||||
| Balance, December 31, 2014 | $ | — | $ | (336 | ) | $ | (25 | ) | $ | (361 | ) | |||||
| Other comprehensive (loss)/gain, net of tax | (2 | ) | (268 | ) | 9 | (261 | ) | |||||||||
| Balance, December 31, 2015 | (2 | ) | (604 | ) | (16 | ) | (622 | ) | ||||||||
| Other comprehensive gain/(loss), net of tax | 10 | 19 | (7 | ) | 22 | |||||||||||
Divestiture of noncontrolling interest(a) | — | 2 | — | 2 | ||||||||||||
| Balance, December 31, 2016 | 8 | (583 | ) | (23 | ) | (598 | ) | |||||||||
| Other comprehensive gain/(loss), net of tax | (11 | ) | 96 | 8 | 93 | |||||||||||
| Balance, December 31, 2017 | $ | (3 | ) | $ | (487 | ) | $ | (15 | ) | $ | (505 | ) | ||||
(a) | Reflects the divestiture of our share of our Taiwan joint venture. See Note 4B. Acquisitions and Divestitures: Divestitures. |
(b) | Reflects the acquisition of the remaining noncontrolling interest in a variable interest entity previously consolidated by Zoetis as the primary beneficiary. See Note 4B. Acquisitions and Divestitures: Divestitures. |
92 |
| 16. | Earnings per Share |
The following table presents the calculation of basic and diluted earnings per share:
| Year Ended December 31, | ||||||||||||
| (MILLIONS OF DOLLARS AND SHARES, EXCEPT PER SHARE DATA) | 2017 | 2016 | 2015 | |||||||||
| Numerator | ||||||||||||
| Net income before allocation to noncontrolling interests | $ | 862 | $ | 819 | $ | 339 | ||||||
| Less: net loss attributable to noncontrolling interests | (2 | ) | (2 | ) | — | |||||||
| Net income attributable to Zoetis Inc. | $ | 864 | $ | 821 | $ | 339 | ||||||
| Denominator | ||||||||||||
| Weighted-average common shares outstanding | 489.918 | 495.715 | 499.707 | |||||||||
| Common stock equivalents: stock options, RSUs, DSUs and PSUs | 3.243 | 2.510 | 2.312 | |||||||||
| Weighted-average common and potential dilutive shares outstanding | 493.161 | 498.225 | 502.019 | |||||||||
| Earnings per share attributable to Zoetis Inc. stockholders—basic | $ | 1.76 | $ | 1.66 | $ | 0.68 | ||||||
| Earnings per share attributable to Zoetis Inc. stockholders—diluted | $ | 1.75 | $ | 1.65 | $ | 0.68 | ||||||
The number of stock options outstanding under the company's Equity Plan that were excluded from the computation of diluted earnings per share, as the effect would have been antidilutive, were approximately 1 million shares as of each year ended December 31, 2017, 2016 and 2015.
| 17. | Commitments and Contingencies |
We and certain of our subsidiaries are subject to numerous contingencies arising in the ordinary course of business. For a discussion of our tax contingencies, see Note 8. Tax Matters.
| A. | Legal Proceedings |
Our non-tax contingencies include, among others, the following:
| • | Product liability and other product-related litigation, which can include injury, consumer, off-label promotion, antitrust and breach of contract claims. |
| • | Commercial and other matters, which can include product-pricing claims and environmental claims and proceedings. |
| • | Patent litigation, which typically involves challenges to the coverage and/or validity of our patents or those of third parties on various products or processes. |
| • | Government investigations, which can involve regulation by national, state and local government agencies in the United States and in other countries. |
Certain of these contingencies could result in losses, including damages, fines and/or civil penalties, and/or criminal charges, which could be substantial.
We believe that we have strong defenses in these types of matters, but litigation is inherently unpredictable and excessive verdicts do occur. We do not believe that any of these matters will have a material adverse effect on our financial position. However, we could incur judgments, enter into settlements or revise our expectations regarding the outcome of certain matters, and such developments could have a material adverse effect on our results of operations or cash flows in the period in which the amounts are paid.
We have accrued for losses that are both probable and reasonably estimable. Substantially all of these contingencies are subject to significant uncertainties and, therefore, determining the likelihood of a loss and/or the measurement of any loss can be complex. Consequently, we are unable to estimate the range of reasonably possible loss in excess of amounts accrued. Our assessments are based on estimates and assumptions that have been deemed reasonable by management, but the assessment process relies on estimates and assumptions that may prove to be incomplete or inaccurate, and unanticipated events and circumstances may occur that might cause us to change those estimates and assumptions.
Amounts recorded for legal and environmental contingencies can result from a complex series of judgments about future events and uncertainties and can rely on estimates and assumptions.
The principal matters to which we are a party are discussed below. In determining whether a pending matter is significant for financial reporting and disclosure purposes, we consider both quantitative and qualitative factors in order to assess materiality, such as, among other things, the amount of damages and the nature of any other relief sought in the proceeding, if such damages and other relief are specified; our view of the merits of the claims and of the strength of our defenses; whether the action purports to be a class action and our view of the likelihood that a class will be certified by the court; the jurisdiction in which the proceeding is pending; any experience that we or, to our knowledge, other companies have had in similar proceedings; whether disclosure of the action would be important to a reader of our financial statements, including whether disclosure might change a reader’s judgment about our financial statements in light of all of the information about the company that is available to the reader; the potential impact of the proceeding on our reputation; and the extent of public interest in the matter. In addition, with respect to patent matters, we consider, among other things, the financial significance of the product protected by the patent.
93 |
PregSure®
We have in total approximately 266 claims in Europe and New Zealand seeking damages related to calves claimed to have died of Bovine Neonatal Pancytopenia (BNP) on farms where PregSure BVD, a vaccine against Bovine Virus Diarrhea (BVD), was used. BNP is a rare syndrome that first emerged in cattle in Europe in 2006. While some earlier studies of BNP suggested a potential association between the administration of PregSure and the development of BNP, a more recent study concluded there is no causal connection between BNP and PregSure BVD. The cause of BNP is not known.
In 2010, we voluntarily stopped sales of PregSure BVD in Europe, and recalled the product at wholesalers while investigations into possible causes of BNP continued. In 2011, after incidences of BNP were reported in New Zealand, we voluntarily withdrew the marketing authorization for PregSure throughout the world.
We have settled approximately 168 of these claims for amounts that are not material individually or in the aggregate. Investigations into possible causes of BNP continue and these settlements may not be representative of any future claims resolutions.
Ulianopolis, Brazil
In February 2012, the Municipality of Ulianopolis (State of Para, Brazil) filed a complaint against Fort Dodge Saúde Animal Ltda. (FDSAL), a Zoetis entity, and five other large companies alleging that waste sent to a local waste incineration facility for destruction, but that was not ultimately destroyed as the facility lost its operating permit, caused environmental impacts requiring cleanup.
The Municipality is seeking recovery of cleanup costs purportedly related to FDSAL's share of all waste accumulated at the incineration facility awaiting destruction, and compensatory damages to be allocated among the six defendants. We believe we have strong arguments against the claim, including defense strategies against any claim of joint and several liability.
At the request of the Municipal prosecutor, in April 2012, the lawsuit was suspended for one year. Since that time, the prosecutor has initiated investigations into the Municipality's actions in the matter as well as the efforts undertaken by the six defendants to remove and dispose of their individual waste from the incineration facility. On October 3, 2014, the Municipal prosecutor announced that the investigation remained ongoing and outlined the terms of a proposed Term of Reference (a document that establishes the minimum elements to be addressed in the preparation of an Environmental Impact Assessment), under which the companies would be liable to withdraw the waste and remediate the area. On March 5, 2015, we presented our response to the prosecutor’s proposed Term of Reference, arguing that the proposed terms were overly general in nature and expressing our interest in discussing alternatives to address the matter. The prosecutor agreed to consider our request to engage a technical consultant to conduct an environmental diagnostic of the contaminated area. On May 29, 2015, we, in conjunction with the other defendant companies, submitted a draft cooperation agreement to the prosecutor, which outlined the proposed terms and conditions for the engagement of a technical consultant to conduct the environmental diagnostic. On August 19, 2016, the parties and the prosecutor agreed to engage the services of a third-party consultant to conduct a limited environmental assessment of the site. The site assessment was conducted during June 2017, and a written report summarizing the results of the assessment was provided to the parties and the prosecutor in November 2017. The report noted that waste is still present on the site and that further environmental assessments are needed before a plan to manage that remaining waste can be prepared. We have not received any further communication from the prosecutor in response to the report.
Lascadoil Contamination in Animal Feed
An investigation by the U.S. Food and Drug Administration (FDA) and the Michigan Department of Agriculture is ongoing to determine how lascadoil, oil for industrial use, made its way into the feed supply of certain turkey and hog feed mills in Michigan. The contaminated feed is believed to have caused the deaths of approximately 50,000 turkeys and the contamination (but not death) of at least 20,000 hogs in August 2014. While it remains an open question as to how the lascadoil made its way into the animal feed, the allegations are that lascadoil intended to be sold for reuse as biofuel was inadvertently sold to producers of soy oil, who in turn, unknowingly sold the contaminated soy oil to fat recycling vendors, who then sold the contaminated soy oil to feed mills for use in animal feed. Indeed, related to the FDA investigation, Shur-Green Farms LLC, a producer of soy oil, recalled certain batches of soy oil allegedly contaminated with lascadoil on October 13, 2014.
During the course of its investigation, the FDA identified the process used to manufacture Zoetis’ Avatec® (lasalocid sodium) and Bovatec® (lasalocid sodium) products as one possible source of the lascadoil, since lascadoil contains small amounts of lasalocid, the active ingredient found in both products. Zoetis has historically sold any and all industrial lascadoil byproduct to an environmental company specializing in waste disposal. The environmental company is contractually obligated to incinerate the lascadoil or resell it for use in biofuel. Under the terms of the agreement, the environmental company is expressly prohibited from reselling the lascadoil to be used as a component in food. The FDA inspected the Zoetis site where Avatec and Bovatec are manufactured, and found no evidence that Zoetis was involved in the contamination of the animal feed.
On March 10, 2015, plaintiffs Restaurant Recycling, LLC (Restaurant Recycling) and Superior Feed Ingredients, LLC (Superior), both of whom are in the fat recycling business, filed a complaint in the Seventeenth Circuit Court for the State of Michigan against Shur-Green Farms alleging negligence and breach of warranty claims arising from their purchase of soy oil allegedly contaminated with lascadoil. Plaintiffs resold the allegedly contaminated soy oil to turkey feed mills for use in feed ingredient. Plaintiffs also named Zoetis as a defendant in the complaint alleging that Zoetis failed to properly manufacture its products and breached an implied warranty that the soy oil was fit for use at turkey and hog mills. Zoetis was served with the complaint on June 3, 2015, and we filed our answer, denying all allegations, on July 15, 2015. On August 10, 2015, several of the turkey feed mills filed a joint complaint against Restaurant Recycling, Superior, Shur-Green Farms and others, alleging claims for negligence, misrepresentation, and breach of warranty, arising out of their alleged purchase and use of the contaminated soy oil. The complaint raises only one count against Zoetis for negligence. We filed an answer to the complaint on November 2, 2015, denying the allegation. On May 16, 2016, two additional turkey producers filed a complaint in the Seventeenth Circuit Court for the State of Michigan against the company, Restaurant Recycling, Superior, Shur-Green Farms and others, alleging claims for negligence and breach of warranties. We filed an answer to the complaint on June 20, 2016, denying the allegations. The Court has consolidated all three cases in Michigan for purposes of discovery and disposition. On July 28, 2017, we filed a motion for summary disposition on the grounds that no genuine issues of material fact exist and that Zoetis is entitled to judgment as a matter of law. On October 19, 2017, the Court granted our motion and dismissed all claims against Zoetis. On October 31, 2017, the plaintiffs filed
94 |
motions for reconsideration of the Court’s decision granting summary disposition. The Court denied all such motions on December 6, 2017, for the same reasons cited in the Court’s original decision. On December 27, 2017, the plaintiffs filed a request with the Michigan Court of Appeals seeking an interlocutory appeal of the lower Court’s decision, which we opposed on January 17, 2018. The matter is stayed pending a decision by the Court of Appeals.
Other Matters
The European Commission published a decision on alleged competition law infringements by several human health pharmaceutical companies on June 19, 2013. One of the involved legal entities is Alpharma LLC (previously having the name Zoetis Products LLC). Alpharma LLC’s involvement is solely related to its human health activities prior to Pfizer's acquisition of King/Alpharma. Zoetis paid a fine in the amount of Euro 11 million (approximately $14 million) and was reimbursed in full by Pfizer in accordance with the Global Separation Agreement between Pfizer and Zoetis, which provides that Pfizer is obligated to indemnify Zoetis for any liabilities arising out of claims not related to its animal health assets. We filed an appeal of the decision on September 6, 2013 to the General Court of the European Union. On September 8, 2016, the General Court upheld the decision of the European Commission. On November 25, 2016, we filed an appeal to the Court of Justice of the European Union and are awaiting a ruling.
| B. | Guarantees and Indemnifications |
In the ordinary course of business and in connection with the sale of assets and businesses, we indemnify our counterparties against certain liabilities that may arise in connection with the transaction or related to activities prior to the transaction. These indemnifications typically pertain to environmental, tax, employee and/or product-related matters and patent-infringement claims. If the indemnified party were to make a successful claim pursuant to the terms of the indemnification, we would be required to reimburse the loss. These indemnifications are generally subject to threshold amounts, specified claim periods and other restrictions and limitations. Historically, we have not paid significant amounts under these provisions and, as of December 31, 2017, recorded amounts for the estimated fair value of these indemnifications were not significant.
C. Purchase Commitments
As of December 31, 2017, we have agreements totaling $442 million to purchase goods and services that are enforceable and legally binding and include amounts relating to contract manufacturing, information technology services and potential milestone payments deemed reasonably likely to occur.
D. Commitments under Operating Leases
We have facilities, vehicles and office equipment under various non-cancellable operating leases with third parties. Total rent expense, net of sublease rental income, was approximately $27 million in 2017, $25 million in 2016 and $26 million in 2015.
Future minimum lease payments under non-cancellable operating leases as of December 31, 2017, follow:
| After | ||||||||||||||||||||||||||||
| (MILLIONS OF DOLLARS) | 2018 | 2019 | 2020 | 2021 | 2022 | 2022 | Total | |||||||||||||||||||||
| Maturities | $ | 35 | $ | 28 | $ | 21 | $ | 17 | $ | 14 | $ | 53 | $ | 168 | ||||||||||||||
| 18. | Segment, Geographic and Other Revenue Information |
| A. | Segment Information |
We manage our operations through two geographic regions. Each operating segment has responsibility for its commercial activities. Within each of these operating segments, we offer a diversified product portfolio, including vaccines, parasiticides, anti-infectives, medicated feed additives and other pharmaceuticals, for both livestock and companion animal customers.
Operating Segments
Our operating segments are the United States and International. Our chief operating decision maker uses the revenue and earnings of the two operating segments, among other factors, for performance evaluation and resource allocation.
Other Costs and Business Activities
Certain costs are not allocated to our operating segment results, such as costs associated with the following:
| • | Other business activities, includes our CSS contract manufacturing results, as well as expenses associated with our dedicated veterinary medicine research and development organization, research alliances, U.S. regulatory affairs and other operations focused on the development of our products. Other R&D-related costs associated with non-U.S. market and regulatory activities are generally included in the international commercial segment. |
| • | Corporate, which is responsible for platform functions such as business technology, facilities, legal, finance, human resources, business development, and communications, among others. These costs also include compensation costs and other miscellaneous operating expenses not charged to our operating segments, as well as interest income and expense. |
| • | Certain transactions and events such as (i) Purchase accounting adjustments, where we incur expenses associated with the amortization of fair value adjustments to inventory, intangible assets and property, plant and equipment; (ii) Acquisition-related activities, where we incur costs associated with acquiring and integrating newly acquired businesses, such as transaction costs and integration costs ; and (iii) Certain significant items, which comprise substantive, unusual items that, either as a result of their nature or size, would not be expected to occur as part of our normal business on a regular basis, such as certain costs related to becoming an independent public company, restructuring |
95 |
charges and implementation costs associated with our cost-reduction/productivity initiatives that are not associated with an acquisition, certain asset impairment charges, certain legal and commercial settlements and the impact of divestiture-related gains and losses.
| • | Other unallocated includes (i) certain overhead expenses associated with our global manufacturing operations not charged to our operating segments; (ii) certain costs associated with business technology and finance that specifically support our global manufacturing operations; (iii) certain supply chain and global logistics costs; and (iv) procurement costs. |
Segment Assets
We manage our assets on a total company basis, not by operating segment. Therefore, our chief operating decision maker does not regularly review any asset information by operating segment and, accordingly, we do not report asset information by operating segment. Total assets were approximately $8.6 billion and $7.6 billion at December 31, 2017, and 2016, respectively.
Selected Statement of Income Information
| Earnings | Depreciation and Amortization(a) | |||||||||||||||||||||||
| Year Ended December 31, | Year Ended December 31, | |||||||||||||||||||||||
| (MILLIONS OF DOLLARS) | 2017 | 2016 | 2015 | 2017 | 2016 | 2015 | ||||||||||||||||||
| U.S. | ||||||||||||||||||||||||
| Revenue | $ | 2,620 | $ | 2,447 | $ | 2,328 | ||||||||||||||||||
| Cost of Sales | 565 | 551 | 551 | |||||||||||||||||||||
| Gross Profit | 2,055 | 1,896 | 1,777 | |||||||||||||||||||||
| Gross Margin | 78.4 | % | 77.5 | % | 76.3 | % | ||||||||||||||||||
| Operating Expenses | 421 | 388 | 389 | |||||||||||||||||||||
| Other (income)/deductions | (3 | ) | — | (2 | ) | |||||||||||||||||||
| U.S. Earnings | 1,637 | 1,508 | 1,390 | $ | 29 | $ | 27 | $ | 24 | |||||||||||||||
| International | ||||||||||||||||||||||||
Revenue(b) | 2,643 | 2,390 | 2,386 | |||||||||||||||||||||
| Cost of Sales | 889 | 833 | 873 | |||||||||||||||||||||
| Gross Profit | 1,754 | 1,557 | 1,513 | |||||||||||||||||||||
| Gross Margin | 66.4 | % | 65.1 | % | 63.4 | % | ||||||||||||||||||
| Operating Expenses | 515 | 501 | 570 | |||||||||||||||||||||
| Other (income)/deductions | (1 | ) | 2 | 2 | ||||||||||||||||||||
| International Earnings | 1,240 | 1,054 | 941 | 44 | 44 | 46 | ||||||||||||||||||
| Total operating segments | 2,877 | 2,562 | 2,331 | 73 | 71 | 70 | ||||||||||||||||||
| Other business activities | (313 | ) | (309 | ) | (293 | ) | 23 | 25 | 26 | |||||||||||||||
| Reconciling Items: | ||||||||||||||||||||||||
| Corporate | (625 | ) | (684 | ) | (606 | ) | 52 | 45 | 40 | |||||||||||||||
| Purchase accounting adjustments | (88 | ) | (99 | ) | (57 | ) | 88 | 84 | 53 | |||||||||||||||
| Acquisition-related costs | (10 | ) | (4 | ) | (21 | ) | — | — | — | |||||||||||||||
Certain significant items(c) | (25 | ) | (57 | ) | (592 | ) | — | 7 | 6 | |||||||||||||||
| Other unallocated | (291 | ) | (181 | ) | (217 | ) | 6 | 8 | 4 | |||||||||||||||
Total Earnings(d) | $ | 1,525 | $ | 1,228 | $ | 545 | $ | 242 | $ | 240 | $ | 199 | ||||||||||||
(a) | Certain production facilities are shared. Depreciation and amortization is allocated to the reportable operating segments based on estimates of where the benefits of the related assets are realized. |
(b) | Revenue denominated in euros was $660 million in 2017, $632 million in 2016, and $593 million in 2015. |
(c) | For 2017, certain significant items primarily includes: (i) charges related to our operational efficiency initiative and supply network strategy of $20 million; (ii) Zoetis stand-up costs of $3 million; (iii) employee termination costs in Europe of $4 million; (iv) income related to an insurance recovery from commercial settlements in Mexico recorded in 2014 and 2016 of $5 million; and (v) charges of $3 million associated with changes to our operating model. Stand-up costs include certain nonrecurring costs related to becoming an independent public company, such as new branding (including changes to the manufacturing process for required new packaging), the creation of standalone systems and infrastructure, site separation, and certain legal registration and patent assignment costs. |
| For 2016, certain significant items primarily includes: (i) Zoetis stand-up costs of $23 million; (ii) charges related to our operational efficiency initiative and supply network strategy of $10 million; (iii) charges related to a commercial settlement in Mexico of $14 million; (iv) charges of $10 million associated with changes to our operating model; (v) a reversal of $1 million related to other restructuring charges/(reversals) and cost-reduction/productivity initiatives; and (vi) an impairment of finite-lived trademarks of $1 million related to a canine pain management product. |
96 |
| For 2015, certain significant items primarily includes: (i) Zoetis stand-up costs of $118 million; (ii) charges related to our operational efficiency initiative and supply network strategy of $373 million, (iii) charges of $93 million of foreign currency losses related to the Venezuela revaluation; (iv) impairment charges of $3 million related to assets held by our joint venture in Taiwan, classified as assets held for sale in 2015 and subsequently sold in 2016, and an impairment of IPR&D assets related to the termination of a canine oncology project of $2 million; and (v) charges due to unusual investor-related activities of $3 million. |
(d) | Defined as income before provision for taxes on income. |
| B. | Geographic Information |
Revenue exceeded $100 million in eight countries outside the United States in 2017, 2016, and 2015. The United States was the only country to contribute more than 10% of total revenue in each year.
Property, plant and equipment, less accumulated depreciation, by geographic region follow:
| As of December 31, | ||||||||
| (MILLIONS OF DOLLARS) | 2017 | 2016 | ||||||
| U.S. | $ | 1,047 | $ | 985 | ||||
| International | 388 | 396 | ||||||
| Property, plant and equipment, less accumulated depreciation | $ | 1,435 | $ | 1,381 | ||||
| C. | Other Revenue Information |
Significant Customers
We sell our livestock products primarily to veterinarians and livestock producers as well as third-party veterinary distributors, and retail outlets who generally sell the products to livestock producers. We sell our companion animal products primarily to veterinarians who then sell the products to pet owners. Sales to our largest customer, a U.S. veterinary distributor, represented approximately 14%, 13% and 14% of total revenue for 2017, 2016, and 2015, respectively.
Revenue by Species
Species revenue are as follows:
| Year Ended December 31, | ||||||||||||
| (MILLIONS OF DOLLARS) | 2017 | 2016 | 2015 | |||||||||
| Livestock: | ||||||||||||
| Cattle | $ | 1,735 | $ | 1,653 | $ | 1,680 | ||||||
| Swine | 621 | 602 | 668 | |||||||||
| Poultry | 479 | 457 | 525 | |||||||||
| Fish | 118 | 90 | 5 | |||||||||
| Other | 84 | 79 | 80 | |||||||||
| 3,037 | 2,881 | 2,958 | ||||||||||
| Companion Animal: | ||||||||||||
| Horses | 151 | 150 | 162 | |||||||||
| Dogs and Cats | 2,075 | 1,806 | 1,594 | |||||||||
| 2,226 | 1,956 | 1,756 | ||||||||||
| Contract Manufacturing | 44 | 51 | 51 | |||||||||
| Total revenue | $ | 5,307 | $ | 4,888 | $ | 4,765 | ||||||
97 |
Revenue by Major Product Category
Revenue by major product category are as follows:
| Year Ended December 31, | ||||||||||||
| (MILLIONS OF DOLLARS) | 2017 | 2016 | 2015 | |||||||||
| Anti-infectives | $ | 1,253 | $ | 1,255 | $ | 1,305 | ||||||
| Vaccines | 1,373 | 1,245 | 1,149 | |||||||||
| Parasiticides | 763 | 659 | 651 | |||||||||
| Medicated feed additives | 475 | 500 | 505 | |||||||||
| Other pharmaceuticals | 1,181 | 988 | 910 | |||||||||
| Other non-pharmaceuticals | 218 | 190 | 194 | |||||||||
| Contract manufacturing | 44 | 51 | 51 | |||||||||
| Total revenue | $ | 5,307 | $ | 4,888 | $ | 4,765 | ||||||
19. Selected Quarterly Financial Data (Unaudited)
| (MILLIONS OF DOLLARS, EXCEPT PER COMMON SHARE DATA) | FIRST | SECOND | THIRD | FOURTH | ||||||||||||
| 2017 | ||||||||||||||||
| Revenue | $ | 1,231 | $ | 1,269 | $ | 1,347 | $ | 1,460 | ||||||||
Costs and expenses(a) | 895 | 924 | 926 | 1,018 | ||||||||||||
| Restructuring charges/(reversals) and certain acquisition-related costs | (1 | ) | — | 8 | 12 | |||||||||||
| Income before provision for taxes on income | 337 | 345 | 413 | 430 | ||||||||||||
Provision for taxes on income(b) | 98 | 98 | 117 | 350 | ||||||||||||
| Net income before allocation to noncontrolling interests | 239 | 247 | 296 | 80 | ||||||||||||
| Net income/(loss) attributable to noncontrolling interests | 1 | — | (2 | ) | (1 | ) | ||||||||||
| Net income attributable to Zoetis | $ | 238 | $ | 247 | $ | 298 | $ | 81 | ||||||||
| Earnings per common share--basic | $ | 0.48 | $ | 0.50 | $ | 0.61 | $ | 0.17 | ||||||||
| Earnings per common share--diluted | $ | 0.48 | $ | 0.50 | $ | 0.61 | $ | 0.16 | ||||||||
| 2016 | ||||||||||||||||
| Revenue | $ | 1,162 | $ | 1,208 | $ | 1,241 | $ | 1,277 | ||||||||
Costs and expenses(a) | 828 | 897 | 904 | 1,026 | ||||||||||||
| Restructuring charges and certain acquisition-related costs | 2 | (21 | ) | 4 | 20 | |||||||||||
| Income before provision for taxes on income | 332 | 332 | 333 | 231 | ||||||||||||
| Provision for taxes on income | 128 | 108 | 96 | 77 | ||||||||||||
| Net income before allocation to noncontrolling interests | 204 | 224 | 237 | 154 | ||||||||||||
| Net loss attributable to noncontrolling interests | — | — | (2 | ) | — | |||||||||||
| Net income attributable to Zoetis | $ | 204 | $ | 224 | $ | 239 | $ | 154 | ||||||||
| Earnings per common share--basic | $ | 0.41 | $ | 0.45 | $ | 0.48 | $ | 0.31 | ||||||||
| Earnings per common share--diluted | $ | 0.41 | $ | 0.45 | $ | 0.48 | $ | 0.31 | ||||||||
(a) | Costs and expenses in the fourth quarter reflect seasonal trends. |
(b) | For the fourth quarter of 2017, includes a provisional net tax charge of approximately $212 million related to the impact of the Tax Act enacted on December 22, 2017. See Note 8. Tax Matters. |
Basic and diluted EPS are computed independently for each of the periods presented. Accordingly, the sum of the quarterly EPS amounts may not agree to the total for the year.
98 |
Zoetis Inc. and Subsidiaries
Schedule II—Valuation and Qualifying Accounts
| Balance, | Balance, | |||||||||||||||
| Beginning of | End of | |||||||||||||||
| (MILLIONS OF DOLLARS) | Period | Additions | Deductions | Period | ||||||||||||
| Year Ended December 31, 2017 | ||||||||||||||||
| Allowance for doubtful accounts | 30 | 3 | (8 | ) | 25 | |||||||||||
| Year Ended December 31, 2016 | ||||||||||||||||
| Allowance for doubtful accounts | $ | 34 | $ | 7 | $ | (11 | ) | $ | 30 | |||||||
| Year Ended December 31, 2015 | ||||||||||||||||
| Allowance for doubtful accounts | 32 | 9 | (7 | ) | 34 | |||||||||||
99 |
Item 9. Changes in and Disagreements With Accountants on Accounting and Financial Disclosure.
None.
| Item 9A. | Controls and Procedures |
Disclosure Controls and Procedures
An evaluation was carried out under the supervision and with the participation of the company's management, including our Chief Executive Officer and Chief Financial Officer, of the effectiveness of the design and operation of our disclosure controls and procedures (as defined in Rules 13a-15(e) and 15d-15(e) under the Securities Exchange Act of 1934). Based upon that evaluation as of December 31, 2017, the company's Chief Executive Officer and Chief Financial Officer concluded that the company's disclosure controls and procedures are effective at a reasonable level of assurance in alerting them in a timely manner to material information required to be disclosed in our periodic reports filed with the SEC.
Management's Report on Internal Control over Financial Reporting
Management is responsible for establishing and maintaining adequate internal control over financial reporting, as such term is defined under Rule 13a-15(f) and 15d-15(f) of the Securities Exchange Act of 1934. Under the supervision and with the participation of management, including the company's Chief Executive Officer and Chief Financial Officer, we conducted an evaluation of the effectiveness of our internal control over financial reporting based on the framework in Internal Control - Integrated Framework (2013) issued by the Committee of Sponsoring Organizations of the Treadway Commission. Based on our evaluation under the framework in Internal Control - Integrated Framework, our management concluded that our internal control over financial reporting was effective as of December 31, 2017. The effectiveness of our internal control over financial reporting as of December 31, 2017, has been audited by KPMG LLP, an independent registered public accounting firm, as stated in its report included herein.
Changes in Internal Controls
During our most recent fiscal quarter, there has not been any change in our internal control over financial reporting (as such term is defined in Rules 13a-15(f) and 15d-15(f) under the Securities Exchange Act of 1934) that has materially affected, or is reasonably likely to materially affect, our internal control over financial reporting.
Item 9B. Other Information.
None.
100 |
PART III
Item 10. Directors, Executive Officers and Corporate Governance.
Information about our directors is incorporated by reference from the discussion under the heading Item 1-Election of Directors in our 2018 Proxy Statement. Information about compliance with Section 16(a) of the Exchange Act is incorporated by reference from the discussion under the heading Ownership of Our Common Stock in our 2018 Proxy Statement. Information about the Zoetis Code of Conduct governing our employees, including our Chief Executive Officer, Chief Financial Officer Principal Accounting Officer and Controller, and the Code of Business Conduct and Ethics for members of our Board of Directors, is incorporated by reference from the discussions under the headings Corporate Governance at Zoetis in our 2018 Proxy Statement. Information regarding the procedures by which our stockholders may recommend nominees to our Board of Directors is incorporated by reference from the discussion under the heading Corporate Governance at Zoetis in our 2018 Proxy Statement. Information about our Audit Committee, including the members of the Committee, and our Audit Committee financial experts, is incorporated by reference from the discussion under the heading Corporate Governance at Zoetis in our 2018 Proxy Statement.
Executive Officers
Juan Ramón Alaix
Age 66
Chief Executive Officer and Director
Mr. Alaix has served as our Chief Executive Officer and Director since July 2012. From 2006 to 2012, he served as President of Pfizer Animal Health, and was responsible for its overall strategic direction and financial performance. Under his leadership, the company grew to become a $4.3 billion enterprise in 2012. Mr. Alaix has more than 35 years’ experience in finance and management, including 20 years in the pharmaceutical industry. He joined Pfizer in 2003 and held various positions, including Regional President of Central/Southern Europe for Pfizer’s pharmaceutical business. Prior to that, Mr. Alaix held various positions with Pharmacia, including as Country President of Spain, from 1998 until Pharmacia’s acquisition by Pfizer in 2003. Earlier in his career he served in general management with Rhône-Poulenc Rorer in Spain and Belgium. In 2013, Mr. Alaix completed a two-year term as President of the International Federation for Animal Health (“IFAH”), now known as HealthforAnimals, and he continues to serve as a member of its board and executive committee. HealthforAnimals represents manufacturers of veterinary medicines, vaccines and other animal health products in both developed and emerging markets. A native of Spain, Mr. Alaix received a graduate degree in economics from the Universidad de Madrid.
Glenn David
Age 46
Executive Vice President and Chief Financial Officer
Mr. David has served as our Executive Vice President and Chief Financial Officer since August 2016. With more than 20 years of experience in finance and operations, Mr. David has played a key role in leading the financial operations for Zoetis since its initial public offering in 2013. He served as our Senior Vice President of Finance Operations from 2013 to 2016 and as acting Chief Financial Officer from April 2014 through August 2014. Mr. David joined Pfizer in 1999 and held various financial roles, including Vice President of Global Finance for Pfizer Animal Health and Vice President of Finance for the U.S. Primary Care franchise prior to the Zoetis initial public offering.
Alejandro Bernal
Age 45
Executive Vice President and Group President, Strategy, Commercial and Business Development
Mr. Bernal has served as our Executive Vice President and Group President, Strategy, Commercial and Business Development since May 2015. From October 2012 through April 2015, he served as our Executive Vice President and Area President of the Europe, Africa and Middle East region. He was Area President of the Europe, Africa and Middle East region for Pfizer’s animal health business unit from 2010 to 2012. Mr. Bernal joined Pfizer in 2000 and held positions of increasing responsibility, including Area President of the Canada and Latin America region; Regional Director of Southwest and Central Latin America; Division Director for Central America and Colombia; Swine and Poultry Team Leader for Mexico; and Swine Product Manager for Northern Latin America for Pfizer Animal Health. Mr. Bernal has resigned from the company effective February 28, 2018.
Heidi C. Chen
Age 51
Executive Vice President, General Counsel and Corporate Secretary
Ms. Chen has served as our Executive Vice President and General Counsel since October 2012, and as our Corporate Secretary since July 2012. Prior to that, Ms. Chen was Vice President and Chief Counsel of Pfizer Animal Health from 2009 to 2012. Ms. Chen joined Pfizer in 1998 and held various legal and compliance positions of increasing responsibility, including lead counsel for Pfizer’s Established Products (generics) business.
Andrew Fenton
Age 54
Executive Vice President and Chief Information Officer
Mr. Fenton has served as our Executive Vice President and Chief Information Officer (CIO) since August 2016, having joined Zoetis as Senior Vice President and CIO in 2014. From November 2013 to September 2014, Andrew was a partner and principal with EY in the Life Sciences practice
101 |
supporting CIO Transformation Services, and from October 2005 to November 2013, he was Senior Vice President and CIO of Warner Chilcott. He has also held senior positions at IBM Global Services and at PricewaterhouseCoopers in the Pharmaceutical Practice.
Catherine A. Knupp
Age 57
Executive Vice President and President of Research and Development
Dr. Knupp has served as our Executive Vice President and President of Research and Development since October 2012. From 2005 to 2012, she served as Vice President of Pfizer’s Veterinary Medicine Research and Development business unit. Dr. Knupp joined Pfizer in July 2001 and held various positions, including Vice President of Pfizer’s Michigan laboratories for Pharmacokinetics, Dynamics and Metabolism.
Roxanne Lagano
Age 53
Executive Vice President and Chief Human Resources Officer
Ms. Lagano has served as our Executive Vice President and Chief Human Resources Officer since November 2012. Prior to joining Zoetis, Ms. Lagano was senior vice president, Global Compensation, Benefits and Wellness for Pfizer. Ms. Lagano joined Pfizer in 1997 and held various positions, including Senior Vice President, Pfizer Global Compensation, Benefits and Wellness; and Senior Director, Business Transactions, Pfizer Worldwide Human Resources.
Clinton A. Lewis, Jr.
Age 51
Executive Vice President and President of International Operations
Mr. Lewis has served as our Executive Vice President and President, International Operations since May 2015. From October 2012 through April 2015, he served as our Executive Vice President and President of U.S. Operations, and from 2007 to 2012, he was President of U.S. Operations for Pfizer Animal Health. Mr. Lewis joined Pfizer in 1988 and held various positions across sales, marketing and general management, including Senior Vice President of Sales, U.S.; General Manager, Pfizer Caribbean; and General Manager, U.S. Anti-Infectives.
Kristin C. Peck
Age 46
Executive Vice President and President of U.S. Operations
Ms. Peck has served as our Executive Vice President and President, U.S. Operations since May 2015. From October 2012 through April 2015, she served as our Executive Vice President and Group President. Ms. Peck joined Pfizer in 2004 and held various positions, including Executive Vice President, Worldwide Business Development and Innovation; Senior Vice President, Worldwide Business Development, Strategy and Innovation; Vice President, Strategic Planning; Chief of Staff to the Vice Chairman; and Senior Director, Strategic Planning. Ms. Peck also served as a member of Pfizer’s Executive Leadership Team.
Roman Trawicki
Age 54
Executive Vice President and President of Global Manufacturing and Supply
Mr. Trawicki has served as our Executive Vice President, Global Manufacturing and Supply since May 2015. He joined Zoetis in January 2015 as President, Global Manufacturing and Supply. From 2009 to 2014, he was GE Healthcare’s General Manager of Global Supply Chain for Medical Diagnostics, where he focused on diagnostics, injectable contrast media and nuclear medicines.
Item 11. Executive Compensation.
Information about director compensation is incorporated by reference from the discussion under the heading Corporate Governance at Zoetis in our 2018 Proxy Statement. Information about executive compensation is incorporated by reference from the discussion under the heading Executive Compensation in our 2018 Proxy Statement.
Item 12. Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters.
Information required by this item is incorporated by reference from the discussion under the heading Ownership of Our Common Stock in our 2018 Proxy Statement.
Item 13. Certain Relationships and Related Transactions, and Director Independence.
Information about certain relationships and transactions with related parties is incorporated by reference from the discussion under the heading Transactions with Related Persons in our 2018 Proxy Statement. Information about director independence is incorporated by reference from the discussion under the heading Corporate Governance at Zoetis-Corporate Governance Principles and Practices-Director Independence in our 2018 Proxy Statement.
102 |
Item 14. Principal Accounting Fees and Services.
Information about the fees for professional services rendered by our independent registered public accounting firm in 2017 and 2016 is incorporated by reference from the discussion under the heading Item 3—Ratification of Appointment of KPMG as our Independent Registered Public Accounting Firm for 2018 in our 2018 Proxy Statement. Our Audit Committee’s policy on pre-approval of audit and permissible non-audit services of our independent registered public accounting firm is incorporated by reference from the discussion under the heading Item 3—Ratification of Appointment of KPMG as our Independent Registered Public Accounting Firm for 2018 in our 2018 Proxy Statement.
See Notes to Consolidated Financial Statements, which are an integral part of these statements.
103 |
PART IV
Item 15. Exhibits, Financial Statement Schedules.
The following entire exhibits are included:
| A. | (1) The financial statements and notes to financial statements are filed as part of this report in Item 8. Financial Statements and Supplementary Data. |
(2) The financial statement schedule is listed in the Index to Financial Statements.
(3) The exhibits are listed in the Index to Exhibits.
Item 16. Form 10-K Summary.
None.
104 |
EXHIBITS
The exhibits listed below and designated with a † are filed with this report. The exhibits listed below and not so designated are incorporated by reference to the documents following the descriptions of the exhibits.
| Share Purchase Agreement, dated as of November 2, 2015, by and among SalarLux Parent S.à.r.l., Salar Invest AS | ||
| and Zoetis Inc. (incorporated by reference to Exhibit 2.1 to Zoetis Inc.'s Current Report on Form 8-K filed on | ||
| November 2, 2015 (File No. 001-35797)) | ||
| Restated Certificate of Incorporation of the Registrant (incorporated by reference to Exhibit 3.1 to Zoetis Inc.'s Quarterly | ||
| Report on Form 10-Q filed on November 10, 2014 (File No. 001-35797)) | ||
| By-laws of the Registrant, amended and restated as of February 19, 2016 (incorporated by reference to Exhibit 3.2 to Zoetis | ||
| Inc.’s 2015 Annual Report on Form 10-K filed on February 24, 2016 (File No. 001-35797)) | ||
| Specimen Class A Common Stock Certificate (incorporated by reference to Exhibit 4.1 of Zoetis Inc.’s registration | ||
| statement on Form S-1 (File No. 333-183254)) | ||
| Indenture, dated as of January 28, 2013, between Zoetis Inc. and Deutsche Bank Trust Company Americas, as trustee | ||
| (incorporated by reference to Zoetis Inc.'s registration statement on Form S-1 (File No. 333-183254)) | ||
| First Supplemental Indenture, dated as of January 28, 2013, between Zoetis Inc. and Deutsche Bank Trust Company | ||
| Americas, as trustee (incorporated by reference to Exhibit 4.3 of Zoetis Inc.'s registration statement on Form S-1 | ||
| (File No. 333-183254)) | ||
| Second Supplemental Indenture, dated November 13, 2015, between Zoetis Inc. and Deutsche Bank Trust Company | ||
| Americas, as trustee (incorporated by reference to Exhibit 4.2 to Zoetis Inc.’s Current Report on Form 8-K filed on | ||
| November 13, 2015 (File No. 001-35797)) | ||
| Third Supplemental Indenture, dated September 12, 2017, between Zoetis Inc. and Deutsche Bank Trust Company Americas, | ||
| as trustee (incorporated by reference to Exhibit 4.2 to Zoetis Inc.’s Current Report on Form 8-K filed on September 12, 2017 | ||
| (File No. 001-35797)) | ||
| Form of 3.450% Senior Notes due 2020 (incorporated by reference to Exhibit 4.2 to Zoetis Inc.’s Current Report on | ||
| Form 8-K filed on November 13, 2015 (File No. 001-35797)) | ||
| Form of 3.250% Senior Notes due 2023 (incorporated by reference to Exhibit 4.3 of Zoetis Inc.'s registration statement on | ||
| Form S-1 (File No. 333-183254)) | ||
| Form of 4.500% Senior Notes due 2025 (incorporated by reference to Exhibit 4.2 to Zoetis Inc.’s Current Report on | ||
| Form 8-K filed on November 13, 2015 (File No. 001-35797)) | ||
| Form of 4.700% Senior Notes due 2043 (incorporated by reference to Exhibit 4.3 of Zoetis Inc.'s registration statement on | ||
| Form S-1 (File No. 333-183254)) | ||
| Form of 3.000% Senior Notes due 2027 (incorporated by reference to Exhibit 4.2 to Zoetis Inc.’s Current Report on Form 8-K | ||
| filed on September 12, 2017 (File No. 001-35797)) | ||
| Form of 3.950% Senior Notes due 2027 (incorporated by reference to Exhibit 4.2 to Zoetis Inc.’s Current Report on Form 8-K | ||
| filed on September 12, 2017 (File No. 001-35797)) | ||
| Global Separation Agreement, dated February 6, 2013, by and between Zoetis Inc. and Pfizer Inc. (incorporated by reference to | ||
| Exhibit 10.1 to Zoetis Inc.’s 2012 Annual Report on Form 10-K filed on March 28, 2013 (File No. 001-35797)) | ||
| Transitional Services Agreement, dated February 6, 2013, by and between Zoetis Inc. and Pfizer Inc. (incorporated by reference | ||
| to Exhibit 10.2 to Zoetis Inc.’s 2012 Annual Report on Form 10-K filed on March 28, 2013 (File No. 001-35797)) | ||
| Tax Matters Agreement, dated February 6, 2013, by and between Zoetis Inc. and Pfizer Inc. (incorporated by reference to | ||
| Exhibit 10.3 to Zoetis Inc.’s 2012 Annual Report on Form 10-K filed on March 28, 2013 (File No. 001-35797)) | ||
| Research and Development Collaboration and License Agreement, dated February 6, 2013, by and between Zoetis Inc. | ||
| and Pfizer Inc. (incorporated by reference to Exhibit 10.4 to Zoetis Inc.’s 2012 Annual Report on Form 10-K filed on | ||
| March 28, 2013 (File No. 001-35797)) | ||
| Employee Matters Agreement (incorporated by reference to Exhibit 10.5 of Zoetis Inc.'s registration statement on Form S-1 | ||
| (File No. 333-183254)) | ||
| Pfizer Inc. 2004 Stock Plan, as Amended and Restated (incorporated by reference to Exhibit 10.6 of Zoetis Inc.'s registration | ||
| statement on Form S-1 (File No. 333-183254))* | ||
105 |
| Pfizer Inc. Amended and Restated Nonfunded Supplemental Retirement Plan, together with all material Amendments | ||
| (incorporated by reference to Exhibit 10.7 of Zoetis Inc.'s registration statement on Form S-1 (File No. 333-183254))* | ||
| Patent and Know-How License Agreement (Zoetis as licensor), dated February 6, 2013, by and between Zoetis Inc. and | ||
| Pfizer Inc. (incorporated by reference to Exhibit 10.8 to Zoetis Inc.’s 2012 Annual Report on Form 10-K filed on | ||
| March 28, 2013 (File No. 001-35797)) | ||
| Patent and Know-How License Agreement (Pfizer as licensor), dated February 6, 2013, by and between Zoetis Inc. and | ||
| Pfizer Inc. (incorporated by reference to Exhibit 10.9 to Zoetis Inc.’s 2012 Annual Report on Form 10-K filed on | ||
| March 28, 2013 (File No. 001-35797)) | ||
| Trademark and Copyright License Agreement, dated February 6, 2013, by and between Zoetis Inc. and Pfizer Inc. | ||
| (incorporated by reference to Exhibit 10.10 to Zoetis Inc.’s 2012 Annual Report on Form 10-K filed on March 28, 2013 | ||
| (File No. 001-35797)) | ||
| Environmental Matters Agreement, dated February 6, 2013, by and between Zoetis Inc. and Pfizer Inc. (incorporated by | ||
| reference to Exhibit 10.13 to Zoetis Inc.’s 2012 Annual Report on Form 10-K filed on March 28, 2013) (File No. 001-35797)) | ||
| Master Manufacturing and Supply Agreement, dated October 1, 2012, by and between Pfizer Inc. and Zoetis Inc. (Pfizer as | ||
| manufacturer) (incorporated by reference to Exhibit 10.14 of Zoetis Inc.'s registration statement on Form S-1 | ||
| (File No. 333-183254)) | ||
| Registration Rights Agreement, dated February 6, 2013, by and between Zoetis Inc. and Pfizer Inc. (incorporated by reference to | ||
| Exhibit 10.15 to Zoetis Inc.’s 2012 Annual Report on Form 10-K filed on March 28, 2013 (File No. 001-35797)) | ||
| Zoetis Inc. 2013 Equity and Incentive Plan (incorporated by reference to Exhibit 10.16 to Zoetis Inc.’s 2012 Annual Report | ||
| on Form 10-K filed on March 28, 2013 (File No. 001-35797))* | ||
| Sale of Business Severance Plan (incorporated by reference to Exhibit 10.17 to Zoetis Inc.’s 2012 Annual Report on | ||
| Form 10-K filed on March 28, 2013 (File No. 001-35797))* | ||
| Revolving Credit Agreement, dated as of December 21, 2016, among Zoetis Inc., the lenders party thereto and JPMorgan | ||
| Chase Bank, N.A., as administrative agent (incorporated by reference to Exhibit 10.1 of Zoetis Inc.'s Current Report | ||
| on Form 8-K filed on December 21, 2016 (File No. 001-35797)) | ||
| Extension Agreement to Revolving Credit Agreement, dated as of December 21, 2017, among Zoetis Inc., the lenders party | ||
| thereto and JPMorgan Chase Bank, N.A., as administrative agent † | ||
| Form of Indemnification Agreement for directors and officers (incorporated by reference to Exhibit 10.19 of Zoetis Inc.'s | ||
| registration statement on Form S-1 (File No. 333-183254)) | ||
| Registration Rights Agreement, dated as of January 28, 2013, by and among Zoetis Inc. and Merrill Lynch, Pierce, Fenner & | ||
| Smith Incorporated, Barclays Capital Inc., J.P. Morgan Securities LLC and Deutsche Bank Securities Inc., as representatives | ||
| of the several initial purchasers (incorporated by reference to Exhibit 10.20 of Zoetis Inc.'s registration statement on Form | ||
| S-1 (File No. 333-183254)) | ||
| Form of Restricted Stock Unit Award agreement (incorporated by reference to Exhibit 10.21 to Zoetis Inc.’s 2012 Annual | ||
| Report on Form 10-K filed on March 28, 2013 (File No. 001-35797))* | ||
| Form of Stock Option Award agreement (incorporated by reference to Exhibit 10.22 to Zoetis Inc.’s 2012 Annual Report | ||
| on Form 10-K filed on March 28, 2013 (File No. 001-35797))* | ||
| Form of Non-Employee Director Deferred Stock Unit Award agreement (incorporated by reference to Exhibit 10.23 | ||
| on Form 10-K filed on March 28, 2013 (File No. 001-35797))* | ||
| Form of Cash Award agreement (incorporated by reference to Exhibit 10.24 to Zoetis Inc.’s 2012 Annual Report on | ||
| Form 10-K filed on March 28, 2013 (File No. 001-35797))* | ||
| Form of Performance Restricted Stock Unit Award Agreement, effective as of February 27, 2015 (incorporated by | ||
| reference to Exhibit 99.1 to Zoetis Inc.’s Current Report on Form 8-K filed on March 4, 2015 (File No. 001-35797))* | ||
| Form of Restricted Stock Unit Award Agreement, effective as of February 27, 2015 (incorporated by reference to | ||
| Exhibit 99.2 to Zoetis Inc.’s Current Report on Form 8-K filed on March 4, 2015 (File No. 001-35797))* | ||
| Form of Stock Option Award Agreement, effective as of February 27, 2015 (incorporated by reference to Exhibit 99.3 | ||
| to Zoetis Inc.’s Current Report on Form 8-K filed on March 4, 2015 (File No. 001-35797))* | ||
| Form of Cash Award Agreement, effective as of February 27, 2015 (incorporated by reference to Exhibit 99.4 to | ||
106 |
| Zoetis Inc.’s Current Report on Form 8-K filed on March 4, 2015 (File No. 001-35797))* | ||
| Non-Employee Director Deferred Compensation Plan (incorporated by reference to Exhibit 10.1 to Zoetis Inc.’s | ||
| Current Report on Form 8-K filed on May 7, 2013 (File No. 001-35797))* | ||
| Zoetis Executive Severance Plan (incorporated by reference to Exhibit 10.1 to Zoetis Inc.’s Quarterly Report on Form 10-Q | ||
| filed on August 14, 2013 (File No. 001-35797))* | ||
| Zoetis Supplemental Savings Plan, as amended and restated, effective September 15, 2014 (incorporated by reference to | ||
| Exhibit 10.4 to Zoetis Inc.'s Quarterly Report on Form 10-Q filed on November 10, 2014 (File No. 001-35797))* | ||
| Zoetis Equity Deferral Plan, effective November 1, 2014 (incorporated by reference to Exhibit 10.5 to Zoetis Inc.’s | ||
| Quarterly Report on Form 10-Q filed on November 10, 2014 (File No. 001-35797))* | ||
| Letter Agreement, dated as of February 3, 2015, by and among Zoetis and Pershing Square Capital Management, L.P. | ||
| and certain affiliates thereof and Sachem Head Capital Management LP and certain affiliates thereof (incorporated by | ||
| reference to Exhibit 99.1 to Zoetis Inc.’s Current Report on Form 8-K filed on February 4, 2015 (File No. 001-35797)) | ||
| Computation of Ratio of Earnings to Fixed Charges † | ||
| Subsidiaries of the Registrant † | ||
| Consent of KPMG LLP † | ||
| Power of Attorney (included as part of signature page) † | ||
| Certification by the Chief Executive Officer Pursuant to Section 302 of the Sarbanes-Oxley Act of 2002 † | ||
| Certification by the Chief Financial Officer Pursuant to Section 302 of the Sarbanes-Oxley Act of 2002 † | ||
| Certification by the Chief Executive Officer Pursuant to 18 U.S.C. Section 1350, as Adopted Pursuant to Section 906 of the | ||
| Sarbanes-Oxley Act of 2002 † | ||
| Certification by the Chief Financial Officer Pursuant to 18 U.S.C. Section 1350, as Adopted Pursuant to Section 906 of the | ||
| Sarbanes-Oxley Act of 2002 † | ||
| EX-101.INS | INSTANCE DOCUMENT | |
| EX-101.SCH | SCHEMA DOCUMENT | |
| EX-101.CAL | CALCULATION LINKBASE DOCUMENT | |
| EX-101.LAB | LABELS LINKBASE DOCUMENT | |
| EX-101.PRE | PRESENTATION LINKBASE DOCUMENT | |
| EX-101.DEF | DEFINITION LINKBASE DOCUMENT | |
| † | Filed herewith |
| * | Management contracts or compensatory plans or arrangements |
107 |
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
Dated: February 15, 2018
| Zoetis Inc. | |
| By: | /S/ JUAN RAMÓN ALAIX |
| Juan Ramón Alaix | |
| Chief Executive Officer and Director | |
We, the undersigned directors and officers of Zoetis Inc., hereby severally constitute Juan Ramón Alaix and Heidi Chen, and each of them singly, our true and lawful attorneys with full power to them and each of them to sign for us, in our names in the capacities indicated below, any and all amendments to this Annual Report on Form 10-K filed with the Securities and Exchange Commission.
Under the requirements of the Securities Exchange Act of 1934, this report was signed by the following persons on behalf of the Registrant and in the capacities and on the date indicated.
| Name | Title | Date | ||
| /S/ JUAN RAMÓN ALAIX | Chief Executive Officer and Director | February 15, 2018 | ||
| Juan Ramón Alaix | (Principal Executive Officer) | |||
| /S/ GLENN DAVID | Executive Vice President and | February 15, 2018 | ||
| Glenn David | Chief Financial Officer (Principal Financial Officer and Principal Accounting Officer) | |||
| /S/ MICHAEL B. MCCALLISTER | Chairman and Director | February 15, 2018 | ||
| Michael B. McCallister | ||||
| /S/ PAUL M. BISARO | Director | February 15, 2018 | ||
| Paul M. Bisaro | ||||
| /S/ FRANK A. D'AMELIO | Director | February 15, 2018 | ||
| Frank A. D’Amelio | ||||
| /S/ SANJAY KHOSLA | Director | February 15, 2018 | ||
| Sanjay Khosla | ||||
| /s/ GREGORY NORDEN | Director | February 15, 2018 | ||
| Gregory Norden | ||||
| /S/ LOUISE M. PARENT | Director | February 15, 2018 | ||
| Louise M. Parent | ||||
| /S/ WILLIE M. REED | Director | February 15, 2018 | ||
| Willie M. Reed | ||||
| /S/ LINDA RHODES | Director | February 15, 2018 | ||
| Linda Rhodes | ||||
| /s/ ROBERT W. SCULLY | Director | February 15, 2018 | ||
| Robert W. Scully | ||||
| /S/ WILLIAM C. STEERE, JR. | Director | February 15, 2018 | ||
| William C. Steere, Jr. | ||||
108 |