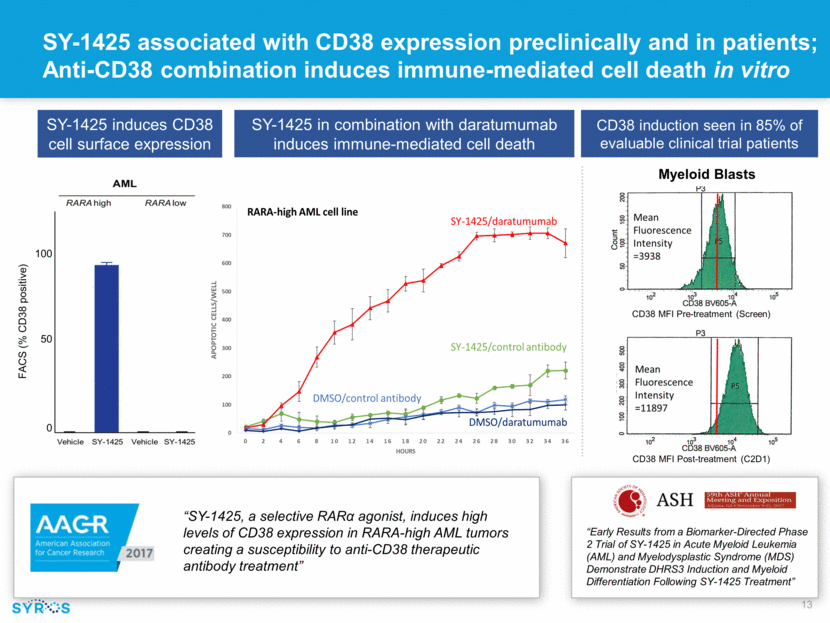
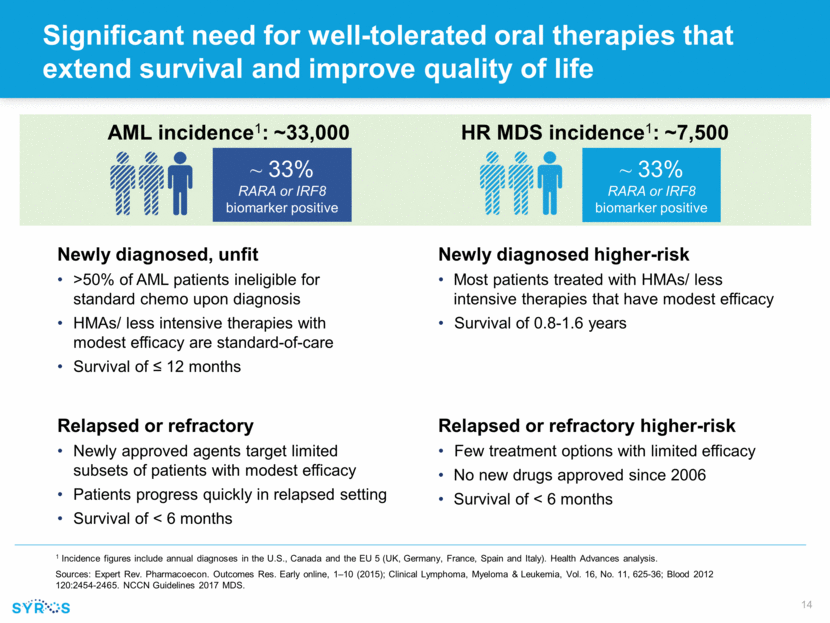
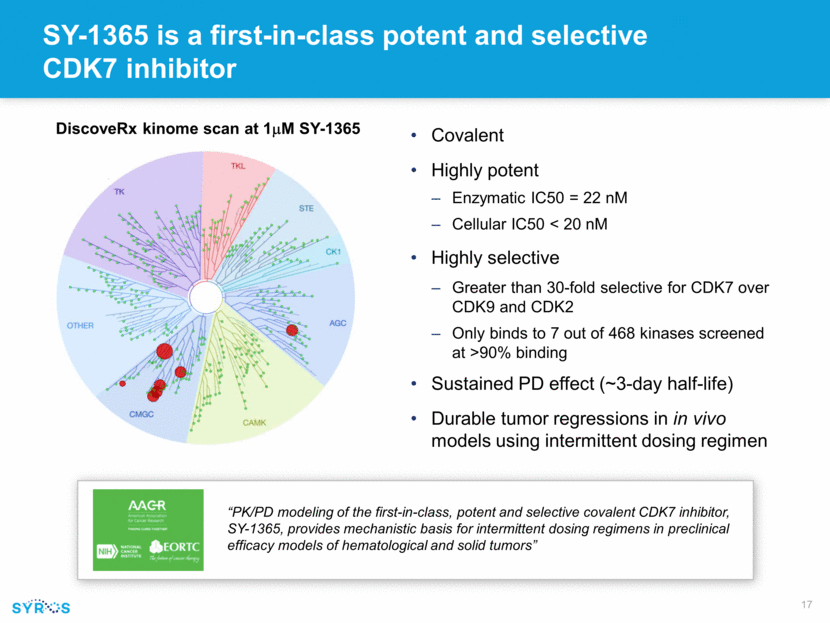
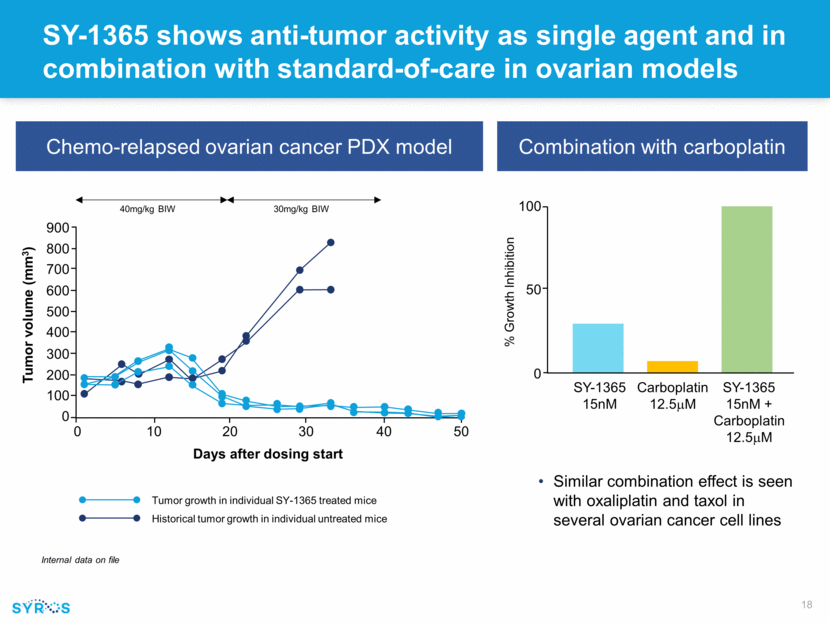
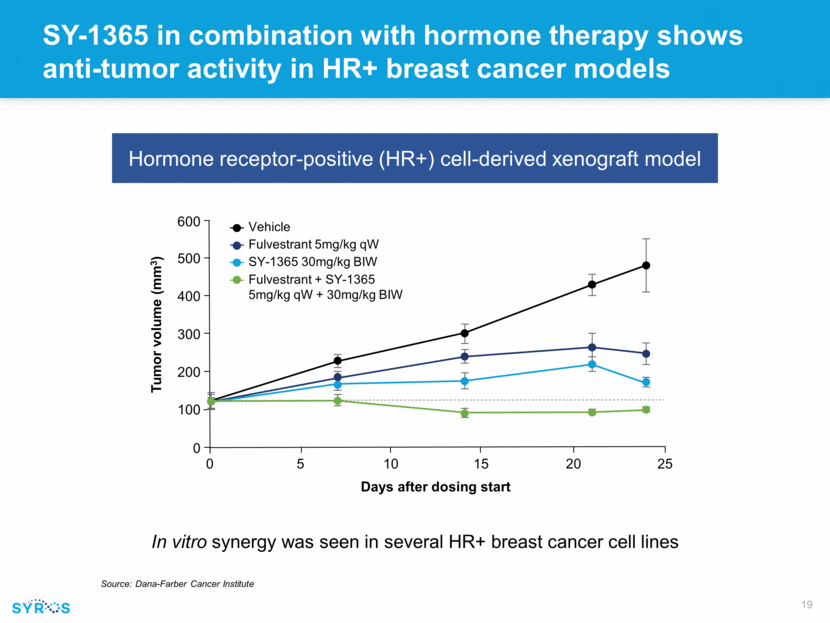
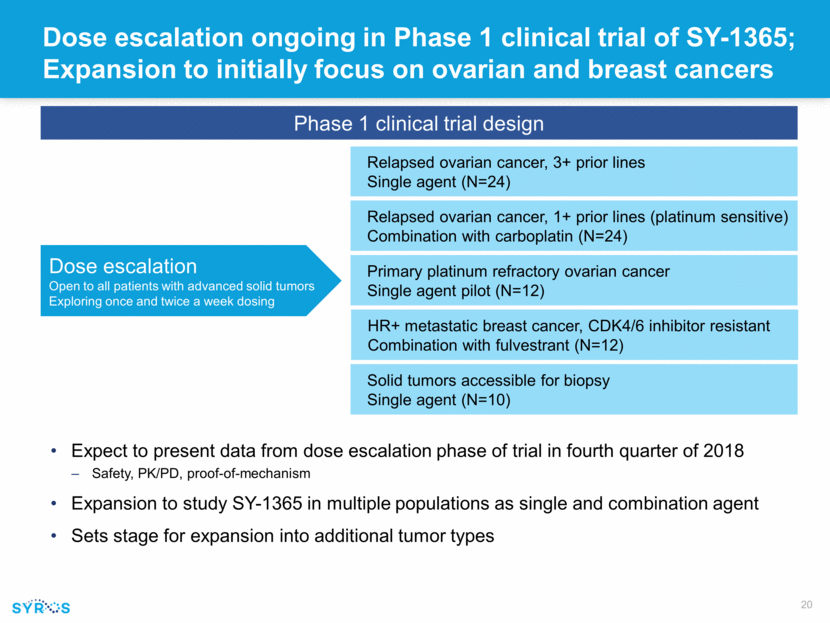
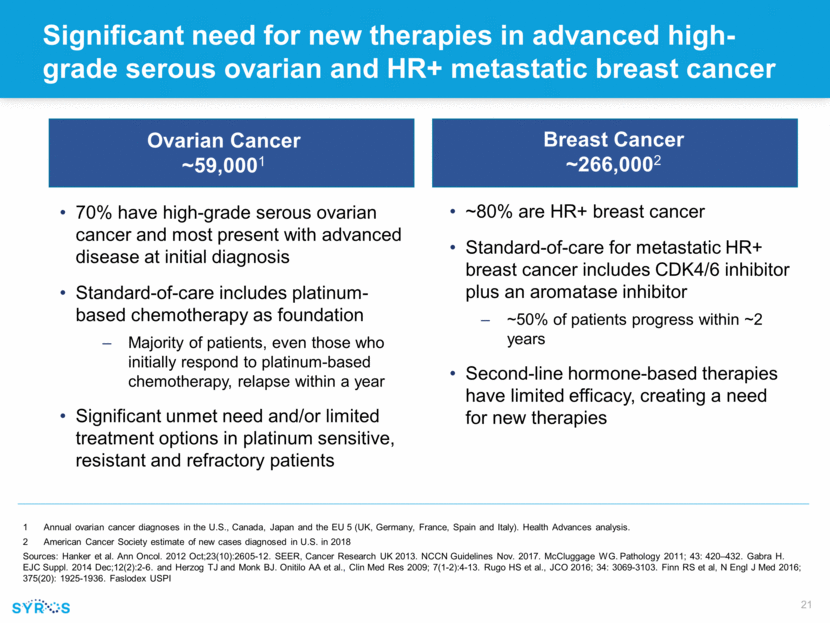
Forward-looking statements This presentation contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995 that involve substantial risks and uncertainties. All statements, other than statements of historical facts, contained in this presentation, including statements regarding our strategy, research and clinical development plans, collaborations, future operations, future financial position, future revenues, projected costs, prospects, plans and objectives of management, are forward-looking statements. The words “anticipate,” “believe,” “estimate,” “expect,” “intend,” “may,” “plan,” “predict,” “project,” “target,” “potential,” “will,” “would,” “could,” “should,” “continue,” and similar expressions are intended to identify forward-looking statements, although not all forward-looking statements contain these identifying words. We may not actually achieve the plans, intentions or expectations disclosed in our forward-looking statements, and you should not place undue reliance on our forward-looking statements. Actual results or events could differ materially from the plans, intentions and expectations disclosed in these forward-looking statements as a result of various important factors, including whether or when Incyte will exercise any of its options or any option exercise fees, milestone payments or royalties under the Incyte collaboration will ever be paid, and Syros’ ability to: advance the development of its programs, including SY-1425 and SY-1365, under the timelines it projects in current and future clinical trials; advance discovery programs to identify drug candidates for IND-enabling studies; obtain and maintain patent protection for its drug candidates and the freedom to operate under third party intellectual property; demonstrate in any current and future clinical trials the requisite safety, efficacy and combinability of its drug candidates; replicate scientific and non-clinical data in clinical trials; successfully develop a companion diagnostic test to identify patients with RARA and IRF8 biomarkers; obtain and maintain necessary regulatory approvals; identify, enter into and maintain collaboration agreements with third parties; manage competition; manage expenses; raise the substantial additional capital needed to achieve its business objectives; attract and retain qualified personnel; and successfully execute on its business strategies; risks described under the caption “Risk Factors” in Syros’ Annual Report on Form 10-K for the year ended December 31, 2017, which is on file with the Securities and Exchange Commission (SEC); and risks described in other filings that we may make with the SEC in the future. Any forward-looking statements contained in this presentation speak only as of the date this presentation is made, and we expressly disclaim any obligation to update any forward-looking statements, whether because of new information, future events or otherwise.