An Expression Makes a World of Difference February 2021 Exhibit 99.1

Forward-looking statements This presentation contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995 that involve substantial risks and uncertainties. All statements, other than statements of historical facts, contained in this presentation, including statements regarding our strategy, research and clinical development plans, collaborations, future operations, future financial position, future revenues, projected costs, prospects, plans and objectives of management, are forward-looking statements. The words “anticipate,” “believe,” “estimate,” “expect,” “intend,” “may,” “plan,” “predict,” “project,” “target,” “potential,” “will,” “would,” “could,” “should,” “continue,” and similar expressions are intended to identify forward-looking statements, although not all forward-looking statements contain these identifying words. We may not actually achieve the plans, intentions or expectations disclosed in our forward-looking statements, and you should not place undue reliance on our forward-looking statements. Actual results or events could differ materially from the plans, intentions and expectations disclosed in these forward-looking statements as a result of various important factors, including our ability to: advance the development of our programs, including SY-1425, SY-2101 and SY-5609, under the timelines we project in current and future clinical trials; demonstrate in any current and future clinical trials the requisite safety, efficacy and combinability of our drug candidates; replicate scientific and non-clinical data in clinical trials; successfully develop a companion diagnostic test to identify patients with the RARA biomarker; obtain and maintain patent protection for our drug candidates and the freedom to operate under third party intellectual property; obtain and maintain necessary regulatory approvals; identify, enter into and maintain collaboration agreements with third parties, including our ability to perform under our collaboration agreements with Incyte Corporation and Global Blood Therapeutics; manage competition; manage expenses; raise the substantial additional capital needed to achieve our business objectives; attract and retain qualified personnel; and successfully execute on our business strategies; risks described under the caption “Risk Factors” in our Annual Report on Form 10-K for the year ended December 31, 2019, our Quarterly Report on Form 10-Q for the quarter ended September 30, 2020, and our Current Report on Form 8-K filed on January 19, 2021, each of which is on file with the Securities and Exchange Commission (SEC); and risks described in other filings that we may make with the SEC in the future. In addition, the extent to which the COVID-19 outbreak continues to impact our workforce and our discovery research, supply chain and clinical trial operations activities, and the operations of the third parties on which we rely, will depend on future developments, which are highly uncertain and cannot be predicted with confidence, including the duration and severity of the outbreak, additional or modified government actions, and the actions that may be required to contain the virus or treat its impact. Any forward-looking statements contained in this presentation speak only as of the date this presentation is made, and we expressly disclaim any obligation to update any forward-looking statements, whether because of new information, future events or otherwise.
Accelerating our vision Targeted hematology therapy franchise Gene control discovery engine Selective CDK inhibitor franchise Fully integrated biopharmaceutical company
Rapidly advancing toward being a commercial-stage company Leading gene control platform 3 clinical programs Experienced leadership team Well-funded beyond multiple data readouts 2 potential NDAs in 2024
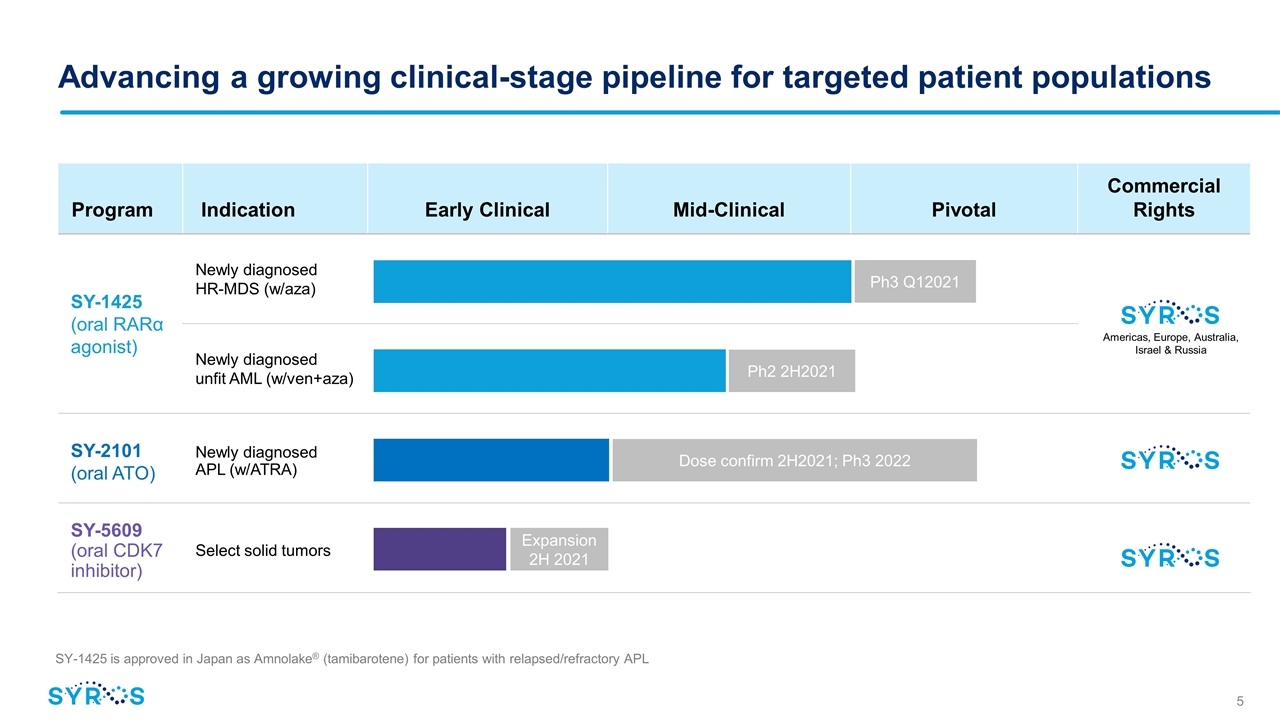
Program Indication Early Clinical IND- Enabling Mid-Clinical Pivotal Pivotal Commercial Rights SY-1425 (oral RARα agonist) Newly diagnosed HR-MDS (w/aza) Newly diagnosed unfit AML (w/ven+aza) SY-2101 (oral ATO) Newly diagnosed APL (w/ATRA) SY-5609 (oral CDK7 inhibitor) Select solid tumors Advancing a growing clinical-stage pipeline for targeted patient populations SY-1425 is approved in Japan as Amnolake® (tamibarotene) for patients with relapsed/refractory APL Americas, Europe, Australia, Israel & Russia Ph3 Q12021 Ph2 2H2021 Dose confirm 2H2021; Ph3 2022 Expansion 2H 2021

SY-1425 Selective oral RARa agonist
Clear vision for SY-1425 in RARA-positive cancers Now Registration strategy in MDS Triplet strategy in unfit AML Next Registration-enabling studies Commercialization Vision Foundation of care for all RARA+ patients
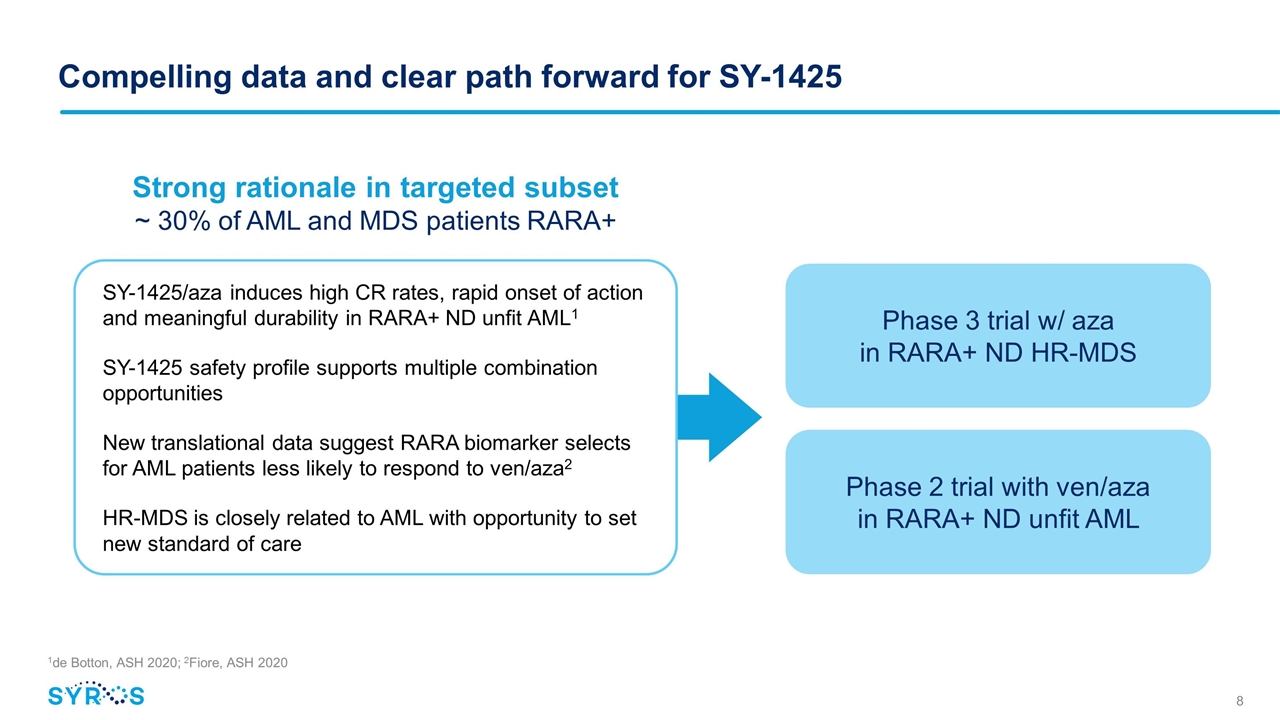
Compelling data and clear path forward for SY-1425 1de Botton, ASH 2020; 2Fiore, ASH 2020 SY-1425/aza induces high CR rates, rapid onset of action and meaningful durability in RARA+ ND unfit AML1 SY-1425 safety profile supports multiple combination opportunities New translational data suggest RARA biomarker selects for AML patients less likely to respond to ven/aza2 HR-MDS is closely related to AML with opportunity to set new standard of care Strong rationale in targeted subset ~ 30% of AML and MDS patients RARA+ Phase 3 trial w/ aza in RARA+ ND HR-MDS Phase 2 trial with ven/aza in RARA+ ND unfit AML
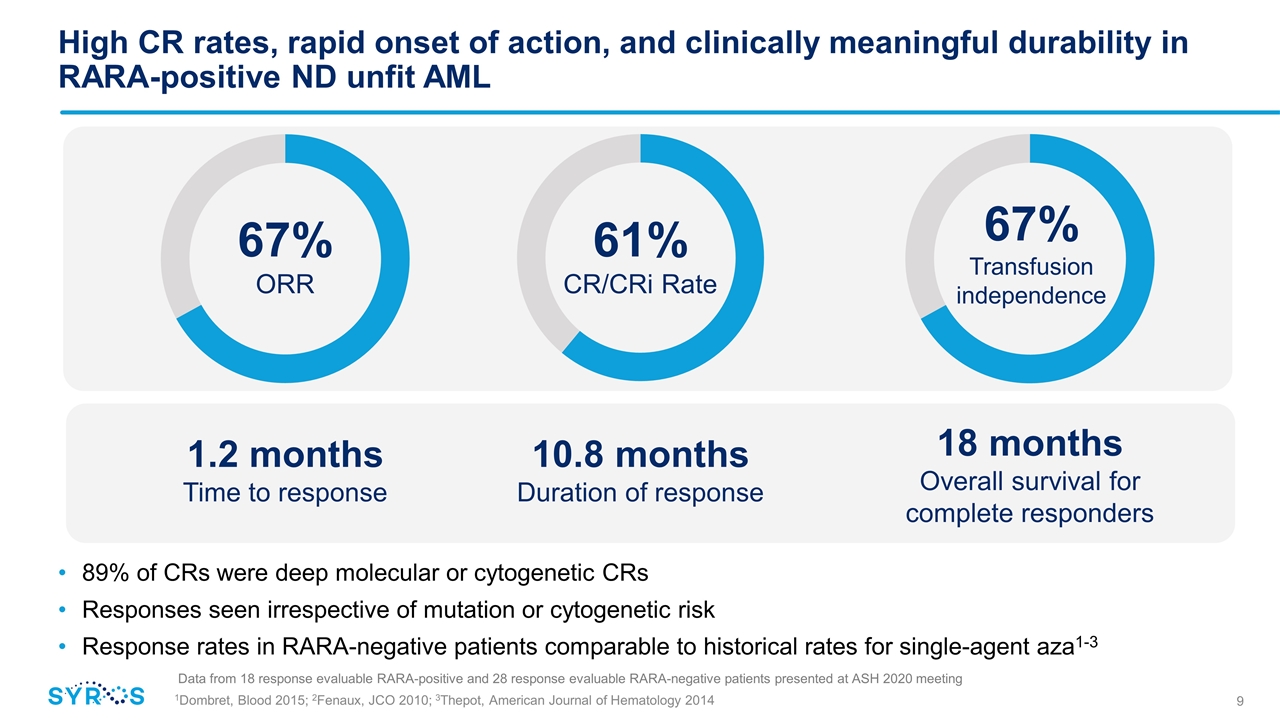
High CR rates, rapid onset of action, and clinically meaningful durability in RARA-positive ND unfit AML 89% of CRs were deep molecular or cytogenetic CRs Responses seen irrespective of mutation or cytogenetic risk Response rates in RARA-negative patients comparable to historical rates for single-agent aza1-3 1.2 months Time to response 10.8 months Duration of response 18 months Overall survival for complete responders Data from 18 response evaluable RARA-positive and 28 response evaluable RARA-negative patients presented at ASH 2020 meeting 61% CR/CRi Rate 67% Transfusion independence 67% ORR 1Dombret, Blood 2015; 2Fenaux, JCO 2010; 3Thepot, American Journal of Hematology 2014
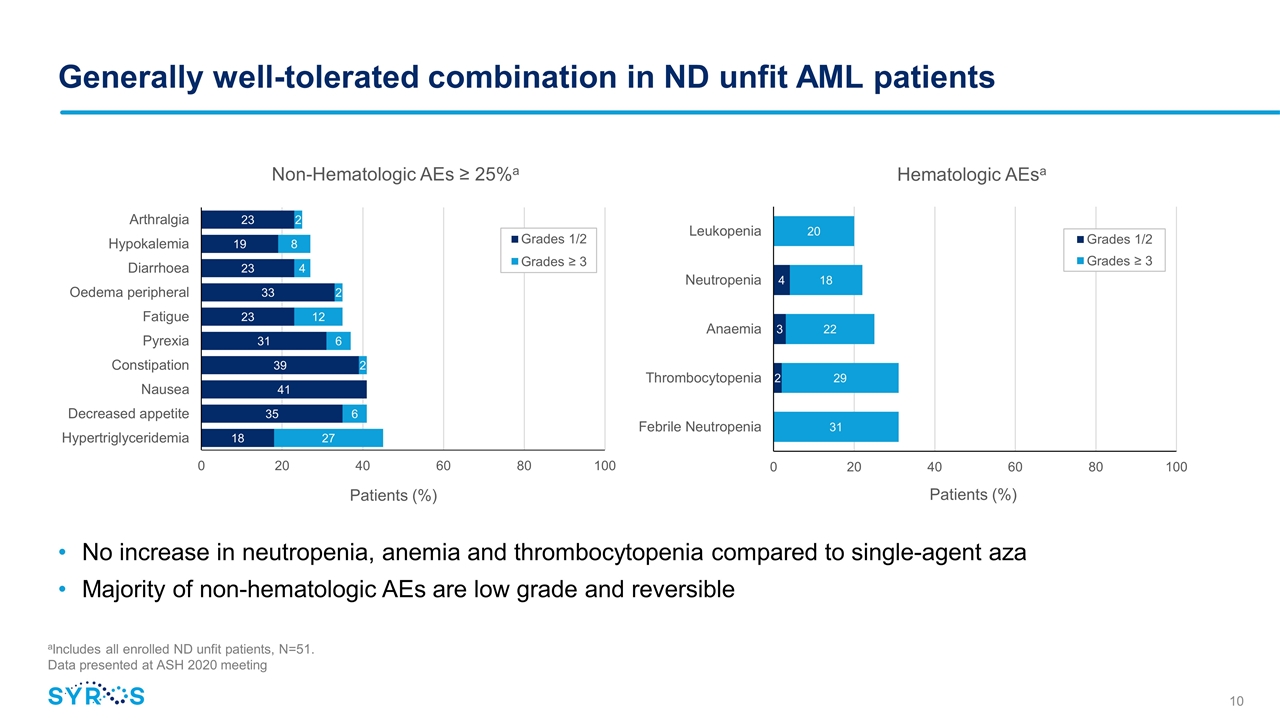
Generally well-tolerated combination in ND unfit AML patients No increase in neutropenia, anemia and thrombocytopenia compared to single-agent aza Majority of non-hematologic AEs are low grade and reversible aIncludes all enrolled ND unfit patients, N=51. Data presented at ASH 2020 meeting
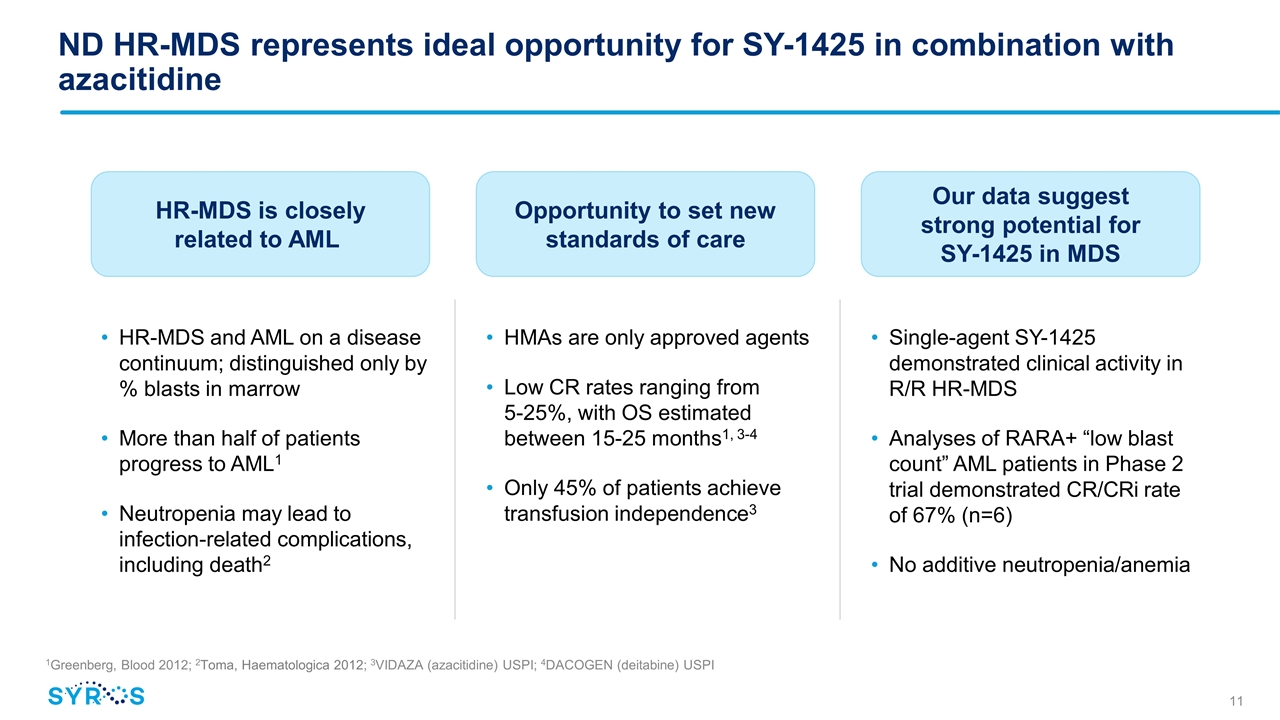
ND HR-MDS represents ideal opportunity for SY-1425 in combination with azacitidine HR-MDS and AML on a disease continuum; distinguished only by % blasts in marrow More than half of patients progress to AML1 Neutropenia may lead to infection-related complications, including death2 HR-MDS is closely related to AML HMAs are only approved agents Low CR rates ranging from 5-25%, with OS estimated between 15-25 months1, 3-4 Only 45% of patients achieve transfusion independence3 Opportunity to set new standards of care Single-agent SY-1425 demonstrated clinical activity in R/R HR-MDS Analyses of RARA+ “low blast count” AML patients in Phase 2 trial demonstrated CR/CRi rate of 67% (n=6) No additive neutropenia/anemia Our data suggest strong potential for SY-1425 in MDS 1Greenberg, Blood 2012; 2Toma, Haematologica 2012; 3VIDAZA (azacitidine) USPI; 4DACOGEN (deitabine) USPI
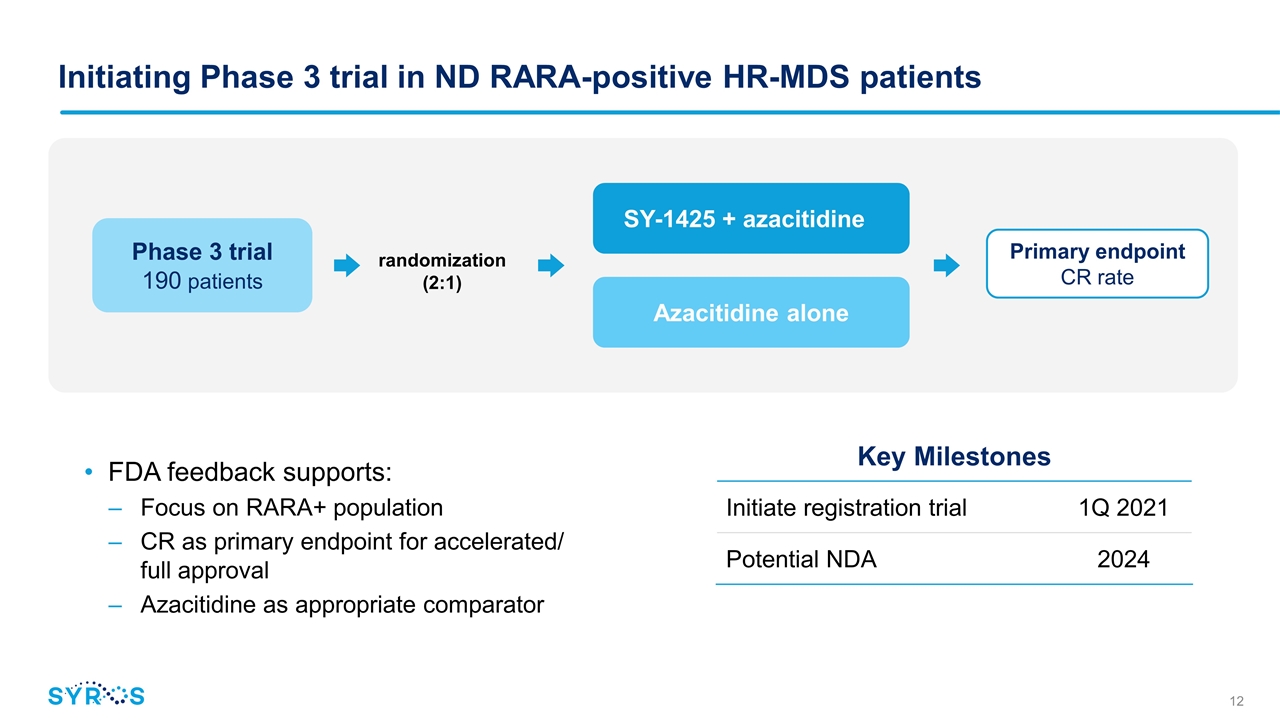
Initiating Phase 3 trial in ND RARA-positive HR-MDS patients FDA feedback supports: Focus on RARA+ population CR as primary endpoint for accelerated/ full approval Azacitidine as appropriate comparator Key Milestones Initiate registration trial 1Q 2021 Potential NDA 2024 Phase 3 trial 190 patients randomization (2:1) SY-1425 + azacitidine Azacitidine alone Primary endpoint CR rate
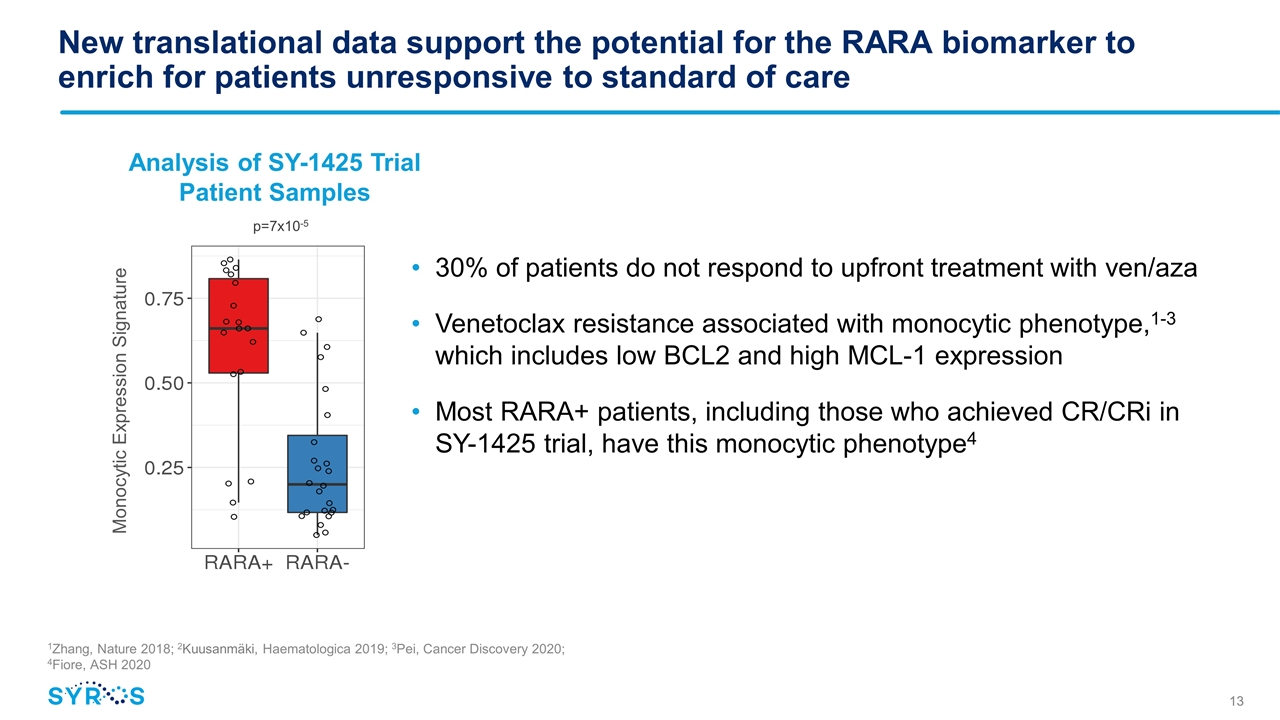
New translational data support the potential for the RARA biomarker to enrich for patients unresponsive to standard of care 30% of patients do not respond to upfront treatment with ven/aza Venetoclax resistance associated with monocytic phenotype,1-3 which includes low BCL2 and high MCL-1 expression Most RARA+ patients, including those who achieved CR/CRi in SY-1425 trial, have this monocytic phenotype4 Analysis of SY-1425 Trial Patient Samples Monocytic Expression Signature p=7x10-5 1Zhang, Nature 2018; 2Kuusanmäki, Haematologica 2019; 3Pei, Cancer Discovery 2020; 4Fiore, ASH 2020
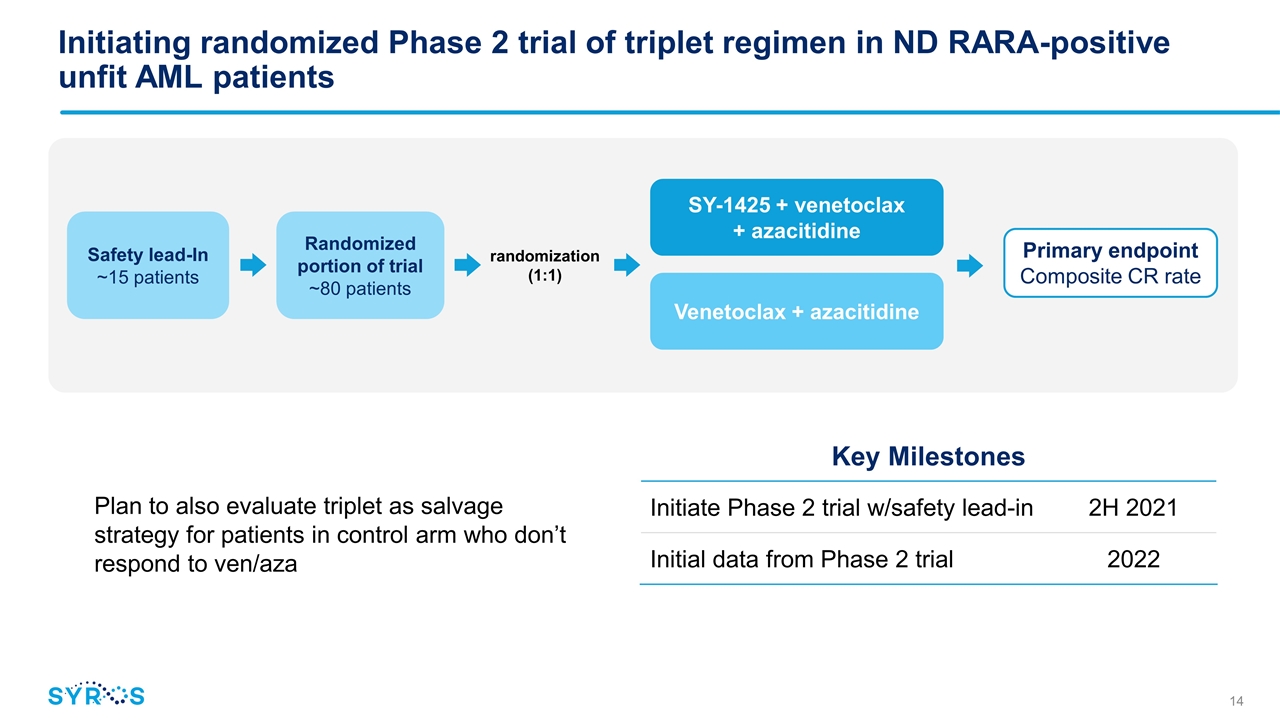
Randomized portion of trial ~80 patients SY-1425 + venetoclax + azacitidine Venetoclax + azacitidine randomization (1:1) Safety lead-In ~15 patients Initiating randomized Phase 2 trial of triplet regimen in ND RARA-positive unfit AML patients Plan to also evaluate triplet as salvage strategy for patients in control arm who don’t respond to ven/aza Key Milestones Initiate Phase 2 trial w/safety lead-in 2H 2021 Initial data from Phase 2 trial 2022 Primary endpoint Composite CR rate
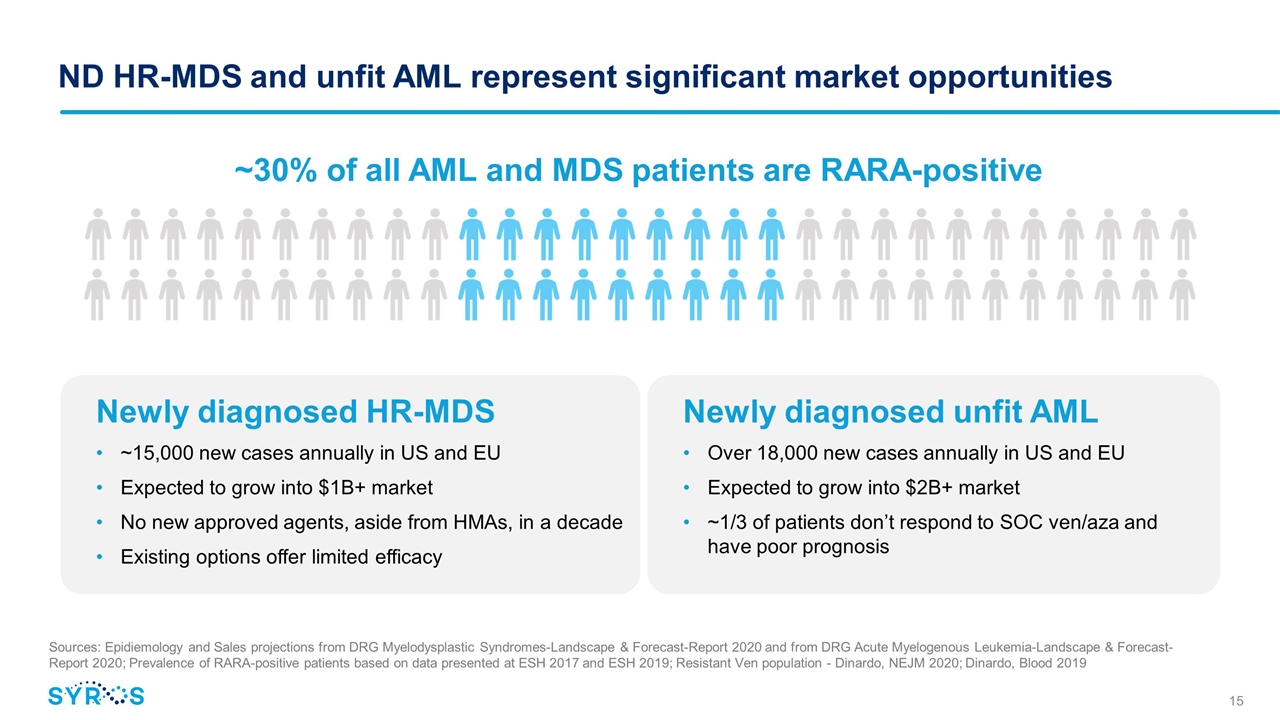
ND HR-MDS and unfit AML represent significant market opportunities ~30% of all AML and MDS patients are RARA-positive Newly diagnosed HR-MDS ~15,000 new cases annually in US and EU Expected to grow into $1B+ market No new approved agents, aside from HMAs, in a decade Existing options offer limited efficacy Sources: Epidiemology and Sales projections from DRG Myelodysplastic Syndromes-Landscape & Forecast-Report 2020 and from DRG Acute Myelogenous Leukemia-Landscape & Forecast-Report 2020; Prevalence of RARA-positive patients based on data presented at ESH 2017 and ESH 2019; Resistant Ven population - Dinardo, NEJM 2020; Dinardo, Blood 2019 Newly diagnosed unfit AML Over 18,000 new cases annually in US and EU Expected to grow into $2B+ market ~1/3 of patients don’t respond to SOC ven/aza and have poor prognosis

SY-2101 Novel oral form of arsenic trioxide
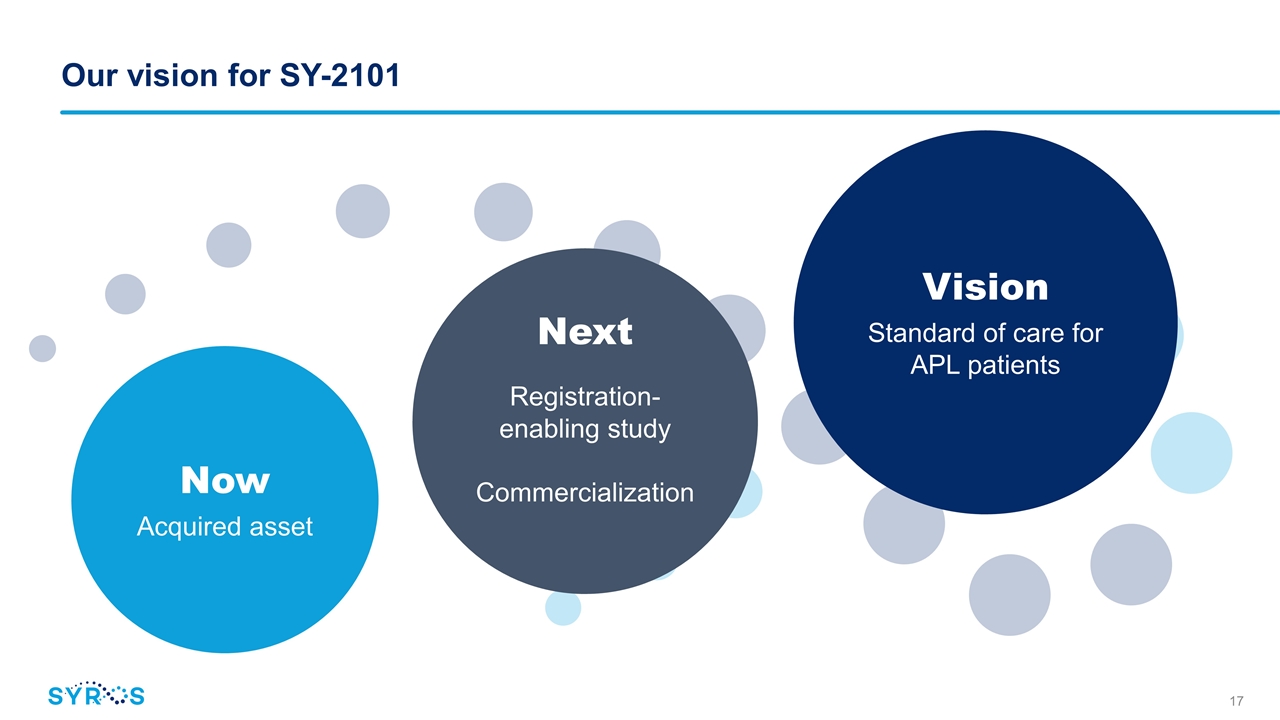
Our vision for SY-2101 Now Acquired asset Next Registration-enabling study Commercialization Vision Standard of care for APL patients

SY-2101: Highly synergistic with our advancing targeted heme franchise Strategic opportunity as we accelerate toward becoming a commercial-stage company Potential to become standard of care in APL Novel oral capsule of arsenic trioxide (ATO) Clinical-stage asset with opportunity for accelerated approval based on molecular CR Potential NDA filing in 2024 Orphan drug designation granted in US and EU Issued patents provide opportunity to extend exclusivity Capitalizes on our expertise to build a leadership position in targeted therapies for hematologic disorders
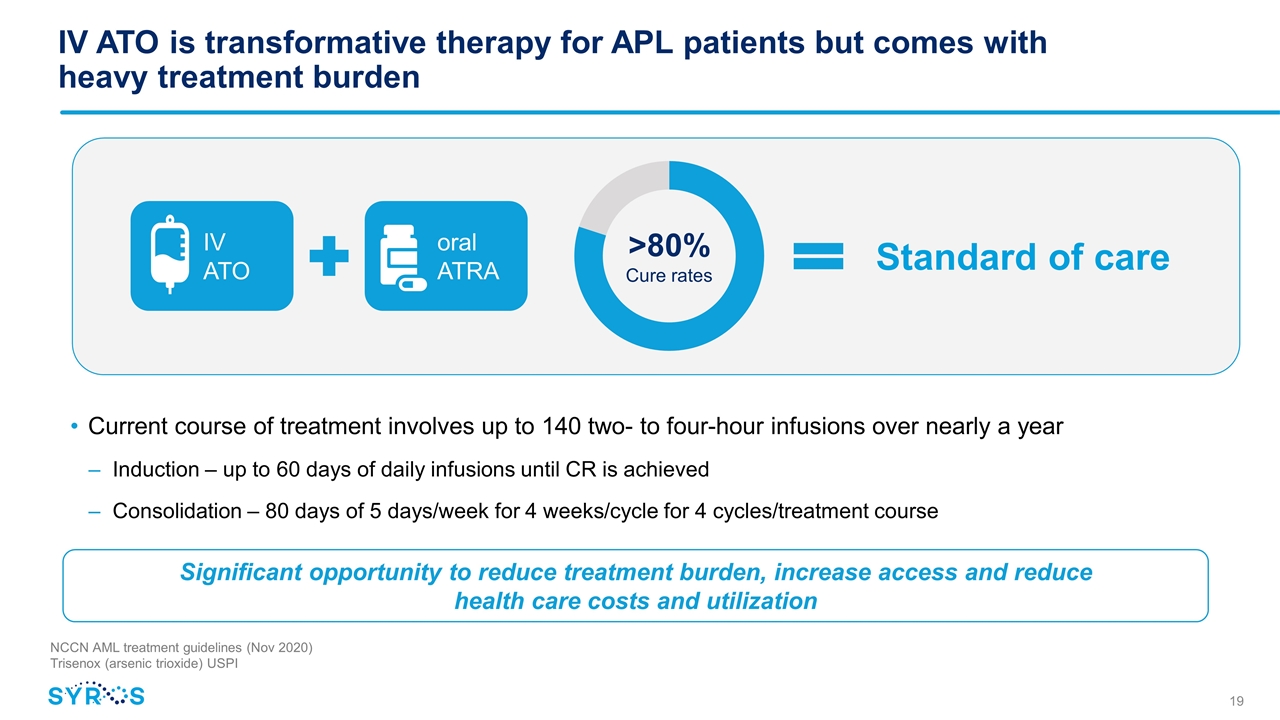
IV ATO is transformative therapy for APL patients but comes with heavy treatment burden >80% Cure rates IV ATO oral ATRA Significant opportunity to reduce treatment burden, increase access and reduce health care costs and utilization Current course of treatment involves up to 140 two- to four-hour infusions over nearly a year Induction – up to 60 days of daily infusions until CR is achieved Consolidation – 80 days of 5 days/week for 4 weeks/cycle for 4 cycles/treatment course NCCN AML treatment guidelines (Nov 2020) Trisenox (arsenic trioxide) USPI Standard of care
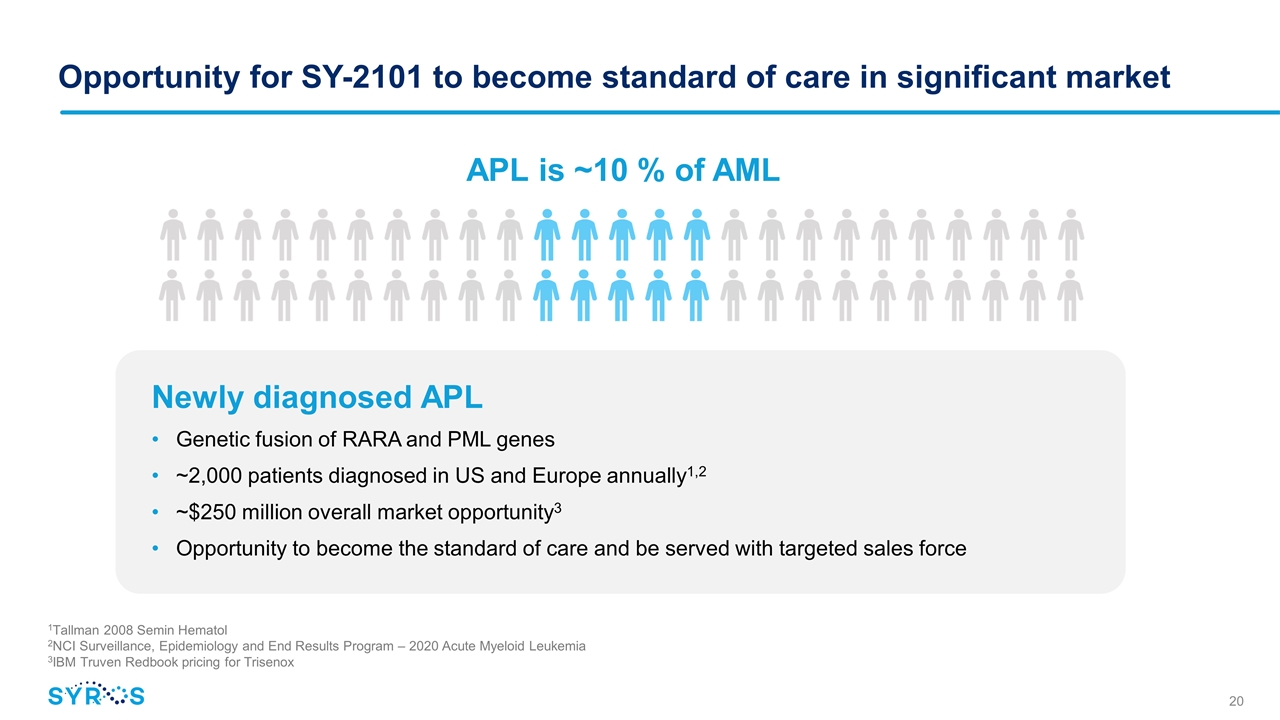
Opportunity for SY-2101 to become standard of care in significant market APL is ~10 % of AML Newly diagnosed APL Genetic fusion of RARA and PML genes ~2,000 patients diagnosed in US and Europe annually1,2 ~$250 million overall market opportunity3 Opportunity to become the standard of care and be served with targeted sales force 1Tallman 2008 Semin Hematol 2NCI Surveillance, Epidemiology and End Results Program – 2020 Acute Myeloid Leukemia 3IBM Truven Redbook pricing for Trisenox
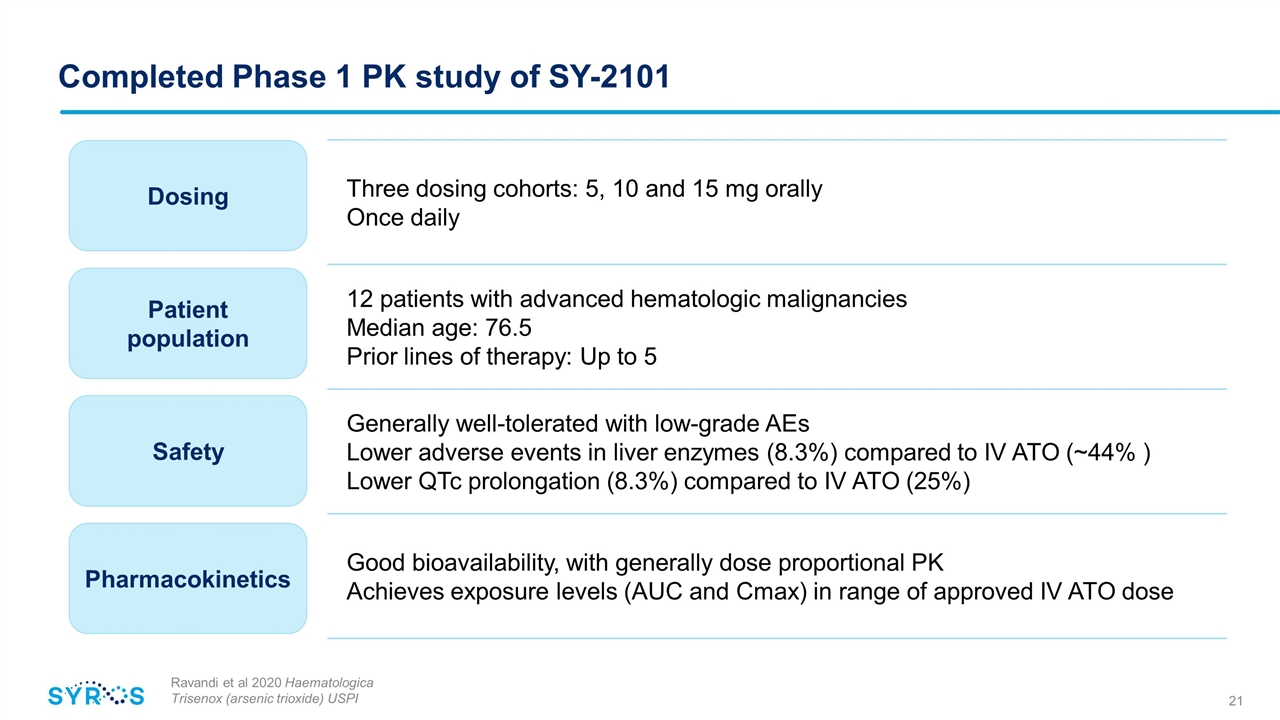
Completed Phase 1 PK study of SY-2101 Ravandi et al 2020 Haematologica Trisenox (arsenic trioxide) USPI Three dosing cohorts: 5, 10 and 15 mg orally Once daily 12 patients with advanced hematologic malignancies Median age: 76.5 Prior lines of therapy: Up to 5 Generally well-tolerated with low-grade AEs Lower adverse events in liver enzymes (8.3%) compared to IV ATO (~44% ) Lower QTc prolongation (8.3%) compared to IV ATO (25%) Good bioavailability, with generally dose proportional PK Achieves exposure levels (AUC and Cmax) in range of approved IV ATO dose Dosing Patient population Safety Pharmacokinetics
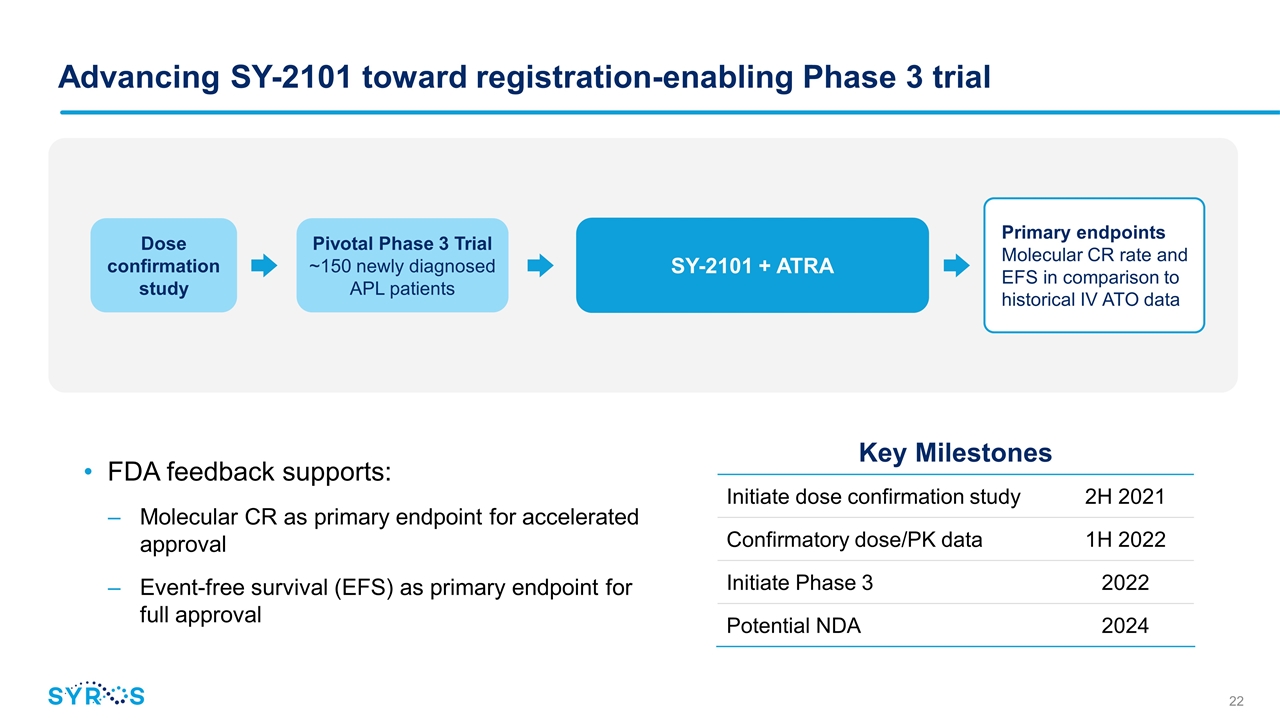
Advancing SY-2101 toward registration-enabling Phase 3 trial FDA feedback supports: Molecular CR as primary endpoint for accelerated approval Event-free survival (EFS) as primary endpoint for full approval Dose confirmation study SY-2101 + ATRA Pivotal Phase 3 Trial ~150 newly diagnosed APL patients Primary endpoints Molecular CR rate and EFS in comparison to historical IV ATO data Key Milestones Initiate dose confirmation study 2H 2021 Confirmatory dose/PK data 1H 2022 Initiate Phase 3 2022 Potential NDA 2024

SY-5609 Selective oral CDK7 inhibitor
Our vision for SY-5609 Now Phase 1 trial Initial combination with fulvestrant Next Phase 1 expansion Additional combinations Vision Transformative targeted therapy for difficult-to-treat cancers
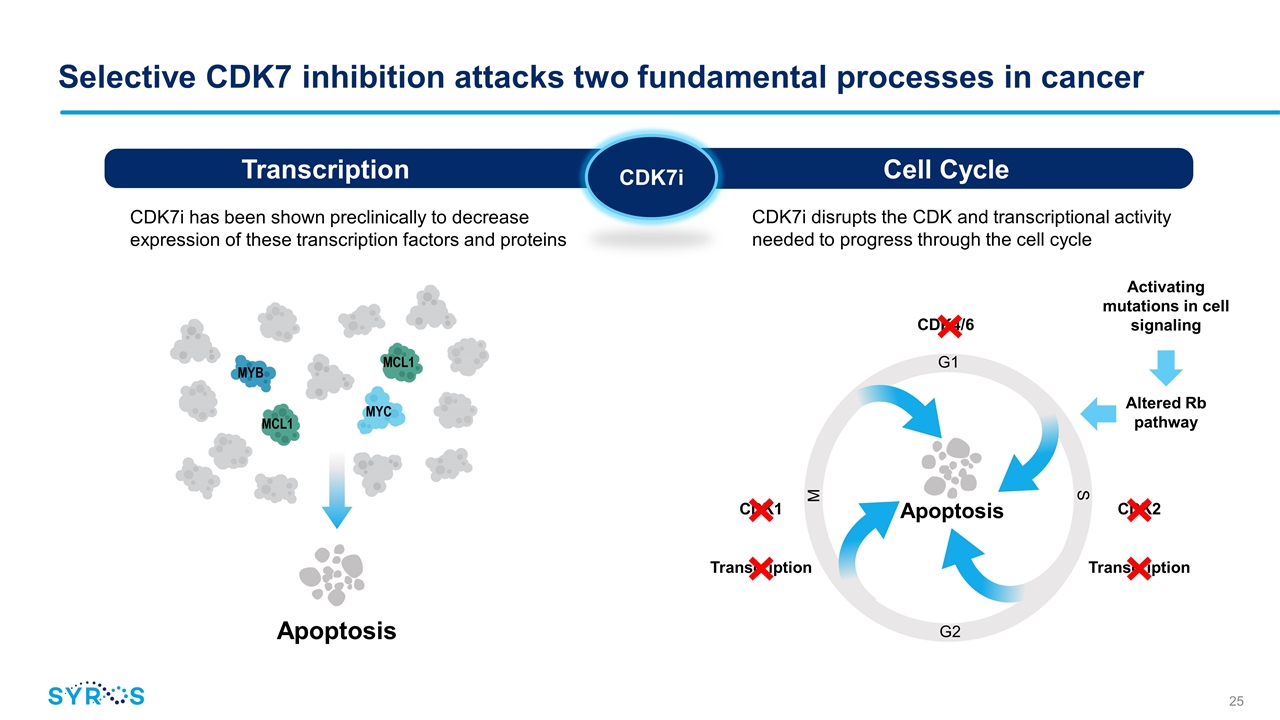
CDK7i disrupts the CDK and transcriptional activity needed to progress through the cell cycle Altered Rb pathway Activating mutations in cell signaling CDK1 CDK2 Selective CDK7 inhibition attacks two fundamental processes in cancer Cell Cycle Transcription CDK7i MYC MYB MCL1 MCL1 Apoptosis CDK7i has been shown preclinically to decrease expression of these transcription factors and proteins Transcription Transcription CDK4/6 M G2 S G1 Apoptosis
Three-pronged development strategy to maximize potential of SY-5609 Compelling preclinical data + Strong mechanistic rationale + High unmet need Leveraging Rb pathway alterations Overcoming CDK4/6 resistance Exploiting transcriptional and cell cycle dependencies
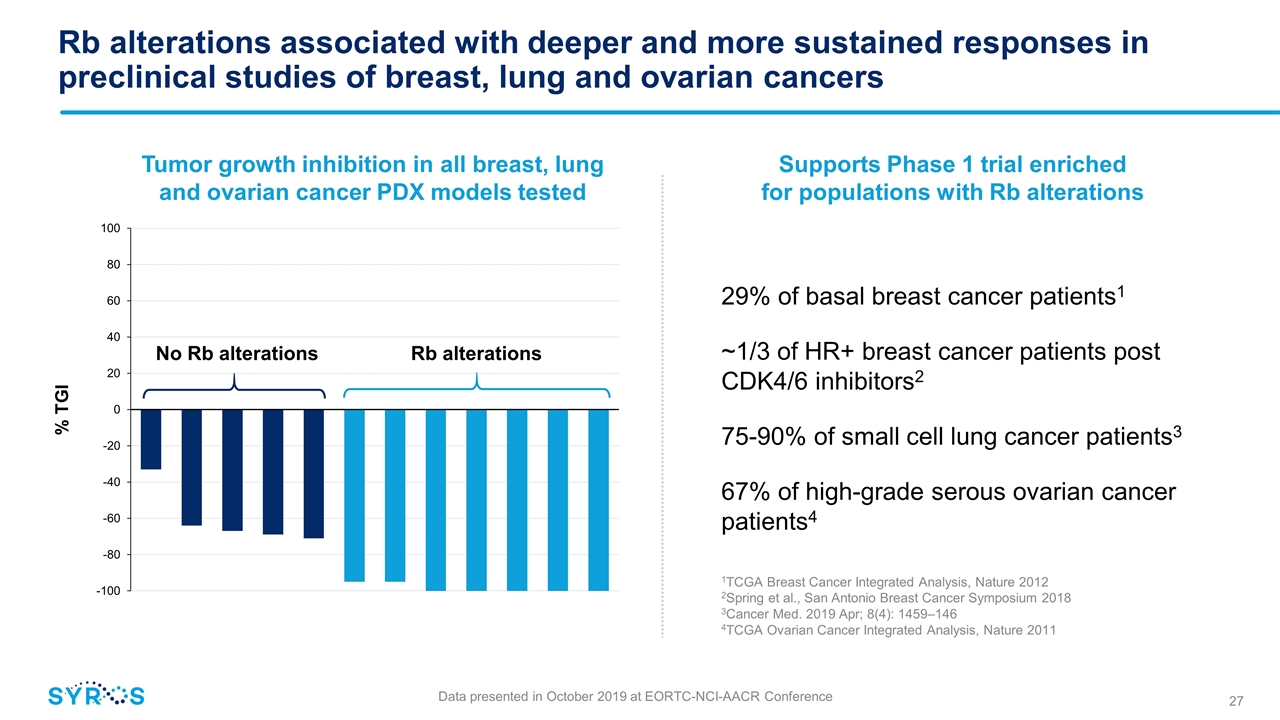
Rb alterations associated with deeper and more sustained responses in preclinical studies of breast, lung and ovarian cancers 29% of basal breast cancer patients1 ~1/3 of HR+ breast cancer patients post CDK4/6 inhibitors2 75-90% of small cell lung cancer patients3 67% of high-grade serous ovarian cancer patients4 Supports Phase 1 trial enriched for populations with Rb alterations 1TCGA Breast Cancer Integrated Analysis, Nature 2012 2Spring et al., San Antonio Breast Cancer Symposium 2018 3Cancer Med. 2019 Apr; 8(4): 1459–146 4TCGA Ovarian Cancer Integrated Analysis, Nature 2011 No Rb alterations Rb alterations % TGI Tumor growth inhibition in all breast, lung and ovarian cancer PDX models tested Data presented in October 2019 at EORTC-NCI-AACR Conference
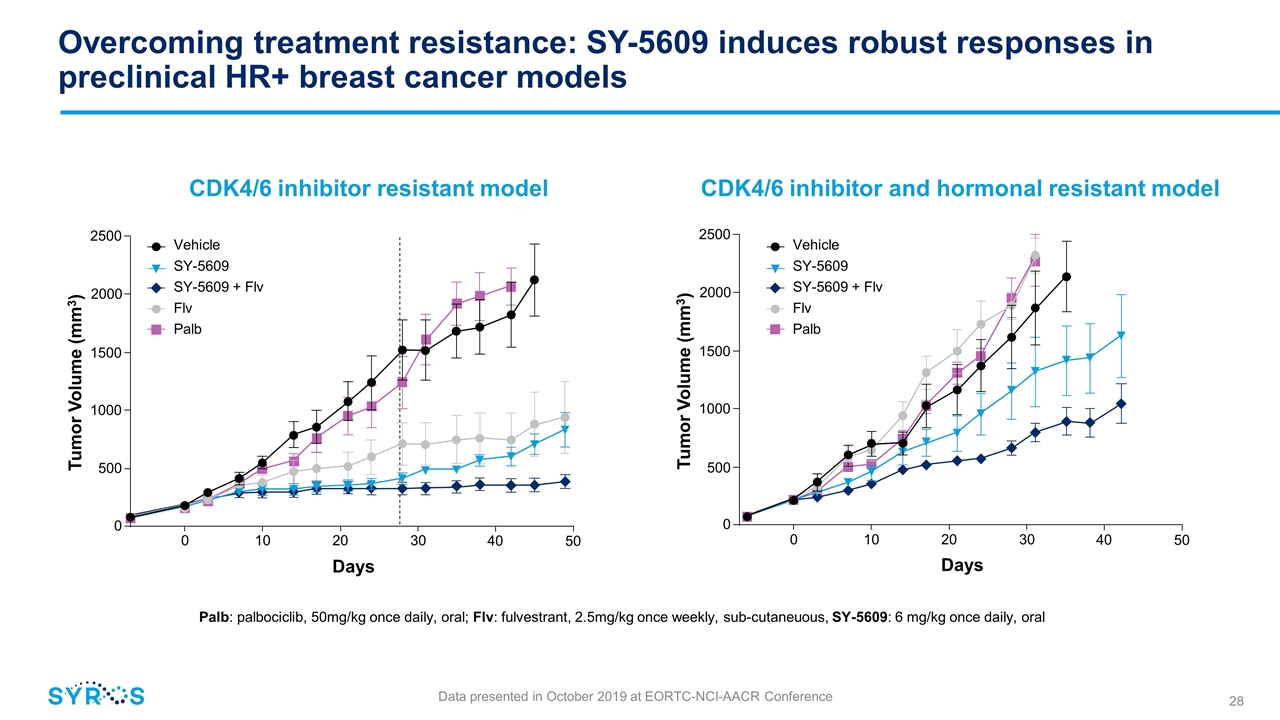
Overcoming treatment resistance: SY-5609 induces robust responses in preclinical HR+ breast cancer models Palb: palbociclib, 50mg/kg once daily, oral; Flv: fulvestrant, 2.5mg/kg once weekly, sub-cutaneuous, SY-5609: 6 mg/kg once daily, oral CDK4/6 inhibitor resistant model CDK4/6 inhibitor and hormonal resistant model 0 10 20 30 Days 40 0 500 1000 1500 2500 Tumor Volume (mm3) 2000 50 Vehicle SY-5609 SY-5609 + Flv Flv Palb Vehicle SY-5609 SY-5609 + Flv Flv Palb 0 10 20 30 Days 40 0 500 1000 1500 2500 Tumor Volume (mm3) 2000 50 Data presented in October 2019 at EORTC-NCI-AACR Conference
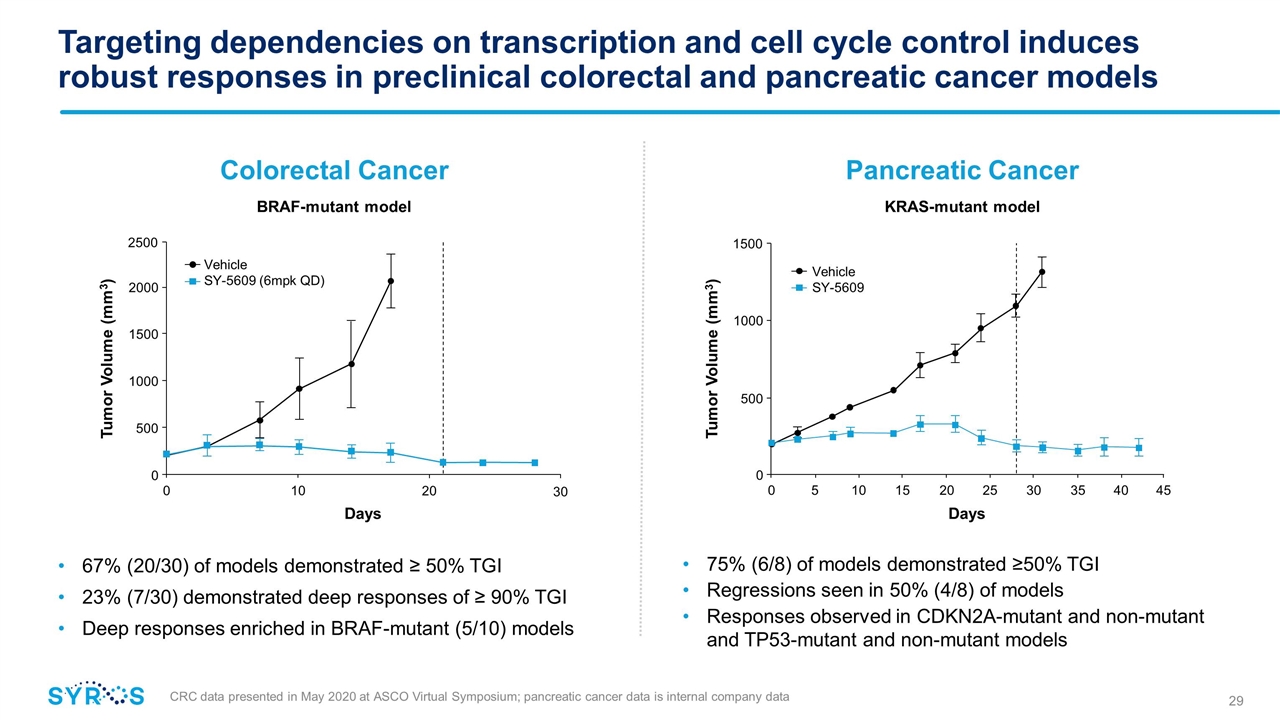
Targeting dependencies on transcription and cell cycle control induces robust responses in preclinical colorectal and pancreatic cancer models 67% (20/30) of models demonstrated ≥ 50% TGI 23% (7/30) demonstrated deep responses of ≥ 90% TGI Deep responses enriched in BRAF-mutant (5/10) models CRC data presented in May 2020 at ASCO Virtual Symposium; pancreatic cancer data is internal company data KRAS-mutant model 75% (6/8) of models demonstrated ≥50% TGI Regressions seen in 50% (4/8) of models Responses observed in CDKN2A-mutant and non-mutant and TP53-mutant and non-mutant models BRAF-mutant model Colorectal Cancer Pancreatic Cancer 0 10 Days 20 0 500 1000 1500 2000 Tumor Volume (mm3) 2500 30 Vehicle SY-5609 (6mpk QD) Vehicle SY-5609 0 10 Days 20 0 500 1000 Tumor Volume (mm3) 1500 45 40 35 30 25 15 5

Dose-escalation Cohort extensions in select populations HR+ breast cancer, post CDK4/6 Expansion Further explore SY-5609 as single and combination agent Advanced solid tumors Single agent Combination with fulvestrant Ongoing Phase 1 dose-escalation trial in select solid tumors Advanced solid tumor populations- breast, colorectal, lung, ovarian, pancreatic, and tumors with Rb alterations Established MTD for continuous daily dosing Additional dose escalation data, including clinical activity, expected in Q3 2021 Expansion phase of Phase 1 trial expected to start in second half of 2021 HR+ breast, post CDK4/6 Alternate dose and schedules
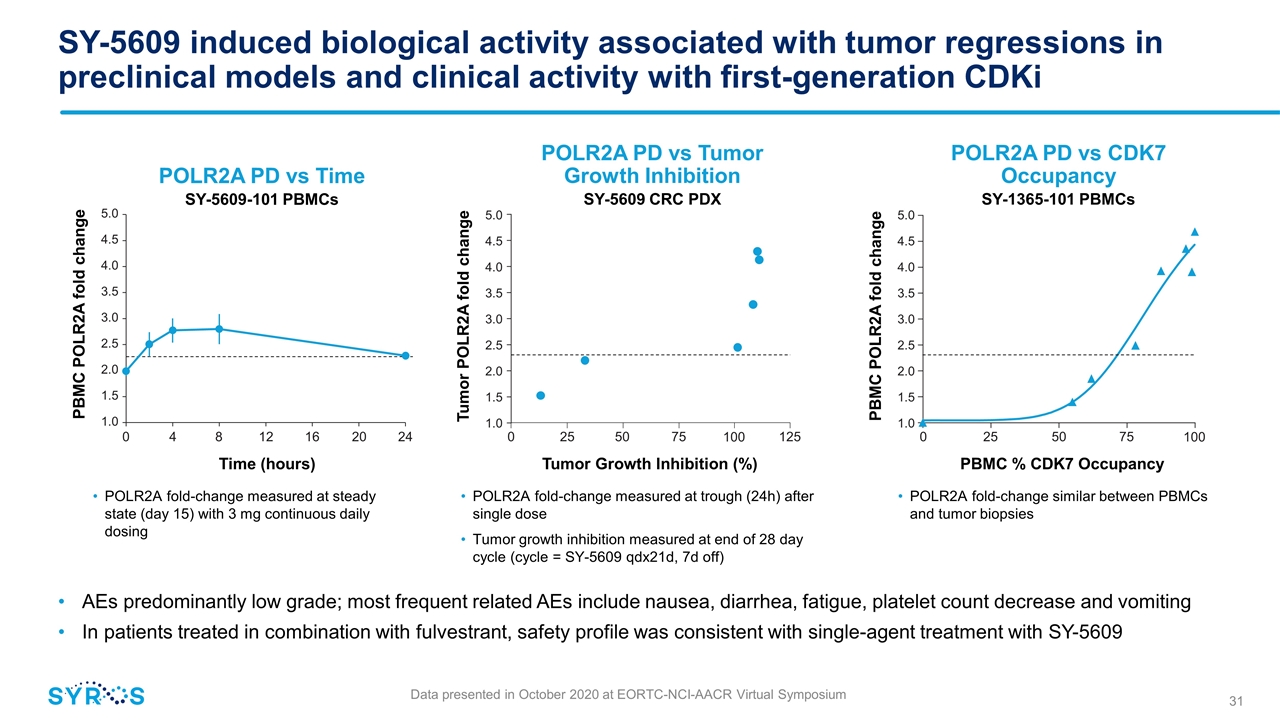
SY-5609 induced biological activity associated with tumor regressions in preclinical models and clinical activity with first-generation CDKi Data presented in October 2020 at EORTC-NCI-AACR Virtual Symposium AEs predominantly low grade; most frequent related AEs include nausea, diarrhea, fatigue, platelet count decrease and vomiting In patients treated in combination with fulvestrant, safety profile was consistent with single-agent treatment with SY-5609 POLR2A fold-change measured at steady state (day 15) with 3 mg continuous daily dosing POLR2A fold-change measured at trough (24h) after single dose Tumor growth inhibition measured at end of 28 day cycle (cycle = SY-5609 qdx21d, 7d off) POLR2A fold-change similar between PBMCs and tumor biopsies 0 4 8 12 16 20 24 1.0 5.0 4.5 4.0 3.5 3.0 2.5 2.0 1.5 Time (hours) PBMC POLR2A fold change POLR2A PD vs Time SY-5609-101 PBMCs Tumor Growth Inhibition (%) 1.0 5.0 4.5 4.0 3.5 3.0 2.5 2.0 1.5 Tumor POLR2A fold change 0 25 50 75 125 100 POLR2A PD vs Tumor Growth Inhibition SY-5609 CRC PDX PBMC % CDK7 Occupancy 1.0 5.0 4.5 4.0 3.5 3.0 2.5 2.0 1.5 PBMC POLR2A fold change 0 25 50 75 100 POLR2A PD vs CDK7 Occupancy SY-1365-101 PBMCs

Gene Control Discovery Engine
Previously unexplored regulatory regions of the genome control expression of genes determining cell function; majority of disease variation found in these regions 98% Patient Impact Medicines that control the expression of genes to provide profound benefit for patients with severe diseases Redefining the power of small molecules to control expression of genes ! Regulatory Genomics Disease Biology Transcriptional Chemistry
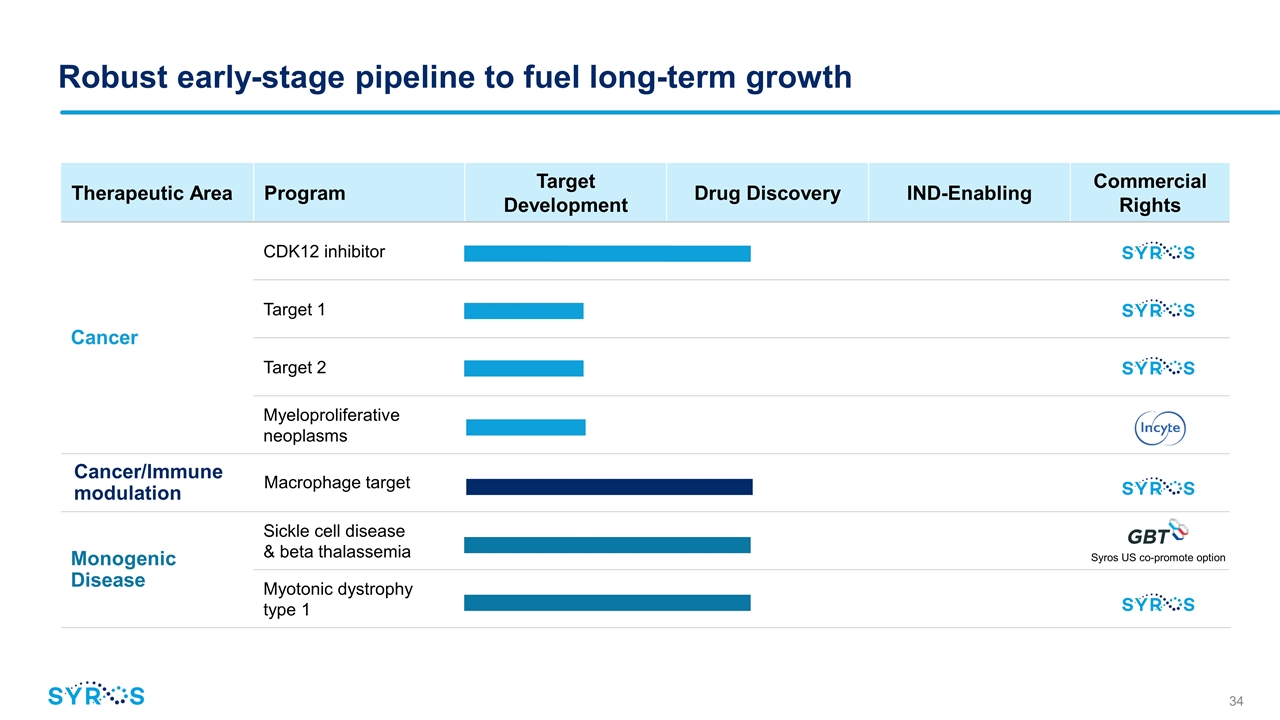
Therapeutic Area Program Target Development Drug Discovery IND-Enabling Commercial Rights Cancer CDK12 inhibitor Target 1 Target 2 Myeloproliferative neoplasms Cancer/Immune modulation Macrophage target Monogenic Disease Sickle cell disease & beta thalassemia Myotonic dystrophy type 1 Robust early-stage pipeline to fuel long-term growth Syros US co-promote option
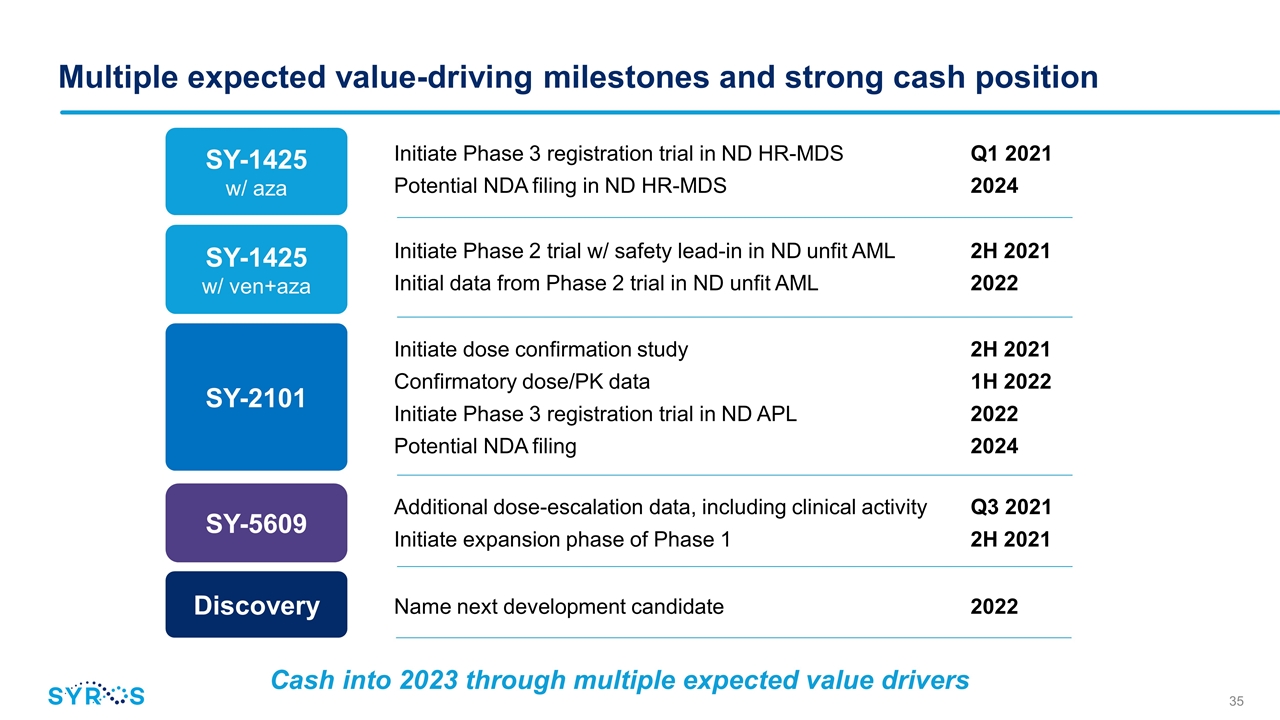
Multiple expected value-driving milestones and strong cash position SY-5609 SY-2101 SY-1425 w/ aza SY-1425 w/ ven+aza Initiate Phase 3 registration trial in ND HR-MDS Q1 2021 Potential NDA filing in ND HR-MDS 2024 Initiate Phase 2 trial w/ safety lead-in in ND unfit AML2H 2021 Initial data from Phase 2 trial in ND unfit AML2022 Initiate dose confirmation study 2H 2021 Confirmatory dose/PK data 1H 2022 Initiate Phase 3 registration trial in ND APL 2022 Potential NDA filing 2024 Additional dose-escalation data, including clinical activity Q3 2021 Initiate expansion phase of Phase 1 2H 2021 Cash into 2023 through multiple expected value drivers Discovery Name next development candidate2022
Rapidly advancing toward our vision Progressing into pivotal studies Preparing for product launches Platform fueling robust pipeline Fully integrated company with medicines that provide a profound benefit for patients Advancing growing clinical-stage pipeline Capital to fund planned operations into 2023 Now Next Vision
Appendix
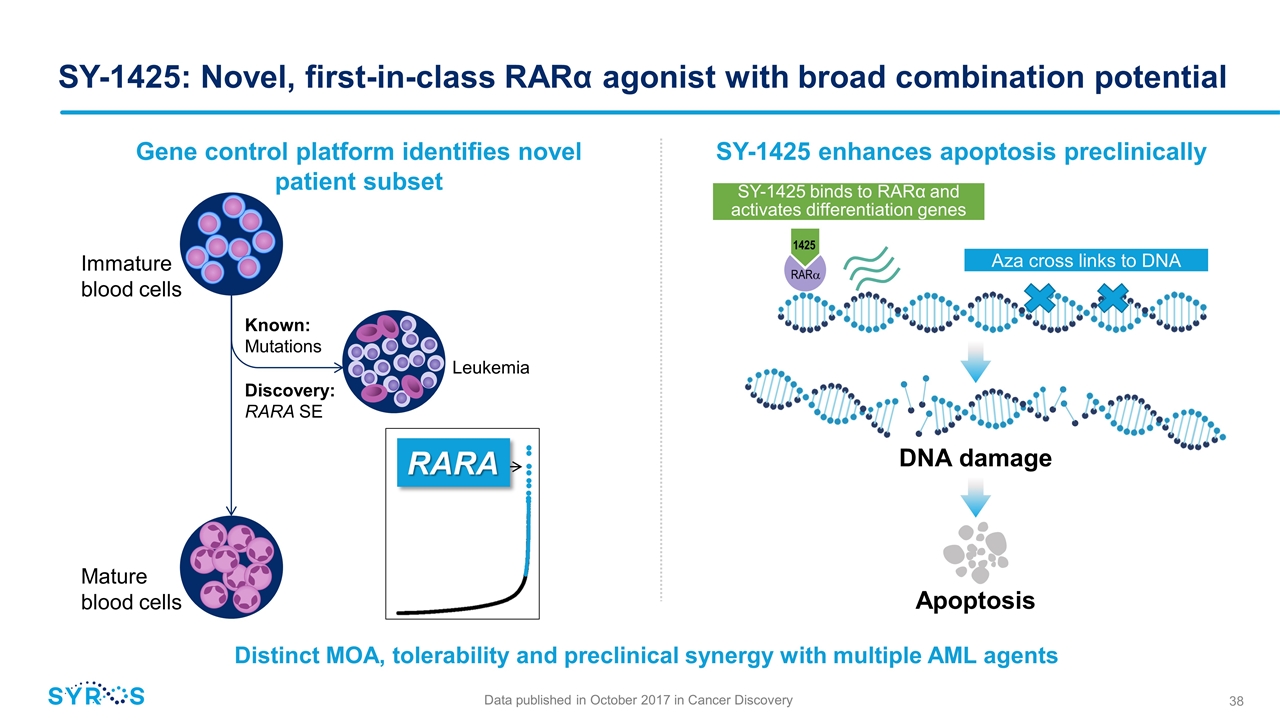
1425 SY-1425 binds to RARα and activates differentiation genes SY-1425: Novel, first-in-class RARα agonist with broad combination potential RARa Aza cross links to DNA SY-1425 enhances apoptosis preclinically Apoptosis DNA damage Gene control platform identifies novel patient subset Distinct MOA, tolerability and preclinical synergy with multiple AML agents Immature blood cells Mature blood cells Discovery: RARA SE Known: Mutations RARA Leukemia Data published in October 2017 in Cancer Discovery
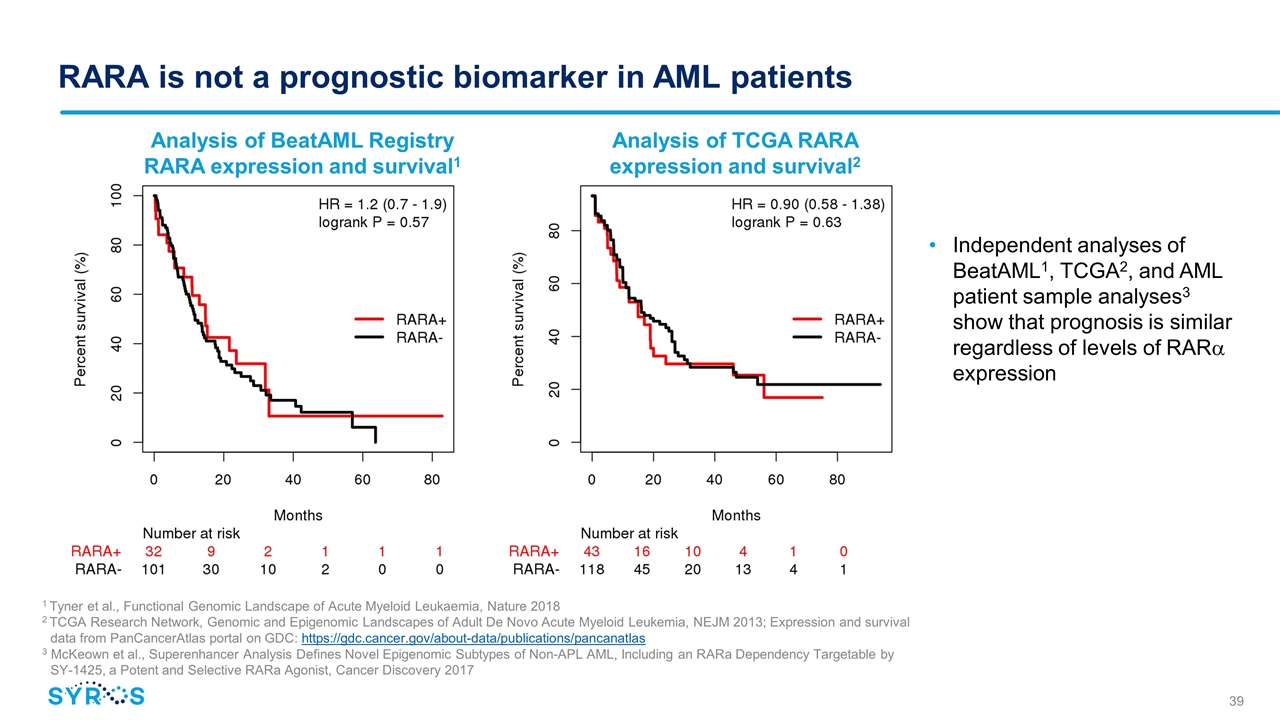
RARA is not a prognostic biomarker in AML patients Analysis of BeatAML Registry RARA expression and survival1 1 Tyner et al., Functional Genomic Landscape of Acute Myeloid Leukaemia, Nature 2018 2 TCGA Research Network, Genomic and Epigenomic Landscapes of Adult De Novo Acute Myeloid Leukemia, NEJM 2013; Expression and survival data from PanCancerAtlas portal on GDC: https://gdc.cancer.gov/about-data/publications/pancanatlas 3 McKeown et al., Superenhancer Analysis Defines Novel Epigenomic Subtypes of Non-APL AML, Including an RARa Dependency Targetable by SY-1425, a Potent and Selective RARa Agonist, Cancer Discovery 2017 Analysis of TCGA RARA expression and survival2 Independent analyses of BeatAML1, TCGA2, and AML patient sample analyses3 show that prognosis is similar regardless of levels of RARa expression
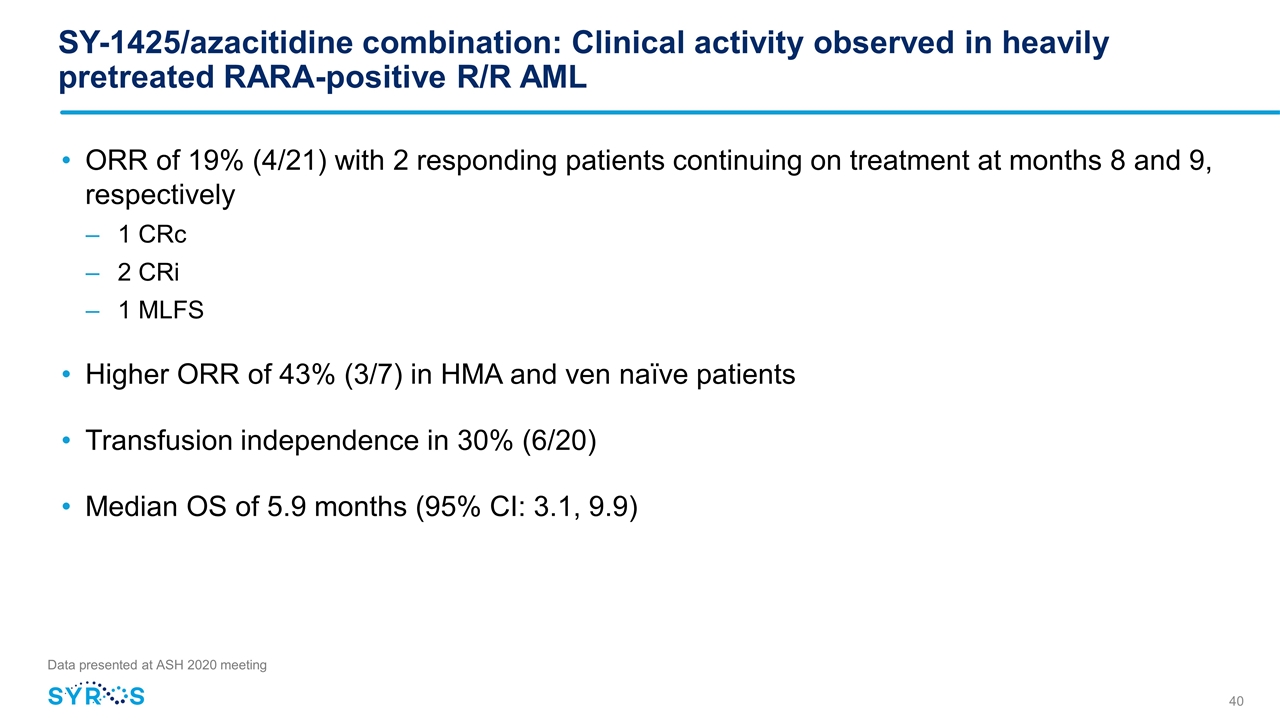
SY-1425/azacitidine combination: Clinical activity observed in heavily pretreated RARA-positive R/R AML ORR of 19% (4/21) with 2 responding patients continuing on treatment at months 8 and 9, respectively 1 CRc 2 CRi 1 MLFS Higher ORR of 43% (3/7) in HMA and ven naïve patients Transfusion independence in 30% (6/20) Median OS of 5.9 months (95% CI: 3.1, 9.9) Data presented at ASH 2020 meeting

SY-5609: Highly selective and potent oral CDK7 inhibitor 0.06 nM potency for CDK7 13,000- to 49,000-fold more selective for CDK7 over CDK2, CDK9 and CDK12 Only 4 of 485 kinases inhibited at ≥ 90% 100-91% Inhibition 90-80% Inhibition 79-71% Inhibition Induced apoptosis in cancer cells but not in non-cancer cells DMSO SY-5609 100nM SY-5609 250nM SY-5609 500nM Non-cancer cells Cancer cells %Annexin V/PI + 60 50 40 30 20 10 0 Data presented in October 2019 at EORTC-NCI-AACR Conference
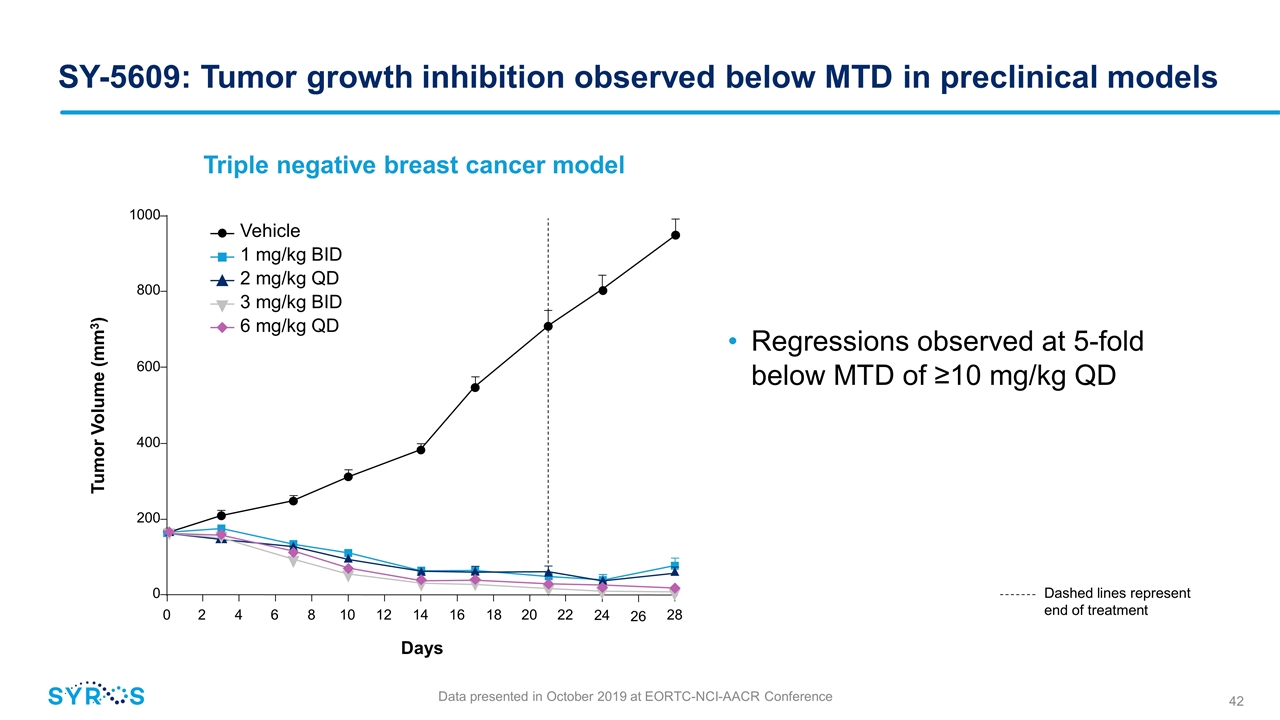
SY-5609: Tumor growth inhibition observed below MTD in preclinical models Regressions observed at 5-fold below MTD of ≥10 mg/kg QD Triple negative breast cancer model Dashed lines represent end of treatment Data presented in October 2019 at EORTC-NCI-AACR Conference Vehicle 1 mg/kg BID 2 mg/kg QD 3 mg/kg BID 6 mg/kg QD 0 2 4 6 8 10 12 14 16 18 20 22 Days 24 26 28 0 200 400 600 800 1000 Tumor Volume (mm3)
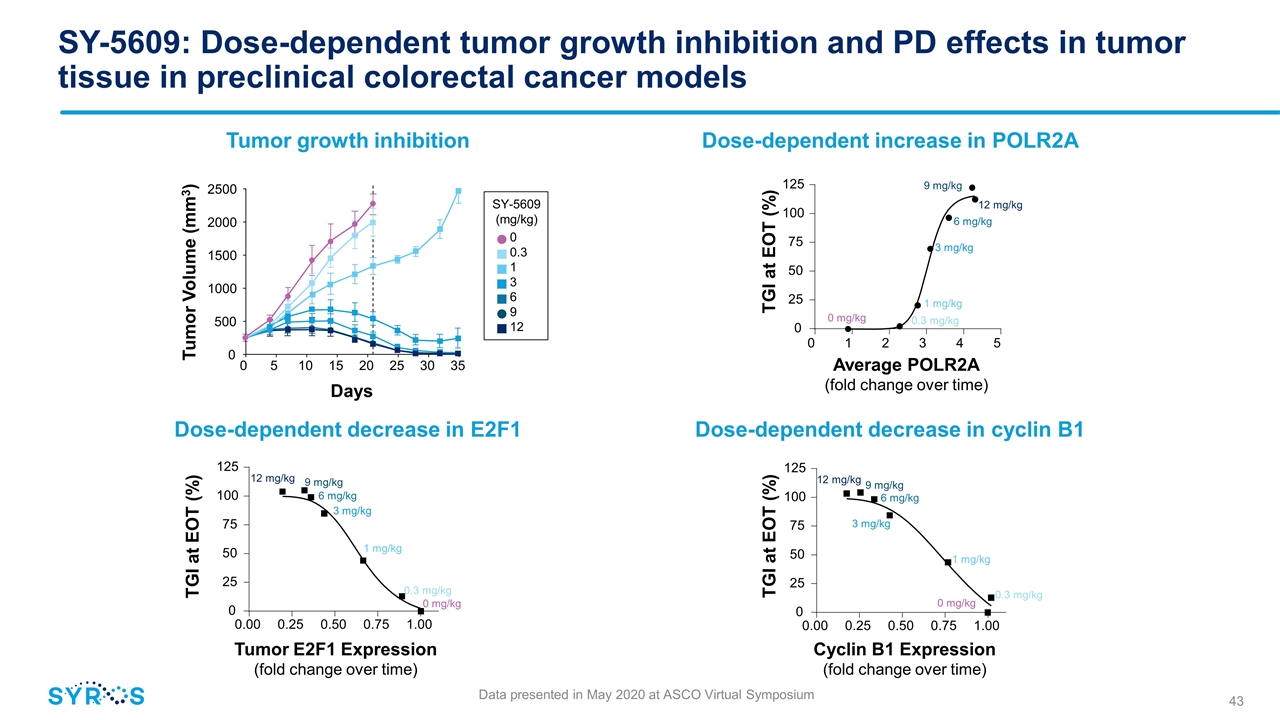
0 0.3 1 3 6 9 12 12 mg/kg SY-5609: Dose-dependent tumor growth inhibition and PD effects in tumor tissue in preclinical colorectal cancer models Dose-dependent increase in POLR2A Tumor growth inhibition Dose-dependent decrease in E2F1 Data presented in May 2020 at ASCO Virtual Symposium Dose-dependent decrease in cyclin B1 0 5 10 15 20 25 30 35 0 500 1000 1500 2000 2500 Days Tumor Volume (mm3) SY-5609 (mg/kg) 0 . 0 0 0 . 2 5 0 . 5 0 0 . 7 5 1 . 0 0 0 2 5 5 0 7 5 100 125 0 . 0 0 0 . 2 5 0 . 5 0 0 . 7 5 1 . 0 0 0 2 5 5 0 7 5 100 125 TGI at EOT (%) Tumor E2F1 Expression (fold change over time) 9 mg/kg 6 mg/kg 3 mg/kg 1 mg/kg 0.3 mg/kg 0 mg/kg TGI at EOT (%) Cyclin B1 Expression (fold change over time) 12 mg/kg 9 mg/kg 6 mg/kg 3 mg/kg 1 mg/kg 0.3 mg/kg 0 mg/kg 0 2 5 5 0 7 5 100 125 0 1 2 3 4 5 12 mg/kg 9 mg/kg 6 mg/kg 3 mg/kg 1 mg/kg 0.3 mg/kg 0 mg/kg TGI at EOT (%) Average POLR2A (fold change over time)
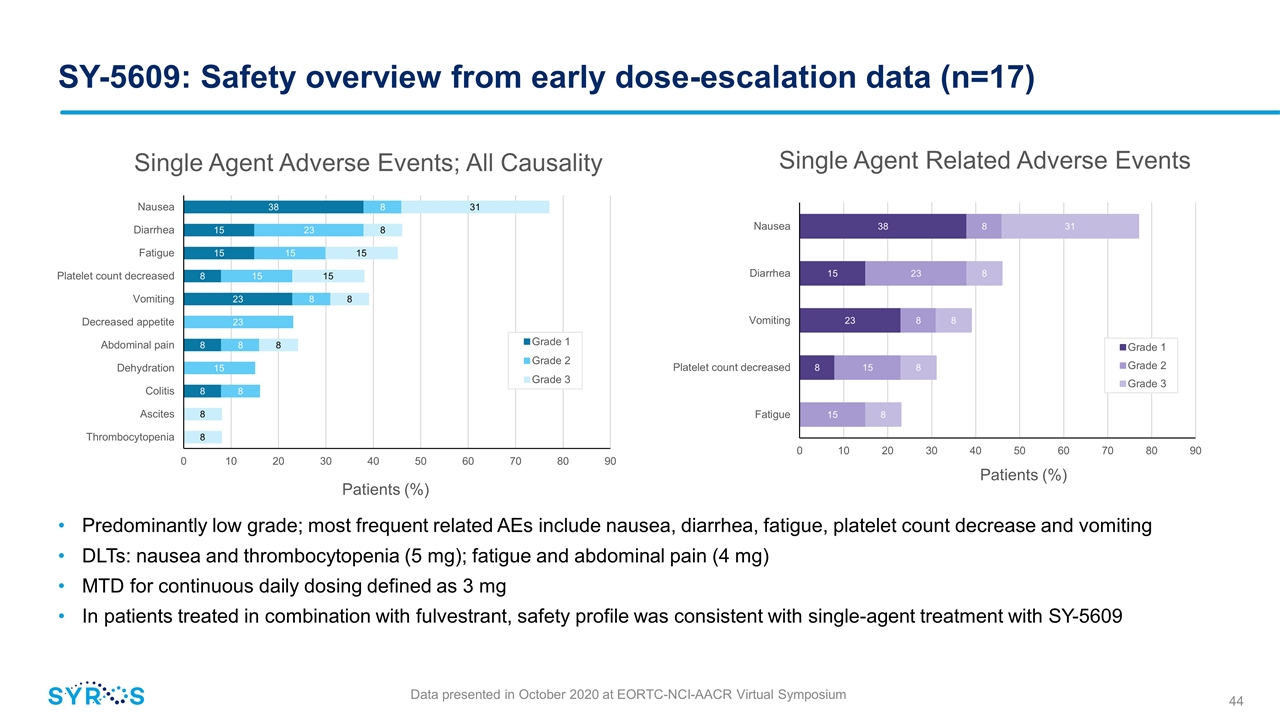
SY-5609: Safety overview from early dose-escalation data (n=17) Single Agent Adverse Events; All Causality Patients (%) Single Agent Related Adverse Events Patients (%) Predominantly low grade; most frequent related AEs include nausea, diarrhea, fatigue, platelet count decrease and vomiting DLTs: nausea and thrombocytopenia (5 mg); fatigue and abdominal pain (4 mg) MTD for continuous daily dosing defined as 3 mg In patients treated in combination with fulvestrant, safety profile was consistent with single-agent treatment with SY-5609 Data presented in October 2020 at EORTC-NCI-AACR Virtual Symposium
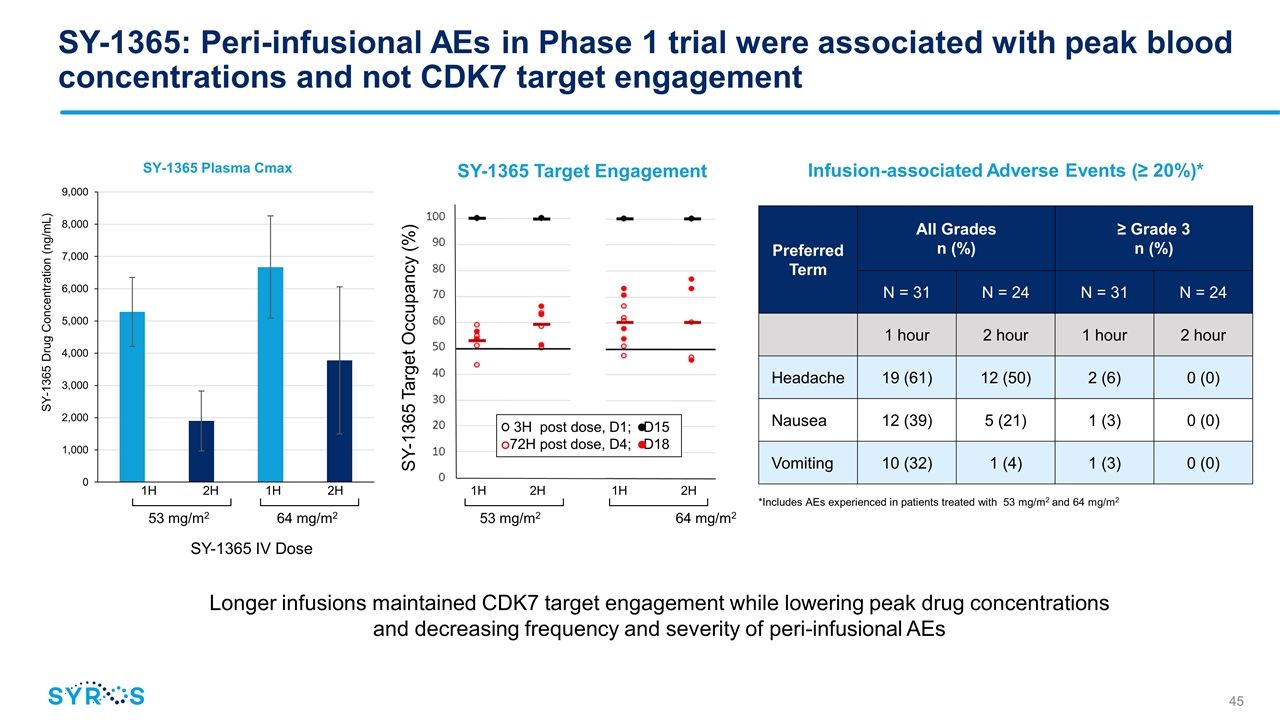
1H2H1H 2H 53 mg/m2 64 mg/m2 SY-1365: Peri-infusional AEs in Phase 1 trial were associated with peak blood concentrations and not CDK7 target engagement Longer infusions maintained CDK7 target engagement while lowering peak drug concentrations and decreasing frequency and severity of peri-infusional AEs 1H2H1H2H 53 mg/m2 64 mg/m2 SY-1365 Target Engagement Preferred Term All Grades n (%) ≥ Grade 3 n (%) N = 31 N = 24 N = 31 N = 24 1 hour 2 hour 1 hour 2 hour Headache 19 (61) 12 (50) 2 (6) 0 (0) Nausea 12 (39) 5 (21) 1 (3) 0 (0) Vomiting 10 (32) 1 (4) 1 (3) 0 (0) Infusion-associated Adverse Events (≥ 20%)* *Includes AEs experienced in patients treated with 53 mg/m2 and 64 mg/m2 SY-1365 IV Dose 3H post dose, D1; D15 72H post dose, D4; D18 SY-1365 Target Occupancy (%)
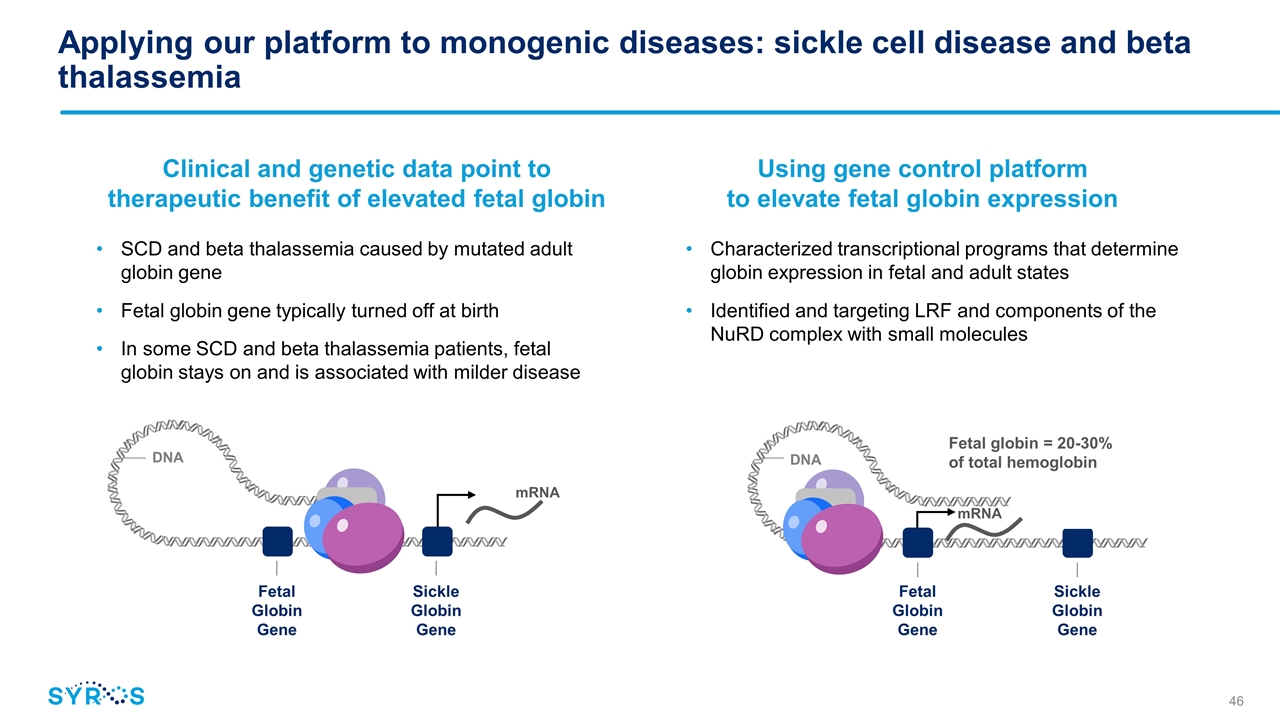
Applying our platform to monogenic diseases: sickle cell disease and beta thalassemia SCD and beta thalassemia caused by mutated adult globin gene Fetal globin gene typically turned off at birth In some SCD and beta thalassemia patients, fetal globin stays on and is associated with milder disease Clinical and genetic data point to therapeutic benefit of elevated fetal globin Using gene control platform to elevate fetal globin expression mRNA Sickle Globin Gene DNA mRNA Fetal Globin Gene Sickle Globin Gene DNA Fetal Globin Gene Characterized transcriptional programs that determine globin expression in fetal and adult states Identified and targeting LRF and components of the NuRD complex with small molecules Fetal globin = 20-30% of total hemoglobin

SY-2101 transaction overview and $90.5 million strategic financing Asset acquisition Upfront cash payment of $12 million Additional regulatory milestone of $6 million in APL indication Aggregate sales milestones of up to $10 million Strategic financing Completed strategic financing yielding $90.5 million in gross proceeds Led by Bain Capital Life Sciences with participation from additional new and existing investors