
An Expression Makes a World of Difference January 2022 Exhibit 99.1

Forward-looking statements This presentation contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995 that involve substantial risks and uncertainties. All statements, other than statements of historical facts, contained in this presentation, including statements regarding our strategy, research and clinical development plans, collaborations, future operations, future financial position, future revenues, projected costs, prospects, plans and objectives of management, are forward-looking statements. The words “anticipate,” “believe,” “estimate,” “expect,” “intend,” “may,” “plan,” “predict,” “project,” “target,” “potential,” “will,” “would,” “could,” “should,” “continue,” and similar expressions are intended to identify forward-looking statements, although not all forward-looking statements contain these identifying words. We may not actually achieve the plans, intentions or expectations disclosed in our forward-looking statements, and you should not place undue reliance on our forward-looking statements. Actual results or events could differ materially from the plans, intentions and expectations disclosed in these forward-looking statements as a result of various important factors, including our ability to: advance the development of our programs, including tamibarotene, SY-2101 and SY-5609, under the timelines we project in current and future clinical trials; demonstrate in any current and future clinical trials the requisite safety, efficacy and combinability of our drug candidates; replicate scientific and non-clinical data in clinical trials; successfully develop a companion diagnostic test to identify patients with the RARA biomarker; obtain and maintain patent protection for our drug candidates and the freedom to operate under third party intellectual property; obtain and maintain necessary regulatory approvals; identify, enter into and maintain collaboration agreements with third parties, including our ability to perform under our collaboration agreements with Incyte Corporation and Global Blood Therapeutics; manage competition; manage expenses; raise the substantial additional capital needed to achieve our business objectives; attract and retain qualified personnel; and successfully execute on our business strategies; risks described under the caption “Risk Factors” in our Annual Report on Form 10-K for the year ended December 31, 2020 and our Quarterly Report on Form 10-Q for the quarter ended September 30, 2021, each of which is on file with the Securities and Exchange Commission (SEC); and risks described in other filings that we may make with the SEC in the future. In addition, the extent to which the COVID-19 outbreak continues to impact our workforce and our discovery research, supply chain and clinical trial operations activities, and the operations of the third parties on which we rely, will depend on future developments, which are highly uncertain and cannot be predicted with confidence, including the duration and severity of the outbreak, additional or modified government actions, and the actions that may be required to contain the virus or treat its impact. Any forward-looking statements contained in this presentation speak only as of the date this presentation is made, and we expressly disclaim any obligation to update any forward-looking statements, whether because of new information, future events or otherwise. All third-party trademarks used in this presentation are the property of their respective owners.
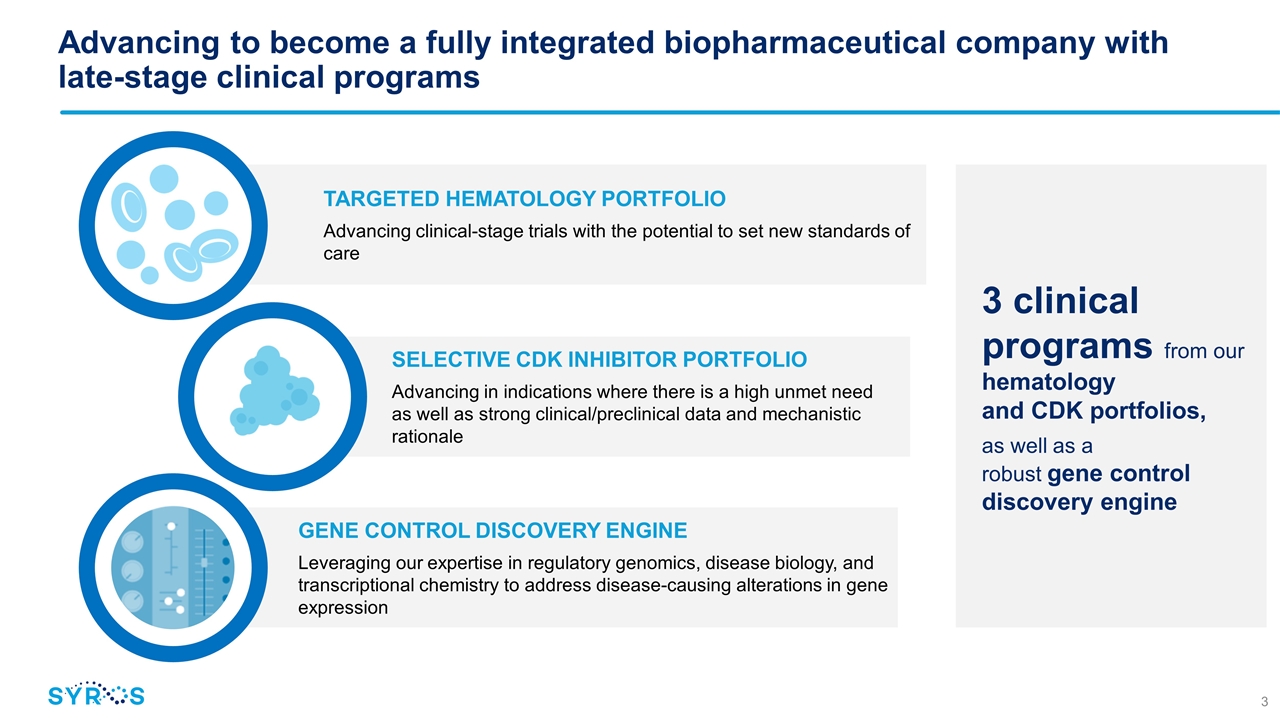
SELECTIVE CDK INHIBITOR PORTFOLIO Advancing in indications where there is a high unmet need as well as strong clinical/preclinical data and mechanistic rationale 3 clinical programs from our hematology and CDK portfolios, as well as a robust gene control discovery engine TARGETED HEMATOLOGY PORTFOLIO Advancing clinical-stage trials with the potential to set new standards of care GENE CONTROL DISCOVERY ENGINE Leveraging our expertise in regulatory genomics, disease biology, and transcriptional chemistry to address disease-causing alterations in gene expression Advancing to become a fully integrated biopharmaceutical company with late-stage clinical programs
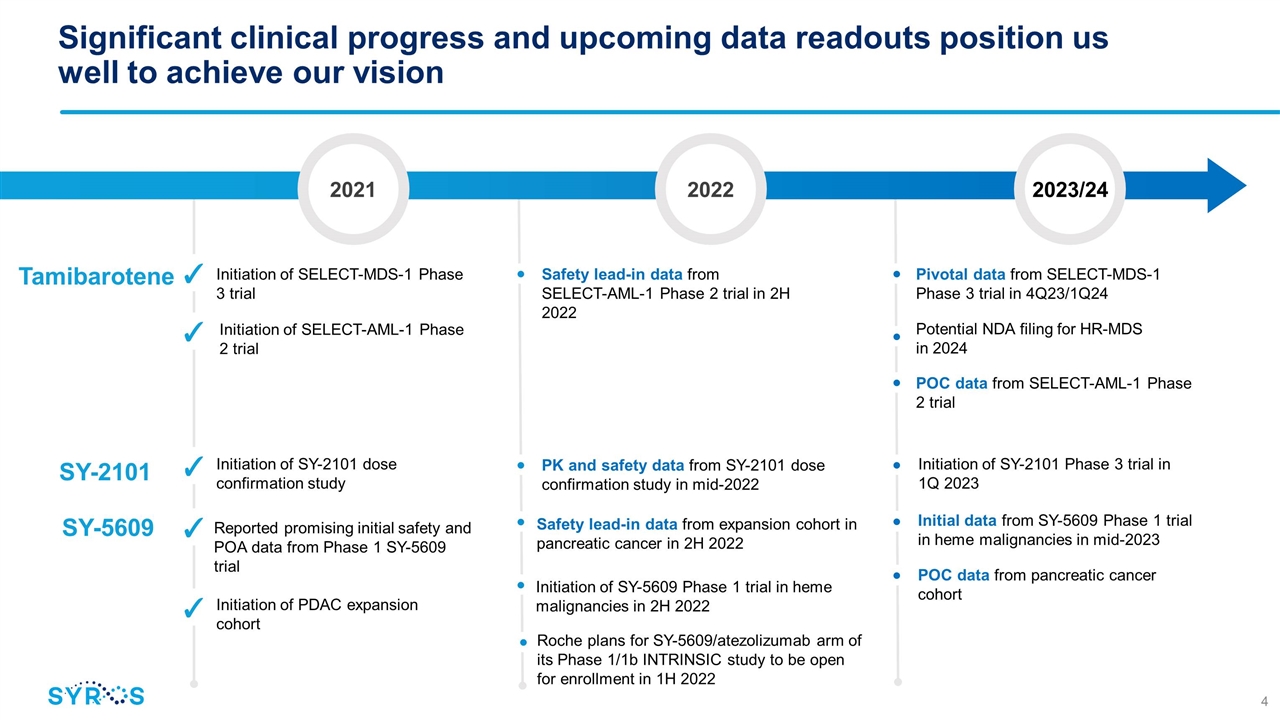
SY-5609 Significant clinical progress and upcoming data readouts position us well to achieve our vision 2021 2022 2023/24 ✓ Initiation of SY-2101 dose confirmation study • • PK and safety data from SY-2101 dose confirmation study in mid-2022 Initiation of SY-2101 Phase 3 trial in 1Q 2023 SY-2101 ✓ Initiation of SELECT-AML-1 Phase 2 trial • Pivotal data from SELECT-MDS-1 Phase 3 trial in 4Q23/1Q24 Potential NDA filing for HR-MDS in 2024 POC data from SELECT-AML-1 Phase 2 trial ✓ Initiation of SELECT-MDS-1 Phase 3 trial • • Safety lead-in data from SELECT-AML-1 Phase 2 trial in 2H 2022 Tamibarotene ✓ Reported promising initial safety and POA data from Phase 1 SY-5609 trial • • • Safety lead-in data from expansion cohort in pancreatic cancer in 2H 2022 Initiation of SY-5609 Phase 1 trial in heme malignancies in 2H 2022 Initial data from SY-5609 Phase 1 trial in heme malignancies in mid-2023 POC data from pancreatic cancer cohort • • ✓ Initiation of PDAC expansion cohort Roche plans for SY-5609/atezolizumab arm of its Phase 1/1b INTRINSIC study to be open for enrollment in 1H 2022 •
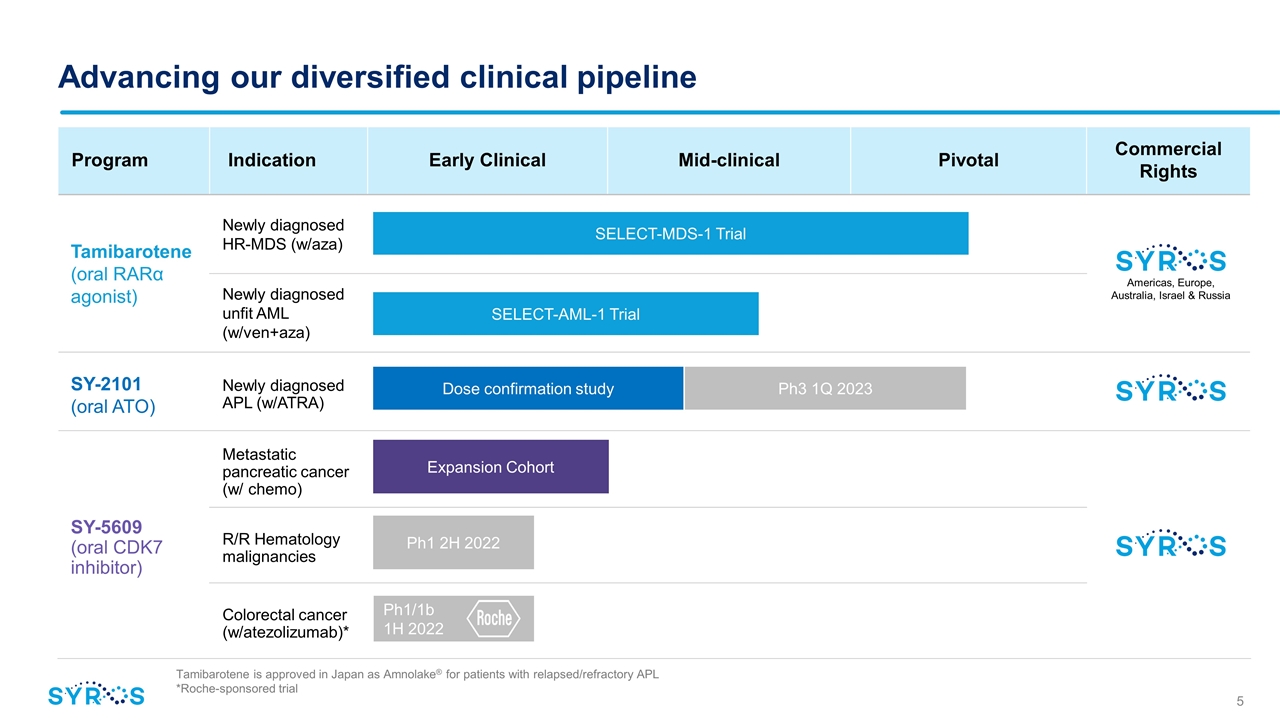
Program Indication Early Clinical IND- Enabling Mid-clinical Pivotal Pivotal Commercial Rights Tamibarotene (oral RARα agonist) Newly diagnosed HR-MDS (w/aza) Newly diagnosed unfit AML (w/ven+aza) SY-2101 (oral ATO) Newly diagnosed APL (w/ATRA) SY-5609 (oral CDK7 inhibitor) Metastatic pancreatic cancer (w/ chemo) R/R Hematology malignancies Colorectal cancer (w/atezolizumab)* Ph1/1b 1H 2022 Advancing our diversified clinical pipeline Tamibarotene is approved in Japan as Amnolake® for patients with relapsed/refractory APL *Roche-sponsored trial Americas, Europe, Australia, Israel & Russia SELECT-AML-1 Trial Ph3 1Q 2023 Dose confirmation study Expansion Cohort SELECT-MDS-1 Trial Ph1 2H 2022

Tamibarotene Selective oral RARa agonist
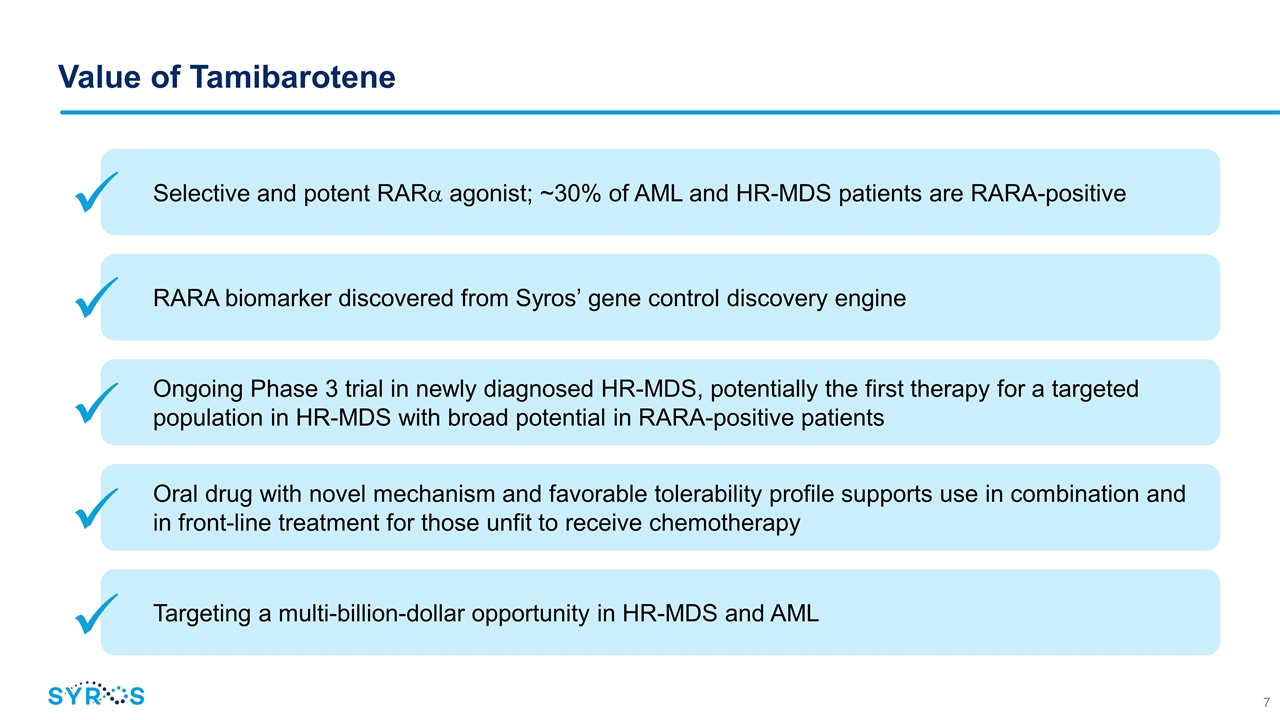
Value of Tamibarotene Selective and potent RARa agonist; ~30% of AML and HR-MDS patients are RARA-positive Ongoing Phase 3 trial in newly diagnosed HR-MDS, potentially the first therapy for a targeted population in HR-MDS with broad potential in RARA-positive patients Oral drug with novel mechanism and favorable tolerability profile supports use in combination and in front-line treatment for those unfit to receive chemotherapy RARA biomarker discovered from Syros’ gene control discovery engine Targeting a multi-billion-dollar opportunity in HR-MDS and AML
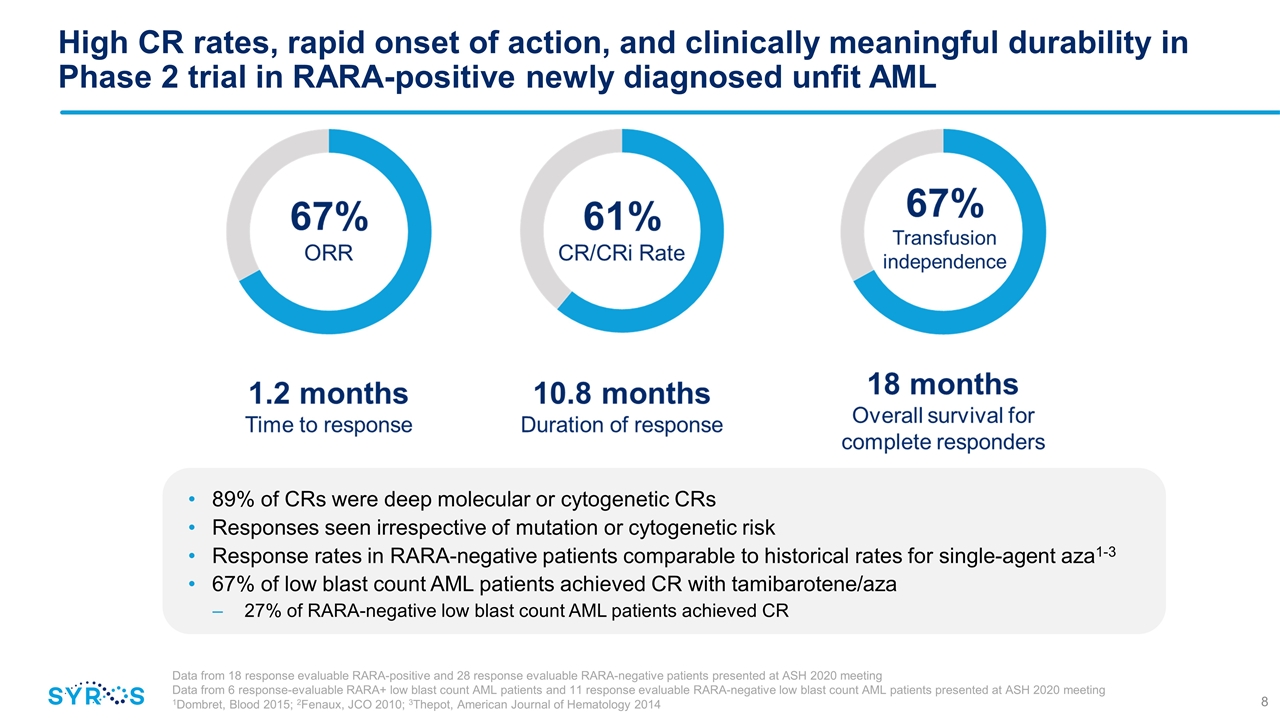
High CR rates, rapid onset of action, and clinically meaningful durability in Phase 2 trial in RARA-positive newly diagnosed unfit AML 89% of CRs were deep molecular or cytogenetic CRs Responses seen irrespective of mutation or cytogenetic risk Response rates in RARA-negative patients comparable to historical rates for single-agent aza1-3 67% of low blast count AML patients achieved CR with tamibarotene/aza 27% of RARA-negative low blast count AML patients achieved CR Data from 18 response evaluable RARA-positive and 28 response evaluable RARA-negative patients presented at ASH 2020 meeting Data from 6 response-evaluable RARA+ low blast count AML patients and 11 response evaluable RARA-negative low blast count AML patients presented at ASH 2020 meeting 1Dombret, Blood 2015; 2Fenaux, JCO 2010; 3Thepot, American Journal of Hematology 2014
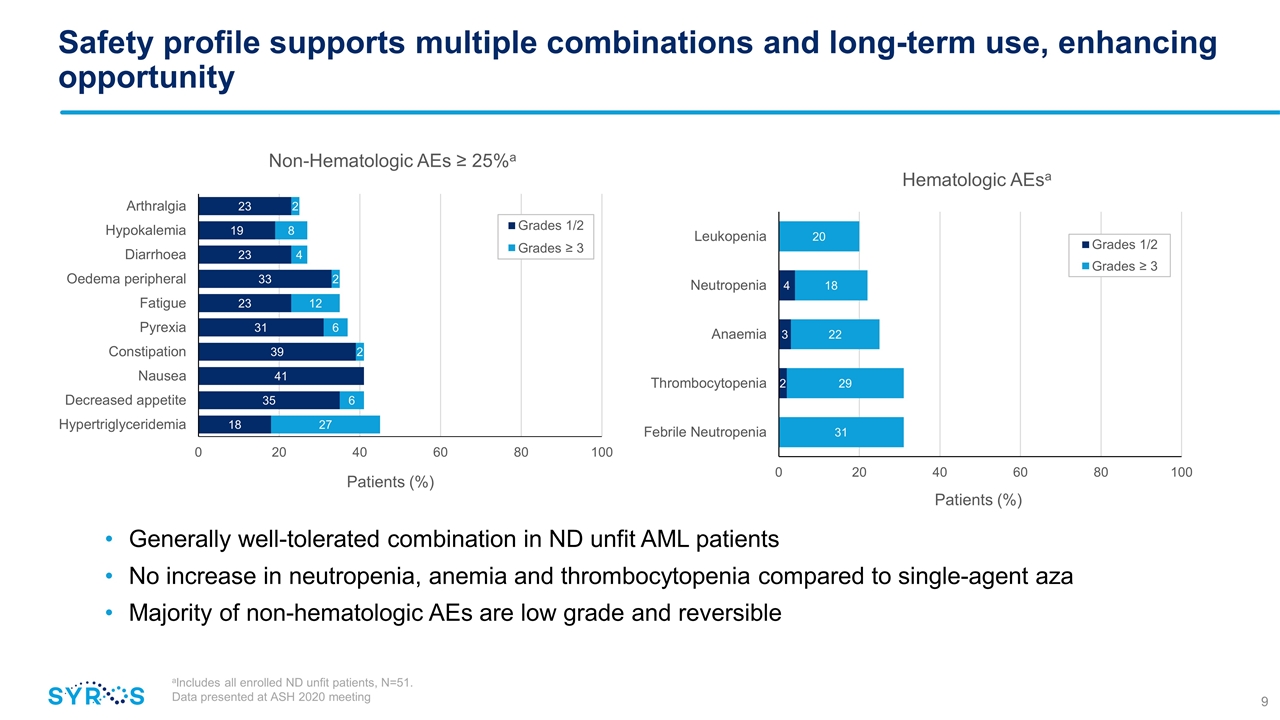
Safety profile supports multiple combinations and long-term use, enhancing opportunity Generally well-tolerated combination in ND unfit AML patients No increase in neutropenia, anemia and thrombocytopenia compared to single-agent aza Majority of non-hematologic AEs are low grade and reversible aIncludes all enrolled ND unfit patients, N=51. Data presented at ASH 2020 meeting
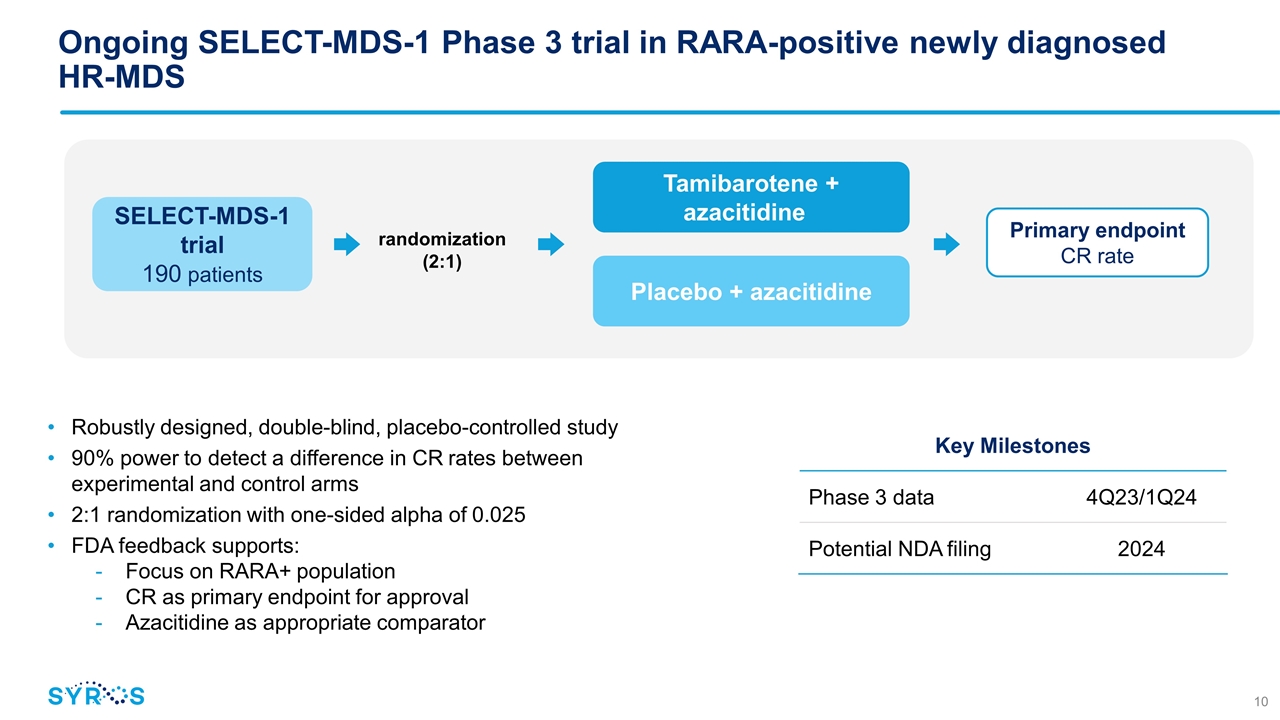
Ongoing SELECT-MDS-1 Phase 3 trial in RARA-positive newly diagnosed HR-MDS Robustly designed, double-blind, placebo-controlled study 90% power to detect a difference in CR rates between experimental and control arms 2:1 randomization with one-sided alpha of 0.025 FDA feedback supports: Focus on RARA+ population CR as primary endpoint for approval Azacitidine as appropriate comparator Key Milestones Phase 3 data 4Q23/1Q24 Potential NDA filing 2024 SELECT-MDS-1 trial 190 patients randomization (2:1) Tamibarotene + azacitidine Placebo + azacitidine Primary endpoint CR rate
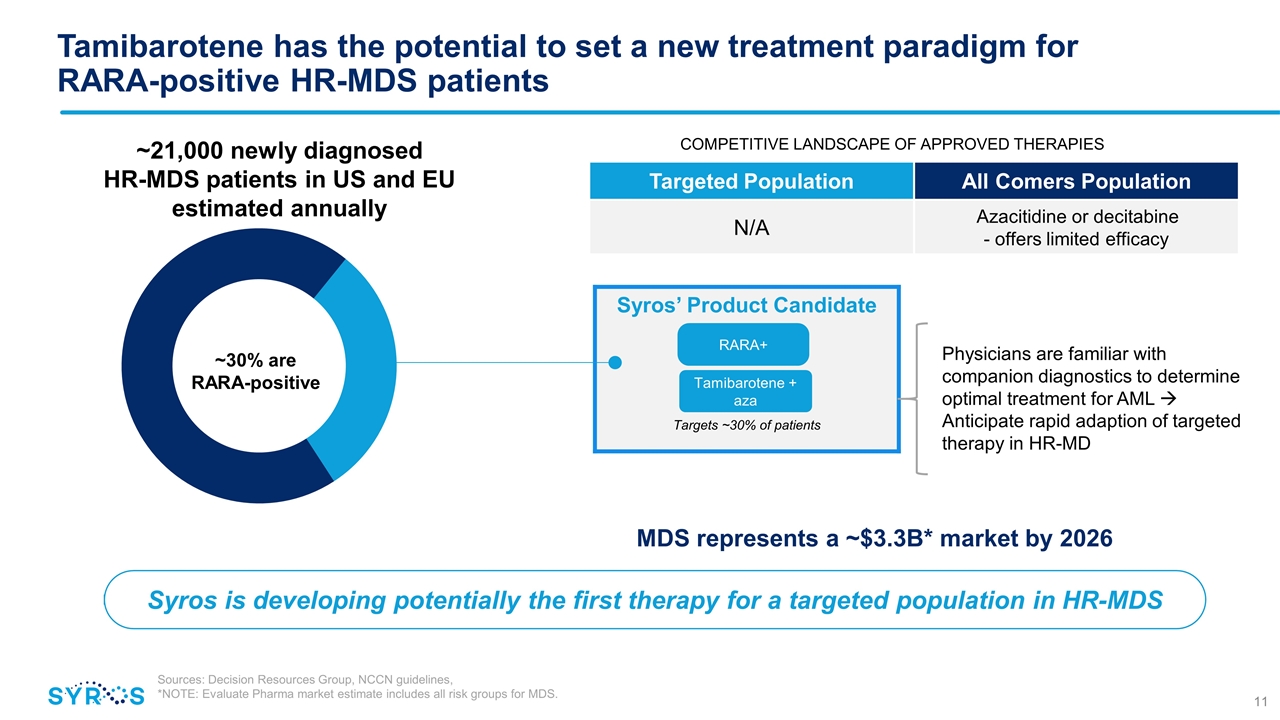
Syros’ Product Candidate Targets ~30% of patients Syros is developing potentially the first therapy for a targeted population in HR-MDS Tamibarotene has the potential to set a new treatment paradigm for RARA-positive HR-MDS patients Physicians are familiar with companion diagnostics to determine optimal treatment for AML à Anticipate rapid adaption of targeted therapy in HR-MD Targeted Population All Comers Population N/A Azacitidine or decitabine - offers limited efficacy COMPETITIVE LANDSCAPE OF APPROVED THERAPIES ~30% are RARA-positive ~21,000 newly diagnosed HR-MDS patients in US and EU estimated annually RARA+ Tamibarotene + aza Sources: Decision Resources Group, NCCN guidelines, *NOTE: Evaluate Pharma market estimate includes all risk groups for MDS. MDS represents a ~$3.3B* market by 2026
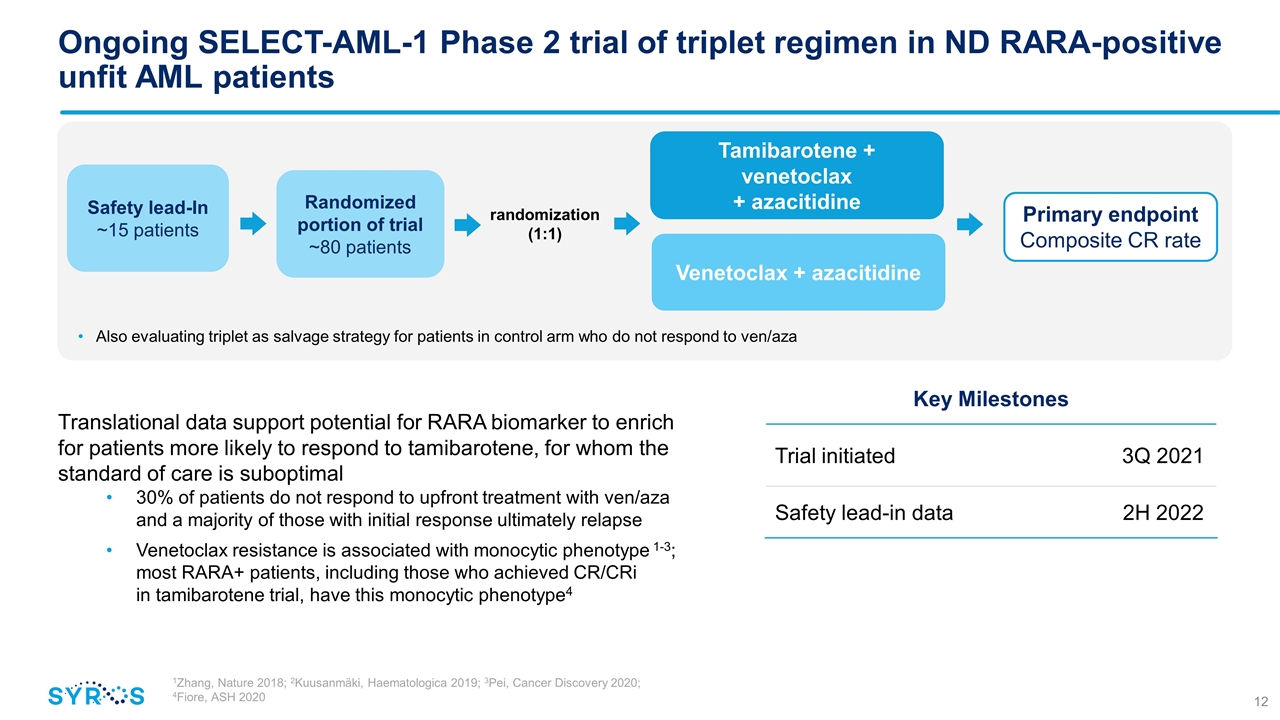
Randomized portion of trial ~80 patients Tamibarotene + venetoclax + azacitidine Venetoclax + azacitidine randomization (1:1) Safety lead-In ~15 patients Ongoing SELECT-AML-1 Phase 2 trial of triplet regimen in ND RARA-positive unfit AML patients Also evaluating triplet as salvage strategy for patients in control arm who do not respond to ven/aza Key Milestones Trial initiated 3Q 2021 Safety lead-in data 2H 2022 Primary endpoint Composite CR rate Translational data support potential for RARA biomarker to enrich for patients more likely to respond to tamibarotene, for whom the standard of care is suboptimal 30% of patients do not respond to upfront treatment with ven/aza and a majority of those with initial response ultimately relapse Venetoclax resistance is associated with monocytic phenotype 1-3; most RARA+ patients, including those who achieved CR/CRi in tamibarotene trial, have this monocytic phenotype4 1Zhang, Nature 2018; 2Kuusanmäki, Haematologica 2019; 3Pei, Cancer Discovery 2020; 4Fiore, ASH 2020
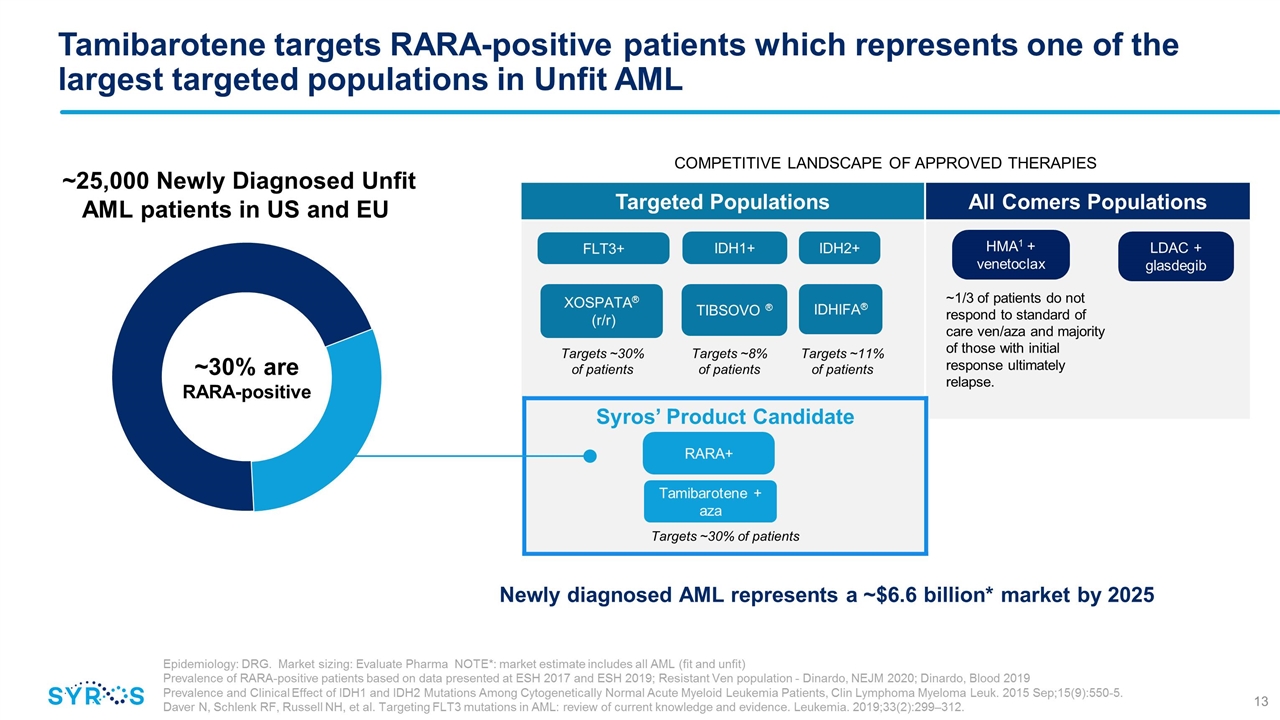
Tamibarotene targets RARA-positive patients which represents one of the largest targeted populations in Unfit AML Targeted Populations All Comers Populations ~30% are RARA-positive LDAC + glasdegib HMA1 + venetoclax XOSPATA® (r/r) FLT3+ TIBSOVO ® IDH1+ IDHIFA® IDH2+ Targets ~8% of patients Targets ~11% of patients Targets ~30% of patients ~1/3 of patients do not respond to standard of care ven/aza and majority of those with initial response ultimately relapse. ~25,000 Newly Diagnosed Unfit AML patients in US and EU Epidemiology: DRG. Market sizing: Evaluate Pharma NOTE*: market estimate includes all AML (fit and unfit) Prevalence of RARA-positive patients based on data presented at ESH 2017 and ESH 2019; Resistant Ven population - Dinardo, NEJM 2020; Dinardo, Blood 2019 Prevalence and Clinical Effect of IDH1 and IDH2 Mutations Among Cytogenetically Normal Acute Myeloid Leukemia Patients, Clin Lymphoma Myeloma Leuk. 2015 Sep;15(9):550-5. Daver N, Schlenk RF, Russell NH, et al. Targeting FLT3 mutations in AML: review of current knowledge and evidence. Leukemia. 2019;33(2):299–312. COMPETITIVE LANDSCAPE OF APPROVED THERAPIES Syros’ Product Candidate Targets ~30% of patients RARA+ Tamibarotene + aza Newly diagnosed AML represents a ~$6.6 billion* market by 2025

SY-2101 Novel oral form of arsenic trioxide
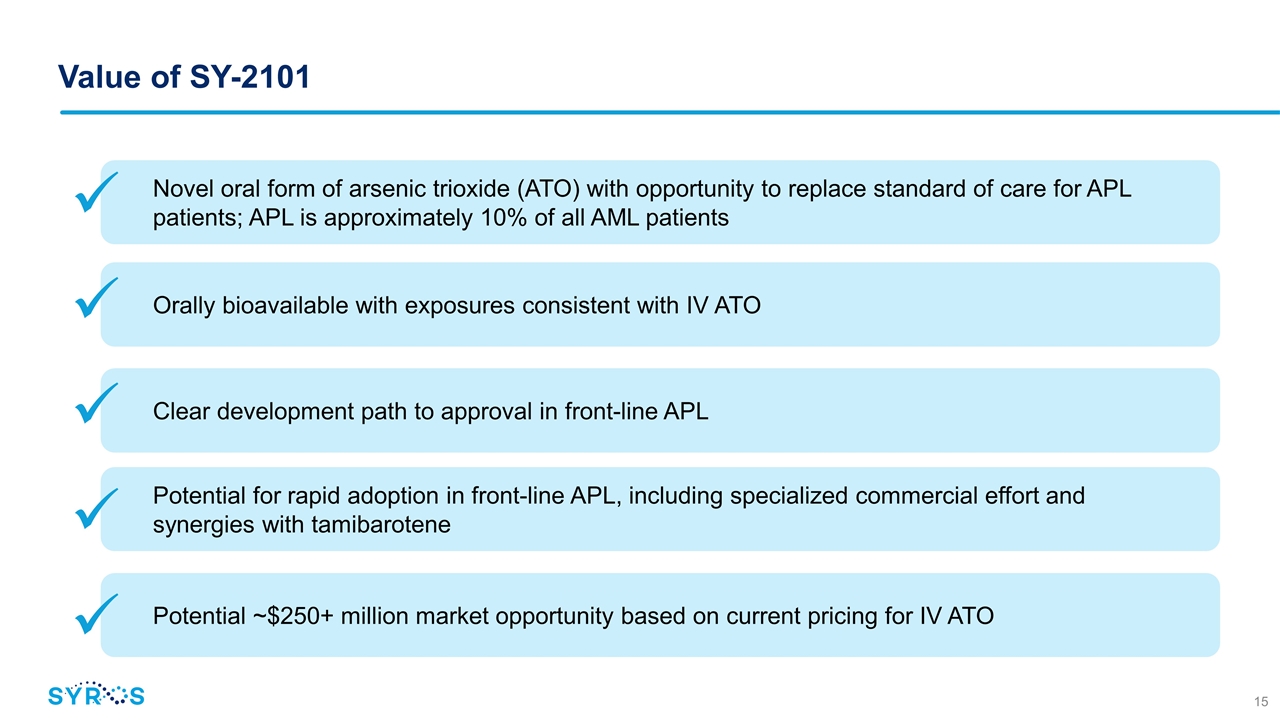
Novel oral form of arsenic trioxide (ATO) with opportunity to replace standard of care for APL patients; APL is approximately 10% of all AML patients Orally bioavailable with exposures consistent with IV ATO Clear development path to approval in front-line APL Potential for rapid adoption in front-line APL, including specialized commercial effort and synergies with tamibarotene Potential ~$250+ million market opportunity based on current pricing for IV ATO Value of SY-2101
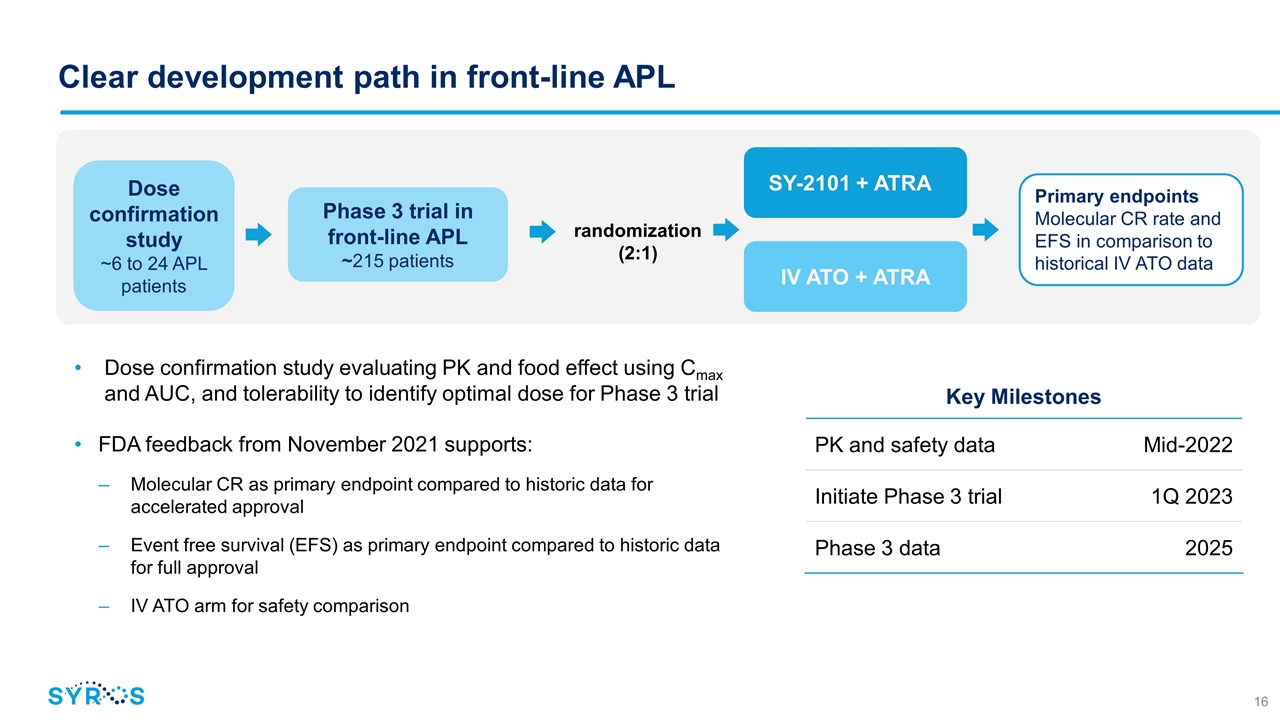
Clear development path in front-line APL Dose confirmation study evaluating PK and food effect using Cmax and AUC, and tolerability to identify optimal dose for Phase 3 trial FDA feedback from November 2021 supports: Molecular CR as primary endpoint compared to historic data for accelerated approval Event free survival (EFS) as primary endpoint compared to historic data for full approval IV ATO arm for safety comparison Phase 3 trial in front-line APL ~215 patients randomization (2:1) SY-2101 + ATRA IV ATO + ATRA Primary endpoints Molecular CR rate and EFS in comparison to historical IV ATO data Dose confirmation study ~6 to 24 APL patients Key Milestones PK and safety data Mid-2022 Initiate Phase 3 trial 1Q 2023 Phase 3 data 2025
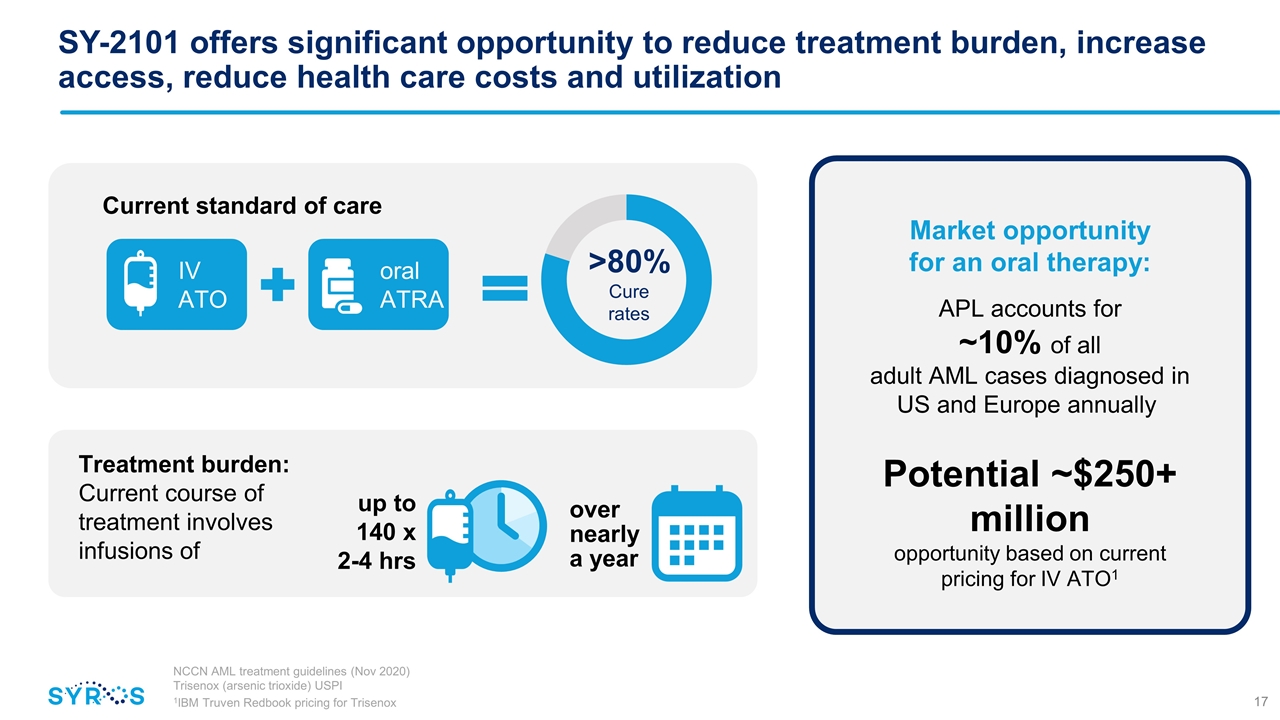
SY-2101 offers significant opportunity to reduce treatment burden, increase access, reduce health care costs and utilization Current standard of care IV ATO oral ATRA >80% Cure rates Treatment burden: Current course of treatment involves infusions of Market opportunity for an oral therapy: APL accounts for ~10% of all adult AML cases diagnosed in US and Europe annually Potential ~$250+ million opportunity based on current pricing for IV ATO1 NCCN AML treatment guidelines (Nov 2020) Trisenox (arsenic trioxide) USPI 1IBM Truven Redbook pricing for Trisenox up to 140 x 2-4 hrs over nearly a year

SY-5609 Highly selective and potent oral CDK7 inhibitor
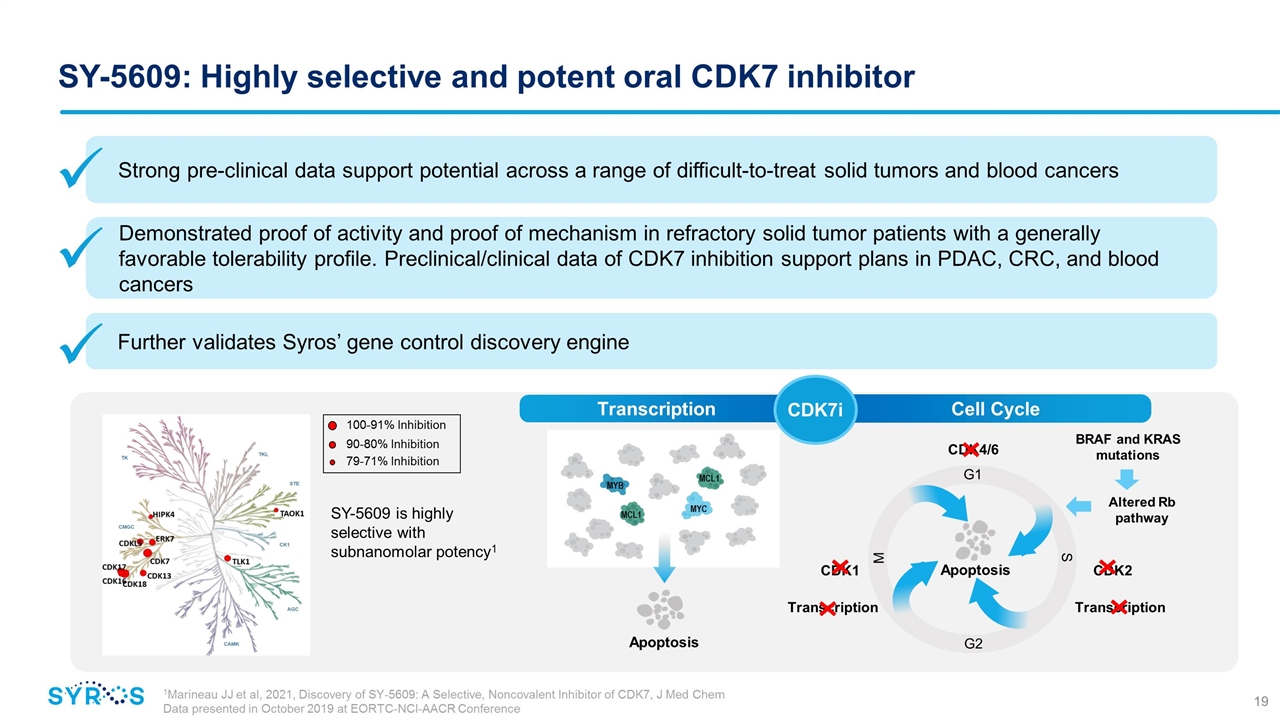
Cell Cycle SY-5609: Highly selective and potent oral CDK7 inhibitor SY-5609 is highly selective with subnanomolar potency1 1Marineau JJ et al, 2021, Discovery of SY-5609: A Selective, Noncovalent Inhibitor of CDK7, J Med Chem Data presented in October 2019 at EORTC-NCI-AACR Conference Transcription CDK7i MYC MYB MCL1 MCL1 Apoptosis Altered Rb pathway BRAF and KRAS mutations CDK1 CDK2 Transcription Transcription CDK4/6 M G2 S G1 Apoptosis Strong pre-clinical data support potential across a range of difficult-to-treat solid tumors and blood cancers Demonstrated proof of activity and proof of mechanism in refractory solid tumor patients with a generally favorable tolerability profile. Preclinical/clinical data of CDK7 inhibition support plans in PDAC, CRC, and blood cancers Further validates Syros’ gene control discovery engine 100-91% Inhibition 90-80% Inhibition 79-71% Inhibition
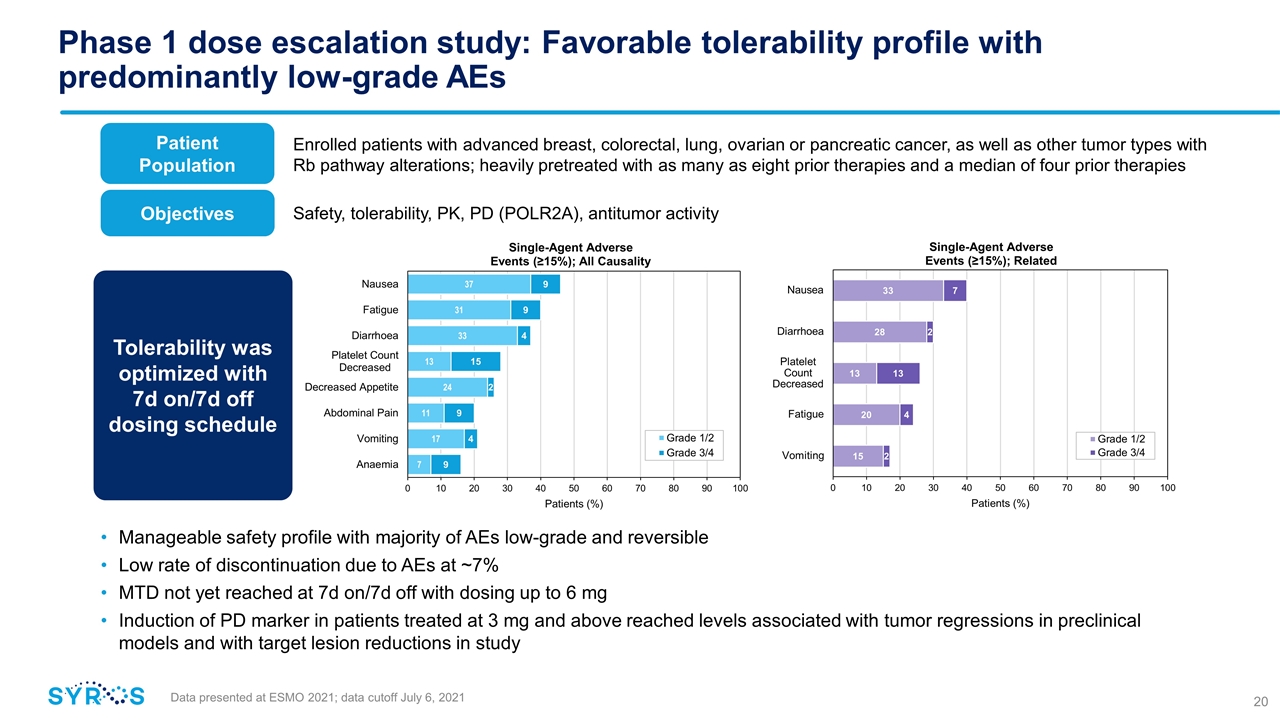
Tolerability was optimized with 7d on/7d off dosing schedule Phase 1 dose escalation study: Favorable tolerability profile with predominantly low-grade AEs Data presented at ESMO 2021; data cutoff July 6, 2021 Manageable safety profile with majority of AEs low-grade and reversible Low rate of discontinuation due to AEs at ~7% MTD not yet reached at 7d on/7d off with dosing up to 6 mg Induction of PD marker in patients treated at 3 mg and above reached levels associated with tumor regressions in preclinical models and with target lesion reductions in study Patient Population Objectives Enrolled patients with advanced breast, colorectal, lung, ovarian or pancreatic cancer, as well as other tumor types with Rb pathway alterations; heavily pretreated with as many as eight prior therapies and a median of four prior therapies Safety, tolerability, PK, PD (POLR2A), antitumor activity
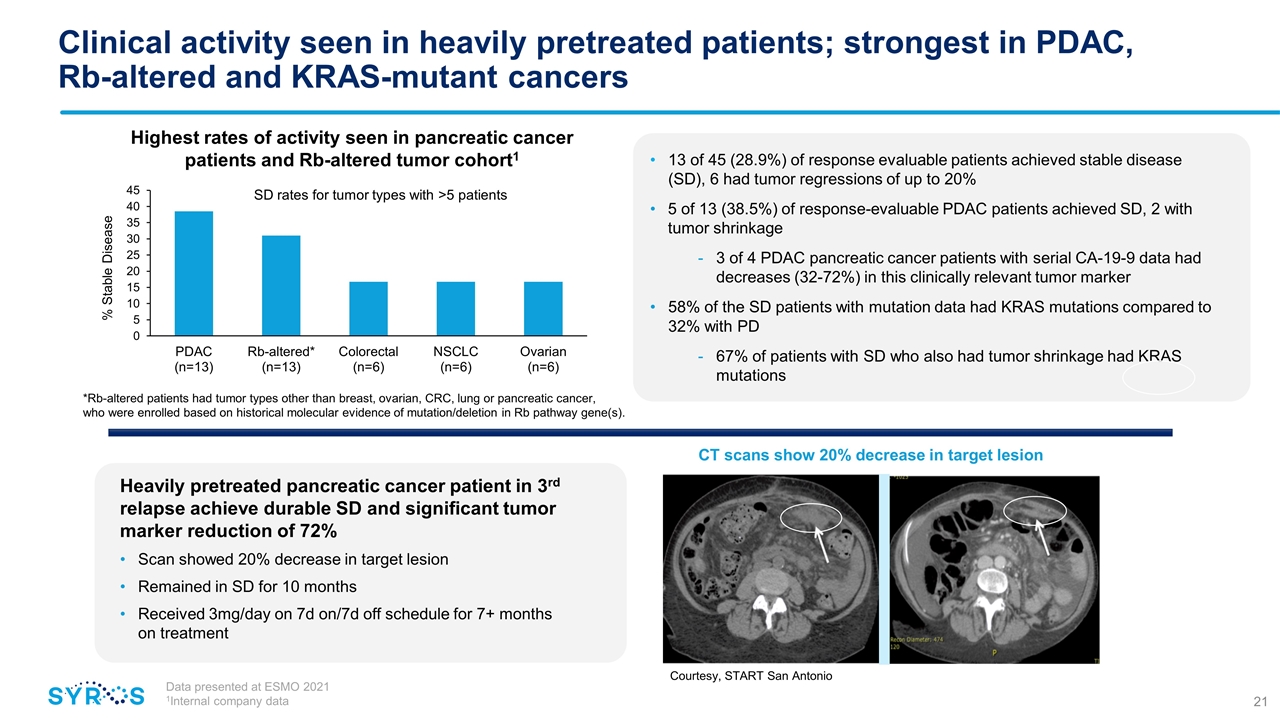
Clinical activity seen in heavily pretreated patients; strongest in PDAC, Rb-altered and KRAS-mutant cancers Data presented at ESMO 2021 1Internal company data Highest rates of activity seen in pancreatic cancer patients and Rb-altered tumor cohort1 *Rb-altered patients had tumor types other than breast, ovarian, CRC, lung or pancreatic cancer, who were enrolled based on historical molecular evidence of mutation/deletion in Rb pathway gene(s). 13 of 45 (28.9%) of response evaluable patients achieved stable disease (SD), 6 had tumor regressions of up to 20% 5 of 13 (38.5%) of response-evaluable PDAC patients achieved SD, 2 with tumor shrinkage 3 of 4 PDAC pancreatic cancer patients with serial CA-19-9 data had decreases (32-72%) in this clinically relevant tumor marker 58% of the SD patients with mutation data had KRAS mutations compared to 32% with PD 67% of patients with SD who also had tumor shrinkage had KRAS mutations Heavily pretreated pancreatic cancer patient in 3rd relapse achieve durable SD and significant tumor marker reduction of 72% Scan showed 20% decrease in target lesion Remained in SD for 10 months Received 3mg/day on 7d on/7d off schedule for 7+ months on treatment Courtesy, Dr. K. Papadopoulos Courtesy, START San Antonio CT scans show 20% decrease in target lesion
Exploring SY-5609 in three distinct approaches based on mechanistic rationale, preclinical data and clinical signals Pancreatic Cancer KRAS mutations are ubiquitous and powerful activators of cell signaling and transcriptional programs Compelling preclinical data and synergy with gemcitabine Single agent SY-5609 showed: Clinical activity in relapsed refractory pancreatic cancer and Rb-altered tumors KRAS mutations associated with clinical activity BRAF mutations, present in 10% of colorectal cancer patients, are powerful activators of cell signaling and transcriptional programs Compelling preclinical data as single agent CDK7 inhibition enhances anti-tumor activity of immunotherapy in preclinical models BRAF-mutant Colorectal Cancer Genetics of heme malignancies point to multiple oncogenic transcriptional, epigenetic, and cell cycle control drivers CDK7 inhibitors demonstrate robust preclinical activity as a single agent and in combination, supporting the potential in a broad range of blood cancers First clinical investigation of CDK7 inhibitor in hematologic malignancies Hematologic Malignancies
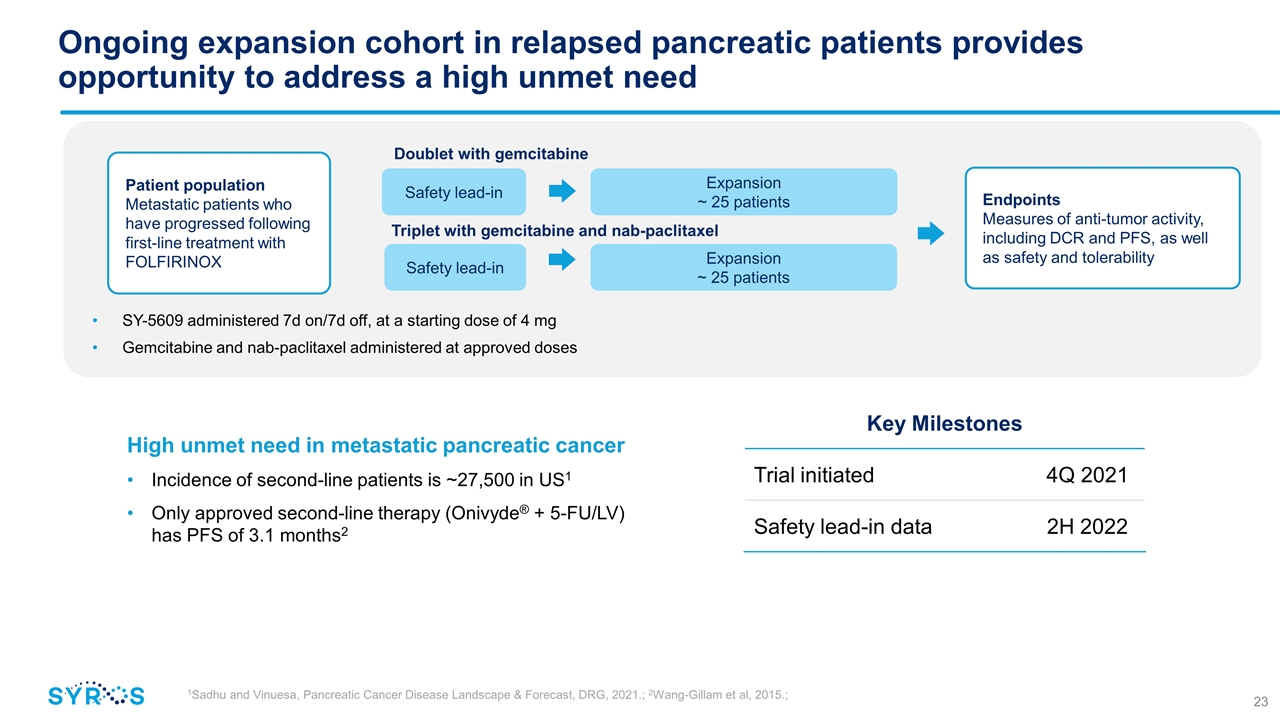
Ongoing expansion cohort in relapsed pancreatic patients provides opportunity to address a high unmet need Expansion ~ 25 patients Endpoints Measures of anti-tumor activity, including DCR and PFS, as well as safety and tolerability Safety lead-in Doublet with gemcitabine Triplet with gemcitabine and nab-paclitaxel Safety lead-in Expansion ~ 25 patients 1Sadhu and Vinuesa, Pancreatic Cancer Disease Landscape & Forecast, DRG, 2021.; 2Wang-Gillam et al, 2015.; High unmet need in metastatic pancreatic cancer Incidence of second-line patients is ~27,500 in US1 Only approved second-line therapy (Onivyde® + 5-FU/LV) has PFS of 3.1 months2 Key Milestones Trial initiated 4Q 2021 Safety lead-in data 2H 2022 SY-5609 administered 7d on/7d off, at a starting dose of 4 mg Gemcitabine and nab-paclitaxel administered at approved doses Patient population Metastatic patients who have progressed following first-line treatment with FOLFIRINOX
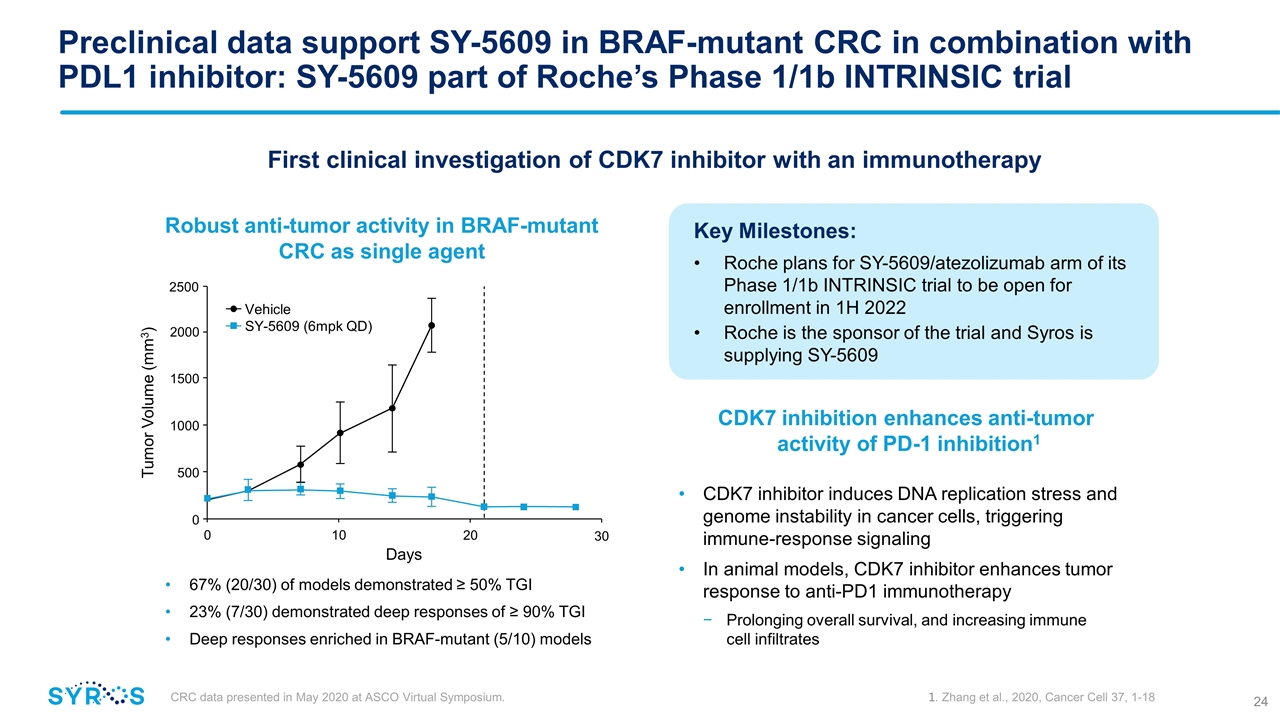
Preclinical data support SY-5609 in BRAF-mutant CRC in combination with PDL1 inhibitor: SY-5609 part of Roche’s Phase 1/1b INTRINSIC trial 67% (20/30) of models demonstrated ≥ 50% TGI 23% (7/30) demonstrated deep responses of ≥ 90% TGI Deep responses enriched in BRAF-mutant (5/10) models Robust anti-tumor activity in BRAF-mutant CRC as single agent 0 10 Days 20 0 500 1000 1500 2000 Tumor Volume (mm3) 2500 30 Vehicle SY-5609 (6mpk QD) CDK7 inhibition enhances anti-tumor activity of PD-1 inhibition1 CDK7 inhibitor induces DNA replication stress and genome instability in cancer cells, triggering immune-response signaling In animal models, CDK7 inhibitor enhances tumor response to anti-PD1 immunotherapy Prolonging overall survival, and increasing immune cell infiltrates CRC data presented in May 2020 at ASCO Virtual Symposium. 1. Zhang et al., 2020, Cancer Cell 37, 1-18 First clinical investigation of CDK7 inhibitor with an immunotherapy Key Milestones: Roche plans for SY-5609/atezolizumab arm of its Phase 1/1b INTRINSIC trial to be open for enrollment in 1H 2022 Roche is the sponsor of the trial and Syros is supplying SY-5609
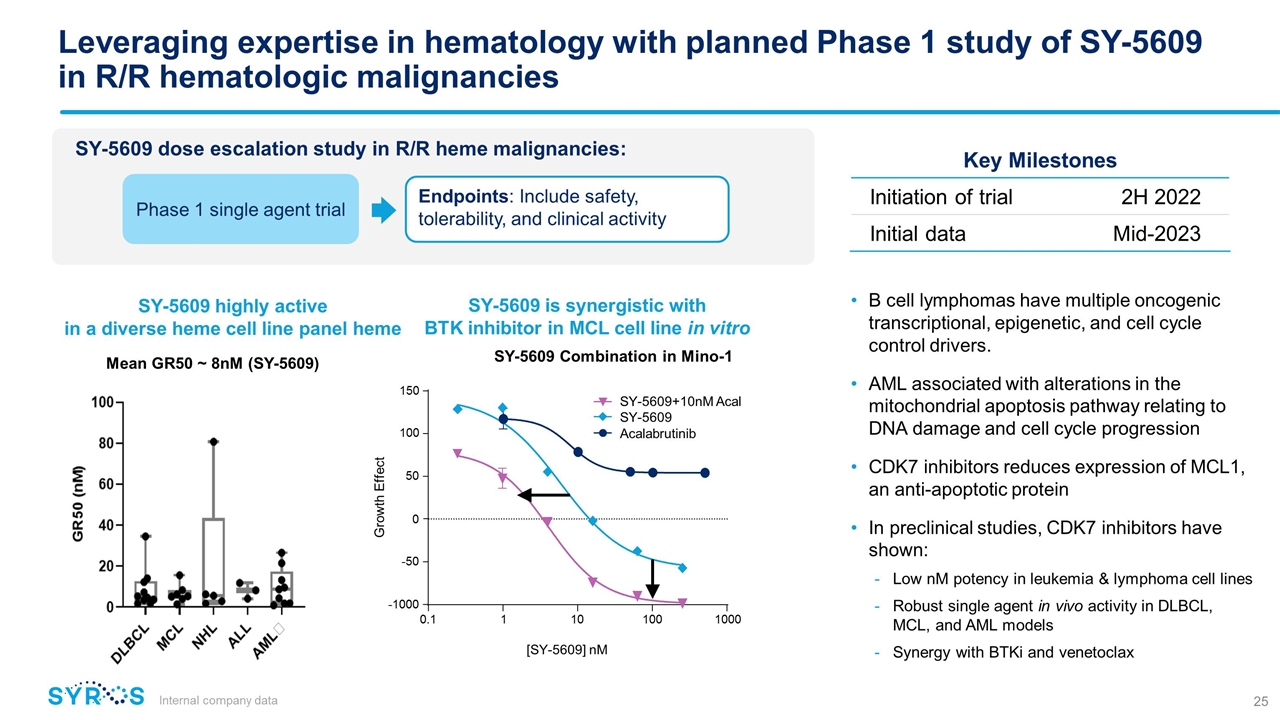
Leveraging expertise in hematology with planned Phase 1 study of SY-5609 in R/R hematologic malignancies Internal company data B cell lymphomas have multiple oncogenic transcriptional, epigenetic, and cell cycle control drivers. AML associated with alterations in the mitochondrial apoptosis pathway relating to DNA damage and cell cycle progression CDK7 inhibitors reduces expression of MCL1, an anti-apoptotic protein In preclinical studies, CDK7 inhibitors have shown: Low nM potency in leukemia & lymphoma cell lines Robust single agent in vivo activity in DLBCL, MCL, and AML models Synergy with BTKi and venetoclax SY-5609 dose escalation study in R/R heme malignancies: Endpoints: Include safety, tolerability, and clinical activity Phase 1 single agent trial Key Milestones Initiation of trial 2H 2022 Initial data Mid-2023 SY-5609 highly active in a diverse heme cell line panel heme SY-5609 is synergistic with BTK inhibitor in MCL cell line in vitro SY-5609 Combination in Mino-1 Mean GR50 ~ 8nM (SY-5609) 50 0.1 1 10 100 1000 -1000 -50 0 100 150 Growth Effect SY-5609+10nM Acal SY-5609 Acalabrutinib [SY-5609] nM

Gene Control Discovery Engine
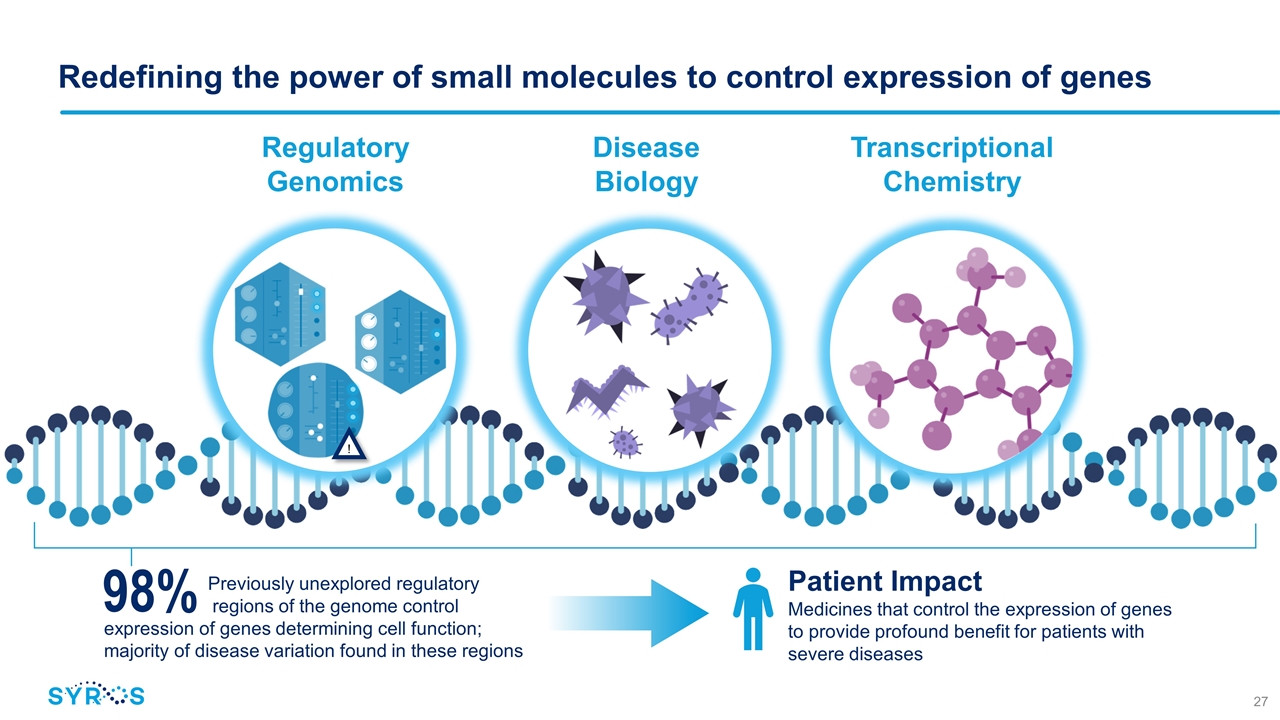
Previously unexplored regulatory regions of the genome control expression of genes determining cell function; majority of disease variation found in these regions 98% Patient Impact Medicines that control the expression of genes to provide profound benefit for patients with severe diseases Redefining the power of small molecules to control expression of genes ! Regulatory Genomics Disease Biology Transcriptional Chemistry
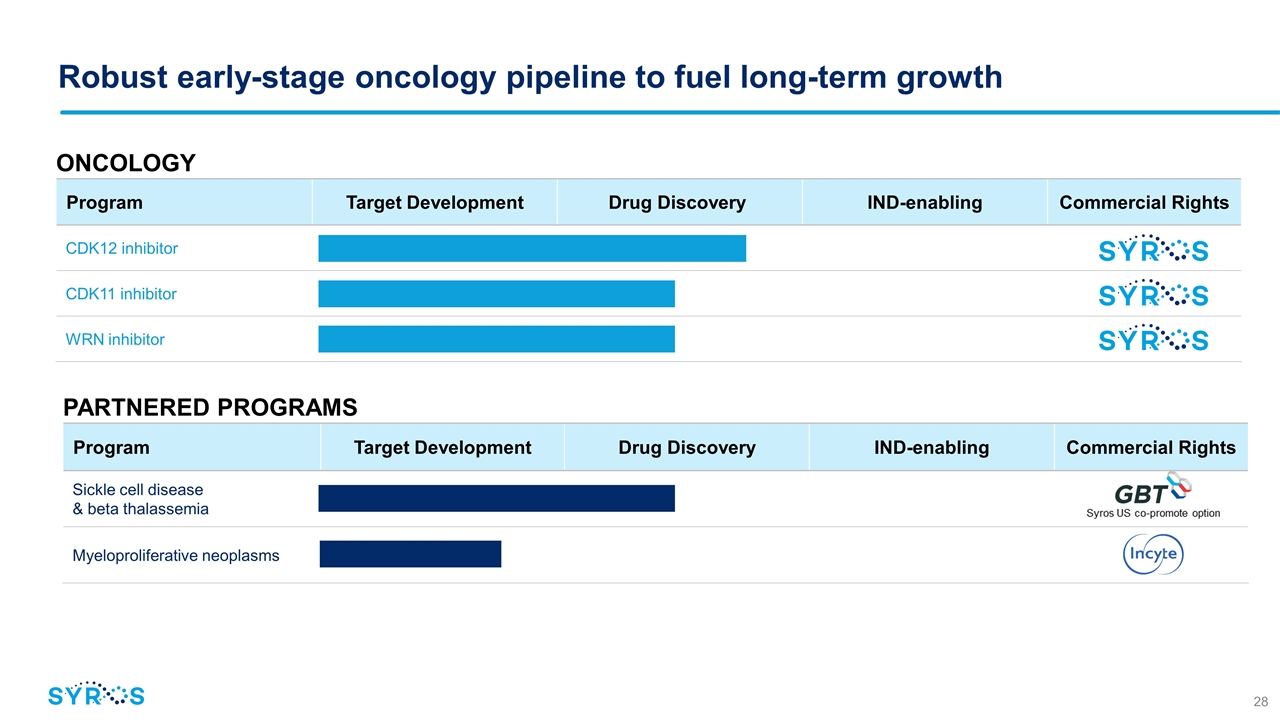
PARTNERED PROGRAMS Program Target Development Drug Discovery IND-enabling Commercial Rights Sickle cell disease & beta thalassemia Myeloproliferative neoplasms Robust early-stage oncology pipeline to fuel long-term growth ONCOLOGY Program Target Development Drug Discovery IND-enabling Commercial Rights CDK12 inhibitor CDK11 inhibitor WRN inhibitor
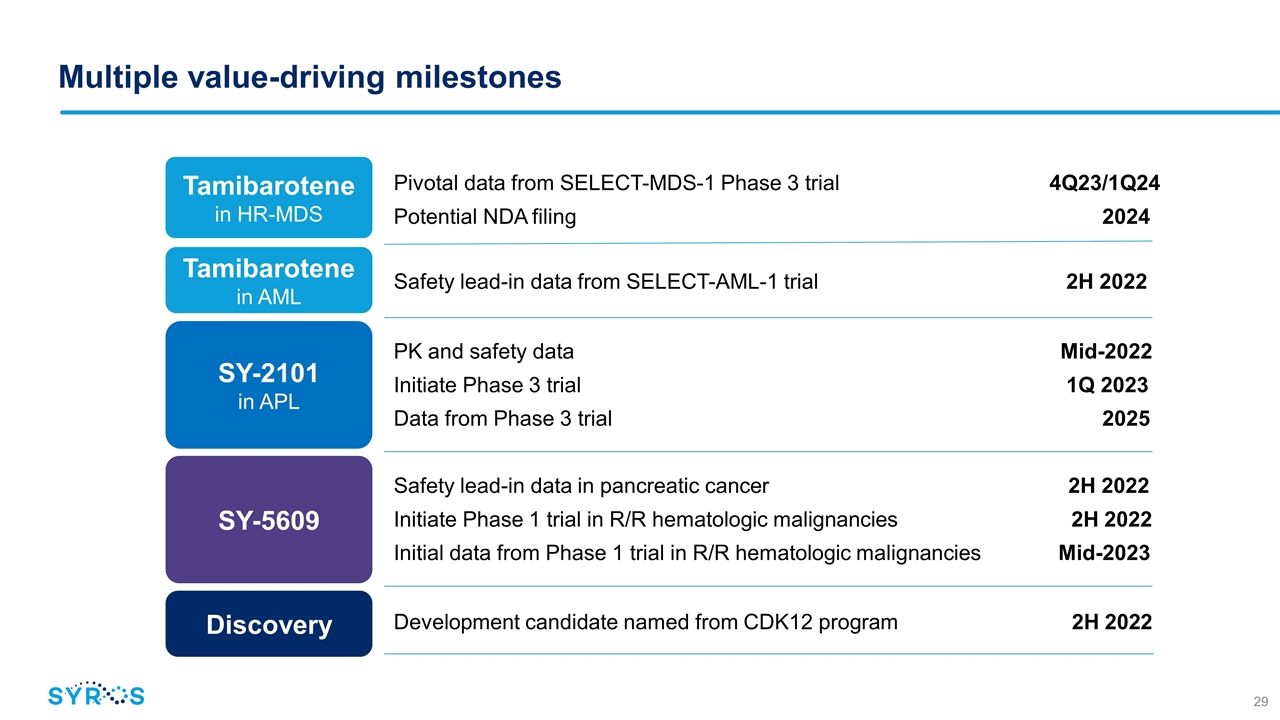
Multiple value-driving milestones SY-5609 SY-2101 in APL Tamibarotene in HR-MDS Tamibarotene in AML Pivotal data from SELECT-MDS-1 Phase 3 trial 4Q23/1Q24 Potential NDA filing 2024 Safety lead-in data from SELECT-AML-1 trial 2H 2022 PK and safety data Mid-2022 Initiate Phase 3 trial 1Q 2023 Data from Phase 3 trial 2025 Safety lead-in data in pancreatic cancer 2H 2022 Initiate Phase 1 trial in R/R hematologic malignancies 2H 2022 Initial data from Phase 1 trial in R/R hematologic malignancies Mid-2023 Discovery Development candidate named from CDK12 program 2H 2022
Rapidly advancing toward our vision Preparing for product launches Platform fueling robust pipeline Fully integrated company with medicines that provide a profound benefit for patients Advancing growing clinical-stage pipeline Advancing in late-stage development Capital to fund planned operations into 2023 Now Next Vision

Appendix
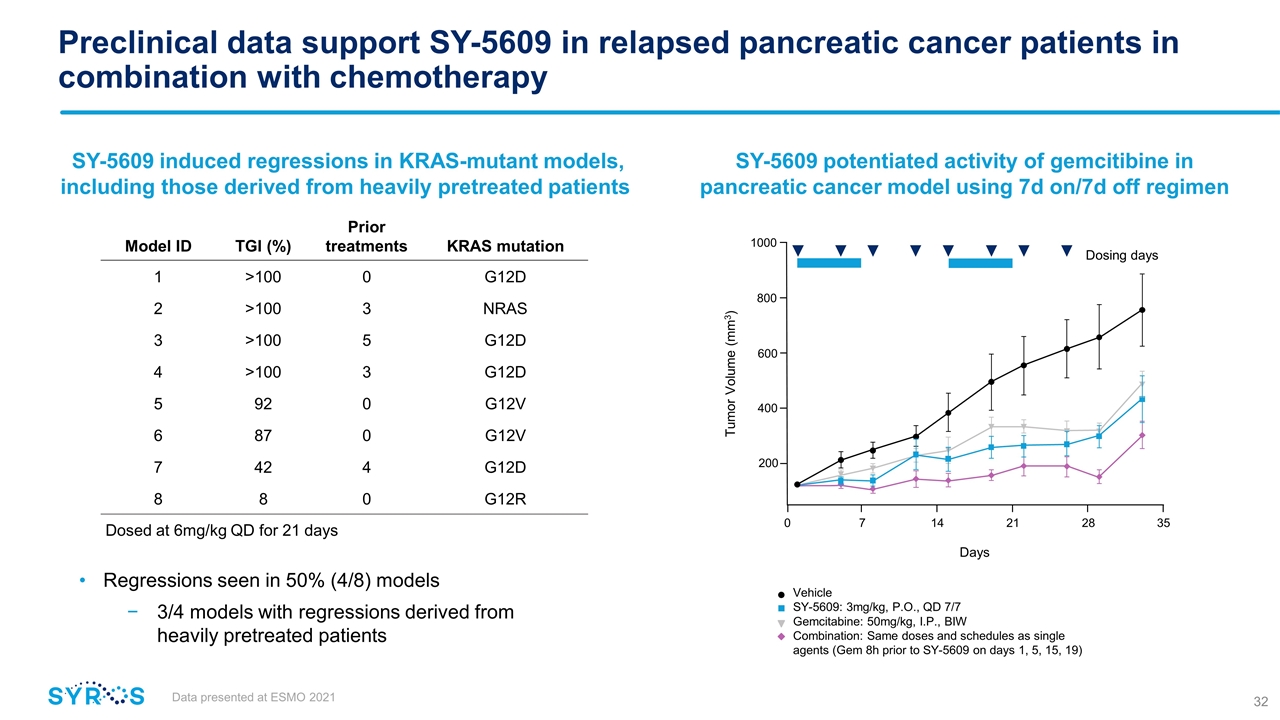
Preclinical data support SY-5609 in relapsed pancreatic cancer patients in combination with chemotherapy SY-5609 potentiated activity of gemcitibine in pancreatic cancer model using 7d on/7d off regimen Vehicle SY-5609: 3mg/kg, P.O., QD 7/7 Gemcitabine: 50mg/kg, I.P., BIW Combination: Same doses and schedules as single agents (Gem 8h prior to SY-5609 on days 1, 5, 15, 19) Data presented at ESMO 2021 SY-5609 induced regressions in KRAS-mutant models, including those derived from heavily pretreated patients Regressions seen in 50% (4/8) models 3/4 models with regressions derived from heavily pretreated patients Model ID TGI (%) Prior treatments KRAS mutation 1 >100 0 G12D 2 >100 3 NRAS 3 >100 5 G12D 4 >100 3 G12D 5 92 0 G12V 6 87 0 G12V 7 42 4 G12D 8 8 0 G12R Dosed at 6mg/kg QD for 21 days 1000 800 600 400 200 Tumor Volume (mm3) 0 7 14 21 28 35 Dosing days Days

































