Exhibit 99.1

Private And Confidential. © 2023 Organicell Regenerative Medicine, Inc. All rights reserved. Investor Presentation June 2023 1

Private And Confidential. © 2023 Organicell Regenerative Medicine, Inc. All rights reserved. No Offering of Securities No offer is made by this Investor Presentation (this "Presentation") to invest in Organicell Regenerative Medicine, Inc .. (the "Company") or to purchase any of its securities . Any offer to make such an investment or purchase and such investment or purchase will be made only pursuant to definitive offering documentation furnished by the Company . Forward - Looking Statements This Presentation includes statements that are, or may be deemed, “forward - looking statements” and information within the “safe harbor” provisions of the Private Securities Litigation Reform Act of 1995 , Section 27 A of the Securities Act of 1933 , as amended (the “Securities Act”) and Section 21 E of the Securities Exchange Act of 1934 , as amended (the “Exchange Act”) . In some cases, you can identify forward - looking statements by terminology including “anticipates,” “believes,” “can,” “continue,” “could,” “estimates,” “expects,” “intends,” “may,” “plans,” “potential,” “predicts,” “should,” “will,” “would” or the negative thereof, other variations thereon or other comparable terminology . We operate in a very competitive and rapidly - changing environment and new risks emerge from time to time . As a result, it is not possible for our management to predict all risks, nor can we assess the impact of all factors on the Company’s business or the extent to which any factor, or combination of factors, may cause actual results to differ materially from those contained in any forward - looking statements we may make . In light of these risks, uncertainties and assumptions, the forward - looking events and circumstances discussed in this Presentation may not occur and actual results could differ materially and adversely from those anticipated or implied in the forward - looking statements . You are cautioned not to place undue reliance upon such forward looking statements as predictions of future events . Although we believe that the expectations reflected in the forward - looking statements are reasonable, we cannot guarantee that the future results, levels of activity, performance or events and circumstances reflected in the forward - looking statements will be achieved or occur . We direct you to the Company’s Annual Report on Form 10 - K for the year ended October 31 , 2022 , our Quarterly Report on Form 10 - Q for the quarter ended January 31 , 2023 , and our current reports on Form 8 - K and our other filings with the Securities and Exchange Commission (the “SEC”) under the Securities Act and the Exchange Act, that may be filed subsequent to the date of this presentation . You may view and obtain these documents at the SEC’s website at www . sec . gov . Any forward - looking statement included in this Presentation speaks only as of the date hereof . Except as required by law, we do not undertake any obligation to update or revise, or to publicly announce any update or revision to, any of the forward - looking statements, whether as a result of new information, future events or any other reason after the date of this Presentation . Disclaimers 2 2
Private And Confidential. © 2023 Organicell Regenerative Medicine, Inc. All rights reserved. Our Mission To transform medicine by developing novel regenerative biologic therapies and nano - technologies to address a broad range of critical medical conditions 3 3
Private And Confidential. © 2023 Organicell Regenerative Medicine, Inc. All rights reserved. Brief History of Regenerative Medicine Definition: Regenerative Medicine replaces or regenerates human cells, tissue or organs, to restore normal function. • Only a few human cell types regenerate naturally : blood cells, liver tissue, skin, fingertip up to age 11 • It’s a novel field of medicine which holds the promise of curing or effectively treating diseases related to aging or injury • 1954: First kidney replacement – risk of rejection reduced later by cyclosporin • 1968: First bone marrow transplant • 1983 Exosomes discovered - we have only understood their function over the last 5 - 7 years • 1998: Isolation of human stem cells • 2007: Discovery of stems cells derived from amniotic fluid and placenta • 2017: First two gene therapies approved by FDA for two rare diseases 4
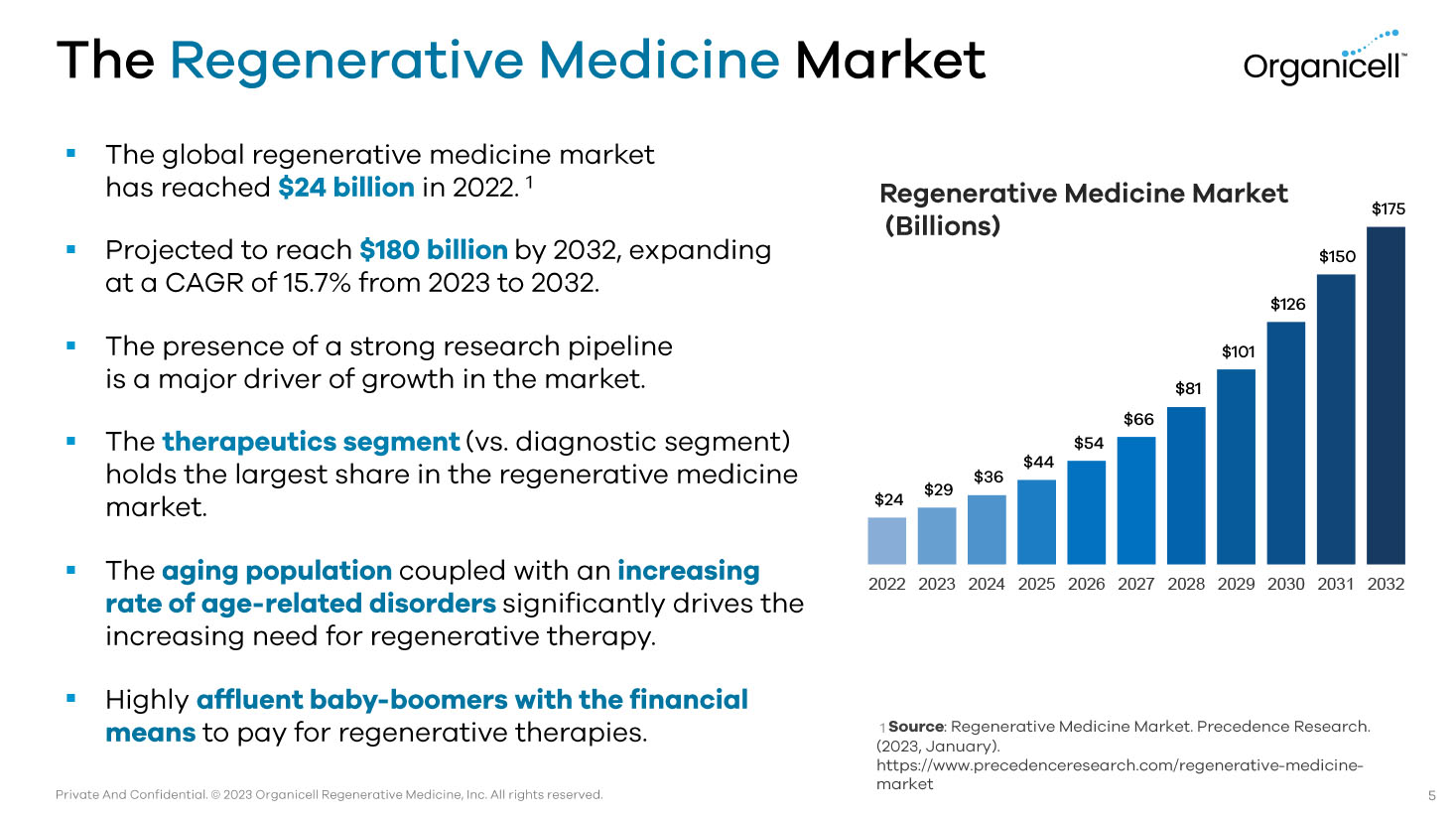
Private And Confidential. © 2023 Organicell Regenerative Medicine, Inc. All rights reserved. The Regenerative Medicine Market ▪ The global regenerative medicine market has reached $24 billion in 2022. 1 ▪ Projected to reach $180 billion by 2032, expanding at a CAGR of 15.7% from 2023 to 2032. ▪ The presence of a strong research pipeline is a major driver of growth in the market. ▪ The therapeutics segment (vs. diagnostic segment) holds the largest share in the regenerative medicine market. ▪ The aging population coupled with an increasing rate of age - related disorders significantly drives the increasing need for regenerative therapy. ▪ Highly affluent baby - boomers with the financial means to pay for regenerative therapies. $24 $29 $36 $44 $54 $66 $81 $101 $126 $150 $175 2022 2023 2024 2025 2026 2027 2028 2029 2030 2031 2032 Regenerative Medicine Market (Billions) 1 Source : Regenerative Medicine Market. Precedence Research. (2023, January). https:// www.precedenceresearch.com /regenerative - medicine - market 5

Private And Confidential. © 2023 Organicell Regenerative Medicine, Inc. All rights reserved. Types of Cellular/Tissue Based Therapies 1. Amniotic fluid - based therapies ( Organicell ) x Technology evaluated since 1910 for safety and efficacy x Tissue processed from human amniotic membrane and fluid, donated by consenting mothers delivering a full - term healthy baby by scheduled Caesarean section x Avoids any ethical or moral concerns, has proven safety record, studies document success in a multitude of systemic and local pathologies 2. Peripheral blood derived therapies (i.e., platelet rich plasma and PPX Ρ ) 3. Bone marrow - derived stem cell therapies 4. Adipose - derived stromal vascular fraction 5. Placental cell therapies 6 Organicell does not use these therapeutics as they have significant manufacturing, regulatory, and clinical challenges

Private And Confidential. © 2023 Organicell Regenerative Medicine, Inc. All rights reserved. Organicell Snapshot Organicell Regenerative Medicine, Inc. A publicly - traded: (OCEL), clinical - stage biopharmaceutical company committed to the research, development, and manufacture of novel regenerative technologies . Unlike most biotech companies, Organicell has: x Multiple opportunities to grow current revenue streams to reduce need for lower valuation capital while conducting the requisite R&D studies to gain FDA approval for our biologic/nanoparticle therapeutics for multiple major diseases. x 5 FDA INDs awarded for clinical trials for COVID, long - haul COVID, knee osteoarthritis, and COPD. x The ability to leverage our technology to create therapies for other serious conditions. 7
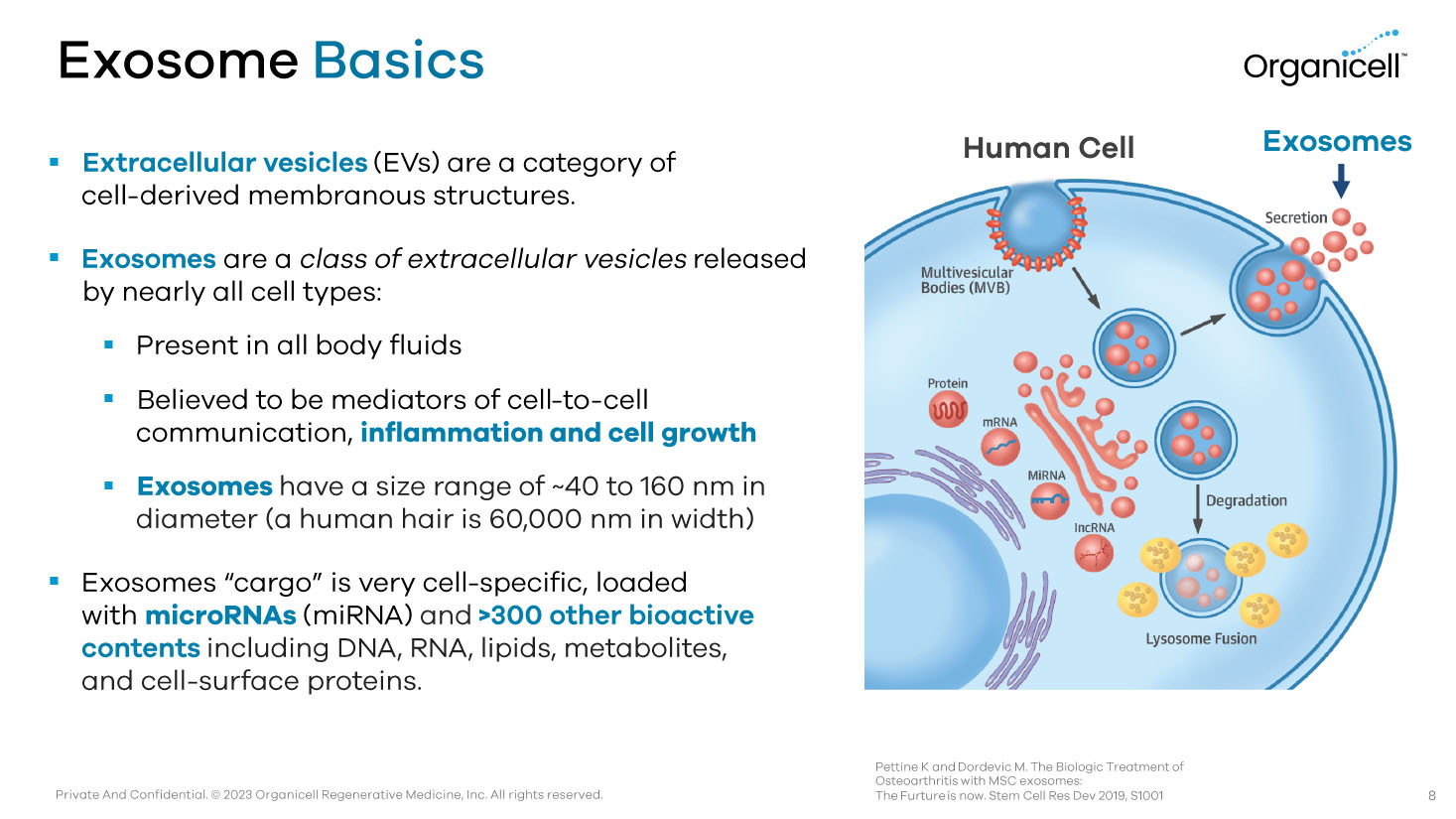
Private And Confidential. © 2023 Organicell Regenerative Medicine, Inc. All rights reserved. Exosome Basics ▪ Extracellular vesicles (EVs) are a category of cell - derived membranous structures. ▪ Exosomes are a class of extracellular vesicles released by nearly all cell types: ▪ Present in all body fluids ▪ Believed to be mediators of cell - to - cell communication, inflammation and cell growth ▪ Exosomes have a size range of ~40 to 160 nm in diameter (a human hair is 60,000 nm in width) ▪ Exosomes “cargo” is very cell - specific, loaded with microRNAs (miRNA) and >300 other bioactive contents including DNA, RNA, lipids, metabolites, and cell - surface proteins. Pettine K and Dordevic M. The Biologic Treatment of Osteoarthritis with MSC exosomes: The Furture is now. Stem Cell Res Dev 2019, S1001 8 Exosomes Human Cell
Private And Confidential. © 2023 Organicell Regenerative Medicine, Inc. All rights reserved. Organicell Technology Platform Exosomes are derived from Amniotic Fluid in a proprietary manner ▪ Very high concentrations of exosomes (i.e., billions per milliliter) ▪ Amniotic fluid is immunologically “neutral” ▪ S calable technology that overcomes the many challenges of working with stem cells Supercharged Anti - Inflammatory Effects ▪ Patent pending process enables the company to concentrate up to 600 billion EVs/Exosomes per milliliter, containing bioactive “cargo” aimed at modulating inflammation Clinical Research to obtain FDA approval for specific conditions ▪ 5 IND’s – Investigative New Drug Filing – approved ▪ 18 Emergency Investigational New Drug applications issued for COVID patients ▪ 2 Clinical trials: ▪ Long - Covid (Enrolling) ▪ Moderate/severe COVID (Completed) Potential of exosome efficacy for immediate commercial applications x PPX Œ autologous exosomes for musculoskeletal conditions x Skin micro - needling to reduce wrinkles by increasing elastin & collagen (via partnership) x Topical dermatological applications to improve skin appearance for commercial sale What Makes Organicell’s Technology Special? 9 9

Private And Confidential. © 2023 Organicell Regenerative Medicine, Inc. All rights reserved. Product Overview x Amniotic Fluid - derived Biologic x Non - HCP/T* x Dose contains approx. 200 billion extracellular vesicles/exosomes x Contains extracellular vesicles, exosomes, bioactive proteins, and hyaluronic acid x Exosomes containing >100 miRNAs and signaling proteins x Amniotic Fluid - derived Biologic x Non - HCP/T* x Pre - diluted mixture for Aesthetic use. x Dose contains approx. 100 - 200 billion extracellular vesicles. x Contains extracellular vesicles, exosomes, proteins, and hyaluronic acid x Exosomes containing >100 miRNA and signaling proteins x Concentrated Amniotic Fluid - derived Biologic. x Non - HCP/T* x Dose contains approx. 400 - 700 billion extracellular vesicles/exosomes x Contains extracellular vesicles, exosomes, bioactive proteins, and hyaluronic acid x Exosomes containing >100 miRNAs and signaling proteins PLATINUM Ƴ Ƴ x Autologous Blood - derived Biologic x Non - HCP/T* x Concentrated EV’s and exosomes from the patient’s own blood . x Acellular product, does not contain platelets like traditional PRP preparations x Dose contains approx. 200 billion nanoparticles and extracellular vesicles Ƴ 10 * Non - human cell, tissue, cellular and tissue - based product
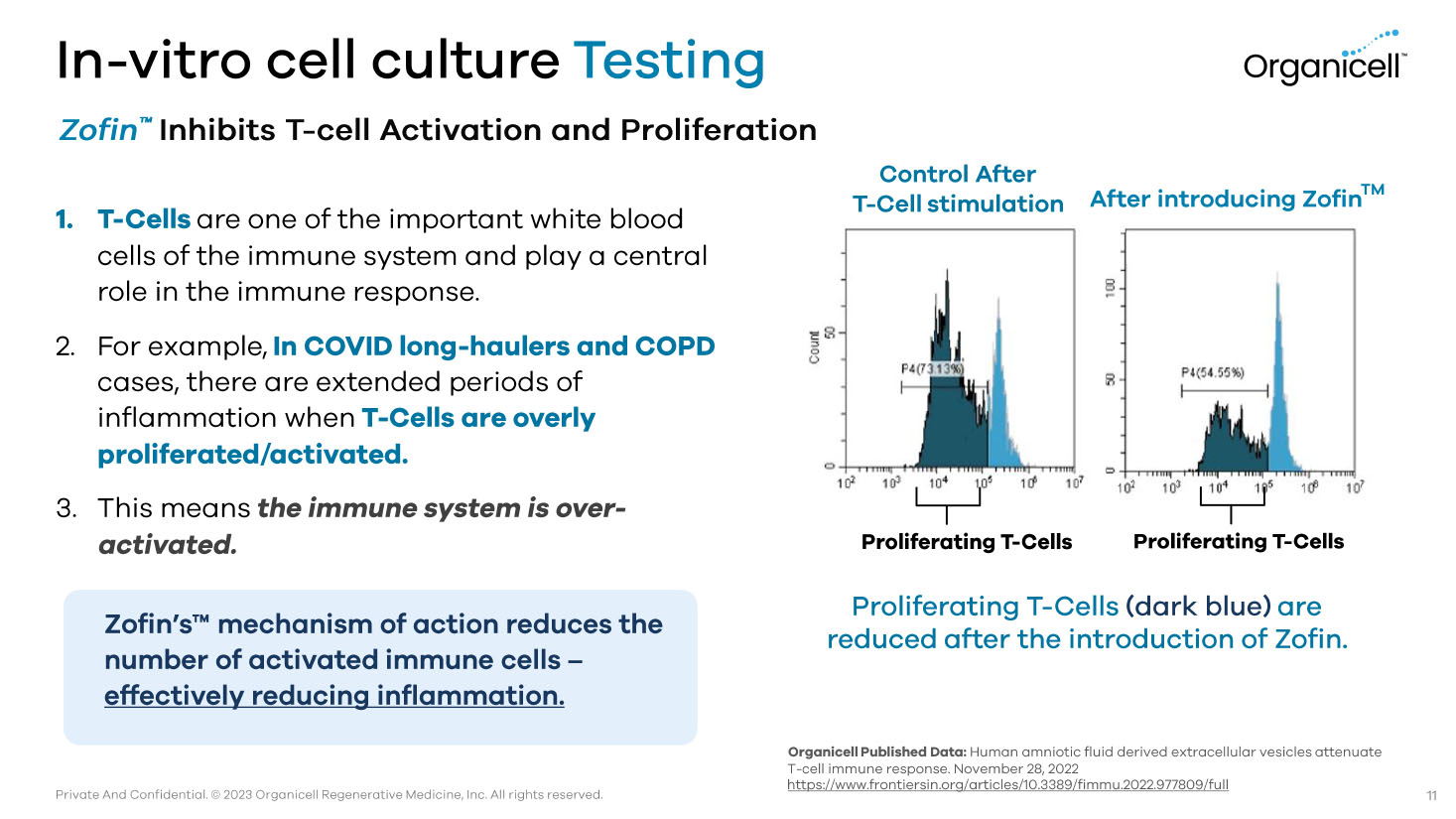
Private And Confidential. © 2023 Organicell Regenerative Medicine, Inc. All rights reserved. In - vitro cell culture Testing Zofin Ƴ Inhibits T - cell Activation and Proliferation 1. T - Cells are one of the important white blood cells of the immune system and play a central role in the immune response. 2. For example, In COVID long - haulers and COPD cases, there are extended periods of inflammation when T - Cells are overly proliferated/activated. 3. This means the immune system is over - activated. Zofin’s Ƴ mechanism of action reduces the number of activated immune cells – effectively reducing inflammation. Organicell Published Data: Human amniotic fluid derived extracellular vesicles attenuate T - cell immune response. November 28, 2022 https://www.frontiersin.org/articles/10.3389/fimmu.2022.977809/full Control After T - Cell stimulation After introducing Zofin TM Proliferating T - Cells Proliferating T - Cells Proliferating T - Cells (dark blue) are reduced after the introduction of Zofin . 11
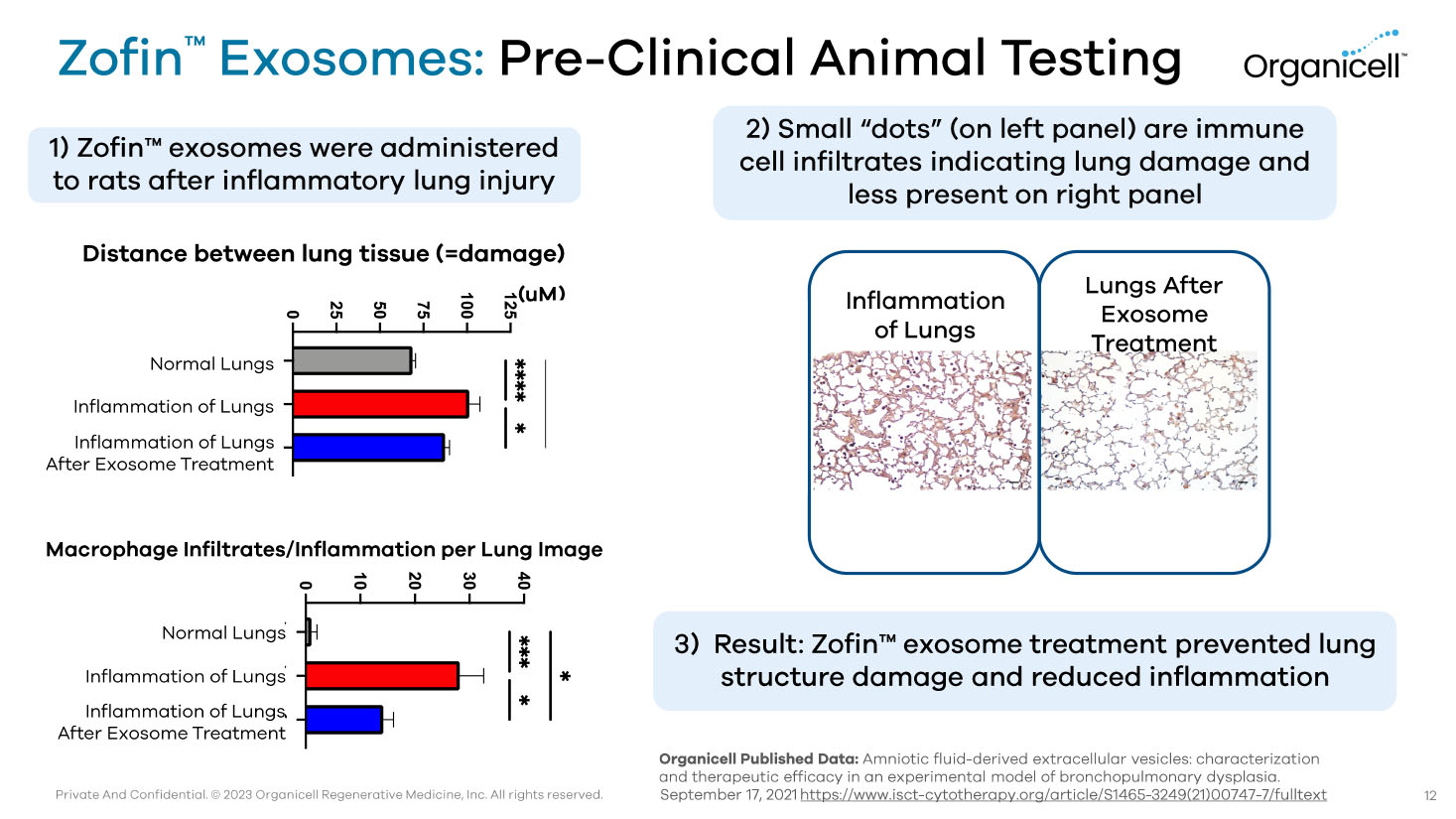
Private And Confidential. © 2023 Organicell Regenerative Medicine, Inc. All rights reserved. Zofin Ƴ Exosomes: Pre - Clinical Animal Testing Normal Lungs Inflammation of Lungs Inflammation of Lungs After Exosome Treatment Inflammation of Lungs Lungs After Exosome Treatment Organicell Published Data: Amniotic fluid - derived extracellular vesicles: characterization and therapeutic efficacy in an experimental model of bronchopulmonary dysplasia. September 17, 2021 https://www.isct - cytotherapy.org/article/S1465 - 3249(21)00747 - 7/fulltext Normal Lungs Inflammation of Lungs Inflammation of Lungs After Exosome Treatment Distance between lung tissue (=damage) ( uM ) Macrophage Infiltrates/Inflammation per Lung Image 12 1) Zofin Ƴ exosomes were administered to rats after inflammatory lung injury 2) Small “dots” (on left panel) are immune cell infiltrates indicating lung damage and less present on right panel 3) Result: Zofin Ƴ exosome treatment prevented lung structure damage and reduced inflammation
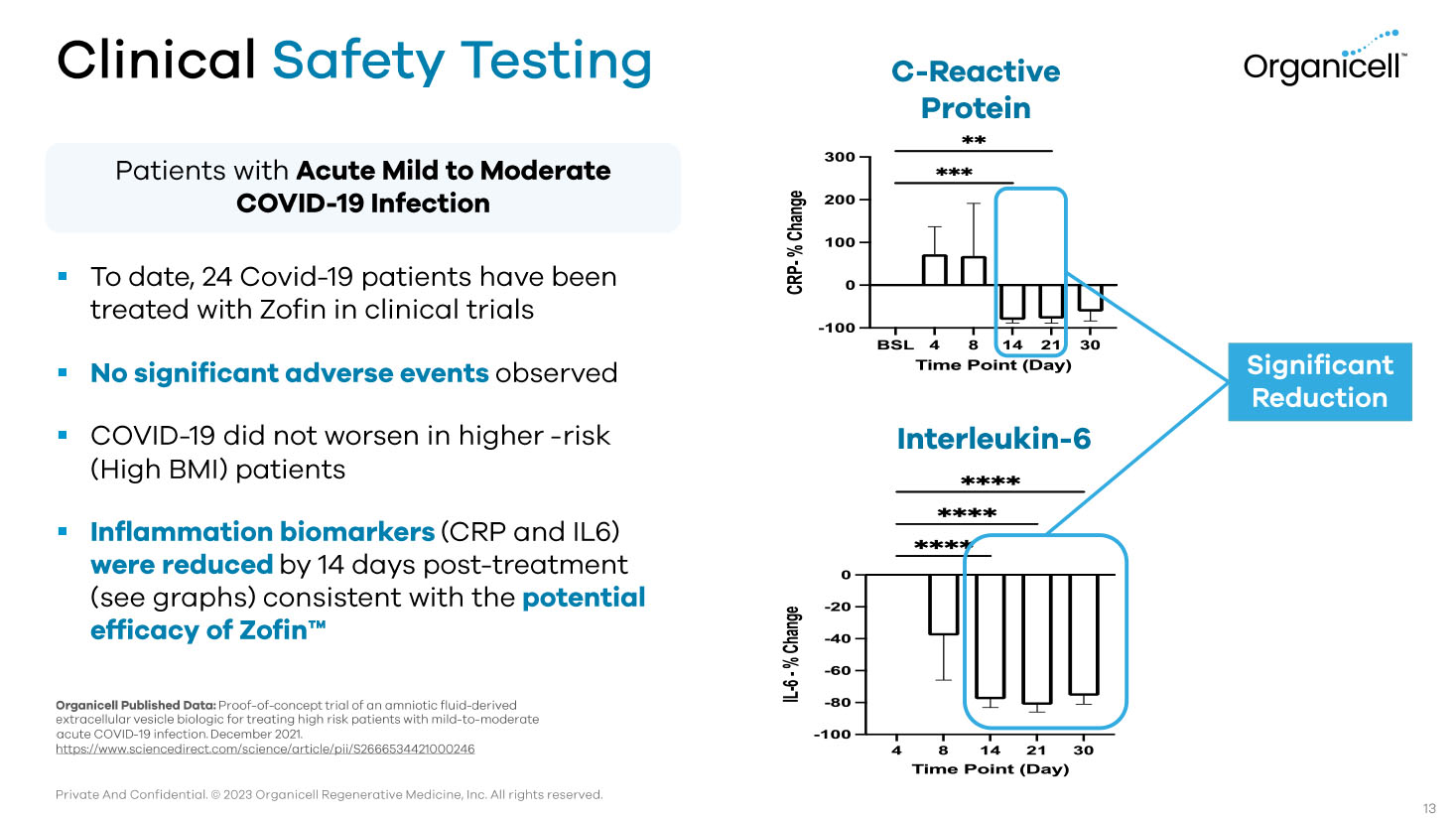
Private And Confidential. © 2023 Organicell Regenerative Medicine, Inc. All rights reserved. Clinical Safety Testing Organicell Published Data: Proof - of - concept trial of an amniotic fluid - derived extracellular vesicle biologic for treating high risk patients with mild - to - moderate acute COVID - 19 infection. December 2021. https://www.sciencedirect.com/science/article/pii/S2666534421000246 Significant Reduction ▪ To date, 24 Covid - 19 patients have been treated with Zofin in clinical trials ▪ No significant adverse events observed ▪ COVID - 19 did not worsen in higher - risk (High BMI) patients ▪ Inflammation biomarkers (CRP and IL6) were reduced by 14 days post - treatment (see graphs) consistent with the potential efficacy of Zofin Ƴ 13 C - Reactive Protein Interleukin - 6 Patients with Acute Mild to Moderate COVID - 19 Infection
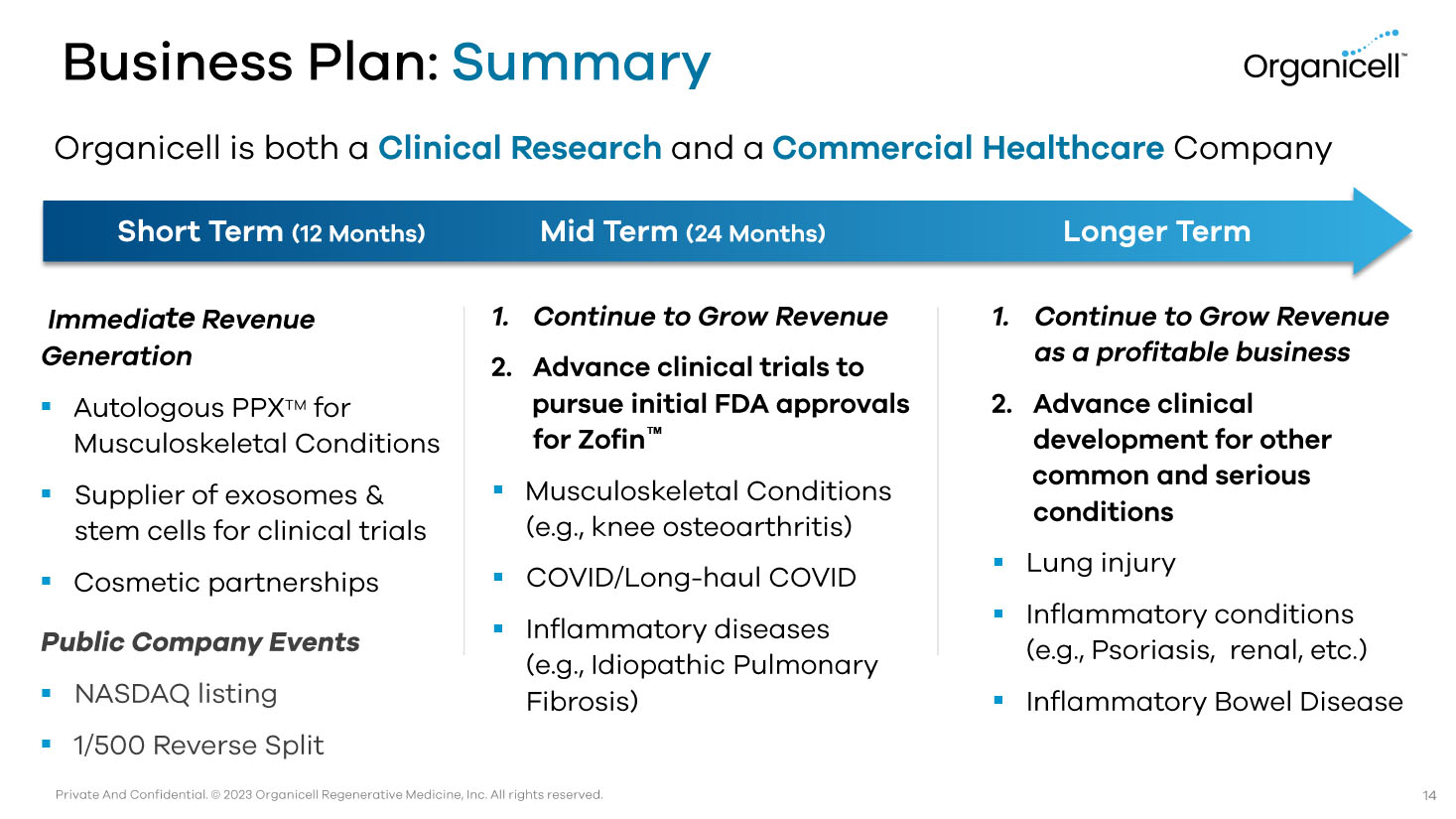
Private And Confidential. © 2023 Organicell Regenerative Medicine, Inc. All rights reserved. Business Plan: Summary Immedia te Revenue Generation ▪ Autologous PPX TM for Musculoskeletal Conditions ▪ Supplier of exosomes & stem cells for clinical trials ▪ Cosmetic partnerships Public Company Events ▪ NASDAQ listing ▪ 1/500 Reverse Split Short Term (12 Months) Mid Term (24 Months) 1. Continue to Grow Revenue 2. Advance clinical trials to pursue initial FDA approvals for Zofin Œ ▪ Musculoskeletal Conditions (e.g., knee osteoarthritis) ▪ COVID/Long - haul COVID ▪ Inflammatory diseases (e.g., Idiopathic Pulmonary Fibrosis) 1. Continue to Grow Revenue as a profitable business 2. Advance clinical development for other common and serious conditions ▪ Lung injury ▪ Inflammatory conditions (e.g., Psoriasis, renal, etc.) ▪ Inflammatory Bowel Disease Longer Term Organicell is both a Clinical Research and a Commercial Healthcare Company 14

Private And Confidential. © 2023 Organicell Regenerative Medicine, Inc. All rights reserved. Understanding Organicell Organicell i s comprised of two arms: Clinical Research and Commercial . To monetize our technology and lab assets through the sale of extracellular vesicle and stem cell (ex - US) products. 1. COVID - 19: Mild/moderate – Complete 2. COVID - 19: Mild/moderate Compassionate use: Ongoing 3. COVID - 19 Long Haulers – Enrolling 4. COVID - 19: Moderate/severe: Active, not recruiting 5. Osteoarthritis – H2 2023 6. COPD: IND approved 7. Additional Trials: In planning stages Our Commercial Products Clinical Research Commercial Products Ƴ FDA - Approved Clinical Trials Key Partnerships PLATINUM Ƴ Ƴ 15

Private And Confidential. © 2023 Organicell Regenerative Medicine, Inc. All rights reserved. Our Strong Distributor Networ k ▪ 8 US Distributors ▪ 2 International Distributors ▪ Partnership with Fountain Life TM 16
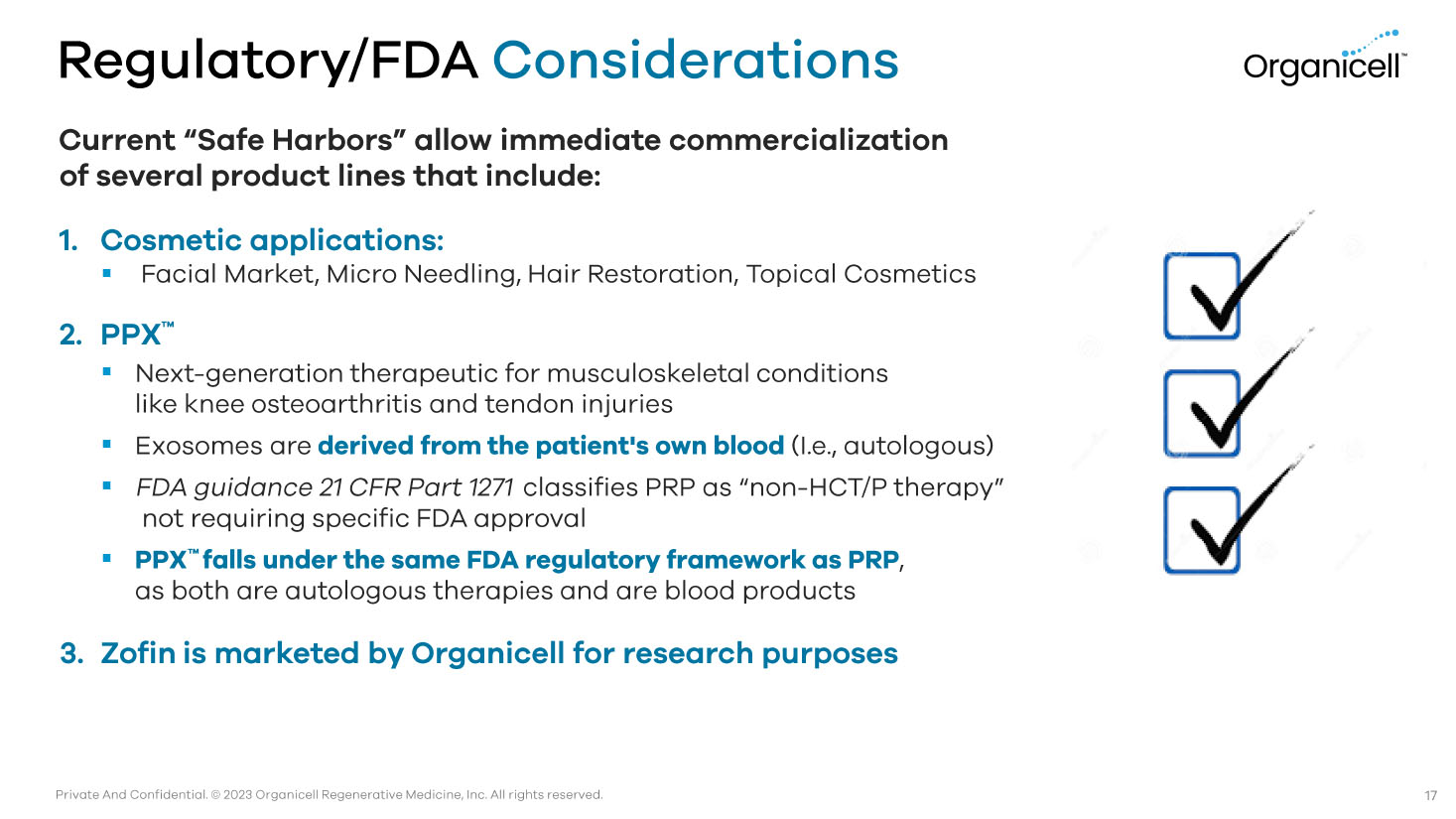
Private And Confidential. © 2023 Organicell Regenerative Medicine, Inc. All rights reserved. Regulatory/FDA Considerations Current “Safe Harbors” allow immediate commercialization of several product lines that include: 1. Cosmetic applications: ▪ Facial Market, Micro Needling, Hair Restoration, Topical Cosmetics 2. PPX Ƴ ▪ Next - generation therapeutic for musculoskeletal conditions like knee osteoarthritis and tendon injuries ▪ Exosomes are derived from the patient's own blood (I.e., autologous) ▪ FDA guidance 21 CFR Part 1271 classifies PRP as “non - HCT/P therapy” not requiring specific FDA approval ▪ PPX Ƴ falls under the same FDA regulatory framework as PRP , as both are autologous therapies and are blood products 3. Zofin is marketed by Organicell for research purposes 17
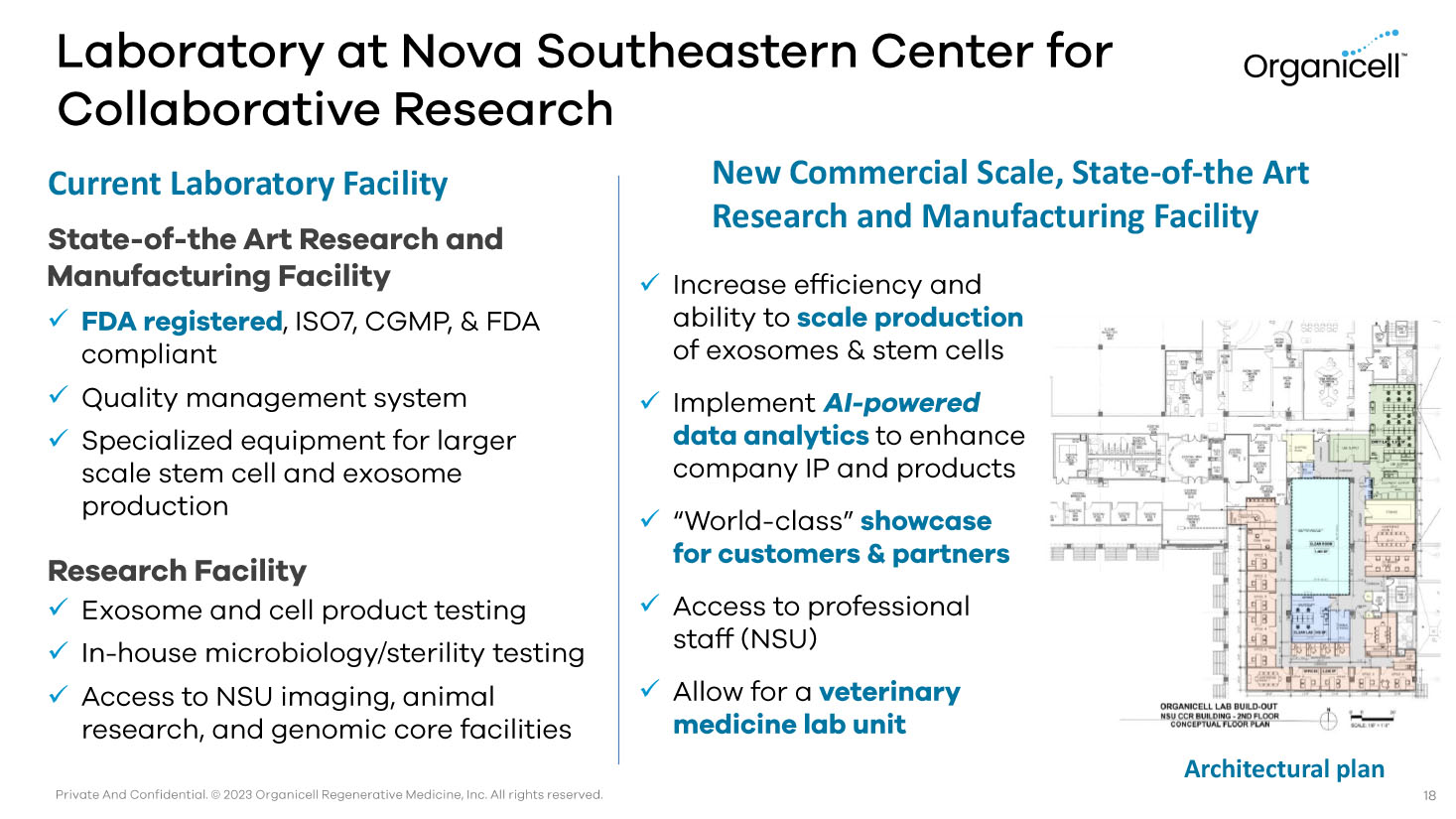
Private And Confidential. © 2023 Organicell Regenerative Medicine, Inc. All rights reserved. Laboratory at Nova Southeastern Center for Collaborative Research Current Laboratory Facility State - of - the Art Research and Manufacturing Facility x FDA registered , ISO7, CGMP, & FDA compliant x Quality management system x Specialized equipment for larger scale stem cell and exosome production Research Facility x Exosome and cell product testing x In - house microbiology/sterility testing x Access to NSU imaging, animal research, and genomic core facilities 18 x Increase efficiency and ability to scale production of exosomes & stem cells x Implement AI - powered data analytics to enhance company IP and products x “World - class” showcase for customers & partners x Access to professional staff (NSU) x Allow for a veterinary medicine lab unit New Commercial Scale, State - of - the Art Research and Manufacturing Facility Architectural plan
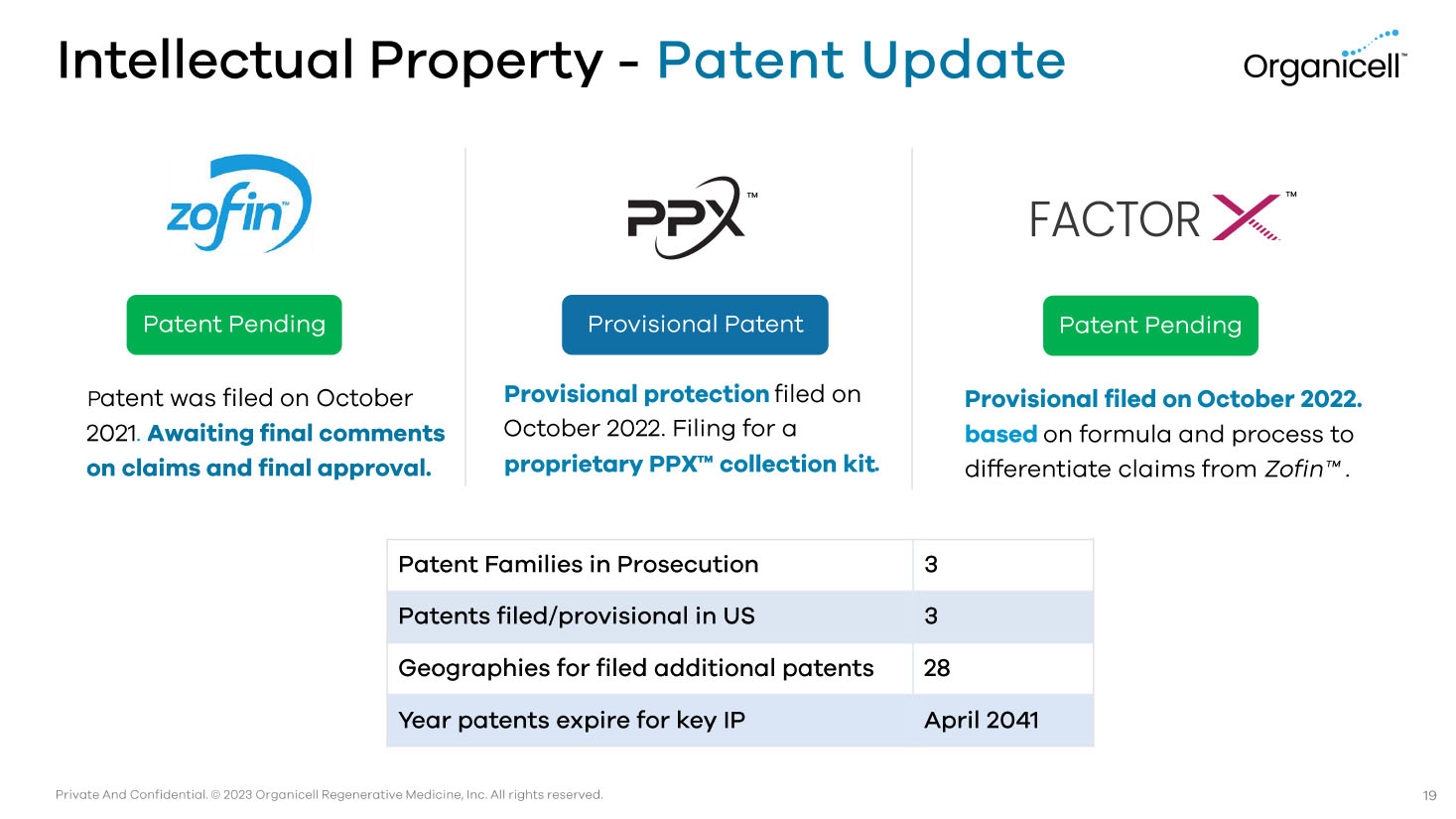
Private And Confidential. © 2023 Organicell Regenerative Medicine, Inc. All rights reserved. P atent was filed on October 2021 . Awaiting final comments on claims and final approval. Provisional protection filed on October 2022. Filing for a proprietary PPX Ƴ collection kit . Provisional filed on October 2022. based on formula and process to differentiate claims from Zofin Ƴ . Intellectual Property - Patent Update Patent Pending Provisional Patent Patent Pending 3 Patent Families in Prosecution 3 Patents filed/provisional in US 28 Geographies for filed additional patents April 2041 Year patents expire for key IP Ƴ Ƴ 19

Private And Confidential. © 2023 Organicell Regenerative Medicine, Inc. All rights reserved. Valuation of Biopharma Companies Acquisition Value of 311 Biopharma companies by stage of research from 2005 to 2020* 1. Before a biopharma company has an FDA approved product, company value is typically linked to achieving research milestones 2. A recent study showed that the mean value at acquisition of biopharma companies is: Pre - clinical stage: $88 million Phase 1: $354 million Phase 2: $683 million Phase 3: $1.8 billion FDA Approval of a lead therapy: $2.5 Billion 3. Biologics typically have higher valuations due to enhanced safety, efficacy and higher FDA - approval success rates *Reference. Value drivers of development stage biopharma companies. European Journal of Health Economics (2022) 23:1287 - 1296 Organicell currently is a Phase 1 company, and will be moving to Phase 2 research in early 2024 20
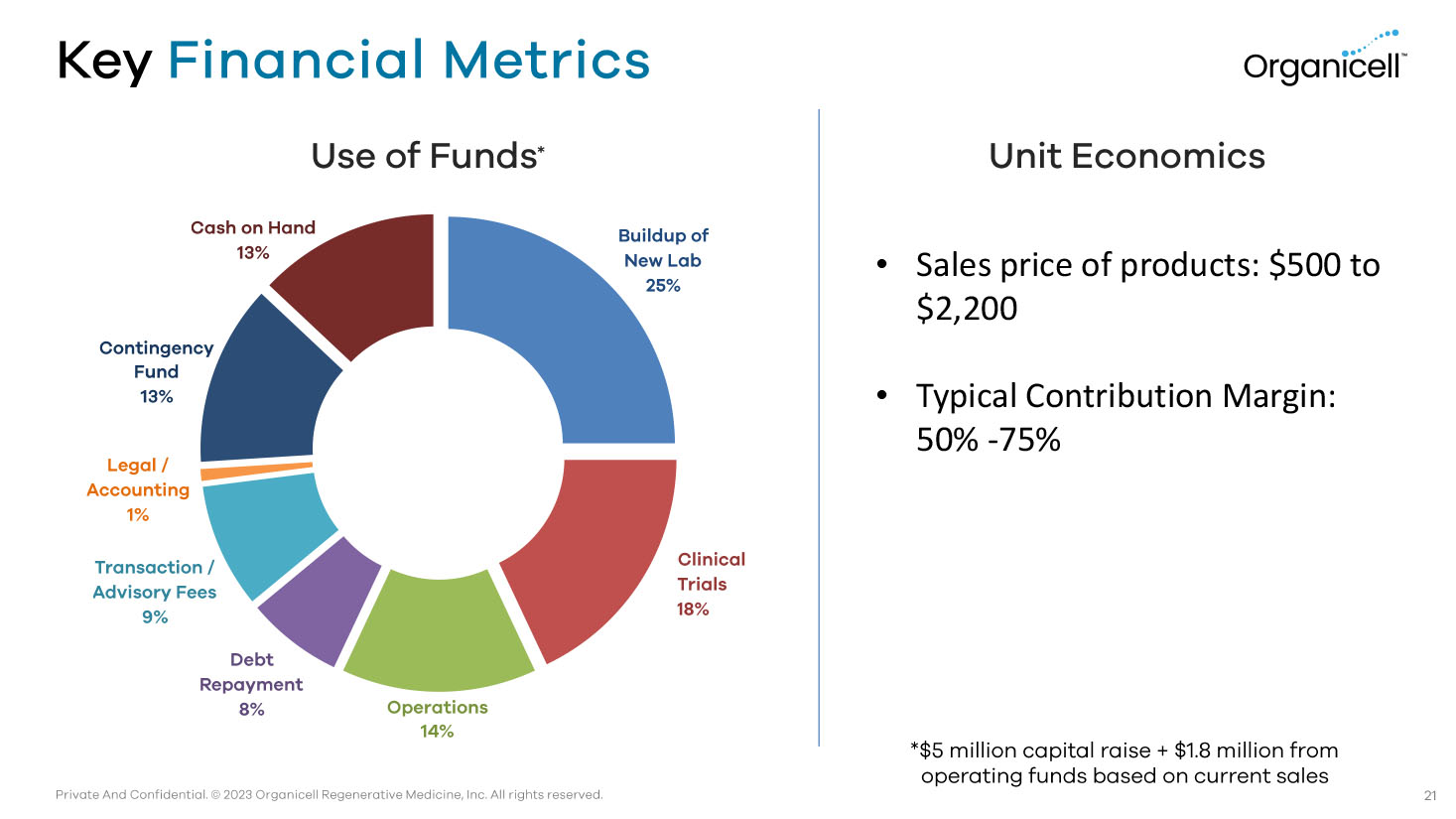
Private And Confidential. © 2023 Organicell Regenerative Medicine, Inc. All rights reserved. Key Financial Metrics Use of Funds * Unit Economics 21 *$5 million capital raise + $1.8 million from operating funds based on current sales Buildup of New Lab 25% Clinical Trials 18% Operations 14% Debt Repayment 8% Transaction / Advisory Fees 9% Legal / Accounting 1% Contingency Fund 13% Cash on Hand 13% • Sales price of products: $500 to $2,200 • Typical Contribution Margin: 50% - 75%

Private And Confidential. © 2023 Organicell Regenerative Medicine, Inc. All rights reserved. Organicell Leadership Team (see Bios in Appendix) Organicell’s world - class leadership team has broad experience in biotech, commercialization of healthcare products, and managing public healthcare companies George C. Shapiro, MD, FACP Chief Medical Officer Ian T. Bothwell Chief Financial Officer Michael A. Bellio , Ph.D Laboratory Director VP of Research and Manufacturing Ivan Santos Biologics Manufacturing Operations Manager Cassie Bennett, Ph.D Quality Assurance Harry Leider, MD, MBA Chief Executive Officer Jackie Domenech Vice President of Operations Howard Golub, MD, Ph.D EVP of Research & Development and Chief Science Officer 22

Private And Confidential. © 2023 Organicell Regenerative Medicine, Inc. All rights reserved. Organicell Medical Advisory Board Organicell’s distinguished Medical Advisory Board has deep expertise in regenerative medicine, anti - aging therapeutics, and innovative orthopedic solutions Mitchell B. Sheinkop , MD Professor Emeritus and former Director of Joint Replacement at Rush Medical Center Craig Ziering, DO Founding Board Member of American Board of Hair Restoration Surgeons Brian Kelly, MD President and Surgeon - in - Chief at the Hospital for Special Surgery Michale J. Barber, MD CEO and Chief Medical Officer of Better Life Age Management Paul Thompson, MD International Expert in Age Management and President of the Thompson Clinic Riley J. Williams III, MD Director of Institute for Cartilage Repair at Hospital for Special Surgery & Team Physician for Brooklyn Nets Julian Gershon, DO Aspen Institute of Anti - Aging and Regenerative Medicine Carolyn DeLucia , MD International Expert in Anti - Aging Therapies and Sexual Wellness William Kapp, MD Chairman and CEO of Landmark Hospitals and CEO of Fountain Life 23

Private And Confidential. © 2023 Organicell Regenerative Medicine, Inc. All rights reserved. Key Investment Considerations x Emerging biotech/commercial company with an advantageous business platform of current revenue streams and diversified clinical trials targeted at accessing large addressable markets x Very large and growing regenerative medicine total addressable market x Expanding market in musculoskeletal conditions, cosmetics, supplier for clinical trials x Five (5) FDA - approved trials/INDs, to date, and our therapeutics demonstrated as safe in humans x Attractive valuation relative to market size and benchmarks x Experienced leadership team with broad expertise in biotech, commercial healthcare products, and public company management 24

© 2023 Organicell Regenerative Medicine, Inc. All rights reserved. Contact: Harry Leider, MD, MBA 443 - 934 - 4906 Harry@organicell.com

Private And Confidential. © 2023 Organicell Regenerative Medicine, Inc. All rights reserved. Leadership Team Bios Harry Leider, MD, MBA Chief Executive Officer Harry Leider, MD, MBA joined Organicell as CEO in June of 2023. Dr. Leider, has over 25 years’ experience as a highly successful C - suite healthcare executive and serial entrepreneur in a variety of innovative high - growth companies. From 2018 to 2022, Dr. Leider was the Chief Medical Officer and Executive Vice President of Gelesis – a biotech company that successfully developed an FDA - cleared therapy for obesity and went public in 2022. Prior to this, he served as the Chief Medical Officer and Group Vice President of Fortune 50 - Walgreens Boots Alliance . Earlier, he was Chief Medical Officer & SVP of Ameritox - a leading national specialty lab company. He has served on the Board of Directors of Alivio Therapeutics, TytoCare , and Mobile Help. In addition, Dr. Leider has been on the faculty of Harvard Medical School and the John Hopkins Carey School of Business. He has published over 25 articles and book chapters on healthcare and leadership and holds 5 patents in laboratory medicine. He received his medical degree from the University of Pennsylvania, an MBA from the University of Washington where he was a Robert Wood Johnson Clinical Scholar, and his undergraduate degree from Pennsylvania State University where he received the most prestigious award at graduation for leadership, academics, and community service. Howard L. Golub, MD, Ph.D Executive Vice President/Chief Science Officer Dr. Howard L. Golub, MD, Ph.D was appointed Executive Vice President/Chief Science Officer in june , 2023. For over 10 years, Dr. Golub was a principal at Care - Safe LLC (a clinical research consulting company whose corporate clients included biotech/drug/medical device companies.) He also currently serves as one of the Clinical Leads for an N.I.H. program designed to develop and accelerate accurate COVID - 19 testing programs and bring them to market. From 2014 to 2016, he was Vice President of Research and Development at Walgreens Co. and developed an innovative program that utilized Walgreen’s massive customer database to power clinical trial patient recruitment. Previously, he was a founder and CEO of CareStat LLC, a 150 person CRO in the Boston Area, which was sold in 2008. Earlier, Dr. Golub served as a serial entrepreneur, where he was one of the founders, and participated as CEO for 3 healthcare companies, two of which were successfully sold. In addition, from 2003 to 2013 he held an adjunct professorship at the Harvard - M.I.T. joint M.S., M.B.A. program and taught a course entitled “Clinical Development for Private Enterprise.” Dr. Golub received his Ph.D. in biomedical engineering and an M.D. from that same Harvard Medical School - M.I.T. joint program. 26
Private And Confidential. © 2023 Organicell Regenerative Medicine, Inc. All rights reserved. Leadership Team Bios Ian T. Bothwell Chief Financial Officer Ian Bothwell joined Organicell as Chief Financial Officer in 2015. From 2003 through November 2015, Mr. Bothwell served in various executive positions for Central Energy GP LLC, the general partner of Central Energy Partners LP, a publicly traded master limited partnership. From July 2007 through November 2015, Mr. Bothwell served as President and a director of Regional Enterprises, Inc. Since April 2007, Mr. Bothwell has served as the President and controlling member of Rover Technologies, LLC, a company formed to provide management solutions to the public transportation industry. Since 2015, Mr. Bothwell has also served as the President and controlling member of CountOnMe Inc., a company that provides software solutions for the educational industry. Mr. Bothwell received his Bachelor of Science in Business Administration from Boston University in 1984 George C. Shapiro, MD FACP Chief Medical Officer Dr. Shapiro has practiced medicine for over 30 years, specializing in Internal Medicine, Cardiology and Age Management Medicine. He is a national expert in age management medicine and led one of the most prominent age management practices in the country as president of Cenegenics New York City. Always challenged by the need for innovative medicine, Dr. Shapiro has patented medical devices as well as explored and innovated new medical protocols. He has long been known as one of New York’s foremost cardiologists, specializing in regenerative medicine and improving longevity, including the genomics of cardiovascular disease. Dr. Shapiro was honored as the recipient of the 10th Annual Alan P. Mintz , M.D. Award, for Clinical Excellence in Age Management Medicine. He is also active in 13 medical societies, he has been a speaker for the Age Management Medicine Group (AMMG,), and is a long - standing member of the AMMG Conference Planning Committee. In addition, Dr. Shapiro was named 2016 - 2020 Top Doctor in New York City. Dr. Shapiro received his MD from New York Medical College. Followed by an internship and residency at Albert Einstein College of Medicine, and a fellowship at Columbia University College of Physicians and Surgeons 27

Private And Confidential. © 2023 Organicell Regenerative Medicine, Inc. All rights reserved. Leadership Team Bios Michael A. Bellio , Ph.D Laboratory Director & VP of Research and Manufacturing Dr. Bellio leads the research, development, and manufacturing of novel biological medicine for the treatment of pulmonary, cardiovascular, and orthopedic degenerative diseases. His teams’ mission is to advance the clinical development and commercialization of extracellular vesicle therapeutics to offer safe and effective solutions to treat diseases. Dr. Bellio earned a PhD in Molecular and Cellular Pharmacology at the University of Miami’s Interdisciplinary Stem Cell Institute (ISCI), where he received comprehensive training in cGMP manufacturing of primary stem cells and extracellular vesicles for pre - clinical and clinical trial applications. At Organicell , he is actively managing both the research and manufacturing arms of the organization while focusing on pharmacy management and the chemistry, manufacturing, and control (CMC) of Organicell’s products for their FDA approved IND clinical trial programs and applications. Jackie Domenech VP of Operations Jackie Domenech manages operations, accounting , and financial reporting for Organicell . Ms. Domenech has over 15 years of operations and accounting management experience with companies in healthcare, media, and technology. Prior to joining Organicell , Ms. Domenech was the Controller of Equisolve , an award - winning digital agency tailored to enhance investor relations. During her tenure, she led a successful transition from an outsourced accounting provider to creating an internal accounting division. She also served as Controller for various startups including MedicFP & MDLive . Most notable is MDLive , a telehealth company acquired by Cigna for $2 billion dollars in 2021. She is passionate about the art of entrepreneurship and has volunteered for an organization in South Florida that focuses on disrupting the market by assisting amateur entrepreneurs with developing proof of concepts and product development. Ms. Domenech received her BA in Accounting from the University of South Florida and plans to attain her CPA licensure in 2024. 28
Private And Confidential. © 2023 Organicell Regenerative Medicine, Inc. All rights reserved. Leadership Team Bios Ivan Santos Biologics Manufacturing Operations Manager Ivan Santos is the Biologics Manufacturing and Operations Manager at Organicell . Experienced in regenerative medicine, Ivan has a strong ability to translate design requirements into actionable plans. He brings valuable experience in biotechnology and neuroscience research and is a key contributor to the development of novel therapeutics at Organicell . Ivan is highly skilled with biologic quantification techniques such as ELISA assays, B radford analysis, flow cytometry, N anosight technology, and is proficient in biologic processing such as exosome isolation, and cell culturing and expansion. He also has extensive experience with MATLAB/Simulink, Wolfram Mathematics, and Python qualitative analysis programing and brings a high level of analytic expertise to Organicell . Cassie Bennett, Ph.D Quality Assurance Dr. Cassie Bennett holds a doctorate in biomedical engineering. She focuses on research, development, and project management within biomedical environments. Her accomplished work has led her to author and co - author numerous peer - reviewed papers, as well as several poster conference presentations. Dr. Bennett received her doctorate from The University of Miami, during which time she worked as a research assistant and lab manager until her graduation. After graduating, she joined the Organicell team to oversee quality control, the development of standard operating procedures, and quality assurance at the biomedical level. 29




























